
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 15 October 2019
Sec. Systems Microbiology
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.02345
Besides the major nutrients, phosphorus (P) is an essential mineral for both the host animal and the porcine gut microbiota. Different strategies including phytase supplementation and more recently lactic acid (LA) are used to enhance the P availability from cereals in pig diets; however, their impact on the gut microbiota has been rarely related to fecal shedding of opportunistic pathogens. The present study investigated the effect of phytase supplementation and the treatment of dietary cereals with 2.5% LA on the fecal microbiome composition of metabolic active bacteria and expression of virulence factor genes of enterotoxigenic Escherichia coli and Clostridium perfringens in growing pigs. Phytase supplementation reduced the fecal abundance of the most abundant Lactobacillaceae family, whereas the LA-treatment of cereals had a stronger impact on the bacterial community, reducing amylolytic, pullulanolytic and hemicellulolytic Lactobacillaceae, Lachnospiraceae and Ruminococcaceae as well as the fecal bacterial species richness (Chao1) and diversity (Shannon index). Mainly the family Clostridiaceae benefited from the decline in the aforementioned families, being enriched by both dietary treatments. Multigroup data integration using sparse partial least squares-discriminant analysis showed that among the most discriminative operational taxonomic units (OTU) especially two unclassified Clostridiaceae-OTUs, one Prevotella copri-like OTU and one OTU within the vadinCA11 group were associated with calcium and P levels but were negatively linked with complex carbohydrates in feces. Heat-stable toxin A (Sta) of enterotoxigenic E. coli and Stx2e of Shiga-toxin producing E. coli were expressed in feces but were similar among feeding groups. Without modifying the total bacterial gene copies and virulence factor expression of E. coli, both dietary phytase supplementation and LA-treatment of cereals drastically altered the bacterial community composition in pig feces. Results thereby allowed for the characterization of bacterial nutrient dependencies, indicating a link between fecal P availability, complex carbohydrate composition and alterations in the predominant genera.
Aside from the major nutrients, the dietary supply of minerals, such as calcium (Ca) and phosphorus (P), modifies the porcine intestinal microbiota (Mann et al., 2014; Heyer et al., 2015). Both Ca and P are essential nutrients for the bacterial cell metabolism, for instance, in being constituents of nucleotides, co-factors, teichoic acids and phospholipids (Metzler and Mosenthin, 2008). We could previously show that different intestinal P availabilities altered the cellulolytic activity (Metzler et al., 2008) and composition of the bacterial microbiota along the gastrointestinal tract of pigs (Metzler-Zebeli et al., 2010, 2013; Mann et al., 2014). Due to environmental concerns associated with high P levels in manure, the total P content of pig diets has been decreased over the past two decades (Oster et al., 2017). This has not only reduced the total P available for the pig but also for the intestinal microbiota. Most P in plant feedstuffs is in the form of phytate-P (Cowieson et al., 2017). Albeit intestinal bacteria can utilize phytate-P, they only increase their phytase activity when the available P in their surroundings becomes low (Dersjant-Li et al., 2015).
As phytate-P cannot be hydrolyzed by mammalian enzymes (Cowieson et al., 2017), different strategies are applied to enhance the dietary P availability and consequently prevent P deficiencies in pigs, mainly dietary supplementation of phytase (Metzler-Zebeli et al., 2010) or (less frequently used) soaking and fermentation of cereals (Blaabjerg et al., 2011, 2012). However, the efficacy of exogenous phytase addition may be lower due to animal (e.g., gastrointestinal pH, digesta passage rate), and feed-related factors (e.g., dietary buffering capacity and pelleting temperature) (Blaabjerg et al., 2011, 2012; Kebreab et al., 2012; Yáñez et al., 2013). For this reason, feed-technological approaches (e.g., soaking and fermentation) that enhance the phytate-P availability in cereal grains prior to feeding to the animals have received more attention lately. For instance, we could recently show that soaking barley grains in lactic acid (LA) reduced the phytate-P content in barley and altered the ruminal microbiota (Metzler-Zebeli et al., 2014, 2015). Aside from this, soaking of cereals in LA may lead to structural changes in other components, such as the starch and hemicellulose fractions (Deckardt et al., 2015), which are key substrates that may alter the fibrolytic bacterial community. Differences in the effects of soaking and fermentation processes on the intestinal microbiota and shedding of virulence factors in feces may be expected, partly due to the lag phase until bacterial fermentation and LA production sets in. In general, LA has bacteriostatic effects when added as feed additive to pig diets, reducing the intestinal abundance of opportunistic pathogens such as enterotoxigenic Escherichia coli and Clostridium perfringens (Suiryanrayna and Ramana, 2015; Koopmans et al., 2016). These pathogens, especially E. coli pathotypes (e.g., Shiga toxin-producing and enterotoxigenic E. coli), C. perfringens, and Campylobacter coli, are responsible for major economic losses in the pig industry (Toledo et al., 2012; Wells et al., 2015; Bardasi et al., 2017) and are a potent source of microbial contaminants in pork products, therefore being a risk factor for foodborne zoonotic diseases (Lamendella et al., 2011). Consequently, dietary strategies should be evaluated whether they increase or decrease intestinal numbers of opportunistic pathogens in order to formulate diets which promote intestinal health and reduce the risk of spreading zoonotic diseases.
Most studies investigating dietary effects on intestinal microbial communities were performed by targeting DNA (Lu et al., 2015). However, the extracted DNA may originate from dead or dormant bacteria and may therefore not represent the metabolically active bacteria, whereas bacterial RNA correlates better to microbial growth and metabolic activity (Westermann et al., 2012; Zhu et al., 2017).
Since hemicellulolytic and proteolytic bacteria previously increased with higher intestinal P availability in pigs (Metzler-Zebeli et al., 2010, 2013), we hypothesized that an increased phytate-P availability in the upper digestive tract due to dietary phytase supplementation or soaking of cereals grains in LA may modify the fibrolytic bacterial community in the distal large intestine and reduce the fecal numbers of common opportunistic pathogens. Due to the capability of LA to modulate the complex carbohydrate fractions in the cereals (Metzler-Zebeli et al., 2014), we further hypothesized that these changes additionally will alter the starch- and fiber-degrading bacterial community. Therefore, this study evaluated the effect of phytase supplementation and the treatment of dietary cereals with 2.5% LA on the fecal composition of metabolic active bacteria and expression of virulence factor genes of enterotoxigenic E. coli and C. perfringens in growing pigs.
Thirty-two castrated male pigs (Large White, 13.1 ± 2.3 kg) with an average age of 6–8 weeks were used in this study. Pigs were obtained from the University research pig farm (University of Veterinary Medicine Vienna) where they were reared from the suckling to post-weaning phase in a similar manner across the four replicate batches. Piglets were weaned at 28 days of age. Prior to the start of the experiment, all pigs received the same feeding regimen (suckling to post-weaning phase). Two days prior to the start of the experiment, pigs were moved to the experimental room and were housed into individual metabolism pens (1.0 × 1.2 m) for the whole experimental period. After this environmental adaptation, pigs were randomly assigned to one of four dietary treatments in a 2 (two phytase levels: 0 and 500 FTU/kg) × 2 (two cereal types: treated versus non-treated) factorial design. In total, there were four replicate batches with eight pigs per replicate batch. Two pigs per replicate batch received the same experimental diet, resulting in eight observations per dietary treatment at the completion of the experiment (Supplementary Figure S1). Each replicate batch consisted of 19 days with the collection of samples from the rectum occurring on days 18 and 19. Pigs had free access to demineralized water throughout the experiment. Pigs were housed in an environmentally controlled room, and room temperature was checked three-times daily to ensure optimal temperature for the pigs. Pigs were weighed at the beginning and end of the experimental period. The health status of the animals was monitored daily.
The diets consisted of wheat, corn and soybean meal (Table 1) and were formulated to meet or exceed the current recommendation for nutrient requirements (Gesellschaft fur Ernahrungsphysiologie [GfE], 2006, National Research Council [NRC], 2012). Pigs were fed one of four following experimental diets: (1) control (Con) diet (Con diet) (2) control diet with phytase (Con-Phy diet) (3) diet containing LA-treated cereals (LA diet) (4) diet with phytase and LA-treated cereals (LA-Phy diet). Two mineral-vitamin premixes were formulated. According to the standard inclusion level of phytase (Dersjant-Li et al., 2015), one premix comprised microbial phytase (500 FTU/kg), whereas the other premix was without microbial phytase. For the diets with LA treatment of the cereal grains, wheat and corn were soaked in a 2.5% LA solution for 48 h, as previously published (Vötterl et al., 2019). The concentration of the LA solution of 2.5% and the incubation time of 48 h of incubation have been selected based on the results of our preceding in vitro study (Vötterl et al., 2019). After the 48 h of incubation, cereals were dried at 70°C for 1 h and afterward at 60°C for 23 h and ground to pass a 5-mm sieve.
Pigs were fed three times daily at 8:00, 12:00, and 16:00 h. Feed allowances were calculated to correspond to 3-times maintenance requirement ([(body weight0.6 × 197)/238.68] × 3) (Gesellschaft fur Ernahrungsphysiologie [GfE], 2006). At feeding, the experimental diets were mixed with water in a ratio of 3: 1 and immediately offered to the pigs. Feed leftovers (feed spillage and feed remainings in the feeding bowls) were collected, dried, and weighed to determine dry matter intake. Diet samples were collected in each replicate run.
On days 18 and 19, pigs were euthanized 2 h after their last feeding via intracardiac injection of T61 (10 ml/kg Embutramide, MSD animal Health, Vienna, Austria) after general anesthesia was induced (Narketan 100 mg/ml, 1 ml/10 kg body weight, Ketaminhydrochlorid, Vétoquinol GmbH, Germany; Stresnil 40 mg/ml; 0.5 ml/10 kg body weight, Azaperon, Elanco Deutschland GmbH, Germany). For the analysis of metabolic active bacteria, fresh feces from the rectum were aseptically collected in 2-ml cryo-tubes (Sarstedt AG & Co., Nümbrecht, Germany), immediately snap-frozen in liquid nitrogen and stored at −80°C until analysis. In one pig, the rectum was empty and digesta could not be collected for microbial analysis.
Diets, freeze-dried digesta and fecal samples were analyzed for dry matter, protein, ash, calcium (Ca) and P according to VDLUFA as previously published (Metzler-Zebeli et al., 2013). The content of neutral detergent fiber (aNDFOM) and acid detergent fiber (ADFOM) were determined using Fiber Therm FT 12 (Gerhardt GmbH & Co., KG, Königswinter, Germany) (Metzler-Zebeli et al., 2014). Resistant (RS) and non-resistant (NRS) Starch contents were analyzed photometrically using enzymatic assay kits (K-RSTAR: Megazyme International Ireland, Ltd., Braz Ireland) according to the manufacturer’s instructions.
