- 1State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, China
- 2School of Food Science and Technology, Jiangnan University, Wuxi, China
- 3Wuxi Translational Medicine Research Center and Jiangsu Translational Medicine Research Institute, Wuxi, China
- 4International Joint Research Laboratory for Probiotics, Jiangnan University, Wuxi, China
- 5National Engineering Research Center for Functional Food, Wuxi, China
- 6(Yangzhou) Institute of Food Biotechnology, Jiangnan University, Yangzhou, China
- 7Beijing Innovation Center of Food Nutrition and Human Health, Beijing Technology and Business University, Beijing, China
Oral exposure to the heavy metal lead (Pb) causes various dysfunctions in animals. However, the influence of gut bacteria on Pb absorption, bioaccumulation, and excretion is largely unknown. In this study, we use a mouse model to investigate the relationship between gut microbiota, Pb-intolerant intestinal microbes and Pb toxicity. First, mice were treated with a broad-spectrum antibiotic cocktail to deplete their gut microbiota, and were then acutely and orally exposed to Pb at 1304 mg/kg for 3 days. Compared to the control mice, antibiotic-treated mice had increased Pb concentrations in the blood and primary organs and decreased Pb fecal concentrations, suggesting that gut microbiota limited the Pb burden that developed from acute oral Pb exposure. Next, three Pb-intolerant gut microbes, Akkermansia muciniphila, Faecalibacterium prausnitzii, and Oscillibacter ruminantium, were orally administered to mice, and their effects against Pb toxicity were evaluated. F. prausnitzii treatment significantly promoted the fecal Pb excretion and reduced Pb concentrations in blood (from 152.70 ± 25.62 μg/dL to 92.20 ± 24.33 μg/dL) and primary tissues. Supplementation with O. ruminantium significantly decreased Pb concentrations in blood (from 152.70 ± 25.62 μg/dL to 104.60 ± 29.85 μg/dL) and kidney (from 7.30 ± 1.08 μg/g to 5.64 ± 0.79 μg/g). Treatment with F. prausnitzii and O. ruminantium also upregulated tight junction (TJ) protein expression and the production of short-chain fatty acids by colonic microbiota, and showed protective effects against liver and kidney toxicity. These results indicate the potential for reducing Pb toxicity by the modulation of gut microbiota.
Introduction
Lead (Pb), a potent environmental toxicant, is ubiquitous in daily life, and poses serious threats to public health. Pb may enter the body by ingestion or inhalation, and ultimately accumulates in blood, soft tissues, and bone. It has been reported that there is no safe threshold for Pb exposure and exposure to even low Pb levels (<10 μg/dL blood) can induce adverse health outcomes (Vorvolakos et al., 2016). The toxicity of Pb in the nervous, hematopoietic, hepatic, and renal systems has been well studied (Flora et al., 2012). However, the effect of Pb on the gut, one of the primary sites of absorption and excretion of metals (James et al., 1985), has received much less attention. To date, studies on the toxic effects of Pb on intestinal physiology have mainly focused on the following aspects. Histopathological observations have proved that Pb exposure can directly lead to local oxidative stress and inflammation in the gut, and caused marked intestinal morphological disorders (Crespo et al., 2006). Abnormal expression of genes such as divalent metal transporter 1 (DMT-1) and metallothioneins (MTs) was also observed in oral Pb-exposed mice, which resulted in impaired uptake of essential metals and other nutrients (Breton et al., 2013; Ebrahimi et al., 2015). Moreover, chronic Pb exposure can damage intestinal epithelial cells and tight junction (TJ) function, and perturbs the synthesis and secretion of intestinal mucin, ultimately disrupting the physical barrier of the gut (Holcombe et al., 1976; Breton et al., 2013).
The microbiota of the mammalian gut is recognized as crucial for the maintenance of the mammalian host’s health (Jandhyala et al., 2015). As oral ingestion is one of the main routes of Pb exposure, gut microbiota are inevitably exposed to this toxic metal. This damages the gut microbial community, as indicated by a decrease in the bacterial alpha diversity and a spatial shift of beta diversity (Wu et al., 2016). Recent reports have shown that both acute and chronic Pb toxicity induce dysbiosis of gut commensal organisms (Zhai et al., 2017; Xia et al., 2018). Metagenomic and metabolomic analysis revealed that some bacterial genera highly associated with the metabolism of energy, lipids, and bile acid (BA), including Ruminococcus and Lachnospiraceae species, are deleteriously affected by Pb exposure (Gao et al., 2017). Conversely, several enteric microorganisms such as Enterococcus (Topcu and Bulat, 2010) and sulfate-reducing bacteria such as Desulfotomaculum acetoxidans (Pado and Pawłowska-Cwiek, 2005) have been demonstrated to bind heavy metal ions such as Pb2+ in vitro. Furthermore, oral administration of probiotic intestinal bacteria, such as L. plantarum (Tian et al., 2012) and Leuconostoc mesenteroides (Yi et al., 2017), were observed to have protective effects against Pb toxicity in mice. Gut microbiota also play an essential role in maintaining intestinal homeostasis, protecting structural integrality of the gut mucosal barrier, and inhibiting the adverse effects of pathogens and xenobiotics on gut function (Jandhyala et al., 2015). However, little is known about the direct effect of whole-gut ecology on the absorption, distribution, and excretion of non-absorbed heavy metals. It is therefore necessary to study the interactions between intestinal microorganisms and Pb toxicity.
Previous studies yielded preliminary data on the effects of oral sub-chronic Pb exposure on the gut bacterial compositions of mice (Zhai et al., 2017). These studies showed that Pb exposure significantly decreased the abundances of microbial genera such as unclassified Lachnospiraceae, unclassified and uncultured Ruminococcaceae, Oscillibacter, Anaerotruncus, Akkermansia, Lachnoclostridium Ruminiclostridium_9, and Rikenellaceae_RC9_gut_group. These effects of were more drastic in the first month of treatment than in the last month. Among the altered microbial genera, the Ruminococcaceae are one of the main mucosa-associated microbe families in the human and murine colon (Nava and Stappenbeck, 2011). This family consists of several gut-resident bacteria such as Faecalibacterium prausnitzii, a commensal bacterium that has been reported to protect against immune disorders (Hornef and Pabst, 2015) and gut barrier hyperpermeability (Laval et al., 2015). In addition, the gut symbiont Oscillibacter has found to produce valeric or butyric acid (Takao et al., 2007; Lee et al., 2012) and Oscillibacter abundance is inversely associated with a wide range of diseases and dysfunctions, such as diarrhea and Crohn’s Disease (CD) (Wang et al., 2015; Fang et al., 2016). A range of studies have shown that Akkermansia muciniphila, a next-generation probiotic, plays an essential role in the regulation of host immune responses and metabolism (Cani and de Vos, 2017; Ottman et al., 2017). An outer membrane protein of the A. muciniphila strain Amuc_1100 has been shown to improve gut barrier functions by its interaction with Toll-like receptor 2 (TLR2) and by further activating the downstream NF-κB pathway (Plovier et al., 2017). In general, specific gut microbes may enhance the repair of intestinal mucosal wounds, maintain the homeostasis of gut immunity, and aid in the alleviation of gut inflammation. Consequently, we hypothesized that Pb exposure may damage the resident gut commensal bacteria, and thus disturb the structure and diversity of gut microbiota, which would further exacerbate heavy-metal absorption and gut barrier dysfunction. The direct oral administration of Pb-intolerant gut microbes may be of potential use for interventions against Pb toxicity.
We therefore assessed the role of the gut microbiota in the bioaccumulation and retention of Pb in mice, following oral Pb exposure. In addition, three Pb-intolerant gut microbes (A. muciniphila, F. prausnitzii, and Oscillibacter ruminantium) were administered to mice, and their effects against Pb toxicity were assessed.
Materials and Methods
Bacterial Strains and Culture
Akkermansia muciniphila ATTC BAA-835 was obtained from the American Type Culture Collection. F. prausnitzii A2-165 and O. ruminantium GH1 were obtained from the Japan Collection of Microorganisms. Other strains were obtained from the Culture Collections of Food Microbiology, Jiangnan University (Wuxi, China). All strains were cultured in de Man, Rogosa and Sharpe agar, Luria-Bertani broth, or Brain Heart Infusion medium (Hopebio Company, Qingdao, China) at 37°C. The cultured biomass was preserved immediately with 30% glycerol as the protectant. Colony counting was conducted to confirm the survival of bacteria for animal treatment.
Animal and Experimental Diet
Six-week-old male C57BL/6 mice were purchased from the Shanghai Laboratory Animal Center (Shanghai, China). The mice had free access to standard commercial mouse chow and drinking water. At the end of the experiment, the mice were sacrificed by CO2 asphyxiation. Livers, kidneys, and other tissues were stored at −80°C prior to analysis. The protocols in this study were approved by the Ethics Committee of Jiangnan University, China (JN No. 20180615-c0801-20-2 and JN No. 20181030-c0301-11-0). All procedures in the study were carried out in accordance with European Community guidelines (Directive 2010/63/EU).
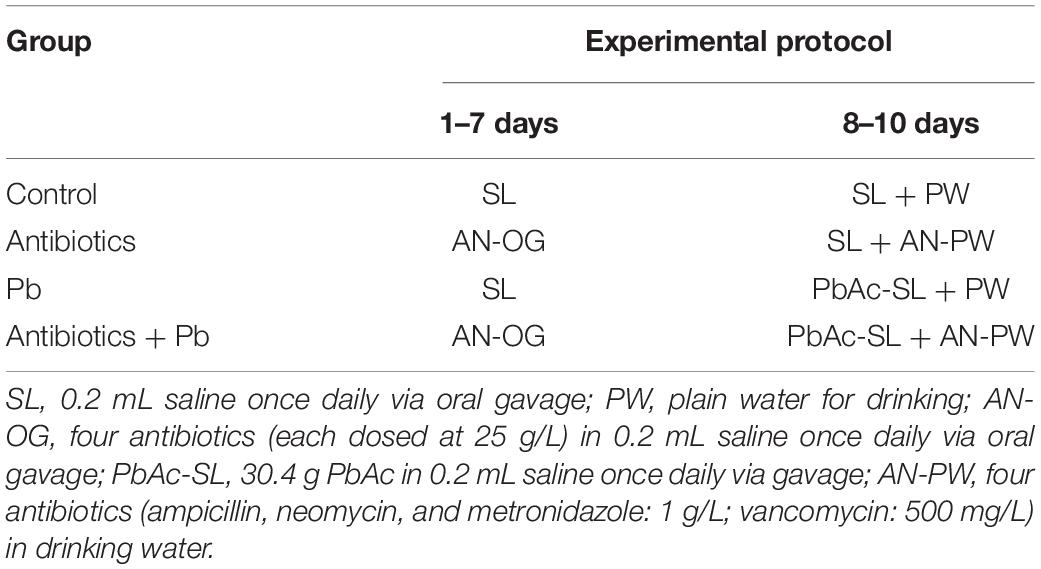
Animal Experimental Design I
Forty mice were randomly divided into four groups (Table 1). In the first 7 days, mice were orally administered a combination of four antibiotics via oral gavage to deplete gut flora (Curtis et al., 2014). Feces were collected before and after antibiotic treatment to confirm depletion of the intestinal microbiota. In the last 3 days, four antibiotics were added to the drinking water and the mice were orally gavaged once daily with a dose of 1304 mg Pb acetate trihydrate (PbAc) per kg body weight. The dose was equivalent to one-fifth of the LD50 of Pb in mice (El-Ghor et al., 2011; Sun et al., 2017). Our preliminary experiment showed that this dose was non-lethal but exhibited a significant toxic effect in mice.
