- 1State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China
- 2College of Plant Science and Technology, Huazhong Agricultural University, Wuhan, China
- 3Hubei Biopesticide Engineering Research Center, Hubei Academy of Agricultural Sciences, Wuhan, China
- 4College of Life Science and Environmental Resources, Yichun University, Yichun, China
Asaia is a bacterial symbiont of sugar-feeding insects that has been shown to be vertically transmitted by maternal transmission and paternal transmission mechanism, and to be horizontally transmitted via co-feeding artificial diet and venereal routes. Here, the first case of plant-mediated horizontal transmission of Asaia between white-backed planthoppers (WBPH), Sogatella furcifera, was reported. In Asaia-infected WBPH, Asaia was detected mostly in salivary glands and to a less extent in stylets. The rice leaf sheaths fed by Asaia-infected WBPH for 12 h were all positive with Asaia, where Asaia persisted for at least 30 d but was localized in the feeding sites only. When confined to Asaia-infected leaf sheaths for 7 d at the sites pre-infested by the Asaia-infected WBPH, all Asaia-free WBPH became infected with Asaia and the acquired Asaia could be vertically transmitted to their offspring. Phylogenetic analysis confirmed an identical Asaia strain in the Asaia-infected donor WBPH, the Asaia-infected leaf sheaths, and the newly infected recipient WBPH. Our findings provide direct evidence for the first time that rice plant can mediate horizontal transmission of Asaia between WBPH, which may contribute to the spread of Asaia in the field WBPH populations.
Introduction
Most insect species harbor heritable symbionts. Recent studies estimate that about 2/3 of terrestrial arthropod species are infected with at least one species of heritable facultative symbiont (Jaenike, 2015). Given the increasing evidence of symbionts' functions in the interactions among host insects, their host plants and the environment (Oliver et al., 2010), it is crucial to know how the symbionts are transmitted.
Symbionts can be transmitted vertically and/or horizontally (Chiel et al., 2009; Chrostek et al., 2017). Vertical transmission occurs in many insect symbionts (Hosokawa et al., 2007), while horizontal transmission also exists in some symbionts (Oliver et al., 2010), which can be realized through parasitism, predation, mating, and feeding (Chiel et al., 2009; Gonella et al., 2015). The feeding route occurs in insects co-feeding on host plant, such as the transmission of Hamiltonella defensa in Sitobion miscanthi (Li et al., 2018), Serratia symbiotica in Acyrthosiphon pisum and Aphis fabae (Pons et al., 2019a; Skaljac et al., 2019), Cardinium in Scaphoideus titanus (Gonella et al., 2015), Wolbachia and Rickettsia in Bemisia tabaci (Caspi-Fluger et al., 2012; Li et al., 2017a,c), or on artificial diet (Crotti et al., 2009; Gonella et al., 2012, 2015), or on honeydew (Pons et al., 2019b).
Asaia is a bacterial symbiont associated with insects that feed on sugar-based diets (Crotti et al., 2010), particularly those in the order Diptera, Hemiptera, Hymenoptera, and Lepidoptera (Favia et al., 2007; Crotti et al., 2009; Li et al., 2017b; Ojha and Zhang, 2019; Zhang et al., 2019a,b). In addition to paternal transmission (Damiani et al., 2008), Asaia can be transmitted vertically via egg smearing (Crotti et al., 2009; Damiani et al., 2010). Asaia can also be horizontally transmitted through feeding route, such as between S. titanus and mosquitoes feeding on artificial diet mixed with Asaia cells (Crotti et al., 2009) and between S. titanus individuals through co-feeding artificial diet (Gonella et al., 2012), and through mating route (Damiani et al., 2008; Gonella et al., 2012). However, plant-mediated horizontal transmission of Asaia has not been observed.
The white-backed planthopper (WBPH), Sogatella furcifera (Hemiptera: Delphacidae), is one of the most destructive insect pests of rice in Asia (Fujita et al., 2013). Both the nymphs and adults co-feed gregariously at the basal parts of rice plants and cause damage by sucking phloem sap from rice leaf sheath (Rubia-Sanchez et al., 2003). During their feeding on rice plants, six distinctive waveforms have been recorded by electrical penetration graph (Lei et al., 2016). WBPH harbors a fungal yeast-like symbiont (Noda et al., 1995), and bacterial symbionts Wolbachia (Noda et al., 2001), Cardinium (Nakamura et al., 2012) and Asaia (Li et al., 2020). Specifically, Asaia has been revealed to play a role in improving WBPH fitness (Li et al., 2019). In a laboratory WBPH population, Asaia exists in all the individual WBPH, while Asaia is vertically transmitted at only 30% in WBPH (Li et al., 2019), indicating the potential for its horizontal transmission. However, there is no direct evidence showing plant-mediated horizontal transmission of Asaia between host insects including WBPH.
The present study was designed to investigate if Asaia can be horizontally transmitted between WBPH via plants (Figure 1), i.e., transmission from Asaia-infected WBPH to rice leaf sheaths and subsequent acquisition by Asaia-free WBPH feeding on the Asaia-infected leaf sheaths. These questions were addressed through dynamic detection of Asaia in this plant-mediated transmission process by fluorescence in situ hybridization (FISH), diagnostic polymerase chain reaction (PCR), and quantitative real-time PCR (qPCR). Also, persistence and distribution of Asaia in the Asaia-infected rice leaf sheaths were determined.
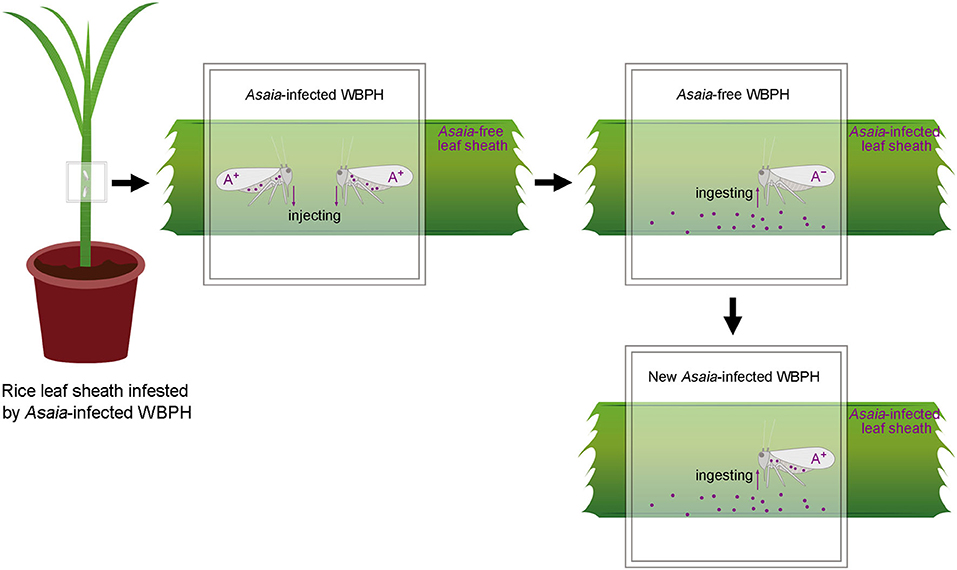
Figure 1. Schematic overview of the rice plant-mediated horizontal transmission of Asaia. A+, Asaia-infected WBPH; A−, Asaia-free WBPH; Dots, Asaia; Black frame, parafilm sachet.
