
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Microbiol. , 04 October 2019
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.02319
 Emily E. Whittle1
Emily E. Whittle1 Simon W. Legood1
Simon W. Legood1 Ilyas Alav1
Ilyas Alav1 Punyawee Dulyayangkul2
Punyawee Dulyayangkul2 Tim W. Overton3
Tim W. Overton3 Jessica M. A. Blair1*
Jessica M. A. Blair1*Gram-negative infections are increasingly difficult to treat because of their impermeable outer membranes (OM) and efflux pumps which maintain a low intracellular accumulation of antibiotics within cells. Historically, measurement of accumulation of drugs or dyes within Gram-negative cells has concentrated on analyzing whole bacterial populations. Here, we have developed a method to measure the intracellular accumulation of ethidium bromide, a fluorescent DNA intercalating dye, in single cells using flow cytometry. Bacterial cells were stained with SYTOTM 84 to easily separate cells from background cell debris. Ethidium bromide fluorescence was then measured within the SYTOTM 84 positive population to measure accumulation. In S. Typhimurium SL1344, ethidium bromide accumulation was low, however, in a number of efflux mutants, accumulation of ethidium bromide increased more than twofold, comparable to previous whole population analysis of accumulation. We demonstrate simultaneous measurement of ethidium bromide accumulation and GFP allowing quantification of gene expression or other facets of phenotype in single cells. In addition, we show here that this assay can be adapted for use with efflux inhibitors, with both Gram-negative and Gram-positive bacteria, and with other fluorescent substrates with different fluorescence spectra.
Antibiotic resistance in Gram-negative bacteria is an ongoing threat. To treat Gram-negative bacterial infections, most antibiotics need to accumulate at high concentrations inside cells in order to reach their targets and be effective. Difficulties arise in obtaining high intracellular levels of antibiotics due to the outer membrane of the Gram-negative bacteria which prevents the access of large and hydrophobic antibiotics into the cell (Nikaido, 1989). Antibiotics that are able to enter through the outer membrane of Gram-negative bacteria can be removed by efflux pumps. Resistance-Nodulation-Division (RND) pumps can extrude a number of antibiotic classes, detergents, and biocides allowing for intrinsic multi-drug resistance (Blair et al., 2015) and are commonly overexpressed in clinical isolates due to mutation (Piddock, 2006; Blair et al., 2015). The outer membrane and efflux systems of Gram-negative bacteria work synergistically to prevent high level accumulation of antibiotics within cells (Krishnamoorthy et al., 2017).
In the literature, there are number of methods used to measure the accumulation of antibiotics and dyes within cells (Blair and Piddock, 2016). These methods are commonly used for assessing accumulation in strains with efflux pump components removed. When measuring accumulation of molecules known to be substrates of efflux pumps, this can provide an indirect assessment of the level of efflux.
Many methods utilize molecules or dyes that are differentially fluorescent when inside and outside cells. In such assays, the fluorescence of cells is measured in a fluorimeter prior to addition of dye and then after dye is added, the increase in fluorescence can be measured as it accumulates within the cell (Coldham et al., 2010; Blair and Piddock, 2016). Examples include DNA intercalating dyes such as ethidium bromide (Olmsted and Kearns, 1977) and Hoechst 33342 (Richmond et al., 2013), which fluoresce when bound to DNA, and nile red and 1,2-dinapthylamine (1,2-DNA) which fluoresce when bound to lipids. These differentially fluorescent compounds are also used for the direct measurement of efflux. The reduction in fluorescence correlated to the removal of dye via efflux pumps. Ethidium bromide is commonly used but nile red (Bohnert et al., 2010) and 1,2-DNA (Bohnert et al., 2011) methods have also been described. An instrument free agar method using ethidium bromide, the cartwheel method, also allows large scale analysis of efflux capacity of strains able to expel the fluorescent dye (Martins et al., 2013).
Accumulation of drugs or other molecules whose fluorescence is not altered by cellular localisation can also be measured although different methods are required. Examples include clinically relevant drugs such as ciprofloxacin (and other naturally fluorescing fluoroquinolones) whose fluorescence is the same inside and outside the cells (Piddock et al., 1999; Blair and Piddock, 2016). To measure accumulation of quinolones, cultures are grown and then incubated with the drug. Cells are washed and lysed, cell debris is removed and the concentration of fluorescent quinolones in the supernatant is measured (Asuquo and Piddock, 1993).
The methods described only measure the ability of a bacteria to accumulate molecules at the bacterial population level. However, the phenotype of a bacterial population is not homogeneous and therefore cells within a population may accumulate different levels of dye/antibiotics. As understanding of heterogeneity and differential gene expression within a bacterial population has increased, it has become desirable to measure the accumulation of molecules at a single cell level. Assessing heterogeneity is important because sub-populations that may accumulate more or less antibiotics than others within a wider population may drive the selection of antimicrobial resistance. Phenotypic variation within a population with regards to efflux may be explained by AcrB bias partitioning between mother and daughter cells during cell division (Bergmiller et al., 2017).
There are a few examples in the literature where fluorescent dyes have been used to measure accumulation in single cells by flow cytometry in certain bacterial species (Sanchez-Romero and Casadesus, 2014; Blair and Piddock, 2016; Hassan et al., 2016; Haynes et al., 2018). Ethidium bromide has been used to measure accumulation in Salmonella enterica (Sanchez-Romero and Casadesus, 2014) and Acinetobacter baumanii (Hassan et al., 2016). Fluorescein diacetate (FDA) is one of a number of substrates tested in the development of a dye retention assay (Haynes et al., 2018) but nile red and rhodamine 6G have also been used to measure accumulation in the yeast species, Saccharomyces cerevisiae expressing Candida albicans efflux pumps (Ivnitski-Steele et al., 2009). Single cell analysis has also been described using a femtoliter droplet array which uses the fluorescent dye fluorescein-di-β-galactopyranoside to assess efflux, as well as being used for the analysis of gene expression (Iino et al., 2012, 2018). The natural fluorescence of fluoroquinolones, in this case fleroxacin and ciprofloxacin, has been harnessed to also measure intracellular accumulation within single cells using deep ultraviolet microscopy with a synchrotron beamline (Kascakova et al., 2012). The methods described for both whole population and single cell analysis of efflux vary with regards to difficulty and accessibility as well as the level of analysis of efflux they provide.
Here, we have developed a simple assay that can be used to measure ethidium bromide accumulation in single cells of a number of Gram-negative organisms and in the Gram positive species Staphylococcus aureus. We demonstrate that this method is useful for assessing the difference in accumulation between efflux proficient and efflux deficient strains, including comparisons with efflux knockout strains and in the presence of efflux inhibitors. We have also adapted this method to measure fluorescent dyes other than ethidium bromide that have previously been described in literature such as nile red and rhodamine 6G.
Unless otherwise stated, all experiments use S. enterica Serovar Typhimurium (hereafter named S. Typhimurium (Brenner et al., 2000). SL1344 and isogenic mutants thereof that have been previously published. Details of all strains are shown in Supplementary Table S1. The construction of Klebsiella pneumoniae ecl8 acrB::Gm is described in the Supplementary Material.
Luria Bertani (Lennox) broth (Sigma Aldrich) was used as the media to grow cultures. 5x Hepes buffered Saline (Alfa Aesar) was diluted to 1x and filter sterilized then used as a buffer for flow cytometry samples. Indole ≥ 99%(Alfa Aesar) was made to a 100 mM stock in 70% ethanol. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (Acros Organics) was made to a 10 mM stock in 80% DMSO. Phenylalanine-Arginine β-Naphthylamide (PaβN) (Sigma-Aldrich) was made to a 1000 μg/ml in H2O.
Information about all dyes used including providers, excitation/emission maxima, laser line, emission filter and channels used (for the Attune NxT Flow cytometer), and references are provided in Supplementary Table S2 of Supplementary Material. SYTOTM84 and SYTOTM9 were both made to a stock concentration of 500 μM in water. A 10 mM stock of both ethidium bromide (EtBr) and rhodamine 6G was made up in water. Nile red was made to a 2.5 mM stock in 70% methanol.
All samples were analyzed using the Attune NxT Flow cytometer and its software package.
Unless otherwise stated, all cultures were incubated in Luria-Bertani broth for 1 h at 37°C, from an overnight culture (4% sub-culture inoculum from overnight culture). Cells were harvested from 300 μL of culture by centrifugation and resuspended in 100 μL 1 × HEPES buffered Saline (HBS) to give a cell count of ∼1 × 106. HBS was used as SYTO dyes are incompatible with phosphate containing buffers. SYTOTM 84 was added to 500 μL of 1 × HBS for a final concentation of 10 μM and ethidium bromide was added to the same sample to give a final concentration of 100 μM. Then 100 μL of bacterial cell suspension was added and incubated for 10 min at room temperature. Cells were analyzed by flow cytometry (Attune Nxt flow cytometer, Life Technologies).
