- 1Health Sciences Division, Department of Microbiology and Immunology, Stritch School of Medicine, Loyola University Chicago, Maywood, IL, United States
- 2Buck Institute for Research on Aging, Novato, CA, United States
- 3Energy & Photon Sciences Directorate, National Synchrotron Light Source II, Brookhaven National Laboratory, Upton, NY, United States
Post-translational modifications (PTM) decorate proteins to provide functional heterogeneity to an existing proteome. The large number of known PTMs highlights the many ways that cells can modify their proteins to respond to diverse stimuli. Recently, PTMs have begun to receive increased interest because new sensitive proteomics workflows and structural methodologies now allow researchers to obtain large-scale, in-depth and unbiased information concerning PTM type and site localization. However, few PTMs have been extensively assessed for functional consequences, leaving a large knowledge gap concerning the inner workings of the cell. Here, we review understanding of N-𝜀-lysine acetylation in bacteria, a PTM that was largely ignored in bacteria until a decade ago. Acetylation is a modification that can dramatically change the function of a protein through alteration of its properties, including hydrophobicity, solubility, and surface properties, all of which may influence protein conformation and interactions with substrates, cofactors and other macromolecules. Most bacteria carry genes predicted to encode the lysine acetyltransferases and lysine deacetylases that add and remove acetylations, respectively. Many bacteria also exhibit acetylation activities that do not depend on an enzyme, but instead on direct transfer of acetyl groups from the central metabolites acetyl coenzyme A or acetyl phosphate. Regardless of mechanism, most central metabolic enzymes possess lysines that are acetylated in a regulated fashion and many of these regulated sites are conserved across the spectrum of bacterial phylogeny. The interconnectedness of acetylation and central metabolism suggests that acetylation may be a response to nutrient availability or the energy status of the cell. However, this and other hypotheses related to acetylation remain untested.
Introduction
The two competing theories for the origin of life (metabolism-first and gene-first) suggest that abiotic chemical reactions were the progenitors of biotic life. These abiotic chemical reactions ultimately formed the basis for metabolism and provided building blocks for the synthesis of both RNA and protein. Over millions of years, cells formed and evolved and, in doing so, generated the diversity of life we observe today. Across phylogeny, the core metabolic pathways necessary for life have been well conserved (Peregrín-Alvarez et al., 2009). In these core pathways, anabolism and catabolism generate a multitude of different metabolites. The potential chemical reactions in which these metabolites can participate greatly exceed cellular metabolic requirements. Therefore, the cell has evolved diverse mechanisms to control these “extra-metabolic” compounds to limit their toxicity or the damage that they impart upon macromolecular structures via non-enzymatic reactions within the cell (Dickinson and Chang, 2011). While cells have evolved means of controlling these “extra-metabolic” compounds, it is thought that accumulation of the resultant damage may be a prototypic driver of aging (Golubev et al., 2017).
Despite the constant possibility of damage due to their own metabolism, bacteria have survived and evolved to adapt to various niches. However, these environments are rarely constant outside of a laboratory setting, and bacteria will often face adverse conditions. In these niches, one of the major challenges is acquisition of nutrients necessary to grow and divide. While some nutrients like carbon may be readily abundant, other nutrients can be scarce, such as iron sequestered by a host (Hood and Skaar, 2012). These conditions may change suddenly, so the bacteria must respond accordingly or perish. It is well established that cells respond to environmental challenges with appropriate regulation of their transcriptional and translational programs (Gottesman, 2017). Whereas transcription and translation are time- and energy-intensive processes, post-translational control over the function of existing proteins can rapidly alter cellular physiology and permit fitness or survival in otherwise suboptimal or lethal conditions (Castaño-Cerezo et al., 2014; Lynch and Marinov, 2015). While small ligands may allosterically regulate proteins, diverse covalent chemical modifications of the existing proteome also occur; these may or may not be reversible (Martin, 2014). Researchers have identified hundreds of chemical modifications of the proteome; these are called post-translational modifications (PTMs). These PTMs can occur on a variety of protein residues and understanding how these modifications affect protein structure and function and whether they interact with one another is critical to generating a complete regulatory picture of the cell.
Many PTMs occur on lysines within proteins, including methylation (Lanouette et al., 2014), propionylation (Chen et al., 2007), butyrylation (Chen et al., 2007), crotonylation (Tan et al., 2011; Sabari et al., 2015), succinylation (Zhang et al., 2011), malonylation (Du et al., 2011; Peng et al., 2011), glutarylation (Tan et al., 2014), 2-hydroxyisobutyrylation (Dai et al., 2014), pupylation (Becker and Darwin, 2017), and N𝜀-lysine acetylation, which is becoming recognized as a prominent modification that is conserved across phylogeny (Kim et al., 2006; Choudhary et al., 2009). While lysine acetylation is best understood as an activating modification of eukaryotic histones (Hebbes et al., 1988), extensive study into the role of lysine acetylation in bacteria only began about a decade ago. Acetylation of certain lysines may have a clear consequence, such as inhibition of enzyme activity due to modification of an active site (Nakayasu et al., 2017), but the functional importance or lack thereof of many acetyllysine modifications are more difficult to discern. To uncover the role of lysine acetylation in these unclear cases, it is helpful to use a model bacterium (e.g., Escherichia coli) with a vast knowledgebase of pathways, protein structure-function relationships, and physiology.
This review will detail what we know concerning lysine acetylation in bacteria with some attention paid to eukaryotic acetylation for context. We will begin by describing the different types of acetylation, the mechanisms that result in acetylation of lysines, and some known effects of lysine acetylation on protein function. We will discuss the importance of mass spectrometry to identify and quantify acetylated proteins. Furthermore, we will explore the possibility that acetylation could be a driver of protein and organismal evolution. Finally, with our deepening understanding of acetylation, we will discuss a possible future for this new field.
Acetylation – From Small Molecules to Proteins
Acetylation is simply the transfer of an acetyl group (CH3CO) onto a molecule. The acetyl group can react with a variety of atoms or functional groups on a target molecule. The atom to which the acetyl group is attached is usually denoted in the name of either the final molecule or the enzyme that performs the acetylation. Acetylation can occur with thiol groups (sulfur), hydroxyl groups (oxygen), and often amino groups (nitrogen).
Acetylation reactions can target small molecules and metabolites, or they can occur on proteins. Multiple metabolic pathways require the transfer of acetyl groups from one metabolite to the next. For example, N-acetylglutamate synthase catalyzes acetylation of L-glutamate, the first step in arginine biosynthesis (Cunin et al., 1986). Acetylation of multiple aminoglycoside antibiotics has been reported to inactivate their bactericidal or bacteriostatic activity (Kawabe et al., 1975; Shaw and Hopwood, 1976; Garneau-Tsodikova and Labby, 2016), and acetylation of the antibiotic chloramphenicol disables its ability to inhibit translation (Bulkley et al., 2010). The chitin of insects, plants, and fungi and the peptidoglycan of the bacterial cell wall are composed of complex polysaccharides (Bernard et al., 2011). These polysaccharides often contain monomers such as N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc), which are monosaccharide derivatives acetylated on the primary amine (N), as their names suggest. However, these can be further acetylated on hydroxyl groups to increase resistance to degradative enzymes, such as lysozyme and autolysins (Bernard et al., 2011).
The most widely studied protein acetylations occur on amino groups, although acetylations on serine, threonine, and histidine residues of proteins also have been detected (Mukherjee et al., 2006, 2007; Paquette et al., 2012; Lee et al., 2015). Two distinct mechanisms for acetylation of amino groups of proteins can occur: lysine acetylation (N𝜀-acetylation) and N-terminal protein acetylation (Nα-acetylation). Lysine acetylation will be discussed in more detail below. Nα-acetylation is very common in eukaryotes, but rare in bacteria with only 47 proteins detected as N-terminally acetylated in E. coli, 117 identified in Pseudomonas aeruginosa, and 145 identified in Acinetobacter baumannii (Ouidir et al., 2015; Kentache et al., 2016; Brown et al., 2017). Acetylation is observed either on the free amino group of methionine or on the exposed amino acid after cleavage of the N-terminal methionine. The modification is usually co-translational in eukaryotes but post-translational in bacteria, mitochondria, and chloroplasts, as the methionine first must be deformylated (Polevoda and Sherman, 2003). Nα-acetylation has been shown to alter protein stability in eukaryotes and is typically irreversible (Helbig et al., 2010; Helsens et al., 2011). E. coli encodes three proteins that have demonstrated Nα-acetyltransferase activity: RimI, RimJ, and RimL. Interestingly, the only known targets of RimI, RimJ, and RimL are the ribosomal proteins S5, L12, and S18, respectively (Yoshikawa et al., 1987; Tanaka et al., 1989). The exact physiological effects of Nα-acetylation in E. coli are not known. There is evidence that RimJ plays a role in ribosome assembly, but it is not known whether this phenotype requires the Nα-acetyltransferase activity of RimJ (Yoshikawa et al., 1987; Roy-Chaudhuri et al., 2008). RimJ also plays a role in transcriptional regulation of the P pilus of uropathogenic E. coli, but the specific target and whether Nα-acetyltransferase activity is required remain unknown (White-Ziegler and Low, 1992; White-Ziegler et al., 2002). One defined role for Nα-acetylation comes from the ESAT-6 virulence factor of Mycobacterium tuberculosis. Cleavage of the N-terminal methionine of ESAT-6 reveals a threonine, which is then acetylated on the amino terminus. This acetylation alters protein–protein interactions with the binding partner CFP-10, which attenuates virulence (Okkels et al., 2004;Lange et al., 2014; Mba Medie et al., 2014).
Until recently, the only known internal residue that could be acetylated was lysine. However, recent work from a few groups found that a family of bacterial effector molecules from certain pathogenic species can acetylate serine, threonine, and histidine in addition to lysine residues. In some Yersinia species, YopJ can act as an acetyltransferase to modify serine and threonine residues of kinases in mammalian host cells to subvert host immunity (Mukherjee et al., 2006, 2007; Mittal et al., 2010; Paquette et al., 2012). Similarly, HopZ3, a YopJ family member from the plant pathogen Pseudomonas syringae, can acetylate plant proteins on lysine, serine, threonine, and histidine residues, as well as bacterial effector proteins AvrB and AvrB3 (Lee et al., 2015). In both cases, the acetylation by YopJ or HopZ3 prevents effective immune signaling, which results in successful bacterial infection. This does not appear to be an exclusively inter-kingdom modification, because O-acetylation of serine and threonine was found to occur on a proteome-wide scale in M. tuberculosis (Birhanu et al., 2017). Finally, O-acetylation of a single serine (S608) in acetyl-CoA synthetase (Acs) regulates the ability of a neighboring lysine (K610) to be acetylated in Streptomyces lividans (VanDrisse and Escalante-Semerena, 2018). The discovery of these unusual acetylated residues opens new questions concerning the prevalence of these modifications and their potential interaction with other PTMs.
N𝜀-Lysine Acetylation
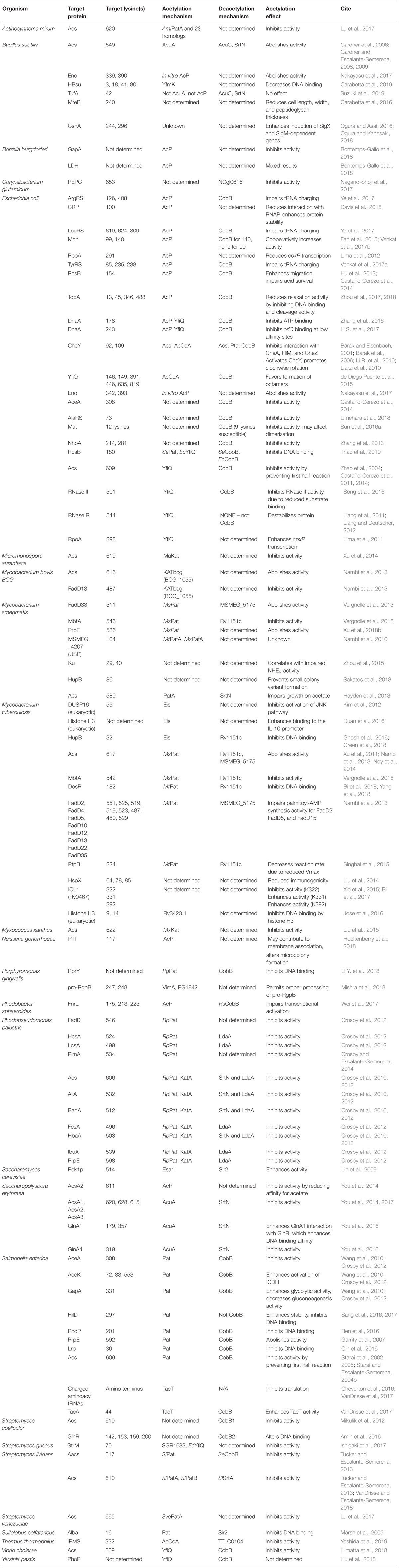
N𝜀-lysine acetylation (hereafter acetylation) is the donation of an acetyl group onto the epsilon amino group of a lysine sidechain (Figure 1). Acetylation increases the size of the side chain and neutralizes the positive charge (+1 to 0) when the pH is less than the pKa of the specific lysine residue. Losing the positive charge and increasing the size of lysine disrupts salt bridges and introduces steric bulk that can alter protein–protein interactions, protein–DNA interactions, stability, and enzymatic activity (Glozak et al., 2005; Yang and Seto, 2008a; Xiong and Guan, 2012). This was first and extensively studied in the context of the histone code of eukaryotes (Phillips, 1963), where acetylation of the lysine rich C-terminal tail reduces protein–DNA binding. This remodels chromatin to activate gene transcription (Allfrey et al., 1964; Gershey et al., 1968; Vidali et al., 1968; Hebbes et al., 1988). However, recent mass spectrometric studies of acetylation have detected acetylation in all three domains of life (Zhao et al., 2003; Marsh et al., 2005; Spange et al., 2009; Zhang et al., 2009). The consortium of acetylated proteins is often called the acetylome. The first bacterial acetylome was reported in 2008 (Yu et al., 2008); since then >50 bacterial acetylomes have been reported (Christensen et al., 2019). This accumulation of bacterial acetylomes has resulted from advances in mass spectrometry-based proteomic approaches.
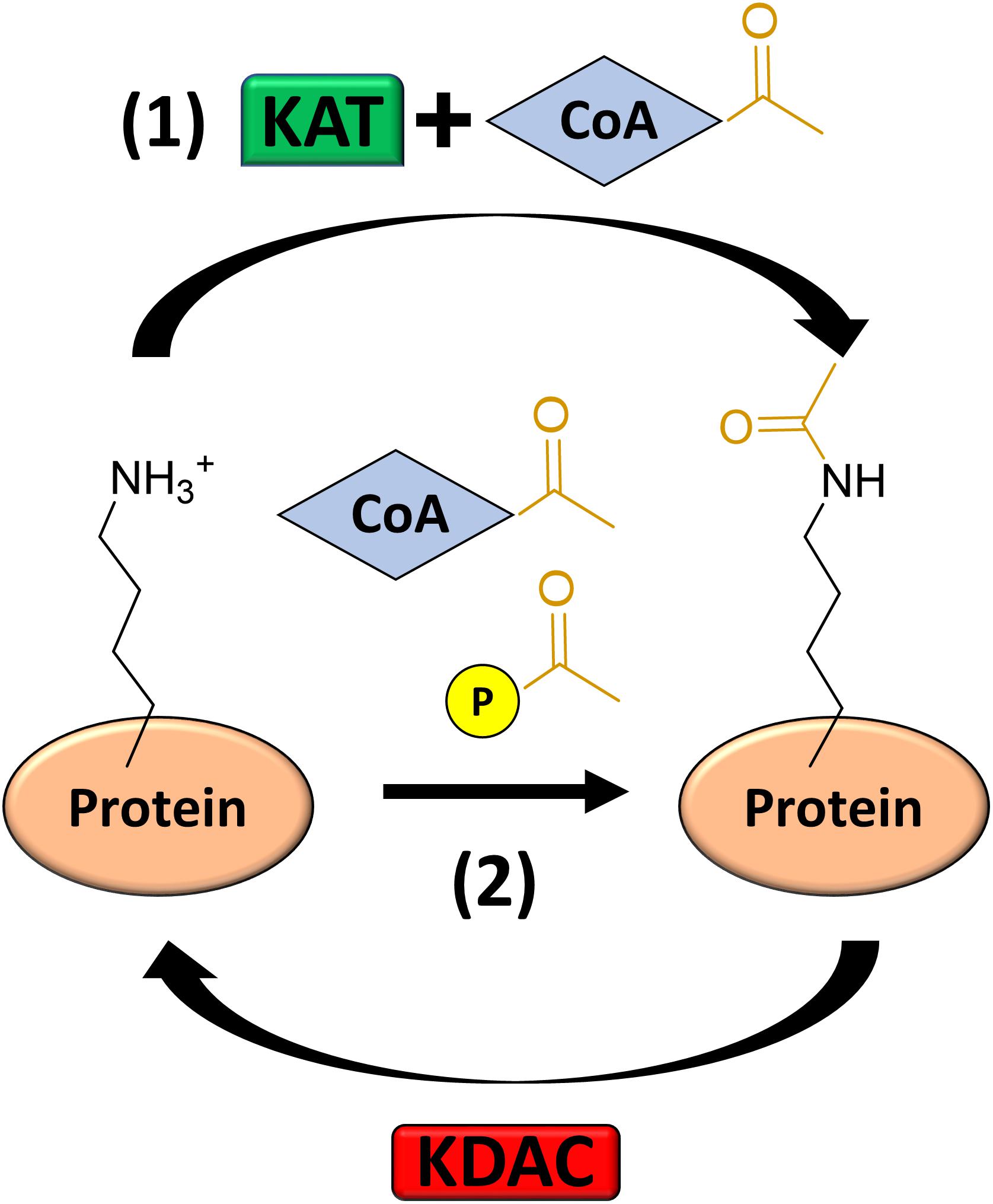
Figure 1. Mechanisms of Acetylation. Acetylation can be catalyzed (1) by a lysine acetyltransferase (KAT) using acetyl-CoA as the acetyl donor or (2) non-enzymatically by acetyl phosphate or acetyl-CoA. Some, but not all, acetylations can be reversed by a lysine deacetylase (KDAC).
