
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 15 March 2021
Sec. Infectious Agents and Disease
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.651217
This article is part of the Research Topic Coinfections of Lyme Disease and Other Tick-Borne Diseases View all 5 articles
The successful treatment of Lyme disease (LD) is contingent on accurate diagnosis. However, current laboratory detection assays lack sensitivity in the early stages of the disease. Because delayed diagnosis of LD incurs high healthcare costs and great suffering, new highly sensitive tests are in need. To overcome these challenges, we developed an internally controlled quantitative PCR (Ter-qPCR) that targets the multicopy terminase large subunit (terL) gene encoded by prophages that are only found in LD-causing bacteria. The terL protein helps phages pack their DNA. Strikingly, the detection limit of the Ter-qPCR was analytically estimated to be 22 copies and one bacterial cell in bacteria spiked blood. Furthermore, significant quantitative differences was observed in terms of the amount of terL detected in healthy individuals and patients with either early or late disease. Together, the data suggests that the prophage-targeting PCR has significant power to improve success detection for LD. After rigorous clinical validation, this new test could deliver a step-change in the detection of LD. Prophage encoded markers are prevalent in many other pathogenic bacteria rendering this approach highly applicable to bacterial identification in general.
Lyme disease (LD) is the most common tick-born disease with approximately 476,000 patients in the United States annually during 2010–2018 (Kugeler et al., 2021). LD is caused by a group of bacteria classified together as the Borrelia burgdorferi sensu lato (s.l.) complex, that comprises a clade of more than 20 species including B. burgdorferi sensu stricto (s.s.) which dominates in United States, and B. garinii and B. afzelii which are prevalent in Europe and Asia. The LD-causing bacteria are generally transmitted to humans after they are bitten by ticks of the Ixodes family infected with LD causing Borrelia. However, recent reports have raised concerns over Borrelia transmission through blood transfusion based on observations that Borrelia can survive and circulate in the human bloodstream (Pavia and Plummer, 2018).
Currently, LD diagnosis is based on the overt clinical manifestation of disease in the form of erythema migrans (EM) skin lesions, commonly known as a ‘bull’s-eye’ rash and a history of tick exposure. Although EM lesions occur in 70 to 80% of infected individuals, only a third of these patients develop the classic ‘bull’s-eye’ rash, and many other types of skin lesions can occur which are easily confused with EM (Chaaya et al., 2016). In addition to the EM uncertainty, other common symptoms of LD such as fatigue, muscle pain, headache, and perceived cognitive dysfunction largely overlap with an array of other diseases, including other tick-borne diseases. One such example is Relapsing Fever (RF), which is caused by close relatives of the LD-causing bacteria, such as Borrelia miyamotoi (Wormser et al., 2019). The two Borrelia ‘groups’ responsible for LD and RF have caused great concern and clinical confusion, as they are morphologically similar and present with almost indistinguishable clinical symptoms (Bergström and Normark, 2018). Despite this, they respond to different antibiotics and treatment regimens (Koetsveld et al., 2017). Another example of confusion surrounding LD is the co-infection caused by Bartonella spp. This genus of bacteria is emerging as an increasingly common human infection (Anderson and Neuman, 1997). Much of the controversy surrounding LD and co-infections with Bartonella and/or B. miyamotoi is due to the lack of a reliable and sensitive diagnostic method to detect and distinguish between the three groups of bacteria, the LD and RF causing Borrelia and Bartonella (Schutzer et al., 2019). Therefore, laboratory tests to determine and distinguish between LD and co-infections play a vital role in the correct diagnosis and consequent treatment with different antibiotics.
Scientists have faced several challenges with LD detection including patients presenting with a delayed antibody response and a low number of Borrelia cells typically found in human clinical samples (Moore et al., 2016). Although it is particularly difficult to diagnose LD early, it is critical, as it is far easier to treat the disease when it is detected at an early stage (Theel et al., 2019). Bacteria-targeting approaches, such as polymerase chain reaction (PCR) detecting the Borrelia chromosomal DNA, can potentially identify early LD but is relatively insensitive detecting only between 30-50% of positive cases, and is therefore deemed to have little clinical utility (Schutzer et al., 2019). The reasons behind the poor sensitivity of the current PCR methods in Lyme detection are twofold; first, the current PCRs target Borrelia genomic DNA regions that have only one copy in each bacterium, such as the bacterial 16S rRNA gene, RecA gene, and the 5S-23S intergenic regions (Brettschneider et al., 1998; Liveris et al., 2012; Waddell et al., 2016; Lohr et al., 2018; Schutzer et al., 2019). Second, at least some Borrelia species are ‘tissue-bound’ and are only transiently found circulating in the blood (Liang et al., 2020).
In response to these diagnostic challenges, we adopted a novel approach, taking advantage of the fact that most pathogenic bacteria carry multiple complete or partial prophages (phages associated with bacteria) (Argov et al., 2019). These prophage sequences can form the bases of a template from which quantitative PCR (qPCR) primers and probes can be designed. It is known that Borrelia carry a large number of linear and circular plasmids (comprising between 33-40% of the Borrelia genome), among which the cp26 and cp32, and the lp54 linear plasmid, are evolutionarily stable (Casjens et al., 2017). Of these paralogous plasmids, cp32 has been experimentally determined to be a Borrelia burgdorferi prophage thus it is highly likely that many of its homologs are also prophages (Eggers and Samuels, 2000).
In this paper we have demonstrated for the first time in Borrelia-related diagnostics that it is possible to overcome the sensitivity challenges associated with LD detection. We highlight the potential of our test to discriminate between healthy volunteers, early LD, and late LD patients. We present data from a systematic and comprehensive study that evaluate the use of the multicopy phage terminase large subunit (terL) gene as a molecular marker for the detection of Borrelia species. The analytical performance of the terL-targeting qPCR (referred to as Ter-qPCR) was thoroughly evaluated, and the test was shown to be able to detect one single Borrelia cell from blood samples. The diagnostic potential was evaluated using a set of blood and serum samples collected from healthy volunteers and individuals who were clinically diagnosed with LD.
In summary, we demonstrate that a quantitative phage-based PCR has the potential to change the diagnosis of LD from blood samples. This approach of detecting bacteria-specific phages may be applicable to infections other than LD such as sepsis caused by Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa etc. (Minasyan, 2019), as long as suitable phages are identifiable.
Each Borrelia species has a distinct amount of species specific variation in its prophage sequences; thus these prophages can be used as a proxy to identify the bacteria because of the tight correlation between them and the exact prophages found in each Borrelia host. As there are multiple prophages per Borrelia cell, the detectable signal is higher for prophages than bacteria. Furthermore, evidence suggests that Borrelia prophages can be released outside the Borrelia cells following encounters with stressors such as antibiotics (Eggers and Samuels, 2000). In this study, we confirmed that Borrelia prophages can escape from the bacterial host cell in a spontaneous manner. Taking advantage of the multicopy and free movement of Borrelia prophages, the approach to target prophages instead of bacteria will bypass the cryptic and tissue-bound feature that typifies human Borrelia infections (Liang et al., 2020). Thus, we have a greater chance of detecting the prophages in blood even when the bacteria may not be present or present in extremely low numbers. In this sense, prophages are somewhat analogous to Borrelia ‘footprints’.
To determine which prophage gene to use as a marker, the B. burgdorferi B31 genome was examined and shown to carry the multicopy terL gene (NC_000948.1). This gene encodes the terL protein which is responsible for packing phage genomes and is essential for phage survival (Sun et al., 2012). The gene was found to be present on seven of the circular plasmids from the cp32 series and on three linear plasmids within B31 genome (Supplementary Information 1). Blastn analysis revealed that the terL homologs are widespread in LD and RF Borrelia spp., including B. miyamotoi. As summarized in Table 1, the terL homologs present in LD Borrelia species are mainly located on the cp32 plasmids with E values of zero and query cover of 100%; thus, primer design specific to species was carried out. There are 13 versions of terL-bearing cp32 plasmids (cp32-1 to cp32-13), all of which are present in the B. burgdorferi spp. In contrast, B. afzelii and B. garinii encode eight and four such cp32 plasmids, respectively.
