
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol. , 12 March 2021
Sec. Microbiotechnology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.633090
This article is part of the Research Topic Nanomicrobiology: Emerging Trends in Microbial Synthesis of Nanomaterials and their Applications View all 10 articles
Biological entities such as green plants, fungi, and lichens are now a days persistently explored for the synthesis of nanoparticles. Lichen-based nanoparticles are also becoming increasingly popular owing to their biocompatibility, eco-friendliness, and cost-effectiveness. The lichen-based metal nanomaterials, particularly synthesized using green chemistry approaches, have turned out to be great substitutes to conventional antimicrobial therapies. Many scientific reports established the significant antimicrobial properties exhibited by the lichen nanoparticles. Therefore, the present mini-review summarizes an overview of lichen-based nanomaterials, their synthesis, their applications, and the molecular mechanism of their potential as broad spectrum antimicrobial agents for biomedical applications.
Microbial pathogenesis is the cause of morbidity and mortality of millions across the globe annually. Soon after the discovery of the antibiotics, they were widely considered as an effective remedy against pathogens and rightly remained so, till the emergence of antibiotic resistance among the microorganisms (Martínez, 2008). However, the recent advancements in nanotechnology led to the development of nanoparticles that have established as potent broad-spectrum antimicrobial agents (Wang et al., 2017). The biosynthesis of nanoparticles through green synthesis method involves bioreduction of metals or metal oxide to their elemental forms with size ranging from 1 to 100 nm. Therefore, this process is gaining considerable attention for its eco-friendliness and cost-effectiveness (Mie et al., 2014; Hussain et al., 2016).
Lichens, the composite organisms that result from a symbiotic association between fungi and algae possess several bioactive compounds (Kambar et al., 2014) and as such have been researched thoroughly and are well known for their bioactivity against many pathogens. Lately, researchers have been exploring the possibilities of using lichens for synthesizing nanoparticles and further utilizing them as antimicrobial agents (Mie et al., 2014).
Many reports are available on the synthesis of nanoparticles from different types of lichens, namely, Parmeliopsis ambigua, Punctelia subrudecta, Evernia mesomorpha, and Xanthoparmelia plitti (Dasari et al., 2013); Parmotrema praesorediosum (Mie et al., 2014); Cetraria islandica (Yıldız et al., 2014; Baláž et al., 2020); Ramalina dumeticola (Din et al., 2015); Acroscyphus sp. and Sticta sp. (Debnath et al., 2016); Parmelia perlata (Leela and Anchana Devi, 2017); Usnea longissima (Siddiqi et al., 2018); Parmotrema tinctorum (Khandel et al., 2018); Parmelia sulcata (Gandhi et al., 2019); Protoparmeliopsis muralis (Alavi et al., 2019); Ramalina sinensis (Safarkar et al., 2020); Cladonia rangiferina (Devasena et al., 2014; Rai and Gupta, 2019); Pseudevernia furfuracea and Lobaria pulmonaria (Goga et al., 2020); Xanthoria elegans, Usnea antarctica, and Leptogium puberulum (Baláž et al., 2020); and Lecanora muralis (Abdullah et al., 2020).
Nanoparticles derived from metals and their oxides such as silver, gold, titanium, cadmium, iron, zinc, and copper have reportedly been synthesized using many lichens (Mie et al., 2014; Çıplak et al., 2018; Bhat, 2018; Alavi et al., 2019; Gandhi et al., 2019). Many of these lichen-based nanoparticles have been reported to exhibit antimicrobial bioactivity against several bacteria and fungi, which could be attributed to their ability to disintegrate the microbial membrane, oxidation of various cellular components, and generation of hydroxyl radicals (Ruparelia et al., 2008; Marambio-Jones and Hoek, 2010). Therefore, the present review highlights the investigation about the utility of lichens as biological laboratories for the sustainable production of antimicrobial metallic nanoparticles.
