- 1Department of Infectious Diseases and Microbiology, University of Lübeck, Lübeck, Germany
- 2Department of Obstetrics and Gynecology, University Hospital of Schleswig-Holstein, University of Lübeck, Lübeck, Germany
- 3Institute of Medical Microbiology, Immunology and Parasitology, University Hospital Bonn, Bonn, Germany
- 4German Center for Infection Research (DZIF), Partner Sites Bonn-Cologne/Hamburg-Lübeck-Borstel-Riems, Lübeck, Germany
- 5Institute of Pharmaceutical Microbiology, University Hospital Bonn, University of Bonn, Bonn, Germany
Ascending Chlamydia trachomatis infection causes functional damage to the fallopian tubes, which may lead to ectopic pregnancy and infertility in women. Treatment failures using the standard regimens of doxycycline and azithromycin have been observed. We tested the polyketide-derived α-pyrone antibiotic Corallopyronin A (CorA) that inhibits the bacterial DNA dependent RNA polymerase and has strong activity against various extracellular and some intracellular bacteria. Extensive testing in cell culture infection models and in an ex vivo human fallopian tube model under different oxygen concentrations was performed to assess the anti-chlamydial efficacy of CorA at physiological conditions. CorA showed high efficacy against C. trachomatis (MICN/H: 0.5 μg/mL for serovar D and L2), C. muridarum (MICN/H: 0.5 μg/mL), and C. pneumoniae (MICN/H: 1 μg/mL) under normoxic (N) and hypoxic (H) conditions. Recoverable inclusion forming units were significantly lower already at 0.25 μg/mL for all tested chlamydiae. CorA at a concentration of 1 μg/mL was also effective against already established C. trachomatis and C. pneumoniae infections (up to 24 h.p.i.) in epithelial cells, while efficacy against C. muridarum was limited to earlier time points. A preliminary study using a C. muridarum genital infection model revealed corresponding limitations in the efficacy. Importantly, in an ex vivo human fallopian tube model, the growth of C. trachomatis was significantly inhibited by CorA at concentrations of 1–2 μg/mL under normoxic and hypoxic conditions. The overall high efficacies of CorA against C. trachomatis in cell culture and an ex vivo human fallopian tube model under physiological oxygen concentrations qualifies this drug as a candidate that should be further investigated.
Introduction
Infections with Chlamydia trachomatis are a major health concern worldwide with more than 100 million new cases per annum (Senior, 2012; Newman et al., 2015). Recurrent infection with C. trachomatis is common and mediates several upper genital tract sequelae including pelvic inflammatory disease (PID), ectopic pregnancy, and infertility in women (Workowski et al., 2015). Some women diagnosed with uncomplicated cervical infection already have a subclinical infection of the upper reproductive tract including the uterus, fallopian tubes, and the ovaries (Workowski et al., 2015).
While the current recommended treatment strategies against urogenital C. trachomatis infections are either a 7-day course of doxycycline or a single dose of azithromycin (Workowski et al., 2015), recent reports suggest that eradication rates using azithromycin are inferior to doxycycline (Geisler et al., 2015). Treatment failures, defined as recurrence of the pathogen after so called efficient antibiotic treatment, are observed in up to 8% of the patients (Horner, 2006).
According to the above mentioned observations, we and others could previously experimentally link impaired efficacies of first-line antimicrobials to low oxygen concentrations (0.5–5.5% O2) (Shima et al., 2011, 2013; Schaffer and Taylor, 2015; Matsuo et al., 2019), which are observed in the human female genital tract (Juul et al., 2007; Dietz et al., 2011). In fact, hypoxia plays a key role in physiological processes as well as pathophysiological conditions, e.g., inflammation associated with bacterial infection (Schaffer and Taylor, 2015), and might thus be one of the reasons accounting for treatment failures in chlamydial infections in patients. Therefore, alternative antimicrobials with high efficacies over a broad range of oxygen concentrations are considered a feasible solution to overcome limitations of current treatment strategies.
Along this line, we analyzed the efficacy of Corallopyronin A (CorA) on infection with Chlamydia spp. CorA was first isolated from Corallococcus coralloides (Irschik et al., 1985). It is a polyketide-derived α-pyrone antibiotic that inhibits the bacterial DNA dependent RNA polymerase (RNAP) (Mukhopadhyay et al., 2008) and thereby impedes bacterial growth. CorA’s anti-bacterial property is mediated through binding to a region outside of the bacterial RNAP active site called the switch region (Srivastava et al., 2011). The antimicrobial efficacy of CorA has been shown mainly for Gram-positive bacteria (Irschik et al., 1985). However, it also eradicates the Gram-negative intracellular bacteria Wolbachia (Schiefer et al., 2012) and Orientia tsutsugamushi (Kock et al., 2018) in vitro and in vivo. We have shown that CorA is a potent antibiotic for the eradication of rifampicin-resistant C. trachomatis serovar L2 in vitro (Shima et al., 2018).
Here, we report high activity of CorA against chlamydiae in epithelial cell lines and a human fallopian tube ex vivo model under normoxic and hypoxic conditions. Conflicting results in a C. muridarum in vivo model have to be further investigated as well as the methods for predicting and transferring antimicrobial efficacy data from the mouse infection model to the situation in human C. trachomatis infections.
Materials and Methods
Bacterial Strains and Cell Culture
Chlamydia trachomatis serovar D (ATCC VR-885), C. trachomatis serovar L2 (ATCC VR-902B), C. muridarum NiggII (ATCC VR-123), and C. pneumoniae CWL029 (ATCC VR-1310) were propagated in HeLa (ATCC CCL-2) or HEp-2 cells (ATCC CCL-23) in vitro. Human fallopian tubes were infected ex vivo with C. trachomatis serovar D. C. muridarum NiggII was used for all in vivo experiments for the genital infection of mice.
Chemicals
Doxycycline was purchased from Sigma-Aldrich (D9891; St. Louis, MO, United States). Purification of CorA was performed as described in previous studies with some modification (Erol et al., 2010; Schiefer et al., 2012) for all in vitro and ex vivo experiments. For the in vivo experiments CorA (purity of 60% by LC/MS) was diluted in dimethyl sulfoxide (DMSO) or ethanol, correcting the mass for the purity to achieve the required concentrations. Intraperitoneal (i.p.) injection was performed by using a mixture of the DMSO-CorA solution (10%) and PBS (90%), while oral administration was given using the ethanol-CorA solution (10%) mixed with Solutol® HS 15 (40%, Sigma-Aldrich) and ddH2O (50%).