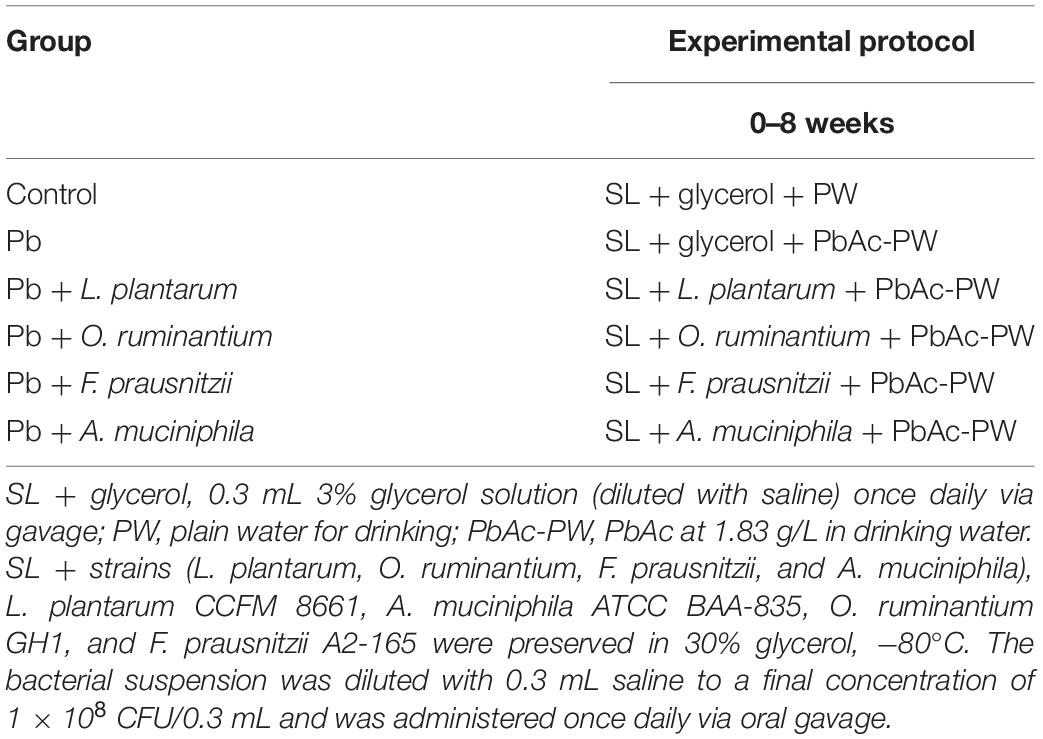
Animal Experimental Design II
For chronic Pb exposure experiments, sixty mice were randomly divided into six groups (Table 2), mice in model group were exposed to Pb in their drinking water (at a dose of 1.83 g PbAc per L of drinking water), as described previously (Tian et al., 2012). All strains were provided as a dose of 1 × 108 CFU once daily by oral gavage (Feng et al., 2019). Feces were collected every 2 weeks for the determination of Pb concentrations or for microbial DNA extraction.
Pb Determination in Tissues, Blood, Urine and Feces
As described previously (Zhai et al., 2019), samples were digested by a microwave digestion system (MARS; CEM, United Kingdom). Pb concentrations in samples were subsequently quantified by atomic absorption spectrophotometry (Spectrum AAS or AA; Varian, United States).
The qPCR-Based Determination of Total Bacterial Number
Microbial genomic DNA was isolated from feces and quantified by spectrophotometry. The primers for mouse genomic DNA were as previously described (Reikvam et al., 2011). The bacterial V3 region of the16S rRNA genes was amplified using primers 16S-F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 16S-R (5′-ATTACCGCGGCTGCTGG-3′). The number of 16S DNA copies was normalized to the number of mouse genomic DNA copies for each sample. Threshold cycle values were used to calculate the number of 16S rRNA gene copies in each sample (Hill et al., 2010).
Biochemical Analyses
The concentrations of malondialdehyde (MDA) and glutathione (GSH) in the tissues and the concentrations of alkaline phosphatase (ALP), aspartate transaminase (AST), urea, and creatinine (CREA) in plasma of mice were measured with assay kits purchased from the Jiancheng Bioengineering Institute (Nanjing, China).
Histopathological Examination
The liver and kidney tissues were fixed in 10% formalin and embedded in paraffin. The tissue slices were subsequently stained with hematoxylin and eosin.
Hematological Assays
The total white blood cell count (WBC), red blood cell count (RBC), eosinophil count, basophil count, mean corpuscular volume (MCV), lymphocyte count, hemoglobin and monocyte content, mean corpuscular hemoglobin (MCH) count, platelet hematocrit (PCT), mean corpuscular hemoglobin concentration (MCHC), and red blood cell distribution width (RDW) of each sample were analyzed with a fully automatic blood analyzer (BC-5000 Vet; Mindary, Shenzhen, China).
The Evaluation of TJ Protein mRNA Expression in Intestinal Tissues
Total RNA was extracted with Trizol reagent and converted to cDNA using Taq-Man reverse transcription reagents (Applied Biosystems; Foster City, CA, United States). Real-time PCR parameters and primer sequences for the targeted mouse genes were as previously described (Zhai et al., 2018).
The Determination of Intestinal Permeability in Mice
After fasting for 6 h, the mice were fed with DX-4000-FITC (sigma) at a dose of 600 mg/kg body weight. After 1 h, 100 mL of blood was taken from the tail vein and centrifuged at 3000 × g for 15 min. The supernatant was mixed with the same volume of PBS (pH 7.3), and the fluorescence intensity of the resulting solution was measured on a fluorescence microplate (SpectraMax; Molecular Devices, San Jose, CA, United States) (Cani et al., 2009).
The Determination of Short-Chain Fatty Acids (SCFAs) in Feces
The concentration of SCFAs was measured by GC-MS (GCMS-QP2010 Ultra system, Shimadzu Corporation, Japan) (Wang et al., 2017). The concentration of total SCFAs was calculated as the sum of acetic, propionic, butyric, isobutyric, and pentanoic acid concentrations.
The Determination of Pb-Binding Abilities of Specific Gut Microbes
As previously described (Halttunen et al., 2008), living cell pellets of tested strains were re-suspended in ultrapure water. containing 50 mg/L Pb2+, and the resulting solutions were incubated for 1 h. The residual Pb2+ concentrations were quantified by atomic absorption spectrophotometry (Spectrum AAS or AA; Varian, United States).
The Determination of the Pb Tolerance of Specific Gut Microbes
The tolerance of the tested strains to Pb was determined by a Minimum Inhibitory Concentration (MIC) assay. PbAc solution was added to MRS or BHI liquid medium to give final Pb2+ concentrations of 0, 200, 250, 500, 1,000, 2,000, 3,000, 4,000, 5,000, and 6,000 mg/L. After inoculation of the activated bacterial liquid into the culture medium (to give a final concentration of 106 CFU/mL), the Pb2+ concentration of each was measured three times. The lowest concentration of Pb2+ that could completely inhibit the growth of intestinal flora was taken to be the MIC (Youravong et al., 2011).
Statistical Analysis
The data were represented as means ± SD. The Differences between each group were assessed by a one-way ANOVA test, followed by Tukey’s multiple-range tests. Statistical analyses and data visualization were conducted in GraphPad Prism (version 7; GraphPad Software Inc., La Jolla, CA, United States), unless otherwise stated.
Results
The Depletion of Gut Microbiota Enhanced Tissue Bioaccumulation of Pb in Acute Pb-Exposed Mice
Compared with control mice, both antibiotics-treated and antibiotics + Pb-treated mice lost weight dramatically and displayed significantly dilated caeca (Supplementary Figures S1A,B). Seven-day oral administration of antibiotics successfully depleted the gut commensals of mice, as indicated by treated mice having a three order of magnitude decrease in their gut microbiota compared to the controls (Supplementary Figure S1C). We also found that all mice treated with the antibiotics had markedly reduced copy numbers of 16S rRNA genes in feces, approximately 500 fold less than the levels in sham-treated mice (Supplementary Figure S1D).
Fecal Pb concentrations were markedly increased in the Pb-treated group (Figure 1A, p < 0.01) compared with the control mice, with fecal Pb concentrations in the control mice being too low to detect (data not shown). Combined antibiotic and Pb treatment significantly reduced fecal Pb concentrations at 8 h, 12 h, and 24 h (2.10 ± 1.10 mg/g, 9.28 ± 4.50 mg/g, and 8.29 ± 3.10 mg/g, respectively) compared with the group that received Pb alone (14.20 ± 2.66 mg/g, 27.10 ± 3.85 mg/g and 14.58 ± 4.34 mg/g, respectively) (Figure 1A, p < 0.01). Accordingly, antibiotic treatment enhanced Pb accumulation in the tissues and blood of the mice (Figures 1B–E).
