
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 14 December 2018
Sec. Infectious Agents and Disease
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.03085
 Tyler K. Nygaard1*
Tyler K. Nygaard1* Timothy R. Borgogna1
Timothy R. Borgogna1 Eli W. Sward1
Eli W. Sward1 Fermin E. Guerra1
Fermin E. Guerra1 Jennifer G. Dankoff1
Jennifer G. Dankoff1 Madison M. Collins1
Madison M. Collins1 Kyler B. Pallister1
Kyler B. Pallister1 Liang Chen2
Liang Chen2 Barry N. Kreiswirth2
Barry N. Kreiswirth2 Jovanka M. Voyich1
Jovanka M. Voyich1Staphylococcus aureus is a common Gram-positive bacteria that is a major cause of human morbidity and mortality. The SaeR/S two-component sensory system of S. aureus is important for virulence gene transcription and pathogenesis. However, the influence of SaeR phosphorylation on virulence gene transcription is not clear. To determine the importance of potential SaeR phosphorylation sites for S. aureus virulence, we generated genomic alanine substitutions at conserved aspartic acid residues in the receiver domain of the SaeR response regulator in clinically significant S. aureus pulsed-field gel electrophoresis (PFGE) type USA300. Transcriptional analysis demonstrated a dramatic reduction in the transcript abundance of various toxins, adhesins, and immunomodulatory proteins for SaeR with an aspartic acid to alanine substitution at residue 51. These findings corresponded to a significant decrease in cytotoxicity against human erythrocytes and polymorphonuclear leukocytes, the ability to block human myeloperoxidase activity, and pathogenesis during murine soft-tissue infection. Analysis of SaeR sequences from over 8,000 draft S. aureus genomes revealed that aspartic acid residue 51 is 100% conserved. Collectively, these results demonstrate that aspartic acid residue 51 of SaeR is essential for S. aureus virulence and underscore a conserved target for novel antimicrobial strategies that treat infection caused by this pathogen.
Staphylococcus aureus (S. aureus) is a Gram-positive bacterium that can cause a wide range of disease in both humans and animals (Nygaard et al., 2008). The diverse pathogenesis of S. aureus can be attributed to the expression of an extensive array virulence factors that are often redundant in function (Guerra et al., 2017). Expression of these virulence genes in vivo is thought to be primarily dictated by the concerted influence of two-component sensory systems that recognize environmental signals and alter gene expression accordingly. The S. aureus genome encodes 16 two-component systems that have been identified by sequence analysis (Cheung et al., 2004). Of these, the SaeR/S two-component system has been shown to be an important mediator of S. aureus virulence by transcriptionally upregulating numerous adhesins, toxins, and immunomodulatory proteins that interact directly with host components to advance pathogenesis (Giraudo et al., 1997; Goerke et al., 2001; Voyich et al., 2009; Nygaard et al., 2010; Borgogna et al., 2018). The mechanisms used by this two-component system to recognize host-specific cues and alter gene transcription in response are not completely understood.
Typical two-component systems are minimally composed of a transmembrane histidine kinase sensor and cognate intracellular response regulator (Groisman, 2016). Recognition of environmental stimulus activates the histidine kinase sensor, inducing autophosphorylation at a histidine residue on the intracellular domain. This phosphate group is then transferred to an aspartic acid residue within the receiver domain of the cognate response regulator. In general, the phosphorylated response regulator then binds to promoter regions within the bacterial genome to mediate gene transcription and promote survival. However, some response regulators such as RcsB from Escherichia coli (Pannen et al., 2016) and SreR from Xanthomonas campestris (Wang et al., 2014) can regulate gene transcription without being phosphorylated. For the SaeR/S two-component system, the need to artificially phosphorylate recombinant SaeR for the in vitro DNA binding activity of this response regulator has not been consistent between studies (Nygaard et al., 2010; Sun et al., 2010). It has also been suggested that different levels of SaeR phosphorylation correspond to the upregulation of distinct groups of SaeR/S regulated genes (Mainiero et al., 2010).
To clarify the importance of potential SaeR phosphorylation sites for mediating S. aureus pathogenesis, we have generated individual single amino acid substitutions at conserved aspartic acid residues of SaeR in the genome of S. aureus PFGE-type USA300. In this report, we assess the significance of these residues for mediating the virulence of a clinically relevant MRSA strain using both in vitro and in vivo models of infection. Results from this study underscore the importance of SaeR aspartic acid residue 51 for virulence gene transcription and demonstrate that the substitution of this single amino acid in the USA300 genome can attenuate pathogenesis of this clinically significant MRSA strain.
Staphylococcus aureus PFGE-type USA300 strain LAC has been described previously (Diep et al., 2006) and the USA300 isogenic deletion mutant of saeR/S (USA300ΔsaeR/S) was generated in previous studies (Nygaard et al., 2010). Bacteria were cultured in an Excella E24 rotary incubator (New Brunswick) at 250 rpm and 37°C. Unless noted otherwise, overnight bacteria cultures grown in 20 mL of tryptic soy broth (TSB; EMD Millipore) were used to start subcultures in 20 mL TSB containing 0.5% glucose (1:100 dilution). Optical density at 600 nm (OD600) was measured using a NanoDrop 2000c Spectrophotometer (ThermoFisher Scientific) and colony forming units (CFUs) were determined by plating diluted samples on tryptic soy agar (TSA; EMD Millipore).
DNA sequence alignment of SaeR homologs was performed using Clustal Omega1 with protein sequences obtained from the NCBI protein data base using the following accession numbers: ABD22784 for USA300, ATX72322 for USA400, EOR90509 for USA100, WP_076742615.1 for MT-0541, AAW53763 for Staphylococcus epidermidis RP62A, Q8CQ17 for Staphylococcus epidermidis 12228, WP_049307325 for Staphylococcus capitis, WP_029378577 for Staphylococcus xylosus, PCQ20359 for Klebsiella pneumonia, NP_346930 for Clostridium acetobutylicum, ACJ50526 for Escherichia coli, and NP_388082 for Bacillus subtilis.
Allelic exchange with plasmid pKOR1 was used to impart genomic mutations in USA300 as previously described (Bae and Schneewind, 2006). All primers used to generate and sequence USA300 mutants are listed in Supplementary Table S1. Primers containing attb sites were used to amplify staphylococcal peroxidase inhibitor (spn) and saeR from the USA300 genome for BP Clonase II (ThermoFisher Scientific) mediated insertion into pKOR1. Spn-check-fwd and rvs primers were used to verify the loss of spn. Site-directed mutagenesis was performed on pKOR1-saeR using saeR-D46A, saeR-D51A, or saeR-D61A primers and PfuUltra II Fusion HS DNA polymerase (Agilent Technologies, Inc.) following the manufacture’s protocol and as previously performed (Ran et al., 2010). The saePQRS operon in generated saeR point mutants was PCR amplified using saePQRS_fwd and rvs primers and sequenced (BigDye Terminator v3.1 Cycle Sequencing Kit) using saeR-seq and saePQRS-seq primers. PCR amplification was performed using saeR-EcoRI-fwd and saeR-XhoI-rvs and cloned into pEPSA5 as previously described (Flack et al., 2014) to generate the complementary plasmid that expresses wt SaeR, pEPSA5-saeR.
Wild-type USA300 and its SaeR mutants were subject to whole genome sequencing. Briefly, genomic DNA was extracted using a Wizard genomic DNA purification kit (Promega, Madison, WI, United States) following by treatment with 20 μg/ml lysostaphin. The DNA library was sequenced on an Illumina NextSeq platform (Illumina, San Diego, CA, United States) with 2 × 150 bp paired-end reads. The reads were mapped against the published NC_007793 (S. aureus USA300_FPR3757) genome using BWA (Li and Durbin, 2009) and Samtools (Li et al., 2009), and the SNPs and InDels were examined using freebayes2. SNPs and InDels were further annotated using snpEff (Cingolani et al., 2012).
Blast analysis of the USA300 saeR (locus_tag SAUSA300_0691) sequence against more than 8,000 S. aureus draft genomes downloaded from the NCBI FTP site3 (dated as June 1, 2018) were extracted, translated into amino acids and aligned using Geneious 11.1.
Staphylococcus aureus transcription analysis using relative quantitative real time RT-PCR was performed as previously described (Voyich et al., 2005, 2009; Nygaard et al., 2010). Briefly, subcultured strains were harvested at mid-exponential (ME; OD600 = 1.5) or early stationary (ES; OD600 = 3.0) growth phases, mechanically disrupted using a FastPrep FP120 cell disrupter (ThermoFisher Scientific), and RNA purified with an RNeasy Kit (Qiagen). TaqMan real-time RT-PCR was performed using previously published primer and probe sets (Nygaard et al., 2010).
