- 1School of Life Sciences, Sun Yat-sen University, Guangzhou, China
- 2Biomedical Center, Sun Yat-sen University, Guangzhou, China
Legionella pneumophila, an environmental bacterium that parasitizes protozoa, is the causative pathogen of Legionnaires' disease. L. pneumophila adopts a distinct biphasic life cycle that allows it to adapt to environmental conditions for survival, replication, and transmission. This cycle consists of a non-virulent replicative phase (RP) and a virulent transmissive phase (TP). Timely and fine-tuned expression of growth and virulence factors in a life cycle-dependent manner is crucial. Herein, we report evidence that CsrA, a key regulator of the switch between the RP and the TP, is dually regulated in a ClpP-dependent manner during the biphasic life cycle of L. pneumophila. First, we show that the protein level of CsrA is temporal during the life cycle and is degraded by ClpP during the TP. The ectopic expression of CsrA in a ΔclpP mutant, but not in the wild type, inhibits both the initiation of the RP in vitro and the invasiveness to Acanthamoeba castellanii, indicating that the ClpP-mediated proteolytic pathway regulates the CsrA protein level. We further show that the temporally expressed IHFB is the transcriptional inhibitor of csrA and is degraded via a ClpP-dependent manner during the RP. During the RP, the level of CsrA is increased by promoting the degradation of IHFB and reducing the degradation of the accumulated CsrA via a ClpP-dependent manner. During the TP, the level of CsrA is decreased by inhibiting the degradation of IHFB and promoting the degradation of the accumulated CsrA via a ClpP-dependent manner as well. In conclusion, our results show that the growth-stage-specific expression level of CsrA is dually regulated by ClpP-dependent proteolysis at both the transcription and protein levels during the biphasic life cycle of L. pneumophila.
Introduction
Legionella pneumophila is a Gram-negative intracellular bacterial pathogen that is the causative agent for most cases of Legionnaires' disease (Fields et al., 2002; Newton et al., 2010; Guyard and Low, 2011). L. pneumophila has a biphasic life cycle that allows it to benefit from the environment of the susceptible host cell and simultaneously ensure its persistence for another infection cycle (Oliva et al., 2018). Within host cells, the bacteria differentiate into two forms—replicative and transmissive—undergoing physiological, morphogenetic, and metabolic changes (Molofsky and Swanson, 2004; Bruggemann et al., 2006). In broth culture, the bacteria enter exponential and post-exponential forms, requiring similar physiological, morphogenetic, and metabolic changes (Byrne and Swanson, 1998; Hammer and Swanson, 1999). The gene expression programs in replicative and transmissive bacteria in vivo are similar to that of exponential and post-exponential bacteria in vitro, respectively, suggesting that the biphasic life cycle is controlled globally by the bacterial growth phase and/or nutrient availability (Oliva et al., 2018). The transition from exponential/replicative phase (RP) to post-exponential/transmissive phase (TP) is governed by a common virulence program (Bruggemann et al., 2006; Faucher et al., 2011). Therefore, the exponential and post-exponential phase in broth cultures has been used to emulate the RP and TP of the L. pneumophila life cycle (Bruggemann et al., 2006).
The biphasic life cycle of L. pneumophila is crucial for the fitness of the pathogen and is linked to its metabolism (Molofsky and Swanson, 2004). L. pneumophila employs at least four distinct two-component systems (TCSs), including LetA/S, PmrA/B, LsqR/ST, and CpxR/A, which govern its differentiation from the replicative to the transmissive state (Gal-Mor and Segal, 2003; Jacobi et al., 2004; Tiaden et al., 2007; Zusman et al., 2007; Altman and Segal, 2008). However, the underlying regulatory cascades and environmental cues controlling this dimorphism are poorly understood. A key regulator of the switch between RP and TP in L. pneumophila is the carbon storage regulator CsrA, a pivotal repressor of transmission traits and activator of replication (Molofsky and Swanson, 2003; Forsbach-Birk et al., 2004). CsrA is a posttranscriptional regulator that represses a variety of post-exponential phase genes in bacteria, which plays important roles in regulating motility, virulence, and metabolism (Vakulskas et al., 2015). CsrA was reported to bind more than 450 mRNA targets in L. pneumophila, altering their translation, transcription, and/or stability (Molofsky and Swanson, 2003; Sahr et al., 2009, 2017). This indicates that CsrA is indispensable and plays an essential role in the life cycle (Molofsky and Swanson, 2003; Sahr et al., 2017). Three TCSs—LetS/LetA, PmrB/PmrA, and LqsS/LqsT/LqsR—have been shown to regulate CsrA activity (Vakulskas et al., 2015). However, the regulatory factors that directly fine-tune the timing of CsrA expression have not yet been identified in L. pneumophila or any other bacterium.
Regulation of gene expression by controlled proteolysis contributes to the survival of pathogenic bacteria during host interaction. This mechanism was first elucidated by the discovery of several global regulatory proteins that are under proteolytic control (Gottesman, 2003; Mahmoud and Chien, 2018). ClpP, the catalytic core of the Clp proteolytic complex, is highly conserved in bacteria and widely involved in many cellular processes by regulating intracellular protein quality (Mahmoud and Chien, 2018). Indeed, ClpP is required for the intracellular proliferation of L. pneumophila in both amoeba and murine macrophages (Li et al., 2010; Zhao et al., 2016) as well as for optimal translocation of several effector proteins (Zhao et al., 2016). ClpP also plays an important role in cell division and the expression of transmission traits of L. pneumophila, suggesting a putative role for ClpP in the regulation of its life cycle (Li et al., 2010). However, the underlying regulatory mechanism affected by ClpP in transition between the RP and the TP is poorly understood.
In this study, we investigated how CsrA expression during the life cycle of L. pneumophila is regulated in a finely tuned and temporal manner that is dependent on ClpP. We found that CsrA is required for bacterial cells in the TP to passage into the RP. We further demonstrated that ClpP regulates the expression of CsrA, thereby controlling the inhibitory function of CsrA during intracellular proliferation. Finally, we showed that IHFB is a transcriptional inhibitor of CsrA. During the RP, the level of IHFB decreases, allowing transcription and elevated protein levels of CsrA. This pairs with a decrease in CsrA clearance, with both effects dependent on ClpP. In contrast, during the TP, ClpP proteolysis degrades accumulated CsrA and allows IHFB to increase, cutting off transcription of CsrA. These findings reveal the temporal regulation mechanism of CsrA by ClpP during the biphasic life cycle of L. pneumophila.
Results
CsrA Is Temporally Expressed During the Life Cycle and Is Regulated by ClpP
In order to assess the protein level of CsrA at different growth phases, we performed proteomic analysis of whole lysates obtained from cultures of L. pneumophila wild-type (WT) strain grown in liquid medium at indicated time points. The results showed that CsrA was not detected during the TP, while high levels of CsrA were detected upon entry into the RP (Figure 1A). Thus, the presence of CsrA during the biphasic life cycle of L. pneumophila is growth-phase-dependent.
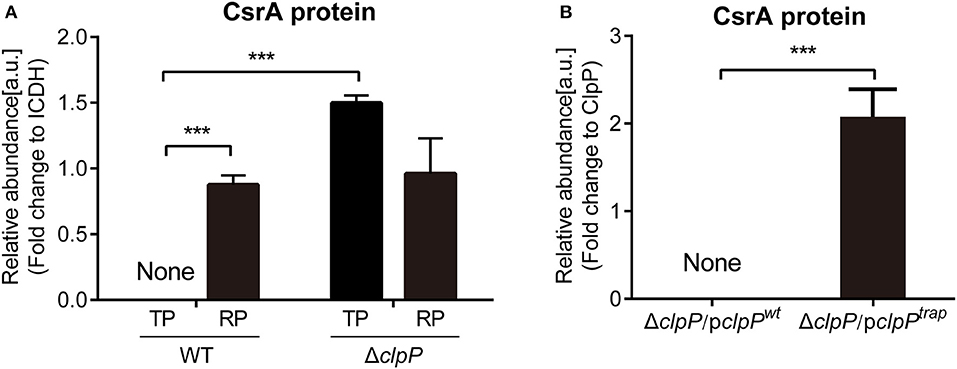
Figure 1. Proteomics analysis of CsrA and identification of CsrA as a substrate of ClpP. (A) The abundance of CsrA in WT and ΔclpP at indicated growth phases. WT and ΔclpP were inoculated into fresh AYE medium at the same initial OD600 values. Bacterial cells in the RP were harvested at an OD600 of 0.7–1.0 and those in the TP were harvested approximately 6 h after the cessation of growth. Total proteins from indicated samples were extracted for proteomic analysis, and the representative peptides were identified by mass spectrometry. ICDH was measured as a loading control. Data represent mean ± SD derived from three independent experiments. ***p < 0.001 were identified by GraphPad Prism. (B) The abundance of CsrA in ΔclpP/pclpPwt and ΔclpP/pclpPtrap in the TP. Bacterial cells were harvested approximately 6 h after the cessation of growth. Bacterial whole-cell lysates from ΔclpP/pclpPwt and ΔclpP/pclpPtrap were prepared and His-tagged proteins were purified by Ni-NTA affinity. Substrates captured inside the proteolytic barrel were co-purified along with the His-tagged ClpP complex and identified by mass spectrometry. ClpP was measured as a loading control. Data represent mean ± SD derived from three independent experiments. ***p < 0.001 were identified by GraphPad Prism.
Our previous work suggested a putative role for the protease ClpP in the regulation of life cycle and virulence in L. pneumophila based on the behavior of a clpP deletion mutant, ΔclpP (Li et al., 2010; Zhao et al., 2016). We designed experiments to determine whether the regulation of CsrA is associated with ClpP. To validate the mutant strain, the whole genomes of WT and ΔclpP were re-sequenced and compared (WT Accession: LP02, PRJNA522676; ΔclpPAccession:XP02, PRJNA522681). The results showed that only the clpP gene had been specifically knocked out (Supplementary Figure S1). Therefore, the clpP allele complemented strain will not be included in the experiments below where indicated.
The protein levels of CsrA in ΔclpP were determined by proteomic analysis of whole lysates of L. pneumophila grown in liquid medium and collected during different growth phases (Supplementary Figure S2). As shown in Figure 1A, CsrA was not detected during the TP in WT cells, while it was highly accumulated in the ΔclpP mutant. During the RP, CsrA were detected in both WT and ΔclpP, with no significant difference between them. These data indicate that ClpP is involved in the temporal regulation of CsrA.
Next, we determined whether CsrA is a substrate of ClpP. ClpPtrap is a proteolytically inactive form of ClpP that will retain but not degrade substrates translocated into its proteolytic chamber (Flynn et al., 2003; Neher et al., 2006; Feng et al., 2013). To generate the ClpPtrap, the active site (serine 110) of ClpP was replaced with an alanine (S110A) (Supplementary Figure S3A). The plasmids expressing His-tagged ClpPwt and ClpPtrap were transformed into ΔclpP to create ΔclpP/pclpPwt and ΔclpP/pclpPtrap, respectively (Supplementary Figure S3A). Expression of the His-tagged recombinant ClpP proteins was verified in each strain (Supplementary Figure S3B). Growth curves were similar for ΔclpP/pclpPwt and WT strains (Supplementary Figure S3C) as well as ΔclpP/pclpPtrap and ΔclpP (Supplementary Figure S3D). The heterologous level of intact or mutated clpP does not interfere with Legionella growth. Thus, we have successfully constructed strains to screen the substrates of ClpP.
Substrates captured inside the proteolytic barrel were co-purified along with the His-tagged ClpP complex and identified by mass spectrometry as previously described (Feng et al., 2013). We found that CsrA was detected in ΔclpP/pclpPtrap, but not in ΔclpP/pclpPwt (Figure 1B, Supplementary Figure S4), indicating that CsrA is a substrate of ClpP. Taken together, these data demonstrated that CsrA is temporally expressed during the life cycle and its regulation is dependent on ClpP.
