
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 06 December 2018
Sec. Microbiotechnology
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.02903
 Stefano Amalfitano1*
Stefano Amalfitano1* Caterina Levantesi1
Caterina Levantesi1 Laurent Garrelly2
Laurent Garrelly2 Donatella Giacosa3
Donatella Giacosa3 Francesca Bersani3
Francesca Bersani3 Simona Rossetti1
Simona Rossetti1During longer-lasting future space missions, water renewal by ground-loaded supplies will become increasingly expensive and unmanageable for months. Space exploration by self-sufficient spacecrafts is thus demanding the development of culture-independent microbiological methods for in-flight water monitoring to counteract possible contamination risks. In this study, we aimed at evaluating total microbial load data assessed by selected early-warning techniques with current or promising perspectives for space applications (i.e., HPC, ATP-metry, qPCR, flow cytometry), through the analysis of water sources with constitutively different contamination levels (i.e., chlorinated and unchlorinated tap waters, groundwaters, river waters, wastewaters). Using a data-driven double-threshold identification procedure, we presented new reference values of water quality based on the assessment of the total microbial load. Our approach is suitable to provide an immediate alert of microbial load peaks, thus enhancing the crew responsiveness in case of unexpected events due to water contamination and treatment failure. Finally, the backbone dataset could help in managing water quality and monitoring issues for both space and Earth-based applications.
Aquatic microbes are retained as primary constituents of all known water sources aboard the international space station (ISS), as well as in future human spaceflights and planetary outposts (Horneck et al., 2010). Since space missions are expected to become longer lasting, space exploration is demanding the development of methods for in-flight monitoring, suitable to face microbial contamination risks within human confined conditions (Yamaguchi et al., 2014; Karouia et al., 2017; Allen et al., 2018). NASA has been developed microbial control strategies to minimize detrimental cell growth during spaceflight by reducing humidity, eliminating free water, and maintaining high-volume exchange and air filtration. The ISS is maintained at around 22°C with a relative humidity of around 60%, with pressure and oxygen concentrations very close to those at sea level on Earth (Pierson et al., 2013).
Currently on ISS, water samples are archived every 6 months for further post-flight analysis. In addition, samples are processed in-flight once every 3 months with the US-supplied Water Microbiology Kit for the quantification of heterotrophic bacteria heterotrophic plate counts (HPC) and the presence of coliforms (Van Houdt et al., 2012). Leaving aside the evidence that the microbial biomass is mainly composed by viable but not cultivable microorganisms (Colwell, 2009), the development of culture-independent methods for space applications is pushed fundamentally by the requirement of timeliness of results and the need to avoid microbial regrowth from analytical wastes. Candidate methods have to comply with limitations in volume, working time, power, safety and microgravity, thus being suitable for automation, lightweight and with minimal consumables. Since first experiments conducted in space, bioluminescence and PCR-based methods have been tested for monitoring the microbial load under microgravity conditions (Guarnieri et al., 1997; Castro et al., 2004; La Duc et al., 2004). Currently, joint scientific and industrial efforts have been focused on developing an on-line self-loading ATP-based monitoring module within an integrated breadboard system to control microbial contamination in water systems during human spaceflights. The module contains ATP-releasing reagents to lyse cells and release ATP, which reacts with d-luciferin in presence of luciferase to produce detectable light signals. The light intensity is then measured as relative light units (RLU), which can be interpreted as a measure of ATP concentration (i.e., H2020 EU project “Biocontamination Integrated Control of Wet Systems for Space Exploration1”). Moreover on the ISS, biomolecular methods and sample processing for DNA extraction and gene sequencing has been tested within dedicated projects (e.g., Genes in Space-3, Wet-lab2) and through customized devices (e.g., miniPCR, MinION, Razor EX PCR) (Boguraev et al., 2017; Karouia et al., 2017; Parra et al., 2017). The quantitative real-time PCR (qPCR) could be applied to assess the absolute bacterial abundance by measuring the number of 16S rRNA housekeeping gene copies in the total DNA extracted from a water sample (Smith and Osborn, 2009). Among the consolidated water monitoring approaches for the direct quantification of aquatic microorganisms, flow cytometry (FCM) has to be also considered, since it was defined as an unparalleled high-throughput technology for single cell counting and characterization in a panoply of applications (Robinson and Roederer, 2015). The basic cytometric detection combines laser light scatter and fluorescence signals, with the ability to discriminate microbial cell subpopulations, phenotypes (e.g., size and shape), and constitutive properties detected upon specific staining procedures (e.g., per-cell nucleic acid content) (Wang et al., 2010).
By largely disregarding recent monitoring techniques and their methodological improvements, current standards for microbiological evaluations are set on the occurrence of few microorganisms, indicators of fecal pollution and hence of the possible co-presence of pathogenic species (European Union, 1998). According to the ISS Medical Operations Requirements Document (Duncan et al., 2008), ISS waters must be free of coliforms (i.e., 0 CFU/100 ml), with a HPC value ≤ 50 CFU/ml, and sample processing and analysis have to follow precise procedural steps using the US Environmental Health System water kit (NASA, 2005). Recently, the space water microbiology was pushed beyond the standardized cultivation-based methods (Moissl-eichinger et al., 2016), also due to the finding that spaceflight microgravity conditions provided conflicting results, with insufficient and largely unpredictable indications on the microbial growth patterns and the virulence of opportunistic human pathogens (Huang et al., 2018).