The emission of SYTOTM 84 was collected in the YL1-H channel. The data was visualized on a plot vs. forward scatter (FSC-H). The SYTOTM 84 dye is used to separate cells from background including cell debris (Figure 1A) and a gate was drawn around the SYTOTM 84+ population to separate out cells (Figure 1A: SYTOTM 84+ is red). 10,000 events were collected in the SYTOTM 84+ gate. The gated SYTOTM 84+ cells were then analyzed for ethidium bromide fluorescence from the BL3-H channel (Figure 1B: EtBr RFU from blue gate). The values that were taken for relative fluorescence units (RFU) is the X-median, where all distibutions were based on a single population. The optimized PMT voltages used were as follows: FSC at 700, SSC at 500, YL1 at 500 and BL3 at 400.
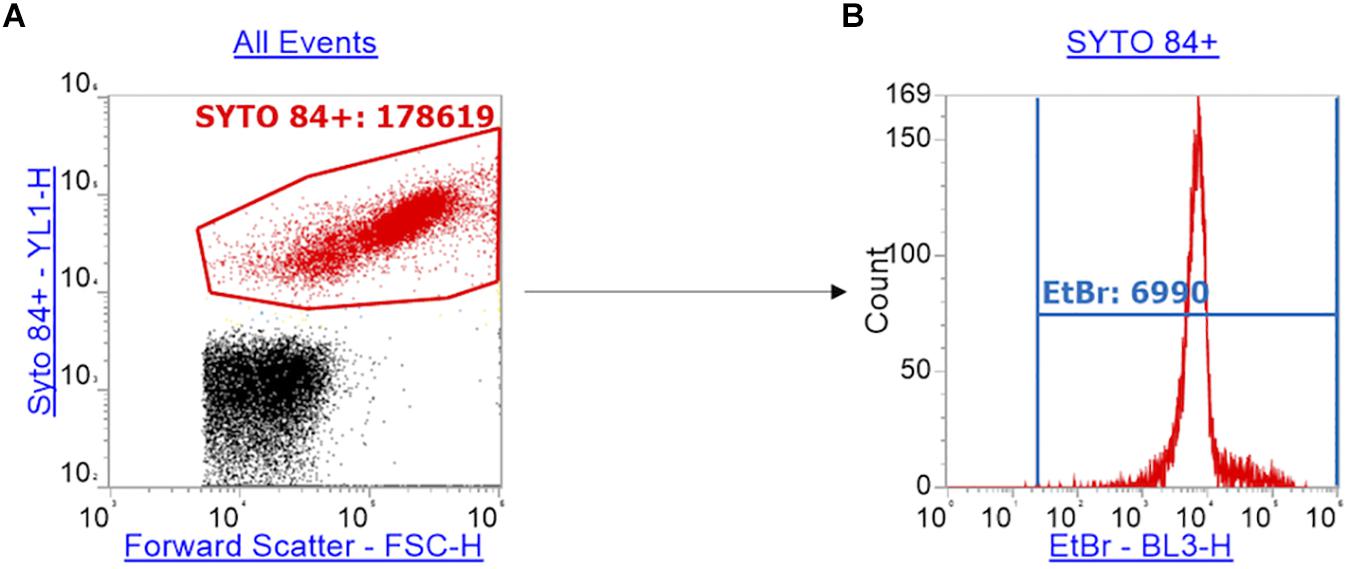
Figure 1. Gating strategy for the measurement of ethidium bromide RFU. Panel (A) shows how cells (red) were separated from background debris (black) by gating SYTOTM 84+ cells (red) using a plot of the orange fluorescence emission channel YL1-H (SYTOTM 84 fluorescence) against forward scatter (FSC-H). (B) Using the red gate of SYTOTM 84+ population, a histogram of ethidium bromide fluorescence per cell was plotted using the emission channel BL3-H. The ethidium bromide gate (blue) gives the x-median of ethidium bromide fluorescence to measure relative accumulation.
The excitation of SYTOTM 84 and ethidium bromide are at different wavelengths and therefore there was limited spillover of the emission spectra of these two fluorophores. However, compensation was still set for the YL1-H and BL3-H parameters against and unstained control using the Attune NxT compensation setup.
The excitation wavelength used for Rhodamine 6G was 488 nm, and so the methods used to measure this dye are the same as measuring accumulation of ethidium bromide.
As above, samples were set up exactly the same apart from the addition of nile red (final concentration of 75 μM) in place of ethidium bromide. Optimisation for concentrations is not shown.
Nile red has an excitation of 549 nm and emission of 628 nm in the presence of phospholipids, and in a neutral lipid environment (tryglycerides), the fluorescence shifts to ex/em of 510/580 nm (Greenspan and Fowler, 1985). For this reason, SYTOTM 9 was used in place of SYTOTM 84. Nile red ex/em overlaps with SYTOTM 84 in both phospholipid and tryglyceride staining. All other sample preparation was the same as with ethidium bromide. The SYTOTM 9 emission channel used was BL2-H for green fluorescence and SYTOTM 9+ cells were gated so as to separate from cell debris. The SYTO 9+ population was then used to measure nile red fluorescence using the YL1-H channel for orange fluorescence. Compensation was set but as with ethidium bromide, there should be no spillover between SYTOTM 9 and nile red emissions.
To analyse the effect of CCCP on dye accumulation in S. Typhimurium, a sub-inhibitory concentration (100 μM) was added to 500 μL of 1 × HBS, followed by ethidium bromide and SYTOTM 84, and SL1344 as above. 100 μM of CCCP was used based on previous direct efflux assays for Gram-negative bacteria (Smith and Blair, 2014). To analyse the effect of CCCP on dye accumulation in S. aureus, 10 μM was added to 500 μL of 1 × HBS, followed by ethidium bromide and SYTOTM 84. Samples could then be analyzed for accumulation after 10 min of incubation. The CCCP concentration for inhibition of pumps in S. aureus was decided based on analysis of the following concentrations: 1 μM, 10 μM, 50 μM and 100 μM and the higher concentrations affected the SYTOTM 84+ population possibly due to cell death. Optimisation for the concentration is not shown.
To analyse the effect of PAβN on nile red accumulation in S. Typhimurium, a concentration of 50 μg/ml was added to 500 μL of 1 × HBS, followed by nile red and SYTOTM 9, and SL1344 as above. We were unable to use ethidium bromide in the presence of PaβN because there was no difference in accumulation in the presence or absence of the RND inhibitor and previous studies suggest this (Lomovskaya et al., 2001; Kern et al., 2006; Viveiros et al., 2008; Machado et al., 2017).
In order to show that measurements of ethidium bromide accumulation can be combined with measurements of GFP, we used a transcriptional reporter plasmid encoding a ramA promoter upstream of a gfp reporter gene. ramA is a transcriptional activator of the acrAB operon, therefore upregulating the AcrAB-TolC efflux pump in response to signals such as indole (Nikaido et al., 2008). When using strains containing GFP transcriptional reporter plasmids, cultures were supplemented with 50 μg/mL of ampicillin and were grown for 2 h (OD600 = 0.6) to correspond to published data reporting increased ramA transcription (Lawler et al., 2013). After 2 h, 200 μL samples of the culture were taken, and to those which were to be induced, a final concentration of 2 mM of indole was added to the culture. This was then incubated for 30 min. Cells were then resuspended in 1x HBS and samples were set up as previously described.
In order to measure accumulation of ethidium bromide in addition to measuring GFP (in this case a transcriptional reporter of ramA), additional gating and compensation was required. In addition to the gating strategy described above for SYTOTM 84 and ethidium bromide (Figures 2A,D), a plot showing YL1-H (SYTOTM 84) vs. BL1-H (for GFP) of SYTOTM 84+ cells was set up (Figures 2B,E). A quadrant gate was then added to this dot plot to specify cells that expressed GFP. The GFP+ population (blue) was then analyzed by a histogram plot (Figures 2C,F) to calculate an X-median RFU for fluorescence of GFP. GFP and ethidium bromide have similar excitation and emission maxima therefore compensation was required to adjust for spillover of emission of ethidium bromide and GFP in both channels.
