
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 28 January 2021
Sec. Microbiotechnology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.637381
This article is part of the Research TopicAdvances in Microbial Biofuel Production View all 5 articles
 Sizwe I. Mhlongo1*
Sizwe I. Mhlongo1* Obinna T. Ezeokoli2
Obinna T. Ezeokoli2 Ashira Roopnarain3
Ashira Roopnarain3 Busiswa Ndaba3
Busiswa Ndaba3 Patrick T. Sekoai4
Patrick T. Sekoai4 Olivier Habimana4
Olivier Habimana4 Carolina H. Pohl2
Carolina H. Pohl2Microbial lipids, also known as single-cell oils (SCOs), are highly attractive feedstocks for biodiesel production due to their fast production rates, minimal labor requirements, independence from seasonal and climatic changes, and ease of scale-up for industrial processing. Among the SCO producers, the less explored filamentous fungi (molds) exhibit desirable features such as a repertoire of hydrolyzing enzymes and a unique pellet morphology that facilitates downstream harvesting. Although several oleaginous filamentous fungi have been identified and explored for SCO production, high production costs and technical difficulties still make the process less attractive compared to conventional lipid sources for biodiesel production. This review aims to highlight the ability of filamentous fungi to hydrolyze various organic wastes for SCO production and explore current strategies to enhance the efficiency and cost-effectiveness of the SCO production and recovery process. The review also highlights the mechanisms and components governing lipogenic pathways, which can inform the rational designs of processing conditions and metabolic engineering efforts for increasing the quality and accumulation of lipids in filamentous fungi. Furthermore, we describe other process integration strategies such as the co-production with hydrogen using advanced fermentation processes as a step toward a biorefinery process. These innovative approaches allow for integrating upstream and downstream processing units, thus resulting in an efficient and cost-effective method of simultaneous SCO production and utilization for biodiesel production.
Biofuels derived from low-cost substrates such as agro-industrial waste are promising alternative sources of liquid energy (Carvalho et al., 2015; Dong et al., 2016). Bio-based fuel can enable energy independence, reduce greenhouse gas emissions, and enhance sustainable economic development (Xia et al., 2011). Unlike fossil-based fuels, which negatively impacts the environment, biodiesel is considered a clean, renewable, and sustainable liquid fuel, which can replace fossil-based diesel (de Jong et al., 2012; Chopra et al., 2016). Biodiesel’s advantages include high energy density, lubricity, fast biodegradation rate, and low greenhouse gas emissions (Vicente et al., 2010). However, the current production of biodiesel relies on conventional sources such as plants and animals, which results in high feedstock costs, competition for arable land, and jeopardizes food security (Jin et al., 2015).
Oils derived from microorganisms, alternatively known as single-cell oils (SCOs), are similar in composition to vegetable oils and animal fats (Carvalho et al., 2015). However, single-cell oils are preferred to plant- and animal-derived oils because it is easy to scale up their production. Also, the production of SCOs is not impacted by factors such as seasonal changes, geographic location, harvest time and transport, which are of concern when using plant and animal materials (Abghari and Chen, 2014; Dong et al., 2016). Single-cell oils are intracellular storage lipids made of triacylglycerols (TAGs), free fatty acids, polar lipids, sterols, hydrocarbons, and pigments (Carvalho et al., 2015; Ochsenreither et al., 2016). All organisms produce SCOs as part of their structural and functional processes, such as forming permeable membranes of cells and organelles (Ochsenreither et al., 2016). Nevertheless, the capacity of microorganisms to produce SCOs can vary from less than 1% to more than 80% (w/w) in dry cell weight (Athenaki et al., 2017). A small number of microorganisms are known as oleaginous microorganisms due to their unique ability to accumulate lipids over 20% (w/w) of their dry cell mass (Carvalho et al., 2015; Dong et al., 2016; Ochsenreither et al., 2016). The ability of oleaginous microorganisms to accumulate large amounts of lipids compared to non-oleaginous microorganisms is not due to a difference in fatty acid biosynthesis (Ochsenreither et al., 2016). Oleaginous microorganisms depend on a continuous supply of acetyl coenzyme A (acetyl-CoA) and nicotinamide adenine dinucleotide phosphate (NADPH) for the production of fatty acids through a reversed β-oxidation under nitrogen-limiting conditions (Ochsenreither et al., 2016). The propensity of microorganisms to accumulate lipids is also primarily determined by their genetic profile, and this may vary even among species or between strains of a given species (Athenaki et al., 2017).
As the body of knowledge is continually expanding in SCO research, it is imperative to update the scientific community with recent advancements. Irrespective of the expansion in SCO research efforts, filamentous fungi remain the less explored SCO producer. However, oleaginous filamentous fungi exhibit several desirable intrinsic features such as a repertoire of hydrolyzing enzymes and a unique pellet morphology that facilitates downstream harvesting, prompting the need for the present work. Hence, this review examines the ability of filamentous fungi in the hydrolysis of various organic feedstocks to produce SCOs. This review also explores the types of mechanisms regulating the synthesis of SCOs from these carbon materials. Furthermore, the paper evaluates the possibilities of integrating SCO processes with other biotechnological processes such as bio-hydrogen production. Future prospects of SCO fermentations are also discussed.
Identifying microorganisms capable of producing a significant amount of SCOs, with compositional similarities to conventional lipids, will be highly beneficial for improving process economics (Athenaki et al., 2017). Mucor circinelloides were the first filamentous fungi used in the commercial production of polyunsaturated fatty acids (PUFAs) (Ratledge, 2004; Čertík et al., 2012). Single-cell oils produced by oleaginous microorganisms such as filamentous fungi are desirable as starting material for biodiesel production due to their high degree of unsaturation. Unfortunately, the large-scale industrialization of microbial SCO has not been achieved due to the high cost of production. Over the years, innovations such as introducing novel fermentation technologies and versatile engineered oleaginous strains have improved the competitiveness of microbial lipid production. Despite all these developments, microbial lipids’ production cost remains high and needs to be reduced to realize its economic viability for use in biodiesels, drop-in biofuels, and other products (Abghari and Chen, 2014).
One strategy that may be exploited to lower PUFA production costs is to integrate microbial oil production with other high-value compounds in a process that uses low-cost agro-industrial waste and lignocellulosic biomass (Ratledge, 2004). Since filamentous fungi produce various hydrolyzing enzymes, allowing them to metabolize complex substrates (Gmoser et al., 2019), they can produce PUFAs from such low-cost substrates. Thus, the use of filamentous fungi as SCO producers presents an opportunity to combine saccharification and fermentation for efficient use of low-cost substrates, such as agro-industrial waste and lignocellulosic substrates, making the process more cost-effective (Martínez et al., 2015).
Several oleaginous filamentous fungi, mostly belonging to two major phyla (Mucoromycota and Ascomycota), have been identified and are taxonomically summarized in Figure 1. Mucor circinelloides was the first species used for the industrial production of γ-linolenic acid (GLA)-rich oil, a precursor for several biologically active compounds (Somashekar et al., 2003; Ratledge, 2013). Over the years, several other oleaginous filamentous fungi have been identified from different sources, including soil, herbaceous plants and animal feeds (Liu et al., 2010; Dey et al., 2011; Banu et al., 2017; Muniraj et al., 2017; Tonato et al., 2018). Particular interest has been placed on Mucoromycetes (formerly Zygomycetes) and Ascomycota species, such as Aspergillus niger, Cunninghamella echinulata, Thermomyces lanuginosus (formerly Humicola lanuginosa), Umbelopsis isabellina (formerly Mortierella isabellina), and Umbelopsis vinacea (formerly Mortierella vinacea), because of their high lipid accumulation (Papanikolaou et al., 2004; Fakas et al., 2008, 2009; Meng et al., 2009).
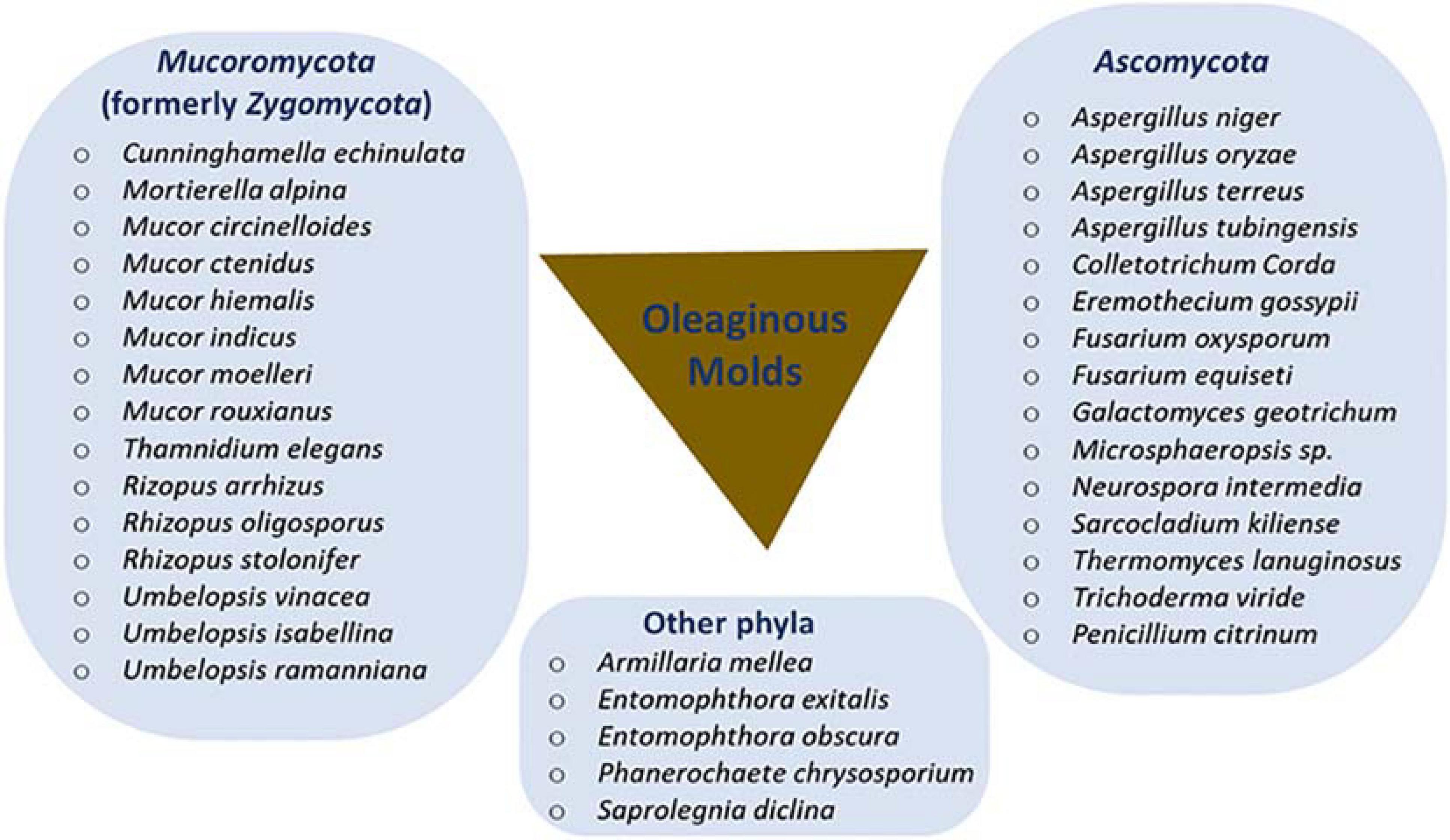
Figure 1. Phyla classification of oleaginous molds. Revised (previous names in parenthesis) taxonomic classifications include genera Umbelopsis (Mortierella), Thermomyces (Humicola), and Eremothecium (Ashbya) as well as the species Mucor moelleri (Zygorhynchus moelleri), Mucor ctenidius (Thamnidium ctenidium), and Rhizopus arrhizus (Mucor rouxii).
Single-cell oils derived from filamentous fungi are highly attractive feedstocks in sustainable biodiesel production since they produce SCOs with similar characteristics to those of conventional vegetable oils. Interestingly, biodiesel produced from microbial oils can be mixed with petroleum diesel at any ratio (Bhanja et al., 2014). Due to the array of benefits associated with filamentous fungi-derived SCOs for third-generation biodiesel production, there has been a surge in screening efforts for promising oleaginous strains (Khot et al., 2012; Abu-Elreesh and Abd-El-Haleem, 2014; Abou-Zeid et al., 2019). Primary considerations when selecting a fungal strain for biodiesel production include elevated total lipid yield, especially high levels of TAGs, and the optimal composition of fatty acids (Khot et al., 2012; Bardhan et al., 2019). In this regard, the degree of branching and unsaturation, as well as chain length, are notable structural features to be considered when selecting fungal SCOs for biodiesel production (Sitepu et al., 2014; Table 1). Oleaginous fungal strains that produce oils that are rich in monounsaturated fatty acids (MUFA) and saturated fatty acids (SFA) can be utilized for biodiesel production, whereas strains rich in polyunsaturated fatty acids (PUFAs) are more desirable for nutraceuticals and pharmaceuticals (Patel et al., 2020). This is due to lipid with elevated levels of PUFAs, which possess more than two double bonds, being susceptible to auto-oxidation. Hence, PUFA rich lipids are more unstable and technically unsatisfactory for biodiesel production. Moreover, PUFA rich lipids results in an unpleasant odor (Knothe, 2012; Patel et al., 2020).
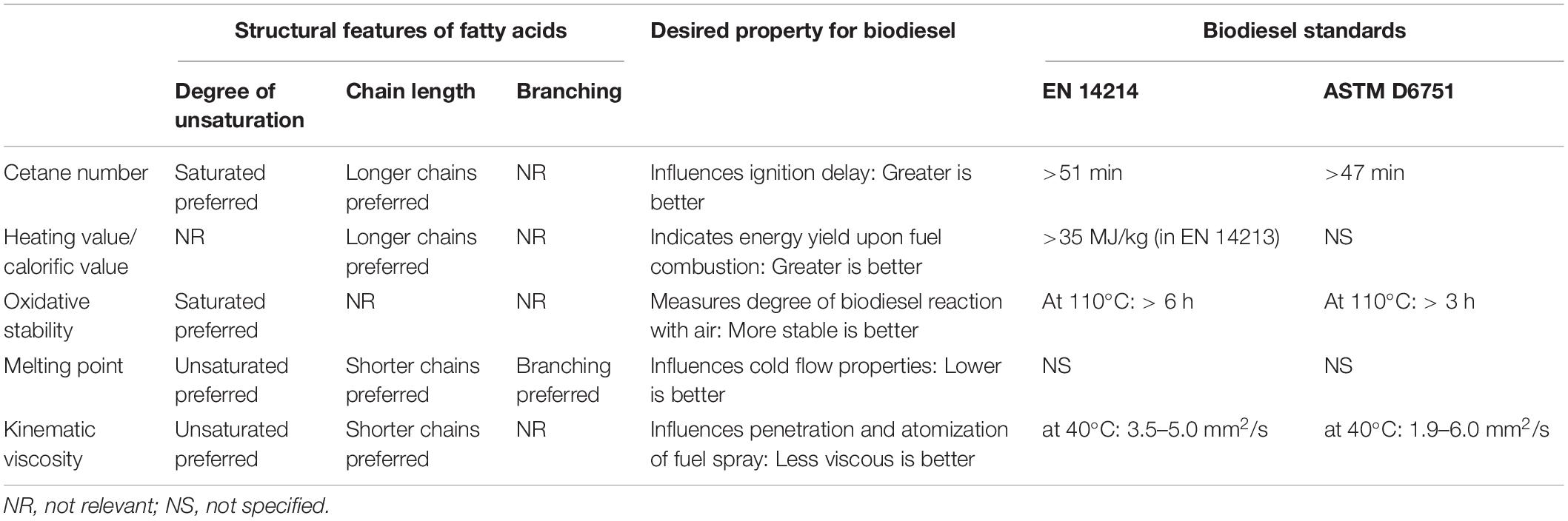
Table 1. Relationship between structural features of fatty acids and selected biodiesel properties as well as biodiesel standards (Rashid et al., 2009; Sitepu et al., 2014; Singh et al., 2019).