In this study, we explored whether alternative methods to assess the total microbial load could be supportive of routine monitoring practices, thus challenging conventional HPC in space applications. Through the analysis of water sources with increasingly different microbial loads (i.e., tap waters, groundwaters, river waters, wastewaters), we aimed to (i) cross-validate early-warning techniques suitable to assess the onboard water microbial load and selected among those consolidated in terrestrial applications (i.e., HPC, ATP-metry, qPCR, FCM), and (ii) propose a data-driven procedure to determine new reference values based on the cultivation-independent assessment of the total microbial load.
By considering the high costs and logistic limitations of water renewal with Earth-supplied resources, we hypothesized that a double-threshold identification procedure could be applicable to identify water microbial contamination events and help counteracting health-risks, which could unexpectedly occur during longer-lasting space missions.
Water samples were collected in one-liter sterile plastic bottles containing pre-dosed sodium thiosulfate (1 ml of 10% solution per bottle), transported in refrigerated boxes and stored at 5 ± 3°C for maximum 24 h before analysis. A total of 35 samples was drawn from five types of water sources known to retain a constitutively different microbial load. Each water type was represented by seven independent samples: chlorinated Tap Water (cTW1-7), unchlorinated Tap Water (uTW1-7), Ground Water (GW1-7), River Water (RW1-7), and Waste Water (WW1-7).
Turbidity was measured in all samples and expressed as nephelometric turbidity units (NTU). The chlorinated tap waters (cTWs = 0.2–0.3 NTU) were collected along the drinking water distribution network, namely at the inlet of water kiosks in seven towns supplied by SMAT in the province of Turin (Italy). All cTWs were disinfected with sodium hypochlorite (final concentration 0.1–0.2 mg/l). Unchlorinated tap waters (uTWs = 0.1–0.5 NTU) were gathered at the granular activated carbon filter outlets of a drinking water treatment plant, following sedimentation, break-point chlorination, chlorine-dioxide peroxidation and clariflocculation with polyhydroxy aluminum chloride. Groundwater samples (GWs = 0.2–7.2 NTU) were collected from wells located in the urban area of Turin (Italy), before any kind of subsequent treatment. River water samples (RW = 0.2–1.8 NTU) were collected from water catchment areas upstream different drinking water treatment plants. Wastewater samples (WWs = 1.0–9.6 NTU) were collected from secondary effluents of activated-sludge wastewater treatment plants.
Heterotrophic plate counts were performed on 90-mm Petri dishes filled with either yeast extract agar (YEA) medium (Sifin Diagnostics, Germany) or Reasoner’s 2A Agar (R2A) medium (Thermo-Fisher Diagnostics, United States) (International Organization for Standardization, 1999; APHA, 2005). Following lab incubation at 22°C for 3 or 7 days, all results derived from the average of two plates, each within the countable range. Depending on the expected microbial concentrations, different volumes (from 1 ml to 100 ml) were analyzed in order to reach a measurable range in terms of colony forming units (CFUs). Volumes up to 1 ml were included in molten medium, while larger volumes (up to 100 ml) were filtered onto cellulose nitrate membranes (0.45-μm pore size; Millipore).
The total intracellular ATP content was measured to estimate the microbial load using the technology developed by GL Biocontrol (Clapiers, France). Briefly, 2 drops of DENDRIAG reagent were added to the water sample (10–50 ml) and measured using the GL Biocontrol instrument to obtain the R1 (RLU) result. Then four drops of DENDRIAG reagent were dispensed into the plastic packaging of the filter and the reactive was backflush by pressing air through the filter. The reagent was pushed into the measuring tube and measured to obtain the R1 (RLU) result. Then, one drop of STANDARD 1000 reagent was added and measured to obtain the R2 (RLU) result. The concentration of intracellular ATP is given in picograms per milliliter, by the following calculations:
With R1 (RLU), sample result; R2 (RLU), sample + standard result; V (ml), volume of water analyzed.
The qPCR Sybr Green assay was utilized to measure the 16S rDNA gene copy number in 25 μl of sample using the CFX96 Touch Real Time PCR Detection System (Bio-Rad, United States). Reactions contained 5 μl of DNA template (from 50 to 5 ng DNA for reaction tube), 12.5 μL of 2X SYBR Green Supermix (Bio-Rad, United States), and primers at required concentrations (Di Cesare et al., 2015). Triplicates samples and no template controls (NTCs) were analyzed. The E. coli 16S rDNA was used as positive control, and standard curves were produced with gene copy numbers from 102 to 106 genes per reaction tube. The concentration of the amplified DNA was determined using NanoDrop spectrophotometer. The gene copy number per μl of solution was calculated according to literature reports (Czekalski et al., 2012). The qPCR results were reported as the mean of measurements of triplicates analysis with standard deviations. Data were analyzed with the CFX ManagerTM software v3.1 (Bio-Rad, Italy).
The aquatic microbial cells were characterized by using the Flow Cytometer A50-micro (Apogee Flow System, Hertfordshire, England) equipped with a solid state laser set at 20 mV and tuned to an excitation wave length of 488 nm. The volumetric absolute counting was carried out on fixed (2% formaldehyde, final concentration) and unfixed water samples, stained with SYBR Green I (1:10000 dilution; Molecular Probes, Invitrogen) or with SYBR Green I and propidium iodide (PI = 10 μg ml−1, f.c.) for 10 min in the dark at room temperature. The light scattering signals (forward and side scatters), the green fluorescence (530/30 nm) and red fluorescence (>610 nm) were acquired for the single cell characterization. A fluorescence threshold was set at 10 units on the green channel. Samples were run at low flow rates to keep the number of events below 1000 events per second. The total number of prokaryotic cells (i.e., total cell counts – TCC) was determined by their signatures in a plot of the side scatter vs. the green fluorescence (Amalfitano et al., 2018). Live and dead cells were differentiated in a plot of green vs. red fluorescence. Viable cells (i.e., intact cell counts – ICC) showed higher green fluorescence signals than the membrane compromised dead cells selectively marked in red by propidium iodide (Grégori et al., 2018). The instrumental settings were kept the same for all samples in order to achieve comparable data. The data were analyzed using the Apogee Histogram Software v2.05.
