
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol., 28 January 2021
Sec. Food Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.631254
This article is part of the Research TopicInsights of Gut Microbiota: Probiotics and Bioactive CompoundsView all 21 articles
Elderly people are an important part of the global population who suffer from the natural processes of senescence, which lead to changes in the gut microbiota composition. These modifications have a great impact on their quality of life, bringing a general putrefactive and inflammatory status as a consequence. Some of the most frequent conditions related to this status are constipation, undernutrition, neurodegenerative diseases, susceptibility to opportunistic pathogens, and metabolic disbalance, among others. For these reasons, there is an increasing interest in improving their quality of life by non-invasive treatments such as the consumption of probiotics, prebiotics, and synbiotics. The aim of the present mini-review is to describe the benefits of these functional supplements/food according to the most recent clinical and pre-clinical studies published during the last decade. In addition, insights into several aspects we consider relevant to improve the quality of future studies are provided.
Worldwide, elderly people (aged 65 or older) represent 12.4% of the global population (Bedani et al., 2016). According to the European Union, the share of people aged 80 years or above is projected to have a two-and-a-half-fold increase between 2019 and 2100, from 5.8 to 14.6% (Eurostat Statistics Explained, 202011).
This rapid evolution has a significant impact on national public health institutes, social services, and health care systems. Consequently, elderly people are gaining increasing interest since they suffer from chronic health conditions that affect their quality of life, leading to a high demand of health services in general (Bedani et al., 2016). For this reason, new options for preserving their health have been investigated, with being functional food a potential option. In this context, probiotics, prebiotics, and synbiotics are worth studying since the scientific evidence about their beneficial effects on gut microbiota homeostasis is constantly increasing. While probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Hill et al., 2014), the term prebiotic is defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (Gibson et al., 2017). From their combination, the term synbiotics arose, which is defined as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” (Swanson et al., 2020).
Several phenomena take place during aging, among them, a low-level systemic inflammation during immunosenescence was described by Guigoz et al. (2008). The term “senescence” refers mainly to non-pathological (biological and physiological) processes dependent on age, while the term “aging” refers to physiological and pathological changes (Rowe, 1997; Troen, 2003). In other words, cellular senescence refers to a permanent state of cell cycle arrest that occurs under different stress factors. Therefore, it works as a cellular defense mechanism that prevents cell damage, and it occurs during different physiological (and sometimes pathological) processes, such as tissue remodeling, cancer, injury, and aging (Calcinotto et al., 2019). During immunosenescence, a global reduction in the ability to cope with a wide range of stressors occurs, with a concomitant progressive increase toward a pro-inflammatory status, a process that seems to be mediated by the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB factor; Salminen et al., 2008). In addition, inflammatory responses may be caused by the leakage of the intestinal barrier, allowing microbial and/or microbial components to filtrate (Shalim et al., 2019). On the other hand, modifications of the T-cell repertoire have been associated with an increase in morbidity caused by infectious diseases (Vasto et al., 2006), and a low activity of natural killer (NK) cells has been reported as well (Jing et al., 2007).
The gut microbiota of elderly subjects also suffer a gradual shift toward a reduced bacterial diversity: a decline in beneficial microorganisms and an increase of facultative anaerobic bacteria. In general, lower levels of Firmicutes (mainly Clostridium cluster XIVa and Faecalibacterium prausnitzii) and Actinobacteria (mainly bifidobacteria), together with increased populations of Proteobacteria, have been found when comparing with adults (Salazar et al., 2017). Elderly people may also have reduced dentition and chewing strength, together with a loss of appetite, which can lead to a limited variety of food ingredients that support the limited microbial diversity (O’Toole and Claesson, 2010). These changes are responsible for a decrease in short chain fatty acids (SCFA) production and shift from a predominantly saccharolytic metabolism (normally observed in adults) toward a predominantly putrefactive metabolism (Woodmansey et al., 2004). SCFAs are volatile fatty acids produced by the gut microbiota in the large bowel from food components that are unabsorbed/undigested in the small intestine. They exert beneficial health effects, such as protection against pathogens and shaping the gut environment, apart from presenting anti-inflammatory properties (Ríos-Covián et al., 2016). Furthermore, they have been associated with the upregulation of the anti-inflammatory cytokines in vitro, together with the induction of CD4+CD25+ Treg cells (Asarat et al., 2016).
In this context, considering that the inflammatory status of this group is highly modulated by the gut microbiota (Guigoz et al., 2008) and that external factors such as diet and lifestyle are crucial for this modulation (O’Toole and Claesson, 2010), functional food turns to be an attractive target to study.
In the present review, we intend to revise the latest studies about the application of probiotics, prebiotics, and synbiotics (solely or in different food matrices) on elderly subjects and the effects these strategies have on their health and the quality of life in general. Besides, some guidelines we consider useful for the development of future products aimed at this part of the population are provided.
According to O’Toole and Jeffery (2015), the composition of microbiota does not suddenly alter at a certain age, but it is a gradual process dependent on several factors, such as gender, location, diet, lifestyle, physical activity, immune system functionality, and the use of medication (O’Toole and Jeffery, 2015; Komanduri et al., 2019). In general, a reduced microbial diversity has been observed, with Bacteroides and Firmicutes as the most dominant phyla (Claesson et al., 2011; Biagi et al., 2012; Odamaki et al., 2016). Many studies have reported a decline in viable counts of Bacteroides with increased age, together with reduced diversity within this genus (Bartosch et al., 2004; Woodmansey et al., 2004; Woodmansey, 2007). This may have a direct impact on digestion since bacteria from this genus are believed to play an important role in the digestion of polysaccharides in the colon (Flint et al., 2012). Furthermore, a decrease in starch and sucrose metabolism, galactose and pyruvate metabolism, and glycolysis/gluconeogenesis has been found using shotgun sequencing, changes that were accompanied by a loss of fibrolytic microorganisms belonging to Eubacterium, Bifidobacterium, and Faecalibacterium genera (Rampelli et al., 2013b). A rise in facultative anaerobes and proteolytic bacteria, such as fusobacteria, propionibacteria, and clostridia, has been reported as well, suggesting a trend toward putrefaction of the large bowel (Woodmansey, 2007). Another characteristic widely observed for this part of the population is a decline in the levels and diversity of bifidobacteria (Woodmansey et al., 2004; Arboleya et al., 2016), possibly leading to a reduced immune responsiveness and an increased susceptibility to gastrointestinal infections (Woodmansey, 2007). In some cases, reduced levels of Clostridium cluster XIVa and Faecalibacterium were described (O’Toole and Claesson, 2010; Salazar et al., 2013, 2019).