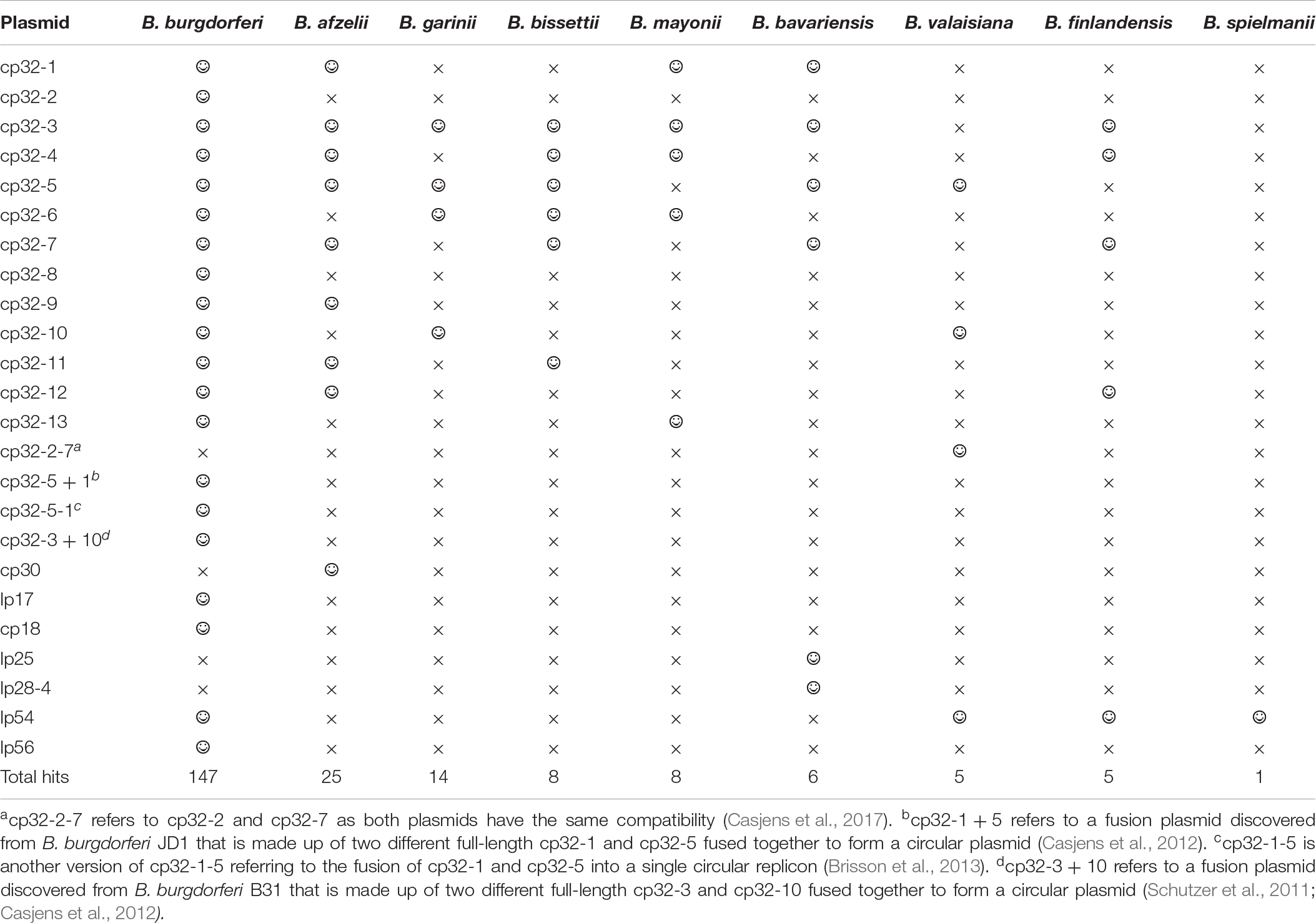
Table 1. The prevalence of the terL homologs among plasmids residing Borrelia species causing Lyme disease (smiley face and ‘ × ’ to denote presence and absence of the terL homologs, respectively).
TerL homologs are also found in RF and B. miyamotoi species (E values ranging from 5e-164 to 0.03 and a query cover ranging from 90% to 5%). Thus, the phage terL gene appears to be a useful marker; indeed, it has previously been used as a marker to reveal the evolutionary relationships within prophages of the environmental Paraburkholderia (Pratama et al., 2018) species and lytic phages of Edwardsiella ictaluri, the causative agent of enteric septicaemia of catfish (Carrias et al., 2011). In summary, the prevalence, amount of variability in the sequence, and multicopy nature of terL suggests that it could be a suitable marker to indicate Borrelia presence. Therefore, we predicted that a qPCR targeting terL homologs will detect Borrelia species to the strain level and distinguish LD Borrelia species from RF species and B. miyamotoi.
To determine the potential of the terL gene as a marker for specific Borrelia species, phylogenetic analyses were carried out using neighbor-joining (NJ) (Figure 1) and likelihood (ML) methods (Supplementary Information 2). Both trees were concordant with each other and demonstrated three well-defined clades of LD, RF and B. miyamotoi, indicating that the terL gene is evolutionary stable and offers resolution at the Borrelia species level. As shown in Figure 1, the terL gene resolves Borrelia into genospecies, revealing an independent sub-group of LD Borrelia species that is well-separated from the other RF Borrelia group (including B. miyamotoi) with statistically a significant bootstrap value.
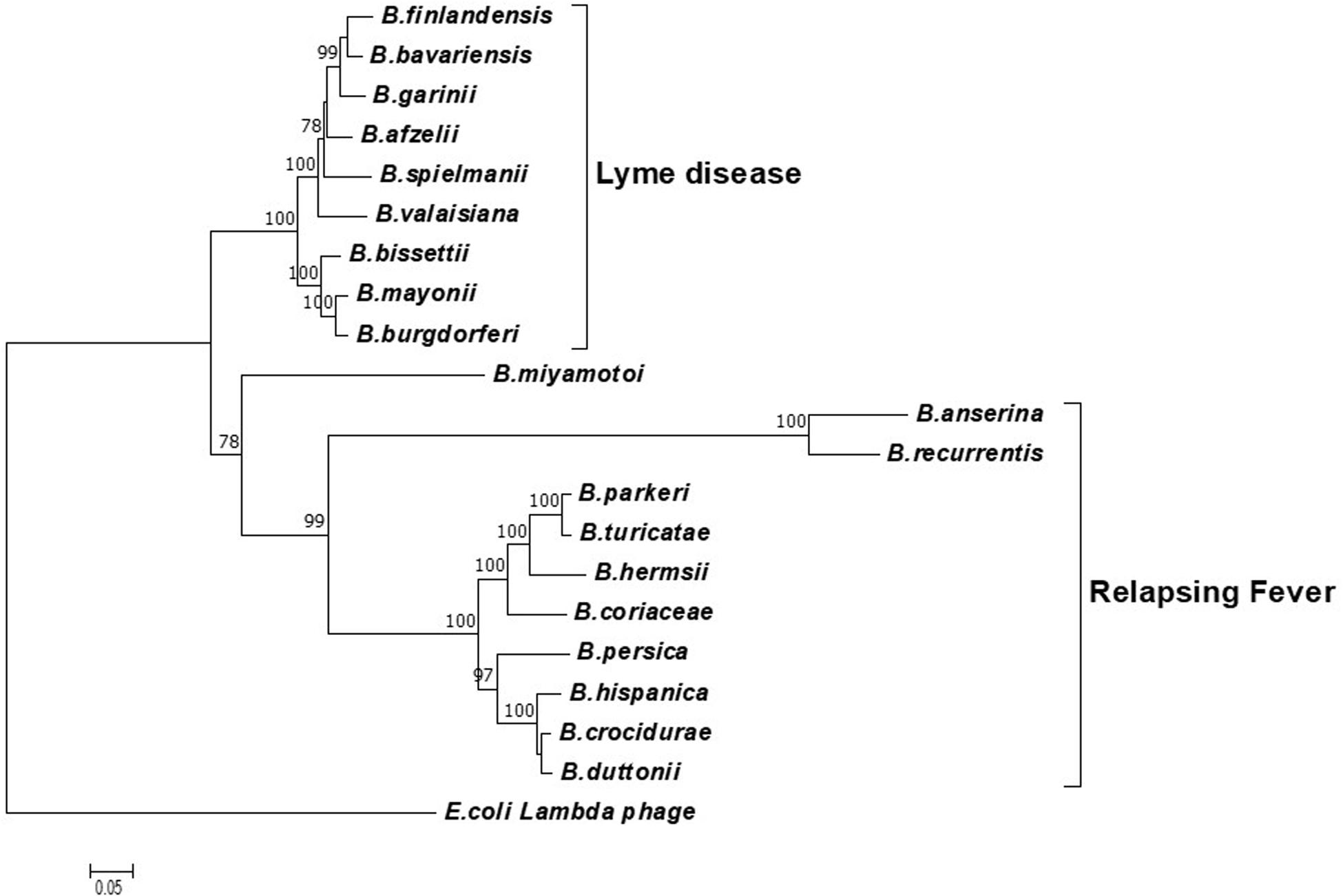
Figure 1. A phylogenetic tree constructed based on the terL nucleotide sequence. The LD and RF Borrelia species and B. miyamotoi are separated into three well-supported clades. The Molecular Evolutionary Genetics Analysis (MEGA) package version 7 was used, and the tree was constructed via the neighbor-joining method. Scale bar represents the units of the number of base substitutions per site. Support for the clades was estimated via bootstrap analysis in MEGA with 3,000 replicates, values are indicated at the nodes (only values greater than 75 are displayed). The terL sequence from phage lambda was used as an outgroup to root the tree.
Encouragingly, and of significant importance to diagnosis and treatment, our analyses show a well-supported resolution within the LD and RF lineages. Moreover, it is important to note that the terL phylogenetic tree largely agrees with the 16S rRNA gene based Borrelia phylogeny. The only exception being that the 16S tree placed B. miyamotoi within the RF clade, while the terL tree places it outside both the LD and RF clades (Takano et al., 2010). This variable phylogenetic position of B. miyamotoi indicates that it is distantly related to both LD and RF, but is more closely related to RF than to LD Borrelia. This detailed resolution offered by the terL gene is useful on two fronts: (1) it reflects the fact that the pathogenesis of B. miyamotoi is distinct to RF (Telford et al., 2015); and (2) as B. miyamotoi is the only RF Borrelia that can be co-transmitted with LD Borrelia species by hard-bodied ticks (Ravagnan et al., 2018), it is really useful to have a molecular marker that can distinguish between LD and RF-causing Borrelia spp., and B. miyamotoi.
To summarize this section, the terL based phylogenetic analysis tightly correlates the Borrelia species. The phylogenetic power of the terL gene combined with the multi-copy nature suggests that it can be developed as a diagnostic marker for accurate identification of LD, and can be used to differentiate LD from related infections and co-infections such as RF and diseases caused by B. miyamotoi.
