
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 23 December 2021
Sec. Microbe and Virus Interactions with Plants
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.696117
Sugarcane smut is a significant sugarcane disease caused by Sporisorium scitamineum and is a large threat to the sugar industry in China and the world. Accordingly, it is important to study the pathogenic mechanism by which this disease occurs to identify effective prevention and control strategies. Gene SsCI72380, which encodes cytochrome P450 sterol 14 alpha-demethylase (CYP51), was screened out from the transcriptome of S. scitamineum. In this study, the functions of gene SsCI72380 were identified via the knockout mutants ΔSs72380+ and ΔSs72380−, which were obtained by polyethylene glycol (PEG)-mediated protoplast transformation technology, as well as the complementary mutants COM72380+ and COM72380−. The results showed that the CYP51 gene SsCI72380 played an important role in sporidial growth, sexual mating/filamentation, hyphae growth, and pathogenicity in S. scitamineum. Gene SsCI72380 may regulate the biosynthesis process of ergosterol by encoding CYP51 enzymes and then affecting the structure and function of the cell membrane. Gene SsCI72380 also played an important role in the response toward different abiotic stresses, including hyperosmotic stress, oxidative stress, and cell wall stress, by regulating the permeability of the cell membrane. In addition, gene SsCI72380 is a new type of pathogenic gene from S. scitamineum that enhances the pathogenicity of S. scitamineum.
Sugarcane smut causes serious economic losses in sugarcane globally and is caused by Sporisorium scitamineum, which belongs to the Basidiomycetes subphylum Melanophila (Nzioki et al., 2010). Sporisorium scitamineum is a two-type fungus. Its life history can be divided into two stages: a stage of a yeast-like basidiospore without pathogenicity, and another stage of a dikaryotic hypha with pathogenicity (Bakkeren et al., 2008; Que et al., 2014). When the teliospore of S. scitamineum infects sugarcane (via sugarcane bud invasion), the pathogenic dikaryotic hypha grows together with the young bud meristem of the sugarcane, and the appressorium grows on the young bud scales of the sugarcane. With the fusion of the two compatible nuclei of the dikaryotic hypha, diploid teliospores are formed, and the sugarcane plants produce black whip symptoms (Taniguti et al., 2015). The teliospores are nearly round, 5–6 μm in diameter, reddish brown or black in color, and can germinate promycelia of different lengths under suitable temperature and humidity conditions. Each promycelia produces four oval and transparent basidiospores, of which two are “+” mating haploids and two are “−” mating haploids. Compatible mating haploids form pathogenic dikaryotic hypha through sexual mating. Therefore, the pathogenicity of S. scitamineum is closely related to its sexual mating and subsequent filamentation (Albert and Schenck, 1996; Chen et al., 2015).
Sporisorium scitamineum is a dimorphic fungus, and the transformation from haploid to diploid is not only a morphological change from yeast-like to mycelium but also an important genetic change (Que et al., 2014). Based on homologous recombination gene knockout technology, Yan et al. (2016) showed that the gene bE of the locus b of S. scitamineum is related to the sexual mating and pathogenicity of this pathogen. After the gene was knocked out, the sexual mating ability of WT17 and WT18 was completely lost, and they were unable to infect sugarcane. Chang et al. (2018) found that the cAMP/PKA signaling pathway can regulate the intracellular reactive oxygen species (ROS) level by limiting the transcription of metabolic enzymes and then regulating the sexual mating ability of S. scitamineum. Deng et al. (2018b) found that the SsKpp2 gene encoding mitogen-activated protein kinase (MAPK) in S. scitamineum affected the sexual mating and mycelial formation of the fungus by regulating tryptophan biosynthesis and the pheromone signal transduction pathway. Zhang et al. (2019) identified an autophagy gene ATG8 in S. scitamineum. The deletion of the ATG8 gene caused the single haploid sporidia of S. scitamineum to be pseudomycelium-like and sensitive to oxidative stress. Based on gene knockout and gene complementation techniques, Sun et al. (2019) found that the gene Ram1 encoding the β-subunit of farnesyl transferase in S. scitamineum regulates the sexual mating, pathogenicity, and cell wall stability of the fungus. Wang et al. (2019) found that the gene SsAgc1 encoding AGC kinase in S. scitamineum is related to the sexual mating and mycelial formation of the fungus, and further inferred that the function of the gene may be related to the synthesis of the small-molecule signal substance tryptophan. In addition, Zhu et al. (2019) reported that the pheromone response factor SsPRF1 was involved in the regulation of sexual mating, mycelial growth, and pathogenicity of the fungus.
Sterol 14α-demethylase is a member of the oldest cytochrome P450 monooxygenase family commonly found in fungi/yeasts, higher plants, and mammals (Yuzo et al., 2000). It is involved in the biosynthesis of fungal ergosterols, phytosterols, and mammalian cholesterol. It is also a target of many azole antifungal drugs (Michael and Galina, 2005; Galina and Michael, 2006). The lack or complete absence of ergosterol biosynthesis will alter the fluidity of the fungal cell membrane and might change the activities of related enzymes on the fungal cell membrane, affecting the function of the fungal cell membrane and inhibiting the growth of fungi (Daum et al., 1998; Lees et al., 1999; Minnebruggen et al., 2010; Felipe et al., 2020). In the study of antifungal mechanism of cinnamaldehyde, under the action of cinnamaldehyde, the expression of the ERG11 gene encoding sterol 14 α-demethylase in Fusarium sambucinum was downregulated, and the ergosterol content in this fungus was decreased by 67.94%, which resulted in cell membrane damage, a slow spore growth rate, and a decrease in pathogenicity (Wei et al., 2020). In the study of antifungal mechanism of Euphorbia humifusa, after treatment with E. humifusa extract (containing 40% flavonoids and 16% tannic acid), the activity of sterol 14 α-demethylase on the cell membrane of Trichophyton rubrum decreased, which affected ergosterol biosynthesis and inhibited the growth of T. rubrum (Li et al., 2014b). The ERG11 gene encoding sterol 14 α-demethylase in the Saccharomyces cerevisiae Y12667 strain is located on chromosome VIII, and its deletion reduced the growth rate of the strain (Chen et al., 2009). However, no studies have been reported on the related genes encoding sterol 14α-demethylase in S. scitamineum.
Based on the previous transcriptome sequencing data of two S. scitamineum isolates Ss16 and Ss47 with different pathogenicities in our laboratory (Wu et al., 2020), a gene, SsCI72380 (GenBank accession no. MZ004860), with a conserved structure encoding sterol 14α-demethylase of the cytochrome P450 family, was screened from the highly pathogenic isolate Ss16 and exhibited a significantly upregulated expression level. The purpose of this study was to explore the biological function of the gene SsCI72380 in S. scitamineum by target gene knockout, gene complementation, phenotype analysis of gene deletion mutants and complements, and pathogenicity identification.
Based on transcriptome sequencing data, a gene SsCI72380 encoding sterol 14α-demethylase was identified as a significantly (p ≤ 0.05) differentially expressed gene in isolates Ss16 (strong pathogenicity) and Ss47 (weak pathogenicity) of S. scitamineum with different pathogenicities (Wu et al., 2020). The protein sequence of SsCI72380 was analyzed using the Compute pI/MW tool1 to determine the theoretical isoelectric point (pI) and molecular weight (MW). Blast comparison based on the amino acid sequences (DNA sequences into amino acid sequences)2 was performed on the NCBI database3 to obtain the conserved domain of the protein encoded by the SsCI72380 gene. Phylogenetic analysis of the protein encoded by the SsCI72380 gene was carried out in MEGA 7, and the phylogenetic tree was drawn using the neighbor-joining method (Saitou and Nei, 1987; Kumar et al., 2016). The Predotar online tool4 was used to predict the subcellular localization.
The wild-type haploid isolates Ss16+ and Ss16− of S. scitamineum were isolated and identified in our laboratory and stored at −80°C (Deng et al., 2018a). The culture medium used in this study included YePSA medium (yeast extract 1%, peptone 2%, sucrose 2%, and agar 2%) and YePS liquid medium (yeast extraction 1%, peptone 2%, sucrose 2%, and pH 7.0), YePS soft medium (yeast extract 1%, peptone 2%, sugar 2%, and agar 0.65%) and YePSS medium (yeast extract 1%, peptone 2%, sugar 2%, D-sorbitol 18.17%, and agar 2%). For the mating/filamentation assay, equal volumes of wild-type, deletion mutant, or complementary mutant haploid sporidia of opposite mating-types were mixed and plated on solid medium in the absence or presence of 5 mM cAMP and 0.02 mM tryptophol and then kept in the dark in a 28°C incubator for 42 h before photographing. For stress tolerance assessment, the sporidial culture at OD600 = 1.0 and its 10-fold serial dilutions were inoculated on YePSA medium in the absence or presence of stress inducers, including 100 μg/ml Congo red (CR), 50 μg/ml SDS, 4 mM H2O2, and 500 mM NaCl, and then incubated in the dark at 28°C for 48 h before assessment and photographing. For the growth assay, sporidia of S. scitamineum wild-type (Ss16+ and Ss16−), deletion mutant (ΔSs72380+ and ΔSs72380−), and complementary mutant (COM72380+ and COM72380−) were cultured in 50 ml of YePS liquid medium at 28°C with shaking at 200 rpm for 24 h. Aliquots of cultured sporidia were then diluted with fresh YePS liquid medium, the cell density was adjusted to 105 cells per ml, and samples were then cultured for another 48 h under the same conditions. The OD 600 was measured with a spectrophotometer (NanoDrop 2000C) every 6 h to monitor the yeast-like (budding) growth of the wild-type, deletion mutant, or complementary mutant strains.
