
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 05 August 2021
Sec. Microbe and Virus Interactions with Plants
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.666761
This article is part of the Research Topic Plants and Microbial Communities: Diversity, Pathogens and Biological Control View all 28 articles
 Changhui Liang1
Changhui Liang1 Wenteng Gao1
Wenteng Gao1 Ting Ge1
Ting Ge1 Xinwei Tan1
Xinwei Tan1 Jiayu Wang1
Jiayu Wang1 Huaxin Liu1
Huaxin Liu1 Yong Wang2
Yong Wang2 Chao Han1
Chao Han1 Qian Xu3*
Qian Xu3* Qunqing Wang1,3*
Qunqing Wang1,3*Sustainable management of plant pathogens is becoming more challenging, and novel solutions are needed. Plant biologically active secondary metabolites are important sources of novel crop protection chemistry. Effective individual compounds of these natural products have the potential to be successful new agrochemicals. In this study, we identified lauric acid (LA) from soybean defense leaf volatiles. LA inhibited the growth of Phytophthora sojae, the causal agent of soybean root rot. It influenced mycelial development, sporangium formation, and zoospore generation and germination by damaging the P. sojae cell membrane. Additionally, we showed that LA and several of its derivatives, such as glycerol monolaurate (GML), had similar biological activities. Both LA and GML were safe to soybean plants when used at less than 0.3 g a.i./plant and could promote soybean growth, implying their potential as eco-friendly biological control agents.
Oomycetes are fungus-like eukaryotic organisms that belong to the class Saprolegniomycetidae of the kingdom Stramenopila (Yutin et al., 2008). They encompass notorious plant disease agents, including Phytophthora, Pythium, and Albugo, and a group of downy mildews (Tyler, 2001). Oomycetes have a negative impact on natural and farm ecosystems due to their strong pathogenicity and infectivity (Kamoun et al., 2015). In addition to the well-known potato late blight caused by Phytophthora infestans, which led to the 19th-century Irish famine, the persistence of sudden oak death caused by Phytophthora ramorum and grape downy mildew caused by Plasmopara viticola demonstrate that oomycete phytopathogens are a persistent threat to subsistence and commercial farming and destructive to native plants (Erwin and Ribeiro, 1996). Soybean (Glycine max L.) root rot caused by Phytophthora sojae is the leading cause of global soybean production loss (Tyler, 2007). Agrochemicals are largely used to control oomycete diseases, resulting in the emergence of resistant strains and resurgence events (Randall et al., 2014). The effective and sustainable control of oomycete-driven diseases requires the identification of novel pharmaceuticals and pesticides to drive the design of fungicides compatible with integrated pest management (IPM) approaches (Gessler et al., 2011).
Environmental-friendly botanical fungicides are valuable because of their higher efficiency, lower residue, and lower toxicity. Multiple interdisciplinary studies and abundant resources have been applied to find new and effective alternatives for the IPM of various oomycete species. Historically, traditional healers have used plants to prevent or cure infections, and active elements for disease control have been identified in various species. For example, artemisinin, present in sweet wormwood (Artemisia annua), a Chinese medicinal plant, is an effective antimalarial agent (Tu, 2011). Medium-chain fatty acids (MCFAs), including octanoic acid (C8), capric acid (C10), and lauric acid (LA) (C12), distilled from virgin coconut oil, inhibit various bacterial, fungal, and viral pathogens and have been widely used in human and veterinary medicine (Kabara et al., 1972; Bartolotta et al., 2001; Rouse et al., 2005; Yang et al., 2009, 2018; Shilling et al., 2013; Fortuoso et al., 2019). For example, capric acid kills Candida albicans quickly and effectively (Bergsson et al., 2001). LA is the most active MCFA at lower concentrations and after longer incubation times (Bergsson et al., 2001; Lalouckova et al., 2021). Additionally, LA has antimicrobial activity against both Gram-positive and Gram-negative pathogens, including Staphylococcus aureus, Streptococcus mutans, Streptococcus pyogenes, Escherichia coli, and Helicobacter pylori (Kabara et al., 1972; Rouse et al., 2005). In addition to their effect on human and animal pathogens, MCFAs also inhibit mycelial growth and spore germination of four plant pathogenic fungi: Alternaria solani, Colletotrichum lagenarium, Fusarium oxysporum f. sp. Cucumerinum, and F. oxysporum f. sp. lycopersici (Liu et al., 2008). These observations suggest that MCFAs may be useful for developing alternative IMP approaches for the control of phytopathogens.
Phytochemicals have a long history as sources of novel agrochemicals. Phytopathologists search for natural products (NPs) that can be developed into pesticides for the treatment of plant diseases. For example, poacic acid, which is commonly found in grass lignocellulosic hydrolyzates, inhibits the growth of Sclerotinia sclerotiorum and A. solani fungi and P. sojae oomycetes (Piotrowski et al., 2015). There is considerable evidence for direct protective effects of chemicals isolated from plant root or leaf exudates and leaf or fruit volatile organic compounds (VOCs) in various organisms. For example, ε-viniferin, isolated from Vitis vinifera canes, has antifungal activity against P. viticola and Botrytis cinerea (Schnee et al., 2013). Secomicromelin, 7-methoxy-8-(4′-methyl-3′-furanyl) coumarin, micromarin B, and isomicromelin, present in Micromelum falcatum fruits, inhibit Pythium insidiosum mycelial growth (Suthiwong et al., 2014). Cuminic acid, present in Cuminum cyminum L. seeds, inhibits Phytophthora capsici mycelial growth and zoospore germination (Wang et al., 2016). Gossypol, which is naturally present in cotton root tissues, has a strong inhibitory activity on the growth of various soil-borne oomycetes and fungi, including Pythium irregulare, Pythium ultimum, and F. oxysporum (Mellon et al., 2014). Several leaf VOCs are produced and emitted rapidly when plants respond to stresses, such as herbivore or mechanical damage or attack by necrotrophic fungi (Cowan, 1999; Scala et al., 2013; Matsui and Koeduka, 2016; Tanaka et al., 2018; Wang et al., 2020). These phytochemicals may contribute directly to plant defense by preventing pathogen invasion (Matsui, 2006; Kishimoto et al., 2008).
