
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 12 February 2021
Sec. Extreme Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.633649
This article is part of the Research Topic Archaea in the Environment: Views on Archaeal Distribution, Activity, and Biogeography View all 10 articles
 Andreas Teske1*
Andreas Teske1* Gunter Wegener2,3
Gunter Wegener2,3 Jeffrey P. Chanton4
Jeffrey P. Chanton4 Dylan White1
Dylan White1 Barbara MacGregor1,5
Barbara MacGregor1,5 Daniel Hoer6,7
Daniel Hoer6,7 Dirk de Beer2
Dirk de Beer2 Guangchao Zhuang8,9,10
Guangchao Zhuang8,9,10 Matthew A. Saxton10,11
Matthew A. Saxton10,11 Samantha B. Joye10
Samantha B. Joye10 Daniel Lizarralde12
Daniel Lizarralde12 S. Adam Soule12
S. Adam Soule12 S. Emil Ruff13,14
S. Emil Ruff13,14Cold seeps and hydrothermal vents are seafloor habitats fueled by subsurface energy sources. Both habitat types coexist in Guaymas Basin in the Gulf of California, providing an opportunity to compare microbial communities with distinct physiologies adapted to different thermal regimes. Hydrothermally active sites in the southern Guaymas Basin axial valley, and cold seep sites at Octopus Mound, a carbonate mound with abundant methanotrophic cold seep fauna at the Central Seep location on the northern off-axis flanking regions, show consistent geochemical and microbial differences between hot, temperate, cold seep, and background sites. The changing microbial actors include autotrophic and heterotrophic bacterial and archaeal lineages that catalyze sulfur, nitrogen, and methane cycling, organic matter degradation, and hydrocarbon oxidation. Thermal, biogeochemical, and microbiological characteristics of the sampling locations indicate that sediment thermal regime and seep-derived or hydrothermal energy sources structure the microbial communities at the sediment surface.
Globally, over 700 marine hydrothermal vent sites are currently known (Beaulieu and Szafranski, 2020), including sedimented hydrothermal systems at coastal and continental margin locations (Price and Giovanelli, 2017). One of the best-studied sedimented hydrothermal systems is Guaymas Basin in the Gulf of California, a young marginal rift basin characterized by active seafloor spreading and rapid deposition of organic-rich sediments from highly productive overlying waters (Lonsdale and Becker, 1985). Buried organic matter is hydrothermally transformed to methane, aliphatic and aromatic hydrocarbons, dissolved inorganic carbon, and ammonia, resulting in well-buffered and nutrient-rich vent fluids (Von Damm et al., 1985) that sustain ample microbial communities. The microbiology and biogeochemistry of hydrothermal sediments in Guaymas Basin have been studied extensively. Sulfur-oxidizing microbial mats occur in visually conspicuous hot spots where sulfide, methane-, and hydrocarbon-rich hydrothermal fluids rise to the sediment surface (McKay et al., 2012; MacGregor et al., 2013). Surficial sediments harbor complex anaerobic microbial communities adapted to these conditions, including thermophilic methane- and alkane-oxidizing archaea (Teske et al., 2002; Biddle et al., 2012; Wegener et al., 2015; Dowell et al., 2016; Laso-Pérez et al., 2016; Wang et al., 2019) and hydrocarbon-oxidizing, free-living or syntrophic sulfate-reducing bacteria (reviewed by Teske, 2019). The diversity of hydrothermal regimes in Guaymas Basin selects for microbial communities with adaptations to different thermal and geochemical niches (McKay et al., 2016; Teske et al., 2016). Here we compare thermal gradients, porewater geochemistry, and microbial community composition in the surface layer of different hydrothermal, cold seep, and background sediments (Table 1) to determine whether sediments with distinct porewater geochemistry and thermal regimes harbor particular bacterial and archaeal populations.
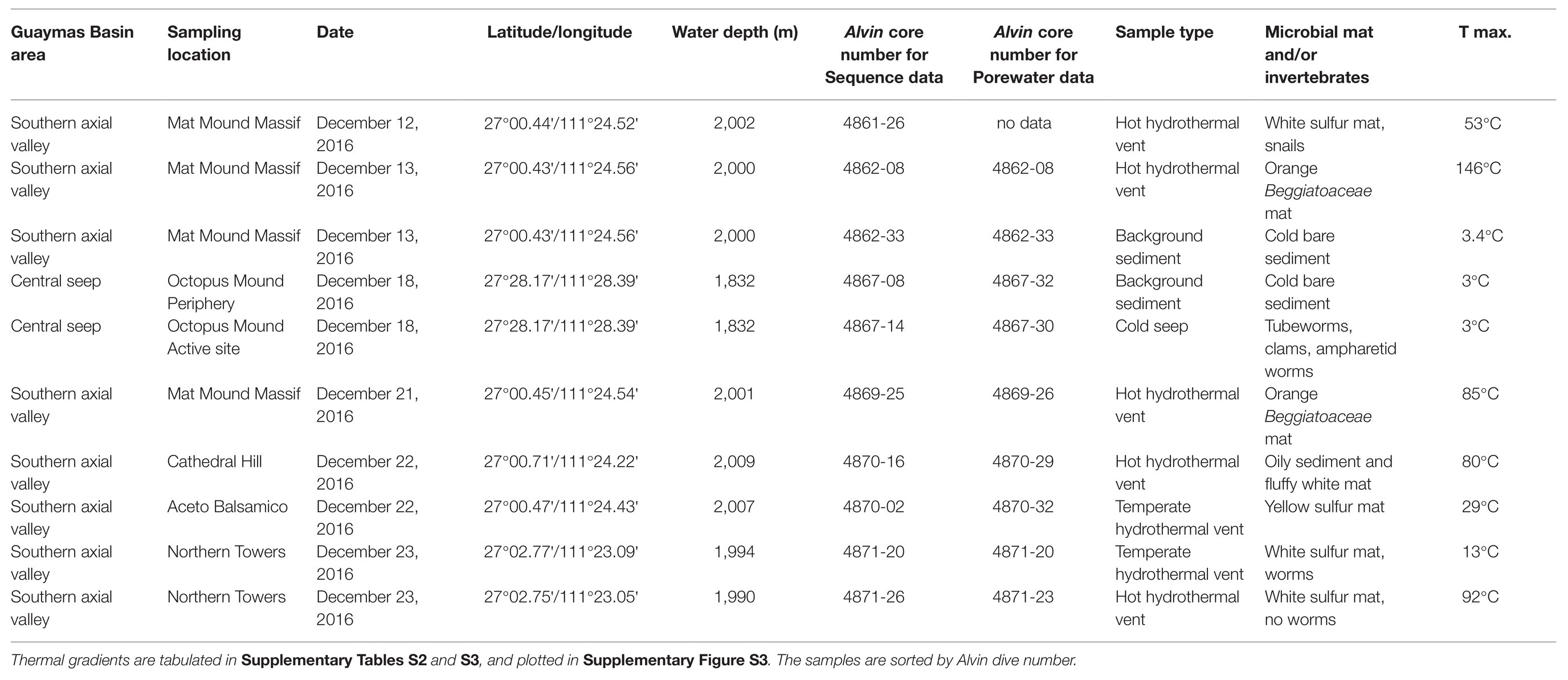
Table 1. Sampling sites and Alvin core numbers for 2016 sediment samples collected during Atlantis cruise AT37-06, used in parallel molecular and biogeochemical analyses.
In contrast to previous small-scale surveys that focused on individual hydrothermal mounds or microbial mats (McKay et al., 2012, 2016; Dowell et al., 2016), the sampling sites are separated by distances of hundreds of meters or several miles (Figure 1). Within a few miles of each other, the greater Guaymas Basin geo-ecosystem includes hydrothermal areas at the southern Guaymas Basin spreading center (Teske et al., 2016), off-axis hydrothermal sites (Teske et al., 2019; Ramírez et al., 2020), hydrothermal mounds just off the northern spreading center (Berndt et al., 2016), and cold seeps on the northern flanking regions and the adjacent Sonora Margin (Geilert et al., 2018). Methane-rich off-axis cold seeps have been documented by deep-tow photography of faunal communities and carbonates, and by in situ measurements of methane anomalies in the deep water column (Lizarralde et al., 2011), and some cold seep sites have been sampled further by multicoring and gravity coring of sediments and dredging of seafloor minerals (Geilert et al., 2018; Núñez-Useche et al., 2018). In contrast to compression-induced seepage on massively sedimented continental margins and plate boundaries (Suess, 2010), seismic surveys have linked the Guaymas Basin cold seeps to deeply buried volcanic sills that extend from the spreading centers (Einsele et al., 1980) up to 50km across the sedimented Guaymas flanking regions (Lizarralde et al., 2011). At specific off-axis locations, shallow hot sills drive hydrothermal fluid and gas circulation (Teske et al., 2019). Yet in most cases, off-axis sills and their seep fluids have cooled off over time, and methane-rich fluids have temperatures near ambient bottom water when they are released at the sediment surface (Lizarralde et al., 2011; Geilert et al., 2018). Guaymas Basin cold seep sites remain to be explored and sampled up close by ROV and submersible. This study concludes by surveying a cold seep site (“Central Seep”) approximately equidistant from Sonora and Baja California on the northern flanking regions (Geilert et al., 2018; Núñez-Useche et al., 2018).
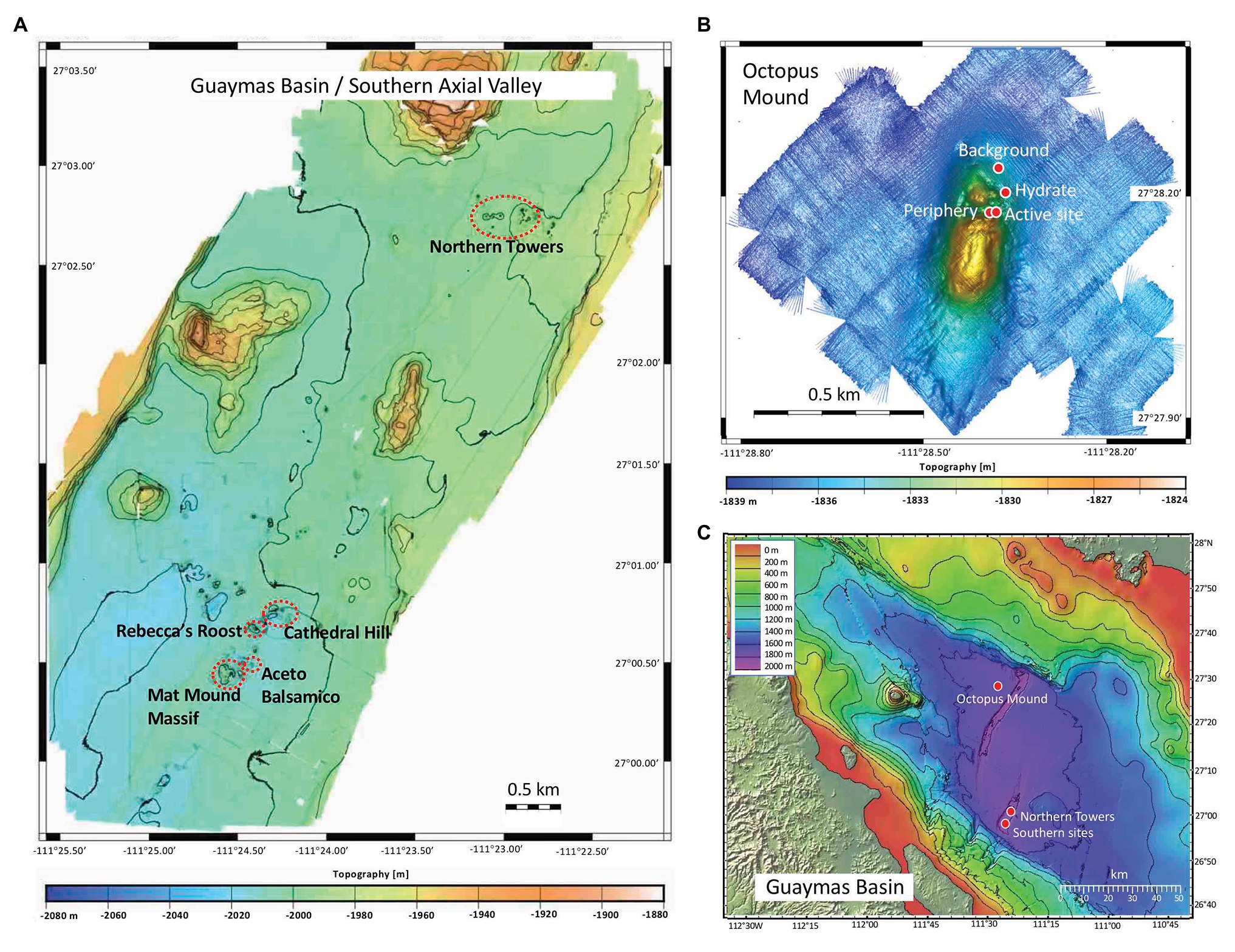
Figure 1. Bathymetric maps of Guaymas Basin and Expedition AT37-06 sampling locations. (A) Guaymas Basin southern axial valley, mapped during Sentry dives 407-409 and 413-417. (B) Central Seep area with Octopus Mound, mapped during Sentry dive 412. (C) Guaymas Basin overview annotated with sampling sites, based on a template courtesy of C. Mortera, UNAM.
Guaymas Basin sites were visited and sampled with R/V Atlantis, HOV Alvin, and AUV Sentry during cruise AT37-06 (December 6–29, 2016). Alvin dives targeted previously explored sampling areas (Teske et al., 2016), or newly identified sites found by AUV Sentry. After Sentry returned from pre-programmed night dives at ca. 6AM, dive data and bathymetries were downloaded and made available in time for the following Alvin dive starting at 8AM. When Sentry performed seafloor photomosaic surveys running ca. 6m above bottom, the resulting images were inspected for microbial mats and potential dive targets, for example, in the “Northern Towers” area of the southern axial trough of Guaymas Basin. Photo coverage of Alvin dives is available at the Alvin frame-grabber site.1 The bathymetry of the hydrothermally active graben segment in southern Guaymas Basin was mapped by AUV Sentry during dives 407-409, 413-417; the bathymetry of Octopus Mound at the Central Seep site was mapped during Sentry dive 412 (Figure 1). Survey height was 65–70m above the bottom. Alvin sampling sites were largely based on Sentry surveys (Supplementary Figure S1).
Thermal profiles were measured in surficial sediments using Alvin’s 50cm or 1-m heat flow probes.2 The 50cm probe was used for hydrothermal sites in the southern axial valley and contains thermal sensors every 10cm, starting 5cm under the attached plastic disk (the “puck”) that limits probe penetration and rests on the seafloor once the probe was inserted. Cold seep sediments of Octopus Mound were profiled using the 1-m probe with thermal sensors every 20cm. After 5–10min of temperature reading stabilization, temperature readings were recorded. Thermal profiles adjacent to sediment cores that were analyzed in this study are shown in Supplementary Figure S3.
DNA was collected from the top 0–1cm of sediment cores after removal of any overlying microbial mat, except for one mat-covered core (4862-08) where draining fluids had sucked the mat into the sediment before slicing. Genomic DNA was extracted from 0.5g of sediment from each sample using the DNeasy PowerLyzer PowerSoil Kit (Cat. No. 12855-100, QIAGEN) and bead-beating at 6ms−1 for 45s using a Bead Ruptor 24 (OMNI International, Kennesaw, GA, United States). Extraction blanks were performed alongside the samples to assess laboratory contamination during the extraction process. DNA concentrations were assessed fluorometrically using a Qubit 2.0 fluorometer (Thermo Fisher Scientific, Canada). Bacterial 16S rRNA gene variable region v3-v4 was amplified using the “universal” primer pair 341F (5'-CCTACGGGAGGCAGCAG-3'; Klindworth et al., 2013) and Pro805R (5'-GACTACNVGGGTATCTAATCC-3'; Takahashi et al., 2014). The archaeal 16S rRNA gene v4-v5 variable region was amplified using the combined forward primers 517F (5'-GCCTAAAGCATCCGTAGC-3', 5'-GCCTAAARCGTYCGTAGC-3', 5'-GTCTAAAGGGTCYGTAGC-3', 5'-GCTTAAAGNGTYCGTAGC-3', 5'-GTCTAAARCGYYCGTAGC-3') and reverse primer 958R (5'-CCGGCGTTGANTCCAATT-3'; Topçuoglu et al., 2016). The primers were modified with Illumina MiSeq overhang adapters. Each PCR reaction consisted of 1–5μl (~10ng) genomic DNA template, 2.5μl of each of the primers (final concentration 1μM), 12.5μl 2X Kapa HiFi HotStart ReadyMix (Kapa Biosystems, Wilmington, MA, United States) and sterile nuclease-free water to make a final volume of 25μl gene amplification was carried out using an initial degradation at 95°C for 3min following 25cycles of denaturation at 95°C for 30s, annealing at 56°C for 45s, and extension at 72°C for 1min, and concluded with a final extension at 72°C for 5min. All PCR reactions were performed in triplicate, pooled, and purified using the NucleoMag NGS Clean-up and Size Select kit (Macherey-Nagel Inc., Bethlehem, PA, United States). The purified PCR products were indexed following the instructions on Illumina’s 16S amplicon library preparation guide. The concentration of dsDNA and the size of the indexed amplicons were verified using the Qubit dsDNA High Sensitivity assay kit on a Qubit 2.0 fluorometer (Thermo Fisher Scientific, Canada) and the Agilent 2100 Bioanalyzer system (Agilent Technologies, Mississauga, ON, Canada), respectively. Indexed amplicons were pooled in equimolar amounts and sequenced using Illumina’s v3 600-cycle (paired-end) reagent kit on a MiSeq benchtop sequencer (Illumina Inc., San Diego, CA, United States).
