- 1National Pathogen Collection Center for Aquatic Animals, Shanghai Ocean University, Shanghai, China
- 2Key Laboratory of Agriculture Ministry for Freshwater Aquatic Genetic Resources, Shanghai Ocean University, Shanghai, China
- 3Guangdong Laboratory for Lingnan Modern Agriculture, South China Agricultural University, Guangzhou, China
Cyprinid herpesvirus 2 (CyHV-2) has caused great economic loss to the crucian carp breeding industry. Upon viral stimulation, eukaryotic cells generally activate the expression of anti-oxidative genes to maintain the intracellular oxidative balance and resist viral infection. Here, intracellular reactive oxygen species (ROS) levels in CyHV-2-infected cells were monitored to show that CyHV-2 induced the increase of intracellular ROS during early infection, and intracellular excessive accumulation of ROS was ameliorated during late infection, which was accompanied by activated expression of genes related to Nrf2 signaling pathway. In order to explore the interaction between CyHV-2 infection and ROS production, RyuF-2 cells were treated with either antioxidant epigallocatechin-3-gallate (EGCG) or berberine hydrochloride (BBH) and then infected with CyHV-2. Both BBH and EGCG could effectively inhibit the amplification of CyHV-2 while inhibiting the accumulation of intracellular ROS. Consistent with this, the oxidant stress-related genes were up-regulated by CyHV-2 infection and down-regulated in cells treated with either BBH or EGCG, through which the production of intracellular ROS was modulated. These results collectively demonstrated that early ROS accumulation favored the replication of CyHV-2, while antioxidants (BBH and EGCG) could inhibit the amplification of CyHV-2 by inhibiting ROS induction.
Introduction
Cyprinid herpesvirus 2 (CyHV-2), belonging to the genus Cyprinivirus and the family Alloherpesviridae, was first isolated and characterized from juvenile goldfish (Carassius auratus auratus) in Japan (Jung and Miyazaki, 1995). Recently, CyHV-2 has emerged as a virulent pathogen for cultured crucian carp (Carassius auratus gibelio) with high mortality and significant economic loss in cultured crucian carp and allogynogenetic gibel carp (Wang et al., 2012; Xu et al., 2013). CyHV-2 is an enveloped DNA virus with a 290.3-kb linear double-stranded DNA genome that encodes approximately 150 genes (Davison et al., 2013). Goldfish are regarded as the natural host for CyHV-2, and the symptoms are generally represented by pale gills and swollen spleens and kidneys. CyHV-2 causes high mortality among juvenile goldfish at water temperatures between 15 and 25°C (Jeffery et al., 2007). Typical symptoms for crucian carp include lethargy and lack of appetite, bleeding and pale gills, pink ascites in the abdominal cavity, and enlarged spleen and kidneys, and death can occur within 1 to 2 days following the onset of clinical signs (Xu et al., 2014). Notably, the symptoms of CyHV-2 infection in crucian carp are significantly more severe than those in goldfish. Currently, neither vaccine nor medicine is available for the control of disease caused by CyHV-2 infection.
Virus generally stimulates the host’s immune system response during the infection process and destroys the homeostasis of the cell. The balance of pro-oxidation/anti-oxidation in the internal environment is very important for maintaining the integrity of cellular structure and function. An excess of bad radical species, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), is often associated with important diseases such as cancer and neurodegeneration (Liguori et al., 2018). Under normal physiological conditions, excessive free radicals will be cleared by the endogenous antioxidant defense through either enzymatic or non-enzymatic pathways. The primary antioxidant enzymes are superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), while the major non-enzymatic antioxidants include bilirubin, α-tocopherol, β-carotene, albumin, and uric acid (Liguori et al., 2018). However, virus infection generally results in a transient ROS induction followed by a continuous and weak antioxidant defense in infected tissues to facilitate viral infection (Kumar et al., 2009; Souza et al., 2011). ROS suppresses the type-I interferon response by oxidizing cysteine 147 on murine stimulator of interferon genes (STING), indicating that ROS orchestrates anti-viral immune responses and can be exploited by viruses to evade cellular defenses (Tao et al., 2020). ROS is usually induced to a higher level immediately following viral attachment and manipulated by the virus to alternate cellular antiviral response; some viruses might modulate the ROS to a normal level after the onset of viral replication for avoiding its adverse effect on viral replication (Baruchel and Wainberg, 1992). ROS is also involved in tissue damage through regulating host inflammatory and immune responses (Akaike, 2001), and virus-induced oxidative stress serves as a common and important pathogenic mechanism for diseases caused by viral infection (Jackson et al., 2010).
Berberine (BBR), the most abundant isoquinoline alkaloid with a concentration range of 4.5–8% in different medical plant species, has been used to treat various inflammatory disorders and related diseases (Wang et al., 2019). Interestingly, low concentrations of berberine (5–20 μM) inhibited increased oxidative stress in 3T3-L1 cells by suppressing ROS production via increased GPx gene expression and GPx activity (Dong et al., 2015), while a higher concentration of berberine (40–160 μmol/l) dose-dependently induced ROS generation, G(2)/M phase arrest, and apoptosis in U266 cells (Hu et al., 2013). Furthermore, BBR has a great potential to reduce the effects of oxidative stress in vivo through markedly reducing Nox2-dependent cytoplasmic and mitochondrial ROS production (Sun et al., 2017).
Epigallocate-chin-3-gallate (EGCG) from green tea extract is a natural antioxidant that confers strong resistance against oxidation and free radicals (Lotito and Fraga, 1998; Yao et al., 2008) and is also regarded as a multifunctional molecule with anti-cancer (Yang and Wang, 2010) and immunity-enhancing properties (Sheikhzadeh et al., 2011). Favorable effects of EGCG have been largely attributed to its scavenging effects on free radicals, inhibition of ROS-generating mechanisms, and upregulation of major antioxidant enzymes including CAT, superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2), and GPx (Potenza et al., 2020).
The understanding of the interplay between virus-induced oxidative stress and anti-oxidative host response has shed light on the application of antioxidants as potential antiviral supplements for better management of some human viral diseases (Lee, 2018). For example, the upregulation of intracellular ROS during the early phase of retroviral infection plays an important role in viral establishment in the host cell, and the treatment of apocynin, an inhibitor of NADPH oxidase, could decrease viral titer in the mouse brain and increase the lifespan of infected mice (Kim and Wong, 2013). Recently, we reported that BBR could systematically impede CyHV-2 replication and protect crucian carp from CyHV-2 challenge in a dose-dependent manner (Su et al., 2021). However, it is not clear whether BBR-mediated modulation of ROS signaling involves the anti-CyHV-2 activity of BBR. In the present study, we monitored the ROS level and progeny virus production of susceptible cells during the infection course of CyHV-2 in the presence of either BBR or EGCG and provided evidence to show that antioxidants could serve as potential therapeutic agents against cyprinid herpesvirus 2 infection.
Materials and Methods
Materials and Reagents
Berberine hydrochloride (BBH) and EGCG standards were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Dimethyl sulfoxide (DMSO) was purchased from Sigma (Shanghai, China). DCFDA/H2DCFDA cellular ROS assay kit was purchased from Abcam (Shanghai, China). M199 medium, FBS serum, penicillin/streptomycin, second antibodies, Pierce BCA Protein Assay Kit, and TRIzol reagent were purchased from Thermo Fisher Scientific (Shanghai, China). PrimeSTAR® Max DNA Polymerase, PrimeScript™ II 1st Strand cDNA Synthesis Kit, TaKaRa MiniBEST Viral RNA/DNA Extraction Kit Ver.5.0, and One-Step SYBR RT-PCR kit were purchased from Takara Bio (Beijing, China). All other reagents were purchased from Sangon Biotech (Shanghai, China). CyHV-2 strain ST-J1 (NCBI: NC_019495) and RyuF-2 cell line were provided by Dr. Motohiko Sano, University of Marine Science and Technology, Tokyo.
Cell Culture
Our cell lines are from the generous sharing of Dr. Motohiko Sano from Tokyo Ocean University. Cryopreserved samples of RyuF-2 cell stock at −150°C were thawed in a 37°C water bath before cell passage and virus propagation. Frozen cells were quickly thawed in a water bath, and the cryopreserved liquid was removed by centrifugation at 850 rpm for 3 min. M199 medium was used to resuspend RyuF-2 cells and supplemented with 10% heat-inactivated FBS and penicillin/streptomycin (50 U/ml/50 mg/ml). The suspension cells are adjusted to a density of 5 by the culture medium and then seeded on a 75-cm2 culture flask/cm2 coated with collagen. The cultured flask was placed in a humidified incubator at 27°C for culture. Before subculturing, the cells were grown to 80% confluence.