Fungal genomic DNA was extracted using a modified CTAB method (Shen et al., 2006). The PCR amplification was performed using Phanta High-Fidelity DNA Polymerase (Vazyme, P505). Purification of DNA fragments was conducted using a FastPure Gel DNA Extraction Mini Kit (Vazyme, DC301). Total RNA was extracted with TRIzol (Vazyme, R401), and HISCRIPT III RT SuperMix (Vazyme, R323) was used for cDNA synthesis. A NanoDrop ND-1000 (Thermo Fischer Scientific, Wilmington, DE, United States) was used for measuring the concentration and purity.
The construction of two fragments for the replacement of the SsCI72380 gene by the Hpt (encodes a phosphotransferase conferring hygromycin resistance) gene was based on previous methods (Chakraborty et al., 1991; Yang and Chung, 2012; Li et al., 2014a; Chang et al., 2018). The flanking DNA of the SsCI72380 gene was PCR-amplified using wild-type S. scitamineum genomic DNA (Ss16+ and Ss16−). The Hpt gene in plasmid pDAN was the template. Gene knockout mutants were obtained by polyethylene glycol (PEG)-mediated protoplast transformation (Cai et al., 2020). The construction process of the deletion mutants is shown in Supplementary Figure S1A. The primer design of the amplified products was derived from the genome sequence LK056684.1 in NCBI. All primers involved in the construction and validation of the knockout mutants are indicated in Table 1.
The complementation of the SsCI72380 gene followed a previous strategy (Chang et al., 2018). The complemented gene not only carries the hygromycin homologous fragment to replace the hygromycin fragment in the knocked out mutants but also carries the zeocin resistance marker gene to screen the complements. The construction process of the complementary mutants is shown in Supplementary Figure S1B. Complementary mutants were obtained by PEG-mediated protoplast methods. All primers involved in the construction and validation of the complementary mutants are listed in Table 1.
We assessed the effect of the SsCI72380 gene on cytochrome P450 sterol 14 alpha-demethylase (CYP51) activity every 12 h over a period of 48 h under haploid sporidia culture conditions (YePS, 28°C, 200 rpm) based on the determination of CYP51 activity. CYP51 activity determination was carried out in accordance with the instructions of the ELISA kit (Shanghai mlbio Enzyme-linked Biotechnology).
The determination method referred to Zhang et al. (2009), with slight modifications. A total of 0.5 g sporidia colonies of the wild-type (Ss16+ and Ss16−), deletion mutant (ΔSs72380+ and ΔSs72380−), or complementary mutant (COM72380+ and COM72380−) collected from YePS liquid medium (cultured at 28°C for 48 h) were saponified by 10 ml saponification solution (50% KOH solution: absolute ethanol = 2:3) in a water bath of 88°C for 3 h. Then the products were added to petroleum ether for extraction. After evaporation drying, samples were dissolved in chloroform. Using ergosterol as an external standard, gas chromatography (GC) was used to determine the ergosterol content and lanosterol content (mg/g). Chromatographic determination conditions: Shimadzu gas chromatograph (GCMS-QP2020); capillary column DB-5 (25.0 m × 0.25 mm × 0.25 μm); temperature program: 195°C, 3 min; rise to 300°C with 5.5°C per min; injector temperature: 280°C; sample volume: 1 μl. Each group of experiments was repeated three times, and each sample was injected twice.
The conductivity measurement method was performed as reported by Duan et al. (2013). Haploid sporidia colonies (0.1 g) of wild-type (Ss16+ and Ss16−), deletion mutant (ΔSs72380+ and ΔSs72380−), or the complementary mutant (COM72380+ and COM72380−) were scraped and placed into 50 ml of sterile ddH2O and cultured at 28°C with shaking at 200 rpm for 2 h. Around 15 ml of each were absorbed separately and centrifuged at a low speed, and the electrical conductivity of the upper liquid (R1) was determined by a conductivity meter (DDS-307A, Shanghai Leici). The remaining spore liquid was boiled in a 100°C water bath for 10 min and then centrifuged at a low speed, and 15 ml was removed to measure the conductivity of the upper liquid (R2). Relative conductivity (%) = (R1/R2) × 100%. Three replicates were set for each sample.
We assessed the transcriptional profile of the SsCI72380 gene every 12 h over a period of 72 h under haploid and mating conditions as well as during the infection process (after inoculation of sugarcane plants of the smut susceptible variety “ROC22”) using quantitative real-time PCR (qRT-PCR). For the qRT-PCR, we used a ChamQ Universal SYBR quantitative PCR (qPCR) Master Mix (Vazyme, Q711), and the reaction was run on a Real-Time PCR System (CFX96, BioRad). Relative expression values were calculated with the 2-ΔΔCt method using ACTIN as an internal control (Livak and Schmittgen, 2001). Three biological repeats each containing three technical replicas for each sample were performed. The primers used in this study are listed in Table 1.
Sporidial colonies of the wild-type (Ss16+ and Ss16−), knockout mutant (ΔSs72380+ and ΔSs72380−), or complementary mutant (COM72380+ and COM72380−) were inoculated into 50 ml of YePS liquid medium and cultured at 28°C with shaking at 200 rpm for 2 days. Sporidia were collected by centrifugation and washed twice with ddH2O, after which they were re-suspended in YePS liquid medium at a final concentration of 2 × 109 spores/ml. Sporidia of opposite mating types were then mixed in equal volumes, after which 200 μl of this mixture was syringe-injected into 4–5 leaves of seedlings of the highly susceptible sugarcane variety ROC22 (20 plants were inoculated in each treatment). A wild-type mixture (Ss16+ and Ss16−) served as a positive control, and sterile water was used as a negative control. Inoculated plants were kept in a greenhouse for 4 months. Investigation of the occurrence of sugarcane smut began after 1 month. At the end of each investigation, the diseased plants were labeled to avoid repeated investigations, black whip symptoms were covered with plastic bags to prevent the spread of teliospores, and the number of diseased plants and the morbidity were calculated.
Images were taken using an Axio Observer Z1 microscope (Zeiss, Jena, Germany) equipped with a sCMOS camera (PCO Edge, Kelheim, Germany).
Data were expressed as the means ± SE. Differences among different treatments were analyzed using GraphPad Prism 8 software (GraphPad, United States).
The sterol 14α-demethylase encoding gene SsCI72380 was previously identified as a significantly (p ≤ 0.05) differentially expressed gene in isolates Ss16 (strong pathogenicity) and Ss47 (weak pathogenicity) of S. scitamineum (Wu et al., 2020). Through NCBI annotation, the gene SsCI72380 coding protein (NCBI: protein accession no. CDU25192.1) is a sterol 14α-demethylase of the cytochrome P450 family and consists of 559 amino acid residues. The isoelectric point (pI) of the protein was 6.58, the MW was 62.1 kDa, the length of the gene was 1,680 bp, and no intron was present (Figure 1A). Phylogenetic analysis showed that the protein encoded by the gene was highly homologous to Sporisorium graminisola conserved hypothetical protein EX895_001104 and Sporisorium reilianum f. sp. reilianum conserved sterol 14 alpha-demethylase, indicating that the SsCI72380 gene is highly conserved in smut fungi (Figure 1B). The predicted subcellular structure of the protein was located on the endoplasmic reticulum (Figure 1C).
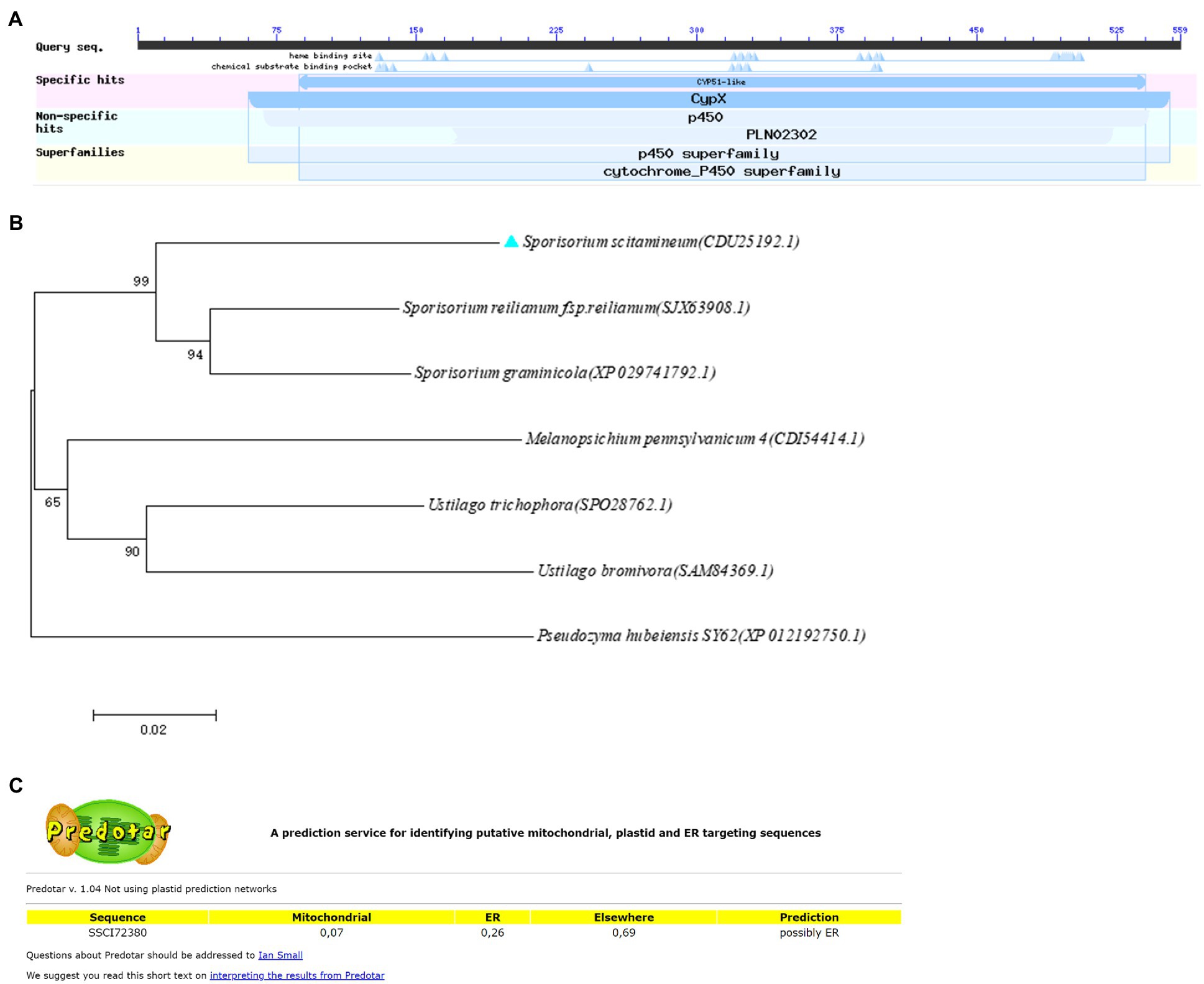
Figure 1. Domain and phylogenetic analysis of SsCI72380 genes encoding sterol 14α-demethylase in Sporisorium scitamineum. (A) Predicted protein encoded by the SSCI72380 gene was sterol 14 alpha-demethylase (p450 family). (B) Phylogenetic tree analysis of SsCI72380 coding proteins; Arabic numerals at the node edge of the tree indicate the degree of credibility of the phylogenetic tree (using 1,000 bootstrap replicates). Sporisorium scitamineum SsCI72380 is denoted by a blue solid triangle. (C) Prediction of the subcellular structural localization of SsCI72380 gene-coding proteases.