To maximize the specificity and sensitivity of our test, we designed a set of primers and a probe to the most conserved regions of terL (to target nine out of 13 terL copies) (Figure 2 and Supplementary Information 3) (Kralik and Ricchi, 2017). The specificity of the primer/probe was confirmed by Blastn and in silico’ PCR1. Positive Ter-qPCR results were also obtained from all the LD Borrelia strains listed in Table 2. No positive Ter-qPCR was observed from B. spielmanii, or RF-causing Borrelia strains as listed in Table 2, or other non-Borrelia bacterial strains tested, along with and human DNA samples (detail in Supplementary Information 4).

Figure 2. Alignment of the terL gene sequence located on the 13 cp32 plasmids (cp32-1 to cp32-13). Identical nucleotides are indicated with an asterisk. Conserved regions of the forward and reverse primers, and probe are highlighted in yellow, green and purple, respectively. Identical primer/probe sequences are present in nine of the terL genes, except for those of the cp32-2, cp32-3, cp32-6, and cp32-13 plasmids.
For clinical diagnosis and treatment, it is essential to understand the Borrelia load present in the patient, which requires absolute quantification. To develop an absolute quantification assay, we cloned the relevant terL fraction into a plasmid (Ter-plasmid) and carried out the Ter-qPCR assay with samples containing background human DNA, this in order to mimic the real clinical samples. We observed a strong linear relationship between the concentration of the Ter-plasmid and Cq (R2 = 0.99) with an amplification efficiency of 99.58% (Figure 3A). B31 DNA dilutions also displayed a robust linear association with Cq values (R2 = 0.999) with an amplification efficiency of 98.64% (Figure 3B). This demonstrates the high efficiency of the Ter-qPCR. A close to 100% amplification efficiency from both serial dilution experiments confirmed that our standard curve assay was robust and repeatable (Bustin et al., 2009).
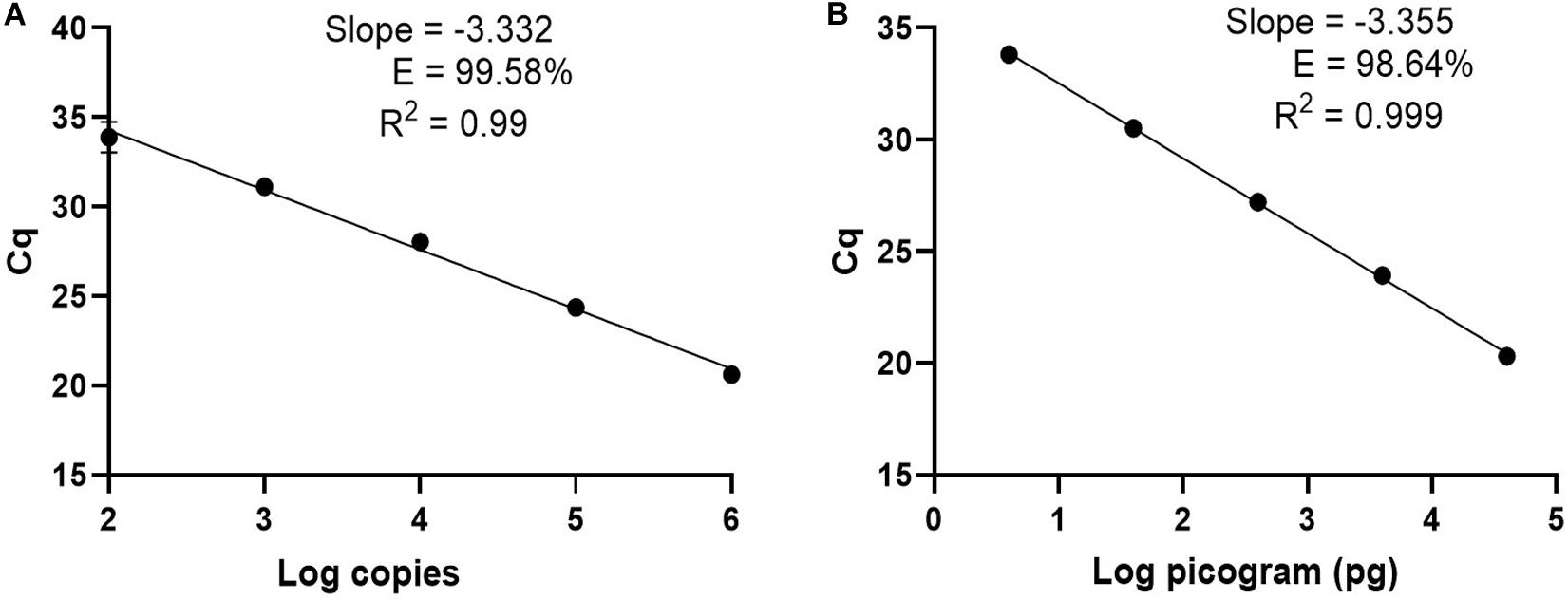
Figure 3. The Ter-qPCR against serial dilutions of the Ter-plasmid and B. burgdorferi B31 DNA to measure the LoD and PCR efficiency: (A) Ter-plasmid and (B) B31 DNA. Cq values were plotted against the log values of serial dilutions of the Ter-plasmid and B31 DNA, respectively. Simple regression analysis was carried out using the Graphpad Prism 8.4.3 software. The slope, coefficient of correlation (R2) and efficiency of the reaction (E) are shown. Each dot represents the average value from triplicate amplifications, along with the SD.
In this study, we defined the analytical limit of detection (LoD) as the lowest concentration where at least 95% of the technical replicates were positive in the Ter-qPCR (Wei et al., 2016). The LoD was calculated from serial dilutions of Ter-plasmids. The proportion of Ter-qPCR positive among replicates was directly correlated with the number of plasmid copies per reaction (Supplementary Figure 5). For example, one copy of the Ter-plasmid led to two positives out of 10 replicates, while 20 and 40 copies generated 9 and 10 positives out of 10 replicates, respectively. Probit analysis via the SPSS package was used to estimate the LoD and was found to be 22 copies per PCR (Pavšič et al., 2016).
Another challenge in detecting bacteria from blood samples is the successful extraction of DNA that forms the PCR target. It is well-known that DNA extraction from blood samples plays a pivotal role in the success of PCR diagnosis of bacterial infections (Opota et al., 2015). It is also true that PCR assays may fail to amplify if bacteria are sparse, or if an inappropriate DNA extraction method was adopted that favored an overwhelming dominance of human DNA in the final DNA product (Gosiewski et al., 2014). To find the most suitable DNA extraction method, three blood DNA extraction methods were applied to blood samples spiked with serial dilutions of Borrelia cells and assessed by qPCR. We determined the best DNA extraction method by assessing each sample in order to determine the highest detectable phage copy number from the Borrelia spiked blood.
To gain an insight into the potential performance of the Ter-qPCR with patients, the Ter-qPCR was applied to Borrelia-spiked blood samples. Consistent copy numbers from technical repeats were recovered from samples with ≥ 1 spike-in Borrelia cell (Figure 4A). This indicates that the Ter-qPCR can potentially detect as low as one Borrelia cell from a blood sample. In contrast, the blood sample with 0.1 of a Borrelia cell (mimicking the scenario of an extremely low level of Borrelia presence in the blood) displayed one copy number out of six repeats (Figure 4A). It is already established that the number of Borrelia cells circulating in the blood is extremely low and is often at the lower end of the detection limit of qPCR (Brettschneider et al., 1998). Therefore, it is common to get one PCR amplification out of technical repeats due to stochastic effect when in low concentration (Primus et al., 2018). To reflect the low and random distribution nature of Borrelia cells in blood, we adopted the following rule for recording copy numbers: the replicate that did not generate a copy number (displayed as ‘failed qPCR’) was scored ‘zero’ (Primus et al., 2018). The ‘zero’ Borrelia presence in blood was manifested in our later study analysing LD patient samples.
