- 1Crop Physiology Laboratory, Department of Plant Science, McGill University, Montreal, QC, Canada
- 2Ravenquest Biomed, Inc., Vancouver, BC, Canada
Legal Cannabis production is now experiencing growing consumer demand due to changing legislation around the world. However, because of heavy restrictions on cannabis cultivation over the past century, little scientific research has been conducted on this crop, in particular around use of members of the phytomicrobiome to improve crop yields. Recent developments in the field of plant science have demonstrated that application of microbes, isolated from the rhizosphere, have enormous potential to improve yields, in particular under stressful growing conditions. This perspective carefully examines the potential for plant growth-promoting rhizobacteria (PGPR) to improve marijuana and hemp yield and quality. It then explores the potential use of PGPR for biological control of plant pathogens, which is particularly interesting given the stringent regulation of pesticide residues on this crop. As an industry-relevant example, biocontrol of powdery mildew, a common and deleterious pathogen affecting cannabis production, is assessed. Finally, two PGPR in genera frequently associated with higher plants (Pseudomonas and Bacillus) were selected as case studies for the potential effects on growth promotion and disease biocontrol in commercial cannabis production.
Introduction
Cannabis production is drawing widespread attention because it can be used as food, fiber, medicine, and a recreational drug (Jiang et al., 2006; Kostic et al., 2008). The specific application and value is largely based on the concentration and composition of cannabinoids in cannabis plants (Sawler et al., 2015). The demand for cannabis is increasing as medical cannabis and cannabis production have been legalized in countries such as Colombia, Mexico, and Canada (Schuermeyer et al., 2014).
In medical cannabis production, the female plant is more desirable than the male for production of cannabinoids, due to higher flower biomass and cannabinoid levels (Potter, 2014). In commercial production, plants are propagated as cuttings from mother plants to produce genetically identical daughter plants to maintain population of desirable genotypes (Potter, 2014). Studies have attempted to determine which elements of cultivation and genetics contribute to cannabis yield and cannabinoid levels/composition. Cannabis yield is influenced by light intensity and plant density (Toonen et al., 2006; Vanhove et al., 2012; Backer et al., 2019). However, little research has been conducted regarding the response of yield and cannabinoid levels/composition to the application of plant-growth promoting rhizobacteria (PGPR), although research has already demonstrated the important role of PGPR on the production of many other crop species (Mabood et al., 2014; Smith et al., 2015). For example, the application of PGPR to plant roots can stimulate crop growth by providing mineral nutrition to plants. PGPR can also improve crop tolerance to abiotic stresses (e.g., drought and salinity) and biotic stress (e.g., plant pathogens) (Yan et al., 2016; Takishita et al., 2018).
Exploitation of PGPR from the phytomicrobiome (plant microbiome) will play an important role in industrial cannabis production, and there is a clear need to better understand the relationship between the phytomicrobiome and cannabis yield, cannabinoid levels/composition and disease resistance. This perspective summarizes knowledge about factors that contribute to cannabis yield and secondary metabolite biosynthesis. In addition, we examine the potential role of PGPR, with a focus on two widely prevalent genera (Pseudomonas and Bacillus), in achieving high yields, desirable cannabinoid profiles, and disease resistance in cannabis.
Strategies to Increase Cannabis Yield and Quality
To achieve optimal quality for medical use, indoor marijuana cultivation aims to maintain highly controlled growth conditions, with stable, high-quality lighting, and temperature and humidity control. Production conditions that influence marijuana yield and cannabinoid concentration include plant genotype and environmental conditions including temperature, water availability, and fertilizer application during the vegetative growth period, photoperiod, light type, and quality and the development stage of the plant (Lydon et al., 1987; Tipparat et al., 2012; Marti et al., 2014; Caplan et al., 2017). At a physiological level, plant growth regulators can also affect cannabinoid accumulation. For instance, application of gibberellic acid (GA3) can increase or decrease the accumulation of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in cannabis leaves while abscisic acid (ABA) and cycocel increase THC content (Mansouri et al., 2011; Singh et al., 2011). The mechanism underlying these effects is not currently understood. One hypothesis is that the application of GA3 contributes to an increase of 1-aminocyclopropane-1-carboxylate (ACC), which subsequently increases ethylene levels in the plant. According to this theory, higher levels of ethylene result in increased THC and CBD contents (Mansouri et al., 2011).
In contrast, industrial/fiber hemp is grown outdoors, with a view to maximum biomass and yield at minimum production cost. Growing conditions, such as temperature, moisture, soil, seeding density, and photoperiod determine the yield and quality of hemp (Vogl et al., 2004; Hoppner and Mange-Hartmann, 2007; Townshend and Boleyn, 2008).
Plant-Growth Promoting Rhizobacteria for Cannabis Production
Plant growth-promoting rhizobacteria are microbes associated with plant roots that promote plant growth by (1) providing enhanced mineral nutrition, (2) producing plant hormones or other molecules that stimulate plant growth and prime plant defenses against biotic and abiotic stresses, or (3) protecting plants against pathogens by affecting survival of pathogenic microorganisms (Podile and Kishore, 2006; Ortíz-Castro et al., 2009; Bhattacharyya and Jha, 2012; Nandal and hooda, 2013; Vacheron et al., 2013; Ahemad and Kibret, 2014; Yan et al., 2016; Rosier et al., 2018). PGPR are well-recognized as promising inputs for sustainable agricultural production (Bhattacharyya and Jha, 2012; Gupta et al., 2015; Backer et al., 2018).
