- 1Frontiers Science Center for Deep Ocean Multispheres and Earth System and College of Marine Life Sciences, Ocean University of China, Qingdao, China
- 2Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
- 3Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao, China
Unraveling the mechanisms structuring microbial community is a central goal in microbial ecology, but a detailed understanding of how community assembly processes relate to living habitats is still lacking. Here, via 16S rRNA gene amplicon sequencing, we investigated the assembly process of microbial communities in different habitats [water verse sediment, free-living (FL) verse particle-associated (PA)] and their impacts on the inter-taxa association patterns in the coastal Bohai Sea, China. The results showed clear differences in the composition and diversity of microbial communities among habitats, with greater dissimilarities between water column and sediment than between FL and PA communities. The microbial community assembly was dominated by dispersal limitation, ecological drift, and homogeneous selection, but their relative importance varied in different habitats. The planktonic communities were mainly shaped by dispersal limitation and ecological drift, whereas homogeneous selection played a more important role in structuring the benthic communities. Furthermore, the assembly mechanisms differed between FL and PA communities, especially in the bottom water with a greater effect of ecological drift and dispersal limitation on the FL and PA fractions, respectively. Linking assembly process to co-occurrence pattern showed that the relative contribution of deterministic processes (mainly homogeneous selection) increased under closer co-occurrence relationships. By contrast, stochastic processes exerted a higher effect when there were less inter-taxa connections. Overall, our findings demonstrate contrasting ecological processes underpinning microbial community distribution in different habitats including different lifestyles, which indicate complex microbial dynamic patterns in coastal systems with high anthropogenic perturbations.
Introduction
Microorganisms are highly diverse in taxonomic composition and metabolic capability, and play important roles in global biogeochemical cycles (Prosser et al., 2007; Falkowski et al., 2008). Intensive efforts have been devoted on the microbial diversity and environmental associations, yet exploring the processes of community assembly is lacking but is crucial for a predictive understanding of their ecosystem functioning (Nemergut et al., 2013; Zhou and Ning, 2017). Deterministic and stochastic processes are two important mechanisms in structuring microbial community assembly (Zhou and Ning, 2017; Liu et al., 2019). On the basis of the niche theory, determinism is developed involving influence of biotic and abiotic factors, which are mainly reflected by species interaction and environmental filtering, respectively (Chesson, 2000). Stochasticity is based on the neutral theory assuming that all species are ecologically equivalent, and a community is controlled by processes such as birth, death, dispersal, and species formation (Chave, 2004). These two processes have distinct effects on microbial communities. Deterministic processes affect the fitness of microbial communities and determine composition and abundance (Li et al., 2019), whereas stochastic processes lead to unpredictable community changes (Tilman, 2004). There is an increasing effort in examining the relative contribution of deterministic and stochastic processes to microbial community assembly in different environments, such as soils (Jiao et al., 2020), freshwater (Mo et al., 2018), hot springs (He et al., 2021), and marine environments (Liu et al., 2020). However, no clear consensus has been obtained (Stegen et al., 2012; Wang et al., 2013; Dini-Andreote et al., 2015). It is considered that geographical scale and environmental gradients control the relative contribution of deterministic and stochastic processes (Hanson et al., 2012; Zhang et al., 2018). Changes in spatial scale can lead to differences in chemical gradients that may have implications for the ecological processes structuring microbial distribution patterns. For example, environmental factors (deterministic processes) including temperature, salinity, and sand size exerted a stronger influence than spatial factors (stochastic processes) on the benthic microeukaryotic communities in marine sandy beaches (up to 12,000 km; Zhang et al., 2018), whereas stochastic processes were the most important process in the assembly of archaeal communities in coastal sediments from the eastern Chinese marginal seas (up to 1,500 km; Liu et al., 2020). These studies suggest that the assembly of microbial community are not conserved among various ecosystems, emphasizing the importance for comparative analyses between environments (Allen et al., 2020; Li et al., 2021a).
The marine environment provides a variety of habitats, such as waters and sediments, which promote divergent adaption by adopting different lifestyles. Regarding water columns, two types of lifestyles exist, namely free-living (FL) and particle-associated (PA). The microbial communities inhabiting these habitats are distinctly different, and are likely assembled under different mechanisms (Wang et al., 2020; Gweon et al., 2021; Li et al., 2022). For example, Wang et al. (2020) found that homogeneous selection had a relatively higher importance in shaping bacterioplankton in the FL fraction, while this process was more important in structuring archaeaplankton of the PA fraction. By contrast, other studies reported that stochastic processes played a larger role in shaping both the FL and PA communities (Gweon et al., 2021; Shi et al., 2022). However, previous studies of marine microbial community assembly have mainly focused on a single habitat and few involve different habitats in a single study. Consequently, despite with an increasing effort devoted to explore the pattern of community assembly, a detailed understanding of how community assembly processes relate to living habitats is rare.
Examining the contribution of deterministic and stochastic processes in microbial community assembly can manifest the ecological strategies of coexisting species (Faust and Raes, 2012; Logares et al., 2020). Recently, correlation-based co-occurrence networks are frequently employed to describe microbial interactions, although they are controversial in reflecting true interactions (Liu et al., 2019). For instance, using the null model and co-occurrence networks, Li et al. (2021b) found that the dominant competition relationships between active bacterial and archaeal taxa could enhance the role of dispersal limitation in community assembly. By contrast, Mo et al. (2020) found that co-occurrence patterns of generalists and specialists were mainly driven by deterministic processes. Therefore, a complex network can reveal the inherent mechanism of microbial interactions after environmental changes and provide system-level insights for community assembly (Berry and Widder, 2014; Banerjee et al., 2018; Lv et al., 2019).
Our analyses were performed in the Bohai Sea (BS), a relatively isolated coastal area in the Chinese coastline, with little exchange with other seas and a long seawater renewal time (1.68 years; Li et al., 2015). The increasing anthropogenic activities have caused serious ecological problems to the BS, such as eutrophication, algal blooms, and hypoxia in the bottom water (Song et al., 2016; Zhai et al., 2019; Zheng et al., 2020). Consistently, microbial communities in the BS have been influenced by hypoxia (Wu et al., 2022), river input (Wang et al., 2021), and heavy metals (Ming et al., 2021). However, the microbial assembly pattern in this highly disturbed ecosystem is largely unknown. Moreover, the BS is a good candidate to investigate the community assembly in the marine environment covering a small spatial scale. In this study, we aimed to investigate whether microbial communities in different habitats (water verse sediment; FL verse PA) are assembled under different ecological processes and whether patterns of community assembly are related to species co-occurrences.
Materials and methods
Samples collection and environmental parameters
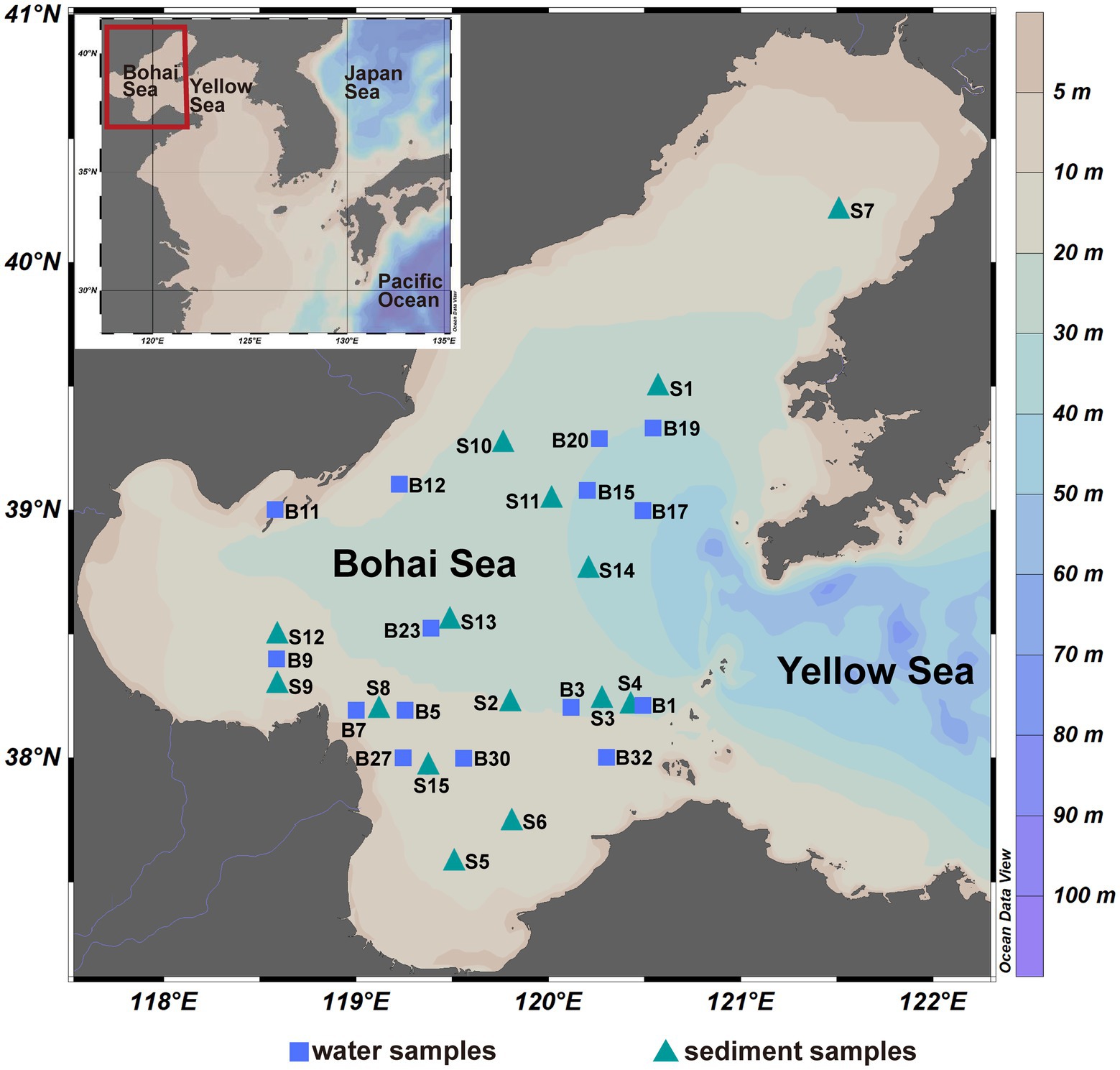
Seawater and surface sediment samples were collected from the Bohai Sea (37°07′~40°56′ N, 117°33′~122°08′ E) of China during July–August 2019, and the location of sites are shown in Figure 1. Surface and bottom seawater samples were collected at 15 sites and were firstly filtered by a 3-μm filter to collect PA communities, and then filtered by a 0.22-μm filter to collect FL communities. The sediment samples (0–2 cm) were collected at 15 sites using a box corer and subsampled using a sterile spatula. The filter and sediment samples were stored at −20°C on board and at −80°C in the laboratory before DNA extraction. A total of 75 samples were finally obtained and divided into five groups according to the lifestyle of microbial community, namely Sur_FL (FL community in surface water), Sur_PA (PA community in surface water), Bot_FL (FL community in bottom water), Bot_PA (PA community in bottom water), and sediment. Salinity (26.6–32.1 PSU), temperature (17.8–28.5°C), pressure (2.9–41.3 dbar), pH (7.33–8.27), dissolved oxygen (3.57–8.96 mg/l), density (16.4–23.0 kg/m3), and fluorescence (0.29–7.82 mg/m3) of the seawater samples (surface and bottom waters) were determined using a Sealogger conductivity-temperature-depth (SBE 25, Sea-Bird Co.). The dissolved inorganic nutrients (nitrate, nitrite, ammonia, phosphate, and silicate) were analyzed by an auto-analyzer (SKALAR, sa3000/5000 chemistry unit).
