
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 08 July 2021
Sec. Systems Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.671699
This article is part of the Research Topic Insect Microbiome: From Diversity To Applications View all 35 articles
The sterile insect technique (SIT) has been developed as a component of area-wide integrated pest management approaches to control the populations of Aedes albopictus, a mosquito vector capable of transmission of dengue, Zika and chikungunya viruses. One of the key factors for the success of SIT is the requirement of high biological quality sterile males, which upon their release would be able to compete with wild males for matings with wild females in the field. In insects, gut bacteriome have played a catalytic role during evolution significantly affecting several aspects of their biology and ecology. Given the importance of gut-associated bacterial species for the overall ecological fitness and biological quality of their hosts, it is of interest to understand the effects of radiation on the gut-associated bacteriome of Ae. albopictus. In this study, the effect of radiation on the composition and density levels of the gut-associated bacterial species at the pupal stage as well as at 1- and 4-day-old males and females was studied using 16S rRNA gene-based next generation sequencing (NGS) and quantitative PCR (qPCR) approaches. Age, diet, sex, and radiation were shown to affect the gut-associated bacterial communities, with age having the highest impact triggering significant changes on bacterial diversity and clustering among pupae, 1- and 4-day-old adult samples. qPCR analysis revealed that the relative density levels of Aeromonas are higher in male samples compared to all other samples and that the irradiation triggers an increase in the density levels of both Aeromonas and Elizabethkingia in the mosquito gut at specific stages. Our results suggest that Aeromonas could potentially be used as probiotics to enhance protandry and sex separation in support of SIT applications against Ae. albopictus, while the functional role of Elizabethkingia in respect to oxidative stress and damage in irradiated mosquitoes needs further investigation.
Mosquito-borne diseases including malaria, dengue, Zika, chikungunya, West Nile, yellow fever, and Japanese encephalitis cause severe burden on public health worldwide (Tolle, 2009). The sterile insect technique (SIT) is a species-specific and environment-friendly approach, which has been proposed and currently tested for the control of mosquito populations and mosquito-borne diseases (Lees et al., 2015; Bourtzis et al., 2016; Bouyer et al., 2020; WHO and IAEA, 2020). An important factor in SIT applications is male mating competitiveness, which is the ability of the mass-reared and radiation-sterilized males to compete with wild males for mating with wild females (Knipling, 1955; Dyck et al., 2020). Several studies have shown that mass-rearing, radiation, handling, marking, and release processes may affect male mating competitiveness and hence, the suppression efficiency of the SIT application (Helinski and Knols, 2008; Ami et al., 2010; Bellini et al., 2013; Maïga et al., 2014; Yamada et al., 2014; Cai et al., 2018; Diallo et al., 2019; Bouyer and Vreysen, 2020; Dyck et al., 2020).
Insects are known to have established diverse symbiotic associations with microbial species (particularly of bacterial origin), which have played a catalytic role during evolution significantly affecting several aspects of their biology and ecology (Dillon and Dillon, 2004; Bourtzis and Miller, 2009; Zchori-Fein and Bourtzis, 2011; Engel and Moran, 2013). During the recent years, a number of studies have characterized the gut-associated microbiota of several insect species, including pests and disease vectors, in an attempt to determine the role of microbial species (mainly bacteria) in host physiology, and also to potentially exploit them as a tool for pest and disease control (Guégan et al., 2018; Itoh et al., 2018; Strand, 2018; Deutscher et al., 2019; Huang et al., 2020; Wang et al., 2020). Due to their importance, any change in the composition or abundance of the bacterial species associated with the insect hosts may have an impact on their physiology. For example, several studies on fruit fly species, which are target for sterile insect technique applications, have shown that changes in the composition and abundance of gut-associated bacterial species may be triggered by irradiation treatments, which in turn may be related to physiological changes affecting the biological quality and the overall fitness of the host (Ami et al., 2010; Gavriel et al., 2010; Cai et al., 2018; Asimakis et al., 2019; Woruba et al., 2019).
However, the biological quality and the ecological fitness of irradiated insects can be restored through probiotic applications as has been shown in two major agricultural tephritid pest species, the Mediterranean fruit fly Ceratitis capitata and the Oriental fruit fly Bactrocera dorsalis (Niyazi et al., 2004; Ami et al., 2010; Yuval et al., 2013; Augustinos et al., 2015; Kyritsis et al., 2017; Cai et al., 2018). Ben-Ami et al. (2010) showed that, upon irradiation, the abundance of Klebsiella oxytoca decreased in the guts of Mediterranean fruit fly. However, the provision of Klebsiella oxytoca as probiotic supplement into the adult diet of irradiated medflies resulted in its successful colonization in the gut, thus enhancing the mating competitiveness and longevity of sterile males (Ami et al., 2010; Gavriel et al., 2010). Similarly, Cai et al. (2018) also found that radiation of Bactrocera dorsalis induced changes in the gut-associated microbiota resulting to a significant reduction in the ecological fitness. However, probiotic applications of the gut associated Klebsiella oxytoca (BD177 strain) were able to rescue these effects and restore the biological quality of irradiated insects.
During the last two decades, symbiotic bacteria have been harnessed for the control of mosquito vector species and mosquito-borne diseases (Flores and O’Neill, 2018; Guégan et al., 2018; Nazni et al., 2019; Zheng et al., 2019; Caputo et al., 2020; Crawford et al., 2020; Huang et al., 2020; Ryan et al., 2020). In parallel, several studies have focused on the characterization of mosquito gut-associated microbiota with an emphasis on bacterial species (for recent reviews see Guégan et al., 2018; Strand, 2018; Scolari et al., 2019). Environmental factors and food resources have been shown to play an important role in the acquisition of bacterial species from breeding sites, which in turn may determine the composition and abundance of bacterial species in the mosquito gastrointestinal tracts (Pastoris et al., 1989; Lindh et al., 2005; Rani et al., 2009; Wang et al., 2011, 2018; Minard et al., 2013a, b; Guégan et al., 2018; Chen et al., 2020; Saab et al., 2020). Mosquito host species, sex and age may also affect the structure of the gut bacteriome and the density levels of its associated bacterial species (Minard et al., 2014, 2018; Guégan et al., 2018; Mancini et al., 2018; Rosso et al., 2018; Wang et al., 2018; Chen et al., 2020). It is also worth noting that many studies have indicated that mosquito-associated bacterial species may affect both metabolism and life history traits including food digestion, supply of vitamins and amino acids, body size, oviposition site choice and egg production, longevity, sex ratio, larval development as well as virus dissemination (Chouaia et al., 2012; Mitraka et al., 2013; Sharma et al., 2013; Coon et al., 2014, 2016a, 2016b; Muturi et al., 2016; Dickson et al., 2017; Guégan et al., 2018).
Aedes albopictus (Diptera: Culicidae) is widespread all over the world contributing to the transmission of dengue, Zika and chikungunya viruses (Gasperi et al., 2012; Weaver et al., 2018). The Insect Pest Control Laboratory of the Joint FAO/IAEA Centre of Nuclear Techniques in Food and Agriculture has been developing the SIT package as a component of area-wide integrated pest management (AW-IPM) approaches to suppress Ae. albopictus populations. Previous studies suggested that a radiation dose (>35 Gy) may negatively affect the longevity and mating competitiveness of Ae. albopictus adult males (Bellini et al., 2013; Madakacherry et al., 2014; Zhang et al., 2016; Du et al., 2019). However, and given the importance of gut-associated bacterial species for the overall ecological fitness and biological quality of their hosts, none of these studies investigated whether the radiation affects the structure of the gut-associated bacteriome, and in the case of a negative effect, whether probiotic applications could restore the ecological fitness as previously observed for other pest species, for example, the tephritid B. dorsalis (Cai et al., 2018). In the present study, Ae. albopictus pupae were exposed at a radiation dose of 40 Gy, a dose which can induce full sterility in females and up to 99% sterility in males (Balestrino et al., 2010; Yamada et al., 2014; Zhang et al., 2015a, 2016; Du et al., 2019). The effect of radiation on the composition and density levels of the gut-associated bacterial species at the pupal stage as well as at 1- and 4-day-old males and females were studied using 16S rRNA gene-based next generation sequencing (NGS) and quantitative PCR (qPCR) approaches. The data produced were also assessed in respect to the age and sex, and they are discussed in the context of mating competitiveness of sterile males and potential microbiota manipulations for the enhancement of SIT applications against the major mosquito vector species Ae. albopictus.
The experiments were conducted at the Joint FAO/IAEA Insect Pest Control Laboratory (hereafter IPCL), Seibersdorf, Austria. Ae. albopictus wild type strain (Guangzhou, China), known as GUA strain (Zhang et al., 2015b), at F13 generation was used in these experiments. Egg hatching, larvae rearing, and adult maintenance was performed as described previously (Zhang et al., 2015b). The colony was maintained under at 26 ± 1°C with a light: dark cycle of 12 h: 12 h, and 60 ± 10% relative humidity.
Bovine-defibrinated blood meals were preheated and then provided to GUA females 7–9 days post emergence. Two days after the blood meal, a plastic 250-ml beaker containing 100 ml sterilized deionized water and a strip of sterilized white filter paper (white creped papers IF C140, Industrial Filtro S.r.l., Cologno Monzese, Italy) was placed in the cage (30 × 30 × 30 cm, BugDorm 1, MegaView, Taichung, Taiwan, China). After maturation, eggs were hatched as described previously (Zhang et al., 2015b). Larvae were fed on modified IAEA liquid larvae diet (Balestrino et al., 2014). Male and female pupae from 24 to 36 h after pupation were separated using an improved Fay and Morlan’s separator (Focks, 1980) and then were irradiated. The irradiation treatment was conducted with a Co-60 Gammacell irradiator 220 (Atomic Energy of Canada Ltd., Canada). The irradiation dose was set to 40 Gy, and the actual dose was measured with Gafchromic® MD-V3 film (Ashland, Bridgewater, New Jersey, United States) and DoseReader 02 (FWT-92D, Far West Technology, Inc., Goleta, Canada). The irradiation dose was set to 40 Gy, a dose which has been shown to induce over 99% sterility on Ae. albopictus males and a reduction in longevity and mating competitiveness (Balestrino et al., 2010; Zhang et al., 2015a, 2016; Du et al., 2019). Due to time and space limitation, materials for pupae and adults were reared and irradiated separately. To minimize variation, pupae and adults of the same batch were taken as control group, respectively.