Among the elderly, age seems to be an important factor that determines the microbiota composition, as observed by Salazar et al. (2019). In this study, the levels of Akkermansia and Lactobacillus for a subgroup of elderly (>80 years old) were significantly higher than those observed in adult (<50 years old) and the younger elderly (50–80 years old) groups, respectively. In this sense, Biagi et al. (2010) found comparable diversity values of the gut microbiota between the elderly and young adults, while centenarians stood out as a separate population, with Bacteroidetes and Firmicutes still dominating the gut microbiota. However, some changes in the relative proportion of Firmicutes subgroups were observed in comparison with the younger adults, with a decrease in the Clostridium cluster XIVa, as observed elsewhere (Bartosch et al., 2004; Zwielehner et al., 2009). In addition, the authors described an increase in bacilli, a rearrangement of the Clostridium cluster IV (lower levels of F. prausnitzii in centenarians than in the younger elderly) and increased Proteobacteria. This last group contains many “pathobionts” bacteria, which, under some circumstances (e.g., inflammation), might induce pathology (Biagi et al., 2010). Some members of this group are Helicobacter hepaticus, segmented filamentous bacteria, Escherichia coli, and Enterococcus faecalis (Jochum and Stecher, 2020). Regarding SCFA production, several butyrate producers were found in lower amounts in centenarians than in other age groups, indicating a general decrease in SCFA levels with age (Salazar et al., 2013, 2019).
Bifidobacteria and lactobacilli have been widely considered health-promoting constituents of the microbiota (Bedani et al., 2016). Different strains of these genera were lately used as probiotics and proved to have many health benefits within the elderly, such as microbiota modulation, improvement of bowel movements, control of opportunistic bacteria, positive effects on mental conditions, stimulation of the innate immune system, increased vitamin intake, among other effects detailed in Supplementary Table S1 according to clinical and pre-clinical trials. Although the impact of prebiotics and synbiotics has been studied to a lesser extent, there are some recent clinical studies indicating positive health benefits as well. Several attempts to isolate probiotic strains from elderly people have been reported; for example, Silvi et al. (2003) isolated Limosilactobacillus fermentum and Bifidobacterium longum strains from elderly people (aged 65–87 years, Italy) as part of an EU-funded project, whose final objective was the future application of the isolated strains to design appropriate functional foods for the elderly. Similarly, Park et al. (2015) isolated L. fermentum as the most frequent species in fecal samples from longevity (>80 years) populations in Korea, highlighting the potential relevance of this particular species for the formulation of probiotic food or supplements for seniors.
Most of the latest research carried out in this field implied clinical trials addressed to healthy elderly people (Supplementary Table S1). In general, the application of commercial probiotics (one strain or a cocktail) was the most chosen strategy among them. The effects observed indicate that the consumption of probiotics may positively impact the gut microbiota by increasing the levels of bifidobacteria or modifying subpopulations of lactobacilli (Nagata et al., 2011; Akatsu et al., 2013; Ostan et al., 2015). Furthermore, probiotics were associated with the ability to promote interactions between key constituents of the microbiota and the host epithelium (Eloe-Fadrosh et al., 2015), enhance the immunity response (Finamore et al., 2019; Yamamoto et al., 2019; Wang et al., 2020) and improve bowel movements (Nagata et al., 2015; Inoue et al., 2018; Aoyagi et al., 2019). Other health benefits were related to their ability to revert age-related increase of opportunistic pathogens, such as Clostridium difficile, involved in antibiotic-associated diarrhea that impact on nutrition and inflammatory status, exerting an important role in pathophysiological processes. In the elderly, C. difficile-associated diarrhea was linked with a reduction on the number of bifidobacteria (Hopkins and MacFarlane, 2002); for this reason, therapies based on the use of probiotics to correct the microbiota imbalance would be promising (Rampelli et al., 2013a). In this direction, Nagamine et al. (2019) reported that probiotics could reduce Clostridium difficile infection (CDI) among elderly patients who underwent proximal femoral fracture surgery, but at this moment, there is not enough information about their mechanisms of action, and the current guidelines do not recommend their administration (McDonald et al., 2018). Notwithstanding the promising results, other studies reported controversial ones, most of them having no significant results (Mallina et al., 2018; Sofian et al., 2019).
When considering the extra-intestinal positive effects, probiotics were able to enhance the oral health of elderly by the control of Candida and hyposalivation, common problems among this group (Ishikawa et al., 2014; Kraft-Bodi et al., 2015; Lee et al., 2019). Other reports have also associated probiotic consumption with positive effects on respiratory tract infections by reducing the duration (Guillemard et al., 2010; Fujita et al., 2013) or accelerating the healing process in patients with acute distal radius fracture (Lei et al., 2016). Although probiotics showed potential effects on bone metabolism in different mouse models (Roberts et al., 2019, 2020), further clinical trials are required to assess this effect on the elderly.
Recently, brain-gut axis has received special attention, since the application of some probiotics on elderly subjects improved mental conditions, such as anxiety and depression, when combined with a 12-week resistance-training program which consisted of classes comprising a warm-up session, resistance training, and a cool-down session (Inoue et al., 2018). Probiotics also promoted mental flexibility and alleviated stress (Kim et al., 2020). Particularly, they were recommended for the treatment of different health conditions as the silent systematic inflammation and neuroinflammation that are frequently observed in the early stage of Alzheimer’s disease (Leblhuber et al., 2018). These authors reported positive results that indicate a probiotic supplement influenced by gut bacteria composition (increased levels F. prausnitzii), tryptophan metabolism and immune response. In this case, in spite of its limitations (a small sample size and the absence of placebo control), it was suggested that the increase of F. praunitzii could mitigate the cerebral accumulation of β-amyloid and lipopolysaccharides, which are overproduced during the pathogenesis of Alzheimer’s disease.
Other effects that probiotics exert on the elderly were associated with augmented levels of vitamins in the blood. For example, Valentini et al. (2015) reported increased concentration of vitamin B12 and folate in serum, accompanied by a reduction in plasma homocysteine in subjects that received a commercial probiotic supplementation. The lack of one of these vitamins may cause megaloblastic anemia and a series of neurological and mental symptoms in the elderly, as both vitamins play a crucial role in the cognitive function (Lokk, 2003). These increases were positively correlated with the change in fecal bifidobacterial concentrations for the subjects with low-grade inflammation. The authors suggested that, since the decrease in homocysteine levels was clinically relevant, the probiotic could provide protective effects against some aging-associated conditions (such as cardiovascular or neurological diseases). However, several limitations can be mentioned: It was an open label study without a placebo group, and it used biological instead of clinical endpoints.
