
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol. , 17 November 2022
Sec. Food Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1001209
This article is part of the Research Topic Targeting Gut Microbiota Modulation by Dietary Supplementation to Improve Metabolic Diseases View all 7 articles
Premature ovarian insufficiency (POI) ranks top in the reproductive disorders that may impair multiple functioning systems, reduce the quality of life and ultimately deprive patients of their fertility among women. Symptoms can be partially alleviated by present hormone replacement therapy that cannot improve conception or decrease occurrence rates of systemic complication. Nutritional dietary supplements are attracting more and more attention because of their safety, bioavailability, and efficacy for well-being. Nutrients in the daily food are composed of carbohydrates, fat and lipoprotein, protein and polypeptide, vitamins, and vegetable or fruits containing phytoestrogens. These are functional nutrients due to the proliferative, anti-inflammatory, anti-oxidant, and mitochondria-protective potential during the course of menopause. Apart from dietary nutrients, microbe-related nutritional substances, including probiotics, prebiotics and the combination-synbiotics, display high potential as well in supporting estrous cycle, ovarian viability and modulating other vital reproductive functions. The present review will discuss dietary and microbial nutrients and their roles and applications in the living body based upon animal or human research, evaluate possible effect mechanisms from molecular, cellular and tissue levels, and provide insights into nutritional therapy for prolonging reproductive lifespan in female patients.
Premature ovarian insufficiency (POI), previously termed as premature ovarian failure, is a common and severe clinical reproductive disorder among women (Hsieh and Ho, 2021). It is primarily characterized by the decrease or loss of ovarian function occurring before the age of 40, with a high incidence of 1% (Ford et al., 2020). Its main clinical manifestations include menstrual disorders (amenorrhea or irregular menstruation), increased pituitary gonadotropin levels, decreased estrogen levels and other perimenopausal changes, especially the decrease in fertility (Di-Battista et al., 2020). In addition, POI patients also have mood changes, hot flashes, sleep disturbances, bone pain and pruritus, and experience long-term harm to the cardiovascular system, skeletal motor system, nervous system and mental health that all together affect women’s fertility (Horton et al., 2019). At present, the only effective treatment for POI is hormone replacement therapy (HRT). Though HRT can alleviate symptoms of low estrogen, rebuild regular menstrual cycle, and lower blood follicle stimulating hormone levels, this therapy may bring potential risks, such as the occurrence of thromboembolic disease, and tumor. Moreover, it hardly slows down the pathological process of ovarian senescence and functional decline, not to mention improving the conception rate of POI patients (European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber et al., 2016). In conclusion, the insufficient understanding of the mechanism of POI leads to the failure of current treatment methods for the effective delay of ovarian function decline. Consequently, it is of great significance to explore the key pathogenic factors involved in the process of POI to improve the efficacy of POI clinical treatment.
In the past decades, the highly increasing incidence of POI is accompanied with the rapid modification in the living lifestyle and deterioration of environment (Golezar et al., 2020). This arouses interest and concerns in the influence of the environmental and life factors on reproductive health, in addition to those genetic, immune or iatrogenic factors (Qin et al., 2015; Golezar et al., 2020). Furthermore, among all these factors, nutrient intake in daily diet of lifestyle is the most accessible and modifiable for POI patients, and remains the least understandable of all factors (Chen et al., 2017). The dietary habit was changed long ago with the improvement in socioeconomic levels worldwide. However, it also brings malnutrition issues to both genders, young and old due to obesity, emaciation, and different types of eating disorders. There are a few studies about the nutrient status and regulation affecting female fertility and menopause (Fontana and Della Torre, 2016; Chiu et al., 2018; Silvestris et al., 2019). Herein, we preliminarily propose the primary dietary factors and nutrients for disease progression, alleviation and control in menopausal course of POI, and expect these considerations may shed light on future clinical protection and treatment of ovarian function (Figure 1 and Table 1).
Figure 1. Primary nutritional factors for progression, alleviation and control of menopause and POI.
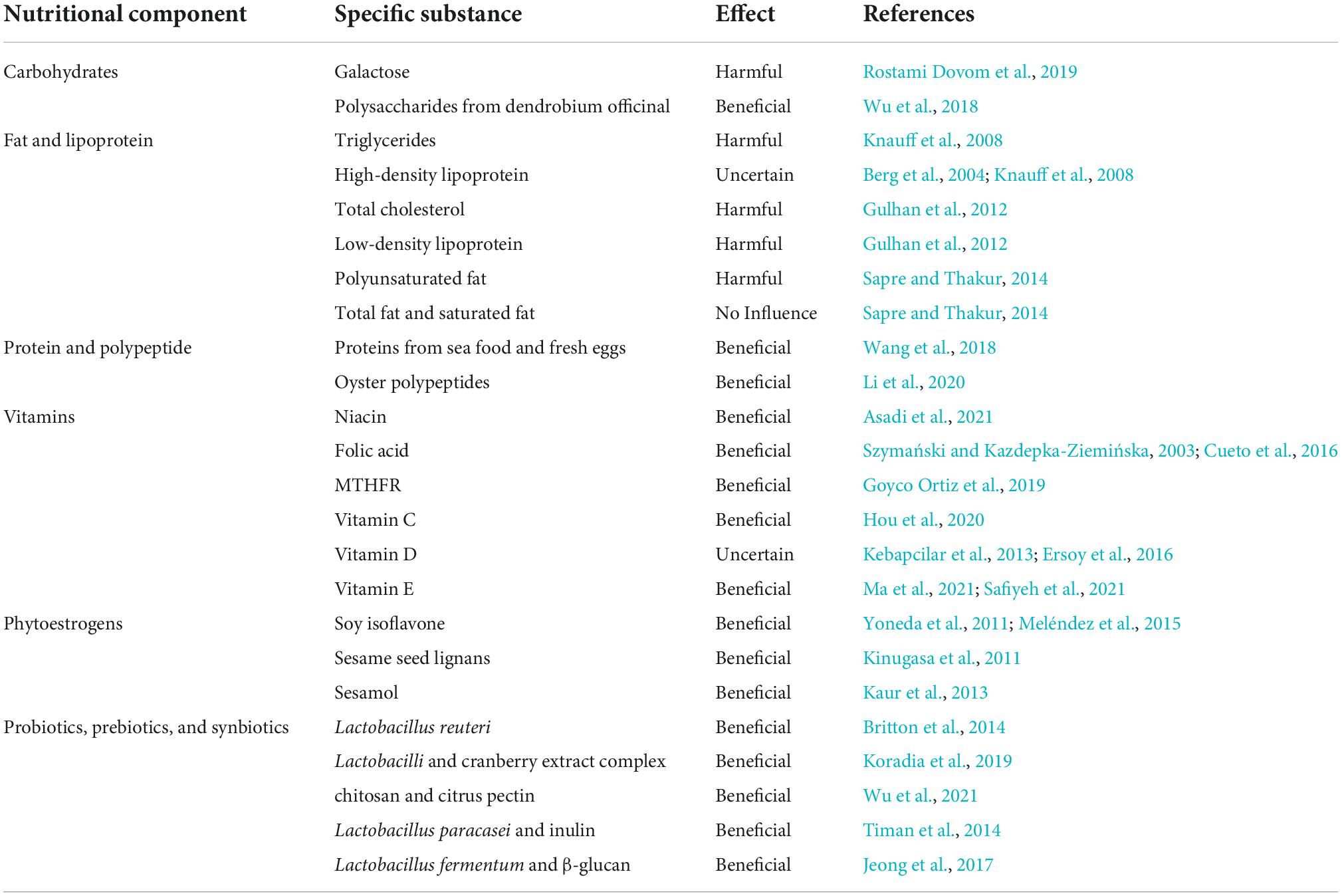
Table 1. Representatives from different nutritional components and their potential effects on menopause and POI.