Raw bacterial 16S rRNA gene amplicon sequence data were analyzed using MetaAmp (Dong et al., 2017), and archaeal raw sequence data were analyzed using DADA2 (Callaghan et al., 2016). Taxonomy of operational taxonomic units (bacterial OTUs, defined at 98% sequence identity) or amplicon sequence variants (archaeal ASVs) was assigned using the SILVA reference database v132 (Quast et al., 2013). Community analyses were performed using VisuaR3, an R-based workflow using the packages vegan, labdsv, tidyverse (stringr, dplyr, ggplot2), UpSetR, and custom scripts (Wickham, 2016, 2018; Oksanen et al., 2012; Roberts, 2012; Conway et al., 2017; Wickham et al., 2018). The original OTU or ASV abundance tables were used to calculate richness and diversity indices, i.e., Inverse Simpson diversity (Hill et al., 2003), Shannon entropy, and Chao1 estimated richness (Chao, 1984) with a subsampling approach to ensure comparability of indices. Dissimilarities between samples were calculated using the Bray-Curtis dissimilarity coefficient (i.e., relative sequence abundance; Bray and Curtis, 1957). The resulting beta-diversity matrices were used for 2D non-metric multidimensional scaling (NMDS) ordinations with 20 random starts (Kruskal, 1964). Stress values below 0.2 indicate that the multidimensional dataset is well represented by the 2D ordination.
Phylogenetic placement of amplicon sequences was conducted using the SILVA database SSU Ref NR 132 (Quast et al., 2013) and the software ARB (Ludwig et al., 2004). ASVs were first added to the SILVA tree using the ARB “quick add” tool, neighboring near full-length sequences (>1,300 nucleotides) were selected and aligned using SINA (Prüsse et al., 2012). The alignment was manually curated based on ribosomal secondary structure and was subsequently used to calculate 100 maximum likelihood phylogenetic trees with the phyML algorithm, of which the most likely tree was automatically selected. We used a positional variability filter including only conserved positions in the alignment with a mutation rate of <3.1%. Finally, amplicon sequences were added to the consensus tree using the same positional variability filter without changing the overall tree topology.
A second set of DNA extractions from sediment was performed with the MO BIO PowerSoil DNA Isolation Kit (QIAGEN, Carlsbad, CA, United States) for the purpose of mcrA gene amplification and sequencing. Amplification was performed with general mcrA primer combination mcrIRD-F (5'-GACCAGTTGTGGTTCGGAAC-3') and mcrIRD-R (5'-ATCTCGAATGGCATTCCCTC-3'; Lever and Teske, 2015). The PCR protocol contained 30cycles of initial denaturation at 95°C for 1min, annealing at 55°C for 1min and extension at 72°C for 1min, and concluded with a final extension at 72°C for 5min. PCR products were purified using the Wizard SV Gel and PCR Cleanup System (Promega Corporation, Madison, WI, United States) and cloned into plasmid vectors using the TOPO TA Cloning Kit (Life Technologies, Carlsbad, CA, United States). These were used to transform One Shot TOP10 Escherichia coli cells (Life Technologies, Carlsbad, CA, United States) which were plated on selective media. Approximately 25 colonies per sample were picked and incubated overnight in SOC medium, then plasmids were extracted using the GeneJET Plasmid Miniprep Kit (Thermo Fisher Scientific, Waltham, MA, United States). Plasmids were then sent to GeneWiz (South Plainfield, NJ, United States) for sequencing. The alignment of representative mcrA gene sequences was created in the MEGA software using the MUSCLE algorithm (Edgar, 2004), and the tree was created from the gene sequence alignment using the neighbor-joining method in MEGA4 (Tamura et al., 2007). The topology of the mcrA phylogeny was tested by 500 bootstrap runs.
For porewater analysis, intact sediment cores were sampled using the Rhizons (Rhizosphere Research Products, Wageningen, NL) as described previously (Seeberg-Elverfeldt et al., 2005). The overlying water was removed from the cores. Holes were drilled into the core at designated sediment sampling depths and pretreated Rhizons (washed twice with HCl and MilliQ water) were injected and suction was applied with syringes for ~30min. For sulfide analysis, 1ml porewater subsamples were fixed with 0.1ml of 0.1M zinc acetate solution to preserve sulfide as zinc sulfide until analysis using the methylene blue method (Cline, 1969). The same fixed porewater sample was used for measuring sulfate concentrations using ion chromatography (Metrohm 930 Compact IC flex oven, Metrosep A PCC HC/4.0 preconcentration column, and Metrosep A Supp 5 Guard/4.0 chromatography column). The concentrations of ammonium, phosphate, and silicate were determined from the same porewater samples using a continuous flow nutrient analyzer (QuAAtro39; Seal Analytical) as published previously (Grasshoff et al., 2009). For combined concentration and δ13C analysis of methane, 2ml sediment subsamples were added to 30ml serum vials containing 2ml of 1M sodium hydroxide solution, sealed with thick butyl rubber stoppers, crimped with aluminum seals, and stored at 4°C. Shipping problems and a resulting shortage of serum vials limited sampling capabilities and only selected sediment cores were sampled for methane. Since cores were retrieved unpressurized, outgassing may have impacted in particular the measurements of methane concentrations near and above saturation. After the cruise, the methane samples were analyzed by headspace gas chromatography-flame ionization detection (GC-FID) at Florida State University (Magen et al., 2014). Additionally, the gas samples were analyzed for δ13CH4 by injecting 0.1–0.5ml of sample into a gas chromatograph interfaced to a Finnigan MAT Delta S isotope ratio Mass Spectrometer inlet system as previously described (Chanton and Liptay, 2000). Values are reported in the per mil (‰) notation relative to Vienna Pee Dee Belemnite (VPDB).
During expedition AT37-06 to Guaymas Basin, AUV Sentry and submersible Alvin mapped and sampled hydrothermally active sediments within the southern Guaymas Basin spreading center, at an off-axis sill-driven vent site (Ringvent) with hybrid seep/vent biota on the northwestern flanking regions near Isla Tortuga (Teske et al., 2019), and at the off-axis Central Seep site on the northwestern Guaymas flanks (Geilert et al., 2018) that is approximately equidistant from the Sonora and Baja California coasts (Figure 1).
Different types of mat-covered sediments and thermal regimes were sampled at these locations (Figure 2). The Mat Mound Massif, a cluster of hydrothermal mounds and edifices, was probed from different angles on previous cruises (e.g., Dowell et al., 2016) but its full extent was only recognized during this expedition. The Aceto Balsamico area is located ca. 150m west of Mat Mound Massif and contains moderately warm sediments covered with lime-yellow mats of sulfur-oxidizing bacteria (Teske et al., 2016). At Cathedral Hill, ca. 200m north of Aceto Balsamico, gradually sloping sediment-covered mounds with extensive microbial mats are topped with hydrothermal edifices (Teske et al., 2016). The Northern Towers area is located ca. 5km northeast from the other, tightly clustered sites, and was not sampled during previous expeditions in 2008/2009 (Teske et al., 2016). It has relatively few hydrothermal sediments and mats but is dominated by massive, steep hydrothermal edifices and chimneys. Here, microbial mats were located by AUV Sentry in photomosaic survey mode, and their coordinates were targeted during subsequent Alvin dives. Alvin push cores from all locations were collected during Expedition AT37-06 on Alvin dives 4861, 4862, 4867, 4869, 4870, and 4871 (Table 1).

Figure 2. Sampling sites for microbial and/or biogeochemical analyses. (A) Extensive white mat at Mat Mound Massif. Dive 4861. (B) Hydrothermal sediment with orange Beggiatoaceae mat, Mat Mound Massif. Dive 4862. (C) Cold seep site “active site” at Octopus Mound. Dive 4867. (D) Hydrothermal sediment with orange Beggiatoaceae mat at Mat Mound Massif. Dive 4869. (E) Summit and slopes at Cathedral Hill. The cores are from the white, fluffy mat area at the bottom of the photo. Dive 4870. (F) Temperate Aceto Balsamico mat with lime-yellow sulfur precipitates. Dive 4870. (G) Temperate “site 2” mat-covered sediment at Northern Towers. Dive 4871. (H) Hot “site 3” mat-covered hydrothermal sediment at Northern Towers. Dive 4871. The sites were photographed from inside Alvin (dives 4862, 4867, 4870) or documented as Alvin screen grabber images (dives 4861, 4869, 4871). Images courtesy of Alvin group, WHOI.
Bathymetric mapping with AUV Sentry and reconnaissance with submersible Alvin at the Central Seep location revealed a mound of 200m north-to-south and 100m east-to-west extension that rises ca. 20m above the seafloor. The base of the mound at its northern end, and nearby sediments harbored extensive cold seep communities and surface-breaching gas hydrates (Figure 3). This mound was informally named “Octopus Mound” for its abundance of cephalopods (Supplementary Figure S1) after it was explored and sampled during Alvin dives 4866 and 4867 (December 17–18, 2016). Surface sediment samples were collected by HOV Alvin within ~100m of each other at the northern tip of Octopus Mound in an area with small-scale topographical diversity, diverse cold seep fauna, seafloor mineral formations, microbial mats, and hydrates (Figure 3). Sampling locations at Octopus Mound (Supplementary Table S1) were chosen based on the presence or absence of seep fauna assemblages (Supplementary Figure S2).
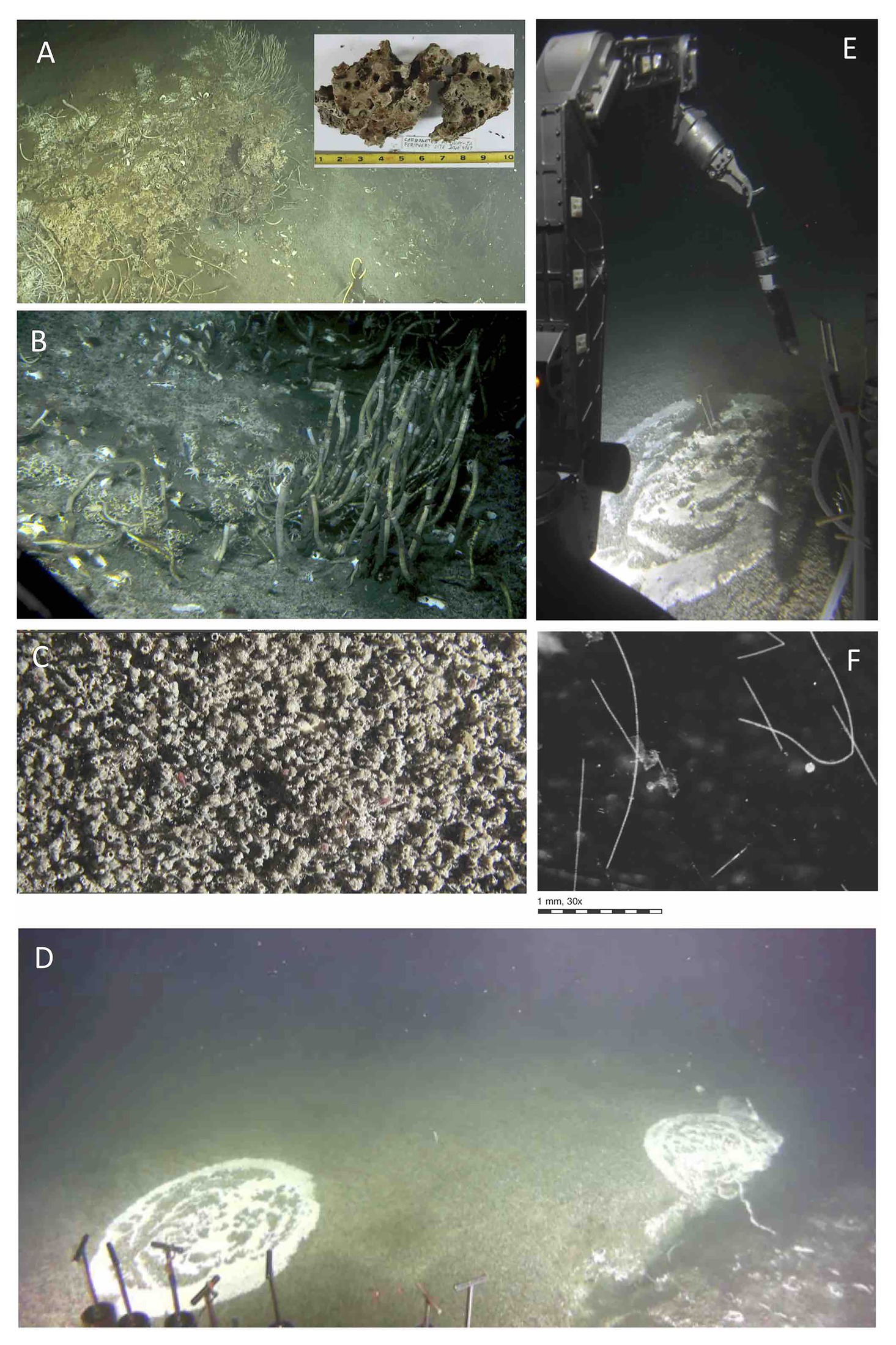
Figure 3. Benthic fauna at Octopus Mound sampling sites. (A) Seafloor carbonate concretions with tube worms at the “active site” near the base of Octopus Mound. Insert, carbonate sample collected at this location during Alvin dive 4867. (B) Seep community with different types of tubeworms resembling Lamellibrachia, galatheid crabs, and clam shells. The original image was underexposed and had to be digitally manipulated, resulting in over-emphasized steel-blue hues instead of olive-green and brown tones. (C) Close-up view of ampharetid worm carpet, with pink worms protruding from several worm tubes. Overall view ~10 × 20cm; video still from Alvin’s bottom camera. (D) Sedimented Hydrate mound, overgrown with an extensive mat of ampharetid worms and circular spots of Beggiatoaceae mats. At the massive fracture to the right, the mat-covered sediment is ca. 0.5m elevated, suggesting rising hydrate underneath. The Beggiatoaceae mat to the left was sampled with Alvin push cores. (E) Coring the Beggiatoaceae mat on top of the hydrate mound during dive 4867, the “hydrate site.” The bottom of the freshly collected core contains white gas hydrate (presumably methane hydrate) that dissipates during transport to the surface. (F) Individual Beggiatoaceae filaments recovered from the hydrate mat are viewed through a dissection binocular. Filament diameters are in the range of 100–160μm, consistent with large, colorless Beggiatoaceae observed previously in Guaymas Basin (McKay et al., 2012; Teske and Salman, 2014). Images A–E courtesy of the Alvin group, WHOI; dissection scope image of filaments by Barbara MacGregor.
The Guaymas Basin cores from these diverse sampling locations can be grouped into three categories based on thermal profiles and microbial mat cover: hot hydrothermal sediments with conspicuous microbial mats and temperatures reaching >50°C within 50cm depth, temperate hydrothermal sediments with microbial mats and a temperature range between 5 and 50°C, and background sediments without visible mats, and a temperature range of 3–5°C (Supplementary Tables S2 and S3, Supplementary Figures S2, S3). These categories were also reflected in the porewater profiles (Figures 4, 5).