Total cell counts were double-checked by epifluorescence microscopy on all samples by following consolidated literature procedures (Porter and Feig, 1980). Briefly, aliquots of fixed samples were filtered through 0.2 μm polycarbonate filters (Ø 25 mm, Millipore) by gentle vacuum (<0.2 bar), and stained for 5 min with DAPI (4′, 6-diamidino-2-phenylindole; 1.5 μg ml−1 final concentration). Filters were stored at −20°C until microscope inspection. Total cell counts were performed by the epifluorescence microscope BX51 (Olympus, Germany) at 1500X magnification by counting a minimum of 300 cells in >10 microscopic fields randomly selected across each filter.
All data were log (x + 1) transformed to facilitate comparability among parameters derived from different methods and waters sources. The non-parametric Kruskal-Wallis test, with Mann-Whitney post-hoc pairwise comparisons, was used to verify whether statistical differences in median values occurred among water groups according to each single parameter. The one-way non-parametric multivariate analysis of variance (PERMANOVA), based on the Euclidean distance measure, was used to test the overall significance of difference between water groups.
A frequency distribution model (FDM) of log-transformed data was applied by using the histogram function of the software PAST v3.20 (Hammer et al., 2001). The bin width (h) was estimated according to the zero-stage rule (Wand, 1997):
where sd is the sample standard deviation and IQ the interquartile range. The number of bins (n) was set at 4 for each single-parameter data series in order to discriminate between two water groups, represented by two bins each and named waters with low microbial load (i.e., first two bins) and high microbial load (i.e., second two bins).
Following the consolidated approach applied to assess upper confidence limits for natural background chemical concentrations (U. S. Environmental Protection Agency [USEPA], 2002), a first confidence threshold (hereafter named warning threshold) was set as the 95th percentile of values assessed from the low microbial load group. A second and higher confidence threshold (hereafter named alarm threshold) was arbitrarily set as the 5th percentile of values assessed from the high microbial load group.
The linear regression model (LRM) was applied in order to cross-validate the FDM thresholds using the possible combinations of log-transformed independent parameters (i.e., HPC-R2A vs. ATP; HPC-R2A vs. qPCR; HPC-R2A vs. FCM-ICC; ATP vs. qPCR; ATP vs. FCM-ICC; qPCR vs. FCM-ICC). Spearman’s correlation coefficients (r) were used for the LRM statistical endorsement. Warning and alarm threshold values, computed by the FDM on single parameters, were applied to each LRM to calculate the corresponding values from linear correlation equations. The mean values (±standard deviation) of four data-driven estimates of warning and alarm thresholds (i.e., one from FDMs plus three from LRMs) of each independent parameter were calculated and presented as new reference values of water quality. All data elaborations were performed by the software PAST v3.20 (Hammer et al., 2001).
The number of heterotrophic bacterial colonies (HPC) varied greatly depending on water origin and across the tested growth conditions, with values ranging from 0 to over 106 CFU/ml. TWs and GWs showed significantly lower values in comparison to RWs and WWs (Supplementary Table S1). Only in TWs, HPC on YEA medium increased significantly passing from 3 to 7 days of incubation (Kruskal-Wallis test, p < < 0.01). After the longer incubation time, there was a statistically significant difference between colony numbers found on YEA and R2A and between all water groups (Kruskal-Wallis test, p < 0.05), though HPC from cTWs was close to the method detection limit in all growth conditions. HPC on R2A medium showed values higher than that on YEA medium.
FDM thresholds were plotted in Figure 1 and reported in Table 1. Overall, we found significant differences between contamination levels of all water groups, assessed in terms of cultivability and tested by PERMANOVA (p < < 0.001).