Here, we analyzed soybean leaf volatiles derived from compatible (disease-producing) and incompatible (successful plant defense) interactions with P. sojae, the causal agent of soybean root and stem rot disease. LA was identified by headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME-GC–MS) among the incompatible interaction-produced volatiles. Both LA and glycerol monolaurate (GML), a chemical compound formed from LA and glycerol, inhibited the mycelial growth of P. sojae in Petri dish assays. GML also disrupted or disintegrated the P. sojae plasma membrane, leading to shrunken mycelia and cytoplasmic electrolyte leakage. We provide a conclusion about the functions and the protective mechanisms of LA as a potential oomycete biological control agent.
Soybean plants were cultivated in a growth chamber at 25℃, with a cycle of 16 h of high light intensity and 8 h of darkness. P. sojae strains were grown on 10% V8 medium (10% V8 juice, 0.02% CaCO3, and 1.5% agar) at 25℃ in the dark. Hyphal plugs were cultured in V8 liquid medium for mycelial harvest. The mycelia were washed with sterile tap water three times and cultured in the darkness at 25℃ for 6 h for zoosporangium incubation and zoospore release. Finally, the concentration of the zoospore suspension was adjusted to 1 × 105 mL.
Soybean leaves inoculated with P. sojae were placed in a 20-mL headspace bottle. An AOC-6000 Multifunctional Autosampler (Shimadzu, Kyoto, Japan) was used for SPME injection, and a GCMS-TQ8040 NX (Shimadzu) was used for detection using the following standard SPME parameters: SPME fiber, FIB-C-WR-95/10; aging temperature, 240℃; aging time before extraction, 30 min; equilibration temperature, 40℃; equilibration time, 5 min; extraction time, 30 min; injection port temperature, 250℃; desorption time, 2 min; and aging time after extraction, 5 min. The GC–MS parameters used were as follows: column, inert cap pure-wax, 30 m × 0.25 mm × 0.25 m; oven program, 50℃(5 min) and 10℃/min 250℃(10 min); carrier gas pressure, 83.5 kPa; injection mode, split; split ratio, 5:1; ion-source temperature, 200℃; interface temperature, 250℃; detector voltage, tuning voltage +0.3 kV; and acquisition mode, MRM.
Hyphal plugs with a 6 mm were cultured in V8 liquid medium containing different concentrations (0.5, 1, 1.5, 2, and 2.5 mM) of LA, its derivatives–GML, methyl laurate (MEL), and ethyl laurate (ETL)–or the same volume of sterile water as a control reference (CK). The inoculated medium was incubated in the darkness at 25℃ for 5 days. Then, the colony diameter was measured, and the mycelium status was observed under a light microscope.
Different concentrations of LA or its derivatives, GML, MEL, and ETL, were added into washed mycelia cultured in V8 liquid medium. After incubation at 25℃ for 6 h, the number of zoosporangia was determined under a light microscope.
Lauric acid and its derivatives were added into the same number of sporangium culture dishes without zoospore release. The zoospores were cultured at 25℃, and the number of zoospores was determined under a light microscope.
A 0.1-mL spore suspension was evenly coated on V8 medium containing different concentrations of LA or its derivatives, GML, MEL, and ETL. After incubation at 25℃ for 4 days, colony formation was analyzed visually.
Mycelia were cultured in V8 liquid medium for 3 days before different concentrations of LA, and its derivatives, GML, MEL, and ETL, were added to the medium. After 1–2 h of treatment, 1/10 of the total volume of propidium iodate (PI) was added to the culture. After 20 min of incubation, the mycelia were rinsed with PBS 2–3 times. The mycelium was observed under a fluorescence microscope.
The same mycelia were placed in liquid V8 medium containing different concentrations of LA or its derivatives, and the concentrations of DNA and protein, and the electrical conductivity, were measured every 2 h and plotted for analysis.
Different concentrations (0.5, 1, 1.5, 2, and 2.5 mM) of LA or its derivatives, GML, MEL, and ETL, were mixed with the same volume of soil. Sterile water was used as a blank control (CK), and each treatment was repeated three times. The growth and development of soybean seedlings were recorded 7 and 14 days after planting.
Different concentrations (0.5, 1, 1.5, 2, and 2.5 mM) of LA or its derivatives, GML, MEL, and ETL, were mixed with the same volume of soil. Sterile water was used as a blank control. The soil was covered with a plastic film; after 15 days, the plastic film was removed, and the soil was ventilated for 2 days. Soybeans were then planted, and their height was measured after 5 days.
Soybean is the main host of P. sojae. Therefore, soybean cultivars that contain resistance genes to P. sojae (Rps) are important resources in agricultural production. Resistance genes provide defense ability against pathogen varieties and induce the emergence of hypersensitive responses and the production of secondary metabolites.
We analyzed the leaf volatiles produced during incompatible soybean–P. sojae interactions. After inoculation with P. sojae, the main constituents of the volatiles produced from the leaves of the susceptible soybean cultivar Williams (without Rps genes) and the resistant cultivar Williams 82 (with the resistance gene Rps1k) were determined by GC–MS. The LA concentration in the leaf volatiles of Williams 82 was significantly higher than that of Williams (Supplementary Figure 1), suggesting that LA contributes to the defense response of soybean to P. sojae.
We measured the radial growth diameters of different P. sojae races grown on V8 medium containing different concentrations of LA; the toxicity of LA to P. sojae was conspicuous (Figure 1 and Supplementary Table 1). After 5 days’ growing on V8 medium with 0.5 mM LA, the diameter of Race2 (strain P6497) hypha was 26.27 mm, and the bacteriostatic rate was 54.21%. Furthermore, the bacteriostatic rates of Race7 (strain P7064), Race17 (strain P7074), and Race19 (strain P7076) were 30.87, 28.22, and 23.33%, respectively. On the medium containing 2.5 mM LA, the bacteriostatic rates for Race2, Race 7, Race17, and Race19 were 64.84, 57.06, 42.16, and 62.86%, respectively. Higher concentrations of LA exacerbated its toxicity to various P. sojae races.