On the other hand, the use of synbiotics (combination of probiotics and prebiotics) has also demonstrated similar beneficial effects on the gut microbiota (Supplementary Table S1). For example, synbiotics proved to increase the number of bifidobacteria and lactobacilli, improve the stool frequency and mucosal integrity, increase butyrate production, diminish pro-inflammatory response, and enhance lipid metabolism (Björklund et al., 2012; Granata et al., 2013; Macfarlane et al., 2013). In addition, synbiotics significantly decreased metabolic syndrome prevalence, several cardiovascular risk factors and markers of insulin resistance in elderly patients (Cicero et al., 2020). Regarding prebiotics, some clinical trials demonstrated that they have positive effects on the gut microbiota composition and immune responses as well (Walton et al., 2012; Alfa et al., 2017).
To sum up, Figure 1 shows, as a graphical representation, the physiological, nutritional and immune targets of intervention generally identified for elderly people and the possible effects of these functional supplements on this population.
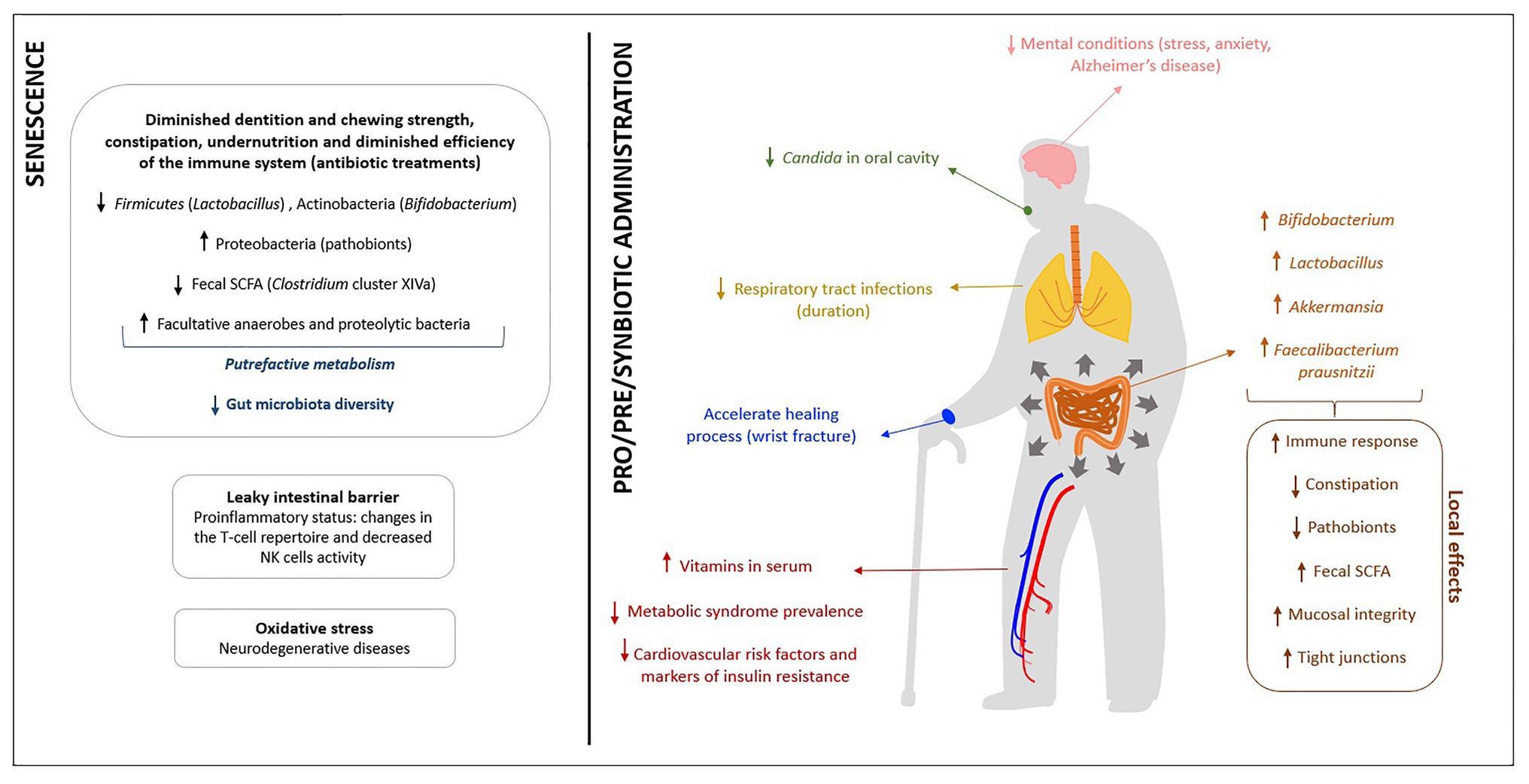
Figure 1. Summary of the main health effects attributed to probiotics, prebiotics and synbiotics in the elderly.
Recently, there have been several studies about the health benefits that probiotics exert on the elderly using different pre-clinical (in vivo) models, as summarized in Supplementary Table S1. In general, the most selected model for studying the advanced age is C57BL/6J mice, aged 18 months or more (Flurkey et al., 2007). With this murine model, along with other in vitro assays, Ahmadi et al. (2020) demonstrated that a probiotic cocktail prevented mice into undergoing a high-fat diet from microbiota dysbiosis, leaky gut, inflammation, and metabolic and physical dysfunctions. In this direction, Jeong et al. (2016) obtained similar results for a probiotic strain of the species Levilactobacillus brevis since the treatment was effective in modulating the gut microbiota, inhibited the expression of inflammatory markers, enhanced colonic tight junctions, and ameliorated colitis and memory impairment. Similarly, Vemuri et al. (2019) demonstrated that a probiotic strain of Lactobacillus acidophilus increased the abundances of beneficial bacteria, such as Akkermansia spp. and Lactobacillus spp., and enhanced the levels of butyrate while downregulating the production of inflammatory cytokines. An interesting result observed by Lee et al. (2016) indicated a probiotic strain provided by female C57BL/6J mice with healthy skin, active folliculogenesis, and hair growth, together with immunomodulation. Probiotics supplementation has also been associated with a positive impact on oxidative stress and inflammation in peripheral tissues in this strain of mice (Ni et al., 2019).
As shown in Supplementary Table S1, there are other murine models that were successfully applied, one of them consisting of using D-galactose to induce premature senescence on Sprague Dawley rats. The results suggest probiotics ameliorated aging-induced metabolic diseases, pathogens growth, microbiota dysbiosis, oxidative stress, inflammation, and alteration of gut metabolites (Hor et al., 2019a,b; Lew et al., 2020). In other works, the BALB/c strain was used, with or without D-galactose injection. Improvement of immunological markers (Molina et al., 2016), modulation of microbiota and protective effects on oxidative stress induced by D-galactose (Zhang et al., 2017) were reported with this model.
