
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 14 December 2020
Sec. Virology
Volume 11 - 2020 | https://doi.org/10.3389/fmicb.2020.583661
Antibiotic resistance continues to be a major global health risk with an increase in multi-drug resistant infections seen across nearly all bacterial diseases. Mycobacterial infections such as Tuberculosis (TB) and Non-Tuberculosis infections have seen a significant increase in the incidence of multi-drug resistant and extensively drug-resistant infections. With this increase in drug-resistant Mycobacteria, mycobacteriophage therapy offers a promising alternative. However, a comprehensive study on the infection dynamics of mycobacteriophage against their host bacteria and the evolution of bacteriophage (phage) resistance in the bacteria remains elusive. We aim to study the infection dynamics of a phage cocktail against Mycobacteria under various pathophysiological conditions such as low pH, low growth rate and hypoxia. We show that mycobacteriophages are effective against M. smegmatis under various conditions and the phage cocktail prevents emergence of resistance for long durations. Although the phages are able to amplify after infection, the initial multiplicity of infection plays an important role in reducing the bacterial growth and prolonging efficacy. Mycobacteriophages are effective against antibiotic-resistant strains of Mycobacterium and show synergy with antibiotics such as rifampicin and isoniazid. Finally, we also show that mycobacteriophages are efficient against M. tuberculosis both under lag and log phase for several weeks. These findings have important implications for developing phage therapy for Mycobacterium.
Prolonged and unsupervised use of antibiotics results in an increase of antibiotic resistance across all bacterial species which is considered a major threat to human health (Keshavjee and Farmer, 2012; Sprenger and Fukuda, 2016). The Mycobacterium genus with over 190 recognized species contains various pathogenic species including M. tuberculosis, M. leprae, M. abscessus, and M. avium complex, etc. (Rastogi et al., 2001). These infections are often difficult to treat owing to their resilient cell walls which render them resistant to prolonged exposure to acidic and alkaline environments (Rastogi et al., 2001). In addition, several mycobacterial species such as M. leprae, M. abscessus, and M. tuberculosis rapidly evolve resistance to antibiotics (Iseman, 1994; Cambau et al., 2018; Luthra et al., 2018; World Health Organisation [WHO], 2019). Similarly, the Mycobacterium avium Complex (MAC) infections are noted to be on the rise, especially in AIDS and pulmonary disease patients (Horsburgh, 1997) and MAC infections are often associated with an increase in mortality (Horsburgh, 1997; Diel et al., 2018). The antibiotic treatment regimen is associated with the distinct and long-lasting changes to the gut flora, often resulting in the depletion of various species of commensal bacteria (Wipperman et al., 2017). Antibiotics are also associated with various adverse side-effects ranging from nausea, vomiting and fever to anaphylaxis, hypersensitivity and photodermatitis (Castells et al., 2019). In order to tackle this growing rate of antibiotic resistance and the side effects of antibiotic treatment regimens, various therapeutic strategies have been proposed, including phage therapy, that has shown tremendous potential against various bacterial pathogens (Kortright et al., 2019).
Phages are viruses that are host specific toward bacteria and are inherently non-pathogenic to humans. They are abundantly present in the natural microflora of the human body (De Paepe et al., 2014; Manrique et al., 2016). A recent non-comparative clinical trial showed that the intravenous delivery of anti-staphylococcal phages in patients with severe infection did not pose any challenges to tolerability and safety (Petrovic Fabijan et al., 2020). Moreover, phages remain infective and lyse antibiotic-resistant bacteria (Kortright et al., 2019). To circumvent the narrow-host range specificity and development of resistance (Labrie et al., 2010), multiple phages are used in phage therapy. Successful compassionate use of phages against antibiotic-resistant bacteria on patients in the United States and Europe has been on the rise and shows their safety and translation potential (Jennes et al., 2017; Schooley et al., 2017).
With over 11,000 mycobacteriophages isolated and more than 1,800 sequenced (Russell and Hatfull, 2017), there is a large library of phages against Mycobacterium. Although the desired lytic phenotype of phages (Pimentel, 2014; Catalão and Pimentel, 2018) for phage therapy brings the number down, development of Bacteriophage Recombineering of Electroporated DNA (BRED) technique (Marinelli et al., 2008) allows rapid conversion of lysogenic phages to lytic phages for phage therapy. Recently, a three-phage cocktail has been successfully used in a clinic for the treatment of a cystic fibrosis patient suffering from Mycobacterium abscessus infection (Dedrick et al., 2019). Prophylactic phage (D29) therapy against TB also showed some success in a preclinical animal model in reducing the burden of infection (Carrigy et al., 2019). A study employing phage therapy in treating subcutaneously injected M. tuberculosis (H37Rv) infection in guinea pigs showed that mycobacteriophages have a therapeutic effect (Sula et al., 1981).
Phage therapy is a strong candidate for treating mycobacterial infections, however, it presents a few physiological hurdles that need to be taken into consideration. Various Mycobacterium species (M. tuberculosis, M. avium complex, M. bovis, and M. leprae) are found to reside intracellularly at low pH conditions which poses a challenge in efficacy of anti-bacterials (Sibling et al., 1987; Oh and Straubinger, 1996; Ehlers and Schaible, 2012; Benítez-Guzmán et al., 2019). M. tuberculosis and M. avium complex also form granulomatous structures, marked by a gradient of hypoxia, nutrient deprivation and acidity (Timm et al., 2003; Voskuil et al., 2004; Lawn and Zumla, 2011; Ehlers and Schaible, 2012; Seto et al., 2020). For a phage to successfully eliminate these bacilli, it needs to be active and efficient under such low pH and hypoxic conditions and should be able to enter into the eukaryotic cells. Since most bacteria enter a non-replicative stationary phase, the phage should also be able to infect and kill bacteria in the stationary phase where most of the current antibiotics fail. Designing a phage therapy that would be effective in such complex physiological environments would necessitate a better understanding of the bacteria-phage dynamics under various conditions. However, to our knowledge, no study has looked at the efficacy of phages over time in such different environments. Hence, there is a need to study the efficacy of phage cocktails in the physiological and pathophysiological conditions in which bacteria is known to survive.
In this study, we have performed phage-bacteria kinetics under various pathophysiological conditions of mycobacterial diseases- low pH, hypoxia and stationary phase. We chose M. smegmatis as our model organism in designing the experiments owing to its relatively fast replication time and therefore, its ability to offer rapid screening and understanding of phage kinetics in extreme host environments. We study the emergence of phage resistance in M. smegmatis and the combinatory effect of antibiotics and phage therapy. We show that mycobacteriophage cocktails are effective against Mycobacterium under the low pH, hypoxic and stationary conditions and showed synergy with antibiotics. Finally, we also find that mycobacteriophages are able to prevent growth of Mycobacterium tuberculosis which encourages further exploration of phages for diseases caused by Mycobacterium.
Middlebrook 7H9 Broth, Middlebrook 7H10 Agar and DMSO were purchased from Sigma (St Louis, MO, United States). Middlebrook 7H11 agar was purchased from BD Difco (NJ, United States). ADC growth supplement, Ziehl-Neelsen stain, rifampicin, and isoniazid were purchased from HiMedia (India). Calcium Chloride, Magnesium Sulfate, Sodium Chloride, Tween 80 and Glycerol were purchased from Fisher Scientific (MA, United States).
Primary Mycobacterium smegmatis (mc2155) or Mycobacterium tuberculosis (H37Ra) cultures were grown in Middlebrook 7H9 broth supplemented with Glycerol, ADC and Tween-80 (0.1% v/v). A log phase primary culture was inoculated into a secondary culture without Tween-80 and supplemented with CaCl2 (2 mM) to promote an efficient infection of phages. Frozen stocks were prepared by adding 300 μL of a late log phase culture to 35% (v/v) final concentration of glycerol and stored at −80°C until future use. All cultures were maintained at 37°C with rotary shaking at 180 rpm. For optical density (OD) measurements, 300 μL of bacterial culture at various time intervals was diluted 10 times in media and pipetted several times to obtain a uniform cell suspension. Readings were taken using a spectrophotometer (Jenway 7205 UV/Visible Spectrophotometer) at 600 nm against a media blank.
Soft agar overlay technique was used to detect and quantify phages. Briefly, soft agar was prepared by adding agar (0.8%) to 7H9 media along with the required supplements. The suspension was autoclaved and allowed to cool down to 42°C. 1 mL of late log phase bacterial culture (OD > 1) and/or 100 μL of phage dilutions was added to 5 mL of the soft agar and was poured onto a 7H10 plate supplemented with CaCl2. The soft agar was allowed to cool down and solidify. The plate was incubated at 37°C overnight.
For verification of phage titers, spot assays were carried out in which late-log phase M. smegmatis cultures (OD > 1) were plated on a Middlebrook 7H10 media plate supplemented with CaCl2 (2 mM) and glycerol to form a bacterial lawn. Phage samples were serially diluted and spotted on to the bacterial lawn. The plates were incubated at 37°C for 24 h and the number of plaques formed at various dilutions was counted.
Five phages [D29, TM4, Che7, PDRPv (Bajpai et al., 2018), and PDRPxv (Sinha et al., 2020)] used in this study were amplified on M. smegmatis. Similarly, three phages (D29, TM4, and DS6A) were amplified on M. tuberculosis. For liquid amplification, phage sample at 10 MOI was added to a log phase culture (with OD between 1 and 2) and incubated for 12–24 h at 37°C with rotary shaking at 180 rpm. The cultures were then centrifuged at 4,000 g for 10 min and the supernatant was collected and filtered through a 0.22 μm syringe filter (Biofil, FPV203030-A). For plate amplification, a soft agar overlay containing log phase M. smegmatis (OD 1–2) was prepared and the phage sample (10 MOI) was spotted on top of the soft agar. The plates were incubated for 12–24 h at 37°C following which, the soft agar was collected into a 50 mL falcon tube. An appropriate amount of Magnesium Sulfate-Tris Chloride-Sodium Chloride Buffer (SM Buffer) was added to the falcon tube to completely immerse the soft agar and the falcon tube was incubated for 2–4 h at 37°C or overnight at 4°C. The falcon tube was then subjected to centrifuge at 4,000 g for 10 min at 4°C and the supernatant was collected and filtered using 0.22 μm syringe filters. The soft agar overlay was used to determine the phage titers.
In order to study the bacterial growth kinetics, 7H9 broth supplemented with ADC and CaCl2 was prepared and inoculated with 50 μL of log-phase secondary bacterial culture (OD 1–2) per 10 mL of the media. The cultures were then incubated at 37°C with rotary shaking at 180 rpm. Periodic measurements of bacterial optical density (OD) were taken. For phage treated samples, a 10 MOI (for M. smegmatis) or 1 for (M. tuberculosis) of each phage in the respective phage solution was added at the appropriate time (either at the start of the experiment (lag phase) or at a specified interval of mid-log phase).