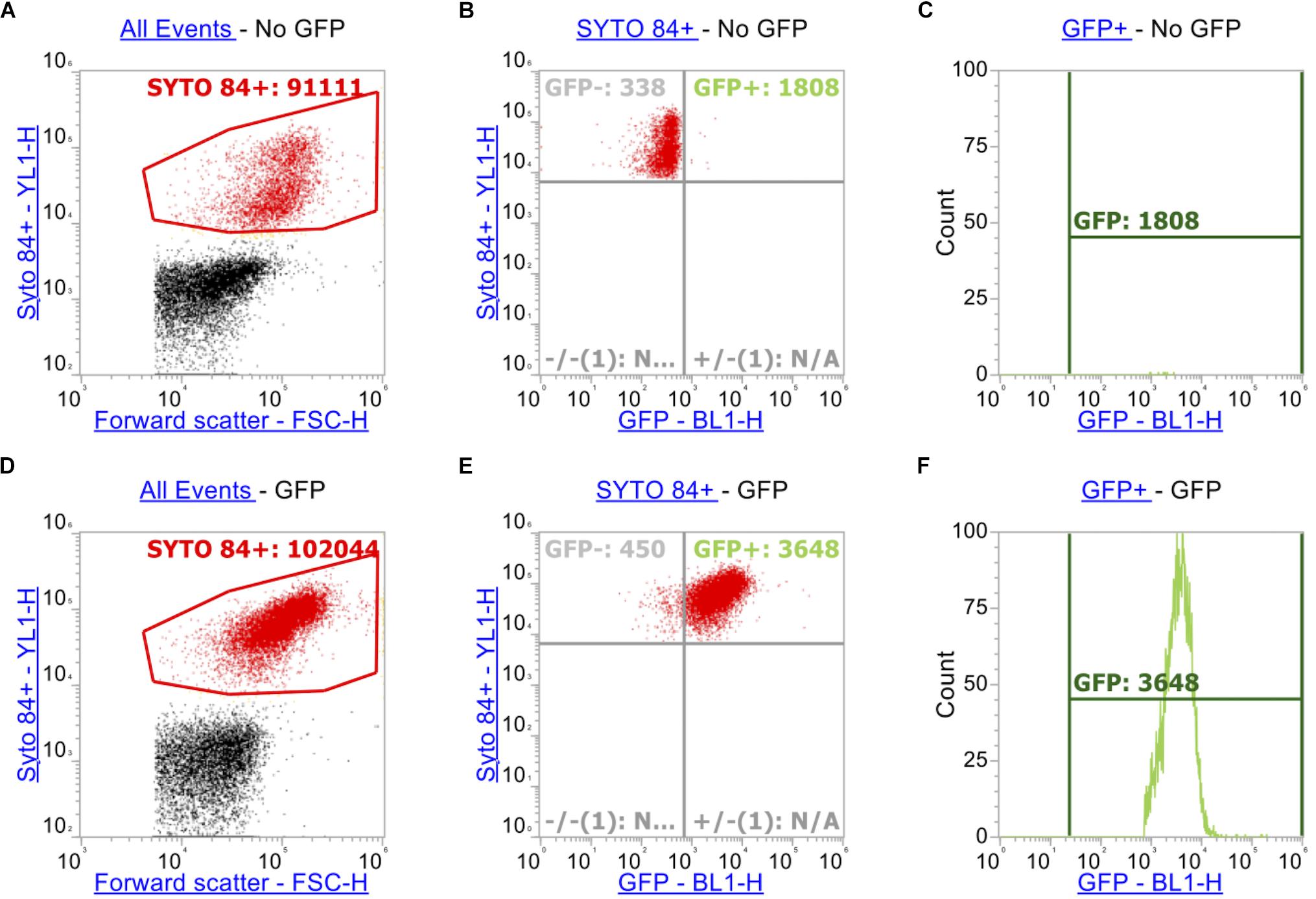
Figure 2. Gating strategy when measuring GFP alongside ethidium bromide. As above, SYTOTM 84+ cells were gated above in red (A,D). Panels (A–C) represents strain SL1344 expressing no GFP. (B,E) Plots YL1-H for SYTOTM 84 against BL1-H for GFP. Only SYTOTM 84+ cells are analyzed in these plots and the GFP+ gate (Blue, right hand side) represents cells that are expressing GFP only and excluding cells fluorescing in BL1-H channel by spillover (C,F).
A one-way ANOVA and Dunnett’s multiple comparison test were used on Prism Graphpad to assess significance and P-values of SL1344 compared to ΔacrB, ΔtolC, ΔacrAE, and Δ4PAP (ΔacrA, ΔacrE, ΔmdtA, ΔmdsA) strains. Unpaired T-tests were run of each wild-type strain of E. coli, K. pneumonia, and Pseudomonas aeruginosa against their efflux deficient strain. Unpaired T-tests were also used for nile red, rhodamine 6G and GFP and also in the presence of inhibitor, CCCP. All P-values and related asterisks are shown in Supplementary Table S3.
Ethidium bromide only fluoresces when bound to DNA therefore the level of fluorescence corresponds to the level of intracellular accumulation. This makes it a powerful tool for analyzing the effects of accumulation in strains that lack efflux pumps. Traditionally, accumulation of substrates such as ethidium bromide has been measured on the level of a whole bacterial population but the method presented here allows accumulation to be measured in single cells within a large population. To optimize this assay to use for flow cytometry, cultures were incubated for 1 h. The DNA intercalating dye SYTOTM 84 was used to distinguish cells from background particulate noise (e.g., cell debris, medium components) and was selected as its fluorescence emission did not overlap the emission of ethidium bromide. A range of concentrations of SYTOTM 84 were tested (0.1 μM, 0.5 μM, 1 μM, 5 μM, 10 μM) but 10 μM was the optimum concentration for use by flow cytometry because there was better separation between cells and debris and a distinct population was visualized (Supplementary Figure S3). To optimize the concentration of ethidium bromide used in the assays, 5 concentrations were tested (10 μM, 25 μM, 50 μM, 75 μM, 100 μM). A 100 μM concentration of ethidium bromide gave the highest mean fluorescence in S. Typhimurium wild type strain SL1344 stained with SYTOTM 84 and the greatest difference between a wild type and ΔacrB. Example plots using the final concentrations for both SYTOTM 84 and ethidium bromide are shown (Figure 3), data from other concentrations can be found in Supplementary Figure S3.
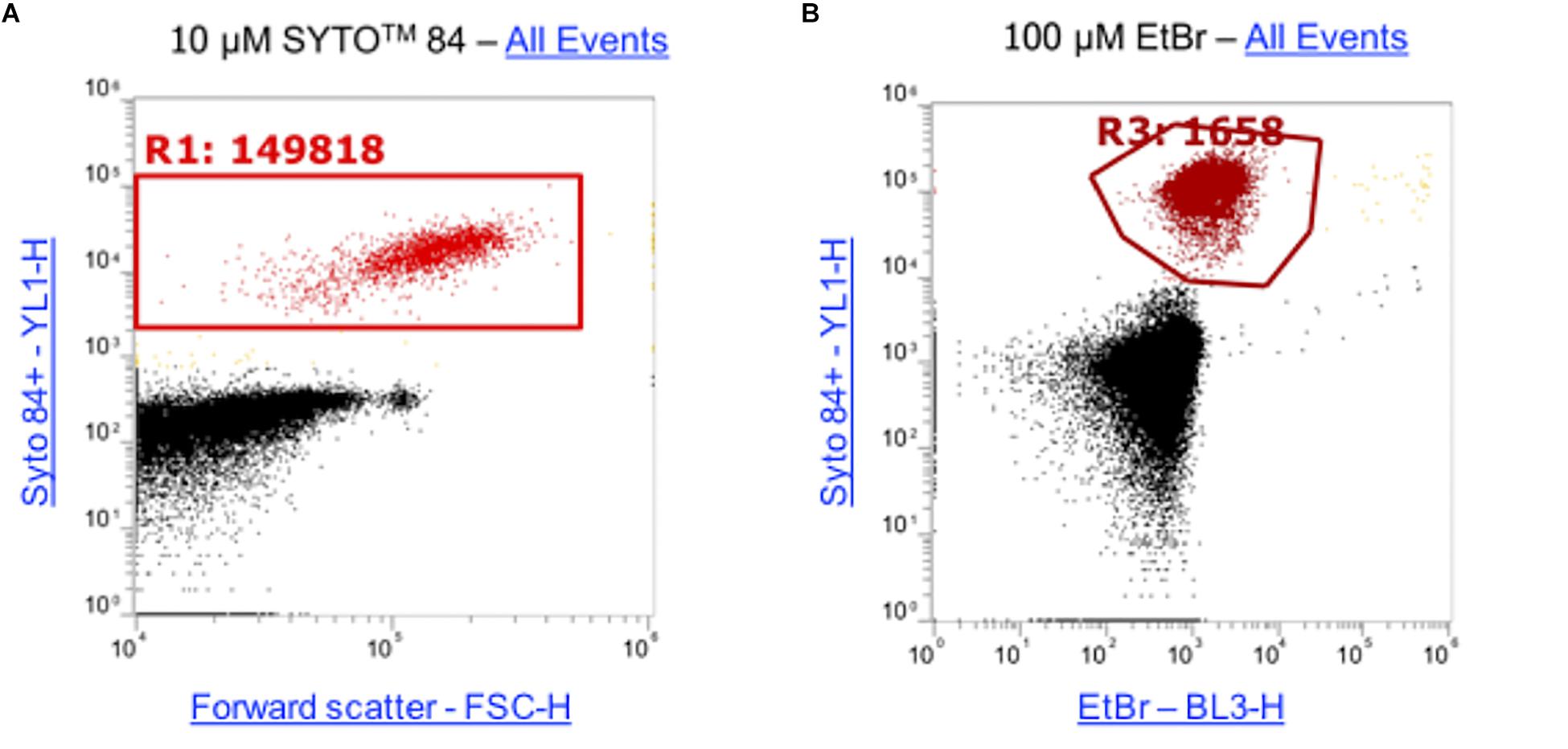
Figure 3. Concentrations of SYTOTM 84 and ethidium bromide used in this assay. (A) 10 μM SYTOTM 84 was used and a population gated (R1) on a orange fluorescence (YL1-H) versus forward scatter (FSC-H) dot plot to identify SYTOTM 84 fluorescence. The concentration was used as it gave the best separation of cells (red) from background cell debris and other particulate noise (black). (B) 100 μM was the optimum concentration of ethidium bromide used as shown via a dot plot of YL1-H (SYTOTM 84) vs BL3-H (ethidium bromide). Both plots show all events.
