- 1Huck Institute of Life Sciences, Penn State University, University Park, PA, United States
- 2School of Biological Sciences, Monash University, Melbourne, VIC, Australia
- 3Department of Zoology, University of Oxford, Oxford, United Kingdom
- 4School of Biological Sciences, The University of Queensland, St. Lucia, QLD, Australia
- 5Institut für Populationsgenetik, Vetmeduni Vienna, Vienna, Austria
- 6Department of Virology, Institut Pasteur, Paris, France
Wolbachia is an intracellular bacterium that blocks virus replication in insects and has been introduced into the mosquito, Aedes aegypti for the biocontrol of arboviruses including dengue, Zika, and chikungunya. Despite ongoing research, the mechanism of Wolbachia-mediated virus blocking remains unclear. We recently used experimental evolution to reveal that Wolbachia-mediated dengue blocking could be selected upon in the A. aegypti host and showed evidence that strong levels of blocking could be maintained by natural selection. In this study, we investigate the genetic variation associated with blocking and use these analyses to generate testable hypotheses surrounding the mechanism of Wolbachia-mediated dengue blocking. From our results, we hypothesize that Wolbachia may block virus replication by increasing the regeneration rate of mosquito cells via the Notch signaling pathway. We also propose that Wolbachia modulates the host’s transcriptional pausing pathway either to prime the host’s anti-viral response or to directly inhibit viral replication.
Introduction
The Aedes aegypti mosquito transmits arboviruses that cause morbidity and mortality globally, including dengue, Zika, and chikungunya (Kraemer et al., 2019). Wolbachia are vertically transmitted, intracellular alpha-proteobacteria that can protect insects from viruses, termed virus “blocking” (Hedges et al., 2008; Teixeira et al., 2008). Wolbachia have been artificially introduced into A. aegypti, where they are stably inherited (Gloria-Soria et al., 2018) and block the transmission of dengue, Zika, and chikungunya viruses (Moreira et al., 2009; Ye et al., 2015; Aliota et al., 2016a, b; Dutra et al., 2016; Tan et al., 2017). Wolbachia-infected mosquitoes have since invaded natural populations of mosquitoes where they have reduced the incidence of locally transmitted dengue (Walker et al., 2011; Hoffmann et al., 2015; Hancock et al., 2016; Jiggins, 2017; Schmidt et al., 2017; O’Neill, 2018; O’Neill et al., 2018).
Despite substantial discoveries, the mechanism of Wolbachia-mediated virus blocking remains unclear. Research using insect cells suggests that blocking impacts early viral genome replication (Rainey et al., 2016; Thomas et al., 2018). Flaviviruses use the host cytoskeleton and alter the endoplasmic reticulum (ER) to form sites of replication, termed replication complexes (RCs) (Lindsey et al., 2018). Wolbachia also modify the ER and rely upon it for protein degradation (Molloy et al., 2016; Geoghegan et al., 2017; White et al., 2017). Moreover, Wolbachia modulates the cytoskeleton to ensure their localization in the cell during mitosis and meiosis (Lindsey et al., 2018). Together, flaviviruses and Wolbachia are likely to compete for the ER and cytoskeleton, along with resources (Caragata et al., 2013). Other pathways have also been shown to affect Wolbachia-mediated blocking but do not seem to be required, e.g., host immune priming (Rancès et al., 2012; Rancès et al., 2013), the RNAi pathway (Terradas et al., 2017b), and host miRNAs (Hussain et al., 2011; Zhang et al., 2013; Rainey et al., 2016), the production of reactive oxygen species (ROS) (Pan et al., 2012, 2018; Wong et al., 2016) and XRN1-mediated virus degradation (Thomas et al., 2018). It is highly likely that viral blocking is multifaceted, encompassing several of these factors, potentially to varying degrees in different Wolbachia-mosquito combinations, and in some cases acting synergistically. The discovery of synergistic mechanisms would be very informative in the choice of Wolbachia strains for disease control.
Recently, we artificially selected upon Wolbachia-infected A. aegypti mosquitoes (wMel.F strain) to test if we could increase or decrease the strength of Wolbachia-mediated dengue blocking (Ford et al., 2019a). Briefly, our selection protocol involved administering a controlled dose of the dengue virus, serotype 3, into blood-fed female mosquitoes by micro-injection and measuring the virus load in the mosquito bodies after 7 days of infection (see materials and methods for full details). We selected for increased blocking in 3 populations of mosquitoes, decreased blocking in another 3 populations and we performed randomized selection from 3 more populations as a control treatment. After selecting for decreased blocking for 4 generations, we were able to isolate mosquitoes that had lost 45% of Wolbachia-mediated blocking (measured as viral loads in the body) compared to randomly selected control populations. We identified that this resulted from genetic variation in A. aegypti and not Wolbachia. Interestingly, we could not increase blocking strength and found evidence to suggest that genotypes exhibiting strong levels of blocking were already at a high frequency due to an inherent fitness advantage. These data indicate the potential for natural selection to maintain blocking. In addition to this, these data provide a great opportunity to gain insight into the mechanism of Wolbachia-mediated dengue blocking. In this previous study, we investigated two candidate genes that had undergone large changes in allele frequencies as a result of selection on blocking. We found one gene whose expression increased with blocking strength. This gene encoded a cadherin protein (AAEL023845). Cadherins are cell-cell adhesion proteins that mediate cell signaling and intracellular trafficking (Yamagata et al., 2018), yet it remains unclear how this gene may be involved in Wolbachia-mediated dengue blocking.
In this study, we investigate 61 A. aegypti genes that exhibited significant changes in allele frequencies between the mosquito populations that we selected for high and low Wolbachia-mediated dengue blocking (Ford et al., 2019a). We then used these data to generate novel and testable hypotheses surrounding the mechanism of Wolbachia-mediated dengue blocking. We found that genes under selection for blocking were significantly enriched for signal transduction and transcription regulation. More specifically, we found that genes involved in neurogenesis, the Notch signaling pathway and cell-cell adhesion are the most commonly selected upon, leading to the hypothesis that Notch-mediated cell replenishment may be important for Wolbachia-mediated viral blocking. We also revealed that the host’s transcriptional pausing pathway could be involved in blocking and find evidence for the host’s oxidative stress response and cytoskeleton, consistent with previous studies.
