
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 25 February 2022
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.819993
This article is part of the Research Topic The Development and Utilization of Novel Antibiotic Alternatives View all 15 articles
The aim of the present study is to investigate the effects of dietary Lycium barbarum polysaccharides (LBPs) supplementation on the growth performance, immune response, serum antioxidant status, and intestinal health of weaned piglets. In total, 24 crossed healthy weaned piglets [Duroc × (Yorkshire × Landrace)], of similar body weight (7.47 ± 0.22 kg), were randomly allocated to three treatment groups: CON (basal diet); LBPs (basal diet plus 4,000 mg/kg LBPs); and antibiotic (ABO, basal diet plus 20 mg/kg flavomycin and 50 mg/kg quinocetone). There were eight pigs per group. The study lasted 28 days. When compared with CON, LBPs or ABO dietary supplementation increased average daily gain (P < 0.05), decreased the ratio of feed to gain and the diarrhea ratio (P < 0.05). Similarly, when compared with CON, LBPs dietary supplementation increased serum immunoglobulin G, immunoglobulin M, interleukin-10, interleukin-2, and tumor necrosis factor-α levels (P < 0.05). Dietary LBPs enhanced the activity of serum total antioxidant capacity and glutathione peroxidase, and decreased malondialdehyde levels (P < 0.05). Principal component analysis showed a distinct separation between CON and LBPs groups, but no differences between ABO and LBPs groups. LBPs addition increased Lactobacillus and Faecalibacterium (P < 0.05) levels, while it decreased Enterococcaceae and Enterobacteriaceae (P < 0.05) levels. Furthermore, when compared with the CON group, LBPs increased villus height (P < 0.05) and the villus height to crypt depth ratio in the duodenum and jejunum (P < 0.05). Thus, dietary supplementation with LBPs improved growth performance, antioxidant capacity and immunity, regulated intestinal microbial composition, and may be used as an efficient antibiotic alternative in weaned piglet feed.
Early weaning increases intestinal permeability and reduces antioxidant capacity and immunity, which reduces feed intake, and increases diarrhea incidence, morbidity, and mortality (Hu et al., 2013; Yin et al., 2014). Diarrhea after weaning is mainly associated with gut microbiome disturbances which may lead to fever and slow growth (Campbell et al., 2013). Antibiotics are widely used in animal feeds to regulate intestinal microorganisms, prevent infection, and improve growth performance (Cook, 2004; Wang W. et al., 2018). However, antibiotics over-dependence has facilitated the emergence of antimicrobial resistance and antimicrobial residues, which affect human health (Li, 2017). In the European Union, antibiotics in feed additives were banned in 2006, whereas, in China, their use ceased in July 2020, therefore, a healthy and pollution-free alternative to antibiotics is required.
Many plant extracts can be used as alternatives to antibiotics (Lu et al., 2010; Pourhossein et al., 2015). Lycium barbarum, as a food and medicine, has been used in Asian countries for thousands of years to induce various health benefits (Donno et al., 2015; Zhao J. et al., 2016). L. barbarum polysaccharides (LBPs) are major bioactive components of L. barbarum and possess distinct bioactivities, including anti-oxidant (Wang et al., 2020; Zhang et al., 2021), anti-tumor (Gong et al., 2020), anti-diabetic (Shimato et al., 2020), immunomodulatory (Feng et al., 2020; Kim et al., 2020), liver protective (Jia et al., 2016), neuroprotective (Zhao Z. et al., 2016), renal protective (Wu et al., 2020), and improved eyesight activities (Zhu et al., 2016). Liu et al. (2021a) demonstrated that variations in the molecular weight of LBPs exerted antioxidant effects on different free radical. Yang et al. (2013) indicated that LBPs treatment may protect intestinal damage by inhibiting oxidative stress and inflammation in rats. Long et al. (2020) reported that dietary supplementation of LBPs could improve the growth performance, immune function, antioxidant capacity, and digestive enzyme activities in broilers. Our previous studies demonstrated that 4,000 mg/kg LBPs dietary supplementation enhanced growth performance, immune status and antioxidant capacity, and improved intestinal microbial populations in weaned piglets (Chen et al., 2020). Based on these favorable effects, we hypothesized that dietary LBPs supplementation could effectively replace antibiotics by improving performance, gastrointestinal tract health, and function in weaned piglets. Therefore, the objective of the current study was to investigate the effects of a 4,000 mg/kg LBPs supplementation on growth performance, diarrhea incidence, serum immunity and antioxidant capacity, intestinal morphology, short-chain fatty acids (SCFAs) levels, and cecum intestinal microflora in weaned pigs.
Experiments were conducted in accordance with Chinese guidelines for animal welfare and experimental protocols. All animal procedures were approved by the Committee of Animal Care at Hunan Agricultural University (Changsha, China) (permit number: CACAHU 2020-00156).
We included 24 crossed healthy weaned piglets [Duroc × (Yorkshire × Landrace)] of similar body weight (BW = 7.47 ± 0.22 kg). Animals were randomly allocated to three treatment groups: CON (basal diet); LBPs (basal diet plus 4,000 mg/kg LBPs); and antibiotic (ABO, basal diet plus 20 mg/kg flavomycin & 50 mg/kg quinocetone). There were eight pigs per group. The basal diet was formulated to satisfy or outstrip National Research Council (National Research Council, 2012) nutrient requirements. Basal diet nutrient levels and ingredients are shown (Table 1).
All pigs were housed in a room with slatted floors. They were fed in individual metabolism cages with a side feeder and a stainless-steel nipple which provided full access to feed and water, respectively. The scale of feeding and feed surplus for each piglet was recorded throughout the study. At study beginning and end, body weights were measured; these data were used to calculate the average daily gain (ADG), average daily feed intake (ADFI), and ratio of feed to gain (F/G). The study lasted for 28 days and diarrhea ratio was monitored daily. Diarrhea ratio (%) was calculated as the number of pigs with diarrhea × the number of days with diarrhea/(the total number of pigs × the number of study days) (Hung et al., 2019).
On the 27th day, blood was collected by anterior vena cava puncture before morning feeding. Blood was centrifuged at 3,000 × g for 15 min at 4°C to isolate serum which was stored at –80°C. All piglets were humanely killed by injection of pentobarbital sodium at study end and the gut, liver, and kidney immediately removed from the abdominal cavity. The intestinal segment and mucosa from the duodenum, jejunum, and ileum were collected and stored at –80°C. An intestinal segment (comprising duodenum, jejunum, and ileum) was fixed in 4% paraformaldehyde-phosphate buffered saline buffer to analyze intestinal morphological structures. Chyme from the ileum, cecum, and colon was collected and stored at –80°C.
Serum immunoglobulins (Ig)A, IgM; IgG, the interleukins, (IL)-2, IL-6, IL-10, IL-1α, and IL-1β; and tumor necrosis factor-α (TNF-α) were measured by using pig-specific ELISA kits (Cusabio Biotechnology Co., Ltd., Wuhan, China).
The activity levels of total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) in serum were determined using respective reagent kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Sections of the duodenum, jejunum, and ileum in each pig were harvested and immediately fixed in 10% formalin, dehydrated in 50% ethanol, embedded paraffin, and sectioned 5 μm for hematoxylin and eosin staining. The sections were scanned using an optical binocular microscope connected to a digital camera (Nikon ECLIPSE 80i). Villus length, crypt depth, and the villus length vs. crypt depth (V/C) ratios were measured from 10 well-oriented villi × 3 sections of each pigs.
According to the manufacturer’s instructions, total genomic DNA was extracted from the chyme of cecum samples using the QIAamp Fast DNA stool mini kit (Qiagen, Hilden, Germany). DNA was checked on 1% agarose gels and concentration and purity were determined using a NanoDrop 2000 UV–vis spectrophotometer (Thermo Fisher Scientific, Wilmington, United States). The V3–V4 hypervariable region of the bacterial 16S rRNA gene was amplified using the following primers; 338 F (5′- ACTCCTACGGGAGGCAGCAG-3′) and 806 R (5′-GGACTACHVGGGTWTCTAAT-3′) on an ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, United States) (Xu et al., 2016). The PCR amplification system and conditions have been previously described (Yang J. et al., 2020). PCR products were extracted from 2% agarose gel and purified using the AxyPrep DNA gel extraction kit (Axygen Biosciences, Union City, CA, United States) according to manufacturer’s instructions and quantified using a Quantus™ Fluorometer (Promega, United States).
Purified amplicons were pooled in equimolar quantities and paired-end sequenced on an Illumina MiSeq PE300 platform/NovaSeq PE250 platform (Illumina, San Diego, CA, United States) according to standard protocols of Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). Raw reads were deposited into the National Center for Biotechnology Information Sequence Read Archive database (Accession Number: SRP342805).
