
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 13 September 2021
Sec. Food Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.708189
 Rosana Basso Kraus1
Rosana Basso Kraus1 Pedro Rassier dos Santos2
Pedro Rassier dos Santos2 Amanda Krummenauer3
Amanda Krummenauer3 Kevin Eduardo Palhares4
Kevin Eduardo Palhares4 Helenice Gonzalez de Lima5
Helenice Gonzalez de Lima5 Sílvia Regina Leal Ladeira6
Sílvia Regina Leal Ladeira6 Giselda Maria Pereira7
Giselda Maria Pereira7 Giniani Carla Dors8
Giniani Carla Dors8 Patrícia da Silva Nascente2*
Patrícia da Silva Nascente2* Rafael Guerra Lund1*
Rafael Guerra Lund1*Bovine colostrum silage (BCS) is a technique used by milk producers for the conservation of bovine colostrum. However, it is necessary to ensure the safety and quality of BCS, as this food will be supplied to the animals. This study aimed to compare the physicochemical and microbiological compositions of colostrum silage at different fermentation times with milk and bovine colostrum (BC) quality parameters. BC samples were obtained from Jersey animals from one dairy farm. The BC samples (n = 21) were placed in 500-mL plastic bottles, stored vertically and anaerobically fermented for periods of 61–437 days. The following parameters of the physicochemical composition of the BCS were evaluated: acidity, protein, total solids and ash, using the methodologies of Adolfo Lutz Institute (2008). The microbiological analysis was developed according to the methodology proposed by Saalfeld et al. (2013), with adaptations. The acidity, total solids and protein over fermentation time (group 1: 61 to 154, group 2: 200 to 273, and group 3: 280 to 437 days) were not significantly different (P > 0.05). The ash content was significantly different (P < 0.05) in groups 1 and 3 and showed a decrease (moderate negative correlation of −0.63) with increasing fermentation time. Positive correlations were observed between total solids and the protein and ash contents. The genus of microorganisms with the highest occurrence was Lactobacillus spp. (95.2% of BCS) and those of lesser occurrence included Escherichia spp., Actinomadura spp., Streptococcus spp. and Leuconostoc spp. (4.8% of BCS). BCS has a physicochemical composition similar to BC and showed changes during the fermentation period; however, the presence of pathogenic microorganisms in BCSs reinforces the need to further explore the quality parameters for BCS to ensure the safety of animals who receive this food.
- Lactobacillus spp. were present in bovine colostrum silages for up to 437 days.
- This is the first study that compares the physicochemical parameters of milk and BC quality with colostrum silage.
- In this study, microbiological determinations were obtained from the fermentation of colostrum in natura.
- A non-significant correlation was found between the degree of acidity and the number of bacterial species.
Bovine colostrum (BC) is the first fluid produced postpartum (Solomons, 2002), and its main function is to provide immunity, increase physiological performance and provide better growth and development to newborn animals (Hurley and Theil, 2011). The composition of BC is superior when compared to the composition of milk in terms of the contents of lipids (BC: 4.6–6.7%; milk: 3.0%), protein (BC: 12.7–18.5%; milk: 3.1%), lactose (BC: 2.0–2.9%; milk: 4.5–5.2%), and total solids (BC: 14.1–27.2%; milk: 12.9%) (Morrill et al., 2012; Saalfeld et al., 2013; Mourad et al., 2014; Zarei et al., 2017). In addition, the presence of immunoglobulins, lactoferrins, lysozyme, leukocytes, cytokines and other immunomodulatory factors stands out. It is important to note that the physicochemical composition of BC differs according to the breed of animal and over the days in which it is excreted (Marnila and Korhonen, 2011; Morrill et al., 2012).
For many reasons, many newborn ruminants do not have access to their mothers’ colostrum immediately after birth: multiple births, acute mastitis, maladaptive maternal behavior mainly at first birth, etc., (Wereme et al., 2001). Therefore, it is necessary to create a colostrum bank for newborn calves that cannot be fed by their own mothers immediately after birth and for the preparation of immune colostrum products that can be used as food supplements or colostrum substitutes to provide effective protection against different enteric diseases in calves and humans (Alexieva et al., 2004).
In addition, dairy cows produce more colostrum than their own calves can consume (El-Fattah et al., 2014), and the concentrations of colostrum immunoglobulin (Ig), for example, are higher in the first postpartum milking and decrease rapidly over time (Alexieva et al., 2004). BC can be stored under refrigeration for up to 91 days (Ramya et al., 2016) to maintain chemical characteristics; however, this method of storage involves expenses with equipment and electricity consumption and affects the concentration of Ig.
Some methods for the preservation of BC have already been investigated: commercial sterilization, pasteurization, freezing, lyophilization (El-Fattah et al., 2014), vacuum evaporation, microwaving, spray drying (Chelack et al., 1993), refrigeration (Ramya et al., 2016), chemical preservatives (Muller and Smallcomb, 1977), drying (Borad and Singh, 2018), membrane filtration, ultrafiltration, microfiltration, high pressure (Gosch et al., 2014), pulsed electric field (Li et al., 2005), and irradiation (Pereira et al., 2014). These techniques have the advantage of preserving BC for a period of time ranging from 21 days to 3 months, but the main disadvantages are physicochemical changes, destruction of nutritional components (Borad and Singh, 2018), cost and the need for equipment.
