
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 27 August 2021
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.663020
This article is part of the Research TopicCarbapenemase-Producing Organisms as Leading Cause of Hospital InfectionsView all 25 articles
 Ana M. Rada1,2*
Ana M. Rada1,2* Elsa De La Cadena3
Elsa De La Cadena3 Carlos A. Agudelo4,5
Carlos A. Agudelo4,5 Christian Pallares3
Christian Pallares3 Eliana Restrepo1
Eliana Restrepo1 Adriana Correa6
Adriana Correa6 María V. Villegas3
María V. Villegas3 Cesar Capataz7
Cesar Capataz7Pseudomonas aeruginosa is an opportunistic Gram-negative pathogen with an increase in the frequency of infections caused by multidrug resistant (MDR) and extensively drug resistant (XDR) strains, limiting the available therapeutic options. The most troublesome resistance is the acquisition and production of carbapenemases such as Verona integron-encoded metallo-β-lactamases (VIM), the most frequent and widespread, and the Klebsiella pneumoniae carbapenemases (KPC), which has continuously spread in the last decade. Its dissemination is linked to their location on mobile genetic elements (MGEs). In Colombia, VIM and KPC have been increasing in its frequency showing major successful dissemination. In this article, we molecularly characterized and analyzed the genetic context of blaVIM and blaKPC in carbapenem-resistant P. aeruginosa (CRPA) isolates from infected and colonized patients in two tertiary-care hospitals, one in Medellín and the other in a municipality close to Medellín, both areas with high carbapenemase endemicity in Colombia (2013–2015). Using whole-genome sequencing (WGS), we identified a remarkable variety of genetic backgrounds in these MDR P. aeruginosa isolates carrying blaKPC–2 and blaVIM–2. There were a diversity of class 1 integron and variations in the gene cassettes associated to blaVIM–2, as well as a possible event of spread of blaKPC–2 mediated by a plasmid that contained part of Tn4401b in one infection case. The dissemination of blaVIM–2 and blaKPC–2 in P. aeruginosa in this area in Colombia has been strongly influenced by successful international clones, carrying these genes and additional determinants of resistance on MGEs, accompanied by gene rearrangement under an antimicrobial selection pressure. These findings emphasize the need to implement control strategies based on rational antibiotic use.
Pseudomonas aeruginosa is an opportunistic Gram-negative pathogen especially in immunocompromised patients capable of causing a wide array of life-threatening infections. In hospitals, P. aeruginosa plays a crucial role in healthcare-associated infections (Pachori et al., 2019). The increasingly frequent infections caused by multidrug resistant (MDR) and extensively drug resistant (XDR) strains with limited therapeutic options are associated with high morbidity and mortality (Magiorakos et al., 2012; Horcajada et al., 2019). The intrinsic resistance is conferred by low outer membrane permeability, expression of efflux pumps, and the production of antibiotic inactivating enzymes. The acquired resistance can occur because of mutational changes or acquisition of resistance genes via horizontal transfer by mobile genetic elements (MGEs), such as integrons, transposons, or plasmids (Horcajada et al., 2019).
In particular, the most troublesome acquired resistance of P. aeruginosa is the production of carbapenemases, which confer resistance to most commercially available β-lactam. The class B carbapenemases, such as Verona integron-encoded metallo-β-lactamases (VIM) and Imipenem metallo-β-lactamases (IMP), are the most frequent (Patel and Bonomo, 2011). The genes encoding IMP and VIM are located on integrons, which also carry other antibiotics resistance genes favoring their worldwide dissemination (Hong et al., 2015; van der Zee et al., 2018). P. aeruginosa carrying class A carbapenemases such as Klebsiella pneumoniae carbapenemases (KPC) have become increasingly important due to their continuous dissemination worldwide including Asia and America during the last decade (Santella et al., 2012; Carrara-Marroni et al., 2015; Hu et al., 2015; Potron et al., 2015). Its dissemination is facilitated by genes encoded on transposable elements and plasmids (Naas et al., 2013; Dai et al., 2016; Hu et al., 2019). In Colombia, VIM-producing P. aeruginosa was first reported in 2006, followed by P. aeruginosa harboring KPC in 2007 (Villegas et al., 2006, 2007). The presence of these carbapenemases in high-risk clones identified by multilocus sequence typing (MLST) as sequence type (ST) 111 (ST111) harboring blaVIM–2 on a class 1 integron such as In59 (Correa et al., 2015; Vanegas et al., 2014), as well as ST308 and ST235 harboring blaKPC–2 on complete or truncated Tn4401b in plasmids or the chromosome, may be the cause of its successful dissemination in Colombia (Cuzon et al., 2011; Abril et al., 2019).
The study of genetic platforms of blaVIM and blaKPC in carbapenem-resistant P. aeruginosa (CRPA) that co-harbor genes conferring resistance to other antibiotics, as well as their wide diversity, is key to understand the role in the dissemination of such resistance determinants among clinical and environmental isolates (Cuzon et al., 2011; Naas et al., 2013; Correa et al., 2015; Abril et al., 2019). In this article, we molecularly characterized and analyzed the genetic context of blaVIM and blaKPC in CRPA isolates from infected and colonized patients in two tertiary-care hospitals, one in Medellín and the other in a municipality close to Medellín, both areas with high carbapenemase endemicity in Colombia (2013–2015).
A collection of CRPA isolates (n = 46) from a surveillance study of carbapenem-resistant Gram-negative bacteria was selected from infected and colonized patients in two tertiary-care hospitals in Colombia between 2013 and 2015. Thirty-eight isolates were recovered from hospital 2 located in a municipality close to Medellin, while the remaining isolates were collected from hospital 1 located in the city of Medellin (143 and 202 beds, respectively; see Supplementary Data Sheet 1). The medical records of infected and colonized patients were reviewed retrospectively. Colonization was defined as a CRPA recovered from a surveillance rectal culture or clinical sample without associated signs or symptoms of disease. Rectal swabs were cultured on a selective chromogenic medium (chromID CARBA; bioMérieux). Infection was defined by an associated clinical syndrome of infection. Colonization and infection were confirmed by the infectious disease services and/or infection control unit. Clinical information such as age, sex, previous hospitalization, days of hospital stay before sampling, use of invasive medical devices, underlying diseases, comorbidities, and antibiotic use was collected from electronic medical records and recorded in a Microsoft Access Database. This study was approved by the Institutional Review Board (IRB) and Ethical Committee in each participating hospital.
Species identification and antimicrobial susceptibility testing was performed using the automated Vitek-2TM system (bioMérieux Marcy-l′Étoile, France). The antimicrobial agents tested included imipenem, meropenem, doripenem, ceftazidime, cefepime, piperacillin/tazobactam, gentamicin, amikacin, and ciprofloxacin. The minimum inhibitory concentration (MIC) results were interpreted following the Clinical and Laboratory Standards Institute (CLSI) breakpoints 2017 (CLSI., 2017). All isolates classified as carbapenem-resistant were tested by PCR assay for the presence of carbapenemases encoding genes including blaVIM, blaKPC, blaNDM, and blaIMP. The primers used for amplification, as well as PCR cycling conditions, have been described elsewhere (Yigit et al., 2001; Dallenne et al., 2010). DNA sequencing was performed on the amplification products of positive PCR, and the results were compared and aligned with reference sequence using the online BLAST database to identify specific alleles.
The characterization by rep-PCR/DiversiLabTM (bioMérieux Marcy-l′Étoile, France) was conducted in 21 isolates of P. aeruginosa carrying the blaKPC or blaVIM gene that complied with DNA in good quantity and quality pos-extraction to determine the genomic relatedness, using >95% similarity to be considered to be of the same rep-PCR type. A total of 16 isolates were selected for whole-genome sequencing (WGS) based on this initial characterization. Total DNA was extracted with the GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific, Waltham, MA, United States). DNA libraries were prepared using a NexteraXT® DNA sample preparation kit and multiplexed with a NexteraXT index primer kit on the Illumina platform (Illumina, San Diego, CA, United States). Genomic libraries were sequenced on a MiSeq sequencer to obtain 250-bp paired-end reads using Kit v2 and v3 (Illumina). The readings were processed to eliminate low-quality bases and contamination with sequences of adapters and later assembled de novo. Cleaning and assembly were carried out using a CLC Genomics Workbench assembler, version 8.5. The genomes were annotated using the RAST server.1 The assemblies were typed on the web server of the Center for Genomic Epidemiology using the MLST 2.0 (Multilocus Sequence Typing)2 (Larsen et al., 2012).
