
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 04 February 2022
Sec. Systems Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.737197
 Jing-Jing Ni1,2
Jing-Jing Ni1,2 Qian Xu1,3
Qian Xu1,3 Shan-Shan Yan1,3
Shan-Shan Yan1,3 Bai-Xue Han1,3
Bai-Xue Han1,3 Hong Zhang1,2
Hong Zhang1,2 Xin-Tong Wei1,3
Xin-Tong Wei1,3 Gui-Juan Feng1,3
Gui-Juan Feng1,3 Min Zhao1,2
Min Zhao1,2 Yu-Fang Pei1,3
Yu-Fang Pei1,3 Lei Zhang1,2*
Lei Zhang1,2*
Evidence supports the observational associations of gut microbiota with a variety of psychiatric disorders, but the causal nature of such associations remains obscure. Aiming to comprehensively investigate their causal relationship and to identify specific causal microbe taxa for psychiatric diseases, we conducted a two-sample Mendelian randomization (MR) analysis of gut microbiome with 15 psychiatric diseases. Specifically, the microbiome genome-wide association study (GWAS) in 18,473 individuals from the MiBioGen study was used as exposure sample, and the GWAS for 15 psychiatric diseases was used as outcome samples. One-hundred ninety bacterial taxa from six levels were available for analysis. At a multiple-testing corrected significance level (phylum P < 5.56 × 10–3, class P < 3.33 × 10–3, order P < 2.63 × 10–3, family P < 1.67 × 10–3, genus P < 4.90 × 10–4, and species P < 3.33 × 10–3), the following eight causal associations from seven bacterial features (one phylum + three classes + one order + one family + one species) were identified: family Prevotellaceae with autism spectrum disorder (P = 5.31 × 10–4), class Betaproteobacteria with bipolar disorder (P = 1.53 × 10–3), class Actinobacteria with schizophrenia (P = 1.33 × 10–3), class Bacteroidia and order Bacteroidales with Tourette syndrome (P = 2.51 × 10–3 and 2.51 × 10–3), phylum Actinobacteria and class Actinobacteria with extroversion (P = 8.22 × 10–4 and 1.09 × 10–3), and species Clostridium innocuum with neuroticism (P = 8.92 × 10–4). Sensitivity analysis showed no evidence of reverse causality, pleiotropy, and heterogeneity. Our findings offered novel insights into the gut microbiota–mediated development mechanism of psychiatric disorders.
Psychiatric disorders are a cluster of complex psychological syndromes in cognition, behavior, or emotion regulation, representing the second leading cause of disability and premature death worldwide (Whiteford et al., 2013). Epidemiological research has shown that the global lifetime incidence of psychiatric disorders in adults ranges between 12.2 and 48.6%, with the overall prevalence varying from 4.3 to 26.4% (Demyttenaere et al., 2004). Certain mental diseases, such as depression, attention deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and schizophrenia (SCZ), account for approximately 12% of the global disease burden (Ochoa-Reparaz et al., 2020). Because they frequently require long-term treatment, their burden was estimated to be $8.5 trillion in 2010 and continuously raised by 41% between 1990 and 2010 (Patel et al., 2016). Thus, there is an urgent need to identify potential causal risk factors for various psychiatric disorders.
The etiologies of psychiatric disorders are largely multi-factorial, including psychological, genetic, and environmental factors. Recently, growing evidence has suggested that the gut microbiota is closely related to host health and is involved in the etiology of a variety of human complex diseases including psychiatric disorders (Chow et al., 2010; Clemente et al., 2012; Cryan et al., 2019). The gut microbiota is a dynamic and complex community of ecological microbes, inhabiting the human intestine, even called a “forgotten organ” (O’Hara and Shanahan, 2006). The microbiota and central nervous system might communicate with each other via the microbiota–gut–brain (MGB) axis, which includes diverse routes including the immune response, the vagus and enteric nerve, and microbiota-derived molecules or metabolites (Cryan et al., 2019). A variety of observational studies have shown that the gut microbiome differs between healthy controls and psychiatric patients (Wang et al., 2011; Strati et al., 2017; Valles-Colomer et al., 2019; Hua et al., 2020). Altered compositions and function of intestinal microbiota were observed in ASD or depression patients (Strati et al., 2017; Valles-Colomer et al., 2019). Further experimental studies also demonstrated the importance of microbiota in the development of psychiatric disorders. For example, fecal microbiota transplantation (FMT) from human donors with ASD into murine induced exacerbated corresponding symptoms (Sharon et al., 2019). However, the causal association between the gut microbiota and psychiatric disorders remains unclear.
Conventionally, the gold standard for inferring a causal association is randomized controlled trials (RCTs). Whereas a RCT is difficult to implement or sometimes even impossible due to ethic restriction. As an alternative, Mendelian randomization (MR) is an efficient method to statistically assess causality from an exposure to an outcome, utilizing genetic variants as instrumental variables (IVs) (Katan, 1986; Smith and Ebrahim, 2003). Because a random assortment of genetic variants occurs during meiosis yielding according to the Mendel’s second law, the selected genetic variants avoid social economic confounding (Emdin et al., 2017). The MR approach is conceptually similar to the RCT study, with only one difference that patients are allocated according to their DNA genotypes. MR analysis relies on three important assumptions: (i) IV is strongly associated with exposure; (ii) IV should be independent of any observed and unobserved confounders of exposure–outcome association; (iii) IV–outcome association is only mediated via exposure rather than any other pathway. In a recent study, utilizing MR, Sanna et al. (2019) identified that propionate, one type of fecal short-chain fatty acid (SCFA), increases the risk of type 2 diabetes, demonstrating the efficacy of microbiota-oriented causal inference via MR analysis.
Two-sample MR analysis can utilize single-nucleotide polymorphism (SNP)–exposure and SNP–outcome associations from independent GWAS analyses and combine them into a single causal estimate. As the number of genome-wide association studies (GWASs) in gut microbiota and psychiatric disorders has increased rapidly, large-scale summary statistics have become more widely available (Demontis et al., 2019; Grove et al., 2019; Howard et al., 2019; Stahl et al., 2019; Kurilshikov et al., 2021), allowing for two-sample MR analysis with significantly improved statistical power.
In this study, we applied a systematic two-sample MR analysis to comprehensively explore whether gut microbiota components have a causal effect on various psychiatric disorders and to identify specific causal bacterial taxa. Specifically, summary statistics of gut microbiota and 15 common psychiatric disorders/traits were derived from large-scale GWAS or genetic consortia.