PGPR-associated yield increases in other crops have been studied extensively. Many investigations have shown that PGPR strains can stimulate the growth of plants, including rice (Etesami et al., 2014), maize (Akladious and Abbas, 2012; Głodowska et al., 2016), soybean (Jayasinghearachchi and Seneviratne, 2004; Ramesh et al., 2014), and wheat (Dilfuza and Zulfiya, 2009). These yield increases have been associated with increased germination percentage (Gholami et al., 2011), seedling vigor (Bharathi et al., 2004), root and shoot growth, and total biomass production (van Loon et al., 1998).
Yield and Quality Enhancements Associated With Plant-Growth Promoting Rhizobacteria
In the case of cannabis production, there is a lack of data about the use of PGPR due to past legal restrictions on production of this crop. There are only two publications (Conant et al., 2017; Pagnani et al., 2018) that report data regarding the benefits of PGPR inoculation on growth and yield of marijuana and hemp. Pagnani et al. (2018) showed that a consortium of PGPR (Azospirillum brasilense, Gluconacetobacter diazotrophicus, Burkholderia ambifaria, and Herbaspirillum seropedicae) improved the growth and physiological status of hemp plants and increased secondary metabolite accumulation and antioxidant activity. Conant et al. (2017) demonstrated that the microbial biostimulant product Mammoth P™ promoted hemp growth at the bloom stage but did not report effects on cannabinoid concentration. Previous studies have shown that PGPR inoculation alters secondary metabolite accumulation in other plant species (Kim et al., 2011; Vacheron et al., 2013; Braga et al., 2016; Mishra et al., 2018); this leads us to hypothesize that PGPR inoculation will alter cannabinoid levels/composition in cannabis. It is critical to determine the effect of PGPR on the yield of cannabis and on the biosynthesis and accumulation of cannabinoids, in particular, in plant tissues or organs at various growth stages.
Our laboratory has already illustrated that bacteria isolated from one plant species can trigger growth promotion and induce stress responses in other species, including crop plants (Smith et al., 2015; Fan et al., 2017; Ricci et al., 2019), which suggest that known PGPR may stimulate growth in cannabis. Moreover, these effects can be induced by inoculating a bacterium or a consortium of bacteria onto plants (Souza et al., 2015). We hypothesize that future research will demonstrate that PGPR-based inoculants can alter (1) cannabinoid accumulation, (2) increase flower yield for marijuana cultivars and seed and fiber yield for hemp cultivars, (3) protect against plant pathogens by production of antimicrobial compounds and priming of plant immune responses, and (4) reduce the impact of abiotic stresses associated with intensive indoor marijuana cultivation (e.g., salinity stress) and challenges associated with climate change, for outdoor hemp cultivation (e.g., drought, high temperatures, flooding).
Biological Control and Disease Resistance Associated With Plant-Growth Promoting Rhizobacteria
Currently, PGPR species of the genera Agrobacterium, Azospirillum, Azotobacter, Bacillus, Burkholderia, Delftia, Paenibacillus, Pantoea, Pseudomonas, Rhizobium, and Serratia are used commercially as biocontrol agents (Glick, 2012). Some of them are already used in the production of various plants, to inhibit diseases via a range of mechanisms (Compant et al., 2005). For instance, Pseudomonas fluorescens controls downy mildew caused by Sclerospora graminicola of pearl millet (Pennisetum glaucum) (Raj et al., 2003) and Bacillus spp. can control bacterial leaf blight of rice caused by Xanthomonas oryzae (Udayashankar et al., 2011). Some Pseudomonas and Bacillus species are used as biological control agents against pests and plant diseases of potato (Hultberg et al., 2010) and sugar beet (Bargabus et al., 2004).
PGPR can help control plant pathogens by (1) direct antagonism against potential pathogens (Beneduzi et al., 2012), (2) competition for space and nutrients (Kumari and Srivastava, 1999), and/or (3) activating induced systemic resistance (ISR) in plants, to prevent infection by specific pathogens (Kloepper et al., 1980, 2004; van Loon et al., 1998; Jetiyanon and Kloepper, 2002; Van et al., 2009; Mishra et al., 2010; Egamberdieva et al., 2017). ISR is mediated by jasmonate (JA)- and ethylene (ET)-sensitive pathways (van Loon et al., 1998; Spoel and Dong, 2012). However, the ability of PGPR strains to elicit ISR appears to depend on the host/rhizobacterium combination (Beneduzi et al., 2012). When successfully activated by PGPR, ISR can enhance the defense capacity of plants by priming for potentiated expression of defense genes (Tjamos et al., 2005). It is clear that PGPR strains, inoculated onto plants, can increase the ability of plants to defend against specific pathogens by eliciting the production of endogenous plant hormones, such as IAA and GA3. Pieterse et al. (2000) found that following the induction of ISR, plants have an enhanced capacity to convert ACC to ethylene, which provides a greater potential to produce ethylene. However, Beneduzi et al. (2012) found that ET- and JA-dependent plant responses can be triggered without a concomitant increase these phytohormones, working instead by enhancing sensitivity to these hormones. Therefore, future research should attempt to determine if the application of PGPR can control infection of cannabis plants by pathogens due to ISR activation via production of plant hormones and/or increased expression of defense-related genes.
Powdery Mildew Control in Indoor Cannabis Cultivation: An Example of Potential Plant-Growth Promoting Rhizobacteria Application
Cannabis can be infected by a plethora of phytopathogens, leading to reduced plant productivity from the seedling to harvest stages (McPartland, 1996; Kusari et al., 2013). For example, Botrytis cinerea and Trichothecium roseum (McPartland, 1996) are commonly found on marijuana plants, especially outdoors, and can seriously damage the plant by attacking leaves, flowers, stems and branches. Indoor-produced cannabis plants are threatened by Trichothecium roseum (McPartland, 1991) and Golovinomyces sp. (Thompson et al., 2017), which attack the leaves and flowers, causing pink rot and powdery mildew diseases, respectively. It is highly desirable to effectively address these threats, to prevent yield losses in cannabis production.