Materials and Methods
Plants and WBPH Populations
Rice plants (var. Taichung Native 1, TN1) used in the experiments included tillering plants and 3-leaf plants. The tillering plants were soil-cultured from seedlings incubated in plastic plates (24 × 18 × 6 cm) containing organic soil (70% peat, 20% humus and 10% vermiculite) and then transplanted to plastic pots (18 cm in height and 18 cm in diameter) in 80-mesh cages in a greenhouse (30 ± 5°C, a photoperiod of 14 L: 10 D). The 3-leaf plants were hydroponically cultured from seeds placed in a glass tube lined with absorbent cotton at the bottom (18 cm in height and 3 cm in diameter, 15 seeds per tube), containing 20 ml rice nutrient solution, and sealed with insect-proof 80-mesh nettings in a phytotron (27 ± 1°C, relative humidity 80 ± 5% and a photoperiod of 14 L: 10 D).
The WBPH population (Lab population) was collected from rice fields in Xing'an (25°3601800 N, 110°4201600 E), China in 2014, and reared on caged rice seedlings in an insectary at the Chinese Academy of Agricultural Sciences (CAAS). Our previous study found that infection of Asaia in this population was 100% (Li et al., 2019). The Lab population served as an Asaia positive WBPH sub-colony. An Asaia negative WBPH sub-colony was established via oral treatment of the Lab population WBPH with tetracycline hydrochloride (Amresco, USA) as described by Li et al. (2019). The infection status of Asaia in these two sub-colonies was checked monthly against 20 randomly selected females and males using PCR with diagnostic primers Asafor and Asarev that amplify 16S rRNA gene (Favia et al., 2007) (Supplementary Figure 1).
Asaia Transmission From WBPH to Rice Plants
To test transmission of Asaia from Asaia-infected WBPH to rice plants, a pair of newly emerged (<12 h) Asaia-infected WBPH adults, deprived of food for 1 h, were confined in a parafilm sachet (5 × 5 cm) attached to the leaf sheath of an Asaia-free tillering rice plant in a cage in the insectary and a total of 11 parafilm sachets were performed. At 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24 or 48 h post WBPH confinement in the sachet (11 treatments in total), the WBPH adults were removed and a 3-cm segment of the WBPH-confined leaf sheath part was collected. The experiment was repeated 30 times, and 30 leaf sheath segments were collected for each treatment. As a negative control, leaf sheath segments were collected from rice plants exposed to the Asaia-free WBPH adults in the same way. Because oviposition of WBPH begins 3.56 d post-emergence on average (Zhu and Cheng, 2001), transmission of Asaia to the rice sheaths via oviposition could be excluded.
Presence of Asaia in the WBPH-exposed leaf sheath segments was qualitatively detected using diagnostic PCR. To this end, total DNA was extracted individually from 20 leaf sheath segments randomly selected from each treatment using the Wizard® Genomic DNA Purification Kit (Promega, USA) according to the manufacturer's protocols. The Asaia-specific primers Asafor and Asarev were used to amplify a sequence of 181 bp of the 16S rRNA gene using 1 μl DNA extract for Asaia detection at conditions: 1 cycle of 94°C for 5 min; 35 cycles of 94°C for 30 s, 62°C for 30 s, and 72°C for 30 s; and a final extension of 72°C for 10 min (Favia et al., 2007). PCR amplified products were visualized on a 2% agarose gel containing GelRed colorant (Biotium, USA). If bands of the expected size were visible on the gels, the PCR products with the expected size were cloned into the pMD-19T plasmid vector (Takara, Japan) and were sequenced. Sterile water was included as a negative control and the DNA samples of WBPH verified by cloning and sequencing were used as a positive control in all PCRs.
Asaia densities in the WBPH-exposed leaf sheath segments were quantified by qPCR with the specific primers Asafor and Asarev using the remaining DNA extract of the 20 samples. To do this, five samples were randomly selected out from the 20 samples and pooled for the quantification. The quantification was performed in three biological repeats (15 samples used in total) each with three technical repetitions. qPCR reactions were conducted with SYBR® Premix Ex Taq™ II (Takara, Japan) in ABI 7500 Real-Time PCR System (Thermo Fisher Scientific, USA). The number of 16S rRNA gene copies of Asaia in the leaf sheath segments was calculated using absolute quantification analysis, following the protocol used in Li et al. (2019). Detailed procedures for qPCR Asaia detection are shown in Supplementary Method 1.
Presence of Asaia in the leaf sheaths and the Asaia-infected WBPH adults was also qualitatively detected using FISH. Twenty to thirty leaf sheath pieces (each 1.0 × 0.5 cm) were cut longitudinally from the rest of collected leaf sheath segments. Twenty to thirty WBPH salivary glands and heads were dissected from the donor Asaia-infected adults in a droplet of phosphate-buffered saline under a stereoscopic microscope. These samples were placed in Carnoy's solution and then hybridized with Asaia-specific Alexa Fluor 488-labeled 16S rDNA probe (A-488: 5′-GTGTAAACCGCCTACGCGCC-3′) (Damiani et al., 2010) using the method described by Li et al. (2020). The final samples were individually mounted on a slide with SlowFade antifade solution and observed under a laser scanning confocal microscope (Zeiss LSM 880, Carl Zeiss, Germany). Asaia-free WBPH and leaf sheaths fed by Asaia-free WBPH treated with the Asaia 16S rDNA probe were used as controls for confirmation of the specificity of Asaia detection.
Persistence of Asaia in Rice Plants
To determine the persistence of Asaia in rice plants following its transmission from WBPH, a pair of newly emerged Asaia-infected WBPH adults starved for 1 h were confined to a leaf sheath in a parafilm sachet for 48 h as described above. This can ensure that Asaia have been transmitted from the WBPH to the plants because the above experiment showed that confinement of a pair of the Asaia-infected WBPH adults for 12 h resulted in Asaia transmission to the WBPH-confined leaf sheaths. A parafilm sachet without WBPH was used as a control. Upon removal of the sachet, the leaf sheath part attached with the sachet was labeled and a segment (~0.4 cm long) of the WBPH-confined leaf sheath part was cut for DNA extraction. This sample collection procedure was run every 5 d for a total period of 30 d. For each collection, three leaf sheath segments were collected. The collected leaf sheath segments were individually measured, each with three technical repetitions, for Asaia densities by qPCR using the specific primers Asafor and Asarev, following the protocol of Li et al. (2019).
