
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 06 January 2023
Sec. Microbial Physiology and Metabolism
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1001750
A correction has been applied to this article in:
Corrigendum: Plastic biodegradation by in vitro environmental microorganisms and in vivo gut microorganisms of insects
Traditional plastics, such as polyethylene (PE), polystyrene (PS), polypropylene (PP), polyvinyl chloride (PVC), polyethylene terephthalate (PET), polyurethane (PUR), and other plastic polymers, are difficult to degrade and are gradually accumulated in the environment to cause a serious environmental problem, which is urgently needed to develop novel treatments or control technology. The biodegradation of plastics has gained great attention due to the advantages of green and safe characteristics. Microorganisms play a vital role in the biodegradation of plastics, including environmental microbes (in vitro) and gut microbes of insects (in vivo). Microbial degradation in environmental conditions in vitro is extremely slow for major plastics at degradation rates on the basis of a month or even a year time, but recent discoveries show that the fast biodegradation of specific plastics, such as PS, PE, and PUR, in some invertebrates, especially insects, could be enhanced at rates on basis of hours; the biodegradation in insects is likely to be gut microbial-dependent or synergetic bioreactions in animal digestive systems. This review comprehensively summarizes the latest 7-year (2016–2022) publications on plastic biodegradation by insects and microorganisms, elucidates the mechanism of plastic degradation in insects and environmental microbes, and highlights the cutting-edge perspectives for the potential applications of plastic biodegradation.
Plastics are flexible materials mainly composed of long polymer chains with superior chemical stability and mechanical properties, which are widely used in the automotive industry, agriculture, construction, packaging, and textiles (Andrady and Neal, 2009; Andrady, 2011; Dris et al., 2015). The largest market for plastics is packaging, which has accelerated its growth because of disposable containers (Geyer et al., 2017). Plastics can be divided into degradable and non-degradable ones based on their degradability in natural environments (Brodhagen et al., 2015; Major et al., 2016). Conventional plastics, including polyethylene (PE), polystyrene (PS), polypropylene (PP), polyvinyl chloride (PVC), polyethylene terephthalate (PET), polyurethane (PUR), and other polymer compounds, have an extremely slow degradation rate in the environments (Wang et al., 2016). According to the European plastics reports of production, demand, and waste data (https://www.plasticseurope.org/en/resources/publications/4312-plastics-facts-2020), global plastic production reached nearly 370 million tons in 2019, which is 247 times more than that of the 1950s. When plastics were invented more than 100 years ago, they were regarded as safe and harmless synthetic organic polymers (Baekeland, 1909). However, plastics are commonly accumulated and distributed in the environment because of their difficult degradation characteristic. An estimated 6.3 billion tons of plastics have been dumped since 1950 (Geyer et al., 2017). In the middle-income and high-income countries, the proportion of plastics in municipal solid waste increased from less than 1% in 1960 to more than 10% in 2005 (Jambeck et al., 2015), which raised huge concerns about plastic pollution, especially in the oceans (Worm et al., 2017).
Plastics can be degraded in natural environments by mechanical, photochemical, thermal, and biochemical mechanisms (Gewert et al., 2015). Photochemistry is the most efficient chemical degradation pathway for plastics in nature. Thermal oxidation proceed slowly at ambient temperature (Gewert et al., 2015), but with the increase in temperature, the thermal oxidation efficiency will also increase with the rising temperature. Taking advantage of the complex enzyme system, microorganisms can effectively degrade plastic polymers and obtain energy from them (Chen et al., 2020), which is considered a more environmentally friendly approach to eliminating plastic waste (Chen et al., 2020), but the microbial degradation rate is extremely slow and it is questionable whether the energy obtained from plastic degradation could support microbial activities, especially growth.
In 1975, a bacterium, Flavobacterium, was discovered to break down nylon in wastewater pools from a nylon factory (Kinoshita et al., 1975). Later, an increasing number of microorganisms had been found to degrade polymers from the natural environments, including soil, seawater, sludge, and compost (Jones et al., 1974; Albertsson et al., 1978; Albertsson and Karlsson, 1988; Pegram and Andrady, 1989; Otake et al., 1995; Ohtake et al., 1998; Artham et al., 2009). The story of insects and plastics begins with consumers' complaints that chocolate-based consumable packaging was being eaten by insects (Terence, 1997). The degradation capacity of insects was reported based on the observation of insects destroying and eating plastic packaging materials. Nowadays, scientists have screened many environmental microbes (in vitro) and gut microbes of insects (in vivo) to degrade plastics (Skariyachan et al., 2016). Thus, this review aims to timely provide new insights and solutions for the environmental pollution problems, which focused on the relationships between plastic biodegradation by insects and environmental microbes.
References were retrieved from ISI Web of Science (http://www.isiknowledge.com), Wiley (https://onlinelibrary.wiley.com/), PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Scopus (http://www.scopus.com/), Springer Link (https://link.springer.com/), and ScienceDirect (http://www.sciencedirect.com/) databases. The following keywords and strings were searched: (synthetic OR non-biodegradable), (plastics particles OR microplastics/nanoplastics), (source OR fate), (exposure pathway OR way), (biodegradation OR plastic degradation), (microorganisms OR microbial OR microbes), (actinomycetes OR actinomycetal), (algae OR algal), (bacteria OR bacterial), (fungi OR fungus OR fungal), (enzyme OR enzymatic OR biocatalysts), (factors), (enzyme OR biocatalysts), and (mechanism OR steps OR processes). Published articles on enzymatic and microbial degradation of non-biodegradable plastics were filtered. References on the degradation of biodegradable plastics were excluded, and the aim was focused on publications within the latest 12 years (2010–2022), except where there is a lack of recent literature on the subject. Also, the keywords of “Plastics” and “Biodegradation” were used for searching back to 1970. Three independent searches were performed, and the consistency of selected papers was confirmed. Data from the search results were reviewed, analyzed, categorized, and expressed in suitable sections to cover the scope of this review.
Usually, the degradation mechanisms of plastics include photooxidation degradation, catalytic degradation, ozone-induced degradation, thermal degradation, mechanical degradation, and biodegradation, among which the final products of biodegradation are CO2 and water, which has the advantages of green environmental protection and low energy consumption (Lu et al., 2013). The plastics in the environments can be degraded into microplastics (MPs) or nanoplastics (NPs) under the action of weathering, cracking, and decomposition, involved in the physical, chemical, and biological processes (Luo et al., 2018). Plastic waste seems to be gone, but it exists everywhere as MPs or NPs, which possesses severe damage potential for health risks (Figure 1). Previous studies reported that specific organisms can turn plastics into small fragments or even NPs (Mateos-Cárdenas et al., 2020). For example, Antarctic krill can turn MPs into NPs through digestive fragmentation (Dawson et al., 2018), and it is a bad sign because NPs are more problematic than MPs based on toxicology. Currently, researchers have found that many bacterial and fungal strains can degrade MPs under laboratory conditions and in the environment (Ru et al., 2020; Yuan et al., 2020). Saini et al. reported the possible biodegradation approaches and techniques for MPs (Miri et al., 2022). All possible approaches that he mentioned include microbial degradation of primary or secondary MPs by using microorganisms or key enzymes (Miri et al., 2022). Plastic-degrading microbes and their degrading plastic types are shown in Table 1. Moreover, the biodegradation of plastics was also summarized, and their inner mechanisms by insects and environmental microbes were highlighted, which will be of great benefit for researchers to investigate safe and efficient treatments for plastic wastes.
Currently, many species of insects were reported to degrade plastics (Table 2), including mealworms (larvae of Tenebrio molitor) (Yang et al., 2018), superworms (larvae of Zophobas atratus) (Peng et al., 2020b), and greater wax moth larvae (Galleria mellonella L) (Jiang et al., 2021b; Wang S. et al., 2022). Notably, Tenebrio molitor exhibited amazing degradation efficiency and possessed a wide selection of plastic types. In a recent study in 2022, the efficiency of mealworms to degrade polyester-PU foam was as high as 67% (Liu et al., 2022). Currently, the widely reported types of plastics degraded by Tenebrio molitor included PE (Brandon et al., 2018), PS (Brandon et al., 2021), Polyester PU (Liu et al., 2022), and PVC (Peng et al., 2020a). Tenebrio molitor larvae not only degrade low-density polyethylene (LDPE) but also linear low-density polyethylene (LLDPE) and high-density polyethylene (HDPE). The depolymerization capability was influenced by plastic type, molecular weight, and branching number (Yang et al., 2022). Yang et al. found that the yellow mealworms can completely degrade PS into CO2 and assimilate it into their biomass (Yang et al., 2015). Zophobas atratus and Tenebrio obscurus have been reported to have a higher degradation capability for PS than yellow mealworms (Peng et al., 2019; Yang Y. et al., 2020). Zophobas atratus larvae can degrade PS and PE but do not generate NPs in their frass (Peng et al., 2020b, 2022), implying that plastic biodegradation by insects is a more environmentally friendly option. Tribolium castaneum was also proven to degrade PS, and Acinetobacter from the larvae of Tribolium castaneum was recently isolated (Wang et al., 2020). Plodia interpunctella can chew PE plastic, but the degradation of plastic is caused by Enterobacter asburiae and Bacillus in the intestinal tract (Graham Bowditch, 1997). The biodegradation of plastic polymer has also been verified by other invertebrates, including lesser waxworm (Achroia grisella) (Kundungal et al., 2019), confused flour beetle (Tribolium confusum) (Kundungal et al., 2019), land snail (Achatina fulica), and other invertebrates (Song et al., 2020). Many studies reported the potential use of earthworms to enhance the decay of biodegradable plastics (Sanchez-Hernandez et al., 2020; Christyraj et al., 2022). Recent studies suggest that earthworms, particularly anecic and endogeic species, may facilitate plastic biodegradation directly and indirectly via their strong impact on soil microbial properties and the intense soil bioturbation (Sanchez-Hernandez et al., 2020; Wang L. et al., 2022). Termites are the major soil insects that can also degrade plastics using their gut microbiota (López-Naranjo et al., 2013; Kumar et al., 2022), but more evidence is needed to be provided.
