
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 20 October 2021
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.748121
Microbial persisters enable the development of certain intrinsic strategies for survival with extreme tolerance to multiple antimicrobials. Porphyromonas gingivalis is considered to be the “keystone” periodontopathogen. Indeed, periodontitis, as a highly common inflammatory disease, is the major cause of severe tooth loss and edentulism in adults globally, and yet it is crucially involved in various systemic comorbidities like diabetes. We have recently revealed P. gingivalis persisters-induced perturbation of immuno-inflammatory responses and effective suppression of this key pathogen by bismuth drugs. This study further explored novel approaches to eradicating P. gingivalis persisters through synergistic combination of colloidal bismuth subcitrate (CBS) with traditional antibiotics. P. gingivalis (ATCC 33277) cells in planktonic and biofilm states were cultured to stationary phase, and then treated with metronidazole (100 mg/L), amoxicillin (100 mg/L), CBS, (100 μM) and combinations of these medications, respectively. Persister survival rate was calculated by colony-forming unit. Cell viability and cytotoxicity of CBS were assessed in human gingival epithelial cells (HGECs). Notably, CBS combined with metronidazole enabled the effective eradication of P. gingivalis persisters in planktonic mode, and nearly eliminated their existence in biofilm mode. Importantly, CBS exhibited no effects on the viability of HGECs, along with minimal cytotoxicity (<5%) even at a high concentration (400 μM). This pioneering study shows that P. gingivalis persisters could be well eliminated via the synergistic combination of CBS with metronidazole. Our findings may contribute to developing novel approaches to tackling periodontitis and inflammatory systemic comorbidities.
Microbial persisters as a tiny subset of microorganisms can enter or be triggered to become a “dormant” and “non-dividing” state, with high tolerance to multiple antimicrobials. These persisters normally take up less than 0.1% of the whole population without heritable genetic mutations, as the descendants of persisters are still sensitive to antimicrobial treatment and generate a similar proportion of persisters (Lewis, 2010; Balaban et al., 2019). As persisters can survive under antimicrobials treatment and resume growth after the cessation of treatment, these noxious cells have been claimed to critically account for the relapse and/or recalcitrance of common infectious/inflammatory diseases in humans (Lewis, 2010; Fauvart et al., 2011).
Periodontitis, as a serious inflammatory disease, is one of the major global oral disease burdens (Pihlstrom et al., 2005; Jin et al., 2011, 2016; Kassebaum et al., 2014; Tonetti et al., 2017). Porphyromonas gingivalis is considered to be the “keystone” periodontopathogen (Hajishengallis et al., 2012) and it critically contributes to the shift of host-microbe symbiosis to dysbiosis even at a low abundance, leading to dysregulated immuno-inflammatory responses and periodontal destruction (Hajishengallis et al., 2011; Honda, 2011). Notably, periodontitis is closely linked with various systemic diseases and disorders, so called inflammatory comorbidities, and indeed P. gingivalis plays essential roles in the etiopathogenesis of these diseases (Hajishengallis, 2015; Hajishengallis and Chavakis, 2021). Recently, our group reported for the first time the profile of P. gingivalis persisters and their underlying survival mechanisms (Li et al., 2018), and surprisingly metronidazole-treated P. gingivalis persisters maintain their virulence factors, such as the ability to adhere to and invade human gingival epithelial cells (HGECs), and perturb immuno-inflammatory responses (Wang et al., 2020). These findings may to some extent account for the hardship to effectively control periodontitis and prevent its recurrence, especially in susceptible individuals. Herein, the working hypothesis is that targeting P. gingivalis persisters might be a critical strategy to tackle periodontitis and other P. gingivalis-related systemic comorbidities like cardiovascular disease (Chistiakov et al., 2016), pancreatic cancer (Fan et al., 2018), and Alzheimer’s disease (Dominy et al., 2019).
More than seventy years have passed since Joseph W. Bigger first named “persisters” in 1944 (Bigger, 1944). To date, persisters have been well-documented in nearly all bacterial species tested (Van den Bergh et al., 2017). Several clinical studies have proven their link to recalcitrant infectious diseases/conditions such as cystic fibrosis (Mulcahy et al., 2010), oral carriage (Lafleur et al., 2010), urinary tract infections (Goneau et al., 2014), and tuberculosis (Jain et al., 2016). Moreover, persisters create a suitable environment for gene transfer and adaptive mutation. For instance, S. Typhimurium persisters can act as a reservoir to promote resistance plasmid transfer among different microorganisms (Bakkeren et al., 2019), and the progeny of E. coli persisters harbor more antibiotic-resistant mutants (Barrett et al., 2019). Furthermore, intracellular bacterial persisters such as Salmonella could manipulate host immune response (Stapels et al., 2018). Due to these annoying roles persisters play in disease onset and development, how to eradicate persisters effectively has drawn a substantial amount of attention over the past two decades. It is known that developing new antibiotics has become rather tough since the 1980s, owing to high costs, being time-consuming, and great uncertainties of the outcomes. So, utilizing different strategies, like potentiating efficiency of conventional antibiotics and re-purposing the usage of classical antimicrobials, could be novel and realizable approaches (Allison et al., 2011; Barraud et al., 2013; Chung and Ko, 2019; Zhao et al., 2020).
Bismuth drugs such as colloidal bismuth subcitrate (CBS) are commonly used for treating Helicobacter pylori infection and related gastrointestinal disorders (Laine et al., 2003; Megraud, 2012; Dore et al., 2016). Other plausible applications of bismuth drugs have been increasingly reported, e.g., inhibiting metallo-β-lactamases-positive bacteria (Wang et al., 2018) and, very recently, suppressing SARS-CoV-2 replication (Yuan et al., 2020). The underlying action mechanisms of bismuth drugs have been increasingly understood in the past few years through adopting newly developed approaches such as metallomics and metalloproteomics. Bismuth binds to the key enzymes in the pathogens and subsequently disrupts the essential pathological pathways (Li et al., 2019; Griffith et al., 2021). Moreover, since bismuth acts as a broad-spectrum inhibitor of metallo-β-lactamases (MBLs), the combination of bismuth drugs with clinically used antibiotics could be an economical and effective alternative to fight against those antibiotic resistant mutations (Wang et al., 2018). Furthermore, bismuth drugs like CBS are frequently employed clinically, and the safety and toxicity issues have been well illustrated, showing that they only exert selective toxicity in microbes but not in human host (Li et al., 2019). We have newly demonstrated the potential effects of bismuth drugs on suppressing P. gingivalis in its various modes (Cheng et al., 2019), while whether these drugs can affect P. gingivalis persisters remains unknown and further investigation is highly warranted.
