- 1College of Animal Science and Technology, Yangzhou University, Yangzhou, China
- 2Laboratory Animal Center of the Academy of Military Medical Sciences, Beijing, China
- 3State Key Laboratory of Animal Nutrition, China Agricultural University, Beijing, China
- 4School of Food Science and Biotechnology, Zhejiang Gongshang University, Hangzhou, China
Either selenium or serine could modulate glucose homeostasis, however, whether there are synergistic effects of selenium with serine on diabetes remains to be unknown. In the present study, eight male db/m mice were used as a control, and 24 male diabetic db/db mice were either orally gavaged with PBS, or with selenomethionine alone, or with both selenomethionine and serine, to investigate the effects of selenomethionine and serine on body weight and glucose level. Furthermore, intestinal microbiota composition was analyzed and fecal microbiota transplantation (FMT) was performed to explore whether microbes mediate the beneficial effects of selenomethionine and serine. The results showed that administration of selenomethionine decreased body weight, adipose tissue weight and serum glucose level in db/db diabetic mice. Importantly, administration of selenomethionine in combination with serine exerted better effects than selenomethionine alone did. Furthermore, a combined administration of selenomethionine and serine restored the microbial composition in diabetic mice. Corynebacterium glutamicum, Bifidobacterium pseudolongum, and Aerococcus urinaeequi were significantly decreased, whereas Lactobacillus murinus was increased in mice in the selenomethionine group and selenomethionine in combination with serine group, when compared with those in the db/db group. FMT decreased body weight and glucose level in db/db mice, further indicating that microbes play critical roles in the beneficial effects of selenomethionine and serine. Thus, we concluded that administration of selenomethionine in combination with serine benefits diabetes via gut microbes. Our results suggested that the synergic application of selenomethionine and serine could be potentially used for the treatment of diabetes.
Introduction
Diabetes mellitus is a metabolic disease increasingly prevalent worldwide. It is characterized by long-term hyperglycemia which results in health problems for a long period. Although many drug portfolios are found to exert the effect of maintaining blood glucose concentrations, most of them are proved to have side effects. Consequently, although increasing new therapies have been successfully explored for diabetes treatment, the seek for novel treatment approaches which can better control glucose level and reduce related complications need to be continuously done.
Selenium, as an essential trace element, exerts critical roles in the maintenance of glucose homeostasis. A deficiency of selenium leads to increased glucose concentration in plasma in healthy rats and further elevated glucose concentration in diabetic individuals (Reddi and Bollineni, 2001). Supplementation with sodium selenite exerts insulin-like effects, and a high-dose of sodium selenite administrated acutely results in hypoglycemia (Becker et al., 1994; Stapleton, 2000). Importantly, selenomethionine, as an organic selenium source, was proved to involve in insulin secretion and glucose homeostasis (Zhao et al., 2021). However, no results have reported the in vivo beneficial effects of selenomethionine on diabetic individuals.
Either selenium or serine could modulate glucose homeostasis. Serine, as a metabolically indispensable amino acid, involved in purine and glutathione synthesis, one-carbon metabolic cycle and lipid metabolism (Zhou et al., 2017). Importantly, serine improves glucose tolerance and insulin sensitivity in high-fat diet-induced mice (Zhou et al., 2018). Interestingly, recent studies found that dietary serine had beneficial effects on nutritional status of selenium and selenoprotein activity (Long et al., 2021; He Y. et al., 2022; Zhou L. et al., 2022). Moreover, the synergistic effects of serine with selenium on the alleviation of oxidative stress were confirmed in vivo and in vitro (Wang et al., 2016; He L. et al., 2022). However, whether a combined administration of selenomethionine and serine has any effects on glucose homeostasis are not explored. It is hypothesized that administration of selenomethionine in combination with serine benefits diabetes via gut microbes. The present work has investigated both administration of selenomethionine alone and in combination with serine on body weight and glucose level in db/db diabetic mice. Furthermore, intestinal microbiota composition was analyzed and fecal microbiota transplantation (FMT) was performed to explore whether microbes mediate the beneficial effects of selenomethionine and serine.
Materials and methods
Animals and experimental design
Eight male db/m mice and 24 male db/db diabetic mice were purchased from Gene&Peace biotech Co., Ltd (Guangzhou, China). The db/m mice were orally gavaged with 0.2 mL PBS and used as control (db/m), while the db/db mice were either orally gavaged with 0.2 mL PBS (db/db), or with selenomethionine (0.16 mg/mL) dissolved in 0.2 mL PBS (SeMet), or with selenomethionine (0.16 mg/mL) and serine (0.20 g/mL) dissolved in 0.2 mL PBS (SeSer). The experiment lasted 21 days. During the experiment, all animals were free to access water and feed, and body weight (BW) was recorded. The study was approved by the Animal Care and Use Committee of China Agricultural University, and conformed to the Guide for the Care and Use of Laboratory Animals.
Sample collection
On day 21, blood samples were collected from the retro-orbital sinus and then centrifuged at 2,500 g at 4°C for 10 min to collect serum. All mice were executed by cervical dislocation, and then inguinal fat, epididymal fat and liver were separated and weighted.
Determination of serum glucose level
Serum glucose level was determined by using the commercially available kits (A154-1-1) from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to manufacturers’ instruction.
Gut microbiota profiling
Freshly collected feces were collected for DNA extraction. The V3–V4 region sequence of bacterial 16S rDNA gene was amplified and PCR reactions were performed as previously described (He L. et al., 2022). The sequencing was performed on the MiSeq Illumina platform, by Novogene Bioinformatics Technology Co., Ltd. (Tianjin, China). Then, the paired-end reads that obtained were assigned to samples based on their unique barcodes. By using USEARCH, the operational taxonomic units (OTUs) were obtained by clustering the high-quality clean tags. Subsequently, by using the Greengenes database and QIIME, representative OTUs were used for α- and β-diversity and principal coordinate analysis (PCoA).