In general, lipids intended as feedstock for biodiesel production must be composed of elevated concentrations of MUFAs such as palmitoleic acid (C16:1) and oleic acids (C18:1), controlled concentrations of SFAs such as palmitic acid (C16:0) and stearic acid (C18:0) and minimal quantities of PUFAs such as linoleic acid (C18:2) and γ-linolenic acid (C18:3) (Miglio et al., 2013). Fatty acid profiles influence various biodiesel properties such as melting point, cetane number, heating value/calorific value, kinematic viscosity and oxidative stability, which greatly affect the performance of the generated biodiesel (Sitepu et al., 2014). The above-mentioned biodiesel properties are regulated by standards (Table 1); hence the screening process must be conducted with utmost care to ensure that the selected strain is ideal for biodiesel production.
Fungal strains that produce lipids with the above-mentioned qualities and that can metabolize low-cost substrates, display a rapid growth rate, and can be easily harvested, are considered more valuable due to the ability of such strains to substantially reduce costs associated with the production process (Sitepu et al., 2014).
An array of detection methods has been established as the first step when screening oleaginous fungi for lipid production potential (Kosa et al., 2017). The second step in the screening process is frequently a qualitative evaluation of the lipids produced by the fungal strain of interest. Traditional methods such as gas chromatography-mass spectrometry (GC-MS) and gas chromatography-flame ionization detection (GC-FID) is mostly utilized for fatty acid analysis (Rumin et al., 2015).
Traditional oleaginous fungi screening methods, which are mostly based on cultivation of strains of interest in shake flasks followed by lipid extraction, quantification and qualitative analysis are feasible when screening a small set of fungal isolates. However, it becomes laborious, costly and inefficient when screening hundreds of strains (Forfang et al., 2017; Kosa et al., 2017). Therefore, high-throughput screening methods such as those described by Kosa et al. (2017) and Yao et al. (2019) have been developed to improve the efficiency of the screening process.
Since the selection of fungal strains with the highest lipid productivity is imperative to improve the cost-effectiveness of the entire biodiesel production process, the adoption of currently available high-throughput screening methods and the development of novel, more rapid screening methods will accelerate the oleaginous fungal screening process and enable the selection of the most promising strains for downstream application in biodiesel generation. Moreover, the integration of automation in the screening process will ensure that more fungal strains can be screened simultaneously, improving the probability of the most promising isolate being selected.
The synthesis of fatty acids in oleaginous microorganisms follows two metabolic routes, de novo (on hydrophilic substrates) or ex novo (on hydrophobic substrate) (Subhash and Mohan, 2011). In this review, we will focus only on de novo lipid synthesis route. The regulation of lipid biosynthesis has been extensively studied in other microorganisms such as yeast, but little has been reported on filamentous fungi. A better understanding of the cellular machinery regulating lipid metabolism in filamentous fungi is essential for improving lipid yields and fatty acid profiles (Laoteng et al., 2011; Vorapreeda et al., 2012; Chen et al., 2015). Although the lipid accumulation process seems to be conserved, there is a considerable variation amongst oleaginous species and even within strains of the same species (Rodríguez-Frómeta et al., 2013). The factors driving these variations are not yet known. Fortunately, genomic data is now available in the literature, and more studies have provided better insights into the regulation of lipid metabolism in oleaginous microorganisms (Vorapreeda et al., 2012).
Lipid biosynthesis involves multiple enzymes, including malic enzymes (MEs), malate transporter, desaturases, ATP-citrate lyase (ACL), adenosine monophosphate deaminase (AMPD), and acetyl-CoA carboxylase 1 (ACC1) (Hao et al., 2014, 2016; Shi et al., 2014; Kerkhoven et al., 2016; Zhao et al., 2016; Zhang et al., 2017; Chang et al., 2019; Wang et al., 2019). Importantly, NADPH and acetyl-CoA (fatty acid precursor) supply are crucial for lipid biosynthesis and accumulation in oleaginous microorganisms (Wang et al., 2019). The major pathways for the generation of NADPH during glucose metabolism include: (i) the pentose phosphate (PP) pathway, with glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase, (ii) the pyruvate/oxaloacetate/malate (POM) cycle, through NADP + dependent malic enzyme, or (iii) the tricarboxylic acid (TCA) cycle via NADP + dependent isocitrate dehydrogenase (Zhao et al., 2016; Wang et al., 2019).
Nutrient imbalances, most typically the excess of carbon source and a limitation in other vital nutrients such as nitrogen, triggers several physiological and metabolic changes leading to the channeling of the carbon flux toward lipid synthesis (Laoteng et al., 2011; Papanikolaou, 2011; Athenaki et al., 2017). Nitrogen starvation results in the activation of adenosine monophosphate (AMP) deaminase, which decreases the concentration of intracellular AMP (Garay et al., 2014; Athenaki et al., 2017). A decrease in mitochondrial AMP concentration alters the Krebs cycle function, resulting in a loss of activity by isocitrate dehydrogenase, which is allosterically activated by intracellular AMP (Papanikolaou, 2011; Tan et al., 2016; Dourou et al., 2018). Isocitrate accumulates in the mitochondria as the production of α-ketoglutarate in the tricarboxylic cycle (TCA) cycle is reduced (Garay et al., 2014). Isocitrate is converted back into citrate, resulting in the carbon flow being redirected toward intra-mitochondrial citric acid (Papanikolaou, 2011; Athenaki et al., 2017). Excess citrate is subsequently secreted to the cytoplasm by an antiport protein, citrate/malate transporters. These transporters facilitate the transport of malate from the cytosol into the mitochondrion in exchange for mitochondrial citrate moving into the cytosol, thus linking the TCA cycle in mitochondria and fatty acid biosynthesis in the cytosol (Ratledge and Wynn, 2002; Zhao et al., 2016; Yang J. et al., 2019). In the cytoplasm, citrate is cleaved by ATP-citrate lyase (ACL) into oxaloacetate (OAA) and acetyl-CoA, which together with NADPH generated in the cytosol, is required for lipid biosynthesis (Ratledge and Wynn, 2002; Zhao et al., 2016; Yang J. et al., 2019; Figure 2). In a study by Vorapreeda et al. (2012), it was observed that ACL was not present in non-oleaginous strains, suggesting that this enzyme only confers an additional route of acetyl-CoA synthesis in oleaginous strains. Similar results were reported by Chen et al. (2015), where they identified 23 lipogenesis-associated genes in the oleaginous fungus, Mortierella alpina, which shared a high similarity of expression patterns to other oleaginous microorganisms, but not with non-oleaginous Aspergillus nidulans.
Oxaloacetate is reduced by malate dehydrogenase to form malate in the cytosol, which is subsequently converted to pyruvate by malic enzyme (ME) (Rodríguez-Frómeta et al., 2013). Malic enzyme plays a critical role in lipid synthesis, and earlier studies suggested that this enzyme is a rate-limiting step in fatty acid synthesis (Hao et al., 2014). Comparative studies have since identified five putative genes encoding MEs. Three of these MEs are located in the mitochondria, while the other two are cytosolic (Rodríguez-Frómeta et al., 2013). An additional route for acetyl-CoA can also be through the generation of glycerol 3-phosphate in glycolysis, which is a building block for the synthesis of phospholipids and triglycerides (Vorapreeda et al., 2012) as well as through the amino acid pathway (Hao et al., 2014).
Nicotinamide adenine dinucleotide phosphate has the reducing power required to convert acetyl-CoA into fatty acids (Figure 2). In this conversion, acetyl-CoA is carboxylated by acetyl-CoA carboxylase (ACC) to form malonyl-CoA (Garay et al., 2014). ACC is a multifunctional complex with components including biotin carboxylase, carboxyltransferase and biotin carboxyl carrier protein domains. This enzyme plays a role in controlling the overall lipid biosynthesis (Laoteng et al., 2011). The condensation of acetyl-CoA and malonyl-CoA follows the carboxylation process by a multi-enzyme complex fatty acid synthase (FAS), which elongates the acyl-CoA chain (Ledesma-Amaro et al., 2014). Acyl-chains are then added to the growing fatty acid chain. This is followed by three successive reactions, namely, reduction, dehydration and reduction, after which saturated fatty acid chains, palmitic acid (16:0) or stearic acid (18:0) are generated as the final product (Muniraj et al., 2015; Santek et al., 2018).
Lipid biosynthesis is complex and cannot be sufficiently gaged through the analysis of single genes or pathways (Chen et al., 2015). Based on the relatively conserved fatty acid pathways in oleaginous microorganisms, the increasing availability of sequence data provides more insight into the molecular mechanisms involved in regulating fatty acid synthesis (Vorapreeda et al., 2012).
Lipid accumulation (the metabolic balance between lipid biosynthesis and degradation) in oleaginous molds can be improved by optimizing culture conditions and substrates and through metabolic engineering. In this section, we provide a review of notable studies in which optimization of cultivation conditions and engineering of genetic components and regulatory mechanisms in the genome of oleaginous filamentous fungi have been conducted in order to increase lipid accumulation.
Storage lipids of oleaginous fungi usually contain TAGs with the right proportion of saturated fatty acids (SFA) during the stationary phase (Aggelis et al., 1995). The lipids are stored within specialized organelles known as lipid granules/lipid droplets. The process of lipid formation in the cell involves the metabolic conversion of the external carbon source into carbohydrates or hydrocarbons and subsequently, lipids (Subhash and Mohan, 2011). Various types of polysaccharides derived from agro-industrial activities are used as carbon sources for SCO production. However, carbon sources such as glucose and starch, which have fundamental biochemical similarities, can lead to differences in lipid accumulation when used for the cultivation of certain oleaginous filamentous fungi (Dyal et al., 2005; Chatzifragkou et al., 2010). Also, the metabolism of various individual sugars can impact the fatty acid composition of storage lipids (Somashekar et al., 2003; Jang et al., 2005; Papanikolaou and Aggelis, 2010).
Other factors which affect the accumulation and fatty acid composition of cellular lipids include temperature, pH, cultivation period, fortification of culture media with specific nutrients such as nitrogen, the inclusion of growth enhancers, agitation, aeration and incorporation of substrates at different concentrations and time points (Hansson and Dostálek, 1988; Kendrick and Ratledge, 1992b; Carvalho et al., 1999; Somashekar et al., 2003; Papanikolaou et al., 2004; Jang et al., 2005; Fakas et al., 2009; Liu et al., 2010; Dey et al., 2011). Some studies investigating optimal values of these cultural parameters for maximum lipid accumulation and fatty acid composition are summarized in Table 2. In most cases, values above or below the optimum led to lower lipid yield or different fatty acid composition. Importantly, the accumulation of lipids is influenced not only by individual factors but a combination of factors and is species/strain-specific. Thus, the optimization of process parameters for maximum lipid yields is usually required and conducted on a strain-by-strain basis (Table 3). Some of these factors that affect lipid accumulation and fatty acid composition are discussed in the following subsections.
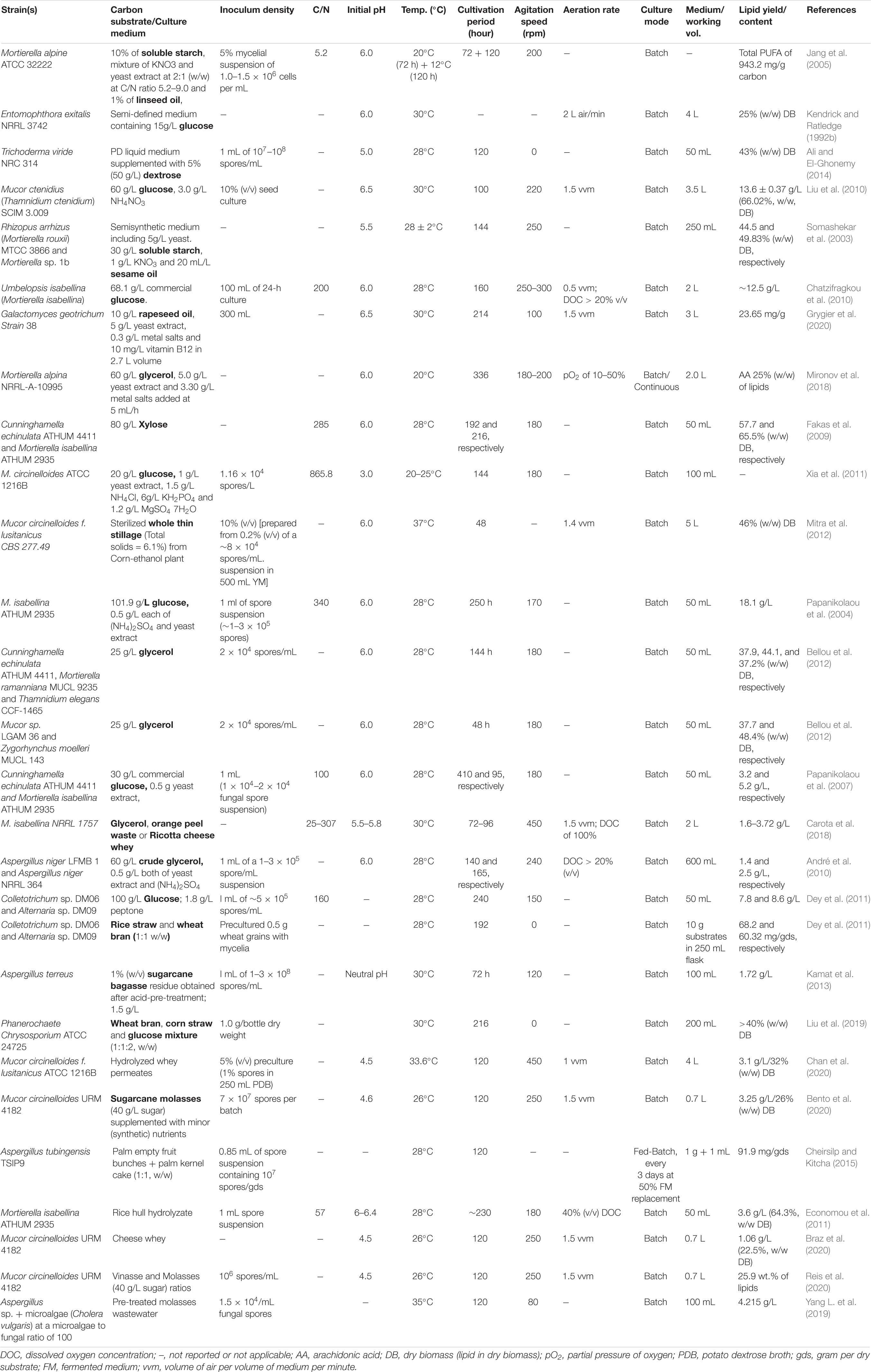
Table 3. Optimal culture conditions employed for maximum lipid production in some oleaginous filamentous fungal studies.
Temperature is a vital factor in regulating fungal fatty acid composition (Neidleman, 1987; Kendrick and Ratledge, 1992b; Mamatha and Venkateswaran, 2010). Lipid fluidity, consequent on the degree of unsaturation, can be influenced by the incubation temperature. Generally, at lower growth temperature, the proportion of unsaturated acids in the total lipid tends to increase (Carvalho et al., 1999). Hence, thermophilic fungi can produce fatty acids that are more saturated than mesophilic fungi (Bruszewski et al., 1972; Kendrick and Ratledge, 1992b). At lower temperatures, thermophilic fungi cannot produce unsaturated fatty acids in sufficient quantities to keep cell membranes in a liquid crystalline state (Neidleman, 1987; Kendrick and Ratledge, 1992b). An early study by Deven and Manocha (1976) on Choanephora cucurbitarum showed that there was increased production of γ-linolenic acid (GLA) at lower growth temperatures with a corresponding increase in the degree of unsaturation of total lipids observed. Kendrick and Ratledge (1992b) investigated the effect of varying temperatures (20–30°C) on fatty acid composition in the oleaginous filamentous fungus, Entomophthora exitalis, under continuous culture conditions with a constant dilution rate of 0.04 h–1. It was observed that the proportion of PUFAs in the total lipids increased proportionally from 18 to 27% (w/w) as the temperature decreased from 30 to 20°C. The observed increase in unsaturation was due to an increase in n−6 PUFA in the phospholipid and sphingo- plus glycolipid fractions. However, the growth of the fungus at a constant dilution rate and temperature (22°C) over a range of dissolved oxygen tension values did not influence lipid unsaturation and triacylglycerol fraction of the lipids. Thus, it was concluded that the observed change in lipid unsaturation is mainly a result of temperature.
