
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 16 July 2019
Sec. Food Microbiology
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.01567
Bile salt hydrolase (BSH) activity, hypo-cholesterolemic effect, and probiotic properties have been reported for Enterococcus strains isolated from animal and human gut and fermented foods but not for strains isolated from environmental niches, like aquatic and terrestrial plants, soil, and water. The present study is the first report on isolation of Enterococcus faecium from rhizospheric soils that harbor the bsh gene, remove cholesterol in vitro, and possess essential and desirable probiotic attributes. Fifteen samples were collected from different sites located in northern, southern, and central regions of India, of which five yielded pure colonies that were named LR2, LR3, ER5, LR13, and VB1. These were identified by 16S rRNA gene sequencing as E. faecium and evaluated for BSH activity, cholesterol-lowering potential in vitro, and probiotic properties. Our results indicated that all the strains were capable of surviving the harsh conditions of the gastrointestinal tract and did not harbor any of the virulence genes. Though all strains showed the presence of bsh and potential for cholesterol removal, E. faecium strain LR13 showed a remarkable cholesterol removal capability and vancomycin susceptibility and possessed most of the desirable and essential attributes of a probiotic. Hence, it seems to be a fairly promising probiotic candidate that needs to be further evaluated in in vivo studies, especially for its hypo-cholesterolemic potential.
Elevated serum cholesterol has become a major lifestyle-related disorder that results in high morbidity and mortality worldwide. Besides chemotherapeutics, natural measures like dietary interventions and, more recently, regular consumption of probiotics have been found to significantly improve the serum lipoprotein profiles. According to the World Health Organization (WHO), probiotics are viable microorganisms that, when consumed in sufficient amounts, deliver several health benefits on the consumer. Probiotics might exert hypo-cholesterolemic effect by several mechanisms: (i) by incorporating cholesterol in their cell membranes; (ii) by binding cholesterol on their cell surfaces, which inhibits formation of cholesterol micelles inside the intestine; and/or (iii) by assimilating cholesterol during growth, which reduces the amount of cholesterol available for intestinal absorption (Lye et al., 2010).
De-conjugation of the bile salts by bacterial bile salt hydrolase (BSH) has been reported as an important mechanism that is physiologically associated with the hypo-cholesterolemic effect of probiotics (Begley et al., 2006; Ha et al., 2006; Park et al., 2007). The WHO has recommended that along with capability for colonization of gastrointestinal (GI) epithelia and resistance to harsh GI conditions, BSH activity should be considered as one of the main criteria for selection of probiotics (FAO/WHO, 2002). Probiotic bacteria include several genera of lactic acid bacteria (LAB) like Lactobacillus, Streptococcus, Lactococcus, Weissella, Pediococcus, Enterococcus (Ehrmann et al., 2002), etc. Enterococcus is an important member of LAB, several strains of which have shown numerous health benefits like regulating the gut microbiota, alleviating obesity, allergy, irritable bowel syndrome and diarrhea (Franz et al., 2011), immunomodulation (Nissen et al., 2009), hypo-cholesterolemic effect (Ejtahed et al., 2011), etc. Over the last few years, some strains of Enterococcus, particularly vancomycin-resistant enterococci (VRE), were recognized as leading nosocomial pathogens harboring multiple genes for antimicrobial resistance and virulence factors (Ogier and Serror, 2008; Rosenthal et al., 2008; Franz et al., 2011; Arias and Murray, 2012). Thus, the genus Enterococcus has been conferred the dual status of a genus associated with both beneficial and pathogenic organisms. Recent studies have demonstrated that food-grade enterococci are safe and can be differentiated from the nosocomial pathogenic enterococcal strains (Montealegre et al., 2015). Currently, enterococcal strains that have been designated by regulatory agencies as commercial probiotics include Enterococcus faecalis Symbioflor®1 (SymbioPharm, Herborn, Germany), Enterococcus faecium SF68® (NCIMB 10415, Cerbios-Pharma SA, Barbengo, Switzerland), and others (Serio et al., 2010; Franz et al., 2011).
Though studies have reported the BSH activity, hypo-cholesterolemic effect, and probiotic properties of Enterococcus strains isolated from animal and human gut (Hlivak et al., 2005; Hyrslova et al., 2016; Zhang et al., 2017) and fermented food products (Guo et al., 2016; Albano et al., 2018; Nami et al., 2019), the BSH activity and cholesterol-lowering ability of Enterococcus isolated from environmental niches, like aquatic and terrestrial plants, soil, and water, have not been reported. Bacteria isolated from environments in which bile salts are absent usually do not demonstrate BSH activity (Elkins et al., 2001; Moser and Savage, 2001; Ahn et al., 2003). Rhizosphere is a narrow region of the soil that is enriched with root secretions and soil microbes. Enterococcus strains isolated from rhizospheric samples might have some useful characteristics as they live in direct contact with soil and plant secretions and, co-evolve with plant pathogenic bacteria and fungi. Though a previous study reported several useful characteristics of rhizospheric-derived E. faecium strains (Klibi et al., 2012), to date, the cholesterol-lowering capability and probiotic potential of E. faecium isolated from rhizospheric soil have not been reported. To the best of our knowledge, our study is the first report on isolation and probiotic characterization of E. faecium isolates from rhizospheric soils, which also exhibited a significant cholesterol-lowering capability, in vitro.