Powdery mildew is a severe fungal disease that damages leaves and buds at all growth stages, and is especially common in indoor cannabis production, due to high humidity levels. Powdery mildew infection causes leaves to senescence prematurely affecting photosynthetic rate and yield, and reducing flower bud quality (McPartland, 1996; McPartland and Cubeta, 1997). Powdery mildew spores destroy the cannabis resin leading to reductions in the medicinal value of marijuana plants (McPartland, 1996). Thus, there is a significant need to develop effective methods to control powdery mildew in cannabis production.
Biological control of plant pathogens, including powdery mildew, provides several advantages over existing chemical control measures. To date, the application of chemical controls such as bicarbonates or refined horticultural oils, has been used to control powdery mildew in other crops (Fernandez et al., 2006). However, these sprays may injure young seedlings, and may have deleterious effects on soil structure (McPartland and Hillig, 2008). Bacillus subtilis has been shown to effectively control strawberry and cucurbit powdery mildew caused by Sphaerotheca macularis (Lowe et al., 2012) and Podosphaera fusca (García-Gutiérrez et al., 2013), respectively, while Pseudomonas aeruginosa can control pea powdery mildew when applied as a foliar spray (Bahadur et al., 2007). These results suggest that inoculating cannabis with PGPR may assist in controlling powdery mildew, representing a substantial advantage over currently available chemical control methods. In addition, fungicide residues could be eliminated on plant parts destined for human consumption (buds for marijuana and seeds for hemp) if an effective biocontrol technology could be applied as a root drench, instead of as a foliar spray.
Examples of Widely Prevalent Phytomicrobiome Members: Pseudomonas and Bacillus for Growth Promotion and Disease Control in Cannabis
Pseudomonas
In general, Pseudomonas spp. show good colonization in numerous ecological niches including soil, water, and plant surfaces (Parret et al., 2003; Humphris et al., 2005; Schreiter et al., 2018) and can inhibit the growth of plant pathogens and promote plant growth. Pseudomonas strains can promote plant growth by producing plant hormones such as IAA and ACC deaminase (Khan et al., 2016) and function as biocontrol agents by producing various pathogen-deterrent compounds, including antibiotics, polysaccharides and siderophores (Beneduzi et al., 2012; Santoyo et al., 2012; Souza et al., 2015). Pseudomonas can induce ISR and to date, experiments with Pseudomonas have concentrated on elucidating the molecular and physiological mechanisms that are the basis of ISR (Kloepper et al., 2004). Hultberg et al. (2010) demonstrated that strains of Pseudomonas can significantly reduce potato late blight disease caused by the oomycete Phytophthora infestans.
Bacillus
Bacillus spp. promote plant growth by (1) excreting cytokinins into the rhizosphere (Arkhipova et al., 2005) and (2) stimulating the synthesis of phytohormones, such as IAA (Shao et al., 2015) and GA3 (Bottini et al., 2004; Idris et al., 2007). Bacillus spores act as biological control agents by inhibiting the growth of various pathogenic microbes (Emmert and Handelsman, 1999; Kumar et al., 2011). Studies have shown that the impact of Bacillus spp. varies among crop species and that the application of Bacillus can improve agronomic traits of crop plants and impart enhanced tolerance to some pathogens (Choudhary, 2011; Lyngwi and Joshi, 2013). Treatment with Bacillus spp. elicited ISR in most of the plant species evaluated and also altered secondary metabolite biosynthesis in plants; both effects contributed to protection against plant diseases (Kloepper et al., 2004). In contrast to Pseudomonas, using Bacillus strains to trigger the ISR pathway in plants is dependent on the ethylene and jasmonate pathways (Santoyo et al., 2012). To date, studies on Bacillus spp. as a biocontrol agents and elicitors of ISR have mainly focused on aspects of microbial ecology, the resilience of plants with activated ISR and direct plant growth promotion (Kloepper et al., 2004).
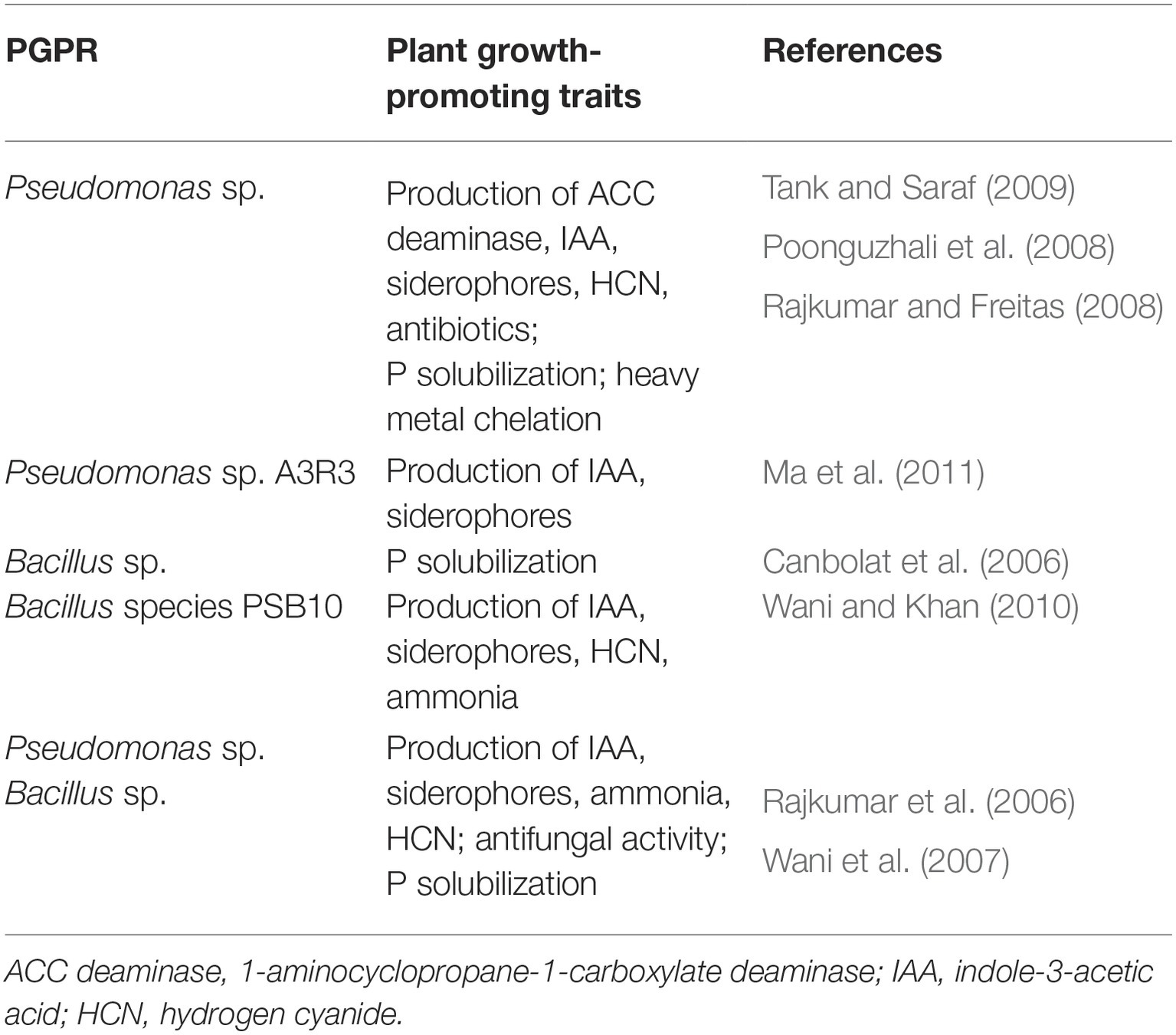
