- 1Department of Veterinary Physiology and Biochemistry, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, India
- 2Department of Veterinary Physiology and Biochemistry, RGSC, Banaras Hindu University, Mirzapur, India
- 3Department of Veterinary Surgery and Radiology, College of Veterinary Sciences, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, India
- 4Department of Animal Biotechnology, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, India
Viral emergence is an unpredictable but obvious event, particularly in the era of climate change and globalization. Efficient management of viral outbreaks depends on pre-existing knowledge and alertness. The potential hotspots of viral emergence often remain neglected and the information related to them is insufficient, particularly for emerging viruses. Viral replication and transmission rely upon usurping the host metabolic machineries. So altered host metabolic pathways can be exploited for containment of these viruses. Metabolomics provides the insight for tracing out such checkpoints. Consequently introspection of metabolic alteration at virus-host interface has evolved as prime area in current virology research. Chromatographic separation followed by mass spectrometry has been used as the predominant analytical platform in bulk of the analyses followed by nuclear magnetic resonance (NMR) and fluorescence based techniques. Although valuable information regarding viral replication and modulation of host metabolic pathways have been extracted but ambiguity often superseded the real events due to population effect over the infected cells. Exploration of cellular heterogeneity and differentiation of infected cells from the nearby healthy ones has become essential. Single cell metabolomics (SCM) emerges as necessity to explore such minute details. Mass spectrometry imaging (MSI) coupled with several soft ionization techniques such as electrospray ionization (ESI), laser ablation electrospray ionization (LAESI), matrix assisted laser desorption/ionization (MALDI), matrix-free laser desorption ionization (LDI) have evolved as the best suited platforms for SCM analyses. The potential of SCM has already been exploited to resolve several biological conundrums. Thus SCM is knocking at the door of virus-host interface.
Introduction
“The risk from viruses is an unanswered question.” is such a meaningful quote with multi-directional implications. The enormous genetic diversity of existing viruses and unpredictability of viral behavior complemented by inadequacy of knowledge about global virome database always poses potential threats over the entire living world to acquire infection from any emerging or re-emerging viruses. Rapid climate change, increased traveling in the era of globalization and frequent mingling of sylvatic and urban lifecycle have also potentiated the viruses for genetic undulation, emergence and re-emergence to become pandemic at occasions (Devaux, 2012). Keen introspection regarding the virus-host interaction is a primordial aspect to reduce the knowledge gap and prepare the answer for the upcoming viral outbreaks. Exploitation of the highly specific nature of the virus-host interaction as well as the virus-cell interaction which are key for completion of viral life cycle and their transmission can offer crucial information to combat emerging viral infections. The fundamental cellular components required for viral replication and the host restriction factors to counter them can be of immense value to decide the checkpoints and therapeutic targets for customizing effective anti-viral mechanisms (Lassen, 2011). Single cell omics tools although in its budding age, still have enough penetration and resolution to introspect at the cellular and sub-cellular level for elucidation of such useful information. Progress in this arena is going on apace which will enable to explore the changing pattern of the viral interactions in cellular heterogeneity under variable ecosystem and host subjects. Suitable theranostics and effective vaccine of broad host specificity by unveiling the common pathways of viral interaction are the need of the hour to counter the ever-ending viral heterogeneity and future pandemics. The security of “One Health” concept in current scenario depends immensely on symbiotic development and convergence of several high throughput techniques such as but not limited to single cell omics, nanotechnology, artificial intelligence and various computational platforms (Minakshi et al., 2019a). The present review will focus toward various developments in single cell metabolomics tools and their few existing and mostly the probable future applications in unveiling the host virus interactions to acquire new armory in the arsenal for combating the emerging viral diseases.
Single Cell Metabolomics (SCM)
“Omics” technologies pertain to the entire sets of molecules expressed at cellular or tissue level, or within an organism under specific set of condition. However cellular heterogeneity in multicellular organisms is often masked in pooled analyses. There the importance of single cell omics can be apprehended because it holds the potential to identify several targets expressed within a single individual cell or a cell population of interest individually at a specified time, or analyze handful of parameters in the same cells over time (Minakshi et al., 2019a). The enormous utility and popularity of omics tools have left almost no stone untouched in any of the biological research arena; the case is also not unlike in unveiling virus host interaction. As single cell omics tools are mostly in its naïve stage, thus ample evidences of their application at virus host interface is currently lacking but surely not too far in future which can be easily predicted from the bulk of omics interventions in terms of genomics, transcriptomics, proteomics, metabolomics and interactomics in the current perspective. Other single cell omics techniques have been employed to explore virus-host interaction in recent years (Dhillon and Li, 2015; Labonté et al., 2015; Drayman et al., 2017, 2019; Zanini et al., 2018; Rosenwasser et al., 2019; Russell et al., 2019; Wang S. et al., 2019). However SCM techniques are yet to start contributing significantly in understanding virus-host interaction.
Metabolites are smaller molecules, usually of lesser than 1.5 kD in size reflecting various cellular pathways which include sugar, lipids, glycolytic products (pyruvate, lactate, etc.), phosphate compounds (AMP, ADP, and ATP etc.), xenobiotics etc., but generally excludes nucleic acids, minerals, and salts (Minakshi et al., 2019a, b). They are arguably the terminal product of the basic central dogma process and provide the most nascent information about the phenotype. Metabolomics refers to the study of entire set of metabolites at a specific time-point. It is advantageous in understanding host-virus interaction in terms of generating immediate information regarding the exact downstream effects and ultimate fates of the analytes. It can yield a snap shot of dynamic and vulnerable host metabolites in response to its viral counterpart modulating each pathways at a particular infection stage. A recent flush of literature in metabolomics targeting virus-host interaction vividly justifies the utility of such analysis in current context (Table 1). Precise sampling methods accompanied by robust and high throughput analytical platforms such as nuclear magnetic resonance (NMR), receptive separation-based methods such as capillary electrophoresis (CE) or liquid chromatography (LC) and gas chromatography (GC) coupled with mass spectrometry (MS), and fluorescence-based techniques have delivered significant precision to “metabolomics.” Parallel progress in metabolite databases and bioinformatics programs have also assisted to generate an array of valuable information in a single go to match the expectations (Minakshi et al., 2019a). However single cell metabolomics (SCM) is obviously beneficial over population study alike other omics techniques to obtain specific temporal information regarding cell differentiation and division, communication, interaction with surroundings, stress responses including stage-specific metabolomic changes during viral infection (Strzelecka et al., 2018).
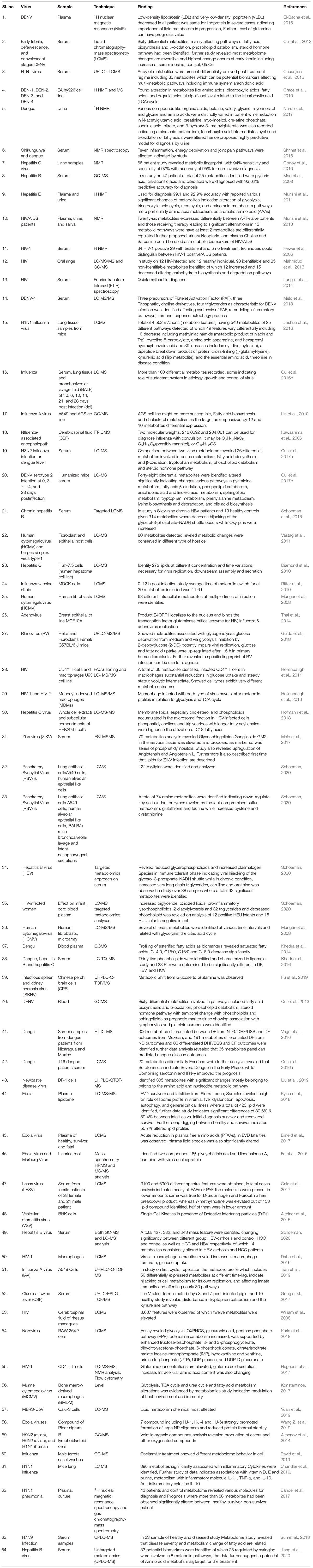
Table 1. List of application and salient findings of metabolomics techniques in emerging viral diseases.
Research related to virus host interaction and information regarding susceptibility, virulence, mechanisms of gene regulation and metabolic pathway modulation under variable environmental factors such as temperature, humidity, light color, intensity and also radiation etc. are mostly lacking that may be useful to combat the incidences of outbreaks from novel viral emergence. Although metabolomics has found its value in virology research but mostly in population study whereas SCM intervention is almost yet to be started to introspect virus-host interaction and is reached upto the level of cell lines only, may be due to the hardship of SCM in terms of single cell isolation and handling, structural diversity and rapid metabolite turnover, lack of amplification opportunity, sensitivity and repeatability of the analytical platforms etc. (Table 1; Minakshi et al., 2019a). However it’s not tough predictions that no longer will SCM be left incumbent considering the worth of information it generates and its ample application in the current context will be reflected in near future.
Sample Preparation Methods for Single Cell Isolation for SCM Analysis
The most common hurdle in SCM analyses is the isolation of single target cell followed by its careful handling and delivery to the downstream analytical platforms because it is directly reflected over the precision of SCM data. Limiting dilutions, manual cell-picking by micromanipulator and laser micro dissection are the common manual methods having relatively low throughput yet applied for single cell analyses where as fluorescence-based cell sorting, high-density microarrays and microfluidics are the most popular automatic high throughput single-cell isolation techniques employed for SCM (Gross et al., 2015; Minakshi et al., 2019b; Figure 1 and Table 2). High cost involvement is a major limitation associated with all of these high end techniques.