Total RNA was extracted using the RNeasy PowerMicrobiome Kit following the manufacturer’s instructions (Qiagen, Hilden, Germany) with some modifications with respect to the bead beating procedure. Frozen feces (250 mg) were weighed into 2-mL screw-cap tubes, containing 0.6 g sterile glass beads (Ø 0.1 mm), 0.4 g ceramic beads (Ø 1.4 mm), 0.55 g ceramic beads (Ø 2.8 mm) and 650 μL guanidinium thiocyanate buffer. TheFastPrep-24 instrument (MP Biomedicals, Heidelberg, Germany) was used for homogenization which consisted of three bead beating steps- each one 1 min at 6.5 m/swith cooling on ice between the bead beating steps. Thereafter, samples were processed according to the manufacturer’s instructions (Qiagen). To remove genomic DNA, the extracted RNA was treated with DNase I (Turbo DNA kit, Life Technologies Limited, Vienna Austria) following the manufacturer’s instructions. The elution volume was 50 μL. The total RNA of each sample was quantified by the Qubit 2.0 Fluorometer (Life Technologies Corporation, CA, United States) using Qubit RNA Assay Kit according to the manufacturer’s instructions as well as qualified with Agilent Bioanalyzer 2100 (Agilent Technologies, Waldbronn, Germany). The Agilent RNA 6000 Nano Assay (Agilent Technologies, Waghäusel-Wiesental, Germany) was used to determine the RNA integrity numbers (RIN), which ranged between 6 and 10. The cDNA synthesis was performed using the High Capacity Reverse Transcription Kit (Life Technologies Foster City, United States) following the manufacturer’s instructions. One μg of total RNA and 0.5 μL of RNase Inhibitor (Qiagen) were added to each reaction. One aliquot of the cDNA was sent for 16S rRNA gene amplicon sequencing, whereas a second aliquot was used for absolute quantification of total bacteria and virulence factors.
Absolute quantification of total bacteria and virulence factor genes (cpa, STa and STx2e) in fecal samples was performed on a Stratagene Mx3000P qPCR system (Agilent Technologies) using previously published primer sets (Supplementary Table S1) (Metzler-Zebeli et al., 2010; Muyzer et al., 1993; Schlegel et al., 2012). Primer sequences were verified with PrimerBLAST1 and tested for efficiencies and specificity using melting curve analysis (Supplementary Table S1). Each 20 μl reaction consisted of 5 ng cDNA, 10 μl Eva Green master mix with low ROX (Biotium, Hayward, CA, United States), 400 nM each of forward and reverse primers, and 10 μl DEPC-treated water (Bioscience) in a 96-well plate in duplicate. The amplification protocol comprised an initial denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for 15 s, primer annealing at 60°C for 30 s, and elongation at 72°C for 30 s, followed by the generation of melting curves with increments of 0.1°C/s between 55 and 95°C. Additionally, negative controls and the reverse transcription controls (RT minus) were also run on each plate.
Standard curves were prepared from 10-fold serial dilutions (107 to 103 molecules/μl) of the purified and quantified PCR products using genomic DNA from pig feces of the present study (Metzler-Zebeli et al., 2016). The final copy number of total bacteria and virulence factor genes was calculated using the following equation: (QM × C × DV)/(S × V), where QM is the quantitative mean of the copy number, C is the DNA concentration of each sample, DV is the dilution volume of isolated DNA, S is the DNA amount (ng) and V is the weight of the sample (g) subjected to DNA extraction. Amplification efficiencies (E = 10(–1/slope)) and coefficient of determination (linearity) can be found in Supplementary Table S1.
The 16S rRNA PCRs, library preparation, and sequencing using the Illumina MiSeq sequencing platform was performed by a commercial provider (Microsynth AG, Balgach, Switzerland) as previously described (Metzler-Zebeli et al., 2018). The V3-V4 hypervariable regions of bacterial 16S rRNA were amplified using the primers 341F-ill (5′-CCTACGGGNGGCWGCAG-3′) and 802R-ill (5′-GACTACHVGGGTATCTAATCC-3′) which produces an amplicon of approximately 460 bp. The 16S rRNA gene PCRs were performed using the KAPA HiFi HotStart PCR Kit (Roche, Baden, Switzerland). The Nextera XT sample preparation kit (Illumina Inc.) was used to construct libraries by ligating sequencing adapters and indices onto purified PCR products. Afterward, equimolar quantities of each library were pooled and sequenced on an Illumina MiSeq sequencing v2 platform using a 250 bp read length paired-end protocol. After sequencing, the overlapping paired-end reads were demultiplexed, trimmed of Illumina adaptor residuals using cutadapt2 and stitched using USEARCH (drive5/com) by Microsynth.
The stitched reads were processed using the software package Quantitative Insights into Microbial Ecology (QIIME, v1.9.4) (Caporaso et al., 2010). Fastq files were quality checked using the ‘split_libraries_fastq.py’ command for non-multiplexed Illumina fastq data with the phred score offset of 33. Chimeric sequences were identified with the UCHIME method and using USEARCH8.1 and gold. fa database, which were subsequently filtered out by the ‘filter_fasta.py’ command (Edgar, 2010; Edgar et al., 2011). Open-reference OTU picking was done at 97% similarity level using UCLUST (Edgar, 2010). Taxonomy was assigned to the OTUs using the Ribosomal Database Project (RDP) naïve Bayesian rRNA classifier and the Greengenes database as reference template (version 13.8) (De Santis et al., 2006). Rare OTUs with less than 10 sequences were excluded. In addition, the sequence identity of OTUs at >0.01% relative abundance was checked using Microbial Nucleotide BLAST3 (Supplementary Table S2). For α-diversity (Shannon, Simpson, and Chao1) measurements, samples were rarefied to a depth of 24,500 sequences. For β-diversity analysis, statistical assessment of dissimilarity matrices (Bray-Curtis) derived from OTU data was performed using the ‘adonis2’ function (PERMANOVA) in the R package ‘vegan’ (version 2.5.2). The PERMANOVA was applied on the Bray-Curtis distance matrices between dietary factors (phytase supplementation, LA treatment of cereals and their interaction) and statistical significance was calculated after 999 random permutations. Clustering of fecal samples from the four dietary treatment groups were visualized in two-dimensional non-metric multidimensional scaling (NMDS) ordination plots obtained with the ‘metaMDS’ function in the vegan R package (Oksanen et al., 2018) in R studio (version 1.0.136). To identify the most discriminant OTUs and nutrients in feces, multigroup supervised DIABLO N-integration networking was performed using the R package ‘mixOmics’ (version 6.3.2) (Rohart et al., 2017). Horizontal sparse partial least squares-discriminant analysis (sPLS-DA) using the ‘block.splsda’ function was applied to integrate the datasets of relative abundances of OTUs and nutrient composition in feces to classify and select key features from each dataset. This enabled the discrimination of treatment groups with the lowest possible error rate, selecting 20 OTUs which were associated with the fecal content of protein, ash, Ca and P forming the “nutrient” dataset and the fecal content of aNDFOM, ADFOM, RS and NRS forming the “carb” dataset. Results were visualized in loading plots and heatmaps for component 1 and 2 as well as in circos plots for component 1 and 2, illustrating the strongest positive and negative correlations among the “OTU”, “nutrients” and “carb” datasets.
Prior to the start of the experiment, a power test analysis estimated according to Kononoff and Hanford (2006) and based on previous data for the intestinal microbiota composition and interaction with the host animal (Heyer et al., 2015; Kelly et al., 2017; Metzler-Zebeli et al., 2013, 2018, 2019; Mann et al., 2014) using the SAS software (version 9.4; SAS Inst. Inc., Cary, NC, United States) was performed to identify the minimum number of observations (n = 6) required for the present pig experiment to reject the null-hypothesis if this was false.
To test for differences in relative abundance of bacterial taxa, only taxa appearing in at least 50% of the fecal samples were considered. The raw read counts from the tables of OTU abundances were collapsed and compositionally normalized such that each sample sums to 1. The relative abundances at the respective taxonomic rank were analyzed. All variables were tested for normal distribution by the Shapiro-Wilk test with the UNIVARIATE procedure in SAS (Version 9.4, SAS Inst. Inc., Cary, NC). To compare differences between dietary treatments, data for α-diversity, bacterial phyla, families and genera as well as selected OTUs were subjected to ANOVA using the MIXED procedure in SAS. The model included the fixed effects of phytase supplementation, LA treatment of grains and their two-way-interaction and replicate as random effect. Pig was the experimental unit. The degrees of freedom were approximated by the Kenward-Rogers method (ddfm = kr). The means were reported as least-squares means ± standard error of the mean (SEM). The differences were considered significant if p < 0.05 and trends at 0.05 < p ≤ 0.10. However, the discussion was mostly based on significant changes between dietary treatments.
All pigs remained healthy throughout the study and ate the same amount of feed across treatment groups (784 ± 20.4 g/day). The LA treatment of cereals reduced the ash, protein, and aNDFOM contents of the diets by 0.5, 1.1 and 1.0%, respectively (Table 1). The LA treatment increased the dry matter (DM) content of feces (p < 0.001), whereas the phytase supplementation tended to reduce it (Table 2; p < 0.10). Treating cereals with LA also increased (p = 0.004) the aNDFOM content in pig feces by 1.9%. Both phytase supplementation and LA treatment of the cereal grains increased the ADFOM content in feces (p < 0.05). The concentration of total, RS and NRS fractions tended to be higher in feces of pigs fed the LA-treated cereals (p < 0.10). When expressing the RS and NRS fractions as proportion of total starch, the RS fraction was smaller with the LA-treated cereals. Moreover, both phytase supplementation and LA treatment decreased the fecal P content, whereas only the phytase supplementation lowered the Ca content and subsequently enhanced the Ca:P ratio in feces (p < 0.001).