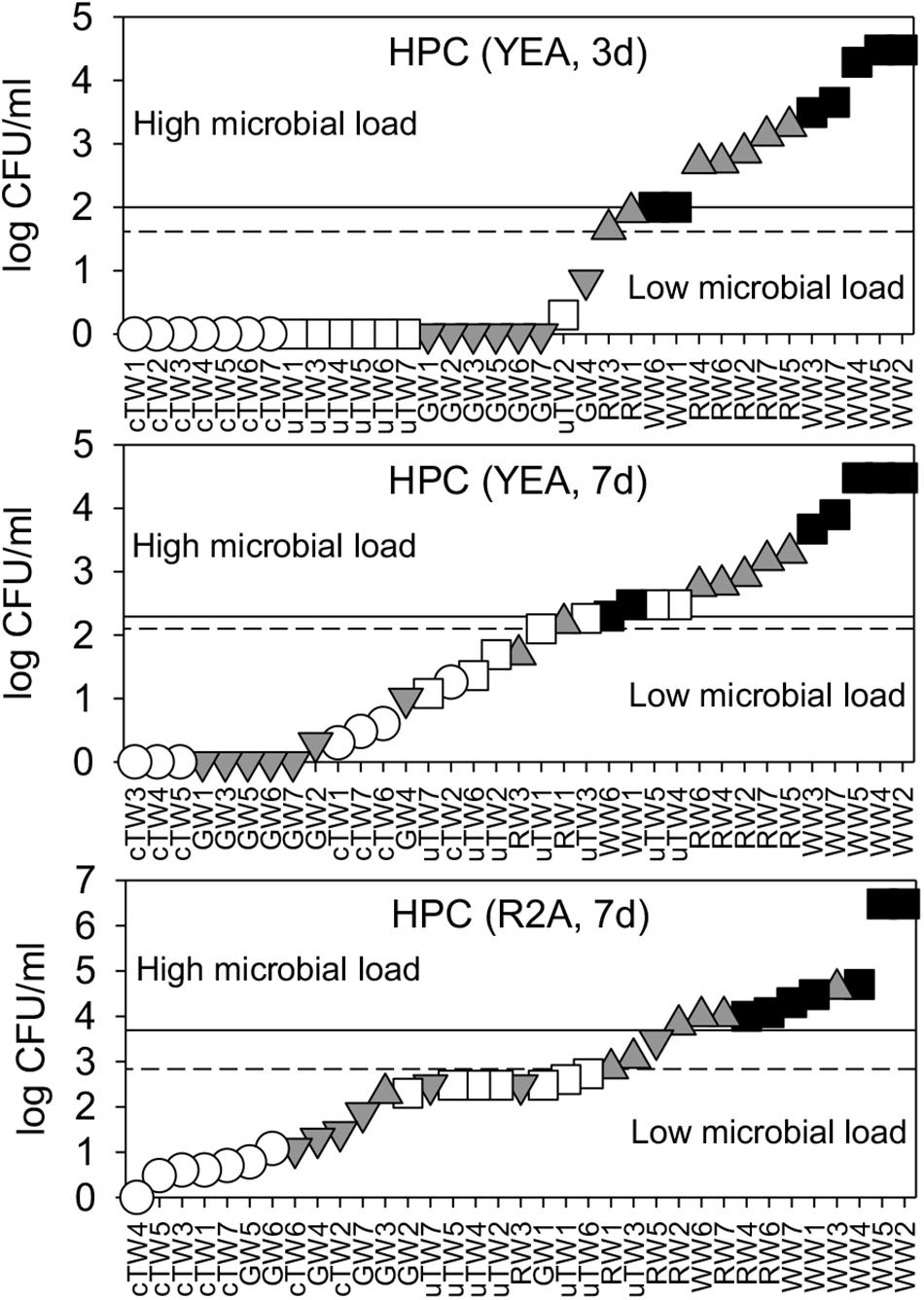
Figure 1. Water microbial load as assessed by plate cultivation on Yeast Agar extract (YEA) and R2A medium, upon 3 days, and 7 days of incubation. HPC data were plotted in ascending rank order on a logarithmic scale in order to better visualize warning (dashed lines) and alarm (solid lines) thresholds, which discriminate waters with low and high microbial load. Samples included chlorinated Tap Waters (cTW1-7), unchlorinated Tap Waters (uTW1-7), Ground Waters (GW1-7), Rivers Waters (RW1-7), and Waste Waters (WW1-7).
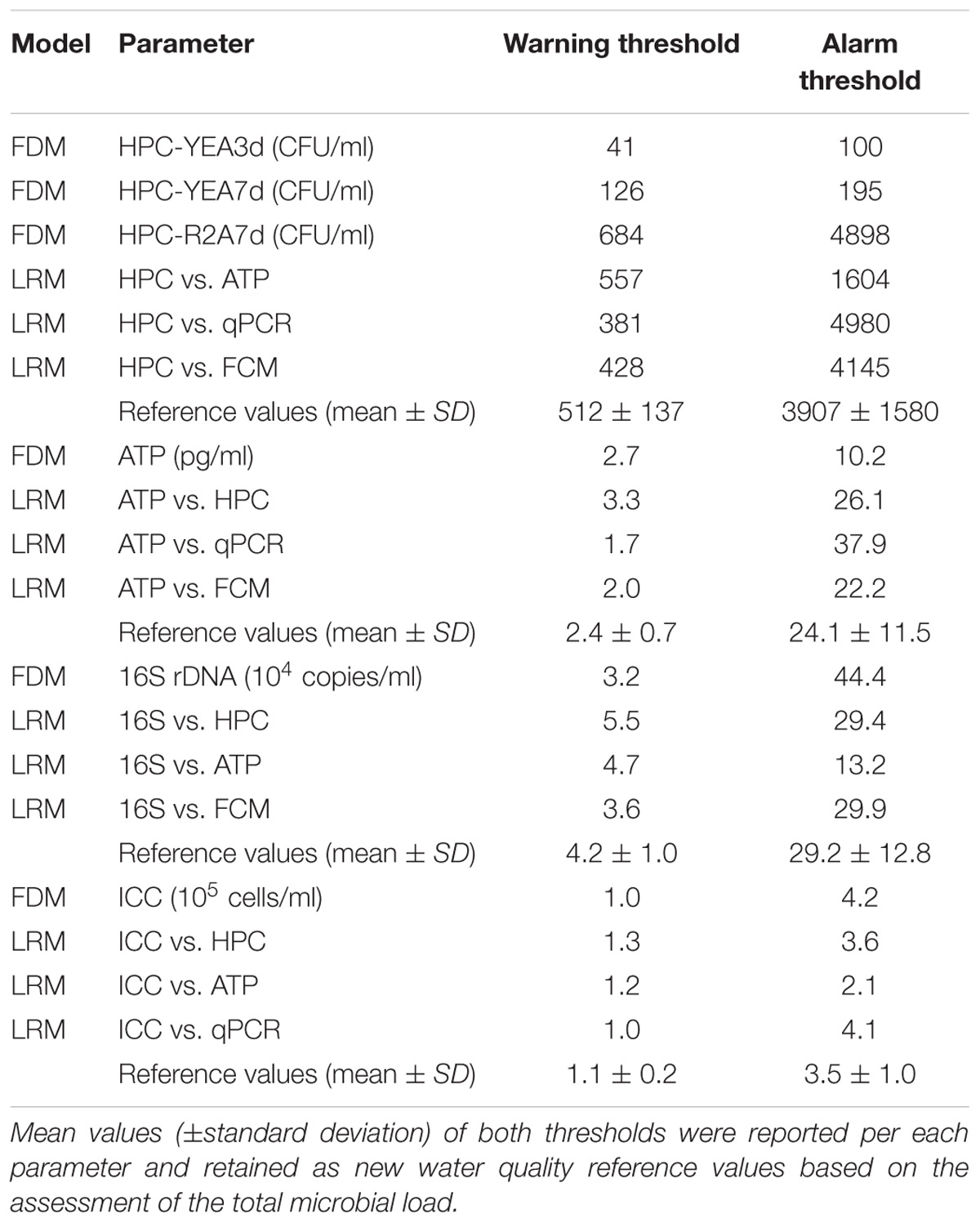
Table 1. Warning and alarm thresholds, computed according to the frequency distribution model (FDM) of each single parameter (see also Figures 1, 2) and the correlation equations of Linear Regression Model (LRM) between pairs of parameters.