Results and Discussion
Gene Ontology Term Enrichment Analysis
We investigated 61 A. aegypti genes that showed significant changes in allele frequencies between the mosquito populations selected for high and low Wolbachia-mediated blocking (Supplementary File 1) in our previous study (Ford et al., 2019a). We explain the generation of these significant genes in section “Materials and Methods.” We tested for enriched gene ontology (GO) terms using the Singular Enrichment Analysis (SEA) available on AgriGo v2 for the AaegL3.3 locus ID (VectorBase) (Figure 1 and Supplementary File 2, generated by AgriGo v2). The most specific GO terms (as measured by “Bgitem,” which is the total number of genes annotated with that GO term in the A. aegypti genome) that were significantly enriched included: “regulation of gene expression” (Fisher test with Benjamini–Hochberg false discovery rate, FDR, P-value adjustments P = 0.018), “nucleobase-containing compound metabolic process” (FDR-corrected P = 0.0113), “cellular macromolecule metabolic process” (FDR-corrected P = 0.0186) and “signal transduction” (FDR-corrected P = 0.0174).
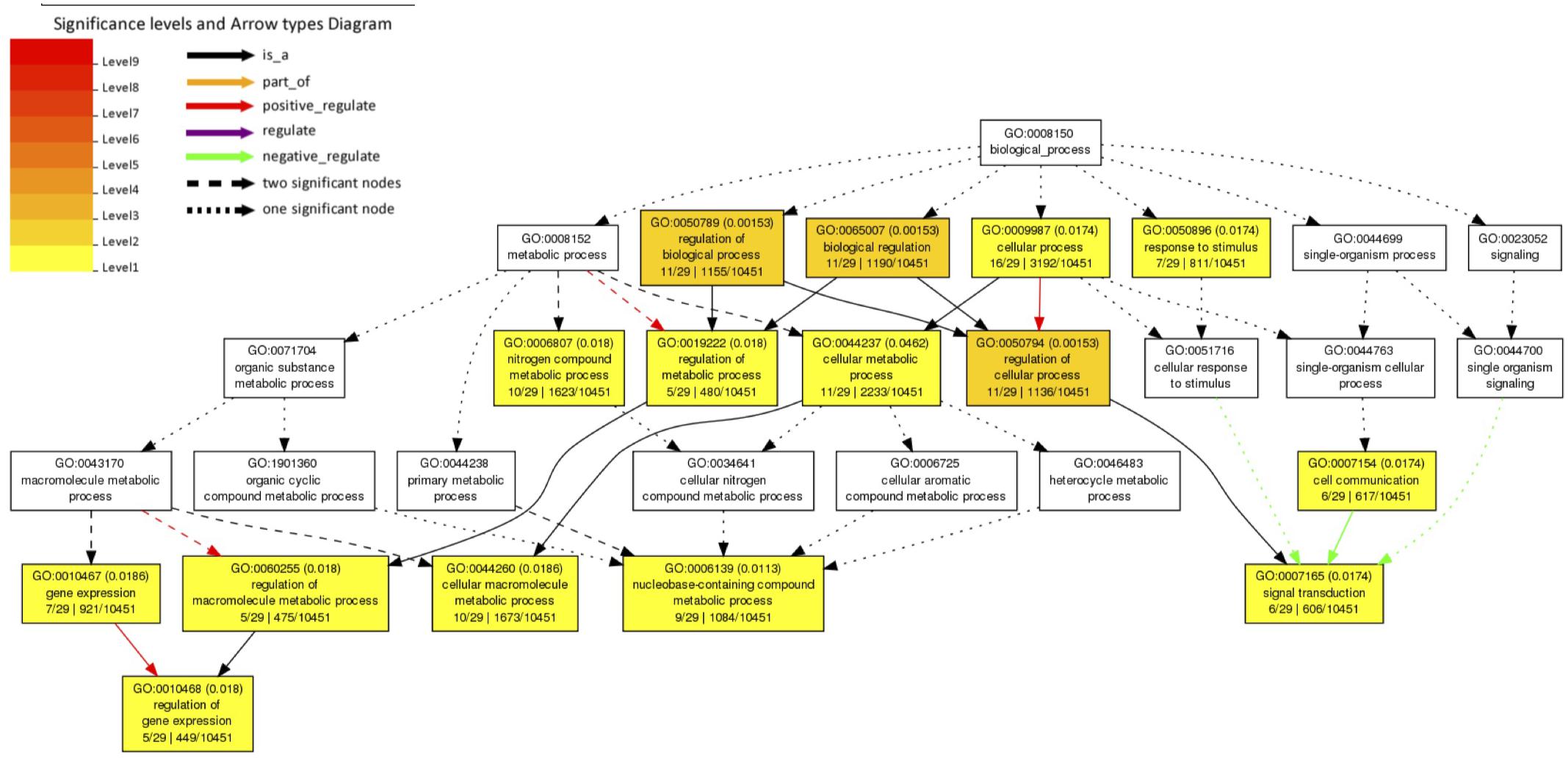
Figure 1. Gene Ontology (GO) term enrichment analysis of A. aegypti genes containing SNPs associated with Wolbachia-mediated dengue blocking. We performed a Singular Enrichment Analysis (SEA) using AgriGo v2 on 61 A. aegypti genes that contained SNPs that were significantly differentiated between mosquito populations selected for high vs. low Wolbachia-mediated dengue blocking (Supplementary File 1) in our previous study (Ford et al., 2019a). The SEA utilised a Fisher test with Benjamini-Hochberg (FDR) P-value adjustments for multiple comparisons. P-values are shown in brackets. GO terms and the gene entries assigned to them are listed in Supplementary File 2.
Cell Replenishment
We then grouped the genes by function, using the OrthoDB, Uniprot, and Interpro databases (Table 1). Consistent with the significant GO term “signal transduction,” we found genes for neurogenesis, the Notch signaling pathway, cell cycle and cell-cell adhesion, including the previously described cadherin gene (AAEL023845). Together, this gene profile suggests that cell replenishment pathways could be important for Wolbachia-mediated dengue blocking. It has been found that the Notch signaling pathway confers resistance to the dengue virus in A. aegypti by controlling host cell regeneration rate whereby mosquitoes with faster cell regeneration in the midgut are significantly more resistant to the dengue virus (Taracena et al., 2018). Moreover, the depletion of the cell-cell adhesion protein, cadherin, is known to downregulate the Notch signaling pathway and so cell replenishment (Sasaki et al., 2007; Hatakeyama et al., 2014). Since we selected upon virus levels following micro-injection, our data suggest that cell replenishment may be important across the whole body.