The biosynthesis of lichen-derived nanoparticles is gaining popularity these days: as the process does not involve use of any toxic chemicals, therefore, they can be safely used as pharmaceuticals (Kowalski et al., 2011). Researchers around the globe have been following different methodologies such as biomechanical and chemical solid-state synthesis for the synthesis of lichen-based nanoparticles (Baláž et al., 2020). Mie et al. (2014) reported the synthesis of silver nanoparticles by the reduction of silver nitrate using aqueous extract of the lichen Parmotrema praesorediosum as a reductant as well as a stabilizer. Nanoparticles were characterized by using ultraviolet (UV)–visible spectroscopy, electron microscopy, energy-dispersive spectroscopy (EDS), and X-ray diffraction (XRD) technique. The cubic structured nanoparticles exhibited an average particle size of 19 nm. Devasena et al. (2014) synthesized magnesium nanoparticles from Cladonia rangiferina with an average size of 23 nm. They used light scattering and UV spectroscopy for characterization of the nanoparticles. Din et al. (2015) successfully synthesized silver nanoparticles by the reduction of silver nitrate with the aqueous extract of the lichen Ramalina dumeticola. The synthesis of silver nanoparticles in the solution was confirmed by UV–visible spectroscopy at 433 nm. Their physical appearance was characterized by transmission electron microscopy (TEM) and XRD techniques, revealing a cubic shape with an average size of 13 nm. Debnath et al. (2016) reported the biogenic synthesis of gold nanoparticles from Acroscyphus sp. and Sticta sp. without the addition of any reducing and stabilizing agent. They were quasi-spherical and prismatic in shapes and characterized by UV–visible, Fourier transform infrared (FT-IR) spectroscopy, powder XRD, and TEM. Çıplak et al. (2018) prepared the lichen-reduced graphene oxide (LrGO) bimetallic nanoparticles nanocomposites (LrGO–AgAu) by using the one-pot approach with Cetraria islandica. The characterization of nanoparticles, so formed, was carried out using techniques such as TEM, scanning electron microscopy (SEM), XRD, and FT-IR.
Baláž et al. (2020) reported the solid-state mechanochemical synthesis of silver nanoparticles using lichens Xanthoria elegans, C. islandica, Usnea antarctica, and Leptogium puberulum. The method involved milling of lichen sample and silver nitrate together in a pulverisette. The milling process was accompanied by recording of XRD pattern, and after the process of milling was complete, the samples were stored in desiccators, and XRD patterns were recorded. TEM analysis and selected area diffraction (SAD) confirmed the formation of silver nanoparticles. Abdullah et al. (2020) used one-pot green synthesis method for the green synthesis of ZnO/TiO2/SiO2 and Fe3O4/SiO2 nanoparticle composites using the lichen Lecanora muralis. XRD, SEM, EDS, and elemental mapping techniques revealed the fabrication of biosynthesized nanostructure. Safarkar et al. (2020) reported the synthesis of iron oxide nanoparticles from the extract of Ramalina sinensis by co-precipitation method. They confirmed the synthesis of nanoparticles by UV spectrophotometer, XRD, FT-IR, and field emission SEM–energy-dispersive X-ray spectrometry (FESEM-EDX). They reported the synthesis of spherical iron oxide nanoparticles with particle size ranging from 31.74 to 53.91 nm, which were observed using FESEM. The visible UV spectra obtained for the iron oxide nanoparticles showed peak in the range of 280–320 nm. The nanoparticles exhibited effective antimicrobial properties against Staphylococcus aureus and Pseudomonas aeruginosa. Goga et al. (2020) used Pseudevernia furfuracea and Lobaria pulmonaria to synthesize silver nanoparticles with an average size of 10 nm (while a few reached 100 nm) by using solid-state mechanochemical synthesis.
Lately, researchers have been making attempts to explore and report antimicrobial properties of the different types of lichen-based nanoparticles (Table 1). Mie et al. (2014) reported the antimicrobial activity of Parmotrema praesorediosum-derived silver nanoparticles against eight types of pathogenic bacteria including gram-positive and gram-negative bacteria. Their results showed that silver nanoparticles synthesized using P. praesorediosum have significant antibacterial activity against gram-negative bacteria. Siddiqi et al. (2018) reported antibacterial activity of Usnea longissima-derived silver nanoparticles against six gram-positive (Staphylococcus aureus, Streptococcus mutans, Streptococcus pyrogenes, Streptococcus viridans, Corynebacterium diphtheriae, and Corynebacterium xerosis) and three gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa). The nanoparticles exhibited significant bioactivity against E. coli and K. pneumoniae, but S. mutans, C. diphtheriae, and P. aeruginosa displayed resistance against them. Baláž et al. (2020) reported that silver nanoparticles produced using Xanthoria elegans, Cetraria islandica, Usnea antarctica, and Leptogium puberulum were excellent antibacterial agents against E. coli and S. aureus. Alavi et al. (2019) observed that Protoparmeliopsis muralis-driven metal (Ag and Cu) and metal oxide (TiO2, ZnO, and Fe3O4) nanoparticles exhibited antibacterial, antibiofilm, antiquorum sensing, and antioxidant abilities against multidrug-resistant bacterium S. aureus and reference bacteria E. coli and P. aeruginosa. Abdullah et al. (2020) examined Lecanora muralis-driven nanocomposites of Fe3O4/SiO2 and ZnO/TiO2/SiO2 for their antimicrobial and antifungal properties and reported that they exhibited good bioactivity against three species of pathogenic bacteria (S. aureus, E. coli, and Pseudomonas spp.) and five species of fungi (Candida albicans, Candida spp., Aspergillus flavus, Aspergillus niger, and Aspergillus terreus).
