- 1Laboratory of Protozoological Biodiversity and Evolution in Wetland, College of Life Sciences, Shaanxi Normal University, Xi’an, China
- 2Key Laboratory for Space Bioscience and Biotechnology, Institute of Special Environmental Biophysics, School of Life Sciences, Northwestern Polytechnical University, Xi’an, China
The morphology and morphogenesis of Pseudosincirra longicirrata nov. gen. and nov. comb., isolated from southern China, were investigated with living observation and protargol staining. Our population is similar to the original population in living characteristics and ciliary patterns. The main determinable morphogenetic features of P. longicirrata nov. comb. are the presence of five frontoventral-transverse cirral anlagen (FVT-anlagen) and a dorsomarginal kinety anlage. According to the origin of FVT-anlagen IV and V in proter, it can be determined that P. longicirrata nov. comb. possesses two frontoventral rows and one right marginal row. Hence, a new genus, Pseudosincirra nov. gen., is proposed, and the diagnosis of P. longicirrata nov. comb. is improved. The new genus is diagnosed as follows: adoral zone of membranelles and undulating membranes is in a Gonostomum pattern; there are three enlarged frontal cirri, one buccal cirrus, and one parabuccal cirrus; postperistomial cirrus and transverse cirri are lacking; there are two more or less long frontoventral rows and one right and two or more left marginal rows; cirri within all rows very widely spaced; dorsal kinety pattern is of Urosomoida type, that is, three dorsal kineties and one dorsomarginal kinety; and caudal cirri are present. Phylogenetic analyses based on the small subunit ribosomal (SSU rDNA) sequence data indicate that P. longicirrata nov. comb. clusters with Deviata and Perisincirra. It is considered that Pseudosincirra nov. gen. and Perisincirra paucicirrata should be assigned to the family Deviatidae; fine cirri, and cirri within all rows being relatively widely spaced, should be considered as plesiomorphies of Deviatidae; and Deviatidae is closely related to Dorsomarginalia or Strongylidium–Hemiamphisiella–Pseudouroleptus.
Introduction
Ciliates are a large group of unicellular eukaryotes, many of which have cosmopolitan distributions (Corliss, 1979; Lynn, 2008). Hypotrichs are the most complex and highly differentiated group of ciliates, have a huge diversity with over 1,000 nominal species, and are abundant in waters (Berger, 1999, 2006, 2008, 2011; Foissner, 1999; Foissner, 2016; Song and Shao, 2017; Paiva, 2020; Shao et al., 2020; Wang et al., 2021). This group is playing an important role in the remineralization of organic material in aquatic ecosystems (Foissner, 1999). Besides, numerous recent studies have also focused on the classification and phylogeny of hypotrichs (Kaur et al., 2019; Park et al., 2020; Zhang et al., 2020; Chen et al., 2021; Jung et al., 2021; Li et al., 2021a,b; Luo et al., 2021; Omar et al., 2021a,b).
Stichotrichida is a group of hypotrichous ciliates that has always been difficult to classify. Its establishment is more due to the variable number and non-grouping of frontoventral-transverse cirral rows in its ciliary pattern (Berger, 2008; Lynn, 2008). Previous studies have shown that the dorsal infraciliature is at least as important as the ventral ciliary pattern for the estimation of the major phylogenetic relationships within the hypotrichs, and its application to the systematics of stichotrichids may prove to be particularly beneficial (Foissner et al., 2002; Berger, 2011).
The genus Perisincirra was erected by Jankowski (1978) with Perisincirra kahli (Grolière, 1975) Jankowski, 1978 as the type species. Unfortunately, some important morphological, morphogenetic, and molecular data are lacking for the type species P. kahli, so its diagnostic characters are insufficient for the systematics and classification of Perisincirra to be resolved. Thus, the inclusion of Perisincirra longicirrata Foissner et al., 2002 in this genus has been questioned. Here, the morphogenesis and phylogenetic position of Perisincirra longicirrata were investigated and discussed to determine whether it belongs to Perisincirra or if it represents a new genus.
Materials and Methods
Sample Site and Cultivation
On 12th September 2018, a mixture of water and silt was collected from a freshwater pond in Shenzhou Peninsula tourist resort (18°40′32.27′′N; 110°20′49.88′′E) in Wanning, China, when the water temperature was 25°C and the pH was about 7.0. The cells of Pseudosincirra longicirrata nov. comb. were isolated from the sample and were cultured at room temperature (25°C) in Petri dishes containing mineral water (Nongfu Spring) with rice grains added to promote the growth of bacteria as food for the ciliates. Although we failed to establish a clonal culture, there were no other hypotrichous morphospecies in the Petri dishes. Therefore, we are certain that the present morphological, morphogenetic, and molecular studies deal solely with Pseudosincirra longicirrata nov. comb.
Morphology
Cells from the cultures were studied in vivo using bright-field and differential interference contrast microscopy at magnifications of 40–1,000 (Bai et al., 2020). The protargol (Sigma-Aldrich) silver staining method was used to reveal the infraciliature and nuclear apparatus (Wilbert, 1975). The drawings of stained specimens were made with a drawing device (Wu et al., 2020). To illustrate the changes occurring during morphogenetical processes, old (parental) ciliary structures are depicted by contour, whereas new ones are shaded black. Terminology follows Berger (2011).
DNA Extraction, PCR Amplification, and Sequencing
Single cell of Pseudosincirra longicirrata nov. comb. was washed three times with sterilized water to remove contaminants and then transferred to a 1.5-ml microfuge tube with a minimum volume of water. Genomic DNA was extracted using a DNeasy Blood & Tissue Kit (Qiagen, Germany) following the manufacturer’s instructions. Small subunit ribosomal (SSU rDNA) amplification from the extracted DNA was carried out using primers 82S-F (5′-GAA ACT GCG AAT GGC TC-3′), 900F (5′-CGA TCA GAT ACC GTC CTA GT-3′), Pro B (5′-GGT TAA AAA GCT CGT AGT-3′), 900R (5′-ACT AGG ACG GTA TCT GAT CG-3′), and 18S-R (5′-GAT CCT TCT GCA GGT TCA CCT AC -3′). The conditions for PCR were as follows: denaturation at 98°C for 2 min; followed by 30 cycles of denaturation at 98°C for 10 s, annealing at 56°C for 15 s, extension at 72°C for 1 min 50 s, and a final extension step at 72°C for 7 min. Sequencing was performed bidirectionally by the Tsingke Biotechnology Co., Ltd. Xi’an Branch.
Phylogenetic Analyses
The SSU rDNA sequence of Pseudosincirra longicirrata nov. comb. and those of 68 other hypotrichs downloaded from GenBank database were used for phylogenetic analyses. Four euplotid species were used as outgroup taxa (for accession numbers, see Figure 5). Sequences were aligned using the GUIDANCE web server1 (Penn et al., 2010). Maximum likelihood (ML) analyses were performed using RAxML-HPC2 on XSEDE v8.2.12 (Stamatakis et al., 2008) on the online server CIPRES Science Gateway2 (Miller et al., 2010). Bayesian inference (BI) analyses were carried out using MrBayes on XSEDE v3.2.7a (Ronquist et al., 2012) on CIPRES Science Gateway with the GTR + I + G model selected by Akaike information criterion (AIC) in MrModeltest v2 (Nylander, 2004). MEGA v5 was used to visualize the tree topologies (Tamura et al., 2011).
Results
ZooBank Registration
The ZooBank LSIDs are as follows:
Present work: urn:lsid:zoobank.org:pub:26F8BE7E-2AB4-40CB-AE5F-003D5019DBDB
Pseudosincirra nov. gen.: urn:lsid:zoobank.org:act: C46FEDCE-946F-4054-91CA-441CC0A42A68
Pseudosincirra longicirrata nov. comb.: urn:lsid:zoobank. org:act:C537D11B-3F9E-4366-B121-59FC1163FB28
Pseudosincirra nov. gen.
Diagnosis
The new genus is diagnosed as follows: adoral zone of membranelles and undulating membranes is in Gonostomum pattern. There are three enlarged frontal cirri, one buccal cirrus, and one parabuccal cirrus. Postperistomial cirrus and transverse cirri are lacking. There are two long frontoventral rows and one right and two or more left marginal rows, and cirri within all rows are widely spaced. Dorsal kineties are in Urosomoida pattern, that is, three dorsal kineties and one dorsomarginal kinety. Caudal cirri are present.
