- 1School of Clinical Medicine, Tsinghua University, Beijing, China
- 2Department of Obstetrics and Gynecology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
- 3School of Life Sciences, Peking University, Beijing, China
- 4Shenzhen Eulikan Biotechnology Co., Ltd, Shenzhen, China
- 5Sichuan Green-house Biotech Co., Ltd, Sichuan, China
- 6Suzhou Turing Microbial Technologies Co., Ltd, Suzhou, China
The high incidence of bacterial vaginosis recurrence is common after treatment with an antibiotic agent and suggests the need for new treatments to prevent this. We conducted a randomized trial to evaluate the ability of maltose gel to treat bacterial vaginosis. Eighteen female rhesus macaques were randomly assigned, in a 2:1 ratio, to receive maltose gel or placebo gel by syringe to the fornix of the vagina for five consecutive days. We used 16S rRNA sequencing data from 70 swab samples of vaginal secretions in two groups in total on days 0, 3, and 5 after medication initiation and days 3 and 5 after medication withdrawal for the study of microbiome composition. We found that, in the placebo control group, there was no significant change in the composition and abundance of vaginal microbiota during the follow-up period. In the maltose gel test group, the abundance of Lactobacillus in the vagina microbiota increased gradually with the prolongation of the treatment time on Days 3 and 5 (ANOVA p = 6.99e−5 < 0.01) but began to decrease after the withdrawal of maltose gel, which was different from that of the control group. Correspondingly, the diversity and abundance of BV-related bacteria, Fusobacterium, Parvimonas, Mobiluncus, Campylobacter, Prevotella, and Sneathia, decreased on Day 0 to Day 5 of medication and increased after drug withdrawal in the maltose gel test group. The study confirms that maltose gel can facilitate the proliferation of Lactobacillus and promote the transition of the vaginal microbiota from BV-related bacteria dominant to Lactobacillus dominant in the rhesus macaque.
Introduction
Bacterial vaginosis (BV) is the most common lower genital tract disorder of fertile women (Mendling et al., 2019) and has been associated with an increased risk of adverse pregnancy outcomes (Leitich and Kiss, 2007; Klebanoff and Brotman, 2018), pelvic inflammatory disease (Taylor et al., 2013), and various sexually transmitted illnesses (STIs; Atashili et al., 2008). Metronidazole and clindamycin are the optimal choices for the treatment of BV worldwide. Although antibiotics represent an effective therapeutic option in the short term, recurrence remains a serious problem (Russo et al., 2019; Cohen et al., 2020). The high incidence of relapses indicates that new agents are needed to treat BV.
Lactobacillus, the most prevalent microbiota in the vagina, protect it from pathogenic infections by producing antimicrobial compounds (e.g., hydrogen peroxide, lactic acid, and bacteriocin-like substances) and by adhering and competing for adhesion sites in the vagina (Borges et al., 2014; Yefet et al., 2020). BV is characterized by shifts in the vaginal microbiota from Lactobacillus dominant to a microbiota with diverse anaerobic bacteria (Srinivasan et al., 2015). In recent years, the use of micro-ecological preparations to restore the dominant position of Lactobacillus has become a new strategy for the treatment and prevention of BV recurrence (Gaziano et al., 2020). A previous study found that individuals with Lactobacillus crispatus-dominant vagina microbiome communities show extremely lower risks of acquiring human immunodeficiency virus (HIV) when compared with individuals with diverse vagina bacterial communities dominated by anaerobes (Gosmann et al., 2017). Recent research also found that Lactobacillus-dominant vagina microbiome shows a protective effect against the human papillomavirus (HPV) infection, and cervical intraepithelial neoplasia (CIN), which is considered to be a precursor of cervical carcinoma (Yang et al., 2018; Mitra et al., 2020).
Prebiotic refers to a food ingredient that cannot be further digested and has a beneficial effect on the host by selectively promoting the growth and/or enhancing the activity of one or several bacteria (Gibson and Roberfroid, 1995). Artificially produced prebiotics includes lactulose, galactooligosaccharides, fructooligosaccharides, maltooligosaccharides, cyclodextrins, lactosaccharose, etc. (Markowiak and Slizewska, 2017). α-Amylases were found in women’s vagina fluid, which can break down glycogen into maltose, maltotriose, and maltotetraose, which are important to support the growth of Lactobacillus (Spear et al., 2014). Though, under low pH environment, α-amylase has reduced activity, there is still detectable maltose generation, which can limit the growth of Lactobacillus at a sustained rate (Spear et al., 2015). Maltose is a single small-molecule compound maltooligosaccharide, which exists in women’s vagina and can be utilized by Lactobacillus (Ganzle and Follador, 2012). Maltose can be used to make maltose gel as a new non-antibiotic agent for the treatment of BV.
The vagina of female rhesus macaques (Macaca mulatta) is colonized by a group of anaerobic bacteria associated with BV in the woman (Chen et al., 2018). Rhesus macaque has been used as a BV animal model to study vaginal microbiota-associated diseases (Spear et al., 2010). Based on the results from the previous animal trials, the topical application of sucrose gel can induce the vaginal microbiota of rhesus macaques to change from BV-state microbiota to Lactobacillus-dominating microbiota (Hu et al., 2015). The current animal trial was designed to assess whether treatment with maltose gel is a better choice for BV than a placebo.
Materials and Methods
Animals and Sample Collection
Rhesus macaques, approximately 4 years of age, were provided by Sichuan Green-house Biotech Co., Ltd., and were deemed to be normal, healthy females at sample collection. Eighteen female rhesus macaques were randomly assigned, in a 2:1 ratio, to receive 0.5 g of maltose gel with maltose concentration of 2.0% (W/V) or 0.5 g of placebo gel by syringe to the fornix of the vagina for 5 consecutive days. The placebo formulation contained the same inactive matrix as maltose gel, which only lacked maltose compared with maltose gel. Both maltose gel (Palavigor Malt. Gel) and placebo gel are provided by Shenzhen Eulikan Biotechnology Co., Ltd. Sampling was scheduled on day 0, day 3, and day 5 during medication and on day 3 and day 5 after withdrawal (hereafter denoted as Day 0, Day 3, Day 5, After 3, and After 5, respectively). All animal studies were reviewed and approved by the Medical Ethics Committee of Beijing Tsinghua Changgung Hospital.
The vaginal discharge was obtained via two swabs. One swab was used to prepare a dry slide for Gram staining, under 1,000× magnification for visual detection. The other swab was quickly plunged into a tube containing 1 ml of PBS solution and stored at −80°C until the total DNA extraction of the vaginal microbiota.
DNA Extraction, PCR Amplification, and Sequencing
The DNA of the sample was extracted through the TIANamp Bacteria DNA Kit (TIANGEN, China) according to the manufacturer’s instructions. This step required additional Lysozyme (Sigma-Aldrich), proteinase K, and RNase A (Sigma-Aldrich), and finally the DNA was washed and stored with 1 × TE buffer. A spectrophotometer was used (Thermo Scientific NanoDrop One) to measure the concentration and purity of the DNA extracts. Then, isolated DNA was stored at −80°C until needed.