Genome-wide association study summary-level statistics for gut microbiota and 15 common psychiatric disorders/traits were obtained from previous studies or consortia. All studies were approved by their respective institutional review boards (IRBs). No new IRB approval was required. A flowchart briefly presents the whole procedure in Figure 1.
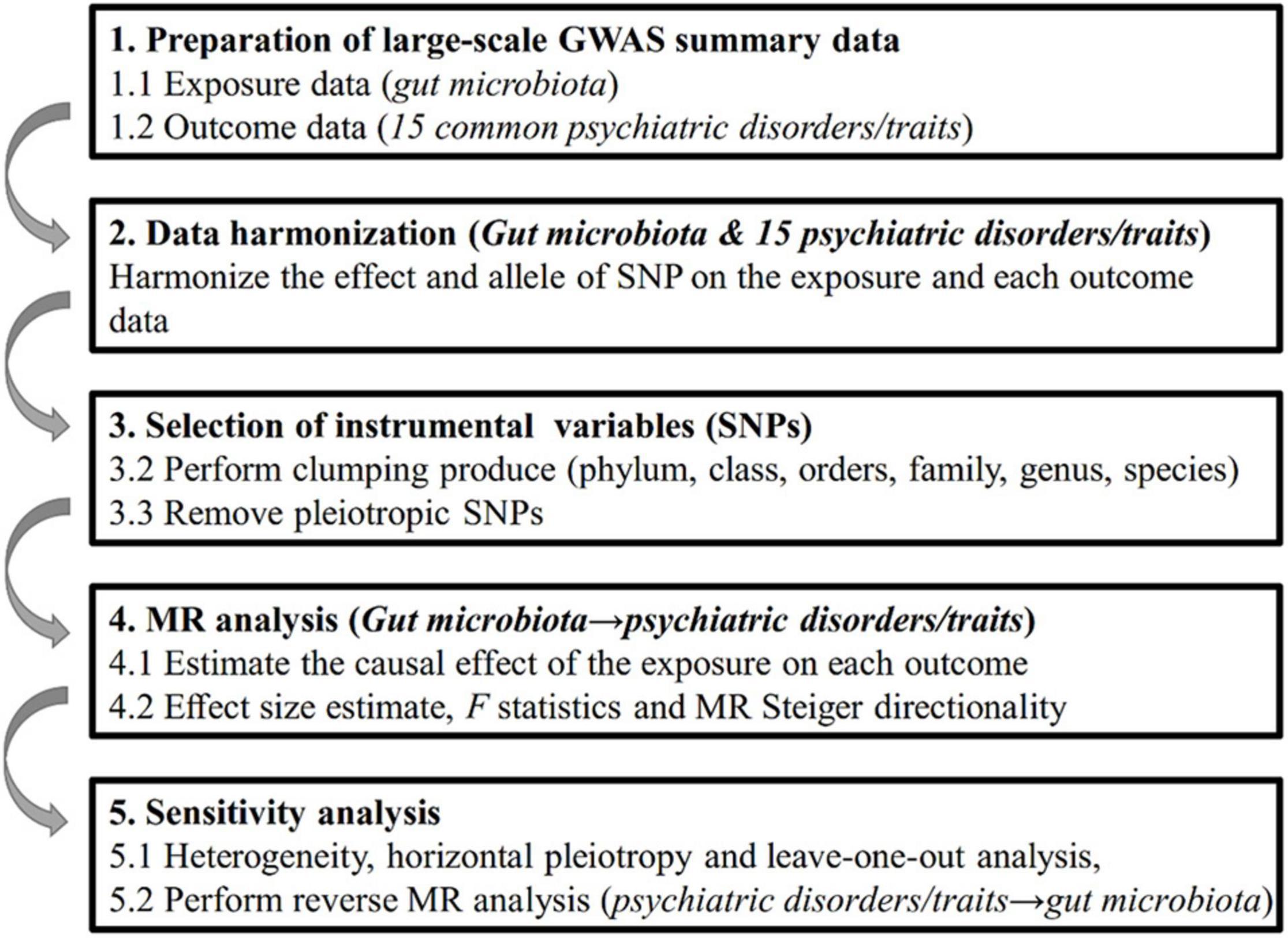
Figure 1. Diagrammatic description of the whole workflow in MR analysis. A flowchart of the whole MR analysis was displayed in this figure.
Genome-wide association study summary statistics of gut microbiota was assessed from the MiBioGen study (Kurilshikov et al., 2021) (1, as of June 28, 2020), which is the largest, multi-ethnic, genome-wide meta-analysis of the gut microbiome to date. Briefly, the MiBioGen study coordinated 16S rRNA gene sequencing profiles and whole-genome genotyping data from 18,473 individuals (25 cohorts) as described elsewhere (Kurilshikov et al., 2021). The microbial composition of distinct cohorts was profiled by targeting three different variable regions of the 16S rRNA gene: V4, V3–V4, and V1–V2, and all microbiome datasets were rarefied to 10,000 reads per cohort. The majority of cohorts used similar imputation procedures using the Michigan Imputation Server or IMPUTE2 software and the Haplotype Reference Consortium 1.0 or 1.1 reference panel. Then, microbiome trait loci mapping was performed to identify genetic loci that affect the relative abundance of microbial taxa. The cutoffs mapping included at least 3,000 effective samples in the presence of at least three cohorts. In total, all available GWAS summary statistics of 190 bacterial taxa were eventually included in the MR analysis.
Genome-wide association study summary statistics for psychiatric disorders were generated from large-scale GWAS or their meta-analysis. We collected as many psychiatric disorders as possible, resulting in 15 disorders (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014; van den Berg et al., 2016; International Obsessive Compulsive Disorder Foundation Genetics Collaborative (Iocdf-Gc) and Ocd Collaborative Genetics Association Studies (OCGAS), 2018; Nagel et al., 2018; Pasman et al., 2018; Demontis et al., 2019; Grove et al., 2019; Howard et al., 2019; Meier et al., 2019; Nievergelt et al., 2019; Sanchez-Roige et al., 2019; Stahl et al., 2019; Watson et al., 2019; Yu et al., 2019; Erlangsen et al., 2020). Criteria to define these disorders are listed in Supplementary Table 1. For each disorder, summary statistics from the largest GWAS were assessed. Detailed descriptions of GWAS for 15 common psychiatric disorders/traits, including the ethnicity, genotyping platform, imputation reference panel, and consortium, are presented in Table 1.
