
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 25 June 2021
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.676414
This article is part of the Research Topic Gram-Negative Pathogenesis View all 9 articles
 Xiaoxuan Feng1,2
Xiaoxuan Feng1,2 Shuai Liu2,3
Shuai Liu2,3 Yang Wang2,3
Yang Wang2,3 Yulin Zhang2,3,4
Yulin Zhang2,3,4 Lingxiao Sun2,3
Lingxiao Sun2,3 Haibo Li2,3,4
Haibo Li2,3,4 Chunlei Wang2,3,4
Chunlei Wang2,3,4 Yingmei Liu2,3,4*
Yingmei Liu2,3,4* Bin Cao1,2,3,4,5*
Bin Cao1,2,3,4,5*Colistin-resistant (Col-R) bacteria are steadily increasing, and are extremely difficult to treat. New drugs or therapies are urgently needed to treat infections caused by these pathogens. Combination therapy with colistin and other old drugs, is an important way to restore the activity of colistin. This study aimed to investigate the activity of colistin in combination with the anti-rheumatic drug auranofin against Col-R Gram-negative bacteria. The results of checkerboard analysis demonstrated that auranofin synergized with colistin against Col-R Gram-negative bacteria. Time-kill assays showed significant synergistic antimicrobial activity of colistin combined with auranofin. Electron microscopy revealed that the combination resulted in more cellular structural alterations compared to each drug alone. Auranofin enhanced the therapeutic effectiveness of colistin in mouse peritoneal infection models. These results suggested that the combination of colistin and auranofin might be a potential alternative for the treatment of Col-R Gram-negative bacterial infections.
Polymyxins (including polymyxin B and colistin) are considered as last resort drugs for the treatment of infections caused by extensively drug-resistant Gram-negative bacteria. Polymyxins act on the outer membrane of Gram-negative bacteria, disrupt the stability of cell membrane, cause osmotic imbalance, and ultimately lead to cell death (Velkov et al., 2010; Berglund et al., 2015). Before 2015, mutations in chromosomal genes, such as pmrAB, phoPQ, and mgrB, were considered as the main causes of high-level polymyxin resistance (Olaitan et al., 2014). However, the discovery of plasmid-encoded mcr-1 gene led to global reports of polymyxin resistance (Liu et al., 2016; Wang R. et al., 2018).
The gradual emergence of the polymyxin-resistant bacterial strains has greatly limited the antibiotic therapy options. It has been shown that repurposing FDA-approved non-antibiotic drugs in combination with polymyxin could be a promising alternative therapeutic strategy (Schneider et al., 2016; Ayerbe-Algaba et al., 2018, 2019; Cannatelli et al., 2018; Tran et al., 2018; Wang Y.M. et al., 2018; Zhou et al., 2018; Falagas et al., 2019; Hussein et al., 2020). To date, a number of FDA-approved non-antibiotic drugs, e.g., azidothymidine (Falagas et al., 2019), oxyclozanide (Ayerbe-Algaba et al., 2019), niclosamide (Ayerbe-Algaba et al., 2018), resveratrol (Cannatelli et al., 2018), mitotane (Tran et al., 2018), sertraline (Hussein et al., 2020), ivacaftor (Schneider et al., 2016), eugenol (Wang Y.M. et al., 2018), pterostilbene (Zhou et al., 2018), in combination with polymyxins have displayed synergistic killing against polymyxin-resistant Gram-negative bacteria. Auranofin is an FDA-approved anti-rheumatoid arthritis drug (Roder and Thomson, 2015). Adverse effects are associated with the long-term (months to years) use of auranofin, including diarrhea (40% of subjects), skin rashes (2–5%), hematologic abnormalities (rare), and proteinuria (5%; Kean et al., 1997). However, the most common side effect, diarrhea, can be easily managed (Suarez-Almazor et al., 2000). Hence, its largely acceptable toxicity paves the way to its repositioning for new and different therapeutic uses. Auranofin exerts a significant antimicrobial activity against numerous Gram-positive bacteria (Aguinagalde et al., 2015; Harbut et al., 2015; Tharmalingam et al., 2019), which is purported to inhibit the thioredoxin reductase (TrxR) of Gram-positive bacteria and disrupt the redox balance, resulting in cell death. However, auranofin alone has limited activity against Gram-negative bacteria (Harbut et al., 2015). Some studies have shown that colistin in combination with auranofin is effective against MDR Gram-negative bacteria (Sun et al., 2016; Torres et al., 2018). A recent study showed that auranofin could restore the activity of colistin against several mcr-1 positive Enterobacteriaceae (Sun et al., 2020). However, it is not known if auranofin can restore the activity of colistin against Colistin-resistant (Col-R) Gram-negative bacteria of different species with different colistin resistance mechanisms. In this study, the in vitro activities of colistin in combination with auranofin were evaluated against a group of clinical Col-R Gram-negative bacteria including Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii. In addition, we explored the therapeutic effectiveness of colistin combined with auranofin in mouse peritoneal infection models.
We used 23 clinical Col-R isolates in this study. Four reference colistin-susceptible (Col-S) K. pneumoniae ATCC 700603, E. coli ATCC25922, P. aeruginosa ATCC27853, and A. baumannii ATCC19606 isolates were also used. Colistin and auranofin were purchased from the National Institutes for Food and Drug Control (Beijing, China). Colistin solutions were prepared in sterile Milli-Q water before the experiments. Stock solutions of auranofin were prepared in dimethyl sulfoxide (DMSO) and then diluted with sterile Milli-Q water to ensure a final DMSO concentration of ≤5% (v/v; Lin et al., 2018).
