
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 07 May 2019
Sec. Fungi and Their Interactions
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.00840
This article is part of the Research Topic Fungal Molecular Systematics, Ranking, and Evolution Using Divergence Time View all 7 articles
 Monika C. Dayarathne1,2,3,4
Monika C. Dayarathne1,2,3,4 Sajeewa S. N. Maharachchikumbura5
Sajeewa S. N. Maharachchikumbura5 E. B. Gareth Jones4
E. B. Gareth Jones4 Wei Dong1,2
Wei Dong1,2 Bandarupalli Devadatha6
Bandarupalli Devadatha6 Jing Yang1
Jing Yang1 Anusha H. Ekanayaka1
Anusha H. Ekanayaka1 Wasana De Silva7
Wasana De Silva7 Vemuri V. Sarma6
Vemuri V. Sarma6 Abdullah M. Al-Sadi5
Abdullah M. Al-Sadi5 Kitiphong Khongphinitbunjong8
Kitiphong Khongphinitbunjong8 Kevin D. Hyde1,2,3
Kevin D. Hyde1,2,3 Rui Lin Zhao9,10*
Rui Lin Zhao9,10*Morphology, phylogeny, and molecular clock analyses were carried out on Savoryellaceae in order to understand the placements of taxa in this family. Ascotaiwania and Neoascotaiwania formed a well-supported separate clade in the phylogeny of concatenated partial SSU, LSU, TEF, and RPB2 gene data. These two genera share similar morphological features, especially in their asexual morphs, indicating that they are congeneric. Hence, we synonymize Neoascotaiwania under Ascotaiwania. Ascotaiwania hughesii (and its asexual morph, Helicoon farinosum) and Monotosporella setosa grouped in a clade sister to Pleurotheciales and are excluded from Ascotaiwania which becomes monophyletic. A novel genus Helicoascotaiwania is introduced to accommodate Ascotaiwania hughesii and its asexual morph, Helicoon farinosum. A novel species, Savoryella yunnanensis is introduced from a freshwater habitat in Yunnan Province, China. Comprehensive descriptions and illustrations are provided for selected taxa in this family. In addition, we provide evolutionary divergence estimates for Savoryellomycetidae taxa and major marine based taxa to support our phylogenetic and morphological investigations. The taxonomic placement of these marine-based taxa is briefly discussed. Our results indicate that the most basal group of marine-based taxa are represented within Lulworthiales, which diverged from ancestral Sordariomycetes around 149 Mya (91–209) and Savoryellomycetidae around 213 Mya (198–303).
The family Savoryellaceae (Savoryellales) was established by Jaklitsch and Réblová (2015) and is typified by the genus Savoryella. Boonyuen et al. (2011) had earlier introduced the order Savoryellales, but without designating a family. According to phylogenetic and molecular clock analyses (Hongsanan et al., 2017; Hyde et al., 2017), the orders Conioscyphales, Fuscosporellales, Pleurotheciales, and Savoryellales cluster together as a distinct clade, with a stem age of 268 Mya. Hence, the order Savoryellales was referred to a new subclass Savoryellomycetidae by Hongsanan et al. (2017) and is supported in the present study. The genus Savoryella, based on morphological features, was previously placed in the Sordariales genera incertae sedis by Jones et al. (2009) and, later, Boonyuen et al. (2011) showed that Savoryella, Ascotaiwania, Ascothailandia, and Canalisporium cluster in the order Savoryellales within Hypocreomycetidae, Sordariomycetes. According to the one fungus-one name concept (Hawksworth, 2011), the genus Canalisporium was recommended for protection over Ascothailandia hence, it was synonymized under Canalisporium based on sequence data (Sri-indrasutdhi et al., 2010). Maharachchikumbura et al. (2016) and Wijayawardene et al. (2018) accepted the placement of Savoryellaceae in the order Savoryellales. With the recent inclusion of the genus Neoascotaiwania, Savoryellaceae comprised four genera: Ascotaiwania, Canalisporium, and Savoryella (Hernández-Restrepo et al., 2017). Taxa presently referred to this family total 40 species (Table 1).
Species of Savoryellaceae are abundant in submerged wood in aquatic habitats, viz fresh, saline or brackish water and some species have been reported from terrestrial woody plants (Linder, 1929; Jones et al., 2015). Ascotaiwania species are isolated from submerged and decaying wood in freshwater habitats (Sivanesan and Chang, 1992; Ranghoo et al., 1999), and are widely distributed in countries such as Ecuador, France, Great Britain, Hong Kong, Malaysia, Mauritius, Taiwan, Thailand, and Australia (Figure 1). Canalisporium species are saprobes mostly on rotten wood and distributed in tropical regions of both hemispheres (Figure 1) (Goh et al., 1998). Further, they occur on woody plants (Rao and de Hoog, 1986; Raja and Shearer, 2007) and submerged, decaying wood in freshwater (Sivichai et al., 1998; Tsui et al., 2001). Canalisporium species have been recorded from Cuba, India, Kenya, Malaysia, Taiwan, Thailand, and Uganda (Holubová-Jechová and Sierra, 1984; Kirk, 1985; Rao and de Hoog, 1986; Matsushima, 1987; Nawawi and Kuthubutheen, 1989; Sri-indrasutdhi et al., 2010). Though Savoryella species are cosmopolitan in distribution, mostly they are common in tropical and subtropical ecosystems (Jones et al., 2016) (Figure 1). Neoascotaiwania species have been documented from forest soil in Spain and decaying wood collected in streams in Taiwan (Chang et al., 1998; Hernández-Restrepo et al., 2017) (Figure 1 and Table 2).

Table 2. Different hosts/substrates of Savoryellaceae species (these species consideration before the family treatment in this study).
Members of Savoryellaceae have perithecial, flask-shaped ascomata with a periphysate ostiole, which is central or eccentric when horizontally lying on the host, papillate or with a short/long neck, conical or cylindrical (Jones and Eaton, 1969; Kohlmeyer and Kohlmeyer, 1979; Eriksson and Hawksworth, 1987; Barr, 1990; Read et al., 1993; Jones and Hyde, 1992; Tsui and Hyde, 2003; Réblová et al., 2016a). Asci of Savoryellaceae are clavate to cylindrical, persistent, with an inamyloid apical ring (Kohlmeyer, 1986; Eriksson and Hawksworth, 1986, 1987; Read et al., 1993; Jones and Hyde, 1992; Tsui and Hyde, 2003; Jones et al., 2009; Boonyuen et al., 2011). Ascomata of Savoryella are long-necked and pale in color while, ascomata of Ascotaiwania are dark-brown to black with a short or long, lateral neck (Chang et al., 1998; Tsui and Hyde, 2003). Ascomatal morphology of Neoascotaiwania also mostly similar to that of Ascotaiwania (Hernández-Restrepo et al., 2017). Further, the sexual morph of Canalisporium (=Ascothailandia), comprises globose, dark brown, ostiolate ascomata (Sri-indrasutdhi et al., 2010). In the protolog of Savoryella, the apical pore or apparatus of ascus was not described (Jones and Eaton, 1969). Later, Jones and Hyde (1992) observed the asci and apical apparatus of Savoryella appendiculata, S. longispora, and S. paucispora. Ultrastructural observations of asci and ascospores with transmission electron microscopy (TEM) by Read et al. (1992) described the ascal apical ring of S. appendiculata and S. longispora as extending subapically on to the side walls of the ascus and similar to that described for Aniptodera chesapeakensis, which comprises retraction of the plasma membrane at the apex. The ascal apical ring of Ascotaiwania is comparatively large and highly developed, while Canalisporium (=Ascothailandia) has a J-prominent apical ring (Sri-indrasutdhi et al., 2010; Zhang et al., 2014). Paraphyses of Savoryella are inconspicuous at maturity while, Ascotaiwania has thin, filiform, septate, up to 2 μm wide paraphyses which deliquesce early and are rarely seen in mature ascomata (Chang et al., 1998; Tsui and Hyde, 2003; Boonyuen et al., 2011). Asci of Ascotaiwania are cylindrical, unitunicate with a prominent J-refractive and well-developed apical apparatus comprises an electron-dense apical ring with a plug. The plug deliquesces prior to ascospore release (Chang et al., 1998; Boonyuen et al., 2011). Asci of Neoascotaiwania are only differed from Ascotaiwania by having a thinner apical ring (Hernández-Restrepo et al., 2017). Ultrastructural studies of S. lignicola revealed that the unitunicate ascus wall comprised an outer, 30–40 nm electron-dense layer and an inner, 420–450 nm, thick, electron-transparent layer (Read et al., 1993).
Savoryella ascospores are ellipsoid to fusiform, with several septa, and versicolorous with brown median cells and hyaline polar cells. The ascospores of Savoryella appendiculata and S. paucispora have mucilaginous sheath around the central cells (Boonyuen et al., 2011). Savoryella appendiculata is the solitary species in this genus with ascospores with polar tetradiate appendages, formed as an outgrowth of the hyaline apical cell of the ascospore on release from the ascus (Jones and Hyde, 1992; Read et al., 1993). Ascotaiwania ascospores often have three or more than three septa, and are versicolored (Chang et al., 1998; Hernández-Restrepo et al., 2017) in which ascospores are surrounded by a thin mucilaginous sheath and an ascospore wall comprising an electron-dense episporium and a less electron-dense mesosporium (Read et al., 1993). Further, external to the episporium is a fibro-granular sheath (Read et al., 1993). Ascospores of Ascothailandia are also fusiform, straight or curved, three euseptate and versicolored (Sri-indrasutdhi et al., 2010). Considering the morphologies of asexual morphs of Savoryellaceae; conidia of Canalisporium species are muriform, flattened dorsoventrally, with a layer of punctually arranged cells, that are aided by a small, thin-walled, cuneiform, pale basal cell (Goh et al., 1998). Conidia possess a single column of vertical septa, and evenly spaced several rows of transverse septa (Boonyuen et al., 2011). The most remarkable feature of the conidia is the presence of narrow canals connecting adjacent cell lumens and surrounded by a highly pigmented ring, observed as a circular disc in lateral view or a barrel shape in dorsiventral view (Moore, 1959; Ellis, 1971, 1976; Ho et al., 2002). Asexual morphs of Ascotaiwania are bactrodesmium-like, monotosporella-like, monodictys-like or trichocladium-like (Ranghoo and Hyde, 1998; Chang, 2001; Tsui and Hyde, 2003; Réblová et al., 2016a). Species in the genera Ascotaiwania, Savoryella, and Canalisporium have common phenotypic characteristics, such as similar ascomata, paraphyses and versicolored ascospores (Boonyuen et al., 2011).
1. Sexual morph perithecial………………………………2
1. Asexual morph hyphomycetous…………Canalisporium
2. Asci comprises massive, refractive, apical ring and ascospores fusiform, with pointed end cells…Ascotaiwania
2. Asci comprises small, discoid, apical pore/ring and ascospores ellipsoid, with rounded end cells….. Savoryella
Note: Key to genera only for the accepted genera in this study.
Herbarium specimens were loaned from the herbaria: BRIP and IMI1. Fresh material was collected on submerged decaying wood of Avicennia marina in Tamil Nadu, India and submerged wood in a small river in Yunnan, China. Specimens were brought to the laboratory in plastic bags and incubated in plastic boxes at 25°C. The samples were processed, examined, and photographed following the method described in Dayarathne et al. (unpublished).
A representative species of 152 of Sordariomycetes (including 24 strains which represent 20 species belonging to the family Savoryellaceae) were selected and Leotia lubrica was selected as the outgroup taxon (Supplementary Table 1) following Samarakoon et al. (2016). Sequence data were obtained from GenBank based on previous literature (Supplementary Table 1) (Samarakoon et al., 2016; Hongsanan et al., 2017). Multiple sequence alignments were generated from four loci (LSU, SSU, RPB2, and TEF1-α) following Hall (1999) and Dayarathne et al. (unpublished). The final alignment consisted of 4,637 characters (LSU = 1,291 bp, SSU = 1,091 bp, RPB2 = 1,092 bp, and TEF1-α = 1,048 bp) and gaps were treated as missing data. Nucleotide substitution models were determined with MrModeltest v. 2.2 (Nylander, 2004). The GTR+I+G model was used in the analysis for phylogenetic and divergence time estimation studies.
Phylogenetic analyses of the sequence data consisted of maximum likelihood (ML) and Bayesian inference analyses (BI). The ML trees were generated following Stamatakis et al. (2008), Miller et al. (2010), and Dayarathne et al. (unpublished). The Bayesian tree was obtained by using MCMC sampling in MrBayes v3.1.2 (Huelsenbeck and Ronquist, 2001; Zhaxybayeva and Gogarten, 2002) using the parameters described by Dayarathne et al. (unpublished). The resulting trees were printed with FigTree v.1.4.0 (Rambaut, 2012) and the final layout was done with Microsoft PowerPoint.
Bayesian analysis was conducted for the estimation of divergence times using molecular data of multi-gene loci and incorporating fossil data and secondary calibration estimations. BEAST v1.8.0 software was used to obtained divergence time estimates were performed using the BEAUti (BEAST package) was aided to prepare XML file including the partitioned alignment. The analysis were generally similar to our previous work of Samarakoon et al. (2016), and the details are as follows. The GTR+I+G was used as models of substitution for each gene regions. Tree priors were set and maximum clade credibility (MCC) trees were obtained as described by Samarakoon et al. (2016). Trees were visualized using FigTree2 and the final layout was optimized with Adobe Illustrator.
A fossil belongs to the subclass Hypocreomycetidae order Hypocreales, Paleoophiocordyceps coccophagus was used in calibrating the tree (Samarakoon et al., 2016). A fossilized male scale for insect (Albicoccidae) parasitized by P. coccophagus from the late Mesozoic (Upper Albian) period in Burmese amber was investigated by Sung et al. (2007) and Sadowski et al. (2016) which is the oldest fossil record as evidence for the fungal-animal parasitism. Morphological characters of the fossil P. coccophagus were reminiscent of asexual morph characteristics of Hirsutella and Hymenostilbe (Sung et al., 2007) that are synonyms of Ophiocordyceps (Ophiocordycipitaceae, Hypocreales; Quandt et al., 2014). Fossil age of P. coccophagus has been estimated as around 99–105 Mya according to the geological timescale (Cruickshank and Ko, 2003). Albian period is estimated as 100–113 Mya according to the geological timescale of Walker et al. (2012). Above fossil data was used to calibrate the crown node of the genus Ophiocordyceps (exponential distribution, offset 100, mean 27.5, with 97.5% credibility interval of 201.4 Mya).
Secondary calibration determination was done according to Samarakoon et al. (2016).
The ML analyses of concatenated sequence alignment with LSU, SSU, RPB2, and TEF1-α dataset generated best scoring RAxML tree (Figure 1) with a final ML optimization likelihood value of -14416.427031. Different parameters for ML analyses are summarized in Supplementary Table 2. For concatenated LSU, SSU, RPB2, and TEF1-α dataset, six simultaneous Markov chains were run for 1.5 M generations and trees were sampled every 100th generation resulting in 15,001 total trees. Of these 13,501 trees were used to calculate the posterior probabilities (PP) in the majority rule consensus tree, after discarding the first 1,500 trees that representing the burn-in phase (10%) of the analysis. Overall, tree topologies obtained from of the ML and BI analyses were similar to each other.
Six distinct clades (Diaporthomycetidae, Hypocreomycetidae, Lulworthiomycetidae, Sordariomycetidae, Savoryellomycetidae, and Xylariomycetidae) were recognized within the phylogenetic tree. Subclass Savoryellomycetidae formed a robust clade (100% ML, 1.00 PP, Figure 2) within the class Sordariomycetes. Five subclades comprising Conioscyphaceae, Fuscosporellaceae, Pleurotheciaceae, Savoryellaceae, and a clade with Ascotaiwania hughesii (DAOM 241947, P2-6) strains and Monotosporella setosa (HKUCC 3713) were recognized within the subclass Savoryellomycetidae.
