
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 24 May 2021
Sec. Food Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.686100
This article is part of the Research TopicFrom Traditional to Modern: Progress of Molds and Yeasts in Fermented-Food ProductionView all 45 articles
The pink-red color of traditional sausages (cured meat) is the result of nitrite addition and the formation of nitrosomyoglobin. However, the pleasant color of processed meat products is a side effect of nitrite addition while the main anticipated goal is to suppress the germination of clostridial spores. The fungus Monascus is known as a producer of oligoketide pigments, which are used in Asian countries, especially in China, for coloring foods, including meat products. Although, different biological activities of Monascus pigments have been tested and confirmed in many studies, their effect on germination of bacterial spores has never been investigated. This study is focused on testing the activity of red yeast rice (RYR) extract, containing monascin, rubropunctatin, rubropunctamine complexes and monascuspiloin as the main pigments, on germination of Clostridium and Bacillus spores. It was found that addition of nitrite alone, at the permitted concentration, had no effect on spore germination. However, the combined effects of nitrite with NaCl, tested after addition of pickling salt, was efficient in inhibiting the germination of C. beijerinckii spores but had no effect on B. subtilis spores. In contrast, total suppression of C. beijerinckii spore germination was reached after addition of RYR extract to the medium at a concentration of 2% v/v. For B. subtilis, total inhibition of spore germination was observed only after addition of 4% v/v RYR extract to the medium containing 1.3% w/w NaCl.
Red yeast rice (RYR) is rice fermented by the fungus Monascus, which is prepared for different applications using different Monascus species (for a recent review see Zhu et al., 2019). RYR has various synonyms such as hong-qu, beni-koji or ang-kak in languages of Asian countries, where the product is popular. In Europe, RYR is only permitted as a food supplement on the condition that it is prepared using Monascus purpureus and the preparation (food supplement) should contain 10 mg of monacolin K, administered daily, in order to guarantee the effect as described in the health claim “Monacolin K from red yeast rice contributes to the maintenance of normal blood cholesterol levels” (Commission Regulation (EU) No 432/2012, 2012). As a dose of 10 mg of monacolin K corresponds to the lowest therapeutically effective dose of statins in prescription drugs, the required amount of monacolin K in RYR food supplements was reconsidered by the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) in 2018 (EFSA Panel on Food Additives and Nutrient Sources added to Food [ANS] et al., 2018) but with no clear conclusion. Nevertheless, the main concern associated with the use of RYR is its potential contamination with citrinin, a mycotoxin whose toxicological effects on people have not been fully elucidated (de Oliveira Filho et al., 2017). By the Commission Regulation (EU) 2019/1901, 2019, the maximum tolerated citrinin concentration in RYR was set to 100 μg/kg.
The fungus Monascus is especially known for its production of red pigments, which are used in certain Asian countries, such as China, Japan or Philippines, for food coloring. As the color red is associated with many different fruits, vegetables and meat products, Monascus pigments are mostly used for coloring cakes or other sweet products, fruit yogurts or other fermented milk products and processed meat. The suitability of Monascus pigments for coloring meat products, particularly with regard to color, texture, smell and other sensory parameters of the products, has already been proven in the scientific literature (Leistner et al., 1991; Fabre et al., 1993; Yu et al., 2015; Seong et al., 2017). In addition, inhibitory effects of Monascus pigments or Monascus extracts on vegetative bacterial cells, e.g., Staphylococcus aureus, Escherichia coli, Bacillus subtilis (Kim et al., 2006; Vendruscolo et al., 2014; Zhao et al., 2016) have been demonstrated. In addition, the safety of Monascus pigments for human consumption has been confirmed in several studies (Bianchi, 2005; Yu et al., 2008; Mohan Kumari et al., 2009).
The aim of the study was to test whether an ethanol extract of RYR, having a red color and containing a mixture of Monascus pigments but without citrinin and monacolin K, might suppress germination of bacterial spores. In traditional food processing, nitrite salts have been added to meat products in order to achieve total inhibition of germination of Clostridium botulinum spores. Nitrite salts are also responsible for the pleasing red color of processed meat products, caused by the formation of nitrosomyoglobin. In the work described for the first time here, Clostridium beijerinckii and Bacillus subtilis spores were used as models of anaerobic and aerobic bacterial spore formers, respectively.
Monascus sp. DBM 4361, isolated from a non-sterile dried red fermented rice sample, was maintained on Potato-Dextrose agar (VWR Chemicals) slants at 4°C. The strain was deposited at the Department of Biochemistry and Microbiology (DBM), University of Chemistry and Technology Prague.
An amount of rice (Giana, Thailand) (150 g) was washed with hot water, then boiled for 1 min. The rice was evenly divided into three autoclavable plastic bags, which were closed with a metal ring and a cotton plug. The use of plastic bags instead of glass vessels enabled manual separation of rice kernels after sterilization without opening of the bags. The cultivation was inspired by soy koji preparation (Lotong and Suwanarit, 1983). The bags were placed in a beaker sealed with aluminum foil and sterilized at 121°C for 20 min. Sterilization was repeated after 24 h to eliminate contamination by spore-forming bacteria. Spores from the Monascus culture (mixture of ascospores and conidia, because the strain formed both asexual and sexual spores, see Figure 1) were transferred to sterile water using a sterile loop. The sterile rice in the bags was inoculated with 5 mL of the spore suspension. Cultivation of the fungus on rice was performed for 10 days at 30°C. The rice was mixed by hand daily.