The minimum inhibitory concentrations (MICs) of colistin and auranofin were determined by the method described in the Clinical and Laboratory Standards Institute, 2019). Colistin or auranofin was prepared with two-fold serial dilutions. A final bacterial suspension at 5 × 105 CFU/mL was added in each well, and incubated with two-fold serial dilutions of colistin or auranofin for 18 h at 37°C. MIC was determined as the lowest concentration that inhibited the visible growth of the bacteria. Colistin resistance mechanisms were analyzed with detection of pmrA, pmrB, phoP, phoQ, mgrB, mcr-1, and mcr-8 genes by PCR (Liu et al., 2016; Wang X. et al., 2018; Ayerbe-Algaba et al., 2019). The sequences of the PCR products were determined by RuiBiotech (Beijing, China).
The synergistic interaction between colistin and auranofin was tested using the checkerboard technique (Flamm et al., 2019). Colistin and auranofin were prepared with two-fold serial dilutions and mixed to create different concentration combinations. A final bacterial suspension at 5 × 105 CFU/mL was added in each well. After incubation for 18 h at 37°C, the optimal fractional inhibitory concentration index (FICI) was calculated as the MIC of the combination divided by that of each compound used alone. FICI ≤ 0.5 denotes synergy, FICI > 0.5–4 denotes no interaction and FICI > 4 denotes antagonism (Odds, 2003).
Time-kill assays were performed to further evaluate the synergistic effect of colistin with auranofin according to Hu’s method (Hu et al., 2019) with minor modifications. The concentration of colistin was used at 2 or 4 mg/L. The concentrations of auranofin were chosen according to the concentrations that showed synergistic effect with colistin in the checkerboard assays. Fresh cultures were mixed with colistin or auranofin alone or a combination and incubated at 37°C with shaking. Thereafter, 10 μL of each suspension was plated on nutrient agar plates for viable bacterial quantification after serial dilution at different time points of incubation. Synergistic activity was defined as a ≥2 log10 decrease in CFU/mL of the combination compared to the most active monotherapy (Doern, 2014).
The impact of colistin in combination with auranofin on the cellular morphology of the high-level Col-R K. pneumoniae 18605 and A. baumannii 13660 was examined by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). For K. pneumoniae 18605, a log phase culture was treated with 2 mg/L colistin, 2 mg/L auranofin, or both for 2 h in cation-adjusted Mueller–Hinton broth (CAMHB). For A. baumannii 13660, a log phase culture was treated with 2 mg/L colistin, 4 mg/L auranofin, or both for 2 h in CAMHB. Bacterial pellets were obtained by centrifugation at 4,000 g for 10 min twice. Next, the pellets were fixed overnight at 4°C with 1 mL 2.5% glutaraldehyde. The fixatives were removed after centrifugation at 4,000 g for 10 min, and finally the bacterial pellets were resuspended in 1 mL PBS. SEM was performed with a HITACHI SU8020 scanning electron microscope. For TEM observation, all images were acquired using a JEM-1200EX microscope.
To further evaluate the in vivo effect of colistin in combination with auranofin, two infection models were established in female ICR mice. 6–8 weeks old mice (weighing an average of 20 g) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. For the bacterial load experiment, A dose of 2 × 106 CFU K. pneumoniae 18605 bacterial suspension was intraperitoneally (i.p.) injected into the mice. After 1 h of infection, mice were i.p. treated with vehicle, colistin sulfate (1 mg/kg) or auranofin (0.5 mg/kg) alone or their combination (n = 8 per group). All mice were euthanized at 14 h post-infection. Peritoneal fluid (PF) was collected by injecting 2 mL sterile saline solution into the peritoneum, followed by gentle massage and aspiration. The spleen was aseptically obtained, weighed and homogenized. Individual samples were serially diluted in sterile saline solution and 0.1 mL aliquots were placed on nutrient agar plates. The colonies were counted after incubation overnight at 37°C. The bacterial loads in PF and spleen between groups were compared using one-way ANOVA and the post hoc Bonferroni test. A p-value < 0.05 was considered significant.
For survival assay, mice were infected i.p. with 1 × 107 CFU of A. baumannii 13660. After 0.5 h of infection, mice (n = 8 per group) were i.p. treated with vehicle or colistin sulfate (1.5 mg/kg) or auranofin (0.5 mg/kg) alone or in combination. The same treatment procedure was then repeated once daily until the end of the study. Survival rates were monitored for 5 days. Log-rank (Mantel–Cox) test was used to compared the survival distributions of different groups. A p-value < 0.05 was considered significant.
The animal experiments were performed in accordance with the national standards for laboratory animals in China, with approval being provided by the Animal Ethical and Welfare Committee of the China-Japan Friendship Hospital (Zryhyy11-20-07-1).
Minimum inhibitory concentrations of colistin and auranofin were tested against a group of 27 strains of four different Gram-negative bacterial species. As shown in Table 1, colistin MICs of the Col-R strains ranged from 4 to 1,024 mg/L, while those for the Col-S reference strains were 0.5–1 mg/L. MICs of auranofin were between 8 and 512 mg/L for all test strains. Mechanisms of resistance to colistin were identified in some of the strains studied (Table 1). Checkerboard assays revealed that the combination of colistin and auranofin showed a significant synergistic effect (FICI ≤ 0.5) against Col-R strains with different colistin resistance mechanisms. Additionally, the colistin MICs of most Col-R strains in the presence of auranofin were reduced to ≤2 mg/L. For all the Col-S reference strains, no interaction effect (FICI > 0.5) was observed.