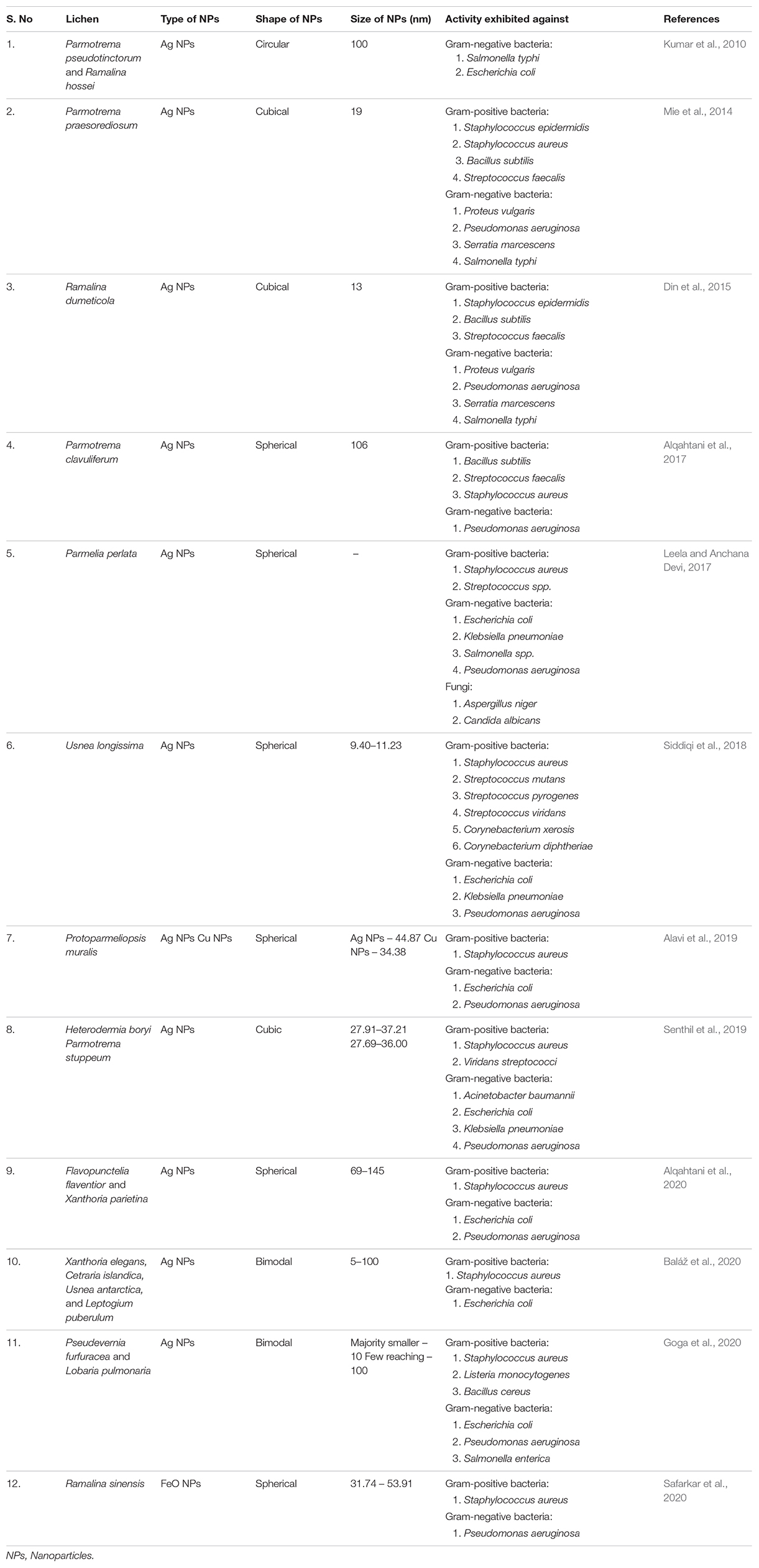
Table 1. Characteristics and antimicrobial activity of Lichen Nanoparticles synthesized by different researchers.