Heparinized venous blood from healthy donors was collected in accordance with the protocol approved by the Institutional Review Board for Human Subjects at Montana State University. All donors provided written informed consent to participate in the study. We adopted protocol described by others (Young et al., 1986) to quantify hemolysis of human blood by S. aureus extracellular proteins. Briefly, freshly drawn human blood was washed three times with 10 times the volume of sterile DPBS then resuspended at a final dilution of 1:200 with sterile DPBS. Sterile-filtered (0.22 um) S. aureus supernatants from 6-h subcultures grown in TSB were combined with washed diluted blood in individual wells of a 96 well plate on ice at a ratio of 1:1. TSB alone and TSB + 0.5% Triton X-100 were also used as negative and positive controls for hemolysis. Samples were then placed in a SpectraMax 190 microplate reader (Molecular Devices) heated to 37°C and absorbance at 630 nm was measured after 6 min of incubation. Percent hemolysis was determined using the following formula: %Hemolysis = (AbsorbanceExperimental – Absorbance TSBcontrol)/(AbsorbanceTriton X – Absorbance TSBcontrol) × 100.
To obtain extracellular proteins from USA300 strains, supernatant from S. aureus sub-cultured for 5 h was sterile-filtered (0.22 um) and stored at -80°C. For human myeloperoxidase (MPO) activity assays, 0.5 ug of recombinant human MPO (R&D Systems) in 50 uL of DPBS was mixed with 50 uL of sterile-filtered S. aureus supernatant for 30 min at room temperature. The MPO-supernatant solution was then exposed to 150 uL of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate reagent set (BD Biosciences) and incubated at 37°C. The oxidation of TMB catalyzed by human MPO was quantified by measuring the OD650 every minute for 30 min using a SpectraMax Paradigm microplate reader (Molecular Devices). The MPO inhibitor sodium azide was used at a concentration of 1 mM.
Human polymorphonuclear leukocytes (neutrophils or PMNs) were isolated under endotoxin-free conditions (<25.0 pg/ml) using freshly drawn heparinized venous blood from healthy donors with written informed consent as previously described (Voyich et al., 2005; Nygaard et al., 2013). Cell viability and purity of preparations were assessed using a FACSCalibur Flow cytometer (BD Biosciences) and only preparations containing ≥ 98% viable PMNs were used. Assays intoxicating PMNs with extracellular S. aureus proteins were performed as previously described (Nygaard et al., 2012; Flack et al., 2014). Briefly, supernatant from S. aureus subcultured for 5 h in TSB was sterile-filtered (0.22 um) and diluted by 1:10 with TSB. To intoxicate PMNs, 20 ul of diluted S. aureus supernatant was combined with 100 uL Roswell Park Memorial Institute (RPMI) 1640 Medium (Corning Cellgro) with 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; Corning Cellgro) containing 1x106 purified human PMNs in a serum coated well of a 96 well plate. Samples were incubated at 37°C for 90 min then stained with propidium iodide (PI; ThermoFisher Scientific) following the manufactures protocol and then analyzed with a FACSCalibur Flow cytometer. Triton-X 100 (0.5%) was used as a positive control for causing PMN plasma membrane permeability. The following formula was used to determine % propidium iodide+: %Propidium Iodide+ = (Mean PI signalExperimental – Mean PI signal untreated)/(Mean PI signalTriton-X – Mean PI signal untreated).
Assays measuring human PMN plasma membrane permeability following the phagocytosis of live S. aureus were performed as previously described (Voyich et al., 2009; Nygaard et al., 2012; Flack et al., 2014). Briefly, subcultured S. aureus was harvested at ME growth by centrifugation (5,000 × g, 5 min, 4°C), washed with DPBS, then opsonized with 50% normal human serum for 15 min at 37°C. Opsonized bacteria were washed with DPBS and then 2 × 107 CFU in 100 uL of DPBS was combined with 100 uL of RPMI/H containing 1 × 106 purified human PMNs in a serum coated well of 96 well plate. Phagocytosis was synchronized by centrifugation (524 × g, 8°C, 8 min) in an Allegra X-15R centrifuge (Beckman Coulter) and samples were incubated at 37°C for 90 min. Following incubation, human PMNs were stained with PI and analyzed using flow cytometry as described above.
All animal studies conformed to National Institute of Health guidelines and were approved by the Animal Care and Use Committee at Montana State University-Bozeman. Female BALB/C mice (8–10 weeks old) with an average weight of 22 g were purchased from Animal Resource Facility at Montana State University (Bozeman, MT, United States). The murine model of soft-tissue infection was performed as previously described (Voyich et al., 2006; Nygaard et al., 2010; Malachowa et al., 2013). Briefly, 12-week-old BALB/C mice were shaved and hair completely removed with NairTM treatment. Two days later, shaved mice (5 per group) were subcutaneously inoculated with 1 × 107 CFUs of S. aureus in 100 uL DPBS. Mice were weighed and abscess size measured at indicated times post-inoculation. The area of soft-tissue infections was determined using the following formula as previously published (Malachowa et al., 2013): Area = π (Length/2) × Width/2.
Analysis of the three-dimensional crystal structure of the two-component response regulator PhoB of Escherichia coli (Solà et al., 1999) indicates that the homologous S. aureus response regulator SaeR is phosphorylated on a conserved aspartic acid at residue 51 (Figure 1A). Aspartic acid residue 46 is also highly conserved in the OmpR family of proteins, suggesting this residue might be important for SaeR function. Analysis of the SaeR sequences extracted from over 8,000 draft S. aureus genomes revealed that aspartic acid residue 51 is 100% conserved. The biological significance of this conservation is heightened by the identification of 50 unique SaeR protein sequences identified from the draft genomes (Supplementary Figure S1).
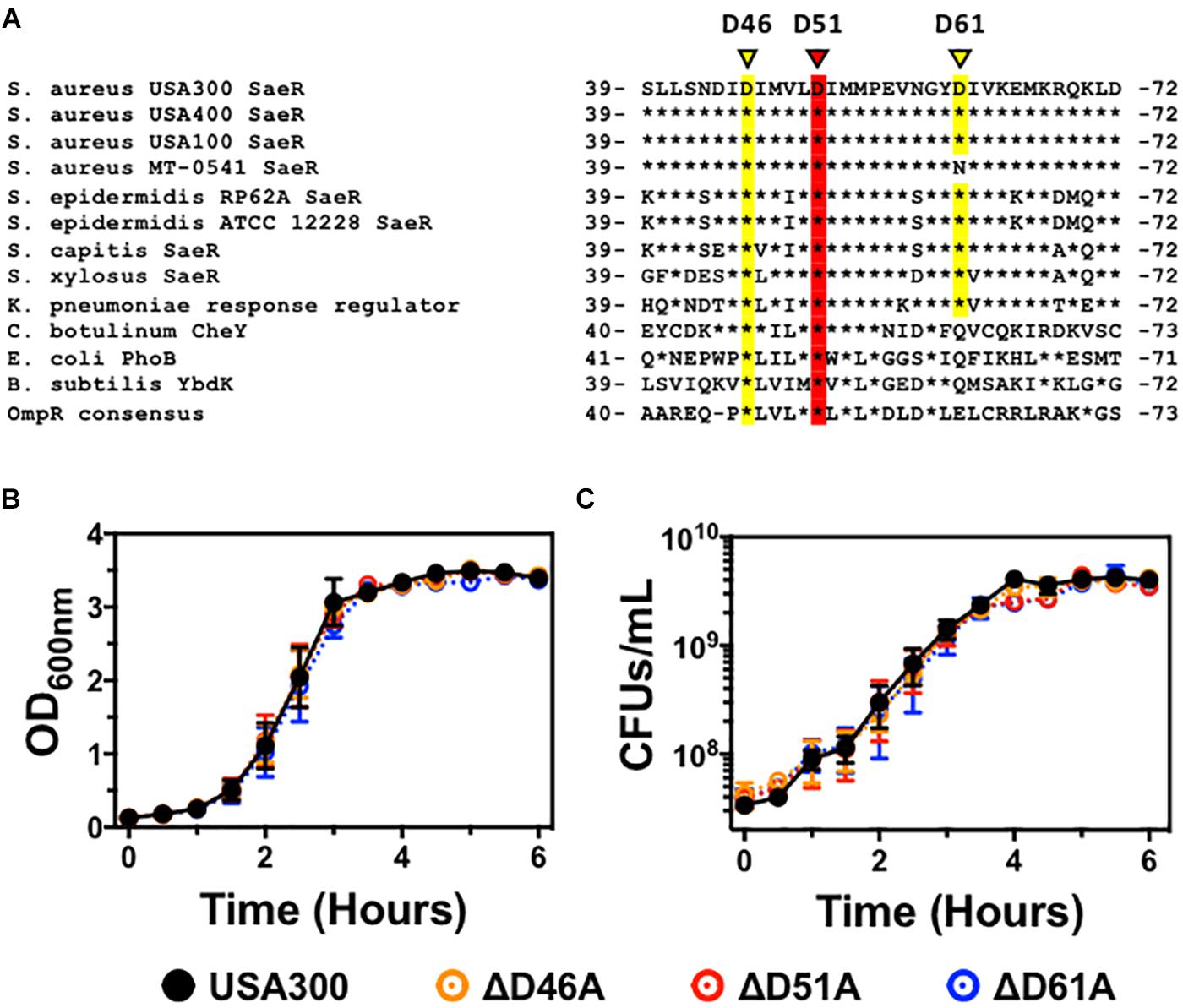
Figure 1. The generation of USA300 genomic point mutations in specific aspartic acid residues of saeR. (A) DNA sequence alignment of saeR homologues from different bacteria highlighting the putative phosphorylation site at conserved aspartic acid residue 51 (red) as well as proximal aspartic acid residues 46 and 61 (yellow). In vitro growth as measured by (B) absorbance at 600 nm, and (C) colony forming units (CFU) per mL of USA300, USA300 with an aspartic acid to alanine point mutation at SaeR residue 46 (ΔD46A), at residue 51 (ΔD51A), or at residue 61 (ΔD61A). Data in panels (B,C) are presented as the mean ± SEM of three independent experiments.