A total of 15 rhizospheric soil samples were collected from different geographical regions of India (Hyderabad, Uttar Pradesh, Haridwar, and New Delhi) following the standard microbiological methods. Briefly, samples were collected in sterilized zipped plastic bags and transported to the laboratory and stored at 10°C. The samples were processed within a week of transport. One gram of the sample was added to screw-capped plastic bottles containing 99 ml of De Man, Rogosa and Sharpe (MRS) broth and incubated at 30°C for 48 h. One milliliter of culture from each bottle was re-inoculated into fresh MRS and again incubated for 48 h. This procedure was repeated thrice and the contents of each bottle were serially diluted and 100 μl of the diluted sample was spread on MRS agar plates that were incubated at 30°C till the colonies became visible. The isolated colonies were picked up randomly. Five cultures that yielded pure colonies were presumptively identified as LAB on the basis of growth on selective medium, Gram-positive staining, and catalase negative test (Holt et al., 1994; Day et al., 2001). The purified cultures were preserved at -80°C and sub-cultured at 30°C prior to use.
The five Gram-positive, catalase-negative LAB isolated from rhizospheric soil were designated as LR2, LR3, ER5, LR13, and VB1. For molecular identification of the LAB, genomic DNA was isolated using a commercial mdi Genomic DNA Miniprep Kit (Ambala Cantt, India). PCR amplification of the gene encoding 16S rRNA was carried out using the universal eubacterial primers in a Thermal Cycler (My CyclerTM, Bio-Rad, United States). Sequences of the forward and reverse primers were 5′AGAGTTTGATCCTGGCTCAG3′ and 5′ACGGCTACCTTGTTACGACTT3′, respectively. The PCR reaction mixture contained 1× PCR buffer (1.5 MgCl2, 1.5 mM KCl, 10 mM Tris–HCL, and 0.1% Triton X-100), 200 μM each of the four dNTPs, 12 U of Taq DNA polymerase (New England Biolabs, Ipswich, MA, United States), 10 pmol of forward and reverse primers, and 100 ng of genomic DNA in a final volume of 25 μl. The PCR reaction cycles were as follows: initial denaturation for 5 min at 95°C followed by 30 consecutive cycles of 30 s at 95°C, 30 s at 58°C, and 90 s at 72°C followed by a final extension for 10 min at 72°C. The PCR amplicons were electrophoresed on 1% agarose gels and visualized under UV light. The PCR amplicons were purified using QIA Quick Gel Extraction Kit (Qiagen, Hilden, Germany) and sequenced at a commercial facility (M/s Invitrogen India, Gurgaon, India). The gene sequences were searched for homology using BLASTN1.
The effect of different temperatures on growth and survival of E. faecium isolates was studied by incubating the isolates in MRS broth at 28, 37, and 45°C. Survival in salt was studied by growing the strains in MRS broth containing different concentrations of NaCl (0.5, 2, 3, 4, 5, 7, and 10%) at 37°C. In each case, the absorbance of the control and inoculated samples was measured at 600 nm after 24 h. The experiments were repeated for each isolate in triplicate and the mean values were reported ± SD.
The resistance of E. faecium isolates to lysozyme was determined following a published method (Zago et al., 2011) and viable cell counts were determined at 0, 0.5, 1, and 2 h. The experiment was repeated for each isolate in triplicate and the mean values were reported ± SD.
Acid tolerance was assessed by incubating the overnight grown E. faecium isolates in three separate flasks of MRS broth with pH adjusted to 1.5, 2, and 3 at 37°C. A 100-μl aliquot was drawn from each flask after 0, 1, and 3 h. Appropriate serial dilutions were made and incubated at 37°C for 18–24 h on MRS plates, and the number of colonies was calculated as log10 CFU/ml. For determining bile tolerance, overnight grown E. faecium strains were incubated in MRS broth containing different concentrations of bile salts (cholic and deoxycholic acid sodium salt; Sigma-Aldrich): 0.06, 0.125, 0.25, 0.5, and 1% (w/v). The cultures were incubated at 37°C, and absorbance was recorded at 600 nm after 24 h. Both the experiments were repeated for each isolate in triplicate, and the mean values were reported ± SD.
Simulated gastric juice was prepared by dissolving 0.3% (w/v) pepsin in saline (0.5%, v/v). The pH was adjusted to 3.0. Overnight grown LAB cultures were centrifuged, and the washed bacterial pellet was suspended in 4 ml of sterile NaCl (0.8%) solution. One milliliter of the cell suspension was mixed with 9 ml of gastric juice (pH 3) and vortexed for 15 s. The cultures were incubated at 200 rpm, and viable cell counts were determined at 0, 2, and 4 h at 37°C. The experiment was repeated for each isolate in triplicate, and the mean values were reported ± SD.
The ability of the LAB to adhere to surfaces was assessed by measuring their cell surface hydrophobicity following the protocol described earlier (Lee et al., 2011). The experiment was repeated for each isolate in triplicate and the mean values were reported ± SD.
The auto-aggregation capability of E. faecium isolates was assessed using a published method (Juárez Tomás et al., 2005). The capability of E. faecium isolates for co-aggregation was quantified by measuring their co-aggregation with Escherichia coli NG9 following a published protocol (Golowczyc et al., 2007). The co-aggregation was expressed as the percentage reduction in the absorbance of the mixed suspension compared to the individual suspensions. Both the experiments were repeated for each isolate in triplicate, and the mean values were reported ± SD.
For assessment of BSH activity, a sterile cork borer was used to punch wells of 6-mm diameter in 0.5% bile salt (cholic and deoxycholic acid sodium salt; Sigma-Aldrich, United States) supplemented MRS plates. Two hundred microliters of MRS broth containing overnight grown culture of E. faecium isolates was added to each well. Plates were kept at 37°C for 24–48 h and visually checked for appearance of white zone of precipitation around the wells.