Mass Spectrometry-Based Proteomics to Study Bacterial Acetylomes
Due to its high sensitivity and high-throughput, high-resolution mass spectrometry has emerged as a powerful technique to study lysine acetylation (Doll and Burlingame, 2015). For example, one can profile the global lysine acetylome of an organism, identifying acetyllysine (Kac) sites within a given proteome. Alternatively, one can use targeted approaches to examine specific acetyllysine sites of biological interest with high sensitivity and quantitative accuracy (Kuo and Andrews, 2013).
To compare the acetylome under different conditions, studies can be designed and mass spectrometric workflows can be optimized to not only identify Kac sites, but also to detect dynamic changes in terms of relative acetylation abundance over time, in isogenic sets of mutants, or in response to different treatments. These methods have been reviewed previously (Carabetta and Cristea, 2017). Such quantitative studies can be quite impactful and relevant. For example, two studies have provided insight into the dynamic changes that occur in response to the nutritional capacity of the bacterial environment (Kuhn et al., 2014; Schilling et al., 2019). Another study characterized the acetylome of drug resistant Salmonella strains, identifying associations between acetylation and drug resistance (Li L. et al., 2018). A recent review described how bacterial protein acetylation might mediate bacterial virulence (Ren et al., 2017). Quantitative mass spectrometric analysis of acetylation sites also has proven helpful in identifying novel lysine acetyltransferases (KATs) (Christensen et al., 2018; Carabetta et al., 2019).
In most mass spectrometry-based workflows, such as those described above, proteins are first subjected to proteolytic digestion, and peptides are immuno-enriched with anti-Kac antibodies before mass spectrometric analysis (Liu et al., 2008; Yu et al., 2008; Kuhn et al., 2014; Schilling et al., 2015, 2019; Xie et al., 2015). However, it is also possible to detect acetylation directly from bacterial whole lysates. For example, a large bacterial acetylation study was carried out by Nakayasu and co-workers to investigate the proteomes and acetylomes of 48 phylogenetically distant bacteria, identifying more than 9,000 acetylated proteins with approximately 190 acetylated proteins per organism, and more than 24,000 acetylated peptides with approximately 508 Kac per organism (Nakayasu et al., 2017). Many of the observed Kac sites were conserved across the phylogenetic spectrum, leading the authors to conclude that lysine acetylation is an evolutionarily conserved mechanism and that bacterial cells use it to regulate central metabolism (Nakayasu et al., 2017).
As mass spectrometric techniques and software have improved, the breadth of targets modified by acetylation has expanded. It is now clear that the extent of acetylation, the mechanisms by which acetylation occurs, the identities of acetylated proteins, and the locations of acetylation on those proteins can vary between organisms; yet, in most cases, there is great conservation in core cellular processes (Nakayasu et al., 2017). Such approaches have shown that acetylation occurs on proteins involved in a wide diversity of cellular process, especially central metabolism and translation (Wang et al., 2010; Weinert et al., 2013a; Baeza et al., 2014; Kuhn et al., 2014; Kosono et al., 2015; Schilling et al., 2015; Kentache et al., 2016; Birhanu et al., 2017; Jers et al., 2017; Nagano-Shoji et al., 2017; Post et al., 2017; Bontemps-Gallo et al., 2018; Gaviard et al., 2018; Sun et al., 2018; Vasileva et al., 2018; Yoshida et al., 2019). As stated above, many of these acetylations are conserved. This conservation has led some to hypothesize that the mechanisms of acetylation and the consequences of those acetylations are also conserved (Nakayasu et al., 2017).
Acetyltransferases in Bacteria
Multiple families of acetyltransferases have been discovered across phylogeny. Many of these families were discovered in relation to histone acetylation in eukaryotes. Two major families of acetyltransferases are the Gcn5-related N-acetyltransferases (GNATs) and MYST families (Yang et al., 1996; Vetting et al., 2005). Two smaller groups of acetyltransferases known as the p300/CBP family and the SRC family also exist, as well as the unique acetyltransferases TAFII250, TFIIIC, Rtt109, and CLOCK (Bannister and Kouzarides, 1996; Mizzen et al., 1996; Spencer et al., 1997; Marmorstein and Roth, 2001). The kinetic mechanisms of these acetyltransferases vary. While GNATs form a ternary complex allowing a direct transfer of the acetyl group, the MYST family can either use this ternary complex mechanism or utilize a ping-pong mechanism that generates an acetylated enzyme intermediate (Yan et al., 2002). In contrast to these larger families, the p300/CBP family has been shown to use a sequential mechanism called the Theorell-Chance mechanism (Liu et al., 2008). Since GNATs are the predominant family present in bacteria, we will focus on them.
The GNATs are present across bacterial phylogeny (Neuwald and Landsman, 1997) and modify a wide range of substrates, including not only proteins, but also small molecules or metabolites (Shaw et al., 1993; Davies and Wright, 1997; Vetting et al., 2005). GNATs are generally small proteins that bind acetyl-CoA (AcCoA). They have low sequence identity, but share a characteristic fold by which they are identified (Favrot et al., 2016). The number of GNATs contained in a given genome can vary wildly with Listeria monocytogenes containing ∼14 GNATs, but Streptomyces lividans containing ∼72 GNATs (Hentchel and Escalante-Semerena, 2015).
The lysine acetylation reaction commonly occurs in a ternary complex between the substrate, AcCoA, and the GNAT, or more specifically KAT. There are three key steps to this acetylation reaction: (1) deprotonation of the target lysine, (2) nucleophilic attack of the lysine on the carbonyl carbon of the acetyl group of AcCoA, and (3) protonation and dissociation of CoA (Vetting et al., 2005). Within the catalytic pocket of the KAT, there is often a catalytic glutamate that acts as a general base to deprotonate the target lysine. In the namesake Gcn5 enzyme of yeast, this glutamate is E173, which can be found conserved in many of the bacterial GNATs (Tanner et al., 1999; Trievel et al., 1999; Liszczak et al., 2011) and some MYST family members in eukaryotes (Yan et al., 2002; Berndsen et al., 2007). However, in the absence of this conserved glutamate, it is hypothesized that a series of residues form a water channel or “proton wire” whereby protons are carried away from active site to other residues or to the solvent (Dyda et al., 2000). Once the lysine is deprotonated, it can perform a nucleophilic attack on the carbonyl carbon of AcCoA, which is bound to the KAT via a positively charged patch that coordinates the CoA moiety via its pyrophosphate and pantothenate moieties (Dutnall et al., 1998; Lin et al., 1999; Tanner et al., 1999; Marmorstein and Roth, 2001; Liu et al., 2008). The newly acetylated substrate dissociates from the KAT, but for the CoA to dissociate, it must first be protonated. Often, a tyrosine in the active site donates a proton to the thiolate of CoA.
In bacteria, GNATs were first characterized as aminoglycoside N-acetyltransferases (Wright and Ladak, 1997; Wolf et al., 1998). Except those that belong to the YopJ effector protein superfamily (Lewis et al., 2011; Ma and Ma, 2016), currently every KAT discovered in bacteria belongs to the GNAT family (Latrasse et al., 2008; Thao and Escalante-Semerena, 2011; Dancy and Cole, 2015). In E. coli, five KATs have been identified with YfiQ (also called Pat, PatZ, or Pka) being the most heavily studied (Wang et al., 2010). Homologs of YfiQ exist in many bacterial species, as described below, but additional KATs with distinctly different structures have been identified, including homologs of the other KATs in E. coli: RimI, YiaC, YjaB, and PhnO (Christensen et al., 2018).
Deacetylases in Bacteria
To make a PTM versatile and energetically efficient, the ability to add and remove the PTM is essential. Lysine acetylation can be reversed via the action of a lysine deacetylase (KDAC). Two types of deacetylases are known: NAD+-dependent sirtuins (Blander and Guarente, 2004) and Zn2+-dependent deacetylases (Marmorstein, 2001; Gregoretti et al., 2004; Yang and Seto, 2008b; Lombardi et al., 2011). Putative homologs of both families are encoded by bacteria, but only a few have been shown to function as true deacetylases (Hildmann et al., 2007; Hentchel and Escalante-Semerena, 2015).
The Zn2+-dependent deacetylases are simple hydrolases that cleave the acetyl group from the lysine to release acetate. The reaction requires that a conserved histidine residue act as a general base to activate a metal-bound water that attacks the carbonyl of the acetyl group. This class of deacetylase has yet to be found in E. coli, but AcuC of Bacillus subtilis (Gardner et al., 2006) and LdaA of Rhodopseudomonas palustris (Crosby et al., 2010) are family members. Enzymes of the hydrolase family can be inhibited by the addition of butyrate (Davie, 2003).
Sirtuins bind NAD+ through a Rossmann fold domain (Sanders et al., 2010) and use the NAD+ as a co-substrate to remove acetyl groups from proteins; the products are then deacetylated lysine, nicotinamide (NAM) and 2′-O-acetyl-ADP-ribose. Since NAD+ levels are linked to nutritional status of the cell, it is thought that sirtuins allow the cell to recognize these changes and adjust acetylation accordingly (Wimpenny and Firth, 1972; Frye, 2000). Furthermore, sirtuins may regulate their own activity by producing NAM. NAM inhibits non-competitively by condensing with an ADP-ribose-like intermediate formed during the deacetylation reaction; this condensation blocks the progression of the reaction (Denu, 2003) at physiological levels (Wimpenny and Firth, 1972; Bitterman et al., 2002; Jackson et al., 2003; Sauve and Schramm, 2003; Zhao et al., 2004; Avalos et al., 2005). NADH also competitively inhibits the reaction at physiological levels (Schmidt et al., 2004; Guarente, 2005; Porcu and Chiarugi, 2005).
In many members of the Enterobacteriaceae, the sirtuin CobB exists as two isoforms. In Salmonella enterica and E. coli, a shorter isoform (CobBS) and the longer isoform (CobBL) are transcribed from two different transcription start sites (Tucker and Escalante-Semerena, 2010; Umehara et al., 2018). These isoforms differ by an N-terminal amphipathic stretch of 37 amino acids. Though the purpose of this N-terminal extension is unknown, both forms of the CobB protein were shown to be biologically active as deacetylases in S. enterica (Tucker and Escalante-Semerena, 2010).
While sirtuins are classically thought of as deacetylases, it appears that they may have a broader specificity than originally thought. Sirtuins in the mitochondria have been reported to desuccinylate (Du et al., 2011; Colak et al., 2013), demalonylate (Du et al., 2011; Peng et al., 2011; Park et al., 2013), dipropionate (Smith and Denu, 2007), deglutarylate (Tan et al., 2014), decrotonylate (Bao et al., 2014), and debutyrylate (Smith and Denu, 2007) lysines in addition to deacetylate. Certain human sirtuins, such as SIRT6, have a preference for removal of long-chain acyl groups from lysines. These long-chain acyl groups can be a long as 16 carbons, as in the case of palmitoylation (Feldman et al., 2013). Sirtuins of archaeon Archaeoglobus fulgidus have similarly been shown to desuccinylate and demyristoylate peptides (Ringel et al., 2014). Bacterial sirtuins have similar abilities to desuccinylate (Colak et al., 2013), dipropionate (Garrity et al., 2007; Sun et al., 2016b), and possibly debutyrylate (Xu et al., 2018a) lysines. Many of these PTMs occur on the same lysine, possibly implicating sirtuins as a general cleanup enzyme for certain lysines. Further complicating the possible role of sirtuins, there is evidence that sirtuins can ADP-ribosylate proteins using NAD+ as a substrate (Frye, 1999).
Effects of Enzymatic Acetylation/Deacetylation on Physiology and Pathogenesis
Several studies have explored the role of enzymatic acetylation on bacterial physiology, often through reverse genetics approaches where the deacetylase, acetyltransferase, or both are deleted or overexpressed. In this section, we will focus on CobB and YfiQ of E. coli and their homologs in S. enterica, as they have been studied the most extensively.
The earliest and thus most comprehensively understood roles for YfiQ and CobB are their opposing effects on the acetylation status of Acs, an enzyme that irreversibly converts acetate into AcCoA via two half reactions. Acs requires lysine 609 (K609) for the first half-reaction, which forms an acetyl-AMP intermediate (Starai et al., 2002). Thus, mutation or modification of this residue leads to a catalytically inactive enzyme (Starai et al., 2002; Starai and Escalante-Semerena, 2004a,b; Sun et al., 2016b). Indeed, an epistasis analysis of Δacs, ΔcobB, and ΔyfiQ mutants showed that regulation of Acs is essential for growth on acetate. In both S. enterica and E. coli, Δacs mutants cannot grow on less than 10 mM acetate as the sole carbon source. This is due to the low affinity of the alternative Pta-AckA pathway for acetate. A ΔcobB mutant phenocopies a Δacs mutant, while the double ΔyfiQΔcobB or ΔpatΔcobB mutants can grow on less than 10 mM acetate (Starai et al., 2003; Castaño-Cerezo et al., 2011). These results confirm that YfiQ/Pat-dependent acetylation of Acs shuts down acetate utilization through Acs and that deacetylation by CobB restores that activity (Starai et al., 2002; Castaño-Cerezo et al., 2011). Acs was found to be regulated in the exact same fashion in more than 15 organisms tested, ranging from bacteria to humans (Table 1) (Kim and Yang, 2011). Beyond Acs, this reversible lysine acetylation has been extended to other AMP-forming acyl-CoA synthetases, which are proteins that share a similar structure and the same conserved acetylated lysine found in Acs. R. palustris possesses 40 of these Acs-like proteins; 10 of them are reversibly acetylated on this conserved lysine (Crosby et al., 2010, 2012). As in Acs, acetylation inactivates these enzymes.
Other proteins have been shown to be targets of both YfiQ and CobB. For example, K501 of RNase II from E. coli is a target of YfiQ and CobB (Song et al., 2016). Acetylation of K501 by YfiQ inhibits substrate binding, which results in reduced exoribonuclease activity. Treatment of RNase II with CobB restores this activity. YfiQ and CobB also reversibly acetylate K201 of PhoP from S. enterica. A member of a two-component regulatory system that responds to low Mg2+ and acid stress, Pat-dependent acetylation of K201 decreases the affinity of PhoP for its DNA binding site and thus reduces expression of PhoP-regulated genes (Ren et al., 2015, 2016). CobB restores DNA binding by deacetylating PhoP. Finally, the highly conserved global transcriptional regulator Lrp (Brinkman et al., 2003; Martínez-Antonio and Collado-Vides, 2003) has been shown to be acetylated on K36 in S. enterica, which prevents DNA binding (Qin et al., 2016). CobB treatment permits DNA binding of Lrp.
While the mechanistic role of reversible lysine acetylation on Acs and a few other proteins is established, multiple phenotypes have been found for Δpat and ΔcobB mutants in S. enterica that are not directly attributed to acetylation of a specific enzyme, but are consistent with the opposing effects of these enzymes. For example, compared to wild-type cells, a Δpat mutant of S. enterica grows more slowly on minimal glucose but more rapidly on minimal citrate, whereas a ΔcobB mutant has the exact opposite phenotype (Wang et al., 2010). The Δpat mutant has reduced flux through glycolysis and the TCA cycle, while a ΔcobB mutant again has the opposite phenotype and promotes gluconeogenesis (Wang et al., 2010). For the latter phenotype, a ΔcobBΔpat double mutant behaves like a Δpat mutant, suggesting that CobB is dispensable in the absence of acetylation via Pat, as expected if the role of CobB was simply to deacetylate Pat-dependent acetylations. Taken together, these results support the hypothesis that Pat and CobB somehow regulate central metabolism. However, the Pat- and CobB-sensitive lysines responsible for these behaviors are not known. Acetylation by Pat was reported on isocitrate lyase (AceA), isocitrate dehydrogenase (Icd), and isocitrate dehydrogenase kinase/phosphatase (AceK), and this acetylation was shown to reduce activity for each protein (Wang et al., 2010); however, these results were called into question when another group could not confirm these findings (Crosby et al., 2012).
YfiQ/Pat, CobB, and their homologs also appear to be involved in certain stress responses. In contrast to the metabolic examples above, some of these effects might occur by modifying different lysines, as deletion of either enzyme sometimes does not result in opposite phenotypes. For example, when exposed to acid stress, both E. coli and Yersinia pestis have reduced survival when either yfiQ or cobB are deleted; in contrast, S. enterica survives better when pat is deleted and more poorly when cobB is deleted (Castaño-Cerezo et al., 2014; Ren et al., 2015; Liu et al., 2018). When exposed to heat stress, survival of E. coli diminishes when yfiQ is deleted but increases when cobB is deleted (Ma and Wood, 2011). In contrast, Y. pestis survival is diminished in both ΔcobB and ΔyfiQ mutants when exposed to heat, cold, or high salt stress (Liu et al., 2018). When exposed to reactive oxygen species, the E. coli ΔcobB mutant has enhanced survival, whereas the ΔyfiQ mutant behaves like its wild-type parent. In a more distantly related organism, M. tuberculosis, loss of its sirtuin (Rv1151c) promotes growth in acidic pH (Bi et al., 2017). Finally, the acetyltransferase PatA in Mycobacterium smegmatis acetylates the Universal Stress Protein (MSMEG_4207) (Nambi et al., 2010). Taken together, the results of these studies suggest a common role for acetylation in stress responses. The pleiotropic phenotypes of ΔyfiQ/pat and ΔcobB mutants provide evidence that the lysines they acetylate and deacetylate control a diverse set of general stress pathways.
The Regulator of Capsule Synthesis B (RcsB) can be reversibly acetylated through YfiQ and CobB in vitro. RcsB, a member of the two-component response regulator family, is a global transcriptional regulator of E. coli and S. enterica, regulating approximately 5 and 20% of their respective genomes (Prüss et al., 2006; Wang et al., 2007). RcsB promotes capsule biosynthesis and biofilm formation, while impairing transcription of genes required for motility. In E. coli and S. enterica, RcsB is acetylated on multiple residues. One of those lysines (K180) can be acetylated in vitro in a YfiQ/Pat-dependent manner and deacetylated by CobB (Thao et al., 2010). K180 is found in the RcsB DNA binding motif, and acetylation of this residue reduces DNA binding, which can then be reversed by CobB. However, there is no evidence to support YfiQ/Pat as the cause of K180 acetylation in vivo, as mutants that delete these KATs have no obvious motility defect (Hu et al., 2013). In contrast, two independent studies found CobB to be an inhibitor of migration in E. coli (Castaño-Cerezo et al., 2014; Gallego-Jara et al., 2017). A ΔcobB mutant has more numerous and longer flagella than the parent strain, which could partially be explained by the ability of CobB to deacetylate RcsB (Gallego-Jara et al., 2017).