Determination of the Minimum Inhibitory Concentration (MIC) for Chlamydiae
A total of 5 × 104 HeLa cells for C. trachomatis and C. muridarum or HEp-2 cells for C. pneumoniae were grown in a 24-well plate (Greiner bio-one, Frickenhausen, Germany) in RPMI1640 (Gibco/Invitrogen, Karlsruhe, Germany) with 5% FCS (Gibco/Invitrogen), non-essential amino acids (GE Healthcare Hyclone, South Logan, UT) and 2 mM glutamine (Lonza, Cologne, Germany) without antibiotics for 24 h under normoxic (20% O2) and hypoxic (2% O2, Toepffer Lab Systems, Göppingen, Germany) conditions. Afterward, cells were infected with C. trachomatis serovar D, C. trachomatis serovar L2, C. muridarum NiggII, or C. pneumoniae CWL029 with an infection rate of 0.5 (50% infected cells in controls). Centrifugation (700 × g, 1 h, 37°C) was used for C. trachomatis serovar D, C. muridarum NiggII, and C. pneumoniae CWL029 infection. In addition, 0.1 μg/mL cycloheximide were used for C. muridarum and C. pneumoniae CWL029 infection. Infected cells were incubated with or without different concentrations of CorA ranging from 0.125 to 2 μg/mL. MICs were determined by the visualization of the growth of chlamydiae after 48 h incubation. Chlamydial inclusions were visualized by immunofluorescence staining with a mouse anti-chlamydial lipopolysaccharide (LPS) antibody (kindly provided by Dr. Helmut Brade, Borstel, Germany) and a polyclonal rabbit FITC-labeled anti-mouse IgG antibody (Dako, Hamburg, Germany).
Testing of Recoverable Chlamydiae
A total of 5 × 104 HeLa for C. trachomatis and C. muridarum or HEp-2 cells for C. pneumoniae was seeded in 24-well plates and cultured for 24 h under normoxic and hypoxic conditions. Afterward, cells were infected with C. trachomatis serovar D, C. trachomatis serovar L2, C. muridarum, or C. pneumoniae CWL029 with an infection rate of 0.5 (50% infected cells in controls). Different concentrations of CorA ranging from 0.25 to 1.5 μg/mL were added at the time of infection. In addition, 1 μg/mL CorA was added at 6, 12, and 24 h post-infection (h.p.i.), to investigate whether an established chlamydial infection can be eradicated. At 30 (C. trachomatis and C. muridarum) or 48 (C. pneumoniae) h.p.i., cells were washed with medium to remove the remaining CorA and recoverable chlamydiae were determined as described (Shima et al., 2011). Chlamydial inclusions were visualized as described above.
Application of CorA in an in vivo Mouse Model of Chlamydia muridarum Infection
Eight-week old female C57BL/6JRj mice (Janvier Labs, France) were synchronized to the same stage of estrous cycle by subcutaneous injection of 2.5 mg medroxyprogesterone acetate (Depo-Clinovir®, Pfizer, New York, NY, United States) per mouse. After 7 days, each mouse was vaginally infected with 106 IFUs C. muridarum. CorA and other antibiotics were applied day 1 to 8 p.i. We used an i.p. injection with DMSO (10%) as solvent or a solvent-mixture of ethanol (10%), Solutol® HS 15 (40%) and ddH2O for oral gavage via feeding tubes (Instech Laboratories, Plymouth Meeting, PA, United States). CorA was given in concentrations of 70 mg/kg BW (once daily) and 35 mg/kg BW (once or twice daily). Other antibiotics were given as follows: doxycycline (50 mg/kg BW i.p., once daily, or 10 mg/kg BW orally, twice daily), azithromycin (40 mg/kg BW, orally, once daily), and amoxicillin (20 mg/kg BW, orally, three times daily). One group was treated with each solvent alone as control. Vaginal swabs (Oxoid Limited, Basingstoke, United Kingdom) were collected at days 3, 7, 10, 14, 17, 21, 28, and 35 p.i. and recovery assay of C. muridarum was performed on HEp-2 cells to determine the bacterial burden. At 43 days post-infection, mice were euthanized and the upper and lower genital tract was macroscopically and microscopically evaluated for the formation of characteristic pathologies.
Scoring of Pathologies
To score pathologies, hydrosalpinx at the upper genital tract were classified as follows based on their size: 0 = none, 1 = microscopically visible, 2 = visible by eye, smaller than the ovaries, 3 = same size as the ovaries, 4 = bigger than the ovaries. A stereomicroscope with a magnification ranging from 4 to 31.5 was used to assess score 1.
Efficacy of CorA Against C. trachomatis Serovar D in the Human Fallopian Tube ex vivo Model
Preparation of human fallopian tubes was performed as described (Jerchel et al., 2012). In brief, the tissue of human fallopian tubes was collected at peripartum sterilization on maternal request during cesarean sections or during hysterectomies due to symptomatic uterine fibroids in the luteal phase. The human fallopian tubes were dissected in a Petri dish containing RPMI1640 (Gibco/Invitrogen) with 5% FCS (Gibco/Invitrogen), non-essential amino acids (GE Healthcare Hyclone), and 2 mM glutamine (Lonza) without antibiotics. After dissection, human fallopian tubes were opened carefully with a small scalpel. The human fallopian tube specimen in culture medium was infected with 5 × 108 IFUs C. trachomatis serovar D with or without CorA for 48 h under normoxic and hypoxic conditions. At 48 h.p.i., cells were washed with medium to remove the remaining CorA and recoverable C. trachomatis serovar D were determined.
Ethics Statement
Experiments with explanted human fallopian tubes were approved by the ethical committee of the University of Lübeck (09-153). Participants were informed and provided written consent. The animal experiments were revised and approved by the Ministry of Energy, Agriculture, the Environment and Rural Areas of Schleswig-Holstein [File Reference V311-7224.122(54-4/13)].
Statistics
Data are indicated as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism statistical software (version 7.03). Continuous variables between two groups were evaluated utilizing paired Student’s t-test. When three or more groups were compared in the experiment, Sidak’s multiple comparison was used if the one-way analysis of variance showed statistical significance. Groups with nominal data were compared using Chi-Square test. P-values ≤ 0.05 were considered as statistically significant.