Raw 16S rRNA gene sequencing reads were demultiplexed, quality-filtered by fastp version 0.20.0 (Chen et al., 2018), and merged by FLASH version 1.2.7 (Magoc and Salzberg, 2011) using the following criteria: (1) the 300 base pair (bp) reads were truncated at any site receiving an average quality score < 20 over a 50 bp sliding window. Truncated reads < 50 bp and reads containing ambiguous characters were also discarded; (2) only overlapping sequences longer than 10 bp were assembled according to their overlapped sequences. The maximum mismatch ratio of the overlap region was 0.2. Reads that could not be assembled were discarded; and (3) samples were distinguished according to the barcode and primers, and the sequence direction was adjusted, exact barcode matching, 2 nucleotide mismatch in primer matching.
Species diversity was evaluated using ACE and Chao richness estimators and Shannon and Simpson diversity indices (Lemieux-labonte et al., 2017). Operational taxonomic units (OTUs), with 97% similarity cutoff (Stackebrandt and Goebel, 1994; Edgar, 2013), were clustered using UPARSE version 7.1 (Edgar, 2013), with chimeric sequences identified and removed. Beta diversity was evaluated using Principal Component Analysis (PCA). Significant differences between samples were evaluated by analysis of similarities (ANOSIM).
We performed gas chromatography (GC) to determine the main SCFAs in intestinal chyme, as described previously (Franklin et al., 2002). Briefly, to isolate supernatants, digesta samples were weighed, vortexed in distilled water, and centrifuged at 12,000 × g for 15 min at 4°C. Supernatants were mixed with 25% metaphosphoric acid at a 9:1 volume ratio, statically reacted for 3–4 h, centrifuged, and filtered. A GC system (GC2014, Shimadzu Corporation, Kyoto, Japan) was used to measure filtered fluids.
Experimental data were analyzed by one-way ANOVA using the General Linear Model procedure of the SPSS software v. 20.0 (SPSS Inc., Chicago, IL, United States). Differences between treatment means were tested using Tukey’s multiple comparison test. Microbe abundance, with significant differences between groups, was assessed by the Kruskal–Wallis test. Results were presented as the mean ± standard error of the mean. P < 0.05 was considered statistically significant.
As shown in Table 2, when compared with the CON group, both LBPs and ABO dietary supplementation significantly increased ADG (P < 0.05) and decreased the F/G (P < 0.05). However, neither dietary LBPs or ABO supplementation had significant effects on initial weight, final weight, or ADFI in weaned piglets (P > 0.05).
As shown (Figure 1), when compared with the CON group, both LBPs and ABO dietary supplementation decreased diarrhea ratios in weaned piglets (P < 0.05), but no significant differences were observed between the LBPs and ABO groups (P > 0.05).
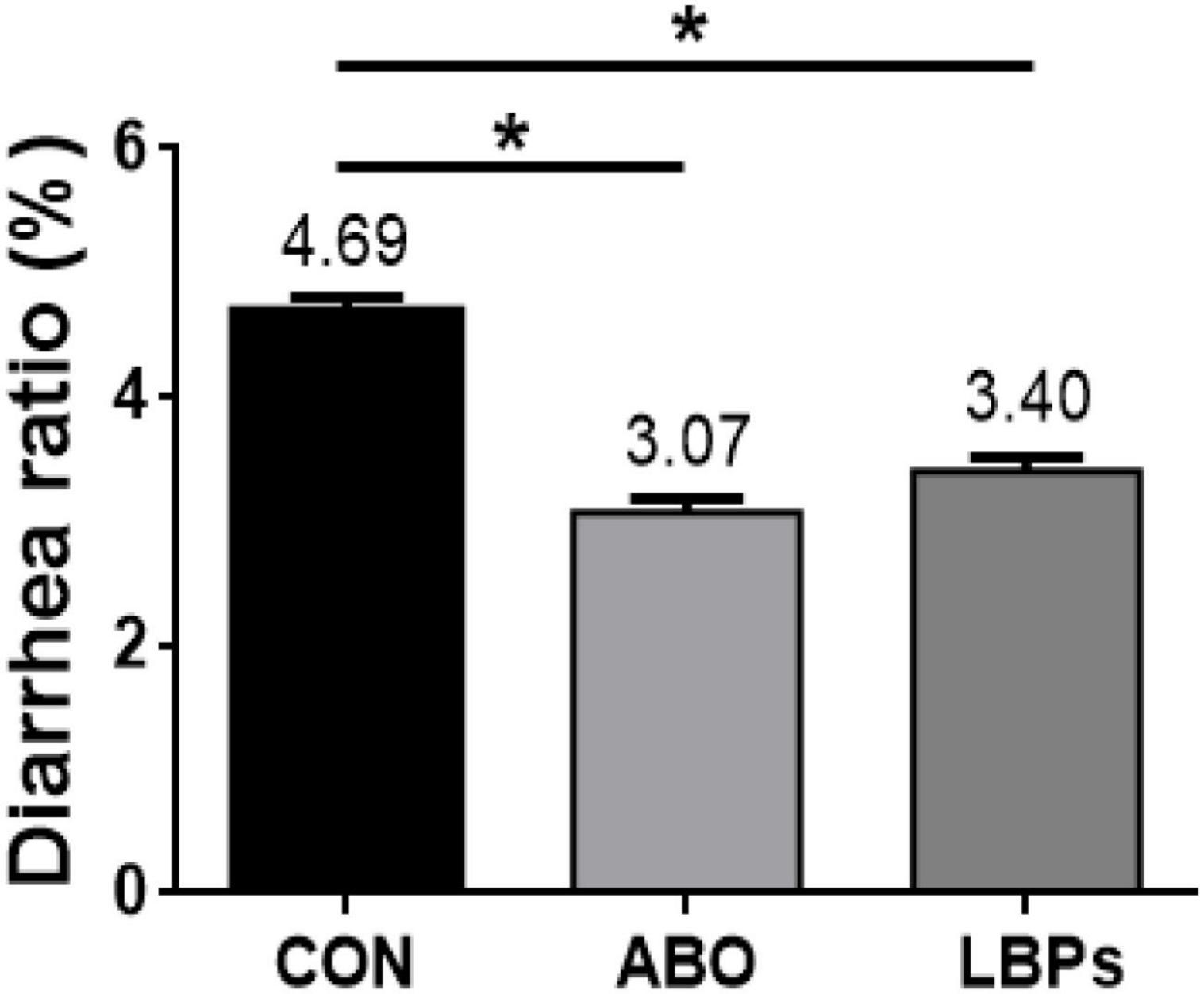
Figure 1. Diarrhea rate of weaned piglets fed MB dietary treatments (%) (n = 8). CON, basal diet; LBPs, basal diet + 4,000 mg/kg LBPs; ABO, basal diet + 20 mg/kg flavomycin + 50 mg/kg quinocetone. Asterisks express statistical differences between different groups: *P < 0.05.
As shown in Table 3, weaned piglets in the LBPs and ABO groups displayed higher IgG and IgM levels than the CON group (P < 0.05), but no significant differences were observed for IgA levels among the groups (P > 0.05). When compared with the CON group, LBPs dietary supplementation significantly increased serum IL-10, IL-2, and TNF-α (P < 0.05) levels, but no significant IL-6, IL-1α, and IL-1β differences were observed between the groups (P > 0.05) (Table 4).
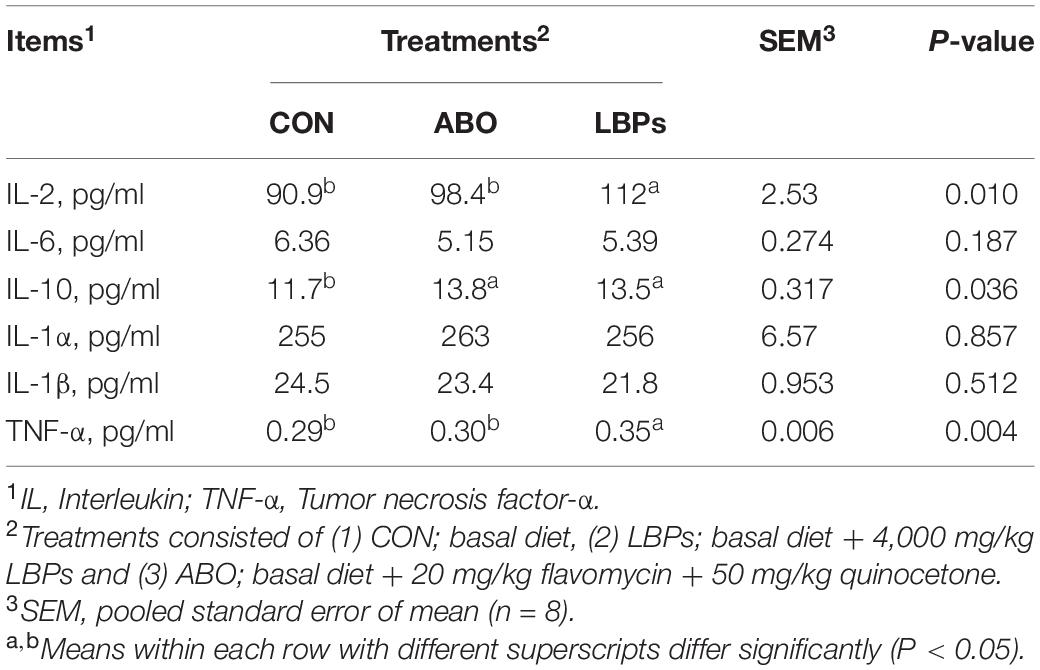
Table 4. Effects of dietary LBPs supplementation on immunologic factors levels in serum of weaned piglets.