The determination of resistance elements was identified using Resfinder 2.13 (Zankari et al., 2012), using an identity percentage higher than 95% and a coverage cutoff greater than 90%. The O-specific antigen analysis was performed in silico in the Pseudomonas aeruginosa serotyper (PAst) program4 (Thrane et al., 2016). The Tn4401 isoforms were determined by BLASTn comparing the region surrounding each blaKPC gene to the sequences of the Tn4401 isoforms as described previously (Naas et al., 2012). Overlapping sequences that comprised the region surrounding blaVIM, the different integrons, and gene cassettes were manually confirmed using BLASTn and BLASTp. The identification of the integrons was investigated using INTEGRALL, the reference database of integron sequences5 (Moura et al., 2009). Likewise, this database was used for registry and the integron number assignment. Easyfig6 was used to compare and visualize the backbone of different MGEs. The BLAST Ring Image Generator (BRIG) software (Alikhan et al., 2011) was applied to align the assembled reads of some sequenced clinical isolates to one reference plasmid carrying blaKPC. The mutations in selected resistance genes (gyrA, gryB, parE, parC, rpoB, pmrA, pmrB, parS, parR, mexX, mexY, mexZ, mexC, oprJ, nfxB, mexT, mexE, mexF, oprN, mexA, mexB, oprM, mexR, nalC, nalD, oprD, ampC, ampD, ampDh2, ampDh3, and ampR) in all genomes annotated were determined with reference to P. aeruginosa PAO1 (accession number NC_002516.2) using a custom pipeline.
The phylogenetic reconstruction of the isolates was carried out by detecting single nucleotide polymorphisms (SNPs) against the reference genome of P. aeruginosa PAO1 (accession number NC_002516.2). Also, we included the reference genome of Pseudomonas putida K72440 (accession number NC-002947.4) as an external group. A SNP matrix (SNP matrix) was constructed and used to reconstruct the phylogeny of the strains with RAxML (Stamatakis, 2014). We used the general time reversal (GTR) model with a GAMMA distribution and Lewis correction for the parameters to determine the best phylogenetic reconstruction by maximum likelihood. We performed 20 runs and chose the one with the best score. In addition, 100 bootstraps were made to support the reconstructions. The trees obtained were visualized by iTOL (Letunic and Bork, 2016).
Comparison of clinical and epidemiological data was performed between colonized and infected patients, as well as between carbapenemase-producing P. aeruginosa (CPPA) and non-CPPA isolates. Fisher’s exact (two tails) or chi-square test was used for qualitative variables and Wilcoxon rank sum test for continuous variables.
The sequence data for the isolates were submitted to the NCBI GenBank database under the BioProject number PRJNA391501.
The 46 isolates of CRPA from the two hospitals were recovered from 41 adult patients; 24 (58.5%) were infected and 17 (41.4%) were colonized, and 7 (17.1%) of them were localized in intensive care unit (ICU) at the time of sampling in the participating hospitals. Most patients were males (68.3%, n = 28) and older adults (median age of 63 years; interquartile range [IQR] = 49–74). The majority of the CRPA isolates from the infected patients were from soft tissue (29.2%, n = 7; see Supplementary Table 1). In general, the most common underlying conditions were hypertension (48.8%, n = 20) and diabetes mellitus (24.4%, n = 10). A total of 19 patients (46.3%) had previous antibiotic exposure, with carbapenems, piperacillin-tazobactam, and glycopeptides being the most frequent (12.2% for each, n = 5; see Supplementary Table 1). Among the isolates recovered, 50% (n = 23) were positive for two of the four carbapenemases-encoding genes evaluated by PCR (2 from hospital 1 and 21 from hospital 2). The genes blaVIM–2, blaKPC–2, and blaVIM–2 plus blaKPC–2 were detected in 47.8% (n = 11), 47.8% (n = 11), and 4.3% (n = 1) isolates, respectively. These isolates were obtained from 19 patients; 10 were infected (n = 3, blaVIM–2; n = 7, blaKPC–2) and 9 were colonized (n = 6, blaVIM–2; n = 2, blaKPC–2; n = 1, blaVIM–2 plus blaKPC–2). Two infected patients were previously colonized by VIM-producing P. aeruginosa (each patient had two isolates). Also, up to three KPC-producing P. aeruginosa isolates were collected from different sources in different days from the same infected patient (see Supplementary Data Sheet 1).
Of the total of CRPA isolates, 91.3, 87.0, and 84.8% were resistant to doripenem, meropenem, and imipenem, respectively. More than half of the isolates were resistant to piperacillin/tazobactam (73.9%), ciprofloxacin (60.8%), cefepime (58.7%), ceftazidime (56.5%), and gentamicin (56.5%). When comparing between CPPA and non-CPPA, the resistance to gentamicin was higher in CPPA than in non-CPPA. Furthermore, more than half of the isolates of both CPPA (86.9%) and non-CPPA (65.2%) were MDR, defined as non-susceptible to at least one antibiotic in three antimicrobial categories (Magiorakos et al., 2012), with amikacin/gentamicin, cefepime, ceftazidime, imipenem, meropenem, doripenem, piperacillin/tazobactam, and ciprofloxacin in CPPA (52.2%) being the most frequent resistance (see Supplementary Table 2).
The initial characterization of CPPA isolates by rep-PCR/diversilab revealed five different rep-PCR-type: two of them harboring blaKPC–2 from hospital 2 (two isolates for each) and three included isolates harboring blaVIM–2 from hospitals 1 and 2 (two isolates for each); the other isolates were unrelated (see Supplementary Figure 1). Based on this characterization, 16 MDR P. aeruginosa isolates were selected for WGS (n = 7, blaKPC–2; n = 9, blaVIM–2). The isolates carrying the blaKPC–2 gene (n = 7) recovered from infected patients exhibited a variety of genetic backgrounds, with five different ST, including the ST309 belonging to the clonal complex (CC) 309 associated with O-antigen serotype O11 (n = 1) and the ST308 belonging to CC308 with O11 serotype (n = 2); other isolates with a singleton sequence type ST313 (n = 1) and belonging to ST699 (n = 2) were associated with other serotypes. Additionally, one new ST was designated as ST3512 (n = 1; see Figure 1). The isolates carrying blaVIM–2 (n = 9) recovered from colonized and infected patients showed four different ST profiles, with ST111 belonging to international CC111 associated with O12 serotype being the most frequent (n = 6), while other isolates belonged to ST1249 (n = 1) and ST1027 (n = 1), and the ST357 (n = 1) belonged to CC357 (Figure 1). Of note, four isolates ST111 genetically related were recovered from rectal swab samples and site of infection (soft tissue) from two patients, suggesting the colonization and infection by the same clone of P. aeruginosa in each patient. In general, the isolates carried other genes that can confer resistance to several antibiotics, including aminoglycosides, sulfonamides, tetracyclines, quinolones, phenicol, fosfomycin, and β-lactam (see Figure 1).
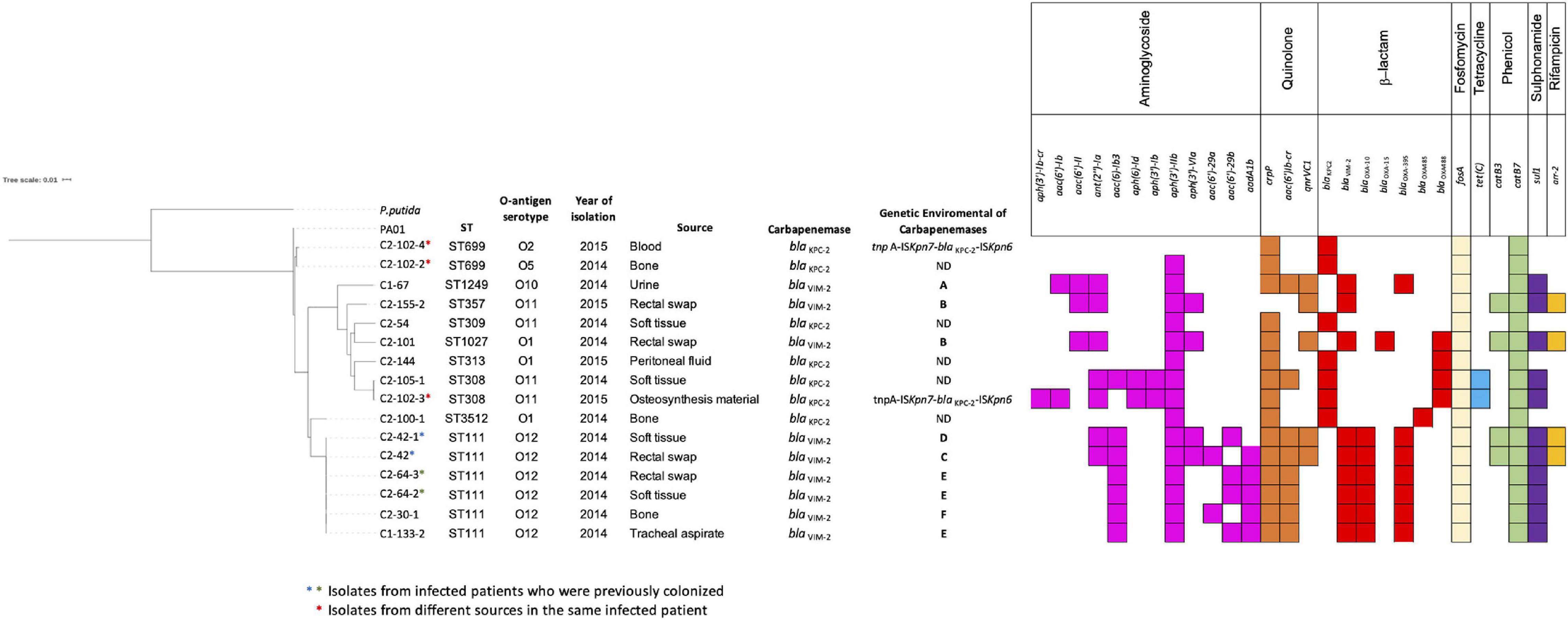
Figure 1. Phylogenetic tree showing the genetic relationships among isolates of Pseudomonas aeruginosa (n = 16). Isolates were characterized by ST, O-antigen serotype, and year of isolation. We indicate carbapenemases genes blaKPC–2 or blaVIM–2 and the genetic environment identified in each isolate. Resistance determinants to aminoglycoside, quinolone, β-lactam, fosfomycin, tetracycline, phenicol, sulfonamide, and rifampicin are grouped and indicated by color (color box indicates presence; blank indicates absence).
