
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 07 May 2021
Sec. Microbiotechnology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.659784
This article is part of the Research Topic Microbial Source Tracking View all 20 articles
Roof-harvested rainwater (RHRW) was investigated for the presence of the human pathogenic bacteria Mycobacterium tuberculosis (M. tuberculosis), Yersinia spp. and Listeria monocytogenes (L. monocytogenes). While Yersinia spp. were detected in 92% (n = 25) of the RHRW samples, and L. monocytogenes and M. tuberculosis were detected in 100% (n = 25) of the samples, a significantly higher mean concentration (1.4 × 103 cells/100 mL) was recorded for L. monocytogenes over the sampling period. As the identification of appropriate water quality indicators is crucial to ensure access to safe water sources, correlation of the pathogens to traditional indicator organisms [Escherichia coli (E. coli) and Enterococcus spp.] and microbial source tracking (MST) markers (Bacteroides HF183, adenovirus and Lachnospiraceae) was conducted. A significant positive correlation was then recorded for E. coli versus L. monocytogenes (r = 0.6738; p = 0.000), and Enterococcus spp. versus the Bacteroides HF183 marker (r = 0.4071; p = 0.043), while a significant negative correlation was observed for M. tuberculosis versus the Bacteroides HF183 marker (r = −0.4558; p = 0.022). Quantitative microbial risk assessment indicated that the mean annual risk of infection posed by L. monocytogenes in the RHRW samples exceeded the annual infection risk benchmark limit (1 × 10–4 infections per person per year) for intentional drinking (∼10–4). In comparison, the mean annual risk of infection posed by E. coli was exceeded for intentional drinking (∼10–1), accidental consumption (∼10–3) and cleaning of the home (∼10–3). However, while the risk posed by M. tuberculosis for the two relevant exposure scenarios [garden hosing (∼10–5) and washing laundry by hand (∼10–5)] was below the benchmark limit, the risk posed by adenovirus for garden hosing (∼10–3) and washing laundry by hand (∼10–3) exceeded the benchmark limit. Thus, while the correlation analysis confirms that traditional indicators and MST markers should be used in combination to accurately monitor the pathogen-associated risk linked to the utilisation of RHRW, the integration of QMRA offers a more site-specific approach to monitor and estimate the human health risks associated with the use of RHRW.
A conservative estimate predicts that 4% of deaths worldwide and 5.7% of the global burden of disease in disability-adjusted life years (DALYs) could be attributed to water, sanitation and hygiene (WASH) related infectious diseases (Yang et al., 2012). The primary aim of Sustainable Development Goal 6 (SDG6) is thus to ensure that the world population has access to safe and affordable water and adequate sanitation services by 2030 (United Nations, 2018). In an effort to achieve this aim, roof-harvested rainwater (RHRW) is being investigated and applied as an alternative, supplementary water source in many countries around the world, including South Africa (Ahmed et al., 2008b; De Kwaadsteniet et al., 2013). However, while RHRW may be used to augment current water supplies (Gould and Nissen-Petersen, 1999), rainwater is exposed to various contamination sources as it traverses the air (e.g., bioaerosols) and during the harvesting process (e.g., debris and animal faecal matter on the catchment surface) (Hamilton et al., 2019).
It is thus well documented that the microbiological quality of RHRW is sub-standard and numerous research groups have reported on the detection of traditional indicator organisms (using culture-based analysis) such as total coliforms, faecal coliforms, Escherichia coli (E. coli) and enterococci species in rainwater (Kaushik et al., 2014; Waso et al., 2018; Hamilton et al., 2019). While the analysis of the indicator groups is routine in water quality monitoring, amongst other pitfalls, a poor correlation has been recorded between traditional indicator organisms and potentially pathogenic microorganisms (Harwood et al., 2005, 2014; Field and Samadpour, 2007). Researchers are thus investigating the use of microbial source tracking (MST) markers to monitor and detect faecal contamination within environmental water samples (Ahmed et al., 2016). Microbial source tracking methods have greatly improved the capacity to detect microorganisms that are host-specific to animals, that occur in water and sediments and unlike faecal indicator bacteria, MST markers are able to differentiate between several sources of faecal contamination (Bradshaw et al., 2016). A few of the common MST markers include the Enterococcus esp gene, enterovirus, Bifidobacterium spp., human-specific Bacteroides HF183, human adenovirus and polyomavirus. Ideal characteristics of these MST markers include: specificity to the target host-group; the marker should be geographically and temporally stable in the target host-group, and; the decay rates of the markers and pathogens present in the relevant water sources should correlate (Ahmed et al., 2015). Moreover, advances in water quality monitoring techniques such as molecular viability and whole community analysis, renders culture-based analyses superfluous and ensures the accurate detection and quantification of MST markers and waterborne pathogens. Using quantitative PCR (qPCR), Savichtcheva et al. (2007) observed a significant positive correlation between the human Bacteroides MST marker versus Salmonella, whilst Viau and Boehm (2011) observed a significant correlation between the human Bacteroides MST marker and Leptospira.
Using molecular detection methods, numerous studies have also identified a variety of opportunistic and pathogenic microorganisms in RHRW. These frequently detected microorganisms include Legionella pneumophila, Pseudomonas aeruginosa, Klebsiella pneumoniae and Salmonella spp. (Hamilton et al., 2019). Ahmed et al. (2017); Strauss et al. (2019) and Reyneke et al. (2020) then employed Illumina amplicon-based sequencing and ethidium monoazide bromide (EMA)-Illumina analysis, to investigate the whole bacterial community in RHRW. Correspondingly, many of the frequently detected bacterial genera such as Legionella, Pseudomonas etc. formed part of this indigenous or core microbial rainwater group. However, a high frequency of detection percentage was also obtained for genera such as Mycobacterium. This genus includes pathogens known to cause serious disease in humans, which is concerning as the utilisation of RHRW as an alternative water source may pose a health risk to the end-user communities. Mycobacterium tuberculosis (M. tuberculosis) was thus predominantly focused on in the current study and as Yersinia spp. and Listeria monocytogenes (L. monocytogenes) have previously been detected in rainwater, these bacterial pathogens were also included (Dobrowsky et al., 2014; Jongman and Korsten, 2016).
The Gram-negative coccobacillus Yersinia genus forms part of the Yersiniaceae family and is comprised of 11 species, three of which are pathogenic to humans: Y. pseudotuberculosis, Y. enterocolitica and Y. pestis. Y. pestis is responsible for causing three different forms of the highly infectious disease known as the plague (pneumonic, bubonic and septicaemic) (Pechous et al., 2016). The plague is transmitted by fleas to various hosts by blood feeding, or by regurgitative transmission of the bacteria (once it has grown in the form of a cohesive biofilm) from the flea host into the bite of the receiving organism and can persist for extended periods of time at low levels in enzootic cycles (Eisen et al., 2006; Hinnebusch et al., 2017; Bosio et al., 2020). Although limited research is available on the presence of Yersinia spp. in RHRW, Dobrowsky et al. (2014) identified the Yersinia genus in rainwater using conventional PCR analysis. There is thus a high probability that these species are present in RHRW systems as bird and rodent faecal matter are often detected on roofing systems and might wash into the harvesting tanks during a rain event. Similarly, M. tuberculosis, which causes tuberculosis, can survive and adapt to hostile or extreme environmental conditions (Cook et al., 2009). It is also capable of adapting to a variety of intracellular human systems such as dendritic cells and macrophages (Cook et al., 2009). However, despite its poor geographical characterisation in terms of abundance in various environments (King et al., 2017), as previously indicated, using Illumina and EMA-Illumina whole-community analysis, the Mycobacterium genus was identified as one of the primary frequently detected genera in rainwater (Ahmed et al., 2017; Strauss et al., 2019; Reyneke et al., 2020). This is a matter of serious concern as 360 000 people were diagnosed with tuberculosis in South Africa in 2019 [World Health Organisation (WHO), 2019] and 781 tuberculosis cases are reported amongst every 100 000 individuals each year (Tadokera et al., 2020). L. monocytogenes is a Gram-positive, psychotropic bacteria that has been detected in the environment and typically occurs in most raw foods. It is responsible for causing listeriosis and from 2017 to 2018, L. monocytogenes sequence type 6 was associated with a listeriosis outbreak in South Africa, that was described by the WHO as the biggest outbreak ever recorded worldwide (Smith et al., 2019). Jongman and Korsten. (2016) then detected L. monocytogenes (using selective culture-based analysis combined with matrix-assisted laser desorption/ionisation time of flight mass spectrometry) in RHRW samples collected from three rural South African villages which rely on RHRW as an alternative water source.
Roof-harvested rainwater thus has the potential to expose vulnerable end-user communities to a myriad of microbial pathogens and opportunistic pathogens (Low, 2002). Quantitative microbial risk assessment (QMRA) can therefore be implemented as a health risk assessment tool and is a technique that has been used for more than two decades to estimate the pathogen-associated risk in drinking water (Pecson et al., 2017). This technique is comprised of four stages, namely (i) hazard identification, (ii) exposure assessment, (iii) dose-response modelling and (iv) risk characterisation. The combination of these stages allows for the construction of a prediction-based analysis model that elucidates the potential health risk associated with specific pathogens based on exposure scenarios associated with a particular environment or activity (Owens et al., 2020). Moreover, using a QMRA framework, it is envisaged that the gap between the level of pathogens in a water source and the treatment required to effectively reduce pathogen-associated risk, can be bridged (Sano et al., 2019).
The primary aim of this study was thus to explore a consortium of RHRW tanks located in a sustainable housing project in Kleinmond, South Africa for the human pathogenic bacterial species M. tuberculosis, Yersinia spp. and L. monocytogenes. Additionally, the presence of the human bacterial pathogens (M. tuberculosis, Yersinia spp. and L. monocytogenes) was correlated to the presence of traditional indicator organisms (i.e., E. coli and Enterococcus spp.) and MST markers (i.e., Bacteroides HF183, adenovirus and Lachnospiraceae). The Bacteroides HF183 marker was selected as the HF183 primer set occurs in all Bacteroides strains of human origin. This marker thus has high specificity for the detection of human faecal matter and sewage in environmental waters (Harwood et al., 2014). Similarly, both Lachnospiraceae and adenovirus have been investigated as indicators of sewage and faecal pollution in environmental waters and could therefore provide valuable information on the health risks associated with the use of various water sources (Newton et al., 2011; McLellan et al., 2013; Sidhu et al., 2013; Rusiñol et al., 2016; Waso et al., 2018). Quantitative PCR analyses were used to identify and quantify the target bacterial pathogens, MST markers and indicator groups, whereafter the health risk associated with the utilisation of the RHRW for potable and various domestic activities (e.g., cleaning the house, laundry, etc.), in the target community, was determined.
The Kleinmond Housing Scheme, Western Cape, South Africa (GPS co-ordinates: 34°20.11′ 81″ S; 19° 00.59′ 74″ E), was used as the sampling site. The sustainable housing project was conceptualised in 2007 in a collaboration between the Overstrand Local Municipality, the Council for Scientific and Industrial Research, the Department of Science and Technology and the Western Cape Provincial Department of Human Settlements. Within this community are 411 houses (40 m2 each), each fitted with a 2000 L aboveground polyethylene rainwater harvesting tank. A random sampling technique was implemented to select five houses, designated A to E (with functioning rainwater harvesting tanks installed), from a collection of houses used in the Dobrowksy et al. (2014) study. Sampling was conducted once a week for five consecutive weeks (August to September 2020), with 5 L of rainwater collected from each RHRW tank (n = 25) using sterile polypropylene bottles as previously described by Waso et al. (2018).