Table 5 presents the differences in serum antioxidant indicators between the treatment groups. Dietary LBPs effectively enhanced serum T-AOC and GSH-Px activities but decreased MDA levels (P < 0.05). No significant differences in SOD activities were observed between the groups (P > 0.05).
The effects of LBPs dietary supplementation on intestinal morphology in piglets at day 28 are shown in Table 6. When compared with the CON group, LBPs increased villus height in the duodenum and ileum (P < 0.05). A distinct decrease in crypt depth in the duodenum of piglets fed ABO was observed when compared with the CON group (P < 0.05). In addition, both LBPs and ABO dietary supplementation increased the V/C in the duodenum and jejunum when compared with the CON group (P < 0.05).
In total, 1,216,334 high-quality sequences were obtained from samples. After clustering at the 97% similarity level, sequences were assigned to 905 OTUs. Firmicutes were the most abundant phylum across all samples, followed by Proteobacteria, Actinobacteriota, Bacteroidota, Spirochaetota, Desulfobacterota, and Patescibacteria (Figure 2). When compared with the CON group, the relative abundance of Firmicutes was significantly increased (P < 0.05) in the ABO and LBPs groups (Figure 3). Alpha diversity analyses indicated that LBPs increased Chao and ACE indices when compared with the CON group (P < 0.05), but no significant differences were observed for Shannon and Simpson indices among the groups (Supplementary Figure 1). PCA showed a distinct separation between the CON and LBPs groups, but no differences between the ABO and LBPs groups (Figure 4A). Hierarchical clustering tree analyses showed that CON microbial composition had mostly gathered in another branch (Figure 4B). From ANOSIM analyses, significant differences were identified in the microbial composition of the study groups; r = 0.2702, P < 0.01 in the CON, LBPs, and ABO groups; r = 0.2907, P < 0.05 for the ABO vs. CON groups; r = 0.4827, P < 0.01 for the LBPs vs. CON groups; and r = 0.0558, P = 0.185 for the ABO vs. LBPs groups). Additionally, Lactobacillus and Faecalibacterium were enriched (Figure 3) in the LBPs group at the genus level (P < 0.05), while Enterobacteriaceae (Figure 3), Enterococcaceae, and Escherichia-Shigella (Supplementary Figure 2) were enriched in the CON group (P < 0.05).
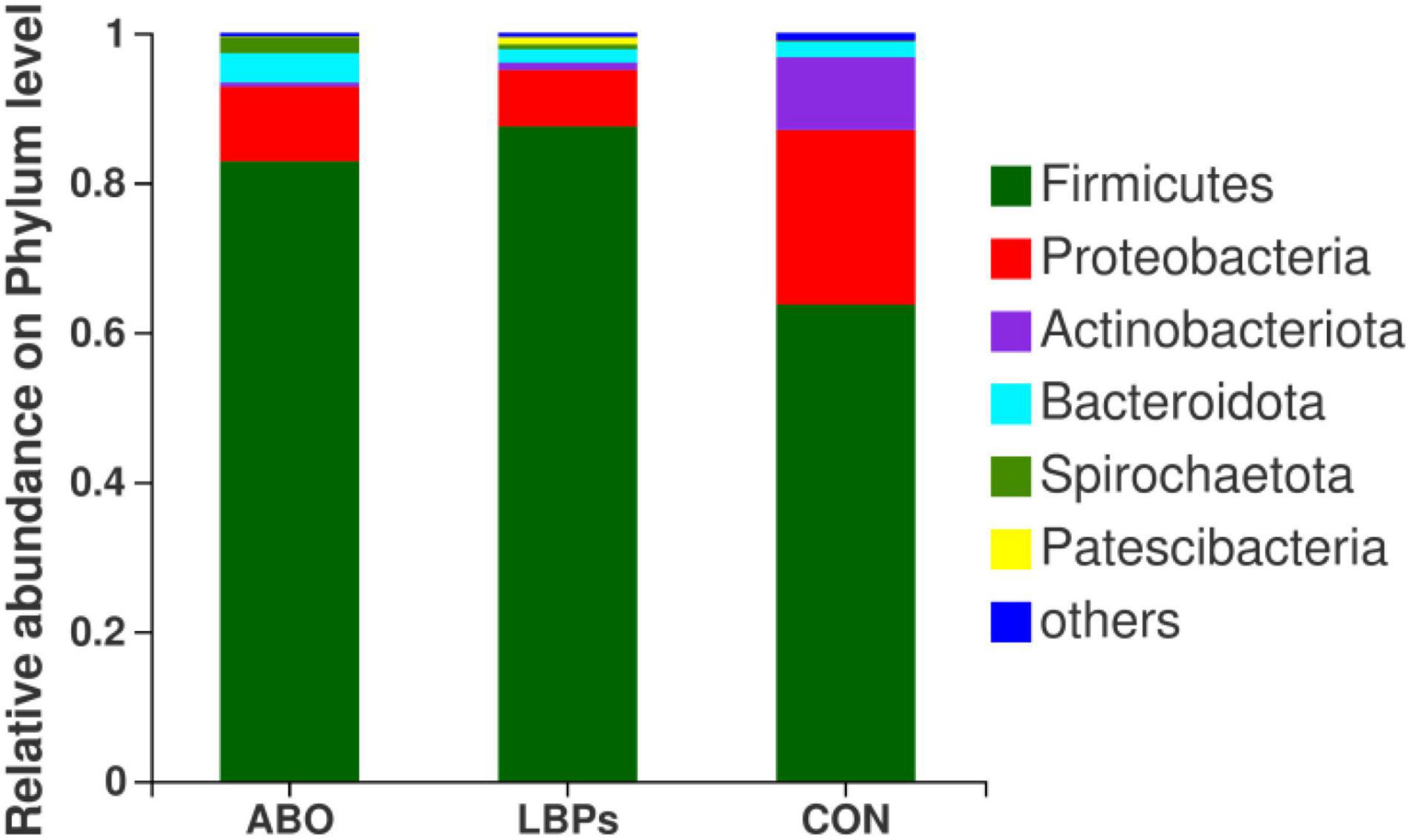
Figure 2. Phylum-level relative abundance of 16S rRNA gene sequences from the cecal digesta of weaned piglets (n = 8). CON, basal diet; LBPs, basal diet + 4,000 mg/kg LBPs; ABO, basal diet + 20 mg/kg flavomycin + 50 mg/kg quinocetone.