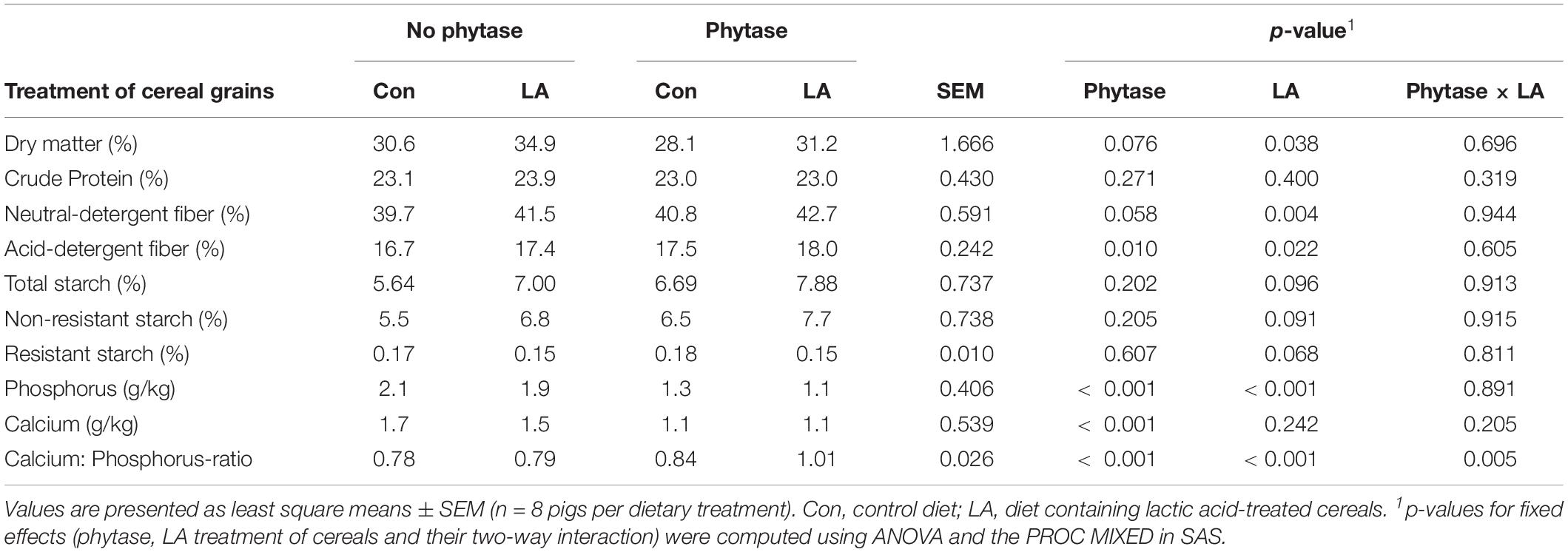
Table 2. Characteristic of feces from pigs fed diets with or without phytase and lactic acid treatment of cereals.
The absolute abundance of metabolically active bacteria in feces amounted to 11.1 log10 gene copies/g feces (Table 3). After quality and chimera filtering, a total of 1,569,539 reads with a mean of 49,048 ± 17,836 sequences per sample and a mean read length of 403.39 ± 35.60 bp remained. Reads were clustered into 3,545 operational taxonomical units (OTUs).
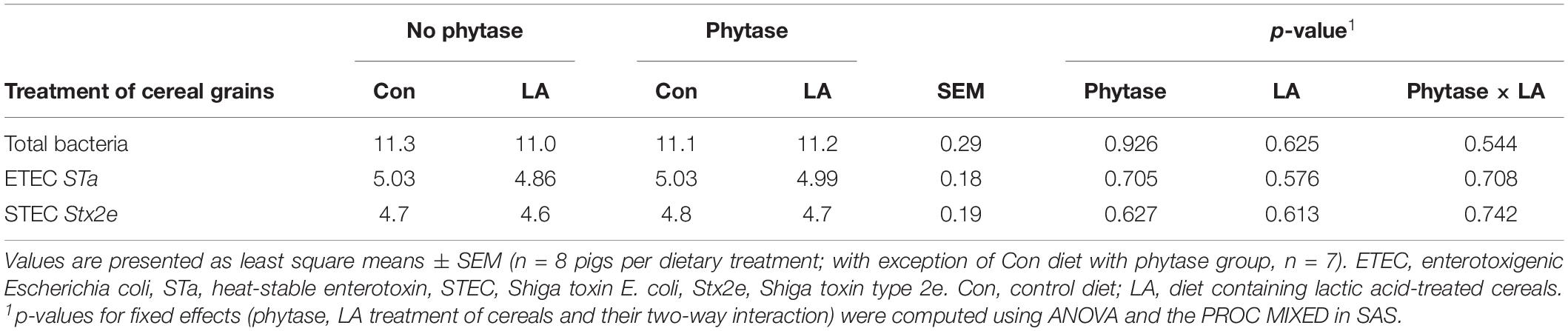
Table 3. Absolute abundance of total bacterial 16S rRNA and virulence factors (log10 gene copies/g) in feces of pigs fed diets with or without phytase and lactic acid treatment of cereals.
The most abundant phyla in feces were Firmicutes (78.8% of all reads), followed by Euryarchaeota (13.0% of all reads) and Bacteroidetes (4.2% of all reads) (Supplementary Table S3). Phylogenetic classification identified 12 families with a mean abundance greater than 0.01% of all reads (Table 4), with Lactobacillaceae (20.1% of all reads), Clostridiaceae (19.3% of all reads), Lachnospiraceae (16.7% of all reads), and Ruminococcaceae (13.0% of all reads) being the dominant families. Accordingly, Lactobacillus and unclassified genera within Clostridiaceae, Lachnospiraceae, and Ruminococcaceae were the dominant genera (Supplementary Table S4). The same trends were also found at operational taxonomic unit (OTU) level with a relative abundance of >0.01% of all reads (Supplementary Table S5).
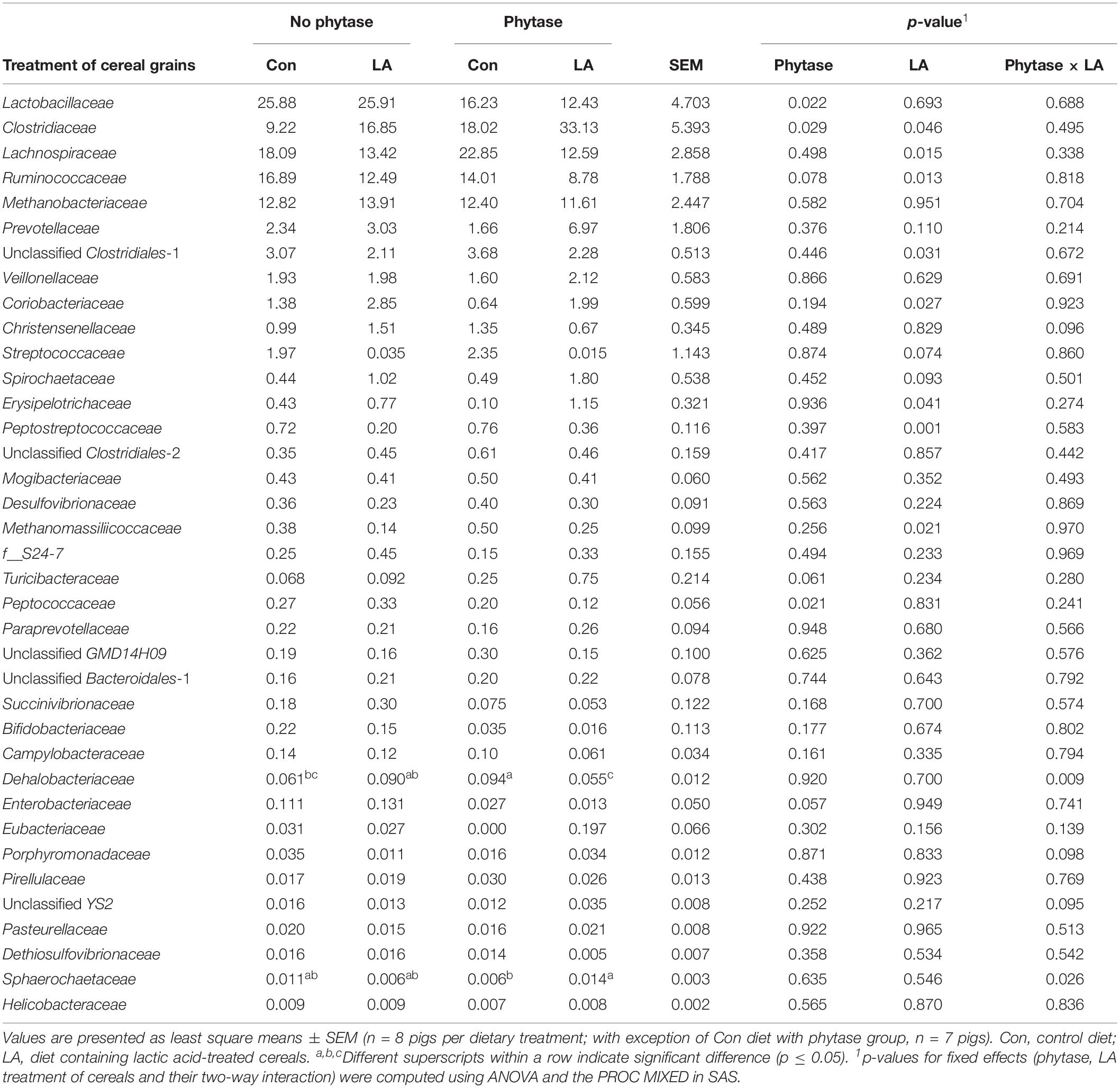
Table 4. Relative abundance (%) of bacterial families (>0.01% of all reads) in feces of pigs fed diets with or without phytase and lactic acid treatment of cereals.
Total bacterial gene copy numbers were similar among treatment groups (Table 3). Separation between treatment groups was detected in the Bray-Curtis derived dissimilarity matrix for the bacterial communities. Accordingly, the communities of pigs fed the diets with the LA-treated cereal grains clustered apart from those of pigs fed diets with non-treated cereal grains (p = 0.031; R2 = 0.064). Similarly, the bacterial communities of pigs fed the diets with and without phytase, respectively, tended to cluster separately (Figure 1 and Supplementary Table S6; p < 0.10). This was also reflected by the α-diversity, showing a lower species richness (Chao 1) and diversity (Shannon) in feces of pigs fed the diets with the LA-treated cereals compared to pigs fed the diets with the non-treated cereals (Table 5; p < 0.05).