Pat has been strongly implicated in S. enterica survival and infection. Pat enhances invasion of the bacteria into HeLa cells and enhances survival of the bacteria within macrophages (Sang et al., 2016). Pat also helps promote survival under acid stress, which may be partially responsible for the macrophage survival phenotype (Ren et al., 2015). Importantly, expression of the Salmonella pathogenicity island (SPI-1) that is required for virulence is enhanced by Pat (Sang et al., 2016). SPI-1 is controlled by transcription factor HilD, and acetylation on K297 of HilD by Pat was suggested to stabilize the protein, which could enhance SPI-1 transcription. The role of Pat in virulence was further established by infecting mice with Δpat and ΔcobB mutants. The Δpat mutants were hypovirulent and caused reduced mortality, implicating Pat and therefore acetylation as necessary for infection (Sang et al., 2016). However, ΔcobB mutants had little effect. The effect by Pat might be due, at least in part, to its ability to acetylate of K201 of PhoP, as a K201Q variant that mimics the constitutively acetylated isoform were hypovirulent (Ren et al., 2016). Like S. enterica, a ΔyfiQ mutant of Yersinia pestis, the causative agent of plague, is hypovirulent in mice (Liu et al., 2018), but unlike S. enterica, the ΔcobB mutant is equally attenuated.
The above examples demonstrate how mechanistically similar machinery has evolved in different organisms to suit their specific niches. In many cases, YfiQ/Pat and CobB act antagonistically to reversibly acetylate a specific lysine. However, in response to certain stresses, loss of either YfiQ/Pat or CobB can be detrimental to the survival of the bacterium. The pathogenicity data from S. enterica and Y. pestis suggest that, while some commonalities may exist between the requirements for acetylation in certain scenarios, there are many cases where YfiQ/Pat and CobB do not always act in opposition. These observations provide strong evidence that these enzymes can and do act independently and should not be considered a system.
Non-Enzymatic Acetylation Via AcP and AcCoA
Whereas KAT-catalyzed acetylation has been known for some time, evidence has accumulated showing that non-enzymatic acetylation is also possible. In vitro, AcCoA and the bacterial metabolite acetyl phosphate (AcP) can non-enzymatically acetylate lysine residues on many proteins. In vivo, the impact that AcP has on the acetylome has been assessed in multiple bacteria. In the cases of E. coli (Weinert et al., 2013a; Kuhn et al., 2014), B. subtilis (Kosono et al., 2015; Suzuki et al., 2019), and Neisseria gonorrhoeae (Post et al., 2017), AcP is the predominant acetyl donor.
Chemical reactions are the basis of all life. While enzymatic catalysis is a primary driver in the kinds of substrates used and products formed, the possible chemical reactions of those substrates and products greatly exceeds those that are catalyzed (Golubev et al., 2017). Many of these uncatalyzed reactions are thought to be deleterious to the cell, causing macromolecular damage, such as oxidation of DNA or proteins. Often, the damage can be caused by products of the cell’s own metabolism, such as reactive oxygen species (Madian and Regnier, 2010). Indeed, non-enzymatic modification has been implicated in multiple human diseases (Brownlee, 2005). As cells have evolved alongside this damaging potential of their own metabolism, it is not surprising that repair pathways exist to mitigate this damage. Perhaps cells have evolved ways to utilize these extra-metabolic occurrences as means to regulate their cellular processes. Specifically, in this context, we will consider acetylation via two acetyl donors, AcCoA and AcP.
The ability of AcCoA and AcP to non-enzymatically modify proteins is not a new idea. AcP has been shown to chemically acetylate amine, thiol, and hydroxyl groups (Di Sabato and Jencks, 1961; Jencks, 1969). In the 1970s, it was discovered that AcP could non-enzymatically acetylate histones, albumin, and polylysine (Ramponi et al., 1975). Due to its high energy nature (ΔG° of -43.1 kJ/mol), which is even greater than that of AcCoA (ΔG° of -31.4 kJ/mol), AcP readily hydrolyzes in water and can acetylate without the need for an enzyme (Koshland, 1952; Voet and Voet, 1990). Furthermore, the concentrations needed to achieve non-enzymatic acetylation in vitro are comparable to or below physiological levels, which in E. coli are around 300–500 μM for AcCoA and between 200 μM and 3 mM for AcP (Vallari and Rock, 1985; Klein et al., 2007; Ramos-Montañez et al., 2010). In vitro acetylation reactions are often carried out at basic pH. Basic pH promotes deacetylation of lysine, which may prime the lysine for acetylation, but also increases the rate at which AcP and AcCoA dissociate (Koshland, 1952; Di Sabato and Jencks, 1961). Susceptibility to non-enzymatic acetylation differs for each lysine and each acetyl donor used. Using glutamate dehydrogenase (GDH) as an example, it was shown a single lysine (e.g., K503) could be acetylated at different rates using two different acetyl donors, AcP and AcCoA. When comparing acetylation by a single acetyl donor, multiple lysines (e.g., K110, K415, and K503) on GDH were acetylated at different rates from one another. These in vitro acetylation reactions with AcP and AcCoA showed that acetylation rates of these specific lysines can vary by three orders of magnitude. Furthermore, some lysines were completely non-reactive under these conditions. These findings suggest that regulation of acetylation depends on the environmental context and thus reactivity of the lysine (Baeza et al., 2015).
Assessment of non-enzymatic acetylation in vivo by AcCoA is difficult. In most cases, it is an essential molecule that if depleted would result in lack of viability. However, attempts to recreate the mitochondrial environment have shown that AcCoA could acetylate proteins at physiological levels in vivo (Wagner and Payne, 2013). Furthermore, attempts to reduce the levels of AcCoA, such as by draining acetyl groups into an acetyl group polymer such as polyhydroxybutyrate, do not avoid any contribution by KATs (Hu, 2013). Work in an organism where AcCoA can be non-essential, such as the reduced genome bacterium Borrelia burgdorferi that solely synthesizes AcCoA to generate mevalonate, a precursor of cell wall lipid I, might be a useful reductionist system to determine whether this truly occurs in vivo (Bontemps-Gallo et al., 2018). Without knowing all possible KATs and eliminating them, it is hard to truly ever say whether an AcCoA-dependent acetylation is truly non-enzymatic in vivo.
Fortunately, modulation or complete elimination of AcP levels is much simpler and less detrimental to many bacteria, which greatly facilitates studying AcP-dependent acetylation. In E. coli and many other bacteria, AcP is generated as an intermediate of the acetate fermentation pathway catalyzed by the reversible enzymes acetate kinase (AckA) and phosphotransacetylase (Pta) (Rose et al., 1954). Pta catalyzes the conversion of AcCoA and inorganic phosphate to AcP and free CoA. AckA then converts AcP into acetate by transferring the phosphoryl group to ADP to generate one molecule of ATP (Figure 2). In the presence of high acetate concentrations (>10 mM), the pathway can run in reverse, synthesizing AcCoA from acetate, ATP, and CoA (Brown et al., 1977; Kumari et al., 1995). By genetically manipulating this pathway, the levels of AcP and thus acetylation can be altered. For example, if cells are grown on glucose, the cells ferment acetate and generate AcP as an intermediate. If one prevents the conversion of AcCoA to AcP by deleting Pta or the entire Pta AckA pathway, AcP cannot be generated. When these mutants are analyzed by western blot, the intensity of the acetylated proteins is greatly reduced to background levels (Weinert et al., 2013a; Kuhn et al., 2014). It is worth noting that acetylation can still be detected by mass spectrometry in Δpta and Δpta ackA mutants, but the relative amount of acetylation and number of acetylated lysines is greatly reduced. On the other hand, by deleting ackA, AcP accumulates to 10–20 mM (Klein et al., 2007; Wolfe, 2010; Weinert et al., 2013a), which can be seen via western blot as a greatly intensified acetylation profile relative to wild-type cells (Weinert et al., 2013a; Kuhn et al., 2014). Mass spectrometry confirms that many more unique lysines on many more unique proteins become acetylated in this mutant, and those lysines that are detected as acetylated in wild-type become further acetylated as the proportion of the total pool of each unique peptide shifts toward acetylated (Weinert et al., 2013a; Kuhn et al., 2014).
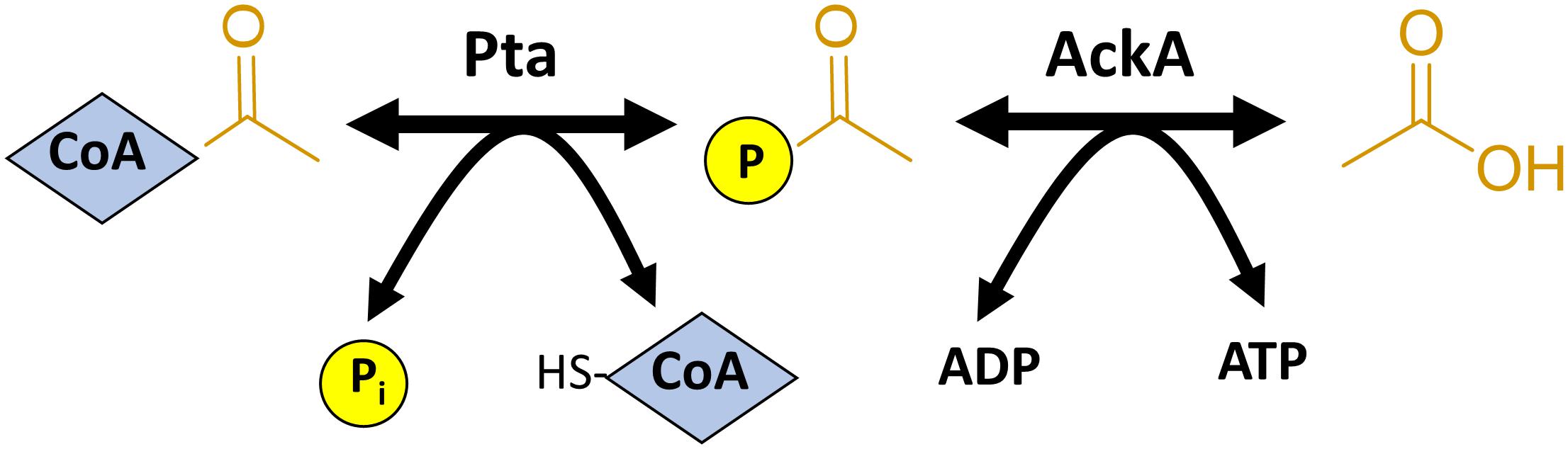
Figure 2. The Pta-AckA acetate fermentation pathway generates AcP as an intermediate. Phosphotransacetylase (Pta) converts AcCoA to AcP by substituting CoA for inorganic phosphate (Pi). Acetate kinase (AckA) converts AcP to acetate, generating an ATP in the process. This pathway is reversible.
Targets of Non-Enzymatic, AcP-Dependent Acetylation
To date, only a handful of groups have tried to directly determine the impact of acetylation by AcP on the acetylome of an organism (Weinert et al., 2013a, 2017; Kuhn et al., 2014; Post et al., 2017; Bontemps-Gallo et al., 2018; Reverdy et al., 2018). This has been achieved by mass spectrometry analysis of wild-type cells and comparing the acetylated proteins to acetylated proteins cells where the levels of AcP have been manipulated, usually by deleting ackA to accumulate AcP in cells or by deleting pta to prevent synthesis of AcP. Due to the prevalence of AcP-dependent acetylation, especially under conditions when cells are exposed to high carbon concentrations, much of the acetylation detected in wild type bacteria is likely a result of acetylation by AcP (Schilling et al., 2015).
These studies result in large data sets with hundreds of lysines acetylated from multiple organisms. Often, to get a picture of what processes these acetylated lysines may be involved in, the modified proteins can be grouped by keyword enrichment, gene ontology term enrichment, and Kegg pathway enrichment. Across these studies and grouping strategies, the modified proteins fall into multiple processes, but the two most common are translation and central metabolism.
From the current E. coli data sets, it appears that most of the ribosomal subunits, most of the aminoacyl-tRNA ligases, and all of the elongation factors are acetylated on multiple lysines in an AcP-dependent manner. In contrast, AcP acetylates only one lysine on one initiation factor (Kuhn et al., 2014; Schilling et al., 2015). Whether any of these acetylations impact the rate or fidelity of translation has yet to be determined. However, it has been shown that charging of tRNAs can be inhibited by AcP-dependent acetylation of certain aminoacyl-tRNA ligases (Venkat et al., 2017a; Ye et al., 2017).
Similarly, most central metabolic enzymes are acetylated in an AcP-dependent manner; this is true for all of the glycolytic pathways of E. coli and B. subtilis (i.e., Embden–Meyerhof–Parnas, Pentose Phosphate, and Entner–Doudoroff). Currently, the functional consequences of most of these acetylations remain largely undetermined. However, it is reported that AcP-dependent acetylation abolishes the activity of the glycolytic enzyme enolase from both E. coli and B. subtilis (Nakayasu et al., 2017) and inhibits the activity of glyceraldehyde 3-phosphate dehydrogenase A from Borrelia burgdorferi (Bontemps-Gallo et al., 2018). The TCA cycle is also highly acetylated by AcP. Thus far, malate dehydrogenase is the rare example of a central metabolic enzyme whose activity is enhanced by AcP-dependent acetylation (Venkat et al., 2017b).
Structural Determinants of Non-Enzymatic Acetylation
While non-enzymatic acetylation does not require a KAT, analysis of the environments that contain acetylated lysines suggest that the mechanism by which this enzyme-independent reaction occurs has many parallels with enzymatic acetylation (Figure 3). While the KAT serves as an AcCoA-binding protein to properly position the acetyl group near the target lysine, non-enzymatic acetylation requires that the protein being acetylated coordinates the CoA group for AcCoA or the phosphate moiety for AcP (Kuhn et al., 2014). Often, the coordinating amino acids are positively charged (Arg and Lys), which make ionic bonds, or polar amino acids that make hydrogen bonds via hydroxyl groups (Ser, Thr, and Tyr) or side chain amides (Gln and Asn) (Kuhn et al., 2014). In contrast to KAT-dependent acetylation, where a glutamate from the KAT typically extracts a proton from the lysine to be acetylated, during non-enzymatic acetylation, the non-enzymatic process requires an internal glutamate or a water molecule (Okanishi et al., 2013; Kuhn et al., 2014). It may be expected that lysines acetylated non-enzymatically may have a reduced pKa, and thus more propensity to lose a proton. However, the pKa has not been found to correlate with reactivity (Baeza et al., 2015). Like KATs and KDACs, there is no clear motif that is predictive of whether a lysine is susceptible to chemical acetylation. This was shown by using AcP to acetylate a peptide library with the sequence GXKZGC, where X and Z are each a standard amino acid with the exception of cysteine, on a SAMDI chip. This method showed that almost every peptide could be acetylated, but selectivity and specificity could be achieved by adding magnesium and salt (Kuhn et al., 2014). The SAMDI results indicated adjacent arginines and lysines favored acetylation; in vivo, however, there is a propensity for glutamates and aspartates to be adjacent to the lysines acetylated by AcP. Similar results were found for AcCoA, where glutamates were found near lysines in vivo (Baeza et al., 2015). For both AcP and AcCoA, there is a tendency for susceptible lysines to be surface-exposed (Kuhn et al., 2014; Baeza et al., 2015). However, due to their relatively small size, AcP and AcCoA can access buried lysines that a KAT could not (Kuhn et al., 2014). Thus, protein structures could have evolved to utilize non-enzymatic acetylation for roles that are impossible for enzymatic acetylation.
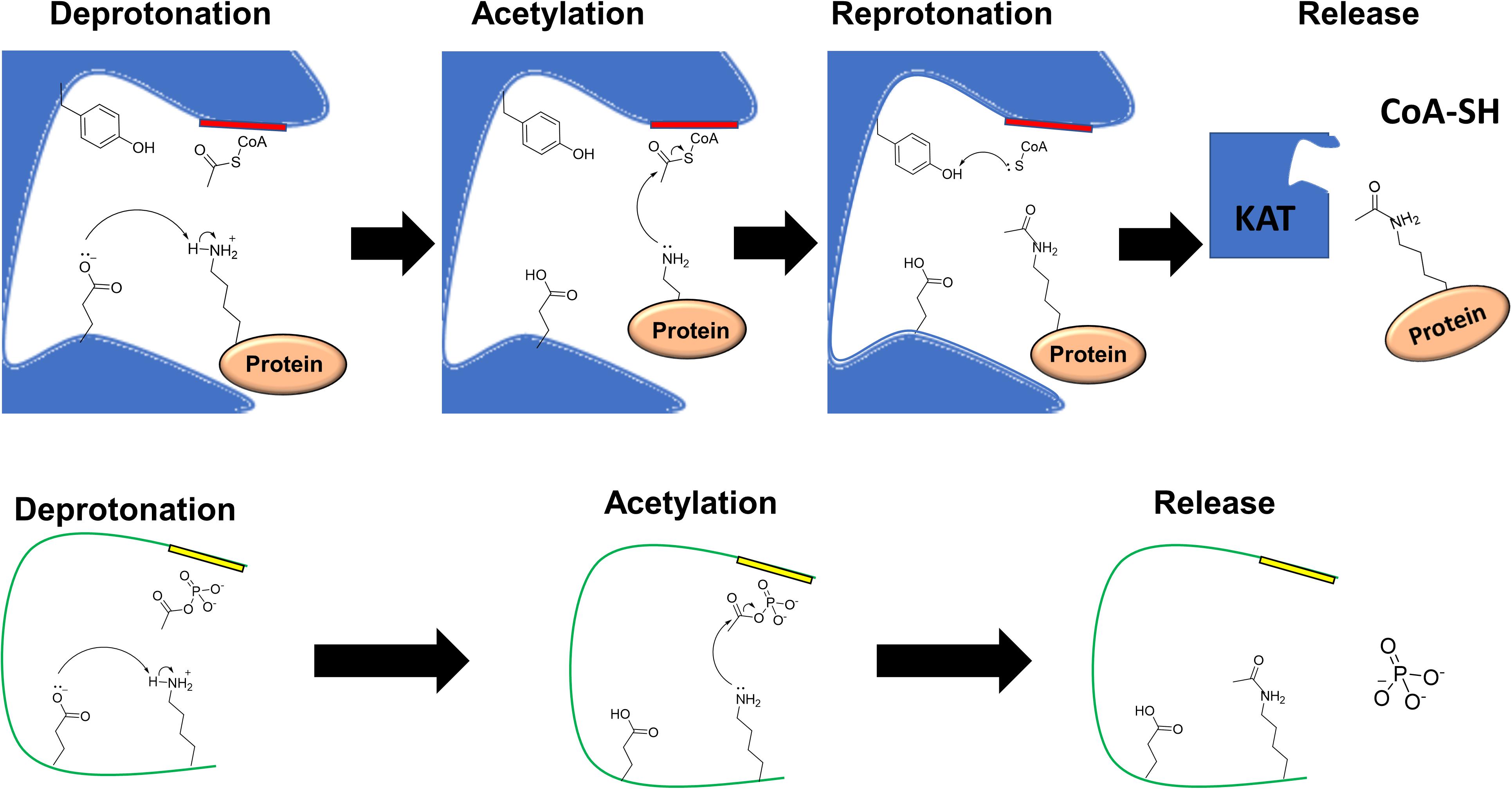
Figure 3. Catalytic mechanisms of enzymatic and non-enzymatic acetylation. (Top) In the enzymatic mechanism, a lysine to be acetylated binds at the acceptor site of a lysine acetyltransferase (KAT) and AcCoA binds at the donor site of the KAT called the P-loop (red) with consensus motif Gln/Arg-x-x-Gly-x-Gly/Ala (Salah Ud-Din et al., 2016). A catalytic glutamate deprotonates the epsilon amino group of the target lysine. The lysine performs a nucleophilic attack on the carbonyl carbon of AcCoA, resulting in acetylation of the lysine. The CoA group becomes protonated by a tyrosine, which regenerates the KAT and facilitates the release of the free CoA and the target protein. (Bottom) In this example of non-enzymatic acetylation, AcP is bound through its negatively charged phosphoryl group to a neighboring patch (yellow) that contains positively charged residues and/or residues that can form hydrogen bonds. In this example, the lysine is deprotonated by a glutamate on the same protein. The lysine performs a nucleophilic attack on the carbonyl carbon of AcP resulting in an acetyllysine. Inorganic phosphate is released as a byproduct of the reaction. A mechanism similar to this can be considered for non-enzymatic AcCoA-dependent acetylation.
