- 1Department of Biology, College of Science, Taif University, Taif, Saudi Arabia
- 2Applied Mycology Group, Environment and AgriFood Theme, Cranfield University, Cranfield, United Kingdom
Pistachio nuts are an economically important commodity produced by many countries. They can be colonized by mycotoxigenic fungi, especially Aspergillus flavus, resulting in contamination with aflatoxins (AFs), especially aflatoxin B1 (AFB1), a Class 1a carcinogen. The objectives were to examine the effect of interactions between the two key abiotic factors, temperature and water activity (aw) on (a) in vitro growth and AFB1 production by four strains of A. flavus isolated from pistachio nuts, on a milled pistachio nut medium modified ionically (NaCl) and non-ionically (glycerol) in the range 20–35°C and 0.995–0.85 aw, (b) colonization of layers of raw pistachio nuts stored at different interacting temperature x aw conditions and on relative AFB1 production and (c) develop models to produce contour maps of the optimal and marginal boundary conditions for growth and AFB1 production by up to 4 strains of this species. On pistachio nut-based media, optimum growth of four strains of A. flavus was at 0.98–0.95 aw and 30–35°C. Optimum AFB1 production was at 30–35°C and 0.98 aw. No significant differences in growth was found on ionic and non-ionically modified media. Colonization of layers of raw pistachio nuts was slower and contamination with AFB1 significantly less than in in vitro studies. Contour maps based on the pooled data for up to four strains (in vitro, in situ) showed the optimum and marginal conditions for growth and AFB1 production. These data can be used to identify those conditions which represent a high, intermediate or low risk of colonization and AFB1 contamination in the pistachio nut processing chain. These results are discussed in the context of the development of appropriate intervention strategies to minimize AFB1 contamination of this economically important commodity.
Introduction
Pistachio nuts (Pistacia vera L.) are an economically important tree nut crop for many countries including the United States, Iran, Southern Europe and China. Of these, Iran and the United States are the major producers and exporters of pistachio nuts (FAOSTAT, 2020). Pistachio nut quality and safety has thus become an important factor because they are prone to infection by species from the Aspergillus section Flavi group, especially A. flavus pre-harvest, if the shells become split early, or due to poor post-harvest drying or storage practices (Kaminiaris et al., 2020). This can result in significant contamination with aflatoxins (AFs). This has resulted in many countries including the EU having strict legislative limits on the maximum contamination levels with aflatoxin B1 (AFB1) or total AFs allowable. Indeed, for a few years Iranian pistachio nuts were excluded for importation into EU countries because of batches consistently exceeding the prevailing legislative limits for AFs (Bui-Klimke et al., 2014). This required action, especially in the harvest and post-harvest phases of the chain to be improved to reduce the contamination levels with these carcinogenic mycotoxins to levels to meet the legislative maximum limits.
Pistachio nuts in various processed forms are very popular in the Middle East and have been imported into most countries including the Kingdom of Saudi Arabia (KSA) (Mozaffari, 2012). Examination of a range of such pistachio nuts from markets in KSA originating from various producers and processors showed that Aspergillus section Flavi strains was present in many of the samples as well as contamination with AFs (Nawar, 2008; Baazeem, 2018). In addition, in other countries including the United States, Iran, Spain and Greece A. flavus has often been the predominant species encountered in pistachio nuts (Ciegler, 1972; Mojtahedi et al., 1979; Fernane et al., 2010a,b; Varga et al., 2011).
There is interest in a better understanding of the key abiotic factors which affect infection and colonization of pistachio nuts pre-harvest during early shell splitting, and post-harvest during drying and storage (Georgiadou et al., 2012). For example, drought stress can lead to increased shell spliting and allow inoculum of A. flavus to colonize the nuts and contaminate them with AFs (Sommer et al., 1986; Doster and Michailides, 1995; Kaminiaris et al., 2020). In addition, harvested pistachio nuts are very hygroscopic, because of their high lipid content, and can re-absorb moisture from the atmosphere, especially during storage or transport that increases the water availability to levels that can become conducive to growth of such xerophilic fungi and AFs contamination. Generally, to maintain and conserve quality, pistachios need to be dried to <6–7% moisture content (= ≤0.70 water activity, aw) and then stored without an increase in water availability during storage or transport and processing phases to avoid the risk of A. flavus colonization and contamination with AFs.
It is thus important to understand the relationship between interacting abiotic factors, especially of aw and temperature on the colonization by A. flavus and contamination of the pistachio nuts with AFB1. Some studies have examined germination, growth and AFB1 production by single strains of A. flavus isolated in Spain on pistachio nut-based media and on stored pistachios (Marin et al., 2012; Aldars-García et al., 2015). Interestingly, having tested different storage m.c. conditions the studies suggested that storage at ≤10% was necessary to minimize AFB1 contamination of pistachios to below the EU legislative levels. However, comparisons were not made between different strains of A. flavus isolated from raw or roasted, salted nuts. While there is a significant amount of data on A. flavus ecology and water relations in maize and peanuts, less is available on pistachio nuts (Giorni et al., 2008; Abdel-Hadi et al., 2012).
The objectives of this study were to examine the effect of interacting abiotic factors of aw x temperature on growth and AFB1 production by four different A. flavus strains isolated from pistachio nuts, on (a) pistachio nut-based media and (b) in stored raw pistachio nuts. The data was used to build boundary models for growth and AFB1 production to identify the overall optimal and marginal conditions which represent the highest and lowest risks for growth and toxin contamination.
Materials and Methods
Aspergillus flavus Strains
Four strains of A. flavus isolated from pistachio nut samples were molecularly identified using two sets of primers (ITS 1/2, ITS 3/4; Supplementary Table 1). They were coded as AB3, AB4, AB5, and AB10 and identification was further confirmed by comparison with a characterized type strain of A. flavus (NRRL 3357) from the Agricultural Research Service Culture Collection (United States), isolated from maize grain (Supplementary Table 2). They were all aflatoxin B1 producers.
Preparation of in vitro Growth Media
The growth medium used was a 3% milled pistachio nut agar (PNA). To prepare this medium, raw unsalted pistachio nuts were milled to a powder in a homogeniser. The milled pistachio powder was then sieved to obtain a uniform size. Thirty grams (30 g) of the pistachio powder and 20 g technical agar (Thermo Fisher Scientific Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) and 0.05 g chloramphenicol (antibacterial agent) was added to 1 L distilled water for the basal medium (0.99 aw). The aw was modified using either the ionic solute NaCl, or the non-ionic solute glycerol, to 0.85, 0.88, 0.90, 0.93, 0.95, 0.98, and 0.995 aw (Lang, 1967; Dallyn and Fox, 1980). For the ionic solute NaCl concentrations were directly added to the PNA media prior to autoclaving. For glycerol-amended media, the glycerol/water mixtures were made up, shaken vigorously and then added like water, to the pistachio nut flour and agar. The treatments were then autoclaved at 121°C for 15 min. After autoclaving, the PNA treatments were cooled, mixed thoroughly, poured into 9 cm sterile Petri plates (17.5–20 ml per plate) and allowed to completely cool and solidify. The aw was measured using an Aqualab 4TE (Decagon Instruments). The media treatments were enclosed in separate closed polyethylene bags and stored at 4°C until use.