In addition, sequencing confirmed the presence of mutations in antimicrobial resistance-associated genes in some of the clinical isolates using P. aeruginosa PAO1 as a reference (Table 1). In general, some mutations in quinolone resistance determining regions (QRDRs) of GyrA, ParC, and ParE detected have been previously linked to fluoroquinolone resistance as well as the overexpression of efflux pump systems (Dunham et al., 2010; Subedi et al., 2018; Do Nascimento et al., 2020). In some isolates, the mexZ, nfxB, mexT, and mexR genes, which regulate the MexXY-OprM, MexCD-OprJ, MexEF-OprN, and MexAB-OprM multidrug efflux systems, revealed the presence of several point mutations predicted to result in several amino acid substitutions associated with resistance previously reported (Monti et al., 2013; López-Causapé et al., 2018b; Neves et al., 2019; Olsson et al., 2020). Regarding AmpC polymorphisms, the T105A substitution was detected in most of the isolates, which has been correlated with more efficient carbapenem and cefepime hydrolysis (Cabot et al., 2012). Furthermore, some mutations detected in PmrA and PmrB were observed previously in colistin-sensitive strains (Snesrud et al., 2018); also, some of the alterations in OprD had been described before with no contribution to carbapenem resistance (Horna et al., 2018; Díaz-Ríos et al., 2021).
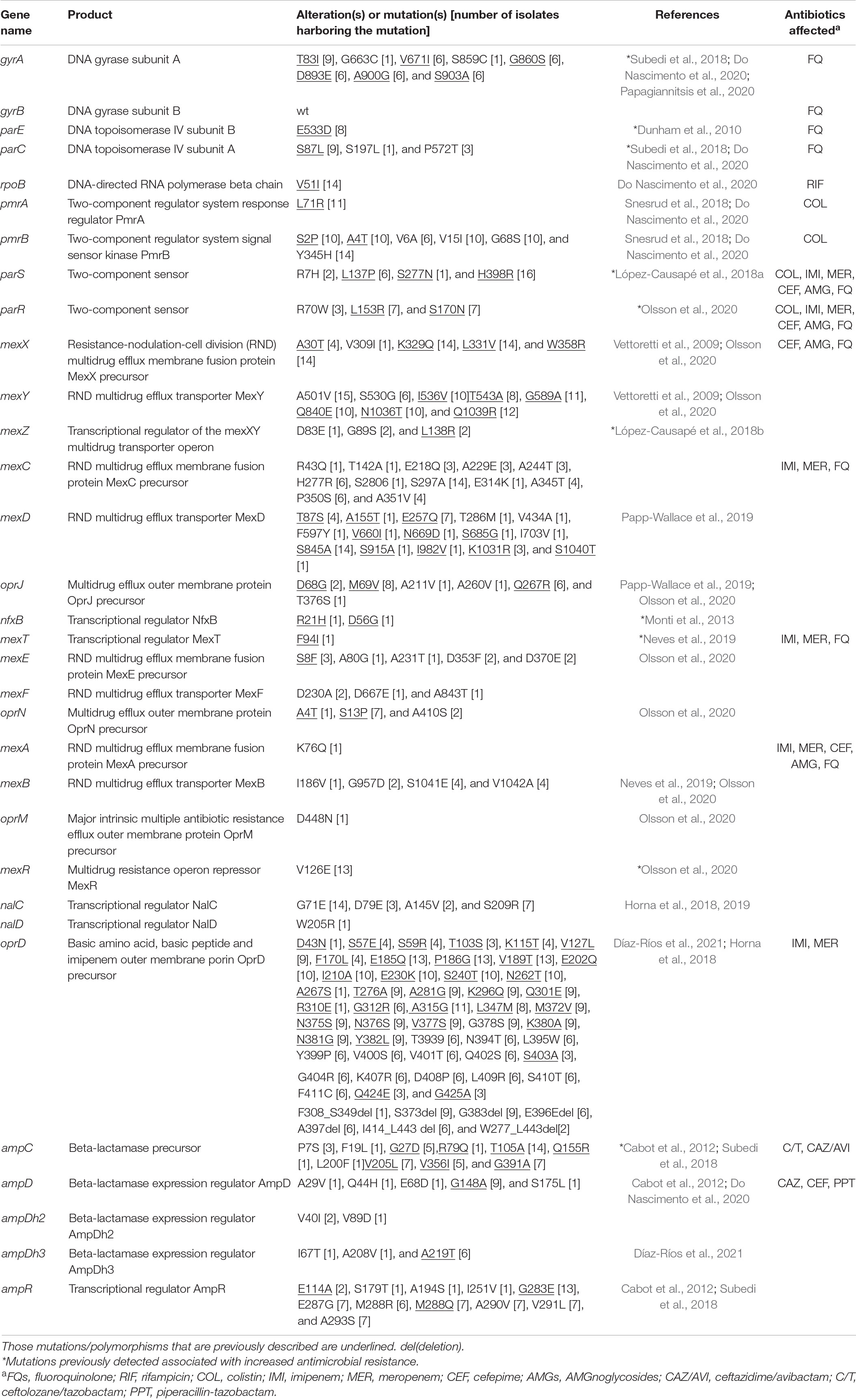
Table 1. Mutations identified in antimicrobial resistance-associated genes of 16 sequenced isolates of Pseudomonas aeruginosa using PAO1 as reference.
The blaVIM–2 gene was associated with six different types of gene cassette arrays encoding resistance to aminoglycosides or chloramphenicol, designated from A to F (see Figure 2). The type E was found in three isolates ST111, the type B in two isolates ST357 and ST1027, and the types A, C, D, and F were found in one isolate each, with different STs (type A—ST1249; types C, D, and F ST111; see Figure 1). We found two of these blaVIM–2 gene cassette arrays within class 1 integrons; the In103 (type A) was first reported in one isolate of P. aeruginosa from Portugal in 2018 (accession number AY954726; Botelho et al., 2018) and a new integron designated as In1545 (type B) including the blaVIM–2-aacA7-catB3-aadB-blaOXA–2Δ:ISAbA125:aphA6 gene cassette. In addition, two isolates recovered from the same patient showed a similar gene cassette to In1545, but differ by the lack of the aacA7 and presence of the upstream region of blaVIM–2 of aac(6′)29a (type C) in one isolate and aac(6′)29b (type D) in the other isolate. Furthermore, we found two cassette arrays (types E and F) similar to a region of In59 previously reported in Colombia (Correa et al., 2015; see Figure 2).