Overall, previous research has shown that these two PGPR genera have strong influences on plant growth promotion through the production of various substances (Table 1; Canbolat et al., 2006; Rajkumar et al., 2006; Wani et al., 2007; Poonguzhali et al., 2008; Rajkumar and Freitas, 2008; Tank and Saraf, 2009; Wani and Khan, 2010; Ma et al., 2011; Ahemad and Kibret, 2014), but their application remains virtually unexplored for cannabis production. Based on the work from our laboratory (Fan et al., 2018; Ricci et al., 2019), Pseudomonas and Bacillus are very common and often dominant bacteria associated with both cultivated and wild plants. Given the results of previous studies (Table 1), it would be very interesting to determine if any strains of these two extremely common PGPR strains have positive influences on cannabis yield and cannabinoid profiles. In addition, further studies should be conducted to investigate the mode-of-action of these two strains, to identify commonalities and unique mechanisms of growth promotion and biocontrol of plant pathogens.
Conclusions and Future Prospects
Cannabis is poised to become an important crop globally; its importance is increasing with the number of countries legalizing the use of cannabis for fiber production and medical applications. It is critical to investigate how to improve cannabis yields and alter cannabinoid concentration and composition. However, because cannabis use has been illegal in most of the world for the past century, there is a great shortage of reliable research data in this area.
The use of PGPR inoculants has contributed to improved yields for many other crops, as a result of nutrient mobilization, hormone production, disease control, and improved stress tolerance. Thus, study of the responses of cannabis to inoculation with PGPR could provide an efficient approach to improve cannabis yield and quality for medical use, and to do so in an environmentally sustainable way. PGPR also have the potential to provide an effective and acceptable strategy for control of key cannabis diseases, without the risks associated with pesticide residue. Overall, elements of the phytomicrobiome have the potential to increase the safety, yield and quality of cannabis.
Author Contributions
DL gathered literature and prepared the manuscript. RB, WR, and DS provided feedback and oversaw progression of the manuscript. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Funding
Funding for this work was provided by a Collaborative Research and Development grant, “Enhanced yield and cannabinoid production of homogenous medical marijuana plants” (award number 517552-17), from the Natural Science and Engineering Council of Canada in collaboration with Ravenquest Biomed.
Conflict of Interest Statement
WR is Chief Executive Officer of Ravenquest Biomed, Inc., a company that produces and sells cannabis products. DS conducts research in collaboration with this company, where the research is funded through the Natural Sciences and Engineering Research Council of Canada, which levers industrial funding.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to acknowledge the editor and anonymous reviewers for the constructive feedback they provided on this article.
References
Ahemad, M., and Kibret, M. (2014). Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J. King Saud Univ. Sci. 26, 1–20. doi: 10.1016/j.jksus.2013.05.001
Akladious, S. A., and Abbas, S. M. (2012). Application of Trichoderma harziunum T22 as a biofertilizer supporting maize growth. Afr. J. Biotechnol. 11, 8672–8683. doi: 10.5897/AJB11.4323
Arkhipova, T., Veselov, S., Melentiev, A., Martynenko, E., and Kudoyarova, G. (2005). Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 272, 201–209. doi: 10.1007/s11104-004-5047-x
Backer, R., Rokem, J. S., Ilangumaran, G., Lamont, J., Praslickova, D., Ricci, E., et al. (2018). Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 9:1473. doi: 10.3389/fpls.2018.01473
Backer, R., Schwinghamer, T., Rosenbaum, P., McCarty, V., Bilodeau, S. E., Lyu, D., et al. (2019). Closing the yield gap for cannabis: a meta-analysis of factors determining cannabis yield. Front. Plant Sci. 10:495. doi: 10.3389/fpls.2019.00495
Bahadur, A., Singh, U., Sarnia, B., Singh, D., Singh, K., and Singh, A. (2007). Foliar application of plant growth-promoting rhizobacteria increases antifungal compounds in pea (Pisum sativum) against Erysiphe pisi. Mycobiology 35, 129–134. doi: 10.4489/MYCO.2007.35.3.129
Bargabus, R., Zidack, N., Sherwood, J., and Jacobsen, B. (2004). Screening for the identification of potential biological control agents that induce systemic acquired resistance in sugar beet. Biol. Control 30, 342–350. doi: 10.1016/j.biocontrol.2003.11.005
Beneduzi, A., Ambrosini, A., and Passaglia, L. M. P. (2012). Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 35, 1044–1051.