Fungal Inoculation and Growth Rate Measurement of Aspergillus flavus Isolates on Milled Pistachio Nut-Based Agar Media
The PNA plates were equilibrated at 25°C and then centrally inoculated with the strains of A. flavus (4 isolates). Inoculum consisted of a conidial spore suspension of each strain made from fresh 5–7 day old growing cultures on PNA at 25°C. The culture surface was gently scraped with a sterile loop and conidia were transferred into sterile 25 ml Universal glass tubes containing 10 ml sterile water +0.1% Tween 80 solution (Tween 80, ACROS organics). The concentration of the spore suspension was determined using a haemocytometer (Olympus BX40 microscope, Microoptical Co.; slide Marienfeld superior, Germany; microscope glass cover slips, No 3, 18 × 18 mm, Chance proper LTD, United Kingdom) and adjusted by dilution with sterile water to 106 spores ml–1. The treatments and replicates were centrally inoculated with 10 μL of the spore suspension. The inoculated treatments and replicates were incubated at 20, 25, 30, and 35°C. Growth was assessed by measuring colony diameters of A. flavus every day for up to 10 days. Measurements of three replicates of each treatment were recorded on an excel sheet. The radial growth rates (mm/d) were obtained by computing the radial extension rate into the y = mx + c equation, where y was the radius (mm), x was day (d) and m was the growth rate (mm/d). c was set to intercept at 0 by assuming that at day 0, the radius was 0.
Colonization of Pistachio Nuts Under Different Temperature × Water Activity Conditions by Strains of Aspergillus flavus
Moisture adsorption curve for pistachio nuts: The pistachio nuts used in this study were gamma irradiated at 12–15 kGys (Synergy Health Sterilization United Kingdom Ltd., Swindon, Wiltshire, United Kingdom) to remove any resident microbiota present. To accurately modifiy the aw of these raw pistachio nuts, a water adsorption curve was developed. The relationship between added water and aw was obtained by adding known amounts of water to 5 g sub-samples of raw pistachio nuts in 25 mL Universal glass bottles. These were shaken, sealed and left at 4°C overnight. After thorough mixing and equilibration at 25°C, the aw was determined for each sub-sample using the Aqualab 4TE water activity meter (Aqualab 4TE; Decagon Devices, Inc., Pullman, WA, United States). This water adsorption curve was subsequently used to accurately determine the amounts of water necessary to obtain the target aw levels in the experimental studies. We used the following modified aw levels of the raw pistachio nuts: 0.88, 0.90, 0.93, 0.95, 0.98, and 0.995 (= 9.5–10, 11–12, 13–14, 18–19, 26–27, and 32–35% m.c.).
Fungal Inoculation and Growth Rate Measurement of Aspergillus flavus Isolates on Pistachio Nuts
Single layers of the raw pistachio nuts were spread into 9 cm sterile Petri plates in a flow bench. These were centrally inoculated with the individual strain (AB3 and AB10) of A. flavus using an agar plug (4 mm diameter) of germinating spores of each strain. The experiment was carried out with three replicates per treatment at 20, 25, 30, and 35°C and carried out twice. The Petri plates were placed in large surface-sterilized plastic chambers where the ERH was maintained with glycerol/water solutions (approx. 2 × 750 mL) to the target aw levels of the nut treatments. The colonization rates were measured on a daily basis for up to 10 day. The glycerol/water solutions were replaced after 5 days with fresh solutions.
Aflatoxin B1 Quantification
Preparation of aflatoxin standards: A 200 μL stock solution of aflatoxins (B1, B2, G1, and G2) standard in methanol containing 250 ng AFB1 was prepared and pipetted into 2 mL Eppendorf tubes for overnight evaporation until dryness in a fume hood similar to the samples.
In vitro Aflatoxin B1 Analyses
Colony Extraction
Initially agar plugs were cut out across the diameter of colonies using a surface sterilized 4 mm diameter cork borer (approx. 4–6). The agar plugs were placed in pre-weighed 2 mL Eppendorf tube and weighed again. Five-hundred μL of high performance liquid chromatography (HPLC)-grade chloroform was added to the tubes and shaken for 30 min using a KS 501 digital orbital shaker (IKA® Werke GmbH & Co., KG, Germany). The chloroform extract was transferred to a new Eppendorf tube, dried gently under air for derivatisation.
Derivatisation of Aflatoxin B1 Extract
Derivatisation of the AFB1 extract was performed according to the AAOC method (Kok, 1994). First, 200 μL hexane was added to the tube followed by 50 μL of triflouroacetic acid. The mixture was vortexed for 30 s and left for 5 min. A mixture of water:acetonitrile (9:1) was then added to the tube, and vortexed for 30 s and left for 10 min to allow for separation of the layers. Then the aqueous layer was filtered using a syringe nylon filter (13 mm × 0.22 μm; Jaytee Biosciences Ltd., United Kingdom) into amber salinized 2 mL HPLC vials (Agilent, United States) before HPLC analysis. All analytical reagents used were HPLC-grade.
Quantification of Aflatoxin B1 With High Performance Liquid Chromatography
A reverse-phase HPLC with fluorescence detection was used to confirm the identity and quantify AFB1. An Agilent 1200 series HPLC system was used for the analysis. It consisted of an in-line degasser, auto sampler, binary pump and a fluorescence detector (excitation and emission wavelengths of 360 and 440 nm, respectively). Separation was achieved using a C18 column (Phenomenex Gemini; 150 × 4.6, 3 μm particle size; Phenomenex, United States) with a Phenomenex Gemini C18 3 mm, 3 μm guard cartridge. Isocratic elution with methanol:water:acetonitrile (30:60:10, v/v/v) as the mobile phase was performed at a flow rate of 1.0 mL/min. The injection volume was 20 μL. A set of standards was injected (1–5 ng AFB1, AFB2, AFG1, and AFG2 per injection) and standard curves were generated by plotting the area underneath the peaks against the amounts of AFB1 standard injected.
Quantification of Aflatoxin B1 in Pistachio Nuts
The dried pistachio nut samples (25 g) were ground and weighed. The background aflatoxin B1 levels in the nuts used in the experiments was 0.015 ng/g. This was taken into account in the final quantification of the results. Acetonitrile/water 60/40 (100 mL) was used as an extraction solvent. The mixture was blended for 3 min and the extract filtered into a smaller sample container. PBS buffer was used for sample dilution, then the diluted extract was passed through an Immunoaffinity Column (IAC; AflaStarTM; Romer Labs, Austria) with a flow rate between 1 and 3 mL/min. The column was rinsed with 2 × 10 mL sterile distilled water. HPLC-grade methanol (1.5–3 mL) was then applied to the column and the eluent was collected in a new vial and left to dry overnight before the derivatisation step as detailed previously.
Statistical Analysis
All experiments were carried out twice. Three replicates per treatment were used in all experiments. Analysis of Variance (ANOVA) was applied to analyze the variation of means with 95% confidence interval. Normal distribution of data was checked by the normality test Kolmogorov–Smirnov using Minitab statistical software. Fisher’s Least Significant Difference (LSD) was used to identify differences between the means with p < 0.05 as the criterion for significant difference using the same statistical software.
Individual fungal growth rate (mm/day) and AFB1 production (ng/mL) as well as the pooled data sets for the different strains on PNA media or on pistachio nuts were fitted using non-linear regression with the software package Statgraphics Centurion 18
Contour maps were built using data from the predicted formula in Sigma Plot 14.