Regulation of AcP-Dependent Acetylation – a Consequence of Fermentation
When the glucose consumption rate depletes free CoA faster than it can be recycled, E. coli will ferment acetate from AcCoA via the Pta-AckA pathway (Figure 4). This occurs even in the presence of oxygen in a process called overflow metabolism, akin to a safety valve that regenerates the limiting pools of CoA (Hollywood and Doelle, 1976; Andersen and von Meyenburg, 1980). However, the production of AcP by Pta is faster than the conversion of AcP to acetate by AckA, leaving a pool of the intermediate AcP (Klein et al., 2007; Keating et al., 2008). Thus, when E. coli cells are grown in the presence of excess carbon such as glucose, the bacteria ferment acetate and produce AcP. Thus, ameliorating the need for cells to ferment reduces acetylation. Such an example is when cells lacking the major glucose transporter EIICBglc are grown in medium supplemented with glucose. These mutants must assimilate glucose through alternative glucose transporters such as the galactose permease GalP. The use of these alternative transporters reduces the rate of glucose flux into the cell. As such, the cells also produce less acetate and, as expected, have reduced acetylation (Steinsiek and Bettenbrock, 2012; Schilling et al., 2015).
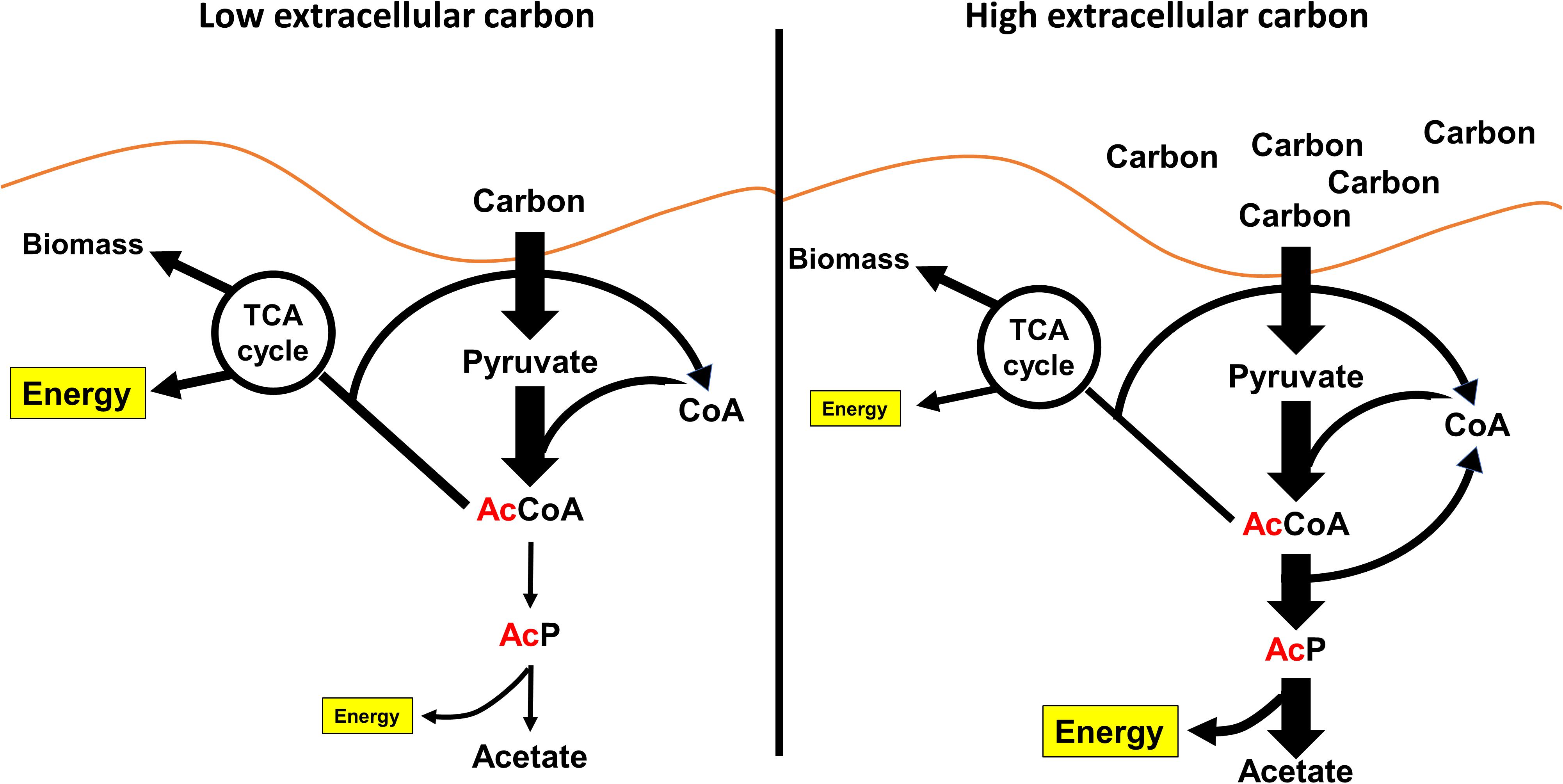
Figure 4. AcP is made as a consequence of overflow metabolism. (Left) When bacteria like E. coli are growing on low concentrations of carbon, much of the carbon is put into the TCA cycle, lipid metabolism, and metabolite biosynthesis. Acetate production is very low. (Right) When the concentration of carbon increases, the flux of carbon through glycolysis depletes the limiting pool of free CoA. To continue to consume carbon, E. coli can regenerate free CoA by fermenting acetate. This results in the production of AcP.
This phenomenon is not exclusive to glucose. Lactate, fructose, and xylose can also potently induce acetylation in E. coli as assessed by anti-acetyllysine western blot (Schilling et al., 2015, 2019; Christensen, 2019). Mass spectrometric analysis has determined the acetylomes of cells grown in xylose, a pentose sugar, and cells grown in glucose, a hexose sugar (Schilling et al., 2019). Glucose and xylose are catabolized through different metabolic pathways; however, each pathway results in acetate fermentation. As such, it may not be surprising that, regardless of carbon source, almost every acetylated lysine was similarly regulated in glucose or xylose. The one exception was xylose isomerase, a protein necessary for catabolism of xylose, which had two lysines differentially acetylated in glucose and xylose. These results suggest that cells regulate AcP-dependent acetylation simply by regulating overflow metabolism and production of AcP.
One other key physiological shift that causes accumulation of acetylations is cessation of growth, such as entry into stationary phase. For both E. coli and N. gonorrhoeae, acetylation increased as the culture entered stationary phase either naturally or when artificially induced (Weinert et al., 2013a; Kuhn et al., 2014; Schilling et al., 2015; Post et al., 2017). We recently determined why acetylation accumulates after E. coli cultures enter into stationary phase. E. coli cultures grown in glucose-supplemented peptide-based media consume both amino acids and glucose during exponential growth; however, most of the glucose is consumed after the culture enters into stationary phase (Christensen et al., 2017).
Compared to exponential phase, two facets of stationary phase make it the perfect growth phase to promote acetylation when excess carbon is present: reduced protein synthesis and reduced respiration. In stationary phase, rates of growth cannot be sustained due to depletion of a limiting nutrient (Reeve et al., 1984). Thus, cells reduce rates of transcription and translation, which greatly reduces the generation of new proteins (Navarro Llorens et al., 2010). Since the rate of protein biosynthesis is reduced, fewer TCA cycle intermediates are used to generate new biomass. This will cause a bottleneck at the TCA cycle, and carbon must enter other pathways. In conjunction with reduced translation, total cellular protein becomes further reduced due to increased protein turnover. When E. coli enters stationary phase, aerobic metabolism is repressed (Nyström, 2004; Navarro Llorens et al., 2010). As such, the carbon flux is driven toward fermentation. The combination of a reduced proteome in stationary phase and the continued metabolism of a fermentable carbon source increase the probability that any susceptible lysine will become acetylated.
In conclusion, data thus far suggests that non-enzymatic AcP-dependent acetylation is an unavoidable consequence of fermentation that accumulates on an aging proteome. Since AcP is made as an intermediate of fermentation, the only means of regulating acetylation would be by regulating fermentation, deacetylation of acetyllysines with a KDAC, and/or evolving protein structures to optimally favor/disfavor acetylation of specific lysines. Because acetate fermentation has evolved as a key mechanism that allows many bacteria to regenerate the limited pool of CoA, this would suggest that E. coli has evolved over millions of years to cope with or utilize this global modification.
A Possible Evolutionary History of Acetylation and Because of Acetylation
Because nearly all life has evolved to utilize activated acetate as a key molecule in metabolism, all organisms must inevitably cope with the potential for protein acetylation. To understand the physiological relevance of acetylation, it is helpful to first imagine how acetylation evolved. As proposed previously, AcP is a relatively simple molecule compared to ATP or AcCoA with higher energy, and as such is likely to be a more ancient energy currency (Ninfa et al., 2000; Wolfe, 2010). Due to the well-understood effects of phosphorylation by AcP, it was proposed that proteins like response regulators could have evolved to accept phosphoryl groups from AcP, and only later would the cell have evolved phosphatases to remove those phosphoryl groups. Finally, some phosphatases would evolve to become kinases to fine-tune these pathways (Wolfe, 2010).
Similarly, the ability for AcP to acetylate proteins also could be a driver of evolution. Acetylation of a catalytic lysine can be extremely detrimental to protein function, and inability to remove these acetylations could result in a dead enzyme. Thus, cells must have evolved deacetylases to ensure that a given lysine would be unmodified despite the presence of endogenously produced reactive metabolites. Contrary to the phosphorylation example, the acetyltransferases are not homologous to the deacetylases and must have evolved independently. Perhaps these acetyltransferases evolved after the development of their primary substrate, AcCoA.
Each acetylated lysine has the potential to be regulatory. Whether these acetylations are due to non-enzymatic mechanisms or catalyzed enzymatically, life must balance the possibility of detrimental acetylation with benign or beneficial acetylations. The best ways to ensure that only optimal lysines are acetylated under a given condition would be to either (1) evolve a lysine deacetylase to remove deleterious modifications or (2) evolve proteins such that they do not have critical lysines susceptible to acetylation. For E. coli, almost all acetylated linear peptides are susceptible to CobB (AbouElfetouh et al., 2015), but not all acetylated E. coli proteins are CobB-sensitive in vivo (Kuhn et al., 2014). The CobB-sensitive lysines tend to be surface-exposed (AbouElfetouh et al., 2015). Indeed, for a lysine to be deacetylated, it almost certainly cannot be buried within the protein structure, unless the acetylated residue becomes accessible through “protein breathing,” large scale conformational changes of the protein structure (Kossiakoff, 1986). However, the extent of breathing in vivo is probably restricted due to molecular crowding in the cytoplasm (Makowski et al., 2008). Thus, the protein must be structured in such a way to favor deacetylation of the target lysine without disrupting the activity of the protein.
If an acetylated lysine blocks protein function and is not accessible to CobB or another deacetylase, the cell must replace that protein by degrading it and synthesizing an entirely new protein. Not all lysines can be acetylated, but those that do become modified can be acetylated at different rates (Baeza et al., 2015; Schilling et al., 2015). As buried lysines would be refractory to CobB, one would also expect that acetylation of these lysines is rarely enzymatic due to the inability for an enzyme to access the lysine. Thus, evolution may have selected for molecular environments that disfavor acetylation of these buried and other critical lysines. However, any changes made over evolution that would disfavor acetylation had to be balanced with a protein sequence that permits proper folding and activity.
Future Thoughts
The study of acetylation is a fast growing field with technological advances in analytical methodologies, such as mass spectrometry, providing a greater depth of analysis. The report of each new acetylome opens more avenues to explore. While some acetylations are certain to be organism-specific, there are bound to be many conserved acetyllysines in critical processes, as we have already observed in central metabolism and translation. Each of these acetyllysines must result from one or more of the recognized acetylation mechanisms, either enzymatic or non-enzymatic. In turn, these mechanisms may themselves be regulated dynamically with the potential for signaling cascades akin to phosphorylation.
Researchers in this new field have two main routes that they can follow. They can investigate the effect of acetylation on individual sites on individual proteins or they can study the effect of global acetylation. The former route can take advantage of the numerous acetylome studies that have yielded data sets containing hundreds of acetylated proteins modified on thousands of unique lysines. The problem is how to prioritize the most functionally relevant acetylation sites. Typically, researchers prioritize by choosing lysines in provocative locations on proteins with easily assessed phenotypes or activities. However, it should be noted that genetic mimic/genetic ablative mutants are often used to compare extreme stoichiometries, i.e., a mimic of the 100% acetylated state versus a mimic of the 100% unacetylated state. The same is true of proteins acetylated by non-canonical amino acid substitution.
Another way to prioritize is to determine the stoichiometry of each acetylated lysine (Baeza et al., 2014; Nakayasu et al., 2014; Weinert et al., 2015, 2017; Meyer et al., 2016; Miyagi, 2017; Wei et al., 2018). A lysine with a large fold change but a low basal stoichiometry may be a less physiologically important acetylation event compared to one with a smaller fold change but a higher basal stoichiometry. Unfortunately, obtaining the stoichiometry of the acetylome is a non-trivial technical challenge for all but a few groups. To make matters worse, most of the existing analyses were performed on highly mutagenized laboratory strains grown under conditions that make physiologically relevant interpretations difficult.
Whereas site-specific or protein-specific investigations can provide lessons concerning the manner in which acetylation influences protein structure/function relationships, such focused studies cannot explain the global effect of simultaneous low stoichiometric acetylation of large numbers of lysines, as occurs by non-enzymatic acetylation. These acetylations greatly expand the acetylome, neutralizing thousands of positive charges, yet the impact of these modifications remains unclear. Furthermore, acetylation is not the only global modification. The proteome can be acylated using many other central metabolites that may compete for the same lysines (Peng et al., 2011; Zhang et al., 2011; Colak et al., 2013; Wagner and Payne, 2013; Weinert et al., 2013b; Kosono et al., 2015; Singhal et al., 2015; Yang et al., 2015; Meyer et al., 2016; Mizuno et al., 2016; Sun et al., 2016b; Basisty et al., 2018; Gaviard et al., 2018; Vasileva et al., 2018; Wei et al., 2018). The potential crosstalk amongst these acylations and between these acylations and other PTMs presents an important challenge as we attempt to truly understand the inner workings of the cell.
Since lysines can be acylated without the use of an enzyme, we would suspect that bacteria would have evolved to utilize this otherwise “wasteful” process. We propose that global acylation may serve many functions. For example, acylations may serve as a feedback mechanism to slow an excessive carbon flux. Acylations could protect proteins against damaging modifications, such as carbonylation, that result in protein misfolding and aggregation (Dalle-Donne et al., 2003; Maisonneuve et al., 2008). Acylations may be less severe modifications that could protect proteins against such harmful lesions. It may also be possible for acetylation to serve as a method for cells to store two carbon subunits. While there are designated carbon storage polymers, such as starch in plants or glycogen in animals, fungi, and bacteria, perhaps acetylation is a primordial form of carbon storage.
Finally, researchers should remember that heterologous expression of a protein in E. coli or any other bacterium carries the possibility that the purified protein preparation may be a heterogeneous mixture decorated by various acylations. Indeed, at least one group has reported that recombinant insulin produced in E. coli is acetylated (Szewczak et al., 2015). The potential for acylations to influence efficacy has great implications for the pharmaceutical industry, but also for any researcher who uses a heterologous system to express their protein of interest. Depending on the protein and the conditions under which the host bacteria are grown, the modified protein may behave in a manner that is not consistent with expectations.
Author Contributions
DC, BS, and AW outlined the review. DC wrote the first draft and designed the figures. XX, NB, JB, and SM helped to complete the review, especially sections focused on structural biology and mass spectrometry. AW did the final edit. BS and AW oversaw the entire process of this study.
Funding
We would like to acknowledge our funding as follows: R01 GM066130 (NIGMS to AW), R01 AI108255 (NIAID subcontract to AW), and R01 AI108255 (NIAID to BS). We also acknowledge support from the NIH shared instrumentation grant for the TripleTOF system at the Buck Institute (1S10 OD016281). The Life Science Biomedical Technology Research resource was primarily supported by the National Institutes of Health, the National Institute of General Medical Sciences (NIGMS) through a Biomedical Technology Research Resource P41 grant (P41GM111244), and by the DOE Office of Biological and Environmental Research (KP1605010). As a National Synchrotron Light Source II facility resource at the Brookhaven National Laboratory, work performed at the LSBR was supported in part by the United States Department of Energy, Office of Science, Office of Basic Energy Sciences Program under contract number and DE-SC0012704 (KC0401040).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge Dr. Misty Kuhn and Kristen Jew of the San Francisco State University for their critical reading of this review.