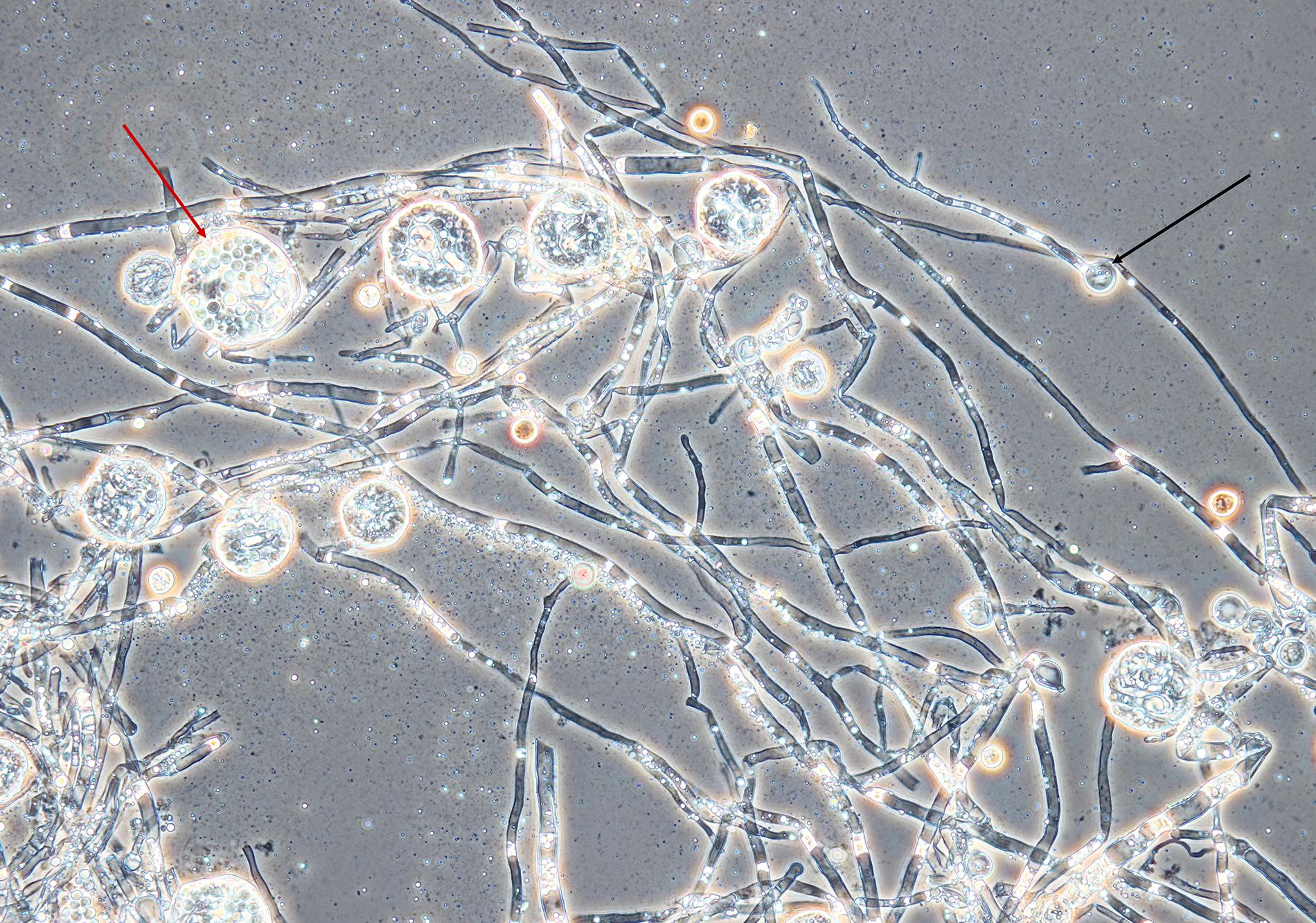
Figure 1. Mycelium, conidia and cleistothecia of Monascus sp. DBM 4361. An example of a conidium is marked with a black arrow, the red arrow shows a cleistothecium with ascospores. The specimen was prepared from the fungus grown on a PDA agar slant for 5 days at 30°C; magnification was 400×.
The RYR (Figure 2) (5 g) was extracted with 25 mL of 70% ethanol and distilled water in 250 mL Erlenmeyer flasks for 1 h, at 30°C, with shaking (laboratory shaker Infors, 100 rpm). The mixture was then filtered through Whatman 1 filter paper. Pooled ethanol extracts from 3 flasks were concentrated using a rotary vacuum evaporator (Boeck) (max. temperature 55°C), so that all ethanol was evaporated. The remaining water extract contained insoluble pigmented particles, which were collected by filtration and dissolved in 96% ethanol. The resulting ethanol extract, having dry matter concentration 9.4 mg/mL, was used for all microbiological assays and was analyzed by HPLC. The pH of pooled water extracts from 3 flasks was measured and shown to be 4.9.
The RYR extract was diluted 200-fold with 96% ethanol to adjust the absorbance to 0.1–1.0 at 330–600 nm. The absorbance of the sample was detected using a spectrophotometer (Varian Cary 50 Bio). The results were represented as an absorbance spectrum or as an absorbance value at a selected wavelength, the dilution factor being taken into consideration. As a blank, 96% ethanol was used.
UHPLC (Agilent Technologies 1260 Infinity II) was used to determine Monascus pigments, citrinin and monacolin K. The following conditions were used: Kinetex Polar C18, 100A, 150 × 4.6 mm column; the mobile phase: 0.025% H3PO4 in water:acetonitrile at a ratio of 60:40; isocratic elution at a flow rate 1.5 mL/min; injection volume 5 μL. For determination of yellow, orange and red pigments, a photodiode detector set at 390, 470, and 500 nm resp. was used. The presence of monacolin K was detected at 237 nm. For the determination of the mycotoxin citrinin, the fluorescence detector setting was 331 nm for excitation and 500 nm for emission. For analysis, the extract sample was diluted 10-fold with 96% ethanol.
The standards of mycotoxin citrinin (Sigma-Aldrich), yellow pigment monascin (Sigma-Aldrich), and orange pigment rubropuctatin (1717 CheMall Corporation) were used as reference samples. A rubropunctamine laboratory standard was prepared from the rubropunctatin standard by reaction with NH4OH (Penta). Unknown yellow, orange and red pigments were identified based on their absorption spectra and quantified as equivalents to their respective standards, i.e. monascin, rubropunctatin and rubropunctamine. For identification of individual compounds in the chromatogram, previous results (Patrovsky et al., 2019) were used.
HPLC-HRMS (Accella 600 Thermo Fisher Scientific) was used to determine molecular weights of the Monascus pigments. The following conditions were used: Luna Omega Polar 1.6 μm, 50 × 2.1 mm Phenomenex column; mobile phase: 0.1% HCOOH in water:methanol; gradient elution (A:B in ratio from 90:10 to 5:95) at a flow rate of 300 μL/min; injection volume 5 μL; ESI-positive mode; LTQ Orbitrap Velos mass analyzer (Thermo Scientific). For the analysis, the extract sample was diluted 200-fold with a solution of 90% water 10% methanol.
Clostridium beijerinckii NCIMB 8052 and Bacillus subtilis DBM 3006 were stored in the form of spore suspensions in sterile distilled water at 4°C. Spores of C. beijerinckii NCIMB 8052 were obtained after 48 h incubation of the culture in 250 mL Erlenmeyer flasks containing 100 mL of TYA medium in an anaerobic chamber (Concept 400, Ruskinn Technology, United Kingdom) at 37°C. TYA medium contained in g/L: glucose 40, yeast extract (Merck) 2, tryptone (Sigma-Aldrich) 6, potassium dihydrogenphosphate 0.5, ammonium acetate 3, magnesium sulfate heptahydrate, 0.3, ferrous sulfate heptahydrate 0.01; the pH of the medium was adjusted prior to sterilization in the autoclave (20 min, 121°C, 0.1 MPa) to 6.8. Spores of B. subtilis DBM 3006 were obtained after 48 h incubation of the culture in 250 mL Erlenmeyer flasks containing 50 mL of MP broth shaken on a rotary shaker (New Brunswick Scientific) at 300 rpm and 30°C. MP broth contained in g/L: meat extract (Roth) 3, peptone (Roth) 5; the pH of the medium was adjusted prior to sterilization in the autoclave (20 min, 121°C) to 7.0. Salts, HCl and NaOH for medium preparation and pH adjustment were purchased from Penta, Czech Republic. After cultivation, spores of both bacterial cultures were harvested by centrifugation (Hettich MIKRO 220R) for 5 min, 5,000 rpm, at 4°C. Spores were washed with sterile water, centrifuged under the same conditions and re-suspended in 20 mL of sterile water. Finally, the spore suspension was pipetted in 1 mL portions into Eppendorf tubes, which were stored at 4°C. All spore handling was performed under aseptic conditions using sterile tools and materials. Spore concentrations were estimated by flow cytometry (Branska et al., 2018) to be 2∗108 spores/mL and 8∗108 spores/mL for C. beijerinckii and B. subtilis, respectively. Prior to inoculation of medium for germination assays, spore suspensions were heat shocked (80°C for 30 s followed by cooling on ice for 2 min) to accelerate germination.