Bharathi, R., Vivekananthan, R., Harish, S., Ramanathan, A., and Samiyappan, R. (2004). Rhizobacteria-based bio-formulations for the management of fruit rot infection in chillies. Crop Prot. 23, 835–843. doi: 10.1016/j.cropro.2004.01.007
Bhattacharyya, P. N., and Jha, D. K. (2012). Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol. 28, 1327–1350. doi: 10.1007/s11274-011-0979-9
Bottini, R., Cassán, F., and Piccoli, P. (2004). Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 65, 497–503. doi: 10.1007/s00253-004-1696-1
Braga, R. M., Dourado, M. N., and Araújo, W. L. (2016). Microbial interactions: ecology in a molecular perspective. Braz. J. Microbiol. 47, 86–98. doi: 10.1016/j.bjm.2016.10.005
Canbolat, M. Y., Bilen, S., Çakmakçı, R., Şahin, F., and Aydın, A. (2006). Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora. Biol. Fertil. Soils 42, 350–357. doi: 10.1007/s00374-005-0034-9
Caplan, D., Dixon, M., and Youbin, Z. (2017). Optimal rate of organic fertilizer during the vegetative-stage for cannabis grown in two coir-based substrates. HortScience 52, 1307–1312. doi: 10.21273/HORTSCI12401-17
Choudhary, D. (2011). Plant growth-promotion (PGP) activities and molecular characterization of rhizobacterial strains isolated from soybean (Glycine max L. Merril) plants against charcoal rot pathogen, Macrophomina phaseolina. Biotechnol. Lett. 33, 2287–2295. doi: 10.1007/s10529-011-0699-0
Compant, S., Duffy, B., Nowak, J., Clément, C., and Barka, E. A. (2005). Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 71, 4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005
Conant, R., Walsh, R., Walsh, M., Bell, C., and Wallenstein, M. (2017). Effects of a microbial biostimulant, Mammoth P™, on Cannabis sativa bud yield. J. Hortic. 4, 1–5. doi: 10.4172/2376-0354.1000191
Dilfuza, E., and Zulfiya, K. (2009). Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol. Fertil. Soils 45, 563–571. doi: 10.1007/s00374-009-0366-y
Egamberdieva, D., Davranov, K., Wirth, S., Hashem, A., and Abd, E. A. (2017). Impact of soil salinity on the plant-growth-promoting and biological control abilities of root associated bacteria. Saudi J. Biol. Sci. 24, 1601–1608. doi: 10.1016/j.sjbs.2017.07.004
Emmert, E. A., and Handelsman, J. (1999). Biocontrol of plant disease: a (Gram-) positive perspective. FEMS Microbiol. Lett. 171, 1–9.
Etesami, H., Hosseini, H. M., and Alikhani, H. A. (2014). Bacterial biosynthesis of 1-aminocyclopropane-1-caboxylate (ACC) deaminase, a useful trait to elongation and endophytic colonization of the roots of rice under constant flooded conditions. Physiol. Mol. Biol. Plants 20, 425–434. doi: 10.1007/s12298-014-0251-5
Fan, D., Schwinghamer, T., and Smith, D. L. (2018). Isolation and diversity of culturable rhizobacteria associated with economically important crops and uncultivated plants in Québec, Canada. Syst. Appl. Microbiol. 41, 629–640. doi: 10.1016/j.syapm.2018.06.004
Fan, X., Zhang, S., Mo, X., Li, Y., Fu, Y., and Liu, Z. (2017). Effects of plant growth-promoting rhizobacteria and N source on plant growth and N and P uptake by tomato grown on calcareous soils. Pedosphere 27, 1027–1036. doi: 10.1016/S1002-0160(17)60379-5
Fernandez, D. E., Beers, E., Brunner, J., Doerr, M., and Dunley, J. (2006). Horticultural mineral oil applications for apple powdery mildew and codling moth, Cydia pomonella (L.). Crop Prot. 25, 585–591. doi: 10.1016/j.cropro.2005.08.014
García-Gutiérrez, L., Zeriouh, H., Romero, D., Cubero, J., De Vicente, A., and Pérez-García, A. (2013). The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate-and salicylic acid-dependent defence responses. Microb. Biotechnol. 6, 264–274. doi: 10.1111/1751-7915.12028
Gholami, A., Shahsavani, S., and Nezarat, S. (2011). The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. Proc. World. Acad. Sci. Eng. Technol. 12, 26–32.
Glick, B. R. (2012). Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:963401. doi: 10.6064/2012/963401
Głodowska, M., Husk, B., Schwinghamer, T., and Smith, D. (2016). Biochar is a growth-promoting alternative to peat moss for the inoculation of corn with a pseudomonad. Agron. Sustain. Dev. 36:21. doi: 10.1007/s13593-016-0356-z
Gupta, G., Parihar, S. S., Ahirwar, N. K., Snehi, S. K., and Singh, V. (2015). Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 07, 1013–1020. doi: 10.4172/1948-5948.1000188
Hoppner, F., and Mange-Hartmann, U. (2007). Yield and quality of fibre and oil of fourteen hemp cultivars in Northern Germany at two harvest dates. Landbauforsch. Volk. 57, 219–232.