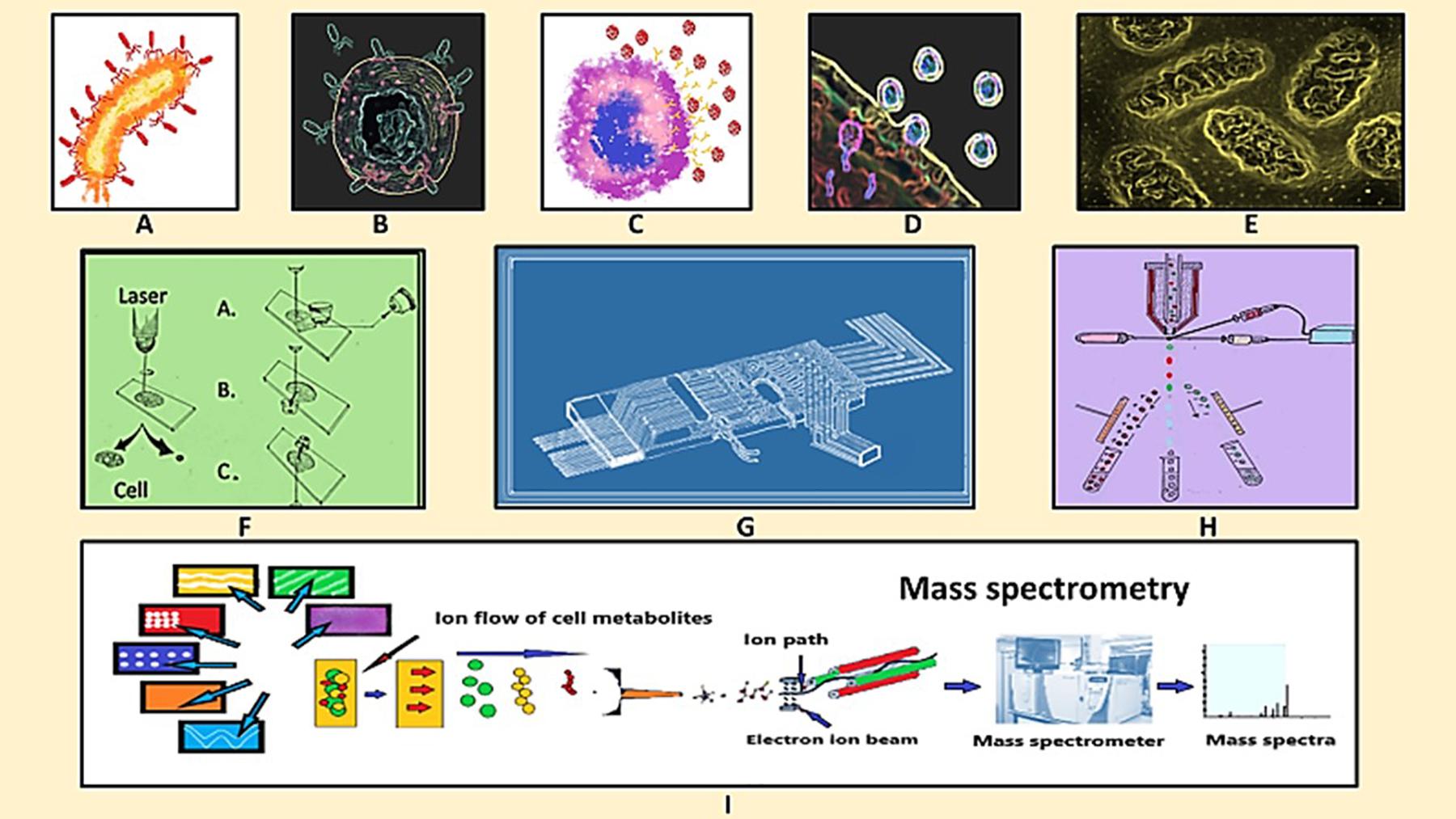
Figure 1. Schematic diagram of virus-host interaction, single cell isolation and single cell metabolomics analysis. (A) Virus infecting the host cell, (B) virus-macrophage interaction, (C) virus encounters immune cell, (D) virus-host cell interaction, (E) metabolic changes at sub-cellular level, (F) Single cell isolation by laser microdisection, (G) microfluidic chip for single cell isolation, (H) fluorescence based cell sorting, and (I) mass spectrometry for single cell metabolomics analysis.
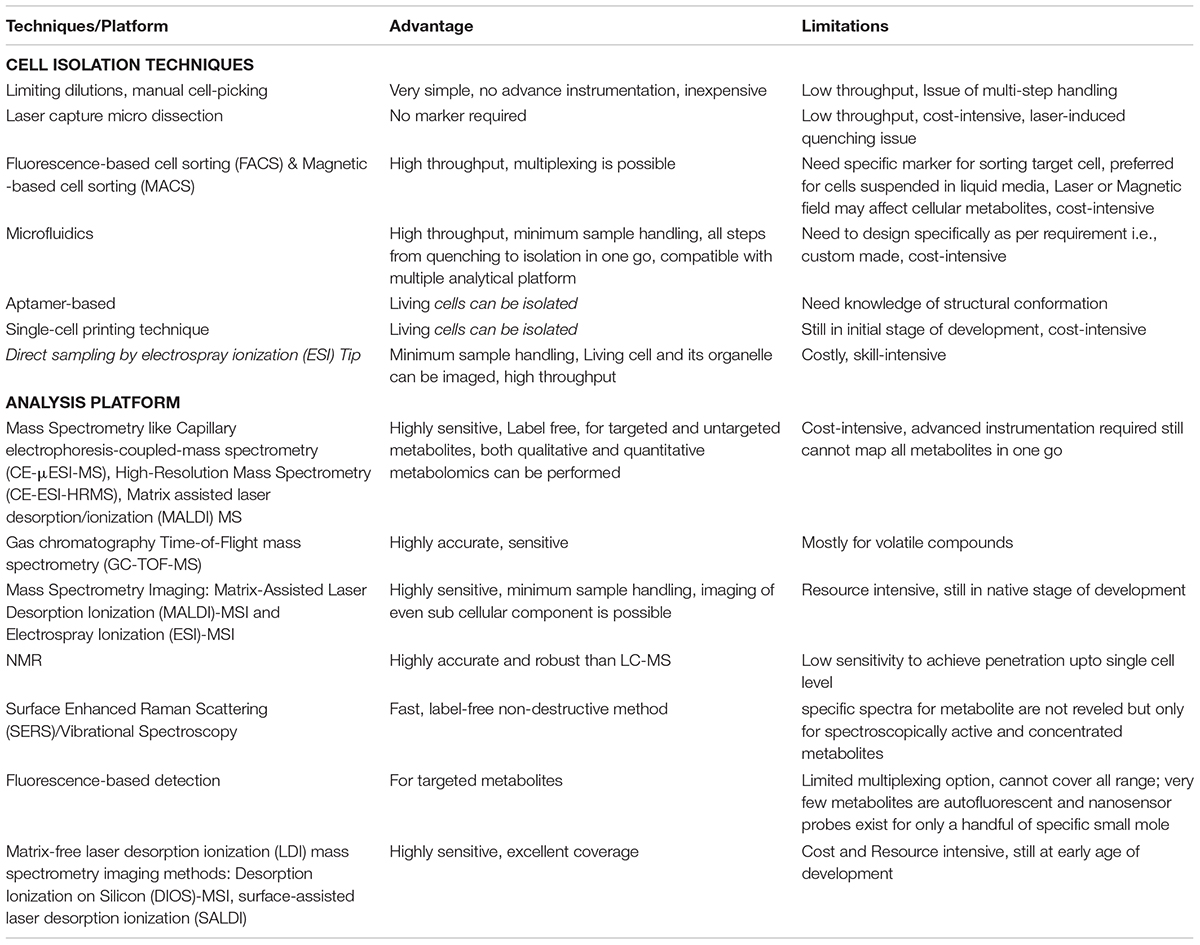
Table 2. Single cell isolation Techniques and platforms for Single cell metabolomics analysis to elucidate Virus-host interaction.
Fluorescence-activated cell sorting isolates the target cells specifically labeled with antibody coupled with a fluorescent tags. This technique prefers the samples that are naturally suspended in liquid media, such as blood, bone marrow cells, semen, ovum, secretions, yeast, protoplast, bacteria, and viruses (Ciuffi et al., 2016; Khalil et al., 2017; Janssens et al., 2018; Singh, 2019). Time-lapse fluorescence imaging is a similar type of fluorescence based single cell analysis technique used for monitoring of cell virus interaction (Drayman et al., 2017).
Immune-magnetic separation using antibody-coated magnetic beads or attached with other ligands for capturing the target cells is also an important single cell isolation method. Dynabeads or MACS (Dynabeads Magnetic Separation Technology) employs such isolation principle (Miltenyi et al., 1990).
Aptamer-based cell isolation relies upon the precise binding of aptamers (single-stranded oligonucleotides, DNA or RNA) to its target using structural conformation and ease the isolation of single cells (Guo et al., 2006; Hu et al., 2016; Delley et al., 2018; Chen, 2019; Kacherovsky et al., 2019). Fluorescent nanodiamonds (FNDs) are diamond nano-crystals which have also been used for single cell labeling for cell tracking and illuminating the cellular processes under different conditions (Prabhakar et al., 2017; Claveau et al., 2018).
Single-cell printing technique like “Block-Cell-Printing” is another example of high throughput single cell isolation technique yet to find ample application (Gross et al., 2013; Zhang et al., 2014; Schoendube et al., 2015).
Microfluidics or Lab-on-a-Chip devices provide excellent opportunity for simultaneous isolation of target single cells along with its handling and manipulation followed by SCM suitable platforms with high end automation (Warrick et al., 2016). Several analytical platforms such as electrophoresis, optical spectroscopy, fluorescence based imaging, or mass spectrometry are compatible with microfluidics based cell isolation. Soft lithographic fabrication onto polydimethylsiloxane (PDMS), glass or silicon chips provides the base of microfluidic devices and hydrodynamic or gravity-driven flow of cells are used to capture the target cells. Microfluidics device installed on a fluorescence microscope has yielded valuable information regarding the kinetics of viral infection in single cells and variability of cellular factors regulating the viral replication (Guo et al., 2017). Hybrid microfluidics using either active or passive approaches or their combination, integration of nano- and microfluidics, digital or virtual microfluidics are the emerging microfluidic platforms which are being recently introduced for high throughput and continuous single cell isolation avoiding the general limitations of the existing technique (Xu et al., 2016; Yan et al., 2016; Wang et al., 2017; Vanderpoorten et al., 2019).
Direct sampling through nano-electrospray ionization tips followed by mass spectrometric analyses method is gaining popularity in recent times due to its minimum sample requirement and processing along with high throughput data generation. Pipette tip column electrospray ionization (PTC-ESI) or ballpoint electrospray ionization (BP-ESI) followed by simultaneous mass spectrometry or live single-cell video-mass spectrometry are the SCM platforms where the technique has been used (Tsuyama et al., 2008; Huang et al., 2012; Ji et al., 2016; Pu et al., 2018).