16S rRNA was sequenced to determine the microbiota of samples. The V1–V2 regions of the 16S rRNA were amplified with universal primers 27F (5'-AGRGTTYGATYCTGGCTCAG-3') and 338R (5'-GCTGCCTCCCGTAGGAGT-3'). All PCR reactions were carried out with Phusion® High-Fidelity PCR MasterMix (New England Biolabs). The PCR products examined with 300–340 bp were chosen and mixed in equal density ratios. Then, the mixture PCR product was purified with the Qiagen Gel Extraction Kit (Qiagen, Germany). Sequencing libraries were generated using a TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, USA) following the manufacturer’s recommendations, and index codes were added. The library quality was assessed on the Qubit 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. Last, the library was sequenced on an Illumina MiSeq platform.
Sequencing Data Processing
The analysis of FASTQ files mainly adopted QIIME2 analysis suites (Bolyen et al., 2019). Firstly, sequencing reads from different samples were separated by duo barcodes with Cutadapt software (v2.8, https://cutadapt.readthedocs.io/en/stable/). Different FASTQ files for the same sample were gathered into one pair of FASTQ files. Then, all the single-sample FASTQ files were imported as QIIME2 artifacts. The raw sequences were denoised using DADA2 software wrapped in QIIME2 (--p-trunc-len-f 200 --p-trunc-len-r 200 --p-max-ee-f 4 --p-max-ee-r 4; Callahan et al., 2016). The denoised feature tables were filtered using QIIME2 feature-table filter-features (--p-min-frequency 10 --p-min-samples 2). The denoised sequences were classified using QIIME2 plugin feature-classifier classify-sklearn with trained Naive Bayes classifier on SILVA (132) taxonomic reference (Yilmaz et al., 2014; Bokulich et al., 2018). Finally, the phylogeny tree and alpha diversity were calculated from the filtered feature table. Further statistic tests and analyses were carried out using R.
Nucleotide Sequence Availability
16S rRNA gene sequences in this study were deposited in the National Center for Biotechnology Information (NCBI) database on BioProject accession number: PRJNA645314.
Results
Diversity of Rhesus Macaque Vaginal Microbiota
Vaginal swabs were collected from 18 rhesus macaques (6 controls, numbered 1–6 and 12 in the test group, numbered 7–18). Due to menstruation and other uncontrollable factors, 8 rhesus macaques (1 control and 7 tests) all completed the collection of vaginal secretions at five time points during the follow-up period. Ten rhesus macaques (5 controls and 5 tests) missed some samples at different time points. We recorded the follow-up data of 18 macaques and collected 70 swab samples of vaginal secretions in two groups (see Supplementary Table 1).
The vaginal microbiota of macaque was polymicrobial, and there was no significant difference between samples from control and test groups initially. The vaginal microbiota of macaques mainly included Lactobacillus, Porphyromonas, Fusobacterium, Corynebacterium, Sneathia, Mobiluncus, Ezakiella, Aerococcus, Streptococcus, Fastidiosipila, Campylobacter, Dialister, Alloprevotella, Parvimonas, Staphylococcus, Peptoniphilus, Atopobium, Facklamia, Helcococcus, Prevotella, Propionimicrobium, Jeotgalicoccus, and Ruminococcaceae, many of which are also found in women with BV (Figure 1A). There was no statistical difference of the mean values of the primary genera between the initial vaginal microbiota (Day 0) of control and test groups (two-tailed Student’s t test, p > 0.05), except for Ezakiella (p = 0.045 < 0.05) and Streptococcus (p = 0.038 < 0.05; Supplementary Table 2). However, when accounting for multiple tests, none of the 23 genera showed a significant difference between control and test groups (FDR > 0.05, Figure 1B; Supplementary Figure 1 and Supplementary Table 2).
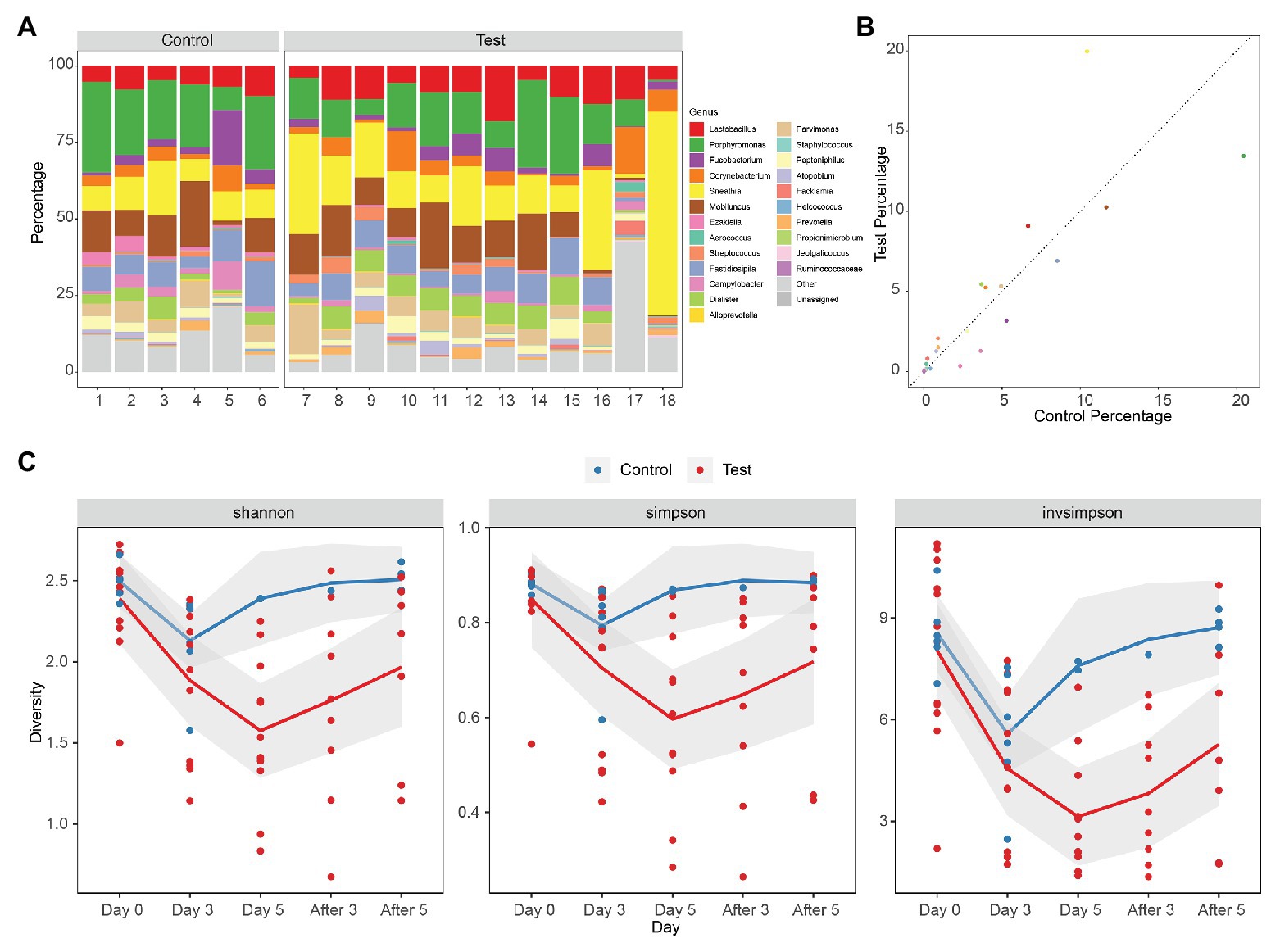
Figure 1. Diversity of rhesus macaque vaginal microbiota in control and test groups. (A) Composition of initial vaginal microbiota (Day 0). (B) The mean values of the primary genera of the initial vaginal microbiota (Day 0). (C) Alpha diversity of microbiota as measured using Shannon index, Simpson, and inverse Simpson of genus level 16S rRNA gene phylotypes. Control, blue; Test, red. The mean values of the five times of follow-up in two groups are connected by blue and red lines, respectively, and the gray area is the standard error of linear regression.