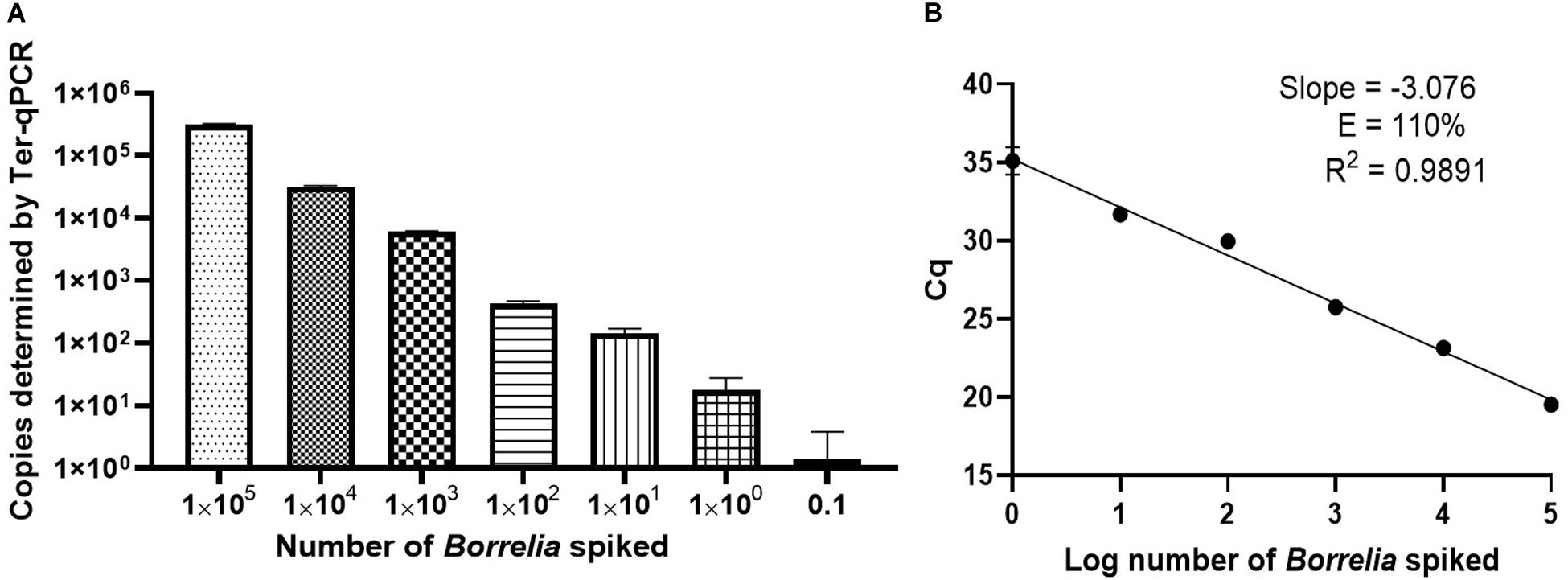
Figure 4. Performance of the Ter-qPCR estimated by examining human blood spiked with tenfold serial dilutions of Borrelia cells (105 to 0.1). (A): bar graph illustrating the number of spike-in Borrelia cells and the resulting copy numbers determined by the Ter-qPCR; (B): Linear regression analysis between the known amount of spiked Borrelia cells and the resulting Cq values revealed a strong linear association. The slope, coefficient of correlation (R2) and efficiency of the reaction (E) are shown. Each dot represents the average value from triplicate repeats along with SD values obtained from two independent experiments.
To better understand the reliability of the Ter-qPCR, simple linear regression analysis was performed. As seen in Figure 4B, a linear association (R2 = 0.9891) was observed between the amount of spiked Borrelia cells and the resulting Cq values, which demonstrates that the signal intensity of Ter-qPCR correlates with the ‘Borrelia load.’ In other words, it appears that the Ter-qPCR can detect as low as one Borrelia per 300 μl blood, which is equivalent to 3.3 Borrelia cells per ml of blood. This is really promising, bearing in mind that evidence from published studies indicates that Borrelia presence in LD patients can range from 1-100 cells/ml (Liveris et al., 2012). Therefore this single cell sensitivity allows the Ter-qPCR test to change the way Borrelia infection is detected (Buchanan et al., 2017). To put this in context, the current practice to circumvent the bottleneck of low numbers of Borrelia circulating in blood is either to culture prior to qPCR or to sample large volumes of blood to artificially increase the amount of PCR templates. Both methods have obvious drawbacks (Brettschneider et al., 1998). Intuitively, by targeting endogenous multi-copy genes, the Ter-qPCR assay offers a much more elegant and reliable way of increasing the amount of PCR template.
We initially chose the phenol method to extract DNA from blood as it is the method of choice when it comes to extracting phage DNA (Pickard, 2009). Although the phenol extraction method is in many ways a gold standard, it is cumbersome. To improve the scaling of the assay, we tested commercial DNA extraction kits, such as the DNeasy Blood and Tissue Kit (a column filtration system) and Maxwell RSC Viral Total Nucleic Acid Purification Kit (a magnetic beads-based system) to see whether they could replace the solvent-based method.
Despite a significant effort, including comparisons with bacterial chromosome-targeting 16S qPCR, the phenol extraction method outperformed the other two commercial methods. Thus, the phenol method was used throughout this study. As shown in Figure 5, regardless of qPCR methods, the phenol approach generated a consistently higher copy number compared to the other two DNA extraction methods. The Ter-qPCR coupled with the phenol method produced significantly higher copy numbers than their counterparts of the 16S qPCR from all spiked samples. Additionally, the Ter-qPCR generated robust copy numbers (three positives out of three replicates) from blood spiked with one Borrelia cell (Figure 5), but the 16S qPCR only displayed amplification once from the triplicate repeats of the same sample. The outstanding sensitivity of Ter-qPCR can also be seen from the markedly lower Cq values (therefore high amounts of PCR target) of the Ter-qPCR than that of the 16S qPCR when both qPCRs were targeting the same B31 genomic DNA (Figure 6B). The competitive advantage of targeting multicopy genes can also be seen in that the copy numbers determined by the Ter-qPCR were consistently higher than the number of spiked Borrelia, while the copy numbers established via the 16S qPCR were numerically about the same as the input Borrelia number (Figure 5).
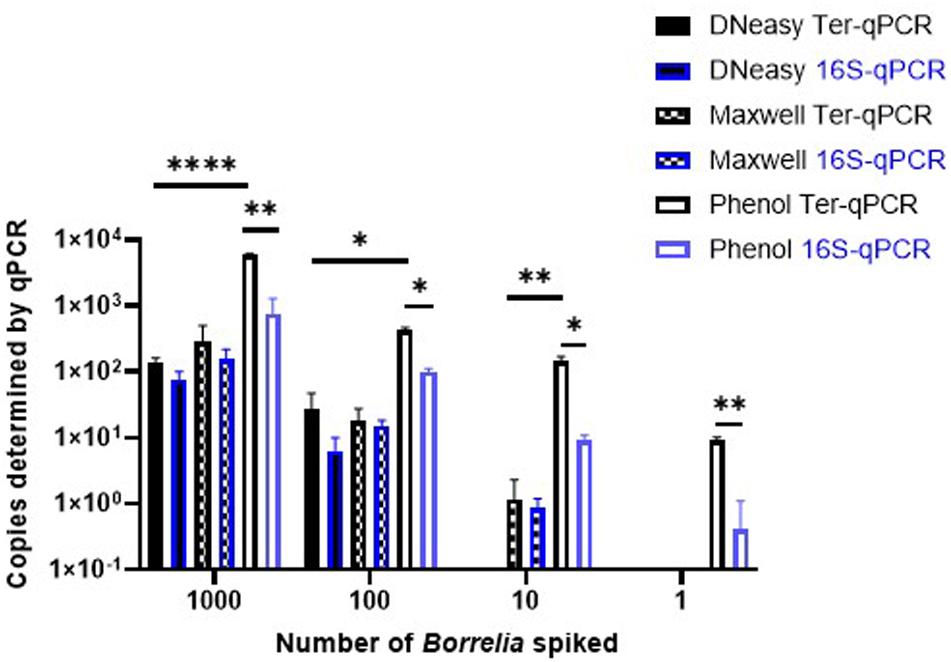
Figure 5. The Ter-qPCR (bars with black border) and 16S qPCR (bars with blue border) against DNA extracted using three methods from human whole blood spiked with tenfold serial dilutions of Borrelia cells (103 to 1). The phenol method, DNeasy Blood and Tissue Kit and the Maxwell RSC Viral Total Nucleic Acid Purification Kit were compared for DNA extraction. The Phenol method coupled with the Ter-qPCR displayed consistently and significantly higher quantitation compared to the other two methods. Copy numbers obtained from the Ter-qPCR were significantly higher than those from the 16S qPCR. Values shown are the means from triplicate repeats along with SD values obtained from two independent experiments. Statistical significance is denoted as *p < 0.05, **p < 0.01, and ****p < 0.0001.
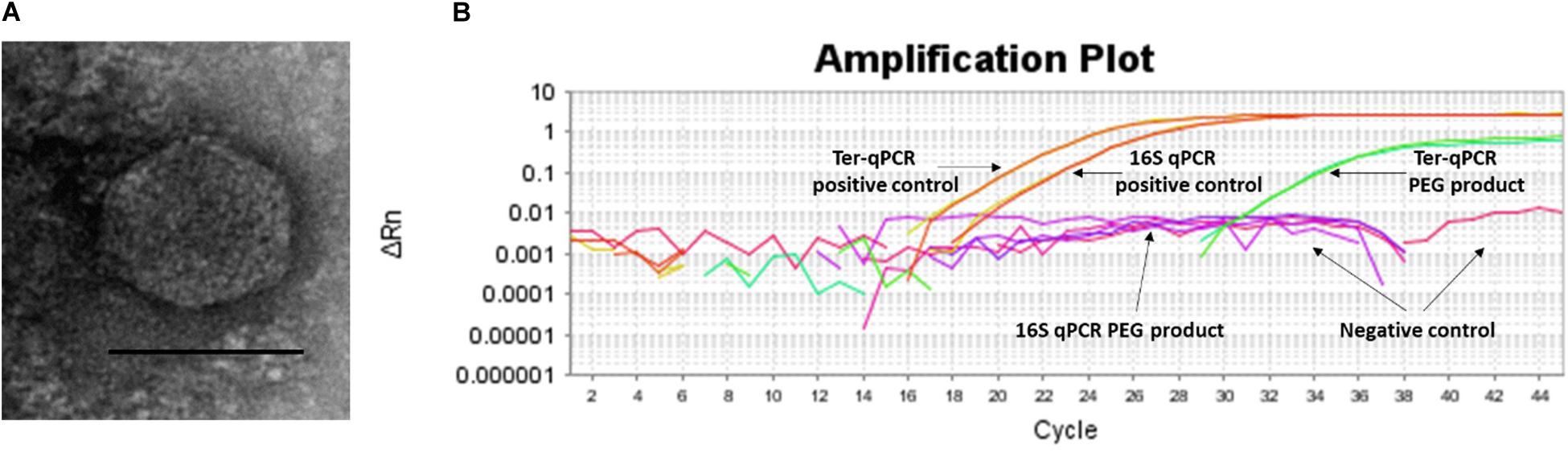
Figure 6. Spontaneous prophage release from B. burgdorferi B31 cultures. (A): Image of the phage-like particles visualized during TEM analysis of the PEG purified cell-free culture filtrates. (B): DNA extracted from the PEG product tested positive during the Ter-qPCR, but negative to the 16S qPCR, indicating the spontaneous release of cp32 prophages. 1 ng of DNA from B31 was used as a positive control (as annotated in panel B).