Analytical Platforms for Single Cell Metabolomics
The precision and coverage of SCM analysis depends upon the “resolution” and “sensitivity” of the analytical platform. Although theoretically SCM analyze the entire set of cellular metabolites but more often it is restricted to qualitative or quantitative analysis of a cluster of metabolites under certain condition. “Targeted” for acquiring information regarding specific metabolite(s) or “untargeted” SCM approach for obtaining the maximum coverage can be chosen according to the requirement and selection of the analytical platform should be done accordingly. Mass Spectrometry Imaging (MSI)-based methods are arguably the most sensitive and thus preferred SCM platform. However, the compromised reliability, restricted quantitation option, and poor resolution for structural characterization are the certain limitations need to be taken care of. Other SCM platforms include but not limited to separation-based methods like capillary electrophoresis (CE), liquid chromatography (LC), or gas chromatography (GC) combined with MS or fluorescent tagging approach which are mostly used for “targeted SCM” due to the sensitivity issue (Minakshi et al., 2019a; Table 2).
Separation-Based Methods Coupled With Mass Spectrometry
Capillary electrophoresis coupled with electrospray ionization and mass spectrometry (CE-μESI-MS) has been employed to analyze single 8-cell embryo of Xenopus laevis to depict more than 80 metabolites (Onjiko et al., 2015). Similarly CE-ESI-TOF-MS has explored over 300 metabolites from the neuronal cell of sea slug Aplysia californica (Nemes et al., 2013). CE-ESI-MS has quantitatively characterized over 15 anionic metabolites (nucleotides and derivatives) from Aplysia sensory neuron with the detection limits in <22 nM range (Liu et al., 2014). High-Resolution Mass Spectrometry (CE-ESI-HRMS) analyses of a single 16-cell stage embryo of Xenopuslaevishasrevealed438 non-redundant protein groups by label-free quantification with a detection limit of ∼75 attomol (∼11 nm) (Lombard-Banek et al., 2016). Targeted automated SCM analysis of neuronal cell by a microchip electrophoresis-mass spectrometric method (MCE-MS) has quantitatively measured dopamine and glutamic acid content within individual cell with detection limits 8.3 and 15.6 nM for the metabolites, respectively. Although the introduction of this platform in virus-host interaction arena is pending but the mentioned evidences has clearly suggested about its potential in SCM analysis.
Nano-flow liquid chromatography-electro spray ionization mass spectrometry (LC-ESI-MS) based analysis has yielded 5 anthocyanins from 2-4picolitervolume of individual Torenia hybrida petal cell (Kajiyama et al., 2006). Gas chromatography Time-of-Flight mass spectrometry (GC-TOF-MS) analyses of Mesembryanthemum crystallinum epidermal bladder cells has identified 194 known and 722 overall molecular features which generate significant pathway information regarding metabolic alterations in salty environment (Barkla and Vera-Estrella, 2015). Thus chromatographic methods have great potential for SCM analyses in combination with MS platforms. Although not in single cell level but GC/MS based metabolomics technique has already been applied for introspection of fatty acid biosynthesis and cholesterol metabolism in A549 and AGS cell lines infected with influenza A virus yielding valuable information regarding susceptibility and cell differentiation in association with viral replication (Lin et al., 2010). Ultra-high-performance liquid chromatography/quadrupole time-of-flight tandem mass spectrometry (UHPLC-QTOF-MS) analyses has depicted significant changes in 305 metabolites during infection of DF-1 cells with the Herts/33 strain of New Castle Disease Virus (Liu et al., 2019). Gut metabolomic analyses of rhesus monkeys by GC-MS and LC-MS during gut virome alteration has been correlated with gut microbiome and the underlying role of metabolites like tryptophan, arginine, and quinine (Li et al., 2019). Although the penetration upto the single cell level is yet to be reached but the evidences are enough to ensure the utility and future of this platform for metabolomic analyses in virology research.
Mass Spectrometry Imaging
Extreme sensitivity, vast coverage and the opportunity of label-free analysis has rendered MSI as one of the most preferred method of SCM analysis, particularly for untargeted approach. Improvement in resolution is going on in terms of reducing the laser spot area by lowering the diameter of optical fiber along with providing multiplexing option by integration of multiple mass analyzers. Matrix-Assisted Laser Desorption Ionization (MALDI)-MSI and Electrospray Ionization (ESI)-MSI are the two conventional platforms applied for SCM analyses (Figure 1).
Polarity-switching option is lacking in MALDI-MSI, it can be run in either ion mode at a time, multiplexing can be added subsequently for increasing the coverage. However, introduction of advanced matrices, such as 9-AA or silver, gold, or titanium and grapheme oxide nanoparticles, have increased the sensitivity by reducing background noise. Multiplex MSI platforms are equipped to directly explore individual intracellular metabolites, even their sub-cellular location irrespective of chromatographic separation. SCM analysis of by MALDI-MS operated in negative ion mode has revealed several metabolites such as ADP, ATP, GTP, and UDP-Glucose etc. from Closterium acerosum (Amantonico et al., 2010). Metabolic analysis of single Yeast cell by coupling microscale sampling and MALDI-MS at negative ion mode detection has elucidated information regarding some valuable metabolites such as ADP, GDP, ATP, GTP, and acetyl-CoA. The detection limit ranged from 5 to 12 attomoles (Amantonico et al., 2008). Pressure probe sampling (in picoliters) coupled with UV-MALDI MS has been applied for shotgun metabolite profiling of organic compounds live single plant cell (Gholipour et al., 2012). High spatial resolution MSI analysis of Maize root epidermal and cortex cell has elucidated several TCA cycle metabolites including their localization with spatial resolution improved to ∼5 μm (Hansen and Lee, 2018). Turkey gut microbiome has been introspected using MALDI-MS platform operated in positive ion mode with nanoparticle microarray and organic matrices (Hansen et al., 2018). Metabolomics variability during virus host interaction in bloom-forming alga Emiliania huxleyi has been evaluated by Plaque assay combined with several MSI techniques (Flow-probe-MSI, MALDI-MSI, UPLC-q-TOF MS, and LC-MS/MC for lipidomics, GC-MS for fatty acid methyl esters) (Schleyer et al., 2019). Fluorescence in situ hybridization (FISH) microscopy combined with high-resolution atmospheric pressure-MALDI-MSI of Bathymodiolus puteoserpensis epithelial cells has also been used to understand host-microbe symbiosis (Geier et al., 2019). Lipidomics profile of infected Aedes albopictus cells during Zika virus infection has been investigated using MALDI-MS platform to identify thirteen infection-specific lipid markers in the mosquitoes (Melo et al., 2016). Thus the mentioned evidences easily suggest about the utility and potential of MALDI-MSI based platform for SCM analyses and can be predicted more often application to evaluate host-virus interaction at single cell level.
Alike MALDI, Electrospray Ionization (ESI) is another important soft ionization technique where the analytes after ionization, evaluated by MS to facilitate metabolite characterization. ESI-MS is equipped with simultaneous detection at both positive and negative ion mode yielding enormous sensitivity. Live nano-ESI-LTQ-MS analyses of Pelargonium zonale leaf cell has depicted over16 metabolites from 1to 5pL of sample (Tejedor et al., 2009). Probe ESI mass spectrometry (PESI-MS) with tungsten probe of 1 μM diameter has detected several metabolites including 6 fructans, 4 lipids and 8 flavone derivatives in single Allium cepa cell (Gong et al., 2014). ESI-Q-TOF-MS operated in either positive or negative-ion mode has yielded status regarding several metabolites such as tryptophan, creatinine, dopamine, and glycine, and cofactors such as lactate and pyruvate to link energy metabolism and immunodeficiency in microgravity (Chakraborty et al., 2018).
Laser ablation electrospray ionization (LAESI) employs mid-infrared (mid-IR) laser to generate gas phase metabolites. Large plant cells or cluster of animal cells are preferred subjects for this platform but high sensitivity, little chemical background and a direct sample delivery to MS platform has made it a desired choice for SCM analysis. Metabolites from single cells or cluster of Allium cepa and Narcissus pseudonarcissus bulb epidermis and single eggs from Lytechinus pictus has been evaluated through LAESI to detect 35 prominent metabolites including anthocyanidins, flavonoids, and their glucosides depicting its potential in SCM analysis (Shrestha and Vertes, 2009). LAESI-MS coupled with ion mobility separation (IMS) has been described as capable of direct profiling and imaging of several biomolecules including metabolites from biological tissues and single cells (Etalo et al., 2018; Paglia and Astarita, 2020). LAESI coupled with a 21 tesla Fourier transform ion cyclotron resonance (21T-FTICR) has capable of elucidating isotopic fine structure of the biomolecules by direct MS analysis and imaging (Stopka et al., 2019).
Live Single-Cell Video-Mass Spectrometry using micro sampling method by video microscopy and micromanipulator guided gold-coated glass capillary nano-ESI tip along with Nano-ESI-Q-TOF platform has demonstrated good potential for SCM analysis (Lapainis et al., 2009; Hiyama et al., 2015).
Non-Mass Spectrometric Methods
Next to mass spectrometry, NMR is a major tool for metabolite analyses. It is even robust and requires less sample processing than the other metabolomics tools. However requirement of large sample volume and the issue of sensitivity render it imperfect for SCM analyses rather it is preferred for tissue sample and biofluid introspection. For example continuous in vivo metabolism has been followed by using NMR (CIVM-NMR) coupled with high-resolution-magic angle spinning to elucidate branched-chain amino acid metabolism in human chronic lymphoid leukemia cells (Judge et al., 2019).
Fluorescence-based sensors are also being applied in targeted SCM analyses. CE with laser-induced fluorescence (LIF) detection has been employed for detection of chiral amino acids and neurotransmitters from mouse brain and neuron cells (Jakó et al., 2014; Qi et al., 2017). Capillary micro sampling MS with fluorescence microscopy has successfully identified 29 metabolites and 54 lipids from Human HepG2/C3Acancer cells (Zhang et al., 2018). Microchip electrophoresis coupled with LIF detection have been employed for identifying ethanol induced metabolite change in mice liver cells to depict elevated hydrogen peroxide along with depleted glutathione and cysteine level in the target cells (Li et al., 2016). However limited multiplexing option has restricted its application mostly within targeted approach.