Etymology
The name is a composite of pseudo- (false, i.e., resembling but not equaling) and suffix (-sincirra) of the genus name Perisincirra Jankowski, 1978. This indicates that Pseudosincirra has a cirral pattern similar to that of Perisincirra and has a feminine gender.
Type of Species
The type of species is Perisincirra longicirrata Foissner et al., 2002.
Remarks
Pseudosincirra longicirrata nov. comb. was previously assigned in Perisincirra as Perisincirra longicirrata. However, according to the origin of frontoventral-transverse cirral anlagen (FVT-anlagen) IV and V (for details, see Divisional Morphogenesis), it can be deduced that right marginal rows 1 and 2 of Pseudosincirra longicirrata nov. comb. described in Foissner et al. (2002) are actually frontoventral rows. It differs from the type species of Perisincirra, P. kahli (Grolière, 1975) Jankowski, 1978, in having two (vs. one) long ventral rows. Hence, a new genus, Pseudosincirra nov. gen., was erected.
Pseudosincirra longicirrata (Foissner et al., 2002) nov. comb.
Improved Diagnosis
The size is 60–130 × 20–50 μm in vivo, with elongate ellipsoidal to bluntly fusiform. There are two macronuclear nodules and 18–26 adoral membranelles. There are two long frontoventral rows comprising 7–16 and 9–18 cirri, respectively. Three left marginal rows are composed of 5–14, 4–12, and 3–10 cirri, from inner to outer row, respectively. There is one right marginal row with 7–16 cirri. There are three frontal, one buccal, one parabuccal, and three caudal cirri. All the cirri are widely spaced and fine, up to 30-μm long.
Voucher Slides
Seven voucher slides (no. GQ2018091202A–G) with protargol-stained specimens were deposited in the Laboratory of Protozoological Biodiversity and Evolution in Wetland, Shaanxi Normal University, China.
Morphological Redescription
The body size is 90–130 × 30–50 μm in vivo (n = 16), usually about 120 × 40 μm; the length-to-width ratio is about 3:1 in live and 2–2.8:1, on average 2.5:1, in protargol preparations due to cell expansion caused by the fixative. The body is usually elongated ellipsoidal in shape, occasionally fusiform with the anterior region slightly more narrowed and the posterior portion indistinctly pointed (Figures 1A,D,E). The body is flexible but not contractile, only slightly flattened dorsoventrally. Invariably, two ellipsoidal macronuclear nodules, about 17 × 9 μm in size (after protargol staining), are located behind the buccal vertex near midline. Usually there are two, sometimes three, globular micronuclei about 2 μm in diameter, two of which are closely associated with macronuclear nodules (Figures 1C,J). A single contractile vacuole without distinct collecting canals, about 13 μm in diameter, is contracting at intervals of about 12 s, located about 33% down the length of the body near the left cell margin. Cortical granules are lacking. The cytoplasm is colorless, usually packed with highly refractive fat globules 1–6 μm across, making cells appear dark under low magnification. Crystals are sparse or lacking. Many individuals have food vacuole with orange contents on the right side of their bodies, about 20 μm across. Locomotion by slowly crawling on substrate was observed; when suspended, swimming while rotating about the longitudinal axis was observed.
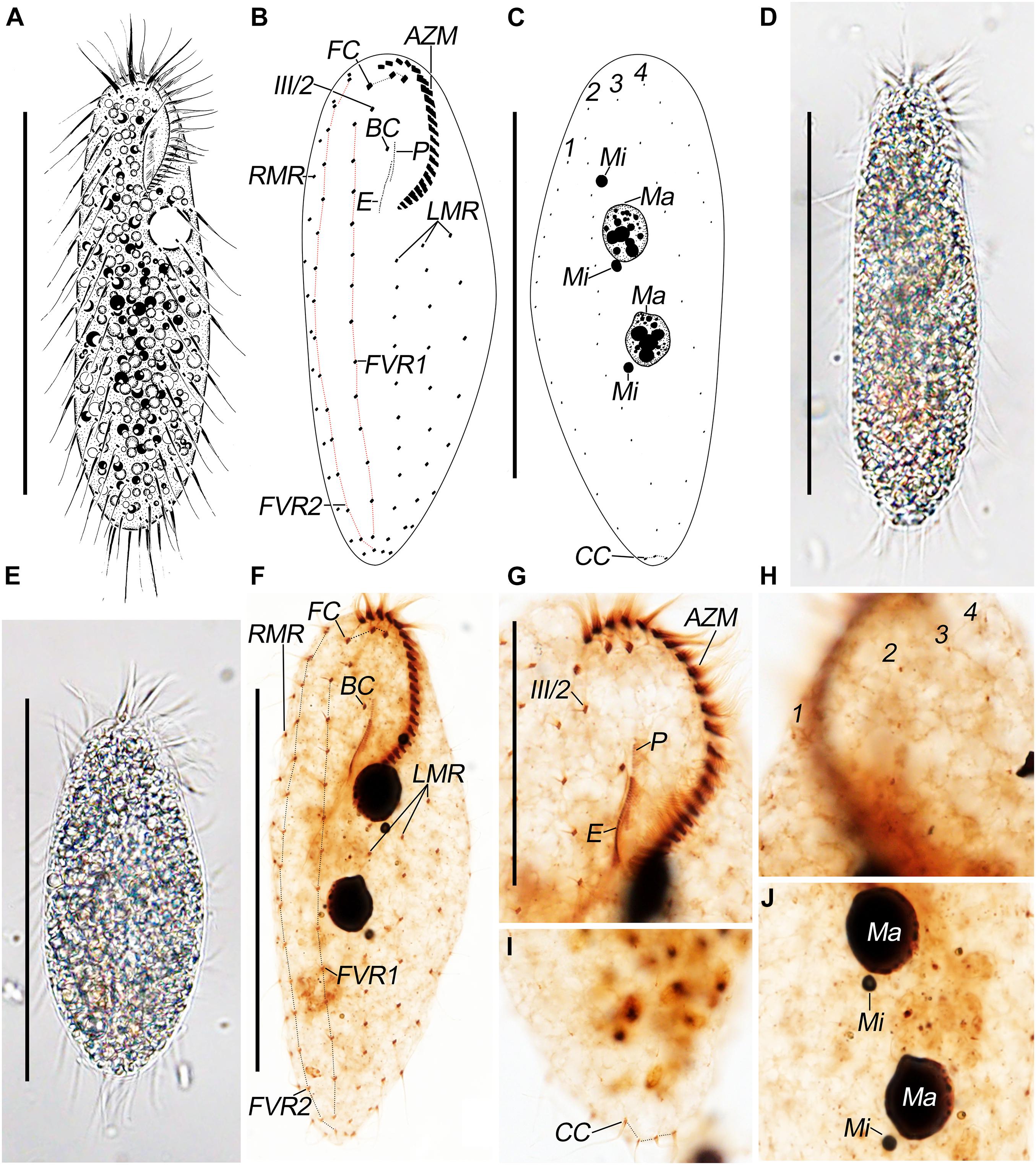
Figure 1. Pseudosincirra longicirrata nov. gen. and nov. comb. morphology. (A) Live and (B,C) after protargol staining. Photomicrographs of (D,E) live and (F–J) after protargol staining. (A) Ventral view of a representative individual. (B,C) Ventral (B) and dorsal (C) views to demonstrate the infraciliature. (D,E) Ventral views of representative individuals to show different body shapes. (F) Ventral view to demonstrate the infraciliature. (G) Ventral view of the anterior end of cell. (H–J) Dorsal views to demonstrate the dorsal kineties (H), caudal cirri (I), and nuclear apparatus (J). AZM, adoral zone of membranelles; BC, buccal cirrus; CC, caudal cirri; E, endoral; FC, frontal cirri; FVR 1, 2, frontoventral rows 1, 2; III/2, cirrus III/2; LMR, left marginal row; Ma, macronuclear nodules; Mi, micronuclei; P, paroral; RMR, right marginal row; 1–3, dorsal kineties; 4, dorsomarginal kinety. Scale bars = 100 μm (A–F) and 40 μm (G).