It is logical that multicopy PCR targets will lead to higher sensitivity when compared to a single-copy PCR target (Luo et al., 2010). We determined whether the prophage encoded genes would provide additional sensitivity to detect infections. Since some prophages can escape from their bacterial hosts either by chemical induction or spontaneous prophage induction (SPI), it is entirely possible that multiple Borrelia prophages could be released into the blood where they magnify the diagnostic signals and thus can be detected. In the case of Borrelia, terL-carrying cp32 prophages have been demonstrated to be susceptible to 1-methyl-3-nitroso-nitroguanidine (MNNG) induction during in vitro culturing (Eggers and Samuels, 1999). We hypothesize that Borrelia prophages are also capable of SPI which increases the chances of Borrelia prophages being induced in the human body.
To test this SPI hypothesis, we conducted morphological and molecular studies to detect phage presence in cell-free filtrates of Borrelia cultures prior and post 36 h incubation. As shown in Figure 6A, transmission electron microscope (TEM) analysis revealed particles morphologically resembling podoviruses (icosahedral heads and very short tails) (Ackermann, 2003) in the polyethylene glycol (PEG)-purified phage fraction derived from Borrelia cultures post 36 h incubation. The PEG product was positive to the Ter-qPCR but negative to the Borrelia 16S qPCR (Figure 6B). The Ter-qPCR positive result therefore indicates the presence of cp32 DNA (prophage) in the PEG product. Meanwhile, the PEG product derived from Borrelia culture prior incubation showed no phage-like particles and was negative to both the Ter-qPCR and the Borrelia 16S qPCR.
Our spontaneous induction data led us to predict that the prophage-based Ter-qPCR would generate a much stronger Borrelia signal than bacteria-based qPCR, because LD Borrelia species are tissue bound and thus can only circulate in blood transiently and in very low numbers (Liang et al., 2020). In contrast, under the scenario of induction, prophages are released into bloodstream, and thus can be a ‘marker’ indicating the presence of Borrelia, even though Borrelia cells can be hiding and not circulating in the blood. This situation of detecting free phage DNA from human blood bears some resemblance to identifying cell-free circulating DNA (cfDNA) as with cancer diagnosis (Phallen et al., 2017). Interestingly, the same challenge also stands in terms of the method of choice for isolating cfDNA (Hufnagl et al., 2013). Phenol chloroform was also highly efficient in recovering cfDNA from clinical samples (Hufnagl et al., 2013). Free phages have been discovered from a range of clinical samples, including blood (Brown-Jaque et al., 2016). Their biological significance remains to be understood. Considering the fact that metabolic active bacteria can better support phage reproduction (Burns et al., 2015), a high level of certain phages in the blood would indicate active infections of their respective bacteria, i.e., a strong terL signal would implicate active Borrelia infection. Given the fact that most pathogenic bacteria carry many inducible (including SPI) prophages, the rational of diagnosing bacteria by detecting their ‘breakaway’ prophages could change the current bacteria-focused paradigm of detecting bacterial infections.
As a final important evaluation step of this work, the Ter-qPCR was applied to 78 individuals belonging to three categories (early LD, late LD patients and healthy volunteers) who were diagnosed by Dr. LT (Cameron et al., 2014). We intended to establish the feasibility of the Ter-qPCR to detect LD. Copy numbers were determined from both blood and serum samples with three technical repeats for each sample type. The technical repeats that did not show detectable copy numbers were scored ‘zero’. The raw data was presented in the Supplementary Table. Visually, there are many more ‘zero’ scores from serum than blood samples, and the mean and median values obtained from blood samples in each category are much higher than those from sera (Supplementary Table). The higher copy numbers determined from the whole blood as compared to the serum samples reflects Borrelia’s intracellular life cycle and the fact that Ter-qPCR can detect both prophages that are inside or outside of the Borrelia cells (Klose et al., 2021). Therefore, whole blood is a more robust sample source from which to diagnose LD using qPCR assay.
Overall, most of samples showed a low terL copy number (<10), which is consistent with the current estimation of a low concentration of Borrelia circulating in the blood (Supplementary Table). It is also common to have variation among technical repeats, for example, three technical repeats of the patient No. 25 showed copy numbers of 10, 2.8, and 0, respectively (Supplementary Table). This qPCR variability is due to the stochastic effect of having a low number of PCR templates, and has been observed in PCR detection of low level bacteria in blood before (Wei et al., 2016). Markedly differences were observed in the mean copy number of early LD (2.4), and late LD (6.9), as well as healthy volunteers (0.8), which suggests a potential positive correlation between the severity of LD and the copy numbers, i.e., higher copy numbers in late LD patients, in contrast to lower copy numbers in early LD patients and healthy volunteers. Also, very encouragingly, the median values from the early (2.0) and late (1.8) LD patients are numerically higher than those of the healthy volunteers (0.7) (Supplementary Table). The fact that some healthy volunteers showed positive with copy numbers, indicates possible asymptomatic Borrelia carriage (Primus et al., 2018). Most importantly, the apparent marked difference in copy numbers from the three categories is supported by statistical analysis. As shown in Figure 7, Mann-Whitney U test revealed significant differences between early LD, late LD patients and healthy volunteers in terms of terL levels determined from whole blood, which offers objective evidence to prove that LD is a fact, and that the early and late LD stages do exist. This statistics-backed difference also suggests the potential application of the Ter-qPCR to distinguish early LD from healthy asymptomatic Borrelia carrier.
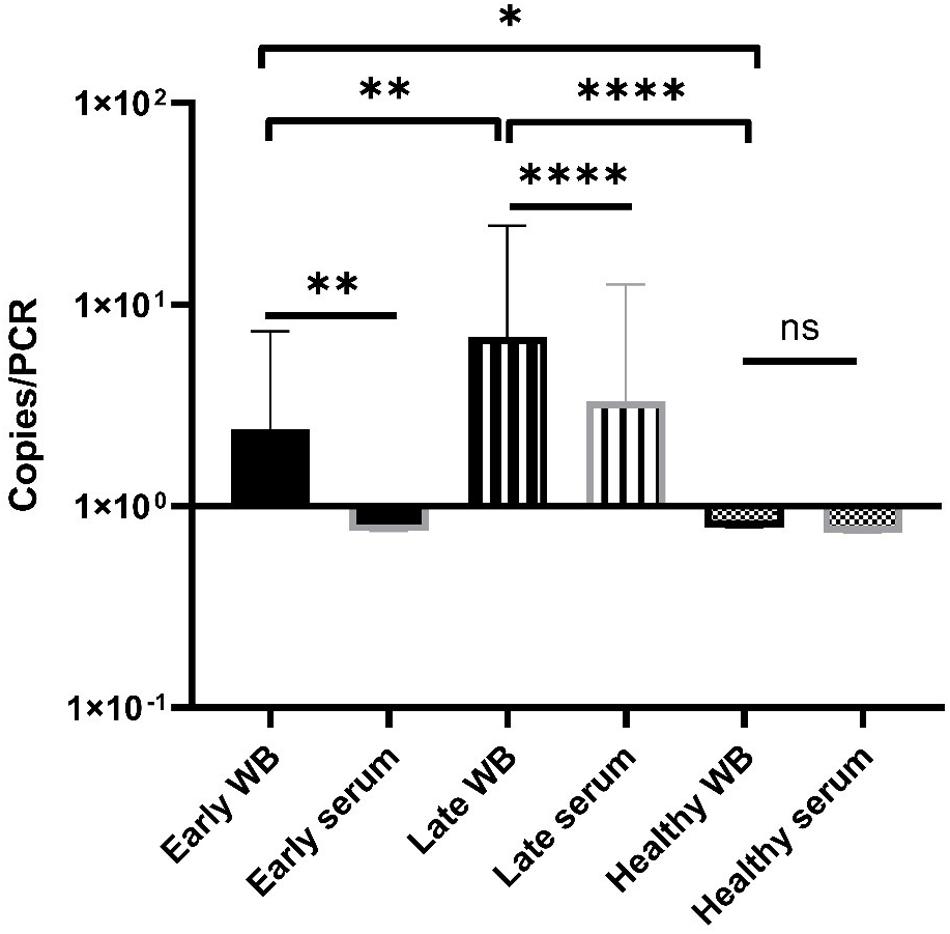
Figure 7. Bar graph displaying the mean terL levels (with SD values) of the three respective study groups from whole blood (black border) and serum (gray border) samples. The Mann-Whitney U test was used to compare differences between early LD patients, late LD patients, and healthy volunteers. The terL copy numbers obtained from the blood samples of late LD patients are significantly higher larger than those of the early LD patients and healthy volunteers, while the terL copy numbers of early LD patients are significantly higher larger than those of healthy volunteers. Statistical significance is denoted as ns (p > 0.05), * (p < 0.05), ** (P < 0.01), *** (P < 0.001), and **** (P < 0.0001).
We conclude that our prophage marker has the ability to differentiate between early and late LD patients. For example, the Ter-qPCR could be used to monitor LD treatment outcomes, to indicate which treatment option may work best, and to help clinicians measure recovery. We are currently validating a terL-based assay targeting RF and B. miyamotoi, respectively, working toward a ‘multiplex’ PCR aiming to detect and differentiate LD Borrelia spp., RF Borrelia spp., and B. miyamoti from a single test.
