- Department of Veterinary Microbiology, Faculty of Veterinary Medicine, Ondokuz Mayis University, Samsun, Turkey
Campylobacteriosis continues to be one of the leading causes of foodborne bacterial zoonotic infections worldwide. Despite its public health importance, the status of this disease in wild birds and the possibility of transmission from wild birds to domestic animals and humans have not been clearly elucidated yet. This article reviews the available literature with the aim of making a comprehensive manuscript on this disease status in wild birds and the possibility of interspecies transmission. Campylobacter has been isolated from various species of wild birds worldwide, with C. jejuni being the most commonly isolated species. The prevalence of Campylobacter in wild birds may vary depending on several factors like geographical location, season, the bird’s health status, bird species, sample type, the method used, and ecological factors. Molecular studies over the past two to three decades have characterized Campylobacter strains isolated from wild birds and have come up with results that fall into two categories. The first are those that report overlapping strains among human, domestic animal, and wild bird isolates. The results of the studies under this category emphasize that wild birds carry strains of Campylobacter, which are indistinguishable from domestic animals and humans and are therefore an important public and animal health concern. In contrast, the studies under the second category highlight significant differences in Campylobacter population structure among these hosts. Despite the controversiality and the inadequacy of current research to draw a full conclusion, the role of wild birds in the epidemiology of Campylobacter should not be undermined as drug-resistant strains, especially resistance to tetracycline and fluoroquinolones, are increasingly documented. In addition, source attribution studies have linked human cases of Campylobacter infections to wild birds. Therefore, the role of wild birds in the epidemiology of Campylobacter infection should not be neglected. However, in order to determine disease status in wild birds and the precise role of wild birds in domestic animals and human health, detail-oriented epidemiological investigations characterizing the genetic relatedness of isolates from the respective species and environment through one health approach are warranted.
Introduction
Campylobacteriosis is currently considered to be the most commonly reported zoonotic bacterial foodborne gastroenteritis worldwide (Silva et al., 2011; Igwaran and Okoh, 2019). Over the past decades, a rise in its incidence has been evidenced in different parts of the world, including both developed and developing countries (Kaakoush et al., 2015; Igwaran and Okoh, 2019). The World Health Organization (WHO) estimated that at least 96 million cases of enteric infections worldwide are associated with Campylobacter species annually (Havelaar et al., 2015). According to the “European Union One Health 2019 zoonoses report,” campylobacteriosis ranked first as the most commonly reported zoonoses in European Union member countries, with 220,682 confirmed human cases in 2019 alone (European Food Safety Authority [EFSA], and European Centre for Disease Prevention and Control [ECDC], 2021). Therefore, campylobacteriosis is a disease of public health concern globally (Igwaran and Okoh, 2019).
Although various animal species, including wild birds, are known sources of Campylobacter infection (Zenebe et al., 2020; Mughini-Gras et al., 2021), poultry is accepted to act as reservoirs of 50–80% of Campylobacter infections in humans, and cattle are considered to act as reservoirs of 20–30% of human infections (European Food Safety Authority [EFSA], 2010). One study conducted in the Baltic States showed that clinical cases of Campylobacter jejuni (C. jejuni) infections in humans were associated with sources from poultry (88.3%), cattle (9.4%), and wild birds (2.3%) (Maësaar et al., 2020). Another study that used multilocus sequence typing (MLST) to determine the infection source linked 64.5% of human C. jejuni infection to poultry, with cattle and wild birds accounting for 25.8 and 2.3%, respectively (Levesque et al., 2013). Thus, poultry and cattle are generally accepted as significant sources of human campylobacter infection (Mughini-Gras et al., 2021). Even though wild bird Campylobacter carriage is much lower than poultry (Maësaar et al., 2020; Zhang and Sahin, 2020), wild birds are known to act as significant reservoirs, implying that they may have a role in spreading the bacteria to the environment (Navarro-Gonzalez et al., 2016; Aksomaitiene et al., 2019; Marotta et al., 2019; Maësaar et al., 2020).
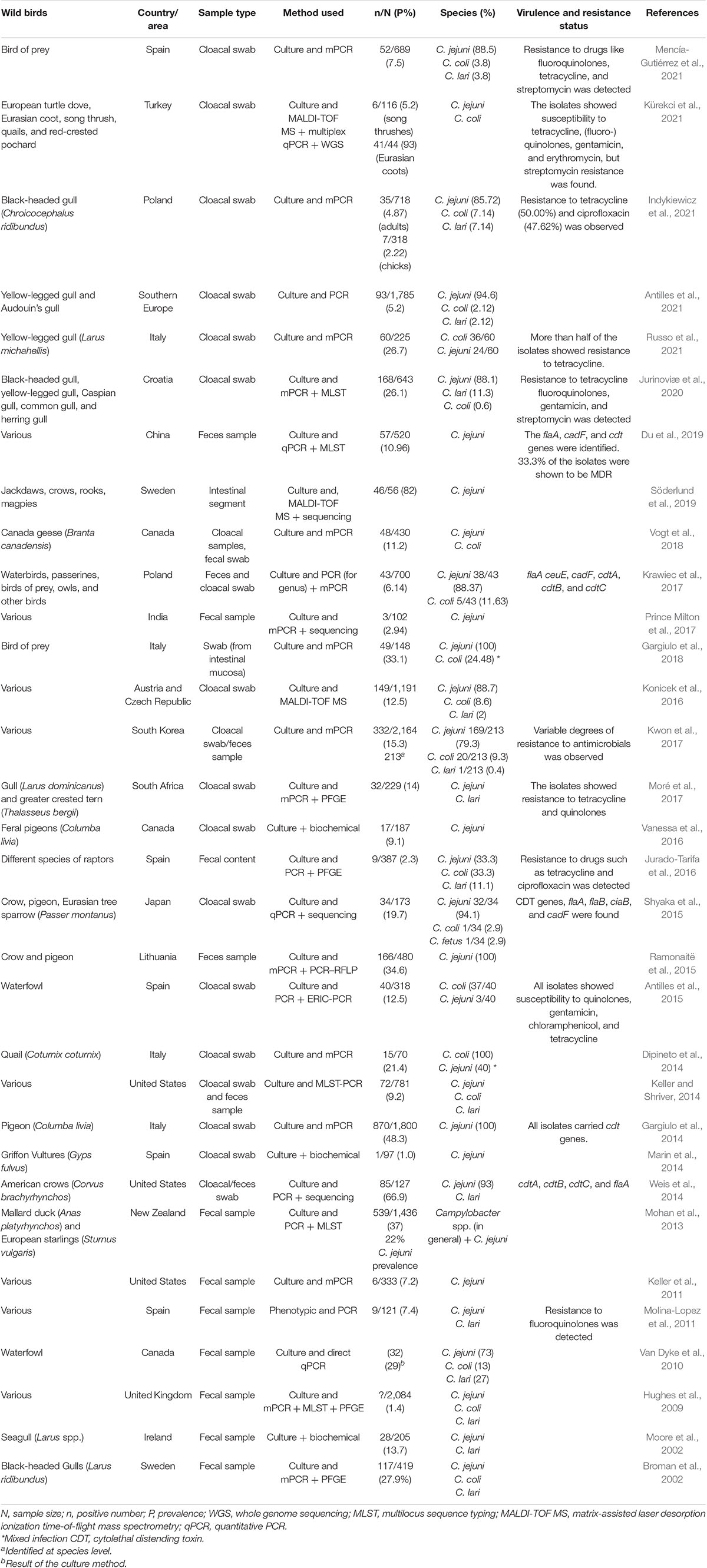
Currently, Campylobacter has been isolated from several species of wild birds (e.g., birds of prey, waterfowl, crows, pigeons, gulls, and others) in different areas in the world, including America, Australia, Asia, Europe, and Africa (Keller et al., 2011; Konicek et al., 2016; Moré et al., 2017; Du et al., 2019; Antilles et al., 2021; Kürekci et al., 2021). In addition to pathogen detection, antibiotic resistance, one of the global challenges of the current century (Sabtu et al., 2015), is also being reported in Campylobacter spp. isolated from wild birds. In particular, resistance to tetracycline and fluoroquinolones is increasingly documented (Jurado-Tarifa et al., 2016; Indykiewicz et al., 2021; Mencía-Gutiérrez et al., 2021; Russo et al., 2021).