Surface Enhanced Raman Scattering (SERS) employs laser in the visible, near-infrared, or near-ultraviolet range for label free detection of the metabolites within the cells (Shalabaeva et al., 2017). SERS has been applied for fast, label-free detection of Chlamydia trahomatis and Neisseria gonorrheoae along with associated extra-cellular metabolite changes (Chen et al., 2018). Raman spectroscopy is a non-destructive method for metabolite analysis but only few functional groups emit detectable Raman signals reducing the applicability of the method for SCM analyses (Smith et al., 2016). Recently, Raman micro-spectroscopy using graded X-ray doses has successfully uncovered several nucleus and cytoplasmic specific metabolic features from single SH-SY5Y human cancer cells (Delfino et al., 2019).
Future SCM Techniques
Secondary Ion Mass Spectrometry Imaging with spatial resolution capacity at the range of 50–100 nm is a potential future tool for SCM as well as sub-cellular metabolite analyses (Svatos, 2011; Kollmer et al., 2012; Gilmore et al., 2019). The matrix-free laser desorption ionization (LDI) methods include Desorption Ionization on Silicon (DIOS)-MSI which is a variant of surface-assisted laser desorption ionization (SALDI), both use soft ionization of analytes followed by MS analyses. LDI-MS platform employing HR-MS along with Orbitrap FT-MS has been used for SCM analysis of microalgae, marine diatoms and freshwater chlorophytes (Baumeister et al., 2019). Matrix-free nanophotonic ionization of analytes on Silicon Nanopost Array chip followed by MSI offers an effective platform for detection of small metabolites at single cell level (Walker et al., 2012; Korte et al., 2016). Desorption Electrospray Ionization is another soft ionization technique having capability to ionize wide variable molecules however poor spatial resolution is a limitation of this technique which can be improved further for SCM analyses. MS coupled with Microfluidic chips or microarray systems and 3D MALDI Mass Spectrometry Imaging are the other potential SCM techniques which can be used for analyzing virus-host interaction in near future. Single-Molecule Array which resembles the digital ELISA format may also be employed for targeted SCM by using specific tagged antibody against the target metabolite(s) (Minakshi et al., 2019a).
Metabolomic Insight at Virus-Host Interface to Combat Emerging Viruses
Zika virus, Dengu virus, Chikungunya virus, HIV, avian influenza, Nipah (NiV) viruses, Ebola and Marburg filoviruses, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome coronavirus (MERS CoV) are the emerging viruses as evidenced in recent times (Afrough et al., 2019). Several of them are having zoonotic importance and emerge from viral spill over from the sylvatic cycle infecting multiple hosts. Metabolomic intervention can trace out some common metabolic pathway(s) in different host range which are also essential for viral replication (Passalacqua et al., 2018; Mayer et al., 2019). For Example, Progression of human cytomegalovirus (HCMV) infection has increased the metabolite flux through glycolysis, citric acid cycle, and pyrimidine nucleotide biosynthesis in human fibroblasts, probably to meet the energy demand for viral replication and supply of macromolecular precursors (Munger et al., 2008). HCMV mediated metabolic reprogramming also includes enhanced lipogenesis through mTOR-sterol regulatory element-binding protein (SREBP)-regulated and other pathways inducing several enzymes such as acetyl-CoA carboxylase, fatty acid synthase, fatty acid elongases etc. The synthesized very long chain and long chain fatty acids are essential for viral envelope production, because inhibition of such pathways result into impaired viral replication. Similarly other DNA viruses such as herpes simplex virus-1 (HSV-1), Kaposi’s sarcoma-associated herpes virus (KSHV), vaccinia virus (VACV) are also found to modulate glycolysis, glutamine metabolism and fatty acid synthesis as common pathways. Thus any substrate analog (e.g., glucose analog 2-deoxyglucose) or enzyme inhibitor which prevents such virus-induced host metabolic reprogramming can be effective against multiple viruses. Although RNA viruses like rhinovirus, hepatitis C virus and several emerging arboviruses such as Zika virus, Dengu virus, Chikungunya virus etc. modulate host metabolic pathways in a little different manner but glycolysis, glutamine metabolism and fatty acid synthesis remain as the prime targets. Similar host enzyme machineries are being exploited by these RNA viruses too. These common metabolic pathway nodes can be exploited for preventive, diagnostic and therapeutic management of emerging viral infections. Further, such exploration can be of interest for therapeutic repurposing of existing drugs to combat any emerging viral outbreak. For example, Remdesivir (GS-5734) is a novel nucleoside analog antiviral prodrug, actually developed for the treatment of single stranded RNA containing Ebola virus disease and Marburg virus infections, has now been suggested as therapeutic option against recent SARS-CoV-2 pandemic. SARS-CoV-2 is also having positive-sense single-stranded RNA genome and Remdesivir gets incorporated into the nascent viral RNA chains leading to inhibition of viral replication through premature termination of RNA transcription (Centers for Disease Control and Prevention, 2020; Liu et al., 2020). However, the toxic effects of such substrate analogs or pathway inhibitors on host cells should be critically analyzed prior to their extensive application. Recently, traditional anti-parasitic drug ivermectin has also found to exhibit in vitro antiviral activity against SARS-CoV-2 infection and warrants rapid further in vivo introspection to combat current COVID-19 pandemic. This is also an example of drug repurposing relying upon the virus-exploited common pathways. Because in earlier research, ivermectin has depicted efficacy against multiple RNA viruses such as DENV 1-4, West Nile Virus, influenza virus, Venezuelan equine encephalitis virus (VEEV) and human immunodeficiency virus-1 (HIV-1) by destabilizing the importin (IMP) α/β1 heterodimer and blocking IMP α/β1-mediated nuclear import of viral proteins (Caly et al., 2020).
Metabolite profiling can also provide an idea about the disease severity and predictive outcome of the viral infection. Serum metabolite analyses of H7N9 infected subjects have revealed that increase in palmitic acid, erucic acid, and phytal level is related to virus-induced repression of fatty acid metabolism and negatively correlated with the disease outcome (Sun et al., 2018).
In arboviral infections, metabolic reprogramming in arthropod vector and vertebrate host takes place in different manner. Zika virus (ZIKV) induced metabolic reprogramming of human foreskin fibroblast cells (HFF-1) shapes differently from C6/36 cells of Aedes albopictus mosquito which serves as the vector of ZIKV infection. Glucose influx has been increased through glycolysis in both the cases, however, it is mainly diverted toward tricarboxylic acid (TCA) cycle and amino acid synthesis in human cells whereas glucose contribution to TCA cycle gets reduced to augment the intermediates of pentose phosphate pathway in mosquito cells. Further, ZIKV infection depletes ATP level in HFF-1 cells leading to elevated AMPK phosphorylation and increased caspase-mediated cell death whereas neither such increase in AMP/ATP and ADP/ATP ratios as well as AMPK phosphorylation nor decreased cell viability has been evidenced in infected C6/36 mosquito cells. Such difference in cell viability may be contributed by both altered pentose phosphate pathway and AMPK activation (Thaker et al., 2019). So metabolic introspection is not only capable of elucidating virus-host interaction but also provides information regarding maintenance and propagation of virus through their arthropod vectors. Understanding of lifecycle of such viruses exploiting metabolic pathways within arthropod vectors can assist to design strategies for preventing their transmission in vertebrate hosts.
Considerable numbers of researches have followed such path providing metabolomic insight at virus-host interface. Chromatography (LC/GC) coupled with tandem mass spectrometry (MS/MS) is the most common method employed for metabolomics introspection followed by NMR, FTIR and fluorescence based methods (Table 1). Most of these experiments have reached upto the level of cell line but significant penetration at single cell level is yet to be achieved in terms of SCM analysis in the context of virus-host interaction. But single-cell analysis may elucidate far precise information regarding viral replication and infection kinetics which is beyond the capacity of population study (Guo et al., 2017). A high-throughput, microfluidics-based platform has been devised to perform kinetic analysis of green fluorescence protein (GFP) tagged polio virus (PV) infections in individual HeLa S3 cells. Monitoring of fluorescence intensity has revealed marked variation in viral replication and infection cycle in each of the GFP-PV infected cells which are uniquely and independently controlled by viral as well as the cellular factors. The time of onset of viral replication varied significantly among the individual infected cells. Similar cellular-factor regulated variable pattern has continued to follow throughout the viral infection cycle upto the cell lysis. The analysis at single cell level has unearthed plethora of valuable information regarding virulence determinants and mechanisms of drug action which have been escaped in population methods (Guo et al., 2017). The mentioned evidence is extremely useful to justify the value of single cell techniques over population measurements and several such SCM introspection at virus-host interface may follow the path in near future. It will not only surmount the heterogeneity issue of samples otherwise succumb to superimposed results but also help to identify the effect of mutation both in virus as well as host, virus homing affinity toward cell types, post-infection survival, progression of disease etc. Although SCM analyses have its own advantage but it must be complemented by the bulk metabolomic analyses of the infected tissue or organ because cell-to-cell communication may have crucial effect in virus infection cycle which may not be reflected in SCM analyses. Thus a combinatorial approach is more beneficial over a standalone SCM introspection so that the real reflection of cellular heterogeneity at virus-host interface as well as the influence of cell communication can be elucidated precisely. The specific information extracted from such analyses like mutation study along with computational modeling may assist in future vaccine formulation, mapping of virus evolution strategy to identify the potential hot-spots of novel viral emergence, their virulence and putative contentment recipes.
However, the inherent challenges of SCM techniques require to be surmounted with diligence and passion for proper utilization of the potential of single-cell analyses. The difficulty in single cell isolation along with rapid turnover, transport and degradation of the cellular metabolites are the key challenges of SCM analyses. Rapid metabolite turnover can produce random noise which may create analytical error and difficulty in differentiating infected cells from the healthy one. Thus normalization of such random error must be performed during the experimental set-up for SCM data acquisition to differentiate the deterministic effects from the stochastic one. Similar type of random fluorescence noise normalization has been performed by Guo et al. (2017) during GFP-PV infected single-cell analyses. Further dealing with minute sample volume, diverse types of metabolites and their femto-molar range concentration demands high sensitivity and throughput of the analytical platform which is essential particularly for SCM analyses. For example, NMR is extremely robust, reliable, requiring minimal sample processing and commonly employed metabolomics analysis platform for population study but low sensitivity has limited the utility of the technique for SCM analyses. Proper analyses of SCM data is another crucial challenge which has been eased out significantly with recent advances in bioinformatics tools and growing metabolite database (Minakshi et al., 2019a). Moreover, integration of SCM with single-cell proteomics, transcriptomics, genomics and interactomics can provide a whole-some image of the life processes which may assist to explore the biological missing links regarding virus infection and transmission cycle.