Rapid and accurate detection of microbial pathogens in blood using qPCR methods is promising, but hampered by the low-level presence of bacteria in circulating blood, false signals and reduced sensitivity due to unspecific amplification of human DNA. The data presented in this study demonstrates for the first time that targeting phage DNA in blood could offer a rapid diagnosis of bacterial infections and could change the paradigm in the field of PCR detection of bacteria in general.
Phylogenetic analysis was constructed using the program Molecular Evolutionary Genetics Analysis (MEGA) 7 according to the previous established method (Shan et al., 2012, 2014). Neighbor-Joining (NJ) with a maximum composite likelihood model and Maximum Likelihood (ML) based on the Tamura-Nei model analyses were conducted on a nucleotide data set of terL genes. Support for clades were estimated using a bootstrap analysis implemented in MEGA using 3, 000 replicates. The trees were rooted with phage Lambda (NC_001416) as an outgroup.
The Borrelia strains used in this study are listed in Table 1. Ten strains were provided by Professor Sven Bergström, Department of Molecular Biology, Umea University, Sweden. Seven strains were purchased from the Pasteur Institute and DSMZ (German Collection of Microorganisms and Cell Cultures GmbH). Two strains were provided by the Center for Disease Control and Prevention (CDC), United States, and two by Cecilia Hizo-Teufel from the German National Reference Centre for Borrelia. Borrelia cells were grown in 15 ml FalconTM conical tubes with a culture volume of 14 ml of Barbour-Stoenner-Kelly (BSK) II medium with 7% rabbit serum (referred to as complete BSKII or c-BSKII) at 35°C without agitation as previously reported (Zuckert, 2007). All culture media was filter sterilized via 0.22 μm pore size filters. Visualization and counting of Borrelia was performed using phase contrast microscopy (Ceti Magnum Trinocular) and a Fuchs Rosenthal Disposable Counting Chamber (C-Chip, NanoEnTek).
A modified phenol-chloroform method was used to extract DNA from blood and serum samples. In brief, samples were treated with ammonium hydroxide (Santino et al., 2008) followed by the classic phenol chloroform DNA extraction method (Sambrook and Russell, 2006). The resulting DNA pellet was air dried for 5 min, dissolved in 40 μl Tris-Cl (10 mM, pH 8.5), and kept at −20°C. DNeasy Blood and Tissue Kit and Maxwell RSC Viral Total Nucleic Acid Purification Kit were used according to the respective manufacturer instructions. The Thermo ScientificTM NanoDropTM One Spectrophotometer was used to measure the quantity and quality of DNA samples.
Primers and probe were designed using the PrimerQuest® Tool based on an alignment of phage terL genes from 13 cp32 plasmids (cp32-1 to cp32-13) carried by B. burgdorferi s.l strains. The resulting Ter-qPCR amplified a 147 bp target region (Shan et al., 2018). The fluorogenic probe was labeled with 6-carboxyfluorescein (FAM) fluorescent reporter dye at the 5′-end, an internal ZENTM Quencher and an Iowa Black Fluorescent Quencher (IBFQ) to the 3′ (5′FAM/ZEN/3′IBFQ). To rule out PCR inhibition and avoid false negatives, the Ter-qPCR was duplexed with an internal amplification control (IAC) qPCR that generated a 145 bp PCR product (Deer et al., 2010). The IAC DNA (accession number FJ357008.1) was synthesized by IDT and added to each Ter-qPCR template. The IAC probe was fluorescently labeled with the fluorescent dye of JOE at the 5′-end. The primers and probe targeting the Borrelia 16S rRNA gene were adopted from a published paper (O’Rourke et al., 2013). All the primers, probes and PrimeTime Gene Expression Master Mix were supplied by IDT.
For absolute quantification, a plasmid carrying the phage terL gene fragment (named as Ter-plasmid) was constructed and used as the standard curve. Specifically, a pair of PCR primers was designed using Primer Blast to amplify a 721 bp region of the phage terL gene (GenBank accession NC_000948), embracing the 147-bp Ter-qPCR product region. The primers were FTer721:AGACTAAGATGCGGGCAAGA and RTer721:TTGCATCAAGAGCGTCATCA. PCRs were carried out in a LabCycler (SensoQuest GmbH) in a total volume of 50 μl, containing 0.25 mM dNTPs, 3 mM MgCl2, 3 μM primers, 50 ng template DNA, 0.5 unit of Taq polymerase (Bioline), and 5 μl 10 × Taq buffer (Bioline). Amplification conditions were: 94°C for 2 min, 30 cycles of 94°C for 30 s, 50°C for 30 s, 72°C for 1 min, with a final extension of 10 min at 72°C. PCR products were gel-purified using a Qiagen gel extraction kit, and subjected to cloning using the NEB® PCR Cloning Kit according to the manufacturer’s instructions. The recombinant Ter-plasmid DNA carrying the 721-bp terL gene was purified using the Qiagen Plasmid Kit from positive clones. The concentration of the Ter-plasmid was converted into DNA copy number on 20 January 2018 (Staroscik, 2004).
The Ter-qPCR duplexed with the IAC assay was conducted in a 20 μl final reaction volume containing 10 μl 2X PrimeTime Master Mix, with each primer and each probe at a final concentration of 0.5 and 0.25 μM, respectively, 4 μl template DNA, 2 μl IAC (200 genome copies) and nuclease-free water. Standard thermal cycling conditions (Applied BiosystemsTM 7500 Fast Real-Time PCR System) were followed with an initial step of 3 min at 95°C (polymerase activation), 45 cycles of 15 s at 95°C (denaturation) and 1 min at 60°C (annealing/extension). The Borrelia 16S qPCR setup was carried out according to the previous report (O’Rourke et al., 2013).
A non-template control (NTC), a positive control of 10 ng B. burgdorferi B31 (labeled as B31) DNA and a standard curve made of a series of five tenfold dilutions of the Ter-plasmid DNA (106–102) were included in each run. The qPCR result was analyzed and quantified according to the standard curve using FAST7500 software v2.3. For the Ter-qPCR to be valid, the IAC signal should always be produced regardless of the presence or absence of template DNA. All samples were tested in triplicate.
The analytical specificity of the Ter-qPCR assay was determined using both in silico and in vitro analyses. In silico analysis of the primer and probe set was carried out using BLAST and Primer-BLAST (Ye et al., 2012), and UCSC in silico PCR2. For in vitro analysis, the Ter-qPCR was applied to DNA extracted from a panel of Borrelia strains that cause LD and RF (Table 1), and microbial species that have been used in the lab, including Clostridium difficile, Clostridium perfringens, Escherichia coli, Pseudomonas aeruginosa, Streptococcus pneumoniae, Staphylococcus aureus, Burkholderia thailandensis, Burkholderia pseudomallei, Haemophilus influenzae and Salmonella enterica. Additionally, human female and male DNA (Promega, G1521 and G1471), and DNA extracted using the phenol method from human whole blood (Cat#: SER-WB10ML from Cambridge Biosciences) were also tested using the Ter-qPCR.
The analytical sensitivity of the Ter-qPCR was firstly evaluated with five tenfold dilutions of the Ter-plasmid (106 to 102) and B31 DNA (40 ng to 4 pg), respectively. Each dilution was tested with three replicates to determine the PCR linearity and amplification efficiency. The LoD was then estimated by testing Ter-plasmid dilutions from 1000 to 100, 80, 60, 40, 20, 10, 5, and 1 copies/PCR. Ten replicates were used for each dilution. To mimic the real situation of analyzing DNA extracted from human samples, all Ter-plasmid serial dilution experiments were conducted with the presence of background human DNA 125 ng per PCR. Probit analysis via the SPSS software was performed to calculate the LoD with 95% probability (Forootan et al., 2017).
Actively growing B31 cultures in the early exponential phase (around 106 spirochaetes/ml) was used to spike human whole blood (in duplicate) to generate a final amount of 105, 104, 103, 102, 10, 1, and 0.1 spike-in B31 cells in 300 μl of blood. DNA extraction was carried out using the modified phenol-chloroform method. To compare the different DNA extraction methods, a subset of the B31-spiked blood samples (corresponding to 103, 102, 10, and 1 B31 cells per 300 μl of blood) were also used for DNeasy and Maxwell DNA extractions, respectively. All the resulting DNAs were analyzed by both the Ter-qPCR and the Borrelia 16S qPCR.
14 ml of early exponential phase, actively growing B31 cultures (7 × 105 -106 cells/ml, dominated by free spirochaetal forms) were spun down (6 000 g for 20 min) and washed twice with sterile PBS. The resulting pellets were resuspended in 14 ml c-BSKII and incubated for 36 h at 35°C. Portions of the B31 suspension prior and post 36 h incubation were centrifuged down, the resulting supernatants were filtered through 0.1 μm pore filters. Extraction of phages from the filtrates were carried out using PEG precipitation (Sambrook and Russell, 2001). The PEG-purified phage product was used for DNA extraction (Sambrook and Russell, 2006) and TEM at the Core Biotechnology Services at the University of Leicester (Shan et al., 2012). The resulting DNA was examined by the Ter-qPCR and the Borrelia 16S qPCR assays.