Due to their significant reservoir role, Campylobacter status in poultry and cattle has been extensively studied, demonstrating the significant attention paid to the risk of acquiring Campylobacter infection from food sources (Mohan, 2015). However, only limited information is available regarding the role of wild birds as a reservoir of pathogens like Campylobacter, which can be related to the difficulty in collecting samples from wild birds (Antilles et al., 2015; Mencía-Gutiérrez et al., 2021). Despite the scarcity of research, the available literature indicates that contamination of equipment and surfaces with wild bird feces can be a risk for human health (French et al., 2009). Wild birds are shown to be one of the leading causes of contamination of surface water (Mulder et al., 2020), and source tracking studies have linked human cases of Campylobacter infections to wild birds (Gardner et al., 2011). For example, a molecular study conducted in the United Kingdom related 476–543 annual clinical cases of human Campylobacter infection to wild birds, emphasizing the importance of wild birds in human campylobacteriosis (Cody et al., 2015). Another molecular study from the United States also linked the human outbreak of Campylobacter infection due to raw peas consumption to wild birds (Kwan et al., 2014).
Despite the public and economic importance of Campylobacter infections, the status of this disease in wild birds and the likelihood of transmission from wild birds to domestic animals and humans have not been clearly determined yet. However, understanding the status of this disease in wild birds and the possibility of interspecies transmission is necessary to designing applicable policies. To date, no article has exclusively reviewed the status of Campylobacter in wild birds and its associated public and animal health significance, except a few articles (Abulreesh et al., 2006; Dhama et al., 2008; Benskin et al., 2009; Whiley et al., 2013; Clark, 2014; Navarro-Gonzalez et al., 2016; Elmberg et al., 2017; Smith et al., 2020) reviewing pathogens in general or in a specific host. Therefore, this article aims to review the available literature on Campylobacter in wild birds and summarize the current understanding of interspecies transmission to show what is currently known about its public and animal health importance.
Historical and General Information on Campylobacter
According to available information, Theodor Escherich was thought to have made the first report on Campylobacter in 1886 (Silva et al., 2011). Despite this, Campylobacter was not recognized as a primary disease-causing agent in humans until the 1970s (Butzler, 2004), which is thought to be due to difficulties in culturing and identifying it (Sheppard and Maiden, 2015). In the case of livestock in general, the diseases due to Campylobacter have been well documented since the beginning of the twentieth century (Butzler, 2004; Hlashwayo et al., 2020). The role of wild birds as carriers of Campylobacter has also long been recognized (Luechtefeld et al., 1980; Kapperud and Rosef, 1983; Kinjo et al., 1983; Fukuyama et al., 1986). Even though it is unclear whether it was the first report, Luechtefeld et al. (1980) reported C. jejuni carriage in migratory waterfowl in samples collected from 1978 to 1980 in Northern Colorado, United States. Following this, several researchers reported Campylobacter carriage in various wild bird species such as pigeons, gulls, crows, starlings, and others (Kinjo et al., 1983; Fukuyama et al., 1986; Ito et al., 1988; Whelan et al., 1988; Fernández et al., 1996).
The currently used Campylobacter genus name (previously known as Vibrio spp.) was proposed by Sébald and Véron for the first time in 1963 (Frasao et al., 2017). The causative agent of campylobacteriosis includes various pathogenic species of Campylobacter, which are small (0.2–0.8 μm × 0.5–5 μm), micro-aerophilic, and spiral Gram-negative bacteria belonging to the family Campylobacteriaceae, class Epsilonproteobacteria, and phylum Proteobacteria (Silva et al., 2011; Muralidharan et al., 2016). Currently, about 53 Campylobacter species and 16 subspecies have been documented (accessed November 20, 2021), (LPSN), including those considered pathogenic to humans and livestock (Humphrey et al., 2007). Among these species, thermophilic Campylobacter (for example, C. jejuni and C. coli) are essential zoonotic pathogens that cause gastroenteritis in humans worldwide (Kreling et al., 2020). The rise in the number of species associated with animal and human infections is believed to be why this bacterium needs significant attention (Igwaran and Okoh, 2019). Most Campylobacter species (except C. gracilis, and C. showae) can move using amphitrichous flagella. If two Campylobacter cells are found together, they appear in an “S” shape, resembling a flying gull’s wing (Silva et al., 2011), and the name Campylobacter, which is taken from the Greek word “campylos,” also describes this “S” shape morphology (Kreling et al., 2020).
Identification and Characterization of Campylobacter
For the identification and characterization of Campylobacter, a variety of phenotypic (e.g., culture) and genotypic methods [e.g., MLST, polymerase chain reaction (PCR)], each with its own pros and cons, have been documented (Eberle and Kiess, 2012). Bacterial culturing and biochemical tests have long been used to characterize pathogens (Ferone et al., 2020). The commonly performed detection method of Campylobacter spp. from avian fecal samples relies on culturing techniques, including directly plating cecal/fecal materials onto selective agar plates or pre-enrichment in “selective enrichment broth” followed by “selective plating.” However, the success of the bacteria recovery rate in the former method depends on the bacteria’s number present, and hence the method may not show satisfactory recovery from animal and avian feces (Abulreesh et al., 2006). Even though pre-enrichment may not always result in a higher recovery rate than direct plating (Zhang and Sahin, 2020), in the case of a low number of bacteria, recovery rates may be improved if pre-enrichment is performed and followed by selective plating (Abulreesh et al., 2006). Since this bacterium requires special conditions (fastidious growth requirements) such as low oxygen concentrations and enriched media, traditional culture-based identification methods are challenging, and species differentiation is also difficult as there are limited biochemical tests (Al Amri et al., 2007; Frasao et al., 2017).
Currently, molecular-based methods such as PCR, MLST, and pulsed-field gel electrophoresis (PFGE) are frequently being used for species identification and genotypic characterization of Campylobacter isolates obtained from wild birds (Keller and Shriver, 2014; Mohan, 2015; Indykiewicz et al., 2021; Kürekci et al., 2021; Mencía-Gutiérrez et al., 2021). Multiplex PCR, a rapid and accurate method that detects multiple-gene presence/absence in a single reaction mixture, is among the most commonly used methods for differentiating different species of Campylobacter (Frasao et al., 2017; Ricke et al., 2019). Several researchers have used this method to identify the species of Campylobacter isolates obtained from wild birds (Keller et al., 2011; Ramonaitë et al., 2015; Kürekci et al., 2021). Therefore, mPCR should be considered when the objective is to identify or differentiate different species of Campylobacter (Frasao et al., 2017).
“Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS)” is another method employed to differentiate Campylobacter at the genus and/or species level (Bessè De et al., 2011; Dudzic et al., 2016). Compared with the morphology-based conventional methods, this method is fast, cost-effective, and more reliable for identifying Campylobacter spp. (Bessè De et al., 2011). Recently, researchers have pointed out the usage of MALDI-TOF MS in the differentiation of Campylobacter isolates obtained from wild birds (Dudzic et al., 2016; Lawton et al., 2018). For example, a more recent study by Kürekci et al. (2021) identified Campylobacter isolates of wild bird origin to genus level using MALDI-TOF MS. The study by Dudzic et al. (2016), who used culture-based detection, MALDI-TOF, and PCR, reported that 35 Campylobacter sp. isolates obtained from pigeons were all confirmed to be Campylobacter at the genus level by MALDI-TOF and contained 16 rRNA genes specific for Campylobacter spp. by PCR. Lawton et al. (2018) comparatively investigated Campylobacter isolates using MALDI-TOF MS, PCR, and WGS and reported 100% agreement in species identification among these methods.
MLST is the sequence-based molecular typing method used for evaluating strain relationships. Although it has a low resolution compared to WGS, MLST can provide highly discriminatory pathogen clustering results by examining the sequences of 7 housekeeping genes (Tong et al., 2021). This technique has been used in assessing Campylobacter strain overlap among different species of wild birds, poultry, and humans (Colles et al., 2008; French et al., 2009; Hughes et al., 2009; Du et al., 2019; Wei et al., 2019; Jurinoviæ et al., 2020). Since the application of MLST is restricted to the sequence type characterization, whole-genome or core-genome MLST techniques are preferred for the accurate differentiation of related strains (Marotta et al., 2020). Currently, technological advancements have enabled the use of a more advanced WGS technique, which reveals an organism’s entire DNA make-up (Franz et al., 2016). WGS provides higher-resolution phylogenetic information than other methods, such as MLST, making it ideal for source attribution studies and comparing strains from different origins (Tong et al., 2021).