This study investigated the synergistic effects of a commonly used bismuth drug (CBS) combined with traditional antibiotics on the eradication of P. gingivalis persisters. Of note, P. gingivalis persisters were effectively eradicated in planktonic mode and nearly eliminated in biofilm mode, by CBS plus metronidazole. Whereas no such significant effects were observed for the combined usage of metronidazole and amoxicillin, or CBS and amoxicillin. Importantly, CBS exhibited no effects on the viability of HGECs, along with minimal cytotoxicity (<5%) even at a high concentration (400 μM). To the best of our knowledge, this is the first study revealing that P. gingivalis persisters could be well eliminated via the synergistic combination of a bismuth drug and metronidazole, thereby inspiring us to develop novel strategies and approaches to better control periodontitis and P. gingivalis-related systemic comorbidities.
P. gingivalis (ATCC 33277) was cultured as previously described by us (Wang et al., 2020). P. gingivalis cells maintained as frozen stock were firstly grown on blood agar plates (44 g/L Columbia agar base, Difco, 5% horse blood, Hemostat, 5 mg/L hemin, Sigma-Aldrich, 1 mg/L vitamin K1, Sigma-Aldrich) in an anaerobic atmosphere composed of 10% H2, 5% CO2, and 85% N2 at 37°C. After 7-day culture, a single colony was picked into liquid trypticase soy broth (30 g/L TSB; Difco) supplemented with yeast extract (5 g/L), vitamin K1 (1 mg/L), and hemin (5 mg/L), and it was then cultured in the same anaerobic conditions.
Antimicrobial susceptibility tests of P. gingivalis to metronidazole (MTZ, Sigma-Aldrich), Amoxicillin (AMX, Sigma-Aldrich), and colloidal bismuth subcitrate (CBS, De-Nol®) were performed as previously described following the CLSI guidelines (Clinical and Laboratory Standards Institute, 2016; Cheng et al., 2019). In brief, serial 2-fold dilutions of MTZ (0–50 mg/L), AMX (0–25 mg/L), or CBS (0–100 mM) were made for P. gingivalis culture broth in 96-well-plate (Thermo Fisher Scientific) with 50 μl in each well. Bacterial suspension (OD600 = 0.1) was added to each well (50 μl). The plates were incubated anaerobically at 37°C for 48 h. Herein, the minimal inhibitory concentration (MIC) was defined as the lowest concentration of antibiotic with no visible growth of P. gingivalis.
Persister assay was performed following our previous protocol with minor modifications (Li et al., 2018). P. gingivalis suspension was diluted in fresh media to OD 600 of 0.1 and incubated to stationary phase (72 h) followed by treatment with MTZ (100 mg/L), AMX (100 mg/L), CBS (100 μM), or different combinations of these medications. At 6, 24, and 48 h, the cultures were washed twice with PBS, followed by 10-fold dilution to 10–7 and 50 μl aliquots of each dilution were plated on blood agar plates for counting colony forming unit (CFU). The survival rate of persisters was then calculated via dividing the CFU of a drug-treated group by the untreated control group. Meanwhile, 3 μl aliquots of each dilution were spotted onto blood agar plates and anaerobically incubated for 7 days until further observation, and recorded.
The heritability of P. gingivalis persister was performed following an established approach (Li et al., 2018). P. gingivalis persisters after 24-h drug treatment were plated on blood agar plates to count CFU. A single colony was inoculated into fresh broth and cultured for 48 h. Then, the bacterial suspension was diluted in fresh media to OD600 of 0.1 and incubated to stationary phase (72 h) followed by treatment with the same drug. This process was repeated three times. The MIC of P. gingivalis recovered from the third treatment against AMX, MTZ, and CBS was determined as aforementioned.
Drug concentration measurements were conducted with modified MIC assay. P. gingivalis in stationary phase (72 h) was treated with MTZ (100 mg/L), AMX (100 mg/L), and CBS (100 μM) for 48 h, respectively. After centrifugation (8,000 g, 10 min) and filtration (0.22 μm), the cultured broth was collected for testing. Herein, the broth was processed with serial 2-fold dilution (10 times) into 96-well-plate (50 μl/well), and P. gingivalis suspension (OD600 = 0.1) was added to each well (50 μl). The plates were incubated anaerobically at 37°C for 48 h. The drug concentration was measured according to the MIC results.
P. gingivalis in mid-exponential phase was diluted (OD600 of 0.1) and seeded onto Thermanoxt plastic coverslips (15 mm diameter, Thermo Fisher Scientific) on the bottom of 12-well plate.
The biofilms were firstly cultured for 72 h to reach maturation, followed by removing the free bacteria and treatment with MTZ (100 mg/L), AMX (100 mg/L), CBS (100 μM), or different combinations of these medications for 24, 48, and 72 h, respectively. After washing with PBS for three times, the biofilms were detected by plate culture. In brief, each plate with biofilm was put into 5 ml fresh broth and vigorously vortexed for 1 min. Then, the broth was 10-fold diluted with fresh broth to 10–7 and 50 μl aliquots of each dilution were plated on blood agar plates for counting CFU. Meanwhile, 3 μl aliquots of each dilution were spotted onto blood agar plates and anaerobically incubated for 7 days until further observation, and recorded.