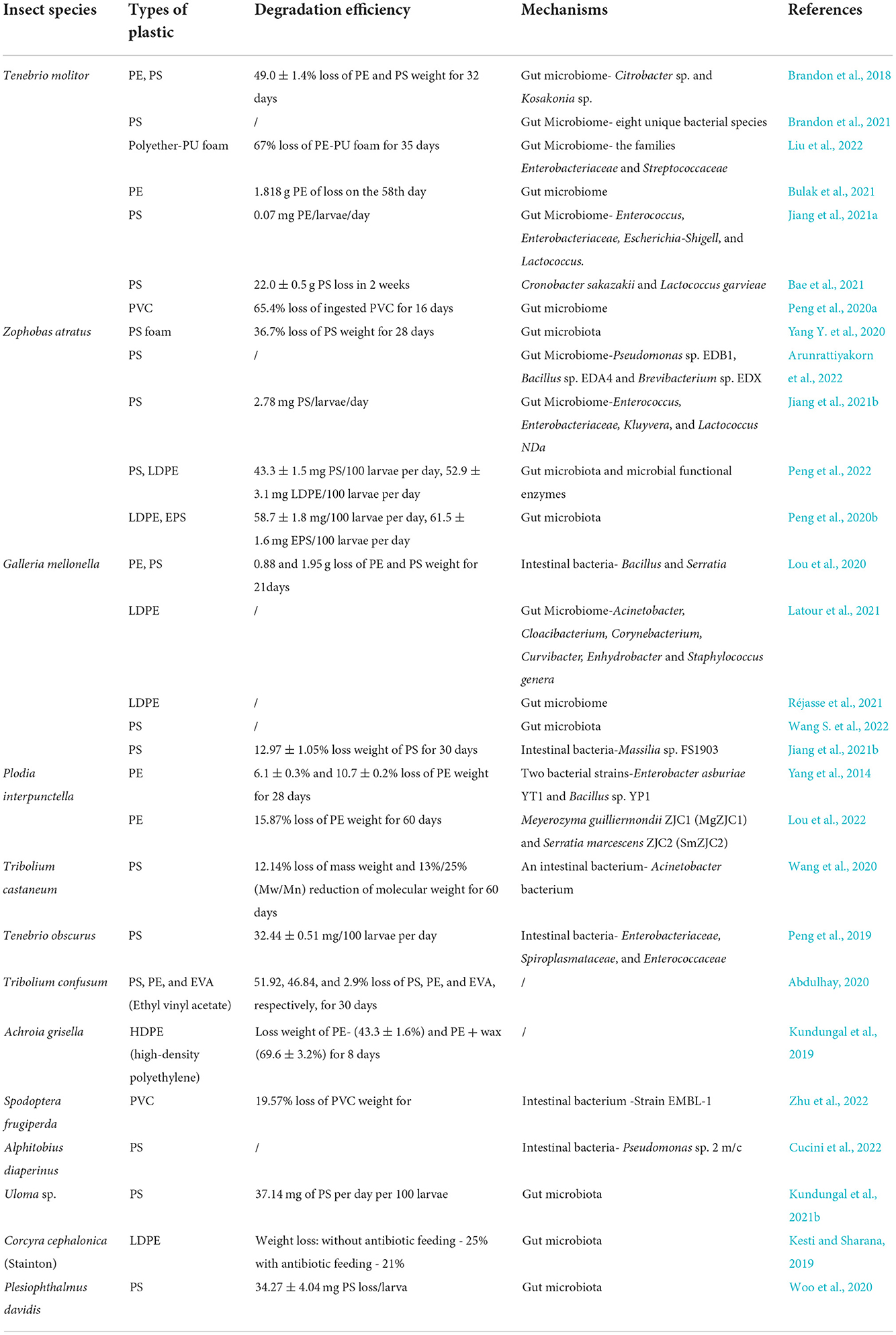
Table 2. The confirmed plastic-degrading insects and their ability to degrade diverse plastic materials.
Due to the different chemical properties of various plastics, the biodegradability in insects is also diverse. Galleria mellonella L. degraded polyethylene (PE) faster than polystyrene (PS) (Lou et al., 2020). Previous studies validated that the yellow mealworms preferred to eat the mixtures of plastics and nutrition to achieve better degradability of plastics (Brandon et al., 2018; Kundungal et al., 2019). A double degradation rate of PS was found when Tenebrio molitor larvae were fed with mixed PS and bran (Brandon et al., 2018). Beeswax can increase the species richness and evenness of the intestinal microbiome in PE-fed larvae (Lou et al., 2020). A continual diet of PS with supplemental nutrition enables better growth and enhanced PS degradation by the beetle larvae, which is similar to lesser waxworms. The ability of Uloma sp. larvae to degrade PS suggests the ubiquitous phenomena of plastic degradation among the beeswax-eating species (Kundungal et al., 2021b). The degradation mechanism is probably involved in those diets with high nutrients that increase the diversity of the intestinal microbiome in worms. Therefore, excellent organisms should be discovered to degrade target plastics, and the best diet formula for blending plastics and nutrition needs to be established. The phyla Actinobacteria (Microbacterium awajiense, Rhodococcus jostii, Mycobacterium vanbaalenii, and Streptomyces fulvissimus) and Firmicutes (Bacillus simplex and Bacillus sp.) isolated from Lumbricus terrestris' gut have been proven to degrade LDPE-MPS with high efficiency-−60% (Huerta Lwanga et al., 2018). Due to different invertebrate species, plastic materials, and evaluation methods, it is difficult to simply describe the differences in the degradation rates of various insects, but specific degradation efficiency data are summarized in Table 2. In addition, except for the invertebrates that confirmed their capabilities of plastic biodegradation, other invertebrates were also reported to eat plastics (Table 3), but their degradation abilities need further studies.
The process of degrading plastics by insects can be divided into five stages based on relevant studies: (1) Plastics are physically chewed by mouthparts and enter the intestinal tract; (2) microbes in the gut adhere to and erode plastic; (3) the plastic is depolymerized into oligomer fragments by oxidation or hydrolysis of enzymes, which are provided by both host and gut microbiome; (4) the host provides bioemulsifying agents enhancing the effectiveness of microbial and host enzymes to attack polymers; (5) the bonds of oligomers are broken to form fatty acids; and (6) fatty acids are decomposed via insect biological metabolism. To seek efficient approaches for plastic biodegradation, the function of intestinal microbiota in insects should be considered. A previous study reported that yellow mealworms lost the ability to degrade PS after inhibiting intestinal bacterial activity with antibiotics, implying that intestinal bacteria play a key role in plastic biodegradation (Yang et al., 2015). The biofilm was formed by the isolated strain YT2 on PS film after a 28-day incubation, and obvious pits and cavities were observed on PS film surfaces, accompanied by the decreasing hydrophobicity and the formation of C–O polar groups. Suspension culture of strain YT2 could degrade 7.4 ± 0.4% of the PS pieces after a 60-day incubation. The molecular weight of the residual PS pieces was lower, and water-soluble intermediates were released, implying the vital effect of mealworm gut bacteria on PS biodegradation and mineralization (Yang et al., 2015). The biodegradation of PP by superworms and yellow mealworms via gut-microbe-dependent depolymerization was also confirmed (Yang S. et al., 2020). Two gut bacteria for PE biodegradation, Bacillus sp. YP1 and Enterobacter asburiae YT1, were isolated from waxworms (Yang et al., 2014). Moreover, the efficiency of PS biodegradation and mineralization in vitro was much lower than that in vivo, suggesting that the accelerated degradation of plastic in insects may be a complex process depending on both the microbiome and the host (Yang et al., 2015). Notably, the physicochemical “treatments” of chewing, ingestion, mixing with intestinal contents, and enzymes secreted by worms may be critical for the rapid degradation of PS in vivo (Yang et al., 2015). Brandon et al. provided evidence that T. molitor secreted one or more emulsifying factor(s) (30–100 kDa) to mediate plastic bioavailability. They also demonstrated that the insect gut microbiome secreted emulsifying factor(s) (< 30 kDa) that enhanced respiration on polystyrene (PS) (Brandon et al., 2021).
In addition to the insects' gut bacteria, gut fungi also can degrade plastics. Recently, Zhang et al. isolated a PE-degrading fungus, Aspergillus flavus, from the intestine of a Wax moth larva (Galleria mellonella), which can degrade HDPE MP to low molecular weight MP after 28 days of culture (Zhang et al., 2020). Moreover, two Laccase-like multicopper oxidase (LMCOS) genes, Afla_053930 and Afla_006190, are upregulated during the degradation process, which is related to PE degradation (Zhang et al., 2020). Enzymes secreted by bacteria and fungi from insects may be the direct reason for plastic degradation. Plastic polymers are mainly depolymerized by extracellular enzymes into short chains or small molecules and then transported to cells for complete oxidation (Amobonye et al., 2021). Bacteria can produce many extracellular enzymes to degrade plastic macromolecules, such as lipases, depolymerase, esterase, proteinase K, cutinase, urease, and dehydrase (Shahnawaz et al., 2019; Taniguchi et al., 2019). For example, the degradation of PET is closely related to PET hydrolase, which can change the polymer chain or ring structure to enhance the efficiency of enzymatic hydrolysis (Kawai et al., 2019). As shown in Figure 2, the potential progress of plastic biodegradation by insects was summarized based on the above references and reports, which provided novel perspectives for the biodegradation treatment of different materials including PE, PS, PP, PET, and PUR.
The importance of insect gut microbes for plastic biodegradation has been well documented earlier, and abundant plastic-degrading microbiota in the environment also play important roles. Recently, different actinomycetes, algae, bacteria, and fungi with the potential to biodegrade various plastic polymers have been investigated. To date, more than 56 species of bacteria and fungi belonging to 25 genera have been reported for polyethylene biodegradation, whose main sources are soil and landfills (Cassone et al., 2020; Kundungal et al., 2021a). Bacillus, Pseudomonas, Streptomyces, Rhodococcus ruber, and other bacteria were found to degrade PE (Park and Kim, 2019; Zhang et al., 2021). PET degradation by Thermobifida fusca, Aspergillus Niger, and Bacillus subtilis is recently reported (Barth et al., 2016). PUR degradation by Curvularia senegalensis and Fusarium solani (Khan et al., 2017) is also discovered. Moreover, the biodegradable bacteria from marine water, such as PE and PVC-degrading Bacillus sp., were recently isolated (Kumari et al., 2019). Actinomycetes including Streptomyces, Rhodococcus ruber, Actinomadura spp., and Thermoactinomyces have been isolated from different environments and confirmed to possess significant plastic biodegradative potentials (Auta et al., 2018; Jabloune et al., 2020; Amobonye et al., 2021). The hydrolytic enzymes they release are one of the main factors responsible for their growth on different plastic polymers and for degrading the high molecular weight compounds to low ones (Gohain et al., 2020). PET, PUR, p-nitrophenyl esters, keratin, rubber diesel, and different chemical additives are found to be degraded by actinomycetes (Singh and Sedhuraman, 2015; Gaytán et al., 2019; Jabloune et al., 2020).