Epidemiology of Campylobacter in Wild Birds
Wild birds are a well-known significant natural reservoir of Campylobacter species (especially thermophilic Campylobacter spp., i.e., C. jejuni, C. coli, and C. lari) (Krawiec et al., 2017; Mencía-Gutiérrez et al., 2021). This bacterium has been isolated from several species of wild birds (birds of prey, waterfowl, crows, pigeons, gulls, geese, and others) in different areas in the world, including Africa, America, Europe, Australia, and Asia, showing its global distribution (Keller et al., 2011; Konicek et al., 2016; Moré et al., 2017; Vogt et al., 2018; Du et al., 2019; Jurinoviæ et al., 2020; Antilles et al., 2021; Kürekci et al., 2021).
Transmission in wild birds is considered to be through fecal–oral, when the birds are foraging near domestic animals, which may result in the spread of pathogen over long distances (Taff and Townsend, 2017). Although avian species, including domestic poultry (Griekspoor et al., 2013) and wild birds (Smith et al., 2020), are known to be asymptomatic carriers of Campylobacter, lower survival rates and/or poor body condition have been encountered in infected birds when compared with healthy birds (Waldenström et al., 2010; Taff and Townsend, 2017). For example, in the study conducted by Taff and Townsend (2017), who compared crows infected with C. jejuni with uninfected crows with the aim of assessing its impact on body condition and survival of crows, the infected crows were found in poor body condition compared to uninfected crows.
Among Campylobacter spp., C. jejuni is the most frequently detected species in several wild bird species, as demonstrated by several researchers (Dipineto et al., 2014; Weis et al., 2014; Krawiec et al., 2017; Gargiulo et al., 2018; Antilles et al., 2021; Indykiewicz et al., 2021; Mencía-Gutiérrez et al., 2021). For example, in one study conducted in Italy, C. jejuni was detected in all isolates (49/49) from birds of prey, and C. jejuni and C. coli (mixed infections) were detected in 12 isolates with the overall Campylobacter sp. prevalence of 33.1% (49/148) (Gargiulo et al., 2018). However, most of the researchers target the thermophilic species due to their public health importance (Table 1), and thus, it seems that attention was not given to other species. A large-scale study by Johansson et al. (2018) assessed the status of Campylobacter in various wild birds (covering a total of 2,278 birds) in the remote area of the Antarctic and sub-Antarctic regions and reported different Campylobacter spp. like C. peloridis, C. subantarcticus, and C. volucris, in addition to C. jejuni and C. lari.
Russo et al. (2021) examined 225 cloacal swabs of yellow-legged gulls (Larus michahellis) in their study conducted in Italy and reported that 60 gulls (26.7%) were carriers of Campylobacter. Antilles et al. (2021) reported a Campylobacter carrier rate of 5.2% (93/1,785) in 1,785 cloacal swabs collected from gulls. In one study conducted in Iran, a relatively higher prevalence was reported in black-headed gulls (63.3%) and starlings (56.6%) compared to other bird species (Malekian et al., 2021). Another study from Turkey examined 183 cloacal swabs obtained from different species of wild birds and found a relatively higher prevalence (93%) in Eurasian coots (Fulica atra) compared to other birds (Kürekci et al., 2021). The study by Mencía-Gutiérrez et al. (2021) analyzed 689 bird of prey samples and reported a 7.5% prevalence. Another study from Poland investigated the Campylobacter prevalence in cloacal samples collected from black-headed gulls (718 adult and 318 chicks) and reported 4.87% (35/718) and 2.22% (7/318) prevalence in adults and chicks, respectively. This study found a non-significant difference among the age groups (adults and chicks) and birds’ habitats (urban and rural). However, a significant difference was found between breeding seasons (Indykiewicz et al., 2021). Broman et al. (2002), who compared the prevalences of C. jejuni isolated from juvenile and adult black-headed gulls, and Mencía-Gutiérrez et al. (2021), who assessed Campylobacter spp. prevalence difference among different age groups of birds of prey, also reported non-significant differences.
The prevalence variation among wild birds may be due to different factors such as location, season, wild bird species, sample type, the method used, ecological factors, and the health status of birds (Mohan et al., 2013; Cody et al., 2015; Mencía-Gutiérrez et al., 2021). For example, the distribution of Campylobacter may differ among wild bird species depending on ecological factors like their feeding habits and pattern of migration (Waldenström et al., 2002; Hald et al., 2016; Gargiulo et al., 2018). To support this argument, researchers have proved that wild birds that eat animal-origin food or forage on the ground near animal farms have a higher risk of acquiring Campylobacter than those foraging far away from the animal’s farm or hunt in the air (Hald et al., 2016). The differences between diurnal and nocturnal birds have also been assessed in several studies (Waldenström et al., 2002; Krawiec et al., 2017; Gargiulo et al., 2018). Gargiulo et al. (2018) compared diurnal and nocturnal birds and reported a statistically significant difference in the prevalence of Campylobacter species among diurnal (39.1%) and nocturnal (18.6%) birds. In contrast, the study conducted in Poland (Krawiec et al., 2017), in Sweden (Waldenström et al., 2002), and in Spain (Mencía-Gutiérrez et al., 2021) reported a higher prevalence in nocturnal birds. The study from New Zealand, which reported a significantly higher Campylobacter spp. prevalence in starlings (46%) than ducks (30%), documented a relatively higher prevalence during the spring and winter months than summer (Mohan et al., 2013). In agreement with this, Mencía-Gutiérrez et al. (2021) reported a significantly higher prevalence in samples they collected during the spring season. In contrast, Hald et al. (2016) reported a significantly higher prevalence during the summer season than the winter season.
Drug Resistance Status
Antibiotic resistance, one of the growing global public health concerns (World Health Organization [WHO], 2020), has also been reported in Campylobacter isolates of wild bird origin (Table 1). Currently, several researchers have reported Campylobacter sp. resistance to different antibiotics (especially tetracycline and fluoroquinolones) (Wei et al., 2015; Jurado-Tarifa et al., 2016; Indykiewicz et al., 2021; Mencía-Gutiérrez et al., 2021; Russo et al., 2021). Multidrug resistance (MDR) Campylobacter isolates have been documented frequently (Wei et al., 2015; Du et al., 2019). Du et al. (2019) found 33.3% MDR in Campylobacter isolated from wild birds, with varying degrees of resistance to antibiotics like streptomycin, tetracycline, gentamicin, and clindamycin at rates of 36.84, 29.82, 29.82, and 28.07%, respectively. Antilles et al. (2021) also found 16.1% resistance to tetracycline in Campylobacter isolates isolated from gulls. In another study by Marotta et al. (2019), relatively higher resistance to tetracycline (19.40%) was recorded, with ciprofloxacin, nalidixic acid, and streptomycin resistance rates being 13.43, 10.45, and 10.45%, respectively.
A recent study by Mencía-Gutiérrez et al. (2021) reported resistance to nalidixic acid, ciprofloxacin, tetracycline, and streptomycin at the rates of 68.9, 68.9, 55.6, and 6.7%, respectively. However, a promising susceptibility to azithromycin (97.62%) and erythromycin (95.24%) was detected in a study conducted in Poland, in which only 50% resistance to tetracycline and 47.62% resistance to ciprofloxacin was reported (Indykiewicz et al., 2021). Similarly, the study from Lithuania reported 87.1% resistance to ciprofloxacin (Aksomaitiene et al., 2019). Another study from Italy reported resistance to tetracycline (12.5%), nalidixic acid (10%), ciprofloxacin (10%), streptomycin (6.7%), and erythromycin (4.2%) (Marotta et al., 2020). To sum up, the antibiotic resistance pattern of Campylobacter in wild birds seems under-investigated, and thus, further studies are warranted. However, as stated above, visible resistance to some antibiotics like tetracycline and fluoroquinolones is increasingly being reported.