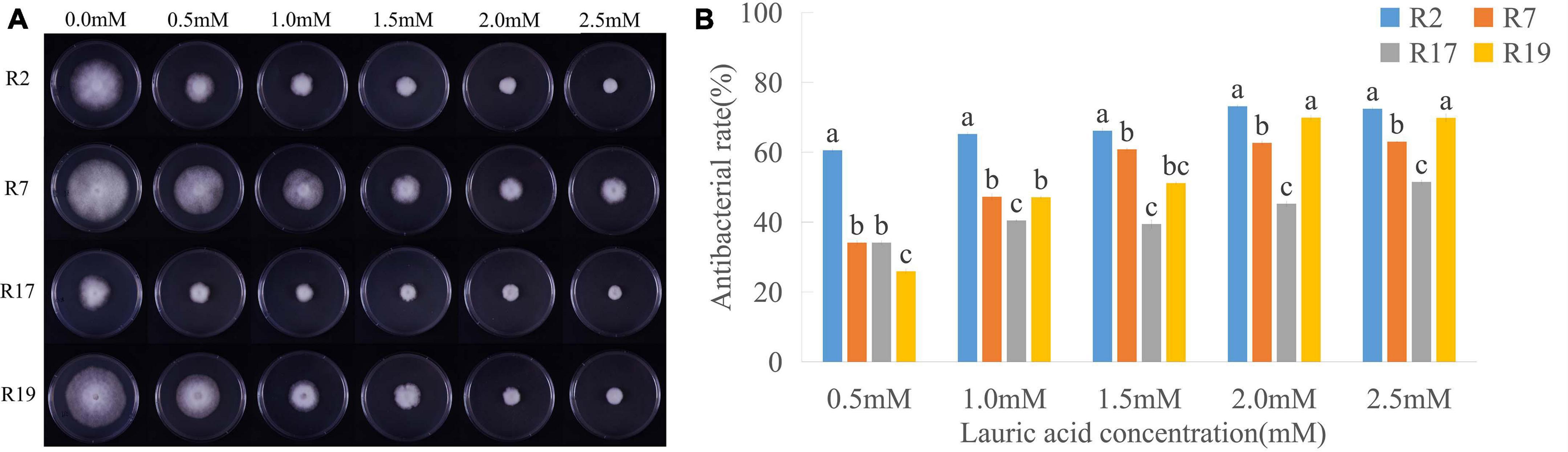
Figure 1. Inhibitory effects of lauric acid (LA) to various P. sojae species. (A) Four different P. sojae races (Race2, Race7, Race17, and, Race19) were cultured on V8 medium with different concentrations of LA for 5 days. The experiment was repeated three times with similar results. (B) The diameter of each colony was measured and statistical analyzed via software Statistical Product and Service Solutions (SPSS). Means with different letters are significantly different (p < 0.05).
Fatty acids can merge into cell membranes, and their hydrophilicity and lipophilicity likely affect their antimicrobial activity. To verify this hypothesis, we analyzed the mycelial growth diameter of P. sojae (Race2) grown under different concentrations of LA and its derivatives, GML, MEL, and ETL. The diameters and bacteriostatic rates of mycelia treated with GML, MEL, and ETL at 0.5 mM were 50.80, 57.53, and 57.37 mm, and 27.32, 17.70, and 17.93%, respectively. For LA, the corresponding values were 45.40 mm and 35.05%, indicating that its toxicity is higher than that of the three derivatives tested (Figure 2 and Supplementary Table 2). The inhibitory effect of LA of P. sojae was the most significant at the lowest concentration (0.5 mM). In summary, the toxicity effects to P. sojae were the highest for LA, followed by GML, ETL, and MEL.
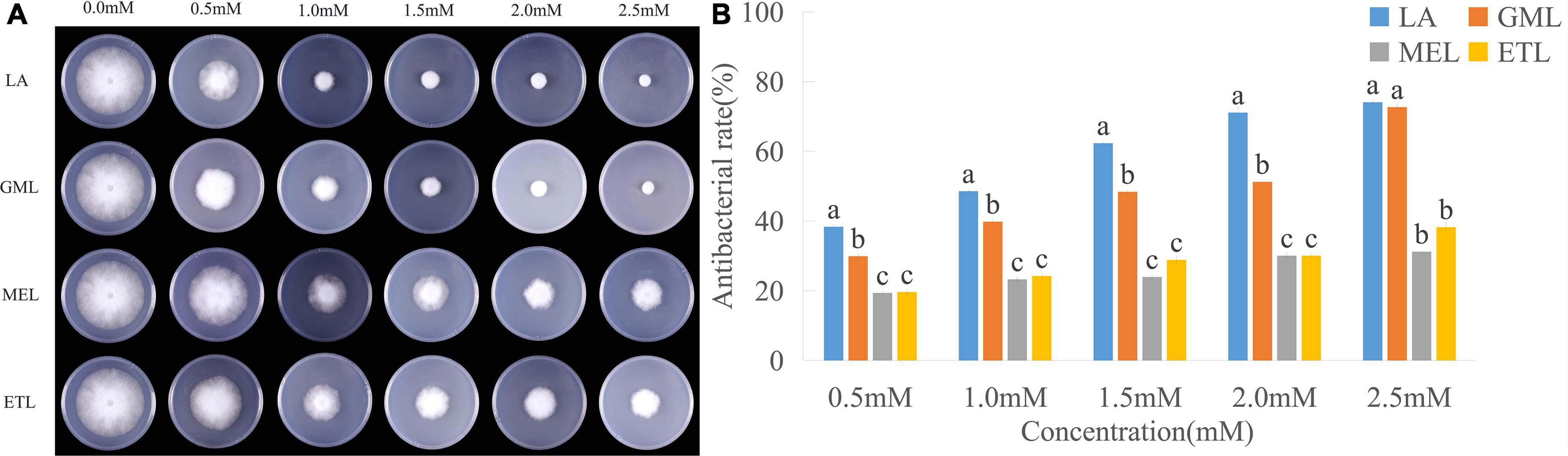
Figure 2. Inhibitory effects of lauric acid (LA) and its derivatives to P. sojae. (A) The mycelia of P. sojae (Race2) were cultured on media containing LA, glycerol monolaurate (GML), methyl laurate (MEL), or ethyl laurate (ETL) for 5 days. (B) The diameter of each colony was measured and statistical analyzed via software Statistical Product and Service Solutions (SPSS). Means with different letters are significantly different (p < 0.05).
We observed the morphology of P. sojae hyphae with an optical microscope. LA treatments led to hyphae with more branches, distortions, bending, and nodules than those in the control group. Moreover, LA-treated hyphae had few zoosporangia (Figure 3).