Results
CorA Has Equivalent MIC Values Against Chlamydiae Under Normoxia and Hypoxia
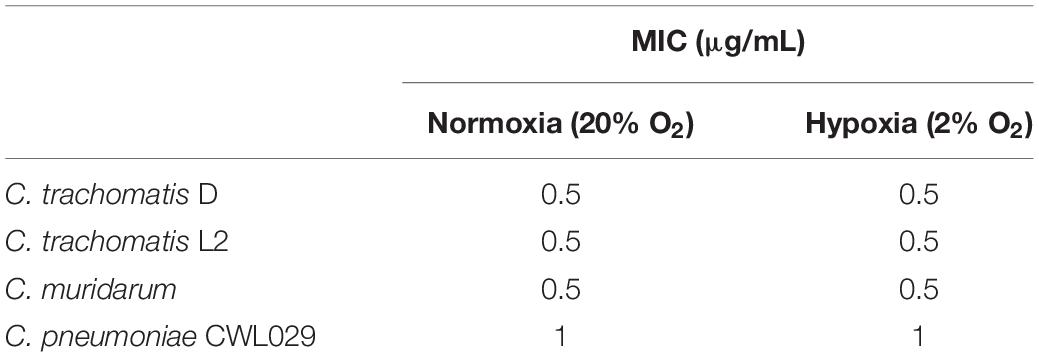
CorA is a promising drug against other intracellular pathogens, such as Wolbachia endobacteria and O. tsutsugamushi (Schiefer et al., 2012; Kock et al., 2018) and, therefore, its efficacy against Chlamydia spp. was determined. The MIC is defined as the minimal inhibitory concentration to prevent visible chlamydial growth in cell culture. We tested the MIC of CorA against several chlamydial species in and investigated whether the MICs of CorA differed under normoxia (20% O2) and hypoxia (2% O2). CorA had high efficacies against C. trachomatis serovars D and L2, C. muridarum and C. pneumoniae. The effectiveness was similar under normoxia and hypoxia (C. trachomatis serovar D: 0.5 μg/mL, C. trachomatis serovar L2: 0.5 μg/mL, C. muridarum: 0.5 μg/mL, and C. pneumoniae: 1 μg/mL) (Table 1 and Figures 1A–C).
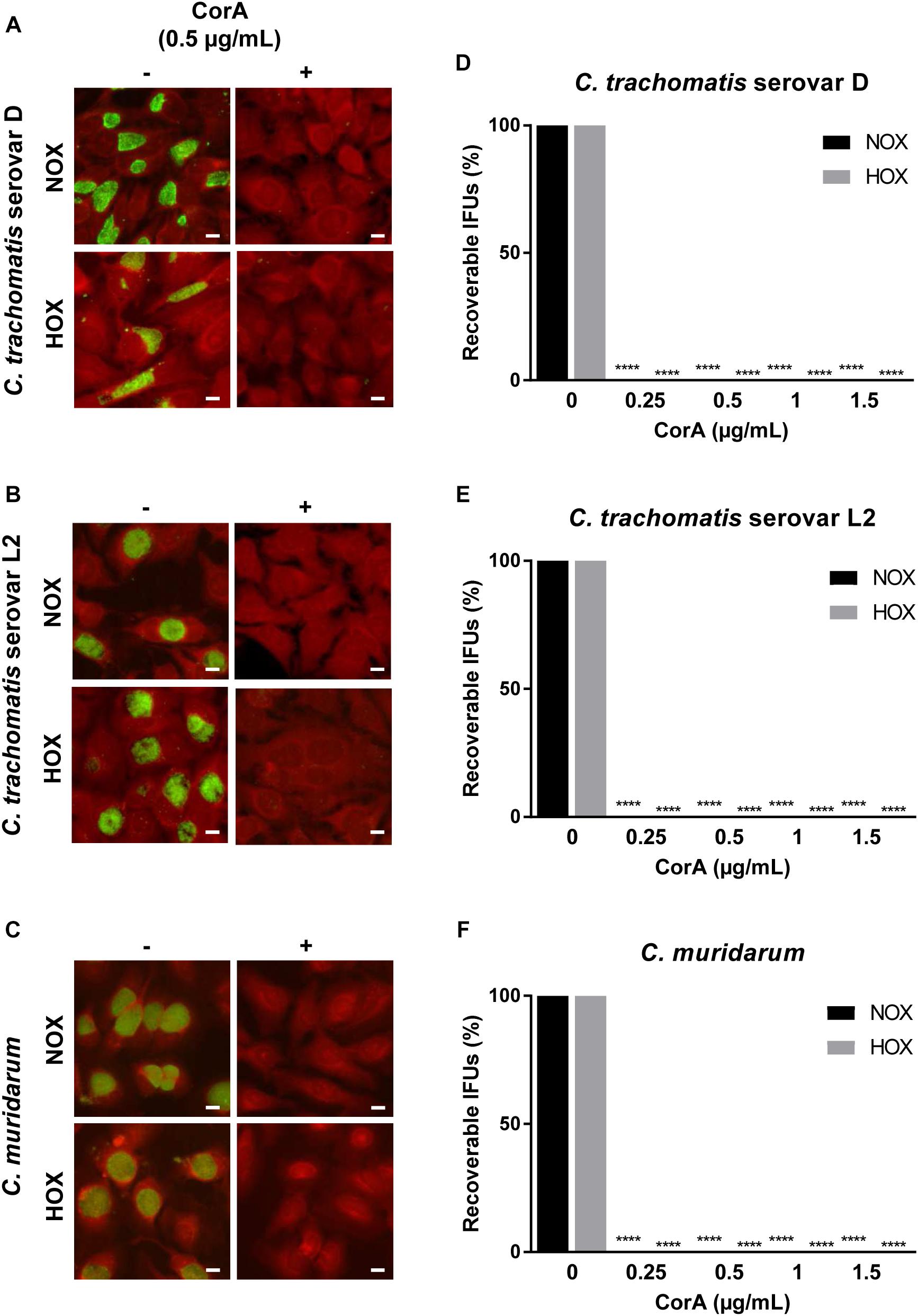
Figure 1. Efficacy of CorA against Chlamydia trachomatis and Chlamydia muridarum in HeLa cells under normoxia and hypoxia. The inhibition of C. trachomatis serovar D (A), serovar L2 (B), and C. muridarum (C) growth in HeLa cells upon exposure to CorA under normoxia and hypoxia. Immunofluorescence staining shows chlamydial inclusions (green) and host cells (red, scale bar = 10 μm). Recoverable IFUs were determined for C. trachomatis serovar D (D), serovar L2 (E), and C. muridarum (F). The numbers of recoverable chlamydiae at the indicated CorA concentrations were calculated as a percentage of the untreated control under normoxia and hypoxia at 30 h post-infection (h.p.i.) (n = 3; mean ± SD, Sidak’s multiple comparison ****p ≤ 0.0001). Nox: Normoxia, Hox: Hypoxia, CorA: Corallopyronin A.
CorA Treatment Significantly Reduces Infectious Progeny of Chlamydiae Under Normoxia and Hypoxia
The inhibition of bacterial RNAP is bactericidal. Therefore, we checked whether the growth inhibition also led to a killing of the bacteria via recovery assay. We analyzed recoverable chlamydiae to investigate the production of infectious EBs under different
CorA concentrations. In this assay we observed a significant reduction in infectious progeny of C. trachomatis serovars D and L2, C. muridarum, and C. pneumoniae treated with CorA in concentrations from 0.25 to 1.5 μg CorA (Figures 1D–F, Supplementary Figure 1A, and Supplementary Table 1) compared to the untreated control. The eradication rate was not significantly different between normoxia and hypoxia.
