
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 07 June 2019
Sec. Plant Pathogen Interactions
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.01296
This article is part of the Research Topic Nematodes in Phytobiomes View all 21 articles
Under more intensified cropping conditions agriculture will face increasing incidences of soil-borne plant pests and pathogens, leading to increasingly higher yield losses world-wide. Soil-borne disease complexes, in particular, are especially difficult to control. In order to better understand soil-borne Meloidogyne-based disease complexes, we studied the volatile-based control mechanism of associated bacteria as well as the rhizospheric microbiome on Ugandan tomato plants presenting different levels of root-galling damage, using a multiphasic approach. The experimental design was based on representative samplings of healthy and infected tomato plants from two field locations in Uganda, to establish species collections and DNA libraries. Root galling symptoms on tomato resulted from a multispecies infection of root-knot nematodes (Meloidogyne spp.). Results revealed that 16.5% of the bacterial strain collection produced nematicidal volatile organic compounds (nVOC) active against Meloidogyne. Using SPME GC-MS, diverse VOC were identified, including sulfuric compounds, alkenes and one pyrazine. Around 28% of the bacterial strains were also antagonistic toward at least one fungal pathogen of the disease complex. However, antagonistic interactions appear highly specific. Nematicidal antagonists included Pseudomonas, Comamonas, and Variovorax and fungicidal antagonists belonged to Bacillus, which interestingly, were primarily recovered from healthy roots, while nematode antagonists were prominent in the rhizosphere and roots of diseased roots. In summary, all antagonists comprised up to 6.4% of the tomato root microbiota. In general, the microbiota of healthy and diseased root endospheres differed significantly in alpha and quantitative beta diversity indices. Bacteria-derived volatiles appear to provide a remarkable, yet wholly unexploited, potential to control Meloidogyne-based soil-borne disease complexes. The highly specific observed antagonism indicates that a combination of volatiles or VOC-producing bacteria are necessary to counter the range of pathogens involved in such complexes.
Agriculture causes long-lasting anthropogenic environmental impacts as it replaces natural vegetation, alters biogeochemical cycles, and decreases biodiversity; this defined a new human-dominated geological epoch, the Anthropocene. The soil disturbance by the conversion of land to agriculture has resulted in species extinctions 100–1,000 times higher than natural rates, and likely constitutes the beginning of the sixth mass extinction in Earth’s history (Lewis and Maslin, 2015). However, much less is known about the loss of microbial diversity (Berg et al., 2017) due to human activity than the loss of macroscopic diversity. In particular, bacteria occupy important niches and roles, linking plant microbial diversity and ecosystem functioning, such as productivity, host plant fitness and resilience (Bais et al., 2004; Tilman et al., 2012; Vandenkoornhuyse et al., 2015; Laforest-Lapointe et al., 2017). Among the most striking direct consequences of agricultural intensification is the elevated presence and impact of soil-borne pests and pathogens (Mendes et al., 2012). Soil-borne diseases are often “microbiome diseases”; they signify the result of a loss of microbial diversity and dysbiosis in soil and consequently in the rhizosphere and endosphere of plants (van Elsas et al., 2012). Once established, bacteria, fungi, and nematode pathogens accumulate, often as a synergistic combination, leading to high yield losses, which prove difficult to control (Oerke, 2006; Bennett et al., 2012). The soil environment is a complex arena, the biological permutations of which are little understood (Bais et al., 2004), especially in areas such as Africa, where assessments and understanding of the microflora and microbiota remain negligible.
Root-knot nematodes Göldi 1892 (RKN, genus Meloidogyne) infect over 5500 host plants, including plant species from nearly every extant known plant family (Trudgill and Blok, 2001). Species of Meloidogyne can be extremely polyphagous, are mainly parthenogenetic, and are highly adapted obligate sedentary plant parasites. They are regarded as the most economically important plant-pathogenic nematode group worldwide (Jones et al., 2013) and in the tropics, are viewed as the most significant biotic threat to crop production (Karssen et al., 2013; Coyne et al., 2018). Host roots are infected by freshly hatched, motile second stage juveniles (J2), which, upon establishing a feeding site behind the root tip, become sedentary, feeding from cells which it modifies to provide a constant supply of nutrients. Infection by RKN often leads to typical symptoms of root damage and gall formation, with above-ground symptoms of stunting, wilting, leaf chlorosis, reduced yield, which are symptomatic of water or nutrient scarcity (Karssen et al., 2013). Nematode-infected plants tend to be more susceptible to other diseases (Coyne et al., 2018). These unspecific above-ground symptoms, however, lead to an excess overuse of fertilizers and ineffective treatment with pesticides (Karungi et al., 2011). Excessive and frequent pesticide applications, in combination with inappropriate handling, increase the risks to human health as well as to water resources and the ecological system (Coyne et al., 2018). The impact of RKN on the host is significantly exaggerated through secondary pathogen infections, such as root rot pathogens, bacterial and fungal wilts, e.g., Fusarium oxysporum and Verticillium dahliae (Back et al., 2002; Karssen et al., 2013). These plant pathogens do not necessarily need the presence of RKN to successfully infect their hosts, but since RKN may act as casual agents, the disease can be seen as Meloidogyne-based disease complex. The interactions between RKN, secondary pathogens, host plant and plant-associated microorganisms lead to the resulting effects on plant health (Karssen et al., 2013). The management of RKN would therefore benefit from a more holistic approach, taking into consideration the management of soil-borne microbial complexes. A deep understanding of the plant-associated microbiomes would be beneficial, given that a proportion of microorganisms are antagonistic toward soil-borne pests and pathogens (Berg et al., 2002). One recently discovered indirect mode of plant disease prevention in bacteria is the production of volatile organic compounds (VOC) (Effmert et al., 2012; Cernava et al., 2015). VOC are semiochemicals that act as “long-range” allelochemicals in soil, which can have growth-promoting or -inhibiting effects on other microorganisms (Effmert et al., 2012) and plants (Ryu et al., 2003). The fact that VOC can have communicational, controlling or inhibitory effects that act inter- and/or intra-specifically, make them a highly interesting field of study for biological control (Effmert et al., 2012; Torto et al., 2018). However, despite their potential, VOC have received only limited attention and are yet to be fully exploited for biocontrol strategies. Our hypothesis was that RKN can be negatively affected and controlled by bacteria-derived VOC in the microbiome.
We selected tomato as a model plant, given its susceptibility to RKN and soil-borne disease complexes, using two RKN-infected field sites in Uganda. Tomatoes in Uganda are a key source of income and food security for smallholders (Ssekyewa, 2006) who often own less than 2 ha of land (Karungi et al., 2011). A major challenge in controlling the RKN-disease complex is the need for simultaneous control of all involved pathogens. Novel mechanisms are urgently needed to address these soil-borne challenges, especially in Africa where the need to sustainably intensify cropping production systems is critical (Bennett et al., 2012; Vanlauwe et al., 2014). Our current study focused on three main objectives: (i) identify the Meloidogyne species present, (ii) screen bacterial strains capable of producing nematicidal VOC or fungicidal metabolites, (iii) analyze the microbiome shift in the root endosphere due to RKN activity. Results of this study will create a better understanding of the soil-pathogen-host interactions in the RKN-disease complex, which will be translated to developing novel control strategies.
Three bulk samples, each comprising ten roots of fruit-bearing tomato plants (Solanum lycopersicum L.) with adhering soil, were collected from two field sites in Uganda in April 2017. Gall formation was categorized according to a root galling index (RGI) from 1 (no visible galling damage) to 5 (severe/lethal damage) (Coyne et al., 2007); two plants were selected for each RGI score from each site during uprooting. Sampling site “Luwero” (0°39′20″ N, 32°24′38″ E, 1187 m) was a rural farmer’s open field with 1-year-old virgin soil. Tomato cv. “Rio Grande,” received unknown application levels of pesticides but included generic fungicides, mainly mancozeb-based and the insecticides cypermethrin and chlorpyriphos. Sampling site “Namulonge” was at the IITA research station (0°31′46″ N, 32°36′45″ E, 1170 m) and consisted of a RKN-infected sandy soil within a concrete tomato outdoor bed with no direct connection to surrounding soils. Tomato cv. “Moneymaker” received no pesticide applications. A 300 g soil sample from each sample site was assessed for pH, nutrient (K, P, Mg, organic matter) content and soil type by “AGROLAB Agrar und Umwelt GmbH” (Sarstedt, Germany) to compare soil composition between the two sampling sites.
Samples were recovered from different microhabitats associated with RKN infection: bulk soil constituting the native bacterial community in the field, rhizosphere representing root-associated bacteria, galled and non-galled rhizoendosphere. Bacterial suspensions were recovered using 0.9% NaCl from a 5 g sub-sample of the bulk soil (soil between tomato plants, three samples/site, n = 6), from rhizosphere (root adhering soil, one/plant, n = 20) and surface sterilized sections of roots from both galled (RE-D, n = 16) and non-galled (RE-H, n = 17) roots. Suspensions were used for DNA extractions for both amplicon analysis and isolation of bacterial strains (for details, see Supplementary Material – Additional Methods). Suspensions were plated onto NA plates (nutrient terestingly agar; Sifin GmbH, Berlin, Germany); in total 260 strains were isolated and screened for nematicidal (see section “Screening for nVOC-Producing Strains”) and fungicidal properties (see section “Bacterial Antagonistic Activity Against Fungal Pathogens”). Extraction of the DNA pellet was conducted using “FastDNA Spin Kit for soil” (MP Biomedical, Eschwege, GER). PCR-products were cleaned with GENECLEAN TurboTM Kit (MP Biomedicals, Eschwege, GER) following the manufacturer’s instructions for genomic DNA. 16S rRNA gene amplifications were carried out in 3 × 30 μl reactions with the Illumina barcode universal bacterial primer set 515f-806r (Caporaso et al., 2011) and PNA Mix (Lundberg et al., 2013) to remove plastid DNA. PCR products of barcoded samples were pooled to equimolarity; sequencing was carried out by Eurofins MWG Operon (Ebersberg, GER1) with an Illumina HiSeq 2500 system (for details, see Supplementary Material – Additional Methods).
Randomly selected females with eggs were dissected from diseased roots. Perineal patterns of females were used for morphological diagnosis; body content of the same crushed females were used for molecular identification using the molecular key method of Adam et al. (2007). Furthermore, the region for NAD dehydrogenase subunit 5 was amplified for genetic determination using the body content of individual females (Janssen et al., 2016). Data were combined to determine species identification. Eggs were used to re-infect tomato seedlings in the fourth-true-leaf stadium to establish pure cultures. J2 of identified pure cultures were partially used in further experiments. For extracting J2, roots were rinsed free of adhering soil. Diseased root sections were chopped coarsely, placed in 1.2% NaOCl solution and blended with a hand blender for 3 min. The suspension was rinsed with tap water on nested 100–25 μm sieves. Eggs were caught on 25 μm sieve and collected into a beaker, which was aerated for 10 days to allow hatching. The J2 suspensions were placed on a Baermann funnel filter for 24 h at room temperature (Supplementary Figure S1A). The resulting ∼30 ml J2 suspension was stored horizontally in 50 ml Sarstedt tubes at 6°C until use.