To study the effects of acidic pH on phage infection dynamics, phage kinetics assay was performed at varying the pH of the media. Briefly, the pH of the prepared media was reduced from 6.8 prior to the experiments using HCl to either a pH of 6.0 or pH of 5.5. The media was filtered and used for future growth kinetics experiments. Bacteria were inoculated into the media at pH 6.8, 6.0, and 5.5. For phage treated samples, a 10 MOI for each phage in the 5-phage cocktail was added at an appropriate time (either at the start of the experiment (lag phase) or at a specified interval of mid-log phase. OD was measured at 600 nm.
For stationary phase experiments, M. smegmatis cultures were grown in 7H9 media supplemented with Glycerol, ADC and CaCl2 (2 mM) until an OD of over 10 was reached. The stationary phase cultures were then either treated with the five-phage cocktail at 10 MOI or left untreated. OD values were obtained at regular intervals at 600 nm.
For the studies involving hypoxia, the cultures were grown in plastic K2-EDTA Vacutainers (BD Cat No. 367525) to generate hypoxia. Briefly, K2-EDTA Vacutainers were obtained from BD and washed with autoclaved sterile water over a period of 24 h to remove the EDTA coating. The vacutainers were then washed with prepared media before experimentation. Upon inoculation, the vacutainers were sealed with the rubber septa caps and parafilm to prevent oxygen diffusion. Samples were collected through a syringe and 20G needle for spectrophotometer readings. Oxygen levels were monitored by adding Methylene Blue (0.01%) to the samples (Boshoff et al., 2004) and measuring the OD at 665 nm.
Ziehl-Neelsen staining was performed periodically to check for contamination and for the confirmation of Mycobacterium spp. as described by the manufacturer. Briefly, the bacterial suspension was spread evenly over the slide and air-dried for 30 min. The slide was then heat-fixed on a hot plate for 15 min at 60°C and then flooded with Carbol Fuchsin stain at 85°C. Care was taken not to overheat or boil the stain and the slide was frequently replenished with additional Carbol Fuchsin stain. The slide was allowed to remain on the hot plate for 10 min following which it was removed and allowed to incubate for an additional 5 min. The stain was washed away under running tap water and flooded with decolorizer for 2 min or until the smear was sufficiently decolorized. The slide was washed under running tap water and treated with Methylene Blue for 30 s. The slide was subjected to one final washing under running tap water to remove the Methylene Blue stain and was air-dried following which, it was observed under a 100× oil immersion objective using an upright fluorescence microscope (Olympus BX53F) for the presence of either pink rod-shaped acid-fast bacteria or blue stained contamination.
The MICs of both rifampicin and isoniazid were determined to establish the antimycobacterial activities of the respective antibiotics against M. smegmatis strain mc2 155. Antibiotic stock solutions were prepared at concentration of 10 mg/mL in sterile deionized H2O for isoniazid and in DMSO for rifampicin. Stocks were stored at −20°C until further usage. Several dilutions were prepared in a 96- well plate for each of the antibiotic stock solutions to obtain various concentrations ranging from 1.5 to 100 μg/mL. A total of 2 × 105 cells of M. smegmatis were added to each of the wells. The 96-well plate was placed in a rotary shaker incubator at 37°C for 48 h and was observed for growth against control be measuring OD at 600 nm. The lowest concentration of antibiotic without bacterial growth was termed as MIC for the respective antibiotic.
In order to study the effect of antibiotics- isoniazid and rifampicin on M. smegmatis growth, 7H9 broth supplemented with ADC and CaCl2 was prepared and inoculated with 50 μL of log-phase secondary bacterial culture per 10 mL of the media. The cultures were then incubated at 37°C with rotary shaking at 180 rpm. Periodic measurements of bacterial OD at 600 nm against a media blank. For antibiotic-treated samples, a final concentration of rifampicin at 40 and 100 μg/mL and isoniazid at 40 and 100 μg/mL were added at the appropriate time (either at lag phase or at a specified interval of mid-log phase). In order to study the potential synergistic relationship between antibiotics and phages, we co-treated bacterial cultures with rifampicin at 20 μg/mL and phage cocktail (at MOIs 0.01, 0.05, and 10) at either the lag phase or at a specified interval of mid-log phase. Periodic OD measurements were taken at 600 nm.
To study the effect of phage cocktail on antibiotic-resistant strain, 7H9 broth supplemented with ADC, CaCl2, and isoniazid (40 μg/mL) was prepared and inoculated with 50 μL of isoniazid-resistant (mc2 4XR1) (Mohan et al., 2015; Padiadpu et al., 2015, 2016) log phase secondary bacterial culture per 10 mL of media. The cultures were then incubated at 37°C with rotary shaking at 180 rpm. Periodic measurements of bacterial OD were taken using a spectrophotometer at 600 nm against a media blank. For phage treated samples, the 5-phage cocktail was added at 10 MOI at an appropriate time (either at lag phase or at a specified interval of mid-log phase).
To determine the phage susceptibility of growing bacterial population after phage exposure, we determined the titers of our phage stocks on this growing population of exposed bacteria. Briefly, bacterial cultures that showed growth post phage (D29, PDRPv, and five-phage cocktail) treatment (in three separate biological replicates) were washed with 1X PBS thrice and were resuspended in fresh 7H9 media containing ADC, glycerol, and CaCl2. Each of these whole cultures were plated on a Middlebrook 7H10 media plate supplemented with CaCl2 (2 mM) and glycerol to form a bacterial lawn. Phage stock samples for all phages were serially diluted and spotted on to the bacterial lawn. The plates were incubated at 37°C for 24 h and the number of plaques formed at various dilutions were counted.
To study the possible synergy between bacteriophages, rifampicin and isoniazid, checkerboard assays were performed. Briefly, M. smegmatis bacterial cultures were seeded into 48-well plates at a seeding density of 105 colony forming units/mL. Respective concentrations of antibiotic and phages were added to the wells and the plates were incubated at 37°C with rotary shaking at 180 rpm for 24–48 h. OD readings were taken at 600 nm using a plate reader (Tecan Spark Multimode Microplate Reader). Fractional Inhibitory Concentrations (FIC) values were calculated as previously described (Hall et al., 1983) using the formula ΣFIC = FICR/I + FICP = (CR/I/MICR/I) + (CP/MICP), where MICR/I and MICP are the MICs of rifampicin/isoniazid and phage cocktail alone, respectively, and CR/I and CP are the MICs of rifampicin/isoniazid and phage cocktail in combination, respectively. Values ≤0.5 were defined as synergistic, values between 0.5 and 4 as no interaction or additive, and values over four as antagonism (Odds, 2003).
To study the stability of the 5-phage cocktail at various pH, we incubated a 108 solution at pH 6.8, 6.0, and 5.5 at 37°C. Samples were collected and titrated against M. smegmatis every 24 h.
All experiments were performed on independent biological replicates. Statistical significance was determined for control and experimental groups using multiple t-test with Holm-Sidak method, with alpha = 0.05. For analysis of multiple groups, 2-way ANOVA was used with Sidak’s multiple comparisons test, with alpha = 0.05. GraphPad (Prism) was used for all statistical analysis. Data points were excluded if contamination was identified using acid-fast staining.
Several lytic Mycobacteriophages were procured and amplified to titers greater than 109 pfu/mL. In order to test their efficacy, M. smegmatis bacterial cultures were infected with phages at the MOI of 10 either in the lag phase or in the mid-log phase. The optical density of the cultures at 600 nm was monitored using a spectrophotometer at various time intervals. Cultures treated with phages D29, TM4, Che7, PDRPv, and PDRPxv individually showed a significant reduction in the optical density after 2–5 h when phages were added at log phase (time of addition shown by red arrows) (Figure 1). Similarly, when the phage infection was carried out at lag phase, no growth was observed for several hours. It is well known that bacteria evolve resistance to phages over time. M. smegmatis cultures infected with phages were also monitored for phage resistance. Bacterial regrowth was observed in all phage treated M. smegmatis cultures. While D29 and Che7 treated cultures showed bacterial regrowth at around 40 h, TM4 treated cultures showed regrowth at 60 h; PDRPv and PDRPxv treated cultures showed regrowth at 83 and 101 h, respectively, (Figure 1). Bacterial cultures infected with phages at an MOI of 30 showed no significant increase in the onset of bacterial regrowth compared to MOI of 10 (Supplementary Figure 1). To test if the bacteria growing after the phage challenge had any altered susceptibility to the phages, we titrated our individual phage stocks on bacterial growths obtained after treatment with D29 and PDRPv. While D29 and PDRPv showed much reduced titers (2–3 log orders) against their respective regrowing bacteria, no significant reduction in susceptibility to other phages was observed (Supplementary Figure 2).
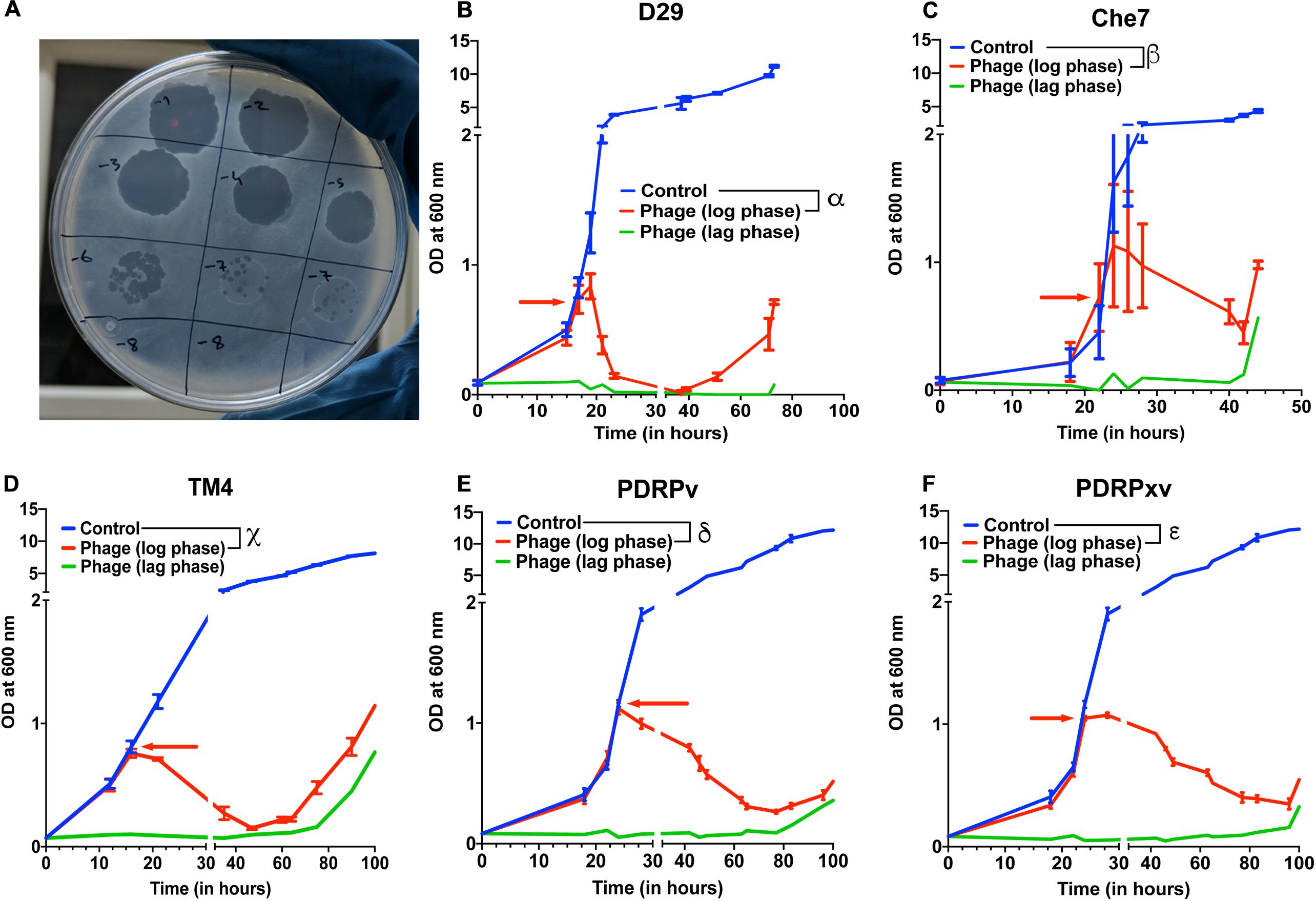
Figure 1. Efficacy of infection and evolution of phage resistance is dependent on individual Mycobacteriophages. (A) Photograph showing spot clearance of a lawn of M. smegmatis after treatment with D29. Growth curves of M. smegmatis in liquid culture in the presence of (B) D29, (C) Che7, (D) TM4, (E) PDRPv, and (F) PDRPxv phage. Red arrow represents the time point at which phage solution was added to the cultures. These experiments were repeated several times (≥2) and the representative graphs were plotted. Multiple t-test with Holm-Sidak method was used to estimate the statistical significance. α represents a significant difference (p < 0.05) between control and D29 treated (log phase) for all time points between 21 and 73 h. β represents a significant difference (p < 0.05) between control and Che7 treated (log phase) for all time points between 28 and 44 h. χ represents a significant difference (p < 0.05) between control and TM4 treated (log phase) for all time points between 21and 105 h. δ represents a significant difference (p < 0.05) between control and PDRPv treated (log phase) for all time points between 28 and 106 h. ε represents a significant difference (p < 0.05) between control and PDRPxv treated (log phase) for all time points between 24 and 106 h.