CorA Eradicates Already Established Chlamydial Infections
The majority of chlamydial infections has an asymptomatic course of infection and remains unnoticed for that reason. As a consequence, antimicrobial treatment is regularly initiated when the infection has already been long established. Therefore, we investigated whether CorA could effectively eradicate already established infections by delaying antimicrobial treatment throughout the chlamydial replication cycle for up to 24 h.p.i. When Chlamydia infected cells were treated with CorA (1.0 μg/mL) there was a significant reduction of infectious progeny for up to 12 h.p.i. in all tested strains. In C. trachomatis serovar D and serovar L2 as well as C. pneumoniae infected cells the reduction was still present if treated 24 h.p.i. (Figures 2A,B, Supplementary Figure 1B, and Supplementary Table 2), whereas the treatment 24 h.p.i. of C. muridarum infected cells led to chlamydial progeny comparable to the untreated control (Figure 2C and Supplementary Table 2). As in the previous experiments, no significant differences were observed between cells infected under normoxia and hypoxia.
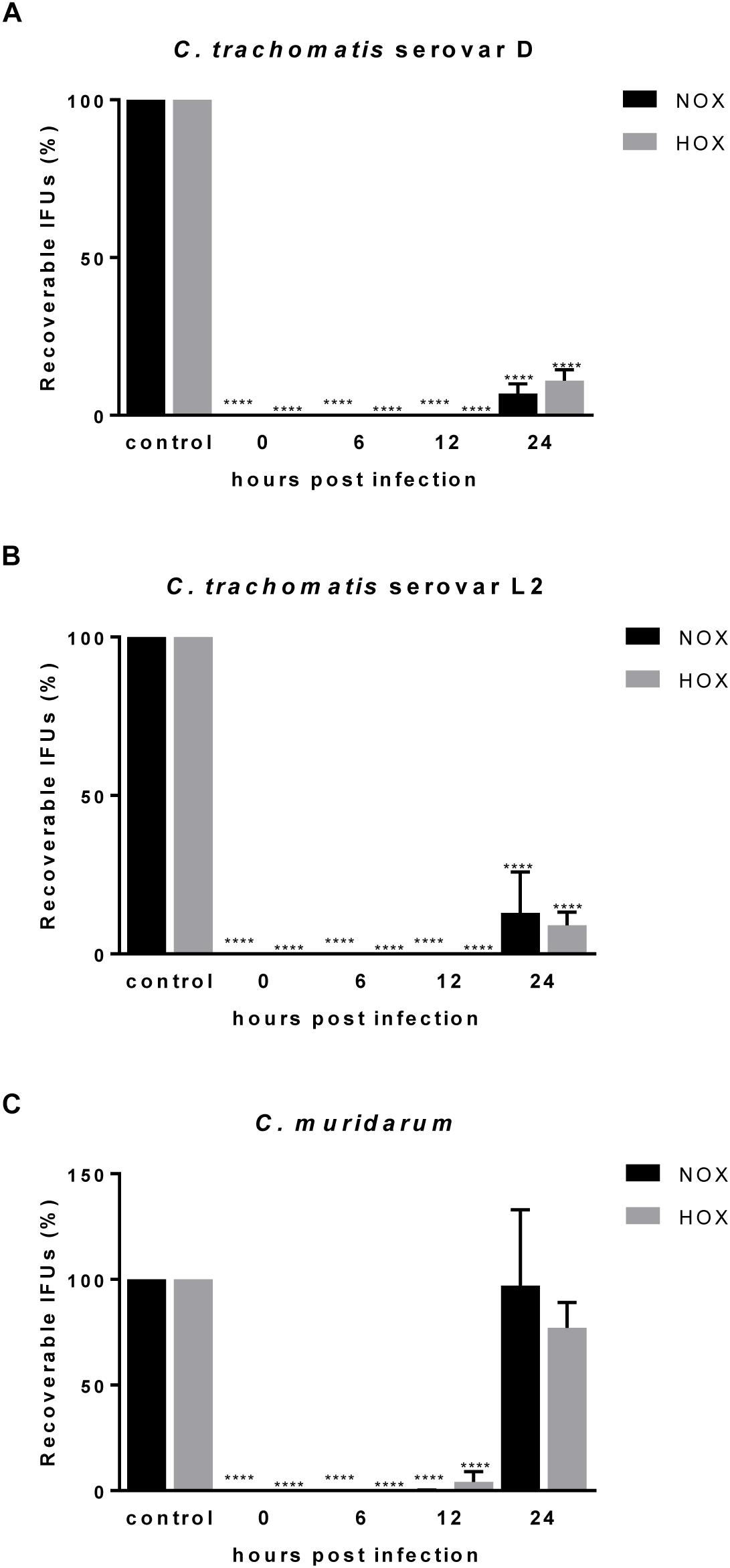
Figure 2. Efficacy of CorA against established C. trachomatis and C. muridarum infection in HeLa cells under normoxia and hypoxia. The eradication rate of C. trachomatis serovar D (A), serovar L2 (B), and C. muridarum (C) in HeLa cells. CorA (1 μg/mL) was added at 0, 6, 12, and 24 h.p.i. The numbers of recoverable chlamydiae in CorA treatment were calculated as a percentage of the untreated control under normoxia and hypoxia at 30 h.p.i. (n = 3; mean ± SD, Sidak’s multiple comparison ****p ≤ 0.0001). Nox: Normoxia, Hox: Hypoxia.
Application of CorA Showed No Impact on a Chlamydia muridarum Infection in vivo
The usage of various animal models in chlamydial infection is broadly accepted, and has successfully been applied in investigations on CorA efficacy against other intracellular bacteria in mice (Schiefer et al., 2012; Kock et al., 2018). Hence, we were interested in the outcome of CorA treatment in an acute C. muridarum mouse infection model in comparison to standard doxycycline treatment. A general overview about the animal experiments is provided in Figure 3A. Oral administration of the anti-chlamydial antibiotics doxycycline, azithromycin, and amoxicillin was tested, and showed that doxycycline and azithromycin prevent hydrosalpinx formation and doxycycline eradicates C. muridarum completely (Supplementary Figure 2). Applying CorA either i.p. or orally, we analyzed bacterial shedding and hydrosalpinx formation during the course of infection. The bacterial burden 3 d.p.i. was comparable between all groups, while only the doxycycline treated mice showed a significantly reduced bacterial burden (Figure 3B and Supplementary Figure 2). Over time, control mice naturally shed C. muridarum over 28 days, with no recoverable pathogen on subsequent days (Figure 3B). C. muridarum was not detectable in doxycycline treated mice from 6 d.p.i. However, i.p. or orally CorA treated mice had a comparable shedding to the control mice (Figure 3B and Supplementary Figure 3A). Mice infected with C. muridarum developed hydrosalpinx on the ovaries with a mean score of 3.25 (Figures 3C,D). Neither the control mice (mean: 2.38) nor the mice treated with CorA (means: 3 and 1.63, respectively, CorA 1x or 2x) showed significant deviations from this pathology score (Figures 3C,D). In contrast, doxycycline completely averted the formation of hydrosalpinx (Figure 3D and Supplementary Figure 3B).
