
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 23 April 2020
Sec. Terrestrial Microbiology
Volume 11 - 2020 | https://doi.org/10.3389/fmicb.2020.00752
Phosphorus solubilizing bacteria (PSB) can promote the level of plant-absorbable phosphorus (P) in agro-ecosystems. However, little attention has been paid to PSB harboring abilities in utilizing multiple phosphorus sources and their potentials for heavy metal immobilization. In this study, we applied the strategy of stepwise acclimation by using Ca3(PO4)2, phytate, FePO4, and AlPO4 as sole P source. We gained 18 PSB possessing abilities of multiple P sources utilization, and these bacteria belonged to eight genera (Acinetobacter, Pseudomonas, Massilia, Bacillus, Arthrobacter, Stenotrophomonas, Ochrobactrum, and Cupriavidus), and clustered to two apparent parts: Gram-positive bacteria and Gram-negative bacteria. The isolate of Acinetobacter pittii gp-1 presented good performance for utilizing Ca3(PO4)2, FePO4, AlPO4, and phytate, with corresponding P solubilizing levels were 250.77, 46.10, 81.99, and 7.91 mg/L PO43–-P, respectively. The PSB A. pittii gp-1 exhibited good performance for solubilizing tricalcium phosphate in soil incubation experiments, with the highest values of water soluble P and available P were 0.80 and 1.64 mg/L, respectively. Additionally, the addition of A. pittii gp-1 could promote the immobilization of lead (Pb), and the highest Pb immobilization efficiency reached 23%. Simultaneously, we found the increases in abundances of both alkaline phosphatase gene (phoD) and β-propeller phytase gene (bpp) in strain gp-1 added soils. Besides, we observed the expression up-regulation of both pyrroloquinoline quinone gene (pqq) and polyphosphate kinases gene (ppk), with the highest relative expression levels of 18.18 and 5.23, respectively. We also found the polyphosphate particles using granule staining. To our knowledge, our findings first suggest that the solubilizing of tricalcium phosphate by phosphorus solubilizing bacterium belonging to Acinetobacter is coupled with the synthesis of polyphosphate. Taken together, A. pittii gp-1 could be a good candidate in improving soil fertility and quality.
Phosphorus (P) is the second limiting nutrient required for plant growth and development involved in important metabolic pathways like nutrient uptake, biological oxidation, and energy metabolism (Nesme et al., 2018). The total P in soil accounts roughly for 0.04–0.1% (w/w), only a very tiny proportion of P (soluble H2PO4– or HPO42–) can directly be assimilated by plants (Chen et al., 2008), as the large portion of P in soils exists in inorganic insoluble form [e.g., Ca3(PO4)2] and organic insoluble/soluble form (e.g., phytate and nucleic acid) (Liu et al., 2015; Neal et al., 2017). The input of P to soil is mainly via fertilization, and both abiotic substance (mainly inorganic P mineral) and organic P compounds (e.g., animal, plant, and microbes residues and wastes) are widely used in agricultural ecosystems (Lim et al., 2007; Jorquera et al., 2013; Fraser et al., 2015), and these extraneously added P including inorganic P (IP) and organic P (OP) get converted into salts and become insoluble by bounding to Ca, Al, Mg, Mn, and Fe (Liu et al., 2015; Reddy et al., 2015). Insoluble IP especially Ca3(PO4)2, AlPO4, and FePO4, and insoluble/soluble OP especially phytate taking up 80% of soil OP need phosphorus-solubilizing microbes (PSM) to transform into orthophosphate which can be absorbed by plants and microbes (Lim et al., 2007; Liu et al., 2014; Fraser et al., 2015). Previous literatures have reported that insoluble IP can be dissolved by low molecular weight organic acids (e.g., citric and gluconic acids) produced and released by both phosphorus solubilizing bacteria (PSB) and fungi (Sashidhar and Podile, 2009; Ogbo, 2010; Patel et al., 2011), and OP can be digested by extracellular enzymes (e.g., phosphatase and phytase) mainly synthesized and secreted by microbes (Tan et al., 2016; Neal et al., 2017). Previous literatures have reported that repeated utilization of fertilizers exacerbates soil quality and lessens phosphorus availability (Bhattacharyya et al., 2015; Liu et al., 2018). To achieve the aim of sustainable agriculture, the application of phosphorus solubilizing microbes with multiple P sources utilizing abilities provides a new approach to improve soil quality.
Phosphorus solubilizing microbes especially PSB are widely distributed in soils, freshwater, seawater, and sediments (Liu et al., 2014, 2017; Zhang et al., 2015), and responsible for the cycling of insoluble P to soluble PO43– ion. Numerous researches have concentrated on the screening of highly efficient PSB, and most PSB are Gram-negative bacteria and belong to Pseudomonas (Misra et al., 2012; Oteino et al., 2015), Acinetobacter (Liu et al., 2014, 2015), Pantoea and Enterobacter (Park et al., 2011; Chen and Liu, 2019), and some PSB are Gram-positive bacteria belonging to Bacillus (Hanif et al., 2015; Wang et al., 2017). PSB have showed good performance for plant growth promotion (Patel et al., 2011; Oteino et al., 2015; Wen et al., 2019) and heavy metal immobilization (Park et al., 2011; Yuan et al., 2017; Chen et al., 2019). However, few studies have been conducted to explore the potentials of PSB for dissolving multiple P sources, and the effect of PSB addition on both soil inorganic and organic P-cycling-related gene abundance remains unknown.
Phospholipids and phytate are major organic P pool in soils, which can be hydrolyzed by phosphatase and phytase, respectively (Maougal et al., 2014; Neal et al., 2017; Irshad and Yergeau, 2018). Previous literatures have reported that alkaline phosphatase is exclusively originated from soil microorganisms, and acid phosphatase is mainly produced and secreted by plants (Krämer and Green, 2000; Fraser et al., 2015). Three prokaryotic genes, phoX, phoA, and phoD, are responsible for encoding alkaline phosphatase (Huang et al., 2009); three genes of bpp encoding β-propeller phytase, ptp encoding protein tyrosine phosphatase, and hap encoding histidine acid phosphatase are found in prokaryote (Lim et al., 2007; Neal et al., 2017). However, in terrestrial ecosystems, phoD-harboring bacteria are more widely distributed than phoX-, phoA-harboring bacteria (Neal et al., 2017; Hu et al., 2018), and bpp-harboring bacteria are more frequently found than ptp-, hap-harboring bacteria (Lim et al., 2007; Neal et al., 2017). In addition, the hydrolysis of inorganic insoluble P requires the participation of small molecular organic acid (e.g., gluconic acid, lactic acid, and citric acid), which can be released by gcd-harboring bacteria (Vyas and Gulati, 2009; Hanif et al., 2015; Rasul et al., 2019). Therefore, the phoD, bpp, and gcd genes could be good biomarkers in evaluating soil inorganic and organic P transformation.
In Gram-negative bacteria, gluconic acid is produced by the direct oxidation of glucose mediated by a membrane-bound glucose dehydrogenase (GDH), which needs pyrroloquinoline quinone (PQQ) as a cofactor in dehydrogenase reactions catalyzed by the so called quinoproteins (Farhat et al., 2013; Wagh et al., 2014). Quinoproteins exist in many bacteria, like alcohol dehydrogenase from Pseudomonas aeruginosa, GDH from Acinetobacter calcoace, methylamine dehydrogenase from Thiobacillus species (Goosen et al., 1992). The PQQ biosynthesis pathway in bacteria involves several genes presenting in a cluster, whose composition in terms of gene number and their organization exhibits considerable distinction in different species (Choi et al., 2008). Polyphosphate kinases (PPK) encoded by ppk gene are responses for the synthesis of polyphosphate considered as a key high-energy compound (Ishige and Noguchi, 2000). Extensive effort has been implemented to determine the pqq gene expression level in the process of inorganic phosphorus solubilization, however, little literature has reported the expression level of PPK gene in the process of inorganic phosphorus solubilization. To enrich the knowledge of phosphorus metabolism during inorganic P solubilizing, the gene expression level of pqq and ppk should be determined.
In this study, we aimed to (i) screen PSB possessing multiple P source utilization capacity, (ii) explore the potential of PSB for lead immobilization, and (iii) determine abundances of P-cycling-related genes in both cells and soils.
The NBRIP medium containing 10 g/L glucose, 0.25 g/L MgSO4⋅7H2O, 5 g/L Ca3(PO4)2/sodium phytate/FePO4/AlPO4, 5 g/L MgCl2⋅7H2O, 0.2 g/L KCl, 0.1 g/L (NH4)2SO4, 2 mL/L trace element solution was applied to acclimate and isolate PSB (Misra et al., 2012). Trace element solution contained (g/L) EDTA, 10; MnSO4⋅H2O, 2.2; FeSO4⋅7H2O, 1.0; CuSO4⋅5H2O, 0.5; CoCl2⋅6H2O, 0.3; Na2MoO4⋅2H2O, 0.2; and CaCl2, 0.1. The initial pH of all mediums was adjusted to 7.0 using 1 mol/L NaOH and 1 mol/L HCl solution.
Five gram of bulk soil collected from Laiyang Experimental Station in Shandong Province, China, were added to 50 mL of sterile water and shaken at 180 rpm for 30 min, and the mixture was kept stand for 10 min. In the first round of acclimation, 10 mL of soil suspension were inoculated to 100 mL of liquid NBRIP medium containing Ca3(PO4)2, and incubated at 30°C with shaking of 180 rpm for 7 days. The microbial suspension was collected and centrifuged at 5000 rpm for 10 min, and then microbial suspension was resuspended with 10 mL of sterile water after precipitation was washed twice. Taking the first round acclimation procedure, the microbial suspension was inoculated to liquid NBRIP containing sodium phytate, FePO4 or AlPO4, respectively, and incubated at the same condition for another 7 days. The acclimated microbial suspension was designated as A1 (NBRIP containing Ca3(PO4)2), A2 (NBRIP containing sodium phytate), A3 (NBRIP containing FePO4), and A4 (NBRIP containing AlPO4), respectively. Additionally, part of the microbial suspension of A1, A2, A3, and A4 were centrifuged at 5000 rpm, and the microbial precipitations were stored at −80°C for subsequent DNA extraction.
Taking stepwise dilution strategy, 0.1 mL of 10–5 and 10–8 diluent of microbial suspension A4 was evenly spread on NBRIP containing Ca3(PO4)2 agar medium and incubated at 30°C for 5 days. The screening strategy for isolation of PSB was by picking colony with clear halo zone. Single colonies were sub-cultured by picking and streaking five times to isolate pure colonies. A total of 18 strains (Ap-1, Ap-2, Ap-3, Ap-4, Ap-6, Ap-8, Ap-9, Ap-10, gp-1, gp-2, gp-3, gp-4, gp-6, gp-7, gp-8, gp-11, gp-12, and gp-15) were gained and identified by simple 16S rRNA gene sequencing in Wuhan Qingke innovation Biotechnology Co., Ltd.