Primers were designed to amplify the complete coding sequence (CCDS) of BSH by retrieving the whole genome sequence of E. faecium and the corresponding sequence of bsh from NCBI, along with some upstream and downstream regions of the gene. Sequences of the forward and reverse primers were BF 5′TGGAATGAGGAAAACTTTGGAG3′ and BR 5′CTGTTTTCTGGATCAACGA3′. The PCR reaction mixture and reaction conditions were the same as for amplification of gene encoding 16S rRNA, except the annealing temperature, which was kept at 55°C. The PCR amplicons were purified, sequenced, and analyzed for protein homology using the same methods as described for sequencing of the 16S rRNA gene. The CCDS of bsh of all the E. faecium isolates were translated using ExPASy2 and aligned using Clustal ‘Ω3.
The capability of E. faecium isolates to assimilate cholesterol in MRS broth supplemented with and without bile salts was assessed using a modified method (Rudel and Morris, 1973). Cholesterol was added to MRS broth supplemented with 6 mM sodium tauroglycocholate at a final concentration of 70 μg/ml. E. faecium strains were incubated in this medium at 37°C for 20 h. The samples were centrifuged; the pellet was washed with distilled water and kept at 80°C in an oven to obtain constant dry weight. One milliliter of the supernatant was added to 2 ml of 95% ethanol followed by 1 ml KOH (33%). The mixture was vortexed and heated at 37°C for 15 min. Then, 3 ml of n-hexane and 2 ml of distilled water were added and vortexed. The phases were allowed to separate at room temperature. Hexane layer was transferred to another test tube and evaporated at 90°C to obtain the residue. The residue was dissolved in 2 ml of o-phthalaldehyde (5 mg/ml) and mixed followed by the addition of 0.5 ml of concentrated sulfuric acid. The mixture was allowed to stand at room temperature for 10–15 min, and the absorbance of inoculated and un-inoculated samples was recorded at 570 nm. A standard curve of absorbance versus different concentrations of cholesterol in MRS (0, 3.91, 7.81, 15.63, 31.25, 62.5, 125, 250, and 500 μg/ml) was obtained. The rate of cholesterol assimilation by various isolates was calculated using the following formula:
The percent cholesterol assimilation was calculated using the formula:
The experiments were repeated for each isolate in triplicate and the mean percentage of cholesterol assimilation was reported ± standard deviation (SD).
The biofilm-forming potential of the enterococcal isolates was determined by crystal violet assay following the published protocols with slight modifications, at different pH values, viz., 4, 5, and 6, and at different time points, viz., 24, 48, and 72 h (Stepanović et al., 2000; Sharma et al., 2018). Briefly, 50 μl of overnight grown E. faecium strains (∼1 × 109 cells/ml) were inoculated in 1 ml of MRS broth, contained in 1.5 ml of polypropylene microcentrifuge tubes (Tarsons, United States). The bacteria were incubated at 37°C for different time periods, to allow the formation of biofilms. After the incubation period, medium was decanted and the microcentrifuge tubes were dried at 55°C for 30 min. One milliliter of 0.1% crystal violet (prepared in isopropanol:methanol:phosphate-buffered saline in the ratio of 1:1:18, v/v) was added to the microcentrifuge tubes and incubated at room temperature for 30 min. After this, crystal violet was removed, followed by two washings with 1 ml of sterile distilled water. Microcentrifuge tubes were further dried at 55°C for 30 min. The dye bound to the biofilm was dissolved in a 200-μl mixture of ethanol and acetone (4:1 v/v), and 100 μl of this mixture was added to a 96-well microtiter plate. Optical density was measured at 540 nm using an ELISA plate reader (Thermo Scientific, United States). Un-inoculated MRS broth was considered as the negative control. The cutoff OD (ODc) was defined as three standard deviations above the mean OD of the negative control. The strains were classified as strong, moderate, or weak biofilm producers based on the ODs of bacterial biofilms, using the following criteria:
The experiment was performed for each strain in triplicate and the average result ± SD was reported. The statistical significance was calculated using the Mann–Whitney U test with R statistical package. A p-value < 0.05 was considered as significant.
The hemolytic activities of E. faecium isolates were determined using the published protocol (Pieniz et al., 2014). Production of the enzyme DNase was determined using the method suggested by Gupta and Malik (2007).
The antibiotic susceptibilities of E. faecium isolates were assessed using the Kirby Bauer disk diffusion method on Muller–Hinton (MH) agar plates. The antibiotic disks (HiMedia, India) for antibiotics that are commonly used for treatment of enterococcal infections were placed on the surface of agar, and plates were incubated at 37°C for 24 h. Results were interpreted as per the guidelines of the Clinical Laboratory Standards Institute (Clinical and Laboratory Standards Institute [CLSI], 2010).
Presence of virulence factors in the genomes of E. faecium isolates was investigated by PCR—amplification of the genes encoding eight major virulence factors, viz., aggregation protein (agg), enterococcal surface protein involved in immune evasion (esp), extracellular metallo-endopeptidase (gelE), cytolysin (cyl), cell wall adhesins (efaAfm), chemotactic for human leukocytes and facilitate conjugation (cpd, cob, and ccf). The primer sequences and the PCR conditions were the same as described in previous studies (Eaton and Gasson, 2001; Vankerckhoven et al., 2004; Sabia et al., 2008) and are presented in Table 1.