The cultivation tests were performed in 20 mL test tubes containing 9.8 mL of medium inoculated with 0.2 mL heat shocked spore suspension of C. beijerinckii. Unmodified TYA medium was used as a positive control. The cultivation tests were performed in triplicate, in the anaerobic chamber, at 37°C for 48 h. Different combinations of agents were added to the TYA medium to test their effect on spore germination (see Table 1).
The cultivation tests were performed on microcultivation plates using the Bioscreen C device (LabSystem) with intermittent shaking (30 s every 3 min) at 30°C for 24 h. Each well of the plate was filled with 196 μL of medium and 4 μL of B. subtilis heat shocked spore suspension. In each well, optical density was measured at 600 nm, every 30 min. Unmodified MP medium was used as a positive control. Each cultivation test was performed in 6 wells. Different combinations of agents were added to the MP medium to test their effect on spore germination (see Table 2).
Clostridium beijerinckii spores were chosen as a model substituting for Clostridium botulinum spores because both species belong to the same Cluster I (sensu stricto) of the Clostridium genus (Cruz-Morales et al., 2019). Heat-shocked spores of C. beijerinckii were inoculated to 2% by volume to the medium in test tubes and were allowed to germinate under anaerobic conditions. The TYA medium and the inoculation ratio were chosen to guarantee reliable spore germination based on previous experience with the strain (Kolek et al., 2016). The compositions of different media were designed (see Table 1) to be able to compare any effect of nitrite salts with the potential effect of the RYR extract, not containing citrinin and monacolin K. Nitrites are only allowed to be added to meat products in a mixture with sodium chloride in the form of NaNO2 (E249 food additive) or KNO2 (E250 food additive), in an amount not exceeding 150 mg of nitrite per 1 kg of a standard meat product (only in some national specialities produced in different EU countries can the amount of nitrite be higher, up to 300 mg/kg, for certain salamis and bacons and up to 500 mg/kg for herrings and sprouts); for a survey of rules valid in the EU for the addition of nitrite/nitrate to meat products (see Honikel, 2008).
Nitrite, in the form of NaNO2, was added to the culture, either independently or as a component of Praganda nitrite pickling salt. In addition, NaNO3 (E251 food additive) was tested because its addition is permitted and applied in cheeses to a maximum concentration of 150 mg/kg, with the aim of suppressing germination of Clostridium butyricum spores. While even the addition of NaNO2 or NaNO3 alone at a concentration of 300 mg/kg had no effect on the germination of C. beijerinckii spores, addition of nitrite pickling salt to the recommended concentration for various products, i.e., 2% (w/w) of the pickling salt or 1.3% (w/w) as recommended for products with low salt content, resulted in total inhibition of spore germination (see Table 3, experiment codes TYA + NaNO2; TYA + NaNO3; TYAN2 and TYAN1). As Clostridium beijerinckii is sensitive to high concentrations of NaCl (Branska et al., 2020), the independent effect of NaCl addition was also tested. While addition of NaCl to 2% w/w suppressed spore germination, 1.3% w/w did not reliably suppress germination in all cases (see Table 3, code TYAS2 and TYAS1).
Addition of 2% v/v RYR extract was tested with standard TYA medium and with medium containing 1.3% w/w NaCl; addition of ethanol to the same concentration (2% v/v) was tested as a control (Table 3, codes TYA + RYR, TYA + Et, TYAS1 + RYR, TYAS1 + Et). While addition of the RYR extract suppressed spore germination, ethanol did not. The RYR extract did not contain citrinin but, because it is known that citrinin has certain antimicrobial properties, the citrinin effect was tested at a concentration of 2,000 μg/kg. This concentration of citrinin reflects the amount that was EU-permitted in an RYR food supplement until 2019, after which the limit was reconsidered and adjusted to 100 μg/kg. Citrinin was tested alone or in combination with RYR (Table 3, codes TYA + cit, TYA + RYR + cit).
Typical test tube growth characteristics of C. beijerinckii exhibiting high turbidity, foam and development of bubbles of fermentation gas (mixture of CO2 and hydrogen) as well as color of the medium after addition of the RYR extract are shown in Figure 3. Only the ability to grow (indicated as + or −) was tested in the C. beijerinckii germination assay. Determination of optical density was not performed in order to not disturb the anaerobic atmosphere. Changed morphology from vegetative cells to spores influences OD values, so comparisons between tests might be misleading. Spores in TYA medium (positive control experiment) started to germinate 16–18 h after inoculation, and in other cases, germination started with different delays.