Using the optimized assay, accumulation of ethidium bromide was measured in wild type Salmonella SL1344 and the isogenic efflux mutants ΔacrB, ΔtolC, ΔacrAE, and a Δ4PAP strain lacking the four periplasmic adaptor protein (PAP) genes (ΔacrA, ΔacrE, ΔmdtA, ΔmdsA).
After measuring 10,000 cells, the X-median of ethidium bromide fluorescence was taken in the distinct population of SYTOTM 84+ cells (Figures 4, 5). Low level fluorescence could be detected in the WT SL1344 with a mean value of 8024 RFU. Fluorescence was sixfold higher in the absence of AcrB, sevenfold higher when the outer membrane channel (TolC) was removed and fourfold higher in both ΔacrAE and Δ4PAP strains. These data are in agreement with previous whole population data showing that efflux deficient strains accumulate more ethidium bromide (Coldham et al., 2010; Blair and Piddock, 2016).
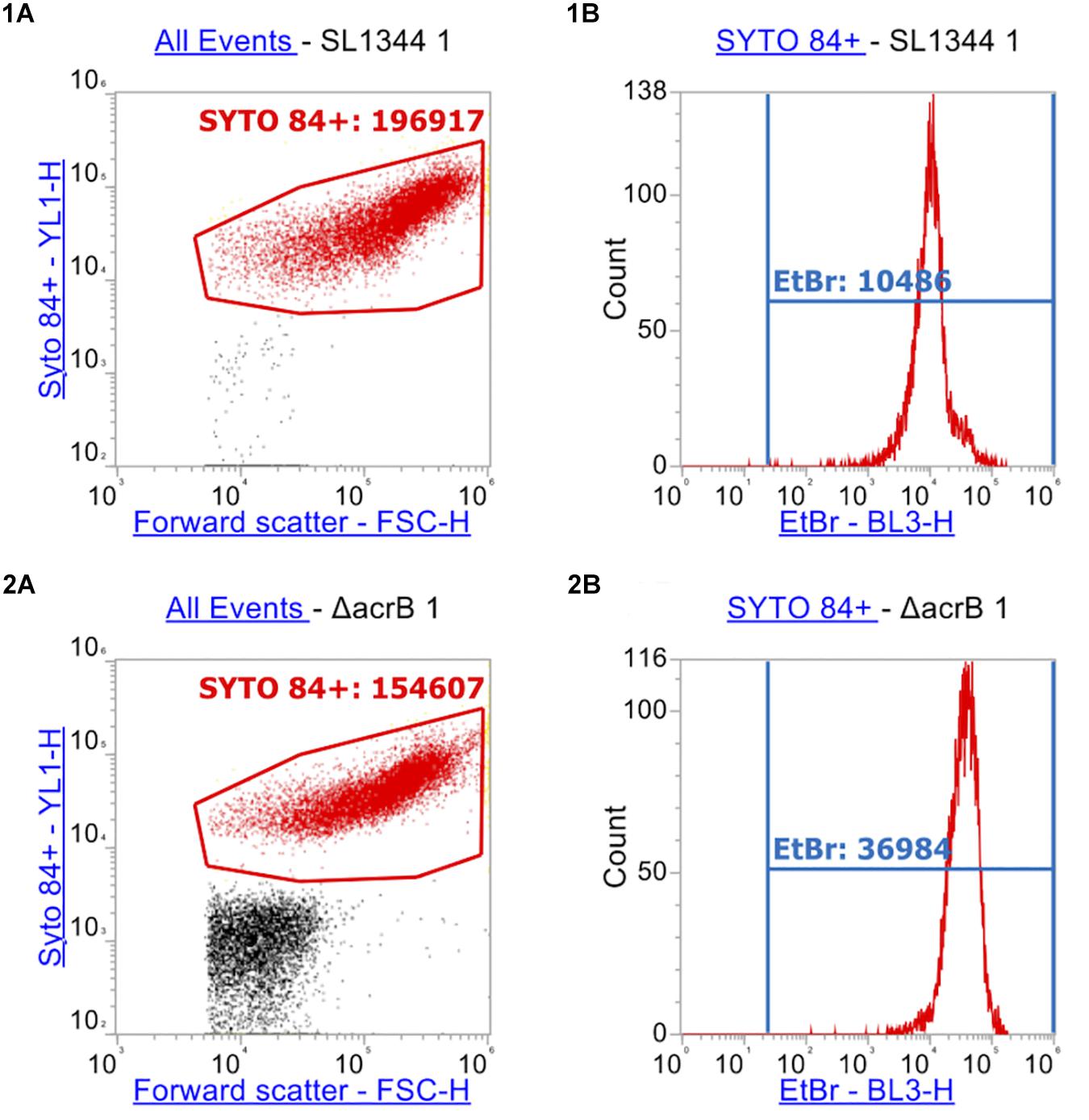
Figure 4. Flow cytometry plots of SL1344 and SL1344 ΔacrB. (1) shows plots of SL1344 and (2) shows plots of efflux mutant SL1344 ΔacrB. (A) Separation of cells from background cell debris using channels YL1-H vs. FSC-H. SYTO 84+ cells are gated (red gate) with the X-median value given. 10,000 events were collected inside the SYTO 84+ gate. From this, the red population is analyzed in (B) plots where the BL3-H channel was used to measure the X-median of ethidium bromide fluorescence in a new gate (EtBr in Blue). Fluorescence of ethidium bromide was only measured from the SYTO 84+ population.