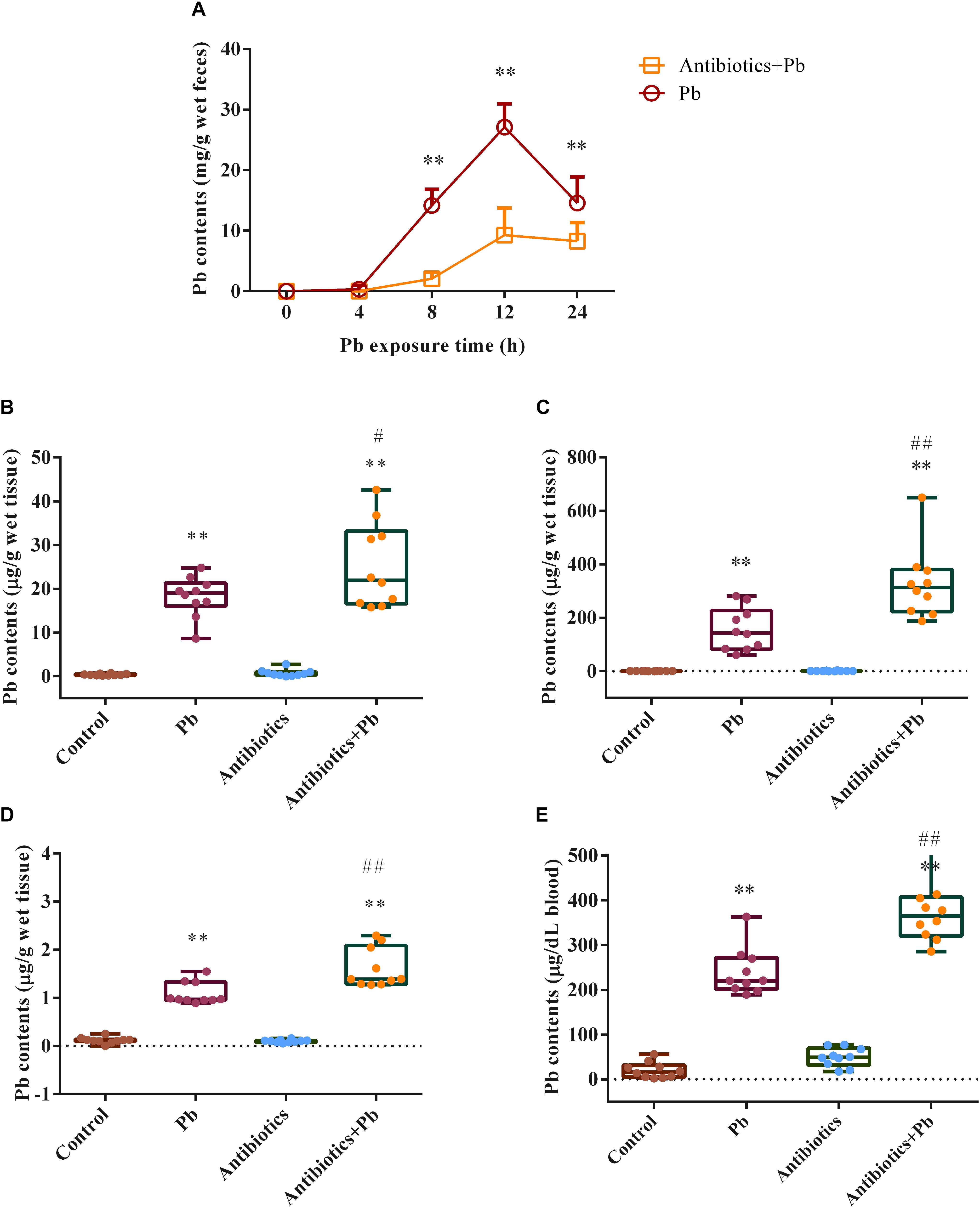
Figure 1. The effects of antibiotic treatment on Pb concentrations in feces, tissues and blood of mice (n = 10). (A). Fecal Pb concentrations in the Pb-treated or Antibiotics + Pb-treated mice at different time points. (B–E). Pb concentrations in the liver (B), kidneys (C), brain (D), and blood (E) of mice. ∗∗p < 0.01 vs. the control group; ## p < 0.01 and #p < 0.05 vs. the Pb group.
The Depletion of Gut Microbiota Aggravated the Disruption of Gut Barrier Function in the Acute Pb-Exposed Mice
As shown in Figure 2, acute Pb exposure led to increased gut permeability in mice, as indicated by the elevated concentrations of DX-4000-FITC in serum (from 0.92 ± 0.23 μg/mL to 1.66 ± 0.38 μg/mL, p < 0.05) and the decreased mRNA expression of ZO-1, ZO-2, occludin and claudin-1 genes in the colon and jejunum of mice (p < 0.05). Also, a greater decline was found in the relative expression of TJ mRNA in antibiotics-treated mice when compared with sham-treated counterparts (Figures 2A,B, p < 0.05).
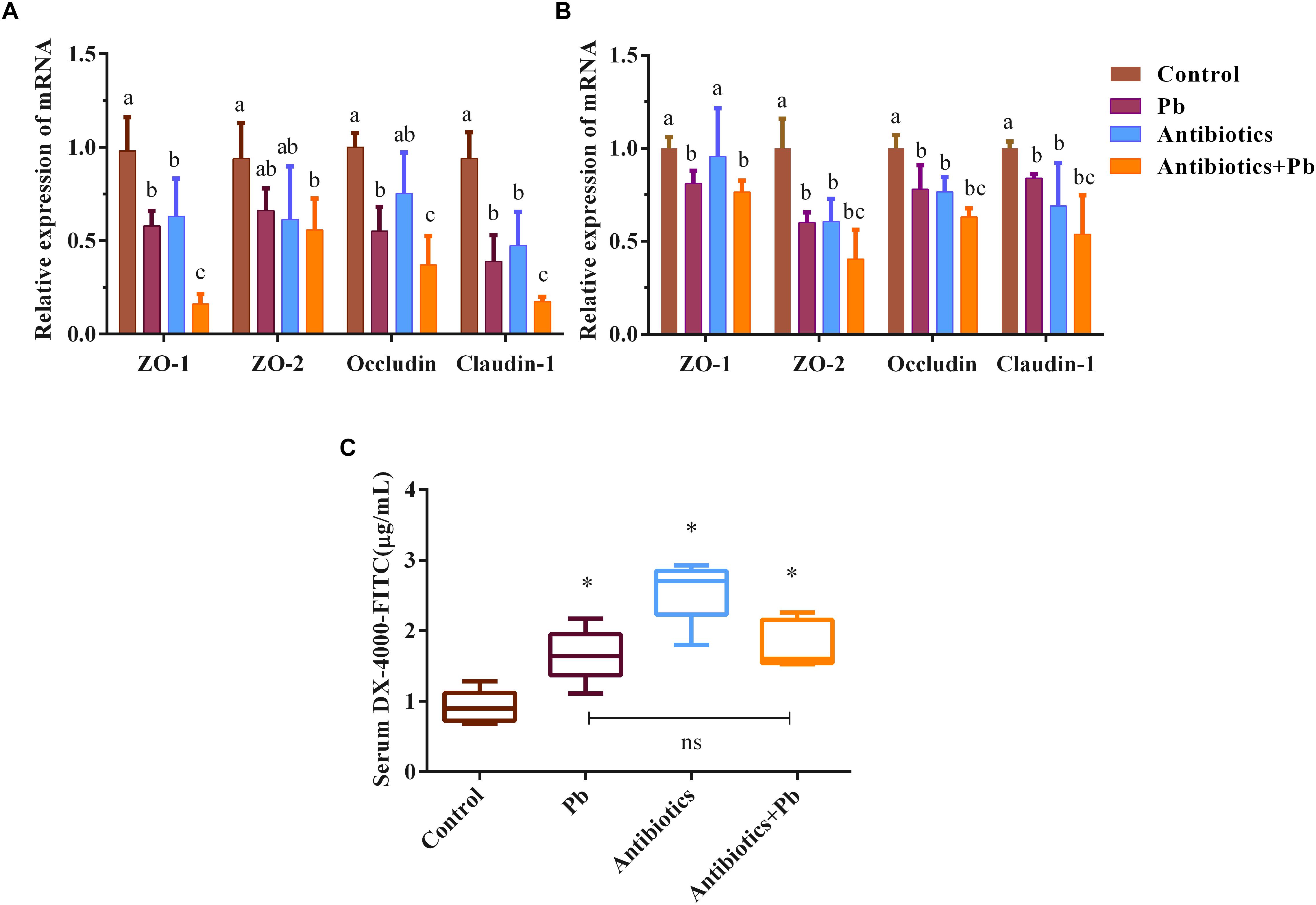
Figure 2. The effects of antibiotic treatment on the gut barrier function of mice (n = 5). mRNA expression of TJ proteins ZO-1, ZO-2, occludin and claudin-1 in representative sections of the colon (A) and small intestine (B) of mice. β-actin was used as the internal control. The data are expressed as the fold-change vs. control group (set to 1). Letters a–c denote statistically significant differences between each group (p < 0.05). (C) DX-4000-FITC levels in the serum of mice, ∗p < 0.05 vs. the control group. “ns” indicates no significant differences (p > 0.05) between groups.
Oral Supplementation of Pb-Intolerant Intestinal Microbes Reduced Pb Burdens and Increased Fecal Pb Excretion in Chronically Pb Exposed-Mice
Chronic Pb exposure induced higher concentrations of Pb in blood, liver, kidney and brain tissues of mice (Figure 3, p < 0.05). Oral supplementation of Pb-intolerant intestinal microbes, such as F. prausnitzii and O. ruminantium, markedly decreased Pb concentrations in the main target organs and blood of mice. However, administration of Pb-intolerant intestinal microbes did not restore the values of basic hematological parameters, except for HGB and MCH (Supplementary Tables S1, S2).