After irradiation, some of the pupae were immediately dissected under the stereomicroscope to isolate the whole gut to understand how irradiation may affect the gut-associated bacterial community. The rest of the pupae were reared separately in sterilized deionized water and placed in cage for adult emergence. Non-fed early emerged adults (both males and females) were collected and their whole gut was dissected upon adult emergence (less than 24 h) in order to see whether potential changes occurred in the pupal gut-associated bacteriome due to radiation could last until the adult stage. The rest of adults were supplied with 10% sucrose solution. To study whether any potential changes in the gut-associated bacteriome due to radiation are naturally restored during the first days of the adulthood, which is important for mating competitiveness and SIT applications, the whole guts from males and females were collected on the 4th day after emergence. Ventral diverticulum and Malpighian tubules were removed from every gut sample.
Samples from 12 groups: male pupae irradiated (MPI), male pupae control (MPC), female pupae irradiated (FPI), female pupae control (FPC), early emerged males irradiated (1DMI), early emerged males control (1DMC), early emerged females irradiated (1DFI), early emerged females control (1DFC), 4 days old males irradiated (4DMI), 4 days old males control (4DMC), 4 days old females irradiated (4DFI), 4 days old females control (4DFC), were collected for dissection. The detail information on the age, diet, sex and radiation treatment of the analyzed samples is shown in Supplementary Table 1. Alive adults which had been anesthetized at 4°C or alive pupae were surface disinfected by dipping in 70% ethanol for 1 min, placed into sterile 1 × PBS (phosphate buffer saline) for rinsing, and then dissected in sterile PBS under a binocular microscope with sterilized needles to get whole guts.
Ten guts per tube were mechanically homogenized using sterile pestles in liquid nitrogen. DNA was extracted following the protocol of DNeasy Blood and Tissue Kit (QIAGEN, Germany), then the contents of two tubes were mixed thus each sample was consisting of 20 guts. DNA was concentrated to > 15 ng/μl according to the original concentration estimated by a NanoDrop 3,000 spectrophotometer and was used for next generation sequencing (NGS) and qPCR analysis. In summary, each group contained four replicates and each replicate included 20 guts.
The NGS analysis was based on the 16S rRNA gene. Two regions of the gene were amplified using the primers U341F (5′-CCTACGGGRSGCAGCAG-30) and 805R (50-GTGCCAGCM GCCGCGGTAA-3′) (V3-V4) and 909F (50-ACTCAAAK GAATWGACGG-30) and 1391R (5′-GACGGGCGGTGWG TRCA-3′) (V6-V8), respectively (Sogin et al., 2006; Cole et al., 2007; Eid et al., 2009; Klindworth et al., 2013). The PCRs, the preparation of the libraries and the sequencing using the Illumina MiSeq platform were performed by Macrogen (Macrogen, Seoul, Korea). The sequences have been deposited to NCBI under the accession number PRJNA682321.
De-multiplexing of the raw sequencing reads was performed followed by their conversion to FASTQ, and standard algorithms were used to trim the Illumina adapters. The sequence reads were prepared for subsequent analysis in usearch v.10 and v.11 (Edgar, 2017). The paired-end reads were assembled and trimmed by length, and the usearch -fastq_mergepairs option in usearch v.11 was used to check for errors and assess quality. Unassembled reads as well as reads which were outside the range of 400–520 bp were not included in the downstream analysis. Using the -fastq_filter in usearch v.11, the quality of the assembled sequences was further improved while unique read sequences and abundances were found with the -fastx_uniques option. The -cluster_otus command was used for the clustering of sequences into operational taxonomic units (OTUs) at 97% similarity (Edgar, 2013), while the -unoise3 option of usearch v.10 was used to remove chimeras (Edgar, 2016b). Taxonomy was assigned against the SILVA 128 release database (Quast et al., 2012; Edgar, 2016a) using the qiime feature-classifier classify-consensus-blast command of qiime2-2020.11 with 0.97% identity (Bolyen et al., 2019; Estaki et al., 2020). The OTUs of chloroplast, Archaea and unassigned OTUs were manually removed and the taxonomy was repeated as mentioned above. No mitochondrial OTUs were detected.
Relative abundance heatmap was created with matrics display in PRIMER version 7 + and was based on the OTU table along with the taxonomy data previously obtained in order to visualize the most dominant OTUs (n = 35) in each sample group at phylum and genus levels. To test the overall statistical differences between the different factors, the bootstrap averages were calculated using the Bray Curtis resemblance matrix of the OTUs abundance results which is based on the analysis of similarity (ANOSIM) pairwise test and plotted in PRIMER version 7 + using number of bootstraps between 50 and 150 per sample.
Based on the NGS results, the three most abundant genera, which also varied greatly among treatments, were quantified by quantitative PCR (qPCR): Aeromonas, Elizabethkingia and Enterococcus. The V3-V4 region of the 16S rRNA gene sequences of these three genera obtained from the NGS analysis were used for BLAST searches in the databases to search the most similar reference sequences. All available 16S rRNA gene sequences of the gut-associated bacteria of the Ae. albopictus GUA strain detected in our previous study (Chen et al., 2020) were also taken as reference for primer specificity. Pairs of genus-specific qPCR primers were designed for these three genera with Primer3plus1. Primer self-complementarity were checked with NCBI primer-blast. We also searched the Silva database to ensure that our primer pairs fit as many target sequences whereas as few untargeted ones. The Ae. albopictus ribosomal protein S6 (rpS6) gene was chosen as reference. The primers used in this analysis along with the annealing temperatures (TA) and melting temperature (TM) are presented in Supplementary Table 2. The amplification was performed using iQTM SYBR® Green Supermix (Bio-Rad, United States). The reaction mixture (15 μl) consisted of 5 ng DNA template and 7.5 μl of 2 × Supermix. qPCR and 200 nM of each primer was performed with a CFX96 Touch Real-Time PCR Detection system (Bio-Rad, United States). The concentration of DNA was diluted, and 5 ng DNA was used in 15 μl reactions. Due to different T(A)s of the primers, housekeeping gene rpS6 and target genes were put on the same wells of different plates. An initial denaturation at 95°C for 2 min was followed by 40 cycles consisting of denaturation at 95°C for 10 s, at annealing temperature for 60 s. The fluorescence collection was performed at the annealing stage. To check and confirm the quality of amplification, a melting profile was generated for the amplicon over a temperature range of 65–95°C. Melting curves for each sample were analyzed after each run to check that there was no primer dimer formation. Four biological replicates were performed for each sample. There were three technical replicates for each biological sample. Relative quantity (Mean ± SEM) of products was calculated using CFX ManagerTM Software (Bio-Rad Laboratories, Inc.). The relative abundance of bacteria was determined by using the 2−ΔΔCT calculation method.
Normalcy of the data was assessed by the D’Agostino-Pearson omnibus normality test of the GraphPad Prism 6.0 software. For the NGS results, alpha-diversity analysis was also performed with Qiime2 and usearch v.11. Pielou.s.evenness and Richness index were used to estimate the number of OTUs per samples whereas Shannon’s and Simpson’s reciprocal [1/Simpson’s Index (D)] indices were used to determine species diversity. The bacterial richness and diversity indices among the samples were compared using Kruskal-Wallis test and Dunn’s multiple comparisons test with the default setting or Two-tailed Mann-Whitney U-test based on different classified groups. Similarities in the structure of bacterial communities and the role of different factors such as age, sex and radiation were assessed using the metric multidimensional scaling (mMDS) plot with bootstrap averages in PRIMER version 7 + and were displayed with a Bray and Curtis matrix based on the square-root transformation of the bacterial OTU abundance data (Clarke and Gorley, 2016). The tests were based on the multivariate null hypothesis via the use of the non-parametric statistical method PERMANOVA (Anderson, 2001).
For qPCR results, Dunn’s multiple comparisons test or Two-tailed Mann-Whitney U-test was used to compare the relative density of the selected bacterial species.
The sequence data of the two 16S rRNA gene regions, amplified by the primer pairs U341F/805R and 909F/1391R, were compared and the analysis indicated that there were no statistically significant differences (Supplementary Table 3). Based on this finding, the sequence data of these two regions were combined and used for all the downstream analysis which resulted to the data, figures and tables presented below.
The effects of age, sex and radiation on the bacterial community composition and diversity in the guts of Ae. albopictus GUA strain, including male and female pupae, 1- and 4- day-old male and female adult mosquitoes, were investigated by 16S rRNA gene sequencing. A total of 506,854 (minimum 30433–maximum 46819) reads, corresponding to 496 (minimum 30–maximum 53) OTUs, were used for analysis in this study after quality filtering of the sequencing results (Table 1 and Supplementary File 1).
Among all the sequenced samples, significant difference on the bacterial community abundance was observed based on species richness indices (Kruskal-Wallis test, df = 11, X2 = 37.15, P < 0.01) (Table 1). The samples were then classified based on treatment (irradiated or non-irradiated), age (pupa, 1- and 4-day-old) and sex (male and female), and, respectively compared based on richness and Shannon indices. The results showed that there were no significant differences on bacterial richness in treatment (Figure 1A, Two-tailed Mann-Whitney U-test, U = 296.5, P = 0.869) or sex (Figure 1C, Two-tailed Mann-Whitney U-test, U = 335, P = 0. 0.337), however, significant difference was observed on age with the highest bacterial diversity being observed in the guts of pupae and those of 4-day-old adults (Figure 1B, Kruskal-Wallis test and Dunn’s multiple comparisons test, x2 = 23.451, df = 2, P < 0.001). Using the Pielou.s.evenness index for assessing the diversity along with species richness, significant differences were detected between samples (x2 = 37.347, df = 11, P < 0.001).
