
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 30 July 2021
Sec. Food Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.711092
This article is part of the Research Topic Postharvest Diseases of Fruit and Vegetable: Methods and Mechanisms of Action View all 12 articles
Subtropical fruit such as avocados (Persea americana), mangoes (Mangifera indica L.), and papayas (Carica papaya L.) are economically important in international trade and predominantly exported to European destinations. These fruits are highly consumed due to their health benefits. However, due to long-distance shipping and the time required to reach the retail department stores, postharvest losses, due to postharvest decay occurring during the supply chain, affect the fruit quality on arrival at the long-distance distribution points. Currently, the use of synthetic fungicide, Prochloraz®, is used at the packing line to reduce postharvest decay and retain the overall quality of mangoes and avocados. Due to the ban imposed on the use of synthetic fungicides on fresh fruit, several studies have focused on the development of alternative technologies to retain the overall quality during marketing. Among the developed alternative technologies for commercial adoption is the use of edible coatings, such as chitosan biocontrol agents and essential oil vapors. The objective of this review is to summarize and analyze the recent advances and trends in the use of these alternative postharvest treatments on anthracnose decay in avocados, mangoes, and papayas.
Avocados are an economically important fruit crop in South Africa, Israel, the United States, Mexico, Peru, Chile, Kenya, and Australia. Papayas and mangoes from Latin American countries, especially from Brazil, are exported in large volumes to the EU. The demand for avocado fruit is rapidly increasing in the last few years due to its global recognition as a “superfruit,” based on nutritional facts and health benefits. The projection for the world avocado market by 2026 was to obtain an overall value of US$21.56 billion (Transparency Market Research Report, 2021). Consumption of avocados has increased in countries; the United States, and the EU and Latin American countries together totaled 1,323,000 tons in 2019 (Transparency Market Research Report, 2021). In 2019, the world export of papayas and mangoes was 2,575,002 and 364,030 tons, which fetched US$3,552,922 and US$295,628,000, respectively (FAOSTAT, 2021).
The export of fruits to the long-distance markets is via refrigerated sea shipment. The entire marketing time frame includes approximately 30 days until it reaches the consumers. During transport, due to cold storage and shelf life at the retail outlet, fruits develop postharvest decay caused by anthracnose (Colletotrichum gloeosporioides Penz and Sacc), which is the most common postharvest fungal disease in avocados (Sanders et al., 2000), mangoes (Lima J. R. et al., 2013), and papayas (Dickman, 1994). C. gloeosporioides infects the fruit via direct penetration, remains quiescent, and causes latent infections (Swinburne, 1983; Siddiqui and Ali, 2014). The latency was broken when the fruit reaches the climacteric peak (Swinburne, 1983). In the ripe fruit, anthracnose symptoms manifest as sunken, dark-brown to black decay lesions; and in the presence of high humidity, prominent pinkish-orange spore masses appear on the lesions (Nelson, 2008). Fruits infected by postharvest fungal pathogens cause decay symptoms, thus affecting the fruit quality, which negatively affects the market value. C. gloeosporioides reportedly infects at an immature stage of the fruit and lives quiescently (Beno-Moualem and Prusky, 2000). Postharvest loss of papayas was reportedly 30–50% due to anthracnose (Bautista-Baños et al., 2013). Avocadoes, mangoes, and papayas are climacteric fruits. Changes occurring during fruit ripening, and ethylene emission activate the infection process, which negatively affects their quality, shelf life, and market value. The previous review written by Sivakumar and Bautista-Baños (2014) highlighted the need for a natural novel fungicide to replace the synthetic fungicide application at the postharvest stage due to higher levels of pesticide residues in the edible portion of the fruit, pathogens developing resistance to fungicides, and the influence of fungicides on environmental footprint. However, still, the synthetic non-systemic fungicide Prochloraz® {N-propyl-N-[2-(2,4,6-trichlorophenoxy) ethyl]-1H-imidazole-1-carboxamide} is applied at the packing line to control anthracnose decay in avocadoes, mangoes, and papayas (Swart et al., 2009; Henriod et al., 2016; Shimshoni et al., 2020). Although the exporting countries comply with the main trade regulations and the food safety requirements, the banning of Prochloraz® fungicide will occur in 2022 due to its hazardous toxicological properties. The major metabolite of 2,4,6-trichlorophenol has been listed as a human carcinogen (Group B2) by the US Environmental Protection Agency (EPA) (Shimshoni et al., 2020; EPA, 2021). The “Farm to Fork Strategy” plays a major role in the European Green Deal set out to reduce reliance on pesticides, increasing organic farming and providing sustainable diets to benefit consumers’ health, which subsequently will help in reducing the medical care costs for treasury funds (EU COM, 2020).
Thus, investigations on numerous alternative postharvest treatments to control decay during the supply chain are ongoing to replace the application of Prochloraz® fungicide at the postharvest stage. Furthermore, chloride-based sanitizers used in the packing line can produce carcinogenic compounds such as chloramines, dichloramines, and trichloromethanes, which have hazardous effects (Schoeny, 2010). The chemicals used in alternative treatments must have minimum toxicological effects on humans, domestic animals, and the environment and must fall under the category of generally recognized as safe (GRAS) by the US Food and Drug Administration (US FDA) and the European Food Safety Authority (EFSA). In addition, the alternative postharvest treatments must also be able to trigger induced defense mechanism of the host (fruit) via its eliciting properties and antimicrobial properties without compromising the fruit quality (Romanazzi et al., 2016). Considering the above, this review summarizes and analyzes the recent advances and trends in the use of these alternative postharvest treatments on the control of anthracnose caused by C. gloeosporioides in subtropical climacteric fruits during marketing based on published research articles from 2014 to date.