References
AbouElfetouh, A., Kuhn, M. L., Hu, L. I., Scholle, M. D., Sorensen, D. J., Sahu, A. K., et al. (2015). The E. coli sirtuin CobB shows no preference for enzymatic and nonenzymatic lysine acetylation substrate sites. Microbiologyopen 4, 66–83. doi: 10.1002/mbo3.223
Allfrey, V. G., Faulkner, R., and Mirsky, A. E. (1964). Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc. Natl. Acad. Sci. U.S.A. 51, 786–794. doi: 10.1073/pnas.51.5.786
Amin, R., Franz-Wachtel, M., Tiffert, Y., Heberer, M., Meky, M., Ahmed, Y., et al. (2016). Post-translational serine/threonine phosphorylation and lysine acetylation: a novel regulatory aspect of the global nitrogen response regulator GlnR in S. coelicolor M145. Front. Mol. Biosci. 3:38. doi: 10.3389/fmolb.2016.00038
Andersen, K. B., and von Meyenburg, K. (1980). Are growth rates of Escherichia coli in batch cultures limited by respiration? J. Bacteriol. 144, 114–123.
Avalos, J. L., Bever, K. M., and Wolberger, C. (2005). Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol. Cell 17, 855–868. doi: 10.1016/j.molcel.2005.02.022
Baeza, J., Dowell, J. A., Smallegan, M. J., Fan, J., Amador-Noguez, D., Khan, Z., et al. (2014). Stoichiometry of site-specific lysine acetylation in an entire proteome. J. Biol. Chem. 289, 21326–21338. doi: 10.1074/jbc.M114.581843
Baeza, J., Smallegan, M. J., and Denu, J. M. (2015). Site-specific reactivity of nonenzymatic lysine acetylation. ACS Chem. Biol. 10, 122–128. doi: 10.1021/cb500848p
Bannister, A. J., and Kouzarides, T. (1996). The CBP co-activator is a histone acetyltransferase. Nature 384, 641–643. doi: 10.1038/384641a0
Bao, X., Wang, Y., Li, X., Li, X. M., Liu, Z., Yang, T., et al. (2014). Identification of ’erasers’ for lysine crotonylated histone marks using a chemical proteomics approach. eLife 3:e02999. doi: 10.7554/eLife.02999
Barak, R., and Eisenbach, M. (2001). Acetylation of the response regulator, CheY, is involved in bacterial chemotaxis. Mol. Microbiol. 40, 731–743. doi: 10.1046/j.1365-2958.2001.02425.x
Barak, R., Yan, J., Shainskaya, A., and Eisenbach, M. (2006). The chemotaxis response regulator CheY can catalyze its own acetylation. J. Mol. Biol. 359, 251–265. doi: 10.1016/j.jmb.2006.03.033
Basisty, N., Meyer, J. G., Wei, L., Gibson, B. W., and Schilling, B. (2018). Simultaneous quantification of the acetylome and succinylome by ’one-pot’ affinity enrichment. Proteomics 18:e1800123. doi: 10.1002/pmic.201800123
Becker, S. H., and Darwin, K. H. (2017). Bacterial proteasomes: mechanistic and functional insights. Microbiol. Mol. Biol. Rev. 81:e00036-16.
Bernard, E., Rolain, T., Courtin, P., Guillot, A., Langella, P., Hols, P., et al. (2011). Characterization of O-acetylation of N-acetylglucosamine: a novel structural variation of bacterial peptidoglycan. J. Biol. Chem. 286, 23950–23958. doi: 10.1074/jbc.M111.241414
Berndsen, C. E., Albaugh, B. N., Tan, S., and Denu, J. M. (2007). Catalytic mechanism of a MYST family histone acetyltransferase. Biochemistry 46, 623–629. doi: 10.1021/bi602513x
Bi, J., Gou, Z., Zhou, F., Chen, Y., Gan, J., Liu, J., et al. (2018). Acetylation of lysine 182 inhibits the ability of Mycobacterium tuberculosis DosR to bind DNA and regulate gene expression during hypoxia. Emerg. Microbes Infect. 7:108. doi: 10.1038/s41426-018-0112-3
Bi, J., Wang, Y., Yu, H., Qian, X., Wang, H., Liu, J., et al. (2017). Modulation of central carbon metabolism by acetylation of isocitrate lyase in Mycobacterium tuberculosis. Sci. Rep. 7:44826. doi: 10.1038/srep44826
Birhanu, A. G., Yimer, S. A., Holm-Hansen, C., Norheim, G., Aseffa, A., Abebe, M., et al. (2017). N𝜀- and O-acetylation in Mycobacterium tuberculosis Lineage 7 and Lineage 4 strains: proteins involved in bioenergetics, virulence and antimicrobial resistance are acetylated. J. Proteome Res. 16, 4045–4059. doi: 10.1021/acs.jproteome.7b00429
Bitterman, K. J., Anderson, R. M., Cohen, H. Y., Latorre-Esteves, M., and Sinclair, D. A. (2002). Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast SIR2 and human SIRT1. J. Biol. Chem. 277, 45099–45107. doi: 10.1074/jbc.m205670200
Blander, G., and Guarente, L. (2004). The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 73, 417–435. doi: 10.1146/annurev.biochem.73.011303.073651
Bontemps-Gallo, S., Gaviard, C., Richards, C. L., Kentache, T., Raffel, S. J., Lawrence, K. A., et al. (2018). Global profiling of lysine acetylation in Borrelia burgdorferi B31 reveals its role in central metabolism. Front. Microbiol. 9:2036. doi: 10.3389/fmicb.2018.02036
Brinkman, A. B., Ettema, T. J., de Vos, W. M., and van der Oost, J. (2003). The Lrp family of transcriptional regulators. Mol. Microbiol. 48, 287–294. doi: 10.1046/j.1365-2958.2003.03442.x
Brown, C. W., Sridhara, V., Boutz, D. R., Person, M. D., Marcotte, E. M., Barrick, J. E., et al. (2017). Large-scale analysis of post-translational modifications in E. coli under glucose-limiting conditions. BMC Genomics 18:301. doi: 10.1186/s12864-017-3676-8
Brown, T. D., Jones-Mortimer, M. C., and Kornberg, H. L. (1977). The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J. Gen. Microbiol. 102, 327–336. doi: 10.1099/00221287-102-2-327
Brownlee, M. (2005). The pathobiology of diabetic complications: a unifying mechanism. Diabetes Metab. Res. Rev. 54, 1615–1625. doi: 10.2337/diabetes.54.6.1615
Bulkley, D., Innis, C. A., Blaha, G., and Steitz, T. A. (2010). Revisiting the structures of several antibiotics bound to the bacterial ribosome. Proc. Natl. Acad. Sci. U.S.A. 107, 17158–17163. doi: 10.1073/pnas.1008685107
Carabetta, V. J., and Cristea, I. M. (2017). Regulation, function, and detection of protein acetylation in bacteria. J. Bacteriol. 199:e00107-17. doi: 10.1128/JB.00107-17
Carabetta, V. J., Greco, T. M., Cristea, I. M., and Dubnau, D. (2019). YfmK is an N𝜀-lysine acetyltransferase that directly acetylates the histone-like protein HBsu in Bacillus subtilis. Proc. Natl. Acad. Sci. U.S.A. 116, 3752–3757. doi: 10.1073/pnas.1815511116
Carabetta, V. J., Greco, T. M., Tanner, A. W., Cristea, I. M., and Dubnau, D. (2016). Temporal regulation of the Bacillus subtilis acetylome and evidence for a role of MreB acetylation in cell wall growth. mSystems 1:e00005-16.
Castaño-Cerezo, S., Bernal, V., Blanco-Catalá, J., Iborra, J. L., and Cánovas, M. (2011). cAMP-CRP co-ordinates the expression of the protein acetylation pathway with central metabolism in Escherichia coli. Mol. Microbiol. 82, 1110–1128. doi: 10.1111/j.1365-2958.2011.07873.x
Castaño-Cerezo, S., Bernal, V., Post, H., Fuhrer, T., Cappadona, S., Sánchez-Díaz, N. C., et al. (2014). Protein acetylation affects acetate metabolism, motility and acid stress response in Escherichia coli. Mol. Syst. Biol. 10:762. doi: 10.15252/msb.20145227
Chen, Y., Sprung, R., Tang, Y., Ball, H., Sangras, B., Kim, S. C., et al. (2007). Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol. Cell. Proteomics 6, 812–819. doi: 10.1074/mcp.m700021-mcp200
Cheverton, A. M., Gollan, B., Przydacz, M., Wong, C. T., Mylona, A., Hare, S. A., et al. (2016). A Salmonella toxin promotes persister formation through acetylation of tRNA. Mol. Cell 63, 86–96. doi: 10.1016/j.molcel.2016.05.002
Choudhary, C., Kumar, C., Gnad, F., Nielsen, M. L., Rehman, M., Walther, T. C., et al. (2009). Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840. doi: 10.1126/science.1175371
Christensen, D. G. (2019). Regulation of Acetyl Phosphate-Dependent Acetylation and Identification of Novel Lysine Acetyltransferases in Escherichia coli. Doctoral Dissertation, Loyola University Chicago, Chicago, IL.
Christensen, D. G., Baumgartner, J. T., Xie, X., Jew, K. M., Basisty, N., Schilling, B., et al. (2019). Mechanisms, detection, and relevance of protein acetylation in prokaryotes. mBio 10:e02708-18. doi: 10.1128/mBio.02708-18
Christensen, D. G., Meyer, J. G., Baumgartner, J. T., D’Souza, A. K., Nelson, W. C., Payne, S. H., et al. (2018). Identification of novel protein lysine acetyltransferases in Escherichia coli. mBio 9:e01905-18. doi: 10.1128/mBio.01905-18
Christensen, D. G., Orr, J. S., Rao, C. V., and Wolfe, A. J. (2017). Increasing growth yield and decreasing acetylation in Escherichia coli by optimizing the carbon-to-magnesium ratio in peptide-based media. Appl. Environ. Microbiol. 83:e03034-16. doi: 10.1128/AEM.03034-16
Colak, G., Xie, Z., Zhu, A. Y., Dai, L., Lu, Z., Zhang, Y., et al. (2013). Identification of lysine succinylation substrates and the succinylation regulatory enzyme CobB in Escherichia coli. Mol. Cell. Proteomics 12, 3509–3520. doi: 10.1074/mcp.M113.031567
Crosby, H. A., and Escalante-Semerena, J. C. (2014). The acetylation motif in AMP-forming Acyl coenzyme A synthetases contains residues critical for acetylation and recognition by the protein acetyltransferase Pat of Rhodopseudomonas palustris. J. Bacteriol. 196, 1496–1504. doi: 10.1128/JB.00004-14
Crosby, H. A., Heiniger, E. K., Harwood, C. S., and Escalante-Semerena, J. C. (2010). Reversible N epsilon-lysine acetylation regulates the activity of acyl-CoA synthetases involved in anaerobic benzoate catabolism in Rhodopseudomonas palustris. Mol. Microbiol. 76, 874–888. doi: 10.1111/j.1365-2958.2010.07127.x
Crosby, H. A., Pelletier, D. A., Hurst, G. B., and Escalante-Semerena, J. C. (2012). System-wide studies of N-lysine acetylation in Rhodopseudomonas palustris reveal substrate specificity of protein acetyltransferases. J. Biol. Chem. 287, 15590–15601. doi: 10.1074/jbc.M112.352104
Cunin, R., Glansdorff, N., Piérard, A., and Stalon, V. (1986). Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50, 314–352.
Dai, L., Peng, C., Montellier, E., Lu, Z., Chen, Y., Ishii, H., et al. (2014). Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nat. Chem. Biol. 10, 365–370. doi: 10.1038/nchembio.1497
Dalle-Donne, I., Giustarini, D., Colombo, R., Rossi, R., and Milzani, A. (2003). Protein carbonylation in human diseases. Trends Mol. Med. 9, 169–176. doi: 10.1016/s1471-4914(03)00031-5
Dancy, B. M., and Cole, P. A. (2015). Protein lysine acetylation by p300/CBP. Chem. Rev. 115, 2419–2452. doi: 10.1021/cr500452k
Davie, J. R. (2003). Inhibition of histone deacetylase activity by butyrate. J. Nutr. 133, 2485S–2493S. doi: 10.1093/jn/133.7.2485S
Davies, J., and Wright, G. D. (1997). Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 5, 234–240. doi: 10.1016/s0966-842x(97)01033-0
Davis, R., Écija-Conesa, A., Gallego-Jara, J., de Diego, T., Filippova, E. V., Kuffel, G., et al. (2018). An acetylatable lysine controls CRP function in E. coli. Mol. Microbiol. 107, 116–131. doi: 10.1111/mmi.13874
de Diego Puente, T., Gallego-Jara, J., Castaño-Cerezo, S., Bernal Sánchez, V., Fernández Espín, V., García de la Torre, J., et al. (2015). The protein acetyltransferase PatZ from Escherichia coli is regulated by autoacetylation-induced oligomerization. J. Biol. Chem. 290, 23077–23093. doi: 10.1074/jbc.M115.649806
Denu, J. M. (2003). Linking chromatin function with metabolic networks: Sir2 family of NAD(+)-dependent deacetylases. Trends Biochem. Sci. 28, 41–48. doi: 10.1016/s0968-0004(02)00005-1
Di Sabato, G., and Jencks, W. P. (1961). Mechanism and catalysis of reactions of acyl phosphates. I. Nucleophilic reactions. J. Am. Chem. Soc. 83, 4393–4400. doi: 10.1021/bi201921a
Dickinson, B. C., and Chang, C. J. (2011). Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 7, 504–511. doi: 10.1038/nchembio.607
Doll, S., and Burlingame, A. L. (2015). Mass spectrometry-based detection and assignment of protein posttranslational modifications. ACS Chem. Biol. 10, 63–71. doi: 10.1021/cb500904b
Du, J., Zhou, Y., Su, X., Yu, J. J., Khan, S., Jiang, H., et al. (2011). Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809. doi: 10.1126/science.1207861
Duan, L., Yi, M., Chen, J., Li, S., and Chen, W. (2016). Mycobacterium tuberculosis EIS gene inhibits macrophage autophagy through up-regulation of IL-10 by increasing the acetylation of histone H3. Biochem. Biophys. Res. Commun. 473, 1229–1234. doi: 10.1016/j.bbrc.2016.04.045
Dutnall, R. N., Tafrov, S. T., Sternglanz, R., and Ramakrishnan, V. (1998). Structure of the yeast histone acetyltransferase Hat1: insights into substrate specificity and implications for the Gcn5-related N-acetyltransferase superfamily. Cold Spring Harb. Symp. Quant. Biol. 63, 501–507.
Dyda, F., Klein, D. C., and Hickman, A. B. (2000). GCN5-related N-acetyltransferases: a structural overview. Annu. Rev. Biophys. Biomol. Struct. 29, 81–103. doi: 10.1146/annurev.biophys.29.1.81
Fan, C., Xiong, H., Reynolds, N. M., and Söll, D. (2015). Rationally evolving tRNAPyl for efficient incorporation of noncanonical amino acids. Nucleic Acids Res. 43:e156. doi: 10.1093/nar/gkv800
Favrot, L., Blanchard, J. S., and Vergnolle, O. (2016). Bacterial GCN5-Related N-Acetyltransferases: from resistance to regulation. Biochemistry 55, 989–1002. doi: 10.1021/acs.biochem.5b01269
Feldman, J. L., Baeza, J., and Denu, J. M. (2013). Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J. Biol. Chem. 288, 31350–31356. doi: 10.1074/jbc.C113.511261
Frye, R. A. (1999). Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem. Biophys. Res. Commun. 260, 273–279. doi: 10.1006/bbrc.1999.0897
Frye, R. A. (2000). Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Commun. 273, 793–798. doi: 10.1006/bbrc.2000.3000
Gallego-Jara, J., Écija Conesa, A., de Diego Puente, T., Lozano Terol, G., and Cánovas Díaz, M. (2017). Characterization of CobB kinetics and inhibition by nicotinamide. PLoS One 12:e0189689. doi: 10.1371/journal.pone.0189689
Gardner, J. G., and Escalante-Semerena, J. C. (2008). Biochemical and mutational analyses of AcuA, the acetyltransferase enzyme that controls the activity of the acetyl coenzyme a synthetase (AcsA) in Bacillus subtilis. J. Bacteriol. 190, 5132–5136. doi: 10.1128/JB.00340-08
Gardner, J. G., and Escalante-Semerena, J. C. (2009). In Bacillus subtilis, the sirtuin protein deacetylase, encoded by the srtN gene (formerly yhdZ), and functions encoded by the acuABC genes control the activity of acetyl coenzyme A synthetase. J. Bacteriol. 191, 1749–1755. doi: 10.1128/JB.01674-08
Gardner, J. G., Grundy, F. J., Henkin, T. M., and Escalante-Semerena, J. C. (2006). Control of acetyl-coenzyme A synthetase (AcsA) activity by acetylation/deacetylation without NAD(+) involvement in Bacillus subtilis. J. Bacteriol. 188, 5460–5468. doi: 10.1128/jb.00215-06
Garneau-Tsodikova, S., and Labby, K. J. (2016). Mechanisms of resistance to aminoglycoside Antibiotics: overview and perspectives. Medchemcomm 7, 11–27. doi: 10.1039/c5md00344j
Garrity, J., Gardner, J. G., Hawse, W., Wolberger, C., and Escalante-Semerena, J. C. (2007). N-lysine propionylation controls the activity of propionyl-CoA synthetase. J. Biol. Chem. 282, 30239–30245. doi: 10.1074/jbc.m704409200
Gaviard, C., Broutin, I., Cosette, P., Dé, E., Jouenne, T., and Hardouin, J. (2018). Lysine succinylation and acetylation in Pseudomonas aeruginosa. J. Proteome Res. 17, 2449–2459. doi: 10.1021/acs.jproteome.8b00210
Gershey, E. L., Vidali, G., and Allfrey, V. G. (1968). Chemical studies of histone acetylation. The occurrence of epsilon-N-acetyllysine in the f2a1 histone. J. Biol. Chem. 243, 5018–5022.