The total DNA of microbial precipitations and soil were extracted using a DNA extraction kit (MoBio, Carlsbad, CA, United States) according to the manufacture’s instruction. The DNA concentrations were measured using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). All DNA samples were stored at −80°C.
To determine the bacterial community composition of microbial precipitations (A1, A2, A3, and A4), the V3–V4 region of bacterial 16S rRNA gene was amplified using the primers 338F (5′- ACT CCT ACG GGA GGC AGC A-3′) and 806R (5′- GGA CTA CHV GGG TWT CTA AT-3′) (Mori et al., 2013). The 16S rRNA gene fragments were amplified under the following condition: an initial denaturation at 95°C for 3 min, 30 cycles of 95°C for 40 s, 58°C for 40 s, and 72°C for 50 s, and a final extension at 72°C for 10 min. The triplicate PCR products were pooled and purified by gel electrophoresis, and quantified using a QuantiFluorTM -ST (Promega, United States). Sequencing was carried out on an Illumina Miseq platform at Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China. The Miseq raw reads were deposited in the National Center for Biotechnology Information (NCBI1) Short Read Archive (SRA) database under accession numbers SRR8731888–SRR8731891.
The raw reads were purified following the pathway of Mothur (Schloss et al., 2009). To minimize the effects of random-sequencing errors, we removed (i) sequences that did not exactly match barcodes and primers; (ii) sequences that contained ambiguous bases call; (iii) sequences with an average quality score <20; and (iv) sequences with maximum homopolymers <10 bp. The purified sequences were clustered into operational taxonomic unit (OTU) at 97% identity against the SILVA v128 reference. The community diversity in different acclimation periods was compared using α-diversity indices including Shannon index, ACE, and Chao1.
The 18 strains were separately inoculated to LB medium and incubated at 30°C with shaking of 180 rpm for 16 h. Strains were collected by centrifuging and washed twice with sterile water, and subsequently resuspended. The optical density (600 nm) was adjusted to 1.0 by dilution of the strain pellets with sterile water. These mixtures were designated as seed cultures (OD = 1.0), and separately inoculated to NBRIP solid and liquid medium containing Ca3(PO4)2, sodium phytate, FePO4, or AlPO4, and incubated at 30°C for 5 days. After incubation, 1 mL of bacterial suspension was collected to determine soluble P concentration, and the diameter of the halo (HD) and the diameter of colony (CD) on solid medium plates were measured (Liu et al., 2015). The PO43–-P content in bacterial suspension was determined using molybdenum blue method (Waterlot, 2018).
To determine P solubilizing capacities of the 18 isolated PSB, soils used in incubation experiment were collected from an uncultivated field in Wuhan, middle of China (30°28′N, 114°21′E). The original pH, TC, TN, water soluble P (WSP), Olsen P, total P, and total lead were 6.92, 0.52%, 0.68%, 0.09 mg/g, 0.22 mg/g, 0.89 mg/g, and 0.57 mg/g, respectively. Three groups with three replicates were designed: 100 g soil + 10 mL seed cultures (S + B group), 95 g soil + 10 mL seed cultures + 5 g Ca3(PO4)2 (S + B + T group), 95 g soil + 10 mL seed cultures + 5 g Ca3(PO4)2 + 10 mL NBRIP liquid medium without P sources (S + B + T + N group). Sterile water was added to adjust soil moisture to 80%, and these experimental groups were incubated at room temperature for 30 days. After incubation, soils were collected to determine physicochemical properties, including the content of WSP and available P (AP). The WAP and AP were extracted by ultrapure water and 0.5 mol L–1 NaHCO3 (pH 8.5), respectively, at a ratio of 1:20 of soil to solution, and the extracted solutions were then filtered through Whatman Grade No. 42 Quantitative Filter Paper (Olsen et al., 1954), and then were measured using molybdenum blue method.
To evaluate the effect of PSB gp-1 inoculation on heavy metal immobilization, the soils containing weight ratio of 1, 2, 3, 4, 5, and 10% Ca3(PO4)2 were spiked with Pb(NO3)2 at a concentration of 500 mg Pb/kg soil and incubated at room temperature for 30 days. No strain gp-1 addition but with Ca3(PO4)2 addition, and without both strain gp-1 and Ca3(PO4)2 addition were used as blank control. Every 5 days, sterile water was added to maintain the moisture value of 80%. After incubation, soils were collected and dried at 60°C, then ground and sieved through a 0.150 mm mesh for available heavy metal evaluation. Heavy metal contaminated soils were extracted with acetic acid (HAc) solution (0.11 mol/L) with a soil to solution ratio of 1:40 and shaken at 180 rpm for 16 h (Yuan et al., 2017). The concentrations of Pb(II) in extraction solutions were measured at an AA240FS atomic-absorption spectrophotometer (Varian Company, United States). Additionally, the content of WSP and AP in heavy metal contaminated soils was determined.
The absolute abundances of P-cycling-related genes in Pb contaminated soil were measured using quantitative PCR (qPCR). Primer sequences for the target genes were applied for qPCR based on previous literatures. Primer bppF (5′–GAC GCA GCC GAY GAY CCN GCN NTN TGG–3′) and primer bppR (5′–CAG GSC GCA NRT CAN CRT TRT T–3′) were employed to amplify bpp gene (Huang et al., 2009); primer ALPS-F730 (5′–CAG TGG GAC GAC CAC GAG GT–3′) and primer ALPS-1101 (5′–GAG GCC GAT CGG CAT GTC G–3′) were applied to amplify phoD gene (Hu et al., 2018); primer gcdF (5′–CGG CGT CAT CCG GGS NTN YRA YRT–3′) and primer gcdR (5′–GGG CAT GTC CAT GTC CCA NAD RTC RTG–3′) were used to amplify gcd gene (Cleton-Jansen et al., 1990). Additionally, primer Eub338 (5′–ACT CCT ACG GGA GGC AGC AG–3′) and primer Eub518 (5′-ATT ACC GCG GCT GG-3′) (Fierer et al., 2005) were selected to amplify 16S rRNA gene. Standard curves were generated using a 10-fold serial dilution of a known amount of recombinant plasmid containing specific gene fragment. Quantitation was performed on three technical replicates with an Applied Biosystems QuantStudio3 Real-time PCR System (Applied Biosystems, Foster City, CA, United States) in a 10 μL reaction system, and was implemented at 95°C for 5 min, followed by 40 cycles of 95°C for 15 s and 55°C for 1 min. The abundances of all genes were expressed as copies per gram of freeze-dried soil. In addition, we employed these primers to amplify bpp, phoD, and gcd from Acinetobacter pittii gp-1.
Genomic DNA- extracted from strain gp-1 was used as template for amplification of the ppk and pqq genes. According to the gene sequences encoding PPK and PQQ in several related bacteria, two conservative DNA fragments were discovered with the DNAMAN program with default parameters for each gene. Primer F1 (5′–ATG ACA GAA GGT GTT GGC CT–3′) and primer R1 (5′–CTA TAT ATC TGT AAT CGT GTG GAC T–3′) were used to amplify the whole pqq gene. While primer F2 (5′–ATG AAT ACA GCG ATT ACA CCA A–3′) and primer R2 (5′–TTA TTT AAT AAT TTC TAA TAG TGC CCT TTG–3′) were employed to amplify the whole ppk gene. The acquired pqq gene had 1155 bp coding a polypeptide of 384 amino acids (accession number: MG820120). The obtained ppk gene had 2079 bp coding a polypeptide of 692 amino acids (accession number: MG820119). The obtained gene sequences were aligned with the closely related sequences extracted by BLAST2 from the GenBank database.
In this assay, the strain gp-1 was inoculated to NBRIP with a shaking speed of 150 rpm at 30°C. The cells were collected at the 0, 6, 9, 12, and 24 h for extracting total RNA by using TriZol reagent. The cDNA synthesis was conducted using a CellAmpTM Direct RT-qPCR Kit (Takara Biotech, Beijing, China). To quantitative analysis of gene expression, primer 16S-F (5′–GCG GAG AGA AGT AGC TTG CT–3′) and primer 16S-R (5′–CCG ACT TAG GCT CAT CTA TTA G–3′) were used to amplify 16S rRNA gene; primer ppk-F (5′–CCA TTT ACC TTA CAT GCT CAG CT–3′) and primer ppk-R (5′–GGT CAA TCT GAA CAC CTG CTT–3′) were applied to amplify ppk gene; primer pqq-F (5′–GAT GCC TTA GCA GGT TCA AA–3′) and primer pqq-R (5′–CTC AAC TGT ATC TGC ATT AAG TT–3′) were employed to amplify pqq gene. The three pairs of primers were designed based on the complete gene sequence data obtained in this assay. The abundance of 16S rRNA gene was used as inner control for copy number estimation. The qPCR was performed using SYBR green PCR Master Mix (Applied Biosystems) and reactions were operated in a real time PCR system (Applied Biosystems 3) in the following steps: denaturation at 94°C for 10 min, followed by 40 cycles of denaturation at 94°C for 15 s, annealing at 60°C for 1 min. Additionally, the concentration of extracellular phosphorus at each time point was also measured. Simultaneously, the granule staining was conducted to investigate the existence of polyphosphate (Poly-P) according to the method described in previous literature (Wan et al., 2017).
Significant differences were obtained by the one-way analysis of variance (one-way ANOVA) with means compared using the Tukey test in IBM SPSS 19. A neighbor-joining phylogenetic tree with 1000 bootstrap replicates was built based on 16S rRNA gene sequence using MEGA6 (Tamura et al., 2013). The immobilized Pb was assessed using the equation: Pb immobilization efficiency = [HAc extractable Pb (Soil + Ca3(PO4)2)−HAc extractable Pb (Soil + Ca3(PO4)2 + PSB)] / HAc extractable Pb (Soil + Ca3(PO4)2) × 100%
During the four acclimation periods, the α-diversity indices including OTU (32–62), ACE (36–64), Chao1 (35–68), and Shannon index (1.30–2.23) decreased (Table 1), suggesting the species diversity gradually decreased. The Good’s coverage of all samples was 99.99%, suggesting the amplicon libraries could represent most of the species in the natural habitat.
The composition of bacterial community at both phylum and genus level presented large differences in four acclimation periods (Figure 1). At the phylum level, Proteobacteria was the dominant phylum in A1 (70.28%) and A2 (94.02%), and Bacteroidetes dominated in A3 (54.74%) and A4 (70.99%) (Figure 1A). while Firmicutes and Deinococcus–Thermus were the secondary level bacteria. At the genus level, Cellvibrio was the first dominant genus in A1 (41.72%) and A2 (52.3%), while Sphingobacterium was the supreme dominant genus in A3 (51.64%) and A4 (70.40%) (Figure 1B). The total relative abundances of Sphingobacterium, Cellvibrio, and Pseudomonas in A3 and A4 could reach to 93.88 and 98.2%, respectively. In these periods, Algoriphagus, Caulobacter, Caulobacteraceae, Cupriavidus, Deinococcus, Devosia, Flavobacterium, Hydrogenophaga, Niveispirillum, Paenibacillus, Phenylobacterium, Pseudomonas, Pseudorhodoferax, Ramlibacter, Rheinheimera, and Sediminibacterium exhibited low abundances. There results indicated that species diversity declined and bacterial community composition changed during long-term acclimation.