The anaerobic fermentation of BC, called bovine colostrum silage (BCS), is a conservation method that has been studied and used (Ferreira et al., 2013; Saalfeld et al., 2013; Azevedo et al., 2014). This method allows BC storage for periods of up to 2 years; is a simple, low-cost process that does not require refrigeration, freezing or the use of additives; and can be used to feed calves (Saalfeld et al., 2013). In addition, BCS provides the transfer of immunoglobulin to calves in a similar manner to BC in natura (Saalfeld et al., 2014).
The objective of this study was to compare the physicochemical and microbiological compositions of colostrum silage at different fermentation times.
Bovine colostrum samples were obtained from Jersey animals (n = 21) from one dairy farm. The property is located in the city of Pelotas, state of Rio Grande do Sul, Brazil. The collections were carried out from March 2018 to April 2019. Table 1 shows the composition data and the somatic cell count (SCC) of milk obtained from the same animal after the end of colostrum production (International Dairy Federation, 2000, 2006).
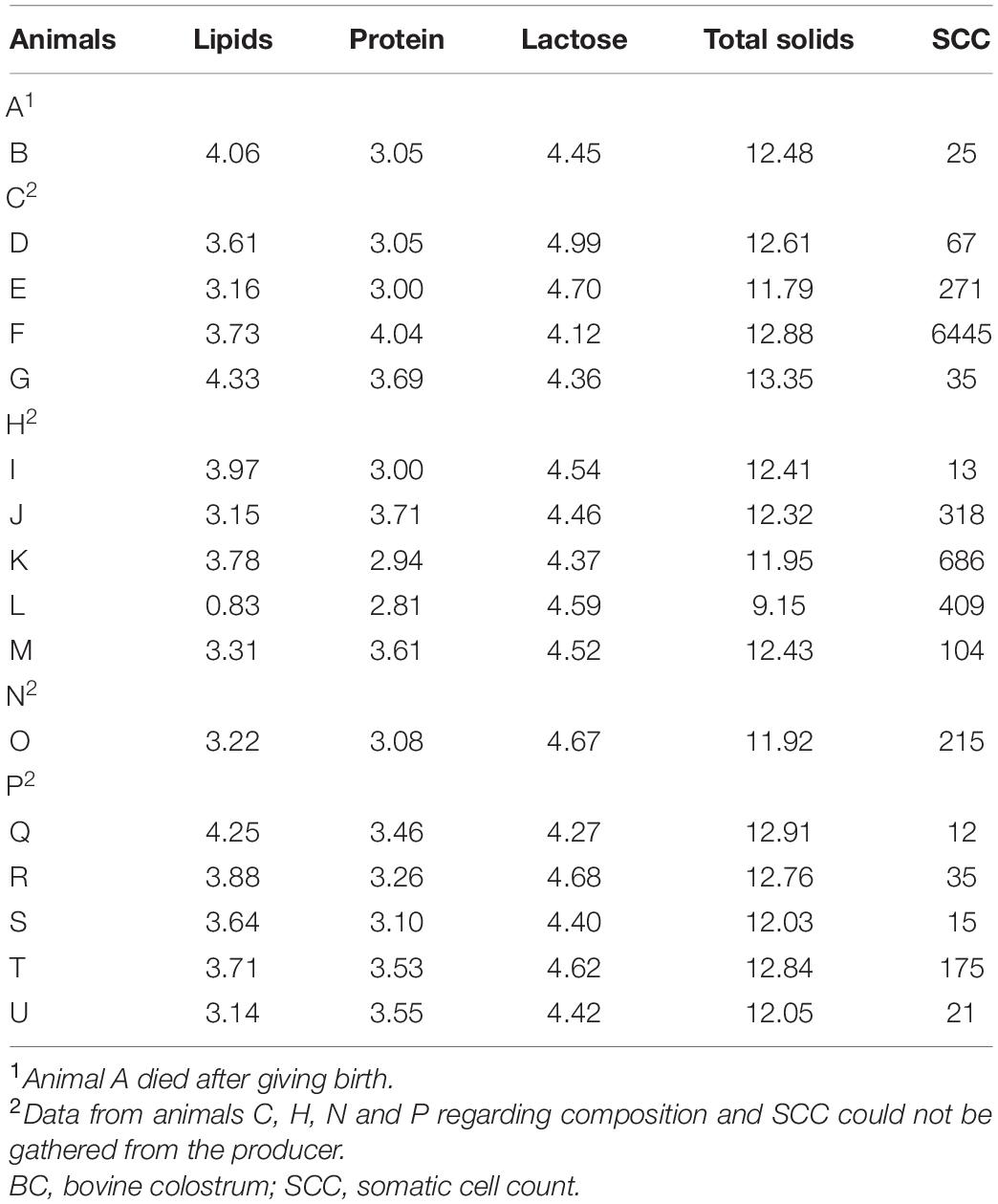
Table 1. Physicochemical composition and somatic cell count of milk produced after BC by Jersey animals (n = 21) on a dairy farm located in southern Brazil in the city of Pelotas.
