
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 13 September 2021
Sec. Infectious Agents and Disease
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.727774
This article is part of the Research Topic Novel Approaches to Rapid Diagnosis and Treatment Monitoring of Active Tuberculosis, Vol II View all 15 articles
 Maria Antonello1†
Maria Antonello1† Rossana Scutari2†
Rossana Scutari2† Calogero Lauricella3
Calogero Lauricella3 Silvia Renica1
Silvia Renica1 Valentina Motta3
Valentina Motta3 Stefania Torri4
Stefania Torri4 Cristina Russo5
Cristina Russo5 Leonarda Gentile5
Leonarda Gentile5 Valeria Cento1
Valeria Cento1 Luna Colagrossi5
Luna Colagrossi5 Giordana Mattana5
Giordana Mattana5 Luigi Ruffo Codecasa6
Luigi Ruffo Codecasa6 Chiara Vismara4
Chiara Vismara4 Francesco Scaglione1,4
Francesco Scaglione1,4 Silvio Marco Veronese3
Silvio Marco Veronese3 Emanuela Bonoldi3
Emanuela Bonoldi3 Alessandra Bandera7,8
Alessandra Bandera7,8 Andrea Gori7,8
Andrea Gori7,8 Ester Mazzola4
Ester Mazzola4 Carlo Federico Perno5,9
Carlo Federico Perno5,9 Claudia Alteri1,9*
Claudia Alteri1,9*Background: Rapid and reliable diagnosis of tuberculosis (TB) represents a diagnostic challenge in compartmentalized extrapulmonary TB infection because of the small number of mycobacteria (MTB) and the frequent lack of fresh samples to perform culture. Here, we estimate the performances of homemade droplet digital PCR (ddPCR)-based assays against culture in 89 biopsies, for those fresh and formalin-fixed and paraffin-embedded (FFPE) subsamples were available.
Methods: MTB diagnosis in fresh subsamples was performed by culture. Fresh subsamples were also analyzed for acid-fast bacilli smear-microscopy (AFB) and Xpert® MTB/RIF (Xpert). MTB examination was repeated in blind in the 89 FFPE subsamples by in-house ddPCR assays targeting the IS6110 and rpoB. Analytical sensitivity of ddPCR assays was evaluated using serial dilution of H37Rv strain. Limit of detection (LOD) was calculated by probit analysis. Results were expressed in copies/106 cells.
Results: IS6110 and rpoB ddPCR assays showed a good linear correlation between expected and observed values (R2: 0.9907 and 0.9743, respectively). Probit analyses predicted a LOD of 17 and 40 copies/106 cells of MTB DNA for IS6110 and rpoB, respectively. Of the 89 biopsies, 68 were culture positive and 21 were culture negative. Considering mycobacterial culture as reference method, IS6110 assay yielded positive results in 67/68 culture-positive samples with a median interquartile range (IQR) of 1,680 (550–8,444) copies/106 cells (sensitivity: 98.5%; accuracy: 98.9). These performances were superior to those reported by the rpoB assay in FFPE subsamples (sensitivity: 66.20%; accuracy: 74.1) and even superior to those reported by Xpert and AFB in fresh subsamples (sensitivity: 79.4 and 33.8%, respectively; accuracy: 84.3 and 49.4, respectively). When Xpert and AFB results were stratified according to mycobacterial load detected by rpoB and IS6110 ddPCR, bacterial load was lower in Xpert and AFB negative with respect to Xpert and AFB-positive samples (p = 0.003 and 0.01 for rpoB and p = 0.01 and 0.11 for IS6110), confirming the poor sensitivity of these methods in paucibacillary disease.
Conclusion: ddPCR provides highly sensitive, accurate, and rapid MTB diagnosis in FFPE samples, as defined by the high concordance between IS6110 assay and culture results. This approach can be safely introduced in clinical routine to accelerate MTB diagnosis mainly when culture results remain unavailable.
Tuberculosis (TB) is a multisystemic disease caused by Mycobacterium tuberculosis complex (MTB). The pathogen primarily infects the lungs (pulmonary TB) but can also affect other structural parts of the human anatomy (extrapulmonary TB), such as lymph nodes, intestines, pleura, skin, and bones (Noussair et al., 2009).
It affects approximately 2 billion people worldwide, especially in developing countries, and stands as the leading cause of death from a single infectious agent [World Health Organization (WHO), 2012].
To reduce the mortality of MTB infection, to prevent its spread, and to start the correct treatment, an early, accurate, and rapid diagnosis is essential.
To date, the conventional approach for MTB diagnosis is mainly based on microscopic detection of acid-fast bacilli in smears (AFB), followed by MTB culture (Ryu, 2015; Caulfield and Wengenack, 2016). While AFB is a laborious method characterized by variable sensitivity (71.4% in lung samples and 24% in extrapulmonary samples) (Karadaǧ et al., 2013), mycobacterium culture is considered the gold standard method for MTB diagnosis as it has high specificity but requires a long time of incubation, up to 8 weeks for a certain negativity (Forbes et al., 2018).
For these reasons, in the case of extrapulmonary MTB infection, the diagnosis is challenging because of the small amount of MTB present at the sites of the disease and the difficulty of obtaining culture results (Golden, 2005; Noussair et al., 2009). In the case of surgically resected tissues fixed in formalin, mycobacterial culture is not feasible at all, and a correct diagnosis based on pathological features and AFB smear is difficult. Acid-fast staining for MTB on formalin-fixed and paraffin-embedded (FFPE) tissue has indeed a very low sensitivity, ranging between 3 and 60% (Fukunaga et al., 2002; Ahmed et al., 2011; Eshete et al., 2011), and some histological findings, like granuloma and necrosis, typically found in many other diseases including sarcoidosis and fungal infections (El-Zammar and Katzenstein, 2007), make MTB diagnosis particularly challenging.
In the last decade, real-time (RT) PCR assays have been introduced in laboratory routine, thanks to their sensitivity and their shortened turnaround time (Forbes and Hicks, 1993; Marchetti et al., 1998; Moure et al., 2019). Most of these methods are based on the detection of multi-copy insertion sequences (IS, such as IS986, IS987, IS1081, and IS6110), which should increase the sensitivity of the assays (Bisognin et al., 2018; Lin et al., 2021). Among these RT-PCR-based assays, Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, United States) (Xpert) is recommended by the World Health Organization (WHO) as rapid molecular diagnostic test for adults and children also in the case of extrapulmonary and FFPE specimens [Lee et al., 2011; Mazzola et al., 2016; Yang et al., 2017; Moure et al., 2019; Nyaruaba et al., 2019; World Health Organization (WHO), 2020], although it has a low diagnostic accuracy in paucibacillary disease (Allahyartorkaman et al., 2019).
Among other molecular platforms, droplet digital PCR (ddPCR) is a highly sensitive method widely used for the detection of a variety of pathogens, thanks to its ability to reliably detect down to a few copies of genomes (Caviglia et al., 2018; Alteri et al., 2020; Chen et al., 2021; Malin et al., 2021). Currently, two reports suggested that homemade ddPCR assays might provide a valid alternative for detecting MTB in extrapulmonary and/or FFPE samples (Yang et al., 2017; Cao et al., 2020), but as far as we know no study has defined the concordance between these molecular assays and the reference culture method.