Distribution of Asaia in Rice Leaf Sheath
To examine the distribution of Asaia in the Asaia-infected leaf sheath, a test arena was designed, where a parafilm sachet containing a pair of newly emerged Asaia-infected WBPH adults was attached to the leaf sheath of an Asaia-free tillering plant and another parafilm sachet lacking WBPH was attached to the same leaf sheath about 3–5 cm below the sachet containing Asaia-infected WBPH adults. An Asaia-free tillering plant attached at the corresponding sites with a parafilm sachet containing a pair of Asaia-free WBPH and a blank sachet was included as the control. All the test rice plants were individually put in an insect-proof cage in the insectary. After 48 h, all the WBPH adults, along with their sachets, were removed. After another 5 d, a 3-cm segment of the WBPH-confined leaf sheath part was collected from each of the leaf sheaths attached with the sachets. The presence of Asaia in each leaf sheath segments was detected using diagnostic PCR with the specific primers Asafor and Asarev, using the method described in Favia et al. (2007). Three biological replicates were performed for each treatment.
Asaia Transmission From Rice Plants to WBPH and Subsequent Vertical Transmission
To evaluate Asaia acquisition by WBPH from Asaia-infected rice plants, one newly emerged Asaia-free WBPH female adult was confined in a parafilm sachet attached to the feeding site of a pair of Asaia-infected WBPH on a tillering plant right after their 48 h infestation and removal from the leaf sheath. The Asaia-free WBPH females were left to feed on the rice sheaths for 1, 3, 5 or 7 d (the treatments) and a total of 30 females were tested in each treatment. Asaia-free WBPH females fed on Asaia-free leaf sheaths were used as a negative control. Twenty recipient females were randomly selected from each treatment for individual DNA extraction and 1 μl of the DNA extraction was used in detection of Asaia presence by diagnostic PCR with the specific primers Asafor and Asarev, using the method described in Favia et al. (2007). After diagnostic PCR, the remaining DNA of five individual WBPH females (five-WBPH-female DNA) in each treatment were randomly selected and pooled for quantification of Asaia using qPCR with the specific primers Asafor and Asarev, following the protocol of Li et al. (2019). The qPCR experiment was biologically repeated three times. The remaining 10 recipient WBPH females from each treatment were dissected for guts to examine Asaia presence using FISH.
Further tests were conducted to examine if the Asaia in WBPH acquired through feeding could be vertically transmitted to the next generation. A pair of the Asaia-free WBPH adults was fed on an Asaia-infected leaf sheath in a sachet for 7 d to ensure acquisition of Asaia. Then the pair of adults was introduced into a glass tube containing Asaia-free 3-leaf rice seedlings. After 24 h, the WBPH adults were removed for Asaia detection individually to confirm the infection of Asaia in the parent adults (F0 generation). If both parents were infected with Asaia, their newly hatched nymphs (F1 generation) were individually transferred to new glass tubes with Asaia-free 3-leaf rice seedlings and allowed to develop ad lib. Twenty females and males randomly selected from the resulting F1 adults were individually examined for the presence of Asaia by PCR with the specific primers Asafor and Asarev, using the method described in Favia et al. (2007). Three pairs of parental WBPH adults (F0) and their offspring (F1) were tested.
Phylogenetic Analysis of Asaia in Relation With Transmission
A multilocus phylogenetic analysis was performed to conform the identity of the Asaia in the Asaia-infected donor WBPH, the Asaia-infected leaf sheath, and the newly infected recipient WBPH. Two Asaia genes: the 16S-23S rDNA internal transcribed spacer (ITS) and the groEL gene, were sequenced following the reported methods (Ruiz et al., 2000; Cleenwerck et al., 2010). The ITS and groEL gene sequences obtained in this study were registered with the GenBank database under accession numbers of MN095200-MN095202 and MN114618-MN114620, respectively. Multiple sequence alignments were performed using the program package Clustal W. The final alignments were manually inspected and corrected. Phylogenetic trees were constructed using the maximum likelihood (ML) method in MEGA v6.0 (Tamura et al., 2013). The ML trees were constructed with HKY + G model for ITS and K2 + I model for the groEL gene from Asaia. Bootstrap analysis of 1,000 replicates was used to deduce confidence levels.
Results
Asaia Transmission From WBPH to Rice Plants
When fed by the Asaia-infected WBPH, rice plants were infected with Asaia (Figures 2A,B; Supplementary Figure 1). Diagnostic PCR showed that the Asaia infection rate of rice plants showed a logistic pattern of increase with the extension of feeding by WBPH (Figure 2A). Feeding for 0.5 h by a pair of the Asaia-infected WBPH adults resulted in an infection rate of 33.3%. The infection rate reached 100% when WBPH feeding was 12 h or more. With qPCR, it is obvious that Asaia density in leaf sheaths also increased with the prolonged feeding by the Asaia-infected WBPH, in a pattern similar to an exponential increase (Figure 2B). During the initial feeding for 0.5–4 h, Asaia densities in leaf sheaths increased slowly, then increased sharply starting from feeding for 6 h.
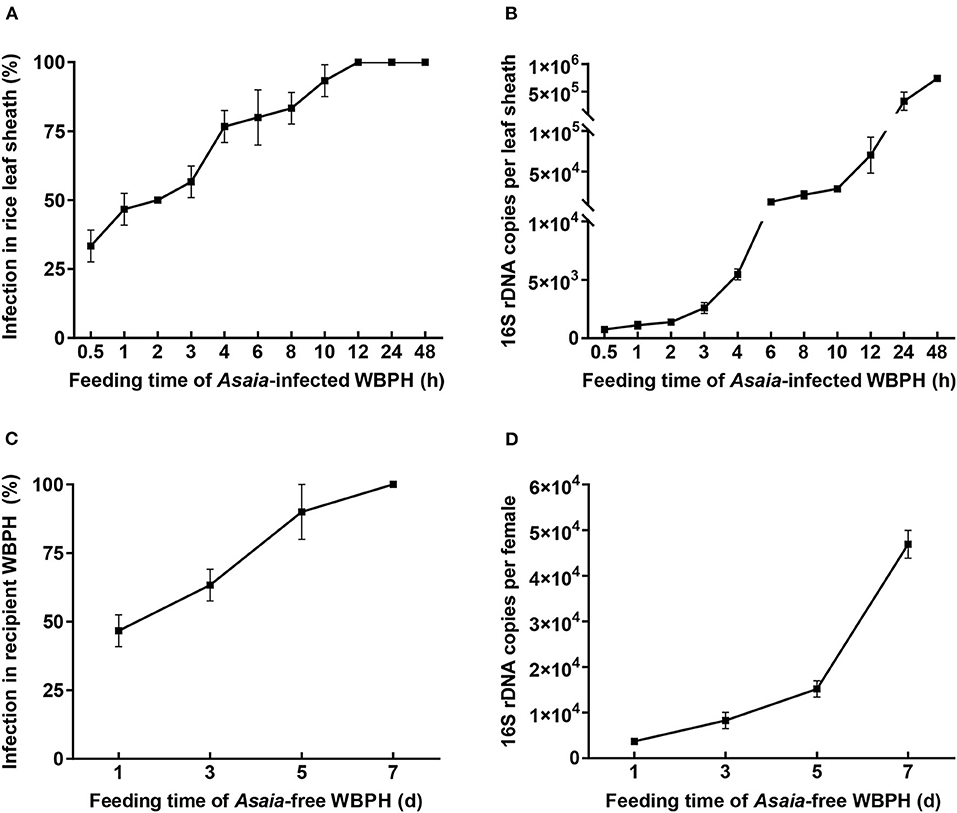
Figure 2. Asaia infection rate and density in leaf sheaths and recipient WBPH. (A) Asaia infection rate in leaf sheaths fed by Asaia-infected WBPH. (B) Asaia density in leaf sheaths fed by Asaia-infected WBPH. (C) Asaia infection rate in the recipient WBPH feeding on Asaia-infected rice leaf sheaths. (D) Asaia density in the recipient WBPH feeding on Asaia-infected rice leaf sheaths. The data are expressed as means ± sd.