To test the effect of phage cocktails, M. smegmatis cultures infected with multiple phages (a cocktail of 3 or a cocktail of 5) at 10 MOI of each phage were monitored for OD at 600 nm (Figure 2). Phage cocktail treatment significantly reduced the optical density of the bacterial cultures compared to controls 5 h post treatment. Bacterial regrowth in cocktail treated cultures prolonged to 105 h in case of 3 phage cocktail (D29, TM4, and Che7) (Figure 2A) and to 322 h in case of 5 phage cocktail (D29, TM4, Che7, PDRPv, and PDRPxv) (Figure 2B). The corresponding Colony Forming Units (CFU)/mL readings were obtained and the viable cell count was observed to be correlating with OD readings (Supplementary Figure 3A). This shows that the cocktail of phages can act synergistically to delay the regrowth of bacteria. Periodic phage titer measurements showed increase in the phage titers as the bacterial OD dropped and phages could be detected upto 400 h although a steady decline in the titer was observed (Supplementary Figure 7A). Subsequent phage stock rechallenge on the growing bacteria in the 5-phage cocktail kinetics showed that all phage stock titers had dropped by >99% (2–3 log order) on the pre-exposed bacteria compared to untreated bacteria (Supplementary Figure 3B). suggesting evolution of the bacteria to partially resist phage mediated lysis. Since the regrowing bacteria were still infected and lysed by the phages, although at much lower efficiencies, it suggests phage tolerance or partial resistance, possibilly due to mutation in the surface receptors. It is important to note that the phages used here belong to different genomic clusters: cluster A (D29, subcluster: A2), cluster K (TM4, subcluster: K2) and cluster B (PDRPv and PDRPxv, both in subcluster B1) (2–6). Cluster information for Che7 is not known. Hence, it is expected that these phages will employ different mechanisms to infect the bacteria which is consistent with delay in regrowth of bacteria (Figure 2) as more phages are added to the cocktail. The 5-phage cocktail was selected and used for future experiments. To determine the time it takes for OD of bacterial cultures to start reducing after phage addition, we took OD readings every 15–20 min. We found that OD of the bacterial cultures starts to drop only after 120–150 min (Supplementary Figure 4) which is consistent with the previously reported latency period of D29 (∼60 min) (Samaddar et al., 2015; Bavda and Jain, 2020) and PDRPxv (∼135 min) in M smegmatis (Sinha et al., 2020).
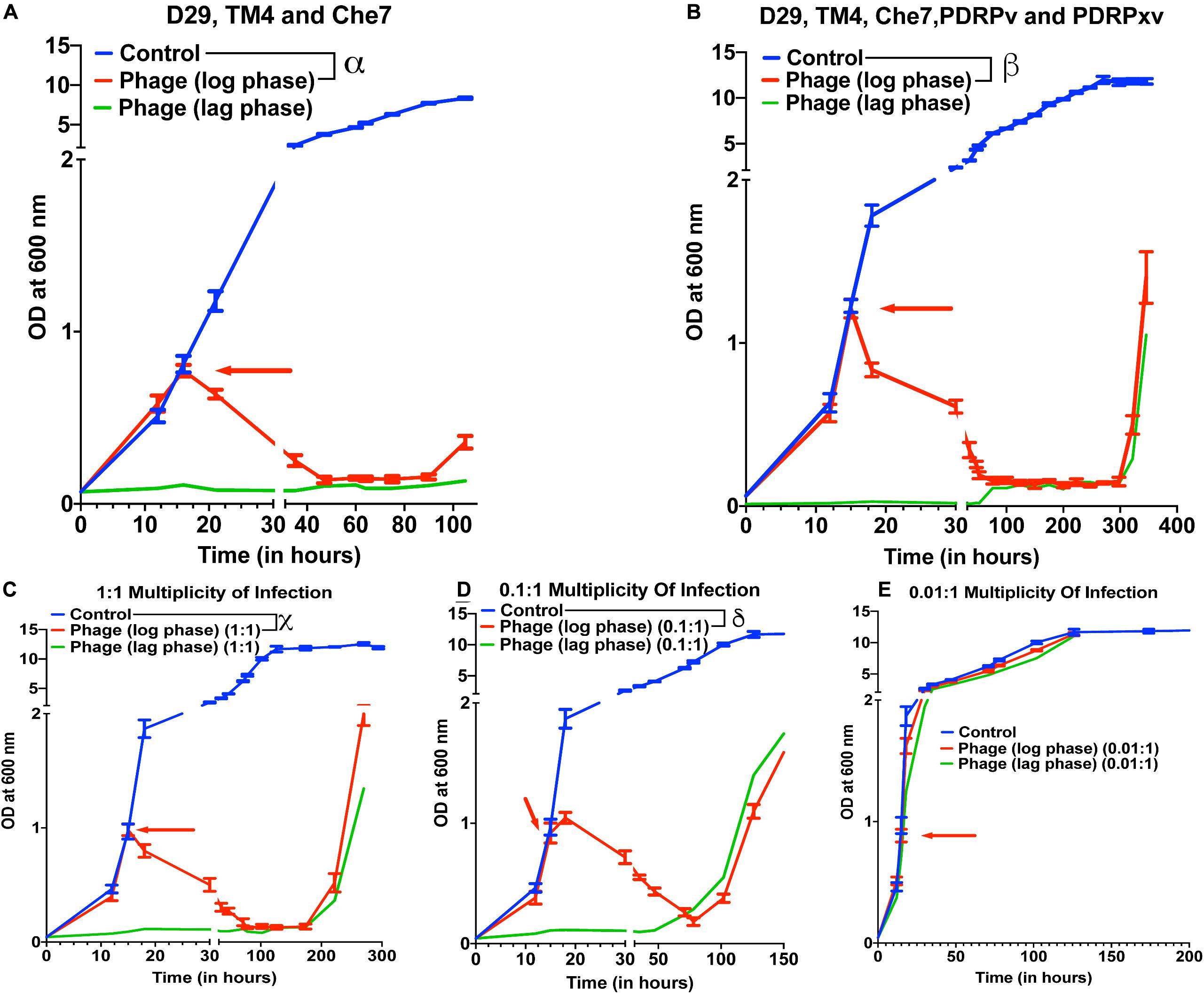
Figure 2. Phage cocktails show synergy compared to individual phage and their efficacy is MOI dependent. Growth kinetics of M. smegmatis cultures treated with (A) 3-phage cocktail (D29, Che7, and TM4) and (B) 5-phage cocktail (D29, TM4, Che7, PDRPv, and PDRPxv) at MOI of 10 for each phage against a non-treated control. Growth kinetics of M. smegmatis cultures treated with a 5-phage cocktail at the MOI of (C) 1, (D) 0.1, and (E) 0.01 for each phage in the phage cocktail. The experiments with MOI of 10 with 3- and 5-phage cocktails were repeated twice and the representative graphs are shown here. Red arrow represents the time point phage solution was added to the cultures. Multiple t-test with Holm-Sidak method was used to estimate the statistical significance (p < 0.05). α represents a significant difference (p < 0.05) between control and three-phage cocktail (D29, TM4, and Che7) treated (log phase) for all time points between 21 and 105 h. β represents a significant difference (p < 0.05) between control and five-phage cocktail (D29, TM4, Che7, PDRPv, and PDRPxv) treated (log phase) for all time points between 18 and 346 h. χ represents a significant difference (p < 0.05) between control and five phage cocktail treated (MOI 1, log phase) for all time points between 18 and 270 h. δ represents a significant difference (p < 0.05) between control and five phage cocktail treated (MOI 0.1, log phase) for all time points between 18 and 174 h.
To test for the efficacy of mycobacteriophages at various Multiplicity of Infection (MOI), M. smegmatis cultures were infected with a MOI of 10, 1, 0.1, and 0.01 of each phage: bacteria in either the lag phase or the mid-log phase. A 10 MOI (Figure 2B) significantly reduced the growth of bacterial culture 3 h post treatment with bacterial regrowth emerging at 322 h. A 1 MOI (Figure 2C) also resulted in a significant reduction of bacterial growth 3 h post treatment, however, regrowth emerged at around 222 h. While a 0.1 multiplicity of infection (Figure 2D) resulted in a reduction in growth, bacterial regrowth was observed at 102 h for mid-log phase treated cultures. A 0.01 MOI did not result in any significant reduction in the bacterial growth (Figure 2E). For all future experiments, we have used the MOI of 10 to evaluate the maximum lysing potential of phages against Mycobacterium.