Similarly, a study by Mamatha and Venkateswaran (2010) also concluded that temperature is the principal regulatory factor in the degree of unsaturation in the lipid profile of Mucor rouxii CFR-G15. In a separate study, Carvalho et al. (1999) observed that the strain Mucor sp. LB-54 accumulated intracellular lipid at 20.73% of dry cell weight, with a GLA content of 15% of total fatty acids, after 5 days of cultivation at 28°C. However, the GLA percentage increased to 24% of total fatty acids as the incubation temperature was reduced to 12°C. In general, as part of their adaptive response to low temperatures, oleaginous filamentous possess relatively high degrees of unsaturation in their lipid profile at lower temperatures (Mamatha and Venkateswaran, 2010).
Nitrogen plays a crucial role in stimulating lipid accumulation. The effect of nitrogen is inverse as the accumulation of lipids by oleaginous fungi is triggered by the exhaustion of the exogenous nitrogen in the culture medium (Chatzifragkou et al., 2010; Xia et al., 2011). However, although the exhaustion of nitrogen allows the conversion of carbon to lipid, it prevents cell proliferation (Somashekar et al., 2003; Dey et al., 2011; Xia et al., 2011). Xia et al. (2011) observed that an increase in the initial nitrogen level in the culture medium coincided with a gradual increase in biomass and glucose consumption by M. circinelloides. However, in media with the highest carbon-to-nitrogen ratio, depletion of nitrogen by M. circinelloides caused the highest lipid accumulation of close to 30% of the dry weight. Dey et al. (2011) evaluated the biomass and lipid yields of two endophytic fungi, Colletotrichum sp. and Alternaria sp., using organic (peptone) and inorganic [(NH4)2SO4, NH4NO3, and KNO3] nitrogen sources. It was observed that nitrogen source influenced biomass and lipid yields of both, with the organic peptone source producing the highest biomass and lipid yield.
In contrast to Liu et al. (2010) and Dey et al. (2011) observed that NH4NO3 was the most suitable nitrogen source for highest biomass and lipid yields in Mucor ctenidius (Thamnidium ctenidium), compared to other inorganic and organic nitrogen sources such as yeast extract, wheat bran, peptone, soybean and corn powder. Elsewhere, KNO3 was the nitrogen source that gave the highest PUFA yield in Mucor rouxii, Mortierella sp., and Mortierella ramanniana strains (Hansson and Dostálek, 1988; Somashekar et al., 2003). The disparity in the suitability of different nitrogen sources observed in the above-highlighted studies can be attributed to strain-specific differences in nutrient requirements as well as differences in other growth conditions employed between studies.
Studies have shown that pH is a stress-inducing factor that affects the accumulation of lipids and their PUFA profiles (Xia et al., 2011; Mironov et al., 2018). In a study by Xia et al. (2011), differences in pH [either acidic (pH 1–3) or alkaline (pH 8.0–10.0)] significantly influenced the cell growth and thus, lipid yields of M. circinelloides, with the highest lipid accumulation observed at a pH of 6.0. Cell growth was totally inhibited at pH values of 1 and 2. Mironov et al. (2018) investigated the effect of pH on the growth of Mortierella alpina (NRRL-A-10995) and the synthesis of lipids and arachidonic acid (AA) in both exponential and stationary phases of growth. The M. alpina strain was initially grown at pH 6.0 for 4 days, after which the pH was adjusted to a pH of 3, 4, 5, 6, 7, or 8 for a further 7 and 14 days. The authors observed that an optimal pH for the growth (biomass accumulation) of M. alpina was 5. Although biomass accumulation remained high between a pH of 5 to 7, a strong inhibition of growth was observed at a pH of 8 as well as at a pH below 5, with a pH of 3 strongly inhibiting the growth of M. alpina. Furthermore, the authors observed that AA synthesis was sensitive to an acidic pH and was inhibited at a pH of 3 (coinciding with inhibition of growth). These observations confirm that pH is a stress factor for cell growth and consequently, lipid accumulation and fatty acid composition.
Filamentous fungi are obligate aerobes; thus, oxygen is required for their cellular functions. Replenishing oxygen in the culture medium is achieved through aeration and/or agitation. Agitation helps to increase surface area for oxygen transport and concentration (dissolved oxygen). However, the rate of agitation needs to be optimized because vigorous agitation may lead to the shearing of cells, while a very low agitation rate may not improve the oxygen concentration sufficiently. Ali and El-Ghonemy (2014) investigated the effect of agitation (at 150 rpm) or the lack thereof on biomass and lipid accumulation of Trichoderma viride. They observed that the highest lipid accumulation (18.35%, w/w dry mass) occurred under static conditions compared to shake cultures (13.89%, w/w dry mass). In contrast, Somashekar et al. (2003) reported that an agitation speed of 250 rpm improved total lipid and GLA yields in both Mortierella sp. and Mucor rouxii. Similarly, lipid accumulation in Mucor ctenidius was higher at an agitation speed of 220 rpm compared to speeds below 220 rpm (Liu et al., 2010). Elsewhere, Kendrick and Ratledge (1992b) observed no difference in lipid accumulation across aeration rates of 150 to 1000 ml/min in Entomophthora exitalis, suggesting that aeration and aeration rates may not be as critical a factor as temperature for the accumulation of lipids in this fungus. Overall, the number of studies in which agitation improves lipid and PUFA profiles of oleaginous fungi appear to be higher in number than those reporting otherwise.
Under nitrogen-limited conditions, excess carbon is diverted to lipid biosynthesis in many oleaginous filamentous fungi (Kavadia et al., 2001; Jang et al., 2005; Chatzifragkou et al., 2010). Carbon substrates are critical for both microbial nutrition, lipid accumulation and fatty acid composition of lipids (Somashekar et al., 2003). To this end, the effect of different carbon-rich substrates and their concentration on lipid accumulation in oleaginous molds have been investigated (Somashekar et al., 2003; Jang et al., 2005; Chatzifragkou et al., 2010; Ali and El-Ghonemy, 2014). Carbon sources (or substrates) used for lipid accumulation studies include molasses, sugar beet, soluble starch, and hydrolyzed carbohydrates such as glucose, sucrose, lactose, and raffinose.
Somashekar et al. (2003) investigated the relative percentage of fatty acids of lipids when M. rouxii and Mortierella sp. were cultivated on glucose, starch, sucrose and lactose. Among these carbon sources, GLA production was maximal in cultures grown on glucose, while lactose poorly promoted biomass and lipid production in both M. rouxii and Mortierella sp. Ali and El-Ghonemy (2014) reported that dextrose, compared to sucrose, lactose, fructose and other simple sugars, promoted the lipid accumulation in Trichoderma viride. The concentration of carbon source can also influence lipid accumulation in oleaginous filamentous fungi. Liu et al. (2010) investigated the effect of varying concentrations of lactose (20–100 g/L) on lipid accumulation in Mucor ctenidius. They observed that lipid and biomass yields increased steadily with lactose concentration, but peaked at 60 g/L, with higher concentrations causing a reduction in biomass and lipid yields.
In addition to carbon substrates, the supplementation with plant oils such as sesame oil, rapeseed oil and linseed oil, have been shown to increase biomass and lipid accumulation as well as alter polyunsaturated fatty acid profiles of lipids produced by oleaginous filamentous fungi (Somashekar et al., 2003; Jang et al., 2005; Grygier et al., 2020). In a study by Somashekar et al. (2003), the biomass and lipid accumulation of both Mortierella rouxii and Mortierella sp. grown on glucose supplemented with sesame oil were all higher than those grown on solely glucose. Surprisingly, there was a cessation of GLA production in the glucose plus sesame oil treatment, suggesting possible repression of Δ6-desaturase, an enzyme responsible for converting linoleic acid (LA, C18:2 ω6) to GLA. Similarly, Grygier et al. (2020) obtained maximum PUFA and higher levels of n−3 fatty acids when the medium was supplemented with 10 g/L rapeseed oil at optimum culture conditions than controls without plant oils. Jang et al. (2005) investigated the influence of soluble starch and glucose on lipid accumulation and fatty acid composition in Mortierella alpina. It was observed that arachidonic acid and total PUFAs production was maximal using 10% soluble starch and that further supplementation with linseed oil (1%, w/v) increased the total PUFA production.
Importantly, oleaginous microorganisms have several technical advantages compared to vegetable oil crops. However, the use of highly refined polysaccharides (i.e., industrial-grade glucose and sucrose) as carbon source may decrease the cost-effectiveness of SCO production in an industrial setting. It is estimated that substrates account for about 60 to 70% of the overall costs in fermentation processes (Papanikolaou and Aggelis, 2011; Tzimorotas et al., 2018). Therefore, carbon substrates derived from waste are now being explored to make this process both cost-effective and environmentally-friendly. To this end, studies utilizing waste materials as carbon sources have been investigated for microbial lipid production. These wastes include potato wastewater, orange peel extracts, sugarcane bagasse residues and cheese whey (Kamat et al., 2013; Muniraj et al., 2013; Carota et al., 2018; Braz et al., 2020).
For an efficient lipid production process, the carbon sources should consist of a high carbon-nitrogen (C/N) ratio (Ratledge, 1993). Several substrates were shown to have an appropriate C/N ratio for the lipid biosynthesis by fungal species. Carota et al. (2018) produced high lipid content in various oleaginous fungi (Aspergillus, Mucor, Mortierella, and Cunninghamella), using orange peel extract and ricotta cheese whey in shake-flasks. Amongst the tested strains, Mortierella exhibited superior performance and generated lipid productivity that ranged from 0.46–1.24 g/L.d. Muniraj et al. (2013) used potato wastewater for microbial lipid production by the oleaginous filamentous fungus, Aspergillus oryzae. In this study, maximum lipid production of up to 3.5 g/L.d was obtained at an optimum dilution ratio of 25%. The authors also observed that the major fatty acids in the lipids consisted of linolenic acid (5.5%), linoleic acid (6.5%), palmitic acid (11.6%), palmitolic acid (15.6%), and stearic acid (19.3%). More recently, sugarcane molasses was successfully used as sole carbon source to produce SCOs, using the filamentous fungus Mucor circinelloides. A maximum lipid productivity of 0.66g/L.d, which was equivalent to a lipid content of 29% (w/w) was attained (Bento et al., 2020). In a similar study, André et al. (2010) demonstrated an innovative approach of converting the biodiesel derived waste glycerol into value-added compounds (biomass, SCOs, and oxalic acid) using two fungal strains (Lentinula edodes and Aspergillus niger). In these experiments the authors used Lentinula edodes strains to produce biomass and two A. niger strains to produce SCO (3.1–3.5 g/L) and oxalic acid (20.5–21.5 g/L) under nitrogen-limiting shake flask conditions. In addition to these studies, other types of feedstocks that have been used in SCO production are shown in Table 2.
Inoculum density and size are factors that may influence lipid accumulation and fatty acid profile (Chen and Liu, 1997). High inoculum density may affect mycelial morphology, growth and product formation, by limiting the diffusion of nutrients within the mycelial floc (Chen and Liu, 1997). On the contrary, the growth of mycelium in smaller and compact inoculum pellets promotes biomass growth and lipid production, because the diffusion limitation of oxygen and other nutrients through the medium is reduced. The effects of inoculum on product formation have been reported in Aspergillus oryzae (Fukushima et al., 1991). Similarly, in a comprehensive study by Chen and Liu (1997), it was observed that the duration of vortexing (for cell homogenization) during inoculum preparation, the mass of the mycelial inoculum and suspension ratio influenced yields of biomass, lipids and GLA content of the lipids in Cunninghamella echinulata. Specifically, the maximum GLA yield of 1.349 mg/L was obtained at a vortex duration of 4 min, a mycelial mass of 1 g and an inoculum suspension of 4%.
Over the years, genetic manipulation of oleaginous filamentous fungi for increased lipid accumulation has involved recombinant DNA technology through RNA interference (RNAi) (Takeno et al., 2005), electroporation (Gutiérrez et al., 2011) and vector-mediated transformations (Zhang et al., 2007; Ando et al., 2009; Chang et al., 2019; Khan et al., 2019a). Unlike in oleaginous yeasts, where the recently developed clustered regularly interspaced short palindromic repeats (CRISPR)/associated protein 9 (CRISPR/Cas9) system for gene editing has been applied to improve fatty acid accumulation (Yao et al., 2020), there is a paucity of studies utilizing CRISPR/Cas9 to engineer oleaginous filamentous fungi for increased lipid accumulation.
With the existing technologies, genetic modifications geared toward increasing lipid accumulation in oil-producing organisms have been performed using different strategies such as the overexpression of fatty acid biosynthetic enzymes, overexpression of TAGs biosynthesis-enhancing enzymes, regulation of related TAG biosynthesis bypass, partial blockage of TAG competing pathways and introduction of multiple genes which enhance lipid accumulation (Zou et al., 1997; Ratledge, 2002; Zhang et al., 2007; Lardizabal et al., 2008; Courchesne et al., 2009; Kamisaka, 2010; Liang and Jiang, 2013; Ledesma-Amaro et al., 2015; Table 4).
Lipid biosynthesis in oleaginous organisms is mediated by various gene products, which collectively work toward the synthesis of fatty acid by converting intracellular carbon. These genes and their associated proteins have been the target of many metabolic engineering studies. The NADP+-dependent malic enzyme (ME) has been shown to be a major source of NADPH which is critical for the synthesis of storage lipids in Mucor circinelloides (Zhang et al., 2007) and other oleaginous fungi (Evans and Ratledge, 1985; Kendrick and Ratledge, 1992a; Wynn and Ratledge, 1997). Zhang et al. (2007) utilized two recombinant strains of M. circinelloides in which the full length ME-coding genes from either M. circinelloides or Mortierella alpina were integrated under the control of the constitutive glyceraldehyde-3-phosphate dehydrogenase gene (GPD1) promoter. Upon cultivation of the two recombinant strains on a high C:N ratio medium in automated controlled submerged-culture bioreactors, a 2- to 3-fold increase in the activities of malic enzyme was observed, with a corresponding increase in the cellular lipid content from 12 to 30%. This increased lipid content coincided with a slight increase in the degree of fatty acid desaturation. These results confirm that the supply of NADPH solely by ME is a rate-limiting step during fatty acid biosynthesis in Mucor circinelloides and Mortierella alpina.
To elucidate the role of the citrate transporter in relation to lipid biosynthesis and accumulation, Yang J. et al. (2019) inserted citrate transporter genes, namely citrate transporter (CT) or tricarboxylate transporter (TCT), into M. circinelloides (CBS 277.49). Compared to the control, overexpression of CT or TCT caused an increase in lipid accumulation of 44% (from 13.0 to 18.8%, w/w, cell dry weight) and 68% (from 13.0 to 21.8%, w/w, cell dry weight), respectively. Moreover, Yang J. et al. (2019) observed that overexpression of the citrate transporter genes activated the downstream enzyme activities in lipid biosynthesis, indicating a greater flux of carbon toward fatty acid biosynthesis. In a separate study to elucidate the role of the malate transporter (MT) in relation to fatty acid synthesis and accumulation in M. circinelloides, Zhao et al. (2016) utilized both MT knockout and MT-overexpressing strains and found that, compared to the control, the lipid content in the overexpression mutant increased by approximately 70% (13 to 22% dry cell weight), while the lipid content in the knockout mutant decreased by 27% (13 to 9.5% dry cell weight). In a follow-up experiment, aimed at elucidating the mechanism by which the MT promotes the transportation of mitochondrial malate and citrate in relation to lipid accumulation, Wang et al. (2019) utilized 13C-labeled glucose metabolic flux analysis of the TCA cycle, glyoxylic acid (GOX) cycle and the pentose phosphate (PP) pathway in M. circinelloides. The authors found that the TCA cycle flux ratio of MT-overexpression strains decreased compared to that of the control strain, although the GOX cycle flux ratio was increased. As expected, a reverse trend was observed for the MT-knockout strain. Taken together, these observations suggest that the GOX cycle might be more effective than the TCA cycle in producing malate and oxaloacetate replenishment during lipid biosynthesis.