The temperature of each sample was measured on-site using a hand-held mercury thermometer; the pH, total dissolved solids, and electrical conductivity were determined using a hand-held Milwaukee Instruments MI806 meter, and the dissolved oxygen was measured using a hand-held Milwaukee Instruments M600 meter (Spraytech, South Africa). All physico-chemical parameters are outlined in Supplementary Table 1 (Supplementary Material). The daily ambient temperature and rainfall data for the duration of the sampling period was obtained from the South African Weather Services (Pretoria, South Africa). A visual representation of the daily ambient temperature and rainfall data is shown in Supplementary Figure 1 (Supplementary Material).
One litre of each RHRW sample (n = 25) was subjected to flocculation as previously described by Dobrowsky et al. (2015). The flocculated samples were filtered through non-charged mixed ester membrane filters with a pore size of 0.45 μM (Merck, Millipore, Billerica, MA, United States) and each filter was placed in a 9 cm petri dish containing 1.5 mL citrate buffer (0.3 M, pH 3.5) (Saarchem, Durban, South Africa) and gently agitated using an orbital platform shaker to remove the cells from the filter. Thereafter, each 1.5 mL concentrated sample was centrifuged at 16,000 × g for 5 min and the resulting pellet was subjected to 6 μM EMA (Biotium, Hayward, CA, United States) treatment as previously described by Reyneke et al. (2017). Ethidium monoazide bromide intercalates with the DNA of cells with compromised membranes or with extracellular DNA. Upon photoactivation, EMA covalently binds to the DNA, which inhibits their amplification in quantification assays (e.g., qPCR) (Emerson et al., 2017). Treatment with EMA was thus done so that the detected gene copies for the pathogens and indicator organisms could be converted into viable cells (may be detected using culture-based analysis) for use in the QMRA analysis. Following EMA treatment, the supernatant containing residual EMA was removed and total genomic DNA extractions (i.e., DNA from intact and presumed viable cells) were performed on the remaining pellet for each of the RHRW samples (n = 25) using the Quick-DNATM Fecal/Soil Microbe Miniprep Kit (Zymo Research, Irvine, CA, United States) according to the manufacturer’s instructions.
Preliminary comparative analysis of EMA-qPCR (intact and presumed viable cells) versus qPCR (whole or total DNA, without EMA), indicated that the EMA treatment may have influenced the detection of the MST markers in the RHRW. Therefore, conventional qPCR was used for the detection of the MST markers (i.e., Bacteroides HF183, Lachnospiraceae, adenovirus) within the RHRW samples. One litre of each RHRW sample (n = 25) was concentrated as described in Section “Rainwater Concentration, EMA Treatment and DNA Extraction for the Detection of Target Pathogens and Indicator Organisms.” Thereafter, each 1.5 mL concentrated sample was centrifuged at 16,000 × g for 5 min and total genomic DNA extractions were performed on the remaining pellet for each of the RHRW samples (n = 25) using the Quick-DNA Fecal/Soil Microbe Miniprep Kit (Zymo Research) according to the manufacturer’s instructions.
All qPCR analyses were conducted using a LightCycler®96 Instrument (Roche Diagnostics, Mannheim, Germany) and the FastStart Essential DNA Green Master/FastStart Essential DNA Probes Master (Roche Diagnostics) in order to quantify gene copies of the target pathogens M. tuberculosis, Yersinia spp. and L. monocytogenes, as well as the MST markers Bacteroides HF183, adenovirus and Lachnospiraceae, and the indicator organisms E. coli and Enterococcus spp. in the RHRW samples. The primers and cycling parameters for each target organism are outlined in Table 1. Each qPCR assay was performed in duplicate. The reaction mixture (final volume of 20 μL) for all the qPCR assays, except the Lachnospiraceae assay, consisted of 10 μL FastStart Essential DNA Green Master (1X), 0.4 μL of the forward and reverse primers (0.2 μM) and 5 μL template DNA. For the Lachnospiraceae assay, the reaction mixture consisted of 10 μL (1X) FastStart Essential DNA Probes Master, 2 μL primer-probe mixture [1 μM of each primer and 0.08 μM of the probe], 3 μL PCR-grade water and 5 μL template DNA. All DNA samples were diluted 10-fold before analysis with the respective qPCR assays in order to minimise PCR inhibitors (Reyneke et al., 2017). For each qPCR reaction, a negative control of sterile milliQ was included in the analysis, while melt curve analysis was included for all SYBR® Green qPCR assays to verify the specificity of the primer sets (temperature increase from 65 to 97 °C at 0.2°C/s and continuous fluorescent signal acquisition at 5 readings/°C) (Reyneke et al., 2017).
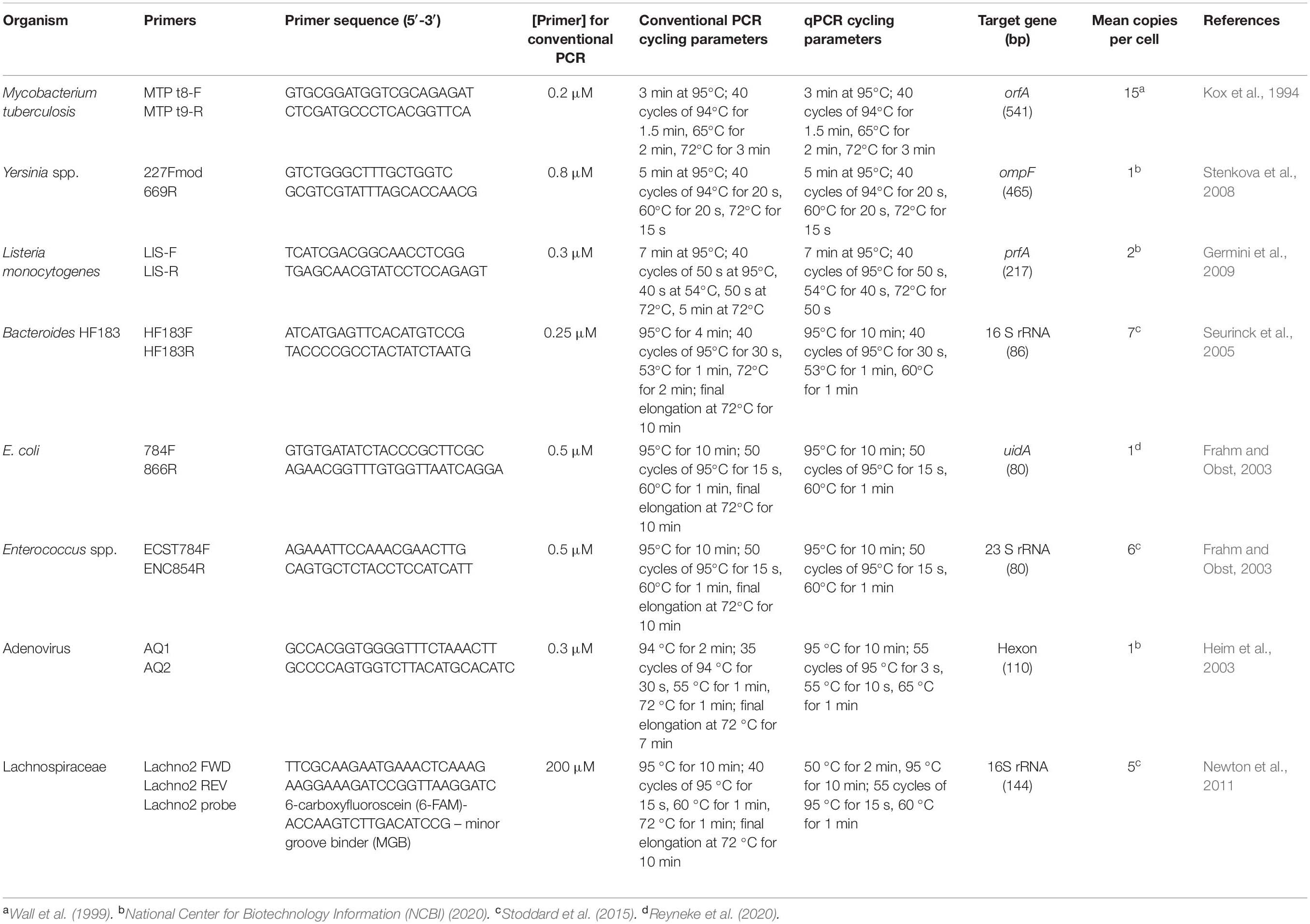
Table 1. Conventional PCR and qPCR primers, cycling parameters and PCR product size of the organisms screened for in the RHRW samples.
Standard curves for the qPCR assays were generated using the methodology outlined in Reyneke et al. (2017). Briefly, conventional PCR assays (Table 1) were performed to amplify the respective target genes using positive control DNA [E. coli ATCC 13706, Enterococcus faecalis (clinical isolate), lyophilised adenovirus (Coris Bioconcept, Gembloux, Belgium), L. monocytogenes ATCC 13932 and Yersinia enterocolitica subsp. enterocolitica ATCC 27729] or DNA extracted from an influent sewage sample collected from a local wastewater treatment plant (M. tuberculosis, Lachnospiraceae and Bacteroides HF183) (Supplementary Table 2, Supplementary Material). A negative control of sterile milliQ was utilised for each PCR reaction.
A standard curve was generated by preparing serial 10-fold dilutions (109 to 100 gene copies/μL) of the PCR products. The lower limit of detection (LLOD) for each qPCR assay was reported as the lowest number of gene copies that was consistently detected in the standard curve (Dobrowsky et al., 2016). The Roche LightCycler®96 Software version 1.1 was utilised for the analysis of the qPCR performance characteristics of the assays.
Based on the detection frequency of the target pathogens, indicators and MST markers obtained using qPCR analysis (Section “Quantitative PCR Analyses”) and the availability of applicable dose-response models, E. coli (representative traditional indicator organism), adenovirus (representative MST marker), L. monocytogenes and M. tuberculosis were selected as the target organisms for the health risk assessment of the RHRW. For use in the QMRA analyses, the detected gene copies (Section “Quantitative PCR Analyses”) were converted to gene copies/100 mL of the original RHRW sample as outlined by Waso et al. (2018). The gene copies/100 mL were then converted to cell equivalents (cells/100 mL) by utilising the number of copies of the target gene present within the host (Table 1). All final concentrations for the EMA-qPCR (intact and presumed viable cells) and conventional qPCR (whole or total DNA) analyses are thus presented as equivalent cells/100 mL original RHRW sample.
The major exposure routes associated with the use of RHRW for several domestic activities in the Kleinmond Housing Scheme site were identified by consulting social survey data reported by Dobrowksy et al. (2014). These activities include washing laundry by hand, cleaning of the home, garden hosing, garden work, washing/bathing, intentional drinking and accidental consumption. The various exposure scenarios (which includes the exposure volume and frequency of occurrence) that were evaluated in the present study are outlined in Supplementary Table 3 (Supplementary Material).