We used a variation of the two clamp VOC assay (TCVA, Cernava et al., 2015): Bacterial strains were streaked on 12-well plates containing NA and incubated at 30°C for 24 h. Each plate had a blank well, containing NA only. Plates were inversed onto another 12-well plate containing ∼100 J2 of M. incognita (provided from Julius Kühn-Institut, Münster, Germany) on 2%-tap water agar. A silicon foil with a 5 mm hole between the two opposing chambers separated the two 12-well plates. The two plates were clamped together to provide airtight test conditions (Supplementary Figure S1B) and then maintained for 24 h at room temperature. Dead J2 were assigned dead if the body was straight and did not react when prodded with a dissection needle. Percentage J2 mortality was calculated after correcting for the blank value of the corresponding plate blank. Bacterial strains were categorized according to their activity: non-active (<10% mortality), slightly active (>10–80%), active (>80–95%), and highly active (>95%). Distributions of the number of bacterial strains within the categories were compared between sampling sites, microhabitat (healthy/diseased root, rhizosphere) and RGI. The experiment was repeated two more times for those samples demonstrating >80% nematicidal activity. The strains showing consistent nematicidal activity were identified by sequencing of 16S rRNA gene.
All 260 isolated bacterial strains were tested for their antagonistic activity against the fungal pathogens Botrytis cinerea, F. oxysporum, Fusarium verticillioides, Sclerotium rolfsii, and V. dahliae (provided by Institute of Environmental Biotechnology Graz) in dual cultures on Waxman agar in three replicates. Antifungal activity was categorized according to: 0 (fungi overgrow bacterial colony), +1 (hyphae reach bacteria, but do not overgrow), +2 (lateral inhibition zone <0.5 cm), and +3 (lateral inhibition zone >0.5 cm). The mean category across the three repeat assessments was calculated. Bacterial strains showing a strong antifungal effect (category +3) were compared using VENN2. DNA of strains with a mean antifungal activity of +3 against at least four pathogens (n = 23, see also Figure 2A) was extracted. A BOX-PCR was carried out to identify clones, resulting in five genotypes. 16S rRNA gene of the resulting five genotypes was amplified and sequenced for identification.
Nematicidal volatile organic compounds were identified using an adapted version of the method from Verginer et al. (2010) (for details, see Supplementary Material – Additional Methods). A total of nine compounds (purity >98%) partially found in the GC-MS samples were tested against M. javanica J2 in a chambered Petri dish, namely decene (10en), undecene (11en), undecane-2-on (11on), dodecene (12en), 2-methoxy-3-methyl pyrazine (2M3MP), 2,5-dimethyl pyrazine (25DP), 5-isobutyl-2,3-dimethyl pyrazine (5I23DP), 2-ethyl-3-methyl pyrazine (2E3MP), and 2-isobutyl-3-methoxy pyrazine (2I3MP) (all Sigma-Aldrich, Darmstadt, Germany). 2M3MP was used as a substitute for 3-methoxy-2,5-dimethyl pyrazine, which was consistently detected in nVOC-volatilome of Pseudomonas koreensis T3GI1 (see also Table 2). The compound 2-undecanone was used as a positive control, due to its known nematicidal effects on M. incognita (Huang et al., 2010) and other nematodes (Gu et al., 2007). On one side of the Petri dish, a 500 μl suspension of M. javanica (∼250 J2) was placed on 8 ml of 2%-tap water agar. On the opposite side, three concentrations (1, 5, and 20 μl) of a single compound were placed on a microscopic slide, which prevented interactions of the compound with the Petri dish plastic. A Petri dish with 20 μl distilled water represented the control. Plates were maintained at room temperature for 24 h and the experiment repeated a further two times and the blank-corrected mortality rate calculated for each compound.
Pre-processing of the reads obtained by the sequencing company of Eurofins MWG Operon was carried out using QIIME 2 (2017.12 release) and QIIME 1 (Caporaso et al., 2010) following the protocol of Schwendner et al. (2017). Demultiplexing, denoising (400 bp length, including phiX and chimera filtering) and generation of ribosomal sequence variants (RSVs) was carried out with the DADA2 algorithm (Callahan et al., 2016). RSVs were summarized in a feature table. The taxonomic analysis was based on a customized naïve-bayes classifier trained on 16S rRNA gene features clustered at 97% similarities within the Silva128 database release and trimmed to a length of 400 bp. Reads for mitochondria and chloroplasts were filtered using QIIME 2 before analysis. The feature table was rarefied to 6,890 reads for core metrics analysis. Alpha diversity indices were analyzed using Pairwise Kruskal–Wallis test and beta diversity indices with PERMANOVA. Bray–Curtis dissimilarity and unweighted UniFrac dissimilarity between habitats were visualized using EMPEROR3. One rhizosphere sample (T4R) was removed due to poor quality. Differential abundances were subjected to ANCOM and Gneiss test, implemented in QIIME 2. Bacterial network of the core microbiome of each habitat (>1% relative abundance within habitat) was visualized using Cytoscape 3.3.0 (Shannon et al., 2003) on order level. The core microbiomes were defined with an occurence of >75% throughout the replicates for each habitat. Mean relative abundance of fungal and nematode antagonists found in this study was calculated for each data set. Bacterial abundances on family level were compared between healthy and diseased root samples to visualize bacterial community shift due to RKN infection.
The combination of identification approaches (SCAR-primer molecular key, morphological examination, NAD5 sequences) identified 10 adult females as M. incognita and two as M. incognita sensu lato. in Luwero (site 1). Just M. incognita was reliably identified from Luwero. In samples from Namulonge (site 2), three females were identified as M. incognita, six as M. javanica and five as M. incognita s. lat. Thus, a multispecies infection was confirmed in Namulonge (see Supplementary Material – Additional Results, Supplementary Figures S2, S3).
Representative bacterial strains from each of the two sampling sites caused similar mean nematode mortality rates through nVOC (32.1 ± 25.4% in Luwero, 30.9 ± 28.1% in Namulonge). Most strains showed no effect (mortality <10%) or only slight nematicidal effects. In total, 43 strains were categorized as active (80–95% mortality; n = 20) or highly active (>95% mortality; n = 23). When comparing the overall nematicidal activity of bacteria from plants of different RGI, a trend was apparent for higher mortality rates of strains from highly diseased plants. Most active and highly active strains (n = 43) were recovered from diseased rhizoendosphere (RE-D) samples, independent of site (Figure 1). Of these 43 strains, six demonstrated repeated nematicidal activity (>80% mean mortality). Five out of these six highly active strains were bacteria isolated from RE-D. Moreover, three of these strains were collected from the same tomato plant (T13), a heavily galled (RGI = 4.5) plant from Namulonge. Just one strain was isolated from healthy, non-galled rhizoendosphere (RE-H) from Luwero. Sequences of the 16S rRNA genes identified the nematicidal bacteria as Pseudomonas koreensis, Comamonas sediminis, Variovorax paradoxus, P. soli and two strains of P. monteilii. Strains with high nematicidal activity showed no high antifungal activity and vice versa (Table 1).
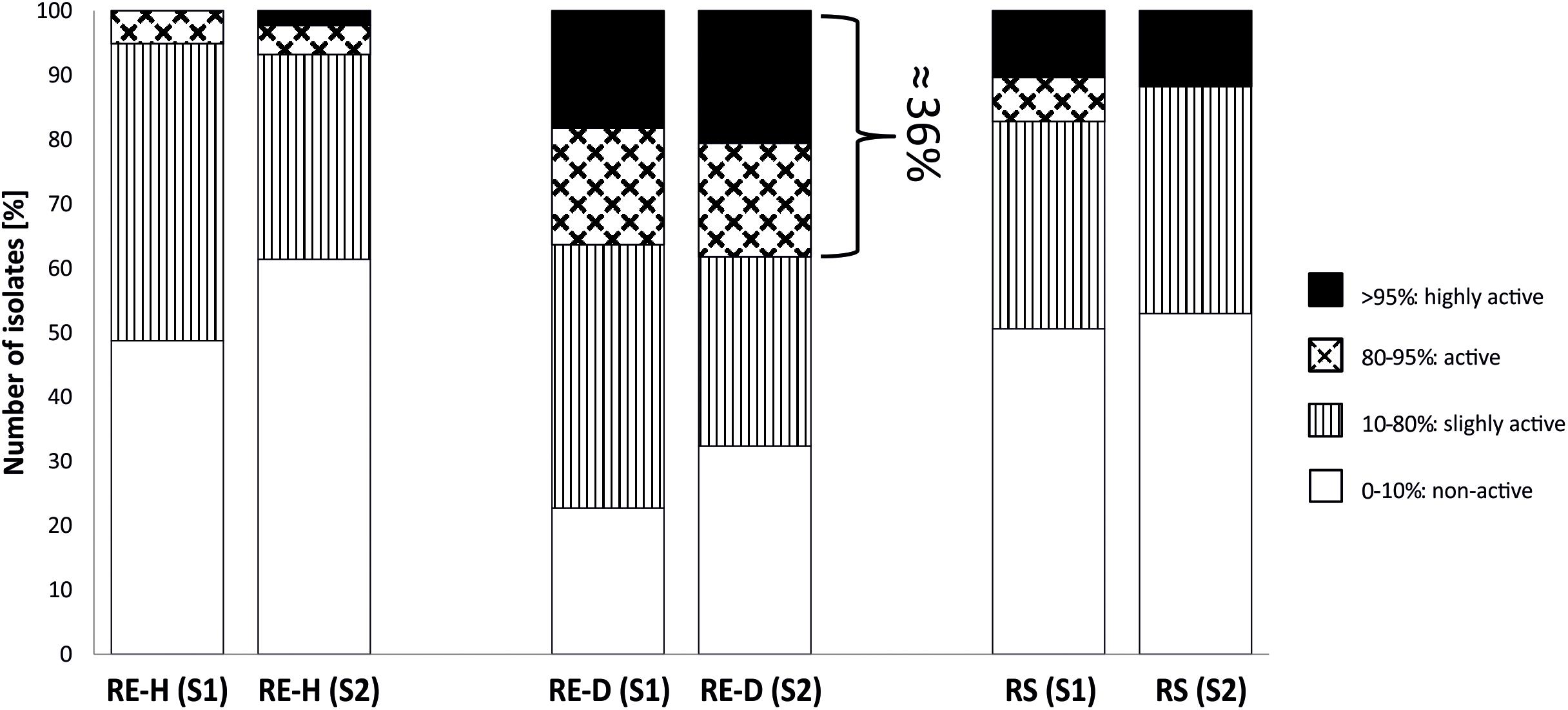
Figure 1. Proportion of nematicidal active bacterial strains. Proportion of nematicidal active strains is highest in diseased root endosphere, independent of site. RE-H, rhizoendosphere healthy; RE-D, rhizoendosphere Meloidogyne-diseased; R, rhizosphere; S1, Site 1, Luwero; S2, Site 2, Namulonge; Pattern refer to the activity category; in RE-D, around 36% of bacterial strains are categorized active or very active (>80% mortality) in nVOC-production.