This study was carried out in accordance with protocols reviewed and approved by the Ethics Committee, Comités de protection des personnes (CPP) with investigator reference of Etude Phelix 01617 V1 and CPP reference of 17031. All participants were diagnosed by Dr. LT according to the ILADS (International Lyme and Associated Diseases Society) guidelines (Cameron et al., 2014). Some of individuals who did not recall a tick bite and had no LD-like symptoms (ILADS guidelines) served as healthy volunteers. All healthy volunteers were also negative to Lyme C6 ELISA that was performed according to manufacturer’s instructions (Immunetics: B. burgdorferi (Lyme) ELISA kit: DK-E352-096). A total of 312 samples (156 whole blood and 156 serum samples) were collected from 78 individuals between April-June 2017 (23 healthy volunteers with no ‘identifiable’ LD symptoms, 13 early stage and 42 late stage LD patients). For everyone involved, two tubes of serum and two tubes of Ethylenediaminetetraacetic acid (EDTA)-treated whole blood (approximately one ml in each tube) were provided. Samples were provided in a coded, de-identified manner to preserve patient anonymity. Informed consent was obtained from all participants. The modified phenol chloroform DNA extraction method was applied to all the samples in a duplicate manner. Therefore, there were four DNA samples generated from one individual, two from the whole blood and two from the serum. Triplicate qPCR was applied to each DNA sample, which led to 12 Ter-qPCR data (six from whole blood, six from serum) expressed in copy numbers according to the standard curve for any one individual.
Graphpad Prism 8.4.3. was used for statistical analysis. Descriptive statistics and the D’Agostino-Pearson normality test were used to assess the data distribution. Mann-Whitney U tests were used to determine the significance of the difference between early, late LD patients and healthy volunteers. Furthermore, Probit analysis was carried out via the SPSS software suite (IBM SPSS Statistics 25) to estimate the LoD. The differences were not considered to be significant when the p-values were greater than 0.05. Linear regressions were used to establish correlations between the serial dilution of Ter-plasmids/B31 DNAs and Cq values.
The Ter-qPCR assay includes a set of oligonucleotide primers and Taqman® probes and plasmid DNA as the standard for in vitro quantitative detection of Borrelia species causing LD. All primers and probes are described in the patent application Ref. P184103.EP.01/T. Other relevant data supporting the findings of the study are available in this article and its Supplementary Information 1–5 and Supplementary Table, or from the corresponding author upon request.
The studies involving human participants were reviewed and approved by Comités de protection des personnes (CPP). The patients/participants provided their written informed consent to participate in this study.
JS and MC co-conceived the initial idea. JS expanded the initial idea into a coherent scientific project, designed primers and probes and performed in-depth data analysis. YJ was responsible for experimentation and optimization, data collecting and initial data interpretation. JS and YJ co-wrote the manuscript. MC proofread the manuscript and provided valuable comments and suggestions. FP helped with bioinformatic and phylogenetic analyses. LT carried out the ethical application, clinical samples selection and some data analysis. All authors contributed to the article and approved the submitted version.
JS, LT, and MC are listed as inventors in the patent application No. PCT/GB2017/053323.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Dr. Andrew Millard and Stacy Guiock for their help in proofreading the manuscript. We gratefully acknowledge the main funding received toward the study from the Phelix Research and Development (Phelix R&D, 37 Langton Street, SW10 0JL London, United Kingdom, the Charity Number 1156666), the ‘Gift’ funding from Lymefonds, Netherlands (ANBI-number: 858578438), and the University of Leicester Drug Discovery and Diagnostics (LD3) spring fund 2018.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.651217/full#supplementary-material
Ackermann, H. W. (2003). Bacteriophage observations and evolution. Res. Microbiol. 154, 245–251. doi: 10.1016/s0923-2508(03)00067-6
Anderson, B. E., and Neuman, M. A. (1997). Bartonella spp. as emerging human pathogens. Clin. Microbiol. Rev. 10, 203–219. doi: 10.1128/cmr.10.2.203-219.1997
Argov, T., Sapir, S. R., Pasechnek, A., Azulay, G., Stadnyuk, O., Rabinovich, L., et al. (2019). Coordination of cohabiting phage elements supports bacteria–phage cooperation. Nat. Commun. 10:5288. doi: 10.1038/s41467-019-13296-x
Bergström, S., and Normark, J. (2018). Microbiological features distinguishing Lyme disease and relapsing fever spirochetes. Wien. Klin. Wochenschr. 130, 484–490. doi: 10.1007/s00508-018-1368-2
Brettschneider, S., Bruckbauer, H., Klugbauer, N., and Hofmann, H. (1998). Diagnostic value of PCR for detection of Borrelia burgdorferi in skin biopsy and urine samples from patients with skin borreliosis. J. Clin. Microbiol. 36, 2658–2665. doi: 10.1128/jcm.36.9.2658-2665.1998
Brisson, D., Zhou, W., Jutras, B. L., Casjens, S., and Stevenson, B. (2013). Distribution of cp32 prophages among Lyme disease-causing spirochetes and natural diversity of their lipoprotein-encoding erp loci. Appl. Environ. Microbiol. 79, 4115–4128. doi: 10.1128/aem.00817-13
Brown-Jaque, M., Muniesa, M., and Navarro, F. (2016). Bacteriophages in clinical samples can interfere with microbiological diagnostic tools. Sci. Rep. 6:33000. doi: 10.1038/srep33000
Buchanan, C. M., Wood, R. L., Hoj, T. R., Alizadeh, M., Bledsoe, C. G., Wood, M. E., et al. (2017). Rapid separation of very low concentrations of bacteria from blood. J. Microbiol. Methods 139, 48–53. doi: 10.1016/j.mimet.2017.05.004
Burns, N., James, C. E., and Harrison, E. (2015). Polylysogeny magnifies competitiveness of a bacterial pathogen in vivo. Evol. Appl. 8, 346–351. doi: 10.1111/eva.12243
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., et al. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622. doi: 10.1373/clinchem.2008.112797
Cameron, D. J., Johnson, L. B., and Maloney, E. L. (2014). Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev. Anti Infect. Ther. 12, 1103–1135. doi: 10.1586/14787210.2014.940900
Carrias, A., Welch, T. J., Waldbieser, G. C., Mead, D. A., Terhune, J. S., and Liles, M. R. (2011). Comparative genomic analysis of bacteriophages specific to the channel catfish pathogen Edwardsiella ictaluri. Virol. J. 8:6. doi: 10.1186/1743-422x-8-6
Casjens, S. R., Gilcrease, E. B., Vujadinovic, M., Mongodin, E. F., Luft, B. J., Schutzer, S. E., et al. (2017). Plasmid diversity and phylogenetic consistency in the Lyme disease agent Borrelia burgdorferi. BMC Genomics 18:165. doi: 10.1186/s12864-017-3553-5
Casjens, S. R., Mongodin, E. F., Qiu, W. G., Luft, B. J., Schutzer, S. E., Gilcrease, E. B., et al. (2012). Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One 7:14. doi: 10.1371/journal.pone.0033280
Chaaya, G., Jaller-Char, J. J., and Ali, S. K. (2016). Beyond the bull’s eye: recognizing Lyme disease. J. Fam. Pract. 65, 373–379.