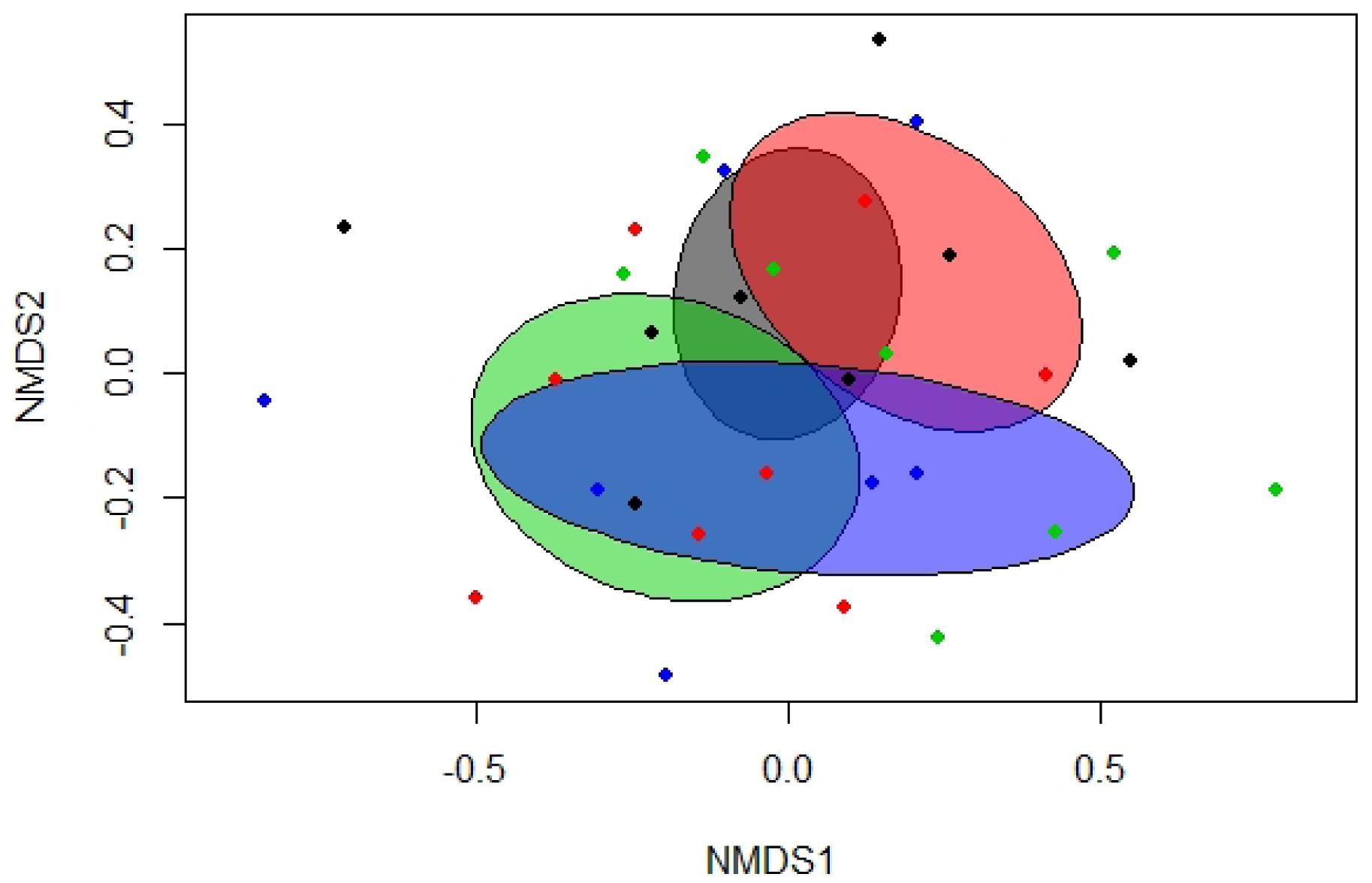
Figure 1. Non-metric multidimensional scaling (NMDS) plot of pairwise Bray-Curtis dissimilarities between bacterial communities in feces of pigs fed diets with or without phytase and lactic acid treatment of cereals (stress level = 0.19). Ellipses represent the standard deviation. Gray, control diet; green, diet containing lactic acid-treated cereals; red, diet with phytase supplementation; and blue, diet with phytase supplementation and lactic acid-treated cereals.
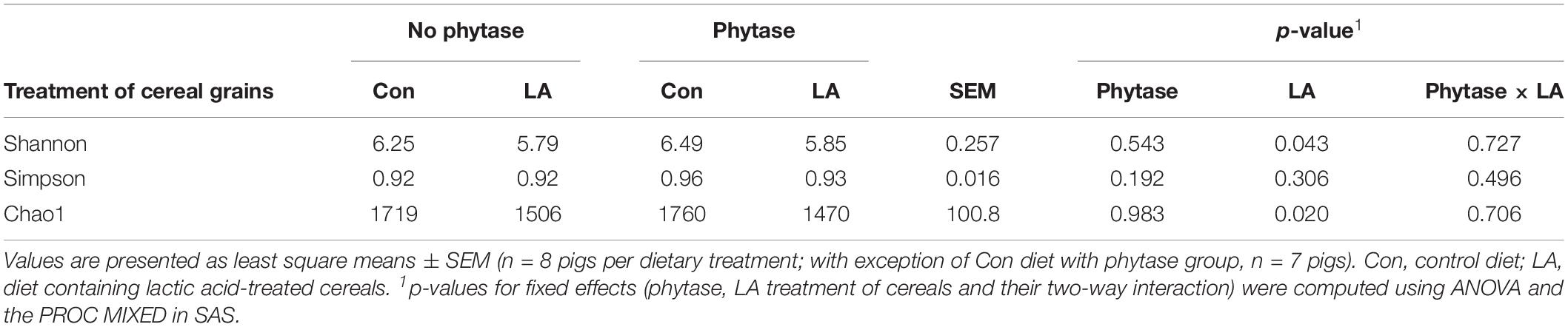
Table 5. The alpha-diversity indices in feces of pigs fed diets with or without phytase and lactic acid treatment of cereals.
Although no differences were detected at phylum level (Supplementary Table S3), the phytase supplementation decreased the relative abundance of Lactobacillaceae and Peptococcaceae by 0.4 and 0.3-fold, whereas it increased the abundance of Clostridiaceae by 1-fold (Table 4; p < 0.05). This was reflected at genera level where the phytase supplementation lowered the abundance of the genus Lactobacillus and increased the abundance of Clostridium (P < 0.05). The LA treatment of grains, in turn, only increased the abundance of Actinobacteria by 0.9-fold at phylum level (p = 0.04), whereas alterations in the relative abundances of bacterial taxa could be found within the Firmicutes without changing the abundance of the phylum as a whole. Overall, the LA treatment of cereals affected 8 bacterial families (>0.01% of all reads) and 13 genera (>0.05% of all reads), with 3 families and 4 genera being increased and 5 families and 9 genera being decreased, respectively (Table 4 and Supplementary Table S4). Drastic changes were observed within the predominant Firmicutes families, with Clostridiaceae increasing, and the other families decreasing in feces of pigs fed the LA diets (p < 0.05).
In using the compositional information of the metabolically active bacteria from the sequencing data, we quantified the abundances of genes for the heat-stable toxin A (STa) and Shiga toxin STEC (Stx2e) of E. coli as well as for α-toxin (cpa) of C. perfringens in order to investigate the impact of phytase supplementation and LA treatment of cereal grains on their fecal expression. In feces, expression of STa (4.9 log10 gene copies/g) and Stx2e (4.7 log10 gene copies/g) was detected (Table 3), whereas cpa cDNA was below detection limit, indicating that no or only few gene transcripts were present in feces. The dietary treatments had no effect on the gene copy numbers of STa and Stx2e.
Supervised sparse partial least squares-discriminant analysis (sPLS-DA) was used to identify the most influential OTUs, and their relation to protein, ash, Ca, and P as well as complex carbohydrate composition (aNDFOM, ADFOM, RS, and NRS) in feces for component 1 and 2. The most influential OTUs and chemical components were visualized in heatmaps (Figures 2A,B) and loading plots (Figures 3, 4). The strongest correlations among the best 20 discriminant OTUs, complex carbohydrates and other nutrients in feces are displayed in circos plots for component 1 (Figure 5A) and component 2 (Figure 5B), respectively. For component 1 (Figure 5A), four OTUs (unclassified Ruminococcaceae-OTU21, VadinCA11-OTU131, unclassified Ruminococcaceae-OTU175, and Prevotella-OTU225) were positively linked to P and Ca, whereby unclassified Ruminococcaceae-OTU21 and Prevotella-OTU225 were additionally positively correlated to fecal ash. In contrast, these four OTUs were negatively correlated to ADFOM and aNDFOM in feces. Moreover, P, Ca, and ash correlated negatively to ADFOM and aNDFOM. For component 2 (Figure 5B), only Peptococcus-OTU85 was positively linked to protein, whereas 11 OTUs (Clostridium-OTU18 and -OTU38, VadinCA11-OTU69, unclassified Clostridiales-OTU71, unclassified [Mogibacteriaceae]-OTU162, Coprococcus-OTU168, unclassified Clostridiaceae-OTU174, unclassified Coriobacteriaceae-OTU183, Blautia-OTU207, unclassified Ruminococcaceae-OTU238, and unclassified Lachnospiraceae-OTU248) were negatively correlated with fecal protein. Additionally, VadinCA11-OTU69, unclassified Clostridiales-OTU71, Coprococcus-OTU168, unclassified Clostridiaceae-OTU174 and unclassified Ruminococcaceae-OTU238 also positively correlated to NRS in feces. Moreover, the protein in feces were negatively linked with RS and NRS in feces.
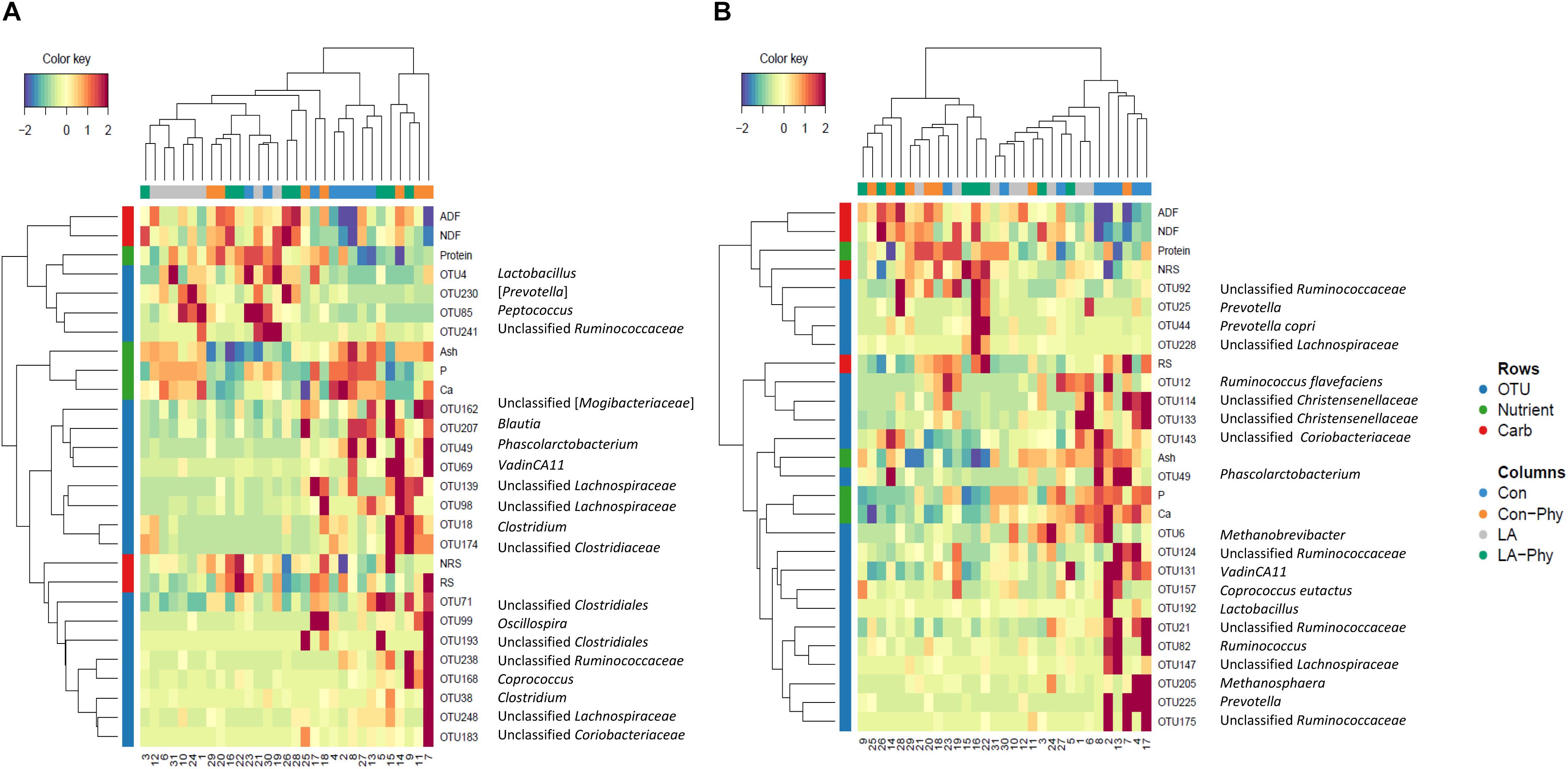
Figure 2. Clustered Image Maps (Euclidean distance, complete linkage) of the multi-omics signature for the identified best discriminant operational taxonomic units (OTUs; n = 20; relative OTU abundance >0.04%), complex carbohydrates (RS, NRS, aNDFOM and ADFOM; n = 4) and nutrients in feces (protein, ash, Ca and P; n = 4) using sparse partial least square-discriminant analysis showing different enrichment between the treatment groups for (A) component 1 and (B) component 2. Con, control diet; LA, diet containing lactic acid- treated cereals; Con-Phy, diet with phytase supplementation; and LA-Phy, diet with phytase and lactic acid-treated cereals.