Virus Infection and Amplification
The virus was saved in normal M199 medium with 2% FBS (2% medium). After CyHV-2 was taken out from −150°C, the titer was quantified by gradient dilution counting. The 2% medium dilution was used to adjust the virus concentration until the multiplicity of infection (MOI) = 1. To infect the RyuF-2 cells with CyHV-2, the supernatant of the cells was removed, and viral inoculum was added immediately for an incubation time of 2 h. After that, the inoculum was replaced with 2% medium. Infected cells were cultured until CPE was developed in 80% of the cells. The remaining cells were scraped off with a spatula, the suspension was collected, and the cells and cell debris were removed by centrifugation at 12,000 g at 4°C for 20 min. The supernatant was filtered with a 0.22-μm filter to collect the virus. The harvested virus was equally divided and placed at −80°C for subsequent experiments. Before the experiment, the gradient dilution method was used to detect the virus titer again, and the virus concentration was adjusted according to the experimental cell density so that MOI = 1. CyHV-2 virus with a known titer was used to infect RyuF-2, which has a density of about 80%. After incubating for 2 h, the virus solution was removed, and the time point was counted as the start of infection 0 h post-infection (p.i.). The cultures were maintained for up to 96 h and were used between 0 and 96 h in culture.
Antioxidant Drug Treatment
Berberine hydrochloride and EGCG were separately dissolved in DMSO to prepare a 1-mg/ml preservation solution. RyuF-2 cells were used for experiments when their cell density was close to 80%. To adjust the concentration of BBH storage solution to 15, 20, and 25 μg/ml, 2% M199 medium was used. EGCG storage solution was diluted to 10, 20, and 30 μg/ml in the same way. The pretreatment was performed on RyuF-2 cells with different concentrations of drugs for 30 min. The drug treatment group kept the drug concentration unchanged and continued to incubate. In the virus infection group, the supernatant was removed and replaced with a virus solution, keeping the drug concentration unchanged. After incubating for 2 h, the virus solution was removed and 2% M199 medium was added with the same drug concentration. The cultures were maintained for up to 72 h and were used between 0 and 72 h in culture.
Measurement of Intracellular Reactive Oxygen Species
The intracellular ROS levels were estimated using a DCFDA/H2DCFDA cellular ROS assay kit according to a classic method (Heo et al., 2018). Briefly, the RyuF-2 cells were seeded into 96-well microtiter plates at 2.5 × 104 cells/well and were allowed to attach for 24 h at 27°C, and 100 μl of 1 × PBS buffer was added. The cells were then washed with 1 × PBS buffer. The 1 × PBS buffer was removed, and the cells were stained by adding 50 μl of the diluted DCFDA solution. Then, the cells were incubated for 45 min at 37°C in the dark. The fluorescence intensity was measured using a fluorescence microtiter ELISA plate reader at excitation and emission wavelengths of 485 and 535 nm, respectively. For cells infected with CyHV-2, cells were collected at 6, 12, 24, 36, 48, 60, 72, and 96 h after infection, and the intracellular ROS levels were quantified using the DCFDA/H2DCFDA cell ROS detection kit. For CyHV-2-infected cells in the presence of 15, 20, and 25 μg/ml BBR or 10, 20, and 30 μg/ml EGCG, the cells were harvested at 24 and 48 h p.i., and the intracellular ROS levels were quantitated using a DCFDA/H2DCFDA cellular ROS assay kit.
Nucleic Acid Extraction and Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction Analysis
The supernatant of infected cells was obtained at different time points after infection for total DNA extraction. Viral DNA was extracted from the supernatant according to the instructions of the Viral Genomic DNA Extraction Kit (Qiagen). The total RNA of the RyuF-2 sample was extracted with 1 ml TRIzol reagent and purified using phenol/chloroform and ethanol precipitation. The NanoDrop Technologies instrument is used to quantify RNA. Each group of samples used 5 μg of RNA as the sample to generate cDNA for real-time quantitative qPCR, which was achieved by the corresponding reverse transcription reaction of PrimeScript RT Master Mix reagent. TB Green Premix Ex Taq II (2x) was used to amplify 1 μl of the resulting cDNA. According to the gene sequence obtained by the analysis of the transcriptome results in the laboratory, Primer Premier 5.1 (Table 1) was used to design a specific primer set for each open reading frame (ORF). The reaction contained 12.5 μl TB Green Premix Ex Taq II (2x) (Takara, Japan), 0.4 μM forward/reverse primers, and 200 ng cDNA. The thermal cycle program included the following: 95°C for 30 s, then 95°C for 5 s and 59°C for 30 min for 39 cycles. The qPCR reaction was repeated three times. In order to construct a standard curve, the DNA extracted from RyuF-2 cells was used for continuous decimal dilution. Primers from β-actin were successfully used in qPCR, using cDNA prepared from infected and uninfected cells as a template. A one-way analysis of variance (ANOVA) was used to calculate statistical significance. Origin 9.0 was used for data analysis and graphing. Values were considered as significant (*) if they had a p-value of 0.01 to 0.05, very significant (**) if they had a p-value of 0.001 to 0.01, and extremely significant (***) if they had a p-value of 0.0001 to 0.001.
Western Blot
Cell samples were collected to extract total protein by boiling with 2X SDS-PAGE protein loading buffer. Thermo Scientific Pierce BCA protein detection kit was used to quantify protein concentration. The protein (20 g) was separated on 10–12% SDS-PAGE and then transferred to PVDF membrane. The membrane was blocked in 1% TBS-T buffer containing 5% skim milk for 1 h at room temperature and then incubated overnight with homemade anti-ORF121 polyclonal antibody (Yu et al., 2019) (1:200 dilution). After washing the membrane with TBS-T three times, horseradish peroxidase-conjugated secondary antibody (diluted 1:1,000) was added and incubated for 2 h at room temperature and then washed with TBS-T three times for 15 min. A chemiluminescence imaging system (Bio-Rad, China) was used to detect the signal with the ECL + western blot kit.
Results
Cyprinid Herpesvirus 2 Induced the Expression of Genes in Keap1-Nrf2 Pathway and Modulated Cellular Reactive Oxygen Species Accumulation During Infection
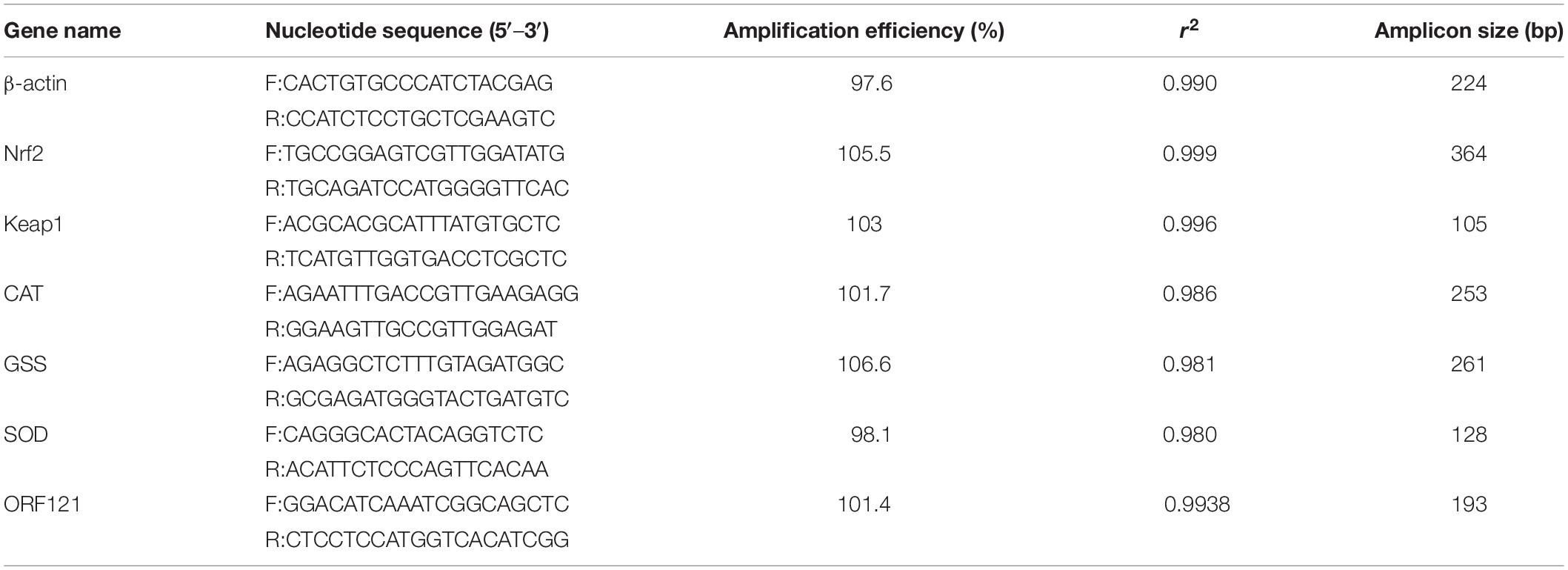
In a recent study to probe host response to viral infection, we identified the differential expression genes in crucian carp cells underlying CyHV-2 infection by transcriptomics analysis (Fei et al., 2020). Primary data analysis of genes involved in the Keap1-Nrf2 pathway indicated that the TPM (transcripts per kilobase million) values of Nrf2, CAT, and SOD2 genes in CyHV-2-infected cells at 48 h post-infection were dramatically higher than those in the negative control (NC), and the values of Keap 1 and GSS were also shown to be slightly higher than those of the control (Figure 1). In order to verify the effect of CyHV-2 infection on the expression of related genes in the Keap1-Nrf2 pathway, we used quantitative real-time RT-PCR analysis to compare the transcriptional expression levels of Keap1, Nrf2, CAT, GSS, and SOD2 in normal cells and CyHV-2-infected cells (Figure 2). At 24, 48, and 72 h after infection with CyHV-2, RyuF-2 cells were collected for total RNA extraction. All qRT-PCR assays were performed in triplicate, with β-actin used as an endogenous control. As shown in Figure 2, CyHV-2 significantly up-regulates all tested genes to higher levels compared to the corresponding low steady-state levels in the control cells. Specifically, the highest induction rate was recorded for Nrf2 and GSS genes at 48 h p.i. (Figures 2B,E). In general, 48 h p.i. seemed to be a time point for all the genes to be upregulated to a peak level.