Bacterial taxa were analyzed at six levels (phylum, class, order, family, genus, and species). A distinct taxon was defined as a feature. Candidate IVs for each feature were selected at the P < 1.0 × 10–5 significance in accordance with the study of Sanna et al. (2019). Then, SNPs associated with each feature were clumped with PLINK (v1.9) to retain only independent SNPs. The linkage disequilibrium (LD) threshold was set to be r2< 0.1, with a clumping window of 500 kb. The 1,000 Genomes Project sequencing data (phase 3) was used to estimate LD.
The horizontal pleiotropy effect, that is, the confounding effect caused by other diseases, is a severe problem and may violate the second assumption in MR analysis. We applied the MR-PRESSO test and the MR-Egger regression test to monitor potential horizontal pleiotropy effect. The MR-PRESSO Outlier test calculates for each SNP a P-value for its pleiotropy significance, whereas the MR-PRESSO Global test calculates a P-value for overall horizontal pleiotropy. SNPs were sorted in an ascending order in terms of their MR-PRESSO Outlier test P-values and were then removed one by one. Each time a SNP was removed from the list, the MR-PRESSO Global test was performed on the remaining SNPs. The recursion was repeated until P-value for the Global test was unsignificant (P > 0.05). The list of the remaining SNPs after removing pleiotropic ones was used for subsequent MR analysis. The significant intercept item of MR-Egger implies the existence of pleiotropy.
To avoid distortion of strand orientation or allele coding, we deleted palindromic SNPs (e.g., with A/T or G/C alleles). In the harmonization process, we aligned alleles to the human genome reference sequence (build 37) and removed ambiguous and duplicated SNPs.
The GWAS summary statistics of gut microbiota and psychiatric disorders/traits were derived from a standardized phenotype (i.e., mean 0 and variance 1). Therefore, we could estimate the proportion of phenotypic variance explained by SNP from summary statistics with the formula 2f(1−f)β2, where f is the effect allele frequency and β is the regression coefficient for gut microbiota and psychiatric disorders/traits.
We performed MR analysis to investigate the causal relationship between microbiome features and the 15 common psychiatric disorders/traits. For features containing only one IV, the Wald ratio test was used to estimate the association between the identified IV and each psychiatric disorder/trait (Burgess et al., 2017). For features containing multiple IVs, five popular MR methods were used: the inverse-variance weighted (IVW) test (Burgess et al., 2013), the maximum likelihood estimator (MLE) (Pierce and Burgess, 2013), the MR-Egger regression (Bowden et al., 2015), the weighted median estimator (WME) (Bowden et al., 2016), and the MR-PRESSO (Verbanck et al., 2018). Each statistical method has its own model assumption, and any violation of the assumption may make the method inferior or even completely invalid. Specific to the five methods investigated: 1) The IVW method assumes no horizontal pleiotropy (Burgess et al., 2013); 2) the MLE (Pierce and Burgess, 2013) assumes the linear correlation of outcome and exposure with jointly normal distribution and allows for uncertainty in both gene–exposure and gene–outcome associations (Burgess et al., 2015); 3) the MR-Egger assumes the presence of pleiotropy in > 50% SNPs (Bowden et al., 2015); 4) the WME assumes the presence of pleiotropy in < 50% SNPs (Bowden et al., 2016); and 5) the MR-PRESSO assumes the presence of pleiotropy but will remove pleiotropic SNPs intrinsically (Verbanck et al., 2018). The IVW method is reported to be slightly more powerful than the others under certain conditions (Bowden et al., 2016). Therefore, the results were mainly based on the IVW method, with the other four methods serving as its complements. Additionally, we established a multiple-testing significance threshold at each feature level (phylum, class, order, family, genus, and species) defined as P < 0.05/n (where n is the effective number of independent bacterial taxa on the corresponding taxonomic level).
To assess robustness of significant results, we performed several sensitivity analyses. The potential heterogeneity was examined by the Q test in the IVW test and the MR-Egger regression. Meanwhile, the leave-one-out analysis was performed to determine whether the causal signal was driven by one SNP. To infer causal direction, we used the MR Steiger directionality (Hemani et al., 2017) test to examine whether the exposure was directionally causal for the outcome. This approach compares the variance explained by IVs for both exposure and outcome. If the IVs explain a greater variance in the exposure than the outcome, then the identified causal association could be considered directionally credible. Furthermore, we calculated F statistics (Burgess and Thompson, 2011) to evaluate the weak instrument bias using the following formula:
where n, k, and R2 are sample size, number of IVs, and the variance explained by IVs, respectively. An F-value less than 10 indicates weak instrument.
We performed an additional reverse MR analysis to explore reverse causality. Significant reverse MR analysis indicates reverse causality from psychiatric disorders/traits (as exposure) to microbiota features (as outcome). The reverse MR analysis procedure was the same as the above MR analysis.
All of the analyses, including MR analyses and sensitivity analyses, were performed with the R packages TwoSampleMR2 (Hemani et al., 2018) and MRPRESSO3 (Verbanck et al., 2018).
After removing palindromic SNPs, we identified 937, 1,576, 1,583, 2,390, 6,525, and 739 SNPs associated with gut microbiota in the phylum, class, order, family, genus, and species levels at the suggestive significance level P < 1.0 × 10–5, respectively (Supplementary Table 2). After clumping and harmonization, the number of IVs associated with each psychiatric disorder varies from 3 to 28. For instance, a total of 2,411 IVs are associated with SCZ, and these IVs are categorized into nine bacteria phyla (123 SNPs), 15 classes (208 SNPs), 19 orders (251 SNPs), 30 families (397 SNPs), 102 genera (1,285 SNPs), and 15 species (177 SNPs), respectively. For SCZ, the genus with the largest number of SNPs is Bifidobacterium (26 SNPs), followed by Roseburia (24 SNPs) and genus with the least number is Senegalimassilia (four SNPs). There is no feature containing only one SNP at any level.
The horizontal pleiotropy effect was evaluated at each taxonomic level. For SCZ, only one of 16 IVs for the order Coriobacteriales was detected as outlier using the MR-PRESSO outlier test. Similarly, at the family level, two out of 15 IVs in family Desulfovibrionaceae and one out of 16 IVs in family Streptococcaceae were identified as outliers. After removing pleiotropic SNPs identified by the MR-PRESSO outlier test and the MR-Egger regression, there is no evidence of horizontal pleiotropy of the remaining IVs (both MR-PRESSO Global test P > 0.05 and MR-Egger regression P > 0.05) (Supplementary Table 3).