Alpha diversity denotes the diversity of the ecosystem or microbiota. The diversity of vaginal microbiota of female rhesus macaques is similar to that of women with BV, which is higher than the diversity of vaginal microbiota of health women (Chen et al., 2018). Alpha diversities of the microbiota of different groups (Day 0, Day 3, Day 5, After 3, and After 5) were measured using the Shannon index, Simpson index, and inverse Simpson index (Invsimpson) from genus-level 16S rRNA gene phylotypes (Figure 1C; Supplementary Table 3). We did not find any significant difference (Shannon index p = 0.33 > 0.05, Simpson index p = 0.29 > 0.05, Invsimpson index p = 0.58 > 0.05) between control and test groups at Day 0 from the diversity indexes, which demonstrated that the initial microbiota diversities of samples from control and test groups were similar (Supplementary Tables 3 and 4). However, when we compared data from different days during treatment (Day 0, Day 3, and Day 5) in control and test groups, we could find that the diversity declined very significantly in test groups (ANOVA: Shannon index p = 0.00024 < 0.01, Simpson index p = 0.0021 < 0.01, Invsimpson index p = 0.000042 < 0.01), but not in control groups (ANOVA: Shannon index p = 0.036 > 0.01, Simpson index p = 0.12 > 0.01, Invsimpson index p = 0.015 > 0.01; Figure 1C). When it came to the days after treatment, we could observe an increasing trend in both control and test groups, although none of them were significant (ANOVA in control: Shannon index p = 0.28 > 0.05, Simpson index p = 0.052 > 0.05, Invsimpson index p = 0.067 > 0.05; ANOVA in test: Shannon index p = 0.34 > 0.05, Simpson index p = 0.47 > 0.05, Invsimpson index p = 0.17 > 0.05). These results indicated that the treatment can significantly reduce the diversity of vagina microbiota of female macaques. Although the diversity index of the treatment group increased after the withdrawal of medication, it remained at a lower level compared with the situation before medication. It indicated that the prebiotics still had a certain lasting effect on maintaining the stability of the bacterial community after drug withdrawal.
A comparison between the test group and the control group showed that the maltose gel could promote the dominance of Lactobacillus in vagina microbiota. In the six samples of the control group (numbers 1–6), there was no significant change in the composition and diversity of vaginal microbiota from Day 0 to Day 5 (Supplementary Table 5), with the percentage of Lactobacillus declining slightly (Day 0 and Day 3, two-sided Student’s t test p = 0.016 > 0.1). In the test group (sample number 7–18), the abundance of Lactobacillus in the vagina microbiota increased gradually with the prolongation of the treatment time on Day 3 and Day 5 (ANOVA p = 6.99e−5 < 0.01; Supplementary Table 6). After drug withdrawal, the abundance of Lactobacillus decreased gradually and was higher than the initial vaginal microbiota on After 3 and After 5 (Figure 2). Based on the above results, the microbiota of the test group was greatly affected by maltose gel. We compared the relative abundance of bacteria on Day 0 and Day 5 in the test group. As shown in Figure 3A, the abundance of Lactobacillus, Fusobacterium, Parvimonas, and Mobiluncus had a significant statistical difference between Day 0 and Day 5 (ANOVA p < 0.01), and the abundance of Campylobacter, Prevotella, and Sneathia had a statistical difference (ANOVA p < 0.05; Supplementary Table 6). By evaluating the changing trend of the above genus at different time points, we found that the abundance of Lactobacillus increased from the beginning of the treatment to Day 5, but began to decrease after the withdrawal of maltose gel (Figure 3B). Correspondingly, the abundance of Fusobacterium, Parvimonas, Mobiluncus, Campylobacter, Prevotella, and Sneathia decreased on Day 0 to Day 5 and increased after drug withdrawal (Figure 3B).
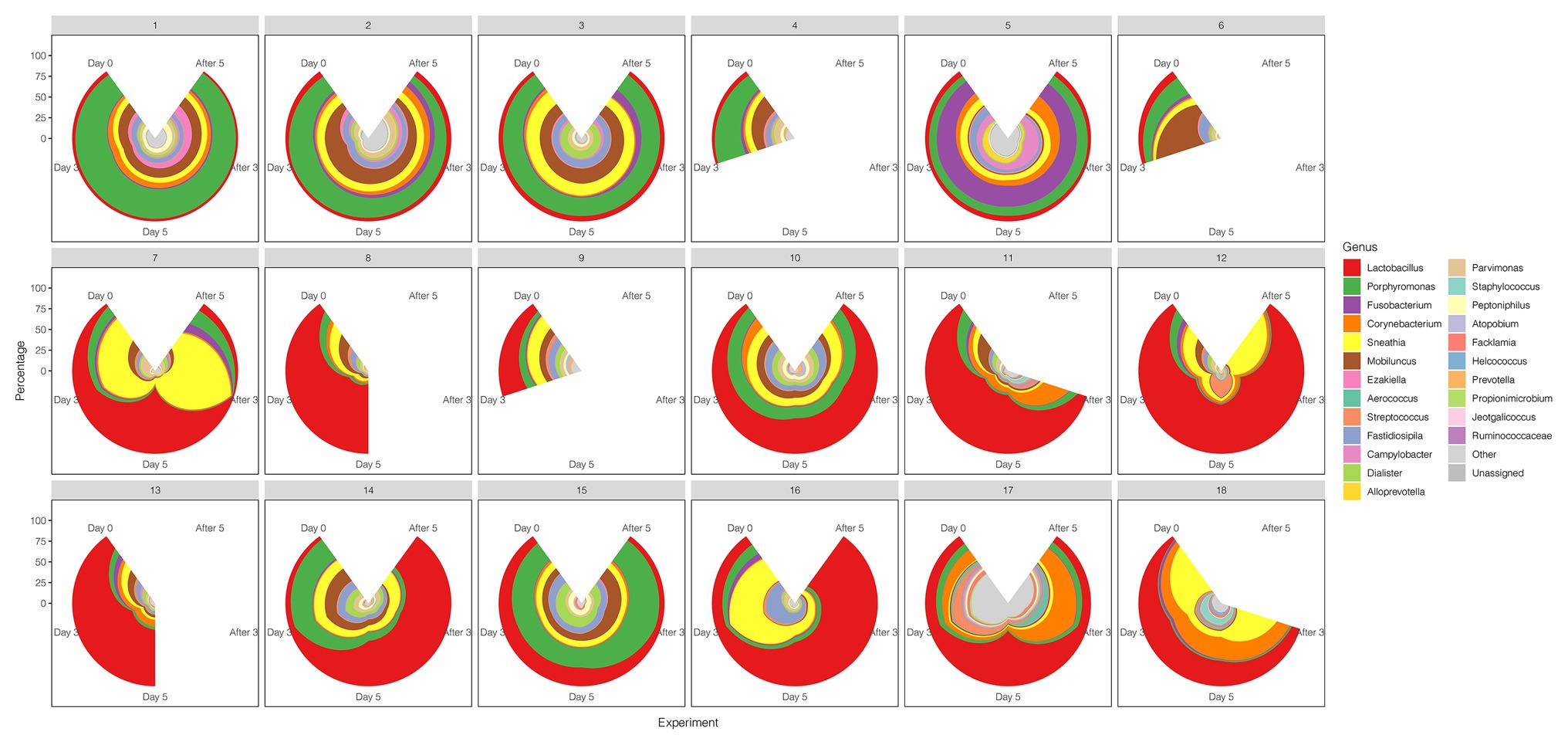
Figure 2. The diversity of the vaginal microbiota during the follow-up of each sample in control and test groups. The radius represents the percentage of the genus.