FISH visualization showed that, in the Asaia-infected WBPH, masses of Asaia coalesced in the accessory salivary gland (Figure 3A) and a small amount of Asaia existed in the stylet (Figure 3B). In the leaf sheaths fed by the Asaia-infected WBPH, Asaia was distributed longitudinally along leaf vein in the sucked points (Figures 3D,E). No fluorescence of Asaia was visualized in the tissues of Asaia-free WBPH or in the leaf sheaths exposed to Asaia-free WBPH (Supplementary Figure 2).
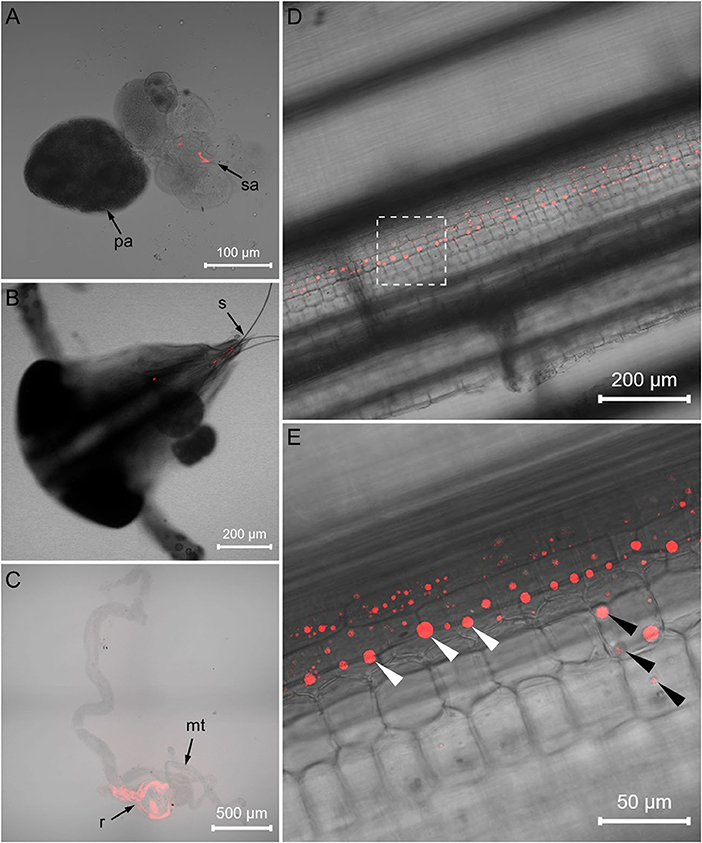
Figure 3. FISH visualization of Asaia in Asaia-infected WBPH and rice leaf sheath. (A) Salivary gland of the Asaia-infected WBPH. (B) Head of the Asaia-infected WBPH. (C) Infected rice leaf sheath. (D) Magnified image of (C). (E) Gut of the newly infected recipient WBPH. pa, primary salivary gland; sa, accessory salivary gland; s, stylet; mt, Malpighian tubule; r, rectum; white arrowheads, Asaia; black arrowheads, sucked points.
Persistence of Asaia in Rice Plants
When rice leaf sheaths were exposed to a pair of Asaia-infected WBPH for only 48 h, Asaia densities in the leaf sheaths showed downward parabola dynamics during a 30-d period post the exposure (Figure 4). Asaia densities peaked at 5 d post exposure and then decreased. Asaia densities in the leaf sheaths at 30 d post exposure was reduced to 21.0% of the initial densities (at 0 d post exposure) and 5.8% of the peak densities (at 5 d post exposure).
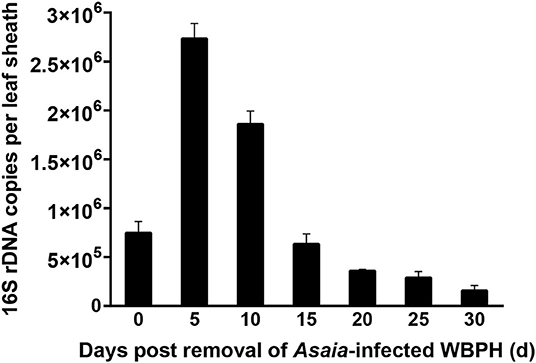
Figure 4. Persistence of Asaia in rice leaf sheaths horizontally transmitted from Asaia-infected WBPH through feeding. The data are expressed as means ± sd.
Distribution of Asaia in Rice Leaf Sheath
Asaia in rice leaf sheath was restricted to the feeding sites, i.e., Asaia did not move in the leaf sheath. PCR detection showed that Asaia was detected only in the leaf sheath segments infested by the Asaia-infected WBPH, not from the leaf sheath segments below the feeding sites, and nor from any of the leaf sheaths in the control (Figure 5).