The Protein Level of CsrA Is Critical for Transition of L. pneumophila From the TP Into the RP
Because csrA is an essential gene, conditional and partial mutants are strongly attenuated for growth (Molofsky and Swanson, 2003; Sahr et al., 2017). Moreover, CsrA controls its own expression in a regulatory feedback loop (Sahr et al., 2009, 2017; Yakhnin et al., 2011). These attributes make the study of CsrA regulation difficult, but also crucial. To sidestep these issues, we constructed plasmids pJB908-csrA to express CsrA and pJB908-gfp to express GFP, both under control of the mip promoter (Supplementary Figure S5A). The transcriptional levels of mip and csrA are consistent in the RP and the TP of L. pneumophila (Bruggemann et al., 2006; Faucher et al., 2011). The plasmids were transformed into WT and ΔclpP to create WT/pcsrA, ΔclpP/pcsrA, WT/pgfp, and ΔclpP/pgfp (Supplementary Figure S5A).
Bacterial inoculum from the TP culture was used to measure the impact of CsrA on the life cycle. Compared to WT/pJB908 and ΔclpP/pJB908, ectopic CsrA did not affect the growth of WT, but significantly prolonged the lag phase of ΔclpP (p < 0.001) and weakened its proliferation (Figure 2A). Meanwhile, GFP control did not affect the growth of either WT or ΔclpP (Supplementary Figure S6), indicating that the effect of CsrA on the growth of ΔclpP is indeed due to the lack of the regulation of CsrA by ClpP, rather than non-specific stress from protein accumulation.
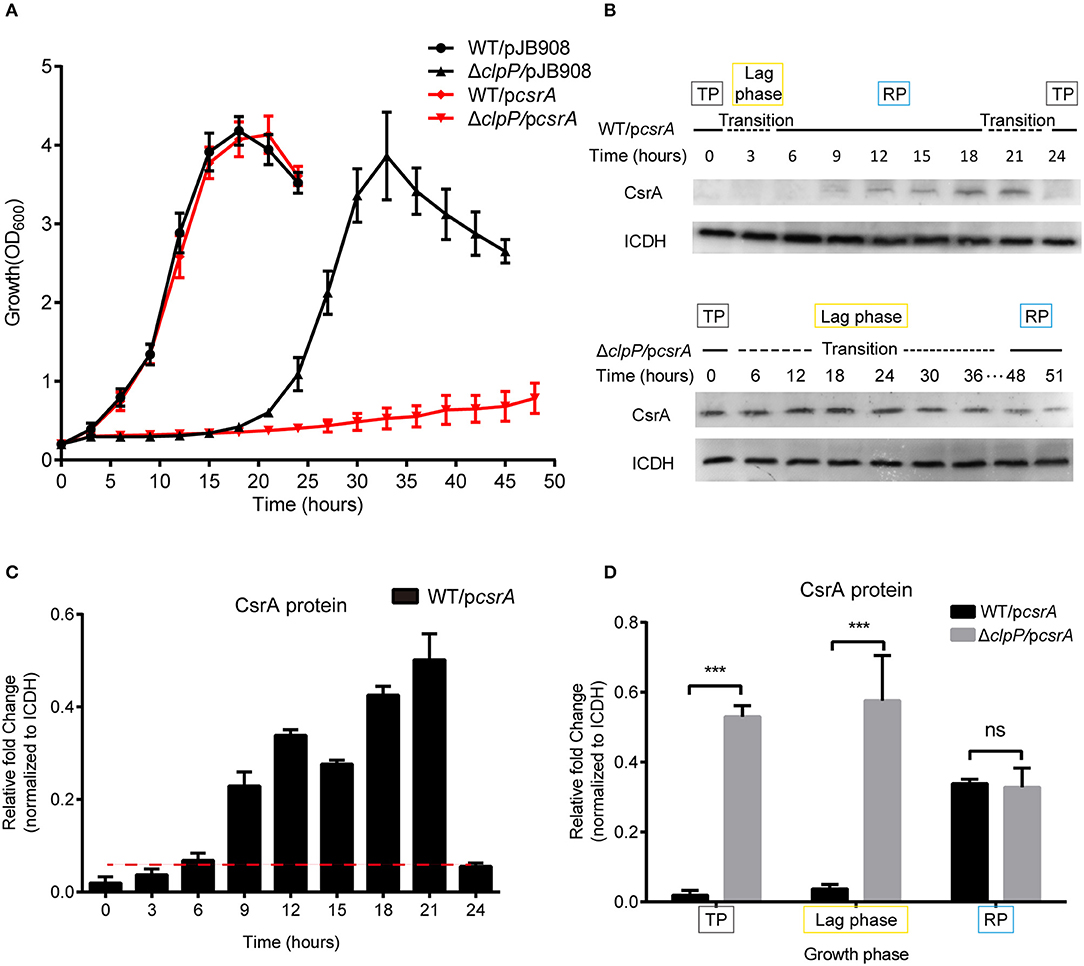
Figure 2. Accumulation of CsrA due to loss of ClpP regulation delays the transition of L. pneumophila from the TP into the RP. (A) Growth curves of L. pneumophila wild-type strain WT (•), the clpP deletion mutant ΔclpP (▴), WT with csrA expression (WT/pcsrA) (), ΔclpP with csrA expression (ΔclpP/pcsrA) (). For negative controls, pJB908 vector was electroporated into WT and ΔclpP to create WT/pJB908, ΔclpP/pJB908, respectively. Bacterial strains in TP (OD600 = 3.0–3.5) were grown in AYE medium at 37°C and samples were taken every 3 h for determination of optical density at 600 nm. (B) Ectopic protein levels of CsrA during the bacterial growth period. Bacterial whole-cell lysates from WT/pcsrA and ΔclpP/pcsrA were prepared and an immunoblot of CsrA was probed with an anti-His tag antibody. ICDH was measured as a loading control. RP refers to the exponential growth of bacteria in AYE broth, and TP refers to the period approximately 6 h after the cessation of growth. Lag phase refers to the transition from transmissive phase to replicative phase in rich medium. (C) Ectopic protein levels of CsrA in WT/pcsrA calculated by ImageJ at the indicated time points. (D) Relative protein levels of CsrA in the RP, lag phase, and TP of WT/pcsrA and ΔclpP/pcsrA calculated by ImageJ. Bacterial cells in the RP were harvested at an OD600 of 0.7–1.0 and those in the TP were harvested approximately 6 h after the cessation of growth. Data represent mean ± SD derived from three independent experiments. ***p < 0.001 were identified by GraphPad Prism.
Lysates were prepared from the indicated time points (Figure 2A) and analyzed by Western blotting. In WT/pcsrA, CsrA was undetected during the TP and the transition from the TP to the RP, but was detected during the RP (Figure 2B), indicating that the level of CsrA is life-cycle-dependent (Figure 2C). In ΔclpP/pcsrA, however, CsrA was detected in both the TP and throughout the transition phase (Figure 2B). Liquid culture observation and quantitative Western blotting analysis showed that, compared to WT/pcsrA, the protein level of CsrA in the clpP mutant significantly increased during the TP and the lag phase, while it was identical during the RP (Figure 2D), indicating that the degradation of CsrA is ClpP-dependent and occurs during the TP. Meanwhile, the upregulation of the protein level of CsrA in WT/pcsrA during the RP indicates that the degradation of CsrA via ClpP is temporally regulated. In the negative control, GFP continuously accumulated and the protein levels were consistent in WT/pgfp and ΔclpP/pgfp at the same growth phase (Supplementary Figures S7A–C), confirming that CsrA is regulated by ClpP. Our analysis indicated that ClpP is expressed throughout the life cycle of L. pneumophila (Supplementary Figure S8), indicating that its protein hydrolysis function is necessary during the biphasic life cycle. These data demonstrate that the accumulation of CsrA delays the transition of L. pneumophila from the TP into the RP, and that the protein level of CsrA is life-cycle-dependent and temporally regulated via ClpP during the transition between the replicative and transmissive forms.
Observation of Morphology Indicates That Accumulation of CsrA During the TP Affects the Transition Into the RP
L. pneumophila cells alternate between different morphogenetic forms, including the slender rods in the RP and the short rods in the TP during its biphasic life cycle (Oliva et al., 2018). The morphological changes of the indicated strains were analyzed during their life cycle (Figure 3). Cells were observed under 100× magnification, and their lengths were measured with ImageJ at different time points during growth. In the WT strains with or without ectopic expression of csrA, the cells showed typical morphology corresponding to each growth phase (Figure 3A, row1 and 2, Figures 3B,C). However, compared to ΔclpP, the changes of cell length in the ΔclpP with accumulation of CsrA occurred much later throughout the indicated time points (Figure 3A, rows 3 and 4, Figures 3D,E), indicating that the accumulation of CsrA due to the loss of the regulation of ClpP (Figure 1B) blocked entry into the RP.
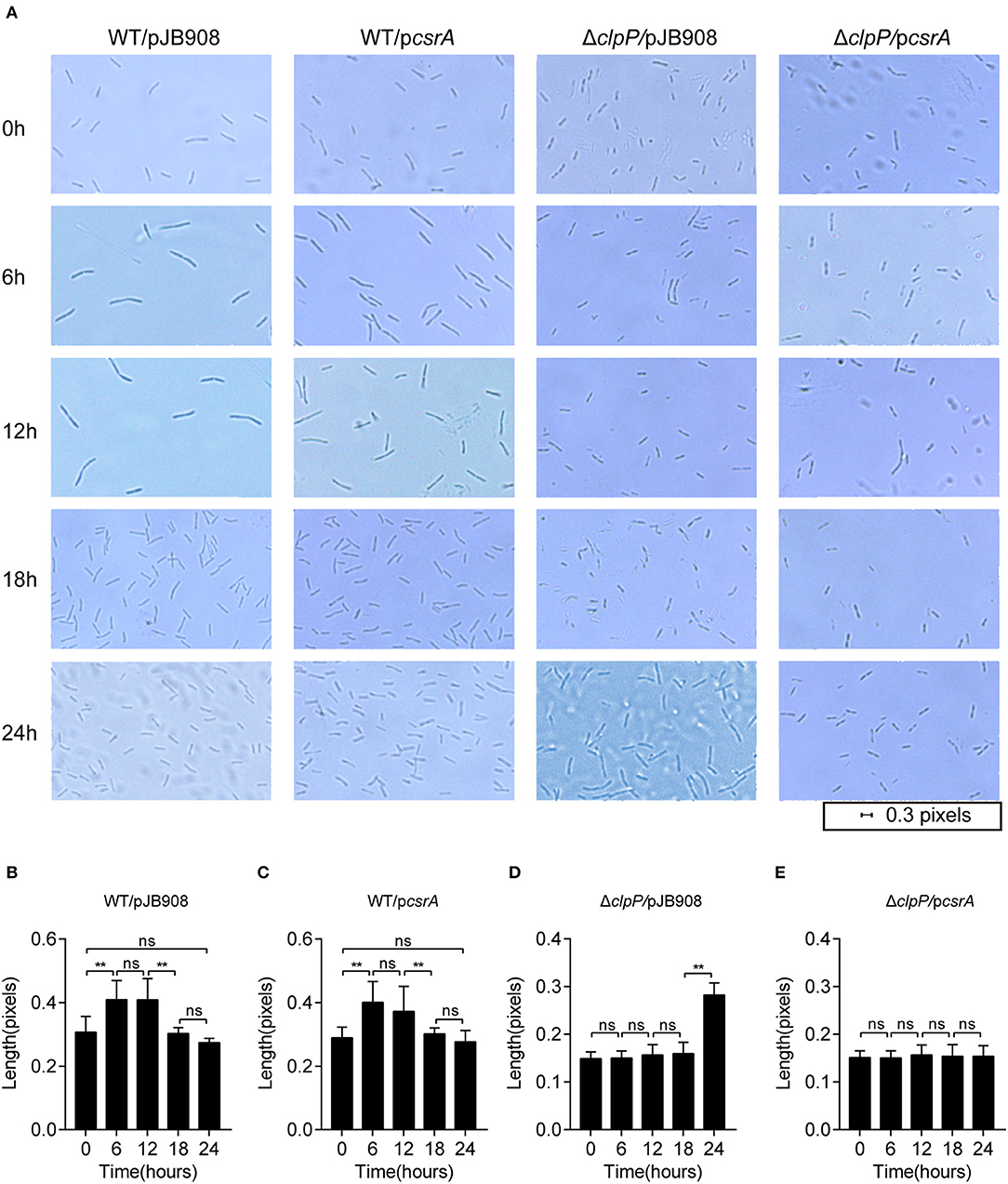
Figure 3. Morphological observation shows that the ClpP-dependent level of CsrA affects entry into the RP. (A) Bacterial morphology of L. pneumophila observed with 100× magnification under oil at the indicated time points. The indicated strains in the TP were inoculated into AYE media to the same initial OD600 of 0.2 with shaking at 37°C. (B–E) Cell length of WT/pJB908 (B), WT/pcsrA (C), ΔclpP/pJB908 (D), and ΔclpP/pcsrA (E) was calculated with ImageJ at the indicated time points. Data represent mean ± SEM derived from three independent experiments. **p < 0.01 were identified by GraphPad Prism. ns means no difference from the wild type.