Interestingly, algae, especially microalgae, also showed the ability to degrade plastic through the toxin systems or enzymes they secrete (Chia et al., 2020). The biological treatment of PE sheets with Anabaena spiroides (blue-green algae), Navicula pupula (diatom), and Scenedesmus dimorphus (green microalga) has been studied. After incubation at room temperature (27 ± 2°C) under light (12:12-h dark and light) for 1 month, Anabaena spiroides showed the most efficient degradation of plastics, which may degrade LDPE with an efficiency of 8.18% (Gopal, 2017). Moreover, when microalgae degrade plastic polymers, the process is involved in the reduction of activation energy to weaken the chemical bonds of PE polymers and consume polymers as a carbon source (Chia et al., 2020; Khoo et al., 2021; Soong et al., 2022). Spirulina sp. could biodegrade PET and PP, but the degradation efficiency was significantly lower, compared to bacteria and fungi (Khoironi et al., 2019). The reason may be that microalgae, unlike bacteria, use atmospheric CO2 as the sole carbon source and sunlight as the main energy source (Dineshbabu et al., 2020). Recently, Phaeodactylum tricornutum was reported for the high-efficiency biodegradation of PET due to its successfully engineered PETase from Ideonella sakaiensis (Moog et al., 2019).
However, the efficiency of plastic degradation by various microorganisms is relatively slow, which hinders the practical application of plastic biodegradation in the industry (Amobonye et al., 2021). Currently, no in vitro technique of plastic degradation fits industrial applications. Thus, genetically engineered microorganisms with delicate designs by integrating efficient plastic-degrading enzymes would be feasible for practical application.
The primary determinant of biodegradable plastic polymers is the property of the bonds linking monomers together. Among the six major types of synthetic plastics (PE, PP, PS, PVC, PUR, and PET), the C–C backbones of PE, PP, PS, and PVC are highly recalcitrant (Figure 3), while PUR and PET with a hydrolyzable backbone are more vulnerable to enzymatic degradation (Chen et al., 2020). Plastic degradation by environmental microbes may be involved in the below steps: (1) Environmental microbes release hydrolase specifically binding to plastic surface receptors and then hydrolyze plastics to molecules; (2) those small molecules of acids or lipids generated from the previous process can enter the microorganism and participate in their physiological metabolic process and are further decomposed into water and carbon dioxide, releasing energy for cell growth (Crawford and Quinn, 2017; Zumstein et al., 2018). Thus, efficient plastic biodegradation can be achieved based on the degradation mechanism.
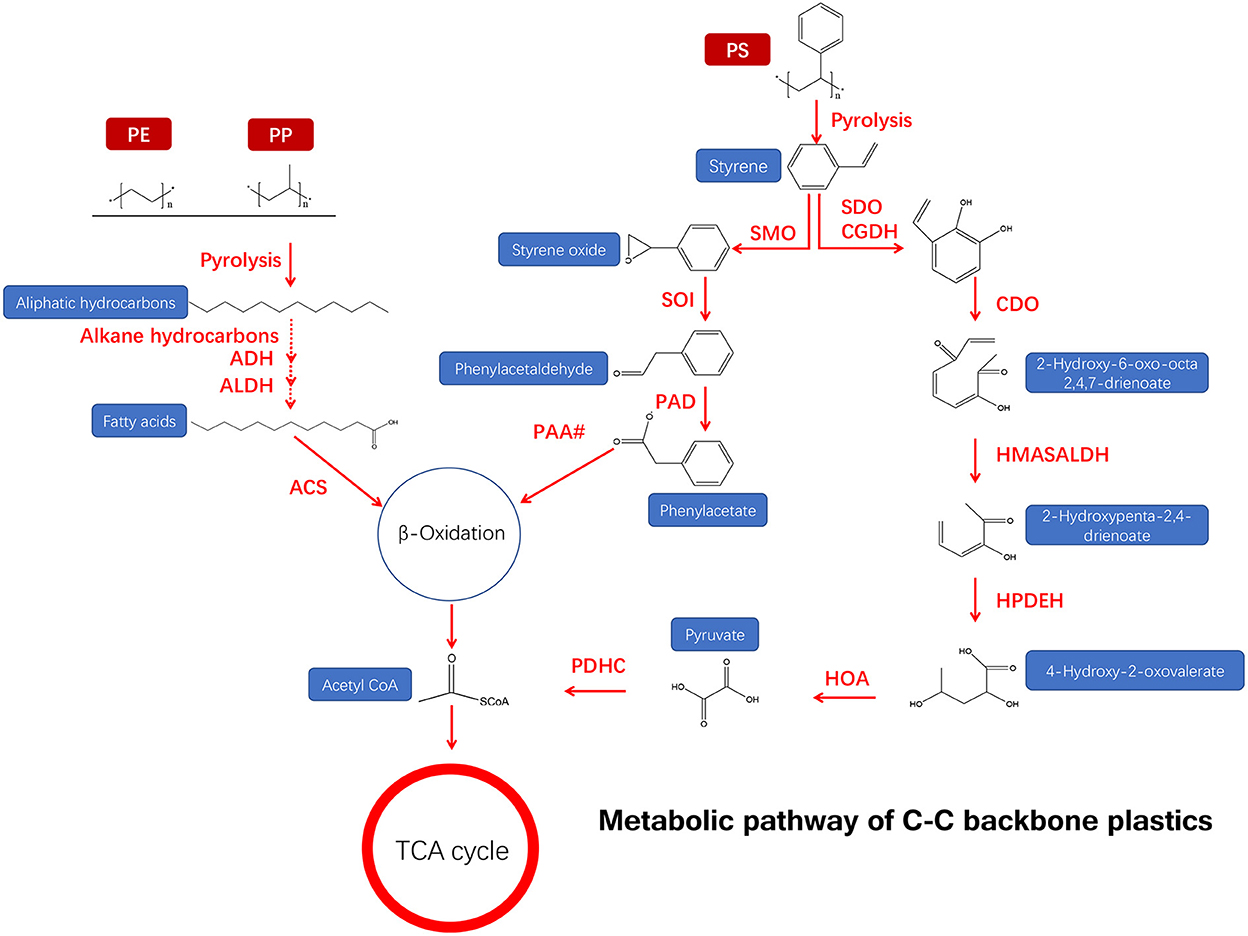
Figure 3. Metabolic pathway of a C–C backbone synthetic plastic material (adapted from previous studies Ru et al., 2020; Ali et al., 2021).
For PE degradation, the biodegradation process usually involves bio-fragmentation of PE polymer by secreted enzymes, followed by bio-assimilation of small lytic fragments by microorganisms (Bonhomme et al., 2003; Montazer et al., 2019). Specifically, the –C–C– groups of a long-chain backbone of PE are oxidized into the –C=O– (carbonyl) group by the microbe, and the small aliphatic hydrocarbons can be transported directly into the cell for degradation (Albertsson et al., 1987, 1998; Desforges et al., 2015).
For PS degradation, the thickness and molecular weight of plastics are the main factors affecting the biodegradation rate (Krueger et al., 2015). Tischler et al. demonstrated that Rhodococcus opacus 1CP, a strain of actinobacterium, can mineralize styrene by styrene oxide via the aerobic phenylacetic acid (PAA) pathway (Tischler et al., 2009), indicating that styrene is degraded by a monooxygenase-catalyzed epoxidation of the vinyl side chain, and styrene oxide further converted it to phenylacetaldehyde with the help of an epoxystyrene isomerase (Tischler et al., 2009). This metabolic pathway is involved in multiple enzymes, including styrene monooxygenase (SMO), phenylacetaldehyde dehydrogenase (PAD), styrene oxide isomerase (SOI), and other enzymes of phenylacetate (PAA#) degradation accessing the tricarboxylic acid cycle (TCA) (Figure 3) (Tischler et al., 2009). The side-chain oxygenation pathway is very common for the aerobic degradation of styrene, which was reported in the proteobacteria genera Pseudomonas and Xanthobacter (O'leary et al., 2002). Moreover, phenylacetaldehyde may be further oxidized to PAA by the action of a phenylacetaldehyde dehydrogenase.
The degradation pathway of PVC is not the same as PE and PP because it contains Cl−. Oxidation of chlorinated hydrocarbons is much more difficult than PE and PP. For example, T. molito can mineralize PE, PP, and PS, but the mineralization of PVC is poor because most PVC is converted to chlorinated intermediates (Peng et al., 2020a). Currently, there are few studies on the mechanism of PVC biodegradation, and most of them just focus on screening certain bacteria that can degrade PVC. Because of the chemical stability and hydrophobicity of the C–C skeleton of PVC, there is no report on the enzymes directly involved in its degradation. The only enzyme found was laccase (Sumathi et al., 2016). Laccase (EC 1.10.3.2) is a kind of oxidoreductase with the ability to oxidize phenolic compounds, which has been widely used in the decomposition of lignin, phenolic substances, and toxic pollutants (Janusz et al., 2020). Sumathi et al. (2016) proved that laccase could break PVC double bonds and generate new C=O bonds, but the specific mechanism was unknown. Based on the degradation pathways of cellulose, lignin, and other macromolecules, it was speculated that oxygen-free radicals in laccase products might attack C–C bonds. The short chain produced by this reaction is degraded by other unknown enzymes, which can be used by microorganisms as a carbon source through the TCA cycle.
Both PVC and PP are highly hydrophobic and resilient to chemical abrasion (Shah et al., 2008), which makes it difficult to degrade them via microbial activity. Although several microbial strains were proven to own the ability to degrade PVC and PP (Sah et al., 2011; Jeyakumar et al., 2013), the essential degradation enzymes and the underlying degradation mechanism remain unknown. Therefore, the depolymerization of PVC and PP should be further studied.
For the ester-linked PET degradation, the PET and PET hydrolase can target the terminal or ring structure of the polymer chains for enzymatic hydrolysis, which increases the hydrophilicity of the PET and improves the subsequent enzymatic hydrolysis efficiency (Kawai et al., 2019). Ideonella Sakaiensis 201-F6, a bacterium from the genus Ideonella, was reported to degrade and assimilate PET (Yoshida et al., 2016) after the generation of PETase and MHETase that efficiently converts PET into environmentally friendly monomers, terephthalic acid, and ethylene glycol (Figure 4) (Yoshida et al., 2016). Notably, this PET hydrolase has 45–53% homology with actinomycete keratinase (Wei et al., 2019) but can completely degrade PET, compared to other PET hydrolases. However, the low stability of PETase limits its wide application. After enzymes digested the ester bond, PET is degraded into MHET. MHET can continue to be hydrolyzed into TPA and EG under the action of MHETase (Peng et al., 2019) and finally enter the tricarboxylic acid cycle (TCA cycle) (Ronkvist et al., 2009).