Conclusion
Rapid development in single cell isolation and handling methods, persistent improvement in sensitivity and resolution of the analytical platforms along with growing database and data interpretation programs are smoothening the hurdles of SCM to introduce it at the virus-host interface as early as possible. The SCM derived precise information regarding the altered metabolism of infected cell over the healthy cells may assist to investigate the virulence, survival, and progression of diseases. The exploration of cellular heterogeneity can illuminate the aspects which remain concealed in population study. Thus SCM holds the promise as a key tool of future to introspect virus-host interaction and assist in efficient management of emerging viral disease.
Author Contributions
MP and MG conceptualized the manuscript. RK and SK wrote the manuscript. MP critically analyzed and improved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Afrough, B., Dowall, S., and Hewson, R. (2019). Emerging viruses and current strategies for vaccine intervention. ClinExpImmunol 196, 157–166. doi: 10.1111/cei.13295
Akpinar, F., Timm, A., and Yin, J. (2015). High-throughput single-cell kinetics of virus infections in the presence of defective interfering particles. J. Virol. 90, 1599–1612. doi: 10.1128/jvi.02190-15
Aksenov, A. A., Sandrock, C. E., Zhao, W., Sankaran, S., Schivo, M., Harper, R., et al. (2014). Cellular scent of influenza virus infection. Chem. Bio. Chem. 15, 1040–1048. doi: 10.1002/cbic.201300695
Amantonico, A., Oh, J. Y., Sobek, J., Heinemann, M., and Zenobi, R. (2008). Mass spectrometric methodfor analyzing metabolites in yeast with single cell sensitivity. Angew. Chem. Int. Ed. Engl. 47, 5382–5385. doi: 10.1002/anie.200705923
Amantonico, A., Urban, P. L., Fagerer, S. R., Balabin, R. M., and Zenobi, R. (2010). Single-cell MALDI-MS as an analytical tool for studying intrapopulation metabolic heterogeneity of unicellular organisms. Anal. Chem. 82, 7394–7400. doi: 10.1021/ac1015326
Banoei, M. M., Vogel, H. J., Weljie, A. M., Kumar, A., Yende, S., and Winston, B. W. (2017). Plasma metabolomics for the diagnosis and prognosis of H1N1 influenza pneumonia. Crit. Care 21:97. doi: 10.1186/s13054-017-1672-7
Barkla, B. J., and Vera-Estrella, R. (2015). Single cell-type comparative metabolomics of epidermal bladder cells from the halophyte Mesembryanthemum crystallinum. Front. Plant Sci. 6:435. doi: 10.3389/fpls.2015.00435
Baumeister, T. U. H., Vallet, M., Kaftan, F., Svatoš, A., and Pohnert, G. (2019). Live single-cell metabolomics with matrix-free laser/desorption ionization mass spectrometry to address microalgal physiology. Front. Plant Sci. 10:172. doi: 10.3389/fpls.2019.00172
Caly, L., Druce, J. D., Catton, M. G., Jans, D. A., and Wagstaff, K. M. (2020). The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 178:104787. doi: 10.1016/j.antiviral.2020.104787
Centers for Disease Control and Prevention (2020). Coronavirus Disease 2019 (COVID-19). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html (accessed April 5, 2020).
Chakraborty, N., Cheema, A., Gautam, A., Donohue, D., Hoke, A., Conley, C., et al. (2018). Gene-metabolite profile integration to understand the cause of spaceflight induced immunodeficiency. NPJ Microgravity 4:4. doi: 10.1038/s41526-017-0038-4
Chandler, J. D., Hu, X., Ko, E. J., Park, S., Lee, Y. T., Orr, M., et al. (2016). Metabolic pathways of lung inflammation revealed by high-resolution metabolomics (HRM) of H1N1 influenza virus infection in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R906–R916. doi: 10.1152/ajpregu.00298.2016
Chen, Y., Premasiri, W. R., and Ziegler, L. D. (2018). Surface enhanced Raman spectroscopy of Chlamydia trachomatis and Neisseria gonorrhoeae for diagnostics, and extra-cellular metabolomics and biochemical monitoring. Sci. Rep. 8:5163.
Chuanjian, L., Zhiting, J., Xuemei, F., Guiya, L., Shasha, L., Chunxia, H., et al. (2012). A metabonomic approach to the effect evaluation of treatment in patients infected with influenza a (H1N1). Talanta 100, 51–56. doi: 10.1016/j.talanta.2012.07.076
Ciuffi, A., Rato, S., and Telenti, A. (2016). Single-cell genomics for virology. Viruses 8:123. doi: 10.3390/v8050123
Claveau, S., Bertrand, J.-R., and Treussart, F. (2018). Fluorescent nanodiamond applications for cellular process sensing and cell tracking. Micromachines 9:247. doi: 10.3390/mi9050247
Cui, L., Fang, J., Ooi, E. E., and Lee, Y. H. (2017a). Serial metabolome changes in a prospective cohort of subjects with influenza viral infection and comparison with dengue fever. J. Proteome Res. 16, 2614–2622. doi: 10.1021/acs.jproteome.7b00173
Cui, L., Hou, J., Fang, J., Lee, Y. H., Costa, V. V., Wong, L. H., et al. (2017b). Serum metabolomics investigation of humanized mouse model of dengue virus infection. J. Virol. 91:e00386-17. doi: 10.1128/JVI.00386-17
Cui, L., Lee, Y. H., Kumar, Y., Xu, F., Lu, K., Ooi, E. E., et al. (2013). Serum metabolome and lipidome changes in adult patients with primary dengue infection. PLoS Negl. Trop. Dis. 7:e2373. doi: 10.1371/journal.pntd.0002373
Cui, L., Lee, Y. H., Thein, T. L., Fang, J., Pang, J., Ooi, E. E., et al. (2016a). Serum metabolomics reveals serotonin as a predictor of severe dengue in the early phase of dengue fever. PLoS Negl. Trop. Dis. 10:e0004607. doi: 10.1371/journal.pntd.0004607
Cui, L., Zheng, D., Lee, Y., Chan, T. K., Kumar, Y., Ho, W. E., et al. (2016b). Metabolomics investigation reveals metabolite mediators associated with acute lung injury and repair in a murine model of influenza pneumonia. Sci. Rep. 6:26076. doi: 10.1038/srep26076
Datta, P. K., Deshmane, S., Khalili, K., Merali, S., Gordon, J. C., Fecchio, C., et al. (2016). Glutamate metabolism in HIV-1 infected macrophages: role of HIV-1 Vpr. Cell Cycle 15, 2288–2298. doi: 10.1080/15384101.2016.1190054
David, J. B., Ding, Y. O., Avinash, V. K., Celeste, T., Michael, S., Dunn, D. T., et al. (2019). Untargeted metabolomics analysis of the upper respiratory tract of ferrets following influenza A virus infection and oseltamivir treatment. Metabolomics 15:33. doi: 10.1007/s11306-019-1499-0
Delfino, I., Ricciardi, V., Manti, L., Lasalvia, M., and Lepore, M. (2019). Multivariate analysis of difference raman spectra of the irradiated nucleus and cytoplasm region of SH-SY5Y human neuroblastoma cells. Sensors 19:3971. doi: 10.3390/s19183971
Delley, C. L., Liu, L., Sarhan, M. F., and Abate, A. R. (2018). Combined aptamer and transcriptome sequencing of single cells. Sci. Rep. 8:2919. doi: 10.1038/s41598-018-21153-y
Devaux, C. A. (2012). Emerging and re-emerging viruses: a global challenge illustrated by Chikungunya virus outbreaks. World J. Virol. 1, 11–22. doi: 10.5501/wjv.v1.i1.11
Dhillon, V., and Li, X. (2015). Single cell genome sequencing for viral-host interactions. J.Comput. Sci. Syst. Biol. 8, 160–165. doi: 10.4172/jcsb.1000183
Diamond, D. L., Syder, A. J., Jacobs, J. M., Sorensen, C. M., Walters, K. A., Sean, C. P., et al. (2010). Temporal proteome and lipidome profiles reveal hepatitis c virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 6:e1000719. doi: 10.1371/journal.ppat.1000719
Drayman, N., Karin, O., Mayo, A., Danon, T., Shapira, L., Rafael, D., et al. (2017). Dynamic proteomics of herpes simplex virus infection. mBio 8:e01612-17. doi: 10.1128/mbio.01612-17
Drayman, N., Patel, P., Vistain, L., and Tay, S. (2019). HSV-1 single-cell analysis reveals the activation of anti-viral and developmental programs in distinct sub-populations. eLife 2019:e46339. doi: 10.7554/eLife.46339
Eisfeld, A. J., Halfmann, P. J., Wendler, J. P., Kyle, J. E., Burnum-Johnson, K. E., Peralta, Z., et al. (2017). Multi-platform ’Omics analysis of human ebola virus disease pathogenesis. Cell Host Microbe 22, 817–829.e8. doi: 10.1016/j.chom.2017.10.011
El-Bacha, T., Struchiner, C. J., Cordeiro, M. T., Almeida, F. C. L. Jr., Marques, E. T., and Da Poian, A. T. (2016). 1H Nuclear magnetic resonance metabolomics of plasma unveils liver dysfunction in dengue patients. J. Virol. 90, 7429–7443. doi: 10.1128/JVI.00187-16
Etalo, D. W., Díez-Simón, C., de Vos, R. C. H., and Hall, R. D. (2018). Laser ablation electrospray ionization-mass spectrometry imaging (LAESI-MS) for spatially resolved plant metabolomics. Methods Mol. Biol. 1778, 253–267. doi: 10.1007/978-1-4939-7819-9_18
Fu, X., Guo, X., Wu, S., Lin, Q., Liu, L., Liang, H., et al. (2019). Non-Targeted UHPLC-Q-TOF/MS-based metabolomics reveals a metabolic shift from glucose to glutamine in cpb cells during ISKNV infection cycle. Metabolitis 9:174. doi: 10.3390/metabo9090174
Fu, X., Wang, Z., Li, L., Dong, S., Li, Z., Jiang, Z., et al. (2016). Novel chemical ligands to ebola virus and marburg virus nucleoproteins identified by combining affinity mass spectrometry and metabolomics approaches. Sci. Rep. 6:29680. doi: 10.1038/srep29680
Gale, T. V., Horton, T. M., Grant, D. S., and Garry, R. F. (2017). Metabolomics analyses identify platelet activating factors and heme breakdown products as Lassa fever biomarkers. PLoS Negl. Trop. Dis. 11:e0005943. doi: 10.1371/journal.pntd.0005943
Geier, B. K., Sogin, E., Michellod, D., Janda, M., Kompauer, M., Spengler, B., et al. (2019). Spatial metabolomics of in situ, host-microbe interactions. bioRxiv [Preprint]. doi: 10.1101/555045
Gholipour, Y., Balsells, R. E., and Nonami, H. (2012). In situ pressure probe sampling and UV-MALDI MSfor profiling metabolites in living single cells. Mass Spectrom. 1:A0003. doi: 10.5702/massspectrometry.A0003
Gilmore, I. S., Sven Heiles, S., and Pieterse, C. L. (2019). Metabolic imaging at the single-cell scale: recent advances in mass spectrometry imaging. Annu. Rev. Anal. Chem. 12, 201–224.