The synergistic effect of colistin in combination with auranofin was tested using time-kill assays against seven selected Col-R strains, namely mcr-8 positive K. pneumoniae C505, mcr-1 positive K. pneumoniae 09-20, mcr-1 positive E. coli 08-85, high-level Col-R K. pneumoniae 18605, E. coli C1157, P. aeruginosa 26587, and A. baumannii 13660. As shown in Figure 1, for all three mcr gene positive isolates, neither colistin nor auranofin monotherapy showed any bacterial killing effect, and the growth curves were similar to that of control. The combination of colistin and auranofin resulted in the elimination of all three mcr gene positive isolates at 4 h, with no regrowth observed for K. pneumoniae C505 and K. pneumoniae 09-20. Interestingly, regrowth was observed for E. coli 08-85 after 8 h of treatment. As shown in Figure 2, for all the high-level Col-R isolates, auranofin monotherapy displayed no antimicrobial activity. Similar to auranofin, colistin monotherapy also could not prevent the growth of all high-level Col-R strains except K. pneumoniae 18605, wherein colistin monotherapy reduced the initial bacterial count slightly in the first 2 h, however, regrowth to the control value was observed at 24 h. When colistin was combined with auranofin, no viable bacterial cells were detected within 2–24 h for all high-level Col-R strains.
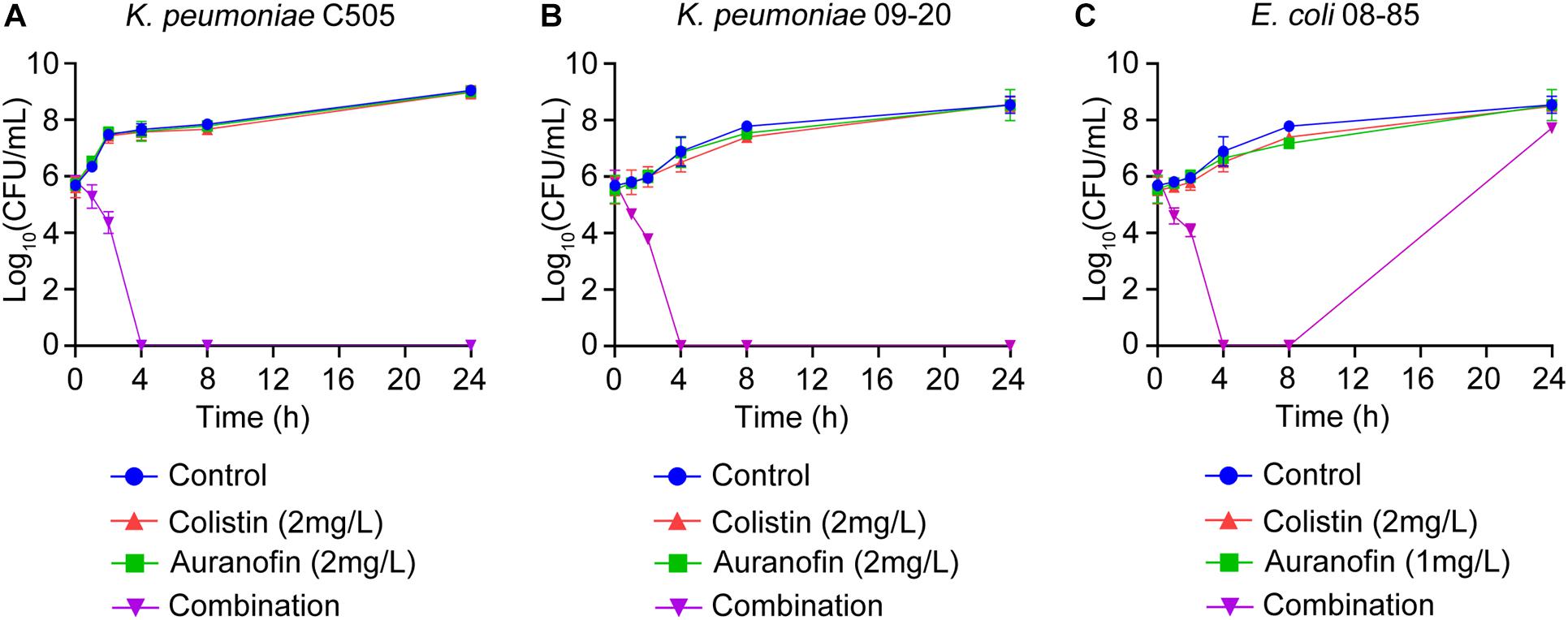
Figure 1. Time-kill assays showing the effects of colistin or auranofin alone or in combination against mcr-8 positive K. pneumoniae C505 (A), mcr-1 positive K. pneumoniae 09-20 (B), and mcr-1 positive E. coli 08-85 (C).