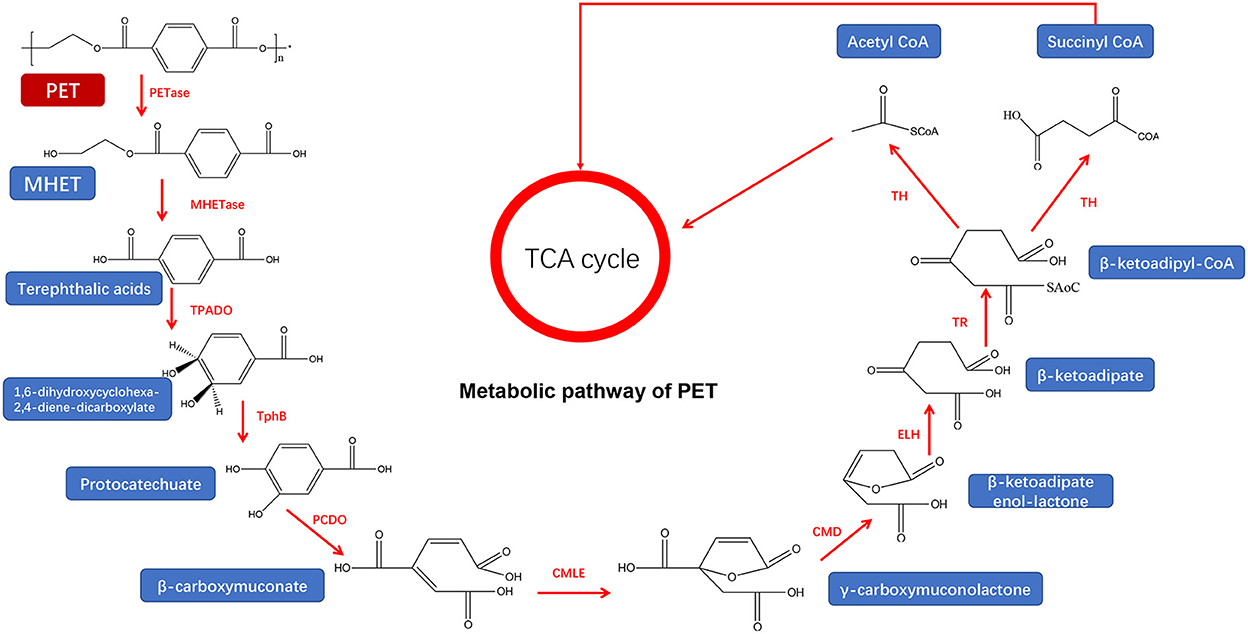
Figure 4. Metabolic pathway of PET (adapted from previous studies Yoshida et al., 2016; Ru et al., 2020).
PUR linked by urethane bonds is composed of di- or polyisocyanate and polyols (Seymour and Kauffman, 1992). Previous studies have reported the enzymes degrading polyester PUR from bacteria (Shah and Green, 1994; Nakajima-Kambe et al., 1995; Howard and Blake, 1998; Stern and Howard, 2000; Howard et al., 2012; Schmidt et al., 2017) and fungi (Crabbe et al., 1994; Russell et al., 2011). PUR can be depolymerized by microbial ureases, esterases, and proteases for hydrolyzing urethane and ester bonds (Figure 5) (Howard, 2012; Loredo-Treviño et al., 2012; Cregut et al., 2013). It has been postulated that proteases can hydrolyze the amide and urethane bonds, while ureases may attack the urea linkages (Labow et al., 1996; Ruiz et al., 1999; Matsumiya et al., 2010). Esterases and proteases can hydrolyze the ester bonds as a major mechanism for PUR depolymerization (Wei and Zimmermann, 2017). According to their localization, PUR-degrading enzymes are divided into membrane-bound and secreted types. The membrane-bound enzymes directly adhere to the PUR surface and hydrolyze the urethane bond, resulting in releasing monomers of the PUR (Cregut et al., 2013). For insoluble PUR, many secretases are released for the degradation of PUR (Wei and Zimmermann, 2017).
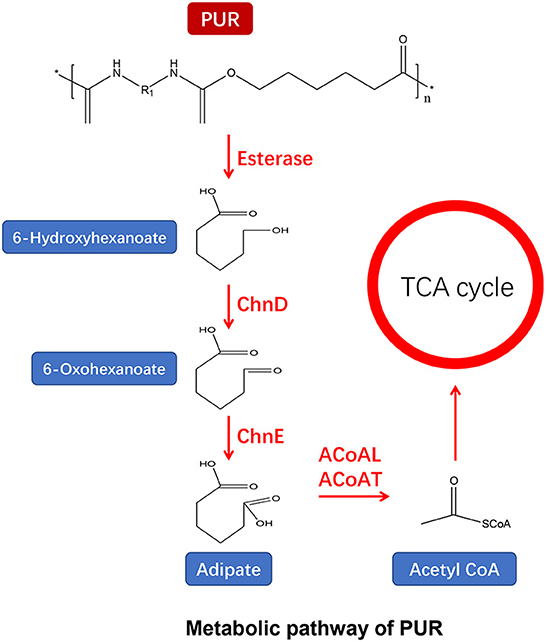
Figure 5. Metabolic pathway of PUR (adapted from previous studies Yoshida et al., 2016; Ru et al., 2020; Ali et al., 2021).
The accumulations of plastics in environments and wild animals are serious and pose risks to human health via the food chain. Thus, the development of biodegradable plastics and the final degradation without toxicity will be urgently needed to solve the problem of white pollution. Compared to traditional methods, the biodegradation of plastics using insects and environmental microbes becomes a potential application in the industrial treatment of plastic waste, but the application of insects for plastic waste treatment is not practicable now. Both insect degradation and microbial degradation are basically environmental science. Enzymatic degradation of PET could have an application future based on cost-effectiveness. This review provides new insights and approaches to solving the problem of plastic pollution from the biodegradation aspects. Due to the different physical and chemical properties of plastics, the degradation efficiency of insects varies greatly. Different types of plastics may affect the growth and development of insects; thus, the degradation toxicity caused by insects should be considered (Sanchez-Hernandez, 2021). More studies on the biodegradable application of plastics by insects and environmental microbes are still recommended. In future, in-depth studies on the following aspects should be considered: (1) Based on the characteristics of plastic-degrading insects, more environmental and gut microbes with a strong degrading ability should be screened to enrich the plastic-degrading insect library. (2) To ensure the normal growth and reproduction of insects, the proportion of plastic in the food diet should be optimized to further improve their degradation efficiency on plastic. (3) With the help of protein engineering and synthetic biology technology, efficient and artificial synthetic microorganisms can be constructed by modifying the plastic-degrading enzymes and designing metabolic pathways. (4) Since human health and the ecological environment harms are inevitably caused by plastics, the in-depth toxicological analysis of plastic-degrading insects and their gut microorganisms should be performed to avoid toxicological risks. (5) And to eliminate the toxicity risks from the source, it is urgent to advocate the use of biodegradable plastics, especially in takeout, e-commerce, and other industries with the widespread use of disposable plastic products.
X-GY and P-PW: investigation. Y-FY and P-PJ: resources. P-PW and W-GL: data analysis. P-PW and D-SP: writing—original draft preparation. D-SP: writing—revision. All authors have read and approved the manuscript.
This study was supported by the High-level Talents Project of Chongqing Medical University (Nos. R4014 and R4020), Research Program of Chongqing Science and Technology Commission (Nos. cstc2019jcyj-zdxmX0035 and CSTCCXLJRC201714), and Program of China–Sri Lanka Joint Center for Water Technology Research and Demonstration by Chinese Academy of Sciences (CAS)/China–Sri Lanka Joint Center for Education and Research by CAS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdulhay, H. (2020). Biodegradation of plastic wastes by confused flour beetle Tribolium confusum Jacquelin du Val larvae. Asian J. Agric. Biol. 8, 201–206. doi: 10.35495/ajab.2019.11.515
Abraham, J., Ghosh, E., Mukherjee, P., and Gajendiran, A. (2017). Microbial degradation of low density polyethylene. Environ. Progress Sustain. Energy 36, 147–154. doi: 10.1002/ep.12467
Albertsson, A., Báanhidi, Z., and Beyer-Ericsson, L. (1978). Biodegradation of synthetic polymers. III. The liberation of 14CO2 by molds like fusarium redolens from 14C labeled pulverized high-density polyethylene. J. Appl. Poly. Sci. 22, 3435–3447. doi: 10.1002/app.1978.070221208
Albertsson, A.-C., Andersson, S. O., and Karlsson, S. (1987). The mechanism of biodegradation of polyethylene. Poly. Degrad. Stab. 18, 73–87. doi: 10.1016/0141-3910(87)90084-X
Albertsson, A.-C., Erlandsson, B., Hakkarainen, M., and Karlsson, S. (1998). Molecular Weight Changes and Polymeric Matrix Changes Correlated with the Formation of Degradation Products in Biodegraded Polyethylene. J. Environ. Poly. Degrad. 6, 187–195. doi: 10.1023/A:1021873631162
Albertsson, A.-C., and Karlsson, S. (1988). The three stages in degradation of polymers—polyethylene as a model substance. J. Appl. Poly. Sci. 35, 1289–1302. doi: 10.1002/app.1988.070350515
Ali, S. S., Elsamahy, T., Al-Tohamy, R., Zhu, D., Mahmoud, Y. A., Koutra, E., et al. (2021). Plastic wastes biodegradation: Mechanisms, challenges and future prospects. Sci. Total Environ. 780, 146590. doi: 10.1016/j.scitotenv.2021.146590
Álvarez-Barragán, J., Domínguez-Malfavón, L., Vargas-Suárez, M., González-Hernández, R., Aguilar-Osorio, G., and Loza-Tavera, H. (2016). Biodegradative activities of selected environmental fungi on a polyester polyurethane varnish and polyether polyurethane foams. Appl. Environ. Microbiol. 82, 5225–5235. doi: 10.1128/AEM.01344-16
Ambika, D. K., Lakshmi, B. K. M., and Hemalatha, K. P. J. (2015). Degradation of low density polythene by Achromobacter denitrificans strain s1, a novel marine isolate. Int. J. Rec. Sci. Res. 6, 5454–5464.