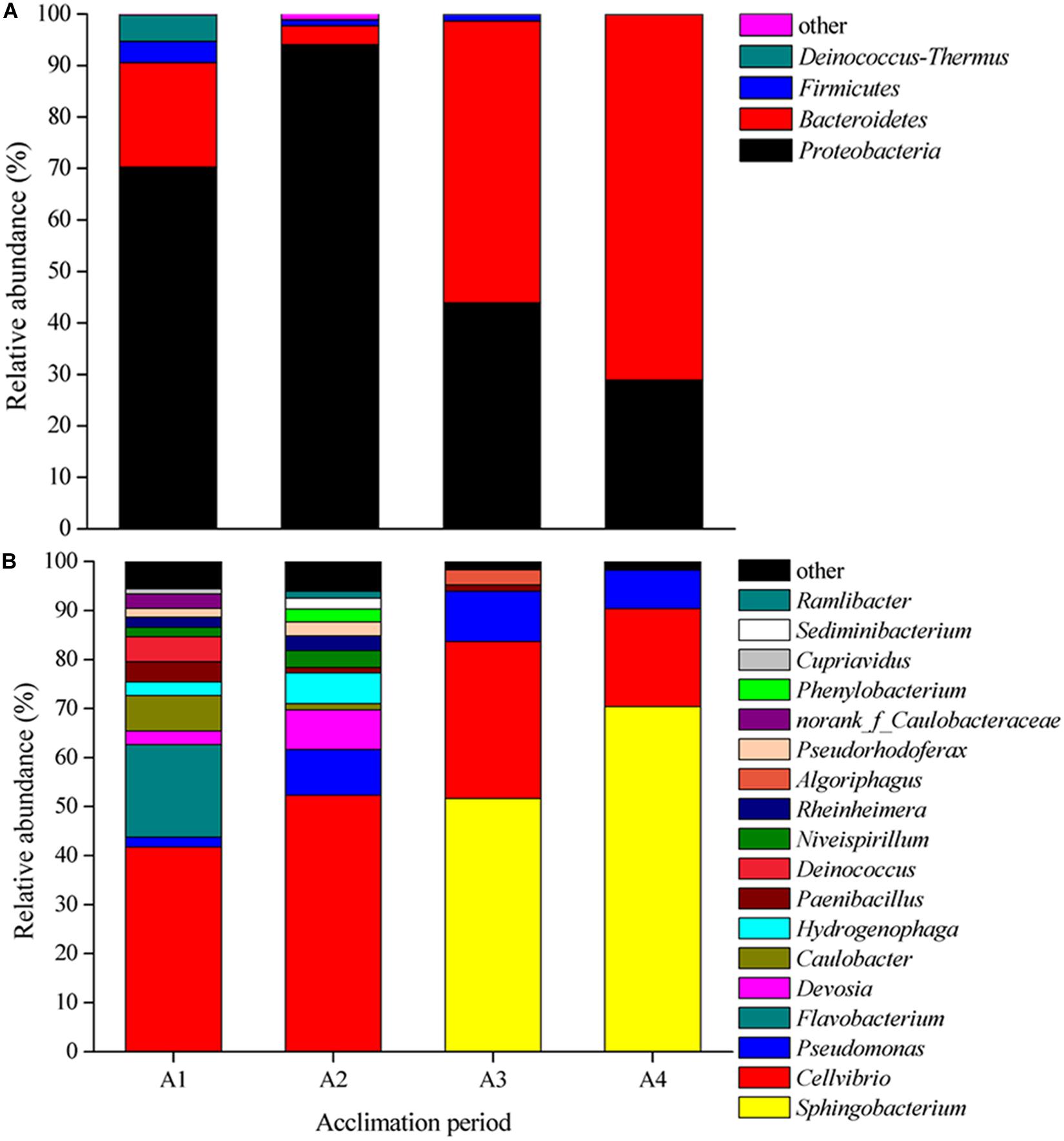
Figure 1. Shifts in bacterial community composition during four acclimation stages. (A) Relative abundance of 16S rRNA gene sequences classified to phylum level. (B) Relative abundance of 16S rRNA gene sequences classified to genus level.
These 18 PSB were identified as Acinetobacter (gp-1), Pseudomonas (gp-2 and Ap-3), Massilia (gp-3 and gp-6), Bacillus (gp-4, gp-7, gp-8, gp-11, gp-15, and Ap-10), Arthrobacter (gp-12), Stenotrophomonas (AP-1, Ap-6, and Ap-9), Ochrobactrum (Ap-2), and Cupriavidus (Ap-4 and Ap-8) based on 16S rRNA gene sequence (Table 2). The colony morphology of most strains presented circular, wet texture, and entire edge with the exception of Bacillus strain, and most of them exhibited off-yellow and crateriform (Table 2). A phylogenetic tree was built to reflect the phylogenetic distance between these PSB (Figure 2). The 18 strains were clustered into two distinct parts: Gram-negative bacteria and Gram-positive bacteria. Besides, these Gram-negative bacteria belonged to Proteobacteria, and Gram-positive bacteria belonged to Firmicutes and Actinobacteria.
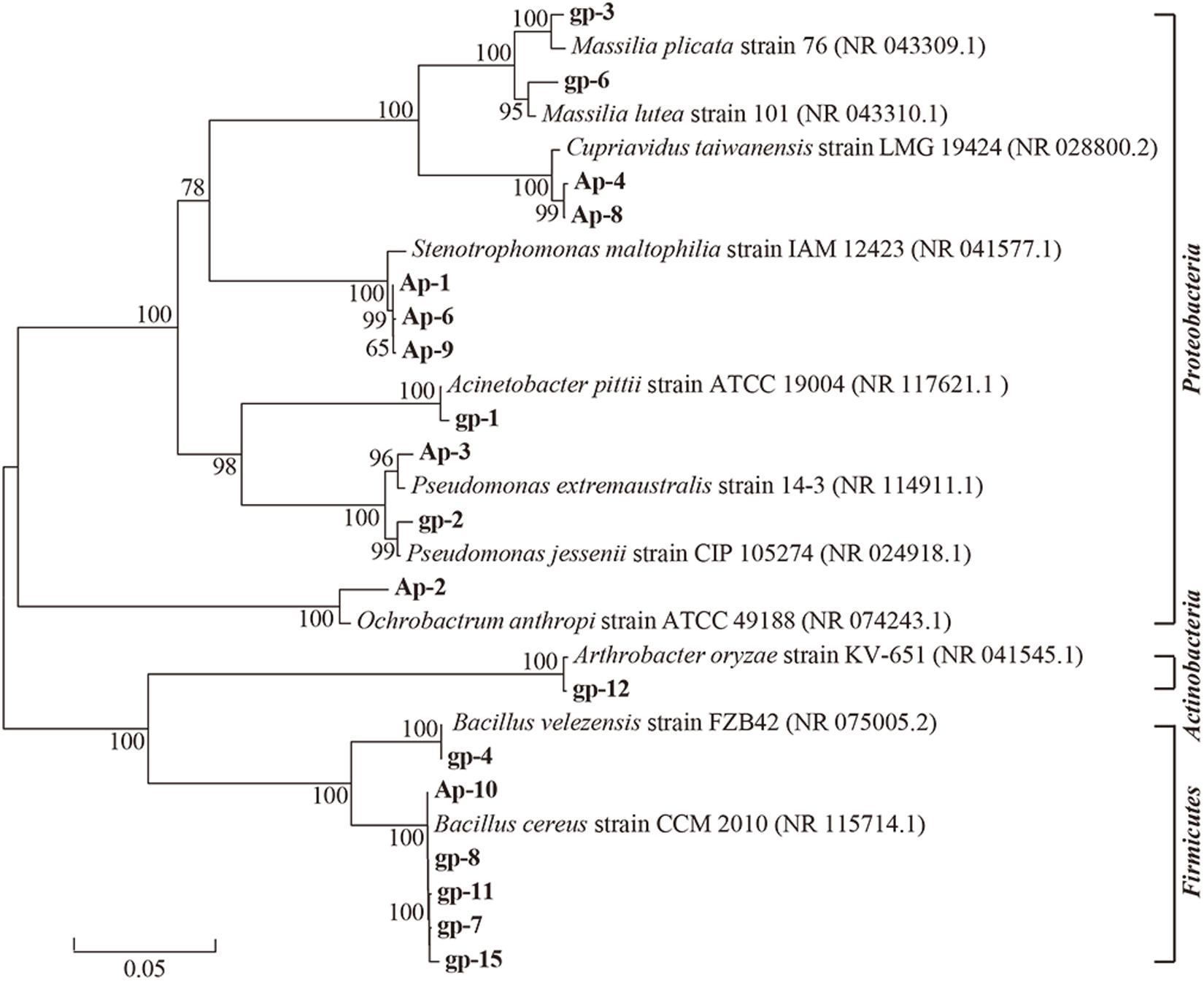
Figure 2. Neighbor-joining phylogenetic tree of 18 phosphorus solubilizing bacteria based on 16S rRNA gene sequences. The numbers at the nodes indicate the levels of bootstrap support based on data for 1000 replicates. The scale bar represents 0.05 sequence divergence.
The 18 PSB presented different abilities to utilize Ca3(PO4)2, phytate, FePO4, and AlPO4 (Figure 3). Basically, Ca3(PO4)2 was the optimal P source for these 18 PSB in liquid medium (Figure 3A), followed by AlPO4 (Figure 3D), FePO4 (Figure 3C), and phytate (Figure 3B). The P dissolving level of these 18 strains in NBRIP liquid medium separately containing Ca3(PO4)2, phytate, FePO4, and AlPO4, were 47.08–250.77, 1.19–7.91, 7.35–46.10, and 14.99–81.99 mg/L PO43–-P, respectively. Additionally, the P solubilizing level represented by HD/CD ratio of these 18 strains in NBRIP solid medium separately containing Ca3(PO4)2, phytate, FePO4, and AlPO4, were 0–4.62 (Figure 3E), 0–6.75 (Figure 3F), 0–13.58 (Figure 3G), and 1.00–8.50 (Figure 3H), respectively. The A. pittii gp-1 presented good performance for utilizing these four types of P sources in both liquid and solid medium, with P dissolving level ranging from 7.91 to 250.77 mg/L and from 3.80 to 7.11 mg/L, respectively. These results suggested that A. pittii gp-1 had great potentials for multiple P sources utilization.