Here, to evaluate the ddPCR-based method as suitable alternative for routine clinical diagnoses of MTB in FFPE samples, the performances of two MTB ddPCR-based assays were compared with the gold-standard culture methods and with the conventional AFB and Xpert in a set of tissues, for those fresh and FFPE subsamples were available.
A total of 89 consecutive biopsies from different anatomical districts of patients with a clinical suspect of TB were retrospectively collected at ASST Grande Ospedale Metropolitano Niguarda (Milan, Italy) between 2013 and 2019. Samples were selected according to clinical suspect of MTB based on radiology findings, cytology reports, and/or medical history of patients including previous MTB treatment [World Health Organization (WHO), 2013]. The distribution of samples against year of collection is reported in Supplementary Table 1. Biopsies were subdivided in two subsamples by trained medical personnel. One fresh subsample was immediately tested for MTB diagnosis by culture methods, using both solid (Lowenstein–Jensen) and liquid (MGIT 960; Becton Dickinson Biosciences, Sparks, MD, United States) media [European Centre for Disease Prevention and Control (ECDC), 2018; Riccardi et al., 2020]. Fresh subsamples were also analyzed for AFB microscopy and Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, United States) (Tortoli et al., 2012). As required by WHO and ECDC guidelines [World Health Organization (WHO), 2012; European Centre for Disease Prevention and Control (ECDC), 2018], all these procedures took place in a Biosafety Level 3 (BSL3) laboratory, with limited access, using required personal protective equipment and following control measures and all procedures to minimize aerosol and droplet formation. The residual sample was stored as formalin fixed and paraffin embedded (FFPE) (Slaoui and Fiette, 2011; Sadeghipour and Babaheidarian, 2019) for alternative diagnosis by histopathological examination and archived in a biobank for later use.
The study was conducted in accordance with the principles of the 1964 Declaration of Helsinki. The related information of the samples was processed by maintaining anonymization measures. Due to the non-interventional nature of the study and according to the applicable relevant national legislation and local rules, approval of the local Research Ethics Committee and informed consent were not mandatory.
MTB detection was repeated in blind in the stored FFPE samples by using ddPCR homemade assays. In brief, after sample deparaffinization (Fu et al., 2016), total DNA was extracted using Maxwell CSC DNA FFPE kit (Promega, Madison, WI, United States) following the instruction of the manufacturer (Sarnecka et al., 2019).
MTB DNA was quantified by means of the QX200TM Droplet Digital PCR System (Biorad) using homemade assays targeting the multicopy gene IS6110 (Forward: 5′-ATCTGGAC CCGCCAA-3′; Reverse: 5′-CCTATCCGTATGGTGGATAA-3′, and HEX Probe: 5′-AGGTCGAGTACGCCTT-3′) and the single-copy gene rpoB (Forward: 5′-GGAGCGCCAAACCG-3′; Reverse: 5′-AGTCCCGGAACCTCAA-3′, and FAM Probe: 5′-TTCGCTAAGCTGCGC-3′). The human albumin was used as housekeeping gene (ddPCRTM Copy Number Assay: ALB, Human, dHsaCNS864404398).
The cycling condition was the following: 95°C (10 min), 39 cycles of 94°C (30 s), and 56°C (1 min), 98°C (10 min), 4°C (∞). A sample was considered “positive and quantifiable” if at least two droplets (in IS6110 or rpoB assay) were observed.
MTB DNA (copies/reaction) was then normalized into number copies/106 cells. In detail, raw data obtained were converted into copies/106 cells according to the following formula: [MTB-DNA (copies/106 cells) = MTB-DNA raw data × 106 cells/(housekeeping gene copies/2)].
To verify the correct performance of MTB detection and quantification, the laboratory-cultured H37Rv strain (previously inactivated by incubation at 95°C for 20 min and sonication at room temperature for 15 min) served as quantitation standards in two independent runs. Five serial dilutions were prepared in order to deposit 104, 103, 102, 10, and 1 copy(ies) of the MTB genome (1 ng = 168,100 copies) in 3 μg of human genomic DNA, corresponding to 106 cells. The first three dilutions were repeated in duplicate, while the remaining ones were in triplicate. To determine the specificity and cross-reactivity of the MTB ddPCR-based assays, the DNA of cultured non-tuberculous strains [M. abscessus subsp. abscessus (MBABAB) and M. abscessus subsp. bolletii (MBABBO)], and negative controls (n = 20) were also added in each run. The negative controls belonged to FFPE samples from subjects without clinical and bacteriological signs of MTB infection [World Health Organization (WHO), 2013].
Coefficient of determination (R2) of MTB quantification was assessed for both IS6110 and rpoB by linear regression analysis by plotting the measured copies of the standards and comparing them with expected values of serial dilutions. The coefficient of variation (CV) was calculated as the standard deviation (SD) of copies/106 cells divided by replicate mean.
The lower limit of blank (LoB) was determined by testing the replicates of the 20 negative controls, according to the following formula: LoB = mean of blank + 1.645 × (SD of blank) (Armbruster and Pry, 2008). The limit of detection (LoD) was determined by probit regression analysis.
Sensitivity and specificity of ddPCR, Xpert, and AFB in terms of the ability to correctly diagnose MTB in tissue samples (pulmonary and extrapulmonary) were evaluated against culture results.
Reproducibility of quantification methods was assessed by intra- and inter-run tests using serial standard dilutions, and the differences between the expected and observed values were expressed as the mean ± SD IS6110 and rpoB copy numbers.
Descriptive statistics were expressed as median values and interquartile range (IQR) for continuous variables and absolute number and frequency (percentage) for categorical variables. To assess significant differences, Fisher exact or Kruskal–Wallis test and Wilcoxon rank sum test were used for categorical and continuous variables, respectively. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS software package for Windows (version 25.0, SPSS INC., Chicago, IL, United States).
The demographic and clinical characteristics of the population included in the study are reported in Table 1. Samples were retrieved mainly by male (57.3%) with a median age of 36 years (IQR: 27–53). As expected, extrapulmonary samples were the majority (85, 95.5%) and mainly from lymphatic system (52, 58.4%) followed by pleural samples (17, 19.1%). Sixty-eight samples (76.4%) were MTB positive because of the positive mycobacterial culture from the fresh sample [median (IQR) days for culture positivity: 11 (13–16)]. The remaining 21 samples were MTB negative because of the negative culture and subsequent different diagnosis. Most of the positive samples (94.1%) belonged to patients receiving their first TB diagnosis at the time of the biopsy, and only 5.9% belonged to patients with a previously MTB-positive known condition.
No differences were found between MTB-positive and -negative samples, with the sole exception of patients aged below 40 years, most frequently found among MTB-positive samples (Table 1). Viral coinfections were quite rare (9.0%) and prevalently found in patients with positive MTB culture (4.5%, no significant data).