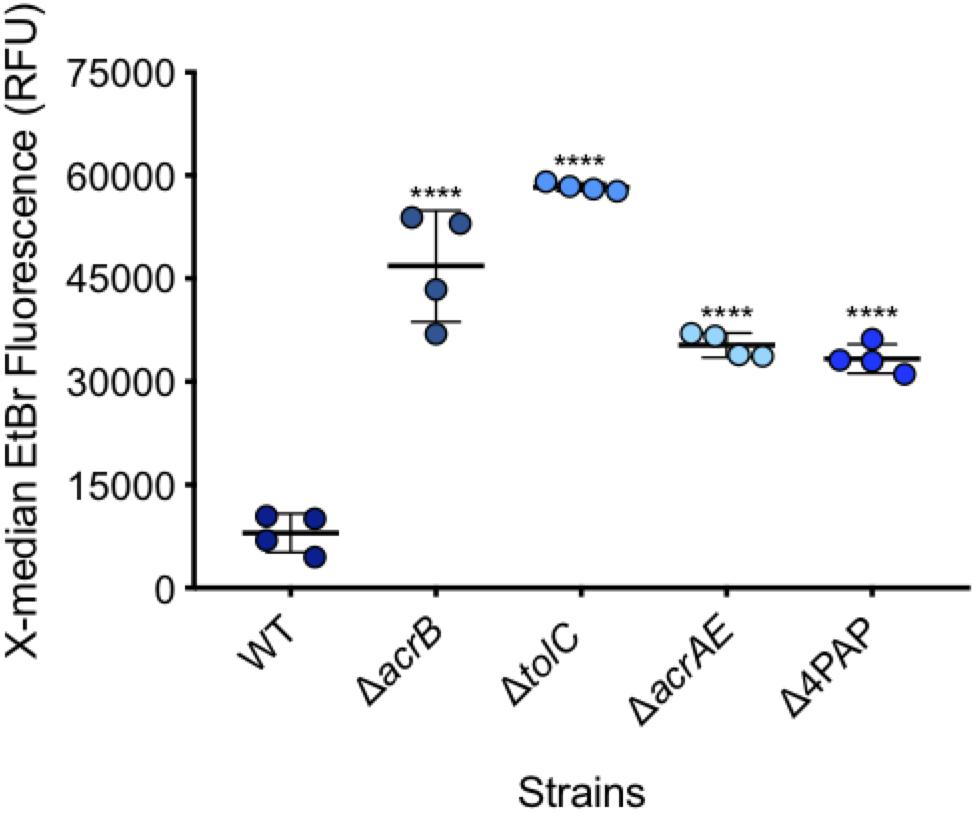
Figure 5. Accumulation of ethidium bromide in WT SL1344 and efflux deficient strains. Single circles represent the X-median value of ethidium bromide fluorescence from 10,000 cells within a biological replicate. 4 biological replicates for each strain are shown, with a long bar to show the mean and standard error of the mean (SEM) error bars. WT had a mean RFU of 8024. This is significantly lower than the mean RFUs of ΔacrB (46824 RFU), ΔtolC (58321 RFU), ΔacrAE (35314 RFU) and D4PAP (33359 RFU). Significance values were based on a one-way ANOVA and Dunnett’s multiple comparison test of all strains compared to WT. All P-values were < 0.0001 which is represented by **** above strain plots in this figure.
Ethidium bromide is commonly used in assays for direct efflux measurement but this assay has also been adapted to use the lipophilic dye, nile red (Bohnert et al., 2010; Bohnert et al., 2011; Blair and Piddock, 2016) and Rhodamine 6G (Ivnitski-Steele et al., 2009). Nile red fluoreces when in the presence of both phospholipids and tryglycerides with different excitation and emission. Here, we also show that the flow cytometry assay described can be adapted for use with these structurally diverse efflux substrates.
A series of concentrations of each dye were tested to determine the optimal concentration. A final sub-inhibitory concentration of 75 μM of nile red was used. The average fluorescence of nile red in SL1344 was 744 RFU while in the absence of AcrB the average fluorescence was twofold higher (1535 RFU, P-value < 0.05) (Figure 6). However, in the absence of TolC, the outer membrane component of the efflux pump, accumulation of nile red was 13-fold higher than SL1344 (9458 RFU, P-value 0.0002).
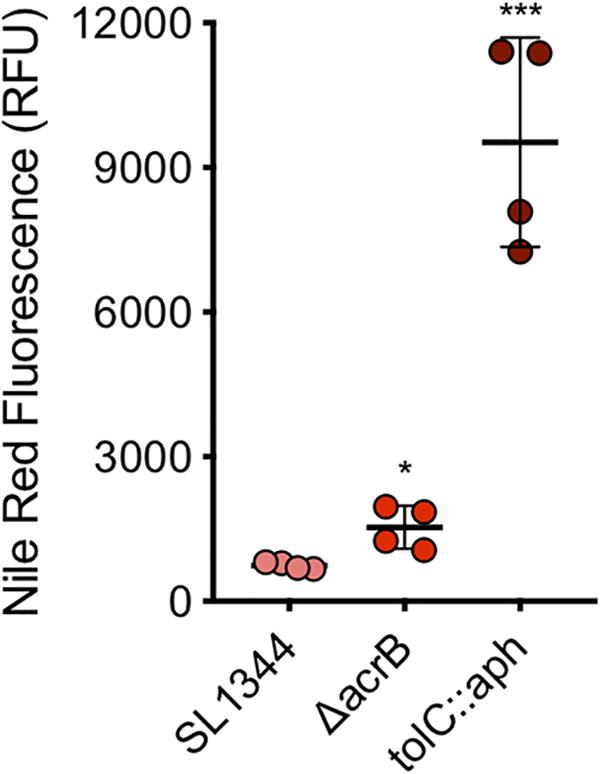
Figure 6. Accumulation of nile red in WT, ΔacrB and tolC::aph in S. Typhimurium. Single circles represent the X-median value of nile red fluorescence from 10,000 cells within a biological replicate using WT (pink), ΔacrB (red) and ΔtolC (dark red). 4 biological replicates for each strain are shown, with a long bar to show the mean and SEM error bars. Significance values were based on an unpaired T-test comparing the efflux deficient strain to WT. ∗ and ∗∗∗ correspond to P-values shown in Supplementary Table S3.
Rhodamine 6G was also tested (Supplementary Figure S4) and accumulation could be measured, however, accumulation between a WT and efflux mutants did not significantly differ. Rhodamine 6G has been reported as a substrate of the AcrAB-TolC pump in S. Typhimurium (Horiyama et al., 2010), however, further optimization such as longer incubation for differences in accumulation may be required.
This assay can also be applied to measure the effect of efflux inhibitors. The accumulation of ethidium bromide was measured in WT SL1344 in the presence of the efflux dissapator Carbonyl Cyanide m-Chlorophenylhydrazine (CCCP). CCCP is a proton motive force inhibitor therefore dissipating the energy source of efflux pumps that require the proton motive force (Mahamoud et al., 2007). The RND pump inhibitor PaβN was used to measure the accumulation in nile red in WT SL1344. Previous studies show that PaβN does not significantly change the MIC or accumulation of ethidium bromide in Gram-negative bacteria (Lomovskaya et al., 2001; Kern et al., 2006; Viveiros et al., 2008; Machado et al., 2017).
A subinhibitory concentration (100 μM) of CCCP was added to SL1344 to measure the level of accumulation when efflux pumps are inhibited, without killing the cells. As with efflux pump gene deletion, there was a significant increase in the level of ethidium bromide accumulation upon the addition of CCCP (Figure 7A).
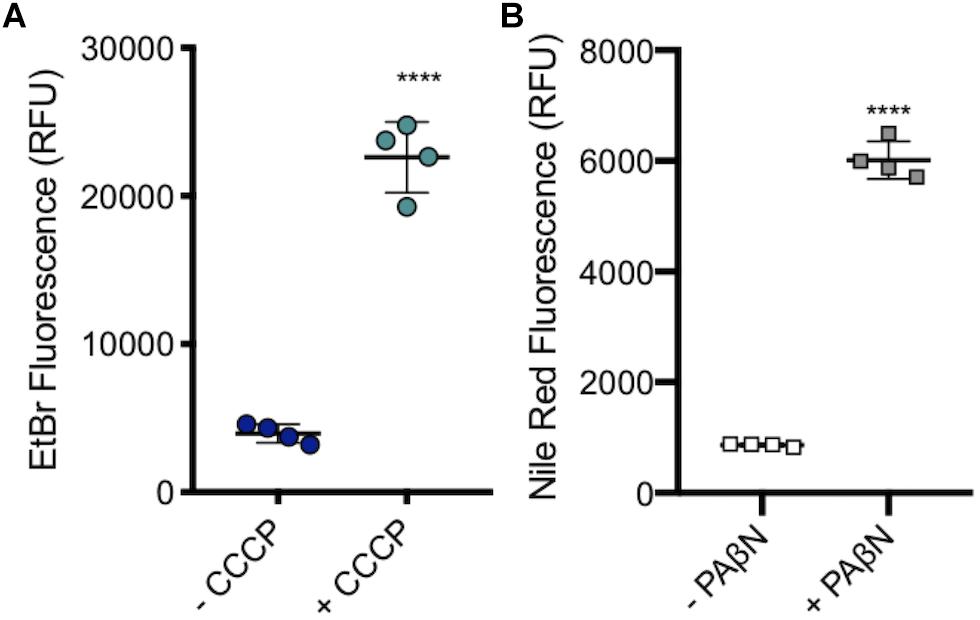
Figure 7. Accumulation of ethidium bromide in SL1344 in the absence and presence of 100 μM CCCP and 50 μg/ml of PAβN. (A) Single dots represent the X-median value of ethidium bromide fluorescence from 10,000 cells within a biological replicate. 4 biological replicates for each condition are shown, with a long bar to show the mean and standard error of the mean (SEM) error bars. WT had a mean RFU of 3936 (blue); +CCCP (green) the RFU increased sixfold to 22596 RFU. (B) Single squares represent the X-median value of nile red fluorescence in the presence and absence of PAβN, from 10,000 cells within a biological replicate. 4 biological replicates for each condition are shown, with a long bar to show the mean and SEM error bars. WT had a mean RFU of 863 (white); + PaβN (gray) RFU showed a sevenfold increase. For both (A) and (B) Significance values were based on an unpaired T-test. The P-values were < 0.0001 which was represented by **** in this figure.