Several species of Mycobacterium exist as intracellular pathogens in acidic and hypoxic environments (Sibling et al., 1987; Oh and Straubinger, 1996; Rastogi et al., 2001; Houben et al., 2006; Benítez-Guzmán et al., 2019; Seto et al., 2020). In order for phage therapy to be efficient against such mycobacterial infections, phages need to retain their activity and infectivity in these extreme environments. We, therefore, evaluated the efficiency of our 5-phage cocktail under low pH conditions normally observed in infected phagosomes (ranges from pH 4–6.5) of mammalian cells. M. smegmatis cultures did not grow at pH 5.0 and below. Hence, we chose pH 5.5 and 6.0 to test the effectiveness of phages as these pH ranges are known to exist in early and late endosomes (Rohde et al., 2007). We tested the phage stability in 7H9 medium with pH 6.8, 6.0, and 5.5. Although there was a progressive loss of phage titers over 300 h, no significant difference in phage activity was observed at lower pH compared to pH of 6.8 (Supplementary Figure 5). Under low pH conditions of 6.0 and 5.5, the mycobacteriophages remain efficient in infecting and killing M. smegmatis and showed regrowth around 300 h, similar to those observed at pH 6.8 (standard pH of 7H9 medium) (Figures 3A,B, Supplementary Figures 6A,B).
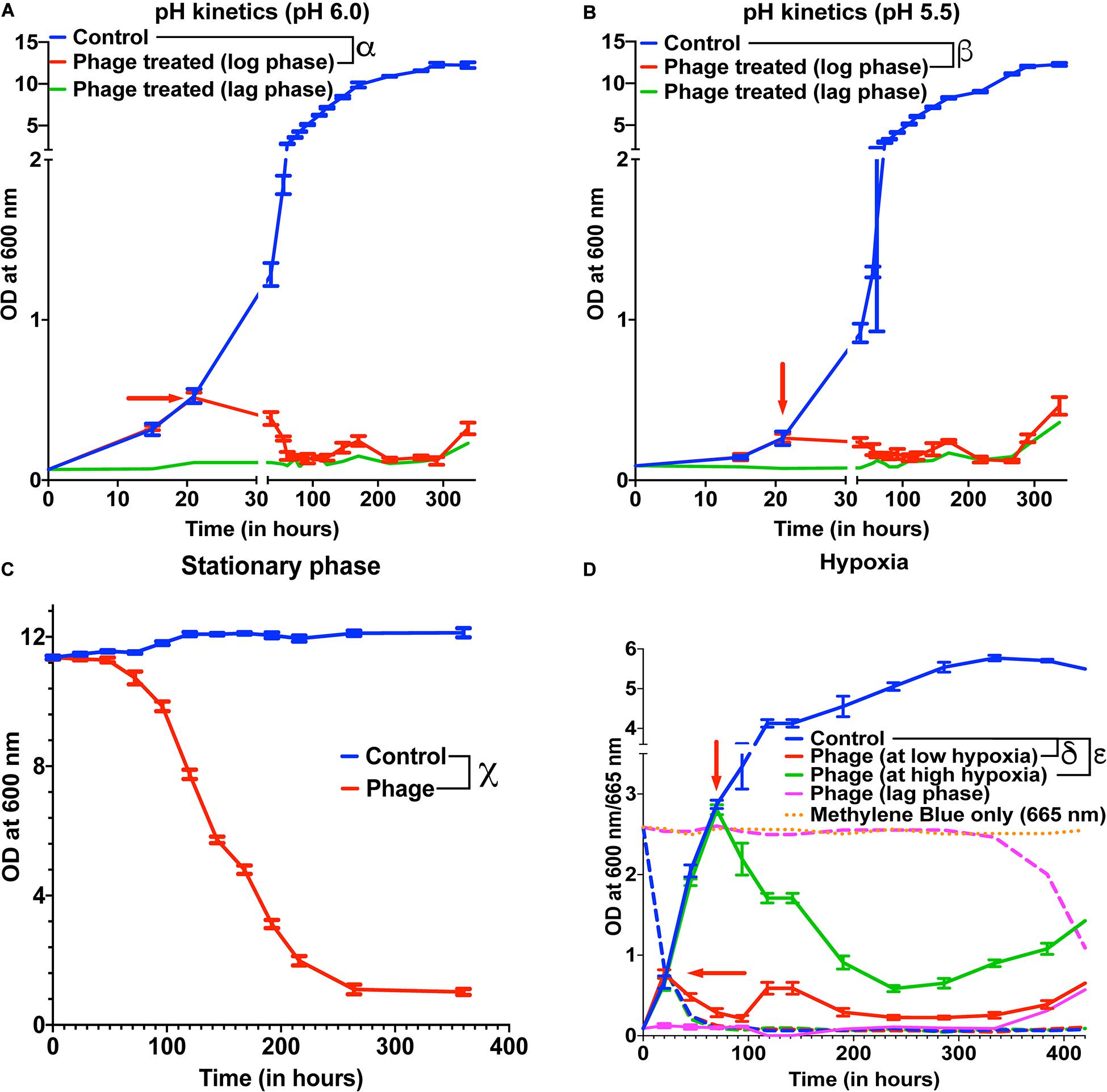
Figure 3. Mycobacteriophages are effective under various pathophysiological conditions. Growth kinetics of M. smegmatis cultures treated with a five-phage cocktail at (A) pH condition of 6.0, (B) pH condition of 5.5, (C) stationary phase, and (D) hypoxic conditions. For hypoxia experiment, solid lines represent bacterial OD at 600 nm and dashed lines are the respective Methylene Blue readings at 665 nm. Red arrow represents the time point phage solution was added to the cultures. These experiments were repeated twice and the representative graphs are shown here. For A, B, and C, multiple t-test with Holm-Sidak method was used to estimate the statistical significance (p < 0.05). α represents a significant difference (p < 0.05) between control and phage cocktail treated (pH 6.0, log phase) for all time points between 36 and 338 h. β represents a significant difference (p < 0.05) between control and phage cocktail treated (pH 5.5, log phase) for all time points between 73 and 338 h. χ represents a significant difference (p < 0.05) between control and phage cocktail treated (stationary phase) for all time points between 24 and 360 h. For D, 2-way ANOVA with Sidak’s multiple comparisons test was used to determine statistical significance (p < 0.05). δ represents a significant difference between control and phage (at low hypoxia) for all time points between 45 and 432 h, and ε represents a significant difference between control and phage (at high hypoxia) for all time points between 94 and 432 h.
Efficacy of the phages under hypoxic conditions was checked by culturing bacteria in sealed vacutainers with septa. Since, there is no exchange of gases in this model, as the bacteria grows over time, the oxygen present in the tube depletes creating a hypoxic environment. This was confirmed by adding methylene blue to the media that turns colorless in the absence of oxygen (Supplementary Figure 8) (Sumitani et al., 2004). We infected cultures with the 5-phage cocktail, at either low hypoxia (intermediate methylene blue absorbance) or high hypoxia (at lowest methylene blue absorbance). We observed lysis of bacteria by the 5-phage cocktail in both the hypoxic conditions 20–25 h post treatment. (Figure 3D and Supplementary Figure 6D). The CFU data showed that while at low hypoxia there was >4 log order reduction, at high hypoxia only about one log order reduction was observed. This could be due to low bacterial growth rates under high hypoxia. Similar to pH experiments, bacterial regrowth was observed in both low and high hypoxia phage treated samples in ∼300 h.
Within the host environment, Mycobacterial species slow their growth rate and often enter a non-replicative state (Voskuil et al., 2004; Houben et al., 2006; Ehlers and Schaible, 2012). This reduced growth state poses a significant hurdle in the treatment of infections with conventional antibiotics (Gutierrez et al., 2017). For phage therapy to be efficient against mycobacterial infections, phages need to retain the infectivity against the slow growing bacteria. To test the effect of phage cocktail against M. smegmatis in stationary phase, M. smegmatis cultures were allowed to reach an OD >10. The stationary phase cultures were then infected with the 5-phage cocktail without any supplementation with nutrients and periodic OD measurements were taken at 600 nm. Significant reduction in OD and CFU were observed in phage treated samples 24 h post treatment, demonstrating that the 5-phage cocktail is effective in stationary phase conditions (Figure 3C, Supplementary Figure 6C). Interestingly, even after 350 h of culture, no growth of bacteria was observed. In periodic phage titer measurements, phages could be detected upto 400 h although a steady decline in the titer was observed (Supplementary Figure 7B).
Tuberculosis infections are conventionally treated by a combination of antibiotics, including the first-line drugs, isoniazid and rifampicin (World Health Organisation [WHO], 2019). To compare our phage kinetics with these antibiotics, we first determined the minimal inhibitory concentration of rifampicin (MIC: 25 μg/mL) and isoniazid (MIC: 15 μg/mL) on M. smegmatis (mc2155). We then treated the cultures with 40 and 100 μg/mL of the respective antibiotic at either lag phase or at mid-log phase. For 40 μg/mL antibiotic concentration, there was no significant reduction of bacterial growth at log phase (Supplementary Figures 9A,B). For 100 μg/mL, we observed only a transient growth inhibition of M. smegmatis compared to the phage treatment (Supplementary Figures 9C,D). The cultures continue to grow even in the presence of high concentration (several times the MIC values) of antibiotics. This growth inhibition was much more pronounced in lag-phase cultures treated with antibiotics than log-phase cultures treated with antibiotics. Antimicrobial susceptibility depends on multiple factors such as inoculum size, metabolic state of the bacteria and cellular respiration rate (Lobritz et al., 2015; Li et al., 2017; Lopatkin et al., 2019). All these factors change as bacterial cells transition from lag phase to exponential growth and antibiotic efficacy may be variable even at the same concentration for different growth states and bacterial numbers. Isoniazid interferes with mycolic acid synthesis and hence is more effective on replicating Mycobacteria but due to high numbers of bacteria in the exponential phase, its efficacy was lower than in the lag phase. These results are also consistent with the previously observed results that isoniazid had reduced efficacy on exponentially growing M. smegmatis cultures (Teng and Dick, 2003).
To check whether treatment with the 5-phage cocktail enhances the efficacy of controlling bacterial growth in antibiotic-treated cultures, we added 20 μg/mL of rifampicin (∼MIC value) along with a 5-phage cocktail solution at an MOI of 0.05, 0.01, or 10 during the lag phase or the mid-log phase. For MOI of 0.01, we did not observe a significant reduction in growth in only phage treated samples at both lag and log phases compared to untreated samples. However, rifampicin and phage cotreated samples showed a significant growth reduction in the log phase 6 h post treatment with bacterial regrowth observed around 224 h (Figure 4A). In the lag-phase bacteria, phage addition at 0.01 MOI had no effect on growth and rifampicin treated bacteria showed growth within 50 h while the lag-phase samples co-treated with both rifampicin and phage cocktail showed no growth emergence upto 254 h. This prominent reduction in bacterial growth in cultures co-treated with rifampicin and phage cocktail could be attributed to antibiotic-phage synergy previously observed in various other bacterial infections including P. aeruginosa infection treatment (Oechslin et al., 2017), S. aureus infection treatment (Chhibber et al., 2013) and A. baumannii infection treatment (Jansen et al., 2018). The effectiveness of a cotreatment at low MOI of phage cocktails and rifampicin concentrations shows phage-antibiotic synergy. While rifampicin binds to RNA polymerase that might interfere with phage amplification, a staggered treatment with phages followed by rifampicin has been shown to be synergistic (Rahman et al., 2011; Tkhilaishvili et al., 2018). Similar observations have been noted for antibiotics that target the DNA replication and translational machinery (Torres-Barceló et al., 2014; Kim et al., 2018). It is possible that the co-incubation of both anti-bacterial agents prevents the emergence of bacteria that is tolerant to the individual anti-bacterials and hence show synergy. For cultures treated with a phage cocktail MOI of 0.05, we observed a similar reduction in growth with bacterial regrowth emerging at 254 h in log-phase treated samples (Supplementary Figure 10A). Interestingly, the phage-antibiotic synergy at log-phase became less pronounced with increase in phage MOI. At MOI of 10, no synergy was observed in log phase cultures but a delay in emergence of bacterial regrowth in lag phase treated cultures was observed (Supplementary Figure 10B). To further test the phage synergy with rifampicin, we performed experiments at different concentration of phage-rifampicin through a checkerboard assay (Figure 4B) and found their interaction to be synergistic in preventing bacterial growth (FIC index of 0.43 (<0.5) at phage MOI 0.1 and rifampicin concentration of 10 μg/mL) (Hall et al., 1983).