The linearity of the ddPCR assays was tested by quantifying serial dilutions of a known amount of MTB DNA. Our method showed a good linear correlation between expected and observed MTB DNA quantification, for both IS6110 (R2 = 0.9907) and rpoB (R2 = 0.9743) (Figures 1A,B). No signal was detected in any of the 20 certainly negative samples tested, nor in the non-tuberculous extract (MBABAB and MBABBO) added in each run (Supplementary Figure 1). An example of Quantasoft panel for IS6110 and rpoB obtained by the positive MTB DNA control, four positive samples, and a negative sample are also reported in Supplementary Figure 2.
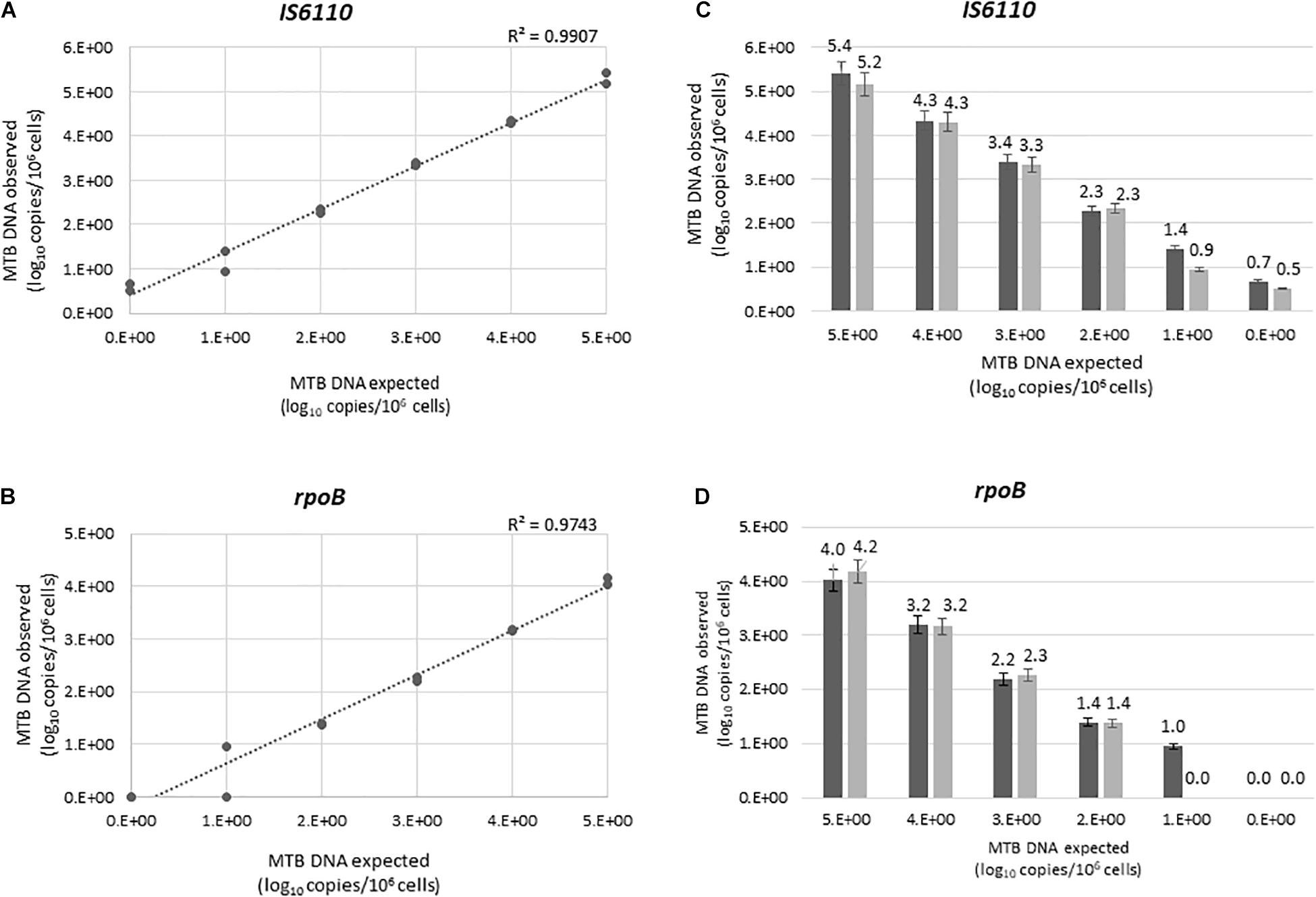
Figure 1. Linear correlations between the expected and the observed IS6110 (A) and rpoB (B) load, expressed as log10 copies/106 cells. IS6110 (C) rpoB (D) load in the first and second experiment were shown in dark and light gray, respectively. Each value was tested in two independent experiments, each led in duplicate. Bars represent mean (+SD).
Intra-run reproducibility analysis confirmed the high reliability of the methods (Figures 1C,D). The mean (±SD) differences between the expected and observed MTB copy number per 106 cells (expressed as log10) were for IS6110 assay: −0.363 ± 0.07 for 105, −0.338 ± 0.02 for 104, −0.338 ± 0.05 for 103, −0.340 ± 0.06 for 102, −0.338 ± 0.12 for 10 copies, −0.348 ± 0.04 for one copy; for rpoB assay: 0.898 ± 0.10 for 105, 0.840 ± 0.05 for 104, 0.802 ± 0.08 for 103, 0.602 ± 0.02 for 102, −0.060 ± 0.40 for 10 copies, −0.348 ± 0.04 for one copy. Mean CVs of the two experiments were 3.48 for IS6110, and 7.85 for rpoB.
The analysis of inter-run reproducibility confirmed the above results. The mean (±SD) differences between the expected and observed MTB copy number per 106 cells (expressed as log10) were for IS6110 assay: −0.292 ± 0.17 for 105, −0.317 ± 0.03 for 104, −0.367 ± 0.04 for 103, −0.307 ± 0.05 for 102, −0.176 ± 0.33 for 10, and −0.584 ± 0.1 for one copy; for rpoB assay: 0.899 ± 0.10 for 105, 0.824 ± 0.02 for 104, 0.764 ± 0.05 for 103, 0.611 ± 0.01 for 102, 0.523 ± 0.67 for 10, −0.090 ± 0.16 for one copy. Mean CV was 9.04 and 24.61 for IS6110 and rpoB, respectively.
All the DNA extracts obtained by the 89 FFPE samples were of high quality and quantity as confirmed by human albumin quantification [median (IQR): 1,760 (1,228–2,200) copies/μl; Supplementary Figure 3].
The overall sensitivity of the ddPCR assays was 98.5 (95.6–100.0) for IS6110 and 66.2 (54.9–77.4) for rpoB. No false-positive results were highlighted among the 21 culture-negative FFPE samples (Table 2).