The formulae used for the calculation of ingestion/inhalation dose, as well as descriptions of the various exposure routes are outlined in Reyneke et al. (2020). The concentration of each respective target organism was obtained from the results of the qPCR analyses. Based on the detection frequency of pathogenic E. coli in RHRW from previous studies, the fraction of E. coli presumed to be human infectious was set at 0.005–0.1 (Reyneke et al., 2020). Additionally, the fraction of detected adenovirus, L. monocytogenes and M. tuberculosis assumed to be infectious to humans were 5.88 × 10–4, 1.00 and 0.66–1.00, respectively (Buchanan et al., 1997; World Health Organisation, 2004; Lyautey et al., 2007; Schijven et al., 2019).
Dose-response models are a set of mathematical expressions which illustrate the probability that an individual will experience an adverse health effect (e.g., infection, death) following exposure to an infectious organism. These models are specifically fitted to the adverse health effects observed in animals or humans that have been exposed to varying doses of infectious microorganisms (Haas et al., 1999; Jones and Su, 2015). Two of the most commonly used dose-response models are the exponential and beta-poisson models. The exponential model assumes that the probability of an organism causing infection is independent of organism dosage, whereas the beta-poisson model assumes that infectivity of an organism is dependent on dose (Buchanan et al., 2000). The exponential dose-response model (Eq. 1) was used to calculate the risk of infection linked to the presence of pathogenic adenovirus (inhalation exposure), M. tuberculosis (inhalation exposure) and L. monocytogenes (ingestion exposure) within the RHRW samples for various exposure scenarios (Couch et al., 1966; Buchanan et al., 1997; Jones et al., 2009):
where Pinf is the probability of infection following a single exposure, k is the parameter which describes the probability of a pathogen surviving the host defence to initiate infection and d is the dose of microorganisms (number of microorganisms that is inhaled/ingested).
The beta-Poisson dose-response model (Eq. 2) was then employed to calculate the risk of infection linked to the presence of enteroinvasive E. coli (ingestion exposure) within the RHRW samples for various exposure scenarios (Haas et al., 1999; Ryan et al., 2014):
where Pinf is the probability of getting infected following a single exposure event, d is the dosage of microorganisms (number of microorganisms that is ingested), α is a shape factor and N50 is the median infective dosage. All parameters associated with the dose-response models are outlined in Table 2.
Lastly, risk characterisation was conducted, whereby the likelihood of infection was calculated for each target pathogen and the corresponding exposure routes as described in Table 2. This was expressed as likely numbers of infections per 10 000 persons per year as previously described by Haas et al. (1999), using Eq. 3:
where P is the probability of infection following n exposure events per year, based on the previously calculated exposure probability of infection (Pinf).
Each exposure scenario was simulated using Monte Carlo analysis in RStudio (version 1.0.153) using 500 000 iterations. Throughout the analyses, the different dose parameters [e.g., pathogen concentrations (Table 2) and ingestion volumes (Supplementary Table 3), amongst others] and exposure events per year (Supplementary Table 3) were sampled randomly based on the corresponding distribution of each parameter. However, the annual risk of infection for adenovirus and M. tuberculosis were only determined for two exposure scenarios: garden hosing (aerosol inhalation) and washing laundry by hand (aerosol ingestion), as inhalation of these organisms is the primary route of infection (World Health Organisation, 2005; Fennelly and Jones-López, 2015).
The relationships between the detected bacterial pathogens, indicator organisms and MST markers enumerated using qPCR analysis, were investigated using Pearson’s correlation analysis and further investigated using Cluster analysis with Ward’s method in StatisticaTM version 12.5 (2014). The Cluster analysis with Ward’s method was specifically applied to visualise the relatedness of the detected bacterial pathogens, indicator organisms and MST markers in the RHRW samples (Waso et al., 2018). Cluster analysis is used to illustrate correlations between organisms. A stronger positive correlation (i.e., a higher correlation coefficient between two organisms), will be represented by a lower linkage distance on a dendrogram (Tilevik, 2017).
The quantification of intact L. monocytogenes, M. tuberculosis and Yersinia spp. cells as well as the indicator organisms E. coli and Enterococcus spp. in the RHRW samples was investigated using EMA-qPCR (intact and presumed viable cells) analysis. The respective performance characteristics of the EMA-qPCR analyses are outlined in Supplementary Table 4 (Supplementary Material).
The LLOD for L. monocytogenes (prfA gene) was 6 gene copies/μL. L. monocytogenes was detected in all RHRW samples (100%, n = 25) at a mean concentration of 1.4 × 103 cells/100 mL (Figure 1). The lowest concentration of L. monocytogenes was detected in sampling 2 with the cell counts in the RHRW tanks ranging from 23 cells/100 mL to 1.8 × 103 cells/100 mL, while the highest concentration of L. monocytogenes was detected in sampling 3, with the cell counts ranging from 2.5 × 103 cells/100 mL to 4.9 × 103 cells/100 mL. The LLOD for M. tuberculosis (orfA gene) was 3 gene copies/μL. M. tuberculosis was detected in all RHRW samples (100%, n = 25) at a mean concentration of 6 cells/100 mL (Figure 1). The lowest concentration of M. tuberculosis was detected in sampling 5, with the cell counts in the RHRW tanks ranging from 1 cell/100 mL to 4 cells/100 mL, while the highest concentration of M. tuberculosis was detected in sampling 2 with the cell counts ranging from 4 cells/100 mL to 16 cells/100 mL. The LLOD for Yersinia spp. (ompF gene) was 11 gene copies/μL. Yersinia spp. were detected in 23 of the RHRW samples (92%, n = 25) at a mean concentration of 24 cells/100 mL (Figure 1). The lowest concentration of Yersinia spp. cells was detected in sampling 5 with the cell counts in the RHRW tanks ranging from 2 cells/100 mL to 11 cells/100 mL, while the highest concentration of Yersinia spp. was detected in sampling 3 with the cell counts ranging from 10 cells/100 mL to 1.5 × 102 cells/100 mL. The LLOD for E. coli (uidA gene) was 2 gene copies/μL. E. coli was detected in all RHRW samples (100%, n = 25) at a mean concentration of 3.1 × 102 cells/100 mL (Figure 1). The lowest concentration of E. coli was detected in sampling 2 with the cell counts in the RHRW tanks ranging from 1.4 × 102 cells/100 mL to 2.9 × 102 cells/100 mL, while the highest concentration of E. coli was detected in sampling 3 and ranged from 3.3 × 102 cells/100 mL to 6.1 × 102 cells/100 mL. The LLOD for Enterococcus spp. (23S rRNA gene) was 1 gene copy/μL. Enterococcus spp. were detected in all RHRW samples (100%, n = 25) at a mean concentration of 6 cells/100 mL (Figure 1). The lowest concentration of Enterococcus spp. was detected in sampling 2 with the cell counts in the RHRW tanks ranging from 2 cells/100 mL to 5 cells/100 mL, while the highest concentration of Enterococcus spp. was detected in sampling 5 and ranged from 2 cells/100 mL to 27 cells/100 mL.
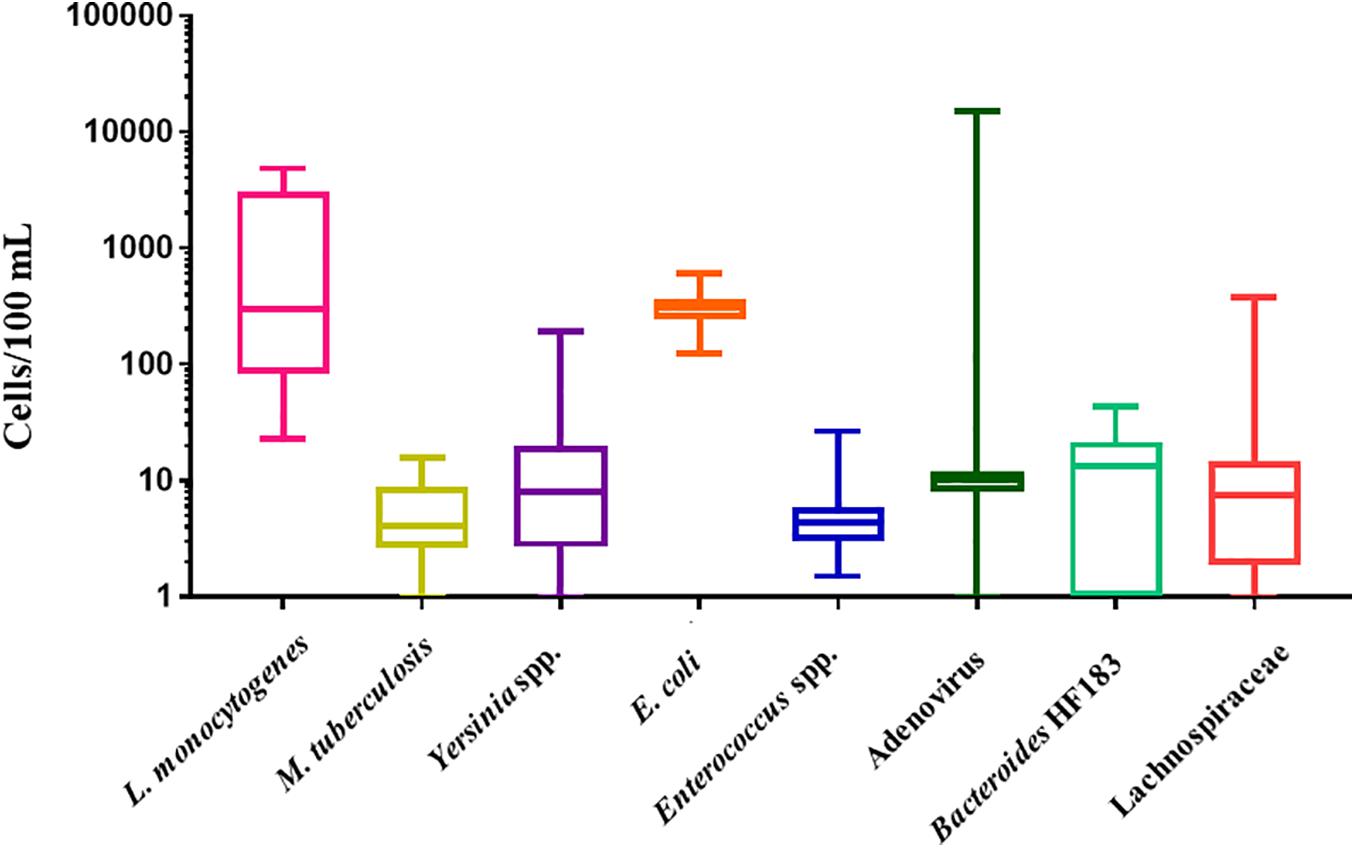
Figure 1. Box and whiskers plot of the concentration (cells/100 mL) for L. monocytogenes, M. tuberculosis, Yersinia spp., E. coli, Enterococcus spp., adenovirus, Bacteroides HF183, and Lachnospiraceae. The whiskers illustrate the minimum and maximum, the outer box illustrates the 1st and 3rd quartiles, and the inner line illustrates the median.