The growth phenotypes were characterized by measuring the transcriptional levels of the RP genes (mip and secE) and the TP genes (flaA and fliA) (Bruggemann et al., 2006) by qRT-PCR. The transcriptional level of life-cycle-dependent genes demonstrates that ΔclpP mutant remains in the TP during the prolonged lag phase (Figure 3B; Supplementary Figure S9A). Expectedly, the TP genes were upregulated while the RP genes were downregulated during the prolonged lag phase in ΔclpP/pcsrA (Supplementary Figure S9B). The transcription levels of the four genes were determined in ΔclpP/pJB908 and ΔclpP/pcsrA at time points of 0, 6, 12, and 18 h. Compared to ΔclpP, TP genes were highly upregulated while RP genes were suppressed with ectopic expression of csrA in ΔclpP (Supplementary Figure S9C). This indicates that the prolonged lag phase of ΔclpP/pcsrA is associated with the accumulation of CsrA (Figure 2B). In conclusion, cell morphology is in agreement with the molecular reality during life cycle transition, indicating that the CsrA control via ClpP is critical for life cycle transition in L. pneumophila.
Accumulation of CsrA During the TP Reduces the Viability of L. pneumophila in the Amoebae Acanthamoeba castellanii
To investigate whether the accumulation of CsrA affects the bacterial infectivity to host cells, L. pneumophila strains in TP were exposed to amoebae A. castellanii and the co-cultures were maintained for 2 h. Then, the extracellular bacteria were cleared and the amoebae were lysed to release L. pneumophila and calculate colony-forming units (CFU) of the infectious bacteria. The survival capability of WT/pcsrA was similar to WT after phagocytosis (Figure 4). However, the survival capability of ΔclpP/pcsrA was significantly (p < 0.01) lower than that of ΔclpP harboring the empty vector (Figure 4). These results indicate that the protein level of CsrA is important for the viability of L. pneumophila after phagocytosis.
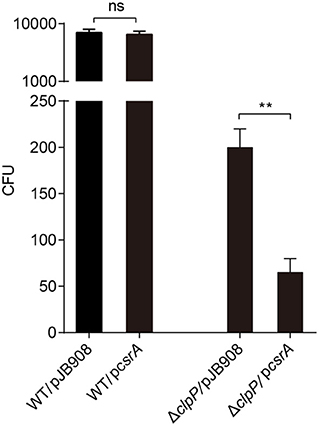
Figure 4. Viability of strains in TP with accumulated CsrA is impaired within the amoebae A. castellanii at the early post infection stage. A. castellanii were infected with TP-phase strains WT/pJB908, ΔclpP/pJB908, WT/pcsrA and ΔclpP/pcsrA at an MOI = 10. pJB908 vector can express the thymine required for growth of bacteria in vivo. Thirty minutes post infection, extracellular bacteria were removed by washing with warm HL5 medium three times. Infected amoebae cells were lysed after 90 min and intracellular bacteria were quantified by determining the CFU. Each time point represents the mean ± SD from three independent experiments. The quantitative data were analyzed using two-way analysis of variance (ANOVA) test by GraphPad Prism. The values that are significantly different are indicated by a bar and asterisk as follows: **p < 0.01. ns means no difference from the wild type.
The Transcription of CsrA Is Temporally Regulated in a ClpP-Dependent Manner
Both the endogenous (Figure 1A) and heterologous protein levels of CsrA (Figures 2B,C) are regulated by ClpP throughout the life cycle of L. pneumophila. We investigated whether the regulation was under transcriptional control by ClpP as well. We found that the transcriptional level of csrA was upregulated in WT upon entry into the RP but was repressed during the TP (Figure 5A), indicating that the transcription of csrA is also temporal. We used qRT-PCR for transcriptional analysis of csrA in WT and ΔclpP. Compared to WT, the transcriptional level of csrA in ΔclpP significantly decreased during the RP, while it was identical during the TP (Figure 5B), suggesting that the transcription of csrA is also life-cycle-dependent and temporally regulated in a ClpP-dependent manner.
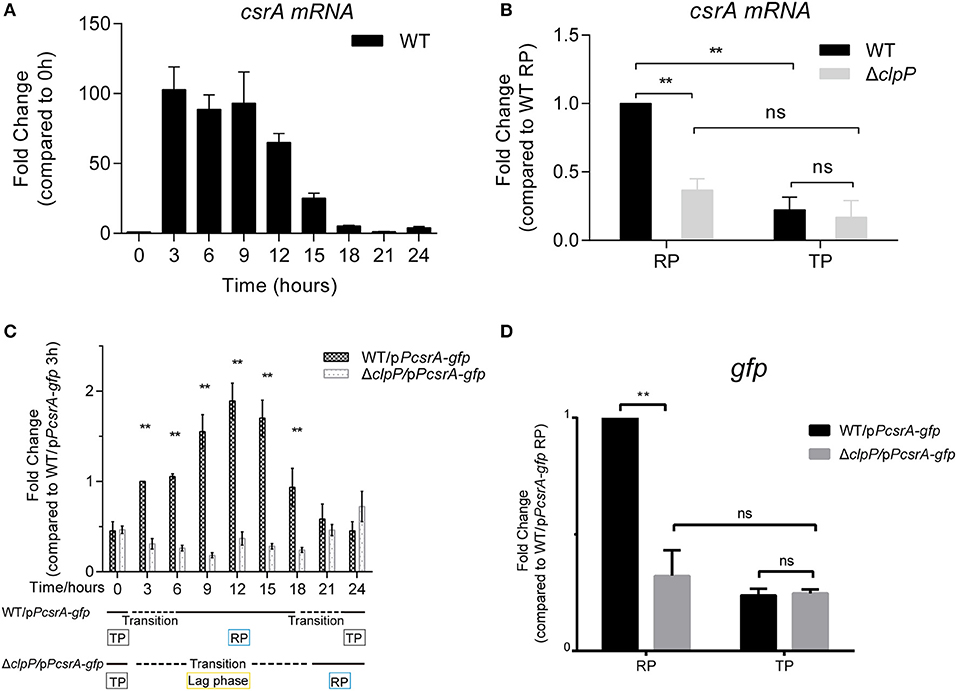
Figure 5. The transcription of csrA is temporally regulated by ClpP during the transition between the replicative and transmissive forms. (A) Transcriptional profile of csrA of WT. Total RNA was prepared from WT at the indicated time points. The transcriptional levels at 0 h were normalized to 1.0. (B) Comparison of the transcriptional levels of csrA in WT and ΔclpP. Bacterial cells in the RP were harvested at an OD600 of 0.7–1.0 and those in the TP were harvested approximately 6 h after the cessation of growth, and the total RNA was prepared. The transcriptional levels of WT at RP were normalized to 1.0. Data represent mean ± SD derived from three independent experiments. **p < 0.01 were identified by GraphPad Prism. (C) Transcriptional profiles of gfp under the control of csrA promoter during the life cycle. Total RNA was prepared from WT and ΔclpP harboring a pPcsrA–gfp reporter plasmid at the indicated time points. Transcriptional levels in WT/pPcsrA-gfp at 3 h were normalized to 1.0. Data represent mean ± SD derived from three independent experiments. **p < 0.01 were identified by GraphPad Prism. (D) Relative transcriptional levels of gfp in the RP and TP calculated by ImageJ. Bacterial cells in the RP were harvested at an OD600 of 0.7–1.0 and those in the TP were harvested approximately 6 h after the cessation of growth. Data represent mean ± SD derived from three independent experiments. **p < 0.01 were identified by GraphPad Prism.
To rule out the influence of self-regulated CsrA, a vector in which gfp is expressed under the control of the csrA promoter was transformed into WT and ΔclpP (Supplementary Figure S5B). The expression of gfp did not affect the growth of WT or ΔclpP (Supplementary Figure S6). As shown in Figure 5C, the transcriptional level of gfp in ΔclpP is consistent throughout the life cycle, while it is life-cycle-dependent in WT. This result confirms that csrA transcription is temporally regulated in a ClpP-dependent manner. Quantitative analysis further supported this conclusion because the gfp level in ΔclpP was significantly downregulated compared to that in WT during the RP, but was identical during the TP (Figure 5D). Taken together, these data indicate that the transcription of CsrA is also temporally regulated in a ClpP-dependent manner.
IHFB Binds Directly to the csrA Promoter Region
The realization that csrA is regulated by ClpP raises the possibility that the expression of csrA is controlled by an unknown transcriptional inhibitor that is degraded by the ClpP-mediated pathway. To test this possibility, we performed bioinformatic analysis of the upstream region of the csrA operon and found an IHF binding site (Figure 6A). IHF is a global transcriptional regulator reported in Escherichia coli, Shigella flexneri, and Caulobacter (Craig and Nash, 1984; Gober and Shapiro, 1990; Porter and Dorman, 1997; Ali Azam et al., 1999; Goodman et al., 1999). Alignment analysis of the known IHF sequences identified the highly conserved IHFB protein as the candidate regulator in L. pneumophila (Figure 6B).
