- 1School of Pharmacy, Naval Medical University, Shanghai, China
- 2Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, China
The vacuole of Candida albicans plays a significant role in many processes including homeostasis control, cellular trafficking, dimorphic switching, and stress tolerance. Thus, understanding the factors affecting vacuole function is important for the identification of new drug targets needed in response to the world’s increasing levels of invasive infections and the growing issue of fungal drug resistance. Past studies have shown that vacuolar proton-translocating ATPases (V-ATPases) play a central role in pH homeostasis and filamentation. Vacuolar protein sorting components (VPS) regulate V-ATPases assembly and at the same time affect hyphal development. As well, vacuolar calcium exchange systems like Yvc1 and Pmc1 maintain cytosolic calcium levels while being affected by V-ATPases function. All these proteins play a role in the virulence and pathogenesis of C. albicans. This review highlights the relationships among V-ATPases, VPS, and vacuolar calcium exchange proteins while summarizing their importance in C. albicans infections.
Introduction
Candida albicans is an opportunistic fungal pathogen generating a high rate of mortality in systemic infections (Jenks et al., 2020). Due to C. albicans’ growing resistance to antifungal drugs, there is a great need to further study the pathways affecting its pathogenesis and virulence in order to discover new potential drug targets (Berman and Krysan, 2020). Vacuoles occupy 10–20% of the yeast cell’s volume and are involved in several cellular functions including ion homeostasis, stress response, cell differentiation, and adaptation to new environments (Armstrong, 2010). Thus, vacuolar function changes can have profound effects on the virulence of C. albicans, and targeting the vacuolar function of C. albicans may provide a new strategy for the development of antifungal drugs (Olsen, 2014).
C. albicans V-ATPases’ Disruption Impairs Vacuolar Acidification and Virulence
pH is a key consideration for pathogenic yeasts like C. albicans as it affects their virulence and dimorphic switching. pH homeostasis is not only required for sensing and responding to ambient pH, but also generating and transducing signals for secreting virulence factors (Patenaude et al., 2013; Du and Huang, 2016). The vacuolar pH is especially important for pathogenesis because vacuoles play a key role in cellular trafficking, and the defects in endosomal trafficking can affect the expression of adhesion and invasion membrane proteins (Kulkarny et al., 2014; Kim et al., 2019).
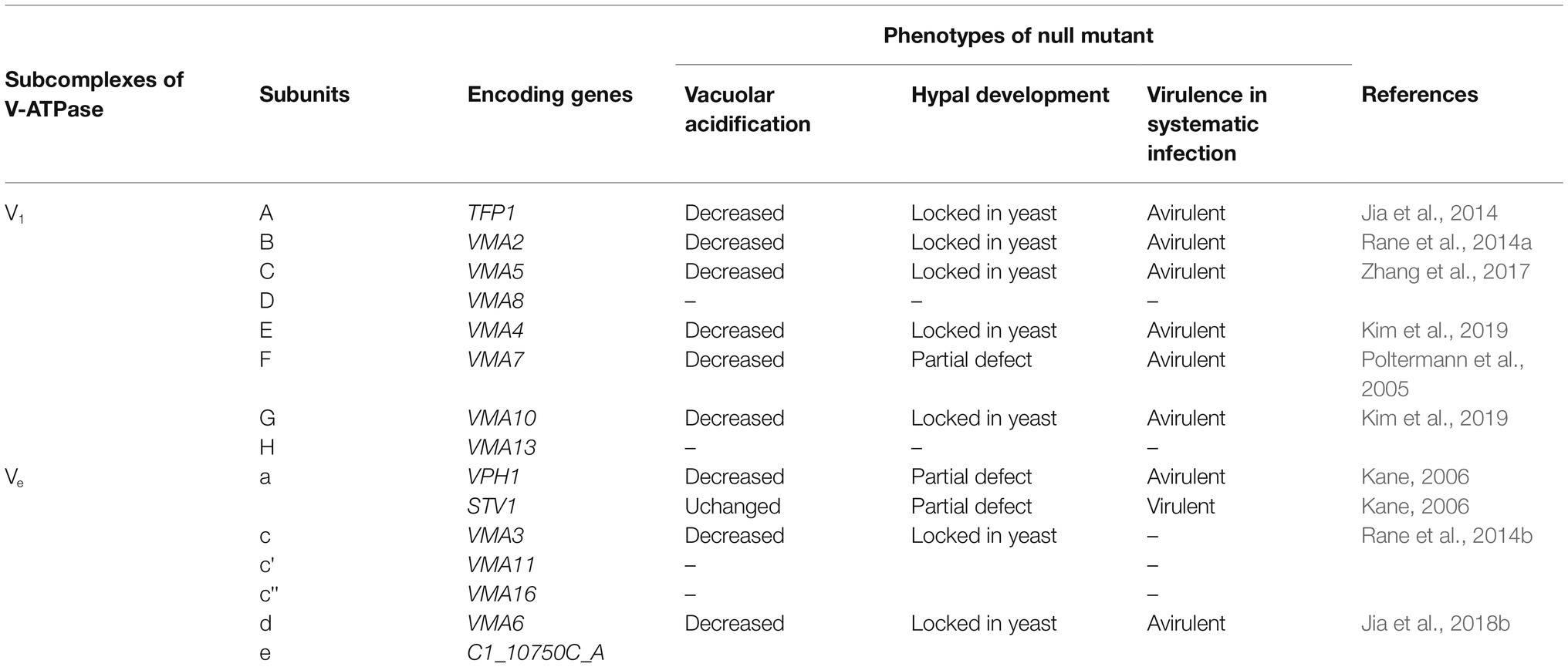
Maintaining vacuolar pH through acidification is the major role of the proton pumps called vacuolar proton-translocating ATPases (V-ATPases), which transport H+ from the cytoplasm into the vacuole (Parra et al., 2014). They contain a peripheral membrane subcomplex V1 and an integral membrane subcomplex V0 (Kane, 2007). The subcomplex V1 consists of subunits A, B, C, D, E, F, G, H, which are encoded by the genes TFP1, VMA2, VMA5, VMA8, VMA4, VMA7, VMA10, and VMA13, respectively. The subcomplex V0 includes subunits a, c, c’, c,” d, e, which are encoded by genes VPH1/STV1, VMA3, VMA11, VMA16, VMA6, and C1_10750C_A (Table 1). For both the non-pathogenic model yeast S. cerevisiae and the pathogenic C. albicans, all subunits are encoded by single genes, except for the subunit a in V0 which is encoded by the paralogs VPH1 and STV1 (Patenaude et al., 2013). The phenotypes of each subunit disruption mutant are summarized in Table 1.