Amobonye, A., Bhagwat, P., Singh, S., and Pillai, S. (2021). Plastic biodegradation: Frontline microbes and their enzymes. Sci. Total Environ. 759, 143536. doi: 10.1016/j.scitotenv.2020.143536
Andrady, A. L. (2011). Microplastics in the marine environment. Marine Pollut. Bull. 62, 1596–1605. doi: 10.1016/j.marpolbul.2011.05.030
Andrady, A. L., and Neal, M. A. (2009). Applications and societal benefits of plastics. Philos. Trans. R. Soc. B. 364, 1977–1984. doi: 10.1098/rstb.2008.0304
Artham, T., Sudhakar, M., Venkatesan, R., Nair, C. M., Murty, K. V. G. K., and Doble, M. (2009). Biofouling and stability of synthetic polymers in sea water. Int. Biodeterior. Biodegrad. 63, 884–890. doi: 10.1016/j.ibiod.2009.03.003
Arunrattiyakorn, P., Ponprateep, S., Kaennonsang, N., Charapok, Y., Punphuet, Y., Krajangsang, S., et al. (2022). Biodegradation of polystyrene by three bacterial strains isolated from the gut of Superworms (Zophobas atratus larvae). J. Appl. Microbiol. 132, 2823–2831. doi: 10.1111/jam.15474
Auta, H. S., Emenike, C. U., Jayanthi, B., and Fauziah, S. H. (2018). Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 127, 15–21. doi: 10.1016/j.marpolbul.2017.11.036
Bae, J., Cho, H. W., Jung, H., Park, J., Yun, S., Ha, S., et al. (2021). Changes in Intestinal Microbiota Due to the Expanded Polystyrene Diet of Mealworms (Tenebrio molitor). Indian J. Microbiol. 61, 130–136. doi: 10.1007/s12088-021-00922-w
Baekeland, L. H. (1909). The synthesis, constitution, and uses of bakelite. J. Ind. Eng. Chem. 1, 149–161. doi: 10.1021/ie50003a004
Barth, M., Honak, A., Oeser, T., Wei, R., Belisario-Ferrari, M. R., Then, J., et al. (2016). A dual enzyme system composed of a polyester hydrolase and a carboxylesterase enhances the biocatalytic degradation of polyethylene terephthalate films. Biotechnol. J. 11, 1082–1087. doi: 10.1002/biot.201600008
Bollinger, A., Thies, S., Knieps-Grünhagen, E., Gertzen, C., Kobus, S., Höppner, A., et al. (2020). A Novel Polyester Hydrolase From the Marine Bacterium Pseudomonas aestusnigri – Structural and Functional Insights. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.00114
Bonhomme, S., Cuer, A., Delort, A. M., Lemaire, J., Sancelme, M., and Scott, G. (2003). Environmental biodegradation of polyethylene. Polymer Degradation and Stability 81, 441–452. doi: 10.1016/S0141-3910(03)00129-0
Brandon, A. M., Gao, S. H., Tian, R., Ning, D., Yang, S. S., Zhou, J., et al. (2018). Biodegradation of Polyethylene and Plastic Mixtures in Mealworms (Larvae of Tenebrio molitor) and Effects on the Gut Microbiome. Environ. Sci. Technol. 52, 6526–6533. doi: 10.1021/acs.est.8b02301
Brandon, A. M., Garcia, A. M., Khlystov, N. A., Wu, W. M., and Criddle, C. S. (2021). Enhanced bioavailability and microbial biodegradation of polystyrene in an enrichment derived from the gut microbiome of tenebrio molitor (Mealworm Larvae). Environ. Sci. Technol. 55, 2027–2036. doi: 10.1021/acs.est.0c04952
Brodhagen, M., Peyron, M., Miles, C., and Inglis, D. A. (2015). Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl. Microbiol. Biotechnol. 99, 1039–1056. doi: 10.1007/s00253-014-6267-5
Bulak, P., Proc, K., Pytlak, A., Puszka, A., Gawdzik, B., and Bieganowski, A. (2021). Biodegradation of different types of plastics by tenebrio molitor insect. Polymers 13, 3508. doi: 10.3390/polym13203508
Calabia, B. P., and Tokiwa, Y. (2006). A novel PHB depolymerase from a thermophilic streptomyces Sp. Biotechnol. Lett. 28, 383–388. doi: 10.1007/s10529-005-6063-5
Cassone, B. J., Grove, H. C., Elebute, O., Villanueva, S. M. P., and Lemoine, C. M. R. (2020). Role of the intestinal microbiome in low-density polyethylene degradation by caterpillar larvae of the greater wax moth, Galleria mellonella. Proc. Biol. Sci. 287, 20200112. doi: 10.1098/rspb.2020.0112
Chaudhary, A. K., and Vijayakumar, R. P. (2020). Studies on biological degradation of polystyrene by pure fungal cultures. Environ. Develop. Sustain. 22, 4495–4508. doi: 10.1007/s10668-019-00394-5
Chen, C.-C., Dai, L., Ma, L., and Guo, R.-T. (2020). Enzymatic degradation of plant biomass and synthetic polymers. Nat. Rev. Chem. 4, 114–126. doi: 10.1038/s41570-020-0163-6
Chia, W. Y., Ying Tang, D. Y., Khoo, K. S., Kay Lup, A. N., and Chew, K. W. (2020). Nature's fight against plastic pollution: Algae for plastic biodegradation and bioplastics production. Environ. Sci. Ecotechnol. 4, 100065. doi: 10.1016/j.ese.2020.100065
Christyraj, J. D. S., Mathews, M. G. R., Subramaniam, R., Yesudhason, B. V., Chelladurai, K. S., and Christyraj, J. R. S. S. (2022). “Importance of Vermicomposting and Vermiremediation Technology in the Current Era,” in Advances in Bioremediation and Phytoremediation for Sustainable Soil Management: Principles, Monitoring and Remediation, ed. J.A. Malik. (Cham: Springer International Publishing) 313–326. doi: 10.1007/978-3-030-89984-4_20
Crabbe, J. R., Campbell, J. R., Thompson, L., Walz, S. L., and Schultz, W. W. (1994). Biodegradation of a colloidal ester-based polyurethane by soil fungi. Int. Biodeteriorat. Biodegrad. 33, 103–113. doi: 10.1016/0964-8305(94)90030-2
Crawford, C. B., and Quinn, B. (2017). “Physiochemical properties and degradation,” in Microplastic Pollutants, eds C. B. Crawford and B. Quinn (Amsterdam: Elsevier), 57–100. doi: 10.1016/B978-0-12-809406-8.00004-9
Cregut, M., Bedas, M., Durand, M. J., and Thouand, G. (2013). New insights into polyurethane biodegradation and realistic prospects for the development of a sustainable waste recycling process. Biotechnol. Adv. 31, 1634–1647. doi: 10.1016/j.biotechadv.2013.08.011
Cucini, C., Funari, R., Mercati, D., Nardi, F., Carapelli, A., and Marri, L. (2022). Polystyrene shaping effect on the enriched bacterial community from the plastic-eating Alphitobius diaperinus (Insecta: Coleoptera). Symbiosis 86, 305–313. doi: 10.1007/s13199-022-00847-y
Danko, A. S., Luo, M., Bagwell, C. E., Brigmon, R. L., and Freedman, D. L. (2004). Involvement of linear plasmids in aerobic biodegradation of vinyl chloride. Appl. Environ. Microbiol. 70, 6092–6097. doi: 10.1128/AEM.70.10.6092-6097.2004
Davidson, T. M. (2012). Boring crustaceans damage polystyrene floats under docks polluting marine waters with microplastic. Mar. Pollut. Bull. 64, 1821–1828. doi: 10.1016/j.marpolbul.2012.06.005
Dawson, A. L., Kawaguchi, S., King, C. K., Townsend, K. A., King, R., Huston, W. M., et al. (2018). Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 9, 1001. doi: 10.1038/s41467-018-03465-9
Desforges, J.-P. W., Galbraith, M., and Ross, P. S. (2015). Ingestion of microplastics by zooplankton in the northeast pacific ocean. Arch. Environ. Contamin. Toxicol. 69, 320–330. doi: 10.1007/s00244-015-0172-5
Dineshbabu, G., Uma, V. S., Mathimani, T., Prabaharan, D., and Uma, L. (2020). Elevated CO2 impact on growth and lipid of marine cyanobacterium Phormidium valderianum BDU 20041– towards microalgal carbon sequestration. Biocatal. Agric. Biotechnol. 25, 101606. doi: 10.1016/j.bcab.2020.101606
Dris, R., Imhof, H., Sanchez, W., Gasperi, J., Galgani, F., Tassin, B., et al. (2015). Beyond the ocean: contamination of freshwater ecosystems with (micro-)plastic particles. Environ. Chem. 12, 539–550. doi: 10.1071/EN14172
Farzi, A., Dehnad, A., and Fotouhi, A. F. (2019). Biodegradation of polyethylene terephthalate waste using Streptomyces species and kinetic modeling of the process. Biocatal. Agric. Biotechnol. 17, 25–31. doi: 10.1016/j.bcab.2018.11.002
Farzi, A., Dehnad, A., Shirzad, N., and Norouzifard, F. (2017). Biodegradation of high density polyethylene using Streptomyces species. J. Coastal Life Med. 5, 474–479. doi: 10.12980/jclm.5.2017J7-94
Gaytán I. Sánchez-Reyes A. Burelo M. Vargas-Suárez M. Liachko I. Press M. . (2019). Degradation of recalcitrant polyurethane and xenobiotic additives by a selected landfill microbial community and its biodegradative potential revealed by proximity ligation-based metagenomic analysis. Front. Microbiol. 10, 2986. doi: 10.3389/fmicb.2019.02986
Gewert, B., Plassmann, M. M., and Macleod, M. (2015). Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 17, 1513–1521. doi: 10.1039/C5EM00207A
Geyer, R., Jambeck, J., and Law, K. (2017). Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782. doi: 10.1126/sciadv.1700782
Gilan, I., Hadar, Y., and Sivan, A. (2004). Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 65, 97–104. doi: 10.1007/s00253-004-1584-8
Gohain, A., Manpoong, C., Saikia, R., and De Mandal, S. (2020). “Chapter 9 - Actinobacteria: diversity and biotechnological applications,” in Recent Advancements in Microbial Diversity, eds. S. De Mandal and P. Bhatt. (Academic Press) 217–231. doi: 10.1016/B978-0-12-821265-3.00009-8