Finally, there are some other murine models used for specific studies. This is the case of Yang et al. (2020), who used SAMP8 mice to study the potential of probiotics to treat deficits of the microbiota-gut-brain axis and cognitive function in aging. On the other hand, transgenic B6 mice were used to analyze the effects probiotics have on Alzheimer’s disease, showing promising results on the glucose metabolism (improved glucose uptake) and on the disease progression (Bonfili et al., 2020).
From the analysis of the information provided in the present mini-review, guidelines to address future studies regarding the role of probiotics, prebiotics and synbiotics in aging could be proposed. Without focusing on specific age groups, the minimum criteria that apply exclusively to probiotic strains for their use in foods and dietary supplements were recently revised (Binda et al., 2020). Similar principles could be considered for the administration of probiotics, prebiotics, or synbiotics to elderly people with special focus on their particular needs.
Special attention should be payed when designing the experiences to ensure the reliability of the clinical trial itself and the correct publication of the results, which should be based on recognized guidelines, as the Good Clinical Practice guidelines of the International Council for Harmonization, ICH-GCP,2 and The Consolidated Standards of Reporting Trials.3 Some of the factors to consider are (i) the choice of adequate controls, (ii) blinding, (iii) design, and (iv) the selection of the elderly population sample (health or disease status, male or female). In order to diminish the variability among studies, researchers should be aware of the effect different ages could cause, since significant differences between young elderly people (65–80 years old), those aged >80 years and centenarians, have been previously reported (Biagi et al., 2010; Salazar et al., 2019). For this reason, homogenous groups are recommended to avoid skewing the results obtained. On balance, nutritional strategies for the elderly should be addressed from a holistic point of view considering their special nutritional needs, the high susceptibility to disease, and the frequent medicine (mainly antibiotics) intake, as a whole (Salazar et al., 2017).
The location where the research takes place has an important influence (elderly living in their homes, in a hospitalized environment, developed or developing countries, etc), since parameters such as diet and lifestyle are of great relevance (O’Toole and Jeffery, 2015; Salazar et al., 2017). In addition, the matrix in which probiotics, prebiotics, or synbiotics are delivered should be chosen carefully because it may have an impact on the overall results. Diminished dentition, chewing strength, and constipation are essential factors to consider when choosing the matrix. The dose, administration schedule, and duration of intervention must be contemplated as well, as too long treatments might be difficult to be followed in practice, this affecting the health effects expected. Therefore, functional food for seniors, containing probiotics, prebiotics, or synbiotics, should cover all these aspects and should be available in the market as more personalized treatments than products for the public in general.
The present work suggests that modifying the gut microbiota of the elderly population by the intake of functional food/supplements as probiotics, prebiotics, or synbiotics may be an effective and non-invasive strategy to counteract the natural consequences of aging, in most cases affected by the extended use of antibiotics, providing a better quality of life. At the same time, these functional products may be suitable, affordable, and economical to most elderly people. However, several concerns should be considered when future studies are addressed, to obtain not only reliable results but also treatments feasible to be applied and practical to be followed.
EA and AB conceptualized and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and Universidad Nacional del Litoral (UNL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.631254/full#supplementary-material
Ahmadi, S., Wang, S., Nagpal, R., Wang, B., Jain, S., Razazan, A., et al. (2020). A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 5:e132055. doi: 10.1172/jci.insight.132055
Ahmed, M., Prasad, J., Gill, H., Stevenson, L., and Gopal, P. (2007). Impact of consumption of different levels of Bifidobacterium lactis HN019 on the intestinal microflora of elderly human subjects. J. Nutr. Health Aging 11, 26–31.
Akatsu, H., Iwabuchi, N., Xiao, J. Z., Matsuyama, Z., Kurihara, R., Okuda, K., et al. (2013). Clinical effects of probiotic Bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. J. Parenter. Enter. Nutr. 37, 631–640. doi: 10.1177/0148607112467819
Alfa, M. J., Strang, D., Tappia, P. S., Graham, M., Van Domselaar, G., Forbes, J. D., et al. (2017). A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin. Nutr. 37, 797–807. doi: 10.1016/j.clnu.2017.03.025
Allen, S. J., Wareham, K., Wang, D., Bradley, C., Sewell, B., Hutchings, H., et al. (2013). A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhea in older people admitted to hospital: a multicentre, randomised, double-blind, placebo-controlled, parallel arm trial. Health Technol. Assess. 17, 1–140. doi: 10.3310/hta17570
Aoyagi, Y., Amamoto, R., Park, S., Honda, Y., Shimamoto, K., Kushiro, A., et al. (2019). Independent and interactive effects of habitually ingesting fermented milk products containing Lactobacillus casei strain shirota and of engaging in moderate habitual daily physical activity on the intestinal health of older people. Front. Microbiol. 10:1477. doi: 10.3389/fmicb.2019.01477
Arboleya, S., Watkins, C., Stanton, C., and Ross, R. P. (2016). Gut bifidobacteria populations in human health and aging. Front. Microbiol. 7:1204. doi: 10.3389/fmicb.2016.01204
Asarat, M., Apostolopoulos, V., Vasiljevic, T., and Donkor, O. (2016). Short-chain fatty acids regulate cytokines and Th17/Treg cells in human peripheral blood mononuclear cells in vitro. Immunol. Investig. 45, 205–222. doi: 10.3109/08820139.2015.1122613
Bartosch, S., Fite, A., Macfarlane, G. T., and McMurdo, M. E. T. (2004). Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 70, 3575–3581. doi: 10.1128/AEM.70.6.3575-3581.2004
Bartosch, S., Woodmansey, E. J., Paterson, J. C. M., McMurdo, M. E. T., and Macfarlane, G. T. (2005). Microbiological effects of consuming a synbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clin. Infect. Dis. 40, 28–37. doi: 10.1086/426027
Bedani, R., Isay Saad, S. M., and Sivieri, K. (2016). “Potential benefits of probiotics, prebiotics, and synbiotics on the intestinal microbiota of the elderly” in Probiotics, prebiotics, Synbiotics: Bioactive foods in health. eds. R. R. Watson and V. R. Preedy (London, UK: Academic Press, Elsevier), 525–538.