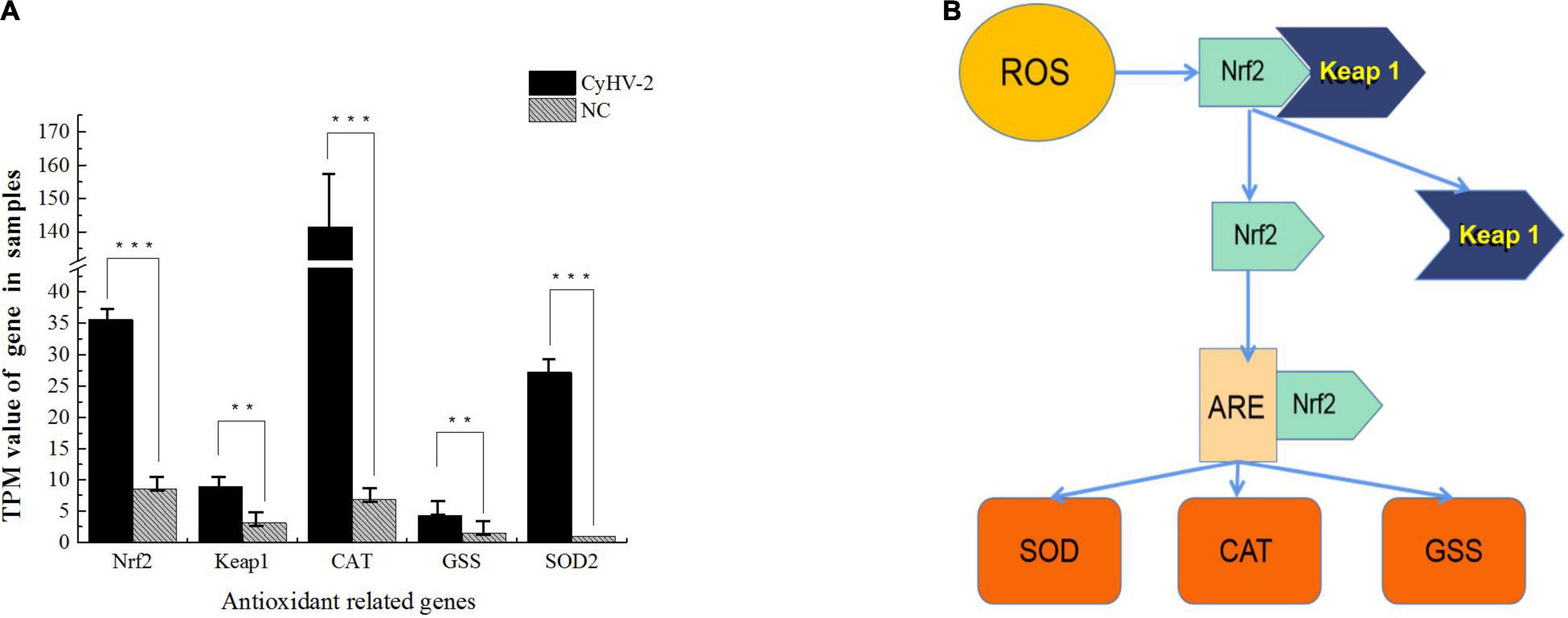
Figure 1. Key molecules of Nrf2 signaling pathway are induced by cyprinid herpesvirus 2 (CyHV-2) in RNA-seq analysis. TPM is transcripts per kilobase million, the transcripts per kilobase of exon model per million mapped reads. TPM is a method of gene expression standardization, which visualizes differential expression analysis. The transcriptome related to CyHV-2 infection was analyzed in the laboratory using the Mega Cloud platform. The differences in the expression of antioxidant-related genes were analyzed and compared between the groups. The result is shown in (A), the difference of TPM values in the Nrf2 signaling pathway identified in transcriptome analysis. According to the known reaction mode of Nrf2-ARE antioxidant element combined with the analysis result, (B) is inferred. (B) Flow chart of proposed Nrf2 signaling pathway induced by CyHV-2.
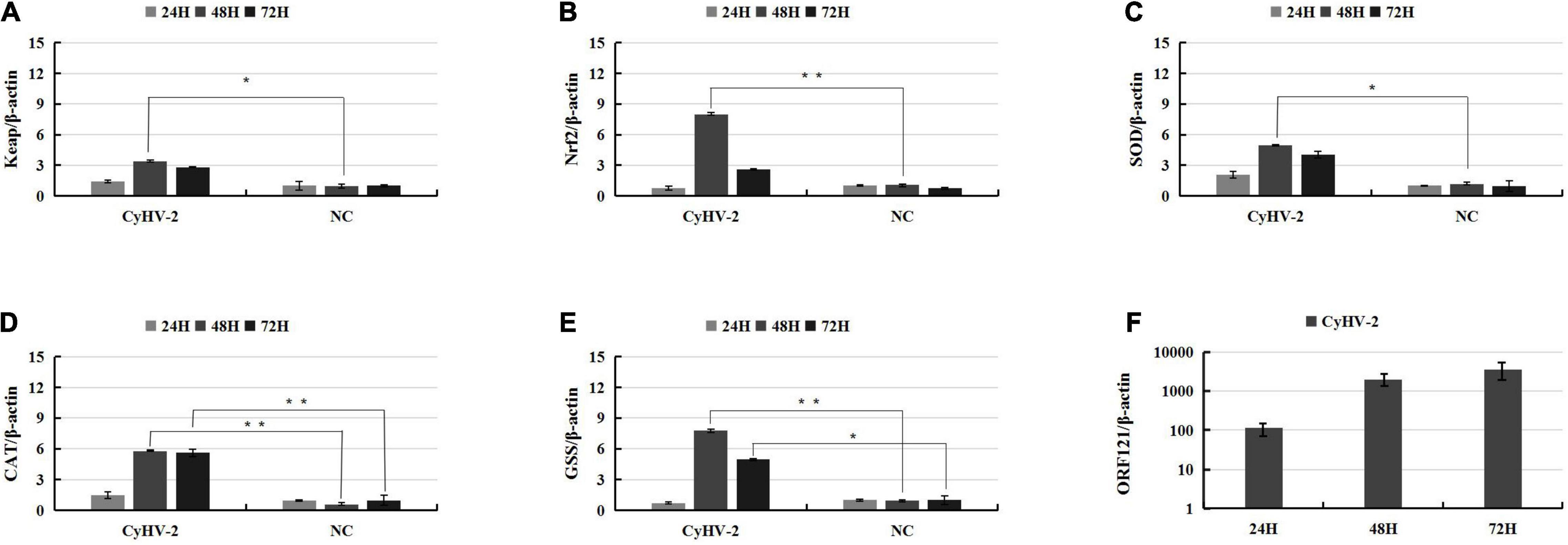
Figure 2. CyHV-2 induces expression of key genes of Nrf2 signaling pathway in RyuF-2 cells. Quantitative real-time RT-PCR analysis was performed to compare the transcriptional expression levels of Keap1 (A), Nrf2 (B), SOD2 (C), CAT (D), GSS (E) and viral ORF121 (F) in infected cells. The mRNA level was normalized against housekeeping gene β-actin. Mock infected cells served as negative control. Similar results were obtained from three independent experiments. Data were represented as Means SD. *P < 0.05, **P < 0.01.