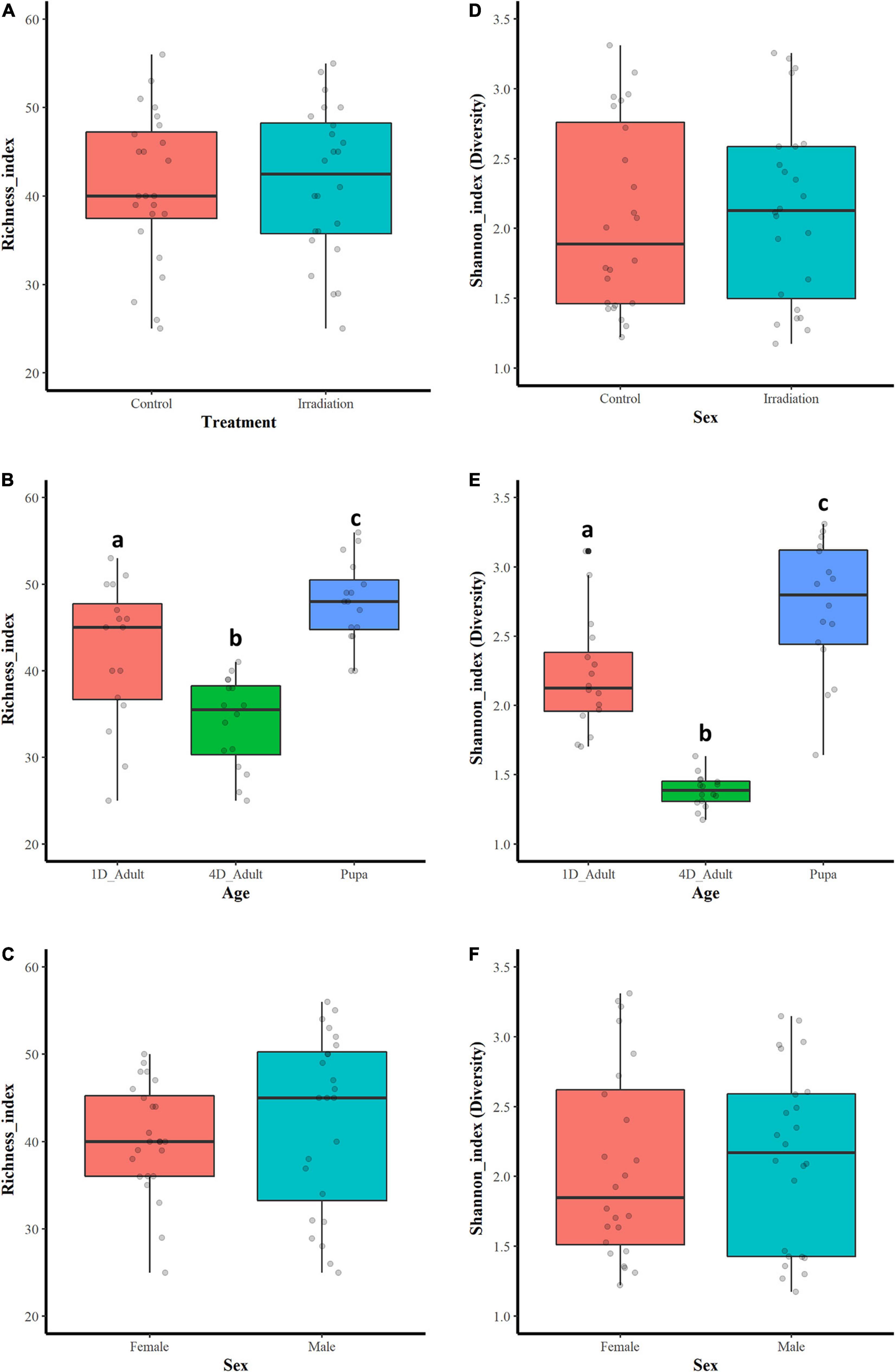
Figure 1. Bacterial community richness and diversity based on the Species richness and Shannon indices. Bacterial richness (A) and diversity (D) of guts between irradiated and non-irradiated samples. Bacterial richness (B) and diversity (E) of guts from pupa, 1- and 4-day-old adults. Bacterial richness (C) and diversity (F) of guts between male and female mosquitoes. Boxes extend between the 25th and 75th percentile. A thick line denotes the median. The whiskers extend up to the most extreme values. The gray circles indicate the number of data points used in each plot. Two-tailed Mann-Whitney U-test was used to compare the bacterial richness and diversity indices in treatment or sex groups. Kruskal-Wallis test and Dunn’s multiple comparisons test were used to compare the bacterial richness and diversity indices in age groups. Significant differences are indicated by different letters.
Regarding the bacterial community diversity, significant differences were observed in the tested samples according to the Shannon (Kruskal-Wallis test, x2 = 37.390, df = 11, P < 0.001) and Simpson indices (Kruskal-Wallis test, x2 = 35.737, df = 11, P < 0.0001) (Table 1). Samples were classified as above-mentioned and comparison was performed based on Shannon index. As shown in Figure 1E, the bacterial diversity was highest in the guts of pupae, followed by 1- and 4-day-old adults (Kruskal-Wallis test and Dunn’s multiple comparisons test, x2 = 34.839, df = 2, P < 0.0001). No significant differences on bacterial diversity of guts with respect to irradiation treatment (Figure 1D, Two-tailed Mann-Whitney U-test, U = 301.5, P = 0.798) and sex (Figure 1F, Two-tailed Mann-Whitney U-test, U = 298, P = 0.846) were observed (Supplementary File 1).
Based on the NGS results, the most abundant gut bacteria (≥ 2%) in Ae. albopictus GUA strain are shown in Figure 2. Based on phylum, the abundance of OUTs was relatively uniform, but also showed some minor differences, in all tested samples (Figure 2A). Bacteroidetes (18.9 and 21.1%), Firmicutes (16.4 and 43.4%), Proteobacteria (55.6 and 31.7%) and Actinobacteria (6.9 and 2.7%), were the four most abundant phyla in pupae and 1-day-old adult mosquitoes, respectively, however, Bacteroidetes, with a relative abundance of up to 86.0%, was the main phylum in the guts of 4-day-old adults. These differences were also observed at the genus level. For example, Elizabethkingia was the genus with the highest relative abundance in 4-day-old adults while Elizabethkingia and Enterococcus were the two relatively high abundant genera in pupae and 1-day-old mosquitoes. In addition, except for female pupae, Aeromonas was also occurred with a high relative abundance in male pupae and 1-day-old mosquitoes. Interestingly, when compared to the corresponding non-irradiated (control) mosquitoes, the irradiated samples exhibited a higher average abundance of Aeromonas (Figure 2B). In contrast, except for 1-day-old male mosquitoes, the average abundance of Enterococcus in the irradiated mosquitoes was lower than their corresponding control samples (Figure 2B).
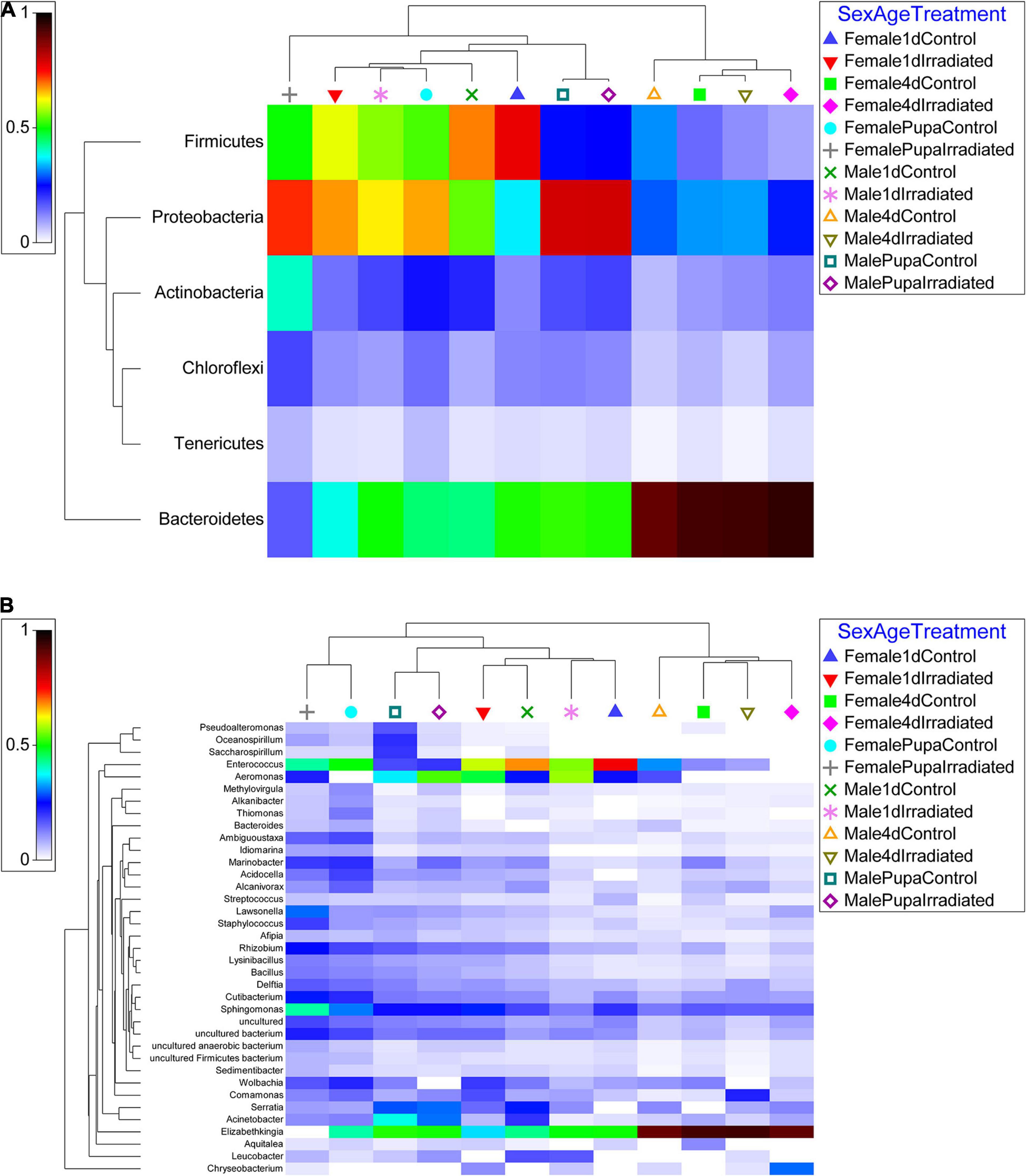
Figure 2. Relative abundance heatmap of the most dominant phylum and genus of all samples examined. (A) Phylum level. (B) Genus level. OTUs abundance results from qiime2 analysis were transformed with square root transformation and averaged based on the number of replicates per sample. The resemblance matrix was conducted with Bray Curtis similarity of the OTUs abundance results.