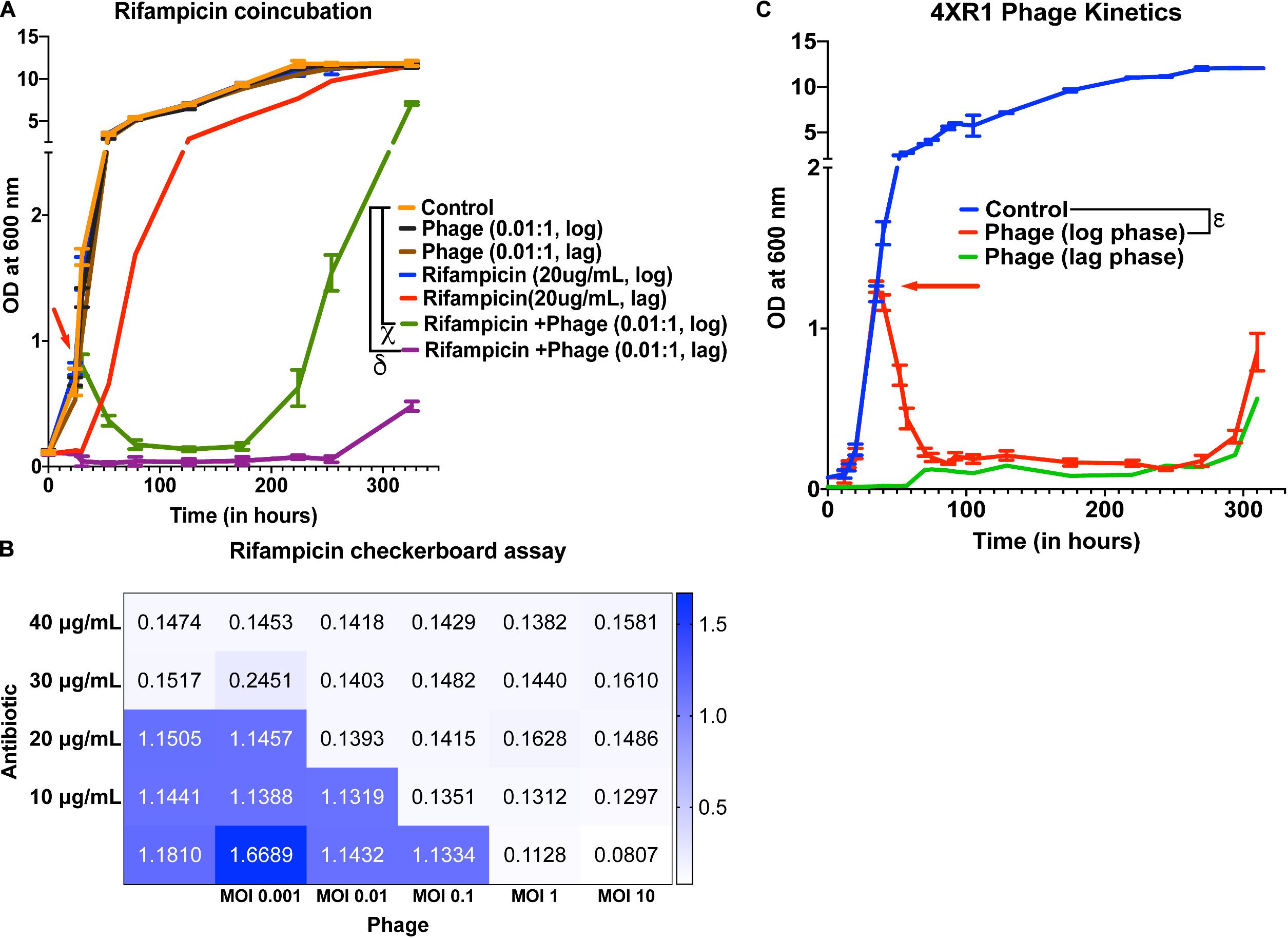
Figure 4. Phage cocktails show synergy with antibiotics and are effective against antibiotic-resistant Mycobacterium. (A) 5-phage cocktail (MOI 0.01) and rifampicin (20 μg/mL)-χ and δ represent a significant difference between control and rifampicin + phage (log phase), and rifampicin + phage (lag phase) for all time points between 30 and 326 h. (B) Checkerboard assay of M. smegmatis cultures treated with rifampicin and 5-phage cocktail (D29, TM4, Che7, PDRPv, and PDRPxv). (C) Growth kinetics of isoniazid-resistant M. smegmatis (4XR1) cultures treated with a 5-phage cocktail. represents a significant difference between control and phage treated (log phase) for all time points between 40 and 310 h. Red arrow represents the time point antibiotic/phage solution was added to the cultures. For A, 2-way ANOVA with Sidak’s multiple comparisons test was used to determine statistical significance (p < 0.05). For C, multiple t-test with Holm-Sidak method was used to estimate the statistical significance (p < 0.05).
Isoniazid-resistant infections are observed to be on the rise across the globe (World Health Organisation [WHO], 2019). We, therefore, looked at the efficacy of phage infection and mycobacteriophages against isoniazid-resistant strain of M. smegmatis (Mohan et al., 2015; Padiadpu et al., 2015, 2016). First, we tested whether individual phages have altered efficiency in infecting and lysing the isoniazid-resistant strain. The phages retained their initial high titers against isoniazid-resistant M. smegmatis and no significant drop in the efficiency of plating was observed (Supplementary Figure 11). Next, we observed that the 5-phage cocktail remains effective in infecting and lysing antibiotic-resistant M. smegmatis (Figure 4C). We observed a sharp decrease in the OD of phage-treated log-phase cultures 5 h post treatment and an extended delay in the growth of lag-phase cultures. We also observed growth of phage treated bacterial cultures at 294 h, similar to the previous experiments. Checkerboard assays against the isoniazid-resistant M. smegmatis indicate the efficacy of phages in the presence of isoniazid antibiotic (Supplementary Figure 12) and the synergy between phages and isoniazid (FIC index of 0.35 (<0.5) at phage MOI 0.01 and isoniazid concentration of 10 μg/mL) (Hall et al., 1983). The checkerboard assay with Mycobacterium in absence of detergent in 48/96 well plates is inherently noisy. We also observed wells that had unexpected absorbance readings due to these clumps. However, overall we see a synergistic response with isoniazid and phages on isoniazid resistant strain.
Next, we checked whether other slow-growing species of Mycobacteria also exhibit similar dynamics with Mycobacteriophages. Although several reports have shown that phages infect and kill M. tuberculosis, whether cocktail of phages can prevent growth of M. tuberculosis strains over long durations and how long it takes for evolution of resistance or bacterial regrowth is not known. In our 5-phage cocktail, two phages (D29 and TM4) are known to infect M. tuberculosis strains. These two phages and another phage DS6A were amplified using M. tuberculosis (H37Ra) as a host because the phages expanded on M. smegmatis had low titer on H37Ra. For infection experiments, a phage cocktail was prepared using these three phages. Since our phage titers against M. tuberculosis strains were low (∼107 PFU/mL) even after several rounds of amplification, we used a low inoculation density (∼104 CFU/mL) of bacteria to perform infection experiments at 10 MOI for each phage. We proceeded to treat the cultures of M. tuberculosis (H37Ra strain) with this three-phage cocktail in both the lag phase (OD ∼0.06) and in the log phase. Phage treatment was successful and efficient in preventing the growth of M. tuberculosis for several weeks (Figure 5A). Emergence of bacterial regrowth was observed after 1368 h (57 days). We also observed reduction in bacterial CFU after phage treatment, consistent with our OD measurements (Figure 5B). These experiments show that phages can be also effective against slow growing Mycobacterium for long durations.
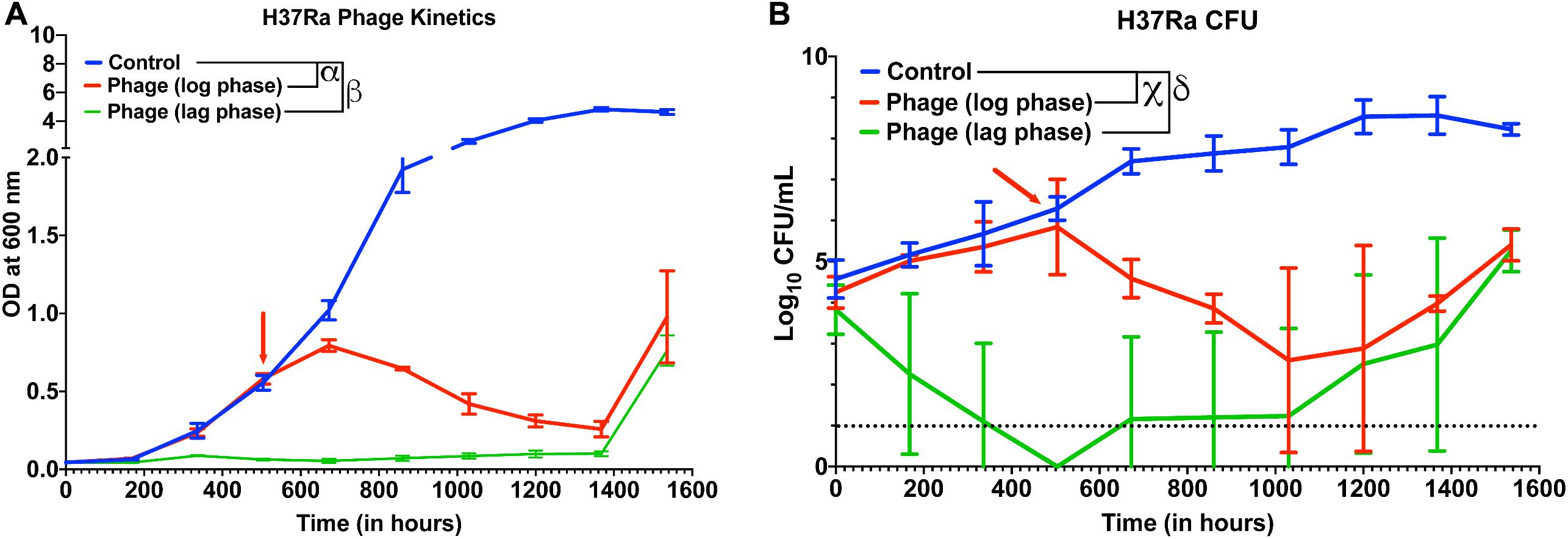
Figure 5. Phage cocktails are effective against M. tuberculosis. (A) Growth kinetics of M. tuberculosis (H37Ra) after treatment with a 3-phage cocktail (D29, Che7, and DS6A). Multiple t-test with Holm-Sidak method was used to estimate the statistical significance (p < 0.05). α represents a significant difference between control and phage treated group (log phase) for all time points between 672 and 1536 h. β represents a significant difference (p < 0.05) between control and phage treated group (lag phase) for all time points between 168 and 1536 h. (B) Colony Forming Unit (CFU) measurements over time of M. tuberculosis cultures treated with a 3-phage cocktail. χ represents a significant difference (p < 0.05) between control and phage (log phase) for all time points between 672 and 1536 h. δ represents a significant difference (p < 0.05) between control and phage (lag phase) for all time points between 168 and 1536 h. Multiple t-test with Holm-Sidak method was used to determine the statistical significance. The detection limit for the assay is represented in black dotted line (10 CFU).