Chitosan, a biodegradable compound, is a polymeric compound of N-acetylglucosamine units joined by b-1,4-glycosidic links. Its production is by the deacetylation of chitin through the alkaline hydrolysis process of acetamide groups using NaOH or KOH (3.7%) at a temperature of 71°C (Gómez-Ríos et al., 2017). The chitosan was registered in the EU as a basic substance for plant protection use (Reg. EU 662014/563), especially in the application for organic agriculture and integrated pest management. Romanazzi et al. (2018) highlighted the contribution of chitosan in the management of postharvest decay of fresh fruit and vegetables due to its antimicrobial, eliciting, and film-forming activities. Rajestary et al. (2021) ran meta-analyses in which it were summarized as clear antimicrobial activity and decay control, while eliciting activity was often clarified and varied among the different studies for time and involved compounds. Romanazzi et al. (2018) showed a list of some of the available chitosan-based commercial products in countries such as Germany, the United States, the United Kingdom, Chile, New Zealand, Italy, Thailand, Poland, and China. Factors such as molecular weight of chitosan formulation, its concentration, method of application, and storage temperature of the fruit affect the efficacy of chitosan application on control of fruit decay (Velázquez-Del Valle et al., 2012). Marques et al. (2016) reported that chitosan, at concentrations of 1.0, 1.5, and 2.0%, inhibited the mycelial growth of C. gloeosporioides by 75, 86, and 90%, respectively; and at higher concentrations, 2.5 and 3.0%, showed a fungicidal effect by resulting in changes in conidial morphology. Marques et al. (2016) further reported that chitosan at concentrations of 1.5 and 2.0% completely arrested the germ tube formation and the germination process in C. gloeosporioides. Controlling the mycelial growth and the germination of C. gloeosporioides by chitosan demonstrates its fungicide potential. The electrostatic forces between its amino-protonated (NH2+) groups and negative residues on cell surfaces of C. gloeosporioides (pathogen) were responsible for the observed antifungal activity facilitated by chitosan (Elsabee and Abdou, 2013; Aloui et al., 2014). Commercial chitosan application (ChitoPlant®) at 1.5%, the formulation that dissolves in water, decreased anthracnose rot in avocados during a storage period of 28 days and after 5 days at marketing simulation conditions (Obianom et al., 2019). The mode of mechanism was attributed to the upregulation of phenylalanine ammonia-lyase, downregulation of lipoxygenase genes, and moderate retention of epicatechin content (90 mg kg–1 FW) in the skin probably due to delayed ripening and ethylene emission that had reduced the anthracnose decay (Obianom et al., 2019). Control of stem-end rot decay in commercial chitosan-coated (ChitoPlant® 1.5%) fruit was due to the upregulation of chitinase genes and higher superoxide dismutase activity (Obianom et al., 2019). Moreover, the non-toxic and antimicrobial properties and biodegradable nature of chitosan made it the most important natural biopolymer material for agriculture biotechnology. Conversely, avocados coated with carboxyl methylcellulose (1%) containing 2% of moringa leaf extract controlled the anthracnose decay by inhibiting the mycelial growth of C. gloeosporioides and maintaining the overall quality and shelf life of the fruits during marketing (Tesfay et al., 2017). Moringa leaf extract incorporated into carboxymethyl cellulose, exposed to gaseous ozone (O3) treatments for 36 h and thereafter, stored at 10°C and 95% relative humidity (RH) for 21 days, extended the shelf life, and maintained the fruit quality of mangoes fruit (‘Keitt’) by reducing the decay (Bambalele et al., 2021). Avocado ‘Maluma Hass’ fruit treated with 1% carboxyl methylcellulose + 10% moringa extract maintained the postharvest quality of fruit stored for 3 weeks at 5.5°C and at the simulated retail shelf conditions for an additional 1 week (Kubheka et al., 2021). Fruits coated with edible coatings form a semi-permeable layer around the fruit and create a modified atmosphere (Ali et al., 2014), and this modifies the internal atmosphere of the fruit, which helped to reduce the decay incidence and severity as compared with the control fruits (Garcia and Davidov-Pardo, 2021). Modified atmospheres created by the coating around the fruit could have reduced the pH (lower) of the skin, which is unfavorable to decay-causing C. gloeosporioides (Yakoby et al., 2000). Ali et al. (2014) recommended the use of the ethanolic extract of propolis (1.5%) and gum arabic (10%) as a biofungicide for the control of anthracnose in papayas. In addition, the edible coatings reduce the rate of respiration, moisture loss, senescence, and weight loss due to the semi-permeable modified atmosphere layer on the fruit surface (Ali et al., 2014). Table 1 summarizes the recent advances on edible coating and additives applied for subtropical fruits avocadoes and mangoes.
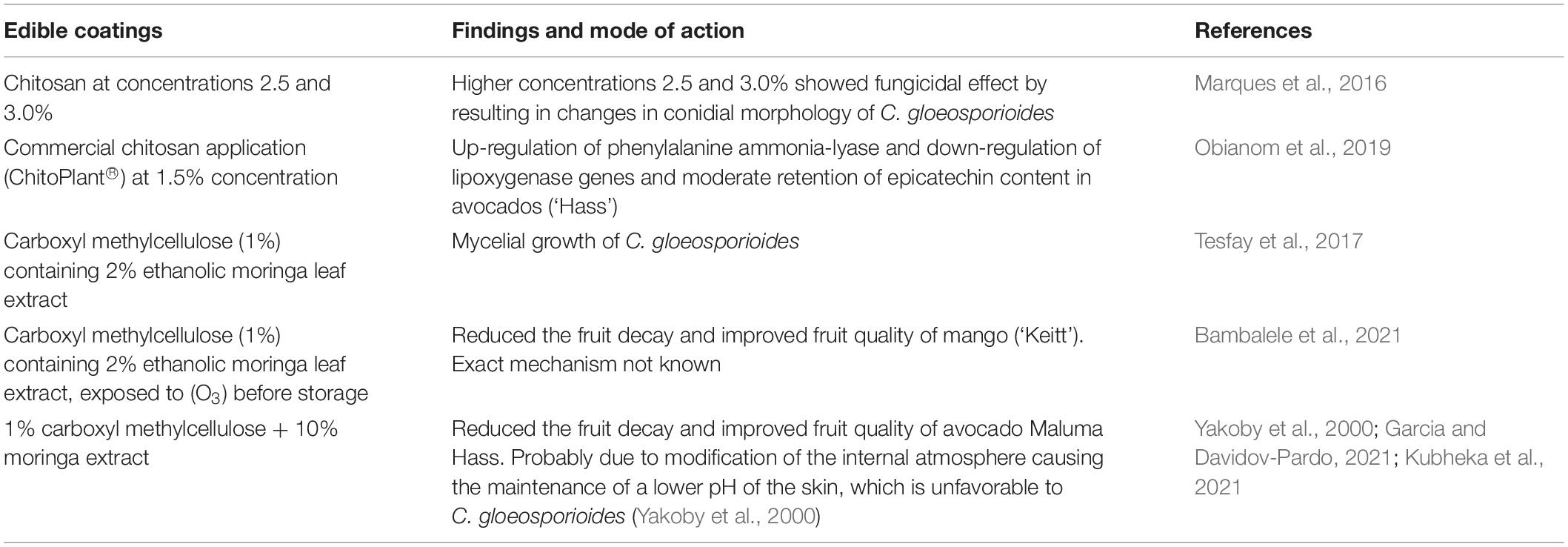
Table 1. Recent advances on edible coating and additives applied for subtropical fruits avocado and mangoes.
Nanoparticles are considered effective fungicides to control fungal pathogens in crops due to their distinctive physical and chemical properties, which do not relate to their bulk properties (De la Rosa-García et al., 2018). The antifungal effects of the nanoparticles are due to the alternation of physicochemical properties (optical, catalytic, and electronic properties) associated with size (De la Rosa-García et al., 2018). Nanoparticles of zinc oxide (ZnO) were evaluated against C. gloeosporioides obtained from papayas and avocadoes (De la Rosa-García et al., 2018). ZnO nanoparticles inhibited the fungal growth of C. gloeosporioides in vitro by causing structural deformation by vacuolar expansion, swelling, and melanization in the spores and mycelia (De la Rosa-García et al., 2018). The mode of action of ZnO nanoparticles is due to the production of reactive oxygen species (ROS) in water suspensions (Applerot et al., 2010). ZnO is listed as GRAS by the US FDA (21CFR182.8991) (FDA, 2021). However, further toxicological studies on the migration of nanoparticles into the fruit and toxicological studies are required to ensure that fruits coated with nanoparticle formulations are safe for consumption.
Research on the incorporation of nanosized edible coatings, such as chitosan, with natural antimicrobials, such as essential oils, to improve fruit health, safety, and shelf life has become popular during the past 5 years. Nanosized chitosan particles are non-toxic, biodegradable, highly permeable through the biological membranes, and cost-effective and have a wide spectrum of antifungal activities, therefore regarded as a suitable natural agent for control of postharvest decay (Meena et al., 2020).
Chitosan coating with silver nanoparticle (chitosan-AgNP), having the size distribution from 10 to 15 nm, significantly inhibited the conidial germination and inhibited the anthracnose decay significantly in mango fruits. Incorporating the silver nanoparticle to chitosan formulation aided in the binding interaction and stabilization by helping the dispersion of the chitosan-AgNP composites in the formulation during application to improve the efficacy (Chowdappa et al., 2014).