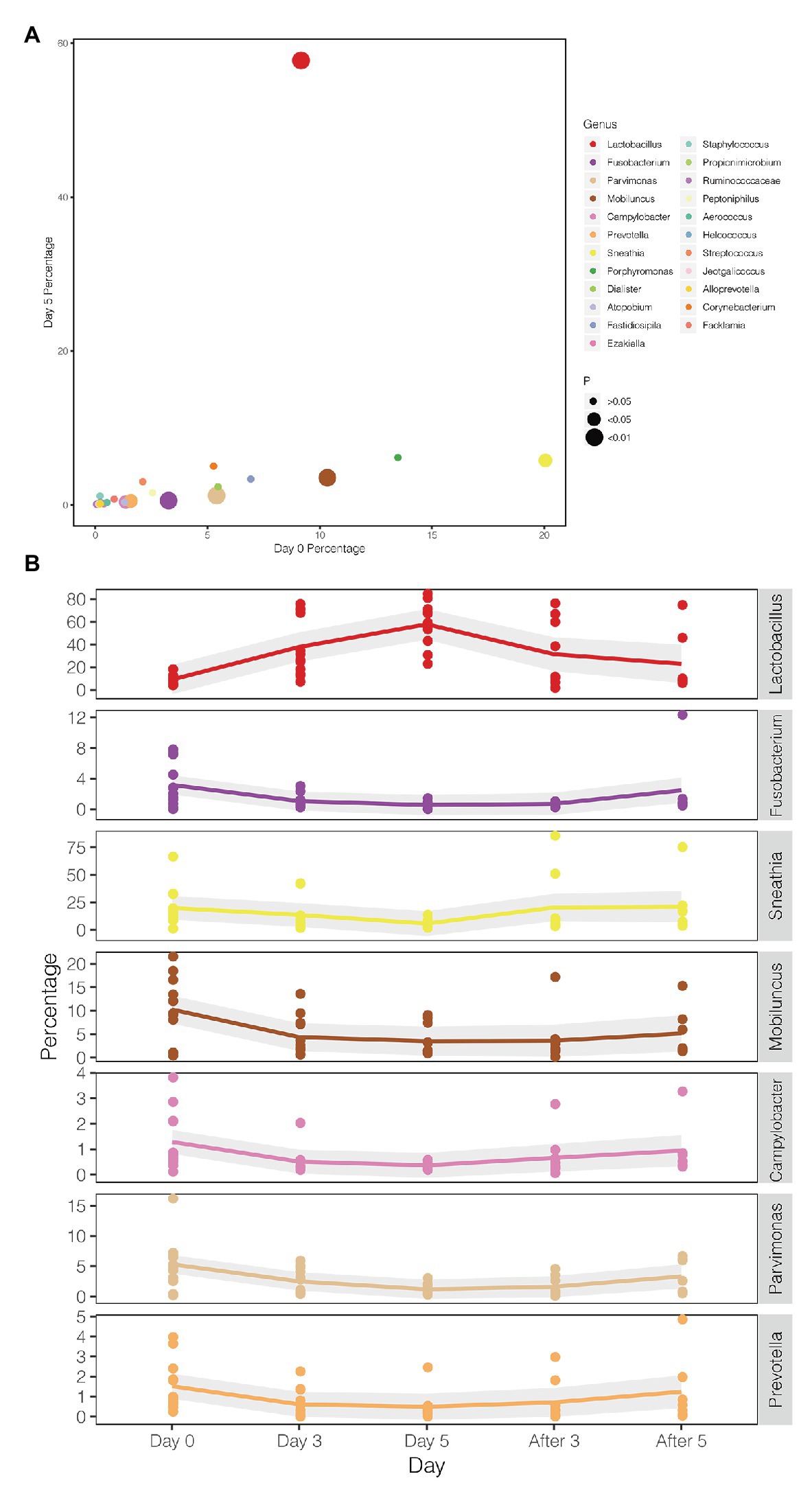
Figure 3. Changes in the abundance of bacteria with statistical difference in test group. (A) The percentage of bacteria on Day 0 and Day 5 in the test group. (B) The mean values of the five follow-up points are connected by different colored lines, respectively, and the gray area denotes the standard error.
Early drug withdrawal might lead to a mild relapse of the BV-like situation in the macaque vagina. When comparing Day 5 data with two follow-ups after withdrawal (After 3 and After 5), the percentage of Lactobacillus in most samples decreased gradually with the prolongation of withdrawal time, the dominant position of Lactobacillus was replaced, and the diversity of vaginal microbiota increased (Figures 1C, 4; Supplementary Table 7). However, the percentage reduction of Lactobacillus in the microbiota was moderate (ANOVA p = 0.016 > 0.01, Supplementary Table 7).