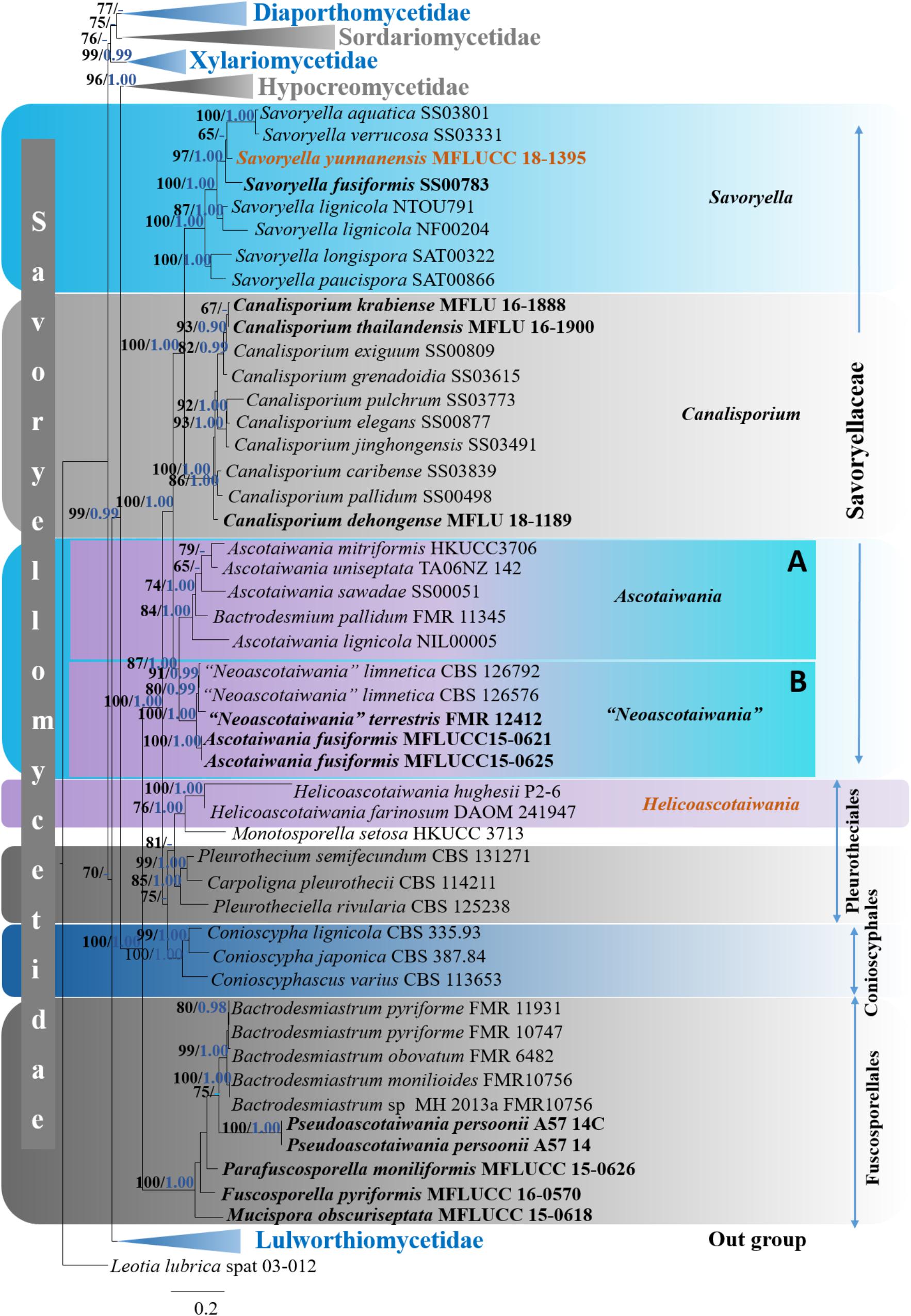
Figure 2. Phylogram generated from maximum likelihood analysis based on combined LSU, SSU, RPB2, and TEF1-α sequence data of selected taxa from Sordariomycetes. One hundred and fifty-two strains are included in the analyses. Single gene analyses were carried out and compared with each species, to compare the topology of the tree and clade stability. The tree was rooted with Leotia lubrica (spat 03-012). Bayesian posterior probabilities (PP, blue) >0.90 and maximum likelihood bootstrap (ML, black) values >60% are given above the nodes. The scale bar indicates 0.2 changes and new isolate is in blue.
The genera Ascotaiwania, “Neoascotaiwania,” Canalisporium and Savoryella formed well-separated subgroups within the family Savoryellaceae that is monophyletic.
Ascotaiwania, Neoascotaiwania clade: Ascotaiwania, Neoascotaiwania, and Bactrodesmium pallidum (FMR 11345) collectively formed a robust clade (100% ML, 1.00 PP, Figure 2) within Savoryellaceae, which splits into two subclades (A & B). Sub clade A comprised Neoascotaiwania limnetica (CBS 126576, CBS 126792), Neoascotaiwania terrestris (CBS 142291), and Ascotaiwania fusiformis (MFLUCC15 0621, MFLUCC15 0625) (Figure 2), while Ascotaiwania mitriformis (HKUCC3706), Ascotaiwania sawadae (SS00051), Bactrodesmium pallidum (FMR 11345), Ascotaiwania lignicola (NIL00005) grouped in subclade B. Canalisporium clade: Canalisporium caribense (SS03839), Canalisporium elegans (SS00877), Canalisporium exiguum (SS00809), Canalisporium grenadoidia (SS03615), Canalisporium jinghongensis (SS03491), Canalisporium pallidum (SS00498) and Canalisporium pulchrum (SS03773) formed a monophyletic clade with strong support (100% ML, 1.00 PP, Figure 2) within Savoryellaceae. Savoryella clade: A newly generated sequence of Savoryella yunnanensis (MFLUCC 18-1395) grouped with Savoryella aquatica (SS03801), Savoryella fusiformis (SS00783), Savoryella lignicola (NF00204, NTOU791), Savoryella longispora (SAT00322), Savoryella paucispora (SAT00866) and Savoryella verrucosa (SS03331). Ascotaiwania hughesii clade: Ascotaiwania hughesii (P2-6, DAOM 241947 “Helicoon farinosum”) and Monotosporella setosa (HKUCC 3713) clustered away from Savoryellaceae but within the subclass Savoryellomycetidae. However, Monotosporella setosa formed a basal lineage to the Ascotaiwania hughesii strains. They shared a sister clade relationship with Pleurotheciaceae (Figure 2).
The evolutionary model based on sequence data linked to fossil data (Figure 3), reveals the divergence of Sordariomycetes at around 325 (265–386) Mya in the Paleozoic era. The major clades representing five sub-classes; Diaporthomycetidae, Hypocreomycetidae, Lulworthiomycetidae, Savoryellomycetidae, Sordariomycetidae, and Xylariomycetidae are well supported in both phylogenetic and the divergence time analyses with median crown ages of 206, 266, 274, 249, 208, 182 Mya, respectively (Figures 3, 4). Lulworthiomycetidae represents the basal clade with stem age of 310 (253–370) Mya that can be considered as the earliest diverged group comprising mostly marine originated taxa. The divergence of the Savoryellaceae crown group occurred 182 Mya, while the three genera Savoryella, Ascotaiwania, and Canalisporium evolved 56–108 Mya (Figure 3 and Table 3). Novel genus Helicoascotaiwania gen. nov. originated 53 (25–88) Mya while, other Pleurotheciales taxa evolved 98 (48–153) (Figure 3).
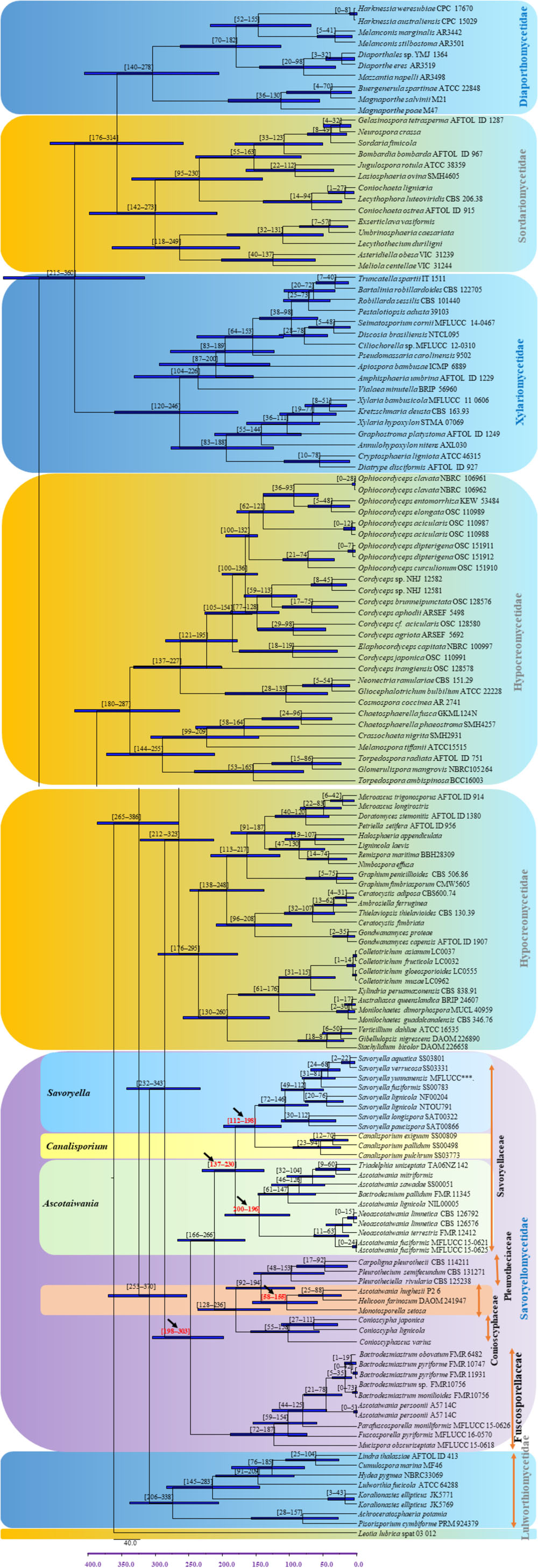
Figure 3. Maximum clade credibility (MCC) tree with divergence times estimates for Savoryellomycetidae using lognormal relaxed clock mode (uncorrelated) in BEAST, with representative families. Geological periods are indicated at the base of the tree.
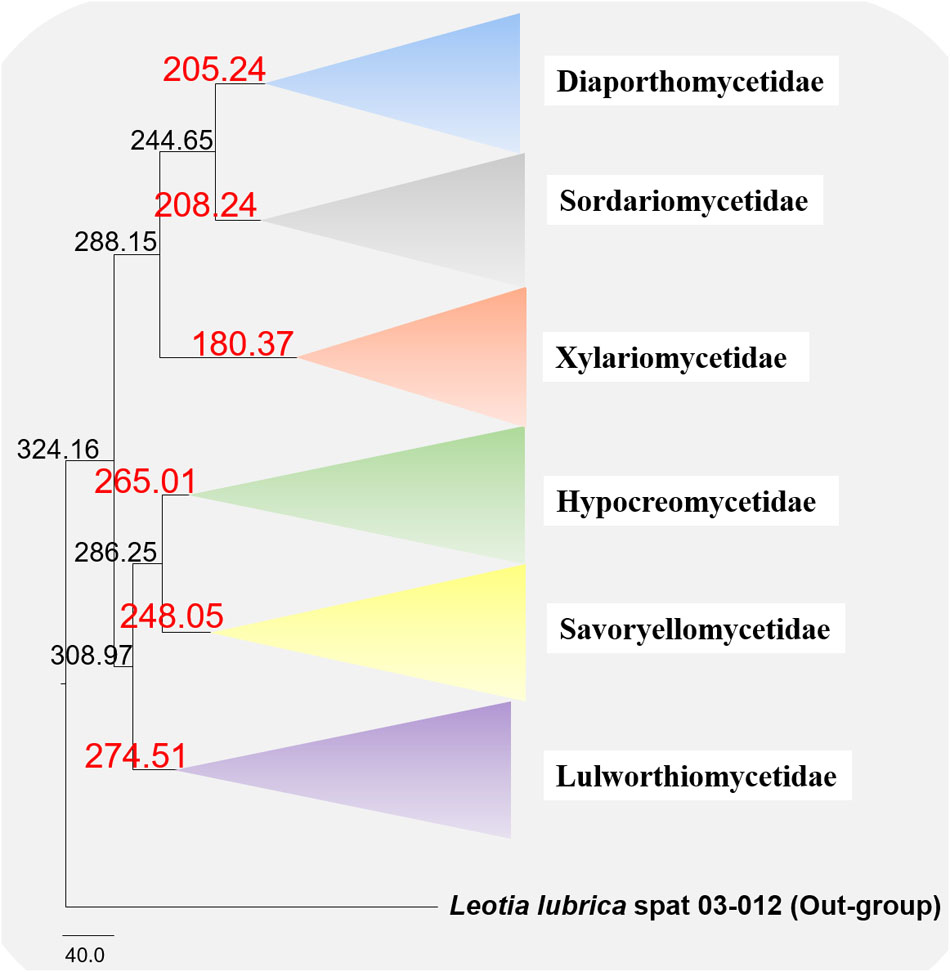
Figure 4. Maximum clade credibility (MCC) tree with divergence times estimates that indicates evolutionary ages of six subclasses estimated using lognormal relaxed clock mode (uncorrelated) in BEAST.
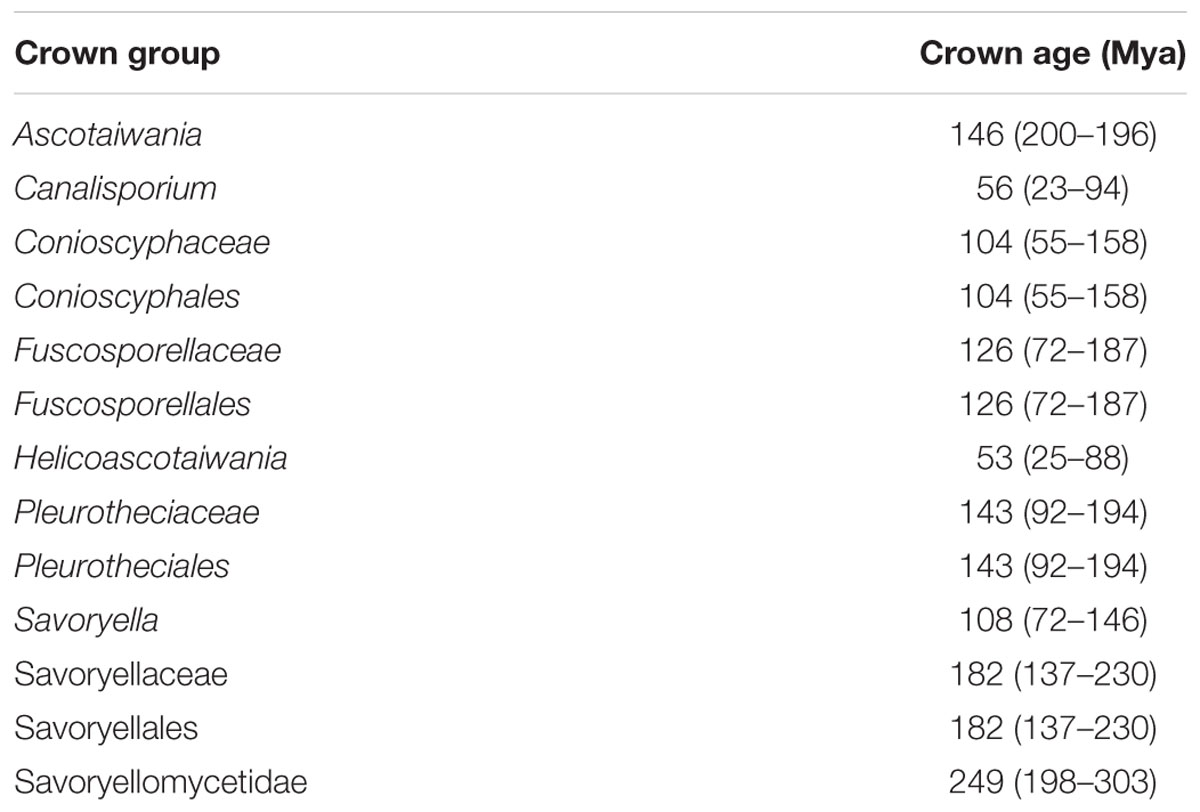
Table 3. Divergence time estimates of different taxa within Savoryellomycetidae from the present study.
Savoryellaceae Jaklitsch & Réblová, in Jaklitsch (2015)
Index Fungorum number: IF 551026; Facesoffungi number: FoF 01283
Description: This family was treated in detail by Maharachchikumbura et al. (2016).