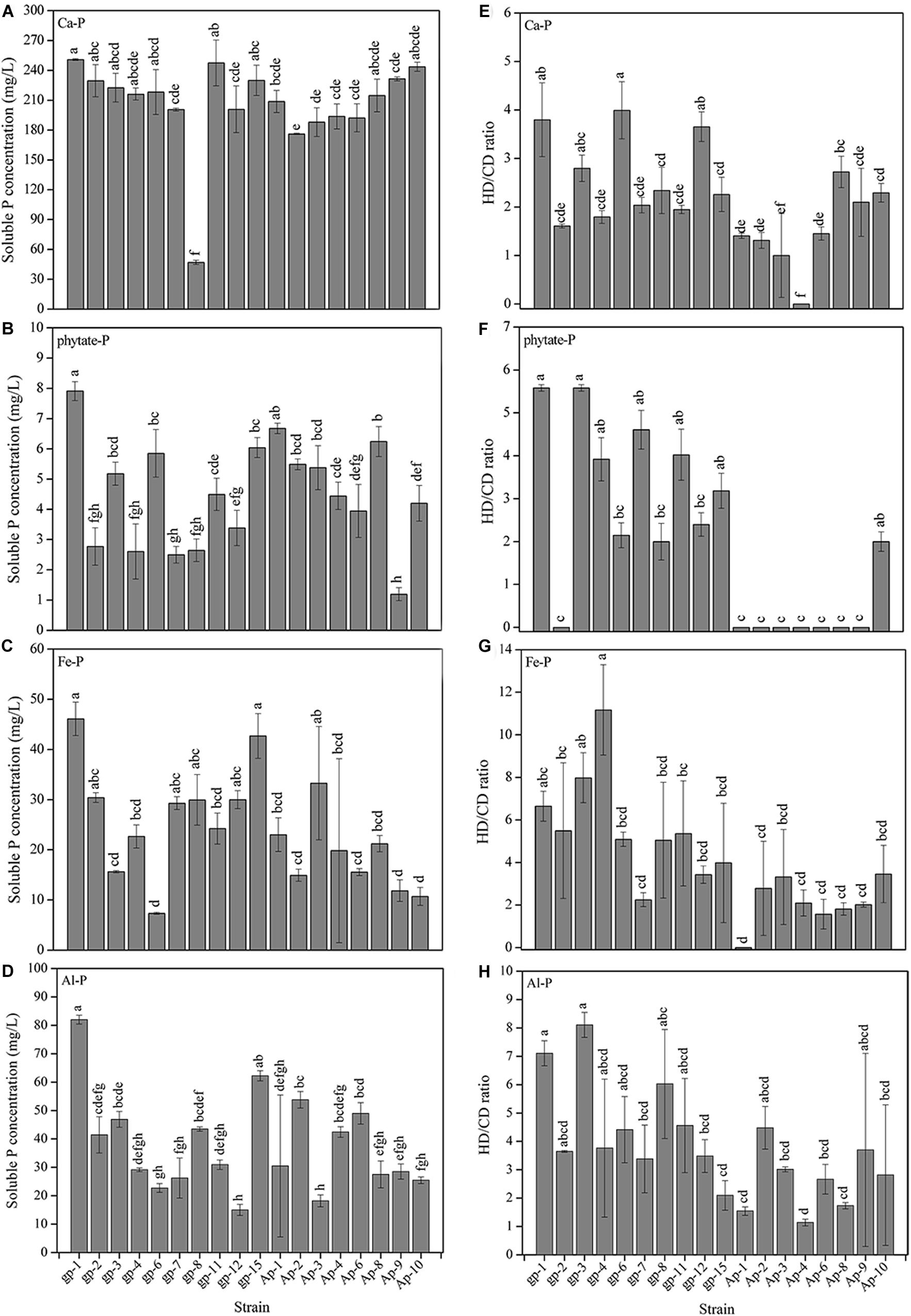
Figure 3. The P solubilizing levels of 18 PSB in liquid NBRIP medium containing Ca3(PO4)2 (A), sodium phytate (B), FePO4 (C), and AlPO4 (D), and in solid NBRIP medium containing Ca3(PO4)2 (E), sodium phytate (F), FePO4 (G), and AlPO4 (H). The results are the mean value of three replicates, error bars represent standard error. The lowercase letters above the columns denote significant level (P < 0.05).
The details of the content of WSP and AP under soil culture conditions after 30 days’ incubation are shown in Table 3. The WSP and AP content achieved the highest value under conditions with A. pittii gp-1 addition in the same soil incubation group. In S + B group, S + B + T group, and S + B + T + N group, the WSP content in soils with A. pittii gp-1 addition were 0.42, 0.52, and 0.80 mg/g, respectively; while AP content in soils with A. pittii gp-1 addition were 0.51, 0.81, and 1.64 mg/g, respectively. Additionally, we found the content of WSP and AP in S + B + T + N group were significantly higher than that in S + B group and S + B + T group (P < 0.05) (Table 3). Most PSB with the addition of Ca3(PO4)2 could also significantly increase the content of WSP and AP. These results indicated that A. pittii gp-1 presented great utilization potential for enriching soil plant-absorbable P in practical condition.
The content of WSP and AP and the Pb immobilization efficiency increased as the addition rate of tricalcium phosphate increased (Figure 4). When the addition ratio of tricalcium phosphate was 10%, the WSP and AP reached the highest value of 0.62 and 1.05 mg/L, respectively. The Pb immobilization efficiency increased from 7.16% at tricalcium phosphate addition ratio of 1 to 22.80% at tricalcium phosphate addition rate of 10%. Additionally, the Pb immobilization efficiency was significantly correlated with WSP (r = 0.987, P < 0.01) and AP (r = 0.990, P < 0.01) (Table 4).
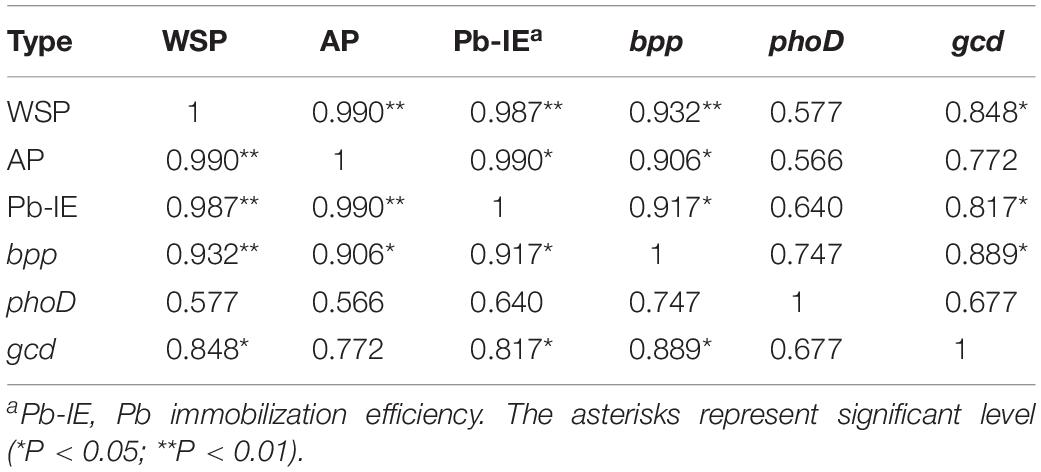
Table 4. Pearson correlation analysis among phosphorus content, Pb immobilization efficiency and gene abundance.
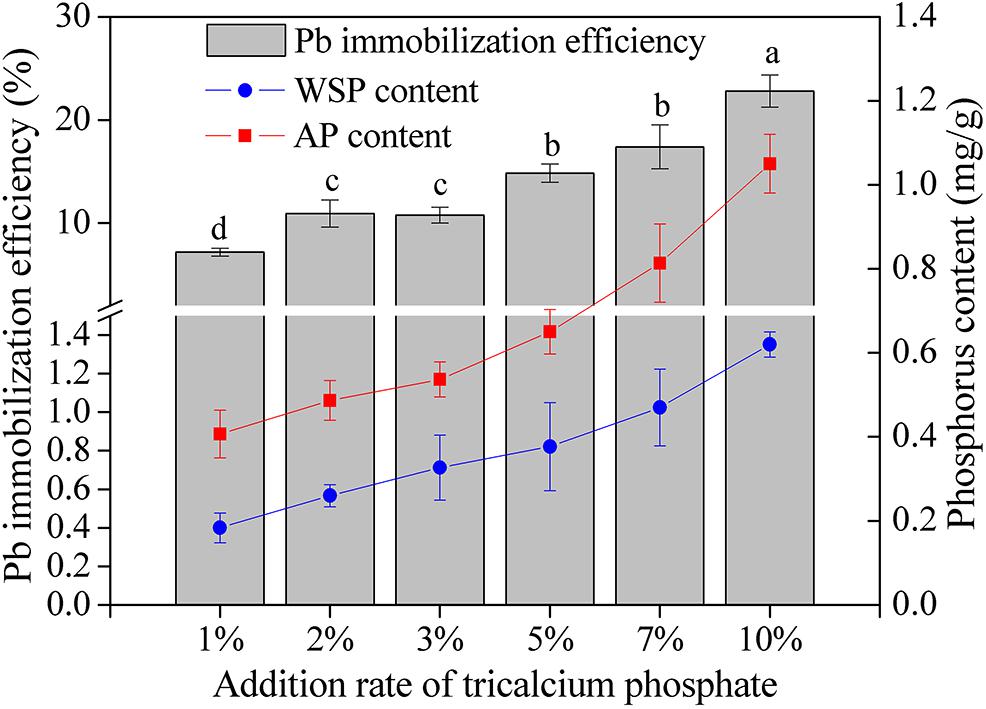
Figure 4. The Pb immobilization efficiency and phosphorus content in soils with different addition rate of tricalcium phosphate. The results are the mean value of three replicates, error bars represent standard error. The lowercase letters above the columns denote significant level (P < 0.05).
The abundances of 16S rRNA genes, gcd, bpp, and phoD in soils without both strain gp-1 and Ca3(PO4)2 addition were 6.37 × 109, 4.08 × 106, 3.38 × 106, and 1.34 × 107 copies/g, respectively. The addition of tricalcium phosphate and A. pittii gp-1 presented different effects on the abundances of P-cycling-related genes (Figure 5). The 16S rRNA gene abundance (6.76 × 109–9.12 × 1011 copies/g) slightly increased as the Ca3(PO4)2 addition ratio increased in soils without gp-1 addition (P > 0.05; Figure 5A). While 16S rRNA gene abundance in soils with gp-1 addition was significantly higher than that in soils without gp-1 addition and in soils without both gp-1 and Ca3(PO4)2 addition (P < 0.05), and the 16S rRNA gene abundance increased from 1.58 × 1011 copies/g at Ca3(PO4)2 addition ratio of 1% to 4.47 × 1011 copies/g at Ca3(PO4)2 addition ratio of 10%. These results indicated that the significant increase of bacterial abundance might be due to the retained PSB A. pittii gp-1. Besides, gcd gene abundance in gp-1 added soils (ranging from 8.64 × 106 copies/g to 2.19 × 108 copies/g) was significantly higher than that in no gp-1 added soils (ranging from 4.11 × 106 copies/g to 1.76 × 107 copies/g) and in soils without both gp-1 and Ca3(PO4)2 addition (P < 0.05; Figure 5B). At the same ratio of tricalcium phosphate addition, we found that bpp gene abundance in soils with gp-1 addition (3.69 × 106–1.26 × 107 copies/g) was higher than that in soils without gp-1 addition (3.36 × 106–5.80 × 106 copies/g) and in soils without both gp-1 and Ca3(PO4)2 addition (P > 0.05; Figure 5C). However, the phoD gene abundance in with gp-1 added soils (1.61 × 107–2.75 × 107 copies/g) was slightly higher than that in without gp-1 added soils (1.33 × 107–1.72 × 107 copies/g) and in without both gp-1 and Ca3(PO4)2 added soils (P > 0.05; Figure 5D). The Pb immobilization efficiency was significantly correlated with bpp (r = 0.917; P < 0.05) and gcd (r = 0.817; P < 0.05) gene abundance (Table 4). These results indicated that P solubilization was responsible for the immobilization of Pb. In addition, gcd could be amplified from A. pittii gp-1 using the primers described above, while bpp and phoD did not. These results implied that the addition of A. pittii gp-1 could increase the abundances of organic P-cycling-related genes.