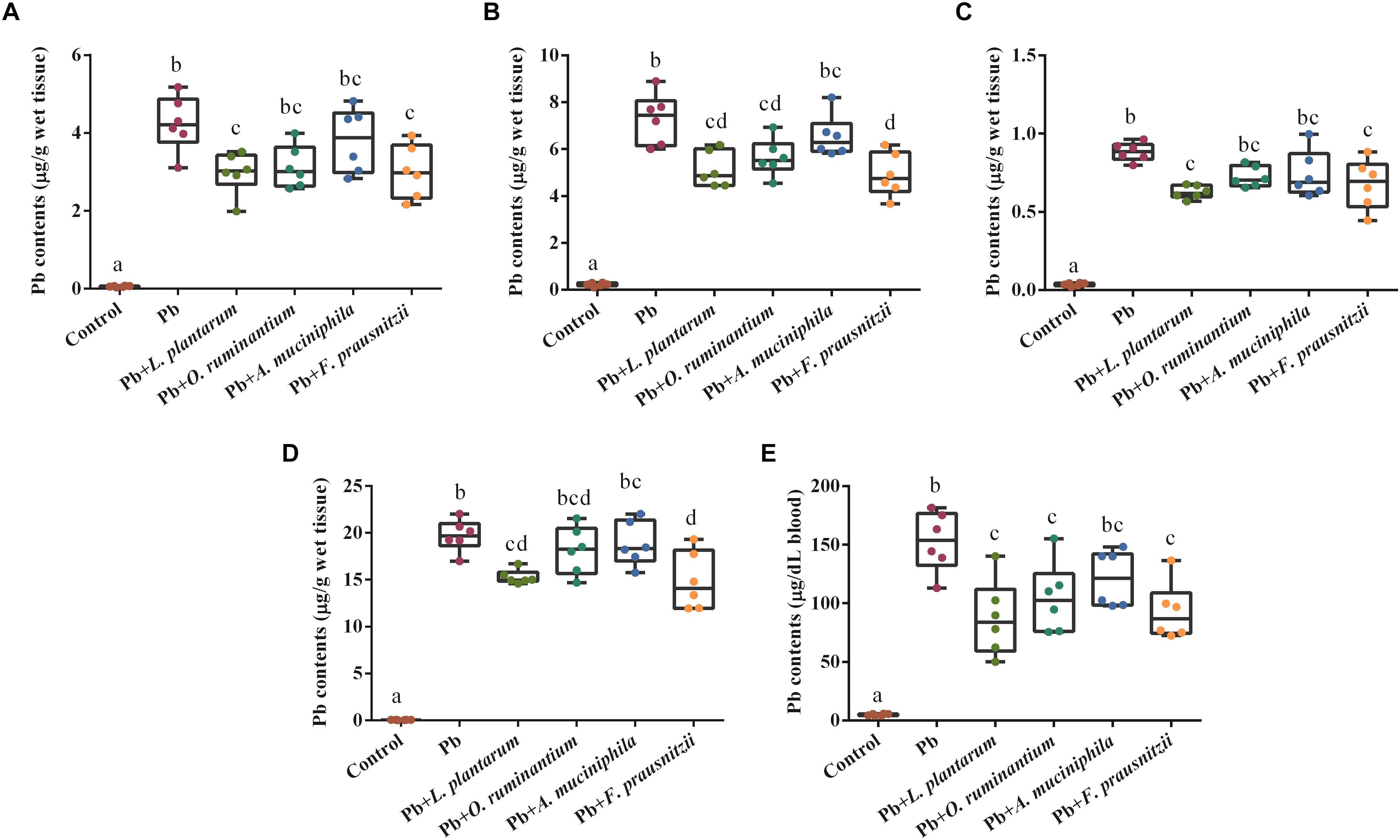
Figure 3. The effects of Pb-intolerant gut microbes on Pb concentrations in the liver (A), kidneys (B), brain (C), small intestine (D), and blood (E) of chronically Pb-exposed mice (n = 6). Letters a–d denote statistically significant differences between each group (p < 0.05).
Oral supplementation of F. prausnitzii after 2 and 4 weeks of Pb exposure significantly promoted fecal Pb excretion (2.11 ± 0.31 mg/g and 2.15 ± 0.50 mg/g, respectively) (Figure 4, p < 0.05). O. ruminantium intervention also induced a higher fecal Pb content after the second week of Pb exposure (2.01 ± 0.66 mg/g) (Figure 4, p < 0.05).
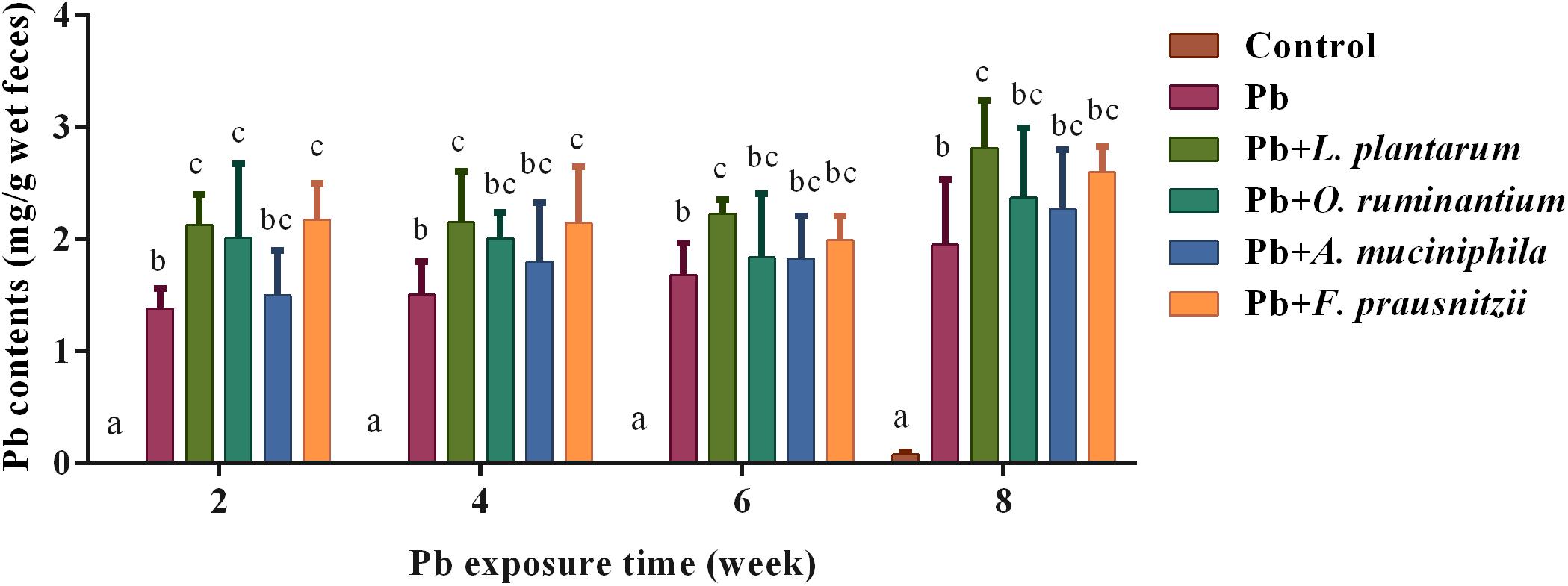
Figure 4. The effects of Pb-intolerant gut microbes on fecal Pb levels in chronically Pb-exposed mice at different time points (n = 6). Letters a–c denote statistically significant differences between each group (p < 0.05).
Oral Supplementation of Pb-Intolerant Intestinal Microbes Alleviated Hepatic and Renal Oxidative Stress in Chronically Pb-Exposed Mice
Although chronic Pb exposure induce no significant pathological lesion in mice (Supplementary Figures S2, S3), marked oxidative damage were observed in the livers and kidneys of the Pb-treated mice, indicated by their increased MDA levels (136.50 ± 25.32 mg/g and 119.80 ± 17.79 mg/g, respectively) and decreased GSH levels (0.96 ± 0.19 mg/g and 1.21 ± 0.22 mg/g, respectively) compared with the control mice (Table 3, p < 0.05). Similar to the effects of treatment with L. plantarum, F. prausnitzii, and O. ruminantium intervention markedly reduced MDA content in renal tissues (70.46 ± 23.11 mg/g and 76.88 ± 22.10 mg/g, respectively). However, there was no significant difference between the Pb-treated group and the Pb + A. muciniphila-treated group (Table 3, p > 0.05).
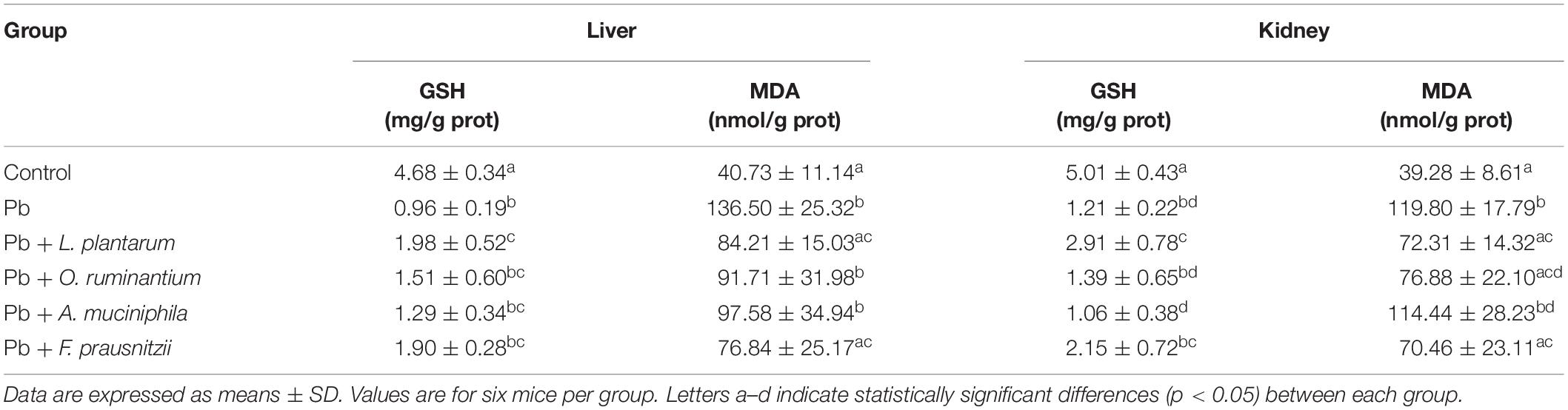
Table 3. The effects of Pb-intolerant gut microbes on the contents of MDA and GSH in the liver and kidneys of chronically Pb-exposed mice.
Chronic Pb exposure significantly increased the concentrations of serum ALP, AST, UREA, and CREA in mice (Table 4, p < 0.05). All treatments with gut microbes, except for A. muciniphila treatment, restored these alterations. Compared with L. plantarum treatment, treatment with F. prausnitzii or O. ruminantium exhibited a similar protective effect on serum ALP, AST, UREA, and CREA.
