
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 01 October 2021
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.743312
This article is part of the Research TopicCarbapenemases in Gram-negative Bacteria: A Global Health Threat and Therapeutic ChallengeView all 19 articles
 Xiangning Huang1†
Xiangning Huang1† Siquan Shen2,3†
Siquan Shen2,3† Qingyu Shi2,3
Qingyu Shi2,3 Li Ding2,3
Li Ding2,3 Shi Wu2,3
Shi Wu2,3 Renru Han2,3
Renru Han2,3 Xun Zhou2,3
Xun Zhou2,3 Hua Yu1*
Hua Yu1* Fupin Hu2,3*
Fupin Hu2,3*Carbapenem-resistant Enterobacterales (CRE) has become a major therapeutic concern in clinical settings, and carbapenemase genes have been widely reported in various bacteria. In Serratia marcescens, class A group carbapenemases including SME and KPC were mostly identified. However, there are few reports of metallo-β-lactamase-producing S. marcescens. Here, we isolated a carbapenem-resistant S. marcescens (S378) from a patient with asymptomatic urinary tract infection which was then identified as an IMP-4-producing S. marcescens at a tertiary hospital in Sichuan Province in southwest of China. The species were identified using MALDI-TOF MS, and carbapenemase-encoding genes were detected using PCR and DNA sequencing. The results of antimicrobial susceptibility testing by broth microdilution method indicated that the isolate S. marcescens S378 was resistant to meropenem (MIC = 32 μg/ml) and imipenem (MIC = 64 μg/ml) and intermediate to aztreonam (MIC = 8 μg/ml). The complete genomic sequence of S. marcescens was identified using Illumina (Illumina, San Diego, CA, United States) short-read sequencing (150 bp paired-end reads); five resistance genes had been identified, including blaIMP–4, blaSRT–2, aac(6′)-Ic, qnrS1, and tet(41). Conjugation experiments indicated that the blaIMP–4-carrying plasmid pS378P was conjugative. Complete sequence analysis of the plasmid pS378P bearing blaIMP–4 revealed that it was a 48,780-bp IncN-type plasmid with an average GC content of 50% and was nearly identical to pP378-IMP (99% nucleotide identity and query coverage).
S. marcescens is recognized to be an important nosocomial pathogen and is usually associated with outbreaks in neonatal wards (Mahlen, 2011; Millán-Lou et al., 2021). The infection caused by S. marcescens can cause nosocomial infection, affecting several parts of the body, such as the meninges, blood, and lungs, leading to a series of infections like central nervous system infections, blood infections (including endocarditis), and nosocomial pneumonia (Mahlen, 2011; da Silva et al., 2021). The emergence of multidrug-resistant (MDR) S. marcescens strains poses a serious threat to public health. One important feature of S. marcescens is its intrinsic and acquired resistance to a large number of antibiotics including ampicillin, nitrofurantoin, tetracycline, macrolides, cefuroxime, cephamycin, fluoroquinolone, and colistin (Sandner-Miranda et al., 2018). The identification of carbapenem-resistant S. marcescens in patients might pose potential spread into the hospital environment and/or to other patients. For example, in 2020, the outbreak of KPC-3-producing S. marcescens among nursing institutions in the United States made it very difficult for clinical treatment (Jimenez et al., 2020). The spread of carbapenem-resistant S. marcescens in a hospital environment is a worrying problem.
The metallo-β-lactamases (MBLs) can hydrolyze nearly all β-lactams, and their activities cannot be inhibited by clinically available β-lactamase inhibitors including avibactam, relebactam, and vaborbactam (Wu et al., 2019; Boyd et al., 2020). IMP-type MBLs were the earliest transferable carbapenemases reported in Gram-negative bacteria (Watanabe et al., 1991). Until now, more than 82 variants of blaIMP have been identified,1 blaIMP–4, as the most reported IMP variant, has often been found in class 1 integrons and carried by multiple plasmid types like HI2, L/M, A/C, and N for dissemination (Lai et al., 2017; Wang et al., 2018; Roberts et al., 2020). However, unlike NDM-type MBLs, blaIMP was not commonly detected among CRE from China (Zhang et al., 2017; Wang et al., 2018; Han et al., 2020). According to a longitudinal large-scale CRE Study in China from 2012 to 2016 (65 hospitals in 25 provinces were included), the common species carrying blaIMP–4 were Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, and Citrobacter freundii (Wang et al., 2018). In 2005, the first clinical isolate of blaIMP–4-positive S. marcescens was identified in Australia (Peleg et al., 2005). In this study, we reported a blaIMP–4 and blaSRT–2 positive S. marcescens containing an IncN-type plasmid in China.