Fecal microbiota transplantation
The db/db diabetic mice were first orally gavaged with 0.2 mg ampicillin, 0.1 mg vancomycin, 0.2 mg neomycin, 0.2 mg streptomycin and 0.2 mg metronidazole dissolved in 0.2 mL PBS for 7 consecutive days as previously described (Zhou X. et al., 2022). Then, the db/db mice were gavaged with 5 mg freshly collected feces suspended in 0.2 mL PBS, either from mice in the db/db group or from those in the SeSer group. The feces were gavaged once daily for 7 consecutive days. Then, body weight and serum glucose level were determined on day 7 and 21.
Statistical analysis
All data was analyzed using a t test or one-way ANOVA followed by S-N-K post hoc test (SPSS18.0). Data were presented as the mean ± SEM. Mean values were considered significantly different when P value < 0.05. Community composition of microbiota and diversity were analyzed using R software (version 3.3.1).
Results
Both administration of selenomethionine alone and in combination with serine decreased body weight and serum glucose level in db/db diabetic mice
As showed in Figure 1, db/db mice had significantly higher initial body weight than those of db/m mice (Figure 1A). At the end of the experiment, both administration of selenomethionine alone and in combination with serine significantly decreased final body weight of db/db mice, however, the body weight of these db/db mice remained to be significantly higher than those of db/m mice (Figure 1B). Both administration of selenomethionine alone and in combination with serine significantly decreased serum glucose level on day 7 and 21, although they remained to be higher than those of db/m mice.
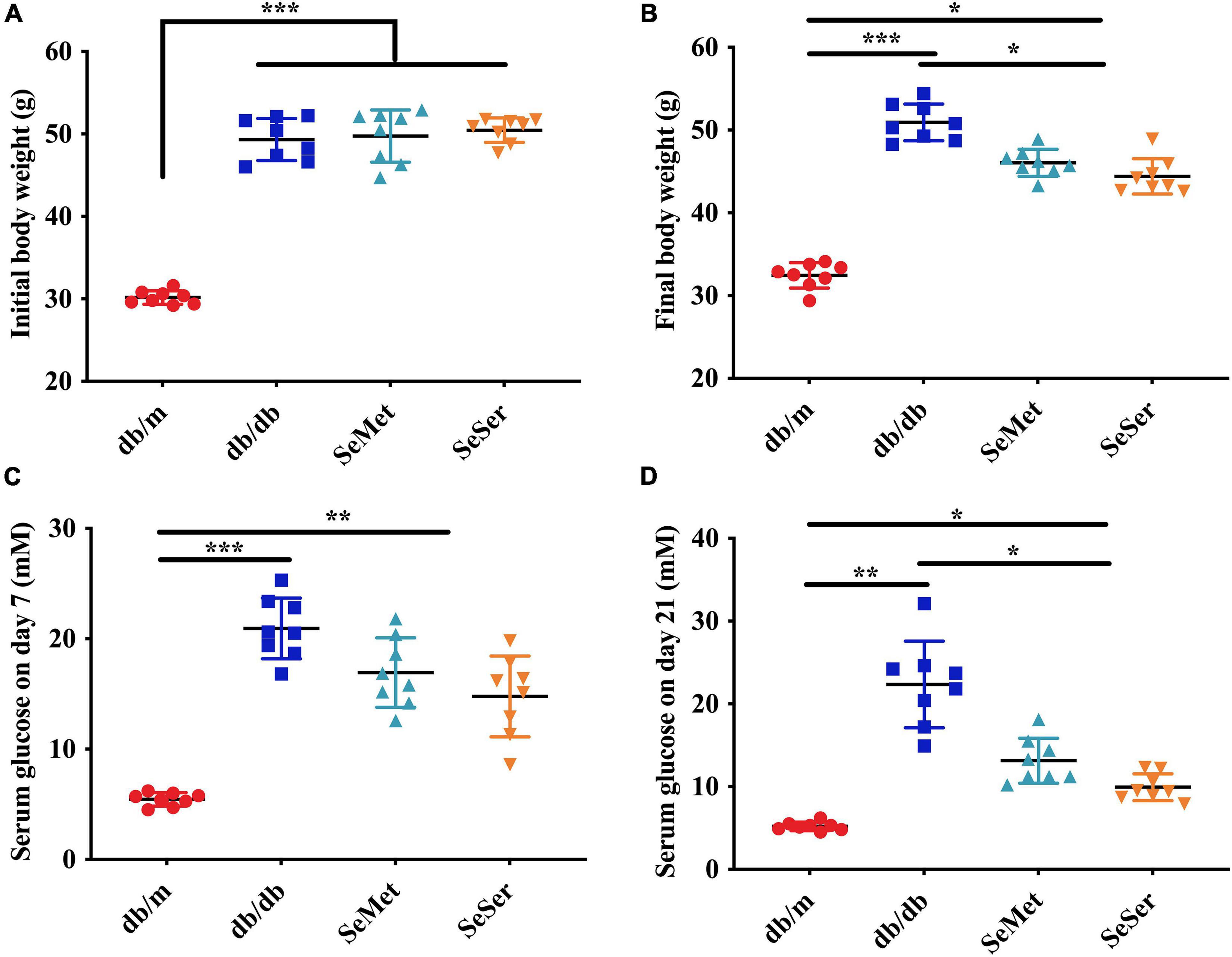
Figure 1. Administration of selenomethionine and serine decreased body weight and serum glucose level in db/db diabetic mice. (A) Initial body weight; (B) final body weight; (C) serum glucose level on day 7; (D) serum glucose level on day 21. Values are expressed as mean ± SEM, n = 8. *P < 0.05, **P < 0.01, ***P < 0.001.