Virulence and Pathogenicity
Even though the pathogenesis of Campylobacter infection is not fully elucidated, several mechanisms are postulated to be involved (Asuming-Bediako et al., 2019), and virulence factors such as adhesion, bacterial invasion, and production of toxin are believed to have a role in its pathogenesis in humans (Kreling et al., 2020). Different virulence genes such as cytolethal distending toxin (CDT) genes, flaA, flaB, ciaB, and cadF have been documented in studies conducted with the aim of understanding the virulence of Campylobacter spp. isolated from the wild birds (Shyaka et al., 2015; Du et al., 2019). The genes encoding CDT (for example, cdtA, cdtB, and cdtC), the only toxin known to be produced by Campylobacter (Kreling et al., 2020), are frequently reported in wild birds (Weis et al., 2014; Shyaka et al., 2015). This toxin has DNAse activity that causes DNA damage (Kreling et al., 2020). As in other bacteria, adhesion to host epithelial cells is known to have a significant role in the pathogenesis of Campylobacter. However, unlike other bacteria (e.g., E. coli and Salmonella), fimbria does not mediate adhesion in the case of Campylobacter (Rubinchik et al., 2012), and the best-known adhesins in this bacterium are Campylobacter adhesion protein fibronectin (CadF) (Bolton, 2015; Kreling et al., 2020). This gene has also been reported in various wild bird species from countries like China (Du et al., 2019), Poland (Krawiec et al., 2017), and Japan (Shyaka et al., 2015). As discussed above, wild birds are considered to act as carriers, with the exception of general signs reported in some species (Taff and Townsend, 2017) that warrant further study, and information is scarce regarding clinical signs, pathogenicity, and pathology of Campylobacter infection in wild birds.
Public and Animal Health Significance
Given the public health significance of Campylobacter, several studies that target comparing the genetic similarity of Campylobacter strains obtained from wild birds with strains circulating among poultry, humans, and other animals have been conducted (Broman et al., 2002, 2004; Waldenström et al., 2007; Colles et al., 2008, 2011; Griekspoor et al., 2013; Wei et al., 2019; Marotta et al., 2020; Zbrun et al., 2021). The results of some of these studies show high levels of host-specific strains (Broman et al., 2002, 2004; Waldenström et al., 2007; Messens et al., 2009; Griekspoor et al., 2013; Marotta et al., 2020). In contrast, some also report that Campylobacter spp. isolated from wild birds share similarities [e.g., sequence types (ST)] with those isolated from humans (French et al., 2009; Wei et al., 2019) and domestic animals (Sippy et al., 2012; Zbrun et al., 2021) and thus may serve as sources of drug-resistant potentially important pathogens even for humans (Cody et al., 2015; Mencía-Gutiérrez et al., 2021).
A molecular study by Colles et al. (2008) compared Campylobacter strains obtained from the wild bird (geese) with starlings and poultry populations using MLST and reported a high host specificity of C. jejuni genotypes obtained from wild geese. Broman et al. (2002) also investigated the genetic similarities between broiler (36 isolates), black-headed gull (Larus ridibundus) (76 isolates), and human (56 isolates) C. jejuni isolates in the same geographical region using PFGE. Their results showed a higher similarity profile between isolates obtained from humans and broiler when compared to isolates of humans and wild bird origin. However, they also emphasized that they found the same macrorestriction profile in 2 gull isolates and 1 human isolate. The result of another study conducted in Switzerland that compared the genetic similarity of C. jejuni isolates from migratory birds (89 isolates) and humans (47 isolates) showed that most of the strains from the migratory bird isolates were not related to the human strains, except the starling and blackbird strains, which showed similarity to some human strains (Broman et al., 2004).
The results of a large study covering 2,084 wild birds in the United Kingdom stated that the transmission pathway of Campylobacter is predominantly from farm animals to wild birds. In this study, 36 C. jejuni isolates were characterized by MLST, and the results showed that wild birds harbor both farm-related and unique C. jejuni strains. Nonetheless, the study did not witness wild bird-specific C. jejuni strains in farm animals (Hughes et al., 2009). Messens et al. (2009) also characterized C. jejuni obtained from wild birds and broilers and reported that the wild bird origin C. jejuni strains are different from broilers.
Unlike the above studies, a study conducted in South Korea has performed the genotypic analysis of Campylobacter species obtained from wild birds using MLST and reported ST similarity among humans and wild wilds (11 C. jejuni ST and 2 C. coli STs shown to be the same to those of human origin). The results of this study highlighted as Campylobacter isolated from wild birds are associated with domestic animal and environmental strains (Wei et al., 2019). A recent study by Zbrun et al. (2021) reported a genotypic similarity between Campylobacter isolated from broilers and wild birds, highlighting the possible role of wild birds in sustaining the epidemiology of this pathogen on farms. The same conclusion was made by Hald et al. (2016).
It has also been shown by Sippy et al. (2012) that wild birds carry Campylobacter isolates that share similarities with the Campylobacter strain known to be pathogenic for livestock. Similarly, in another study conducted in China, phylogenetic analysis of C. jejuni strains in different species of wild bird was performed, and it was determined that wild birds share the same ST with human-origin C. jejuni, indicating that this bacterium may be transmissible between different species (Du et al., 2019).
In a study conducted in Alaska, United States, C. jejuni isolates obtained from sick humans, environment, and wild birds during an outbreak of human campylobacteriosis in association with consumption of raw peas showed an indistinguishable PFGE, and the outbreak was linked with contamination from wild bird feces (Gardner et al., 2011). The outbreak of C. jejuni infection in children has also been linked to drinking “milk from bottles with bird-pecked tops” (Riordan et al., 1993). In another study from the United Kingdom, researchers investigated the role of wild birds as the source of human Campylobacter infection for nearly 10 years and found that wild birds accounted for 476 (2.1%) to 543 (3.5%) human cases per year (Cody et al., 2015).
As French et al. (2009) noted, the likely route of bird-to-human transmission can be equipment or surface contamination with wild birds’ fecal material (like in parks and children’s playgrounds). In this case, young children are more likely to be at risk because of frequent hand–mouth contact, which may expose them to swallowing infective material (French et al., 2009). In addition, wild birds are shown to be one of the leading sources of surface water contamination with Campylobacter spp. In a study conducted in the Netherlands that linked more than 90% of recreational water-origin Campylobacter isolates to wild birds, the risk of Campylobacter transmission by swimming in recreational water areas was emphasized (Mulder et al., 2020). In another study conducted in Canada that compared the similarity between Campylobacter strains (C. lari) isolated from river water and waterfowl, 100% homology was reported, and the likely risk of surface water contamination due to waterfowl was highlighted (Van Dyke et al., 2010). The study from Finland pointed out that swimming in natural water is independently related to sporadic campylobacteriosis (Schönberg-Norio et al., 2004). A similar finding was documented in a recent study by Mughini-Gras et al. (2021), who indicated that open-water swimming areas are a risk factor for human Campylobacter infections. Outbreaks of human campylobacteriosis that occurred in Norway in 1994 and 1995 were also suspected to be associated with drinking water contaminated with pink-footed geese feces (Varslot et al., 1996).
In summary, what we understand from research done so far seems controversial and inadequate to draw a complete conclusion about the risk of interspecies transmission of Campylobacter and warrants further comprehensive epidemiological investigations. As described in a framework proposed by Smith et al. (2020), criteria such as bacterial shedding pattern and bacterial survival in the environment need to be elucidated to better understand the possibility of transmission from wild birds to other hosts. Nevertheless, the detection of Campylobacter in wild birds is not neglectable from a public and animal health point of view. The main reason for this can be the isolation of drug-resistant Campylobacter species from various wild birds (Wei et al., 2015; Aksomaitiene et al., 2019; Du et al., 2019; Mencía-Gutiérrez et al., 2021). Furthermore, the results of the source attribution studies discussed above deserve public health attention. Therefore, despite the existing controversiality and the necessity of future studies, the detection of this pathogen in wild birds demonstrates their reservoir potential and the transmission of antibiotic-resistant pathogenic Campylobacter to domestic animals, and they may also play a role in Campylobacter transmission to humans by causing environmental contamination that may threaten public health (Gardner et al., 2011; Sippy et al., 2012; Du et al., 2019; Wei et al., 2019). To break the transmission chain, possible prevention and control interventions should target each transmission stage, and multi-sectoral collaborative epidemiological studies should be employed to monitor potential reservoirs using modern molecular techniques continuously.