To determine if these aspartic acid residues in the receiver domain of SaeR are important for S. aureus pathogenesis, we used allelic recombination to induce substitutions of specific nucleic acids within the S. aureus genome as previously described by others (Bae and Schneewind, 2006; Villaruz et al., 2009; Mairpady Shambat et al., 2016). Using this technique, we generated USA300 strains with an aspartic acid to alanine substitution at the conserved SaeR residue 51 (USA300ΔsaeR-D51A) or the proximal conserved residue 46 (USA300ΔsaeR-D46A). In addition, we generated an aspartic acid to alanine substitution at the less maintained residue 61 of SaeR (USA300ΔsaeR-D61A) to serve as a control for this study. Primary DNA sequencing analysis and additional whole genome sequencing analysis verified the targeted amino acid substitutions that were generated in the three mutants and showed that there were no additional SNPs or InDels when compared to the wild-type strain. As with previously generated isogenic deletion mutants of saeR/S in S. aureus (Voyich et al., 2009; Nygaard et al., 2010), no differences during in vitro growth could be detected in these SaeR point mutants relative to the parental USA300 strain (Figures 1B,C).
The SaeR/S two-component system plays an essential role during pathogenesis by upregulating S. aureus virulence gene transcription (Giraudo et al., 1997; Goerke et al., 2001; Voyich et al., 2009; Nygaard et al., 2010). It is thought that this process requires the activation of SaeR via phosphorylation at an aspartic acid residue within the receiver domain of this response regulator. Indeed, others have indicated that aspartic acid residue 51 of SaeR is necessary for the transcription of α-hemolysin (Hla) using an artificial plasmid overexpression system in S. aureus strain Newman lacking wild-type saeR/S (Mainiero et al., 2010). However, the transcription of saeR that is under a positive feedback loop by the SaeR/S two-component system was actually enhanced in the absence of aspartic acid 51 while no difference in SaeR/S regulated coagulase A transcription could be demonstrated (Mainiero et al., 2010). This study suggested that different levels of SaeR phosphorylation corresponds to the up-regulation of specific sets of virulence genes, prompting us to also examine the influence of the highly conserved SaeR aspartic acid residue 46 and partially conserved aspartic acid residue 61 on SaeR/S-mediated virulence gene transcription.
To resolve the importance of these SaeR aspartic acid residues for virulence gene expression in clinically relevant MRSA, we examined the transcript abundance of numerous adhesins, toxins, and immunomodulatory proteins in the USA300 aspartic acid point mutant strains using relative quantitative real time RT-PCR (Figure 2). Compared to USA300, we observed substantial decreases in the abundance of transcripts encoding various adhesins, toxins, and immunomodulatory proteins in a USA300 isogenic deletion mutant of saeR/S (USA300ΔsaeR/S) relative to the USA300 wt (Figures 2A,B). The virulence gene transcription profile for USA300ΔsaeR-D51A was almost identical to that of USA300ΔsaeR/S. In contrast, the transcript abundance of virulence genes in USA300ΔsaeR-D46A and USA300ΔsaeR-D61A was comparable to the USA300Δ parental wt (Figures 2A,B). Reintroduction of wild-type SaeR to USA300ΔsaeR-D51A rescued defects in gene transcription (Supplementary Figure S2), supporting results indicating aspartic acid residue 51 of SaeR is required for the upregulation of virulence gene expression by this response regulator.
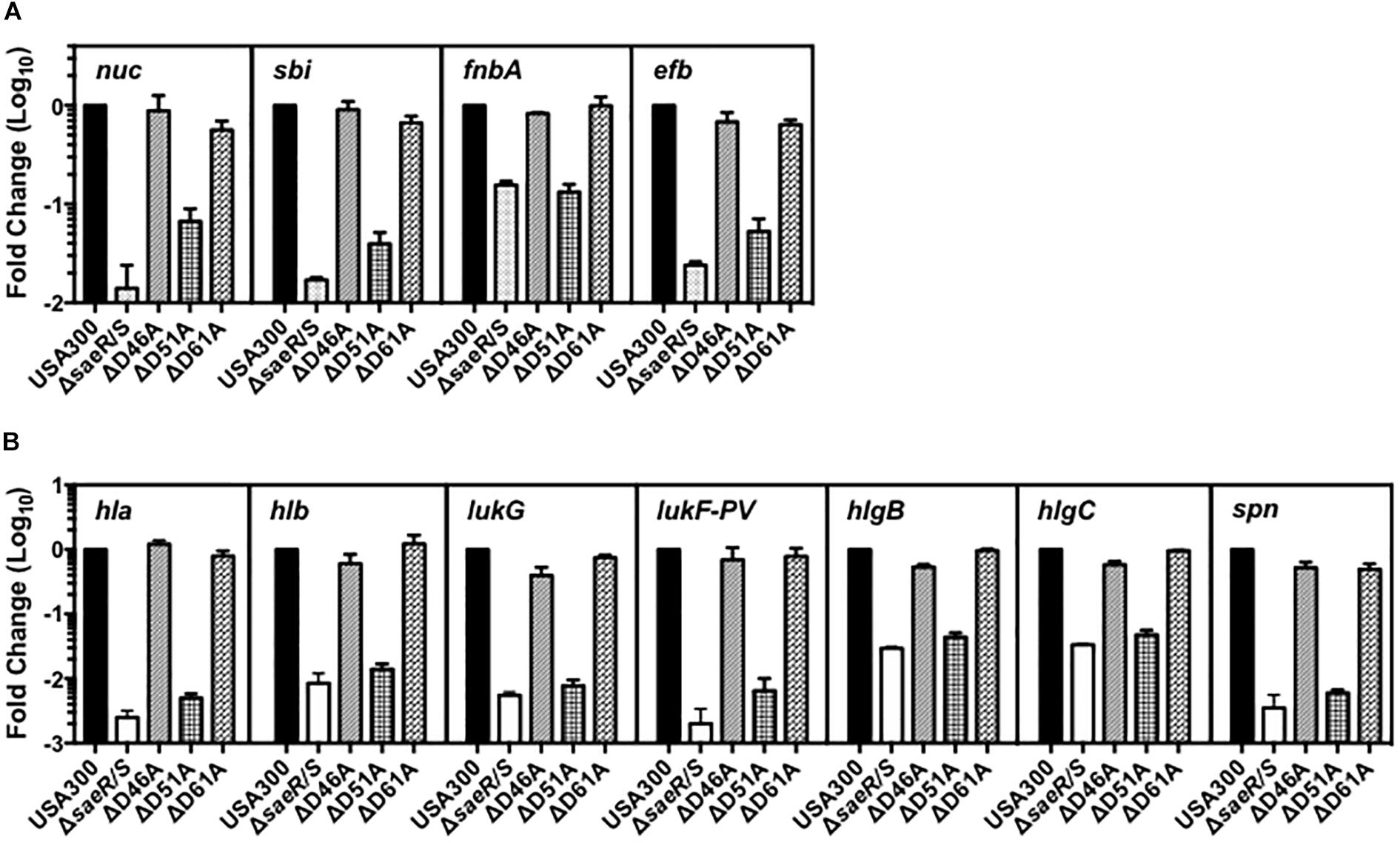
Figure 2. Aspartic acid residue 51 of SaeR is important for the transcription of numerous virulence genes in USA300. Taqman® RT-PCR analysis of USA300, an isogenic deletion mutant of saeR/S in USA300 (USA300ΔsaeR/S), and USA300 genomic point mutants that confer aspartic acid to alanine substitutions at SaeR residue 46 (ΔD46A), 51 (ΔD51A), or 61 (ΔD61A) during growth in vitro. Transcriptional analysis was performed at (A) mid-exponential growth for nuclease (nuc), the second binder of IgG (sbi), fibronectin-binding protein A (fnbA), and the extracellular fibrinogen-binding protein (efb) or at (B) early-stationary growth for γ-hemolysin (hla), γ-hemolysin (hlb), leukocidin subunit G (lukG), the Panton-Valentine leukocidin subunit F (lukF-PV), γ-hemolysin component B (hlgB),γ-hemolysin component C (hlgC), and the staphylococcal peroxidase inhibitor (spn). All panels show the mean ± SEM of at least two separate experiments and are presented as fold change relative to USA300 wt.