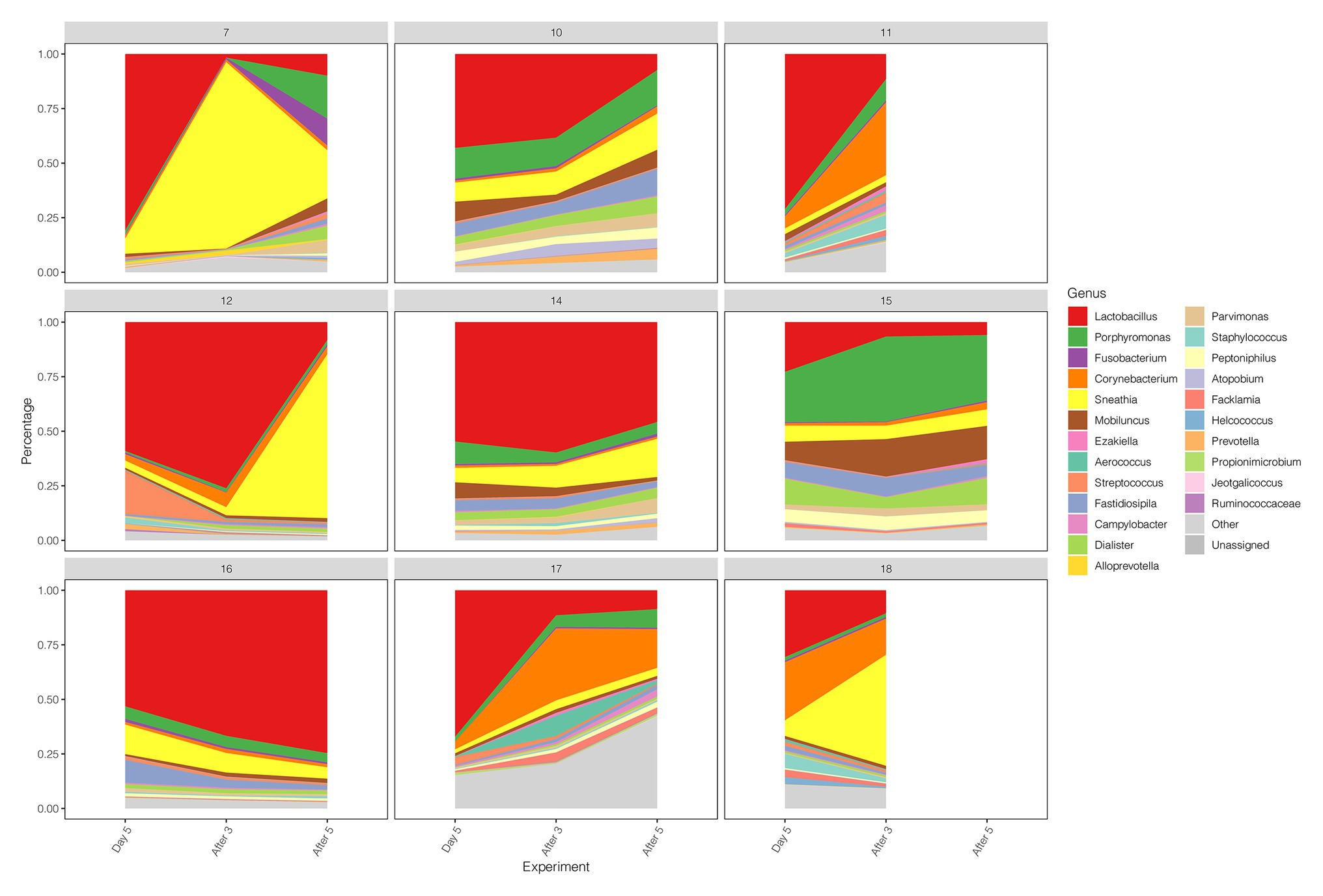
Figure 4. Diversity and abundance of vaginal microbiota between Day 5, After 3, and After 5 in test group.
We compared the diversity and abundance of Lactobacillus during follow-up between the control and test group. Lactobacillus sequences (mostly L. johnsonii) were found in rhesus macaques, and others include L. crispatus, L. iners, L. jensenii, L. mucosae, L. murinus, L. reuteri, L. ruminis, etc. (Figure 5; Supplementary Table 8). In the placebo gel control group, there was little change in the abundance of Lactobacillus. However, the abundance of Lactobacillus increased during the treatment period and gradually decreased after the withdrawal of the drug in the test group.
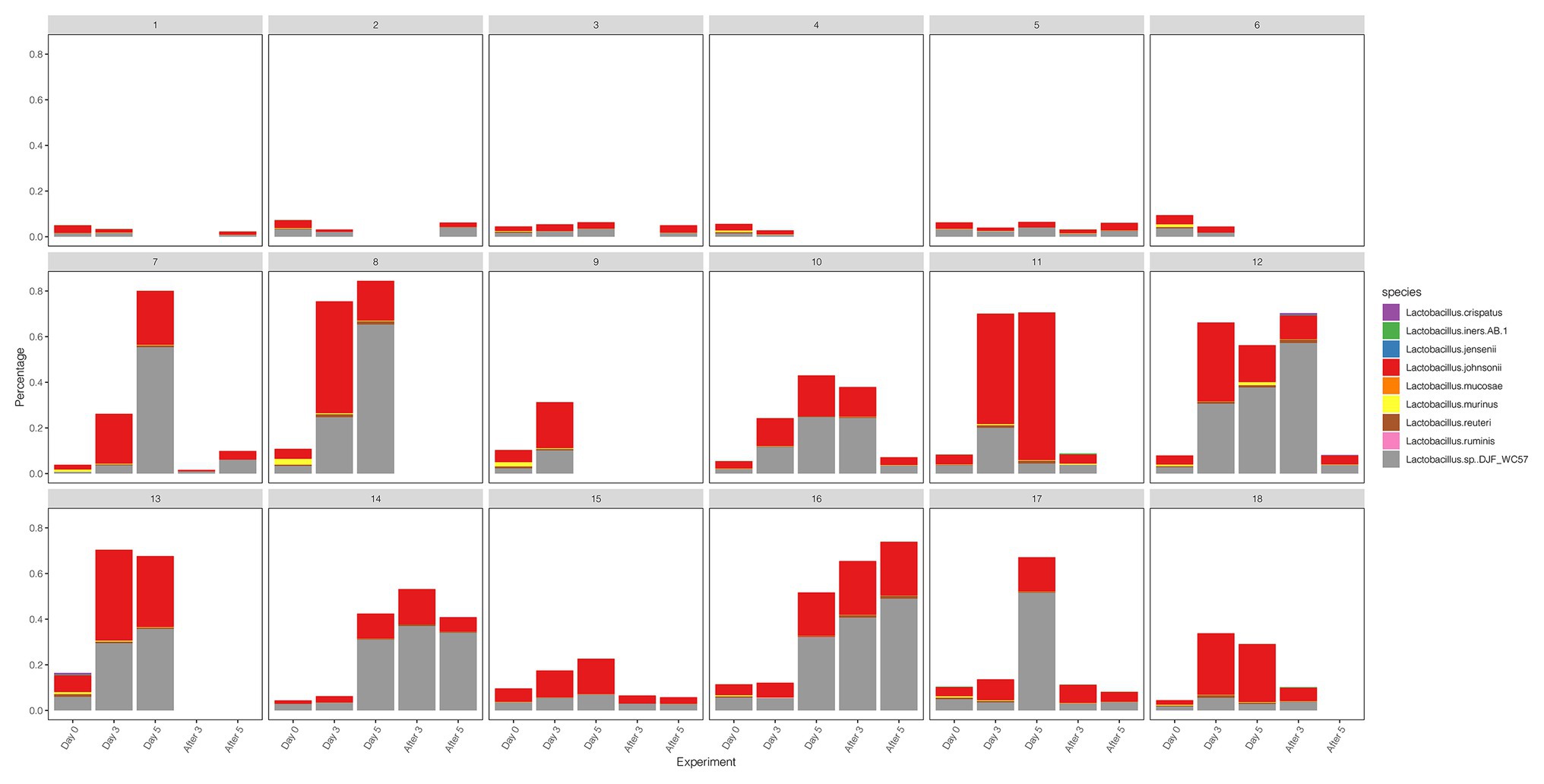
Figure 5. The diversity and abundance of the Lactobacillus during the follow-up of each sample in control and test groups.