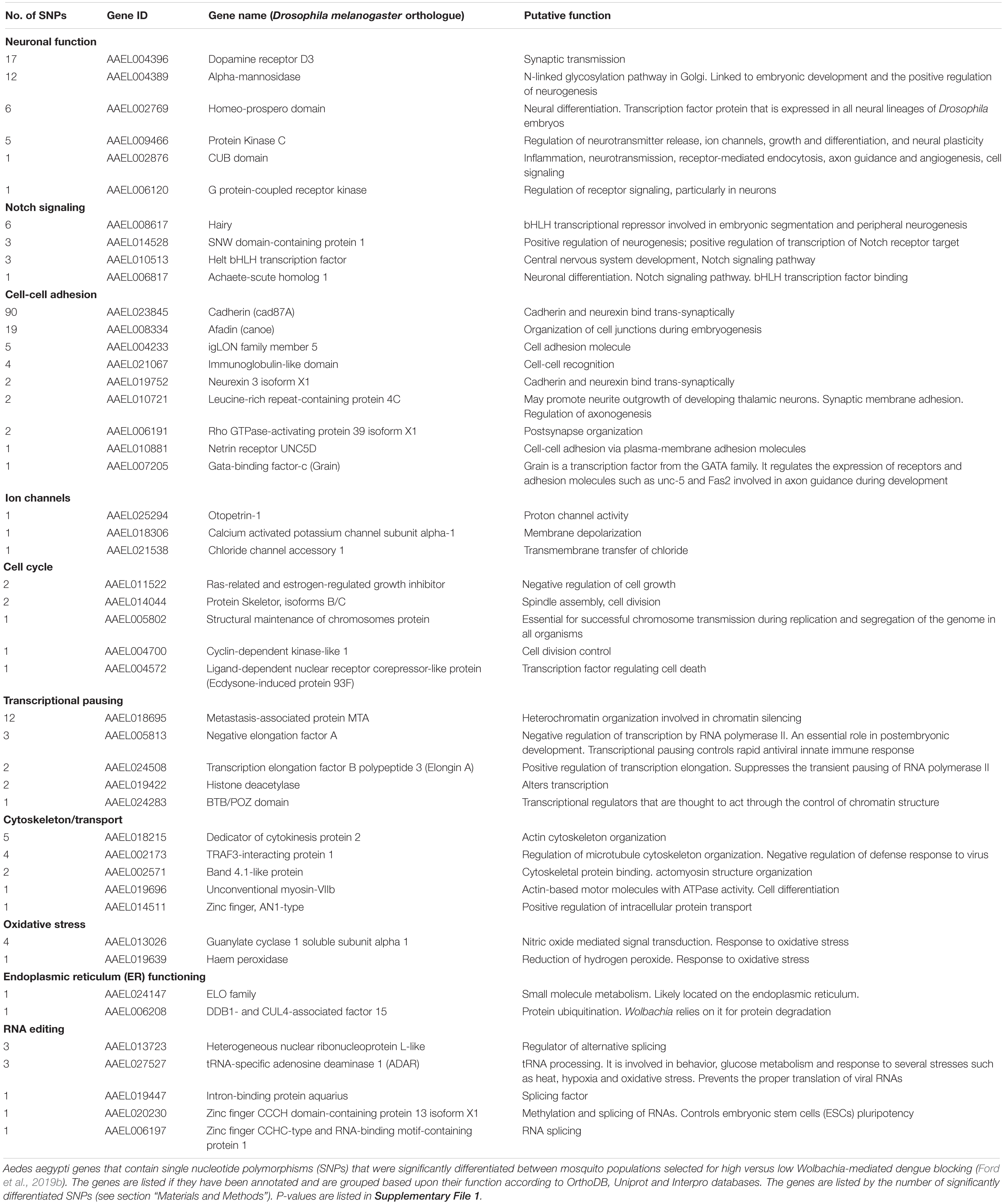
Table 1. Functional groups of annotated genes under selection for Wolbachia-mediated dengue blocking.
Based on these data, we hypothesize that Wolbachia could block virus by increasing cell-cell adhesion and host cell regeneration rate via Notch signaling. This hypothesis would be consistent with our previous observation that the reduction in viral blocking was associated with reduced expression of the cell-cell adhesion gene, cadherin (Ford et al., 2019a). Moreover, the involvement of the Notch signaling pathway could also explain the inherent fitness cost that we observed in mosquitoes with weaker viral blocking (Ford et al., 2019a). Since the Notch signaling pathway is important for both insect development and cell replenishment, we speculate that reduced activity of this pathway might not only reduce virus-blocking, but may reduce fitness by slowing both development and the replenishment of damaged cells as an adult. Additionally, it has been shown that reduced Notch signaling significantly impairs A. aegypti fecundity and fertility (Chang et al., 2018). How Wolbachia might alter the Notch signaling pathway is unknown, but Wolbachia has been found to control stem cell behavior and stimulate mitosis in nematodes (Foray et al., 2018). Importantly, little is known about whether this occurs within insects. Although nematode-Wolbachia interactions are likely to be very different to insect-Wolbachia interactions, we may expect methods of cell-to-cell transmission to share similarities and so this could be a fruitful area of research. What we do know is that Wolbachia infection has been found to increase the metabolic rate of mosquitoes, which may be linked to increased cell turnover (Evans et al., 2009).
It is unclear whether neurogenesis specifically plays a role in Wolbachia-mediated blocking or whether the gene annotations exhibit a bias from Drosophila developmental studies and these genes instead function in cell proliferation and differentiation more generally (Taracena et al., 2018). The Zika virus has, however, been found to dysregulate neurogenesis in humans through the Notch signaling pathway (Ferraris et al., 2019) and by degrading adherens junction proteins (Yoon et al., 2017). Although this has not been shown with the dengue virus, it does upregulate the expression of cell-cell junction genes in humans (Afroz et al., 2016). Wolbachia is also known to cause neuropathology (Min and Benzer, 1997; Moreira et al., 2011) and control host factors during neurogenesis (Albertson et al., 2009). Critically, if Wolbachia-mediated virus blocking involves pathways by which viruses cause neuropathology in humans then it could select for viruses with altered disease severity. This exemplifies the importance of understanding the mechanism of blocking.
Transcriptional Pausing
Consistent with the significant GO term “regulation of gene expression,” we found a number of genes involved in transcriptional pausing. The transcriptional pausing pathway plays a role in the Drosophila antiviral response by priming the rapid transcription of immune genes involved in RNA silencing, autophagy, JAK/STAT, Toll and Imd pathways along with components of Toll receptors (Xu et al., 2012). Transcriptional pausing can also directly restrict viral transcription in mammalian cells (Fujinaga et al., 2004; Rao et al., 2006). Transcriptional pausing involves Negative and Positive Elongation Factors (NELF and P-TEFb) and is associated with open chromatin structures. Here, we find genetic variation associated with blocking in genes encoding NELF and P-TEFb (AAEL005813 and AAEL024508, respectively) and genes involved in chromatin-structure mediated silencing (AAEL018695, AAEL024283, and AAEL019422). We hypothesize that Wolbachia-mediated viral blocking could trigger the host’s transcriptional pausing pathway to prime the antiviral response or to directly inhibit virus transcription.