A carbapenem-resistant S. marcescens (S378) was isolated from a urine sample from a patient with asymptomatic urinary tract infection at a tertiary hospital in Sichuan Province in southwest of China. Species identification was performed using MALDI-TOF MS (bioMérieux, France). Phenotypic and genotypic detection of carbapenemases was performed using imipenem-EDTA double-disk synergy test and NG-Test Carba 5, respectively. The existence of the carbapenemase genes (KPC, NDM, OXA, IMP, and VIM) was confirmed by PCR-based sequencing, as previously described (Gülmez et al., 2008; Woodford et al., 2008; Feng et al., 2016; Ferreira et al., 2020; Solgi et al., 2020; Nikibakhsh et al., 2021). The broth microdilution method recommended by the Clinical and Laboratory Standards Institute (CLSI) was used as a reference for determining the minimal inhibition concentration with quality control and interpretation of the results according to CLSI M100-31th breakpoints for all agents with the exception of tigecycline, polymyxin, and cefoperazone–sulbactam (Clinical and Laboratory Standards Institute, 2021). Cefepime–zidebactam and cefepime–tazobactam referred to criteria for cefepime in CLSI. Tigecycline MICs were interpreted using US FDA MIC breakpoints for Enterobacterales (FDA, 2021), and polymyxin MICs were interpreted using the European Committee for Antimicrobial Susceptibility Testing (EUCAST) MIC breakpoints for Enterobacterales. E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as controls for testing antimicrobial susceptibility.
A conjugation experiment was performed to explore the transferability of the plasmid. Briefly, the blaIMP–4-positive isolate S. marcescens S378 was used as the donor, while the E. coli J53 (azide resistant) was used as the recipient strain. Conjugants were selected on Mueller–Hinton (MH) agar supplemented with azide (100 μg/ml) and ampicillin (50 μg/ml). The presence of the blaIMP–4 gene and other resistance genes in conjugants was confirmed by antimicrobial susceptibility, PCR, and DNA sequencing. The plasmids of the blaIMP–4-containing conjugants were extracted using the Qiagen Midi kit (Qiagen, Hilden, Germany) and sequenced using Illumina NovaSeq (Illumina, San Diego, CA, United States) short-read sequencing (150-bp paired-end reads). The sequencing reads were trimmed with sickle (GitHub) and de novo assembled using SPAdes 3.12.0. To evaluate and compare the assembly results, Pilon 1.18 is used for basic calibration. Open reading frame prediction and annotation were done with RAST version 2.02 and BLAST3 at NCBI; the plasmid replicon was determined using the PCR-based replicon typing method (Carattoli et al., 2005). Plasmid comparisons were performed using BRIG4 (Alikhan et al., 2011).
Genomic DNA was extracted using a Genomic DNA Isolation Kit (Qiagen, Hilden, Germany) and sequenced using Illumina (Illumina, San Diego, CA, United States) short-read sequencing (150-bp paired-end reads). Sequences were de novo assembled using SPAdes 3.12.0. Antimicrobial resistance gene analysis and draft genome annotation were performed using BacWGSTdb.5
S. marcescens S378 was isolated from a urine specimen of a 76-year-old male patient who was admitted to the hospital for treatment of chronic obstructive pulmonary disease. During hospitalization, the patient was dizzy accompanied by shortness of breath and aggravated after activity. CT showed inflammatory changes in the lungs. The patient suffered from subarachnoid hemorrhage and has been cured. Comorbidities of the elderly patient included diabetes mellitus II, hypertension, acute cerebral infarction, occlusion of right internal carotid artery, chronic obstructive pulmonary disease, and asymptomatic urinary tract infection. The interventional operation was performed for this patient to deal with acute cerebral infarction, and on the third day after the operation, he developed a fever and one extended-spectrum β-lactamase-negative K. pneumoniae was isolated from the sputum. With suspicion of pneumonia, the patient was started administering intravenous moxalactam (1 g Q12) for 5 days and ceftazidime (1 g Q8h) for 4 days. Subsequently, the patient’s body temperature returned to normal and the infection was controlled. On the 20th day, S. marcescens S378 was isolated from the urine causing asymptomatic urinary tract infection. No antibiotic was given for this strain because this Serratia marcescens was considered to only colonize in the urinary tract. The patient was recovered and discharged 26 days after admission.
The antimicrobial susceptibility profiles of S. marcescens S378 are presented in Table 1. The isolate was susceptible to tigecycline (MIC = 0.5 μg/ml), amikacin (MIC = 16 μg/ml), and Trimethoprim-sulfamethoxazole (MIC ≤ 0.25 μg/L), intermediate to aztreonam (MIC = 8 μg/ml), but resistant to cefoperazone–sulbactam (MICs > 128 μg/ml), meropenem (MIC = 32 μg/ml), imipenem (MIC = 64 μg/ml), ceftazidime-avibactam (MIC ≥ 32 μg/ml), levofloxacin (MIC = 2 μg/ml), and ciprofloxacin (MIC = 1 μg/ml).