PaβN, at 50 μg/ml, was added to SL1344 to measure the level of nile red accumulation in the absence of RND pump function, without killing the bacterial cells. Experimental data showed that there was a significant increase in the accumulation of nile red in SL1344 cells that were treated with PaβN, a similar result to the addition of CCCP and efflux gene deleted strains. This shows that this method could be used to screen for putative efflux inhibitors (Figure 7B).
To answer complex biological questions it is often desirable to measure two phenotypic characteristics in the same sample in parallel and doing this in single cells can be particularly powerful. The ethidium bromide accumulation assay described can be combined with measurement of green fluorescence, and therefore could be adapted to measure alongside fluorophores such as from GFP or a FITC labeled antibody, to allow this complexity. Excitation of both ethidium bromide and GFP was at 488 nm but their emission walevelengths differ so both can be analyzed simultaneously.
To illustrate this we have used the wild-type S. Typhimurium SL1344 transformed with a transcriptional reporter plasmid encoding the promoter of the ramA gene fused to gfp (pMW82-ramA) to show it is possible to monitor dye accumulation and gene expression in parallel in single cells (Figure 8). RamA is a positive regulator of the efflux pump genes acrAB. GFP fluorescence corresponding to ramA transcription and ethidium bromide accumulation were measured in the same cells, in the absence and presence of indole, a known inducer of ramA (Nikaido et al., 2008; Lawler et al., 2013). Upon addition of indole there was a twofold increase in GFP fluorescence showing increased transcription of ramA, and this was associated with significantly lower accumulation of ethidium bromide per cell within the same population, presumably through induction of the AcrAB efflux pump.
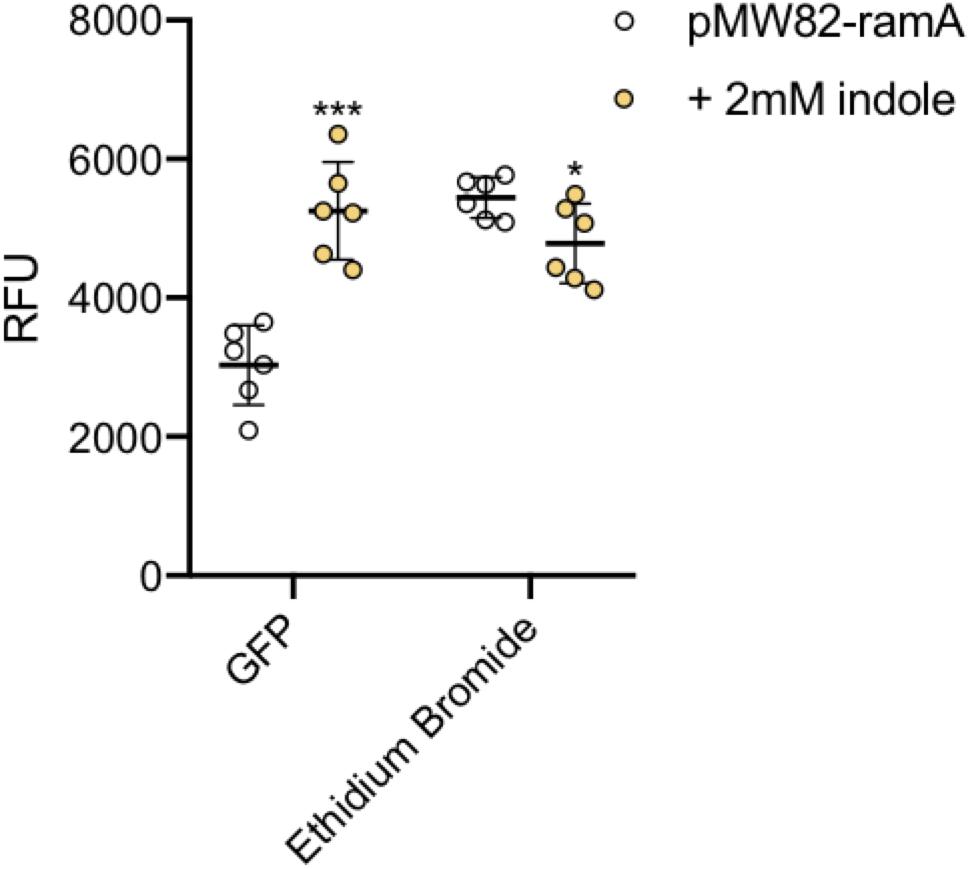
Figure 8. Accumulation of ethidium bromide and fluorescence of GFP. The ethidium bromide and GFP fluorescence (x-axis) were measured in SL1344 pMW82-ramA in the absence (white) and presence (orange) of 2 mM indole. 6 biological replicates for each strain are shown, with a long bar to show the mean and standard error of the mean (SEM) error bars. Ethidium bromide fluorescence of the strains is shown and is consistent with results above. The X-median RFU for GFP is shown without (white) and with (orange) the addition of indole in strains containing a pMW82-ramA transcriptional reporter. Without indole, the mean RFU is 2963 but with indole it is 5230 RFU. Unpaired T-tests were run to compare fluorescence of GFP and ethidium bromide with and without indole. The P-value for GFP (∗∗∗) was 0.0001; The P-value for EtBr (∗) was 0.0315.
Salmonella Typhimurium is one of many Gram-negative organisms that are of concern with regards to antimicrobial resistance and where efflux has been shown to contribute to multi-drug resistance in clinical isolates. For this reason, and to confirm the use of this assay in measuring accumulation in other species, WT and efflux mutants of Escherichia coli, K. pneumoniae and P. aeruginosa strains were also tested. For each of the three Gram-negative species tested, a significant increase in accumulation could be seen in the absence of efflux (Figure 9A). This shows that this assay can be used with many species of Gram-negative bacteria to test substrate accumulation.