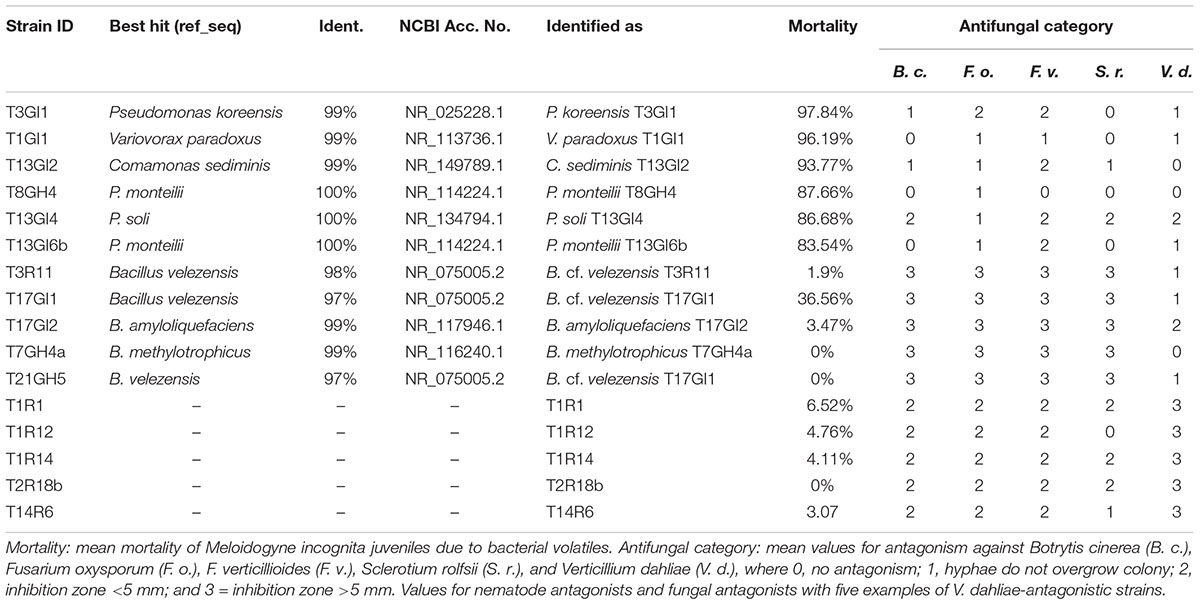
Table 1. Identification of bacterial strains with antagonistic properties against either root-knot nematodes or fungal pathogens.
The volatilomes of the nVOC-producing strains mainly consisted of alkenes, sulfuric compounds, alcohols, ketones, and aldehydes. Additionally, one pyrazine (3-methoxy-2,5-dimethyl pyrazine) was consistently detected in P. koreensis T3GI1, the strain with the highest nematicidal effect (Table 2). 1-undecene was one of the main components of the volatilome of C. sediminis, P. monteilii, and P. soli. Except for P. monteilii T8GH4, dimethyl disulfide was the main component of the volatilome but strangely, was also found in the blank (Table 2). When comparing the overall number of mass spectra ion counts, the total ionic counts of bacterial volatilomes were up to sevenfold higher (in P. soli T13GI4) than in the blank (Table 2). When individually assessing 2-undecanone, several alkenes and pyrazine derivates against J2, just three compounds, 2-undecanone (11on), 2-methoxy-3-methyl pyrazine (2m3mp), and 2-ethyl-3-methyl pyrazine (2e3mp), gave significantly higher mortality rates than the blank mortality after 1-day incubation using quantities of 5 and 20 μl. Only 11on and 2m3mp gave significantly higher mortality rates than the blank when using 1 μl of compound (Supplementary Figure S4). At the highest concentration, live J2 were mainly found surrounded by or within a mass of dead J2 individuals. Three more compounds showed nematicidal effects at 20 μl following incubation for 4 days, namely 2-isobutyl-3-methyl pyrazine (67.8%), 5-isobutyl-2,3-dimethyl pyrazine (44%), and 2-ethyl-3-methyl pyrazine (50.2%).
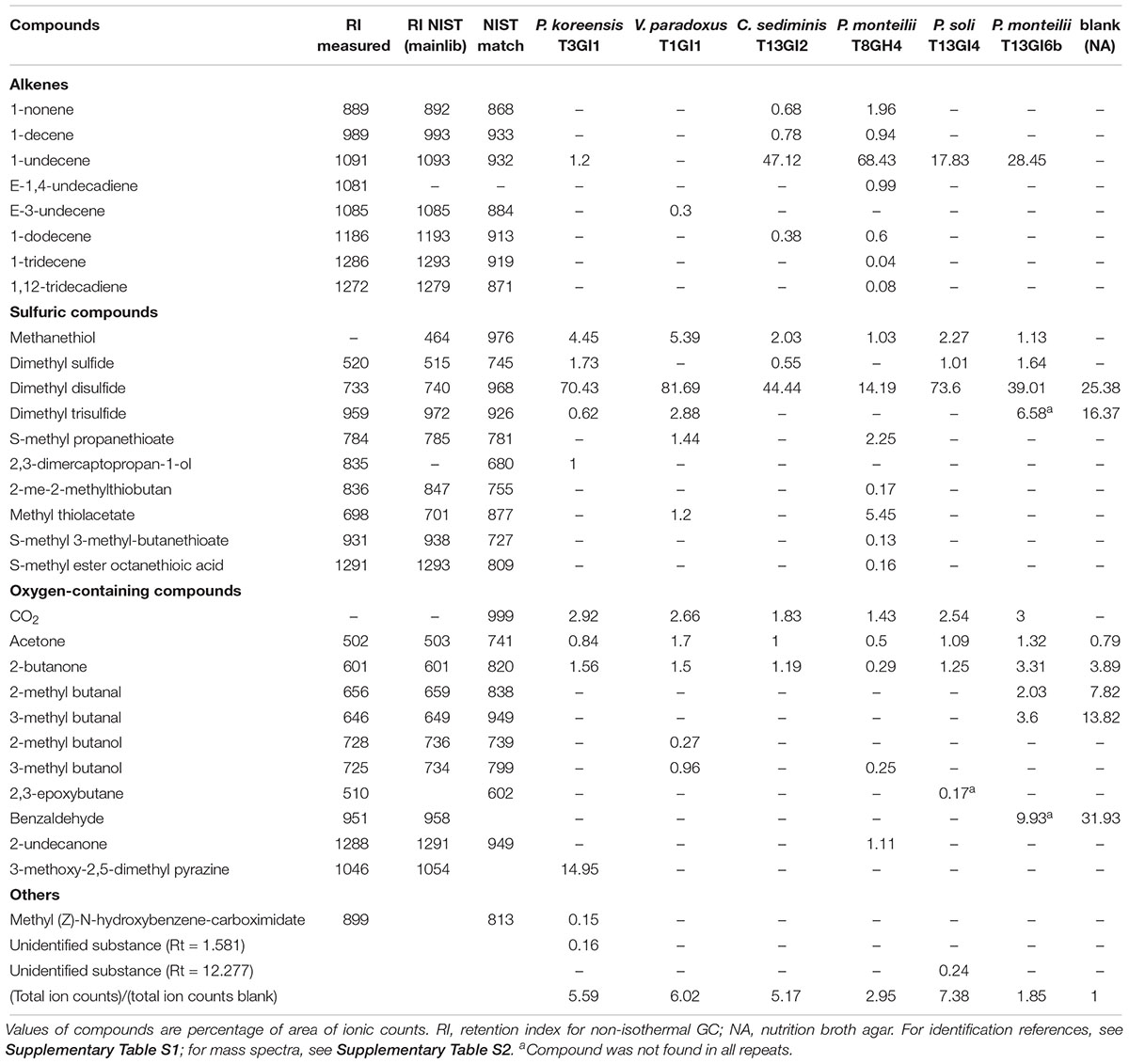
Table 2. SPME GC-MS: volatilome composition of nematicidal VOC-producing bacterial strains associated with root-knot nematode infection in Ugandan tomato.
Of 260 bacterial strains 72 showed a high antagonistic effect on at least one fungal pathogen tested Botrytis cinerea, F. oxysporum, F. verticillioides, S. rolfsii, and V. dahliae (Figure 2A). Strains showing a fungal antagonistic effect were mostly isolated from RE-H (n = 30 strains) rather than from RE-D (n = 14 strains) samples. Antagonists of V. dahliae were recovered only from the root adhering soil, or “rhizosphere.” Further analysis was carried out only with strains showing antagonism toward at least four different plant pathogenic fungi (n = 23, Figure 2A), thus Verticillium antagonists were excluded. Five separate genotypes were revealed by analyzing the BOX-PCR patterns of the 23 strains that complied with this requirement. Sequencing the 16S rRNA gene of these strains identified them all as members of the Bacillus amyloliquefaciens complex, namely one strain of B. amyloliquefaciens (T17GI2), one strain of B. methylotrophicus (T7GH4), and three strains of B. velezensis (T3R11, T17GI1, T21GH5). None of these strains showed high antagonistic activity against V. dahliae (Table 1).
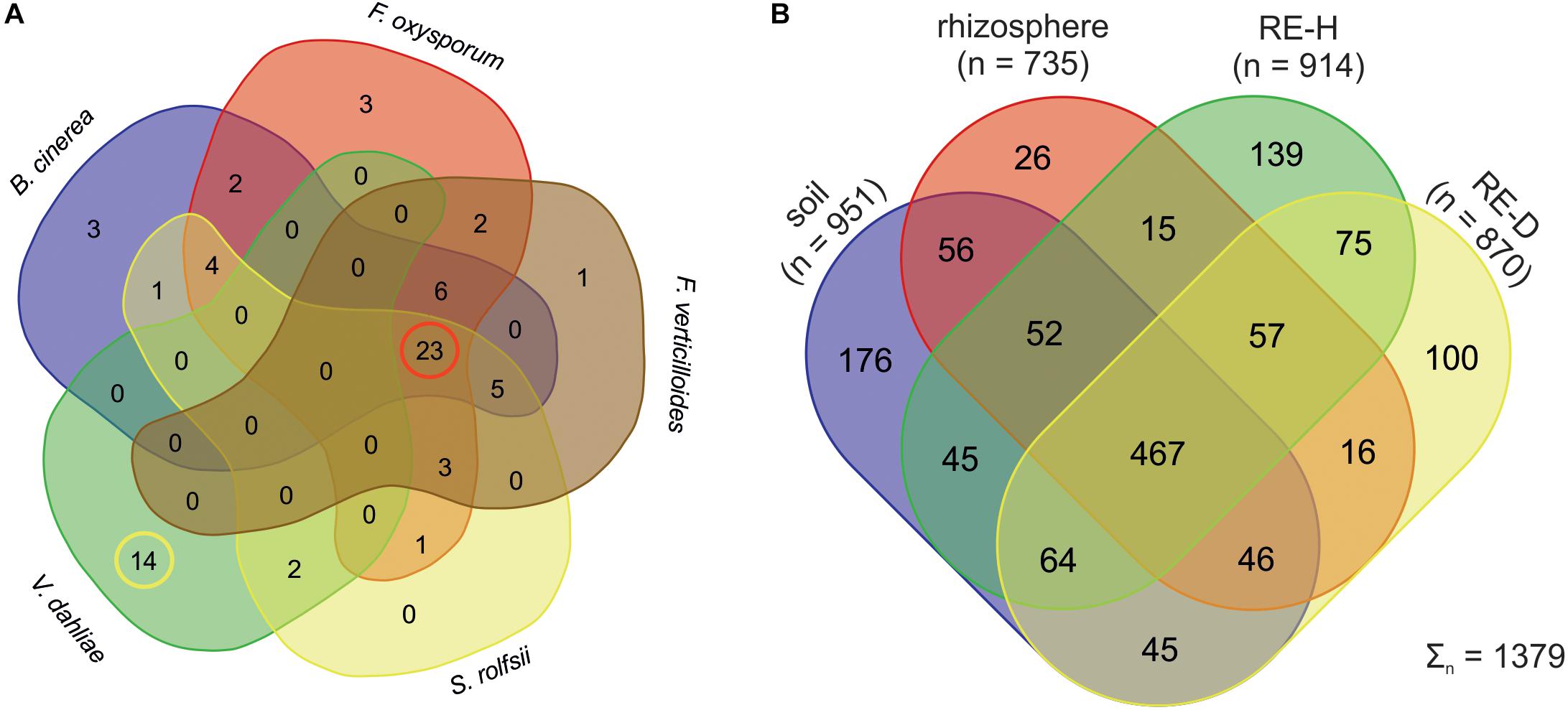
Figure 2. (A) Venn diagram of bacterial strains showing high antagonism against fungal pathogens. Strains that showed high antagonism against Botrytis cinerea, Fusarium oxysporum, F. verticillioides, and Sclerotium rolfsii did not show high effects against Verticillium dahliae. Red circle: active strains against four fungal pathogens. Yellow circle: Verticillium antagonists. (B) Venn Diagram displaying the number of taxa on genus level of bacterial strains. Rhizosphere sample have the least microhabitat-specific taxa. Endosphere samples have more specific than shared taxa.