Staphylococcus aureus expresses numerous hemolysins under direct transcriptional regulation by the SaeR/S two-component system (Voyich et al., 2009; Nygaard et al., 2010) that lyse mammalian erythrocytes including Hla, Hlb, and HlgA/B (Salgado-Pabón et al., 2014; Spaan et al., 2015). Real time RT-PCR analysis indicated aspartic acid residue 51 of SaeR is essential for the transcriptional upregulation of these hemolysins (Figure 2). To determine the importance of this putative SaeR phosphorylation site for hemolysis caused by S. aureus, we first qualitatively assessed the ability of USA300, USA300ΔsaeR/S, USA300ΔsaeR-D46A, USA300ΔsaeR-D51A, and USA300ΔsaeR-D61A to lyse human red blood cells during growth on agar (Figure 3A). The zone of hemolysis generated by USA300ΔsaeR/S on agar was decreased relative to USA300 (Figure 3A), corresponding to observations in this study (Figure 2) and in previously published reports (Voyich et al., 2009; Nygaard et al., 2010) that demonstrate a decrease in transcript abundance of various S. aureus hemolysins when this two-component system is absent. A reduced hemolysis of human erythrocytes during growth on agar of USA300ΔsaeR-D51A paralleled that of USA300ΔsaeR/S, supporting the notion that aspartic acid residue 51 is essential for SaeR/S activity. In contrast, the zone of hemolysis for USA300ΔsaeR-D46A and USA300ΔsaeR-D61A was indistinguishable from that caused by USA300.
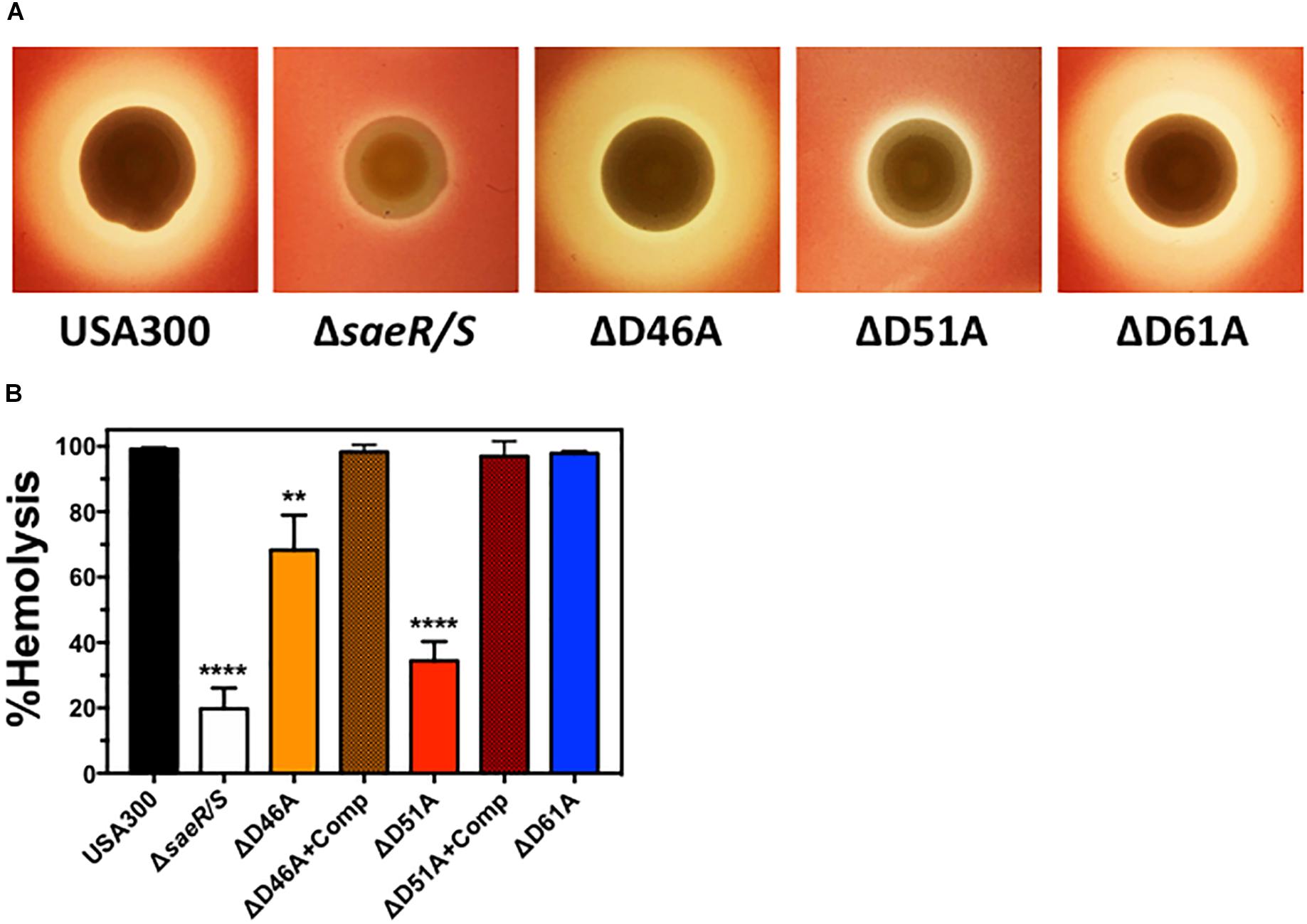
Figure 3. Aspartic acid residues 46 and 51 of SaeR are important for the hemolysis of human erythrocytes by USA300. (A) Zone of hemolysis caused by USA300, an isogenic deletion mutant of saeR/S in USA300 (USA300ΔsaeR/S), or USA300 expressing SaeR with aspartic acid to alanine substitutions at residue 46 (ΔD46A), 51 (ΔD51A), or 61 (ΔD61A) during growth on agar containing 2.5% human red blood cells. (B) Percent hemolysis of human red blood cells in solution exposed to 6 h supernatants from USA300, USA300ΔsaeR/S, ΔD46A, ΔD51A, ΔD61A or ΔD46A and ΔD51A complemented with a plasmid expressing wt SaeR (ΔD46A+Comp or ΔD51A+comp, respectively). All panels represent at least 3 separate experiments. Panels (B,C) show the mean ± SEM with ∗∗P ≤ 0.01 and ∗∗∗∗P ≤ 0.0001 relative to USA300 as determined by one-way ANOVA with Dunnett’s multiple comparison test.
To quantify the hemolysis caused by aspartic acid point mutants of SaeR, we measured the turbidity of human erythrocytes in solution after being combined with extracellular proteins produced by these strains. Corresponding to hemolysis during growth on agar, significantly less human red blood cells were lysed by filtered supernatants from USA300ΔsaeR/S or USA300ΔsaeR-D51A relative to the USA300 parental strain (Figure 3B). In addition, extracellular proteins produced by USA300ΔsaeR-D46A generated significantly less hemolysis than USA300. In support of these findings, the reintroduction of wild-type SaeR to USA300ΔsaeR-D46A or USA300ΔsaeR-D51A rescued the hemolytic activity of supernatants from these strains to levels observed for USA300. Taken together, these findings show that aspartic acid residue 51 of SaeR is necessary for causing the hemolysis of human erythrocytes that is facilitated by the SaeR/S two-component system. In addition, SaeR aspartic acid residue 46 appears to play a significant but less important role than residue 51 mediating hemolysis caused by USA300.