The adoral zone of membranelles is about 27% of the body length in vivo and 30% on average in protargol preparations due to strong cell expansion caused by the fixative. Oral apparatus is in Gonostomum pattern. The adoral zone is composed of 22–26 membranelles (n = 16) with cilia up to 17-μm long. The endoral is located behind and parallel to the paroral and are almost equal in length (Figures 1B,F,G; Table 1).

Table 1. Morphometric characterization of the Chinese population of Pseudosincirra longicirrata nov. gen. and nov. comb.
The ciliary pattern on the ventral side is rather constant although the number of cirri within rows is variable. All cirri are rather long and conspicuous, with cilia of the frontal cirri and the marginal cirri about 20-μm long. Constantly, there are three frontal cirri with the rightmost one in front of cirrus III/2. Buccal cirrus is ahead of the endoral and slightly behind the anterior end of the paroral. There are two long frontoventral rows; the inner row (FVR1) is composed of 13–16 cirri and commences slightly behind the level of cirrus III/2, and the outer row (FVR2) consists of 14–18 cirri and commences at the approximately same level as the rightmost frontal cirrus; both rows terminate at the posterior end of the cell. There are three widely spaced left marginal rows, commencing near the proximal end of the adoral zone, composed of 10–13, 10–12, and 7–9 cirri, from inner to outer row, respectively. Invariably, there is one right marginal row, with 12–16 cirri, which is almost bipolar (Figures 1B,F,G). All cirri are fine, and most frontoventral and marginal cirri are composed of four basal bodies and widely spaced.
Dorsal bristles are about 5-μm long; constantly arranged in four kineties; and composed of 12–14, 12–16, 11–13, and 5–8 dikinetids, respectively. Dorsal kineties 2 and 3 are almost bipolar; kinety 1 commences about 20% down the length of the body; kinety 4 (dorsomarginal kinety) terminates about 35% down the length of body. Usually, there are three caudal cirri, one at the posterior end of each of dorsal kineties 1–3; sometimes, there are four caudal cirri (in about 25% of the individuals), two at the posterior end of dorsal kinety 1 and one at the posterior end of each of dorsal kineties 2 and 3 (Figures 1C,H,I).
Divisional Morphogenesis
Development of the Ventral Ciliature
The oral primordium for the opisthe appears in the postoral area between the inner left marginal row and frontoventral row 1. With the proliferation of basal bodies, the oral primordium lengthens posteriorly and differentiates posteriad (Figures 2A–D, 4A,B). Three streaks are formed anteriorly to the right of the oral primordium (Figures 2C,D, 4C,E).
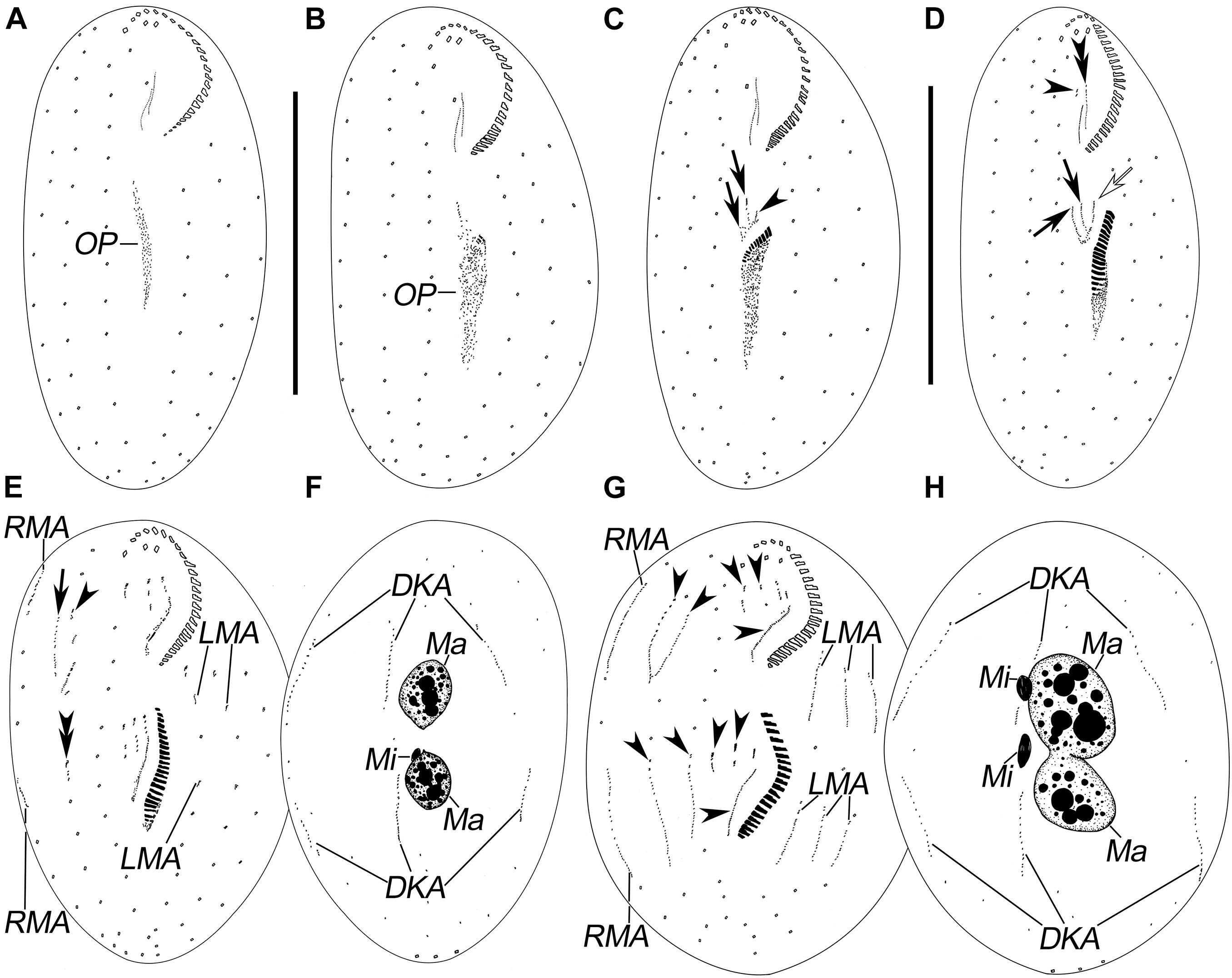
Figure 2. Morphogenesis of Pseudosincirra longicirrata nov. gen., nov. comb. after protargol staining. (A,B) Ventral views of very early dividers showing the newly formed oral primordium. (C) Ventral view of an early divider; arrows mark the FVT-anlagen and arrowhead points to the undulating membranes anlage. (D) Ventral view of a slightly later phase; arrows display the FVT-anlagen, hollow arrow denotes the undulating membrane anlage, and arrowhead and double arrowhead mark the dedifferentiation of buccal cirrus and the parental paroral and endoral, respectively. (E,F) Ventral and dorsal views of a later divider; the arrow shows anlage V in the proter, arrowhead denotes anlage IV in the proter, and double arrowhead marks anlage V in the opisthe. (G,H) Ventral and dorsal views of a cell in prometaphase; arrowheads demonstrate the anlagen I–V in the proter and opisthe, respectively. DKA, dorsal kinety anlagen; FVT-anlagen, frontoventral-transverse cirral anlagen; LMA, left marginal anlagen; Ma, macronuclear nodules; Mi, micronuclei; OP, oral primordium; RMA, right marginal anlagen. Scale bars = 100 μm.
In the proter, the parental adoral zone remains intact. Anlage I forms from the dedifferentiated parental paroral and endoral, anlage II develops from the dedifferentiated parental buccal cirrus, and anlage III is generated from the dedifferentiated parental cirrus III/2 (as shown in Figures 2D,E, 4D). Up to this point, three FVT-anlagen are formed in each filial product.
Soon after, in the proter, anlage IV is generated within the frontoventral row 2, and anlage V develops de novo to the right of frontoventral row 2. In the opisthe, anlagen IV and V are generated within frontoventral rows 1 and 2, respectively (Figures 2E,G, 4F). Then, in the proter, anlagen IV and V migrate to the mid-region of the body (Figures 2G, 3A, 4H,I).