The BC samples (n = 21) were placed in 500-mL plastic bottles, stored vertically in a dry environment with natural light (light/dark), and anaerobically fermented for the period shown in Supplementary Table 1 (minimum of 61 days and a maximum of 437 days). Supplementary Table 1 also presents the animal information. Supplementary Table 2 shows the average temperature in each fermentation period.
Shortly after the fermentation period, the samples of BCS were removed from the containers, homogenized in a blender for 30 s, transferred to sterile 80-mL plastic containers and stored at −4°C until the time of analysis.
The following parameters of the physicochemical composition of the silage were evaluated: acidity (No. 427/IV), protein (No. 037/IV), total solids (No. 429/IV), and ash (No. 437/IV), using the methodologies of Adolfo Lutz Institute (2008). The physicochemical analyses were performed in duplicate.
The microbiological analysis was developed according to the methodology proposed by Saalfeld et al. (2013), with adaptations. BCS was sown in Chapman agar (Kasvi, Brazil), MacConkey agar (Himedia, France) and brain heart infusion agar (Kasvi, Brazil) as culture media. Thereafter, the plates were aerobically incubated at 37°C for 72 h. Then, the identification of isolated microorganisms was carried out using the Gram stain technique and biochemical tests (Barrow and Feltham, 1993). The presumptive identification of Lactobacillus spp. was performed on selective agar for Lactobacillus (Acumedia, United States), only observing the growth of these microorganisms.
The Kruskal–Wallis test, Dunn’s posttest and Spearman’s correlation analyses of variance were performed to determine the influence of the fermentation time (group 1: 61 to 154, group 2: 200 to 273, and group 3: 280 to 437 days) on the variables acidity, total solids, protein and ash. The Kruskal–Wallis test was used to assess the relationship between the acidity content (group 1: 7.5 to 19, group 2: 21 to 26.5, and group 3: 29 to 35°D) and the genera of the microorganisms identified. Statistical analyses were performed in BioEstat software and in the R environment, with a 95% significance level (P < 0.05).
