
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 21 December 2018
Sec. Infectious Agents and Disease
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.03131
This article is part of the Research TopicNovel Approaches to Rapid Diagnosis and Treatment Monitoring of Active TuberculosisView all 7 articles
 Yuan-yong Liu1,2†
Yuan-yong Liu1,2† Wei Sha3†
Wei Sha3† Shiqiang Xu2
Shiqiang Xu2 Xu-wei Gui3
Xu-wei Gui3 Liliang Xia2
Liliang Xia2 Ping Ji2
Ping Ji2 Shujun Wang2
Shujun Wang2 Guo-ping Zhao4
Guo-ping Zhao4 Xiao Zhang1*
Xiao Zhang1* Yingying Chen2*
Yingying Chen2* Ying Wang2,4
Ying Wang2,4Identification of HLA-restricted peptides derived from mycobacterial antigens that are endowed with high affinity and strong antigenicity is not only of interest in tuberculosis (TB) diagnostics and treatment efficacy evaluation, but might also provide potential candidates for the development of therapeutic vaccines against drug-resistant TB. Our previous work demonstrated that lipoprotein Z (LppZ) displayed high immunogenicity and antigenicity in active TB patients. In the present study, ten HLA-A2-restricted LppZ peptides (LppZp1-10) were predicted by bioinformatics, among which LppZp7 and LppZp10 were verified to possess high affinity to HLA-A2 molecules using T2 cell-based affinity binding assay. Moreover, results from ELISpot assay showed that both LppZp7 and LppZp10 peptides were able to induce more IFN-γ producing cells upon ex vivo stimulation of PBMC from HLA-A2+ active TB (ATB) patients as compared to those from healthy controls (HCs). Also, the numbers of LppZp7 and LppZp10-specific IFN-γ producing cells exhibited positive correlations with those of ESAT-6 peptide (E6p) or CFP-10 peptide (C10p) in ATB. Interestingly, stimulation with LppZp7/p10 mixture was able to induce higher intracellular expression of IFN-γ and IL-2 cytokines in CD8+ and CD4+ T cells from ATB as compared to HC, associated with lower expression of TNF-α in both CD8+ and CD4+ T cells. Taken together, HLA-A2-restricted LppZp7 and LppZp10 peptides display high immunoreactivity in HLA-matched ATB patients demonstrated by high responsiveness in both CD8+ and CD4+ T cells. With the ability to induce strong antigen-specific cellular responses, LppZp7 and LppZp10 are of potential value for the future applications in the prevention and control of TB.
Tuberculosis (TB) is the leading cause of death from the single infectious agent, Mycobacterium tuberculosis (M.tb). It is still one of the most severe global health problems. In 2016, there were an estimated 10.4 million newly onset TB patients and 1.04 million death (WHO, 2017). The number of new TB cases in China was nearly 0.9 million, ranking fourth worldwide after India, Indonesia, and Nigeria (WHO, 2017). The complexity of its pathogenesis and pathology makes the prevention and control of TB more challenging. For instance, accurate and rapid diagnosis for TB is still insufficient. The rapid increase of multiple drug-resistant (MDR) and extensively drug-resistant (XDR) TB in clinic leads to the longer duration of the treatment and the use of more expensive drugs whereas less efficacy, increasing the economic burden as well as the morbidity of global TB (Basak et al., 2016). Bacille Calmette-Guérin (BCG) is the only vaccine available for the prevention of TB, which is widely used in high-incidence regions. But its protection is demonstrated to be limited between 5 and 10 years (Cernuschi et al., 2018). Therefore, to develop new vaccines either for the prevention of TB or the immunotherapy of drug-resistant TB in the future is worthy of exploration.
T lymphocytes are considered to be the main cellular component to exert protection against M.tb. Unlike macrophages possessing phagocytosis ability through the direct clearance of bacilli (Sartain et al., 2006), T cells eliminate M.tb largely by lysing infected cells (Flynn and Chan, 2001). Both CD8+ and CD4+ T cells are involved in the immune protection offered by TB vaccine (Li et al., 2009). They initiate cellular immune responses through recognizing antigenic peptides presented by HLA class I and II molecules on host antigen presenting cells, respectively. Compared to the whole antigens, antigenic peptides have more advantages in the preparation and commercial applications, such as high purity in synthesis and containing less harmful antigenic components (Mollica et al., 2013). Early secretary antigenic target protein 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) are known as two immunodominant antigens derived from M.tb. The artificially synthesized antigenic peptide pools are used in M.tb antigen-specific IGRA assay, including T.SPOT®.TB and QFT® for the immune diagnosis of TB, which can avoid the interference of endogenous endotoxin existing in the purified proteins. However, these two antigens are still not good enough in neither TB diagnosis (Hemmati et al., 2016) nor vaccine development (Jeon et al., 2013). Gao et al. (2009) has recently reported that HLA-restricted mycobacterial peptides-based DNA vaccine could potentially trigger increased Th1 immune response in a mouse model. A CFP21-derived HLA-A2-restricted epitope could enhance the activity of cytotoxic T lymphocytes (Lv et al., 2010). However, only fusion protein containing CFP21 could enhance the protection against M.tb infection (Wang et al., 2011). Hence, to screen new mycobacterial antigenic peptides with high affinity and strong antigenicity is still in urgent need. Among all HLA-A alleles, HLA-A2 is the most common subtype in Asian population with an estimated frequency of more than 40% (Mehra et al., 2001). Therefore, identification of HLA-A2-restricted epitopes derived from M.tb proteins is of large application in the future.
Mycobacterial lipoprotein Z (LppZ), encoded by rv3006, is a newly identified antigens with good antigenicity in active TB patients (Xiao et al., 2016). It is a conserved lipoprotein with enzymes and metabolic activities in various mycobacterial strains (Sutcliffe and Harrington, 2004), including M.bovis and M.tb. Being one of the most immunogenic proteins with high antibody-to-protein ratio among culture filtrate proteins (Malen et al., 2008), it is reported to be sero-reactive in both cavitary and non-cavitary TB patients with sero-diagnostic potential for TB (Sartain et al., 2006). Our previous work has also reported that LppZ-specific IgA levels in TB patients and latent TB infection (LTBI) individuals were dramatically higher than that in healthy controls (HCs). LppZ-specific IgA level decreased substantially along with the anti-TB treatment (Xiao et al., 2017). What’s more, its potential as a new TB vaccine candidate antigen was evaluated with enhanced immune protection against M.tb challenge in mouse models (under revision manuscript). In the present study, potential HLA-A2 epitopes derived from LppZ were predicted by bioinformatic tools. Their immunoreactivity in HLA-A2+ active TB patients were further determined. Our study thus intended to provide key evidence for the construction of peptide-based TB vaccine and diagnostic approach.
Active tuberculosis (ATB) patients were included in the study. All ATB patients were in-patients from Shanghai Pulmonary Hospital affiliated to Tongji University School of Medicine, and were confirmed based on medical history, chest radiograph (X-ray and CT), acid-fast bacilli (AFB) smear or sputum culture. All the patients were both HIV- and HBV-negative. They have signed voluntary informed consent before being enrolled in this study. Both HCs and LTBIs were from healthy blood donors undergoing annual physical examination in Ruijin Hospital (Shanghai, China). They had no medical history or disease symptoms. LTBI subjects were detected by ESAT-6 or CFP-10 induced IFN-γ releasing cells, and were defined as more than 6 SFUs. Those subjects were eliminated from this study. All individuals involved in this study were adults who had been vaccinated with BCG Shanghai strain (Shanghai Institute of Biological Products Co., Ltd., Shanghai, China) during childhood. This study was approved by the Ethical Committee of Shanghai Jiao Tong University School of Medicine.
The HLA-A2-restricted LppZ peptides were predicted on the website http://www-bimas.cit.nih.gov/molbio/hla_bind. ESAT-6 peptide mixture (E6p), CFP-10 peptide mixture (C10p) (Yang et al., 2017), HLA-A2-restricted LppZ peptides (LppZp1-10) and HLA-A2-restricted OVA66 peptide L235 (sequence FLPDHINIV) (Jin et al., 2005) were synthesized by Sangon Biotech (Shanghai, China). The peptides were purified by high performance liquid chromatography (HPLC). The purity of the peptides was more than 95%. These peptides were stored at −80°C after lyophilization in DMSO (Sigma) and diluted in endotoxin free PBS (GIBCO, New York, NY, United States) when used.