Results
Effect of Temperature × Water Activity on Growth and Contour Maps for Optimum and Marginal Conditions for Aspergillus flavus Strains on Milled Pistachio Nut-Based Media Modified Ionically or Non-ionically
An example of the effect of temperature x aw on the relative growth rates of A. flavus strain AB10 on the PNA solute modified media is shown in Figure 1. Optimum conditions for growth were between 30–35°C and 0.95–0.98 aw. Growth rates were similar on both NaCl- and glycerol-amended PNA media although the strain was slightly more sensitive to the ionic solute at marginal conditions for growth (e.g., 0.90 aw).
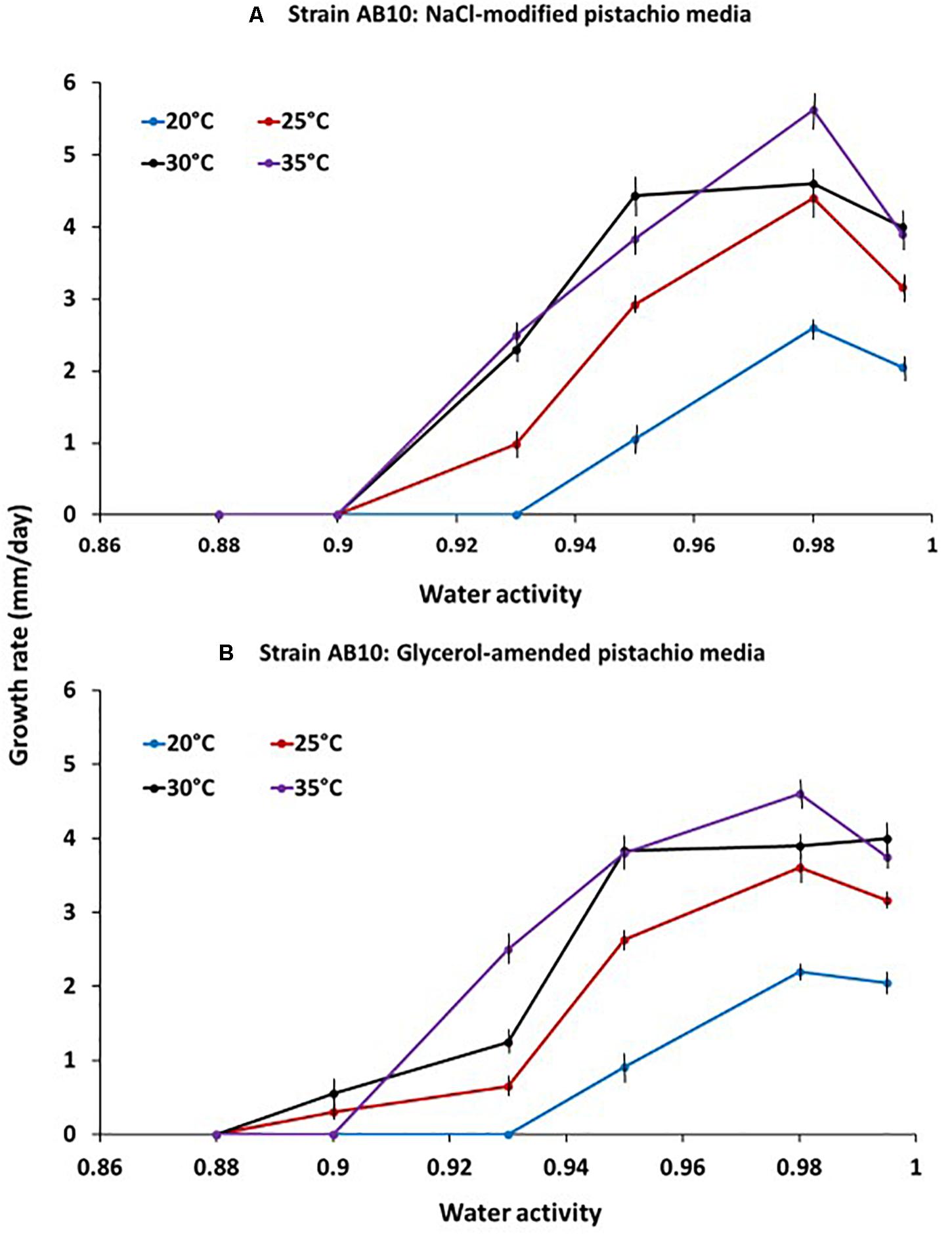
Figure 1. Effect of temperature x water activity on growth of A. flavus strain AB10 on a milled pistachio nut agar medium modified with either (A) NaCl or (B) glycerol. Based on growth over a 10 days incubation period. Bars represent the Standard Error of the means.
The contour maps developed for this strain (AB10) and for the pooled data for the four strains examined (AB3, AB4, AB5, and AB10) are shown in Figure 2. This clearly shows that on both solute modified media optimum growth rates are at >30°C and >0.98 aw for strain AB10 (Figures 2A,B) and also for the pooled data for the 4 strains examined (Figures 2C,D). Boundary conditions were close to 0.90–0.91 aw over the temperature range examined for both types of solute-modified PNA media.
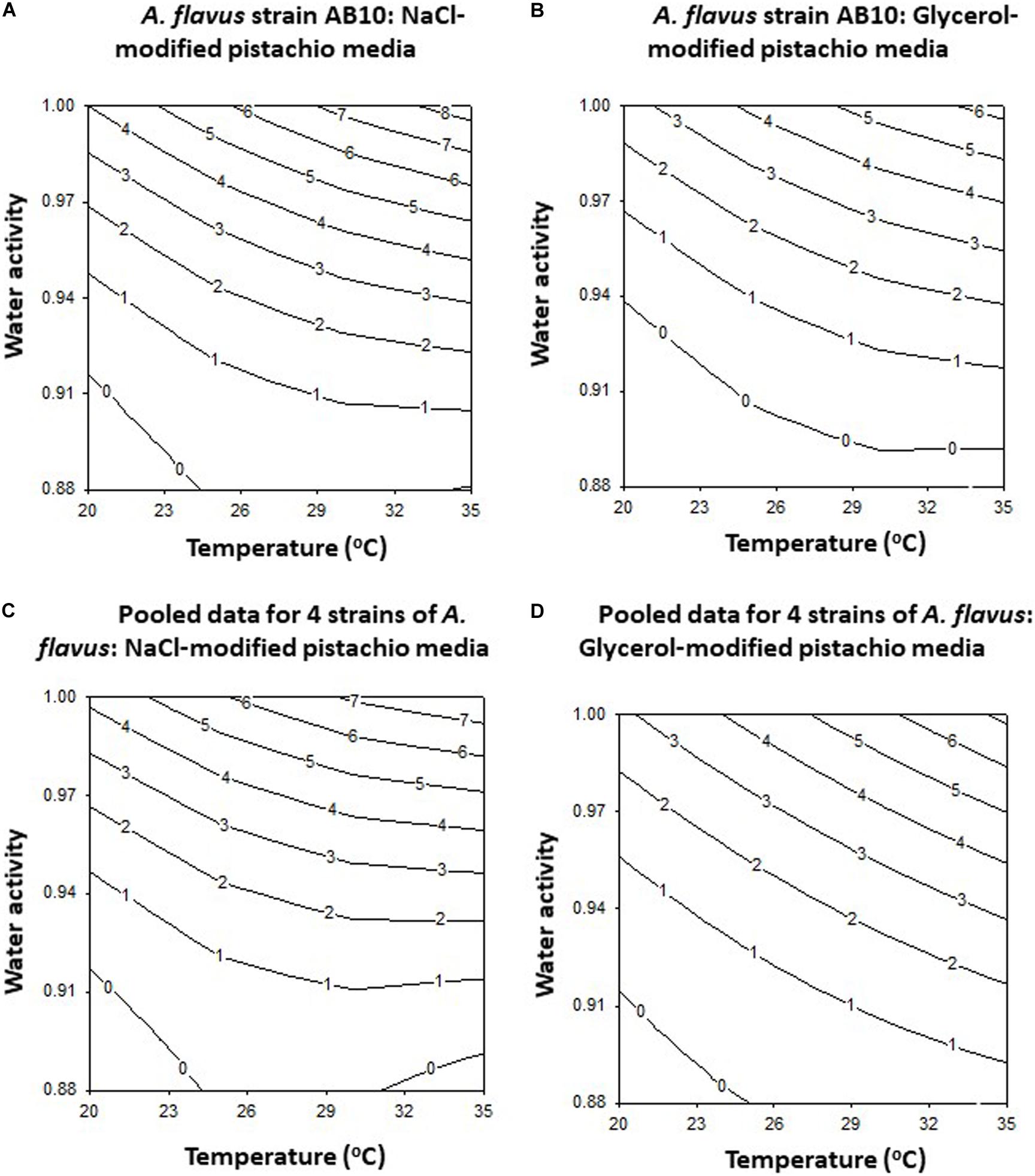
Figure 2. The contour maps for growth of A. flavus strain AB10 on (A) NaCl and (B) glycerol-amended pistachio nut agar media. The pooled data for 4 strains (AB3, AB4, AB5, and AB10) is shown for solute-modified media for (C) NaCl and (D) glycerol.