Hultberg, M., Bengtsson, T., and Liljeroth, E. (2010). Late blight on potato is suppressed by the biosurfactant-producing strain Pseudomonas koreensis 2.74 and its biosurfactant. BioControl 55, 543–550. doi: 10.1007/s10526-010-9289-7
Humphris, S. N., Bengough, A. G., Griffiths, B. S., Kilham, K., Rodger, S., Stubbs, V., et al. (2005). Root cap influences root colonisation by Pseudomonas fluorescens SBW25 on maize. FEMS Microbiol. Ecol. 54, 123–130. doi: 10.1016/j.femsec.2005.03.005
Idris, E. E., Iglesias, D. J., Talon, M., and Borriss, R. (2007). Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant Microbe In. 20, 619–626. doi: 10.1094/MPMI-20-6-0619
Jayasinghearachchi, H., and Seneviratne, G. (2004). A bradyrhizobial-Penicillium spp. biofilm with nitrogenase activity improves N2 fixing symbiosis of soybean. Biol. Fert. Soil 40, 432–434. doi: 10.1007/s00374-004-0796-5
Jetiyanon, K., and Kloepper, J. W. (2002). Mixtures of plant growth-promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol. Control 24, 285–291. doi: 10.1016/S1049-9644(02)00022-1
Jiang, H. E., Li, X., Zhao, Y. X., Ferguson, D. K., Hueber, F., Bera, S., et al. (2006). A new insight into Cannabis sativa (Cannabaceae) utilization from 2500-year-old Yanghai Tombs, Xinjiang, China. J. Ethnopharmacol. 108, 414–422. doi: 10.1016/j.jep.2006.05.034
Khan, A. L., Halo, B. A., Elyassi, A., Ali, S., Al-Hosni, K., Hussain, J., et al. (2016). Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron. J. Biotechnol. 21, 58–64. doi: 10.1016/j.ejbt.2016.02.001
Kim, Y. C., Leveau, J., Gardener, B. B. M., Pierson, E. A., Pierson, L. S., and Ryu, C. M. (2011). The multifactorial basis for plant health promotion by plant-associated bacteria. Appl. Environ. Microbiol. 77, 1548–1555. doi: 10.1128/AEM.01867-10
Kloepper, J. W., Ryu, C. M., and Zhang, S. (2004). Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94, 1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259
Kloepper, J. W., Schroth, M. N., and Miller, T. D. (1980). Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 70, 1078–1082. doi: 10.1094/Phyto-70-1078
Kostic, M., Pejic, B., and Skundric, P. (2008). Quality of chemically modified hemp fibers. Bioresour. Technol. 99, 94–99. doi: 10.1016/j.biortech.2006.11.050
Kumar, A., Prakash, A., and Johri, B. N. (2011). “Bacillus as PGPR in crop ecosystem” in Bacteria in agrobiology: Crop ecosystems. ed. D. K. Maheshwari (Berlin, Heidelberg: Springer), 37–59.
Kumari, V., and Srivastava, J. (1999). Molecular and biochemical aspects of rhizobacterial ecology with emphasis on biological control. World J. Microbiol. Biotechnol. 15, 535–543. doi: 10.1023/A:1008958912647
Kusari, P., Kusari, S., Spiteller, M., and Kayser, O. (2013). Endophytic fungi harbored in Cannabis sativa L.: diversity and potential as biocontrol agents against host plant-specific phytopathogens. Fungal Divers. 60, 137–151. doi: 10.1007/s13225-012-0216-3
Lowe, A., Rafferty-McArdle, S. M., and Cassells, A. C. (2012). Effects of AMF-and PGPR-root inoculation and a foliar chitosan spray in single and combined treatments on powdery mildew disease in strawberry. Agric. Food Sci. 21, 28–38. doi: 10.23986/afsci.4997
Lydon, J., Teramura, A. H., and Coffman, C. B. (1987). UV-B radiation effects on photosynthesis, growth and cannabinoid production of two Cannabis sativa chemotypes. Photochem. Photobiol. 46, 201–206.
Lyngwi, N. A., and Joshi, S. (2013). “Economically important Bacillus and related genera: a mini review” in Biology of useful plants and microbes. ed. A. Sen (New Delhi: India, Narosa Publishing House), 33–43.
Ma, Y., Rajkumar, M., Luo, Y., and Freitas, H. (2011). Inoculation of endophytic bacteria on host and non-host plants—effects on plant growth and Ni uptake. J. Hazard. Mater. 195, 230–237. doi: 10.1016/j.jhazmat.2011.08.034
Mabood, F., Zhou, X., and Smith, D. L. (2014). Microbial signaling and plant growth promotion. Can. J. Plant Sci. 94, 1051–1063. doi: 10.4141/cjps2013-148
Mansouri, H., Asrar, Z., and Amarowicz, R. (2011). The response of terpenoids to exogenous gibberellic acid in Cannabis sativa L. at vegetative stage. Acta Physiol. Plant. 33, 1085–1091. doi: 10.1007/s11738-010-0636-1
Marti, G., Schnee, S., Andrey, Y., Simoes-Pires, C., Carrupt, P. A., Wolfender, J. L., et al. (2014). Study of leaf metabolome modifications induced by UV-C radiations in representative Vitis, Cissus and Cannabis species by LC-MS based metabolomics and antioxidant assays. Molecules 19, 14004–14021. doi: 10.3390/molecules190914004
McPartland, J. M., and Cubeta, M. A. (1997). New species, combinations, host associations and location records of fungi associated with hemp (Cannabis sativa). Mycol. Res. 101, 853–857. doi: 10.1017/S0953756297003584
McPartland, J. M., and Hillig, K. W. (2008). Differentiating powdery mildew from false powdery mildew. J. Ind. Hemp 13, 78–87. doi: 10.1080/15377880801898758
Mishra, J., Fatima, T., and Arora, N. K. (2018). “Role of secondary metabolites from plant growth-promoting rhizobacteria in combating salinity stress” in Plant microbiome: Stress response. eds. D. Egamberdieva and P. Ahmad (Singapore: Springer), 127–163.