The antimicrobial properties of lichen nanomaterials corroborate their ability to disintegrate microbial cellular barriers (cell wall and membranes), which enable them to penetrate the cytoplasm and disintegrate cellular components and genetic material, which eventually halt their metabolic function (Figure 1; Slavin et al., 2017). However, possible mechanisms of antibacterial activity of lichen nanoparticles have been proposed such as (i) interference during cell wall synthesis, (ii) cellular stress by reactive oxygen species (ROS), (iii) interference in protein synthesis, (iv) disruption of transcription process, (v) disruption of primary metabolic pathways, (vi) inculcation with genetic material, and (vii) alteration in cell signaling process (Dhand et al., 2016). However, studies highlight that the antimicrobial efficacy and molecular mechanism of lichen nanomaterials depend on (i) type of nanomaterial, (ii) shape and size, (iii) microbial membrane composition, and (iv) physicochemical condition (pH, temperature, presence of co-ions, biofilm formation, etc.) (Sánchez-López et al., 2020).
Siddiqi et al. (2018) demonstrated the antimicrobial property of Usnea longissima-driven silver nanoparticles through the denaturation of ribosomes that leads to the inactivation of enzymes and proteins, which ultimately stops their metabolic function and results in bacterial apoptosis. Alavi et al. (2019) critically investigated Protoparmeliopsis muralis lichen aqueous extract-assisted green synthesis of silver, copper, titanium oxide, zinc oxide, and iron oxide nanoparticles and their associated antibacterial properties. Total antioxidant capacity (TAC) and 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) antioxidant assay were used to determine the antioxidant property of P. muralis lichen. Results clearly indicated that the copper and silver nanoparticles show superior antioxidant and antimicrobial properties over other nanoparticles. Alqahtani et al. (2020) reported that Xanthoria parietina- and Flavopunctelia flaventior-based silver nanoparticles exhibited greater antibacterial activity against gram-negative bacteria as compared with gram-positive bacteria. This could be attributed to greater penetration of nanoparticles in gram-negative bacteria than that in gram-positive because of a thinner layer of peptidoglycan in the cell wall. Safarkar et al. (2020) reported antimicrobial properties of iron oxide nanoparticle synthesis from Ramalina sinensis extract. A study highlights potential antimicrobial efficacy of synthesized nanoparticles against gram-positive and gram-negative bacteria. Electrostatic interaction of positively charged iron nanomaterial and negatively charged bacterial cells may lead to oxidation of bacterial membranes by iron ions, inducing oxidative stress in microbial cells. Production of ROS in stressed microbial cell may further trigger free radical formation. Synthesized free radicals can degenerate various cellular components and may lead to cell death.
Lichen-mediated nanoparticles are reported as stable, cost-effective, and biocompatible, which make them an ideal candidate for antimicrobial agents. Owing to their unique physical and chemical properties, they exhibit efficacy against a wide spectrum of pathogenic microorganisms such as gram-positive and gram-negative strains of bacteria and some species of fungi. Cost-effectiveness and cellular toxicity are some key concerns that are required to be critically investigated before exploring their antimicrobial candidature widely in pharmaceuticals. The environmental fate of engineered lichen nanomaterials is another big challenge for the sustainable usage of nanotechnology for biological and environmental applications. Therefore, their green synthesis not only can reduce cost of production but also can enhance the associated biocompatibility for living beings.
MB prepared the description plan of this review article. RR, SS, and BS carried out the manuscript writing and figure charting. All authors in the manuscript have contributed substantially in the writing of the manuscript and therefore approve it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge the funding agency BDT, GOI (No. BT/IN/Indo-US/Foldscope/39/2015 dated 20/03/2018) for the present review study.