Temperature × Water Activity Abiotic Factors on Aflatoxin B1 Production by Strains of Aspergillus flavus on Milled Pistachio Nut-Based Media Modified Ionically or Non-ionically
The relative production of AFB1 by strain AB10 of A. flavus on the PNA media modified with NaCl or glycerol as an example is presented in Figure 3. This clearly shows that maximum AFB1 was produced at 30°C and 0.99 aw. No toxin was produced at 0.90 aw over the time periods of our experiments at the temperatures examined. This was followed by production over a wider aw range at 25°C. Interestingly less AFB1 was produced at 35°C despite the faster colonization rate observed on PNA-media modified with NaCl or glycerol. In addition at a marginal temperature for growth (20°C) more AFB1 was produced at 0.95 and 0.98 aw.
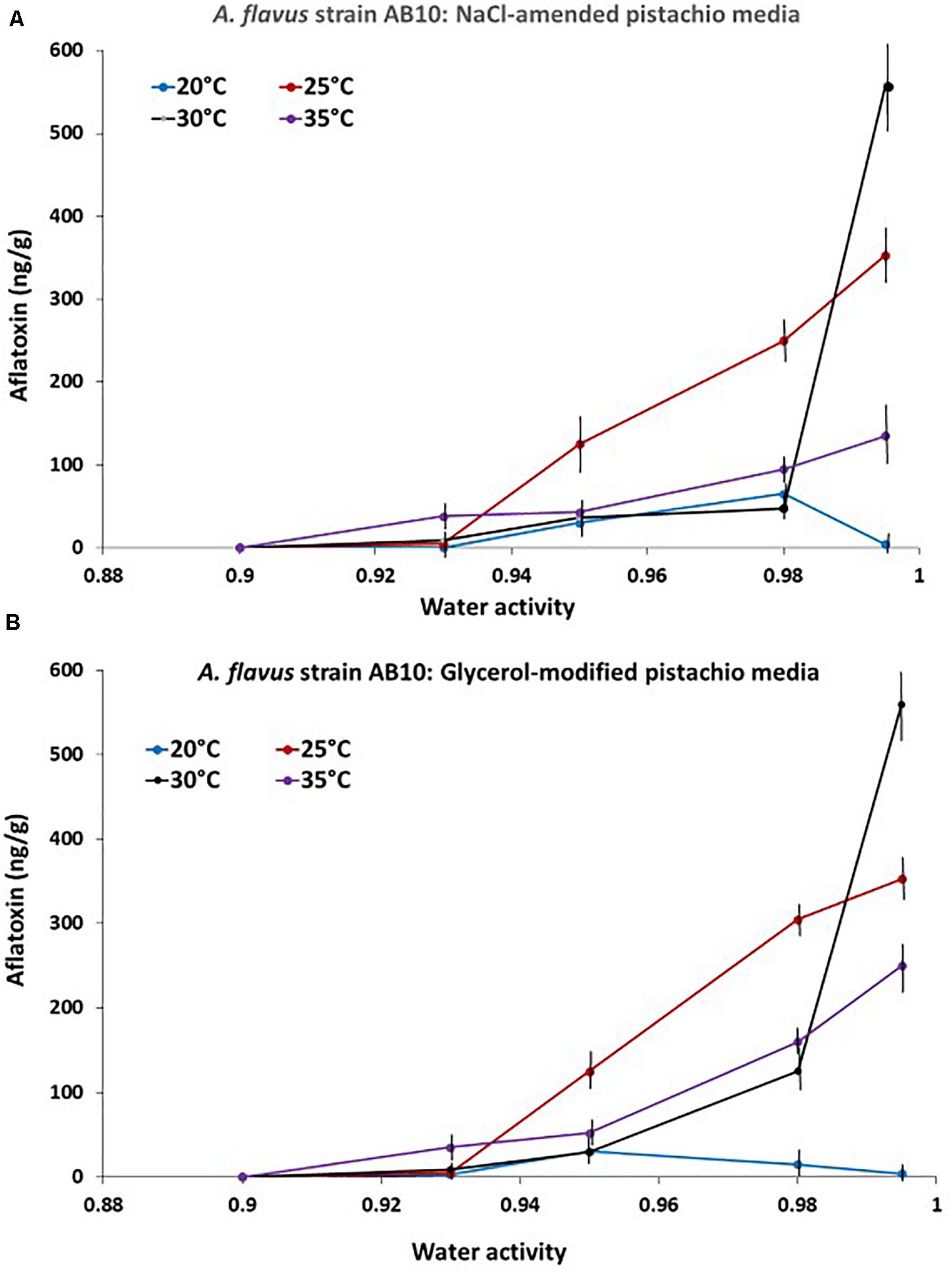
Figure 3. Effect of temperature x water activity on aflatoxin B1 production by A. flavus strain AB10 on a milled pistachio nut agar medium modified with either (A) NaCl or (B) glycerol. Quantification was done after 10 days incubation. Bars represent the Standard Error of the means.
The data was used to develop the models for the contour maps for optimum and marginal AFB1 production conditions for the 4 strains together on both solute-modified PNA media (Figures 4A,B). This shows that the predicted AFB1 production would be optimum over a wide temperature range of 25–35°C at >0.98 aw.