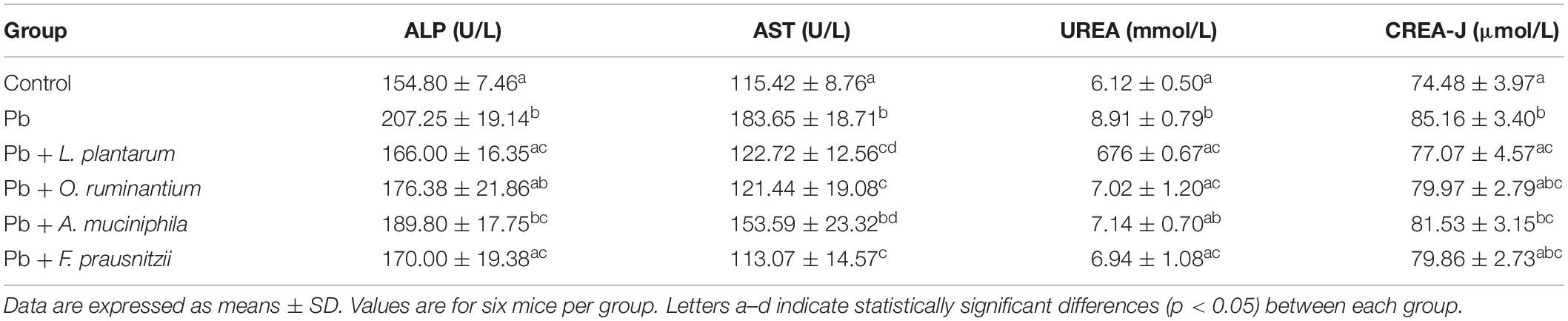
Table 4. The effects of Pb-intolerant gut microbes on the values of haematogenic immunity parameters in chronically Pb- exposed mice.
Oral Supplementation of Pb-Intolerant Intestinal Microbes Relieved Intestinal Barrier Impairment Caused by Chronic Pb Exposure in Mice
The expression of TJ proteins in the colon and jejunum tissues of Pb-exposed mice decreased significantly compared to control mice (Figure 5, p < 0.05). Oral supplementation of F. prausnitzii or O. ruminantium significantly increased the expression of ZO-1, occludin and claudin-1 proteins in the colon and small intestine. Treatment with A. muciniphila had no influence on the expression of these TJ genes.
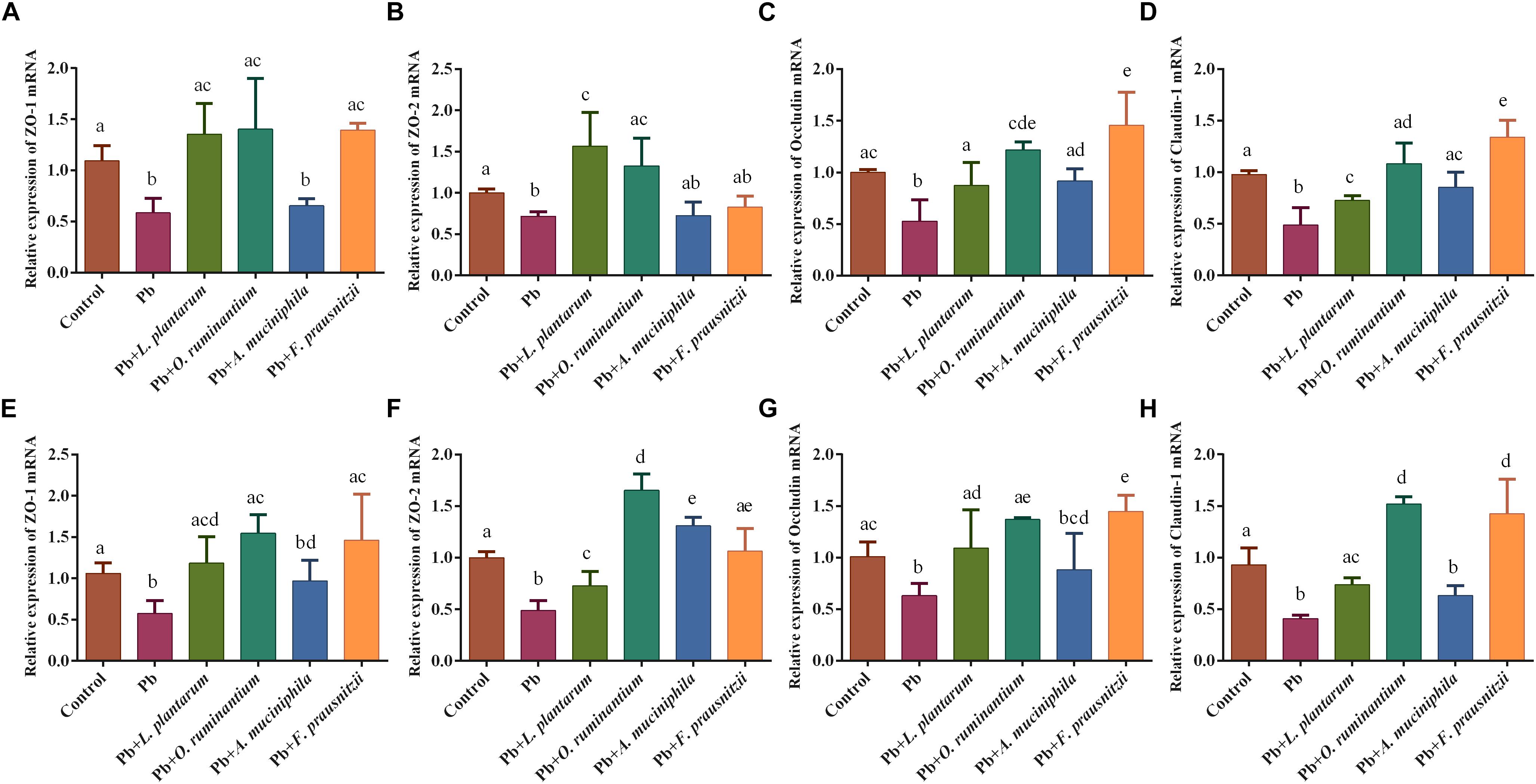
Figure 5. The effects of Pb-intolerant gut microbes on mRNA expression of TJ genes in representative sections of colon (A–D) and small intestine (E–H) of chronic Pb-exposed mice (n = 6). Letters a–e denote statistically significant differences between each group (p < 0.05).
The levels of colonic gut bacterial metabolite SCFAs, such as acetic acid and butyric acid, were significantly reduced in the feces of Pb-exposed mice (61.26 ± 4.61 μmol/g and 3.01 ± 0.65 μmol/g, respectively) compared to the control group (38.54 ± 3.35 μmol/g and 6.95 ± 0.98 μmol/g, respectively) (Figure 6, p < 0.05). This reduction was reversed by the oral supplementation of Pb-intolerant intestinal microbes. Although a clear trend was observed, statistically significant differences were only found between the Pb-exposed group and F. prausnitzii- or O. ruminantium-treated groups for acetic acid and butyric acid levels and total SCFA content (Figure 6, p < 0.05).
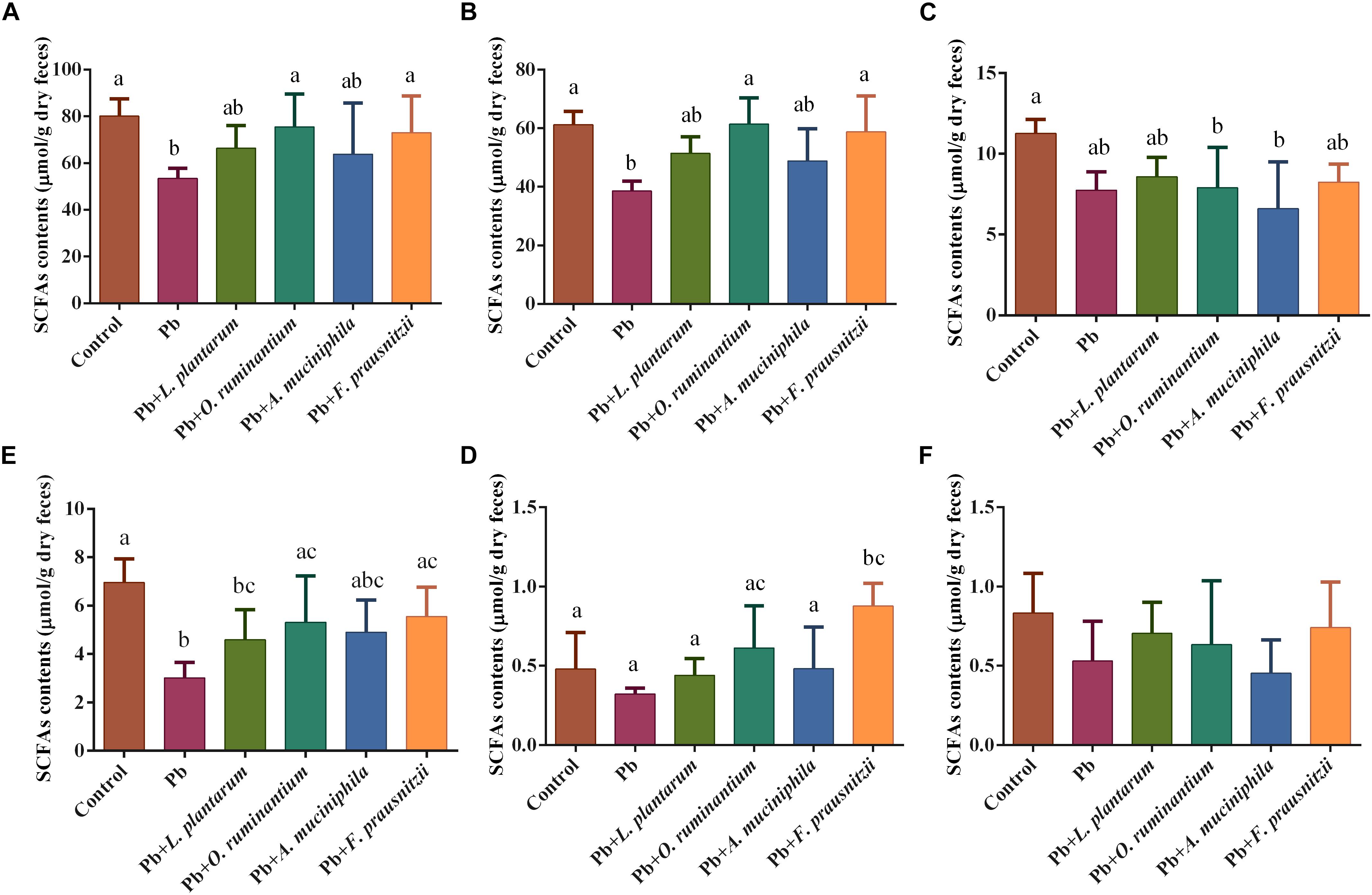
Figure 6. The effects of Pb-intolerant gut microbes on the total colonic contents of SCFAs (A), acetic acid (B), propionic acid (C), butyric acid (D), isobutyric acid (E), and valeric acid (F) based on dry weights, of chronic Pb-exposed mice. Data are expressed as means ± SD (n = 6). Letter a–c refer to significant differences between each group (p < 0.05).