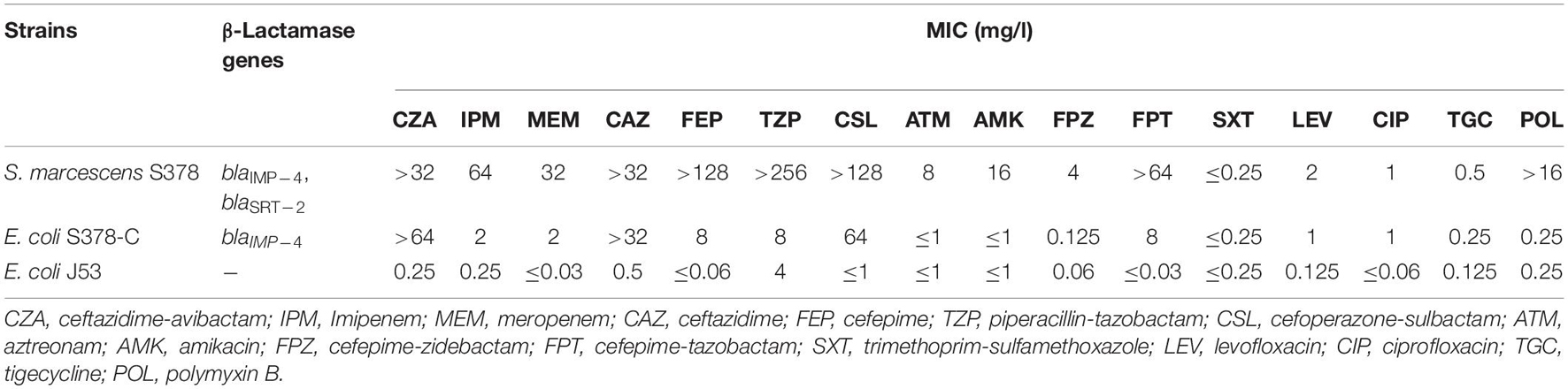
Table 1. Susceptibility of S. marcescens clinical isolate, conjugant and recipient to antimicrobial agents.
PCR-based sequencing demonstrated the presence of blaIMP–4 in S. marcescens strain S378. The blaIMP–4-carrying plasmid was successfully transferred from S. marcescens strain S378 to E. coli J53, making the conjugants resistant to ceftazidime and ceftazidime–avibactam but intermediate to imipenem and meropenem. Compared with the recipient E. coli J53, the meropenem, imipenem, and ceftazidime–avibactam MICs of conjugants increased at least 60-, 8-, and 256-fold, respectively (Table 1).
According to the whole-genome sequencing (WGS) analysis, many resistance genes had been identified, including the β-lactamase genes blaIMP–4 and blaSRT–2, the aminoglycoside resistance genes aac(6′)-Ic, the fluoroquinolone resistance gene qnrS1, and the tetracycline resistance gene tet(41). According to the sequencing results of pS378P, it was a 48,780-bp plasmid (Figure 1), belonging to the IncN type, with an average GC content of 50%. This targeted plasmid contained 43 open reading frames (ORFs). Only two resistance genes were identified in pS378P, blaIMP–4, and qnrS1, conferring resistance to carbapenems and quinolones, respectively. Blast comparison indicates that pS378P in this study shares extensive similarity with pP378-IMP (99% nucleotide identity and query coverage), an IncN-type blaIMP–4 carrying plasmid with the length of 51,207 bp in a carbapenem-resistant P. aeruginosa strain P378 isolated from a teaching hospital in Chongqing China in 2016 (Feng et al., 2016). Like the source of our strain, they were all isolated from urine specimen. pP378-IMP and pS378P both possess the conserved IncN1-type backbone regions, the tra genes and kikA-korB for conjugal transfer, and IS1 remnant (Figure 1). There are two major genetic differences between the backbones of pS378P and pP378-IMP. First, pP378-IMP contains an intact anti-restriction gene combination ccgA I, ccgAII, ccgC, and ccgD (located around the 4.1–4.6-kb nucleotide positions of pP378-IMP), while only ccgAI and ccgAII genes were found in pS378P. Second, compared with the plasmid pP378-IMP, the class one integron in pS378P is incomplete that an insertion sequence, IS6100, was truncated (Figure 2).