Carbohydrates provide the most abundant life-supporting energy source for all living creatures. They are typically classified into monosaccharide, oligosaccharide, and polysaccharide (Kikkeri et al., 2010). Galactose is a kind of commonly seen monosaccharides reported in the POI and related infertile diseases (Bandyopadhyay et al., 2003). Dovom et al. claimed that it exerted a toxicity effect on ovaries based on systematic review analysis (Rostami Dovom et al., 2019). Therefore, galactose may be applied for induction of POI animal model under necessary concentration, appropriate time and exposure. Galactose may also inhibit follicular migration toward the gonadal region and lead to decreased ovarian reserves and estradiol synthesis (Rostami Dovom et al., 2019). Therefore, abnormal sugar galactose metabolism is related with POI onset and progression.
Polysaccharides from dendrobium officinal (DO) induced a protective effect on POI process in natural aging rodents (Wu et al., 2018). They were administered at 70 mg/kg through oral route and helped these rats regain normal weight and alleviate pathological changes to the ovary, such as vessel proliferation reduction and follicular decrease. DO-oriented polysaccharides reduced inflammatory insults in the ovarian tissues by regulating the NF-κB and p53/Bcl-2 signaling pathways and upregulated activities of glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) and decreased malondialdehyde (MDA) concentration in the serum to exert anti-oxidative effects. At the same time, they also manipulated the mitochondrial function and restored the ovarian cell viability (Wu et al., 2018). The significance of carbohydrates for POI onset or progression should be evaluated further.
Fat and high body weight may be relevant to control of POI severity. Several studies reported early menopause was associated with low body mass index (Tao et al., 2015; Szegda et al., 2017). The exact mechanisms are unclear. Lipoproteins are transportable forms of fat that actively participate in metabolic activities in the human body (Hoeke et al., 2016). Knauff et al. (2008) performed lipid profiles in POI patients and identified higher triglycerides (TG) concentration and moderately lower high-density lipoprotein (HDL) level in the serum were correlated with free androgen index increase and sex hormone binding globulin loss. These findings indicated that a TG increase might lead to an insulin sensitivity decrease (Knauff et al., 2008). In another retrospective clinical study, Gulhan et al. (2012) similarly found that young female patients who presented POI syndromes without any previous hormone intake history showed higher total cholesterol (TC) and low-density lipoprotein (LDL) concentrations in the serum in comparison with normal females of close epidemiological features. The results implicated that undesirable lipid alteration was triggered under deprived estrogen conditions in female POI patients (Gulhan et al., 2012). However, the concentration level of HDL is very inconsistent among different studies, with some reporting elevated levels, and others showing decreased levels in menopause women (Berg et al., 2004). As to the impact of fat intake on menopause, differences may be caused due to the various kinds of fat, including polyunsaturated, total and saturated fat. Sapre and Thakur (2014) reported that high consumption of polyunsaturated fats was related to earlier menopause onset; in the meantime, total fat and saturated fat intake barely influenced menopausal period and age. Nevertheless, it is unclear whether different fat types in the daily diet may affect POI pathogenesis in a certain way.
High protein intake was reported to be useful to postpone the early arrival of menopause (Taneja et al., 2019). Wang et al. (2018) found that consumption of seafood (around 3 days/week) and fresh eggs (more than 4 days/week) was positively correlated with late menopausal onset. Several other researches also supported similar conclusions. Dorjgochoo et al. (2008) claimed that high protein consumption was beneficial to delay menopause and prolong the reproductive function. The European Prospective Investigation into Cancer and Nutrition (EPIC) also conducted a cohort study, in which early menopausal occurrence was inversely associated with protein intake level (Gold et al., 2013). In addition to direct protein consumption, polypeptide is also significant for protecting ovarian function and delaying ovarian aging (Wang et al., 2021). Li et al. (2020) reported that oyster polypeptides, purified from oyster via enzymolysis, were able to protect against oxidative stress and inflammation to exert a therapeutic effect on POI because of their DPPH (2,2-diphenyl-1-picrylhydrazyl) radical-scavenger ability. Oyster polypeptides could correct the abnormal estrous cycle, and enhance serum follicle stimulating hormone (FSH) and luteinizing hormone (LH) concentrations. Further, they increased primordial follicular count and distribution, and significantly alleviated ovarian cell death by mimicking SOD clearance. The ovarian cell protection and survival supported by oyster polypeptides was activated upon regulating the death receptor BCL-2-dependent signaling pathway (Li et al., 2020). Therefore, appropriate protein and peptide intake may be a potential way to affect POI progression and severity.