Regarding the beta diversity of the bacterial communities, the sequenced samples were classified according to irradiation treatment (hereafter treatment), age or sex. The results clearly revealed that bacterial communities differed significantly in respect to these three independent factors (Table 2, PERMANOVA; Treatment: P = 0.004; Age: P = 0.001; Sex: P = 0.001). The metric multidimensional scaling (mMDS) showed that unique community clusters were formed separately between the irradiated and non-irradiated samples (Figure 3A). Similarly, the formation of distinct clusters among pupae, 1- and 4-day-old adults (Figure 3B) or between male and female mosquitoes (Figure 3C) were, respectively observed.
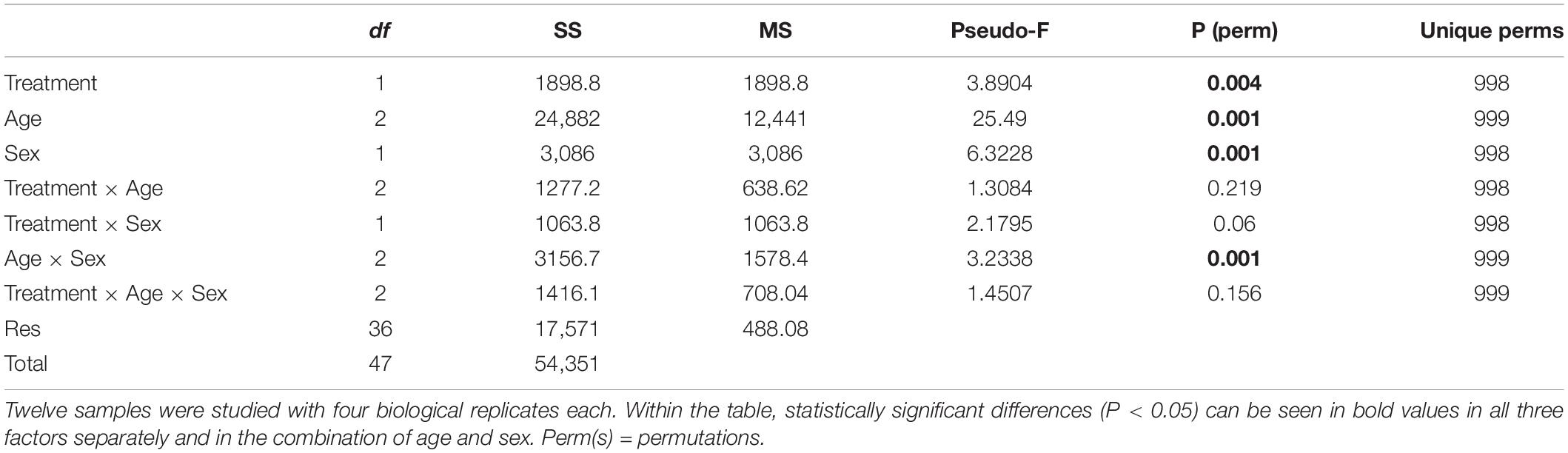
Table 2. PERMANOVA table of results for all three factors and their combinations for genera level abundance.
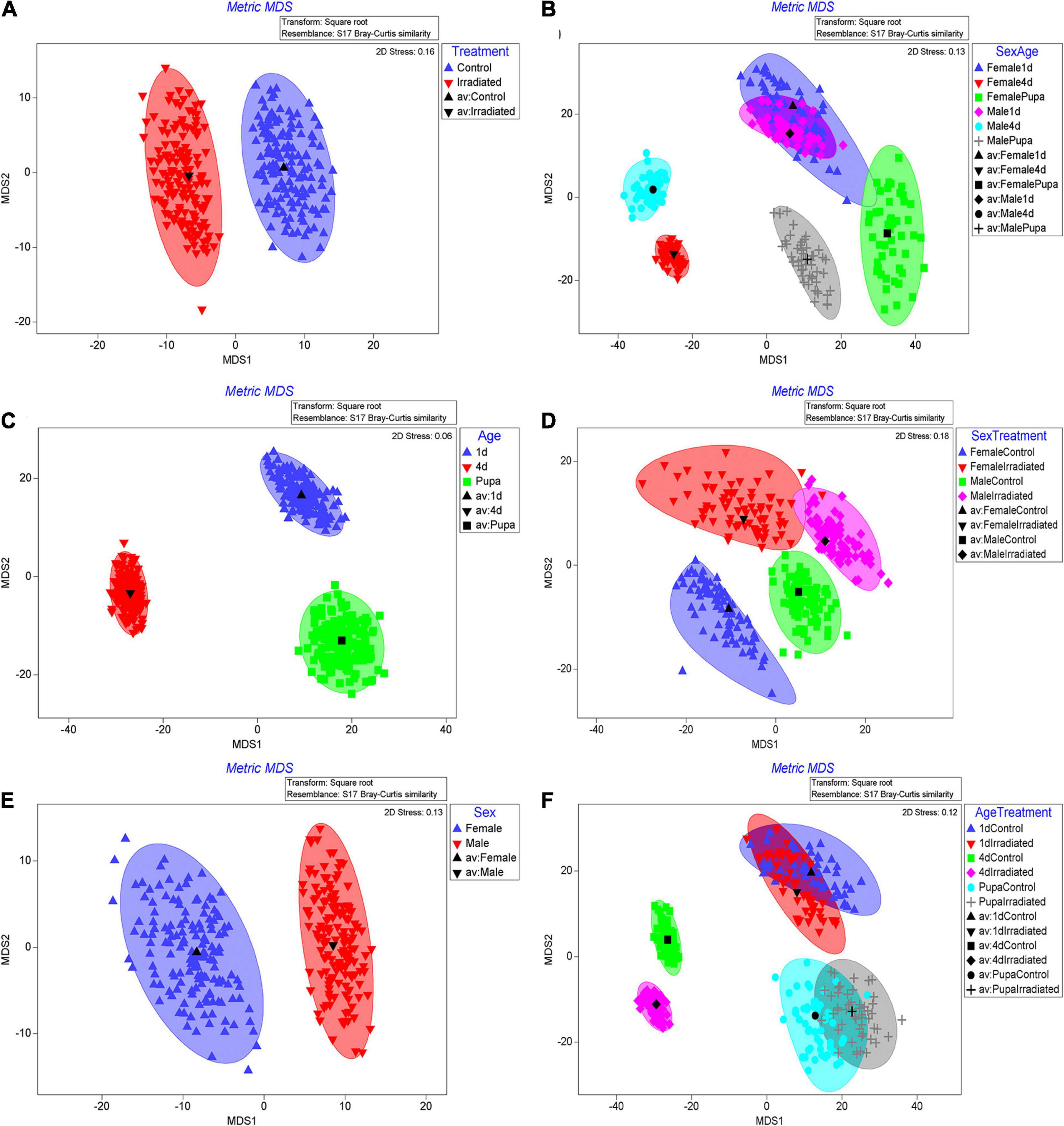
Figure 3. Bootstrap averages with Metric multidimensional scaling (mMDS) of bacterial communities based on relative abundances of OTUs originating from treatment, age or sex groups (A–C) or combinations of them (D–F). OTUs abundance results from qiime2 analysis were transformed with square root transformation and the resemblance matrix was conducted with Bray Curtis similarity. Bootstrap averages were analyzed with 5 (D,F), 75 (E) and 150 (A–C) bootstraps per sample indicated by the number of points in the plots.
Strong clustering of bacterial communities was observed only when age and sex factors combined (Table 2, PERMANOVA; Age × Sex: P = 0.001). Results of mMDS confirmed the clusters (Figures 3D–F). However, three factor combinations did not show such a high clustering (Table 2, PERMANOVA, P = 0.156). In addition, the combination between Age and Sex factors or Treatment and Sex factors also did not present high clustering (Table 2, PERMANOVA, Treatment × Age: P = 0.219, Treatment × Sex: P = 0.060).
Based on qPCR results, the relative abundance of the three taxa is presented in Figure 4. In general, all three bacterial taxa showed clear and distinct patterns among different developmental stages, ages, and sexes. The relative abundance of Aeromonas in the midgut of pupae, with or without irradiation, showed no significant difference compared to either 1- or 4-day-old adult mosquito for both males and females (Dunn’s multiple comparisons test, P > 0.05) except for the female pupae which exhibited a lower density than 1-day-old female adults (Dunn’s multiple comparisons test, P < 0.05). Interestingly, irradiated 1-day-old adult mosquitoes had a higher Aeromonas density than in 4-day-old mosquitoes (Dunn’s multiple comparisons test, P < 0.05), but this pattern was not observed in the control groups (Dunn’s multiple comparisons test, P > 0.05). One-day-old adult mosquitoes showed no significant difference regarding Elizabethkingia abundance compared to either pupae or 4-day-old males and females, irrespective to irradiation treatment (Dunn’s multiple comparisons test, P > 0.05). However, 4-day-old adults had a higher Elizabethkingia density than their respective pupae (Dunn’s multiple comparisons test, P < 0.05). No significant difference on Enterococcus density was observed among pupae, 1- and 4-day-old female adults, regardless of irradiation treatment (Dunn’s multiple comparisons test, P > 0.05). In respect to male mosquitoes, 1-day-old male adults exhibited higher Enterococcus density than pupae (Dunn’s multiple comparisons test, P < 0.05). Four-day-old males showed no significant difference compared to either pupae or 1-day-old males (Dunn’s multiple comparisons test, P > 0.05). When combined the data from same sex, males had a higher Aeromonas density than females in both treatment and control groups (Two-tailed Mann-Whitney U-test, P < 0.05), but this was not observed for Elizabethkingia (Two-tailed Mann-Whitney U-test, P > 0.05) or Enterococcus (Two-tailed Mann-Whitney U-test, P > 0.05).