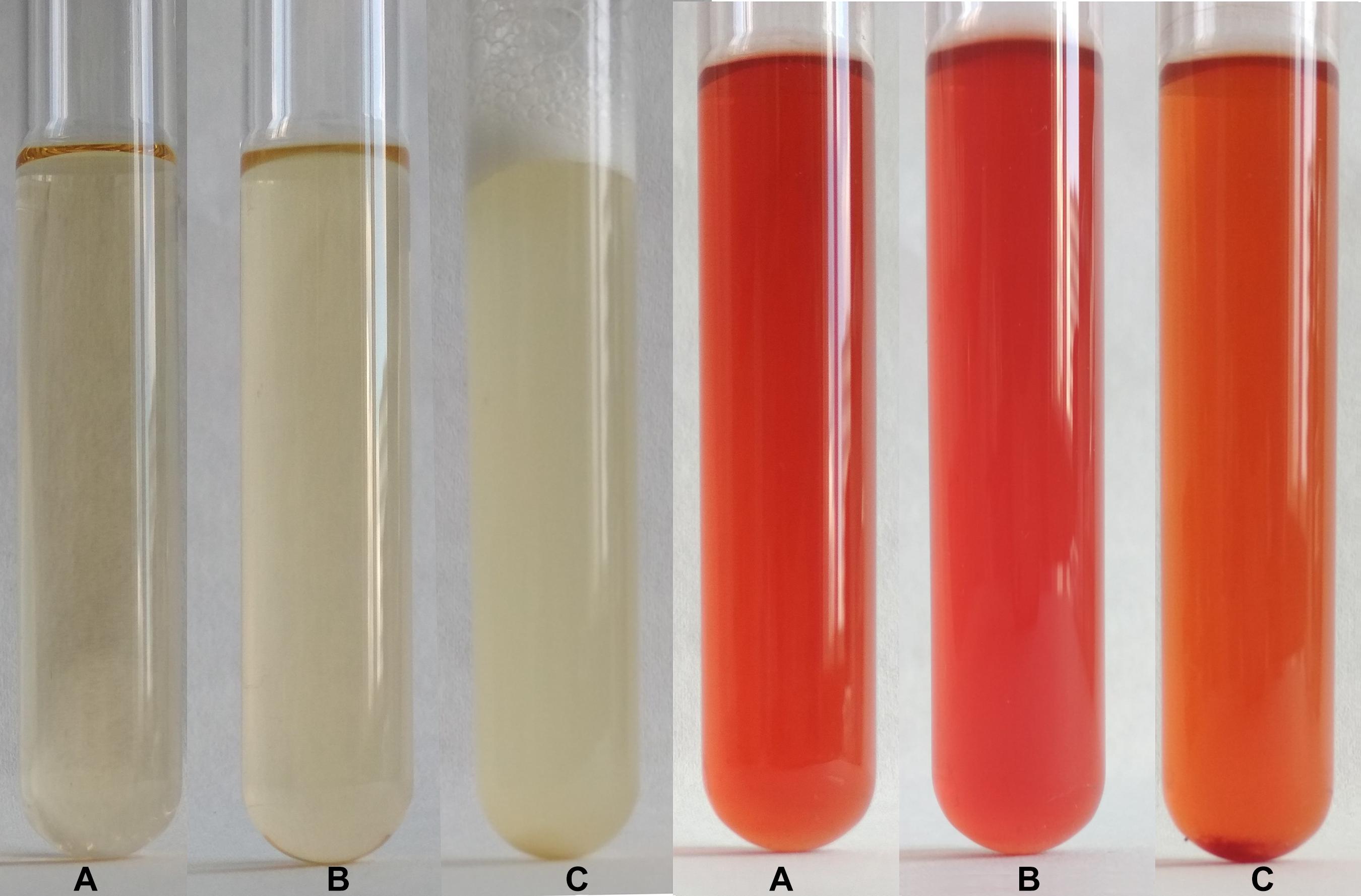
Figure 3. Demonstration of growth in test tubes. Positive control, i.e., outgrowth of C. beijerinckii spores in TYA medium is shown on the left while suppression of spore germination after addition of RYR extract to 2% v/v is shown on the right. A test tube with medium was photographed (A) prior to inoculation, (B) after inoculation, and (C) after 48 h cultivation.
To follow the growth of spore formers after germination, Bacillus subtilis germination assays were performed, even if these spores do not normally occur in meat products (Figure 4). Nevertheless, B. subtilis spores are more resistant to adverse environmental effects in comparison with the C. beijerinckii spores, therefore the design of experiments had to be different (Table 2) in order to inhibit germination. Total suppression of spore germination was achieved only after addition of the RYR extract to 4% v/v in medium containing 1.3% w/w NaCl (Figure 4D, MPS1 + RYR2). In other cases, growth was always detected, even if, in some cases, germination was delayed by up to 10 h (Figures 4B,C, MP + RYR2; MP + RYR1 + Cit). Surprisingly, addition of nitrite pickling salt to the recommended 2% w/w did not inhibit germination of the spores (Figure 4A, MPN2).
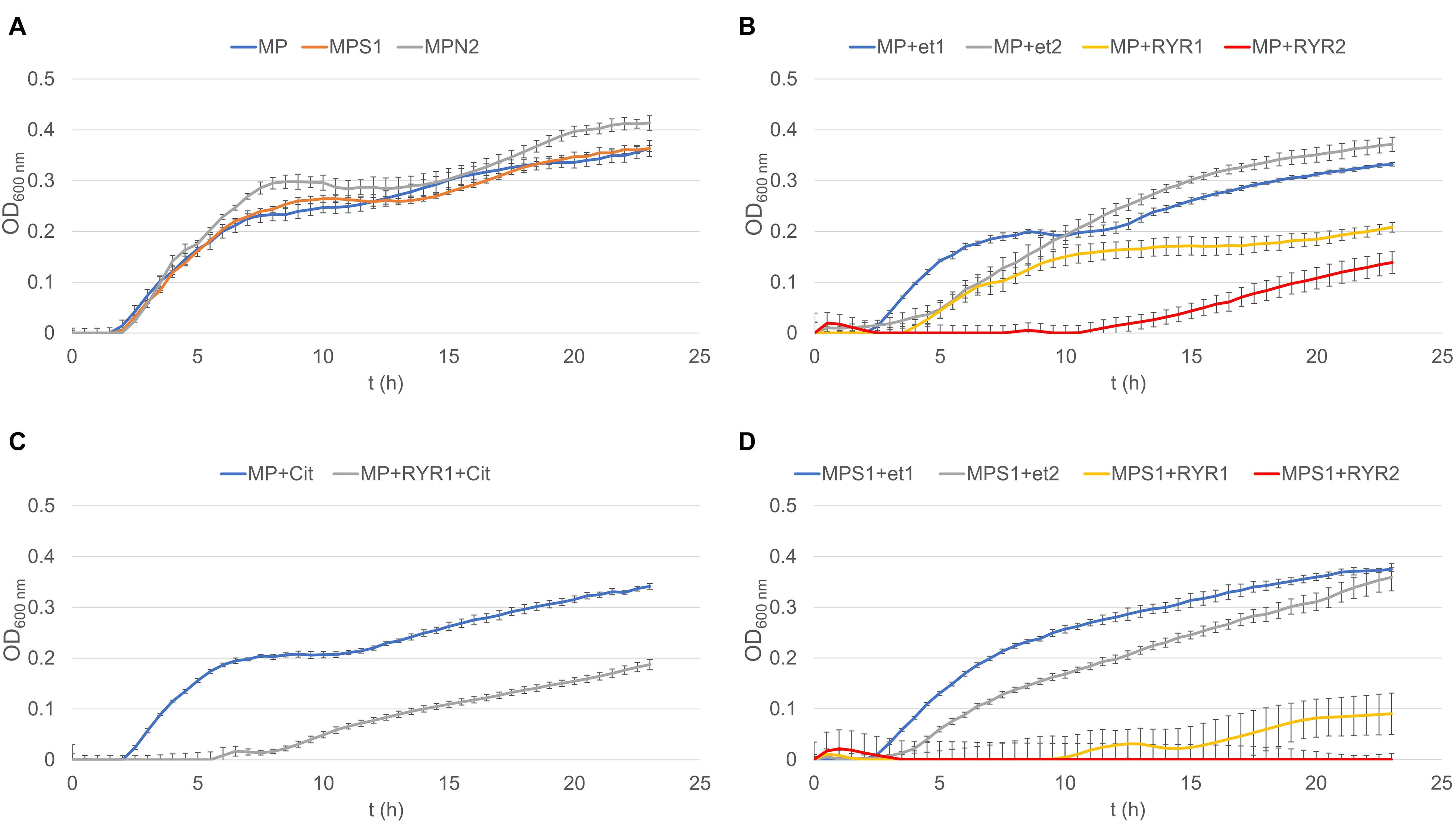
Figure 4. Growth curves of B. subtilis using different compositions of culture medium (for the experiment design see Table 2). (A) Shows control experiment in MP medium and demonstrates the influence of NaCl to 1.3% w/w (MPS1) and pickling nitrite salt to 2.0% w/w (MPN2). (B) Shows the effect of ethanol at concentrations of 1.92 and 3.84% v/v to MP medium (MP + Et1, MP + Et2) and addition of RYR ethanol extract to 1.92 and 3.84% v/v (MP + RYR1, MP + RYR2). (C) Shows the citrinin effect independently (MP + cit) or in combination with RYR extract (MP + RYR1 + cit). (D) Shows the combined influence of NaCl (1.3% w/w) and ethanol (1.92 and 3.84% v/v); (MPS1 + Et1, MPS1 + Et2) or RYR ethanol extract (2 and 4% v/v); (MPS1 + RYR1, MPS1 + RYR2).