Table 1. Primers used for detection of genes for virulence factors in E. faecium strains isolated from rhizosphere.
The experiments were performed for each isolate in triplicate and the results were reported as mean ± SD of triplicates. The values of the mean and SD were calculated using Microsoft Excel.
Fifteen samples of rhizospheric soils were collected from different regions of India to isolate potential probiotic strains from LAB. Of these, five pure cultures named LR2, LR3, ER5, LR13, and VB1 were presumptively identified as LAB. The results of 16S rRNA gene sequencing and homology search using BLAST confirmed that these strains were E. faecium. The GenBank accession numbers of strains LR2, LR3, ER5, LR13, and VB1 are MK461568, MK461567, MK461570, MK461571, and MK461569, respectively.
Appearance of a white zone of precipitation around the wells in bile-supplemented MRS indicated that E. faecium strain LR2 produced the enzyme BSH, while E. faecium strains LR3 ER5, LR13, and VB1 did not.
The primer pair (BF and BR) used for amplification of bsh resulted in the desired amplicon of 1.157 kbp in all the isolates. The purification, sequencing, and BLAST analysis of the PCR amplicons confirmed that these encoded for the bsh gene in E. faecium isolates. Multiple sequence alignment of the translated bsh revealed that the amino acid sequences of BSH of all E. faecium strains were identical (Figure 1).

Figure 1. Multiple sequence alignment (MSA) of amino acid sequences of bile salt hydrolases (BSH) present in E. faecium LR2, LR3, ER5, LR13, and VB1 strains.
Though all the E. faecium isolates assimilated > 50% of the cholesterol present in the medium, the percentage of cholesterol assimilation increased in the presence of bile salt. Of all the strains, E. faecium LR13 showed a significantly higher capability than other strains to assimilate cholesterol from the medium, i.e., 76% in medium without bile salts and 98% in medium with bile salts (Table 2).
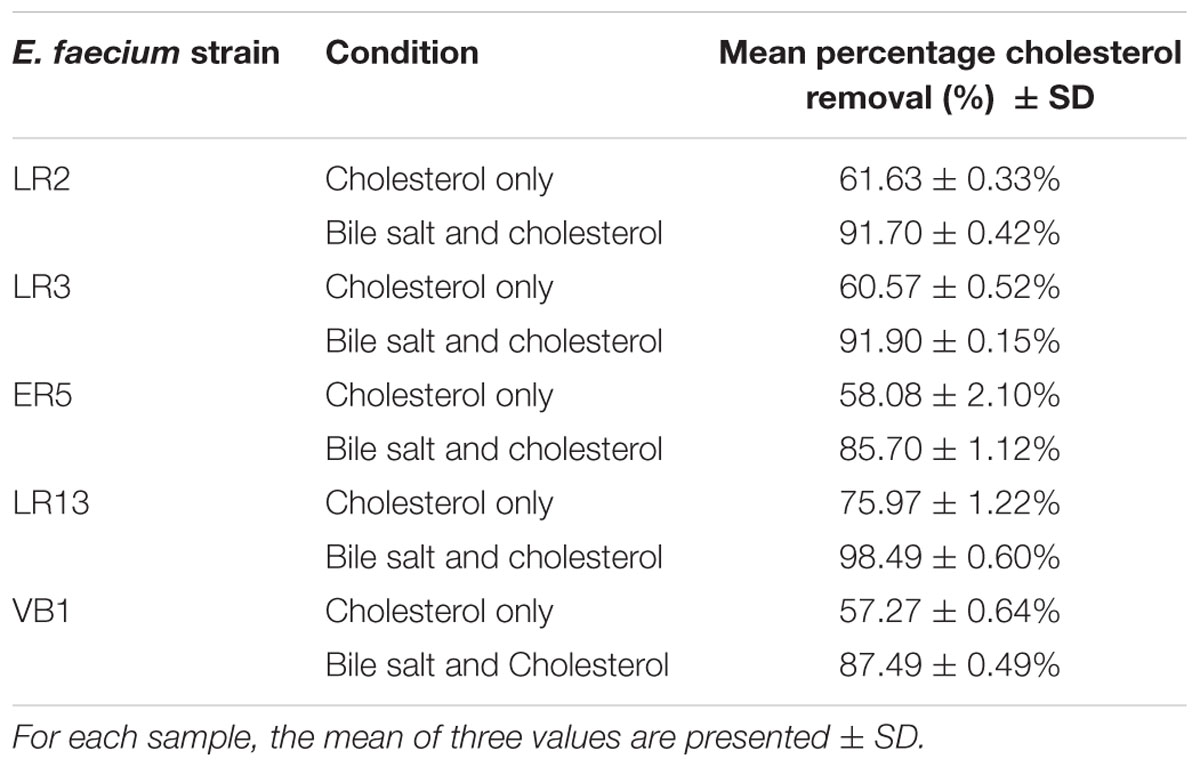
Table 2. Mean percentage cholesterol removal in vitro by E. faecium strains in MRS medium with and without bile salt.
All the five E. faecium isolates grew well in the temperature range of 28–45°C with optimum growth observed at 37°C. Similarly, all the isolates thrived in increasing concentrations of salt, with viabilities affected by not more than 0.5 log CFU as the salt concentration increased from 0.5 to 10%. Also, all the isolates showed high tolerance to lysozyme, with average percent survival being 75–80% even after 2 h of incubation (results not shown).
None of the E. faecium strain survived at pH 1.5 and 2.0. However, all the strains maintained their viability at pH 3.0 even after 3 h (Table 3). Similarly, all strains of E. faecium survived well in simulated gastric juice (pH 3.0) even after 3 h (Table 4).