Physical soil composition was comparable at both sampling sites; soil type was loamy sand (Table 3). Amplicon libraries of different tomato microhabitats (rhizosphere; RE-H: rhizoendosphere healthy; RE-D: rhizoendosphere diseased) as well as from surrounding soil were analyzed. When taxa were compared at the genera level between all four microhabitats, a total of 1,379 taxa were found with 467 taxa present in all microhabitats. The rhizosphere presented the smallest number of microhabitat-specific taxa, while RE-H and RE-D had more microhabitat-specific than shared taxa (Figure 2B). When visualizing beta diversity with a PCoA-plot of Bray–Curtis dissimilarities, samples from the same sampling site did not cluster. Axis 1 (16.45% variation explained) showed no two-dimensional clustering of the samples in combination with axis 2 (12.96%) or axis 3 (8.11%), but samples clearly cluster habitat-specific with axis 2 and 3 (Supplementary Figures S5A–C). The combination of all three axes 1–3 shows a clear clustering of soil, rhizosphere and RE-D, while RE-H samples overlap with rhizosphere and RE-D (Figure 3A).
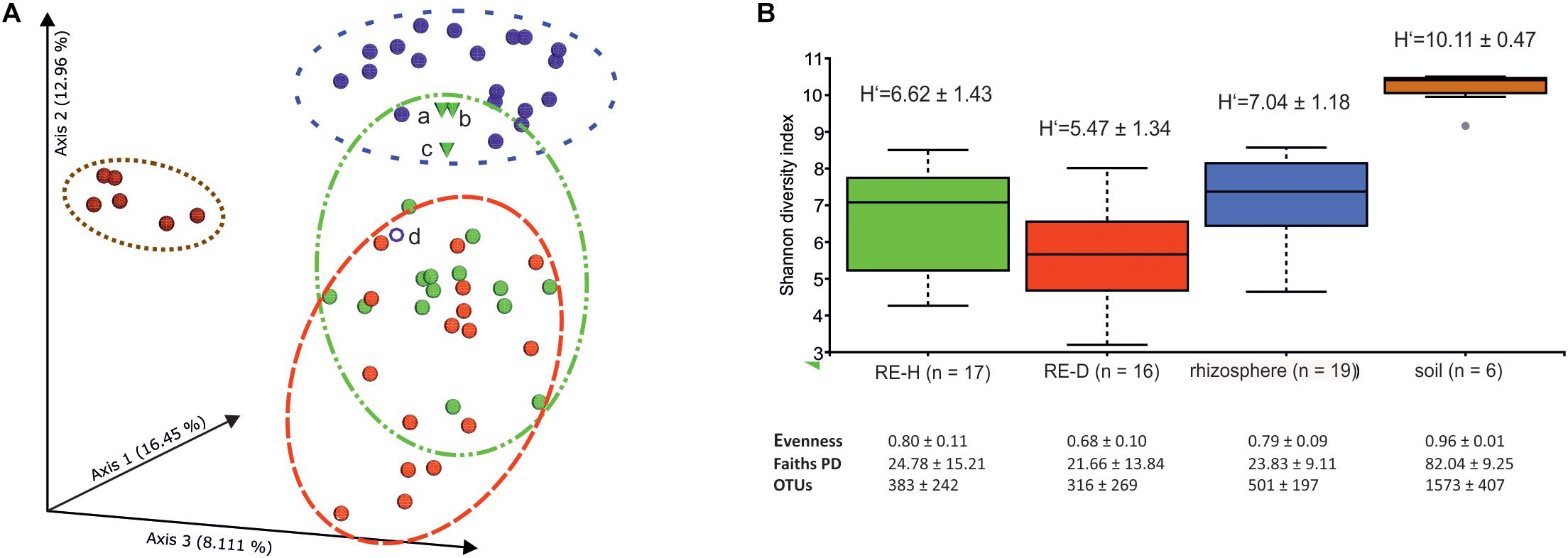
Figure 3. (A) PCoA plot of Bray–Curtis dissimilarity between microhabitats. PCoA-plot shows clear clustering in soil (brown) in rhizosphere (blue) while healthy rhizoendosphere (green) sampling points (a-c) overlap with Meloidogyne-diseased rhizoendosphere (red) and rhizosphere; sample point d (rhizosphere) does not cluster with other rhizosphere samples and was removed in statistical analysis. (B) Barplots of Shannon diversity of microhabitats. Alpha diversity is highest in soil and lowest in diseased rhizoendosphere (galls). Alpha diversity index values are given.
Shannon alpha diversity of the four microhabitats showed a high alpha diversity in soil and a decline in alpha diversity from rhizosphere to RE-H to RE-D (Figure 3B). Pairwise Kruskal–Wallis test for alpha diversity indices showed highly significant (p < 0.01) differences in alpha diversity between all microhabitats in most cases. Only rhizosphere and RE-H samples failed to differ significantly in all tested alpha diversity indices (Figure 3B and Table 4). Pairwise PERMANOVA test of beta diversity indices significantly differed between all microhabitats, except qualitative indices (Jaccard and unweighted UniFrac distance) in RE-H and RE-D samples (Table 4).
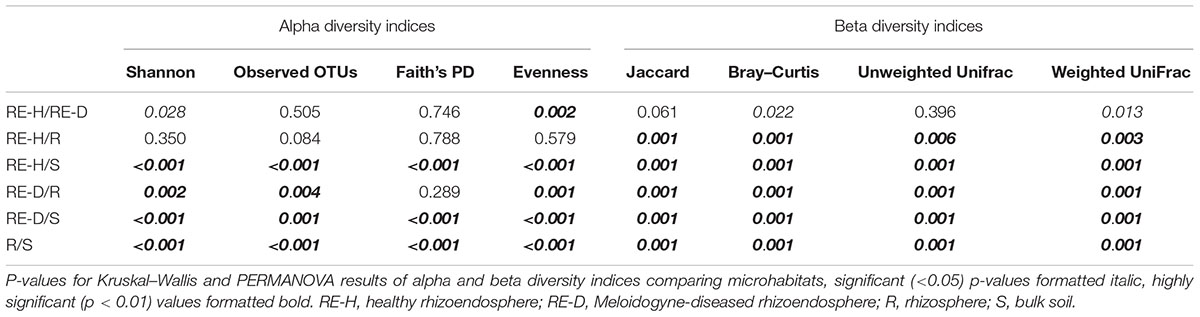
Table 4. P-values of diversity indices reveal significant microhabitat-specific bacterial community differences in root-knot nematode-diseased tomato roots.
Amplicon analysis of the soil samples revealed a total of 763 taxa at the genus level in Luwero (site 1) and 782 in Namulonge (site 2), with 594 taxa shared between sites. The most abundant classes in soil were Alphaproteobacteria (13.7%), Planctomycetacia (7.2%), and Bacilli (6.2%) (Figure 4). Caulobacterales, Cytophagales, Rhodocyclales, and Rhodospirillales are shared between soil and rhizosphere samples (Figure 5).
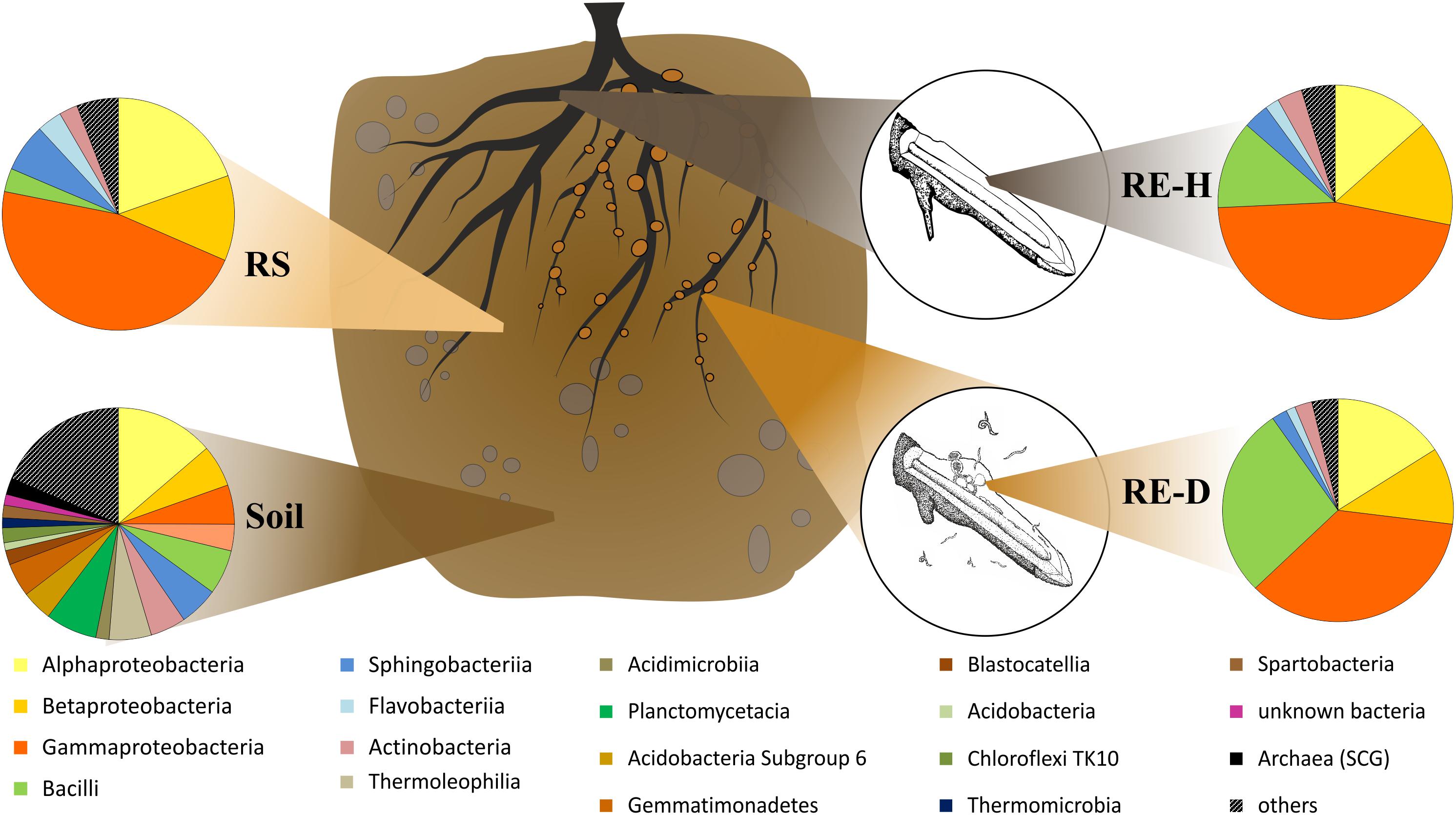
Figure 4. Microbial composition on class level within the four microhabitats. In diseased rhizoendosphere (RE-D), mainly Bacilli and Gammaproteobacteria change in their abundances compared to healthy rhizoendosphere (RE-D). RE-D, Meloidogyne-diseased rhizoendosphere; RE-H, healthy rhizoendosphere; RS, rhizosphere.