T2 cells were purchased from ATCC and were maintained in Iscove’s Modified Dulbecco’s Media (IMDM) (GIBCO) supplemented with 10% fetal bovine serum (FBS) (Millipore, Burlington, MA, United States), 100 units/mL penicillin (GIBCO) and 100 μg/mL streptomycin (GIBCO). T2 cells were harvested and washed with IMDM serum-free medium. The cell concentration was adjusted to 1 × 106 cells/mL, and were then stimulated with 10 μg/mL peptides in the presence of 3 μg/mL beta 2 microglobulin (β2-M) (Sigma-Aldrich, St. Louis, MO, United States) and incubated in 5% CO2 incubator for 4 h at 37°C. The expression of HLA-A2 molecules on T2 cells was determined by PE-conjugated mouse anti-human HLA-A2 (clone: BB7.2) (Abcam, United Kingdom), and cells were then incubated for 30 min in the dark at 4°C. After wash and resuspended in PBS, T2 cells were acquired by flow cytometer (BD FACSCanto II, San Jose, CA, United States). Binding ability of predicted HLA-A2-restricted LppZ peptides to T2 cells were determined based on the mean fluorescence intensity (MFI) values of HLA-A2 molecule expression. Peptide L235 was considered as a positive control, PBS (no antigenic peptide) as a negative control.
T2 cells were stimulated with HLA-A2-restricted LppZ peptides in different concentrations (0, 0.4, 2, 10 μg/mL) in the presence of β2-M for 4 h. The expression levels of HLA-A2 molecules on T2 cells were determined by flow cytometry as mentioned above. The binding affinity of LppZ-derived peptides to HLA-A2 molecules was defined by the concentration at which it displayed 50% of the maximum MFI (MFImax) of labeled HLA-A2 molecules.
For binding stability assay, T2 cells were stimulated LppZ-derived peptides at optimal concentration (10 μg/mL) in the presence of β2-M for 4 h. The MFIs of HLA-A2 molecule expression on T2 cells were detected at 0, 2, 4, and 6 h separately after 4 h stimulation. The binding stability of LppZ-derived peptides to HLA-A2 molecules was defined by the difference between MFI0hr and MFI6hr (Δ MFI = MFI0 h - MFI6 h).
Whole blood (10 mL) was collected in tubes containing ethylene diamine tetraacetic acid (EDTA). Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-hypaque density gradient centrifugation with LymphoprepTM solution (AXIS-SHIELD Poc AS, Oslo, Norway) according to the manufacturer’s recommendation. The mononuclear cell layer was carefully transferred to a new 15 mL conical tube and washed twice with RPMI 1640 medium (GIBCO) by centrifuging at 486 × g for 10 min at room temperature. PBMCs were re-suspended at a concentration of 2.5 × 106/mL in RPMI 1640 culture medium containing 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin.
Add 1.0 ml of lysing solution (BD) to a tube containing 100 μl anti-coagulant whole blood. Gently vortex tube immediately, and incubate at room temperature in the dark for 15 min. Centrifuge at 200 × g for 5 min, and carefully aspirate supernatent. Add 1.0 ml FACS buffer (PBS containing 2% FBS), centrifuge again and carefully aspirate supernatent. The pellet was resuspended in FACS buffer containing PE-conjugated mouse anti-human HLA-A2 monoclonal antibody, and incubate in the dark for 40 min at 4°C. Cells were then washed and resuspended in PBS. The samples were further analyzed by flow cytometer (BD FACSCanto II). Only HLA-A2 positive individuals were recruited in this study.
Antigen-specific IFN-γ releasing levels were determined by using an enzyme-linked immunospot (ELISpot) assay according to the manufacturer’s instructions (U-CyTech, Utrecht, Netherlands). Briefly, 96-well PVDF plates (Millipore) were coated with anti-human IFN-γ coating antibody overnight at 4°C. The wells were blocked for 1 h at 37°C. 2.5 × 105 PBMCs in 100 μL RPMI 1640 culture medium were inoculated in each well and stimulated with HLA-A2-restricted LppZ peptides, E6p or C10p pools (2 μg/mL per peptide). RPMI 1640 culture medium served as a negative control and 2.5 μg/mL phytohemagglutinin (PHA) (Sigma-Aldrich) was used as a positive control. After 20 h incubation at 37°C, the plates were incubated with biotin-labeled detection antibody at 37°C for 1 h and subsequently HRP-conjugated streptavidin working solution for another 1 h. AEC substrate solution was added to each well for 30 min in the dark at room temperature. Color development was stopped by thoroughly rinsing both sides of the PVDF membrane with demineralized water. The plates were dried in the dark at room temperature. The spots were counted by C.T.L. ImmunoSpot® S6 Ultra Analyzer (Cellular Technology Limited, Shaker Heights, OH, United States). The number of antigen-specific IFN-γ-producing cells was calculated based on spot-forming units (SFUs) per 2.5 × 105 PBMCs after deducting the background SFUs detected by the paired negative control wells.
T cell functionality was determined by intracellular cytokine staining (ICS) after antigen stimulation as described in a previous study (Xiao et al., 2016). Freshly isolated PBMCs were inoculated in the 96-well U-bottom plates (Corning, PA, United States) at 1 × 106 /well. Cells were stimulated with E6p and C10p peptides pool (E6C10p), HLA-A2-restricted LppZp7/p10 peptides pool (2 μg/mL per peptide), and incubated in 5% CO2 incubator for 20 h at 37°C. GolgiStop (BD Bioscience) was added 4 hrs before the end of ex vivo stimulation. For surface staining, cells were labeled with Pacific Blue-conjugated mouse anti-human CD3, PE-Cy7-conjugated mouse anti-human CD8, and FITC-conjugated mouse anti-human CD45RO (all from BD Bioscience) for 40 min in the dark at 4°C. After washing with FACS buffer, cells were fixed with Cytofix/Cytoperm solution and permeated with Fix/Perm working solution (BD Biosciences). Cells were then stained with PerCP-Cy5.5-conjugated mouse anti-human IFN-γ (BD Biosciences), APC-conjugated mouse anti-human IL-2 (BioLegend, San Diego, CA, United States) and PE-conjugated mouse anti-human TNF-α (eBioscience, San Diego, CA, United States) antibodies for 40 min in the dark at 4°C. After wash and resuspended in PBS, cells were acquired with flow cytometer (BD FACSCanto II) in 2 h. Data were analyzed by using FlowJo software 10 (FlowJo LLC, Treestar Inc., Ashland, OR, United States), and the doublet events were excluded by FSC-A/FSC-H gating.
All the data are shown as mean ± SEM. Statistical analyses were performed by using GraphPad Prism 7 software (Graphpad software Inc., La Jolla, CA, United States). Statistical differences were assessed by the unpaired t-test for the data with Gaussian distribution and by the Mann–Whitney test for those with non-Gaussian distribution.
HLA-A2 positive (HLA-A2+) ATB patients (N = 15) and HCs (N = 20) were involved in this study (Table 1). HLA-A2+ ATB patients (age: 44 ± 16.25; female/male: 7/8) included new onset patients (N = 11) and the retreated patients (N = 4). Ten (66.66%) were sputum smear positive, and eight (53.33%) were diagnosed with pulmonary cavitation (Table 2).
LppZ is a 38 kDa protein with 373 amino acid residues1. HLA-A2-restricted peptides were predicted on the website http://www-bimas.cit.nih.gov/molbio/hla_bind. Ten LppZ peptides were screened out (Table 3) with the predicted binding affinity score. The higher the score was, the higher the affinity to HLA-A2 molecule was theoretically.
Ten predicted HLA-A2-restricted LppZ peptides were subjected to a binding ability assay. T2 cells were stimulated with individual peptide at 10 μg/mL in vitro, and the expression level of HLA-A2 molecules on T2 cells was determined. L235 is a peptide derived from OVA66, which is already proved to display high ability to the HLA-A2 molecules on T2 cell surface (Jin et al., 2005). No peptide presented the background of HLA-A2 expression on T2 cells without peptide stimulation. The results showed that among ten predicted peptides, LppZp1, LppZp7, LppZp9, and LppZp10 stabilized HLA-A2 expression on T2 cells after 4-h incubation with MFI values similar to the positive peptide control L235 (Figure 1), whereas incubation of T2 cells with the resting predicted peptides displayed significantly lower MFI values of HLA-A2 expression than that of L235 (p = 0.0001). Therefore, LppZp1, LppZp7, LppZp9, and LppZp10 exhibit strong binding ability to HLA-A2 molecules among predicted LppZ peptides.