HGECs (CELLnTEC, Bern, Switzerland) were cultured and seeded into 96-well-plate (5 × 103 cells/well) following our established protocol (Wang et al., 2020). After adhesion, cells were treated with different concentrations of CBS (0–400 μM) for 24, 48, and 72 h. Cell viability was evaluated with CyQUANTTM MTT Cell Proliferation Assay Kit (Thermo Fisher Scientific Inc., United States), and the cytotoxicity was measured via Pierce CyQUANTTM LDH Cytotoxicity Assay Kit (Thermo Fisher Scientific Inc., United States) concurrently according to the instructions of the products.
All experiments were undertaken for at least three independent repeats. Data were presented as the mean ± standard deviation (SD) for the results of independent experiments. GraphPad Prism 8 was used to take statistical calculations and obtain statistical graphs. Inter-group difference was compared using the One-Way or Two-way Analysis of Variance (ANOVA) and multiple comparisons by Tukey’s test as appropriate. Statistical significance was considered when p < 0.05.
There was a biphasic pattern of killing curves against P. gingivalis, and a small subset of survival persisters existed after treatments with metronidazole (MTZ, 100 mg/L), Amoxicillin (AMX, 100 mg/L), and colloidal bismuth subcitrate (CBS, 100 μM) for 48 h (Figure 1A). In addition, these drugs remained effective against P. gingivalis during the experimental period (Table 1).
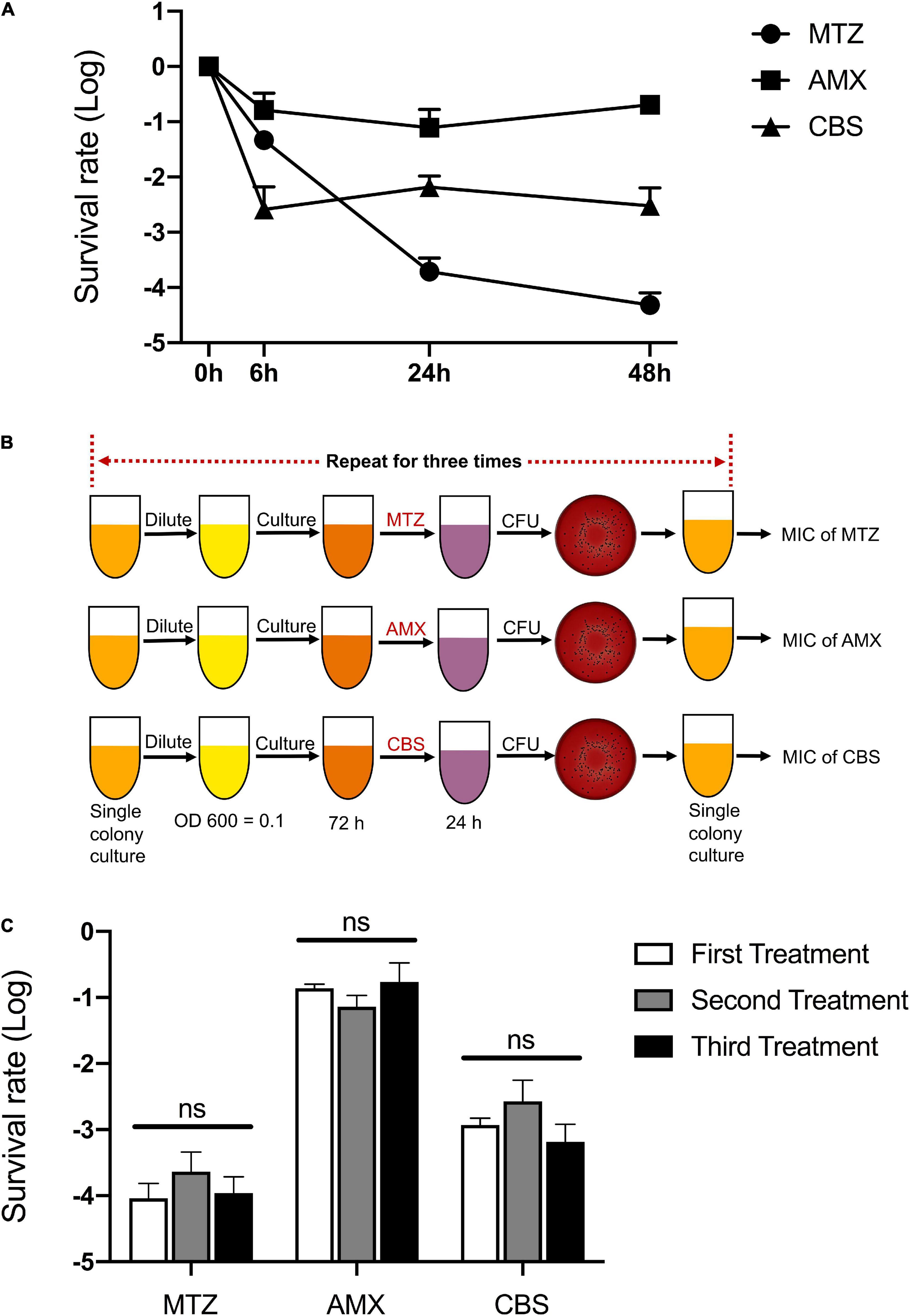
Figure 1. Multiple antimicrobial tolerance of P. gingivalis persisters. (A) The biphasic killing curves of P. gingivalis persisters after treatments with metronidazole (MTZ, 100 mg/L), Amoxicillin (AMX, 100 mg/L), and colloidal bismuth subcitrate (CBS, 100 μM) for 48 h, respectively. (B) Workflow of the three repeated treatments by MTZ, AMX, and CBS. (C) The survival rate of P. gingivalis persisters following the three repeated treatments by MTZ, AMX, and CBS. Data present the mean ± standard deviation (SD) of the results from three independent experiments. ns: no significance.
In order to verify whether the surviving species contain resistant mutations, the treatments were repeated three times and the survival rates were calculated following every 24-h treatment. The MIC value of each antimicrobial was then tested after the last testing (Figure 1B). Notably, both survival rate and MIC value of P. gingivalis persisters remained unchanged (Figure 1C and Table 2).