DNA extraction and sequencing
Total DNA was extracted from the seawater and sediment samples using the DNeasy PowerSoil Kit (QIAGEN, Germany) according to the manufacturer’s protocols. The primer pair 515F/806R (Walters et al., 2016) was used to amply the hypervariable V4 region of the 16S rRNA gene for bacteria and archaea. PCRs were conducted in a 20 μl reaction system, containing 0.4 μl of TransStart FastPfu polymerase, 2 μl of 2.5 mM dNTPs, 0.8 μl of 5 μM each primer, 4 μl of TransStart 5× FastPfu buffer, and 10 ng of template DNA. The PCR amplification conditions were as follows: 95°C for 3 min, followed by 35 cycles at 95°C for 30 s, 55°C for 30 s, 72°C for 45 s, and a final extension at 72°C for 10 min. The amplified PCR products were extracted from 2% agarose gel and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States). The purified amplicons were sequenced on the Illumina Miseq PE300 platform (Illumina, San Diego, United States), performed by Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China. The raw sequencing data had been deposited in National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database under accession number PRJNA855144, and in the NODE database under accession number OEP003571.
Sequencing data processing
The raw reads were firstly quality-filtered by fastp version 0.20.0 (Chen et al., 2018) to discard those of low average quality (<20) and short length (<50 bp), with any mismatch to barcodes and a maximum of two mismatches to primers. The high-quality reads were then merged by FLASH version 1.2.7 (Magoč and Salzberg, 2011). The merged reads were clustered into operational taxonomic units (OTUs) at a 3% dissimilarity level using UPARSE (Edgar, 2013). To reduce the potential of PCR bias, singleton OTUs were removed for downstream analyses. Taxonomic information for each OTU was assigned against the SILVA database (version 138)1 using the Ribosomal Database Project (RDP) classifier version 2.22 (Wang et al., 2007) with a confidence threshold of 0.7. Chloroplast and mitochondrial 16S rRNA genes were removed from further analyses.
Statistical analyses
All analyses were performed in the R software version 4.13 unless otherwise indicated.
To evaluate the diversity of microbial communities, alpha and beta diversity indices were calculated using the “vegan” package (Jari Oksanen et al., 2020). Comparison of the alpha diversity indices including Chao1, Shannon, ACE, and observed species was analyzed using the Kruskal–Wallis test. The beta diversity was estimated by Bray–Curtis distances. The community dissimilarity among different habitats was visualized by the non-metric multidimensional scaling analysis (NMDS). Statistical significance between groups was tested by permutational multivariate analysis (PERMANOVA) and analysis of similarities (ANOSIM). In order to evaluate the resources available to different microbial communities, we calculated the community-level niche breadth by the “niche.width “function of the “spaa” package (Zhang, 2016a), which is the average of niche breadth of a community (Wu et al., 2018).
The relationship between microbial communities and environmental factors was revealed by a Mantel test based on Pearson’s correlations. A dissimilarity matrix of community composition was calculated based on Bray–Curtis distances, which was carried out by the “linkET” package (Huang, 2021). A null model analysis was carried out using the statistical framework with 999 randomizations by Stegen et al. (2013) to quantify the relative contribution of deterministic and stochastic processes, namely homogeneous selection, heterogeneous selection, homogeneous dispersal, dispersal limitation, and drift. The beta nearest taxon index (βNTI) and the Bray–Curtis-based Raup-Crick metric (RCbray) are the two most important parameters of the framework to distinguish different ecological processes, calculated using the “picante” package (Kembel et al., 2010). The |βNTI| values higher than 2 indicate that community structure is mainly influenced by deterministic processes, where the βNTI values less than −2 or higher than 2 represent homogeneous and heterogeneous selection, respectively. The |βNTI| values falling within the range of −2 to 2 indicate that stochastic processes dominate the community assembly, and the specific stochastic processes can be further inferred by combining the RCbray values. For |βNTI| < 2, RCbray < −0.95, RCbray > 0.95, and |RCbray| < 0.95 represent homogenizing dispersal and dispersal limitation and ecological drift, respectively. The neutral community model (NCM; Sloan et al., 2006) was further used to evaluate the potential roles of neutral processes in the assembly process by predicting the relationship between OTU relative abundance and their occurrence frequency. R2 is used as the coefficient of determination to assess the goodness of fit to the NCM, with higher values indicating that community assembly is more influenced by stochastic processes. The estimated migration rate (m) represents the probability of stochastic loss of OTUs in the community being replaced by dispersal from the metacommunity, with smaller values indicating that the community is more affected by dispersal limitation. The values of R2 and m were calculated by the “MicEco” package (Russel, 2021).
Meta-community co-occurrence network based on Spearman correlation coefficients was constructed using the “igraph” (Csardi and Nepusz, 2006) and “psych” (Revelle, 2021) packages, and visualized using Gephi version 0.9.2.3 To reduce the complexity of the network for a better visualization, only the top 500 OTUs were used for network construction. The pairwise Spearman’s rank correlations were calculated to visualize the associations between species (at OTU level), with correlation coefficients |r| > 0.7 and false discovery rate-corrected p value < 0.01 being considered as a valid relationship. The topological features of the network including network level (average degree, clustering coefficient, average path length, modularity, graph density, network diameter, betweenness centralization, and degree centralization) and node level (node degree, node transitivity, betweenness centrality, and closeness centrality) were calculated. The subnetwork of different communities was extracted from the meta-community network.
Results
Composition of microbial communities
Illumina sequencing generated a total of 1,373,925 clean reads, which were clustered into 15,846 OTUs at a 97% sequence similarity level. The flattening of the species accumulation curve indicated that the sequencing data were able to recover most of the local species (Supplementary Figure 1). The sequences were taxonomically diverse and affiliated with a total of 78 phyla. The dominant phyla were Proteobacteria (39.4%), Bacteroidota (19.1%), Actinobacteriota (7.3%), and Cyanobacteria (6.0%), which accounted for more than half of the community (Figure 2). These four phyla varied considerably between water depths and between water column and sediment. They showed a decreasing trend from surface water (88.0%), bottom water (71.0%) to sediment (40.8%). As expected, the abundance of Cyanobacteria especially Synechococcus peaked in surface seawater (11.1%) and decreased in bottom water (3.8%) and sediment (0.1%; Figure 2; Supplementary Figure 2). By contrast, archaeal phyla Crenarchaeota and Thermoplasmatota, mainly represented by Nitrosopumilaceae and Marine Group II, respectively, were more abundant in bottom water than in surface water (Kruskal–Wallis test, p < 0.001). Compared to seawater, the sediment samples were relatively enriched with Planctomycetota, Desulfobacterota, Chloroflexi, Acidobacteriota, and Nitrospirota (Kruskal–Wallis test, p < 0.001). Abundant orders in the sediment samples included Anaerolineales (Chloroflexi), Thermoanaerobaculales (Acidobacteriota), Desulfobacterales (Desulfobacterota), and MSBL9 (Planctomycetota).
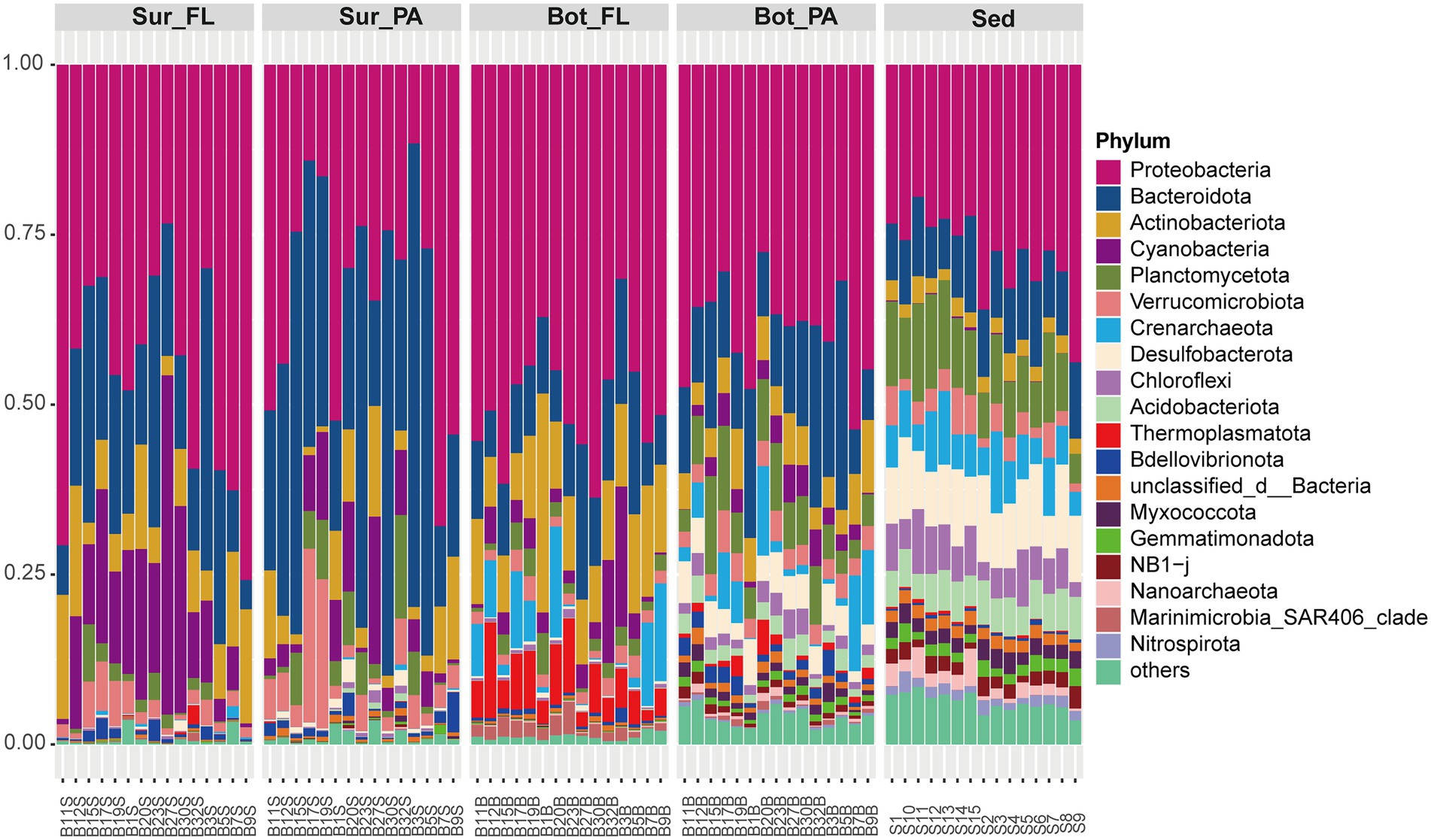
Figure 2. Composition of microbial communities at the phylum level. Sur_FL, FL community in surface water; Sur_PA, PA community in surface water; Bot_FL, FL community in bottom water; Bot_PA, PA community in bottom water; Sed, sedimentary community.