In addition to the GOX and TCA cycle flux ratios, Wang et al. (2019) observed a relatively higher flux ratio of the PP pathway in MT-overexpressing strains, which suggest that the PP pathway possibly plays a significant role in supplying NADPH for fatty acid synthesis in M. circinelloides. However, a lack of significant difference in the ME flux between the mutant strains and parental control suggested that ME is not a limiting factor for fatty acid synthesis in M. circinelloides. This observation agrees with earlier findings by Wynn and Ratledge (1997), who investigated the role of ME on lipid accumulation in Aspergillus nidulans.
Chang et al. (2019) explored the role of AMPD expression on lipid accumulation in Mortierella alpina under fermentation conditions by utilizing recombinant homologous AMPD-overexpressing M. alpina strains. It was observed that the overexpression of homologous AMPD in M. alpina favored lipid synthesis above cell growth. In addition, a concomitant reduction (by 40–60%) in intracellular adenosine monophosphate (AMP) and a 1.9 to 2.7-fold increase in citrate content compared with the control was observed at the early stages of fermentation. The increase in citrate suggests the provision of more precursors for fatty acid synthesis, while the decrease in AMP level indicates a metabolic reprogramming, which involves the channeling of more carbon toward lipid synthesis pathways.
β-oxidation is the main catabolic pathway of fatty acid degradation. Because lipid accumulation is the metabolic balance between lipidogenesis and degradation, the activity of β-oxidases competes with the accumulation of lipids. The β-oxidation pathway consists of a four-step cycle that eliminates two carbons (an acetyl-CoA molecule) from the acyl-CoA chain during each cycle (Courchesne et al., 2009). Thus, by blocking this lipid biosynthesis -competing pathway and by increasing the availability of lipid precursors, such as acetyl-CoA, lipid accumulation can be significantly enhanced in oleaginous filamentous fungi (Ledesma-Amaro et al., 2015; Díaz-Fernández et al., 2017). To this end, Ledesma-Amaro et al. (2015) constructed fatty-acyl coenzyme A oxidase (encoded by POX1) and acyl-CoA oxidase (encoded by FOX1) knock-out mutants of Ashbya gossypii, using A. gossypii strains which had been integrated with heterologous ACL1 and ACL2 genes (from Yarrowia lipolytica) for the expression of ATP-citrate lyase activity (the wild-type A. gossypii strain lack ACL). By blocking the first step of β -oxidation through the knock-out of POX1, the authors observed an increase in lipid accumulation (from ∼15% in the wild type to 70% in the mutant) in media containing 1% glucose and 2% oleic acid after 7 days of cultivation. Thus, demonstrating that blockage of lipid accumulation competing pathways in oleaginous filamentous fungi is a viable approach for enhancing the yields of SCO.
Other lipid biosynthetic-related enzymes which determine the degree of saturation and accumulation of specific fatty acids of interest are desaturases, such as those that catalyze the conversion of oleic acid to linoleic acid or gamma-linoleic acid (GLA, 18:3, n−6). Toward enhancement of GLA production in M. circinelloides, Zhang et al. (2017) constructed recombinant strains with homologous overexpression of Δ6 desaturases. It was observed that this increased the GLA proportion by 38% of the total fatty acids and the yield by 33%, thereby demonstrating that the Δ6 desaturase-overexpressing M. circinelloides strain can potentially be used for enhanced microbial GLA production. In turn, GLA can be further converted to dihomo-gamma linolenic acid (DGLA, 20:3, n−6), an elongated product of GLA catalyzed by the enzyme Δ6 elongase (D6E) or γ-linolenic acid elongase (Yazawa et al., 2007). In a study by Khan et al. (2019b), a D6E overexpression strain of M. circinelloides (generated from M. circinelloides CBS277.49) showed an increase of up to 5.75% DLGA. Thus, this demonstrated that the overexpression of D6E in M. circinelloides through genetic engineering can be used for enhanced production of DLGA, an important nutritional and medical fatty acid.
Biodiesel production typically involves multiple processing steps that are energy-intensive and contribute to high operating costs (Chopra et al., 2016). Filamentous fungi possess several traits that can be manipulated in the unit process consolidation. Some of the most attractive traits of filamentous fungi include their high growth rates and the ability to secrete an array of hydrolytic enzymes. Such traits confer filamentous fungi’s unique ability to colonize and degrade various substrates (Jun et al., 2011; Zhao et al., 2017). Microbial lipid production from low-cost lignocellulosic and agro-waste material is a promising avenue (Jin et al., 2015), however, these substrates are recalcitrant, and require a pretreatment step (e.g., steam explosion) before they can be hydrolyzed and fermented. During this process, various inhibitory by-products such as organic acids, phenolic compounds and sugar degradation products are generated, which may negatively affect microbial growth (Yang et al., 2011; Zhao et al., 2017; Valdés et al., 2020). The advantage of using filamentous fungi is that they exhibit higher tolerance to these inhibitory by-products (Abghari and Chen, 2014; Zhao et al., 2017).
Enzymatic hydrolysis is another significant cost factor in biofuel processes accounting for about 15.7% of the total cellulosic ethanol production cost (Yang et al., 2010; Fang et al., 2016). On-site production of enzymes by filamentous fungi has the potential to drive down biomass to SCO production (Ahamed and Vermette, 2008; Jun et al., 2011; Fang et al., 2016). Some of the advantages of on-site enzyme production include using low-cost substrates, eliminating the cost of separation, concentration, storage, and transportation (Fang et al., 2016; Zhao et al., 2017). Furthermore, on-site enzyme production by filamentous fungi is stimulated by the lignocellulosic substrate to be hydrolyzed, resulting in increased enzymatic hydrolysis specificity for that particular substrate (Fang et al., 2016).
Multi-stage enzymatic hydrolysis using mixed fungal cultures of Trichoderma reesei and Aspergillus niger has been reported in SCO production as an excellent approach to achieve an optimum on-site production of cellulases (Yang et al., 2010; Zhao et al., 2017). Various cellulases produced by filamentous fungi can degrade the substrate synergistically. Zhao et al. (2017) showed that the synergistic interaction of enzymes produced by a mixed culture of T. reesei and A. niger had a cellulose hydrolysis potential, which was two or three times higher than that seen in monocultures. Notably, T. reesei produced a full set of cellulases in these mixed cultures, but its β-glucosidase activity was lower than required to achieve efficient hydrolysis. The complementary partner, A. niger, produced a strong activity of β-glucosidase, which allowed for efficient hydrolysis (Jun et al., 2011; Zhao et al., 2017). Mixed culture fermentations are preferred over monoculture fermentations because they provide better substrate utilization, increased productivity, resistance to contaminants, and better adaptability to changing conditions (Ahamed and Vermette, 2008).
Yang et al. (2010) explored a three-stage hydrolysis (6, 6, and 12 h) configuration to shorten the hydrolysis time. They found that in the three-stage hydrolysis, the yields produced from steam-exploded corn stover reached 70.2% in 24 h, whereas single-stage hydrolysis required 72 h to achieve the same yield. Further to this, Yang et al. (2011) extended the evaluation of the three-stage hydrolysis, and observed that the hydrolysis of steam-exploded corn stover increased by approximately 37% compared to single-stage hydrolysis, reaching 85.1% in 30 h. To demonstrate the on-site three-stage hydrolysis with the production of SCOs, Fang et al. (2016) evaluated a mixed culture system composed of T. reesei and A. niger during the conversion of corn stover to SCOs by Mortierella isabellina. In this study, a three-stage enzymatic hydrolysis process produced the highest lipid content of 57.34%. In a similar study, Zhao et al. (2017) looked at the potential of Trichosporon cutaneum in producing SCO for biodiesel production, when grown on steam pretreated corn stover. For this, they evaluated various mixed cultures of T. reesei and A. niger as on-site producers of cellulose enzymes, before fermentation using T. cutaneum. Their results agreed with earlier studies where they found that using a mixed culture of T. reesei and A. niger resulted in enhanced on-site cellulase production (Zhao et al., 2017). Moreover, the fermentation time was reduced from 72 h to 30 h, yielding about 74% at a cellulase dosage of 20 FPIU/g (Zhao et al., 2017). These results were encouraging and showed that multi-stage enzymatic hydrolysis could improve the yield and enzymatic hydrolysis time with less cellulase usage. Another interesting observation was that the on-site production of cellulases performed well than when Celluclast, a commercial cellulase, was added together with A. niger monoculture. This strongly suggests that the cellulase enzymes produced on-site are highly specific for the substrate, resulting in enhanced hydrolysis (Zhao et al., 2017).
Over the years, efforts in developing industrial production of fungal lipids have led to the establishment of two fermentation strategies, submerged fermentation and solid-state fermentation (Čertík et al., 2012; Ochsenreither et al., 2016).
Submerged fermentation (SmF) are widely used in large-scale microbial lipid production facilities (Liu et al., 2019) and is performed with soluble nutrients submerged in liquid and typically involves three key steps, i.e., fermentation, cell separation, oil extraction and refining (Čertík et al., 2012; Lima et al., 2019). SmF offers several advantages, including (i) better monitoring of process parameters, (ii) a homogeneous medium which allows for efficient heat and mass transfer, and (iii) the fungal inoculum remains submerged and distributed evenly, thus favoring nutrient absorption (Lima et al., 2019). Another advantage of SmF is that it facilitates product recovery through simple filtration or centrifugation.
Fungal morphology can be modulated by cultivation conditions such as pH, dissolved oxygen, and nutrient supply (Chan et al., 2018). The fungal morphologies can range from free suspended mycelia to pellets. In SmF, fungi typically assume a pellet morphology which yields a high surface area to volume ratio and high-density products from fermentation broth with better biomass recovery via filtration (Xia et al., 2011; Chan et al., 2018). In addition, pelleted growth minimizes mating and occluding of the bioreactor impellers, reduces viscosity of the fermentation broth and allows better mass transfer of nutrients, and oxygen (Chan et al., 2018). Therefore, fungal hyphal growth induced by SmF conditions provides a cost-effective downstream harvesting option instead of a highly expensive centrifugation process, which is an obstacle for the microbe to fuel production approach (Xia et al., 2011). Although fungal hyphal growth may result in cheaper harvesting of fermentation products, controlling this morphology in fermentation processes might be difficult and may cause problems with oxygen transfer in liquid media. Other limitations in SmF processing conditions include the consumption of substantial amounts of water and electricity for aeration, stirring and temperature control (Colla et al., 2016; Liu et al., 2019). These inevitably result in additional costs that exceed the value of the biodiesel produced.
Solid-state fermentation (SSF) is a promising alternative to submerged fermentation. It is gaining increasing attention as a preferred fermentation strategy for fungal-derived microbial oils (Čertík et al., 2012). In SSF configuration, the fermentation of a solid substrate proceeds in the absence of water with moisture supporting the growth and metabolic activity of the fermenting microorganism (Lizardi-Jiménez and Hernández-Martínez, 2017). The solid medium in SSF better simulates the natural habitats of fungi, results in lower wastewater output, reduced energy requirements, easy aeration and smaller bioreactor volume (Qiao et al., 2018; Liu et al., 2019). SSF exhibits several other advantages over submerged fermentation, including resistance of the fermenting microorganisms to catabolic repression when the substrate is present in abundance and the use of low-cost agro-industrial wastes (Čertík et al., 2012; Lizardi-Jiménez and Hernández-Martínez, 2017). Since various waste substrates offer different nutrient compositions, selecting the most suitable fungal species that will transform the waste materials into high-quality fatty acids that are compatible as precursors of biodiesel and other value-added products is required (Čertík et al., 2012).
Many studies on SSF focus on process and reactor design, while there are currently limited studies detailing the physiological mechanisms underlying fungal behavior in SSF (Biesebeke et al., 2002; Cheirsilp and Kitcha, 2015). Fungi exhibit hyphal growth allowing it to penetrate and colonize the solid substrate in SSF effectively. Fungal mycelia cover the surface of the substrate with the assistance of its enzymes, which helps the fungus to penetrate and colonize the substrate (Biesebeke et al., 2002). The close association of fungi with the moist substrate allows them to utilize the bound water of their substrate and their growth is not limited by the lack of free water in an SSF setup (Cheirsilp and Kitcha, 2015). Limited water content in SSF minimizes the risk of bacterial contamination in the process (Čertík et al., 2012).
However, fungal hyphal growth may also lead to gradients in substrate concentration, enzymes, water content and temperature, and the presence of substrate-air interface (Biesebeke et al., 2002). Besides, limited water content results in heat removal problems. This can be effectively addressed when there is a better understanding of oxygen consumption by fungi in SSF conditions. Uptake rates of oxygen give a better reflection of the metabolic activities related to heat production. It is well known that heat production is proportional to the respiration rate and controlling temperature to an optimum level is a difficult but essential part of SSF (Biesebeke et al., 2002). Biesebeke et al. (2002) measured the respiration rate of Aspergillus oryzae cultivated in wheat kernels at varying temperatures. They established that the optimum temperature for fungal respiration was between 30 and 35°C. Furthermore, they established which factor contributed to the cooling problem between the dense mycelium on the surface and the abundant aerial mycelium. The densely packed mycelium layers limited oxygen diffusion, while less diffusion limitation was observed in the aerial mycelium. This observation suggested that cooling problems in fungal SSF might be solved by selecting fungi that do not produce densely packed mycelium.
Process consolidation strategies that remove single operational units are recognized as the future of microbial lipid production at industrial scale. Qiao et al. (2018) reported that M. circinelloides can produce cellulases and convert lignocellulosic material to yield high lipid content without prior pretreatment process in SSF. The authors noted that the accumulation of lipids in the 6 days of fermentation was coupled with an increase in cellulase activity. This suggested that the conversion of lignocellulosic material to lipids directly depends on enzyme production and hydrolysis of complex substrate to fermentable sugars with high cellulase activity resulting in enhanced lipid production (Qiao et al., 2018).
Previous studies have shown that an integrated onsite enzyme production and fermentation of substrate to microbial lipids can be achieved by sequential combination of both SSF and SmF (Cunha et al., 2012; Melikoglu and Webb, 2013). Cunha et al. (2012) used a combined fermentation strategy system where they utilized SSF to stimulate the production of cellulases for the hydrolysis of cellulose. The cellulose hydrolyzate was subsequently fermented using SmF. Melikoglu and Webb (2013) also evaluated the potential of a combined fermentation system by first producing hydrolytic enzymes from waste bread, through a SSF process and subsequently shifting to SmF to produce value-added chemicals. Recently, Gmoser et al. (2019) reported a two-step fermentation strategy by cultivating Neurospora intermedia in thin stillage to initiate fungal biomass, enzyme production and hydrolysis in a SmF process, before switching to SSF (Gmoser et al., 2019).
Single-cell oil recovery is aimed at efficiently separating the desired oils from other cellular components, such as proteins and polysaccharides. For efficient oil extraction, time of extraction, associated costs, the characteristics of the desired lipids as well as of the microorganisms have to be considered (Figure 3; Mustafa and Turner, 2011). In addition, when selecting the extraction method, the solvents to be used for extraction must also be considered. The choice of solvents is dependent on the polarity of lipids to be extracted. For instance, lipids such as TAGs, due to their neutrality, can be extracted using non-polar solvents such as hexane, while polar solvents such as alcohols are used for phospholipid extraction. However, solvent mixtures have been shown to enhance extraction efficiency (Byreddy et al., 2015). This is attributed to a polar solvents’ ability to release lipids from protein-lipid complexes and subsequent dissolution in the non-polar solvent.