However, the EU does not permit food products or packaging that possesses silver nanoparticles (AgNPs) without any authorization. Allowable limits, based on the EFSA (EFSA, 2011), must not exceed 0.05 mg L–1 in water and 0.05 mg kg–1 in food. This emphasizes that the testing for antimicrobial effectiveness should comply with the current regulations. Furthermore, incorporations of antimicrobials, such as essential oils, in edible coatings or films are commercially adoptable due to the cost-effectiveness of low volumes of essential oils, effective diffusion efficiency, and prolonged antimicrobial activity during marketing.
Within essential oils, the US FDA Regulatory Agency (FDA, 2021) considers thyme oil (TO) as GRAS. Nanostructured edible coatings based on chitosan (3%)–thyme essential oil (5%) nanoparticles showed a synergetic effect on the control of anthracnose in avocadoes (cv. Hass) by arresting the growth of C. gloeosporioides (Correa-Pacheco et al., 2017). The drawback of this study was that there was no comparison of the efficacy of the antifungal activity of chitosan nanoparticles-essential oil coating with the currently used commercial Prochloraz® fungicide, and it was not tested on the avocado cultivar Fuerte, which is highly susceptible to anthracnose decay. In addition, nano or microencapsulation of essential oils prevents the degradation of the active compounds and their stability during unfavorable environmental conditions, such as heat, oxygen, light, pressure, moisture, and pH (Majeed et al., 2015).
The efficacy of the chitosan nanoparticles-essential oil coating also depends on the release rate and migration of active components of essential oils from the edible coating matrix. Additional investigations are required on the influence of incorporation of chitosan nano formulation-TO on fruit quality by measuring the gas exchange barrier, water vapor transmission, tensile and transparent properties of the coatings, internal and external fruit quality, and organoleptic characteristics (Flores-López et al., 2016). Chitosan nanoparticle-essential oil coatings can affect the ripening pattern, flesh color, weight loss, fruit firmness, and degradation of phenolic compounds and antioxidant property of the subtropical fruits avocadoes, papayas, and mangoes. Furthermore, the inclusion of essential oils inside of chitosan nanoparticles helps in releasing and reducing the active compounds (Mohammadi et al., 2015).
In addition, chitosan nanomaterials contain functionalizing agents, such as Cu, Zn, and salicylic acid (SA), which were added to improve the upregulation of antioxidant-defense enzymes in the plant cells, regulate the plant cellular homeostasis, and provide protection against diseases (Saharan et al., 2015; Kumaraswamy et al., 2019). The Cu–chitosan nanoparticles were tested for postharvest application (Meena et al., 2020). The mode of action was due to induced protonation of amino groups of chitosan because of acidic pH that released Cu ions from chitosan nanostructures to perform the antifungal activity. The Cu2+ ions showed an antifungal activity by improving the production of highly reactive hydroxyl radicals that eventually harm the biomolecules of the fungal cells (Meena et al., 2020). Chitosan/nano-silica coating enhanced the activities of antioxidant enzymes (superoxide dismutase, catalase, and ascorbate peroxidase) and reduced the generation of superoxide anion (O2–) and hydrogen peroxide (H2O2) (Song et al., 2016). Nitric oxide-releasing chitosan nanoparticles also reduced ethylene production and ROS and increased the antioxidant enzyme activity and antioxidant capacity in fruits (Ma et al., 2019).
Biopolymer chitosan is a suitable alternative to synthetic polymeric materials due to its biodegradable nature and negative impact on the environment. Chitosan and TiO2 nanocomposite films maintained the quality of climacteric fruit by photodegradation of ethylene activity in the presence of UV light, which caused delayed fruit ripening (Kaewklin et al., 2018). Furthermore, a combination of 3% CaCl2 and nano-chitosan improved the antioxidant capacity by improving the hydroxyl radical (∙OH) scavenging capacity and possibly could have acted as “signalling compounds” triggering the increase in antioxidant activities (enzymatic and non-enzymatic) in tissues (Jin et al., 2012; Nguyen et al., 2020). Table 2 summarizes the recent developments in nanomaterial antifungal properties.
Most essential oils are under the category of GRAS by the US FDA (FDA, 2021). Nanoemulsions of orange essential oil (Citrus sinensis) and xoconostle (Opuntia oligacantha) extract controlled anthracnose, increased the retention of fruit firmness in avocados, and improved the antioxidant activity due to the signaling effect of the active compounds of essential oils (Cenobio-Galindo et al., 2019). Furthermore, basil oil incorporated with beeswax coating increased shelf life and reduced anthracnose development in mango cv. Willard (Karunanayake et al., 2020). Rosemary pepper and Noni essential oils effectively controlled the C. gloeosporioides isolated from the papaya fruit (Dias et al., 2020). Noni essential oils totally inhibited conidial germination at 3,000 ppm, while rosemary pepper inhibited conidial germination at 5,000 ppm. The inhibition of mycelial growth of C. gloeosporioides occurred at 4,000- and 6,000-ppm concentrations with Noni and rosemary pepper essential oils (Dias et al., 2020). Savory oil with the active chemical compound carvacrol (71.2%) completely controlled the mycelial growth of C. gloeosporioides obtained from the papaya fruit (Sarkosh et al., 2018). C. gloeosporioides isolated from mangoes was effectively controlled by thymol-based essential oil (Chillet et al., 2018). Essential oils also cause structural and functional damage in the microbial cell by affecting the membrane permeability, causing leakage of cellular constituents, and dissipating the H+ and K+ ion gradients (Sarkosh et al., 2018).
Obianom and Sivakumar (2018) illustrated that the incorporation of 0.1% (v/v) TO in half-strength Prochloraz® (reduced concentration) completely reduced the anthracnose rot in avocado cv. Fuerte. The incorporation of TO had induced the activity of defense enzymes and resulted in decay reduction (Obianom and Sivakumar, 2018). The growers prefer the TO application in the aqueous phase, as it is easy to implement on the packing line.
Spray-dried TO microencapsulated with modified starch/agave fructan microcapsules, 0.10 and 0.20 g filled in nylon bags (4 × 4 cm) (antifungal sachets), controlled the mycelial growth of C. gloeosporioides and reduced the anthracnose incidence in mangoes during storage in boxes, which were sealed with parafilm for 9 days at 20 ± 2°C (Esquivel-Chávez et al., 2021). Active components of TO together triggered the induced defense mechanism by upregulation of phenylalanine ammonia-lyase, chitinase, and β-1,3-glucanase enzymes in the fruit and exocarp while downregulating the lipoxygenase enzymes, which helped to retain a moderately higher concentration of epicatechin on the exocarp and delayed the fruit ripening (Bill et al., 2017). Furthermore, C. gloeosporioides isolated from the papaya fruit (‘Sekaki’) exposed to lemongrass oil vapor at a concentration of 28 μL L–1 containing geranial (45.6%) and neral (34.3%) as the major components for 18 h and stored for 9 days controlled the anthracnose decay due to fungicidal properties (Ali et al., 2015). Table 3 summarizes the recent developments in application of essential oils volatile components on the control of postharvest decay in avocado, mango and papaya. In addition, the starch film incorporated with carvacrol (750 mg L–1) and thymol (750 mg L–1) in the starch film showed complete inhibition of C. gloeosporioides (in vitro) (Ochoa-Velasco et al., 2021). The synergetic effect of the chemical constituents of essential oil components is due to the increased phenolic compounds in the molecule structure and could have facilitated the binding of these compounds to the proteins, preferably the cell membrane of the pathogens, which could have facilitated the membrane disintegration and loss of cellular content (Ochoa-Velasco et al., 2021). Furthermore, an antimicrobial transparent flexible trilayer low-density polyethylene (LDPE) film containing organically modified layered double hydroxides (OLDHs) and plant bioactive TOTO showed a remarkable reduction in anthracnose disease events in Hass cultivar (Kesavan Pillai et al., 2020). The barrier and antifungal properties of nanocomposite film helped to release the volatile bioactive release in a controlled manner; thus, the synergistic effect of LDPE–OLDH–TO nanocomposite film can act as a prospective strategy to control anthracnose disease in avocados with modification of headspace gas composition (Kesavan Pillai et al., 2020).