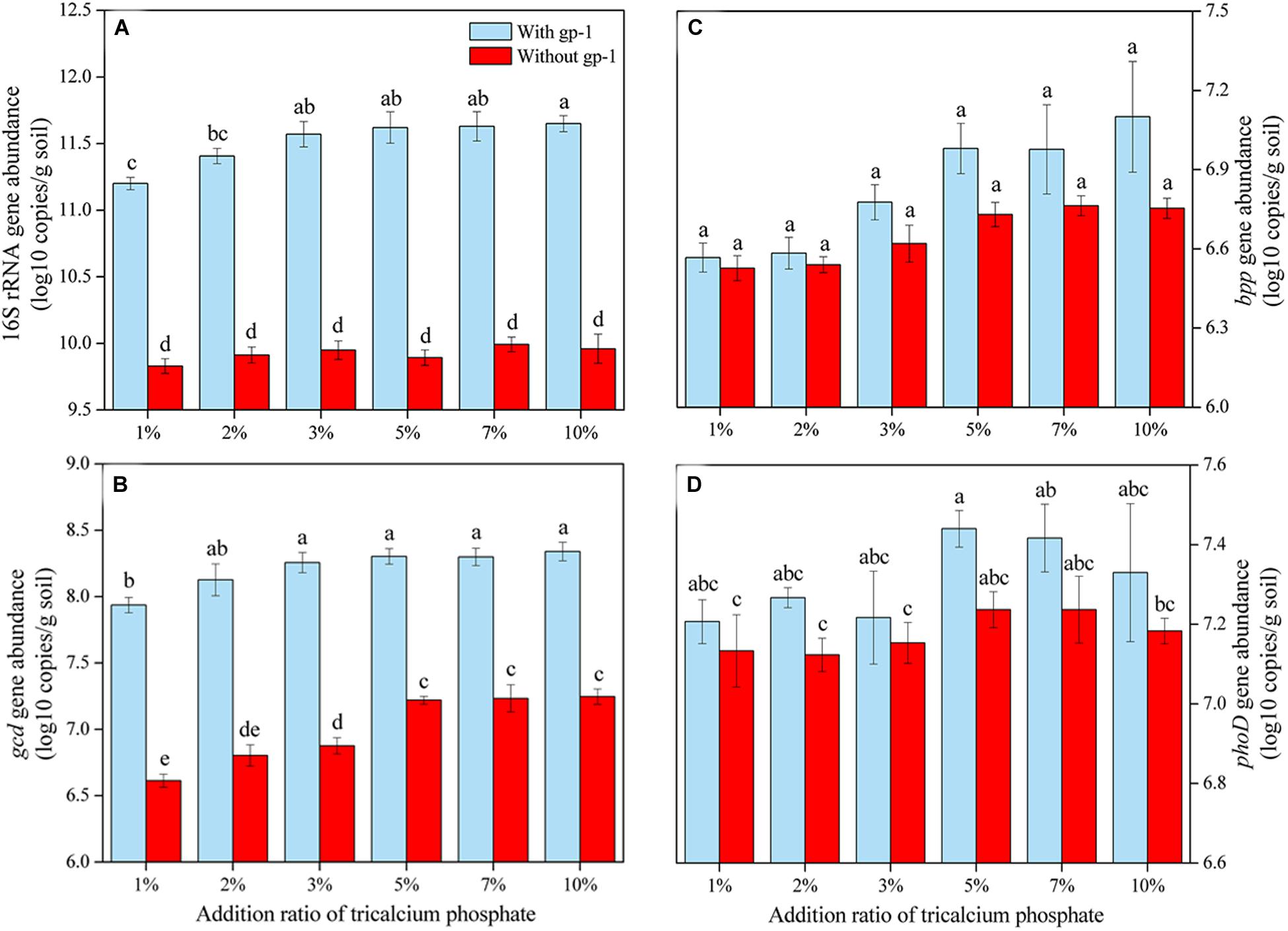
Figure 5. The abundances of 16S rRNA gene (A), gcd gene (B), bpp gene (C), and phoD gene (D) in soils with different addition rate of tricalcium phosphate. The results are the mean value of three replicates, error bars represent standard error. The lowercase letters above the columns denote significant level (P < 0.05).
The complete sequences of ppk and pqq genes in A. pittii gp-1 presented the highest similarities with complete sequences of ppk and pqq genes in Acinetobacter lactucae strain OTEC-02 (accession number: CP020015.1) (Figure 6), with corresponding similarities of 96.01% (Figure 6A) and 94.98% (Figure 6B), respectively. The expression levels of P-cycling-related genes and concentration of free phosphorus presented dynamic change during strain gp-1 incubation (Figure 7). The relative expression level of pqq gene significantly (P < 0.05) increased 18.18 times at the 6th h, and then declined to 4.99 times at the 24th h (Figure 7A). While the relative expression level of ppk gene dramatically (P < 0.05) increased, and achieved the highest value of 5.23 times at the 24th h. Similarly, the concentration of extracellular phosphorus remarkably increased from 0.29 mg/L at the initial period to 74.58 mg/L at the 24th h (P < 0.05; Figure 7B). The extracellular phosphorus concentration was positively correlated with the relative expression levels of ppk (r = 0.951; P < 0.05) and pqq (r = 0.495; P > 0.05). In addition, granule staining presented positive reaction (Figure 7C), suggesting Poly-P existed in A. pittii gp-1 cells. These results implied that ppk and pqq genes were closely correlated with phosphorus cycling, and the process of extracellular inorganic insoluble phosphorus solubilization was coupled with the process of polyphosphate synthesis.
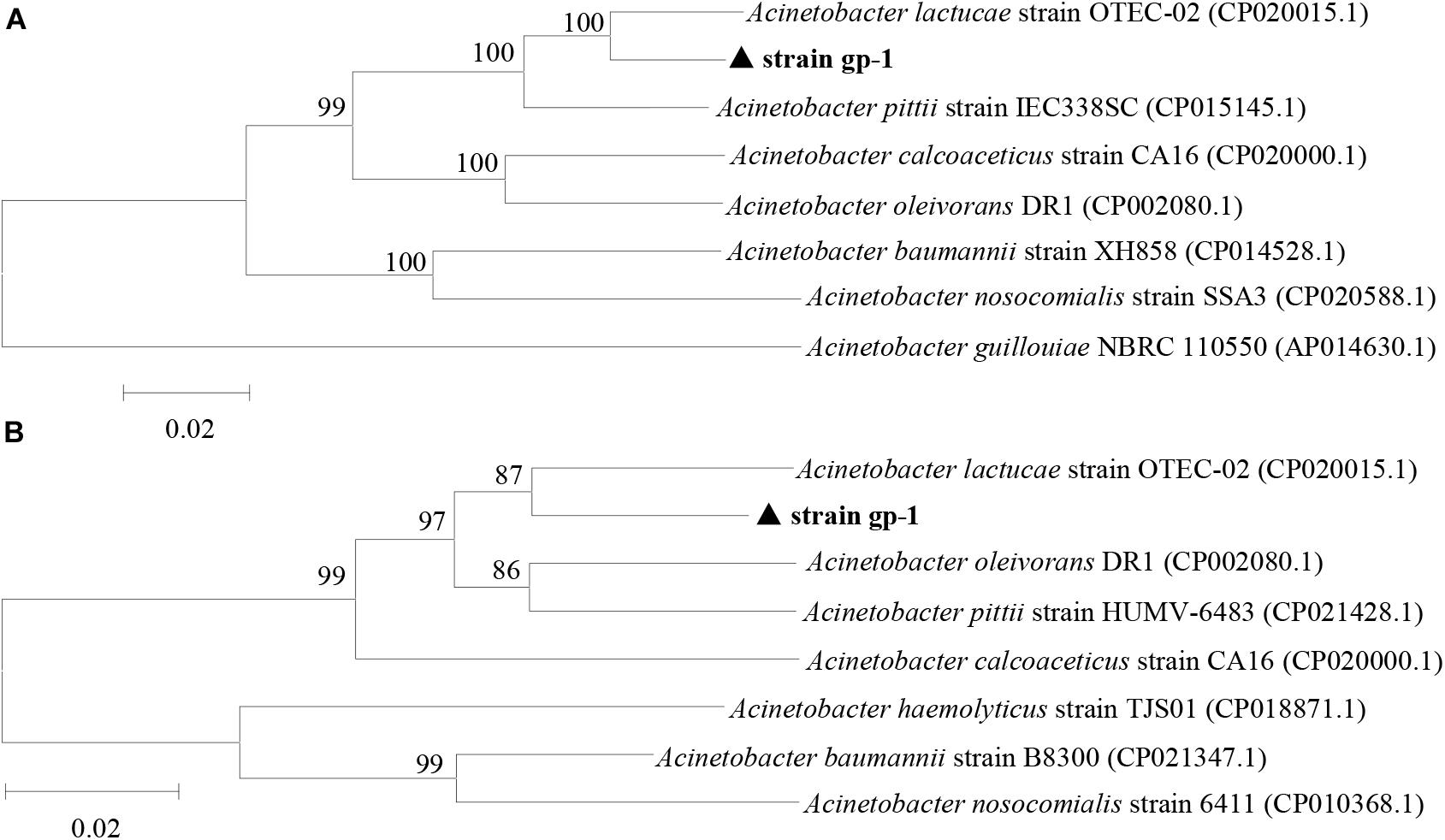
Figure 6. Neighbor-joining phylogenetic tree of ppk gene (A) and pqq gene (B) based on sequence similarity. The numbers at the nodes indicate the levels of bootstrap support based on data for 1000 replicates. The scale bar represents 2% sequence divergence.