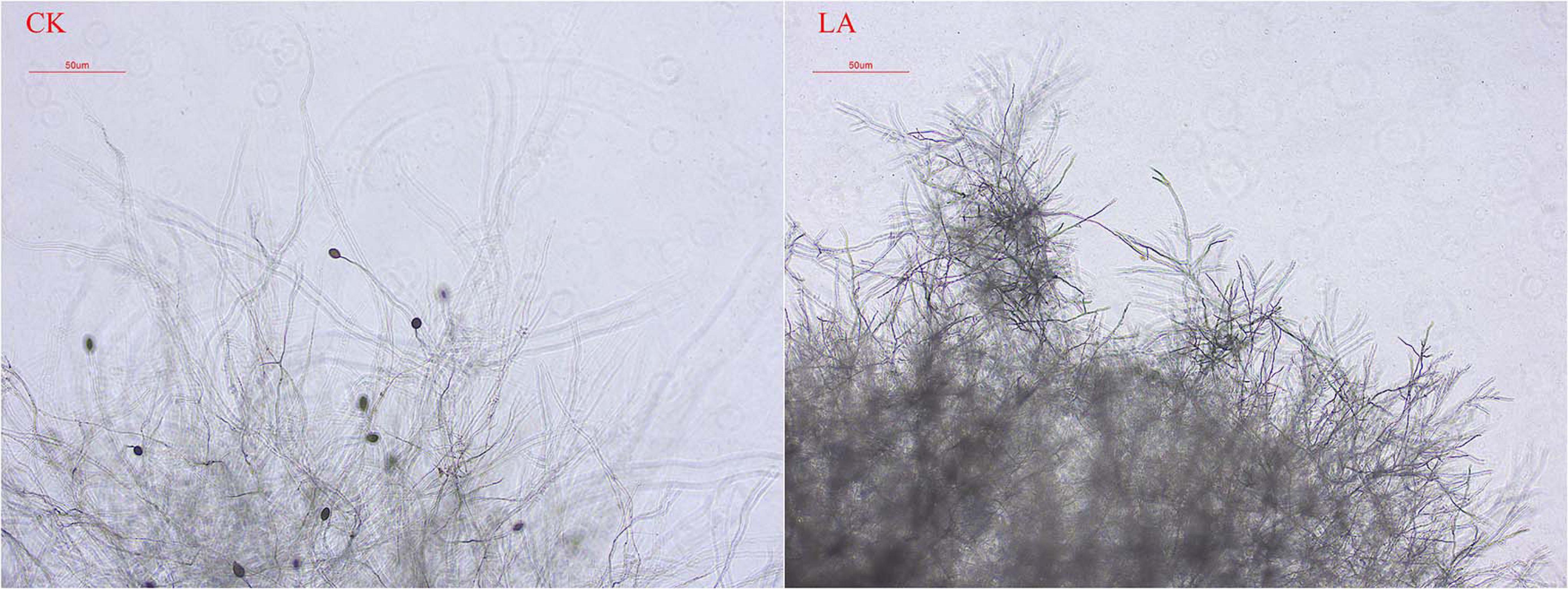
Figure 3. Effect of LA on P. sojae mycelial morphology. Mycelia were treated with LA or the same volume of water (CK) for 2 h before their morphology was analyzed. Mycelial growth after LA treatment was disorderly, the terminal branch number increased significantly, and the formation of zoosporangium was inhibited. In CK, the mycelium grew normally and expanded without any distortion, bending, or increased terminal branching. The experiment was repeated three times with similar results.
To explore how LA and its derivatives affect P. sojae zoosporangium formation and zoospore release, we determined the number of zoosporangia formed and calculated the inhibition effect of each chemical. The number of zoosporangia was reduced with 0.16 mM LA or GML when compared with the water control; no zoosporangia were observed with 0.64 mM treatments. With 1.28 mM MEL or ETL, a small number of zoosporangia were obtained (Supplementary Table 3).
Zoosporangia formed in the water could not release zoospores when the LA or GML concentration increased to 0.64 mM. Interestingly, almost no zoospores were released from zoosporangia treated with 0.32 mM GML, which was significantly lower than what we observed for LA, MEL, or ETL (Supplementary Table 3).
To explore the influence of LA and its derivatives on P. sojae zoospore germination, we evenly daubed zoospore suspensions onto media containing various concentrations of LA, GML, MEL, or ETL. Compared to the V8 medium control, LA and GML at 0.08 mM significantly inhibited zoospore germination, and few zoospores could form individual colonies (Figure 4). However, MEL and ETL had weak inhibitory effects on zoospore germination at the maximum concentration of 1.28 mM (Figure 4 and Supplementary Table 4).