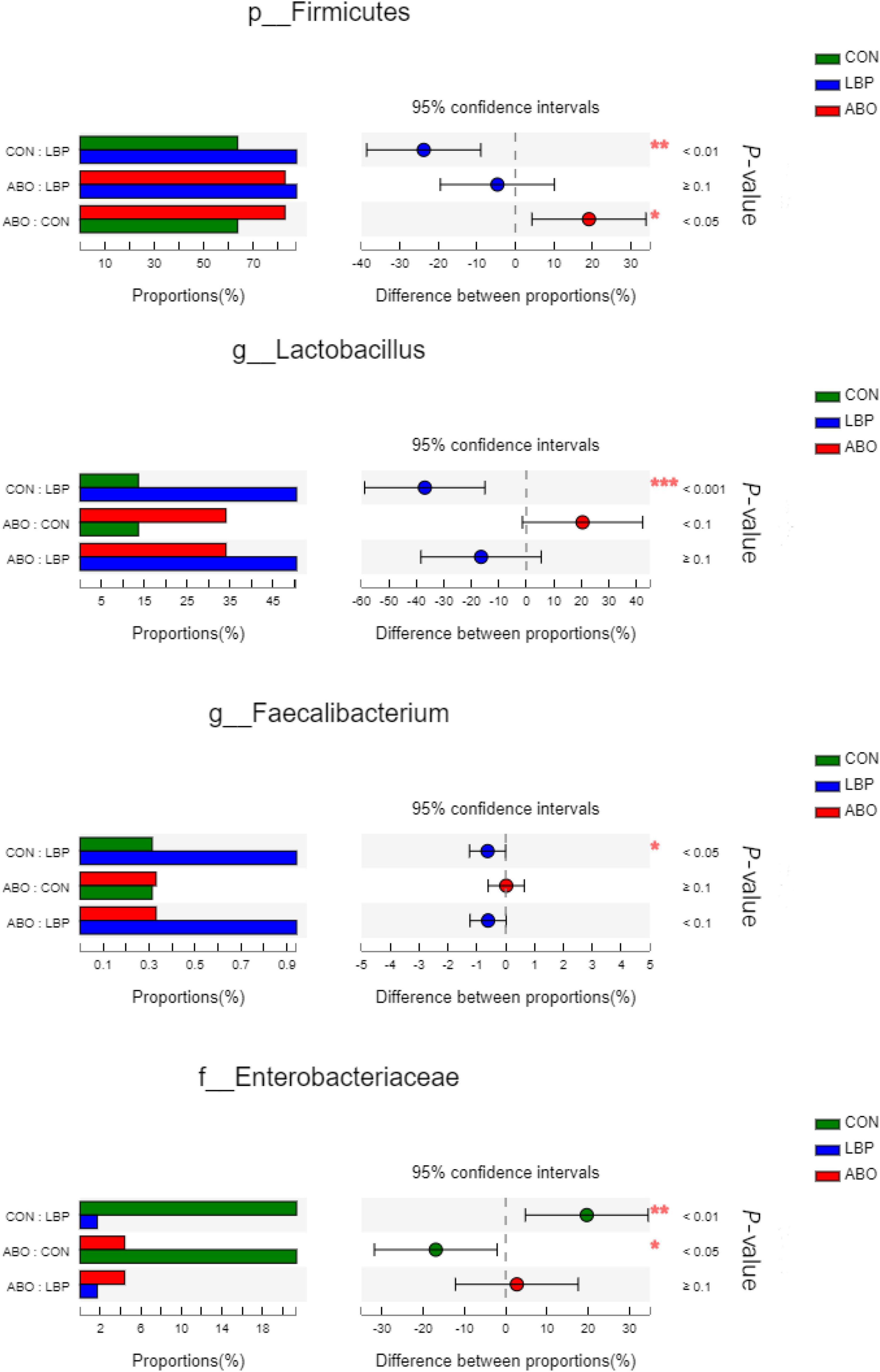
Figure 3. Comparative analysis of 3 most relative abundances of gut microbiota (n = 8). Kruskal–Wallis test followed by Tukey test was used to evaluate the statistical significance. Asterisks express statistical differences between different groups: *0.01 < P ≤ 0.05, **0.001 < P ≤ 0.01, ***P ≤ 0.001. CON, basal diet; LBP, basal diet + 4,000 mg/kg LBPs; ABO, basal diet + 20 mg/kg flavomycin + 50 mg/kg quinocetone.
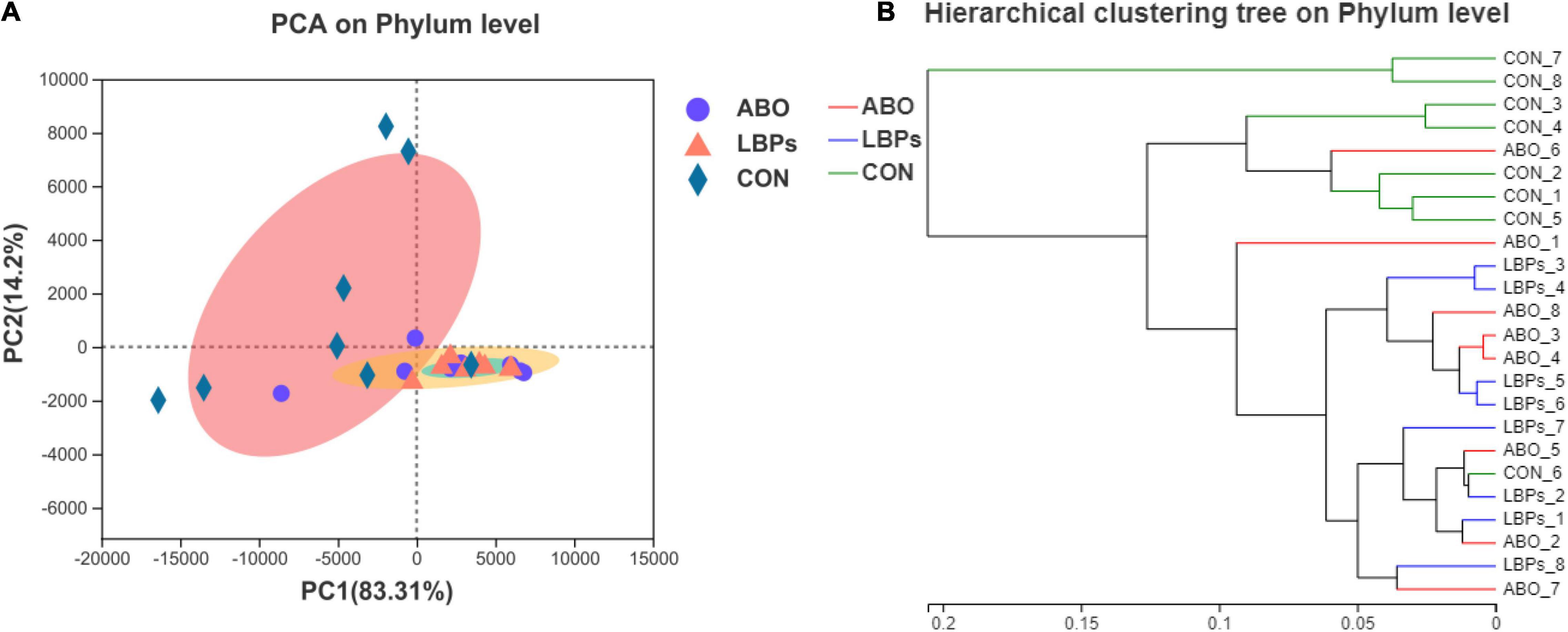
Figure 4. (A) Principal Component Analysis (PCA) of bacterial communities in the cecal digesta of weaned piglets (based on the Bray–Curtis distance) (n = 8). (B) Analysis of hierarchical clustering tree on Phylun level showed that the microbial composition of CON was almost entirely gathered in another branch. CON, basal diet; LBPs, basal diet + 4,000 mg/kg LBPs; ABO, basal diet + 20 mg/kg flavomycin + 50 mg/kg quinocetone.
Total SCFAs, as well as acetic, propionic, isobutyric, butyric, isopentoic, and valeric acid levels in the cecum, ileum, and colon are shown in Table 7. When compared with the CON group, dietary both LBPs and ABO supplementation increased acetic, propionic and butyric acid levels, and total SCFAs, in the cecum (P < 0.05). However, no significant differences were observed in total ileum SCFAs or each SCFAs across groups (P > 0.05). Piglets fed the LBPs diet showed increased isobutyric and isopentoic acid levels in the colon when compared with the other groups (P < 0.05).