Mishra, M., Kumar, U., Mishra, P. K., and Prakash, V. (2010). Efficiency of plant growth promoting rhizobacteria for the enhancement of Cicer arietinum L. growth and germination under salinity. Adv. Biol. Res. 2, 92–96.
Nandal, M., and Hooda, R. (2013). Plant growth promoting rhizobiacteria: a review article. Int. J. Curr. Res. 5, 3863–3871.
Ortíz-Castro, R., Contrerascornejo, H. A., Macíasrodríguez, L., and Lópezbucio, J. (2009). The role of microbial signals in plant growth and development. Plant Signal. Behav. 4, 701–712. doi: 10.4161/psb.4.8.9047
Pagnani, G., Pellegrini, M., Galieni, A., D’egidio, S., Matteucci, F., Ricci, A., et al. (2018). Plant growth-promoting rhizobacteria (PGPR) in Cannabis sativa ‘Finola’ cultivation: an alternative fertilization strategy to improve plant growth and quality characteristics. Ind. Crop. Prod. 123, 75–83. doi: 10.1016/j.indcrop.2018.06.033
Parret, A. H., Schoofs, G., Proost, P., and De, M. R. (2003). Plant lectin-like bacteriocin from a rhizosphere-colonizing Pseudomonas isolate. J. Bacteriol. 185, 897–908. doi: 10.1128/JB.185.3.897-908.2003
Pieterse, C. M., Pelt, J. A. V., Ton, J., Parchmann, S., Mueller, M. J., Buchala, A. J., et al. (2000). Rhizobacteria-mediated induced systemic resistance (ISR) in Arabidopsis requires sensitivity to jasmonate and ethylene but is not accompanied by an increase in their production. Physiol. Mol. Plant Pathol. 125, 652–661. doi: 10.1006/pmpp.2000.0291
Podile, A. R., and Kishore, G. K. (2006). “Plant growth-promoting rhizobacteria” in Plant-associated bacteria. ed. S. S. Gnanamanickam (Dordrecht, Netherlands: Springer), 195–230.
Poonguzhali, S., Madhaiyan, M., and Sa, T. (2008). Isolation and identification of phosphate solubilizing bacteria from Chinese cabbage and their effect on growth and phosphorus utilization of plants. J. Microbiol. Biotechnol. 18, 773–777.
Potter, D. J. (2014). A review of the cultivation and processing of cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test. Anal. 6, 31–38. doi: 10.1002/dta.1531
Raj, S. N., Chaluvaraju, G., Amruthesh, K., Shetty, H. S., Reddy, M., and Kloepper, J. W. (2003). Induction of growth promotion and resistance against downy mildew on pearl millet (Pennisetum glaucum) by rhizobacteria. Plant Dis. 87, 380–384. doi: 10.1094/PDIS.2003.87.4.380
Rajkumar, M., and Freitas, H. (2008). Effects of inoculation of plant-growth promoting bacteria on Ni uptake by Indian mustard. Bioresour. Technol. 99, 3491–3498. doi: 10.1016/j.biortech.2007.07.046
Rajkumar, M., Nagendran, R., Lee, K. J., Lee, W. H., and Kim, S. Z. (2006). Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 62, 741–748. doi: 10.1016/j.chemosphere.2005.04.117
Ramesh, A., Sharma, S. K., Sharma, M. P., Yadav, N., and Joshi, O. P. (2014). Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 73, 87–96. doi: 10.1016/j.apsoil.2013.08.009
Ricci, E., Schwinghamer, T., Fan, D., Smith, D. L., and Gravel, V. (2019). Growth promotion of greenhouse tomatoes with Pseudomonas sp. and Bacillus sp. biofilms and planktonic cells. Appl. Soil Ecol. 138, 61–68. doi: 10.1016/j.apsoil.2019.02.009
Rosier, A., Medeiros, F. H., and Bais, H. P. (2018). Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 428, 35–55. doi: 10.1007/s11104-018-3679-5
Santoyo, G., Orozco-Mosqueda, M. D. C., and Govindappa, M. (2012). Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Biocontrol Sci. Tech. 22, 855–872. doi: 10.1080/09583157.2012.694413
Sawler, J., Stout, J. M., Gardner, K. M., Hudson, D., Vidmar, J., Butler, L., et al. (2015). The genetic structure of marijuana and hemp. PLoS One 10:e0133292. doi: 10.1371/journal.pone.0133292
Schreiter, S., Babin, D., Smalla, K., and Grosch, R. (2018). Rhizosphere competence and biocontrol effect of Pseudomonas sp. RU47 independent from plant species and soil type at the field scale. Front. Microbiol. 9:97. doi: 10.3389/fmicb.2018.00097
Schuermeyer, J., Salomonsen-Sautel, S., Price, R. K., Balan, S., Thurstone, C., Min, S. J., et al. (2014). Temporal trends in marijuana attitudes, availability and use in Colorado compared to non-medical marijuana states: 2003-2011. Drug Alcohol Depend. 140, 145–155. doi: 10.1016/j.drugalcdep.2014.04.016
Shao, J., Xu, Z., Zhang, N., Shen, Q., and Zhang, R. (2015). Contribution of indole-3-acetic acid in the plant growth promotion by the rhizospheric strain Bacillus amyloliquefaciens SQR9. Biol. Fertil. Soils 51, 321–330. doi: 10.1007/s00374-014-0978-8
Singh, R. K., Ali, S. A., Nath, P., and Sane, V. A. (2011). Activation of ethylene-responsive p-hydroxyphenylpyruvate dioxygenase leads to increased tocopherol levels during ripening in mango. J. Exp. Bot. 62, 3375–3385. doi: 10.1093/jxb/err006