To further investigate the functions of SsCI72380, gene deletion and complementary strains were constructed as described in the Materials and Methods. Using wild-type S. scitamineum genomic DNA (Ss16+ and Ss16−) as template, each fragment was amplified by PCR with the primer pairs SsCI72380-LB-F/R and SsCI72380-RB-F/R. The band sizes were 1,101 and 1,052 bp (Figure 2A), and a fragment fusion (Figure 2B) was used to transform the fragments into S. scitamineum wild-type protoplasts with the primer pairs SsCI72380-LB-F/Hpt-LB-226 and SsCI72380-RB-F/Hpt-RB-R, the band sizes of which were about 3 and 2.5 Kb, respectively. As expected, two deletion mutants (ΔSs72380+ and ΔSs72380−) were obtained. The SsCI72380 deletion mutants were confirmed with PCR using the primer pairs SsCI72380-JC-F/SsCI72380-JC-R and SsCI72380-LB-F/Hpt-RB-R. Use of the primer pair SsCI72380-JC-F/SsCI72380-JC-R resulted in a 439 bp band from the SsCI72380 gene in the wild-type (Ss16+ and Ss16−), but not in the deletion mutants, while the primer pair SsCI72380-LB-F/Hpt-RB-R produced a 4,149 bp band corresponding to the inserted HPT gene from the deletion mutants (Figures 2C–F). The SsCI72380 complementary mutants (COM72380+ and COM72380−) were confirmed with PCR using the primer pairs Zeocin-JC-F/Zeocin-JC-R (583 bp) and SsCI72380-JC-F/SsCI72380-JC-R (439 bp; Figure 2G).
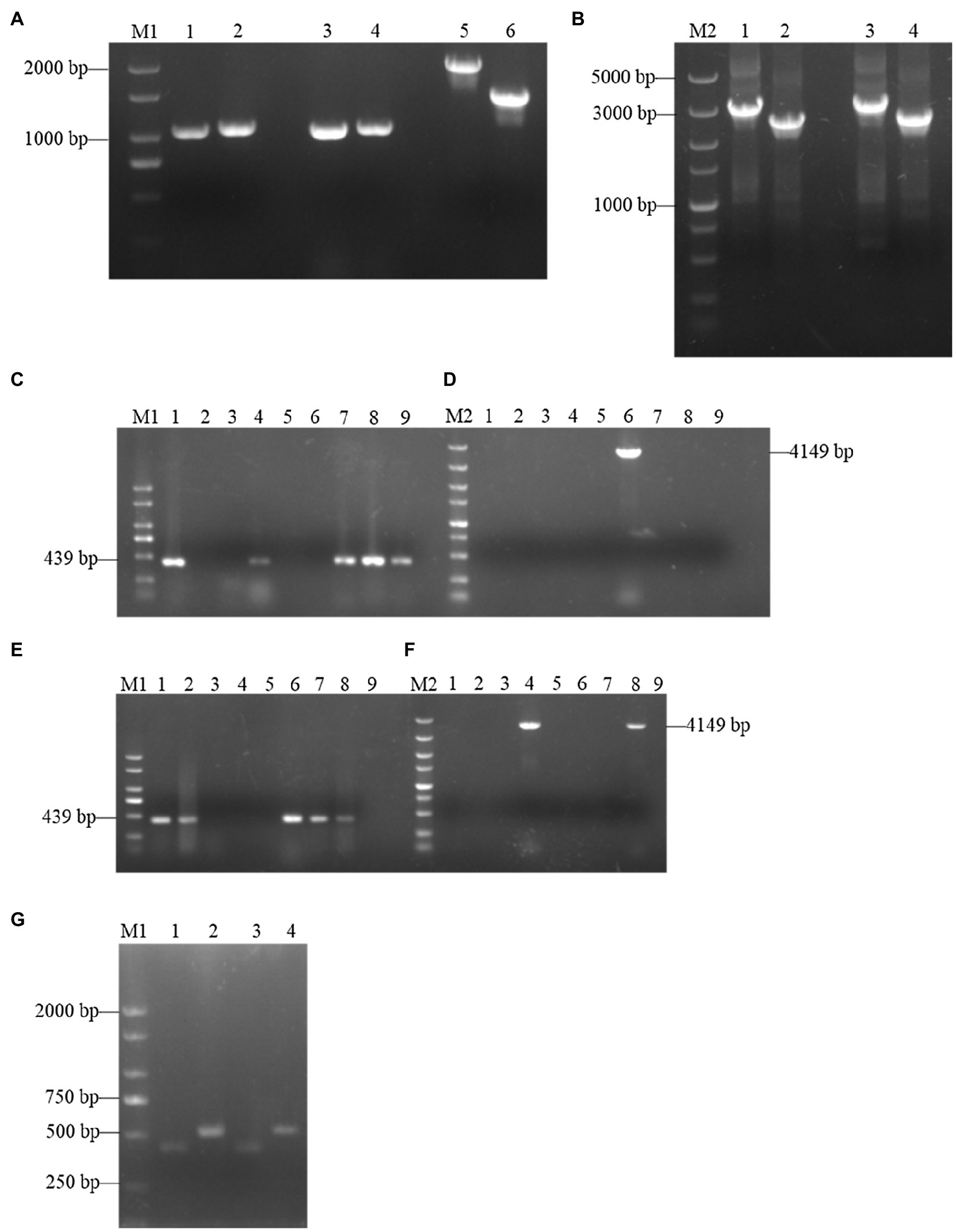
Figure 2. Construction and validation of SsCI72380 gene knockout and complementary mutants. Lane M1 is the 2,000 marker, and lane M2 is the 5,000 marker. (A) PCR amplification. Lanes 1, 3 and 2, 4 represent the right and left borders of the wild-type Ss16+ and Ss16−, respectively; lanes 5 and 6 represent the two overlapping HPT fragments with primer pairs Hpt-LB-F/226 and Hpt-RB-225/R, the band sizes of which were about 2 and 1.5 Kb, respectively. (B) Fusion-PCR. Lanes 1 and 3 represent the fusion fragments of the left borders of the wild-type Ss16+ and Ss16− with the left HPT fragments, and lanes 2 and 4 represent the fusion fragments of the right borders of the wild-type Ss16+ and Ss16− with the right HPT fragments, respectively. (C,D) The knockout mutant ΔSs72380+ in the Ss16+ background was confirmed by PCR. In (C,D), lanes 2–9 are transformants, lane 1 represents the wild-type. (C,D) denote that lane 6 was the deletion mutant as the internal primer pair SsCI72380-JC-F/SsCI72380-JC-R was unable to produce a 439 bp band, while the external primer pair SsCI72380-LB-F/Hpt-RB-R produced a 4,149 bp band corresponding to the inserted HPT gene from the deletion mutant. (E,F) The knockout mutant ΔSs72380− in the Ss16− background was confirmed by PCR. In (E,F), lanes 2–9 are transformants, lane 1 represents the wild-type. In the same way, (E,F) denote that lane 4 was the deletion mutant. (G) Electrophoretic validation of complementary mutants (COM72380+ and COM72380−) positive transformants. Lanes 1 and 2 were verified by complementary mutant electrophoresis in ΔSs72380+ background, lanes 3 and 4 were verified by complementary mutant electrophoresis in ΔSs72380− background, lanes 1 and 3 were the SsCI72380 target gene fragment, and lanes 2 and 4 were the target gene fragment of zeocin.
It was observed that the haploid colony morphology of the SsCI72380 gene knockout mutant (ΔSs72380+ and ΔSs72380−) on the YePSA plates was not different from that of the wild-type strains (Ss16+ and Ss16−) and complementary mutants (COM72380+ and COM72380−; Figure 3A). In contrast, the growth rate of the knockout mutants (ΔSs72380+ and ΔSs72380−) was found to be lower than that of the wild-type strains (Ss16+ and Ss16−), and the growth rate of the complementary mutants (COM72380+ and COM72380−) basically returned to that of the wild-type strains (Figures 3B,C).