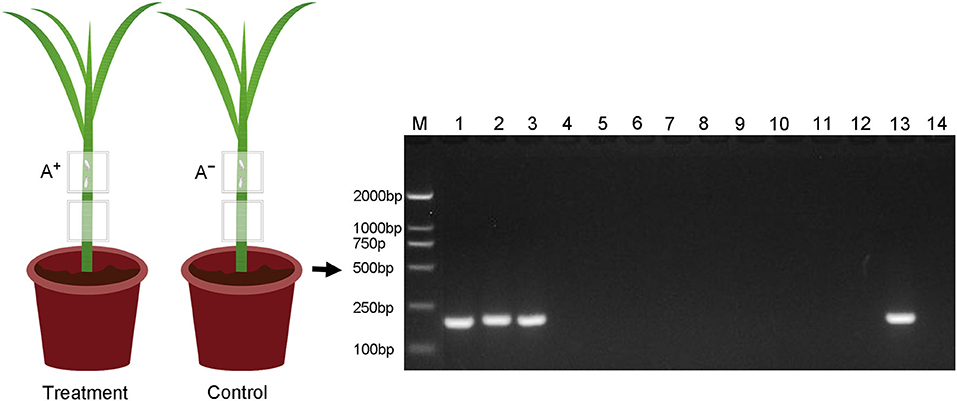
Figure 5. Detection of Asaia in different parts of the rice leaf sheaths fed by Asaia-infected and Asaia-free WBPH or not. M, DNA marker; Lanes 1–3, the upper leaf sheath segments fed by Asaia-infected WBPH (the treatment); lanes 4–6, the lower leaf sheath segments from the treatment without WBPH feeding; lanes 7–9, the upper leaf sheath segments fed by Asaia-free WBPH (the control); lanes 10–12, the lower leaf sheath segments from the control without WBPH feeding; lane 13, positive control of PCR; lane 14, negative control of PCR; A+, Asaia-infected WBPH; A−, Asaia-free WBPH.
Asaia Transmission From Rice Plants to WBPH and Subsequent Vertical Transmission
When the Asaia-free WBPH females were confined to the Asaia-infected rice sheaths, Asaia was successfully transmitted from the plants to the WBPH (Figures 2C,D; Supplementary Figure 1). The transmission was a feeding-time dependent process, with Asaia infection rates in the WBPH increasing almost linearly from 46.7% at feeding for 1 d to 100% at feeding for 7 d (Figure 2C). Asaia densities in the WBPH increased with the extension of feeding in an exponential manner (Figure 2D), being 12.6 times more at feeding for 7 d than at feeding for 1 d. FISH visualization showed heavy presence of Asaia in the rectum of the WBPH fed on Asaia-infected rice plants for 7 d (Figure 3C).
Vertical transmission of Asaia was measured in the WBPH that acquired Asaia from the Asaia-infected rice plants during a 7-d feeding period. The results showed that 40.0% of F1 female and 38.3% of F1 male WBPH adults were Asaia positive (Supplementary Figure 1). There was no significant sexual difference in the vertical transmission rate (t = 0.223, df = 4, P = 0.835).
Phylogenetic Analysis of Asaia in Relation With Transmission
The Asaia sequences of ITS and groEL gene were used in the phylogenetic analysis. These genetic analyses revealed an identical Asaia strain in the Asaia-infected donor WBPH, the Asaia-infected leaf sheaths, and the newly infected recipient WBPH (Figure 6). These results show that the Asaia symbionts remain consistent during the plant-mediated horizontal transmission.
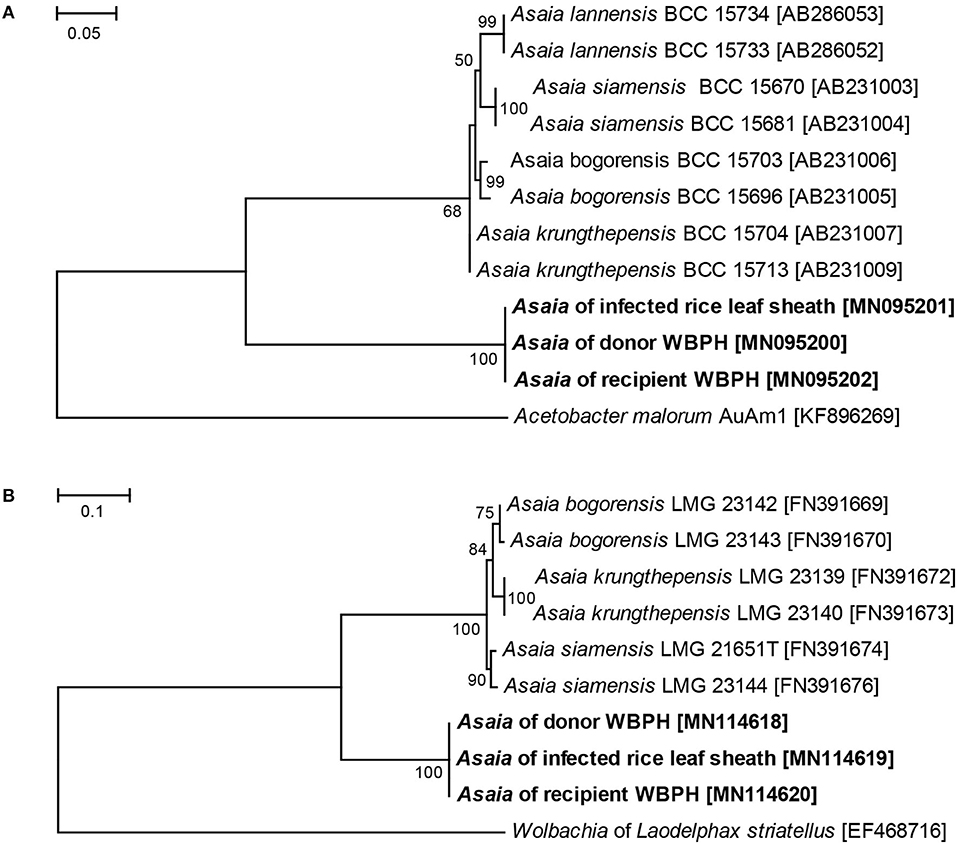
Figure 6. ML phylogenetic analysis of Asaia strains detected in different hosts. (A) ML tree based on Asaia ITS sequences. (B) ML tree based on Asaia groEL gene sequences. Acetobacter malorum AuAm1 and Wolbachia of Laodelphax striatellus were used as outgroups. Accession numbers of the sequences retrieved from GenBank are shown in square brackets. Sequences obtained in this study are shown in bold. The percent bootstrap values are shown at the nodes.
Discussion
The horizontal transmission routes of symbionts have emerged as a focus of symbiont research over the past two decades (Moran and Dunbar, 2006; Jaenike et al., 2007; Caspi-Fluger et al., 2012; Ahmed et al., 2015; Li et al., 2017a,c, 2018; Pons et al., 2019a,b; Skaljac et al., 2019). Symbionts can be horizontally transmitted through such routes as parasitism, predation, mating, and feeding (Gonella et al., 2015). Previous studies have revealed that Asaia can be horizontally transmitted through co-feeding artificial diet (Crotti et al., 2009; Gonella et al., 2012) and through mating (Damiani et al., 2008; Gonella et al., 2012). Our current study provides evidence for the first time that Asaia can be horizontally transmitted via plant.