Phage therapy has been successfully used in the treatment of various bacterial infections including P. aeruginosa urinary tract infection (Khawaldeh et al., 2011), multidrug-resistant A. baumannii infection (Schooley et al., 2017), P. aeruginosa aortic graft infection (Chan et al., 2018), and more recently, M. abscessus infection of a 15-year old cystic fibrosis patient (Dedrick et al., 2019). Although phage therapy holds strong potential, there is little understanding of phage-host dynamics in disease environments. In this study, we studied the host-phage infection dynamics under various physiological and disease-mimicking conditions.
We chose mycobacterial species as our phage host, as persistent and multidrug-resistant mycobacterial infections have been increasing in incidence over the past decades and there is a need for new therapy against such infections (Iseman, 1994; Gygli et al., 2017; Aslam et al., 2018; Cambau et al., 2018; Luthra et al., 2018). For phage therapy to be successful and efficient against mycobacterial infections, the phages need to retain their infectious activity in these extreme environments and infect non-replicative stationary phase bacteria. D29 is a well-known phage against M. tuberculosis known to produce superoxide radicals in oxygen-rich environments, which synergizes with phage mediated lysis to accelerate the killing of Mycobacterium (Samaddar et al., 2015). However, it is observed to be ineffective in hypoxic conditions (Swift et al., 2014). TM4, on the other hand, is shown to be efficient in infecting M. tuberculosis during stationary phases and hypoxic conditions (Swift et al., 2014). Biochemical analysis revealed that a peptidoglycan motif in the tape measure protein of TM4 phage confers an advantage in infecting bacteria in stationary phase (Piuri and Hatfull, 2006). This complementary nature of phages suggests that a cocktail of phages would be effective in the elimination of mycobacterial infections across a broad range of environments and growth states. Although research has been carried out on individual mycobacteriophages under specific environments, limited information exists which solidifies our understanding of phage cocktail infection kinetics and evolution of phage resistance under different disease-mimicking conditions.
In this study, we show that a 5-phage cocktail is efficient against M. smegmatis at various pH, low oxygen availability and different growth rate of bacteria. Furthermore, for the first time, our study shows the M. tuberculosis growth kinetics in presence of phage cocktail over long durations. We found that a 3-phage cocktail of D29, TM4 and DS6A was successful in preventing as well as reducing the growth of M. tuberculosis (H37Ra) for several weeks (Figure 5).
We also found that two frontline TB antibiotics-isoniazid and rifampicin were limited in their ability to control the growth of M. smegmatis in the exponential phase of bacterial growth (Supplementary Figure 9). The more striking result from our data was the synergy of phages with rifampicin and effectiveness of phages on isoniazid-resistant M. smegmatis (Figures 4A–C). This finding stands in line with observations of synergy during phage-antibiotic combination treatments for other bacterial systems (Comeau et al., 2007; Chhibber et al., 2013; Oechslin et al., 2017; Jansen et al., 2018). Although phage therapy holds promise in treating bacterial infections and antibiotic resistance, bacteria are known to develop resistance against phages through various mechanisms (Labrie et al., 2010). However, unlike antibiotics which have limited classes and require scrutinized drug design, phages are abundant in nature (Edwards and Rohwer, 2005) and the mycobacteriophage repository alone is over 10,000 in number (Russell and Hatfull, 2017), providing a much larger repertoire of arsenal against bacterial infections. However, when designing a phage cocktail, the phage resistance needs to be considered. The phage-bacterial dynamics and evolution of partial resistance described here are some the first studies performed on mycobacterial species and provide useful information for future exploration of phages for therapeutic use. We found that the bacteria eventually evolve partial resistance to the phage or phage cocktail as is seen by regrowth of the bacteria after phage challenge even though the phages can be detected in the medium at all times. Subsequent challenge of our phage stocks on this regrowing population showed a drop of 2–3 log orders (>99%) in titers suggesting evolution of the bacteria to resist phage mediated lysis. The mechanisms of this resistance are not clear and need further investigation. Bacterial hosts are known to develop resistance against phages through various mechanisms including genetic mutations, hypermutable loci, the blockage of the injection of phage DNA, cleavage of injected phage DNA, interference with phage assembly, abortive infection and other phage defense systems such as the R-M system, DISARM system or the CRISPR-Cas system (Azam and Tanji, 2019; Turkington et al., 2019). Partial resistance or tolerance could also be achieved by mutations in the surface receptors following phage exposure (Latino et al., 2016; Wright et al., 2019). Whether Mycobacterium spp. utilize these mechanisms to overcome the phage challenge is not clear and requires further research. In our studies, we observed a strong correlation between the delay in the emergence of phage resistance and the number of phages used in our phage cocktail.
Our phage infection at different MOI suggests that it is important to deliver a high number of phages at the site of infection for therapy to be successful (Figures 2C,D). Several approaches have been developed to deliver active phages to the deep lung such as vibrating mesh nebulizer, soft mist inhalers and dry powder inhalers (Puapermpoonsiri et al., 2009; Vandenheuvel et al., 2013; Liu et al., 2016; Carrigy et al., 2017; Agarwal et al., 2018). Similarly, delivery to macrophages by direct phagocytosis of phages (Jończyk-Matysiak et al., 2015) or through nanoparticles/microparticles can result in targeting of the intracellular niche of the bacteria (Fenaroli et al., 2014; Nieth et al., 2015; Agarwal et al., 2018). However, the current understanding of the diffusion of phages across the granuloma remains limited and merits further studies in animal models.
A limitation to this study is that most experiments were performed on non-pathogenic, fast growing M. smegmatis and may not translate to slow growing pathogenic species such as M. tuberculosis. Although we show that phages can infect and lyse M. tuberculosis for long durations, further studies are warranted in testing the efficiency of these phages on such slow growing pathogenic species for longer durations and in acidic and hypoxic conditions. However, efficacy of mycobacteriophages in lysing intracellular slow growing mycobacteria in vitro as well as in vivo has been demonstrated by other groups. Preliminary reports on D29 incubation with H37Rv infected mouse macrophages show reduction in intracellular bacterial burden, however, penetration of D29 inside the macrophage was reported to be low (Lapenkova et al., 2018). It is critical for the phages to directly encounter bacteria to be effective. Phage delivery inside infected mammalian cells can be facilitated by using carriers, for instance by adsorbing on non-pathogenic bacteria. This led to enhanced intracellular localization of phages in vitro in M. avium and M. tuberculosis infected macrophages and reduction in bacterial load in a mouse model of disseminated M. avium infection (Broxmeyer et al., 2002; Danelishvili et al., 2006). Bactericidal efficiency of phages has also been shown in vivo in a M. ulcerans infected mouse footpad model (Trigo et al., 2013). Another challenge for phage therapy is the human immunological response. Although phages are known to elicit immune response (Ooi et al., 2019), previous studies have shown that phages and host immune response can synergistically result in the elimination of acute and chronic bacterial infections (Leung and Weitz, 2017; Roach et al., 2017; Waters et al., 2017). This is further supported by the successful treatment of several patients after phage administration in clinics.