Gram Stain of Vaginal Microbiota Before and After Gel Treatment
We stained the vaginal secretions with Gram obtained during different periods of follow-up. In this longitudinal analysis, we select respectively Subject 5 and Subject 16 in control and test groups to display a dynamic morphology under 1,000× magnification. There was almost no significant change in the morphology of Subject 5 before and after treatment (Figure 6A). The morphology of Subject 16 did not change significantly within 3 days after medication (Day 0, Day 1, and Day 2). In Figure 6B, Lactobacillus (green arrows) gradually appeared in the microscope field on Day 3 and Day 4, and the content of Day 4 increased compared with Day 3. Compared with Day 4, there were fewer cocci or vibrio in the microscopic field on Day 5 and only a large number of Gram-positive macrobacillus (mostly Lactobacillus). This indicates that Lactobacillus is predominant in the vagina. During a follow-up of 4 days after withdrawal (After 1, After 2, After 3, and After 4), the number of Lactobacillus decreased gradually.
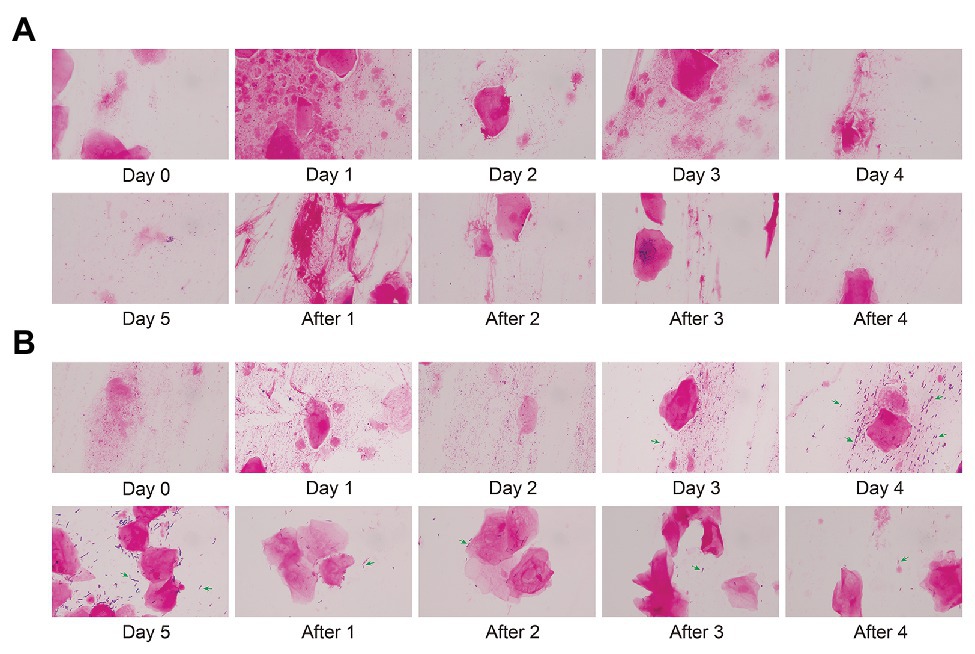
Figure 6. Gram stain of vaginal microbiota before and after gel treatment. (A) The morphology of Subject 5 (control group) under 1,000× magnification after gram staining during placebo gel treatment and withdrawal. (B) The morphology of Subject 16 (test group) during maltose gel treatment and withdrawal. Green arrows indicate Lactobacillus.
Discussion
Diversity of Rhesus Macaque Vaginal Microbiota
This study suggests that the rhesus macaque genital microbiota was relatively polymicrobial, mainly including Porphyromonas, Mobiluncus, Sneathia, Fastidiosipila, Prevotella, Atopobium, Lactobacillus, etc. The microbial profile is characterized by relatively low levels of Lactobacillus and high numbers of Gram-negative bacteria. At the same time, we observed that the background bacteria of the initial vaginal sample (Day 0) after Gram staining consisted of a large number of dense Gram-negative microbacilli and clue cells with unclear boundaries under 1,000× magnification. These microbial features are consistent with previous reports in macaque vagina and similar to the characteristics of BV in women (Spear et al., 2010; Amaral et al., 2017). Considering the characteristics of low Lactobacillus and high BV-related bacteria in macaque vagina, macaque has been gradually used as animal models to study human BV in recent years (Spear et al., 2010; Amaral et al., 2017; Chen et al., 2018).
Healthy vaginal flora is composed of more than 90% Lactobacillus (mainly L. crispatus, L. iners, L. gasseri, or L. jensenii; Ravel et al., 2011). BV occurs when there is a shift in this flora to include a greater proportion of mixed anaerobic bacteria, such as the Gardnerella, Prevotella, and Atopobium species (Force et al., 2020). Although the rhesus macaque is one of the most extensively used nonhuman primate models for human diseases (Simmons, 2016), the main difference between the human BV state and macaque vagina is that the abundance of Gardnerella vaginalis (dominant bacteria) in the former is significantly higher than this in the latter. Additionally, the Lactobacillus (non-dominant bacteria) in the macaque vagina is mainly L. johnsonii, which is not consistent with the Lactobacillus (often L. iners) in BV. Therefore, the microbiota naturally present in primates share great similarities with human pathogens, but the distinction of the predominant microbiota between macaque and human highlights the need for cautious interpretation of the results of animal model studies (Chen et al., 2018).
Prebiotic Maltose Gel Can Induce the Transition From Polymicrobial State of BV to Lactobacillus Dominant
Compared with Day 0, the alpha diversity index (Shannon index, Simpson index, and Invsimpson) declined very significantly in test groups on Day 3 and Day 5 during maltose gel therapy, and the abundance of Lactobacillus was significantly higher with the prolongation of the treatment time than the placebo gel group. At the same time, the abundance of several BV-related bacteria, Fusobacterium, Parvimonas, Mobiluncus, Campylobacter, Prevotella, and Sneathia, decreased on Day 0 to Day 5. The results indicate that prebiotic maltose gel can promote the growth of Lactobacillus and induce the polymicrobial state of BV to Lactobacillus dominant state, which results in the inhibition of BV bacteria in rhesus macaque vagina.
The composition of the human vaginal microbiota ranges from Lactobacillus dominant to a microbiota with diverse anaerobic bacteria, most evident with the common condition BV, which can be associated with a myriad of symptoms and adverse health outcomes (Falconi-McCahill, 2019). Furthermore, after treatment with an antibiotic agent, 20–75% of women have recurrent BV within 3 months (Bradshaw et al., 2006). Antibiotics are the first choice for the treatment of BV in the clinic (Sherrard et al., 2018; Cohen et al., 2020). However, while antibiotics inhibit BV-related bacteria, they may also eliminate Lactobacillus (Goldstein et al., 2015; Anisimova and Yarullina, 2019). This may be one reason for the high recurrence rate of BV. Vaginal Lactobacillus spp. provide broad-spectrum protection by producing lactic acid, bacteriocins, and biosurfactants, and by adherence to the mucosa that forms barriers against pathogenic infection (Kovachev, 2018). The presence of Lactobacillus is an important factor in the prevention of BV infection than antibiotics.