The Keap1-Nrf2 pathway regulates both mitochondrial and cytosolic ROS production (Kovac et al., 2015); thus, the above data predicted that CyHV-2 infection might interfere with ROS synthesis and activate host oxidative stress response. To clarify whether CyHV-2 infection disturbed the host cellular redox balance, we monitored the ROS level following CyHV-2 infection using DCFDA/H2DCFDA cellular ROS assay. Following CyHV-2 infection at a MOI = 1, the ROS level in RyuF-2 cells was dramatically induced to a higher level with maximum ROS accumulation at 24 h p.i. and then gradually reversed to a constant and lower level (Figure 3A). Successful replication of CyHV-2 in RyuF-2 cells was reflected by the titration of progeny virus from the virus supernatant after infection by quantitating the genome copy number using a real-time polymerase chain reaction (PCR) analysis. As shown in Figure 3B, the produced CyHV-2 in supernatant significantly rose to a higher level of more than 107 genome copy number/ml since 24 h p.i. and then gradually increased until late infection of 96 h p.i. The experiments indicated that higher ROS accumulation was only induced at early phase of CyHV-2 infection, and the high level of ROS was reversed during late infection.
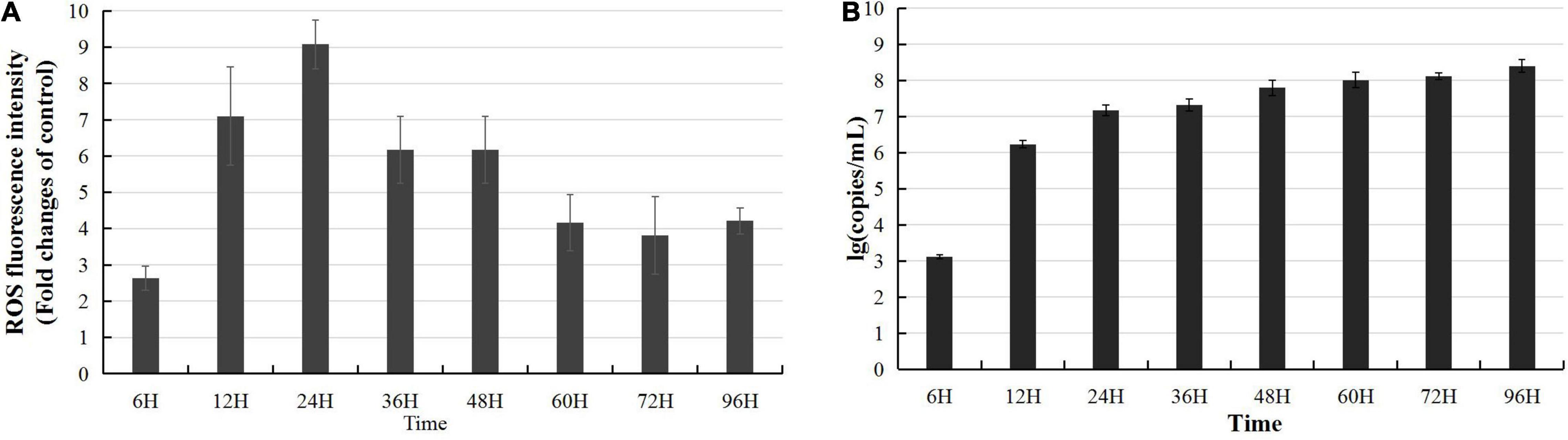
Figure 3. Modulation of reactive oxygen species (ROS) accumulation during CyHV-2 infection in RyuF-2 cells. CyHV-2 with MOI = 1 was used to infect RyuF-2. Cell samples and supernatant after infection were obtained at 6, 12, 24, 36, 48, 60, 72, and 92 h after infection. The DCFDA/H2DCFDA cellular ROS assay kit is used to detect changes in the accumulation of intracellular ROS after virus infection. The control group cells were used as the normalization standard to reduce the differences between groups. The results are shown in (A). After infection, the supernatant was used to obtain total DNA for detection of virus amplification. The early gene ORF121 was used as a representative gene for detection of CyHV-2 replication, as shown in (B). The RT-PCR results were processed using the absolute quantification standard program, and the data were expressed as the mean SD.
The Effects of Antioxidants on the Keap1-Nrf2 Pathway During Cyprinid Herpesvirus 2 Infection
Epigallocatechin-3-gallate, the most abundant polyphenolic substance in green tea, typically plays a critical role in free radical scavenging owing to its antioxidant nature and subsequently exerts a large number of medical activities in cancer control, viral diseases, bacterial diseases, neurodegenerative diseases, etc. (Xu et al., 2017). Thus, we were interested to test the effect of EGCG on the CyHV-2-mediated upregulation of Keap1-Nrf2 pathway. For this purpose, RyuF-2 cells were infected with CyHV-2 at a MOI = 1 in the presence of 10 μg/ml EGCG. At 24, 48, and 72 h post-infection, the infected cells were harvested for total RNA extraction and subjected to gene expression assay by quantitative real-time RT-PCR analysis. MOCK-infected cells treated with 10 μg/ml EGCG served as negative control, and CyHV-2-infected cells without EGCG treatment served as positive control. As shown in Figure 4, the treatment of MOCK-infected cells with EGCG could result in an increased expression of Keap1, Nrf2, GSS, and CAT gene; in comparison to CyHV-2-infected cell controls, the treatment of CyHV-2-infected cells with EGCG could result in a significant increase of Nrf2 at 48 h p.i., Keap 1 at 72 h p.i., GSS at all three time points, and CAT at 72 h p.i. Thus, EGCG could enhance the expression of key genes in the Keap1-Nrf2 pathway except for SOD gene, which was independent of CyHV-2 infection.
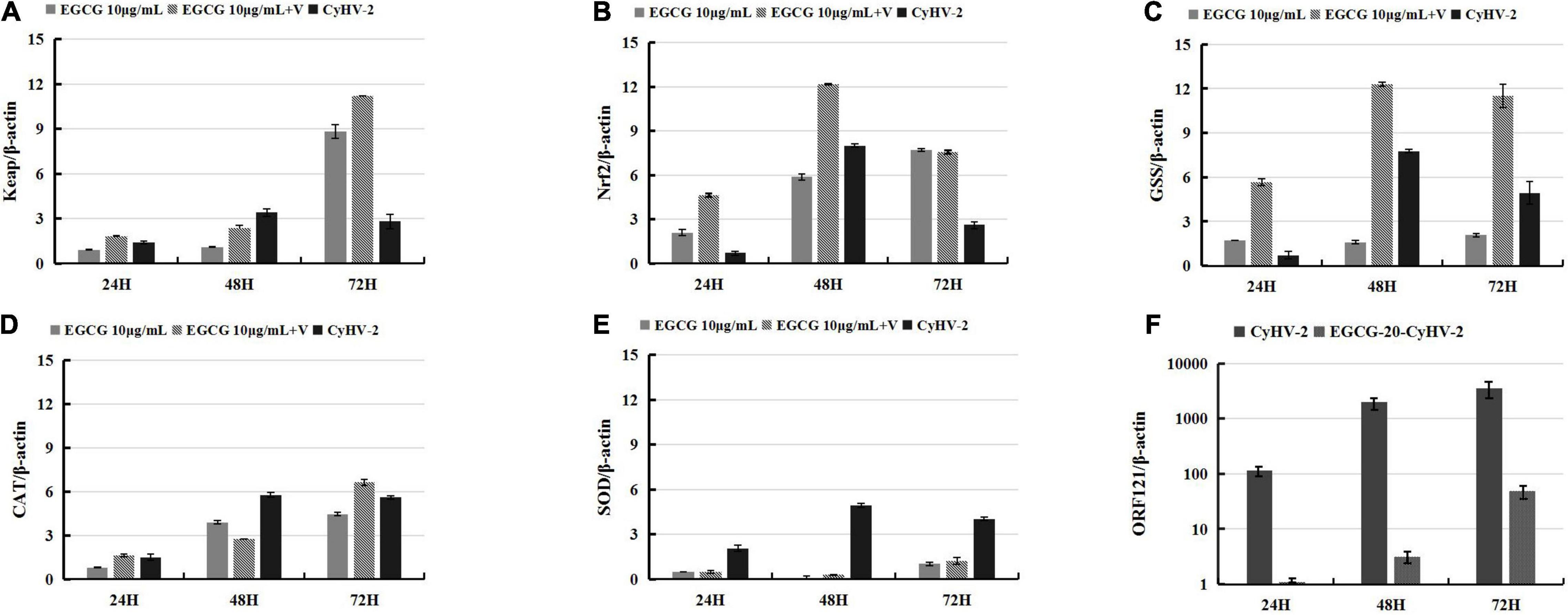
Figure 4. Epigallocate-chin-3-gallate (EGCG) enhanced the CyHV-2-induced expression of genes of Nrf2 signaling pathway in RyuF-2 cells. Quantitative real-time RT-PCR analysis was performed to compare the transcriptional expression levels of Keap1 (A), Nrf2 (B), GSS (C), CAT (D), SOD2 (E) and viral ORF121 (F) in infected cells. The mRNA level was normalized against housekeeping gene β-actin. MOCK-infected cells treated with 10 μg/ml EGCG and CyhV-2-infected cells without EGCG treatment served as controls. Similar results were obtained from three independent experiments. Data were represented as means SD.