In summary, we demonstrate the efficacy of phages under various pH ranges (6.8, 6.0, and 5.5), stationary phase as well as under hypoxic conditions. We show that phages are effective in infecting and lysing antibiotic resistant bacteria and show synergy with antibiotics. We further show that a three-phage cocktail is effective in preventing the growth of slow growing M. tuberculosis for several weeks.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
RA conceived the idea. YK and RA designed the research. YK and PS performed the research. YK and RA analyzed the data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Ramanujan fellowship (SB/S2/RJN-036/2017, Department of Science and Technology, India), Bill & Melinda Gates Foundation (OPP1210498), Indian Institute of Science, Mr. Lakshmi Narayanan financial support. Department of Biotechnology (DBT/2017/IISc/941) supported PS salary.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge Dr. Sujoy K. Das (Bose Institute, Kolkata), Dr. Urmi Bajpai (Acharya Narendra Dev College, Delhi), Dr. V.N. Azger Dusthackeer (National Institute for Research in Tuberculosis, Chennai) and Dr. Graham Hatfull (University of Pittsburgh) for providing us with mycobacteriophages. We are grateful toward Dr. Amit Singh (Indian Institute of Science, Bengaluru) for providing bacterial strains. We thank Dr. Nagasuma Chandra for kindly providing us with the isoniazid-resistant M. smegmatis. We thank the Molecular Reproduction, Development and Genetics, and Materials Engineering common facility for instrument access. The manuscript has been released as a pre-print at BioRxiv (Kalapala et al., 2020).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.583661/full#supplementary-material
Agarwal, R., Johnson, C. T., Imhoff, B. R., Donlan, R. M., McCarty, N. A., and García, A. J. (2018). Inhaled bacteriophage-loaded polymeric microparticles ameliorate acute lung infections. Nat. Biomed. Eng. 2, 841–849. doi: 10.1038/s41551-018-0263-5
Aslam, B., Wang, W., Arshad, M. I., Khurshid, M., Muzammil, S., Rasool, M. H., et al. (2018). Antibiotic resistance: a rundown of a global crisis. Infect. Drug Resist. 11, 1645–1658. doi: 10.2147/IDR.S173867
Azam, A. H., and Tanji, Y. (2019). Bacteriophage-host arm race: an update on the mechanism of phage resistance in bacteria and revenge of the phage with the perspective for phage therapy. Appl. Microbiol. Biotechnol. 103, 2121–2131. doi: 10.1007/s00253-019-09629-x
Bajpai, U., Mehta, A. K., Eniyan, K., Sinha, A., Ray, A., Virdi, S., et al. (2018). Isolation and characterization of bacteriophages from India, with lytic activity against Mycobacterium tuberculosis. Can. J. Microbiol. 64, 483–491. doi: 10.1139/cjm-2017-0387
Bavda, V. R., and Jain, V. (2020). Deciphering the Role of Holin in Mycobacteriophage D29 Physiology. Front. Microbiol. 11:883. doi: 10.3389/fmicb.2020.00883
Benítez-Guzmán, A., Esquivel-Solís, H., Romero-Torres, C., Arriaga-Díaz, C., and Gutiérrez-Pabello, J. A. (2019). Genetically related Mycobacterium bovis strains displayed differential intracellular growth in bovine macrophages. Vet. Sci. 6:81. doi: 10.3390/vetsci6040081
Boshoff, H. I. M., Myers, T. G., Copp, B. R., McNeil, M. R., Wilson, M. A., and Barry, C. E. (2004). The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism. Novel insights into drug mechanisms of action. J. Biol. Chem. 279, 40174–40184. doi: 10.1074/jbc.M406796200
Broxmeyer, L., Sosnowska, D., Miltner, E., Chacón, O., Wagner, D., McGarvey, J., et al. (2002). Killing of mycobacterium avium and mycobacterium tuberculosis by a mycobacteriophage delivered by a nonvirulent mycobacterium: a model for phage therapy of intracellular bacterial pathogens. J. Infect. Dis. 186, 1155–1160. doi: 10.1086/343812
Cambau, E., Saunderson, P., Matsuoka, M., Cole, S. T., Kai, M., Suffys, P., et al. (2018). Antimicrobial resistance in leprosy: results of the first prospective open survey conducted by a WHO surveillance network for the period 2009–15. Clin. Microbiol. Infect. 24, 1305–1310. doi: 10.1016/j.cmi.2018.02.022
Carrigy, N. B., Chang, R. Y., Leung, S. S. Y., Harrison, M., Petrova, Z., Pope, W. H., et al. (2017). Anti-Tuberculosis Bacteriophage D29 Delivery with a Vibrating Mesh Nebulizer. Jet Nebulizer, and Soft Mist Inhaler. Pharm. Res. 34, 2084–2096. doi: 10.1007/s11095-017-2213-4
Carrigy, N. B., Larsen, S. E., Reese, V., Pecor, T., Harrison, M., Kuehl, P. J., et al. (2019). Prophylaxis of Mycobacterium tuberculosis H37Rv Infection in a preclinical mouse model via inhalation of nebulized bacteriophage D29. Antimicrob. Agents Chemother. 63: AAC.00871-19. doi: 10.1128/AAC.00871-19
Castells, M., Khan, D. A., and Phillips, E. J. (2019). Penicillin allergy. N. Engl. J. Med. 381, 2338–2351. doi: 10.1056/NEJMra1807761
Catalão, M. J., and Pimentel, M. (2018). Mycobacteriophage lysis enzymes: targeting the mycobacterial cell envelope. Viruses 10:428. doi: 10.3390/v10080428
Chan, B. K., Turner, P. E., Kim, S., Mojibian, H. R., Elefteriades, J. A., and Narayan, D. (2018). Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol. Med. Public Heal. 2018, 60–66. doi: 10.1093/emph/eoy005
Chhibber, S., Kaur, T., and Kaur, S. (2013). Co-Therapy Using Lytic Bacteriophage and Linezolid: effective treatment in eliminating methicillin resistant Staphylococcus aureus (MRSA) from diabetic foot infections. PLoS One 8:e56022. doi: 10.1371/journal.pone.0056022
Comeau, A. M., Tétart, F., Trojet, S. N., Prère, M.-F., and Krisch, H. M. (2007). Phage-Antibiotic Synergy (PAS): beta-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One 2:e799. doi: 10.1371/journal.pone.0000799
Danelishvili, L., Young, L. S., and Bermudez, L. E. (2006). Infection As Delivered by a Nonvirulent Mycobacterium. Microb. Drug Resist 12, 1-6.
De Paepe, M., Leclerc, M., Tinsley, C. R., and Petit, M. A. (2014). Bacteriophages: an underestimated role in human and animal health? Front. Cell. Infect. Microbiol. 5:39. doi: 10.3389/fcimb.2014.00039
Dedrick, R. M., Guerrero-Bustamante, C. A., Garlena, R. A., Russell, D. A., Ford, K., Harris, K., et al. (2019). Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 25, 730–733. doi: 10.1038/s41591-019-0437-z
Diel, R., Lipman, M., and Hoefsloot, W. (2018). High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect. Dis. 18:206. doi: 10.1186/s12879-018-3113-x
Edwards, R. A., and Rohwer, F. (2005). Viral metagenomics. Nat. Rev. Microbiol. 3, 504–510. doi: 10.1038/nrmicro1163
Ehlers, S., and Schaible, U. E. (2012). The granuloma in tuberculosis: dynamics of a host-pathogen collusion. Front. Immunol. 3:411. doi: 10.3389/fimmu.2012.00411
Fenaroli, F., Westmoreland, D., Benjaminsen, J., Kolstad, T., Skjeldal, F. M., Meijer, A. H., et al. (2014). Nanoparticles as drug delivery system against Tuberculosis in Zebrafish Embryos: direct Visualization and Treatment. ACS Nano 8, 7014–7026. doi: 10.1021/nn5019126
World Health Organisation [WHO] (2019). Global Tuberculosis Report. Geneva: WHO, doi: 10.1016/j.icrp.2009.12.007
Gutierrez, A., Jain, S., Bhargava, P., Hamblin, M., Lobritz, M. A., and Collins, J. J. (2017). Understanding and sensitizing density-dependent persistence to quinolone antibiotics. Mol. Cell 68, 1147.e–1154.e. doi: 10.1016/j.molcel.2017.11.012
Gygli, S. M., Borrell, S., Trauner, A., and Gagneux, S. (2017). Antimicrobial resistance in Mycobacterium tuberculosis: mechanistic and evolutionary perspectives. FEMS Microbiol. Rev. 41, 354–373. doi: 10.1093/femsre/fux011
Hall, M. J., Middleton, R. F., and Westmacott, D. (1983). The fractional inhibitory concentration (FIC) index as a measure of synergy. J. Antimicrob. Chemother. 11, 427–433. doi: 10.1093/jac/11.5.427
Horsburgh, C. R. (1997). Epidemiology of Mycobacterium avium complex disease. Am. J. Med. 102, 11–15. doi: 10.1016/S0002-9343(97)00038-7
Houben, E. N. G., Nguyen, L., and Pieters, J. (2006). Interaction of pathogenic mycobacteria with the host immune system. Curr. Opin. Microbiol. 9, 76–85. doi: 10.1016/j.mib.2005.12.014
Iseman, M. D. (1994). Evolution of drug-resistant tuberculosis: a tale of two species. Proc. Natl. Acad. Sci. U.S.A. 91, 2428–2429. doi: 10.1073/pnas.91.7.2428
Jansen, M., Wahida, A., Latz, S., Krüttgen, A., Häfner, H., Buhl, E. M., et al. (2018). Enhanced antibacterial effect of the novel T4-like bacteriophage KARL-1 in combination with antibiotics against multi-drug resistant Acinetobacter baumannii. Sci. Rep. 8:14140. doi: 10.1038/s41598-018-32344-y
Jennes, S., Merabishvili, M., Soentjens, P., Pang, K. W., Rose, T., Keersebilck, E., et al. (2017). Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury-a case report. Crit. Care 21:129. doi: 10.1186/s13054-017-1709-y
Jończyk-Matysiak, E., Łusiak-Szelachowska, M., Kłak, M., Bubak, B., Międzybrodzki, R., Weber-Dąbrowska, B., et al. (2015). The effect of bacteriophage preparations on intracellular killing of bacteria by phagocytes. J. Immunol. Res. 2015:482863. doi: 10.1155/2015/482863
Kalapala, Y. C., Sharma, P. R., and Agarwal, R. (2020). Antimycobacterial potential of mycobacteriophage under pathophysiological conditions. bioRxiv [Preprint]. doi: 10.1101/2020.05.16.100123
Keshavjee, S., and Farmer, P. E. (2012). Tuberculosis, drug resistance, and the history of modern medicine. N. Engl. J. Med. 367, 931–936. doi: 10.1056/NEJMra1205429
Khawaldeh, A., Morales, S., Dillon, B., Alavidze, Z., Ginn, A. N., Thomas, L., et al. (2011). Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection. J. Med. Microbiol. 60, 1697–1700. doi: 10.1099/jmm.0.029744-0
Kim, M., Jo, Y., Hwang, Y. J., Hong, H. W., Hong, S. S., Park, K., et al. (2018). Phage-Antibiotic Synergy via Delayed Lysis. Appl. Environ. Microbiol. 84:02085-18. doi: 10.1128/AEM.02085-18
Kortright, K. E., Chan, B. K., Koff, J. L., and Turner, P. E. (2019). Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25, 219–232. doi: 10.1016/j.chom.2019.01.014
Labrie, S. J., Samson, J. E., and Moineau, S. (2010). Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327. doi: 10.1038/nrmicro2315
Lapenkova, M. B., Smirnova, N. S., Rutkevich, P. N., and Vladimirsky, M. A. (2018). Evaluation of the Efficiency of Lytic Mycobacteriophage D29 on the Model of M. tuberculosis-Infected Macrophage RAW 264 Cell Line. Bull. Exp. Biol. Med. 164, 344–346. doi: 10.1007/s10517-018-3986-0
Latino, L., Midoux, C., Hauck, Y., Vergnaud, G., and Pourcel, C. (2016). Pseudolysogeny and sequential mutations build multiresistance to virulent bacteriophages in pseudomonas aeruginosa. Microbiology 162, 748–763. doi: 10.1099/mic.0.000263