The number of colonies of all the isolates decreased as the concentration of bile salt increased from 0.06 to 1%, but all survived in concentration of 0.5% bile salt even after 2–4 h (Table 5).
The antibiotic susceptibility profile of E. faecium isolates revealed that all the isolates were sensitive to gentamicin but resistant to kanamycin and erythromycin. Also, all the isolates were sensitive to amoxicillin, amoxicillin–clavulanic acid, and ciprofloxacin, except ER5 and VB1. Vancomycin resistance was observed in LR2, ER5, and VB1, while LR3 and LR13 were susceptible to this antibiotic (Table 6).
All the E. faecium isolates showed a moderate ability to form biofilms at pH 4, 5, and 6 after time intervals of 24, 48, and 72 h (Table 7). It was also observed that none of the E. faecium isolates harbored any of the genes for virulence investigated in this study.
The present study is the first report on isolation of E. faecium from rhizospheric soils and assessment of their probiotic potential. The isolates were collected from sites located in northern, southern, and central regions of India. Rhizospheric soil represents an interesting ecological niche for isolation of probiotic bacteria as these bacteria directly co-evolve with plant pathogenic bacteria and fungi, and are competent to survive in the naturally microbe-enriched human GI tract. The five pure rhizospheric isolates were confirmed by 16S rRNA gene sequencing as E. faecium and evaluated for BSH activity, cholesterol-lowering capability, and probiotic properties.
It is important to evaluate the BSH activity of bacteria proposed as probiotics, because BSH has been recommended by the WHO as an important criterion for selection of probiotics. Results of the BSH phenotypic assay indicated that BSH was present only in strain LR2 and absent in other strains. However, PCR amplification and sequence analysis confirmed that bsh gene was present in all the strains. This non-concordance between the BSH phenotypic assay and presence of bsh gene indicates that phenotypic assay might not be a good indicator for screening of BSH activity in E. faecium. The presence of bsh in all rhizospheric E. faecium isolates was an interesting observation because BSH activity has rarely been reported from bacteria isolated from rhizospheric soil. Also, the fact that the amino acid sequences of BSH were identical in all the isolates indicates that these proteins are highly conserved in E. faecium.
All the rhizospheric E. faecium isolates showed a remarkable capability to assimilate cholesterol, which increased further in the presence of bile salts. In comparison to others, E. faecium isolate LR13 showed a significantly higher capability to assimilate cholesterol, i.e., 75% in medium without bile salts and 98% in medium with bile salts. This capability was much higher than the in vitro cholesterol removal capability of 42–55% reported previously for a probiotic E. faecium strain VC223 (Albano et al., 2018). This suggested that rhizospheric enterococci may be considered as good probiotic candidates, provided they satisfy other essential criteria for probiotic potential.
There are several characteristics that are essential for a probiotic strain to confer beneficial effects on the consumer, like adherence to the epithelial cells of the gut, tolerance to lysozyme, gastric juice, low pH, bile, and safety for human consumption. All the strains of E. faecium were salt tolerant and showed optimum growth at 37°C. Lysozyme disrupts the cell wall of Gram-positive bacteria. Since lysozyme is present in the human saliva; it is the first assault that probiotic bacteria must overcome to reach the human gut. It was observed that all the isolates of E. faecium grew well in high concentration of lysozyme (100 mg/l), even after contact with lysozyme for 2 h. Another attribute that probiotics need to possess to survive in the human gut is the low pH of the stomach, which might range from 1.5 to 2.0 and the gastric acid (pH 3.0) for at least 3–4 h. Though E. faecium isolates did not tolerate very low pH, i.e., 1.5 and 2.0, they survived at pH 3.0 for 3–4 h. Similarly, all the isolates survived in simulated gastric juice, even after 24 h. Thus, our results are in concordance with the results of previous studies (Banwo et al., 2013; Nami et al., 2014, 2018). After their passage through the stomach, probiotic bacteria have to combat the bile juices present in the intestine. A good tolerance to 0.3–0.5% bile helps the probiotic in easy colonization of the host gut (Luo et al., 2012). Thus, it was important to evaluate the survival of probiotic organisms in the presence of bile salts. All the isolates of E. faecium survived fairly in 0.5% bile salt, even after exposure for 2–4 h. Once inside the intestine, putative probiotics should adhere to intestinal mucosa, which facilitates transient colonization and impedes their removal by peristalsis. The biofilm potential of bacteria reflects their potential for cell adherence (Boris et al., 1997). All the isolates of E. faecium showed a moderate biofilm potential at pH 6, which is similar to the physiological pH of the intestine. The ability of probiotic bacteria to auto-aggregate helps in adherence to the epithelial cells and mucosal surfaces (Collado et al., 2008), while the ability to co-aggregate helps in excluding pathogenic bacteria from colonizing the human gut (Abushelaibi et al., 2017). Hence, these characteristics are also important classifiers for probiotics. All the isolates of E. faecium showed a moderate auto- and co-aggregation capabilities. None of the E. faecium isolates showed hemolytic or DNase activity, indicating their safety in potential probiotic preparations.