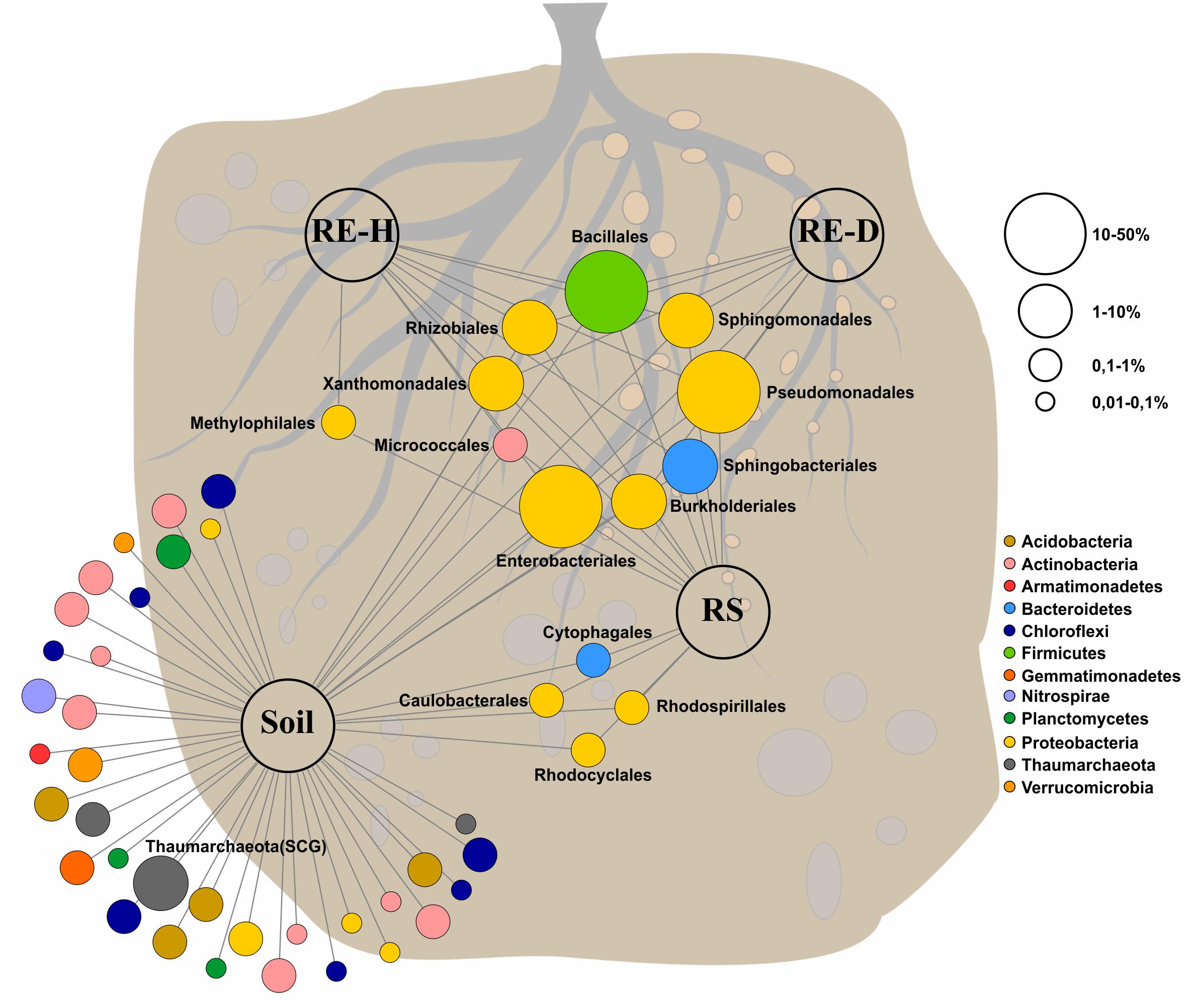
Figure 5. Feature network of core taxa (≥80% of samples) at class level (>1% relative abundance) found within the four microhabitats. Bacterial community in soil is very diverse, all endosphere taxa belong to the core microbiome, Methylophilales are <1% abundant in diseased endosphere. Core microbiome covers around 90% of all abundant orders except for soil (soil: 68.9%; rhizosphere: 92.7%; healthy rhizoendosphere/RE-H: 91.27%; Meloidogyne-diseased rhizoendosphere/RE-D: 91.24%).
All plant-associated samples were dominated by Gammaproteobacteria. The classes most abundant in the rhizosphere were Gamma- (47.4%), Alpha- (19.8%), and Betaproteobacteria (11.6%) (Figure 5). The most abundant bacterial genera in the rhizosphere were Pseudomonas spp. (25.5%), unidentified Enterobacteriaceae species (17.2%) and Sphingobium spp. (8.2%). Endosphere samples mainly consisted of orders of the core microbiome (Figure 5).
Differential abundance analysis using Gneiss proved that the “habitat” category is the main driver of variance within our dataset, changes of the microbiome composition through higher RGI were not significant. Enterobacteriaceae, Burkholderiaceae, Pasteuriaceae, and Rhizobiaceae were identified as the main drivers of microbiome changes in rhizoendosphere (Supplementary Figures S6A–D). When comparing core taxa (present in >75% of the samples) on family level, these families showed a strong abundance shift (Figure 6).
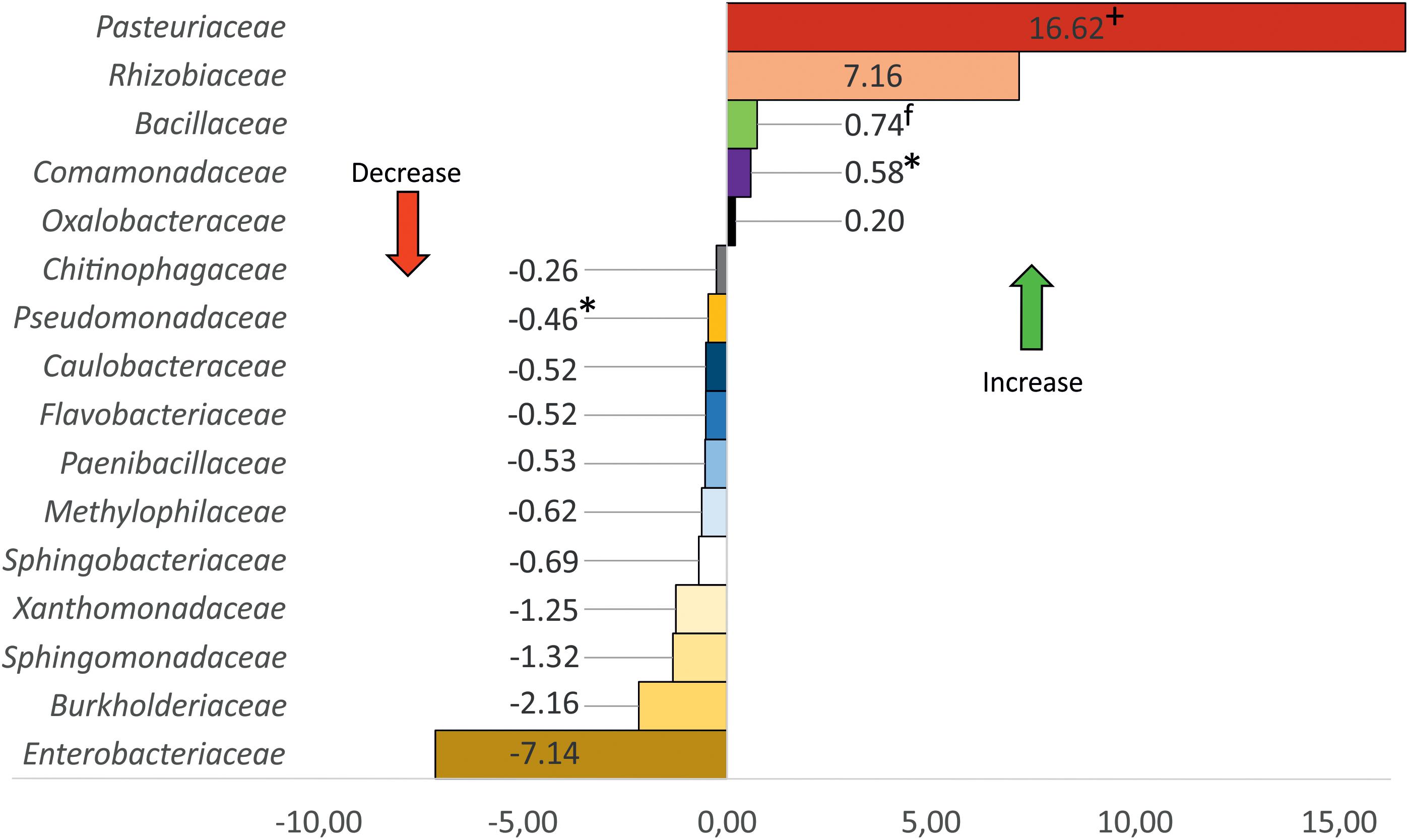
Figure 6. Changes of relative abundance of bacteria families due to root-knot nematode (Meloidogyne spp.) infection. Only families with >1% relative abundance shown. +, obligate nematode parasites; f, fungi antagonists; ∗, nematode antagonists found in this study.
Antagonists differ in their overall abundance between microhabitats. Bacillus spp. had the highest mean abundance in RE-H (4.1%), Variovorax spp. in RE-D (1%), Comamonas spp. (0.3%), and Pseudomonas spp. (22%) across the 19 rhizosphere samples. Taxa showing >98% identity with 16S rRNA sequences of isolated antagonists were represented by 6% of the root endosphere microbiome (Table 5). Focusing on the three most abundant antagonistic genera (Pseudomonas, Pasteuria, Bacillus), no direct correlation between their abundance and RGI was detected (Figure 7). Pseudomonas was especially abundant in diseased plants with a RGI of 5, whereas Pasteuria showed highest abundance in moderately diseased (RGI = 3 ± 1) plants (Figure 7).