Vitamins are a class of micro-organic substances only from food and maintain some vital physiological activities and functions in the human body. They do not provide energy but regulate metabolic process. Many vitamins play a key role in supporting female health, such as vitamin B, C, D, E, and some coenzymes (Chavarro and Schlaff, 2018).
Niacin belongs to the Vitamin B family, metabolizes cellular energy, and directly impacts normal physiology (Ye et al., 2019). In addition to many neurological and dermatitis diseases, niacin also reduced ovarian cell death by inhibiting oxidative stress to improve follicle development (Asadi et al., 2021). Interestingly, niacin was also able to modify POI phenotype and recover female POI mice induced by chemotherapy or radiation stimulation. The authors further confirmed the addition of 10 Mmol niacin could decrease the expression level of follicle arrest marker FOXO3 and increase the expression level of oocyte marker DDX4 to exert the ovarian protective effects (Asadi et al., 2021). Folic acid is a kind of water-soluble vitamin B and is important to protein synthesis, erythrocyte division and growth. A clinical study reported based on a small sample that females of low fertility showed a higher pregnancy rate after receiving 400-μg folic acid consumption for 12 weeks in comparison with placebo treatment (Westphal et al., 2006). Folic acid consumption reduced the pregnancy time for healthy females and improved the quality and maturation of oocytes from a couple of cohort studies (Szymański and Kazdepka-Ziemińska, 2003; Cueto et al., 2016). MTHFR could strongly affect the folate cycle and help a POI patient who simultaneously had non-Hodgkin’s lymphoma and repeat pregnancy losses, successfully deliver a healthy male baby (Goyco Ortiz et al., 2019).
Vitamin C is a natural antioxidant and is actively involved in stem cell fate regulation (DiTroia et al., 2019). It was reported to modify the biological characteristics of human amniotic epithelial cells (hAECs) and significantly stimulated the ovarian marker expression in the ovary tissues from vitamin C-treated hAECs transplanted POI mice. The possible effecting mechanism was the vitamin C dependent paracrine excretion of several important growth factors, such as hepatocyte growth factor and epidermal growth factor (Hou et al., 2020).
Vitamin D belongs to fat-soluble vitamins and is vital for life support by regulating calcium and phosphate metabolism (Bischoff-Ferrari et al., 2005). Its active form, 1,25-dihydroxy vitamin D3 is localized in many organs, such as kidney, liver, brain and ovary (Xu et al., 2016). In the female reproductive system, vitamin D can regulate anti-Müllerian hormone (AMH) secretion in the granulosa cells and consequently affect FSH level (Simpson et al., 2020). However, its role in POI pathogenesis is controversial as some claimed it did not influence pathophysiological process of POI, while others reported vitamin D deficiency was correlated with coagulation and was a primary factor for POI due to follicular dysgenesis and aromatase level decrease (Kebapcilar et al., 2013; Ersoy et al., 2016). Neither did previous researches report any confirmative findings on the vitamin D supplementation for POI treatment. It was found that vitamin D intake at 50,000 IU per week, for around 2 months, was irrelevant to pregnant improvement clinically (Aflatoonian et al., 2014). IVF outcome was not improved either by increasing serum vitamin D level in egg recipient patients (Rudick et al., 2014). Therefore, the interpretation of the significance of vitamin D for POI patients remains vague and still need further investigation.
Vitamin E is well-known beneficial factor for reproductive system because it increases ovarian volume and stimulates proliferation primary follicles (Peh et al., 2016). Vitamin E is mainly composed of tocopherols and tocotrienols that are important for free radical clearance under physiological and pathological conditions (Abraham et al., 2019). Deficiency of vitamin E might lead to ovarian cell death and developmental abnormality in a female rodent model (Das and Chowdhury, 1999). In a clinical study, vitamin E was identified to be related with the onset of POI among 40 patients. The active form of vitamin E-α tocopherol concentration was significantly higher in females with normal menstruation than POI patients. Further analysis showed that this could be associated with the decreased expression level of AMH and indicated ovarian reserve capability was compromised in the POI pathogenesis (Das and Chowdhury, 1999; Ma et al., 2021). In another randomized controlled trial study, vitamin E at 400IU was given to POI patients for 3 months and significantly increased antral follicle count and mean ovarian volume, in addition to the improvement in AMH levels (Safiyeh et al., 2021). The vitamin E supplementation was potentially useful to alleviate POI process and barely exerted side effects.
Phytoestrogens are not indeed part of estrogens but function similarly by providing a high affinity of estrogen receptor β as hormonal supplements (Wei et al., 2017). They typically involve isoflavones from soy, and lignans from sesame, both of which are important and ordinary food types in our daily diet. Several studies confirmed the reduction effect of phytoestrogen consumption on TC, LDL, and plasma lipids among females after menopause (de Kleijn et al., 2002; Kreijkamp-Kaspers et al., 2005). Isoflavone intake from soy consumption also significantly alleviated iliac artery atherosclerosis reduced the size of coronary artery plaques for those females who consumed soy food before and after menopause within 5 years. The beneficial effect was not obvious for those who started to have soy food as daily diet after menopause (Meléndez et al., 2015). Yoneda et al. (2011) noted that soy isoflavone might reduce hot flushes in a menopausal rodent setting by ovariectomy. The effect was based on the production of isoflavan equol by gut microbiota and estrogen-like mechanism (Yoneda et al., 2011). Sesame seed lignans, one of the components in sesame seed oil increased β-oxidation of fatty acids and prohibited cholesterol absorption in a mouse model (Kinugasa et al., 2011). Sesamol is a strong antioxidant and primary component of sesame. It could ameliorate memory, attenuate anxious emotion, and decrease oxidant insults in the central nervous system. In the meantime, sesamol also regulated the lipid constituent in the serum and decreased inflammatory effects by downregulating tumor necrosis factor-α (TNF-α) concentration in the serum in comparison with menopausal rats after ovariectomy (Kaur et al., 2013).