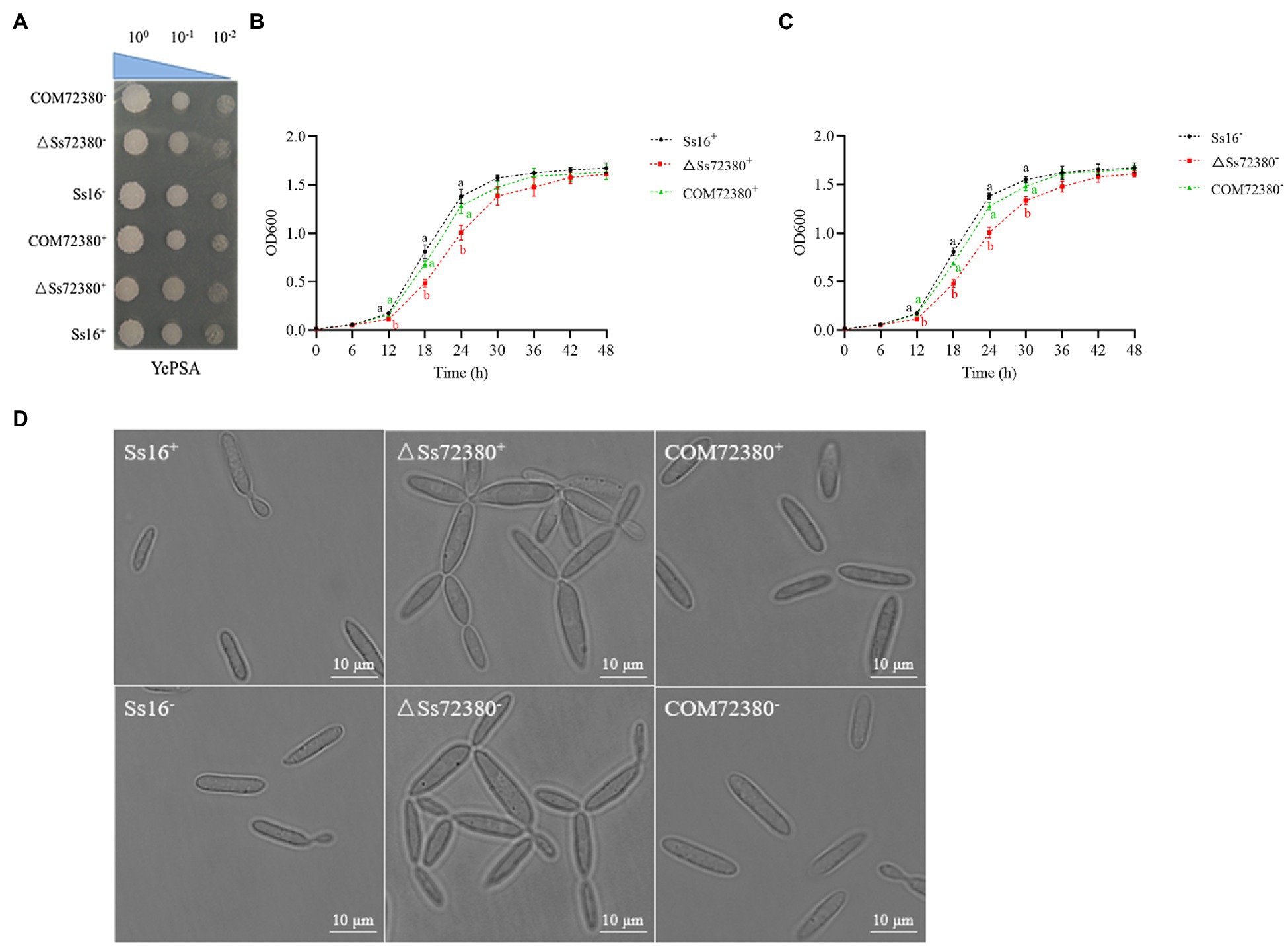
Figure 3. Effects of the SsCI72380 gene on the haploid phenotype of S. scitamineum. (A) Haploid colonies of wild-types (Ss16+ and Ss16−), knockout mutants (ΔSs72380+ and ΔSs72380−), and complementary mutants (COM72380+ and COM72380−) were spotted on YePSA plates and incubated at 28°C for 48 h. (B,C) Haploid growth curve, each sample had three replicates. Bars indicate the SE. Different lowercase letters represent a difference at the 0.05 level. (D) Microscopic images of sporidia of WT, deletion mutants, and complementary mutants (YePS liquid medium, 28°C, 200 rpm, 48 h, observed under 100× oil immersion microscopy).
The morphological changes in the haploid spores of the SsCI72380 gene knockout mutant (ΔSs72380+ and ΔSs72380−) were observed under a microscope. Most of the knockout mutants were pseudomycelium-like, while the wild-type (Ss16+ and Ss16−) and complementary mutants (COM72380+ and COM72380−) were long oval rods or two connected long rods at the mitotic stage (Figure 3D).
As shown in Figure 4, based on the determination of the sexual mating ability of S. scitamineum on YePSA plates, it was found that the sexual mating ability between the two knockout mutants (ΔSs72380+ and ΔSs72380−) was significantly weakened, and almost no white villous hyphae were produced. The sexual mating ability between the knockout mutant and the wild-type (Ss16+ and ΔSs72380− or Ss16− and ΔSs72380+) was also significantly weaker than that between the wild-type (Ss16+ and Ss16−), and the sexual mating ability between the complementing mutants (COM72380+ and COM72380−) almost recovered to that between the wild-type. The addition of previously reported small-molecule signaling substances (tryptophol or cAMP) involved in the sexual mating of S. scitamineum (Deng et al., 2018b; Wang et al., 2019) promoted the sexual mating ability of the wild-type and complemented mutants, but could not restore the sexual mating defect of the knockout mutants.
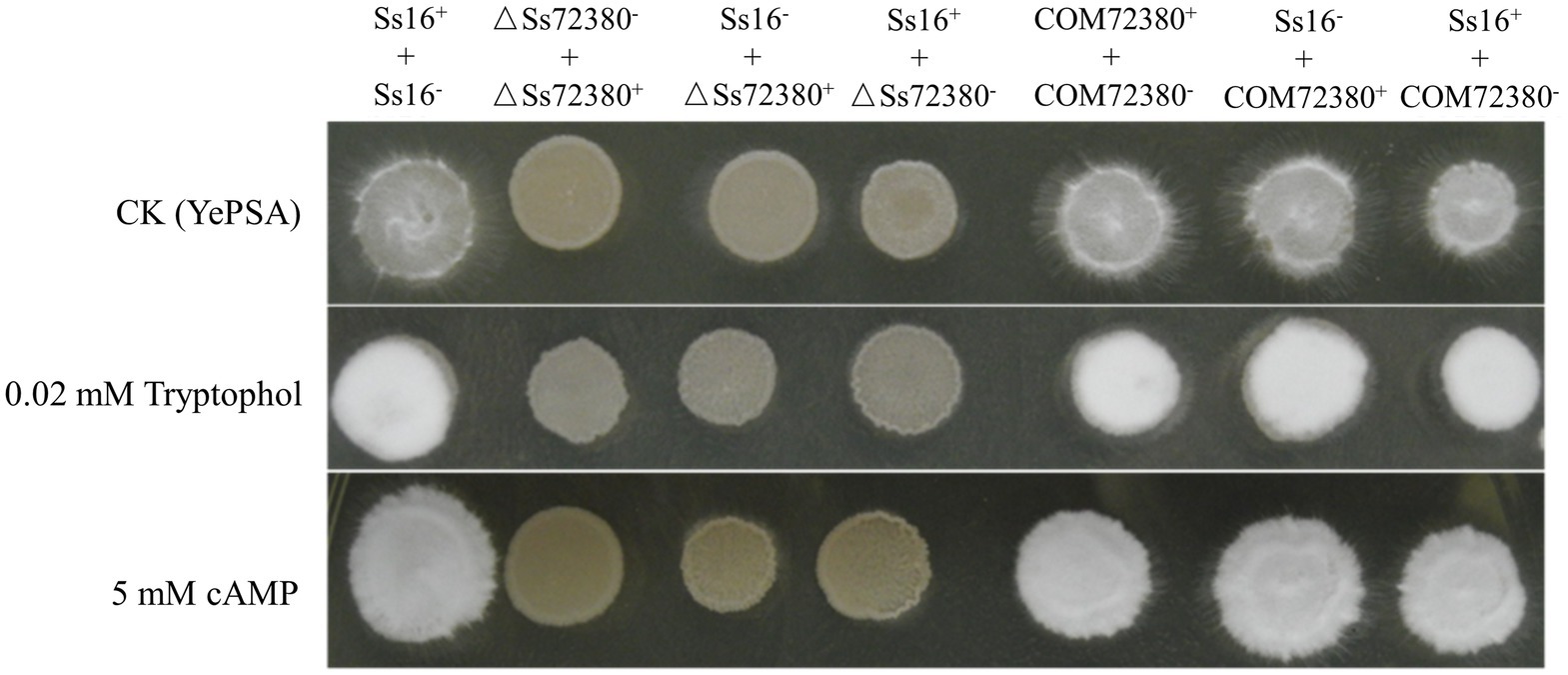
Figure 4. Effect of the SsCI72380 gene on the sexual mating of S. scitamineum. Sexual mating was assessed on YePSA plates supplemented with cAMP (5 mM) or tryptophol (0.02 mM). Photographs were taken 42 h after inoculation. The control was an untreated culture.
In the sporidia growth process of S. scitamineum, the CYP51 activity of the knockout mutants (ΔSs72380+ and ΔSs72380−) was always significantly lower than that of the wild-type (Ss16+ and Ss16−) and complemented mutants (COM72380+ and COM72380−), while the CYP51 activity of the complemented mutants (COM72380+ and COM72380−) was almost equal to that of the wild-type (Ss16+ and Ss16−; Figure 5A).

Figure 5. Effect of the SsCI72380 gene on cytochrome P450 sterol 14 alpha-demethylase (CYP51) activity (A), ergosterol content (B), lanosterol content (C), and the conductivity (D) in S. scitamineum. Bars indicate the SE derived from three independent replicates. In the same group, different lowercase letters represent a difference at the 0.05 level.