Conclusion and Future Perspectives
Currently, various wild bird species have been proven to be significant natural reservoirs of thermophilic Campylobacter species. In particular, C. jejuni, one of the major causes of foodborne infections worldwide, is the most frequently isolated species. Despite their reservoir role, the wild bird’s ability to transmit this pathogen to another host is not fully elucidated yet. Some studies have found overlapping strains among human, domestic animal, and wild bird isolates, while others have found significant differences in Campylobacter population structure among these hosts. In addition to pathogen detection, drug-resistant Campylobacter isolates, particularly resistance to tetracycline and fluoroquinolones, are documented. Source attribution studies have also linked human cases of Campylobacter infections to wild birds. Therefore, the role of wild birds in the epidemiology of Campylobacter should not be undermined. The currently available literature has focused on bacterial detection and, to some extent, antimicrobial resistance and comparative analysis of pathogen population structure in animals and humans. However, in order to determine disease status in wild birds and the precise role of wild birds in domestic animals and human health, detail-oriented molecular epidemiological studies characterizing the genetic relatedness of isolates from the respective species and environment through one health approach are warranted. In addition, determining bacterial survival in the environment, infective dose, and pathogen shading patterns in various bird species may play an essential role in clarifying the possibility of interspecies transmission. The study focusing on the clinical patterns of Campylobacter in infected wild birds also deserves attention from a conservation perspective.
Author Contributions
NA: conceptualization, collecting the available data, and writing. TG: conceptualization and writing. Both authors have read and approved final version for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abulreesh, H. H., Paget, T. A., and Goulder, R. (2006). Campylobacter in waterfowl and aquatic environments: incidence and methods of detection. Environ. Sci. Technol. 40, 7122–7131. doi: 10.1021/es060327l
Aksomaitiene, J., Ramonaite, S., Tamuleviciene, E., Novoslavskij, A., Alter, T., and Malakauskas, M. (2019). Overlap of antibiotic resistant Campylobacter jejuni MLST genotypes isolated from humans, broiler products, dairy cattle and wild birds in Lithuania. Front. Microbiol. 10:1377. doi: 10.3389/fmicb.2019.01377
Al Amri, A., Senok, A. C., Ismaeel, A. Y., Al-Mahmeed, A. E., and Botta, G. A. (2007). Multiplex PCR for direct identification of Campylobacter spp. in human and chicken stools. J. Med. Microbiol. 56, 1350–1355. doi: 10.1099/jmm.0.47220-0
Antilles, N., García-Bocanegra, I., Alba-Casals, A., López-Soria, S., Pérez-Méndez, N., Saco, M., et al. (2021). Occurrence and antimicrobial resistance of zoonotic enteropathogens in gulls from southern Europe. Sci. Total Environ. 763:143018. doi: 10.1016/j.scitotenv.2020.143018
Antilles, N., Sanglas, A., and Cerdà-Cuéllar, M. (2015). Free-living Waterfowl as a Source of Zoonotic Bacteria in a Dense Wild Bird Population Area in Northeastern Spain. Transbound. Emerg. Dis. 62, 516–521. doi: 10.1111/tbed.12169
Asuming-Bediako, N., Kunadu, A. P. H., Abraham, S., and Habib, I. (2019). Campylobacter at the Human-Food Interface: the African Perspective. Pathogens 8:87. doi: 10.3390/pathogens8020087
Benskin, C. M. W. H., Wilson, K., Jones, K., and Hartley, I. R. (2009). Bacterial pathogens in wild birds: a review of the frequency and effects of infection. Biol. Rev. 84, 349–373. doi: 10.1111/j.1469-185X.2008.00076.x
Bessè De, E., Solecki, O., Sifré, E., Labadi, L., and Mé Graud, F. (2011). Identification of Campylobacter species and related organisms by matrix assisted laser desorption ionization†“time of flight (MALDI-TOF) mass spectrometry. Clin. Microbiol. Infect. 17, 1735–1739. doi: 10.1111/j.1469-0691.2011.03468.x
Bolton, D. J. (2015). Campylobacter virulence and survival factors. Food Microbiol. 48, 99–108. doi: 10.1016/j.fm.2014.11.017
Broman, T., Palmgren, H., Bergström, S., Sellin, M., Waldenström, J., Danielsson-Tham, M. L., et al. (2002). Campylobacter jejuni in black-headed gulls (Larus ridibundus): prevalence, genotypes, and influence on C. jejuni epidemiology. J. Clin. Microbiol. 40, 4594–4602. doi: 10.1128/JCM.40.12.4594-4602.2002
Broman, T., Waldenström, J., Dahlgren, D., Carlsson, I., Eliasson, I., and Olsen, B. (2004). Diversities and similarities in PFGE profiles of Campylobacter jejuni isolated from migrating birds and humans. J. Appl. Microbiol. 96, 834–843. doi: 10.1111/j.1365-2672.2004.02232.x
Butzler, J. P. (2004). Campylobacter, from obscurity to celebrity. Clin. Microbiol. Infect. 10, 868–876. doi: 10.1111/j.1469-0691.2004.00983.x
Clark, L. (2014). “Disease Risks Posed by Wild Birds Associated with Agricultural Landscapes,” in The Produce Contamination Problem: Causes and Solutions: Second Edition, eds C. P. G. K. R. Matthews and G. M. Sapers (Cambridge: Academic Press), 139–165. doi: 10.1016/B978-0-12-404611-5.00007-5
Cody, A. J., McCarthy, N. D., Bray, J. E., Wimalarathna, H. M. L., Colles, F. M., Rensburg, M. J. J., et al. (2015). Wild bird-associated Campylobacter jejuni isolates are a consistent source of human disease, in Oxfordshire, United Kingdom. Environ. Microbiol. Rep. 7:782. doi: 10.1111/1758-2229.12314
Colles, F. M., Ali, J. S., Sheppard, S. K., McCarthy, N. D., and Maiden, M. C. J. (2011). Campylobacter populations in wild and domesticated Mallard ducks (Anas platyrhynchos). Environ. Microbiol. Rep. 3:574. doi: 10.1111/j.1758-2229.2011.00265.x
Colles, F. M., Dingle, K. E., Cody, A. J., and Maiden, M. C. J. (2008). Comparison of Campylobacter populations in wild geese with those in starlings and free-range poultry on the same farm. Appl. Environ. Microbiol. 74, 3583–3590. doi: 10.1128/AEM.02491-07
Dhama, K., Mahendran, M., and Tomar, S. (2008). Pathogens transmitted by migratory birds: threat perceptions to poultry health and production. Int. J. Poult. Sci. 7, 516–525. doi: 10.3923/ijps.2008.516.525
Dipineto, L., Russo, T. P., Gargiulo, A., Borrelli, L., De Luca Bossa, L. M., Santaniello, A., et al. (2014). Prevalence of enteropathogenic bacteria in common quail (Coturnix coturnix). Avian Pathol. 43, 498–500. doi: 10.1080/03079457.2014.966055
Du, J., Luo, J., Huang, J., Wang, C., Li, M., Wang, B., et al. (2019). Emergence of genetic diversity and multi-drug resistant campylobacter jejuni from wild birds in beijing, china. Front. Microbiol. 10:2433. doi: 10.3389/fmicb.2019.02433
Dudzic, A., Urban-Chmiel, R., Stêpień-Pyśniak, D., Dec, M., Puchalski, A., and Wernicki, A. (2016). Isolation, identification and antibiotic resistance of Campylobacter strains isolated from domestic and free-living pigeons. Br. Poult. Sci. 57, 172–178. doi: 10.1080/00071668.2016.1148262
Eberle, K. N., and Kiess, A. S. (2012). Phenotypic and genotypic methods for typing Campylobacter jejuni and Campylobacter coli in poultry. Poult. Sci. 91, 255–264. doi: 10.3382/ps.2011-01414
European Food Safety Authority [EFSA] (2010). Scientific Opinion on Quantification of the risk posed by broiler meat to human campylobacteriosis in the EU; Scientific Opinion on Quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 8:1437. doi: 10.2903/j.efsa.2010.1437
European Food Safety Authority [EFSA], and European Centre for Disease Prevention and Control [ECDC] (2021). The European Union One Health 2019 Zoonoses Report. EFSA J. 19:e06406. doi: 10.2903/j.efsa.2021.6406
Elmberg, J., Berg, C., Lerner, H., Waldenström, J., and Hessel, R. (2017). Potential disease transmission from wild geese and swans to livestock, poultry and humans: a review of the scientific literature from a One Health perspective. Infect. Ecol. Epidemiol. 7:1300450. doi: 10.1080/20008686.2017.1300450
Fernández, H., Gesche, W., Montefusco, A., and Schlatter, R. (1996). Wild birds as reservoir of thermophilic enteropathogenic Campylobacter species in southern Chile. Mem. Inst. Oswaldo Cruz 91, 699–700. doi: 10.1590/S0074-02761996000600007