Studies have shown that the structure of the V-ATPases in vacuoles plays an important role in pH balance and ion homeostasis (Veses et al., 2008). Factors affecting V-ATPase assembly and cellular trafficking also have strong influences on calcium ion homeostasis. All these functions are required for virulence and pathogenesis in C. albicans. The deletion of any one of the genes encoding subunits of the V-ATPases creates a Vma deficient (Vma−, vacuolar membrane ATPase activity) phenotype. Vma− S. cerevisiae and C. albicans demonstrate similar functional patterns, showing increased sensitivity to high pH, heavy metal ions, and antifungal drugs (Kane, 2007). S. cerevisiae cells with the Vma− phenotype also show slower growth compared to wild-type cells even at pH 5 and have defects in sporulation and germination (Kane, 2006).
The case is similar for C. albicans; several studies have established the necessity of V-ATPases subunits in maintaining vacuolar pH and virulence. The vma4 and vma10 null mutants of C. albicans both show non-acidic compartments and attenuated virulence. Protease secretion is also defective in the null mutants, and this compromises their ability in host cell degradation and in immune evasion (Kim et al., 2019). When VMA2 expression is repressed, vacuolar acidification is inhibited causing abnormal vacuolar morphology, and autophagy is delayed as visualized by monitoring Ape1-GFP localization. The mutant shows the Vma− growth phenotype and is avirulent in the C. elegans infection model (Rane et al., 2014a). The vma5 and vma7 null mutants of C. albicans are found to have the same defects in vacuolar acidification and are avirulent in a mouse model of systemic candidiasis (Poltermann et al., 2005; Zhang et al., 2017). C. albicans VMA3 is found to be functionally similar to S. cerevisiae VMA3 and, when its expression is disrupted, results in the loss of V-ATPase activity and vacuolar acidity. In addition, loss of VMA3 results in significantly attenuated macrophage killing (Rane et al., 2013). The deletion of Tfp1, the putative C. albicans homologue of S. cerevisiae Vma1, can cause a defect in vacuolar acidification and strongly reduces virulence (Jia et al., 2014). VPH2 encodes the homologue of Vma12, which is one of the V-ATPases assembly factors, and VMA6 encodes subunit d required for V1 domain assembly. Disruption of either of these two genes elevates vacuolar pH and weakens the virulence of C. albicans (Jia et al., 2018b).
There is a different case for the VPH1 and STV1 genes, as they both encode for a subunit of V0. Both the VPH1 and STV1 genes need to be deleted to show a full Vma− phenotype (Kane, 2006). However, the vph1 null mutant is unable to acidify vacuolar compartments and is avirulent, while the stv1 null mutants can have their functions compensated by Vph1 and are shown to be virulent. This study shows that Vph1 plays a more important role in maintaining virulence for C. albicans than Stv1, although there is functional redundancy between the two isoforms that makes the effects of losing either one of them less significant than a regular Vma− phenotype (Patenaude et al., 2013).