Table 2. MICs of MTZ, AMX, and CBS against P. gingivalis persisters following 3-time treatments and recoveries#.
No colony formation of P. gingivalis was observed after 24-h treatment of CBS plus MTZ, strongly indicating that its persisters were effectively eradicated through this combined approach. Indeed, it was much stronger than the traditionally used MTZ plus AMX or CBS plus AMX, as the persister cells survived even after 48-h treatments by these two sets of drugs (Figures 2A,B). Next, we further explored whether the combined usage of MTZ and CBS could reduce the anti-persister concentration of these drugs. Interestingly, it was highly achievable to get rid of the P. gingivalis persisters at relatively low dosages (25 mg/L of MTZ + 100 μM CBS; 50 mg/L of MTZ + 50 μM CBS) (Figure 2C).

Figure 2. CBS plus MTZ eradicated P. gingivalis persisters in planktonic mode. (A) The survival rate of P. gingivalis persisters after treatments with MTZ (100 mg/L), AMX (100 mg/L), and CBS (100 μM) or different combinations of these medications for 6, 24, and 48 h, respectively. Data represent the mean ± SD of three independent experiments. Black arrows: not detected. (B) Plate culture of P. gingivalis persisters after 24-h treatment. (C) Combined usage of CBS and MTZ against P. gingivalis persisters with different concentrations of the drugs. (B,C) Show one randomly chosen result from three independent experiments.
It was found that single use of MTZ, AMX, or CBS was unable to eradicate P. gingivalis persisters in biofilm mode even after 72-h treatment (Figure 3A). Whereas these persisters were dramatically suppressed by the combined treatment of MTZ and CBS, thereby leaving a lower survival rate as compared to MTZ plus AMX or CBS plus AMX (Figures 3B,C).

Figure 3. CBS plus MTZ dramatically suppressed P. gingivalis persisters in biofilm mode. P. gingivalis cells in biofilm mode were treated with MTZ (100 mg/L), AMX (100 mg/L), and CBS (100 μM) or different combinations of these medications for 24, 48, and 72 h, respectively. (A) The survival rate of P. gingivalis persisters after single drug treatment. (B) The survival rate of P. gingivalis persisters after 72-h treatment, with reference to the most effective group (MTZ + CBS). *P < 0.05; **P < 0.01; ns: no significance. Data in (A,B) represent the mean ± SD of three independent experiments. (C) Plate culture of P. gingivalis persisters after 72-h treatment, randomly chosen from three independent experiments.
Furthermore, the potential effects of CBS on HGECs were tested with different concentrations of CBS for 24, 48, and 72 h. Of note, the growth of HGECs was not affected (Figure 4A) and there was negligible cellular cytotoxicity (Figure 4B).
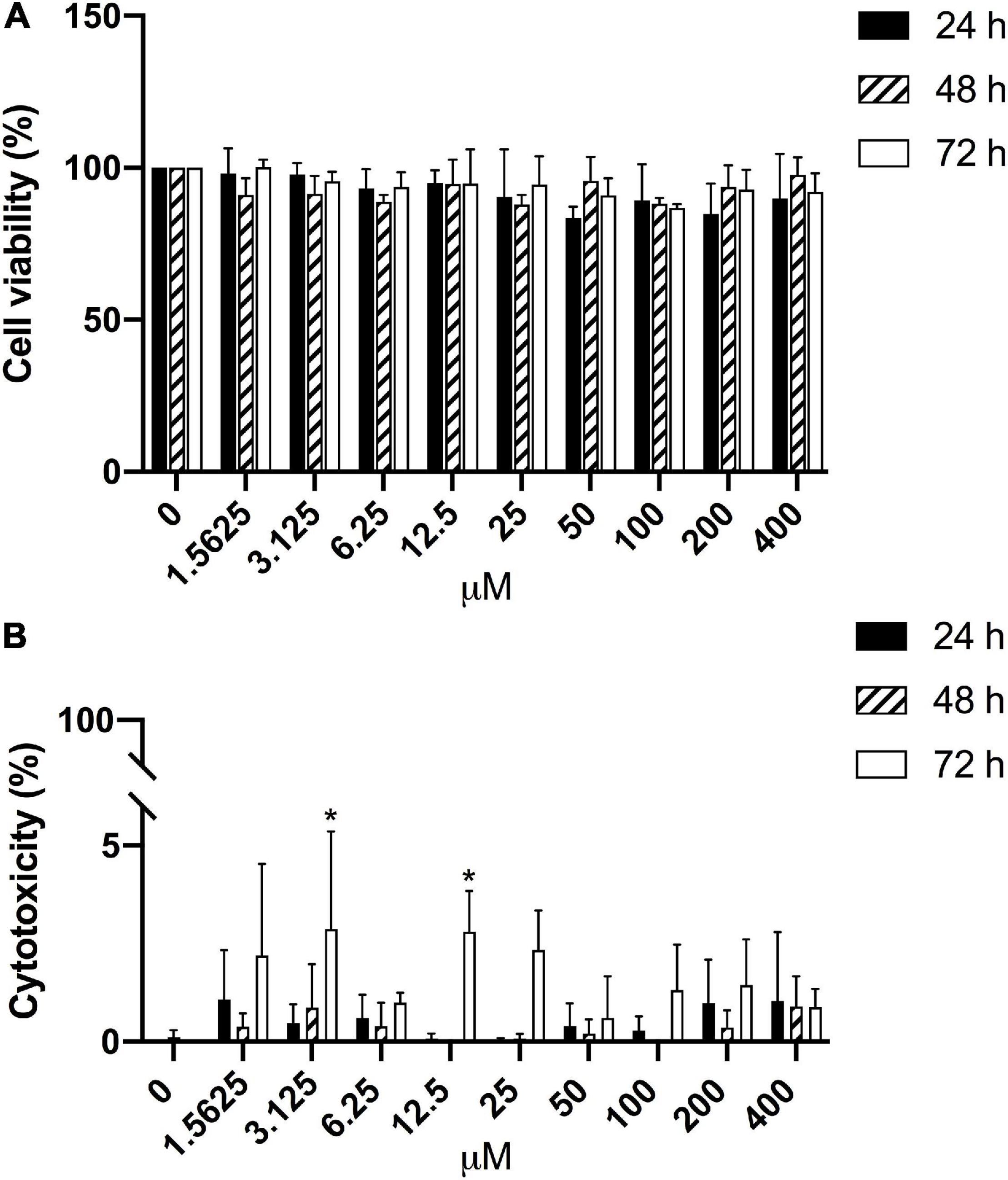
Figure 4. Negligible effects of CBS on HGECs. HGECs were treated with different concentrations of CBS for 24, 48, and 72 h, respectively. Cell viability and cytotoxicity were analyzed, with reference to the untreated group (0 μM). (A) Cell viability results from MTT test. No significant difference was found among different groups. (B) Cell cytotoxicity results from LDH test. *P < 0.05. Data represent the mean ± SD of three independent experiments.