Cytoskeleton/Transport
The dengue virus utilizes the cytoskeleton to enter/exit the cell and recruit resources to RCs (Lindsey et al., 2018). Simultaneously, Wolbachia utilize the cytoskeleton for successful transmission during mitosis and meiosis and so may interfere with the virus life-cycle (Lindsey et al., 2018). In our dataset, we identified many genes with putative links to the cytoskeleton, including genes related to microtubules (AAEL002173), actin (AAEL018215), and associated motors (AAEL019696). Of particular interest is the gene TRAF3-interacting protein 1 (AAEL002173). TRAF3 has been implicated in an antiviral response in the sand fly, Lutzomyia longipalpis (Martins-da-Silva et al., 2018).
Oxidative Stress
Oxidative stress is a by-product of Wolbachia infection and can trigger anti-microbial host responses (Lindsey et al., 2018). We find variation associated with blocking in a gene that reduces hydrogen peroxide (AAEL019639) and in another that responds to nitric oxide (AAEL013026). Changes in these genes could either: remove ROS, e.g., to enable the host to tolerate Wolbachia infection; or prevent the removal of ROS, e.g., to trigger an anti-microbial response, or stimulate mitosis and so increase cell regeneration (Taracena et al., 2018).
Conclusion
By reviewing A. aegypti genes isolated by artificial selection, we present new hypotheses for the mechanism of Wolbachia-mediated dengue blocking. Signal transduction and the regulation of transcription were significantly enriched in this dataset, corresponding to large sets of genes for cell regeneration and the transcriptional pausing pathway. Our data led to two main hypotheses: (1) efficient regeneration of mosquito cells increases resistance against flaviviruses and Wolbachia could promote this by modulating adherens junctions and Notch signaling; and (2) Wolbachia could trigger the host’s transcriptional pausing pathway to either prime the hosts’ anti-viral response or inhibit viral transcription. Consistent with previous studies, we also found evidence that oxidative stress and the cytoskeleton are likely to be involved in blocking.
It is important to note that, as with many genome-wide association studies, we do not expect that every gene identified in this study will be required, or even involved in, Wolbachia-mediated blocking. We present this discussion to propose candidate pathways and hence shape the direction of future experimental studies in the broader field. The relevance of each pathway for diverse Wolbachia strains, dengue virus serotypes, other viruses and mosquito populations is yet to be explored. Moreover, these pathways were identified in response to injection with the dengue virus and so their role in midgut resistance to an infectious blood meal will be investigated.
Materials and Methods
Ethics
The evolution experiment was carried out at Monash University in Melbourne. The Monash University Human Research Ethics Committee gave ethical approval for human volunteers to provide blood meals to mosquitoes not infected with dengue virus (permit: CF11/0766-2011000387). One volunteer was involved throughout the study and provided written consent.
Mosquitoes
For the evolution experiment we used a population of A. aegypti that were infected with the wMel.F strain of Wolbachia (Hoffmann et al., 2011; Walker et al., 2011) and had been maintained in the laboratory for 33 generations. Every three generations, they were outcrossed with Wolbachia-free mosquitoes collected from Queensland, Australia, to maintain standing genetic variation (Hoffmann et al., 2011; Terradas et al., 2017a). Mosquitoes collected for outcrossing were replaced every six generations. During outcrossing, ∼30% of males from the laboratory population were replaced with males from the collected population. The wild-type populations used in this study to measure gene expression were the AFM line obtained from Zhiyong Xi, collected 12 months prior by Pablo Manrique in Merida, Mexico.
Dengue Virus
For the evolution experiment, we used dengue virus serotype 3, isolated from Cairns (Ritchie et al., 2013; Ye et al., 2016). Virus was grown within C6/36 Aedes albopictus cells following standard methods (Terradas et al., 2017a). Cells were grown to 80% confluency at 26°C in T175 tissue culture flasks containing 25 ml RPMI 1640 media (Life Technologies) supplemented with 10% fetal bovine serum (Life Technologies), 2% HEPES (Sigma-Aldrich) and 1% Glutamax (Life Technologies). The media was then replaced with 25 ml RPMI supplemented with 2% fetal bovine serum, 2% HEPES and 1% Glutamax, and 20 μl virus was added. After 7 days, cells were scraped off and the suspension was centrifuged at 3,200 g for 15 min at 4°C. The supernatant was frozen in single-use aliquots at −80°C, and all experiments were conducted using these aliquots. Virus titres were measured from a thawed aliquot by: (1) mixing 20 μl with 200 μl of TRIzol (Invitrogen); (2) extracting the RNA following the manufacturer’s protocol and treating with DNAse I (Sigma-Aldrich); and (3) quantifying dengue virus RNA using quantitative reverse-transcription PCR (RT-qPCR) (see section “Dengue Virus Quantification”). Three independent extractions were performed and two replicates of each extraction were measured to generate an average value of 1.80 × 106 genomic copies of the dengue virus per ml.
Dengue Virus Quantification
Dengue virus was quantified via RT-qPCR using the LightCycler 480 (Roche). We used the TaqMan Fast Virus 1-Step Master Mix (Thermo Fisher Scientific) in a total reaction volume of 10 μl, following the manufacturer’s instructions (Terradas et al., 2017a). The list of primers and probes is given in Supplementary Table 1. The temperature profile used was: 50°C for 10 min; 95°C for 20 s; 35 cycles of 95°C for 3 s; 60°C for 30 s; 72°C for 1 s; and 40°C for 10 s. Data were analyzed using absolute quantification where the dengue virus copy number per sample was calculated from a reference curve. This reference curve was made up from known quantities of the genomic region of the virus that the primers amplify. This genomic region had previously been cloned into the pGEM-T plasmid (Promega) and transformed into Escherichia coli (Ye et al., 2014). After growing E. coli in liquid Luria broth (LB) overnight at 37°C, we extracted the plasmid using the PureYield Plasmid Midiprep System kit (Promega) and linearized it by restriction digest. We then purified the plasmid using phenol-chloroform extraction, resuspended in 20 μl of UltraPure distilled water (Invitrogen) and quantified it by Qubit. A dilution series of 107, 106, 105, 104, 103, 102, and 101 copies of the genomic fragment was created and frozen as single-use aliquots. All assays measuring viral load used these aliquots, and three replicates of the dilution series were run on every 96-well plate to create a reference curve for dengue virus quantification.