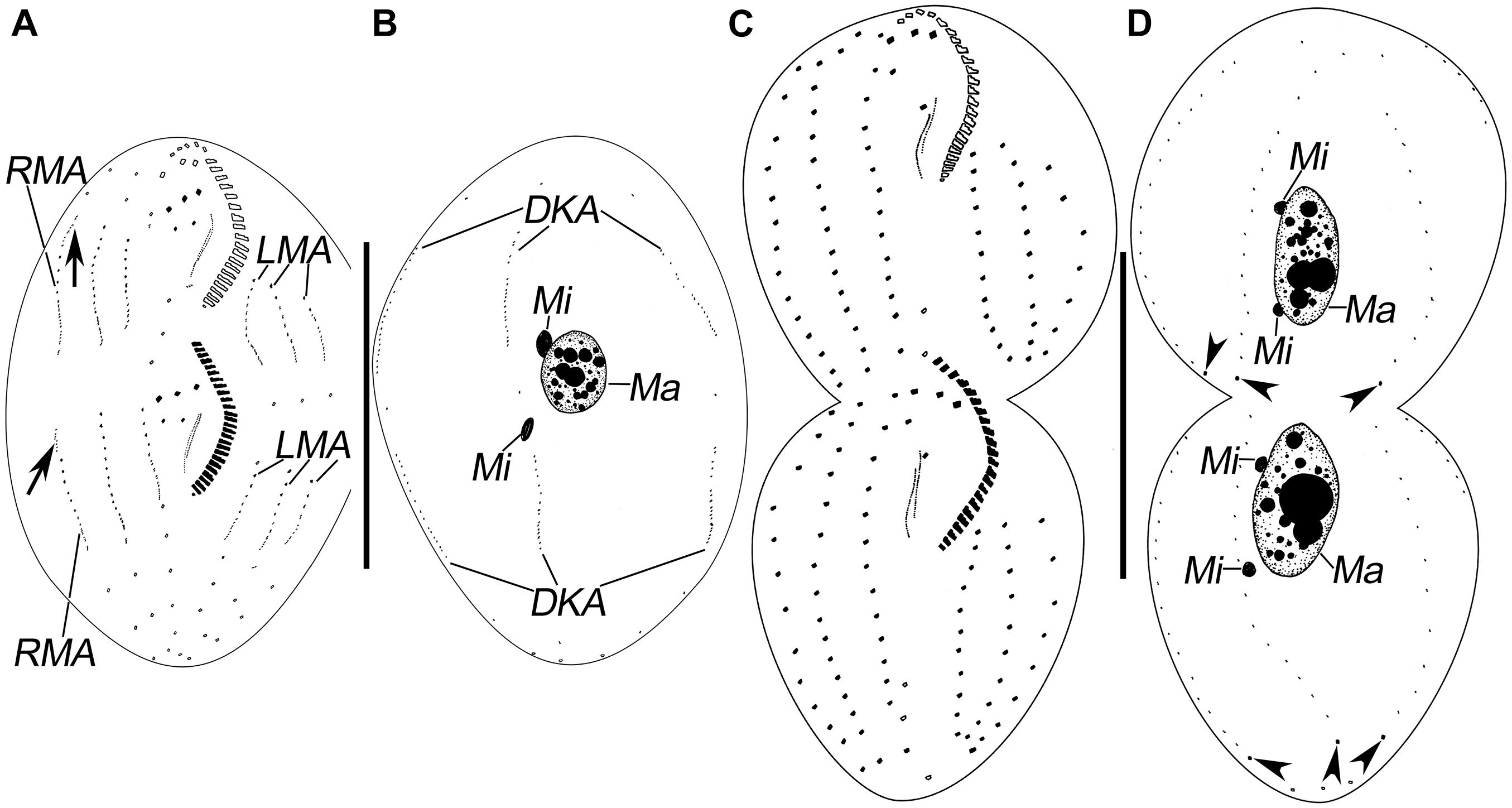
Figure 3. Morphogenesis of Pseudosincirra longicirrata nov. gen. and nov. comb. after protargol staining. (A,B) Ventral and dorsal views of a middle divider; arrows show the dorsomarginal kinety anlagen. (C,D) Ventral and dorsal views of a late divider to show the infraciliature; arrowheads point to the caudal cirri. DKA, dorsal kinety anlagen; LMA, left marginal anlagen; Ma, macronuclear nodules; Mi, micronuclei; RMA, right marginal anlagen. Scale bars = 100 μm.
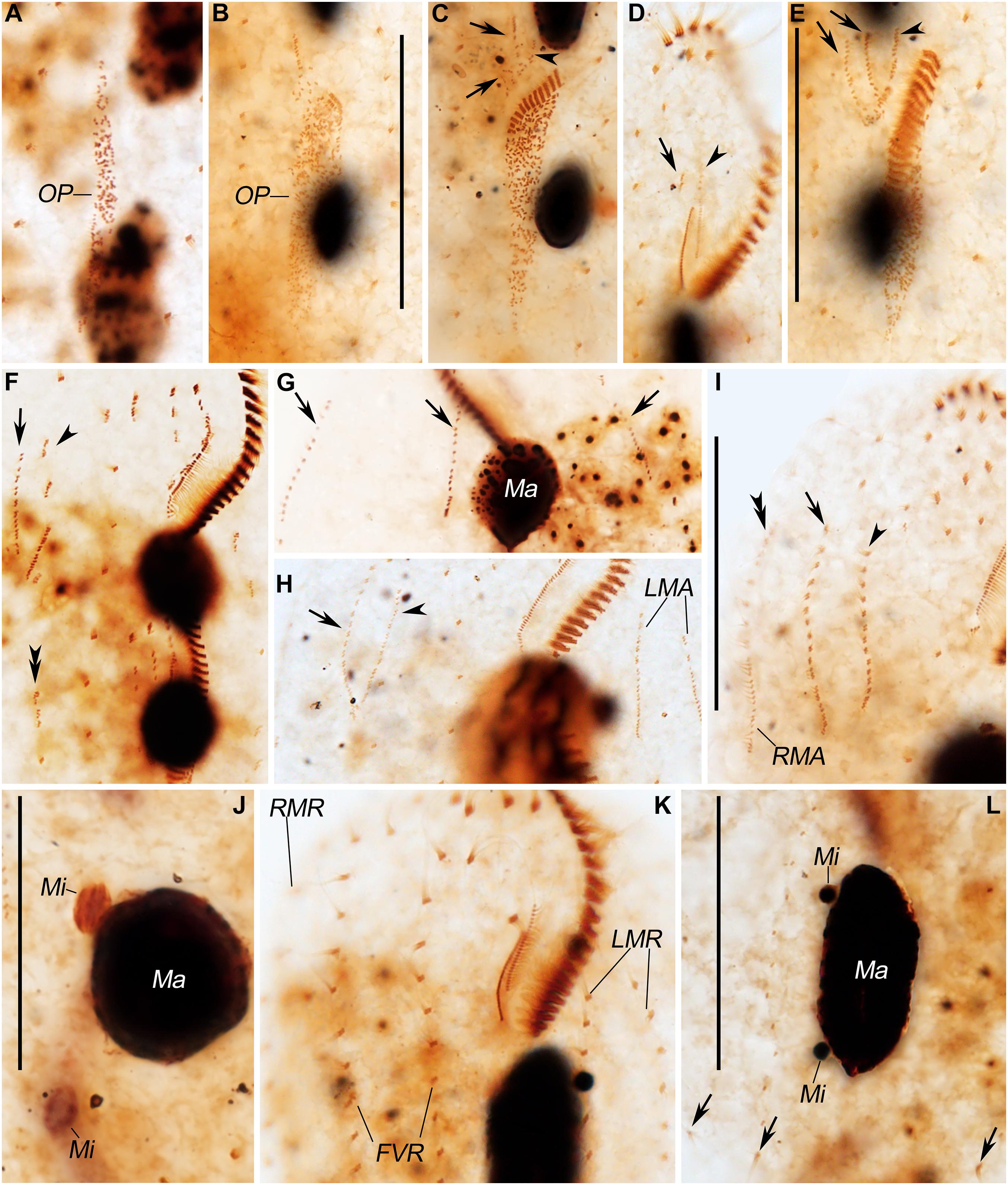
Figure 4. Photomicrographs of Pseudosincirra longicirrata nov. gen. and nov. comb. during morphogenesis after protargol staining. (A,B) Ventral views of very early phases showing the newly formed oral primordium. (C) Ventral view of an early divider; arrows display the FVT-anlagen, while arrowhead marks the undulating membrane anlage. (D,E) Ventral views of a slightly later divider. In panel (D), the arrow and arrowhead mark the dedifferentiation of buccal cirrus and the parental paroral and endoral, respectively. Arrows and arrowhead in panel (E) show the FVT-anlagen and undulating membrane anlage, respectively. (F,H,I) Ventral views; arrows mark anlage V in the proter, arrowheads denote anlage IV in the proter, double arrowhead in panel (F) reveals anlage V in the opisthe, and double-arrowhead in panel (I) points to the dorsomarginal kinety anlage. (G) Dorsal view; arrows demonstrate the dorsal kinety anlagen. (J) Dorsal view of the same cell as shown in panel (I), showing the fusion of the macronuclear nodules. (K,L) Ventral and dorsal views of a late divider, arrows in panel (L) indicate the newly formed caudal cirri. FVR, frontoventral rows; FVT-anlagen, frontoventral-transverse cirral anlagen; LMA, left marginal anlagen; LMR, left marginal row; Ma, macronuclear nodules; Mi, micronuclei; OP, oral primordium; RMA, right marginal anlagen; RMR, right marginal row. Scale bars = 40 μm.