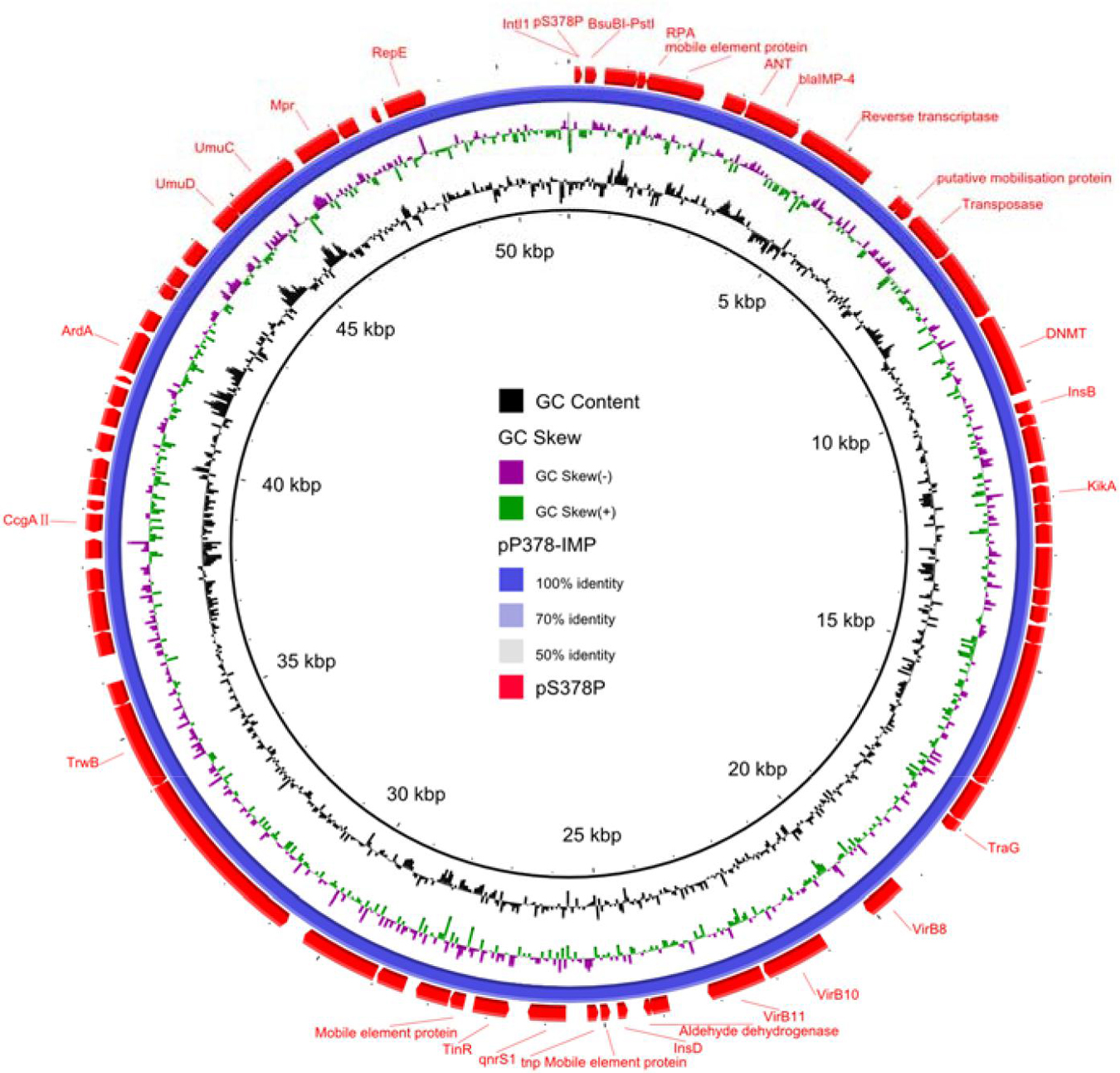
Figure 1. Circular comparison between plasmid pS378P (MZ643942, in this study) and plasmid pP378-IMP (KX711879). The different colors indicated different plasmids and are listed in the color key.