As well as the genes directly coding for the components of V-ATPase, V-ATPase also require ergosterol to function properly. Erg mutants with disruptions in the last step of ergosterol synthesis also show a Vma− phenotype with an inability to grow in alkaline medium and failure to acidify the vacuole (Zhang and Rao, 2010). This suggests that ergosterol is necessary for V-ATPase activity. Other lipids may play a role in controlling V-ATPase activity as well. Sphingolipids with a C26 acyl group are critical for the activity of V-ATPase (Chung et al., 2003), and deletion of either Sur4 and Fen1, which are critical for sphingolipid biosynthesis, results in a milder version of the Vma− phenotype (Kane, 2006).
The deletion of genes coding vacuolar protein sorting components (VPS) like Vps28 and Vps32 also give rise to similar phenotypes to Vma− with enhanced sensitivity to alkaline pH and weakened virulence (Cornet et al., 2005). The null mutants of a subset of VPS genes like VPS34 or VPS15 abolish the uptake of quinacrine into the vacuole and lead to increased sensitivity to high pH with reduced V-ATPase activity due to a vacuolar acidification defect (Sambade et al., 2005). Certain VPS proteins like Vps34 are found to directly interact with Vma7 and may control the assembly of V-ATPase, so the vps34 null mutant has the same phenotypes as the vma7 null mutant in terms of vacuolar acidification and lower virulence (Poltermann et al., 2005).
Overall, the Vma− phenotype highlights the vacuole’s role in maintaining ion homeostasis, and morphological transformation can also be impaired when V-ATPases or vacuolar trafficking pathways are defective.
Hyphal Growth Defects and Cell Wall Changes Through V-ATPases Inactivation
C. albicans are more capable of blocking phagosomal maturation and acidification when they have normal filamentation, and C. albicans invasion into oral and gastrointestinal tract epithelia involve hyphal form cells (Zhang and Rao, 2010), so filamentation could be used as a trait to assess virulence. The loss of hyphal growth can have several different vacuole-related genetic causes (Chen et al., 2020). Vma− mutants exhibit different degrees of defects in hyphal development. The vma3 and vma7 null mutants have essentially no filamentous growth in liquid Spider medium while filaments can be induced from wild-type cells. The deletion of TFP1, VPH2, or VMA6 also gives dramatic attenuation of C. albicans filamentous growth (Jia et al., 2014). In addition, the vph1 mutant shows deficiencies in hyphae formation while the stv1 null mutant has more normal filamentation. Interestingly, different hyphal development defects correspond with the inability to acidify vacuoles in the vph1 mutant but to lesser extent in the stv1 mutant. A link between vacuolar pH and hyphal formation is thus evident, and, as antifungal drugs that disrupt vacuolar pH also block hyphal growth, this suggests the V-ATPases may assist the signaling that induces hyphal formation (Patenaude et al., 2013).
In addition, the decreased activity of V-ATPase may influence cell wall synthesis through a reduction in the transport of secretory vesicles (Marshansky and Futai, 2008). For instance, vph2 or vma6 null mutants are hypersensitive to cell wall stresses and their cell wall composition changes significantly; the mutants contain more chitin and less β-1,3-glucan and phosphomannan (Jia et al., 2018b).
Hyphal Development Defects Caused by Disruption of Vacuolar Trafficking Genes
Hyphal formation in C. albicans is not only related to the vacuolar pH regulated by V-ATPases, but also related to vacuolar trafficking. Vacuolar trafficking involves the exchange of substances or vesicles between the vacuole and the endoplasmic reticulum, Golgi, mitochondria and other organelles, and is essential for maintaining the virulence of C. albicans (Bianchi et al., 2019). In S. cerevisiae, Vps21 was found to mediate vacuolar trafficking via an endosomal route, and a vps21 deletion in C. albicans causes a mild reduction in hyphal growth and virulence. Although the null mutant of aps3 alone does not produce an avirulent strain with a loss of filamentation, loss of function for both VPS21 and APS3 shows synthetic effects, generating pseudohyphae without vacuolated compartments and causing a significant decrease in virulence. This suggests that VPS21 and APS3 mediate vacuolar trafficking through distinct pathways and that the APS3 pathway is more significant when endosomal trafficking is disrupted (Palmer, 2010). The vps34 null mutant also has faulty vacuolar trafficking, with enlarged vacuoles and significantly less hyphal growth (Bruckmann et al., 2000). The vps11 null mutant has defects in filamentation and secreting proteases, and is completely unable to kill macrophages, resulting in a decrease in virulence (Palmer et al., 2003, 2005). Disruption of VPS1 by a regulatable tetracycline promoter produces defective filamentation and markedly reduced biofilm formation (Bernardo et al., 2008). It appears that disruption of vacuolar trafficking prevents vacuolation, compromises the regulation of turgor pressure that helps to provide a force for directional hyphal elongation, and prevents necessary factors like V-ATPase subunits from localizing in the vacuole. All these could be reasons why vacuolar trafficking is critical for fungal filamentation (Palmer, 2010).