Causal association between each pair of bacterial taxon and psychiatric disorder is tested by five MR methods. To take into account multiple-testing correction, the significance threshold for various taxa levels was set to the following: phylum P = 5.56 × 10–3 (0.05/9), class P = 3.33 × 10–3 (0.05/15), order P = 2.63 × 10–3 (0.05/19), family P = 1.67 × 10–3 (0.05/30), genus P = 4.90 × 10–4 (0.05/102), and species P = 3.33 × 10–3 (0.05/15).
A total of eight causal associations from seven bacterial features to six psychiatric disorders/traits were identified by the IVW method (Table 2), including family Prevotellaceae with ASD (PIVW = 5.31 × 10–4), class Betaproteobacteria with bipolar disorder (BD) (PIVW = 1.53 × 10–3), class Actinobacteria with SCZ (PIVW = 1.33 × 10–3), class Bacteroidia (PIVW = 2.51 × 10–3), and order Bacteroidales (PIVW = 2.51 × 10–3) with Tourette syndrome (TS), phylum Actinobacteria (PIVW = 8.22 × 10–4) and class Actinobacteria (PIVW = 1.09 × 10–3) with extroversion, and species Clostridium innocuum with neuroticism (NEU) (PIVW = 8.92 × 10–4). Scatter plots across various tests are displayed in Figure 2 and Supplementary Figure 1. In total, 234 SNPs are included as IVs of gut microbiota to calculate the causal relationship with psychiatric disorders/traits (Supplementary Table 4). Seven of the eight causal associations are cross-validated by more than two MR tests, demonstrating the robustness of our results. All MR methods produced consistent direction of effect estimates, which strengthens the confidence toward true association (Table 2). Specifically, four bacterial features showed positive causal direction with ASD, BD, SCZ, and NEU, with regression coefficients ranging from 0.03 to 0.24. A total of four bacterial features showed a negative causal direction with TS and extroversion, whose regression coefficients are between −0.46 and −0.07. Of note, genus is a sub-category of family; therefore, the sets of SNPs contained in genus and its relevant family may heavily overlap. For instance, in SCZ, the SNPs of genus Bacteroides are within the family Bacteroidaceae. Besides, 10 more bacterial features were identified by only one of the five MR tests, as listed in Supplementary Table 5.
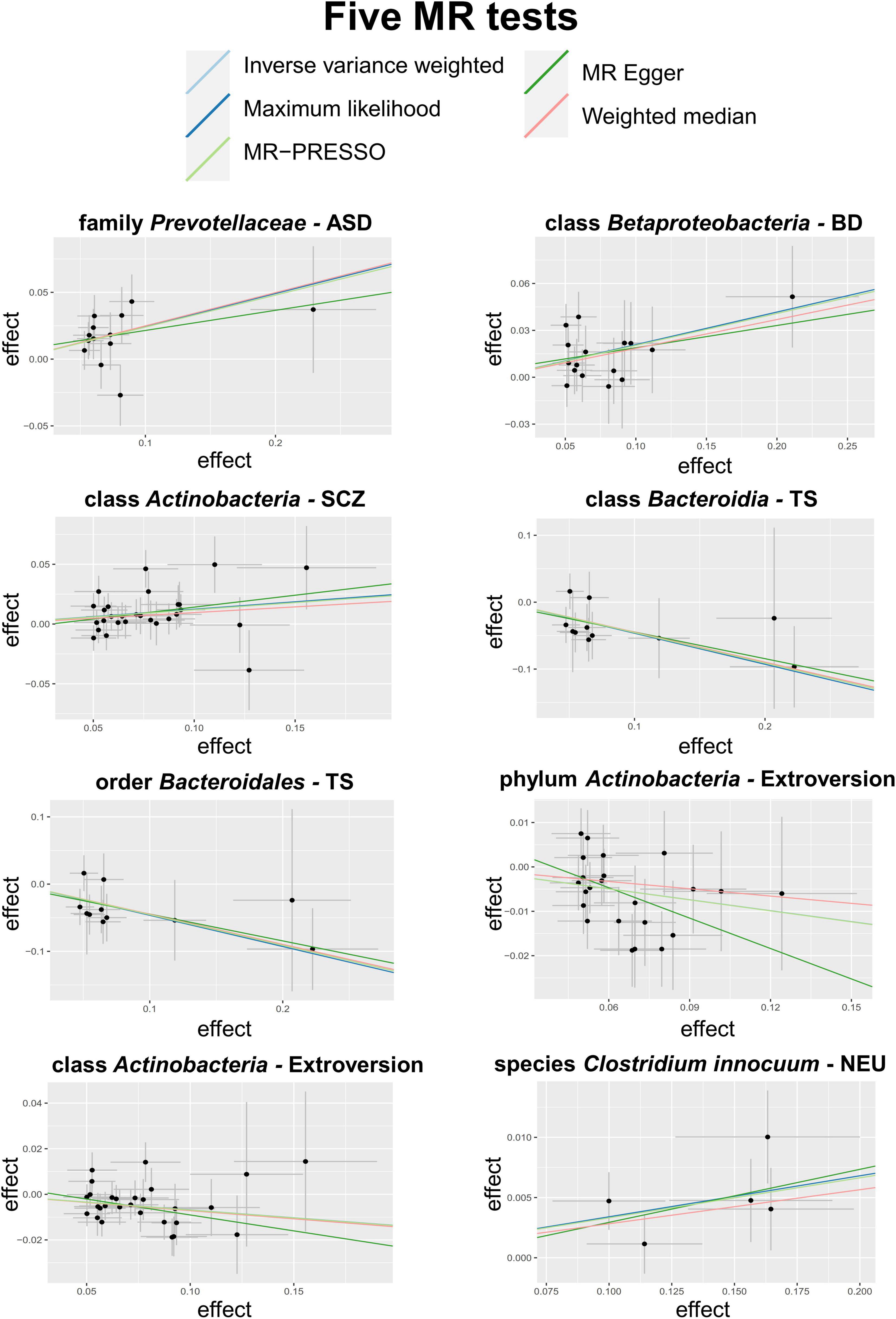
Figure 2. Scatter plots of the 5 MR tests in 8 causal associations from 7 bacterial features to 6 psychiatric disorders/traits. SNP effects were plotted into lines for the inverse-variance weighted test (light blue line), MR-Egger regression (green line), weighted median estimator (red line), MR-PRESSO (light green line) and maximum likelihood estimator (blue line). The slope of the line corresponded to the causal estimation.