Gopal, R. (2017). Biodegradation of polyethylene by green photosynthetic microalgae. J. Bioremed. Biodegr. 8, 2. doi: 10.4172/2155-6199.1000381
Graham Bowditch, T. (1997). Penetration of Polyvinyl Chloride and Polypropylene Packaging Films by Ephestia cautella (Lepidoptera: Pyralidae) and Plodia interpunctella (Lepidoptera: Pyralidae) Larvae, and Tribolium confusum (Coleoptera: Tenebrionidae) Adults. J. Econ. Entomol. 90, 1028–1031
Hadad, D., Geresh, S., and Sivan, A. (2005). Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J. Appl. Microbiol. 98, 1093–1100. doi: 10.1111/j.1365-2672.2005.02553.x
Howard, G. T. (2002). Biodegradation of polyurethane: a review. Int. Biodeterior. Biodegrad. 49, 245–252. doi: 10.1016/S0964-8305(02)00051-3
Howard, G. T. (2012). “Polyurethane Biodegradation,” in Microbial Degradation of Xenobiotics, ed. S.N. Singh. (Berlin, Heidelberg: Springer Berlin Heidelberg) 371–394. doi: 10.1007/978-3-642-23789-8_14
Howard, G. T., and Blake, R. C. (1998). Growth of Pseudomonas fluorescens on a polyester–polyurethane and the purification and characterization of a polyurethanase–protease enzyme. Int. Biodeterior. Biodegrad. 42, 213–220. doi: 10.1016/S0964-8305(98)00051-1
Howard, G. T., Norton, W. N., and Burks, T. (2012). Growth of Acinetobacter gerneri P7 on polyurethane and the purification and characterization of a polyurethanase enzyme. Biodegradation 23, 561–573. doi: 10.1007/s10532-011-9533-6
Huerta Lwanga, E., Thapa, B., Yang, X., Gertsen, H., Salánki, T., Geissen, V., et al. (2018). Decay of low-density polyethylene by bacteria extracted from earthworm's guts: A potential for soil restoration. Sci. Total Environ. 624, 753–757. doi: 10.1016/j.scitotenv.2017.12.144
Hung, C.-S., Zingarelli, S., Nadeau, L. J., Biffinger, J. C., Drake, C. A., Crouch, A. L., et al. (2016). Carbon Catabolite Repression and Impranil Polyurethane Degradation in Pseudomonas protegens Strain Pf-5. Appl. Environ. Microbiol. 82, 6080–6090. doi: 10.1128/AEM.01448-16
Jabloune, R., Khalil, M., Ben Moussa, I. E., Simao-Beaunoir, A. M., Lerat, S., Brzezinski, R., et al. (2020). Enzymatic Degradation of p-Nitrophenyl Esters, Polyethylene Terephthalate, Cutin, and Suberin by Sub1, a Suberinase Encoded by the Plant Pathogen Streptomyces scabies. Microb. Environ. 35, ME19086. doi: 10.1264/jsme2.ME19086
Jambeck, J. R., Geyer, R., Wilcox, C., Siegler, T. R., Perryman, M., Andrady, A., et al. (2015). Marine pollution. Plastic waste inputs from land into the ocean. Science 347, 768–771. doi: 10.1126/science.1260352
Janusz, G., Pawlik, A., Swiderska-Burek, U., Polak, J., Sulej, J., Jarosz-Wilkołazka, A., et al. (2020). Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 21, 966. doi: 10.3390/ijms21030966
Jeyakumar, D., Chirsteen, J., and Doble, M. (2013). Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresource Technol. 148, 78–85. doi: 10.1016/j.biortech.2013.08.074
Jiang, S., Su, T., Zhao, J., and Wang, Z. (2021a). Biodegradation of polystyrene by tenebrio molitor, galleria mellonella, and zophobas atratus larvae and comparison of their degradation effects. Polymers 13, 3539. doi: 10.3390/polym13203539
Jiang, S., Su, T., Zhao, J., and Wang, Z. (2021b). Isolation, identification, and characterization of polystyrene-degrading bacteria from the gut of galleria mellonella (Lepidoptera: Pyralidae) Larvae. Front. Bioeng. Biotechnol. 9, 736062. doi: 10.3389/fbioe.2021.736062
Jones, P., Prasad, D., Heskins, M., Morgan, M., and Guillet, J. (1974). Biodegradability of photodegraded polymers. I. Development of experimental procedures. Environ. Sci. Technol. 8, 919–923. doi: 10.1021/es60095a010
Kawai, F., Kawabata, T., and Oda, M. (2019). Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 103, 4253–4268. doi: 10.1007/s00253-019-09717-y
Kesti, S., and Sharana, S. (2019). First report on biodegradation of low density polyethylene by rice moth larvae, Corcyra cephalonica (Stainton). Holistic Appr. Environ. 9, 79–83. doi: 10.33765/thate.9.4.2
Khan, S., Nadir, S., Shah, Z. U., Shah, A. A., Karunarathna, S. C., Xu, J., et al. (2017). Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ. Pollut. 225, 469–480. doi: 10.1016/j.envpol.2017.03.012
Khoironi, A., Anggoro, S., and Sudarno, S. (2019). Evaluation of the interaction among microalgae spirulina sp, plastics polyethylene terephthalate and polypropylene in freshwater environment. J. Ecol. Eng. 20, 161–173. doi: 10.12911/22998993/108637
Khoo, K. S., Ho, L. Y., Lim, H. R., Leong, H. Y., and Chew, K. W. (2021). Plastic waste associated with the COVID-19 pandemic: Crisis or opportunity? J. Hazard Mater. 417, 126108. doi: 10.1016/j.jhazmat.2021.126108
Kinoshita, S., Kageyama, S., Iba, K., Yamada, Y., and Okada, H. (1975). Utilization of a Cyclic Dimer and Linear Oligomers of ε-Aminocaproic Acid by Achrornobacter guttatus KI 72. Agric. Biol. Chem. 39, 1219–1223. doi: 10.1080/00021369.1975.10861757
Krueger, M. C., Hofmann, U., Moeder, M., and Schlosser, D. (2015). Potential of wood-rotting fungi to attack polystyrene sulfonate and its depolymerisation by gloeophyllum trabeum via hydroquinone-driven fenton chemistry. PLoS ONE 10, e0131773. doi: 10.1371/journal.pone.0131773
Kumar, A., Kalleshwaraswamy, C., Sharma, R., Sharma, P., and Poonia, A. (2022). “Biodegradation of plastic using termites and their gut microbiota: a mini review,” in IOP Conference Series: Earth and Environmental Science, 1057, 012016. doi: 10.1088/1755-1315/1057/1/012016
Kumari, A., Chaudhary, D. R., and Jha, B. (2019). Destabilization of polyethylene and polyvinylchloride structure by marine bacterial strain. Environ. Sci. Pollut. Res. Int. 26, 1507–1516. doi: 10.1007/s11356-018-3465-1
Kundungal, H., Gangarapu, M., Sarangapani, S., Patchaiyappan, A., and Devipriya, S. P. (2019). Efficient biodegradation of polyethylene (HDPE) waste by the plastic-eating lesser waxworm (Achroia grisella). Environ. Sci. Pollut. Res. Int. 26, 18509–18519. doi: 10.1007/s11356-019-05038-9
Kundungal, H., Gangarapu, M., Sarangapani, S., Patchaiyappan, A., and Devipriya, S. P. (2021a). Role of pretreatment and evidence for the enhanced biodegradation and mineralization of low-density polyethylene films by greater waxworm. Environ. Technol. 42, 717–730. doi: 10.1080/09593330.2019.1643925
Kundungal, H., Synshiang, K., and Devipriya, S. P. (2021b). Biodegradation of polystyrene wastes by a newly reported honey bee pest Uloma sp. larvae: An insight to the ability of polystyrene-fed larvae to complete its life cycle. Environ. Chall. 4, 100083. doi: 10.1016/j.envc.2021.100083
Labow, R. S., Erfle, D. J., and Santerre, J. P. (1996). Elastase-induced hydrolysis of synthetic solid substrates: poly(ester-urea-urethane) and poly(ether-urea-urethane). Biomaterials 17, 2381–2388. doi: 10.1016/S0142-9612(96)00088-9
Latour, S., Noël, G., Serteyn, L., Sare, A. R., Massart, S., Delvigne, F., et al. (2021). Multi-omics approach reveals new insights into the gut microbiome of Galleria mellonella (Lepidoptera:Pyralidae) exposed to polyethylene diet. bioRxiv.2021.2006.2004.446152. doi: 10.1101/2021.06.04.446152
Liu, J., Liu, J., Xu, B., Xu, A., Cao, S., Wei, R., et al. (2022). Biodegradation of polyether-polyurethane foam in yellow mealworms (Tenebrio molitor) and effects on the gut microbiome. Chemosphere 304, 135263. doi: 10.1016/j.chemosphere.2022.135263
López-Naranjo, E. J., Alzate-Gaviria, L. M., Hernández-Zárate, G., Reyes-Trujeque, J., Cupul-Manzano, C. V., and Cruz-Estrada, R. H. (2013). Effect of biological degradation by termites on the flexural properties of pinewood residue/recycled high-density polyethylene composites. J. Appl. Poly. Sci. 128, 2595–2603. doi: 10.1002/app.38212
Loredo-Treviño, A., Gutiérrez-Sánchez, G., Rodríguez-Herrera, R., and Aguilar, C. N. (2012). Microbial enzymes involved in polyurethane biodegradation: a review. J. Polym. Environ. 20, 258–265. doi: 10.1007/s10924-011-0390-5
Lou, H., Fu, R., Long, T., Fan, B., Guo, C., Li, L., et al. (2022). Biodegradation of polyethylene by Meyerozyma guilliermondii and Serratia marcescens isolated from the gut of waxworms (larvae of Plodia interpunctella). Sci. Total Environ. 853, 158604. doi: 10.1016/j.scitotenv.2022.158604
Lou, Y., Ekaterina, P., Yang, S. S., Lu, B., Liu, B., Ren, N., et al. (2020). Biodegradation of polyethylene and polystyrene by greater wax moth larvae (Galleria mellonella L.) and the effect of co-diet supplementation on the core gut microbiome. Environ. Sci. Technol. 54, 2821–2831. doi: 10.1021/acs.est.9b07044
Lu, C., Liu, L., Li, J., Du, G., and Chen, J. (2013). Isolation and characterization of a microorganism degrading starch/polyethylene blends. Chin. J. Appl. Environ. Biol. 19, 683–687.