The vps11 null mutant has reduced function in secreting proteases and lipases and is completely unable to kill macrophages (Palmer et al., 2003; Veses et al., 2008). Studies also found that C. albicans lacking VPS1 expression, as well as the vps4 null mutant, have reduced secretion of aspartyl proteases and phospholipase (Bernardo et al., 2008; Lee et al., 2009). The vps4 mutant causes greatly decreased virulence in both a mouse tail model of disseminated infection and in a C. elegans model of infection. The null mutant also has decreased macrophage killing ability and causes less tissue damage to epithelial cells, showing that the pre-vacuolar secretory pathway plays a role in several virulence-related aspects in C. albicans (Rane et al., 2014b).
In addition to vesicle trafficking, vacuolar calcium and iron transport are crucial for C. albicans virulence and hyphal formation. Some proteins involved in vacuolar ion transport are shown in Figure 1. Ca2+ participates in various signaling pathways to mediate cellular responses, and a low cytosolic Ca2+ concentration is required (Li et al., 2018). The vacuole is the site for calcium storage to maintain the optimum intracellular calcium level. The major vacuolar importer and exporters are the Ca2+ channel Yvc1, the Ca2+ pump Pmc1 and the Ca2+/H+ exchanger Vcx1, which are all vital to Ca2+ homeostasis (Cunningham, 2011).
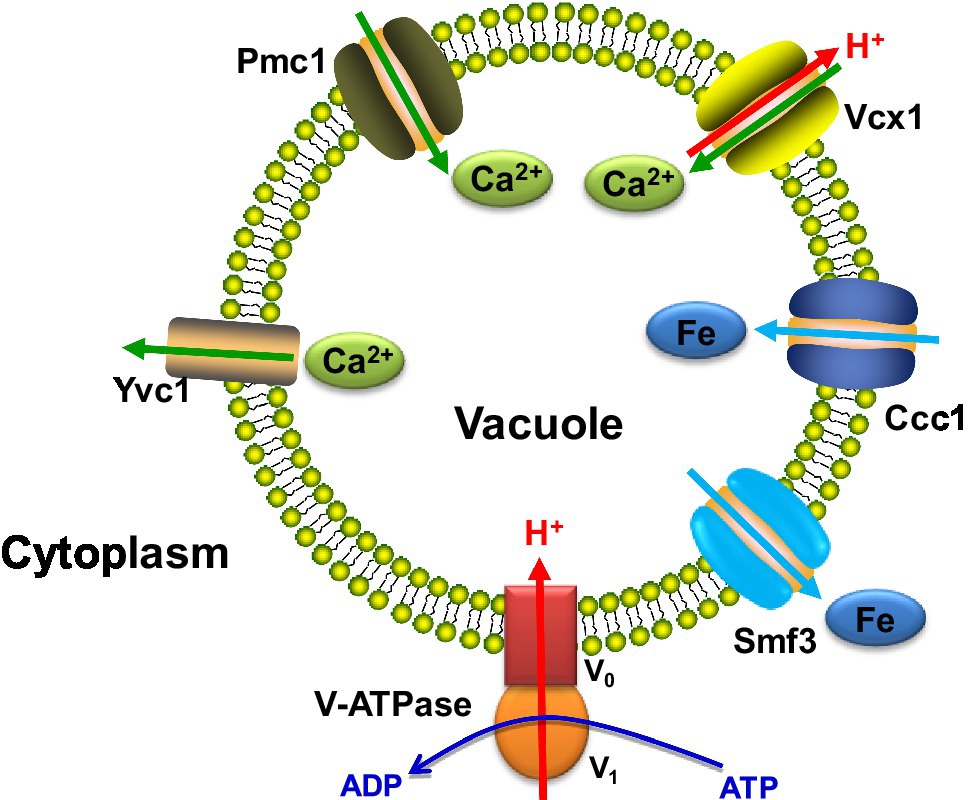
Figure 1. The major ion transporters located on the vacuolar membrane in C. albicans. V-ATPase, pumps H+ from the cytoplasm to the vacuole. Yvc1, transports Ca2+ from the vacuole to the cytoplasm. Pmc1, transports Ca2+ from the cytoplasm to the vacuole. Vcx1, the Ca2+/H+ exchanger. Ccc1, the vacuolar iron importer. Smf3, the vacuolar iron exporter. The arrows represent the direction of ion transport.