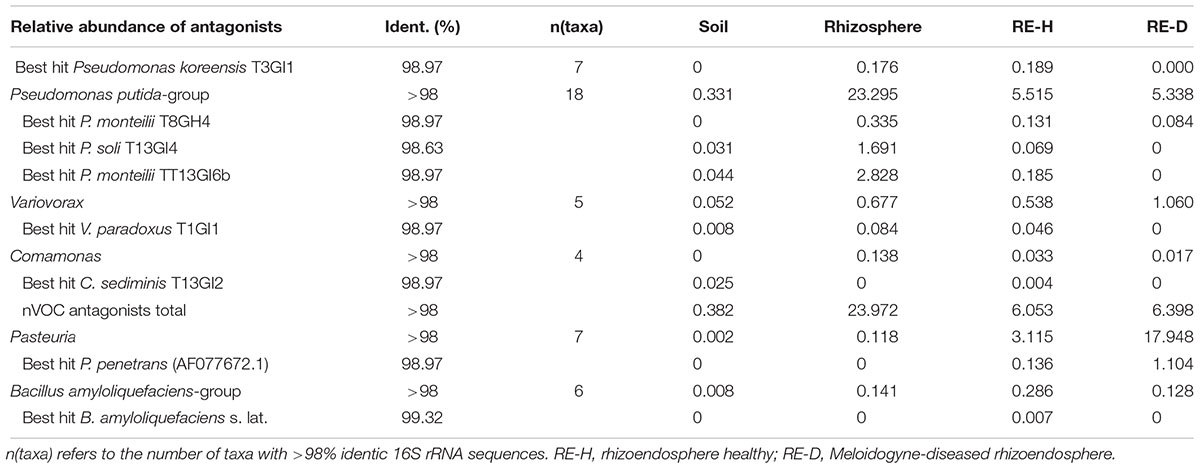
Table 5. Relative abundance of taxa most similar to 16S rRNA sequences of isolated bacterial antagonists in different microhabitats.
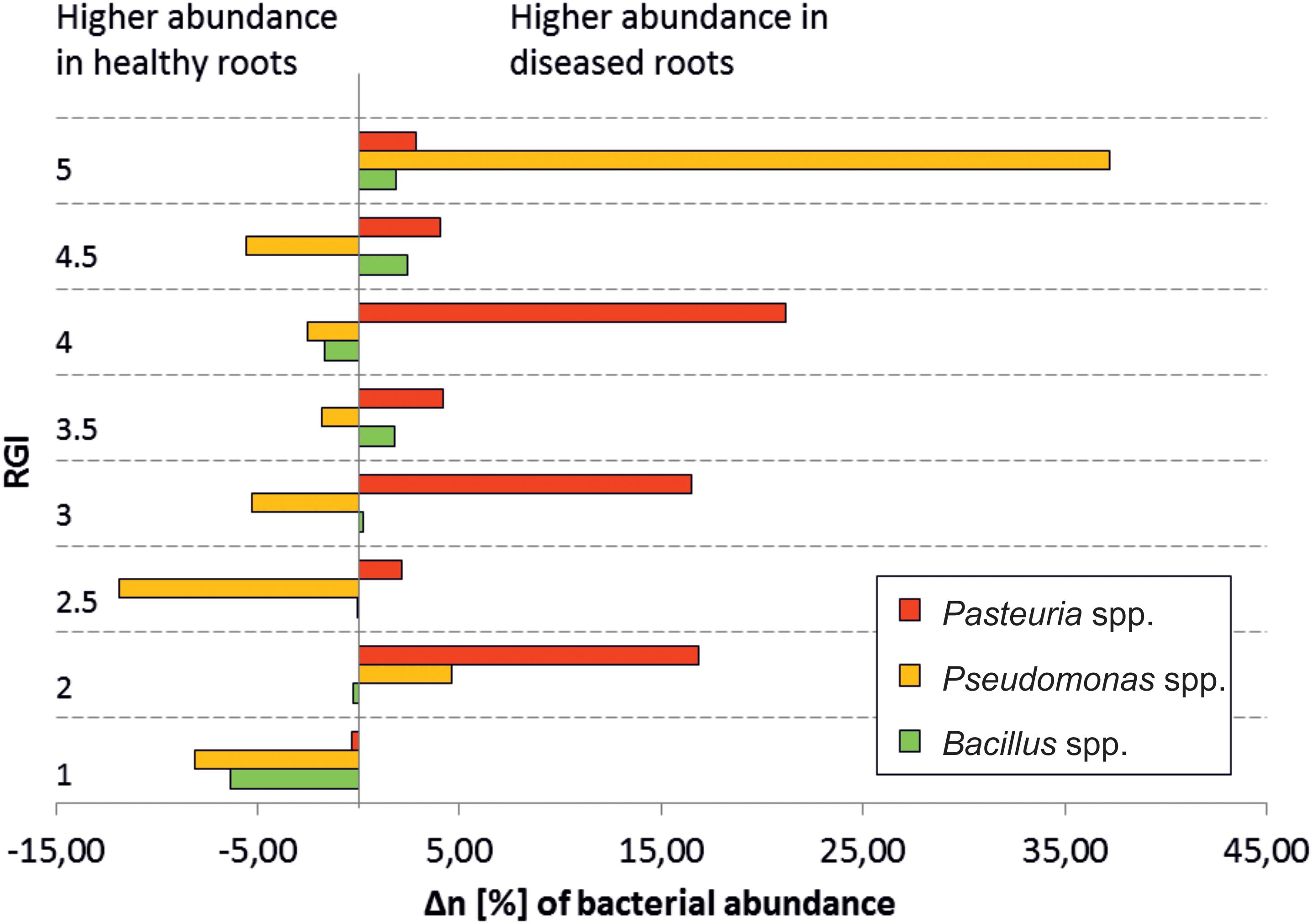
Figure 7. Abundance of selected nematode-antagonistic genera in relation to severity of Meloidogyne-infection. ΔrA, difference of relative abundances, = abundance (diseased root) – abundance (healthy root); RGI, root galling index; 1, symptomless; 5, severely damaged roots; antagonistic genera are generally higher in healthy roots, except for obligate nematode parasites Pasteuria spp. The high abundance of Pseudomonas in the RG5 plant may be due to saprophytic species, which already degrade the plant material.
In our comprehensive study of soil-borne diseases of tomato from Uganda, we discovered novel principles, which help to explain the disease complex and offer new potential strategies as to how to suppress them. Diseased tomatoes suffered from a multispecies infection of Meloidogyne. We clearly identified two species (M. incognita, M. javanica) but results indicate that more species are involved (Adam et al., 2007; Janssen et al., 2016).
Our novel VOC-based screening method toward RKN resulted in the identification of several nematicidal antagonists, namely Comamonas sediminis, P. koreensis, P. monteilii, P. soli, and Variovorax paradoxus. P. koreensis and P. monteilii have known potential for biocontrol against oomycetes (Hultberg et al., 2010a,b) and fungi in general through VOC, respectively (Dharni et al., 2014). In contrast, P. soli and C. sediminis have not previously been identified as potential biocontrol candidates. However, the closely related C. acidovorans has shown antagonistic effects toward plant pathogens (El-Banna, 2007; Liu et al., 2007). Variovorax paradoxus has great potential for bioremediation, biotechnology (Satola et al., 2013), and plant-protection (Chen et al., 2013). Variovorax spp. and Comamonas spp. occurred in relatively low abundance in endosphere samples (Table 5). The question remains as to whether they can become sufficiently abundant to affect RKN through their nematicidal nVOC and support the plant indirectly, or if they inherit direct plant-promoting traits.
A considerable aspect of the cultivable bacteria isolated in our study demonstrated their negative impact on RKN by producing nVOC. VOC produced by the bacterial community – among other factors – may contribute to the overall suppressiveness toward RKN of different soils. This may also explain the efficacy of RKN management methods that promote bacterial growth and diversity, e.g., the use of soil amendments (Viaene et al., 2013). We identified six nVOC-producing strains and 72 potential fungal antagonists. However, our tests did not result in any single strain that controlled both RKN and phytopathogenic fungi to any great extent. Furthermore, individual antagonists tended to attain high abundances in different, separate microhabitats. Fungal antagonists were mainly isolated from, and abundant in, RE-H, as supported by the amplicon data, indicating an important role of these strains for host-plant protection. The strong antagonistic effects on fungal pathogens by members of the B. amyloliquefaciens complex is well-known (Chowdhury et al., 2015). The strains found in our study did not effectively inhibit growth of V. dahliae in dual cultures, although in vitro antagonism toward V. dahliae was been reported for other Bacillus strains (Danielsson et al., 2007). The antimicrobial activity of their metabolites is well-studied, showing that induced systemic resistance (ISR) and antimicrobial metabolite production are their main mechanism of plant protection (Chowdhury et al., 2015). Our findings exclude nVOC of Bacillus strains as a controlling component for RKN, though they may have a repellent effect in vitro. We question that Bacillus spp. in the root endosphere are enriched from surrounding soil, because Bacillus spp. are a part of the core microbiome of the tomato seeds itself (Bergna et al., 2018). In this case, susceptibility of tomatoes toward diseases may also be a consequence of declining bacterial diversity through breeding practices or the inability to establish native antagonists.
One component of our most effective nVOC-producing strain was a pyrazine, which have known antimicrobial effects (Haidar et al., 2016; Kusstatscher et al., 2017), we therefore focused on different pyrazines in the single compound test. We found that the effect of pyrazines on RKN appears dependent on their functional groups. Although all pyrazines were not as effective as 2-undecanone, they may enhance the nematicidal effect of the total volatilome of P. koreensis T3GI1. Alkenes were consistently found in high concentrations in the nVOC spectra but they showed no nematicidal effect. This may be a result of their hydrophobicity, as J2 were protected by a thicker water layer in the single compound test, than in the modified TCVA-screening. Therefore, hydrophobic compounds would be less able to affect the nematode cuticle. We also found several sulfuric compounds with strong odors (e.g., dimethyl sulfide, octanethioic acid S-methylester). Nematicidal properties may mostly arise from dimethyl disulfide, which is an effective nematicide (Gómez-Tenorio et al., 2015) and was the main volatilome component in all strains except P. monteilii T8GH4 in our assessment. Sulfur is generally regarded to be in low concentrations in tropical soils (Blair et al., 1980). As elemental sulfur is known to reduce RKN densities in non-sterile soil (Rumiani et al., 2016), VOC of the microbial sulfur metabolism may enhance RKN control. Although our study revealed the potential of VOC in suppressing multispecies plant diseases, in order to be able to translate this concept into plant protection a greater understanding and more research is needed.