Ferone, M., Gowen, A., Fanning, S., and Scannell, A. G. M. (2020). Microbial detection and identification methods: bench top assays to omics approaches. Compr. Rev. Food Sci. Food Saf. 19, 3106–3129. doi: 10.1111/1541-4337.12618
Franz, E., Gras, L. M., and Dallman, T. (2016). Significance of whole genome sequencing for surveillance, source attribution and microbial risk assessment of foodborne pathogens. Curr. Opin. Food Sci. 8, 74–79. doi: 10.1016/j.cofs.2016.04.004
Frasao, B., da, S., Marin, V. A., and Conte-Junior, C. A. (2017). Molecular Detection, Typing, and Quantification of Campylobacter spp. in Foods of Animal Origin. Compr. Rev. Food Sci. Food Saf. 16, 721–734. doi: 10.1111/1541-4337.12274
French, N. P., Midwinter, A., Holland, B., Collins-Emerson, J., Pattison, R., Colles, F., et al. (2009). Molecular Epidemiology of Campylobacter jejuni Isolates from Wild-Bird Fecal Material in Children’s Playgrounds. Appl. Environ. Microbiol. 75:779. doi: 10.1128/AEM.01979-08
Fukuyama, M., Kamimura, T., Itoh, T., Saito, K., Takahashi, M., Sakai, S., et al. (1986). Distribution of Campylobacter jejuni in wild birds and serogroup of isolates by slide agglutination technique. Jpn. J. Vet. Sci. 48, 487–493. doi: 10.1292/jvms1939.48.487
Gardner, T. J., Fitzgerald, C., Xavier, C., Klein, R., Pruckler, J., Stroika, S., et al. (2011). Outbreak of Campylobacteriosis Associated With Consumption of Raw Peas. Clin. Infect. Dis. 53, 26–32. doi: 10.1093/cid/cir249
Gargiulo, A., Fioretti, A., Russo, T. P., Varriale, L., Rampa, L., Paone, S., et al. (2018). Occurrence of enteropathogenic bacteria in birds of prey in Italy. Lett. Appl. Microbiol. 66, 202–206. doi: 10.1111/lam.12836
Gargiulo, A., Russo, T. P., Schettini, R., Mallardo, K., Calabria, M., Menna, L. F., et al. (2014). Occurrence of enteropathogenic bacteria in Urban pigeons (Columba livia) in Italy. Vector Borne Zoonotic Dis. 14, 251–255. doi: 10.1089/vbz.2011.0943
Griekspoor, P., Colles, F. M., McCarthy, N. D., Hansbro, P. M., Ashhurst-Smith, C., Olsen, B., et al. (2013). Marked host specificity and lack of phylogeographic population structure of Campylobacter jejuni in wild birds. Mol. Ecol. 22, 1463–1472. doi: 10.1111/mec.12144
Hald, B., Skov, M. N., Nielsen, E. M., Rahbek, C., Madsen, J. J., Wainø, M., et al. (2016). Campylobacter jejuni and Campylobacter coli in wild birds on Danish livestock farms. Acta Vet. Scand. 58:11. doi: 10.1186/s13028-016-0192-9
Havelaar, A. H., Kirk, M. D., Torgerson, P. R., Gibb, H. J., Hald, T., Lake, R. J., et al. (2015). World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 12:1001923. doi: 10.1371/journal.pmed.1001923
Hlashwayo, D. F., Sigaúque, B., and Bila, C. G. (2020). Epidemiology and antimicrobial resistance of Campylobacter spp. in animals in Sub-Saharan Africa: a systematic review. Heliyon 6:e03537. doi: 10.1016/j.heliyon.2020.e03537
Hughes, L. A., Bennett, M., Coffey, P., Elliott, J., Jones, T. R., Jones, R. C., et al. (2009). Molecular epidemiology and characterization of Campylobacter spp. isolated from wild bird populations in northern england. Appl. Environ. Microbiol. 75, 3007–3015. doi: 10.1128/AEM.02458-08
Humphrey, T., O’Brien, S., and Madsen, M. (2007). Campylobacters as zoonotic pathogens: a food production perspective. Int. J. Food Microbiol. 117, 237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006
Igwaran, A., and Okoh, A. I. (2019). Human campylobacteriosis: a public health concern of global importance. Heliyon 5:e02814. doi: 10.1016/j.heliyon.2019.e02814
Indykiewicz, P., Andrzejewska, M., Minias, P., Śpica, D., and Kowalski, J. (2021). Prevalence and Antibiotic Resistance of Campylobacter spp. in Urban and Rural Black-Headed Gulls Chroicocephalus ridibundus. EcoHealth 18, 147–156. doi: 10.1007/s10393-021-01540-0
Ito, K., Kubokura, Y., Kaneko, K., Totake, Y., and Ogawa, M. (1988). Occurrence of Campylobacter jejuni in free-living wild birds from Japan. J. Wildl. Dis. 24, 467–470. doi: 10.7589/0090-3558-24.3.467
Johansson, H., Ellström, P., Artursson, K., Berg, C., Bonnedahl, J., Hansson, I., et al. (2018). Characterization of Campylobacter spp. isolated from wild birds in the Antarctic and Sub-Antarctic. PLoS One 13:e0206502. doi: 10.1371/journal.pone.0206502
Jurado-Tarifa, E., Torralbo, A., Borge, C., Cerdà-Cuéllar, M., Ayats, T., Carbonero, A., et al. (2016). Genetic diversity and antimicrobial resistance of Campylobacter and Salmonella strains isolated from decoys and raptors. Comp. Immunol. Microbiol. Infect. Dis. 48, 14–21. doi: 10.1016/j.cimid.2016.07.003
Jurinoviæ, L., Duvnjak, S., Kompes, G., Šoprek, S., Šimpraga, B., Krstuloviæ, F., et al. (2020). Occurrence of Campylobacter jejuni in Gulls Feeding on Zagreb Rubbish Tip, Croatia; Their Diversity and Antimicrobial Susceptibility in Perspective with Human and Broiler Isolates. Pathogens 9:695. doi: 10.3390/pathogens9090695
Kaakoush, N. O., Castaño-Rodríguez, N., Mitchell, H. M., and Man, S. M. (2015). Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 28, 687–720. doi: 10.1128/CMR.00006-15
Kapperud, G., and Rosef, O. (1983). Avian wildlife reservoir of Campylobacter fetus subsp. jejuni, Yersinia spp., and Salmonella spp. in Norway. Appl. Environ. Microbiol. 45, 375–380. doi: 10.1128/aem.45.2.375-380.1983
Keller, J. I., Gregory Shriver, W., Waldenström, J., Griekspoor, P., and Olsen, B. (2011). Prevalence of Campylobacter in wild birds of the mid-atlantic region, USA. J. Wildl. Dis. 47, 750–754. doi: 10.7589/0090-3558-47.3.750
Keller, J. I., and Shriver, W. G. (2014). Prevalence of three Campylobacter species, C. jejuni, C. coli, and C. lari, using multilocus sequence typing in wild birds of the mid-atlantic region, USA. J. Wildl. Dis. 50, 31–41. doi: 10.7589/2013-06-136
Kinjo, T., Morishige, M., Minamoto, N., and Fukushi, H. (1983). Prevalence of Campylobacter jejuni in feral pigeons. Jpn. J. Vet. Sci. 45, 833–835. doi: 10.1292/jvms1939.45.833
Konicek, C., Vodrážka, P., Barták, P., Knotek, Z., Hess, C., Raèka, K., et al. (2016). Detection of zoonotic pathogens in wild birds in the cross-border region Austria –Czech Republic. J. Wildl. Dis. 52, 850–861. doi: 10.7589/2016-02-038
Krawiec, M., Woźniak-Biel, A., Bednarski, M., and Wieliczko, A. (2017). Antimicrobial Susceptibility and Genotypic Characteristic of Campylobacter spp. Isolates from Free-Living Birds in Poland. Vector Borne Zoonotic Dis. 17, 755–763. doi: 10.1089/vbz.2017.2116
Kreling, V., Falcone, F. H., Kehrenberg, C., and Hensel, A. (2020). Campylobacter sp.: pathogenicity factors and prevention methods—new molecular targets for innovative antivirulence drugs? Appl. Microbiol. Biotechnol. 104, 10409–10436. doi: 10.1007/s00253-020-10974-5
Kürekci, C., Sakin, F., Epping, L., Knüver, M.-T., Semmler, T., and Stingl, K. (2021). Characterization of Campylobacter spp. Strains Isolated From Wild Birds in Turkey. Front. Microbiol. 12:712106. doi: 10.3389/fmicb.2021.712106
Kwan, P. S. L., Xavier, C., Santovenia, M., Pruckler, J., Stroika, S., Joyce, K., et al. (2014). Multilocus sequence typing confirms wild birds as the source of a Campylobacter outbreak associated with the consumption of raw peas. Appl. Environ. Microbiol. 80, 4540–4546. doi: 10.1128/AEM.00537-14
Kwon, Y. K., Oh, J. Y., Jeong, O. M., Moon, O. K., Kang, M. S., Jung, B. Y., et al. (2017). Prevalence of Campylobacter species in wild birds of South Korea. Avian Pathol. 46, 474–480. doi: 10.1080/03079457.2017.1315048
Lawton, S. J., Weis, A. M., Byrne, B. A., Fritz, H., Taff, C. C., Townsend, A. K., et al. (2018). Comparative analysis of Campylobacter isolates fromwild birds and chickens using MALDI-TOF MS, biochemical testing, and DNAsequencing. J. Vet. Diagn. Invest. 30, 354–361. doi: 10.1177/1040638718762562
Levesque, S., Fournier, E., Carrier, N., Frost, E., Arbeit, R. D., and Michaud, S. (2013). Campylobacteriosis in urban versus rural areas: a case-case study integrated with molecular typing to validate risk factors and to attribute sources of infection. PLoS One 8:e83731. doi: 10.1371/journal.pone.0083731
LPSN. Genus Campylobacter. Available online at: https://www.bacterio.net/genus/campylobacter (accessed November 20, 2021).