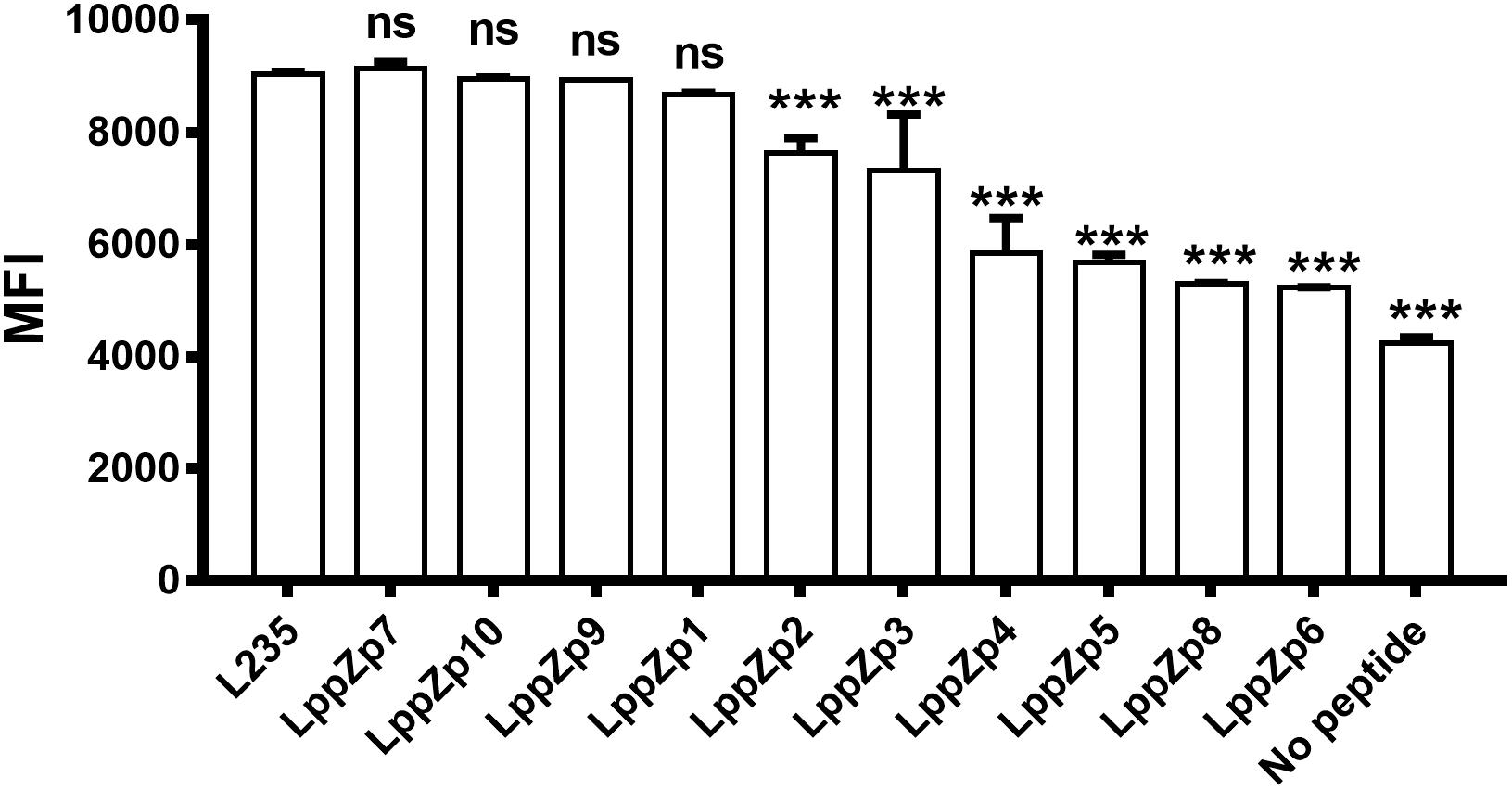
Figure 1. Binding capacity of HLA-A2-restricted LppZ peptides to T2 cell. The P-value was calculated using the Mann–Whitney test. ∗∗∗p ≤ 0.001. L235, positive control; No peptide, negative control.
In order to further determine the binding affinity of LppZp1, LppZp7, LppZp9, and LppZp10 to HLA-A2 molecules more quantitatively, peptides at different concentrations (0, 0.4, 2, and 10 μg/mL) were incubated with T2 cells in the presence of β2-M and the corresponding MFI values were detected. The results showed that along with the increase in peptide concentration, the expression levels of HLA-A2 molecules on T2 cells augmented in parallel (Figure 2). However, four LppZ peptides displayed different increasing curves. At low concentration level (2 μg/mL), LppZp9 (3025) and LppZp1 (2380) exerted higher MFI values than LppZp7 (2172) and LppZp10 (1652), whereas LppZp7 (3831) and LppZp10 (3679.5) exhibited dramatically increased affinities to HLA-A2 molecules than LppZp9 (3216) and LppZp1 (2938) at the concentration of 10 μg/mL. Both LppZp9 and LppZp1 have reached the MFI platform at 2 μg/mL. Peptide concentrations of LppZp1 (0.65 μg/mL) and LppZp9 (0.49 μg/mL) at 50% MFImax were lower LppZp10 (2.27 μg/mL) and LppZp7 (1.54 μg/mL).
Peptide binding stability is another criteria to define the binding affinity of specific peptides. The assay was performed through determining the HLA-A2 expression levels at different time points after the incubation with T2 cells. It was shown that the MFI values of HLA-A2 molecules on T2 cells displayed a dramatic decrease along with the duration of incubation times both in LppZ peptides and L235 positive peptide. Four LppZ peptides could be subgrouped into two according to the HLA-A2 expression patterns: LppZp7 and LppZp10 peptides displayed higher binding stability than L235 peptides whereas binding stability of LppZp1 and LppZp9 peptides were lower (Figure 3).
Considering the results from T2 binding affinity and stability assays, LppZp7 and LppZp10 were classified as peptides binding better to HLA-A2 molecule. We thus defined LppZp7 and LppZp10 as candidate peptides for further clinical evaluation of cellular immune responses in TB patients.
Our previous work has demonstrated that LppZ was an immune dominant antigens inducing strong immune responses in ATB patients and LTBI cohorts (Xiao et al., 2017). Whether LppZp7 and LppZp10, with strong and stable binding ability to HLA-A2 molecule defined in the above mentioned in vitro T2 binding screening assays, possess the similar ability to trigger cellular immune response was further investigated. IFN-γ ELISpot assay was adapted to define the immunoreactivity of these two LppZ-derived peptides in ATB patients as well as in HCs. As shown in Figure 4, both LppZp7 and LppZp10 have triggered more IFN-γ producing cells in ATB patients (51.93 ± 10.43 and 45.60 ± 9.10 SFUs/2.5 × 105 PBMCs, respectively) than HCs (12.15 ± 3.55 and 1.65 ± 3.48 SFUs/2.5 × 105 PBMCs, respectively) with statistical significance (p = 0.0003) (Figure 4B). Furthermore, IFN-γ secretion induced by HLA-A2-restricted peptides pool was abolished by addition of BB7.2 mAb and CD8 depletion (Supplementary Figure S1).