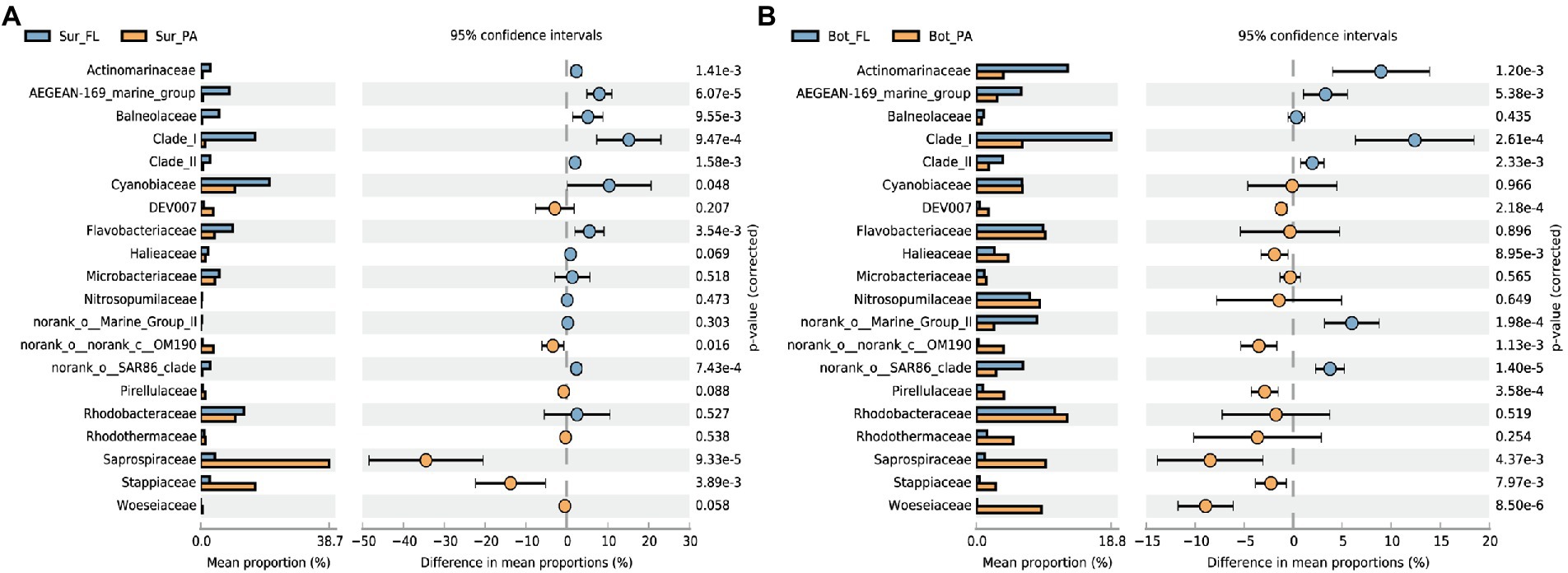
Significant compositional changes were also observed between the FL and PA communities (Figure 3; Supplementary Figure 3). The lifestyle-based community variance appeared to be more evident in bottom water than in surface water, with more phyla being enriched in PA than FL (Supplementary Figure 3). At a finer taxonomical level, Actinomarinaceae, Clade I (SAR11), and AEGEAN-169 marine group were more abundant in the FL fraction, whereas the PA community especially in surface water was relatively enriched by Saprospiraceae belonging to Chitinophagales in Bacteroidota, followed by Stappiaceae (Figure 3A). In the bottom water, more microbial families were enriched in PA than in FL fractions, and the PA-enriched families were also dominant in the sediment, such as Woeseiaceae, Pirellulaceae, and Halieaceae (Figure 3B).
Diversity of microbial communities
ACE, observed species, Chao 1, and Shannon indices exhibited similar variation trends, with the highest diversity level found in sediment, followed by bottom water and surface water (Kruskal–Wallis test, p < 0.001; Figures 4A,B; Supplementary Figures 4A,B). In bottom water, the alpha diversity indices were significantly higher in the PA than the FL fractions (p < 0.001). No similar trend was observed in the surface water, where the average value of Shannon index appeared to be higher in FL than in PA communities though with no statistical significance (Figure 4B).
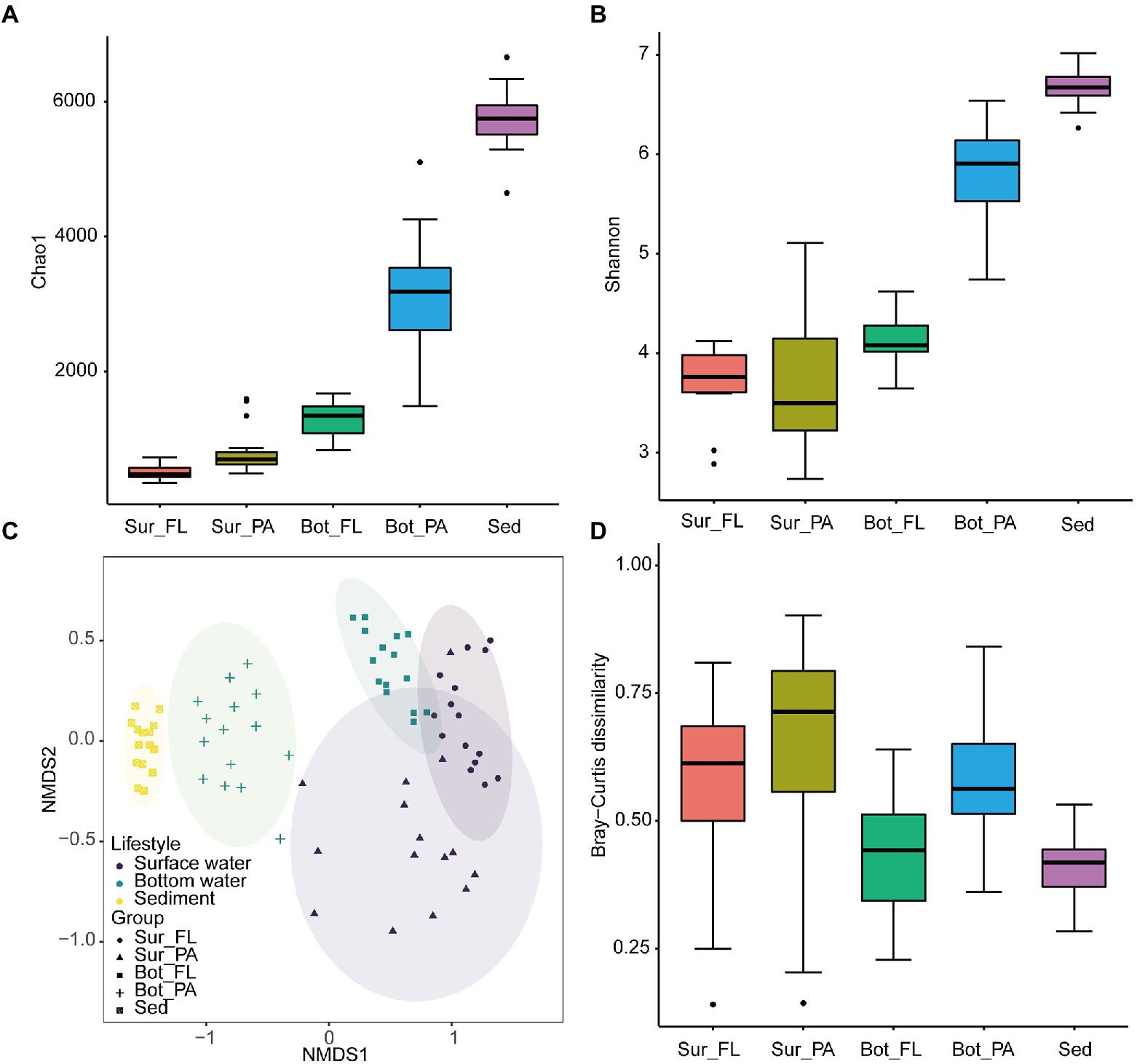
Figure 4. Alpha and beta diversity of microbial communities at the OTU level. (A) Chao1 index of different communities; (B) Shannon index of different communities; (C) NMDS plot of different communities; (D) Beta diversity index of different communities.
NMDS analysis based on the Bray–Curtis distance showed that the microbial communities can be grouped into five clusters, i.e., Sur_FL, Sur_PA, Bot_FL, Bot_PA, sediment (Figure 4C). The pairwise PERMANOVA analysis further supported this clustering pattern and showed that the microbial community was more variable between habitats (sediment verse water) than between lifestyles (FL verse PA; Supplementary Table 1). Although separated from seawater samples, the sedimentary community showed a high similarity to the Bot_PA community. The Sur_FL community was more similar to the Bot_FL than to the Sur_PA community (Supplementary Table 2; Supplementary Figure 5), which indicated that niche partitioning by lifestyle was more evident than that by water depth. Furthermore, there was a greater level of intra-group variation for planktonic than benthic communities, and the PA communities exhibited higher heterogeneity compared to those in the FL fraction (Figure 4D).
Niche breadth of microbial communities
The community-level niche breadth values varied significantly among habitats (Kruskal–Wallis test, p < 0.001; Figure 5). The niche breadth was highest in sediment, moderate in bottom water, and narrowest in surface water (Figure 5A). In surface water, the niche breadth values were significantly higher for the FL community than for the PA community (Figure 5B; p < 0.001); however, an opposite trend was observed for the niche breadth values in bottom water (Figure 5B; p < 0.001).
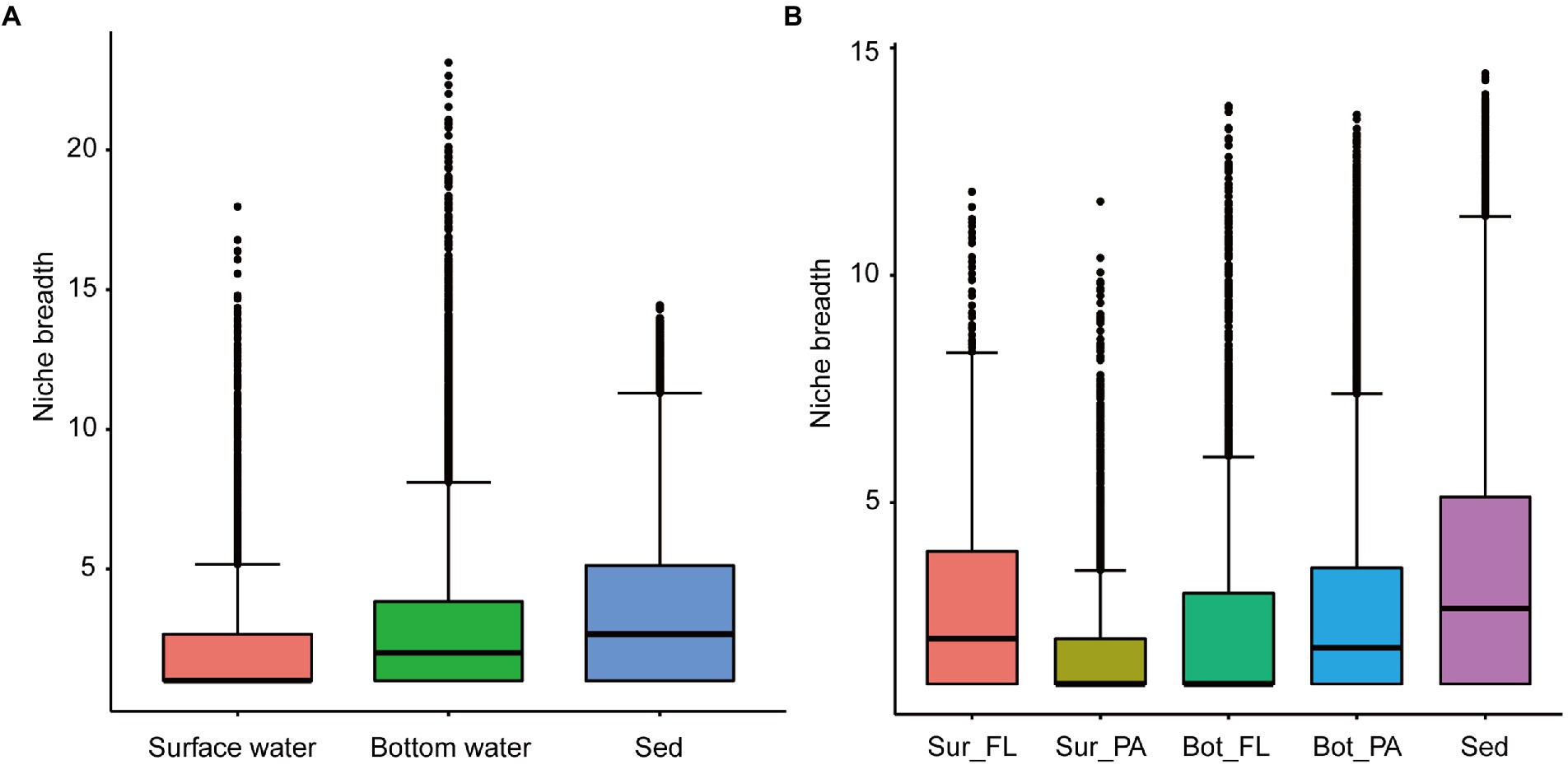
Figure 5. Niche breadth of microbial communities. (A) Comparison of habitat niche breadth of surface water, bottom water, and sediment; (B) Comparison of habitat niche breadth of Sur_FL, Sur_PA, Bot_FL, Bot_PA, and sediment.