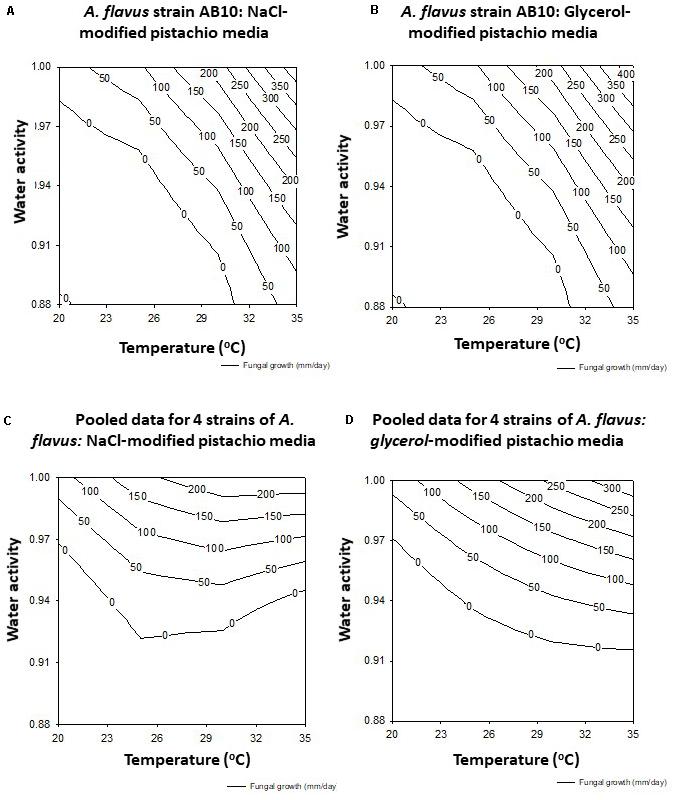
Figure 4. Contour maps for aflatoxin B1 production (ng/g) by strain AB10 on milled pistachio nut agar modified with (A) NaCl) or (B) glycerol. The pooled data for four strains (AB3, AB4, AB5, AB10) on the same media is shown in (C,D) respectively.
Effect of Temperature × Water Activity on Colonization Rates and Contour Maps of Growth Profiles for the Aspergillus flavus Strains on Raw Pistachio Nuts
The colonization rates of layers of raw pistachio nuts by strains AB3 and AB10 were examined (Figure 5). Both strains showed similar behavior with growth rate increasing as temperature or aw were increased. There was no growth observed at 0.90 aw when incubated at 25°C. Strain AB10 showed slightly faster colonization rates than strain AB3. There was no significant difference between the growth rate of the two strains at 25 and 30°C and 0.95 and 0.98 aw. However, at 35°C growth was almost double that at 30°C at all aw levels tested for both strains.
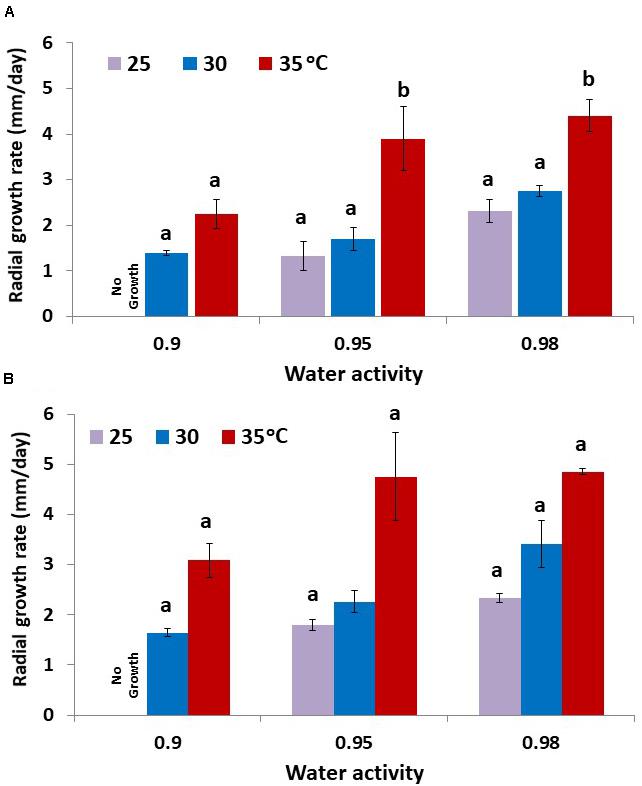
Figure 5. Colonization of layers of raw pistachio nuts by A. flavus strain (A) AB3 and (B) AB10 at different water activity levels and different temperatures. Data are means of three replicates. Bars represent the Standard Error of the means. Different letters indicate significant differences (p < 0.05).
The contour maps show the profiles for optimum and marginal conditions of colonization of layers of pistachio nuts (Figure 6). Colonization rates were best at 30–35°C and over a wide range of aw conditions with maximum at >0.95–0.98 aw for the pooled data for the two strains (AB3 and AB10; Figure 6).
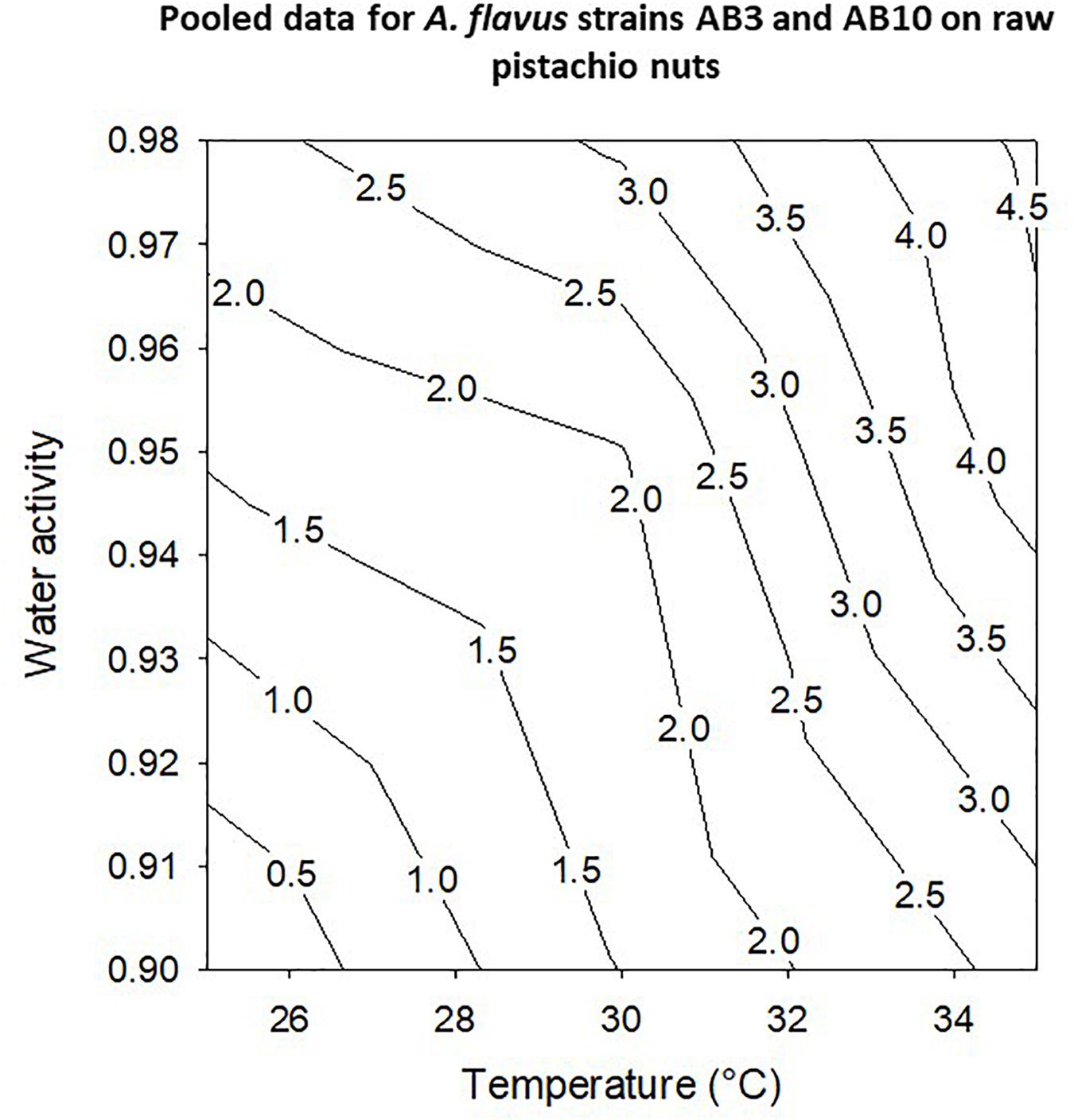
Figure 6. Contour maps for pooled data for growth of A. flavus strains AB3 and AB10 on layers of raw pistachio nuts using growth rates over periods of 10 days.