Godoy, M. M. G., Lopes, E. P. A., Silva, R. O., Hallwass, F., Koury, L. C. A., and Moura, I. M. (2010). Hepatitis C virus infection diagnosis using metabonomics. J. Viral. Hepat. 17, 854–858. doi: 10.1111/j.1365-2893.2009.01252.x
Gong, W., Jia, J., Zhang, B., Mi, S., Zhang, L., Xie, X., et al. (2017). Serum metabolomic profiling of piglets infected with virulent classical swine fever virus. Front. Microbiol. 8:731. doi: 10.3389/fmicb.2017.00731
Gong, X., Zhao, Y., Cai, S., Fu, S., Yang, C., Zhang, S., et al. (2014). Single cell analysis with probe ESI-mass spectrometry: detection of metabolites at cellular and subcellular levels. Anal. Chem. 86, 3809–3816. doi: 10.1021/ac500882e
Grace, B., Sheryl, M. C., Boon, P. L., Mah, L. N., and Sam, F. Y. L. (2010). Metabolomics approach for investigation of effects of dengue virus infection using the EA. hy926 cell line. J. Proteome Res. 9, 6523–6534. doi: 10.1021/pr100727m
Gross, A., Schoendube, J., Zimmermann, S., Steeb, M., Zengerle, R., and Koltay, P. (2015). Technologies for single-cell isolation. Int. J. Mol. Sci. 16, 16897–16919. doi: 10.3390/ijms160816897
Gross, A., Schöndube, J., Niekrawitz, S., Streule, W., Riegger, L., Zengerle, R., et al. (2013). Single-cell printer. J. Lab. Autom. 18, 504–518. doi: 10.1177/2211068213497204
Guido, A., Gualdonia, K. A., Mayera, A. M. K., Katharina, K., Alexander, P., Philip, K., et al. (2018). Rhinovirus induces an anabolic reprogramming in host cell metabolism essential for viral replication. Proc. Natl. Acad. Sci. U.S.A. 115, E7158-E7165. doi: 10.1073/pnas.1800525115
Guo, F., Li, S., Caglar, M. U., Mao, Z., Liu, W., Woodman, A., et al. (2017). Single-cell virology: on-chip investigation of viral infection dynamics. Cell Rep. 21, 1692–1704. doi: 10.1016/j.celrep.2017.10.051
Guo, K. T., SchÄfer, R., Paul, A., Gerber, A., Ziemer, G., and Wendel, H. P. (2006). A new technique for the isolation and surface immobilization of mesenchymal stem cells from whole bone marrow using high-specific dna aptamers. Stem Cells 24, 2220–2231. doi: 10.1634/stemcells.2006-0015
Hansen, R. L., and Lee, Y. J. (2018). High spatial resolution mass spectrometry imaging: toward single cell metabolomics in plant tissues. Chem. Rec. 18, 65–77. doi: 10.1002/tcr.201700027
Hansen, R. L., Dueñas, M. E., Looft, T., and Lee, Y. J. (2018). Nanoparticle microarray for high-throughput microbiome metabolomics using matrix-assisted laser desorption ionization mass spectrometry. Anal. Bioanal. Chem. 411, 147–156. doi: 10.1007/s00216-018-1436-5
Hegedus, A., Kavanagh, W. M., Khan, M. B., Dias Zeidler, J., Da Poian, A. T., and El-Bacha et al. (2017). Evidence for altered glutamine metabolism in human immunodeficiency virus type 1 infected primary human cd4+ t cells. AIDS Res. Hum. Retroviruses 33, 1236–1247. doi: 10.1089/aid.2017.0165
Hewer, R., Vorster, J., Steffens, F. E., and Meyer, D. (2006). Applying biofluid 1H NMR-based metabonomic techniques to distinguish between HIV-1 positive/AIDS patients on antiretroviral treatment and HIV-1 negative. J. Pharm. Biomed. Anal. 41, 1442–1446.
Hiyama, E., Ali, A., Amer, S., Harada, T., Shimamoto, K., Furushima, R., et al. (2015). Direct lipido-metabolomics of single floating cells for analysis of circulating tumor cells by live single cell mass spectrometry. Anal. Sci. 31, 1215–1217. doi: 10.2116/analsci.31.1215
Hofmann, S., Krajewski, M., Scherer, C., Scholz, V., Mordhorst, V., Truschow, P., et al. (2018). Complex lipid metabolic remodeling is required for efficient hepatitis C virus replication. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1863, 1041–1056. doi: 10.1016/j.bbalip.2018.06.002
Hollenbaugh, J. A., Montero, C., Schinazi, R. F., Munger, J., and Kim, B. (2016). Metabolic profiling during HIV-1 and HIV-2 infection of primary human monocyte-derived macrophages. Virology 491, 106–114. doi: 10.1016/j.virol.2016.01.023
Hollenbaugh, J. A., Munger, J., and Kim, B. (2011). Metabolite profiles of human immunodeficiency virus infected CD4+ T cells and macrophages using LC-MS/MS analysis. Virology 415, 153–159. doi: 10.1016/j.virol.2011.04.007
Hu, P., Zhang, W., Xin, H., and Deng, G. (2016). Single cell isolation and analysis. Front. Cell Dev. Biol. 4:116 doi: 10.3389/fcell.2016.00116
Huang, Y.-Q., You, J.-Q., Yuan, B.-F., and Feng, Y.-Q. (2012). Sample preparation and direct electrospray ionization on a tip column for rapid mass spectrometry analysis of complex samples. Analyst 137:4593. doi: 10.1039/c2an35856e
Jakó, T., Szabó, E., Tábi, T., Zachar, G., Csillag, A., and Szökõ, É (2014). Chiral analysis of amino acid neurotransmitters and neuromodulators in mouse brain by CE-LIF. Electrophoresis 35, 2870–2876. doi: 10.1002/elps.201400224
Janssens, S., Schotsaert, M., Manganaro, L., Dejosez, M., Simon, V., García-Sastre, A., et al. (2018). FACS-mediated isolation of neuronal cell populations from virus-infected human embryonic stem cell-derived cerebral organoid cultures. Curr. Protoc. Stem Cell Biol. 48:e65. doi: 10.1002/cpsc.65
Ji, B., Xia, B., Gao, Y., Ma, F., Ding, L., and Zhou, Y. (2016). Generating electrospray ionization on ballpoint tips. Anal. Chem. 88, 5072–5079. doi: 10.1021/acs.analchem.5b03990
Jiang, Y., Li, Y., Zhou, L., and Zhang, D. (2020). Comparative metabolomics unveils molecular changes and metabolic networks of syringin against hepatitis B mice by untargeted mass spectrometry. RSC Advances 10, 461–473. doi: 10.1039/c9ra06332c
Joshua, D. C., Xin, H., Eun, J. K., Soojin, P., Young, T. L., Micheal, O., et al. (2016). Metabolic pathways of lung inflammation revealed by high-resolution metabolomics (HRM) of H1N1 influenza virus infection in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, 906–916.
Judge, M. T., Wu, Y., Tayyari, F., Hattori, A., Glushka, J., Ito, T., et al. (2019). Continuous in vivo metabolism by NMR. Front. Mol. Biosci. 30:26. doi: 10.3389/fmolb.2019.00026
Kacherovsky, N., Cardle, I. I., Cheng, E. L., Yu, J. L., Baldwin, M. L., Salipante, S. J., et al. (2019). Traceless aptamer-mediated isolation of CD8+ T cells for chimeric antigen receptor T-cell therapy. Nat. Biomed. Eng. 3, 783–795. doi: 10.1038/s41551-019-0411-6
Kajiyama, S., Harada, K., Fukusaki, E., and Kobayashi, A. (2006). Single cell-based analysis of toreniapetal pigments by a combination of ArFexcimer laser micro sampling and nano-high performance liquid chromatography (HPLC)-mass spectrometry. J. Biosci. Bioeng. 102, 575–578.
Karla, D. P., Jia, L., Goodfellow, I., Kolawole, A. O., Arche, J. R., Maddox, R. J., et al. (2018). Central carbon metabolism is an intrinsic factor for optimal replication of a norovirus. bioRxiv [Preprint]. doi: 10.1101/434019
Kawashima, H., Oguchi, M., Ioi, H., Amaha, M., Yamanaka, G., Kashiwagi, Y., et al. (2006). Primary biomarkers in cerebral spinal fluid obtained from patients with influenza-associated encephalopathy analyzed by metabolomics. Int. J. Neurosci. 116, 927–936. doi: 10.1080/00207450600550519
Khalil, J. Y. B., Langlois, T., Andreani, J., Sorraing, J.-M., Raoult, D., Camoin, L., et al. (2017). Flow cytometry sorting to separate viable giant viruses from amoeba co-culture supernatants. Front. Cell. Infect. Microbiol. 6:202. doi: 10.3389/fcimb.2016.00202
Khedr, A., Hegazy, M. A., Kammouna, A. K., and Shehata, M. A. (2016). Phospholipidomic identification of potential serum biomarkers in dengue fever, hepatitis B and hepatitis C using liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Chromatography B Analyt. Technol. Biomed. Life Sci. 1009–1010, 44–54. doi: 10.1016/j.jchromb.2015.12.011
Khedra, A., Hegazyb, M., Kamala, A., and Shehatab, M. A. (2014). Profiling of esterified fatty acids as biomarkers in the blood of dengue fever patients using a microliter-scale extraction followed by gas chromatography and mass spectrometry. J. Sep. Sci. 38, 316–324. doi: 10.1002/jssc.201400749
Kollmer, F., Paul, W., Krehl, M., and Niehuis, E. (2012). Ultra high spatial resolution SIMS with cluster ions—approaching the physical limits. Surf. Interface Anal. 45, 312–314. doi: 10.1002/sia.5093
Konstantinos, K. (2017). Metabolic Programming in Murine Cytomegalovirus Infected Macrophages. Ph.D. thesis, University of Edinburgh, Edinburgh.