Correlations of microbial communities with environmental factors
To further investigate the underlying mechanisms that structure the microbial community in different habitats, partial Mantel test was performed to correlate distance-corrected dissimilarities of microbial community with those of environmental factors (Figure 6). A detailed description of the physical and chemical properties in surface and bottom waters is shown in Supplementary Table 3. A variety of environmental factors showed correlations with the different microbial communities. In surface water, the FL community was positively correlated with nitrate (r = 0.40, p < 0.01) and pH (r = 0.23, p < 0.05), whereas the PA community showed no significant correlations with environmental factors. In bottom water, pressure (r = 0.52, p < 0.01), and nitrite (r = 0.26, p < 0.05) correlated to the FL community, while temperature (r = 0.38, p < 0.05) and density (r = 0.28, p < 0.05) correlated to the PA community.
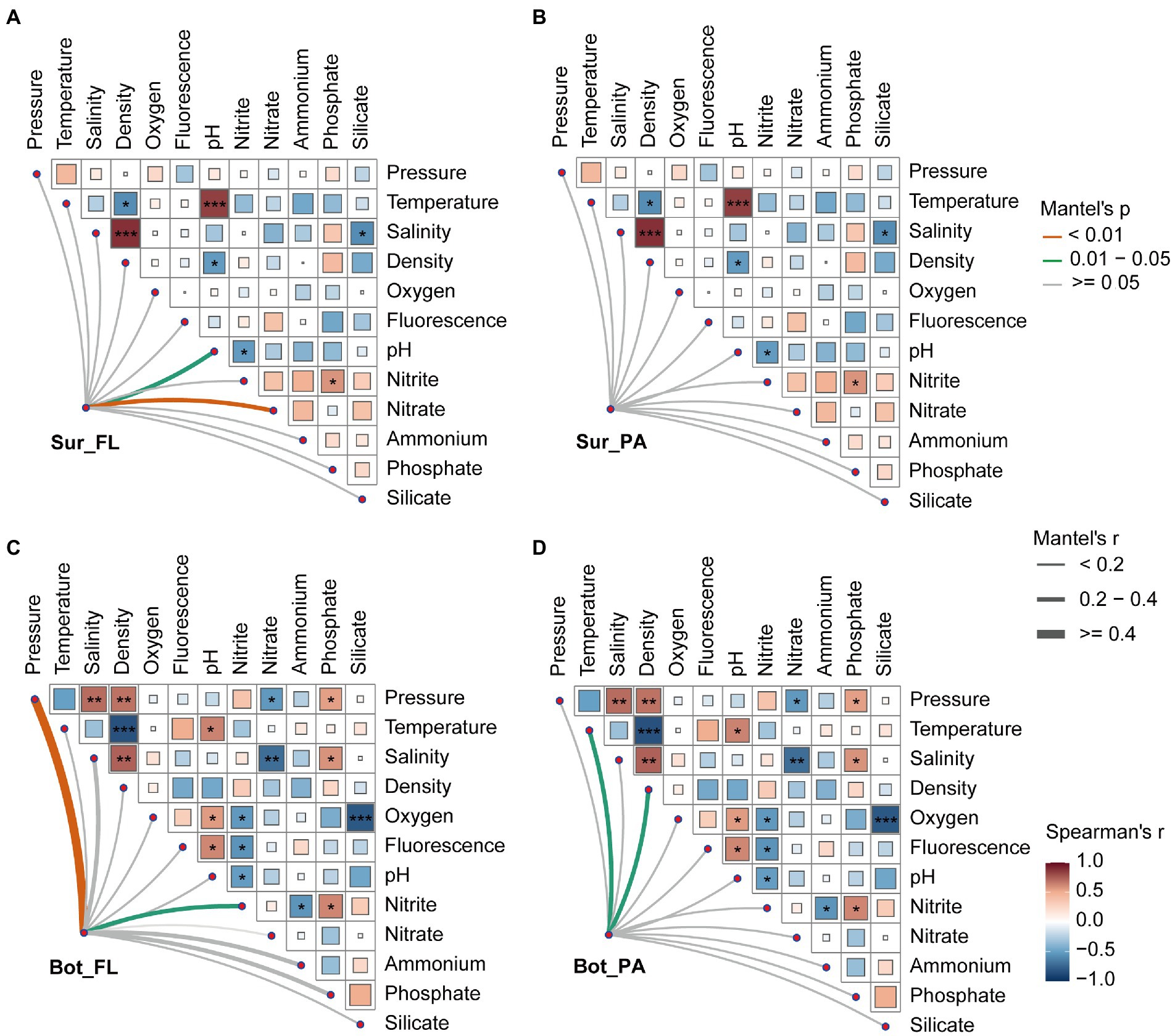
Figure 6. Relationships between microbial community and environmental factors in surface and bottom waters. (A) Sur_FL community; (B) Sur_PA community; (C) Bot_FL community; (D) Bot_PA community. Pairwise comparisons of environmental factors are shown, with a color gradient denoting Spearman’s correlation coefficients.
Assembly process of microbial communities
The |βNTI| values showed that FL and PA communities in surface and bottom waters were mainly influenced by stochastic factors, whereas the effect of deterministic processes was more important than stochastic processes in the benthic communities (Figure 7A). Dispersal limitation and ecological drift of stochastic processes dominated the assembly of microbial communities in surface and bottom waters, but homogeneous selection of deterministic processes was overwhelmingly dominant in the sedimentary community with a proportion of 93.3%. Similarly, the relative contributions of ecological processes to the assembly of communities in different habitats were distinct (Figure 7B). For example, dispersal limitation exerted a great role in Sur_FL (55.2%), Sur_PA (52.4%), and Bot_PA (57.1%) communities, whereas ecological drift contributed a large fraction to the community assembly in Bot_FL (74.3%). Moreover, homogeneous selection had a higher effect on communities in bottom water (10.5% for Bot_FL and 31.4% for Bot_PA) than in surface water (7.6% for Sur_FL and 0.95% for Sur_PA). Homogenizing dispersal and heterogeneous selection played minor roles in the communities.
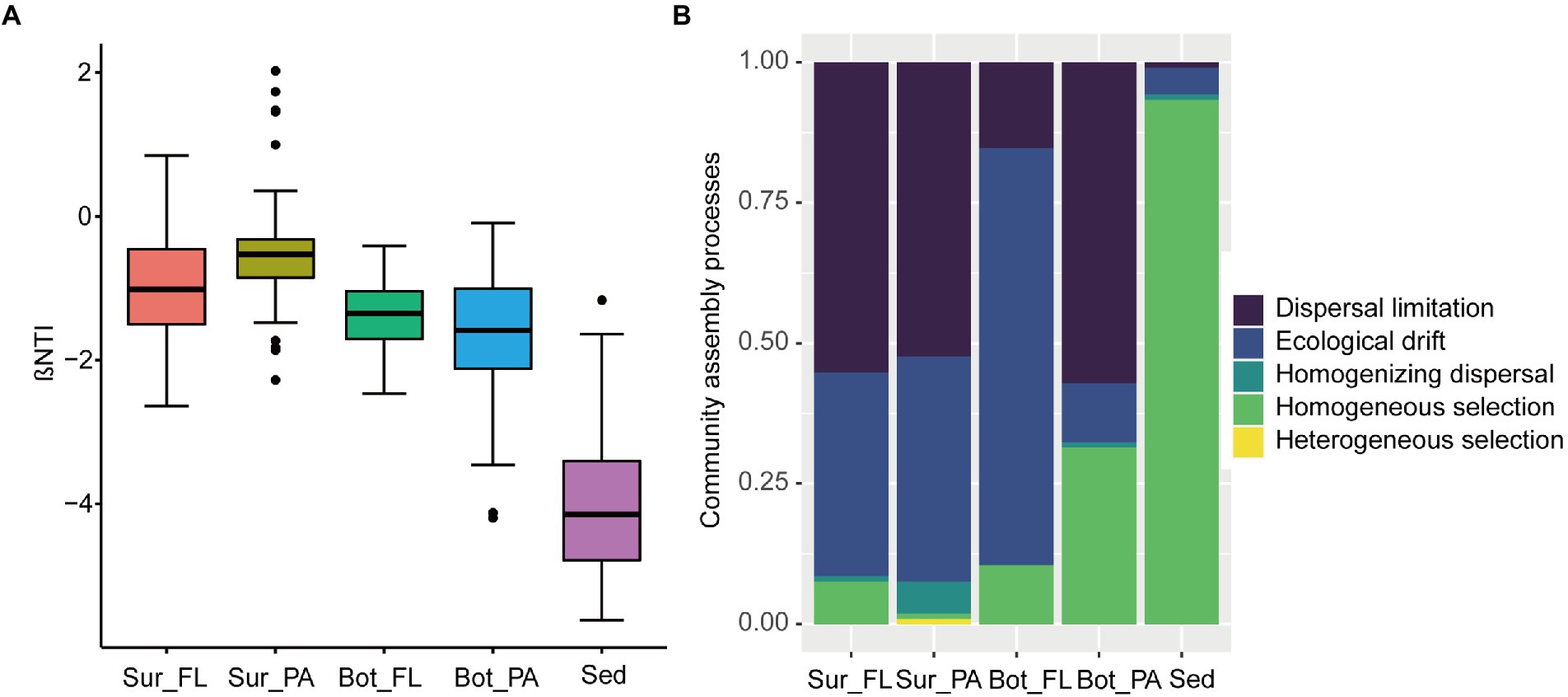
Figure 7. Community assembly mechanisms in different communities. (A) βNTI values for different communities; (B) The assembly processes of different communities with the null model.
The NCM was also performed to verify the results from the null model. The NCM fitted well to the community assembly, showing that stochasticity played an unneglectable role in the assembly of the microbial communities. The degree of fit (R2) was higher in seawater than in sediment, but the migration rates (m) exhibited an opposite trend (Supplementary Table 4), which were in line with the null model analysis. In general, both deterministic and stochastic processes affected the microbial community assembly, and their relative contribution varied across microbial communities in different habitats.
Co-occurrence pattern of microbial communities
A co-occurrence network of 442 nodes and 21,188 edges was generated for the microbial community based on Spearman’s correlation coefficients (Figure 8A). The network consisted of 6 modules, of which modules 1, 2, 3, and 4 accounted for 46.4%, 10.4%, 11.8%, and 30.3% of the whole network, respectively (Figure 8A). The nodes in the network were assigned to 37 identified phyla, accounting for 85.3% of all nodes. Nanoarchaeota (22.9%), Planctomycetota (10.6%), Patescibacteria (10.0%), Proteobacteria (7.0%), Verrucomicrobiota (4.8%), Chloroflexi (4.5%), and Bacteroidota (3.4%) mainly occupied the nodes. The majority of edges (98.0%) in the network were positive, indicating a predominantly cooperative relationship across the microbial community.