Table 2 shows the physicochemical compositions of BCS at different fermentation times. The acidity contents ranged from 10.5 to 33.5°D, 11.5 to 35°D, and 8 to 30°D, with averages of 20.8, 22.7, and 20.8°D in groups 1, 2, and 3 (61 to 154, 200 to 273, and 280 to 437 days), respectively. The protein contents obtained ranged from 5.1 to 13.2%, 4.3 to 14%, and 3.5 to 13.7%, with averages of 10.9, 9.5, and 7.3% in groups 1, 2, and 3 (61 to 154, 200 to 273, and 280 to 437 days), respectively. The total solids reached percentages of 8.1 to 22.3%, 8 to 21%, and 6.8 to 19.5%, with averages of 16.7, 14.2, and 11.6% in groups 1, 2, and 3 (61 to 154, 200 to 273, and 280 to 437 days), respectively. The ash contents varied from 1.19 to 1.33%, 1.03 to 1.29%, and 0.45 to 1.27%, with averages of 1.29, 1.17, and 0.98% in groups 1, 2, and 3 (61 to 154, 200 to 273, and 280 to 437 days), respectively.
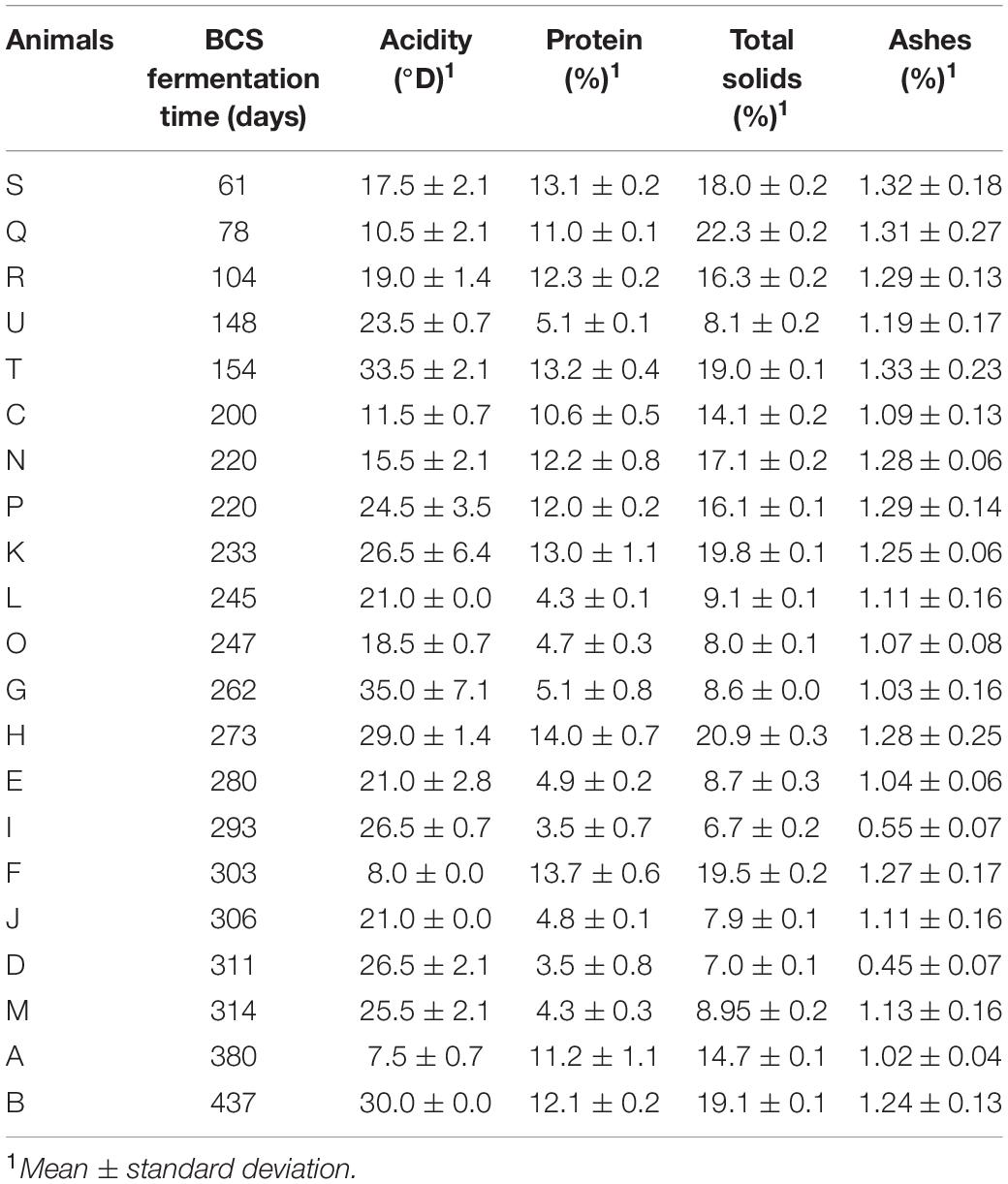
Table 2. Physicochemical composition of BCS resulting from the fermentation of BC of Jersey animals (n = 21) from one dairy farm located in southern Brazil in the city of Pelotas.
When analyzing the averages of the fermentation time groups, it was noted that the acidity increased until the fermentative time of group 2 (200–273 days), followed by a reduction in group 3 (280–437 days), and the percentages of protein, total solids and ash decreased over the fermentation time. The present work found variations in the acidity, protein, total solids and ash results, and this behavior may be related to the longer fermentation period.
When comparing the different fermentation time groups (group 1: 61 to 154, group 2: 200 to 273, and group 3: 280 to 437 days) for the variables acidity, total solids and protein, no significant differences were observed (P > 0.05) (Table 3), meaning that these components were not influenced by fermentation time. A significant difference was observed in ash content (P < 0.05) in groups 1 and 3 (61 to 154 days and 280 to 437 days, respectively), decreasing (moderate negative correlation of −0.63) with increasing fermentation time. When verifying the influence between the studied variables and the fermentation time (group 1: 61 to 154, group 2: 200 to 273, and group 3: 280 to 437 days), it was observed that the total solids (0.77) and protein percentage (0.76) were positively correlated with the ash content, indicating that as the ash concentration increased, so did the total solids and protein percentage. There was also a positive correlation (0.85) between protein concentration and the total solids: as the protein concentration increased, the total solids increased.
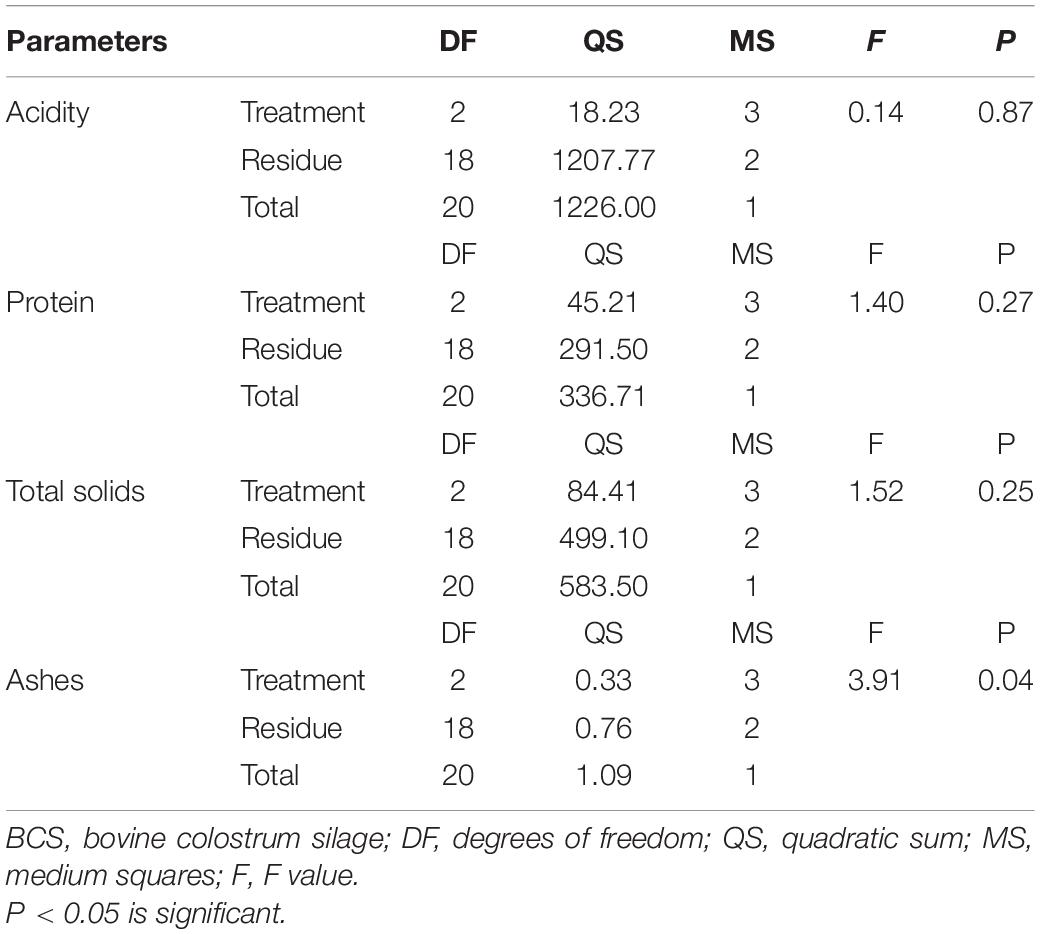
Table 3. Analysis of variance of protein, ash, total solids, and acidity concentrations at different BCS fermentation times.