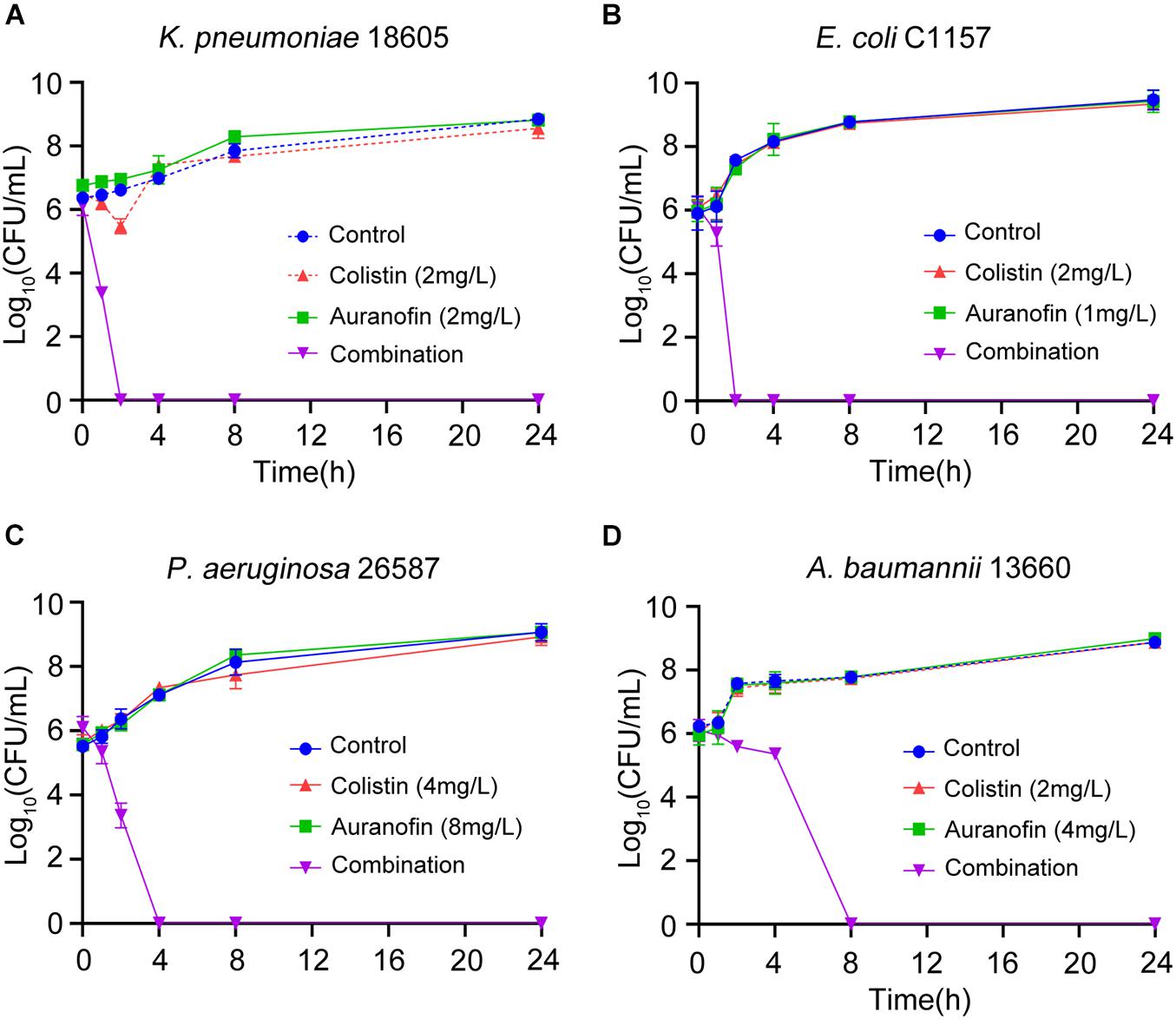
Figure 2. Time-kill assays showing the effects of colistin or auranofin alone or in combination against high-level Col-R K. pneumoniae 18605 (A), E. coli C1157 (B), P. aeruginosa 26587 (C), and A. baumannii 13660 (D).
We performed SEM and TEM experiments to determine the morphological changes of K. pneumoniae 18605 and A. baumannii 13660 induced by colistin, auranofin, or both. For K. pneumoniae 18605, SEM images showed that auranofin monotherapy displayed no morphological changes (Figure 3C) compared to the control group (Figure 3A). Membrane blebbing was evidently observed with colistin monotherapy (Figure 3B). The combination treatment caused large-scale membrane disruptions (Figure 3D). By TEM, treatment with auranofin monotherapy did not show any impact on the cellular morphology (Figure 3G) compared to the control group (Figure 3E). However, minor protrusions were caused by colistin monotherapy (Figure 3F). With the combination, bacterial cell surface was extensively disrupted and showed cell lysis (Figure 3H). For A. baumannii 13660, SEM images showed that colistin (Supplementary Figure 1B) and auranofin (Supplementary Figure 1C) monotherapy displayed no morphological changes compared to the control group (Supplementary Figure 1A). The combination treatment (Supplementary Figure 1D) resulted in a significant reduction in cell length, and the cell surface was more uneven compared to the control group (Supplementary Figure 1A). For TEM, auranofin monotherapy (Supplementary Figure 1G) did not affected the cell surface. Membrane blebbing was observed with colistin monotherapy (Supplementary Figure 1F). Bacterial cells treated with the combination (Supplementary Figure 1H) were much shorter in length compared to the control group (Supplementary Figure 1E).

Figure 3. SEM and TEM images of high-level Col-R K. pneumoniae 18605 after treatment with 2 mg/L colistin alone (B,F), 2 mg/L auranofin alone (C,G), or combination (D,H) for 2 h. (A,E) represent the control condition.
The in vivo efficacy of colistin in combination with auranofin was tested using two murine models of K. pneumoniae 18605 and A. baumannii 13660 infection. The dose of auranofin was selected based on the in vitro synergistic potentiation (Table 1 and Figures 1, 2), and was much lower than the maximum tolerated dose (Harbut et al., 2015). Colistin sulfate was dosed at approximately the human equivalent dose (Li et al., 2018). As shown in Figure 4, we evaluated the bacterial loads in the PF and spleen of mice. Neither colistin nor auranofin monotherapy showed antimicrobial activity against the infected bacteria (Figures 4A,B). In contrast, the combination of colistin and auranofin proved efficacious, with almost 10-fold bacterial load reduction (p < 0.0001) in the PF and spleen than that of the control group (Figures 4A,B). In the A. baumannii 13660 infection mouse model, monotherapy treatments administered 0.5 h post-infection did not demonstrate any significant survival beyond that of the control group (Figure 4C). However, animals receiving colistin and auranofin combination therapy daily for 5 days could rescue 50% of those treated (Figure 4C).