The initial study on “persister” dates back to the 1940s, while highly focused and intensive investigations on these noxious survivors started from the beginning of the twenty-first century (Lewis, 2001). Meanwhile, the widely used indwelling devices and increased numbers of immuno-compromised patients resulted in the explosion of chronic infections and inflammation, partly due to the action of various microbial persisters and current lack of relevant combating approaches (Lewis, 2010; Fauvart et al., 2011). It still remains a considerable challenge to effectively and predictably tackle persisters and related diseases (Putrins et al., 2015). Moreover, persisters also critically account for the emerging antimicrobial resistance worldwide (Barrett et al., 2019). It is therefore an urgent matter to seek novel strategies and approaches to eradicating persisters powerfully and precisely.
The past two decades have witnessed a booming increase in scientific research in the field of microbial persisters. Various novel agents have been developed against these tough persister cells. For instance, NH125, a well-known inhibitor of WalK, enables the successful removal of methicillin resistant Staphylococcus aureus persisters through inducing rapid membrane permeabilization (Kim et al., 2016). One unresolved issue is that plenty of time and money need to be invested for developing a new drug. Metallodrugs have been increasingly employed in medical healthcare. Of them, bismuth drugs have been routinely applied for managing patients with Helicobacter pylori-induced diseases (Marshall et al., 1988; Laine et al., 2003). Of note, our group has recently provided the first evidence that bismuth drugs can markedly suppress P. gingivalis in its planktonic, biofilm, and intracellular states (Cheng et al., 2019).
This study extended to investigate the potential effects of bismuth drug (CBS) on P. gingivalis persisters. Firstly, a single usage of MTZ, AMX, or CBS left a subset of survival persisters but not resistant mutations, and this outcome was not due to drugs failure. Importantly, the synergistic combination of CBS and MTZ completely eradicated the persister cells of P. gingivalis, while the MTZ plus AMX and CBS plus AMX could not. Furthermore, reduced dosages of both CBS and MTZ remained to totally eradicate P. gingivalis persisters. Thus, CBS could be an excellent alternative to potentiate the efficiency of MTZ for tackling P. gingivalis persisters.
In fact, a majority of the microbes are able to form biofilms, and therefore biofilm-related infections and inflammation are highly difficult to control (Lewis, 2007). Various underlying mechanisms have been proposed for explaining the high tolerance of biofilms to antimicrobials (Paraje, 2011; Singh et al., 2016; Teirlinck et al., 2017; Subramani and Jayaprakashvel, 2019; Uruen et al., 2020). Whereas it has been verified that the presence of persister cells crucially accounts for it (Lewis, 2001; Spoering and Lewis, 2001). Herein, Lewis sets a notable model to explain the biofilm-related recalcitrance of chronic infections that the complex microenvironment with microbial biofilms contributes to the formation of persisters. Indeed, antimicrobials along with the immune system could eradicate all the microorganisms outside the biofilms but frequently fail to eliminate the persisters within the biofilms, and these tough persisters could regrow following the termination of antimicrobial treatments (Lewis, 2007, 2010). As such, tackling persisters in the biofilms is critical for effective control of biofilm-related infections and inflammatory diseases. P. gingivalis, as the keystone periodontopathogen, co-aggregates with other oral pathogens to form multi-species biofilms to enhance survival capacity and pathogenicity (Bostanci and Belibasakis, 2012). Currently, it is rather challenging to suppress P. gingivalis in its biofilm mode. Our group has proved that bismuth drugs inhibit the formation of P. gingivalis biofilms and meanwhile significantly disrupt the mature biofilms (Cheng et al., 2019), while it remains unclear whether P. gingivalis persisters in their biofilm state could be suppressed. In this study, we found that single usage of MTZ, AMX, and CBS on the matured P. gingivalis biofilm was rather disappointing. Our study highlighted that CBS plus MTZ remarkably suppressed P. gingivalis persisters in their biofilm mode, with reference to MTZ plus AMX or CBS plus AMX. Further study is needed to refine the protocol for maximizing the anti-persister effectiveness in oral biofilms.
It is known that the serum concentration of bismuth after intake of one tablet of CBS can only reach a rather low level (44.5 μg/L or 0.2129 μM) (Vanhoe et al., 1993). In addition, the standard use of bismuth subcitrate, metronidazole, and tetracycline (BMT) for targeting Helicobacter pylori generates a relatively low concentration of blood bismuth (16.9 μg/L or 0.081 μM) (Guiard et al., 2019). Taken together, these findings indicate that such oral dosage of bismuth is far away from providing effective control of P. gingivalis in terms of its MIC (1,306 μg/L or 6.25 μM). Nevertheless, higher bismuth concentration in the serum would cause severe side-effects, such as encephalopathy, nephropathy, and osteoarthropathy (Slikkerveer and de Wolff, 1989; Cengiz et al., 2005). Thus, an appropriate approach is to deliver the bismuth drugs for topical application, such as through mouthwash, dentifrice, applying gels, or direct delivery to periodontal lesions, to enhance treatment efficiency while reducing side-effects. Our findings suggest CBS may be promising for treating periodontal diseases via local delivery mode, owing to its minimal effects on host cells like HGECs and negligible cellular cytotoxicity. Further translational studies should investigate the feasibility of combined usage of bismuth drugs with commonly used antibiotics for oral/periodontal healthcare in clinical practice.