The absorption spectrum of the RYR extract is shown in Figure 5. Values of absorption found by spectrophotometric analysis at 390, 470, and 500 nm, corresponding to assumed absorption maxima of yellow, orange and red pigments, were 98, 58, and 70, respectively. Monascin (yellow), rubropunctatin (orange) and rubropunctamine (red) were identified in the RYR extract by UHPLC analysis (Figure 6), while their analogs with seven carbon side chains, i.e., ankaflavin (yellow), monascorubrin (orange) and monascorubramine (red) were not detected; neither was citrinin or monacolin K. The detected yellow pigments were quantified as monascin equivalents (1m220 mg/L), orange pigments as rubropunctatin equivalents (336 mg/L) and red pigments as rubropunctamine equivalents (408 mg/L). However, other compounds labeled as yellow I and red I-VI were found and for their putative identification, HPLC-MS analysis and already published m/z data on different Monascus metabolites were used (for survey of the Monascus pigments data see Chen et al., 2019). While red I-red VI are probably rubropunctamine derivatives that were formed by the reaction of rubropunctatin with available amino group containing compounds, yellow I was identified as monascuspiloin (m/z 360.4).
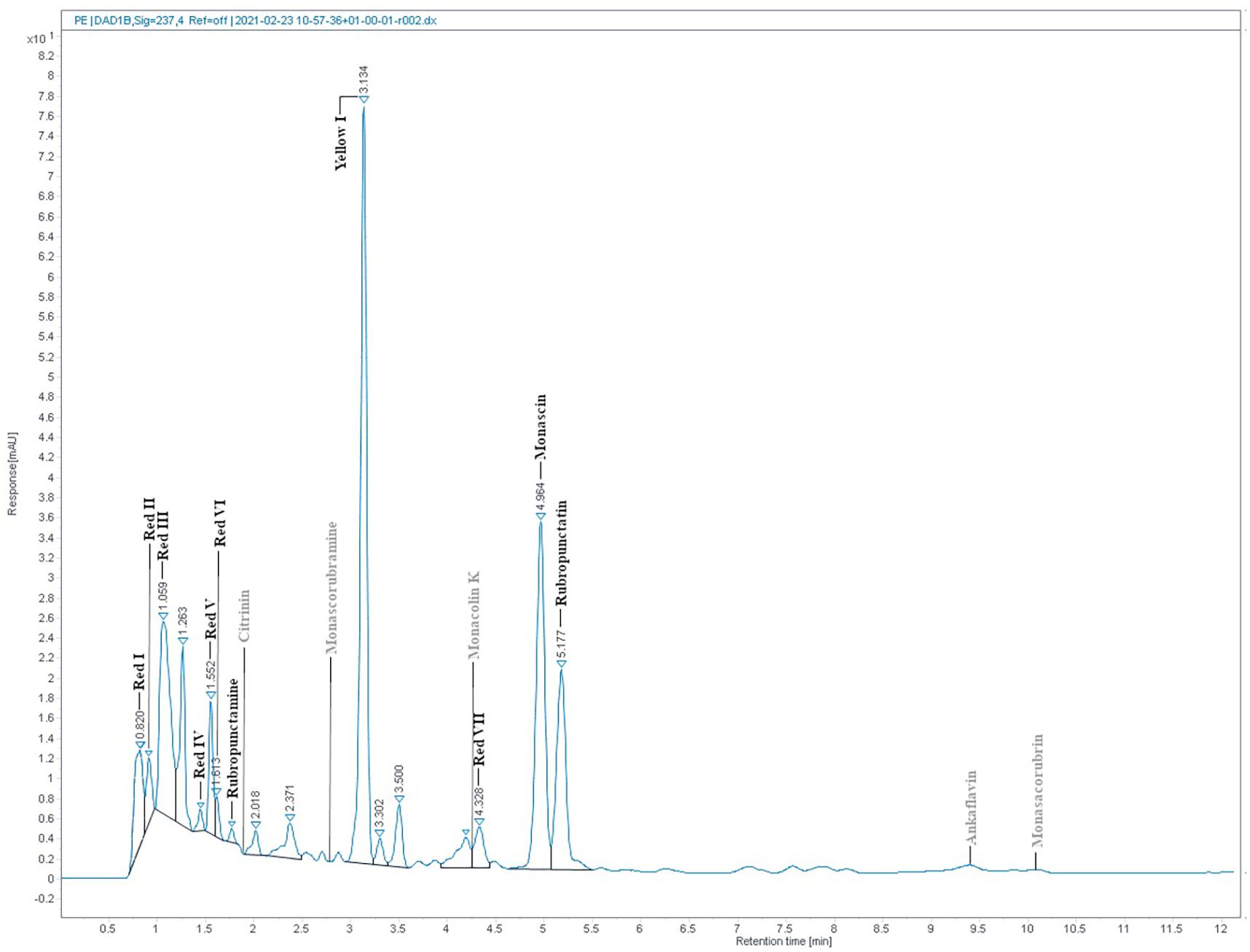
Figure 6. RYR extract chromatogram from UHPLC analysis. Detected compounds are given in black while expected but undetected compounds are given in gray color. Conditions of UHPLC analysis are given in section “RYR Extract Analysis.”
The RYR extract was added to TYA and MP medium to concentrations of 2 and 4% v/v, respectively, and the concentrations of pigments added to the medium are shown in Table 4. The water extract of the RYR had a pH 4.9 but the pH of TYA or MP medium was 6.8 or 7.0. To determine whether the pigment profile was the same as the original RYR extract in the culture medium, samples of medium containing RYR mixed in a 1:1 volume ratio were analyzed by UHPLC. As expected, rubropunctatin was absent in the samples while monascin and monascuspiloin remained (data not shown).