Symbionts colonize different organs in host insects, principally the salivary glands and guts, which are key organs involved in the horizontal transmission of symbionts (Chouaia et al., 2014). Our FISH visualization results showed Asaia in the salivary glands and stylets of the Asaia-infected donor WBPH (Figures 3A,B) and in the guts of the newly infected recipient WBPH (Figure 3C), which is consistent with Asaia localization in mosquitoes and leafhopper (Favia et al., 2007; Crotti et al., 2009; Gonella et al., 2012), and also in the leaf sheaths infested by the Asaia-infected donor WBPH (Figures 3D,E). Further, the Asaia in the newly infected recipient WBPH was vertically transmitted to their offspring at ca. 39%, corresponding to our previous detection of 30% heritable transmission (Li et al., 2019). In contrast, field WBPH populations are infected with Asaia at as high as 83.3% and in the laboratory population, at 100% (Li et al., 2020). These results indicate that horizontal transmission does occur in Asaia, especially in the case of laboratory population where the insects are confined in a cage. Furthermore, the phylogenetic analysis (Figure 6) revealed that the Asaia strain in the newly infected recipient WBPH is exactly the one from the Asaia-infected donor WBPH. Similar results were previously obtained with Wolbachia during horizontal transmission between two species of insect hosts (Ahmed et al., 2015; Li et al., 2017a). In addition, there is a possibility of transmission via honeydew. However, PCR revealed that the honeydews collected from the parafilm sachets with Asaia-infected WBPH were negative with Asaia (Supplementary Figure 1); and if honeydew contains Asaia, FISH will show massive fluorescence signals instead of dotted fluorescence signals as shown in this study (Figures 3D,E). Therefore, transmission via honeydew can be excluded. From these results, it is certain that plant-mediated horizontal transmission of Asaia does occur: Asaia harbored in the salivary glands of the Asaia-infected WBPH are injected with saliva via the stylet into the leaf sheaths, thus producing Asaia-infected rice plants; then the Asaia therein are ingested via the stylet into the gut of the recipient WBPH when they feed on the feeding sites of the Asaia-infected WBPH (Figure 1). The Asaia in the gut of the newly infected recipient WBPH finally cross the numerous physical and biochemical barriers to the salivary glands.
In this study, Asaia was quickly and efficiently transmitted from the Asaia-infected WBPH to rice plants. Akin to the transmission of a virus by virus-infected WBPH to rice plants (Lei et al., 2016), Asaia transmission is also a process that depends on the extent of feeding by the Asaia-infected WBPH (Figures 2A,B). Besides, symbiont transmission differs in their association with hosts. In contrast to the slow transmission of H. defensa by the aphids S. miscanthi (Li et al., 2018), Asaia transmission is quick; the difference may be that Asaia is an extracellular symbiont (Favia et al., 2007) while H. defensa is an extracellular and intracellular symbiont (Dykstra et al., 2014; Chrostek et al., 2017).
In the infected rice plants, Asaia persisted for as long as 30 d, like Rickettsia persisting in cotton leaves (Li et al., 2017c), although less than the 50-d persistence of Wolbachia in cotton leaves (Li et al., 2017a). The extended persistence may help enhance the chance of horizontal transmission of symbiont in the field. During the persistence of Asaia in the leaf sheaths, Asaia density experienced a downward parabola dynamic, peaking at 5 d post infestation by the Asaia-infected WBPH (Figure 4), indicating Asaia might have self-multiplied in plant tissues. It may be reasoned that the dynamic change of Asaia density in the leaf sheaths is a result of plant defense, in which the plants may take some time to mobilize certain chemicals to fight against or even eliminate the invading symbionts (Li et al., 2017c). However, this reasoning needs to be further investigated in future.
It is surprising that, in this study, Asaia couldn't be detected in the lower segment of the same leaf sheath that was infested with the Asaia-infected WBPH in an upper leaf sheath segment (Figure 5), indicating that Asaia is restricted locally to the WBPH feeding sites. This result is consistent with the localized infection of H. defensa in wheat leaves (Li et al., 2018), while contrary to that reported for Rickettsia (Caspi-Fluger et al., 2012; Li et al., 2017c), where Rickettsia move within the phloem and can be detected in the lower adjacent cotton leaves. The Asaia-free WBPHs were all infected with Asaia after feeding on Asaia-infected rice leaves for 7 d (Figure 2C), however this occurred in this study when they were confined to the feeding sites of the Asaia-infected WBPHs. The localized distribution of Asaia in the infected leaf sheath may limit its transmission, while this may be circumvented, to certain extent, by the efficient Asaia inoculation from the donor WBPH to rice plant, the extended Asaia persistence in rice plant, and the gregarious feeding habit of WBPH. The interconnection of these factors may enhance plant-mediated horizontal transmission of Asaia in nature.
Horizontal transmission may serve as a compensatory event to vertical transmission to rescue symbiont loss (Casiraghi et al., 2004) or replace the symbiont (Moran and Yun, 2015) in the host insect to help maintain the symbiont population in nature. In plant-mediated horizontal transmission, plants act as “springboards” for the symbionts to be spread to more host insects (Frago et al., 2012). Plant-mediated transmission of symbiont in WBPH has not been reported previously. Thus, the current results have broadened our understanding of symbiont transmission routes in WBPH. Whether horizontal transmission of Asaia occurs between Asaia-infected WBPH and other planthopper species needs to be investigated in the future, as Asaia has been detected in the small brown planthopper (Laodelphax striatellus) (Li et al., 2017b; Zhang et al., 2019b) and the brown planthopper (N. lugens) (Ojha and Zhang, 2019; Zhang et al., 2019a). Although most symbionts are transmitted vertically (Hosokawa et al., 2007), a recent study shows that, during plant-mediated horizontal transmission, symbionts orally secreted from the herbivorous hosts help suppress plant defenses (Moran and Yun, 2015). Thus, symbionts in herbivorous insects can not only confer numerous ecologically relevant traits to their hosts directly (Oliver et al., 2010), but also influence their hosts indirectly through involvement in insect-plant interactions (Beck et al., 2018) as a component of insect saliva (Zhang et al., 2017).
Conclusions
Altogether, the current results have provided novel evidence showing the plant-mediated horizontal transmission of the bacterial symbiont Asaia between WBPH. The Asaia in the Asaia-infected donor WBPH is transmitted to rice plants quickly and efficiently when the insects feed on the leaf sheaths, and the Asaia in the infected leaf sheaths can also be efficiently acquired by the Asaia-free recipient WBPH when the insects feed on the leaf sheath sites pre-infested by the donor WBPH. The efficient Asaia inoculation from the donor WBPH to rice plants, the extended Asaia persistence in rice plants, and the gregarious feeding habit of WBPH can circumvent the limiting effects of the localized infection of Asaia in rice plants. Furthermore, the Asaia acquired by the Asaia-free recipient WBPH can be vertically transmitted to their offspring. Thus, the horizontal transmission via feeding route, coupled with vertical transmission, can help maintain Asaia in the field WBPH populations.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, MN094401; https://www.ncbi.nlm.nih.gov/genbank/, MN094402; https://www.ncbi.nlm.nih.gov/genbank/, MN094403; https://www.ncbi.nlm.nih.gov/genbank/, MN095200; https://www.ncbi.nlm.nih.gov/genbank/, MN095201; https://www.ncbi.nlm.nih.gov/genbank/, MN095202; https://www.ncbi.nlm.nih.gov/genbank/, MN114618; https://www.ncbi.nlm.nih.gov/genbank/, MN114619; https://www.ncbi.nlm.nih.gov/genbank/, MN114620.