It is apparent that metronidazole forms nitro radicals and generates toxic metabolites when entering into anaerobes, subsequently disrupting the DNA of microbial cells (Hernandez Ceruelos et al., 2019; Weir and Le, 2021). Anaerobes, meanwhile, could develop various intrinsic strategies to contract the drug action, such as overexpressing antioxidant enzymes like thioredoxin and SOD (Wassmann et al., 1999; Leitsch et al., 2016). Indeed, our previous work has proved that bismuth can continuously inhibit the activities of thioredoxin and SOD (Cheng et al., 2019). Thus, we suppose that the effective eradication of P. gingivalis persisters by CBS plus metronidazole could be due to their synergistic action on the oxidation-reduction reaction. Moreover, such a synergistic combination might be applicable to other anaerobic pathogens for tackling common chronic infections and inflammation. On the other hand, novel approaches need to be developed for refining the drug vehicles and maximizing the effectiveness. Our group has recently explored nano-based antimicrobials and anti-inflammatory agents, such as nanoparticle-encapsulated chlorhexidine and nanoparticle-encapsulated baicalein (Li et al., 2016, 2017). These findings are inspiring for further development along this line.
This study indicates the existence of multi-drug tolerant P. gingivalis persisters, which is not due to antimicrobial resistance and drug failure. Notably, a synergistic combination of CBS and metronidazole sufficiently eliminates P. gingivalis persisters in planktonic mode, and remarkably suppresses their survival rates in biofilm mode. This combination is more effective than the commonly used metronidazole plus amoxicillin, or CBS plus amoxicillin. Importantly, CBS has minimal cytotoxic effects on HGECs and their viability is not affected. Our findings demonstrate that the synergistic combination of CBS and metronidazole enables the effective eradication of P. gingivalis persisters. This work may contribute to developing novel approaches to tackling P. gingivalis for effective control of periodontitis and common inflammatory comorbidities. Further investigation can be extended to tackle other pathogens for better care of common immuno-inflammatory diseases.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
LJ conceived the project and revised the manuscript. CW and LJ designed the study. CW, XL, and TC performed the experiments, collected, and analyzed the data. CW drafted the manuscript. HS and LJ made a critical review of the manuscript. All authors contributed to the interpretation of the results and approved the final version of the manuscript.
This study was supported by the Hong Kong Research Grants Council (GRF No. 17119819 to LJ), the Research Impact Fund (R7070-18 to HS), and the Modern Dental Laboratory/The University of Hong Kong (HKU) Endowment Fund to LJ.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to Joyce Yau and Becky Cheung at the Central Research Laboratories, Faculty of Dentistry, The University of Hong Kong for their technical assistance and great support.
Allison, K. R., Brynildsen, M. P., and Collins, J. J. (2011). Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473, 216–220. doi: 10.1038/nature10069
Bakkeren, E., Huisman, J. S., Fattinger, S. A., Hausmann, A., Furter, M., Egli, A., et al. (2019). Salmonella persisters promote the spread of antibiotic resistance plasmids in the gut. Nature 573, 276–280. doi: 10.1038/s41586-019-1521-8
Balaban, N. Q., Helaine, S., Lewis, K., Ackermann, M., Aldridge, B., Andersson, D. I., et al. (2019). Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 17, 441–448. doi: 10.1038/s41579-019-0196-3
Barraud, N., Buson, A., Jarolimek, W., and Rice, S. A. (2013). Mannitol enhances antibiotic sensitivity of persister bacteria in Pseudomonas aeruginosa biofilms. PLoS One 8:e84220. doi: 10.1371/journal.pone.0084220
Barrett, T. C., Mok, W. W. K., Murawski, A. M., and Brynildsen, M. P. (2019). Enhanced antibiotic resistance development from fluoroquinolone persisters after a single exposure to antibiotic. Nat. Commun. 10:1177. doi: 10.1038/s41467-019-09058-4
Bigger, J. W. (1944). Treatment of staphylococcal infections with penicillin. Lancet 244, 497–500. doi: 10.1016/S0140-6736(00)74210-3
Bostanci, N., and Belibasakis, G. N. (2012). Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 333, 1–9. doi: 10.1111/j.1574-6968.2012.02579.x
Cengiz, N., Uslu, Y., Gok, F., and Anarat, A. (2005). Acute renal failure after overdose of colloidal bismuth subcitrate. Pediatr. Nephrol. 20, 1355–1358. doi: 10.1007/s00467-005-1993-7
Cheng, T., Lai, Y. T., Wang, C., Wang, Y., Jiang, N., Li, H., et al. (2019). Bismuth drugs tackle Porphyromonas gingivalis and attune cytokine response in human cells. Metallomics 11, 1207–1218. doi: 10.1039/c9mt00085b
Chistiakov, D. A., Orekhov, A. N., and Bobryshev, Y. V. (2016). Links between atherosclerotic and periodontal disease. Exp. Mol. Pathol. 100, 220–235. doi: 10.1016/j.yexmp.2016.01.006
Chung, E. S., and Ko, K. S. (2019). Eradication of persister cells of Acinetobacter baumannii through combination of colistin and amikacin antibiotics. J. Antimicrob. Chemother. 74, 1277–1283. doi: 10.1093/jac/dkz034
Clinical and Laboratory Standards Institute (2016). Performance Standards for Antimicrobial Susceptibility Testing CLSI supplement M100, 26th Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
Dominy, S. S., Lynch, C., Ermini, F., Benedyk, M., Marczyk, A., Konradi, A., et al. (2019). Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5:eaau3333. doi: 10.1126/sciadv.aau3333
Dore, M. P., Lu, H., and Graham, D. Y. (2016). Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 65, 870–878. doi: 10.1136/gutjnl-2015-311019
Fan, X., Alekseyenko, A. V., Wu, J., Peters, B. A., Jacobs, E. J., Gapstur, S. M., et al. (2018). Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut 67, 120–127. doi: 10.1136/gutjnl-2016-312580
Fauvart, M., De Groote, V. N., and Michiels, J. (2011). Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 60, 699–709. doi: 10.1099/jmm.0.030932-0
Goneau, L. W., Yeoh, N. S., Macdonald, K. W., Cadieux, P. A., Burton, J. P., Razvi, H., et al. (2014). Selective target inactivation rather than global metabolic dormancy causes antibiotic tolerance in uropathogens. Antimicrob. Agents Chemother. 58, 2089–2097. doi: 10.1128/aac.02552-13
Griffith, D. M., Li, H., Werrett, M. V., Andrews, P. C., and Sun, H. (2021). Medicinal chemistry and biomedical applications of bismuth-based compounds and nanoparticles. Chem. Soc Rev. doi: 10.1039/d0cs00031k [Epub ahead of print].