Both administration of selenomethionine alone and in combination with serine decreased liver and fat weight in db/db diabetic mice
As showed in Figure 2, mice in the SeMet and SeSer groups had significantly lower liver weight, inguinal and epididymal fat weight than those in the db/db group, however, the weight of liver, inguinal and epididymal fat of mice in the SeMet and SeSer groups remained to be significantly higher than those of mice in the db/m group.
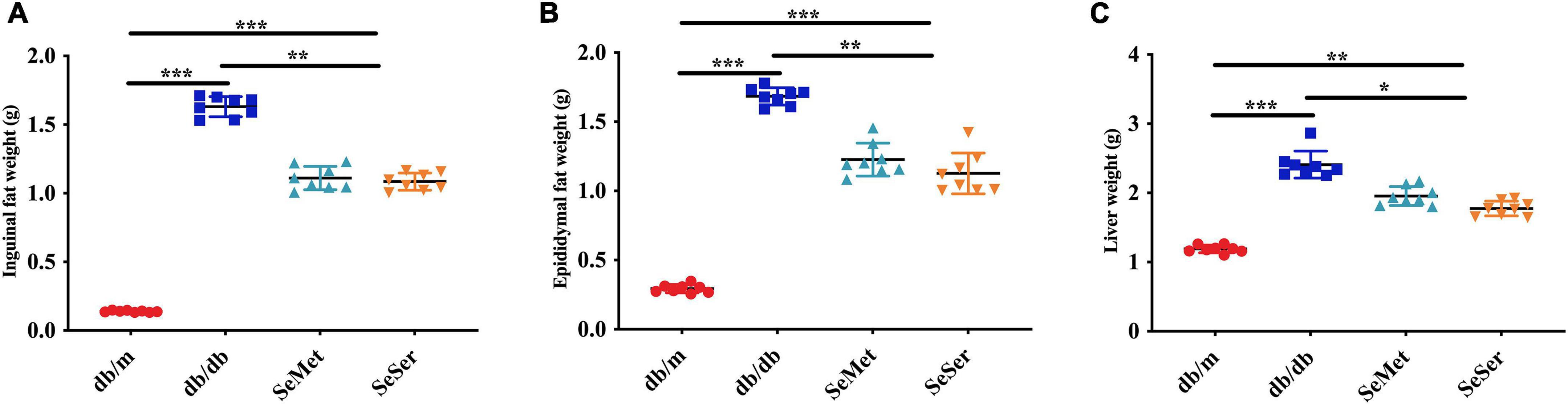
Figure 2. Administration of selenomethionine and serine decreased liver and fat weight in db/db diabetic mice. (A) Inguinal fat weight; (B) epididymal fat weight; (C) liver weight. Values are expressed as mean ± SEM, n = 8. *P < 0.05, **P < 0.01, ***P < 0.001.
Both administration of selenomethionine alone and in combination with serine increased alpha-diversity of gut microbiota in db/db diabetic mice
We compared the microbial population in the feces of mice from the four treatment groups by using the 16S rDNA phylogenetic approach. The Venn diagram results showed that 516 OTUs were universal to all treatment groups among a total of 1432 detected OTUs (Figure 3A). There were 37 unique OTUs in the db/m group, 433 unique OTUs in the db/db group, 21 unique OTUs in the SeMet group and 131 unique OTUs in the SeSer group. The α-diversity, as indicated by the Simpson and Shannon index, was significantly higher in mice in the SeMet and SeSer groups than those in the db/db group, whereas no significant difference was observed among the SeMet, SeSer and db/m group (Figures 3B,C).