Family type: Savoryella E.B.G. Jones & R.A. Eaton (1969)
Note: Savoryellales taxa share a set of characters including immersed, semi-immersed to superficial, non-stromatic, heavily pigmented, coriaceous ascomata, mostly lying horizontally to the host, partly deliquescing, paraphyses, unitunicate asci comprises non-amyloid apical annulus, and fusiform to ellipsoidal, transversely septate ascospores with hyaline end cells and brown median cells (Jones and Eaton, 1969; Jones and Hyde, 1992; Tsui and Hyde, 2003; Jones et al., 2009; Boonyuen et al., 2011). Different types of asexual morphs (based on morphological observations only) have been experimentally linked to Ascotaiwania and Neoascotaiwania species such as, monotosporella-like in A. sawada (Sivichai et al., 1998) and A. mitriformis (Ranghoo and Hyde, 1998), monodictys-like in A. lignicola (Chang, 2001), trichocladium-like in A. hsilio (Chang, 2001), and bactrodesmium-like asexual morphs for Neoascotaiwania species (Hernández-Restrepo et al., 2017). These various asexual morphs have led to confusion in the classification of various sexual genera in the family. Canalisporium species comprise muriform conidia (sexual morph – Ascothailandia; Sri-indrasutdhi et al., 2010), but not all species have been sequenced. Therefore, Savoryellales asexual morphs are associated with dematiaceous hyphomycetes characterized by semi-macronematous conidiophores (conidiophores that are only slightly morphologically different from the vegetative hyphae) and monoblastic conidiogenous cells generating brown, thick-walled, dictyoseptate conidia that are transversely septate or cheiroid. The distant position of Helicoon farinosum (asexual morph of Ascotaiwania hughesii) (Fallah et al., 1999), from the rest of members of Savoryellales was confirmed by phylogenetic analysis (Boonyuen et al., 2011; Réblová et al., 2012).
Savoryella E.B.G. Jones & R.A. Eaton (1969)
Index Fungorum number: IF 4870; Facesoffungi number: FoF 05391
Description: Please see Maharachchikumbura et al. (2016).
Type species: Savoryella lignicola E.B.G. Jones & R.A. Eaton (1969)
Note: Savoryella was introduced by Jones and Eaton (1969) and is typified by S. lignicola. Savoryella is a widespread genus of lignicolous ascomycetes in aquatic habitats. All species in the genus comprise ellipsoidal, three septate ascospores whose central cells are brown, and end cells are hyaline, with or without polar appendages (Jones et al., 2016). Savoryella was variously assigned to higher orders based on morphological observations: the order Sphaeriales family incertae sedis by Kohlmeyer and Kohlmeyer (1979); ascomycetes incertae sedis by Kohlmeyer (1986); Eriksson and Hawksworth (1986); Amphisphaeriaceae (Eriksson and Hawksworth, 1987) and Sordariales (Jones and Hyde, 1992). Barr (1990) established this genus within the order Halosphaeriales based on morphology (the catenophyses-like paraphyses) and ultrastructural observations, but these placements have all been rejected (Jones et al., 2016). Savoryella elongata and S. longispora referred to the order Hypocreales, subclass Hypocreomycetidae by Cai et al. (2006) and Vijaykrishna et al. (2006), based on a phylogenetic analysis of large subunit nuclear ribosomal DNA sequence data with low statistical support. Based on phenotypic characters, Jones et al. (2009) placed the Savoryella in Sordariales genera incertae sedis. Although the asexual morphs of Savoryella are still unknown (Boonyuen et al., 2011), dark brown, 3–5-septate conidia were observed from the host surface and in living cultures obtained from ascospore isolates of two S. limnetica strains (Chang et al., 1998). A later phylogenetic study by Réblová et al. (2016b), confirmed S. limnetica as an Ascotaiwania species. The genus currently comprises twelve species (Jones and Eaton, 1969; Hyde, 1994; Abdel-Wahab and Jones, 2000). Savoryella limnetica introduced by Chang et al. (1998) was latterly synonymized as Neoascotaiwania limnetica based on multi-gene analyses (Hernández-Restrepo et al., 2017). Key to Savoryella species is shown below.
1. Asci 8-spored…………………………………………2
1. Asci 2-spored……………………………. S. paucispora
2. Ascospores lack appendages…………………………. 3
2. Ascospores with polar appendages…….. S. appendiculata
3. Ascospores straight……………………………………4
3. Ascospores slightly curverd or curved, fusiform………………………………….. S. curvispora
4. Ascospores with smooth walls……………………….. 5
4. Ascospores with markedly verrucose walls…S. verrucosa
5. Ascospores often wider than 12 μm……………………6
5. Ascospores often narrower than 12 μm………………7
6. Ascospores ellipsoidal or irregular………S. yunnanensis
6. Ascospores ellipsoidal, brown or broadly ellipsoidal, dark brown………………………………………………9
7. Average width of ascospores <9 μm, freshwater species (ascospores 25 ± 35–6 ± 9 ± 6 μm) 27–32 × 12–17 μm………………………………………S. fusiformis
7. Average width of ascospores >8 ± 5 μm, intertidal or freshwater species……………………………………8
8. Mean length of ascospores <35 μm, intertidal or freshwater species (ascospores 24 ± 37 8 ± 14 μm)………S. lignicola
8. Mean length of ascospores >35 μm, intertidal species (ascospores 34 ± 47–7 ± 5 ± 12 μm)………S. longispora
9. Ascospores ellipsoidal, brown…………………………10
9. Ascospores broadly ellipsoidal, dark brown.. S. grandispora
10. Found in marine habitat, ascomata (160–305 × 184–225 μm), asci (170–212 × 15–25), and ascospores (32–45 × 15–18 μm)…………………………………S. melanospora
10. Found in freshwater habitat ascomata (195–260 × 91–130 μm), asci (106–140 × 26–34), and ascospores (29–38 × 13.5–17 μm)…………………………S. aquatica
Sequence data are available only for S. aquatica, S. fusiformis, S. lignicola, S. longispora, S. verrucosa, and S. paucispora. Phylogenetic analyses showed Savoryella members formed a well-defined (ML 100%, 1.00 PP) clade within order Savoryellales (Boonyuen et al., 2011; Jones et al., 2016; Hernández-Restrepo et al., 2017).
Savoryella lignicola E.B.G. Jones & R.A. Eaton (1969), Figure 5

Figure 5. Savoryella lignicola (IMI 129784, IMI 129785, holotype). (a) Herbarium specimen. (b,c) Ascomata on host. (d,e) Ascomata. (f) Paraphyses. (g–i) Asci. (j–n) Ascospores. Scale bars: (c) 200 μm, (d,e) 100 μm, (g–i) 50 μm, (j–n) 20 μm.
Index Fungorum: IF 338840; Facesoffungi number: FoF 05392
Saprobic on submerged wood in water cooling towers, rivers, streams, and marine environments. Sexual morph: Ascomata 180–320 × 120–150 μm perithecoid, subglobose to globose or ellipsoidal, superficial, semi-immersed or immersed, ostiollate, papillate, membranous, light to dark brown, with a long-neck, up to 80–165 μm, brown, periphysate. Peridium brown, composed of several layers of thick-walled angular cells forming textura angularis. Paraphyses present, but sparse. Asci 128–180 × 16–24 μm, 2–8-spored, cylindrical or clavate, short-pedicellate, unitunicate, persistent, with an apical truncate non-amyloid apical ring with a pore. Ascospores 20–34 × 8–12 μm, 1–2 seriate, ellipsoidal, 3-septate, not distinctly constricted at the septa, central cells brown, apical cells smaller and hyaline. Asexual morph: Undetermined (modified description of Maharachchikumbura et al., 2016).
Material examined: United Kingdom, Flintshire, Connah’s Quay, on Scots pine test-blocks placed for 168 days amongst the packing timber of a water-cooling tower at Connah’s Quay, 16 June 1966 and 1 December 1966, IMI 129784, IMI 129785 type.
Note: Savoryella lignicola was initially isolated from a water-cooling tower in Great Britain and has now been reported as a cosmopolitan species (Jones and Eaton, 1969; Ho et al., 1997; Jones et al., 2016). This is the sole Savoryella taxon detailed from both marine and freshwater environments (Ho et al., 1997; Luo et al., 2004). Though the marine and freshwater isolates of S. lignicola are morphologically alike, it is doubtful whether they are same species (Ho et al., 1997). Molecular data are available only for two S. lignicola strains described from mangrove wood from Malaysia and submerged Nypa fruticans fronds from Thailand, with no molecular data for the freshwater strain of S. lignicola (Ho et al., 1997; Boonyuen et al., 2011). Therefore, molecular data should be obtained from collections from freshwater habitats to establish whether they are the same species or not. Savoryella lignicola morphologically resembles S. fusiformis and S. longispora (Boonyuen et al., 2011). However, these taxa can easily be distinguished by measurements of length/width ratio of ascospores and molecular data (Ho et al., 1997; Boonyuen et al., 2011).
Savoryella aquatica K.D. Hyde (1993)
Index Fungorum: IF 361052; Facesoffungi number: FoF 05393
Description: Please see Jones and Hyde (1992).
Material examined: Australia, Atherton Tablelands, Koah, Clohesy River, on submerged wood, October 1990, K.D. Hyde, BRIP 19327, holotype.
Note: Savoryella aquatica differs from S. lignicola and S. longispora, by having wider ascospores (13.5–17 μm vs. 7.5–12 μm). It differs from S. appendiculata, which has appendaged ascospores, S. paucispora, with two-spored asci, and S. verrucosa that have distinctly verrucose ascospore walls (Jones and Hyde, 1992). However, no ascomata of S. aquatica were present in the type material of the species. Therefore, epitypification is needed for future studies.
Savoryella grandispora K.D. Hyde (1994), Figure 6

Figure 6. Savoryella grandispora (BRIP 20918, holotype). (a) Herbarium specimen. (b) Ascomata on host. (c) Section through an ascoma. (d) Peridium. (e) Ascus. (f) Apical ring. (g) Paraphyses. (h–j) Ascospores. Scale bars: (c) 200 μm, (e) 50 μm, (d,g) 20 μm, (f–j) 10 μm.
Index Fungorum: IF 362061; Facesoffungi number: FoF 05394
Ascomata 200–250 × 90–135 μm, immersed or superficial, coriaceous, pyriform, brown to dark brown, papillate, solitary or gregarious. Necks cylindrical, brown, pale and slightly tapering toward the apex, periphysate. Peridium 20–40 μm wide, thin, brown, of textura epidermoidea. Paraphyses sparse, septate. Asci 80–100 × 12–16 μm, 8-spored, cylindrical, pedicellate, unitunicate, persistent, with a J-apical ring. Ascospores 22–28 × 5–8 μm, uniseriate, fusiform, curved, 3-septate, slightly constricted at the septa, smooth, thin-walled; central cells brown, hyaline to pale brown (description generated from herbarium material observation and observations of Ho et al., 1997).
Material examined: Malaysia, State Negara, Lipur Lentang Nature Reserve, on submerged wood, October 1991, K.D. Hyde, BRIP 20918, holotype.
Note: Savoryella grandispora is a distinctive species in the genus with the largest ascospores (46–58 × 14–16 μm) among Savoryella species (Hyde, 1993, 1994). Savoryella grandispora has similar morphological features to S. aquatica (Hyde, 1993). Savoryella grandispora is distinguished in having longer ascospores which are light-brown as compared to the dark brown ascospores of S. aquatica (Hyde, 1994). However, the sequence data are unavailable for S. grandispora to compare their phylogenetic affinities.
Savoryella longispora E.B.G. Jones & K.D. Hyde (1992), Figure 7

Figure 7. Savoryella longispora (AMH-9997, isotype). (a) Ascomata semi-immersed on decaying wood of Avicennia marina. (b,c) Squash mounts of ascomata. (d,e) Immature asci. (f) Peridium. (g–i). Ascospores. Scale bars: (b,c) 100 μm, (d,e) 50 μm, (f–i) 10 μm.
Index Fungorum: IF 358210; Facesoffungi number: FoF 05395
Saprobic on decaying wood of Avicennia marina. Ascomata 175–340 × 205–250 μm ( = 341 × 231 μm, n = 5), globose to subglobose or ellipsoidal, immersed, semi-immersed or superficial, ostiolate, papillate, membranous, and light to dark brown. Necks 115–160 × 55–60 μm ( = 137 × 64 μm, n = 5), brown, with periphyses. Peridium 10–25 μm ( = 18, n = 5), brown, one-layered, comprises several layers of thick-walled angular cells forming textura angularis. Paraphyses present, sparse. Asci 150–200 × 15–25 μm, ( = 245 × 186 μm, n = 15), 8-spored, cylindrical or clavate, short-stalked, unitunicate, persistent, with truncate, J-apical ring containing a pore. Ascospores 34–45 × 7.5–12 μm ( = 39 × 10 μm, n = 5), uni- or biseriate, ellipsoidal, 3-septate, slightly constricted at the septa, central cells brown, apical cells smaller and hyaline (description generated from observation of herbarium material and the description of Jones and Hyde, 1992).
Material examined: INDIA: Tamil Nadu, Muthupet mangroves (10.4°N 79.5°E), on decaying wood of Avicennia marina (Acanthaceae), 30 March 2016, B. Devadatha (AMH-9997).
Note: Savoryella longispora was first reported from intertidal mangrove wood from Malaysia (Jones and Hyde, 1992). Both Savoryella longispora and S. lignicola share similar morphological characteristics but S. longispora is distinguished in having longer (34–45 μm vs. 24–36 μm) and narrower ascospores (7.5–12 μm vs. 8–12 μm) when compared to S. lignicola (Abdel-Wahab and Jones, 2000).
Savoryella yunnanensis W. Dong, Dayarathne & K.D. Hyde sp. nov., Figure 8

Figure 8. Savoryella yunnanensis (MFLU 18-1203, holotype) (a,b) Appearance of black ascomata superficial on host. (c) Vertical section of ascoma. (d) Structure of peridium. (e–g) unitunicate asci. (h) Paraphyses. (i–l) Ascospores. (m) Colony on PDA (from front). (n) Colony on PDA (from below). Scale bars: (c,e–g,i–l) 20 μm, (d,h) 10 μm.
Index Fungorum: IF555621; Facesoffungi number: FoF 05484
Etymology: Species epithet derived according to the geographical location, Yunnan province where the taxon found.
Saprobic on decaying wood submerged in freshwater. Sexual morph: Ascomata 70–90 μm high, 110–130 μm diameter, black, scattered or solitary, superficial, ellipsoidal, unilocular, thin-walled, laterally ostiolate, lying horizontal to the host surface with a short, hyaline neck. Peridium 15–20 μm thick, comprising several layers of dark brown to brown, thick-walled cells of textura angularis. Paraphyses ca. 8 μm diameter, numerous, swollen cylindrical, unbranched, hyaline, septate, constricted at the septa. Asci 100–135 × 25–30 μm ( = 115 × 27 μm, n = 10), 8-spored, unitunicate, clavate to narrow ellipsoidal, with short or without pedicellate. Ascospores 27–32 × 12–17 μm ( = 30 × 15 μm, n = 15), biseriate, straight or slightly curved, hyaline when young, central cells dark brown when old, end cells hyaline, 1-septate, slightly constricted at the septa, ellipsoidal or irregular, thin-walled, without sheaths or appendages. Asexual morph: Undetermined.
Culture characteristics: On PDA, colony circular, reaching 5 mm in 45 days at 25°C, gray to brown from above, gray from below, surface rough, dry, edge entire.
Material examined: China, Yunnan Province, Dehong, on submerged wood in a stream, 25 November 2017, W. Dong, H40C (MFLU 18-1203, holotype), ex-type living culture, MFLUCC 18-1395; on submerged wood in a small river, 25 November 2017, W. Dong H40C (HKAS 101726, isotype), living culture KUMCC 18-0076.
Note: Savoryella yunnanensis is morphologically most similar to Savoryella lignicola. However, it differs by possessing shorter and wider asci (128–180 × 16–24 μm vs. 100–135 × 25–30 μm) and wider ascospores (24.5–33.5 × 8.5–12.5 μm vs. 27–32 × 12–17 μm) and the shape of ascospores (ellipsoidal vs. ellipsoidal or irregular) (Jones and Eaton, 1969). Savoryella yunnanensis formed a well-supported (65% ML) separate lineage basal to a clade comprising Savoryella aquatica and Savoryella verrucosa. Base pair difference of S. yunnanensis and S. verrucosa for LSU only 4 bp out of 940 bp (<1%), SSU 30 bp out of 1,073 bp (2.79%), RPB2 116 bp out of 964 (12.03%) which are in the required range to consider them as two distinct species.