Conventionally, the extraction of lipids from microbes is conducted using Soxhlet or Folch techniques (Neto et al., 2019). These techniques firstly involve biomass recovery from the broth through centrifugation and subsequent drying before solvent extraction. Oil recovery from dry biomass is considered to be efficient, however, there are economical and safety shortcomings involved. Drying of biomass involves extensive energy use and may also lead to substantial compound degradation due to high reaction temperatures (Xu et al., 2011; Neto et al., 2019). Soxhlet extraction requires extended durations of extraction and high solvent quantities (Somashekar et al., 2001). However, the method has been improved for the enhancement of extraction capabilities. For instance, a microwave-assisted Soxhlet extraction has been more efficient by reducing extraction time and solvent consumption than Soxhlet extraction alone (García-Ayuso et al., 2000). Therefore, the cost is also considerably reduced. Even though Soxhlet and Folch extraction methods have been used for SCO recovery, they have been found to be not as efficient in extracting single oil classes compared to total lipid extraction (McNichol et al., 2012). Therefore, both methods require modifications when the focus is to recover oils from single-cell microorganisms (Ochsenreither et al., 2016).
Cell disruption methods have the potential to enhance oil recovery yields (Koubaa et al., 2020). The most investigated technologies include bead milling, high-pressure homogenization (HPH), ultrasound- and microwave-assisted extraction (UAE and MAE, respectively). The degree of cell disruption is dependent on various factors such as the type of microorganism tested, the concentration of the microorganism in the growth medium, as well as cell wall composition and structure. Among cell disruption technologies for recovering oil, bead milling is considered the most efficient method (Koubaa et al., 2020). It involves the use of beads for grinding biomass against the solid surfaces during violent agitation (Dong et al., 2016). High-pressure homogenization involves passing a suspension through a high-pressure valve. Although the mechanisms responsible for cell disruption when using HPH are still vague, it has been hypothesized that the high-velocity effects of the fluid passing through the valve as well as differential pressure between the valve cause cell wall damage and subsequent release of the intracellular compounds (Samarasinghe et al., 2012). Zhou et al. (2013) investigated UAE of SCOs from Mortierella isabellina. The yield of lipids recovered was approximately 109.88 mg/g, which was 1.35-fold the yield of the acid-heating method, suggesting that UAE might be a cost-effective and sustainable method for SCO extraction. Thus, UAE can have the potential for large-scale oil extraction technology from microorganisms (Zhou et al., 2013).
Microwave application for the disruption of microbial cells has also been extensively studied (Woo et al., 2000; Campanha et al., 2007; Dong et al., 2016). The major mechanism involved in the microwave extraction is heating due to the rise in pressure and temperature. The phenomena are responsible for damaging cell walls, releasing intracellular compounds. Zhou et al. (2018) studied the efficiency of extraction and the quality of SCO from M. isabellina using ultrasound in combination with the microwave extraction method. It was observed that the combined extraction method improved the quality and yield of SCO. This can potentially translate to cost reduction due to reduced time and energy usage for extraction.
Nanoparticles can be used to maximize oil recovery (Dong et al., 2016; Negin et al., 2016). However, the application of nanoparticles for SCO recovery is still in its infancy. Depending on the process conditions during extraction, various nanoparticles perform differently due to their characteristics such as particle size and surface area to volume ratio, leading to different levels of lipid recovery. According to Bjørnar (2012), the efficacy of these nanoparticles is due to their small size, making it possible for the nanoparticles to penetrate through pore spaces where conventional recovery techniques cannot, thus, resulting in higher recovery. Therefore, more research devoted to nanoparticle behavior during SCO recovery is pertinent for developing fast and convenient extraction methods. Furthermore, research work on the use of “green” extraction solvents such as terpenes in place of the currently used solvents (e.g., hexane, chloroform, etc.) is necessary to promote eco-friendly oil extraction methods.
The advantages and disadvantages of different cell disruption techniques for oil recovery are summarized in Table 5.
The selection of a suitable cell disruption technology for oil recovery and cost-benefit analysis toward the final product requires more research attention. Presently, most studies on energy consumption of conventional methods for different processes have primarily focused on time and temperature reduction (Chemat et al., 2017; Postma et al., 2017; Roohinejad et al., 2018; Gaudino et al., 2019). The lack of information on energy usage makes it difficult to estimate overall costs for scaling-up the process.
Although it has been established that several fungal species can accumulate SCOs, which may be harvested and utilized for value-added product generation, high fermentation costs have often restricted industrial application of such species (Kothri et al., 2020; Patel et al., 2020). However, the combination of fungal SCO production with several other biotechnological applications, in a biorefinery concept, could help minimize costs (Kothri et al., 2020). A biorefinery refers to a facility that utilizes various technologies to enable complete processing solutions, thereby resulting in an array of bioproducts with minimal waste generation. Such bioproducts include biomaterials (e.g., pulps, fibers), biofuels (e.g., biogas, biodiesel, bioethanol), and various bioactive compounds (e.g., enzymes, anti-bacterial agents), which collectively contribute to improving the value derived from the feedstock in use (Yousuf et al., 2018). Filamentous fungi have received attention as promising candidates for adoption in biorefineries, due to their ability to synthesize a multitude of macromolecules such as SCOs, enzymes, structural polymers, and compounds with pharmaceutical and nutraceutical applications (Reis et al., 2019).
In comparison to algal biorefineries, minimal studies have been conducted to test the feasibility of oleaginous fungi for biodiesel production in biorefineries, and in most instances, such studies were only laboratory-based. One such study, conducted by Kamat et al. (2013), tested two fungal species, isolated from a mangrove for their ability to produce lipids, biodiesel, xylitol and xylanase from sugarcane bagasse. The synthesized lipids were well within the international standards for use as a biodiesel feedstock and the xylanase was reported to have the potential for application in the seafood industry. Carvalho et al. (2019) tested M. circinelloides for its ability to produce SCOs from sugarcane bagasse hydrolyzate. They found that M. circinelloides was able to synthesize fuel-grade ethyl esters and PUFAs for potential use in the nutraceutical industry. Moreover, they found that the fungal biomass presented with lipolytic activity and the fungal lipids contained carotenoids indicating that M. circinelloides has the potential to generate several high-value products. While research on fungal biorefineries is less prominent, there is a substantial motivation for more research to be channeled in this direction as the extracted biomass may still be utilized for bioethanol, biomethane or biohydrogen production. For example, Bagy et al. (2014) tested a two-stage system where six strains of oleaginous fungi were cultivated in sugarcane molasses for biodiesel production (first stage). The spent fungal culture medium was then utilized for biohydrogen production (second stage) by Clostridium acetobutylicum ATCC 824 to improve the process’s economic feasibility.
Biohydrogen has numerous advantages over other biofuels such as biogas and bioethanol. The main advantages are that biohydrogen has elevated energy density and the significant emission from its utilization in a hydrogen fuel cell, is solely water. However, its commercial production is impeded due to elevated production costs. Biohydrogen is produced during dark fermentation. The by-product of the production process is a liquid waste (HPLW), which can be utilized as a feedstock for lipid production (Sarma et al., 2015). Hence, the integration of biohydrogen and lipid generation in a biorefinery may offset the elevated costs associated with sole production of biohydrogen. One such concept was proposed by Béligon et al. (2018) where they tested the feasibility of an immersed membrane bioreactor containing individual reactor compartments for biohydrogen and SCO production. In their study, the first bioreactor compartment was structured to promote dark fermentation of lignocellulosic matter to biohydrogen and volatile fatty acids (VFA). The second compartment comprised oleaginous yeast, Cryptococcus curvatus, which has the ability to convert the VFA-rich effluent from the first compartment to SCOs. A similar concept could be used when integrating oleaginous filamentous fungi into biorefineries.
It is therefore proposed that more studies be aimed at testing the economic and technical feasibility of integrating biohydrogen and SCO production, particularly from oleaginous filamentous fungi (Figure 4). Like with the study by Béligon et al. (2018), it is suggested that low-cost lignocellulosic material be utilized as the feedstock for the proposed biorefinery. This will substantially reduce input costs and improve economic viability. Filamentous fungi may find applications in all stages of the biorefinery process due to their ability to produce an array of extracellular enzymes (Troiano et al., 2020). Thus, they may be utilized for the biological pretreatment of the lignocellulosic substrate prior to dark fermentation, which would enable the breakdown of the lignocellulosic structure. This can improve the accessibility of cellulose and hemicellulose, and maximize biohydrogen production (Liu and Qu, 2019). Facultative or obligate anaerobic fungal strains may also be utilized as an inoculum during the biohydrogen production process as proven in a study by Nkemka et al. (2015), where bioaugmentation with the fungal strain, Piromyces rhizinflata, resulted in increased initial biohydrogen and biomethane production rates in a two-stage reactor. Increased initial biogas production rates will in turn, reduce the required digestion time, which will reduce the cost implications of the process (Nkemka et al., 2015). Oleaginous fungal species may then be utilized to convert the effluent, after biohydrogen production, into SCOs. A combination of low and high-value SCOs will improve the cost-effectiveness of the process. The upgrading of the final effluent after extraction of the lipid-rich cells will also be beneficial in improving economic viability (Jin et al., 2015). A cost-intensive aspect of SCO production is the harvesting of biomass prior to lipid extraction. The use of oleaginous fungi for SCO production is beneficial since fungal mycelia’s entanglement decreases medium viscosity and eases cell harvesting (Reis et al., 2019). A recent study by Yang L. et al. (2019) showed that the co-cultivation of oleaginous algae and fungi aided in harvesting the resulting lipids and improved lipid quality in comparison to mono-cultivation. This could be another strategy that can be adopted in a SCO biorefinery to improve the quality of SCOs with downstream economic implications.
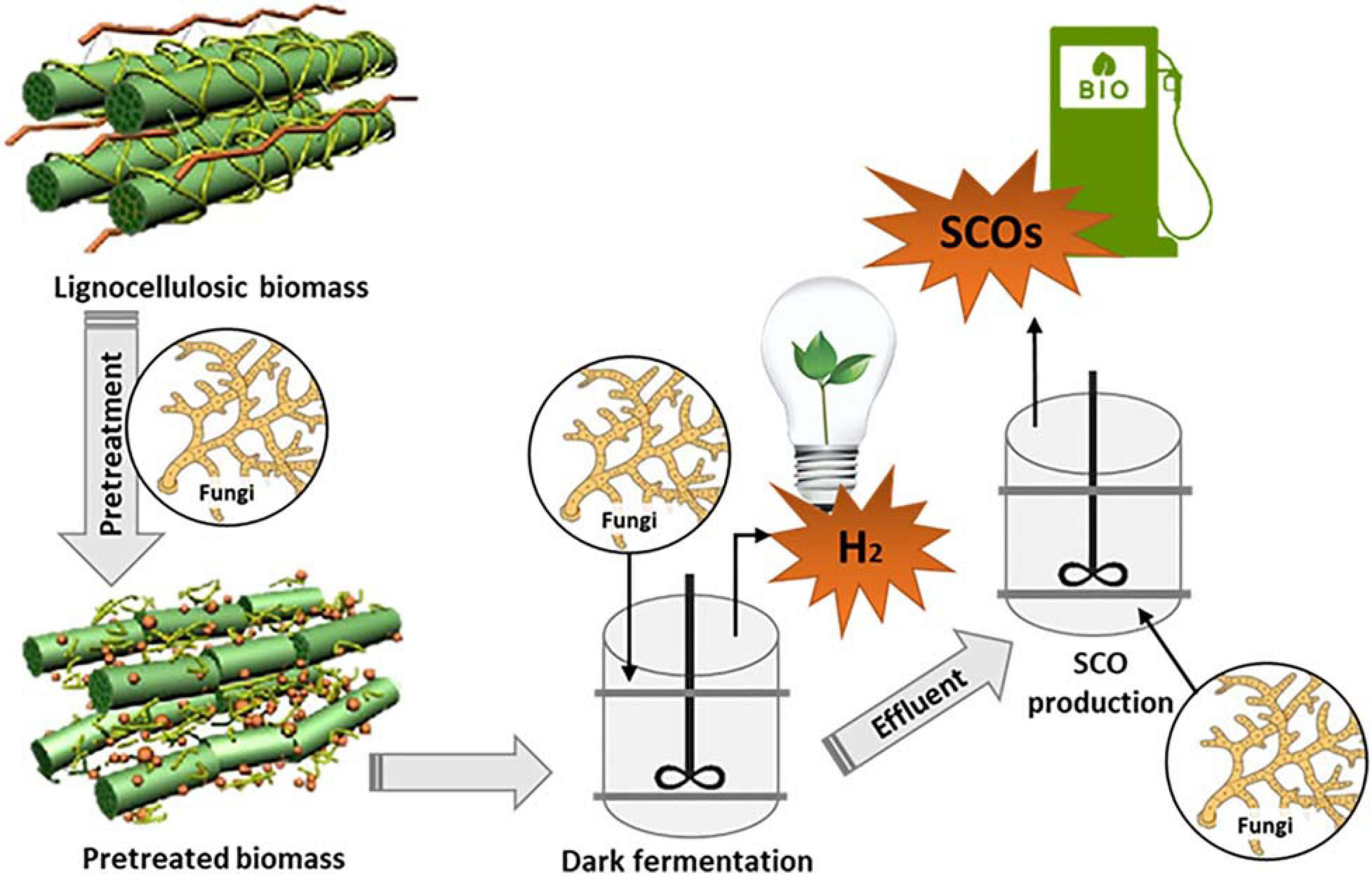
Figure 4. Combining biohydrogen (H2) and single cell oil (SCO) production to increase the efficiency and economic feasibility of the process.
Overall, several factors influence the success of an SCO biorefinery. Such factors include strain selection, choice of feedstock, method of substrate pre-treatment, the configuration of the production process, method of cell harvesting and lipid recovery as well as the number and type of co-products generated. Various strategies can be put into place to address the factors mentioned above, such as strain genetic modification, optimization of culture conditions and technological improvements of process configurations (Jin et al., 2015). All in all, such strategies are aimed at overcoming cost implications, which is the major bottleneck in SCO production.
Single-cell oils have the potential to provide a sustainable alternative for obtaining PUFA-rich lipids. Hence, researchers have been focusing on increasing the yield of SCO for its commercial realization. For SCOs to be considered commercially feasible, the production process must be conducted at ideal conditions for optimal efficiency and minimum costs. This could be achievable by making use of relatively inexpensive substrates such as agro-industrial waste. Despite, low-cost starting material, the process of SCO production in combination with downstream processing still makes it cost-intensive. Hence, the paper also discussed possibilities of reducing costs by utilizing SCO co-production with other prospective metabolic products such as biohydrogen in a biorefinery approach to balance production cost, thereby circumventing overall cost challenges. Aside from improving economic feasibility, a biorefinery approach will also help achieve several sustainable development goals and promote a sustainable circular economy. In addition, the quest for developing tolerant, robust and high yielding microbial strains for efficient oil production would also play a vital role for a sustainable holistic approach.
SM conceptualized and prepared the initial outline of the manuscript. SM, OE, AR, BN, and PS wrote and edited the first draft of the manuscript. OH and CP were responsible for critical review, literature gathering, and overall improvement of the manuscript. All authors read and approved the final draft of the manuscript.
The Authors would like to thank the University of KwaZulu Natal for assistance with publication fees.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abghari, A., and Chen, S. (2014). Yarrowia lipolytica as an oleaginous cell factory platform for production of fatty acid-based biofuel and bioproducts. Front. Energy Res. 2:21. doi: 10.3389/fenrg.2014.00021
Abou-Zeid, A. M., Abd El-Zaher, E. H., Saad-Allah, K. M., and Ahmed, R. U. (2019). Screening and optimization of some Egyptian soil-born fungi for lipids production as possible source for biofuel. Egypt J. Exp. Biol. 15, 243–250.