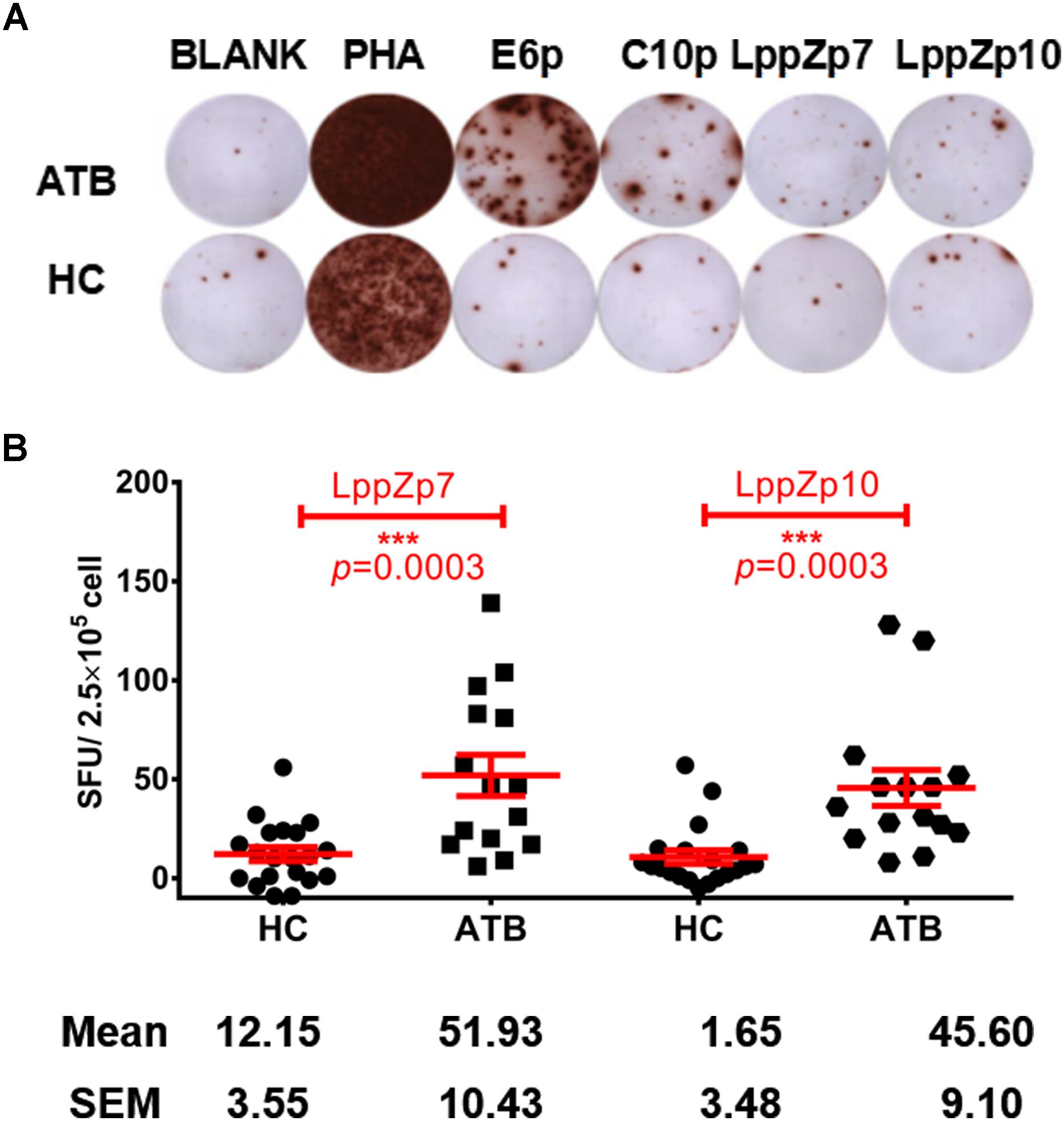
Figure 4. LppZp7 and LppZp10-specific IFN-γ releasing levels in HLA-A2+ population. (A) A representative of the IFN-γ ELISpot assay. PBMCs from one ATB patient and one HC were stimulated with ESAT-6 peptide pool (E6p), CFP-10 peptide pool (C10p), LppZp7 or LppZp10, and PHA (as the positive control). No stimulant blank was used as blank control. (B) The numbers of IFN-γ secreting cells (per 250,000 PBMCs) from ATB patients (N = 15) and HCs (N = 20) following stimulation with indicated LppZ peptides determined by ELISpot. The P-value was calculated using the Mann-Whitney test. ∗∗∗p ≤ 0.001.
ESAT-6 and CFP-10 are two well-known immune dominant antigens specifically existing in M.tb. They are subjected to immunological diagnosis for M.tb infection. We further compared the response levels between LppZp7 or LppZp10 peptides and ESAT-6 (E6p) or CFP-10 (C10p) peptide pools in ATB patients. Our results showed that the immune responses to E6p and C10p in ATB patients (102 ± 16.35 and 89.87 ± 18.77 SFUs/2.5 × 105 PBMCs, respectively) were significantly higher than those in HC group (2.556 ± 0.669 and 2.556 ± 0.747 SFUs/2.5 × 105 PBMCs, respectively) (p = 0.001) (Figure 5A). More importantly, there existed significantly positive correlations between LppZp10 and E6p/C10p (r > 0.7) (Figure 5B) as well as between LppZp7 and E6p/C10p (r > 0.8) (Figure 5C) in ATB population. Since ATB patients responded to LppZ-derived HLA-A2-restricted peptides in a consistent manner as to E6p or C10p, receiver operating characteristic curve (ROC curve) analyses were performed to further evaluate the diagnostic potential of LppZ peptides-specific IFN-γ production in ATB subjects. It was demonstrated that LppZ peptides-induced cellular responses could also discriminated ATB patients from HCs with the AUC value of 0.8317∼0.8933. The sensitivity of LppZp7 and LppZp10 were 53.33 and 46.67%, respectively while the specificity was 95.00% (Figure 6, red dot and blue square). Those peptides have exhibited higher specificity than E6p or C10p (Figure 6, black dot and square), although the sensitivity was lower. These results, however, indicate that HLA-A2-restricted LppZp7 and LppZp10 peptides display apparent immunoreactivity equivalent to E6p and C10p in ATB patients.
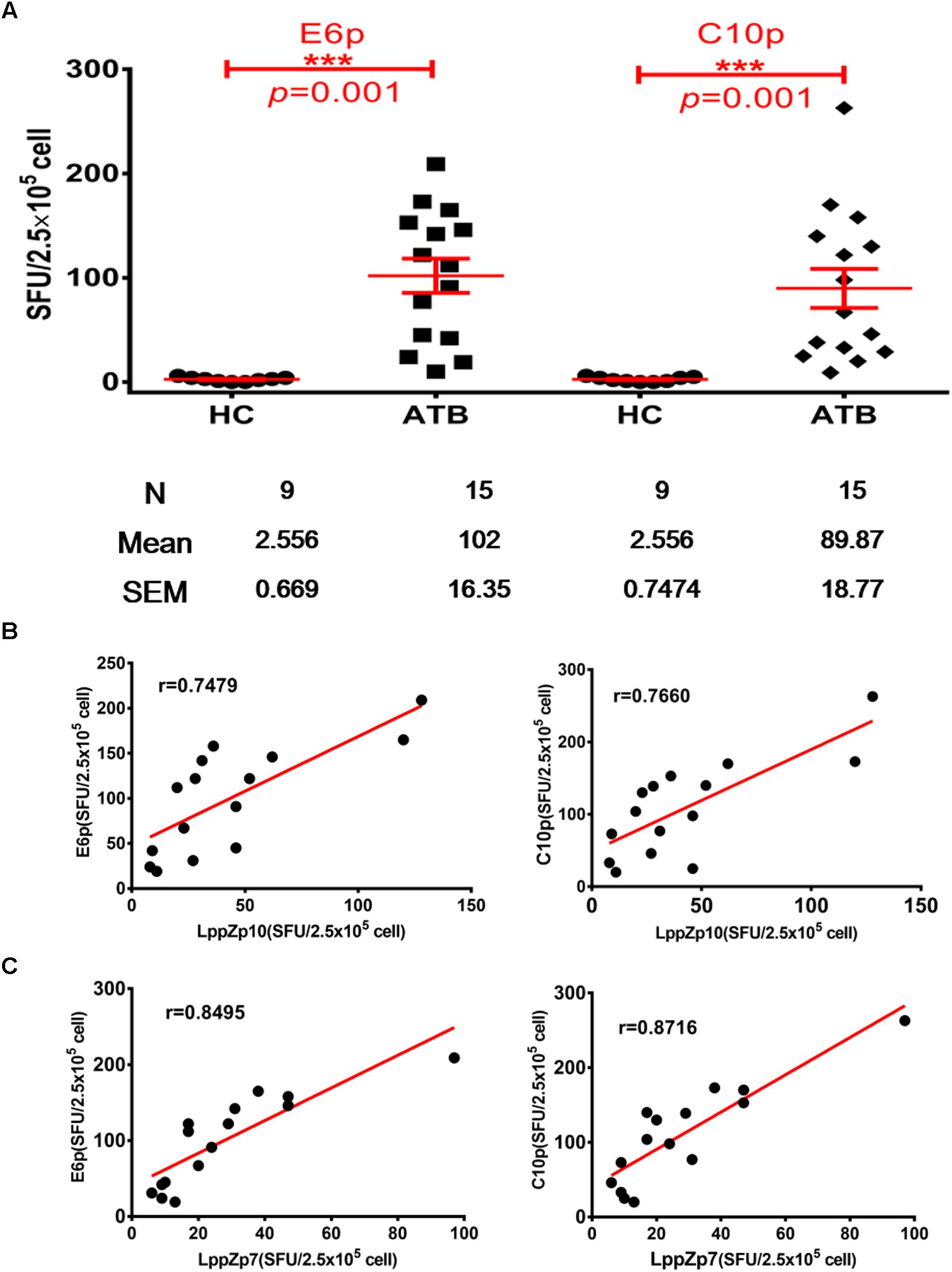
Figure 5. Correlations between HLA-A2-restricted LppZ peptide-specific and E6p or C10p specific IFN-g release. (A) Numbers of E6p- or C10p-specific IFN-γ producing cells from HLA-A2+ ATB patients (N = 15) and HC (N = 20). The P-value was calculated using the Mann–Whitney test. (B) Correlation analysis between the LppZp10-specific IFN-γ releasing level and that to E6p (left, r = 0.7479, p = 0.0039) or C10p (right, r = 0.7660, p = 0.0022) in ATB patients (N = 15). (C) Correlation analysis between the LppZp7-specific IFN-γ releasing level and that E6p (left, r = 0.8495, p = 0.0001) or C10p (right, r = 0.8716, p = 0.0001) in ATB patients (N = 15). The correlation coefficient r and the P-value were calculated using the Mann–Whitney test. ∗∗∗p ≤ 0.001.