Ascotaiwania Sivan. & H.S. Chang (1992)
Index Fungorum: IF 25163; Facesoffungi number: FoF 05693
= Neoascotaiwania Hern.-Restr., R.F. Castañeda & Guarro (2017)
Saprobic on decaying plant parts including wood, petioles, twigs, prop roots. Sexual morph: Ascomata singled to grouped, partly to completely immersed in the host substrate, oblique or horizontal, dark brown to black globose, with a lateral short to long, erect, periphysate beak. Peridium composed of pseudoparenchymatous cells. Paraphyses filiform, hyaline, simple to rarely branched, deliquescing early. Asci cylindrical, 8-spored, pedicellate, functionally unitunicate, with a distinct non-amyloid apical annulus. Ascospores uniseriate to overlapping biseriate in the ascus, fusoid, transversely 5- or 3- or 7-septate, not constricted, straight to somewhat curved, smooth, with larger brown central cells and smaller hyaline to subhyaline end cells (modified description of Sivanesan and Chang, 1992). Asexual morph: brachysporiella-like, bactrodesmium-like, monodictys-like or monotosporella-like or trichocladium-like. Conidiophores micronematous to semi-macronematous or mononematous, flexuous, smooth, thin-walled, hyaline or thick walled and light brown to brown, occasionally indistinct. Conidiogenous cells erect or prostrate, holoblastic or monoblastic, integrated or determinate, mostly intercalary or terminal directly differentiate and developed at random point regions of hyphae, sometimes septate, smooth and thick-walled, cylindrical, flask-shaped, pale to dark brown some are arising from brown 2- or 3-septate rhizoids. Conidia solitary, frequently aggregate, globose, subglobose to obpyriform or ellipsoidal or subspherical, some aggregates, 1–4 septate, slightly constricted at the septa or constricted at the septa, sometimes a prominent scar on the conidium at the point of session from the conidiogenous cell, dark reddish brown to almost dark, smooth, the basal cells are mid-brown while the upper cell is blackish brown to black (modified description of Rao and de Hoog, 1986).
Type species: Ascotaiwania lignicola Sivan. & H.S. Chang (1992)
Note: Ascotaiwania was introduced by Sivanesan and Chang (1992) for an ascomycete with dark-brown to black ascomata having short or long lateral necks, asci with a comparatively massive J-apical ring and 5- or 3- or 7-septate pigmented ascospores with hyaline polar cells with A. lignicola as the type species. The family placement of Ascotaiwania has been widely debated (Ranghoo et al., 1999; Hernández-Restrepo et al., 2017). Ascotaiwania currently comprises 12 species. Initially, Ascotaiwania comprised taxa with only 7-septate ascospore (Sivanesan and Chang, 1992). Later, taxa with 3- or 5-septate ascospores were accepted in the genus, i.e., A. hsilio, A. hughesii, A. palmicola, A. pallida, A. pennisetorum, A. persoonii, and A. sawada (Chang et al., 1998) and subsequently, A. mauritiana, A. mitriformis, and A. wulai (Ranghoo and Hyde, 1998; Fallah et al., 1999; Hyde and Goh, 1999; Hernández-Restrepo et al., 2017). However, A. persoonii was synonymized under Pseudoascotaiwania persoonii within the order Fuscosporellales by Yang et al. (2016).
Asexual morphs of Ascotaiwania species have different morphologies (Hernández-Restrepo et al., 2017), such as monodictys-like comprising multicellular dark brown conidia in A. lignicola (Chang, 2001) (Figure 10); trichocladium-like, 1-septate conidia, which have dark brown apical cells and smaller, hyaline basal cells in A. hsilio (Chang, 2001); monotosporella-like in taxa in A. mitriformis (Ranghoo and Hyde, 1998) and A. sawada (Sivichai et al., 1998); and Helicoon farinosum in A. hughesii (Fallah et al., 1999). Jones et al. (2015), Réblová et al. (2016b), and Hernández-Restrepo et al. (2017) established that Ascotaiwania is polyphyletic and their asexual morph is phylogenetically more informative than the sexual morph. Recent studies by Yang et al. (2016) revealed Ascotaiwania fusiformis (asexual morph), clustered as a sister species to A. limnetica (=Neoascotaiwania limnetica). In our phylogenetic analyses, it grouped more closely to Neoascotaiwania terrestris, an asexual morph introduced by Hernández-Restrepo et al. (2017). Hernández-Restrepo et al. (2017) differentiated Neoascotaiwania from Ascotaiwania in having 3-septate ascospores, asci with a thinner, non-amyloid apical ring, and a bactrodesmium-like asexual morph. Phylogenetic analysis with a larger number of taxa (Figure 2) shows that both Neoascotaiwania and Ascotaiwania cluster in a single clade with high statistical support (100% ML, 1.00 PP). Two subclades (A and B in Figure 2) can be recognized within this clade. However, both clades comprise species with overlapping morphologies, i.e., Neoascotaiwania limnetica (subclade B) which is the type of Neoascotaiwania has three septate ascospores, while A. sawada also has three septate ascospores (subclade A) (Figure 9). Further, species with bactrodesmium-like asexual morphs, such as Ascotaiwania uniseptata, Bactrodesmium pallidum, Neoascotaiwania terrestris are distributed in both subclades (Figure 9). Therefore, we believe that species morphologies of both of those subclades are insufficient to distinguish them as two distinct genera, hence, we synonymize Neoascotaiwania under Ascotaiwania.
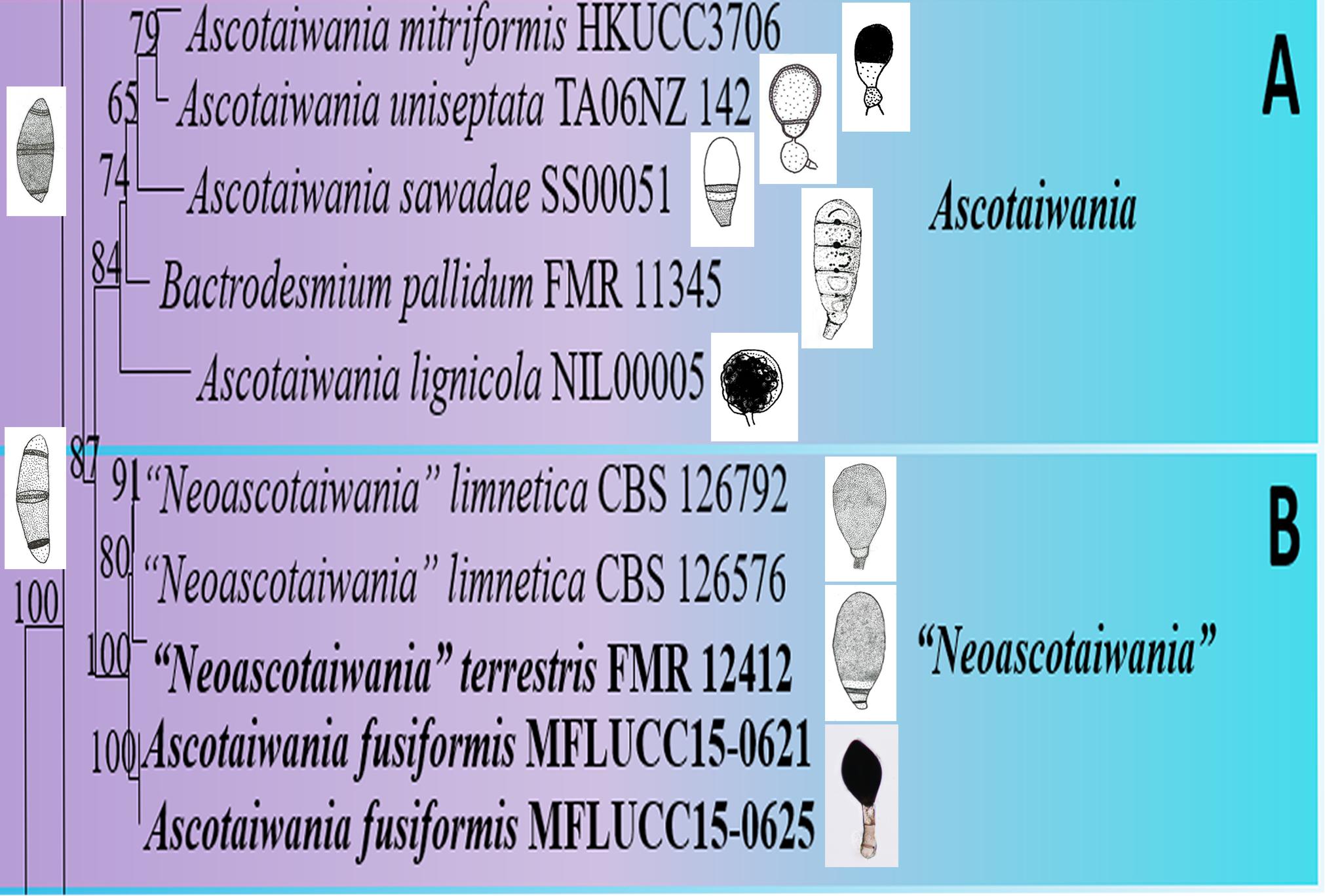
Figure 9. Conidial and ascospore character comparison of (A) Ascotaiwania and (B) “Neoascotaiwania” species.
Asexual morph Bactrodesmium pallidum nested in subclade B (Figure 2) with, A. mitriformis, A. sawadae, A. lignicola and Triadelphia uniseptata. Ascotaiwania mitriformis and A. sawadae showed monotosporella-like asexual morphs (Ranghoo and Hyde, 1998) while, A. lignicola (Chang, 2001) has trichocladium-like asexual morphs (Figure 10). Triadelphia uniseptata resembles B. pallidum in producing holoblastic, brown, septate conidia but B. pallidum differs by having sporodochial conidiomata and slightly differentiated, hyaline conidiophores (Holubová-Jechová, 1972; Tsui and Hyde, 2003; Réblová et al., 2016a). However, both B. pallidum and Triadelphia uniseptata clustered with Ascotaiwania species. According to both morpho-molecular data, this was supported by previous studies of Boonyuen et al. (2011) and Réblová et al. (2016b). Among Helicoon species, only Helicoon farinosum has been referred to the Savoryellaceae and it has been experimentally linked as the asexual morph of Ascotaiwania hughesii by rDNA data (Fallah et al., 1999). The Monotosporella state of Ascotaiwania sawada and A. mitriformis were regarded as different species from M. setosa and were regarded as two different taxa (Ranghoo and Hyde, 1998; Sivichai et al., 1998). In the analysis of the large subunit nuclear ribosomal DNA sequences by Ranghoo et al. (1999) they were separated as individual species, who established the connection between Ascotaiwania and Monotosporella. While later analyses with a higher number of taxon sampling (Boonyuen et al., 2011; Yang et al., 2016; Hernández-Restrepo et al., 2017) showed that M. setosa clustered away from the Ascotaiwania clade and formed a basal lineage to A. hugesii (P2-6, DAOM 241947) within Pleurotheciales. This is supported in our study (Figures 2, 3). We exclude A. hugesii and Monotosporella setosa from Ascotaiwania. Therefore, the genus Ascotaiwania is no longer polyphyletic.
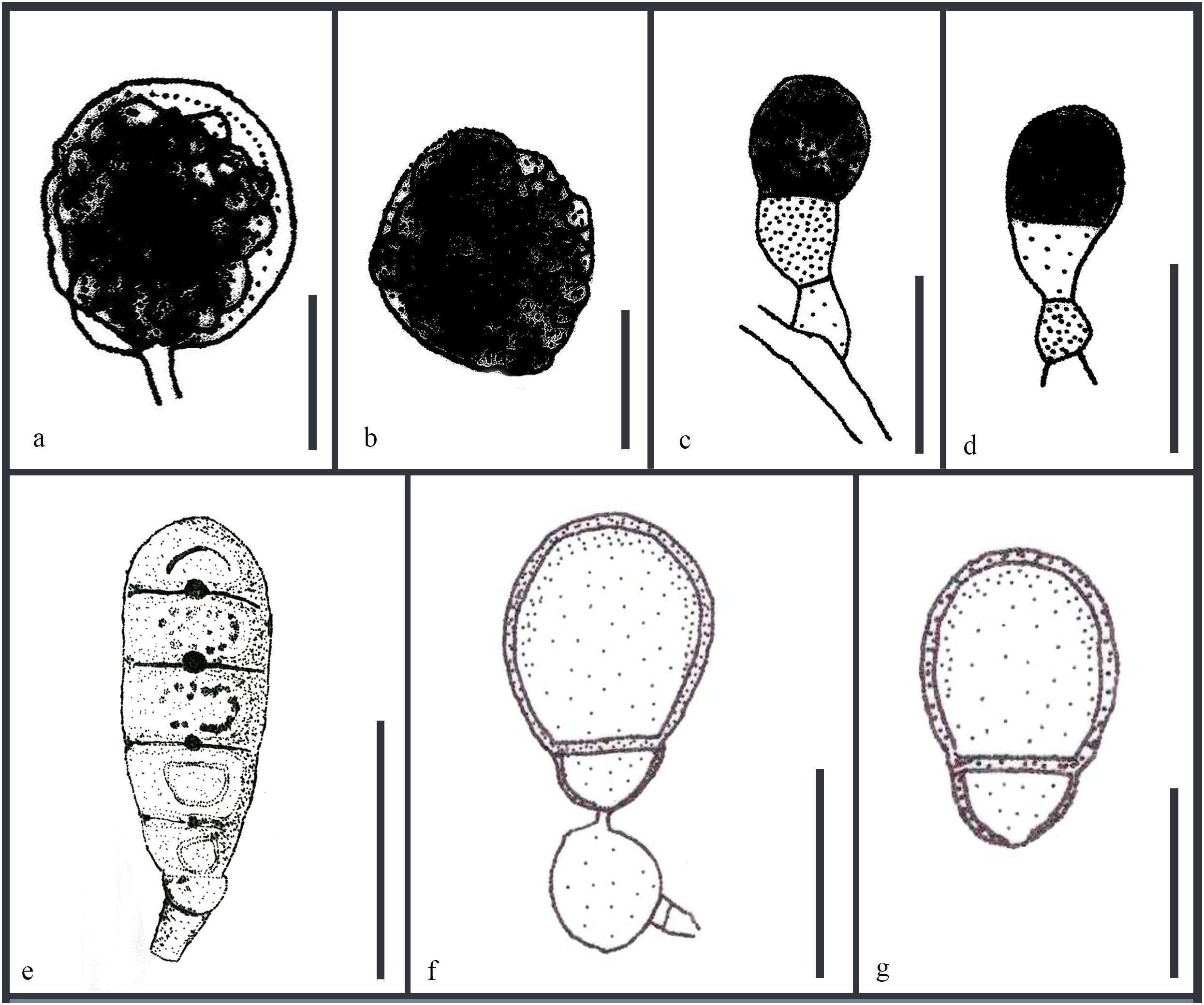
Figure 10. Revised drawings of different asexual morphs of Ascotaiwania. Conidiogenous cells and conidia of (a,b) A. lignicola (Chang, 2001) (c,d) A. mitriformis (Ranghoo and Hyde, 1998) (e) B. pallidum (Tsui and Hyde, 2003) (f,g) A. uniseptata (Kirk, 1983). Scale bars: (a,b,e) 20 μm, (c,d,f,g) 10 μm.