Korte, A. R., Stopka, S. A., Morris, N., Razunguzwa, T., and Vertes, A. (2016). Large-scale metabolite analysis of standards and human serum by laser desorption ionization mass spectrometry from silicon nanopost arrays. Anal. Chem. 88, 8989–8996. doi: 10.1021/acs.analchem.6b01186
Kylea, J. E., Burnum-Johnsona, K. E., Wendlera, J. P., Eisfeldb, A. J., Halfmannb, P. J., Watanabec, T., et al. (2018). Plasma lipidome reveals critical illness and recovery from human Ebola virus disease. Proc. Natl. Acad. Sci. U.S.A. 116, 3919–3928. doi: 10.1073/pnas.1815356116
Labonté, J. M., Swan, B. K., Poulos, B., Luo, H., Koren, S., Hallam, S. J., et al. (2015). Single-cell genomics-based analysis of virus–host interactions in marine surface bacterioplankton. ISME J. 9, 2386–2399. doi: 10.1038/ismej.2015.48
Lapainis, T., Rubakhin, S. S., and Sweedler, J. V. (2009). Capillary electrophoresis with electrospray ionizationmass spectrometric detection for single-cell metabolomics. Anal. Chem. 81, 5858–5864. doi: 10.1021/ac900936g
Li, H., Li, H., Wang, J., Guo, L., Fan, H., Zheng, H., et al. (2019). The altered gut virome community in rhesus monkeys is correlated with the gut bacterial microbiome and associated metabolites. Virol J. 16:105. doi: 10.1186/s12985-019-1211-z
Li, Q., Chen, P., Fan, Y., Wang, X., Xu, K., Li, L., et al. (2016). Multicolor fluorescence detection-based microfluidic device for single-cell metabolomics: simultaneous quantitation of multiple small molecules in primary liver cells. Anal. Chem. 88, 8610–8616. doi: 10.1021/acs.analchem.6b01775
Lin, S., Liu, N., Yang, Z., Song, W., Wang, P., Chen, H., et al. (2010). GC/MS-based metabolomics reveals fatty acid biosynthesis and cholesterol metabolism in cell lines infected with influenza A virus. Talanta 83, 262–268. doi: 10.1016/j.talanta.2010.09.019
Liu, J., Cao, R., Xu, M., Wang, X., Zhang, H., Hu, H., et al. (2020). Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 6:16. doi: 10.1038/s41421-020-0156-0
Liu, J.-X., Aerts, J. T., Rubakhin, S. S., Zhang, X.-X., and Sweedler, J. V. (2014). Analysis of endogenous nucleotides by single cell capillary electrophoresis-mass spectrometry. Analyst 139, 5835–5842. doi: 10.1039/c4an01133c
Liu, P., Yin, Y., Gong, Y., Qiu, X., Sun, Y., Tan, L., et al. (2019). In vitro and in vivo metabolomic profiling after infection with virulent newcastle disease virus. Viruses 11:962. doi: 10.3390/v11100962
Lombard-Banek, C., Reddy, S., Moody, S. A., and Nemes, P. (2016). Label-free quantification of proteins in single embryonic cells with neural fate in the cleavage-stage frog (Xenopuslaevis) embryo using capillary electrophoresis electrospray ionization high-resolution mass spectrometry (CE-ESI-HRMS). Mol. Cell. Proteomics 15, 2756–2768. doi: 10.1074/mcp.m115.057760
Lungile, S., Francois, S., Tjaart, P. J. K., and Debra, M. (2014). Mid-ATR-FTIR spectroscopic profiling of HIV/AIDS sera for novel systems diagnostics in global health. J. Integr. Biol. 18, 513–523. doi: 10.1089/omi.2013.0157
Mahmoud, A. G., Pranab, K. M., Richard, J. J., Mauricio, R., Robert, E. B., Masoumeh, S., et al. (2013). Metabolomics reveals differential levels of oral metabolites in hiv-infected patients: toward novel diagnostic targets. J. Integr. Biol. 17, 5–15. doi: 10.1089/omi.2011.0035
Mao, Y., Huang, X., Yu, K., Qu, H., Chang-xiao, L., and Cheng, Y. (2008). Metabonomic analysis of hepatitis B virus-induced liver failure: identification of potential diagnostic biomarkers by fuzzy support vector machine. J. Zhejiang Univ. Sci. B 9, 474–481. doi: 10.1631/jzus.B0820044
Mayer, K. A., Stöckl, J., Zlabinger, G. J., and Gualdoni, G. A. (2019). Hijacking the supplies: metabolism as a novel facet of virus-host interaction. Front. Immunol. 10:1533. doi: 10.3389/fimmu.2019.01533
Melo, C. F. O. R., de Oliveira, D. N., Lima, E., de, O., Guerreiro, T. M., Esteves, C. Z., et al. (2016). A lipidomics approach in the characterization of zika-infected mosquito cells: potential targets for breaking the transmission cycle. PLoS One 11:e0164377. doi: 10.1371/journal.pone.0164377
Melo, C. F. O. R., Delafiori, J., Dabaja, M. Z., Diogo, N. O., Tatiane, M. G., Tatiana, E. C., et al. (2018). The role of lipids in the inception, maintenance and complications of dengue virus infection. Sci. Rep. 8:11826. doi: 10.1038/s41598-018-30385-x
Melo, C. F. O. R., Delafiori, J., de Oliveira, D. N., Guerreiro, T. M., Esteves, C. Z., Lima, E. D. O., et al. (2017). Serum metabolic alterations upon zikainfection. Front. Microbiol. 8:1954. doi: 10.3389/fmicb.2017.01954
Miltenyi, S., Muller, W., Weichel, W., and Radbruch, A. (1990). High gradient magnetic cell separation with MACS. Cytometry 11, 231–238. doi: 10.1002/cyto.990110203
Minakshi, P., Ghosh, M., Kumar, R., Patki, H. S., Saini, H. M., Ranjan, K., et al. (2019a). “Single-cell metabolomics: technology and applications,” in Single-Cell Omics, eds D. Barh and V. Azevedo (Cambridge, MA: Academic Press), 319–353. doi: 10.1016/b978-0-12-814919-5.00015-4
Minakshi, P., Kumar, R., Ghosh, M., Saini, H. M., Ranjan, K., Brar, B., et al. (2019b). “Single-Cell proteomics: technology and applications,” in Single-Cell Omics, eds D. Barh and V. Azevedo (Cambridge, MA: Academic Press), 283–318. doi: 10.1016/b978-0-12-814919-5.00014-2
Munger, J., Bennett, B. D., Parikh, A., Feng, X. J., McArdle, J., Rabitz, H. A., et al. (2008). Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26, 1179–1186. doi: 10.1038/nbt.1500
Munshi, S. U., Rewari, B. B., Bhavesh, N. S., and Jameel, S. (2013). Nuclear magnetic resonance based profiling of biofluids reveals metabolic dysregulation in HIV-infected persons and those on anti-retroviral therapy. PLoS One 8:e64298. doi: 10.1371/journal.pone.0064298
Munshi, S. U., Taneja, S., Bhavesh, N. S., Shastri, J., Aggarwal, R., and Jameel, S. (2011). Metabonomic analysis of hepatitis E patients shows deregulated metabolic cycles and abnormalities in amino acid metabolism. J.Viral. Hepat. 18, 591–602. doi: 10.1111/j.1365-2893.2011.01488.x
Nemes, P., Rubakhin, S., Aerts, J., and Sweedler, J. (2013). Qualitative and quantitative metabolomic investigation of single neurons by capillary electrophoresis electrospray ionization mass spectrometry. Nat. Protoc. 8, 783–799. doi: 10.1038/nprot.2013.035
Nurul, S., Hasnah, O., Tang, T. H., and Abdel-Hamid, Z. A. H. (2017). Metabolomics approach for multibiomarkers determination to investigate dengue virus infection in human patients. Acta Biochim. Pol. 64, 215–219. doi: 10.18388/abp.2015_1224
Onjiko, R. M., Moody, S. A., and Nemes, P. (2015). Single-cell mass spectrometry reveals small moleculesthat affect cell fates in the 16-cell embryo. Proc. Natl. Acad. Sci. U.S.A. 112, 6545–6550. doi: 10.1073/pnas.1423682112
Paglia, G., and Astarita, G. (eds). (2020). “Ion mobility-mass spectrometry”, in Methods in Molecular Biology (New York, NY: Springer), XI, 312.