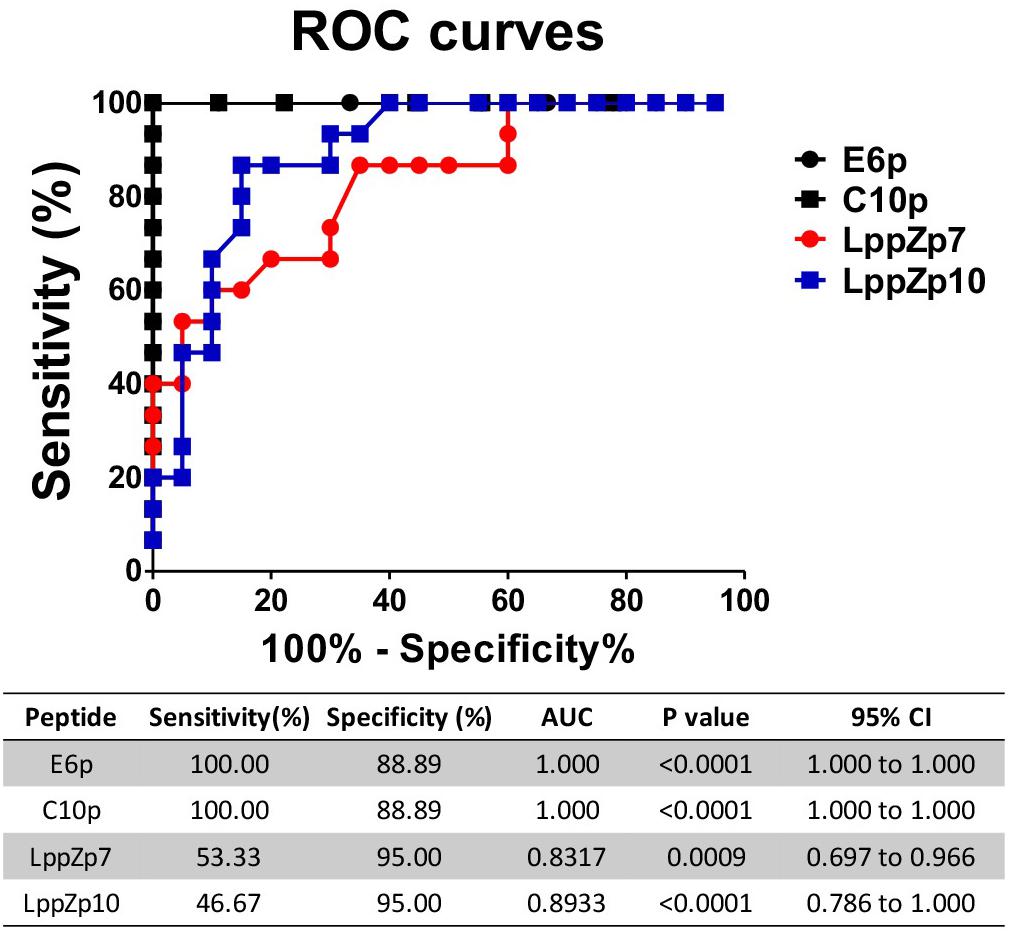
Figure 6. Receiver operating characteristic curve analyses of antigen specific IFN-γ producing cells between HC individuals and TB patients. The P-values were calculated using Mann–Whitney test. AUCs were listed in the table.
Cytokine production is one of the key signatures for T cell functionality. The ability of HLA-A2-restricted LppZ peptides to trigger CD8+ T cell response was further studied through detecting the production of Th1-type cytokines. PBMCs from HLA-A2+ ATB patients were stimulated ex vivo with peptide LppZp7, LppZp10 or the combination of LppZp7 and LppZp10 peptides (LppZp7/p10) and Th1-type cytokines were detected by ICS. RPMI1640 culture medium served as unstimulation control which defined the background cytokine release level (data not shown). The results showed that although the numbers of cytokine-secreting CD8+ T cells stimulated with two peptide alone were few (data not shown), stimulation of LppZp7/p10 induced more frequencies of IL-2+CD8+ T cells (0.197 ± 0.129%) and IFN-γ+CD8+ T cells (2.67 ± 2.39%) in ATB patients than those in HCs (IL-2+CD8+ T cells: 0.079 ± 0.058%, p = 0.0114; IFN-γ+CD8+ T cells: 0.957 ± 0.517%, p = 0.0445). However, the frequency of TNF-α secreting CD8+ T cells (0.548 ± 0.486%) in ATB group was significantly lower than that in HCs (2.498 ± 1.82%) (p = 0.0003) (Figure 7A). Nevertheless, HLA-A2-restricted LppZp7/p10 peptides were able to induce apparent CD8+ T cell immune responses in ATB patients.