Lawn, S. D., and Zumla, A. I. (2011). Tuberculosis. Lancet 378, 57–72. doi: 10.1016/S0140-6736(10)62173-3
Leung, C. Y., and Weitz, J. S. (2017). Modeling the synergistic elimination of bacteria by phage and the innate immune system. J. Theor. Biol. 429, 241–252. doi: 10.1016/j.jtbi.2017.06.037
Li, J., Xie, S., Ahmed, S., Wang, F., Gu, Y., Zhang, C., et al. (2017). Antimicrobial activity and resistance: Influencing factors. Front. Pharmacol. 8:364. doi: 10.3389/fphar.2017.00364
Liu, K., Yang, W., Dong, X., Cong, L., Li, N., Li, Y., et al. (2016). Inhalation Study of Mycobacteriophage D29 Aerosol for Mice by Endotracheal Route and Nose-Only Exposure. J. Aerosol. Med. Pulm. Drug Deliv. 29, 393–405. doi: 10.1089/jamp.2015.1233
Lobritz, M. A., Belenky, P., Porter, C. B. M., Gutierrez, A., Yang, J. H., Schwarz, E. G., et al. (2015). Antibiotic efficacy is linked to bacterial cellular respiration. Proc. Natl. Acad. Sci. U.S.A. 112, 8173–8180. doi: 10.1073/pnas.1509743112
Lopatkin, A. J., Stokes, J. M., Zheng, E. J., Yang, J. H., Takahashi, M. K., You, L., et al. (2019). Bacterial metabolic state more accurately predicts antibiotic lethality than growth rate. Nat. Microbiol. 4, 2109–2117. doi: 10.1038/s41564-019-0536-0
Luthra, S., Rominski, A., and Sander, P. (2018). The role of antibiotic-target-modifying and antibiotic-modifying enzymes in mycobacterium abscessusdrug resistance. Front. Microbiol. 9:2179. doi: 10.3389/fmicb.2018.02179
Manrique, P., Bolduc, B., Walk, S. T., Van Oost, J. D., De Vos, W. M., and Young, M. J. (2016). Healthy human gut phageome. Proc. Natl. Acad. Sci. U.S.A. 113, 10400–10405. doi: 10.1073/pnas.1601060113
Marinelli, L. J., Piuri, M., Swigoňová, Z., Balachandran, A., Oldfield, L. M., van Kessel, J. C., et al. (2008). BRED: a simple and powerful tool for constructing mutant and recombinant bacteriophage genomes. PLoS One 3:e3957. doi: 10.1371/journal.pone.0003957
Mohan, A., Padiadpu, J., Baloni, P., and Chandra, N. (2015). Complete Genome Sequences of a Mycobacterium smegmatis Laboratory Strain (MC2 155) and Isoniazid-Resistant (4XR1/R2) Mutant Strains. Genome Announc. 3, 4–5. doi: 10.1128/genomeA.01520-14.Copyright
Nieth, A., Verseux, C., Barnert, S., Süss, R., and Römer, W. (2015). A first step toward liposome-mediated intracellular bacteriophage therapy. Expert Opin. Drug Deliv. 12, 1411–1424. doi: 10.1517/17425247.2015.1043125
Odds, F. C. (2003). Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. doi: 10.1093/jac/dkg301
Oechslin, F., Piccardi, P., Mancini, S., Gabard, J., Moreillon, P., Entenza, J. M., et al. (2017). Synergistic Interaction Between Phage Therapy and Antibiotics Clears Pseudomonas Aeruginosa Infection in Endocarditis and Reduces Virulence. J. Infect. Dis. 215, 703–712. doi: 10.1093/infdis/jiw632
Oh, Y. K., and Straubinger, R. M. (1996). Intracellular fate of Mycobacterium avium: use of dual-label spectrofluorometry to investigate the influence of bacterial viability opsonization on phagosomal pH phagosome-lysosome interaction. Infect. Immun. 64, 319–325. doi: 10.1128/iai.64.1.319-325.1996
Ooi, M. L., Drilling, A. J., Morales, S., Fong, S., Moraitis, S., MacIas-Valle, L., et al. (2019). Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to staphylococcus aureus. JAMA Otolaryngol. Head Neck Surg. 145, 723–729. doi: 10.1001/jamaoto.2019.1191
Padiadpu, J., Baloni, P., Anand, K., Munshi, M. H., Thakur, C., Mohan, A., et al. (2016). Identifying and tackling emergent vulnerability in drug-resistant mycobacteria. ACS Infect. Dis. 2, 592–607. doi: 10.1021/acsinfecdis.6b00004
Padiadpu, J., Baloni, P., and Chandra, N. (2015). Gene expression profiles of wild-type and isoniazid-resistant strains of Mycobacterium smegmatis. Data Br. 4, 186–189. doi: 10.1016/j.dib.2015.05.006
Petrovic Fabijan, A., Lin, R. C. Y., Ho, J., Maddocks, S., Ben Zakour, N. L., Iredell, J. R., et al. (2020). Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 5, 465–472. doi: 10.1038/s41564-019-0634-z
Pimentel, M. (2014). Genetics of Phage Lysis. Microbiol. Spectr 2:MGM2-0017-2013. doi: 10.1128/microbiolspec.MGM2-0017-2013
Piuri, M., and Hatfull, G. F. (2006). A peptidoglycan hydrolase motif within the mycobacteriophage TM4 tape measure protein promotes efficient infection of stationary phase cells. Mol. Microbiol. 62, 1569–1585. doi: 10.1111/j.1365-2958.2006.05473.x
Puapermpoonsiri, U., Spencer, J., and van der Walle, C. F. (2009). A freeze-dried formulation of bacteriophage encapsulated in biodegradable microspheres. Eur. J. Pharm. Biopharm. 72, 26–33. doi: 10.1016/j.ejpb.2008.12.001
Rahman, M., Kim, S., Kim, S. M., Seol, S. Y., and Kim, J. (2011). Characterization of induced Staphylococcus aureus bacteriophage SAP-26 and its anti-biofilm activity with rifampicin. Biofouling 27, 1087–1093. doi: 10.1080/08927014.2011.631169
Rastogi, N., Legrand, E., and Sola, C. (2001). The Mycobacteria: an introduction to nomenclature and pathogenesis. OIE Rev. Sci. Tech. 20, 21–54. doi: 10.20506/rst.20.1.1265
Roach, D. R., Leung, C. Y., Henry, M., Morello, E., Singh, D., Di Santo, J. P., et al. (2017). Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe 22, 38.e–47.e. doi: 10.1016/j.chom.2017.06.018
Rohde, K., Yates, R. M., Purdy, G. E., and Russell, D. G. (2007). Mycobacterium tuberculosis and the environment within the phagosome. Immunol. Rev. 219, 37–54. doi: 10.1111/j.1600-065X.2007.00547.x
Russell, D. A., and Hatfull, G. F. (2017). PhagesDB: the actinobacteriophage database. Bioinformatics 33, 784–786. doi: 10.1093/bioinformatics/btw711
Samaddar, S., Grewal, R. K., Sinha, S., Ghosh, S., Roy, S., and Das Gupta, S. K. (2015). Dynamics of mycobacteriophage-mycobacterial host interaction: evidence for secondary mechanisms for host lethality. Appl. Environ. Microbiol. 82, 124–133. doi: 10.1128/AEM.02700-15
Schooley, R. T., Biswas, B., Gill, J. J., Hernandez-Morales, A., Lancaster, J., Lessor, L., et al. (2017). Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 61:e00954-17. doi: 10.1128/AAC.00954-17
Seto, S., Morimoto, K., Yoshida, T., Hiramatsu, M., Hijikata, M., Nagata, T., et al. (2020). Proteomic profiling reveals the architecture of granulomatous lesions caused by tuberculosis and mycobacterium avium complex lung disease. Front. Microbiol. 10:3081. doi: 10.3389/fmicb.2019.03081
Sibling, L. D., Franzblau, S. G., and Krahenbuhl, J. L. (1987). Intracellular fate of Mycobacterium leprae in normal and activated mouse macrophages. Infect. Immun. 55, 680–685. doi: 10.1128/iai.55.3.680-685.1987
Sinha, A., Eniyan, K., Manohar, P., Ramesh, N., and Bajpai, U. (2020). Characterization and genome analysis of B1 sub-cluster mycobacteriophage PDRPxv. Virus Res. 279:197884. doi: 10.1016/j.virusres.2020.197884
Sprenger, M., and Fukuda, K. (2016). New mechanisms, new worries. Science 351, 1263–1264. doi: 10.1126/science.aad9450
Sula, L., Sulová, J., and Stolcpartová, M. (1981). Therapy of experimental tuberculosis in guinea pigs with mycobacterial phages DS-6A. GR-21 T, My-327. Czech. Med. 4, 209–214.
Sumitani, M., Takagi, S., Tanamura, Y., and Inoue, H. (2004). Oxygen indicator composed of an organic/inorganic hybrid compound of methylene blue, reductant, surfactant and saponite. Anal. Sci. 20, 1153–1157. doi: 10.2116/analsci.20.1153
Swift, B. M. C., Gerrard, Z. E., Huxley, J. N., and Rees, C. E. D. (2014). Factors affecting phage D29 infection: a tool to investigate different growth states of mycobacteria. PLoS One 9:e106690. doi: 10.1371/journal.pone.0106690
Teng, R., and Dick, T. (2003). Isoniazid resistance of exponentially growing Mycobacterium smegmatis biofilm culture. FEMS Microbiol. Lett. 227, 171–174. doi: 10.1016/S0378-1097(03)00584-6
Timm, J., Post, F. A., Bekker, L. G., Walther, G. B., Wainwright, H. C., Manganelli, R., et al. (2003). Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc. Natl. Acad. Sci. U.S.A. 100, 14321–14326. doi: 10.1073/pnas.2436197100
Tkhilaishvili, T., Lombardi, L., Klatt, A. B., Trampuz, A., and Di Luca, M. (2018). Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int. J. Antimicrob. Agents 52, 842–853. doi: 10.1016/j.ijantimicag.2018.09.006
Torres-Barceló, C., Arias-Sánchez, F. I., Vasse, M., Ramsayer, J., Kaltz, O., and Hochberg, M. E. (2014). A window of opportunity to control the bacterial pathogen Pseudomonas aeruginosa combining antibiotics and phages. PLoS One 9:e0106628. doi: 10.1371/journal.pone.0106628
Trigo, G., Martins, T. G., Fraga, A. G., Longatto-Filho, A., Castro, A. G., Azeredo, J., et al. (2013). Phage therapy is effective against infection by mycobacterium ulcerans in a murine footpad model. PLoS Negl. Trop. Dis. 7:e2183. doi: 10.1371/journal.pntd.0002183
Turkington, C. J. R., Morozov, A., Clokie, M. R. J., and Bayliss, C. D. (2019). Phage-Resistant Phase-Variant Sub-populations Mediate Herd Immunity Against Bacteriophage Invasion of Bacterial Meta-Populations. Front. Microbiol. 10:1473. doi: 10.3389/fmicb.2019.01473
Vandenheuvel, D., Singh, A., Vandersteegen, K., Klumpp, J., Lavigne, R., and Van den Mooter, G. (2013). Feasibility of spray drying bacteriophages into respirable powders to combat pulmonary bacterial infections. Eur. J. Pharm. Biopharm. 84, 578–582. doi: 10.1016/j.ejpb.2012.12.022
Voskuil, M. I., Visconti, K. C., and Schoolnik, G. K. (2004). Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis 84, 218–227. doi: 10.1016/j.tube.2004.02.003
Waters, E. M., Neill, D. R., Kaman, B., Sahota, J. S., Clokie, M. R. J., Winstanley, C., et al. (2017). Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa. Thorax 72, 666–667. doi: 10.1136/thoraxjnl-2016-209265
Wipperman, M. F., Fitzgerald, D. W., Juste, M. A. J., Taur, Y., Namasivayam, S., Sher, A., et al. (2017). Antibiotic treatment for Tuberculosis induces a profound dysbiosis of the microbiome that persists long after therapy is completed. Sci. Rep. 7:10767. doi: 10.1038/s41598-017-10346-6
Keywords: phage therapy, antimicrobial resistance (AMR), tuberculosis, mycobacterium, antibiotic synergy
Citation: Kalapala YC, Sharma PR and Agarwal R (2020) Antimycobacterial Potential of Mycobacteriophage Under Disease-Mimicking Conditions. Front. Microbiol. 11:583661. doi: 10.3389/fmicb.2020.583661
Received: 15 July 2020; Accepted: 16 November 2020;
Published: 14 December 2020.
Edited by:
Robert Czajkowski, University of Gdańsk, PolandReviewed by:
Paras Jain, Intellectual Ventures, United StatesCopyright © 2020 Kalapala, Sharma and Agarwal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachit Agarwal, cmFjaGl0QGlpc2MuYWMuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.