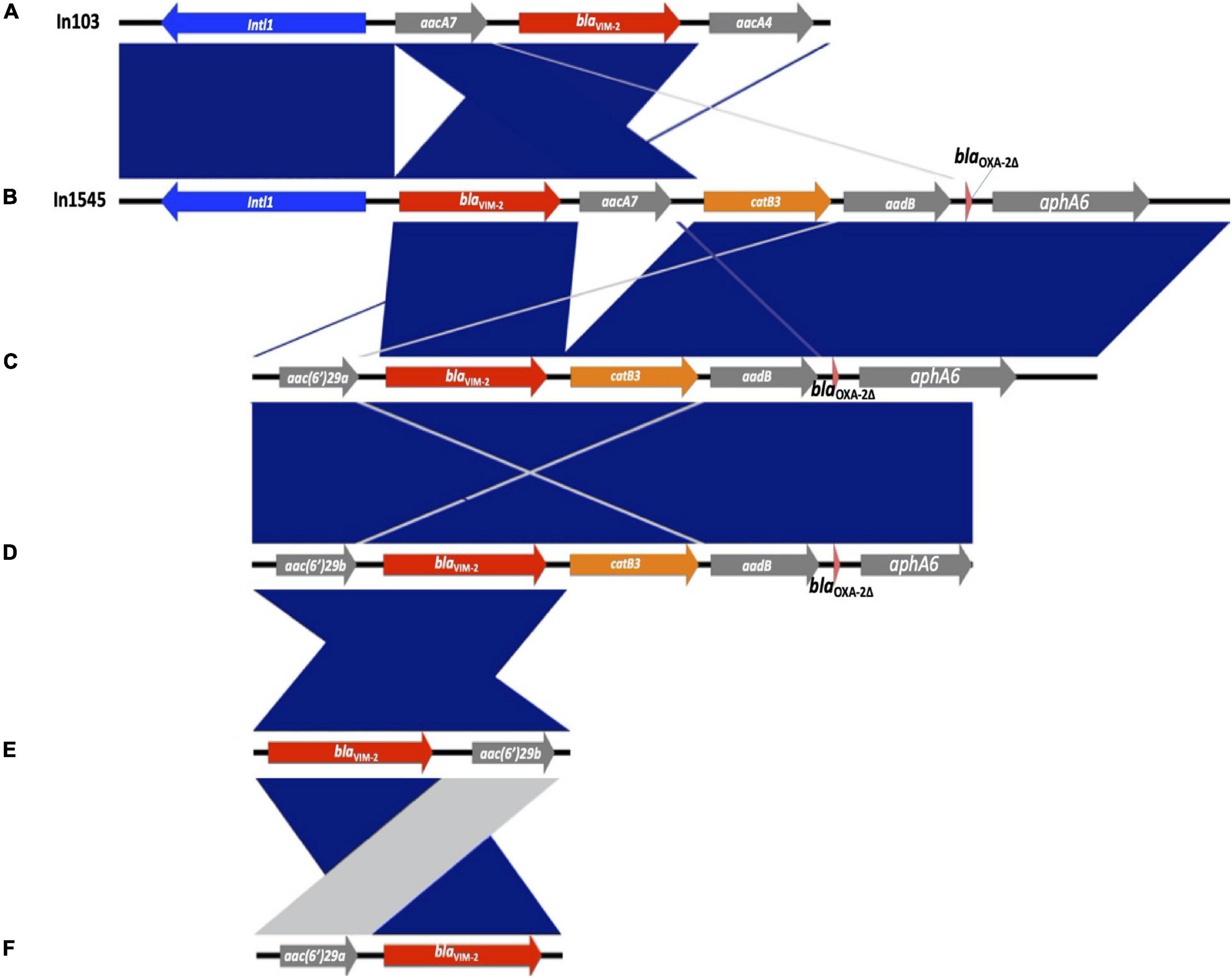
Figure 2. Genetic comparison of the blaVIM–2-containing class 1 integrons detected in this study and gene cassette arrays. The different types of gene cassette arrays identified are designated from (A) to (F). Genes are denoted by horizontal arrows with their corresponding transcription orientations. blaVIM–2 is shown with red arrows. Figure was created using EasyFig.
Interestingly, in six isolates, the class 1 integrons were associated with the other gene cassette; the In1237 was detected in one isolate from hospital 2 (C2-42), containing the gene cassette qnrvc-gcu165-arr2-dfrA22e, which confers resistance to fluoroquinolones, rifampicin, and trimethoprim. This integron was previously identified in P. aeruginosa in France in 2016 (accession number KU984332; Janvier et al., 2017). Also, a new integron designated as In2011 was detected in five isolates (C1-133-2, C2-64-3, C2-64-2, C2-30-1, and C2-42-1) from hospitals 1 and 2, containing the gene cassette gcu183-aacA4′-aadA1Δ32 -blaOXA–10, which confers resistance to aminoglycosides and β-lactam. This integron carries a gene cassette gcu183 without a recognized function yet (Liapis et al., 2019). Additionally, the gene cassette structures of In1237 and In2011 were detected in three (C2-42-1, C2-155-2, and C2-101) and one (C2-42) isolates from hospital 2, respectively.
The sequence analysis of class 1 integrons with both gene cassette arrays and promotor showed that In103 contained strong Pc variant PcS, which contributes significantly to the expression of gene cassettes and the IntI1 variant IntI1 R–32_H39, while In1545 and In2011 contain the weak Pc variant PcH1 and the IntI1 variant IntI1R–32_H39, which has been associated with an efficient excision activity (Jové et al., 2010). However, it has been suggested that this ability might compensate for the weak expression of antibiotic resistance and could enhance the capacity of the integron to adapt to antibiotic pressure and thus represent a survival advantage (Vinué et al., 2011).
The blaKPC–2 gene was not detected in the genome in five of the seven isolates of P. aeruginosa with positive PCR suggesting the loss of the plasmid or the blaKPC–2 gene during storage or subculture processing prior to sequencing. However, in two of the three isolates recovered from the same patient, the blaKPC–2 gene was detected in the genome. The strains were isolated from a patient in hospital 2 with chronic pelvic osteomyelitis with the first strain an MDR P. aeruginosa (ST699/C2-102-2 strain). Subsequently, osteomyelitis treatment failed due to the retention of the osteosynthesis material, with a second isolate of MDR P. aeruginosa (ST308/C2-102-3 strain) from the osteosynthesis material. A third relapse of the infectious occurred, and an MDR P. aeruginosa (ST699/C2-102-4 strain) was isolated in blood cultures. The timeline of the antimicrobial treatment and bacterial isolates of this case are shown in Figure 3.
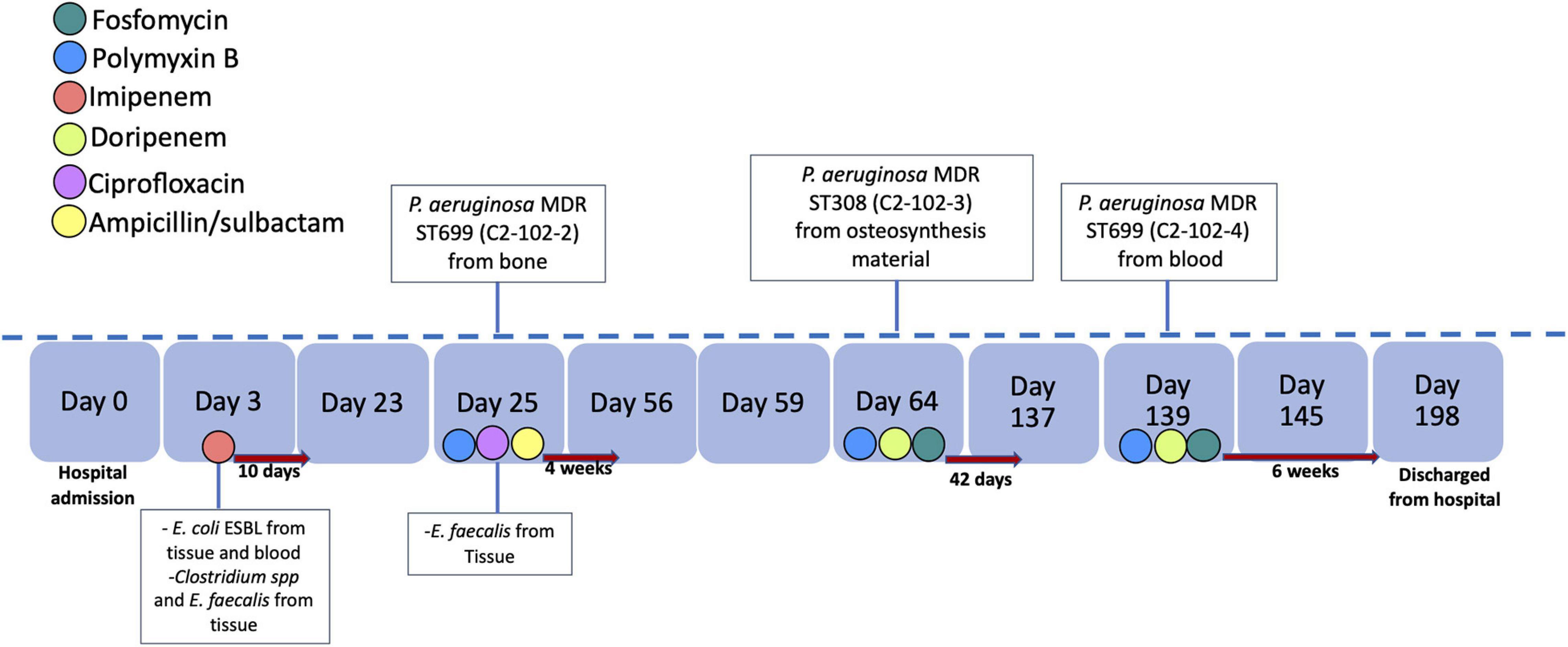
Figure 3. Timeline of antimicrobial treatment and bacterial identification. The antibiotics are indicated by color and the time of treatment with a red arrow to the right. We indicate the bacteria and source of isolation in the day of identification.
The isolates recovered from the osteosynthesis material (C2-102-3) and blood (C2-102-4) belonging to ST308 and ST699 carried crpP, fosA, and catb7 conferring, respectively, quinolone, fosfomycin, and chloramphenicol resistance. Additionally, the C2-102-3 isolate carried tetC, sul1, aph(3″)-Ib, aac(6′)Ib-cr, ant(2″)Ia, aph(6)-Id, and aac(6)-Ib3 conferring tetracyclin, sulfonamide, and aminoglycoside resistance, respectively (see Figure 1). Also, a new integron was detected and designated as In2012 including aadB (ant(2″)-Ia) and aacA4′ (aac(6)-Ib3) gene cassettes, and containing the variants PcH1 and IntI1R–32_H39. Despite the different genetic backgrounds of C2-102-3 and C2-102-4 isolates described above, both isolates carried blaKPC–2 on a transposon similar to Tn4401b identified previously in the pCOL1 plasmid from P. aeruginosa COL-1 in Colombia (accession number KC609323.1; Naas et al., 2013), without the resolvase gene (tnpR). Therefore, the GCGCT target site duplication (TSD) only was detected downstream to the ISKpn6 gene (see Figure 4A). Additionally, this ΔTn4401b was flanked at both ends by terminal inverted repeats (TIRs) of 90 bp followed downstream by tpnR and upstream by vapC-tnpA-merP-merT-merR genes.