Figure 3. Efficacy of CorA against C. muridarum in vivo. Experimental setup of all conducted mouse experiments including sampling time points (A). Bacterial shedding of C. muridarum in comparison to different treatments calculated from infectious progeny from vaginal swabs. (n = 4, Pairwise Wilcoxon rank sum test *p < 0.05, **p < 0.01) (B). Formation of characteristic pathologies following infection with C. muridarum and treatment with corresponding substances (white arrows = hydrosalpinx, scale bar = 5 mm) (C). Percentage of the observed pathology scores for all groups of mice (n = 8, Chi-Square test *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001) (D). CorA: Corallopyronin A.
CorA Inhibits the Growth of C. trachomatis Serovar D in an ex vivo Fallopian Tube Model
We aimed to test the efficacy of CorA against C. trachomatis as close as possible to the human in vivo setting. Therefore, we applied an ex vivo fallopian tube model that has been previously established for the analysis of host-pathogen interactions (Jerchel et al., 2012). Initially, we checked whether hypoxia affects the infectious outcome. We found that equal amounts of C. trachomatis could be recovered from infected fallopian tubes under normoxia and hypoxia (Supplementary Figure 4). We then tested whether CorA effectively inhibits the growth of C. trachomatis in this model. At 1 μg/mL CorA 2 ± 5% (normoxia) and 77 ± 60% (hypoxia) of C. trachomatis serovar D survived (Figure 4 and Supplementary Table 3). However, when we treated with 2 μg/mL CorA, growth of C. trachomatis serovar D was completely blocked in the ex vivo fallopian tube model under normoxia and hypoxia (Figure 4 and Supplementary Table 3).
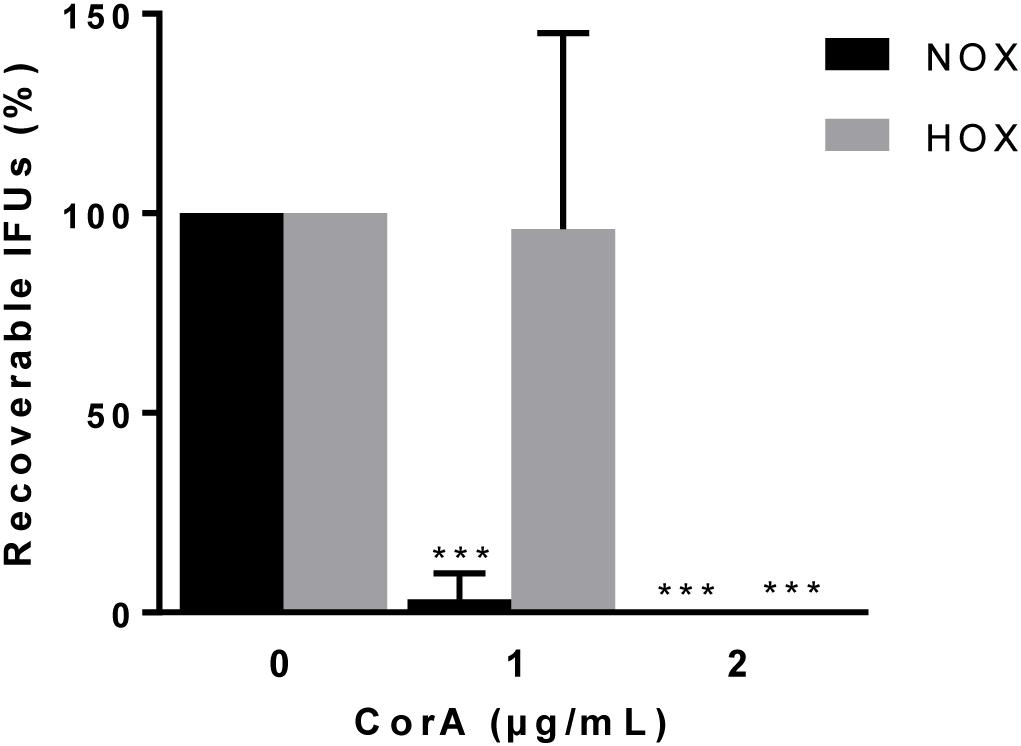
Figure 4. Efficacy of CorA against C. trachomatis serovar D in ex vivo culture of primary human fallopian tubes under normoxia and hypoxia. The inhibition rate of CorA on C. trachomatis serovar D growth under normoxia and hypoxia. The numbers of recoverable C. trachomatis serovar D under CorA treatment were calculated as a percentage of the untreated control at 48 h.p.i. (Normoxia n = 5; mean ± SD, Hypoxia n = 4; mean ± SD, Sidak’s multiple comparison ***p ≤ 0.001). Nox: Normoxia, Hox: Hypoxia, CorA: Corallopyronin A.
Discussion
More than 70% of C. trachomatis infections are asymptomatic, bearing the risk for progression during the acute infection phase or chronically over years (Molano et al., 2005). If appropriate therapy is not given or treatment is delayed in infected women, the pathogen may ascend from the lower female genital tract to the upper genital tract causing salpingitis and PID. Both are known risk factors for developing fallopian tube scarring, potentially leading to ectopic pregnancy and tubal infertility (Briceag et al., 2015; Hafner, 2015).
Doxycycline (100 mg orally twice a day for 7 days) or azithromycin (1 g orally in a single dose) are first line drugs to treat urogenital chlamydial infections and treatment is successful in >95% (Lau and Qureshi, 2002; Workowski et al., 2015). However, for longer periods following application of either doxycycline or azithromycin, 8% treatment failure has been observed (Fortenberry et al., 1999; Golden et al., 2005). Reduced intracellular activities of doxycycline and azithromycin were demonstrated under hypoxic conditions (Shima et al., 2011, 2013) and oxygen concentrations range from 0.5 to 5.5% in the cervix and vagina (Juul et al., 2007; Dietz et al., 2011). We therefore hypothesized that this is one of the possible reasons accounting for the insufficient eradication of C. trachomatis in the urogenital tract. Accordingly, we here present data on an alternative antimicrobial, CorA, with focus on its efficacy against intracellular C. trachomatis infection in cell culture and a human fallopian tube model, both under different oxygen environments and in vivo.