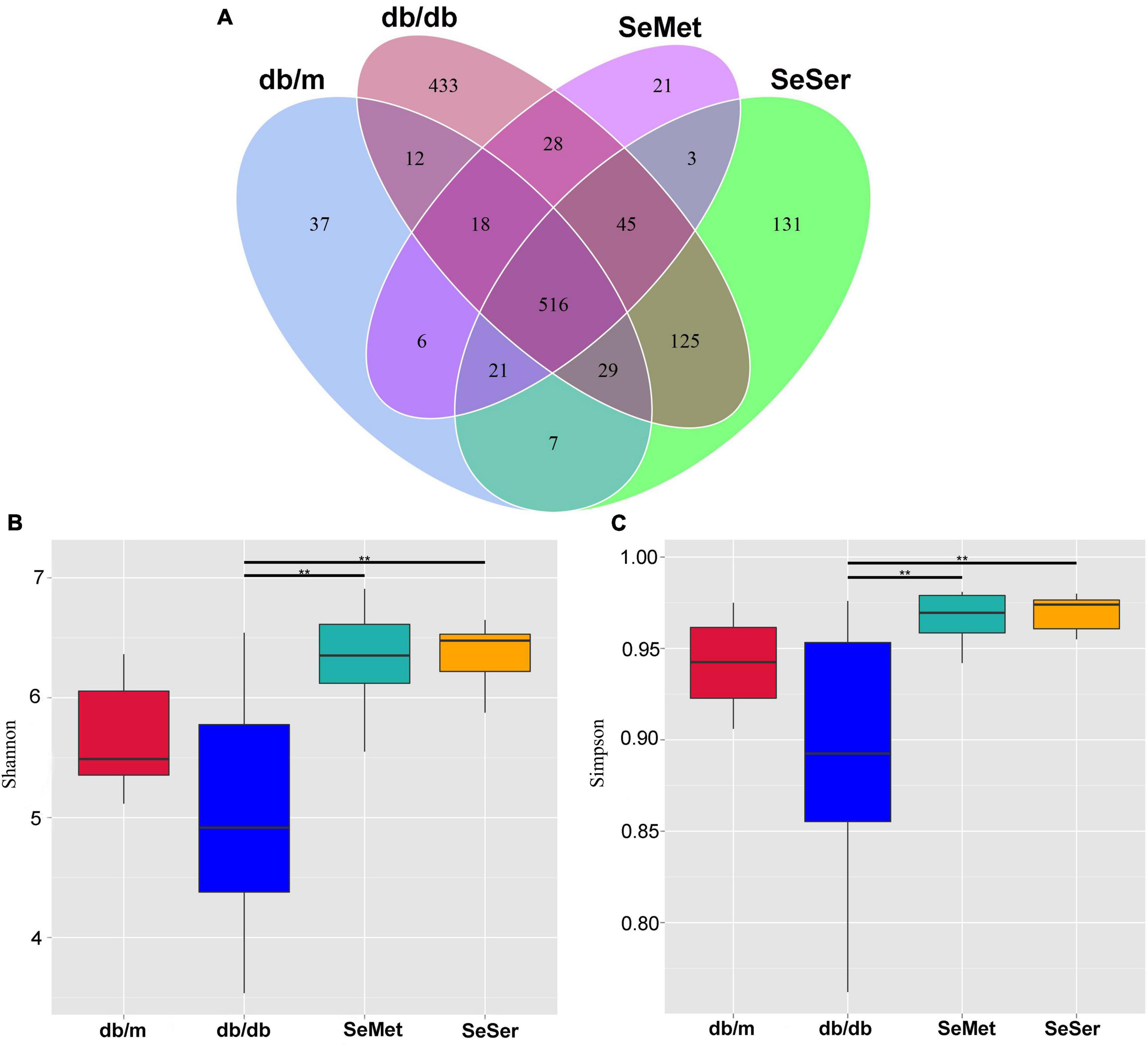
Figure 3. Administration of selenomethionine and serine increased alpha-diversity of gut microbiota in db/db diabetic mice. (A) Venn diagram of OTUs; (B) Shannon index; (C) Simpson index. Values are expressed as mean ± SEM, n = 8. **P < 0.01.
Both administration of selenomethionine alone and in combination with serine altered microbiota composition in db/db diabetic mice
The beta diversity at the species-level in mice in the four treatment groups were significantly different from each other (Figure 4A). Moreover, the results of the unweighted Unifrac PCoA analysis showed that the microbial structure in the feces of mice from db/db group was separated from the other three groups (Figure 4B). At the phylum level, mice in the db/db group had decreased abundance of Bacteroidetes and Firmicutes and increased abundance of Actinobacteriota, when compared with those in the db/m group; Administration of selenomethionine alone and in combination with serine retrieved these changes (Figure 4C). At the species level, mice in the db/db group had increased abundance of Corynebacterium glutamicum, Bifidobacterium pseudolongum, and Aerococcus urinaeequi, and decreased abundance of Lactobacillus murinus and Lactobacillus johnsonii; Administration of selenomethionine alone and in combination with serine