Evolution Experiment
We selected for low and high Wolbachia-mediated dengue blocking alongside a control treatment where mosquitoes were selected at random. Each treatment included three independent populations generated from an ancestral population of mosquitoes using a random number generator (Kawecki et al., 2012). For each generation, eggs were hatched in trays (30 cm × 40 cm × 8 cm) containing 2 l of autoclaved reverse osmosis water to achieve 150–200 larvae per tray. Larvae were fed ground TetraMin tablets and reared under controlled conditions of temperature (26 ± 2°C), relative humidity (∼70%) and photoperiod (12 h:12 h light:dark). After pupation, pupae were placed within 30 cm × 30 cm × 30 cm cages in cups containing autoclaved reverse osmosis water for eclosion to achieve ∼450 mosquitoes per cage. Mosquitoes were fed 10% sucrose water from dental wicks. When mosquitoes were 5–7 d old, each population was allowed to blood-feed on a human volunteer in a random order. Females that fed were separated into cups enclosed with mesh that contained moist filter paper to provide an oviposition site. Mosquitoes were fed 10% sucrose water from cotton wool.
After 4 d, eggs were collected, numbered and dried following a standard protocol for short-term egg storage (Zheng et al., 2015). The number of each set of eggs was written on the cups of the corresponding female. Egg collection was done before infection with dengue to prevent vertical transmission of the virus (Joshi et al., 2002). Between 40 and 70 females from each of the high- and low-blocking populations were anesthetized with CO2, injected with 69 nl of the dengue virus stock (equaling ∼124 genomic copies of dengue; see section “Dengue Virus”) and returned to their numbered cups. Virus was delivered at a speed of 46 nl s–1 into the thorax using a pulled glass capillary needle and a manual microinjector (Nanoject II; Drummond Scientific). This controlled the infection dose by removing the variation that would have resulted from oral feeding, to ensure successful artificial selection. This method also ensured a sufficient number of infected mosquitoes to select between.
At 7 d post-infection, females were anesthetized with CO2, placed into individual wells of 96-well plates containing 50 μl extraction buffer and homogenized with a 3-mm glass bead. The extraction buffer was made up of squash buffer [10 mM Tris (pH 8.2), 1 mM EDTA and 50 mM NaCl] (Yeap et al., 2014) with proteinase K at a concentration of 12.5 μl ml–1 (Bioline). Samples were then incubated for 5 min at 56°C and 5 min at 95°C. We then measured the viral load per mosquito using RT-qPCR (see section “Dengue Virus Quantification”). This method was used for rapid phenotype determination of a large number of samples. Mosquitoes were then ranked in order: (1) from the lowest viral load in the high-blocking populations; (2) from the highest viral load in the low-blocking populations; and (3) using a random number generator in the random population. Eggs from each mosquito were hatched into separate cups of autoclaved reverse osmosis water. The next day, larvae were taken from cups in rank order until ∼200 larvae were collected for each replicate population. On average, offspring were taken from six mosquitoes per replicate population per generation. This was done to impose the strongest selection pressure possible while ensuring that enough mosquitoes would be reared for selection in the subsequent generation. At this point, the protocol was repeated. In total, four rounds of selection were completed.
Genomic Analysis
DNA was extracted from 90 individual mosquitoes from the ancestral population and each evolved population after 4 generations of selection. We extracted DNA from the TRIzol reagent (Invitrogen) using a modified version of the manufacturer’s protocol with additional washing steps using phenol, chloroform and isoamylalcohol. DNA was sequenced using an Illumina HiSeq 3000 with 150-base pair paired-end reads.
FastQC version 0.11.4 was used with default settings to check the quality of the raw reads. To minimize false positives, Trimmomatic version 0.36 was used to trim the 3′ ends if the quality was <20, and the reads were discarded if trimming resulted in reads that were <50 base pairs in length (<0.5%). We mapped the resulting reads to the A. aegypti assembly (Liverpool AGWG-AaegL5) using BWA MEM 2.2.1, and checked for quality using Qualimap version 2.2.1. Indel realignment was completed using GATK version 3.8.0. Duplicates were removed using Picard version 2.17.8, and reads with poor mapping quality were removed using SAMtools 1.6 and filtering via hex flags: −q 20 (only include reads with a mapping quality of ≥20); −f 0 × 002 (only include reads with all of the flags mapped in a proper pair); −F 0 × 004 (only include reads with none of the flags unmapped); and −F 0 × 008 (only include reads with none of the flags mate unmapped). Around 10% of reads were PCR duplicates (615,305,021) and ∼58% of reads failed mapping quality filters (3,655,353,869). The quality was checked using Qualimap.
Single nucleotide polymorphisms (SNPs) were called using PoPoolation2 based on a minimum coverage of 20 and a maximum coverage of 200. Coverage was ∼46 after duplicate and low-quality mapping were removed (∼51 before). We ran an alternative method to call variants to cross-check the output from the above method. Variants were called for each sample using the GATK HaplotypeCaller tool (gatk-4.0.8.1) using default settings except for ploidy, which was set to 10. A multi-sample variant file was then created merging the vcf files using the “bcftools merge” command. SNPs from the original analysis were retained if at least one population had the same SNP called by GATK.
We identified SNPs that were significantly differentiated between treatments (see section “Statistics”) and annotated them with gene information using gene transfer format files and bedtools intersect (bedtools version 2.25). Annotation files were downloaded from VectorBase (AaegL5.1). Information on A. aegypti gene function was collected by searching VectorBase gene IDs on OrthoDB (Kriventseva et al., 2018).
Statistics
We tested for differences in allele frequency between treatments using generalized linear models that were applied to replicate level major and minor allele counts. Using R version 3.2.21, we fitted these single-SNP models using the glm() function in R and assumed a binomial error structure. To aid interpretation, we conducted these analyzes in a pairwise fashion, analyzing differentiation between all possible pairs of selection treatments (that is, high versus low, high versus random, and low versus random populations). To assess the genome-wide significance of these models, and to account for the P-value inflation that occurs in single-SNP analyses of evolve and resequence data, we estimated an empirical significance threshold based on exhaustive permutation of our experimental data (Jha et al., 2016). We estimated a permutation-based P-value threshold that corresponded to a genome-wide FDR of 5% by re-running our genome scan on all possible permutations of our pairwise contrasts between high and low, high and random, and low and random populations. In each case, there were nine possible permutations excluding the observed arrangement of the six replicate populations. For each permuted dataset, we refitted our linear model to all SNPs and estimated the number of significant SNPs.