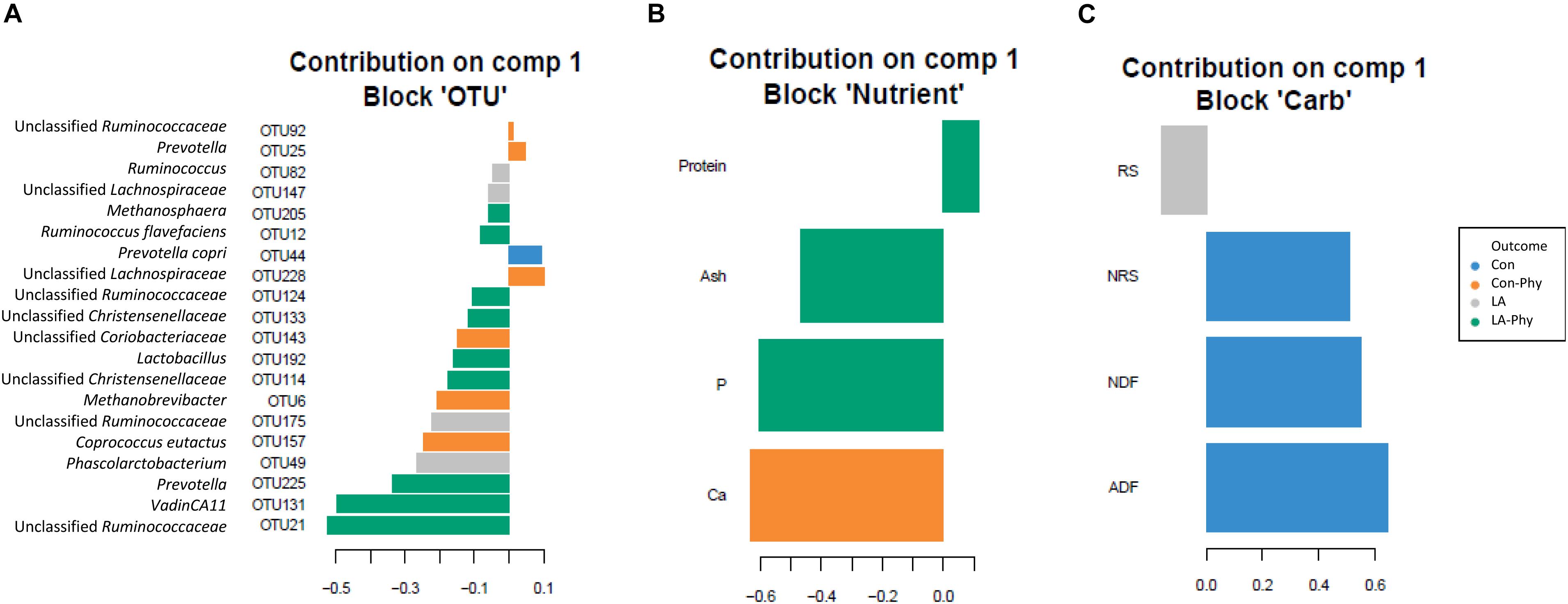
Figure 3. Loading plots of sparse partial least square-discriminant analysis showing the best discriminant bacterial operational taxonomic units (OTU; n = 20; relative OTU abundance >0.04%) and complex carbohydrates (RS, NRS, aNDFOM and ADFOM; n = 4) or nutrients in feces (protein, ash, Ca and P; n = 4). The sPLS-DA identified the best discriminant bacterial OTUs on component 1 at more than 0.04% relative abundance of all (A) OTUs, (B) nutrients and (C) carbohydrates (method = “median”). Colors indicate the treatment groups where the mean abundance of the OTUs, nutrients and carbohydrates is maximal. Con, control diet; LA, diet containing lactic acid- treated cereals; Con-Phy, diet with phytase supplementation; and LA-Phy, diet with phytase and lactic acid-treated cereals.
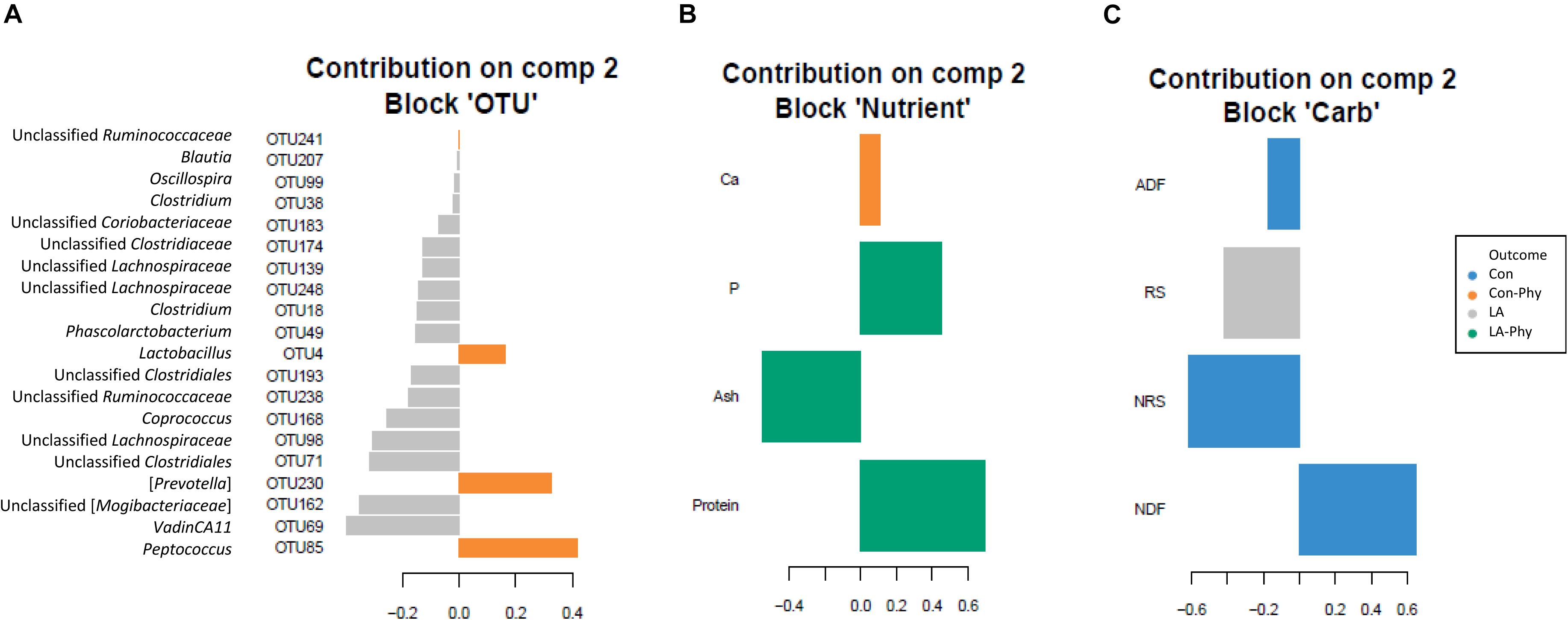
Figure 4. Loading plots of sparse partial least square-discriminant analysis showing (A) the best discriminant bacterial operational taxonomic units (OTU; n = 20; relative OTU abundance >0.04%) and (B) complex carbohydrates (RS, NRS, aNDFOM and ADFOM; n = 4) or (C) nutrients in feces (protein, ash, Ca and P; n = 4). Colors indicate the treatment groups where the mean abundance of the OTUs, nutrients and carbohydrates is maximal. Con, control diet; LA, diet containing lactic acid- treated cereals; Con-Phy, diet with phytase supplementation; and LA-Phy, diet with phytase and lactic acid-treated cereals.