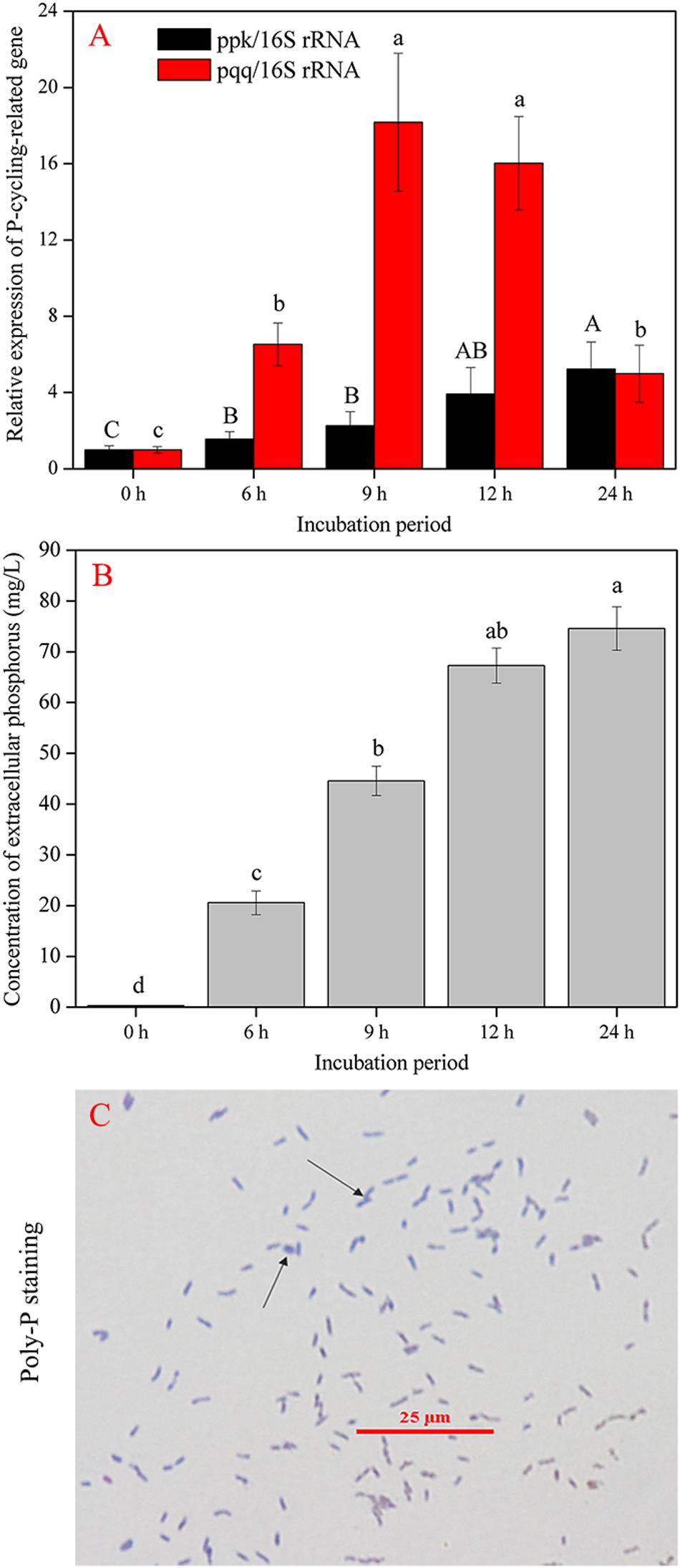
Figure 7. Gene abundance and phosphorus determination during 24 h. (A) shows the shift in P-cycling-related gene abundance. (B) reflects the concentration of extracellular phosphorus. (C) shows the granular staining. The results are the mean value of three replicates, error bars represent standard error. The lowercase letters above the columns denote significant level (P < 0.05).
In this study, we found big shifts in bacterial community composition during four acclimation stages. Some PSB have been identified belonging to Proteobacteria, Bacteroidetes, Firmicutes, and Deinococcus–Thermus (Vyas and Gulati, 2009; Shrivastava et al., 2010; Hanif et al., 2015; Li et al., 2017), which were also the major bacterial phylum in our study. Some genera, such as Sphingobacterium and Cellvibrio, have not been reported as PSB, however, they were the major genus in four acclimation stages. This might be due to they are potential PSB could not be cultured at present, or they are closely related with PSB.
After four rounds of acclimation, we gained 18 PSB belonging to eight genera including Acinetobacter, Arthrobacter, Bacillus, Cupriavidus, Massilia, Ochrobactrum, Pseudomonas, and Stenotrophomonas. Previous literatures have reported that some PSB belong to these genera (Yu et al., 2011; Imran et al., 2014; Liu et al., 2014; Silva et al., 2017), but most studies have not reported PSB possessing multiple P sources utilizing abilities. For instance, strain gp-1 identified as Acinetobacter genus in our study presented good performance for solubilizing Ca3(PO4)2, FePO4, and AlPO4, and for digesting phytate, with corresponding P solubilizing levels were 250.77, 46.10, 81.99, and 7.91 mg/L PO43–-P, respectively. The P solubilizing capacity of A. pittii gp-1 is higher than that of other reported bacteria, such as Acinetobacter baylyi W16 for Ca3(PO4)2 (91 mg/L) (Yu et al., 2011), Enterobacter sp. C for Ca3(PO4)2 (100 mg/L) (Melo et al., 2018), Bacillus sp. AB066338 for Ca3(PO4)2 (137 mg/L) (Liu et al., 2014), Burkholderia seminalis. PSB7 for Ca3(PO4)2 (145 mg/L) (Panhwar et al., 2014), Pantoea dispersa Cav.cy3 for Ca3(PO4)2, FePO4, and AlPO4 (<50 mg/L) (Chen et al., 2014), Pseudomonas sp. for phytate (0.40 mg/L) (Maougal et al., 2014), and Paenibacillus elgii B56 for AlPO4 (16 mg/L) and Bacillus megaterium B119 for phytate (4.9 mg/g) (Oliveira et al., 2009). Some PSB present higher P solubilizing ability than that of A. pittii gp-1, such as Pseudomonas trivialis BIHB 745 for Ca3(PO4)2 (827 mg/L) (Vyas and Gulati, 2009), Serratia marcescens RP8 for Ca3(PO4)2 (974 mg/L) (Misra et al., 2012), Acinetobacter sp. ASL12 for Ca3(PO4)2 (717 mg/L) (Liu et al., 2014), Bacillus cereus SPC09 for phytate (579.01 mg/L) (Zhang et al., 2015), Saccharomyces cerevisiae CICIMY008 for phytate (93 mg/L) (Chen et al., 2016), Burkholderia cepacia B116 for phytate (52.7 mg/L) (Oliveira et al., 2009). However, the multiple P sources utilizing ability promises gp-1 itself easily get used to environment, and therefore exhibits great potential for phosphate chemical industry and agro-ecosystems. Additionally, the stepwise acclimation by using Ca3(PO4)2, phytate, FePO4, and AlPO4 provides a useful approach to obtain PSB possessing multiple P sources utilizing capacity.
Many studies have reported that PSB contribute to the immobilization of heavy metal. For instance, PSB Pantoea sp. CS2-B1 and Enterobacter cloacae SM1-B1 can immobilize Pb (Park et al., 2011); Enterobacter sp. could enhance Pb immobilization (Chen et al., 2019); Serratia marcescens OPDB3-6-1 shows good capacity for the immobilization of Pb, Cd, and Cu (Zhu et al., 2019); Pseudomonas sp. strain PG-12 exhibits good performance for Pb immobilization (Manzoor et al., 2019); Leclercia adecarboxylata B3 and Pseudomonas putida F2-1 can turn soluble Pb(II) into insoluble form (Teng et al., 2019). The promotion mechanism of heavy metal immobilization by PSB has been acknowledged, namely PSB produce soluble phosphorus and metabolite, and then these products could bind with heavy metal ions and turn into insoluble form (Park et al., 2011; Yuan et al., 2017; Teng et al., 2019; Zhu et al., 2019). The Pb immobilization efficiency was significantly correlated with soil available P and WSP in this study, which is similar with the findings that Pb immobilized amount and available phosphorus present significant correlation (Park et al., 2011; Yuan et al., 2017). Additionally, some studies have reported that the addition of inorganic phosphorus can increase the abundance of P-cycling-related gene (Silva et al., 2017; Hu et al., 2018; Wei et al., 2019), and alter bacterial community composition (Lagos et al., 2016; Luo et al., 2017; Wei et al., 2019). However, the effect of PSB addition on indigenous organic P-cycling-related gene abundance is rarely reported. To our knowledge, we first report that the addition of A. pittii gp-1 can significantly increase the abundance of inorganic P-cycling-related gcd-harboring bacteria via direct input of A. pittii gp-1, and slightly increase the abundances of organic P-cycling-related bpp-harboring and phoD-harboring bacterial communities via changing indigenous bacterial community. Additionally, limited study has revealed the relationship between Pb immobilization and P-cycling-related gene abundance in soils with PSB addition. The Pb immobilization efficiency was significantly positively correlated with gcd-harboring bacterial abundance and bpp-harboring bacterial abundance, suggesting gcd-harboring bacteria and bpp-harboring bacteria might be responsible for Pb immobilization. This finding might be due to on the one hand A. pittii gp-1 can survive from harsh condition; on the other hand, A. pittii gp-1 can dissolve inorganic phosphorus for enriching the soil available phosphorus, therefore causing changes in bacterial community composition and abundances of P-cycling-related genes. Previous literatures have reported that bacteria in Acinetobacter genus harbor heavy metal resistance gene Saffarian et al., 2017; Zhou et al., 2019), and phytase encoded by bpp-harboring bacteria presents high resistance to heavy metal damage (Yung et al., 2014).
To explain the process of phosphorus metabolism of A. pittii gp-1, we quantified the expression of polyphosphate kinase gene (ppk) and pyrroloquinoline quinone (pqq). Our results revealed that the transformation of insoluble tricalcium phosphate into soluble phosphorus involved in a strong expression of pqq gene. This finding is in line with previous reports that the expression of pqq gene is closely correlated with the solubilization of inorganic phosphorus (Ogut et al., 2010; Wagh et al., 2014; Oteino et al., 2015). We also observed an increasing expression level of ppk gene, which was closely correlated with the content of extracellular P. In addition, we found the existence of polyphosphate by using granular staining. To our knowledge, this is the first report that the solubilization of inorganic insoluble phosphorus is coupled with the synthesis of polyphosphate. It has been reported that polyphosphate regarded as high-energy compound could be hydrolyzed when phosphorus accumulating bacteria undergo undernourished conditions (Wan et al., 2017; Li and Dittrich, 2019; Zhong et al., 2019). Previous literatures have reported that some bacteria belonging to Acinetobacter genus harbor the ability to synthesize polyphosphate and could be regarded as phosphorus accumulating bacteria (Keating et al., 2016; Han et al., 2018). Therefore, we can conclude that one part of soluble phosphorus can be used for the formation of bacterial substance (e.g., DNA and RNA), and another part of soluble phosphorus can be applied for the synthesis of polyphosphate during inorganic phosphorus solubilization. However, the process of polyphosphate synthesis should be inhibited or blocked when use PSB in agro-ecosystems, since polyphosphate is not plant-absorbable phosphorus.