In addition to daily nutrient supplemented in the diet, some beneficial living organisms are also getting increasingly attraction due to their potential influence on female health (Han et al., 2021), especially POI (Wang et al., 2020). They are termed as probiotics (Milani et al., 2017). Probiotics promote digestion and absorption of nutrients, improve immune function, maintain the structural balance of intestinal flora, and enhance the antioxidant level of human body. A number of probiotics are applied in food supplement and medical treatment. For female health, a close correlation is proposed between hormone levels and microbiota from either gut or vagina (Anahtar et al., 2018). Gut microbiota were regarded as an alternate metabolic supplier of estrogens in patients with leiomyoma and breast carcinoma, and were mainly enriched in E. coli, Clostridium sp., Lactobacillus sp., and Bacteroides (Kwa et al., 2016; Critchley et al., 2020). Considering these phenomena, the regulatory effect of probiotics may also be exerted by modulating gut microbiota in an indirect style through changing metabolic activity and efficiency of several vegetable constituents with estrogenic or antiestrogenic properties, termed as phytoestrogens, such as soy isoflavones, lignans, or urolithins mentioned in the previous section (Seyed Hameed et al., 2020).
Probiotics are involved in the regulation of premenstrual syndrome, urogenital infection and osteoporosis. Estrogen deficiency is a primary reason of osteoporosis related to bone inflammation and resorption after POI (Samad et al., 2020). Previous studies indicate that gut microbiota show a potential influence on bone formation and metabolism. Bone increase was noticed in mice, rats and birds that received prebiotic therapy (Scholz-Ahrens and Schrezenmeir, 2007; Weaver et al., 2011; Yang et al., 2013). Nevertheless, probiotics were less known as to their roles in bone regulation. Probiotic Lactobacillus reuteri inhibited upregulation of bone marrow CD4+ T-lymphocytes and consequent osteoclast activation in vitro and in ovariectomized rodent study. Britton et al. (2014) assumed that Lactobacillus reuteri could directly deactivate T cells and inhibit TNF-α production, and indirectly deactivate stromal cells via T cells to reduce osteoclast cytokine formation, and therefore increase bone metabolic activity and reduce bone loss. In a randomized controlled trial consisting of 60 postmenopausal women ranging from 40 to 60 years, probiotics also enhanced the metabolic activity of isoflavones to alleviate urogenital problems such as dry vagina and bad sex experience after menopause for 16-week consecutive treatment. The effect was comparable to low-dose vaginal estrogen, the gold standard therapy for genitourinary syndrome of menopause (Ribeiro et al., 2018). Szulińska et al. (2018) reported in a 12-week randomized clinical trial that multispecies probiotic bacteria at 1 × 1010 colony forming units (CFU) concentration affected gut permeability and cardiometabolic parameters, such as lipopolysaccharide levels, insulin, glucose, uric acid, fat mass, subcutaneous fat and insulin-resistant index. Koradia et al. (2019) found that Lactobacilli and cranberry extract complex could prevent recurrent urinary tract infections in female patients before or around menopause for consecutive 26 weeks in a pilot clinical study.
Another type of microbiota-related substances are termed prebiotics. Prebiotics are fermented dietary fibers which contain certain modifications, both in the component and/or bioactivity in the intestinal microbiota providing beneficial effects on health status of the human body (Alenaizan et al., 2021). At present, prebiotics mainly include bifidogenic, non-digestible oligosaccharides (especially galactooligosaccharides, inulin and its hydrolytic product oligofructose). Plenty of nutritional prebiotics may facilitate the bioabsorption of calcium, such as caseinophosphopeptides (CPP), proteolytic outcome of casein that together construct soluble composites with calcium (Ilesanmi-Oyelere and Kruger, 2020). Besides, some complex organic acids, like malic or citric acid can add to the percentage of dissolvable composites, bioabsorbable calcium in the intestine. The potential mechanisms of prebiotic-dependent calcium metabolism include that the acid metabolic products, such as lactic, acetic and propionic acid, from bacteria fermented carbohydrates in the colon lowered the regional pH to elevate the luminal level of calcium ions and to increase passive bioabsorption of calcium. Further, electrical charges might be modified via Ca2+-H+ composites. Therefore, the prebiotics played a protective role in osteoporosis prevention and inhibition after female menopause (Smejkal et al., 2003; de Vrese, 2009). Wu et al. (2021) showed that chitosan and citrus pectin, two kinds of prebiotics, enhanced glucose tolerance, reduced dyslipidemia, and elevated serum arginine, propionate, leucine and butyrate levels. In addition, chitosan was more useful to modulate gut microbiota and consequently to affect the postmenopausal symptoms (Wu et al., 2021).
Some combination attempts of probiotics and prebiotics were also carried out to produce synbiotics (O’Connor et al., 2021). Timan et al. (2014) showed that Lactobacillus paracasei and inulin enhanced the bioactive availability of isoflavones that were beneficial for alleviating menopausal issues. Jeong et al. (2017) reported that using synbiotics composed of Lactobacillus fermentum and β-glucan from cauliflower mushroom as probiotics and prebiotics could reduce skin temperature of rodent tails and maintain 17β-estradiol level in the serum and uterine index. The synbiotics dependent mechanisms were further validated as they activated hepatic insulin signaling, followed by AMPK phosphorylation in estrogen-deficient rats (Jeong et al., 2017). These studies preliminarily reflected the beneficial roles of probiotics, prebiotics and synbiotics in regulating menopausal syndromes and their primary pathophysiological process. The promising findings will be vital for the investigation into POI pathogenesis and treatment by microbial nutrient strategy.