Instrumental variables for each identified features can explain 1.95–5.54% of the variance in each feature and 0.003–0.53% of the variance in the corresponding psychiatric disorders/traits, respectively. The F statistics for all IVs are larger than 10, indicating no evidence of weak instrument bias. Furthermore, the MR Steiger directionality test revealed that the variances explained by included SNPs of bacterial exposure are larger than psychiatric outcome, implying the true causal associations directionally (Supplementary Table 3). Q statistics of the IVW test and the MR-Egger regression showed no evidence of heterogeneity at the identified results (Supplementary Table 3). Forest plots of causal effects using single SNP showed that none of them is extremely significant for association with psychiatric disorders/traits (Supplementary Figures 2A, 3A), and the leave-one-out sensitivity analysis demonstrated no single SNP driving the causal association signal (Supplementary Figures 2B, 3B).
The results of the reverse MR analysis, as listed in the Supplementary Table 6, showed no evidence of causal effect from psychiatric disorders/traits to identified bacterial features after multiple-testing correction (P < 0.05/18 = 2.78 × 10–3).
Some factors, such as chronic bowel diseases, may affect the association between gut microbiota and psychiatric disorders. To check the potential influence of confounding factors, we identified several sub-types of chronic bowel disease, including irritable bowel syndrome, inflammatory bowel disease, ulcerative colitis, Crohn’s disease, bowel problem, other non-infective gastroenteritis and colitis, and other functional intestinal disorders categories. We retrieved the associations of the identified IVs with each type in the United Kingdom Biobank summary statistics through the GeneATLAS website4. After multiple-testing correction, the results showed that none of the associations is significant, indicating limited confounding effect of chronic bowel diseases, as listed in the Supplementary Table 7. We further excluded IVs associated with any one of the chronic bowel diseases at a nominal level (P < 0.05) and re-perform the MR analysis using the remaining IVs. The results remain significant at the eight identified causal associations. Meanwhile, neither the MR-PRESSO test nor the MR-Egger regression test showed evidence of horizontal pleiotropy (both P > 0.05). Together, these results implied that the identified causal associations were unlikely to be mediated by chronic bowel disease.
In the current study, we conducted MR analyses to evaluate the potential causality between the gut microbiota and 15 psychiatric disorders/traits. Using large-scale summary statistics from microbiome GWAS and 15 psychiatric disorders/traits GWAS, we identified seven bacterial features that were causally associated with six psychiatric disorders/traits.
The positive association between family Prevotellaceae and ASD is in line with previous findings (Qiao et al., 2018; Dan et al., 2020). Prevotellaceae is characterized as a propionate-producing bacterium. Previous studies have found that propionate, as an enteric metabolite produced by gut microbiota, induced social abnormalities, cognitive impairments, sensorimotor dysfunction, and exacerbated ASD symptoms after intracerebroventricular injection (Shultz et al., 2015). Other studies have also manifested the consistent inference that changes in brain tissue after propionate administration result in conditions similar to ASD patients, such as reactive astrogliosis and oxidative stress (Thomas et al., 2012).
In accordance with the previous studies, class Actinobacteria, as a gram-positive bacterium, showed a positive causal association with SCZ, and class Betaproteobacteria has a positive direction on BD. For instance, patients with SCZ and other psychotic disorders have a higher abundance of Actinobacteria (Zheng et al., 2016; Li et al., 2020; Vindegaard et al., 2020), which is also supported by animal models (Dunphy-Doherty et al., 2018). Members of Betaproteobacteria were found to be more abundant in a mouse model of psychiatric diseases, whereas they were strongly correlated with increased gut permeability and intestinal chronic inflammation in humans, which may affect mental health or brain development via the MGB axis (Bauerl et al., 2018; Chen et al., 2018).
Consistent with previous literature, our result showed that class Bacteroidia and its child taxon, order Bacteroidales, have a negative effect on TS. Both Bacteroidia and Bacteroidales are correlated with the tryptophan hydroxylase-II (TPH2) serotonin pathway (Liu et al., 2020). In animal experiments, deficits and excess of TPH2 activity may induce significant behavioral disturbances and catalepsy, whereas the human TPH2 gene is related to psychiatric disorders (Kulikova and Kulikov, 2019). Moreover, serotonin, one of the main brain neurotransmitters, plays an important role in promoting immunity and reducing inflammation in mucosal infections (Gao J. et al., 2018). Thus, decreased serotonin concentrations in the brain and cerebrospinal fluid in Tourette patients might be implicated in the pathogenesis of TS (Mossner et al., 2007). In a FMT study, the abundance of Bacteroides coprocola was reduced in TS patients, but its restoration could improve tic symptoms (Zhao et al., 2020). Personality traits, such as extroversion and NEU, affect fundamental behavior patterns and have been related to mental disorders. In the present study, phylum Actinobacteria and its child taxon, order Actinobacteria, both have a negative effect direction on extroversion, whereas species Clostridium innocuum has a positive effect on NEU.
In addition to the above causal associations identified by IVW test, several intriguing results were identified by other MR tests, including families Christensenellaceae and Methanobacteriaceae, both of which have negative effects on OCD and SA. These associations are broadly supported by previous studies. For instance, it is accepted that SCFA butyrate might suppress the inflammation and oxidative damages in colon and brain, alleviating cognitive impairments, behavioral disorders, and gastrointestinal disorders (Peruzzotti-Jametti and Pluchino, 2018). Family Christensenellaceae, as a gram-negative, strictly anaerobic, and SCFA-producing taxon (Waters and Ley, 2019), could increase the concentration of butyrate in colon, potentially alleviating colitis-related OCD behaviors by diet in humans (Nagpal et al., 2019). It was also found to be positively correlated with cognitive ability in mice (Gao L. et al., 2018). Whereas Methanobacteriaceae, a dominant methanogenic archaeon, can increase levels of SCFAs in the colon (Samuel and Gordon, 2006) and likely has beneficial psychological effects via SCFAs such as migraine reduction in elderly women, which could partly explain the protective effect of this taxon against suicide (Chen et al., 2019).
There are many population-based observational studies and their meta-analyses for the association of gut microbiota with psychiatric disorders (Strati et al., 2017; Ma et al., 2019; Valles-Colomer et al., 2019; Hua et al., 2020; Iglesias-Vazquez et al., 2020; Nikolova et al., 2021). Among them, Nikolova et al. (2021) meta-analyzed 34 case-control studies in a total of 1,519 psychiatric patients versus 1,429 normal controls and found no difference in the diversity of gut microbiota. The meta-analysis attempts to resolve a controversial scientific question such as if an association between two conditions exists, whereas the causal nature of such association is unknown. Fundamentally different from meta-analysis, MR analysis, on the other hand, is an approach statistically inferring the causal nature of an association observed in a cross-sectional study.