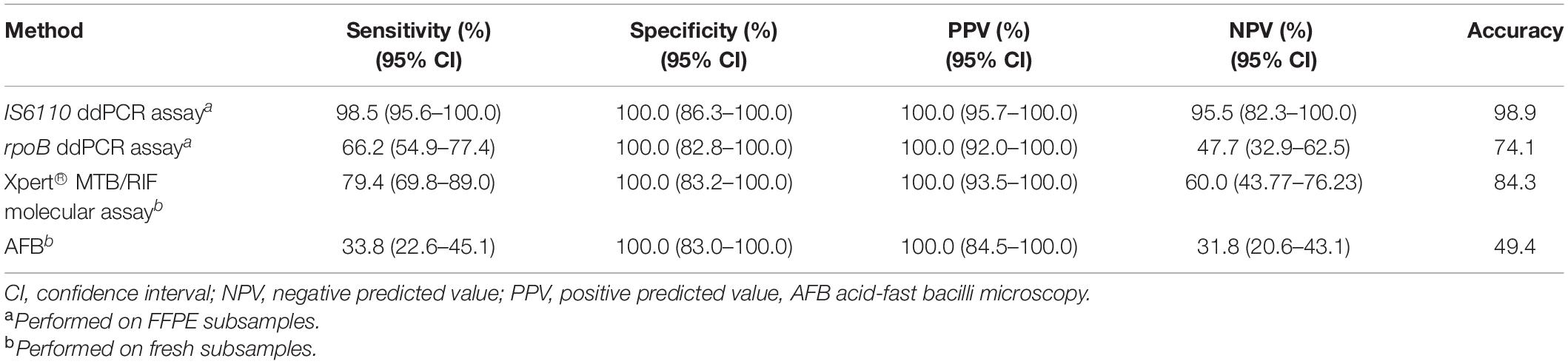
Table 2. Performances of homemade ddPCR assays, Xpert® MTB/RIF molecular assay, and AFB against MTB culture.
Among the 68 positive mycobacterial cultures, ddPCR yielded 67/68 (98.5%) positive results for IS6110 with a median (IQR) of 1,680 (550–8,444) copies/106 cells, and 45/68 (66.2%) positive results for rpoB with a median (IQR) of 308 (99–1,419) copies/106 cells. One sample was negative for both rpoB and IS6110, 45 samples were positive for both IS6110 and rpoB (double positive), and 22 were positive only for IS6110 (single positive). Double-positive with respect to single-positive samples were characterized by higher mycobacterial loads [IS6110 copies/106 cells, median (IQR): 3,578 (1,352–11,983) vs. 308 (99–1,419), p = 0.001], but not by a shorter time to positive culture [days, median (IQR): 13 (11–16) vs. 12 (10–15), p = 0.687].
When used in fresh subsamples, Xpert and AFB had a sensitivity of 79.4% (69.8–89.0) and 33.8% (22.6–45.1), respectively, lower than that observed with ddPCR in FFPE subsamples. As for ddPCR, no false-positive results were highlighted (Table 2). To define if the bacterial load can influence the sensitivity of Xpert and AFB, qPCR and smear results were stratified according to rpoB and IS6110 load detected by ddPCR-assays. As expected, rpoB and IS6110 loads were significantly lower in Xpert-negative with respect to Xpert-positive samples [rpoB load, median (IQR): 107 (97–210) vs. 414 (110–2,651), p = 0.003; IS6110 load, median (IQR): 941 (361–1,412) vs. 2,892 (552–10,709), p = 0.01, Figures 2A,B]. Superimposable data were found for smear results (Figures 2C,D). No significant differences in the IS6110 or rpoB loads were found against time of the first MTB diagnosis, anatomical districts, age, or sex of patients (Supplementary Figures 4A–H).
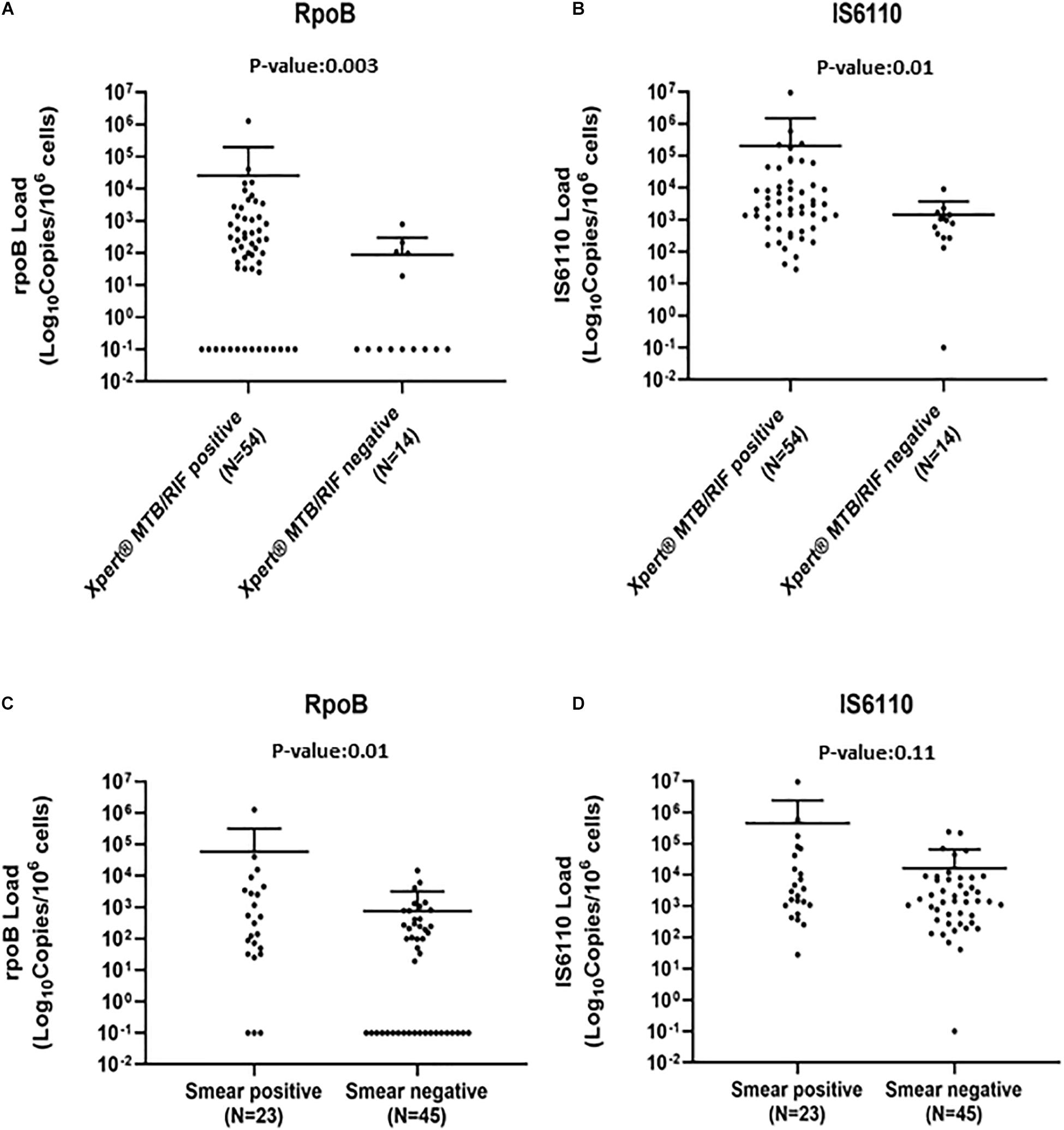
Figure 2. Mycobacteria tuberculosis (MTB) rpoB and IS6110 load against Xpert (A,B) and acid-fast bacilli (AFB) smear (C,D) results. Each value was represented by a dot; bars represent median and interquartile range (IQR). p-Values were calculated by Wilcoxon rank sum test and Kruskal–Wallis where necessary.