Abu-Elreesh, G., and Abd-El-Haleem, D. (2014). Promising oleaginous filamentous fungi as biodiesel feed stocks: screening and identification. Eur. J. Exp. Biol. 4, 576–582.
Aggelis, G., Komaitis, M., Papanikolaou, S., and Papadopoulos, G. (1995). A mathematical model for the study of lipid accumulation in oleaginous microorganisms. I. Lipid accumulation during growth of Mucor circinelloides CBS 172-27 on a vegetable oil. Grasas Aceites 46, 169–173. doi: 10.3989/gya.1995.v46.i3.921
Ahamed, A., and Vermette, P. (2008). Enhanced enzyme production from mixed cultures of Trichoderma reesei RUT-C30 and Aspergillus niger LMA grown as fed batch in a stirred tank bioreactor. Biochem. Eng. J. 42, 41–46. doi: 10.1016/j.bej.2008.05.007
Ali, T. H., and El-Ghonemy, D. H. (2014). Optimization of culture conditions for the highest lipid production from some oleaginous fungi for biodiesel preparation. Asian J. Appl. Sci. 2, 2321–2893.
Ando, A., Sumida, Y., Negoro, H., Suroto, D. A., Ogawa, J., Sakuradani, E., et al. (2009). Establishment of Agrobacterium tumefaciens-mediated transformation of an oleaginous fungus, Mortierella alpina 1S-4, and its application for eicosapentaenoic acid producer breeding. Appl. Environ. Microbiol. 75, 5529–5535. doi: 10.1128/AEM.00648-09
André, A., Diamantopoulou, P., Philippoussis, A., Sarris, D., Komaitis, M., and Papanikolaou, S. (2010). Biotechnological conversions of bio-diesel derived waste glycerol into added-value compounds by higher fungi: production of biomass, single cell oil and oxalic acid. Ind. Crops Prod. 31, 407–416. doi: 10.1016/j.indcrop.2009.12.011
Athenaki, M., Gardeli, C., Diamantopoulou, P., Tchakouteu, S. S., Sarris, D., Philippousis, A., et al. (2017). Lipids from yeasts and fungi: physiology, production and analytical considerations. J. Appl. Microbiol. 124, 336–367. doi: 10.1111/jam.13633
Bagy, M. M. K., Abd-Alla, M. H., Morsy, F. M., and Hassan, E. A. (2014). Two stage biodiesel and hydrogen production from molasses by oleaginous fungi and Clostridium acetobutylicum ATCC 824. Int. J. Hydrog. Energy 39, 3185–3197. doi: 10.1016/j.ijhydene.2013.12.106
Banu, B. S., Kuncham, R., Azeem, M. A., and Bharath, M. R. (2017). Screening and identification of oleaginous moulds for lipid production. J. Environ. Biol. 38, 697–701. doi: 10.22438/jeb/38/4/MRN-618
Bardhan, P., Gohain, M., Daimary, N., Kishor, S., Chattopadhyay, P., Gupta, K., et al. (2019). Microbial lipids from cellulolytic oleaginous fungus Penicillium citrinum PKB20 as a potential feedstock for biodiesel production. Ann. Microbiol. 69, 1135–1146. doi: 10.1007/s13213-019-01494-3
Béligon, V., Noblecourt, A., Christophe, G., Lebert, A., Larroche, C., and Fontanille, P. (2018). Proof of concept for biorefinery approach aiming at two bioenergy production compartments, hydrogen and biodiesel, coupled by an external membrane. Biofuels 9, 163–174. doi: 10.1080/17597269.2016.1259142
Bellou, S., Moustogianni, A., Makri, A., and Aggelis, G. (2012). Lipids containing polyunsaturated fatty acids synthesized by zygomycetes grown on glycerol. Appl. Biochem. Biotechnol. 166, 146–158. doi: 10.1007/s12010-011-9411-z
Bento, H. B. S., Carvalho, A. K. F., Reis, C. E. R., and De Castro, H. F. (2020). Single cell oil production and modification for fuel and food applications: assessing the potential of sugarcane molasses as culture medium for filamentous fungus. Ind. Crops Prod. 145:112141. doi: 10.1016/j.indcrop.2020.112141
Bhanja, A., Minde, G., Magdum, S., and Kalyanraman, V. (2014). Comparative studies of oleaginous fungal strains (Mucor circinelloides and Trichoderma reesei) for effective wastewater treatment and bio-oil production. Biotechnol. Res. Int. 2014:479370. doi: 10.1155/2014/479370
Biesebeke, R., Ruijter, G., Rahardjo, Y. S., Hoogschagen, M. J., Heerikhuisen, M., Levin, A., et al. (2002). Aspergillus oryzae in solid-state and submerged fermentations. Progress report on a multi-disciplinary project. FEMS Yeast Res. 2, 245–248. doi: 10.1111/j.1567-1364.2002.tb00089.x
Bjørnar, E. (2012). The Potential of Hydrophilic Silica Nanoparticles for EOR Purposes. Masters thesis, Norwegian University of Science and Technology, Norway.
Braz, C. A., Carvalho, A. K. F., Bento, H. B. S., Reis, C. E. R., and De Castro, H. F. (2020). Production of value-added microbial metabolites: oleaginous fungus as a tool for valorization of dairy by-products. Bioenergy Res. 13, 963–973. doi: 10.1007/s12155-020-10121-y
Bruszewski, T. E., Fergus, C. L., and Mumma, R. O. (1972). Thermophilic fungi: IV. The lipid composition of six species. Lipids 7, 695–698. doi: 10.1007/BF02533117
Byreddy, A. R., Gupta, A., Barrow, C. J., and Puri, M. (2015). Comparison of cell disruption methods for improving lipid extraction from Thraustochytrid strains. Mar. Drugs 13, 5111–5127. doi: 10.3390/md13085111
Campanha, N. H., Pavarina, A. C., Brunetti, I. L., Vergani, C. E., Machado, A. L., and Spolidorio, D. M. P. (2007). Candida albicans inactivation and cell membrane integrity damage by microwave irradiation. Mycoses 50, 140–147. doi: 10.1111/j.1439-0507.2006.01339.x
Carota, E., Crognale, S., D’Annibale, A., and Petruccioli, M. (2018). Bioconversion of agro-industrial waste into microbial oils by filamentous fungi. Process Saf. Environ. Prot. 117, 143–151. doi: 10.1016/j.psep.2018.04.022
Carvalho, A. K., Rivaldi, J. D., Barbosa, J. C., and de Castro, H. F. (2015). Biosynthesis, characterization and enzymatic transesterification of single cell oil of Mucor circinelloides–a sustainable pathway for biofuel production. Bioresour. Technol. 181, 47–53. doi: 10.1016/j.biortech.2014.12.110
Carvalho, A. K. F., Bento, H. B. S., Reis, C. E. R., and De Castro, H. F. (2019). Sustainable enzymatic approaches in a fungal lipid biorefinery based in sugarcane bagasse hydrolysate as carbon source. Bioresource Technol. 276, 269–275. doi: 10.1016/j.biortech.2018.12.118
Carvalho, P., de, O., de Oliveira, J. G., and Pastore, G. M. (1999). Enhancement of gamma-linolenic acid production by the fungus Mucor SP LB-54 by growth temperature. Rev. Microbiol. 30, 170–175. doi: 10.1590/s0001-37141999000200016
Čertík, M., Adamechová, Z., and Laoteng, K. (2012). Microbial production of γ-linolenic acid: submerged vs. solid-state fermentations. Food Sci. Biotechnol. 21, 921–926. doi: 10.1007/s10068-012-0121-2
Chan, L. G., Cohen, J. L., and de Moura Bell, J. M. L. N. (2018). Conversion of agricultural streams and food-processing by-products to value-added compounds using filamentous fungi. Annu. Rev. Food Sci. Technol. 9, 503–523. doi: 10.1146/annurev-food-030117-012626
Chan, L. G., Dias, F. F. G., Saarni, A., Cohen, J., Block, D., Taha, A. Y., et al. (2020). Scaling up the bioconversion of cheese whey permeate into fungal oil by Mucor circinelloides. J. Am. Oil Chem. Soc. 97, 703–716. doi: 10.1002/aocs.12372
Chang, L., Tang, X., Lu, H., Zhang, H., Chen, Y. Q., Chen, H., et al. (2019). Role of adenosine monophosphate deaminase during fatty acid accumulation in oleaginous fungus Mortierella alpina. J. Agric. Food Chem. 67, 9551–9559. doi: 10.1021/acs.jafc.9b03603
Chatzifragkou, A., Fakas, S., Galiotou-Panayotou, M., Komaitis, M., Aggelis, G., and Papanikolaou, S. (2010). Commercial sugars as substrates for lipid accumulation in Cunninghamella echinulata and Mortierella isabellina fungi. Eur. J. Lipid Sci. Technol. 112, 1048–1057. doi: 10.1002/ejlt.201000027
Cheirsilp, B., and Kitcha, S. (2015). Solid state fermentation by cellulolytic oleaginous fungi for direct conversion of lignocellulosic biomass into lipids: fed-batch and repeated-batch fermentations. Ind. Crops Prod. 66, 73–80. doi: 10.1016/j.indcrop.2014.12.035
Chemat, F., Rombaut, N., Sicaire, A. G., Meullemiestre, A., Fabiano-Tixier, A. S., and Abert-Vian, M. (2017). Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 34, 540–560. doi: 10.1016/j.ultsonch.2016.06.035
Chen, H., Hao, G., Wang, L., Wang, H., Gu, Z., Liu, L., et al. (2015). Identification of a critical determinant that enables efficient fatty acid synthesis in oleaginous fungi. Sci. Rep. 5:11247. doi: 10.1038/srep11247
Chen, H. C., and Liu, T. M. (1997). Inoculum effects on the production of γ-linolenic acid by the shake culture of Cunninghamella echinulata CCRC 31840. Enzyme Microb. Technol. 21, 137–142. doi: 10.1016/S0141-0229(96)00262-1
Chopra, J., Dineshkumar, R., Bhaumik, M., Dhanarajan, G., Kumar, R., and Sen, R. (2016). Integrated in situ transesterification for improved biodiesel production from oleaginous yeast: a value proposition for possible industrial implication. RSC Adv. 6, 70364–70373. doi: 10.1039/c6ra14003c
Colla, L. M., Primaz, A. L., Benedetti, S., Loss, R. A., Lima, M. D., Reinehr, C. O., et al. (2016). Surface response methodology for the optimization of lipase production under submerged fermentation by filamentous fungi. Braz. J. Microbiol. 47, 461–467. doi: 10.1016/j.bjm.2016.01.028
Courchesne, N. M. D., Parisien, A., Wang, B., and Lan, C. Q. (2009). Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J. Biotechnol. 141, 31–41. doi: 10.1016/j.jbiotec.2009.02.018
Cunha, F. M., Esperança, M. N., Zangirolami, T. C., Badino, A. C., and Farinas, C. S. (2012). Sequential solid-state and submerged cultivation of Aspergillus niger on sugarcane bagasse for the production of cellulase. Bioresour. Technol. 112, 270–274. doi: 10.1016/j.biortech.2012.02.082
de Jong, B., Siewers, V., and Nielsen, J. (2012). Systems biology of yeast: enabling technology for development of cell factories for production of advanced biofuels. Curr. Opin. Biotechnol. 23, 624–630. doi: 10.1016/j.copbio.2011.11.021
Deven, J. M., and Manocha, M. S. (1976). Effect of various cultural conditions on the fatty acid and lipid composition of Choanephora cucurbitarum. Can. J. Microbiol. 22, 443–449. doi: 10.1139/m76-070
Dey, P., Banerjee, J., and Maiti, M. K. (2011). Comparative lipid profiling of two endophytic fungal isolates - Colletotrichum sp. and Alternaria sp. having potential utilities as biodiesel feedstock. Bioresour. Technol. 102, 5815–5823. doi: 10.1016/j.biortech.2011.02.064
Díaz-Fernández, D., Aguiar, T. Q., Martín, V. I., Romaní, A., Silva, R., Domingues, L., et al. (2019). Microbial lipids from industrial wastes using xylose-utilizing Ashbya gossypii strains. Bioresour. Technol. 293:122054. doi: 10.1016/j.biortech.2019.122054
Díaz-Fernández, D., Lozano-Martínez, P., Buey, R. M., Revuelta, J. L., and Jiménez, A. (2017). Utilization of xylose by engineered strains of Ashbya gossypii for the production of microbial oils. Biotechnol. Biofuels 10, 1–12. doi: 10.1186/s13068-016-0685-9
Dong, T., Knoshaug, E. P., Pienkos, P. T., and Laurens, L. M. (2016). Lipid recovery from wet oleaginous microbial biomass for biofuel production: a critical review. Appl. Energy 177, 879–895. doi: 10.1016/j.apenergy.2016.06.002
Dourou, M., Aggeli, D., Papanikolaou, S., and Aggelis, G. (2018). Critical steps in carbon metabolism affecting lipid accumulation and their regulation in oleaginous microorganisms. Appl. Microbiol. Biotechnol. 102, 2509–2523. doi: 10.1007/s00253-018-8813-z
Dyal, S. D., Bouzidi, L., and Narine, S. S. (2005). Maximizing the production of γ-linolenic acid in Mortierella ramanniana var. ramanniana as a function of pH, temperature and carbon source, nitrogen source, metal ions and oil supplementation. Food Res. Int. 38, 815–829. doi: 10.1016/j.foodres.2005.04.002
Economou, C. N., Aggelis, G., Pavlou, S., and Vayenas, D. V. (2011). Single cell oil production from rice hulls hydrolysate. Bioresour. Technol. 102, 9737–9742. doi: 10.1016/j.biortech.2011.08.025
Evans, C. T., and Ratledge, C. (1985). Possible regulatory roles of ATP:citrate lyase, malic enzyme, and AMP deaminase in lipid accumulation by Rhodosporidium toruloides CBS 14. Can. J. Microbiol. 31, 1000–1005. doi: 10.1139/m85-189
Fakas, S., Papanikolaou, S., Batsos, A., Galiotou-Panayotou, M., Mallouchos, A., and Aggelis, G. (2009). Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy 33, 573–580. doi: 10.1016/j.biombioe.2008.09.006
Fakas, S., Papanikolaou, S., Galiotou-Panayotou, M., Komaitis, M., and Aggelis, G. (2008). Organic nitrogen of tomato waste hydrolysate enhances glucose uptake and lipid accumulation in Cunninghamella echinulata. J. Appl. Microbiol. 105, 1062–1070. doi: 10.1111/j.1365-2672.2008.03839.x
Fang, H., Zhao, C., and Chen, S. (2016). Single cell oil production by Mortierella isabellina from steam exploded corn stover degraded by three-stage enzymatic hydrolysis in the context of on-site enzyme production. Bioresour Technol. 216, 988–995. doi: 10.1016/j.biortech.2016.06.051
Forfang, K., Zimmermann, B., Kosa, G., Kohler, A., and Shapaval, V. (2017). FTIR spectroscopy for evaluation and monitoring of lipid extraction efficiency for oleaginous fungi. PLoS One 12:e0170611. doi: 10.1371/journal.pone.0170611
Fukushima, Y., Okada, K., Itoh, H., Fukase, T., and Motai, H. (1991). Protease production in continuous culture by Aspergillus oryzae: effect of morphological changes of mycelia on resistance against shear force. Hukkokogaku Kaishi 69, 441–446. doi: 10.1016/0922-338X(91)90067-Q
Garay, L. A., Boundy-Mills, K. L., and German, J. B. (2014). Accumulation of high-value lipids in single-cell microorganisms: a mechanistic approach and future perspectives. J. Agric. Food Chem. 62, 2709–2727. doi: 10.1021/jf4042134
García-Ayuso, L. E., Velasco, J., Dobarganes, M. C., and De Castro, M. L. (2000). Determination of the oil content of seeds by focused microwave-assisted soxhlet extraction. Chromatographia 52, 103–108. doi: 10.1007/bf02490801
Gaudino, E. C., Cravotto, G., Manzoli, M., and Tabasso, S. (2019). From waste biomass to chemicals and energy via microwave-assisted processes. Green Chem. 21, 1202–1235. doi: 10.1039/C8GC03908A
Gmoser, R., Sintca, C., Taherzadeh, M. J., and Lennartsson, P. R. (2019). Combining submerged and solid state fermentation to convert waste bread into protein and pigment using the edible filamentous fungus N. intermedia. Waste Manag. 97, 63–70. doi: 10.1016/j.wasman.2019.07.039