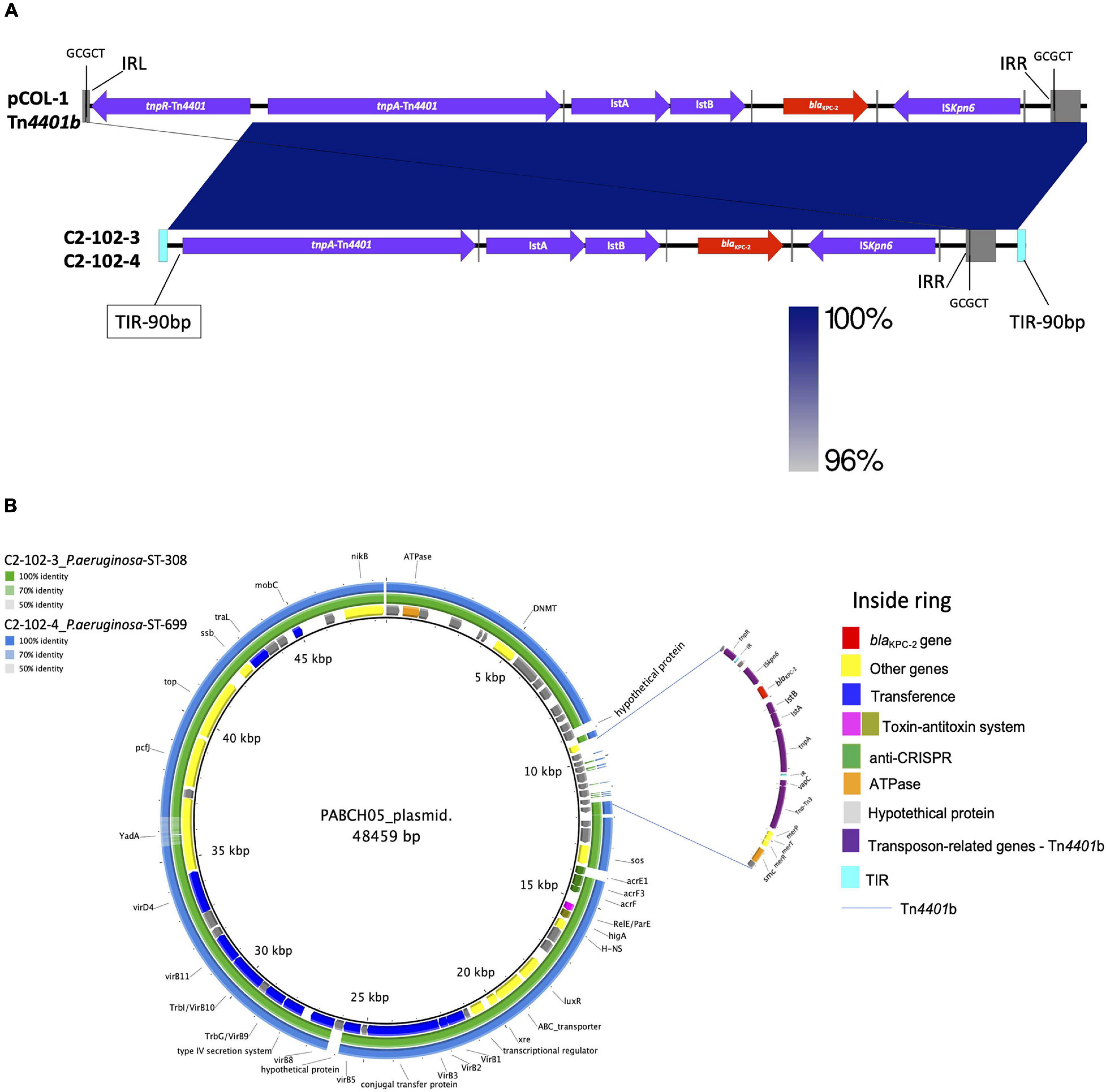
Figure 4. (A) Comparison of Tn4401b containing blaKPC–2 gene identified in two isolates of P. aeruginosa from the same patient (C2-102-3 and C2-102-4) with isoform Tn4401b from P. aeruginosa COL-1 from Colombia (accession number KC609323.1) (18). Gray squares represent left and right inverted repeats (IRL and IRR, respectively) delimiting Tn4401b, flanked by GCGCT target site duplications (TSDs). Genes are denoted by horizontal arrows with their corresponding transcription orientations. Insertion sequences: Iskpn6 and Iskpn7 with IRL and IRR sequences (gray line); transposase tnpA and resolvase, tnpR. (B) BLAST Ring Image Generator (BRIG 0.95 and BLASTN v2.2.29) of comparison of the annotated plasmid from P. aeruginosa PABCH05 strain, Boston, MA, United States (accession number CP056099.1), with C2-102-3 and C2-102-4 isolates sequenced in this study. The internal ring shows the resistance and structural genes of the plasmid of the PABCH05 strain indicated by different colors (right panel). Green ring and blue ring correspond to the BLASTn result of C2-102-3 and C2-102-4 contigs relative to the plasmid reference (inside ring), respectively. The Tn4401b without the resolvase (tnpR) gene and surrounding is indicated by the blue lines. The transposon is flanked at both ends by terminal inverted repeats (TIRs) of 90 bp followed downstream by tnpR and upstream by vapC-tnpA-merP-merT-merR genes.
Interestingly, a BLAST analysis revealed that 2 and 3 contigs obtained by de novo assembly of the C2-102-3 and C2-102-4 isolates, respectively, showed similarities to the backbone of a plasmid from the P. aeruginosa PABCH05 strain recovered in Boston, MA, United States (accession number CP056099.1), albeit only the region in the contigs that contained ΔTn4401b and the surrounding not matched with the plasmid (see Figure 4B). These findings suggest a novel plasmid likely generated through transposition and homologous recombination events. Our finding supports the notion that the blaKPC–2 gene could have been horizontally transferred by this plasmid between different strains of P. aeruginosa in the same patient during the time of infection.
This study provides new data supporting the genetic diversity and differences in the genetic context of blaVIM–2 and blaKPC–2 of MDR P. aeruginosa isolates recovered from colonized and infected patients from two tertiary care hospitals, one in Medellín and the second located in a municipality close to Medellín, both areas with high carbapenemase endemicity in Colombia. We identified a remarkable variety of genetic backgrounds of P. aeruginosa isolates carrying blaKPC2 or blaVIM2, diversity of the genetic environment of blaVIM, as well as a possible event of spread of blaKPC–2 mediated by a plasmid associated with a structure that contained part of Tn4401b in isolates from an infected case. This molecular heterogeneity suggests the potential of these resistant determinants to disseminate mediated by different MGE in P. aeruginosa in Colombia.
According to previous studies in Colombia, blaVIM and blaKPC have been the most frequent carbapenemase genes detected in P. aeruginosa and are widely disseminated in the country (Pacheco et al., 2014; Saavedra et al., 2014; Vanegas et al., 2014; Correa et al., 2015). In this study, we detected equal frequency of isolates of CRPA carrying blaVIM–2 and blaKPC–2 (n = 11, 47.8% for each), in contrast to other countries where blaVIM–2 is widely spread (Hong et al., 2015). We detected that most isolates carrying blaVIM–2 were recovered from colonized patients, while blaKPC–2 was mostly from infected patients. Furthermore, we found one isolate co-harboring blaKPC–2 and blaVIM–2 from a patient with urinary tract colonization, which was previously described in infected patients (Correa et al., 2012, 2015; Vanegas et al., 2014; Pacheco et al., 2019). Studies of molecular characterization in other countries and in Colombia previously focused mainly on infections caused by CPPA (Correa et al., 2015; Vanegas et al., 2014), in contrast to our study where we found colonization with CRPA harboring VIM and KPC, which is a major infection control concern (Abdalhamid et al., 2016). Interestingly, all the patients who were colonized by CRPA had a record of previous hospitalization, and most of them were referred from other hospital localized in different municipalities near Medellin and more than half had previous antibiotic exposure (see Supplementary Table 1). Antimicrobial pressure is a risk factor associated with the colonization of XDR P. aeruginosa in previous studies (Buhl et al., 2015).