ATP concentrations varied over more than 6 log units, with a sharp increase from tap waters (range 1.5–24.1 × 10−3 pg ATP/ml) to waste waters (range 1.9–59.0 × 102 pg ATP/ml) (Figure 2A). Apart from TWs and GWs which showed similar values (Kruskal-Wallis test, p > 0.05), the differences in the mean ATP content among water groups were greater than would be expected by chance (Kruskal-Wallis test, p < 0.05).
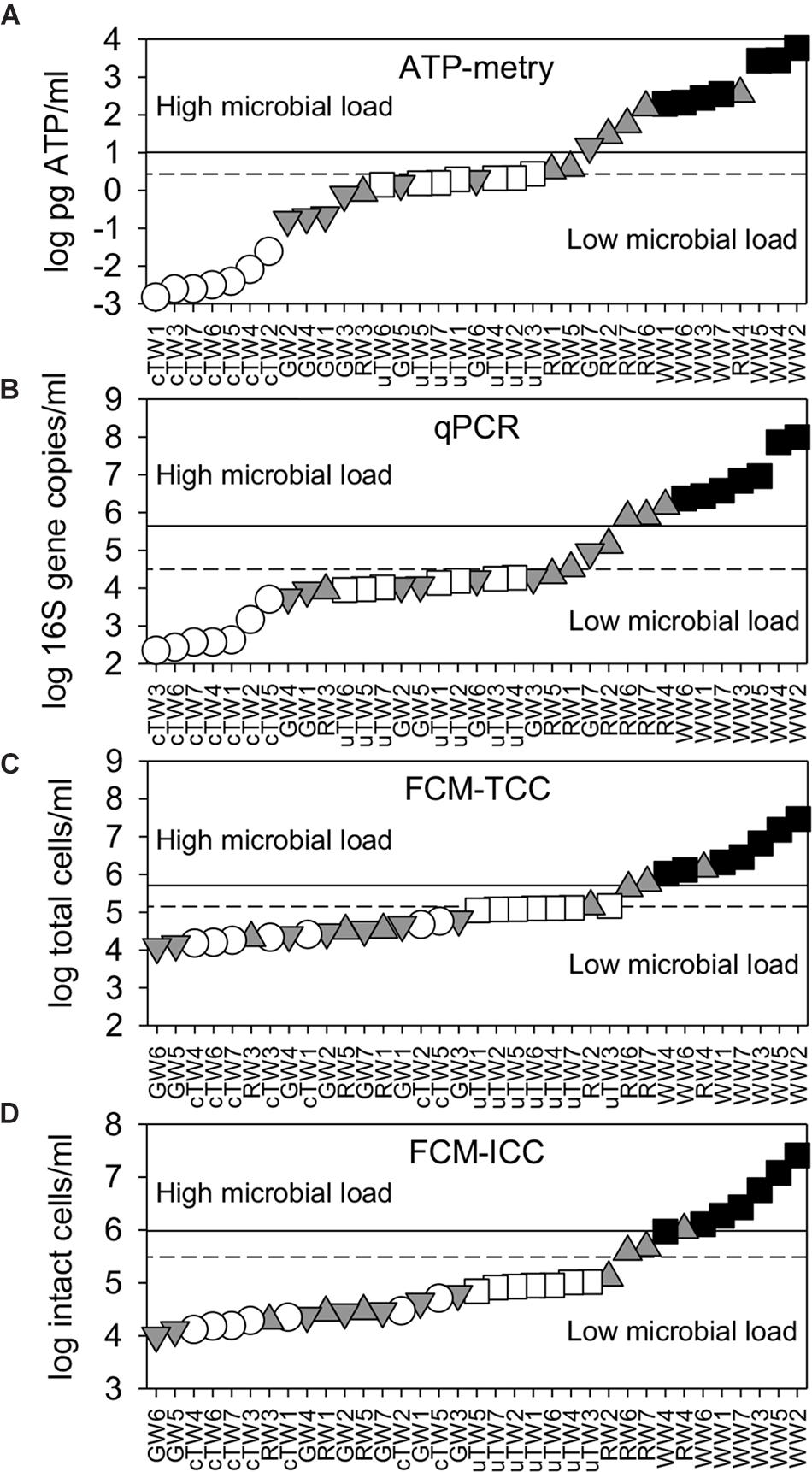
Figure 2. Water microbial load as assessed by alternative parameters [i.e., (A) ATP content; (B) 16S rRNA gene abundance; (C) total cell counts, TCC; and (D) intact cell counts, ICC]. All data were plotted in ascending rank order on a logarithmic scale in order to better visualize warning (dashed lines) and alarm (solid lines) thresholds, which discriminate waters with low and high microbial load. Samples included chlorinated Tap Waters (cTW1-7), unchlorinated Tap Waters (uTW1-7), Ground Waters (GW1-7), Rivers Waters (RW1-7), and Waste Waters (WW1-7).