Finally, anlage I produces the left frontal cirrus and undulating membranes, anlage II generates the middle frontal cirrus and buccal cirrus, anlage III develops into the right frontal cirrus and cirrus III/2, while anlagen IV and V generate frontoventral rows 1 and 2, respectively (Figures 3A,C, 4K).
Development of Marginal Rows and Dorsal Kineties
The marginal row anlagen and dorsal kinety anlagen develop intrakinetally within the parental marginal rows and dorsal kineties 1–3 in each daughter cell, respectively. These anlagen enlarge by proliferation of basal bodies and stretch in both directions to eventually replace the parental rows (Figures 2E–H, 3A,B, 4G–I). In addition, a short streak of basal bodies, i.e., the dorsal kinety 4 anlage, develops ahead of the anteriormost portion of the right marginal anlage in both the proter and the opisthe (Figures 3A, 4I). One or two caudal cirri are formed at the posterior end of dorsal kinety anlage 1, and constantly, one caudal cirrus is formed at the posterior end of each dorsal kineties anlagen 2 and 3 (Figures 3D, 4L).
Division of Nuclear Apparatus
In this process, the two macronuclear nodules fuse into a single mass (Figures 3B, 4J). At later stages of morphogenesis, the mass splits and is distributed between the proter and the opisthe (Figures 3D, 4L). The micronuclei divide mitotically (Figures 2H, 3B,D, 4J,L).
Phylogenetic Analyses Based on SSU rDNA Gene Sequences
The SSU rDNA sequence of Pseudosincirra longicirrata nov. comb. was deposited in GenBank with the accession number OK173050. The length and GC content of the new sequence are 1,613 bp and 45.38%, respectively. Since the topologies of ML tree and BI tree are basically the same, only the ML tree is shown with nodal support for both algorithms (Figure 5).
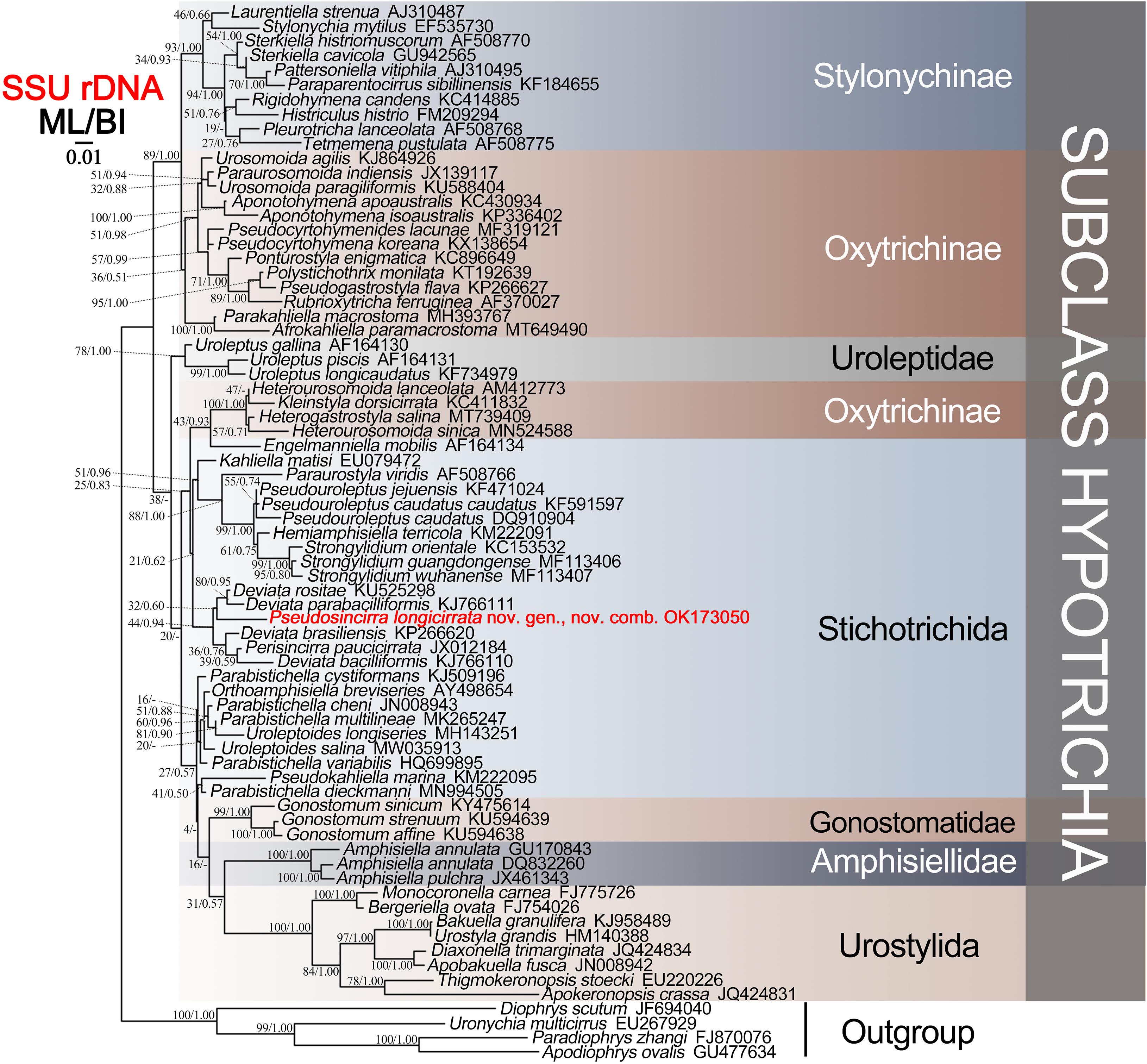
Figure 5. Maximum likelihood (ML) tree based on the SSU rDNA sequence data. The newly sequenced Pseudosincirra longicirrata nov. gen. and nov. comb. are indicated in red. Numbers at the nodes represent the bootstrap values of ML and the posterior probabilities of Bayesian analysis (BI), respectively. A hyphen “-” indicates the disagreement between the BI tree and the reference ML tree. All branches are drawn to scale. The scale bar corresponds to 0.01 expected substitutions per site.
The phylogenetic analyses showed that Pseudosincirra longicirrata nov. comb. groups with Deviata baciliformis (Gelei, 1954) Eigner, 1995; D. brasiliensis Siqueira-Castro et al., 2009; D. parabaciliformis Li et al., 2014; D. rositae Küppers et al., 2007; and Perisincirra paucicirrata Foissner et al., 2002. The SSU rDNA sequence similarities of Pseudosincirra longicirrata nov. comb. to D. baciliformis, D. brasiliensis, D. parabaciliformis, D. rositae, and P. paucicirrata are 94.2, 93.2, 94.3, 91.5, and 91.2%, respectively.