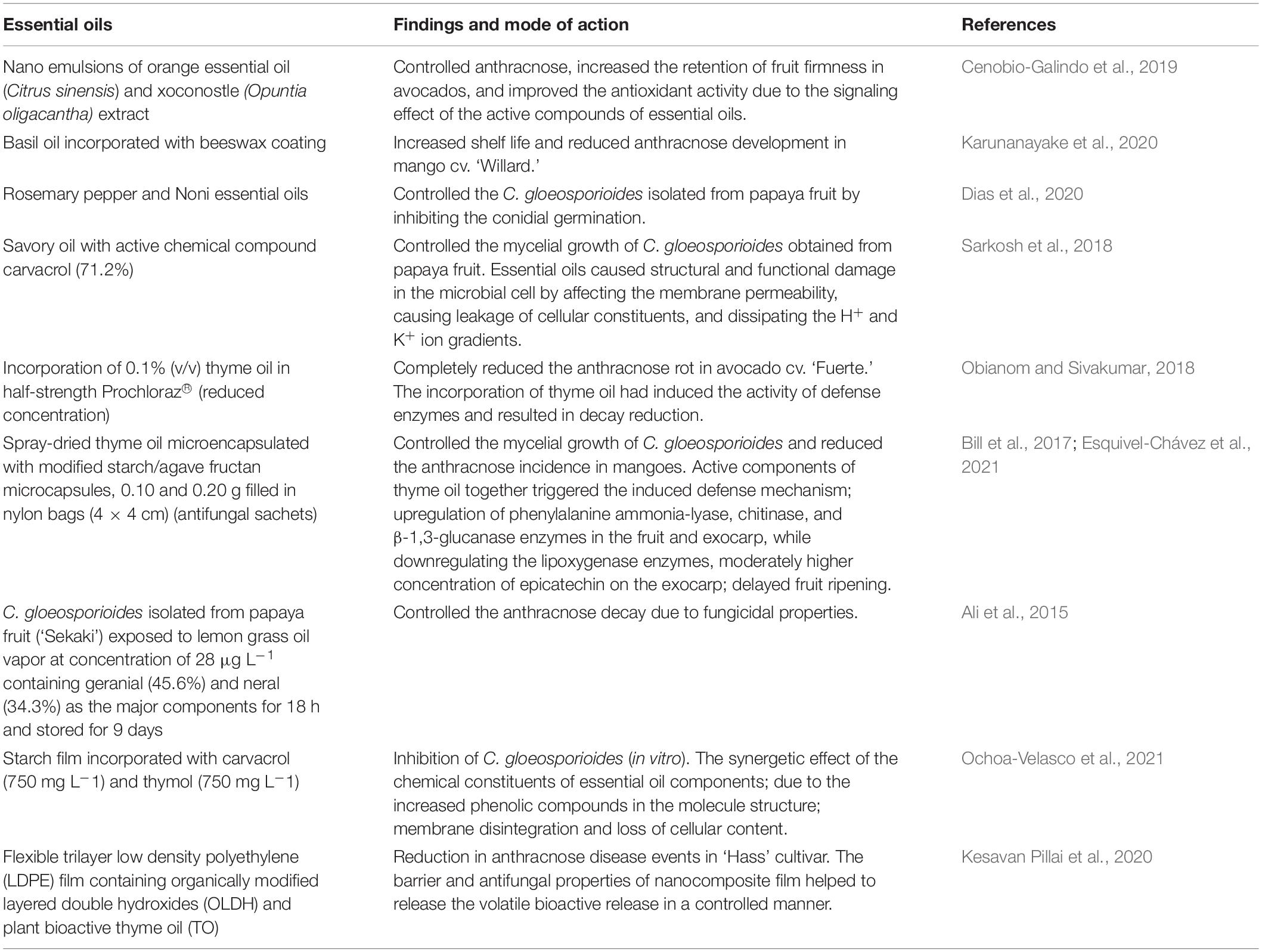
Table 3. Summary of recent developments in essential oils and incorporation of essential oils into wax or films on antifungal properties against C. gloeosporioides.
The use of biocontrol agents is for the managing of postharvest decay of fruit as an alternative treatment to synthetic fungicides. The ideal antagonist should be able to survive adverse environmental conditions, should use an affordable growth substrate for mass production, should not infect the host plant, or should not produce metabolites that are toxic for consumers; it must be resistant to the commonly used pesticides and well suited with other chemical and physical postharvest treatments (Wilson and Wisniewski, 1989). The efficiency of biocontrol products could be improved during an integrated control strategy to reduce fungicide input (Govender et al., 2005). Biocontrol products, Biosave-100 and Biosave-110, were commercially used as a postharvest treatment especially for apples: Bio-Save [biocontrol agent (b.a) Pseudomonas syringae], Boni Protect (b.a Aureobasidium pullulans), Candifruit (b.a Candida sake), Nexy (b.a Candida oleophila), Pantovital (b.a Pantoea agglomerans), Shemer (b.a Metschnikowia fructicola), and Yield Plus (b.a i. Cryptococcus albidus) (Sharma et al., 2009; Spadaro and Droby, 2016; Janisiewicz and Jurick, 2017). However, commercial biocontrol products are limited to the control of anthracnose decay in avocados. Biocontrol agent Debaryomyces nepalensis completely controlled the anthracnose decay in mangoes at 1×107 cells mL–1 concentration at 15°C for 30 and 40 days (Konsue et al., 2020). Postharvest treatment with D. nepalensis reduced the anthracnose decay by delaying the ripening and senesce of the fruit caused due to an increase in cell membrane permeability and less lipid peroxidation (malondialdehyde content) than the control during storage (Konsue et al., 2020). The yeast, Torulaspora indica DMKU-RP31, controlled the controlling anthracnose and stem-end rot in mango fruits, and the volatiles produced by the yeast strain were responsible for the antifungal property (Konsue et al., 2020). Furthermore, dipping of the mangoes in Metschnikowia pulcherrima suspension (1.0 × 108 cfu mL–1), SA solution (50 mg L–1), or calcium chloride (CaCl2) solution (1.0 g L–1) reduced the anthracnose symptoms in fruit by inducing the defense-related enzymes phenylalanine ammonia-lyase, chitinase, and β-1,3-glucanase compared with the control treatment (Tian et al., 2018). Yeast Meyerozyma caribbica in sodium alginate coating controlled the anthracnose (C. gloeosporioides Pa14) in avocado fruit (Iñiguez-Moreno et al., 2020). The sodium alginate coating helped as a matrix to trap the yeast and the volatile compounds, especially 1-butanol, 3-methyl- and phenethyl alcohol, and ethyl acetate, to arrest the invasion of the pathogen (Iñiguez-Moreno et al., 2020). The alcohols cause destruction of plasma membrane due to accelerated degeneration of protein molecules that eventually affect the metabolism and cell lysis (McDonnell and Russell, 1999). With ripening, the yeast population increased on the fruit surface and provided residual protection against anthracnose (Iñiguez-Moreno et al., 2020). Besides, M. caribbica also showed the mode of action due to competition for nutrients, biofilm development, and production of β-1,3-glucanase and chitinase enzymes (Iñiguez-Moreno et al., 2020).