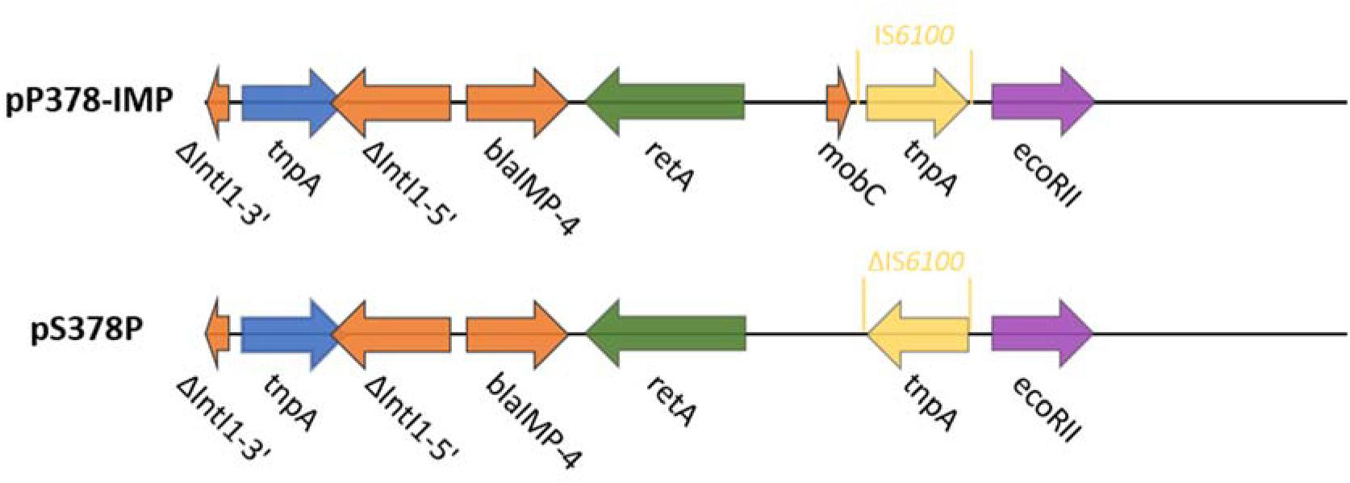
Figure 2. Plasmid accessory resistance regions. The genetic environment comparison of blaIMP–4 between pP378-IMP and pS378P. Genes are denoted by arrows and colored based on gene function classification.
According to a previous epidemiological study, the most frequent carbapenemases found in S. marcescens species belong to the class A group, including chromosomal location SME type or KPC-2 (Dabos et al., 2019). blaSRT–2, an AmpC-type β-lactamase gene, was first reported in a S. marcescens strain in 2004. Almost all subsequent reports about it are related to S. marcescens. Moreover, in S. marcescens, blaSRT–2 often appears with different resistance genes, such as blaCTX–M–3, blaTEM–1, aminoglycoside AAC (6′)-Ic, and blaKPC–2 (Wu et al., 2004; Yu et al., 2008; Srinivasan and Rajamohan, 2019; Quezada-Aguiluz et al., 2020). blaIMP–4 was first identified in Acinetobacter spp. in 2001 from Hong Kong, China (Chu et al., 2001), and had spread rapidly around the world (Espedido et al., 2008; Lee et al., 2017, 2018; Ghaith et al., 2018), but unlike KPC-type and NDM-type carbapenemases among CRE (NDM-type MBL remains predominant), blaIMP–4 has not been frequently detected, especially in S. marcescens (Xiong et al., 2016; Han et al., 2020). In 2018, the first report of blaIMP–4- and blaVIM–2-producing S. marcescens was published in Egypt (Ghaith et al., 2018). This is a clinical retrospective study. A total of 40 strains of S. marcescens were isolated from March to August 2015, of which 42.5% was IMP-4-positive and 37.5% was VIM-2-positive. Just like the strain in our study, they all showed resistance to meropenem and ceftazidime. Our study demonstrates the emergence of carbapenemase-producing S. marcescens, expressing blaIMP–4 and blaSRT–2 β-lactamase genes in China.
S. marcescens is featured by its rapid acquisition of antibiotic resistance, mainly due to the acquisition of plasmid (Mahlen, 2011). However, comprehensive analysis for the genome sequence carrying blaIMP–4 is rare. According to current reports, although different types of plasmids had been detected, the IncN type remains predominant in China, especially for the transmission of blaIMP–4 in recent years, and this type of plasmid usually presents a broad host range (Feng et al., 2016; Wang et al., 2017; Xu et al., 2020), compared with the most identical plasmid pP378-IMP. The strains in both studies all harbored a conjugative blaIMP–4-carrying plasmid, which accounts for the carbapenem resistance phenotype. In both plasmids, the blaIMP–4 gene was found in class 1 integron, identical to the IMP-4-carrying plasmids before (Xu et al., 2020; Liu et al., 2021). Class 1 integrons are responsible for the transmission of the blaIMP gene; so far, many Class 1 integrons carrying the blaIMP–4 gene have been reported, such as In 809, 823, 1,456, 1,460, and 1,589 (Lee et al., 2017; Matsumura et al., 2017; Wang et al., 2017; Dolejska et al., 2018). In addition to integrons and the conjugative plasmids, the insertion sequence also plays an important role in the transmission of resistant genes. Previous studies about blaIMP–4-carrying plasmids emphasized the role of the IS26 mobile element, which may play an important role in the dissemination of IMP-4 in different plasmids (Xu et al., 2020; Liu et al., 2021); the corresponding situation also exists in our strains. Timely determination of the resistance mechanism and the transmission mechanism of resistance genes is very important for clinical anti-infective treatment and controlling the wide spread of these multi-drug resistant bacteria.