The performance characteristics of the qPCR (whole or total DNA) analyses of the MST markers adenovirus, Lachnospiraceae and Bacteroides HF183 are outlined in Supplementary Table 4 (Supplementary Material).
The LLOD for Bacteroides HF183 (16S rRNA gene) was 3 gene copies/μL. Bacteroides HF183 was detected in 19 of the RHRW samples (76%, n = 25) at a mean concentration of 13 cells/100 mL (Figure 1). The lowest concentration of Bacteroides HF183 was detected in sampling 2 with the cell counts in the RHRW tanks ranging from 0 cells/100 mL to 16 cells/100 mL, while the highest concentration of Bacteroides HF183 cells was detected in sampling 5 and ranged from 0 cells/100 mL to 44 cells/100 mL. The LLOD for adenovirus (Hexon gene) was 5 gene copies/μL. Adenovirus was detected in 24 of the RHRW samples (96%, n = 25) at a mean concentration of 6.6 × 102 cells/100 mL (Figure 1). The lowest concentration of adenovirus was detected in sampling 1 with the cell counts in the RHRW tanks ranging from 0 cells/100 mL to 9 cells/100 mL, while the highest concentration of adenovirus cells was detected in sampling 3 and ranged from 9 cells/100 mL to 1.5 × 104 cells/100 mL. The LLOD for Lachnospiraceae (16S rRNA gene) was 1 gene copy/μL. Lachnospiraceae was detected in 20 of the RHRW samples (80%, n = 25) at a mean concentration of 24 cells/100 mL (Figure 1). The lowest concentration of Lachnospiraceae was detected in sampling 4 with the cell counts in the RHRW tanks ranging from 0 cells/100 mL to 10 cells/100 mL, while the highest concentration of Lachnospiraceae cells was detected in sampling 2 and ranged from 2 cells/100 mL to 3.7 × 102 cells/100 mL.
Pearson’s correlation and Cluster analysis was used to correlate and visualise the relatedness of the pathogenic bacterial species, indicator organisms and MST markers detected in the RHRW samples (Table 3 and Figure 2). Results indicated that a significant positive correlation was recorded for E. coli versus L. monocytogenes (r = 0.6738; p = 0.000); and Enterococcus spp. versus the Bacteroides HF183 marker (r = 0.4071; p = 0.043), while a significant negative correlation was recorded for M. tuberculosis versus the Bacteroides HF183 marker (r = −0.4558; p = 0.022) (Table 3). However, despite no significant correlation being observed, based on the cluster analysis, adenovirus was then related to E. coli (r = 0.1938; p = 0.353) and L. monocytogenes (r = 0.2517; p = 0.225) (Figure 2). Additionally, the MST marker Lachnospiraceae clustered with the target pathogens M. tuberculosis (r = −0.0445; p = 0.833) and Yersinia spp. (r = −0.0810; p = 0.700) (Figure 2).
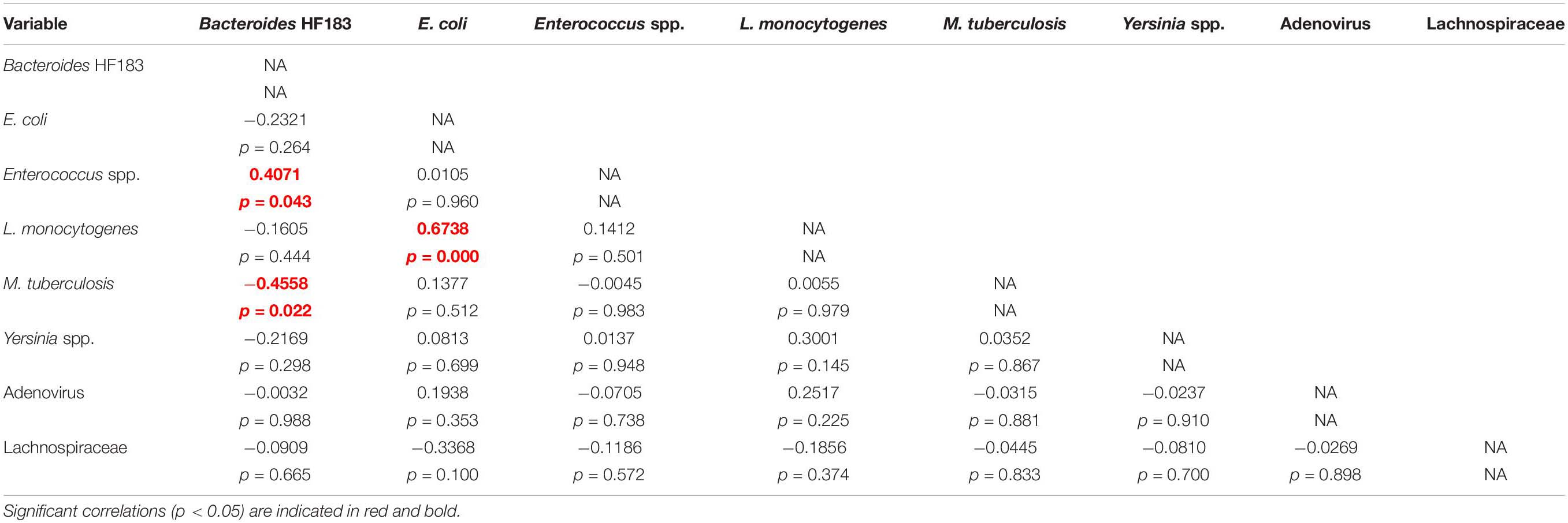
Table 3. Summary of the correlations observed between the target pathogens, MST markers and indicator organisms detected in the RHRW samples.
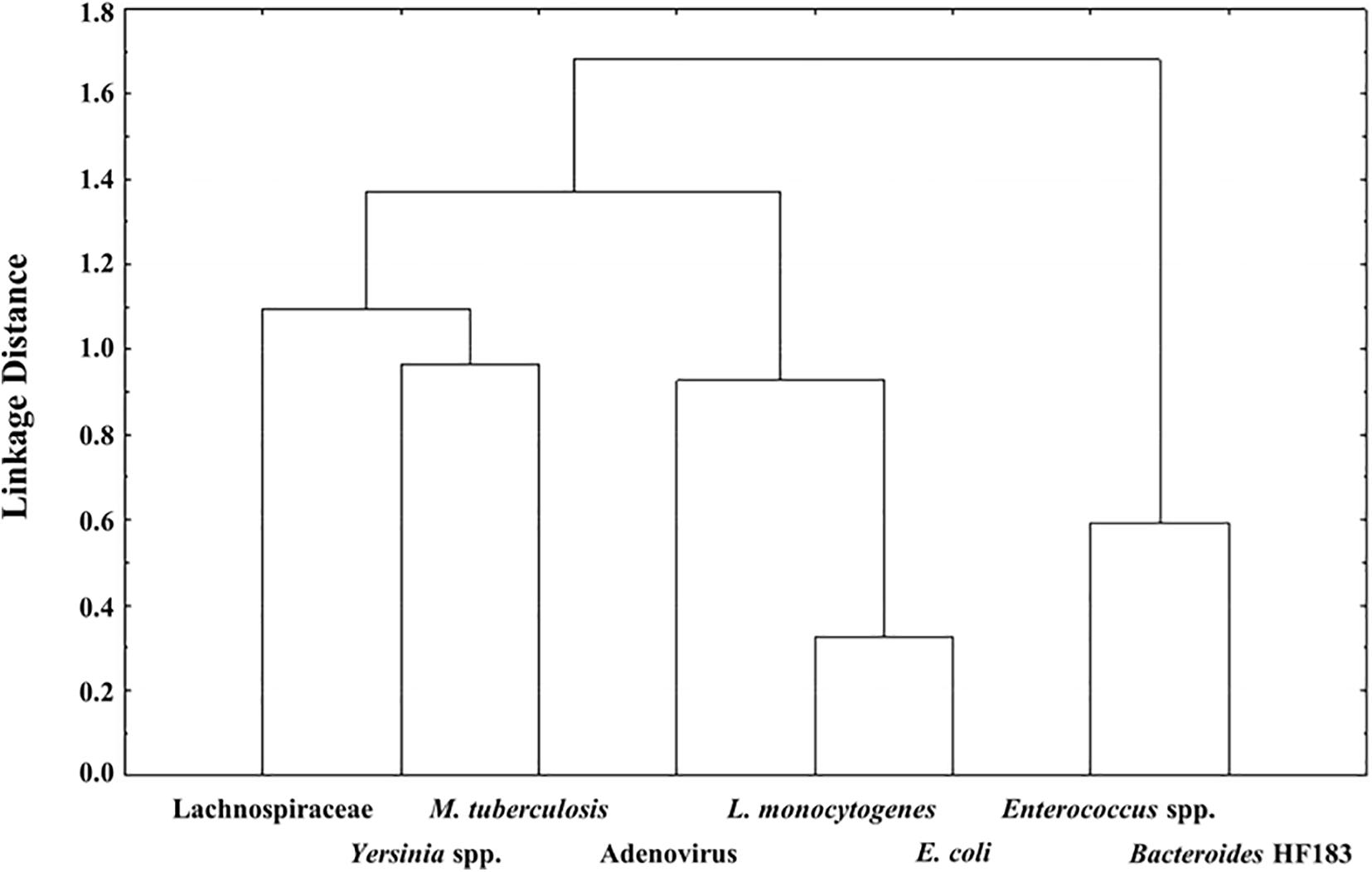
Figure 2. Dendrogram of the Cluster Analysis with Ward’s Methods of target pathogens versus the MST markers and indicator organisms detected in the RHRW samples.
The annual infection risks linked to the utilisation of untreated RHRW for each exposure scenario, based on the presence of pathogenic adenovirus, L. monocytogenes, M. tuberculosis and E. coli (Figure 3) were determined. This was done by comparing all annual risks to a hypothetical benchmark value of 1 × 10–4 which represents the benchmark annual risk of infection for drinking water (Regli et al., 1991).