Results of this preliminary study clearly revealed that the ddPCR-based assay was non-inferior to the reference culture method for the detection of MTB in tissue biopsies, even when FFPE samples were considered. Moreover, the performances of IS6110 ddPCR assay in detecting MTB in FFPE subsamples were superior to the standard Xpert® MTB/RIF and AFB microscopy used for tuberculosis case detection in fresh subsamples, as well as defined by the sensitivities obtained in the 89 in-blind analyzed biopsies (sensitivity: 98.5 vs. 79.4 vs. 33.8%, respectively).
Due to the low sensitivity and specificity of AFB microscopy when compared with culture method (Karadaǧ et al., 2013; Molicotti et al., 2014; Pk et al., 2017; Bahr et al., 2018), molecular methods (as Xpert® MTB/RIF assay) were introduced to improve the speed and specificity of TB diagnosis mainly in the context of extrapulmonary and FFPE samples, when bacterial load is low, and culture is not even possible. Sensitivities of Xpert® MTB/RIF and its ultra version reported in fresh non-FFPE clinical samples, so far, are always higher than 60% (Tortoli et al., 2012; Sauzullo et al., 2016; Lee et al., 2017; Bahr et al., 2018; Dorman et al., 2018; Sarfaraz et al., 2018; Sulis et al., 2018; Aydemir et al., 2019; Kohli et al., 2021) and can reach more than 90% only in some body districts (Mazzola et al., 2016). The few data available regarding the Xpert assay performances in FFPE samples are based on a few clinical biopsies and reported a wide range of sensitivities, which were not even concordant (from 97.6% in the case of the Ultra version to 53.2% in the case of the first MTB/RIF assay) (Seo et al., 2014; Du et al., 2019; Njau et al., 2019; Budvytiene and Banaei, 2020; Huang et al., 2020). Some of these reports also suggest different sensitivities against the sites of biopsies (i.e., lymph node vs. non-lymph nodes sites) (Polepole et al., 2017).
Hence, performing an accurate, quantitative, and sensitive MTB diagnosis is still a challenge. ddPCR is a third-generation PCR technology that allows an absolute quantification of nucleic acid molecules. This methodology is widely used to diagnose infectious diseases for its good accuracy, sensitivity, and specificity (Caviglia et al., 2018; Alteri et al., 2020; Chen et al., 2021; Malin et al., 2021), and has been successfully applied in different samples and clinical settings like SARS-CoV-2 (Alteri et al., 2020), HPV (Malin et al., 2021), HBV (Caviglia et al., 2018), or HIV (Strain et al., 2013), as some examples.
To the best of our knowledge, this is the first study that compared the performances of ddPCR with the MTB culture, recognized to be the gold standard for the MTB diagnosis, thanks to the availability of one fresh and one FFPE subsamples from the same biopsy.
In brief, we defined the performances of two duplex ddPCR assays targeting the multi-copy MTB gene IS6110 or the single-copy MTB gene rpoB, respectively, and the human albumin as housekeeping gene. Introducing a reference gene in the MTB ddPCR assay was helpful in measuring and reducing the errors from variations among the samples, defining efficiency of DNA extraction and amplifications. The use of the reference gene was also important for normalizing and providing accurate quantification of MTB copy numbers, expressed in our study as per 106 human cells.
By comparing the performances of IS6110 and rpoB ddPCR assays, we confirmed that targeting a multicopy gene like IS6110 guarantees a sensitive and reliable MTB detection (LOD 17 vs. 40 copies/106 human cells) (Bahador et al., 2005; Kolia-Diafouka et al., 2019). According to the approach by Armbruster and Pry (2008), the LoB were 0 copies/reaction for both IS6110 and rpoB. Probit analysis predicted a LoD of 14 copies/106 cells for IS6110 and 40 copies/106 cells for rpoB.
This ddPCR assay was non-inferior to the reference culture method, failing to find bacilli in only one 12-day culture-positive biopsy. This biopsy resulted in MTB negative by both Xpert and AFB methods, suggesting the presence of a paucibacillary TB disease. In line with this hypothesis, the negative ddPCR result could be caused by the absence of MTB inclusions in the FFPE subsample.
Both ddPCR assays allow to detect MTB where AFB and Xpert failed. Indeed, when the rpoB and IS6110 loads detected by ddPCR were reported against AFB and Xpert results, the copies of bacilli were lower in AFB/Xpert-negative samples with respect to AFB/Xpert-positive samples, thus, highlighting the high efficiency of ddPCR assay in diagnosing also low bacterial loads.
About paucibacillary TB disease, maintaining two mycobacterial targets like rpoB and IS6110 might allow to easily discriminate between high (characterized by double positive results) and low (characterized by single positive result) mycobacteria loads. This approach can also be used as a proxy measure for monitoring anti-TB treatment efficacy over time.
No difference in sensitivity and specificity of the ddPCR-based assays was found between patients with and without a history of tuberculosis, nor a difference was found against sex, age, or site of biopsies. Both assays maintain the ability to rule out MTB from culture-negative samples (specificity: 100%).
Our results are consistent with other published papers (Patterson et al., 2018; Song et al., 2018; Cho et al., 2020) reporting the rapid detection of MTB DNA in clinical or cultural samples by ddPCR system. In 2017, Yang et al. (2017) used a IS6110 ddPCR assay to quantify MTB DNA in the whole blood of patients with pulmonary and extrapulmonary TB, proving that this technique can have the potential to be included in TB routine diagnosis. Two years later, Nyaruaba et al. (2019) stated that ddPCR technology offers enormous advantages for MTB diagnosis, such as unparalleled sensitivity, high precision, and absolute quantification, over common molecular diagnostic platforms like the qPCR. In 2020, Cao et al. (2020) used a homemade IS6110 ddPCR assay for MTB diagnosis in FFPE samples. Recently, a single dye duplex ddPCR protocol targeting two different MTB IS demonstrates the superiority of this system with respect to qPCR in the detection of MTB in different culture isolates (Nyaruaba et al., 2020).
This preliminary study has some limitations that need to be discussed. First, the monocentric nature of the study prevented us to collect a large number of clinical samples by different body districts, limiting the possibility to draw certain statements. A multicenter study with larger sample size could be helpful and supportive in confirming the results obtained, including the sensitivity and the specificity here reported. Moreover, the Xpert method was only performed on fresh subsamples. This avoided the possibility to compare the performances of ddPCR assay and Xpert method in FFPE subsamples. Ultra-version of the Xpert® MTB/RIF, developed to improve the detection of paucibacillary disease, was not available at the time of the study, and thus, its performance on both fresh and FFPE samples was not assessed. No clinical follow-up was available, and thus, no correlation between mycobacteria load and clinical outcome can be performed. In addition, the negative control population was not selected against other diseases like HIV, asthma, Leishmania, toxoplasma, diabetes, and neither this information was retrospectively available. Even if in this study all positive ddPCR results were confirmed to be positive by culture, these molecular assays are unable to discriminate between viable or non-viable bacilli.