Luo, Y., Zhou, Q., Zhang, H., Pan, X., Chen, T. U., Lianzhen, L. I., et al. (2018). Pay attention to research on microplastic pollution in soil for prevention of ecological and food chain risks. Bull. Chin. Acad. Sci. 33, 1021–1030. doi: 10.16418/j.issn.1000-3045.2018.10.003
Mahdi, M. S., Ameen, R. S., and Ibrahim, H. K. (2016). Study on degradation of nylon 6 by thermophilic bacteria Anoxybacillus rupiensis Ir3 (JQ912241). Int. J. Adv. Res. Biol. Sci. 3, 200–209. doi: 10.22192/ijarbs.2016.03.09.027
Major, I., Fuenmayor, E., and Mcconville, C. (2016). The production of solid dosage forms from non-degradable polymers. Curr. Pharm. Des. 22, 2738–2760. doi: 10.2174/1381612822666160217141049
Mateos-Cárdenas, A., O'halloran, J., Van Pelt, F. N. a. M., and Jansen, M.a.K. (2020). Rapid fragmentation of microplastics by the freshwater amphipod Gammarus duebeni (Lillj.). Scientific Rep. 10, 12799. doi: 10.1038/s41598-020-69635-2
Matsumiya, Y., Murata, N., Tanabe, E., Kubota, K., and Kubo, M. (2010). Isolation and characterization of an ether-type polyurethane-degrading micro-organism and analysis of degradation mechanism by Alternaria sp. J. Appl. Microbiol. 108, 1946–1953. doi: 10.1111/j.1365-2672.2009.04600.x
Miri, S., Saini, R., Davoodi, S. M., Pulicharla, R., Brar, S. K., and Magdouli, S. (2022). Biodegradation of microplastics: Better late than never. Chemosphere 286, 131670. doi: 10.1016/j.chemosphere.2021.131670
Montazer, Z., Habibi Najafi, M. B., and Levin, D. B. (2019). Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can. J. Microbiol. 65, 224–234. doi: 10.1139/cjm-2018-0335
Moog, D., Schmitt, J., Senger, J., Zarzycki, J., Rexer, K.-H., Linne, U., et al. (2019). Using a marine microalga as a chassis for polyethylene terephthalate (PET) degradation. Microbial. Cell Factor. 18, 171. doi: 10.1186/s12934-019-1220-z
Muhonja, C. N., Makonde, H., Magoma, G., and Imbuga, M. (2018). Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE 13, e0198446. doi: 10.1371/journal.pone.0198446
Nakajima-Kambe, T., Onuma, F., Kimpara, N., and Nakahara, T. (1995). Isolation and characterization of a bacterium which utilizes polyester polyurethane as a sole carbon and nitrogen source. FEMS Microbiol. Lett. 129, 39–42. doi: 10.1111/j.1574-6968.1995.tb07554.x
Novotný, C., Malachová, K., Adamus, G., Kwiecień, M., Lotti, N., Soccio, M., et al. (2018). Deterioration of irradiation/high-temperature pretreated, linear low-density polyethylene (LLDPE) by Bacillus amyloliquefaciens. Int. Biodeterior. Biodegrad. 132, 259–267. doi: 10.1016/j.ibiod.2018.04.014
Ohtake, Y., Kobayashi, T., Asabe, H., Murakami, N., and Ono, K. (1998). Oxidative degradation and molecular weight change of LDPE buried under bioactive soil for 32–37 years. J. Appl. Poly. Sci. 70, 1643–1648. doi: 10.1002/(SICI)1097-4628(19981128)70:9<1643::AID-APP1>3.0.CO;2-V
O'leary, N. D., O'connor, K. E., and Dobson, A. D. (2002). Biochemistry, genetics and physiology of microbial styrene degradation. FEMS Microbiol. Rev. 26, 403–417. doi: 10.1111/j.1574-6976.2002.tb00622.x
Otake, Y., Kobayashi, T., Asabe, H., Murakami, N., and Ono, K. (1995). Biodegradation of low-density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. J. Appl. Poly. Sci. 56, 1789–1796. doi: 10.1002/app.1995.070561309
Paço, A., Duarte, K., Da Costa, J. P., Santos, P. S. M., Pereira, R., Pereira, M. E., et al. (2017). Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 586, 10–15. doi: 10.1016/j.scitotenv.2017.02.017
Park, S. Y., and Kim, C. G. (2019). Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 222, 527–533. doi: 10.1016/j.chemosphere.2019.01.159
Pegram, J. E., and Andrady, A. L. (1989). Outdoor weathering of selected polymeric materials under marine exposure conditions. Polymer Degrad. Stabil. 26, 333–345. doi: 10.1016/0141-3910(89)90112-2
Peng, B.-Y., Chen, Z., Chen, J., Yu, H., Zhou, X., Criddle, C. S., et al. (2020a). Biodegradation of Polyvinyl Chloride (PVC) in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Environ. Int. 145, 106106. doi: 10.1016/j.envint.2020.106106
Peng, B.-Y., Li, Y., Fan, R., Chen, Z., Chen, J., Brandon, A. M., et al. (2020b). Biodegradation of low-density polyethylene and polystyrene in superworms, larvae of Zophobas atratus (Coleoptera: Tenebrionidae): Broad and limited extent depolymerization. Environ. Pollut. 266, 115206. doi: 10.1016/j.envpol.2020.115206
Peng, B.-Y., Sun, Y., Wu, Z., Chen, J., Shen, Z., Zhou, X., et al. (2022). Biodegradation of polystyrene and low-density polyethylene by Zophobas atratus larvae: Fragmentation into microplastics, gut microbiota shift, and microbial functional enzymes. J. Cleaner Prod. 367, 132987. doi: 10.1016/j.jclepro.2022.132987
Peng, B. Y., Su, Y., Chen, Z., Chen, J., Zhou, X., Benbow, M. E., et al. (2019). Biodegradation of Polystyrene by Dark (Tenebrio obscurus) and Yellow (Tenebrio molitor) Mealworms (Coleoptera: Tenebrionidae). Environ. Sci. Technol. 53, 5256–5265. doi: 10.1021/acs.est.8b06963
Pramila, R., and Ramesh, K. V. (2015). Potential biodegradation of low density polyethylene (LDPE) by Acinetobacter baumannii. Afr. J Bacteriol. Res. 7, 24–28. doi: 10.5897/jbr2015.0152
Réjasse, A., Waeytens, J., Deniset-Besseau, A., Crapart, N., Nielsen-Leroux, C., and Sandt, C. (2021). Plastic biodegradation: do Galleria mellonella larvae - bio-assimilate polyethylene? A spectral histology approach using isotopic labelling and infrared microspectroscopy. bioRxiv.2021.2010.2008.463624. doi: 10.1101/2021.10.08.463624
Ren, L., Men, L., Zhang, Z., Guan, F., Tian, J., Wang, B., et al. (2019). Biodegradation of Polyethylene by Enterobacter sp. D1 from the Guts of Wax Moth Galleria mellonella. Int. J. Environ. Res. Public Health 16, 1941. doi: 10.3390/ijerph16111941
Riudavets, J., Salas, I., and Pons, M. J. (2007). Damage characteristics produced by insect pests in packaging film. J. Stored Prod. Res. 43, 564–570. doi: 10.1016/j.jspr.2007.03.006
Ronkvist, Å. M., Xie, W., Lu, W., and Gross, R. A. (2009). Cutinase-Catalyzed Hydrolysis of Poly(ethylene terephthalate). Macromolecules 42, 5128–5138. doi: 10.1021/ma9005318
Ru, J., Huo, Y., and Yang, Y. (2020). Microbial degradation and valorization of plastic wastes. Front. Microbiol. 11, 442–442. doi: 10.3389/fmicb.2020.00442
Ruiz, C., Main, T., Hilliard, N. P., and Howard, G. T. (1999). Purification and characterization of twopolyurethanase enzymes from Pseudomonas chlororaphis. Int. Biodeterior. Biodegrad. 43, 43–47. doi: 10.1016/S0964-8305(98)00067-5
Russell, J. R., Huang, J., Anand, P., Kucera, K., Sandoval, A. G., Dantzler, K. W., et al. (2011). Biodegradation of polyester polyurethane by endophytic fungi. Appl. Environ. Microbiol. 77, 6076–6084. doi: 10.1128/AEM.00521-11
Sah, A., Negi, H., Kapri, A., Anwar, S., and Goel, R. (2011). Comparative shelf life and efficacy of LDPE and PVC degrading bacterial consortia under bioformulation. Ekologija 57, 55–61. doi: 10.6001/ekologija.v57i2.1885
Sanchez-Hernandez, J. C. (2021). A toxicological perspective of plastic biodegradation by insect larvae. Compar. Biochem. Physiol. Part C. 248, 109117. doi: 10.1016/j.cbpc.2021.109117
Sanchez-Hernandez, J. C., Capowiez, Y., and Ro, K. S. (2020). Potential Use of Earthworms to Enhance Decaying of Biodegradable Plastics. ACS Sustain. Chem. Eng. 8, 4292–4316. doi: 10.1021/acssuschemeng.9b05450
Sangale, M. K., Shahnawaz, M., and Ade, A. B. (2019). Gas chromatography-Mass Spectra analysis and deleterious potential of fungal based polythene-degradation products. Scientific Rep. 9, 1599. doi: 10.1038/s41598-018-37738-6
Sangeetha Devi, R., Rajesh Kannan, V., Nivas, D., Kannan, K., Chandru, S., and Robert Antony, A. (2015). Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar. Pollut. Bull. 96, 32–40. doi: 10.1016/j.marpolbul.2015.05.050
Sathya, R., Ushadevi, T., and Panneerselvam, A. (2012). Plastic degrading actinomycetes isolated from mangrove sediments. Int. J. Curr. Res. 4, 1–3. Available online at: https://www.journalcra.com/sites/default/files/issue-pdf/1864.pdf
Schmidt, J., Wei, R., Oeser, T., Dedavid E Silva, L. A., Breite, D., Schulze, A., et al. (2017). Degradation of Polyester Polyurethane by Bacterial Polyester Hydrolases. Polymers 9, 65. doi: 10.3390/polym9020065
Seymour, R. B., and Kauffman, G. B. (1992). Polyurethanes: A class of modern versatile materials. J. Chem. Educ. 69, 909. doi: 10.1021/ed069p909
Shah, A. A., Hasan, F., Hameed, A., and Ahmed, S. (2008). Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 26, 246–265. doi: 10.1016/j.biotechadv.2007.12.005
Shah, S., and Green, J. R. (1994). Disease susceptibility genes and the sib-pair method: a review of recent methodology. Ann. Hum. Genet. 58, 381–395. doi: 10.1111/j.1469-1809.1994.tb00734.x
Shahnawaz, M., Sangale, M. K., and Ade, A. B. (2019). “Bacteria as Key Players of Plastic Bioremediation,” in Bioremediation Technology for Plastic Waste, eds. M. Shahnawaz, M.K. Sangale and A.B. Ade. (Singapore: Springer Singapore) 45–69. doi: 10.1007/978-981-13-7492-0