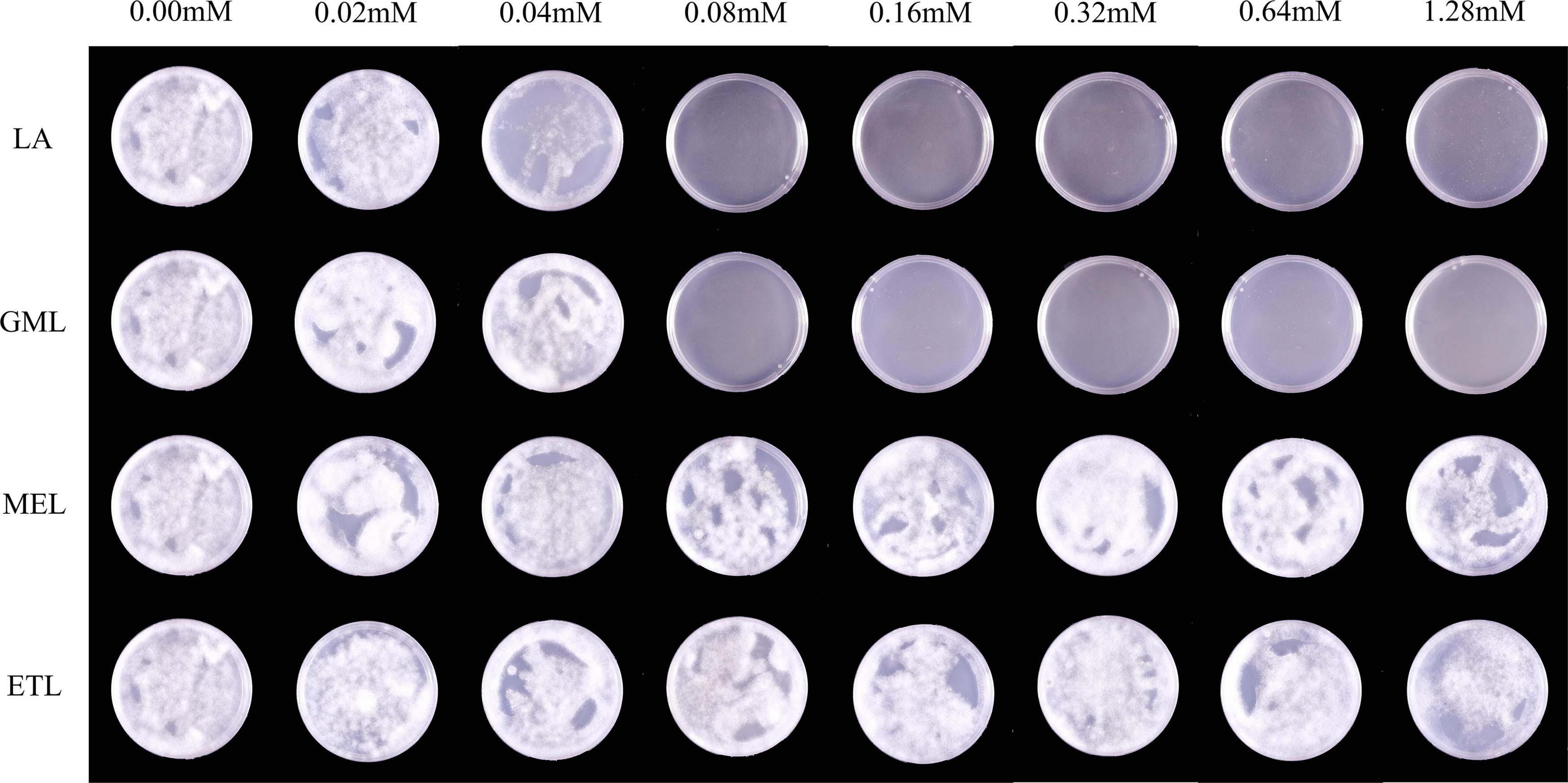
Figure 4. The effects of LA and its derivatives on P. sojae zoospore germination. To observe zoospore germination, zoospore suspensions were smeared on V8 medium containing LA, GML, MEL, or ETL at different concentrations. The experiment was repeated three times with similar results. Means with different letters are significantly different (Statistical Product and Service Solutions (SPSS), p < 0.05).
Propidium iodate is a DNA-binding and a cell-membrane–impermeable dye, usually used as a marker for membrane integrity and cell viability; PI cannot cross the membrane of live cells but can penetrate and stain the cell nucleus red if the cell membrane is damaged. We PI-stained P. sojae hyphae nuclei after exposure to LA and observed that the cell membrane was severely damaged, resulting in red fluorescence in the nucleus (Figure 5A).
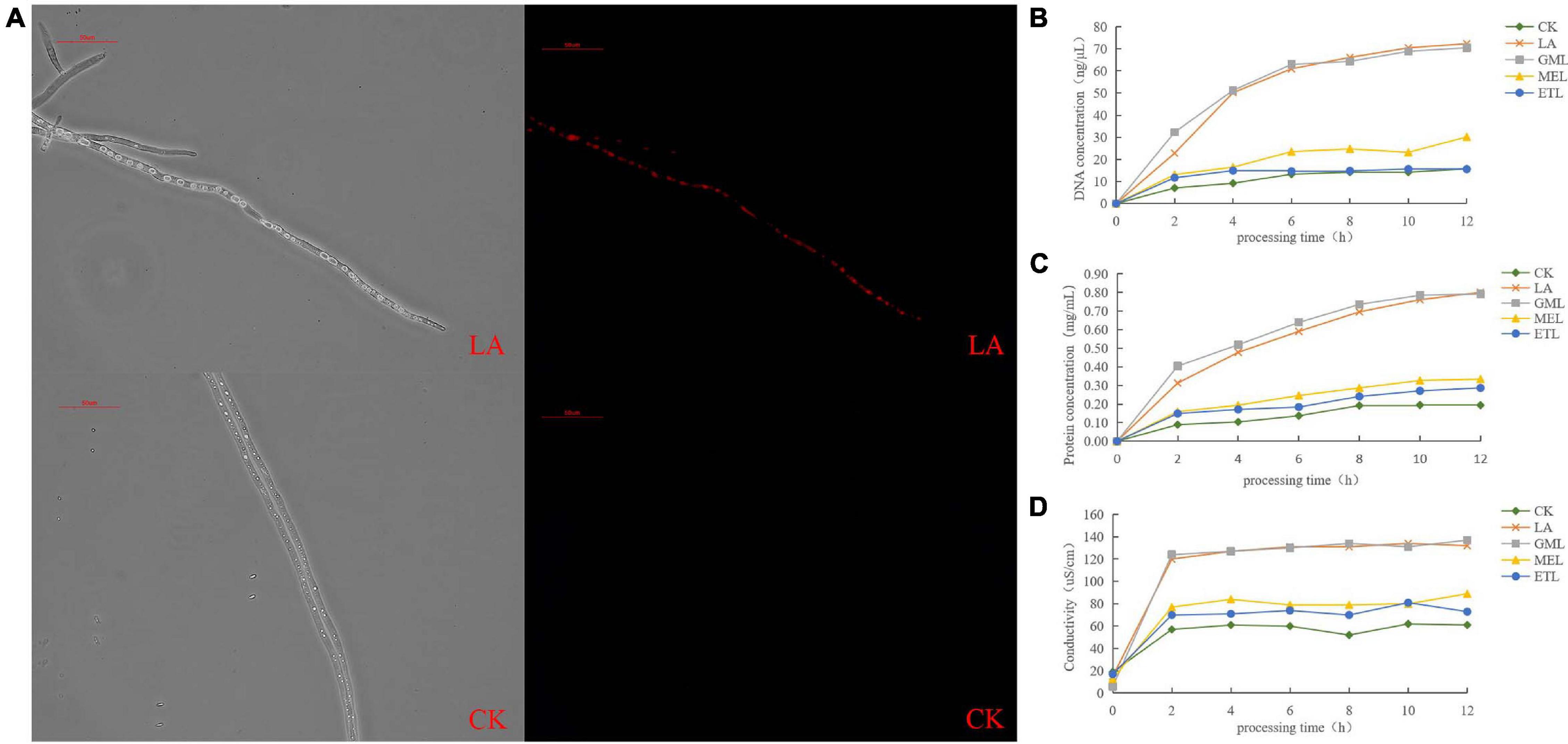
Figure 5. Effect of LA on P. sojae cell membrane integrity. (A) P. sojae hyphae nuclei stained with propidium iodide (PI) after exposure to LA (upper image) or the same amount of water (lower image) and observed under a microscope. (B) Effects of LA and its derivatives, GML, MEL, or ETL, on DNA content. (C) Effects of LA GML, MEL, and ETL on protein content. (D) Effects of LA, GML, MEL, and ETL on solution conductivity. Means with different letters are significantly different (SPSS, p < 0.05).