Moreover, some disadvantages of ddPCR over other molecular methods need to be mentioned: (1) the system is not widely available; (2) ddPCR implementation is more complex than other standard molecular methods and needs specialized personnel; and (3) the cost per ddPCR reaction is not cheaper than other standard molecular methods (Hindson et al., 2013; Campomenosi et al., 2016).
In spite of these limitations, our study showed preliminary evidence regarding the highly sensitive, accurate, and rapid MTB diagnosis in FFPE samples by ddPCR methods, as defined by the high concordance between IS6110 assay and culture results. The quantitative approach of ddPCR and its performances independent of body districts make this system able to differentiate high bacillary load multisystemic disseminated condition from paucibacillary anatomically compartmentalized TB. Considering these results, the ddPCR approach can be safely introduced in the clinical routine to accelerate MTB diagnosis with respect to culture, as well as to provide reliable results when culture remains unavailable, like in the case of FFPE samples.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
CA, MA, RS, CFP, and EM contributed to the conception and design of the study. ST, CV, FS, SMV, EB, and EM provided the samples. MA and RS designed and conducted the experiments with the help of CL, SR, VM, ST, LG, LC, and GM. CR, VC, CV, LRC, AB, and AG helped with the clinical characterization of the patients. MA, RS, and CA analyzed and interpreted the data and wrote the manuscript. CA, CFP, EM, AB, and AG critically revised the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by the Italian “Ministero dell’Istruzione, dell’Università e della Ricerca” (Grant No. ARS01_00530).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.727774/full#supplementary-material
Supplementary Figure 1 | Quantasoft panel for IS6110 (green) and rpoB (blue) of all 20 negative samples, 2 non-tuberculous extracts [M. abscessus subsp. abscessus (MBABAB) and M. abscessus subsp. bolletii (MBABBO)], and 1 positive control.
Supplementary Figure 2 | Quantasoft panel for IS6110 (green) and rpoB (blue) of positive control (Pos Ctrl; H37Rv), four positive samples (repeated in duplicate), and a negative sample (repeated in duplicate).
Supplementary Figure 3 | Human albumine quantification in the 89 samples, expressed as copies/μl. Dots represent MTB positive culture samples, while triangles represent MTB negative culture samples; bars represent median and interquartile range (IQR).
Supplementary Figure 4 | MTB rpoB and IS6110 load against new and past MTB diagnoses (A,B), sex (C,D), age (E,F), and anatomical compartments (G,H). Each value was represented by a dot; bars represent median and interquartile range (IQR). p-Values were calculated by Wilcoxon rank sum test and Kruskal–Wallis where necessary.
Ahmed, H. G. E., Nassar, A. S., and Ginawi, I. (2011). Screening for tuberculosis and its histological pattern in patients with enlarged lymph node. Patholog. Res. Int. 2011, 1–4. doi: 10.4061/2011/417635
Allahyartorkaman, M., Mirsaeidi, M., Hamzehloo, G., Amini, S., Zakiloo, M., and Nasiri, M. J. (2019). Low diagnostic accuracy of Xpert MTB/RIF assay for extrapulmonary tuberculosis: A multicenter surveillance. Sci. Rep. 9:55112. doi: 10.1038/s41598-019-55112-y
Alteri, C., Cento, V., Antonello, M., Colagrossi, L., Merli, M., Ughi, N., et al. (2020). Detection and quantification of SARS-CoV-2 by droplet digital PCR in real-time PCR negative nasopharyngeal swabs from suspected COVID-19 patients. PLoS One 15:236311. doi: 10.1371/journal.pone.0236311
Armbruster, D. A., and Pry, T. (2008). Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 29(Suppl. 1), S49–S52.
Aydemir, O., Karakece, E., Koroglu, M., Altindis, M., and Terzi, H. A. (2019). Comparison of the GeneXpert® MTB/RIF test and conventional methods in the diagnosis of mycobacterium tuberculosis. Clin. Lab. 65, 1–6. doi: 10.7754/Clin.Lab.2018.180613
Bahador, A., Etemadi, H., Kazemi, B., Ghorbanzadeh, R., Nakhjavan, F. A., and Nejad, Z. A. (2005). Performance Assessment of IS1081-PCR for direct detection of tuberculous pleural effusion:compared to rpoB-PCR. Res. J. Agric. Biol. Sci. 2005, 142–145.
Bahr, N. C., Nuwagira, E., Evans, E. E., Cresswell, F. V., Bystrom, P. V., Byamukama, A., et al. (2018). Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. Lancet Infect. Dis. 18, 68–75. doi: 10.1016/S1473-3099(17)30474-7
Bisognin, F., Lombardi, G., Lombardo, D., Carla Re, M., and Dal Monte, P. (2018). Improvement of Mycobacterium tuberculosis detection by Xpert MTB/RIF Ultra: A head-to-head comparison on Xpert-negative samples. PLoS One 13:201934. doi: 10.1371/journal.pone.0201934
Budvytiene, I., and Banaei, N. (2020). Simple processing of formalin-fixed paraffin-embedded tissue for accurate testing with the xpert MTB/RIF assay. J. Clin. Microbiol. 58:19. doi: 10.1128/JCM.01905-19
Campomenosi, P., Gini, E., Noonan, D. M., Poli, A., D’Antona, P., Rotolo, N., et al. (2016). A comparison between quantitative PCR and droplet digital PCR technologies for circulating microRNA quantification in human lung cancer. BMC Biotechnol. 60:2927. doi: 10.1186/s12896-016-0292-7
Cao, Z., Wu, W., Wei, H., Gao, C., Zhang, L., Wu, C., et al. (2020). Using droplet digital PCR in the detection of Mycobacterium tuberculosis DNA in FFPE samples. Int. J. Infect. Dis. 99, 77–83. doi: 10.1016/j.ijid.2020.07.045
Caulfield, A. J., and Wengenack, N. L. (2016). Diagnosis of active tuberculosis disease: From microscopy to molecular techniques. J. Clin. Tuberc. Other Mycobact. Dis. 4, 33–43. doi: 10.1016/j.jctube.2016.05.005
Caviglia, G. P., Abate, M. L., Tandoi, F., Ciancio, A., Amoroso, A., Salizzoni, M., et al. (2018). Quantitation of HBV cccDNA in anti-HBc-positive liver donors by droplet digital PCR: A new tool to detect occult infection. J. Hepatol. 69, 301–307. doi: 10.1016/j.jhep.2018.03.021
Chen, B., Jiang, Y., Cao, X., Liu, C., Zhang, N., and Shi, D. (2021). Droplet digital PCR as an emerging tool in detecting pathogens nucleic acids in infectious diseases. Clin. Chim. Acta 517, 156–161. doi: 10.1016/j.cca.2021.02.008
Cho, S. M., Shin, S., Kim, Y., Song, W., Hong, S. G., Jeong, S. H., et al. (2020). A novel approach for tuberculosis diagnosis using exosomal DNA and droplet digital PCR. Clin. Microbiol. Infect. 26:942. doi: 10.1016/j.cmi.2019.11.012
Dorman, S. E., Schumacher, S. G., Alland, D., Nabeta, P., Armstrong, D. T., King, B., et al. (2018). Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect. Dis. 18, 76–84. doi: 10.1016/S1473-3099(17)30691-6
Du, W. L., Song, J., Wang, J. G., Liu, Z. C., Li, K., Wang, Y. X., et al. (2019). Performance of xpert MTB/RIF Ultra and Xpert MTB/RIF for the diagnosis of tuberculosis through testing of formalin-fixed Paraffin-embedded Tissues. Biomed. Environ. Sci. 32, 922–925. doi: 10.3967/bes2019.115
El-Zammar, O. A., and Katzenstein, A. L. A. (2007). Pathological diagnosis of granulomatous lung disease: a review. Histopathology 50, 289–310. doi: 10.1111/j.1365-2559.2006.02546.x
Eshete, A., Zeyinudin, A., Ali, S., Abera, S., and Mohammed, M. (2011). M. tuberculosis in Lymph node biopsy paraffin-embedded sections. Tuberc. Res. Treat. 2011, 1–5. doi: 10.1155/2011/127817
European Centre for Disease Prevention and Control (ECDC) (2018). Handbook on tuberculosis laboratory diagnostic methods in the European Union. Solna Municipality: ECDC.