Biagi, E., Candela, M., Fairweather-Tait, S., Franceschi, C., and Brigidi, P. (2012). Ageing of the human metaorganism: the microbial counterpart. Age (Dordr.) 34, 247–267. doi: 10.1007/s11357-011-9217-5
Biagi, E., Nylund, L., Candela, M., Ostan, R., Bucci, L., Pini, E., et al. (2010). Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5:e10667. doi: 10.1371/journal.pone.0010667
Binda, S., Hill, C., Johansen, E., Obis, D., Pot, B., Sanders, M. E., et al. (2020). Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front. Microbiol. 11:1662. doi: 10.3389/fmicb.2020.01662
Björklund, M., Ouwehand, A. C., Forssten, S. D., Nikkilä, J., Tiihonen, K., Rautonen, N., et al. (2012). Gut microbiota of healthy elderly NSAID users is selectively modified with the administration of Lactobacillus acidophilus NCFM and lactitol. Age (Dordr.) 34, 987–999. doi: 10.1007/s11357-011-9294-5
Bonfili, L., Cecarini, V., Gogoi, O., Berardi, S., Scarpona, S., Angeletti, M., et al. (2020). Gut microbiota manipulation through probiotics oral administration restores glucose homeostasis in a mouse model of Alzheimer’s disease. Neurobiol. Aging 87, 35–43. doi: 10.1016/j.neurobiolaging.2019.11.004
Butler, C. C., Lau, M., Gillespie, D., Owen-Jones, E., Lown, M., Wootton, M., et al. (2020). Effect of probiotic use on antibiotic administration among care home residents: a randomized clinical trial. JAMA 324, 47–56. doi: 10.1001/jama.2020.8556
Calcinotto, A., Kohli, J., Zagato, E., Pellegrini, L., Demaria, M., and Alimonti, A. (2019). Cellular senescence: aging, cancer, and injury. Physiol. Rev. 99, 1047–1078. doi: 10.1152/physrev.00020.2018
Cicero, A. F. G., Fogacci, F., Bove, M., Giovannini, M., and Borghi, C. (2020). Impact of a short-term synbiotic supplementation on metabolic syndrome and systemic inflammation in elderly patients: a randomized placebo-controlled clinical trial. Eur. J. Nutr. doi: 10.1007/s00394-020-02271-8 [Epub ahead of print]
Claesson, M. J., Cusack, S., O’Sullivan, O., Greene-Diniz, R., De Weerd, H., Flannery, E., et al. (2011). Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. U. S. A. 108, 4586–4591. doi: 10.1073/pnas.1000097107
Eloe-Fadrosh, E. A., Brady, A., Crabtree, J., Drabek, E. F., Ma, B., Mahurkar, A., et al. (2015). Functional dynamics of the gut microbiome in elderly people during probiotic consumption. mBio 6, e00231–e00215. doi: 10.1128/mBio.00231-15
Finamore, A., Roselli, M., Donini, L. M., Brasili, D. E., Rami, R., Carnevali, P., et al. (2019). Supplementation with Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 mixture improves immunity in elderly humans (over 75 years) and aged mice. Nutrition 63–64, 184–192. doi: 10.1016/j.nut.2019.02.005
Flint, H. J., Scott, K. P., Duncan, S. H., Louis, P., and Forano, E. (2012). Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3, 289–306. doi: 10.4161/gmic.19897
Flurkey, K., Currer, J. M., and Harrison, D. E. (2007). “The mouse in aging research” in The mouse in biomedical research. 2nd Edn. eds. J. G. Fox, S. Barthold, M. Davisson, C. Newcomer, F. Quimby, and A. Smith (Burlington, MA: American College Laboratory Animal Medicine, Elsevier), 637–672.
Fonollá, J., Gracián, C., Maldonado-Lobón, J. A., Romero, C., Bédmar, A., Carrillo, J. C., et al. (2017). Effects of Lactobacillus coryniformis K8 CECT5711 on the immune response to influenza vaccination and the assessment of common respiratory symptoms in elderly subjects: a randomized controlled trial. Eur. J. Nutr. 58, 83–90. doi: 10.1007/s00394-017-1573-1
Fujita, R., Iimuro, S., Shinozaki, T., Sakamaki, K., Uemura, Y., Takeuchi, A., et al. (2013). Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: a multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. Am. J. Infect. Control 41, 1231–1235. doi: 10.1016/j.ajic.2013.04.005
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., et al. (2017). Expert consensus document: the international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502. doi: 10.1038/nrgastro.2017.75
Granata, M., Brandi, G., Borsari, A., and Gasbarri, R., and Di Gioia, D. (2013). Synbiotic yogurt consumption by healthy adults and the elderly: the fate of bifidobacteria and LGG probiotic strain. Int. J. Food Sci. Nutr. 64, 162–168. doi: 10.3109/09637486.2012.718742
Guigoz, Y., Doré, J., and Schiffrin, E. J. (2008). The inflammatory status of old age can be nurtured from the intestinal environment. Curr. Opin. Clin. Nutr. Metab. Care 11, 13–20. doi: 10.1097/MCO.0b013e3282f2bfdf
Guillemard, E., Tondu, F., Lacoin, F., and Schrezenmeir, J. (2010). Consumption of a fermented dairy product containing the probiotic lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 103, 58–68. doi: 10.1017/S0007114509991395
Hatakka, K., Ahola, A. J., Yli-Knuuttila, H., Richardson, M., Poussa, T., Meurman, J. H., et al. (2007). Probiotics reduce the prevalence of oral Candida in the elderly a randomized controlled trial. J. Dent. Res. 86, 125–130. doi: 10.1177/154405910708600204
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Hopkins, M. J., and MacFarlane, G. T. (2002). Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J. Med. Microbiol. 51, 448–454. doi: 10.1099/0022-1317-51-5-448
Hor, Y. Y., Lew, L. C., Jaafar, M. H., Lau, A. S. Y., Ong, J. S., Kato, T., et al. (2019a). Lactobacillus sp. improved microbiota and metabolite profiles of aging rats. Pharmacol. Res. 146:104312. doi: 10.1016/j.phrs.2019.104312
Hor, Y. Y., Ooi, C. H., Khoo, B. Y., Choi, S. B., Seeni, A., Shamsuddin, S., et al. (2019b). Lactobacillus strains alleviated aging symptoms and aging-induced metabolic disorders in aged rats. J. Med. Food 22, 1–13. doi: 10.1089/jmf.2018.4229