Recently published findings demonstrate that S. aureus prevents the generation of reactive oxygen species by human polymorphonuclear leukocytes (neutrophils or PMNs) via SPIN, a protein under strong SaeR/S regulation that binds directly to the active site of human MPO and inhibits peroxidase activity of this enzyme (Guerra et al., 2016; de Jong et al., 2017). To determine if the putative phosphorylation site at aspartic acid residue 51 of SaeR is required for the inhibition of MPO activity by USA300, we assessed the activity of human MPO in the presence of extracellular proteins produced by USA300, an isogenic deletion mutant of SPIN in USA300 (USA300Δspn), USA300ΔsaeR/S, USA300ΔsaeR-D46A, USA300ΔsaeR-D51A, and USA300ΔsaeR-D61A (Figure 4). Congruent with previously published findings (de Jong et al., 2017), a strong inhibition of human MPO activity was observed in the presence of the MPO inhibitor azide or extracellular proteins produced by USA300 but not extracellular proteins produced by USA300ΔsaeR/S or by USA300Δspn (Figures 4A,B). As with USA300ΔsaeR/S and USA300Δspn, extracellular proteins produced by USA300ΔsaeR-D51A did not inhibit human MPO activity (Figures 4A,B). Reintroduction of wild-type SaeR to USA300ΔsaeR-D51A rescued the inhibition of human MPO activity by extracellular proteins produced from this strain (Figure 4C). In contrast, supernatant from USA300ΔsaeR-D46A and USA300ΔsaeR-D61A blocked the activity of human MPO.
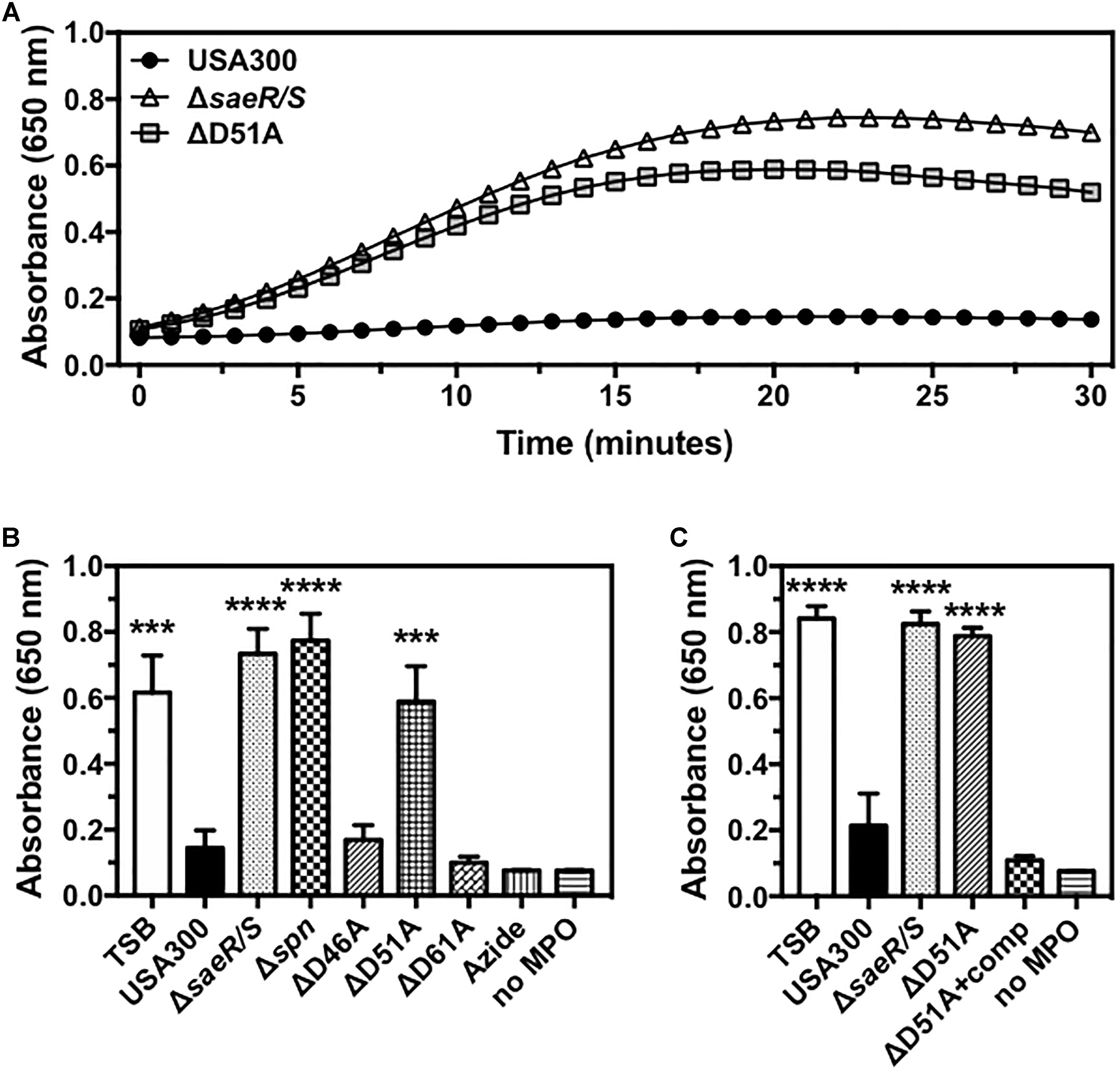
Figure 4. Aspartic acid residue 51 of SaeR is essential for the inhibition of human myeloperoxidase activity by USA300. (A) Human myeloperoxidase (MPO) activity in the presence of extracellular proteins produced by USA300, an isogenic deletion mutant of saeR/S in USA300 (ΔsaeR/S), or an aspartic acid to alanine substitution at residue 51 of SaeR in USA300 (ΔD51A) as measured by absorbance at 650 nm following exposure to 3,3′,5,5′-Tetramethylbenzidine (TMB) and hydrogen peroxide. (B) The activity of human MPO in the presence of extracellular proteins produced by USA300, ΔsaeR/S, an isogenic deletion mutant of SPIN in USA300 (Δspn), ΔD51A, or USA300 with an aspartic acid to alanine substitution at residue 46 (ΔD46A) or 61 (ΔD61A) of SaeR. Peroxidase activity in the presence of the MPO inhibitor sodium azide (Azide) or samples lacking MPO (no MPO) were included as negative controls. (C) Experiments in panel (B) were repeated using a D51A point mutant of saeR complemented with a plasmid expressing wt SaeR (ΔD51A+comp). All panels show the mean ± SEM of at least three independent experiments. For panels (B,C), absorbance is shown at 20 min with ∗∗∗P < 0.001 and ∗∗∗∗P < 0.0001 relative to USA300 as determined by one-way ANOVA with Dunnett’s multiple comparisons test.
Previous studies have demonstrated that the SaeR/S two-component system upregulates S. aureus toxins that target human PMNs (Gauduchon et al., 2001; Voyich et al., 2009; Nygaard et al., 2010; Ventura et al., 2010; DuMont et al., 2011; Malachowa et al., 2011). To assess the importance of aspartic acid residues within the receiver domain of SaeR for mediating toxicity against PMNs, we first examined the ability of extracellular proteins produced by D46A, D51A, and D61A point mutants of SaeR to cause human PMN plasma membrane permeability as measured by propidium iodide staining (Figure 5A). No plasma membrane permeability was observed for PMNs exposed to extracellular proteins produced by USA300ΔsaeR-D51A, indicating aspartic acid residue 51 of SaeR is necessary for the toxicity of USA300 against human PMNs. A significant reduction in PMN plasma membrane permeability was also observed for extracellular proteins produced by USA300ΔsaeR-D46A, though this difference was less than that observed for USA300ΔsaeR-D51A. In support of these findings, complementation of USA300ΔsaeR-D46A, or USA300ΔsaeR-D51A with wild-type SaeR rescued the lytic capacity of extracellular proteins produced by these strains against human PMNs. Corresponding to the toxicity of extracellular proteins produced by these strains, significantly less PMN plasma membrane permeability was observed following phagocytosis of live USA300ΔsaeR/S, USA300ΔsaeR-D51A, and USA300ΔsaeR-D46A relative to USA300 (Figure 5B). These results demonstrate that SaeR aspartic acid residue 51 and, to a lesser extent, aspartic acid residue 46 are important for activity of this two-component system.