Forbes, B. A., and Hicks, K. E. S. (1993). Direct detection of Mycobacterium tuberculosis in respiratory specimens in a clinical laboratory by polymerase chain reaction. J. Clin. Microbiol. 31, 1688–1694. doi: 10.1128/jcm.31.7.1688-1694.1993
Forbes, B. A., Miller, M. B., Banaei, N., Brown-Elliott, B. A., Das, S., Salfinger, M., et al. (2018). Laboratory Detection and Identification of Mycobacteria. M48-A. 2nd ed. Annapolis Junction, MD: CLSI.
Fu, Y. C., Liao, I. C., Chen, H. M., and Yan, J. J. (2016). Detection of Mycobacterium tuberculosis complex in paraffin-embedded tissues by the new automated Abbott RealTime MTB Assay. Ann. Clin. Lab. Sci. 46, 412–417.
Fukunaga, H., Murakami, T., Gondo, T., Sugi, K., and Ishihara, T. (2002). Sensitivity of acid-fast staining for Mycobacterium tuberculosis in formalin-fixed tissue. Am. J. Respir. Crit. Care Med. 166, 994–997. doi: 10.1164/rccm.2111028
Hindson, C. M., Chevillet, J. R., Briggs, H. A., Gallichotte, E. N., Ruf, I. K., Hindson, B. J., et al. (2013). Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods 2013, 1003–1005. doi: 10.1038/nmeth.2633
Huang, S., Qin, M., Shang, Y., Fu, Y., Liu, Z., Dong, Y., et al. (2020). Performance of Xpert MTB/RIF in diagnosis of lymphatic tuberculosis from fresh and formaldehyde-fixed and paraffin embedded lymph nodes. Tuberculosis 124:101967. doi: 10.1016/j.tube.2020.101967
Karadaǧ, A., Usta, E., Bilgin, K., Güney, A. K., Eroǧlu, C., and Günaydin, M. (2013). Comparison of culture, real-time DNA amplification assay and Ehrlich-Ziehl-Neelsen for detection of Mycobacterium tuberculosis. Balkan Med. J. 30, 13–15. doi: 10.5152/balkanmedj.2012.061
Kohli, M., Schiller, I., Dendukuri, N., Yao, M., Dheda, K., Denkinger, C. M., et al. (2021). Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 2021:CD012768. doi: 10.1002/14651858.CD012768.pub3
Kolia-Diafouka, P., Carrère-Kremer, S., Lounnas, M., Bourdin, A., Kremer, L., Van de Perre, P., et al. (2019). Detection of Mycobacterium tuberculosis in paucibacillary sputum: performances of the Xpert MTB/RIF ultra compared to the Xpert MTB/RIF, and IS6110 PCR. Diagn. Microbiol. Infect. Dis. 94, 365–370. doi: 10.1016/j.diagmicrobio.2019.02.008
Lee, H. S., Park, K. U., Park, J. O., Chang, H. E., Song, J., and Choe, G. (2011). Rapid, sensitive, and specific detection of Mycobacterium tuberculosis complex by real-time PCR on paraffin-embedded human tissues. J. Mol. Diagnostics 13, 390–394. doi: 10.1016/j.jmoldx.2011.02.004
Lee, J., Choi, S. M., Lee, C. H., Lee, S. M., Yim, J. J., Yoo, C. G., et al. (2017). The additional role of Xpert MTB/RIF in the diagnosis of intrathoracic tuberculous lymphadenitis. J. Infect. Chemother. 23, 381–384. doi: 10.1016/j.jiac.2017.03.001
Lin, C. R., Wang, H. Y., Lin, T. W., Lu, J. J., Hsieh, J. C. H., and Wu, M. H. (2021). Development of a two-step nucleic acid amplification test for accurate diagnosis of the Mycobacterium tuberculosis complex. Sci. Rep. 11:5750. doi: 10.1038/s41598-021-85160-2
Malin, K., Louise, B. M., Gisela, H., Mats, K. G., and Gabriella, L.-L. (2021). Optimization of droplet digital PCR assays for the type-specific detection and quantification of five HPV genotypes, including additional data on viral loads of nine different HPV genotypes in cervical carcinomas. J. Virol. Methods 294:114193. doi: 10.1016/j.jviromet.2021.114193
Marchetti, G., Gori, A., Catozzi, L., Vago, L., Nebuloni, M., Rossi, M. C., et al. (1998). Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: Comparison of four amplification assays. J. Clin. Microbiol. 36, 1512–1517. doi: 10.1128/jcm.36.6.1512-1517.1998
Mazzola, E., Arosio, M., Nava, A., Fanti, D., and Gesu, G. (2016). Performance of real-time PCR Xpert ® MTB/RIF in diagnosing extrapulmonary tuberculosis. Infez. Med. 4, 304–309.