Ghosh, S., Padmanabhan, B., Anand, C., and Nagaraja, V. (2016). Lysine acetylation of the Mycobacterium tuberculosis HU protein modulates its DNA binding and genome organization. Mol. Microbiol. 100, 577–588. doi: 10.1111/mmi.13339
Glozak, M. A., Sengupta, N., Zhang, X., and Seto, E. (2005). Acetylation and deacetylation of non-histone proteins. Gene 363, 15–23. doi: 10.1016/j.gene.2005.09.010
Golubev, A., Hanson, A. D., and Gladyshev, V. N. (2017). Non-enzymatic molecular damage as a prototypic driver of aging. J. Biol. Chem. 292, 6029–6038. doi: 10.1074/jbc.R116.751164
Gottesman, S. (2017). Stress reduction, bacterial style. J. Bacteriol. 199:e00433-17. doi: 10.1128/JB.00433-17
Green, K. D., Biswas, T., Pang, A. H., Willby, M. J., Reed, M. S., Stuchlik, O., et al. (2018). Acetylation by Eis and deacetylation by Rv1151c of Mycobacterium tuberculosis HupB: biochemical and structural insight. Biochemistry 57, 781–790. doi: 10.1021/acs.biochem.7b01089
Gregoretti, I. V., Lee, Y. M., and Goodson, H. V. (2004). Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J. Mol. Biol. 338, 17–31. doi: 10.1016/j.jmb.2004.02.006
Guarente, L. (2005). Calorie restriction and SIR2 genes–towards a mechanism. Mech. Ageing Dev. 126, 923–928. doi: 10.1016/j.mad.2005.03.013
Hayden, J. D., Brown, L. R., Gunawardena, H. P., Perkowski, E. F., Chen, X., and Braunstein, M. (2013). Reversible acetylation regulates acetate and propionate metabolism in Mycobacterium smegmatis. Microbiology 159, 1986–1999. doi: 10.1099/mic.0.068585-0
Hebbes, T. R., Thorne, A. W., and Crane-Robinson, C. (1988). A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 7, 1395–1402. doi: 10.1002/j.1460-2075.1988.tb02956.x
Helbig, A. O., Gauci, S., Raijmakers, R., van Breukelen, B., Slijper, M., Mohammed, S., et al. (2010). Profiling of N-acetylated protein termini provides in-depth insights into the N-terminal nature of the proteome. Mol. Cell. Proteomics 9, 928–939. doi: 10.1074/mcp.M900463-MCP200
Helsens, K., Van Damme, P., Degroeve, S., Martens, L., Arnesen, T., Vandekerckhove, J., et al. (2011). Bioinformatics analysis of a Saccharomyces cerevisiae N-terminal proteome provides evidence of alternative translation initiation and post-translational N-terminal acetylation. J. Proteome Res. 10, 3578–3589. doi: 10.1021/pr2002325
Hentchel, K. L., and Escalante-Semerena, J. C. (2015). Acylation of biomolecules in prokaryotes: a widespread strategy for the control of biological function and metabolic stress. Microbiol. Mol. Biol. Rev. 79, 321–346. doi: 10.1128/MMBR.00020-15
Hildmann, C., Riester, D., and Schwienhorst, A. (2007). Histone deacetylases–an important class of cellular regulators with a variety of functions. Appl. Microbiol. Biotechnol. 75, 487–497. doi: 10.1007/s00253-007-0911-2
Hockenberry, A. M., Post, D. M. B., Rhodes, K. A., Apicella, M., and So, M. (2018). Perturbing the acetylation status of the Type IV pilus retraction motor, PilT, reduces Neisseria gonorrhoeae viability. Mol. Microbiol. 110, 677–688. doi: 10.1111/mmi.13979
Hollywood, N., and Doelle, H. W. (1976). Effect of specific growth rate and glucose concentration on growth and glucose metabolism of Escherichia coli K-12. Microbios 17, 23–33.
Hood, M. I., and Skaar, E. P. (2012). Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10, 525–537. doi: 10.1038/nrmicro2836
Hu, L. (2013). Understanding the Posttranslational Regulation of the Response Regulator RcsB and Acetyl Phosphate as an Acetyl Group Donor in E. coli. Doctoral Dissertation, Loyola University Chicago, Chicago, IL.
Hu, L. I., Chi, B. K., Kuhn, M. L., Filippova, E. V., Walker-Peddakotla, A. J., Bäsell, K., et al. (2013). Acetylation of the response regulator RcsB controls transcription from a small RNA promoter. J. Bacteriol. 195, 4174–4186. doi: 10.1128/JB.00383-13
Ishigaki, Y., Akanuma, G., Yoshida, M., Horinouchi, S., Kosono, S., and Ohnishi, Y. (2017). Protein acetylation involved in streptomycin biosynthesis in Streptomyces griseus. J. Proteomics 155, 63–72. doi: 10.1016/j.jprot.2016.12.006
Jackson, M. D., Schmidt, M. T., Oppenheimer, N. J., and Denu, J. M. (2003). Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases. J. Biol. Chem. 278, 50985–50998. doi: 10.1074/jbc.m306552200
Jencks, W. P. (1969). Catalysis in Chemistry and Enzymology. New York, NY: McGraw-Hill Book Company.
Jers, C., Ravikumar, V., Lezyk, M., Sultan, A., Sjöling,Å., Wai, S. N., et al. (2017). The global acetylome of the human pathogen Vibrio cholerae V52 reveals lysine acetylation of major transcriptional regulators. Front. Cell. Infect. Microbiol. 7:537. doi: 10.3389/fcimb.2017.00537
Jose, L., Ramachandran, R., Bhagavat, R., Gomez, R. L., Chandran, A., Raghunandanan, S., et al. (2016). Hypothetical protein Rv3423.1 of Mycobacterium tuberculosis is a histone acetyltransferase. FEBS J. 283, 265–281. doi: 10.1111/febs.13566
Kawabe, H., Kondo, S., Umezawa, H., and Mitsuhashi, S. (1975). R factor-mediated aminoglycoside antibiotic resistance in Pseudomonas aeruginosa: a new aminoglycoside 6′-N-acetyltransferase. Antimicrob. Agents Chemother. 7, 494–499. doi: 10.1128/aac.7.5.494
Keating, D. H., Shulla, A., Klein, A. H., and Wolfe, A. J. (2008). Optimized two-dimensional thin layer chromatography to monitor the intracellular concentration of acetyl phosphate and other small phosphorylated molecules. Biol. Proced. Online 10, 36–46. doi: 10.1251/bpo141
Kentache, T., Jouenne, T., Dé, E., and Hardouin, J. (2016). Proteomic characterization of Nα- and N𝜀-acetylation in Acinetobacter baumannii. J. Proteomics 144, 148–158. doi: 10.1016/j.jprot.2016.05.021
Kim, G. W., and Yang, X. J. (2011). Comprehensive lysine acetylomes emerging from bacteria to humans. Trends Biochem. Sci. 36, 211–220. doi: 10.1016/j.tibs.2010.10.001
Kim, K. H., An, D. R., Song, J., Yoon, J. Y., Kim, H. S., Yoon, H. J., et al. (2012). Mycobacterium tuberculosis Eis protein initiates suppression of host immune responses by acetylation of DUSP16/MKP-7. Proc. Natl. Acad. Sci. U.S.A. 109, 7729–7734. doi: 10.1073/pnas.1120251109
Kim, S. C., Sprung, R., Chen, Y., Xu, Y., Ball, H., Pei, J., et al. (2006). Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 23, 607–618. doi: 10.1016/j.molcel.2006.06.026
Klein, A. H., Shulla, A., Reimann, S. A., Keating, D. H., and Wolfe, A. J. (2007). The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators. J. Bacteriol. 189, 5574–5581. doi: 10.1128/jb.00564-07
Koshland, D. E. Jr. (1952). Effect of catalysts on the hydrolysis of acetyl phosphate. Nucleophilic displacement mechanisms in enzymatic reactions. J. Am. Chem. Soc. 74, 2286–2292. doi: 10.1021/ja01129a035
Kosono, S., Tamura, M., Suzuki, S., Kawamura, Y., Yoshida, A., Nishiyama, M., et al. (2015). Changes in the acetylome and succinylome of Bacillus subtilis in response to carbon source. PLoS One 10:e0131169. doi: 10.1371/journal.pone.0131169
Kossiakoff, A. A. (1986). Protein dynamics investigated by neutron diffraction. Methods Enzymol. 131, 433–447. doi: 10.1016/0076-6879(86)31051-6
Kuhn, M. L., Zemaitaitis, B., Hu, L. I., Sahu, A., Sorensen, D., Minasov, G., et al. (2014). Structural, kinetic and proteomic characterization of acetyl phosphate-dependent bacterial protein acetylation. PLoS One 9:e94816. doi: 10.1371/journal.pone.0094816
Kumari, S., Tishel, R., Eisenbach, M., and Wolfe, A. J. (1995). Cloning, characterization, and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 177, 2878–2886. doi: 10.1128/jb.177.10.2878-2886.1995
Kuo, Y. M., and Andrews, A. J. (2013). Quantitating the specificity and selectivity of Gcn5-mediated acetylation of histone H3. PLoS One 8:e54896. doi: 10.1371/journal.pone.0054896
Lange, S., Rosenkrands, I., Stein, R., Andersen, P., Kaufmann, S. H., and Jungblut, P. R. (2014). Analysis of protein species differentiation among mycobacterial low-Mr-secreted proteins by narrow pH range Immobiline gel 2-DE-MALDI-MS. J. Proteomics 97, 235–244. doi: 10.1016/j.jprot.2013.06.036
Lanouette, S., Mongeon, V., Figeys, D., and Couture, J. F. (2014). The functional diversity of protein lysine methylation. Mol. Syst. Biol. 10:724. doi: 10.1002/msb.134974
Latrasse, D., Benhamed, M., Henry, Y., Domenichini, S., Kim, W., Zhou, D. X., et al. (2008). The MYST histone acetyltransferases are essential for gametophyte development in Arabidopsis. BMC Plant Biol. 8:121. doi: 10.1186/1471-2229-8-121
Lee, J., Manning, A. J., Wolfgeher, D., Jelenska, J., Cavanaugh, K. A., Xu, H., et al. (2015). Acetylation of an NB-LRR plant immune-effector complex suppresses immunity. Cell Rep. 13, 1670–1682. doi: 10.1016/j.celrep.2015.10.029
Lewis, J. D., Lee, A., Ma, W., Zhou, H., Guttman, D. S., and Desveaux, D. (2011). The YopJ superfamily in plant-associated bacteria. Mol. Plant Pathol. 12, 928–937. doi: 10.1111/j.1364-3703.2011.00719.x
Li, L., Wang, W., Zhang, R., Xu, J., Wang, R., Wang, L., et al. (2018). First acetyl-proteome pro?ling of Salmonella Typhimurium revealed involvement of lysine acetylation in drug resistance. Vet. Microbiol. 226, 1–8. doi: 10.1016/j.vetmic.2018.09.024
Li, R., Gu, J., Chen, Y. Y., Xiao, C. L., Wang, L. W., Zhang, Z. P., et al. (2010). CobB regulates Escherichia coli chemotaxis by deacetylating the response regulator CheY. Mol. Microbiol. 76, 1162–1174. doi: 10.1111/j.1365-2958.2010.07125.x
Li, S., Zhang, Q., Xu, Z., and Yao, Y. F. (2017). Acetylation of lysine 243 inhibits the oriC binding ability of DnaA in Escherichia coli. Front. Microbiol. 8:699. doi: 10.3389/fmicb.2017.00699
Li, Y., Krishnan, K., and Duncan, M. J. (2018). Post-translational regulation of a Porphyromonas gingivalis regulator. J. Oral Microbiol. 10:1487743. doi: 10.1080/20002297.2018.1487743
Liang, W., and Deutscher, M. P. (2012). Post-translational modification of RNase R is regulated by stress-dependent reduction in the acetylating enzyme Pka (YfiQ). RNA 18, 37–41. doi: 10.1261/rna.030213.111
Liang, W., Malhotra, A., and Deutscher, M. P. (2011). Acetylation regulates the stability of a bacterial protein: growth stage-dependent modification of RNase R. Mol. Cell 44, 160–166. doi: 10.1016/j.molcel.2011.06.037
Liarzi, O., Barak, R., Bronner, V., Dines, M., Sagi, Y., Shainskaya, A., et al. (2010). Acetylation represses the binding of CheY to its target proteins. Mol. Microbiol. 76, 932–943. doi: 10.1111/j.1365-2958.2010.07148.x
Liimatta, K., Flaherty, E., Ro, G., Nguyen, D. K., Prado, C., and Purdy, A. E. (2018). A putative acetylation system in Vibrio cholerae modulates virulence in arthropod hosts. Appl. Environ. Microbiol. 84:e01113-18. doi: 10.1128/AEM.01113-18
Lima, B. P., Antelmann, H., Gronau, K., Chi, B. K., Becher, D., Brinsmade, S. R., et al. (2011). Involvement of protein acetylation in glucose-induced transcription of a stress-responsive promoter. Mol. Microbiol. 81, 1190–1204. doi: 10.1111/j.1365-2958.2011.07742.x
Lima, B. P., Thanh Huyen, T. T., Bäsell, K., Becher, D., Antelmann, H., and Wolfe, A. J. (2012). Inhibition of acetyl phosphate-dependent transcription by an acetylatable lysine on RNA polymerase. J. Biol. Chem. 287, 32147–32160. doi: 10.1074/jbc.M112.365502
Lin, Y., Fletcher, C. M., Zhou, J., Allis, C. D., and Wagner, G. (1999). Solution structure of the catalytic domain of GCN5 histone acetyltransferase bound to coenzyme A. Nature 400, 86–89. doi: 10.1038/21922
Lin, Y. Y., Lu, J. Y., Zhang, J., Walter, W., Dang, W., Wan, J., et al. (2009). Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell 136, 1073–1084. doi: 10.1016/j.cell.2009.01.033
Liszczak, G., Arnesen, T., and Marmorstein, R. (2011). Structure of a ternary Naa50p (NAT5/SAN) N-terminal acetyltransferase complex reveals the molecular basis for substrate-specific acetylation. J. Biol. Chem. 286, 37002–37010. doi: 10.1074/jbc.M111.282863
Liu, F., Yang, M., Wang, X., Yang, S., Gu, J., Zhou, J., et al. (2014). Acetylome analysis reveals diverse functions of lysine acetylation in Mycobacterium tuberculosis. Mol. Cell. Proteomics 13, 3352–3366. doi: 10.1074/mcp.M114.041962
Liu, W., Tan, Y., Cao, S., Zhao, H., Fang, H., Yang, X., et al. (2018). Protein acetylation mediated by YfiQ and CobB is involved in the virulence and stress response of Yersinia pestis. Infect. Immun. 86:e00224-18. doi: 10.1128/IAI.00224-18
Liu, X., Wang, L., Zhao, K., Thompson, P. R., Hwang, Y., Marmorstein, R., et al. (2008). The structural basis of protein acetylation by the p300/CBP transcriptional coactivator. Nature 451, 846–850. doi: 10.1038/nature06546
Liu, X. X., Liu, W. B., and Ye, B. C. (2015). Regulation of a protein acetyltransferase in Myxococcus xanthus by the coenzyme NADP. J. Bacteriol. 198, 623–632. doi: 10.1128/JB.00661-15
Lombardi, P. M., Cole, K. E., Dowling, D. P., and Christianson, D. W. (2011). Structure, mechanism, and inhibition of histone deacetylases and related metalloenzymes. Curr. Opin. Struct. Biol. 21, 735–743. doi: 10.1016/j.sbi.2011.08.004
Lu, Y. X., Liu, X. X., Liu, W. B., and Ye, B. C. (2017). Identification and characterization of two types of amino acid-regulated acetyltransferases in actinobacteria. Biosci. Rep. 37:BSR20170157. doi: 10.1042/BSR20170157
Lynch, M., and Marinov, G. K. (2015). The bioenergetic costs of a gene. Proc. Natl. Acad. Sci. U.S.A. 112, 15690–15695. doi: 10.1073/pnas.1514974112
Ma, K. W., and Ma, W. (2016). YopJ family effectors promote bacterial infection through a unique acetyltransferase activity. Microbiol. Mol. Biol. Rev. 80, 1011–1027. doi: 10.1128/mmbr.00032-16
Ma, Q., and Wood, T. K. (2011). Protein acetylation in prokaryotes increases stress resistance. Biochem. Biophys. Res. Commun. 410, 846–851. doi: 10.1016/j.bbrc.2011.06.076
Madian, A. G., and Regnier, F. E. (2010). Proteomic identification of carbonylated proteins and their oxidation sites. J. Proteome Res. 9, 3766–3780. doi: 10.1021/pr1002609
Maisonneuve, E., Fraysse, L., Lignon, S., Capron, L., and Dukan, S. (2008). Carbonylated proteins are detectable only in a degradation-resistant aggregate state in Escherichia coli. J. Bacteriol. 190, 6609–6614. doi: 10.1128/JB.00588-08
Makowski, L., Rodi, D. J., Mandava, S., Minh, D. D., Gore, D. B., and Fischetti, R. F. (2008). Molecular crowding inhibits intramolecular breathing motions in proteins. J. Mol. Biol. 375, 529–546. doi: 10.1016/j.jmb.2007.07.075
Marmorstein, R. (2001). Structure of histone deacetylases: insights into substrate recognition and catalysis. Structure 9, 1127–1133. doi: 10.1016/s0969-2126(01)00690-6
Marmorstein, R., and Roth, S. Y. (2001). Histone acetyltransferases: function, structure, and catalysis. Curr. Opin. Genet. Dev. 11, 155–161. doi: 10.1016/s0959-437x(00)00173-8
Marsh, V. L., Peak-Chew, S. Y., and Bell, S. D. (2005). Sir2 and the acetyltransferase, Pat, regulate the archaeal chromatin protein, Alba. J. Biol. Chem. 280, 21122–21128. doi: 10.1074/jbc.m501280200
Martin, B. L. (ed.). (2014). “Regulation by covalent modification,” in eLS, (Midsomer Norton: JWS Ltd.).