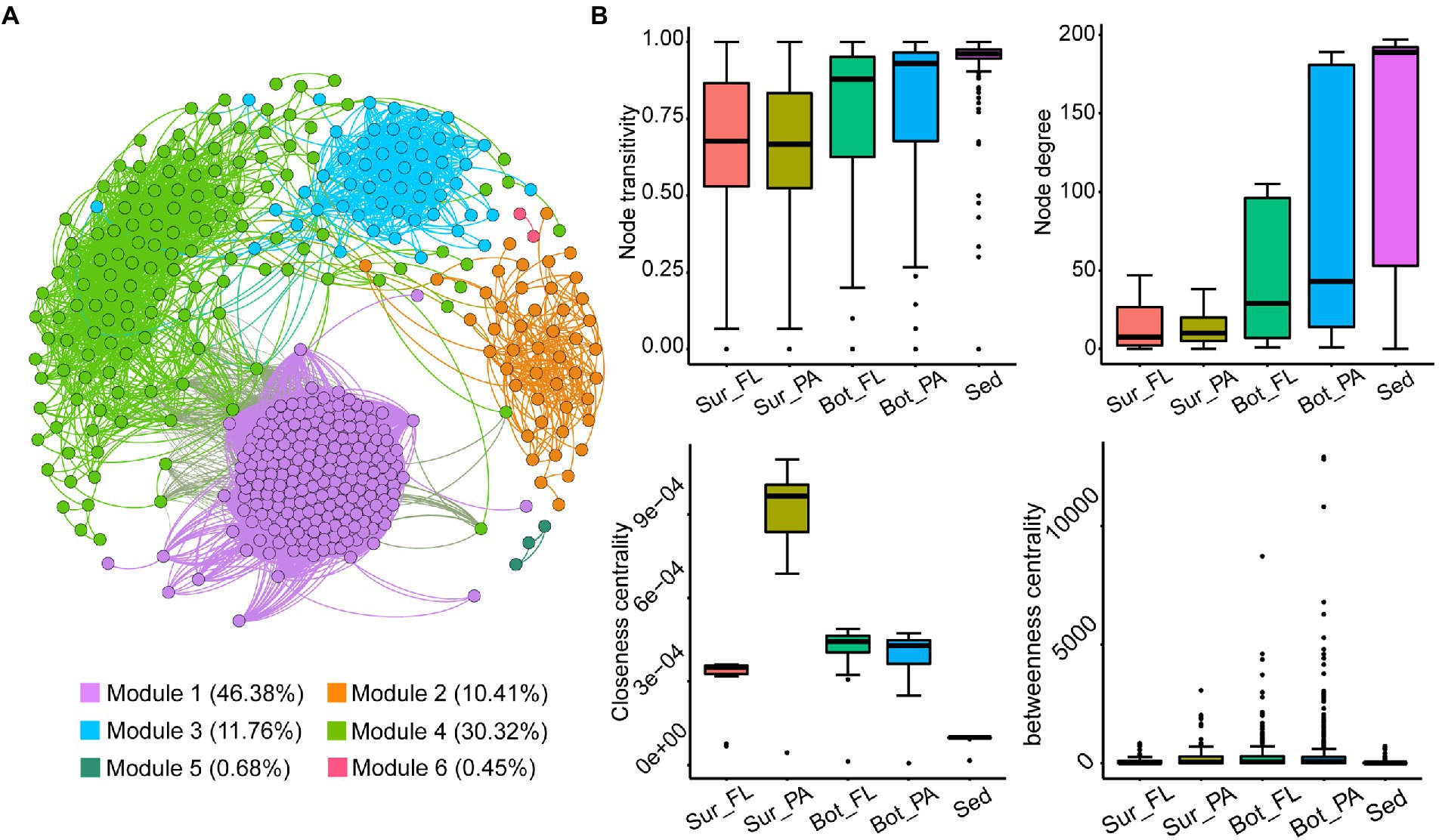
Figure 8. Co-occurrence networks of the microbial community in all samples and topological properties. (A) OTU level co-occurrence network; (B) Comparison of node-level topological features among subcommunities.
To estimate the extent of species co-existence in different habitats, network topology parameters for each of the community groups were calculated (Figure 8B; Supplementary Table 5). The network of different communities demonstrated distinct co-occurrence patterns. Comparison of the network topological parameters showed the complexity of the networks increased from seawaters (surface and bottom waters) to sediment. Specifically, the sediment network had higher clustering coefficient, average degree and graph density, and lower average path length and network diameter than the networks in seawater, suggesting that the benthic community was more interconnected. In seawater, higher clustering coefficient, average degree, and graph density were observed in the bottom water than in surface water. PA communities consistently exhibited much closer interconnections than FL communities, which was confirmed by higher values of the topological parameters. In addition, the node-level topology showed an increased trend in node degree and node transitivity from the surface water to the sediment, and the lowest closeness centrality and betweenness centrality were observed in the sediment network.
Discussion
Exploring the mechanisms that govern community assembly has been a central task in microbial ecology. In this study, we examined the assembly mechanism of FL and PA communities in seawater of the BS and compared it with that of the sediment populations. We found that the dissimilarities in community between water column and sediment were greater than those between FL and PA communities. Dispersal limitation, homogeneous selection, and ecological drift were the dominant processes in microbial community assembly but showed obvious difference among habitats. Our results suggest that ecological processes underlying the microbial community reflect the influence of environmental homogeneity and the potential influence of human disturbance, niche selection, and putative interspecies interactions.
Varying compositions of microbial community in different habitats
In line with some studies in the BS (Chen et al., 2020; Guo et al., 2022; Wu et al., 2022), Proteobacteria, Bacteroidetes, and Cyanobacteria were found to be dominant groups and were abundant in both water and sediments. These groups have been characterized as the important groups in eutrophic coastal ecosystems (Klawonn et al., 2016; Gomes et al., 2019; Zhang et al., 2019; Zhao et al., 2021). However, clear differences in the relative abundance of several dominant microbial phyla were seen between water and sediment samples. Cyanobacteria especially Synechococcus and the proteobacterial SAR11 clade were abundant in the water samples, supporting the global importance of Synechococcus as a clade of primary producer, especially at coastal sites (Nagarkar et al., 2021), and the cosmopolitan nature of SAR11 in the global ocean (Giovannoni, 2017). The sedimentary communities were featured by higher abundances of Desulfobacterota, Planctomycetota, Chloroflexi, Acidobacteriota, and Nitrospirota, probably because they have enhanced capability in mineralization of organic matter and degradation of pollutants under anoxic conditions (Petro et al., 2017). For example, Desulfobacterota and Acidobacteriota are involved in the sulfate and/or sulfur-related processes (Chen et al., 2020) and nitrate reduction processes (Kielak et al., 2016), respectively. Previous studies have reported increased abundance of sulfate-reducing bacteria of Desulfobacterota in coastal than in open ocean sediments (Liu et al., 2015). The capability of Desulfobacterota in degrading organic pollutants (Ayangbenro et al., 2018) indicated that anthropogenic activities can lead to considerable organic pollutants in BS, which may affect the composition pattern of a microbial community.
FL and PA communities have been demonstrated to be compositionally different (Ortega-Retuerta et al., 2013; Bižić-Ionescu et al., 2014; Rieck et al., 2015; Roth Rosenberg et al., 2021), which was also the case in the BS. In addition to this information, we found that the size fractioned community dissimilarities were greater than those caused by depth. The indication was that there was a strong niche partition by lifestyle in the shallow basin, which may be created by river-transported particles and algal growth. This was reflected by a higher proportion of Chitinophagales (represented by an unclassified genus) in the PA community. Chitinophagales belonged to Bacteroidetes containing members associated with algal-derived organic matter degradation (Krüger et al., 2019). Indeed, members of Chitinophagales have been found to be positively correlated with algal biomass productivity (Steichen et al., 2020). Attachment to particles may also provide a shelter for microbes, facilitating escape from the influence of changing environments in seawater (Yung et al., 2016). This was consistent with our finding that the PA communities especially in the surface water were less affected by environmental factors compared to its FL counterpart. It was notable that the PA communities in the bottom water were more similar to the sedimentary communities with increased abundance of Chloroflexi and Desulfobacterota in favor of anoxic conditions. This was not expected since the bottom water samples did not reach hypoxia. Alternatively, we hypothesized that the sediment resuspension event (Wang et al., 2014) may bring the surface sediment-associated cells into the bottom water layers. The residence time and ecological effect of the sedimentary dwellers in the bottom water need further characterization.
Differences in community composition reflect habitat differentiation, which in turn affects the diversity of microbial communities. As expected, the diversity of microbial community in different habitats was distinct. According to the “more individuals hypothesis,” higher resource availability would increase the number of individuals and thus sustain higher microbial diversity (Storch et al., 2018; Furness et al., 2021). In our study, the microbial alpha diversity was higher in sediment than in waters. The sedimentary communities were developed under the effect of long-term sediment accumulation, deposition, and erosion. Therefore, microbial colonization and enrichment on nutrient-rich substrates may be an important reason for the high diversity of the sedimentary community. Similarly, particles can provide a nutrient-richer environment relative to the surrounding water (Azam and Malfatti, 2007), which may have resulted in a higher species diversity in the bottom water PA community (Bižić-Ionescu et al., 2014; Rieck et al., 2015; Yung et al., 2016). Alternatively, this more diverse PA community in bottom water may be contributed by resuspension of sediment-associated cells as noted before. Such a hypothesis was supported by the observation of a similar alpha diversity level between the FL and PA community in surface water. The over-representation of Chitinophagales may have resulted in the relatively less diverse surface PA community. In our study, the microbial community with high alpha diversity tended to have a wider niche breadth, whereas the community with a wider niche breadth tended to have lower beta diversity. As expected, the narrowest niche breadth was observed in the surface PA community, which implied that the number of available niches for the PA community to colonize was relatively limited. An increase in niche breadth would raise the number of taxa at a particular site and increase the probability of the same taxon being present at different sites, resulting in an increased alpha diversity and a decreased inter-site beta diversity (Okie et al., 2015). Additionally, different microbial taxa have distinct ecological niches, and only a subset of a regional collection of species can survive and reproduce under local conditions (Okie et al., 2015). Therefore, niche selection may be one of the reasons for the lower Shannon index in the surface PA community predominated by Chitinophagales.
Ecological processes underlying the community assembly of seawater and sediment
Deterministic and stochastic processes are believed to jointly determine the assembly of microbial communities, but gaps remain with regard to their relative importance (Stegen et al., 2013; Dini-Andreote et al., 2015; Zhou and Ning, 2017; Vass et al., 2020). Contrary to the macroecological theory in which the relative impact of stochasticity increases with resource supply (Chase, 2010), the less effect of stochasticity in nutrient-rich sediments compared to seawater suggested a negative resource supply-stochasticity relationship. Such a relationship has also been observed in soil ecosystems, implying that the relationship between resource supply and stochasticity is dependent on the extent of physicochemical gradients that impose selection (Dini-Andreote et al., 2015). In addition to physicochemical conditions, stochasticity may also be affected by the interaction between dispersal and selection strength (Evans et al., 2016) as well as spatial scale (Martiny et al., 2011).
Previous studies have demonstrated that dispersal limitation played an important role in community assembly (Nemergut et al., 2013; Stegen et al., 2015; Liu et al., 2020). This ecological process was considered to be resulted from large spatial distance and/or steer environmental variance (Zhou and Ning, 2017). The dispersal capacity of microbes is generally thought to be higher in seawater than in sediment (Zinger et al., 2014; Liu et al., 2020). However, our results revealed a higher effect of dispersal limitation in seawater, especially in the bottom PA fraction. One plausible explanation for this is that the planktonic bacteria in BS seawater are largely affected by particles as noted earlier. This was similar to the previous report that dispersal limitation played a more important role in the assembly process of the bacterial community on microplastics than those in water and sediments (Zhang et al., 2022). Association with particles can decrease the chance of dispersal. Although particles may also spread in seawater, the abundant Chloroplast 16S rRNA gene sequences in the PA fraction (data not shown) reflected high algal biomass due to eutrophication. The potential large particle size of eukaryotic algae may have increased the possibility of dispersal limitation. Difference in environmental conditions and micro-niches provided by the particulate matter would act as a hindrance and make it more difficult for microbial dispersal (Okie et al., 2015). Alternatively, due to frequent human activity-derived disturbance, the planktonic microorganisms may have evolved to tolerate the environmental dynamics and thus respond weakly to deterministic factors (Liu et al., 2020). Across water depths, increased effect of dispersal limitation with water depth due to wind decay has been found in the assembly of bacterial community in the East China Sea (Wu et al., 2018). However, we showed here that dispersal limitation exerted a greater influence in the surface than the bottom FL community. The shallow water depth of BS causing similar wind effect across depths and the potential effect of algae in the surface water may act as possible explanations.