Abdullah, M. S., Kolo, K., and Sajadi, S. M. (2020). Greener pathway toward the synthesis of lichen−based ZnO@TiO2@SiO2 and Fe3O4@SiO2nanocomposites and investigation of their biological activities. Food Sci. Nutrit. 8, 4044–4054. doi: 10.1002/fsn3.1661
Alavi, M., Karimi, N., and Valadbeigi, T. (2019). Antibacterial, Antibiofilm, Antiquorum Sensing, Antimotility, and Antioxidant Activities of Green Fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via Protoparmeliopsismuralis Lichen Aqueous Extract against Multi-Drug-Resistant Bacteria. ACS Biomater. Sci. Engine. 5, 4228–4243. doi: 10.1021/acsbiomaterials.9b00274
Alqahtani, M. A., Al Othman, M. R., and Mohammed, A. E. (2020). Bio fabrication of silver nanoparticles with antibacterial and cytotoxic abilities using lichens. Sci. Rep. 10:16781. doi: 10.1038/s41598-020-73683-z
Alqahtani, M. A., Mohammed, A. E., Daoud, S. I, Alkhalifah, D. H. M., and Albrahim, J. S. (2017). Lichens (Parmotrema clavuliferum) extracts: Bio-mediator in silver nanoparticles formation and antibacterial potential. J. Bionanosci. 11, 410–415. doi: 10.1166/jbns.2017.1457
Baláž, M., Goga, M., Hegedüs, M., Daneu, N., Kováčová, M., Tkáčiková, L., et al. (2020). Biomechanochemical Solid-State Synthesis of Silver Nanoparticles with Antibacterial Activity Using Lichens. ACS Sustain. Chem. Engine. 8, 13945–13955. doi: 10.1021/acssuschemeng.0c03211
Bhat, M. (2018). Antibacterial activities of nanoparticles from foliose lichens: a review. Int. J. Basic Appl. Biol. 5, 10–11.
Çıplak, Z., Gökalp, C., Getiren, B., Yıldız, A., Yıldız, and Nuray. (2018). Catalytic performance of Ag, Au and Ag-Au nanoparticles synthesized by lichen extract. Green Process. Synthesis 7, 433–440. doi: 10.1515/gps-2017-0074
Dasari, S., Suresh, K. A., Rajesh, M., Reddy, C., Hemalatha, C. S., Wudayagiri, R., et al. (2013). Biosynthesis, Characterization, Antibacterial and Antioxidant Activity of Silver Nanoparticles Produced by Lichens. J. Bionanosci. 7, 237–244. doi: 10.1166/jbns.2013.1140
Debnath, R., Purkayastha, D. D., Hazra, S., Ghosh, N. N., Bhattacharjee, C. R., and Rout, J. (2016). Biogenic synthesis of antioxidant, shape selective gold nanomaterials mediated by high altitude lichens. Mater. Lett. 169, 58–61. doi: 10.1016/j.matlet.2016.01.072
Devasena, T., Ashok, V., Dey, N., and Arul Prakash, F. (2014). Phytosynthesis of magnesium nanoparticles using lichens. World J. Pharmaceut. Res. 3, 4625–4632.
Dhand, V., Soumya, L., Bharadwaj, S., Chakra, S., Bhatt, D., and Sreedhar, B. (2016). Green synthesis of silver nanoparticles using Coffea Arabica seed extract and its antibacterial activity. Mat. Sci. Eng. C. 58, 36–43. doi: 10.1016/j.msec.2015.08.018
Din, L. B., Mie, R., Samsudin, M. W., Ahmad, A., and Ibrahim, N. (2015). Biomimetic synthesis of silver nanoparticles using the lichen Ramalinadumeticola and the antibacterial activity. Malays. J. Anal. Sci. 19, 369–376.
Gandhi, A. D., Murugan, K., and Umamahesh, K. (2019). Lichen Parmeliasulcata mediated synthesis of gold nanoparticles: an eco-friendly tool against Anopheles stephensi and Aedesaegypti. Environ. Sci. Pollut. Res. 26, 23886–23898. doi: 10.1007/s11356-019-05726-6
Goga, M., Baláž, M., and Daneu, N. (2020). Biological activity of selected lichens and lichen-based Ag nanoparticles prepared by a green solid-state mechano chemical approach. Mater. Sci. Engine. C 119:111640. doi: 10.1016/j.msec.2020.111640
Hussain, I., Singh, N. B., Singh, A., Singh, H., and Singh, S. C. (2016). Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 38, 545–560. doi: 10.1007/s10529-015-2026-7
Kambar, Y., Vivek, M., Manasa, M., and Mallikarjun, N. (2014). Antimicrobial activity of Leptogiumburnetiae, Ramalinahossei, Roccellamontagnei and Heterodermiadiademata. Int. J. Pharm. Phytopharm. Res. 4, 164–168.