Martínez-Antonio, A., and Collado-Vides, J. (2003). Identifying global regulators in transcriptional regulatory networks in bacteria. Curr. Opin. Microbiol. 6, 482–489. doi: 10.1016/j.mib.2003.09.002
Mba Medie, F., Champion, M. M., Williams, E. A., and Champion, P. A. (2014). Homeostasis of N-α-terminal acetylation of EsxA correlates with virulence in Mycobacterium marinum. Infect. Immun. 82, 4572–4586. doi: 10.1128/IAI.02153-14
Meyer, J. G., D’Souza, A. K., Sorensen, D. J., Rardin, M. J., Wolfe, A. J., Gibson, B. W., et al. (2016). Quantification of lysine acetylation and succinylation stoichiometry in proteins using mass spectrometric data-independent acquisitions (SWATH). J. Am. Soc. Mass Spectrom. 27, 1758–1771. doi: 10.1007/s13361-016-1476-z
Mikulik, K., Felsberg, J., Kudrnáèová, E., Bezoušková, S., Setinová, D., Stodùlková, E., et al. (2012). CobB1 deacetylase activity in Streptomyces coelicolor. Biochem. Cell Biol. 90, 179–187. doi: 10.1139/o11-086
Mishra, A., Roy, F., Dou, Y., Zhang, K., Tang, H., and Fletcher, H. M. (2018). Role of acetyltransferase PG1842 in gingipain biogenesis in Porphyromonas gingivalis. J. Bacteriol. 200:e00385-18. doi: 10.1128/JB.00385-18
Mittal, R., Peak-Chew, S. Y., Sade, R. S., Vallis, Y., and McMahon, H. T. (2010). The acetyltransferase activity of the bacterial toxin YopJ of Yersinia is activated by eukaryotic host cell inositol hexakisphosphate. J. Biol. Chem. 285, 19927–19934. doi: 10.1074/jbc.M110.126581
Miyagi, M. (2017). Site-specific quantification of lysine acetylation using isotopic labeling. Methods Enzymol. 586, 85–95. doi: 10.1016/bs.mie.2016.09.029
Mizuno, Y., Nagano-Shoji, M., Kubo, S., Kawamura, Y., Yoshida, A., Kawasaki, H., et al. (2016). Altered acetylation and succinylation profiles in Corynebacterium glutamicum in response to conditions inducing glutamate overproduction. Microbiologyopen 5, 152–173. doi: 10.1002/mbo3.320
Mizzen, C. A., Yang, X. J., Kokubo, T., Brownell, J. E., Bannister, A. J., Owen-Hughes, T., et al. (1996). The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell 87, 1261–1270. doi: 10.1016/s0092-8674(00)81821-8
Mukherjee, S., Hao, Y. H., and Orth, K. (2007). A newly discovered post-translational modification–the acetylation of serine and threonine residues. Trends Biochem. Sci. 32, 210–216. doi: 10.1016/j.tibs.2007.03.007
Mukherjee, S., Keitany, G., Li, Y., Wang, Y., Ball, H. L., Goldsmith, E. J., et al. (2006). Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312, 1211–1214. doi: 10.1126/science.1126867
Nagano-Shoji, M., Hamamoto, Y., Mizuno, Y., Yamada, A., Kikuchi, M., Shirouzu, M., et al. (2017). Characterization of lysine acetylation of a phosphoenolpyruvate carboxylase involved in glutamate overproduction in Corynebacterium glutamicum. Mol. Microbiol. 104, 677–689. doi: 10.1111/mmi.13658
Nakayasu, E. S., Burnet, M. C., Walukiewicz, H. E., Wilkins, C. S., Shukla, A. K., Brooks, S., et al. (2017). Ancient regulatory role of lysine acetylation in central metabolism. mBio 8:e01894-17. doi: 10.1128/mBio.01894-17
Nakayasu, E. S., Wu, S., Sydor, M. A., Shukla, A. K., Weitz, K. K., Moore, R. J., et al. (2014). A method to determine lysine acetylation stoichiometries. Int. J. Proteomics 2014:730725. doi: 10.1155/2014/730725
Nambi, S., Basu, N., and Visweswariah, S. S. (2010). cAMP-regulated protein lysine acetylases in Mycobacteria. J. Biol. Chem. 285, 24313–24323. doi: 10.1074/jbc.M110.118398
Nambi, S., Gupta, K., Bhattacharyya, M., Ramakrishnan, P., Ravikumar, V., Siddiqui, N., et al. (2013). Cyclic AMP-dependent protein lysine acylation in mycobacteria regulates fatty acid and propionate metabolism. J. Biol. Chem. 288, 14114–14124. doi: 10.1074/jbc.M113.463992
Navarro Llorens, J. M., Tormo, A., and Martínez-García, E. (2010). Stationary phase in gram-negative bacteria. FEMS Microbiol. Rev. 34, 476–495. doi: 10.1111/j.1574-6976.2010.00213.x
Neuwald, A. F., and Landsman, D. (1997). GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem. Sci. 22, 154–155. doi: 10.1016/s0968-0004(97)01034-7
Ninfa, A. J., Jiang, P., Atkinson, M. R., and Peliska, J. A. (2000). Integration of antagonistic signals in the regulation of nitrogen assimilation in Escherichia coli. Curr. Top. Cell. Regul. 36, 31–75.
Noy, T., Xu, H., and Blanchard, J. S. (2014). Acetylation of acetyl-CoA synthetase from Mycobacterium tuberculosis leads to specific inactivation of the adenylation reaction. Arch. Biochem. Biophys. 550–551, 42–49. doi: 10.1016/j.abb.2014.04.004
Nyström, T. (2004). Stationary-phase physiology. Annu. Rev. Microbiol. 58, 161–181. doi: 10.1146/annurev.micro.58.030603.123818
Ogura, M., and Asai, K. (2016). Glucose induces ECF sigma factor genes, sigX and sigM, independent of cognate anti-sigma factors through acetylation of CshA in Bacillus subtilis. Front. Microbiol. 7:1918. doi: 10.3389/fmicb.2016.01918
Ogura, M., and Kanesaki, Y. (2018). Newly identified nucleoid-associated-like protein YlxR regulates metabolic gene expression in Bacillus subtilis. mSphere 3:e00501-18. doi: 10.1128/mSphere.00501-18
Okanishi, H., Kim, K., Masui, R., and Kuramitsu, S. (2013). Acetylome with structural mapping reveals the significance of lysine acetylation in Thermus thermophilus. J. Proteome Res. 12, 3952–3968. doi: 10.1021/pr400245k
Okkels, L. M., Müller, E. C., Schmid, M., Rosenkrands, I., Kaufmann, S. H., Andersen, P., et al. (2004). CFP10 discriminates between nonacetylated and acetylated ESAT-6 of Mycobacterium tuberculosis by differential interaction. Proteomics 4, 2954–2960. doi: 10.1002/pmic.200400906
Ouidir, T., Jarnier, F., Cosette, P., Jouenne, T., and Hardouin, J. (2015). Characterization of N-terminal protein modifications in Pseudomonas aeruginosa PA14. J. Proteomics 114, 214–225. doi: 10.1016/j.jprot.2014.11.006
Paquette, N., Conlon, J., Sweet, C., Rus, F., Wilson, L., Pereira, A., et al. (2012). Serine/threonine acetylation of TGFβ-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc. Natl. Acad. Sci. U.S.A. 109, 12710–12715. doi: 10.1073/pnas.1008203109
Park, J., Chen, Y., Tishkoff, D. X., Peng, C., Tan, M., Dai, L., et al. (2013). SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol. Cell 50, 919–930. doi: 10.1016/j.molcel.2013.06.001
Peng, C., Lu, Z., Xie, Z., Cheng, Z., Chen, Y., Tan, M., et al. (2011). The first identification of lysine malonylation substrates and its regulatory enzyme. Mol. Cell. Proteomics 10:M111.012658. doi: 10.1074/mcp.M111.012658
Peregrín-Alvarez, J. M., Sanford, C., and Parkinson, J. (2009). The conservation and evolutionary modularity of metabolism. Genome Biol. 10:R63. doi: 10.1186/gb-2009-10-6-r63
Phillips, D. M. (1963). The presence of acetyl groups of histones. Biochem. J. 87, 258–263. doi: 10.1042/bj0870258
Polevoda, B., and Sherman, F. (2003). N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins. J. Mol. Biol. 325, 595–622. doi: 10.1016/s0022-2836(02)01269-x
Porcu, M., and Chiarugi, A. (2005). The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol. Sci. 26, 94–103. doi: 10.1016/j.tips.2004.12.009
Post, D. M. B., Schilling, B., Reinders, L. M., D’Souza, A. K., Ketterer, M. R., Kiel, S. J., et al. (2017). Identification and characterization of AckA-dependent protein acetylation in Neisseria gonorrhoeae. PLoS One 12:e0179621. doi: 10.1371/journal.pone.0179621
Prüss, B. M., Besemann, C., Denton, A., and Wolfe, A. J. (2006). A complex transcription network controls the early stages of biofilm development by Escherichia coli. J. Bacteriol. 188, 3731–3739. doi: 10.1128/jb.01780-05
Qin, R., Sang, Y., Ren, J., Zhang, Q., Li, S., Cui, Z., et al. (2016). The bacterial two-hybrid system uncovers the involvement of acetylation in regulating of Lrp Activity in Salmonella Typhimurium. Front. Microbiol. 7:1864. doi: 10.3389/fmicb.2016.01864
Ramos-Montañez, S., Kazmierczak, K. M., Hentchel, K. L., and Winkler, M. E. (2010). Instability of ackA (acetate kinase) mutations and their effects on acetyl phosphate and ATP amounts in Streptococcus pneumoniae D39. J. Bacteriol. 192, 6390–6400. doi: 10.1128/JB.00995-10
Ramponi, G., Manao, G., and Camici, G. (1975). Nonenzymatic acetylation of histones with acetyl phosphate and acetyl adenylate. Biochemistry 14, 2681–2685. doi: 10.1021/bi00683a018
Reeve, C. A., Bockman, A. T., and Matin, A. (1984). Role of protein degradation in the survival of carbon-starved Escherichia coli and Salmonella Typhimurium. J. Bacteriol. 157, 758–763.
Ren, J., Sang, Y., Lu, J., and Yao, Y. F. (2017). Protein acetylation and its role in bacterial virulence. Trends Microbiol. 25, 768–779. doi: 10.1016/j.tim.2017.04.001
Ren, J., Sang, Y., Ni, J., Tao, J., Lu, J., Zhao, M., et al. (2015). Acetylation regulates survival of Salmonella enterica serovar Typhimurium under acid stress. Appl. Environ. Microbiol. 81, 5675–5682. doi: 10.1128/AEM.01009-15
Ren, J., Sang, Y., Tan, Y., Tao, J., Ni, J., Liu, S., et al. (2016). Acetylation of lysine 201 inhibits the DNA-binding ability of PhoP to regulate Salmonella virulence. PLoS Pathog. 12:e1005458. doi: 10.1371/journal.ppat.1005458
Reverdy, A., Chen, Y., Hunter, E., Gozzi, K., and Chai, Y. (2018). Protein lysine acetylation plays a regulatory role in Bacillus subtilis multicellularity. PLoS One 13:e0204687. doi: 10.1371/journal.pone.0204687
Ringel, A. E., Roman, C., and Wolberger, C. (2014). Alternate deacylating specificities of the archaeal sirtuins Sir2Af1 and Sir2Af2. Protein Sci. 23, 1686–1697. doi: 10.1002/pro.2546
Rose, I. A., Grunberg-Manago, M., Korey, S. R., and Ochoa, S. (1954). Enzymatic phosphorylation of acetate. J. Biol. Chem. 211, 737–756.
Roy-Chaudhuri, B., Kirthi, N., Kelley, T., and Culver, G. M. (2008). Suppression of a cold-sensitive mutation in ribosomal protein S5 reveals a role for RimJ in ribosome biogenesis. Mol. Microbiol. 68, 1547–1559. doi: 10.1111/j.1365-2958.2008.06252.x
Sabari, B. R., Tang, Z., Huang, H., Yong-Gonzalez, V., Molina, H., Kong, H. E., et al. (2015). Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol. Cell 58, 203–215. doi: 10.1016/j.molcel.2015.02.029
Sakatos, A., Babunovic, G. H., Chase, M. R., Dills, A., Leszyk, J., Rosebrock, T., et al. (2018). Posttranslational modification of a histone-like protein regulates phenotypic resistance to isoniazid in mycobacteria. Sci. Adv. 4:eaao1478. doi: 10.1126/sciadv.aao1478
Salah Ud-Din, A. I., Tikhomirova, A., and Roujeinikova, A. (2016). Structure and functional diversity of GCN5-related N-acetyltransferases (GNAT). Int. J. Mol. Sci. 17:E1018. doi: 10.3390/ijms17071018
Sanders, B. D., Jackson, B., and Marmorstein, R. (2010). Structural basis for sirtuin function: what we know and what we don’t. Biochim. Biophys. Acta 1804, 1604–1616. doi: 10.1016/j.bbapap.2009.09.009
Sang, Y., Ren, J., Ni, J., Tao, J., Lu, J., and Yao, Y. F. (2016). Protein acetylation is involved in Salmonella enterica serovar Typhimurium virulence. J. Infect. Dis. 213, 1836–1845. doi: 10.1093/infdis/jiw028
Sang, Y., Ren, J., Qin, R., Liu, S., Cui, Z., Cheng, S., et al. (2017). Acetylation regulating protein stability and DNA-binding ability of HilD, thus modulating Salmonella Typhimurium virulence. J. Infect. Dis. 216, 1018–1026. doi: 10.1093/infdis/jix102
Sauve, A. A., and Schramm, V. L. (2003). Sir2 regulation by nicotinamide results from switching between base exchange and deacetylation chemistry. Biochemistry 42, 9249–9256. doi: 10.1021/bi034959l
Schilling, B., Basisty, N., Christensen, D. G., Sorensen, D., Orr, J. S., Wolfe, A. J., et al. (2019). Global lysine acetylation in Escherichia coli results from growth conditions that favor acetate fermentation. J. Bacteriol. 201:e00768-18. doi: 10.1128/JB.00768-18
Schilling, B., Christensen, D., Davis, R., Sahu, A. K., Hu, L. I., Walker-Peddakotla, A., et al. (2015). Protein acetylation dynamics in response to carbon overflow in Escherichia coli. Mol. Microbiol. 98, 847–863. doi: 10.1111/mmi.13161
Schmidt, M. T., Smith, B. C., Jackson, M. D., and Denu, J. M. (2004). Coenzyme specificity of Sir2 protein deacetylases: implications for physiological regulation. J. Biol. Chem. 279, 40122–40129. doi: 10.1074/jbc.m407484200
Shaw, K. J., Rather, P. N., Hare, R. S., and Miller, G. H. (1993). Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57, 138–163.
Shaw, W. V., and Hopwood, D. A. (1976). Chloramphenicol acetylation in Streptomyces. J. Gen. Microbiol. 94, 159–166. doi: 10.1099/00221287-94-1-159
Singhal, A., Arora, G., Virmani, R., Kundu, P., Khanna, T., Sajid, A., et al. (2015). Systematic analysis of mycobacterial acylation reveals first example of acylation-mediated regulation of enzyme activity of a bacterial phosphatase. J. Biol. Chem. 290, 26218–26234. doi: 10.1074/jbc.M115.687269
Smith, B. C., and Denu, J. M. (2007). Acetyl-lysine analog peptides as mechanistic probes of protein deacetylases. J. Biol. Chem. 282, 37256–37265. doi: 10.1074/jbc.m707878200
Song, L., Wang, G., Malhotra, A., Deutscher, M. P., and Liang, W. (2016). Reversible acetylation on Lys501 regulates the activity of RNase II. Nucleic Acids Res. 44, 1979–1988. doi: 10.1093/nar/gkw053
Spange, S., Wagner, T., Heinzel, T., and Krämer, O. H. (2009). Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int. J. Biochem. Cell Biol. 41, 185–198. doi: 10.1016/j.biocel.2008.08.027
Spencer, T. E., Jenster, G., Burcin, M. M., Allis, C. D., Zhou, J., Mizzen, C. A., et al. (1997). Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389, 194–198. doi: 10.1038/38304
Starai, V. J., Celic, I., Cole, R. N., Boeke, J. D., and Escalante-Semerena, J. C. (2002). Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science 298, 2390–2392. doi: 10.1126/science.1077650
Starai, V. J., and Escalante-Semerena, J. C. (2004a). Acetyl-coenzyme A synthetase (AMP forming). Cell. Mol. Life Sci. 61, 2020–2030.
Starai, V. J., and Escalante-Semerena, J. C. (2004b). Identification of the protein acetyltransferase (Pat) enzyme that acetylates acetyl-CoA synthetase in Salmonella enterica. J. Mol. Biol. 340, 1005–1012. doi: 10.1016/j.jmb.2004.05.010
Starai, V. J., Garrity, J., and Escalante-Semerena, J. C. (2005). Acetate excretion during growth of Salmonella enterica on ethanolamine requires phosphotransacetylase (EutD) activity, and acetate recapture requires acetyl-CoA synthetase (Acs) and phosphotransacetylase (Pta) activities. Microbiology 151, 3793–3801. doi: 10.1099/mic.0.28156-0
Starai, V. J., Takahashi, H., Boeke, J. D., and Escalante-Semerena, J. C. (2003). Short-chain fatty acid activation by acyl-coenzyme A synthetases requires SIR2 protein function in Salmonella enterica and Saccharomyces cerevisiae. Genetics 163, 545–555.