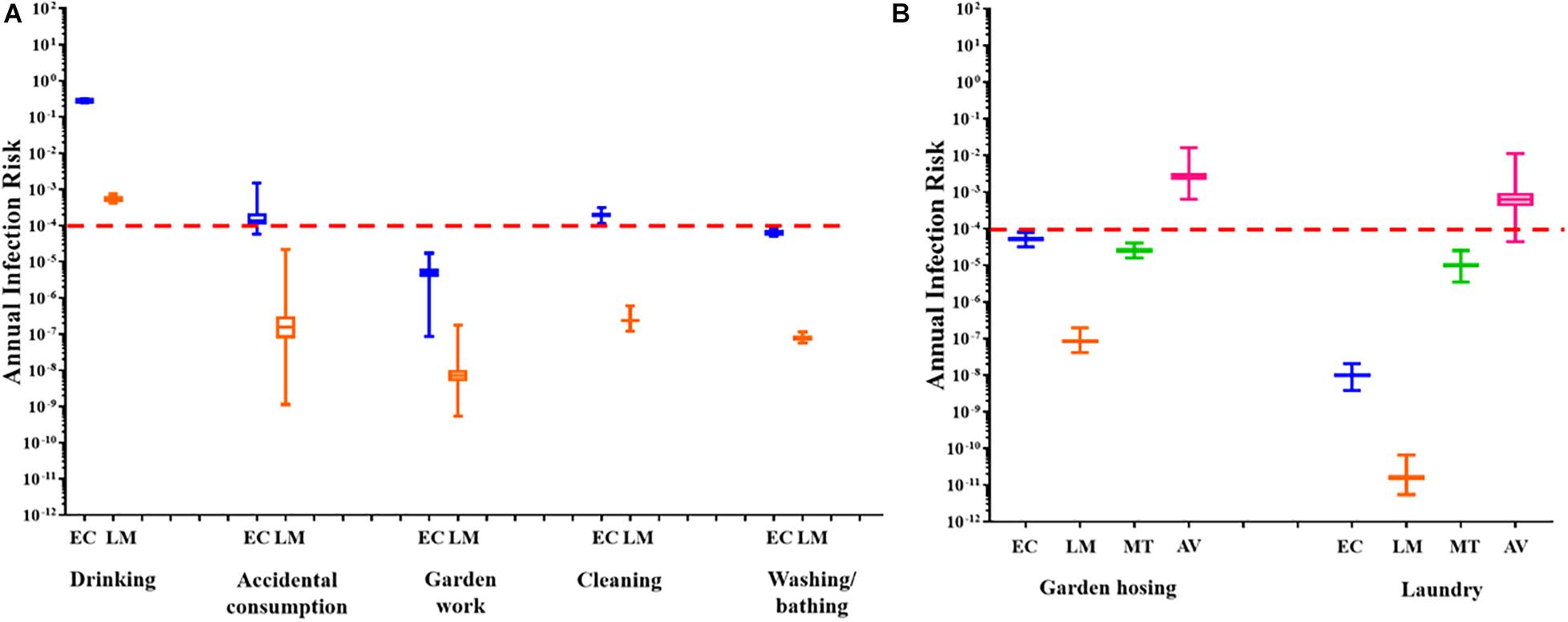
Figure 3. (A) Annual health risk associated with the use of RHRW in the community for ingestion scenarios based on the presence of E. coli (EC; blue) and L. monocytogenes (LM; orange). (B) Annual health risk associated with the use of RHRW in the community for inhalation scenarios based on the presence of E. coli (EC; blue), L. monocytogenes (LM; orange), M. tuberculosis (MT; green) and adenovirus (AV; pink). The whiskers illustrate the minimum and maximum, the outer box illustrates the 1st and 3rd quartiles, and the inner line illustrates the median. The benchmark limit (1 × 10– 4) is indicated by the dashed red line.
For all the sampling sessions, the mean annual risk of infection posed by E. coli for garden hosing (∼10–4), garden work (∼10–5), washing laundry by hand (10–8) and washing/bathing (∼10–4) was below the annual infection risk benchmark limit (Figures 3A,B). Similarly, the mean annual risk of infection posed by L. monocytogenes for garden hosing (∼10–7), garden work (∼10–8), washing laundry by hand (10–11) and washing/bathing (∼10–7) was below the annual infection risk benchmark limit (Figures 3A,B). In comparison, while the annual risk of infection posed by L. monocytogenes for accidental consumption and cleaning of the home were below (∼10–7) the benchmark limit, the annual risk of infection posed by E. coli for these two exposure scenarios exceeded the recommended benchmark limit (∼10–3). Additionally, the annual risk of infection posed by the intentional drinking of RHRW, was exceeded for both E. coli and L. monocytogenes (Figure 3A). The annual risk of infection for M. tuberculosis and adenovirus was only determined for two exposure scenarios: garden hosing (aerosol inhalation) and washing laundry by hand (aerosol ingestion volume was used as a substitute for aerosol inhalation volume to represent a worst-case scenario). Analysis of the mean annual risk of infection posed by M. tuberculosis in the RHRW samples indicated that both garden hosing (∼10–5) and washing laundry by hand (∼10–5) were below the annual infection risk benchmark limit (Figure 3B). In contrast, analysis of the mean annual risk of infection posed by adenovirus in the RHRW samples indicated that both garden hosing (∼10–3) and washing laundry by hand (∼10–3) exceeded the annual infection risk benchmark limit (Figure 3B).
Ethidium monoazide bromide-qPCR analyses indicated that intact (viable) cells of the human pathogens L. monocytogenes and M. tuberculosis were detected in all (100%) the RHRW samples, while Yersinia spp. were detected in 92% of the samples. L. monocytogenes has frequently been detected in soil, plant and surface water samples, as well as in sewage, slaughterhouse waste, human and animal faeces (Weis and Seeliger, 1975; Farber and Peterkin, 1991). Correspondingly, in a study conducted by Lyautey et al. (2007), L. monocytogenes was isolated repeatedly from surface water over a 5-month sampling period using a culture-based selective enrichment and isolation procedure. Similarly, Colburn et al. (1990) isolated L. monocytogenes from 62% (n = 37) of samples extracted from fresh or low-salinity river water, which drained into the Humboldt-Arcata Bay (California). These results emphasise the ability of L. monocytogenes to survive in environments that are characterised by physical (e.g., solar irradiation, temperature) and chemical (e.g., pH, oxygen concentration, nutrients) variation. Additionally, Linke et al. (2014) observed a significant link between the presence of Listeria spp. in soil samples (collected from 12 different areas in Austria from 2007 to 2009) and the abiotic conditions of the soil (e.g., pH, moisture, type of soil) and found that Listeria spp. were more regularly isolated from soil samples characterised by neutral pH, low moisture, or having a consistency made up of sand and humus. Moreover, the same authors noticed that seasonal changes had an effect on the prevalence of Listeria spp. in soil, with the lowest cell counts recorded in July. Several strains of L. monocytogenes have also been found to survive for months to several years in food processing plants, including those used to produce dairy, meat, fish and ready-to-eat products (Ferreira et al., 2014). It is thus hypothesised that the high frequency of detection of the ubiquitous organism L. monocytogenes in the rainwater samples, may be due to its ability to survive across a wide range of temperatures and resist several environmental stresses (Lyautey et al., 2007).
Similar to L. monocytogenes, Yersinia spp. are extensively distributed in the environment with common reservoirs identified as wild rodents, livestock, wild animals, water and soil (Kapperud, 1975; Mollaret et al., 1979). In 2014 and 2019 two outbreaks of yersiniosis were caused by Y. enterocolitica O9 (Norway) and Y. enterocolitica O3 (Sweden and Denmark), respectively, with both outbreaks linked to the consumption of fresh salad/vegetables (MacDonald et al., 2016; Espenhain et al., 2019). Traceback investigations into the outbreak linked to Y. enterocolitica O9 indicated that the factory did not regularly change the water in the rinsing tanks, used for the processing of the salad mixes, which was subsequently identified as the likely cause of the Y. enterocolitica O9 contamination of the food products (MacDonald et al., 2016). Interestingly, Yersinia spp. are carried by most mammals, but generally do not cause serological or histopathological responses in these hosts (Pocock et al., 2001). However, close contact between rodents (e.g., house mice) and humans or livestock, has resulted in rodents being identified as significant vectors of Yersinia spp. infection (Pocock et al., 2001). Therefore, the detection of Yersinia spp. in the RHRW samples is hypothesised to have originated from mice and other rodents’ faecal matter or bodily fluids being deposited on the catchment surface or in the rainwater harvesting tanks.
In comparison, while M. tuberculosis is capable of adapting to hostile or extreme environmental conditions (Cook et al., 2009), the presence and persistence of this bacterium in the environment, and its possible role in the cause and distribution of community-acquired tuberculosis, has been a continuous debate since the start of the 20th century (Velayati et al., 2015). However, it is known that transmission of this bacterium from the environment is possibly due to its ability to persist under various environmental conditions. For example, tuberculosis bacilli have been isolated from wooden tongue depressors over an 88-day time-period, woollen household carpet over a 19-day sampling period, and both dry and moist soil for up to 4 weeks post initial contamination (Velayati et al., 2015). Additionally, it has been suggested that water and soil can become contaminated with M. tuberculosis through sputum from infected individuals (coughing sputum) (Velayati et al., 2015). The presence of M. tuberculosis within the rainwater tank samples may thus have resulted from sputum contaminated soil, or other debris, being deposited into the rainwater harvesting tanks. This is a cause for serious concern as South Africa forms part of the top six countries around the world that are burdened by a high incidence of tuberculosis (Tadokera et al., 2020). Within the Overberg District Municipality (region where the sampling site is located), from 2011 to 2015, tuberculosis-related deaths were reported as the number one cause of mortality for individuals between 25 and 64 years of age (Health Systems Trust, 2019). Additionally, individuals living in poverty-stricken areas have been identified as being at higher risk of contracting tuberculosis, as these individuals generally live in crowded conditions and lack access to basic healthcare (Foster et al., 2015).
Escherichia coli and Enterococcus spp. are generally employed as indicators of faecal pollution by warm-blooded animals (Field and Samadpour, 2007; Harwood et al., 2014) and based on the high frequency of detection (100 and 99%, respectively) of these indicator organisms in the RHRW samples, the hypothesis that the rainwater may be contaminated with the faecal matter of rodents and animals, amongst others, that was deposited on the rooftops or in the gutter systems, is thus confirmed. However, as previously indicated, MST markers are frequently used in combination with traditional indicator organisms, as these markers are able to differentiate between several sources of faecal contamination. Amongst the most promising MST markers are members of the Bacteroides spp. as these organisms are limited to the digestive tract of both humans and warm-blooded animals, where they dominate in the natural gut microflora (Ravaliya et al., 2014) and are subsequently detected in high concentrations in host faecal matter (Fogarty et al., 2003; Ge et al., 2010). Of particular interest is the HF183 marker, which is conserved among Bacteroides strains of human origin and has exhibited high specificity for the detection of human faecal matter and sewage contamination in environmental waters (Harwood et al., 2014). A high frequency of the Bacteroides HF183 marker (76%, n = 25) was subsequently detected within the RHRW samples in the current study. Personal communication with a few residents of the Kleinmond Housing Scheme site indicated that in order to prevent pets from scavenging household waste, garbage bags are regularly placed on top of the rainwater harvesting tanks (Waso et al., 2016). It is thus hypothesised that household waste stored on top of the tanks could potentially have introduced human faecal matter (e.g., from babies nappies/diapers) into the rainwater tanks. Similarly, adenovirus and Lachnospiraceae are ubiquitously distributed in the environment, particularly in areas contaminated with sewage or human faeces (World Health Organisation, 2005; Newton et al., 2011). Therefore, the detection of adenovirus and Lachnospiraceae in the RHRW samples (96 and 80%, respectively) may have also occurred through the introduction of household waste into the rainwater tanks.