Affecting various signaling pathways, berberine has been shown to decrease the activity of xanthine oxidase, COX2, and superoxide dismutase and subsequently reduce ROS levels (Kaboli et al., 2014). As an alternative antioxidant, BBR was subjected for analysis on its effect on the CyHV-2-mediated upregulation of the Keap1-Nrf2 pathway. For this purpose, RyuF-2 cells were infected with CyHV-2 at a MOI = 1 in the presence of 20 μg/ml BBH. At 24, 48, and 72 h post-infection, the infected cells were harvested for total RNA extraction and subjected to gene expression assay by quantitative real-time RT-PCR analysis. MOCK-infected cells treated with 20 μg/ml BBH served as negative control, and CyHV-2 infected cells without BBH treatment served as positive control. As shown in Figure 5, in contrast to MOCK-infected cells treated with BBH, CyHV-2 infection resulted in an increased expression of all tested genes; compared with CyHV-2-infected control cells, the treatment of CyHV-2-infected cells with BBH could result in a significant increase of Nrf2, Keap 1, and GSS at 48 h p.i. The gene expression level was not affected at 48 h p.i. for CAT and at 72 h p.i. for SOD. The results even suggested that these two genes might be downregulated at other time points (Figures 5D,E). Overall, the above experiments suggested that antioxidants, such as EGCG and BBH, could efficiently activate the Keap1-Nrf2 pathway that was induced to a higher level by CyHV-2 challenge in RyuF-2 cells.
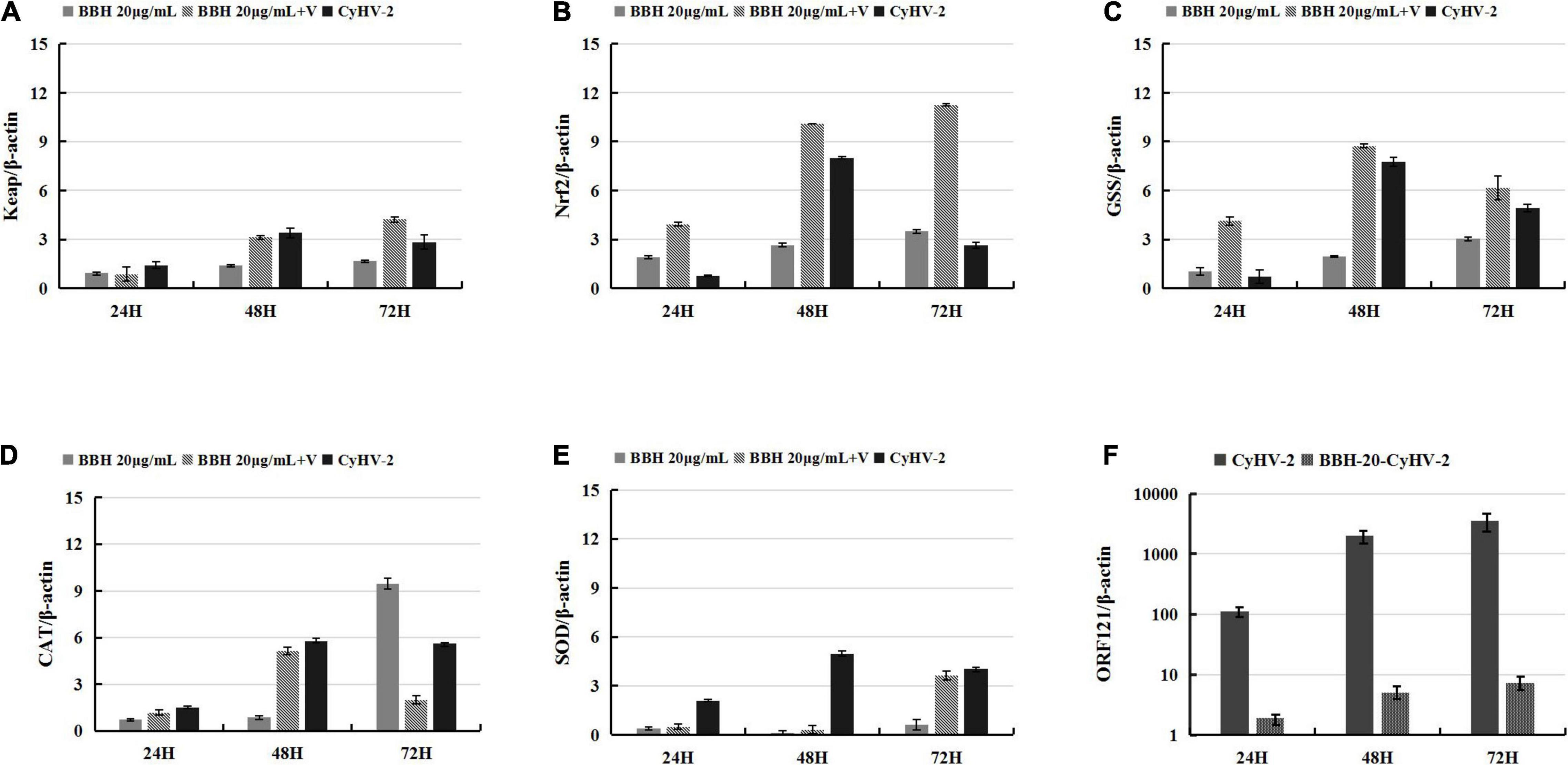
Figure 5. Berberine hydrochloride (BBH) enhanced the CyHV-2-induced expression of genes of Nrf2 signaling pathway in RyuF-2 cells. Quantitative real-time RT-PCR analysis was performed to compare the transcriptional expression levels of Keap1 (A), Nrf2 (B), GSS (C), CAT (D), SOD2 (E) and viral ORF121 (F) in infected cells. The mRNA level was normalized against housekeeping gene β-actin. MOCK-infected cells treated with 20 μg/ml BBH and CyhV-2-infected cells without BBH treatment served as controls. Similar results were obtained from three independent experiments. Data were represented as means SD.
The Effects of Antioxidants on Cyprinid Herpesvirus 2-Modulated Reactive Oxygen Species and Viral Replication in RyuF-2 Cells
The above experiments indicated that CyHV-2 modulated ROS level in infected cells and could induce dramatic ROS accumulation during early infection. We are interested to know what will happen if the ROS accumulation is inhibited by antioxidants such as BBR and EGCG during CyHV-2 infection. RyuF-2 cells were treated with various doses of BBH (0, 15, 20, and 25 μg/ml BBH) or EGCG (0, 10, 20, and 30 μg/ml EGCG) and subjected to CyHV-2 infection at a MOI = 1. At 24 and 48 h post-infection, the cells were harvested for DCFDA/H2DCFDA cellular ROS assay, in which the ROS level was reflected by the fluorescence strength detected from the cellular extract. The NC group referred to normal cells without antioxidant treatment and viral infection. Cells infected with CyHV-2 and treated with 0 μg/ml antioxidant served as positive control (CyHV-2 group). As shown in Figure 6, both BBH and EGCG effectively counteracted the accumulation of ROS induced by CyHV-2 infection at 24 and 48 h post-infection. In contrast to the dramatic increase of ROS level at indicated time points post-infection, treatment with BBH (Figure 6A) or EGCG (Figure 6B) resulted in low ROS accumulation in CyHV-2-infected cells, which was close to the level of the NC group.