Ascotaiwania fusiformis Jing Yang, Bhat & K.D. Hyde (2016), Figure 11
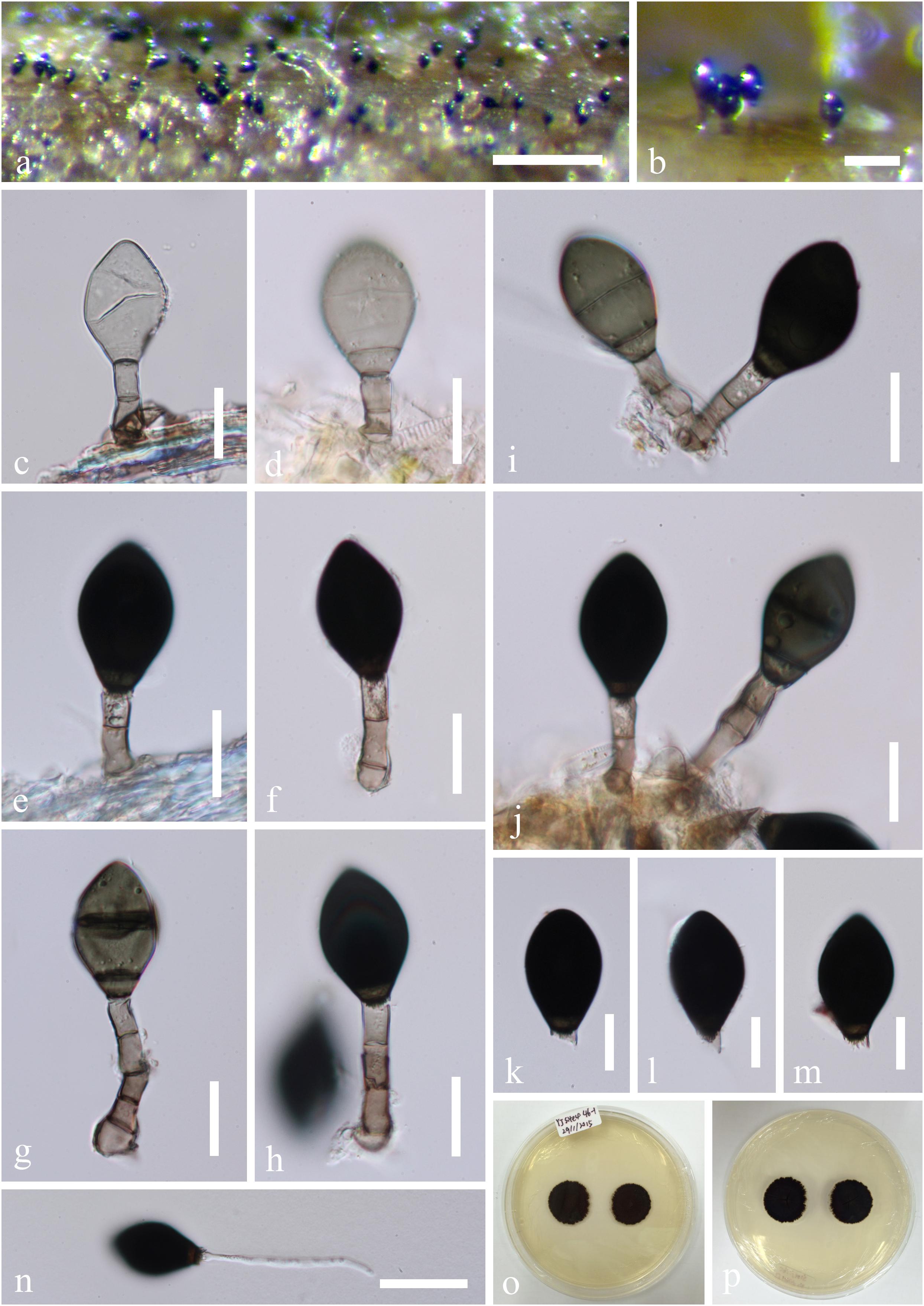
Figure 11. Ascotaiwania fusiformis (MFLU15-1156, holotype). (a) Colonies on the substrate. (b–g) Conidiophores, developing and mature conidia. (h–k). Conidiophores and conidiogenous cell. (l–q) Conidia. (r) Germinated conidium on PDA. (s,t) Culture, from above (s), from below (t). Scale bars: (a) 50 μm, (b) 15 μm, (c–k) = 20 μm, (l–q) 15 μm, (r) 40 μm.
Index Fungorum: IF 552294; Facesoffungi number: FoF 02429
Description: Please see Yang et al. (2016).
Material examined: Thailand, Prachuap Khiri Khan Province, on decaying submerged wood in a stream, 25 December 2014, Jaap van Strien, Site4- 27-1, MFLU 15-1156, holotype, ex-type living culture, MFLUCC 15-0621; ibid., HKAS 95048, isotype.
Note: In our phylogenetic analyses, Ascotaiwania fusiformis grouped in a clade comprising A. limnetica and A. terrestris. Ascotaiwania fusiformis morphologically resembles Monotosporella species and the brachysporiella-like asexual morphs of Ascotaiwania (Sadowski et al., 2012; Réblová et al., 2016b). Ascotaiwania fusiformis is easily distinguishable from Monotosporella rhizoidea in the absence of lobe-like swollen conidiogenous cells (Rao and de Hoog, 1986), while it can be separated from Monotosporella clavata which has clavate, 4–6-septate conidia, by it’s fusiform and less septate conidia (Yanna and Hyde, 2002). Ascotaiwania fusiformis differs from Monotosporella doerfeltii by larger conidia with dark basal cells (Sadowski et al., 2012). Brachysporiella-like asexual morphs of A. limnetica have (3–) 5–6 septate conidia, while A. fusiformis usually has 2-septate conidia (Réblová et al., 2016b). Ascotaiwania terrestris morphologically differs from A. fusiformis in having (2–)3–4(–5)- septate, ellipsoidal to obovoid conidia (Hernández-Restrepo et al., 2017).
Ascotaiwania limnetica (Hern.-Restr., R.F. Castañeda & Gené) Dayarathne, Hyde KD comb. nov.
Index Fungorum: IF555623
Basionym: Savoryella limnetica H.S. Chang & S.Y. Hsieh, in Chang, Hsieh, Jones (1998)
Synonym: Ascotaiwania limnetica (H.S. Chang & S.Y. Hsieh) Réblová & J. Fourn., in Réblová, Seifert, Fournier & Štìpánek (2015) [2016]
Descriptions and illustrations: Hernández-Restrepo et al. (2017)
Ascotaiwania terrestris (Hern.-Restr., R.F. Castañeda & Guarro) Dayarathne, Hyde KD comb. nov.
Index Fungorum: IF555622
Basionym: Neoascotaiwania terrestris Hern.-Restr., R.F. Castañeda & Guarro, in Hernández-Restrepo et al. (2017)
Descriptions and illustrations: Chang et al. (1998) and Réblová et al. (2016b).
Ascotaiwania uniseptata (Berk. & Broome) P.M. Kirk (1983), Figure 10
Index Fungorum: IF 109271
Basionym: Sporidesmium uniseptatum Berk. & Broome (1859)
Description and illustrations: Please see Kirk (1983)
Note: In our phylogram, Ascotaiwania uniseptata (=Triadelphia uniseptata), a dematiaceous hyphomycete, clustered in the Ascotaiwania clade with affinities to A. mitriformis. This placement was supported by the previous studies of Réblová et al. (2016b), Yang et al. (2016), and Hernández-Restrepo et al. (2017). The genus Triadelphia typified by T. heterospora, was introduced to include fungi from freshwater and brackish water habitats (Shearer and Crane, 1971). Triadelphia heterospora is characterized by sub globose to subglobose, subhyaline to dematiaceous conidiogenous cells, schizolytic conidial secession while conidia generated blastically from a single locus, brown, or versicolorous conidia often with one or two end cells which are lighter than the middle ones, septate, often with brighter bands masking some septa (Shearer and Crane, 1971). Triadelphia heterospora was described with dimorphic conidia (Constantinescu and Samson, 1982). There are 22 species records in Index Fungorum while one species was synonymized under Pithomyces3. Phylogenetic position of its type species is unknown. Only a few species of Triadelphia; T. heterospora, T. pulvinata, T. moubasheri have molecular data in GenBank and they have affinities to species in the Microascales (Edathodu et al., 2013; Wijayawardene et al., 2017, 2018). Triadelphia species resemble Bactrodesmiastrum in having cylindrical to lageniform, aggregated; conidiogenous cells (Réblová et al., 2016b). The morphology of Ascotaiwania uniseptata is illustrated in Figure 10. Réblová et al. (2016b) referred Ascotaiwania uniseptata to the Savoryellales and this is supported by Yang et al. (2016), who demonstrated that Triadelphia is polyphyletic. The continuation of this generic name, and inclusion of its species within the genus, needs additional sampling. However, T. heterospora, the generic type has no sequence data existing in GenBank. By considering the morphological similarities and phylogenetic togetherness, Boonyuen et al. (unpublished) proposed the inclusion of this species under Ascotaiwania and introduced the new family Triadelphialaceae to accommodate other Triadelphia species.
Bactrodesmium pallidum M.B. Ellis (1959), Figure 10
Index Fungorum: IF 293575
Basionym: Bactrodesmium pallidum M.B. Ellis (1959)
Description and illustrations: Please see Tsui and Hyde (2003)
Note: Bactrodesmium is typified by B. abruptum isolated from dead wood in the United Kingdom (Berkeley and Broome, 1865). The taxonomy of the genus is doubtful and presently known to be polyphyletic (Hernández-Restrepo et al., 2013). It has been placed in several orders and families: Helotiales (Koukol and Koláøová, 2010), Massarineae (Tanaka et al., 2015) and currently placed in Pleosporales genera incertae sedis (Wijayawardene et al., 2018). However, there is no designated type material or living culture for the type species. Furthermore, there is a limited number of cultures available (Hernández-Restrepo et al., 2013). Tsui and Hyde (2003) described the morphological characters of B. pallidum isolated from wood submerged in a river in Japan (Figure 10). There are 61 species recorded under Bactrodesmium while five species have synonyms under Melanommataceae and Magnaporthaceae4. Therefore, the bactrodesmium-like taxa are polyphyletic. Phylogenetic analyses by Hernández-Restrepo et al. (2013) showed that B. pallidum grouped with Ascotaiwania and this was supported in our study. Hence, depending on present morphological and molecular data, we suggest the accommodation of B. pallidum within Ascotaiwania.
1. Asexual morphs, bactrodesmium-like or brachysporiella-like, monodictys-like, monotosporella-like or trichocladium-like……………………………………. 2
1. Sexual morphs only……………………………………6
2. Asexual morphs, bactrodesmium-like, brachysporiella-like or triadelphia-like……………………………………. 3
2. Asexual morphs, monodictys-like, monotosporella-like or trichocladium-like……………………………………. 5
3. Conidia fusiform, obovoid to broadly obovoid, 1–2-septate at all the stages……………………………………….. 4
3. Conidia ellipsoidal, obovoid, mostly 3–4-septate……………………………………A. terrestris
4. Conidia 1-septate near the base, constricted at the septum, upper cell dark and thick-walled, lower cell thin-walled……………………………………A. uniseptata
4. Conidia 2-septate, seen as uniseptate when mature, not constricted at the septum, subhyaline when young, becoming olive to dark brown, paler at the basal cell………………………………………A. fusiformis
5. Asexual morphs, monodictys-like……………A. lignicola
5. Asexual morphs, trichocladium-like……………A. hsilio
5. Asexual morphs, monotosporella-like…………………………………………A. sawada
6. Ascospores with polar appendages…………A. palmicola
6. Ascospores without polar appendages…………………4
7. Ascospores 3-septate………………………………….. 5
7. Ascospores 5–7-septate……………………………….. 7
8. Ascospores slightly constricted at the septa…………….. 6
8. Ascospores not constricted at the septa…A. pennisetorum
9. Peridium comprising textura intricata, hyaline at the inside and brown outwardly, fusing at the outside with the host cells, composed of brown thick walled textura angularis ………………………………………………A. pallida
9. Peridium with opaque walls and sparse pores, the inner layer comprising thin-walled, hyaline, flattened cells, outward grading into small protruding cells, outer layer consisting of dark brown, polyhedral, flattened cells of textura prismatica…………………………A. limnetica
10. Ascospores shorter than 40 μm (19–30 μm). A. mauritiana
10. Ascospores 55 μm or longer (53 ± 62 μm)………A. wulai
Canalisporium Nawawi & Kuthub. (1989)
= Ascothailandia Sri-indr., Boonyuen, Sivichai & E.B.G. Jones (2010)
Index Fungorum: IF 11041; Facesoffungi number: FoF 05485
Description: Please refer Goh et al. (1998) and Sri-indrasutdhi et al. (2010).
Type species: Canalisporium caribense (Hol.-Jech. & Mercado) Nawawi & Kuthub. (1989)
= Berkleasmium caribense Hol.-Jech. & Mercado (1984)
Note: Canalisporium species are characterized by having scattered, punctiform, pulvinate, granular, black, glistening sporodochia, which contain acrogenous, holoblastic conidia that developed in a hyaline gelatinous sheath (Goh et al., 1998). Conidia of Canalisporium species are muriform, however, they differ from those of Berkleasmium species in being flattened dorsoventrally, comprising a single layer of regularly arranged cells, which are supported by a small, thin-walled, cuneiform, pale basal cell (Sri-indrasutdhi et al., 2010). At present, 15 species are recognized in Canalisporium (Zhao et al., 2012; Hyde et al., unpublished). The monotypic genus Ascothailandia was established by Sri-indrasutdhi et al. (2010) for the sexual morph of C. grenadoideum in a phylogenetic tree based on six species of Canalisporium, with the type species, and confirmed that Ascothailandia and Canalisporium are congeneric (Sri-indrasutdhi et al., 2010). Canalisporium has priority as it has fewer number of name changes and is an earlier name. Jones et al. (2016) and Réblová et al. (2016a) recommended the conservation of the generic name Canalisporium over Ascothailandia.
Canalisporium caribense (Hol.-Jech. & Mercado) Nawawi & Kuthub. (1989), Figure 12
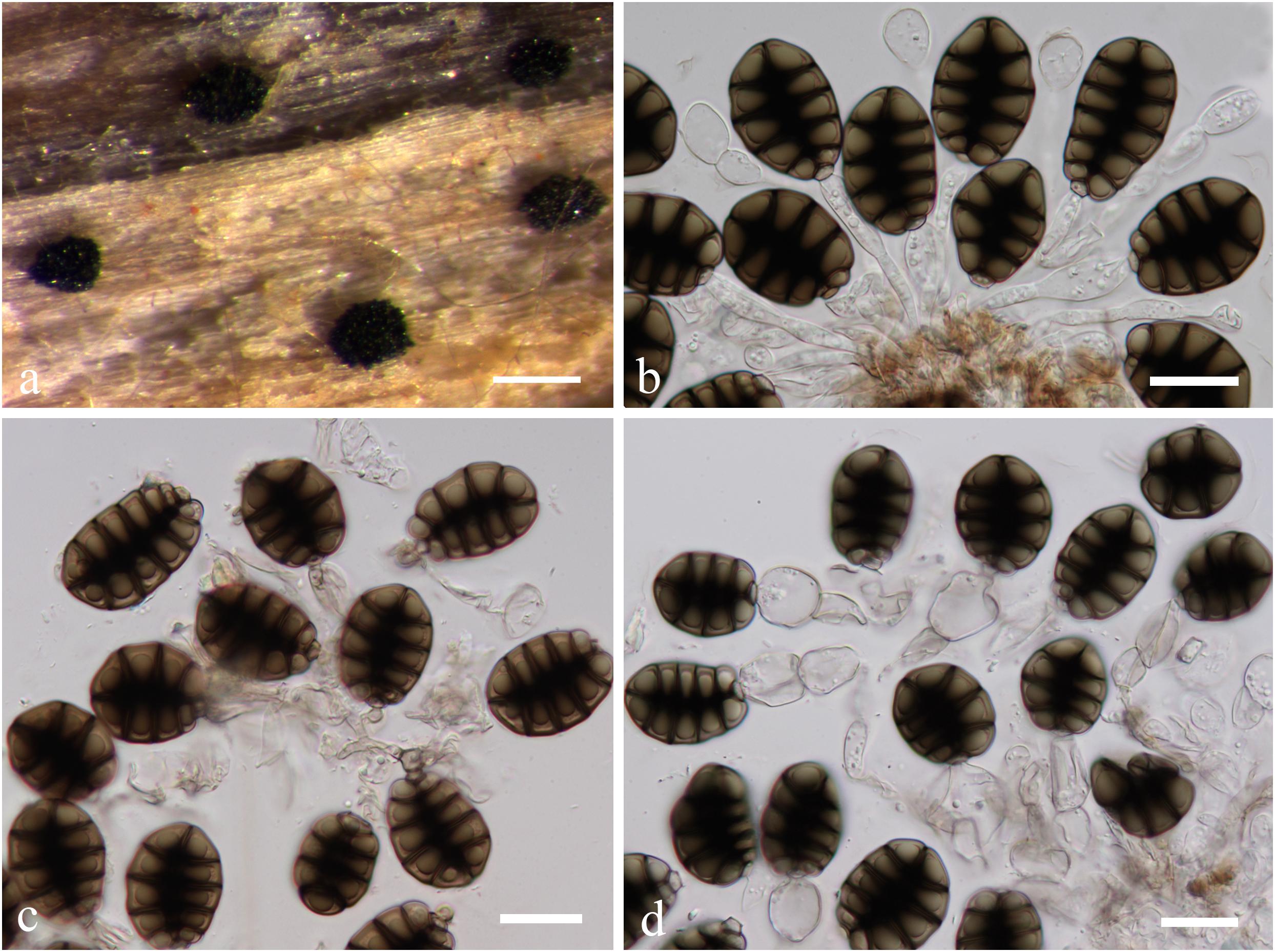
Figure 12. Canalisporium caribense(MFLU15-3581, holotype). (a) Substrate. (b–d) Sporodochia on wood. (e,f) Squash mount of a sporodochium. (g) Conidiophores. (h,i) Vesiculate conidiogenous cell. (j) Germinated conidium on nature substrate. (k) Germinated conidium on PDA medium. (l–o) Conidia. Scale bars: (b) 500 μm, (c,d) 50 μm, (e,i–k) 20 μm, (f) 30 μm, (g,h) 15 μm, (l–o) 10 μm.