Deer, D. M., Lampel, K. A., and Gonzalez-Escalona, N. (2010). A versatile internal control for use as DNA in real-time PCR and as RNA in real-time reverse transcription PCR assays. Lett. Appl. Microbiol. 50, 366–372. doi: 10.1111/j.1472-765x.2010.02804.x
Eggers, C., and Samuels, D. S. (2000). Molecular evidence for a new bacteriophage of Borrelia burgdorferi. J. Bacteriol. 181, 7308–7313. doi: 10.1128/JB.181.23.7308-7313.1999
Eggers, C. H., and Samuels, D. S. (1999). Molecular evidence for a new bacteriophage of Borrelia burgdorferi. J. Bacteriol. 181, 7308–7313. doi: 10.1128/JB.181.23.7308-7313.1999
Forootan, A., Sjöback, R., Björkman, J., Sjögreen, B., Linz, L., and Kubista, M. (2017). Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR). Biomol. Detect. Quantif. 12, 1–6. doi: 10.1016/j.bdq.2017.04.001
Gosiewski, T., Szała, L., Pietrzyk, A., Brzychczy-Włoch, M., Heczko, P. B., and Bulanda, M. (2014). Comparison of methods for isolation of bacterial and fungal DNA from human blood. Curr. Microbiol. 68, 149–155. doi: 10.1007/s00284-013-0451-1
Hufnagl, C., StöCher, M., Moik, M., Geisberger, R., and Greil, R. (2013). A modified phenol-chloroform extraction method for isolating circulating cell free DNA of tumor patients. J. Nucleic Acids Invest. 4:e1. doi: 10.4081/jnai.2013.4282
Klose, M., Scheungrab, M., Luckner, M., Wanner, G., and Linder, S. (2021). FIB-SEM-based analysis of Borrelia intracellular processing by human macrophages. J. Cell Sci. 134:jcs252320. doi: 10.1242/jcs.252320
Koetsveld, J., Draga, R. O. P., Wagemakers, A., Manger, A., Oei, A., Visser, C. E., et al. (2017). In vitro susceptibility of the relapsing-fever spirochete Borrelia miyamotoi to antimicrobial agents. Antimicrob. Agents Chemother. 61, e535–e517. doi: 10.1128/AAC.00535-17
Kralik, P., and Ricchi, M. (2017). A basic guide to real time PCR in microbial diagnostics: definitions, parameters, and everything. Front. Microbiol. 8:108. doi: 10.3389/fmicb.2017.00108
Kugeler, K., Schwartz, A., Delorey, M., Mead, P., and Hinckley, A. (2021). Estimating the frequency of lyme disease diagnoses, United States, 2010–2018. Emerg. Infect. Dis. J. 27, 616–619. doi: 10.3201/eid2702.202731
Liang, L., Wang, J., Schorter, L., Nguyen Trong, T. P., Fell, S., Ulrich, S., et al. (2020). Rapid clearance of Borrelia burgdorferi from the blood circulation. Parasit. Vectors 13:191. doi: 10.1186/s13071-020-04060-y
Liveris, D., Schwartz, I., Mckenna, D., Nowakowski, J., Nadelman, R., Demarco, J., et al. (2012). Comparison of five diagnostic modalities for direct detection of Borrelia burgdorferi in patients with early Lyme disease. Diagn. Microbiol. Infect. Dis. 73, 243–245. doi: 10.1016/j.diagmicrobio.2012.03.026
Lohr, B., Fingerle, V., Norris, D. E., and Hunfeld, K.-P. (2018). Laboratory diagnosis of Lyme borreliosis: current state of the art and future perspectives. Crit. Rev. Clin. Lab. Sci. 55, 219–245. doi: 10.1080/10408363.2018.1450353
Luo, R. F., Scahill, M. D., and Banaei, N. (2010). Comparison of single-copy and multicopy real-time PCR targets for detection of Mycobacterium tuberculosis in paraffin-embedded tissue. J. Clin. Microbiol. 48, 2569–2570. doi: 10.1128/jcm.02449-09
Minasyan, H. (2019). Sepsis: mechanisms of bacterial injury to the patient. Scand. J. Trauma Resusc. Emerg. Med. 27:19. doi: 10.1186/s13049-019-0596-4
Moore, A., Nelson, C., Molins, C., Mead, P., and Schriefer, M. (2016). Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of lyme disease, United States. Emerg. Infect. Dis. 22, 1169–1177. doi: 10.3201/eid2207.151694
Opota, O., Jaton, K., and Greub, G. (2015). Microbial diagnosis of bloodstream infection: towards molecular diagnosis directly from blood. Clin. Microbiol. Infect. 21, 323–331. doi: 10.1016/j.cmi.2015.02.005
O’Rourke, M., Traweger, A., Lusa, L., Stupica, D., Maraspin, V., Barrett, P. N., et al. (2013). Quantitative detection of Borrelia burgdorferi sensu lato in erythema migrans skin lesions using internally controlled duplex real time PCR. PLoS One 8:e63968. doi: 10.1371/journal.pone.0063968
Pavia, C. S., and Plummer, M. M. (2018). Transfusion-associated Lyme disease – although unlikely, it is still a concern worth considering. Front. Microbiol. 9:2070. doi: 10.3389/fmicb.2018.02070
Pavšič, J., Žel, J., and Milavec, M. (2016). Assessment of the real-time PCR and different digital PCR platforms for DNA quantification. Anal. Bioanal. Chem. 408, 107–121. doi: 10.1007/s00216-015-9107-2
Phallen, J., Sausen, M., Adleff, V., Leal, A., Hruban, C., White, J., et al. (2017). Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 9:eaan2415. doi: 10.1126/scitranslmed.aan2415
Pickard, D. J. (2009). Preparation of bacteriophage lysates and pure DNA. Methods Mol. Biol. 502, 3–9. doi: 10.1007/978-1-60327-565-1_1
Pratama, A. A., Chaib De Mares, M., and Van Elsas, J. D. (2018). Evolutionary history of bacteriophages in the genus Paraburkholderia. Front. Microbiol. 9:835. doi: 10.3389/fmicb.2018.00835
Primus, S., Akoolo, L., Schlachter, S., Gedroic, K., Rojtman, A. D., and Parveen, N. (2018). Efficient detection of symptomatic and asymptomatic patient samples for Babesia microti and Borrelia burgdorferi infection by multiplex qPCR. PLoS One 13:e0196748. doi: 10.1371/journal.pone.0196748
Ravagnan, S., Tomassone, L., Montarsi, F., Krawczyk, A. I., Mastrorilli, E., Sprong, H., et al. (2018). First detection of Borrelia miyamotoi in Ixodes ricinus ticks from northern Italy. Parasit. Vectors 11:130. doi: 10.1186/s13071-018-2713-z
Sambrook, J., and Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
Sambrook, J., and Russell, D. W. (2006). Purification of nucleic acids by extraction with phenol:chloroform. Cold Spring Harb. Protoc. 2006:db.rot4455. doi: 10.1101/pdb.prot4455
Santino, I., Berlutti, F., Pantanella, F., Sessa, R., and Del Piano, M. (2008). Detection of Borrelia burgdorferi sensu lato DNA by PCR in serum of patients with clinical symptoms of Lyme borreliosis. FEMS Microbiol. Lett. 283, 30–35. doi: 10.1111/j.1574-6968.2008.01134.x
Schutzer, S. E., Body, B. A., Boyle, J., Branson, B. M., Dattwyler, R. J., Fikrig, E., et al. (2019). Direct diagnostic tests for Lyme disease. Clin. Infect. Dis. 68, 1052–1057. doi: 10.1093/cid/ciy614
Schutzer, S. E., Fraser-Liggett, C. M., Casjens, S. R., Qiu, W.-G., Dunn, J. J., Mongodin, E. F., et al. (2011). Whole-genome sequences of thirteen isolates of Borrelia burgdorferi. J. Bacteriol. 193:1018. doi: 10.1128/jb.01158-10
Shan, J., Clokie, M. R., and Teulières, L. (2018). Phage-Based Detection of Borreliosis and Means Therefor. Google Patent No. PCT/GB2017/053323.
Shan, J., Korbsrisate, S., Withatanung, P., Adler, N. L., Clokie, M. R. J., and Galyov, E. E. (2014). Temperature dependent bacteriophages of a tropical bacterial pathogen. Front. Microbiol. 5:599. doi: 10.3389/fmicb.2014.00599
Shan, J., Patel, K. V., Hickenbotham, P. T., Nale, J. Y., Hargreaves, K. R., and Clokie, M. R. (2012). Prophage carriage and diversity within clinically relevant strains of Clostridium difficile. Appl. Environ. Microbiol. 78, 6027–6034. doi: 10.1128/aem.01311-12
Staroscik, A. (2004). Calculator for Determining the Number of Copies of a Template. Available online at: http://cels.uri.edu/gsc/cndna.html (accessed January 20, 2018).
Sun, S., Gao, S., Kondabagil, K., Xiang, Y., Rossmann, M. G., and Rao, V. B. (2012). Structure and function of the small terminase component of the DNA packaging machine in T4-like bacteriophages. Proc. Natl. Acad. Sci. U.S.A. 109, 817–822. doi: 10.1073/pnas.1110224109
Takano, A., Goka, K., Une, Y., Shimada, Y., Fujita, H., Shiino, T., et al. (2010). Isolation and characterization of a novel Borrelia group of tick-borne borreliae from imported reptiles and their associated ticks. Environ. Microbiol. 12, 134–146. doi: 10.1111/j.1462-2920.2009.02054.x
Telford, S. R. III, Goethert, H. K., Molloy, P. J., Berardi, V. P., Chowdri, H. R., Gugliotta, J. L., et al. (2015). Borrelia miyamotoi disease: neither Lyme disease nor relapsing fever. Clin. Lab. Med. 35, 867–882. doi: 10.1016/j.cll.2015.08.002
Theel, E. S., Aguero-Rosenfeld, M. E., Pritt, B., Adem, P. V., and Wormser, G. P. (2019). Limitations and confusing aspects of diagnostic testing for neurologic Lyme disease in the United States. J. Clin. Microbiol. 57, 01406–01418. doi: 10.1128/JCM.01406-18
Waddell, L. A., Greig, J., Mascarenhas, M., Harding, S., Lindsay, R., and Ogden, N. (2016). The accuracy of diagnostic tests for lyme disease in humans, a systematic review and meta-analysis of north American research. PLoS One 11:e0168613. doi: 10.1371/journal.pone.0168613
Wei, B., Chen, L., Kibukawa, M., Kang, J., Waskin, H., and Marton, M. (2016). Development of a PCR Assay to detect low level Trypanosoma cruzi in blood specimens collected with PAXgene blood DNA tubes for clinical trials treating chagas disease. PLoS Negl. Trop. Dis. 10:e0005146. doi: 10.1371/journal.pntd.0005146
Wormser, G. P., Shapiro, E. D., and Fish, D. (2019). Borrelia miyamotoi: an emerging tick-borne pathogen. Am. J. Med. 132, 136–137. doi: 10.1016/j.amjmed.2018.08.012
Ye, J., Coulouris, G., Zaretskaya, I., Cutcutache, I., Rozen, S., and Madden, T. L. (2012). Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13:134. doi: 10.1186/1471-2105-13-134
Keywords: prophages, Lyme disease, diagnosis, multicopy, Borrelia, qPCR
Citation: Shan J, Jia Y, Teulières L, Patel F and Clokie MRJ (2021) Targeting Multicopy Prophage Genes for the Increased Detection of Borrelia burgdorferi Sensu Lato (s.l.), the Causative Agents of Lyme Disease, in Blood. Front. Microbiol. 12:651217. doi: 10.3389/fmicb.2021.651217
Received: 08 January 2021; Accepted: 16 February 2021;
Published: 15 March 2021.
Edited by:
Jie Feng, Lanzhou University Medical College, ChinaReviewed by:
Yang Wu, Fudan University, ChinaCopyright © 2021 Shan, Jia, Teulières, Patel and Clokie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinyu Shan, anM0MDFAbGUuYWMudWs=; Martha R. J. Clokie, bXJqYzFAbGUuYWMudWs=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.