When relating the physical–chemical composition of milk obtained after the production of BC with the parameters of milk quality through Normative Instruction numbers 76 and 77 of 2018 (BRAZIL, 2018a,b), it is possible to state that of the 21 milk samples, only one sample did not meet the requirements for the levels of lipids, protein and total solids; two samples did not present the minimum lactose concentration; and all samples were within the limits established for the SCC.
Supplementary Table 3 identifies the microorganisms isolated in BCS at different fermentation times. Lactobacillus spp. Were isolated in 20 of the 21 silages; in addition, from 61 to 200 days of fermentation, the microorganisms identified were Staphylococcus spp., Lactobacillus spp., Escherichia spp., Enterococcus spp. And Bacillus spp. From 220 to 273 days, other genera of organisms appeared, such as Corynebacterium spp., and the genera Staphylococcus spp. and Lactobacillus spp. continued to grow. From 280 to 437 days, the organisms identified were Actinomadura spp., Leuconostoc spp., Lactococcus spp., and Streptococcus spp.; Staphylococcus spp., Lactobacillus spp. and Corynebacterium spp. still remained, and, again, the presence of Bacillus spp. and Enterococcus spp. was identified, as in the period from 61 to 200 days.
The genus of microorganisms with the highest occurrence was Lactobacillus spp. (95.2% of BCS), and the genera of lesser occurrence were Escherichia spp., Actinomadura spp., Streptococcus spp. and Leuconostoc spp. (4.8% of BCS), as illustrated in Figure 1. There was a reduction in the number of genera of microorganisms isolated from BCS, but it was not possible to observe a significant difference (P > 0.05) between the acidity groups (group 1: 7.5 to 19, group 2: 21 to 26.5, and group 3: 29 to 35°D).
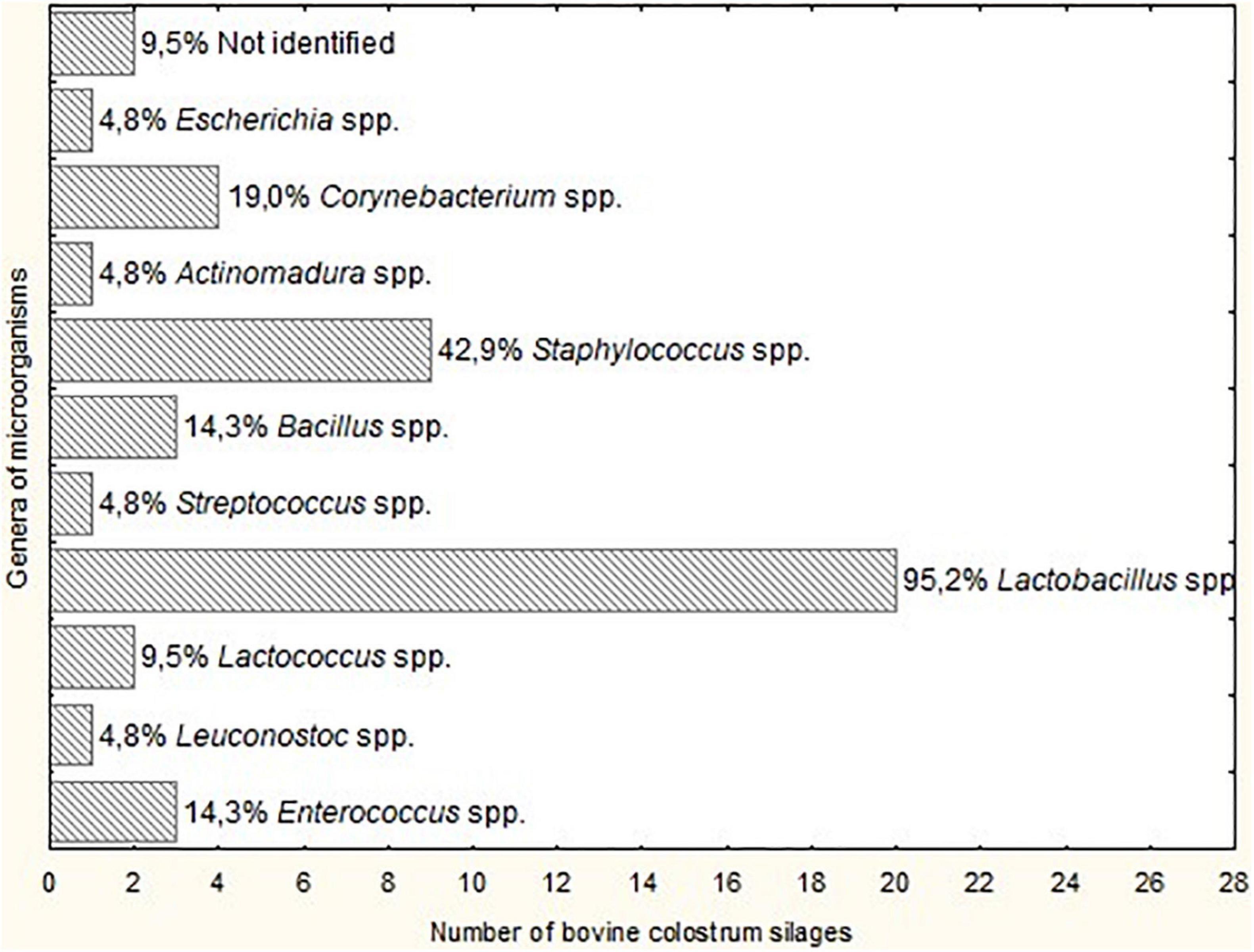
Figure 1. Genera of microorganisms isolated from Bovine colostrum silage (BCS) at different fermentation times.
This study was the first to compare the physicochemical and microbiological parameters of milk quality with those obtained from BCS at different fermentation times. It is worth mentioning that the BC samples were acquired directly from milk producers and represent the handling performed in the daily lives of these producers. For this reason, it was decided not to carry out experimental planning with defined time and temperatures since the development of BCS in the properties occurs at room temperature and for as long as the producer needs. Aiming to reproduce the conditions carried out by the producers, another limitation was found in the present study in relation to the number of samples (n = 21).