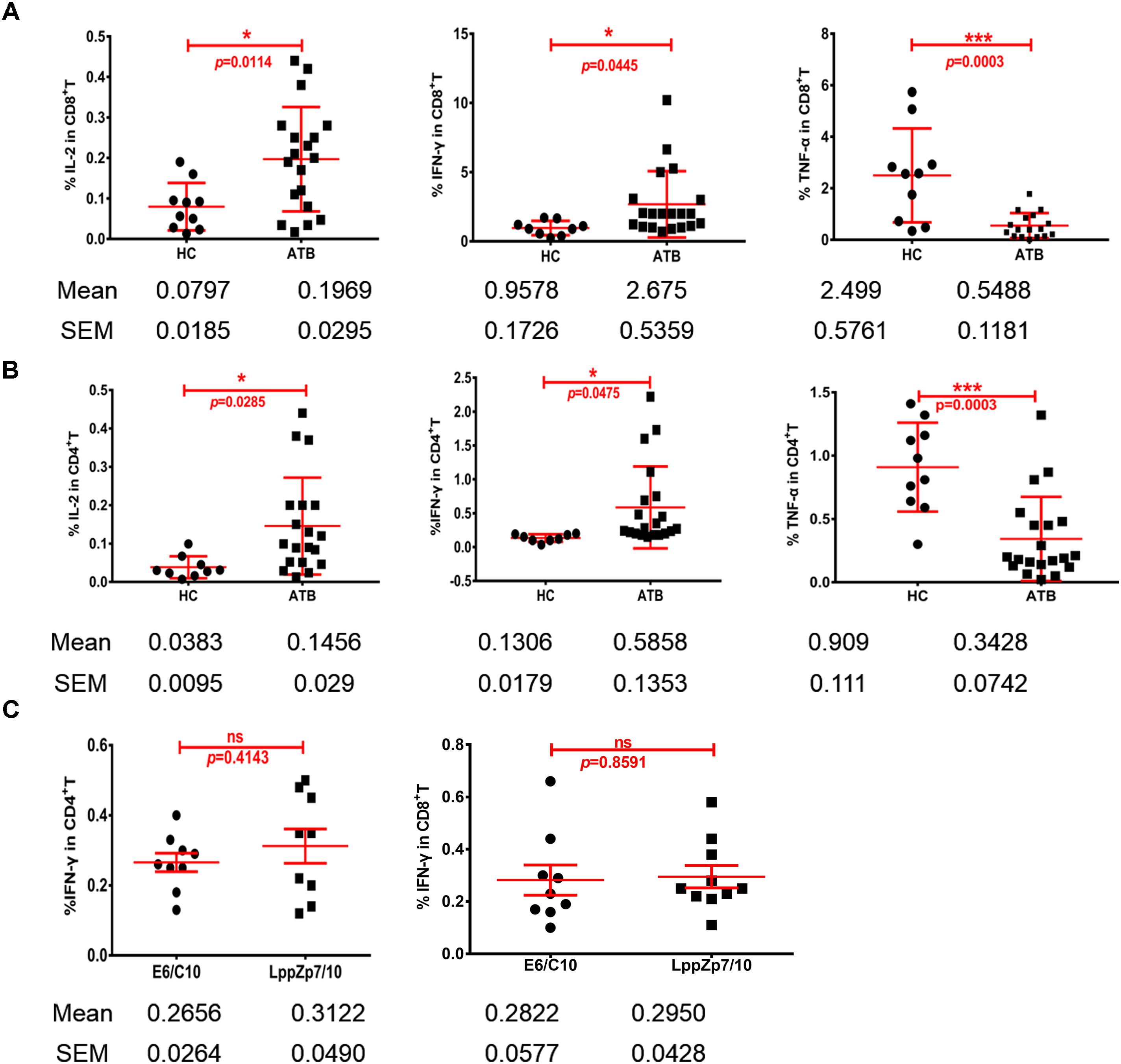
Figure 7. HLA-A2-restricted LppZ peptides induce antigen specific responses in both CD8+ and CD4+ T cells. (A) LppZp7/10-specific IFN-γ and IL-2 release in CD8+ T cells from ATB patients and HCs. (B) LppZp7/10-specific production of IFN-γ and IL-2 in CD4+ T cell from ATB patients and HCs. (C) Comparison of IFN-γ releasing levels between E6p/C10p and LppZp7/10 stimulation in peripheral CD4+ and CD8+ T cells from ATB patients. ∗p ≤ 0.05; ∗∗∗p ≤ 0.001.
More interestingly, the generation of Th1-type cytokine was also detectable in CD4+ T cells from HLA-A2+ ATB patients. The cytokine release pattern in CD4+T cells was quite similar with that in CD8+T cells. Significantly higher frequencies of IL-2+CD4+ T cells (0.145 ± 0.126%) and IFN-γ+CD4+ T cells (0.58 ± 0.60%) were observed in ATB patients when compared with those in HC group (IL-2+CD4+ T cells: 0.039 ± 0.030%, p = 0.0285; IFN-γ+CD4+ T cells: 0.134 ± 0.056%, p = 0.0475) (Figure 7B). We have also compared the secretion levels of IFN-γ in CD4+ and CD8+ T cells upon either LppZp7/10 mixture or E6p/C10p stimulation in HLA-A2+ ATB patients. There were no difference between two types of antigenic peptide stimulation (Figure 7C).
These results demonstrate that LppZp7 and LppZp10 peptides induce not only CD8+ T cell responses, but also CD4+ T cell responses.
Although M.tb specific ESAT-6 and CFP-10 are recognized as the most immunogenic antigens that are successfully used in the immunodiagnosis of TB (Lewinsohn et al., 2010), their application in vaccine development is less promising due to strong inflammatory reactions especially in patients receiving immunosuppressive agents or lymphocytopenia patients (Kobashi et al., 2008). Screening new mycobacterial antigens is thus still of great significance in both the development of TB vaccines and new immunodiagnostic tools, as well as in the potential application of immunotherapy against TB.
Th1 cytokines release CD4 T are considered as main factor against M.tb infection, while recent study showed that CD8 T cells mediated immune responses can also protect mouse from H37Rv challenge (Hu et al., 2017). HLA-A2-restricted peptides are usually considered to induce cytotoxic activity in CD8+ T lymphocytes. Furthermore, peptide vaccines are one of the most promising strategies for the prevention and treatment of tumors (Sahin et al., 2017; Tsuchiya et al., 2017). The predicted candidate antigenic peptides are further determined by experimental investigation and clinical trials against tumors (Chheda et al., 2018). LppZ is one of the proteins identified by our group with high immunogenicity and antigenicity (Xiao et al., 2016, 2017). Our unpublished data also indicated that LppZ vaccination is protective in mouse models (under revision manuscript). Our present study might provide another choice represented by LppZ peptides that are dominant mycobacterial antigens with good immunogenicity while less inflammatory responses, thus making LppZ peptides new candidate peptides in the application of immunotherapy for the refractory TB in the future.
In this study, we have successfully mapped two HLA-A2-restricted LppZ epitopes (LppZp7 and LppZp10) by bioinformatics and affinity binding assay. In fact, computational prediction highlighted LppZp1 with the highest binding score. However, results from T2 binding affinity assay indicated that LppZp1 displayed similar binding ability to HLA-A2 molecules as LppZp7 and LppZp10, but its binding stability was less than other two LppZ peptides. Multiple molecules are involved in HLA class I-restricted antigen presentation, among which transporters associated with antigen processing (TAPs) are responsible for the transportation of cytosol peptides into endoplasmic reticulum. A recent investigation on Gaussian process (GP) model has shown that the P1, P2, P3, and P9 residues are pivotal positions that dominate TAP-peptide recognition (Ren et al., 2011) while P2 and P9 residues are anchoring residues for binding to HLA class I molecules. We then evaluated the hydrophobicity and hydrophilicity of amino acid residues on these sites (Zhu et al., 2016). LppZp7 possesses hydrophobic residues (A1L2A3 and L9) at all anchor positions while LppZp1 holds 3 (K1L2 and L9) and LppZp10 (V1M2) has only 2 hydrophobic residues, respectively. Surprisingly, more hydrophilic amino acid residues were found in LppZp7 (D5 and Q7S8) and LppZp10 (G4C5 and S8) than in LppZp1 (D4). Therefore, the similarity in anchoring residues with HLA-A2 molecules is not sufficient enough for the precision prediction of peptides. While anchoring residues direct the binding of the peptides to the corresponding HLA molecules, the remaining residues might affect the molecular interaction between peptides and other molecules involved in antigen presentation, which finally determine the binding properties of peptides to HLA molecules and the presentation on cell surface. Therefore, computational prediction combined with T2 cell-based experimental screening remains the reliable strategy to determine the antigen peptide. Similar results have also been reported in other studies (Mizukoshi et al., 2011; Bai et al., 2018).
Whether LppZp7 and LppZp10 possess the ability to trigger immunoreactivity in TB patients was further investigated. LppZp7 and LppZp10-specific IGRA assays were performed in ATB patients and HCs. Consistent with the results from LppZ protein (Xiao et al., 2017), both LppZp7 and LppZp10 peptides induced more dramatic cellular immune responses in HLA-A2+ ATB patients than in HC counterparts (Figure 4). What is more, the responsiveness to both LppZp7 and LppZp10 displays significantly positive correlations to E6p/C10p in ATB group, demonstrating that the LppZ-derived peptides bear comparable immunogenicity to ESAT-6 and CFP-10, which confirmed the immunoreactivity of the whole protein in our previous study (Xiao et al., 2017).