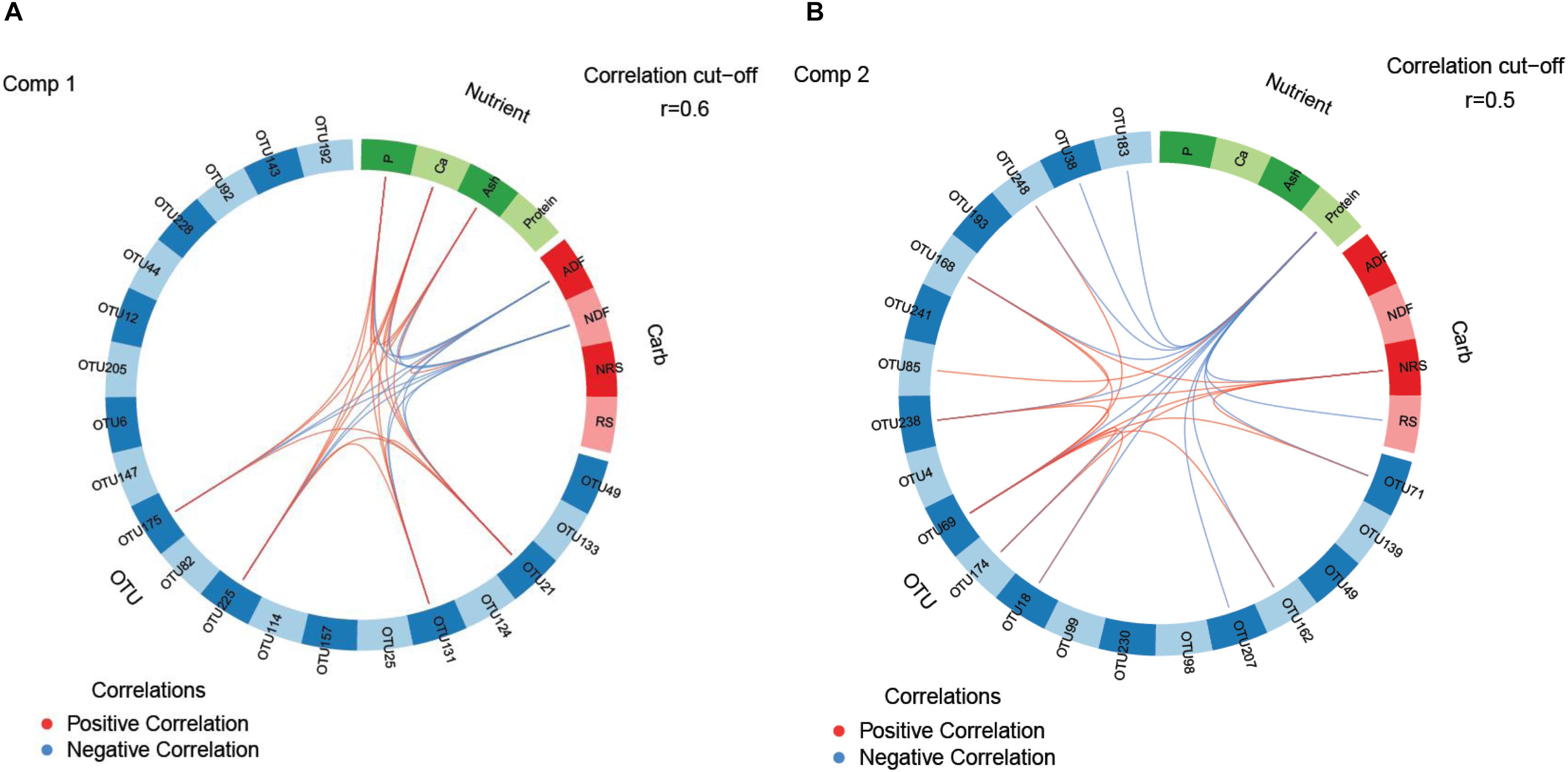
Figure 5. The circos plots of sparse partial least square-discriminant analysis displaying correlations between the identified best discriminant bacterial operational taxonomic units (OTU; n = 20; relative OTU abundance >0.04%), complex carbohydrates (RS, NRS, aNDFOM and ADFOM; n = 4) and nutrients in feces (protein, ash, Ca and P; n = 4) for (A) component 1 and (B) component 2. Positive and negative correlations (| r| > 0.6 for component 1 and | r| > 0.5 for component 2) are displayed by red and blue links, respectively. OTU4 Lactobacillus; OTU6 Methanobrevibacter; OTU12 Ruminococcus flavefaciens; OTU18 Clostridium; OTU21 Ruminococcaceae; OTU25 Prevotella; OTU38 Clostridium; OTU44 Prevotella copri; OTU49 Phascolarctobacterium; OTU69 VadinCA11; OTU71 Unclassified Clostridiales; OTU82 Ruminococcus; OTU85 Peptococcus; OTU92 Unclassified Ruminococcaceae; OTU98 Unclassified Lachnospiraceae; OTU99 Oscillospira; OTU114 Unclassified Christensenellaceae; OTU124 Unclassified Ruminococcaceae; OTU131 VadinCA11; OTU133 Unclassified Christensenellaceae; OTU139 Unclassified Lachnospiraceae; OTU143 Unclassified Coriobacteriaceae; OTU147 Unclassified Lachnospiraceae; OTU157 Coprococcus eutactus; OTU162 Unclassified [Mogibacteriaceae]; OTU168 Coprococcus; OTU174 Unclassified Clostridiaceae; OTU175 Unclassified Ruminococcaceae; OTU183 Unclassified Coriobacteriaceae; OTU192 Lactobacillus; OTU193 Unclassified Clostridiales; OTU205 Methanosphaera; OTU207 Blautia; OTU225 Prevotella; OTU228 Unclassified Lachnospiraceae; OTU230 [Prevotella]; OTU238 Unclassified Ruminococcaceae; OTU241 Unclassified Ruminococcaceae; OTU248 Unclassified Lachnospiraceae.
Albeit not being representative for the bacterial community in the small intestine and proximal large intestine, the fecal microbiota of pigs is qualitatively similar and hence representative for the bacterial microbiome composition in the more distal parts of the large intestine (Zhao et al., 2015). Therefore, characterization of the bacterial community in feces is valid and informative to our understanding of nutrient-microbiota interactions, contributing to the discovery of cause-and-effect relationships between dietary changes and expression and shedding of virulence factor genes of common opportunistic pathogens in pigs. Due to the present manipulation of the intestinal P availability by both phytase supplementation and pre-treatment of the cereals with 2.5% LA together with changes in the complex carbohydrate fraction due to the LA-treatment of grains (Metzler-Zebeli et al., 2014; Metzler-Zebeli et al., 2015), we predicted changes mainly in the proteolytic and fibrolytic fecal bacterial communities as previously observed in pigs and ruminants (Metzler-Zebeli et al., 2014; Metzler-Zebeli et al., 2015; Metzler-Zebeli et al., 2018). The present results showed that both dietary treatments caused distinct community composition profiles of the metabolically active bacteria in feces of pigs; however, without affecting total bacterial gene copy numbers or virulence factor expression of E. coli and C. perfringens. Notably, all major bacterial families were either affected by the dietary phytase or LA-treated cereals, whereby more taxa were modulated by the LA-treated cereals than by the phytase supplementation. This was reflected in the α-diversity analysis showing that only the LA treatment of cereals impacted species richness (Chao1) and evenness (Shannon). However, as we did not observe an increase in opportunistic pathogens, this decrease in diversity may not have weakened the stability of the bacterial community in the distal large intestine. We could associate several bacterial OTUs with the fecal nutrient concentrations, showing that the bacterial community adapted rapidly to changes in nutrient availabilities in the distal large intestine. Digestion and fermentation continuously advance throughout the digestive tract; therefore, bacterial abundances observed in feces are a consequence of alterations in the digestive and fermentative processes occurring proximally. Part of the observed dietary effects may be therefore linked to subsequent alterations in the microbe-microbe-interactions and cross-feeding of primary fermentation metabolites throughout the lower intestine (Flint et al., 2015). This relationship was supported by the present correlations between the most discriminant OTUs and fecal concentrations of Ca, P, protein and complex carbohydrate fractions.
While the LA treatment of cereals released the phytate-bound phosphate groups pre-feeding (Metzler-Zebeli et al., 2014; Vötterl et al., 2018), the supplemental phytase was only activated in the stomach when reaching their pH optima (Zhang et al., 2010). Although the chemical composition of feces can only be indicative for changes in intestinal absorption, present results suggest that the phytase was more efficient to improve the intestinal P availability than the LA treatment of cereal grains, whereby combining dietary phytase and LA-treated cereals acted synergistically on reducing the fecal P content. Notably, fecal Ca was not similarly affected by both treatments as indicated by the varying Ca:P ratio among the four treatment groups. Indeed, the Phy × LA interaction for the Ca:P ratio suggested that more Ca was retained in large intestinal digesta and feces with the combination of dietary phytase and LA-treated cereals, which may have been due to buffering properties of Ca, binding to dietary residuals or bacterial incorporation (Metzler and Mosenthin, 2008). Feces became enriched with aNDFOM and ADFOM with the LA-treated cereals, indicating low fermentability of the residual fiber fractions. Since feces also contained more NRS, changes in gut motility and a subsequently higher intestinal passage may be a possible explanation for these observations, modifying the time available for bacterial substrate utilization and proliferation (Le Goff et al., 2002; Van Leeuwen et al., 2006; Wilfart et al., 2007; Metzler-Zebeli et al., 2019). Therefore, it suggests the fecal transit time and available substrates are considered as vital factors influencing bacterial substrate utilization and proliferation, especially enteric pathogens. This may be additionally supported by the high fecal DM content in pigs fed the LA-treated cereals, which may contribute to reducing the risk of diarrhea as more water is absorbed.
Both dietary phytase supplementation and LA-treatment of grains led to dramatic alterations in the dominant families, all belonging to the Firmicutes phylum. The main beneficiary of the community shifts was the family Clostridiaceae, becoming especially enriched in feces when both treatments were combined. This diverse and versatile family comprises a plethora of metabolic capabilities including starch-degrading, fibrolytic and proteolytic capacities as well as many important porcine enteric pathogens, e.g., C. perfringens. Against this background, the limited classification of many Clostridiaceae genera and species makes it difficult to predict their metabolic dependencies within the present bacterial communities and their role for host health. Correlations displayed in the circos plots indicated negative relationships of Clostridium-OTU18 and -OTU38, both representing species related to C. saccharolyticum, and fecal protein. C. saccharolyticum mainly utilizes di- and oligosaccharides (Murray et al., 1982). Their fecal enrichment may have been therefore an indirect result of cross-feeding of sugars released by other hemicellulolytic and cellulolytic species. Similar metabolic preferences may explain the negative relationships between dietary protein and other best discriminant OTUs observed in the circos plots. This assumption is supported by the positive link of Coprococcus-OTU168, unclassified Ruminococcaceae-OTU238, unclassified Clostridiales-OTU71 and VacinCA11-OTU69 with NRS, indicating amylolytic capabilities.
The importance of intestinal P availability for bacterial proliferation (Metzler and Mosenthin, 2008) was indicated by the decline of the predominant Lactobacillus and lesser abundant butyrate-producing Dorea in feces, both saccharolytic taxa, after feeding pigs the phytase-containing diets. Previous findings in pigs (Metzler-Zebeli et al., 2010) and rats (Ten Bruggencate et al., 2004) showed that the intestinal abundance of the Lactobacillus group may be rather related to the Ca than to the P availability in digesta. This may have been also the case in the present study as the dietary phytase supplementation reduced both the fecal Ca and P content. Several underlying modes of action may explain this finding and may be related to buffering properties of the insoluble Ca-P complex, which forms in the intestinal lumen at pH values above 5 (Govers and Van der Meer, 1993) and precipitates for bacterial cells cytotoxic fatty and bile acids (Kurdi et al., 2006). Also feasible is a modification in the mucosal attachment of Lactobacillus due to a variation in free Ca ions as shown in vitro (Lim and Ahn, 2012). Other Ca- or P-related dependencies in bacterial metabolism and enzyme activation were also likely but it requires a more functional approach (e.g., metatranscriptomics) to predict the affected metabolic pathways. Although Lactobacillus- and Dorea-OTUs were not among the most influential OTUs linked to fecal Ca and P, the sPLS-DA identified Prevotella copri-related OTU225, two Ruminococcaceae-OTUs (OTU21 and OTU175) and OTU131 belonging to the archaeal VadinCA11 group to have depended on the fecal Ca and P concentration. Since the methanogenic VadinCA11-OTU131 was also positively linked to Prevotella-OTU225, this may indicate cross-feeding of hydrogen which stimulated their abundance rather than the fecal Ca and P availability. Additionally, the inverse relationship of these most discriminant OTUs and fecal Ca and P with fecal aNDFOM and ADFOM concentrations may support depression in bacterial fiber degradation with lower Ca and P availability in the large intestine as has been reported for rumen bacteria under low rumen P availability (Harder et al., 2015).