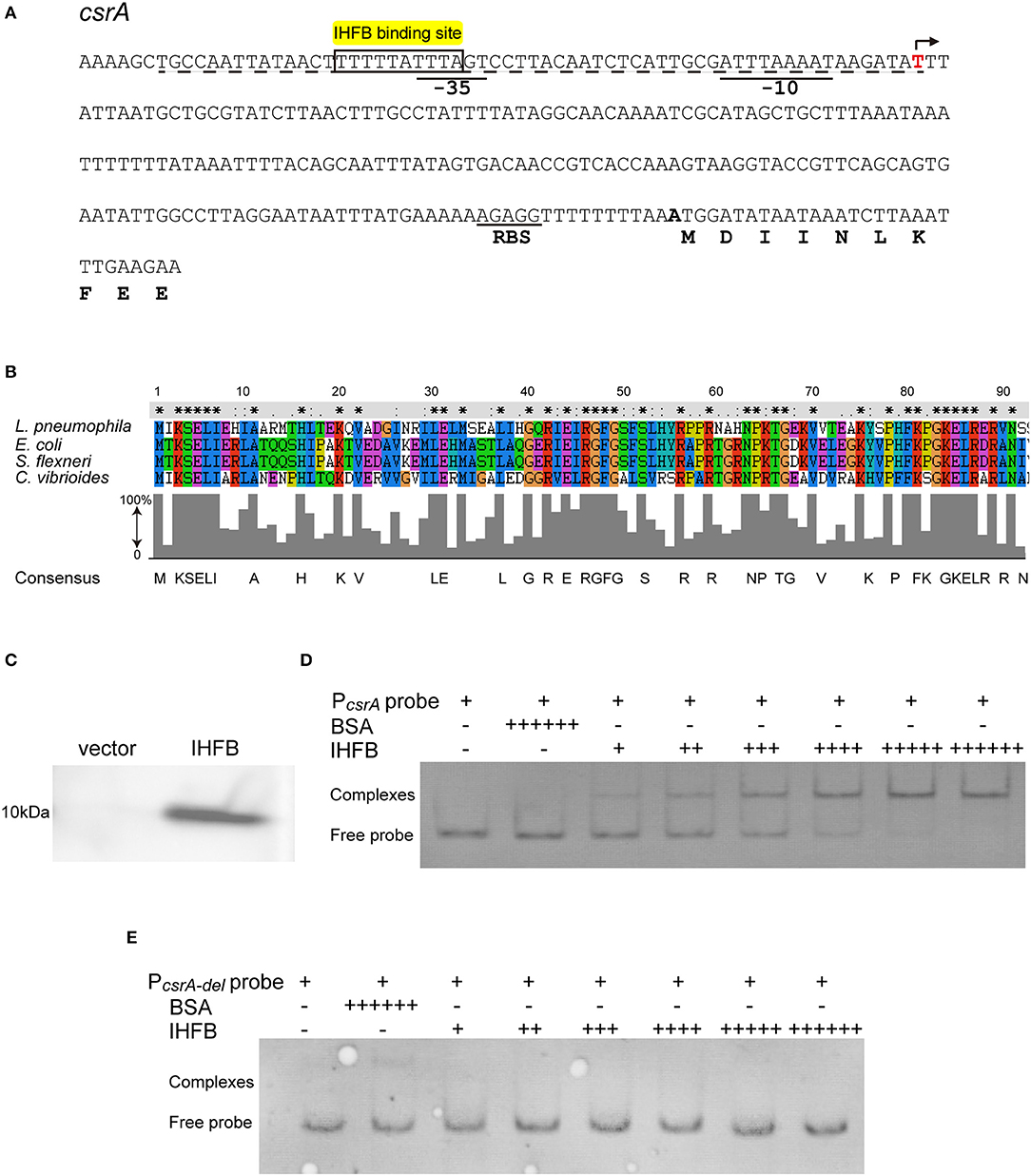
Figure 6. IHFB binds directly to the regulatory region of csrA. (A) Map of the promoter regions of csrA and positions of putative IHF binding sites. The transcriptional start site is indicated by an angled arrow. Possible −10 site, −35 site, and RBS site sequences are underlined. Predicted IHFB binding sites are labeled in yellow. The dashed line indicates the 60 bp DNA fragment that is deleted in the “PcsrA-del” probe. Bioinformatics analysis was performed using Softberry software. (B) Sequence alignment of the putative IHFB from L. pneumophila with other prokaryotic IHF proteins. Numbers indicate the positions of amino acids in the sequences. Identical or similar residues are labeled with asterisks or periods, respectively. (C) Immunoblot of IHFB expressed in the BL21 strain of E. coli. The coding regions of IHFB were PCR amplified and fused to the expression vector pET-28a by the Gibson assembly method. The fusion gene constructs were transformed into E. coli strain BL21. Expression of IHFB was induced by IPTG to a final concentration of 10 μM. Proteins extracted from equivalent numbers of recombinant bacteria (1 × 109 cells) were loaded onto an SDS-PAGE gel and IHFB was detected using an anti-His tag antibody. (D,E) EMSA analysis of in vitro binding of IHFB to the promoter region of csrA (PcsrA probe) (D) and the promoter region deleted 60 bp upstream from the transcriptional start site of csrA (PcsrA-del probe) (E). An equivalent amount of the csrA probe DNA (200 ng) was added to every lane. BSA is used as a negative control for normalization, in which the amount is excessive (+ + + + ++). The first two lanes contain no IHFB (–) and the amount of IHFB is gradually increased (+). Each + sign indicates 200 ng of protein.
To determine if the IHFB protein binds to the csrA promoter region, we performed DNA binding electrophoretic mobility shift assay (EMSA). First, the ihfB gene was fused with a His-tag to produce a recombinant fusion protein (Figure 6C). Then, serial concentrations of the fusion protein were incubated with the 600-bp-long DNA fragment upstream of the csrA start codon. The same fragment with a 60-bp deletion in the 5′ noncoding region was used as a control. As shown in Figure 6D, recombinant IHFB produced a gel shift with the full length of the DNA fragment in a concentration-dependent manner, whereas IHFB incubated with the truncated fragment did not produce a gel shift (Figure 6E). These data indicate the direct binding of IHFB to the promoter region of csrA.
IHFB Is a Transcriptional Inhibitor That Regulates the Temporal Transcription of csrA
Since transcription of csrA in WT is temporally regulated (Figures 5B,D) and IHFB binds directly to the promoter region of csrA (Figure 6), we hypothesized that IHFB is a transcriptional regulator of csrA. To test this, we investigated the effect of ihfB deletion on the transcription of csrA. An ihfB deletion strain (ΔihfB) was constructed using a non-polar deletion strategy. The growth kinetics of the ΔihfB mutant in AYE were similar to WT and ihfB-complemented strains (Figure 7A), indicating that IHFB is not essential for L. pneumophila growth in vitro. qRT-PCR results showed that the transcriptional level of csrA in the ΔihfB mutant was persistent during the entire life cycle (Figure 7B), whereas in WT, it was downregulated during the TP (Figure 7B, time points 18 to 21). These results indicate that IHFB inhibits the transcription of csrA during the TP.
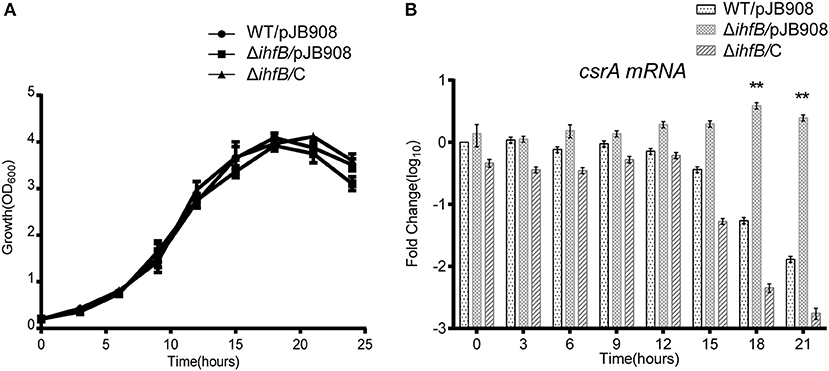
Figure 7. IHFB is a transcriptional inhibitor of csrA. (A) Growth curves of WT (•), ihfB deletion mutant ΔihfB (■), and the complemented strain ΔihfB/C (▴). For negative controls, pJB908 vector was electroporated into WT and ΔihfB to create WT/pJB908 and ΔihfB/pJB908, respectively. Bacterial inoculum from the TP culture was inoculated into AYE media to the same initial OD600 of 0.2 at time zero. Bacterial cells were grown in AYE medium at 37°C and samples were taken every 3 h for determination of optical density at 600 nm. (B) Relative transcriptional profiles of csrA in WT, ΔihfB, and the complemented strain ΔihfB/C during the life cycle. Total RNA was prepared at the indicated time points. Transcriptional level of csrA was detected by qRT-PCR. The transcriptional levels of csrA in WT/pJB908 at 0 h were normalized to 0 by taking log10. Data represent mean ± SD derived from three independent experiments. **p < 0.01 were identified by GraphPad Prism.
IHFB Is Temporally Expressed During the Life Cycle and Is Degraded by ClpP During the RP
We measured the protein level of IHFB during different growth phases using proteomic analysis of whole cell lysates obtained from cultures of WT and ΔclpP grown in liquid medium. By quantifying the IHFB-representative peptide (Supplementary Figure S10), we found that the protein level of IHFB in ΔclpP was significantly (p < 0.01) higher than WT during the RP, whereas the protein level of IHFB in ΔclpP was similar to WT during the TP (Figure 8A). These data indicate that endogenous IHFB is temporally expressed and that its protein level is regulated in a ClpP-dependent manner.
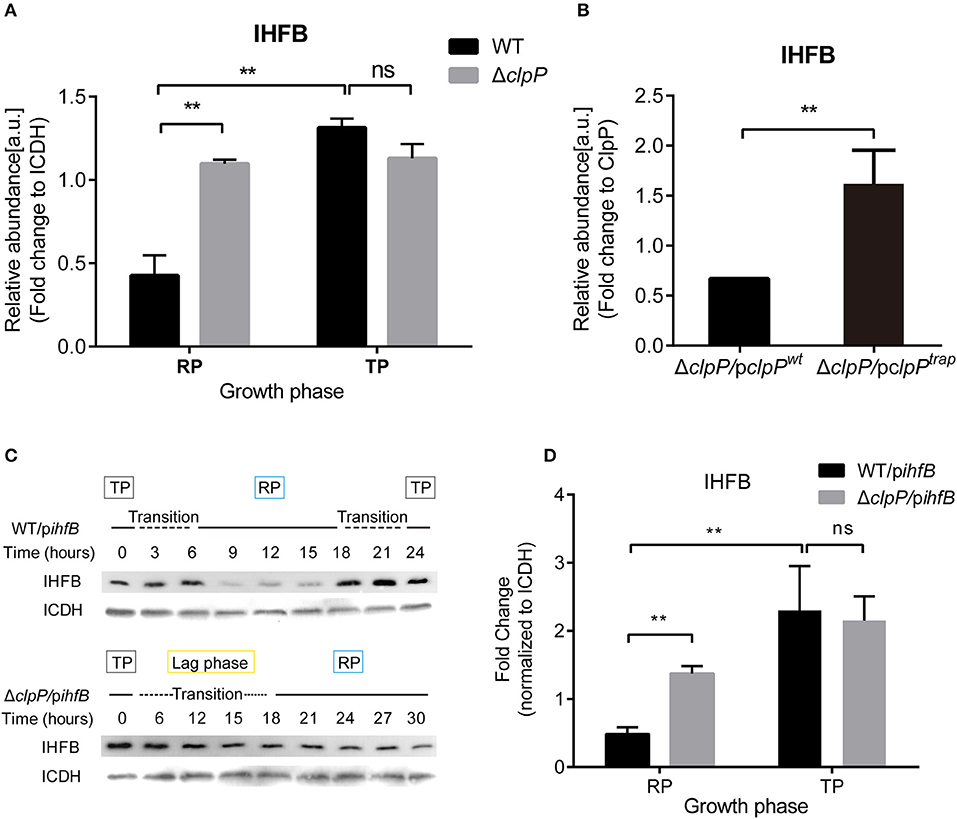
Figure 8. IHFB is temporally expressed and is degraded by ClpP during the RP. (A) The abundance of IHFB in WT and ΔclpP determined by proteomics analysis. WT and ΔclpP were cultured in fresh AYE medium at the same initial OD600 values. Bacterial cells in the RP were harvested at an OD600 of 0.7–1.0 and those in the TP were harvested approximately 6 h after the cessation of growth. Total proteins from indicated samples were extracted for proteomic analysis. Bacterial whole-cell lysates from WT and ΔclpP were prepared and identified by mass spectrometry. ICDH was measured as a loading control. (B) The abundance of IHFB in ΔclpP/pclpPwt and ΔclpP/pclpPtrap were identified by ClpPtrap proteomic analysis. Bacterial cells in TP were harvested approximately 6 h after the cessation of growth. Whole-cell lysates from ΔclpP/pclpPwt and ΔclpP/pclpPtrap were prepared and His-tagged ClpP proteins were purified by Ni-NTA affinity. Substrates captured inside the proteolytic barrel were co-purified along with the His-tagged ClpP complex and identified by mass spectrometry. ClpP was measured as a loading control. (C) IHFB is degraded by ClpP during the RP. Whole-cell lysates were prepared from equal amounts of cells of WT/pihfB and ΔclpP/pihfB at indicated time points, and an immunoblot of IHFB was performed using an anti-His tag antibody. ICDH was probed as a loading control. (D) Relative protein levels of IHFB in the RP and the TP calculated by ImageJ. Bacterial cells in the RP were harvested at an OD600 of 0.7–1.0 and those in the TP were harvested approximately 6 h after the cessation of growth. The quantitative data were analyzed using two-way analysis of variance (ANOVA) test by GraphPad Prism. The values that are significantly different are indicated by a bar and asterisk as follows: **p < 0.01.
We applied ClpP trapping analysis to determine whether IHFB is a substrate of ClpP (Supplementary Figure S11). Using the protein level of ClpP as a reference, we identified that although IHFB was captured in both ΔclpP/pclpPwt and ΔclpP/pclpPtrap, significantly more IHFB was detected in ΔclpP/pclpPtrap than in ΔclpP/pclpPwt (Figure 8B). We interpret that ΔclpP/pclpPwt captures and degrades IHFB, whereas ΔclpP/pclpPtrap accumulates IHFB because it cannot degrade the captured IHFB. Our results suggest that IHFB is a substrate of ClpP. Thus, ClpP may directly control IHFB, the negative transcriptional regulator of csrA.