In summary, we first identified a blaIMP–4 and blaSRT–2 co-positive S. marcescens strain from a human urine sample in China. The patient was accompanied by many underlying diseases such as diabetes, emphysema, diabetic peripheral neuropathy, and atherosclerosis, and multiple antimicrobial substances were used in the course of treatment; since such risk factors for MDR bacteria are commonly present in high-risk populations, it seems justified to screen Gram-negative bacilli for carbapenemases in these patients with high-risk factors based on our routine antimicrobial susceptibility testing and molecular biotechnology. Moreover, to date, S. marcescens and many other Enterobacteriaceae bacteria that are not often reported might still be a neglected source of undetected carbapenemase allocation.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: GenBank MZ643942.
The study protocol was approved by the Institutional Review Board of Huashan Hospital, Fudan University (No. 2018-408).
FH and HY designed the study. SS and XH collected clinical samples and performed the experiments. LD, SW, RH, and XZ analyzed the data. SS and XH wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Mega-project for Innovative Drugs (2019ZX09721001-006-004), the National Natural Science Foundation of China (81871690), the Shanghai Antimicrobial Surveillance Network (3030231003), and the China Antimicrobial Surveillance Network (2018QD100). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alikhan, N. F., Petty, N. K., Ben Zakour, N. L., and Beatson, S. A. (2011). BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402
Boyd, S. E., Livermore, D. M., Hooper, D. C., and Hope, W. W. (2020). Metallo-β-lactamases: structure, function, epidemiology, treatment options, and the development pipeline. Antimicrob. Agents Chemother. 64:e00397-e20. doi: 10.1128/aac.00397-20
Carattoli, A., Bertini, A., Villa, L., Falbo, V., Hopkins, K. L., and Threlfall, E. J. (2005). Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63, 219–228.
Chu, Y. W., Afzal-Shah, M., Houang, E. T., Palepou, M. I., Lyon, D. J., Woodford, N., et al. (2001). IMP-4, a novel metallo-beta-lactamase from nosocomial Acinetobacter spp. Collected in Hong Kong between 1994 and 1998. Antimicrob. Agents Chemother. 45, 710–714. doi: 10.1128/aac.45.3.710-714.2001
Clinical and Laboratory Standards Institute (2021). Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100, 31th Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
da Silva, K. E., Rossato, L., Jorge, S., de Oliveira, N. R., Kremer, F. S., Campos, V. F., et al. (2021). Three challenging cases of infections by multidrug-resistant Serratia marcescens in patients admitted to intensive care units. Braz. J. Microbiol. 52, 1341–1345. doi: 10.1007/s42770-021-477-474
Dabos, L., Patiño-Navarrete, R., Nastro, M., Famiglietti, A., Glaser, P., Rodriguez, C. H., et al. (2019). SME-4-producing Serratia marcescens from Argentina belonging to clade 2 of the S. Marcescens phylogeny. J. Antimicrob. Chemother. 74, 1836–1841. doi: 10.1093/jac/dkz115
Dolejska, M., Papagiannitsis, C., Medvecky, M., Davidova-Gerzova, L., and Valcek, A. J. A. (2018). Characterization of the complete nucleotide sequences of IMP-4-encoding plasmids, belonging to diverse inc families, recovered from Enterobacteriaceae isolates of wildlife origin. Antimicrob. Agents Chemother. 62:e2434-e17. doi: 10.1128/aac.02434-17
Espedido, B. A., Partridge, S. R., and Iredell, J. R. (2008). blaIMP–4 in different genetic contexts in Enterobacteriaceae isolates from Australia. Antimicrob. Agents Chemother. 52, 2984–2987. doi: 10.1128/aac.01634-07
FDA (2021). Tigecycline-Injection Products. Available online at: https://www.fda.gov/drugs/development-resources/tigecycline-injection-products.