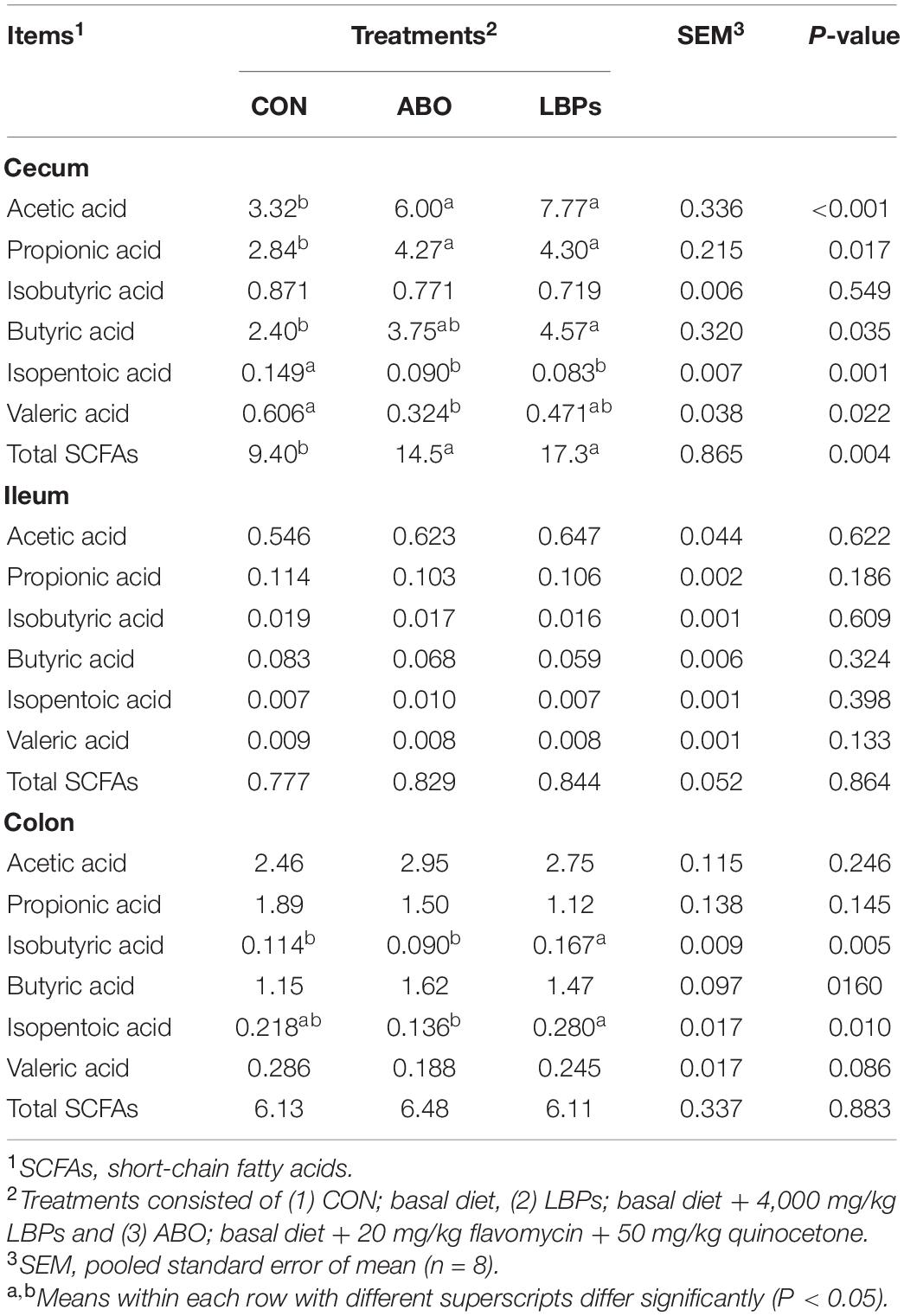
Table 7. Effects of dietary LBPs supplementation on short-chain fatty acids in intestinal contents of weaned piglets (μg/kg).
Weaning stress causes intestinal and immune system dysfunction and reduces pig growth and health (Campbell et al., 2013). Numerous studies have reported that plant-derived polysaccharides (e.g., Achyranthes bidentata and Ganoderma lucidum polysaccharides) improve immune responses, maintain intestinal structure integrity, balance intestinal microbiota, and reduce diarrhea, which promote pig growth (Li et al., 2015; Hou et al., 2021). In this study, dietary supplementation with either LBPs or ABO increased ADG and decreased the F/G, which may have been attributed to immune response stimulation by LBPs (Zhu et al., 2020). Tan et al. (2019) reported that LBPs, when added to hybrid grouper (Epinephelus lanceolatus ♂ × E. fuscoguttatus ♀) diets, inhibited hepatic inflammatory responses, increased antioxidant enzyme activity, and improved growth performance and feed efficiency. The intestine has crucial roles in nutrient absorption and defenses against external pathogens (Halas et al., 2010). Wang et al. (2019) reported that dietary LBPs improved intestinal morphology and nutrient absorption in young rats. In addition, Hsieh et al. (2021) indicated that dietary LBPs improved gastric microbiota by increasing gastric Bifdobacterium levels in rats. Therefore, these and our evidence may be mediated by the promotional effects of LBPs on growth performance.
Diarrhea incidence has been used as an index to reflect gut health, with a lower diarrheal incidence beneficial for gut health (Pierce et al., 2005). Qiao et al. (2013) reported that diarrheal incidence in piglets was decreased by supplementing medicinal Aloe vera polysaccharides. In our study, dietary LBPs or ABO supplementation reduced diarrheal incidence in weaned piglets. Nagy et al. (1992) reported piglet diarrhea after weaning is related to some pathogen levels in the intestine. We previously demonstrated that weaned piglets fed 4,000 mg/kg LBPs had a decreased relative abundance of Escherichia coli and Firmicutes in the ileum and cecum (Chen et al., 2020). Also, intestinal pH is associated with the proliferation of probiotic microbes, preventing post weaning diarrhea, and maintaining gut enzyme activity (Beuria et al., 2005; Guggenbuhl et al., 2007). Furthermore, Xia et al. (2020) found that dietary LBPs supplementation increased the abundance of Roseburia faecis, Prevotella spp., Butyricicoccus pullicaecorum, and Eubacterium uniforme in mice, which generated particular SCFAs. Thus, LBPs appear to reduce diarrhea incidence in weaned piglets by modulating gut microbiota composition.
Immunoglobulins reflect the immune status of the animal (Yuan et al., 2015; Wang Y. et al., 2018). Hao et al. (2015) reported that the major serum Igs, IgA, IgG, and IgM, were key humoral immunity components in all mammals; they enhance monocyte macrophage phagocytosis and inhibit pathogenic virus and microorganism reproduction (Heidebrecht and Kulozik, 2019; Planchais and Mouquet, 2020). In our study, dietary LBPs supplementation increased serum IgG and IgM levels in weaned piglets. Similarly, Long et al. (2020) reported that broilers fed 2,000 mg/kg LBPs increased serum IgA and IgG levels. Furthermore, the immunoenhancing effects of LBPs may stimulate IL-2 and TNF-α gene expression in human monocytes (Lu and Zhang, 2002). In our study, LBPs dietary supplementation enhanced serum IL-2, IL-10, and TNF-α production in agreement with Ding et al. (2019a), who reported that LBPs administration increased IL-2, IL-6, IL-1, TNF-α, and interferon-γ levels in mice. Littringer et al. (2018) reported that IL-2 and TNF-α were secreted mainly through T helper cells. Wang et al. (2021) found that polysaccharides from traditional Chinese medicines, such as Artemisia rupestris L., Astragalus, L. barbarum, and G. lucidum regulated immune cell functions and metabolism by activating macrophages and T/B lymphocyte signal pathways. Thus, dietary LBPs appeared to improve the health status of piglets by activating the immune system.
Weaning decreases antioxidation capacity by increasing free radical levels and disrupting oxidative balance (Burke et al., 2009; Yin et al., 2014). Antioxidant parameters such as SOD, GSH-Px, T-AOC, and MDA are routinely used to evaluate antioxidation properties (Hao et al., 2015). SOD degrades superoxide radicals and thus functions as an antioxidant (Urso and Clarkson, 2003). The reduction reaction of lipid peroxides is catalyzed by GSH-Px, and total antioxidative capacity is reflected by T-AOC levels (Aleryani et al., 1998; Tao et al., 2006). MDA is an indicator of lipid peroxidation and reflects the severity of free radical attack on cells (Jiang et al., 2016). It was reported that plant polysaccharides could alleviate this oxidative stress (Liu et al., 2018; Chen and Huang, 2019). In our study, LBPs dietary supplementation increased T-AOC and GSH-Px levels but decreased MDA production. Yang F. et al. (2020) reported that LBPs dietary supplementation relieved oxidative stress in high fat diet-induced obese mice. Liu et al. (2021b) found that LBPs supplementation reduced myocardial oxidative stress via activation of the nuclear factor erythroid-2 antioxidant signal pathway. Furthermore, plants containing flavonoids, phenolic compounds, ascorbic acid, and tocopherol were shown to exhibit antioxidant effects (Wang et al., 2008). It is documented that LBPs were rich in these abovementioned agents (Peng and Tian, 2001). Therefore, we speculated that the antioxidant effects of LBPs may be associated with these components, however, more research is required in this area.
A healthy mucosal structure is key for digestion, physiological function, and growth (Pluske et al., 1996). After weaning, significant changes occur in villus height, crypt depth, and V/C ratios (Cheng et al., 2017). A large V/C ratio represents a greater absorptive efficiency in the small intestine for nutrients, and increased resistance toward disease (Pu et al., 2018). Wang et al. (2019) reported that compound polysaccharide supplementation increased villus height and V/C ratios in the duodenum of young rats. In our study, LBPs dietary supplementation increased villus height and V/C ratios in the duodenum of weaned piglets. Thus, LBPs improved intestinal morphology, maintained intestinal integrity, and promoted intestinal absorption.