Low or limited dispersal can introduce stochasticity by increasing ecological drift (Hanson et al., 2012; Stegen et al., 2013; Dini-Andreote et al., 2015), whereas high dispersal rates result in higher microbial biomass and species diversity and can introduce mass effects (Evans et al., 2016). Consistently, in this study, stochasticity contributed by ecological drift was a vital aspect of community assembly in seawater, particularly in FL communities. And, our result was in line with the hypothesis that drift will become dominant under weak selection pressure and low species diversity (Chase and Myers, 2011). Indeed, the lower alpha diversity of FL relative to PA communities in bottom water leads to a stronger effect of ecological drift on the FL community assembly.
The sediment habitats are often more heterogeneous and have a lower regional connectivity, decreasing the probability of active dispersal in sedimentary microorganisms (Zinger et al., 2014). Thus, it was considered that sedimentary microorganisms may have a lower dispersal capacity, which may enhance the impact of dispersal limitation on community assembly (Östman et al., 2010). However, both the null model and neutral model showed that the sedimentary community experienced lower dispersal limitation compared to the seawater community. The sediments could have mobility due to transport by water currents (Liu et al., 2018), causing the sediment resuspension event facilitating cell dispersal. Another potential reason may be that microbial communities can be constrained by niche breadth. Niche breadth generally represents the range or variety of conditions defining a species’ niche and the adaptability of a species to its environment is defined by niche breadth (Sexton et al., 2017). On this basis, the degree of niche breadth may be determined by the microbial sensitivity/resistance in response to environmental gradients, thus reflecting the importance of environmental filtering (deterministic process). Indeed, previous studies have shown that species with wider niche breadths are influenced less by environmental factors (Pandit et al., 2009; Wu et al., 2018). In this study, microbial communities inhabiting sediments have significantly higher niche breadth than those in seawater, which may be determined by the small spatial scale-covered and low environmental variances. Thus, the influence of deterministic factors was expected to be low. However, homogeneous selection was the most important ecological process explaining more than 90% of the sedimentary community variation. The indication of homogeneous selection is that similarity in some sedimentary factors such as the high content of organic matter and pollutions (Gao et al., 2014; Zhao et al., 2018) may have led to habitat homogenization (Liu et al., 2020; Zhang et al., 2021) and select similar communities across samples, although environmental factors of the sediment samples were not measured here. Therefore, despite high deterministic contribution, the consistent selection pressures driven by consistent environmental conditions underpin the similarity of community composition in the sediment and lead to high niche breadth values. This selection minimizes the role of stochastic processes and drives similarity in local community structures (Evans et al., 2016).
Linking community assembly processes and co-occurrence patterns: Homogeneous selection enhances the connectivity of microbial communities
Microorganisms occupying a specific ecological niche can form complex interaction networks (Faust and Raes, 2012). Hence, interspecific interactions are important factors structuring microbial community assembly (Yuan et al., 2019; Ortiz et al., 2021). Many studies have shown that diverse microbial groups can influence other taxonomic groups by interactions (Morriën et al., 2017; Chen et al., 2019; Trivedi et al., 2020). Co-occurrence networks have been frequently used to infer potential interactions, although topology-based system approach did not reflect true inter-taxa correlations (Liu et al., 2019). Nodes in the constructed network were comprised of many rare taxa such as Nanoarchaeota and Patescibacteria. This implied that rare species could play key roles in maintaining the microbial community structure, in line with previous studies in soil (Lupatini et al., 2014) and deep-sea sediments (Zhang et al., 2021). The majority of connections (>96%) in the networks were positive, likely indicative of more frequent putative cooperation than competition. On the other hand, the co-occurrence of many microorganisms may reflect cross-feeding, co-aggregation, co-colonization, and niche overlap (Faust and Raes, 2012; Faust et al., 2015; Shi et al., 2019). For example, Bacteroidetes, one of the dominant groups in samples of this study, can degrade polysaccharides (Gómez-Pereira et al., 2011) and their degradation products can promote the growth of other heterotrophic bacteria (Nagata et al., 2003). Additionally, the high-complexity networks tend to have greater stability in communities (Mougi and Kondoh, 2012). The relatively high community stability indicated by the more clustered network structures and stronger connections in sediments compared to seawater may be facilitated by efficient putative resource and information transfer between species (Morriën et al., 2017). Consistent with previous studies (Zhang et al., 2016b; Xu et al., 2018), the network of the PA communities in seawater was more complex than the FL communities, which indicated that PA communities exhibited higher stability than the FL communities. The reasons for this may be that PA communities are physically closer to their collaborators or competitors within the relatively confined particle space, thus facilitating putative metabolic cooperation and genetic exchange (Zhang et al., 2014, 2016b; Dang and Lovell, 2016). And, the large amount of nutrients on the particles could create more suitable micro-niches for the PA communities (Xu et al., 2018); these particulate micro-niches might play a vital role in shaping the structure of PA communities (Zhang et al., 2016b).
Determining the linkages between community assembly and species coexistence is essential to understand the mechanisms that maintain community diversity (Vályi et al., 2016; Jiao et al., 2020; Li et al. 2021a). By constructing the linkage between assembly processes and species co-occurrence in microbial communities, Jiao et al. (2020) found that the microbial co-occurrences tended to be higher when agricultural soil microbial communities were mainly driven by dispersal limitation. By contrast, Li et al. (2021a) found that deterministic processes (mainly heterogeneous selection) became a less dominant ecological process in the intermonsoon communities in the Eastern Indian Ocean as the co-occurrence increased. These linkages were thought to be largely related to environmental filtering (Jiao et al., 2020; Li et al., 2021a). In this study, an opposite trend was observed that stochastic processes become less dominant as the connection of microbial communities increased. The reason may be that the different mechanisms selected for microbial community assembly in different habitats, thus causing microbial communities in specific ecological niches to change their coexistence strategies. The contemporary coexistence theory highlights that coexistence depends on niche differences and fitness differences (HilleRisLambers et al., 2012). The wider community-level niche breadth observed in sediments would favor coexistence of different species by reducing direct competition, namely niche complementarity (Konopka et al., 2015). Compared to seawater, the sedimentary community had higher diversity, accompanied by the high positive correlation (99.3%) in the network. Therefore, microbial associations could contribute to microbial assembly by acting as a selective force to promote diversity.
Conclusion
In this study, we compared the diversity, community composition, assembly processes, and co-occurrence patterns of microbial communities in different marine habitats in BS, including those between seawater and sediment and between FL and PA lifestyles. The most dominant taxa were Proteobacteria, Bacteroidota, Actinobacteriota, and Cyanobacteria. The community alpha diversity showed a gradual increase from surface water to sediment, which was accompanied with the decreased beta diversity and the increased average niche breadth values. Furthermore, we provided evidence that the community of different living habitats was structured by different ecological processes. In contrast to a higher contribution of stochastic processes in seawater community, the sedimentary community was mainly shaped by deterministic processes. Concomitant with a decrease in the contribution of stochastic processes from surface water to sediment, the degree of microbial associations increased. We also showed distinct assembly pattern between the FL and PA communities. The dynamics of microbial communities and assembly patterns may be contributed by the local environmental conditions, ocean currents, and to a larger extent by human activities. Overall, our results demonstrated habitat-specific community assembly and potential linkages with association patterns, underscoring the importance for an integrated understanding of microbial community dynamics.
Data availability statement
The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov PRJNA855144 and https://www.biosino.org/node, OEP003571.
Author contributions
JiwL and X-HZ designed the experiments. JinL and XW analyzed the data and wrote the manuscript. JiaL and XL carried out the laboratory experiments. JiwL, X-HZ, XW, and JinL revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (nos. U1806211, 92051115, and 41976101), the Fundamental Research Funds for the Central Universities (nos. 202141009 and 202172002), and the National Key Research and Development Program of China (no. 2018YFE0124100). Data and samples were collected onboard of R/V Beidou implementing the open research cruise NORC2019-01 supported by the NSFC Shiptime Sharing Project (no. 41849901).
Acknowledgments
We thank all of the scientists and crews on the cruise for their assistance with sampling during the cruises. We also thank Guodong Song from Ocean University of China for providing sediment samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1003623/full#supplementary-material
Footnotes
References
Allen, R., Hoffmann, L. J., Larcombe, M. J., Louisson, Z., and Summerfield, T. C. (2020). Homogeneous environmental selection dominates microbial community assembly in the oligotrophic South Pacific gyre. Mol. Ecol. 29, 4680–4691. doi: 10.1111/mec.15651
Ayangbenro, A. S., Olanrewaju, O. S., and Babalola, O. O. (2018). Sulfate-reducing bacteria as an effective tool for sustainable acid mine bioremediation. Front. Microbiol. 9:1986. doi: 10.3389/fmicb.2018.01986
Azam, F., and Malfatti, F. (2007). Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 5, 782–791. doi: 10.1038/nrmicro1747
Banerjee, S., Schlaeppi, K., and van der Heijden, M. G. A. (2018). Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 16, 567–576. doi: 10.1038/s41579-018-0024-1
Berry, D., and Widder, S. (2014). Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 5:219. doi: 10.3389/fmicb.2014.00219
Bižić-Ionescu, M., Zeder, M., Ionescu, D., Orlić, S., Fuchs, B. M., Grossart, H. P., et al. (2014). Comparison of bacterial communities on limnic versus coastal marine particles reveals profound differences in colonization. Environ. Microbiol. 17, 3500–3514. doi: 10.1111/1462-2920.12466
Chase, J. M. (2010). Stochastic community assembly causes higher biodiversity in more productive environments. Science 328, 1388–1391. doi: 10.1126/science.1187820
Chase, J. M., and Myers, J. A. (2011). Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B Biol. Sci. 366, 2351–2363. doi: 10.1098/rstb.2011.0063
Chave, J. (2004). Neutral theory and community ecology. Ecol. Lett. 7, 241–253. doi: 10.1111/j.1461-0248.2003.00566.x
Chen, Q., Fan, J., Su, J., Ming, H., Sun, Z., Li, M., et al. (2020). Spatial distribution characteristics of bacterial community structure and gene abundance in sediments of the Bohai Sea. Acta Oceanol. Sin. 39, 69–78. doi: 10.1007/s13131-020-1554-8
Chen, L., Jiang, Y., Liang, C., Luo, Y., Xu, Q., Han, C., et al. (2019). Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 7:77. doi: 10.1186/s40168-019-0693-7
Chen, S., Zhou, Y., Chen, Y., and Gu, J. (2018). Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890. doi: 10.1093/bioinformatics/bty560
Chesson, P. (2000). Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366. doi: 10.1146/annurev.ecolsys.31.1.343
Csardi, G., and Nepusz, T. (2006). The igraph software package for complex network research. InterJ. Complex Syst. 1695: 1–6.