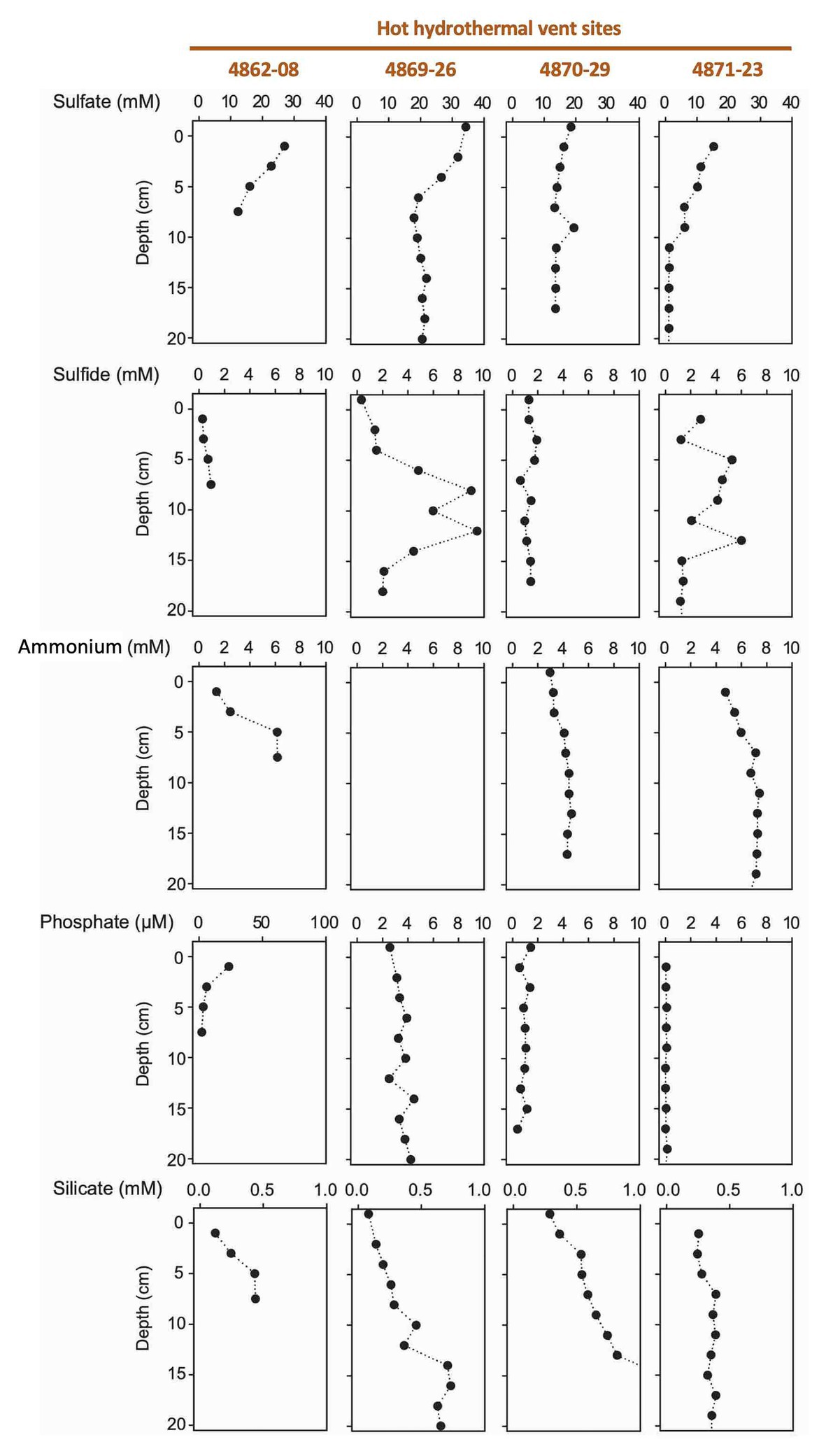
Figure 4. Porewater concentration profiles of sulfate, sulfide, ammonium, phosphate, and silicate in four hydrothermal cores.
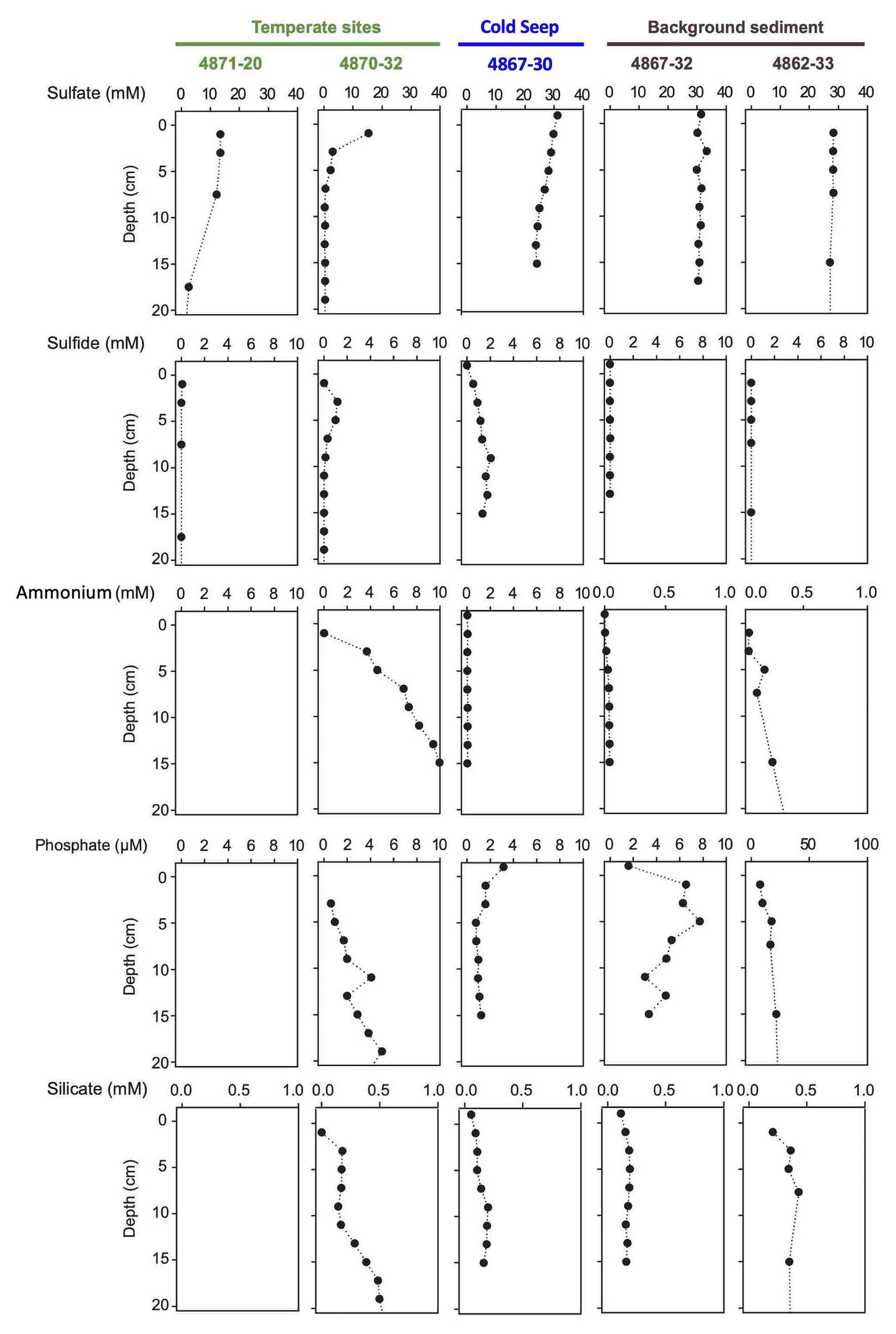
Figure 5. Porewater concentration profiles of sulfate, sulfide, ammonium, phosphate, and silicate in two temperate cores, one seep core, and two background cores without visible microbial mats. Empty panels indicate data gaps.
The hot hydrothermal sediments (cores 4862-08, 4869-26, 4870-29, 4871-23) are characterized by steeply increasing temperatures into the range of >80°C by 50cm depth (Supplementary Table S1, Supplementary Figure S3) and thick microbial mats covering the seafloor (Supplementary Figure S2). The extracted porewater contained high sulfide (1–10mM) and ammonium (up to 7mM) concentrations (Figure 4). Elevated concentrations of dissolved silicate (>0.2mM) suggest hydrothermal dissolution and mobilization of solid silicate phases, and irregular sulfate profiles most likely represent seawater inmixing (Figure 4). Cores in the “temperate” sediment category (cores 4870-32 and 4871-20) had moderate in situ temperatures of 10–30°C. The ammonium concentration of core 4870-32 (5–10mM; no ammonium data for core 4871-20) was even higher than observed at the hot hydrothermal sites. Sulfate is rapidly depleted below the sediment surface, indicating the absence of seawater sulfate inmixing; sulfide appears to be limited to surficial sediments (Figure 5). In the “background” category, Alvin cores 4862-33 and 4867-32 have temperatures near bottom seawater (3–4°C). These cores have significantly lower ammonium and silicate concentrations, in combination with near-seawater sulfate concentrations; sulfide was only found in micromolar traces (Figure 4B). Sediment core 4867-30 from the off-axis Octopus Mound sampling site does not fit easily into these categories; it is uniformly at bottom water temperature (2.9°C), lacks hydrothermal signatures such as high ammonium or silicate concentrations, and shows only moderate sulfate depletion; yet the core was strongly sulfidic (Figure 5).
Alvin dives 4866 and 4867 provided an opportunity to inspect different faunal assemblages and geochemical settings, and to collect sediment push cores from closely spaced targets at the edge of Octopus Mound. As determined with the Alvin heat flow probe, all sampling sites at Octopus Mound had in situ temperatures between 2.9°C at the seawater interface, increasing towards local maxima of 2.95–3.03°C at different shallow sediment depths as determined with the Alvin heat flow probe (Supplementary Table S3). The “active site,” named for its conspicuous benthic invertebrate community, harbors a cold seep assemblage of tube worms, seep clams, and dense populations of ampharetid worms covering the sediment surface (Figures 3A,B,C). Sampling holes after core removal were deep black, indicative of sulfidic, strongly reducing conditions. The geochemically analyzed core 4867-30 was collected at this location. The “periphery” site was selected to sample the edge of this conspicuous seep community, at a distance of ~2m. The “background” site lacked visible seep fauna. The “gas hydrate site” (Figures 3D,E,F) was initially sampled for its conspicuous circular microbial mat consisting of large, filamentous sulfur-oxidizing bacteria of the family Beggiatoaceae (Teske and Salman, 2014), with ca. 100 μm filament diameter (Figure 3F). On closer inspection, the sulfur-oxidizing Beggiatoaceae mat was growing on top of an ampharetid worm assemblage that was spreading over the entire hydrate area (Figures 3D,E). When push cores were removed from the sediment, the bottom end contained white hydrates, presumably methane hydrate, which dissociated during transport to the surface (Figure 3E). Sequence-based and geochemical analyses for these cores are briefly summarized in Supplementary Table S1.
Porewater nutrient profiles set the Octopus Mound sediments apart from their hydrothermal counterparts (Figure 5). The concentrations of ammonium were near 50 μM (“active” core 4867-30 and “background” core 4867-32), approximately two orders of magnitudes lower than measured in porewaters from the hydrothermal sites. Independently obtained ammonium profiles from nearby multicorer and gravity cores collected by the R/V Sonne expedition in 2015 yielded similar ammonium concentrations, around 30 μM in surficial sediments, and increasing to 300 μM at 4m depth (Geilert et al., 2018). Phosphate concentrations remained below 2 μm in the active core and 10 μm in the background core sediments, respectively. Porewater silicate concentrations in the Alvin push cores were in the range of 100–200 μm, near the bottom water concentration of ca. 175 μM in Guaymas Basin (Campbell and Gieskes, 1984), and much below the elevated dissolved silicate concentrations of 0.5–2mM that characterize the porewater of hydrothermal Guaymas Basin cores (Figure 5).
Methane at Octopus Mound is predominantly biogenic, as indicated by Octopus Mound δ13C-CH4 values from −68 to −76‰ (Figure 6A), falling between the biogenic methane of Sonora Margin cold seeps (ca. −80 ‰, Vigneron et al., 2015) and the mixed biogenic/thermogenic methane at the off-axis Ringvent site (ca. −60 ‰, Teske et al., 2019). Methane concentrations in the Octopus Mound sediment cores were often supersaturated, ranging from 0.6 to 1.8mM at the active site, and between 3.6 and 8.3mM at the hydrate site (Figure 6B). Methane concentrations in sediments at the periphery and background sampling areas were below detection. A comparison to hydrothermal conditions is provided by two hydrothermal cores from the Northern Tower area, 4871-04 and 4871-27 (Figure 6A). Due to data gaps, these two cores were not included in broader biogeochemical and microbiological comparisons, but their microbial mat cover and their temperature maxima >100°C (Supplementary Table S2) identify them as hydrothermal. Their δ13C-CH4 profiles approach the values of previously studied hydrothermal cores dominated by thermogenic methane, and their multi-millimolar methane concentrations are typical for hydrothermal methane-saturated sediments of Guaymas Basin (McKay et al., 2016). As illustrated by this comparison, biogenic methane dominates at Octopus Mound, whereas thermogenic methane is either entirely absent or limited to a minimal contribution.
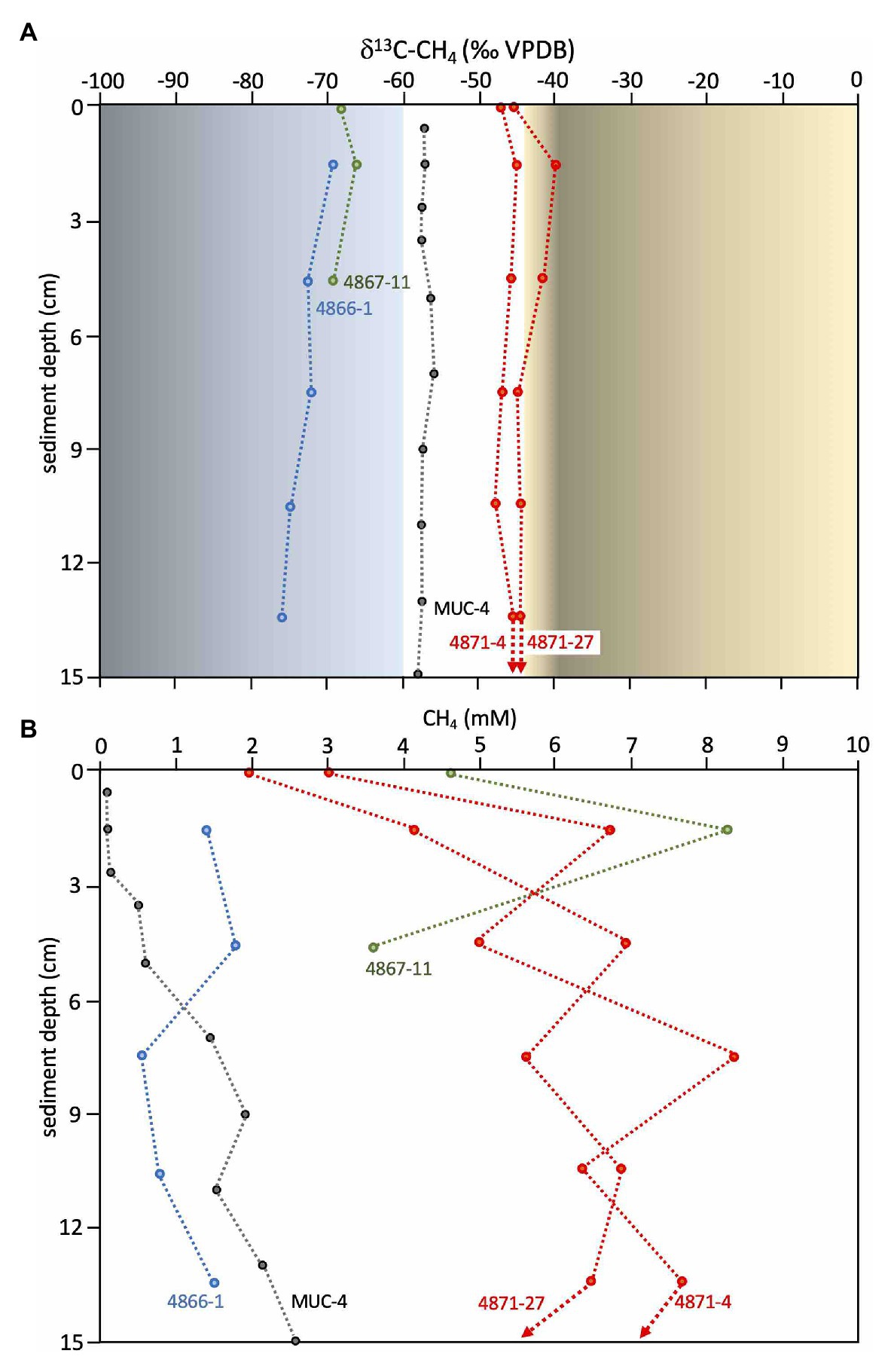
Figure 6. Methane isotopic values and concentrations. (A) Methane δ13CH4 values (VPDB) for Octopus Mound cores 4866-1 (active site, in blue) and 4867-11 (hydrate site, in green), and the nearby sediment push core MUC-4 (black), previously collected and measured independently at Central Seep (Geilert et al., 2018). Methane δ13CH4 values (VPDB) for hydrothermal cores from the Northern Towers area (4871-4 and 4871-27) are plotted in red. Orange shading indicates the range of δ13CH4 values for thermogenic methane in hydrothermal sediments in the southern Guaymas axial valley (McKay et al., 2016). Blue shading indicates the range of δ13CH4 values for microbially produced methane in cold sediments of the Sonora Margin (Vigneron et al., 2015, Teske et al., 2019), delimited at 60‰ based on Schoell (1982) and Simoneit et al. (1986). (B) Methane concentrations for the same samples. Data points for supernatant samples are plotted at 0cm depth. δ13CH4 values and their standard deviations, and methane concentrations are tabulated in Supplementary Table S4.