IHFB protein stability might also be regulated in a ClpP-dependent manner. To test this possibility, ihfB was fused with a C-terminal His-tag in plasmid pJB908-ihfB, which was transformed into WT and ΔclpP (Supplementary Figure S5C). The expression of ihfB did not affect the growth kinetics of WT or ΔclpP (Supplementary Figure S6). The protein levels of IHFB were detected by Western blotting using an anti-His antibody. As shown in Figure 8C, IHFB persistently accumulated in both strains during the TP and transition. However, the amount of IHFB in WT decreased significantly during the RP compared to ΔclpP, indicating that the protein level of IHFB is regulated in a ClpP-dependent manner. The GFP control continuously accumulated and the protein levels were consistent at the same growth phase between WT/pgfp and ΔclpP/pgfp (Supplementary Figures S7A–C). Quantitative analysis confirmed that the protein level of IHFB in ΔclpP was significantly (p < 0.01) higher than WT during the RP, but similar during the TP (Figure 8D).
ClpP-Mediated Degradation of CsrA Protein During the TP Is IHFB-Independent
We test whether the ClpP-dependent inhibition of csrA transcription by IHFB and the ClpP-mediated degradation of CsrA protein were independent processes. The plasmid pJB908-csrA, in which the expression of csrA is controlled by the mip promoter, was transformed into ΔihfB, resulting in the strain ΔihfB/pcsrA. To ensure that IHFB did not affect the expression of the mip promoter-controlled gene, we used gfp under the control of mip promoter in WT and ΔihfB. The result showed that the expression of gfp was consistent in both strains at the same growth phase (Supplementary Figures S7A,B,D). The expression profile of csrA was then measured in ΔihfB/pcsrA. Similar to the protein level of CsrA in WT (Figure 2B), in a new life cycle of ΔihfB/pcsrA, CsrA was undetected during the TP but was upregulated during the RP (Figure 9). This indicated that the protein level of accumulated CsrA was temporally regulated in the absence of ihfB. Combining with the result in Figure 2B, these data indicate that the ClpP-dependent transcriptional inhibition of csrA by IHFB does not affect the ClpP-mediated degradation of CsrA during the biphasic life cycle of L. pneumophila.
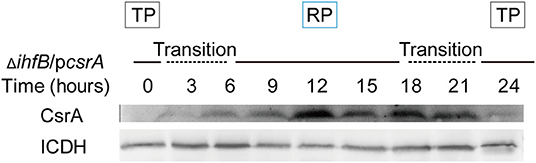
Figure 9. Expression of CsrA in ΔihfB during the life cycle reveals that the degradation of CsrA during the TP is IHFB-independent. Bacterial whole-cell lysates were prepared from ΔihfB/pcsrA and an immunoblot of CsrA was performed using an anti-His tag antibody. ICDH was probed as a loading control.
In conclusion, our study demonstrates that the expression of CsrA is temporally regulated in a ClpP-dependent manner both at the protein and transcriptional levels during the biphasic life cycle of L. pneumophila (Figure 10). Specifically, during the RP (Figure 10A), the accumulation of CsrA is promoted in a ClpP-dependent manner by degrading the transcriptional inhibitor IHFB to promote the transcription of csrA and reducing the degradation of the CsrA protein. When nutrients become limiting (Figure 10B), the level of CsrA is decreased in a ClpP-dependent manner by ceasing the degradation of IHFB to inhibit the transcription of csrA and promoting the degradation of the accumulated CsrA.
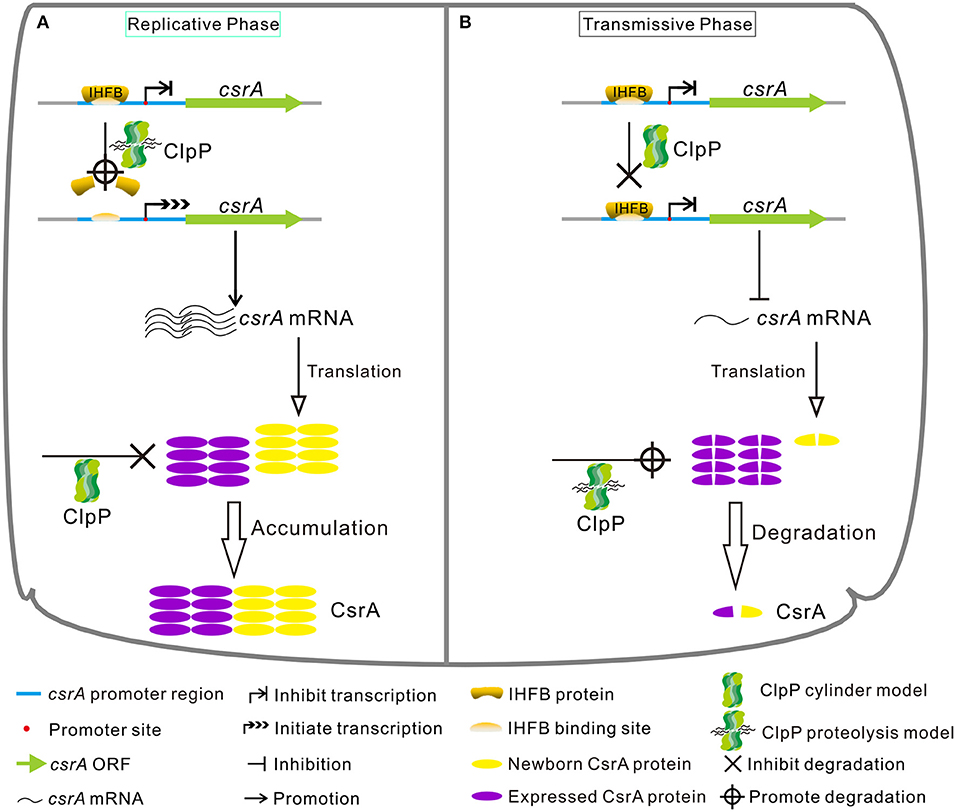
Figure 10. Model of regulatory cascade of the expression of CsrA during the biphasic life cycle of L. pneumophila. (A) During the replicative phase, the transcription of csrA increases due to the degradation of the transcription inhibitor IHFB via ClpP, while the CsrA protein is avoided to be degraded by ClpP via an unknown mechanism, resulting in the accumulation of CsrA. (B) During the transmissive phase, IHFB binds to inhibit the transcription of csrA, while the accumulated CsrA is degraded by ClpP, leading to the decrease of intracellular CsrA. See text for details.
Discussion
To adapt to various harsh environmental conditions in vitro and in host cells, L. pneumophila adopts a biphasic life cycle, allowing it to switch between an RP and a transmissive/virulent phase, and exclusively expresses genes required for the transition (Molofsky and Swanson, 2004; Bruggemann et al., 2006). Although several regulators, such as LetA/S, RpoS, PmrA, CpxR, rsmX/Y/Z, and LqsR, that govern the Legionella life cycle have been characterized (Gal-Mor and Segal, 2003; Bachman and Swanson, 2004; Jacobi et al., 2004), the network involved in controlling the life cycle cascade is still incompletely understood. L. pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. It controls the switch from replicative to transmissive virulence phase of infection (Molofsky and Swanson, 2003). It has been demonstrated that CsrA-dependent repression of transmission traits is alleviated by the LetS/LetA TCS and that RsmY/Z link the LetS/LetA TCS and CsrA to the control of replication vs. transmission phases (Sahr et al., 2009). Though the majority of LetS/LetA-regulatory effects depend on RsmY/Z, regulation of several motility genes does not. Analysis of the transcriptional programs of the ΔletA, ΔletS, and ΔrsmYZ strains revealed that the switch to TP is partially blocked (Sahr et al., 2009). These data suggest that there may be other regulatory pathways involved in the regulation of CsrA. The results of this study indicate that the temporal expression of CsrA is dually regulated in a ClpP-dependent manner. This extends our understanding of how the bacteria manipulate their life cycle by using multiple strategies. Given that ClpP and CsrA are highly conserved in all L. pneumophila strains sequenced so far (Supplementary Figures S12, S13), the underlying mechanism of the life cycle control by CsrA and ClpP may be representative of the genus Legionella or Gram-negative bacteria in general.
Regulated proteolysis of native regulatory proteins is required for bacteria to maintain quality control and undergo cell-cycle progression, physiological transitions, and adaptations needed for survival and persistence (Mahmoud and Chien, 2018). In the aquatic dimorphic organism Caulobacter crescentus, ClpP regulates the swarmer-to-stalked transition (Joshi and Chien, 2016). Bacillus subtilis requires proteolysis by ClpXP to initiate a sporulation program from mature spores to dead spores (Tan et al., 2015). In E. coli, ClpP regulates the transition from logarithmic growth to stationary phase (Hengge, 2009). However, the stage of life cycle control that ClpP is involved in is still unclear. We found that the growth curve and bacterial morphology observed in the ΔclpP mutant revealed a prolonged lag phase compared to WT (Figures 2A, 3). Further detection of the transcriptional level of life-cycle-dependent genes demonstrated that the prolonged lag phase of the ΔclpP mutant remained in the TP (Figure 3B; Supplementary Figure S9A). Ectopic expression further revealed that intracellular accumulation of CsrA delayed the transition of L. pneumophila from the TP into the RP. Temporal expression of CsrA was dually regulated in a ClpP-dependent manner during the transition between the replicative and transmissive forms. Therefore, the growth defects of ΔclpP may be caused by insufficient control of CsrA. It is noteworthy that, because csrA is essential for Legionella growth, all studies on the regulation of csrA expression were performed using an ectopic expression strategy (Fettes et al., 2001; Sahr et al., 2009, 2017; Yakhnin et al., 2011), including the present study. Likewise, ClpP is essential for bacteria to acclimate to their niche for growth. For example, the loss of clpP in Streptococcus pneumoniae, cyanobacteria Synechococcus, and Salmonella enterica serovar Typhimurium is responsible for growth defects at reduced temperatures (Porankiewicz et al., 1998; Robertson et al., 2002; Knudsen et al., 2014) although the mechanism is unknown. Our finding that the regulation of CsrA by ClpP affects growth of L. pneumophila suggests a shared regulatory strategy by these bacteria.
The impaired growth of the clpP mutant L. pneumophila is associated with high levels of CsrA (Figures 2A,B, 3C,E, 4). This is different from S. enterica serovar Typhimurium in which the impaired growth at low temperature due to the clpP deletion is associated with high levels of stationary-phase-specific sigma factor RpoS (Knudsen et al., 2014). In addition, ClpP indirectly regulates CsrA through RpoS to reduce the virulence in S. enterica serovar Typhimurium (Knudsen et al., 2013). Previous work has shown increased expression of RpoS in an L. pneumophila csrA mutant (Forsbach-Birk et al., 2004), which is in line with the observations in S. enterica serovar Typhimurium (Knudsen et al., 2013). However, RpoS is not involved in the growth-phase-dependent resistance to stress in L. pneumophila. Rather, it likely regulates the genes that enable the bacteria to survive within protozoa (Hales and Shuman, 1999). Our data also show that the impaired growth of the clpP mutant of L. pneumophila is not associated with high levels of RpoS (data not shown), but with high levels of CsrA. These data suggest that ClpP is involved in the regulation of bacterial growth by a different pathway in L. pneumophila than in S. enterica serovar Typhimurium.