Dang, H., and Lovell, C. R. (2016). Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 80, 91–138. doi: 10.1128/mmbr.00037-15
Dini-Andreote, F., Stegen, J. C., van Elsas, J. D., and Salles, J. F. (2015). Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. 112, E1326–E1332. doi: 10.1073/pnas.1414261112
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Evans, S., Martiny, J. B. H., and Allison, S. D. (2016). Effects of dispersal and selection on stochastic assembly in microbial communities. ISME J. 11, 176–185. doi: 10.1038/ismej.2016.96
Falkowski, P. G., Fenchel, T., and Delong, E. F. (2008). The microbial engines that drive Earth’s biogeochemical cycles. Science 320, 1034–1039. doi: 10.1126/science.1153213
Faust, K., Lima-Mendez, G., Lerat, J. S., Sathirapongsasuti, J. F., Knight, R., Huttenhower, C., et al. (2015). Cross-biome comparison of microbial association networks. Front. Microbiol. 6:1200. doi: 10.3389/fmicb.2015.01200
Faust, K., and Raes, J. (2012). Microbial interactions: from networks to models. Nat. Rev. Microbiol. 10, 538–550. doi: 10.1038/nrmicro2832
Furness, E. N., Garwood, R. J., Mannion, P. D., and Sutton, M. D. (2021). Productivity, niche availability, species richness, and extinction risk: untangling relationships using individual-based simulations. Ecol. Evol. 11, 8923–8940. doi: 10.1002/ece3.7730
Gao, X., Zhou, F., and Chen, C.-T. A. (2014). Pollution status of the Bohai Sea: an overview of the environmental quality assessment related trace metals. Environ. Int. 62, 12–30. doi: 10.1016/j.envint.2013.09.019
Giovannoni, S. J. (2017). SAR11 bacteria: the Most abundant plankton in the oceans. Ann. Rev. Mar. Sci. 9, 231–255. doi: 10.1146/annurev-marine-010814-015934
Gomes, J., Khandeparker, R., Meena, R. M., and Ramaiah, N. (2019). Bacterial community composition markedly altered by coastal hypoxia. Indian J. Microbiol. 59, 200–208. doi: 10.1007/s12088-019-00790-5
Gómez-Pereira, P. R., Schüler, M., Fuchs, B. M., Bennke, C., Teeling, H., Waldmann, J., et al. (2011). Genomic content of uncultured Bacteroidetes from contrasting oceanic provinces in the North Atlantic Ocean. Environ. Microbiol. 14, 52–66. doi: 10.1111/j.1462-2920.2011.02555.x
Guo, X., Song, G., Li, Y., Zhao, L., and Wang, J. (2022). Switch of bacteria community under oxygen depletion in sediment of Bohai Sea. Front. Mar. Sci. 9:833513. doi: 10.3389/fmars.2022.833513
Gweon, H. S., Bowes, M. J., Moorhouse, H. L., Oliver, A. E., Bailey, M. J., Acreman, M. C., et al. (2021). Contrasting community assembly processes structure lotic bacteria metacommunities along the river continuum. Environ. Microbiol. 23, 484–498. doi: 10.1111/1462-2920.15337
Hanson, C. A., Fuhrman, J. A., Horner-Devine, M. C., and Martiny, J. B. H. (2012). Beyond biogeographic patterns: processes shaping the microbial landscape. Nat. Rev. Microbiol. 10, 497–506. doi: 10.1038/nrmicro2795
He, Q., Wang, S., Hou, W., Feng, K., Li, F., Hai, W., et al. (2021). Temperature and microbial interactions drive the deterministic assembly processes in sediments of hot springs. Sci. Total Environ. 772:145465. doi: 10.1016/j.scitotenv.2021.145465
HilleRisLambers, J., Adler, P. B., Harpole, W. S., Levine, J. M., and Mayfield, M. M. (2012). Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst. 43, 227–248. doi: 10.1146/annurev-ecolsys-110411-160411
Huang, H. (2021). linkET: everything is linkable. R package version 0.0.3.2. Available at: https://github.com/Hy4m/linkET
Jari Oksanen, F., Blanchet, G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2020). Vegan: Community ecology package. R package version 2.5-7. Available at: https://CRAN.R-project.org/package=vegan
Jiao, S., Yang, Y., Xu, Y., Zhang, J., and Lu, Y. (2020). Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 14, 202–216. doi: 10.1038/s41396-019-0522-9
Kembel, S. W., Cowan, P. D., Helmus, M. R., Cornwell, W. K., Morlon, H., Ackerly, D. D., et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. doi: 10.1093/bioinformatics/btq166
Kielak, A. M., Barreto, C. C., Kowalchuk, G. A., van Veen, J. A., and Kuramae, E. E. (2016). The ecology of Acidobacteria: moving beyond genes and genomes. Front. Microbiol. 7:744. doi: 10.3389/fmicb.2016.00744
Klawonn, I., Nahar, N., Walve, J., Andersson, B., Olofsson, M., Svedén, J. B., et al. (2016). Cell-specific nitrogen- and carbon-fixation of cyanobacteria in a temperate marine system (Baltic Sea). Environ. Microbiol. 18, 4596–4609. doi: 10.1111/1462-2920.13557
Konopka, A., Lindemann, S., and Fredrickson, J. (2015). Dynamics in microbial communities: unraveling mechanisms to identify principles. ISME J. 9, 1488–1495. doi: 10.1038/ismej.2014.251
Krüger, K., Chafee, M., Ben Francis, T., Glavina del Rio, T., Becher, D., Schweder, T., et al. (2019). In marine Bacteroidetes the bulk of glycan degradation during algae blooms is mediated by few clades using a restricted set of genes. ISME J. 13, 2800–2816. doi: 10.1038/s41396-019-0476-y
Li, Y., Gao, Y., Zhang, W., Wang, C., Wang, P., Niu, L., et al. (2019). Homogeneous selection dominates the microbial community assembly in the sediment of the three gorges reservoir. Sci. Total Environ. 690, 50–60. doi: 10.1016/j.scitotenv.2019.07.014
Li, D., Li, J. L., Yin, L. Z., Luo, X. Q., Ahmad, M., Fang, B. Z., et al. (2022). Habitat-dependent prokaryotic microbial community, potential keystone species, and network complexity in a subtropical estuary. Environ. Res. 212:113376. doi: 10.1016/j.envres.2022.113376
Li, M., Mi, T., He, H., Chen, Y., and Yu, Z. (2021b). Active bacterial and archaeal communities in coastal sediments: biogeography pattern, assembly process and co-occurrence relationship. Sci. Total Environ. 750:142252. doi: 10.1016/j.scitotenv.2020.142252
Li, L., Pujari, L., Wu, C., Huang, D., Wei, Y., Guo, C., et al. (2021a). Assembly processes and co-occurrence patterns of abundant and rare bacterial community in the eastern Indian Ocean. Front. Microbiol. 12:616956. doi: 10.3389/fmicb.2021.616956
Li, Y., Wolanski, E., and Zhang, H. (2015). What processes control the net currents through shallow straits? A review with application to the Bohai Strait, China. Estuar. Coast. Shelf Sci. 158, 1–11. doi: 10.1016/j.ecss.2015.03.013
Liu, K., Liu, Y., Hu, A., Wang, F., Chen, Y., Gu, Z., et al. (2020b). Different community assembly mechanisms underlie similar biogeography of bacteria and microeukaryotes in Tibetan lakes. FEMS Microbiol. Ecol. 96:fiaa071. doi: 10.1093/femsec/fiaa071
Liu, J., Liu, X., Wang, M., Qiao, Y., Zheng, Y., and Zhang, X.-H. (2015). Bacterial and archaeal communities in sediments of the north Chinese marginal seas. Microb. Ecol. 70, 105–117. doi: 10.1007/s00248-014-0553-8
Liu, J., Meng, Z., Liu, X., and Zhang, X.-H. (2019). Microbial assembly, interaction, functioning, activity and diversification: a review derived from community compositional data. Marine Life Sci. Technol. 1, 112–128. doi: 10.1007/s42995-019-00004-3
Liu, T., Zhang, A. N., Wang, J., Liu, S., Jiang, X., Dang, C., et al. (2018). Integrated biogeography of planktonic and sedimentary bacterial communities in the Yangtze River. Microbiome 6:16. doi: 10.1186/s40168-017-0388-x
Liu, J., Zhu, S., Liu, X., Yao, P., Ge, T., and Zhang, X.-H. (2020). Spatiotemporal dynamics of the archaeal community in coastal sediments: assembly process and co-occurrence relationship. ISME J. 14, 1463–1478. doi: 10.1038/s41396-020-0621-7
Logares, R., Deutschmann, I. M., Junger, P. C., Giner, C. R., Krabberød, A. K., Schmidt, T. S. B., et al. (2020). Disentangling the mechanisms shaping the surface ocean microbiota. Microbiome 8:55. doi: 10.1186/s40168-020-00827-8
Lupatini, M., Suleiman, A. K. A., Jacques, R. J. S., Antoniolli, Z. I., de Siqueira Ferreira, A., Kuramae, E. E., et al. (2014). Network topology reveals high connectance levels and few key microbial genera within soils. Front. Environ. Sci. 2:10. doi: 10.3389/fenvs.2014.00010
Lv, X., Zhao, K., Xue, R., Liu, Y., Xu, J., and Ma, B. (2019). Strengthening insights in microbial ecological networks from theory to applications. mSystems 4, e00124–e00119. doi: 10.1128/msystems.00124-19
Magoč, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Martiny, J. B. H., Bohannan, B. J. M., Brown, J. H., Colwell, R. K., Fuhrman, J. A., Green, J. L., et al. (2006). Microbial biogeography: putting microorganisms on the map. Nat. Rev. Microbiol. 4, 102–112. doi: 10.1038/nrmicro1341
Martiny, J. B. H., Eisen, J. A., Penn, K., Allison, S. D., and Horner-Devine, M. C. (2011). Drivers of bacterial β-diversity depend on spatial scale. Proc. Natl. Acad. Sci. 108, 7850–7854. doi: 10.1073/pnas.1016308108
Ming, H., Fan, J., Liu, J., Su, J., Wan, Z., Wang, Y., et al. (2021). Full-length 16S rRNA gene sequencing reveals spatiotemporal dynamics of bacterial community in a heavily polluted estuary, China. Environ. Pollut. 275:116567. doi: 10.1016/j.envpol.2021.116567
Mo, Y., Zhang, W., Wilkinson, D. M., Yu, Z., Xiao, P., and Yang, J. (2020). Biogeography and co-occurrence patterns of bacterial generalists and specialists in three subtropical marine bays. Limnol. Oceanogr. 66, 793–806. doi: 10.1002/lno.11643
Mo, Y., Zhang, W., Yang, J., Lin, Y., Yu, Z., and Lin, S. (2018). Biogeographic patterns of abundant and rare bacterioplankton in three subtropical bays resulting from selective and neutral processes. ISME J. 12, 2198–2210. doi: 10.1038/s41396-018-0153-6
Morriën, E., Hannula, S. E., Snoek, L. B., Helmsing, N. R., Zweers, H., de Hollander, M., et al. (2017). Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 8:14349. doi: 10.1038/ncomms14349
Mougi, A., and Kondoh, M. (2012). Diversity of interaction types and ecological community stability. Science 337, 349–351. doi: 10.1126/science.1220529
Nagarkar, M., Wang, M., Valencia, B., and Palenik, B. (2021). Spatial and temporal variations in Synechococcus microdiversity in the Southern California coastal ecosystem. Environ. Microbiol. 23, 252–266. doi: 10.1111/1462-2920.15307
Nagata, T., Meon, B., and Kirchman, L. (2003). Microbial degradation of peptidoglycan in seawater. Limnol. Oceanogr. 48, 745–754. doi: 10.4319/lo.2003.48.2.0745
Nemergut, D. R., Schmidt, S. K., Fukami, T., O’Neill, S. P., Bilinski, T. M., Stanish, L. F., et al. (2013). Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 77, 342–356. doi: 10.1128/mmbr.00051-12
Okie, J. G., Van Horn, D. J., Storch, D., Barrett, J. E., Gooseff, M. N., Kopsova, L., et al. (2015). Niche and metabolic principles explain patterns of diversity and distribution: theory and a case study with soil bacterial communities. Proc. R. Soc. B Biol. Sci. 282:20142630. doi: 10.1098/rspb.2014.2630
Ortega-Retuerta, E., Joux, F., Jeffrey, W. H., and Ghiglione, J. F. (2013). Spatial variability of particle-attached and free-living bacterial diversity in surface waters from the Mackenzie River to the Beaufort Sea (Canadian Arctic). Biogeosciences 10, 2747–2759. doi: 10.5194/bg-10-2747-2013
Ortiz, A., Vega, N. M., Ratzke, C., and Gore, J. (2021). Interspecies bacterial competition regulates community assembly in the C. elegans intestine. ISME J. 15, 2131–2145. doi: 10.1038/s41396-021-00910-4
Östman, Ö., Drakare, S., Kritzberg, E. S., Langenheder, S., Logue, J. B., and Lindström, E. S. (2010). Regional invariance among microbial communities. Ecol. Lett. 13, 118–127. doi: 10.1111/j.1461-0248.2009.01413.x
Pandit, S. N., Kolasa, J., and Cottenie, K. (2009). Contrasts between habitat generalists and specialists: an empirical extension to the basic metacommunity framework. Ecology 90, 2253–2262. doi: 10.1890/08-0851.1
Petro, C., Starnawski, P., Schramm, A., and Kjeldsen, K. U. (2017). Microbial community assembly in marine sediments. Aquat. Microb. Ecol. 79, 177–195. doi: 10.3354/ame01826
Prosser, J. I., Bohannan, B. J. M., Curtis, T. P., Ellis, R. J., Firestone, M. K., Freckleton, R. P., et al. (2007). The role of ecological theory in microbial ecology. Nat. Rev. Microbiol. 5, 384–392. doi: 10.1038/nrmicro1643
Revelle, W. (2021). Psych: Procedures for personality and psychological research, Northwestern University, Evanston, Illinois, USA.