As assessed by qPCR, the abundance of 16S rDNA copies varied from 2.2 × 102 copies/ml (with minimum values found in chlorinated tap waters) to 9.8 × 107 copies/ml (with maximum values found in WWs) (Figure 2B). GWs showed similar values to uTWs and RWs (Kruskal-Wallis, p > 0.10), but there were statistically significant differences among all other water groups (Kruskal-Wallis, p < 0.05).
As assessed by flow cytometry, TCC ranged over 3 log units, passing from 1.2 × 104 cells/ml (minimum values in GWs) to 2.9 × 107 cells/ml (maximum values in WWs) (Figure 2C). WWs showed a different mean value from all other groups (Kruskal-Wallis test, p < 0.01), while GWs did not show any statistical difference with cTWs and RWs (Kruskal-Wallis test, p > 0.07). Total cell counts double-checked by epifluorescence microscopy were similar and well correlated with FCM data points on the 1:1 log-log line (Spearman’s r = 0.91, p << 0.001; data not shown). On average, the great majority of total cells comprised membrane-intact cells (ICC = 84.1 ± 10.3% of TCC), with percentages lower in tap waters (78.1 ± 11.9%) and higher in ground waters (91.7 ± 5.0%). Given the limited variation range, ICC followed patterns very similar to TCC on the log scale (Figure 2D). FDM thresholds were plotted in Figure 2 and reported in Table 1. Overall, we found significant differences between the average contamination levels of all water groups, also using the combination of alternative parameters (i.e., ATP, 16S rDNA, ICC) (PERMANOVA, p < 0.01).
Over the monitored wide range of microbial contamination levels, positive linear correlations were observed between all parameters and methods applied in this study. HPC on R2A showed the best correlations with other parameters in comparison to HPC on YEA. The best fit was found in the log-log linear relation between ATP and HPC-R2A (Spearman’s r = 0.91) and qPCR data (Spearman’s r = 0.97), while the weakest between ATP and FCM data (Spearman’s r = 0.81). HPC-R2A and ICC showed higher correlation coefficients than HPC-YEA and TCC, respectively. Thus, they were used in correlation plots against all other parameters (Figure 3).
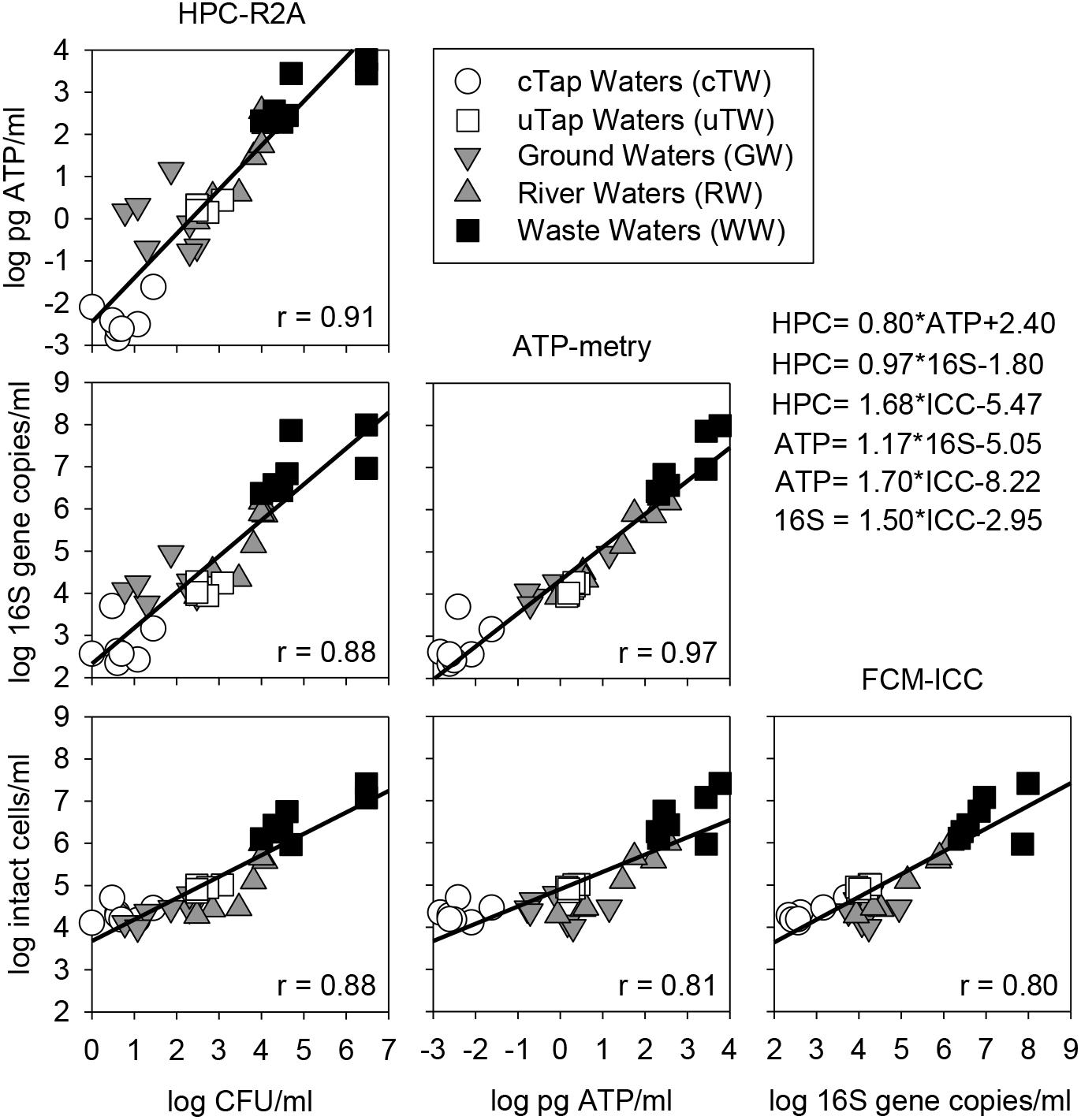
Figure 3. Correlation matrix (log-log) among alternative parameters to assess microbial contamination in waters of different origin (i.e., cTW and uTW, chlorinated and unchlorinated tap waters; GW, ground waters; RW, river waters; WW, waste waters). Equations of Linear Regression Models (LRMs) are reported, along with Sperman’s correlation coefficients (r).