The safety of the enterococci isolates as good probiotics was also evaluated with regard to presence of virulence factors and antibiotic profile. The E. faecium isolates were evaluated for the presence of several virulence genes, viz., esp, gelE, cyl, efaAfm, cpd, cob, and ccf. As reported for another E. faecium probiotic strain SF68, none of the isolates harbored genes for virulence factors and, thus, may be considered as safe probiotic candidates (Kayser, 2003). The FAO/WHO (2002) recommended that, as a safety measure, the antibiotic resistance profile of a proposed probiotic should also be evaluated. The antibiotic susceptibility testing of E. faecium strains revealed that isolates ER5 and VBI were resistant to β-lactams, fluoroquinolones, kanamycin, and vancomycin, except gentamycin; hence, they might not be safe for human consumption. Many Enterococcus strains show an intrinsic resistance for β-lactam antibiotics (Lopes et al., 2005). Earlier studies reported a higher rate of aminoglycoside resistance in enterococcal strains isolated from dairy products (Hammad et al., 2015; Aspri et al., 2017); however, our results indicated that though all the rhizospheric soil isolates were resistant to kanamycin, they were susceptible to gentamycin. Thus, based on the susceptibility for the most common antibiotics used against Enterococcus infections (Favaro et al., 2014), it may be inferred that E. faecium isolates LR2 and LR13 might be considered as potential probiotic candidates.
In this study, we have reported, for the first time, isolation and characterization of E. faecium from rhizospheric soil samples collected from different parts of India. Our results indicated that all the rhizospheric isolates showed a fair survival in the harsh conditions of the GI tract and did not harbor any of the virulence genes. Though all isolates showed potential for cholesterol removal, E. faecium isolate LR13 showed remarkable cholesterol removal capability and vancomycin susceptibility and possessed most of the desirable and essential attributes of a probiotic. Hence, it seems to be a fairly promising probiotic candidate that needs to be further evaluated in in vivo studies, especially for its hypo-cholesterolemic potential.
The datasets generated for this study can be found in the GenBank, MK461568, MK461567, MK461570, MK461571, and MK461569.
NS designed the study. NS, AM, MK, and SM carried out the experiments. NS, MK, and JV drafted the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
NS is thankful to the Science and Engineering Research Board (SERB) of the Department of Science & Technology (SB/YS/LS-156/2014) for the fellowship.
Abushelaibi, A., Al-Mahadin, S., El-Tarabily, K., Shah, N. P., and Ayyash, M. (2017). Characterization of potential probiotic lactic acid bacteria isolated from camel milk. LWT Food Sci. Technol. 79, 316–325. doi: 10.1155/2018/7970463
Ahn, Y. T., Kim, G. B., Lim, K. S., Baek, Y. J., and Kim, H. U. (2003). Deconjugation of bile salts by Lactobacillus acidophilus isolates. Int. Dairy J. 13, 303–311. doi: 10.1016/s0958-6946(02)00174-7
Albano, C., Morandi, S., Silvetti, T., Casiraghi, M. C., Manini, F., and Brasca, M. (2018). Lactic acid bacteria with cholesterol-lowering properties for dairy applications: In vitro and in situ activity. J. Dairy Sci. 101, 10807–10818. doi: 10.3168/jds.2018-15096
Arias, C. A., and Murray, B. E. (2012). The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. 10:266. doi: 10.1038/nrmicro2761
Aspri, M., Bozoudi, D., Tsaltas, D., Hill, C., and Papademas, P. (2017). Raw donkey milk as a source of Enterococcus diversity: assessment of their technological properties and safety characteristics. Food Control 73, 81–90. doi: 10.1016/j.foodcont.2016.05.022
Banwo, K., Sanni, A., and Tan, H. (2013). Technological properties and probiotic potential of Enterococcus faecium strains isolated from cow milk. J. Appl. Microbiol. 114, 229–241. doi: 10.1111/jam.12031
Begley, M., Hill, C., and Gahan, C. G. (2006). Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 72, 1729–1738. doi: 10.1128/aem.72.3.1729-1738.2006
Boris, S., Suarez, J. E., and Barbes, C. (1997). Characterization of the aggregation promoting factor from Lactobacillus gasseri, avaginal isolate. J. Appl. Microbiol. 83, 413–420. doi: 10.1046/j.1365-2672.1997.00250.x
Clinical and Laboratory Standards Institute [CLSI] (2010). Performance Standards for Antimicrobial Susceptibility Testing. 20th Informational Supplement (M100–S20). Wayne, PA: Clinical and Laboratory Standards Institute.
Collado, M. C., Meriluoto, J., and Salminen, S. (2008). Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 226, 1065–1073. doi: 10.1007/s00217-007-0632-x
Day, A. M., Sandoe, J. A., Cove, J. H., and Phillips-Jones, M. K. (2001). Evaluation of a biochemical test scheme for identifying clinical isolates of Enterococcus faecalis and Enterococcus faecium. Lett. Appl. Microbiol. 33, 392–396. doi: 10.1046/j.1472-765X.2001.01017.x
Eaton, T. J., and Gasson, M. J. (2001). Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 67, 1628–1635. doi: 10.1128/AEM.67.4.1628-1635.2001
Ehrmann, M. A., Kurzak, P., Bauer, J., and Vogel, R. F. (2002). Characterization of Lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 92, 966–975. doi: 10.1046/j.1365-2672.2002.01608.x
Ejtahed, H. S., Mohtadi-Nia, J., Homayouni-Rad, A., Niafar, M., Asghari-Jafarabadi, M., Mofid, V., et al. (2011). Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J. Dairy Sci. 94, 3288–3294. doi: 10.3168/jds.2010-4128
Elkins, C. A., Moser, S. A., and Savage, D. C. (2001). Genes encoding bile salt hydrolases and conjugated bile salt transporters in Lactobacillus johnsonii 100-100 and other Lactobacillus species. Microbiology 147, 3403–3412. doi: 10.1099/00221287-147-12-3403
FAO/WHO (2002). Guidelines for the Evaluation of Probiotics in Food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. Rome: FAO.