Author Contributions
FL, HH, and MH conceived the idea, experimental design, and wrote the manuscript. FL and YH carried out the experiments. FL and MH analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Key R&D Program of China (2016YFD0300700 and 2018YFD0200300).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Haiyan Zhang and Siwen Zhao for assistance in the experiments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.593485/full#supplementary-material
References
Ahmed, M. Z., Li, S. J., Xue, X., Yin, X. J., Ren, S. X., Jiggins, F. M., et al. (2015). The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLoS Pathog. 11:e1004672. doi: 10.1371/journal.ppat.1004672
Beck, J. J., Alborn, H. T., Block, A. K., Christensen, S. A., Hunter, C. T., Rering, C. C., et al. (2018). Interactions among plants, insects, and microbes: elucidation of inter-organismal chemical communications in agricultural ecology. J. Agric. Food. Chem. 66, 6663–6674. doi: 10.1021/acs.jafc.8b01763
Casiraghi, M., Bain, O., Guerrero, R., Martin, C., Pocacqua, V., Gardner, S. L., et al. (2004). Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Int. J. Parasitol. 34, 191–203. doi: 10.1016/j.ijpara.2003.10.004
Caspi-Fluger, A., Inbar, M., Mozes-Daube, N., Katzir, N., Portnoy, V., Belausov, E., et al. (2012). Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc. Biol. Sci. 279, 1791–1796. doi: 10.1098/rspb.2011.2095
Chiel, E., Zchori-Fein, E., Inbar, M., Gottlieb, Y., Adachi-Hagimori, T., Kelly, S. E., et al. (2009). Almost there: transmission routes of bacterial symbionts between trophic levels. PLoS ONE 4:e0004767. doi: 10.1371/journal.pone.0004767
Chouaia, B., Gaiarsa, S., Crotti, E., Comandatore, F., Esposti, M. D., Ricci, I., et al. (2014). Acetic acid bacteria genomes reveal functional traits for adaptation to life in insect guts. Genome Biol. Evol. 6, 912–920. doi: 10.1093/gbe/evu062
Chrostek, E., Pelz-Stelinski, K., Hurst, G. D. D., and Hughes, G. L. (2017). Horizontal transmission of intracellular insect symbionts via plants. Front. Microbiol. 8:2237. doi: 10.3389/fmicb.2017.02237
Cleenwerck, I., De Vos, P., and De Vuyst, L. (2010). Phylogeny and differentiation of species of the genus Gluconacetobacter and related taxa based on multilocus sequence analyses of housekeeping genes and reclassification of Acetobacter xylinus subsp. sucrofermentans as Gluconacetobacter sucrofermentans (Toyosaki et al. 1996) sp. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 60, 2277–2283. doi: 10.1099/ijs.0.018465-0
Crotti, E., Damiani, C., Pajoro, M., Gonella, E., Rizzi, A., Ricci, I., et al. (2009). A versatile acetic acid bacterial symbiont, capable of cross–colonizing insects of phylogenetically distant genera and orders. Environ. Microbiol. 11, 3252–3264. doi: 10.1111/j.1462-2920.2009.02048.x
Crotti, E., Rizzi, A., Chouaia, B., Ricci, I., Favia, G., Alma, A., et al. (2010). Acetic acid bacteria, new emerging symbionts of insects. Appl. Environ. Microbiol. 76, 6963–6970. doi: 10.1128/AEM.01336-10
Damiani, C., Ricci, I., Crotti, E., Rossi, P., Rizzi, A., Scuppa, P., et al. (2008). Paternal transmission of symbiotic bacteria in malaria vectors. Curr. Biol. 18, R1087–R1088. doi: 10.1016/j.cub.2008.10.040
Damiani, C., Ricci, I., Crotti, E., Rossi, P., Rizzi, A., Scuppa, P., et al. (2010). Mosquito-bacteria symbiosis: the case of Anopheles gambiae and Asaia. Microb. Ecol. 60, 644–654. doi: 10.1007/s00248-010-9704-8
Dykstra, H. R., Weldon, S. R., Martinez, A. J., White, J. A., Hopper, K. R., Heimpel, G. E., et al. (2014). Factors limiting the spread of the protective symbiont Hamiltonella defensa in Aphis craccivora Aphids. Appl. Environ. Microbiol. 80, 5818–5827. doi: 10.1128/AEM.01775-14
Favia, G., Ricci, I., Damiani, C., Raddadi, N., Crotti, E., Marzorati, M., et al. (2007). Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl. Acad. Sci. U. S. A. 104, 9047–9051. doi: 10.1073/pnas.0610451104
Frago, E., Dicke, M., and Godfray, H. C. J. (2012). Insect symbionts as hidden players in insect-plant interactions. Trends Ecol. Evol. 27, 705–711. doi: 10.1016/j.tree.2012.08.013
Fujita, D., Kohli, A., and Horgan, F. G. (2013). Rice resistance to planthoppers and leafhoppers. Crit. Rev. Plant. Sci. 32, 162–191. doi: 10.1080/07352689.2012.735986
Gonella, E., Crotti, E., Rizzi, A., Mandrioli, M., Favia, G., Daffonchio, D., et al. (2012). Horizontal transmission of the symbiotic bacterium Asaia sp. in the leafhopper Scaphoideus stitanus Ball (Hemiptera: Cicadellidae). BMC Microbiol. 12:S4. doi: 10.1186/1471-2180-12-S1-S4
Gonella, E., Pajoro, M., Marzorati, M., Crotti, E., Mandrioli, M., Pontini, M., et al. (2015). Plant-mediated interspecific horizontal transmission of an intracellular symbiont in insects. Sci. Rep. 5:5811. doi: 10.1038/srep15811
Hosokawa, T., Kikuchi, Y., and Fukatsu, T. (2007). How many symbionts are provided by mothers, acquired by offspring, and needed for successful vertical transmission in an obligate insect-bacterium mutualism? Mol. Ecol. 16, 5316–5325. doi: 10.1111/j.1365-294X.2007.03592.x
Jaenike, J. (2015). Heritable symbionts contribute to host plant adaptation. Funct. Ecol. 29, 1371–1372. doi: 10.1111/1365-2435.12547
Jaenike, J., Polak, M., Fiskin, A., Helou, M., and Minhas, M. (2007). Interspecific transmission of endosymbiotic Spiroplasma by mites. Biol. Lett. 3, 23–25. doi: 10.1098/rsbl.2006.0577
Lei, W., Li, P., Han, Y., Gong, S., Yang, L., and Hou, M. (2016). EPG recordings reveal differential feeding behaviors in Sogatella furcifera in response to plant virus infection and transmission success. Sci. Rep. 6:30240. doi: 10.1038/srep30240