Molicotti, P., Bua, A., and Zanetti, S. (2014). Cost-effectiveness in the diagnosis of tuberculosis: Choices in developing countries. J. Infect. Dev. Ctries. 8, 24–38. doi: 10.3855/jidc.3295
Moure, Z., Castellví, J., Sánchez-Montalvá, A., Pumarola, T., and Tórtola, M. T. (2019). The role of molecular techniques for the detection of mycobacterium tuberculosis complex in paraffin-embedded biopsies. Appl. Immunohistochem. Mol. Morphol. 27, 77–80. doi: 10.1097/PAI.0000000000000533
Njau, A. N., Gakinya, S. M., Sayed, S., and Moloo, Z. (2019). Xpert® MTB/RIF assay on formalin-fixed paraffin-embedded tissues in the diagnosis of extrapulmonary tuberculosis. Afr. J. Lab. Med. 8:748. doi: 10.4102/ajlm.v8i1.748
Noussair, L., Bert, F., Leflon-Guibout, V., Gayet, N., and Nicolas-Chanoine, M. H. (2009). Early diagnosis of extrapulmonary tuberculosis by a new procedure combining broth culture and PCR. J. Clin. Microbiol. 47, 1452–1457. doi: 10.1128/JCM.00066-09
Nyaruaba, R., Mwaliko, C., Kering, K. K., and Wei, H. (2019). Droplet digital PCR applications in the tuberculosis world. Tuberculosis 117, 85–92. doi: 10.1016/j.tube.2019.07.001
Nyaruaba, R., Xiong, J., Mwaliko, C., Wang, N., Kibii, B. J., Yu, J., et al. (2020). Development and evaluation of a single dye duplex droplet digital PCR assay for the rapid detection and quantification of mycobacterium tuberculosis. Microorganisms 8:8050701. doi: 10.3390/microorganisms8050701
Patterson, B., Morrow, C., Singh, V., Moosa, A., Gqada, M., Woodward, J., et al. (2018). Detection of bacilli in Mycobacterium tuberculosis bio-aerosols from untreated TB patients. Gates Open Res. 1:11. doi: 10.12688/gatesopenres.12758.2
Pk, M., Mp, N., and Dn, M. (2017). Performance of GeneXpert assay in detecting pulmonary tuberculosis and rifampicin resistance in patients attending kitui county hospital, kenya. J. Tropical Dis. Public Health 2017:246. doi: 10.4172/2329-891X.1000246
Polepole, P., Kabwe, M., Kasonde, M., Tembo, J., Shibemba, A., O’Grady, J., et al. (2017). Performance of the Xpert MTB/RIF assay in the diagnosis of tuberculosis in formalin-fixed, paraffin-embedded tissues. Int. J. Mycobacteriol. 6, 87–93. doi: 10.4103/2212-5531.201892
Riccardi, N., Ferrarese, M., Castellotti, P., Mazzola, E., Sozzi, F., Rigatti, P., et al. (2020). A rare case of multi-focal human TB after BCG instillation for non-muscle-invasive bladder cancer. Urol. J. 87, 199–202. doi: 10.1177/0391560319860396
Ryu, Y. J. (2015). Diagnosis of pulmonary tuberculosis: Recent advances and diagnostic algorithms. Tuberc. Respir. Dis. 78, 64–71. doi: 10.4046/trd.2015.78.2.64
Sadeghipour, A., and Babaheidarian, P. (2019). Making formalin-fixed, paraffin embedded blocks. Methods Mol. Biol. 1897, 253–268. doi: 10.1007/978-1-4939-8935-5_22
Sarfaraz, S., Iftikhar, S., Memon, Y., Zahir, N., Hereker, F. F., and Salahuddin, N. (2018). Histopathological and microbiological findings and diagnostic performance of GeneXpert in clinically suspected tuberculous lymphadenitis. Int. J. Infect. Dis. 76, 73–81. doi: 10.1016/j.ijid.2018.08.020
Sarnecka, A. K., Nawrat, D., Piwowar, M., Ligęza, J., Swadźba, J., and Wójcik, P. (2019). DNA extraction from FFPE tissue samples – a comparison of three procedures. Wspolczesna Onkol. 23, 52–58. doi: 10.5114/wo.2019.83875
Sauzullo, I., Maria Rodio, D., Facchinetti, S., Puggioni, G., De Angelis, M., Goldoni, P., et al. (2016). Diagnostic accuracy of Xpert MTB/RIF versus smear microscopy in the early diagnosis of tuberculosis in the real life of the “Umberto I” Hospital in Rome. New Microbiol. 39, 304–306.
Seo, A. N., Park, H. J., Lee, H. S., Park, J. O., Chang, H. E., Nam, K. H., et al. (2014). Performance characteristics of nested polymerase chain reaction vs real-time polymerase chain reaction methods for detecting mycobacterium tuberculosis complex in paraffin-embedded human tissues. Am. J. Clin. Pathol. 142, 384–390. doi: 10.1309/AJCP2QZRH4ZNPRDD
Slaoui, M., and Fiette, L. (2011). Histopathology procedures: from tissue sampling to histopathological evaluation. Methods Mol. Biol. 691, 69–82. doi: 10.1007/978-1-60761-849-2_4
Song, N., Tan, Y., Zhang, L., Luo, W., Guan, Q., Yan, M. Z., et al. (2018). Detection of circulating Mycobacterium tuberculosis-specific DNA by droplet digital PCR for vaccine evaluation in challenged monkeys and TB diagnosis article. Emerg. Microbes. Infect. 7:76. doi: 10.1038/s41426-018-0076-3
Strain, M. C., Lada, S. M., Luong, T., Rought, S. E., and Gianella, S. (2013). Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One 8:55943. doi: 10.1371/journal.pone.0055943
Sulis, G., Agliati, A., Pinsi, G., Bozzola, G., Foccoli, P., Gulletta, M., et al. (2018). Xpert MTB/RIF as add-on test to microscopy in a low tuberculosis incidence setting. Eur. Respir. J. 51:2017. doi: 10.1183/13993003.02345-2017
Tortoli, E., Russo, C., Piersimoni, C., Mazzola, E., Dal Monte, P., Pascarella, M., et al. (2012). Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur. Respir. J. 40, 442–447. doi: 10.1183/09031936.00176311
World Health Organization (WHO). (2013). Definitions and reporting framework for tuberculosis. Geneva: WHO.
Yang, J., Han, X., Liu, A., Bai, X., Xu, C., Bao, F., et al. (2017). Use of digital droplet PCR to detect Mycobacterium tuberculosis DNA in whole blood-derived DNA samples from patients with pulmonary and extrapulmonary tuberculosis. Front. Cell. Infect. Microbiol. 7:369. doi: 10.3389/fcimb.2017.00369
Keywords: MTB, ddPCR, MTB diagnosis, tuberculosis, extrapulmonary TB
Citation: Antonello M, Scutari R, Lauricella C, Renica S, Motta V, Torri S, Russo C, Gentile L, Cento V, Colagrossi L, Mattana G, Codecasa LR, Vismara C, Scaglione F, Veronese SM, Bonoldi E, Bandera A, Gori A, Mazzola E, Perno CF and Alteri C (2021) Rapid Detection and Quantification of Mycobacterium tuberculosis DNA in Paraffinized Samples by Droplet Digital PCR: A Preliminary Study. Front. Microbiol. 12:727774. doi: 10.3389/fmicb.2021.727774
Received: 19 June 2021; Accepted: 16 August 2021;
Published: 13 September 2021.
Edited by:
Xiao-Yong Fan, Fudan University, ChinaReviewed by:
Amit Singh, All India Institute of Medical Sciences, IndiaCopyright © 2021 Antonello, Scutari, Lauricella, Renica, Motta, Torri, Russo, Gentile, Cento, Colagrossi, Mattana, Codecasa, Vismara, Scaglione, Veronese, Bonoldi, Bandera, Gori, Mazzola, Perno and Alteri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia Alteri, Y2xhdWRpYS5hbHRlcmlAdW5pbWkuaXQ=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.