Different ST profiles (n = 5) were identified among the isolates harboring blaKPC–2 analyzed by both rep-PCR/diversilab and WGS (n = 7). ST309 is a potential high-risk clone reported in the isolates of P. aeruginosa from Mexico, carrying blaKPC–2 and important virulence factors involved in colonization and dissemination, also described in two extensively drug-resistant isolates from US carrying blaGES–19 and blaGES–26 (Morales-Espinosa et al., 2017; Khan et al., 2019). ST308, a clone associated with higher virulence, was reported before in Colombia and other countries of South America, Europe, Asia, and Oceania (Cuzon et al., 2011; Del Barrio-Tofiño et al., 2020). The isolates belonging to ST309 and ST308 were associated with the serotype O11, documented in several high-risk clones also (Del Barrio-Tofiño et al., 2020). Other isolates belonging to ST313 and ST699 were associated with other serotypes, all previously described from different continents, widely disseminated (Libisch et al., 2008; Ji et al., 2013; Cholley et al., 2014). Likewise, among the isolates harboring blaVIM–2 (n = 9), various ST profiles (n = 4) were identified, with a main linage ST111 associated with the O12 serotype, the second more widespread high-risk clone after ST235, which disseminated in different Colombian cities and has been reported in other countries of America, Europe, and Asia (Vanegas et al., 2014; Correa et al., 2015; Del Barrio-Tofiño et al., 2020). Interestingly, isolates that belonged to ST111 from rectal swabs and sites of infection from two patients from hospital 2 were genetically related. Other isolates belonged to ST1249 described previously (Vanegas et al., 2014), and ST357 and ST1027 reported in other countries (Hrabák et al., 2011; Horna et al., 2019; Nawfal Dagher et al., 2019; Khan et al., 2020). These findings reflect a variety of genetic backgrounds of MDR P. aeruginosa isolates carrying blaKPC2 or blaVIM2 due to the dissemination of successful international clones and the emergence of other clones in this area of Colombia, associated to the widespread dissemination mediated by MGEs.
Our analysis of WGS revealed that blaVIM–2 was associated with different gene cassette arrays encoding resistance to other antibiotics such as aminoglycosides and chloramphenicol, among isolates with different ST (see Figure 2). Some isolates carried blaVIM–2-containing class 1 integrons including In103 (ST1249) and a new integron designated In1545 (ST357 and ST1027) whose cassette genes were detected in two isolates ST111 from the same patient but differ by the lack of aacA7, as well as a different upstream region of blaVIM–2 for each isolate (see Figure 2), suggesting gene cassette rearrangement. Previous studies demonstrated that under antimicrobial pressure, the IntI-mediated rearrangement can generate integron variants (Barraud and Ploy, 2015). Additionally, we found coexistence with infrequent or new integrons with other gene cassettes that confer resistance to fluoroquinolones, rifampicin, trimethoprim, and β-lactam (In2011 and In1237). This is consistent with previous studies from other countries that showed a high prevalence of class 1 integrons with a high diversity of gene cassettes among MDR P. aeruginosa isolates (Botelho et al., 2018). Likewise, the detection of some mutational mechanisms of resistance showed the propensity to develop the MDR phenotype in the isolates of the study.
Another important finding in our study are the isolates recovered at different times from the same patient that showed heterogeneous genetic backgrounds but the same location of the blaKPC–2 gene within a transposon similar to Tn4401b of the pCOL1 plasmid (Naas et al., 2013), without the resolvase (encoded by gene tnpR). Supported by the clinical data and BLAST analysis, we hypothesized that the blaKPC–2 gene could have been horizontally transferred by one plasmid that carried the transposon between the different strains of P. aeruginosa in the same patient during the infection period (see Figure 4B). There are a few reports of blaKPC inter- and intraspecies transfer within patients (Goren et al., 2010; Adler et al., 2016), but some studies have demonstrated in vivo acquisition of an insertion sequence or plasmid harboring blaKPC–2 among Enterobacterales (Ding et al., 2016). The acquisition also suggests that under antimicrobial pressure, the transposition of insertion sequences or the movement of plasmid among coinfected strains may emerge. In our case, these events could have occurred because the patient had broad-spectrum antimicrobial therapy and several infection relapses secondary to the osteosynthesis material. Future long read sequencing studies are required to confirm the complete sequence of this plasmid.
Overall, in this study, most of the patients colonized and infected by CRPA were older adults (>63 years old), with different underlying conditions, with various medical devices and broad antibiotic exposure, mainly to carbapenems, piperacillin-tazobactam, and glycopeptides (see Supplementary Table 1). Exposure to broad-spectrum antibiotics has been described as the main factor related to carbapenems resistance (Richter et al., 2019). Furthermore, a multidrug resistance phenotype was detected in more than half of CPPA and non-CPPA isolates, a phenomenon locally described only in CPPA (Vanegas et al., 2014). These differences might be explained by the inclusion of isolates recovered from rectal swabs in colonized patients in this study, giving the possibility of acquisition of different resistance genes in these isolates because the gastrointestinal tract is the main source of resistant Enterobacterales and can play a key role in the spread of antibiotic resistance by horizontal transmission (Abdalhamid et al., 2016).
Some limitations of this study include that only isolates from two hospitals were analyzed, and most of these were collected from a single institution, limiting the extrapolation of the results. On the other hand, five P. aeruginosa isolates could have lost the blaKPC gene or the plasmid that contained it during storage or subculture processing prior to sequencing. Therefore, it was not possible to define the genetic environment of the blaKPC gene in those isolates.
In conclusion, the dissemination of blaVIM–2 and blaKPC–2 in P. aeruginosa in this area in Colombia has been strongly influenced by successful international clones and emergence of new clones carrying these genes, as well as the presence of resistance determinants in integrons, transposable elements, and plasmids, accompanied by gene rearrangement likely through transposition and homologous recombination. We postulate that the antimicrobial pressure may have played an important role. Infection control strategies and rational antibiotic use may help limit the spread. In addition, surveillance of colonization patients may also limit the subsequent infection and dissemination of these bacteria.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
ED and AR made the significant contributions to laboratory processing. CC, CA, and CP made the collection and analysis of clinical data. AR performed the sequence data processing, analysis and wrote the manuscript, which was reviewed by all authors.
This study was supported by the Colombian Agency for Science, Technology, and Innovation (COLCIENCIAS), grant 1115-5693-3375 (ER) and the Institución Universitaria Colegio Mayor de Antioquia, Grant FCS46-2016 (AR). The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
MV has received grant support from the Merck Inc., Pfizer, and WestQuímica.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the Fundación Clínica del Norte and Clínica Universitaria Bolivariana for providing clinical and microbiological information and sending isolates.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.663020/full#supplementary-material
Abdalhamid, B., Elhadi, N., Alabdulqader, N., Alsamman, K., and Aljindan, R. (2016). Rates of gastrointestinal tract colonization of carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa in hospitals in Saudi Arabia. New Microbes. New Infect. 10, 77–83. doi: 10.1016/j.nmni.2016.01.014
Abril, D., Marquez-Ortiz, R. A., Castro-Cardozo, B., Moncayo-Ortiz, J. I., Olarte Escobar, N. M., and Corredor Rozo, Z. L. (2019). Genome plasticity favours double chromosomal Tn4401b-blaKPC–2 transposon insertion in the Pseudomonas aeruginosa ST235 clone. BMC Microbiol. 19:45. doi: 10.1186/s12866-019-1418-6
Adler, A., Khabra, E., Paikin, S., and Carmeli, Y. (2016). Dissemination of the blaKPC gene by clonal spread and horizontal gene transfer: comparative study of incidence and molecular mechanisms. J. Antimicrob. Chemother. 71, 2143–2146. doi: 10.1093/jac/dkw106
Alikhan, N. F., Petty, N. K., Ben Zakour, N. L., and Beatson, S. A. (2011). BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 12:402. doi: 10.1186/1471-2164-12-402
Barraud, O., and Ploy, M. C. (2015). Diversity of class 1 Integron gene cassette rearrangements selected under antibiotic pressure. J. Bacteriol. 197, 2171–2178. doi: 10.1128/JB.02455-14
Botelho, J., Grosso, F., Quinteira, S., Brilhante, M., Ramos, H., and Peixe, L. (2018). Two decades of blaVIM-2-producing Pseudomonas aeruginosa dissemination: an interplay between mobile genetic elements and successful clones. J. Antimicrob. Chemother. 73, 873–882. doi: 10.1093/jac/dkx517
Buhl, M., Peter, S., and Willmann, M. (2015). Prevalence and risk factors associated with colonization and infection of extensively drug-resistant Pseudomonas aeruginosa: a systematic review. Expert. Rev. Anti. Infect. Ther. 13, 1159–1170. doi: 10.1586/14787210.2015.1064310
Cabot, G., Ocampo Sosa, A. A., Dominguez, M. A., Gago, J. F., Juan, C., Tubau, F., et al. (2012). Genetic markers of widespread extensively drug-resistant Pseudomonas aeruginosa high-risk clones. Antimicrob. Agents Chemother. 56, 6349–6357. doi: 10.1128/AAC.01388-12
Carrara-Marroni, F. E., Cayô, R., Streling, A. P., da Silva, A. C., Palermo, R. L., Romanin, P., et al. (2015). Emergence and spread of KPC-2-producing Pseudomonas aeruginosa isolates in a Brazilian teaching hospital. J. Glob. Antimicrob. Resist. 3, 304–306. doi: 10.1016/j.jgar.2015.07.002
Cholley, P., Ka, R., Guyeux, C., Thouverez, M., Guessennd, N., and Ghebremedhin, B. (2014). Population structure of clinical Pseudomonas aeruginosa from West and Central African countries. PloS One 9:e107008. doi: 10.1371/journal.pone.0107008
CLSI. (2017). Performance standards for antimicrobial susceptibility testing: Twenty-seven informational supplement– M100-S27. Wayne, PA: CLSI.