The ergosterol content of the SsCI72380 knockout mutants (ΔSs72380+ and ΔSs72380−) was significantly lower than that of the wild-type (Ss16+ and Ss16−) and complemented mutants (COM72380+ and COM72380−), while the ergosterol content of the complemented mutants (COM72380+ and COM72380−) was almost the same as that of the wild-type (Ss16+ and Ss16−), in addition, the content of lanosterol, CYP51 substrate, showed the opposite trend, indicating that the biosynthesis of ergosterol in S. scitamineum requires the participation of the SsCI72380 gene (Figures 5B–C).
The conductivity of the SsCI72380 gene knockout mutants (ΔSs72380+ and ΔSs72380−) was significantly higher than that of the wild-type (Ss16+ and Ss16−) and complementary mutants (COM72380+ and COM72380−), and the conductivity of the complementary mutants (COM72380+ and COM72380−) was almost the same as that of the wild-type (Ss16+ and Ss16−), indicating that the SsCI72380 gene is involved in the regulation of cell membrane stability in S. scitamineum (Figure 5D).
We examined the tolerance toward various stressful conditions in the wild-type and mutant sporidia, including cell wall stress (SDS or Congo red), hyperosmotic stress (NaCl), and oxidative stress (H2O2; Figure 6). The growth rate of the SsCI72380 gene knockout mutants (ΔSs72380+ and ΔSs72380−) in YePSA medium was slower than that of the wild-type (Ss16+ and Ss16−) and complemented mutants (COM72380+ and COM72380−). On YePSA medium supplemented with Congo red, SDS, NaCl, and H2O2, the growth rate of the SsCI72380 gene knockout mutants was further slower than that of the wild-type and complemented mutants (especially at low concentrations), and the growth rate of the complemented mutants was basically the same as that of the wild-type (Figure 6). The results showed that the SsCI72380 gene was involved in physiological processes such as hyperosmotic, oxidative, or cell wall integrity (CWI) stress responses in S. scitamineum.
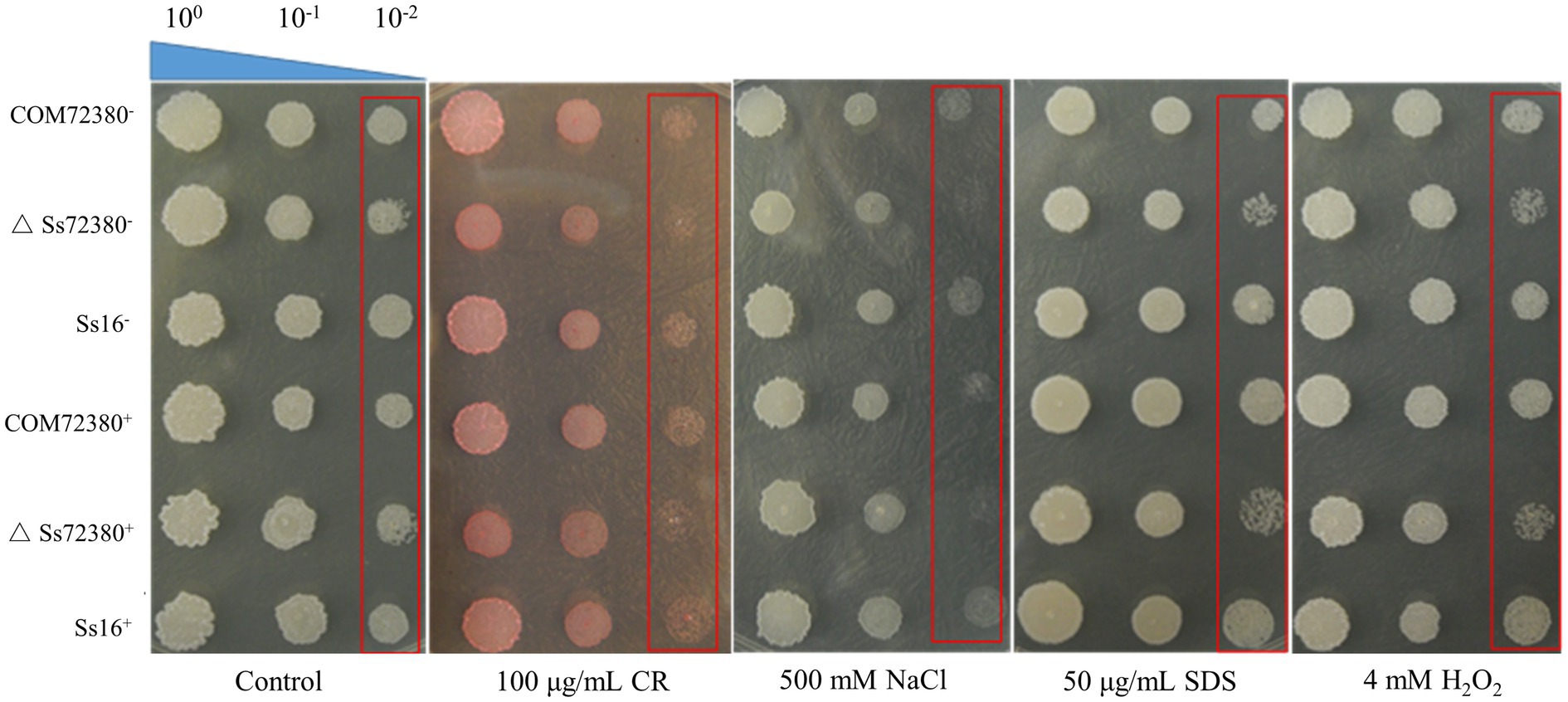
Figure 6. Effect of the SsCI72380 gene on abiotic stress in S. scitamineum. Serially diluted cells of WT (Ss16+ and Ss16−), deletion mutants (ΔSs16+ and ΔSs16−), or complementary mutants (COM72380+ and COM72380−) were spotted onto YePSA plates supplemented with H2O2 (4 mM), NaCl (500 mM), SDS (50 μg/ml), or Congo red (CR; 100 μg/ml). Samples were incubated at 28°C for 48 h before examination.
The SsCI72380 gene expression of the tested strains was determined by qRT-PCR, and the results are shown in Figure 7. During the growth of the haploid sporidia of S. scitamineum, the expression of the SsCI72380 gene in the wild-type (Ss16+ and Ss16−) and complementary mutants (COM72380+ and COM72380−) increased with the growth of the sporidia, while the expression of the SsCI72380 gene in the knockout mutants (ΔSs72380+ and ΔSs72380−) was undetectable (Figure 7A). In the sexual mating stage, the expression of the SsCI72380 gene first increased and then decreased with culture time, and the maximal expression occurred at around 60 h. The expression level of the SsCI72380 gene between the wild-type (Ss16+ and Ss16−) and complementing mutants (COM72380+ and COM72380−) or between the wild-type and complementing mutants (Ss16+ and COM72380− or COM72380+ and Ss16−) was significantly higher than that between the wild-type and knockout mutants (Ss16+ and ΔSs72380− or Ss16− and ΔSs72380+) or between the complementary mutants and knockout mutants (COM72380+ and ΔSs72380− or COM72380− and ΔSs72380+), while the expression level of the SsCI72380 gene was not detected between the knockout mutants (ΔSs72380+ and ΔSs72380−; Figure 7B). During the process of sugarcane bud infection, the expression of the SsCI72380 gene was similar to that in the process of sexual mating (Figure 7C).
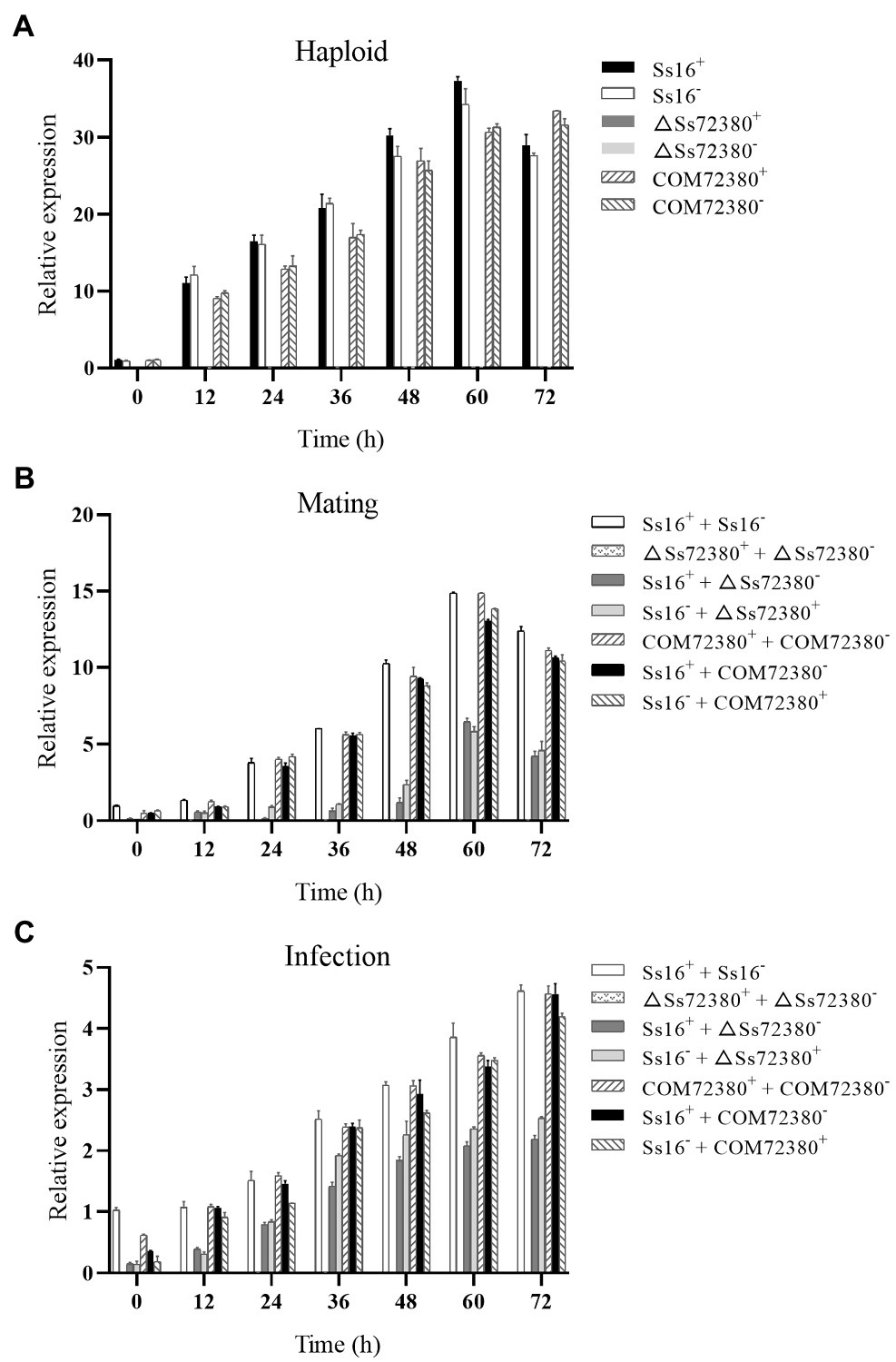
Figure 7. Expression analysis of SsCI72380 during various stages. (A) Gene expression levels under sporidial growth. (B) Gene expression levels under sexual mating conditions. (C) Gene expression levels under sugarcane bud infection. SsCI72380 expression at 0 h was normalized to one. Bars indicate the SE. The analyses were repeated three times.