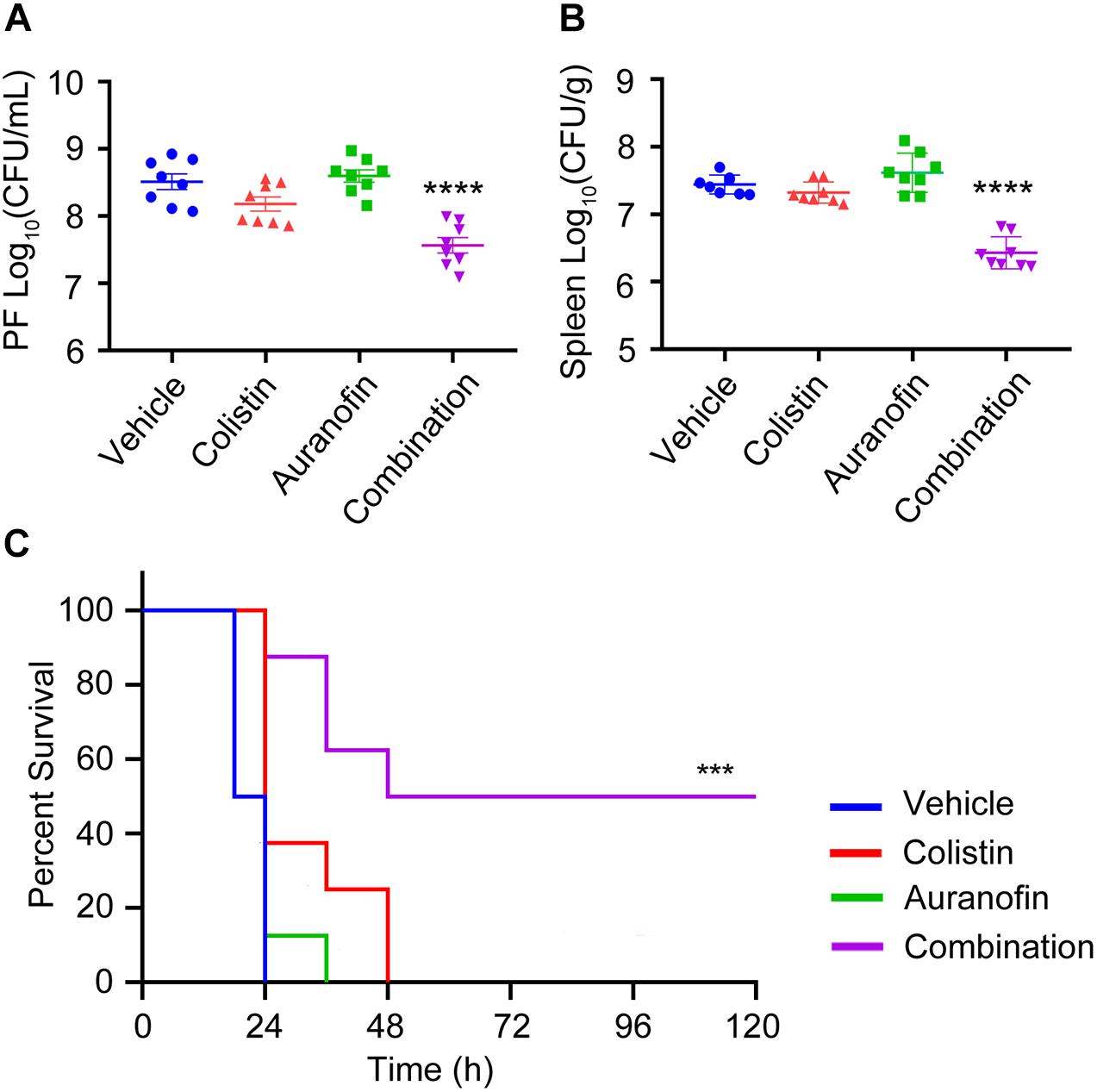
Figure 4. The combination of colistin and auranofin shows potency in vivo. (A,B) Mice were infected by high-level Col-R K. pneumoniae 18605 and received single dose of i.p. administration of vehicle, colistin sulfate, auranofin, or their combination (n = 8 per group). Bacterial loads in the PF (A) and spleen (B) are shown. (C) Survival curves showing combination efficacies in the peritoneal infection model. Mice were infected by a lethal dose of high-level Col-R A. baumannii 13660 and treated with one dose at 0.5 h post infection, followed by once-daily treatment with i.p. vehicle, colistin sulfate, auranofin, or the combination (n = 8 per group). ****indicates p ≤ 0.0001, ***indicates p ≤ 0.001, compared with the control group.
As a drug of last resort, colistin was used to treat infections caused by MDR Gram-negative bacteria. However, with the consumption of colistin, the number of Col-R strains increased (Bialvaei and Samadi Kafil, 2015). Since the discovery of mcr-1 plasmid and its spread worldwide (Liu et al., 2016; Wang R. et al., 2018), the problem of colistin resistance has aroused widespread concern. Hence, it is important to restore the activity of colistin through combination therapy. In this study, we evaluated the potential application of colistin combined with auranofin to treat infections caused by Col-R isolates.