Saalfeld et al. (2013) carried out BCS with 60 days of anaerobic fermentation, and the percentages of lipids, protein, dry matter and ash remained throughout the period that was evaluated. Azevedo et al. (2014) evaluated the influence of BC milking day on BCS fermentation, and the BC obtained from the second and third milkings showed a 90.5% BCS disposal rate due to the microorganisms from the BC that cause putrid appearance and odor, expansion or even rupture of the packaging due to the high production of gases. Therefore, improper fermentations favor the growth of pathogenic organisms, in addition to lactic acid bacteria, and the fermentation temperature has an effect on the degradation speed of some parameters, such as pH, lactic acid, acidity, and lactose. Higher temperatures (32.5°C) cause lower physical-chemical contents than room temperature (17.4 to 21.5 and 22.5°C) and benefit the growth of pathogenic microorganisms (Ferreira et al., 2013).
The behavior of wash concentration over the fermentation time was already expected and can be observed in fermentative processes that use milk, such as yogurt and fermented milk (Souza et al., 2013; Andrade et al., 2015). The studies in the literature on BCS used shorter fermentation times (Ferreira et al., 2013; Saalfeld et al., 2013); for this reason, the physicochemical composition did not behave similarly to the present study. In addition, fermentation temperature also influences physicochemical concentrations. A temperature of 32.5°C resulted in lower rates and greater reductions in pH, acidity and concentration of lactic acid; however, the storage of BC at 22°C or room temperature (17.4–21.5°C) resulted in higher concentrations of these components (Ferreira et al., 2013).
Azevedo et al. (2014) reported the occurrence of Enterobacteriaceae, Staphylococcus spp. and fungi in some BCS fermented for 33 days at 25°C. Ferreira et al. (2013) observed lower counts of lactic acid bacteria and Enterobacteriaceae when fermentation was carried out at higher temperatures (32.5°C), and the ambient temperature (17.4–21.5°C) had a significant influence on the development of microorganisms in the BCS.
Our findings could not be compared with the results of Saalfeld et al. (2016) because according to the methodology used in that study, the colostrum was inoculated with the bacteria to check whether the fermentation of BC inhibited microbial growth, and in the present study, we isolated the bacteria from the newly fermented colostrum.
The presence of pathogenic organisms such as Staphylococcus spp., Streptococcus spp., and Corynebacterium spp. in the BCS must be limited; however, there is no legislation in force in Brazil for BC or BCS. Normative Instruction number 76 (BRAZIL, 2018a) limits bacterial and somatic cell counts for milk and can be used as a basis for the control of microorganisms in BC and BCS. Limitations were found regarding physicochemical and microbiological determinations due to the absence of standardized methodologies for BC and BCS, and for this reason, methods applied to milk were used.
There were some limitations in the present study, such as the sample size, which could impair the power of the statistical analysis; all samples of BCS were collected from one farm; and the absence of colostrum regulations, including specifications about the concentrations of microorganism genera that would be allowed in the silage as well as the variation of the fermentation time, had an influence on the microorganisms present.