To test whether SsCI72380 is required for S. scitamineum pathogenicity, the highly susceptible sugarcane variety ROC22 was inoculated by injection with mixed fungal sporidia of different combinations (of opposite mating types) as follows: ∆SsCI72380+ + ∆SsCI72380−, ∆SsCI72380− + Ss16+, ∆SsCI72380+ + Ss16−, COM72380+ + COM72380−, Ss16+ + COM72380−, and Ss16− + COM72380+, as well as with the wild-type Ss16+ + Ss16− combination as a positive, and sterile water was used as a negative control. The combinations containing knockout mutants (∆SsCI72380+ + ∆SsCI72380−, ∆SsCI72380− + Ss16+, and ∆SsCI72380+ + Ss16−) displayed significantly reduced pathogenicity, with only 12, 26, and 23% of the infected seedlings showing black whip symptoms, respectively. In contrast, the combinations without knockout mutants (COM72380+ + COM72380−, Ss16+ + COM72380−, and Ss16− + COM72380+) showed significantly high incidence rates, and the wild-type Ss16+ + Ss16− mixture led to 76% of the infected seedlings having black whip symptoms (Figure 8). Therefore, we conclude that SsCI72380 is required for the full pathogenicity of S. scitamineum.
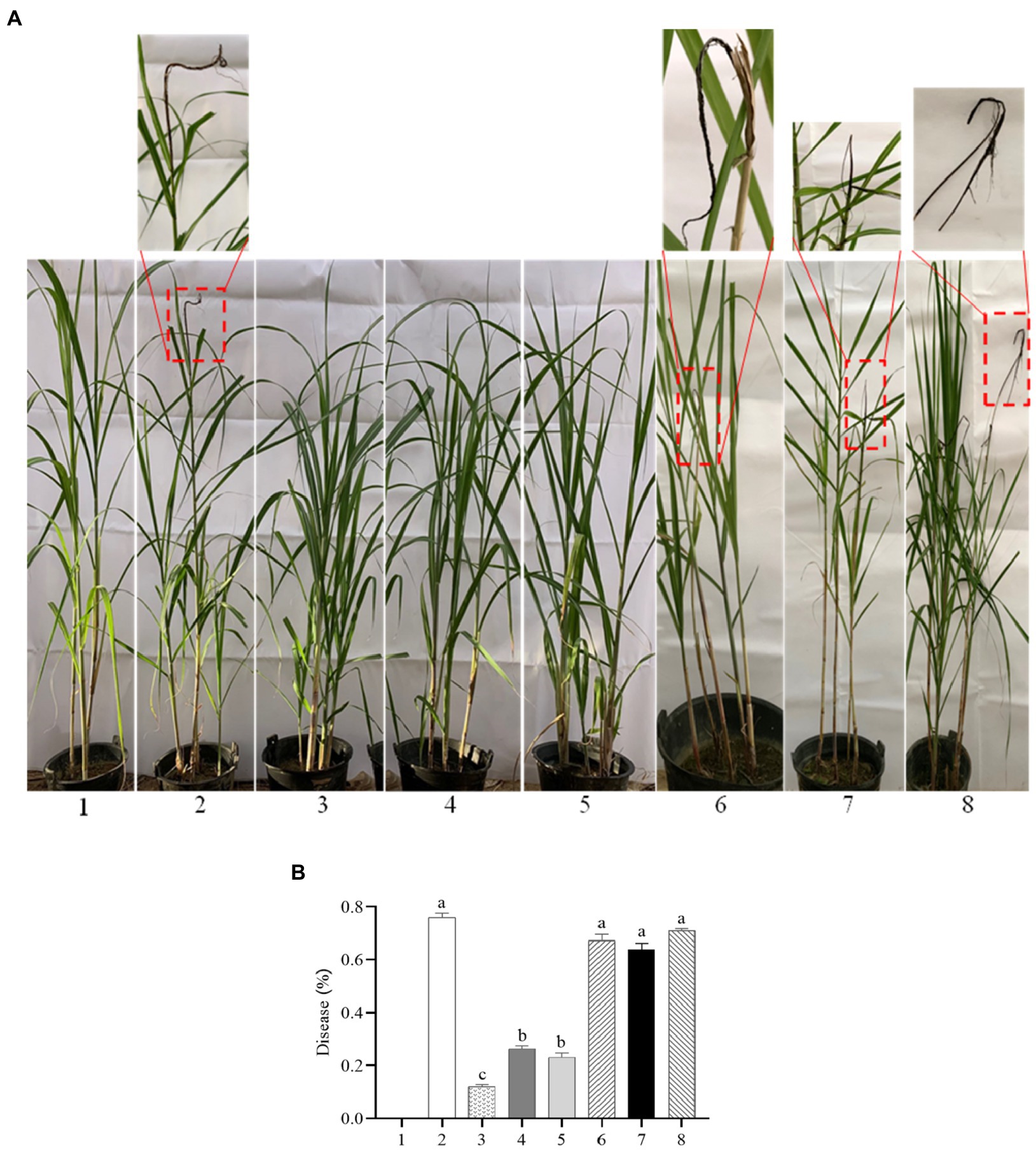
Figure 8. SsCI72380 gene involved in the pathogenicity of S. scitamineum. (A) Symptoms of smut whip. (B) Incidence. Numbers indicate: sterile water (1), Ss16+ + Ss16− (2), ΔSsCI72380+ + ΔSsCI72380− (3), Ss16+ + ΔSsCI72380− (4), Ss16− + ΔSsCI72380+ (5), COM72380+ + COM72380− (6), Ss16+ + COM72380− (7), and Ss16− + COM72380+ (8) inoculation, respectively. Bars indicate the SE. Different lowercase letters represent a difference at the 0.05 level.
In this study, based on the transcriptome sequencing data of different pathogenic isolates of S. scitamineum (Wu et al., 2020), a gene SsCI72380 encoding sterol 14α-demethylase was screened from the most enriched pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. The phylogenetic tree showed that the protein encoded by the gene was highly conserved among smut fungi. In addition, the subcellular localization predicted that the protein encoded by the gene might be located on the endoplasmic reticulum (The prediction results of subcellular localization by different tools are inconsistent, and some predictions are located on the plasma membrane, which needs further research). Among the biosynthetic pathways of ergosterol in biological S. cerevisiae, sterol 14α-demethylase is involved in the synthesis of ergosterol (Jordá and Puig, 2020). Ergosterol is a common sterol compound found in fungi that is mainly involved in the biological processes of fungal cell membrane fluidity, structural maintenance, sexual development, cell viability, and growth (Alvarez et al., 2007; Jin et al., 2008; Abe et al., 2009; Martínez et al., 2011). In Aspergillus fumigatus, there are two homologous genes of the yeast ERG11 gene (encoding sterol 14α-demethylase), namely erg11a and erg11b. Deletion of erg11b reduces ergosterol content (Hu et al., 2007). Sterol 14α-demethylase is a key enzyme in ergosterol biosynthesis. The synthesis of ergosterol is partially blocked, which will destroy the membrane structure of fungi and reduce the growth rate of fungi. In yeast, deletion of the gene encoding the enzyme can change the cell proliferation status and decrease the growth rate (Odds et al., 2003; Chen et al., 2009). Gene ERG11 knockout mutants in Candida albicans showed obvious filament defects, morphological changes in the hyphae, and decreased pathogenicity (Wu et al., 2018). However, the genes encoding sterol 14α-demethylase and their biological functions in S. scitamineum have not been reported.