Favaro, L., Basaglia, M., Casella, S., Hue, I., Dousset, X., de Melo Franco, B. D. G., et al. (2014). Bacteriocinogenic potential and safety evaluation of non-starter Enterococcus faecium strains isolated from home made white brine cheese. Food Microbiol. 38, 228–239. doi: 10.1016/j.fm.2013.09.008
Franz, C. M., Huch, M., Abriouel, H., Holzapfel, W., and Gálvez, A. (2011). Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 151, 125–140. doi: 10.1016/j.ijfoodmicro.2011.08.014
Golowczyc, M. A., Mobili, P., Garrote, G. L., Abraham, A. G., and De Antoni, G. L. (2007). Protective action of Lactobacillus kefir carrying S-layer protein against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 118, 264–273. doi: 10.1016/j.ijfoodmicro.2007.07.042
Guo, L., Li, T., Tang, Y., Yang, L., and Huo, G. (2016). Probiotic properties of Enterococcus strains isolated from traditional naturally fermented cream in China. Microb. Biotechnol. 9, 737–745. doi: 10.1111/1751-7915.12306
Gupta, H., and Malik, R. K. (2007). Incidence of virulence in bacteriocin-producing enterococcal isolates. Le Lait 87, 587–601. doi: 10.1051/lait:2007031
Ha, C. G., Cho, J. K., Lee, C. H., Chai, Y. G., Ha, Y., and Shin, S. H. (2006). Cholesterol lowering effect of Lactobacillus plantarum isolated from human feces. J. Microbiol. Biotechnol. 16, 1201–1209. doi: 10.1080/09168451.2018.1497939
Hammad, A. M., Hassan, H. A., and Shimamoto, T. (2015). Prevalence, antibiotic resistance and virulence of Enterococcus spp. in Egyptian fresh raw milk cheese. Food Control 50, 815–820. doi: 10.1016/j.foodcont.2014.10.020
Hlivak, P., Odraska, J., Ferencik, M., Ebringer, L., Jahnova, E., and Mikes, Z. (2005). One-year application of probiotic strain Enterococcus faecium M-74 decreases serum cholesterol levels. Bratisl Lek Listy 106, 67–72.
Holt, J. G., Krieg, N. R., Sneath, P. H., Staley, J. T., and Williams, S. T. (1994). Bergey’s Manual of Determinative Bacteriology, 9th Edn. Baltimore, MD: Lippincott Williams and Wilkins.
Hyrslova, I., Krausova, G., Bartova, J., Kolesar, L., Jaglic, Z., Stankova, B., et al. (2016). Characterization of Enterococcus faecium CCDM 922 in respect of its technological and probiotic properties. Int. J. Curr. Microbiol. Appl. Sci. 5, 474–482. doi: 10.20546/ijcmas.2016.505.049
Juárez Tomás, M. S., Wiese, B., and Nader-Macías, M. E. (2005). Effects of culture conditions on the growth and auto-aggregation ability of vaginal Lactobacillus johnsonii CRL 1294. J. Appl. Microbial. 99, 1383–1391. doi: 10.1111/j.1365-2672.2005.02726.x
Kayser, F. H. (2003). Safety aspects of enterococci from the medical point of view. Int. J. Food Microbial. 88, 255–262. doi: 10.1016/s0168-1605(03)00188-0
Klibi, N., Slimen, N. B., Fhoula, I., López, M., Slama, K. B., Daffonchio, D., et al. (2012). Genotypic diversity, antibiotic resistance and bacteriocin production of enterococci isolated from rhizospheres. Microb. Environ. 27, 533–537. doi: 10.1264/jsme2.ME12041
Lee, J., Yun, H. S., Cho, K. W., Oh, S., Kim, S. H., Chun, T., et al. (2011). Evaluation of probiotic characteristics of newly isolated Lactobacillus spp.: immune modulation and longevity. Int. J. Food Microbiol. 148, 80–86. doi: 10.1016/j.ijfoodmicro.2011.05.003
Lopes, M. F. S., Ribeiro, T., Abrantes, M., Marques, J. J. F., Tenreiro, R., and Crespo, M. T. B. (2005). Antimicrobial resistance profiles of dairy and clinical isolates and type strains of enterococci. Int. J. Food Microb. 103, 191–198. doi: 10.1016/j.ijfoodmicro.2004.12.025
Luo, Y., Ma, B. C., Zou, L. K., Cheng, J. G., Cai, Y. H., Kang, J. P., et al. (2012). Identification and characterization of lactic acid bacteria from forest musk deer feces. Afr. J. Microbiol. Res. 6, 5871–5881. doi: 10.5897/AJMR12.807
Lye, H. S., Rusul, G., and Liong, M. T. (2010). Removal of cholesterol by Lactobacilli via incorporation and conversion to coprostanol. J. Dairy Sci. 93, 1383–1392. doi: 10.3168/jds.2009-2574
Montealegre, M. C., Singh, K. V., and Murray, B. E. (2015). Gastrointestinal tract colonization dynamics by different Enterococcus faecium clades. J. Infect. Dis. 213, 1914–1922. doi: 10.1093/infdis/jiv597
Moser, S. A., and Savage, D. C. (2001). Bile salt hydrolase activity and resistance to toxicity of conjugated bile salts are unrelated properties in Lactobacilli. Appl. Environ. Microbiol. 67, 3476–3480. doi: 10.1128/aem.67.8.3476-3480.2001