restored these changes (Figure 4D). Analysis with the linear discriminant analysis (LDA) effect size (LEfSe) method showed that the db/db group was characterized by Actinobacteriota, while the SeMet group was characterized by Enterobacterales and the SeSer group was characterized by Campilobacterota (Figure 4E).
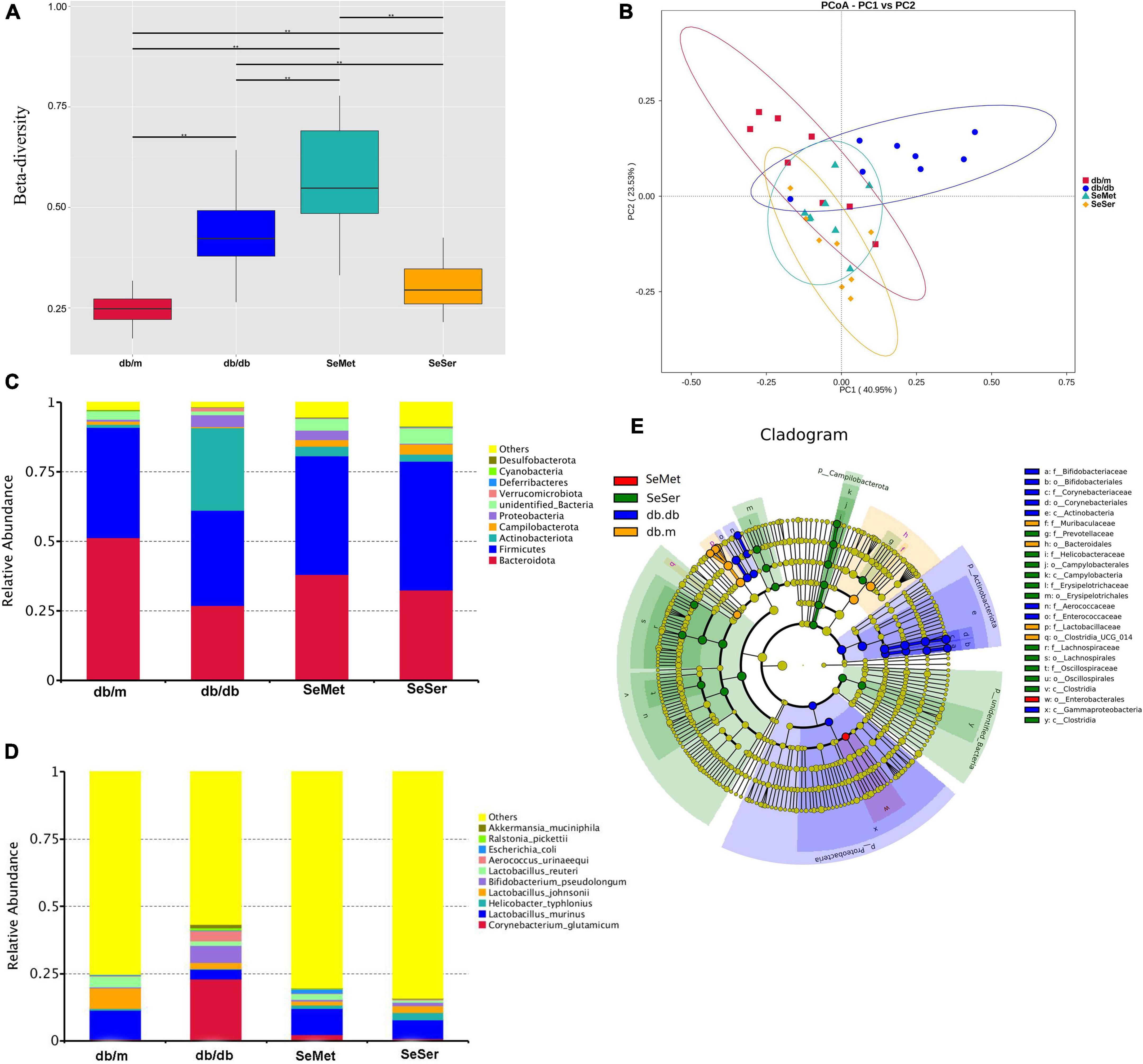
Figure 4. Administration of selenomethionine and serine altered microbiota composition in db/db diabetic mice. (A) Beta-diversity at the species-level; (B) PCoA plot of themicrobiota based on a weighted UniFrac metric; (C) relative abundance of predominant bacteria at the phylum level; (D) relative abundance of predominant bacteria at the species level; (E) taxonomic cladogram generated from LEfSe of metagenomic sequencing data. Values are expressed as mean ± SEM, n = 8. *P < 0.05, **P < 0.01.
We further analyzed the difference of microbe abundance in the db/db, SeMet and SeSer groups by using T-test analysis. As shown in Figure 5, the results showed that C. glutamicum, B. pseudolongum, and A. urinaeequi were significantly decreased, whereas L. murinus was increased in mice in the SeMet and SeSer groups, when compared with those in the db/db group.
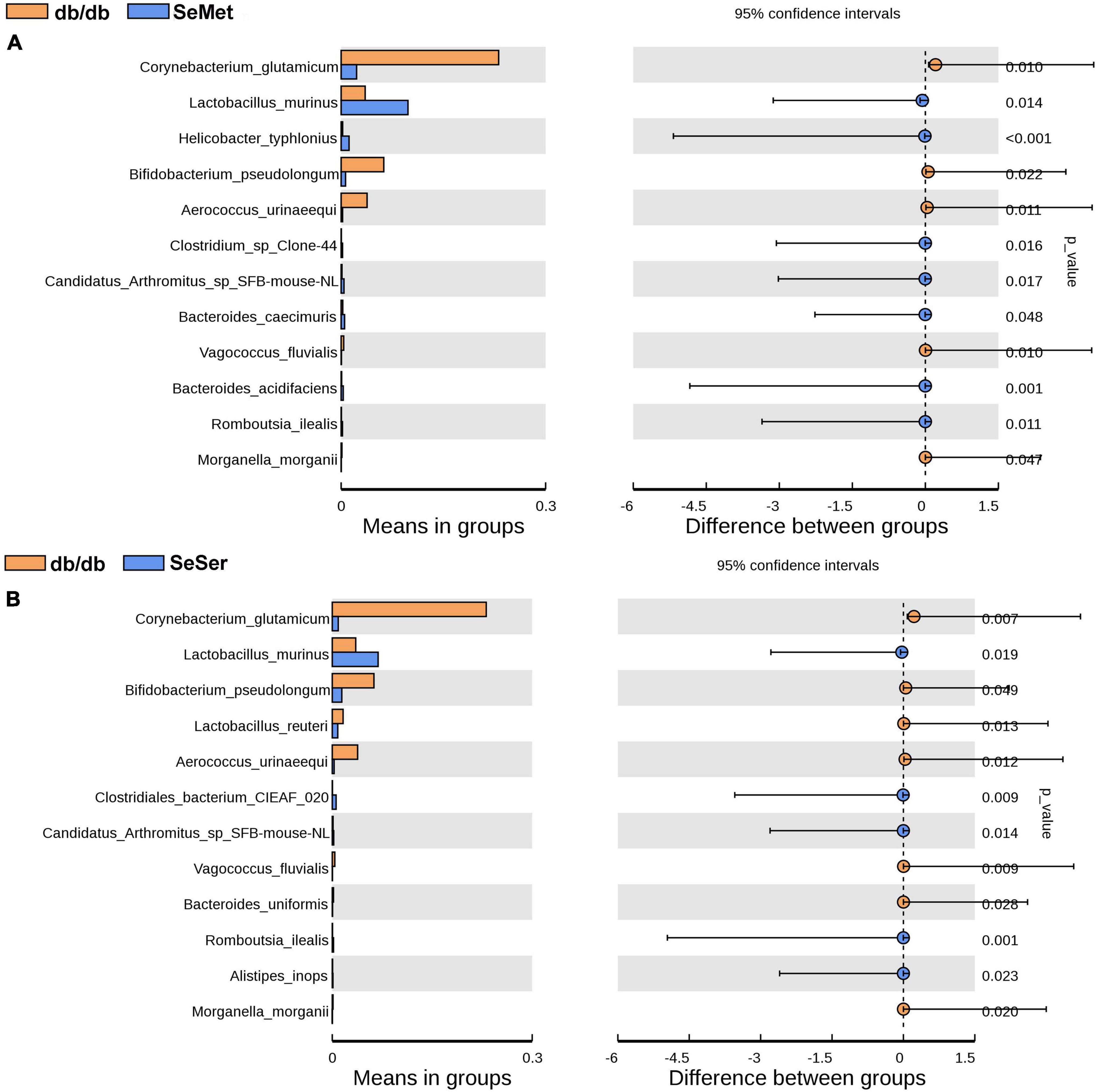
Figure 5. The difference of microbe abundance in the db/db, SeMet and SeSer groups by using T-test analysis. (A) Difference between the db/db and SeMet group; (B) difference between the db/db and SeSer group.