Steinsiek, S., and Bettenbrock, K. (2012). Glucose transport in Escherichia coli mutant strains with defects in sugar transport systems. J. Bacteriol. 194, 5897–5908. doi: 10.1128/JB.01502-12
Sun, M., Guo, H., Lu, G., Gu, J., Wang, X., Zhang, X. E., et al. (2016a). Lysine acetylation regulates the activity of Escherichia coli S-adenosylmethionine synthase. Acta Biochim. Biophys. Sin. 48, 723–731. doi: 10.1093/abbs/gmw066
Sun, M., Xu, J., Wu, Z., Zhai, L., Liu, C., Cheng, Z., et al. (2016b). Characterization of protein lysine propionylation in Escherichia coli: global profiling, dynamic change, and enzymatic regulation. J. Proteome Res. 15, 4696–4708. doi: 10.1021/acs.jproteome.6b00798
Sun, X. L., Yang, Y. H., Zhu, L., Liu, F. Y., Xu, J. P., Huang, X. W., et al. (2018). The lysine acetylome of the nematocidal bacterium Bacillus nematocida and impact of nematode on the acetylome. J. Proteomics 177, 31–39. doi: 10.1016/j.jprot.2018.02.005
Suzuki, S., Kondo, N., Yoshida, M., Nishiyama, M., and Kosono, S. (2019). Dynamic changes in lysine acetylation and succinylation of the elongation factor Tu in Bacillus subtilis. Microbiology 165, 65–77. doi: 10.1099/mic.0.000737
Szewczak, J., Bierczyñska-Krzysik, A., Piejko, M., Mak, P., and Stadnik, D. (2015). Isolation and characterization of acetylated derivative of recombinant insulin Lispro produced in Escherichia coli. Pharm. Res. 32, 2450–2457. doi: 10.1007/s11095-015-1637-y
Tan, M., Luo, H., Lee, S., Jin, F., Yang, J. S., Montellier, E., et al. (2011). Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146, 1016–1028. doi: 10.1016/j.cell.2011.08.008
Tan, M., Peng, C., Anderson, K. A., Chhoy, P., Xie, Z., Dai, L., et al. (2014). Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 19, 605–617. doi: 10.1016/j.cmet.2014.03.014
Tanaka, S., Matsushita, Y., Yoshikawa, A., and Isono, K. (1989). Cloning and molecular characterization of the gene rimL which encodes an enzyme acetylating ribosomal protein L12 of Escherichia coli K12. Mol. Gen. Genet. 217, 289–293. doi: 10.1007/bf02464895
Tanner, K. G., Trievel, R. C., Kuo, M. H., Howard, R. M., Berger, S. L., Allis, C. D., et al. (1999). Catalytic mechanism and function of invariant glutamic acid 173 from the histone acetyltransferase GCN5 transcriptional coactivator. J. Biol. Chem. 274, 18157–18160. doi: 10.1074/jbc.274.26.18157
Thao, S., Chen, C. S., Zhu, H., and Escalante-Semerena, J. C. (2010). N𝜀-lysine acetylation of a bacterial transcription factor inhibits Its DNA-binding activity. PLoS One 5:e15123. doi: 10.1371/journal.pone.0015123
Thao, S., and Escalante-Semerena, J. C. (2011). Control of protein function by reversible N?-lysine acetylation in bacteria. Curr. Opin. Microbiol. 14, 200–204. doi: 10.1016/j.mib.2010.12.013
Trievel, R. C., Rojas, J. R., Sterner, D. E., Venkataramani, R. N., Wang, L., Zhou, J., et al. (1999). Crystal structure and mechanism of histone acetylation of the yeast GCN5 transcriptional coactivator. Proc. Natl. Acad. Sci. U.S.A. 96, 8931–8936. doi: 10.1073/pnas.96.16.8931
Tucker, A. C., and Escalante-Semerena, J. C. (2010). Biologically active isoforms of CobB sirtuin deacetylase in Salmonella enterica and Erwinia amylovora. J. Bacteriol. 192, 6200–6208. doi: 10.1128/JB.00874-10
Tucker, A. C., and Escalante-Semerena, J. C. (2013). Acetoacetyl-CoA synthetase activity is controlled by a protein acetyltransferase with unique domain organization in Streptomyces lividans. Mol. Microbiol. 87, 152–167. doi: 10.1111/mmi.12088
Umehara, T., Kosono, S., Söll, D., and Tamura, K. (2018). Lysine acetylation regulates alanyl-tRNA synthetase activity in Escherichia coli. Genes 9:E473. doi: 10.3390/genes9100473
Vallari, D. S., and Rock, C. O. (1985). Pantothenate transport in Escherichia coli. J. Bacteriol. 162, 1156–1161.
VanDrisse, C. M., and Escalante-Semerena, J. C. (2018). In Streptomyces lividans, acetyl-CoA synthetase activity is controlled by O-serine and N. Mol. Microbiol. 107, 577–594. doi: 10.1111/mmi.13901
VanDrisse, C. M., Parks, A. R., and Escalante-Semerena, J. C. (2017). A toxin involved in Salmonella persistence regulates its activity by acetylating its cognate antitoxin, a modification reversed by CobB sirtuin deacetylase. mBio 8:e00708-17. doi: 10.1128/mBio.00708-17
Vasileva, D., Suzuki-Minakuchi, C., Kosono, S., Yoshida, M., Okada, K., and Nojiri, H. (2018). Proteome and acylome analyses of the functional interaction network between the carbazole-degradative plasmid pCAR1 and host Pseudomonas putida KT2440. Environ. Microbiol. Rep. 10, 299–309. doi: 10.1111/1758-2229.12639
Venkat, S., Gregory, C., Gan, Q., and Fan, C. (2017a). Biochemical characterization of the lysine acetylation of tyrosyl-tRNA synthetase in Escherichia coli. Chembiochem 18, 1928–1934. doi: 10.1002/cbic.201700343
Venkat, S., Gregory, C., Sturges, J., Gan, Q., and Fan, C. (2017b). Studying the lysine acetylation of malate dehydrogenase. J. Mol. Biol. 429, 1396–1405. doi: 10.1016/j.jmb.2017.03.027
Vergnolle, O., Xu, H., and Blanchard, J. S. (2013). Mechanism and regulation of mycobactin fatty acyl-AMP ligase FadD33. J. Biol. Chem. 288, 28116–28125. doi: 10.1074/jbc.M113.495549
Vergnolle, O., Xu, H., Tufariello, J. M., Favrot, L., Malek, A. A., Jacobs, W. R., et al. (2016). Post-translational acetylation of MbtA modulates mycobacterial siderophore biosynthesis. J. Biol. Chem. 291, 22315–22326. doi: 10.1074/jbc.m116.744532
Vetting, M. W., de Carvalho, L. P., Yu, M., Hegde, S. S., Magnet, S., Roderick, S. L., et al. (2005). Structure and functions of the GNAT superfamily of acetyltransferases. Arch. Biochem. Biophys. 433, 212–226. doi: 10.1016/j.abb.2004.09.003
Vidali, G., Gershey, E. L., and Allfrey, V. G. (1968). Chemical studies of histone acetylation. The distribution of epsilon-N-acetyllysine in calf thymus histones. J. Biol. Chem. 243, 6361–6366.
Wagner, G. R., and Payne, R. M. (2013). Widespread and enzyme-independent N𝜀-acetylation and N𝜀-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J. Biol. Chem. 288, 29036–29045. doi: 10.1074/jbc.M113.486753
Wang, Q., Zhang, Y., Yang, C., Xiong, H., Lin, Y., Yao, J., et al. (2010). Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 327, 1004–1007. doi: 10.1126/science.1179687
Wang, Q., Zhao, Y., McClelland, M., and Harshey, R. M. (2007). The RcsCDB signaling system and swarming motility in Salmonella enterica serovar Typhimurium: dual regulation of flagellar and SPI-2 virulence genes. J. Bacteriol. 189, 8447–8457. doi: 10.1128/jb.01198-07
Wei, L., Meyer, J. G., and Schilling, B. (2018). Quantification of site-specific protein lysine acetylation and succinylation stoichiometry using data-independent acquisition mass spectrometry. J. Vis. Exp. 134:57209. doi: 10.3791/57209
Wei, W., Liu, T., Li, X., Wang, R., Zhao, W., Zhao, G., et al. (2017). Lysine acetylation regulates the function of the global anaerobic transcription factor FnrL in Rhodobacter sphaeroides. Mol. Microbiol. 104, 278–293. doi: 10.1111/mmi.13627
Weinert, B. T., Iesmantavicius, V., Moustafa, T., Schölz, C., Wagner, S. A., Magnes, C., et al. (2015). Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol. Syst. Biol. 11:833. doi: 10.15252/msb.156513
Weinert, B. T., Iesmantavicius, V., Wagner, S. A., Schölz, C., Gummesson, B., Beli, P., et al. (2013a). Acetyl-phosphate is a critical determinant of lysine acetylation in E. coli. Mol. Cell 51, 265–272. doi: 10.1016/j.molcel.2013.06.003
Weinert, B. T., Schölz, C., Wagner, S. A., Iesmantavicius, V., Su, D., Daniel, J. A., et al. (2013b). Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 4, 842–851. doi: 10.1016/j.celrep.2013.07.024
Weinert, B. T., Satpathy, S., Hansen, B. K., Lyon, D., Jensen, L. J., and Choudhary, C. (2017). Accurate quantification of site-specific acetylation stoichiometry reveals the impact of sirtuin deacetylase CobB on the E. coli acetylome. Mol. Cell. Proteomics 16, 759–769. doi: 10.1074/mcp.M117.067587
White-Ziegler, C. A., Black, A. M., Eliades, S. H., Young, S., and Porter, K. (2002). The N-acetyltransferase RimJ responds to environmental stimuli to repress pap fimbrial transcription in Escherichia coli. J. Bacteriol. 184, 4334–4342. doi: 10.1128/jb.184.16.4334-4342.2002
White-Ziegler, C. A., and Low, D. A. (1992). Thermoregulation of the pap operon: evidence for the involvement of RimJ, the N-terminal acetylase of ribosomal protein S5. J. Bacteriol. 174, 7003–7012. doi: 10.1128/jb.174.21.7003-7012.1992
Wimpenny, J. W., and Firth, A. (1972). Levels of nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide in facultative bacteria and the effect of oxygen. J. Bacteriol. 111, 24–32.
Wolf, E., Vassilev, A., Makino, Y., Sali, A., Nakatani, Y., and Burley, S. K. (1998). Crystal structure of a GCN5-related N-acetyltransferase: Serratia marcescens aminoglycoside 3-N-acetyltransferase. Cell 94, 439–449.
Wolfe, A. J. (2010). Physiologically relevant small phosphodonors link metabolism to signal transduction. Curr. Opin. Microbiol. 13, 204–209. doi: 10.1016/j.mib.2010.01.002
Wright, G. D., and Ladak, P. (1997). Overexpression and characterization of the chromosomal aminoglycoside 6′-N-acetyltransferase from Enterococcus faecium. Antimicrob. Agents Chemother. 41, 956–960. doi: 10.1128/aac.41.5.956
Xie, L., Wang, X., Zeng, J., Zhou, M., Duan, X., Li, Q., et al. (2015). Proteome-wide lysine acetylation profiling of the human pathogen Mycobacterium tuberculosis. Int. J. Biochem. Cell Biol. 59, 193–202. doi: 10.1016/j.biocel.2014.11.010
Xiong, Y., and Guan, K. L. (2012). Mechanistic insights into the regulation of metabolic enzymes by acetylation. J. Cell Biol. 198, 155–164. doi: 10.1083/jcb.201202056
Xu, H., Hegde, S. S., and Blanchard, J. S. (2011). Reversible acetylation and inactivation of Mycobacterium tuberculosis acetyl-CoA synthetase is dependent on cAMP. Biochemistry 50, 5883–5892. doi: 10.1021/bi200156t
Xu, J. Y., Xu, Z., Liu, X., Tan, M., and Ye, B. C. (2018a). Protein acetylation and butyrylation regulate the phenotype and metabolic shifts of the endospore-forming Clostridium acetobutylicum. Mol. Cell. Proteomics 17, 1156–1169. doi: 10.1074/mcp.RA117.000372
Xu, J. Y., Zhao, L., Liu, X., Hu, H., Liu, P., Tan, M., et al. (2018b). Characterization of the lysine acylomes and the substrates regulated by Protein Acyltransferase in Mycobacterium smegmatis. ACS Chem. Biol. 13, 1588–1597. doi: 10.1021/acschembio.8b00213
Xu, J. Y., You, D., Leng, P. Q., and Ye, B. C. (2014). Allosteric regulation of a protein acetyltransferase in Micromonospora aurantiaca by the amino acids cysteine and arginine. J. Biol. Chem. 289, 27034–27045. doi: 10.1074/jbc.M114.579078
Yan, Y., Harper, S., Speicher, D. W., and Marmorstein, R. (2002). The catalytic mechanism of the ESA1 histone acetyltransferase involves a self-acetylated intermediate. Nat. Struct. Biol. 9, 862–869.
Yang, H., Sha, W., Liu, Z., Tang, T., Liu, H., Qin, L., et al. (2018). Lysine acetylation of DosR regulates the hypoxia response of Mycobacterium tuberculosis. Emerg. Microbes Infect. 7:34. doi: 10.1038/s41426-018-0032-2
Yang, M., Wang, Y., Chen, Y., Cheng, Z., Gu, J., Deng, J., et al. (2015). Succinylome analysis reveals the involvement of lysine succinylation in metabolism in pathogenic Mycobacterium tuberculosis. Mol. Cell. Proteomics 14, 796–811. doi: 10.1074/mcp.M114.045922
Yang, X. J., Ogryzko, V. V., Nishikawa, J., Howard, B. H., and Nakatani, Y. (1996). A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382, 319–324. doi: 10.1038/382319a0
Yang, X. J., and Seto, E. (2008a). Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol. Cell 31, 449–461. doi: 10.1016/j.molcel.2008.07.002
Yang, X. J., and Seto, E. (2008b). The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol. 9, 206–218. doi: 10.1038/nrm2346
Ye, Q., Ji, Q. Q., Yan, W., Yang, F., and Wang, E. D. (2017). Acetylation of lysine ?-amino groups regulates aminoacyl-tRNA synthetase activity in Escherichia coli. J. Biol. Chem. 292, 10709–10722. doi: 10.1074/jbc.M116.770826
Yoshida, A., Yoshida, M., Kuzuyama, T., Nishiyama, M., and Kosono, S. (2019). Protein acetylation on 2-isopropylmalate synthase from Thermus thermophilus HB27. Extremophiles 23, 377–378. doi: 10.1007/s00792-019-01090-y
Yoshikawa, A., Isono, S., Sheback, A., and Isono, K. (1987). Cloning and nucleotide sequencing of the genes rimI and rimJ which encode enzymes acetylating ribosomal proteins S18 and S5 of Escherichia coli K12. Mol. Gen. Genet. 209, 481–488. doi: 10.1007/bf00331153
You, D., Wang, M. M., and Ye, B. C. (2017). Acetyl-CoA synthetases of Saccharopolyspora erythraea are regulated by the nitrogen response regulator GlnR at both transcriptional and post-translational levels. Mol. Microbiol. 103, 845–859. doi: 10.1111/mmi.13595
You, D., Yao, L. L., Huang, D., Escalante-Semerena, J. C., and Ye, B. C. (2014). Acetyl coenzyme A synthetase is acetylated on multiple lysine residues by a protein acetyltransferase with a single Gcn5-type N-acetyltransferase (GNAT) domain in Saccharopolyspora erythraea. J. Bacteriol. 196, 3169–3178. doi: 10.1128/JB.01961-14
You, D., Yin, B. C., Li, Z. H., Zhou, Y., Yu, W. B., Zuo, P., et al. (2016). Sirtuin-dependent reversible lysine acetylation of glutamine synthetases reveals an autofeedback loop in nitrogen metabolism. Proc. Natl. Acad. Sci. U.S.A. 113, 6653–6658. doi: 10.1073/pnas.1525654113
Yu, B. J., Kim, J. A., Moon, J. H., Ryu, S. E., and Pan, J. G. (2008). The diversity of lysine-acetylated proteins in Escherichia coli. J. Microbiol. Biotechnol. 18, 1529–1536.
Zhang, J., Sprung, R., Pei, J., Tan, X., Kim, S., Zhu, H., et al. (2009). Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol. Cell. Proteomics 8, 215–225. doi: 10.1074/mcp.M800187-MCP200
Zhang, Q., Zhou, A., Li, S., Ni, J., Tao, J., Lu, J., et al. (2016). Reversible lysine acetylation is involved in DNA replication initiation by regulating activities of initiator DnaA in Escherichia coli. Sci. Rep. 6:30837. doi: 10.1038/srep30837
Zhang, Q. F., Zhang, Q., Gu, J., Gong, P., Wang, X. D., Wang, X., et al. (2013). Reversibly acetylated lysine residues play important roles in the enzymatic activity of Escherichia coli N-hydroxyarylamine O-acetyltransferase. FEBS J. 280, 1966–1979. doi: 10.1111/febs.12216
Zhang, Z., Tan, M., Xie, Z., Dai, L., Chen, Y., and Zhao, Y. (2011). Identification of lysine succinylation as a new post-translational modification. Nat. Chem. Biol. 7, 58–63. doi: 10.1038/nchembio.495
Zhao, K., Chai, X., and Marmorstein, R. (2003). Structure of a Sir2 substrate, Alba, reveals a mechanism for deacetylation-induced enhancement of DNA binding. J. Biol. Chem. 278, 26071–26077. doi: 10.1074/jbc.m303666200
Zhao, K., Harshaw, R., Chai, X., and Marmorstein, R. (2004). Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD(+)-dependent Sir2 histone/protein deacetylases. Proc. Natl. Acad. Sci. U.S.A. 101, 8563–8568. doi: 10.1073/pnas.0401057101
Zhou, Q., Gomez Hernandez, M. E., Fernandez-Lima, F., and Tse-Dinh, Y. C. (2018). Biochemical basis of E. coli topoisomerase I relaxation activity reduction by nonenzymatic lysine acetylation. Int. J. Mol. Sci. 19:E1439. doi: 10.3390/ijms19051439
Zhou, Q., Zhou, Y. N., Jin, D. J., and Tse-Dinh, Y. C. (2017). Deacetylation of topoisomerase I is an important physiological function of E. coli CobB. Nucleic Acids Res. 45, 5349–5358. doi: 10.1093/nar/gkx250
Keywords: acetylation, lysine acetyltransferase, bacteria, mass spectrometry, proteomics
Citation: Christensen DG, Xie X, Basisty N, Byrnes J, McSweeney S, Schilling B and Wolfe AJ (2019) Post-translational Protein Acetylation: An Elegant Mechanism for Bacteria to Dynamically Regulate Metabolic Functions. Front. Microbiol. 10:1604. doi: 10.3389/fmicb.2019.01604
Received: 30 April 2019; Accepted: 26 June 2019;
Published: 12 July 2019.
Edited by:
Boris Macek, University of Tübingen, GermanyReviewed by:
Feng Ge, Institute of Hydrobiology (CAS), ChinaSaori Kosono, The University of Tokyo, Japan
Copyright © 2019 Christensen, Xie, Basisty, Byrnes, McSweeney, Schilling and Wolfe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Birgit Schilling, YnNjaGlsbGluZ0BidWNraW5zdGl0dXRlLm9yZw==; Alan J. Wolfe, YXdvbGZlQGx1Yy5lZHU=
 David G. Christensen
David G. Christensen Xueshu Xie
Xueshu Xie Nathan Basisty
Nathan Basisty James Byrnes
James Byrnes Sean McSweeney
Sean McSweeney Birgit Schilling
Birgit Schilling Alan J. Wolfe
Alan J. Wolfe