In this study, we found big changes in diversity and composition of bacterial community during four acclimation periods. Cellvibrio was dominant genus in the first and second rounds of acclimation, while Sphingobacterium was the dominant genus in the third and fourth rounds of acclimation. A total of 18 PSB belonging to Acinetobacter, Arthrobacter, Bacillus, Cupriavidus, Massilia, Ochrobactrum, Pseudomonas, and Stenotrophomonas presented multiple phosphorus sources utilizing capabilities. The isolate A. pittii gp-1 exhibited good performance for utilizing Ca3(PO4)2, phytate, FePO4, and AlPO4 in both solid and liquid medium. Additionally, strain gp-1 could significantly increase the content of soil available phosphorus and presented good capacity in immobilizing Pb. Simultaneously, the addition of A. pittii gp-1 could increase the abundance of P-cycling-related genes including gcd, bpp, and phoD. To our knowledge, we first report that the solubilization of tricalcium phosphate by phosphorus solubilizing bacterium belonging to Acinetobacter is coupled with the synthesis of polyphosphate.
Therefore, soil-derived A. pittii gp-1 possessing multiple P sources utilizing ability and Pb immobilization capacity exhibits great potentials in agro-ecosystems.
The datasets generated for this study can be found in NCBI BioProject PRJNA527148, accession numbers SRR8731888–SRR8731891.
WW designed the whole experiment. WW, YQ, HW, WZ, HH, JT, and YW conducted all the experiments. WW analyzed the data and wrote the manuscript. DH revised the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (grant number 31070087) and the Fundamental Research Funds for the Central Universities (grant numbers 2662015PY016 and 2662015PY116).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Bhattacharyya, P., Nayak, A. K., Shahid, M., Tripathi, R., Mohanty, S., Kumar, A., et al. (2015). Effects of 42-year long-term fertilizer management on soil phosphorus availability, fractionation, adsorption-desorption isotherm and plant uptake in flooded tropical rice. Crop J. 3, 387–395. doi: 10.1016/j.cj.2015.03.009
Chen, H., Zhang, J., Tang, L., Su, M., Tian, D., Zhang, L., et al. (2019). Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 127, 395–401. doi: 10.1016/j.envint.2019.03.068
Chen, Q., and Liu, S. (2019). Identification and characterization of the phosphate-solubilizing bacterium Pantoea sp. S32 in reclamation soil in Shanxi, China. Front. Microbiol. 10:2171. doi: 10.3389/fmicb.2019.02171
Chen, X., Xiao, Y., Shen, W., Govender, A., Zhang, L., Fan, Y., et al. (2016). Display of phytase on the cell surface of Saccharomyces cerevisiae to degrade phytate phosphorus and improve bioethanol production. Appl. Microbiol. Biotechnol. 100, 2449–2458. doi: 10.1007/s00253-015-7170-4
Chen, Y., Fan, J. B., Du, L., Xu, H., Zhang, Q. H., and He, Y. Q. (2014). The application of phosphate solubilizing endophyte Pantoea dispersa triggers the microbial community in red acidic soil. Appl. Soil Ecol. 84, 235–244. doi: 10.1016/j.apsoil.2014.05.014
Chen, Z., Ma, S., and Liu, L. (2008). Studies on phosphorus solubilizing activity of a strain of phosphobacteria isolated from chestnut type soil in China. Bioresour. Technol. 99, 6702–6707. doi: 10.1016/j.biortech.2007.03.064
Choi, O., Kim, J., Kim, J. G., Jeong, Y., Moon, J. S., Park, C. S., et al. (2008). Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens. Plant Physiol. 146, 657–668. doi: 10.1104/pp.107.112748
Cleton-Jansen, A. M., Goosen, N., Fayet, O., and van de Putte, P. (1990). Cloning, mapping, and sequencing of the gene encoding Escherichia coli quinoprotein glucose dehydrogenase. J. Bacteriol. 172, 6308–6315. doi: 10.1128/jb.172.11.6308-6315.1990
Farhat, M. B., Fourati, A., and Chouayekh, H. (2013). Coexpression of the pyrroloquinoline quinone and glucose dehydrogenase genes from Serratia marcescens CTM 50650 conferred high mineral phosphate-solubilizing ability to Escherichia coli. Appl. Biochem. Biotechnol. 170, 1738–1750. doi: 10.1007/s12010-013-0305-0
Fierer, N., Jackson, J. A., Vilgalys, R., Jackson, R. B. (2005). Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl. Environ. Microbiol. 71, 4117–4120. doi: 10.1128/AEM.71.7.4117-4120.2005
Fraser, T. D., Lynch, D. H., Bent, E., Entz, M. H., and Dunfield, K. E. (2015). Soil bacterial phoD gene abundance and expression and long-term management. Soil Biol. Biochem. 88, 137–147. doi: 10.1016/j.soilbio.2015.04.014
Goosen, N., Huinen, R. G. M., and Van de Putte, P. (1992). A 24-amino-acid polypeptide is essential for the biosynthesis of the coenzyme pyrrolo-quinoline-quinone. J. Bacteriol. 174, 1426–1427. doi: 10.1128/jb.174.4.1426-1427.1992
Han, Y. H., Fu, T., Wang, S. S., Yu, H. T., Xiang, P., Zhang, W. X., et al. (2018). Efficient phosphate accumulation in the newly isolated Acinetobacter Junii strain LH4. 3 Biotech. 8:313. doi: 10.1007/s13205-018-1338-4
Hanif, M. K., Hameed, S., Imran, A., Naqqash, T., Shahid, M., and van Elsas, J. D. (2015). Isolation and characterization of a β-propeller gene containing phosphobacterium Bacillus subtilis strain KPS-11 for growth promotion of potato (Solanum tuberosum L.). Front. Microbiol. 6:583. doi: 10.3389/fmicb.2015.00583
Hu, Y., Xia, Y., Sun, Q., Liu, K., Chen, X., Ge, T., et al. (2018). Effects of long-term fertilization on phoD-harboring bacterial community in Karst soils. Sci. Total Environ. 62, 53–63. doi: 10.1016/j.scitotenv.2018.01.314
Huang, H., Shi, P., Wang, Y., Luo, H., Shao, N., Wang, G., et al. (2009). Diversity of beta-propeller phytase genes in the intestinal content of grass crap provides insight into the release of major phosphorus from phytate in nature. Appl. Environ. Microbio. 75, 1508–1516. doi: 10.1128/AEM.02188-08
Imran, A., Saadalla, M. J. A., Khan, S. U., Mirza, M. S., Malik, K. A., and Hafeez, F. Y. (2014). Ochrobactrum sp Pv272 exhibits multiple traits of plant growth promotion, biodegradation and N-acyl-homoserine-lactone quorum sensing. Ann. Microbiol. 64, 1797–1806. doi: 10.1007/s13213-014-0824-0
Irshad, U., and Yergeau, E. (2018). Bacterial subspecies variation and nematode grazing change P dynamics in the wheat rhizosphere. Front. Microbiol. 9:1990. doi: 10.3389/fmicb.2018.01990
Ishige, K., and Noguchi, T. (2000). Inorganic polyphosphate kinase and adenylate kinase participate in the polyphosphate: AMP phosphotransferase activity of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 97, 14168–14171. doi: 10.1073/pnas.011518098
Jorquera, M. A., Saavedra, N., Maruyama, F., Richardson, A. E., Crowley, D. E., del, C., et al. (2013). Phytate addition to soil induces changes in the abundance and expression of Bacillus β-propeller phytase genes in the rhizosphere. FEMS Microbiol. Ecol. 83, 352–360. doi: 10.1111/j.1574-6941.2012.01480.x
Keating, C., Chin, J. P., Hughes, D., Manesiotis, P., Cysneiros, D., Mahony, T., et al. (2016). Biological phosphorus removal during high-rate, low-temperature, anaerobic digestion of wastewater. Front. Microbiol. 7:226. doi: 10.3389/fmicb.2016.00226
Krämer, S., and Green, D. M. (2000). Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biol. Biochem. 32, 179–188. doi: 10.1016/s0038-0717(99)00140-6
Lagos, L. M., Acuña, J. J., Maruyama, F., Ogram, A., de la Luz Mora, M., and Jorquera, M. A. (2016). Effect of phosphorus addition on total and alkaline phosphomonoesterase-harboring bacterial populations in ryegrass rhizosphere microsites. Biol. Fertil. Soils 52, 1007–1019. doi: 10.1007/s00374-016-1137-1
Li, J., and Dittrich, M. (2019). Dynamic polyphosphate metabolism in cyanobacteria responding to phosphorus availability. Environ. Microbiol. 21, 572–583. doi: 10.1111/1462-2920.14488
Li, Y., Ai, M. J., Sun, Y., Zhang, Y. Q., and Zhang, J. Q. (2017). Spirosoma lacussanchae sp. nov., a phosphate-solubilizing bacterium isolated from a freshwater reservoir. Int. J. Syst. Evol. Microbiol. 67, 3144–3149. doi: 10.1099/ijsem.0.001778
Lim, B. L., Yeung, P., Cheng, C., and Hill, J. E. (2007). Distribution and diversity of phytate-mineralizing bacteria. ISME J. 1, 321–330. doi: 10.1038/ismej.2007.40
Liu, F. P., Liu, H. Q., Zhou, H. L., Dong, Z. G., Bai, X. H., Bai, P., et al. (2014). Isolation and characterization of phosphate-solubilizing bacteria from betel nut (Areca catechu) and their effects on plant growth and phosphorus mobilization in tropical soils. Bio. Fertil. Soils 50, 927–937. doi: 10.1007/s00374-014-0913-z
Liu, J., Cade-Menun, B. J., Yang, J., Hu, Y., Liu, C. W., Tremblay, J., et al. (2018). Long-term land use affects phosphorus speciation and the composition of phosphorus cycling genes in agricultural soils. Front. Microbiol. 9:1643. doi: 10.3389/fmicb.2018.01643
Liu, Y., Cao, X., Li, H., Zhou, Z., Wang, S., Wang, Z., et al. (2017). Distribution of phosphorus-solubilizing bacteria in relation to fractionation and sorption behaviors of phosphorus in sediment of the three gorges reservoir. Environ. Sci. Pollut. Res. 24, 17679–17687. doi: 10.1007/s11356-017-9339-0
Liu, Z., Li, Y. C., Zhang, S., Fu, Y., Fan, X., Patel, J. S., et al. (2015). Characterization of phosphate-solubilizing bacteria isolated from calcareous soils. Appl. Soil Ecol. 96, 217–224. doi: 10.1016/j.apsoil.2015.08.003
Luo, G., Ling, N., Nannipieri, P., Chen, H., Raza, W., Wang, M., et al. (2017). Long-term fertilisation regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol. Fertil. Soils 53, 375–388. doi: 10.1007/s00374-017-1183-3
Manzoor, M., Abid, R., Rathinasabapathi, B., De Oliveira, L. M., da Silva, E., Deng, F., et al. (2019). Metal tolerance of arsenic-resistant bacterial and their ability to promote plant growth of Pteris vittata in Pb-contaminated soil. Sci. Total Environ. 660, 18–24. doi: 10.1016/j.scitotenv.2019.01.013
Maougal, R. T., Brauman, A., Plassard, C., Abadie, J., Djekoun, A., and Drevon, J. J. (2014). Bacterial capacities to mineralize phytate increase in the rhizosphere of nodulated common bean (Phaseolus vulgaris) under P deficiency. Eur. J. Soil Biol. 62, 8–14. doi: 10.1016/j.ejsobi.2014.02.006
Melo, J., Carvalho, L., Correia, P., de Souza, S. B., Dias, T., Santana, M., et al. (2018). Conventional farming disrupts cooperation among phosphate solubilising bacteria isolated from Carica papaya’s rhizosphere. Appl. Soil Ecol. 124, 284–288. doi: 10.1016/j.apsoil.2017.11.015
Misra, N., Gupta, G., and Jha, P. N. (2012). Assessment of mineral phosphate-solubilizing properties and molecular characterization of zinc-tolerant bacteria. J. Basic Microb. 52, 549–558. doi: 10.1002/jobm.201100257
Mori, H., Maruyama, F., Kato, H., Toyoda, A., Dozono, A., Ohtsubo, Y., et al. (2013). Design and experimental application of a novel non-degenerate universal primer set that amplifies prokaryotic 16S rRNA genes with a low possibility to amplify eukaryotic rRNA genes. DNA Res. 21, 217–227. doi: 10.1093/dnares/dst052
Neal, A. L., Rossmann, M., Brearley, C., Akkari, E., Guyomar, C., Clark, I. M., et al. (2017). Land-use influences phosphatase gene microdiversity in soils. Environ. Microbiol. 19, 2740–2753. doi: 10.1111/1462-2920.13778
Nesme, T., Metson, G. S., and Bennett, E. M. (2018). Global P flows through agricultural trade. Glob. Environ. Change 50, 133–141. doi: 10.1016/j.gloenvcha.2018.04.004
Ogbo, F. C. (2010). Conversion of cassava wastes for biofertilizer production using phosphate solubilizing fungi. Bioresour. Technol. 101, 4120–4124. doi: 10.1016/j.biortech.2009.12.057
Ogut, M., Er, F., and Kandemir, N. (2010). Phosphate solubilization potentials of soil Acinetobacter strains. Biol. Fert. Soils 46, 707–715. doi: 10.1007/s00374-010-0475-7
Oliveira, C. A., Alves, V. M. C., Marriel, I. E., Gomes, E. A., Scotti, M. R., Carneiro, N. P., et al. (2009). Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol. Biochem. 41, 1782–1787. doi: 10.1016/j.soilbio.2008.01.012
Olsen, S. R., Cole, C. V., Watanbe, F. S., and Dean, L. A. (1954). Estimation of Available Phosphorus in Soils by Extraction With Sodium Bicarbonate. Washington, DC: USDA.