Fecal microbiota transplantation decreased body weight and glucose level in db/db diabetic mice
The db/db mice in the CONT and FMT group had similar body weight at the start of the experiment (Figure 6A), while we observed no significant changes in body weight after FMT for 7 days (Figure 6B). However, db/db mice in the FMT group had significantly decreased body weight on day 21, when compared with those in the CONT group (Figure 6C). Moreover, db/db mice in the FMT group had significantly decreased glucose level on day 7 (Figure 6D) and day 21 (Figure 6E) when compared with those in the CONT group.
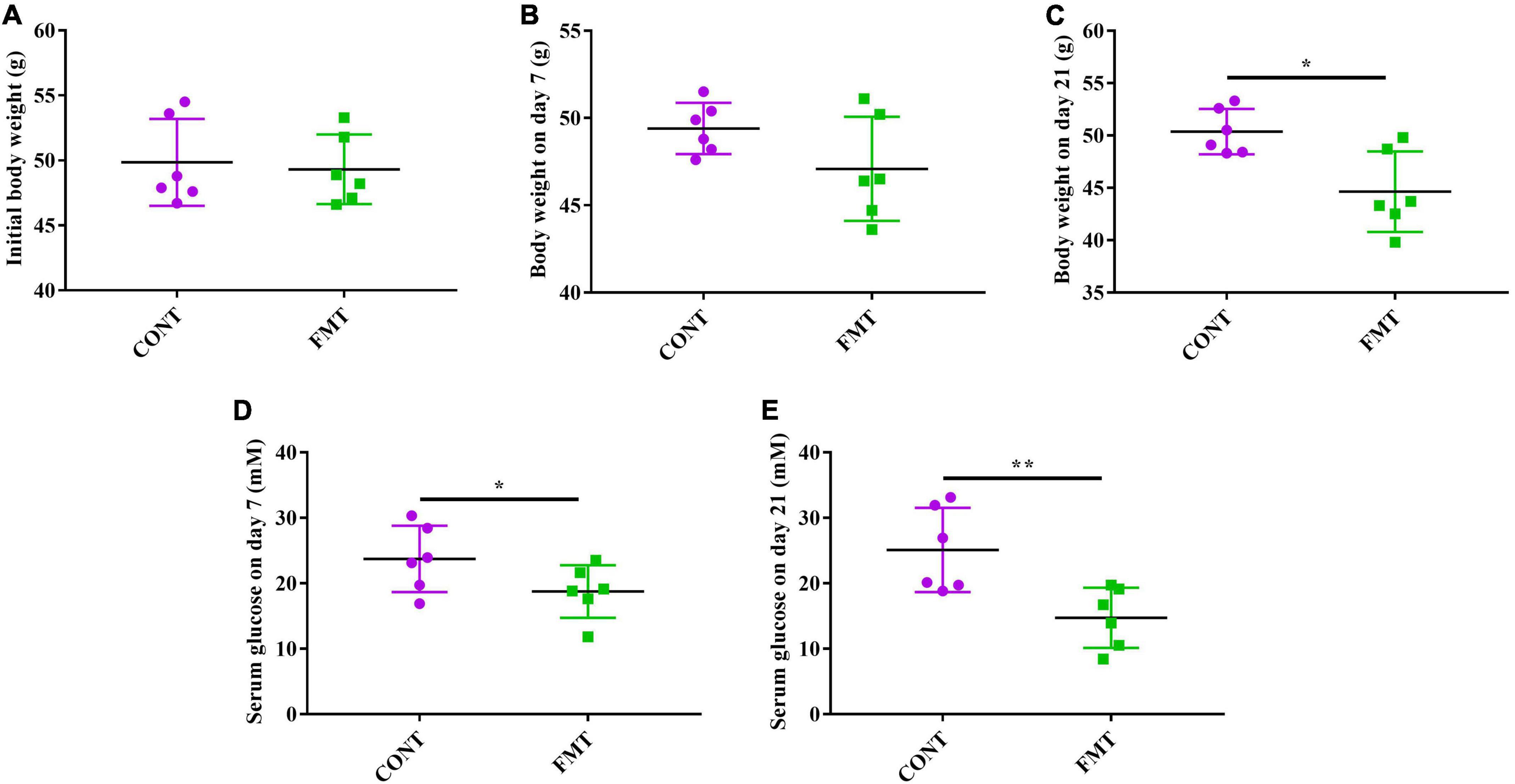
Figure 6. FMT decreased body weight and glucose level in db/db diabetic mice. (A) Initial body weight; (B) body weight on day 7; (C) Body weight on day 21; (D) serum glucose level on day 7; (E) serum glucose level on day 21. Values are expressed as mean ± SEM, n = 6. *P < 0.05, **P < 0.01.