The taxonomic composition of bacterial and archaeal 16S rRNA gene sequences recovered from surficial sediments is distinctly different for hot hydrothermal sediments, temperate hydrothermal sediments, and background sediments (Figure 7). All sequence-based analyses have to be qualified by the fact that they are based on sequence frequencies, which are derived from the microbial community but do not necessarily represent it in identical proportions, and do not provide independent quantifications.
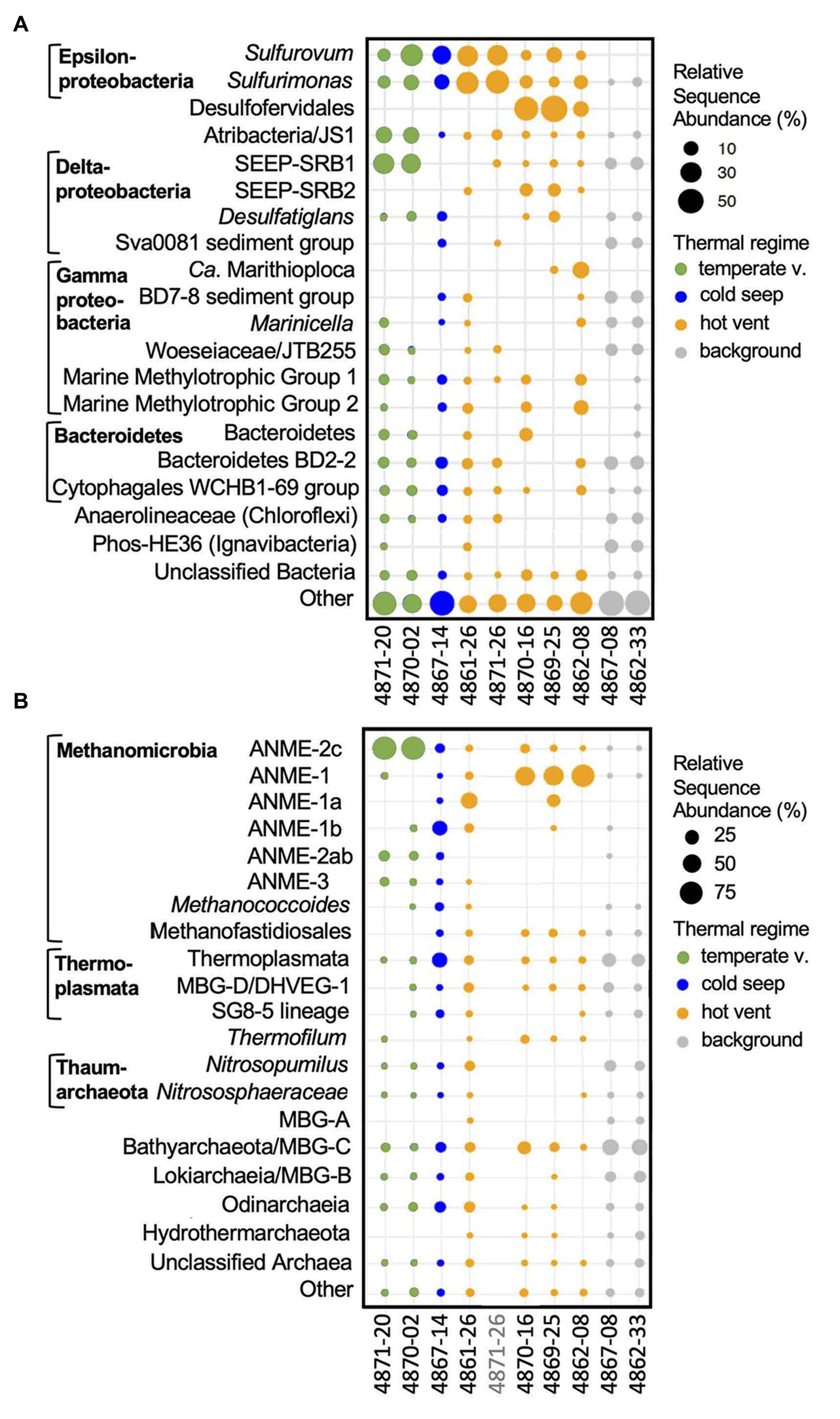
Figure 7. Bacterial and archaeal community composition of Guaymas Basin sediment cores. The size of the dots indicates the relative sequence abundance of microbial clades based on 16S rRNA gene amplicon sequences. The 20 most abundant bacterial (A) and archaeal (B) family-level lineages are shown. Less abundant clades are summarized as “Other.” When appropriate, taxa are annotated to supplement the automated SILVA identifications. DNA from core 4871-26 had run out after several sequencing attempts before archaeal sequencing could be finalized.
Sequences of the sulfur-oxidizing chemoautotrophic genera Sulfurovum and Sulfurimonas within the Epsilonproteobacteria (Campbell et al., 2006) are broadly shared among sulfide-rich hydrothermal sediment cores of Guaymas Basin (Figure 7A). Mat-forming filamentous sulfur-oxidizing bacteria of the family Beggiatoaceae were abundant in situ (Figure 2), but the mats were removed for separate analyses before sediment slicing and DNA extraction. Therefore, Beggiatoaceae sequences (here assigned to Ca. Marithioploca, Figure 7A) were found in high abundance only in a single core (core 4862-08) where draining fluids had sucked the overlying mat into the surficial sediment. Sulfate-reducing bacteria are dominated by thermophiles: Sequences of the Ca. Desulfofervidus auxilii lineage occur in high frequency in the hot hydrothermal cores 4869-25 and 4870-16, and in reduced proportions in hydrothermal sediment core 4862-08. Ca. Desulfofervidus is the thermophilic bacterial syntroph of methane- and short-chain alkane-oxidizing archaea that dominates in enrichment cultures at temperatures of 37–60°C (Holler et al., 2011; Laso-Pérez et al., 2016; Krukenberg et al., 2018; Hahn et al., 2020). Interestingly, Ca. Desulfofervidus sequences were not recovered from core 4861-26, collected from an extensive mat area covered with white sulfur precipitates (Figure 2A) The intermediate temperatures there (T max. near 50°C, Table 1) may not suffice to sustain Ca. Desulfofervidus populations; the core smelled strongly sulfidic upon shipboard recovery but detailed geochemical data are not available. Sequences assigned to mesophilic sulfate-reducing bacteria of the SEEP-SRB1 cluster within the Desulfobacteraceae (Schreiber et al., 2010) account for smaller proportions of hydrothermal core sequences, and are absent in 4861-25. Sulfate-reducing bacteria of the SEEP-SRB2 cluster, an uncultured, presumably mesophilic and syntrophic lineage that may participate in short-chain alkane oxidation (Kleindienst et al., 2012; Krukenberg et al., 2018), occur in four out of five of the hot hydrothermal cores, except 4871-26 (Figure 7A). Heterotrophic, anaerobic phyla with fermentative potential (Atribacteria, Bacteroidetes, Chloroflexi) are represented by sequences from the hot hydrothermal cores, but were generally recovered from the temperature cores and background sediments (Figure 7A). Sequences of aerobic methylotroph MMG1 and MMG2 groups (Ruff et al., 2013) occur in hot and temperate hydrothermal cores (Figure 7A).
Sequences assigned to the dominant archaeal group, ANME-1, were recovered at the highest relative abundance from the fully mat-covered cores 4862-08, 4869-25, and 4870-16 (Figure 7B); other anaerobic methane-oxidizing archaea (ANME) types were found as well (most consistently, ANME-2c) but appear more frequently in the temperate hydrothermal cores. The most consistently found methanogenic group are methylotrophic Methanofastidiosales (Nobu et al., 2016b), previously detected as “Guaymas euryarchaeotal group” in Guaymas Basin hydrothermal sediments (Dhillon et al., 2005). Crenarchaeotal sequences assigned to the heterotrophic, thermophilic sulfur-reducing, and moderately acidophilic genus Thermofilum (Zillig et al., 1983) occur in smaller but consistently detected proportions. Uncultured sediment-associated archaea within the Thermoplasmatales, and the Bathyarchaeota and Asgardarchaeota are found in the hot and the temperate hydrothermal cores (Figure 7B).
These sediments share the epsilonproteobacterial sequences of the high-temperature hydrothermal cores, but they consistently lack Ca. Desulfofervidus (Figure 7A). Surface samples of cool cores 4870-2 and 4871-20 are characterized by frequent recovery of sequences attributed to the sulfate-reducing bacterial SEEP-SRB1 group (Knittel et al., 2003) which include the mesophilic syntrophic partners of ANME-1 methane-oxidizing archaea (Schreiber et al., 2010), and to the fermentative, mesophilic Atribacteria (Nobu et al., 2016a; Katayama et al., 2020) which are common inhabitants of cold seeps (Ruff et al., 2015; Chakraborty et al., 2020) and anaerobic subsurface sediments (Starnawski et al., 2017). Sequences from the aromatics-degrading sulfate-reducing Desulfatiglans lineage were recovered from all temperate cores, as well as from several hot hydrothermal and background samples, consistent with its cosmopolitan distribution (Jochum et al., 2018). The archaeal sequences recovered were predominantly assigned to sulfate-dependent ANME-2c methane oxidizers for the Northern Towers and Aceto Balsamico cores, and ANME-1b methane oxidizers for the Octopus Mound core (Figure 7B). Most other archaeal groups are shared with the background sediment, including members of the Bathyarchaeia, Thermoplasmata, Lokiarchaea, Marine Benthic Group D (renamed Thermoprofundales, Zhou et al., 2019), the uncultured SG8-5 Thermoplasmatales lineage found in estuarine sediments (Lazar et al., 2017), Thaumarchaeota of the aerobic, ammonia-oxidizing families Nitrosopumilaceae and Nitrososphaeraceae (Stieglmeier et al., 2014), methylotrophic methanogens of the uncultured Methanofastidiosales (Nobu et al., 2016b) and of the genus Methanococcoides (Liu and Whitman, 2008), and small proportions of ANME-2ab archaea. Some of these widely shared archaeal sequences show a comparatively spotty occurrence pattern in the hot hydrothermal samples, suggesting that strong hydrothermal conditions are selecting against them (Figure 7B). A preference for moderate over hot hydrothermal conditions characterizes the Lokiarchaeota and Odinarchaeia within the Asgardarchaeota (Zaremba-Niedzwiedzka et al., 2017) that were found recently in metagenomic surveys of Guaymas Basin hydrothermal sediments (Dombrowski et al., 2018; Seitz et al., 2019).
Sequences of the sulfur-oxidizing microaerobic and nitrate-reducing chemoautotrophic genus Sulfurimonas occurred in reduced relative abundance in the background cores 4862-33 and 4867-08; the related sulfur-reducing genus Sulfurovum was no longer detected (Figure 7A). Sulfate-reducing bacteria were represented by SEEP-SRB-1, Desulfatiglans, and the Sva0081 group of uncultured Desulfobacteraceae that are hypothesized to scavenge H2 in marine sediments (Dyksma et al., 2018). Uncultured marine sediment lineages that were frequently found include the deep-sea sediment lineage BD2-2 within the Bacteroidetes (Li et al., 1999) and the deep-sea sediment lineage BD7-8 within the Gammaproteobacteria (Li et al., 1999), the PHOS-HE36 clade (Dabert et al., 2001) within the Ignavibacteria (Iino et al., 2010), the cosmopolitan sediment-associated gammaproteobacterial JTB255 lineage (Woeseiaceae, Mussmann et al., 2017; Hoffmann et al., 2020), the WCHB1-69 lineage within the Cytophagales (Dojka et al., 1998), and diverse unclassified Anaerolineae. Based on the spotty detection of their sequences in vent and seep cores, these bacteria may tolerate some hydrothermal or seep activity, unless selected against by other extreme conditions, or they may be generally abundant in the surrounding sediment and thus occur as relic DNA in the seep cores. Sequences of aerobic methylotroph groups MMG1 and MMG2 (Ruff et al., 2013) were not detected (4867-08) or occurred in reduced proportions (4862-33; Figure 7A). The archaeal sequences resembled those from the temperate cores, with increased relative representation of the Bathyarchaeota and Thermoplasmatales, and the addition of Marine Benthic Group A, a sediment-dwelling sister lineage of the marine Thaumarchaeota (Lauer et al., 2016). Thus, the Guaymas Basin background sediments harbor the five globally distributed marine benthic archaeal groups of MBG-A, MBG-B (Lokiarchaeota), MBG-C (Bathyarchaeota), MBG-D (Thermoprofundales), and MBG-E (Thermoplasmata subgroup) that were originally discovered in cold North Atlantic seafloor sediments (Vetriani et al., 1999).
As previously observed for the geochemical characteristics of Octopus Mound sediments, the Octopus Mound seep core 4867-14 does not fit easily into these three categories; it differs from the background sediments by not having SEEP-SRB1 sequences among the 20 most frequently detected bacterial taxa (Figure 7A), from temperate sediments by its lower proportion of ANME-2c sequences, and from all sediments by its high frequency of ANME-1b sequences (Figure 7B).
Non-metric multidimensional scaling ordination plots show distinct clustering patterns for bacterial and archaeal communities that are consistent with geochemical characteristics (Figure 8). The strongest separation occurs between cold background cores 4862-33 and 4867-08, which cluster together in archaeal and bacterial NMDS despite their geographical distance of ca. 50km, and all other cores. Communities at hot and temperate sites are clearly separated from each other. The hot hydrothermal cores 4862-08, 4870-16, and 4869-25 stand apart from the temperate cores 4871-20, 4870-02, and the Octopus Mound core 4867-14, while the remaining hydrothermal cores (4871-26 and 4861-26) fall in between (Figure 8). Interestingly, core 4861-26 is the coolest of the hot hydrothermal cores, with a bottom temperature of 55°C (Supplementary Figure S3), and this intermediate thermal regime is consistent with its intermediate position in both archaeal and bacterial NMDS plots. Within the archaeal NMDS plots, the Octopus Mound core 4867-14 clusters apart from the cool hydrothermal sites (Figures 8D,E,F), consistent with its distinctive habitat characteristics. Except for the two background cores 4862-33 and 4867-08, no cores show overlapping NMDS clustering. These results suggest that sites with similar geochemistry can harbor microbial communities that diverge to some extent. Additional variables that were not measured could have an impact, or dynamic selection pressures due to rapidly changing hydrothermal regimes allow stochasticity to play an important role in site-specific community assembly (McKay et al., 2016). While these results contrast with a previous survey of Guaymas Basin microbial diversity that emphasized community overlap among hydrothermal sites within a constrained sampling area (Meyer et al., 2013), this survey covers a greater habitat range with distinct thermal and geochemical regimes, and thus distinct microbial communities.