A balanced intestinal microbiota is critical for good gut health and nutrition. We observed that some changes had occurred in intestinal microbial composition and metabolism of cecal digesta across groups. PCA revealed that microbial composition and structures were distinct between the CON and LBPs groups, but no differences were determined between the ABO and LBPs groups. Some polysaccharides selectively stimulate the growth and metabolic activity of particular intestinal bacteria associated with health and well-being (Wang et al., 2019). We previously showed that LBPs dietary supplementation decreased the relative abundance of E. coli and Firmicutes in the ileum and cecum of pigs (Chen et al., 2020). Similar observations by Zhu et al. (2015) showed that polymannuronate addition to broiler diets increased lactic acid bacteria and decreased cecal E. coli levels. Furthermore, increased E. coli levels may be associated with an increased rate of diarrhea (Zhao et al., 2015). Interestingly, in our study, LBPs dietary supplementation reduced the relative abundance of Escherichia-Shigella, Enterococcaceae, and Enterobacteriaceae in the cecum, and also decreased the diarrhea ratio index. A previous study demonstrated that Lactobacillus could protect the intestine by producing antimicrobial agents that suppressed pathogen colonization (Yu et al., 2018). In the current study, dietary supplemental LBPs promoted Faecalibacterium and Lactobacillus levels. Similarly, Zhao et al. (2015) reported that mulberry leaf polysaccharide dietary supplementation reduced the relative abundance of E. coli and promoted Lactobacilli and Bifidobacteria abundance in weaned piglets. Furthermore, Zhu et al. (2020) also reported that LBPs dietary supplementation increased Proteobacteria and Firmicutes abundance, while reducing Bacteroidetes ratios in mice. These results indicated that LBPs could modulate gut microbiota composition and maintain the health of intestinal communities, which may underlie increased growth performance in animal models.
The intestinal digesta contains considerable microbial metabolites and fermentation products that reflect microbial activity and intestinal health (Diao et al., 2014). SCFAs are key metabolites that gut microbiota use to limit inflammation and maintain intestinal integrity to promote gut health (Liu et al., 2020). Thorburn et al. (2015) showed that acetic acid inhibited the histone deacetylase HDAC9.39 to promote regulatory T cell differentiation, with propionic acid enhancing the generation of macrophage. Cresci et al. (2010) reported that butyric acid promoted gut immune responses and preserved intestinal barrier integrity. In addition, intestinal pH was associated with the proliferation of probiotic microbes, the prevention of post weaning diarrhea, and the maintenance of gut enzyme activity (Beuria et al., 2005; Guggenbuhl et al., 2007). Furthermore, LBPs increased SCFAs production which reduced gut environment pH and inhibited E. coli levels in vivo and in vitro studies (Knudsen et al., 2012; Ding et al., 2019b). In our study, LBPs enhanced acetic, propionic, and butyric acid production, and total SCFAs in the cecum, and also promoted Faecalibacterium which produced butyrate and generated anti-inflammatory properties (Wan et al., 2019). Therefore, LBPs may have active roles in host immunity and health by modulating gut microbiota and promoting SCFAs production.
The present research demonstrated that dietary LBPs supplementation improved growth performance, antioxidant capacity and immunity, and reduced diarrhea incidence in weaned piglets. These LBPs effects were associated with a regulatory input on intestinal microbial composition, microbial metabolite production, and intestinal morphology integrity. Thus, LBPs may be used as efficient antibiotic alternatives in weaned piglet feed.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The animal study was reviewed and approved by the Committee of Animal Care at Hunan Agricultural University (Changsha, China) (permit number: CACAHU 2020-00156).
YXY: conceptualization, formal analysis, and writing—original draft. JC: methodology, validation, and resources. FW: data curation. MY: software. BT: funding acquisition. YLY: investigation and supervision. ZY: methodology, project administration, and writing—review and editing. All authors have read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (U20A2054 and 32072745), the Earmarked Fund for China Agriculture Research System (CARS-35), the Hunan Provincial Key Research and Development Project (2020NK2031), the Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-CXRC-038), the Youth Science Foundation Project of Hunan Agricultural University (19QN01), and the Open Foundation of CAS Key Laboratory of Agro-ecological Processes in Subtropical Region, Institute of Subtropical Agriculture (ISA2020101).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.819993/full#supplementary-material
Aleryani, S., Milo, E., Rose, Y., and Kostka, P. (1998). Superoxide-mediated decomposition of biological S-nitrosothiols. J. Biol. Chem. 273, 6041–6045. doi: 10.1074/jbc.273.11.6041
Beuria, T. K., Santra, M. K., and Panda, D. (2005). Sanguinarine blocks cytokinesis in bacteria by inhibiting FtsZ assembly and bundling. Biochemistry 44, 16584–16593. doi: 10.1021/bi050767
Burke, N. C., Scaglia, G., Boland, H. T., and Swecker, W. S. (2009). Influence of two-stage weaning with subsequent transport on body weight, plasma lipid peroxidation, plasma selenium, and on leukocyte glutathione peroxidase and glutathione reductase activity in beef calves. Vet. Immunol. Immunopathol. 127, 365–370. doi: 10.1016/j.vetimm.2008.11.017
Campbell, J. M., Crenshaw, J. D., and Polo, J. (2013). The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol 4, 124–127. doi: 10.1186/2049-1891-4-19
Chen, F., and Huang, G. (2019). Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 125, 906–908. doi: 10.1016/j.ijbiomac.2018.12.134
Chen, J., Long, L., Jiang, Q., Kang, B., Li, Y., and Yin, J. (2020). Effects of dietary supplementation of Lycium barbarum polysaccharides on growth performance, immune status, antioxidant capacity and selected microbial populations of weaned piglets. J. Anim. Physiol. Anim. Nutr. 104, 1106–1115. doi: 10.1111/jpn.13247
Chen, S., Zhou, Y., Chen, Y., and Gu, J. (2018). Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, 884–890. doi: 10.1093/bioinformatics/bty560
Cheng, W., Lu, J., Li, B., Lin, W., Zhang, Z., Wei, X., et al. (2017). Effect of functional oligosaccharides and ordinary dietary fiber on intestinal microbiota diversity. Front. Microbiol. 8:1750. doi: 10.3389/fmicb.2017.01750
Cook, M. E. (2004). Antibodies: alternatives to antibiotics in improving growth and feed efficiency. J. Appl. Poult. Res 13, 106–119. doi: 10.1093/japr/13.1.106
Cresci, G. A., Thangaraju, M., Mellinger, J. D., Liu, K., and Ganapathy, V. (2010). Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest. Surg. 14, 449–461. doi: 10.1007/s11605-009-1045-x
Diao, H., Zheng, P., Yu, B., He, J., Mao, X., Yu, J., et al. (2014). Effects of dietary supplementation with benzoic acid on intestinal morphological structure and microflora in weaned piglets. Livest. Sci. 167, 249–256. doi: 10.1016/j.livsci.2014.05.029
Ding, Y., Yan, Y., Chen, D., Ran, L., Mi, J., Lu, L., et al. (2019a). Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 10, 3671–3683. doi: 10.1039/c9fo00638a
Ding, Y., Yan, Y., Peng, Y., Chen, D., Mi, J., Lu, L., et al. (2019b). In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 125, 751–760. doi: 10.1016/j.ijbiomac.2018.12.081
Donno, D., Beccaro, G. L., Mellano, M. G., Cerutti, A. K., and Bounous, G. (2015). Goji berry fruit (Lycium spp.): antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods 18, 1070–1085. doi: 10.1016/j.jff.2014.05.020
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/NMETH.2604
Feng, L., Xiao, X., Liu, J., Wang, J., Zhang, N., Bing, T., et al. (2020). Immunomodulatory effects of Lycium barbarum polysaccharide extract and its uptake behaviors at the cellular level. Molecules 25:1351. doi: 10.3390/molecules25061351
Franklin, M. A., Mathew, A. G., Vickers, J. R., and Clift, R. A. (2002). Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs 24 days of age. J. Anim. Sci. 80, 2904–2910. doi: 10.2527/2002.80112904x
Gong, G., Liu, Q., Deng, Y., Dang, T., Dai, W., Liu, T., et al. (2020). Arabinogalactan derived from Lycium barbarum fruit inhibits cancer cell growth via cell cycle arrest and apoptosis. Int. J. Biol. Macromol. 149, 639–650. doi: 10.1016/j.ijbiomac.2020.01.251