Luechtefeld, N. A. W., Blaser, M. J., Reller, L. B., and Wang, W. L. L. (1980). Isolation of Campylobacter fetus subsp. jejuni from migratory waterfowl. J. Clin. Microbiol. 12:406. doi: 10.1128/jcm.12.3.406-408.1980
Maësaar, M., Tedersoo, T., Meremaë, K., and Roasto, M. (2020). The source attribution analysis revealed the prevalent role of poultry over cattle and wild birds in human campylobacteriosis cases in the Baltic States. PLoS One 15:e0235841. doi: 10.1371/journal.pone.0235841
Malekian, M., Shagholian, J., and Hosseinpour, Z. (2021). Pathogen Presence in Wild Birds Inhabiting Landfills in Central Iran. EcoHealth 18, 76–83. doi: 10.1007/s10393-021-01516-0
Marin, C., Palomeque, M. D., Marco-Jiménez, F., and Vega, S. (2014). Wild griffon vultures (Gyps fulvus) as a source of Salmonella and Campylobacter in eastern Spain. PLoS One 9:e94191. doi: 10.1371/journal.pone.0094191
Marotta, F., Garofolo, G., Di Marcantonio, L., Di Serafino, G., Neri, D., Romantini, R., et al. (2019). Antimicrobial resistance genotypes and phenotypes of Campylobacter jejuni isolated in Italy from humans, birds from wild and urban habitats, and poultry. PLoS One 14:e0223804. doi: 10.1371/journal.pone.0223804
Marotta, F., Janowicz, A., Marcantonio, L., Di Ercole, C., Donato, G., Di Garofolo, G., et al. (2020). Molecular Characterization and Antimicrobial Susceptibility of C. jejuni Isolates from Italian Wild Bird Populations. Pathogens 9:304. doi: 10.3390/pathogens9040304
Mencía-Gutiérrez, A., Martín-Maldonado, B., Pastor-Tiburón, N., Moraleda, V., González, F., García-Peña, F. J., et al. (2021). Prevalence and Antimicrobial Resistance of Campylobacter from Wild Birds of Prey in Spain. Comp. Immunol. Microbiol. Infect. Dis. 79:101712. doi: 10.1016/j.cimid.2021.101712
Messens, W., Herman, L., De Zutter, L., and Heyndrickx, M. (2009). Multiple typing for the epidemiological study of contamination of broilers with thermotolerant Campylobacter. Vet. Microbiol. 138, 120–131. doi: 10.1016/j.vetmic.2009.02.012
Mohan, V. (2015). Faeco-prevalence of Campylobacter jejuni in urban wild birds and pets in New Zealand. BMC Res. Notes 8:1. doi: 10.1186/1756-0500-8-1
Mohan, V., Stevenson, M., Marshall, J., Fearnhead, P., Holland, B. R., Hotter, G., et al. (2013). Campylobacter jejuni colonization and population structure in urban populations of ducks and starlings in New Zealand. Microbiologyopen 2, 659–673. doi: 10.1002/mbo3.102
Molina-Lopez, R. A., Valverdú, N., Martin, M., Mateu, E., Obon, E., Cerdà-Cuéllar, M., et al. (2011). Wild raptors as carriers of antimicrobial-resistant Salmonella and Campylobacter strains. Vet. Rec. 168:565. doi: 10.1136/vr.c7123
Moore, J. E., Gilpin, D., Crothers, E., Canney, A., Kaneko, A., and Matsuda, M. (2002). Occurrence of Campylobacter spp. and Cryptosporidium spp. in seagulls (Larus spp.). Vector Borne Zoonotic Dis. 2, 111–114. doi: 10.1089/153036602321131913
Moré, E., Ayats, T., Ryan, P. G., Naicker, P. R., Keddy, K. H., Gaglio, D., et al. (2017). Seabirds (Laridae) as a source of Campylobacter spp., Salmonella spp. and antimicrobial resistance in South Africa. Environ. Microbiol. 19, 4164–4176. doi: 10.1111/1462-2920.13874
Mughini-Gras, L., Pijnacker, R., Coipan, C., Mulder, A. C., Veludo, A. F., de Rijk, S., et al. (2021). Sources and transmission routes of campylobacteriosis: a combined analysis of genome and exposure data. J. Infect. 82, 216–226. doi: 10.1016/j.jinf.2020.09.039
Mulder, A. C., Franz, E., de Rijk, S., Versluis, M. A. J., Coipan, C., Buij, R., et al. (2020). Tracing the animal sources of surface water contamination with Campylobacter jejuni and Campylobacter coli. Water Res. 187:116421. doi: 10.1016/j.watres.2020.116421
Muralidharan, M., Ghosh, A., Singhvi, N., Dhanaraj, P. S., Lal, R., Dutt Patel, D., et al. (2016). Identification of Genus Campylobacter up to Species Level Using Internal Features of 16S rRNA Gene Sequences. Mol. Genet. Microbiol. 31, 187–196. doi: 10.3103/S0891416816030071
Navarro-Gonzalez, N., Ugarte-Ruiz, M., Domínguez, L., and Ruiz-Fons, F. (2016). “A European Perspective on the Transmission of Foodborne Pathogens at the Wildlife–Livestock–Human Interface,” in Food Safety Risks from Wildlife, eds M. Jay-Russell and M. Doyle (Cham: Springer) 59–88. doi: 10.1007/978-3-319-24442-6_3
Prince Milton, A. A., Agarwal, R. K., Priya, G. B., Saminathan, M., Aravind, M., Reddy, A., et al. (2017). Prevalence of Campylobacter jejuni and Campylobacter coli in captive wildlife species of India. Iran. J. Vet. Res. 18, 177–182.