Pb-Binding and Pb-Sensitivity of Bacterial Strains
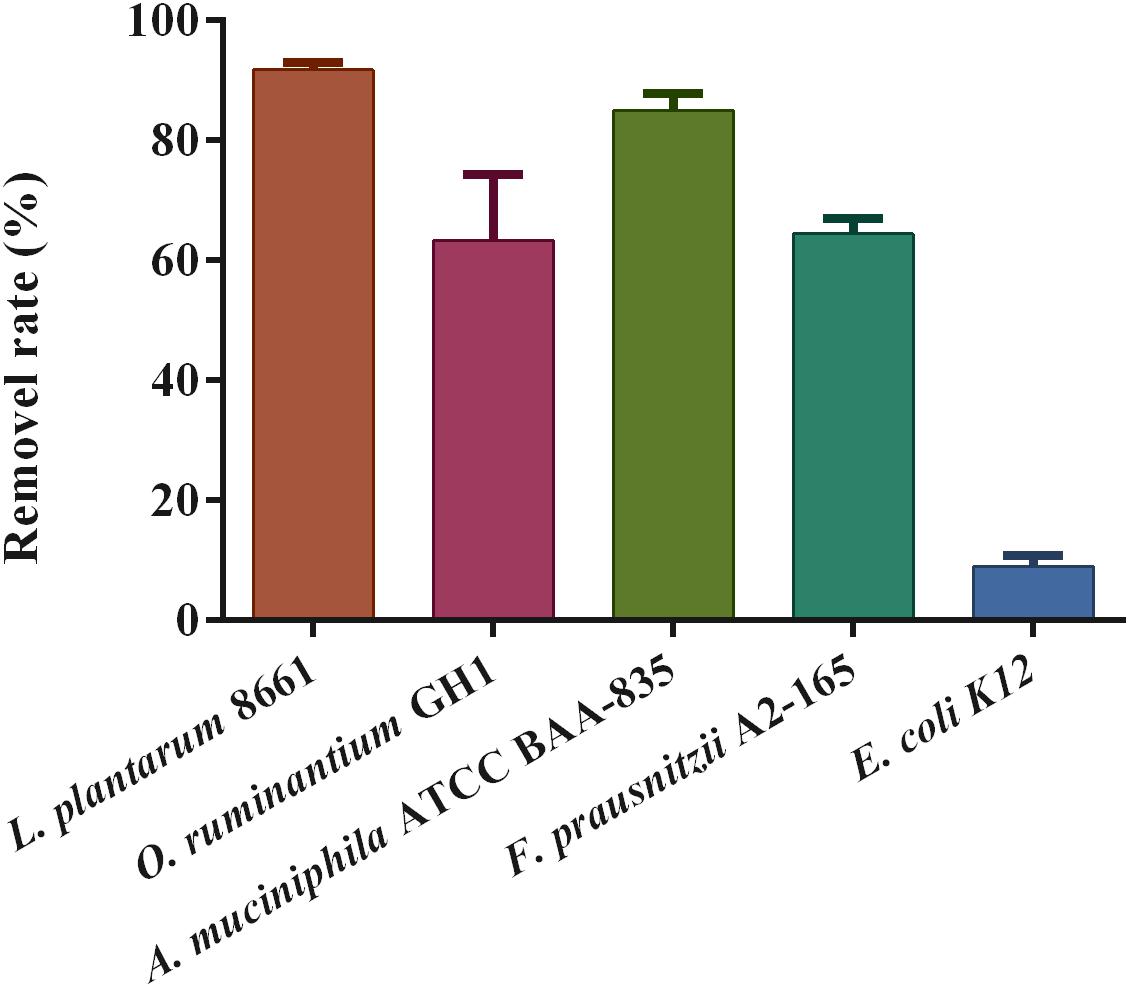
The Pb-sensitivity of the tested intestinal bacteria varied significantly (Table 5). When compared with E. coli K12, a resident bacterium in the gut, F. prausnitzii A2-165, A. muciniphila ATCC BAA-835, and O. ruminantium GH1 can be considered to be sensitive (intolerant) to Pb. Among the three Pb-intolerant gut microbes, F. prausnitzii A2-165 had the highest Pb tolerance, which is in close proximity to the Pb-tolerant strain.
The examination of the Pb removal rate showed that these strains have extensive differences in Pb-adsorption capacity, ranging from 9.02 ± 1.73% to 91.81 ± 1.16%. The three Pb-intolerant gut microbe strains showed a high adsorption capacity for Pb2+ compared with E. coli K12 (Figure 7). Among these, the binding capacity of F. prausnitzii A2-165 and O. ruminantium GH1 was markedly lower than that of A. muciniphila.
Discussion
Gut commensals are recognized as an important factor in maintaining host health. However, the role of the gut microbiome in Pb detoxification has not been well addressed. In this study, we observed that the intestinal absorption and tissue accumulation of Pb were exacerbated in mice lacking intestinal microbiota (Figure 1), suggesting that gut microorganisms can play a role in limiting the bioavailability of Pb.
The protective effects of gut microbiota against Pb absorption may be due to the following reasons. First, consistent with an earlier report (Reikvam et al., 2011), our results showed that the cecum is greatly enlarged in antibiotics-treated animals (Supplementary Figure S1C). This may prolong the retention of Pb in the gut and increase the absorption of this heavy metal. Second, it has been reported that compared with normal mice, gut commensal depletion by antibiotics increases gut permeability (Wu et al., 2014) and inhibits the expression of TJ proteins (Shi et al., 2018). Similar results were also observed in our acute Pb-exposure trial (Figure 2). Antibiotic treatment also elevates the expression of MT1 and MT2, which are metal-binding proteins with a high affinity for divalent cations crossing the apical membrane (Loumbourdis et al., 2007). This could in turn exacerbate the permeation of Pb through the gut barrier. Third, Pb undergoes enterohepatic circulation in the body and can be released into the gut from the liver via bile (Zhai et al., 2019). Antibiotic cocktail treatment decreases fecal BA excretion and leads to more effective BA conservation by strongly elevating the expression of the intestinal apical sodium-dependent bile salt transporter (Out et al., 2015), indicating elevated Pb reabsorption in the distal ileum and bioaccumulation in the liver. Finally, gut microbes such as Lactobacillus, Bifidobacterium (Teemu et al., 2008), and Enterococcus (Topcu and Bulat, 2010) were proved to bind and remove heavy metals via their surface compounds such as capsular polysaccharides and teichoic acids. This indicates that gut microorganisms may function as a biological barrier to directly compete with Pb absorption by the gut, thus limiting the bioavailability of this toxic metal.
Previous results indicated that among the entire gut microbiota population, some specific microbes, such as Ruminiclostridium_9, Oscillibacter, Akkermansia and others were extremely sensitive to Pb exposure (Zhai et al., 2017). We thus hypothesized that oral supplementation with these strains may be protective against Pb toxicity. From this study, we have shown that oral administration of F. prausnitzii and O. ruminantium had profound effects on relevant biomarkers – i.e., Pb content, gut barrier integrity, oxidation resistance, and the production of SCFA – showing that these bacteria significantly alleviated Pb-induced toxicity in mice, while A. muciniphila treatment failed to provide this protection.
The protective effects of F. prausnitzii treatment against chronic Pb exposure may be explained as follows. First, the increased gut permeability in different models is markedly associated with the reduced abundance of F. prausnitzii in feces of mice (Leclercq et al., 2013; Machiels et al., 2014), and the supplementation of F. prausnitzii can exert protective effects on the intestinal mucosal barrier (Laval et al., 2015). The mechanism may involve gene regulation controlling the intercellular barrier, the enhancement of SCFAs production, and benign interactions with gut microorganisms. Accordingly, our results showed that F. prausnitzii treatment strengthened the expression of TJ proteins such as ZO-1, occludin and claudin-1 in Pb-exposed mice (Figure 5). This was in line with an earlier finding that the expression levels of claudin-4 and F11r in the colon of mice were significantly increased after treatment with this bacterial strain (Laval et al., 2015). We also observed that F. prausnitzii treatment significantly increased the concentrations of SCFAs in colonic products (Figure 6). This is in accordance with a previous report showing the ability of F. prausnitzii to produce butyric acid or other SCFAs (Machiels et al., 2014). Upregulation of SCFAs, especially butyric acid, can reduce the pH of the intestinal lumen and trigger a number of signaling effects including the regulation of mucin synthesis and secretion (Paassen et al., 2009), further reinforcing the intestinal barrier (Wong et al., 2006). An earlier report provided direct in vivo evidence that F. prausnitzii was able to utilize acetate produced by B. thetaiotaomicron and that this symbiotic relationship exerted far-reaching and beneficial influences on the regulation of the bowel mucus barrier (Wrzosek et al., 2013). It is apparent that breaches in this physical barrier can aggravate the absorption of heavy metals, which indicates that enhancement of the biological barrier by F. prausnitzii treatment can prevent leakage and keep exogenous toxicants such as Pb out of the body, thus limiting the physical harm of Pb. Second, chronic Pb exposure directly induces the apoptosis of epithelial cells and marked local bowel inflammation, resulting in systemic disorders and elevated Pb absorption in the gut. Interestingly, levels of F. prausnitzii are inversely correlated with inflammatory markers such as IL-6 in low-grade inflammatory disease, indicating that this beneficial bacterium may play an inhibitory role in local intestinal inflammation and infection (Furet et al., 2010). Other studies have demonstrated that this normally abundant bacterium strongly induces the expression of microbial anti-inflammatory molecules (MAM) such as IL-10 in human and mouse dendritic cells and blocks NF-κB signals, ultimately supporting the homeostasis of the mucosal immune barrier (Hornef and Pabst, 2015; Rossi et al., 2016). This immunomodulatory effect of F. prausnitzii may effectively maintain the integrity of the epithelial barrier, thus minimizing Pb toxicity in the body. Third, a lowered abundance of fecal F. prausnitzii is observed in patients with inflammatory diseases such as infectious colitis and IBD (Sokol et al., 2009; Duboc et al., 2013), usually accompanied by an elevated levels of hepatic BAs and diminished levels of fecal unconjugated BAs. Consistent with the protective effects of L. plantarum CCFM8661 (Zhai et al., 2019), the fact that F. prausnitzii administration could facilitate fecal Pb excretion may be partly due to its role in regulating BA hepato-enteric circulation. F. prausnitzii treatment may inhibit BA re-uptake and promote the hepatic synthesis of BAs by repression of the enterohepatic FXR–FGF15 axis (Jia et al., 2018). This can limit the reflux of Pb from the gut to the liver via bile, thus giving rise to an increase in the biliary Pb output. In addition, the surface of Gram-positive bacteria contain negatively charged groups such as carboxyl, hydroxyl, and phosphate groups, and these can bind metals (Teemu et al., 2008). This may explain the observation that the supplementation of F. prausnitzii, a Gram-positive resident bacteria in the gut, significantly increased fecal Pb excretion due to its good Pb sequestration capability, and further mitigated chronic Pb burden for the mice when Pb was orally introduced (Figure 4). Recently, F. prausnitzii has been reported to provide full protection against arsenic toxicity in a murine model (Coryell et al., 2018), similar to our results.