Upon a hypotonic shock, vacuolar Yvc1 releases Ca2+ into the cytosol. A study on Yvc1’s importance for C. albicans shows that the yvc1 null mutant has a much weaker calcium pulse under alkaline pH or hypertonic shock, and a second fluctuation, where Yvc1 releases vacuolar calcium in response to the stimuli to increase cytosolic calcium levels, is reduced. Yvc1 thus plays a part in mediating the increase of cytoplasmic calcium levels after external stimuli.
In addition, the yvc1 null mutant shows a reduction in hyphal development, producing mainly pseudohyphae, and has defects in biofilm development and hyphal polarized growth. Yvc1 has a role in activating expression of hypha-specific genes during hyphal growth, and the virulence of C. albicans without Yvc1 is highly attenuated in a mouse model of systemic infection. Also, the damage ability of the yvc1 null mutant is significantly decreased compared to WT during invasion of human epithelial cells.
Yvc1 mediates stress resistance after stimulation by controlling cytoplasmic calcium levels and the subsequent activation of calcium signaling pathways, and it has a role in hyphal growth and re-orientation to host cells. These observations suggest why this putative vacuolar Ca2+ channel has an important part in maintaining the virulence of C. albicans (Yu et al., 2014).
Pmc1 and Vxc1 sequester Ca2+ ions into the vacuole (Cunningham, 2011). The C. albicans pmc1 null mutant was severely impaired when CaCl2 concentrations are high, while the vxc1 null mutant is unaffected. This suggests a significant role of Pmc1 in calcium homeostasis and stress tolerance. Also, the loss of PMC1 impairs the cell’s ability to form hyphae, and this negatively affects the pmc1 null mutant’s biofilm development, which is both related to the high calcium concentration caused by the loss of calcium detoxification performed by Pmc1. Furthermore, the pmc1 null mutant is avirulent in a mouse model of disseminated infection, while the vxc1 null mutant shows no difference compared to wild type in these aspects. Pmc1, with its calcium mediation function, has proved to be essential for the pathogenicity and virulence of C. albicans (Luna-Tapia et al., 2019).
Iron homeostasis has been found to be critical for the regulation of commensalism and pathogenicity of C. albicans (Noble et al., 2017; Tripathi et al., 2020). Because they maintain the major iron pools in fungi, mitochondria and vacuoles play central roles in modulating intracellular iron homeostasis. The vacuolar iron importer Ccc1 and exporter Smf3 are confirmed to regulate both cellular iron levels and hyphal development in C. albicans. However, the hyphal development and virulence deficiencies caused by CCC1 and SMF3 knockouts are not as significant as those caused by the disruption of the mitochondrial iron transporter MRS4. In addition, CCC1 disruption could rescue the filamentous development and virulence in the mrs4Δ/Δ mutant, which suggests an opposing influence of Mrs4 and Ccc1 on iron homeostasis (Xu et al., 2014).
V-ATPases Subunits and Assembly Factors Maintain Vacuolar Calcium Homeostasis
Vacuolar calcium channels have been found to be affected by other regulators, especially those related to V-ATPases. For example, the absence of the assembly factor Vph2 of the V-ATPase and the loss of Tfp1, the subunit a of the V1 domain, causes abnormal localization of Yvc1 and leads to the disruption of calcium transport from the vacuoles to the cytosol (Peng et al., 2020). The vph2 null mutant has attenuated pathogenicity (Jia et al., 2018b), and the tfp1 null mutant has significantly increased cytosolic calcium levels, indicating its importance in ion homeostasis (Jia et al., 2015). The tfp1 pmc1 double mutant has increased disruption in calcium homeostasis compared to the pmc1 null mutant alone. The vph2 or vma6 null mutants give rise to abnormal localization of Tfp1, which consequently affects the vacuolar calcium channel Yvc1 (Jia et al., 2018a). Overall, the proteins involved in vacuolar protein or ion transport mentioned above are critical for maintaining the pathogenicity of C. albicans.