CorA binds to the bacterial RNAP, a highly conserved haloenzyme in bacteria with low homology to RNAP in eukaryotic cells. Thus, this antimicrobial is specific to prokaryotic RNAP (Irschik et al., 1985; Campbell et al., 2001; Belogurov et al., 2009). While CorA was originally described to inhibit the growth of Gram-positive bacteria (MIC value: between 0.1 and 10 μg/mL) (Irschik et al., 1985), it has recently been shown that it is also highly active against Gram-negative intracellular O. tsutsugamushi (Kock et al., 2018) and Wolbachia endobacteria in vitro and in vivo (Schiefer et al., 2012). The structure and preponderance of efflux pumps of many Gram-negative bacteria seems to be a barrier to CorA (Irschik et al., 1985). Therefore, it was proposed that it might be effective against Wolbachia because they are not able to synthesize lipopolysaccharides (Schiefer et al., 2012). Chlamydial LPS has a unique and atypical structure of its lipid A (Kosma, 1999) and lacks O-antigen polysaccharide side chains (Rund et al., 1999). Further, Chlamydia do not encode for TolC, an outer membrane tunnel of multidrug resistance efflux pumps that has been implicated in CorA export in E. coli (Mariner et al., 2011). Therefore, we hypothesized that CorA might also be effective against obligate intracellular chlamydiae.
In this study, MICs against C. trachomatis serovars D and L2 were 0.5 μg/mL under normoxia and hypoxia, while recovery of infectious EBs was limited already at 0.25 μg/mL. Using recovery assays, we could further show strong CorA activity against chlamydiae during an established infection under both normoxia and hypoxia. Our data indicate that CorA has a high efficacy against C. trachomatis, wherein the activity of CorA appears not to be influenced by the environmental oxygen availability in cell culture, an important finding given the described impact of oxygen on standardly prescribed antibiotics (Shima et al., 2011, 2013). Furthermore, we have already described CorA as a potent substance against rifampin-resistant C. trachomatis L2 serovars (Shima et al., 2018), thereby enhancing the application area of CorA. We were curious whether efficacy is also observed against other pathogenic Chlamydia and observed that CorA inhibits C. muridarum in a similar concentration, while C. pneumoniae CWL029 is inhibited at a MIC of 1 μg/mL (Table 1 and Supplementary Figure 1). This indicates a general activity of CorA across the Chlamydia genus. While treatment throughout the first hours of the infection has equal efficacies, we observed enhanced chlamydial recovery if treated after 24 h for C. muridarum (Figure 2), indicating that CorA acts mainly against the replicative reticulate bodies of the chlamydial cell cycle. In favor of this assumption, no increase in recovery is observed if treatment takes place 24 h.p.i. in C. pneumoniae infection (Supplementary Figure 1B), reflecting a longer duration of its life cycle and, thus, later redifferentiation to elementary bodies.
We tested the impact of CorA in an in vivo model against the murine pathogen C. muridarum, as it successfully infects the genital tract of female mice (Barron et al., 1981). Since the MIC against several chlamydial species is comparable to the MIC of CorA against Wolbachia, our mouse experiments used the same doses or even double of published CorA doses (Schiefer et al., 2012). In these experiments, CorA has been shown to effectively target Wolbachia, essential intracellular endosymbionts of filarial nematodes, after intraperitoneal administration (Schiefer et al., 2012).
Although doxycycline was able to eradicate C. muridarum in our in vivo model, no comparable effect was achieved when CorA was administered, neither i.p. nor orally. As with amoxicillin, the resulting hydrosalpinx were comparable to infected control animals. A wide range of factors need to be considered when addressing the in vivo results. Thus, in contrast to the human pathogens C. trachomatis and C. pneumoniae, the CorA treatment that was initiated 24 h after infection was also not sufficient in depleting the murine pathogen C. muridarum in vitro (Figure 2C). The start of the treatment in the in vivo model mimics the same conditions, by infecting the mice and starting the treatment 24 h after infection. Thus, the timing of the treatment in conjunction with strain-specific life cycle kinetics may contribute to the missing in vivo activity of CorA.
Further reasons for the lack of treatment success in our mouse model may be partially attributed to differences in available treatment settings compared to studies with other pathogens. While successful Wolbachia treatment is performed over a course of 2–4 weeks (Schiefer et al., 2012; Schiefer et al., unpublished data), resulting in a long lasting antibiotic pressure on the bacteria, this cannot be performed in the short, but intensive infection with C. muridarum. In the case of the treatment of O. tsutsugamushi the treatment duration is comparable to our setting. However, treatment was applied directly at the site of infection (Kock et al., 2018) and is thus not comparable to our model.
While CorA availability in the plasma of mice has been shown earlier (Shima et al., 2018), impaired drug penetration to the infected tissues is a problem in urogenital diseases (Muller et al., 2004). Thus, a short duration of high plasma levels of CorA in combination with limited drug penetration may eventually lead to a blood-tissue disequilibrium preventing successful treatment of C. muridarum infection in the mouse. This may have been exacerbated by the formulation used. Although CorA dosages were adjusted taking into account the 60% purity of CorA, a liquid formulation able to fully dissolve CorA at high concentrations and suitable for in vivo experiments is needed to achieve good genital tissue penetration.
While in vivo treatment of C. muridarum infection in the mouse is hampered during classical treatment regimens (e.g., oral administration), future experiments could make use of other application strategies, e.g., direct administration of CorA to the vagina. Local administration of metronidazole as gels and tablets is commonly used in the clinics for the treatment of bacterial vaginosis in females (Menard, 2011), but no models exist for such treatment of STIs.
Severe pathological sequels of C. trachomatis infections of the female genital tract frequently take place in the fallopian tubes. These are muscular conduits connecting the ovaries with the uterus and therefore play a key role for fertilization and transport of the developing blastocyst to the uterus. C. trachomatis infection causes salpingitis, which leads to functional damage of the fallopian tubes (Hafner, 2015). Explanted fallopian tubes have been shown to provide a powerful tool for the investigation of chlamydial infection (Roth et al., 2010; Jerchel et al., 2012; Kessler et al., 2012). As the whole organ is infected in vivo in ascending genital infections, our ex vivo system provides a model that mirrors the in vivo human state, and provides additional value in performing infection experiments with C. trachomatis in comparison to human cell lines and experimental animal models (Cooper et al., 1990; Jerchel et al., 2012; Kessler et al., 2012). In this study, we could show that the growth of C. trachomatis was completely blocked by 2 μg/mL CorA treatment in the fallopian tube ex vivo model under hypoxia, while 1 μg/mL was sufficient under normoxia. This difference in effectivity might be related to the complexity of the tissue structure of the fallopian tube and diverging behavior compared to a monolayer cell culture. As CorA is effective in reasonable doses in fallopian tubes, it might have the potential to eradicate C. trachomatis not only in cell-based approaches, but also in vivo in genital infection in humans as such.