Figure 13. Sexual morph of Helicoascotaiwania hughesii (micro slides of holotype ILLS 53605). (a,b) Herbarium material. (c) Peridium. (d) Neck region. (e–g) Ascospores. Scale bars: (d) 100 μm, (c) 20 μm, (e–g) 10 μm.
Index Fungorum: IF 125432; FacesofFungi number: FoF 05486
Description: Please see Maharachchikumbura et al. (2016).
Material examined: Thailand, Chiang Rai Province, a stream flowing in Tham Luang Nang Non-Cave, on submerged wood, 25 November 2014, J. Yang (MFLU15-3581, holotype).
Note: Canalisporium caribense, the type species of this genus, is the latest name for Berkleasmium caribense (Holubová-Jechová and Sierra, 1984). Conidia of Canalisporium comprises septal canals, which is unique amongst taxa of hyphomycetes (Sri-indrasutdhi et al., 2010). Canalisporium caribense, however, has thick septa banded so that the canals are obscured by this heavy pigmentation, which is common in the freshwater environment (Nawawi and Kuthubutheen, 1989). The characteristic darkened pigmentation around the septa of the conidia is rather variable (Sri-indrasutdhi et al., 2010).
1. Conidiophore absent……………………C. panamense
1. Conidiophore present…………………………………2
2. Conidia with three small cells at the base and a single cell at the apex………………………………….. C. kenyense
2. Conidia with a single cell at the base and one (rarely), two or multi cells at the apex………………………………3
3. Conidia with a column of longitudinal septa, scattered, pale olivaceous with clearly visible septa and canals, septa thin and not banded……………………………C. pallidum
3. Conidia with a single, double, or four to five columns of longitudinal septa, light brown to dark brown, septa often thick and brightly banded, canals obscured or not usually visible…………………………………………………4
4. Conidia comprising a column of longitudinal septa…………………………………………………5
4. Conidia comprising two or multi columns of longitudinal septa…………………………………………………7
5. Conidia with 3–6(–7) rows of transverse septa…………6
5. Conidia with 2–3(–4) rows of transverse septa.. C. exiguum
6. Conidia 20–51 × 12–29 × (8–)10–16 μm, with 3–6(–7) rows of transverse septa………………………………8
6. Conidia with 25–50 × 13–19 × 6–10 μm, 4–5 rows of transverse septa………………………………………7
7. Conidia 25–34 × 13–19 × 6–10 μm, with 4–5 rows of transverse septa………………………………C. nigrum
7. Conidia 39–60 × 13–17.5 μm, with 4–5 rows of transverse septa…………………………………C. thailandensis
8. Conidia 24–51 × 15–29 × (8–)10–16 μm, with 3–6(–7) rows of transverse septa……………………C. caribense
8. Conidia 20–30 × 12–19 μm, with 3–5 rows of transverse septa……………………………………C. dehongense
8. Conidia 27–50 × 22–32 μm, 4–6 rows of transverse septa………………………………………C. krabiense
9. Conidia regularly with two columns of longitudinal septa…………………………………………………10
9. Conidia irregularly with 4–5 columns of longitudinal septa…………………………………………………11
10. Conidia with 2–4 rows, 1 cell at the apex, 22–35 × 15–23 × 10–10.5 μm, 2.5–5 wide………………C. variabile
10. Conidia with 2–4 rows, 1–4 cells at the apex, 25–33 × 20–28 × 7.5–11.5 μm, up to 25 μm long and 1.5–2 μm wide…………………………………C. jinghongenses
11. Conidia with 3–9 rows, 1–3 cells at the apex, 12.5–63 × 8–32 × 4–17 μm………………………………………12
11. Conidia with 5–8 rows, 1–5 cells at the apex, 32–58 × 25–38 × 10–13 μm……………………………C. elegans
11. Conidia with 4–6 rows, 3–4 cells at the apex, 27.5–37.5 × 24–27.5 × 17.5–22.5 μm…………C. grenadoidia
12. Conidia 25–63 × (16–)20–32 × 12–17 μm…C. pulchrum
12. Conidia 12.5–20 × 8–12 × 4–6 μm……C. microsporum
Helicoon Morgan, J. Cincinnati. Soc. Nat. Hist. 15: 49 (1892)
Note: Morgan (1892) introduced the genus Helicoon, an aero-aquatic hyphomycete characterized by the production of non-proliferating, cylindrical, barrel-shaped conidia (Figure 14). The conidia are borne on distinct conidiophores, which can be very short and inconspicuous (Fisher, 1977; Goos et al., 1986). According to MycoBank (Crous et al., 2004), NCBI5 (2018) and Wijayawardene et al. (2017) the genus Helicoon has 20 accepted species. Linder (1929) included seven of these in his monograph on helicosporous fungi. Nine species were listed by Moore (1955) in his key, and Abdullah (1980) described seven species from United Kingdom. Goos et al. (1986) included eight species in their key. The genus Helicoon was shown to be polyphyletic (Tsui and Berbee, 2006) although the sexual morph of Helicoon is presently unknown (Goos et al., 1986). Ascotaiwania hughesii was experimentally connected with a Helicoon asexual morph recognized as conspecific with H. farinosum (Fallah et al., 1999). Helicoon farinosum comprises hyaline, coiled, septate conidia that formed holoblastically on short denticles (Fallah et al., 1999) (Figure 14). In our phylogenetic analyses, Ascotaiwania hughesii along with its asexual morph H. farinosum grouped within Pleurotheciales and this is accepted by Réblová et al. (2012). Hence, we exclude Helicoon farinosum from Savoryellales. This is the only representative with helicosporous conidia in the Pleurotheciales and in subclass Savoryellomycetidae. Helicoon sessile (type species), was linked with Orbilia of the Orbiliales (Orbiliomycetes) (Pfister, 1997). The internal transcribed spacer sequence of H. sessile (U72605, Pfister, 1997) is identical to the sequence of Sarocladium kiliense in the Hypocreales (KP132606). Other phylogenetically related groups are Tubeufiaceae (H. gigantisporum), Pleosporales (H. richonis) or Dothideomycetes (H. fuscosporum). We could not obtain any type of material of H. fuscosporum.
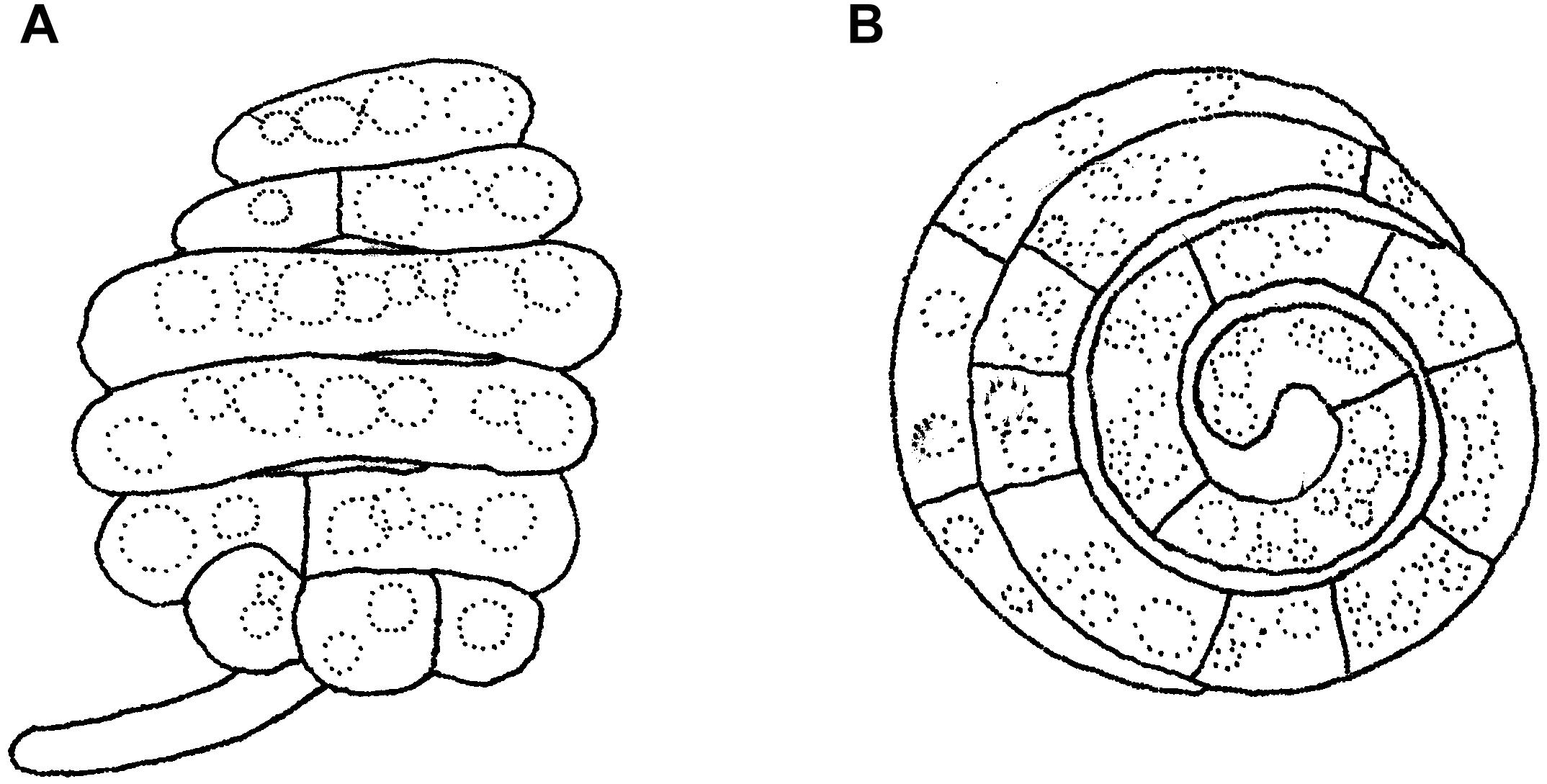
Figure 14. Asexual morph of Helicoascotaiwania hughesii. (A,B) Helicoid conidia. Scale bars: (A,B) = 20 μm (redrawn from Goos et al., 1986).
Monotosporella S. Hughes, Can. J. Bot. 36: 786 (1958)
Note: Hughes (1978) proposed Monotosporella for a hyphomycete with conidia produced by percurrent proliferation of conidiophores, isolated from wood in New Zealand and is typified by Monotosporella setosa. Subsequently, Monotosporella was synonymized under Brachysporiella; a genus comprising conidiophores that branch at the apex (Ellis, 1959). Later, Hughes (1958) suggested that Monotosporella should be different from Brachysporiella, because the lateral branches were not observed in any of the earlier introduced Monotosporella species. Seifert and Gams (2011) showed that Monotosporella is heterogenous while, Wijayawardene et al. (2012) listed Monotosporella under Melanommataceae. Presently, five species have been designated in Monotosporella (Index Fungorum, 2017). Sivichai et al. (1998) observed affinities between the asexual state of Ascotaiwania and Monotosporella setosa. Further, monotosporella-like asexual morphs have been reported for A. mitriformis and A. sawadae (Ranghoo and Hyde, 1998; Sivichai et al., 1998). The asexual morph Ascotaiwania fusiformis resembles Monotosporella species (Yang et al., 2016). Our phylogenetic analysis showed that M. setosa does not group in Savoryellaceae but within the order Pleurotheciales (please see Figure 19 in Tian et al., 2015, for the complete illustration of M. setosa). Hernández-Restrepo et al. (2013) concurred with Tian et al. (2015), and therefore we exclude Monotosporella from Savoryellaceae.
Helicoascotaiwania Dayarathne, Maharachch. & K.D. Hyde gen. nov.
Index Fungorum: IF555625; Facesoffungi number: FoF 05487
Etymology: Name referring the similar morphologies to the genus Ascotaiwania and Helicoon.
Saprobic on decaying wood. Sexual morph: Ascomata perithecial, immersed, scattered, pyriform to obpyriform, black, straight or incline, with erumpent necks, membranous. Necks central, conical to cylindrical, rounded at apex, with a periphysate ostiolate. Peridium comprising several cell layers of brown, textura angularis in surface view, with 7–9(–12) compressed brown cells. Paraphyses 1.5–3 μm wide, branched, septate. Asci 8-spored, unitunicate, cylindrical, persistent, tapering to a long pedicel, with a J-apical ring. Ascospores uniseriate, fusiform, straight or curved, smooth-walled, 3-septate, central cells brown, polar cells hyaline, with a rounded apex and a wide base. Asexual morph: Colonies inconspicuous, white, powdery. Conidiophores micronematous, mononematous, subtle, rarely branched, hyaline, straight or flexuous, tapering upward, smooth. Conidiogenous cells monoblastic, integrated, terminal, determinate, denticulate. Conidia acrogenous, hyaline. Conidial filament coiled five to eight times in three planes to form a subglobose to ellipsoidal spore body (description based on our observations, Goos et al., 1986; Fallah et al., 1999).
Type species: Helicoascotaiwania hughesii (Fallah, J.L. Crane & Shearer) Dayarathne & K.D. Hyde
Helicoascotaiwania hughesii (Fallah, J.L. Crane & Shearer) Dayarathne & K.D. Hyde comb. nov., Figure 13
Index Fungorum: IF555626; Facesoffungi number: FoF 05488
= Ascotaiwania hughesii Fallah, J.L. Crane & Shearer, Can. J. Bot. 77(1): 89 (1999)
Saprobic on decaying wood. Sexual morph: Ascomata 580–630 × 348–380 μm, immersed in decorticated wood, scattered, obpyriform, straight or tilted, with protruding beaks, membranous, black. Necks 200–225 × 100–140 μm, central, membranous, cylindrical to conical, broadly rounded at apex, periphysate, ostiolate. Peridium 40–45 μm wide, composed of several layers, brown, of textura angularis in surface view, composed of seven to nine compressed brown cells; cells 4.5–9 × 1.5–2 μm. Paraphyses 1.5–3 μm wide, branched, septate. Asci 263–347 × 9–10 μm, unitunicate, long-cylindrical, eight spored, persistent, tapering gradually to a long stalk, with an apical ring, 9–13.5 μm, J-, staining blue in aqueous cotton blue. Ascospores 18–30 × 6–8 μm, fusiform, uniseriate, straight or curved, smooth-walled, three septate, central cells brown, 8–10 μm long; end cells hyaline, rounded at apex, 4.5–5.5 μm wide at the base. Asexual morph: Colonies inconspicuous, white, powdery. Conidiophores micronematous, mononematous, inconspicuous, rarely branched, hyaline, straight or flexuous, tapering upward, smooth. Conidiogenous cells monoblastic, integrated, terminal, determinate, denticulate. Conidia acrogenous, hyaline. Conidial filament coiled five to eight times in three planes to form a subglobose to ellipsoidal spore body (modified description of Fallah et al., 1999 and based on our observations).
Material examined: United States, Wisconsin, Vilas Co., Sparkling Lake, UTM Zone 16, 291184 m E, 5099214 m N, 46°00’87”N, 89 41° ’83”W, on submerged wood, 8 August 1994, PMF P2-6, (micro slides of holotype ILLS 53605).