Conclusion and Perspectives
In summary, studies have found C. albicans virulence is affected by several aspects of vacuolar function including vacuolar pH, vacuolar trafficking, calcium homeostasis, and iron homeostasis. The master pump V-ATPase maintains vacuolar pH and is crucial for pathogenesis and virulence, and its loss of activity also affects hyphal growth and calcium channel function. As well, the calcium and iron channels are necessary for filamentation and biofilm development in C. albicans. Vacuolar trafficking also controls vacuolar morphology, V-ATPase activity, autophagy, and hyphal growth, elaborating the role of VPS genes in the pathogenicity of C. albicans. This vacuolar trafficking process also involves the interaction of multiple protein families, such as Rho/Rab GTPases, guanylate exchange factors, the HOPS (homotypic fusion and vacuole protein sorting) complex, and the SNARE (soluble NSF attachment protein receptor) complex; this extensive system has not been detailed in this focused review (Bröcker et al., 2010). Moreover, vacuolar fusion can influence hyphal compartments; the highly fragmented vacuoles in C. albicans enable hyphal extensions and septation with reduced branching frequencies. These interconnected pathways may have further potential as targets for future antifungal drug discovery. Thus, further research is still needed to fully understand both morphogenesis and the role of vacuoles in the mechanisms behind pathogenesis and virulence in C. albicans.
Author Contributions
LY conceived and wrote the review. QL and YJ conceived and searched the references. All authors contributed to the article and approved the submitted version.
Funding
This review is funded by the National Natural Science Foundation of China (82173867), Shanghai Science and Technology Innovation Action Plan, International Science and Technology Cooperation Project (21430713000), Shanghai Science and Technology Support Project in the Field of Biomedicine Project (19431901300), Shanghai Sailing Program (19YF1458800) and the key project of the National Natural Science Foundation of China (81830106), and Shanghai Pujiang Program (21PJD081).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Malcolm Whiteway (Concordia University) for critical reading of our manuscript.
References
Armstrong, J. (2010). Yeast vacuoles: more than a model lysosome. Trends Cell Biol. 20, 580–585. doi: 10.1016/j.tcb.2010.06.010
Berman, J., and Krysan, D. J. (2020). Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 18, 319–331. doi: 10.1038/s41579-020-0415-y
Bernardo, S. M., Khalique, Z., Kot, J., Jones, J. K., and Lee, S. A. (2008). Candida albicans VPS1 contributes to protease secretion, filamentation, and biofilm formation. Fungal Genet. Biol. 45, 861–877. doi: 10.1016/j.fgb.2008.01.001
Bianchi, F., Van’t Klooster, J. S., Ruiz, S. J., and Poolman, B. (2019). Regulation of amino acid transport in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 83:e00024-19. doi: 10.1128/MMBR.00024-19
Bröcker, C., Engelbrecht-Vandré, S., and Ungermann, C. (2010). Multisubunit tethering complexes and their role in membrane fusion. Curr. Biol. 20, R943–R952. doi: 10.1016/j.cub.2010.09.015
Bruckmann, A., Kunkel, W., Hartl, A., Wetzker, R., and Eck, R. (2000). A phosphatidylinositol 3-kinase of Candida albicans influences adhesion, filamentous growth and virulence. Microbiology 146, 2755–2764. doi: 10.1099/00221287-146-11-2755
Chen, H., Zhou, X., Ren, B., and Cheng, L. (2020). The regulation of hyphae growth in Candida albicans. Virulence 11, 337–348. doi: 10.1080/21505594.2020.1748930
Chung, J. H., Lester, R. L., and Dickson, R. C. (2003). Sphingolipid requirement for generation of a functional v1 component of the vacuolar ATPase. J. Biol. Chem. 278, 28872–28881. doi: 10.1074/jbc.M300943200
Cornet, M., Bidard, F., Schwarz, P., Da Costa, G., Blanchin-Roland, S., Dromer, F., et al. (2005). Deletions of endocytic components VPS28 and VPS32 affect growth at alkaline pH and virulence through both RIM101-dependent and RIM101-independent pathways in Candida albicans. Infect. Immun. 73, 7977–7987. doi: 10.1128/IAI.73.12.7977-7987.2005