Furthermore, cell-free supernatant of Bacillus atrophaeus strain B5 inhibited the mycelial growth and conidial germination of C. gloeosporioides in vitro and reduced the severity and incidence of anthracnose disease on avocado fruit. B. atrophaeus strain B5 harbors a gene that expressed the biosynthesis of antibiotics, mainly surfactin, bacillomycin, and iturin, which probably could have shown antifungal activities against C. gloeosporioides (Guardado-Valdivia et al., 2018). Inhibition of spore germination is vital to control the inoculum load at an early stage of decay development. The lipopeptides fengycins and iturins showed a higher antifungal activity than surfactin (Ongena and Jacques, 2008; Meena and Kanwar, 2015). Some lipopeptides (surfactin and other cyclic lipopeptides) were shown to elicit induced systemic resistance (ISR) in the host (Ongena and Jacques, 2008; Raaijmakers et al., 2010; Gond et al., 2015). Since the biocontrol agents do not fall under the category of GRAS compounds, the metabolites in the cell-free supernatants can be of great interest for the control of postharvest decay in avocado, mango, or papaya fruits (Guardado-Valdivia et al., 2018).
Marine yeast Debaryomyces hansenii 1R11CB strain and marine bacteria Stenotrophomonas rhizophila KM02 strain were identified as the suitable antagonistic agents to anthracnose (C. gloeosporioides) in mangoes (‘Ataulfo’) and reduced the decay incidence to 56 and 89%, respectively (Hernandez Montiel et al., 2017). The authors suggest that the possible mechanisms could be (i) antagonists competing for nutrients such as glucose, sucrose, and fructose with C. gloeosporioides due to their rapid growth rate, which affects the infection process of the pathogen by colonizing the hyphae of Colletotrichum; or (ii) by secreting chitinase to degrade β-glucan, mannoprotein, and chitin, respectively, of the cell wall components of C. gloeosporioides (Vero et al., 2013; Liu et al., 2016); or (iii) by inducing the host defense mechanism by triggering the antioxidants, peroxidase, catalase, and superoxide dismutase and of disease-defense enzyme phenylalanine ammonia-lyase, chitinase, and β-1,3-glucanase indirectly and preventing the entry of C. gloeosporioides (Wei et al., 2016; Wu et al., 2019). Furthermore, yeast Wickerhamomyces anomalus reduced the anthracnose (C. gloeosporioides) incidence in avocado fruit and provided effective protection against anthracnose (Campos-Martínez et al., 2016). W. anomalus showed mycoparasitism with C. gloeosporioides hyphae and caused the loss of turgor pressure and eventually yeast gaining entry into the fungal cells via cell walls (Lima N. B. et al., 2013; Campos-Martínez et al., 2016). Table 4 summarizes the recent developments in biocontrol agent with antifungal properties against C. gloeosporioides infecting mangoes and avocados.
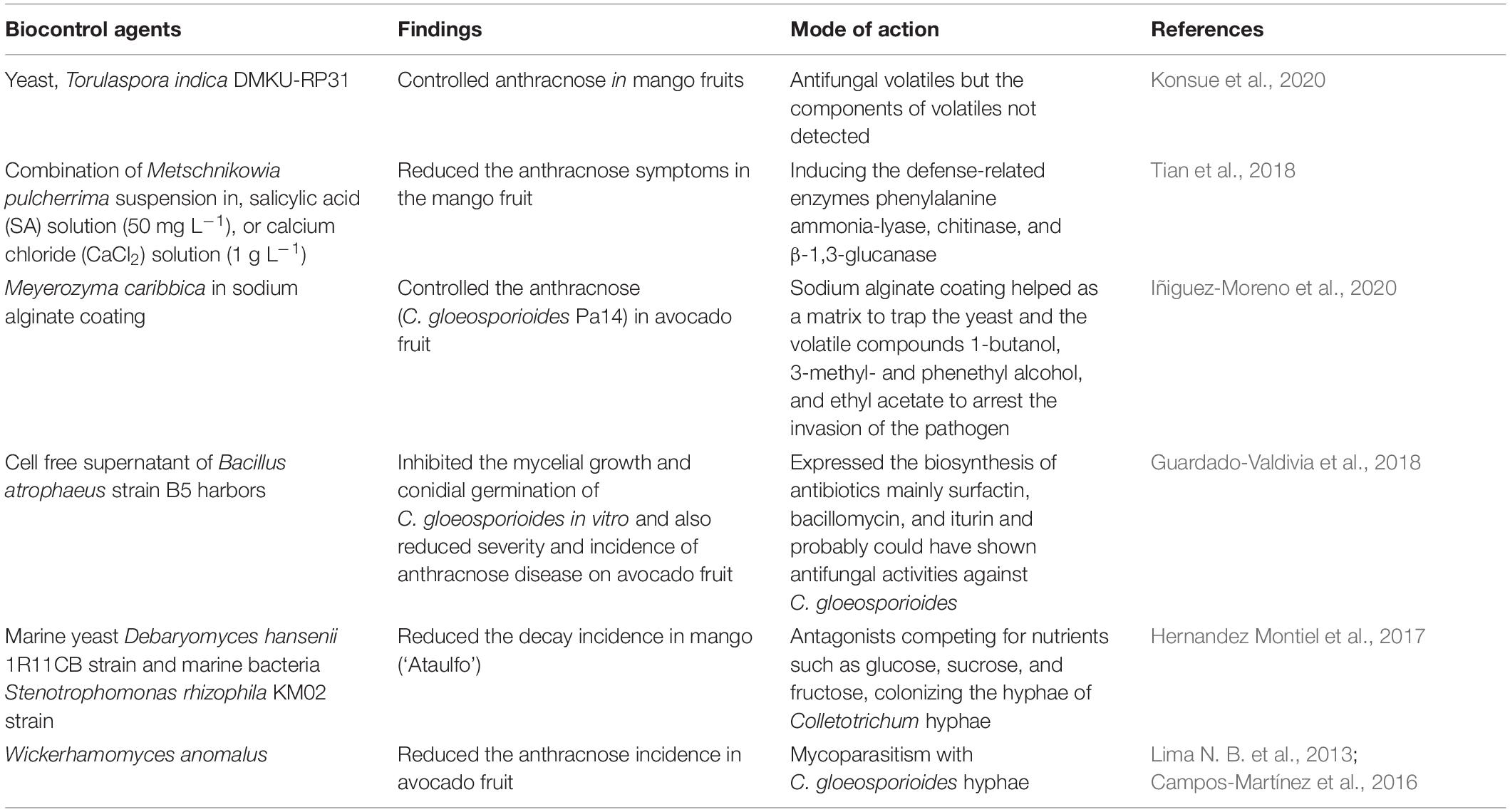
Table 4. Summary of recent developments in biocontrol agent with antifungal properties against Colletotrichum gloeosporioides infecting mango and avocados.
Alternative treatments, edible coatings, films and additives, nanomaterials, essential oils, and biocontrol agents could be more effective during integrated combination via a “hurdle concept” due to their different mechanisms. The alternative products replacing the commercial synthetic fungicides should not affect the fruit quality or sensory properties of the fruit. In addition, during the application, the product should not affect the environment and other organisms that are agriculturally friendly. Furthermore, the practical application, reliability of the product, commercialization, registration, and cost-effectiveness of the treatments are important before recommending the treatments to the avocado, mango, and papaya industries.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The SARChI Research Chair grant for Phytochemical Food Network to Improve Nutritional Quality for Consumers, supported by the NRF (National Research Foundation) grant number 98352, was gratefully acknowledged.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ali, A., Cheong, C. K., and Zahid, N. (2014). Composite effect of propolis and gum arabic to control postharvest anthracnose and maintain quality of papaya during storage. Int. J. Agric. Biol. 16, 1117–1122.