This study has advantages in several aspects. First, the identified causal relationship may provide candidate bacteria for subsequent functional studies. Second, we comprehensively studied up to 15 common psychiatric disorders. In a previous study, Zhuang et al. (2020) studied three psychiatric disorders and revealed that order Enterobacteriales and family Enterobacteriaceae were causally associated with a higher risk of schizophrenia, and increased class Bacilli was causally associated with a higher risk of major depressive disorder.
There are also certain limitations in this study. First, gut microbiota GWAS is still in its infancy in terms of sample size; therefore, the number of associated loci is relatively small compared with that for psychiatric disorders. Second, because of the small sample size and insufficient power for microbiome GWAS, there may not be enough IVs for certain bacterial features at genus or species level. As a compromise, we analyzed features at a higher level (phylum, class, order, or family). When microbiome GWAS will eventually be equipped with sufficient sample size, these more specific features will hopefully be identified at a finer resolution (Thomas, 2019). Third, to maximize sample size and statistical power, GWAS of gut microbiota and psychiatric disorders/traits analyzed in this study might originate from multi-ancestry samples. Thus, the results should be interpreted with caution.
In conclusion, we comprehensively assessed the potential causal association between gut microbiota and a series of psychiatric disorders/traits. Four bacterial features showed positive causal direction with ASD, BD, SCZ, and NEU, whereas another four bacterial features showed a negative causal direction with TS and extroversion. This study may be useful in providing new insights into the development mechanism of microbiota-mediated psychiatric disorders.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by all studies were approved by respective institutional review boards (IRBs). No new IRB approval was required. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
LZ and Y-FP designed the study. LZ, QX, and J-JN collected the data. J-JN and QX analyzed the data. S-SY, B-XH, HZ, X-TW, G-JF, Y-FP, MZ, QX, and J-JN performed the literature search. J-JN drafted the early version of the manuscript. LZ and Y-FP jointly supervised the study. All authors were involved in writing the manuscript and had final approval of the submitted and published versions.
Y-FP and LZ were partially supported by the funding from the National Natural Science Foundation of China (31771417 and 31571291) and a project funded by the Priority Academic Program Development (PAPD) of Jiangsu higher education institutions. The numerical calculations in this manuscript have been done on the supercomputing system of the National Supercomputing Center in Changsha.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We appreciate all the volunteers who participated in this study. We would like to thank the MiBioGen study for releasing the gut microbiota GWAS summary statistics and large-scale consortia or studies including HRC, PGC, United Kingdom Biobank, iPSYCH, ICC, ANGI, GCAN/WTCCC3, IOCDF-GC, OCGAS, and GPC for releasing psychiatric disorders/traits GWAS summary statistics.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.737197/full#supplementary-material
ADHD, attention deficit/hyperactivity disorder; ASD, autism spectrum disorder; SCZ, schizophrenia; FMT, fecal microbiota transplantation; MGB, microbiota–gut–brain; RCT, randomized controlled trial; MR, Mendelian randomization; IV, instrumental variable; SNP, nucleotide polymorphism; SCFA, short-chain fatty acid; GWAS, genome-wide association study; IRB, institutional review board; LD, linkage disequilibrium; IVW, inverse-variance weighted; MLE, maximum likelihood estimator; WME, weighted median estimator; BD, bipolar disorder; TS, Tourette syndrome; NEU, neuroticism; OCD, obsessive-compulsive disorder; SA, suicide attempt; TPH2, tryptophan hydroxylase-II.
Bauerl, C., Collado, M. C., Diaz Cuevas, A., Vina, J., and Perez Martinez, G. (2018). Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett. Appl. Microbiol. 66, 464–471. doi: 10.1111/lam.12882
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int. J. Epidemiol. 44, 512–525. doi: 10.1093/ije/dyv080
Bowden, J., Davey Smith, G., Haycock, P. C., and Burgess, S. (2016). Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. doi: 10.1002/gepi.21965
Burgess, S., and Thompson, S. G. (2011). Bias in causal estimates from mendelian randomization studies with weak instruments. Stat. Med. 30, 1312–1323. doi: 10.1002/sim.4197
Burgess, S., Butterworth, A., and Thompson, S. G. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665. doi: 10.1002/gepi.21758
Burgess, S., Scott, R. A., Timpson, N. J., Davey Smith, G., Thompson, S. G., and Consortium, E.-I. (2015). Using published data in mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 30, 543–552. doi: 10.1007/s10654-015-0011-z
Burgess, S., Small, D. S., and Thompson, S. G. (2017). A review of instrumental variable estimators for mendelian randomization. Stat. Methods Med. Res. 26, 2333–2355. doi: 10.1177/0962280215597579
Chen, J., Wang, Q., Wang, A., and Lin, Z. (2019). Structural and functional characterization of the gut microbiota in elderly women with migraine. Front. Cell Infect. Microbiol. 9:470. doi: 10.3389/fcimb.2019.00470
Chen, Y. J., Wu, H., Wu, S. D., Lu, N., Wang, Y. T., Liu, H. N., et al. (2018). Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J. Gastroenterol. Hepatol. 33, 1844–1852. doi: 10.1111/jgh.14281
Chow, J., Lee, S. M., Shen, Y., Khosravi, A., and Mazmanian, S. K. (2010). Host-bacterial symbiosis in health and disease. Adv. Immunol. 107, 243–274. doi: 10.1016/B978-0-12-381300-8.00008-3
Clemente, J. C., Ursell, L. K., Parfrey, L. W., and Knight, R. (2012). The impact of the gut microbiota on human health: an integrative view. Cell 148, 1258–1270. doi: 10.1016/j.cell.2012.01.035
Cryan, J. F., O’Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013.
Dan, Z., Mao, X., Liu, Q., Guo, M., Zhuang, Y., Liu, Z., et al. (2020). Altered gut microbial profile is associated with abnormal metabolism activity of autism spectrum disorder. Gut. Microbes. 11, 1246–1267. doi: 10.1080/19490976.2020.1747329
Demontis, D., Walters, R. K., Martin, J., Mattheisen, M., Als, T. D., Agerbo, E., et al. (2019). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 51, 63–75. doi: 10.1038/s41588-018-0269-7
Demyttenaere, K., Bruffaerts, R., Posada-Villa, J., Gasquet, I., Kovess, V., Lepine, J. P., et al. (2004). Prevalence, severity, and unmet need for treatment of mental disorders in the world health organization world mental health surveys. JAMA. 291, 2581–2590. doi: 10.1001/jama.291.21.2581
Dunphy-Doherty, F., O’Mahony, S. M., Peterson, V. L., O’Sullivan, O., Crispie, F., Cotter, P. D., et al. (2018). Post-weaning social isolation of rats leads to long-term disruption of the gut microbiota-immune-brain axis. Brain Behav. Immun. 68, 261–273. doi: 10.1016/j.bbi.2017.10.024
Emdin, C. A., Khera, A. V., and Kathiresan, S. (2017). Mendelian randomization. JAMA. 318, 1925–1926.