Guiard, E., Lelievre, B., Rouyer, M., Zerbib, F., Diquet, B., Megraud, F., et al. (2019). Bismuth concentrations in patients treated in real-life practice with a bismuth subcitrate-metronidazole-tetracycline preparation: the SAPHARY study. Drug Saf. 42, 993–1003. doi: 10.1007/s40264-019-00821-6
Hajishengallis, G. (2015). Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 15, 30–44. doi: 10.1038/nri3785
Hajishengallis, G., and Chavakis, T. (2021). Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 21, 426–440. doi: 10.1038/s41577-020-00488-6
Hajishengallis, G., Darveau, R. P., and Curtis, M. A. (2012). The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10, 717–725. doi: 10.1038/nrmicro2873
Hajishengallis, G., Liang, S., Payne, M. A., Hashim, A., Jotwani, R., Eskan, M. A., et al. (2011). Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10, 497–506. doi: 10.1016/j.chom.2011.10.006
Hernandez Ceruelos, A., Romero-Quezada, L. C., Ruvalcaba Ledezma, J. C., and Lopez Contreras, L. (2019). Therapeutic uses of metronidazole and its side effects: an update. Eur. Rev. Med. Pharmacol. Sci. 23, 397–401. doi: 10.26355/eurrev_201901_16788
Honda, K. (2011). Porphyromonas gingivalis sinks teeth into the oral microbiota and periodontal disease. Cell Host Microbe 10, 423–425. doi: 10.1016/j.chom.2011.10.008
Jain, P., Weinrick, B. C., Kalivoda, E. J., Yang, H., Munsamy, V., Vilcheze, C., et al. (2016). Dual-reporter mycobacteriophages (Φ2DRMs) reveal preexisting mycobacterium tuberculosis persistent cells in human sputum. mBio 7, e01023–16. doi: 10.1128/mBio.01023-16
Jin, L. J., Armitage, G. C., Klinge, B., Lang, N. P., Tonetti, M., and Williams, R. C. (2011). Global oral health inequalities: task group–periodontal disease. Adv. Dent. Res. 23, 221–226. doi: 10.1177/0022034511402080
Jin, L. J., Lamster, I. B., Greenspan, J. S., Pitts, N. B., Scully, C., and Warnakulasuriya, S. (2016). Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral. Dis. 22, 609–619. doi: 10.1111/odi.12428
Kassebaum, N. J., Bernabe, E., Dahiya, M., Bhandari, B., Murray, C. J., and Marcenes, W. (2014). Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J. Dent. Res. 93, 1045–1053. doi: 10.1177/0022034514552491
Kim, W., Fricke, N., Conery, A. L., Fuchs, B. B., Rajamuthiah, R., Jayamani, E., et al. (2016). NH125 kills methicillin-resistant Staphylococcus aureus persisters by lipid bilayer disruption. Future Med. Chem. 8, 257–269. doi: 10.4155/fmc.15.189
Lafleur, M. D., Qi, Q., and Lewis, K. (2010). Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob. Agents Chemother. 54, 39–44. doi: 10.1128/AAC.00860-09
Laine, L., Hunt, R., El-Zimaity, H., Nguyen, B., Osato, M., and Spenard, J. (2003). Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North Am. Trial. Am. J. Gastroenterol. 98, 562–567. doi: 10.1111/j.1572-0241.2003.t01-1-07288.x
Leitsch, D., Muller, J., and Muller, N. (2016). Evaluation of Giardia lamblia thioredoxin reductase as drug activating enzyme and as drug target. Int. J. Parasitol. Drugs Drug. Resist. 6, 148–153. doi: 10.1016/j.ijpddr.2016.07.003
Lewis, K. (2001). Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45, 999–1007. doi: 10.1128/AAC.45.4.999-1007.2001
Lewis, K. (2007). Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 5, 48–56. doi: 10.1038/nrmicro1557
Lewis, K. (2010). Persister cells. Annu. Rev. Microbiol. 64, 357–372. doi: 10.1146/annurev.micro.112408.134306
Li, H., Wang, R., and Sun, H. (2019). Systems approaches for unveiling the mechanism of action of bismuth drugs: new medicinal applications beyond Helicobacter Pylori infection. Acc. Chem. Res. 52, 216–227. doi: 10.1021/acs.accounts.8b00439
Li, P., Fung, Y. E., Yin, X., Seneviratne, C. J., Che, C. M., and Jin, L. (2018). Controlled cellular redox, repressive hemin utilization and adaptive stress responses are crucial to metronidazole tolerance of Porphyromonas gingivalis persisters. J. Clin. Periodontol. 45, 1211–1221. doi: 10.1111/jcpe.13002
Li, X., Luo, W., Ng, T. W., Leung, P. C., Zhang, C., Leung, K. C., et al. (2017). Nanoparticle-encapsulated baicalein markedly modulates pro-inflammatory response in gingival epithelial cells. Nanoscale 9, 12897–12907. doi: 10.1039/c7nr02546g
Li, X., Wong, C. H., Ng, T. W., Zhang, C. F., Leung, K. C., and Jin, L. (2016). The spherical nanoparticle-encapsulated chlorhexidine enhances anti-biofilm efficiency through an effective releasing mode and close microbial interactions. Int. J. Nanomedicine 11, 2471–2480. doi: 10.2147/IJN.S105681
Marshall, B. J., Goodwin, C. S., Warren, J. R., Murray, R., Blincow, E. D., Blackbourn, S. J., et al. (1988). Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet 2, 1437–1442. doi: 10.1016/s0140-6736(88)90929-4