Correa, A., Del Campo, R., Perenguez, M., Blanco, V. M., Rodríguez-Baños, M., and Perez, F. (2015). Dissemination of high-risk clones of extensively drug-resistant Pseudomonas aeruginosa in colombia. Antimicrob. Agents Chemother. 59, 2421–2425. doi: 10.1128/AAC.03926-14
Correa, A., Montealegre, M. C., Mojica, M. F., Maya, J. J., Rojas, L. J., and De La Cadena, E. P. (2012). First report of a Pseudomonas aeruginosa isolate coharboring KPC and VIM carbapenemases. Antimicrob. Agents Chemother. 56, 5422–5423. doi: 10.1128/AAC.00695-12
Cuzon, G., Naas, T., Villegas, M. V., Correa, A., Quinn, J. P., and Nordmann, P. (2011). Wide dissemination of Pseudomonas aeruginosa producing beta-lactamase blaKPC–2 gene in Colombia. Antimicrob. Agents Chemother. 55, 5350–5353. doi: 10.1128/AAC.00297-11
Dai, X., Zhou, D., Xiong, W., Feng, J., Luo, W., and Luo, G. (2016). The IncP-6 Plasmid p10265-KPC from Pseudomonas aeruginosa carries a novel ΔISEc33-Associated blaKPC–2 Gene Cluster. Front. Microbiol. 7:310. doi: 10.3389/fmicb.2016.00310
Dallenne, C., Da Costa, A., Decré, D., Favier, C., and Arlet, G. (2010). Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 65, 490–495. doi: 10.1093/jac/dkp498
Del Barrio-Tofiño, E., López-Causapé, C., and Oliver, A. (2020). Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally-acquired β-lactamases: 2020 update. Int. J. Antimicrob. Agents 56:106196. doi: 10.1016/j.ijantimicag.2020.106196
Díaz-Ríos, C., Hernández, M., Abad, D., Álvarez-Montes, L., Varsaki, A., Iturbe, D., et al. (2021). New Sequence Type ST3449 in Multidrug-Resistant Pseudomonas aeruginosa Isolates from a Cystic Fibrosis Patient. Antibiotics 10:491. doi: 10.3390/antibiotics10050491
Ding, B., Shen, Z., Hu, F., Ye, M., Xu, X., and Guo, Q. (2016). In vivo acquisition of carbapenemase gene blaKPC–2 in multiple species of Enterobacteriaceae through horizontal transfer of insertion sequence or plasmid. Front. Microbiol. 7:1651. doi: 10.3389/fmicb.2016.01651
Do Nascimento, A., Medeiros Filho, F., Pauer, H., Antunes, L., Sousa, H., Senger, H., et al. (2020). Characterization of a SPM-1 metallo-beta-lactamase-producing Pseudomonas aeruginosa by comparative genomics and phenotypic analysis. Sci. Rep. 10:13192. doi: 10.1038/s41598-020-69944-6
Dunham, S. A., McPherson, C. J., and Miller, A. A. (2010). The relative contribution of efflux and target gene mutations to fluoroquinolone resistance in recent clinical isolates of Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 29, 279–288. doi: 10.1007/s10096-009-0852-z
Goren, M. G., Carmeli, Y., Schwaber, M. J., Chmelnitsky, I., Schechner, V., and Navon-Venezia, S. (2010). Transfer of carbapenem-resistant plasmid from Klebsiella pneumoniae ST258 to Escherichia coli in patient. Emerg. Infect. Dis. 16, 1014–1017. doi: 10.3201/eid1606.091671
Hong, D. J., Bae, I. K., Jang, I. H., Jeong, S. H., Kang, H. K., and Lee, K. (2015). Epidemiology and characteristics of metallo-β-Lactamase-producing Pseudomonas aeruginosa. Infect. Chemother. 47, 81–97. doi: 10.3947/ic.2015.47.2.81
Horcajada, J. P., Montero, M., Oliver, A., Sorlí, L., Luque, S., Gómez-Zorrilla, S., et al. (2019). Epidemiology and treatment of multidrug-resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 32, e19–e31. doi: 10.1128/CMR.00031-19
Horna, G., Amaro, C., Palacios, A., Guerra, H., and Ruiz, J. (2019). High frequency of the exoU+/exoS+ genotype associated with multidrug-resistant “high-risk clones” of Pseudomonas aeruginosa clinical isolates from Peruvian hospitals. Sci. Rep. 9:10874. doi: 10.1038/s41598-019-47303-4
Horna, G., López, M., Guerra, H., Saénz, Y., and Ruiz, J. (2018). Interplay between MexAB-OprM and MexEF-OprN in clinical isolates of Pseudomonas aeruginosa. Sci. Rep. 8:16463. doi: 10.1038/s41598-018-34694-z
Hrabák, J., Cervená, D., Izdebski, R., Duljasz, W., Gniadkowski, M., and Fridrichová, M. (2011). Regional spread of Pseudomonas aeruginosa ST357 producing IMP-7 metallo-β-lactamase in Central Europe. J. Clin. Microbiol. 49, 474–475. doi: 10.1128/JCM.00684-10
Hu, Y. Y., Gu, D. X., Cai, J. C., Zhou, H. W., and Zhang, R. (2015). Emergence of KPC-2-producing Pseudomonas aeruginosa sequence type 463 isolates in Hangzhou. China. Antimicrob. Agents Chemother. 59, 2914–2917. doi: 10.1128/AAC.04903-14
Hu, Y. Y., Wang, Q., Sun, Q. L., Chen, G. X., and Zhang, R. (2019). A novel plasmid carrying carbapenem-resistant gene blaKPC–2 in Pseudomonas aeruginosa. Infect. Drug Resist. 12, 1285–1288. doi: 10.2147/IDR.S196390
Janvier, F., Otto, M. P., Jové, T., Mille, A., Contargyris, C., and Meaudre, E. (2017). A case of multiple contamination with methylase ArmA-producing pathogens. J. Antimicrob. Chemother. 72, 618–620. doi: 10.1093/jac/dkw418
Ji, J., Wang, J., Zhou, Z., Wang, H., Jiang, Y., and Yu, Y. (2013). Multilocus sequence typing reveals genetic diversity of carbapenem- or ceftazidime-nonsusceptible Pseudomonas aeruginosa in China. Antimicrob. Agents Chemother. 57, 5697–5700. doi: 10.1128/AAC.00970-13
Jové, T., Da Re, S., Denis, F., Mazel, D., and Ploy, M. C. (2010). Inverse correlation between promoter strength and excision activity in class 1 integrons. PLoS Genet. 6:e1000793. doi: 10.1371/journal.pgen.1000793
Khan, A., Tran, T. T., Rios, R., Hanson, B., Shropshire, W. C., Sun, Z., et al. (2019). Extensively Drug-Resistant Pseudomonas aeruginosa ST309 harboring tandem guiana extended spectrum β-lactamase enzymes: A newly emerging threat in the united states. Open Forum. Infect. Dis. 6:ofz273. doi: 10.1093/ofid/ofz273
Khan, M., Stapleton, F., Summers, S., Rice, S. A., and Willcox, M. (2020). Antibiotic resistance characteristics of Pseudomonas aeruginosa isolated from keratitis in Australia and India. Antibiotics 9:600. doi: 10.3390/antibiotics9090600
Larsen, M. V., Cosentino, S., Rasmussen, S., Friis, C., Hasman, H., Marvig, R. L., et al. (2012). Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 50, 1355–1361. doi: 10.1128/JCM.06094-11
Letunic, I., and Bork, P. (2016). Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44, W242–W245. doi: 10.1093/nar/gkw290
Liapis, E., Bour, M., Triponney, P., Jové, T., Zahar, J. R., Valot, B., et al. (2019). Identification of diverse Integron and plasmid structures carrying a novel carbapenemase among Pseudomonas species. Front. Microbiol. 10:404. doi: 10.3389/fmicb.2019.00404
Libisch, B., Watine, J., Balogh, B., Gacs, M., Muzslay, M., Szabó, G., et al. (2008). Molecular typing indicates an important role for two international clonal complexes in dissemination of VIM-producing Pseudomonas aeruginosa clinical isolates in Hungary. Res. Microbiol. 159, 162–168. doi: 10.1016/j.resmic.2007.12.008
López-Causapé, C., Cabot, G., Del Barrio-Tofiño, E., and Oliver, A. (2018a). The Versatile Mutational Resistome of Pseudomonas aeruginosa. Front. Microbiol. 9:685. doi: 10.3389/fmicb.2018.00685
López-Causapé, C., Rubio, R., Cabot, G., and Oliver, A. (2018b). Evolution of the Pseudomonas aeruginosa Aminoglycoside Mutational Resistome In Vitro and in the Cystic Fibrosis Setting. Antimicrob Agents Chemother 62, e2517–e2583. doi: 10.1128/AAC.02583-17
Magiorakos, A. P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas, M. E., Giske, C. G., et al. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18, 268–281. doi: 10.1111/j.1469-0691.2011.03570.x
Monti, M. R., Morero, N. R., Miguel, V., and Argaraña, C. E. (2013). nfxB as a novel target for analysis of mutation spectra in Pseudomonas aeruginosa. PloS One 8:e66236. doi: 10.1371/journal.pone.0066236