Discussion
Establishment of the New Genus
In terms of having three more or less long cirral rows right in the cell midline, two or more cirral rows left of the cell midline, and a dorsomarginal kinety, Pseudosincirra nov. gen. should be compared with six genera, namely, Afrokahliella Berger, 2011; Fragmocirrus Foissner, 2000; Idiodeviata Foissner, 2016; Kahliella Corliss, 1960; Neogeneia Eigner, 1995; and Parakahliella Berger et al., 1985.
The new genus differs from Afrokahliella in having a Gonostomum-patterned (vs. Oxytricha-patterned) adoral zone of membranelles, fine (vs. moderately thick) cirri, very widely (vs. narrowly) spaced cirri in all rows, and caudal cirri at the end of dorsal kineties 1–3 (vs. at the end of dorsal kineties 1 and 2 only) (Berger, 2011).
Compared with Fragmocirrus, the new genus has a Gonostomum-patterned (vs. Oxytricha-patterned) adoral zone of membranelles and undulating membranes, a single buccal cirrus and a single parabuccal cirrus (vs. a buccal row and a parabuccal row), transverse cirri absent (vs. present), fine cirri (vs. moderately thick), cirri within all rows very widely (vs. narrowly) spaced, and caudal cirri at the end of dorsal kineties 1–3 (vs. at the end of dorsal kineties 1 and 2 only) (Berger, 2011).
Pseudosincirra nov. gen. differs from Idiodeviata in the presence (vs. absence) of caudal cirri and in having three (vs. one) dorsal kineties (Foissner, 2016).
Kahliella resembles Pseudosincirra nov. gen. in terms of the structure of its adoral zone of membranelles and undulating membranes, but can be separated from the latter by having three (vs. two) frontoventral rows, moderately thick (vs. fine) cirri and cirri within all rows narrowly (vs. widely) spaced, parental left marginal rows retained (vs. resorbed) in postdividers, and caudal cirri absent (vs. present) (Berger, 2011).
Pseudosincirra nov. gen. can be separated from Neogeneia by having a Gonostomum-patterned (vs. Oxytricha-patterned) adoral zone of membranelles, fine (vs. moderately thick) cirri, and parental right and left marginal cirri not retained (vs. retained) in postdividers (Berger, 2011).
Pseudosincirra nov. gen. differs from Parakahliella in having a Gonostomum-patterned (vs. Oxytricha-patterned) adoral zone of membranelles and undulating membranes, fine (vs. moderately thick) cirri, all rows with very widely (vs. narrowly) spaced cirri, a single buccal and a single parabuccal cirrus (vs. a buccal row and a parabuccal row), parental dorsal kineties absorbed (vs. retained) in postdividers, and caudal cirri at the end of dorsal kineties 1–3 (vs. at the end of dorsal kineties 1 and 2 only) (Berger, 2011).
Pseudosincirra nov. gen. resembles Perisincirra in having widely spaced cirri in all rows, but can be separated from the latter by having two (vs. one) long ventral rows (Berger, 2011).
Identification of the Chinese Population of Pseudosincirra longicirrata nov. comb. and Comparison With Two African Populations
Pseudosincirra longicirrata nov. comb. was first reported by Foissner et al. (2002) as Perisincirra longicirrata based on populations discovered in Benin and Namibia. The present population differs from the Benin and Namibian populations in having the following: more adoral membranelles (22–26 vs. 18–22); a smaller ratio of adoral zone length to body length (24–37% and average 30 vs. 29–44% and average 37%); more cirri in the frontoventral rows 1 and 2 (13–16 and 14–18 vs. 7–12 and 9–14) and in the middle left marginal row (10–12 vs. 4–10); usually three, sometimes four, caudal cirri (vs. invariably three caudal cirri); two or three micronuclei (vs. one or two micronuclei); and shorter cilia on the ventral side (20 vs. 30-μm long). However, we consider these differences to be population-dependent and therefore not significant for species-level separation. The identity of the present population is therefore not in doubt.
Morphogenesis
Previous studies on Pseudosincirra longicirrata nov. comb. were limited to morphology, so this is the first report of its morphogenesis (Foissner et al., 2002). According to the origin of FVT-anlagen IV and V in the proter, it can be deduced that right marginal rows 1 and 2 described in Foissner et al. (2002) are actually frontoventral rows. Hence, only one right marginal row is present. Considering the formative mode of FVT-anlagen IV and V, we compare P. longicirrata nov. comb. with some similar species that possess three clearly differentiated frontal cirri and at least two more or less long frontoventral rows and whose FVT-anlagen development is well known, i.e., Afrokahliella paramacrostoma Li et al., 2021a; Deviata abbrevescens Eigner, 1995; D. baciliformis; D. brasiliensis; D. parabaciliformis; Fragmocirrus espeletiae Foissner, 2000; Khaliella simplex (Horváth, 1934) Berger, 2011; Neogeneia hortualis Eigner, 1995; and Parakahliella macrostoma (Foissner, 1982) Berger et al., 1985 (for details, see Table 2). In all these genera, not all the FVT-anlagen IV–VI in both daughter cells develop within the corresponding parental frontoventral rows IV–VI, as in Parastrongylidium oswaldi (Aescht and Foissner, 1992). We speculated that this point may be phylogenetically informative pending greater taxon sampling and the availability of further information on molecular phylogeny of these species.
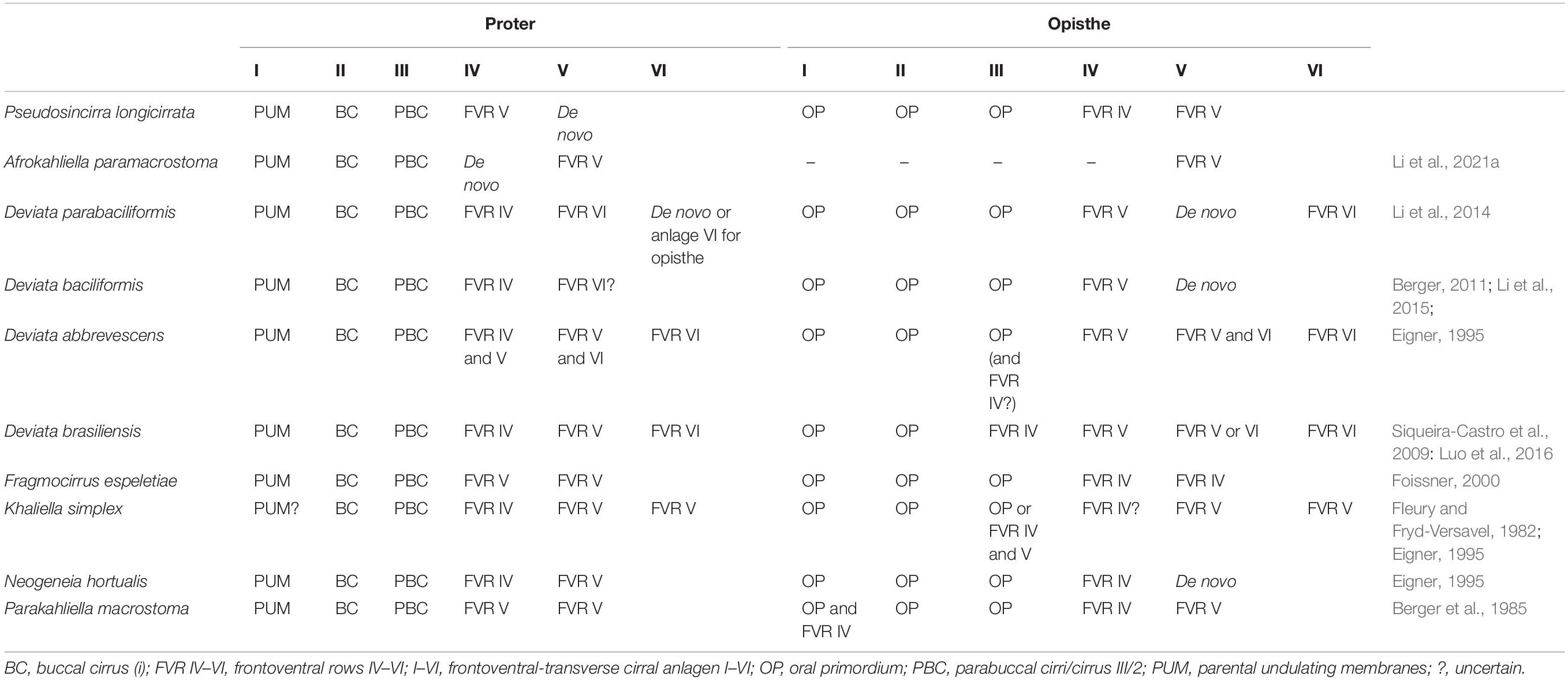
Table 2. Morphogenetic comparison of the Chinese population of Pseudosincirra longicirrata nov. gen. and nov. comb. with similar species showing the origin of frontoventral-transverse cirral anlagen.