Compared to F. prausnitzii treatment, the protective effects of O. ruminantium administration against Pb toxicity was less significant. O. ruminantium markedly reversed Pb-induced alterations in gene expression levels of TJ proteins, and the concentrations of total SCFAs and butyric acid (Figures 5, 6), which may be closely related to its capability of producing butyric acid (Lee et al., 2012). Recent studies have demonstrated that butyric acid, a major source of energy for intestinal epithelial cells, can increase the mucus-layer replenishment rate (Gaudier et al., 2004) and induce the differentiation of Treg/Tr1 cells via inhibition of histone deacetylation. This short chain fatty acid can also suppress inflammatory responses in the gut by downregulating the expression of INF-γ, TLR2, and TNF-α (Nicholas et al., 2013; Elce et al., 2017). Different inflammatory disorders such as IBD, enteric infections, and necrotizing enterocolitis (Lechuga and Ivanov, 2017) are usually manifested by increased permeability of the epithelial barrier. This suggests that the communication between the SCFAs induced by O. ruminantium and the immune response in intestinal mucosa may facilitate the repair of the intestinal barrier degradation caused by Pb exposure, thus limiting the uptake and toxicity of Pb in the host. However, this strain showed no obvious effect on renal and fecal Pb content (Figure 1), which may be because of its weaker Pb-binding capacity. Thus, the limited Pb-absorption ability of O. ruminantium means it cannot provide the same protection as F. prausnitzii in chronic Pb-exposed mice.
In contrast to both F. prausnitzii and O. ruminantium treatment, A. muciniphila treatment failed to ameliorate Pb toxicity (Figures 3–6). This may be closely connected with the reported opposing effects of A. muciniphila in regulating intestinal barrier function. Several studies have reported that A. muciniphila can restore mucus layer thickness and alleviate the intestinal inflammation reaction via pili-like proteins, and improve inflammation-induced loss of barrier integrity, thus reducing gut barrier disruption (Ottman et al., 2017; Chelakkot et al., 2018). In contrast, other experiments demonstrated that in DSS-treated or pathogen-infected colitis models, commensal A. muciniphila could drive alterations in mucosal constituents, disturb the homeostasis of the mucus layer and further exacerbate inflammation due to excessive degradation of mucin (Ganesh et al., 2013; Seregin et al., 2017). Supporting our results, a recent study showed that oral administration of A. muciniphila failed to exhibit protective effects against both acute and chronic cadmium toxicity (Feng et al., 2019). In this study, we also found that A. muciniphila ATCC BAA-835 was extremely sensitive to Pb (Table 5), which limits the viability of this strain when interacting with Pb in the gut and restricts its role in reducing Pb toxicity.
Conclusion
Collectively, these results show that gut microbiota can limit the absorption and accumulation of Pb in host tissues. The oral administration of Pb-intolerant intestinal microbes such as F. prausnitzii and O. ruminantium significantly reduced Pb accumulation and alleviated Pb toxicity in mice, indicating that the modulation of gut microbiota may be important for the reduction of Pb toxicity burden in the host.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The animal study was reviewed and approved by the Ethics Committee of Jiangnan University, China.
Author Contributions
QZ and WC designed the experiments. QZ, DQ, and YY carried out the experiments. DQ and SF developed the analysis tools. QZ and DQ wrote the manuscript. LY, FT, JZ, HZ, and WC revised the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China Program [Nos. 31530056, 31820103010, and 31871773]; National Key Research and Development Project [No. 2018YFC1604206]; Projects of Innovation and Development Pillar Program for Key Industries in Southern Xinjiang of Xinjiang Production and Construction Corps [2018DB002]; National First-Class Discipline Program of Food Science and Technology [JUFSTR20180102]; the BBSRC Newton Fund Joint Centre Award; and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.03161/full#supplementary-material
References
Breton, J., Clère, K. L., Daniel, C., Sauty, M., Nakab, L., Chassat, T., et al. (2013). Chronic ingestion of cadmium and lead alters the bioavailability of essential and heavy metals, gene expression pathways and genotoxicity in mouse intestine. Arch. Toxicol. 87, 1787–1795. doi: 10.1007/s00204-013-1032-6
Cani, P. D., Possemiers, S., Van de Wiele, T., Guiot, Y., Everard, A., Rottier, O., et al. (2009). Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103. doi: 10.1136/gut.2008.165886
Cani, P. D., and de Vos, W. M. (2017). Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front. Microbiol. 8:1765. doi: 10.3389/fmicb.2017.01765
Chelakkot, C., Choi, Y., Kim, D. K., Park, H. T., Ghim, J., Kwon, Y., et al. (2018). Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 50:e450. doi: 10.1038/emm.2017.282
Coryell, M., McAlpine, M., Pinkham, N. V., McDermott, T. R., and Walk, S. T. (2018). The gut microbiome is required for full protection against acute arsenic toxicity in mouse models. Nat. Commun. 9:5424. doi: 10.1038/s41467-018-07803-9
Crespo, S., Nonnotte, G., Colin, D. A., Leray, C., and Aubree, A. (2006). Morphological and functional alterations induced in trout intestine by dietary cadmium and lead. J. Fish Biol. 28, 69–80. doi: 10.1111/j.1095-8649.1986.tb05143.x
Curtis, M. M., Hu, Z. P., Klimko, C., Narayanan, S., Deberardinis, R., and Sperandio, V. (2014). The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microb. 16, 759–769. doi: 10.1016/j.chom.2014.11.005
Duboc, H., Rajca, S., Rainteau, D., Benarous, D., Maubert, M.-A., Quervain, E., et al. (2013). Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 62, 531–539. doi: 10.1136/gutjnl-2012-302578
Ebrahimi, R., Faseleh Jahromi, M., Liang, J. B., Soleimani Farjam, A., Shokryazdan, P., and Idrus, Z. (2015). Effect of dietary lead on intestinal nutrient transporters mRNA expression in broiler chickens. Biomed. Res. Int. 2015:149745. doi: 10.1155/2015/149745
Elce, A., Amato, F., Zarrilli, F., Calignano, A., Troncone, R., Castaldo, G., et al. (2017). Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells. Benef. Microb. 8, 1–8. doi: 10.3920/BM2016.0197
El-Ghor, A. A., Noshy, M. M., and Eid, J. I. (2011). Lead acetate and arsenic trioxide induce instability of microsatellites at three different fragile sites (6q21, 9q32-9q33 and 15p14) within the genome of the rat. Mutat. Res. 726, 195–199. doi: 10.1016/j.mrgentox.2011.10.003
Fang, X., Wang, X., Yang, S., Meng, F., Wang, X., Wei, H., et al. (2016). Evaluation of the microbial diversity in amyotrophic lateral sclerosis using high-throughput sequencing. Front. Microbiol. 7:1479. doi: 10.3389/fmicb.2016.01479
Feng, S., Liu, Y., Huang, Y., Zhao, J., Zhang, H., Zhai, Q., et al. (2019). Influence of oral administration of Akkermansia muciniphila on the tissue distribution and gut microbiota composition of acute and chronic cadmium exposure mice. FEMS Microbiol. Lett. 366:fnz160. doi: 10.1093/femsle/fnz160
Flora, G., Gupta, D., and Tiwari, A. (2012). Toxicity of lead: a review with recent updates. Interdiscip. Toxicol. 5, 47–58. doi: 10.2478/v10102-012-0009-2
Furet, J.-P., Kong, L.-C., Tap, J., Poitou, C., Basdevant, A., Bouillot, J.-L., et al. (2010). Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59, 3049–3057. doi: 10.2337/db10-0253
Ganesh, B. P., Klopfleisch, R., Loh, G., and Blaut, M. (2013). Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella typhimurium-infected gnotobiotic mice. PLoS One 8:e74963. doi: 10.1371/journal.pone.0074963
Gao, B., Chi, L., Mahbub, R., Bian, X., Tu, P., Ru, H., et al. (2017). Multi-omics reveals that lead exposure disturbs gut microbiome development, key metabolites and metabolic pathways. Chem. Res. Toxicol. 30, 996–1005. doi: 10.1021/acs.chemrestox.6b00401
Gaudier, E., Jarry, A., Blottière, H. M., De, C. P., Buisine, M. P., Aubert, J. P., et al. (2004). Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastr. 287, 1168–1174. doi: 10.1152/ajpgi.00219.2004
Halttunen, T., Collado, M. C., El-Nezami, H., Meriluoto, J., and Salminen, S. (2008). Combining strains of lactic acid bacteria may reduce their toxin and heavy metal removal efficiency from aqueous solution. Lett. Appl. Microbiol. 46, 160–165. doi: 10.1111/j.1472-765X.2007.02276.x
Hill, D. A., Hoffmann, C., Abt, M. C., Du, Y., Kobuley, D., Kirn, T. J., et al. (2010). Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 3, 148–158. doi: 10.1038/mi.2009.132
Holcombe, G. W., Benoit, D. A., Leonard, E. N., and McKim, J. M. (1976). Long-term effects of lead exposure on three generations of brook trout (Salvelinus fontinalis). J. Fish. Res. Board Can. 33, 1731–1741. doi: 10.1139/f76-220
Hornef, M. W., and Pabst, O. (2015). Real friends: Faecalibacterium prausnitzii supports mucosal immune homeostasis. Gut 65, 365–367. doi: 10.1136/gutjnl-2015-310027
James, H. M., Hilburn, M. E., and Blair, J. A. (1985). Effects of meals and meal times on uptake of lead from the gastrointestinal tract in humans. Hum. Toxicol. 4, 401–407. doi: 10.1177/096032718500400406