Taken together, our data indicate that CorA is highly active against C. trachomatis in cervical epithelial cells and human fallopian tubes, under normoxia as well as hypoxia. This provides an advantage over currently used antibiotics that have been shown to lose efficacy under normal physiologic oxygen tension. Thus, CorA should be further developed as an in vivo treatment option for chlamydial infections. In addition, there is a range of anti-bacterial compounds produced from myxobacteria besides CorA (Schaberle et al., 2014; Herrmann et al., 2017) including several derivates of CorA (Schaberle et al., 2015), which should be screened for their efficacy against Chlamydia.
Author Contributions
NL, SG, SL, KS, IK, FH, and BH performed the experiments. JR, SG, NL, AH, KP, AS, BH, and IK contributed to the conception of the work. NL, SG, and KS performed statistical analysis. NL, SG, KS, and JR prepared the first draft of the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the DFG Research Training Group (RTG) 1743, the University of Lübeck (E10-2013), and the German Centre for Infection Research (Deutsches Zentrum für Infektionsforschung; DZIF), and grant # TTU09_703 (Preclinical Development of Corallopyronin A).
Conflict of Interest Statement
AH, AS, and KP hold patents for Corallopyronin A in the United States and European Union (US 9168244 B2, US 9687470 B2, and EP 2704708 B1).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully thank Siegrid Pätzmann, Kristin Wischnat, and Anke Hellberg for their technical assistance. The Corallopyronin A used in the study was produced and kindly provided by the Bio Pilot Plant of the Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany. Furthermore, we thank the Gemeinsame Tierhaltung (GTH) of the University of Lübeck for their support in conducting the animal experiments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00943/full#supplementary-material
References
Barron, A. L., White, H. J., Rank, R. G., Soloff, B. L., and Moses, E. B. (1981). A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J. Infect. Dis. 143, 63–66. doi: 10.1093/infdis/143.1.63
Belogurov, G. A., Vassylyeva, M. N., Sevostyanova, A., Appleman, J. R., Xiang, A. X., Lira, R., et al. (2009). Transcription inactivation through local refolding of the RNA polymerase structure. Nature 457, 332–335. doi: 10.1038/nature07510
Briceag, I., Costache, A., Purcarea, V. L., Cergan, R., Dumitru, M., Briceag, I., et al. (2015). Fallopian tubes–literature review of anatomy and etiology in female infertility. J. Med. Life 8, 129–131.
Campbell, E. A., Korzheva, N., Mustaev, A., Murakami, K., Nair, S., Goldfarb, A., et al. (2001). Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104, 901–912. doi: 10.1016/s0092-8674(01)00286-0
Cooper, M. D., Rapp, J., Jeffery-Wiseman, C., Barnes, R. C., and Stephens, D. S. (1990). Chlamydia trachomatis infection of human fallopian tube organ cultures. J. Gen. Microbiol. 136, 1109–1115. doi: 10.1099/00221287-136-6-1109
Dietz, I., Jerchel, S., Szaszák, M., Shima, K., and Rupp, J. (2011). When oxygen runs short: the microenvironment drives host-pathogen interactions. Microbes Infect. 14, 311–316. doi: 10.1016/j.micinf.2011.11.003
Erol, O., Schäberle, T. F., Schmitz, A., Rachid, S., Gurgui, C., El Omari, M., et al. (2010). Biosynthesis of the myxobacterial antibiotic corallopyronin A. Chembiochem 11, 1253–1265. doi: 10.1002/cbic.201000085
Fortenberry, J. D., Brizendine, E. J., Katz, B. P., Wools, K. K., Blythe, M. J., and Orr, D. P. (1999). Subsequent sexually transmitted infections among adolescent women with genital infection due to Chlamydia trachomatis. Neisseria gonorrhoeae, or Trichomonas vaginalis. Sex. Transm. Dis. 26, 26–32. doi: 10.1097/00007435-199901000-00005
Geisler, W. M., Uniyal, A., Lee, J. Y., Lensing, S. Y., Johnson, S., Perry, R. C., et al. (2015). Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N. Engl. J. Med. 373, 2512–2521. doi: 10.1056/NEJMoa1502599
Golden, M. R., Whittington, W. L., Handsfield, H. H., Hughes, J. P., Stamm, W. E., Hogben, M., et al. (2005). Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N. Engl. J. Med. 352, 676–685. doi: 10.1056/nejmoa041681
Hafner, L. M. (2015). Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections. Contraception 92, 108–115. doi: 10.1016/j.contraception.2015.01.004
Herrmann, J., Fayad, A. A., and Muller, R. (2017). Natural products from myxobacteria: novel metabolites and bioactivities. Nat. Prod. Rep. 34, 135–160. doi: 10.1039/c6np00106h
Horner, P. (2006). The case for further treatment studies of uncomplicated genital Chlamydia trachomatis infection. Sex. Transm. Infect. 82, 340–343. doi: 10.1136/sti.2005.019158
Irschik, H., Jansen, R., Hofle, G., Gerth, K., and Reichenbach, H. (1985). The corallopyronins, new inhibitors of bacterial RNA synthesis from Myxobacteria. J. Antibiot. 38, 145–152. doi: 10.7164/antibiotics.38.145
Jerchel, S., Knebel, G., Konig, P., Bohlmann, M. K., and Rupp, J. (2012). A human fallopian tube model for investigation of C. trachomatis infections. J. Vis. Exp. 66:e4036. doi: 10.3791/4036
Juul, N., Jensen, H., Hvid, M., Christiansen, G., and Birkelund, S. (2007). Characterization of in vitro chlamydial cultures in low-oxygen atmospheres. J. Bacteriol. 189, 6723–6726. doi: 10.1128/jb.00279-07
Kessler, M., Zielecki, J., Thieck, O., Mollenkopf, H. J., Fotopoulou, C., and Meyer, T. F. (2012). Chlamydia trachomatis disturbs epithelial tissue homeostasis in fallopian tubes via paracrine Wnt signaling. Am. J. Pathol. 180, 186–198. doi: 10.1016/j.ajpath.2011.09.015