Oteino, N., Lally, R. D., Kiwanuka, S., Lloyd, A., Ryan, D., Germaine, K. J., et al. (2015). Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 6:745. doi: 10.3389/fmicb.2015.00745
Panhwar, Q. A., Naher, U. A., Shamshuddin, J., Othman, R., Latif, M. A., and Ismail, M. R. (2014). Biochemical and molecular characterization of potential phosphate-solubilizing bacteria in acid sulfate soils and their beneficial effects on rice growth. PLoS One 9:e97241. doi: 10.1371/journal.pone.0097241
Park, J. H., Bolan, N., Megharaj, M., and Naidu, R. (2011). Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. J. Hazard. Mater. 185, 829–836. doi: 10.1016/j.jhazmat.2010.09.095
Patel, D. K., Murawala, P., Archana, G., and Kumar, G. N. (2011). Repression of mineral phosphate solubilizing phenotype in the presence of weak organic acids in plant growth promoting fluorescent Pseudomonads. Bioresour. Technol. 102, 3055–3061. doi: 10.1016/j.biortech.2010.10.041
Rasul, M., Yasmin, S., Suleman, M., Zaheer, A., Reitz, T., Tarkka, M. T., et al. (2019). Glucose dehydrogenase gene containing phosphobacteria for biofortification of phosphorus with growth promotion of rice. Microbiol. Res. 223–225, 1–12. doi: 10.1016/j.micres.2019.03.004
Reddy, C. S., Achary, V. M., Manna, M., Singh, J., Kaul, T., and Reddy, M. K. (2015). Isolation and molecular characterization of thermostable phytase from Bacillus subtilis (BSphyARRMK33). Appl. Biochem. Biotechnol. 175, 3058–3067. doi: 10.1007/s12010-015-1487-4
Saffarian, A., Touchon, M., Mulet, C., Tournebize, R., Passet, V., Brisse, S., et al. (2017). Comparative genomic analysis of Acinetobacter strains from murine colonic crypts. BMC Genomics 18:525. doi: 10.1186/s12864-017-3925-x
Sashidhar, B., and Podile, A. R. (2009). Transgenic expression of glucose dehydrogenase in Azotobacter vinelandii enhances mineral phosphate solubilization and growth of sorghum seedlings. Microbial. Biotechnol. 2, 521–529. doi: 10.1111/j.1751-7915.2009.00119.x
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mother: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1155/2012/605289
Shrivastava, M., Rajpurohit, Y. S., Misra, H. S., and D’Souza, F. S. (2010). Survival of phosphate-solubilizing bacteria against DNA damage agents. Can. J. Microbiol. 56, 822–830. doi: 10.1139/W10-067
Silva, U. C., Medeiros, J. D., Leite, L. R., Morais, D. K., Cuadros-Orellana, S., Oliveira, C. A., et al. (2017). Long-term rock phosphate fertilization impacts the microbial communities of maize rhizosphere. Front. Microbiol. 8:1266. doi: 10.3389/fmicb.2017.01266
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Tan, H., Wu, X., Xie, L., Huang, Z., Peng, W., and Gan, B. (2016). Identification and characterization of a mesophilic phytase highly resilient to high-temperatures from fungus-garden associated metagenome. Appl. Microbiol. Biotechnol. 100, 2225–2241. doi: 10.1007/s00253-015-7097-9
Teng, Z., Shao, W., Zhang, K., Huo, Y., and Li, M. (2019). Characterization of phosphate solubilizing bacteria isolated from heavy metal contaminated soils and their potential for lead immobilization. J. Environ. Manage. 231, 189–197. doi: 10.1016/j.jenvman.2018.10.012
Vyas, P., and Gulati, A. (2009). Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 9:174. doi: 10.1186/1471-2180-9-174
Wagh, J., Shah, S., Bhandari, P., Archana, G., and Kumar, G. N. (2014). Heterologous expression of pyrroloquinoline quinone (pqq) gene cluster confers mineral phosphate solubilization ability to Herbaspirillum seropedicae Z67. Appl. Microbiol. Biotechnol. 98, 5117–5129. doi: 10.1007/s00253-014-5610-1
Wan, W., He, D., and Xue, Z. (2017). Removal of nitrogen and phosphorus by heterotrophic nitrification-aerobic denitrification of a denitrifying phosphorus-accumulating bacterium Enterobacter cloacae HW-15. Ecol. Eng. 99, 199–208. doi: 10.1016/j.ecoleng.2016.11.030
Wang, Z., Xu, G., Ma, P., Lin, Y., Yang, X., and Cao, C. (2017). Isolation and characterization of a phosphorus-solubilizing bacterium from rhizosphere soils and its colonization of Chinese Cabbage (Brassica campestris ssp. chinensis). Front. Microbiol. 8:1270. doi: 10.3389/fmicb.2017.01270
Waterlot, C. (2018). Alternative approach to the standard, measurements and testing program used to establish phosphorus fractionation in soils. Analytic Climica Acta 1003, 26–33. doi: 10.1016/j.aca.2017.11.059
Wei, X., Hu, Y., Razavi, B. S., Zhou, J., Shen, J., Nannipieri, P., et al. (2019). Rare taxa of alkaline phosphomonoesterase-harboring microorganisms mediate soil phosphorus mineralization. Soil Biol. Biochem. 131, 62–70. doi: 10.1016/j.soilbio.2018.12.025
Wen, Z. L., Yang, M. K., Du, M. H., Zhong, Z. Z., Lu, Y. T., Wang, G. H., et al. (2019). Enrichments/derichments of root-associated bacteria related to plant growth and nutrition caused by the growth of an EPSPS-transgenic maize line in the field. Front. Microbiol. 10:1335. doi: 10.3389/fmicb.2019.01335
Yu, X., Liu, X., Zhu, T. H., Liu, G. H., and Mao, C. (2011). Isolation and characterization of phosphate-solubilizing bacteria walnut and their effect on growth and phosphorus mobilization. Biol. Fertil. Soils 47, 437–446. doi: 10.1007/s00374-011-0548-2
Yuan, Z., Yi, H., Wang, T., Zhang, Y., and Zhu, X. (2017). Application of phosphate solubilizing bacteria in immobilization of Pb and Cd in soil. Environ. Sci. Pollut. Res. 24, 21877–21884. doi: 10.1007/s11356-017-9832-5
Yung, M. C., Ma, J., Salemi, M. R., Phinney, B. S., Bowman, G. R., and Jiao, Y. (2014). Shotgun proteomic analysis unveils survival and detoxification strategies by Caulobacter crescentus during exposure to uranium, chromium, and cadmium. J. Proteome Res. 13, 1833–1847. doi: 10.1021/pr400880s
Zhang, S., Liao, S. A., Yu, X., Lu, H., Xian, J. A., Guo, H., et al. (2015). Microbial diversity of mangrove sediment in Shenzhen Bay and gene cloning characterization of an isolated phytase-producing strain of SPC09 B. cereus. Appl. Microbiol. Biotechnol. 99, 5339–5350. doi: 10.1007/s00253-015-6405-8
Zhong, C., Zhang, P., Liu, C., Liu, M., Chen, W., Fu, J., et al. (2019). The PolS-PolR two-component system regulates genes involved in poly-P metabolism and phosphate transport in Microlunatus phosphovorus. Front. Microbiol. 10:2127. doi: 10.3389/fmicb.2019.02127
Zhou, Q., Wang, M., Zhong, X., Liu, P., Xie, X., Wangxiao, J., et al. (2019). Dissemination of resistance genes in duck/fish polyculture ponds in Guangdong Province: correlations between Cu and Zn and antibiotic resistance genes. Environ. Sci. Pollut. R. 26, 8182–8193. doi: 10.1007/s11356-018-04065-2
Keywords: Acinetobacter pittii gp-1, multiple phosphorus source utilizing capacity, Pb immobilization, P-cycling-related gene, phosphorus solubilizing bacteria, ppk and pqq genes
Citation: Wan W, Qin Y, Wu H, Zuo W, He H, Tan J, Wang Y and He D (2020) Isolation and Characterization of Phosphorus Solubilizing Bacteria With Multiple Phosphorus Sources Utilizing Capability and Their Potential for Lead Immobilization in Soil. Front. Microbiol. 11:752. doi: 10.3389/fmicb.2020.00752
Received: 05 November 2019; Accepted: 30 March 2020;
Published: 23 April 2020.
Edited by:
Jaak Truu, University of Tartu, EstoniaReviewed by:
Usman Irshad, COMSATS University Islamabad, PakistanCopyright © 2020 Wan, Qin, Wu, Zuo, He, Tan, Wang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donglan He, aGRsQG1haWwuc2N1ZWMuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.