The MST markers (Bacteroides HF183, adenovirus and Lachnospiraceae) and traditional indicator organisms (E. coli and Enterococcus spp.) were then statistically correlated to the human pathogenic species (M. tuberculosis, Yersinia spp. and L. monocytogenes) detected in the rainwater. Results showed significant positive correlations for E. coli versus L. monocytogenes (r = 0.6738; p = 0.000); and Enterococcus spp. versus the Bacteroides HF183 marker (r = 0.4071; p = 0.043), while a significant negative correlation was observed for M. tuberculosis versus the Bacteroides HF183 marker (r = −0.4558; p = 0.022) (Table 3). The significant positive correlation recorded between E. coli and L. monocytogenes, could be explained by the fact that these organisms share several common reservoirs (e.g., water, soil, human and animal faeces) and could thus have entered the rainwater tank via a common source. Based on the cluster analysis (Figure 2), adenovirus was then also related to L. monocytogenes and E. coli (albeit not significantly), which is hypothesised to be due to the common occurrence of all three groups in faecal matter (Weis and Seeliger, 1975; Farber and Peterkin, 1991; Pocock et al., 2001; Waso et al., 2018). Similarly, the significant correlation and clustering observed between Enterococcus spp. and the Bacteroides HF183 marker confirms results of previous studies where indicator organisms positively correlated with MST markers (Korajkic et al., 2018; Waso et al., 2018). This is hypothesised to be due to the common occurrence of these indicators and MST markers in the gut of humans and warm-blooded animals, and consequently, in host faecal matter (Field and Samadpour, 2007; Ahmed et al., 2008a; Harwood et al., 2014). Interestingly, a significant negative correlation was observed between M. tuberculosis and the Bacteroides HF183 marker (r = −0.4558; p = 0.022). Research has indicated that during tuberculosis infection and the implementation of subsequent treatment strategies, the gut microbiota is altered significantly (Namasivayam et al., 2018). Consequently, a decrease in the diversity of Bacteroides spp. present in the gut has been observed during M. tuberculosis infection (Namasivayam et al., 2018), which could possibly elucidate the significant negative correlation observed between M. tuberculosis and the Bacteroides HF183 marker in the current study.
A QMRA framework was then applied to assess the health risk associated with the consumption of RHRW containing pathogenic E. coli, adenovirus, L. monocytogenes and M. tuberculosis for potable and several domestic activities (exception of M. tuberculosis and adenovirus where only two potential inhalation exposure scenarios were assessed). Results of the QMRA for L. monocytogenes indicated that the annual benchmark for infection risk was only exceeded for intentional drinking, while the risk associated with the use of the rainwater contaminated with L. monocytogenes for each of the remaining domestic activities was below the annual infection risk benchmark limit (<1 × 10–4). Similarly, for E. coli, the risk associated with the use of the RHRW for the domestic activities garden hosing, garden work, washing laundry by hand and washing/bathing, was below the annual infection risk benchmark limit. In contrast, the risk associated with intentional drinking, accidental consumption and cleaning of the home exceeded the annual infection risk benchmark limit (1 × 10–4) for untreated rainwater and thus posed a possible risk of infection by E. coli. This is concerning as results of a social survey conducted by Dobrowksy et al. (2014) indicated that 70% of individuals residing in the Kleinmond Housing Scheme site use the RHRW for cleaning, whilst 24% use it for drinking (without treatment). Consumption of rainwater contaminated with enteroinvasive E. coli pathotypes and L. monocytogenes could therefore significantly increase the occurrence of gastrointestinal disease in developing countries, particularly in sub-Saharan Africa (Ryan et al., 2014; Robins-Browne et al., 2016). Moreover, it should be noted that while the L. monocytogenes sequence type 6 subtype (predominantly associated with the major listeriosis outbreak in South Africa in 2017 and 2018) (Smith et al., 2019) was not analysed for in the current study, consumption of untreated RHRW could potentially lead to an increase in the number of listeriosis cases in the end-user community.
The annual risk of infection for adenovirus and M. tuberculosis was only calculated for two exposure scenarios (i.e., garden hosing and washing laundry by hand) which are linked to the inhalation of water particles, as infection with human adenovirus and M. tuberculosis bacilli primarily results in respiratory infections rather than gastrointestinal illness (World Health Organisation, 2005; Fennelly and Jones-López, 2015). Although it is possible to contract M. tuberculosis by consuming water contaminated with this bacterium, tuberculosis infection is initiated when droplet nuclei containing M. tuberculosis are inhaled and reach the alveoli of the lungs (Centers for Disease Control and Prevention, 2016). While the QMRA for M. tuberculosis, for garden hosing and washing laundry by hand, was below the annual infection risk benchmark limit, the QMRA for adenovirus exceeded the annual infection risk benchmark limit for both garden hosing (∼10–3) and washing laundry by hand (∼10–3). Adenovirus was selected for the QMRA analysis as this group of viruses are prevalent in high numbers in a wide range of water environments and have shown to be highly resistant to processes of disinfection and purification (Van Heerden et al., 2003). However, while the results obtained for M. tuberculosis are similar to data obtained by Hamilton et al. (2017), who observed that the annual risk of infection posed by the Mycobacterium avium complex (a group of bacteria related to M. tuberculosis), was below the benchmark value of 1 × 10–4, the health risks posed by M. tuberculosis in rainwater need to be further investigated. A significantly high incidence of tuberculosis is reported for the Western Cape region of South Africa and immune-compromised individuals have been identified as highly vulnerable to infection with M. tuberculosis (Sester et al., 2014). The M. tuberculosis QMRA results obtained in the current study may thus be an underestimation of the risk associated for immune-compromised individuals residing in the end-user communities, who rely on RHRW as a primary water source.
The frequent detection of L. monocytogenes, M. tuberculosis and Yersinia spp. in the RHRW samples verifies that human pathogenic species are able to survive in rainwater which can pose a serious health risk to low- and middle-income communities, who routinely utilise RHRW as a sustainable water source. In addition, results of the correlation analysis confirm that traditional indicator organisms and MST markers should be used in combination to monitor RHRW quality, as both indicator groups correlated with the human pathogens (i.e., E. coli versus L. monocytogenes; M. tuberculosis versus Bacteroides HF183), as well as with each other (i.e., Enterococcus spp. versus Bacteroides HF183). Nonetheless, additional research should be conducted to assess the correlation of a broader range of human pathogenic species to the presence of several indicator organism groups (e.g., total coliforms, faecal coliforms) and MST markers (e.g., polyomavirus, Bifidobacterium spp., human mitochondrial DNA), in order to fully elucidate the environmental distribution and relationships between the various indicator groups and human pathogens.
The QMRA analysis then indicated that the use of RHRW containing L. monocytogenes, adenovirus, and E. coli poses a health risk to end-user communities, particularly when used for intentional drinking (E. coli and L. monocytogenes), cleaning of the home (E. coli), garden hosing (adenovirus), washing laundry by hand (adenovirus), or when accidentally consumed (E. coli). However, while the QMRA results indicated that the concentration of M. tuberculosis obtained in the current study did not pose a health-risk to the end-user community, further research should be conducted, taking into consideration the approximate percentage of immune-compromised individuals living in South Africa and who utilise RHRW, in order to accurately estimate the risk associated with the use of RHRW for potable and domestic activities. This can ultimately determine or predict the potential of various available point-of-use treatment technologies (e.g., filtration, solar disinfection, chlorination, solar pasteurisation) to effectively reduce the estimated health risk to within the benchmark limit.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JD, BR, and WK conceived and designed the experiments. JD performed the experiments. JD, BR, and MW analysed the data. WK and SK contributed reagents, materials, and analysis tools. JD, BR, MW, and WK compiled the manuscript. SK edited the manuscript. All authors contributed to the article and approved the submitted version.
This project has received funding from the European Union’s Horizon 2020 – Research and Innovation Programme under grant agreement no. 688928 (WATERSPOUTT H2020-Water-5c). The authors acknowledge the financial assistance provided by the National Research Foundation (NRF) of South Africa toward the student scholarship. Opinions communicated and all conclusions arrived at are those of the authors and are not necessarily to be attributed to the NRF.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.659784/full#supplementary-material
Ahmed, W., Goonetilleke, A., and Gardner, T. (2008a). Alternative indicators for detection and quantification of faecal pollution. Water (Australia) 39, 46–49. doi: 10.HH/j.l472-765X.2007.02287.x
Ahmed, W., Hamilton, K., Gyawali, P., Toze, S., and Haas, C. (2016). Evidence of avian and possum fecal contamination in rainwater tanks as determined by microbial source tracking approaches. Appl. Environ. Microbiol. 82, 4379–4386. doi: 10.1128/AEM.00892-16
Ahmed, W., Harwood, V., Nguyen, K., Young, S., Hamilton, K., and Toze, S. (2015). Utility of Helicobacter spp. associated GFD markers for detecting avian faecal pollution in natural waters of two continents. Water Res. 88, 613–622. doi: 10.1016/j.watres.2015.10.050
Ahmed, W., Huygens, F., Goonetilleke, A., and Gardner, T. (2008b). Real-time PCR detection of pathogenic microorganisms in roof-harvested rainwater in Southeast Queensland. Australia. Appl. Environ. Microbiol. 74, 5490–5496. doi: 10.1128/AEM.00331-08
Ahmed, W., Staley, C., Hamilton, K. A., Beale, D. J., Sadowsky, M. J., Toze, S., et al. (2017). Amplicon-based taxonomic characterization of bacteria in urban and peri-urban roof-harvested rainwater stored in tanks. Sci. Total Environ. 576, 326–334. doi: 10.1016/j.scitotenv.2016.10.090
Bosio, C. F., Jarrett, C. O., Scott, D. P., Fintzi, J., and Hinnebusch, J. (2020). Comparison of the transmission efficiency and plague progression dynamics associated with two mechanisms by which fleas transmit Yersinia pestis. PLoS Pathogens 16:e1009092. doi: 10.1371/journal
Bradshaw, J. K., Snyder, B. J., Oladeinde, A., Spidle, D., Berrang, M. E., Meinersmann, R. J., et al. (2016). Characterizing relationships among fecal indicator bacteria, microbial source tracking markers, and associated waterborne pathogen occurrence in stream water and sediments in a mixed land use watershed. Water Res. 101, 498–509. doi: 10.1016/j.watres.2016.05.014
Buchanan, R. L., Damert, W. G., Whiting, R. C., and van Schothorst, M. (1997). Use of epidemiologic and food survey data to estimate a purposefully conservative dose-response relationship for Listeria monocytogenes levels and incidence of listeriosis. J. Food Prot. 60, 918–922. doi: 10.4315/0362-028x-60.8.918
Buchanan, R. L., Smith, J. L., and Long, W. (2000). Microbial risk assessment: dose-response relations and risk characterisation. Int. J. Food Microbiol. 58, 159–172. doi: 10.1016/s0168-1605(00)00270-1
Centers for Disease Control and Prevention (2016). Latent Tuberculosis Infection Resources. Available online at: https://www.cdc.gov/tb/. (Accessed March 13, 2021)
Colburn, K. G., Kaysner, C. A., Abeyta, C., and Wekell, M. M. (1990). Listeria species in a California coast estuarine environment. Appl. Environ. Microbiol. 56, 2007–2011. doi: 10.1128/AEM.56.7.2007-2011.1990
Cook, G. M., Berney, M., Gebhard, S., Heinemann, M., Cox, R. A., Danilchanka, O., et al. (2009). Physiology of Mycobacteria. Adv. Microb. Physiol. 55, 81–182,318–9. doi: 10.1016/S0065-2911(09)05502-7
Couch, R. B., Cate, T. R., Douglas, R. G., Gerone, P. J., and Knight, V. (1966). Effect of route of inoculation on experimental respiratory viral disease in volunteers and evidence for airborne transmission. Microbiol. Mol. Biol. Rev. 30, 517–529.