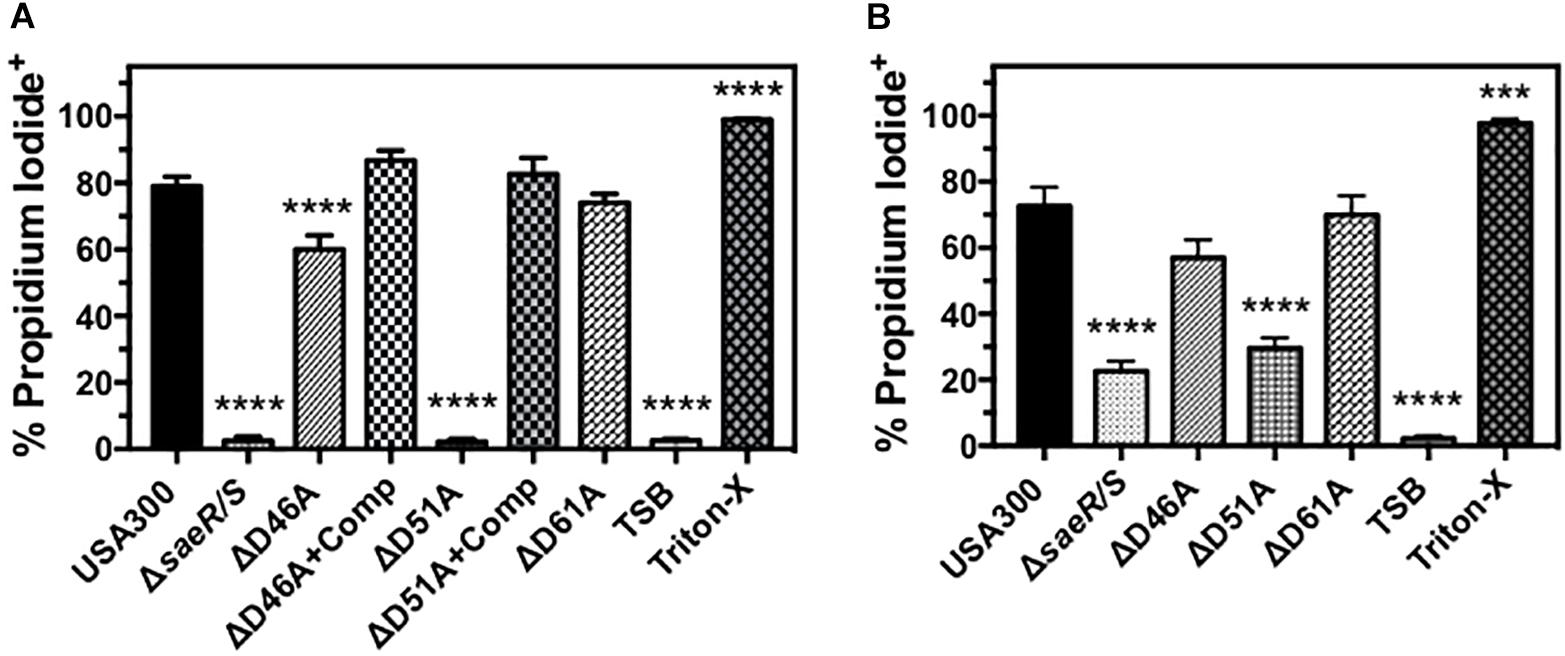
Figure 5. Human PMN plasma membrane permeability caused by USA300 is significantly influenced by aspartic acid residues 46 and 51 of SaeR. (A) Flow cytometry was used to assess the percentage of purified human PMNs permeable to propidium iodide 90 min after exposure to 5 h supernatants from USA300, an isogenic deletion mutant of saeR/S in USA300 (USA300ΔsaeR/S), or USA300 expressing SaeR with aspartic acid to alanine substitutions at residue 46 (ΔD46A), 51 (ΔD51A), 61 (ΔD61A), and ΔD46A or ΔD51A complemented with plasmids expressing wt SaeR (ΔD46A+Comp or ΔD51A+comp, respectively). (B) The percentage of propidium iodide positive human PMNs 90 min after the phagocytosis of live Staphylococcus aureus at a ratio of 20 colony forming units per PMN. For these experiments, PMNs were also treated with tryptic soy broth (TSB) alone or TSB with 0.5% Triton X-100 (Triton-X). All panels show the mean ± SEM of at least 5 independent experiments. ∗∗∗P ≤ 0.001 and ∗∗∗∗P ≤ 0.0001 relative to USA300 as determined by one-way ANOVA with Dunnett’s multiple comparison test.
Previously published findings demonstrate the upregulation of virulence gene transcription in vivo by the SaeR/S two-component system is essential for the pathogenesis of S. aureus during both systemic and localized infection (Voyich et al., 2009; Nygaard et al., 2010). Transcriptional analysis of USA300ΔsaeR-D51A in this report (Figure 2) indicates aspartic acid residue 51 of SaeR is required for upregulating S. aureus virulence gene transcription mediated by SaeR/S in vitro. To determine if potential SaeR phosphorylation sites are important for pathogenesis in vivo, we assessed the virulence of USA300, USA300ΔsaeR/S, USA300ΔsaeR-D46A, USA300ΔsaeR-D51A, and USA300ΔsaeR-D61A during a murine model of soft-tissue infection (Figure 6). In a manner indistinguishable from USA300ΔsaeR/S, soft-tissue infections caused by USA300ΔsaeR-D51A were significantly smaller than infections caused by USA300 (Figure 6A) and did not exhibit the open dermonecrotic lesions characteristic of USA300 pathogenesis (Figure 6B) that is attributed to the high expression of Hla by this strain (Kennedy et al., 2010). In addition, a significant decrease in the weight of mice following infection with USA300 was not observed following inoculation with USA300ΔsaeR/S or USA300ΔsaeR-D51A (Figure 6C). These findings could not be explained by differences in inoculum concentration given to mice for each S. aureus strain tested (Figure 6D). As opposed to USA300ΔsaeR-D51A, no differences could be distinguished between soft-tissue infections caused by USA300ΔsaeR-D46A or USA300ΔsaeR-D61A relative to USA300 (Figures 6A–C). These results demonstrate that USA300 pathogenesis during murine soft-tissue infection is dependent upon aspartic acid residue 51 of SaeR while aspartic acid residues 46 and 61 are not essential for causing disease.
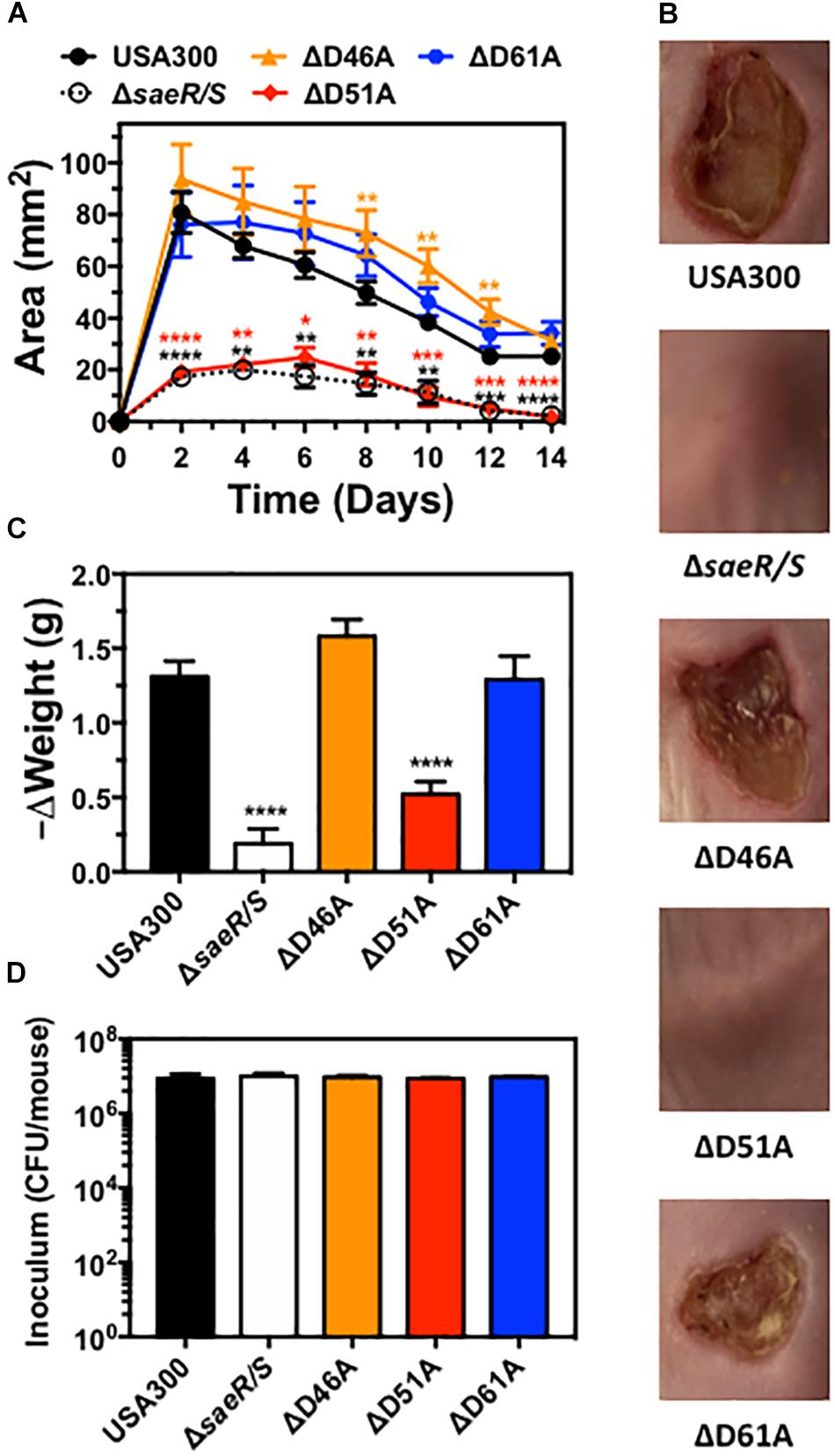
Figure 6. Aspartic acid residue 51 of SaeR significantly influences the pathogenesis of murine soft-tissue infections caused by USA300. (A) Area of soft-tissue infection caused by USA300, an isogenic deletion mutant of saeR/S in USA300 (ΔsaeR/S), or USA300 with an aspartic acid to alanine substitution at SaeR residue 46 (ΔD46A), 51 (ΔD51A) or 61 (ΔD61A). (B) Representative images of soft-tissue infections from experiments in panel (A) on day 6 post-inoculation. (C) Change in the weight of mice during experiments in panel (A) between days 0 and 2 post-inoculation. (D) Inoculum of each strain given to mice in panels (A-C). Panels (A), (C), and (D) show the mean ± SEM of two independent experiments. ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001 and ∗∗∗∗P ≤ 0.0001 relative to USA300 as determined by one-way ANOVA with Dunnett’s multiple comparison test.