Erlangsen, A., Appadurai, V., Wang, Y., Turecki, G., Mors, O., Werge, T., et al. (2020). Genetics of suicide attempts in individuals with and without mental disorders: a population-based genome-wide association study. Mol. Psychiatry 25, 2410–2421. doi: 10.1038/s41380-018-0218-y
Gao, J., Xu, K., Liu, H., Liu, G., Bai, M., Peng, C., et al. (2018). Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell Infect. Microbiol. 8:13. doi: 10.3389/fcimb.2018.00013
Gao, L., Li, J., Zhou, Y., Huang, X., Qin, X., and Du, G. (2018). Effects of baicalein on cortical proinflammatory cytokines and the intestinal microbiome in senescence accelerated mouse prone 8. ACS Chem. Neurosci. 9, 1714–1724. doi: 10.1021/acschemneuro.8b00074
Grove, J., Ripke, S., Als, T. D., Mattheisen, M., Walters, R. K., Won, H., et al. (2019). Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51, 431–444. doi: 10.1038/s41588-019-0344-8
Hemani, G., Tilling, K., and Davey Smith, G. (2017). Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 13:e1007081. doi: 10.1371/journal.pgen.1007081
Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., et al. (2018). The MR-base platform supports systematic causal inference across the human phenome. Elife 7:e34408. doi: 10.7554/eLife.34408
Howard, D. M., Adams, M. J., Clarke, T. K., Hafferty, J. D., Gibson, J., Shirali, M., et al. (2019). Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352. doi: 10.1038/s41593-018-0326-7
Hua, X., Zhu, J., Yang, T., Guo, M., Li, Q., Chen, J., et al. (2020). The gut microbiota and associated metabolites are altered in sleep disorder of children with autism spectrum disorders. Front. Psychiatry 11:855. doi: 10.3389/fpsyt.2020.00855
Iglesias-Vazquez, L., Van Ginkel Riba, G., Arija, V., and Canals, J. (2020). Composition of gut microbiota in children with autism spectrum disorder: a systematic review and meta-analysis. Nutrients 12:792. doi: 10.3390/nu12030792
International Obsessive Compulsive Disorder Foundation Genetics Collaborative (Iocdf-Gc) and Ocd Collaborative Genetics Association Studies (OCGAS) (2018). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol. Psychiatry 23, 1181–1188. doi: 10.1038/mp.2017.154
Katan, M. B. (1986). Apolipoprotein E isoforms, serum cholesterol, and cancer. Lancet 1, 507–508. doi: 10.1016/s0140-6736(86)92972-7
Kulikova, E. A., and Kulikov, A. V. (2019). Tryptophan hydroxylase 2 as a therapeutic target for psychiatric disorders: focus on animal models. Exp. Opin. Ther. Targets 23, 655–667. doi: 10.1080/14728222.2019.1634691
Kurilshikov, A., Medina-Gomez, C., Bacigalupe, R., Radjabzadeh, D., Wang, J., Demirkan, A., et al. (2021). Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 53, 156–165. doi: 10.1038/s41588-020-00763-1
Li, S., Zhuo, M., Huang, X., Huang, Y., Zhou, J., Xiong, D., et al. (2020). Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ 8:e9574. doi: 10.7717/peerj.9574
Liu, G., Chong, H. X., Chung, F. Y., Li, Y., and Liong, M. T. (2020). Lactobacillus plantarum DR7 modulated bowel movement and gut microbiota associated with dopamine and serotonin pathways in stressed adults. Int. J. Mol. Sci. 21:4608. doi: 10.3390/ijms21134608
Ma, B., Liang, J., Dai, M., Wang, J., Luo, J., Zhang, Z., et al. (2019). Altered gut microbiota in chinese children with autism spectrum disorders. Front. Cell Infect. Microbiol. 9:40. doi: 10.3389/fcimb.2019.00040
Meier, S. M., Trontti, K., Purves, K. L., Als, T. D., Grove, J., Laine, M., et al. (2019). Genetic variants associated with anxiety and stress-related disorders: a genome-wide association study and mouse-model study. JAMA Psychiatry 76, 924–932. doi: 10.1001/jamapsychiatry.2019.1119
Mossner, R., Muller-Vahl, K. R., Doring, N., and Stuhrmann, M. (2007). Role of the novel tryptophan hydroxylase-2 gene in tourette syndrome. Mol. Psychiatry 12, 617–619. doi: 10.1038/sj.mp.4002004
Nagel, M., Jansen, P. R., Stringer, S., Watanabe, K., de Leeuw, C. A., Bryois, J., et al. (2018). Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat. Genet. 50, 920–927. doi: 10.1038/s41588-018-0151-7
Nagpal, R., Neth, B. J., Wang, S., Craft, S., and Yadav, H. (2019). Modified mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 47, 529–542. doi: 10.1016/j.ebiom.2019.08.032
Nievergelt, C. M., Maihofer, A. X., Klengel, T., Atkinson, E. G., Chen, C. Y., Choi, K. W., et al. (2019). International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat. Commun. 10:4558. doi: 10.1038/s41467-019-12576-w
Nikolova, V. L., Smith, M. R. B., Hall, L. J., Cleare, A. J., Stone, J. M., and Young, A. H. (2021). Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry 78, 1343–1354. doi: 10.1001/jamapsychiatry.2021.2573
Ochoa-Reparaz, J., Ramelow, C. C., and Kasper, L. H. A. (2020). gut feeling: the importance of the intestinal microbiota in psychiatric disorders. Front. Immunol. 11:510113. doi: 10.3389/fimmu.2020.510113
O’Hara, A. M., and Shanahan, F. (2006). The gut flora as a forgotten organ. EMBO Rep. 7, 688–693. doi: 10.1038/sj.embor.7400731
Pasman, J. A., Verweij, K. J. H., Gerring, Z., Stringer, S., Sanchez-Roige, S., Treur, J. L., et al. (2018). GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat. Neurosci. 21, 1161–1170. doi: 10.1038/s41593-018-0206-1
Patel, V., Chisholm, D., Parikh, R., Charlson, F. J., Degenhardt, L., Dua, T., et al. (2016). Addressing the burden of mental, neurological, and substance use disorders: key messages from disease control priorities, 3rd edition. Lancet 387, 1672–1685. doi: 10.1016/S0140-6736(15)00390-6
Peruzzotti-Jametti, L., and Pluchino, S. (2018). Targeting mitochondrial metabolism in neuroinflammation: towards a therapy for progressive multiple sclerosis. Trends Mol. Med. 24, 838–855. doi: 10.1016/j.molmed.2018.07.007