Our study provides the first in-depth analysis of the influence of RKN on the bacterial community under field conditions. Results of Tian et al. (2015), which studied microbiomes of RKN-diseased tomatoes under controlled, greenhouse conditions differ heavily from our study in terms of the dominating taxa, microbiome composition, and microbiome shift due to RKN infection. These differences are not entirely surprising, however, given that our study was performed on plants removed from fields following natural infection by RKN, compared with the controlled pot environment and artificial inoculation of RKN by Tian et al. (2015). Some of this variance may also be due to cultivar differences (Rybakova et al., 2017) but which is not expected to be apparent at this taxonomic level. Infection with nematodes was correlated with a strong bacterial community shift in tomato roots, with a microbiome from healthy plants differing from infected roots, even though this was not necessarily dependent upon the RGI. Regarding the beta diversity, only quantitative indices revealed significant differences between RE-D and RE-H. Thus, nematode feeding site (NFS) induction would appear to have a greater impact on the abundance of bacterial taxa that are present and highly abundant in both RE-D and RE-H (Figure 6), rather than the microhabitat-specific colonization pattern of low-abundant taxa (Figure 2B). A higher diversity of endophytes due to RKN infection (Tian et al., 2015) could not be confirmed; cultivable bacteria and habitat-specific taxa were more abundant in RE-H than in RE-D. Further, RE-H and rhizosphere did not significantly differ in alpha diversity indices. This appears to be extraordinary: the rhizosphere is regarded as a biodiversity hotspot and root endosphere diversity in tomato was found to be lower than in rhizosphere. Nevertheless, both rhizosphere and to a lesser extent root endosphere diversity are influenced by the surrounding soil (Bergna et al., 2018). Since tomato are not indigenous to Uganda, the rhizoendophytic alpha diversity may be raised because of the combination of tomato core microbiota and an uptake of native soil bacteria. Interestingly, alpha diversity in RE-D is significantly lowered compared to RE-H despite comparable numbers of species. Therefore, RKN may favor roots with lower microbiome diversity for NFS selection. Diversity of endophytic microbiota is regarded as a key factor for plant health (Berg et al., 2017) but RGI and Shannon diversity do not clearly correlate with healthy and diseased roots (data not shown). Furthermore, RE-D microbiomes of moderately damaged plants (RGI 2–3.5) show a more asymmetric composition with fewer but more dominant taxa than the severely damaged plants (RGI = 4.5–5). This may be due to delayed establishment of slower growing saprophytic or commensal species, although, Tian et al. (2015) also found a specific enrichment of some bacterial groups in the NFS, which indicated specific association of these groups with the NFS and nematode pathogenesis. Our results indicate that the changed physiological conditions of plant cells at the NFS is responsible for microbiome changes. The microbial community within the NFS is influenced by microbes that are able to adhere to the nematode cuticle (Elhady et al., 2017). This is most obvious when looking at the abundance of obligate parasites of plant pathogenic nematodes, such as Pasteuria spp. within RE-D samples (six samples with >20% relative abundance). Their abundance did not correlate with RGI (Figure 7), maybe because it is dependent on a successful transportation adhered to the cuticle of RKN to the NFS. The increase of Rhizobiaceae in RKN galls seems to be a constant effect (Hallmann et al., 2001; Tian et al., 2015). There are three possible explanations: (i) Rhizobiaceae have a preference to attach to the nematode surface during soil migration, as reported for Neorhizobium (Elhady et al., 2017); (ii) it is a side effect of NFS induction, since RKN manipulate the gene expression of plant hormones and nodulation factors (Gheysen and Fenoll, 2002; Jones et al., 2013); (iii) it contributes to a defense reaction of the host plant, since Rhizobiaceae are known to closely interact with plants. The latter two hypotheses are connected to the regulation of plant flavonoids, which have several important functions in the plant-nematode interaction (Hutangura et al., 1999; Weston and Mathesius, 2013) and there is evidence that RKN counteract the effects of Rhizobium, since RKN reduce nodulation in legumes (Kimenju et al., 1999). Enterobacteriaceae and Burkholderiaceae were the families with the highest negative shift in abundance in RE-D. Whether there was a lower abundance at the NFS beforehand, or this was lowered following RKN infection remains unclear. However, Enterobacteriaceae and Burkholderiaceae appear to be less competitive following the physiological and physical changes that occur as galls develop in the roots. Due to the high abundance of Enterobacteriaceae (31.8%) and Burkholderiaceae (5.3%) in RE-H their abundance shift is most obvious. Still, when examining the abundance changes separately, the percentage change in abundances is higher in low-abundant taxa, such as Caulobacterales or Methylophilales (Supplementary Table S3).
Managing the RKN disease complexes through biocontrol requires a detailed knowledge on the antagonists and their effects. Since antagonistic strains of bacteria are here shown to prefer different microhabitats, they would likely affect RKN at different stages of their life cycle, which would indicate the need for a more holistic consortia of biological control agents. Further, some bacterial strains are known to impact on multiple targets, such as both fungi and RKN (Adam et al., 2014). As we did not observe this in the current study, we hypothesize that the fungal antagonists in our study would affect RKN with alternative mode of action than with nVOC.
Our results indicate that VOC and plant-associated microbial diversity offers promise for RKN-defense management. Based on our data, we suggest three methods for RKN-control: (i) application of a consortia containing bacterial, fungi and nematode antagonists; (ii) application of sulfur-containing fertilizers to enhance sulfur-containing VOC in the rhizosphere for J2-reduction; (iii) screening and application of Rhizobiaceae-strains that produce nematicidal metabolites to take advantage of their increased abundance in galls. The combination of different management methods can lead to synergistic beneficial effects in tropical climates (Viaene et al., 2013). Implementing environmentally sensitive biocontrol strategies in agricultural programs, especially on smallholder farms, is an alternative to the harmful and often unspecific toxic biocides, toward preserving the stability and diversity of macro- and microhabitats. It would also help alleviate agriculture-related health issues, hunger and social conflicts while simultaneously providing economic and nutritional needs of the local people.
The datasets generated and/or analyzed during the current study are available in the European Nucleotide Archive (ENA) under project no. PRJEB28436 under the accession numbers ERS2856266–ERS2856324. Reference sequences of Meloidogyne incognita (KJ476151.1), M. javanica (KP202352.1), M. arenaria (KP202350.1), M. ethiopica (KU372360), and M. chitwoodi (KJ476150.1) are publicly available at the NCBI database (https://www.ncbi.nlm.nih.gov/) under the corresponding accession numbers.
DC, GB, and AW designed the study. AW, JT, DC, RG, and GB performed the sample process. AW and JT analyzed the data. AW and GB wrote the manuscript. All authors improved and approved the final manuscript.
This work was undertaken as part of the research project “IITA – Healthy seedling systems for a safer, more productive vegetables in East Africa” (F37139), funded by the Austrian Development Agency (ADA) to DC and GB.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Günther Raspotnig (Graz), Johannes Hallmann (Münster), and Wim Bert (Ghent) for their expertise in nematode identification. We also thank Tobija Glawogger, Nikolina Todorovic, Jelena Gagic, Ingrid Matzer, Barbara Fetz, Isabella Wrolli, Monica Schneider-Trampitsch, and Doreen Nampamya (Graz, Kampala) for their help in the laboratory. Alexander Mahnert, Henry Müller, and Tomislav Cernava (Graz) supported the bioinformatic analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01296/full#supplementary-material
Adam, M., Heuer, H., and Hallmann, J. (2014). Bacterial antagonists of fungal pathogens also control root-knot nematodes by induced systemic resistance of tomato plants. PLoS One 9:e90402. doi: 10.1371/journal.pone.0090402
Adam, M. A. M., Phillips, M. S., and Blok, V. C. (2007). Molecular diagnostic key for identification of single juveniles of seven common and economically important species of root-knot nematode (Meloidogyne spp.). Plant Pathol. 56, 190–197. doi: 10.1111/j.1365-3059.2006.01455.x
Back, M. A., Haydock, P. P. J., and Jenkinson, P. (2002). Disease complexes involving plant parasitic nematodes and soilborne pathogens. Plant Pathol. 51, 683–697. doi: 10.1046/j.1365-3059.2002.00785.x
Bais, H. P., Park, S. W., Weir, T. L., Callaway, R. M., and Vivanco, J. M. (2004). How plants communicate using the underground information superhighway. Trends Plant Sci. 9, 26–32. doi: 10.1016/j.tplants.2003.11.008
Bennett, A. J., Bending, G. D., Chandler, D., Hilton, S., and Mills, P. (2012). Meeting the demand for crop production: the challenge of yield decline in crops grown in short rotations. Biol. Rev. 87, 52–71. doi: 10.1111/j.1469-185X.2011.00184.x
Berg, G., Köberl, M., Rybakova, D., Müller, H., Grosch, R., and Smalla, K. (2017). Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 93, 1–9. doi: 10.1093/femsec/fix050
Berg, G., Roskot, N., Steidle, A., Eberl, L., Zock, A., and Smalla, K. (2002). Plant-dependent genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different Verticillium host plants. Appl. Environ. Microbiol. 68, 3328–3338. doi: 10.1128/AEM.68.7.3328
Bergna, A., Cernava, T., Rändler, M., Grosch, R., Zachow, C., and Berg, G. (2018). Tomato seeds preferably transmit plant beneficial endophytes. Phytobiomes. J. 2, 183–193. doi: 10.1094/PBIOMES-06-18-0029-R
Blair, G. J., Mamaril, C. P., and Ismunadji, M. (1980). “Sulfur deficiency in soils in the tropics as a constraint to food production,” in Priorities for Alleviating Soil-Related Constraints to Food Production in the Tropics, ed. M. Drosdoff (Los Baños: International Rice Research Institute), 233–251.
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13:581. doi: 10.1038/nmeth.3869
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335.
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Lozupone, C. A., Turnbaugh, P. J., et al. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. U.S.A. 108, 4516–4522. doi: 10.1073/pnas.1000080107
Cernava, T., Aschenbrenner, I. A., Grube, M., Liebminger, S., and Berg, G. (2015). A novel assay for the detection of bioactive volatiles evaluated by screening of lichen-associated bacteria. Front. Microbiol. 6:398. doi: 10.3389/fmicb.2015.00398
Chen, L., Dodd, I. C., Theobald, J. C., Belimov, A. A., and Davies, W. J. (2013). The rhizobacterium Variovorax paradoxus 5C-2, containing ACC deaminase, promotes growth and development of Arabidopsis thaliana via an ethylene-dependent pathway. J. Exp. Bot. 64, 1565–1573. doi: 10.1093/jxb/ert031
Chowdhury, S. P., Hartmann, A., Gao, X. W., and Borriss, R. (2015). Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42 - A review. Front. Microbiol. 6:780. doi: 10.3389/fmicb.2015.00780
Coyne, D. L., Cortada, L., Dalzell, J. J., Claudius-cole, A. O., Haukeland, S., Luambano, N., et al. (2018). Plant-parasitic nematodes and food security in Sub-Saharan Africa. Annu. Rev. Phytopathol. 56, 1–23. doi: 10.1146/annurev-phyto-080417-45833
Coyne, D. L., Nicol, J. M., and Claudius-Cole, B. (2007). Practical plant nematology: A field and laboratory guide. Cotonou: SP-IPM Secretariat, International Institute of Tropical Agriculture (IITA).