We performed a SEA on the AgriGO v2 website using the 61 A. aegypti genes associated with Wolbachia-mediated dengue blocking listed in the Supplementary File 1. We used the AaegL3.3 reference genome and performed a Fisher test, correcting for multiple comparisons using the Hochberg (FDR) method. We set the significance level to 0.05 and the minimum number of mapping entries to 5. We set the gene ontology type to Generic GO_slim, which includes a reduced version of the terms in the whole GO terms.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.nature.com/articles/s41564-019-0533-3#additional-information.
Author Contributions
SF and EM designed the study and wrote up the manuscript. SF, MJ, LS, and CK generated the data. IA, AS, SA, SC, and SF identified genes under selection. SF described the genetic data, performed the follow-up statistical analyses, and interpreted the results. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant (APP1103804) from the National Health and Medical Research Council of Australia to EM.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01456/full#supplementary-material
Footnotes
References
Afroz, S., Giddaluru, J., Abbas, M. M., and Khan, N. (2016). Transcriptome meta-analysis reveals a dysregulation in extra cellular matrix and cell junction associated gene signatures during Dengue virus infection. Sci. Rep. 6:33752. doi: 10.1038/srep33752
Albertson, R., Casper-Lindley, C., Cao, J., Tram, U., and Sullivan, W. (2009). Symmetric and asymmetric mitotic segregation patterns influence Wolbachia distribution in host somatic tissue. J. Cell Sci. 122(Pt 24), 4570–4583. doi: 10.1242/jcs.054981
Aliota, M. T., Peinado, S. A. I, Velez, D., and Osorio, J. E. (2016a). The wMel strain of Wolbachia reduces transmission of zika virus by Aedes aegypti. Sci. Rep. 6:28792. doi: 10.1038/srep28792
Aliota, M. T., Walker, E. C., Uribe Yepes, A., Velez, I. D., Christensen, B. M., and Osorio, J. E. (2016b). The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Negl. Trop. Dis. 10:e0004677. doi: 10.1371/journal.pntd.0004677
Caragata, E. P., Rancès, E., Hedges, L. M., Gofton, A. W., Johnson, K. N., O’Neill, S. L., et al. (2013). Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 9:e1003459. doi: 10.1371/journal.ppat.1003459
Chang, C. H., Liu, Y. T., Weng, S. C., Chen, I. Y., Tsao, P. N., Shiao, S.-H., et al. (2018). The non-canonical Notch signaling is essential for the control of fertility in Aedes aegypti. PLoS Negl. Trop. Dis. 12:e0006307. doi: 10.1371/journal.pntd.0006307
Dutra, H. L., Rocha, M. N., Dias, F. B., Mansur, S. B., Caragata, E. P., and Moreira, L. A. (2016). Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 19, 771–774. doi: 10.1016/j.chom.2016.04.021
Evans, O., Caragata, E. P., McMeniman, C. J., Woolfit, M., Green, D. C., Williams, C. R., et al. (2009). Increased locomotor activity and metabolism of Aedes aegypti infected with a life-shortening strain of Wolbachia pipientis. J. Exp. Biol. 212(Pt 10), 1436–1441. doi: 10.1242/jeb.028951
Ferraris, P., Cochet, M., Hamel, R., Gladwyn-Ng, I., Alfano, C., Diop, F., et al. (2019). Zika virus differentially infects human neural progenitor cells according to their state of differentiation and dysregulates neurogenesis through the Notch pathway. Emerg. Microbes Infect. 8, 1003–1016. doi: 10.1080/22221751.2019.1637283
Foray, V., Pérez-Jiménez, M. M., Fattouh, N., and Landmann, F. (2018). Wolbachia control stem cell behavior and stimulate germline proliferation in filarial nematodes. Dev. Cell 45, 198–211.e3. doi: 10.1016/j.devcel.2018.03.017
Ford, S. A., Allen, S. L., Ohm, J. R., Sigle, L. T., Sebastian, A., Albert, I., et al. (2019a). Selection on Aedes aegypti alters Wolbachia-mediated dengue virus blocking and fitness. Nat. Microbiol. 4, 1832–1839. doi: 10.1038/s41564-019-0533-3
Ford, S. A., Allen, S. L., Ohm, J. R., Sigle, L. T., Sebastian, A., Albert, I., et al. (2019b). Data From: Selection on Aedes Aegypti Alters Wolbachia-Mediated Dengue Virus Blocking and Fitness. Cape Town: Dryad.
Fujinaga, K., Irwin, D., Huang, Y., Taube, R., Kurosu, T., and Peterlin, B. M. (2004). Dynamics of human immunodeficiency virus transcription: P-TEFb phosphorylates RD and dissociates negative effectors from the transactivation response element. Mol. Cell Biol. 24, 787–795. doi: 10.1128/mcb.24.2.787-795.2004
Geoghegan, V., Stainton, K., Rainey, S. M., Ant, T. H., Dowle, A. A., Larson, T., et al. (2017). Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat. Commun. 8:526. doi: 10.1038/s41467-017-00610-8
Gloria-Soria, A., Chiodo, T. G., and Powell, J. R. (2018). Lack of evidence for natural Wolbachia infections in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 55, 1354–1356. doi: 10.1093/jme/tjy084
Hancock, P. A., White, V. L., Ritchie, S. A., Hoffmann, A. A., and Godfray, H. C. (2016). Predicting Wolbachia invasion dynamics in Aedes aegypti populations using models of density-dependent demographic traits. BMC Biol. 14:96. doi: 10.1186/s12915-016-0319-5
Hatakeyama, J., Wakamatsu, Y., Nagafuchi, A., Kageyama, R., Shigemoto, R., and Shimamura, K. (2014). Cadherin-based adhesions in the apical endfoot are required for active Notch signaling to control neurogenesis in vertebrates. Development 141, 1671–1682. doi: 10.1242/dev.102988
Hedges, L. M., Brownlie, J. C., O’Neill, S. L., and Johnson, K. N. (2008). Wolbachia and virus protection in insects. Science 322:702. doi: 10.1126/science.1162418
Hoffmann, A. A., Montgomery, B. L., Popovici, J., Iturbe-Ormaetxe, I., Johnson, P. H., Muzzi, F., et al. (2011). Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457. doi: 10.1038/nature10356