Ibrahim, F., Ruvio, S., Granlund, L., Salminen, S., Viitanen, M., and Ouwehand, A. C. (2010). Probiotics and immunosenescence: cheese as a carrier. FEMS Immunol. Med. Microbiol. 59, 53–59. doi: 10.1111/j.1574-695X.2010.00658.x
Inoue, T., Kobayashi, Y., Mori, N., Sakagawa, M., Xiao, J. Z., Moritani, T., et al. (2018). Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benefic. Microbes 9, 843–853. doi: 10.3920/BM2017.0193
Ishikawa, K. H., Mayer, M. P. A., Miyazima, T. Y., Matsubara, V. H., Silva, E. G., Paula, C. R., et al. (2014). A multispecies probiotic reduces oral Candida colonization in denture wearers. J. Prosthodont. 24, 194–199. doi: 10.1111/jopr.12198
Jeong, J. J., Kim, K. A., Hwang, Y. J., Han, M. J., and Kim, D. H. (2016). Anti-inflammaging effects of Lactobacillus brevis OW38 in aged mice. Benefic. Microbes 7, 707–718. doi: 10.3920/BM2016.0016
Jing, Y., Gravenstein, S., Rao Chaganty, N., Chen, N., Lyerly, K. H., Joyce, S., et al. (2007). Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp. Gerontol. 42, 719–732. doi: 10.1016/j.exger.2007.01.009
Jochum, L., and Stecher, B. (2020). Label or concept – what is a pathobiont? Trends Microbiol. 28, 789–792. doi: 10.1016/j.tim.2020.04.011
Kim, C. -S., Cha, L., Sim, M., Jung, S., Chun, W. Y., Baik, H. W., et al. (2020). Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. A Biol. Sci. Med. Sci. 76, 32–40. doi: 10.1093/gerona/glaa090
Kobayashi, Y., Kuhara, T., Oki, M., and Xiao, J. Z. (2019). Effects of Bifidobacterium breve a1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benefic. Microbes 10, 511–520. doi: 10.3920/BM2018.0170
Komanduri, M., Gondalia, S., Scholey, A., and Stough, C. (2019). The microbiome and cognitive aging: a review of mechanisms. Psychopharmacology 236, 1559–1571. doi: 10.1007/s00213-019-05231-1
Kraft-Bodi, E., Jørgensen, M. R., Keller, M. K., Kragelund, C., and Twetman, S. (2015). Effect of probiotic bacteria on oral Candida in frail elderly. J. Dent. Res. 94, 181S–186S. doi: 10.1177/0022034515595950
Lahtinen, S. J., Forssten, S., Aakko, J., Granlund, L., Rautonen, N., Salminen, S., et al. (2012). Probiotic cheese containing Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus NCFM® modifies subpopulations of fecal lactobacilli and Clostridium difficile in the elderly. Age (Dordr.) 34, 133–143. doi: 10.1007/s11357-011-9208-6
Lahtinen, S. J., Tammela, L., Korpela, J., Parhiala, R., Ahokoski, H., Mykkänen, H., et al. (2009). Probiotics modulate the Bifidobacterium microbiota of elderly nursing home residents. Age (Dordr.) 31, 59–66. doi: 10.1007/s11357-008-9081-0
Leblhuber, F., Steiner, K., Schuetz, B., Fuchs, D., and Gostner, J. M. (2018). Probiotic supplementation in patients with Alzheimer’s dementia ‐ an explorative intervention study. Curr. Alzheimer Res. 15, 1106–1113. doi: 10.2174/1389200219666180813144834
Lee, X., Vergara, C., and Lozano, C. P. (2019). Severity of Candida-associated denture stomatitis is improved in institutionalized elders who consume Lactobacillus rhamnosus SP1. Aust. Dent. J. 64, 229–236. doi: 10.1111/adj.12692
Lee, J., Yang, W., Hostetler, A., Schultz, N., Suckow, M. A., Stewart, K. L., et al. (2016). Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 16:69. doi: 10.1186/s12866-016-0686-7
Lei, M., Hua, L. M., and Wang, D. W. (2016). The effect of probiotic treatment on elderly patients with distal radius fracture: a prospective double-blind, placebo-controlled randomised clinical trial. Benefic. Microbes 7, 631–637. doi: 10.3920/BM2016.0067
Lew, L. C., Hor, Y. Y., Jaafar, M. H., Lau, A. S. Y., Khoo, B. Y., Sasidharan, S., et al. (2020). Effects of potential probiotic strains on the fecal microbiota and metabolites of D-galactose-induced aging rats fed with high-fat diet. Probiotics Antimicrob. Proteins 12, 545–562. doi: 10.1007/s12602-019-09545-6
Liu, Y. -P., Liu, X., and Dong, L. (2012). Lactulose plus live binary Bacillus subtilis in the treatment of elders with functional constipation. Zhonghua yi xue za zhi 92, 2961–2964. doi: 10.3760/cma.j.issn.0376-2491.2012.42.003
Lokk, J. (2003). News and views on folate and elderly persons. J. Gerontol. A Biol. Sci. Med. Sci. 58, M354–M361. doi: 10.1093/gerona/58.4.M354
Macfarlane, S., Cleary, S., Bahrami, B., Reynolds, N., and Macfarlane, G. T. (2013). Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment. Pharmacol. Ther. 38, 804–816. doi: 10.1111/apt.12453
Makino, S., Ikegami, S., Kume, A., Horiuchi, H., Sasaki, H., and Orii, N. (2010). Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 104, 998–1006. doi: 10.1017/S000711451000173X
Mallina, R., Craik, J., Briffa, N., Ahluwalia, V., Clarke, J., and Cobb, A. G. (2018). Probiotic containing Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophilus (ACTIMEL) for the prevention of Clostridium difficile associated diarrhea in the elderly with proximal femur fractures. J. Infect. Public Health 11, 85–88. doi: 10.1016/j.jiph.2017.04.001
Mañé, J., Pedrosa, E., Lorén, V., Gassull, M. A., Espadaler, J., Cuñé, J., et al. (2011). A mixture of Lactobacillus plantarum CECT 7315 and CECT 7316 enhances systemic immunity in elderly subjects. A dose-response, double-blind, placebo-controlled, randomized pilot trial. Nutr. Hosp. 26, 228–235. doi: 10.3305/nh.2011.26.1.5112
Maneerat, S., Lehtinen, M. J., Childs, C. E., Forssten, S. D., Alhoniemi, E., Tiphaine, M., et al. (2013). Consumption of Bifidobacterium lactis bi-07 by healthy elderly adults enhances phagocytic activity of monocytes and granulocytes. J. Nutr. Sci. 2, 1–10. doi: 10.1017/jns.2013.31
Manzoni, M. S. J., Rossi, E. A., Pauly-Silveira, N. D., Pinto, R. A., Roselino, M. N., Carlos, I. Z., et al. (2017). Consumption effect of a synbiotic beverage made from soy and yacon extracts containing Bifidobacterium animalis ssp. lactis BB-12 on the intestinal polyamine concentrations in elderly individuals. Food Res. Int. 99, 495–500. doi: 10.1016/j.foodres.2017.06.005