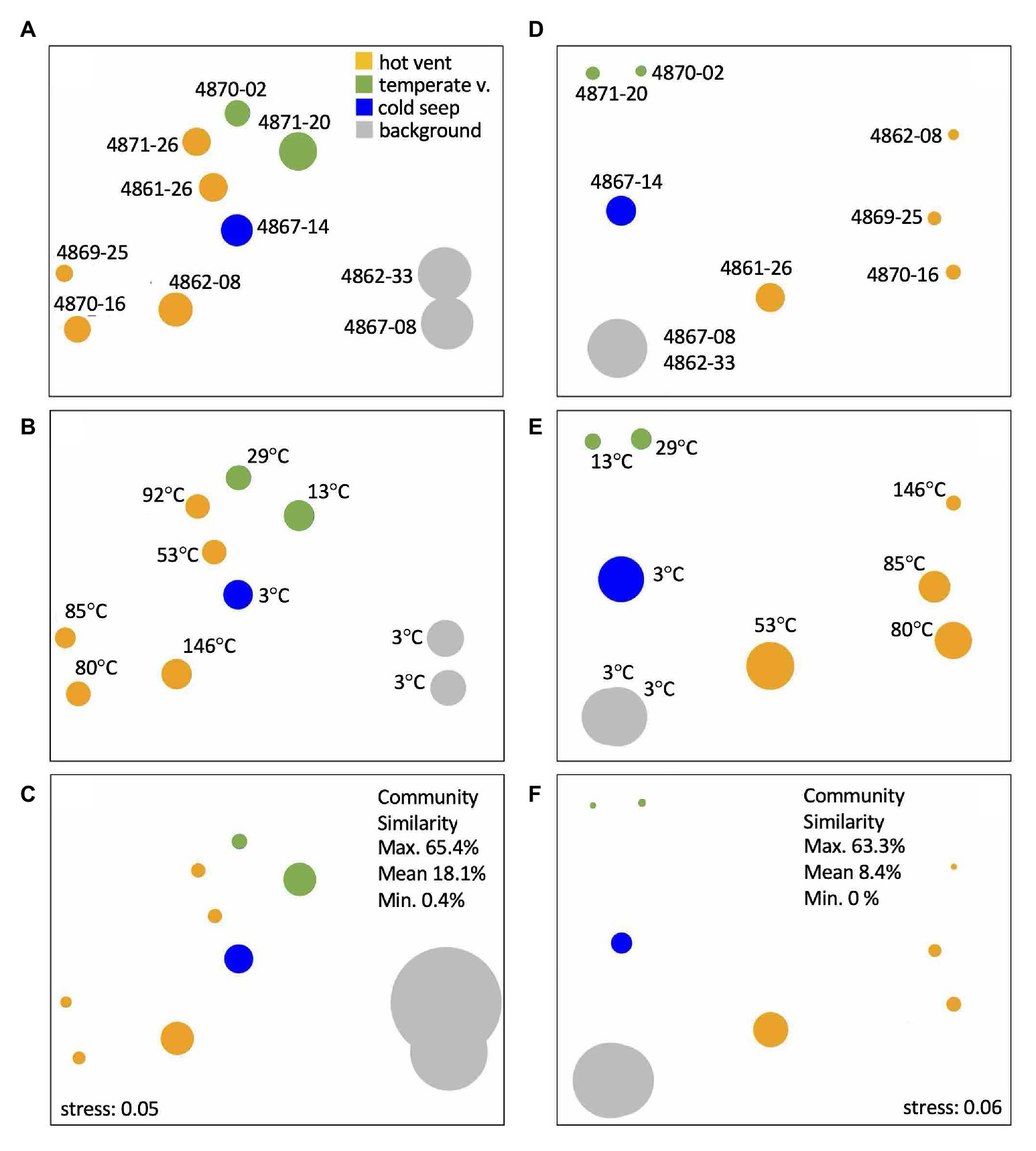
Figure 8. Non-metric multidimensional scaling (NMDS) ordination plots based on 16S rRNA gene amplicons for bacterial operational taxonomic units (OTUs; A-C) and archaeal amplicon sequence variants (ASVs; D-F) from the upper cm of sediment cores, color-coded by geochemical cluster, and annotated with core number (A,D) and in situ temperature at 50cm depth (B,E). In plots (A,D), circle size represents observed OTU richness. In plots (B,E), circle size represents Shannon entropy. In plots (C,F), circle size represents Inverse Simpson evenness.
In contrast to previously sampled hydrothermal Guaymas Basin sediment communities, which were apparently dominated by ANME (Teske et al., 2002), the surface layer of the Octopus Mound site and several of the hydrothermal sites reported here have yielded 16S rRNA gene sequences of aerobic methanotrophic and methylotrophic bacteria (Figure 7A). Their detection could be linked to the sampling scheme; these aerobes are more likely to be detected in the top 0–1cm layer sampled here than in surficial samples of multiple centimeters that are predominantly anoxic. The sequences are affiliated with the cultured methanotrophic genus Methyloprofundus (Tavormina et al., 2015), and the uncultured lineages Marine Methylotrophic Groups 2 and 3 within the Gammaproteobacteria (Ruff et al., 2013). These groups contain gene sequences from New Zealand cold seeps, the Håkon-Mosby mud volcano, methylotrophic mussel endobionts, and diverse seafloor sediments (Figure 9). Some Guaymas Basin OTUs are specifically related to OTUs from methane-rich seeps and mud volcanoes, the Hikurangi Margin in New Zealand that harbors abundant amphetarid worm communities (Ruff et al., 2013), and the Håkon-Mosby Mud volcano in the Norwegian Arctic Ocean (Ruff et al., 2019).
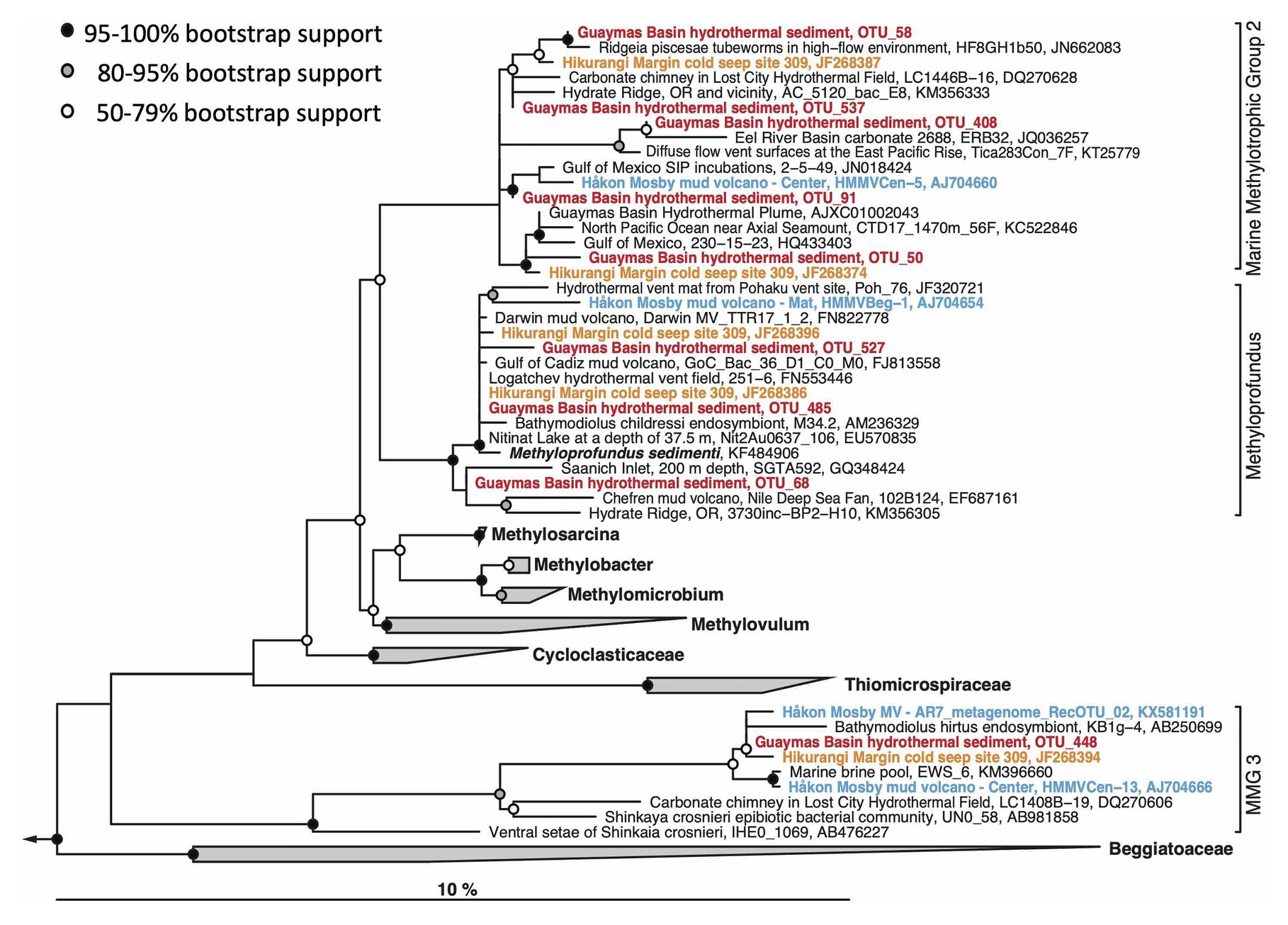
Figure 9. Maximum likelihood phylogeny of gammaproteobacterial methylotrophic and methanotrophic bacterial OTUs. The tree was calculated using near full-length sequences; partial 16S rRNA gene sequences (ca. 450 nucleotide positions) obtained with primers 341F and Pro805 were added without changing the tree topology. The Methyloprofundus branch is synonymous with Marine Methylotroph Group 1 (MMG1), a sister lineage to MMG2 and MMG3. Sequences used for the phylogeny are listed in Supplementary Table S5 for easy retrieval.
Analogous to bacterial methanotrophs, the archaeal anaerobic methane oxidizers also show considerable phylogenetic complexity (Figure 10). Within the broadly defined ANME-1 archaea, some Guaymas ASVs were affiliated with ANME-1a and 1b, closely related subgroups that co-occur in diverse cold seeps (Knittel et al., 2005). The ASVs assigned by the SILVA pipeline to the generic “ANME-1” category (Figure 7B) are phylogenetically divergent and affiliate with two different ANME-1 lineages, the uncultured and presumably thermolerant ANME-1 Guaymas lineage that occurs consistently in hydrothermal Guaymas sediments (see data compilation in Dowell et al., 2016), and a cluster termed “ANME-1a Guaymas II” that was cultured in methane-oxidizing enrichments at 50°C (Holler et al., 2011) and also appears consistently in Guaymas hydrothermal sediments (Dowell et al., 2016). Thus, the ANME-1 archaea that are specifically recovered from hydrothermal cores (4862-08, 4869-25, and 4870-16) belong to lineages that are either cultured thermophiles or show a preference for hydrothermal sediments (Figure 10). In contrast, sequences affiliated with the diverse ANME-2 subgroups were recovered predominantly from temperate cores (Figure 6B), consistent with previous observations (McKay et al., 2016). The ANME-3 subgroup, previously identified in cold seep sediments of an arctic mud volcano (Niemann et al., 2006; Lösekann et al., 2007), is also preferentially found in temperate and cold seep sediments (Figure 7B).
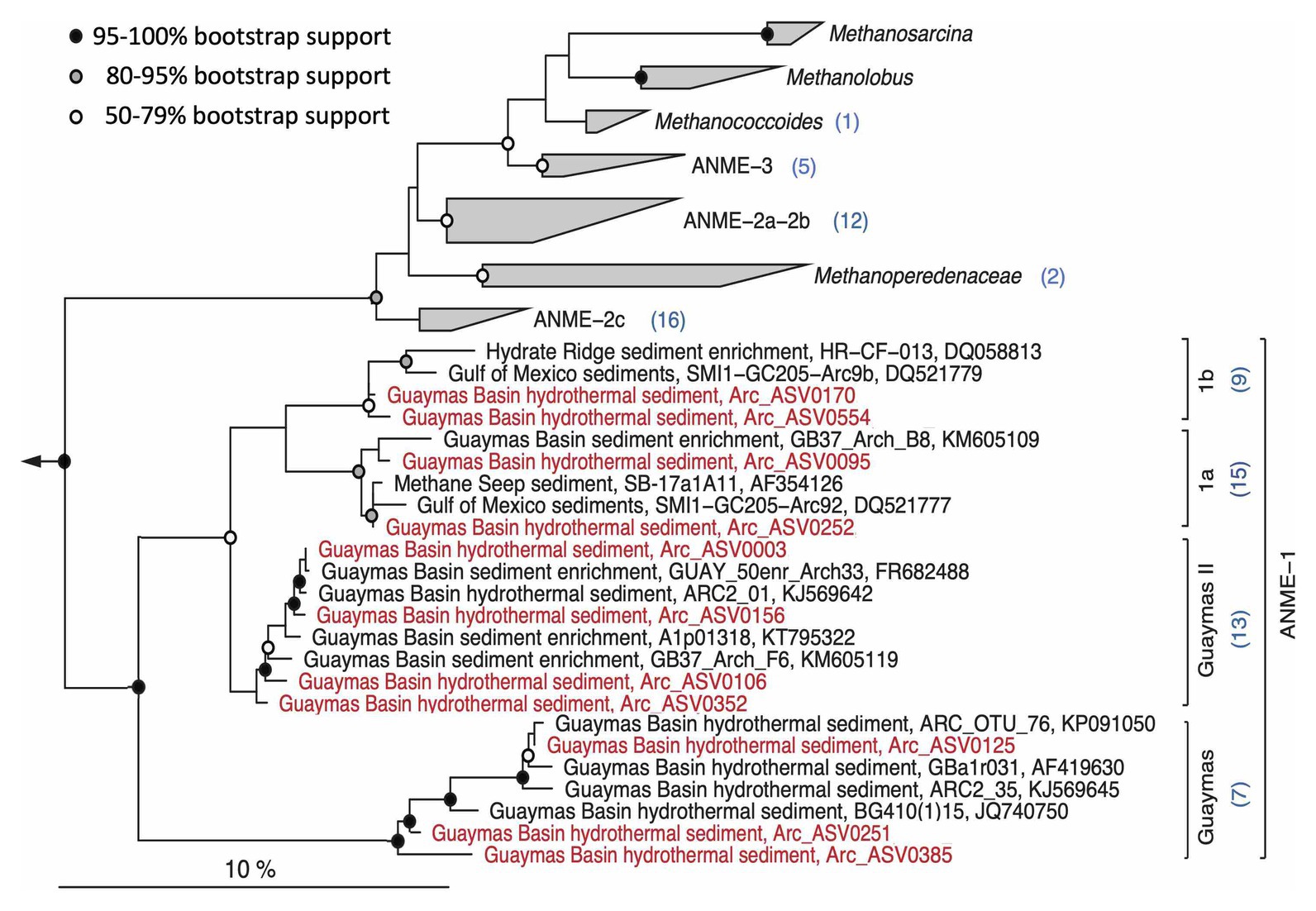
Figure 10. Maximum likelihood phylogeny of ANME-1 archaea and related ANMEs and methanogens. The tree was calculated using near full-length sequences; partial 16S rRNA gene sequences (ca. 450 nucleotide positions) obtained with primers 517F and 958R were added without changing the tree topology. The annotation numbers in parentheses indicate the number of representative ASVs for different clades. Sequences used for the phylogeny are listed in Supplementary Table S5 for easy retrieval.
To further target anaerobic methanogenic and methane-oxidizing archaea at the Octopus Mound site, mcrA gene sequences that are diagnostic for these archaea were PCR-amplified and surveyed (Figure 11). The sediments at the ampharetid-dominated “active site,” sampled by Alvin core 4866-1, yielded mcrA sequences of the ANME-2ab clades; below-surface layers of core 4867-26 from the nearby “periphery site” yielded ANME-1; and the surficial centimeters of core 4867-11 from the hydrate site yielded ANME-1 and ANME-2 sequences (Figure 11). No mcrA gene sequences of cultured methanogenic genera or families were detected.