Hoffmann, A. A., Ross, P. A., and Rašič, G. (2015). Wolbachia strains for disease control: ecological and evolutionary considerations. Evol. Appl. 8, 751–768. doi: 10.1111/eva.12286
Hussain, M., Frentiu, F. D., Moreira, L. A., O’Neill, S. L., and Asgari, S. (2011). Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 108, 9250–9255. doi: 10.1073/pnas.1105469108
Jha, A. R., Zhou, D., Brown, C. D., Kreitman, M., Haddad, G. G., and White, K. P. (2016). Shared genetic signals of hypoxia adaptation in Drosophila and in high-altitude human populations. Mol. Biol. Evol. 33, 501–517. doi: 10.1093/molbev/msv248
Jiggins, F. M. (2017). The spread of Wolbachia through mosquito populations. PLoS Biol. 15:e2002780. doi: 10.1371/journal.pbio.2002780
Joshi, V., Mourya, D. T., and Sharma, R. C. (2002). Persistence of dengue-3 virus through transovarial transmission passage in successive generations of Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 67, 158–161. doi: 10.4269/ajtmh.2002.67.158
Kawecki, T. J., Lenski, R. E., Ebert, D., Hollis, B., Olivieri, I., and Whitlock, M. C. (2012). Experimental evolution. Trends Ecol. Evol. 27, 547–560. doi: 10.1016/j.tree.2012.06.001
Kraemer, M. U. G., Reiner, R. C., Brady, O. J., Messina, J. P., Gilbert, M., Pigott, D. M., et al. (2019). Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 4, 854–863. doi: 10.1038/s41564-019-0376-y
Kriventseva, E. V., Kuznetsov, D., Tegenfeldt, F., Manni, M., Dias, R., Simão, F. A., et al. (2018). OrthoDB v10: sampling the diversity of animal, plant, fungal, protist, bacterial and viral genomes for evolutionary and functional annotations of orthologs. Nucleic Acids Res. 47, D807–D811. doi: 10.1093/nar/gky1053
Lindsey, A. R. I., Bhattacharya, T., Newton, I. L. G., and Hardy, R. W. (2018). Conflict in the intracellular lives of endosymbionts and viruses: a mechanistic look at Wolbachia-mediated pathogen-blocking. Viruses 10:141. doi: 10.3390/v10040141
Martins-da-Silva, A., Telleria, E. L., Batista, M., Marchini, F. K., Traub-Csekö, Y. M., and Tempone, A. J. (2018). Identification of secreted proteins involved in nonspecific dsRNA-mediated lutzomyia longipalpis LL5 cell antiviral response. Viruses 10:43. doi: 10.3390/v10010043
Min, K. T., and Benzer, S. (1997). Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. U.S.A. 94, 10792–10796. doi: 10.1073/pnas.94.20.10792
Molloy, J. C., Sommer, U., Viant, M. R., and Sinkins, S. P. (2016). Wolbachia modulates lipid metabolism in Aedes albopictus mosquito cells. Appl. Environ. Microbiol. 82, 3109–3120. doi: 10.1128/AEM.00275-16
Moreira, L. A., Iturbe-Ormaetxe, I., Jeffery, J. A., Lu, G., Pyke, A. T., Hedges, L. M., et al. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278. doi: 10.1016/j.cell.2009.11.042
Moreira, L. A., Ye, Y. H., Turner, K., Eyles, D. W., McGraw, E. A., and O’Neill, S. L. (2011). The wMelPop strain of Wolbachia interferes with dopamine levels in Aedes aegypti. Parasit. Vectors 4:28. doi: 10.1186/1756-3305-4-28
O’Neill, S. L. (2018). The use of Wolbachia by the World Mosquito Program to interrupt transmission of Aedes aegypti transmitted viruses. Adv. Exp. Med. Biol. 1062, 355–360. doi: 10.1007/978-981-10-8727-1_24
O’Neill, S. L., Ryan, P. A., Turley, A. P., Wilson, G., Retzki, K., Iturbe-Ormaetxe, I., et al. (2018). Scaled deployment of Wolbachia to protect the community from Aedes transmitted arboviruses [version 1; referees: 1 approved]. Gates Open Res. 2:36. doi: 10.12688/gatesopenres.12844.1
Pan, X., Pike, A., Joshi, D., Bian, G., McFadden, M. J., Lu, P., et al. (2018). The bacterium Wolbachia exploits host innate immunity to establish a symbiotic relationship with the dengue vector mosquito Aedes aegypti. ISME J. 12, 277–288. doi: 10.1038/ismej.2017.174
Pan, X., Zhou, G., Wu, J., Bian, G., Lu, P., Raikhel, A. S., et al. (2012). Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 109, E23–E31. doi: 10.1073/pnas.1116932108
Rainey, S. M., Martinez, J., McFarlane, M., Juneja, P., Sarkies, P., Lulla, A., et al. (2016). Wolbachia blocks viral genome replication early in infection without a transcriptional response by the endosymbiont or host small RNA pathways. PLoS Pathog. 12:e1005536. doi: 10.1371/journal.ppat.1005536
Rancès, E., Johnson, T. K., Popovici, J., Iturbe-Ormaetxe, I., Zakir, T., Warr, C. G., et al. (2013). The toll and Imd pathways are not required for Wolbachia-mediated dengue virus interference. J. Virol. 87, 11945–11949. doi: 10.1128/JVI.01522-13
Rancès, E., Ye, Y. H., Woolfit, M., McGraw, E. A., and O’Neill, S. L. (2012). The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 8:e1002548. doi: 10.1371/journal.ppat.1002548
Rao, J. N., Neumann, L., Wenzel, S., Schweimer, K., Rösch, P., and Wöhrl, B. M. (2006). Structural studies on the RNA-recognition motif of NELF E, a cellular negative transcription elongation factor involved in the regulation of HIV transcription. Biochem. J. 400, 449–456. doi: 10.1042/BJ20060421
Ritchie, S. A., Pyke, A. T., Hall-Mendelin, S., Day, A., Mores, C. N., Christofferson, R. C., et al. (2013). An explosive epidemic of DENV-3 in Cairns, Australia. PLoS One 8:e68137. doi: 10.1371/journal.pone.0068137
Sasaki, N., Sasamura, T., Ishikawa, H. O., Kanai, M., Ueda, R., Saigo, K., et al. (2007). Polarized exocytosis and transcytosis of Notch during its apical localization in Drosophila epithelial cells. Genes Cells 12, 89–103. doi: 10.1111/j.1365-2443.2007.01037.x