Feng, W., Zhou, D., Wang, Q., Luo, W., Zhang, D., Sun, Q., et al. (2016). Dissemination of IMP-4-encoding pIMP-HZ1-related plasmids among Klebsiella pneumoniae and Pseudomonas aeruginosa in a Chinese teaching hospital. Sci. Rep. 6:33419. doi: 10.1038/srep33419
Ferreira, R. L., Rezende, G. S., Damas, M. S. F., Oliveira-Silva, M., Pitondo-Silva, A., Brito, M. C. A., et al. (2020). Characterization of KPC-producing Serratia marcescens in an intensive care unit of a Brazilian tertiary hospital. Front. Microbiol. 11:956. doi: 10.3389/fmicb.2020.00956
Ghaith, D. M., Zafer, M. M., Ismail, D. K., Al-Agamy, M. H., Bohol, M. F. F., Al-Qahtani, A., et al. (2018). First reported nosocomial outbreak of Serratia marcescens harboring blaIMP–4 and blaVIM–2 in a neonatal intensive care unit in Cairo, Egypt. Infect. Drug Resist. 11, 2211–2217. doi: 10.2147/idr.S174869
Gülmez, D., Woodford, N., Palepou, M. F., Mushtaq, S., Metan, G., Yakupogullari, Y., et al. (2008). Carbapenem-resistant Escherichia coli and Klebsiella pneumoniae isolates from Turkey with OXA-48-like carbapenemases and outer membrane protein loss. Int. J. Antimicrob. Agents 31, 523–526. doi: 10.1016/j.ijantimicag.2008.01.017
Han, R., Shi, Q., Wu, S., Yin, D., Peng, M., Dong, D., et al. (2020). Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant Enterobacteriaceae isolated from adult and children patients in China. Front. Cell. Infect. Microbiol. 10:314. doi: 10.3389/fcimb.2020.00314
Jimenez, A., Abbo, L. M., Martinez, O., Shukla, B., Sposato, K., Iovleva, A., et al. (2020). KPC-3-producing Serratia marcescens outbreak between acute and long-term care facilities, Florida, USA. Emerg. Infect. Dis. 26, 2746–2750. doi: 10.3201/eid2611.202203
Lai, K., Ma, Y., Guo, L., An, J., Ye, L., and Yang, J. (2017). Molecular characterization of clinical IMP-producing Klebsiella pneumoniae isolates from a Chinese tertiary Hospital. Ann. Clin. Microbiol. Antimicrob. 16:42. doi: 10.1186/s12941-017-0218-9
Lee, J. H., Bae, I. K., Lee, C. H., and Jeong, S. (2017). Molecular characteristics of first IMP-4-producing Enterobacter cloacae sequence type 74 and 194 in Korea. Front. Microbiol. 8:2343. doi: 10.3389/fmicb.2017.02343
Lee, J. H., Lee, C. H., and Bae, I. K. (2018). Emergence of IMP-4-producing Enterobacter aerogenes clinical isolate. Clin. Lab. 64, 1323–1326. doi: 10.7754/Clin.Lab.2018.180211
Liu, W., Dong, H., Yan, T., Liu, X., Cheng, J., Liu, C., et al. (2021). Molecular characterization of blaIMP–4-carrying Enterobacterales in henan province of china. Front. Microbiol. 12:626160. doi: 10.3389/fmicb.2021.626160
Mahlen, S. D. (2011). Serratia infections: from military experiments to current practice. Clin. Microbiol. Rev. 24, 755–791. doi: 10.1128/cmr.00017-11
Matsumura, Y., Peirano, G., Motyl, M. R., Adams, M. D., Chen, L., Kreiswirth, B., et al. (2017). Global molecular epidemiology of IMP-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 61:e02729-16.
Millán-Lou, M. I., López, C., Bueno, J., Pérez-Laguna, V., Lapresta, C., Fuertes, M. E., et al. (2021). Successful control of Serratia marcescens outbreak in a neonatal unit of a tertiary-care hospital in Spain. Enferm. Infecc. Microbiol. Clin. 16:S0213-005X(21)00186-5. doi: 10.1016/j.eimc.2021.05.003
Nikibakhsh, M., Firoozeh, F., Badmasti, F., Kabir, K., and Zibaei, M. (2021). Molecular study of metallo-β-lactamases and integrons in Acinetobacter baumannii isolates from burn patients. BMC Infect. Dis. 21:782. doi: 10.1186/s12879-021-06513-w
Peleg, A. Y., Franklin, C., Bell, J. M., and Spelman, D. W. (2005). Dissemination of the metallo-beta-lactamase gene blaIMP–4 among gram-negative pathogens in a clinical setting in Australia. Clin. Infect. Dis. 41, 1549–1556. doi: 10.1086/497831
Quezada-Aguiluz, M., Lincopan, N., Cerdeira, L., Fuga, B., Silva, F., Barrera, B., et al. (2020). Draft genome sequence of a multidrug-resistant KPC-2 and SRT-2 co-producing Serratia marcescens strain isolated from a hospitalised patient in Chile. J. Glob. Antimicrob. Resist. 21, 1–2. doi: 10.1016/j.jgar.2020.02.004