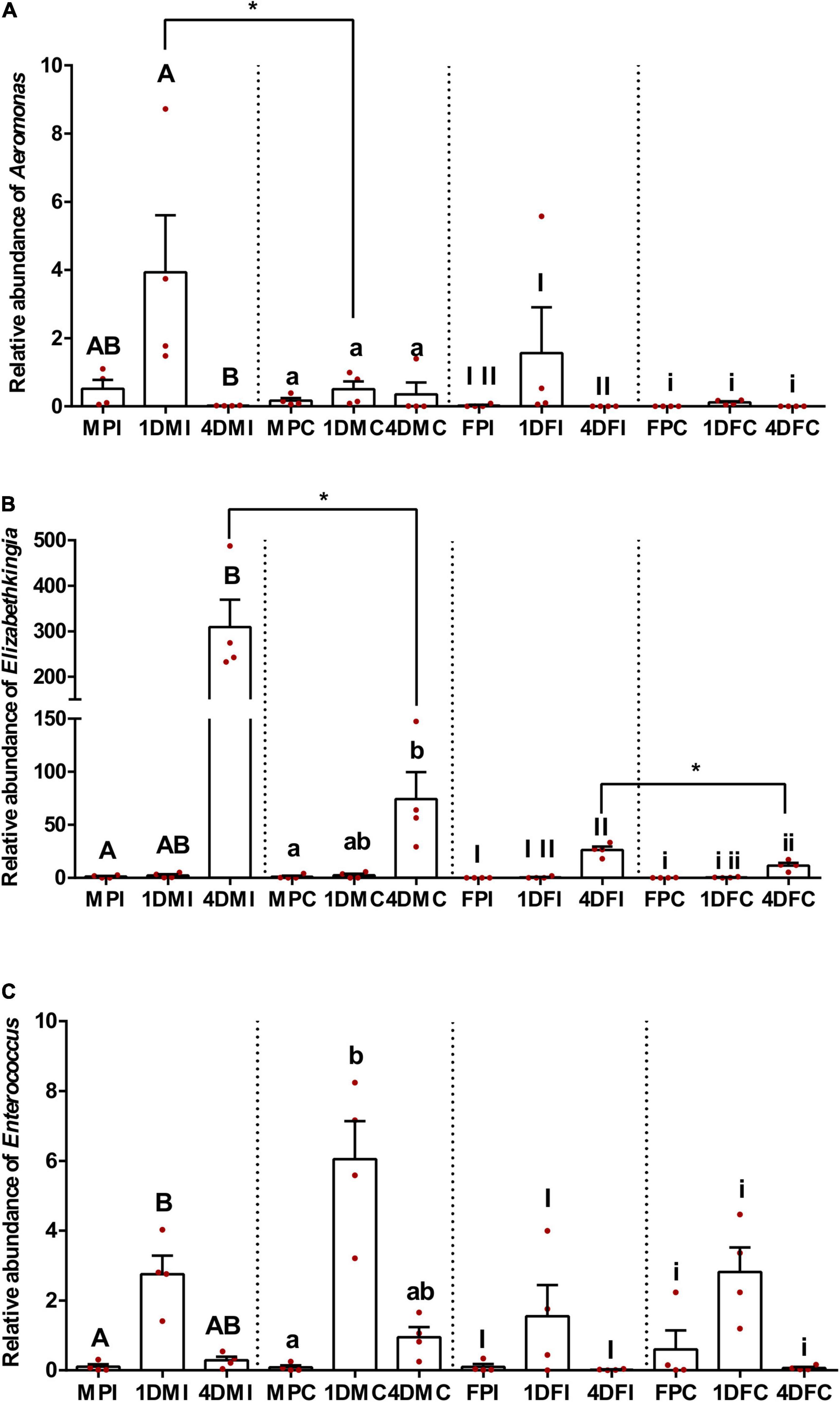
Figure 4. Relative abundance of three major bacterial groups based on qPCR. (A) Aeromonas; (B) Elizabethkingia; (C) Enterococcus. Dots represent biological replicates, each one as the mean of three technical replicates. Relative abundance data (n = 4 for each sample, Mean ± S.E.M) are presented relative to the housekeeping gene rps6. Within (A–C) samples of the same sex and irradiation/control, values followed by different lowercase letters or capital letters or Roman numbers were statistically different using Kruskal-Wallis test and Dunn’s multiple comparisons test (P < 0.05). Two-tailed Mann-Whitney U-test was performed between the irradiated and control samples of the same developmental stage/age/sex. Only the significantly different results are shown in the figures. * indicates P < 0.05.
The effect of irradiation on the abundance of the three bacterial taxa was compared to the respective control groups of the same sex and developmental stage/age. Increased Aeromonas density was only observed in the irradiated 1-day-old adult males when compared to non-irradiated ones (Two-tailed Mann-Whitney U-test, P < 0.05). Similar results were found in 4-day-old irradiated adults which had higher Elizabethkingia density than the controls in both males and females (Two-tailed Mann-Whitney U-test, P < 0.05). No significant difference was observed on the Enterococcus density between irradiated and non-irradiated samples (Two-tailed Mann-Whitney U-test, P > 0.05). Detailed information about the statistical analysis is shown in Supplementary Tables 4, 5.
Sterile insect technique (SIT), as a component of area-wide integrated pest management (AW-IPM) programmes, has been successfully applied to suppress or even locally eradicate insect populations of several major agricultural and livestock pest species during the last seventy years (Vreysen et al., 2007; Dyck et al., 2021). Based on these successful applications, recent efforts have focused on the development and validation of the SIT package to suppress populations of mosquito disease vectors including Ae. albopictus and Ae. aegypti (Lees et al., 2015; Bourtzis et al., 2016; Bouyer and Vreysen, 2020). Indeed, several small-scale field trials have provided quite encouraging results suggesting that SIT has the potential to suppress Aedes mosquito populations (Bellini et al., 2013; Kittayapong et al., 2018, 2019; Zheng et al., 2019). One of the key elements for the successful application of SIT to suppress populations of insect pests and disease vectors, including mosquitoes, is the production of high-quality sterile males (Bouyer and Vreysen, 2020; Dyck et al., 2021).
It has been shown that mass rearing, handling, transport, release and sterilization may affect the male mating competitiveness (Bellini et al., 2013; Madakacherry et al., 2014; Maïga et al., 2014; Yamada et al., 2014; Diallo et al., 2019; Du et al., 2019; Zheng et al., 2019; Bouyer and Vreysen, 2020; Dyck et al., 2021). In addition, several studies have suggested that changes in the male mating competitiveness may be associated with radiation-induced changes in the gut-associated bacteriome of SIT target species (Ami et al., 2010; Gavriel et al., 2010; Cai et al., 2018; Asimakis et al., 2019; Woruba et al., 2019). Using 16S rRNA gene-based sequencing approaches, the present study investigated the potential effect of age, sex and radiation on the gut-associated bacteriome of the laboratory-reared Ae. albopictus GUA strain. Our results clearly showed that all three factors can affect the gut-associated bacterial communities with age having the highest impact triggering significant changes on bacterial diversity and clustering among pupae, 1- and 4-day-old adult samples. It is also worth noting that the qPCR results revealed that the relative density levels of Aeromonas are higher in male samples compared to all other samples and that the irradiation triggers an increase in the density levels of both Aeromonas and Elizabethkingia in the mosquito gut at specific stages.
Previous studies on fruit flies have clearly shown that age plays an important role in shaping their gut-associated bacteriome (Ami et al., 2010; Hamden et al., 2013; Augustinos et al., 2015; Malacrinò et al., 2018). The present study confirmed that age is a critical factor in shaping the bacterial communities in Ae. albopictus guts too (Figures 3C,D). Actinobacteria, Bacteroidetes, Firmicutes and Proteobacteria were found to be the four main phyla in pupae and 1-day-old adult mosquitoes while Bacteroidetes was detected as the main phylum in 4-day-old adults (Figure 2A). However, age-dependent differences may be reflecting the distinct feeding behaviors during different developmental stages. In Diptera insects, once larva becomes pupa, it does not feed anymore until adult emergence (Truman, 2019). This indicates that all the metabolic activities taking place during the pupal stage are supported from nutrients produced through gut bacterial-mediated decomposition of larva-acquired intestinal contents. It is interesting to note that during the dissection of mosquito pupal guts, partial remnants of intestinal contents were observed in some samples and this may explain why higher bacterial diversity was observed at the pupal stage compared to adults (Figure 1E). Teneral mosquito adults (<24 h old) do not feed and their guts are usually empty indicating that the intestinal contents have been digested after eclosion. Four-day-old adult mosquitoes are only provided with sugar solution which may explain why teneral adults may present lower bacteria diversity compared to pupae (Figure 1E). It is noteworthy that Elizabethkingia is the most abundant bacterium in 4-day-old adults (Figure 2B), which is in line with previous studies showing that this bacterial species is mainly involved in the digestion of blood and sugar (Wang et al., 2011; Chen et al., 2015, 2020).
Sex may also affect the composition of mosquito gut-associated bacteriomes and differences have mainly been attributed to different food sources and nutrients (Minard et al., 2013a, 2014; Mancini et al., 2018). For blood-sucking insects such as mosquitoes, females require animal blood, which contains essential nutrients for the maturation of eggs (Dodd, 1995; Barrozo, 2019). Intestinal bacterial species are involved in the digestion of blood to produce these essential nutrients and this may explain why the abundance of key bacterial species increases after a blood meal (Strand, 2018). Unlike females, males usually feed only on honey or sugar throughout their entire lifetime. Previous studies have documented clear differences between the bacterial communities associated with male and female mosquitoes (Minard et al., 2018; Rosso et al., 2018; Wang et al., 2018; Chen et al., 2020). The present study also confirmed the different structure of the gut-associated bacterial communities present in Ae. albopictus male and female mosquitoes (Figure 3C).