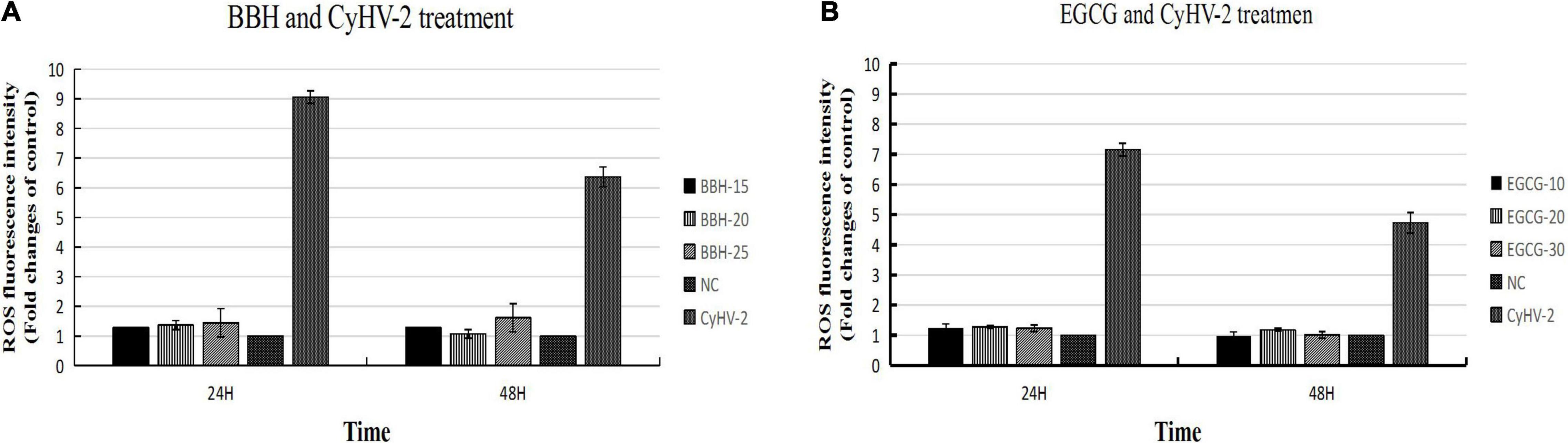
Figure 6. BBH and EGCG antagonized the ROS induction mediated by CyHV-2 infection in RyuF-2 cells. RyuF-2 cells were treated with 15, 20, and 25 μg/ml BBH (A) or 10, 20, and 30 μg/ml EGCG (B) and infected with CyHV-2 at a MOI = 1. At 24 and 48 h p.i., cells were harvested for ROS detection as reflected by the strength of fluorescence signal. MOCK-infected cells served as the negative control and CyhV-2-infected cells without BBH or EGCG treatment served as positive control. Similar results were obtained from three independent experiments. Data were represented as means SD.
The above experiments indicated that ROS accumulation was efficiently inhibited by BBH or EGCG during CyHV-2 infection. Then, it will be interesting to know the effect of these antioxidants on CyHV-2 replication. For this purpose, we determined the progeny virus in the supernatants of infected cells by quantifying the viral genome copy number through real-time PCR analysis. The antiviral activity of antioxidants in vitro was evaluated according to the reduced level of progeny virus produced from CyHV-2-infected RyuF-2 cells in the presence of different concentrations of EGCG or BBH. The supernatants of infected RyuF-2 cells, in the presence of BBH at 0, 15, 20, and 25 μg/ml or EGCG at 0, 10, 20, and 30 μg/ml, were collected at 8, 24, and 48 h post-infection for total viral genome extraction and quantification. As shown in Figure 7, both BBH and EGCG dose-dependently suppressed the production of progeny virus from infected cells, and the overall efficiency of these two antioxidants was similar at 48 h post-infection. At 8 h post-infection, the treatment of EGCG (Figure 7B) displayed a lower level of CyHV-2 (Figure 7A) in supernatants than that of BBH, suggesting a more robust effect of EGCG in antagonizing viral replication at this time point.
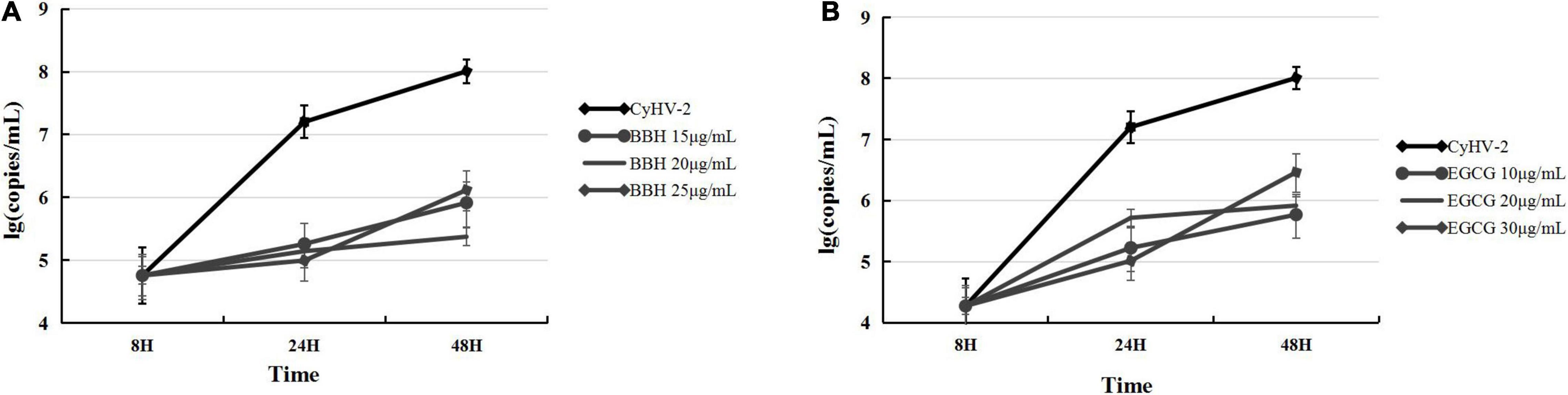
Figure 7. BBH and EGCG inhibited the production of progeny virus in RyuF-2 cells infected with CyHV-2. RyuF-2 cells were treated with 15, 20, and 25 μg/ml BBH (A) or 10, 20, and 30 μg/ml EGCG (B) and infected with CyHV-2 at a MOI = 1. At 8, 24, and 48 h p.i., supernatants of infected cells were harvested for viral genome copy quantification by real-time polymerase chain reaction (PCR) analysis. CyhV-2-infected cells without BBH or EGCG treatment served as positive control. Similar results were obtained from three independent experiments. Data were represented as means SD.
To confirm the antiviral effects of BBH and EGCG, we continued to investigate the transcriptional and translational expression levels of ORF121, an immediately early gene encoded by CyHV-2 (Tang et al., 2020). The infected RyuF-2 cells in the presence of 20 μg/ml BBH or 10 μg/ml EGCG were harvested at 24, 48, and 72 h post-infection, and total protein samples were extracted from the infected cells for ORF121 detection by western blot analysis; total RNA samples were extracted from the infected cells for ORF121 mRNA detection by real-time RT-PCR analysis. As shown in Figure 8A, the RyuF-2 cells infected with CyHV-2 resulted in robust transcriptional expression of ORF121, which lasted until 72 h post-infection; treatment with either BBH or EGCG significantly suppressed the transcriptional expression of ORF121 throughout the infection course. As shown in Figures 8B,C, in contrast to the strong signal of ORF121 protein from CyHV-2-infected RyuF-2 cells in the immunoblot analysis, the treatment of BBH or EGCG at indicated concentrations demonstrated a dramatic decrease of protein signal, which indicated that either BBH or EGCG was effective in inhibiting viral protein synthesis.