Grygier, A., Myszka, K., Juzwa, W., Białas, W., and Rudzińska, M. (2020). Galactomyces geotrichum mold isolated from a traditional fried cottage cheese produced omega-3 fatty acids. Int. J. Food Microbiol. 319:8503. doi: 10.1016/j.ijfoodmicro.2019.108503
Gutiérrez, A., López-García, S., and Garre, V. (2011). High reliability transformation of the basal fungus Mucor circinelloides by electroporation. J. Microbiol. Methods 84, 442–446. doi: 10.1016/j.mimet.2011.01.002
Hansson, L., and Dostálek, M. (1988). Effect of culture conditions on mycelial growth and production of γ-linolenic acid by the fungus Mortierella ramanniana. Appl. Microbiol. Biotechnol. 28, 240–246. doi: 10.1007/BF00250448
Hao, G., Chen, H., Du, K., Huang, X., Song, Y., Gu, Z., et al. (2014). Increased fatty acid unsaturation and production of arachidonic acid by homologous over-expression of the mitochondrial malic enzyme in Mortierella alpina. Biotechnol. Lett. 36, 1827–1834. doi: 10.1007/s10529-014-1546-x
Hao, G., Chen, H., Gu, Z., Zhang, H., Chen, W., and Chen, Y. Q. (2016). Metabolic engineering of Mortierella alpina for enhanced arachidonic acid production through the NADPH-supplying strategy. Appl. Environ. Microbiol. 82, 3280–3288. doi: 10.1128/AEM.00572-16
Hussain, S. A., Garcia, A., Khan, M. A. K., Nosheen, S., Zhang, Y., Koffas, M. A. G., et al. (2020). Increased accumulation of medium-chain fatty acids by dynamic degradation of long-chain fatty acids in Mucor circinelloides. Genes 11, 1–21. doi: 10.3390/genes11080890
Hussain, S. A., Hameed, A., Khan, M. A. K., Zhang, Y., Zhang, H., Garre, V., et al. (2019). Engineering of fatty acid synthases (FASs) to boost the production of medium-chain fatty acids (MCFAs) in Mucor circinelloides. Int. J. Mol. Sci. 20, 1–14. doi: 10.3390/ijms20030786
Jang, H., Der Lin, Y. Y., and Yang, S. S. (2005). Effect of culture media and conditions on polyunsaturated fatty acids production by Mortierella alpina. Bioresour. Technol. 96, 1633–1644. doi: 10.1016/j.biortech.2004.12.027
Jin, M., Slininger, P. J., Dien, B. S., Waghmode, S., Moser, B. R., Orjuela, A., et al. (2015). Microbial lipid-based lignocellulosic biorefinery: feasibility and challenges. Trends Biotechnol. 33, 43–54. doi: 10.1016/j.tibtech.2014.11.005
Jun, H., Kieselbach, T., and Jönsson, L. J. (2011). Enzyme production by filamentous fungi: analysis of the secretome of Trichoderma reesei grown on unconventional carbon source. Microb. Cell Fact 10:68. doi: 10.1186/1475-2859-10-68
Kamat, S., Khot, M., Zinjarde, S., RaviKumar, A., and Gade, W. N. (2013). Coupled production of single cell oil as biodiesel feedstock, xylitol and xylanase from sugarcane bagasse in a biorefinery concept using fungi from the tropical mangrove wetlands. Bioresour. Technol. 135, 246–253. doi: 10.1016/j.biortech.2012.11.059
Kamisaka, Y. (2010). “Preparation of oleaginous yeasts by genetic modification and its potential applications,” in Biocatalysis and Biomolecular Engineering, eds C. T. Hou and J.-F. Shaw (Hoboken, NJ: John Wiley & Sons), 57–65. doi: 10.1002/9780470608524.ch4
Kavadia, A., Komaitis, M., Chevalot, I., Blanchard, F., Marc, I., and Aggelis, G. (2001). Lipid and γ-linolenic acid accumulation in strains of zygomycetes growing on glucose. J. Am. Oil Chem. Soc. 78, 341–346. doi: 10.1007/s11746-001-0266-3
Kendrick, A., and Ratledge, C. (1992a). Desaturation of polyunsaturated fatty acids in Mucor circinelloides and the involvement of a novel membrane-bound malic enzyme. Eur. J. Biochem. 209, 667–673. doi: 10.1111/j.1432-1033.1992.tb17334.x
Kendrick, A., and Ratledge, C. (1992b). Lipid formation in the oleaginous mould Entomophthora exitalis grown in continuous culture: effects of growth rate, temperature and dissolved oxygen tension on polyunsaturated fatty acids. Appl. Microbiol. Biotechnol. 37, 18–22. doi: 10.1007/BF00174196
Kerkhoven, E. J., Pomraning, K. R., Baker, S. E., and Nielsen, J. (2016). Regulation of amino-acid metabolism controls flux to lipid accumulation in Yarrowia lipolytica. NPJ Syst. Biol. Appl. 2, 1–7. doi: 10.1038/npjsba.2016.5
Khan, M. A. K., Yang, J., Hussain, S. A., Zhang, H., Garre, V., and Song, Y. (2019a). Genetic modification of Mucor circinelloides to construct stearidonic acid producing cell factory. Int. J. Mol. Sci. 20:1683. doi: 10.3390/ijms20071683
Khan, M. A. K., Yang, J., Hussain, S. A., Zhang, H., Liang, L., Garre, V., et al. (2019b). Construction of DGLA producing cell factory by genetic modification of Mucor circinelloides. Microb. Cell Fact. 18:64. doi: 10.1186/s12934-019-1110-4
Khot, M., Kamat, S., Zinjarde, S., Pant, A., Chopade, B., and Ravikumar, A. (2012). Single cell oil of oleaginous fungi from the tropical mangrove wetlands as a potential feedstock for biodiesel. Microb. Cell Fact. 11:71. doi: 10.1186/1475-2859-11-71
Knothe, G. (2012). Fuel properties of highly polyunsaturated fatty acid methyl esters. Prediction of fuel properties of algal biodiesel. Energy Fuels 26, 5265–5273. doi: 10.1021/ef300700v
Kosa, G., Kohler, A., Tafintseva, V., Zimmermann, B., Forfang, K., Afseth, N. K., et al. (2017). Microtiter plate cultivation of oleaginous fungi and monitoring of lipogenesis by high-throughput FTIR spectroscopy. Microb. Cell Fact. 16, 1–12. doi: 10.1186/s12934-017-0716-7
Kothri, M., Mavrommati, M., Elazzazy, A. M., Baeshen, M. N., Moussa, T. A., and Aggelis, G. (2020). Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett. 367: fnaa028.
Koubaa, M., Imatoukene, N., Drévillon, L., and Vorobiev, E. (2020). Current insights in yeast cell disruption technologies for oil recovery: a review. Chem. Eng. Proc. 150:107868. doi: 10.1016/j.cep.2020.107868
Laoteng, K., Čertík, M., and Cheevadhanarak, S. (2011). Mechanisms controlling lipid accumulation and polyunsaturated fatty acid synthesis in oleaginous fungi. Chem. Pap. 65, 97–103. doi: 10.2478/s11696-010-0097-4
Lardizabal, K., Effertz, R., Levering, C., Mai, J., Pedroso, M. C., Jury, T., et al. (2008). Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol. 148, 89–96. doi: 10.1104/pp.108.123042
Ledesma-Amaro, R., Lozano-Martínez, P., Jiménez, A., and Revuelta, J. L. (2015). Engineering Ashbya gossypii for efficient biolipid production. Bioengineered 6, 119–123. doi: 10.1080/21655979.2015.1011525
Ledesma-Amaro, R., Santos, M. A., Jiménez, A., and Revuelta, J. L. (2014). Strain design of Ashbya gossypii for single-cell oil production. Appl. Environ. Microbiol. 80, 1237–1244. doi: 10.1128/AEM.03560-13
Liang, M. H., and Jiang, J. G. (2013). Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology. Prog. Lipid Res. 52, 395–408. doi: 10.1016/j.plipres.2013.05.002
Lima, L. G. R., Gonçalves, M. M. M., Couri, S., Melo, V. F., Sant’Ana, G. C. F., and da Costa, A. C. A. (2019). Lipase production by Aspergillus niger C by submerged fermentation. Braz. Arch. Biol. Technol. 62, 1–14. doi: 10.1590/1678-4324-2019180113
Liu, G., Lin, Q., Jin, X., Wang, X., and Zhao, Y. (2010). Screening and fermentation optimization of microbial lipid-producing molds from forest soils. Afr. J. Microbiol. Res. 4, 1462–1468.
Liu, G., and Qu, Y. (2019). Engineering of filamentous fungi for efficient conversion of lignocellulose: tools, recent advances and prospects. Biotechnol. Adv. 37, 519–529. doi: 10.1016/j.biotechadv.2018.12.004
Liu, L., Song, J., Li, Y., Li, P., and Wang, H. (2019). Robust and cost-saving static solid cultivation method for lipid production using the chlamydospores of Phanerochaete chrysosporium. Biotechnol. Biofuels 12:123. doi: 10.1186/s13068-019-1464-1
Lizardi-Jiménez, M. A., and Hernández-Martínez, R. (2017). Solid state fermentation (SSF): diversity of applications to valorize waste and biomass. 3 Biotech. 7:44. doi: 10.1007/s13205-017-0692-y
Lozano-Martínez, P., Buey, R. M., Ledesma-Amaro, R., Jiménez, A., and Revuelta, J. L. (2017). Engineering Ashbya gossypii strains for de novo lipid production using industrial by-products. Microb. Biotechnol. 10, 425–433. doi: 10.1111/1751-7915.12487
Lozano-Martínez, P., Ledesma-Amaro, R., and Revuelta, J. L. (2016). Engineering Ashbya gossypii for the production of ricinoleic and linoleic acid. Chem. Eng. Trans. 49, 253–258. doi: 10.3303/CET1649043
Mamatha, S. S., and Venkateswaran, G. (2010). Differential temperature effect on the production of enhanced gamma linolenic acid in Mucor rouxii CFR-G15. Indian J. Microbiol. 50, 52–56. doi: 10.1007/s12088-010-0053-6
Martínez, E. J., Raghavan, V., González-Andrés, F., and Gómez, X. (2015). New biofuel alternatives: integrating waste management and single cell oil production. Int. Mol. Sci. 16, 9385–9405. doi: 10.3390/ijms16059385
McNichol, J., MacDougall, K. M., Melanson, J. E., and McGinn, P. J. (2012). Suitability of soxhlet extraction to quantify microalgal fatty acids as determined by comparison with in situ transesterification. Lipids 47, 195–207. doi: 10.1007/s11745-011-3624-3
Melikoglu, M., and Webb, C. (2013). “Use of waste bread to produce fermentation products,” in Food Industry Wastes, eds M. Kosseva and C. Webb (San Diego: Academic Press), 61–76. doi: 10.1016/B978-0-12-391921-2.00004-4
Meng, X., Yang, J., Xu, X., Zhang, L., Nie, Q., and Xian, M. (2009). Biodiesel production from oleaginous microorganisms. Renew. Energy 34, 1–5. doi: 10.1016/j.renene.2008.04.014
Miglio, R., Palmery, S., Salvalaggio, M., Carnelli, L., Capuano, F., and Borrelli, R. (2013). Microalgae triacylglycerols content by FT-IR spectroscopy. J. Appl. Phycol. 25, 1621–1631. doi: 10.1007/s10811-013-0007-6
Mironov, A. A., Nemashkalov, V. A., Stepanova, N. N., Kamzolova, S. V., Rymowicz, W., and Morgunov, I. G. (2018). The effect of ph and temperature on arachidonic acid production by glycerol-Grown Mortierella alpina NRRL-A-10995. Fermentation 4:17. doi: 10.3390/fermentation4010017
Mitra, D., Rasmussen, M. L., Chand, P., Chintareddy, V. R., Yao, L., Grewell, D., et al. (2012). Value-added oil and animal feed production from corn-ethanol stillage using the oleaginous fungus Mucor circinelloides. Bioresour. Technol. 107, 368–375. doi: 10.1016/j.biortech.2011.12.031
Muniraj, I. K., Uthandi, S. K., Hu, Z., Xiao, L., and Zhan, X. (2015). Microbial lipid production from renewable and waste materials for second-generation biodiesel feedstock. Environ. Technol. Rev. 4, 1–16. doi: 10.1080/21622515.2015.1018340
Muniraj, I. K., Xiao, L., Hu, Z., and Zhan, X. (2017). Screening and characterization of oleaginous fungi from Irish soil for growth under low carbon substrates. Int. J. Curr. Microbiol. Appl. Sci. 6, 772–781. doi: 10.20546/ijcmas.2017.612.082
Muniraj, I. K., Xiao, L., Hu, Z., Zhan, X., and Shi, J. (2013). Microbial lipid production from potato processing wastewater using oleaginous filamentous fungi Aspergillus oryzae. Water Res. 47, 3477–3483. doi: 10.1016/j.watres.2013.03.046
Mustafa, A., and Turner, C. (2011). Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Analyt. Chim. Acta 703, 8–18. doi: 10.1016/j.aca.2011.07.018
Negin, C., Ali, S., and Xie, Q. (2016). Application of nanotechnology for enhancing oil recovery–A review. Petroleum 2, 324–333. doi: 10.1021/acs.iecr.9b03693
Neidleman, S. L. (1987). Effects of temperature on lipid unsaturation. Biotechnol. Genet. Eng. Rev. 5, 245–268. doi: 10.1080/02648725.1987.10647839
Neto, C. D., Sydney, E. B., Candeo, E. S., de Souza, E. B. S., Camargo, D., Sydney, A. C. N., et al. (2019). New method for the extraction of single-cell oils from wet oleaginous microbial biomass: efficiency, oil characterisation and energy assessment. Waste Biomass Valor. 11, 3443–3452. doi: 10.1007/s12649-019-00705-x
Nkemka, V. N., Gilroyed, B., Yanke, J., Gruninger, R., Vedres, D., McAllister, T., et al. (2015). Bioaugmentation with an anaerobic fungus in a two-stage process for biohydrogen and biogas production using corn silage and cattail. Bioresour. Technol. 185, 79–88. doi: 10.1016/j.biortech.2015.02.100
Ochsenreither, K., Glück, C., Stressler, T., Fischer, L., and Syldatk, C. (2016). Production strategies and applications of microbial single cell oils. Front. Microbiol. 7:1539. doi: 10.3389/fmicb.2016.01539
Papanikolaou, S. (2011). Oleaginous yeasts: biochemical events related with lipid synthesis and potential biotechnological applications. Ferment. Technol. 1:e103. doi: 10.4172/2167-7972.1000e103
Papanikolaou, S., and Aggelis, G. (2010). Yarrowia lipolytica: a model microorganism used for the production of tailor-made lipids. Eur. J. Lipid Sci. Technol. 112, 639–654. doi: 10.1002/ejlt.200900197
Papanikolaou, S., and Aggelis, G. (2011). Lipids of oleaginous yeasts. Part I: biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 113, 1031–1051. doi: 10.1002/ejlt.201100014
Papanikolaou, S., Galiotou-Panayotou, M., Fakas, S., Komaitis, M., and Aggelis, G. (2007). Lipid production by oleaginous Mucorales cultivated on renewable carbon sources. Eur. J. Lipid Sci. Technol. 109, 1060–1070. doi: 10.1002/ejlt.200700169
Papanikolaou, S., Komaitis, M., and Aggelis, G. (2004). Single cell oil (SCO) production by Mortierella isabellina grown on high-sugar content media. Bioresour. Technol. 95, 287–291. doi: 10.1016/j.biortech.2004.02.016
Patel, A., Karageorgou, D., Rova, E., Katapodis, P., Rova, U., Christakopoulos, P., et al. (2020). An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 8:434. doi: 10.3390/microorganisms8030434
Postma, P. R., Suarez-Garcia, E., Safi, C., Yonathan, K., Olivieri, G., Barbosa, M. J., et al. (2017). Energy-efficient bead milling of microalgae: effect of bead size on disintegration and release of proteins and carbohydrates. Bioresour. Technol. 224, 670–679. doi: 10.1016/j.biortech.2016.11.071
Qiao, W., Tao, J., Luo, Y., Tang, T., Miao, J., and Yang, Q. (2018). Microbial oil production from solid-state fermentation by a newly isolated oleaginous fungus, Mucor circinelloides Q531 from mulberry branches. R. Soc. Open Sci. 5:180551. doi: 10.1098/rsos.180551
Rashid, U., Anwar, F., and Knothe, G. (2009). Evaluation of biodiesel obtained from cottonseed oil. Fuel Process. Technol. 90, 1157–1163. doi: 10.1016/j.fuproc.2009.05.016
Ratledge, C. (1993). Single-cell oils-have they a biotechnological future?İ Trends Biotechnol. 11, 278–284.