De Kwaadsteniet, M., Dobrowsky, P. H., van Deventer, A., Khan, W., and Cloete, T. E. (2013). Domestic rainwater harvesting: Microbial and chemical water quality and point-of-use treatment systems. Water Air Soil Pollut. 224, 961–971. doi: 10.1007/s11270-013-1629-7
Dobrowksy, P. H., Mannel, D., De Kwaadsteniet, M., Prozesky, H., Khan, W., and Cloete, T. E. (2014). Quality assessment and primary uses of harvested rainwater in Kleinmond. South Africa. Water SA 40, 401–406. doi: 10.4314/wsa.v40i3.2
Dobrowsky, P. H., De Kwaadsteniet, M., Cloete, T. E., and Khan, W. (2014). Distribution of indigenous bacterial pathogens and potential pathogens associated with roof-harvested rainwater. Appl. Environ. Microbiol. 80, 2307–2316. doi: 10.1128/AEM.04130-13
Dobrowsky, P. H., Khan, S., Cloete, T. E., and Khan, W. (2016). Molecular detection of Acanthamoeba spp., Naegleria fowleri and Vermamoeba (Hartmannella) vermiformis as vectors for Legionella spp. in untreated and solar pasteurized harvested rainwater. Parasit. Vectors 9:539. doi: 10.1186/s13071-016-1829-2
Dobrowsky, P. H., Lombard, M., Cloete, W. J., Saayman, M., Cloete, T. E., Carstens, M., et al. (2015). Efficiency of microfiltration systems for the removal of bacterial and viral contaminants from surface and rainwater. Water Air Soil Pollut. 226:33. doi: 10.1007/s11270-015-2317-6
Eisen, R. J., Bearden, S. W., Wilder, A. P., Montenieri, J. A., Antolin, M. F., and Gage, K. L. (2006). Early-phase transmission of Yersinia pestis by unblocked fleas as a mechanism explaining rapidly spreading plague epizootics. Proc. Natl. Acad. Sci. U.S.A. 103, 15380–15385. doi: 10.1073/pnas.0606831103
Emerson, J. B., Adams, R. I., Betancourt Román, C. M., Coil, D. A., Dahlhausen, K., Ganz, H. H., et al. (2017). Schrödinger’s microbes: tools for distinguishing the living from the dead in microbial ecosystems. Microbiome. 5, 86. doi: 10.1186/s40168-017-0285-3
Espenhain, L., Riess, M., Müller, L., Colombe, S., Ethelberg, S., Litrup, E., et al. (2019). Cross-border outbreak of Yersinia enterocolitica O3 associated with imported fresh spinach, Sweden and Denmark, March 2019. Euro Surveill. 24:1900368. doi: 10.2807/1560-7917.ES.2019.24.24.1900368
Farber, J. M., and Peterkin, P. I. (1991). Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55, 476–511. doi: 10.1016/B978-0-12-811444-5.00006-3
Fennelly, K. P., and Jones-López, E. (2015). Quantity and quality of inhaled dose predicts immunopathology in tuberculosis. Front. Microbiol. 6:313. doi: 10.3389/fimmu.2015.00313
Ferreira, V., Wiedmann, M., Teixeira, P., and Stasiewicz, W. J. (2014). Listeria monocytogenes persistence in food-associated environments: epidemiology, strain characteristics, and implications for public health. J. Food Prot. 77, 150–170. doi: 10.4315/0362-028X.JFP-13-150
Field, K. G., and Samadpour, M. (2007). Fecal source tracking, the indicator paradigm, and managing water quality. Water Res. 41, 3517–3538. doi: 10.1016/j.watres.2007.06.056
Fogarty, L., Haack, S., Wolcott, M., and Whitman, R. (2003). Abundance and characteristics of the recreational water quality indicator bacteria Escherichia coli and enterococci in gull feces. J. Appl. Microbiol. 94, 865–878. doi: 10.1046/j.1365-2672.2003.01910.x
Foster, N., Vassal, A., Cleary, S., Cunnama, L., Churchyard, G., and Sinanovic, E. (2015). The economic burden of TB diagnosis and treatment in South Africa. Soc. Sci. Med. 130, 42–50. doi: 10.1016/j.socscimed.2015.01.046
Frahm, E., and Obst, U. (2003). Application of the fluorogenic probe technique (TaqMan PCR) to the detection of Enterococcus spp. and Escherichia coli in water samples. J. Microbiol. Methods. 52, 123–131. doi: 10.1016/s0167-7012(02)00150-1
Ge, Z., Nevers, M. B., Schwab, D. J., and Whitman, R. L. (2010). Coastal loading and transport of Escherichia coli at an embayed beach in Lake Michigan. Environ. Sci. Technol. 44, 6731–6737. doi: 10.1021/es100797r
Germini, A., Masola, A., Carnevali, P., and Marchelli, R. (2009). Simultaneous detection of Escherichia coli O175:H7. Salmonella spp., and Listeria monocytogenes by multiplex PCR. Food Control 20, 733–738. doi: 10.1016/j.foodcont.2008.09.010
Gould, J., and Nissen-Petersen, E. (1999). Rainwater Catchment Systems for Domestic Supply. London: Intermediate Technology Publications.
Haas, C. N., Rose, J. B., and Gerba, C. P. (1999). Quantitative Microbial Risk Assessment, 2nd Edn. New York, NY: John Wiley & Sons, Inc.
Hamilton, K., Reyneke, B., Waso, M., Clements, T., Ndlovu, T., Khan, W., et al. (2019). A global review of the microbiological quality and potential health risks associated with roof-harvested rainwater tanks. npj Clean Water 2:7. doi: 10.1038/s41545-019-0030-5
Hamilton, K. A., Ahmed, W., Toze, S., and Haas, C. N. (2017). Human health risks for Legionella and Mycobacterium avium complex (MAC) from potable and non-potable uses of roof-harvested rainwater. Water Res. 119, 288–303. doi: 10.1016/j.watres.2017.04.004
Harwood, V. J., Levine, A. D., Scott, T. M., Chivukula, V., Lukasik, J., Farrah, S. R., et al. (2005). Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl. Environ. Microbiol. 71, 3163–3170. doi: 10.1128/AEM.71.6.3163-3170.2005
Harwood, V. J., Staley, C., Badgley, B. D., Borges, K., and Korajkic, A. (2014). Microbial source tracking markers for detection of fecal contamination in environmental waters: relationships between pathogens and human health outcomes. FEMS Microbiol. Rev. 38, 1–40. doi: 10.1111/1574-6976.12031
Health Systems Trust. (2019). Western Cape Province: Population Distribution, Sub-district Boundaries and Health Facility Locations. Available online at: https://www.hst.org.za/publications/District%20Health%20Barometers/16%20(Section%20B)%20Western%20Cape%20Province%20DHP20172018.pdf (Accessed March 18, 2021)
Heim, A., Ebnet, C., Harste, G., and Pring-Åkerblom, P. (2003). Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 70, 228–239. doi: 10.1002/jmv.10382
Hinnebusch, B. J., Jarrett, C. O., and Bland, D. M. (2017). “Fleaing” the plague: adaptations of Yersinia pestis to its insect vector that lead to transmission. Annu. Rev. Microbiol. 71, 215–232. doi: 10.1146/annurev-micro-090816-093521
Jones, M. R., Masago, Y., Bartrand, T., Haas, C. N., Nicas, M., and Rose, J. B. (2009). Characterising the risk of infection from Mycobacterium tuberculosis in commercial passenger aircraft using quantitative microbial risk assessment. Risk Anal. 29, 355–365. doi: 10.1111/j.1539-6924.2008.01161.x
Jones, R. M., and Su, Y. (2015). Dose-response models for selected respiratory infectious agents: Bordetella pertussis, group a Streptococcus, rhinovirus and respiratory syncytial virus. BMC Infect. Dis. 15:90. doi: 10.1186/s12879-015-0832-0
Jongman, M., and Korsten, L. (2016). Microbial quality and suitability of roof-harvested rainwater in rural villages for crop irrigation and domestic use. J. Water Health 14, 961–971. doi: 10.2166/wh.2016.058
Kapperud, G. (1975). Yersinia enterocolitica in small rodents from Norway, Sweden and Finland. APMIS 83, 335–342. doi: 10.1111/j.1699-0463.1975.tb00110.x
Kaushik, R., Balasubramanian, R., and Dunstan, H. (2014). Microbial quality and phylogenetic diversity of fresh rainwater and tropical freshwater reservoir. PLoS One 9:e100737. doi: 10.1371/journal.pone.0100737
King, H. C., Kera-Butler, T., James, P., Oakley, B. B., Erenso, G., Aseffa, A., et al. (2017). Environmental reservoirs of pathogenic Mycobacteria across the Ethiopian biogeographical landscape. PLoS One 12:e0173811. doi: 10.1371/journal.pone.0173811
Korajkic, A., McMinn, B. R., and Harwood, V. J. (2018). Relationships between microbial indicators and pathogens in recreational water settings. Int. J. Environ. Res. Public Health 15:2842. doi: 10.3390/ijerph15122842
Kox, L. F. F., Rhienthong, D., Miranda, A. M., Udomsantisuk, N., Ellis, K., van Leeuwen, J., et al. (1994). A more reliable PCR for detection of Mycobacterium tuberculosis in clinical samples. J. Clin. Microbiol. 32, 672–678. doi: 10.1128/jcm.32.3.672-678.1994
Linke, K., Ruckerl, I., Brugger, K., Karpiskova, R., Walland, J., Muri-Klinger, S., et al. (2014). Reservoirs of Listeria species in three environmental ecosystems. Appl. Environ. Microbiol. 80, 5583–5592. doi: 10.1128/AEM.01018-14
Low, C. S. (2002). Appropriate Microbial Indicator Tests for Drinking Water in Developing Countries and Assessment of Ceramic Water Filters. Ph.D. dissertation. Massachusetts, MA: Massachusetts Institute of Technology.
Lyautey, E., Lapen, D. R., Wilkes, G., McCleary, K., Pagotto, F., Tyler, K., et al. (2007). Distribution and characteristics of Listeria monocytogenes isolates from surface waters of the South Nation River Watershed, Ontario, Canada. Appl. Environ. Microbiol. 73, 5401–5410. doi: 10.1128/AEM.00354-07
MacDonald, E., Einöder-Moreno, M., Borgen, K., Brandal, L. T., Diab, L., and Fossli, O. (2016). National outbreak of Yersinia enterocolitica infections in military and Civilian populations associated with consumption of mixed salad, Norway, 2014. Euro Surveill. 21:34. doi: 10.2807/1560-7917.ES.2016.21.34.30321
McLellan, S. L., Newton, R. J., Vandewalle, J. L., Shanks, O. C., Huse, S. M., Eren, A. M., et al. (2013). Sewage reflects the distribution of human faecal Lachnospiraceae. Environ. Microbiol. 15, 2213–2227. doi: 10.1111/1462-2920.12092
Mollaret, H. H., Bercovier, H., and Alonso, J. M. (1979). Summary of data received at the WHO reference centre for Yersinia enterocolitica. Contrib. Microbiol. Immunol. 5, 174–184.