As in many insect species, Ae. albopictus is characterized by the phenomenon of protandry, male mosquitoes develop faster than female ones (Zhang et al., 2015b). In addition, it has been shown that Aeromonas, as well as Klebsiella and yeast, can accelerate the larval development in the mosquito species Culex pipiens (Díaz-Nieto et al., 2016). Interestingly, our study showed that Aeromonas bacteria are present in higher densities in Ae. albopictus males compared to females in both irradiation and control groups, while no difference was observed in respect to Elizabethkingia and Enterococcus taxa (Supplementary Table 2). These results suggest that Aeromonas maybe a contributing factor for the different developmental rate observed in Ae. albopictus males and females (Supplementary Table 2). Further experimental work is needed to test this hypothesis and see whether Aeromonas could be used as probiotic to further enhance the protandry phenomena in Ae. albopictus thus enhancing sex separation efficiency and male recovery in support of SIT applications against this major vector species.
Several studies have shown that radiation may affect the diversity and abundance of gut-associated bacteria in insects (Ami et al., 2010; Lauzon and Potter, 2012; Yuval et al., 2013; Cai et al., 2018; Asimakis et al., 2019; Woruba et al., 2019). However, our results suggest that irradiation of Ae. albopictus pupae does not affect the overall abundance and diversity of the gut-associated bacterial community (Table 2 and Figures 1A,D), but it rather triggers a shift to its actual composition (Table 2 and Figure 3). It is worth noting that 4-day-old non-irradiated mosquitoes have significant higher abundance of Elizabethkingia when compared to their irradiated counterparts (Supplementary Table 2), whereas this difference is not observed in pupae and 1-day-old mosquitoes. The Elizabethkingia’s genome is properly equipped to metabolize sugars present in the host intestine (Kukutla et al., 2014). This has been confirmed by an increase of Elizabethkingia’s density levels in the intestine of 14-day-old male Ae. albopictus adults, which have been fed on sugar solution only, by more than 30 times when compared to 1-day-old males (Chen et al., 2020). In addition, Elizabethkingia encodes an antioxidant protein HemS (heme-degrading protein), which is known to reduce the oxidative damage during blood digestion (Kukutla et al., 2014). Previous studies have shown that irradiation increases the abundance of oxygen free radicals in the intestine of insects causing oxidative damages (Zaghloul et al., 2013; Ali et al., 2017; Wang et al., 2019). Based on this observation, the higher abundance of Elizabethkingia detected in the irradiated adults in the present study may indicate that irradiated mosquitoes use this bacterium to remove the oxygen free radicals to reduce any potential oxidative damage. In addition, the genome of Elizabethkingia encodes for several hemolysins, and with their hemolytic activity this bacterial species can participate in the digestion of red blood cells (Kukutla et al., 2014). The sharp increase of the density levels of this bacterium in the guts of female mosquitoes after a blood meal is also in agreement with this (Chen et al., 2020).
In conclusion, the effects of age (pupa, 1- and 4-day-old adults), sex (female and male) and radiation on the gut-associated bacterial species of lab-reared Ae. albopictus were investigated in the present study using 16S rRNA gene-based next generation sequencing approaches. The results showed that age, perhaps in conjunction with the diet, is a key factor that can shape the diversity and structure of the mosquito gut-associated bacteriome. In addition, sex and radiation may also affect the bacteria community, even though no significant impact was observed on richness and diversity. In addition, our data suggested that Aeromonas could potentially be used as probiotics to enhance protandry and sex separation in support of SIT applications against Aedes albopictus, while the functional role of Elizabethkingia in respect to oxidative stress and damage in irradiated mosquitoes needs further investigation.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA682321 and https://dataverse.harvard.edu/dataset.xhtml?persistentId=, doi: 10.7910/DVN/HEL3PS
DZ and SC performed the experiments, analyzed the data, and drafted the manuscript. AA-A performed the bioinformatic analysis, interpreted the data, contributed to the drafting, and critical revision of the manuscript. KB conceived the study, designed the experiments, interpreted the data, contributed to the drafting, and critically revised the manuscript. All authors approved the final version of the manuscript.
This study was supported by the Joint FAO/IAEA Insect Pest Control Subprogramme. DZ was supported by the National Natural Science Foundation of China (82002168), the 6th Nuclear Energy R&D Project (20201192), the China Postdoctoral Innovation Program (BX20180394), and the China Postdoctoral Science Foundation (2018M643317).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Jeremie Gilles for his overall support to this project and Prof. George Tsiamis for his advice on parts of the bioinformatic analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.671699/full#supplementary-material
Ali, A., Rashid, M. A., Huang, Q. Y., and Lei, C.-L. (2017). Influence of UV-A radiation on oxidative stress and antioxidant enzymes in Mythimna separata (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 24, 8392–8398. doi: 10.1007/s11356-017-8514-7
Ami, E. B., Yuval, B., and Jurkevitch, E. (2010). Manipulation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. ISME J. 4, 28–37. doi: 10.1038/ismej.2009.82
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Asimakis, E. D., Khan, M., Stathopoulou, P., Caceres, C., Bourtzis, K., and Tsiamis, G. (2019). The effect of diet and radiation on the bacterial symbiome of the melon fly, Zeugodacus cucurbitae (Coquillett). BMC Biotechnol. 19:88. doi: 10.1186/s12896-019-0578-7
Augustinos, A. A., Kyritsis, G. A., Papadopoulos, N. T., Abd-Alla, A. M. M., Cáceres, C., and Bourtzis, K. (2015). Exploitation of the Medfly gut microbiota for the enhancement of sterile insect technique: use of Enterobacter sp. in Larval Diet-Based Probiotic Applications. PLoS One 10:e0136459. doi: 10.1371/journal.pone.0136459
Balestrino, F., Medici, A., Candini, G., Carrieri, M., Maccagnani, B., Calvitti, M., et al. (2010). Gamma ray dosimetry and mating capacity studies in the laboratory on Aedes albopictus males. J. Med. Entomol. 47, 581–591. doi: 10.1093/jmedent/47.4.581
Balestrino, F., Puggioli, A., Gilles, J. R. L., and Bellini, R. (2014). Validation of a new larval rearing unit for Aedes albopictus (Diptera: Culicidae) mass rearing. PLoS One 9:e91914. doi: 10.1371/journal.pone.0091914
Barrozo, R. B. (2019). Food recognition in hematophagous insects. Curr. Opin. Insect Sci. 34, 55–60. doi: 10.1016/j.cois.2019.03.001
Bellini, R., Balestrino, F., Medici, A., Gentile, G., Veronesi, R., and Carrieri, M. (2013). Mating competitiveness of Aedes albopictus radio-sterilized males in large enclosures exposed to natural conditions. J. Med. Entomol. 50, 94–102. doi: 10.1603/ME11058
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Bourtzis, K., Lees, R. S., Hendrichs, J., and Vreysen, M. J. B. (2016). More than one rabbit out of the hat: radiation, transgenic and symbiont-based approaches for sustainable management of mosquito and tsetse fly populations. Acta Trop. 157, 115–130. doi: 10.1016/j.actatropica.2016.01.009
Bouyer, J., and Vreysen, M. J. B. (2020). Yes, irradiated sterile male mosquitoes can be sexually competitive! Trends Parasitol. 36, 877–880. doi: 10.1016/j.pt.2020.09.005
Bouyer, J., Yamada, H., Pereira, R., Bourtzis, K., and Vreysen, M. J. B. (2020). Phased conditional approach for mosquito management using sterile insect technique. Trends Parasitol. 36, 325–336. doi: 10.1016/j.pt.2020.01.004
Cai, Z., Yao, Z., Li, Y., Xi, Z., Bourtzis, K., Zhao, Z., et al. (2018). Intestinal probiotics restore the ecological fitness decline of Bactrocera dorsalis by irradiation. Evol. Appl. 11, 1946–1963. doi: 10.1111/eva.12698
Caputo, B., Moretti, R., Manica, M., Serini, P., Lampazzi, E., Bonanni, M., et al. (2020). A bacterium against the tiger: preliminary evidence of fertility reduction after release of Aedes albopictus males with manipulated Wolbachia infection in an Italian urban area. Pest Manag. Sci. 76, 1324–1332. doi: 10.1002/ps.5643
Chen, S., Bagdasarian, M., and Walker, E. D. (2015). Elizabethkingia anophelis: molecular Manipulation and Interactions with Mosquito Hosts. Appl. Environ. Microbiol. 81, 2233–2243. doi: 10.1128/aem.03733-14
Chen, S., Zhang, D., Augustinos, A., Doudoumis, V., Bel Mokhtar, N., Maiga, H., et al. (2020). Multiple factors determine the structure of bacterial communities associated With Aedes albopictus under artificial rearing conditions. Front. Microbiol. 11:605. doi: 10.3389/fmicb.2020.00605
Chouaia, B., Rossi, P., Epis, S., Mosca, M., Ricci, I., Damiani, C., et al. (2012). Delayed larval development in Anopheles mosquitoes deprived of Asaia bacterial symbionts. BMC Microbiol. 12:S2. doi: 10.1186/1471-2180-12-S1-S2
Clarke, K. R., and Gorley, R. N. (2016). Getting started with PRIMER v7. Available online at: http://updates.primer-e.com/primer7/manuals/Getting_started_with_PRIMER_7.pdf (accessed 2021).