LppZp7 and LppZp10 are HLA class I-restricted peptides. They were expected to induce cytokine production in CD8+ T cells. Although single peptide could hardly generate the secretion of Th1-type cytokines in CD8+ T cells (data not shown), ex vivo combinational treatment of LppZp7 and LppZp10 led to more IFN-γ+ and IL-2+ release CD8+ T cells in ATB patients than those in HC group. As a typical chronic infectious disease characterized as delayed type hypersensitivity responses, persistent mycobacterial antigens can stimulate T cells to release cytokines such as IFN-γ, IL-2 and TNF-α continuously. IFN-γ is one of the key signature cytokines for CD8+ T cell functionality. Mycobacterial antigen-specific IFN-γ level is considered to be closely related to M.tb infection state. Hinks et al. (2012) found that the number of specific IFN-γ cells upon the stimulation of antigens from M.tb RD1 region was related to bacilli status in patients. Studies from Park (Park and Shim, 2017) and our group (Wu et al., 2017) indicated that the number of antigen-specific IFN-γ producing cells decreased along with the anti-TB treatment and the subsequent decrease in bacilli burden. Out of our expectation, LppZ peptide-induced TNF-α secretion was significantly lower in CD8+ T cells from ATB patients when compared to HC group. It is believed that protection and pathology in TB are modulated to certain extent by TNF-α. The decreased secretion level of TNF-α in ATB population to some extent represents the pathological status of TB patients (Dorhoi and Kaufmann, 2014). Besides, loss of TNF-α might also due to the T cell exhaustion in TB patients (Jayaraman et al., 2016). It has been reported that the levels of TNF-α are lower in the pathogenesis of bronchitis and sepsis as well (Wada et al., 1991; Marchant et al., 1996). Increasing antigen-specific TNF-α response might be used as one of the criteria for successful vaccination in TB vaccine development (Maggioli et al., 2016).
Interestingly, upon LppZ peptides ex vivo stimulation, not only cytokine production in CD8+ T cell, but also in CD4+ T cells was observed. LppZp7 and LppZp10 are HLA-A2-restricted peptides whereas antigens that trigger specific CD4+T cell responses are often MHC class II molecules restricted. One possibility lies in the fact that LppZp7 and LppZp10 can bind to MHC class II molecules as well with weak ability. However, blockade with BB7.2 mAb revoked cytokine release (Supplementary Figure S1). Thus, the most possibility is that cytokines produced by activated CD8+ T cells can function on CD4+ T cells, which reflects by-stand effects of HLA class I-restricted peptides. It is reported that T cell vaccine-mediated immune protection on autoimmune encephalomyelitis model is orchestrated by CD4+ and CD8+ T cells (Fiebiger et al., 2015). Although the exact mechanism needs to be clarified further, our results thus suggest that HLA class I-restricted peptides can still effectively trigger the activation of CD4+ T cells, which is expected to play synergistic roles in anti-TB immunity. The roles of CD8+ T cells have been reported as well (Hu et al., 2017).
In summary, we have identified HLA-A2-restricted LppZp7 and LppZp10 peptides with good binding capacity and binding stability. They also exert higher immunoreactivity in ATB patients with antigen-specific CD8+ and CD4+ T cell responses. With the ability of LppZp7 and LppZp10 to trigger strong immune responses against TB, their potential value in vaccine development and diagnosis are worthy of further exploration.
This study was carried out in accordance with the recommendations of the Ethical Committee of Shanghai Jiao Tong University School of Medicine with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Ethical Committee of Shanghai Jiao Tong University School of Medicine.
YW and YC designed the experiments. Y-yL, WS, and YC conducted the experiments. YW, YC, XZ, G-pZ, Y-yL, WS, and SX analyzed the data. WS and X-wG collected the samples and clinical data. LX, PJ, and SW contributed reagents, materials, and analysis tools. Y-yL, XZ, YC, and YW wrote the manuscript.
This work was supported by grants from Chinese National Mega Science and Technology Program on Infectious Diseases (2018ZX10731301-001-004, 2018ZX10302301-002-002), the National Natural Science Foundation of China (81501361), Shanghai Institute of Immunology, the Fundamental Research Funds for the Central Universities (17X100040012), Project of Shanghai Municipal Health and Family Planning Commission (201840280), Clinical Research Plan of SHDC (16CR1028B) and the Innovation Project for graduate students of Shanghai Jiao Tong University School of Medicine.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.03131/full#supplementary-material
Bai, X., Wang, D., Liu, Y., Xiao, L., Liang, Y., Yang, Y., et al. (2018). Novel epitopes identified from Mycobacterium tuberculosis antigen Rv2629induces cytotoxic T lymphocyte response. Immunol. Lett. 203, 21–28. doi: 10.1016/j.imlet.2018.06.005
Basak, S., Singh, P., and Rajurkar, M. (2016). Multidrug resistant and extensively drug resistant bacteria: a study. J. Pathog. 2016:4065603. doi: 10.1155/2016/4065603
Cernuschi, T., Malvolti, S., Nickels, E., and Friede, M. (2018). Bacillus Calmette-Guerin (BCG) vaccine: a global assessment of demand and supply balance. Vaccine 36, 498–506. doi: 10.1016/j.vaccine.2017.12.010
Chheda, Z. S., Kohanbash, G., Okada, K., Jahan, N., Sidney, J., Pecoraro, M., et al. (2018). Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J. Exp. Med. 215, 141–157. doi: 10.1084/jem.20171046
Dorhoi, A., and Kaufmann, S. H. (2014). Tumor necrosis factor alpha in mycobacterial infection. Semin. Immunol. 26, 203–209. doi: 10.1016/j.smim.2014.04.003
Fiebiger, B. M., Maamary, J., Pincetic, A., and Ravetch, J. V. (2015). Protection in antibody- and T cell-mediated autoimmune diseases by antiinflammatory IgG Fcs requires type II FcRs. Proc. Natl. Acad. Sci. U.S.A. 112, E2385–E2394. doi: 10.1073/pnas.1505292112
Flynn, J. L., and Chan, J. (2001). Immunology of tuberculosis. Annu. Rev. Immunol. 19, 93–129. doi: 10.1146/annurev.immunol.19.1.93
Gao, H., Li, K., Yu, S., and Xiong, S. (2009). A novel DNA vaccine containing multiple TB-specific epitopes cast in a natural structure elicits enhanced Th1 immunity compared with BCG. Microbiol. Immunol. 53, 541–549. doi: 10.1111/j.1348-0421.2009.00157.x
Hemmati, M., Seghatoleslam, A., Rasti, M., Ebadat, S., Naghibalhossaini, F., and Mostafavi-Pour, Z. (2016). Additive effect of recombinant Mycobacterium tuberculosis ESAT-6 protein and ESAT-6/CFP-10 fusion protein in adhesion of macrophages through fibronectin receptors. J. Microbiol. Immunol. Infect. 49, 249–256. doi: 10.1016/j.jmii.2014.06.002
Hinks, T. S., Varsani, N., Godsiff, D. T., Bull, T. C., Nash, K. L., McLuckie, L., et al. (2012). High background rates of positive tuberculosis-specific interferon-gamma release assays in a low prevalence region of UK: a surveillance study. BMC Infect. Dis. 12:339. doi: 10.1186/1471-2334-12-339
Hu, Z., Wong, K. W., Zhao, H. M., Wen, H. L., Ji, P., Ma, H., et al. (2017). Sendai virus mucosal vaccination establishes lung-resident memory CD8 T cell immunity and boosts BCG-primed protection against TB in mice. Mol. Ther. 25, 1222–1233. doi: 10.1016/j.ymthe.2017.02.018
Jayaraman, P., Jacques, M. K., Zhu, C., Steblenko, K. M., Stowell, B. L., Madi, A., et al. (2016). TIM3 mediates T cell exhaustion during Mycobacterium tuberculosis infection. PLoS Pathog. 12:e1005490. doi: 10.1371/journal.ppat.1005490
Jeon, Y. L., Nam, Y. S., You, E., Yang, J. J., Kim, M. J., Cho, S. Y., et al. (2013). Factors influencing discordant results of the QuantiFERON-TB Gold In-tube test in patients with active TB. J. Infect. 67, 288–293. doi: 10.1016/j.jinf.2013.06.005
Jin, S., Wang, Y., Wang, S. J., Zhang, H. Z., Li, M. X., Chen, Y., et al. (2005). [Identification of a novel HLA-A2-restrictive CTL epitope of an ovary cancer-associated antigen OVA66]. Chin. J. Cell. Mol. Immunol. 21, 233–236, 242.