In this study, the knockout mutants and complementary mutants of gene SsCI72380 encoding sterol 14α-demethylase were obtained from S. scitamineum by gene knockout and gene complementation techniques. The CYP51 activity, electrical conductivity, ergosterol content, and lanosterol content in the wild-type strains, knockout mutants, and complementary mutants were determined. The CYP51 activity of the knockout mutants decreased much more than the wild-type strains. However, the CYP51 activity did not decrease to zero in the knockout mutants. It is speculated that there should be additional genes encoding CYP51 involved in the regulation of CYP51 activity. In fact, through NCBI annotation, we found another gene (GenBank accession no. LK056650.1) encoding CYP51 in S. scitamineum. There are similar phenomena in some fungi. For example, there are two genes encoding CYP51 in A. fumigatus and three genes encoding CYP51 in Fusarium graminearum (Hu et al., 2007; Fan et al., 2013); the electrical conductivity of the knockout mutants increased significantly compared with that of the wild-type strains; the content of ergosterol decreased significantly compared with that of the wild-type strains. The ergosterol content of the mutants was not zero, possibly due to the existence of additional genes encoding CYP51. Similarly, A. fumigatus exists two genes, ERG11A and ERG11B, encoding CYP51. Deletion of ERG11A has no effect on ergosterol content, while deletion of ERG11B significantly reduces ergosterol content. The double deletion of ERG11A and ERG11B leads to the death of the fungus (Hu et al., 2007); and the content of lanosterol, the substrate of CYP51, showed the opposite change. The CYP51 activity of the knockout mutants decreased significantly, resulting in the accumulation of the lanosterol. There is a similar phenomenon in S. cerevisiae. In the knockout mutant of the gene ERG11 encoding CYP51, the content of lanosterol is significantly higher than that of the wild-type (Chen et al., 2009). However, the CYP51 activity, electrical conductivity, ergosterol contents, and lanosterol contents of the complementary mutants were consistent with those of the wild-type strains. It is speculated that the deletion of this gene does not inhibit ergosterol biosynthesis, it reduces ergosterol production in S. scitamineum and partially affect the integrity of membrane structure and function in S. scitamineum. Our findings corroborate previous reports that the deletion of the sterol 14α-demethylase gene in some fungi partially blocks ergosterol synthesis and leads to the destruction of fungal cell membrane structure (Hu et al., 2007).
The development of fungal pathogenicity is closely related to the growth and morphology of the spores. Aflcla4 knockout mutants of the pathogenic filamentous fungus Aspergillus flavus indicated abnormal branching during mycelial growth and significantly reduced conidia production, resulting in a significant decline in pathogenicity and virulence (Qin et al., 2020). The deletion mutant of CfMKK1 (encoding mitogen-activated protein kinase) of Colletotrichum fructicola significantly reduced the growth rate of the spores, failed to form conidiospore appressorium, and reduced the pathogenicity (Xiao and Li, 2020). The knockout mutants of gene Cla4 (encoding PAK family kinases) in Ustilago maydis were forked in haploid morphology, which reduced the sexual compatibility and pathogenicity of U. maydis (Leveleki et al., 2004). In this study, we found that the growth rate of the knockout mutants of gene SsCI72380 in S. scitamineum was slower than that of the wild-type strains. Under abiotic stresses such as H2O2, NaCl, SDS, and Congo red, the growth rate of the knockout mutants was even lower than that of the wild-type strains, and the growth rate of the complementary mutants was basically the same as that of the wild-type strains. Through S. scitamineum haploid morphological observation and comparison, it was found that the haploid spores of the knockout mutant showed a series of connected forked branches, while the spores of the wild-type and the complementary mutant showed a single short rod or two short rod cells connected together due to the division stage. A pathogenicity test showed that the incidence rate of sugarcane smut in the mixed inoculation group containing SsCI72380 gene knockout mutants (∆SsCI72380+ + ∆SsCI72380−, Ss16+ + ∆SsCI72380−, or Ss16− + ∆SsCI72380+) was significantly lower than that in the wild-type mixed inoculation group (Ss16+ + Ss16−). Our findings indicate that the deletion of gene SsCI72380 affects the growth rate, morphology, and pathogenicity of S. scitamineum. This is similar to previous studies on pathogenic genes in A. flavus (Qin et al., 2020), C. fructicola (Xiao and Li, 2020), and U. maydis (Leveleki et al., 2004) in pathogenic filamentous fungus.
As a dimorphic fungus, the pathogenicity of S. scitamineum is closely related to sexual mating and the formation of dikaryotic hypha (Que et al., 2014). Previous studies have focused on signaling pathways and small-molecule signaling substances, such as tryptophol or cAMP, which are associated with sexual mating in S. scitamineum (Chang et al., 2018; Wang et al., 2019). In this study, the SsCI72380 knockout mutants showed significantly decreased sexual mating ability, and their sexual mating ability was not restored after the addition of small-molecule signaling substances such as tryptophol or cAMP. It is suggested that the gene SsCI72380 may not be involved in the synthesis or transport of small-molecule signaling substances related to sexual mating in S. scitamineum. The gene SsCI72380 is mainly involved in the biosynthesis of ergosterol in the cell membrane, regulates the integrity of cell membrane structure and function, and affects the growth rate and morphological changes of S. scitamineum, thus weakening its sexual mating ability and pathogenicity development. This differs completely from previous studies on the pathogenic mechanism of S. scitamineum based on sexual mating by the synthesis of small-molecule signal substances or the regulation of the signaling pathway (Chang et al., 2018; Deng et al., 2018b; Sun et al., 2019; Wang et al., 2019; Zhang et al., 2019). Therefore, the SsCI72380 gene found in this study is a new type of pathogenic gene of S. scitamineum – a finding that contributes to the elucidation of the pathogenic mechanism of S. scitamineum.
In conclusion, PEG-mediated protoplast transformation technology was used to successfully obtain SsCI72380 knockout mutants and complementary mutants of S. scitamineum. Comparative analysis of gene SsCI72380 expression level, CYP51 activity, ergosterol content, lanosterol content, conductivity, growth rate, spore morphology, and abiotic stress showed that gene SsCI72380 is mainly involved in ergosterol biosynthesis, regulating the integrity of cell membrane structure and function, and its mutation affects the growth rate and spore morphology, and weakening the sexual mating ability, thus reducing the pathogenicity of S. scitamineum. In addition, this study showed that gene SsCI72380 constitutes a new type of pathogenic gene of S. scitamineum that enhances the pathogenicity of S. scitamineum.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, MZ004860.
WS conceived and designed the experimental plan. HL, YC, and HB performed the experiments. HL, YC, and WS analyzed the data and wrote the manuscript. WS, YC, HL, QD, and JC revised the paper. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Earmarked Fund for National Natural Science Foundation of China (31771861 and 32172063) and Guangdong Provincial Team of Technical System Innovation for Sugarcane Sisal Hemp Industry (2021KJ104-07).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank C. Q. Chang (South China Agricultural University) for sharing the vector plasmid pDAN.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.696117/full#supplementary-material
Supplementary Figure S1 | The schematic diagram of the mutant construction process. (A) The construction process of deletion mutants. (B) The construction process of complementary mutants.
1. ^https://web.expasy.org/compute_pi
2. ^https://www.novopro.cn/tools/translate.html
3. ^https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome
Abe, F., Usui, K., and Hiraki, T. (2009). Fluconazole modulates membrane rigidity, heterogeneity, and water penetration into the plasma membrane in Saccharomyces cerevisiae. Biochemistry 48, 8494–8504. doi: 10.1021/bi900578y
Albert, H. H., and Schenck, S. (1996). PCR amplification from a homolog of the bE mating-type gene as a sensitive assay for the presence of Ustilago scitaminea DNA. Plant Dis. 80, 1189–1192. doi: 10.1094/PD-80-1189
Alvarez, F. J., Douglas, L. M., and Konopka, J. B. (2007). Sterol-rich plasma membrane domains in fungi. Eukaryot. Cell 6, 755–763. doi: 10.1128/EC.00008-07
Bakkeren, G., Kämper, J., and Schirawski, J. (2008). Sex in smut fungi: structure, function and evolution of mating-type complexes. Fungal Genet. Biol. 45, S15–S21. doi: 10.1016/j.fgb.2008.04.005
Cai, E. P., Mei, D., Zhang, X. M., Sun, X., Li, L. Y., Wu, R. R., et al. (2020). A gene knockout method based on protoplast transformation with two PCR fragments in Sporisorium scitamineum. Mycosystema 39, 2314–2327. doi: 10.13346/j.mycosystema.200273
Chakraborty, B. N., Patterson, N. A., and Kapoor, M. (1991). An electroporation based system for high-efficiency transformation of germinated conidia of filamentous fungi. Can. J. Microbiol. 37, 858–863. doi: 10.1139/m91-147
Chang, C. Q., Cai, E. P., Deng, Y. Z., Mei, D., Qiu, S. X., Chen, B. S., et al. (2018). cAMP/PKA signalling pathway regulates redox homeostasis essential for Sporisorium scitamineum mating/filamentation and virulence. Environ. Microbiol. 21, 959–971. doi: 10.1111/1462-2920.14496
Chen, L. S., Liu, C. D., Tsay, J. G., and Chen, R. S. (2015). PCR-mediated detection of Sporisorium scitamineum in sugarcane based on the b E mating-type gene sequence. Tropical Plant Pathol. 40, 65–69. doi: 10.1007/s40858-015-0009-9
Chen, S. H., Sheng, C. Q., Xu, X. H., Jiang, Y. Y., Zhang, W. N., and He, C. (2009). Deletion and verification of the correct recombination of the ERG11 gene in yeast Y12667. Life Sci. Res. 2, 116–121. doi: 10.1007/978-1-4020-9623-5_5
Daum, G., Lees, N. D., Bard, M., and Dickson, R. (1998). Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast 14, 1471–1510. doi: 10.1002/(SICI)1097-0061(199812)14:16<1471::AID-YEA353>3.0.CO;2-Y
Deng, Q. Q., Xu, G. H., Dou, Z. M., and Shen, W. K. (2018a). Identification of three Sporisorium scitamineum pathogenic races in mainland China. Int. J. Agric. Biol. 20, 799–802. doi: 10.17957/IJAB/15.0566
Deng, Y. Z., Zhang, B., Chang, C. Q., Wang, Y. X., Lu, S., Sun, S. Q., et al. (2018b). The MAP kinase SsKpp2 is required for mating/filamentation in Sporisorium scitamineum. Front. Microbiol. 9:2555. doi: 10.3389/fmicb.2018.02555
Duan, Y. B., Ge, C. Y., Liu, S. M., Chen, C. J., and Zhou, M. G. (2013). Effect of phenylpyrrole fungicide fludioxonil on morphological and physiological characteristics of Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 106, 61–67. doi: 10.1016/j.pestbp.2013.04.004
Fan, J., Urban, M., Parker, J. E., Brewer, H. C., Kelly, S. L., Hammond-Kosack, K. E., et al. (2013). Characterization of the sterol 14α-demethylases of Fusarium graminearum identifies a novel genus-specific CYP51 function. New Phytol. 198, 821–835. doi: 10.1111/nph.12193
Felipe, R. P. M., Rhayssa, F., Veronica, D. S. C., Gregório, N. Q., Sabrina, M. L. C., Mateus, G. D. G., et al. (2020). New method for rapid identification and quantification of fungal biomass using ergosterol autofluorescence. Talanta 219:121238. doi: 10.1016/j.talanta.2020.121238
Galina, I. L., and Michael, R. W. (2006). Sterol 14α-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim. Biophys. Acta 1770, 467–477. doi: 10.1016/j.bbagen.2006.07.018
Hu, W. Q., Sillaots, S., Lemieux, S., Davison, J., Kauffman, S., Breton, A., et al. (2007). Essential gene identification and drug target prioritization in Aspergillus fumigatus. PLoS Pathog. 3:e24. doi: 10.1371/journal.ppat.0030024
Jin, H., McCaffery, J. M., and Grote, E. (2008). Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast. J. Cell Biol. 180, 813–826. doi: 10.1083/jcb.200705076
Jordá, T., and Puig, S. (2020). Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Gene 11:795. doi: 10.3390/genes11070795
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Lees, N. D., Bard, M., and Kirsch, D. R. (1999). Biochemistry and molecular biology of sterol synthesis in Saccharomyces cerevisiae. Crit. Rev. Biochem. Mol. Biol. 34, 33–47.