Nutrition is vital for female health throughout the lifespan, especially for post-menopause period. Primary nutrients, such as carbohydrates, fat and lipoprotein, protein and polypeptide, vitamins, and phytoestrogens, have displayed huge potential and enjoyable outcomes in the application for menopausal intervention of POI, both in animal and clinical studies. The dietary nutrients may delay the menopause by affecting various aspects, from improving ovarian viability and function directly to counteracting oxidative stress, inflammation, aging and systemic endocrinal disorders. A latest meta-analysis suggested an association between POI and an increased risk of type 2 diabetes mellitus (T2DM) (Anagnostis et al., 2019). There is increasing evidence indicating that the gut microbiota is included in the pathophysiological process of insulin resistance. Thus, it leads to the progression of T2DM via influencing the contents of some vital metabolites. Nevertheless, it remains to be further validated whether modification of gut microbiota in POI patients results in serum metabolites out of balance, which in turn predisposes to menopause manifestation and related health concerns. Moreover, the therapeutic effects were not consistent in all researches and even among different time points in a single research study. This indicates the individual difference is significant and further work should be emphasized on precision nutrition with individual nutrient needs for optimal female reproduction, like POI treatment. Still, microbial nutrition is rarely discussed in POI patients or animals, possibly due to the unclear pathogenesis of POI. Especially, the correlation between probiotics, prebiotics and synbiotics and POI remains poorly investigated. It should also be a major theme to work on in the future. From the present work, dietary and microbial nutrients are important and promising for regulating female reproductive disorder, like POI, and therefore, optimal lifestyle intervention by diet is promising and deserves more efforts and attention to provide better and healthier life for female individuals.
QH wrote the manuscript, reviewed the literature, and collected the information. YD and Z-JC revised the manuscript. All authors approved the final manuscript.
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81971343 and 82171623), National Key Research and Development Program of China (No. 2021YFC2701104), Innovative Research Team of High-Level Local Universities in Shanghai (No. SHSMUZLCX20210200), and Shanghai Commission of Science and Technology (Nos. 21XD1401900 and 20DZ2270900).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abraham, A., Kattoor, A. J., Saldeen, T., and Mehta, J. L. (2019). Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 59, 2831–2838. doi: 10.1080/10408398.2018.1474169
Aflatoonian, A., Arabjahvani, F., Eftekhar, M., and Sayadi, M. (2014). Effect of vitamin D insufficiency treatment on fertility outcomes in frozen-thawed embryo transfer cycles: a randomized clinical trial. Iran J. Reprod. Med. 12, 595–600.
Alenaizan, A., Borca, C. H., Karunakaran, S. C., Kendall, A. K., Stubbs, G., Schuster, G. B., et al. (2021). X-ray fiber diffraction and computational analyses of stacked hexads in supramolecular polymers: insight into self-assembly in water by prospective prebiotic nucleobases. J. Am. Chem. Soc. 143, 6079–6094. doi: 10.1021/jacs.0c12010
Anagnostis, P., Christou, K., Artzouchaltzi, A. M., Gkekas, N. K., Kosmidou, N., Siolos, P., et al. (2019). Early menopause and premature ovarian insufficiency are associated with increased risk of type 2 diabetes: a systematic review and meta-analysis. Eur. J. Endocrinol. 180, 41–50. doi: 10.1530/EJE-18-0602
Anahtar, M. N., Gootenberg, D. B., Mitchell, C. M., and Kwon, D. S. (2018). Cervicovaginal microbiota and reproductive health: the virtue of simplicity. Cell Host Microbe 23, 159–168. doi: 10.1016/j.chom.2018.01.013
Asadi, N., Izadi, M., Aflatounian, A., Esmaeili-Dehaj, M., Rezvani, M. E., and Hafizi, Z. (2021). Chronic niacin administration ameliorates ovulation, histological changes in the ovary and adiponectin concentrations in a rat model of polycystic ovary syndrome. Reprod. Fertil. Dev. 33, 447–454. doi: 10.1071/RD20306
Bandyopadhyay, S., Chakrabarti, J., Banerjee, S., Pal, A. K., Goswami, S. K., Chakravarty, B. N., et al. (2003). Galactose toxicity in the rat as a model for premature ovarian failure: an experimental approach readdressed. Hum. Reprod. 18, 2031–2038. doi: 10.1093/humrep/deg414
Berg, G., Mesch, V., Boero, L., Sayegh, F., Prada, M., Royer, M., et al. (2004). Lipid and lipoprotein profile in menopausal transition. effects of hormones, age and fat distribution. Horm. Metab. Res. 36, 215–220. doi: 10.1055/s-2004-814450
Bischoff-Ferrari, H. A., Willett, W. C., Wong, J. B., Giovannucci, E., Dietrich, T., and Dawson-Hughes, B. (2005). Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293, 2257–2264. doi: 10.1001/jama.293.18.2257
Britton, R. A., Irwin, R., Quach, D., Schaefer, L., Zhang, J., Lee, T., et al. (2014). Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell Physiol. 229, 1822–1830. doi: 10.1002/jcp.24636
Chavarro, J. E., and Schlaff, W. D. (2018). Introduction: impact of nutrition on reproduction: an overview. Fertil. Steril. 110, 557–559. doi: 10.1016/j.fertnstert.2018.07.023
Chen, H., Cheng, R., and Xu, L. Z. (2017). Correlation between dietary nutrition and premature ovarian failure. Sichuan Da Xue Xue Bao Yi Xue Ban 48, 575–578.