Discussion
Selenium has been proved to lower blood glucose level in several rodent models. In STZ-induced diabetic rats, dietary supplemented with inorganic selenium for 24 weeks significantly lowered serum glucose concentration (Becker et al., 1996). Additionally, dietary selenium improved glucose intolerance in high-fat diet-fed mice (Wang et al., 2022). However, diabetic rats also showed resistance to selenium, as sodium selenite only decreased glucose concentration for the first two weeks, while they had no further effects for the last four weeks of the experiment (McNeill et al., 1991). Importantly, dietary supplemented with high dose of different types of selenium may exert contrary effects. For instance, selenite can alleviate hyperglycemia via serving as an insulin mimic, whereas selenite inhibit insulin signaling in diabetic mice (Pinto et al., 2011; Steinbrenner, 2013; Zhou et al., 2015). Selenium compounds are classified as organic and inorganic. Compared with inorganic selenium, organic selenium is proved to be more easily absorbed and with better biocompatibility. In the present study, we explored the effects of selenomethionine, organic selenium, on glucose homeostasis by using the db/db diabetic mice. According to our results, the beneficial effects of selenomethionine on the control of glucose level were further confirmed. Moreover, the body weight and adipose tissue weight were both decreased in diabetic mice after the administration of selenomethionine. These results suggested that selenomethionine could be potentially used for the treatment of diabetes.
Serine has been proved to exert many beneficial effects including maintaining oxidative status and alleviating inflammatory response in several animal models (Zhang et al., 2018; Zhou et al., 2021). Particularly, serine improved glucose tolerance and insulin sensitivity in high-fat diet-fed mice (Zhou et al., 2018). However, it did not significantly lower body weight and adipose weight/body weight ratio. Recently, a study showed the synergistic effect of serine and selenocompounds on glutathione peroxidase concentration (Wang et al., 2016), which indicated that serine could be a potential candidate for combined selenium administration. We further explored the combined effects of serine and selenium on glucose level in db/db diabetic mice, and the results showed that administration of selenomethionine in combination with serine significantly decreased body weight and glucose level. Notably, they showed better effects than administration of selenomethionine alone, although the difference did not reach significance. Our results further confirmed the synergistic effect of serine and selenocompounds on maintaining the glucose homeostasis in diabetic mice.
For decades, increasing evidences suggested that gut microbiota play critical roles in type 2 diabetes. As an essential micronutrient, selenium can play critical roles in oxidative stress and immune function through modulating gut microbiota composition (Ferreira et al., 2021). Serine can also regulate the composition of gut microbiota and alleviated inflammation (Zhang et al., 2018). However, whether selenomethionine and serine can improve the microbial composition in diabetic mice were not previously explored. Our results suggested that selenomethionine and serine restored alpha and beta diversity which were decreased in db/db diabetic mice. Furthermore, selenomethionine and serine retrieved the abundance of microbiota composition. Notably, administration of selenomethionine in combination with serine increased the abundance of L. murinus and L. reuteri, which are generally considered to be beneficial microbes (Goldstein et al., 2015). FMT, namely stool transplantation, is an approach that transplants feces from a donor into another recipient to directly change the intestinal microbiota of the recipient to restore the composition, in order to gain beneficial treatment effects (Wang et al., 2019). To confirm that microbes mediate the beneficial effect of selenomethionine and serine on glucose homeostasis in diabetic mice, we further transplanted the freshly collected feces from mice treated with selenomethionine and serine to db/db diabetic mice. We found that db/db diabetic mice had decreased body weight and serum glucose level after FMT, which further proved that selenomethionine and serine alleviated hyperglycemia via modulating gut microbiota.
Conclusion
In conclusion, our results suggested that administration of selenomethionine decreased body weight, adipose tissue weight and serum glucose level in db/db diabetic mice. Importantly, administration of selenomethionine in combination with serine exerted better effects than selenomethionine alone. Furthermore, a combined administration of selenomethionine and serine restored the microbial composition in diabetic mice. FMT decreased body weight and glucose concentration in db/db mice, further indicating that microbes play critical roles in the beneficial effects of selenomethionine and serine. Thus, we concluded that administration of selenomethionine in combination with serine benefits diabetes via gut microbes.
Data availability statement
The data presented in the study are deposited in the NCBI SRA database repository, accession number is PRJNA880689.
Ethics statement
The animal study was reviewed and approved by the Animal Care and Use Committee of China Agricultural University, and conformed to the Guide for the Care and Use of Laboratory Animals.
Author contributions
XC and YY designed the experiments and contributed to the literature search, interpretation, writing, and proofreading of the manuscript. JC performed the animal experiments. XC and JC analyzed the 16S rRNA data. All authors approved and contributed the submitted version.
Funding
This work was funded by Natural Science Foundation of Zhejiang Province (LQ19C200002) and National Natural Science Foundation of China (32102559).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Becker, D. J., Reul, B., Ozcelikay, A. T., Briechard, S. M., and Henquin, J. C. (1994). Oral selenium improves glucose-homeostasis of insulin-deficient diabetic rats. Diabetologia 37, A132–A132. doi: 10.1007/BF00400407
Becker, D. J., Reul, B., Ozcelikay, A. T., Buchet, J. P., Henquin, J. C., and Brichard, S. M. (1996). Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenic enzymes in diabetic rats. Diabetologia 39, 3–11.