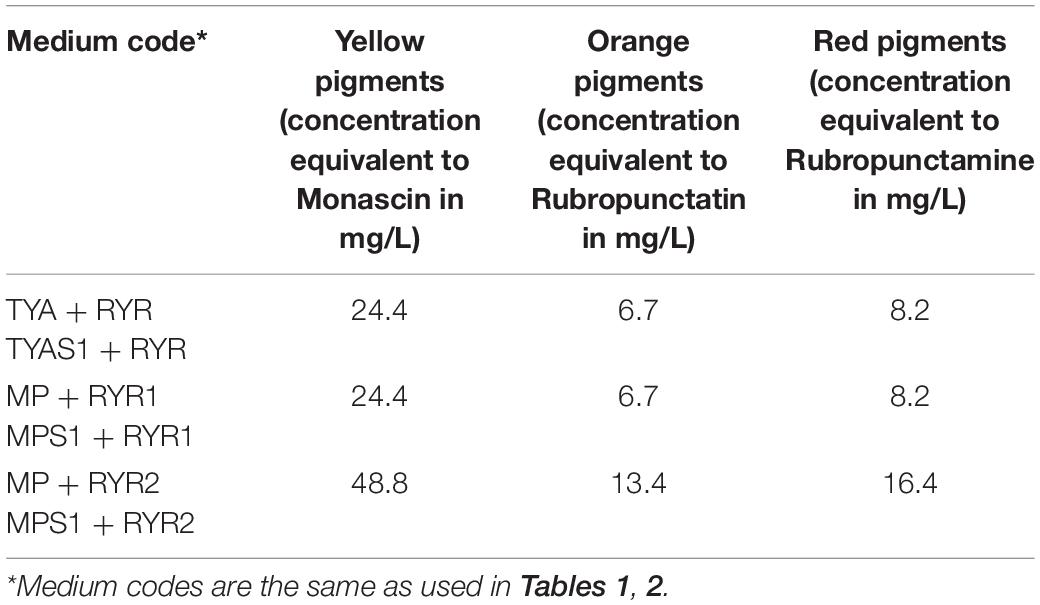
Table 4. Concentration of pigments quantified as monascin, rubropunctatin, and rubropunctamine equivalents added to TYA and MP media.
Nitrite and nitrate addition to meat products, together with NaCl, is traditional in European countries and is considered to be of low impact on human health even if cancerogenic nitrosamines can be formed in the acidic environment of the human stomach after ingestion of nitrite/nitrate containing food (Honikel, 2008; EFSA Panel on Food Additives Nutrient Sources added to Food [ANS] et al., 2017). It is believed that the benefits of stable red color, antioxidant and antimicrobial effects of nitrite/nitrate outweigh potential risks. Nevertheless, within this study it was found that addition of nitrite or nitrate alone, to the permitted concentration, did not suppress spore germination. Similar observations were documented in other studies for the germination of Clostridium perfringens (Labbe and Duncan, 1970), Clostridium botulinum (Sofos et al., 1979) or cheese associated clostridia including C. beijerinckii (Ávila et al., 2014) spores. However, combined effect of nitrite with NaCl tested after addition of the pickling salt both at the standard concentration of pickling salt (2% w/w) and at the level recommended for low salt products (1.3% w/w) was efficient in inhibiting the germination of C. beijerinckii spores but had no effect on germination of B. subtilis spores. However, total suppression of germination of C. beijerinckii spores was also achieved after addition of RYR extract to TYA medium, to a concentration of 2% v/v while total suppression of germination of B. subtilis spores was only achieved after addition of 4% v/v RYR extract to MP containing 1.3% w/w NaCl. These results suggest that the RYR extract might substitute for nitrite salts in inhibiting germination of Clostridium spores.
Within the study, the ethanol effect on bacterial spore germination was confirmed (Setlow et al., 2002) as well as the effect of NaCl (Nagler et al., 2014) and the synergistic effect of different agents (Nerandzic et al., 2015). Even if the RYR extract did not contain citrinin, its effect at 2,000 μg/L (the permitted concentration of citrinin in RYR food supplements in the EU until 2019) was tested, but if applied independently, had no effect on spore germination.
Monascus sp. DBM 4361, used for the preparation of RYR, was not classified on the species level but it might be M. pilosus because its characteristics, i.e., no citrinin production and formation of both conidia and ascospores, corresponds with an already described strain, M. pilosus MS-1, also isolated from the RYR (Feng et al., 2016). The absence of citrinin production was also found in M. pilosus NBRC4520 (Higa et al., 2020). In the RYR extract, there were found three of six iconic Monascus pigments; in particular monascin, rubropunctatin and rubropunctamine, together with rubropunctamine complexes with different amino group-containing compounds and monascuspiloin, a yellow pigment with a structure similar to monascin that has already been described as the metabolite of M. pilosus M93 (Chen et al., 2012). Interestingly, only monascin and rubropunctatin, i.e., pigments with a shorter five carbon side chain, but not their analogs (ankaflavin and monascorubrin with seven carbon side chains) were found. During the biosynthesis of pigments by M. ruber M7 (Chen et al., 2017), MrPigJ and MrPigK subunits of fatty acid synthase were found as the proteins responsible for the integration of β-ketooctanoic or β-ketodecanoic acid moieties into the structure of the pigments. However, the selection of the particular fatty acid moiety was considered to be random or not yet understood. Homologous genes to MrPigJ, MrPigK were described in M. purpureus (MpFasA2, MpFasB2) (Balakrishnan et al., 2013) and other Monascus strains (Guo et al., 2019) but not in M. pilosus. Even in the newest review of azaphilone biosynthesis (Pavesi et al., 2021), factors that determine the selection of particular fatty acid moieties are not described.
For the detection of substances in Monascus extracts, it is not sufficient to use the absorption spectrum or to determine absorbance values of the extract at the absorption maxima of individual pigments, typically at 390, 470, and 500 nm. It is really necessary to analyze extracts by HPLC or other analytical method (cf. Figures 5, 6). Notwithstanding, the determination of individual compounds in Monascus extracts is difficult because it depends inter alia on the pH of the extract (Shi et al., 2016).