Nami, Y., Abdullah, N., Haghshenas, B., Radiah, D., Rosli, R., and Khosroushahi, A. Y. (2014). Probiotic assessment of Enterococcus durans 6HL and Lactococcus lactis 2HL isolated from vaginal microflora. J. Med. Microb. 63, 1044–1051. doi: 10.1099/jmm.0.074161-0
Nami, Y., Haghshenas, B., Vaseghi Bakhshayesh, R., Mohammadzadeh Jalaly, H., Lotfi, H., Eslami, S., et al. (2018). Novel autochthonous lactobacilli with probiotic aptitudes as a main starter culture for p robiotic fermented milk. LWT 98, 85–93. doi: 10.1016/j.lwt.2018.08.035
Nami, Y., Vaseghi Bakhshayesh, R., Mohammadzadeh Jalaly, H., Lotfi, H., Eslami, S., and Hejazi, M. A. (2019). Probiotic properties of Enterococcus isolated from artisanal dairy products. Front. Microbiol. 10:300. doi: 10.3389/fmicb.2019.00300
Nissen, L., Chingwaru, W., Sgorbati, B., Biavati, B., and Cencic, A. (2009). Gut health promoting activity of new putative probiotic/protective Lactobacillus spp. strains: a functional study in the small intestinal cell model. Int. J. Food Microbiol. 135, 288–294. doi: 10.1016/j.ijfoodmicro.2009.08.027
Ogier, J. C., and Serror, P. (2008). Safety assessment of dairy microorganisms: the Enterococcus genus. Int. J. Food Microbiol. 126, 291–301. doi: 10.1016/j.ijfoodmicro.2007.08.017
Park, Y. H., Kim, J. G., Shin, Y. W., Kim, S. H., and Whang, K. Y. (2007). Effect of dietary inclusion of Lactobacillus acidophilus ATCC 43121 on cholesterol metabolism in rats. J. Microbiol. Biotechnol. 17, 655–662.
Pieniz, S., Andreazza, R., Anghinoni, T., Camargo, F., and Brandelli, A. (2014). Probiotic potential, antimicrobial and antioxidant activities of Enterococcus durans strain LAB18s. Food Control 37, 251–256. doi: 10.1016/j.foodcont.2013.09.055
Rosenthal, V. D., Maki, D. G., Mehta, A., Álvarez-Moreno, C., Leblebicioglu, H., Higuera, F., et al. (2008). International nosocomial infection control consortium report, data summary for 2002-2007, issued January 2008. Am. J. Infect. Control 36, 627–637. doi: 10.1016/j.ajic.2008.03.003
Rudel, L. L., and Morris, M. D. (1973). Determination of cholesterol using o-phthalaldehyde. J. Lipid Res. 14, 364–366.
Sabia, C., De Niederhäusern, S., Guerrieri, E., Messi, P., Anacarso, I., Manicardi, G., et al. (2008). Detection of bacteriocin production and virulence traits in vancomycin-resistant enterococci of different sources. J. Appl. Microbiol. 104, 970–979. doi: 10.1111/j.1365-2672.2007.03612.x
Serio, A., Chaves-López, C., Paparella, A., and Suzzi, G. (2010). Evaluation of metabolic activities of enterococci isolated from pecorino abruzzese cheese. Int. Dairy J. 20, 459–464. doi: 10.1016/j.idairyj.2010.02.005
Sharma, P., Kaur, S., Kaur, R., Kaur, M., and Kaur, S. (2018). Proteinaceous secretory metabolites of probiotic human commensal Enterococcus hirae 20c, E. faecium 12a and L12b as antiproliferative agents against cancer cell lines. Front. Microbiol. 9:948. doi: 10.3389/fmicb.2018.00948
Stepanović, S., Vuković, D., Dakić, I., Saviæ, B., and Švabiæ-Vlahoviæ, M. (2000). A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbial. Methods 40, 175–179. doi: 10.1016/s0167-7012(00)00122-6
Vankerckhoven, V., Van Autgaerden, T., Vael, C., Lammens, C., Chapelle, S., Rossi, R., et al. (2004). Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J. Clin. Microbiol. 42, 4473–4479. doi: 10.1128/JCM.42.10.4473-4479.2004
Zago, M., Fornasari, M. E., Carminati, D., Burns, P., Suàrez, V., Vinderola, G., et al. (2011). Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol. 28, 1033–1040. doi: 10.1016/j.fm.2011.02.009
Keywords: bile salt hydrolase, Enterococcus faecium, cholesterol-lowering, probiotics, hypo-cholesterolemia
Citation: Singhal N, Maurya AK, Mohanty S, Kumar M and Virdi JS (2019) Evaluation of Bile Salt Hydrolases, Cholesterol-Lowering Capabilities, and Probiotic Potential of Enterococcus faecium Isolated From Rhizosphere. Front. Microbiol. 10:1567. doi: 10.3389/fmicb.2019.01567
Received: 15 March 2019; Accepted: 24 June 2019;
Published: 16 July 2019.
Edited by:
Haifeng Zhao, South China University of Technology, ChinaReviewed by:
Jorge Reinheimer, National University of the Littoral, ArgentinaCopyright © 2019 Singhal, Maurya, Mohanty, Kumar and Virdi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neelja Singhal, bmVlbGphMzBAZ21haWwuY29t; Manish Kumar, bWFuaXNoQHNvdXRoLmR1LmFjLmlu; Jugsharan Singh Virdi, dmlyZGlfZHVzY0ByZWRpZmZtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.