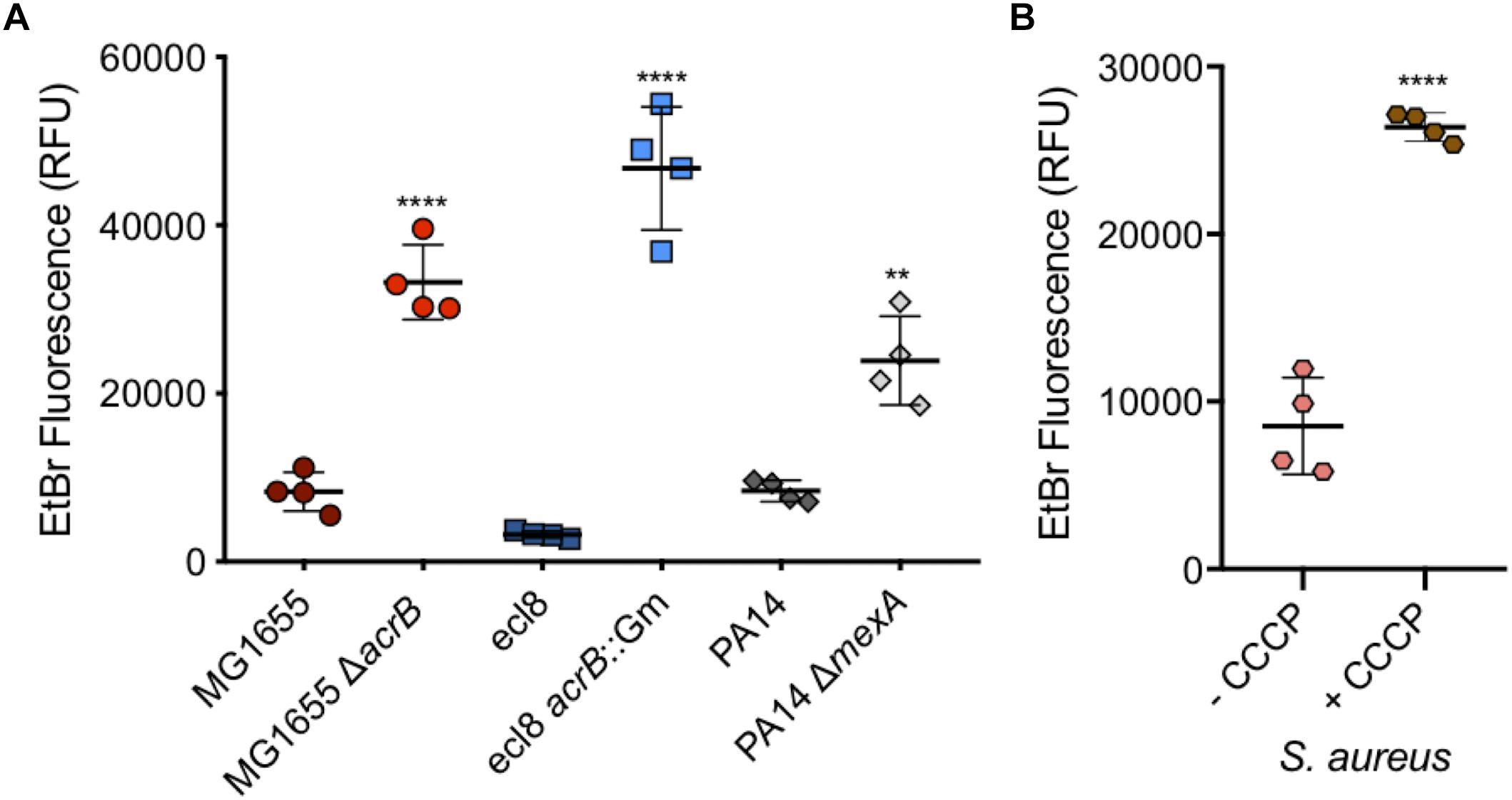
Figure 9. Accumulation of ethidium bromide in WT and efflux mutants of Escherichia coli, Klebsiella pneumonia, and P. aeruginosa, and in Staphylococcus aureus with CCCP. (A) E. coli MG1655 (dark red circles) had an average fluorescence of 8310 RFU and was significantly lower (fourfold) than its ΔacrB (red circles) equivalent at 33233 RFU. Klebsiella pneumoniae ecl8 (dark blue squares) had an average RFU of 3225, 14-fold lower than its acrB::Gm inactivated strain with a RFU of 46764. P. aeruginosa strain PA14 (gray diamonds) had ethidium bromide fluorescence of 8401 RFU which was significantly lower than the fluorescence for its ΔmexA strain at RFU (threefold difference). (B) S. aureus ethidium bromide accumulation was measured in the absence (pink) and presence (brown) of CCCP. In the presence of CCCP, accumulation was threefold higher (P < 0.0001). 4 biological replicates for each strain are shown, with a long bar to show the mean and standard error of the mean (SEM) error bars. Statistical tests were unpaired T-tests between individual WT and efflux deficient strain. Results were considered significantly different if p-value ≤ 0.05. ∗∗ and **** correspond to P-values shown in Supplementary Table S3.
Ethidium bromide accumulation was also measured in S. aureus when efflux pumps were inhibited by CCCP (Figure 9B). This confirmed that this assay can also be used when measuring accumulation in Gram-positive bacteria.
Traditionally measurement of dye or drug accumulation has focused on measurement of whole bacterial populations and this approach has been critical in the investigation of efflux pumps and assessing potential efflux inhibitors. It is now evident that populations of bacteria are not homogeneous in phenotype, and that stochastic gene expression leads to a heterogeneous population and studies have shown its importance in measuring accumulation (Sanchez-Romero and Casadesus, 2014; Davis and Isberg, 2016). For this reason, especially when assessing inhibition of efflux, it may be important to analyse accumulation at the single cell level. If a population of cells with inhibited efflux contains a sub-population that accumulates low concentrations of drug, this may lead to acquisition of mutations and therefore resistance. The assay described here allows the measurement of substrate accumulation in single cells and therefore strengthens our understanding of a cell ability to accumulate drug/dye across a population.
The variations of the assay described highlight its versatility. Not only were we able to show the accumulation of ethidium bromide in S. enterica Serovar Typhimurium, but also its use in other Gram-negative species such as E. coli (Bohnert et al., 2008), K. pneumoniae (Srinivasan et al., 2014) and P. aeruginosa (Mao et al., 2002; Stoitsova et al., 2008) and the Gram-positive organism, S. aureus. The P. aeruginosa mexA mutant only showed a slight (but significant) increase in ethidium bromide accumulation as compared to the wild type. This organism has an intrinsically impermeable membrane and a large number of efflux systems (Hancock, 1998; Breidenstein et al., 2011) therefore removing a single efflux gene (mexA) may not have as a dramatic effect as in the other organisms tested here. This highlights a potential difficulty when assessing drug accumulation in Gram-negatives such as P. aeruginosa. Some species such as E. coli have more than one pump that export ethidium bromide. Previous studies showed the application of nile red (Bohnert et al., 2010) in efflux assays and we have shown that our flow cytometry assay can be adapted to work with this substrate. In future, our assay could be adapted to measure accumulation of many other fluorescent subtrates and may therefore also be used to identify if a substrate is exported by a specific efflux pump.
A large number of antimicrobial resistant clinical isolates often have high expression of efflux pumps making it an attractive new target for therapeutics. The use of CCCP, which stops efflux through the proton motive force, shows that this assay has potential applications in efflux inhibitor screening. Finally, coupling our assay to Fluorescence-Activated Cell Sorting (FACS) will permit physical selection of single cells with particular efflux properties, potentially in combination with other phenotypic markers such as gene expression, aiding screening applications.
The datasets generated for this study are available on request to the corresponding author.
JB and EW designed this assay. EW performed all experiments apart from S. aureus experiments performed by SL and nile red experiments performed by IA, and analyzed all the data. PD constructed the Klebsiella pneumoniae ecl8 mutant strain. EW, JB, and TO wrote the manuscript.
EW was funded by the AAMR Wellcome Trust DTP grant 108876/B/15/Z at the University of Birmingham. IA was funded by the MIBTP2 BBSRC BB/M01116X/1 at the University of Birmingham. JB and SL were funded by the BBSRC grant BB/M02623X/1 (David Phillips Fellowship to JB).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02319/full#supplementary-material
Asuquo, A. E., and Piddock, L. J. (1993). Accumulation and killing kinetics of fifteen quinolones for Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 31, 865–880. doi: 10.1093/jac/31.6.865
Bergmiller, T., Andersson, A. M. C., Tomasek, K., Balleza, E., Kiviet, D. J., Hauschild, R., et al. (2017). Biased partitioning of the multidrug efflux pump AcrAB-TolC underlies long-lived phenotypic heterogeneity. Science 356, 311–315. doi: 10.1126/science.aaf4762
Blair, J. M., and Piddock, L. J. (2016). How to measure export via bacterial multidrug resistance efflux pumps. MBio 7:e840-16. doi: 10.1128/mBio.00840-16
Blair, J. M., Smith, H. E., Ricci, V., Lawler, A. J., Thompson, L. J., and Piddock, L. J. (2015). Expression of homologous RND efflux pump genes is dependent upon AcrB expression: implications for efflux and virulence inhibitor design. J. Antimicrob. Chemother. 70, 424–431. doi: 10.1093/jac/dku380
Bohnert, J. A., Karamian, B., and Nikaido, H. (2010). Optimized nile red efflux assay of AcrAB-TolC multidrug efflux system shows competition between substrates. Antimicrob. Agents Chemother. 54, 3770–3775. doi: 10.1128/AAC.00620-10
Bohnert, J. A., Schuster, S., Seeger, M. A., Fahnrich, E., Pos, K. M., and Kern, W. V. (2008). Site-directed mutagenesis reveals putative substrate binding residues in the Escherichia coli RND efflux pump AcrB. J. Bacteriol. 190, 8225–8229. doi: 10.1128/JB.00912-08
Bohnert, J. A., Schuster, S., Szymaniak-Vits, M., and Kern, W. V. (2011). Determination of real-time efflux phenotypes in Escherichia coli AcrB binding pocket phenylalanine mutants using a 1,2’-dinaphthylamine efflux assay. PLoS One 6:e21196. doi: 10.1371/journal.pone.0021196
Breidenstein, E. B., de la Fuente-Nunez, C., and Hancock, R. E. (2011). Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 19, 419–426. doi: 10.1016/j.tim.2011.04.005
Brenner, F. W., Villar, R. G., Angulo, F. J., Tauxe, R., and Swaminathan, B. (2000). Salmonella nomenclature. J. Clin. Microbiol. 38, 2465–2467.