Effect of Temperature × Water activity on Aflatoxin B1 (ng/g) Production of Aspergillus flavus Strains and Contour Maps on Raw Pistachio Nuts
The effect of aw × temperature on AFB1 production by the AB10 strain on raw pistachio nuts is presented in Figure 7. Overall, the production of AFB1 on pistachio nuts was at least 10–15x lower than that produced on PNA media. AFB1 production was significantly higher at 0.98 aw and 30 and 35°C when compared with the other conditions examined. No AFB1 was detected at 35°C except at 0.98 aw for strain AB10.
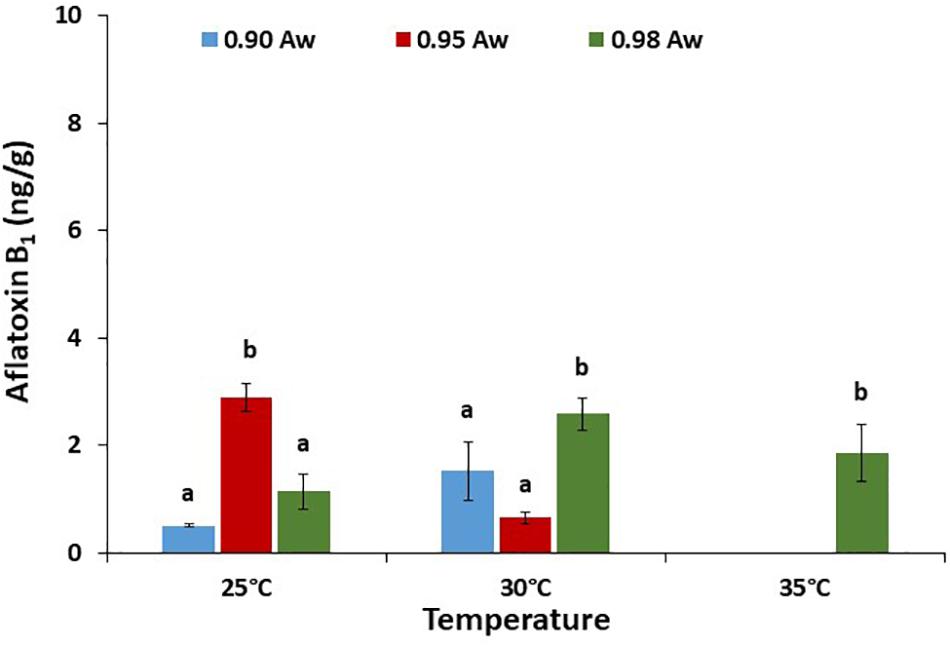
Figure 7. Aflatoxin B1 production (ng/g) by A. flavus strain AB10 grown on a layer of raw pistachio nuts at different water activities and temperatures. Data are means of three replicates. Bars represent Standard Error of the means. Different letters indicate significant difference (p < 0.05).
The modeling of the contour maps for joining similar production levels across the range of conditions examined showed that for strain AB10 the optimum was around 25–35°C and >0.98 aw (Figure 8A). However, when the data for the two strains were combined the optimum conditions were slightly different with optimum production predicted at >30°C and >0.98 aw (Figure 8B). For the AB10 strain and the pooled data for both strains suggests that some production will occur at around 0.91–0.90 aw across a range of temperatures.
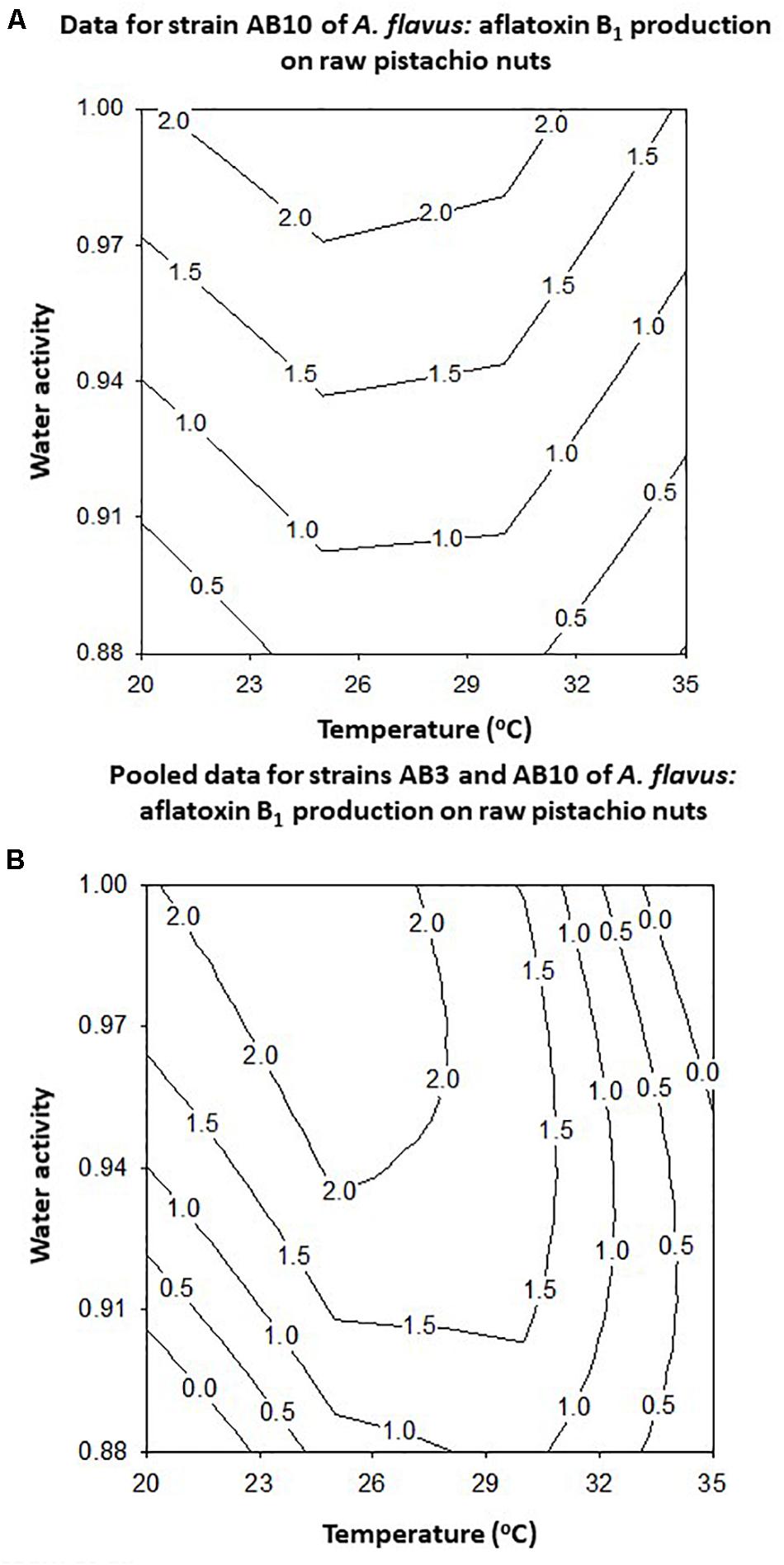
Figure 8. Contour maps for the effect of temperature x water activity on aflatoxin B1 by A. flavus strain AB10 (A) and for the pooled data for strains AB3 and AB10 (B) produced after 10 days incubation on layers of raw pistachio nuts.