Morales-Espinosa, R., Delgado, G., Espinosa, L. F., Isselo, D., Méndez, J. L., and Rodriguez, C. (2017). Fingerprint analysis and identification of strains ST309 as a potential high risk clone in a Pseudomonas aeruginosa population isolated from children with bacteremia in mexico city. Front. Microbiol. 8:313. doi: 10.3389/fmicb.2017.00313
Moura, A., Soares, M., Pereira, C., Leitão, N., Henriques, I., and Correia, A. (2009). INTEGRALL: a database and search engine for integrons, integrases and gene cassettes. Bioinformatics. 25, 1096–1098. doi: 10.1093/bioinformatics/btp105
Naas, T., Bonnin, R. A., Cuzon, G., Villegas, M. V., and Nordmann, P. (2013). Complete sequence of two KPC-harbouring plasmids from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 68, 1757–1762. doi: 10.1093/jac/dkt094
Naas, T., Cuzon, G., Truong, H. V., and Nordmann, P. (2012). Role of ISKpn7 and deletions in blaKPC gene expression. Antimicrob. Agents Chemother. 56, 4753–4759. doi: 10.1128/AAC.00334-12
Nawfal Dagher, T., Al-Bayssari, C., Diene, S. M., Azar, E., and Rolain, J. M. (2019). Emergence of plasmid-encoded VIM-2-producing Pseudomonas aeruginosa isolated from clinical samples in Lebanon. New Microbes. New Infect. 29:100521. doi: 10.1016/j.nmni.2019.100521
Neves, P. R., Perdigão Neto, L. V., Ruedas Martins, R. C., Ramos, J. F., Leite, G., Rossi, F., et al. (2019). Carbapenem-resistant Pseudomonas aeruginosa carrying blaVIM–36 assigned to ST308: Indicated non-virulence in a Galleria mellonella model. J. Global Antimicrob. Resist. 16, 92–97. doi: 10.1016/j.jgar.2018.09.007
Olsson, A., Wistrand-Yuen, P., Nielsen, E. I., Friberg, L. E., Sandegren, L., Lagerbäck, P., et al. (2020). Efficacy of antibiotic combinations against multidrug-resistant pseudomonas aeruginosa in automated time-lapse microscopy and static time-kill experiments. Antimicrob. Agents Chemother. 64, e2111–e2119. doi: 10.1128/AAC.02111-19
Pacheco, R., Osorio, L., Correa, A. M., and Villegas, M. V. (2014). Prevalencia de bacterias Gram negativas portadoras del gen blaKPC en hospitales de Colombia. Biomédica 34, 81–90. doi: 10.7705/biomedica.v34i0.1642
Pacheco, T., Bustos-Cruz, R. H., Abril, D., Arias, S., Uribe, L., and Rincón, J. (2019). Pseudomonas aeruginosa Coharboring BlaKPC–2 and BlaVIM–2 Carbapenemase Genes. Antibiotics 8:98. doi: 10.3390/antibiotics8030098
Pachori, P., Gothalwal, R., and Gandhi, P. (2019). Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes. Dis. 6, 109–119. doi: 10.1016/j.gendis.2019.04.001
Papagiannitsis, C. C., Verra, A., Galani, V., Xitsas, S., Bitar, I., Hrabak, J., et al. (2020). Unravelling the Features of Success of VIM-Producing ST111 and ST235 Pseudomonas aeruginosa in a Greek Hospital. Microorganisms 8:1884. doi: 10.3390/microorganisms8121884
Papp-Wallace, K. M., Zeiser, E. T., Becka, S. A., Park, S., Wilson, B. M., Winkler, M. L., et al. (2019). Ceftazidime-avibactam in combination with fosfomycin: a novel therapeutic strategy against multidrug-resistant pseudomonas aeruginosa. J. Infect. Dis. 220, 666–676. doi: 10.1093/infdis/jiz149
Patel, G., and Bonomo, R. A. (2011). Status report on carbapenemases: challenges and prospects. Expert. Rev. Anti. Infect. Ther. 9, 555–570. doi: 10.1586/eri.11.28
Potron, A., Poirel, L., and Nordmann, P. (2015). Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int. J. Antimicrob. Agents. 45, 568–585. doi: 10.1016/j.ijantimicag.2015.03.001
Richter, S. E., Miller, L., Needleman, J., Uslan, D. Z., Bell, D., and Watson, K. (2019). Risk factors for development of carbapenem resistance among Gram-negative rods. Open Forum. Infect. Dis. 6:ofz027. doi: 10.1093/ofid/ofz027
Saavedra, S. Y., Duarte, C., Gonzaalez, M. N., and Realpe, M. E. (2014). Caracterización de aislamientos de Pseudomonas aeruginosa productores de carbapenemasas en siete departamentos de Colombia. Biomédica 34:1685. doi: 10.7705/biomedica.v34i0.1685
Santella, G., Cittadini, R., Papalia, M., Vera Ocampo, C., Del Castillo, M., Vay, C., et al. (2012). First clonal spread of KPC-producing Pseudomonas aeruginosa in Buenos Aires. Argent. Infect. Genet. Evol. 12, 2003–2005. doi: 10.1016/j.meegid.2012.03.022
Snesrud, E., Maybank, R., Kwak, Y. I., Jones, A. R., Hinkle, M. K., and McGann, P. (2018). Chromosomally Encoded mcr-5 in Colistin-Nonsusceptible Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 62, e618–e679.
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Subedi, D., Vijay, A. K., Kohli, G. S., Rice, S. A., and Willcox, M. (2018). Association between possession of ExoU and antibiotic resistance in Pseudomonas aeruginosa. PloS one 13:e0204936. doi: 10.1371/journal.pone.0204936
Thrane, S. W., Taylor, V. L., Lund, O., Lam, J. S., and Jelsbak, L. (2016). Application of whole-genome sequencing data for o-specific antigen analysis and in silico serotyping of pseudomonas aeruginosa isolates. J. Clin. Microbiol. 54, 1782–1788. doi: 10.1128/JCM.00349-16
van der Zee, A., Kraak, W. B., Burggraaf, A., Goessens, W., Pirovano, W., Ossewaarde, J. M., et al. (2018). Spread of carbapenem resistance by transposition and conjugation among Pseudomonas aeruginosa. Front. Microbiol. 9:2057. doi: 10.3389/fmicb.2018.02057
Vanegas, J. M., Cienfuegos, A. V., Ocampo, A. M., López, L., del Corral, H., and Roncancio, G. (2014). Similar frequencies of Pseudomonas aeruginosa isolates producing KPC and VIM carbapenemases in diverse genetic clones at tertiary-care hospitals in Medellín. Colomb. J. Clin. Microbiol. 52, 3978–3986. doi: 10.1128/JCM.01879-14
Vettoretti, L., Plésiat, P., Muller, C., El Garch, F., Phan, G., Attrée, I., et al. (2009). Efflux unbalance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 53, 1987–1997. doi: 10.1128/AAC.01024-08
Villegas, M. V., Lolans, K., Correa, A., Kattan, J. N., Lopez, J. A., and Quinn, J. P. (2007). First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing beta-lactamase. Antimicrob. Agents Chemother. 51, 1553–1555. doi: 10.1128/AAC.01405-06
Villegas, M. V., Lolans, K., del Rosario Olivera, M., Suarez, C. J., Correa, A., Queenan, A. M., et al. (2006). First detection of metallo-beta-lactamase VIM-2 in Pseudomonas aeruginosa isolates from Colombia. Antimicrob. Agents Chemother. 50, 226–229. doi: 10.1128/AAC.50.1.226-229.2006
Vinué, L., Jové, T., Torres, C., and Ploy, M. C. (2011). Diversity of class 1 integron gene cassette Pc promoter variants in clinical Escherichia coli strains and description of a new P2 promoter variant. Int. J. Antimicrob. Agents 38, 526–529. doi: 10.1016/j.ijantimicag.2011.07.007
Yigit, H., Queenan, A. M., Anderson, G. J., Domenech-Sanchez, A., Biddle, J. W., and Steward, C. D. (2001). Novel carbapenem- hydrolyzing beta-lactamase. KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45, 1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001
Keywords: Pseudomonas aeruginosa, genetic diversity, blaKPC–2, blaVIM–2, integron, plasmid
Citation: Rada AM, De La Cadena E, Agudelo CA, Pallares C, Restrepo E, Correa A, Villegas MV and Capataz C (2021) Genetic Diversity of Multidrug-Resistant Pseudomonas aeruginosa Isolates Carrying blaVIM–2 and blaKPC–2 Genes That Spread on Different Genetic Environment in Colombia. Front. Microbiol. 12:663020. doi: 10.3389/fmicb.2021.663020
Received: 02 February 2021; Accepted: 19 July 2021;
Published: 27 August 2021.
Edited by:
Paul G. Higgins, University of Cologne, GermanyReviewed by:
Remy A. Bonnin, Université Paris-Saclay, FranceCopyright © 2021 Rada, De La Cadena, Agudelo, Pallares, Restrepo, Correa, Villegas and Capataz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana M. Rada, YW5hLnJhZGFAY29sbWF5b3IuZWR1LmNv
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.