Smith, D. L., Subramanian, S., Lamont, J. R., and Bywater-Ekegärd, M. (2015). Signaling in the phytomicrobiome: breadth and potential. Front. Plant Sci. 6:709. doi: 10.3389/fpls.2015.00709
Souza, R. D., Ambrosini, A., and Passaglia, L. M. P. (2015). Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 38, 401–419. doi: 10.1590/S1415-475738420150053
Spoel, S. H., and Dong, X. (2012). How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 12, 89–100. doi: 10.1038/nri3141
Takishita, Y., Charron, J. B., and Smith, D. L. (2018). Biocontrol rhizobacterium Pseudomonas sp. 23S induces systemic resistance in tomato (Solanum lycopersicum L.) against bacterial canker Clavibacter michiganensis subsp. michiganensis. Front. Microbiol. 9:2119. doi: 10.3389/fmicb.2018.02119
Tank, N., and Saraf, M. (2009). Enhancement of plant growth and decontamination of nickel-spiked soil using PGPR. J. Basic Microbiol. 49, 195–204. doi: 10.1002/jobm.200800090
Thompson, G., Tuscano, J., Dennis, M., Singapuri, A., Libertini, S., Gaudino, R., et al. (2017). A microbiome assessment of medical marijuana. Clin. Microbiol. Infect. 23, 269–270. doi: 10.1016/j.cmi.2016.12.001
Tipparat, P., Natakankitkul, S., Chamnivikaipong, P., and Chutiwat, S. (2012). Characteristics of cannabinoids composition of Cannabis plants grown in Northern Thailand and its forensic application. Forensic Sci. Int. 215, 164–170. doi: 10.1016/j.forsciint.2011.05.006
Tjamos, S. E., Flemetakis, E., Paplomatas, E. J., and Katinakis, P. (2005). Induction of resistance to Verticillium dahliae in Arabidopsis thaliana by the biocontrol agent K-165 and pathogenesis-related proteins gene expression. Mol. Plant Microbe In. 18, 555–561. doi: 10.1094/MPMI-18-0555
Toonen, M., Ribot, S., and Thissen, J. (2006). Yield of illicit indoor cannabis cultivation in the Netherlands. J. Forensic Sci. 51, 1050–1054. doi: 10.1111/j.1556-4029.2006.00228.x
Townshend, J. M., and Boleyn, J. M. (2008). “Plant density effect on oil seed yield and quality of industrial hemp cv. Fasamo in Canterbury” in Seed symposium: Seeds for futures. eds. C. R. McGill and J. S. Rowarth (Palmerston North, New Zealand: Agronomy Society of New Zealand).
Udayashankar, A., Nayaka, S. C., Reddy, M., and Srinivas, C. (2011). Plant growth-promoting rhizobacteria mediate induced systemic resistance in rice against bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae. Biol. Control 59, 114–122. doi: 10.1016/j.biocontrol.2011.06.010
Vacheron, J., Desbrosses, G., Bouffaud, M. L., Touraine, B., Moënne-Loccoz, Y., Muller, D., et al. (2013). Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 4:356. doi: 10.3389/fpls.2013.00356
van Loon, L. C., Bakker, P. A., and Pieterse, C. M. (1998). Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 36, 453–483.
Van, D. E. S., Van Wees, S. C., and Pieterse, C. M. (2009). Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 41, 1581–1588. doi: 10.1016/j.phytochem.2009.06.009
Vanhove, W., Surmont, T., Van, D. P., and De, R. B. (2012). Yield and turnover of illicit indoor cannabis (Cannabis spp.) plantations in Belgium. Forensic Sci. Int. 220, 265–270. doi: 10.1016/j.forsciint.2012.03.013
Vogl, C. R., Lissek-Wxsolf, G., and Surböck, A. (2004). Comparing hemp seed yields (Cannabis sativa L.) of an on-farm scientific field experiment to an on-farm agronomic evaluation under organic growing conditions in lower Austria. J. Ind. Hemp 9, 37–49. doi: 10.1300/J237v09n01_05
Wani, P. A., and Khan, M. S. (2010). Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem. Toxicol. 48, 3262–3267. doi: 10.1016/j.fct.2010.08.035
Wani, P. A., Khan, M. S., and Zaidi, A. (2007). Synergistic effects of the inoculation with nitrogen-fixing and phosphate-solubilizing rhizobacteria on the performance of field-grown chickpea. J. Plant Nutr. Soil Sci. 170, 283–287. doi: 10.1002/jpln.200620602
Keywords: cannabis, cannabinoids, plant-growth promoting rhizobacteria, powdery mildew, biological control
Citation: Lyu D, Backer R, Robinson WG and Smith DL (2019) Plant Growth-Promoting Rhizobacteria for Cannabis Production: Yield, Cannabinoid Profile and Disease Resistance. Front. Microbiol. 10:1761. doi: 10.3389/fmicb.2019.01761
Edited by:
Ivan Baccelli, Istituto per la Protezione Sostenibile delle Piante, ItalyReviewed by:
Zisis Vryzas, Democritus University of Thrace, GreeceAnelise Beneduzi, Fundação Estadual de Pesquisa Agropecuária (Fepagro), Brazil
Copyright © 2019 Lyu, Backer, Robinson and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donald L. Smith, ZG9uYWxkLnNtaXRoQG1jZ2lsbC5jYQ==
 Dongmei Lyu
Dongmei Lyu Rachel Backer
Rachel Backer W. George Robinson2
W. George Robinson2 Donald L. Smith
Donald L. Smith