Singh, M. J., and Sedhuraman, P. (2015). Biosurfactant, polythene, plastic, and diesel biodegradation activity of endophytic Nocardiopsis sp. mrinalini9 isolated from Hibiscus rosasinensis leaves. Bioresour. Bioprocess. 2, 2. doi: 10.1186/s40643-014-0034-4
Skariyachan, S., Manjunatha, V., Sultana, S., Jois, C., Bai, V., and Vasist, K. S. (2016). Novel bacterial consortia isolated from plastic garbage processing areas demonstrated enhanced degradation for low density polyethylene. Environ. Sci. Pollut. Res. Int. 23, 18307–18319. doi: 10.1007/s11356-016-7000-y
Song, Y., Qiu, R., Hu, J., Li, X., Zhang, X., Chen, Y., et al. (2020). Biodegradation and disintegration of expanded polystyrene by land snails Achatina fulica. Sci. Total Environ. 746, 141289. doi: 10.1016/j.scitotenv.2020.141289
Soong, Y.-H., Sobkowicz, M., and Xie, D. (2022). Recent Advances in Biological Recycling of Polyethylene Terephthalate (PET) Plastic Wastes. Bioengineering 9, 98. doi: 10.3390/bioengineering9030098
Stern, R. V., and Howard, G. T. (2000). The polyester polyurethanase gene (pueA) from Pseudomonas chlororaphis encodes a lipase. FEMS Microbiol. Lett. 185, 163–168. doi: 10.1111/j.1574-6968.2000.tb09056.x
Sudhakar, M., Doble, M., Murthy, P. S., and Venkatesan, R. (2008). Marine microbe-mediated biodegradation of low- and high-density polyethylenes. Int. Biodeterior. Biodegrad. 61, 203–213. doi: 10.1016/j.ibiod.2007.07.011
Sumathi, T., Viswanath, B., Sri Lakshmi, A., and Saigopal, D. V. (2016). Production of laccase by cochliobolus sp. isolated from plastic dumped soils and their ability to degrade low molecular weight PVC. Biochem. Res. Int. 2016, 9519527. doi: 10.1155/2016/9519527
Syranidou, E., Karkanorachaki, K., Amorotti, F., Repouskou, E., Kroll, K., Kolvenbach, B., et al. (2017). Development of tailored indigenous marine consortia for the degradation of naturally weathered polyethylene films. PLoS ONE 12, e0183984. doi: 10.1371/journal.pone.0183984
Taniguchi, I., Yoshida, S., Hiraga, K., Miyamoto, K., Kimura, Y., and Oda, K. (2019). Biodegradation of PET: Current status and application aspects. ACS Catalysis 9, 4089–4105. doi: 10.1021/acscatal.8b05171
Terence, G. B. (1997). Penetration of polyvinyl chloride and polypropylene packaging films by ephestia cautella (lepidoptera: pyralidae) and plodia interpunctella (Lepidoptera: Pyralidae) Larvae, and Tribolium confusum (Coleoptera: Tenebrionidae) Adults. J. Econ. Entomol. 90, 1028–1031. doi: 10.1093/jee/90.4.1028
Tischler, D., Eulberg, D., Lakner, S., Kaschabek, S. R., Van Berkel, W. J. H., and Schlömann, M. (2009). Identification of a novel self-sufficient styrene monooxygenase from Rhodococcus opacus 1CP. J. Bacteriol. 191, 4996–5009. doi: 10.1128/JB.00307-09
Tseng, M., Yang, S. F., Hoang, K. C., Liao, H. C., Yuan, G. F., and Liao, C. C. (2009). Actinomadura miaoliensis sp. nov., a thermotolerant polyester-degrading actinomycete. Int. J. System. Evolut. Microbiol. 59, 517–520. doi: 10.1099/ijs.0.001479-0
Umamaheswari, S., and Subramani, M. (2017). GCMS analysis of pseudomonas sp., mediated degradation of polystyrene. Ann. Biol. Res. 8, 8–11.
Volke-Sepúlveda, T., Saucedo-Castañeda, G., Gutiérrez-Rojas, M., Manzur, A., and Favela-Torres, E. (2002). Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus niger. J. Appl. Poly. Sci. 83, 305–314. doi: 10.1002/app.2245
Wang, J., Tan, Z., Peng, J., Qiu, Q., and Li, M. (2016). The behaviors of microplastics in the marine environment. Mar. Environ. Res. 113, 7–17. doi: 10.1016/j.marenvres.2015.10.014
Wang, L., Peng, Y., Xu, Y., Zhang, J., Liu, C., Tang, X., et al. (2022). Earthworms' degradable bioplastic diet of polylactic acid: easy to break down and slow to excrete. Environ. Sci. Technol. 56, 5020–5028. doi: 10.1021/acs.est.1c08066
Wang, S., Shi, W., Huang, Z., Zhou, N., Xie, Y., Tang, Y., et al. (2022). Complete digestion/biodegradation of polystyrene microplastics by greater wax moth (Galleria mellonella) larvae: Direct in vivo evidence, gut microbiota independence, and potential metabolic pathways. J. Hazardous Mater. 423, 127213. doi: 10.1016/j.jhazmat.2021.127213
Wang, Z., Xin, X., Shi, X., and Zhang, Y. (2020). A polystyrene-degrading Acinetobacter bacterium isolated from the larvae of Tribolium castaneum. Sci. Total Environ. 726, 138564. doi: 10.1016/j.scitotenv.2020.138564
Wei, R., Song, C., Gräsing, D., Schneider, T., Bielytskyi, P., Böttcher, D., et al. (2019). Conformational fitting of a flexible oligomeric substrate does not explain the enzymatic PET degradation. Nature Communications 10, 5581. doi: 10.1038/s41467-019-13492-9
Wei, R., and Zimmermann, W. (2017). Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: how far are we? Microb. Biotechnol. 10, 1308–1322. doi: 10.1111/1751-7915.12710
Woo, S., Song, I., and Cha, H. J. (2020). Fast and Facile Biodegradation of Polystyrene by the Gut Microbial Flora of Plesiophthalmus davidis Larvae. Appl. Environ. Microbiol. 86, e01361–e01320. doi: 10.1128/AEM.01361-20
Worm, B., Lotze, H. K., Jubinville, I., Wilcox, C., and Jambeck, J. (2017). Plastic as a Persistent Marine Pollutant. Ann. Rev. Environ. Resour. 42, 1–26. doi: 10.1146/annurev-environ-102016-060700
Yamada-Onodera, K., Mukumoto, H., Katsuyaya, Y., Saiganji, A., and Tani, Y. (2001). Degradation of polyethylene by a fungus, Penicillium simplicissimum YK. Poly. Degrad. Stability 72, 323–327. doi: 10.1016/S0141-3910(01)00027-1
Yang, J., Yang, Y., Wu, W. M., Zhao, J., and Jiang, L. (2014). Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 48, 13776–13784. doi: 10.1021/es504038a
Yang, S., Ding, M., Ren, X., Zhang, Z., Li, M., Zhang, L., et al. (2022). Impacts of physical-chemical property of polyethylene on depolymerization and biodegradation in yellow and dark mealworms with high purity microplastics. Sci. Total Environ. 828, 154458. doi: 10.1016/j.scitotenv.2022.154458
Yang, S., Ding, M.-Q., He, L., Zhang, C.-H., Li, Q.-X., Xing, D., et al. (2020). Biodegradation of polypropylene by yellow mealworms (Tenebrio molitor) and superworms (Zophobas atratus) via gut-microbe-dependent depolymerization. Sci. Total Environ. 756, 144087. doi: 10.1016/j.scitotenv.2020.144087
Yang, S.-S., Brandon, A. M., Andrew Flanagan, J. C., Yang, J., Ning, D., Cai, S.-Y., et al. (2018). Biodegradation of polystyrene wastes in yellow mealworms (larvae of Tenebrio molitor Linnaeus): Factors affecting biodegradation rates and the ability of polystyrene-fed larvae to complete their life cycle. Chemosphere 191, 979–989. doi: 10.1016/j.chemosphere.2017.10.117
Yang, Y., Wang, J., and Xia, M. (2020). Biodegradation and mineralization of polystyrene by plastic-eating superworms Zophobasatratus. Sci. Total Environ. 708, 135233. doi: 10.1016/j.scitotenv.2019.135233
Yang, Y., Yang, J., Wu, W. M., Zhao, J., Song, Y., Gao, L., et al. (2015). Biodegradation and mineralization of polystyrene by plastic-eating mealworms: Part 1. Chemical and physical characterization and isotopic tests. Environ. Sci. Technol. 49, 12080–12086. doi: 10.1021/acs.est.5b02661
Yoshida, S., Hiraga, K., Takehana, T., Taniguchi, I., Yamaji, H., Maeda, Y., et al. (2016). A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351, 1196–1199. doi: 10.1126/science.aad6359
Yuan, J., Ma, J., Sun, Y., Zhou, T., Zhao, Y., and Yu, F. (2020). Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 715, 136968. doi: 10.1016/j.scitotenv.2020.136968
Zhang, J., Gao, D., Li, Q., Zhao, Y., Li, L., Lin, H., et al. (2020). Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 704, 135931. doi: 10.1016/j.scitotenv.2019.135931
Zhang, Y., Jiao, Y., Li, Z., Tao, Y., and Yang, Y. (2021). Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Sci. Total Environ. 771, 145418. doi: 10.1016/j.scitotenv.2021.145418
Zhu, P., Shen, Y., Li, X., Liu, X., Qian, G., and Zhou, J. (2022). Feeding preference of insect larvae to waste electrical and electronic equipment plastics. Sci. Total Environ. 807, 151037. doi: 10.1016/j.scitotenv.2021.151037
Zumstein, M. T., Schintlmeister, A., Nelson, T. F., Baumgartner, R., Woebken, D., Wagner, M., et al. (2018). Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Sci. Adv. 4, eaas9024. doi: 10.1126/sciadv.aas9024
Keywords: enzyme, gut microbes, insects, invertebrate, plastic biodegradation
Citation: Yang X-G, Wen P-P, Yang Y-F, Jia P-P, Li W-G and Pei D-S (2023) Plastic biodegradation by in vitro environmental microorganisms and in vivo gut microorganisms of insects. Front. Microbiol. 13:1001750. doi: 10.3389/fmicb.2022.1001750
Received: 24 July 2022; Accepted: 22 November 2022;
Published: 06 January 2023.
Edited by:
Hua Xiang, Institute of Microbiology (CAS), ChinaReviewed by:
Kumar Ponnuchamy, Alagappa University, IndiaCopyright © 2023 Yang, Wen, Yang, Jia, Li and Pei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: De-Sheng Pei, cGVpZHNAY3FtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.