In addition to our finding that ClpP degrades CsrA, we revealed that the protein level of IHFB, the transcriptional inhibitor of CsrA, is also controlled by ClpP (Figure 8). Originally classified as an architectural protein in E. coli, IHF is also a transcriptional regulator that appears to be conserved in function, as homologs of IHF are involved in the regulation of gene expression in a number of closely and distantly related bacteria, including pathogens (Goosen and van de Putte, 1995; Fyfe and Davies, 1998; Dorman et al., 2001; Sieira et al., 2004; Mangan et al., 2006; Stonehouse et al., 2008; Perez-Rueda et al., 2009; Arvizu-Gomez et al., 2011). This suggests that IHF plays a role in virulence. For example, ihf in C. crescentus is required for temporal activation of flagellar genes during its life cycle and it promotes efficient chromosomal replication (Gober and Shapiro, 1990; Quon et al., 1996; Porter and Dorman, 1997; Siam et al., 2003). In L. pneumophila, although ihfB is not required for growth in vitro (Figure 7A), our unpublished data show that ΔihfB mutants fail to grow in A. castellani, consistent with the finding that IHF is required for full virulence in amoebae (Morash et al., 2009). IHF is temporally expressed at minimal and maximal levels during the RP and TP, respectively (Morash et al., 2009), further suggesting a role for IHF in virulent phenotypes. Interestingly, IHF was also found to directly bind to and activate transcription of RsmY and RsmZ, two non-coding regulatory RNAs responsible for the de-repression of CsrA-repressed transcripts associated with the progression to the TP (Pitre et al., 2013). How ClpP is involved in temporal control of the IHF-sRNA-CsrA cascade is worthy of investigation in the future.
We performed a BLAST search of the L. pneumophila genome, and a set of potential IHF binding sites were identified in the upstream sequences of approximately 300 L. pneumophila genes (data not shown), including several regulators of post-exponentially expressed genes (letA, letE, fleQ, rpoS, and ihfA) (Morash et al., 2009) and effector-encoding genes. Moreover, CsrA is reported to control the expression of over 40 Dot/Icm substrates (Sahr et al., 2009, 2017) and is essential for intracellular growth (Morash et al., 2009). Lack of intracellular growth and infection efficiency of L. pneumophila in A. castellanii were observed in ΔclpP mutants (Figure 4), ΔihfB mutants, and ΔcsrA mutants (Forsbach-Birk et al., 2004; Morash et al., 2009; Li et al., 2010). These results indicate that the action of ClpP on CsrA and IHFB may play a more integral role in the regulation of the life cycle and virulence of L. pneumophila.
Based on our results and previous studies, we propose a model for the network of the life cycle controlled by CsrA, whose expression is regulated simultaneously by ClpP and IHFB (Figure 10). When bacteria are in a nutrient-rich environment, ClpP promotes the degradation of the response regulator CsrA (Figures 1, 2A,B). Therefore, L. pneumophila can normally enter the RP from the TP, initiating a new cycle. During the RP (Figure 10A), ClpP promotes the expression of CsrA by reducing the amount of the transcriptional inhibitor IHFB while ceasing degradation of CsrA protein. When nutrients become limiting (Figure 10B), ClpP promotes accumulation of IHFB to inhibit the transcription of csrA, and simultaneously promotes the degradation of accumulated CsrA to govern the transition from the RP to the TP. Overall, CsrA likely acts as a biphasic switch during the Legionella life cycle. It is finely regulated at dual levels to achieve control of metabolism/replication, motility, and virulent traits of L. pneumophila.
Materials and Methods
Bacterial Strains, Plasmids, Primers, and Media
The bacterial strains, plasmids, and primers used in this work are listed in Supplementary Tables S1, S2, respectively. All L. pneumophila strains were cultured on buffered charcoal yeast extract (BCYE) plates, or in N-(2-acetamido)-2-aminoethanesulfonic acid (ACES)-buffered yeast extract (AYE) medium, supplemented with thymidine (100 μg/ml) (Feeley et al., 1979) when required. E. coli DH5α and E. coli BL21(DE3), used as host strains for cloning strategies and recombinant protein expression, respectively, were grown in Luria-Bertani (LB) broth and agar at 37°C. For liquid culture, AYE broth was inoculated with TP bacteria grown in the previous cycle to a final OD600 of 0.2 and incubated at 37°C with vigorous shaking. RP bacteria were harvested at an OD600 of 0.7–1.0 and TP bacteria were harvested approximately 6 h after the cessation of growth, which is at an approximate OD600 of 3.0–3.5. Ampicillin (amp) was added to a final concentration of 100 μg/ml, kanamycin (kan) to 50 μg/ml, chloramphenicol (cm) to 34 μg/ml, and IPTG to 10 μM for E. coli, and chloramphenicol to 5 μg/ml for L. pneumophila. A. castellanii (ATCC 30234) was grown in proteose yeast extract glucose medium (PYG) at 30°C (Segal and Shuman, 1999). To ascertain CFU, serial dilutions of bacteria were incubated on BCYE for 4 days and resultant colonies were counted. Bacto yeast exact and proteose peptone were obtained from Becton Dickinson Biosciences. All other reagents were from Sigma Co., unless specified otherwise. All primers were synthesized by Ruibiotech Co., China. All restriction enzymes were purchased from New England Biolabs. All DNA cloning was carried out in the E. coli DH5a strain using standard molecular techniques. The protein concentration was determined using Bradford's protein assay reagent (Bio-Rad).
Construction of Mutants and Plasmids
ihfB deletion strain was constructed by utilizing an in-frame gene replacement suicide vector (pBRDX) strategy (LeBlanc et al., 2008). Briefly, upstream and downstream flanking sequences of ihfB were amplified by PCR using the PΔihfB-F1/PΔihfB-R1 and PΔihfB-F2/PΔihfB-R2 primer pairs, respectively. The PCR products were mixed and then used as templates for the subsequent fusion PCR using the PΔihfB-F1/PΔihfB-R2 primers. Fusion PCR products were digested with BglII and BamHI and sub-cloned into the pBRDX vector, creating pBRDXΔihfB. Then, pBRDXΔihfB was introduced into the WT strain by electroporation, and chloramphenicolR+ colonies were selected on BCYET-Cm plates. Transformants were inoculated into AYET and then incubated on BCYET containing 10% sucrose for 3 days at 37°C to select for strains devoid of the vector backbone. Positive colonies (ΔihfB) were confirmed by PCR and sequencing.
Complementation Assay
For complementation experiments, a RSF1010 pKB5-derived vector pJB908 was utilized as the cloning backbone (Sexton et al., 2004). To construct the complementing strain of ihfB, ihfB gene and its promoter region were amplified by PCR with the PihfB-CF/PihfB-CR primer pair and cloned into pJB908. The resulting plasmid pJB908-ihfB was electroporated into ΔihfB to create the complemented strain ΔihfB/C. For negative controls, pJB908 vector was electroporated into WT, ΔclpP, and ΔihfB to create WT/pJB908, ΔclpP/pJB908, and ΔihfB/pJB908, respectively.
DNA Library Preparation and Whole-Genome Sequencing
For whole-genome sequencing, genomic (g) DNA of WT (LP02) and ΔclpP (XP02) were prepared from bacterial cultures using the Bacterial DNA kit (Omega Co.) and was used to construct gDNA library for genomic sequencing. Paired-end sequences and a read length of 100 bases were obtained from an Illumina HiSeq 2500. Sequence reads were mapped to a reference genome using genome alignment software BWA (Li and Durbin, 2009); single nucleotide polymorphisms (SNPs) and Insertion & Deletion (InDel) were searched using GATK (McKenna et al., 2010), and detection of all potential chromosome structural variation (SV) site in the whole genome was carried out by chromosome structure variation analysis software DELLY (Rausch et al., 2012) (Annoroad, China).
In vivo Trapping of ClpP Substrate
The ClpP trapping system was constructed according to the previous report with minor modification (Feng et al., 2013). Briefly, to generate the ClpPtrap, the active site (serine 110) of ClpP was replaced with an alanine (S110A). The plasmids expressing His-tagged ClpPwt and ClpPtrap were transformed into ΔclpP, respectively, to create ΔclpP/pclpPwt and ΔclpP/pclpPtrap. The ΔclpP/pclpPwt and ΔclpP/pclpPtrap strains in TP were grown in 100 ml of AYE at 37°C to an OD600 of 0.2. To screen accumulated substrates of ClpPtrap during the whole life cycle, bacterial whole-cell lysates from ΔclpP/pclpPwt and ΔclpP/pclpPtrap in the TP (harvested approximately 6 h after the cessation of growth) were prepared and His-tagged proteins were purified with Ni-NTA affinity column (GE Healthcare) following the manufacturer's instructions. Substrates captured inside the proteolytic barrel were co-purified along with the His-tagged ClpP complex and identified by mass spectrometry to identify substrates of ClpP in the WT background. ClpP (Protein Accession: Q5ZUD9) was calculated as a loading control because the amount of ClpP determines how much the substrate is bound.
Proteomic Analysis (LC-MS)
For each sample, 100 μg of protein was reduced with 10 mM dithiothreitol (DTT) at 37°C for 45 min and iodoacetamide (IAM) was then added to a final concentration of 15 mM, with incubation at room temperature for 1 h in the dark. The samples were then diluted with 100 mM ammonium bicarbonate buffer and digested with trypsin (1:50, trypsin/lysate ratio) for 16 h at 37°C. Digests were centrifuged through 3-kDa filter tubes so that only digested peptides can go through. Peptide concentrations were determined with a modified Lowry Protein Assay Kit (Sangon Biotech. Co.). Twenty micrograms of peptides was desalted on Pierce C18 Spin Columns (Thermo Fisher Scientific, Co.) according to the manufacturer's instructions. Peptides were analyzed with the Q Exactive HF-Orbitrap MS (Thermo Fisher Scientific, Co). For each sample, the same amounts of peptides from total protein were separated on the analytical column with a 70-min linear gradient at a flow rate of 400 nl/min (0–3% B in 3 min; 3–8% B in 4 min; 8–32% B in 44 min; 32–99% B in 5 min; 99% B for 4 min, 3% B for 10 min). The spectra were acquired in the positive ionization mode by data-dependent methods consisting of a full MS scan in high mass accuracy FT-MS mode at 60,000 resolutions, with the precursor ion scan recorded over the m/z range of 350–1500. Database searching of all LC-MS/MS raw files was performed in Proteome Discoverer 2.2 (Thermo Fisher Scientific, Co). MASCOT 2.2.4 and SEQUEST were used for database searching against the Uniprot L. pneumophila database (L. pneumophila subsp. pneumophila strain Philadelphia 1/ATCC 33152/DSM 7513 proteome, last modified: October 26, 2018; 2930 proteins). Proteomics analysis of the peptide data of CsrA (Protein Accession: Q5ZV47) and IHFB (Protein Accession: Q5ZRC7) in the RP and TP of WT and ΔclpP are shown in Supplementary Table S3; ICDH (Protein Accession: Q5ZXB6) was calculated as a loading control. Proteomics analysis of the peptide data of CsrA and IHFB purified by the ClpP trapping system is shown in Supplementary Table S4; ClpP (Protein Accession: Q5ZUD9) was calculated as a loading control.
Construction of the Plasmids That Ectopically Express CsrA, IHFB, and GFP
To avoid the interference of transcriptional regulation of self-promoter, the plasmids for ectopic expression of CsrA, IHFB, and GFP were constructed. To this end, the sequence of mip promoter region and csrA gene was amplified by PCR using the Ppmip-F/Ppmip-R1 and PcsrA-F/PcsrA-R primer pairs. The PCR products were mixed and then used as templates for the subsequent fusion PCR using the Ppmip-F/PcsrA-R primers. Fusion PCR products were digested with SacI and SphI and sub-cloned into the pJB908 vector, creating plasmid pJB908-csrA. Likewise, the same strategy was employed to create plasmid PJB908-ihfB using primer pairs Ppmip-F/Ppmip-R2 and PihfB-F/PihfB-R and plasmid PJB908-gfp using primer pairs Ppmip-F/Ppmip-R3 and Pmgfp-F/Pmgfp-R. A hexa-histidine tag was added to the C-terminus of the protein in both the resulting recombinant plasmids pJB908-csrA and PJB908-ihfB during cloning. The resultant plasmid pJB908-csrA was electroporated into WT, ΔclpP, and ΔihfB to create strain WT/pcsrA, ΔclpP/pcsrA, and ΔihfB/pcsrA, respectively. The resultant plasmid pJB908-ihfB was electroporated into WT and ΔclpP to create strain WT/pihfB and ΔclpP/pihfB. The resultant plasmid pJB908-gfp was electroporated into WT, ΔclpP, and ΔihfB to create strain WT/pgfp, ΔclpP/pgfp, and ΔihfB/pgfp, respectively.