After the addition of the RYR extract to medium, the original pH of the RYR extract changed from 4.9 to 6.8 or 7.0, resulting in the reaction of rubropunctatin with available amino group containing compounds, such as amino acids. It is possible that this reaction might contribute to the suppression of spore germination in the medium. Amino acids such as L-alanine or glycine are known germinants (factors stimulating germination) of bacterial spores (Setlow, 2014; Bhattacharjee et al., 2016) and if their amount was decreased it might affect germination. In addition, it was reported that Monascus red pigment derivatives had pronounced effects on the growth of Gram positive bacteria, including B. subtilis (Kim et al., 2006). The assumed cause of inhibition was adsorption of pigment derivatives onto the surface of cells, limiting oxygen uptake, where their MIC values were found to be 4–8 μg/L. In our assay, rubropunctatin and a mixture of red pigment derivatives, to concentrations of 13.4 mg/L and 16.4 mg/L, respectively, were added to MP culture medium used for outgrowth of B. subtilis spores (see Table 4) and B. subtilis was cultured under aerobic conditions; the above aerobic effect (Kim et al., 2006) might also apply here. Orange and red Monascus pigments, at concentrations of 10–20 mg/L, inhibited growth of Gram negative bacteria (Vendruscolo et al., 2014), which corresponds with our findings. Yellow pigments monascin and monascuspiloin, detected in the RYR, were found to have anticancerogenic effects (Akihisa et al., 2005; Chen et al., 2012; Chiu et al., 2012) but their antibacterial effect was never tested.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
MH performed cultivation experiments, prepared samples for analyses, participated in analysis, performed data evaluation, and revised the manuscript. MP assisted with cultivation experiments, preparation of samples for analyses, participated in analysis, and revised the manuscript. BB developed the UHPLC method for the pigments analysis and revised the manuscript. PP conceived the study, designed and coordinated it, analyzed the data, and wrote the manuscript. All authors read and approved the final manuscript version.
Financial support from specific University of Chemistry and Technology in Prague research, Project No. MEYS No 8-SVV/2021.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Akihisa, T., Tokuda, H., Ukiya, M., Kiyota, A., Yasukawa, K., Sakamoto, N., et al. (2005). Anti-tumor-initiating effects of monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem. Biodivers 2, 1305–1309. doi: 10.1002/cbdv.200590101
EFSA Panel on Food Additives and Nutrient Sources added to Food [ANS], Younes, M., Aggett, P., Aguilar, F., Crebelli, R., and Dusemund, B. (2018). Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 16:e05368.
Ávila, M., Gómez-Torres, N., Hernández, M., and Garde, S. (2014). Inhibitory activity of reuterin, nisin, lysozyme and nitrite against vegetative cells and spores of dairy-related Clostridium species. Int. J. Food Microbiol. 172, 70–75. doi: 10.1016/j.ijfoodmicro.2013.12.002
Balakrishnan, B., Karki, S., Chiu, S.-H., Kim, H.-J., Suh, J.-W., Nam, B., et al. (2013). Genetic localization and in vivo characterization of a Monascus azaphilone pigment biosynthetic gene cluster. Appl. Microbiol. Biotechnol. 97, 6337–6345. doi: 10.1007/s00253-013-4745-9
Bhattacharjee, D., McAllister, K. N., and Sorg, J. A. (2016). Germinants and their receptors in Clostridia. J. Bacteriol. 198, 2767–2775. doi: 10.1128/JB.00405-16
Bianchi, A. (2005). Extracts of Monascusus purpureus beyond statins —Profile of efficacy and safety of the use of extracts of Monascus purpureus. Chin. J. Integr. Med. 11, 309–313. doi: 10.1007/BF02835797
Branska, B., Fořtová, L., Dvořáková, M., Liu, H., Patakova, P., Zhang, J., et al. (2020). Chicken feather and wheat straw hydrolysate for direct utilization in biobutanol production. Renewable Energy 145, 1941–1948. doi: 10.1016/j.renene.2019.07.094
Branska, B., Pechacova, Z., Kolek, J., Vasylkivska, M., and Patakova, P. (2018). Flow cytometry analysis of Clostridium beijerinckii NRRL B-598 populations exhibiting different phenotypes induced by changes in cultivation conditions. Biotechnol. Biofuels 11:99. doi: 10.1186/s13068-018-1096-x
Chen, R.-J., Hung, C.-M., Chen, Y.-L., Wu, M.-D., Yuan, G.-F., and Wang, Y.-J. (2012). Monascuspiloin induces apoptosis and autophagic cell death in human prostate cancer cells via the Akt and AMPK signaling pathways. J. Agric. Food. Chem. 60, 7185–7193. doi: 10.1021/jf3016927
Chen, W., Chen, R., Liu, Q., He, Y., He, K., Ding, X., et al. (2017). Orange, red, yellow: biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 8, 4917–4925. doi: 10.1039/c7sc00475c
Chen, W., Feng, Y., Molnár, I., and Chen, F. (2019). Nature and nurture: confluence of pathway determinism with metabolic and chemical serendipity diversifies Monascus azaphilone pigments. Nat. Product Rep. 36, 561–572. doi: 10.1039/C8NP00060C
Chiu, H.-W., Fang, W.-H., Chen, Y.-L., Wu, M.-D., Yuan, G.-F., Ho, S.-Y., et al. (2012). Monascuspiloin enhances the radiation sensitivity of human prostate cancer cells by stimulating endoplasmic reticulum stress and inducing autophagy. PLoS One 7:e40462. doi: 10.1371/journal.pone.0040462
Commission Regulation (EU) 2019/1901, (2019). Commission Regulation (EU) 2019/1901 of 7 November 2019 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Citrinin in Food Supplements Based on Rice Fermented with Red Yeast Monascus Purpureus. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2019:293:FULL&from=EN
Commission Regulation (EU) No 432/2012, (2012). Commission Regulation (EU) No 432/2012 of 16May 2012 Establishing a List of Permitted Health ClaimsMade on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0432&from=CS
Cruz-Morales, P., Orellana, C. A., Moutafis, G., Moonen, G., Rincon, G., Nielsen, L. K., et al. (2019). Revisiting the evolution and taxonomy of Clostridia, a phylogenomic update. Genome Biol. Evol. 11, 2035–2044. doi: 10.1093/gbe/evz096
de Oliveira Filho, J. W. G., Islam, M. T., Ali, E. S., Uddin, S. J., Santos, J. V. D. O., de Alencar, M. V. O. B., et al. (2017). A comprehensive review on biological properties of citrinin. Food Chem. Toxicol. 110, 130–141. doi: 10.1016/j.fct.2017.10.002
EFSA Panel on Food Additives Nutrient Sources added to Food [ANS], Aguilar, F., Crebelli, R., Di Domenico, A., Dusemund, B., and Frutos, M. J. (2017). Re-evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. EFSA J 15:e04786. doi: 10.2903/j.efsa.2017.4786
Fabre, C. E., Santerre, A. L., Loret, M. O., Baberian, R., Pareilleux, A., Goma, G., et al. (1993). Production and food applications of the red pigments of Monascus ruber. J. Food Sci. 58, 1099–1102. doi: 10.1111/j.1365-2621.1993.tb06123.x
Feng, Y., Chen, W., and Chen, F. (2016). A Monascus pilosus MS-1 strain with high-yield monacolin K but no citrinin. Food Sci. Biotechnol. 25, 1115–1122. doi: 10.1007/s10068-016-0179-3
Guo, X., Li, Y., Zhang, R., Yu, J., Ma, X., Chen, M., et al. (2019). Transcriptional regulation contributes more to Monascus pigments diversity in different strains than to DNA sequence variation. World J. Microbiol. Biotechnol. 35, 1–13. doi: 10.1007/s11274-019-2711-0
Higa, Y., Kim, Y.-S., Altaf-Ul-Amin, M., Huang, M., Ono, N., and Kanaya, S. (2020). Divergence of metabolites in three phylogenetically close Monascus species (M. pilosus, M. ruber, and M. purpureus) based on secondary metabolite biosynthetic gene clusters. BMC Genomics 21:679. doi: 10.1186/s12864-020-06864-9
Honikel, K.-O. (2008). The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 78, 68–76. doi: 10.1016/j.meatsci.2007.05.030
Kim, C., Jung, H., Kim, Y. O., and Shin, C. S. (2006). Antimicrobial activities of amino acid derivatives of monascus pigments. FEMS Microbiol. Lett. 264, 117–124. doi: 10.1111/j.1574-6968.2006.00451.x
Kolek, J., Branska, B., Drahokoupil, M., Patakova, P., and Melzoch, K. (2016). Evaluation of viability, metabolic activity and spore quantity in clostridial cultures during ABE fermentation. FEMS Microbiol. Lett. 363:fnw031. doi: 10.1093/femsle/fnw031
Labbe, R. G., and Duncan, C. L. (1970). Growth from spores of Clostridium perfringens in the presence of sodium nitrite. Appl. Microbiol. 19, 353–359.