Prebiotics are chemically stable, which can significantly improve the growth and function of microbiota (Singh et al., 2017). The study confirms that maltose gel can facilitate the proliferation of Lactobacillus and promote the vaginal microbiota from BV-related bacteria dominant to Lactobacillus dominant in the rhesus macaque. Our results showed that once the prebiotic maltose gel was discontinued, the levels of Lactobacillus decreased gradually, accompanied by the increase of diversity of the vaginal microbiota. After all, the prebiotic maltose gel is a favorable carbon source for Lactobacillus. Lactobacillus abundance shall decline when carbon source supplies are reduced. In addition, the macaques had high levels of BV-related bacteria in their vagina, and low levels of Lactobacillus originally, which might be the “normal” state of the female macaques. When we artificially interfered with the vaginal microbiome of macaques with prebiotics, it led to the growth of Lactobacillus and the decline of BV-related bacteria. Once the intervention ceases, the physiological regulation of macaques may begin to play a role in promoting “health,” tending towards the initial bacterial state. The current trial evaluated 5 days of treatment and 5 days of follow-up; the further study should be considered to assess the longer-term sustainability of colonization and prevention of BV. Long-term use of maltose gel may maintain the dominance of Lactobacillus.
Glycogen in the vaginal epithelial cells is catabolized by human α-amylase to maltose, maltotriose, and α-dextrins, which are then metabolized to lactic acid by Lactobacillus species (Spear et al., 2014). This creates an acidic environment (pH, 3.5–4.5) conducive for the growth of Lactobacillus at the expense of other anaerobic bacterial species (Amabebe and Anumba, 2018). In our previous research, sucrose gel has a good performance in promoting the proliferation of Lactobacillus (Hu et al., 2015), but then we found that sucrose needs to be stable at around pH 5.5. When sucrose gel is used in the vagina, it will interfere with the normal pH of the vagina. However, maltose is more stable in an environment of pH 3.8, and as a decomposition product of glycogen under natural vagina environment, it is more suitable for selection as a vaginal prebiotic (Spear et al., 2015). Besides, unlike antibiotics, prebiotic substances rarely cause allergies (Pretorius et al., 2018) and do not provoke resistance. While prebiotics may be inferior to antibiotics against pathogens, the properties mentioned above make them a natural substitute for antibiotics (Markowiak and Slizewska, 2017). Based on the advantage above, prebiotic maltose gel may be possible to treat female BV clinically in the future. Notably, different prebiotics will stimulate the growth of different indigenous bacteria (Markowiak and Slizewska, 2017). Lactobacillus in the macaque reproductive tract is mainly L. johnsonii, and also L. crispatus, L. iners, L. jensenii, etc., which are common in the human vagina. Therefore, it may require in vitro and in vivo experiments to prove whether maltose gel is suitable for the treatment of human BV before it can be clinically applied in the future. One of the shortcomings of this study is that the number of macaques is a bit small. However, as our preliminary experiment, this study has confirmed that prebiotic maltose gel can promote the growth of Lactobacillus and induce a shift in the polymicrobial state of BV to Lactobacillus dominant. Then, we will expand the sample size for more detailed experimental verification in the future.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by Medical Ethics Committee of Beijing Tsinghua Changgung Hospital.
Author Contributions
All authors were involved in the conception and design of the study. Q-qZ processed the samples and performed 16S rRNA gene sequencing. Q-qZ, Z-hL, and LZ analyzed the 16S rRNA data. L-lL, GH, and G-lL were responsible for maltose gel administration and follow-up in macaques. Q-qZ, Z-hL, and Q-pL contributed to study design and wrote the manuscript. Q-qZ and YW contributed to obtaining images of vaginal secretions after Gram staining. YC and WW helped coordinate and edit the manuscript. All authors reviewed and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81671409) and Beijing Natural Science Foundation of China (7202239).
Conflict of Interest
L-lL was employed by the company Shenzhen Eulikan Biotechnology Co., Ltd., China. GH and G-lL were employed by the company Sichuan Green-house Biotech Co., Ltd., China. YC and WW were employed by the company Suzhou Turing Microbial Technologies Co., Ltd., China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Zhong-ming Zeng for his coordination work for the experiment. We also thank Shenzhen Eulikan Biotechnology Co., Ltd. for providing maltose gel (Palavigor Malt. Gel) and placebo gel and Sichuan Green-house Biotech Co., Ltd. for access to the macaques. Thanks to Qiao-ying Shi for the assistance with Gram staining of the secretions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.594065/full#supplementary-material
References
Amabebe, E., and Anumba, D. O. C. (2018). The vaginal microenvironment: the physiologic role of lactobacilli. Front. Med. 5:181. doi: 10.3389/fmed.2018.00181
Amaral, W. Z., Lubach, G. R., Kapoor, A., Proctor, A., Phillips, G. J., Lyte, M., et al. (2017). Low lactobacilli abundance and polymicrobial diversity in the lower reproductive tract of female rhesus monkeys do not compromise their reproductive success. Am. J. Primatol. 79:e22691. doi: 10.1002/ajp.22691
Anisimova, E. A., and Yarullina, D. R. (2019). Antibiotic resistance of LACTOBACILLUS strains. Curr. Microbiol. 76, 1407–1416. doi: 10.1007/s00284-019-01769-7
Atashili, J., Poole, C., Ndumbe, P. M., Adimora, A. A., and Smith, J. S. (2008). Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 22, 1493–1501. doi: 10.1097/QAD.0b013e3283021a37
Bokulich, N. A., Kaehler, B. D., Rideout, J. R., Dillon, M., Bolyen, E., Knight, R., et al. (2018). Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:90. doi: 10.1186/s40168-018-0470-z
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Borges, S., Silva, J., and Teixeira, P. (2014). The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 289, 479–489. doi: 10.1007/s00404-013-3064-9
Bradshaw, C. S., Morton, A. N., Hocking, J., Garland, S. M., Morris, M. B., Moss, L. M., et al. (2006). High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 193, 1478–1486. doi: 10.1086/503780