Warning and alarm thresholds, computed according to the FDM of each single parameter and the LRM correlations equations between all pairs of data (Figure 3), were summarized in Table 1. The mean values (±standard deviation) of warning and alarm thresholds were proposed as new reference values based on the microbial load assessed by each independent parameter (Table 1).
For preflight and inflight waters, HPC acceptability levels were developed through space analytical experience to mitigate risks to crew health and to maintain the integrity of water treatment systems (e.g., prevention of biofouling in water recirculation and distribution network; microbial growth on hardware components) (Van Houdt et al., 2012; Pierson et al., 2013). Microbial specifications and monitoring requirements for ISS waters have fixed the limit of HPC ≤ 50 CFU/ml to meet the onboard quality standards in United States and Russian segments (Duncan et al., 2008).
When considering the cultivation conditions relatively more similar to those of the United States water kit (i.e., HPC on YEA after 3 incubation days at 22°C), TWs and GWs samples accomplished the HPC limit of 50 CFU/ml, with warning and alarm thresholds, respectively, lower and higher of that established for space requirements. However, the HPC thresholds were considerably higher if estimated from other cultivation conditions (Supplementary Table S1). In drinking water legislation and guidelines, maximum HPC limits can vary from 20 CFU/ml to 500 CFU/ml depending on local regulations and sampling locations (Allen et al., 2004).
In line with recently published data (Gensberger et al., 2015; Diduch et al., 2016), we found that HPC were influenced significantly by the type of carbon sources in the cultivation medium (i.e., HPC-R2A > HPC-YEA), the time of incubation (i.e., HPC at 3 days < HPC at 7 days), and the initial microbial load level (Figure 1). These results are critical when considering that the ISS is provided with four different supplied waters from the space agencies of United States (NASA), Europe (ESA), Russia (Roscosmos; Russian Federal Space Agency), and Japan (JAXA; Japanese Aerospace Exploration Agency). Water microbial communities (e.g., phylogenetic structure) and local treatment requirements (e.g., addition of different concentration of chlorine, silver, or iodine as biocide agents) may differ considerably, so as HPC outcomes. Further differences in microbial cell cultivability under space conditions are likewise expected owing to microgravity and varying cosmic radiation levels, as it was found either for specific bacterial suspended cultures (Kacena et al., 1999) or for strains isolated from built environments on Earth and cultivated on the ISS (Coil et al., 2016). Experiments have been conducted to improve the speed and efficiency of microbial cultivation assays on the ISS using disposable simple devices and microfluidic systems2,3. Major advantages arise from target-specific isolation and characterization of different types of microorganisms in pure cultures, including water-borne pathogens (Boitard et al., 2015). However, the HPC reliability for total microbial load assessments in space waters might fall far below the acceptable reproducibility levels, unless other cultivation-independent techniques are applied to provide confirmatory data.
Based on a 20 years’ experience on space microbial monitoring, ATP-metry has been retained a consistent approach for estimating the viable microbial biomass in water samples (Guarnieri et al., 1997; La Duc et al., 2004). By offering feasible automation options for space applications, we found that ATP-metry allowed to consistently discriminate water types according to their constitutive microbial contamination levels, also showing a wider variation range in comparison to the other selected parameters (Figure 2). The highest ratio between alarm and warning thresholds was also observed (Table 1).
In drinking water and food industries, routine ATP measurements were added upon commercially available ATP assay kits and compared in-depth to standard cultivation-based outcomes (Hammes et al., 2010; van der Wielen and van der Kooij, 2010; Bottari et al., 2015). One caveat is that community structure variations, with a natural succession of microbial cells with different ATP content (e.g., prokaryotic and eukaryotic cells), may be overlooked owing to the ataxonomic resolution of ATP assays. Therefore, the microbial load evaluations based on ATP-metry could be further strengthened by complimenting with specific cell-targeting parameters (e.g., biomolecular information, total cell counts, cell size measurements) (Siebel et al., 2008; Vang et al., 2014).
In space research, the successful application of biomolecular assays was found to rely on procedural improvements for extracting cell nucleic acids and selecting appropriate control samples (e.g., with the same amplification efficiency as the target sequence under microgravity conditions), along with instrumental developments (Yamaguchi et al., 2014).