Ratledge, C. (2002). Regulation of lipid accumulation in oleaginous microorganisms. Biochem. Soc. Trans. 30, A101–A101. doi: 10.1042/bst030a101
Ratledge, C. (2004). Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86, 807–815. doi: 10.1016/j.biochi.2004.09.017
Ratledge, C. (2013). Microbial oils: an introductory overview of current status and future prospects. OCL Oilseeds Fats Crop Lipids 20:D602. doi: 10.1051/ocl/2013029
Ratledge, C., and Wynn, J. P. (2002). The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 51, 1–51. doi: 10.1016/s0065-2164(02)51000-5
Reis, C. E. R., Giovanna, R. F., Bento, H. S. B., Carvalho, A. K. F., Alves, T. M., and de Castro, H. F. (2020). Sugarcane byproducts within the biodiesel production chain: vinasse and molasses as feedstock for oleaginous fungi and conversion to ethyl esters. Fuel 277:118064. doi: 10.1016/j.fuel.2020.118064
Reis, R. C. E., Bento, H. B., Carvalho, A. K., Rajendran, A., Hu, B., and De Castro, H. F. (2019). Critical applications of Mucor circinelloides within a biorefinery context. Crit. Rev. Biotechnol. 39, 555–570. doi: 10.1080/07388551.2019.1592104
Rodríguez-Frómeta, R. A., Gutiérrez, A., Torres-Martínez, S., and Garre, V. (2013). Malic enzyme activity is not the only bottleneck for lipid accumulation in the oleaginous fungus Mucor circinelloides. Appl. Microbiol. Biotechnol. 97, 3063–3072. doi: 10.1007/s00253-012-4432-2
Roohinejad, S., Parniakov, O., Nikmaram, N., Greiner, R., and Koubaa, M. (2018). “Energy saving food processing,” in Sustainable Food Systems from Agriculture to Industry, ed. C. M. Galanakis (Cambridge, MA: Academic Press), 191–243. doi: 10.1016/b978-0-12-811935-8.00006-8
Rumin, J., Bonnefond, H., Saint-Jean, B., Rouxel, C., Sciandra, A., Bernard, O., et al. (2015). The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol. Biofuels 8:42. doi: 10.1186/s13068-015-0220-4
Samarasinghe, N., Fernando, S., Lacey, R., and Faulkner, W. B. (2012). Algal cell rupture using high pressure homogenization as a prelude to oil extraction. Renew. Energy 48, 300–308. doi: 10.1016/j.renene.2012.04.039
Santek, M. I., Beluhan, S., and Santek, B. (2018). “Production of microbial lipids from lignocellulosic biomass,” in Advances in Biofuels and Bioenergy, eds M. Nageswara-Rao and J. Soneji (London: Intechopen), doi: 10.5772/intechopen.74013
Sarma, S. J., Pachapur, V., Brar, S. K., Le Bihan, Y., and Buelna, G. (2015). Hydrogen biorefinery: potential utilization of the liquid waste from fermentative hydrogen production. Renew. Sustain. Energy Rev. 50, 942–951. doi: 10.1016/j.rser.2015.04.191
Shi, S., Chen, Y., Siewers, V., and Nielsen, J. (2014). Improving production of malonyl coenzyme A-derived metabolites by abolishing Snf1-dependent regulation of Acc1. mBio 5:e01130-14. doi: 10.1128/mBio.01130-14
Singh, D., Sharma, D., Soni, S. L., Sharma, S., and Kumari, D. (2019). Chemical compositions, properties, and standards for different generation biodiesels: a review. Fuel 253, 60–71. doi: 10.1016/j.fuel.2019.04.174
Sitepu, I. R., Garay, L. A., Sestric, R., Levin, D., Block, D. E., German, J. B., et al. (2014). Oleaginous yeasts for biodiesel: current and future trends in biology and production. Biotechnol. Adv. 32, 1336–1360. doi: 10.1016/j.biotechadv.2014.08.003
Somashekar, D., Venkateshwaran, G., Sambaiah, K., and Lokesh, B. R. (2003). Effect of culture conditions on lipid and gamma-linolenic acid production by mucoraceous fungi. Process. Biochem. 38, 1719–1724. doi: 10.1016/S0032-9592(02)00258-3
Somashekar, D., Venkateshwaran, G., Srividya, C., Sambaiah, K., and Lokesh, B. R. (2001). Efficacy of extraction methods for lipid and fatty acid composition from fungal cultures. World J. Microbiol. Biotechnol. 17, 317–320. doi: 10.1023/A:1016792311744
Subhash, G. V., and Mohan, S. V. (2011). Biodiesel production from isolated oleaginous fungi Aspergillus sp. using corncob waste liquor as a substrate. Bioresour. Technol. 102, 9286–9290. doi: 10.1016/j.biortech.2011.06.084
Takeno, S., Sakuradani, E., Tomi, A., Inohara-Ochiai, M., Kawashima, H., Ashikari, T., et al. (2005). Improvement of the fatty acid composition of an oil-producing filamentous fungus, Mortierella alpina 1S-4, through RNA interference with Δ12-desaturase gene expression. Appl. Environ. Microbiol. 71, 5124–5128. doi: 10.1128/AEM.71.9.5124-5128.2005
Tamano, K., Bruno, K. S., Karagiosis, S. A., Culley, D. E., Deng, S., Collett, J. R., et al. (2013). Increased production of fatty acids and triglycerides in Aspergillus oryzae by enhancing expressions of fatty acid synthesis-related genes. Appl. Microbiol. Biotechnol. 97, 269–281. doi: 10.1007/s00253-012-4193-y
Tan, K. W. M., Lin, H., Shen, H., and Lee, Y. K. (2016). Nitrogen-induced metabolic changes and molecular determinants of carbon allocation in Dunaliella tertiolecta. Sci. Rep. 6, 1–13. doi: 10.1038/srep37235
Tonato, D., Marcuz, C., Vendruscolo, R. G., Bevilacqua, C., Jacques, R. J. S., Wagner, R., et al. (2018). Production of polyunsaturated fatty acids by microorganisms isolated in the Brazilian Pampa biome. Braz. J. Chem. Eng. 35, 835–846. doi: 10.1590/0104-6632.20180353s20170155
Troiano, D., Orsat, V., and Dumont, M. J. (2020). Status of filamentous fungi in integrated biorefineries. Renew. Sust. Energ. Rev. 117:109472. doi: 10.1016/j.rser.2019.109472
Tzimorotas, D., Afseth, N. K., Lindberg, D., Kjørlaug, O., Axelsson, L., and Shapaval, V. (2018). Pretreatment of different food rest materials for bioconversion into fungal lipid-rich biomass. Bioprocess Biosyst. Eng. 41, 1039–1049. doi: 10.1007/s00449-018-1933-0
Valdés, G., Mendonça, R. T., and Aggelis, G. (2020). Lignocellulosic biomass as a substrate for oleaginous microorganisms: a review. Appl. Sci. 10:7698. doi: 10.3390/app10217698
Vicente, G., Bautista, F. L., Gutiérrez, F. J., Rodríguez, R., Martínez, V., Rodríguez-Frómeta, R. A., et al. (2010). Direct transformation of fungal biomass from submerged cultures into biodiesel. Energy Fuels 24, 3173–3178. doi: 10.1021/ef9015872
Vorapreeda, T., Thammarongtham, C., Cheevadhanarak, S., and Laoteng, K. (2012). Alternative routes of acetyl-CoA synthesis identified by comparative genomic analysis: involvement in the lipid production of oleaginous yeast and fungi. Microbiology 158, 217–228. doi: 10.1099/mic.0.051946-0
Wang, L., Zhang, H., Zhang, Y., and Song, Y. (2019). 13C metabolic flux analysis on roles of malate transporter in lipid accumulation of Mucor circinelloides. Microb. Cell Fact. 18, 1–14. doi: 10.1186/s12934-019-1207-9
Woo, I. S., Rhee, I. K., and Park, H. D. (2000). Differential damage in bacterial cells by microwave radiation on the basis of cell wall structure. Appl. Environ. Microbiol. 66, 2243–2247. doi: 10.1128/aem.66.5.2243-2247.2000
Wynn, J. P., and Ratledge, C. (1997). Malic enzyme is a major source of NADPH for lipid accumulation by Aspergillus nidulans. Microbiology 143, 253–257. doi: 10.1099/00221287-143-1-253
Xia, C., Zhang, J., Zhang, W., and Hu, B. (2011). A new cultivation method for microbial oil production: cell pelletization and lipid accumulation by Mucor circinelloides. Biotechnol. Biofuels 4:15. doi: 10.1186/1754-6834-4-15
Xu, L., Brilman, D. W. W., Withag, J. A., Brem, G., and Kersten, S. (2011). Assessment of a dry and a wet route for the production of biofuels from microalgae: energy balance analysis. Bioresour. Technol. 102, 5113–5122. doi: 10.1016/j.biortech.2011.01.066
Yang, J., Khan, M. A. K., López-García, S., Nosheen, S., Nazir, Y., Zhang, H., et al. (2020). Improved SDA production in high lipid accumulating strain of Mucor circinelloides WJ11 by genetic modification. Am. J. Biochem. Biotechnol. 16:147. doi: 10.3844/ajbbsp.2020.138.147
Yang, J., Li, S., Kabir Khan, M. A., Garre, V., Vongsangnak, W., and Song, Y. (2019). Increased lipid accumulation in Mucor circinelloides by overexpression of mitochondrial citrate transporter genes. Ind. Eng. Chem. Res. 58, 2125–2134. doi: 10.1021/acs.iecr.8b05564
Yang, L., Li, H., and Wang, Q. (2019). A novel one-step method for oil-rich biomass production and harvesting by co-cultivating microalgae with filamentous fungi in molasses wastewater. Bioresour. Technol. 275, 35–43. doi: 10.1016/j.biortech.2018.12.036
Yang, J., Zhang, X., Yong, Q., and Yu, S. (2010). Three-stage hydrolysis to enhance enzymatic saccharification of steam-exploded corn stover. Bioresour. Technol. 101, 4930–4935. doi: 10.1016/j.biortech.2009.09.079
Yang, J., Zhang, X., Yong, Q., and Yu, S. (2011). Three-stage enzymatic hydrolysis of steam-exploded corn stover at high substrate concentration. Bioresour. Technol. 102, 4905–4908.
Yao, F., Liu, S. C., Wang, D. N., Liu, Z. J., Hua, Q., and Wei, L. J. (2020). Engineering oleaginous yeast Yarrowia lipolytica for enhanced limonene production from xylose and lignocellulosic hydrolysate. FEMS Yeast Res. 20, 1–9. doi: 10.1093/femsyr/foaa046
Yao, Q., Chen, H., Wang, S., Tang, X., Gu, Z., Zhang, H., et al. (2019). An efficient strategy for screening polyunsaturated fatty acid-producing oleaginous filamentous fungi from soil. J. Microbiol. Methods 158, 80–85. doi: 10.1016/j.mimet.2018.12.023
Yazawa, H., Iwahashi, H., Kamisaka, Y., Kimura, K., Aki, T., Ono, K., et al. (2007). Heterologous production of dihomo-γ-linolenic acid in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 73, 6965–6971. doi: 10.1128/AEM.01008-07
Yousuf, A., Ethiraj, B., Khan, M. R., and Pirozzi, D. (2018). “Fungal biorefinery for the production of single cell oils as advanced biofuels,” in Fungal Biorefineries. Fungal Biology, eds S. Kumar, P. Dheeran, M. Taherzadeh, and S. Khanal (Cham: Springer), 185–213. doi: 10.1007/978-3-319-90379-8_9
Zhang, Y., Adams, I. P., and Ratledge, C. (2007). Malic enzyme: the controlling activity for lipid production? Overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiology 153, 2013–2025. doi: 10.1099/mic.0.2006/002683-0
Zhang, Y., Luan, X., Zhang, H., Garre, V., Song, Y., and Ratledge, C. (2017). Improved γ-linolenic acid production in Mucor circinelloides by homologous overexpressing of delta-12 and delta-6 desaturases. Microb. Cell Fact. 16, 10–12. doi: 10.1186/s12934-017-0723-8
Zhao, C., Fang, H., and Chen, S. (2017). Single cell oil production by Trichosporon cutaneum from steam-exploded corn stover and its upgradation for production of long-chain α,ω-dicarboxylic acids. Biotechnol. Biofuels 10:202. doi: 10.1186/s13068-017-0889-7
Zhao, L., Cánovas-Márquez, J. T., Tang, X., Chen, H., Chen, Y. Q., Chen, W., et al. (2016). Role of malate transporter in lipid accumulation of oleaginous fungus Mucor circinelloides. Appl. Microbiol. Biotechnol. 100, 1297–1305. doi: 10.1007/s00253-015-7079-y
Zhao, L., Tang, X., Luan, X., Chen, H., Chen, Y. Q., Chen, W., et al. (2015). Role of pentose phosphate pathway in lipid accumulation of oleaginous fungus Mucor circinelloides. RSC Adv. 5, 97658–97664. doi: 10.1039/c5ra20364c
Zhou, C., Sun, D., Sun, X., Zhu, C., and Wang, Q. (2018). Combining ultrasound and microwave to improve the yield and quality of single-cell oil from Mortierella isabellina NTG1— 121. J. Am. Oil Chem. Soc. 95, 1535–1547. doi: 10.1002/aocs.12134
Zhou, C., Zhu, C., and Ren, Y. (2013). Optimization of ultrasound-assisted extraction of single cell oil from Mortierella isabellina. Sci. Technol. 48, 2188–2195. doi: 10.1080/01496395.2013.796562
Keywords: single-cell oils, filamentous fungi, biorefinery, feedstock, biodiesel
Citation: Mhlongo SI, Ezeokoli OT, Roopnarain A, Ndaba B, Sekoai PT, Habimana O and Pohl CH (2021) The Potential of Single-Cell Oils Derived From Filamentous Fungi as Alternative Feedstock Sources for Biodiesel Production. Front. Microbiol. 12:637381. doi: 10.3389/fmicb.2021.637381
Received: 03 December 2020; Accepted: 07 January 2021;
Published: 28 January 2021.
Edited by:
Debarati Paul, Amity University, IndiaReviewed by:
Sirasit Srinuanpan, Chiang Mai University, ThailandCopyright © 2021 Mhlongo, Ezeokoli, Roopnarain, Ndaba, Sekoai, Habimana and Pohl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sizwe I. Mhlongo, TWhsb25nb1M5QHVrem4uYWMuemE=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.