Growth Curve Assay and Bacterial Morphology Observation
Fresh L. pneumophila cells were inoculated into 5 ml of AYE(T) medium and were cultured to the TP at 37°C. Then, the cultures were transferred into 50 ml of AYE in flasks, incubated to the TP, and then diluted into new flasks to similar optical densities at an approximate OD600 of 0.2 at time zero. Cultures were grown at 37°C with shaking. To measure the growth curve, 1 ml of the cells was sampled every 3 h for measurement of absorbance at 600 nm. Bacterial cells for morphological observation were sampled at 0, 6, 12, 18, and 24 h after L. pneumophila cells were transferred into 50 ml of AYE. Light microscopic images of Legionella cells were captured at a 100-fold oil microscope using Leica LAS-EZ optical microscopy equipped with a camera. The length of the cells was presented by ImageJ. At least three sections of each sample were photographed, and one typical photograph was selected to represent. To ensure conformity, multiple replicates on different days were examined.
Fluorescence Intensity Analysis of mip Promoter
The pJB908-gfp plasmid was transferred into the WT strain and the clpP mutant ΔclpP, respectively, to construct WT/pgfp and ΔclpP/pgfp strains. The culture operation of the bacteria is the same as the experimental procedure of growth curve assay. Bacteria were collected at OD600 of 1.0, 2.0, 3.0, and 4.0, respectively, from the liquid AYE for fluorescence intensity analysis. After the analysis of fluorescence intensity, the bacterial cells were plated on AYE plates from each period to detect bacterial viability. Bacterial cells were centrifuged at 5°C and 4,000× g. The cell pellets were resuspended in 20 ml of PBS buffer and were centrifuged at 4,000× g for 5 min at 4°C. This step was repeated once to ensure that the medium is completely removed. Then, the pellets were resuspended with PBS buffer and the cell suspension concentration was adjusted to approximately 108 bacteria/ml with a spectrophotometer. The fluorescence intensity of the excitation fluorescence spectrophotometer was 488 nm, the absorption wavelength was 507 nm, and the fluorescence intensity was measured using PBS buffer as a blank control.
RNA Isolation, cDNA Preparation, and qRT-PCR
RNA for real-time quantitative PCR (qRT-PCR) was prepared using an Eastep® super Kit following the manufacturer's protocols (Promega Co.) and treated with DNase I according to the manufacturer's instructions (Promega Co.) prior to cDNA preparation. cDNA was prepared using GoScript™ reverse transcription system as described by the manufacturer (Promega Co.). qRT-PCR was performed in a 20-μl reaction volume using an Applied Biosystems Step One Plus 96-well reverse transcription-PCR system with Power SYBR Green PCR Master Mix following the manufacturer's instructions (Applied Biosystems Co.). 16S rRNA was used as the reference sample in all comparative threshold cycle (ΔΔCT) experiments. All qRT-PCR primers were tested for amplification efficiency. qRT-PCR data were analyzed using Step One System software and GraphPad Prism. Primers used in qRT-PCR experiments are shown in Supplementary Table S2. All analysis was performed in biological triplicate.
Bacterial Infectivity in A. castellanii
The A. castellanii cells were seeded onto a 24-well plate (5 × 105 per well) and allowed to adhere for 2 h prior to infection. L. pneumophila cells were grown for 20 h in AYE broth at 37°C with shaking, diluted in HL5, and were used to infect amoebae at an MOI of 10. Thirty minutes post infection, extracellular bacteria were removed by washing three times with warm HL5 medium (Tiaden et al., 2007). At the indicated time points, culture supernatant was removed and the amoebae cells were lysed with 0.04% Triton. The supernatant and the lysates were combined, and serial dilutions were prepared and aliquots were plated on BCYE plates for CFU counting (Al-Khodor et al., 2008). All experiments were performed in triplicate at 30°C.
Protein Isolation and Western Blotting
Total cell extracts of L. pneumophila were prepared at various time points after growth at 37°C. Briefly, bacterial cell pellets were resuspended in 1 ml of lysis buffer and sonicated for 2 min. The cells were then centrifuged for 1 h at 12,000× g. The protein-containing supernatant was removed and the protein concentration was measured using a commercial kit (Biorad Co.). Samples were normalized for protein loading and run on a 15% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) as described previously (Laemmli, 1970). Western blotting was carried out as described elsewhere (Towbin et al., 1979). The levels of CsrA or IHFB were immunoblotted with anti-His tag antibody. ICDH was probed as a loading control.
Expression and Purification of IHFB
The coding regions of IHFB were PCR amplified and fused to the expression vector pET-28a by Gibson assembly method. The fusion gene constructs were transformed into E. coli strain BL21. For protein expression, 5 ml of overnight culture of the E. coli cells harboring the appropriate plasmid was transferred to 500 ml of LB medium with 50 μg/ml kanamycin and grown until OD600 of 0.4–0.6 was reached. After adding IPTG (isopropyl thio-d-galactopyranoside) to a final concentration of 10 μM, the cultures were further incubated in a shaker at 20°C for 16–18 h. Bacterial cells were harvested by spinning at 5000× g, resuspended in lysis buffer (25 mM Tris–HCl and 500 mM NaCl), and lysed by sonication. The soluble fractions were collected by centrifugation at 12,000× g for 30 min at 4°C. His-tagged proteins were purified with Ni-NTA affinity column (GE Healthcare Co.) following the manufacturer's instructions.
Electrophoretic Mobility Shift Assay (EMSA)
EMSA was performed as previously described (Altman and Segal, 2008), with a few modifications. The His-tagged IHFB protein was purified with Ni-NTA affinity column (GE Healthcare) following the manufacturer's instructions. The regulatory region of csrA (~600 bp) was amplified by PCR with primers listed in Supplementary Table S2. Increasing amounts of the purified protein were mixed with 200 ng of the csrA promoter region probe in buffer containing 10 mM Tris–HCl (pH 7.5), 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, 0.1 mM dithiothreitol, 0.1 mg/ml bovine serum albumin, 0.5 mg/ml herring sperm DNA, and 5% glycerol. The binding reaction was carried out for 30 min at room temperature, and samples were then loaded onto 6% polyacrylamide 0.5× Tris–acetate–EDTA gel in 0.5× Tris–acetate–EDTA running buffer. Following electrophoresis at 4°C, the gel was transferred to nylon membrane and fixed by UV cross-linking.
GFP Reporter Assay
To confirm the regulation of csrA transcription by IHFB via a ClpP-dependent manner, a fragment containing 600 bp of the csrA RBS (Heuner et al., 1995), the putative σ70 promoter and transcriptional start site was amplified using primers PPcsrA-F and PPcsrA-R. The fragment was ligated into pJB908 directly 5′ of gfp as described (Hammer and Swanson, 1999). The plasmid pJB908-PcsrA-gfp was transformed into WT and ΔclpP, respectively, generating WT/pPcsrA-gfp and ΔclpP/pPcsrA-gfp.
Statistical Analysis
Basic statistical analysis was performed using Excel. One-way ANOVA was performed using GraphPad Prism followed by a post-hoc Student–Newman–Keul's test. Morphological length of bacteria and quantitative analysis of Western blot were performed using ImageJ. The alignment of amino acid sequences was performed using the online NCBI BLAST.
Data Availability Statement
The datasets generated for this study can be found in the LP02 (https://www.ncbi.nlm.nih.gov/sra/SRX5381953[accn]), XP02 (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA522681).
Author Contributions
YL conceived the project. YL and ZG designed the experiments. ZG, QL, PY, XP, and DS performed the experiments. YL and ZG prepared the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Natural Science Foundation of Guangdong province (No. 2016A030311036) and Guangdong key area R&D project (No. 2018B020205002) to YL.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02495/full#supplementary-material
References
Ali Azam, T., Iwata, A., Nishimura, A., Ueda, S., and Ishihama, A. (1999). Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol. 181, 6361–6370.
Al-Khodor, S., Price, C. T., Habyarimana, F., Kalia, A., and Abu Kwaik, Y. (2008). A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol. Microbiol. 70, 908–923. doi: 10.1111/j.1365-2958.2008.06453.x
Altman, E., and Segal, G. (2008). The response regulator CpxR directly regulates expression of several Legionella pneumophilaicm/dot components as well as new translocated substrates. J. Bacteriol. 190, 1985–1996. doi: 10.1128/JB.01493-07
Arvizu-Gomez, J. L., Hernandez-Morales, A., Pastor-Palacios, G., Brieba, L. G., and Alvarez-Morales, A. (2011). Integration host factor (IHF) binds to the promoter region of the phtD operon involved in phaseolotoxin synthesis in P. syringae pv. phaseolicola NPS3121. BMC Microbiol. 11:90. doi: 10.1186/1471-2180-11-90
Bachman, M. A., and Swanson, M. S. (2004). The LetE protein enhances expression of multiple LetA/LetS-dependent transmission traits by Legionella pneumophila. Infect. Immun. 72, 3284–3293. doi: 10.1128/IAI.72.6.3284-3293.2004
Bruggemann, H., Hagman, A., Jules, M., Sismeiro, O., Dillies, M. A., Gouyette, C., et al. (2006). Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila. Cell Microbiol. 8, 1228–1240. doi: 10.1111/j.1462-5822.2006.00703.x
Byrne, B., and Swanson, M. S. (1998). Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66, 3029–3034.
Craig, N. L., and Nash, H. A. (1984). E. coli integration host factor binds to specific sites in DNA. Cell 39, 707–716. doi: 10.1016/0092-8674(84)90478-1
Dorman, C. J., McKenna, S., and Beloin, C. (2001). Regulation of virulence gene expression in Shigella flexneri, a facultative intracellular pathogen. Int. J. Med. Microbiol. 291, 89–96. doi: 10.1078/1438-4221-00105
Faucher, S. P., Mueller, C. A., and Shuman, H. A. (2011). Legionella pneumophila transcriptome during intracellular multiplication in human macrophages. Front. Microbiol. 2:60. doi: 10.3389/fmicb.2011.00060
Feeley, J. C., Gibson, R. J., Gorman, G. W., Langford, N. C., Rasheed, J. K., Mackel, D. C., et al. (1979). Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10, 437–441.
Feng, J., Michalik, S., Varming, A. N., Andersen, J. H., Albrecht, D., Jelsbak, L., et al. (2013). Trapping and proteomic identification of cellular substrates of the ClpP protease in Staphylococcus aureus. J. Proteome Res. 12, 547–558. doi: 10.1021/pr300394r
Fettes, P. S., Forsbach-Birk, V., Lynch, D., and Marre, R. (2001). Overexpresssion of a Legionella pneumophila homologue of the E. coli regulator csrA affects cell size, flagellation, and pigmentation. Int. J. Med. Microbiol. 291, 353–360. doi: 10.1078/1438-4221-00141
Fields, B. S., Benson, R. F., and Besser, R. E. (2002). Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15, 506–526. doi: 10.1128/CMR.15.3.506-526.2002
Flynn, J. M., Neher, S. B., Kim, Y. I., Sauer, R. T., and Baker, T. A. (2003). Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol. Cell. 11, 671–683. doi: 10.1016/S1097-2765(03)00060-1
Forsbach-Birk, V., McNealy, T., Shi, C., Lynch, D., and Marre, R. (2004). Reduced expression of the global regulator protein CsrA in Legionella pneumophila affects virulence-associated regulators and growth in Acanthamoeba castellanii. Int. J. Med. Microbiol. 294, 15–25. doi: 10.1016/j.ijmm.2003.12.003
Fyfe, J. A., and Davies, J. K. (1998). An AT-rich tract containing an integration host factor-binding domain and two UP-like elements enhances transcription from the pilEp1 promoter of Neisseria gonorrhoeae. J. Bacteriol. 180, 2152–2159.
Gal-Mor, O., and Segal, G. (2003). Identification of CpxR as a positive regulator of icm and dot virulence genes of Legionella pneumophila. J. Bacteriol. 185, 4908–4919. doi: 10.1128/JB.185.16.4908-4919.2003
Gober, J. W., and Shapiro, L. (1990). Integration host factor is required for the activation of developmentally regulated genes in Caulobacter. Genes. Dev. 4, 1494–1504. doi: 10.1101/gad.4.9.1494
Goodman, S. D., Velten, N. J., Gao, Q., Robinson, S., and Segall, A. M. (1999). In vitro selection of integration host factor binding sites. J. Bacteriol. 181, 3246–3255.