Chiu, Y. H., Chavarro, J. E., and Souter, I. (2018). Diet and female fertility: doctor, what should I eat? Fertil. Steril. 110, 560–569. doi: 10.1016/j.fertnstert.2018.05.027
Critchley, H. O. D., Babayev, E., Bulun, S. E., Clark, S., Garcia-Grau, I., Gregersen, P. K., et al. (2020). Menstruation: science and society. Am. J. Obstet. Gynecol. 223, 624–664. doi: 10.1016/j.ajog.2020.06.004
Cueto, H. T., Riis, A. H., Hatch, E. E., Wise, L. A., Rothman, K. J., Sørensen, H. T., et al. (2016). Folic acid supplementation and fecundability: a Danish prospective cohort study. Eur. J. Clin. Nutr. 70, 66–71. doi: 10.1038/ejcn.2015.94
Das, P., and Chowdhury, M. (1999). Vitamin E-deficiency induced changes in ovary and uterus. Mol. Cell Biochem. 198, 151–156. doi: 10.1023/a:1006954032164
de Kleijn, M. J., van der Schouw, Y. T., Wilson, P. W., Grobbee, D. E., and Jacques, P. F. (2002). Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S.women: the Framingham study. J. Nutr. 132, 276–282. doi: 10.1093/jn/132.2.276
de Vrese, M. (2009). Health benefits of probiotics and prebiotics in women. Menopause Int. 15, 35–40. doi: 10.1258/mi.2009.009008
Di-Battista, A., Moysés-Oliveira, M., and Melaragno, M. I. (2020). Genetics of premature ovarian insufficiency and the association with X-autosome translocations. Reproduction 160, R55–R64. doi: 10.1530/REP-20-0338
DiTroia, S. P., Percharde, M., Guerquin, M. J., Wall, E., Collignon, E., Ebata, K. T., et al. (2019). Maternal vitamin C regulates reprogramming of DNA methylation and germline development. Nature 573, 271–275. doi: 10.1038/s41586-019-1536-1
Dorjgochoo, T., Kallianpur, A., Gao, Y. T., Cai, H., Yang, G., Li, H., et al. (2008). Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women’s Health Study. Menopause 15, 924–933. doi: 10.1097/gme.0b013e3181786adc
Ersoy, E., Ersoy, A. O., Yildirim, G., Buyukkagnici, U., Tokmak, A., and Yilmaz, N. (2016). Vitamin D levels in patients with premature ovarian failure. Ginekol. Pol. 87, 32–36. doi: 10.17772/gp/57839
European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber, L., Davies, M., Anderson, R., Bartlett, J., Braat, D., et al. (2016). ESHRE guideline: management of women with premature ovarian insufficiency. Hum. Reprod. 31, 926–937. doi: 10.1093/humrep/dew027
Fontana, R., and Della Torre, S. (2016). The deep correlation between energy metabolism and reproduction: a view on the effects of nutrition for women fertility. Nutrients 8:87. doi: 10.3390/nu8020087
Ford, E. A., Beckett, E. L., Roman, S. D., McLaughlin, E. A., and Sutherland, J. M. (2020). Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction 159, R15–R29. doi: 10.1530/REP-19-0201
Gold, E. B., Crawford, S. L., Avis, N. E., Crandall, C. J., Matthews, K. A., Waetjen, L. E., et al. (2013). Factors related to age at natural menopause: longitudinal analyses from SWAN. Am. J. Epidemiol. 178, 70–83. doi: 10.1093/aje/kws421
Golezar, S., Keshavarz, Z., Ramezani Tehrani, F., and Ebadi, A. (2020). An exploration of factors affecting the quality of life of women with primary ovarian insufficiency: a qualitative study. BMC Womens Health 20:163. doi: 10.1186/s12905-020-01029-y
Goyco Ortiz, L. E., Servy, E. J., and Menezo, Y. J. R. (2019). A successful treatment with 5 methyltetrahydrofolate of a 677 TT MTHFR woman suffering premature ovarian insufficiency post a NHL (non-Hodgkin’s lymphoma) and RPL (repeat pregnancy losses). J. Assist. Reprod. Genet. 36, 65–67. doi: 10.1007/s10815-018-1332-0
Gulhan, I., Bozkaya, G., Uyar, I., Oztekin, D., Pamuk, B. O., and Dogan, E. (2012). Serum lipid levels in women with premature ovarian failure. Menopause 19, 1231–1234. doi: 10.1097/gme.0b013e318254102b
Han, Q., Wang, J., Li, W., Chen, Z. J., and Du, Y. (2021). Androgen-induced gut dysbiosis disrupts glucolipid metabolism and endocrinal functions in polycystic ovary syndrome. Microbiome 9:101. doi: 10.1186/s40168-021-01046-5
Hoeke, G., Kooijman, S., Boon, M. R., Rensen, P. C., and Berbée, J. F. (2016). Role of brown fat in lipoprotein metabolism and atherosclerosis. Circ. Res. 118, 173–182.
Horton, J., Sterrenburg, M., Lane, S., Maheshwari, A., Li, T. C., and Cheong, Y. (2019). Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: a systematic review and meta-analysis. Hum. Reprod. Update 25, 592–632. doi: 10.1093/humupd/dmz012
Hou, S., Ding, C., Shen, H., Qian, C., Zou, Q., Lu, J., et al. (2020). Vitamin C improves the therapeutic potential of human amniotic epithelial cells in premature ovarian insufficiency disease. Stem Cell Res. Ther. 11:159. doi: 10.1186/s13287-020-01666-y
Hsieh, Y. T., and Ho, J. Y. P. (2021). Thyroid autoimmunity is associated with higher risk of premature ovarian insufficiency-a nationwide Health Insurance Research Database study. Hum. Reprod. 36, 1621–1629. doi: 10.1093/humrep/deab025
Ilesanmi-Oyelere, B. L., and Kruger, M. C. (2020). The role of milk components, Pro-, Pre-, and synbiotic foods in calcium absorption and bone health maintenance. Front. Nutr. 7:578702. doi: 10.3389/fnut.2020.578702
Jeong, S. Y., Kang, S., Hua, C. S., Ting, Z., and Park, S. (2017). Synbiotic effects of β-glucans from cauliflower mushroom and Lactobacillus fermentum on metabolic changes and gut microbiome in estrogen-deficient rats. Genes Nutr. 12:31. doi: 10.1186/s12263-017-0585-z
Kaur, A., Jindal, S., Kaur, I. P., and Chopra, K. (2013). Effect of sesamol on the pathophysiological changes induced by surgical menopause in rodents. Climacteric 16, 426–437. doi: 10.3109/13697137.2012.696292
Kebapcilar, A. G., Kulaksizoglu, M., Ipekci, S. H., Korkmaz, H., Kebapcilar, L., Akyurek, F., et al. (2013). Relationship between mean platelet volume and low-grade systemic coagulation with vitamin D deficiency in primary ovarian insufficiency. Arch. Gynecol. Obstet. 288, 207–212. doi: 10.1007/s00404-013-2735-x
Kikkeri, R., Kamena, F., Gupta, T., Hossain, L. H., Boonyarattanakalin, S., Gorodyska, G., et al. (2010). Ru(II) glycodendrimers as probes to study lectin-carbohydrate interactions and electrochemically measure monosaccharide and oligosaccharide concentrations. Langmuir 26, 1520–1523. doi: 10.1021/la9038792