Guggenbuhl, P., Seon, A., Quintana, A. P., and Nunes, C. S. (2007). Effects of dietary supplementation with benzoic acid (VeVo Vitall®) on the zootechnical performance, the gastrointestinal microflora and the ileal digestibility of the young pig. Livest. Sci. 108, 218–221. doi: 10.1016/j.livsci.2007.01.068
Halas, D., Hansen, C. F., Hampson, D. J., Mullan, B. P., Kim, J. C., Wilson, R. H., et al. (2010). Dietary supplementation with benzoic acid improves apparent ileal digestibility of total nitrogen and increases villous height and caecal microbial diversity in weaner pigs. Anim. Feed Sci. Technol. 160, 137–147. doi: 10.1016/j.anifeedsci.2010.07.001
Hao, R., Li, Q., Zhao, J., Li, H., Wang, W., and Gao, J. (2015). Effects of grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livest. Sci. 178, 237–242. doi: 10.1016/j.livsci.2015.06.004
Heidebrecht, H., and Kulozik, U. (2019). Fractionation of casein micelles and minor proteins by microfiltration in diafiltration mode. study of the transmission and yield of the immunoglobulins IgG, IgA and IgM. Int. Dairy J. 93, 1–10. doi: 10.1016/j.idairyj.2019.01.009
Hou, G., Peng, W., Wei, L., Li, R., Huang, X., and Yin, Y. (2021). Probiotics and Achyranthes bidentata polysaccharides improve growth performance via promoting intestinal nutrient utilization and enhancing immune function of weaned pigs. Animals 11:2617. doi: 10.3390/ani11092617
Hsieh, S., Lian, Y., Lin, I., Yang, Y., Tinkov, A. A., Skalny, A. V., et al. (2021). Combined Lycium babarum polysaccharides and C-phycocyanin increase gastric Bifidobacterium relative abundance and protect against gastric ulcer caused by aspirin in rats. Nutr. Metab. 18:4. doi: 10.1186/s12986-020-00538-9
Hu, C., Xiao, K., Luan, Z., and Song, J. (2013). Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 91, 1094–1101. doi: 10.2527/jas.2012-5796
Hung, D., Cheng, Y., Chen, W., Hua, K., Pietruszka, A., Dybus, A., et al. (2019). Bacillus licheniformis-fermented products reduce diarrhea incidence and alter the fecal microbiota community in weaning piglets. Animals 9:1145. doi: 10.3390/ani9121145
Jia, L., Li, W., Li, J., Li, Y., Song, H., Luan, Y., et al. (2016). Lycium barbarum polysaccharide attenuates high-fat diet-induced hepatic steatosis by up-regulating SIRT1 expression and deacetylase activity. Sci. Rep. 6, 1–11. doi: 10.1038/srep36209
Jiang, W., Wen, H., Liu, Y., Jiang, J., Wu, P., Zhao, J., et al. (2016). Enhanced muscle nutrient content and flesh quality, resulting from tryptophan, is associated with anti-oxidative damage referred to the Nrf2 and TOR signalling factors in young grass carp (Ctenopharyngodon idella): avoid tryptophan deficiency or excess. Food Chem. 199, 210–219. doi: 10.1016/j.foodchem.2015.12.003
Kim, H. J., Lee, J., Kim, S. C., Seo, J. Y., Hong, S. B., and Park, Y. I. (2020). Immunostimulating activity of Lycium chinense miller root extract through enhancing cytokine and chemokine production and phagocytic capacity of macrophages. J. Food Biochem. 44:e13215. doi: 10.1111/jfbc.13215
Knudsen, K. E., Hedemann, M. S., and Lærke, H. N. (2012). The role of carbohydrates in intestinal health of pigs. Anim. Feed Sci. Technol. 173, 41–53. doi: 10.1016/j.anifeedsci.2011.12.020
Lemieux-labonte, V., Simard, A., Willis, C. K., and Lapointe, F. (2017). Enrichment of beneficial bacteria in the skin microbiota of bats persisting with white-nose syndrome. Microbiome 5:115. doi: 10.1186/s40168-017-0334-y
Li, J. (2017). Current status and prospects for in-feed antibiotics in the different stages of pork production - a review. Asian Austral. J. Anim. 30, 1667–1673. doi: 10.5713/ajas.17.0418
Li, X., He, L., Yang, Y., Liu, F., Cao, Y., and Zuo, J. (2015). Effects of extracellular polysaccharides of Ganoderma lucidum supplementation on the growth performance, blood profile, and meat quality in finisher pigs. Livest. Sci. 178, 187–194. doi: 10.1016/j.livsci.2015.04.001
Littringer, K., Moresi, C., Rakebrandt, N., Zhou, X., Schorer, M., Dolowschiak, T., et al. (2018). Common features of regulatory T cell specialization during Th1 responses. Front. Immunol. 9:1344. doi: 10.3389/fimmu.2018.01344
Liu, J., Pu, Q., Qiu, H., Di, D., Liu, J., Pu, Q., et al. (2021a). Polysaccharides isolated from Lycium barbarum L. by integrated tandem hybrid membrane technology exert antioxidant activities in mitochondria. Ind. Crops Prod. 168:113547. doi: 10.1016/j.indcrop.2021.113547
Liu, J., Zhao, G., He, L., Wang, Z., Zibrila, A. I., Niu, B., et al. (2021b). Lycium barbarum polysaccharides inhibit ischemia/reperfusion-induced myocardial injury via the Nrf2 antioxidant pathway. Toxicol. Rep. 8, 657–667. doi: 10.1016/j.toxrep.2021.03.019
Liu, Y., Sun, Y., Huang, G., Liu, Y., Sun, Y., and Huang, G. (2018). Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 111, 780–786. doi: 10.1016/j.ijbiomac.2018.01.086
Liu, Y., Wang, X., Chen, Q., Luo, L., Ma, M., Xiao, B., et al. (2020). Camellia sinensis and Litsea coreana ameliorate intestinal inflammation and modulate gut microbiota in dextran sulfate sodium-induced colitis mice. Mol. Nutr. Food Res. 64:1900943. doi: 10.1002/mnfr.201900943
Long, L., Kang, B., Jiang, Q., and Chen, J. (2020). Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poultry. Sci. 99, 744–751. doi: 10.1016/j.psj.2019.10.043
Lu, G., and Zhang, S. (2002). Effects of Lycium barbarum polysaccharide on cytokine expression in human monocytes. Acta Nutr. Sin. 24, 67–69. doi: 10.1038/sj.cr.7290131
Lu, T., Piao, X., Zhang, Q., Wang, D., Piao, X., and Kim, S. (2010). Protective effects of Forsythia suspensa extract against oxidative stress induced by diquat in rats. Food Chem. Toxicol. 48, 764–770. doi: 10.1016/j.fct.2009.12.018
Magoc, T., and Salzberg, S. L. (2011). Flash: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Nagy, B., Arp, L. H., Moon, H. W., and Casey, T. A. (1992). Colonization of the small intestine of weaned pigs by enterotoxigenic Escherichia coli that lack known colonization factors. Vet. Pathol. 29, 239–246. doi: 10.1177/030098589202900308
National Research Council (2012). Nutrient Requirements of Swine, 11th Edn. Washington, DC: National Academy Press.
Peng, X., and Tian, G. (2001). Structural characterization of the glycan part of glycoconjugate LbGp2 from Lycium barbarum L. Carbohydr. Res. 331, 95–99. doi: 10.1016/S0008-6215(00)00321-9
Pierce, M., Callan, J., Mccarthy, P., and O’doherty, V. (2005). Performance of weanling pigs offered low or high lactose diets supplemented with avilamycin or inulin. Anim. Sci. 80, 313–318. doi: 10.1079/asc40900313
Planchais, C., and Mouquet, H. (2020). Easy pan-detection of human IgA immunoglobulins. J. Immunol. Methods 484:112833. doi: 10.1016/j.jim.2020.112833
Pluske, J., Williams, I., and Aherne, F. (1996). Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim. Sci. 62, 131–144.
Pourhossein, Z., Qotbi, A. A., Seidavi, A., Laudadio, V., Centoducati, G., and Tufarelli, V. (2015). Effect of different levels of dietary sweet orange (Citrus sinensis) peel extract on humoral immune system responses in broiler chickens. Anim. Sci. J. 86, 105–110. doi: 10.1111/asj.12250
Pu, J., Chen, D., Tian, G., He, J., Zheng, P., Mao, X., et al. (2018). Protective effects of benzoic acid, Bacillus coagulans, and oregano oil on intestinal injury caused by enterotoxigenic Escherichia coli in weaned piglets. Biomed. Res. Int. 2018:1829632. doi: 10.1155/2018/1829632
Qiao, J., Li, H., Zheng, C., Feng, Z., and Wang, W. (2013). Dietary supplementation with Aloe vera polysaccharide enhances the growth performance and immune function of weaned piglets. J. Anim. Feed Sci. 22, 329–334. doi: 10.22358/jafs/65921/2013
Shimato, Y., Hattori, T., and Ohno, T. (2020). Hypoglycemic activity and the mechanisms of Lycium bark extract in db/db mice. Biol. Pharm. Bull. 43, 946–950. doi: 10.1248/bpb.b19-00814
Stackebrandt, E., and Goebel, B. (1994). Taxonomic note: a place for DNA-DNA reassociation and 16s rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Micr. 44, 846–849. doi: 10.1099/00207713-44-4-846
Tan, X., Sun, Z., Ye, C., Lin, H., Tan, X., Sun, Z., et al. (2019). The effects of dietary Lycium barbarum extract on growth performance, liver health and immune related genes expression in hybrid grouper (Epinephelus lanceolatus♂ × E. fuscoguttatus♀) fed high lipid diets. Fish Shellf. Immun. 87, 847–852. doi: 10.1016/j.fsi.2019.02.016
Tao, X., Xu, Z., and Wang, Y. (2006). Effects of dietary fluoride levels on growth, serum indexes and antioxidant systems in growing pigs. Turk. J. Vet. Anim. Sci. 30, 65–70.