McDonald, L. C., Gerding, D. N., Johnson, S., Bakken, J. S., Carroll, K. C., Coffin, S. E., et al. (2018). Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 66, 987–994. doi: 10.1093/cid/ciy149
Molina, V., Médici, M., Villena, J., Font, G., and Pía Taranto, M. (2016). Dietary supplementation with probiotic strain improves immune-health in aged mice. Open J. Immunol. 06, 73–78. doi: 10.4236/oji.2016.63008
Moro-Garcia, M. A., Alonso-Arias, R., Baltadjieva, M., Benitez, C. F., Barrial, M. A. F., Ruisánchez, E. D., et al. (2013). Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age (Dordr.) 35, 1311–1326. doi: 10.1007/s11357-012-9434-6
Moroti, C., Souza Magri, L., De Rezende Costa, M., Cavallini, D. C. U., and Sivieri, K. (2012). Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 11, 1–8. doi: 10.1186/1476-511X-11-29
Nagamine, T., Matsumoto, Y., and Nakamura, M. (2019). Combination probiotics may prevent Clostridium difficile infection among elderly patients undergoing an orthopedic surgerya. Biosci. Microbiota. Food Health 38, 31–33. doi: 10.12938/bmfh.18-009
Nagata, S., Asahara, T., Ohta, T., Yamada, T., Kondo, S., Bian, L., et al. (2011). Effect of the continuous intake of probiotic-fermented milk containing Lactobacillus casei strain Shirota on fever in a mass outbreak of norovirus gastroenteritis and the faecal microflora in a health service facility for the aged. Br. J. Nutr. 106, 549–556. doi: 10.1017/S000711451100064X
Nagata, S., Asahara, T., Wang, C., Suyama, Y., Chonan, O., Takano, K., et al. (2015). The effectiveness of Lactobacillus beverages in controlling infections among the residents of an aged care facility: a randomized placebo-controlled double-blind trial. Ann. Nutr. Metab. 68, 51–59. doi: 10.1159/000442305
Ni, Y., Yang, X., Zheng, L., Wang, Z., Wu, L., Jiang, J., et al. (2019). Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol. Nutr. Food Res. 63, 1–14. doi: 10.1002/mnfr.201900603
O’Toole, P. W., and Claesson, M. J. (2010). Gut microbiota: changes throughout the lifespan from infancy to elderly. Int. Dairy J. 20, 281–291. doi: 10.1016/j.idairyj.2009.11.010
O’Toole, P. W., and Jeffery, I. B. (2015). Gut microbiota and aging. Science 350, 1214–1215. doi: 10.1126/science.aac8469
Odamaki, T., Kato, K., Sugahara, H., Hashikura, N., Takahashi, S., Xiao, J., et al. (2016). Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 16:90. doi: 10.1186/s12866-016-0708-5
Ostan, R., Béné, M. C., Spazzafumo, L., Pinto, A., Donini, L. M., Pryen, F., et al. (2015). Impact of diet and nutraceutical supplementation on inflammation in elderly people. Results from the RISTOMED study, an open-label randomized control trial. Clin. Nutr. 35, 812–818. doi: 10.1016/j.clnu.2015.06.010
Park, J. -S., Shin, E., Hong, H., Shin, H. -J., Cho, Y. -H., Ahn, K. -H., et al. (2015). Characterization of Lactobacillus fermentum PL9988 isolated from healthy elderly Korean in a longevity village. J. Microbiol. Biotechnol. 25, 1510–1518. doi: 10.4014/jmb.1505.05015
Rampelli, S., Candela, M., Severgnini, M., Biagi, E., Turroni, S., Roselli, M., et al. (2013a). A probiotics-containing biscuit modulates the intestinal microbiota in the elderly. J. Nutr. Health Aging 17, 166–172. doi: 10.1007/s12603-012-0372-x
Rampelli, S., Candela, M., Turroni, S., Biagi, E., Collino, S., Franceschi, C., et al. (2013b). Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging (Albany NY) 5, 902–912. doi: 10.18632/aging.100623
Ríos-Covián, D., Ruas-Madiedo, P., Margolles, A., Gueimonde, M., De los Reyes-Gavilán, C. G., and Salazar, N. (2016). Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 7:185. doi: 10.3389/fmicb.2016.00185
Roberts, J., Darby, T., Fernandes, L., Jones, R., and Drissi, H. (2019). Probiotic supplements accelerate bone repair and prevent systemic bone loss following femoral fracture (FS09-04-19). Curr. Dev. Nutr. 3:nzz044.FS09-04-19. doi: 10.1093/cdn/nzz044.FS09-04-19
Roberts, J. L., Liu, G., Darby, T. M., Fernandes, L. M., Diaz-Hernandez, M. E., Jones, R. M., et al. (2020). Bifidobacterium adolescentis supplementation attenuates fracture-induced systemic sequelae. Biomed. Pharmacother. 132:110831. doi: 10.1016/j.biopha.2020.110831
Salazar, N., Arboleya, S., Fernández-Navarro, T., de los Reyes-Gavilán, C. G., Gonzalez, S., and Gueimonde, M. (2019). Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: a cross-sectional study. Nutrients 11, 1–11. doi: 10.3390/nu11081765
Salazar, N., López, P., Valdés, L., Margolles, A., Suárez, A., Patterson, Á. M., et al. (2013). Microbial targets for the development of functional foods accordingly with nutritional and immune parameters altered in the elderly. J. Am. Coll. Nutr. 32, 399–406. doi: 10.1080/07315724.2013.827047
Salazar, N., Valdés-Varela, L., González, S., Gueimonde, M., and de los Reyes-Gavilán, C. G. (2017). Nutrition and the gut microbiome in the elderly. Gut Microbes 8, 82–97. doi: 10.1080/19490976.2016.1256525
Salminen, A., Huuskonen, J., Ojala, J., Kauppinen, A., Kaarniranta, K., and Suuronen, T. (2008). Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 7, 83–105. doi: 10.1016/j.arr.2007.09.002
Shalim, C. P., Yoewono, A., Utomo, Y., and Kuswardhani, R. T. (2019). The role of probiotic supplementation on the immune system in elderly. Int. J. Med. Biomed. Stud. 3, 250–255. doi: 10.32553/ijmbs.v3i8.492
Silvi, S., Verdenelli, M. C., Orpianesi, C., and Cresci, A. (2003). EU project Crownalife: functional foods, gut microflora and healthy ageing: isolation and identification of Lactobacillus and Bifidobacterium strains from faecal samples of elderly subjects for a possible probiotic use in functional foods. J. Food Eng. 56, 195–200. doi: 10.1016/S0260-8774(02)00249-2