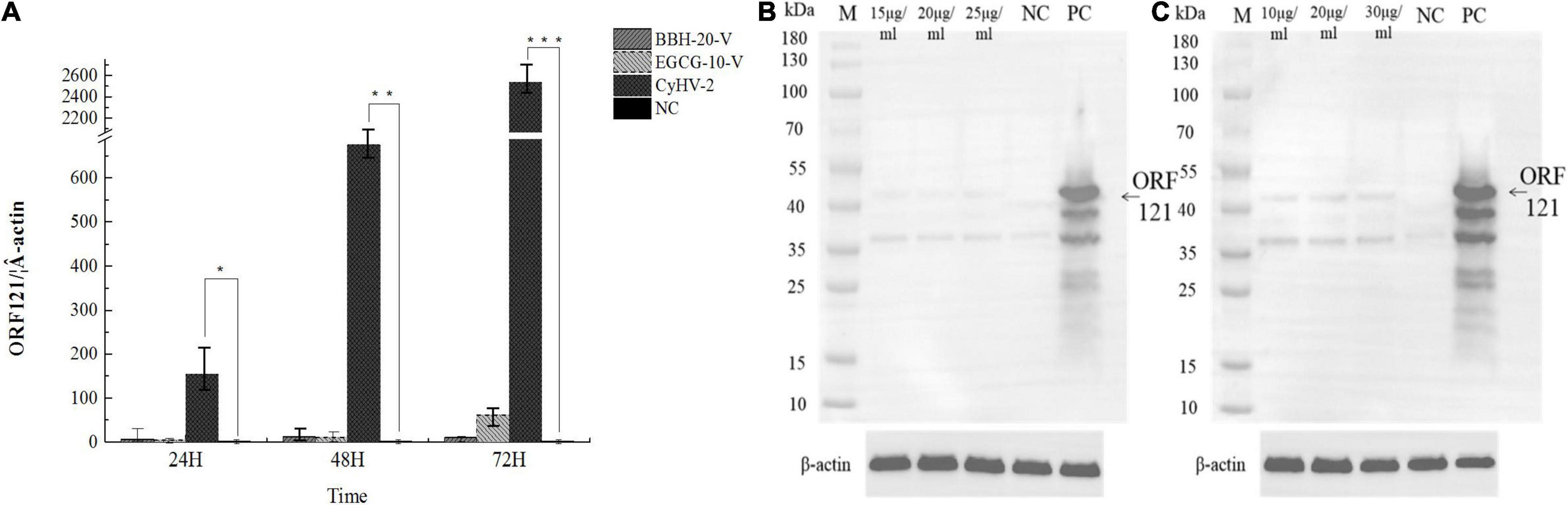
Figure 8. Inhibition of viral gene transcription and viral protein synthesis by either BBH or EGCG. RyuF-2 cells were treated with 15, 20, and 25 μg/ml BBH or 10, 20, and 30 μg/ml EGCG and infected with CyHV-2 at a MOI = 1. At 24, 48, and 72 h p.i., infected cells treated with 20 μg/ml BBH or 10 μg/ml EGCG were harvested for viral gene expression analysis with the ORF121 as target by real-time RT-PCR analysis (A). At 72 h p.i., infected cells treated with 15, 20, and 25 μg/ml BBH (B) or 10, 20, and 30 μg/ml EGCG (C) were harvested for protein expression analysis by western blot, which targeted ORF121 with a home-made anti-ORF121 polyclonal antibody. Real-time RT-PCR results were obtained from three independent experiments, and data were presented as means SD. *P < 0.05; **P < 0.01. MOCK-infected cells served as the negative control and CyhV-2-infected cells without BBH or EGCG treatment served as the positive control. Housekeeping protein β-actin served as the internal control in the western blot analysis.
Thus, the above experiments suggested that BBH and EGCG could significantly block CyHV-2-induced ROS accumulation, inhibit viral gene transcription and protein synthesis, and reduce the production of progeny virus.
Discussion
A virus needs to keep oxidative stress at a level optimal for viral reproduction, which is usually higher than normal; viral infection thus generally induces oxidative stress in infected cells, which must be in tight control in order to minimize the potentially detrimental effects of ROS on the overall health of the infected host cell (Lee, 2018). Although infection-initiated oxidative stress plays a key role in the activation of innate immunity to fight off pathogenic microbes, a number of viruses were shown to induce oxidative stress on purpose to facilitate their replication inside the cell, which at least included influenza virus, respiratory syncytial virus (RSV), human metapneumovirus (hMPV), dengue virus (DENV), rotavirus, herpes simplex virus, Zika virus, and HIV (McCord et al., 2020). CyHV-2 infection in crucian carp remains a challenge for the aquaculture section worldwide, and the lack of vaccine or medicine in the field urgently required the development of a novel antiviral strategy (Su et al., 2021). Nrf2 and its principal negative regulator Keap1 play a central role in the maintenance of intracellular redox homeostasis and regulation of inflammation. The purpose of this study is to evaluate the effects of antioxidants (BBH and EGCG) on CyHV-2 infection in crucian carp cells, which is based on the knowledge that CyHV-2 regulates the Keap1-Nrf2 pathway (Figures 1, 2) and modulates ROS accumulation in infected cells (Figure 3).
The Nrf2-dependent antioxidative pathway is regarded as an adaptive cellular response to oxidative and electrophilic stress and a responsive host defense system if caused by a viral infection. In a study to apply Nrf2 agonists as anti-rotaviral drugs, initial induction of Nrf2 concurred with a virus-induced early burst of oxidative stress, and rotavirus infection was determined to be sensitive to antioxidant treatments (Patra et al., 2020). NrF2 belongs to the CNC family and is mainly involved in the elimination of cell endogenous ROS. In a normal cell environment, Nrf2 and Keap1 combine through the Neh2 structure of Nrf2 to promote ubiquitination of Nrf2 and degrade it through the proteasome pathway. It has been found that Nrf2 can regulate hundreds of different genes involved in the maintenance of cell homeostasis, including heme oxygenase-1, NADPH quinone reductase, superoxide dismutase, and other antioxidant enzymes related to ROS elimination (Figure 1B). Our data indicated that BBH and EGCG could efficiently activate the Nrf2-dependent antioxidative pathway in normal RyuF-2 cells (Figures 4, 5), as well as CyHV-2-infected cells (Figures 4, 5); the induction of ROS accumulation during early viral infection (Figure 3) could be abolished through treatment of BBH or EGCG (Figure 6). Thus, CyHV-2-mediated induction could be modulated by antioxidants, which was consistent with reports from other viruses (McCord et al., 2020). As expected, either BBH or EGCG efficiently blocks viral gene transcription (Figure 8A), viral protein synthesis (Figure 8B), and progeny virus production (Figure 7). Thus, our study is the first to successfully apply antioxidants to counter fish herpes virus infection in vitro.
It is generally believed that virus has evolved to gain the ability to manipulate the Nrf2 pathway and control the ROS level to its favor. However, it is still not understood how the early ROS accumulation was produced for CyHV-2, neither is the mechanism involved in maintaining low ROS level during late infection known. Based on our data, the treatment of antioxidants seemed to upregulate the cytoprotective and detoxifying genes of the Keap1-Nrf2 pathway (Figures 4, 5), which is also targeted and regulated by CyHV-2 (Figures 1, 2). Antioxidants like EGCG and BBH should be beneficial not only for disruption of the ROS-dependent steps of the viral life cycle but also for the direct amelioration of the stressed conditions of the infected host cells. Our data thus supported the idea that Nrf2 modulators might be able to serve as a promising supplement for viral diseases by therapeutic modulation of virus-induced oxidative stress (Lee, 2018). Although the transcriptional regulation of the host Keap1-Nrf2 pathway by CyHV-2 merits further investigation, complex regulatory mechanisms were believed to occur in oxidative stress-related pathogenesis, such as the reduction of mitochondrial damage, regulation of calcium ions in the cell, programmed cell death, and autophagy (Liu et al., 2021).
Conclusion
Cyprinid herpesvirus 2 activated the Keap1-Nrf2 pathway and modulated ROS accumulation in host cells, especially during early infection. Antioxidants such as BBH and EGCG further enhanced the expression of genes involved in the Keap1-Nrf2 pathway and abolished the ROS-induction effect of CyHV-2 in infected cells. In the presence of antioxidants, viral gene expression and protein synthesis were dramatically inhibited resulting in decreased replication efficacy of CyHV-2. The current study should shed light on developing antioxidants as environmentally friendly reagents toward fighting the disease caused by CyHV-2 infection in aquaculture.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material of referenced article (Fei et al., 2020), further inquiries can be directed to the corresponding author. The previously analysed transcriptome data can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI BioProject, PRJNA596297, and PRJNA596598.
Author Contributions
LL, RT, and CL conceptualized the study. RT and CL made contributions in the methodology and wrote the original draft. RT, CL, MS, and JZ performed the investigation. LL reviewed and edited the manuscript, supervised the study, administrated the project, and acquired funding. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Technology Innovation Action Program of Shanghai, China (no. 17391902100) and the China Agriculture Research System of MOF and MARA (no. CARS-45-19).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge the technical support from the Experimental Center of College of Fishery and Life Science, Shanghai Ocean University.