Roberts, L. W., Catchpoole, E., Jennison, A. V., Bergh, H., Hume, A., Heney, C., et al. (2020). Genomic analysis of carbapenemase-producing Enterobacteriaceae in Queensland reveals widespread transmission of blaIMP–4 on an IncHI2 plasmid. Microb. Genom. 6:e000321. doi: 10.1099/mgen.0.000321
Sandner-Miranda, L., Vinuesa, P., Cravioto, A., and Morales-Espinosa, R. (2018). The genomic basis of intrinsic and acquired antibiotic resistance in the genus Serratia. Front Microbiol 9:828. doi: 10.3389/fmicb.2018.00828
Solgi, H., Nematzadeh, S., Giske, C. G., Badmasti, F., Westerlund, F., Lin, Y. L., et al. (2020). Molecular epidemiology of OXA-48 and NDM-1 producing Enterobacterales species at a university hospital in Tehran, Iran, Between 2015 and 2016. Front. Microbiol. 11:936. doi: 10.3389/fmicb.2020.00936
Srinivasan, V. B., and Rajamohan, G. (2019). Genome analysis of urease positive Serratia marcescens, co-producing SRT-2 and AAC(6′)-Ic with multidrug efflux pumps for antimicrobial resistance. Genomics 111, 653–660. doi: 10.1016/j.ygeno.2018.04.001
Wang, Q., Wang, X., Wang, J., Ouyang, P., Jin, C., Wang, R., et al. (2018). Phenotypic and genotypic characterization of carbapenem-resistant Enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012-2016). Clin. Infect. Dis. 67(Suppl. 2), S196–S205. doi: 10.1093/cid/ciy660
Wang, Y., Lo, W.-U., Lai, R. W.-M., Tse, C. W.-S., Lee, R. A., Luk, W.-K., et al. (2017). IncN ST7 epidemic plasmid carrying blaIMP–4 in Enterobacteriaceae isolates with epidemiological links to multiple geographical areas in China. J. Antimicrob. Chemother. 72, 99–103. doi: 10.1093/jac/dkw353
Watanabe, M., Iyobe, S., Inoue, M., and Mitsuhashi, S. (1991). Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 35, 147–151. doi: 10.1128/aac.35.1.147
Woodford, N., Zhang, J., Warner, M., Kaufmann, M. E., Matos, J., Macdonald, A., et al. (2008). Arrival of Klebsiella pneumoniae producing KPC carbapenemase in the United Kingdom. J. Antimicrob. Chemother. 62, 1261–1264. doi: 10.1093/jac/dkn396
Wu, L. T., Tsou, M. F., Wu, H. J., Chen, H. E., Chuang, Y. C., and Yu, W. L. (2004). Survey of CTX-M-3 extended-spectrum beta-lactamase (ESBL) among cefotaxime-resistant Serratia marcescens at a medical center in middle Taiwan. Diagn. Microbiol. Infect. Dis. 49, 125–129. doi: 10.1016/j.diagmicrobio.2004.02.004
Wu, W., Feng, Y., Tang, G., Qiao, F., McNally, A., and Zong, Z. (2019). NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin. Microbiol. Rev. 32, e00115-18. doi: 10.1128/cmr.00115-18
Xiong, J., Déraspe, M., Iqbal, N., Ma, J., Jamieson, F. B., Wasserscheid, J., et al. (2016). Genome and plasmid analysis of blaIMP–4-carrying Citrobacter freundii B38. Antimicrob. Agents Chemother. 60, 6719–6725. doi: 10.1128/aac.00588-16
Xu, J., Lin, W., Chen, Y., and He, F. (2020). Characterization of an IMP-4-producing Klebsiella pneumoniae ST1873 strain recovered from an infant with a bloodstream infection in China. Infect. Drug Resist. 13, 773–779. doi: 10.2147/idr.S247341
Yu, W. L., Ko, W. C., Cheng, K. C., Chen, H. E., Lee, C. C., and Chuang, Y. C. (2008). Institutional spread of clonally related Serratia marcescens isolates with a novel AmpC cephalosporinase (S4): a 4-year experience in Taiwan. Diagn. Microbiol. Infect. Dis. 61, 460–467. doi: 10.1016/j.diagmicrobio.2008.03.010
Keywords: Serratia marcescens, blaIMP–4, blaSRT–2, IncN plasmid, class 1 integron
Citation: Huang X, Shen S, Shi Q, Ding L, Wu S, Han R, Zhou X, Yu H and Hu F (2021) First Report of blaIMP–4 and blaSRT–2 Coproducing Serratia marcescens Clinical Isolate in China. Front. Microbiol. 12:743312. doi: 10.3389/fmicb.2021.743312
Received: 18 July 2021; Accepted: 01 September 2021;
Published: 01 October 2021.
Edited by:
Mary Marquart, University of Mississippi Medical Center, United StatesReviewed by:
Hua Zhou, Zhejiang University, ChinaCopyright © 2021 Huang, Shen, Shi, Ding, Wu, Han, Zhou, Yu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Yu, eXZodWEyMDAyQDE2My5jb20=; Fupin Hu, aHVmdXBpbkBmdWRhbi5lZHUuY24=
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.