Leveleki, L., Mahlert, M., Sandrock, B., and Bolker, M. (2004). The PAK family kinase Cla4 is required for budding and morphogenesis in Ustilago maydis. Mol. Microbiol. 54, 396–406. doi: 10.1111/j.1365-2958.2004.04296.x
Li, M. H., Xie, X. L., Lin, X. F., Shi, J. X., Ding, Z. J., Ling, J. F., et al. (2014a). Functional characterization of the gene FoOCH1 encoding a putative α-1,6-mannosyltransferase in Fusarium oxysporum f. sp. cubense. Fungal Genet. Biol. 65, 1–13. doi: 10.1016/j.fgb.2014.01.005
Li, Z. J., Zhao, M. Y., Dawuti, G., and Aibai, S. (2014b). Action of Euphorbia humifusa effective fraction on membrane biosynthesis. Acta Pharm. Sin. 49, 273–276. doi: 10.16438/j.0513-4870.2014.02.009
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using realtime quantitative PCR and the 2-ΔΔCt method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Martínez, M. F., Pascual-Ahuir, A., and Proft, M. (2011). Repression of ergosterol biosynthesis is essential for stress resistance and is mediated by the Hog1 MAP kinase and the Mot3 and Rox1 transcription factors. Mol. Microbiol. 79, 1008–1023. doi: 10.1111/j.1365-2958.2010.07502.x
Michael, R. W., and Galina, I. L. (2005). Sterol 14α-demethylase, an abundant and essential mixed-function oxidase. Biochem. Biophys. Res. Commun. 338, 418–422. doi: 10.1016/j.bbrc.2005.08.118
Minnebruggen, G. V., Francois, Y., Cammue, B. P. A., Thevissen, K., and Shroot, B. (2010). A general overview on past, present and future antimycotics. Open Mycol. J. 4, 22–32. doi: 10.2174/1874437001004010022
Nzioki, H. S., Jamoza, J. E., Olweny, C. O., and Rono, J. K. (2010). Characterization of physiologic races of sugarcane smut (Ustilago scitaminea) in Kenya. Afr. J. Microbiol. Res. 4, 1694–1697. doi: 10.1016/j.chom.2010.07.004
Odds, F. C., Brown, A. J. P., and Gow, N. A. R. (2003). Antifungal agents: mechanisms of action. Trends Microbiol. 11, 272–279. doi: 10.1016/S0966-842X(03)00117-3
Qin, L., Li, X. W., Li, D., Zhao, J. R., Wang, S. H., and Yuan, J. (2020). Protein kinase Cla4 regulates morphology development, aflatoxin biosynthesis and pathogenicity of Aspergillus flavus. J. Fungus, 1–15. doi: 10.13346/j.mycosystema.200199
Que, Y. X., Xu, L., Wu, Q. B., Liu, Y. F., Ling, H., Liu, Y. H., et al. (2014). Genome sequencing of Sporisorium scitamineum provides insights into the pathogenic mechanisms of sugarcane smut. BMC Genomics 15:996. doi: 10.1186/1471-2164-15-996
Saitou, N., and Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425. doi: 10.1093/oxfordjournals.molbev.a040454
Shen, W. K., Zhou, G. H., Deng, H. H., and Zhou, L. Y. (2006). Detection of sugarcane ratoon stunting disease pathogen with polymerase chain reaction (PCR) and nucleotide sequence analysis. Chinese Agric. Sci. Bull. 22:413. doi: 10.3969/j.issn.1000-6850.2006.12.098
Sun, S. Q., Deng, Y. Z., Cai, E. P., Yan, M. X., Jiang, Z. D., Chen, B., et al. (2019). The farnesyltransferase β-subunit Ram1 regulates Sporisorium scitamineum mating, pathogenicity and cell wall integrity. Front. Microbiol. 10:976. doi: 10.3389/fmicb.2019.00976
Taniguti, L. M., Schaker, P. D. C., Benevenuto, J., Peters, L. P., Carvalho, G., Palhares, A., et al. (2015). Complete genome sequence of Sporisorium scitamineum and biotrophic interaction transcriptome with sugarcane. PLoS One 10:e129318. doi: 10.1371/journal.pone.0129318
Wang, Y., Deng, Y. Z., Cui, G. B., Huang, C. W., Zhang, B., Chang, C. Q., et al. (2019). The AGC kinase SsAgc1 regulates Sporisorium scitamineum mating/filamentation and pathogenicity. mSphere 4, e00259–e00219. doi: 10.1128/mSphere.00259-19
Wei, J., Bi, Y., Xue, H., Wang, Y., Zong, Y., and Prusky, D. (2020). Antifungal activity of cinnamaldehyde against Fusarium sambucinum involves inhibition of ergosterol biosynthesis. J. Appl. Microbiol. 129, 256–265. doi: 10.1111/jam.14601
Wu, J., Li, H. Z., Deng, Q. Q., Chen, J. W., and Shen, W. K. (2020). Transcriptomic analysis of Sporisorium scitamineum isolates with different pathogenicity. J. Huazhong Agric. Univ. 33, 40–44. doi: 10.13300/j.cnki.hnlkxb.2020.03.007
Wu, Y. Q., Wu, M. Y., Wang, Y. Y., Chen, Y. S., Gao, J., and Ying, C. M. (2018). ERG11 couples oxidative stress adaptation, hyphal elongation, and virulence in Candida albicans. FEMS Yeast Res. 18:foy057. doi: 10.1093/femsyr/foy057
Xiao, Y., and Li, H. (2020). MAPKK-encoding gene CfMKK1 in Colletotrichum fructicola is required for its growth and pathogenicity. J. Microbiol., 1–12. doi: 10.13343/j.cnki.wsxb.20200124
Yan, M. X., Zhu, G. N., Lin, S. Y., Xian, X. Y., Chang, C. Q., Xi, P. G., et al. (2016). The mating-type locus b of the sugarcane smut Sporisorium scitamineum is essential for mating, filamentous growth and pathogenicity. Fungal Genet. Biol. 86, 1–8. doi: 10.1016/j.fgb.2015.11.005
Yang, S. L., and Chung, K. R. (2012). The NADPH oxidase-mediated production of hydrogen peroxide (H2O2) and resistance to oxidative stress in the necrotrophic pathogen Alternaria alternata of citrus. Mol. Plant Pathol. 13, 900–914. doi: 10.1111/j.1364-3703.2012.00799.x
Yuzo, Y., Yuri, A., Mitsuhide, N., and Osamu, G. (2000). Sterol 14-demethylase P450 (CYP51) provides a breakthrough for the discussion on the evolution of cytochrome P450 gene superfamily. Biochem. Biophys. Res. Commun. 273, 799–804. doi: 10.1006/bbrc.2000.3030
Zhang, B., Cui, G. B., Chang, C. Q., Wang, Y. X., Zhang, H. Y., Chen, B. S., et al. (2019). The autophagy gene ATG8 affects morphogenesis and oxidative stress tolerance in Sporisorium scitamineum. J. Integr. Agric. 18, 1024–1034. doi: 10.1016/S2095-3119(18)62109-4
Zhang, Z., He, X., Li, W., Lu, Y., and Zhang, B. (2009). Regulation role of sterol C-24 methyltransferase and sterol C-8 isomerase in the ergosterol biosynthesis of Saccharomyces cerevisiae. Acta Microbiol Sin. 49, 1063–1068. doi: 10.13343/j.cnki.wsxb.2009.08.009
Keywords: Sporisorium scitamineum, cytochrome P450 sterol 14 alpha-demethylase, ergosterol, sexual mating, pathogenicity
Citation: Li H, Cai Y, Deng Q, Bao H, Chen J and Shen W (2021) Cytochrome P450 Sterol 14 Alpha-Demethylase Gene SsCI72380 Is Required for Mating/Filamentation and Pathogenicity in Sporisorium scitamineum. Front. Microbiol. 12:696117. doi: 10.3389/fmicb.2021.696117
Received: 16 April 2021; Accepted: 01 December 2021;
Published: 23 December 2021.
Edited by:
Massimo Reverberi, Sapienza University of Rome, ItalyReviewed by:
Jennifer Alcaíno, University of Chile, ChileCopyright © 2021 Li, Cai, Deng, Bao, Chen and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wankuan Shen, d2tzaGVuNjlAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.