Li, F., Hua, H., Ali, A., and Hou, M. (2019). Characterization of a bacterial symbiont Asaia sp. in the white-backed planthopper, Sogatella furcifera, and its effects on host fitness. Front. Microbiol. 10:2179. doi: 10.3389/fmicb.2019.02179
Li, F., Li, P., Hua, H., Hou, M., and Wang, F. (2020). Diversity, tissue localization and infection pattern of bacterial symbionts of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Microb. Ecol. 79, 720–730. doi: 10.1007/s00248-019-01433-4
Li, Q., Fan, J., Sun, J., Wang, M. Q., and Chen, J. (2018). Plant-mediated horizontal transmission of Hamiltonella defensa in the wheat aphid Sitobion miscanthi. J. Agric. Food. Chem. 66, 13367–13377. doi: 10.1021/acs.jafc.8b04828
Li, S., Zhou, C., Chen, G., and Zhou, Y. (2017b). Bacterial microbiota in small brown planthopper populations with different rice viruses. J. Basic Microbiol. 57, 590–596. doi: 10.1002/jobm.201700004
Li, S. J., Ahmed, M. Z., Lv, N., Shi, P. Q., Wang, X. M., Huang, J. L., et al. (2017a). Plant-mediated horizontal transmission of Wolbachia between whiteflies. ISME J. 11:1019–1028. doi: 10.1038/ismej.2016.164
Li, Y. H., Ahmed, M. Z., Li, S. J., Lv, N., Shi, P. Q., Chen, X. S., et al. (2017c). Plant-mediated horizontal transmission of Rickettsia endosymbiont between different whitefly species. FEMS Microbiol. Ecol. 93, 1–9. doi: 10.1093/femsec/fix138
Moran, N. A., and Dunbar, H. E. (2006). Sexual acquisition of beneficial symbionts in aphids. Proc. Natl. Acad. Sci. U. S. A. 103, 12803–12806. doi: 10.1073/pnas.0605772103
Moran, N. A., and Yun, Y. (2015). Experimental replacement of an obligate insect symbiont. Proc. Natl. Acad. Sci. U. S. A. 112, 2093–2096. doi: 10.1073/pnas.1420037112
Nakamura, Y., Yukuhiro, F., Matsumura, M., and Noda, H. (2012). Cytoplasmic incompatibility involving Cardinium and Wolbachia in the white-backed planthopper Sogatella furcifera (Hemiptera Delphacidae). Appl. Entomol. Zool. 47, 273–283. doi: 10.1007/s13355-012-0120-z
Noda, H., Koizumi, Y., Zhang, Q., and Deng, K. (2001). Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem. Mol. Biol. 31, 727–737. doi: 10.1016/S0965-1748(00)00180-6
Noda, H., Nakashima, N., and Koizumi, M. (1995). Phylogenetic position of yeast-like symbiotes of rice planthoppers based on partial 18S rDNA sequences. Insect Biochem. Mol. Biol. 25, 639–646. doi: 10.1016/0965-1748(94)00107-S
Ojha, A., and Zhang, W. (2019). A comparative study of microbial community and dynamics of Asaia in the brown planthopper from susceptible and resistant rice varieties. BMC Microbiol. 19:139. doi: 10.1186/s12866-019-1512-9
Oliver, K. M., Degnan, P. H., Burke, G. R., and Moran, N. A. (2010). Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 55, 247–266. doi: 10.1146/annurev-ento-112408-085305
Pons, I., Renoz, F., Noël, C., and Hance, T. (2019a). Circulation of the cultivable symbiont Serratia symbiotica in aphids is mediated by plants. Front. Microbiol. 10:764. doi: 10.3389/fmicb.2019.00764
Pons, I., Renoz, F., Noël, C., and Hance, T. (2019b). New insights into the nature of symbiotic associations in aphids: infection process, biological effects, and transmission mode of cultivable Serratia symbiotica bacteria. Appl. Environ. Microbiol. 2:85. doi: 10.1128/AEM.02445-18
Rubia-Sanchez, E., Suzuki, Y., Arimura, K., Miyamoto, K., Matsumura, M., and Watanabe, T. (2003). Comparing Nilaparvata lugens (Stal) and Sogatella furcifera (Horvath) (Homoptera: Delphacidae) feeding effects on rice plant growth processes at the vegetative stage. Crop Prot. 22, 967–974. doi: 10.1016/S0261-2194(03)00112-1
Ruiz, A., Poblet, M., Mas, A., and Guillamon, J. M. (2000). Identification of acetic acid bacteria by RFLP of PCR-amplified 16S rDNA and 16S-23S rDNA intergenic spacer. Int. J. Syst. Evol. Micr. 50, 1981–1987. doi: 10.1099/00207713-50-6-1981
Skaljac, M., Vogel, H., Wielsch, N., Mihajlovic, S., and Vilcinskas, A. (2019). Transmission of a protease-secreting bacterial symbiont among pea aphids via host plants. Front. Physiol. 10:438. doi: 10.3389/fphys.2019.00438
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA 6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Zhang, J. H., Yu, N., Xu, X. X., and Liu, Z. W. (2019a). Community structure, dispersal ability and functional profiling of microbiome existing in fat body and ovary of the brown planthopper, Nilaparvata lugens. Insect Sci. 26, 683–694. doi: 10.1111/1744-7917.12575
Zhang, X., Li, T. P., Zhou, C. Y., Zhao, D. S., Zhu, Y. X., Bing, X. L., et al. (2019b). Antibiotic exposure perturbs the bacterial community in the small brown planthopper Laodelphax striatellus. Insect Sci. 27, 895–907. doi: 10.1111/1744-7917.12675
Zhang, Y., Fan, J., Francis, F., and Chen, J. (2017). Watery saliva secreted by the grain aphid Sitobion avenae stimulates aphid resistance in wheat. J. Agric. Food. Chem. 65, 8798–8805. doi: 10.1021/acs.jafc.7b03141
Keywords: Asaia, Sogatella furcifera, plant-mediated, horizontal transmission, symbiont
Citation: Li F, Hua H, Han Y and Hou M (2020) Plant-Mediated Horizontal Transmission of Asaia Between White-Backed Planthoppers, Sogatella furcifera. Front. Microbiol. 11:593485. doi: 10.3389/fmicb.2020.593485
Received: 10 August 2020; Accepted: 06 November 2020;
Published: 30 November 2020.
Edited by:
Mariana Mateos, Texas A&M University, United StatesReviewed by:
Tong Zhang, South China Agricultural University, ChinaMorten Schiøtt, Technical University of Denmark, Denmark
Copyright © 2020 Li, Hua, Han and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maolin Hou, bWFvbGluaG91X2lwcEAxNjMuY29t; bWxob3VAaXBwY2Fhcy5jbg==
 Fei Li
Fei Li Hongxia Hua2
Hongxia Hua2 Maolin Hou
Maolin Hou