The in vitro results showed that auranofin exhibited significant synergy with colistin against Col-R Gram-negative bacteria including mcr positive strains. Similarly, a recent study reported that auranofin restored the activity of colistin against bacteria carrying mcr-1 gene or its variants/homologs (Sun et al., 2020). Two previous studies reported that with the addition of auranofin, the effects of colistin, and auranofin against MDR Gram-negative bacteria were significantly increased (Sun et al., 2016; Torres et al., 2018).
In vivo study showed that colistin combined with auranofin improved the mice survival rate, and reduced the bacterial loads of K. pneumoniae 18605 in the PF and spleen of mice (Figures 4A,B). In vitro study on K. pneumoniae 18605 strain showed that the combined application of both colistin and auranofin at 2 mg/L dose could significantly reduce the initial bacterial count to the limit of detection (Figure 2A). However, the reduction of bacterial loads in vivo was much less than that in in vitro study. This discrepancy might be related to the pharmacokinetic properties of colistin and auranofin. The peak plasma concentration of colistin in mice treated with a single dose of colistin sulfate (2.5 mg/kg) was 3.08 mg/L (Li et al., 2018). Capparelli et al. (2017) have found that oral administration of 6 mg of auranofin daily for 7 days could result in a mean Cmax value of 0.312 mg/L in plasma, which is far below its in vitro concentration of 2 mg/L used in this study. Using Monte–Carlo simulations, it has been shown that daily oral administration of 21 mg of auranofin could result in Cmax of auranofin’s plasma concentration ranging between 0.4 and 1.6 mg/L (Capparelli et al., 2017). When mice were intraperitoneally administered with a single dose of 1 mg/kg colistin sulfate and 0.5 mg/kg auranofin, the plasma concentration of each compound might not reach the corresponding in vitro concentration. Therefore, the combination therapy showed less antimicrobial activity in vivo than in vitro. The safety of auranofin in clinical use is well established. Auranofin has been extensively used in clinical setting at the FDA-approved human dose (6 mg/day), a steady-state blood gold concentration of 3.5 μM would be reached in 12 weeks (Debnath et al., 2012). Patients with rheumatoid arthritis who were treated with auranofin (6 mg/day), had shown a safe toxicity profile (Suarez-Almazor et al., 2000). Patients with relapsed chronic lymphocytic leukemia who received 9 and 12 mg of auranofin daily for at least 28 days, followed by up to 21 mg/day, were well-tolerated (Clinical Trails registration no. NCT01419691). Given that the course of antimicrobial therapy is much shorter than that of treatment for rheumatoid arthritis or chronic lymphocytic leukemia, auranofin could be safely used to treat bacterial infections.
Scanning electron microscopy and TEM of K. pneumoniae 18605 and A. baumannii 13660 cells revealed that auranofin alone had no influence on cellular morphology (Figures 3C,G and Supplementary Figures 1C,G). Membrane blebbing was evidently observed when the cells were treated with colistin alone (Figure 3B and Supplementary Figure 1F). Similar changes have been observed in E. coli (Koike et al., 1969), P. aeruginosa (Hussein et al., 2020) after treatment with colistin alone. The bacterial outer membrane of K. pneumoniae 18605 was dramatically disrupted by the combination of colistin with auranofin, and showed cell lysis (Figures 3D,H). For A. baumannii 13660, the combination affected the overall structure of the strain, leading to an extensive shortening in the length of the bacteria (Supplementary Figures 1D,H). These observations suggested that auranofin may directly enhance the structural alteration effects of colistin.
Auranofin directly inhibited the TrxR in S. aureus and M. tuberculosis, leading to disruption of thiol-redox homeostasis and cell death (Harbut et al., 2015). However, the presence of glutathione system in several Gram-negative bacteria could maintain the redox balance and render auranofin ineffective (Harbut et al., 2015). A previous study has reported that the permeability barrier of outer membrane is the reason for the lack of activity of auranofin against Gram-negative bacteria (Thangamani et al., 2016). For the mcr-1 positive bacteria, auranofin was reported that irreversibly inhibited the function of MCR-1 via displacement of Zn (II) cofactors from the active site, thereby resensitizing mcr-1 positive bacteria to colistin (Sun et al., 2020). MCR-1 is a phosphoethanolamine (PEA) transferase, which confers colistin resistance by adding the PEA moiety to lipid A (Wang Y. et al., 2017). Further research is needed to identify the exact mode of action of auranofin when combined with colistin against high-lever Col-R pathogens.
In summary, auranofin enhanced the in vitro and in vivo antimicrobial activities of colistin against Col-R Gram-negative bacteria. As auranofin is currently available, it can be easily used for antimicrobial therapy instead of developing new antimicrobial drugs. There is great potential for this novel combination to treat infections caused by Col-R Gram-negative bacteria.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by Animal Ethical and Welfare Committee of the China-Japan Friendship Hospital (Zryhyy11-20-07-1).
BC and YL conceived and designed the study. XF, SL, YW, YZ, LS, HL, and CW carried out the experiments. XF performed the statistical analysis and wrote the draft. All authors read and approved the final version of the manuscript.