Goosen, N., and van de Putte, P. (1995). The regulation of transcription initiation by integration host factor. Mol. Microbiol. 16, 1–7. doi: 10.1111/j.1365-2958.1995.tb02386.x
Gottesman, S. (2003). Proteolysis in bacterial regulatory circuits. Annu. Rev. Cell Dev. Biol. 19, 565–587. doi: 10.1146/annurev.cellbio.19.110701.153228
Guyard, C., and Low, D. E. (2011). Legionella infections and travel associated legionellosis. Travel Med. Infect. Dis. 9, 176–186. doi: 10.1016/j.tmaid.2010.05.006
Hales, L. M., and Shuman, H. A. (1999). The L. pneumophilarpoS gene is required for growth within Acanthamoeba castellanii. J. Bacteriol. 181, 4879–4889.
Hammer, B. K., and Swanson, M. S. (1999). Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33, 721–731. doi: 10.1046/j.1365-2958.1999.01519.x
Hengge, R. (2009). Proteolysis of sigmaS (RpoS) and the general stress response in Escherichia coli. Res. Microbiol. 160, 667–676. doi: 10.1016/j.resmic.2009.08.014
Heuner, K., Bender-Beck, L., Brand, B. C., Luck, P. C., Mann, K. H., Marre, R., et al. (1995). Cloning and genetic characterization of the flagellum subunit gene (flaA) of Legionella pneumophila serogroup 1. Infect. Immun. 63, 2499–2507.
Jacobi, S., Schade, R., and Heuner, K. (2004). Characterization of the alternative sigma factor sigma54 and the transcriptional regulator FleQ of Legionella pneumophila, which are both involved in the regulation cascade of flagellar gene expression. J. Bacteriol. 186, 2540–2547. doi: 10.1128/JB.186.9.2540-2547.2004
Joshi, K. K., and Chien, P. (2016). Regulated proteolysis in bacteria: Caulobacter. Annu. Rev. Genet. 50, 423–445. doi: 10.1146/annurev-genet-120215-035235
Knudsen, G. M., Nielsen, M. B., Thomsen, L. E., Aabo, S., Rychlik, I., and Olsen, J. E. (2014). The role of ClpP, RpoS and CsrA in growth and filament formation of Salmonella enterica serovar Typhimurium at low temperature. BMC Microbiol. 14:208. doi: 10.1186/s12866-014-0208-4
Knudsen, G. M., Olsen, J. E., Aabo, S., Barrow, P., Rychlik, I., and Thomsen, L. E. (2013). ClpP deletion causes attenuation of Salmonella Typhimurium virulence through mis-regulation of RpoS and indirect control of CsrA and the SPI genes. Microbiology 159, 1497–1509. doi: 10.1099/mic.0.065797-0
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. doi: 10.1038/227680a0
LeBlanc, J. J., Brassinga, A. K., Ewann, F., Davidson, R. J., and Hoffman, P. S. (2008). An ortholog of OxyR in Legionella pneumophila is expressed postexponentially and negatively regulates the alkyl hydroperoxide reductase (ahpC2D) operon. J. Bacteriol. 190, 3444–3455. doi: 10.1128/JB.00141-08
Li, H., and Durbin, R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Li, X. H., Zeng, Y. L., Gao, Y., Zheng, X. C., Zhang, Q. F., Zhou, S. N., et al. (2010). The ClpP protease homologue is required for the transmission traits and cell division of the pathogen Legionella pneumophila. BMC Microbiol. 10:54. doi: 10.1186/1471-2180-10-54
Mahmoud, S. A., and Chien, P. (2018). Regulated proteolysis in bacteria. Annu. Rev. Biochem. 87, 677–696. doi: 10.1146/annurev-biochem-062917-012848
Mangan, M. W., Lucchini, S., Danino, V., Croinin, T. O., Hinton, J. C., and Dorman, C. J. (2006). The integration host factor (IHF) integrates stationary-phase and virulence gene expression in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 59, 1831–1847. doi: 10.1111/j.1365-2958.2006.05062.x
McKenna, A., Hanna, M., Banks, E., Sivachenko, A., Cibulskis, K., Kernytsky, A., et al. (2010). The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. doi: 10.1101/gr.107524.110
Molofsky, A. B., and Swanson, M. S. (2003). Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol. Microbiol. 50, 445–461. doi: 10.1046/j.1365-2958.2003.03706.x
Molofsky, A. B., and Swanson, M. S. (2004). Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53, 29–40. doi: 10.1111/j.1365-2958.2004.04129.x
Morash, M. G., Brassinga, A. K., Warthan, M., Gourabathini, P., Garduno, R. A., Goodman, S. D., et al. (2009). Reciprocal expression of integration host factor and HU in the developmental cycle and infectivity of Legionella pneumophila. Appl. Environ. Microbiol. 75, 1826–1837. doi: 10.1128/AEM.02756-08
Neher, S. B., Villen, J., Oakes, E. C., Bakalarski, C. E., Sauer, R. T., Gygi, S. P., et al. (2006). Proteomic profiling of ClpXP substrates after DNA damage reveals extensive instability within SOS regulon. Mol. Cell 22, 193–204. doi: 10.1016/j.molcel.2006.03.007
Newton, H. J., Ang, D. K., van Driel, I. R., and Hartland, E. L. (2010). Molecular pathogenesis of infections caused by Legionella pneumophila. Clin. Microbiol. Rev. 23, 274–298. doi: 10.1128/CMR.00052-09
Oliva, G., Sahr, T., and Buchrieser, C. (2018). The life cycle of L. pneumophila: cellular differentiation is linked to virulence and metabolism. Front. Cell Infect. Microbiol. 8:3. doi: 10.3389/fcimb.2018.00003
Perez-Rueda, E., Janga, S. C., and Martinez-Antonio, A. (2009). Scaling relationship in the gene content of transcriptional machinery in bacteria. Mol. Biosyst. 5, 1494–1501. doi: 10.1039/b907384a
Pitre, C. A., Tanner, J. R., Patel, P., and Brassinga, A. K. (2013). Regulatory control of temporally expressed integration host factor (IHF) in Legionella pneumophila. Microbiology 159, 475–492. doi: 10.1099/mic.0.062117-0
Porankiewicz, J., Schelin, J., and Clarke, A. K. (1998). The ATP-dependent Clp protease is essential for acclimation to UV-B and low temperature in the cyanobacterium Synechococcus. Mol. Microbiol. 29, 275–283. doi: 10.1046/j.1365-2958.1998.00928.x
Porter, M. E., and Dorman, C. J. (1997). Positive regulation of Shigella flexneri virulence genes by integration host factor. J. Bacteriol. 179, 6537–6550. doi: 10.1128/jb.179.21.6537-6550.1997
Quon, K. C., Marczynski, G. T., and Shapiro, L. (1996). Cell cycle control by an essential bacterial two-component signal transduction protein. Cell 84, 83–93. doi: 10.1016/S0092-8674(00)80995-2
Rausch, T., Zichner, T., Schlattl, A., Stutz, A. M., Benes, V., and Korbel, J. O. (2012). DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 28, i333–i339. doi: 10.1093/bioinformatics/bts378
Robertson, G. T., Ng, W. L., Foley, J., Gilmour, R., and Winkler, M. E. (2002). Global transcriptional analysis of clpP mutations of type 2 Streptococcus pneumoniae and their effects on physiology and virulence. J. Bacteriol. 184, 3508–3520. doi: 10.1128/JB.184.13.3508-3520.2002
Sahr, T., Bruggemann, H., Jules, M., Lomma, M., Albert-Weissenberger, C., Cazalet, C., et al. (2009). Two small ncRNAs jointly govern virulence and transmission in Legionella pneumophila. Mol. Microbiol. 72, 741–762. doi: 10.1111/j.1365-2958.2009.06677.x
Sahr, T., Rusniok, C., Impens, F., Oliva, G., Sismeiro, O., Coppee, J. Y., et al. (2017). The Legionella pneumophila genome evolved to accommodate multiple regulatory mechanisms controlled by the CsrA-system. PLoS Genet. 13:e1006629. doi: 10.1371/journal.pgen.1006629
Segal, G., and Shuman, H. A. (1999). Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 67, 2117–2124.
Sexton, J. A., Pinkner, J. S., Roth, R., Heuser, J. E., Hultgren, S. J., and Vogel, J. P. (2004). The Legionella pneumophila PilT homologue DotB exhibits ATPase activity that is critical for intracellular growth. J. Bacteriol. 186, 1658–1666. doi: 10.1128/JB.186.6.1658-1666.2004
Siam, R., Brassinga, A. K., and Marczynski, G. T. (2003). A dual binding site for integration host factor and the response regulator CtrA inside the Caulobacter crescentus replication origin. J. Bacteriol. 185, 5563–5572. doi: 10.1128/JB.185.18.5563-5572.2003
Sieira, R., Comerci, D. J., Pietrasanta, L. I., and Ugalde, R. A. (2004). Integration host factor is involved in transcriptional regulation of the Brucella abortusvirB operon. Mol. Microbiol. 54, 808–822. doi: 10.1111/j.1365-2958.2004.04316.x
Stonehouse, E., Kovacikova, G., Taylor, R. K., and Skorupski, K. (2008). Integration host factor positively regulates virulence gene expression in Vibrio cholerae. J. Bacteriol. 190, 4736–4748. doi: 10.1128/JB.00089-08
Tan, I. S., Weiss, C. A., Popham, D. L., and Ramamurthi, K. S. (2015). A quality-control mechanism removes unfit cells from a population of sporulating bacteria. Dev. Cell 34, 682–693. doi: 10.1016/j.devcel.2015.08.009
Tiaden, A., Spirig, T., Weber, S. S., Bruggemann, H., Bosshard, R., Buchrieser, C., et al. (2007). The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA. Cell Microbiol. 9, 2903–2920. doi: 10.1111/j.1462-5822.2007.01005.x
Towbin, H., Staehelin, T., and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76, 4350–4354. doi: 10.1073/pnas.76.9.4350
Vakulskas, C. A., Potts, A. H., Babitzke, P., Ahmer, B. M., and Romeo, T. (2015). Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol. Mol. Biol. Rev. 79, 193–224. doi: 10.1128/MMBR.00052-14
Yakhnin, H., Yakhnin, A. V., Baker, C. S., Sineva, E., Berezin, I., Romeo, T., et al. (2011). Complex regulation of the global regulatory gene csrA: CsrA-mediated translational repression, transcription from five promoters by Eσ70 and EσS, and indirect transcriptional activation by CsrA. Mol. Microbiol. 81, 689–704. doi: 10.1111/j.1365-2958.2011.07723.x
Zhao, B. B., Li, X. H., Zeng, Y. L., and Lu, Y. J. (2016). ClpP-deletion impairs the virulence of Legionella pneumophila and the optimal translocation of effector proteins. BMC Microbiol. 16:174. doi: 10.1186/s12866-016-0790-8
Keywords: Legionella pneumophila, biphasic life cycle, CsrA, ClpP, IHFB
Citation: Ge Z, Long Q, Yuan P, Pan X, Shen D and Lu Y (2019) The Temporal Expression of Global Regulator Protein CsrA Is Dually Regulated by ClpP During the Biphasic Life Cycle of Legionella pneumophila. Front. Microbiol. 10:2495. doi: 10.3389/fmicb.2019.02495
Received: 01 August 2019; Accepted: 16 October 2019;
Published: 07 November 2019.
Edited by:
Ulrike Kappler, University of Queensland, AustraliaReviewed by:
Michele S. Swanson, University of Michigan, United StatesArwa Abu Khweek, Birzeit University, Palestine
Copyright © 2019 Ge, Long, Yuan, Pan, Shen and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-jun Lu, bHV5akBtYWlsLnN5c3UuZWR1LmNu
 Zhen-huang Ge1,2
Zhen-huang Ge1,2 Yong-jun Lu
Yong-jun Lu