Rieck, A., Herlemann, D. P. R., Jürgens, K., and Grossart, H. P. (2015). Particle-associated differ from free-living bacteria in surface waters of the Baltic Sea. Front. Microbiol. 6:1297. doi: 10.3389/fmicb.2015.01297
Roth Rosenberg, D., Haber, M., Goldford, J., Lalzar, M., Aharonovich, D., Al-Ashhab, A., et al. (2021). Particle-associated and free-living bacterial communities in an oligotrophic sea are affected by different environmental factors. Environ. Microbiol. 23, 4295–4308. doi: 10.1111/1462-2920.15611
Russel, J. (2021). MicEco: various functions for microbial community data. R package version 0.9.16. Available at: https://github.com/Russel88/MicEco
Sexton, J. P., Montiel, J., Shay, J. E., Stephens, M. R., and Slatyer, R. A. (2017). Evolution of ecological niche breadth. Annu. Rev. Ecol. Evol. Syst. 48, 183–206. doi: 10.1146/annurev-ecolsys-110316-023003
Shi, Y., Fan, K., Li, Y., Yang, T., He, J.-S., and Chu, H. (2019). Archaea enhance the robustness of microbial co-occurrence networks in Tibetan plateau soils. Soil Sci. Soc. Am. J. 83, 1093–1099. doi: 10.2136/sssaj2018.11.0426
Shi, J., Zuo, Y., Qu, W., Liu, X., Fan, Y., Cao, P., et al. (2022). Stochastic processes shape the aggregation of free-living and particle-attached bacterial communities in the Yangtze River estuary, China. J. Basic Microbiol. doi: 10.1002/jobm.202100666
Sloan, W. T., Lunn, M., Woodcock, S., Head, I. M., Nee, S., and Curtis, T. P. (2006). Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 8, 732–740. doi: 10.1111/j.1462-2920.2005.00956.x
Song, N., Wang, N., Lu, Y., and Zhang, J. (2016). Temporal and spatial characteristics of harmful algal blooms in the Bohai Sea during 1952–2014. Cont. Shelf Res. 122, 77–84. doi: 10.1016/j.csr.2016.04.006
Stegen, J. C., Lin, X., Fredrickson, J. K., Chen, X., Kennedy, D. W., Murray, C. J., et al. (2013). Quantifying community assembly processes and identifying features that impose them. ISME J. 7, 2069–2079. doi: 10.1038/ismej.2013.93
Stegen, J. C., Lin, X., Fredrickson, J. K., and Konopka, A. E. (2015). Estimating and mapping ecological processes influencing microbial community assembly. Front. Microbiol. 6:370. doi: 10.3389/fmicb.2015.00370
Stegen, J. C., Lin, X., Konopka, A. E., and Fredrickson, J. K. (2012). Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 6, 1653–1664. doi: 10.1038/ismej.2012.22
Steichen, S. A., Gao, S., Waller, P., and Brown, J. K. (2020). Association between algal productivity and phycosphere composition in an outdoor Chlorella sorokiniana reactor based on multiple longitudinal analyses. J. Microbial. Biotechnol. 13, 1546–1561. doi: 10.1111/1751-7915.13591
Storch, D., Bohdalková, E., and Okie, J. (2018). The more-individuals hypothesis revisited: the role of community abundance in species richness regulation and the productivity-diversity relationship. Ecol. Lett. 21, 920–937. doi: 10.1111/ele.12941
Tilman, D. (2004). Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. 101, 10854–10861. doi: 10.1073/pnas.0403458101
Trivedi, P., Leach, J. E., Tringe, S. G., Sa, T., and Singh, B. K. (2020). Plant-microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol. 18, 607–621. doi: 10.1038/s41579-020-0412-1
Vályi, K., Mardhiah, U., Rillig, M. C., and Hempel, S. (2016). Community assembly and coexistence in communities of arbuscular mycorrhizal fungi. ISME J. 10, 2341–2351. doi: 10.1038/ismej.2016.46
Vass, M., Székely, A. J., Lindström, E. S., and Langenheder, S. (2020). Using null models to compare bacterial and microeukaryotic metacommunity assembly under shifting environmental conditions. Sci. Rep. 10:2455. doi: 10.1038/s41598-020-59182-1
Walters, W., Hyde, E. R., Berg-Lyons, D., Ackermann, G., Humphrey, G., Parada, A., et al. (2016). Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 1, e00009–e00015. doi: 10.1128/msystems.00009-15
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/aem.00062-07
Wang, Y., Pan, J., Yang, J., Zhou, Z., Pan, Y., and Li, M. (2020). Patterns and processes of free-living and particle-associated bacterioplankton and archaeaplankton communities in a subtropical river-bay system in South China. Limnol. Oceanogr. 65, S161–S179. doi: 10.1002/lno.11314
Wang, J., Shen, J., Wu, Y., Tu, C., Soininen, J., Stegen, J. C., et al. (2013). Phylogenetic beta diversity in bacterial assemblages across ecosystems: deterministic versus stochastic processes. ISME J. 7, 1310–1321. doi: 10.1038/ismej.2013.30
Wang, H., Wang, A., Bi, N., Zeng, X., and Xiao, H. (2014). Seasonal distribution of suspended sediment in the Bohai Sea, China. Cont. Shelf Res. 90, 17–32. doi: 10.1016/j.csr.2014.03.006
Wang, C., Zhang, H., Liu, P., Wang, Y., Sun, Y., Song, Z., et al. (2021). Divergent patterns of bacterial community structure and function in response to estuarine output in the middle of the Bohai Sea. Front. Microbiol. 12:630741. doi: 10.3389/fmicb.2021.630741
Wu, C., Kan, J., Narale, D. D., Liu, K., and Sun, J. (2022). Dynamics of bacterial communities during a seasonal hypoxia at the Bohai Sea: coupling and response between abundant and rare populations. J. Environ. Sci. 111, 324–339. doi: 10.1016/j.jes.2021.04.013
Wu, W., Lu, H. P., Sastri, A., Yeh, Y. C., Gong, G. C., Chou, W. C., et al. (2018). Contrasting the relative importance of species sorting and dispersal limitation in shaping marine bacterial versus protist communities. ISME J. 12, 485–494. doi: 10.1038/ismej.2017.183
Xu, H., Zhao, D., Huang, R., Cao, X., Zeng, J., Yu, Z., et al. (2018). Contrasting network features between free-living and particle-attached bacterial communities in Taihu Lake. Microb. Ecol. 76, 303–313. doi: 10.1007/s00248-017-1131-7
Yuan, H., Mei, R., Liao, J., and Liu, W. T. (2019). Nexus of stochastic and deterministic processes on microbial community assembly in biological systems. Front. Microbiol. 10:1536. doi: 10.3389/fmicb.2019.01536
Yung, C. M., Ward, C. S., Davis, K. M., Johnson, Z. I., and Hunt, D. E. (2016). Insensitivity of diverse and temporally variable particle-associated microbial communities to bulk seawater environmental parameters. Appl. Environ. Microbiol. 82, 3431–3437. doi: 10.1128/aem.00395-16
Zhai, W., Zhao, H., Su, J., Liu, P., Li, Y., and Zheng, N. (2019). Emergence of summertime hypoxia and concurrent carbonate mineral suppression in the central Bohai Sea, China. Eur. J. Vasc. Endovasc. Surg. 124, 2768–2785. doi: 10.1029/2019jg005120
Zhang, J. (2016a). Spaa: SPecies association analysis. R package version 0.2.2. Available at: https://CRAN.R-project.org/package=spaa
Zhang, W., Chen, L., Chen, H., Liu, W., and Yang, Y. (2022). Geographic dispersal limitation dominated assembly processes of bacterial communities on microplastics compared to water and sediment. Microb. Ecol. 88:e0048222. doi: 10.1128/aem.00482-22
Zhang, J., Chen, M., Huang, J., Guo, X., Zhang, Y., Liu, D., et al. (2019). Diversity of the microbial community and cultivable protease-producing bacteria in the sediments of the Bohai Sea, Yellow Sea and South China Sea. PLoS One 14:e0215328. doi: 10.1371/journal.pone.0215328
Zhang, W., Pan, Y., Yang, J., Chen, H., Holohan, B., Vaudrey, J., et al. (2018). The diversity and biogeography of abundant and rare intertidal marine microeukaryotes explained by environment and dispersal limitation. Environ. Microbiol. 20, 462–476. doi: 10.1111/1462-2920.13916
Zhang, Y., Xiao, W., and Jiao, N. (2016b). Linking biochemical properties of particles to particle-attached and free-living bacterial community structure along the particle density gradient from freshwater to open ocean. J. Geophys. Res. Biogeo. 121, 2261–2274. doi: 10.1002/2016jg003390
Zhang, Y., Yao, P., Sun, C., Li, S., Shi, X., Zhang, X., et al. (2021). Vertical diversity and association pattern of total, abundant and rare microbial communities in deep-sea sediments. Mol. Ecol. 30, 2800–2816. doi: 10.1111/mec.15937
Zhang, Y., Zhao, Z., Dai, M., Jiao, N., and Herndl, G. J. (2014). Drivers shaping the diversity and biogeography of total and active bacterial communities in the South China Sea. Mol. Ecol. 23, 2260–2274. doi: 10.1111/mec.12739
Zhao, D., Gao, P., Xu, L., Qu, L., Han, Y., Zheng, L., et al. (2021). Disproportionate responses between free-living and particle-attached bacteria during the transition to oxygen-deficient zones in the Bohai seawater. Sci. Total Environ. 791:148097. doi: 10.1016/j.scitotenv.2021.148097
Zhao, J., Ran, W., Teng, J., Liu, Y., Liu, H., Yin, X., et al. (2018). Microplastic pollution in sediments from the Bohai Sea and the Yellow Sea, China. Sci. Total Environ. 640-641, 637–645. doi: 10.1016/j.scitotenv.2018.05.346
Zheng, L., Zhai, W., Wang, L., and Huang, T. (2020). Improving the understanding of central Bohai Sea eutrophication based on wintertime dissolved inorganic nutrient budgets: roles of North Yellow Sea water intrusion and atmospheric nitrogen deposition. Environ. Pollut. 267:115626. doi: 10.1016/j.envpol.2020.115626
Zhou, J., and Ning, D. (2017). Stochastic community assembly: does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 81, e00002–e00017. doi: 10.1128/mmbr.00002-17
Keywords: microbial community, community assembly, co-occurrence pattern, habitat differentiation, Bohai Sea
Citation: Liu J, Wang X, Liu J, Liu X, Zhang X-H and Liu J (2022) Comparison of assembly process and co-occurrence pattern between planktonic and benthic microbial communities in the Bohai Sea. Front. Microbiol. 13:1003623. doi: 10.3389/fmicb.2022.1003623
Edited by:
Alexander Eiler, University of Oslo, NorwayReviewed by:
Fanghua Liu, Guangdong Institute of Eco-Environmental and Soil Sciences (CAS), ChinaJihua Liu, Shandong University, China
Jun Gong, Sun Yat-sen University, China
Copyright © 2022 Liu, Wang, Liu, Liu, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiwen Liu, bGl1aml3ZW5Ab3VjLmVkdS5jbg==
†These authors have contributed equally to this work
 Jinmei Liu
Jinmei Liu Xiaolei Wang
Xiaolei Wang Jiao Liu
Jiao Liu Xiaoyue Liu1
Xiaoyue Liu1 Xiao-Hua Zhang
Xiao-Hua Zhang Jiwen Liu
Jiwen Liu