Thorburn, A. N., Mckenzie, C. I., Shen, S., Stanley, D., Macia, L., Mason, L. J., et al. (2015). Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 6:7320. doi: 10.1038/ncomms8320
Urso, M. L., and Clarkson, P. M. (2003). Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189, 41–54. doi: 10.1016/S0300-483X(03)00151-3
Wan, Y., Wang, F., Yuan, J., Li, J., Jiang, D., Zhang, J., et al. (2019). Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 68, 1417–1429. doi: 10.1136/gutjnl-2018-317609
Wang, D., Liu, Y., and Zhao, W. (2021). The adjuvant effects on vaccine and the immunomodulatory mechanisms of polysaccharides from traditional Chinese medicine. Front. Mol. Biosci. 8:655570. doi: 10.3389/fmolb.2021.655570
Wang, H., Li, Y., Liu, J., Di, D., Liu, Y., and Wei, J. (2020). Hepatoprotective effect of crude polysaccharide isolated from Lycium barbarum L. against alcohol-induced oxidative damage involves Nrf2 signaling. Food Sci. Nutr. 8, 6528–6538. doi: 10.1002/fsn3.1942
Wang, L., Piao, X., Kim, S., Piao, X., Shen, Y., and Lee, H. (2008). Effects of Forsythia suspensa extract on growth performance, nutrient digestibility, and antioxidant activities in broiler chickens under high ambient temperature. Poult. Sci. 87, 1287–1294. doi: 10.3382/ps.2008-00023
Wang, M., Xie, Z., Li, L., Chen, Y., Li, Y., Wang, Y., et al. (2019). Supplementation with compound polysaccharides contributes to the development and metabolic activity of young rat intestinal microbiota. Food Funct. 10, 2658–2675. doi: 10.1039/c8fo02565g
Wang, W., Chen, J., Zhou, H., Wang, L., Ding, S., Wang, Y., et al. (2018). Effects of microencapsulated Lactobacillus plantarum and fructooligosaccharide on growth performance, blood immune parameters, and intestinal morphology in weaned piglets. Food Aag. Immunol. 29, 84–94. doi: 10.1080/09540105.2017.1360254
Wang, Y., Dong, Z., Song, D., Zhou, H., Wang, W., Miao, H., et al. (2018). Effects of microencapsulated probiotics and prebiotics on growth performance, antioxidative abilities, immune functions, and caecal microflora in broiler chickens. Food Aag. Immunol. 29, 859–869. doi: 10.1080/09540105.2018.1463972
Wu, Q., Liu, L., Wang, X., Lang, Z., Meng, X., Guo, S., et al. (2020). Lycium barbarum polysaccharides attenuate kidney injury in septic rats by regulating Keap1-Nrf2/are pathway. Life Sci. 242:117240. doi: 10.1016/j.lfs.2019.117240
Xia, W., Li, X., Khan, I., Yin, L., Su, L., Leong, W. K., et al. (2020). Lycium berry polysaccharides strengthen gut microenvironment and modulate gut microbiota of the mice. Evid-Based Compl. Alt. 2020:809702. doi: 10.1155/2020/8097021
Xu, N., Tan, G., Wang, H., and Gai, X. (2016). Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 74, 1–8. doi: 10.1016/j.ejsobi.2016.02.004
Yang, F., Wei, Y., Liao, B., Wei, G., Qin, H., Pang, X., et al. (2020). Effects of Lycium barbarum polysaccharide on endoplasmic reticulum stress and oxidative stress in obese mice. Front. Pharmacol. 11:742. doi: 10.3389/fphar.2020.00742
Yang, J., Wang, C., Huang, K., Zhang, M., Wang, J., and Pan, X. (2020). Compound Lactobacillus sp. administration ameliorates stress and body growth through gut microbiota optimization on weaning piglets. App. Microbiol. Biot. 104, 6749–6765. doi: 10.1007/s00253-020-10727-4
Yang, X., Bai, H., Cai, W., Li, J., Zhou, Q., Wang, Y., et al. (2013). Lycium barbarum polysaccharides reduce intestinal ischemia/reperfusion injuries in rats. Chem-Biol. Interact. 204, 166–172. doi: 10.1016/j.cbi.2013.05.010
Yin, J., Wu, M., Xiao, H., Ren, W., Duan, J., Yang, G., et al. (2014). Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 92, 612–619. doi: 10.2527/jas.2013-6986
Yu, M., Mu, C., Zhang, C., Yang, Y., Su, Y., and Zhu, W. (2018). Marked response in microbial community and metabolism in the ileum and cecum of suckling piglets after early antibiotics exposure. Front. Microbiol. 9:1166. doi: 10.3389/fmicb.2018.01166
Yuan, W., Jin, H., Ren, Z., Deng, J., Zuo, Z., Wang, Y., et al. (2015). Effects of antibacterial peptide on humoral immunity in weaned piglets. Food Agr. Immunol. 26, 682–689. doi: 10.1080/09540105.2015.1007448
Zhang, F., Zhang, X., Gu, Y., Wang, M., Guo, S., Liu, J., et al. (2021). Hepatoprotection of Lycii fructus polysaccharide against oxidative stress in hepatocytes and larval zebrafish. Oxid. Med. Cell Longev. 2021:3923625. doi: 10.1155/2021/3923625
Zhao, J., Ge, L., Xiong, W., Leong, F., Huang, L., and Li, S. (2016). Advanced development in phytochemicals analysis of medicine and food dual purposes plants used in China (2011-2014). J. Chromatogr. 1428, 39–54. doi: 10.1016/j.chroma.2015.09.006
Zhao, X., Li, L., Luo, Q., Ye, M., Luo, G., and Kuang, Z. (2015). Effects of mulberry (Morus alba L.) leaf polysaccharides on growth performance, diarrhea, blood parameters, and gut microbiota of early-weanling pigs. Livest. Sci. 177, 88–94. doi: 10.1016/j.livsci.2015.03.001
Zhao, Z., Yu, H., Liu, B., Wang, H., Luo, Q., and Ding, X. (2016). Antioxidative mechanism of Lycium barbarum polysaccharides promotes repair and regeneration following cavernous nerve injury. Neural. Regen. Res. 11, 1312–1321. doi: 10.4103/1673-5374.189197
Zhu, W., Li, D., Wang, J., Wu, H., Xia, X., Bi, W., et al. (2015). Effects of polymannuronate on performance, antioxidant capacity, immune status, cecal microflora, and volatile fatty acids in broiler chickens. Poult. Sci. 94, 345–352. doi: 10.3382/ps/pev006
Zhu, W., Zhou, S., Liu, J., Mclean, R. J., and Chu, W. (2020). Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharmacother. 121:109591. doi: 10.1016/j.biopha.2019.109591
Zhu, Y., Zhao, Q., Gao, H., Peng, X., Wen, Y., and Dai, G. (2016). Lycium barbarum polysaccharides attenuates N-methy-N-nitrosourea-induced photoreceptor cell apoptosis in rats through regulation of poly (ADP-ribose) polymerase and caspase expression. J. Ethnopharmacol. 191, 125–134. doi: 10.1016/j.jep.2016.05.037
Keywords: antioxidant, growth performance, immune, intestinal health, Lycium barbarum polysaccharides, weaned piglets
Citation: Yin Y, Wang F, Yang M, Tan B, Yin Y, Chen J and Yang Z (2022) Lycium barbarum Polysaccharides as Antibiotic Substitutes Improve Growth Performance, Serum Immunity, Antioxidant Status, and Intestinal Health for Weaned Piglets. Front. Microbiol. 12:819993. doi: 10.3389/fmicb.2021.819993
Received: 22 November 2021; Accepted: 30 December 2021;
Published: 25 February 2022.
Edited by:
Wang Jiajun, Northeast Agricultural University, ChinaReviewed by:
Tarique Hussain, Nuclear Institute for Agriculture and Biology, PakistanCopyright © 2022 Yin, Wang, Yang, Tan, Yin, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe Yang, emhleWFuZ0BodW5hdS5lZHUuY24=; Jiashun Chen, anNjaGVuQGh1bmF1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.