This work was funded by the Natural Science Foundation of China (No. 81970010/H0104), National Key Research and Development Program of China (2018YFC1200102), the Ministry of Science and Technology of China (2017ZX10103004), and the CAMS Innovation Fund for Medical Sciences (CIFMS 2018-I2M-1-003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Hui Wang and Xuefu You for the kind gift of the E. coli clinical isolates.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.676414/full#supplementary-material
Aguinagalde, L., Díez-Martínez, R., Yuste, J., Royo, I., Gil, C., Lasa, I., et al. (2015). Auranofin efficacy against MDR Streptococcus pneumoniae and Staphylococcus aureus infections. J. Antimicrob. Chemother. 70, 2608–2617. doi: 10.1093/jac/dkv163
Ayerbe-Algaba, R., Gil-Marqués, M. L., Jiménez-Mejías, M. E., Sánchez-Encinales, V., Parra-Millán, R., Pachón-Ibáñez, M. E., et al. (2018). Synergistic Activity of Niclosamide in Combination With Colistin Against Colistin-Susceptible and Colistin-Resistant Acinetobacter baumannii and Klebsiella pneumoniae. Front. Cell. Infect. Microb. 8:348. doi: 10.3389/fcimb.2018.00348
Ayerbe-Algaba, R., Gil-Marqués, M. L., Miró-Canturri, A., Parra-Millán, R., Pachón-Ibáñez, M. E., Jiménez-Mejías, M. E., et al. (2019). The anthelmintic oxyclozanide restores the activity of colistin against colistin-resistant Gram-negative bacilli. Internat. J. Antimicrob. Agents 54, 507–512. doi: 10.1016/j.ijantimicag.2019.07.006
Berglund, N. A., Piggot, T. J., Jefferies, D., Sessions, R. B., Bond, P. J., and Khalid, S. (2015). Interaction of the antimicrobial peptide polymyxin B1 with both membranes of E. coli: a molecular dynamics study. PLoS Computat. Biol. 11:e1004180. doi: 10.1371/journal.pcbi.1004180
Bialvaei, A. Z., and Samadi Kafil, H. (2015). Colistin, mechanisms and prevalence of resistance. Curr. Med. Res. Opin. 31, 707–721. doi: 10.1185/03007995.2015.1018989
Cannatelli, A., Principato, S., Colavecchio, O. L., Pallecchi, L., and Rossolini, G. M. (2018). Synergistic Activity of Colistin in Combination With Resveratrol Against Colistin-Resistant Gram-Negative Pathogens. Front. Microb. 9:1808. doi: 10.3389/fmicb.2018.01808
Capparelli, E. V., Bricker-Ford, R., Rogers, M. J., McKerrow, J. H., and Reed, S. L. (2017). Phase I Clinical Trial Results of Auranofin, a Novel Antiparasitic Agent. Antimicrob. Agents Chemother. 61:1. doi: 10.1128/aac.01947-16
Debnath, A., Parsonage, D., Andrade, R. M., He, C., Cobo, E. R., Hirata, K., et al. (2012). A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat. Med. 18, 956–960. doi: 10.1038/nm.2758
Doern, C. D. (2014). When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microb. 52, 4124–4128. doi: 10.1128/JCM.01121-14
Falagas, M. E., Voulgaris, G. L., Tryfinopoulou, K., Giakkoupi, P., Kyriakidou, M., Vatopoulos, A., et al. (2019). Synergistic activity of colistin with azidothymidine against colistin-resistant Klebsiella pneumoniae clinical isolates collected from inpatients in Greek hospitals. Internat. J. Antimicrob. Agents 53, 855–858. doi: 10.1016/j.ijantimicag.2019.02.021
Flamm, R. K., Rhomberg, P. R., Lindley, J. M., Sweeney, K., Ellis-Grosse, E. J., and Shortridge, D. (2019). Evaluation of the Bactericidal Activity of Fosfomycin in Combination with Selected Antimicrobial Comparison Agents Tested against Gram-Negative Bacterial Strains by Using Time-Kill Curves. Antimicrob. Agents Chemother. 63:5. doi: 10.1128/AAC.02549-18
Harbut, M. B., Vilchèze, C., Luo, X., Hensler, M. E., Guo, H., Yang, B., et al. (2015). Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl Acad. Sci. U S A 112, 4453–4458. doi: 10.1073/pnas.1504022112
Hu, Y., Liu, Y., and Coates, A. (2019). Azidothymidine Produces Synergistic Activity in Combination with Colistin against Antibiotic-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 63:1. doi: 10.1128/aac.01630-18
Hussein, M., Schneider-Futschik, E. K., Paulin, O. K. A., Allobawi, R., Crawford, S., Zhou, Q. T., et al. (2020). Effective Strategy Targeting Polymyxin-Resistant Gram-Negative Pathogens: Polymyxin B in Combination with the Selective Serotonin Reuptake Inhibitor Sertraline. ACS Infect. Dis. 6, 1436–1450. doi: 10.1021/acsinfecdis.0c00108
Clinical and Laboratory Standards Institute (2019). Performance standards for antimicrobial susceptibility testing. Wayne, PA: CLSI.