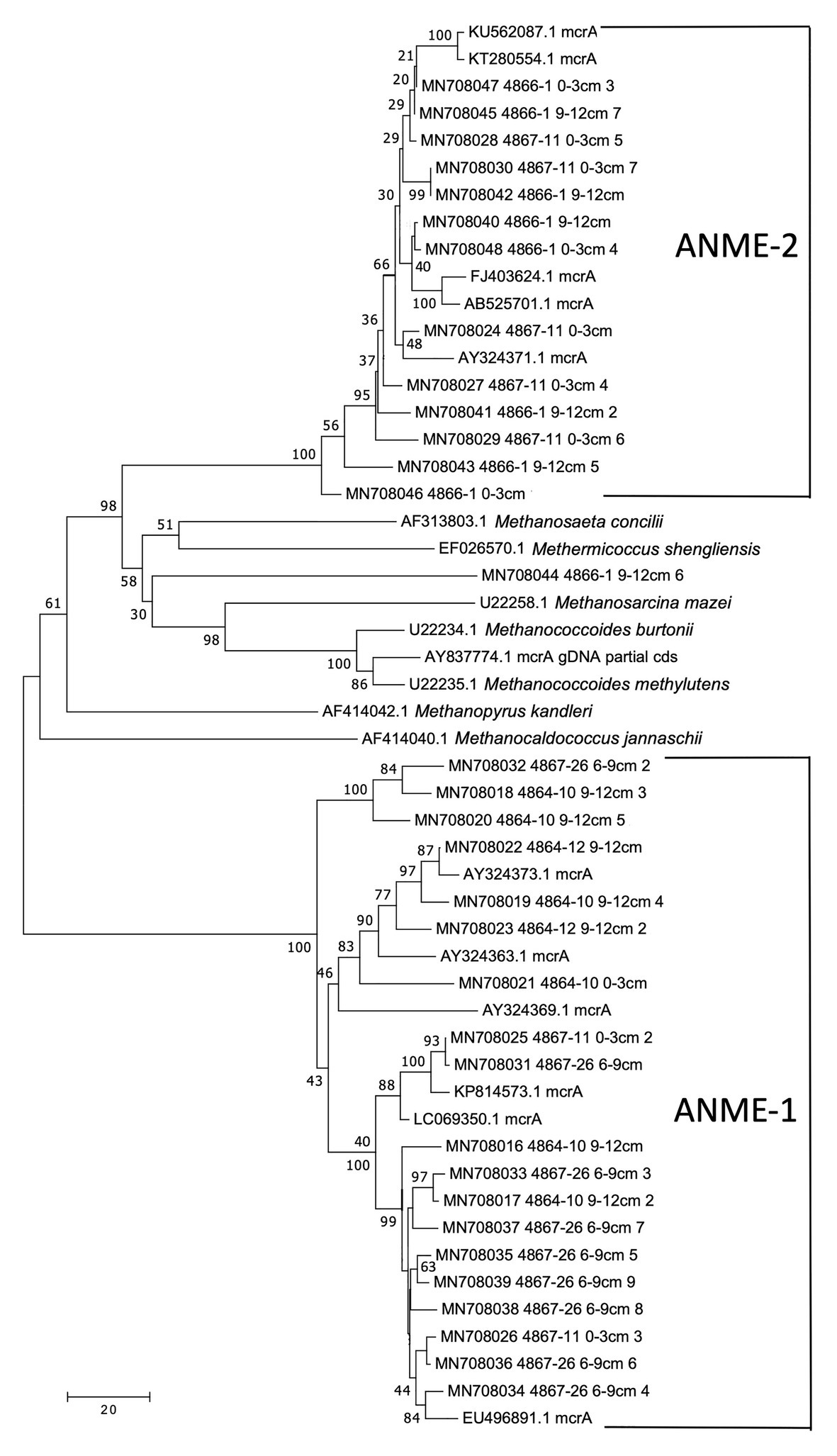
Figure 11. Distance phylogeny of mcrA genes recovered from Octopus Mound sediments. Taxon labels begin with the Genbank number, followed by the Alvin Dive and core number, and (if applicable) the number of multiple clones from the same location and core that is represented by this sequence. Additional mcrA genes come from Ringvent sediments sampled during Alvin dive 4864 (Teske et al., 2019). Bootstrap numbers were obtained by 500 replicates.
The strong contrasts between different Guaymas Basin hydrothermal sites and the Octopus Mound cold seeps in seafloor microbial community, benthic life, thermal characteristics, and porewater geochemistry highlight the diversity of geo-ecosystems in the greater Guaymas Basin region. A closer look at the thermal and biogeochemical parameters of these different sites shows that they are not always tightly coupled, suggesting underlying transitions or hybrids between these environmental regimes.
With ammonium concentrations in the 5–10mM range, silicate concentrations in the 0.5–2mM range, and high sulfide concentrations of 1–3mM, the hot hydrothermal sediment cores share key signatures of Guaymas Basin hydrothermal fluids (von Damm et al., 1985). Interestingly, these porewater indicators are not always linked to high temperatures. High ammonium and silicate concentrations are also observed in temperate hydrothermal sediments (core 4870-32 and 4871-20), suggesting that rising fluids with these hydrothermal signatures have cooled down locally before reaching the sediment surface. The lack of seawater sulfate below the upper 5cm of sediment (Figure 5) indicates that the sediments are not cooled by seawater inmixing near the surface. Instead, the hydrothermal fluids must have cooled down considerably during migration through the sediments. These conditions are not indicative of a transient regime, but remain stable over many years, as observed in the Aceto Balsamico area where cool or temperate, sulfidic sediments with high acetate porewater concentrations and a conspicuous sulfur-rich lime-yellow surface layer were documented and sampled during previous cruises in 2008 and 2009 (Teske et al., 2016). These sediments were found in the same location and sampled again in 2016 and 2018. The resulting microbial niche is very different from the hydrothermal mixing regime and steep thermal gradients that characterize hot sediments overgrown with thick Beggiatoaceae mats (McKay et al., 2012, 2016).
Hydrothermal porewater signatures even persist in attenuated form in cores that, based on cold temperature and lack of microbial mats, have been categorized as background sediments when they were collected during Alvin dives. For example, the “background” core 4862-33 from the southern Guaymas Basin had ammonium and silicate concentrations of 300–400μM, one order of magnitude above ammonium and double the silicate concentrations for the Octopus Mound background core 4867-08. Residual hydrothermal influence is also reflected in the slightly elevated sediment temperature, 3.4°C for core 4862-33 compared to 2.9°C for all Octopus Mound cores. Based on these observations, background sediments in the strict sense (no measurable hydrothermal influence at all) may have to be collected several miles away from hydrothermal features (Teske et al., 2019).
The Octopus Mound site does not fall along the spectrum between hydrothermal sediments and background endmembers. Its uniformly cold temperatures profiles, extensive carbonate concretions, high sulfide and methane concentrations, white filamentous sulfur-oxidizing mats, chemosynthetic clams, and shallow methane hydrates are reminiscent of cold seeps at the base of the nearby Sonora Margin (Simoneit et al., 1990; Paull et al., 2007; Cruaud et al., 2017). Its δ13C-CH4 values are distinct from thermogenic methane in hydrothermal Guaymas Basin sediments (δ13C ≈ −38 to 43‰; McKay et al., 2016). Instead, they occupy an intermediate position between the biogenic methane at Sonora Margin seeps (δ13C ≈ −80‰; Vigneron et al., 2015) and mixed biogenic/thermogenic methane at the off-axis Ringvent site (δ13C ≈ −60 ‰; Teske et al., 2019). Interestingly, Octopus Mound methane is isotopically lighter than porewater methane from cores collected elsewhere in the Central Seep area and other off-axis seep areas of Guaymas Basin (δ13C ≈ −55 to −58‰ in MUC04; Geilert et al., 2018), and from deep subsurface sediments (δ13C ≈ −40 to −65‰; Simoneit et al., 1986), suggesting additional biogenic contributions to the porewater methane pool.
Seafloor methane undergoes microbial oxidation, as shown by the presence of methane-derived carbonates, and ANME of different types. Carbonate samples collected at the base of Octopus Mound during Alvin dive 4867 showed the same morphology (Figure 3) as previously dredged carbonates from this location with δ13C isotopic values of −46.6 and −44.7‰ (Geilert et al., 2018), and −45.2 to −47.6‰ (Núñez-Useche et al., 2018) that indicate the incorporation of methane-derived carbon. The sediments of Octopus Mound are populated with ANME archaea, as shown by 16S rRNA gene profiling (Figure 7B) and mcrA gene analysis (Figure 11). ANME-2 prefers surficial sediments with lower sulfide concentrations and tolerates some degree of oxygen exposure (Knittel et al., 2005; Rossel et al., 2011) that would be consistent with bio-irrigation by ampharetid worms, as shown by the identification of ANME-2ab archaea as the dominant ANME type in Ampharetid mats of the Hikurangi Margin seeps in New Zealand (Ruff et al., 2013). ANME-1 is generally found in reduced and sulfidic sediments (Rossel et al., 2011), consistent with the thick mat of sulfide-oxidizing Beggiatoaceae at the surface of the hydrate site, and with reducing conditions below the sediment surface in the “periphery” site, where bio-irrigating worms are absent.
The observation that the thermally and geochemically based core categories are generally reflected in NMDS analyses of bacterial and archaeal community composition should not be taken for granted but requires an explanation. In situ temperatures in the upper centimeter of hydrothermally active sediments and at the mat interface – the source of the bacterial and archaeal sequences – are almost always moderate or cool, mostly at around 5°C, or averaging ~10°C when high-temperature outliers are included (McKay et al., 2012). The integrated impact of the geochemical regime within the entire sediment core – a composite of electron acceptors and donors, nutrients, and carbon sources – shapes the community composition at the sediment surface. In this view, the sediment surface may function as an integrator for microbial cells that may originate deeper within the sediment and thus reflect its average geochemical regime; these cells may move upwards with rising hydrothermal and seep fluids and accumulate at the sediment surface, as recently proposed for seep microbiota (Chakraborty et al., 2020). Consistently, microbial cell numbers (Meyer et al., 2013), cell densities of cultivable thermophiles (Teske et al., 2009), microbial lipid concentrations (Guezennec et al., 1996; Teske et al., 2002; Schouten et al., 2003), and microbial activities such as sulfate reduction rates (Weber and Jørgensen, 2002; Dowell et al., 2016) and acetate oxidation rates (Zhuang et al., 2019) are strongly focused towards maximum values within the top 3–5cm of surficial sediment. These patterns are consistent with geochemical observations that hydrothermal activity mobilizes biomass and organic carbon from the deeper sediments towards the sediment/water interface (Lin et al., 2017).
Similar to a previous study of the hydrothermal core community in Guaymas Basin sediments (Cruaud et al., 2017), sulfate-reducing and methane-oxidizing microbial groups emerge as indicator species of hydrothermal activity: the facultatively syntrophic, hydrogen-oxidizing sulfate reducer Ca. Desulfofervidus (Krukenberg et al., 2016), the uncultured, presumably syntrophic sulfate-reducing and alkane-oxidizing SEEP-SRB2 lineage (Krukenberg et al., 2018), and the thermophilic ANME-1 lineages (Figure 9) that are distinct from the cold-seep ANME-1a and ANME-1b groups (Biddle et al., 2012) appear characteristic for hot hydrothermal sediments, consistent with a synopsis of published 16S rRNA genes from Guaymas Basin (Dowell et al., 2016). In contrast, members of the SEEP-SRB1 lineage within the Desulfobacteraceae were present in hot hydrothermal core samples, but are more frequently found in cool or background sediment cores. Interestingly, the SEEP-SRB1 bacteria were not detected in Octopus Mound core 4867-14, although the thermal conditions would have been suitable. Methane-oxidizing syntrophic SEEP-SRB1 bacteria (Schreiber et al., 2010) could be outcompeted in this location, if bio-irrigation by mat-like assemblages of ampharetid polychaetes (Figure 3) favors aerobic methane oxidation by widespread gammaproteobacterial methylotrophs, which occur in 4867-14 and in other cores (Figure 5).
The archaeal spectrum in the diverse Guaymas Basin samples overlaps with the benthic archaeal core community of ANME-1, Thermoplasmatales (Marine Benthic Group D), Lokiarchaeota, and Bathyarchaeota that was previously proposed for Guaymas Basin hydrothermal sediments (Cruaud et al., 2017). While benthic anaerobic archaea predominate, they coexist with members of the Thaumarchaeota (Nitrosopumilaceae) in several hydrothermal sediments. Although aerobic, ammonia-oxidizing Nitrosopumilaceae (Qin et al., 2017) dominate archaeal communities in the water column, ammonium provided by hydrothermal fluids or by nitrate-reducing Beggiatoaceae mats enriches these archaea in surficial hydrothermal sediments as well (Winkel et al., 2014). These microbial groups with different physiologies and requirements may coexist in surficial hydrothermal sediments since this habitat provides an interface with moderate temperatures where electron donors and carbon sources from the sediment overlap with electron acceptors from the water column and create highly compressed or co-existing biogeochemical niches (Schutte et al., 2018; Buckley et al., 2019). Hydrothermal circulation in surficial sediments with microbial mats (Gundersen et al., 1992; Teske et al., 2016) can transport anaerobic or thermophilic subsurface archaea to the seawater interface and mix them with aerobic Thaumarchaeota (Qin et al., 2017). The moderate temperatures just below the sediment/seawater interface would be compatible with mesophilic microorganisms, and at the same time allow the accumulation of thermophiles and hyperthermophiles that grow at higher temperatures a few centimeters downcore (Teske et al., 2009). Since surficial hydrothermal sediments harbor diverse mesophiles as well as thermophiles, they represent an attractive target to explore the highly diverse microbiota of Guaymas Basin, with outstanding potential for evolutionary and physiological discoveries (Dombrowski et al., 2018; Seitz et al., 2019).
The contrasting hydrothermal regimes and microbial communities in Guaymas Basin reflect deeply sourced fluid and gas flow that is driven by underlying sill emplacement and local hydrothermal temperature gradients. Hydrothermal features in the southern Guaymas Basin spreading center are linked to underlying shallow sills (Lonsdale and Becker, 1985; Teske et al., 2016); the high local variability within hydrothermal areas indicates further differentiation of fluid and gas transport to the sediment surface (Ondréas et al., 2018). High-resolution bathymetries combined with shallow subbottom seismic profiles penetrating ca. 30–60m below the sediment surface show that the southern vent sites, including Mat Mound Massif (synonymous with the “Orpheus” site, Ondréas et al., 2018), Rebecca’s Roost, Cathedral Hill, and Aceto Balsamico, are linked to small sub-circular seafloor depressions with massive subsurface hydrothermal precipitate formation and lithification that creates convoluted flow paths skirting surface-breaching hydrothermal edifices. These complex and relatively shallow subsurface flow paths are consistent with frequent observations of hydrothermal hot spots and microbial mats at the base or the lower slope of these hydrothermal mounds (Dowell et al., 2016; Teske et al., 2016). The collapsed depressions are thought to facilitate the release of soluble light hydrocarbon (gas, oil, and condensates) that are transported by hydrothermal fluids towards shallow sediments where they accumulate (Ondréas et al., 2018). This setting has produced a vast patchwork of hydrothermal sediments and microbial mats, frequently visited by microbiological surveys (e.g., Teske et al., 2002; Cruaud et al., 2017; Dombrowski et al., 2018), including this study. In contrast, hydrothermal circulation at the northern sites, such as the Northern Towers area, would be linked to deeper faults and remobilize deeper hydrocarbons (Ondréas et al., 2018); we speculate that the resulting network of subsurface flow paths would be more channelized and less diversified compared to the southern area, and therefore generate large hydrothermal edifices, but fewer microbial mats and seafloor hot spots, as indicated by the Sentry survey of this area. Multichannel seismic surveys along the entire southern axial trough of Guaymas Basin would be needed to further substantiate such a scenario. Interestingly, a difference in subsurface hydrothermal circulation is suggested by the δ13C-CH4 isotopic values of hydrothermal sediments in the Northern Towers area that are slightly heavier (near −45‰, Figure 6A) than hydrothermal methane in the main sampling area of the southern axial trough (−39 to −43‰, McKay et al., 2016). This difference between the two sampling areas has been noticed previously (Teske et al., 2016), and could indicate the impact of isotopically heavier deep subsurface methane in the Northern Towers area (Simoneit et al., 1986).
The setting is entirely different at Octopus Mound. Multichannel seismic profiles show a gas pipe rising from a deeply buried sill underneath Octopus Mound that appears to funnel deeply sourced methane towards the sediment surface and into hydrate reservoirs (indicated by extensive shallow bottom simulating reflectors) around the mound (Teske et al., 2018). Deep sill-driven methane seepage is the default mechanism of hydrocarbon and methane mobilization across the spreading center and flanking regions of Guaymas Basin (Lizarralde et al., 2011). Yet, significant local variability with regard to sill depth, age, and thermal stage can also characterize the off-axis sites. For example, the deep and presumably old sill intrusion, cold seepage and predominantly biogenic δ13C-CH4 isotope values at Octopus Mound contrast with the shallow, recently emplaced sill, locally high temperatures and heavier, thermogenically influenced δ13C-CH4 isotope values at Ringvent (Teske et al., 2019), and with similar thermogenically influenced δ13C-CH4 values at diverse off-axis seep locations of Guaymas Basin (Geilert et al., 2018). Another factor is the localized biological modification of isotopic signatures; the Octopus Mound data indicate that biogenic methane sources are augmenting subsurface-derived methane at active seep locations. Given its compact size, habitat diversity, and geochemical contrasts, Octopus Mound and its surrounding seep sediments and shallow hydrates in the Central Seep area provide a rewarding model system for the study of sill-driven cold seepage in Guaymas Basin.
The 16S rRNA miseq data are deposited under Sequence Read Archive project PRJNA626075 at Genbank. The methyl coenzyme M reductase alpha subunit gene sequences are deposited at GenBank under nucleotide accession numbers MN708024 to MN708048. The geochemical data are available from the Biological and Chemical Oceanography Data Management Office at the Woods Hole Oceanographic Institution under Project Number 474317.