Danielsson, J., Reva, O., and Meijer, J. (2007). Protection of oilseed rape (Brassica napus) toward fungal pathogens by strains of plant-associated Bacillus amyloliquefaciens. Microb. Ecol. 54, 134–140. doi: 10.1007/s00248-006-9181-9182
Dharni, S., Sanchita, S., Maurya, A., Samad, A., Srivastava, S. K., Sharma, A., et al. (2014). Purification, characterization, and in vitro activity of 2,4-Di- tert-butylphenol from Pseudomonas monteilii psf84: conformational and molecular docking studies. J. Agric. Food Chem. 62, 6138–6146. doi: 10.1021/jf5001138
Effmert, U., Kalderás, J., Warnke, R., and Piechulla, B. (2012). Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 38, 665–703. doi: 10.1007/s10886-012-0135-135
El-Banna, N. M. (2007). Antifungal activity of Comamonas acidovorans isolated from water pond in south Jordan. Afr. J. Biotechnol. 6, 2216–2219. doi: 10.5897/ajb2007.000-2347
Elhady, A., Giné, A., Topalovic, O., Jacquiod, S., Sørensen, S. J., Sorribas, F. J., et al. (2017). Microbiomes associated with infective stages of root-knot and lesion nematodes in soil. PLoS One 12:e0177145. doi: 10.1371/journal.pone.0177145
Gheysen, G., and Fenoll, C. (2002). Gene expression in nematode feeding sites. Annu. Rev. Phytopathol. 40, 191–219. doi: 10.1146/annurev.phyto.40.121201.093719
Gómez-Tenorio, M. A., Zanón, M. J., de Cara, M., Lupión, B., and Tello, J. C. (2015). Efficacy of dimethyl disulfide (DMDS) against Meloidogyne sp. and three formae speciales of Fusarium oxysporum under controlled conditions. Crop Prot. 78, 263–269. doi: 10.1016/j.cropro.2015.09.013
Gu, Y. Q., Mo, M. H., Zhou, J. P., Zou, C. S., and Zhang, K. Q. (2007). Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 39, 2567–2575. doi: 10.1016/j.soilbio.2007.05.011
Haidar, R., Roudet, J., Bonnard, O., Dufour, M. C., Corio-Costet, M. F., Fert, M., et al. (2016). Screening and modes of action of antagonistic bacteria to control the fungal pathogen Phaeomoniella chlamydospora involved in grapevine trunk diseases. Microbiol. Res. 192, 172–184. doi: 10.1016/j.micres.2016.07.003
Hallmann, J., Quadt-Hallmann, A., Miller, W. G., Sikora, R. A., and Lindow, S. E. (2001). Endophytic colonization of plants by the biocontrol agent Rhizobium etli G12 in relation to Meloidogyne incognita infection. Phytopathology 91, 415–422. doi: 10.1094/PHYTO.2001.91.4.415
Huang, Y., Xu, C. K., Ma, L., Zhang, K. Q., Duan, C. Q., and Mo, M. H. (2010). Characterisation of volatiles produced from Bacillus megaterium YFM3.25 and their nematicidal activity against Meloidogyne incognita. Eur. J. Plant Pathol. 126, 417–422. doi: 10.1007/s10658-009-9550-z
Hultberg, M., Alsberg, T., Khalil, S., and Alsanius, B. (2010a). Suppression of disease in tomato infected by Pythium ultimum with a biosurfactant produced by Pseudomonas koreensis. BioControl 55, 435–444. doi: 10.1007/s10526-009-9261-9266
Hultberg, M., Bengtsson, T., and Liljeroth, E. (2010b). Late blight on potato is suppressed by the biosurfactant-producing strain Pseudomonas koreensis 2.74 and its biosurfactant. BioControl 55, 543–550. doi: 10.1007/s10526-010-9289-9287
Hutangura, P., Mathesius, U., Jones, M. G. K., and Rolfe, B. G. (1999). Auxin induction is a trigger for root gall formation caused by root-knot nematodes in white clover and is associated with the activation of the flavonoid pathway. Funct. Plant Biol. 26, 221–231.
Janssen, T., Karssen, G., Verhaeven, M., Coyne, D., and Bert, W. (2016). Mitochondrial coding genome analysis of tropical root-knot nematodes (Meloidogyne) supports haplotype based diagnostics and reveals evidence of recent reticulate evolution. Sci. Rep. 6, 1–13. doi: 10.1038/srep22591
Jones, J. T., Haegeman, A., Danchin, E. G. J., Gaur, H. S., Helder, J., Jones, M. G. K., et al. (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 14, 946–961. doi: 10.1111/mpp.12057
Karssen, G., Wesemael, W., and Moens, M. (2013). “Root-knot nematodes,” in Plant Nematology, eds R. N. Perry and M. Moens (Wallingford, UK: CAB International), 73–108. doi: 10.1079/9781780641515.0073
Karungi, J., Kyamanywa, S., Adipala, E., and Erbaugh, M. (2011). “Pesticide utilisation, regulation and future prospects in small scale horticultural crop production systems in a developing country,” in Pesticides in the Modern World, ed. M. Stoytcheva (London: InTechopen), 19–34.
Kimenju, J. W., Karanja, N. K., and Macharia, I. (1999). Plant parasitic nematodes associated with common bean in Kenya and the effect of Meloidogyne infection on bean nodulation. Afr. Crop Sci. J. 7, 503–510. doi: 10.4314/acsj.v7i4.27744
Kusstatscher, P., Cernava, T., Liebminger, S., and Berg, G. (2017). Replacing conventional decontamination of hatching eggs with a natural defense strategy based on antimicrobial, volatile pyrazines. Sci. Rep. 7, 1–8. doi: 10.1038/s41598-017-13579-13577
Laforest-Lapointe, I., Paquette, A., Messier, C., and Kembel, S. W. (2017). Leaf bacterial diversity mediates plant diversity and ecosystem function relationships. Nature 546, 145–147. doi: 10.1038/nature22399
Lewis, S. L., and Maslin, M. A. (2015). Defining the anthropocene. Nature 519, 171–180. doi: 10.1038/nature14258
Liu, W., Sutton, J. C., Grodzinski, B., Kloepper, J. W., and Reddy, M. S. (2007). Biological control of pythium root rot of chrysanthemum in small-scale hydroponic units. Phytoparasitica 35:159. doi: 10.1007/BF02981111
Lundberg, D. S., Yourstone, S., Mieczkowski, P., Jones, C. D., and Dangl, J. L. (2013). Practical innovations for high-throughput amplicon sequencing. Nat. Methods 10:999. doi: 10.1038/nmeth.2634
Mendes, R., Kruijt, M., de Bruijn, I., Dekkers, E., van der Voort, M., Schneider, J. H. M., et al. (2012). Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332, 1097–1100. doi: 10.1126/science.1203980
Oerke, E.-C. (2006). Crop losses to pests. J. Agric. Sci. 144, 31–43. doi: 10.1017/S0021859605005708
Rumiani, M., Karegar, A., Hamzehzarghani, H., and Banihashemi, Z. (2016). Effect of elemental sulfur on the root-knot nematode. Meloidogyne incognita, activities in cucumber plants. Iran. J. Plant Pathol. 52, 85–98.
Rybakova, D., Mancinelli, R., Wikström, M., Birch-Jensen, A. S., Postma, J., Ehlers, R. U., et al. (2017). The structure of the Brassica napus seed microbiome is cultivar-dependent and affects the interactions of symbionts and pathogens. Microbiome 5:104. doi: 10.1186/s40168-017-0310-316
Ryu, C.-M., Farag, M. A., Hu, C.-H., Reddy, M. S., Wei, H.-X., Paré, P. W., et al. (2003). Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 100, 4927–4932. doi: 10.1073/pnas.0730845100
Satola, B., Wübbeler, J. H., and Steinbüchel, A. (2013). Metabolic characteristics of the species Variovorax paradoxus. Appl. Microbiol. Biotechnol. 97, 541–560. doi: 10.1007/s00253-012-4585-z
Schwendner, P., Mahnert, A., Koskinen, K., Moissl-Eichinger, C., Barczyk, S., Wirth, R., et al. (2017). Preparing for the crewed Mars journey: microbiota dynamics in the confined Mars500 habitat during simulated Mars flight and landing. Microbiome 5:129. doi: 10.1186/s40168-017-0345-348
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. doi: 10.1101/gr.1239303.metabolite
Ssekyewa, C. (2006). Incidence, Distribution and Characteristics of Major Tomato Leaf Curl and Mosaic Virus Diseases in Uganda. PhD-thesis. Faculty of Bioscience Engineering, Ghent University, Ghent.
Tian, B. Y., Cao, Y., and Zhang, K. Q. (2015). Metagenomic insights into communities, functions of endophytes, and their associates with infection by root-knot nematode. Meloidogyne incognita, in tomato roots. Sci. Rep. 5, 1–15. doi: 10.1038/srep17087
Tilman, D., Reich, P. B., and Isbell, F. (2012). Biodiversity impacts ecosystem productivity as much as resources, disturbance, or herbivory. Proc. Natl. Acad. Sci. U.S.A. 109, 10394–10397. doi: 10.1073/pnas.1208240109
Torto, B., Cortada-Gonzalez, L., Murungi, L. K., Haukeland, S., and Coyne, D. (2018). Management of cyst and root knot nematodes: a chemical ecology perspective. J. Agric. Food Chem. 66, 8672–8678. doi: 10.1021/acs.jafc.8b01940
Trudgill, D. L., and Blok, V. C. (2001). Apomicitc, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopathol. 39, 53–77. doi: 10.1146/annurev.phyto.39.1.53
van Elsas, J. D., Chiurazzi, M., Mallon, C. A., Elhottova, D., Kristufek, V., and Salles, J. F. (2012). Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. U.S.A. 109, 1159–1164. doi: 10.1073/pnas.1109326109
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A., and Dufresne, A. (2015). The importance of the microbiome of the plant holobiont. New Phytol. 206, 1196–1206. doi: 10.1111/nph.13312
Vanlauwe, B., Coyne, D., Gockowski, J., Hauser, S., Huising, J., Masso, C., et al. (2014). Sustainable intensification and the African smallholder farmer. Curr. Opin. Environ. Sustain. 8, 15–22. doi: 10.1016/j.cosust.2014.06.001
Verginer, M., Leitner, E., and Berg, G. (2010). Production of volatile metabolites by grape-associated microorganisms. J. Agric. Food Chem. 58, 8344–8350. doi: 10.1021/jf100393w
Viaene, N., Coyne, D. L., and Davies, K. G. (2013). “Biological and Cultural Management,” in Plant Nematology, eds M. Moens and R. N. Perry (Wallingford, UK: CAB International), 383–410. doi: 10.1079/9781780641515.0383
Keywords: root-knot nematodes, tomato microbiome, biocontrol, antagonists, Pseudomonas, Comamonas, Variovorax, Bacillus
Citation: Wolfgang A, Taffner J, Guimarães RA, Coyne D and Berg G (2019) Novel Strategies for Soil-Borne Diseases: Exploiting the Microbiome and Volatile-Based Mechanisms Toward Controlling Meloidogyne-Based Disease Complexes. Front. Microbiol. 10:1296. doi: 10.3389/fmicb.2019.01296
Received: 06 March 2019; Accepted: 23 May 2019;
Published: 07 June 2019.
Edited by:
Jesús Mercado-Blanco, Instituto de Agricultura Sostenible (IAS), SpainReviewed by:
Miguel Talavera, Andalusian Institute for Research and Training in Agriculture, Fisheries, Food and Ecological Production (IFAPA), SpainCopyright © 2019 Wolfgang, Taffner, Guimarães, Coyne and Berg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriele Berg, Z2FicmllbGUuYmVyZ0B0dWdyYXouYXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.