To further investigate the potential damage to the cell membrane, we analyzed cell substance leakage in P. sojae mycelia treated with LA and its derivatives, GML, MEL, and ETL. Total DNA and protein concentrations were monitored every 2 h in the growth media. DNA and protein contents increased significantly over time and gradually flattened out in LA and GML solutions but changed only slightly in CK, MEL, or ETL solutions (Figures 5B, C). The conductibility of the five tested solutions increased in the first 2 h, but it was significantly higher in LA and GML than in CK, MEL, or ETL (Figure 5D). The results indicated that the P. sojae DNA and proteins penetrated the solution upon treatment with LA or GML, probably because the cell membrane was damaged or destroyed.
To understand the security of LA and its derivatives on host plants, we applied different concentrations of LA, GML, MEL, or ETL in the pot-planting holes; soybean seedling height was measured at 7 and 14 days after planting. When LA and GML concentrations were lower than 0.3 g a.i./plant, the treatments promoted soybean growth, whereas the application of 0.3 g a.i./plant did not influence plant growth, and there was no significant difference in height compared with the control group. At 0.6 g a.i./plant, MEL and ETL treatments did not significantly affect plant growth compared to the control at the two time points. However, concentrations higher than 0.6 g a.i./plant were harmful to plants and inhibited their growth (Supplementary Table 5).
The mycelium and oospores of P. sojae formed on V8 medium were homogenized using a blender and mixed with potted soil before LA, and its derivatives, GML, MEL, or ETL, were added to the pots. Seeds of the P. sojae–susceptible soybean cultivar Williams (lacking Rps resistance genes) were sowed in the processed soil, and soybean height was measured after 5 days. Potted soybean plants grown in soil treated with LA or its derivatives had similar growth patterns than those in sterilized soil (CK2). However, most seedlings were killed by P. sojae in inoculated soil when plants were planted without LA, GML, MEL, or ETL treatments (CK1) (Figure 6).

Figure 6. Potted experiment of P. sojae control by LA and its derivatives. The soil was treated with LA, GML, MEL, or ETL. In CK1, no agent added to the soil. In CK2, no P. sojae mycelium was added to the soil. Plant height was measured 5 days after planting. The experiment was repeated three times with similar results. Means with different letters are significantly different (SPSS, p < 0.05).
Because of the expanding global population and the increasingly stringent environmental, toxicological, and regulatory requirements, plant pathogen control remains a constant need, and the products suitable for phytopathogen management are further limited. NPs are primary or secondary metabolites produced by living cells and have been an important source and template for the development of novel environmental-friendly agrochemicals. The metabolites synthesized in response to pathogen infection, such as leaf VOCs, have been particularly important and have functional benefits in multiple aspects of plant defense. Several VOCs are produced and emitted rapidly when plants respond to biotic stress, and such compounds require further exploration as leads for novel crop protection chemistry. VOCs tend to be chemically complex, and few are used directly for agricultural pathogen control. Nevertheless, it is technically possible to identify the effective constituents in VOCs and modify them as commercial products, transforming many NPs into agriculturally suitable molecules for pathogen control.
Here, we identified the components of soybean leaf volatiles produced in incompatible interactions and compatible interactions with P. sojae and found that LA was emitted specificity in a resistant cultivar. As previously reported, LA inhibits the mycelial growth of phytopathogenic fungi. Additionally, LA significantly reduces the R. solani and P. ultimum mycelial growth in agar culture at the concentration of 100 μm or greater, whereas no fungal growth occurred in liquid culture at concentrations greater than 50 μm (Walters et al., 2003). In this study, P. sojae mycelia growth was suppressed by LA, and the formation of zoosporangium was inhibited. This study provides the first report of the activity of LA against a Phytophthora pathogen and indicates the need for further research of its mechanism of action.
Lauric acid-treated mycelia grew disorderly, and the number of terminal branches increased significantly. Furthermore, the plasma membrane of P. sojae was disrupted or disintegrated by LA. MCFAs and monoglycerides are single-chain lipid amphiphiles that interact with phospholipid membranes as part of various biological activities. For example, they can have membrane-disruptive behavior against microbial pathogens on the human skin surface (Valle-González et al., 2018). LA has a minimum bactericidal concentration to E. coli at 1 mM (Kim and Rhee, 2013). Here, we found that LA can inhibit P. sojae growth at 0.5 mM, which means it has a greater effect on Phytophthora than on E. coli. Additionally, the concentration of cell substances, including DNA and protein, in water culture increased after LA treatment, and PI staining of the nucleus indicated that the P. sojae cell membrane was damaged.
It has been proposed that the hydrophilic and lipophilic characteristics of fatty acid derivatives affect their antibacterial activities according to their ability to incorporate into the bacterial cell membrane (Park et al., 2018; Valle-González et al., 2018). To verify this hypothesis, we selected three kinds of LA derivatives esterified with different non–fatty acid moieties and investigated whether the antibacterial activity from their precursor (i.e., LA) was retained or lost. GML had comparative bacteriostatic and bactericidal effects against P. sojae to LA, whereas MEL and ETL had weaker inhibitory activity. The antimicrobial properties of LA, GML, and their ester derivatives may be attributed to physicochemical processes and their interference with various cellular processes. GML is 200 times more effective than LA in bactericidal activity (Schlievert and Peterson, 2012), but, in this study, LA had similar effects to P. sojae, which suggests differences in the inhibitory mechanisms involved.