Passalacqua, K. D., Lu, J., Goodfellow, I., Kolawole, A. O., Arche, J. R., Maddox, R. J., et al. (2018). Central carbon metabolism is an intrinsic factor for optimal replication of a norovirus. bioRxiv [Preprint]. doi: 10.1101/434019
Prabhakar, N., Khan, M. H., Peurla, M., Chang, H.-C., Hänninen, P. E., and Rosenholm, J. M. (2017). Intracellular trafficking of fluorescent nanodiamonds and regulation of their cellular toxicity. ACS Omega 2, 2689–2693. doi: 10.1021/acsomega.7b00339
Pu, F., Chiang, S., Zhang, W., and Ouyang, Z. (2018). Direct sampling mass spectrometry for clinical analysis. Analyst 144, 1034–1051. doi: 10.1039/c8an01722k
Qi, M., Philip, M. C., Yang, N., and Sweedler, J. V. (2017). Single cell neurometabolomics. ACS Chem. Neurosci. 9, 40–50. doi: 10.1021/acschemneuro.7b00304
Ritter, J. B., Wahl, A. S., Freund, S., and Genezel, Y. (2010). Metabolic effects of influenza virus infection in cultured animal cells: intra- and extracellular metabolite profiling. BMC Syst. Biol. 4:61. doi: 10.1186/1752-0509-4-61
Rosenwasser, S., Sheyn, U., Frada, M. J., Pilzer, D., Rotkopf, R., and Vardi, A. (2019). Unmasking cellular response of a bloom-forming alga to viral infection by resolving expression profiles at a single-cell level. PLoS Pathog. 15:e1007708. doi: 10.1371/journal.ppat.1007708
Russell, A. B., Elshina, E., Kowalsky, J. R., teVelthuis, A. J. W., and Bloom, J. D. (2019). Single-cell virus sequencing of influenza infections that trigger innate immunity. J. Virol. 93:e00500-19. doi: 10.1128/jvi.00500-19
Schleyer, G., Shahaf, N., Ziv, C., Dong, Y., Meoded, R. A., Helfrich, E. J. N., et al. (2019). In plaque-mass spectrometry imaging of a bloom-forming alga during viral infection reveals a metabolic shift towards odd-chain fatty acid lipids. Nat. Microbiol. 4, 527–538. doi: 10.1038/s41564-018-0336-y
Schoeman, J. C. (2020). Virus-host Metabolic Interactions: Using Metabolomics to Probe Oxidative Stress, Inflammation and Systemic Immunity. Ph.D. thesis, University of Leiden, Chicago, IL.
Schoeman, J. C., Hou, J., Harms, A. C., Vreeken, R. J., Berger, R., Hankemeier, T., et al. (2016). Metabolic characterization of the natural progression of chronic hepatitis B. Genome Med. 8:64. doi: 10.1186/s13073-016-0318-8
Schoendube, J., Wright, D., Zengerle, R., and Koltay, P. (2015). Single-cell printing based on impedance detection. Biomicrofluidics 9:014117. doi: 10.1063/1.4907896
Shalabaeva, V., Lovato, L., La Rocca, R., Messina, G. C., Dipalo, M., Miele, E., et al. (2017). Time resolved and label free monitoring of extracellular metabolites by surface enhanced Raman spectroscopy. PLoS One 12:e0175581. doi: 10.1371/journal.pone.0175581
Shrestha, B., and Vertes, A. (2009). In situ metabolic profiling of single cells by laser ablation electrospray ionization mass spectrometry. Anal. Chem. 81, 8265–8271. doi: 10.1021/ac901525g
Shrinet, J., Shastri, J., and Gaind, R. (2016). Serum metabolomics analysis of patients with chikungunya and dengue mono/co-infections reveals distinct metabolite signatures in the three disease conditions. Sci. Rep. 6:36833. doi: 10.1038/srep36833
Singh, A. M. (2019). An efficient protocol for single-cell cloning human pluripotent stem cells. Front. Cell Dev. Biol. 7:11. doi: 10.3389/fcell.2019.00011
Smith, R., Wright, K. L., and Ashton, L. (2016). Raman spectroscopy: an evolving technique for live cell studies. Analyst 141, 3590–3600. doi: 10.1039/c6an00152a
Stopka, S. A., Samarah, L., Shaw, J. B., Liyu, A. V., Velièkoviæ, D., Agtuca, B. J., et al. (2019). Ambient metabolic profiling and imaging of biological samples with Ultrahigh molecular resolution using laser ablation electrospray ionization 21 tesla FTICR Mass spectrometry. Anal. Chem. 91, 5028–5035. doi: 10.1021/acs.analchem.8b05084
Strzelecka, P. M., Ranzoni, A. M., and Cvejic, A. (2018). Dissecting human disease with single-cell omics: application in model systems and in the clinic. Dis. Model. Mech. 11:dmm036525. doi: 10.1242/dmm.036525
Sun, X., Song, L., Feng, S., Li, L., Yu, H., Wang, Q., et al. (2018). Fatty acid metabolism is associated with disease severity after h7n9 infection. EBioMedicine 33, 218–229. doi: 10.1016/j.ebiom.2018.06.019
Svatos, A. (2011). Single cell metabolomics comes of age: new developments in mass spectrometry profiling and imaging. Anal. Chem. 83, 5037–5044. doi: 10.1021/ac2003592
Tejedor, M. L., Mizuno, H., Tsuyama, N., Harada, T., and Masujima, T. (2009). Direct single-cell molecular analysis of plant tissues by video mass spectrometry. Anal. Sci. 25, 1053–1055.
Thai, M., Nicholas, A. G., Braas, D., Nehil, M., Komisopoulou, E., and Kurdistani, S. K. (2014). Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 19, 694–670. doi: 10.1016/j.cmet.2014.03.009
Thaker, S. K., Chapa, T., Garcia, G. Jr., Gong, D., Schmid, E. W., Arumugaswami, V., et al. (2019). Differential metabolic reprogramming by zika virus promotes cell death in human versus mosquito cells. Cell Metab. 29, 1206–1216.e4. doi: 10.1016/j.cmet.2019.01.024
Tian, X., Zhang, K., Min, J., Chen, C., Cao, Y., Ding, C., et al. (2019). Metabolomic analysis of influenza a virus A/WSN/1933 (H1N1) infected A549 cells during first cycle of viral replication. Viruses 11:1007. doi: 10.3390/v11111007
Tsuyama, N., Mizuno, H., Tokunaga, E., and Masujima, T. (2008). Live single-cell molecular analysis by video-mass spectrometry. Anal. Sci. 24, 559–561. doi: 10.2116/analsci.24.559
Vanderpoorten, O., Peter, Q., Challa, P. K., Keyser, U. F., Baumberg, J., Kaminski, C. F., et al. (2019). Scalable integration of nano-, and microfluidics with hybrid two-photon lithography. Microsyst. Nanoeng. 5:40. doi: 10.1038/s41378-019-0080-3
Vastag, L., Koyuncu, E., Grady, S. L., Shenk, T. E., and Rabinowitz, J. D. (2011). Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 7:e1002124. doi: 10.1371/journal.ppat.1002124
Voge, N. V., Perera, R., Mahapatra, S., Gresh, L., Balmaseda, A., Loroño-Pino, M. A., et al. (2016). Metabolomics-based discovery of small molecule biomarkers in serum associated with dengue virus infections and disease outcomes. PLoS Negl. Trop. Dis. 10:e0004449. doi: 10.1371/journal.pntd.0004449
Walker, B. N., Stolee, J. A., and Vertes, A. (2012). Nanophotonic ionization for ultratrace and single-cell analysis by mass spectrometry. Anal.Chem. 84, 7756–7762. doi: 10.1021/ac301238k
Wang, H., Chen, L., and Sun, L. (2017). Digital microfluidics: a promising technique for biochemical applications. Front. Mech. Eng. 12:510–525. doi: 10.1007/s11465-017-0460-z
Wang, S., Zhang, Q., Hui, H., Agrawal, K., and Karris, M. A. Y. (2019). An atlas of immune cell exhaustion in HIV-infected individuals revealed by single-cell transcriptomics. bioRxiv [Preprint]. doi: 10.1101/678763
Wang, Z., Liang, H., Cao, H., Zhang, B., Li, J., Wang, W., et al. (2019). Efficient ligand discovery from natural herbs by integrating virtual screen, affinity mass spectrometry and targeted metabolomics. Analyst 144, 2881–2890. doi: 10.1039/c8an02482k
Warrick, J. W., Timm, A., Swick, A., and Yin, J. (2016). Tools for single-cell kinetic analysis of virus-host interactions. PLoS One 11:e0145081. doi: 10.1371/journal.pone.0145081
William, R., Wikoff, Siuzdak, G., Howard, S., and Fox, J. (2008). Metabolomic analysis of the cerebrospinal fluid reveals changes in phospholipase expression in the CNS of SIV-infected macaques. Clin. Invest. 118, 2661–2669. doi: 10.1172/JCI34138
Xu, L., Brito, I. L., Alm, E. J., and Blainey, P. C. (2016). Virtual microfluidics for digital quantification and single-cell sequencing. Nat. Methods 13, 759–762. doi: 10.1038/nmeth.3955
Yan, S., Zhang, J., Yuan, D., and Li, W. (2016). Hybrid microfluidics combined with active and passive approaches for continuous cell separation. Electrophoresis 38, 238–249. doi: 10.1002/elps.201600386
Yuan, S., Chu, H., Chan, J. F. W., Ye, Z. W., Wen, L., Yan, B., et al. (2019). SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 10:120. doi: 10.1038/s41467-018-08015-x
Zanini, F., Robinson, M. L., Croote, D., Sahoo, M. K., Sanz, A. M., Ortiz-Lasso, E., et al. (2018). Virus-inclusive single-cell RNA sequencing reveals the molecular signature of progression to severe dengue. Proc. Natl. Acad. Sci. U.S.A. 115, E12363-E12369. doi: 10.1073/pnas.1813819115
Zhang, K., Chou, C. K., Xia, X., Hung, M. C., and Qin, L. (2014). Block-Cell-Printing for live single-cell printing. Proc. Natl. Acad. Sci. U.S.A. 111, 2948–2953. doi: 10.1073/pnas.1313661111
Keywords: single cell metabolomics, virus-host interaction, emerging viruses, mass spectrometry, imaging
Citation: Kumar R, Ghosh M, Kumar S and Prasad M (2020) Single Cell Metabolomics: A Future Tool to Unmask Cellular Heterogeneity and Virus-Host Interaction in Context of Emerging Viral Diseases. Front. Microbiol. 11:1152. doi: 10.3389/fmicb.2020.01152
Received: 28 January 2020; Accepted: 06 May 2020;
Published: 03 June 2020.
Edited by:
Helene Dutartre, UMR5308 Centre International de Recherche en Infectiologie (CIRI), FranceReviewed by:
Mireille Laforge, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceAnurag Kulkarni, Oxford BioMedica, United Kingdom
Copyright © 2020 Kumar, Ghosh, Kumar and Prasad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mayukh Ghosh, Z2hvc2gubWF5dWtoODdAZ21haWwuY29t; Minakshi Prasad, bWluYWtzaGkuYWJ0QGdtYWlsLmNvbQ==; bWluYWtzaGkuYWJ0QGx1dmFzLmVkdS5pbg==
 Rajesh Kumar1
Rajesh Kumar1 Mayukh Ghosh
Mayukh Ghosh Minakshi Prasad
Minakshi Prasad