Discussion
This study has examined both growth and AFB1 production by a group of strains of A. flavus isolated from pistachio nuts. This has provided useful data in relation to relative colonization and AFB1 production in pistachio nut-based media modified with both salt and a non-ionic solute, glycerol. This was to reflect both raw unsalted and roasted salted pistachio nuts to identify relative similarities and differences in terms of strain resilience and effects on toxin production. Changes in water availability, regardless of the solutes used had no significant differential effect on growth or indeed AFB1 production. The contour maps for both these treatments in relation to temperature x aw interacting abiotic factors were similar, for both optimum and marginal conditions, using 1 or all 4 A. flavus strains.
Previous studies by Aldars-García et al. (2017a) examined initial germination, growth and AFB1 production on 3% pistachio extract medium. However, the studies were limited to 25°C and 0.85 and 0.87 aw which were marginal for growth and toxin production. The time for germination, growth rates and time to visible colonies was estimated. They suggested that even at these aw levels initiation of AFB1 production could occur within 6 days. This suggests that at 30–35°C where germination rates, growth and toxin production are much more rapid, toxin contamination might occur even earlier. Perhaps a kinetic study at 30–35°C and these aw levels would provide useful data to predict the risks of AFB1 contamination. However, the present study has shown that toxin production rates were significantly higher on the milled PNA media than in raw pistachio nuts. Thus, care is needed in using such predictions of relative risks based on temperature x aw conditions using in vitro studies. More data sets are required on relative AFB1 production by A. flavus strains in stored pistachio nuts to obtain more accurate predictions of risks relative to the legislative limits (Aldars-García et al., 2017b).
In the present study, A. flavus strains were able to grow effectively when incubated at 25–35°C, over a range of aw levels, both in vitro and in situ. However, the range of conditions for AFB1 production was narrower with optimum production at 30°C in modified media, at 0.98 and 0.955 aw; and at 35°C + 0.98 aw by strain AB3 on raw pistachio nuts. Studies by Marin et al. (2012) examined the effect of 10–30% m.c. of pistachio nuts and storage temperatures of 10–42°C and inoculated with an A. flavus strain. They stored the pistachio nuts for longer periods of time (up to 60 days) and used kinetic and probability models to identify growth/no growth and toxin/no toxin conditions. The probability model showed good predictions for growth/no growth after 60 days incubation. Similarly, the probability for AFs presence was predicted in 89% of the cases. They thus suggested that the EU legislative limits would be exceeded if pistachio nuts were stored for up to one-month at 20°C if the m.c. was >10–12%. The present study included relevant aw levels equivalent to approx. 10–30% m.c. We obtained little AFB1 contamination at approx. ≥0.90 aw. However, our studies were limited to 10 days and longer storage periods would have perhaps provided more information on the absolute minima for growth and toxin production. However, we were able to obtain data on different strains which suggest that the overall ecological behavior was relatively similar.
In addition, it is clear that colonization rates in vitro were generally more rapid than that observed in situ. In addition, AFB1 production was significantly higher on the milled PNA media when compared with that produced in situ. This could be because of the better access to the nutrients in the milled pistachio nut-based media allowing the strains to grow faster and produce more toxins. In situ, the surface area is different and initial colonization and access to the nutrients may be slower or delayed. It was interesting that these strains isolated from pistachio nuts could all grow well at 35°C. Usually A. flavus strains from maize and peanuts are more sensitive to this temperature with optima closer to 28–30°C (Giorni et al., 2008; Abdel-Hadi et al., 2012; Bhatnagar et al., 2018). Thus, ecologically this may be important when examining intervention strategies to reduce AFs contamination of pistachio nuts during drying, storage and processing phases. In addition, the good tolerance of 35°C suggests resilience also in relation to climate-related more extreme abiotic factors (Medina et al., 2017).
Previous studies by Fernane et al. (2010a, b) showed that about 70% of A. flavus strains from a total of 204 were able to produce AFB1 and AFB2 on pistachio nuts. Aldars-García et al. (2015) used a predictive modeling approach to predict potential AFB1 contamination of pistachio nuts based on environmental conditions. They found that using this approach it was possible to predict 70–81% of samples correctly with regard to growth and in 67–81% of the cases contamination with AFB1 based on the models developed from their in vitro studies. Recently a mechanistic model has been developed using the available data on the life cycle of A. flavus ecologically as well as the pistachio development and the prevailing abiotic conditions (Kaminiaris et al., 2020). Ecological data on a wider number of strains isolated from pistachio nuts obtained in the present study might also be beneficial for the further development of the “AFLA-PISTACHIO” model for improving the accuracy and validation of evaluating the relative risks in different pistachio-producing countries.