Glycerol monolaurate has a potent antimicrobial activity, and LA has the ability to convert into GML, which can destroy the lipid membrane of bacteria (Jin et al., 2021). The high levels of activity of capric and lauric acids, and particularly that of monocaprin, are notable and suggest that these lipids have specific antichlamydial activities (Bergsson et al., 1998). A recent study demonstrated that the potencies of saturated FAs increased sharply by lowering the pH, and a decrease of only 0.5 pH units could cause a change from non-lethal to lethal conditions. Conversely, the bactericidal action of GLM was not pH-dependent (Sun et al., 2003). That means GML is more environmentally stable and likely more suitable for crop protection.
As the main component of coconut oil and breast milk, LA is usually part of regular diets and feed additives, showing potent antimicrobial effects and lack of toxicity (Zeiger et al., 2017; Khan et al., 2020; Zhang et al., 2020). In the host security test, with concentrations lower than 0.3 g a.i./plant, both LA and GML could promote soybean growth; however, higher concentrations may negatively influence host growth.
None of the natural and eco-friendly chemical alternatives currently registered and available have the full spectrum of activity and versatility of methyl bromide as preplant soil fumigants (Duniway, 2002). Based on the results described here, LA and GML have potent antimicrobial effects and positive effects regulating plant growth. Hence, they may be promising substitutes for traditional anti-Phytophthora agents.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
QW and QX designed the experiments. CH, TG, and WG wrote the manuscript, performed the experiments, and analyzed the data. YW performed the GC–MS experiments and analyzed the data. QX, JW, and XT participated in manuscript revision or experiments. QW revised the manuscript and provided the funding for this research. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Key R&D projects in Shandong Province (2019GNC106060), Major Basic Research Program of the Shandong Natural Science Foundation 2019JZZY020608, National Natural Science Foundation of China (31972249), and National Key Research and Development Project of China (No. 2016YFD0100602).
YW was employed by company Shimadzu (China) Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the Phytohormone Analysis Platform, Agronomy College of SDAU, for providing technical support and suggestions for this work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.666761/full#supplementary-material
Bartolotta, S., Garcia, C. C., Candurra, N. A., and Damonte, E. B. (2001). Effect of fatty acids on arenavirus replication: inhibition of virus production by lauric acid. Arch. Virol. 146, 777–790. doi: 10.1007/s007050170146
Bergsson, G., Arnfinnsson, J., Karlsson, S. M., Steingrimsson, O., and Thormar, H. (1998). In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 42, 2290–2294. doi: 10.1128/aac.42.9.2290
Bergsson, G., Arnfinnsson, J., Steingrimsson, O., and Thormar, H. (2001). In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 45, 3209–3212. doi: 10.1128/aac.45.11.3209-3212.2001
Cowan, M. (1999). Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 12, 564–582. doi: 10.1128/cmr.12.4.564
Duniway, J. M. (2002). Status of Chemical Alternatives to Methyl Bromide for Pre-Plant Fumigation of Soil. Phytopathology 92, 1337–1343. doi: 10.1094/phyto.2002.92.12.1337
Erwin, D. C., and Ribeiro, O. K. (1996). Phytophthora Diseases Worldwide. USA: The American Phytopathological Society.
Fortuoso, B. F., Dos Reis, J. H., Gebert, R. R., Barreta, M., Griss, L. G., Casagrande, R. A., et al. (2019). Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: impact on health, performance and meat quality. Microb. Pathog. 129, 161–167. doi: 10.1016/j.micpath.2019.02.005
Gessler, C., Pertot, I., and Perazzolli, M. (2011). Plasmopara viticola: a review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 50, 3–44.
Jin, X., Zhou, J., Richey, G., Wang, M., Hong, S. M. C., and Hong, S. H. (2021). Undecanoic Acid, Lauric Acid, and N-Tridecanoic Acid Inhibit Escherichia coli Persistence and Biofilm Formation. J. Microbiol. Biotechnol. 31, 130–136. doi: 10.4014/jmb.2008.08027
Kabara, J. J., Swieczkowski, D. M., Conley, A. J., and Truant, J. P. (1972). Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 2, 23–28. doi: 10.1128/aac.2.1.23
Kamoun, S., Furzer, O., Jones, J. D., Judelson, H. S., Ali, G. S., Dalio, R. J., et al. (2015). The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 16, 413–434. doi: 10.1111/mpp.12190
Khan, H. U., Aamir, K., Sisinthy, S. P., Nagojappa, N. B. S., and Arya, A. (2020). Food additive “lauric acid” possess non-toxic profile on biochemical, haematological and histopathological studies in female Sprague Dawley (SD) rats. PeerJ 8:e8805. doi: 10.7717/peerj.8805
Kim, S. A., and Rhee, M. S. (2013). Marked synergistic bactericidal effects and mode of action of medium-chain fatty acids in combination with organic acids against Escherichia coli O157:H7. Appl. Environ. Microbiol. 79, 6552–6560. doi: 10.1128/aem.02164-13
Kishimoto, K., Matsui, K., Ozawa, R., and Takabayashi, J. (2008). Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry 69, 2127–2132. doi: 10.1016/j.phytochem.2008.04.023
Lalouckova, K., Skrivanova, E., Rondevaldova, J., Frankova, A., Soukup, J., and Kokoska, L. (2021). In vitro antagonistic inhibitory effects of palm seed crude oils and their main constituent, lauric acid, with oxacillin in Staphylococcus aureus. Sci. Rep. 11:177.
Liu, S., Ruan, W., Li, J., Xu, H., Wang, J., Gao, Y., et al. (2008). Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 166, 93–102. doi: 10.1007/s11046-008-9124-1
Matsui, K. (2006). Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 9, 274–280. doi: 10.1016/j.pbi.2006.03.002
Matsui, K., and Koeduka, T. (2016). Green Leaf Volatiles in Plant Signaling and Response. Subcell. Biochem. 86, 427–443. doi: 10.1007/978-3-319-25979-6_17
Mellon, J. E., Dowd, M. K., Beltz, S. B., and Moore, G. G. (2014). Growth inhibitory effects of gossypol and related compounds on fungal cotton root pathogens. Lett. Appl. Microbiol. 59, 161–168. doi: 10.1111/lam.12262
Park, K. M., Lee, S. J., Yu, H., Park, J. Y., Jung, H. S., Kim, K., et al. (2018). Hydrophilic and lipophilic characteristics of non-fatty acid moieties: significant factors affecting antibacterial activity of lauric acid esters. Food Sci. Biotechnol. 27, 401–409. doi: 10.1007/s10068-018-0353-x
Piotrowski, J. S., Okada, H., Lu, F., Li, S. C., Hinchman, L., Ranjan, A., et al. (2015). Plant-derived antifungal agent poacic acid targets beta-1,3-glucan. Proc. Natl. Acad. Sci. U. S. A. 112, E1490–E1497.