Cole, J. R., Chai, B., Farris, R. J., Wang, Q., Kulam-Syed-Mohideen, A. S., McGarrell, D. M., et al. (2007). The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res. 35, D169–D172. doi: 10.1093/nar/gkl889
Coon, K. L., Brown, M. R., and Strand, M. R. (2016a). Gut bacteria differentially affect egg production in the anautogenous mosquito Aedes aegypti and facultatively autogenous mosquito Aedes atropalpus (Diptera: Culicidae). Parasit. Vectors 9:375. doi: 10.1186/s13071-016-1660-9
Coon, K. L., Brown, M. R., and Strand, M. R. (2016b). Mosquitoes host communities of bacteria that are essential for development but vary greatly between local habitats. Mol. Ecol. 25, 5806–5826. doi: 10.1111/mec.13877
Coon, K. L., Vogel, K. J., Brown, M. R., and Strand, M. R. (2014). Mosquitoes rely on their gut microbiota for development. Mol. Ecol. 23, 2727–2739. doi: 10.1111/mec.12771
Crawford, J. E., Clarke, D. W., Criswell, V., Desnoyer, M., Cornel, D., Deegan, B., et al. (2020). Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol. 38, 482–492. doi: 10.1038/s41587-020-0471-x
Deutscher, A. T., Chapman, T. A., Shuttleworth, L. A., Riegler, M., and Reynolds, O. L. (2019). Tephritid-microbial interactions to enhance fruit fly performance in sterile insect technique programs. BMC Microbiol. 19:287. doi: 10.1186/s12866-019-1650-0
Diallo, S., Seck, M. T., Rayaissé, J. B., Fall, A. G., Bassene, M. D., Sall, B., et al. (2019). Chilling, irradiation and transport of male Glossina palpalis gambiensis pupae: effect on the emergence, flight ability and survival. PLoS One 14:e0216802. doi: 10.1371/journal.pone.0216802
Díaz-Nieto, L. M., D´Alessio, C., Perotti, M. A., and Berón, C. M. (2016). Culex pipiens development is greatly influenced by native bacteria and exogenous yeast. PLoS One 11:e0153133. doi: 10.1371/journal.pone.0153133
Dickson, L. B., Jiolle, D., Minard, G., Moltini-Conclois, I., Volant, S., Ghozlane, A., et al. (2017). Carryover effects of larval exposure to different environmental bacteria drive adult trait variation in a mosquito vector. Sci. Adv. 3:e1700585. doi: 10.1126/sciadv.1700585
Dillon, R. J., and Dillon, V. M. (2004). T HE G UT B ACTERIA OF I NSECTS: nonpathogenic interactions. Annu. Rev. Entomol. 49, 71–92. doi: 10.1146/annurev.ento.49.061802.123416
Dodd, C. (1995). Why do Insects Bite? A review of blood sucking behaviour. J. R. Army Med. Corps 141, 151–156. doi: 10.1136/jramc-141-03-05
Du, W., Hu, C., Yu, C., Tong, J., Qiu, J., Zhang, S., et al. (2019). Comparison between pupal and adult X-ray radiation, designed for the sterile insect technique for Aedes albopictus control. Acta Trop. 199, 105110. doi: 10.1016/j.actatropica.2019.105110
Dyck, V. A., Hendrichs, J., and Robinson, A. S. (eds) (2020). Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd Edn. Boco Raton, FL: CRC Press.
Dyck, V. A., Hendrichs, J., and Robinson, A. S. (eds) (2021). Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd Edn. Boco Raton, FL: CRC Press. doi: 10.1201/9781003035572
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Edgar, R. C. (2016a). SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. Bioinformatics. 1–20. doi: 10.1101/074161 Available online at: https://www.biorxiv.org/content/10.1101/074161v1.full.pdf.
Edgar, R. C. (2016b). UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv [Preprint]. doi: 10.1101/081257
Edgar, R. C. (2017). SEARCH_16S: a new algorithm for identifying 16S ribosomal RNA genes in contigs and chromosomes. bioRxiv [Preprint]. doi: 10.1101/124131
Eid, J., Fehr, A., Gray, J., Luong, K., Lyle, J., Otto, G., et al. (2009). Real-time DNA sequencing from single polymerase molecules. Science 323, 133–138. doi: 10.1126/science.1162986
Engel, P., and Moran, N. A. (2013). The gut microbiota of insects – diversity in structure and function. FEMS Microbiol. Rev. 37, 699–735. doi: 10.1111/1574-6976.12025
Estaki, M., Jiang, L., Bokulich, N. A., McDonald, D., González, A., Kosciolek, T., et al. (2020). QIIME 2 enables comprehensive end-to-end analysis of diverse microbiome data and comparative studies with publicly available data. Curr. Protoc. Bioinforma 70:e100. doi: 10.1002/cpbi.100
Flores, H. A., and O’Neill, S. L. (2018). Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 16, 508–518. doi: 10.1038/s41579-018-0025-0
Focks, D. A. (1980). An improved separator for the developmental stages, sexes, and species of mosquitoes (Diptera: Culicidae). J. Med. Entomol. 17, 567–568. doi: 10.1093/jmedent/17.6.567
Gasperi, G., Bellini, R., Malacrida, A. R., Crisanti, A., Dottori, M., and Aksoy, S. (2012). A new threat looming over the Mediterranean basin: emergence of viral diseases transmitted by Aedes albopictus mosquitoes. PLoS Negl. Trop. Dis. 6:e1836. doi: 10.1371/journal.pntd.0001836
Gavriel, S., Gazit, Y., and Yuval, B. (2010). Effect of diet on survival, in the laboratory and the field, of sterile male Mediterranean fruit flies. Entomol. Exp. Appl. 135, 96–104. doi: 10.1111/j.1570-7458.2010.00972.x
Guégan, M., Zouache, K., Démichel, C., Minard, G., Tran Van, V., Potier, P., et al. (2018). The mosquito holobiont: fresh insight into mosquito-microbiota interactions. Microbiome 6:49. doi: 10.1186/s40168-018-0435-2
Hamden, H., M’saad Guerfali, M., Fadhl, S., Saidi, M., and Chevrier, C. (2013). Fitness improvement of mass-reared sterile males of Ceratitis capitata (Vienna 8 strain) (Diptera: Tephritidae) after gut enrichment with probiotics. J. Econ. Entomol. 106, 641–647. doi: 10.1603/EC12362
Helinski, M. E. H., and Knols, B. G. J. (2008). Mating competitiveness of male Anopheles arabiensis mosquitoes irradiated with a partially or fully sterilizing dose in small and large laboratory cages. J. Med. Entomol. 45, 698–705. doi: 10.1603/0022-2585(2008)45[698:mcomaa]2.0.co;2
Huang, W., Wang, S., and Jacobs-Lorena, M. (2020). Use of microbiota to fight mosquito-borne disease. Front. Genet. 11:196. doi: 10.3389/fgene.2020.00196
Itoh, H., Tago, K., Hayatsu, M., and Kikuchi, Y. (2018). Detoxifying symbiosis: microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat. Prod. Rep. 35, 434–454. doi: 10.1039/C7NP00051K
Kittayapong, P., Kaeothaisong, N., Ninphanomchai, S., and Limohpasmanee, W. (2018). Combined sterile insect technique and incompatible insect technique: sex separation and quality of sterile Aedes aegypti male mosquitoes released in a pilot population suppression trial in Thailand. Parasit. Vectors 11:657. doi: 10.1186/s13071-018-3214-9
Kittayapong, P., Ninphanomchai, S., Limohpasmanee, W., Chansang, C., Chansang, U., and Mongkalangoon, P. (2019). Combined sterile insect technique and incompatible insect technique: the first proof-of-concept to suppress Aedes aegypti vector populations in semi-rural settings in Thailand. PLoS Negl. Trop. Dis. 13:e0007771. doi: 10.1371/journal.pntd.0007771
Klindworth, A., Pruesse, E., Schweer, T., Peplies, J., Quast, C., Horn, M., et al. (2013). Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41:e1. doi: 10.1093/nar/gks808
Knipling, E. F. (1955). Possibilities of insect control or eradication through the use of sexually sterile males1. J. Econ. Entomol. 48, 459–462. doi: 10.1093/jee/48.4.459
Kukutla, P., Lindberg, B. G., Pei, D., Rayl, M., Yu, W., Steritz, M., et al. (2014). Insights from the genome annotation of Elizabethkingia anophelis from the malaria vector Anopheles gambiae. PLoS One 9:e97715. doi: 10.1371/journal.pone.0097715
Kyritsis, G. A., Augustinos, A. A., Cáceres, C., and Bourtzis, K. (2017). Medfly gut microbiota and enhancement of the sterile insect technique: similarities and differences of Klebsiella oxytoca and Enterobacter sp. AA26 probiotics during the larval and adult stages of the VIENNA 8D53+ genetic sexing strain. Front. Microbiol. 8:2064. doi: 10.3389/fmicb.2017.02064
Lauzon, C. R., and Potter, S. E. (2012). Description of the irradiated and nonirradiated midgut of Ceratitis capitata Wiedemann (Diptera: Tephritidae) and Anastrepha ludens Loew (Diptera: Tephritidae) used for sterile insect technique. J. Pest Sci. 85, 217–226. doi: 10.1007/s10340-011-0410-1
Lees, R. S., Gilles, J. R., Hendrichs, J., Vreysen, M. J., and Bourtzis, K. (2015). Back to the future: the sterile insect technique against mosquito disease vectors. Curr. Opin. Insect Sci. 10, 156–162. doi: 10.1016/j.cois.2015.05.011
Lindh, J. M., Terenius, O., and Faye, I. (2005). 16S rRNA Gene-Based Identification of Midgut Bacteria from Field-Caught Anopheles gambiae Sensu Lato and A. funestus mosquitoes reveals new species related to known insect symbionts. Appl. Environ. Microbiol. 71, 7217–7223. doi: 10.1128/AEM.71.11.7217-7223.2005
Madakacherry, O., Lees, R. S., and Gilles, J. R. L. (2014). Aedes albopictus (Skuse) males in laboratory and semi-field cages: release ratios and mating competitiveness. Acta Trop. 132, S124–S129. doi: 10.1016/j.actatropica.2013.11.020
Maïga, H., Damiens, D., Niang, A., Sawadogo, S. P., Fatherhaman, O., Lees, R. S., et al. (2014). Mating competitiveness of sterile male Anopheles coluzzii in large cages. Malar. J. 13:460.