Sofian, M., Eghbal, E., Ghaznavi-Rad, E., Ramezani, A., and Mohaghegh, P. (2019). The effect of probiotic yogurt on the frequency of Clostridium difficile in old hospitalized patients. J. Arak Uni. Med. Sci. 22, 52–65. doi: 10.32598/JAMS.22.4.50
Swanson, K. S., Gibson, G. R., Hutkins, R., Reimer, R. A., Reid, G., Verbeke, K., et al. (2020). The international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 17, 687–701. doi: 10.1038/s41575-020-0344-2
Tannock, G. W., Tiong, I. S., Priest, P., Munro, K., Taylor, C., Richardson, A., et al. (2011). Testing probiotic strain Escherichia coli Nissle 1917 (Mutaflor) for its ability to reduce carriage of multidrug-resistant E. coli by elderly residents in long-term care facilities. J. Med. Microbiol. 60, 366–370. doi: 10.1099/jmm.0.025874-0
Toda, T., Kosaka, H., Terai, M., Mori, H., Benno, Y., and Yamori, Y. (2005). Effects of fermented milk with Lactococcus lactis subsp. cremoris FC on defecation frequency and fecal microflora in healthy elderly volunteers. Nippon Shokuhin Kagaku Kogaku Kaishi 52, 243–250. doi: 10.3136/nskkk.52.243
Valentini, L., Pinto, A., Bourdel-Marchasson, I., Ostan, R., Brigidi, P., Turroni, S., et al. (2015). Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota ‐ the “RISTOMED project”: randomized controlled trial in healthy older people. Clin. Nutr. 34, 593–602. doi: 10.1016/j.clnu.2014.09.023
Van Puyenbroeck, K., Hens, N., Coenen, S., Michiels, B., Beunckens, C., Molenberghs, G., et al. (2012). Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am. J. Clin. Nutr. 95, 1165–1171. doi: 10.3945/ajcn.111.026831
Vasto, S., Malavolta, M., and Pawelec, G. (2006). Age and immunity. Immun. Ageing 3:2. doi: 10.1186/1742-4933-3-2
Vemuri, R., Gundamaraju, R., Shinde, T., Perera, A. P., Basheer, W., Southam, B., et al. (2019). Lactobacillus acidophilus DDS-1 modulates intestinal-specific microbiota, short-chain fatty acid and immunological profiles in aging mice. Nutrients 11, 1–23. doi: 10.3390/nu11061297
Vulevic, J., Drakoularakou, A., Yaqoob, P., Tzortzis, G., and Gibson, G. R. (2008). Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 88, 1438–1446. doi: 10.3945/ajcn.2008.26242
Vulevic, J., Juric, A., Walton, G. E., Claus, S. P., Tzortzis, G., Toward, R. E., et al. (2015). Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 114, 586–595. doi: 10.1017/S0007114515001889
Walton, G. E., van den Heuvel, E. G. H. M., Kosters, M. H. W., Rastall, R. A., Tuohy, K. M., and Gibson, G. R. (2012). A randomised crossover study investigating the effects of galacto-oligosaccharides on the faecal microbiota in men and women over 50 years of age. Br. J. Nutr. 107, 1466–1475. doi: 10.1017/S0007114511004697
Wang, P., Yin, X., Chen, G., Li, L., Le, Y., Xie, Z., et al. (2020). Perioperative probiotic treatment decreased the incidence of postoperative cognitive impairment in elderly patients following non-cardiac surgery: a randomised double-blind and placebo-controlled trial. Clin. Nutr. 40, 64–71. doi: 10.1016/j.clnu.2020.05.001
Woodmansey, E. J. (2007). Intestinal bacteria and ageing. J. Appl. Microbiol. 102, 1178–1186. doi: 10.1111/j.1365-2672.2007.03400.x
Woodmansey, E. J., McMurdo, M. E. T., Macfarlane, G. T., and Macfarlane, S. (2004). Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl. Environ. Microbiol. 70, 6113–6122. doi: 10.1128/AEM.70.10.6113-6122.2004
Yamamoto, Y., Saruta, J., Takahashi, T., To, M., Shimizu, T., Hayashi, T., et al. (2019). Effect of ingesting yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on influenza virus-bound salivary IgA in elderly residents of nursing homes: a randomized controlled trial. Acta Odontol. Scand. 77, 517–524. doi: 10.1080/00016357.2019.1609697
Yang, X., Yu, D., Xue, L., Li, H., and Du, J. (2020). Probiotics modulate the microbiota–gut–brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 10, 475–487. doi: 10.1016/j.apsb.2019.07.001
Zaharoni, H., Rimon, E., Vardi, H., Friger, M., Bolotin, A., and Shahar, D. R. (2011). Probiotics improve bowel movements in hospitalized elderly patients ‐ the proage study. J. Nutr. Health Aging 15, 215–220. doi: 10.1007/s12603-010-0323-3
Zhang, J., Zhao, X., Jiang, Y., Zhao, W., Guo, T., Cao, Y., et al. (2017). Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir. J. Dairy Sci. 100, 6025–6041. doi: 10.3168/jds.2016-12480
Zhao, J., Tian, F., Yan, S., Zhai, Q., Zhang, H., and Chen, W. (2018). Lactobacillus plantarum CCFM10 alleviating oxidative stress and restoring the gut microbiota in d-galactose-induced aging mice. Food Funct. 9, 917–924. doi: 10.1039/C7FO01574G
Zwielehner, J., Liszt, K., Handschur, M., Lassl, C., Lapin, A., and Haslberger, A. G. (2009). Combined PCR-DGGE fingerprinting and quantitative-PCR indicates shifts in fecal population sizes and diversity of Bacteroides, bifidobacteria and Clostridium cluster IV in institutionalized elderly. Exp. Gerontol. 44, 440–446. doi: 10.1016/j.exger.2009.04.002
Keywords: microbiota, elderly, probiotic, prebiotic, synbiotic, health
Citation: Ale EC and Binetti AG (2021) Role of Probiotics, Prebiotics, and Synbiotics in the Elderly: Insights Into Their Applications. Front. Microbiol. 12:631254. doi: 10.3389/fmicb.2021.631254
Received: 19 November 2020; Accepted: 04 January 2021;
Published: 28 January 2021.
Edited by:
Eugenia Bezirtzoglou, Democritus University of Thrace, GreeceReviewed by:
Parameth Thiennimitr, Chiang Mai University, ThailandCopyright © 2021 Ale and Binetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana G. Binetti, YW5hYmluZXR0aUBmaXEudW5sLmVkdS5hcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.