AT headed the R/V Atlantis expedition in December 2016, collected thermal profiles and samples, compiled the biological seafloor observations, developed the concept for this manuscript, and wrote the manuscript with input from all authors. Porewater geochemical analyses for ammonium, phosphate, silicate, sulfate, and sulfide were supervised and compiled by GW, and by JC for methane. DW isolated DNA from Octopus Mound samples, amplified the mcrA genes, and constructed the mcrA gene phylogeny. BM kept core records and core photographs throughout the cruise, photographed filamentous Beggiatoaceae from the hydrate site, and selected coring sites and recorded thermal profiles during Alvin dive 4869. MS and DH measured the thermal sediment gradients during dives 4866 and 4867, respectively. DH also inspected Sentry survey images for microbial mats and suitable sampling sites, and took Alvin video footage of cephalopod residents at Octopus Mound. DB and GZ measured thermal profiles during Alvin dives 4862 and 4871 and selected coring sites, respectively. SJ co-organized the 2016 Atlantis expedition. SAS co-wrote the Sentry proposal for this cruise, compiled the Sentry bathymetry, and together with DL developed the interpretation of the Octopus Mound site. SER extracted DNA from surface samples, performed 16S rRNA gene sequence-based community characterizations and statistical comparisons, and inferred phylogenetic trees.
Research on Guaymas Basin in the Teske lab is supported by NSF Molecular and cellular Biology grant 1817381 “Collaborative Research: Next generation physiology: a systems-level understanding of microbes driving carbon cycling in marine sediments”. Sampling in Guaymas Basin was supported by collaborative NSF Biological Oceanography grants 1357238 and 1357360 “Collaborative Research: Microbial carbon cycling and its interaction with sulfur and nitrogen transformations in Guaymas Basin hydrothermal sediments” to AT and SJ, respectively. SER was supported by an AITF/Eyes High Postdoctoral Fellowship and start-up funds provided by the Marine Biological Laboratory.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Alvin and Sentry teams for a stellar performance during Guaymas Basin cruise AT37-06. We also thank Martina Alisch (MPI Bremen) and Claire Wilson (FSU) for generating the porewater data and Carmen Li (University of Calgary), Aleksey Morozov, Nicole Robichaud, Hilary Morrison, and Sherlynette Pérez Castro (MBL) for support with amplicon sequencing. AT highly appreciates the safe and conducive writing environment provided by the Hanse Institute for Advanced Studies (Hanse Wissenschaftskolleg) in Delmenhorst during the COVID-19 pandemic.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.633649/full#supplementary-material
Beaulieu, S. E., and Szafranski, K. M. (2020). InterRidge Global Database of active submarine hydrothermal vent fields. Version 3.4. PANGAEA. doi: 10.1594/PANGAEA.917894
Berndt, C., Hensen, C., Mortera-Gutierrez, C., Sarkar, S., Geilert, S., Schmidt, M., et al. (2016). Rifting under steam – how magmatism triggers methane venting from sedimentary basins. Geology 44, 767–770. doi: 10.1130/G38049.1
Biddle, J. F., Cardman, Z., Mendlovitz, H., Albert, D. B., Lloyd, K. G., Boetius, A., et al. (2012). Anaerobic oxidation of methane at different temperature regimes in Guaymas Basin hydrothermal sediments. ISME J. 6, 1018–1031. doi: 10.1038/ismej.2011.164
Bray, J. R., and Curtis, J. T. (1957). An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27, 326–349.
Buckley, A., MacGregor, B. J., and Teske, A. (2019). Identification, expression and activity of candidate nitrite reductases from orange Beggiatoaceae, Guaymas Basin. Front. Microbiol. 10:644. doi: 10.3389/fmicb.2019.00644
Callaghan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Campbell, B., Engel, A. S., Porter, M. L., and Takai, K. (2006). The versatile epsilon-proteobacteria: key players in sulphidic habitats. Nat. Rev. Microbiol. 4, 458–468. doi: 10.1038/nrmicro1414
Campbell, A. T., and Gieskes, J. M. (1984). Water column anomalies associated with hydrothermal activity in the Guaymas Basin, Gulf of California. Earth Planet. Sci. Lett. 68, 57–72.
Chakraborty, A., Ruff, S. E., Dong, X., Ellefson, E. D., Li, C., Brooks, J. M., et al. (2020). Hydrocarbon seepage in the deep seabed links subsurface and seafloor biospheres. Proc. Natl. Acad. Sci. U. S. A. 117, 11029–11037. doi: 10.1073/pnas.2002289117
Chanton, J., and Liptay, K. (2000). Seasonal variation in methane oxidation in a landfill cover soil as determined by an in situ stable isotope technique. Global Biogeochem. Cycles 14, 51–60.
Chao, A. (1984). Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 11, 265–270.
Cline, J. D. (1969). Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14, 454–458.
Conway, J. R., Lex, A., and Gehlenborg, N. (2017). UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics 33, 2938–2940. doi: 10.1093/bioinformatics/btx364
Cruaud, P., Vigneron, A., Pignet, P., Caprais, J. -C., Lesongeur, F., Toffin, L., et al. (2017). Comparative study of Guaymas Basin microbiomes: cold seeps vs. hydrothermal vents sediments. Front. Mar. Sci. 4:417. doi: 10.3389/fmars.2017.00417
Dabert, P., Sialve, B., Delgenes, J. P., Moletta, R., and Godon, J. J. (2001). Characterization of the microbial 16S rDNA diversity of an aerobic phosphorus-removal ecosystem and monitoring of its transition to nitrate respiration. Appl. Microbiol. Biotechnol. 55, 500–509. doi: 10.1007/s002530000529
Dhillon, A., Lever, M., Lloyd, K. G., Albert, D. B., Sogin, M. L., and Teske, A. (2005). Methanogen diversity evidenced by molecular characterization of methyl coenzyme M reductase A (mcrA) genes (mcrA) in hydrothermal sediments of the Guaymas Basin. Appl. Environ. Microbiol. 71, 4592–4601.
Dojka, M. A., Hugenholtz, P., Haack, S. K., and Pace, N. R. (1998). Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl. Environ. Microbiol. 64, 3869–3877. doi: 10.1128/AEM.64.10.3869-3877.1998
Dombrowski, N., Teske, A. P., and Baker, B. J. (2018). Extensive metabolic versatility and redundancy in microbially diverse, dynamic Guaymas Basin hydrothermal sediments. Nat. Commun. 9:4999. doi: 10.1038/s41467-018-07418-0
Dong, X., Kleiner, M., Sharp, C. E., Thorson, E., Li, C., Liu, D., et al. (2017). Fast and simple analysis of miseq amplicon sequencing data with MetaAmp. Front. Microbiol. 8:1461. doi: 10.3389/fmicb.2017.01461
Dowell, F., Cardman, Z., Dasarathy, S., Kellermann, M. Y., McKay, L. J., MacGregor, B. J., et al. (2016). Microbial communities in methane and short alkane-rich hydrothermal sediments of Guaymas Basin. Front. Microbiol. 7:17. doi: 10.3389/fmicb.2016.00017
Dyksma, S., Pjevac, P., Ovanesov, K., and Mussmann, M. (2018). Evidence for H2 consumption by uncultured Desulfobacterales in coastal sediments. Environ. Microbiol. 20, 450–461. doi: 10.1111/1462-2920.13880
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Einsele, G., Gieskes, J. M., Curray, J., Moore, D. M., Aguago, E., Aubry, M. P., et al. (1980). Intrusion of basaltic sills into highly porous sediments and resulting hydrothermal activity. Nature 283, 441–445.
Geilert, S., Hensen, C., Schmidt, M., Liebetreu, V., Scholz, V., Doll, M., et al. (2018). Transition from hydrothermal vents to cold seeps records timing of carbon release in the Guaymas Basin, Gulf of California. Biogeosciences 15, 5715–5731. doi: 10.5194/bg-2018-12
Grasshoff, K., Kremling, K., and Ehrhardt, M. (2009). Methods of seawater analysis. Weinheim, Germany: John Wiley & Sons.
Guezennec, J. G., Dussauze, J., Bian, M., Rocchicioli, F., Ringelberg, D., Hedrick, D. B., et al. (1996). Bacterial community structure from Guaymas Basin, Gulf of California, as determined by analysis of phospholipid ester-linked fatty acids. J. Mar. Biotechnol. 4, 165–175.
Gundersen, J. K., Jørgensen, B. B., Larsen, E., and Jannasch, H. W. (1992). Mats of giant sulphur bacteria on deep-sea sediments due to fluctuating hydrothermal flow. Nature 360, 454–456.
Hahn, C., Laso-Pérez, R., Volcano, F., Vaziourakis, K. -M., Stokke, R., Steen, I. H., et al. (2020). Candidatus Ethanoperedens, a thermophilic genus of archaea mediating the anaerobic oxidation of ethane. MBio 11:e00600-20. doi: 10.1128/mBio.00600-20
Hill, T. C. J., Walsh, K. A., Harris, J. A., and Moffett, B. F. (2003). Using ecological diversity measures with bacterial communities. FEMS Microbiol. Ecol. 43, 1–11. doi: 10.1111/j.1574-6941.2003.tb01040.x
Hoffmann, K., Bienhold, C., Buttigieg, P. L., Knittel, K., Laso-Pérez, R., Rapp, J. Z., et al. (2020). Diversity and metabolism of Woeseiales bacteria, global members of marine sediment communities. ISME J. 14, 1042–1056. doi: 10.1038/s41396-020-0588-4
Holler, T., Widdel, F., Knittel, K., Amann, R., Kellermann, M. Y., Hinrichs, K. -U., et al. (2011). Thermophilic anaerobic oxidation of methane by marine microbial consortia. ISME J. 5, 1946–1956. doi: 10.1038/ismej.2011.77
Iino, T., Mori, K., Uchino, Y., Nakagawa, T., Harayama, S., and Suzuki, K. (2010). Ignavibacterium album gen. nov., sp. nov., a moderately thermophilic anaerobic bacterium isolated from microbial mats at a terrestrial hot spring and proposal of Ignavibacteria classis nov., for a novel lineage at the periphery of green sulfur bacteria. Int. J. Syst. Evol. Microbiol. 60, 1376–1382. doi: 10.1099/ijs.0.012484-0
Jochum, L. M., Schreiber, L., Marshall, I. P. G., Jørgensen, B. B., Schramm, A., and Kjeldsen, K. U. (2018). Single-cell genomics reveals a diverse metabolic potential of uncultivated Desulfatiglans-related Deltaproteobacteria widely distributed in marine sediment. Front. Microbiol. 9:2038. doi: 10.3389/fmicb.2018.02038
Katayama, T., Nobu, M. K., Kusada, H., Meng, X. -Y., Hosogi, N., Uematsu, K., et al. (2020). Isolation of a member of the candidate phylum Atribacteria reveals a unique cell membrane structure. Nat. Commun. 11:6382. doi: 10.1038/s41467-020-20149-5
Kleindienst, S., Ramette, A., Amann, R., and Knittel, K. (2012). Distribution and in situ abundance of sulfate-reducing bacteria in diverse marine hydrocarbon seep sediments. Environ. Microbiol. 14, 2689–2710. doi: 10.1111/j.1462-2920.2012.02832.x
Klindworth, A., Prüsse, E., Schweer, T., Peplies, J., Quast, C., Horn, M., et al. (2013). Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41:e1. doi: 10.1093/nar/gks808
Knittel, K., Boetius, A., Lemke, A., Eilers, H., Lochte, K., Pfannkuche, O., et al. (2003). Activity, distribution, and diversity of sulfate reducers and other bacteria in sediments above gas hydrate (Cascadia margin, Oregon). Geomicrobiol J. 20, 269–294. doi: 10.1080/01490450303896
Knittel, K., Lösekann, T., Boetius, A., Kort, R., and Amann, R. (2005). Diversity and distribution of Methanotrophic Archaea at cold seeps. Appl. Environ. Microbiol. 71, 467–479. doi: 10.1128/AEM.71.1.467-479.2005
Krukenberg, V., Harding, K., Richter, M., Glöckner, F. -O., Gruber-Vodicka, H. R., Adam, B., et al. (2016). Candidatus Desulfofervidus auxilii, a hydrogenotrophic sulfate-reducing bacterium involved in the thermophilic anaerobic oxidation of methane. Environ. Microbiol. 18, 3073–3091. doi: 10.1111/1462-2920.13283
Krukenberg, V., Riedel, D., Gruber-Vodicka, H. R., Buttigieg, P. L., Tegetmeyer, H. E., Boetius, A., et al. (2018). Gene expression and ultrastructure of meso- and thermophilic methanotrophic consortia. Environ. Microbiol. 20, 1651–1666. doi: 10.1111/1462-2920.14077
Kruskal, J. B. (1964). Nonmetric multidimensional scaling: a numerical method. Psychometrika 29, 115–129.
Laso-Pérez, R., Wegener, G., Knittel, K., Widdel, F., Harding, K. J., Krukenberg, V., et al. (2016). Thermophilic archaea activate butane via alkyl-coenzyme M formation. Nature 539, 396–401. doi: 10.1038/nature20152
Lauer, A., Sørensen, K. B., and Teske, A. (2016). Phylogenetic characterization of marine benthic Archaea in organic-poor sediments of the Eastern Equatorial Pacific Ocean (ODP site 1225). Microorganisms 4:32. doi: 10.3390/microorganisms4030032
Lazar, C. S., Baker, B. J., Seitz, K. W., and Teske, A. P. (2017). Genomic reconstruction of multiple lineages of uncultured benthic archaea suggests distinct biogeochemical roles and ecological niches. ISME J. 11, 1118–1129. doi: 10.1038/ismej.2016.189
Lever, M. A., and Teske, A. (2015). Methane-cycling archaeal diversity in hydrothermal sediment investigated by general and group-specific functional gene and 16S rRNA gene PCR primers. Appl. Environ. Microbiol. 81, 1426–1441. doi: 10.1128/AEM.03588-14
Li, L., Kato, C., and Horikoshi, K. (1999). Bacterial diversity in deep-sea sediments from different depths. Biodivers. Conserv. 8, 659–677.
Lin, Y. -S., Koch, B. P., Feseker, T., Ziervogel, K., Goldhammer, T., Schmidt, F., et al. (2017). Near-surface heating of young rift sediment causes mass production and discharge of reactive dissolved organic matter. Sci. Rep. 7:44864. doi: 10.1038/srep44864
Liu, Y., and Whitman, W. B. (2008). Metabolic, phylogenetic and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 1125, 171–189. doi: 10.1196/annals.1419.019
Lizarralde, D., Soule, A., Seewald, J., and Proskurowski, G. (2011). Carbon release by off-axis magmatism in a young sedimented spreading centre. Nat. Geosci. 4, 50–54. doi: 10.1038/ngeo1006
Lonsdale, P., and Becker, K. (1985). Hydrothermal plumes, hot springs, and conductive heat flow in the southern trough of guaymas basin. Earth Planet. Sci. Lett. 73, 211–225.