Molecular Phylogeny
Pseudosincirra longicirrata nov. comb. falls in the Deviata baciliformis + D. brasiliensis + D. parabaciliformis + D. rositae + Perisincirra paucicirrata clade, the close relationship between these six species being supported by having fine cirri, i.e., cirri in the ventral and marginal rows are mostly composed of two or four cilia and cirri within all rows are relatively widely spaced. The presence or absence of dorsomarginal kineties and the number of dorsal kineties vary in these species and other species in the family Deviatidae Foissner, 2016. We agree with Foissner (2016) that “the feature of presence/absence of dorsomarginal kineties evolved several times independently” and posit that Pseudosincirra and Perisincirra paucicirrata should be assigned to Deviatidae. Furthermore, we suggest that the possession of fine cirri and the relatively widely spaced cirri within all rows should be considered as plesiomorphies of this family/group and added in the diagnosis of the family.
Some species of the family Deviatidae cluster with Strongylidium–Hemiamphisiella–Pseudouroleptus, which is close to Dorsomarginalia and Dorsomarginalian species in present and previous studies (Lu et al., 2020; Wang et al., 2020; Ma et al., 2021; Vd’ačný and Foissner, 2021). Hence, we disagree with Foissner (2016) that Deviatidae is possibly sister to the non-dorsomarginalian Kahliellidae Tuffrau, 1979, but closely related to Dorsomarginalia or Strongylidium–Hemiamphisiella–Pseudouroleptus.
Data Availability Statement
The data presented in the study are deposited in the GenBank database, accession number OK173050.
Author Contributions
QG and QT collected the samples and carried out almost all of the experiments (preparations, illustrations, micrographs, etc.). All authors did the identification of the species and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of China (Project number: 32070428) and the China Postdoctoral Science Foundation (Project number: 2021M 692010).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to express their sincere thanks to Alan Warren (NHM, London) for his helpful critique in enhancing the language of this manuscript.
Footnotes
References
Aescht, E., and Foissner, W. (1992). Biology of a high-rate activated sludge plant of a pharmaceutical company. Arch. Hydrobiol. Suppl. 90, 207–251.
Bai, Y., Wang, R., Song, W., Suzuki, T., and Hu, X. Z. (2020). Redescription of five tintinnine ciliates (Alveolata: Ciliophora: Oligotrichea) from coastal waters of Qingdao. China. Mar. Life Sci. Technol. 2, 209–221. doi: 10.1007/s42995-020-00034-2
Berger, H. (1999). Monograph of the Oxytrichidae (Ciliophora, Hypotrichia). Monogr. Biol. 78, 1–1080. doi: 10.1007/978-94-011-4637-1
Berger, H. (2006). Monograph of the Urostyloidea (Ciliophora, Hypotricha). Monogr. Biol. 85, 1–1304. doi: 10.1007/1-4020-5273-1_1
Berger, H. (2008). Monograph of the amphisiellidae and trachelostylidaE (Ciliophora. Hypotricha). Monogr. Biol. 88, 1–737. doi: 10.1007/978-1-4020-8917-6
Berger, H. (2011). Monograph of the gonostomatidae and Kahliellidae (Ciliophora, Hypotricha). Monogr. Biol. 90, 1–741. doi: 10.1007/978-94-007-0455-8
Berger, H., Foissner, W., and Adam, H. (1985). Morphological variation and comparative analysis of morphogenesis in Parakahliella macrostoma (Foissner, 1982) nov. gen. and Histriculus muscorum (Kahl, 1932), (Ciliophora, Hypotrichida). Protistologica 21, 295–311.
Chen, L. Y., Liu, Y. Q., Long, Y. L., Lyu, J., Feng, C. Y., Ning, Y. Z., et al. (2021). Morphology and molecular phylogeny of two new soil ciliates, Hemiurosomoida warreni nov. spec. and Hemiurosoma clampi nov. spec. (Ciliophora, Hypotrichia) from Tibet. Eur. J. Protistol. 77:125746. doi: 10.1016/j.ejop.2020.125746
Corliss, J. O. (1979). The Ciliated Protozoa: Characterization, Classification and Guide to the Literature, 2nd Edn. New York, NY: Pergamon Press, 455.
Eigner, P. (1995). Divisional morphogenesis in Deviata abbrevescens nov. gen., nov. spec., Neogeneia hortualis nov. gen., nov. spec., and Kahliella simplex (Horvath) Corliss and redefinition of the Kahliellidae (Ciliophora, Hypotrichida). Eur. J. Protistol. 31, 341–366. doi: 10.1016/S0932-4739(11)80098-2
Fleury, A. A., and Fryd-Versavel, G. (1982). Aspects de la morphogenèse chez Kahliella (Cilié hypotriche). Protistologica 18, 135–145.
Foissner, W., Berger, H., and Schaumburg, J. (1999). Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer Landesamtes für Wasserwirtschaft. 3, 1–79.
Foissner, W. (2000). Notes on ciliates (Protozoa, Ciliophora) from Espeletia trees and Espeletia soils of the Andean Páramo, with descriptions of Sikorops espeletiae nov. spec. and Fragmocirrus espeletiae nov. gen., nov. spec. Stud. Neotrop Fauna Environ. 35, 52–79. doi: 10.1076/0165-0521(200004)35:1;1-m;ft052
Foissner, W. (2016). Terrestrial and semiterrestrial ciliates (Protozoa, Ciliophora) from Venezuela and Galápagos. Denisia 35, 1–912.
Foissner, W., Agatha, S., and Berger, H. (2002). Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha region and the Namib desert. Denisia 5, 1–1063. doi: 10.1111/j.1469-7998.1989.tb02561.x
Jankowski, A. V. (1978). The revision of the system of Polyhymenophora class (Spirotrichia). Tezisky Dokl. Zool. Inst. Akad. Nauk SSSR. 39–40.
Jung, J. H., Omar, A., Park, M. H., Nguyen, T. V., Jung, Y. H., Yang, H. M., et al. (2021). Anteholosticha foissneri n. sp. a marine hypotrich ciliate (Ciliophora: Spirotrichea) from Vietnam: morphology, morphogenesis, and molecular phylogeny. Eur. J. Protistol. 78:125768. doi: 10.1016/j.ejop.2021.125768
Kaur, H., Shashi, Negi, R. K., and Kamra, K. (2019). Morphological and molecular characterization of Neogastrostyla aqua nov. gen., nov. spec. (Ciliophora, Hypotrichia) from River Yamuna, Delhi; comparison with Gastrostyla-like genera. Eur. J. Protistol. 68, 68–79. doi: 10.1016/j.ejop.2019.01.002
Küppers, G. C., Lopretto, E. C., and Claps, M. C. (2007). Description of Deviata rositae n. sp., a new ciliate species (Ciliophora, Stichotrichia) from Argentina. J. Eukaryot. Microbiol. 54, 443–447. doi: 10.1111/j.1550-7408.2007.00284.x
Li, F. C., Li, Y. B., Lyu, Z., Mei, Y. M., Gao, S. W., and Shao, C. (2015). On morphology and morphogenesis of a soil hypotrichous ciliate, Deviata bacilliformis (Gelei, 1954) Eigner, 1995 (Protozoa, Ciliophora). Acta Hydrobiol. Sin. 39, 1255–1260. doi: 10.7541/2015.164
Li, F. C., Lyu, Z., Yi, Z. Z., Al-Farraj, S. A., Al-Rasheid, K. A. S., and Shao, C. (2014). Taxonomy and phylogeny of two species of the genus Deviata (Protista, Ciliophora) from China, with description of a new soil form, Deviata parabacilliformis sp. nov. Int. J. Syst. Evol. Microbiol. 64, 3775–3785. doi: 10.1099/ijs.0.068031-0
Li, J. B., Li, L. N., Wang, J. Y., Zhu, E. K., and Shao, C. (2021a). Morphology, morphogenesis and molecular phylogeny of a novel soil ciliate, Afrokahliella paramacrostoma n. sp. (Ciliophora, Hypotrichia). Eur. J. Protistol. 77:125748. doi: 10.1016/j.ejop.2020.125748
Li, J. B., Wang, J. Y., Wang, Y. R., Ma, J. Y., and Shao, C. (2021b). Morphology, ontogenesis and molecular phylogeny of a new saline soil ciliate, Uroleptoides salina nov. spec. (Ciliophora, Hypotrichia). Eur. J. Protistol. 78:125766. doi: 10.1016/j.ejop.2021.125766
Lu, X. T., Wang, Y. Y., Al-Farraj, S. A., El-Serehy, H., Huang, J., and Shao, C. (2020). The insights into the systematic relationship of Gastrostyla-affinitive genera, with report on a new saline soil ciliate genus and new species (Protozoa, Ciliophora). BMC Evol. Biol. 20:92. doi: 10.1186/s12862-020-01659-8
Luo, X. T., Bourland, W. A., Song, W. B., and Huang, J. (2021). New contributions to the taxonomy of urostylid ciliates (Ciliophora, Hypotrichia), with establishment of a new genus and new species. Eur. J. Protistol. 80:125810. doi: 10.1016/j.ejop.2021.125810
Luo, X. T., Fan, Y. B., Hu, X. Z., Miao, M., Al-Farraj, S. A., and Song, W. B. (2016). Morphology, ontogeny, and molecular phylogeny of two freshwater species of Deviata (Ciliophora, Hypotrichia) from southern China. J. Eukaryot. Microbiol. 63, 771–785. doi: 10.1111/jeu.12324
Lynn, D. H. (2008). The Ciliated Protozoa: Characterization, Classification, and Guide to the Literature. Dordrecht: Springer.