Namasivayam, S., Sher, A., Glickman, M. S., and Wipperman, M. F. (2018). The microbiome and tuberculosis: early evidence for cross talk. mBio 9, e01420-18. doi: 10.1128/mBio.01420-18
National Center for Biotechnology Information (NCBI) (2020). COVID-19 is an Emerging, Rapidly Evolving Situation. Available online at: https://www.ncbi.nlm.nih.gov/ (Accessed October 28, 2020)
Newton, R. J., Vandewalle, J. L., Borchardt, M. A., Gorelick, M. H., and McLellan, S. L. (2011). Lachnospiraceae and Bacteroidales alternative fecal indicators reveal chronic human sewage contamination in an urban harbor. Appl. Environ. Microbiol. 77, 6972–6981. doi: 10.1128/aem.05480-11
Owens, C. E. L., Angles, M. L., Cox, P. T., Byleveld, P. M., Osborne, N. J., and Rahman, B. (2020). Implementation of quantitative microbial risk assessment (QMRA) for public drinking water supplies: systematic review. Water Res. 174:115614. doi: 10.1016/j.watres.2020.115614
Pechous, R. D., Sivaraman, V., Stasulli, N. M., and Goldman, W. E. (2016). Pneumonic plague: the darker side of Yersinia pestis. Trends Microbiol. 24, 190–197. doi: 10.1016/j.tim.2015.11.008
Pecson, B. M., Triolo, S. C., Olivieri, S., Chen, E. C., Pisarenko, A. N., Yang, C., et al. (2017). Reliability of pathogen control in direct potable reuse: performance evaluation and QMRA of a full-scale 1 MGD advanced treatment train. Water Res. 122, 258–268. doi: 10.1016/j.watres.2017.06.014
Pocock, M. J. O., Searle, J. B., Betts, W. B., and White, P. C. L. (2001). Patterns of infection by Salmonella and Yersinia spp. in commensal house mouse (Mus musculus domesticus) populations. J. Appl. Microbiol. 90, 755–760. doi: 10.1046/j.1365-2672.2001.01303.x
Ravaliya, K., Gentry-Shields, J., Garcia, S., Heredia, N., Fabiszewski de Aceituno, A., Bartz, F. E., et al. (2014). Use of Bacteroidales microbial source tracking to monitor fecal contamination in fresh produce production. Appl. Env. Microbiol. 80, 612–617. doi: 10.1128/AEM.02891-13
Regli, S., Rose, J. B., Haas, C. N., and Gerba, C. P. (1991). Modelling the risk from Giardia and viruses in drinking water. J. Am. Water Works Assoc. 83, 76–84.
Reyneke, B., Hamilton, K. A., Fernández-Ibáñez, P., Polo-López, M. I., McGuigan, K. G., Khan, S., et al. (2020). EMA-Illumina 16S metagenomic sequencing informs risk assessment analysis of water treatment systems. Sci. Total Environ. 743:140717. doi: 10.1016/j.scitotenv.2020.140717
Reyneke, B., Ndlovu, T., Khan, S., and Khan, W. (2017). Comparison of EMA-, PMA- and DNase qPCR for the determination of microbial cell viability. Appl. Microbiol. Biotechnol. 101, 7371–7383. doi: 10.1007/s00253-017-8471-6
Robins-Browne, R. M., Holt, K. E., Ingle, D. J., Hocking, D. M., Yang, J., and Tauschek, M. (2016). Are Escherichia coli pathotypes still relevant in the era of whole-genome sequencing? Front. Cell. Infect. Microbiol. 6:141. doi: 10.3389/fcimb.2016.00141
Rusiñol, M., Moriarty, E., Lin, S., Bofill-Mas, S., and Gilpin, B. (2016). Human-, ovine-, and bovine-specific viral source tracking tools to discriminate between the major fecal sources in agricultural waters. Food Environ Virol. 8, 34–45. doi: 10.1007/s12560-015-9223-3
Ryan, M. O., Haas, C. N., Gurian, P. L., Gerba, C. P., Panzl, B. M., and Rose, J. B. (2014). Application of quantitative microbial risk assessment for selection of microbial reduction targets for hard surface disinfectants. Am. J. Infect. Control. 42, 1165–1172. doi: 10.1016/j.ajic.2014.07.024
Sano, D., Haas, C. N., and Rose, J. B. (2019). A QMRA Framework for Sanitation Treatment Decisions. Available online at: https://www.waterpathogens.org/book/a-QMRA-framework-for-sanitation-treatment -decisions (Accessed March 12, 2020)
Savichtcheva, O., Okayama, N., and Okabe, S. (2007). Relationships between Bacteroides 16S rRNA genetic markers and presence of bacterial enteric pathogens and conventional fecal indicators. Water Res. 41, 3615–3628. doi: 10.1016/j.watres.2007.03.028
Schijven, J., Teunis, P., Suylen, T., Ketelaars, H., Hornstra, L., and Rutjes, S. (2019). QMRA of adenovirus in drinking water at a drinking water treatment plant using UV and chlorine dioxide disinfection. Water Res. 158, 34–45. doi: 10.1016/j.watres.2019.03.090
Sester, M., van Leth, F., Bruchfeld, J., Bumbacea, D., Cirillo, D. M., Dilektasli, A. G., et al. (2014). Risk assessment of tuberculosis in immunocompromised patients–A TBNET study. Am. J. Respir. Crit. Care Med. 190:10. doi: 10.1164/rccm.201405-0967OC
Seurinck, S., Defoirdt, T., Verstraete, W., and Siciliano, S. D. (2005). Detection and quantification of the human-specific HF183 Bacteroides 16S rRNA genetic marker with real-time PCR for assessment of human fecal pollution in freshwater. Environ. Microbiol. 7, 249–259. doi: 10.1111/j.1462-2920.2004.00702.x
Sidhu, J. P., Ahmed, W., Gernjak, W., Aryal, R., McCarthy, D., Palmer, A., et al. (2013). Sewage pollution in urban stormwater runoff as evident from the widespread presence of multiple microbial and chemical source tracking markers. Sci. Total Environ. 463, 488–496. doi: 10.1016/j.scitotenv.2013.06.020
Smith, A. M., Tau, N. P., Smouse, S. L., Allam, M., Ismail, A., Ramalwa, N. R., et al. (2019). Outbreak of Listeria monocytogenes in South Africa, 2017-2018: laboratory activities and experiences associated with whole-genome sequencing analysis of isolates. Foodborne Pathog. Dis. 16, 524–530. doi: 10.1089/fpd.2018.2586
Stenkova, A. M., Issaeva, M., and Rasskazov, V. A. (2008). Development of a multiplex PCR procedure for detection of Yersinia genus with identification of pathogenic species (Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica). Mol. Gen. Microbiol. Virol. 23, 119–125. doi: 10.3103/S0891416808030038
Stoddard, S. F., Smith, B. J., Hein, R., Roller, B. R. K., and Schmidt, T. M. (2015). rrnDB: Improved Tools for Interpreting rRNA Gene Abundance in Bacteria and Archaea and a new Foundation for Future Development. Available online at: https://rrndb.umms.med.umich.edu (Accessed October 15, 2020).
Strauss, A., Reyneke, B., Waso, M., Ndlovu, T., Brink, C. J., Khan, S., et al. (2019). EMA-amplicon-based taxonomic characterisation of the viable bacterial community present in untreated and SODIS treated roof-harvested rainwater. Environ. Sci. Water Res. and Technol. 5, 91–101. doi: 10.1039/C8EW00613J
Tadokera, R., Bekker, L., Kreiswirth, B. N., Mathema, B., and Middelkopp, K. (2020). TB transmission is associated with prolonged stay in a low-socioeconomic high burdened TB and HIV community in Cape Town, South Africa. BMC Infect. Dis. 20:120. doi: 10.1186/s12879-020-4828-z
Tilevik, A. (2017). Evaluation of Clustering Methods for Analyzing Drug Cytokine profiles. Ph.D. thesis. Sweden, SE: Karlstad University.
United Nations (2018). Sustainable Development Goal 6: Synthesis Report on Water and Sanitation. New York, NY: United Nations Publications.
Van Heerden, J., Ehlers, M. M., Van Zyl, W. B., and Grabow, W. O. K. (2003). Incidence of adenoviruses in raw and treated water. Water Res. 37, 3704–3708. doi: 10.1016/S0043-1354(03)00245-8
Velayati, A. A., Farnia, P., Mozafari, M., Malekshahian, D., Farahbod, A. M., Seif, S., et al. (2015). Identification and genotyping of Mycobacterium tuberculosis isolated from water and soil samples of a metropolitan city. Chest 147, 1094–1102. doi: 10.1378/chest.14-0960
Viau, E. J., and Boehm, A. B. (2011). Quantitative PCR-based detection of pathogenic Leptospira in Hawai’ian coastal streams. J. Water Health 9, 637–646. doi: 10.2166/wh.2011.064
Wall, S., Ghanekar, K., McFadden, J., and Dale, J. W. (1999). Context-sensitive transposition of IS6110 in Mycobacteria. Microbiology 145, 3169–3176. doi: 10.1099/00221287-145-11-3169
Waso, M., Khan, S., and Khan, W. (2018). Microbial source tracking markers associated with domestic rainwater harvesting systems: correlation to indicator organisms. Environ. Res. 161, 446–455. doi: 10.1016/j.envres.2017.11.043
Waso, M., Ndlovu, T., Dobrowsky, P. H., Khan, S., and Khan, W. (2016). Presence of microbial and chemical source tracking markers in roof-harvested rainwater and catchment systems for the detection of fecal contamination. Environ. Sci. Pollut. Res. 23, 16987–17001. doi: 10.1007/s11356-016-6895-7
Weis, J., and Seeliger, H. P. R. (1975). Incidence of Listeria monocytogenes in nature. Appl. Microbiol. 30, 29–32.
World Health Organisation (WHO) (2004). “Pathogenic Mycobacteria in water–a guide to public health consequences, monitoring and management,” in Emerging Issues in Water and Infectious Disease Series, eds S. Pedley, J. Bartram, G. Rees, A. Dufour, and J. Cotruvo (London: IWA Publishing).
World Health Organisation (WHO) (2005). Recreation and Disease. Plausibility of Associated Infections: Acute Effects, Sequelae and Mortality. Geneva: World Health Organisation.
World Health Organisation (WHO) (2019). Global Tuberculosis Report 2019. Geneva: World Health Organization.
Keywords: microbial source tracking markers, human pathogenic bacteria, traditional indicator organisms, QMRA, rainwater
Citation: Denissen JK, Reyneke B, Waso M, Khan S and Khan W (2021) Human Pathogenic Bacteria Detected in Rainwater: Risk Assessment and Correlation to Microbial Source Tracking Markers and Traditional Indicators. Front. Microbiol. 12:659784. doi: 10.3389/fmicb.2021.659784
Received: 28 January 2021; Accepted: 09 April 2021;
Published: 07 May 2021.
Edited by:
Georg H. Reischer, Vienna University of Technology, AustriaReviewed by:
Svetoslav Todorov, University of São Paulo, BrazilCopyright © 2021 Denissen, Reyneke, Waso, Khan and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wesaal Khan, d2VzYWFsQHN1bi5hYy56YQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.