Randall, E., Young, V., Sierotzki, H., Scalliet, G., Birch, P. R., Cooke, D. E., et al. (2014). Sequence diversity in the large subunit of RNA polymerase I contributes to Mefenoxam insensitivity in Phytophthora infestans. Mol. Plant Pathol. 15, 664–676. doi: 10.1111/mpp.12124
Rouse, M. S., Rotger, M., Piper, K. E., Steckelberg, J. M., Scholz, M., Andrews, J., et al. (2005). In vitro and in vivo evaluations of the activities of lauric acid monoester formulations against Staphylococcus aureus. Antimicrob. Agents Chemother. 49, 3187–3191. doi: 10.1128/aac.49.8.3187-3191.2005
Scala, A., Allmann, S., Mirabella, R., Haring, M. A., and Schuurink, R. C. (2013). Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 14, 17781–17811. doi: 10.3390/ijms140917781
Schlievert, P. M., and Peterson, M. L. (2012). Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS One 7:e40350. doi: 10.1371/journal.pone.0040350
Schnee, S., Queiroz, E. F., Voinesco, F., Marcourt, L., Dubuis, P. H., Wolfender, J. L., et al. (2013). Vitis vinifera canes, a new source of antifungal compounds against Plasmopara viticola, Erysiphe necator, and Botrytis cinerea. J. Agric. Food Chem. 61, 5459–5467. doi: 10.1021/jf4010252
Shilling, M., Matt, L., Rubin, E., Visitacion, M. P., Haller, N. A., Grey, S. F., et al. (2013). Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J. Med. Food 16, 1079–1085. doi: 10.1089/jmf.2012.0303
Sun, C. Q., O’Connor, C. J., and Roberton, A. M. (2003). Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 36, 9–17. doi: 10.1016/s0928-8244(03)00008-7
Suthiwong, J., Sriphana, U., Thongsri, Y., Promsuwan, P., Prariyachatigul, C., and Yenjai, C. (2014). Coumarinoids from the fruits of Micromelum falcatum. Fitoterapia 94, 134–141. doi: 10.1016/j.fitote.2014.02.004
Tanaka, T., Ikeda, A., Shiojiri, K., Ozawa, R., Shiki, K., Nagai-Kunihiro, N., et al. (2018). Identification of a Hexenal Reductase That Modulates the Composition of Green Leaf Volatiles. Plant Physiol. 178, 552–564. doi: 10.1104/pp.18.00632
Tu, Y. (2011). The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 17, 1217–1220. doi: 10.1038/nm.2471
Tyler, B. M. (2001). Genetics and genomics of the oomycete-host interface. Trends Genet. 17, 611–614. doi: 10.1016/s0168-9525(01)02517-3
Tyler, B. M. (2007). Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol. Plant Pathol. 8, 1–8. doi: 10.1111/j.1364-3703.2006.00373.x
Valle-González, E. R., Jackman, J. A., Yoon, B. K., Park, S., Sut, T. N., and Cho, N. J. (2018). Characterizing how acidic pH conditions affect the membrane-disruptive activities of lauric acid and glycerol monolaurate. Langmuir 34, 13745–13753. doi: 10.1021/acs.langmuir.8b02536
Walters, D. R., Walker, R. L., and Walker, K. C. (2003). Lauric Acid Exhibits Antifungal Activity Against Plant Pathogenic Fungi. J. Phytopathol. 151, 228–230. doi: 10.1046/j.1439-0434.2003.00713.x
Wang, X., Wang, S., Yi, J., Li, Y., Liu, J., Wang, J., et al. (2020). Three Host Plant Volatiles, Hexanal, Lauric Acid, and Tetradecane, are Detected by an Antenna-Biased Expressed Odorant Receptor 27 in the Dark Black Chafer Holotrichia parallela. J. Agric. Food Chem. 68, 7316–7323. doi: 10.1021/acs.jafc.0c00333
Wang, Y., Sun, Y., Zhang, Y., Zhang, X., and Feng, J. (2016). Antifungal activity and biochemical response of cuminic acid against phytophthora capsici leonian. Molecules 21:756. doi: 10.3390/molecules21060756
Yang, D., Pornpattananangkul, D., Nakatsuji, T., Chan, M., Carson, D., Huang, C. M., et al. (2009). The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes. Biomaterials 30, 6035–6040. doi: 10.1016/j.biomaterials.2009.07.033
Yang, H.-T., Chen, J.-W., Rathod, J., Jiang, Y.-Z., Tsai, P.-J., Hung, Y.-P., et al. (2018). Lauric Acid Is an Inhibitor of Clostridium difficile Growth in Vitro and Reduces Inflammation in a Mouse Infection Model. Front. Microbiol. 8:2635. doi: 10.3389/fmicb.2017.02635
Yutin, N., Makarova, K. S., Mekhedov, S. L., Wolf, Y. I., and Koonin, E. V. (2008). The deep archaeal roots of eukaryotes. Mol. Biol. Evol. 25, 1619–1630. doi: 10.1093/molbev/msn108
Zeiger, K., Popp, J., Becker, A., Hankel, J., Visscher, C., Klein, G., et al. (2017). Lauric acid as feed additive - An approach to reducing Campylobacter spp. in broiler meat. PLoS One 12:e0175693. doi: 10.1371/journal.pone.0175693
Keywords: lauric acid, leaf volatile compounds, Phytophthora sojae, cell membrane damage, biological control
Citation: Liang C, Gao W, Ge T, Tan X, Wang J, Liu H, Wang Y, Han C, Xu Q and Wang Q (2021) Lauric Acid Is a Potent Biological Control Agent That Damages the Cell Membrane of Phytophthora sojae. Front. Microbiol. 12:666761. doi: 10.3389/fmicb.2021.666761
Received: 11 February 2021; Accepted: 24 June 2021;
Published: 05 August 2021.
Edited by:
Yong Wang, Guizhou University, ChinaReviewed by:
Maofeng Jing, Nanjing Agricultural University, ChinaCopyright © 2021 Liang, Gao, Ge, Tan, Wang, Liu, Wang, Han, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Xu, eHVxaWFuQHNkYXUuZWR1LmNu; Qunqing Wang, d2FuZ3F1bnFpbmdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.