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J., and Holmes, S. P. (2016). DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Chen, Z., Yeoh, Y. K., Hui, M., Wong, P. Y., Chan, M. C. W., Ip, M., et al. (2018). Diversity of macaque microbiota compared to the human counterparts. Sci. Rep. 8:15573. doi: 10.1038/s41598-018-33950-6
Cohen, C. R., Wierzbicki, M. R., French, A. L., Morris, S., Newmann, S., Reno, H., et al. (2020). Randomized trial of Lactin-V to prevent recurrence of bacterial vaginosis. N. Engl. J. Med. 382, 1906–1915. doi: 10.1056/NEJMoa1915254
Falconi-McCahill, A. (2019). Bacterial vaginosis: a clinical update with a focus on complementary and alternative therapies. J. Midwifery Womens Health 64, 578–591. doi: 10.1111/jmwh.13013
Force, U. S. P. S. T., Owens, D. K., Davidson, K. W., Krist, A. H., Barry, M. J., Cabana, M., et al. (2020). Screening for bacterial Vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force Recommendation Statement. JAMA 323, 1286–1292. doi: 10.1001/jama.2020.2684
Ganzle, M. G., and Follador, R. (2012). Metabolism of oligosaccharides and starch in lactobacilli: a review. Front. Microbiol. 3:340. doi: 10.3389/fmicb.2012.00340
Gaziano, R., Sabbatini, S., Roselletti, E., Perito, S., and Monari, C. (2020). Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front. Microbiol. 11:718. doi: 10.3389/fmicb.2020.00718
Gibson, G. R., and Roberfroid, M. B. (1995). Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125, 1401–1412. doi: 10.1093/jn/125.6.1401
Goldstein, E. J., Tyrrell, K. L., and Citron, D. M. (2015). Lactobacillus species: taxonomic complexity and controversial susceptibilities. Clin. Infect. Dis. 60(Suppl. 2), S98–S107. doi: 10.1093/cid/civ072
Gosmann, C., Anahtar, M. N., Handley, S. A., Farcasanu, M., Abu-Ali, G., Bowman, B. A., et al. (2017). Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV Acquisition in young South African women. Immunity 46, 29–37. doi: 10.1016/j.immuni.2016.12.013
Hu, K. T., Zheng, J. X., Yu, Z. J., Chen, Z., Cheng, H., Pan, W. G., et al. (2015). Directed shift of vaginal microbiota induced by vaginal application of sucrose gel in rhesus macaques. Int. J. Infect. Dis. 33, 32–36. doi: 10.1016/j.ijid.2014.12.040
Klebanoff, M. A., and Brotman, R. M. (2018). Treatment of bacterial vaginosis to prevent preterm birth. Lancet 392, 2141–2142. doi: 10.1016/S0140-6736(18)32115-9
Kovachev, S. (2018). Defence factors of vaginal lactobacilli. Crit. Rev. Microbiol. 44, 31–39. doi: 10.1080/1040841X.2017.1306688
Leitich, H., and Kiss, H. (2007). Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 21, 375–390. doi: 10.1016/j.bpobgyn.2006.12.005
Markowiak, P., and Slizewska, K. (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9:1021. doi: 10.3390/nu9091021
Mendling, W., Palmeira-de-Oliveira, A., Biber, S., and Prasauskas, V. (2019). An update on the role of Atopobium vaginae in bacterial vaginosis: what to consider when choosing a treatment? A mini review. Arch. Gynecol. Obstet. 300, 1–6. doi: 10.1007/s00404-019-05142-8
Mitra, A., MacIntyre, D. A., Ntritsos, G., Smith, A., Tsilidis, K. K., Marchesi, J. R., et al. (2020). The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat. Commun. 11:1999. doi: 10.1038/s41467-020-15856-y
Pretorius, R., Prescott, S. L., and Palmer, D. J. (2018). Taking a prebiotic approach to early immunomodulation for allergy prevention. Expert. Rev. Clin. Immunol. 14, 43–51. doi: 10.1080/1744666X.2018.1411191
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S., McCulle, S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U. S. A. 108(Suppl. 1), 4680–4687. doi: 10.1073/pnas.1002611107
Russo, R., Karadja, E., and De Seta, F. (2019). Evidence-based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: a double blind, placebo controlled, randomised clinical trial. Benefic. Microbes 10, 19–26. doi: 10.3920/BM2018.0075
Sherrard, J., Wilson, J., Donders, G., Mendling, W., and Jensen, J. S. (2018). 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int. J. STD AIDS 29, 1258–1272. doi: 10.1177/0956462418785451
Simmons, H. A. (2016). Age-associated pathology in rhesus macaques (Macaca mulatta). Vet. Pathol. 53, 399–416. doi: 10.1177/0300985815620628
Singh, S. P., Jadaun, J. S., Narnoliya, L. K., and Pandey, A. (2017). Prebiotic oligosaccharides: special focus on Fructooligosaccharides, its biosynthesis and bioactivity. Appl. Biochem. Biotechnol. 183, 613–635. doi: 10.1007/s12010-017-2605-2
Spear, G. T., French, A. L., Gilbert, D., Zariffard, M. R., Mirmonsef, P., Sullivan, T. H., et al. (2014). Human alpha-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by lactobacillus. J. Infect. Dis. 210, 1019–1028. doi: 10.1093/infdis/jiu231
Spear, G. T., Gilbert, D., Sikaroodi, M., Doyle, L., Green, L., Gillevet, P. M., et al. (2010). Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res. Hum. Retrovir. 26, 193–200. doi: 10.1089/aid.2009.0166
Spear, G. T., McKenna, M., Landay, A. L., Makinde, H., Hamaker, B., French, A. L., et al. (2015). Effect of pH on cleavage of glycogen by vaginal enzymes. PLoS One 10:e0132646. doi: 10.1371/journal.pone.0132646
Srinivasan, S., Morgan, M. T., Fiedler, T. L., Djukovic, D., Hoffman, N. G., Raftery, D., et al. (2015). Metabolic signatures of bacterial vaginosis. mBio 6, e00204–e00215. doi: 10.1128/mBio.00204-15
Taylor, B. D., Darville, T., and Haggerty, C. L. (2013). Does bacterial vaginosis cause pelvic inflammatory disease? Sex. Transm. Dis. 40, 117–122. doi: 10.1097/OLQ.0b013e31827c5a5b
Yang, X., Da, M., Zhang, W., Qi, Q., Zhang, C., and Han, S. (2018). Role of Lactobacillus in cervical cancer. Cancer Manag. Res. 10, 1219–1229. doi: 10.2147/CMAR.S165228
Yefet, E., Colodner, R., Strauss, M., Gam Ze Letova, Y., and Nachum, Z. (2020). A randomized controlled open label crossover trial to study vaginal colonization of orally administered Lactobacillus reuteri RC-14 and rhamnosus GR-1 in pregnant women at high risk for preterm labor. Nutrients 12:1141. doi: 10.3390/nu12041141
Keywords: maltose gel, vaginal microbiota, Lactobacillus , bacterial vaginosis, rhesus macaque
Citation: Zhang Q-q, Liu Z-h, Liu L-l, Hu G, Lei G-l, Wang Y, Cao Y, Wu W, Zhang L and Liao Q-p (2020) Prebiotic Maltose Gel Can Promote the Vaginal Microbiota From BV-Related Bacteria Dominant to Lactobacillus in Rhesus Macaque. Front. Microbiol. 11:594065. doi: 10.3389/fmicb.2020.594065
Edited by:
Tingtao Chen, Nanchang University, ChinaReviewed by:
Ming Li, Dalian Medical University, ChinaBuzhen Tan, Second Affiliated Hospital of Nanchang University, China
Copyright © 2020 Zhang, Liu, Liu, Hu, Lei, Wang, Cao, Wu, Zhang and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin-ping Liao, cWlucGluZ19saWFvQDE2My5jb20=; Lei Zhang, ZHJfemxAaG90bWFpbC5jb20=
 Qiong-qiong Zhang
Qiong-qiong Zhang Zhi-heng Liu3
Zhi-heng Liu3