Ali, A., Wee Pheng, T., and Mustafa, M. A. (2015). Application of lemongrass oil in vapour phase for the effective control of anthracnose of ‘Sekaki’ papaya. J. Appl. Microbiol. 118, 1456–1464. doi: 10.1111/jam.12782
Aloui, H., Khwaldia, K., Licciardello, F., Mazzaglia, A., Muratore, G., Hamdi, M., et al. (2014). Efficacy of the combined application of chitosan and locust bean gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int. J. Food Microbiol. 170, 21–28. doi: 10.1016/j.ijfoodmicro.2013.10.017
Applerot, G., Abu-Mukh, R., Irzh, A., Charmet, J., Keppner, H., Laux, E., et al. (2010). Decorating parylene-coated glass with ZnO nanoparticles for antibacterial applications: a comparative study of sonochemical, microwave, and microwave-plasma coating routes. ACS Appl. Mater. Inter. 2, 1052–1059. doi: 10.1021/am900825h
Bambalele, N., Mditshwa, A., Tesfay, S. Z., and Magwaza, L. S. (2021). Moringa leaf extract infused into carboxymethyl cellulose edible coating combined with gaseous ozone as postharvest treatments maintain quality and extend shelf life of mango fruit. Acta Hortic. 1306, 225–232. doi: 10.17660/actahortic.2021.1306.28
Bautista-Baños, S., Sivakumar, D., Bello-Pérez, A., and VillanueVa, R. (2013). A review of the management alternatives for controlling fungi on papaya fruit during the postharvest supply chain. Crop Prot. 49, 8–20. doi: 10.1016/j.cropro.2013.02.011
Beno-Moualem, D., and Prusky, D. B. (2000). Early events during quiescent infection development by Colletotrichum gloeosporioides in unripe avocado fruits. Phytopathology 90, 553–559. doi: 10.1094/phyto.2000.90.5.553
Bill, M., Korsten, L., Remize, F., Glowacz, M., and Sivakumar, D. (2017). Effect of thyme oil vapours exposure on phenylalanine ammonia-lyase (PAL) and lipoxygenase (LOX) genes expression, and control of anthracnose in ‘Hass’ and ‘Ryan’ avocado fruit. Sci. Hortic. 224, 232–237. doi: 10.1016/j.scienta.2017.06.026
Campos-Martínez, A., Velázquez-del Valle, M. G., Flores-Moctezuma, H. E., Suárez-Rodríguez, R., Ramírez-Trujillo, J. A., et al. (2016). Antagonistic yeasts with potential to control Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. and Colletotrichum acutatum J.H. Simmonds on avocado fruits. Crop Prot. 89, 101–104. doi: 10.1016/j.cropro.2016.07.001
Cenobio-Galindo, A. J., Juan, O., Abigail, R.-M., and María, L. C. (2019). Influence of bioactive compounds incorporated in a nanoemulsion as coating on avocado fruits (Persea americana) during postharvest storage: antioxidant activity. Physicochem. Chang. Struct. Eval. Antioxid. 8:500. doi: 10.3390/antiox8100500
Chillet, M., Minier, J., Ducrog, M., and Meile, J. C. (2018). Postharvest treatment of mango: potential use of essential oil with thymol to control anthracnose development. Fruits 73, 153–157. doi: 10.17660/th2018/73.3.2
Chowdappa, P., Shivakumar, G. C., Chethana, S., and Madhura, S. (2014). Antifungal activity of chitosan-silver nanoparticle composite against Colletotrichum gloeosporioides associated with mango anthracnose. Afr. J. Microbiol. Res. 8, 1803–1812. doi: 10.5897/ajmr2013.6584
EU COM (2020). European Commission Brussels, 20.5.2020. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions a Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. Available Online at: https://ec.europa.eu/info/sites/default/files/communication-annex-farm-fork-green-deal_en.pdf (accessed May 14, 2021).
Correa-Pacheco, Z. N., Bautista-Baños, S., Valle-Marquina, M. A., and Hernández-López, M. (2017). The effect of nanostructured chitosan and chitosan-thyme essential oil coatings on Colletotrichum gloeosporioides growth in vitro and on cv Hass avocado and fruit quality. J. Phytopathol. 165, 297–305. doi: 10.1111/jph.12562
De la Rosa-García, S. C., Martínez-Torres, P., Gómez-Cornelio, S., Corral-Aguado, M. A., Quintana, P., and Gómez-Ortíz, M. (2018). Antifungal activity of ZnO and MgO nanomaterials and their mixtures against Colletotrichum gloeosporioides strains from tropical fruit. J. Nanomater. 2018:3498527.
Dias, B. L., Costa, P. F., Dakin, M. S., de Souza Carlos Mou rao, D., Dias, F. R., de Sousa, R. R., et al. (2020). Control of papaya fruits anthracnose by essential oils of medicinal plants associated to different coatings. J. Med. Plants Res. 14, 239–246. doi: 10.5897/jmpr2019.6838
Dickman, M. B. (1994). “Papaya anthracnose,” in Compendium of Tropical Fruit Diseases, eds R.C. Ploetz, G.A. Zentmyer, W.T. Nishijima, K.G. Rohrbach, and H.D. Ohr (Minneapolis, MN: American Phytopathological Society), 58–59.
EFSA (2011). European Food Safety Authority (EFSA) scientific committee. scientific opinion on guidance on the risk assessment of the application of nanoscience and nanotechnology in the food and feed chain. EFSA J. 9:2140. doi: 10.2903/j.efsa.2011.2140
Elsabee, M. Z., and Abdou, E. S. (2013). Chitosan based edible films and coatings: a review. Mat. Sci. Eng. C 33, 1819–1841. doi: 10.1016/j.msec.2013.01.010
EPA (2021). United States Environmental Agency. Integrated Risk Information System (IRIS). Available Online at: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=122. (accessed May 13, 2020).