Kock, F., Hauptmann, M., Osterloh, A., Schaberle, T. F., Poppert, S., Frickmann, H., et al. (2018). Orientia tsutsugamushi is highly susceptible to the RNA polymerase switch region inhibitor corallopyronin a in vitro and in vivo. Antimicrob. Agents Chemother. 62, e1732-17. doi: 10.1128/AAC.01732-17
Lau, C. Y., and Qureshi, A. K. (2002). Azithromycin versus doxycycline for genital chlamydial infections: a meta-analysis of randomized clinical trials. Sex. Transm. Dis. 29, 497–502. doi: 10.1097/00007435-200209000-00001
Mariner, K., Mcphillie, M., Trowbridge, R., Smith, C., O’neill, A. J., Fishwick, C. W., et al. (2011). Activity of and development of resistance to corallopyronin A, an inhibitor of RNA polymerase. Antimicrob. Agents Chemother. 55, 2413–2416. doi: 10.1128/AAC.01742-10
Matsuo, J., Sakai, K., Okubo, T., and Yamaguchi, H. (2019). Chlamydia pneumoniae enhances interleukin 8 (IL-8) production with reduced azithromycin sensitivity under hypoxia. APMIS 127, 131–138. doi: 10.1111/apm.12924
Menard, J. P. (2011). Antibacterial treatment of bacterial vaginosis: current and emerging therapies. Int. J. Womens Health 3, 295–305. doi: 10.2147/IJWH.S23814
Molano, M., Meijer, C. J., Weiderpass, E., Arslan, A., Posso, H., Franceschi, S., et al. (2005). The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J. Infect. Dis. 191, 907–916. doi: 10.1086/428287
Mukhopadhyay, J., Das, K., Ismail, S., Koppstein, D., Jang, M., Hudson, B., et al. (2008). The RNA polymerase “switch region” is a target for inhibitors. Cell 135, 295–307. doi: 10.1016/j.cell.2008.09.033
Muller, M., Dela Pena, A., and Derendorf, H. (2004). Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob. Agents Chemother. 48, 1441–1453. doi: 10.1128/aac.48.5.1441-1453.2004
Newman, L., Rowley, J., Vander Hoorn, S., Wijesooriya, N. S., Unemo, M., Low, N., et al. (2015). Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi: 10.1371/journal.pone.0143304
Roth, A., Konig, P., Van, Z. G., Klinger, M., Hellwig-Burgel, T., Daubener, W., et al. (2010). Hypoxia abrogates antichlamydial properties of IFN-gamma in human fallopian tube cells in vitro and ex vivo. Proc. Natl. Acad. Sci. U.S.A. 107, 19502–19507. doi: 10.1073/pnas.1008178107
Rund, S., Lindner, B., Brade, H., and Holst, O. (1999). Structural analysis of the lipopolysaccharide from Chlamydia trachomatis serotype L2. J. Biol. Chem. 274, 16819–16824.
Schaberle, T. F., Lohr, F., Schmitz, A., and Konig, G. M. (2014). Antibiotics from myxobacteria. Nat. Prod. Rep. 31, 953–972. doi: 10.1039/c4np00011k
Schaberle, T. F., Schmitz, A., Zocher, G., Schiefer, A., Kehraus, S., Neu, E., et al. (2015). Insights into structure-activity relationships of bacterial RNA polymerase inhibiting corallopyronin derivatives. J. Nat. Prod. 78, 2505–2509. doi: 10.1021/acs.jnatprod.5b00175
Schaffer, K., and Taylor, C. T. (2015). The impact of hypoxia on bacterial infection. FEBS J. 282, 2260–2266. doi: 10.1111/febs.13270
Schiefer, A., Schmitz, A., Schaberle, T. F., Specht, S., Lammer, C., Johnston, K. L., et al. (2012). Corallopyronin A specifically targets and depletes essential obligate Wolbachia endobacteria from filarial nematodes in vivo. J. Infect. Dis. 206, 249–257. doi: 10.1093/infdis/jis341
Senior, K. (2012). Chlamydia: a much underestimated STI. Lancet Infect. Dis. 12, 517–518. doi: 10.1016/s1473-3099(12)70161-5
Shima, K., Klinger, M., Solbach, W., and Rupp, J. (2013). Activities of first-choice antimicrobials against gamma interferon-treated Chlamydia trachomatis differ in hypoxia. Antimicrob. Agents Chemother. 57, 2828–2830. doi: 10.1128/AAC.02211-12
Shima, K., Ledig, S., Loeper, N., Schiefer, A., Pfarr, K., Hoerauf, A., et al. (2018). Effective inhibition of rifampin-resistant Chlamydia trachomatis by the novel DNA-dependent RNA-polymerase inhibitor Corallopyronin A. Int. J. Antimicrob. Agents 52, 523–524. doi: 10.1016/j.ijantimicag.2018.07.025
Shima, K., Szaszak, M., Solbach, W., Gieffers, J., and Rupp, J. (2011). Impact of a low-oxygen environment on the efficacy of antimicrobials against intracellular Chlamydia trachomatis. Antimicrob. Agents Chemother. 55, 2319–2324. doi: 10.1128/AAC.01655-10
Srivastava, A., Talaue, M., Liu, S., Degen, D., Ebright, R. Y., Sineva, E., et al. (2011). New target for inhibition of bacterial RNA polymerase: ‘switch region’. Curr. Opin. Microbiol. 14, 532–543. doi: 10.1016/j.mib.2011.07.030
Keywords: Corallopyronin A, C. trachomatis, antibiotic treatment, novel antibiotics, RNA polymerase inhibitor
Citation: Loeper N, Graspeuntner S, Ledig S, Kaufhold I, Hoellen F, Schiefer A, Henrichfreise B, Pfarr K, Hoerauf A, Shima K and Rupp J (2019) Elaborations on Corallopyronin A as a Novel Treatment Strategy Against Genital Chlamydial Infections. Front. Microbiol. 10:943. doi: 10.3389/fmicb.2019.00943
Received: 01 October 2018; Accepted: 15 April 2019;
Published: 07 May 2019.
Edited by:
Sven Hammerschmidt, University of Greifswald, GermanyReviewed by:
Barbara Susanne Sixt, Umeå University, SwedenAmanda J. Brinkworth, Washington State University, United States
Copyright © 2019 Loeper, Graspeuntner, Ledig, Kaufhold, Hoellen, Schiefer, Henrichfreise, Pfarr, Hoerauf, Shima and Rupp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Rupp, amFuLnJ1cHBAdWtzaC5kZQ==
†These authors have contributed equally to this work
 Nathalie Loeper
Nathalie Loeper Simon Graspeuntner
Simon Graspeuntner Svea Ledig1
Svea Ledig1 Andrea Schiefer
Andrea Schiefer Kenneth Pfarr
Kenneth Pfarr Kensuke Shima
Kensuke Shima Jan Rupp
Jan Rupp