Pierce, B. L., and Burgess, S. (2013). Efficient design for mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am. J. Epidemiol. 178, 1177–1184. doi: 10.1093/aje/kwt084
Qiao, Y., Wu, M., Feng, Y., Zhou, Z., Chen, L., and Chen, F. (2018). Alterations of oral microbiota distinguish children with autism spectrum disorders from healthy controls. Sci. Rep. 8:1597. doi: 10.1038/s41598-018-19982-y
Samuel, B. S., and Gordon, J. I. (2006). A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc. Natl. Acad. Sci. U.S.A. 103, 10011–10016. doi: 10.1073/pnas.0602187103
Sanchez-Roige, S., Palmer, A. A., Fontanillas, P., Elson, S. L., The 23andMe Research Team, the Substance Use Disorder Working Group of the Psychiatric Genomics Consortium Adams, M. J., et al. (2019). Genome-wide association study meta-analysis of the alcohol use disorders identification test (AUDIT) in two population-based cohorts. Am. J. Psychiatry 176, 107–118. doi: 10.1176/appi.ajp.2018.18040369
Sanna, S., van Zuydam, N. R., Mahajan, A., Kurilshikov, A., Vich Vila, A., Vosa, U., et al. (2019). Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51, 600–605. doi: 10.1038/s41588-019-0350-x
Schizophrenia Working Group of the Psychiatric Genomics Consortium. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. doi: 10.1038/nature13595
Sharon, G., Cruz, N. J., Kang, D. W., Gandal, M. J., Wang, B., Kim, Y. M., et al. (2019). Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177, 1600–1618.e17. doi: 10.1016/j.cell.2019.05.004
Shultz, S. R., Aziz, N. A., Yang, L., Sun, M., MacFabe, D. F., and O’Brien, T. J. (2015). Intracerebroventricular injection of propionic acid, an enteric metabolite implicated in autism, induces social abnormalities that do not differ between seizure-prone (FAST) and seizure-resistant (SLOW) rats. Behav. Brain Res. 278, 542–548. doi: 10.1016/j.bbr.2014.10.050
Smith, G. D., and Ebrahim, S. (2003). ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 32, 1–22. doi: 10.1093/ije/dyg070
Stahl, E. A., Breen, G., Forstner, A. J., McQuillin, A., Ripke, S., Trubetskoy, V., et al. (2019). Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 51, 793–803. doi: 10.1038/s41588-019-0397-8
Strati, F., Cavalieri, D., Albanese, D., De Felice, C., Donati, C., Hayek, J., et al. (2017). New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5:24.
Thomas, H. (2019). Mendelian randomization reveals causal effects of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 198–199. doi: 10.1038/s41575-019-0133-y
Thomas, R. H., Meeking, M. M., Mepham, J. R., Tichenoff, L., Possmayer, F., Liu, S., et al. (2012). The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J. Neuroinflam. 9:153.
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632. doi: 10.1038/s41564-018-0337-x
van den Berg, S. M., de Moor, M. H., Verweij, K. J., Krueger, R. F., Luciano, M., Arias Vasquez, A., et al. (2016). Meta-analysis of genome-wide association studies for extraversion: findings from the genetics of personality consortium. Behav. Genet. 46, 170–182. doi: 10.1007/s10519-015-9735-5
Verbanck, M., Chen, C. Y., Neale, B., and Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698. doi: 10.1038/s41588-018-0099-7
Vindegaard, N., Speyer, H., Nordentoft, M., Rasmussen, S., and Benros, M. E. (2020). Gut microbial changes of patients with psychotic and affective disorders: a systematic review. Schizophr Res. 234, 1–10.
Wang, L., Christophersen, C. T., Sorich, M. J., Gerber, J. P., Angley, M. T., and Conlon, M. A. (2011). Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl. Environ. Microbiol. 77, 6718–6721. doi: 10.1128/AEM.05212-11
Waters, J. L., and Ley, R. E. (2019). The human gut bacteria christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 17:83. doi: 10.1186/s12915-019-0699-4
Watson, H. J., Yilmaz, Z., Thornton, L. M., Hubel, C., Coleman, J. R. I., Gaspar, H. A., et al. (2019). Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 51, 1207–1214. doi: 10.1038/s41588-019-0439-2
Whiteford, H. A., Degenhardt, L., Rehm, J., Baxter, A. J., Ferrari, A. J., Erskine, H. E., et al. (2013). Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet 382, 1575–1586. doi: 10.1016/s0140-6736(13)61611-6
Yu, D., Sul, J. H., Tsetsos, F., Nawaz, M. S., Huang, A. Y., Zelaya, I., et al. (2019). Interrogating the genetic determinants of tourette’s syndrome and other tic disorders through genome-wide association studies. Am. J. Psychiatry 176, 217–227. doi: 10.1176/appi.ajp.2018.18070857
Zhao, H. J., Luo, X., Shi, Y. C., Li, J. F., Pan, F., Ren, R. R., et al. (2020). The efficacy of fecal microbiota transplantation for children with tourette syndrome: a preliminary study. Front. Psychiatry 11:554441. doi: 10.3389/fpsyt.2020.554441
Zheng, P., Zeng, B., Zhou, C., Liu, M., Fang, Z., Xu, X., et al. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796. doi: 10.1038/mp.2016.44
Keywords: Mendelian randomization (MR), gut microbiota (GM), psychiatric disorders, causal relationship, species Clostridium innocuum
Citation: Ni J-J, Xu Q, Yan S-S, Han B-X, Zhang H, Wei X-T, Feng G-J, Zhao M, Pei Y-F and Zhang L (2022) Gut Microbiota and Psychiatric Disorders: A Two-Sample Mendelian Randomization Study. Front. Microbiol. 12:737197. doi: 10.3389/fmicb.2021.737197
Received: 06 July 2021; Accepted: 14 December 2021;
Published: 04 February 2022.
Edited by:
Anil Kumar Puniya, National Dairy Research Institute (ICAR), IndiaReviewed by:
Amit K. Singh, Albany Medical College, United StatesCopyright © 2022 Ni, Xu, Yan, Han, Zhang, Wei, Feng, Zhao, Pei and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, bHpoYW5nNkBzdWRhLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.