The physicochemical composition of BCS showed changes during the fermentation period. Nevertheless, pathogenic microorganisms were present at different times of fermentation. The presence of these organisms does not meet the milk quality parameters. Thus, it is recommended to use mild temperatures (17–22.5°C) for a minimum period of 35 days to carry out the silage. With regard to future perspectives, a course on good practices will be held, reinforcing cleaning and hygiene techniques before and after milking to reduce microbiological contamination of BCS.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
RK contributed to antimicrobial evaluation and drafted the manuscript. PS and AK contributed to the physicochemical and microbiological analyses. KP contributed to the collection and identification of colostrum samples and microbiological analysis. HL and SL contributed to the conception of this study and supervised the laboratory work. GP contributed to the statistical analyses. GD and PN contributed to the design of this study and conception and the data interpretation. RL coordinated the study and contributed to critical reading of the manuscript. All the authors have read the final manuscript and approved the submission.
This study was financed in part by the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES) – Finance Code 001.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.708189/full#supplementary-material
Adolfo Lutz Institute. (2008). Physicochemical Methods for Food Analysis. Pages 819-877 in Physicochemical Methods for Food Analysis. São Paulo, BR: Adolfo Lutz Institute.
Alexieva, B., Markova, T., and Nikolova, E. (2004). Bovine colostrum – The promising nutraceutical. Czech J. Food Sci. 22, 73–79. doi: 10.17221/3409-CJFS
Andrade, E. H. P., Silva, N. M. A., Resende, M. F. S., Souza, M. R., Fonseca, L. M., Cerqueira, M. M. O. P., et al. (2015). Microbiological and physical-chemical characteristics of fermented milk beverages. [in Portugese]. Arq. Bras. Med. Vet. Zootec. 67, 1735–1742. doi: 10.1590/1678-4162-8066
Azevedo, R. A., Guimarães, F., Viegas, C. R., Almeida, P. N. M., Geraseev, L. C., Pinto, M. S., et al. (2014). [Colostrum silage: microbiological risks and characterization of pH as a function of day of collection.] Rev. Bras. Med. Vet. 36, 271–276.
Barrow, G. I., and Feltham, R. K. A. (1993). “Cowan and Steel’s manual for the identification of medical bactéria,” in Pages 50 – 164 in Cowan and Steel’s Manual for the Identification of Medical Bactéria, (United Kingdom: Cambridge University Press).
Borad, S. G., and Singh, A. K. (2018). Colostrum immunoglobulins: processing, preservation and application aspects. Int. Dairy J. 85, 201–210. doi: 10.1016/j.idairyj.2018.05.016
BRAZIL (2018a). [Normative Instruction (IN) Number 76, of November 26, 2018. Technical Regulation of Identify and Quality of Refrigerated Raw Milk. Ministry of Agriculture, Livestock and Supply, 2018.]. Avaliable online at: http://sistemasweb.agricultura.gov.br/sislegis/action/detalhaAto.do?method=consultarLegislacaoFederal (accessed July 17, 2020).
BRAZIL (2018b). Normative Instruction (IN) Number 77, of November 26, 2018. Criteria and Procedures for the Production, Packaging, Preservation, Transport and Reception of Raw Milk in Establishments Registered With the Official Inspection Service. Ministry of Agriculture, Livestock and Supply, 2018.]. Avaliable online at: http://sistemasweb.agricultura.gov.br/sislegis/action/detalhaAto.do?method=consultarLegislacaoFederal (accessed July 17, 2020).
Chelack, B. J., Morley, P. S., and Hainess, D. M. (1993). Evaluation of methods for dehydration of bovine colostrum for total replacement of normal colostrum in calves. Can. Vet. J. 34, 407–412.
EAPEL (Agroclimatology Station of Pelotas) (2020). Agroclimatological Bulletin, Laboratory of Agrometeorology. Avaliable online at: http://agromet.cpact.embrapa.br/estacao/boletim.php (accessed Aug. 26, 2020).
El-Fattah, A. M. A., Rabo, F. H. R. A., El-Dieb, S. M., and El-Kashef, H. A. S. (2014). Preservation methods of buffalo and bovine colostrum as a source of bioactive components. Int. Dairy J. 39, 24–27. doi: 10.1016/j.idairyj.2014.04.008
Ferreira, L. S., Silva, J. T., Paula, M. R., Soares, M. C., and Bittar, C. M. M. (2013). Colostrum silage: fermentative, microbiological and nutritional dynamics of colostrum fermented under anaerobic conditions at different temperatures. Acta Scientiarum. 35, 395–401. doi: 10.4025/actascianimsci.v35i4.19870
Gosch, T., Apprich, S., Kneifel, W., and Novalin, S. (2014). A combination of microfiltration and high pressure treatment for the elimination of bacteria in bovine colostrum. Int. Dairy J. 34, 41–46. doi: 10.1016/j.idairyj.2013.06.014
Hurley, W. L., and Theil, P. K. (2011). Perspectives on immunoglobulins in colostrum and milk. Nutrients 3, 442–474. doi: 10.3390/nu3040442
International Dairy Federation (2000). “International Standard 141C:2000 Whole milk - determination of milk fat, protein and lactose content,” in Guidance on the Operation of Mid-Infrared Instruments, Brussels.