Ma, J. Y., Zhao, Y., Zhang, T. Y., Al-Rasheid, K. A. S., Song, W. B., and Shao, C. (2021). Cell-division pattern and phylogenetic analyses of a new ciliate genus Parasincirra n. g. (Protista, Ciliophora, Hypotrichia), with a report of a new soil species, P. sinica n. sp. from northwest China. BMC Ecol. Evo. 21:21. doi: 10.1186/s12862-020-01730-4
Miller, M. A., Pfeiffer, W., and Schwartz, T. (2010). “Creating the CIPRES science gateway for inference of large phylogenetic trees,” in Proceedings of the Gateway Computing Environments Workshop (GCE), (New Orleans, LA), 1–8. doi: 10.1109/GCE.2010.5676129
Nylander, J. A. A. (2004). MrModeltest, Version 2.2. Program distributed by the Author. Evolutionary Biology Centre. Uppsala: Uppsala University, doi: 10.4236/bio.2004.48074
Omar, A., Moon, J. H., and Jung, J. H. (2021a). Molecular phylogeny of a new gonostomatid ciliate revealing a discrepancy between interphasic and cell divisional patterns (Ciliophora, Hypotricha). Eur. J. Protistol. 79:125794. doi: 10.1016/j.ejop.2021.125794
Omar, A., Moon, J. H., Nam, S. W., and Jung, J. H. (2021b). Tunicothrix halophila n. sp., a secondarily oligomerized parabirojimid hypotrich (Ciliophora, Spirotrichea) from hypersaline costal water in Korea. Front. Microbiol. 12:691361. doi: 10.3389/fmicb.2021.691361
Paiva, T. D. S. (2020). Systematic redefinition of the Hypotricha (Alveolata, Ciliophora) based on combined analyses of morphological and molecular characters. Protist 171:125755. doi: 10.1016/j.protis.2020.125755
Park, K. M., Jung, J. H., Kim, J. H., Min, G. S., and Kim, S. (2020). Morphology, morphogenesis, and molecular phylogeny of a new freshwater ciliate, Gonostomum jangbogoensis n. sp. (Ciliophora, Hypotricha), from Victoria Land, Antarctica. Eur. J. Protistol. 73:125669. doi: 10.1016/j.ejop.2019.125669
Penn, O., Privman, E., Ashkenazy, H., Landan, G., Graur, D., and Pupko, T. (2010). GUIDANCE: a web server for assessing alignment confidence scores. Nucleic Acids Res. 38, W23–W28. doi: 10.1093/nar/gkq443
Ronquist, F., Teslenko, M., Mark, P. V. D., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Shao, C., Chen, X. M., and Jiang, J. M. (2020). Hypotrichous Ciliates in China. Beijing: Science Press, 429.
Siqueira-Castro, I. C. V., Paiva, T. D. S., and Silva-Neto, I. D. D. (2009). Morphology of Parastrongylidium estevesi comb. nov. and Deviata brasiliensis sp. nov. (Ciliophora: Stichotrichia) from a sewage treatment plant in Rio de Janeiro, Brazil. Zoologia 26, 774–786. doi: 10.1590/S1984-46702009000400024
Song, W. B., and Shao, C. (2017). Ontogenetic Patterns of Hypotrich Ciliates (in Chinese). Beijing: Science Press.
Stamatakis, A., Hoover, P., and Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML Web-servers. Syst. Biol. 57, 758–771. doi: 10.1080/10635150802429642
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. doi: 10.1093/molbev/msr121
Vd’ačný, P., and Foissner, W. (2021). Morphology and ontogenesis of two new Hemiholosticha species (Ciliophora, Hypotrichia, Hemiholostichidae nov. fam.). Eur. J. Protistol. 77:125763. doi: 10.1016/j.ejop.2020.125763
Wang, J. Y., Zhang, T. T., Li, F. C., Warren, A., Li, Y. B., and Shao, C. (2021). A new hypotrich ciliate, Oxytricha xianica sp. nov., with notes on the morphology and phylogeny of a Chinese population of Oxytricha auripunctata Blatterer & Foissner, 1988 (Ciliophora, Oxytrichidae). Mar. Life Sci. Technol. 3, 303–312. doi: 10.1007/s42995-020-00089-1
Wang, J. Y., Zhao, Y., Lu, X. T., Lyu, Z., Warren, A., and Shao, C. (2020). Does the Gonostomum-patterned oral apparatus in Hypotrichia carry a phylogenetic signal? Evidence from morphological and molecular data based on extended taxon sampling using three nuclear genes (Ciliophora, Spirotrichea). Sci. China Life Sci. 64, 311–322. doi: 10.1007/s11427-020-1667-3
Wilbert, N. (1975). Eine verbesserte technik der Protargolimprägnation für Ciliaten. Mikrokosmos 64, 171–179.
Wu, T., Li, Y. Q., Lu, B. R., Warren, A., Shen, Z., and Song, W. B. (2020). Morphology, taxonomy and molecular phylogeny of three marine peritrich ciliates, including two new species: Zoothamnium apoarbuscula n. sp. and Z. apohentscheli n. sp. (Protozoa, Ciliophora, Peritrichia). Mar. Life Sci. Technol. 2, 334–348. doi: 10.1007/s42995-020-00046-y
Keywords: Hypotrichia, morphogenesis, new genus, phylogenetic analyses, Pseudosincirra nov. gen.
Citation: Gao Q, Shao C, Tang Q and Li J (2021) Redescription, Morphogenesis, and Molecular Phylogeny of Pseudosincirra longicirrata nov. comb., With Establishment of a New Genus Pseudosincirra nov. gen. (Ciliophora, Hypotrichia). Front. Microbiol. 12:777540. doi: 10.3389/fmicb.2021.777540
Received: 15 September 2021; Accepted: 28 October 2021;
Published: 30 November 2021.
Edited by:
Xinpeng Fan, East China Normal University, ChinaReviewed by:
Lei Wu, South China Normal University, ChinaXuming Pan, Harbin Normal University, China
Yuanjun Zhao, Chongqing Normal University, China
Copyright © 2021 Gao, Shao, Tang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Shao, c2hhb2NoZW5Ac25udS5lZHUuY24=; Jingbao Li, bGlqaW5nYmFvQG53cHUuZWR1LmNu
†These authors have contributed equally to this work
 Qi Gao1†
Qi Gao1† Chen Shao
Chen Shao