Cunningham, K. W. (2011). Acidic calcium stores of Saccharomyces cerevisiae. Cell Calcium 50, 129–138. doi: 10.1016/j.ceca.2011.01.010
Du, H., and Huang, G. (2016). Environmental pH adaption and morphological transitions in Candida albicans. Curr. Genet. 62, 283–286. doi: 10.1007/s00294-015-0540-8
Jenks, J. D., Cornely, O. A., Chen, S. C., Thompson, G. R. 3rd, and Hoenigl, M. (2020). Breakthrough invasive fungal infections: who is at risk? Mycoses 63, 1021–1032. doi: 10.1111/myc.13148
Jia, C., Yu, Q., Xu, N., Zhang, B., Dong, Y., Ding, X., et al. (2014). Role of TFP1 in vacuolar acidification, oxidative stress and filamentous development in Candida albicans. Fungal Genet. Biol. 71, 58–67. doi: 10.1016/j.fgb.2014.08.012
Jia, C., Zhang, K., Yu, Q. L., Zhang, B., Xiao, C. P., Dong, Y. J., et al. (2015). Tfp1 is required for ion homeostasis, fluconazole resistance and N-Acetylglucosamine utilization in Candida albicans. BBA-Mol. Cell. Res. 1853, 2731–2744. doi: 10.1016/j.bbamcr.2015.08.005
Jia, C., Zhang, K., Zhang, D., Yu, Q., Xiao, C., Dong, Y., et al. (2018a). Effects of disruption of PMC1 in the tfp1/mutant on calcium homeostasis, oxidative and osmotic stress resistance in Candida albicans. Mycopathologia 183, 315–327. doi: 10.1007/s11046-017-0216-7
Jia, C., Zhang, K., Zhang, D., Yu, Q., Zhao, Q., Xiao, C., et al. (2018b). Roles of VPH2 and VMA6 in localization of V-ATPase subunits, cell wall functions and filamentous development in Candida albicans. Fungal Genet. Biol. 114, 1–11. doi: 10.1016/j.fgb.2018.03.001
Kane, P. M. (2006). The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol. Mol. Biol. Rev. 70, 177–191. doi: 10.1128/MMBR.70.1.177-191.2006
Kane, P. M. (2007). The long physiological reach of the yeast vacuolar H+-ATPase. J. Bioenerg. Biomembr. 39, 415–421. doi: 10.1007/s10863-007-9112-z
Kim, S. W., Park, Y. K., Joo, Y. J., Chun, Y. J., Hwang, J. Y., Baek, J. H., et al. (2019). Subunits of the vacuolar H+-ATPase complex, Vma4 and Vma10, are essential for virulence and represent potential drug targets in Candida albicans. Fungal Biol. 123, 709–722. doi: 10.1016/j.funbio.2019.06.002
Kulkarny, V. V., Chavez-Dozal, A., Rane, H. S., Jahng, M., Bernardo, S. M., Parra, K. J., et al. (2014). Quinacrine inhibits Candida albicans growth and filamentation at neutral pH. Antimicrob. Agents Chemother. 58, 7501–7509. doi: 10.1128/AAC.03083-14
Lee, S. A., Jones, J., Hardison, S., Kot, J., Khalique, Z., Bernardo, S. M., et al. (2009). Candida albicans VPS4 is required for secretion of aspartyl proteases and In vivo virulence. Mycopathologia 167, 55–63. doi: 10.1007/s11046-008-9155-7
Li, Y., Sun, L., Lu, C., Gong, Y., Li, M., and Sun, S. (2018). Promising antifungal targets against Candida albicans based on ion homeostasis. Front. Cell. Infect. Microbiol. 8:286. doi: 10.3389/fcimb.2018.00286
Luna-Tapia, A., DeJarnette, C., Sansevere, E., Reitler, P., Butts, A., Hevener, K. E., et al. (2019). The Vacuolar Ca(2+) ATPase pump Pmc1p is required for Candida albicans pathogenesis. mSphere 4:e00715-18. doi: 10.1128/mSphere.00715-18
Marshansky, V., and Futai, M. (2008). The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr. Opin. Cell Biol. 20, 415–426. doi: 10.1016/j.ceb.2008.03.015
Noble, S. M., Gianetti, B. A., and Witchley, J. N. (2017). Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 15, 96–108. doi: 10.1038/nrmicro.2016.157
Olsen, I. (2014). Attenuation of Candida albicans virulence with focus on disruption of its vacuole functions. J. Oral Microbiol. 6:23898. doi: 10.3402/jom.v6.23898
Palmer, G. E. (2010). Endosomal and AP-3-dependent vacuolar trafficking routes make additive contributions to Candida albicans hyphal growth and pathogenesis. Eukaryot. Cell 9, 1755–1765. doi: 10.1128/EC.00029-10
Palmer, G. E., Cashmore, A., and Sturtevant, J. (2003). Candida albicans VPS11 is required for vacuole biogenesis and germ tube formation. Eukaryot. Cell 2, 411–421. doi: 10.1128/EC.2.3.411-421.2003