International Dairy Federation (2006). “Milk - enumeration of somatic cells,” in Part 2: Guidance on the Operation of Fluoro-Opto-Electronic Counters, Brussels.
Li, S., Bomser, J. A., and Zhang, Q. H. (2005). Effects of pulsed electric fields and heat treatment on stability and secondary structure of bovine immunoglobulin G. J. Agric. Food Chem. 53, 663–670. doi: 10.1021/jf048923r
Marnila, P., and Korhonen, H. (2011). Milk/Colostrum. Pages 591 – 597 in Encyclopedia of Dairy Sciences, 2 Edn. Finland: UE, doi: 10.1016/B978-0-12-374407-4.00322-8
Morrill, K. M., Conrad, E., Lago, A., Campbell, J., Quigley, J., and Tyler, H. (2012). Nationwide evaluation of quality and composition of colostrum on dairy farms in the United States. J. Dairy Sci. 95, 3997–4005. doi: 10.3168/jds.2011-5174
Mourad, G., Bettache, G., and Samir, M. (2014). Composition and nutritional value of raw milk. Issues Bio. Sci. Pharma. Res. 2, 115–122. doi: 10.15739/ibspr.005
Muller, L. D., and Smallcomb, J. (1977). Laboratory evaluation of several chemicals for preservation of excesso colostrum. J. Dairy Sci. 60, 627–631. doi: 10.3168/jds.S0022-0302(77)83911-8
Pereira, R. V., Bicalho, M. L., Machado, V. S., Lima, S., Teixeira, A. G., Warnick, L. D., et al. (2014). Evaluation of the effects of ultravioleta light on bacterial contaminants inoculated into whole milk and colostrum, ando n colostrum immunoglobulin G. J. Dairy Sci. 97, 2866–2875. doi: 10.3168/jds.2013-7601
Ramya, S. B., Ramasamy, D., and Dhineshkumar, V. (2016). Effects of refrigeration, deep freezing-spray drying and pasteurization on lgG bovine colostrum preservation. Int. J. Dairy Process Res. 3, 35–37. doi: 10.19070/2379-1578-160010
Saalfeld, M. H., Pereira, D. I. B., Borchardt, J. L., Sturbelle, R. T., Rosa, M. C., Guedes, M. C., et al. (2014). Evaluation of the transfer of immunoglobulin from colostrum anaerobic fermentation (colostrum silage) to newborn calves. Anim. Sci. J. 85, 963–967. doi: 10.1111/asj.12229
Saalfeld, M. H., Pereira, D. I. B., Silveira, K. R. K., Schramm, R., Valente, J. S. S., Borchardt, J. L., et al. (2013). Anaerobically fermented colostrum: an alternative for feeding calves. Rev. Cienc. Rural. 43, 1636–1641. doi: 10.1590/S0103-84782013000900016
Saalfeld, M. H., Pereira, D. I. B., Valente, J. S. S., Borchardt, J. L., Weissheimer, C. F., Gularte, M. A., et al. (2016). Effect of anaerobic bovine colostrum fermentation on bacteria growth inhibition. Rev. Cienc. Rural. 46, 2152–2157. doi: 10.1590/0103-8478cr20160393
Solomons, N. W. (2002). Modulation of the imune system and the response against pathogens with bovine colostrum concentrates. Eur. J. Clin. Nut. 56, S24–S28. doi: 10.1038/sj.ejcn.1601480
Souza, A. H. P., Costa, G. A. N., Miglioranza, L. H. S., Furlaneto-Maia, L., and Oliveira, A. F. (2013). Microbiological, physical, chemical and sensory chracteristics of milk fermented with Lactobacillus plantarum. Acta Scientiarum. 35, 125–131. doi: 10.4025/actascihealthsci.v35i1.11939
Wereme, A. N., Strabel, M., Grongnet, J., and Piot, M. (2001). Immunoglobulin G absorption from pooled maternal colostrum, commercial poder and freeze-dried colostrum by newborn calves. Anim. Res. 50, 315–323. doi: 10.1051/animres:2001133
Keywords: bovine colostrum, silage colostrum, storage colostrum, bacteriological examination, quality
Citation: Kraus RB, dos Santos PR, Krummenauer A, Palhares KE, Lima HG, Ladeira SRL, Pereira GM, Dors GC, Nascente PS and Lund RG (2021) Bovine Colostrum Silage: Physicochemical and Microbiological Characteristics at Different Fermentation Times. Front. Microbiol. 12:708189. doi: 10.3389/fmicb.2021.708189
Received: 11 May 2021; Accepted: 17 August 2021;
Published: 13 September 2021.
Edited by:
Lisa Solieri, University of Modena and Reggio Emilia, ItalyReviewed by:
Aly Farag El Sheikha, Jiangxi Agricultural University, ChinaCopyright © 2021 Kraus, dos Santos, Krummenauer, Palhares, Lima, Ladeira, Pereira, Dors, Nascente and Lund. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrícia da Silva Nascente, cGF0dHNuQGdtYWlsLmNvbQ==; Rafael Guerra Lund, cmFmYWVsLmx1bmRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.