Kinugasa, C., Naemura, A., Hyodo, K., Nakai, Y., Katsuta, M., and Yamamoto, J. (2011). Experimental antithrombotic effects of sesame seed whole grains and extracts. Blood Coagul. Fibrinolysis 22, 526–531. doi: 10.1097/MBC.0b013e328347b085
Knauff, E. A., Westerveld, H. E., Goverde, A. J., Eijkemans, M. J., Valkenburg, O., van Santbrink, E. J., et al. (2008). Lipid profile of women with premature ovarian failure. Menopause 15, 919–923. doi: 10.1097/gme.0b013e31816b4509
Koradia, P., Kapadia, S., Trivedi, Y., Chanchu, G., and Harper, A. (2019). Probiotic and cranberry supplementation for preventing recurrent uncomplicated urinary tract infections in premenopausal women: a controlled pilot study. Expert Rev. Anti. Infect. Ther. 17, 733–740. doi: 10.1080/14787210.2019.1664287
Kreijkamp-Kaspers, S., Kok, L., Bots, M. L., Grobbee, D. E., and van der Schouw, Y. T. (2005). Dietary phytoestrogens and plasma lipids in Dutch postmenopausal women; a cross-sectional study. Atherosclerosis 178, 95–100. doi: 10.1016/j.atherosclerosis.2004.06.002
Kwa, M., Plottel, C. S., Blaser, M. J., and Adams, S. (2016). The intestinal microbiome and estrogen receptor-positive female Breast cancer. J. Natl. Cancer Inst. 108:djw029. doi: 10.1093/jnci/djw029
Li, Y., Qiu, W., Zhang, Z., Han, X., Bu, G., Meng, F., et al. (2020). Oral oyster polypeptides protect ovary against d-galactose-induced premature ovarian failure in C57BL/6 mice. J. Sci. Food Agric. 100, 92–101. doi: 10.1002/jsfa.9997
Ma, L., Chen, G., Xu, W., Chen, P., Lan, Y., Huang, Y., et al. (2021). The relationship between vitamin E level and premature ovarian insufficiency. J. Obstet. Gynaecol. Res. 47, 1481–1486. doi: 10.1111/jog.14659
Meléndez, G. C., Register, T. C., Appt, S. E., Clarkson, T. B., Franke, A. A., and Kaplan, J. R. (2015). Beneficial effects of soy supplementation on postmenopausal atherosclerosis are dependent on pretreatment stage of plaque progression. Menopause 22, 289–296. doi: 10.1097/GME.0000000000000307
Milani, C., Duranti, S., Bottacini, F., Casey, E., Turroni, F., Mahony, J., et al. (2017). The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 81:e00036-17. doi: 10.1128/MMBR.00036-17
O’Connor, L. E., Gahche, J. J., Herrick, K. A., Davis, C. D., Potischman, N., and Vargas, A. J. (2021). Nonfood prebiotic, probiotic, and synbiotic use has increased in US Adults and Children From 1999 to 2018. Gastroenterology 161, 476–486.e3. doi: 10.1053/j.gastro.2021.04.037
Peh, H. Y., Tan, W. S., Liao, W., and Wong, W. S. (2016). Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol. Ther. 162, 152–169. doi: 10.1016/j.pharmthera.2015.12.003
Qin, Y., Jiao, X., Simpson, J. L., and Chen, Z. J. (2015). Genetics of primary ovarian insufficiency: new developments and opportunities. Hum. Reprod. Update 21, 787–808. doi: 10.1093/humupd/dmv036
Ribeiro, A. E., Monteiro, N. E. S., Moraes, A. V. G., Costa-Paiva, L. H., and Pedro, A. O. (2018). Can the use of probiotics in association with isoflavone improve the symptoms of genitourinary syndrome of menopause? results from a randomized controlled trial. Menopause 26, 643–652. doi: 10.1097/GME.0000000000001279
Rostami Dovom, M., Noroozzadeh, M., Mosaffa, N., Zadeh-Vakili, A., Piryaei, A., and Ramezani Tehrani, F. (2019). Induced premature ovarian insufficiency by using D galactose and its effects on reproductive profiles in small laboratory animals: a systematic review. J. Ovarian Res. 12:96. doi: 10.1186/s13048-019-0565-6
Rudick, B. J., Ingles, S. A., Chung, K., Stanczyk, F. Z., Paulson, R. J., and Bendikson, K. A. (2014). Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil. Steril. 101, 447–452. doi: 10.1016/j.fertnstert.2013.10.008
Safiyeh, F. D., Mojgan, M., Parviz, S., Sakineh, M. A., and Behnaz, S. O. (2021). The effect of selenium and vitamin E supplementation on anti-Mullerian hormone and antral follicle count in infertile women with occult premature ovarian insufficiency: a randomized controlled clinical trial. Complement Ther. Med. 56:102533. doi: 10.1016/j.ctim.2020.102533
Samad, N., Nguyen, H. H., Ebeling, P. R., and Milat, F. (2020). Musculoskeletal health in premature ovarian insufficiency. Part Two: Bone. Semin. Reprod. Med. 38, 289–301. doi: 10.1055/s-0041-1722849
Sapre, S., and Thakur, R. (2014). Lifestyle and dietary factors determine age at natural menopause. J. Midlife Health 5, 3–5. doi: 10.4103/0976-7800.127779
Scholz-Ahrens, K. E., and Schrezenmeir, J. (2007). Inulin and oligofructose and mineral metabolism: the evidence from animal trials. J. Nutr. 137, 2513S–2523S. doi: 10.1093/jn/137.11.2513S
Seyed Hameed, A. S., Rawat, P. S., Meng, X., and Liu, W. (2020). Biotransformation of dietary phytoestrogens by gut microbes: a review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol. Adv. 43:107576. doi: 10.1016/j.biotechadv.2020.107576
Silvestris, E., Lovero, D., and Palmirotta, R. (2019). Nutrition and female fertility: an interdependent correlation. Front. Endocrinol. 10:346. doi: 10.3389/fendo.2019.00346
Simpson, S., Seifer, D. B., Shabanova, V., Lynn, A. Y., Howe, C., Rowe, E., et al. (2020). The association between anti-Müllerian hormone and vitamin 25(OH)D serum levels and polycystic ovarian syndrome in adolescent females. Reprod. Biol. Endocrinol. 18:118. doi: 10.1186/s12958-020-00676-y
Smejkal, C., Kolida, S., Bingham, M., Gibson, G., and McCartney, A. (2003). Probiotics and prebiotics in female health. J. Br. Menopause Soc. 9, 69–74. doi: 10.1258/136218003100322224
Szegda, K. L., Whitcomb, B. W., Purdue-Smithe, A. C., Boutot, M. E., Manson, J. E., Hankinson, S. E., et al. (2017). Adult adiposity and risk of early menopause. Hum. Reprod. 32, 2522–2531. doi: 10.1093/humrep/dex304
Szulińska, M., Łoniewski, I., van Hemert, S., Sobieska, M., and Bogdański, P. (2018). Dose-Dependent effects of multispecies probiotic supplementation on the lipopolysaccharide (LPS) level and cardiometabolic profile in obese postmenopausal women: a 12-Week randomized clinical trial. Nutrients 10:773. doi: 10.3390/nu10060773
Szymański, W., and Kazdepka-Ziemińska, A. (2003). Effect of homocysteine concentration in follicular fluid on a degree of oocyte maturity. Ginekol. Pol. 74, 1392–1396.