Schmidt, T. L., Barton, N. H., Rašič, G., Turley, A. P., Montgomery, B. L., Iturbe-Ormaetxe, I., et al. (2017). Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 15:e2001894. doi: 10.1371/journal.pbio.2001894
Tan, C. H., Wong, P. J., Li, M. I., Yang, H., Ng, L. C., and O’Neill, S. L. (2017). wMel limits zika and chikungunya virus infection in a Singapore Wolbachia-introgressed Ae. aegypti strain, wMel-Sg. PLoS Negl. Trop. Dis. 11:e0005496. doi: 10.1371/journal.pntd.0005496
Taracena, M. L., Bottino-Rojas, V., Talyuli, O. A. C., Walter-Nuno, A. B., Oliveira, J. H. M., Angleró-Rodriguez, Y. I., et al. (2018). Regulation of midgut cell proliferation impacts Aedes aegypti susceptibility to dengue virus. PLoS Negl. Trop. Dis. 12:e0006498. doi: 10.1371/journal.pntd.0006498
Teixeira, L., Ferreira, A., and Ashburner, M. (2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6:e2. doi: 10.1371/journal.pbio.1000002
Terradas, G., Allen, S. L., Chenoweth, S. F., and McGraw, E. A. (2017a). Family level variation in Wolbachia-mediated dengue virus blocking in Aedes aegypti. Parasit Vectors 10:622. doi: 10.1186/s13071-017-2589-3
Terradas, G., Joubert, D. A., and McGraw, E. A. (2017b). The RNAi pathway plays a small part in Wolbachia-mediated blocking of dengue virus in mosquito cells. Sci. Rep. 7:43847. doi: 10.1038/srep43847
Thomas, S., Verma, J., Woolfit, M., and O’Neill, S. L. (2018). Wolbachia-mediated virus blocking in mosquito cells is dependent on XRN1-mediated viral RNA degradation and influenced by viral replication rate. PLoS Pathog. 14:e1006879. doi: 10.1371/journal.ppat.1006879
Walker, T., Johnson, P. H., Moreira, L. A., Iturbe-Ormaetxe, I., Frentiu, F. D., McMeniman, C. J., et al. (2011). The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476, 450–453. doi: 10.1038/nature10355
White, P. M., Serbus, L. R., Debec, A., Codina, A., Bray, W., Guichet, A., et al. (2017). Reliance of Wolbachia on high rates of host proteolysis revealed by a genome-wide RNAi Screen of Drosophila cells. Genetics 205, 1473–1488. doi: 10.1534/genetics.116.198903
Wong, Z. S., Brownlie, J. C., and Johnson, K. N. (2016). Impact of ERK activation on fly survival and Wolbachia-mediated protection during virus infection. J. Gen. Virol. 97, 1446–1452. doi: 10.1099/jgv.0.000456
Xu, J., Grant, G., Sabin, L. R., Gordesky-Gold, B., Yasunaga, A., Tudor, M., et al. (2012). Transcriptional pausing controls a rapid antiviral innate immune response in Drosophila. Cell Host Microbe 12, 531–543. doi: 10.1016/j.chom.2012.08.011
Yamagata, M., Duan, X., and Sanes, J. R. (2018). Cadherins interact with synaptic organizers to promote synaptic differentiation. Front. Mol. Neurosci. 11:142. doi: 10.3389/fnmol.2018.00142
Ye, Y. H., Carrasco, A. M., Frentiu, F. D., Chenoweth, S. F., Beebe, N. W., van den Hurk, A. F., et al. (2015). Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLoS Negl. Trop. Dis. 9:e0003894. doi: 10.1371/journal.pntd.0003894
Ye, Y. H., Chenoweth, S. F., Carrasco, A. M., Allen, S. L., Frentiu, F. D., van den Hurk, A. F., et al. (2016). Evolutionary potential of the extrinsic incubation period of dengue virus in Aedes aegypti. Evolution 70, 2459–2469. doi: 10.1111/evo.13039
Ye, Y. H., Ng, T. S., Frentiu, F. D., Walker, T., van den Hurk, A. F., O’Neill, S. L., et al. (2014). Comparative susceptibility of mosquito populations in North Queensland, Australia to oral infection with dengue virus. Am. J. Trop. Med. Hyg. 90, 422–430. doi: 10.4269/ajtmh.13-0186
Yeap, H. L., Axford, J. K., Popovici, J., Endersby, N. M., Iturbe-Ormaetxe, I., Ritchie, S. A., et al. (2014). Assessing quality of life-shortening Wolbachia-infected Aedes aegypti mosquitoes in the field based on capture rates and morphometric assessments. Parasit Vectors 7:58. doi: 10.1186/1756-3305-7-58
Yoon, K. J., Song, G., Qian, X., Pan, J., Xu, D., Rho, H. S., et al. (2017). Zika-virus-encoded NS2A disrupts mammalian cortical neurogenesis by degrading adherens junction proteins. Cell Stem Cell 21, 349–358.e6. doi: 10.1016/j.stem.2017.07.014
Zhang, G., Hussain, M., O’Neill, S. L., and Asgari, S. (2013). Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 110, 10276–10281. doi: 10.1073/pnas.1303603110
Zheng, M. L., Zhang, D. J., Damiens, D. D., Lees, R. S., and Gilles, J. R. (2015). Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes aegypti and Aedes albopictus (Diptera: Culicidae) - II - Egg storage and hatching. Parasit Vectors 8:348. doi: 10.1186/s13071-015-0951-x
Keywords: disease control, Aedes aegypti, Wolbachia pipientis, genetic variation, evolution
Citation: Ford SA, Albert I, Allen SL, Chenoweth SF, Jones M, Koh C, Sebastian A, Sigle LT and McGraw EA (2020) Artificial Selection Finds New Hypotheses for the Mechanism of Wolbachia-Mediated Dengue Blocking in Mosquitoes. Front. Microbiol. 11:1456. doi: 10.3389/fmicb.2020.01456
Received: 24 December 2019; Accepted: 04 June 2020;
Published: 07 July 2020.
Edited by:
Juan-Carlos Saiz, Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA), SpainReviewed by:
Rafael Maciel-de-Freitas, Oswaldo Cruz Foundation (Fiocruz), BrazilMatthew Aliota, University of Minnesota, Twin Cities, United States
Corey Lee Brelsfoard, Texas Tech University, United States
Copyright © 2020 Ford, Albert, Allen, Chenoweth, Jones, Koh, Sebastian, Sigle and McGraw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suzanne A. Ford, c3V6YW5uZS5hYmlnYWlsLmZvcmRAZ21haWwuY29t; Elizabeth A. McGraw, ZWFtN0Bwc3UuZWR1
 Suzanne A. Ford
Suzanne A. Ford Istvan Albert
Istvan Albert Scott L. Allen4,5
Scott L. Allen4,5 Matthew Jones
Matthew Jones Cassandra Koh
Cassandra Koh Aswathy Sebastian
Aswathy Sebastian Leah T. Sigle
Leah T. Sigle