Leistner, L., Fink-Gremmels, J., and Dresel, J. (1991). “Monascus extract–A possible alternative to nitrite in meats,” in Proceedings of 37th International Congress of Meat Science and Technology, (Kulmbach: Federal Centre for Meat Research), 1252–1256.
Lotong, N., and Suwanarit, P. (1983). Production of soy sauce koji mold spore inoculum in plastic bags. Appl. Environ. Microbiol. 46, 1224–1226. doi: 10.1128/AEM.46.5.1224-1226.1983
Mohan Kumari, H. P., Akhilender Naidu, K., Vishwanatha, S., Narasimhamurthy, K., and Vijayalakshmi, G. (2009). Safety evaluation of Monascus purpureus red mould rice in albino rats. Food Chem. Toxicol. 47, 1739–1746. doi: 10.1016/j.fct.2009.04.038
Nagler, K., Setlow, P., Li, Y.-Q., and Moeller, R. (2014). High salinity alters the germination behavior of Bacillus subtilis spores with nutrient and nonnutrient germinants. Appl. Environ. Microbiol. 80, 1314–1321. doi: 10.1128/AEM.03293-13
Nerandzic, M. M., Sankar, C. T., Setlow, P., and Donskey, C. J. (2015). A cumulative spore killing approach: synergistic sporicidal activity of dilute peracetic acid and ethanol at low pH against Clostridium difficile and Bacillus subtilis spores. Open Forum Infect. Dis. 3:ofv206. doi: 10.1093/ofid/ofv206
Patrovsky, M., Sinovska, K., Branska, B., and Patakova, P. (2019). Effect of initial pH, different nitrogen sources, and cultivation time on the production of yellow or orange Monascus purpureus pigments and the mycotoxin citrinin. Food Sci. Nutr. 7, 3494–3500. doi: 10.1002/fsn3.1197
Pavesi, C., Flon, V., Mann, S., Leleu, S., Prado, S., and Franck, X. (2021). Biosynthesis of azaphilones: a review. Nat. Prod. Rep. doi: 10.1039/D0NP00080A
Seong, P. N., Ba, H. V., Kim, Y. S., Kang, S. M., Cho, S. H., Kim, J. H., et al. (2017). Effects of additions of Monascus and laccaic acid on the color and quality properties of nitrite-free emulsion sausage during refrigerated storage. Korean J. Food Sci. Anim. Resour. 37, 10–17. doi: 10.5851/kosfa.2017.37.1.10
Setlow, B., Loshon, C. A., Genest, P. C., Cowan, A. E., Setlow, C., and Setlow, P. (2002). Mechanisms of killing spores of Bacillus subtilis by acid, alkali and ethanol. J. Appl. Microbiol. 92, 362–375. doi: 10.1046/j.1365-2672.2002.01540.x
Setlow, P. (2014). Germination of spores of Bacillus species: what we know and do not know. J. Bacteriol. 196, 1297–1305. doi: 10.1128/JB.01455-13
Shi, K., Chen, G., Pistolozzi, M., Xia, F., and Wu, Z. (2016). Improved analysis of Monascus pigments based on their pH-sensitive UV-Vis absorption and reactivity properties. Food Addit Contam. Part A 33, 1396–1401. doi: 10.1080/19440049.2016.1214289
Sofos, J., Busta, F., and Allen, C. (1979). Sodium nitrite and sorbic acid effects on Clostridium botulinum spore germination and total microbial growth in chicken frankfurter emulsions during temperature abuse. Appl. Environ. Microbiol. 37, 1103–1109. doi: 10.1128/AEM.37.6.1103-1109.1979
Vendruscolo, F., Tosin, I., Giachini, A. J., Schmidell, W., and Ninow, J. L. (2014). Antimicrobial activity of Monascus pigments produced in submerged fermentation. J. Food Process. Preserv. 38, 1860–1865. doi: 10.1111/jfpp.12157
Yu, C.-C., Wang, J.-J., Lee, C.-L., Lee, S.-H., and Pan, T.-M. (2008). Safety and mutagenicity evaluation of nanoparticulate red mold rice. J. Agric. Food Chem. 56, 11038–11048. doi: 10.1021/jf801335u
Yu, X., Wu, H., and Zhang, J. (2015). Effect of Monascus as a nitrite substitute on color, lipid oxidation, and proteolysis of fermented meat mince. Food Sci. Biotechnol. 24, 575–581. doi: 10.1007/s10068-015-0075-2
Zhao, G.-P., Li, Y.-Q., Yang, J., and Cui, K.-Y. (2016). Antibacterial characteristics of orange pigment extracted from Monascus pigments against Escherichia coli. Czech J. Food Sci. 34, 197–203. doi: 10.17221/430/2015-CJFS
Keywords: Monascus, red yeast rice, bacterial spores germination, nitrite, Clostridium beijerinckii, Bacillus subtilis
Citation: Husakova M, Plechata M, Branska B and Patakova P (2021) Effect of a Monascus sp. Red Yeast Rice Extract on Germination of Bacterial Spores. Front. Microbiol. 12:686100. doi: 10.3389/fmicb.2021.686100
Received: 26 March 2021; Accepted: 27 April 2021;
Published: 24 May 2021.
Edited by:
Wanping Chen, Georg-August-University Goettingen, GermanyReviewed by:
Yanli Feng, Hubei Normal University, ChinaCopyright © 2021 Husakova, Plechata, Branska and Patakova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petra Patakova, cGV0cmEucGF0YWtvdmFAdnNjaHQuY3o=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.