Esquivel-Chávez, F., Colín-Chávez, C., Virgen-Ortiz, J. J., Martínez-Téllez, M. A., Avena-Bustillos, R. J., Peña-Madrigal, G., et al. (2021). Control of mango decay using antifungal sachets containing of thyme oil/modified starch/agave fructan microcapsules. Future Foods 3:100008. doi: 10.1016/j.fufo.2020.100008
FAOSTAT (2021). Food and Agricultural Organization of the United Nations. Available Online at: http://www.fao.org/faostat/en/#data/QC (accessed May 13, 2021)
FDA (2021). United States Food & Drug Administration, CFR-Code of Federal Regulations Title 21. Available Online at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=182&showFR=1 (accessed April 21, 2021)
Flores-López, M. L., Cerqueira, M. A., de Rodríguez, D. J., and Vincente, A. A. (2016). Perspectives on utilization of edible coatings and nano-laminate coatings for extension of postharvest storage of fruits and vegetables. Food Eng. Rev. 8, 292–305. doi: 10.1007/s12393-015-9135-x
Garcia, F., and Davidov-Pardo, G. (2021). Recent advances in the use of edible coatings for preservation of avocados: a review. J. Food Sci. 86, 6–15. doi: 10.1111/1750-3841.15540
Gómez-Ríos, D., Barrera-Zapata, R., and Ríos-Estepa, R. (2017). Comparison of process technologies for chitosan production from shrimp shell waste: a techno-economic approach using Aspen Plus®. Food Bioprod. Process. 103, 49–57. doi: 10.1016/j.fbp.2017.02.010
Gond, S. K., Bergen, M. S., Torres, M. S., and White, J. F. (2015). Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 172, 79–87. doi: 10.1016/j.micres.2014.11.004
Govender, V., Korsten, L., and Sivakumar, D. (2005). Semi-commercial evaluation of Bacillus licheniformis to control mango postharvest diseases in South Africa. Postharvest Biol. Technol. 38, 57–65. doi: 10.1016/j.postharvbio.2005.04.005
Guardado-Valdivia, L., Tovar-Pérez, E., Chacón-López, A., López-García, U., Gutiérrez-Martínez, P., Stoll, A., et al. (2018). Identification and characterization of a new Bacillus atrophaeus strain B5 as biocontrol agent of postharvest anthracnose disease in soursop (Annona muricata) and avocado (Persea americana). Microbiol. Res. 210, 26–32. doi: 10.1016/j.micres.2018.01.007
Henriod, R., Diczbalis, Y., Sole, D., Stice, K. N., and Tora, L. (2016). Investigation into various fungicides and alternative solutions for controlling postharvest diseases in papaya fruit. Acta Hortic. 1111, 113–118. doi: 10.17660/actahortic.2016.9789462611054.17
Hernandez Montiel, L. G., Zulueta Rodriguez, R., Angulo, C., Rueda Puente, E. O., Quiñonez Aguilar, E. E., and Galicia, R. (2017). Marine yeasts and bacteria as biological control agents against anthracnose on mango. J. Phytopathol. 165, 833–840. doi: 10.1111/jph.12623
Iñiguez-Moreno, M., Ragazzo-Sánchez, J. A., Barros-Castillo, J. C., Sandoval-Contreras, T., and Calderón-Santoyo, M. (2020). Sodium alginate coatings added with Meyerozyma caribbica: postharvest biocontrol of Colletotrichum gloeosporioides in avocado (Persea americana Mill. cv. Hass). Postharvest Biol. Techol. 163:111123. doi: 10.1016/j.postharvbio.2020.111123
Janisiewicz, W. J., and Jurick, W. M. (2017). “Sustainable approaches to control postharvest diseases of apples,” in Achieving Sustainable Cultivation of Apples, ed. K. Evans (Washington: Burleigh Dodds Science Publishing), 307–336. doi: 10.19103/as.2016.0017.15
Jin, P., Wang, H., Zhang, Y., Huang, Y., Wang, L., and Zheng, Y. (2017). UV-C enhances resistance against gray mold decay caused by Botrytis cinerea in strawberry fruit. Sci. Horticult. 225, 106–111. doi: 10.1016/j.scienta.2017.06.062
Jin, P., Wu, X., Xu, F., Wang, X., Wang, J., and Zheng, Y. (2012). Enhancing antioxidant capacity and reducing decay of Chinese bayberries by essential oils. J. Agric. Food Chem. 60, 3769–3775. doi: 10.1021/jf300151n
Kaewklin, P., Siripatrawan, U., Suwanagul, A., and Lee, Y. S. (2018). Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 112, 523–529. doi: 10.1016/j.ijbiomac.2018.01.124
Karunanayake, K. O. L. C., Liyanage, K. C. M., Jayakody, L. K. R. R., and Somaratne, S. (2020). Basil oil incorporated beeswax coating to increase shelf life and reduce anthracnose develop-ment in mango cv. Willard. Ceylon J. Sci. 49, 355–361. doi: 10.4038/cjs.v49i5.7802
Kesavan Pillai, Sivakumar, D., Sinha Ray, S., Obianom, P., Eggers, S., and Mhlabeni, T. (2020). “Active nanocomposite films based on low density polyethylene/organically modified layered double hydroxides/thyme oil to retain retail shelf life and quality of hass avocados [Conference presentation],” in AIP Conference Proceedings, (College Park, MD: American Institute of Physics).
Konsue, W., Dethoup, T., and Limtong, S. (2020). Biological control of fruit rot and anthracnose of postharvest mango by antagonistic yeasts from economic crops leaves. Microorganisms 8:317. doi: 10.3390/microorganisms8030317
Kubheka, S. F., Tesfay, S. Z., Mditshwa, A., and Magwaza, L. S. (2021). Efficacy of carboxymethyl cellulose and gum arabic edible coatings in combination with moringa leaf extract in improving postharvest quality of ‘Maluma’ avocado fruit. Acta Hortic. 1306, 293–300. doi: 10.17660/actahortic.2021.1306.37
Kumaraswamy, R. V., Kumari, S., Choudhary, R. C., Sharma, S. S., Pal, A., Raliya, R., et al. (2019). Salicylic acid functionalized chitosan nanoparticle: a sustainable biostimulant for plant. Int. J. Biol. Macromol. 123, 59–69. doi: 10.1016/j.ijbiomac.2018.10.202
Lima, J. R., Gondim, D. M. F., Oliveira, J. T. A., Oliveira, F. S. A., Gonçalves, L. R. B., and Viana, F. M. P. (2013). Use of killer yeast in the management of postharvest papaya anthracnose. Postharvest Biol. Technol. 83, 5–64.
Lima, N. B., de, A., Batista, M. V., De Morais Jr. M. A., Barbosa, M. A. G., Michereff, S. J., et al. (2013). Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil. Fungal Divers. 61, 75–88. doi: 10.1007/s13225-013-0237-6
Liu, Y., Wang, R., Cao, Y., Chen, C., Bai, F., Xu, T., et al. (2016). Identification and antagonistic activity of endophytic bacterial strain Paenibacillus sp. 5 L8 isolated from the seeds of maize (Zea mays L. Jingke 968). Ann. Microbiol. 66, 653–660. doi: 10.1007/s13213-015-1150-x
Ma, Y., Fu, L., Hussain, Z., Huang, D., and Zhu, S. (2019). Enhancement of storability and antioxidant systems of sweet cherry fruit by nitric oxide-releasing chitosan nanoparticles (GSNO-CS NPs). Food Chem. 285, 10–21. doi: 10.1016/j.foodchem.2019.01.156
Majeed, H., Bian, Y., Ali, B., Jamil, A., Majeed, U., Khan, Q. F., et al. (2015). Essential oil encapsulations: uses, procedures, and trends. RSC Adv. 5, 58449–58463.
Marques, K. M., Galati, V. C., Fernandes, J. D. R., Guimarães, J. E. R., Silva, J. P., Mattiuz, B. H., et al. (2016). Use of chitosan for the control of postharvest anthracnose and quality in avocados. Acta Hortic. 1120, 225–232. doi: 10.17660/actahortic.2016.1120.34
McDonnell, G., and Russell, A. D. (1999). Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12, 147–179. doi: 10.1128/cmr.12.1.147
Meena, K. R., and Kanwar, S. S. (2015). Lipopeptides as antifungal and antibacterial agents: applications in food safety and therapeutics. BioMed Res. Int. 2015:473050.
Meena, M., Pilania, S., Pal, A., Mandhania, S., Bhushan, B., Kumar, S., et al. (2020). Cu-chitosan nano-net improves keeping quality of tomato by modulating physio-biochemical responses. Sci. Rep. 10:21914.