Conclusion
This study has shown that optimum interacting abiotic conditions of temperature × aw for growth and AFB1 production by up to four strains of A. flavus isolated from pistachio nuts was very different from the ecology of strains colonizing maize and peanuts. It was interesting to note that there was no difference in growth or toxin production in PNA media modified with salt or the non-ionic solute glycerol. This also suggests that these strains have evolved to have better resilience to elevated temperatures although the water stress tolerance may well be similar. In addition, AFB1 production on raw pistachio nuts was much lower than on milled pistachio nut media. The contour maps clearly show the optimum and marginal conditions of these two interacting abiotic factors on both growth and toxin production. It is recommended that the safe storage m.c. for pistachio nuts is <8–10% for medium term storage under ambient temperature conditions (20–25°C). However, they are very hygroscopic because of their fatty acid content. Thus, they can easily re-adsorb moisture during bulk transport through different climatic regions and poor storage, resulting in a deterioration in quality and an increase in toxin contamination.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AB carried out the research work. EG-C carried out the modeling work. AM and NM supervised the research and drafted the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Taif University Researchers Supporting Project Number (TURSP-2020/295), Taif University, Taif, Saudi Arabia for funding this research which was carried out in the Applied Mycology Group, Cranfield University, United Kingdom.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.624007/full#supplementary-material
References
Abdel-Hadi, A., Schmidt-Heydt, M., Parra, R., Geisen, R., and Magan, N. (2012). A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J. R. Soc. Interface 9, 757–767. doi: 10.1098/rsif.2011.0482
Aldars-García, L., Sanchis, V., Ramos, A. J., and Marín, S. (2015). An attempt to model the probability of growth and aflatoxin B1 production of Aspergillus flavus under non-isothermal conditions in pistachio nuts. Food Microbiol. 51, 117–129. doi: 10.1016/j.fm.2015.05.013
Aldars-García, L., Sanchis, V., Ramos, A. J., and Marin, S. (2017a). Single vs multiple-spore inoculum effect on growth kinetic parameters and modeled probabilities of growth and aflatoxin B1 production of Aspergillus flavus on pistachio extract agar. Int. J. Food Microbiol. 243, 28–35. doi: 10.1016/j.ijfoodmicro.2016.11.026
Aldars-García, L., Sanchis, V., Ramos, A. J., and Marín, S. (2017b). Time-course of germination, initiation of mycelium proliferation and probability of visible growth and detectable AFB1 production of an isolate of Aspergillus flavus on pistachio extract agar. Food Microbiol. 64, 104–111. doi: 10.1016/j.fm.2016.12.015
Baazeem, A. (2018). Ecology, Climate Change and Control Strategies for Aspergillus flavus Colonisation and Aflatoxin Contamination Of Pistachio Nuts. PhD thesis, Applied Mycology Group, Cranfield University, Cranfield.
Bhatnagar, D., Rajasekaran, K., Gilbert, M., Cary, J. W., and Magan, N. (2018). Research advances to safeguard food and feed supply from aflatoxin contamination. World Mycotoxin J. 11, 47–72. doi: 10.3920/wmj2017.2283
Bui-Klimke, T. R., Guclu, H., Kensler, T. W., Yuan, J.-M., and Wu, F. (2014). Aflatoxin regulations and global pistachio trade: insights from social network analysis. PLoS One 9:e92149. doi: 10.1371/journal.pone.0092149
Ciegler, A. (1972). Bioproduction of ochratoxin A and penicillic acid by members of the Aspergillus ochraceus group. Can. J. Microbiol. 18, 631–636. doi: 10.1139/m72-100
Dallyn, H., and Fox, A. (1980). “Spoilage of materials of reduced water activity by xerophilic fungi,” in Microbial Growth and Survival in Extremes of Environment, eds G. W. Gould and J. E. L. Corry (London, New York: Academic press), 129–139.
Doster, M. A., and Michailides, T. J. (1995). The relationship between date of hull splitting and decay of pistachio nuts by Aspergillus species. Plant Dis. 79, 766–769. doi: 10.1094/pd-79-0766
Fernane, F., Cano-Sancho, G., Sanchis, V., Marín, S., and Ramos, A. J. (2010a). Aflatoxins and ochratoxin A in pistachios sampled in Spain: occurrence and presence of mycotoxigenic fungi. Food Add. Contam. Part B Surveill. 3, 185–192. doi: 10.1080/19440049.2010.497257
Fernane, F., Sanchis, V., Marın, S., and Ramos, A. J. (2010b). First report on mould and mycotoxin contamination of pistachios sampled in algeria. Mycopathologia 170, 423–429. doi: 10.1007/s11046-010-9332-3
Georgiadou, M., Dimou, A., and Yanniotis, S. (2012). Aflatoxin contamination in pistachio nuts: a farm to storage study. Food Control 26, 580–586. doi: 10.1016/j.foodcont.2012.02.014
Giorni, P., Battilani, P., and Magan, N. (2008). Effect of solute and matric potential on in vitro growth and sporulation of strains from a new population of Aspergillus flavus isolated in Italy. Fungal Ecol. 1, 102–108. doi: 10.1016/j.funeco.2008.07.001
Kaminiaris, M. D., Camardo Leggieri, M., Tsitsigiannis, D. I., and Battilani, P. (2020). AFLA-PISTACHIO: development of a mechanistic model to predict the aflatoxin contamination of pistachio nuts. Toxins 12:445. doi: 10.3390/toxins12070445
Kok, W.Th (1994). Derivatization reactions for the determination of aflatoxins by liquid chromatography with fluorescence detection. J Chromatogr. B Biomed. Sci. Appl. 659, 127–137. doi: 10.1016/0378-4347(94)00152-9
Lang, A.R.G. (1967). Osmotic coefficients and water potentials of sodium chloride solutions from 0–40°C. Aust. J. Chem. 20, 2017–2023. doi: 10.1071/CH9672017
Marin, S., Ramos, A. J., and Sanchis, V. (2012). Modelling Aspergillus flavus growth and aflatoxins production in pistachio nuts. Food Microbiol. 32, 378–388. doi: 10.1016/j.fm.2012.07.018
Medina, A., Akbar, A., Baazeem, A., Rodriguez, A., and Magan, N. (2017). Climate change, food security and mycotoxins: do we know enough? Fungal Biol. Rev. 31, 143–154. doi: 10.1016/j.fbr.2017.04.002
Mojtahedi, H., Rebie, C. J., Lubben, A., Steyn, M., and Danesh, D. (1979). Toxic aspergilli from pistachio nuts. Mycopathologia 67, 123–127.
Mozaffari, A. S. (2012). Global pistachio production and marketing challenges in Iran. Acta Hortic. 963, 133–141. doi: 10.17660/actahortic.2012.963.23
Nawar, L. S. (2008). Prevention and control of fungi contaminated stored pistachio nuts imported to Saudi Arabia. Saudi J. Biol. Sci. 15, 105–112.
Sommer, N. F., Buchanan, J. R., and Fortlage, R. J. (1986). Relation of early splitting and tattering of pistachio nuts to aflatoxin in the orchard. Phytopathology 76, 692–694. doi: 10.1094/phyto-76-692
Keywords: water relations, growth, mycotoxins, boundary conditions, optimum conditions, aflatoxins, pistachios
Citation: Baazeem A, Garcia-Cela E, Medina A and Magan N (2021) Interacting Abiotic Factors Affect Growth and Aflatoxin B1 Production Profiles of Aspergillus flavus Strains on Pistachio-Based Matrices and Pistachio Nuts. Front. Microbiol. 11:624007. doi: 10.3389/fmicb.2020.624007
Received: 30 October 2020; Accepted: 17 December 2020;
Published: 20 January 2021.
Edited by:
Lin Lin, Jiangsu University, ChinaReviewed by:
Zuzana Hruska, Mississippi State University, United StatesMassimo Reverberi, Sapienza University of Rome, Italy
Abhishek Kumar Dwivedy, Banaras Hindu University, India
Copyright © 2021 Baazeem, Garcia-Cela, Medina and Magan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naresh Magan, bi5tYWdhbkBjcmFuZmllbGQuYWMudWs=
†Present address: Esther Garcia-Cela, School of Life and Medical Sciences, University of Hertfordshire, Hatfield, United Kingdom
 Alaa Baazeem
Alaa Baazeem Esther Garcia-Cela
Esther Garcia-Cela Angel Medina
Angel Medina Naresh Magan
Naresh Magan