Khandel, P., Shahi, S. K., Kanwar, L., Yadaw, R. K., and Soni, D. K. (2018). Biochemical profiling of microbes inhibiting silver nanoparticles using symbiotic organisms. Int. J. Pharm. Sci. Invent. 9, 273–285.
Kowalski, M., Hausner, G., and Piercey-Normore, M. D. (2011). Bioactivity of secondary metabolites and thallus extracts from lichen fungi. Mycoscience 52, 413–418. doi: 10.1007/s10267-011-0118-3
Kumar, S. V. P., Kekuda, T. R. P., Vinayaka, K. S., and Yogesh, M. (2010). Synergistic efficacy of lichen extracts and silver nanoparticles against bacteria causing food poisoning. Asian J. Res. Chem. 3, 67–70.
Leela, K., and Anchana Devi, C. (2017). A study on the applications of silver nanoparticle synthesized using the aqueous extract and the purified secondary metabolites of Lichen Parmeliaperlata. Int. J. Pharm. Sci. Invent. 6, 42–59.
Marambio-Jones, C., and Hoek, E. M. V. (2010). A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. Nanopart Res. 12, 1531–1551. doi: 10.1007/s11051-010-9900-y
Martínez, J. L. (2008). Antibiotics and Antibiotic Resistance Genes in Natural Environments. Science 321, 365–367. doi: 10.1126/science.1159483
Mie, R., Samsudin, M. W., Din, L. B., Ahmad, A., Ibrahim, N., and Adnan, S. N. A. (2014). Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. Int. J. Nanomed. 9, 121–127. doi: 10.2147/ijn.s52306
Rai, H., and Gupta, R. K. (2019). Biogenic fabrication, characterization, and assessment of antibacterial activity of silver nanoparticles of a high altitude Himalayan lichen-Cladoniarangiferina (L.) Weber ex FH Wigg. Trop. Plant Res. 6, 293–298. doi: 10.22271/tpr.2019.v6.i2.037
Ruparelia, J. P., Chatterjee, A. K., Duttagupta, S. P., and Mukherji, S. (2008). Strain specificity in antimicrobial activity of silver and copper nanoparticles. ActaBiomaterialia 4, 707–716. doi: 10.1016/j.actbio.2007.11.006
Safarkar, R., Rajaei, G. E., and Khalili-Arjagi, S. (2020). The study of antibacterial properties of iron oxide nanoparticles synthesized using the extract of lichen Ramalinasinensis. Asian J. Nanosci. Mater. 3, 157–166.
Sánchez-López, E., Esteruelas, G., Bonilla, L., Lopez-Machado, A. L., Galindo, R., and Camins, A. (2020). Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 10:292.
Senthil, P. S., Ramanujam, J. R., and Sudha, S. S. (2019). Antibacterial activity of silver nanoparticles synthesized by using lichens Heterodermia boryi and Parmotrema stuppeum. Int. J. Pharm. Biol. Sci. 9, 1397–1402.
Siddiqi, K. S., Rashid, M., Rahman, A., Tajuddin, Husen, A., and Rehman, S. (2018). Biogenic fabrication and characterization of silver nanoparticles using aqueous-ethanolic extract of lichen (Usnealongissima) and their antimicrobial activity. Biomater. Res. 22:23.
Slavin, Y. N., Asnis, J., Häfeli, U. O., and Bach, H. (2017). Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15:65. doi: 10.1186/s12951-017-0308-z
Wang, L., Hu, C., and Shao, L. (2017). The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 12, 1227–1249. doi: 10.2147/ijn.s121956
Keywords: lichens, antimicrobial, nanoparticles, green synthesis, applications
Citation: Rattan R, Shukla S, Sharma B and Bhat M (2021) A Mini-Review on Lichen-Based Nanoparticles and Their Applications as Antimicrobial Agents. Front. Microbiol. 12:633090. doi: 10.3389/fmicb.2021.633090
Received: 24 November 2020; Accepted: 03 February 2021;
Published: 12 March 2021.
Edited by:
Sougata Ghosh, RK University, IndiaReviewed by:
Sejal Shah, RK University, IndiaCopyright © 2021 Rattan, Shukla, Sharma and Bhat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mamta Bhat, bWFtdGFiaGF0MTJvY3RAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.