Bacterial pathogens must recognize environmental cues and respond appropriately to cause disease. For S. aureus, the SaeR/S two-component system plays an important role during pathogenesis by sensing host-specific signals and up-regulating virulence gene expression in response (Geiger et al., 2008; Zurek et al., 2014). In this report, we show that the substitution of the putative SaeR phosphorylation site at aspartic acid residue 51 completely ameliorates SaeR/S-mediated virulence. Specifically, we observed a dramatic decrease in the transcript abundance of various hemolysins and leukocidins in USA300 lacking aspartic acid residue 51 of SaeR. These findings correspond to a decrease in the ability of this strain to lyse human erythrocytes and PMNs. SaeR aspartic acid residue 51 was also shown to be important for the transcription of the human MPO inhibitor SPIN and USA300 lacking SaeR aspartic acid residue 51 did not inhibit human MPO activity. Moreover, we show that the virulence of USA300ΔsaeR-D51A during murine models of soft-tissue infection paralleled the transcript abundance of Hla in this strain, consistent with previous findings demonstrating this toxin is a major S. aureus virulence determinant in this model (Kennedy et al., 2010). Collectively, these findings indicate that SaeR residue 51 is essential for S. aureus evasion of innate immunity and support other studies that suggest only phosphorylated SaeR has DNA binding activity (Sun et al., 2010).
We also observed a more subtle decrease in the toxicity of USA300 against human erythrocytes and PMNs in the absence of the conserved aspartic acid residue 46 of SaeR. It is not clear if substitution of this residue simply perturbs the receiver domain structure of SaeR to diminish activity or if aspartic acid residue 46 plays a more direct role in the phosphorylation state of this response regulator. Regardless, a reduction in the lytic capacity of USA300ΔsaeR-D46A suggests this conserved residue also influences SaeR function and will be further examined in future studies.
This study demonstrates that the substitution of a single amino acid residue in the SaeR response regulator of USA300 renders this highly pathogenic strain avirulent, highlighting the critical importance of two-component sensory systems for bacterial pathogenesis. Indeed, others have shown that point mutations in the dimerization interface of the histidine kinase sensor AgrC (Mairpady Shambat et al., 2016) or in the P2 promoter region of the Agr two-component system (Villaruz et al., 2009) have a profound effect on the lytic capacity and colonization potential of S. aureus while a point mutation in the histidine kinase sensor LiaS of Streptococcus pyogenes contributes to the carrier phenotype of this bacterium (Flores et al., 2017). Interestingly, no natural mutations of aspartic acid residue 51 could be identified in over 8,000 S. aureus genomes, suggesting this putative phosphorylation site is imperative for the fitness of this pathogen. Taken together, these studies indicate that novel therapeutic strategies that inhibit the activation of this highly conserved response regulator would impede the expression of multiple virulence factors and effectively block different aspects of pathogenesis resulting in clearance of S. aureus by innate immune mechanisms.
TN and JV contributed to the conception and design of this study. TN, TB, ES, FG, JD, MC, KP, LC, and BK performed the experiments and data analysis. TN and JV wrote and prepared the manuscript for submission. All authors read and approved this manuscript.
This work was supported by the U.S. National Institutes of Health (Grants NIH-R01A1090046, GM110732, R21AI128295, and U54GM115371), the Montana University System Research Initiative (51040-MUSRI2015-03), the Montana State University Agriculture Experiment Station, and an equipment grant from Murdock Charitable Trust.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Dr. Frank DeLeo (Laboratory of Bacteriology, Rocky Mountain Laboratories, National Institute of Health, Hamilton, MT, United States) and Dr. Justin Prigge (Department of Microbiology and Immunology, Montana State University, Bozeman, MT, United States) for technical support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.03085/full#supplementary-material
Bae, T., and Schneewind, O. (2006). Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55, 58–63. doi: 10.1016/j.plasmid.2005.05.005
Borgogna, T. R., Hisey, B., Heitmann, E., Obar, J. J., Meissner, N., and Voyich, J. M. (2018). Secondary Bacterial pneumonia by Staphylococcus aureus following influenza a infection is SaeR/S dependent. J. Infect. Dis. 218, 809–813. doi: 10.1093/infdis/jiy210
Cheung, A. L., Bayer, A. S., Zhang, G., Gresham, H., and Xiong, Y. Q. (2004). Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 40, 1–9. doi: 10.1016/S0928-8244(03)00309-2
Cingolani, P., Platts, A., Wang, L. L., Coon, M., Nguyen, T., Wang, L., et al. (2012). A program for annotating and predicting the effects of single nucleotide polymorphisms. SnpEff. Fly 6, 80–92. doi: 10.4161/fly.19695
de Jong, N. W. M., Ramyar, K. X., Guerra, F. E., Nijland, R., Fevre, C., Voyich, J. M., et al. (2017). Immune evasion by a staphylococcal inhibitor of myeloperoxidase. Proc. Natl. Acad. Sci. U.S.A. 114, 9439–9444. doi: 10.1073/pnas.1707032114
Diep, B. A., Gill, S. R., Chang, R. F., Phan, T. H., Chen, J. H., Davidson, M. G., et al. (2006). Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367, 731–739. doi: 10.1016/S0140-6736(06)68231-7
DuMont, A. L., Nygaard, T. K., Watkins, R. L., Smith, A., Kozhaya, L., Kreiswirth, B. N., et al. (2011). Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol. Microbiol. 79, 814–825. doi: 10.1111/j.1365-2958.2010.07490.x
Flack, C. E., Zurek, O. W., Meishery, D. D., Pallister, K. B., Malone, C. L., Horswill, A. R., et al. (2014). Differential regulation of staphylococcal virulence by the sensor kinase SaeS in response to neutrophil-derived stimuli. Proc. Natl. Acad. Sci. U.S.A. 111, E2037–E2045. doi: 10.1073/pnas.1322125111
Flores, A. R., Olsen, R. J., Cantu, C., Pallister, K. B., Guerra, F. E., Voyich, J. M., et al. (2017). Increased pilus production conferred by a naturally occurring mutation alters hostpathogen interaction in favor of carriage in Streptococcus pyogenes. Infect. Immun. 85:e00949-16. doi: 10.1128/IAI.00949-16
Gauduchon, V., Werner, S., Prévost, G., Monteil, H., and Colin, D. A. (2001). Flow cytometric determination of Panton-Valentine leucocidin S component binding. Infect. Immun. 69, 2390–2395. doi: 10.1128/IAI.69.4.2390-2395.2001
Geiger, T., Goerke, C., Mainiero, M., Kraus, D., and Wolz, C. (2008). The virulence regulator sae of Staphylococcus aureus: promoter activities and response to phagocytosis-related signals. J. Bacteriol. 190, 3419–3428. doi: 10.1128/JB.01927-07
Giraudo, A. T., Cheung, A. L., and Nagel, R. (1997). The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Arch. Microbiol. 168, 53–58. doi: 10.1007/s002030050469
Goerke, C., Fluckiger, U., Steinhuber, A., Zimmerli, W., and Wolz, C. (2001). Impact of the regulatory loci agr, sarA and sae of Staphylococcus aureus on the induction of α-toxin during device-related infection resolved by direct quantitative transcript analysis. Mol. Microbiol. 40, 1439–1447. doi: 10.1046/j.1365-2958.2001.02494.x