Megraud, F. (2012). The challenge of Helicobacter pylori resistance to antibiotics: the comeback of bismuth-based quadruple therapy. Therap. Adv. Gastroenterol. 5, 103–109. doi: 10.1177/1756283X11432492
Mulcahy, L. R., Burns, J. L., Lory, S., and Lewis, K. (2010). Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 192, 6191–6199. doi: 10.1128/JB.01651-09
Paraje, M. (2011). Antimicrobial resistance in biofilms. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2, 736–734. doi: 10.1016/j.ijantimicag.2009.12.011
Pihlstrom, B. L., Michalowicz, B. S., and Johnson, N. W. (2005). Periodontal diseases. Lancet 366, 1809–1820. doi: 10.1016/S0140-6736(05)67728-8
Putrins, M., Kogermann, K., Lukk, E., Lippus, M., Varik, V., and Tenson, T. (2015). Phenotypic heterogeneity enables uropathogenic Escherichia coli to evade killing by antibiotics and serum complement. Infect. Immun. 83, 1056–1067. doi: 10.1128/IAI.02725-14
Singh, R., Sahore, S., Kaur, P., Rani, A., and Ray, P. (2016). Penetration barrier contributes to bacterial biofilm-associated resistance against only select antibiotics, and exhibits genus-, strain- and antibiotic-specific differences. Pathog. Dis. 74:ftw056. doi: 10.1093/femspd/ftw056
Slikkerveer, A., and de Wolff, F. A. (1989). Pharmacokinetics and toxicity of bismuth compounds. Med. Toxicol. Adverse Drug Exp. 4, 303–323. doi: 10.1007/BF03259915
Spoering, A. L., and Lewis, K. (2001). Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183, 6746–6751. doi: 10.1128/JB.183.23.6746-6751.2001
Stapels, D. A. C., Hill, P. W. S., Westermann, A. J., Fisher, R. A., Thurston, T. L., Saliba, A. E., et al. (2018). Salmonella persisters undermine host immune defenses during antibiotic treatment. Science 362, 1156–1160. doi: 10.1126/science.aat7148
Subramani, R., and Jayaprakashvel, M. (2019). “Bacterial quorum sensing: biofilm formation, survival behaviour and antibiotic resistance,” in Implication of Quorum Sensing and Biofilm Formation in Medicine, Agriculture and Food Industry, ed. P. V. Bramhachari (Singapore: Springer), 21–37.
Teirlinck, E., Samal, S. K., Coenye, T., and Braeckmans, K. (2017). “Penetrating the bacterial biofilm,” in Functionalized Nanomaterials for the Management of Microbial Infection, eds R. Boukherroub, S. Szunerits, and D. Drider (Boston, MA: Elsevier), 49–76.
Tonetti, M. S., Jepsen, S., Jin, L., and Otomo-Corgel, J. (2017). Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J. Clin. Periodontol. 44, 456–462. doi: 10.1111/jcpe.12732
Uruen, C., Chopo-Escuin, G., Tommassen, J., Mainar-Jaime, R. C., and Arenas, J. (2020). Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics (Basel) 10:3. doi: 10.3390/antibiotics10010003
Van den Bergh, B., Fauvart, M., and Michiels, J. (2017). Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol. Rev. 41, 219–251. doi: 10.1093/femsre/fux001
Vanhoe, H., Versieck, J., Vanballenberghe, L., and Dams, R. (1993). Bismuth in human serum: reference interval and concentrations after intake of a therapeutic dose of colloidal bismuth subcitrate. Clin. Chim. Acta 219, 79–91. doi: 10.1016/0009-8981(93)90199-e
Wang, C., Cheng, T., Li, X., and Jin, L. (2020). Metronidazole-Treated Porphyromonas gingivalis persisters invade human gingival epithelial cells and perturb innate responses. Antimicrob. Agents Chemother. 64, e02529–19. doi: 10.1128/AAC.02529-19
Wang, R., Lai, T. P., Gao, P., Zhang, H., Ho, P. L., Woo, P. C., et al. (2018). Bismuth antimicrobial drugs serve as broad-spectrum metallo-beta-lactamase inhibitors. Nat. Commun. 9:439. doi: 10.1038/s41467-018-02828-6
Wassmann, C., Hellberg, A., Tannich, E., and Bruchhaus, I. (1999). Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J. Biol. Chem. 274, 26051–26056. doi: 10.1074/jbc.274.37.26051
Weir, C. B., and Le, J. K. (2021). “Metronidazole,” in StatPearls [Internet]. (Treasure Island FL: StatPearls Publishing). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK539728/
Yuan, S., Wang, R., Chan, J. F., Zhang, A. J., Cheng, T., Chik, K. K., et al. (2020). Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters. Nat. Microbiol. 5, 1439–1448. doi: 10.1038/s41564-020-00802-x
Keywords: Porphyromonas gingivalis, persisters, eradication, bismuth drugs, metronidazole
Citation: Wang C, Li X, Cheng T, Sun H and Jin L (2021) Eradication of Porphyromonas gingivalis Persisters Through Colloidal Bismuth Subcitrate Synergistically Combined With Metronidazole. Front. Microbiol. 12:748121. doi: 10.3389/fmicb.2021.748121
Received: 27 July 2021; Accepted: 24 September 2021;
Published: 20 October 2021.
Edited by:
Fabian Cieplik, University Medical Center Regensburg, GermanyReviewed by:
Olivier Huck, Université de Strasbourg, FranceCopyright © 2021 Wang, Li, Cheng, Sun and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijian Jin, bGpqaW5AaGt1Lmhr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.