Taneja, C., Gera, S., Kim, S. M., Iqbal, J., Yuen, T., and Zaidi, M. (2019). FSH-metabolic circuitry and menopause. J. Mol. Endocrinol. 63, R73–R80. doi: 10.1530/JME-19-0152
Tao, X., Jiang, A., Yin, L., Li, Y., Tao, F., and Hu, H. (2015). Body mass index and age at natural menopause: a meta-analysis. Menopause 22, 469–474. doi: 10.1097/GME.0000000000000324
Timan, P., Rojanasthien, N., Manorot, M., Sangdee, C., and Teekachunhatean, S. (2014). Effect of synbiotic fermented milk on oral bioavailability of isoflavones in postmenopausal women. Int. J. Food. Sci. Nutr. 65, 761–767. doi: 10.3109/09637486.2014.908169
Wang, J., Xu, J., Han, Q., Chu, W., Lu, G., Chan, W. Y., et al. (2020). Changes in the vaginal microbiota associated with primary ovarian failure. BMC Microbiol. 20:230. doi: 10.1186/s12866-020-01918-0
Wang, L., Tang, J., Wang, L., Tan, F., Song, H., Zhou, J., et al. (2021). Oxidative stress in oocyte aging and female reproduction. J. Cell Physiol. 236, 7966–7983. doi: 10.1002/jcp.30468
Wang, M., Gong, W. W., Hu, R. Y., Wang, H., Guo, Y., Bian, Z., et al. (2018). Age at natural menopause and associated factors in adult women: findings from the China Kadoorie Biobank study in Zhejiang rural area. PLoS One 13:e0195658. doi: 10.1371/journal.pone.0195658
Weaver, C. M., Martin, B. R., Nakatsu, C. H., Armstrong, A. P., Clavijo, A., McCabe, L. D., et al. (2011). Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J. Agric Food Chem. 59, 6501–6510. doi: 10.1021/jf2009777
Wei, J., Chen, J. R., Pais, E. M. A., Wang, T. Y., Miao, L., Li, L., et al. (2017). Oxyresveratrol is a phytoestrogen exerting anti-inflammatory effects through NF-κB and estrogen receptor signaling. Inflammation 40, 1285–1296. doi: 10.1007/s10753-017-0572-y
Westphal, L. M., Polan, M. L., and Trant, A. S. (2006). Double-blind, placebo-controlled study of fertilityblend: a nutritional supplement for improving fertility in women. Clin. Exp. Obstet Gynecol. 33, 205–208.
Wu, X., Kim, M. J., Yang, H. J., and Park, S. (2021). Chitosan alleviated menopausal symptoms and modulated the gut microbiota in estrogen-deficient rats. Eur. J. Nutr. 60, 1907–1919. doi: 10.1007/s00394-020-02382-2
Wu, Y. Y., Liang, C. Y., Liu, T. T., Liang, Y. M., Li, S. J., Lu, Y. Y., et al. (2018). Protective roles and mechanisms of polysaccharides from dendrobium officinal on natural aging-induced premature ovarian failure. Biomed. Pharmacother. 101, 953–960. doi: 10.1016/j.biopha.2018.03.030
Xu, J., Hennebold, J. D., and Seifer, D. B. (2016). Direct vitamin D3 actions on rhesus macaque follicles in three-dimensional culture: assessment of follicle survival, growth, steroid, and antimüllerian hormone production. Fertil. Steril. 106, 1815–1820.e1. doi: 10.1016/j.fertnstert.2016.08.037
Yang, L. C., Wu, J. B., Lu, T. J., and Lin, W. C. (2013). The prebiotic effect of Anoectochilus formosanus and its consequences on bone health. Br. J. Nutr. 109, 1779–1788. doi: 10.1017/S0007114512003777
Ye, L., Cao, Z., Lai, X., Wang, W., Guo, Z., Yan, L., et al. (2019). Niacin fine-tunes energy homeostasis through canonical GPR109A signaling. FASEB J. 33, 4765–4779. doi: 10.1096/fj.201801951R
Keywords: premature ovarian insufficiency, female fitness, nutrition, diet, microbiota, menopause
Citation: Han Q, Chen Z-J and Du Y (2022) Dietary supplementation for female infertility: Recent advances in the nutritional therapy for premature ovarian insufficiency. Front. Microbiol. 13:1001209. doi: 10.3389/fmicb.2022.1001209
Received: 23 July 2022; Accepted: 01 November 2022;
Published: 17 November 2022.
Edited by:
Lin Shi, Shaanxi Normal University, ChinaReviewed by:
Shimeng Huang, Jiangsu Academy of Agricultural Sciences, ChinaCopyright © 2022 Han, Chen and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanzhi Du, ZHV5ekBzanR1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.