Ramonaitë, S., Novoslavskij, A., Zakarienë, G., Aksomaitienë, J., and Malakauskas, M. (2015). High Prevalence and Genetic Diversity of Campylobacter jejuni in Wild Crows and Pigeons. Curr. Microbiol. 71, 559–565. doi: 10.1007/s00284-015-0881-z
Ricke, S. C., Feye, K. M., Chaney, W. E., Shi, Z., Pavlidis, H., and Yang, Y. (2019). Developments in rapid detection methods for the detection of foodborne campylobacterin the United States. Front. Microbiol. 10:3280. doi: 10.3389/fmicb.2018.03280
Riordan, T., Humphrey, T. J., and Fowles, A. (1993). A point source outbreak of campylobacter infection related to bird-pecked milk. Epidemiol. Infect. 110, 261. doi: 10.1017/S0950268800068187
Rubinchik, S., Seddon, A., and Karlyshev, A. V. (2012). Molecular mechanisms and biological role of Campylobacter jejuni attachment to host cells. Eur. J. Microbiol. Immunol. 2, 32–40. doi: 10.1556/EuJMI.2.2012.1.6
Russo, T. P., Pace, A., Varriale, L., Borrelli, L., Gargiulo, A., Pompameo, M., et al. (2021). Prevalence and Antimicrobial Resistance of Enteropathogenic Bacteria in Yellow-Legged Gulls (Larus michahellis) in Southern Italy. Animals 11:275. doi: 10.3390/ani11020275
Sabtu, N., Enoch, D. A., and Brown, N. M. (2015). Antibiotic resistance: what, why, where, when and how? Br. Med. Bull. 116, 105–113. doi: 10.1093/bmb/ldv041
Schönberg-Norio, D., Takkinen, J., Hänninen, M.-L., Katila, M.-L., Kaukoranta, S.-S., Mattila, L., et al. (2004). Swimming and Campylobacter Infections1. Emerg. Infect. Dis. 10;1474. doi: 10.3201/eid1008.030924
Sheppard, S. K., and Maiden, M. C. J. (2015). The Evolution of Campylobacter jejuni and Campylobacter coli. Cold Spring Harb. Perspect. Biol. 7:a018119. doi: 10.1101/cshperspect.a018119
Shyaka, A., Kusumoto, A., Chaisowwong, W., Okouchi, Y., Fukumoto, S., Yoshimura, A., et al. (2015). Virulence characterization of Campylobacter jejuni isolated from resident wild birds in Tokachi area, Japan. J. Vet. Med. Sci. 77, 967–972. doi: 10.1292/jvms.15-0090
Silva, J., Leite, D., Fernandes, M., Mena, C., Gibbs, P. A., and Teixeira, P. (2011). Campylobacter spp. As a foodborne pathogen: a review. Front. Microbiol. 2:200. doi: 10.3389/fmicb.2011.00200
Sippy, R., Sandoval-Green, C. M. J., Sahin, O., Plummer, P., Fairbanks, W. S., Zhang, Q., et al. (2012). Occurrence and molecular analysis of Campylobacter in wildlife on livestock farms. Vet. Microbiol. 157, 369–375. doi: 10.1016/j.vetmic.2011.12.026
Smith, O. M., Snyder, W. E., and Owen, J. P. (2020). Are we overestimating risk of enteric pathogen spillover from wild birds to humans? Biol. Rev. 95, 652–679. doi: 10.1111/brv.12581
Söderlund, R., Skarin, H., Börjesson, S., Sannö, A., Jernberg, T., Aspán, A., et al. (2019). Prevalence and genomic characteristics of zoonotic gastro-intestinal pathogens and ESBL/pAmpC producing Enterobacteriaceae among Swedish corvid birds. Infect. Ecol. Epidemiol. 9:1701399. doi: 10.1080/20008686.2019.1701399
Taff, C. C., and Townsend, A. K. (2017). Campylobacter jejuni infection associated with relatively poor condition and low survival in a wild bird. J. Avian Biol. 48, 1071–1076. doi: 10.1111/jav.01282
Tong, S., Ma, L., Ronholm, J., Hsiao, W., and Lu, X. (2021). Whole genome sequencing of Campylobacter in agri-food surveillance. Curr. Opin. Food Sci. 39, 130–139. doi: 10.1016/j.cofs.2020.12.020
Van Dyke, M. I., Morton, V. K., McLellan, N. L., and Huck, P. M. (2010). The occurrence of Campylobacter in river water and waterfowl within a watershed in southern Ontario, Canada. J. Appl. Microbiol. 109, 1053–1066. doi: 10.1111/j.1365-2672.2010.04730.x
Vanessa, G. R., Fairbrother, J. H., Donald, T., Harel, J., Côté, N., and Julie, A. (2016). Prevalence and risk factors for Campylobacter spp., Salmonella spp., Coxiella burnetii, and Newcastle disease virus in feral pigeons (Columba livia) in public areas of Montreal, Canada. Can. J. Vet. Res. 80:81.
Varslot, M., Resell, J., and Fostad, I. G. (1996). Vannbaaren campylobacter-infeksjon-trolig foraarsaket av kortnebbgjess. Tidsskr Nor Laegeforen 116, 3366–3369.
Vogt, N. A., Pearl, D. L., Taboada, E. N., Mutschall, S. K., Janecko, N., Reid-Smith, R., et al. (2018). Epidemiology of Campylobacter, Salmonella and antimicrobial resistant Escherichia coli in free-living Canada geese (Branta canadensis) from three sources in southern Ontario. Zoonoses Public Health 65, 873–886. doi: 10.1111/zph.12511
Waldenström, J., Axelsson-Olsson, D., Olsen, B., Hasselquist, D., Griekspoor, P., Jansson, L., et al. (2010). Campylobacter jejuni colonization in wild birds: results from an infection experiment. PLoS One 5:e9082. doi: 10.1371/journal.pone.0009082
Waldenström, J., Broman, T., Carlsson, I., Hasselquist, D., Achterberg, R. P., Wagenaar, J. A., et al. (2002). Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl. Environ. Microbiol. 68, 5911–5917. doi: 10.1128/AEM.68.12.5911-5917.2002
Waldenström, J., On, S. L. W., Ottvall, R., Hasselquist, D., and Olsen, B. (2007). Species diversity of campylobacteria in a wild bird community in Sweden. J. Appl. Microbiol. 102, 424–432. doi: 10.1111/j.1365-2672.2006.03090.x
Wei, B., Cha, S. Y., Kang, M., and Jang, H. K. (2015). Dissemination of multidrug-resistant Campylobacter in wild birds from South Korea. Int. J. Antimicrob. Agents 45, 197–198. doi: 10.1016/j.ijantimicag.2014.10.007
Wei, B., Kang, M., and Jang, H. K. (2019). Genetic characterization and epidemiological implications of Campylobacter isolates from wild birds in South Korea. Transbound. Emerg. Dis. 66, 56–65. doi: 10.1111/tbed.12931
Weis, A. M., Miller, W. A., Byrne, B. A., Chouicha, N., Boyce, W. M., and Townsend, A. K. (2014). Prevalence and pathogenic potential of Campylobacter isolates from free-living, human-commensal American crows. Appl. Environ. Microbiol. 80, 1639–1644. doi: 10.1128/AEM.03393-13
Whelan, C. D., Monaghan, P., Girdwood, R. W. A., and Fricker, C. R. (1988). The significance of wild birds (Larus sp.) in the epidemiology of Campylobacter infections in humans. Epidemiol. Infect. 101, 259–267. doi: 10.1017/S0950268800054170
Whiley, H., van den Akker, B., Giglio, S., and Bentham, R. (2013). The Role of Environmental Reservoirs in Human Campylobacteriosis. Int. J. Environ. Res. Public Health 10:5886. doi: 10.3390/ijerph10115886
World Health Organization [WHO] (2020). Antimicrobial Resistance. Available online at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (Accessed October 18, 2021)
Zbrun, M. V., Rossler, E., Olivero, C. R., Soto, L. P., Zimmermann, J. A., Frizzo, L. S., et al. (2021). Possible reservoirs of thermotolerant Campylobacter at the farm between rearing periods and after the use of enrofloxacin as a therapeutic treatment. Int. J. Food Microbiol. 340:109046. doi: 10.1016/j.ijfoodmicro.2021.109046
Zenebe, T., Zegeye, N., and Eguale, T. (2020). Prevalence of Campylobacter species in human, animal and food of animal origin and their antimicrobial susceptibility in Ethiopia: a systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 19:61. doi: 10.1186/s12941-020-00405-8
Keywords: Campylobacter, public health, wild birds, animal health, foodborne infections
Citation: Ahmed NA and Gulhan T (2022) Campylobacter in Wild Birds: Is It an Animal and Public Health Concern? Front. Microbiol. 12:812591. doi: 10.3389/fmicb.2021.812591
Received: 10 November 2021; Accepted: 14 December 2021;
Published: 10 February 2022.
Edited by:
Hosny El-Adawy, Institut für Bakterielle Infektionen und Zoonosen, Friedrich Loeffler Institut, GermanyReviewed by:
Marwa Ibrahim Abd El-Hamid, Zagazig University, EgyptRhiannon Wallace, Agriculture and Agri-Food Canada (AAFC), Canada
Shahzad Ali, University of Veterinary and Animal Sciences, Pakistan
Copyright © 2022 Ahmed and Gulhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nejash A. Ahmed, bmVqYXNoLmFiZGVsYTIwMjBAZ21haWwuY29t; Timur Gulhan, dGltdXIuZ3VsaGFuQG9tdS5lZHUudHI=
 Nejash A. Ahmed*
Nejash A. Ahmed* Timur Gulhan
Timur Gulhan