Palmer, G. E., Kelly, M. N., and Sturtevant, J. E. (2005). The Candida albicans vacuole is required for differentiation and efficient macrophage killing. Eukaryot. Cell 4, 1677–1686. doi: 10.1128/EC.4.10.1677-1686.2005
Parra, K. J., Chan, C. Y., and Chen, J. (2014). Saccharomyces cerevisiae Vacuolar H+ -ATPase regulation by disassembly and reassembly: one structure and multiple signals. Eukaryot. Cell 13, 706–714. doi: 10.1128/EC.00050-14
Patenaude, C., Zhang, Y., Cormack, B., Kohler, J., and Rao, R. (2013). Essential role for vacuolar acidification in Candida albicans virulence. J. Biol. Chem. 288, 26256–26264. doi: 10.1074/jbc.M113.494815
Peng, L., Yu, Q., Zhu, H., Zhu, N., Zhang, B., Wei, H., et al. (2020). The V-ATPase regulates localization of the TRP ca(2+) channel Yvc1 in response to oxidative stress in Candida albicans. Int. J. Med. Microbiol. 310:151466. doi: 10.1016/j.ijmm.2020.151466
Poltermann, S., Nguyen, M., Gunther, J., Wendland, J., Hartl, A., Kunkel, W., et al. (2005). The putative vacuolar ATPase subunit Vma7p of Candida albicans is involved in vacuole acidification, hyphal development and virulence. Microbiology. 151, 1645–1655. doi: 10.1099/mic.0.27505-0
Rane, H. S., Bernardo, S. M., Hayek, S. R., Binder, J. L., Parra, K. J., and Lee, S. A. (2014a). The conƒtribution of Candida albicans vacuolar ATPase subunit V(1)B, encoded by VMA2, to stress response, autophagy, and virulence is independent of environmental pH. Eukaryot. Cell 13, 1207–1221. doi: 10.1128/EC.00135-14
Rane, H. S., Bernardo, S. M., Raines, S. M., Binder, J. L., Parra, K. J., and Lee, S. A. (2013). Candida albicans VMA3 is necessary for V-ATPase assembly and function and contributes to secretion and Filamentation. Eukaryot. Cell 12, 1369–1382. doi: 10.1128/EC.00118-13
Rane, H. S., Hardison, S., Botelho, C., Bernardo, S. M., Wormley, F. Jr., and Lee, S. A. (2014b). Candida albicans VPS4 contributes differentially to epithelial and mucosal pathogenesis. Virulence 5, 810–818. doi: 10.4161/21505594.2014.956648
Sambade, M., Alba, M., Smardon, A. M., West, R. W., and Kane, P. M. (2005). A genomic screen for yeast vacuolar membrane ATPase mutants. Genetics 170, 1539–1551. doi: 10.1534/genetics.105.042812
Tripathi, A., Liverani, E., Tsygankov, A. Y., and Puri, S. (2020). Iron alters the cell wall composition and intracellular lactate to affect Candida albicans susceptibility to antifungals and host immune response. J. Biol. Chem. 295, 10032–10044. doi: 10.1074/jbc.RA120.013413
Veses, V., Richards, A., and Gow, N. A. (2008). Vacuoles and fungal biology. Curr. Opin. Microbiol. 11, 503–510. doi: 10.1016/j.mib.2008.09.017
Xu, N., Dong, Y., Cheng, X., Yu, Q., Qian, K., Mao, J., et al. (2014). Cellular iron homeostasis mediated by the Mrs4-Ccc1-Smf3 pathway is essential for mitochondrial function, morphogenesis and virulence in Candida albicans. Biochim. Biophys. Acta 1843, 629–639. doi: 10.1016/j.bbamcr.2013.12.009
Yu, Q., Wang, F., Zhao, Q., Chen, J., Zhang, B., Ding, X., et al. (2014). A novel role of the vacuolar calcium channel Yvc1 in stress response, morphogenesis and pathogenicity of Candida albicans. Int. J. Med. Microbiol. 304, 339–350. doi: 10.1016/j.ijmm.2013.11.022
Zhang, K., Jia, C., Yu, Q., Xiao, C., Dong, Y., Zhang, M., et al. (2017). Contribution of VMA5 to vacuolar function, stress response, ion homeostasis and autophagy in Candida albicans. Future Microbiol. 12, 1147–1166. doi: 10.2217/fmb-2017-0029
Keywords: Candida albicans, vacuolar proton-translocating ATPases, vacuolar protein sorting components, vacuolar Ca2+ channel, virulence
Citation: Lv Q, Yan L and Jiang Y (2021) The Importance of Vacuolar Ion Homeostasis and Trafficking in Hyphal Development and Virulence in Candida albicans. Front. Microbiol. 12:779176. doi: 10.3389/fmicb.2021.779176
Edited by:
Lele Zhu, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Sandra Paiva, University of Minho, PortugalZeeshan Fatima, Amity University Gurgaon, India
Copyright © 2021 Lv, Yan and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Yan, eWxhbnNtbXVAc2luYS5jb20=
 Quanzhen Lv
Quanzhen Lv Lan Yan
Lan Yan Yuanying Jiang2
Yuanying Jiang2