Mohammadi, A., Hashemi, M., and Hosseini, S. M. (2015). Postharvest treatment of nanochitosan-based coating loaded with Zataria multiflora essential oil improves antioxidant activity and extends shelf life of cucumber. Innov. Food Sci. Emerg. 33, 580–588. doi: 10.1016/j.ifset.2015.10.015
Nelson, S. (2008). Anthracnose of Avocado. College of Tropical Agriculture and Human Resources (CTAHR) Publication, 1-6. Available Online at: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/pd-58.pdf. (accessed March 07, 2021)
Nguyen, V. T. B., Nguyen, D. H. H., and Nguyen, H. V. H. (2020). Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biol. Technol. 162:111103. doi: 10.1016/j.postharvbio.2019.111103
Obianom, C., and Sivakumar, D. (2018). Differential response to combined Prochloraz and thyme oil drench treatment in avocados against the control of anthracnose and stem-end rot. Phytoparasitica 46, 273–281. doi: 10.1007/s12600-018-0663-9
Obianom, C., Romanazzi, G., and Sivakumar, D. (2019). Effects of chitosan treatment on avocado postharvest diseases and expression of phenylalanine ammonia-lyase, chitinase and lipoxygenase genes. Postharvest Biol. Technol. 147, 214–221. doi: 10.1016/j.postharvbio.2018.10.004
Ochoa-Velasco, C. E., Pérez-Pérez, J. C., Varillas-Torres, J. M., Navarro-Cruz, A. R., Hernández-Carranza, P., Munguía-Pérez, R., et al. (2021). Starch edible films/coatings added with carvacrol and thymol: in vitro and in vivo evaluation against Colletotrichum gloeosporioides. Foods 10:175. doi: 10.3390/foods10010175
Ongena, M., and Jacques, P. (2008). Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16, 115–125. doi: 10.1016/j.tim.2007.12.009
Raaijmakers, J. M., De Bruijn, I., Nybroe, O., and Ongena, M. (2010). Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol. Rev. 34, 1037–1062. doi: 10.1111/j.1574-6976.2010.00221.x
Rajestary, S., Landi, L., and Romanazzi, G. (2021). Chitosan and postharvest decay of fresh fruit: meta-analysis of disease control and antimicrobial and eliciting activities. Compr. Rev. Food Sci. Food Saf. 20, 563–582. doi: 10.1111/1541-4337.12672
Romanazzi, G., Feliziani, E., and Sivakumar, D. (2018). Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: eliciting, antimicrobial and film-forming properties. Front. Microbiol. 9:2745.
Romanazzi, G., Sanzani, S. M., Bi, Y., Tian, S., Gutiérrez Martínez, P., and Alkan, N. (2016). Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 122, 82–94. doi: 10.1016/j.postharvbio.2016.08.003
Saharan, V., Sharma, G., Yadav, M., Choudhary, M. K., Sharma, S. S., Pal, A., et al. (2015). Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int. J. Biol. Macromol. 75, 346–353. doi: 10.1016/j.ijbiomac.2015.01.027
Sanders, G. M., Korsten, L., and Wehner, F. C. (2000). Market survey of postharvest diseases and incidence of Colletotrichum gloeosporioides on avocado and mango fruit in South Africa. Trop. Sci. 40, 192–198.
Sarkosh, A., Schaffer, B., Vargas, A. I., Palmateer, A. J., Lopez, P., Soleymani, A., et al. (2018). Antifungal activity of five plant-extracted essential oils against anthracnose in papaya fruit. Biol. Agric. Hortic. 34, 18–26. doi: 10.1080/01448765.2017.1358667
Schoeny, R. (2010). Disinfection by-products: a question of balance. Environ. Health Perspect. 118, A466–A467.
Sharma, R. R., Singh, D., and Singh, R. (2009). Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol. Control 50, 205–221. doi: 10.1016/j.biocontrol.2009.05.001
Shimshoni, J. A., Bommuraj, V., Chen, Y., Sperling, R., Barel, S., Feygenberg, O., et al. (2020). Postharvest fungicide for avocado fruits: antifungal efficacy and peel to pulp distribution kinetics. Foods 9:124. doi: 10.3390/foods9020124
Siddiqui, Y., and Ali, A. (2014). “Colletotrichum gloeosporioides (Anthracnose) (ED Bautista-Banos),” in Post Harvest Decay: Control Strategies, ed. S. Bautista-Banos. Amsterdam: Elsevier.
Sivakumar, D., and Bautista-Baños, S. (2014). A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 64, 27–37. doi: 10.1016/j.cropro.2014.05.012
Song, H., Yuan, W., Jin, P., Wang, W., Wang, X., Yang, L., et al. (2016). Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Biol. Technol. 119, 41–48. doi: 10.1016/j.postharvbio.2016.04.015
Spadaro, D., and Droby, S. (2016). Development of biocontrol products for postharvest diseases of fruit: the importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 47, 39–49. doi: 10.1016/j.tifs.2015.11.003
Swart, S. H., Serfontein, J. J., Swart, G., and Labuschagne, C. (2009). Chemical control of post-harvest diseases of mango: the effect of fludioxonil and prochloraz on soft brown rot, stem-end rot and anthracnosE. Acta Hortic. 820, 503–510. doi: 10.17660/actahortic.2009.820.64
Swinburne, T. R. (1983). “Quiescent infections in post-harvest diseases,” in Post Harvest Pathology of Fruits and Vegetables, ed. C. Dennis (London: Academic Press).
Tesfay, S. Z., Magwaza, L. S., Mbili, N., and Mditshwa, A. (2017). Carboxyl methylcellulose (CMC) containing moringa plant extracts as new postharvest organic edible coating for Avocado (Persea americana Mill.) fruit. Sci. Hortic. 226, 201–207. doi: 10.1016/j.scienta.2017.08.047
Tian, Y. Q., Li, W., Jiang, Z. T., Jing, M. M., and Shao, Y. Z. (2018). The preservation effect of Metschnikowia pulcherrima yeast on anthracnose of postharvest mango fruits and the possible mechanism. Food Sci. Biotechnol. 27, 95–105. doi: 10.1007/s10068-017-0213-0
Transparency Market Research Report (2021). Global Avocado Market to Reach US$21.56 bn by 2026, Increasing Health Consciousness Among People to Promote Growth. Available Online at: https://www.transparencymarketresearch.com/pressrelease/avocado-market.htm (accessed March 01, 2021)
Velázquez-Del Valle, M. G., Hernández-Lauzardo, A. N., Guerra-Sanchez, G., and Mariaca-Gaspar, G. I. (2012). Chitosan as an alternative to control phytopathogenic fungi on fruits and vegetables in Mexico. Afr. J. Microbiol. Res. 6, 6606–6611.
Vero, S., Garmendia, G., Belén González, M., Bentancur, O., and Wisniewski, M. (2013). Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple (Malus × domestica). FEMS Yeast Res. 13, 189–199. doi: 10.1111/1567-1364.12021
Wei, Y., Xu, M., Wu, H., Tu, S., Pan, L., and Tu, K. (2016). Defense response of cherry tomato at different maturity stages to combined treatment of hot air and Cryptococcus laurentii. Postharvest Biol. Technol. 117, 177–186. doi: 10.1016/j.postharvbio.2016.03.001
Wilson, C. L., and Wisniewski, M. E. (1989). Biological control of postharvest diseases of fruits and vegetables: an emerging technology. Annu. Rev. Phytopathol. 27, 425–441. doi: 10.1146/annurev.py.27.090189.002233
Wu, S., Zhen, C., Wang, K., and Gao, H. (2019). Effects of Bacillus subtilis CF-3 VOCs combined with heat treatment on the control of Monilinia fructicola in peaches and Colletotrichum gloeosporioides in litchi fruit. J. Food Sci. 84, 3418–3428. doi: 10.1111/1750-3841.14949
Keywords: chitosan, edible coatings, essential oil vapors, latent infections, biocontrol agents
Citation: Sivakumar D, Tuna Gunes N and Romanazzi G (2021) A Comprehensive Review on the Impact of Edible Coatings, Essential Oils, and Their Nano Formulations on Postharvest Decay Anthracnose of Avocados, Mangoes, and Papayas. Front. Microbiol. 12:711092. doi: 10.3389/fmicb.2021.711092
Received: 17 May 2021; Accepted: 07 July 2021;
Published: 30 July 2021.
Edited by:
Khamis Youssef, Agricultural Research Center, EgyptReviewed by:
Asgar Ali, University of Nottingham Malaysia Campus, MalaysiaCopyright © 2021 Sivakumar, Tuna Gunes and Romanazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dharini Sivakumar, U2l2YWt1bWFyREB0dXQuYWMuemE=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.