Note: Helicoascotaiwania hughesii (=Ascotaiwania hughesii) in reminiscent of A. sawada and A. palmicola in having 3-septate ascospores. It is distinguished from A. sawada by shorter ascospores with a cylindric-fusoid shape. The second septum of the ascospores is demarcated relatively closer to the apex in A. hughesii than that of A. sawada. The asci of A. hughesii are longer and narrower than those in A. sawada (Fallah et al., 1999). Ascomata of Ascotaiwania hughesii are larger and less rounded than in A. palmicola while the ascospores are longer and broader in A. hughesii. Ascospore appendages are present in A. palmicola but absent in A. hughesii. Fallah et al. (1999) reported that ascospores of A. hughesii germinated and formed white colonies and conidia were formed several days later, and all colonies were identified as belonging to the genus Helicoon (Helicoon farinosum). They also observed ascomata on the natural substratum, which discharged ascospores of A. hughesii among the conidia of H. farinosum. Further, sequences generated from both sexual morph and asexual morph strains did not clade with other Ascotaiwania members or within Savoryellales. They formed a well-supported clade within Pleurotheciaceae and the same result observed by Boonyuen et al. (2011); Yang et al. (2016), and Hernández-Restrepo et al. (2017). With the current phylogenetic placement and morphological differences of the asexual morph, we introduce a novel genus, Helicoascotaiwania to accommodate A. hughesii within Pleurotheciaceae.
Savoryellaceae is a fascinating family with taxa distributed in terrestrial and aquatic microhabitats (Boonyuen et al., 2011). There are several phylogenetic studies on this family, however, the family is not well resolved (Boonyuen et al., 2011). For instance, Ascotaiwania species was polyphyletic and the sexual and asexual morph links of certain species are not well-defined (Boonyuen et al., 2011). In our phylogram, the orders Conioscyphales, Fuscosporellales, Pleurotheciales, and Savoryellales clustered within the subclass Savoryellomycetidae, in a highly supported clade. In our phylogenetic analyses, Savoryellaceae formed a monophyletic group, with species belonging to Ascotaiwania, Canalisporium, Neoascotaiwania, Savoryella, Bactrodesmium pallidum, and Triadelphia uniseptata. Similar results were observed by Boonyuen et al. (2011) and Réblová et al. (2016b). Canalisporium and Savoryella are well-established monophyletic genera while Ascotaiwania was polyphyletic. Here, we resolved the polyphyly of Ascotaiwania by excluding A. hughesii (=Helicoascotaiwania hughesii) from the genus. We observed and re-described the type of A. hughesii (=H. hughesii) and provide a detailed description. Taxa previously referred to Neoascotaiwania comprise similar morphologies to species of Ascotaiwania and both taxa formed a monophyletic clade with high statistical support (100% ML, 1.00 PP), which indicates that Neoascotaiwania should be synonymized under Ascotaiwania. However, when comparing the available morphological data from different asexual morphs of Ascotaiwania it is clear that asexual morph characters are more informative in species recognition in Ascotaiwania when compared to their sexual morph, a view advanced by Hernández-Restrepo et al. (2017). Therefore, several name changes are undertaken in this study and summarized in Table 4. Savoryella species formed a well-supported (100% ML) monophyletic clade within Savoryellaceae. We introduced a novel species S. yunnanensis collected from China. Savoryella yunnanensis showed close phylogenetic affinities to S. aquatica, S. fusiformis and S. verrucosa. Savoryella yunnanensis morphologically resembles S. lignicola, however, the two species are phylogenetically distinct. Their bases pair differences of LSU 6 out of 826 (<1%), SSU 46 out of 1075 (4.27%), 12 out of 885 (1.3%) which are in adequate range to consider them as two distinct species as recommended by Jeewon and Hyde (2016). We observed the types of S. aquatica, S. grandispora, and S. lignicola, and report that the holotype of S. aquatica is in poor condition that has deprived us from observing any ascomata on the host substrate.
The divergence time estimations in the present study are congruent with former revisions (Beimforde et al., 2014; Pérez-Ortega et al., 2016; Samarakoon et al., 2016; Hyde et al., 2017). Our molecular clock analyses indicate that divergence times (crown age) of Sordariomycetes (325 Mya), is in agreement to those of the latest studies (317 Mya in Pérez-Ortega et al., 2016 and 309 Mya in Beimforde et al., 2014, 347 Mya in Samarakoon et al., 2016, 341 MYA in Hyde et al., 2017; Hongsanan et al., 2017). The divergence of Dothideomycetes crown group at 366 (400–492) Mya occurred before Sordariomycetes (325 Mya) (Liu et al., 2017). In our study, divergence times for most orders of Sordariomycetes are between 20 and 200 Mya, while divergence times for most families are between 10 and 100 Mya, which are consistent with those of Hyde et al. (2017). According to the available divergence time estimates of the marine ascomycete taxa, Koralionastetaceae and Lulworthiaceae might be the earliest with the stem age of 215 (145–283) Mya while the earliest marine lineage of Dothideomycete is reported for Halottiaceae with the stem age of 186 (133–135) (Liu et al., 2017).
Hongsanan et al. (2017) and Hyde et al. (2017) suggested that stem age of the subclass Sordariomycetes falls in the range of 250–289 Mya hence, Savoryellales is upgraded to subclass Savoryellomycetidae as its emergence goes back to early Mesozoic (201–252 Mya). This is supported by our study also and showed divergence time with stem age of 287 (232–343) for Sordariomycetes. The subclass Savoryellomycetidae includes four orders (Conioscyphales, Fuscosporellales, Pleurotheciales, and Savoryellales). The stem age of Pleurotheciales is around 143 Mya while it was reported as 139 Mya in Hongsanan et al. (2017); Conioscyphales, the sister clade of Pleurotheciales, evolved at the stem age of 180 Mya. Further, Fuscosporellales and Savoryellales have evolved at the stem ages of 249 Mya and 213 Mya, respectively. Therefore, order level status within Savoryellomycetidae range from 143–249 Mya, with families having crown ages of 104–182 Mya.
The occurrence of marine ascomycetes along with terrestrial or freshwater taxa provide evidence for the migration of ascomycetes from land to the marine environment (Spatafora et al., 1998; Vijaykrishna et al., 2006). Savoryellaceae is a good example for the above hypothesis comprising many terrestrial and freshwater genera as well as transition species such as S. lignicola (Pinruan et al., 2002; Vijaykrishna et al., 2006; Jones et al., 2009; Sakayaroj et al., 2011; Suetrong et al., 2015). The orders Koralionastetales, and Lulworthiales have co-evolved with a divergent age of 215 Mya which represents the most basal group. Additionally, the well-adapted marine taxa in the Halosphaeriaceae (order Microascales) and the marine order Torpedosporales showed divergence at about 171–241 Mya (Hongsanan et al., 2017). Vijaykrishna et al. (2006) documented that the Halosphaeriaceae evolved around 100 Mya, which is in agreement with the divergence time of 47–130 Mya in our study. However, molecular clock analyses provide various divergence time estimates for marine lineages and confirmed their evolution at different periods. The discovery of the extent new Caledonian species identified as Monotosporella setosa found developing on semi-solidified resin flows of Agathis ovata (Araucariaceae), is the first record of a Monotosporella species from modern resin substrates (Sadowski et al., 2012). Thus, the fossil age of this species could help to determine the exact phylogenetic placement of other Monotosporella species in the future. Most of the divergence time estimations have been conducted with mainly terrestrial representatives rather than those of marine origin and we recommend a thorough analysis of a wider range of marine fungal taxa.
AA-S, EJ, KH, KK, MD, RZ, SM, and VS planned the experiments. BD, WD, JY, MD, and WD conducted the experiments. AE, MD, and WD analyzed the data. BD, MD, SM, and EJ wrote the manuscript. All authors approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the National Research Council of Thailand (NRCT) for a grant entitled Diseases of mangrove trees and maintenance of good forestry practice (Grant No. 60201000201) and Thailand Research Fund (TRF) (Grant No. RSA59800680). VS would like to offer his sincere gratitude to the grant of Ministry of Earth Sciences, Government of India, under (Sanction order: MOES/36/OO1S/Extra/40/2014/PC-IV dt.14.1.2015), District Forest Office, Tiruvarur and PCCF, Forest Department of Tamil Nadu, India for providing permission to collect samples, Department of Biotechnology, Pondicherry University for providing the laboratory facilities and BD thanks Ministry of Earth Sciences, Government of India for providing a fellowship. EJ was supported under the Distinguished Scientist Fellowship Program (DSFP), King Saud University, Saudi Arabia. Dr. Shaun Pennycook was acknowledged for the help in Latin diagnosis for the fungal names. MD offers her deepest gratitude to Dr. Putarak Chomnunti, Dr. Saranyaphat Boonmee, Nuwanthika Wijesinghe, Rashika Brahmanage, and Milan Samarakoon for their helpful comments and advice.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00840/full#supplementary-material
Abdel-Wahab, M. A., and Jones, E. B. G. (2000). Three new marine ascomycetes from driftwood in Australia sand dunes. Mycoscience 41, 379–388. doi: 10.1007/BF02463951
Barr, M. E. (1990). Prodromus to nonlichenized, pyrenomycetous members of class Hymenoascomycetes. Mycotaxon 39, 43–184.
Beimforde, C., Feldberg, K., Nylinder, S., Rikkinen, J., Tuovila, H., Dörfelt, H., et al. (2014). Estimating the phanerozoic history of the ascomycota lineages: combining fossil and molecular data. Mol. Phylogenet. Evol. 78, 386–398. doi: 10.1016/j.ympev.2014.04.024
Berkeley, M. J., and Broome, C. E. (1865). Notices of British fungi (1038–1062). Ann. Mag. Nat. Hist. 15, 400–404. doi: 10.1080/00222936508681827
Boonyuen, N., Chuaseeharonnachai, C., Suetrong, S., Sri-Indrasutdhi, V., Sivichai, S., Jones, E. G., et al. (2011). Savoryellales (Hypocreomycetideae, Sordariomycetes): a novel lineage of aquatic ascomycetes inferred from multiple-gene phylogenies of the genera Ascotaiwania, Ascothailandia and Savoryella. Mycologia 103, 1351–1350. doi: 10.3852/11-102
Bussaban, B., Lumyong, S., Lumyong, P., McKenzie, E. H. C., and Hyde, K. D. (2001). A synopsis of the genus Berkleasmium with two new species and new records of Canalisporium caribense from Zingiberaceae in Thailand. Fungal Divers. 8, 73–85.
Cai, L., Hyde, K. D., and Tsui, C. K. M. (2006). Genera of freshwater fungi. Fungal Divers. Res. Ser. 18, 1–261.
Chang, H. S. (2001). Trichocladium anamorph of Ascotaiwania hsilio and monodictys-like anamorphic states of Ascotaiwania lignicola. Fungal Sci. 16, 35–38.
Chang, H. S., Hsieh, S. Y., and Jones, E. B. G. (1998). New freshwater species of Ascotaiwania and Savoryella from Taiwan. Mycol. Res. 102, 709–718. doi: 10.1017/s0953756297005637
Constantinescu, O., and Samson, R. A. (1982). Triadelphia, a Pleomorphic Genus of Hyphomycetes [Fungi, New Taxa]. Ithaca, NY: Mycotaxon.
Crous, P. W., Gams, W., Stalpers, J. A., Robert, V., and Stegehuis, G. (2004). MycoBank: an online initiative to launch mycology into the 21st century. Stud. Mycol. 50, 19–22.
Cruickshank, R. D., and Ko, K. (2003). Geology of an amber locality in the Hukawng Valley, Northern Myanmar. J. Asian Earth Sci. 21, 441–455. doi: 10.1016/S1367-9120(02)00044-5
Dulymamode, R., Cannon, P. F., Hyde, K. D., and Peerally, A. (2001). Four new Ascomycete species from endemic Pandanus of Mauritius. Fungal Divers. 8, 87–96.
Edathodu, J., Al-Abdely, H. M., AlThawadi, S., and Wickes, B. L. (2013). Invasive fungal infection due to Triadelphia pulvinata in a patient with acute myeloid leukemia. J. Clin. Microbiol. 51, 3426–3429. doi: 10.1128/JCM.00457-13
Eriksson, O. E., and Hawksworth, D. L. (1986). An alphabetical list of the generic names of ascomycetes. Syst. Ascomycetum 5, 3–111.
Eriksson, O. E., and Hawksworth, D. L. (1987). Notes on ascomycete systematics, Nos. 225–463. Syst. Ascomycetum 6, 111–165.
Fallah, P. M., Crane, J. L., and Shearer, C. A. (1999). Freshwater ascomycetes: two new species of Ascotaiwania from North America. Can. J. Bot. 77, 87–92. doi: 10.1139/b98-202
Ferrer, A., and Shearer, C. A. (2005). New records and a new species of Canalisporium from aquatic habitats in Panama. Mycotaxon 93, 179–188.
Fisher, P. J. (1977). Ecological Studies on Aero-aquatic Hyphomycetes. Ph.D. Thesis, University of Exeter, Exeter.
Goh, T. K., Ho, W. H., Hyde, K. D., Whitton, S. R., and Umali, T. E. (1998). New records and species of Canalisporium (Hyphomycetes), with a revision of the genus. Can. J. Bot. 76, 142–152. doi: 10.1139/b97-164
Goh, T. K., and Hyde, K. D. (1999). Fungi on submerged wood and bamboo in the Plover Cove Reservoir, Hong Kong. Fungal Divers. 3, 57–85.
Goos, R. D., Abdullah, S. K., Fisher, P. J., and Webster, J. (1986). The anamorph genus Helicoon. Trans. Br. Mycol. Soc. 87, 115–122. doi: 10.1016/s0007-1536(86)80010-9
Hall, T. A. (1999). “BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT,” in Proceedings of the Nucleic Acids Symposium Series 41:95–98 (London: Information Retrieval Ltd.), c1979–c2000.
Hawksworth, D. L. (2011). A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. IMA Fungus 2:155. doi: 10.5598/imafungus.2011.02.02.06
Hernández-Restrepo, M., Gené, J., Castañeda-Ruiz, R. F., Mena-Portales, J., Crous, P. W., and Guarro, J. (2017). Phylogeny of saprobic microfungi from Southern Europe. Stud. Mycol. 86, 53–97. doi: 10.1016/j.simyco.2017.05.002
Hernández-Restrepo, M., Mena-Portales, J., Gené, J., Cano, J., and Guaarro, J. (2013). New Bactrodesmiastrum and Bactrodesmium from decaying wood in Spain. Mycologia 105, 172–180. doi: 10.3852/12-004
Ho, W. H., Hyde, K. D., and Hodgkiss, I. J. (1997). Ascomycetes from tropical freshwater habitats: the genus Savoryella, with two new species. Mycol. Res. 101, 803–809. doi: 10.1017/s0953756296003310
Ho, W. H., Yanna, Hyde, K. D., and Hodgkiss, I. J. (2002). Seasonality and sequential occurrence of fungi on wood submerged in Tai Po Kau Forest Stream, Hong Kong. In: Fungal Succession (eds. K.D. Hyde and E.B.G. Jones). Fungal Divers. 10, 21–43.
Holubová-Jechová, V. (1972). Lignicolous hyphomycetes from Czechoslovakia 2, Bactrodesmium. Folia Geobot. 7, 407–418. doi: 10.1007/BF02854768
Holubová-Jechová, V., and Sierra, A. M. (1984). Studies on hyphomycetes from Cuba II. Hyphomycetes from the Isla de la Juventudeská. Česká Mykol. 38, 96–120.
Hongsanan, S., Maharachchikumbura, S. S., Hyde, K. D., Samarakoon, M. C., Jeewon, R., Zhao, Q., et al. (2017). An updated phylogeny of sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers. 84, 25–41. doi: 10.1007/s13225-017-0384-2
Huelsenbeck, J. P., and Ronquist, F. (2001). MRBAYES: bayesian inference of phylogeny. Bioinformatics 17, 754–755. doi: 10.1093/bioinformatics/17.8.754
Hughes, S. J. (1958). Revisiones hyphomycetum aliquot cum appendice de nominibus rejiciendis. Can. J. Bot. 36, 727–836. doi: 10.1139/b58-067
Hughes, S. J. (1978). New Zealand fungi 25. Miscellaneous species. N. Z. J. Bot. 16, 311–370. doi: 10.1080/0028825x.1978.10425143
Hyde, K. D. (1993). Tropical Australian freshwater fungi. V. Bombardia sp., Jahnula australiensis sp. nov., Savoryella lignicola and S. aquatica sp. nov. Aust. Syst. Bot. 5, 161–167.