Despite an enrichment of feces with aNDFOM and, as trends, starch fractions, the decline in the fecal abundances of Prevotella, Lachnospiraceae and Ruminococcaceae, which comprise many amylolytic, pullulanolytic and fibrolytic species (Kraler et al., 2016; La Reau et al., 2016), with the LA-treated cereals may have indicated that their preferred substrate became depleted in the distal large intestine. With substrate availability being a major factor for population dynamics (Kelly et al., 2017), the lower proportional RS contribution to total starch in feces of pigs fed the LA-treated grains would support this assumption. Fittingly, the circos plot for component 2 illustrated positive relationships between the NRS content and saccharolytic bacteria, such as Coprococcus, Lachnospiraceae-, Ruminococcaceae- and Clostridium saccharobutylicum-like OTUs, and methanogens of the VadinCA11 group. In addition, it is feasible that the fecal composition of aNDFOM differed due to the LA treatment (Metzler-Zebeli et al., 2014) which was potentiated by the progressing digestion and fermentation throughout the large intestine, thereby contributing to the lower fecal abundances of Prevotella, Lachnospiraceae and Ruminococcaceae.
Previously, we found a positive relationship between increased dietary Ca and P levels and the intestinal abundance of proteolytic bacterial groups including Clostridium cluster I, Enterobacteriaceae, Campylobacter and Helicobacter (Metzler-Zebeli et al., 2013). Although these taxa are considered commensals in the gastrointestinal tract of pigs, they also comprise important opportunistic pathogens. While being only a tendency for a difference, present results confirmed our hypothesis and supported the previously reported link between intestinal P availability and Enterobacteriaceae numbers (Metzler-Zebeli et al., 2013). Feeding the LA-treated grains also decreased the fecal P content. Nevertheless, the P in digesta of the distal large intestine with the LA-treated cereals was obviously sufficient to support proliferation of Enterobacteriaceae, Campylobacteraceae and Helicobacteraceae. Results for E. coli virulence factor gene expression showed that enterotoxigenic and shiga-toxin-producing E. coli were present but equally expressed their virulence genes among pig groups. Despite the predominance of the whole Clostridiaceae family, expression of cpa of C. perfringens was not detectable. Therefore, it may be assumed that despite the present changes in the bacterial communities including the reduced bacterial diversity with the LA-treated cereals, this did not lead to temporal instability and depletion of distinct commensal species that suppress virulence factor gene expression, resulting in a more colitogenic commensal composition (Frosali et al., 2015). The greatest effect that we found on opportunistic pathogens was the rise in Erysipelotrichaceae with the LA-treated cereals. However, this increase appeared to be related to other nutrients than P as phytase supplementation did not modify their abundance.
The present results demonstrate that both dietary phytase supplementation and LA-treatment of cereals caused specific alterations in the fecal viable bacterial community composition, whereas only the LA-treated grains reduced the bacterial diversity. Although not altered by the present dietary treatments, virulence factor expression in feces has implications for health risk assessment to reduce disease transmission within a pig herd and spreading of foodborne zoonotic diseases. The sPLS-DA further allowed the characterization of bacterial nutrient dependencies in the large intestine, indicating a link between the P availability and complex carbohydrate composition in feces and alterations in the predominant genera belonging to Clostridiaceae, Lactobacillaceae, Lachnospiraceae, Ruminococcaceae and Prevotellaceae.
The datasets generated for this study can be found in the NCBI Bioproject databank (PRJNA522345).
The animal study was reviewed and approved by the Institutional Ethics Committee of the University of Veterinary Medicine Vienna (Vienna, Austria) and the national authority according to paragraph 8 of the Law for Animal Experiments, Tierversuchsgesetz (TVG) (BMWFW-68.205/0158-WF/V/3b/2016).
BM-Z and QZ conceived and designed the experiments. JK, JV, and BM-Z performed the experiments. JK and BM-Z analyzed the data and wrote the manuscript. BM-Z edited and finalized the manuscript. All authors read and approved the final version of the manuscript.
The project received funding from the Tandem Ph.D. Program of the University of Veterinary Medicine, Vienna, Austria.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge A. Sener, A. Dockner, M. Hollmann, M. Wild, S. Sharma, and T. Enzinger for assistance with laboratory analysis and the experiment. The first author is grateful of the ASEAN-European Academic University Network (ASEA UNINET) under the Austrian Agency for International Cooperation (Österreichischer Austauschdienst, OEAD) for financial support for her Ph.D. program.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02345/full#supplementary-material
ADFOM, acid detergent fiber exclusive of residual ash; aNDFOM, alpha amylase-stable neutral detergent fiber exclusive of residual ash; Ca, calcium; Con, control diet; Con-Phy, control diet with 500 FTU/kg phytase; Cpa, Clostridium perfringens alpha toxin; DM, dry matter; LA, diet containing lactic acid treated cereals; LA-Phy, diet with 500 FTU/kg phytase and lactic acid-treated cereals; NRS, non-resistant starch; OTU, operational taxonomic units; P, phosphorus; RS, resistant starch; STa, heat-stable enterotoxin of enterotoxigenic Escherichia coli; Stx2e, shiga toxin type 2e of shiga-toxin producing E. coli.
Bardasi, L., Taddei, R., Fiocchi, I., Pelliconi, M. F., Ramini, M., Toschi, E., et al. (2017). Shiga toxin-producing Escherichia Coli in slaughtered pigs and pork products. Ital. J. Food Sci. 6, 1–8. doi: 10.4081/ijfs.2017.6584
Blaabjerg, K., Jorgensen, H., Tauson, A. H., and Poulsen, H. D. (2011). The presence of inositol phosphates in gastric pig digesta is affected by time after feeding a nonfermented or fermented liquid wheat- and barley-based diet. J. Anim. Sci. 89, 3153–3162. doi: 10.2527/jas.2010-3358
Blaabjerg, K., Strathe, A. B., and Poulsen, H. D. (2012). Modelling phytate degradation kinetics in soaked wheat and barley. Anim. Feed Sci. Technol. 175, 48–56. doi: 10.1016/j.anifeedsci.2012.03.018
Caporaso, J., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Cowieson, A. J., Ruckebusch, J., Sorbara, J. O. B., Wilson, J. W., Guggenbuhl, P., Tanadini, L., et al. (2017). A systematic view on the effect of microbial phytase on ileal amino acid digestibility in pigs. Anim. Feed Sci. Tech. 231, 138–149. doi: 10.1016/j.anifeedsci.2017.07.007
De Santis, T. Z., Hugenholtz, P., Larsen, N., Rojas, M., Brodie, E. L., Keller, K., et al. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072. doi: 10.1128/AEM.03006-05
Deckardt, K., Metzler-Zebeli, B. U., and Zebeli, Q. (2015). Processing barley grain with lactic acid and tannic acid ameliorates rumen microbial fermentation and degradation of dietary fibre in vitro. J. Sci. Food Agric. 96, 223–231. doi: 10.1002/jsfa.7085
Dersjant-Li, Y., Awati, A., Schulze, H., and Partridge, G. (2015). Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 95, 878–896. doi: 10.1002/jsfa.6998
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. doi: 10.1093/bioinformatics/btq461
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., and Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
Flint, H., Duncan, S., Scott, K., and Louis, P. (2015). Links between diet, gut microbiota composition and gut metabolism. Proc. Nutri. Soc. 74, 13–22. doi: 10.1017/s0029665114001463
Frosali, S., Pagliari, D., Gambassi, G., Landolfi, R., Pandolfi, F., and Cianci, R. (2015). How the intricate interaction among Toll-Like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J. Immunol. Res. 2015:489821. doi: 10.1155/2015/489821
Gesellschaft fur Ernahrungsphysiologie [GfE], (2006). Empfehlungen Zur Energie- und Nährstoffversorgung Von Schweinen. Frankfurt: DLG-Verlag.
Govers, M. J., and Van der Meer, R. (1993). Effects of dietary calcium and phosphate on the intestinal interactions between calcium, phosphate, fatty acids, and bile acids. Gut 34, 365–370. doi: 10.1136/gut.34.3.365
Harder, H., Khol-Parisini, A., Metzler-Zebeli, B. U., Klevenhusen, F., and Zebeli, Q. (2015). Treatment of grain with organic acids at 2 different dietary phosphorus levels modulates ruminal microbial community structure and fermentation patterns in vitro. J. Dairy Sci. 98, 8107–8120. doi: 10.3168/jds.2015-9913
Heyer, C., Weiss, E., Schmucker, S., Rodehutscord, M., Hoelzle, L., Mosenthin, R., et al. (2015). The impact of phosphorus on the immune system and the intestinal microbiota with special focus on the pig. Nutr. Res. Rev. 28, 67–82. doi: 10.1017/S0954422415000049
Kebreab, E., Hansen, A. V., and Strathe, A. (2012). Animal production for efficient phosphate utilization: from optimized feed to high efficiency livestock. Curr. Opin. Biotech. 23, 872–877. doi: 10.1016/j.copbio.2012.06.001
Kelly, J., Daly, K., Moran, A., Ryan, S., Bravo, D., and Shirazi-Beechey, S. (2017). Composition and diversity of mucosa-associated microbiota along the entire length of the pig gastrointestinal tract; dietary influences. Environ. Microbiol. 19, 1425–1438. doi: 10.1111/1462-2920.13619
Kononoff, P. J., and Hanford, K. J. (2006). Technical note: estimating statistical power of mixed models used in dairy nutrition experiments. J. Dairy Sci. 89, 3968–3971. doi: 10.3168/jds.s0022-0302(06)72439-0
Koopmans, S. J., Bikker, P., and van Krimpen, M. M. (2016). Inoculated Fermented Corn as Dietary Performance and Health Enhancer in Pigs: Literature and in Vitro Study. Wageningen: Wageningen University & Research.