Coldham, N. G., Webber, M., Woodward, M. J., and Piddock, L. J. (2010). A 96-well plate fluorescence assay for assessment of cellular permeability and active efflux in Salmonella enterica serovar Typhimurium and Escherichia coli. J Antimicrob. Chemother. 65, 1655–1663. doi: 10.1093/jac/dkq169
Davis, K. M., and Isberg, R. R. (2016). Defining heterogeneity within bacterial populations via single cell approaches. Bioessays 38, 782–790. doi: 10.1002/bies.201500121
Greenspan, P., and Fowler, S. D. (1985). Spectrofluorometric studies of the lipid probe, nile red. J. Lipid Res. 26, 781–789.
Hancock, R. E. (1998). Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin. Infect. Dis. 27(Suppl. 1), S93–S99.
Hassan, K. A., Cain, A. K., Huang, T., Liu, Q., Elbourne, L. D., Boinett, C. J., et al. (2016). Fluorescence-based flow sorting in parallel with transposon insertion site sequencing identifies multidrug efflux systems in Acinetobacter baumannii. MBio 7:e1200-e16. doi: 10.1128/mBio.01200-16
Haynes, M. K., Garcia, M., Peters, R., Waller, A., Tedesco, P., Ursu, O., et al. (2018). High-throughput flow cytometry screening of multidrug efflux systems. Methods Mol. Biol. 1700, 293–318. doi: 10.1007/978-1-4939-7454-2_16
Horiyama, T., Yamaguchi, A., and Nishino, K. (2010). TolC dependency of multidrug efflux systems in Salmonella enterica serovar Typhimurium. J. Antimicrob. Chemother. 65, 1372–1376. doi: 10.1093/jac/dkq160
Iino, R., Hayama, K., Amezawa, H., Sakakihara, S., Kim, S. H., Matsumono, Y., et al. (2012). A single-cell drug efflux assay in bacteria by using a directly accessible femtoliter droplet array. Lab Chip. 12, 3923–3929.
Iino, R., Sakakihara, S., Matsumoto, Y., and Nishino, K. (2018). Large-scale femtoliter droplet array for single cell efflux assay of bacteria. Methods Mol. Biol. 1700, 331–341. doi: 10.1007/978-1-4939-7454-2_18
Ivnitski-Steele, I., Holmes, A. R., Lamping, E., Monk, B. C., Cannon, R. D., and Sklar, L. A. (2009). Identification of Nile red as a fluorescent substrate of the Candida albicans ATP-binding cassette transporters Cdr1p and Cdr2p and the major facilitator superfamily transporter Mdr1p. Anal. Biochem. 394, 87–91. doi: 10.1016/j.ab.2009.07.001
Kascakova, S., Maigre, L., Chevalier, J., Refregiers, M., and Pages, J. M. (2012). Antibiotic transport in resistant bacteria: synchrotron UV fluorescence microscopy to determine antibiotic accumulation with single cell resolution. PLoS One 7:e38624. doi: 10.1371/journal.pone.0038624
Kern, W. V., Steinke, P., Schumacher, A., Schuster, S., von Baum, H., and Bohnert, J. A. (2006). Effect of 1-(1-naphthylmethyl)-piperazine, a novel putative efflux pump inhibitor, on antimicrobial drug susceptibility in clinical isolates of Escherichia coli. J. Antimicrob. Chemother. 57, 339–343. doi: 10.1093/jac/dki445
Krishnamoorthy, G., Leus, I. V., Weeks, J. W., Wolloscheck, D., Rybenkov, V. V., and Zgurskaya, H. I. (2017). Synergy between active efflux and outer membrane diffusion defines rules of antibiotic permeation into gram-negative bacteria. MBio 8:e1172-e17. doi: 10.1128/mBio.01172-17
Lawler, A. J., Ricci, V., Busby, S. J., and Piddock, L. J. (2013). Genetic inactivation of acrAB or inhibition of efflux induces expression of ramA. J. Antimicrob. Chemother. 68, 1551–1557. doi: 10.1093/jac/dkt069
Lomovskaya, O., Warren, M. S., Lee, A., Galazzo, J., Fronko, R., Lee, M., et al. (2001). Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45, 105–116. doi: 10.1128/aac.45.1.105-116.2001
Machado, D., Fernandes, L., Costa, S. S., Cannalire, R., Manfroni, G., Tabarrini, O., et al. (2017). Mode of action of the 2-phenylquinoline efflux inhibitor PQQ4R against Escherichia coli. PeerJ 5:e3168. doi: 10.7717/peerj.3168
Mahamoud, A., Chevalier, J., Alibert-Franco, S., Kern, W. V., and Pages, J. M. (2007). Antibiotic efflux pumps in Gram-negative bacteria: the inhibitor response strategy. J. Antimicrob. Chemother. 59, 1223–1229. doi: 10.1093/jac/dkl493
Mao, W. M., Warren, M. S., Black, D. S., Satou, T., Murata, T., Nishino, T., et al. (2002). On the mechanism of substrate specificity by resistance nodulation division (RND)-type multidrug resistance pumps: the large periplasmic loops of MexD from Pseudomonas aeruginosa are involved in substrate recognition. Mol. Microbiol. 46, 889–901. doi: 10.1046/j.1365-2958.2002.03223.x
Martins, M., McCusker, M. P., Viveiros, M., Couto, I., Fanning, S., Pages, J. M., et al. (2013). A simple method for assessment of mdr bacteria for over-expressed efflux pumps. Open Microbiol. J. 7, 72–82. doi: 10.2174/1874285801307010072
Nikaido, E., Yamaguchi, A., and Nishino, K. (2008). AcrAB multidrug efflux pump regulation in Salmonella enterica serovar Typhimurium by RamA in response to environmental signals. J. Biol. Chem. 283, 24245–24253. doi: 10.1074/jbc.m804544200
Nikaido, H. (1989). Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob. Agents Chemother. 33, 1831–1836. doi: 10.1128/aac.33.11.1831
Olmsted, J. III, and Kearns, D. R. (1977). Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry 16, 3647–3654. doi: 10.1021/bi00635a022
Piddock, L. J. (2006). Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19, 382–402. doi: 10.1128/cmr.19.2.382-402.2006
Piddock, L. J., Jin, Y. F., Ricci, V., and Asuquo, A. E. (1999). Quinolone accumulation by Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli. J. Antimicrob. Chemother. 43, 61–70.
Richmond, G. E., Chua, K. L., and Piddock, L. J. (2013). Efflux in Acinetobacter baumannii can be determined by measuring accumulation of H33342 (bis-benzamide). J. Antimicrob. Chemother. 68, 1594–1600. doi: 10.1093/jac/dkt052
Sanchez-Romero, M. A., and Casadesus, J. (2014). Contribution of phenotypic heterogeneity to adaptive antibiotic resistance. Proc. Natl. Acad. Sci. U.S.A. 111, 355–360. doi: 10.1073/pnas.1316084111
Smith, H. E., and Blair, J. M. (2014). Redundancy in the periplasmic adaptor proteins AcrA and AcrE provides resilience and an ability to export substrates of multidrug efflux. J. Antimicrob. Chemother. 69, 982–987. doi: 10.1093/jac/dkt481
Srinivasan, V. B., Singh, B. B., Priyadarshi, N., Chauhan, N. K., and Rajamohan, G. (2014). Role of novel multidrug efflux pump involved in drug resistance in Klebsiella pneumoniae. PLoS One 9:e96288. doi: 10.1371/journal.pone.0096288
Stoitsova, S. O., Braun, Y., Ullrich, M. S., and Weingart, H. (2008). Characterization of the RND-type multidrug efflux pump MexAB-OprM of the plant pathogen Pseudomonas syringae. Appl. Environ. Microbiol. 74, 3387–3393. doi: 10.1128/AEM.02866-07
Keywords: flow cytometry, accumulation, efflux, ethidium bromide, gram-negative
Citation: Whittle EE, Legood SW, Alav I, Dulyayangkul P, Overton TW and Blair JMA (2019) Flow Cytometric Analysis of Efflux by Dye Accumulation. Front. Microbiol. 10:2319. doi: 10.3389/fmicb.2019.02319
Received: 25 July 2019; Accepted: 23 September 2019;
Published: 04 October 2019.
Edited by:
Henrietta Venter, University of South Australia, AustraliaReviewed by:
Byron Brehm-Stecher, Iowa State University, United StatesCopyright © 2019 Whittle, Legood, Alav, Dulyayangkul, Overton and Blair. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica M. A. Blair, Si5NLkEuQmxhaXJAYmhhbS5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.