Malacrinò, A., Campolo, O., Medina, R. F., and Palmeri, V. (2018). Instar- and host-associated differentiation of bacterial communities in the Mediterranean fruit fly Ceratitis capitata. PLoS One 13:e0194131. doi: 10.1371/journal.pone.0194131
Mancini, M. V., Damiani, C., Accoti, A., Tallarita, M., Nunz, E., Cappelli, A., et al. (2018). Estimating bacteria diversity in different organs of nine species of mosquito by next generation sequencing. BMC Microbiol. 10:126. doi: 10.1186/s12866-018-1266-9
Minard, G., Mavingui, P., and Moro, C. (2013a). Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit. Vectors 6:146. doi: 10.1186/1756-3305-6-146
Minard, G., Tran, F.-H., Dubost, A., Tran-Van, V., Mavingui, P., and Valiente Moro, C. (2014). Pyrosequencing 16S rRNA genes of bacteria associated with wild tiger mosquito Aedes albopictus: a pilot study. Front. Cell. Infect. Microbiol. 4:59. doi: 10.3389/fcimb.2014.00059
Minard, G., Tran, F. H., Raharimalala, F. N., Hellard, E., Ravelonandro, P., Mavingui, P., et al. (2013b). Prevalence, genomic and metabolic profiles of Acinetobacter and Asaia associated with field-caught Aedes albopictus from Madagascar. FEMS Microbiol. Ecol. 83, 63–73. doi: 10.1111/j.1574-6941.2012.01455.x
Minard, G., Tran, F.-H., Tran Van, V., Fournier, C., Potier, P., Roiz, D., et al. (2018). Shared larval rearing environment, sex, female size and genetic diversity shape Ae. albopictus bacterial microbiota. PLoS One 13:e0194521. doi: 10.1371/journal.pone.0194521
Mitraka, E., Stathopoulos, S., Siden-Kiamos, I., Christophides, G. K., and Louis, C. (2013). Asaia accelerates larval development of Anopheles gambiae. Pathog. Glob. Health 107, 305–311. doi: 10.1179/2047773213Y.0000000106
Muturi, E. J., Bara, J. J., Rooney, A. P., and Hansen, A. K. (2016). Midgut fungal and bacterial microbiota of Aedes triseriatus and Aedes japonicus shift in response to La Crosse virus infection. Mol. Ecol. 25, 4075–4090. doi: 10.1111/mec.13741
Nazni, W. A., Hoffmann, A. A., NoorAfizah, A., Cheong, Y. L., Mancini, M. V., Golding, N., et al. (2019). Establishment of Wolbachia Strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr. Biol. 29, 4241–4248.e5. doi: 10.1016/j.cub.2019.11.007
Niyazi, N., Lauzon, C. R., and Shelly, T. E. (2004). Effect of probiotic adult diets on fitness components of sterile male Mediterranean fruit Flies (Diptera: Tephritidae) under laboratory and field cage conditions. J. Econ. Entomol. 97, 1570–1580. doi: 10.1603/0022-0493-97.5.1570
Pastoris, M. C., Passi, C., and Maroli, M. (1989). Evidence of Legionella pneumophila in some arthropods and related natural aquatic habitats. FEMS Microbiol. Lett. 62, 259–263. doi: 10.1111/j.1574-6968.1989.tb03700.x
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Rani, A., Sharma, A., Rajagopal, R., Adak, T., and Bhatnagar, R. K. (2009). Bacterial diversity analysis of larvae and adult midgut micro-flora using culture-dependent and culture-independent methods in lab-reared and field-collected Anopheles stephensi-an Asian malarial vector. BMC Microbiol. 9:96. doi: 10.1186/1471-2180-9-96
Rosso, F., Tagliapietra, V., Albanese, D., Pindo, M., Baldacchino, F., Arnoldi, D., et al. (2018). Reduced diversity of gut microbiota in two Aedes mosquitoes species in areas of recent invasion. Sci. Rep. 8:16091. doi: 10.1038/s41598-018-34640-z
Ryan, P. A., Turley, A. P., Wilson, G., Hurst, T. P., Retzki, K., Brown-Kenyon, J., et al. (2020). Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 3:1547. doi: 10.12688/gatesopenres.13061.2
Saab, S. A., Dohna, H. Z., Nilsson, L. K. J., Onorati, P., Nakhleh, J., Terenius, O., et al. (2020). The environment and species affect gut bacteria composition in laboratory co-cultured Anopheles gambiae and Aedes albopictus mosquitoes. Sci. Rep. 10:3352. doi: 10.1038/s41598-020-60075-6
Scolari, F., Casiraghi, M., and Bonizzoni, M. (2019). Aedes spp. and their microbiota: a review. Front. Microbiol. 10:2036. doi: 10.3389/fmicb.2019.02036
Sharma, A., Dhayal, D., Singh, O. P., Adak, T., and Bhatnagar, R. K. (2013). Gut microbes influence fitness and malaria transmission potential of Asian malaria vector Anopheles stephensi. Acta Trop. 128, 41–47. doi: 10.1016/j.actatropica.2013.06.008
Sogin, M. L., Morrison, H. G., Huber, J. A., Welch, D. M., Huse, S. M., Neal, P. R., et al. (2006). Microbial diversity in the deep sea and the underexplored “rare biosphere.”. Proc. Natl. Acad. Sci. U.S.A. 103, 12115–12120. doi: 10.1073/pnas.0605127103
Strand, M. R. (2018). Composition and functional roles of the gut microbiota in mosquitoes. Curr. Opin. Insect Sci. 28, 59–65. doi: 10.1016/j.cois.2018.05.008
Tolle, M. A. (2009). Mosquito-borne Diseases. Curr. Probl. Pediatr. Adolesc. Health Care 39, 97–140. doi: 10.1016/j.cppeds.2009.01.001
Truman, J. W. (2019). The evolution of insect metamorphosis. Curr. Biol. 29, R1252–R1268. doi: 10.1016/j.cub.2019.10.009
Vreysen, M. J. B., Robinson, A. S., and Hendrichs, J. (eds) (2007). Area-Wide Control of Insect Pests. Dordrecht: Springer. doi: 10.1007/978-1-4020-6059-5
Wang, L., Cheng, W., Meng, J., Speakmon, M., Qiu, J., Pillai, S., et al. (2019). Hypoxic environment protects cowpea bruchid (Callosobruchus maculatus) from electron beam irradiation damage: hypoxia increases bruchid radiotolerance. Pest Manag. Sci. 75, 726–735. doi: 10.1002/ps.5172
Wang, S., Wang, L., Fan, X., Yu, C., Feng, L., and Yi, L. (2020). An insight into diversity and functionalities of gut microbiota in insects. Curr. Microbiol. 77, 1976–1986. doi: 10.1007/s00284-020-02084-2
Wang, X., Liu, T., Wu, Y., Zhong, D., Zhou, G., Su, X., et al. (2018). Bacterial microbiota assemblage in Aedes albopictus mosquitoes and its impacts on larval development. Mol. Ecol. 27, 2972–2985. doi: 10.1111/mec.14732
Wang, Y., Gilbreath, T. M., Kukutla, P., Yan, G., and Xu, J. (2011). Dynamic gut microbiome across life history of the Malaria Mosquito Anopheles gambiae in Kenya. PLoS One 6:e24767. doi: 10.1371/journal.pone.0024767
Weaver, S. C., Charlier, C., Vasilakis, N., and Lecuit, M. (2018). Zika, Chikungunya, and other emerging vector-borne viral diseases. Annu. Rev. Med. 69, 395–408. doi: 10.1146/annurev-med-050715-105122
WHO and IAEA (2020). TDR | Guidance Framework for Testing the Sterile Insect Technique as a Vector Control Tool Against Aedes-borne Diseases. Geneva: WHO.
Woruba, D. N., Morrow, J. L., Reynolds, O. L., Chapman, T. A., Collins, D. P., and Riegler, M. (2019). Diet and irradiation effects on the bacterial community composition and structure in the gut of domesticated teneral and mature Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). BMC Microbiol. 19:281. doi: 10.1186/s12866-019-1649-6
Yamada, H., Vreysen, M. J., Gilles, J. R., Munhenga, G., and Damiens, D. D. (2014). The effects of genetic manipulation, dieldrin treatment and irradiation on the mating competitiveness of male Anopheles arabiensis in field cages. Malar. J. 13:318.
Yuval, B., Ben-Ami, E., Behar, A., Ben-Yosef, M., and Jurkevitch, E. (2013). The Mediterranean fruit fly and its bacteria - potential for improving sterile insect technique operations: bacteria of Ceratitis capitata and the SIT. J. Appl. Entomol. 137, 39–42. doi: 10.1111/j.1439-0418.2010.01555.x
Zaghloul, Y. S., Elakhdar, E. A. H., and Abbassy, S. A. (2013). Studies on the effect of E-Selen as antioxidant in ameliorating the physiological status of gamma-irradiated Mediterranean fruit fly, Ceratits capitata (Wied.). J. Nucl. Technol. Appl. Sci. 1, 299–311.
Zchori-Fein, E., and Bourtzis, K. (2011). Manipulative Tenants: Bacteria Associated with Arthropods, 1st Edn. Boco Raton, FL: CRC Press.
Zhang, D., Lees, R. S., Xi, Z., Bourtzis, K., and Gilles, J. R. L. (2016). Combining the Sterile Insect Technique with the Incompatible Insect Technique: III-Robust Mating Competitiveness of Irradiated Triple Wolbachia-Infected Aedes albopictus Males under Semi-Field Conditions. PLoS One 11:e0151864. doi: 10.1371/journal.pone.0151864
Zhang, D., Lees, R. S., Xi, Z., Gilles, J. R. L., and Bourtzis, K. (2015a). Combining the Sterile Insect Technique with Wolbachia-Based Approaches: II- A Safer Approach to Aedes albopictus Population Suppression Programmes, Designed to Minimize the Consequences of Inadvertent Female Release. PLoS One 10:e0135194. doi: 10.1371/journal.pone.0135194
Zhang, D., Zheng, X., Xi, Z., Bourtzis, K., and Gilles, J. R. L. (2015b). Combining the sterile insect technique with the incompatible insect technique: I-impact of Wolbachia infection on the fitness of triple-and double-infected strains of Aedes albopictus. PLoS One 10:e0121126. doi: 10.1371/journal.pone.0121126
Keywords: gut bacteriome, Elizabethkingia, Aeromonas, 16S rRNA gene, Aedes albopictus
Citation: Zhang D, Chen S, Abd-Alla AMM and Bourtzis K (2021) The Effect of Radiation on the Gut Bacteriome of Aedes albopictus. Front. Microbiol. 12:671699. doi: 10.3389/fmicb.2021.671699
Received: 24 February 2021; Accepted: 14 June 2021;
Published: 08 July 2021.
Edited by:
Chrysoula C. Tassou, Institute of Technology of Agricultural Products, Hellenic Agricultural Organisation DIMITRA, GreeceReviewed by:
Guido Favia, University of Camerino, ItalyCopyright © 2021 Zhang, Chen, Abd-Alla and Bourtzis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kostas Bourtzis, Sy5Cb3VydHppc0BpYWVhLm9yZw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.