Lösekann, T., Knittel, K., Nadalig, T., Fuchs, B., Niemann, H., Boetius, A., et al. (2007). Diversity and abundance of aerobic and anaerobic methane oxidizers at the Haakon Mosby Mud Volcano, Barents Sea. Appl. Environ. Microbiol. 73, 3348–3362. doi: 10.1128/AEM.00016-07
Ludwig, W., Strunk, O., Westram, R., Richter, L., Meier, H., Yadhukumar, A., et al. (2004). ARB: a software environment for sequence data. Nucleic Acids Res. 32, 1363–1371. doi: 10.1093/nar/gkh293
MacGregor, B. J., Biddle, J. F., Siebert, J. R., Staunton, E., Hegg, E., Matthysse, A. G., et al. (2013). Why orange Guaymas Basin Beggiatoa spp. are orange: single-filament genome-enabled identification of an abundant octaheme cytochrome with hydroxylamine oxidase, hydrazine oxidase and nitrite reductase activities. Appl. Environ. Microbiol. 79, 1183–1190. doi: 10.1128/AEM.02538-12
Magen, C., Lapham, L. L., Pohlman, J. W., Marshall, K., Bosman, S., Casso, M., et al. (2014). A simple headspace equilibration method for measuring dissolved methane. Limnol. Oceanogr. Methods 12, 637–650. doi: 10.4319/lom.2014.12.637
McKay, L., Klokman, V., Mendlovitz, H., LaRowe, D., Zabel, M., Hoer, D., et al. (2016). Thermal and geochemical influences on microbial biogeography in the hydrothermal sediments of Guaymas Basin, Gulf of California. Environ. Microbiol. Rep. 8, 150–161. doi: 10.1111/1758-2229.12365
McKay, L. J., MacGregor, B. J., Biddle, J. F., Mendlovitz, H. P., Hoer, D., Lipp, J. S., et al. (2012). Spatial heterogeneity and underlying geochemistry of phylogenetically diverse orange and white Beggiatoa mats in Guaymas Basin hydrothermal sediments. Deep-Sea Res. I 67, 21–31. doi: 10.1016/j.dsr.2012.04.011
Meyer, S., Wegener, G., Lloyd, K. G., Teske, A., Boetius, A., and Ramette, A. (2013). Microbial habitat connectivity across spatial scales and hydrothermal temperature gradients at Guaymas Basin. Front. Microbiol. 4:207. doi: 10.3389/fmic.2013.00207
Mussmann, M., Pjevac, P., Krüger, K., and Dyksma, S. (2017). Genomic repertoire of the Woeseiaceae/JTB255, cosmopolitan and abundant core members of microbial communities in marine sediments. ISME J. 11, 1276–1281. doi: 10.1038/ismej.2016.185
Niemann, H., Lösekann, T., de Beer, D., Elvert, M., Nadalig, T., Knittel, K., et al. (2006). Novel microbial communities of the Haakon Mosby mud volcano and their role as a methane sink. Nature 443, 854–858. doi: 10.1038/nature05227
Nobu, M. K., Dodsworth, J. A., Murugapiran, S. K., Rinke, C., Gies, E. A., Webster, G., et al. (2016a). Phylogeny and physiology of candidate phylum ‘Atribacteria’ (OP9/JS1) inferred from cultivation-independent genomics. ISME J. 10, 273–286. doi: 10.1038/ismej.2015.97
Nobu, M. K., Narihiro, T., Kuroda, K., Mei, R., and Liu, W. T. (2016b). Chasing the elusive Euryarchaeota class WSA2: genomes reveal a uniquely fastidious methyl-reducing methanogen. ISME J. 10, 2478–2487. doi: 10.1038/ismej.2016.33
Núñez-Useche, F., Canet, C., Liebetrau, V., Puig, T. P., Ponciano, A. C., Alfonso, P., et al. (2018). Redox conditions and authigenic mineralization related to cold seeps in central Guaymas Basin, Gulf of California. Mar. Petrol. Geol. 95, 1–15. doi: 10.1016/j.marpetgeo.2018.04.010
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O’Hara, R. B., et al. (2012). vegan: Community Ecology Package. Available at: http://cran.r-project.org/package=vegan (Accessed December 30, 2020).
Ondréas, H., Scalabrin, C., Fouquet, Y., and Godfroy, A. (2018). Recent high-resolution mapping of Guaymas hydrothermal fields (Southern Trough). BSGF – Earth Sciences Bulletin 189:6. doi: 10.1051/bsgf/2018005
Paull, C. K., Ussler, W. III., Peltzer, E. T., Brewer, P. G., Keaten, R., Mitts, P. J., et al. (2007). Authigenic carbon entombed in methane-soaked sediments from the northeastern transform margin of the Guaymas Basin, Gulf of California. Deep-Sea Res. II 54, 1240–1267. doi: 10.1016/j.dsr2.2007.04.009
Price, R. E., and Giovanelli, D. (2017). “A review on the geochemistry and microbiology of marine shallow-water hydrothermal vents” in Reference Module in Earth Systems and Environmental Sciences. Elsevier.
Prüsse, E., Peplies, J., and Glöckner, F. O. (2012). SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28, 1823–1829. doi: 10.1093/bioinformatics/bts252
Qin, W., Heal, K. R., Ramdasi, R., Kobelt, J. N., Martens-Habbena, W., Bertagnolli, A. D., et al. (2017). Nitrosopumilus maritimus gen. Nov., sp. nov., Nitrosopumilus cobalaminigenes sp. nov., Nitrosopumilus oxyclinae sp. nov., and Nitrosopumilus ureiphilus sp. nov., four marine ammonia-oxidizing archaea of the phylum Thaumarchaeota. Int. J. Syst. Evol. Microbiol. 67, 5067–5079. doi: 10.1099/ijsem.0.002416
Quast, C., Prüsse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Ramírez, G. A., McKay, L. J., Fields, M. W., Buckley, A., Mortera, C., Hensen, C., et al. (2020). The Guaymas Basin subseafloor sedimentary Archaeome reflects complex environmental histories. iScience 23:101459. doi: 10.1016/j.isci.2020.101459
Roberts, D. W. (2012). labdsv: Ordination and Multivariate Analysis for Ecology [Internet]. Available at: http://cran.r-project.org/package=labdsv (Accessed December 30, 2020).
Rossel, P. E., Elvert, M., Ramette, A., Boetius, A., and Hinrichs, K. -U. (2011). Factors controlling the distribution of anaerobic methanotrophic communities in marine environments: evidence from intact polar membrane lipids. Geochim. Cosmochim. Acta 75, 164–184. doi: 10.1016/j.gca.2010.09.031
Ruff, S. E., Arnds, J., Knittel, K., Amann, R., Wegener, G., et al. (2013). Microbial communities of deep-sea methane seeps at Hikurangi Continental Margin (New Zealand). PLoS One 8:e72627. doi: 10.1371/journal.pone.0072627
Ruff, S. E., Biddle, J. F., Teske, A., Knittel, K., Boetius, A., and Ramette, A. (2015). Global dispersion and local diversification of the methane seep microbiome. Proc. Natl. Acad. Sci. U. S. A. 112, 4015–4020. doi: 10.1073/pnas.1421865112
Ruff, S. E., Felden, J., Gruber-Vodicka, H. R., Marcon, Y., Knittel, K., Ramette, A., et al. (2019). In situ development of a methanotropic microbiome in deep-sea sediments. ISME J. 13, 197–213. doi: 10.1038/s41396-018-0263-1
Schoell, M. (1982). “Stable isotopic analyses of interstitial gases in quaternary sediments from the Gulf of California” in Initial reports of the Deep Sea drilling project. Vol. 64. eds. J. Curray and D. Moore (Texas, TX: Ocean Drilling Program, College Station), 815–817.
Schouten, S., Wakeham, S. G., Hopmans, E. C., and Sinninghe Damste, J. S. (2003). Biogeochemical evidence that thermophilic archaea mediate the anaerobic oxidation of methane. Appl. Environ. Microbiol. 69, 1680–1686. doi: 10.1128/aem.69.3.1680-1686.2003
Schreiber, L., Holler, T., Knittel, K., Meyerdierks, A., and Amann, R. (2010). Identification of the dominant sulfate-reducing bacterial partner of anaerobic methanotrophs of the ANME-2c clade. Environ. Microbiol. 12, 2327–2340. doi: 10.1111/j.1462-2920.2010.02275.x
Schutte, C., Teske, A., MacGregor, B., Salman-Carvalho, V., Lavik, G., Hach, P., et al. (2018). Filamentous giant Beggiatoaceae from Guaymas Basin are capable of both denitrification and dissimilatory nitrate reduction to ammonium (DNRA). Appl. Environ. Microbiol. 84:e02860-17. doi: 10.1128/AEM.02860-17
Seeberg-Elverfeldt, J., Schlüter, M., Feseker, T., and Kölling, M. (2005). Rhizon sampling of porewaters near the sediment-water interface of aquatic systems. Limnol. Oceanogr. Methods 3, 361–371. doi: 10.4319/lom.2005.3.361
Seitz, K. W., Dombrowski, N., Eme, L., Spang, A., Lombard, J., Sieber, J., et al. (2019). Asgard Archaea capable of anaerobic hydrocarbon cycling. Nature Commun. 10:1822. doi: 10.1038/s41467-019-09364-x
Simoneit, B. R. T., Lonsdale, P., Edmond, J. M., and Shanks, W. C. III. (1990). Deep-water hydrocarbon seeps in Guaymas Basin, Gulf of California. Appl. Geochem. 5, 41–49.
Simoneit, B. R. T., Summerhayes, C. P., and Meyers, P. A. (1986). Sources and hydrothermal alteration of organic matter in quaternary sediments: a synthesis of studies from the central Gulf of California. Mar. Petrol. Geol. 3, 282–297.
Starnawski, P., Bataillon, T., Ettema, T. J. G., Jochum, L. M., Schreiber, L., Chen, X., et al. (2017). Microbial community assembly and evolution in subseafloor sediment. Proc. Natl. Acad. Sci. U. S. A. 114, 2940–2945. doi: 10.1073/pnas.1614190114
Stieglmeier, M., Klingl, A., Alves, R. J., Rittmann, S. K., Melcher, M., Leisch, N., et al. (2014). Nitrososphaera viennensis gen. nov., sp. nov., an aerobic and mesophilic, ammonia-oxidizing archaeon from soil and a member of the archaeal phylum Thaumarchaeota. Int. J. Syst. Evol. Microbiol. 64, 2738–2752. doi: 10.1099/ijs.0.063172-0
Suess, E. (2010). “Marine cold seeps” in Handbook of hydrocarbon and lipid microbiology. ed. K. N. Timmis (Berlin, Heidelberg: Springer-Verlag), 188–302.
Takahashi, S., Tomita, J., Nishioka, K., Hisada, T., and Nishijima, M. (2014). Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS One 9:e105592. doi: 10.1371/journal.pone.0105592
Tamura, K., Dudley, J., Nei, M., and Kumar, S. (2007). MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599. doi: 10.1093/molbev/msm092
Tavormina, P. L., Hatzenpichler, R., McGlynn, S., Chadwick, G., Dawson, K. S., Connon, S. A., et al. (2015). Methyloprofundus sedimentii gen. nov. sp. nov., an obligate methanotroph from ocean sediment belonging to the “deep sea-1” clade of marine methanotrophs. Int. J. Syst. Evol. Microbiol. 65, 251–259. doi: 10.1099/ijs.0.062927-0
Teske, A. (2019). “Hydrocarbon-degrading anaerobic microbial communities in natural oil seeps” in Microbial communities utilizing hydrocarbons and lipids: Members, metagenomics and ecophysiology, handbook of hydrocarbon and lipid microbiology. ed. T. J. McGenity (Cham: Springer).
Teske, A., de Beer, D., McKay, L., Tivey, M. K., Biddle, J. F., Hoer, D., et al. (2016). The Guaymas Basin hiking guide to hydrothermal mounds, chimneys and microbial mats: complex seafloor expressions of subsurface hydrothermal circulation. Front. Microbiol. 7:75. doi: 10.3389/fmicb.2016.00075
Teske, A., Edgcomb, V., Rivers, A. R., Thompson, J. R., de Vera Gomez, A., Molyneaux, S. J., et al. (2009). A molecular and physiological survey of a diverse collection of hydrothermal vent Thermococcus and Pyrococcus isolates. Extremophiles 13, 905–915. doi: 10.1007/s00792-009-0278-7
Teske, A., Hinrichs, K. -U., Edgcomb, V., de Vera Gomez, A., Kysela, D., Sylva, S. P., et al. (2002). Microbial diversity in hydrothermal sediments in the Guaymas Basin: evidence for anaerobic methanotrophic communities. Appl. Environ. Microbiol. 68, 1994–2007. doi: 10.1128/aem.68.4.1994-2007.2002
Teske, A., Lizarralde, D., and Höfig, T. W. (2018). Expedition 385 scientific prospectus: Guaymas Basin tectonics and biosphere. College Station, TX: International Ocean Discovery Program.
Teske, A., McKay, L. J., Ravelo, A. C., Aiello, I., Mortera, C., Núñez-Useche, F., et al. (2019). Characteristics and evolution of sill-driven off-axis hydrothermalism in Guaymas Basin – the Ringvent site. Sci. Rep. 9:13847. doi: 10.1038/s41598-019-50200-5
Teske, A., and Salman, V. (2014). “The family Beggiatoaceae. Chapter 6” in The prokaryotes – Gammaproteobacteria. 4th Edn. eds. E. Rosenberg, E. F. DeLong, F. Thompson, S. Lory, and E. Stackebrandt (Berlin/Heidelberg: Springer-Verlag), 93–134.
Topçuoglu, B. D., Stewart, L. C., Morrison, H. G., Butterfield, D. A., Huber, J. A., and Holden, J. F. (2016). Hydrogen limitation and syntrophic growth among natural assemblages of thermophilic methanogens at deep-sea hydrothermal vents. Front. Microbiol. 7:1240. doi: 10.3389/fmicb.2016.01240
Vetriani, C., Jannasch, J. W., MacGregor, B. J., Stahl, D. A., and Reysenbach, A. -L. (1999). Population structure and phylogenetic characterization of marine benthic archaea in deep-sea sediments. Appl. Environ. Microbiol. 65, 4375–4384. doi: 10.1128/AEM.65.10.4375-4384.1999
Vigneron, A., L’Haridon, S., Godfroy, A., Roussel, E. G., Cragg, B. A., Parkes, R. J., et al. (2015). Evidence of active methanogen communities in shallow sediments of the Sonora margin cold seeps. Appl. Environ. Microbiol. 81, 3451–3459. doi: 10.1128/AEM.00147-15
Von Damm, K. L., Edmond, J. M., Measures, C. I., and Grant, B. (1985). Chemistry of submarine hydrothermal solutions at Guaymas Basin, Gulf of California. Geochim. Cosmochim. Acta 49, 2221–2237.
Wang, Y., Feng, X., Natarajan, V. P., Xiao, X., and Wang, F. (2019). Diverse anaerobic methane-and multi-carbon alkane-metabolizing archaea coexist and show activity in Guaymas Basin hydrothermal sediment. Environ. Microbiol. 21, 1344–1355. doi: 10.1111/1462-2920.14568
Weber, A., and Jørgensen, B. B. (2002). Bacterial sulfate reduction in hydrothermal sediments of the Guaymas Basin, Gulf of California, Mexico. Deep Sea Res. I 149, 827–841. doi: 10.1016/S0967-0637(01)00079-6
Wegener, G., Krukenberg, V., Riedel, D., Tegetmeyer, H. E., and Boetius, A. (2015). Intracellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature 526, 587–590. doi: 10.1038/nature15733
Wickham, H. (2016). Ggplot2: Elegant graphics for data analysis. 2nd Edn. New York: Springer International Publishing.
Wickham, H. (2018). stringr: Simple, Consistent Wrappers for Common String Operations. Available at: https://www.rdocumentation.org/packages/stringr (Accessed December 30, 2020).
Wickham, H., François, R., Henry, L., and Müller, K. (2018). dplyr: A Grammar of Data Manipulation. Available at: https://dplyr.tidyverse.org (Accessed December 30, 2020).
Winkel, M., de Beer, D., Lavik, G., Peplies, J., and Mussmann, M. (2014). Close association of active nitrifyers with Beggiatoa mats covering deep-sea hydrothermal sediments. Environ. Microbiol. 16, 1612–1626. doi: 10.1111/1462-2920.12316
Zaremba-Niedzwiedzka, K., Caceres, E. F., Saw, J. H., Bäckström, D., Juzokaite, L., Vancaester, E., et al. (2017). Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature 541, 353–358. doi: 10.1038/nature21031
Zhou, Z., Liu, Y., Lloyd, K. G., Pan, J., Yang, Y., Gu, J. -D., et al. (2019). Genomic and transcriptomic insights into the ecology and metabolism of benthic archaeal cosmopolitan Thermoprofundales (MBG-D archaea). ISME J. 13, 885–901. doi: 10.1038/s41396-018-0321-8
Zhuang, G. -C., Montgomery, A., Samarkin, V. A., Song, M., Liu, J., Schubotz, F., et al. (2019). Generation and utilization of volatile fatty acids and alcohols in hydrothermally altered sediments in the Guaymas Basin, Gulf of California. Geophys. Res. Lett. 46, 2637–2646. doi: 10.1029/2018GL081284
Keywords: cold seep, hydrothermal sediment, porewater profiles, bacteria, archaea, Guaymas Basin
Citation: Teske A, Wegener G, Chanton JP, White D, MacGregor B, Hoer D, de Beer D, Zhuang G, Saxton MA, Joye SB, Lizarralde D, Soule SA and Ruff SE (2021) Microbial Communities Under Distinct Thermal and Geochemical Regimes in Axial and Off-Axis Sediments of Guaymas Basin. Front. Microbiol. 12:633649. doi: 10.3389/fmicb.2021.633649
Received: 25 November 2020; Accepted: 12 January 2021;
Published: 12 February 2021.
Edited by:
Axel Schippers, Federal Institute for Geosciences and Natural Resources, GermanyReviewed by:
Takuro Nunoura, Japan Agency for Marine-Earth Science and Technology (JAMSTEC), JapanCopyright © 2021 Teske, Wegener, Chanton, White, MacGregor, Hoer, de Beer, Zhuang, Saxton, Joye, Lizarralde, Soule and Ruff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Teske, dGVza2VAZW1haWwudW5jLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.