Kean, W. F., Hart, L., and Buchanan, W. W. (1997). Auranofin. Br. J. Rheumatol. 36, 560–572. doi: 10.1093/rheumatology/36.5.560
Koike, M., Iida, K., and Matsuo, T. (1969). Electron microscopic studies on mode of action of polymyxin. J. Bacteriol. 97, 448–452. doi: 10.1128/JB.97.1.448-452.1969
Li, Y., Lin, X., Yao, X., Huang, Y., Liu, W., Ma, T., et al. (2018). Synergistic Antimicrobial Activity of Colistin in Combination with Rifampin and Azithromycin against Escherichia coli Producing MCR-1. Antimicrob. Agents Chemother. 62:12. doi: 10.1128/aac.01631-18
Lin, Y.-W., Yu, H. H., Zhao, J., Han, M.-L., Zhu, Y., Akter, J., et al. (2018). Polymyxin B in Combination with Enrofloxacin Exerts Synergistic Killing against Extensively Drug-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother 2:6. doi: 10.1128/AAC.00028-18
Liu, Y.-Y., Wang, Y., Walsh, T. R., Yi, L.-X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet. Infect.Dis. 16, 161–168. doi: 10.1016/S1473-3099(15)00424-7
Odds, F. C. (2003). Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. doi: 10.1093/jac/dkg301
Olaitan, A. O., Morand, S., and Rolain, J.-M. (2014). Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front. Microb. 5:643. doi: 10.3389/fmicb.2014.00643
Roder, C., and Thomson, M. J. (2015). Auranofin: repurposing an old drug for a golden new age. Drugs R&D 15, 13–20. doi: 10.1007/s40268-015-0083-y
Schneider, E. K., Azad, M. A. K., Han, M.-L., Tony Zhou, Q., Wang, J., Huang, J. X., et al. (2016). An “Unlikely” Pair: The Antimicrobial Synergy of Polymyxin B in Combination with the Cystic Fibrosis Transmembrane Conductance Regulator Drugs KALYDECO and ORKAMBI. ACS Infect. Dis. 2, 478–488. doi: 10.1021/acsinfecdis.6b00035
Suarez-Almazor, M. E., Spooner, C. H., Belseck, E., and Shea, B. (2000). Auranofin versus placebo in rheumatoid arthritis. Cochrane Database Syst. Rev. 2:CD002048. doi: 10.1002/14651858.CD002048
Sun, H., Zhang, Q., Wang, R., Wang, H., Wong, Y.-T., Wang, M., et al. (2020). Resensitizing carbapenem- and colistin-resistant bacteria to antibiotics using auranofin. Nat. Commun. 11:5263. doi: 10.1038/s41467-020-18939-y
Sun, W., Weingarten, R. A., Xu, M., Southall, N., Dai, S., Shinn, P., et al. (2016). Rapid antimicrobial susceptibility test for identification of new therapeutics and drug combinations against multidrug-resistant bacteria. Emerg. Microb. Infect. 5:e116. doi: 10.1038/emi.2016.123
Thangamani, S., Mohammad, H., Abushahba, M. F. N., Sobreira, T. J. P., Hedrick, V. E., Paul, L. N., et al. (2016). Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci. Rep. 6:22571. doi: 10.1038/srep22571
Tharmalingam, N., Ribeiro, N. Q., da Silva, D. L., Naik, M. T., Cruz, L. I., Kim, W., et al. (2019). Auranofin is an effective agent against clinical isolates of Staphylococcus aureus. Future Med. Chem. 11, 1417–1425. doi: 10.4155/fmc-2018-0544
Torres, N. S., Montelongo-Jauregui, D., Abercrombie, J. J., Srinivasan, A., Lopez-Ribot, J. L., Ramasubramanian, A. K., et al. (2018). Antimicrobial and Antibiofilm Activity of Synergistic Combinations of a Commercially Available Small Compound Library With Colistin Against Pseudomonas aeruginosa. Front. Microb. 9:2541. doi: 10.3389/fmicb.2018.02541
Tran, T. B., Wang, J., Doi, Y., Velkov, T., Bergen, P. J., and Li, J. (2018). Novel Polymyxin Combination With Antineoplastic Mitotane Improved the Bacterial Killing Against Polymyxin-Resistant Multidrug-Resistant Gram-Negative Pathogens. Front. Microb. 9:721. doi: 10.3389/fmicb.2018.00721
Velkov, T., Thompson, P. E., Nation, R. L., and Li, J. (2010). Structure–activity relationships of polymyxin antibiotics. J. Med. Chem. 53, 1898–1916. doi: 10.1021/jm900999h
Wang, R., van Dorp, L., Shaw, L. P., Bradley, P., Wang, Q., Wang, X., et al. (2018). The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 9:1179. doi: 10.1038/s41467-018-03205-z
Wang, X., Wang, Y., Zhou, Y., Li, J., Yin, W., Wang, S., et al. (2018). Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg. Microb. Infect. 7:122. doi: 10.1038/s41426-018-0124-z
Wang, Y.-M., Kong, L.-C., Liu, J., and Ma, H.-X. (2018). Synergistic effect of eugenol with Colistin against clinical isolated Colistin-resistant strains. Antimicrob. Resist. Infect.Control 7:17. doi: 10.1186/s13756-018-0303-7
Wang, Y., Tian, G.-B., Zhang, R., Shen, Y., Tyrrell, J. M., Huang, X., et al. (2017). Prevalence, risk factors, outcomes, and molecular epidemiology of mcr-1-positive Enterobacteriaceae in patients and healthy adults from China: an epidemiological and clinical study. Lancet. Infect. Dis. 17, 390–399. doi: 10.1016/S1473-3099(16)30527-8
Keywords: colistin, auranofin, colistin-resistant, repurposing, combination therapy, synergistic effect
Citation: Feng X, Liu S, Wang Y, Zhang Y, Sun L, Li H, Wang C, Liu Y and Cao B (2021) Synergistic Activity of Colistin Combined With Auranofin Against Colistin-Resistant Gram-Negative Bacteria. Front. Microbiol. 12:676414. doi: 10.3389/fmicb.2021.676414
Received: 05 March 2021; Accepted: 03 June 2021;
Published: 25 June 2021.
Edited by:
Haruyoshi Tomita, Gunma University, JapanReviewed by:
Emanuele Durante Mangoni, University of Campania Luigi Vanvitelli, ItalyCopyright © 2021 Feng, Liu, Wang, Zhang, Sun, Li, Wang, Liu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingmei Liu, bHltMTM2MDEwNjMyMjNAMTI2LmNvbQ==; Bin Cao, Y2FvYmluX2JlbkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.