Groisman, E. A. (2016). Feedback control of two-component regulatory systems. Annu. Rev. Microbiol. 70, 103–124. doi: 10.1146/annurev-micro-102215-095331
Guerra, F. E., Addison, C. B., de Jong, N. W. M., Azzolino, J., Pallister, K. B., van Strijp, J. A. G., et al. (2016). Staphylococcus aureus SaeR/S-regulated factors reduce human neutrophil reactive oxygen species production. J. Leukoc. Biol. 100, 1–6. doi: 10.1189/jlb.4VMAB0316-100RR
Guerra, F. E., Borgogna, T. R., Patel, D. M., Sward, E. W., and Voyich, J. M. (2017). Epic Immune battles of history: neutrophils vs. Staphylococcus aureus. Front. Cell. Infect. Microbiol. 7:286. doi: 10.3389/fcimb.2017.00286
Kennedy, A. D., Wardenburg, J. B., Gardner, D. J., Long, D., Whitney, A. R., Braughton, K. R., et al. (2010). Targeting of Alpha-Hemolysin by active or passive immunization decreases severity of USA300 Skin infection in a mouse model. J. Infect. Dis. 202, 1050–1058. doi: 10.1086/656043
Li, H., and Durbin, R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Mainiero, M., Goerke, C., Geiger, T., Gonser, C., Herbert, S., and Wolz, C. (2010). Differential target gene activation by the Staphylococcus aureus two-component system saeRS. J. Bacteriol. 192, 613–623. doi: 10.1128/JB.01242-09
Mairpady Shambat, S., Siemens, N., Monk, I. R., Mohan, D. B., Mukundan, S., Krishnan, K. C., et al. (2016). A point mutation in AgrC determines cytotoxic or colonizing properties associated with phenotypic variants of ST22 MRSA strains. Sci. Rep. 6:31360. doi: 10.1038/srep31360
Malachowa, N., Kobayashi, S. D., Braughton, K. R., and DeLeo, F. R. (2013). Mouse model of Staphylococcus aureus skin infection. Methods Mol. Biol. 1031, 109–116. doi: 10.1007/978-1-62703-481-4_14
Malachowa, N., Whitney, A. R., Kobayashi, S. D., Sturdevant, D. E., Kennedy, A. D., Braughton, K. R., et al. (2011). Global changes in staphylococcus aureus gene expression in human blood. PLoS One 6:e18617. doi: 10.1371/journal.pone.0018617
Nygaard, T. K., DeLeo, F. R., and Voyich, J. M. (2008). Community-associated methicillin-resistant Staphylococcus aureus skin infections: advances toward identifying the key virulence factors. Curr. Opin. Infect. Dis. 21, 147–152. doi: 10.1097/QCO.0b013e3282f64819
Nygaard, T. K., Pallister, K. B., DuMont, A. L., DeWald, M., Watkins, R. L., Pallister, E. Q., et al. (2012). Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One 7:e36532. doi: 10.1371/journal.pone.0036532
Nygaard, T. K., Pallister, K. B., Ruzevich, P., Griffith, S., Vuong, C., and Voyich, J. M. (2010). SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J. Infect. Dis. 201, 241–254. doi: 10.1086/649570
Nygaard, T. K., Pallister, K. B., Zurek, O. W., and Voyich, J. M. (2013). The impact of α-toxin on host cell plasma membrane permeability and cytokine expression during human blood infection by CA-MRSA USA300. J. Leukoc. Biol. 94, 971–979. doi: 10.1189/jlb.0213080
Pannen, D., Fabisch, M., Gausling, L., and Schnetz, K. (2016). Interaction of the RcsB response regulator with auxiliary transcription regulators in Escherichia coli. J. Biol. Chem. 291, 2357–2370. doi: 10.1074/jbc.M115.696815
Ran, Y., Liu, M., Zhu, H., Nygaard, T. K., Browne, D. E., Fabian, M., et al. (2010). Spectroscopic identification of heme axial ligands in HtsA that are involved in heme acquisition by Streptococcus pyogenes. Biochemistry 49, 2834–2842. doi: 10.1021/bi901987h
Salgado-Pabón, W., Herrera, A., Vu, B. G., Stach, C. S., Merriman, J. A., Spaulding, A. R., et al. (2014). Staphylococcus aureus β-toxin production is common in strains with the β-toxin gene inactivated by bacteriophage. J. Infect. Dis. 210, 784–792. doi: 10.1093/infdis/jiu146
Solà, M., Gomis-Rüth, F. X., Serrano, L., González, A., and Coll, M. (1999). Three-dimensional crystal structure of the transcription factor PhoB receiver domain. J. Mol. Biol. 285, 675–687. doi: 10.1006/jmbi.1998.2326
Spaan, A. N., Reyes-Robles, T., Badiou, C., Cochet, S., Boguslawski, K. M., Yoong, P., et al. (2015). Staphylococcus aureus Targets the Duffy Antigen Receptor for Chemokines (DARC) to Lyse Erythrocytes. Cell Host Microbe 18, 363–370. doi: 10.1016/j.chom.2015.08.001
Sun, F., Li, C., Jeong, D., Sohn, C., He, C., and Bae, T. (2010). In the Staphylococcus aureus two-component system sae, the response regulator SaeR Binds to a direct repeat sequence and DNA binding requires phosphorylation by the sensor kinase SaeS. J. Bacteriol. 192, 2111–2127. doi: 10.1128/JB.01524-09
Ventura, C. L., Malachowa, N., Hammer, C. H., Nardone, G. A., Robinson, M. A., Kobayashi, S. D., et al. (2010). Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One 5:e11634. doi: 10.1371/journal.pone.0011634
Villaruz, A. E., Bubeck Wardenburg, J., Khan, B. A., Whitney, A. R., Sturdevant, D. E., Gardner, D. J., et al. (2009). A point mutation in the agr locus rather than expression of the Panton-Valentine leukocidin caused previously reported phenotypes in Staphylococcus aureus pneumonia and gene regulation. J. Infect. Dis. 200, 724–734. doi: 10.1086/604728
Voyich, J. M., Braughton, K. R., Sturdevant, D. E., Whitney, A. R., Said-Salim, B., Porcella, S. F., et al. (2005). Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175, 3907–3919. doi: 10.4049/jimmunol.175.6.3907
Voyich, J. M., Otto, M., Mathema, B., Braughton, K. R., Whitney, A. R., Welty, D., et al. (2006). Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J. Infect. Dis. 194, 1761–1770. doi: 10.1086/509506
Voyich, J. M., Vuong, C., DeWald, M., Nygaard, T. K., Kocianova, S., Griffith, S., et al. (2009). The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J. Infect. Dis. 199, 1698–1706. doi: 10.1086/598967
Wang, F. F., Deng, C. Y., Cai, Z., Wang, T., Wang, L., Wang, X. Z., et al. (2014). A three-component signalling system fine-tunes expression kinetics of HPPK responsible for folate synthesis by positive feedback loop during stress response of Xanthomonas campestris. Environ. Microbiol. 16, 2126–2144. doi: 10.1111/1462-2920.12293
Young, J. D. E., Leong, L. G., DiNome, M. A., and Cohn, Z. A. (1986). A semiautomated hemolysis microassay for membrane lytic proteins. Anal. Biochem. 154, 649–654. doi: 10.1016/0003-2697(86)90042-4
Keywords: Staphylococcus aureus, saeR/S, virulence, pathogenesis, two-components system, neutrophil, toxin, transcription
Citation: Nygaard TK, Borgogna TR, Sward EW, Guerra FE, Dankoff JG, Collins MM, Pallister KB, Chen L, Kreiswirth BN and Voyich JM (2018) Aspartic Acid Residue 51 of SaeR Is Essential for Staphylococcus aureus Virulence. Front. Microbiol. 9:3085. doi: 10.3389/fmicb.2018.03085
Received: 05 October 2018; Accepted: 29 November 2018;
Published: 14 December 2018.
Edited by:
Konstantin V. Korotkov, University of Kentucky, United StatesReviewed by:
Shivani Agarwal, Northwestern University, United StatesCopyright © 2018 Nygaard, Borgogna, Sward, Guerra, Dankoff, Collins, Pallister, Chen, Kreiswirth and Voyich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tyler K. Nygaard, dHlsZXIubnlnYWFyZEBtc3UubW9udGFuYS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.