Jandhyala, S. M., Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M., and Nageshwar Reddy, D. (2015). Role of the normal gut microbiota. World J. Gastroenterol. 21, 8787–8803. doi: 10.3748/wjg.v21.i29.8787
Jia, W., Xie, G., and Jia, W. (2018). Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastrol. Hepat. 15, 111–128. doi: 10.1038/nrgastro.2017.119
Laval, L., Martin, R., Natividad, J. N., Chain, F., Miquel, S., Maredsous, C., et al. (2015). Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microb. 6, 1–9. doi: 10.4161/19490976.2014.990784
Lechuga, S., and Ivanov, A. I. (2017). Disruption of the epithelial barrier during intestinal inflammation: quest for new molecules and mechanisms. Biochim. Biophys. Acta 1864, 1183–1194. doi: 10.1016/j.bbamcr.2017.03.007
Leclercq, S., Timary, P. D., Neyrinck, A., Cani, P., and Delzenne, N. (2013). O3 ∗ free oral communications 3: alcohol use and liver disease. Alcohol Alcohol. 48, i36–i37. doi: 10.1093/alcalc/agt099
Lee, G. H., Kumar, S., Lee, J. H., Chang, D. H., Kim, D. S., Choi, S. H., et al. (2012). Genome sequence of Oscillibacter ruminantium strain GH1, isolated from rumen of Korean native cattle. J. Bacteriol. 194:6362. doi: 10.1128/JB.01677-12
Loumbourdis, N. S., Kostaropoulos, I., Theodoropoulou, B., and Kalmanti, D. (2007). Heavy metal accumulation and metallothionein concentration in the frog Rana ridibunda after exposure to chromium or a mixture of chromium and cadmium. Environ. Pollut. 145, 787–792. doi: 10.1016/j.envpol.2006.05.011
Machiels, K., Joossens, M., Sabino, J., De Preter, V., Arijs, I., Eeckhaut, V., et al. (2014). A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63, 1275–1283. doi: 10.1136/gutjnl-2013-304833
Nava, G. M., and Stappenbeck, T. S. (2011). Diversity of the autochthonous colonic microbiota. Gut Microb. 2, 99–104. doi: 10.4161/gmic.2.2.15416
Nicholas, A., Clarissa, C., Xiying, F., Stanislav, D., Joris, V. D. V., Paul, D., et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455. doi: 10.1038/nature12726
Ottman, N., Reunanen, J., Meijerink, M., Pietilä, T. E., Kainulainen, V., Klievink, J., et al. (2017). Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One 12:e0173004. doi: 10.1371/journal.pone.0173004
Out, C., Patankar, J. V., Doktorova, M., Boesjes, M., Bos, T., de Boer, S., et al. (2015). Gut microbiota inhibit Asbt-dependent intestinal bile acid reabsorption via Gata4. J. Hepatol. 63, 697–704. doi: 10.1016/j.jhep.2015.04.030
Paassen, N. B., Vincent, A., Puiman, P., van der Sluis, M., Bouma, J., Boehm, G., et al. (2009). The regulation of the intestinal mucin MUC2 expression by short chain fatty acids: implications for epithelial protection. Biochem J. 420, 211–219. doi: 10.1016/S0016-5085(09)60189-X
Pado, R., and Pawłowska-Cwiek, L. (2005). The uptake and accumulation of iron by the intestinal bacterium Desulfotomaculum acetoxidans DSM 771. Folia Biol. 53, 79–81. doi: 10.3409/1734916054663519
Plovier, H., Everard, A., Druart, C., Depommier, C., Van Hul, M., Geurts, L., et al. (2017). A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113. doi: 10.1038/nm.4236
Reikvam, D. H., Erofeev, A., Sandvik, A., Grcic, V., Jahnsen, F. L., Gaustad, P., et al. (2011). Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One 6:e17996. doi: 10.1371/journal.pone.0017996
Rossi, O., van Berkel, L. A., Chain, F., Tanweer Khan, M., Taverne, N., Sokol, H., et al. (2016). Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci. Rep. 6:18507. doi: 10.1038/srep18507
Seregin, S. S., Golovchenko, N., Schaf, B., Chen, J., Pudlo, N. A., Mitchell, J., et al. (2017). NLRP6 protects IL10–/– mice from colitis by limiting colonization of Akkermansia muciniphila. Cell Rep. 19, 733–745. doi: 10.1016/j.celrep.2017.03.080
Shi, Y., Kellingray, L., Zhai, Q., Gall, G. L., Narbad, A., Zhao, J., et al. (2018). Structural and functional alterations in the microbial community and immunological consequences in a mouse model of antibiotic-induced dysbiosis. Front. Microbiol. 9:1948. doi: 10.3389/fmicb.2018.01948
Sokol, H., Seksik, P., Furet, J. P., Firmesse, O., Nion-Larmurier, I., Beaugerie, L., et al. (2009). Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 15, 1183–1189. doi: 10.1002/ibd.20903
Sun, B., Zhang, X., Yin, Y., Sun, H., Ge, H., and Li, W. (2017). Effects of sulforaphane and vitamin E on cognitive disorder and oxidative damage in lead-exposed mice hippocampus at lactation. J. Trace Elem. Med. Bio. 44, 88–92. doi: 10.1016/j.jtemb.2017.06.004
Takao, I., Koji, M., Kenji, T., Ken-Ichiro, S., and Shigeaki, H. (2007). Oscillibacter valericigenes gen. nov., sp. nov., a valerate-producing anaerobic bacterium isolated from the alimentary canal of a Japanese Corbicula clam. Int. J. Syst. Evol. Microbiol. 57, 1840–1845. doi: 10.1099/ijs.0.64717-0
Teemu, H., Seppo, S., Jussi, M., Raija, T., and Kalle, L. (2008). Reversible surface binding of cadmium and lead by lactic acid and Bifidobacteria. Int. J. Food Microbiol. 125, 170–175. doi: 10.1016/j.ijfoodmicro.2008.03.041
Tian, F., Zhai, Q., Zhao, J., Liu, X., Wang, G., Zhang, H., et al. (2012). Lactobacillus plantarum CCFM8661 alleviates lead toxicity in mice. Biol. Trace Elem. Res. 150, 264–271. doi: 10.1007/s12011-012-9462-1
Topcu, A., and Bulat, T. (2010). Removal of cadmium and lead from aqueous solution by Enterococcus faecium strains. J. Food Sci. 75, T13–T17. doi: 10.1111/j.1750-3841.2009.01429.x
Vorvolakos, T., Arseniou, S., and Samakouri, M. (2016). There is no safe threshold for lead exposure: a literature review. Psychiatriki 27, 204–214. doi: 10.22365/jpsych.2016.273.204
Wang, A., Ling, Z., Yang, Z., Kiela, P. R., Wang, T., Wang, C., et al. (2015). Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: a pilot study. PLoS One 10:e0126312. doi: 10.1371/journal.pone.0126312
Wang, L., Hu, L., Yan, S., Jiang, T., Fang, S., Wang, G., et al. (2017). Effects of different oligosaccharides at various dosages on the composition of gut microbiota and short-chain fatty acids in mice with constipation. Food Funct. 8:1966. doi: 10.1039/c7fo00031f
Wong, J. M., De, S. R., Kendall, C. W., Emam, A., and Jenkins, D. J. (2006). Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40, 235–243. doi: 10.1097/00004836-200603000
Wrzosek, L., Miquel, S., Noordine, M.-L., Bouet, S., Joncquel Chevalier-Curt, M., Robert, V., et al. (2013). Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 11:61. doi: 10.1186/1741-7007-11-61
Wu, J., Wen, X. W., Faulk, C., Boehnke, K., Zhang, H., Dolinoy, D. C., et al. (2016). Perinatal lead exposure alters gut microbiota composition and results in sex-specific bodyweight increases in adult mice. Toxicol. Sci. 151, 324–333. doi: 10.1093/toxsci/kfw046
Wu, Y. Y., Hsu, C. M., Chen, P. H., Fung, C. P., and Chen, L. W. (2014). Toll-like receptor stimulation induces nondefensin protein expression and reverses antibiotic-induced gut defense impairment. Infect. Immun. 82, 1994–2005. doi: 10.1128/IAI.01578-14
Xia, J., Lu, L., Jin, C., Wang, S., Zhou, J., Ni, Y., et al. (2018). Effects of short term lead exposure on gut microbiota and hepatic metabolism in adult zebrafish. Comp. Biochem. Phys. C 209, 1–8. doi: 10.1016/j.cbpc.2018.03.007
Yi, Y. J., Lim, J. M., Gu, S., Lee, W. K., Oh, E., Lee, S. M., et al. (2017). Potential use of lactic acid bacteria Leuconostoc mesenteroides as a probiotic for the removal of Pb(II) toxicity. J. Microbiol. 55, 296–303. doi: 10.1007/s12275-017-6642-x
Youravong, N., Carlen, A., Teanpaisan, R., and Dahlén, G. (2011). Metal-ion susceptibility of oral bacterial species. Lett. Appl. Microbiol. 53, 324–328. doi: 10.1111/j.1472-765X.2011.03110.x
Zhai, Q., Cen, S., Li, P., Tian, F., Zhao, J., Zhang, H., et al. (2018). Effects of dietary selenium supplementation on intestinal barrier and immune responses associated with its modulation of gut microbiota. Environ. Sci. Tech. Let. 5, 724–730. doi: 10.1021/acs.estlett.8b00563
Zhai, Q., Li, T., Yu, L., Xiao, Y., Feng, S., Wu, J., et al. (2017). Effects of subchronic oral toxic metal exposure on the intestinal microbiota of mice. Sci. Bull. 62, 831–840. doi: 10.1016/j.scib.2017.01.031
Keywords: Pb toxicity, gut bacteria, intestinal barrier, oxidative stress, short chain fatty acids
Citation: Zhai Q, Qu D, Feng S, Yu Y, Yu L, Tian F, Zhao J, Zhang H and Chen W (2020) Oral Supplementation of Lead-Intolerant Intestinal Microbes Protects Against Lead (Pb) Toxicity in Mice. Front. Microbiol. 10:3161. doi: 10.3389/fmicb.2019.03161
Received: 21 November 2019; Accepted: 31 December 2019;
Published: 22 January 2020.
Edited by:
Zongxin Ling, Zhejiang University, ChinaReviewed by:
Tingtao Chen, Nanchang University, ChinaDiling Chen, Guangdong Academy of Science, China
Jiufu Qin, Technical University of Denmark, Denmark
Copyright © 2020 Zhai, Qu, Feng, Yu, Yu, Tian, Zhao, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Chen, Y2hlbndlaTY2QGppYW5nbmFuLmVkdS5jbg==
†These authors have contributed equally to this work
 Qixiao Zhai
Qixiao Zhai Dingwu Qu
Dingwu Qu Saisai Feng
Saisai Feng Yaqi Yu
Yaqi Yu Leilei Yu
Leilei Yu Fengwei Tian
Fengwei Tian Jianxin Zhao
Jianxin Zhao Hao Zhang1,2,5,6
Hao Zhang1,2,5,6 Wei Chen
Wei Chen