Ferreira, R., Sena-Evangelista, K., Azevedo, E., Pinheiro, F. I., and Pedrosa, L. (2021). Selenium in human health and gut microflora: Bioavailability of selenocompounds and relationship with diseases. Front. Nutr. 8:685317. doi: 10.3389/fnut.2021.685317
Goldstein, E. J., Tyrrell, K. L., and Citron, D. M. (2015). Lactobacillus species: Taxonomic complexity and controversial susceptibilities. Clin. Infect. Dis. 60(Suppl. 2), S98–S107. doi: 10.1093/cid/civ072
He, Y., Liu, Y., Guan, P., He, L., and Zhou, X. (2022). Serine administration improves selenium status, oxidative stress, and mitochondrial function in longissimus dorsi muscle of piglets with intrauterine growth retardation. Biol. Trace Elem. Res. 94, 43–49. doi: 10.1007/s12011-022-03304-5
He, L., Zhou, X., Liu, Y., Zhou, L., and Li, F. (2022). Fecal miR-142a-3p from dextran sulfate sodium-challenge recovered mice prevents colitis by promoting the growth of Lactobacillus reuteri. Mol. Ther. 30, 388–399. doi: 10.1016/j.ymthe.2021.08.025
Long, J., Liu, Y., Zhou, X., and He, L. (2021). Dietary serine supplementation regulates selenoprotein transcription and selenoenzyme activity in pigs. Biol. Trace Elem. Res. 199, 148–153. doi: 10.1007/s12011-020-02117-8
McNeill, J. H., Delgatty, H. L., and Battell, M. L. (1991). Insulinlike effects of sodium selenate in streptozocin-induced diabetic rats. Diabetes 40, 1675–1678. doi: 10.2337/diab.40.12.1675
Pinto, A., Speckmann, B., Heisler, M., Sies, H., and Steinbrenner, H. (2011). Delaying of insulin signal transduction in skeletal muscle cells by selenium compounds. J. Inorg. Biochem. 105, 812–820. doi: 10.1016/j.jinorgbio.2011.03.010
Reddi, A. S., and Bollineni, J. S. (2001). Selenium-deficient diet induces renal oxidative stress and injury via TGF-beta1 in normal and diabetic rats. Kidney Int. 59, 1342–1353. doi: 10.1046/j.1523-1755.2001.0590041342.x
Steinbrenner, H. (2013). Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radic. Biol. Med. 65, 1538–1547. doi: 10.1016/j.freeradbiomed.2013.07.016
Wang, J. W., Kuo, C. H., Kuo, F. C., Wang, Y. K., Hsu, W. H., Yu, F. J., et al. (2019). Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 118(Suppl. 1), S23–S31.
Wang, Q., Sun, L. C., Liu, Y. Q., Lu, J. X., Han, F., and Huang, Z. W. (2016). The synergistic effect of serine with selenocompounds on the expression of SelP and GPx in HepG2 cells. Biol. Trace Elem. Res. 173, 291–296. doi: 10.1007/s12011-016-0665-8
Wang, Y., Liu, B., Wu, P., Chu, Y., Gui, S., Zheng, Y., et al. (2022). Dietary selenium alleviated mouse liver oxidative stress and NAFLD induced by obesity by regulating the KEAP1/NRF2 pathway. Antioxidants 11:349. doi: 10.3390/antiox11020349
Zhang, H., Hua, R., Zhang, B., Zhang, X., Yang, H., and Zhou, X. (2018). Serine alleviates dextran sulfate sodium-induced colitis and regulates the gut microbiota in mice. Front. Microbiol. 9:3062. doi: 10.3389/fmicb.2018.03062
Zhao, L., Carmean, C. M., Landeche, M., Chellan, B., and Sargis, R. M. (2021). Selenomethionine modulates insulin secretion in the MIN6-K8 mouse insulinoma cell line. FEBS Lett. 595, 3042–3055. doi: 10.1002/1873-3468.14232
Zhou, J., Xu, G., Bai, Z., Li, K., Yan, J., Li, F., et al. (2015). Selenite exacerbates hepatic insulin resistance in mouse model of type 2 diabetes through oxidative stress-mediated JNK pathway. Toxicol. Appl. Pharmacol. 289, 409–418. doi: 10.1016/j.taap.2015.10.019
Zhou, L., Feng, Y., Liu, Y., He, L., Zhou, X., and Yin, Y. (2022). Serine supplementation in the diets of late gestating and lactating sows improves selenium nutritional status in sows and their offspring. Biol. Trace Elem. Res. 200, 609–614. doi: 10.1007/s12011-021-02661-x
Zhou, X., He, L., Wu, C., Zhang, Y., Wu, X., and Yin, Y. (2017). Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice. Mol. Nutr. Food Res. 61, 1–13. doi: 10.1002/mnfr.201700262
Zhou, X., He, L., Zuo, S., Zhang, Y., Wan, D., Long, C., et al. (2018). Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 488–498. doi: 10.1016/j.bbadis.2017.11.009
Zhou, X., Liu, Y., Xiong, X., Chen, J., Tang, W., He, L., et al. (2022). Intestinal accumulation of microbiota-produced succinate caused by loss of microRNAs leads to diarrhea in weanling piglets. Gut Microbes 14:2091369. doi: 10.1080/19490976.2022.2091369
Keywords: diabetes, microbe, selenium, selenomethionine, serine
Citation: Cui X, Chen J and Yang Y (2022) Administration of selenomethionine in combination with serine benefits diabetes via gut microbiota. Front. Microbiol. 13:1007814. doi: 10.3389/fmicb.2022.1007814
Received: 31 July 2022; Accepted: 31 August 2022;
Published: 12 October 2022.
Edited by:
Yongwen Zhu, South China Agricultural University, ChinaReviewed by:
Shaaban Saad Elnesr, Fayoum University, EgyptFuguang Xue, Jiangxi Agricultural University, China
Copyright © 2022 Cui, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuexi Yang, eXVleGkueWFuZ0B6amdzdS5lZHUuY24=
†These authors have contributed equally to this work
 Xiaoyan Cui
Xiaoyan Cui Jingqing Chen
Jingqing Chen Yuexi Yang4*
Yuexi Yang4*