Kraler, M., Ghanbari, M., Domig, K., Schedle, K., and Kneifel, W. (2016). The intestinal microbiota of piglets fed with wheat bran variants as characterized by 16S rRNA next-generation amplicon sequencing. Arch. Anim. Nutr. 70, 173–189. doi: 10.1080/1745039x.2016.1160534
Kurdi, P., Kawanishi, K., Mizutani, K., and Yokota, A. (2006). Mechanism of growth inhibition by free bile acids in Lactobacilli and Bifidobacteria. J. Bacteriol. 188, 1979–1986. doi: 10.1128/jb.188.5.1979-1986.2006
La Reau, A. J., Meier-Kolthoff, J. P., and Suen, G. (2016). Sequence-based analysis of the genus Ruminococcus resolves its phylogeny and reveals strong host association. Microb. Genome. 2:e000099. doi: 10.1099/mgen.0.000099
Lamendella, R., Domingo, J. W., Ghosh, S., Martinson, J., and Oerther, D. B. (2011). Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiol. 11:103. doi: 10.1186/1471-2180-11-103
Le Goff, G., van Milgen, J., and Noblet, J. (2002). Influence of dietary fibre on digestive utilization and rate of passage in growing pigs, finishing pigs and adult sows. Anim. Sci. 74, 503–515. doi: 10.1017/S1357729800052668
Lim, S. M., and Ahn, D. H. (2012). Factors affecting adhesion of lactic acid bacteria to caco-2 cells and inhibitory effect on infection of Salmonella typhimurium. J. Microbiol. Biotechnol. 22, 1731–1739. doi: 10.4014/jmb.1208.08049
Lu, Y., Hugenholtz, P., and Batstone, D. J. (2015). Evaluating DNA extraction methods for community profiling of pig hindgut microbial community. PLoS One 10:e0142720. doi: 10.1371/journal.pone.0142720
Mann, E., Schmitz-Esser, S., Zebeli, Q., Wagner, M., Ritzmann, M., and Metzler-Zebeli, B. U. (2014). Mucosa-associated bacterial microbiome of the gastrointestinal tract of weaned pigs and dynamics linked to dietary calcium-phosphorus. PLoS One 9:e86950. doi: 10.1371/journal.pone.0086950
Metzler, B. U., and Mosenthin, R. (2008). A review of interactions between dietary fiber and the gastrointestinal microbiota and their consequences on intestinal phosphorus metabolism in growing pigs. Asian Australas. J. Anim. 21, 603–615. doi: 10.5713/ajas.2008.r.03
Metzler, B. U., Mosenthin, R., Baumgärtel, T., and Rodehutscord, M. (2008). The effect of dietary phosphorus and calcium level, phytase supplementation, and ileal infusion of pectin on the chemical composition and carbohydrase activity of fecal bacteria and the level of microbial metabolites in the gastrointestinal tract of pigs. J. Anim. Sci. 86, 1544–1555. doi: 10.2527/jas.2007-0267
Metzler-Zebeli, B. U., Deckardt, K., Schollenberger, M., Rodehutscord, M., and Zebeli, Q. (2014). Lactic acid and thermal treatments trigger the hydrolysis of myo-inositol hexakisphosphate and modify the abundance of lower myo-inositol phosphates in barley (hordeum vulgare L.). PLoS One 9:e101166. doi: 10.1371/journal.pone.0101166
Metzler-Zebeli, B. U., Ertl, R., Grüll, D., Molnar, T., and Zebeli, Q. (2016). Enzymatically modified starch up-regulates expression of incretins and sodium-coupled monocarboxylate transporter in jejunum of growing pigs. Animal 11, 1180–1188. doi: 10.1017/s1751731116002615
Metzler-Zebeli, B. U., Hooda, S., Pieper, R., Zijlstra, R. T., van Kessel, A. G., Mosenthin, R., et al. (2010). Nonstarch polysaccharides modulate bacterial microbiota, pathways for butyrate production, and abundance of pathogenic Escherichia coli in the pig gastrointestinal tract. Appl. Environ. Microbiol. 76, 3692–3701. doi: 10.1128/aem.00257-10
Metzler-Zebeli, B. U., Khol-Parisini, A., Gruber, L., and Zebeli, Q. (2015). Microbial populations and fermentation profiles in rumen liquid and solids of holstein cows respond differently to dietary barley processing. J. Appl. Microbiol. 119, 1502–1514. doi: 10.1111/jam.12958
Metzler-Zebeli, B. U., Lawlor, P. G., Magowan, E., and Zebeli, Q. (2018). Interactions between metabolically active bacteria and host gene expression at the cecal mucosa in pigs of diverging feed efficiency. J. Anim. Sci. 96, 2249–2264. doi: 10.1093/jas/sky118
Metzler-Zebeli, B. U., Mann, E., Schmitz-Esser, S., Wagner, M., Ritzmann, M., and Zebeli, Q. (2013). Changing dietary calcium-phosphorus level and cereal source selectively alters abundance of bacteria and metabolites in the upper gastrointestinal tracts of weaned pigs. Appl. Environ. Microbiol. 79, 7264–7272. doi: 10.1128/aem.02691-13
Metzler-Zebeli, B. U., Newman, M. A., Ladinig, A., Kandler, W., Grüll, D., and Zebeli, Q. (2019). Transglycosylated starch accelerated intestinal transit and enhanced bacterial fermentation in the large intestine using a pig model. Br. J. Nutr. 122, 1–13. doi: 10.1017/S0007114519000849
Murray, W. D., Khan, A. W., and van den Berg, L. (1982). Clostridium saccharolyticum sp. nov., a saccharolytic species from sewage sludge. Int. J. Syst. Bacteriol. 32, 132–135. doi: 10.1099/00207713-32-1-132
Muyzer, G., de Waal, E. C., and Uitterlinden, A. G. (1993). Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59, 695–700.
National Research Council [NRC] (2012). Nutrient Requirements of Swine. Washington, D.C: Academy Press.
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2018). Vegan: Community Ecology Package R Package Version 2. Available at: https://CRAN.R-project.org/package=vegan (accessed October 25, 2018).
Oster, M., Gerlinger, C., Heide, K., Just, F., Borgelt, L., Wolf, P., et al. (2017). Lower dietary phosphorus supply in pigs match both animal welfare aspects and resource efficiency. Ambio 47, 20–29. doi: 10.1007/s13280-017-0969-8
Rohart, F., Gautier, B., Singh, A., and Lê Cao, K.-A. (2017). Mixomics: an r package for ‘Omics feature selection and multiple data integration. PLoS Comput. Biol. 13:e1005752. doi: 10.1371/journal.pcbi.1005752
Schlegel, B. J., Nowell, V. J., Parreira, V. R., Soltes, G., and Prescott, J. F. (2012). Toxin-associated and other genes in Clostridium perfringens type A isolates from bovine clostridial abomasitis (BCA) and Jejunal Hemorrhage Syndrome (JHS). Can. J. Vet. Res. 76, 248–254.
Suiryanrayna, M., and Ramana, J. (2015). A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotech. 6:45. doi: 10.1186/s40104-015-0042-z
Ten Bruggencate, S. J. M., Bovee-Oudenhoven, I. M. J., Lettink-Wissink, M. L. G., Katan, M. B., and van der Mee, R. R. (2004). Dietary fructo-oligosaccharides and inulin decrease resistance of rats to Salmonella: protective role of calcium. Gut 53, 530–535. doi: 10.1136/gut.2003.023499
Toledo, A., Gómez, D., Cruz, C., Carreón, R., López, J., Giono, S., et al. (2012). Prevalence of virulence genes in Escherichia coli strains isolated from piglets in the suckling and weaning period in Mexico. J. Med. Microbiol. 61, 148–156. doi: 10.1099/jmm.0.031302-0
Van Leeuwen, P., van Gelder, A. H., de Leeuw, J. A., and van der Klis, J. D. (2006). An animal model to study digesta passage in different compartments of the gastro-intestinal tract (GIT) as affected by dietary composition. Curr. Nutr. Food Sci. 2, 97–105. doi: 10.2174/157340106775472001
Vötterl, J. C., Zebeli, Q., Hennig-Pauka, I., and Metzler-Zebeli, B. U. (2019). Soaking in lactic acid lowers the phytate-phosphorus content and increases the resistant starch in wheat and corn grains. Anim. Feed Sci. Tech. 252, 115–125. doi: 10.1016/j.anifeedsci.2019.04.013
Vötterl, J. C., Zebeli, Q., and Metzler-Zebeli, B. U. (2018). “Effects of soaking wheat and corn in lactic acid on the phytate-phosphorus and resistant starch content,” in Proceedings of the Society of Nutrition Physiology: 72nd Conference 13th - 15th March 2018 in Göttingen, Frankfurt. doi: 10.1016/j.anifeedsci.2019.04.013
Wells, J. E., Berry, E. D., Kalchayanand, N., Rempel, L. A., Kim, M., and Oliver, W. T. (2015). Effect of lysozyme or antibiotics on faecal zoonotic pathogens in nursery pigs. J. Appl. Microbiol. 118, 1489–1497. doi: 10.1111/jam.12803
Westermann, A. J., Gorski, S. A., and Vogel, J. (2012). Dual RNA-seq of pathogen and host. Nat. Rev. Microbiol. 10, 618–630. doi: 10.1038/nrmicro2852
Wilfart, A., Montagne, L., Simmins, H., Noblet, J., and Milgen, J. (2007). Digesta transit in different segments of the gastrointestinal tract of pigs as affected by insoluble fibre supplied by wheat bran. Br. J. Nutr. 98, 54–62. doi: 10.1017/S0007114507682981
Yáñez, J., Landero, J., Owusu-Asiedu, A., Cervantes, M., and Zijlstra, R. (2013). Growth performance, diet nutrient digestibility, and bone mineralization in weaned pigs fed pelleted diets containing thermostable phytase. J. Anim. Sci. 91, 745–754. doi: 10.2527/jas.2011-4949
Zhang, G., Dong, X., Wang, Z., Zhang, Q., Wang, H., and Tong, J. (2010). Purification, characterization, and cloning of a novel phytase with low pH optimum and strong proteolysis resistance from Aspergillus ficuum NTG-23. Bioresour. Technol. 101, 4125–4131. doi: 10.1016/j.biortech.2010.01.001
Zhao, W., Wang, Y., Liu, S., Huang, J., Zhai, Z., He, C., et al. (2015). The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS One 10:e0117441. doi: 10.1371/journal.pone.0117441
Keywords: fecal microbiome, phytase, lactic acid, virulence factor expression, pig, metabolically active bacteria
Citation: Klinsoda J, Vötterl J, Zebeli Q and Metzler-Zebeli BU (2019) Lactic Acid Treatment of Cereals and Dietary Phytase Modified Fecal Microbiome Composition Without Affecting Expression of Virulence Factor Genes in Growing Pigs. Front. Microbiol. 10:2345. doi: 10.3389/fmicb.2019.02345
Received: 13 June 2019; Accepted: 26 September 2019;
Published: 15 October 2019.
Edited by:
Yanhong Liu, University of California, Davis, United StatesReviewed by:
Jinxin Liu, University of California, Davis, United StatesCopyright © 2019 Klinsoda, Vötterl, Zebeli and Metzler-Zebeli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara U. Metzler-Zebeli, QmFyYmFyYS5NZXR6bGVyQHZldG1lZHVuaS5hYy5hdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.