References
Akaike, T. (2001). Role of free radicals in viral pathogenesis and mutation. Rev. Med. Virol. 11, 87–101. doi: 10.1002/rmv.303
Baruchel, S., and Wainberg, M. A. (1992). The role of oxidative stress in disease progression in individuals infected by the human immunodeficiency virus. J. Leukoc. Biol. 52, 111–114. doi: 10.1002/jlb.52.1.111
Davison, A. J., Kurobe, T., Gatherer, D., Cunningham, C., Korf, I., Fukuda, H., et al. (2013). Comparative genomics of carp herpesviruses. J. Virol. 87, 2908–2922. doi: 10.1128/JVI.03206-12
Dong, S.-F., Yasui, N., Negishi, H., Kishimoto, A., Sun, J.-N., and Ikeda, K. (2015). Increased oxidative stress in cultured 3T3-L1 cells was attenuated by berberine treatment. Nat. Product Commun. 10:1934578X1501000626. doi: 10.1177/1934578X1501000626
Fei, Y., Han, M., Chu, X., Feng, Z., Yu, L., Luo, Y., et al. (2020). Transcriptomic and proteomic analyses reveal new insights into the regulation of immune pathways during cyprinid herpesvirus 2 infection in vitro. Fish Shellf. Immunol. 106, 167–180. doi: 10.1016/j.fsi.2020.07.044
Heo, J.-R., Lee, G.-A., Kim, G.-S., Hwang, K.-A., and Choi, K.-C. (2018). Phytochemical-induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis and differentiation in malignant melanoma cells. Phytomedicine 39, 100–110. doi: 10.1016/j.phymed.2017.12.006
Hu, H.-y., Li, K.-p., Wang, X.-j., Liu, Y., Lu, Z.-g., Dong, R.-h., et al. (2013). Set9, NF-κB, and microRNA-21 mediate berberine-induced apoptosis of human multiple myeloma cells. Acta Pharmacol. Sin. 34, 157–166. doi: 10.1038/aps.2012.161
Jackson, A. C., Kammouni, W., Zherebitskaya, E., and Fernyhough, P. (2010). Role of oxidative stress in rabies virus infection of adult mouse dorsal root ganglion neurons. J. Virol. 84, 4697–4705. doi: 10.1128/JVI.02654-09
Jeffery, K., Bateman, K., Bayley, A., Feist, S., Hulland, J., Longshaw, C., et al. (2007). Isolation of a cyprinid herpesvirus 2 from goldfish, Carassius auratus (L.), in the UK. J. Fish Dis. 30, 649–656. doi: 10.1111/j.1365-2761.2007.00847.x
Jung, S., and Miyazaki, T. (1995). Herpesviral haematopoietic necrosis of goldfish, Carassius auratus (L.). J. Fish Dis. 18, 211–220. doi: 10.1111/j.1365-2761.1995.tb00296.x
Kaboli, P. J., Rahmat, A., Ismail, P., and Ling, K.-H. (2014). Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur. J. Pharmacol. 740, 584–595. doi: 10.1016/j.ejphar.2014.06.025
Kim, S. J., and Wong, P. K. (2013). ROS upregulation during the early phase of retroviral infection plays an important role in viral establishment in the host cell. J. Gen. Virol. 94(Pt 10):2309. doi: 10.1099/vir.0.055228-0
Kovac, S., Angelova, P. R., Holmström, K. M., Zhang, Y., Dinkova-Kostova, A. T., and Abramov, A. Y. (2015). Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta Gen. Sub. 1850, 794–801. doi: 10.1016/j.bbagen.2014.11.021
Kumar, S., Misra, U. K., Kalita, J., Khanna, V. K., and Khan, M. Y. (2009). Imbalance in oxidant/antioxidant system in different brain regions of rat after the infection of Japanese encephalitis virus. Neurochem. Int. 55, 648–654. doi: 10.1016/j.neuint.2009.06.008
Lee, C. (2018). Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxidat. Med. Cell. Long. 2018:6208067. doi: 10.1155/2018/6208067
Liguori, I., Russo, G., Curcio, F., Bulli, G., Aran, L., Della-Morte, D., et al. (2018). Oxidative stress, aging, and diseases. Clin. Interven. Aging 13:757. doi: 10.2147/CIA.S158513
Liu, Y., Yang, X., Liu, Y., Jiang, T., Ren, S., Chen, J., et al. (2021). NRF2 signalling pathway: new insights and progress in the field of wound healing. J. Cell. Mol. Med. 25, 5857–5868. doi: 10.1111/jcmm.16597
Lotito, S. B., and Fraga, C. G. (1998). (+)-Catechin prevents human plasma oxidation. Free Radic. Biol. Med. 24, 435–441. doi: 10.1016/S0891-5849(97)00276-1
McCord, J. M., Hybertson, B. M., Cota-Gomez, A., Geraci, K. P., and Gao, B. (2020). Nrf2 activator PB125® as a potential therapeutic agent against COVID-19. Antioxidants 9:518. doi: 10.3390/antiox9060518
Patra, U., Mukhopadhyay, U., Mukherjee, A., Sarkar, R., and Chawla-Sarkar, M. (2020). Progressive rotavirus infection downregulates Redox-Sensitive transcription factor Nrf2 and Nrf2-Driven transcription units. Oxidat. Med. Cell. Long. 2020:7289120. doi: 10.1155/2020/7289120
Potenza, M. A., Iacobazzi, D., Sgarra, L., and Montagnani, M. (2020). The intrinsic virtues of EGCG, an extremely good cell guardian, on prevention and treatment of diabesity complications. Molecules 25:3061. doi: 10.3390/molecules25133061
Sheikhzadeh, N., Nofouzi, K., Delazar, A., and Oushani, A. K. (2011). Immunomodulatory effects of decaffeinated green tea (Camellia sinensis) on the immune system of rainbow trout (Oncorhynchus mykiss). Fish Shellf. Immunol. 31, 1268–1269. doi: 10.1016/j.fsi.2011.09.010
Souza, F. N., Monteiro, A. M., dos Santos, P. R., Sanchez, E. M. R., Blagitz, M. G., Latorre, A. O., et al. (2011). Antioxidant status and biomarkers of oxidative stress in bovine leukemia virus-infected dairy cows. Vet. Immunol. Immunopathol. 143, 162–166. doi: 10.1016/j.vetimm.2011.05.028
Su, M., Tang, R., Wang, H., and Lu, L. (2021). Suppression effect of plant-derived berberine on cyprinid herpesvirus 2 proliferation and its pharmacokinetics in Crucian carp (Carassius auratus gibelio). Antiv. Res. 186:105000. doi: 10.1016/j.antiviral.2020.105000
Sun, Y., Yuan, X., Zhang, F., Han, Y., Chang, X., Xu, X., et al. (2017). Berberine ameliorates fatty acid-induced oxidative stress in human hepatoma cells. Sci. Rep. 7, 1–11. doi: 10.1038/s41598-017-11860-3
Tang, R., Lu, L., Wang, B., Yu, J., and Wang, H. (2020). Identification of the immediate-early genes of cyprinid herpesvirus 2. Viruses 12:994. doi: 10.3390/v12090994
Tao, L., Lemoff, A., Wang, G., Zarek, C., Lowe, A., Yan, N., et al. (2020). Reactive oxygen species oxidize STING and suppress interferon production. ELife 9:e57837. doi: 10.7554/eLife.57837.sa2
Wang, J., Wang, L., Lou, G.-H., Zeng, H.-R., Hu, J., Huang, Q.-W., et al. (2019). Coptidis Rhizoma: a comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharmaceut. Biol. 57, 193–225. doi: 10.1080/13880209.2019.1577466
Wang, L., He, J., Liang, L., Zheng, X., Jia, P., Shi, X., et al. (2012). Mass mortality caused by Cyprinid Herpesvirus 2 (CyHV-2) in Prussian carp (Carassius gibelio) in China. Bull. Eur. Assoc. Fish Pathol. 32, 164–173.
Xu, J., Xu, Z., and Zheng, W. (2017). A review of the antiviral role of green tea catechins. Molecules 22:1337. doi: 10.3390/molecules22081337
Xu, J., Zeng, L., Zhang, H., Zhou, Y., Ma, J., and Fan, Y. (2013). Cyprinid herpesvirus 2 infection emerged in cultured gibel carp, Carassius auratus gibelio in China. Vet. Microbiol. 166, 138–144. doi: 10.1016/j.vetmic.2013.05.025
Xu, L., Podok, P., Xie, J., and Lu, L. (2014). Comparative analysis of differential gene expression in kidney tissues of moribund and surviving crucian carp (Carassius auratus gibelio) in response to cyprinid herpesvirus 2 infection. Arch. Virol. 159, 1961–1974. doi: 10.1007/s00705-014-2011-9
Yang, C. S., and Wang, X. (2010). Green tea and cancer prevention. Nutr. Cancer 62, 931–937. doi: 10.1080/01635581.2010.509536
Yao, K., Yin, Y.-L., Chu, W., Liu, Z., Deng, D., Li, T., et al. (2008). Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J. Nutr. 138, 867–872. doi: 10.1093/jn/138.5.867
Keywords: Keap1-Nrf2 pathway, BBH, EGCG, CyHV-2, ROS
Citation: Lu C, Tang R, Su M, Zou J and Lu L (2022) Induction of Reactive Oxygen Species Is Necessary for Efficient Onset of Cyprinid Herpesvirus 2 Replication: Implications for Novel Antiviral Strategy With Antioxidants. Front. Microbiol. 12:792655. doi: 10.3389/fmicb.2021.792655
Received: 10 October 2021; Accepted: 02 December 2021;
Published: 28 January 2022.
Edited by:
Yongqun Oliver He, University of Michigan, United StatesReviewed by:
Sonam Popli, University of Toledo, United StatesMd. Golzar Hossain, Bangladesh Agricultural University, Bangladesh
Copyright © 2022 Lu, Tang, Su, Zou and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqun Lu, bHFsdkBzaG91LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Cuiyu Lu
Cuiyu Lu Ruizhe Tang
Ruizhe Tang Meizhen Su2
Meizhen Su2 Liqun Lu
Liqun Lu