Hyde, K. D. (1994). The genus Savoryella from freshwater habitats, including S. grandispora sp. nov. Mycoscience 35, 59–61. doi: 10.1007/BF02268529
Hyde, K. D., and Goh, T. K. (1999). Fungi on submerged wood from the River Coln, England. Mycol. Res. 103, 1561–1574. doi: 10.1017/s0953756299008989
Hyde, K. D., Maharachchikumbura, S. S., Hongsanan, S., Samarakoon, M. C., Lücking, R., Pem, D., et al. (2017). The ranking of fungi: a tribute to David L. Hawksworth on his 70th birthday. Fungal Divers. 84, 1–23. doi: 10.1007/s13225-017-0383-3
Index Fungorum (2017). Retrieved 24 June 2018, from http://wwwindexfungorumorg/Names/Namesasp.
Jaklitsch, W. M., and Réblová, M. (2015). Savoryellaceae Jaklitsch & Réblová. Index Fungorum 209:1. doi: 10.1007/978-3-319-23534-9_1
Jeewon, R., and Hyde, K. D. (2016). Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 7, 1669–1677. doi: 10.5943/mycosphere/7/11/4
Jones, E. B. G., and Eaton, R. A. (1969). Savoryella lignicola gen. et sp. nov. from water cooling towers. Trans. Br. Mycol. Soc. 52, 161–174.
Jones, E. B. G., and Hyde, K. D. (1992). Taxonomic studies on Savoryella Jones et Eaton (Ascomycotina). Bot Mar. 35, 83–92. doi: 10.1515/botm.1992.35.2.83
Jones, E. B. G., Sakayaroj, J., Suetrong, S., Somrithipol, S., and Pang, K. L. (2009). Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers. 35, 1–187.
Jones, E. B. G., Suetrong, S., Sakayaroj, J., and Bahkali, A. H. (2015). Classification of marine ascomycota, basidiomycota, blastocladiomycota and chytridiomycota. Fungal Divers. 73, 1–72. doi: 10.1007/s13225-015-0339-4
Jones, E. B. G., To-anun, C., Suetrong, S., and Boonyuen, N. (2016). Mycosphere Essays 12. Progress in the classification of the water cooling tower ascomycete Savoryella and a tribute to John Savory: a review. Mycosphere 7, 570–581. doi: 10.5943/mycosphere/7/5/4
Kirk, P. M. (1983). New or interesting microfungi X. Hyphomycetes on Laurus nobilis leaf litter. Mycotaxon 18, 259–298.
Kirk, P. M. (1985). New or interesting microfungi. XIV. Dematiaceous hyphomycetes from Mt. Kenya. Mycotaxon 23, 305–352.
Kohlmeyer, J. (1986). Taxonomic Studies of the Marine Ascomycotina. The Biology of Marine Fungi. Cambridge: Cambridge University Press, 234–257.
Kohlmeyer, J., and Kohlmeyer, E. (1979). Marine mycology. The Higher Fungi. New York, NY: Academic Press.
Koukol, O., and Kolárová, Z. (2010). Bactrodesmium gabretae (anamorphic Helotiales), a new sporodochial species described from spruce needles. Nova Hedwigia 91, 243–248. doi: 10.1127/0029-5035/2010/0091-0243
Linder, D. H. (1929). A monograph of the helicosporous Fungi Imperfecti. Ann. Missouri Bot. Gard. 16, 227–388.
Liu, J. K., Hyde, K. D., Jeewon, R., Phillips, A. J., Maharachchikumbura, S. S., Ryberg, M., et al. (2017). Ranking higher taxa using divergence times: a case study in Dothideomycetes. Fungal Divers. 84, 75–99. doi: 10.1007/s13225-017-0385-1
Luo, J., Yin, J., Cai, L., Zhang, K., and Hyde, K. D. (2004). Freshwater fungi in Lake Dianchi, a heavily polluted lake in Yunnan, China. Fungal Divers. 16, 93–112.
Maharachchikumbura, S. S., Hyde, K. D., Jones, E. G., McKenzie, E. H. C., Bhat, J. D., Dayarathne, M. C., et al. (2016). Families of Sordariomycetes. Fungal Divers. 79, 1–317. doi: 10.1007/s13225-016-0369-6
Manimohan, P., Amritha, M., and Sairabanu, N. K. (2011). A comparison of diversity of marine fungi on three co-habiting mangrove plants. Mycosphere 2, 533–538.
Miller, M. A., Pfeiffer, W., and Schwartz, T. (2010). “Creating the CIPRES science gateway for inference of large phylogenetic tree,” in Proceedings of the Gateway Computing Environments Workshop (GCE)., November 14, 2010, New Orleans, LA, 1–8.
Nawawi, A., and Kuthubutheen, A. J. (1989). Canalisporium, a new genus of lignicolous hyphomycetes from Malaysia. Mycotaxon 34, 475–487. doi: 10.1139/b97-164
Nylander, J. A. A. (2004). MrModel test v. 2. Program. Uppsala: Evolutionary Biology Centre, Uppsala University.
Pérez-Ortega, S., Garrido-Benavent, I., Grube, M., Olmo, R., and de los Ríos, A. (2016). Hidden diversity of marine borderline lichens and a new order of fungi: Collemopsidiales (Dothideomyceta). Fungal Divers. 80, 285–300. doi: 10.1007/s13225-016-0361-1
Pfister, P. (1997). Castor, pollux and life histories of fungi. Mycologia 89, 1–23. doi: 10.2307/3761168
Pinruan, U., Jones, E. B. G., and Hyde, K. D. (2002). Aquatic fungi from peat swamp palms: Jahnula appendiculata sp. nov. Sydowia 54, 242–247.
Quandt, C. A., Kepler, R. M., Gams, W., and Araújo, J. P. M. (2014). Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus 5, 121–134. doi: 10.5598/imafungus.2014.05.01.1
Raja, H. A., and Shearer, C. A. (2007). Freshwater ascomycetes: Aliquandostipite minuta (Jahnulales, Dothideomycetes), a new species from Florida. Mycoscience 48, 395–398. doi: 10.1007/S10267-007-0375-3
Rambaut, A. (2012). Fig. Tree. Tree Figure Drawing Tool, v. 1.4.0. Available at: http://tree.bio.ed.ac.uk/software/figtree/ (accessed August, 2018).
Ranghoo, V. M., and Hyde, K. D. (1998). Ascomycetes from freshwater habitats: Ascolacicola aquatica gen. et sp. nov. and a new species of Ascotaiwania from wood submerged in a reservoir in Hong Kong. Mycologia 90, 1055–1062. doi: 10.2307/3761280
Ranghoo, V. M., Hyde, K. D., Liew, E. C. Y., and Spatafora, J. W. (1999). Family placement of Ascotaiwania and Ascolacicola based on DNA sequences from the large subunit rRNA gene. Fungal Divers. 2, 159–168.
Read, S. J., Hsieh, S. Y., Jones, E. B. G., Moss, S. T., and Chang, H. S. (1992). Paraliomyces lentiferus: an ultrastructural study of a little- known marine ascomycete. Can. J. Bot. 70, 2223–2232. doi: 10.1139/b93-028
Read, S. J., Jones, E. B. G., and Moss, S. T. (1993). Taxonomic studies of marine Ascomycotina: ultrastructure of the asci, ascospores and appendages of Savoryella species. Can. J. Bot. 71, 273–283. doi: 10.1139/b93-028
Réblová, M., Miller, A. N., Rossman, A. Y., and Seifert, K. A. (2016a). Recommendations for competing sexual-asexually typified generic names in Sordariomycetes (except Diaporthales, Hypocreales, and Magnaporthales). IMA Fungus 7, 131–153. doi: 10.5598/imafungus.2016.07.01.08
Réblová, M., Seifert, K. A., Fournier, J., and Štěpánek, V. (2016b). Newly recognized lineages of perithecial ascomycetes: the new orders Conioscyphales and Pleurotheciales. Persoonia 37, 57–81. doi: 10.3767/003158516X689819
Réblová, M., Seifert, K. A., Fournier, J., and Štěpánek, V. (2012). Phylogenetic classification of Pleurothecium and Pleurotheciella gen. nov. and its dactylaria-like anamorph (Sordariomycetes) based on nuclear ribosomal and protein-coding genes. Mycologia 104, 1299–1314. doi: 10.3852/12-035
Sadowski, E. M., Beimforde, C., Gube, M., and Rikkinen, J. (2012). The anamorphic genus Monotosporella (Ascomycota) from Eocene amber and from modern Agathis resin. Fungal Biol. 116, 1099–1110. doi: 10.1016/j.funbio.2012.08.003
Sadowski, E. M., Schmidt, A. R., Rudall, P. J., Simpson, D. A., Gröhn, C., Wunderlich, J., et al. (2016) Graminids from Eocene Baltic amber. Rev. Palaeobot. Palynol. 233, 161–168. doi: 10.1016/j.revpalbo.2016.02.005
Sakayaroj, J., Pang, K. L., and Jones, E. B. G. (2011). Multi-gene phylogeny of the Halosphaeriaceae: its ordinal status, relationships between genera and morphological character evolution. Fungal Divers. 46, 87–109. doi: 10.1007/s13225-010-0072-y
Samarakoon, M. C., Hyde, K. D., Promputtha, I., and Hongsanan, S. (2016). Evolution of Xylariomycetidae (Ascomycota: Sordariomycetes). Mycosphere 7, 1746–1761. doi: 10.5943/mycosphere/7/11/9
Seifert, K. A., and Gams, W. (2011). The genera of Hyphomycetes-2011 update. Persoonia Mol. Phylogeny Evol. Fungi 27, 119–129. doi: 10.3767/003158511x617435
Shearer, C. A., and Crane, J. L. (1971). Fungi of the chesapeake bay and its tributaries. I. Patuxent River. Mycologia 63, 237–260. doi: 10.2307/3757758
Sivanesan, A., and Chang, H. S. (1992). Ascotaiwania, a new amphisphaeriaceous ascomycete genus on wood from Taiwan. Mycol. Res. 96, 481–484. doi: 10.1016/S0953-7562(09)81094-0
Sivichai, S., Hywel-Jones, N. L., and Jones, E. B. G. (1998). Lignicolous freshwater Ascomycota from Thailand 1. Ascotaiwania sawada and its anamorph state Monotosporella. Mycoscience 39, 307–311. doi: 10.1007/BF02464013
Spatafora, J., Volkmann-Kohlmeyer, B., and Kohlmeyer, J. (1998). Independent terrestrial origins of the Halosphaeriales (marine Ascomycota). Am. J. Bot. 85, 1569–1580. doi: 10.2307/2446483
Sri-indrasutdhi, V., Boonyuen, N., Suetrong, S., Chuaseeharonnachai, C., Sivichai, S., and Jones, E. B. G. (2010). Wood inhabiting freshwater fungi from Thailand: Ascothailandia gen. et sp. nov., Canalisporium sp. nov. with a key to Canalisporium species (Sordariomycetes Ascomycota). Mycoscience 51, 411–420. doi: 10.1007/s10267-010-0055-6
Stamatakis, A., Hoover, P., and Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 57, 758–771. doi: 10.7872/crym/v36.iss3.2015.319
Suetrong, S., Klaysuban, A., Sakayaroj, J., and Preedanon, S. (2015). Tirisporellaceae, a new family in the order Diaporthales (Sordariomycetes, Ascomycota) Cryptogam. Mycology 36, 319–330. doi: 10.3114/sim.2007.57.01
Sung, G. H., Hywel-Jones, N. L., Sung, J. M., and Luangsaard, J. J. (2007). Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 57, 5–59. doi: 10.1016/j.simyco.2015.10.002
Tanaka, K., Hirayama, K., Yonezawa, H., and Sato, G. (2015). Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud. Mycol. 82, 75–136. doi: 10.5598/imafungus.2011.02.02.01
Tian, Q., Liu, J. K., Hyde, K. D., Wanasinghe, D. N., Boonmee, S., Jayasiri, S. C., et al. (2015). Phylogenetic relationships and morphological reappraisal of Melanommataceae (Pleosporales). Fungal Divers. 74, 267–324. doi: 10.1007/s13225-015-0350-9
Tibpromma, S., Hyde, K. D., McKenzie, E. H., Bhat, D. J., Phillips, A. J., Wanasinghe, D. N., et al. (2018). Fungal diversity notes 840–928: micro-fungi associated with Pandanaceae. Fungal Divers. 93, 1–160. doi: 10.1007/s13225-018-0408-6
Tsui, C. K. M., and Berbee, M. L. (2006). Phylogenetic relationships and convergence of helicosporous fungi inferred from ribosomal DNA sequences. Mol. Phylogenet. Evol. 39, 587–597. doi: 10.1016/j.ympev.2006.01.025
Tsui, C. K. M., and Hyde, K. D. (2003). Freshwater mycology. Fungal Divers. Res. Ser. 10, 1–350. doi: 10.2307/1468086
Tsui, C. K. M., Hyde, K. D., and Hodgkiss, I. J. (2001). Longitudinal and temporal distribution of freshwater ascomycetes and dematiaceous hyphomycetes on submerged wood in the Lam Tsuen River, Hong Kong. J. N. Am. Benthol. Soc. 20, 533–549. doi: 10.2307/1468086
Vijaykrishna, D., Jeewon, R., and Hyde, K. D. (2006). Molecular taxonomy, origins and evolution of freshwater ascomycetes. Fungal Divers. 23, 351–390.
Walker, J. D., Geissman, J. W., Bowring, S. A., and Babcock, L. E. (2012). Data from: geologic Time Scale v. 4.0. Geol. Soc. Am. Bull. 125, 259–272. doi: 10.1130/2012.CTS004R3C
Wijayawardene, D. N. N., McKenzie, E. H. C., and Hyde, K. D. (2012). Towards incorporating anamorphic fungi in a natural classification – Checklist and notes for 2011. Mycosphere 3, 157–228. doi: 10.5943/mycosphere/3/2/5
Wijayawardene, N. N., Hyde, K. D., Lumbsch, H. T., Liu, J. K., Maharachchikumbura, S. S., Ekanayaka, A. H., et al. (2018). Outline of Ascomycota- 2017. Fungal Divers. 88, 167–263. doi: 10.1007/s13225-018-0394-8
Wijayawardene, N. N., Hyde, K. D., Rajeshkumar, K. C., Hawksworth, D. L., Madrid, H., Kirk, P. M., et al. (2017). Notes for genera: ascomycota. Fungal Divers. 86, 1–594. doi: 10.1007/s13225-017-0386-0
Wong, M. K. M., and Hyde, K. D. (2001). Fungi on grasses: new species of Ascotaiwania, Diaporthe and Oxydothis (Ascomycetes). Cryptogam. Mycol. 22, 19–28. doi: 10.1016/S0181-1584(01)01055-7
Yang, J., Maharachchikumbura, S. S. N., Bhat, D. J., and Hyde, K. D. (2016). Fuscosporellales, a new order of aquatic and terrestrial hypocreomycetidae (Sordariomycetes). Cryptogam. Mycol. 37, 449–475. doi: 10.7872/crym/v37.iss4.2016.449
Yanna, H., and Hyde, K. D. (2002). New saprobic fungi on fronds of palms from northern Queensland, Australia. Aust. Syst. Bot. 15, 755–764.
Zhang, H., Zhou, D. Q., and Wang, M. (2014). Canalisporium in freshwater habitats. Adv. Mater. Res. 889, 1593–1599. doi: 10.4028/www.scientific.net/AMR.889-890.1593
Zhao, G., Yu, P., and Liu, X. (2012). Cancellidium and Canalisporium (Hyphomycetes) from China. Nova Hedwigia 96, 221–236. doi: 10.1127/0029-5035/2012/0048
Keywords: freshwater, marine, morphology, phylogeny, Savoryellomycetidae, taxonomy
Citation: Dayarathne MC, Maharachchikumbura SSN, Jones EBG, Dong W, Devadatha B, Yang J, Ekanayaka AH, De Silva W, Sarma VV, Al-Sadi AM, Khongphinitbunjong K, Hyde KD and Zhao RL (2019) Phylogenetic Revision of Savoryellaceae and Evidence for Its Ranking as a Subclass. Front. Microbiol. 10:840. doi: 10.3389/fmicb.2019.00840
Received: 14 January 2019; Accepted: 02 April 2019;
Published: 07 May 2019.
Edited by:
Baokai Cui, Beijing Forestry University, ChinaReviewed by:
Somayeh Dolatabadi, Westerdijk Fungal Biodiversity Institute, NetherlandsCopyright © 2019 Dayarathne, Maharachchikumbura, Jones, Dong, Devadatha, Yang, Ekanayaka, De Silva, Sarma, Al-Sadi, Khongphinitbunjong, Hyde and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Lin Zhao, emhhb3J1aWxpbkBnbWFpbC5jb20=; emhhb3JsQGltLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.