Kobashi, Y., Mouri, K., Yagi, S., Obase, Y., Miyashita, N., Okimoto, N., et al. (2008). Clinical utility of the QuantiFERON TB-2G test for elderly patients with active tuberculosis. Chest 133, 1196–1202. doi: 10.1378/chest.07-1995
Lewinsohn, D. A., Lobato, M. N., and Jereb, J. A. (2010). Interferon-gamma release assays: new diagnostic tests for Mycobacterium tuberculosis infection, and their use in children. Curr. Opin. Pediatr. 22, 71–76. doi: 10.1097/MOP.0b013e3283350301
Li, D., Molldrem, J. J., and Ma, Q. (2009). LFA-1 regulates CD8+ T cell activation via T cell receptor-mediated and LFA-1-mediated Erk1/2 signal pathways. J. Biol. Chem. 284, 21001–21010. doi: 10.1074/jbc.M109.002865
Lv, H., Gao, Y., Wu, Y., Zhai, M., Li, L., Zhu, Y., et al. (2010). Identification of a novel cytotoxic T lymphocyte epitope from CFP21, a secreted protein of Mycobacterium tuberculosis. Immunol. Lett. 133, 94–98. doi: 10.1016/j.imlet.2010.07.007
Maggioli, M. F., Palmer, M. V., Thacker, T. C., Vordermeier, H. M., McGill, J. L., Whelan, A. O., et al. (2016). Increased TNF-alpha/IFN-gamma/IL-2 and decreased TNF-alpha/IFN-gamma production by central memory T cells are associated with protective responses against bovine tuberculosis following BCG vaccination. Front. Immunol. 7:421. doi: 10.3389/fimmu.2016.00421
Malen, H., Softeland, T., and Wiker, H. G. (2008). Antigen analysis of Mycobacterium tuberculosis H37Rv culture filtrate proteins. Scand. J. Immunol. 67, 245–252. doi: 10.1111/j.1365-3083.2007.02064.x
Marchant, A., Amraoui, Z., Gueydan, C., Bruyns, C., Le Moine, O., Vandenabeele, P., et al. (1996). Methylprednisolone differentially regulates IL-10 and tumour necrosis factor (TNF) production during murine endotoxaemia. Clin. Exp. Immunol. 106, 91–96. doi: 10.1046/j.1365-2249.1996.d01-799.x
Mehra, N. K., Jaini, R., Rajalingam, R., Balamurugan, A., and Kaur, G. (2001). Molecular diversity of HLA-A∗02 in Asian Indians: predominance of A∗0211. Tissue Antigens 57, 502–507. doi: 10.1034/j.1399-0039.2001.057006502.x
Mizukoshi, E., Nakamoto, Y., Arai, K., Yamashita, T., Sakai, A., Sakai, Y., et al. (2011). Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology 53, 1206–1216. doi: 10.1002/hep.24149
Mollica, A., Stefanucci, A., and Costante, R. (2013). Strategies for developing tuberculosis vaccines: emerging approaches. Curr. Drug Targets 14, 938–951. doi: 10.2174/1389450111314090002
Park, I. N., and Shim, T. S. (2017). Qualitative and quantitative results of interferon-gamma release assays for monitoring the response to anti-tuberculosis treatment. Korean J. Intern. Med. 32, 302–308. doi: 10.3904/kjim.2016.199
Ren, Y., Wu, B., Pan, Y., Lv, F., Kong, X., Luo, X., et al. (2011). Characterization of the binding profile of peptide to transporter associated with antigen processing (TAP) using Gaussian process regression. Comput. Biol. Med. 41, 865–870. doi: 10.1016/j.compbiomed.2011.07.004
Sahin, U., Derhovanessian, E., Miller, M., Kloke, B. P., Simon, P., Lower, M., et al. (2017). Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226. doi: 10.1038/nature23003
Sartain, M. J., Slayden, R. A., Singh, K. K., Laal, S., and Belisle, J. T. (2006). Disease state differentiation and identification of tuberculosis biomarkers via native antigen array profiling. Mol. Cell. Proteomics 5, 2102–2113. doi: 10.1074/mcp.M600089-MCP200
Sutcliffe, I. C., and Harrington, D. J. (2004). Lipoproteins of Mycobacterium tuberculosis: an abundant and functionally diverse class of cell envelope components. FEMS Microbiol. Rev. 28, 645–659. doi: 10.1016/j.femsre.2004.06.002
Tsuchiya, N., Yoshikawa, T., Fujinami, N., Saito, K., Mizuno, S., Sawada, Y., et al. (2017). Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology 6:e1346764. doi: 10.1080/2162402X.2017.1346764
Wada, H., Ohiwa, M., Kaneko, T., Tamaki, S., Tanigawa, M., Takagi, M., et al. (1991). Plasma level of tumor necrosis factor in disseminated intravascular coagulation. Am. J. Hematol. 37, 147–151. doi: 10.1002/ajh.2830370302
Wang, C., Chen, Z., Fu, R., Zhang, Y., Chen, L., Huang, L., et al. (2011). A DNA vaccine expressing CFP21 and MPT64 fusion protein enhances BCG-induced protective immunity against Mycobacterium tuberculosis infection in mice. Med. Microbiol. Immunol. 200, 165–175. doi: 10.1007/s00430-011-0188-z
Wu, J. B., Chen, Y., Ji, P., Li, Y., Wang, S. J., Shen, H., et al. (2017). [Dynamic properties and clinical significance of antigen specific T cell responses during anti-tuberculosis treatment]. Curr. Immunol. 37, 198–205.
Xiao, J., Xiong, Y., Chen, Y., Xiao, Y., Ji, P., Li, Y., et al. (2017). Determination of lipoprotein Z-Specific IgA in tuberculosis and latent tuberculosis infection. Front. Cell. Infect. Microbiol. 7:495. doi: 10.3389/fcimb.2017.00495
Xiao, Y., Sha, W., Tian, Z., Chen, Y., Ji, P., Sun, Q., et al. (2016). Adenylate kinase: a novel antigen for immunodiagnosis and subunit vaccine against tuberculosis. J. Mol. Med. 94, 823–834. doi: 10.1007/s00109-016-1392-5
Yang, X., Wu, J. B., Liu, Y., Xiong, Y., Ji, P., Wang, S. J., et al. (2017). Identification of mycobacterial bacterioferritin B for immune screening of tuberculosis and latent tuberculosis infection. Tuberculosis 107, 119–125. doi: 10.1016/j.tube.2017.08.005
Zhu, C., Gao, Y., Li, H., Meng, S., Li, L., Francisco, J. S., et al. (2016). Characterizing hydrophobicity of amino acid side chains in a protein environment via measuring contact angle of a water nanodroplet on planar peptide network. Proc. Natl. Acad. Sci. U.S.A. 113, 12946–12951. doi: 10.1073/pnas.1616138113
Keywords: tuberculosis, mycobacterial lipoprotein Z, HLA-A2-restricted peptide, antigenicity, CD8+ and CD4+ T cells responses
Citation: Liu Y-y, Sha W, Xu S, Gui X-w, Xia L, Ji P, Wang S, Zhao G-p, Zhang X, Chen Y and Wang Y (2018) Identification of HLA-A2-Restricted Mycobacterial Lipoprotein Z Peptides Recognized by T Cells From Patients With Active Tuberculosis Infection. Front. Microbiol. 9:3131. doi: 10.3389/fmicb.2018.03131
Received: 26 January 2018; Accepted: 04 December 2018;
Published: 21 December 2018.
Edited by:
Diane Ordway, Colorado State University, United StatesReviewed by:
Roberto Nisini, Istituto Superiore di Sanità (ISS), ItalyCopyright © 2018 Liu, Sha, Xu, Gui, Xia, Ji, Wang, Zhao, Zhang, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Zhang, emhhbmd4aWFvQGN1c3QuZWR1LmNu Yingying Chen, eWluZ3lpbmcuY2hlbkBzaHNtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.