In this study, the abundance of 16S rRNA gene copies was significantly different among water types, also showing significant correlations with values of total microbial load assessed by the other parameters (Figure 2). The estimated threshold values allowed discriminating waters with low and high microbial load (Table 1). Despite showing puzzling low values in TWs and GWs (on average 0.39 ± 0.07 16S rDNA/cell), the 16S rDNA per-cell ratio was highly variable among water types and consistent with literature data (Klappenbach, 2001; Matturro et al., 2013). In view of recent technological developments of molecular methods for space applications, we found that qPCR could be considered as a sensible method for water monitoring, although time-to-results can rise up to several hours from sampling (Lopez-Roldan et al., 2013). Advantages and limitations of the 16S rDNA targeting PCR procedures were reviewed extensively within the context of molecular techniques used to generate data for biomonitoring (Smith and Osborn, 2009; Porter and Hajibabaei, 2018). In particular, it was underlined that current protocols are definitely more informative when used to quantify the occurrence of target functional genes and species of interest (e.g., human pathogens and microorganisms of habitability concerns) rather than estimating the total microbial load (Smith and Osborn, 2009; Sohier et al., 2014).
Flow cytometry has been included in the roadmaps of space agencies for monitoring spaceflight-associated requirements (Crucian and Sams, 2005). Though it was not yet specifically tested for onboard water quality assessments, a customized FCM platform was already successfully tested on board the ISS to assess physiological adaptations of astronauts’ blood cells to microgravity (Crucian and Sams, 2012; Dubeau-Laramée et al., 2014; Phipps et al., 2014). In multiple full-scale terrestrial applications, detailed reasons were recognized and meticulously described to argue that FCM could represent a suitable alternative for routine microbiological water monitoring (Van Nevel et al., 2017).
Our results were in line with published data in terms of both total and intact cell counts assessed from different water types including drinking waters (Vital et al., 2012), ground waters (Amalfitano et al., 2014), river waters (Boi et al., 2016), and wastewaters (Foladori et al., 2010). Evidences of significant cross-correlation among microbial quantification techniques are widely reported in literature (Siebel et al., 2008; Vital et al., 2012). Accordingly, we found significant correlations between FCM data and results from epifluorescence microscopy, along with the water microbial load assessed by HPC, ATP-metry, and qPCR (Figure 3).
However, unexpected low TCC values particularly in some RWs and WWs could originate from the presence of suspended cell aggregates (i.e., verified by microscopic direct observations), which are acquired as single events (Casentini et al., 2016; Liu et al., 2016). Moreover, the cytometric evaluations are susceptible to increased background levels and debris found in very clean waters (Hammes et al., 2008), with possible TCC over-estimations in TWs. This could partly explain why TCC showed the lower data variation (i.e., 3 log units) in comparison to the other parameters among water types with such different origin and contamination levels. Accordingly, the alarm threshold was only three times higher than the warning threshold. Therefore, FCM routine analysis and developed protocols still require a thorough calibration and validation of their performances and drawbacks for space applications.
The total microbial load has been retained a key driver of microbial alterations due to varying environmental factors and water treatment settings in numerous applications on either natural or engineered aquatic systems (Osman et al., 2008; Harry et al., 2016; Besmer et al., 2017). In this study, all selected early-warning monitoring techniques showed pioneering potential applicability to space and human-confined environments, given the necessity to overcome some basic drawbacks of cultivation-based approaches (i.e., time-to-result up to several days from sampling; growth of opportunistic microorganisms from stored analytical wastes).
The appropriate consideration of microorganisms in human-confined habitats and their interactions with the space environment are essential to start designing a self-sufficient spacecraft for safe and successful future missions (Pierson, 2001). In extremely confined habitability conditions, as those found onboard crewed space-crafts and during longer-lasting flight missions, water renewal by ground-loaded supplies could be increasingly expensive and unmanageable for months (McCleskey et al., 2012). Therefore, water consumption needs and human health issues may fundamentally rely on a timely detection of unexpected microbiological contamination events, e.g., owing to failure of onboard water recycling and disinfection treatments.
Here we presented a data-driven double-threshold procedure intended to identify new reference values of water quality, using alternative cultivation-independent parameters suitable for (near) real-time assessments of the total microbial load. In current water regulations on Earth, several procedures have been recommended to distinguish between elements of geogenic and anthropogenic origin, elucidate the spatial distribution of chemical elements, identify the source of pollution, and estimate the related risks for human health and activities. The detection of anomalies in the concentration of major and trace chemical elements is one of the main tasks in the adopted statistical approaches (Reimann et al., 2005; Biddau et al., 2017; Stefania et al., 2018). Probability plots, in combination with a data pre-selection, were proposed to graphically represent trends and discontinuities, also identifying data exceeding fixed percentile values (generally the 90th, 95th, and 97.7th percentile outliers) (Preziosi et al., 2014). In this study, we followed a similar approach but using the microbial load data assessed by different methodologies in order to determine warning and alarm threshold values, respectively, set on the 95th and 5th percentiles of values found in the water samples with low and high microbial load.
By fundamentally relying on the number and distribution of the available data, the described procedure was not intended to provide fixed limits nor the risks associated with water microbial contamination events. The application of alternative parameters into regulatory water quality monitoring is carefully considered but still prevented by methodological and procedural issues, including inter-laboratory reproducibility, prioritization of water contaminants, and cross-validation of applied methodologies (Chapman, 1996).
The identified threshold values (see Table 1) could constitute novel reference values, in view of data deriving from real space conditions and human-confined environments. It is worth noting that two drinking water samples (i.e., Russian potable spring waters), analyzed after 5-years exposure to ISS microgravity conditions by using ATP-metry and flow cytometry with the same full methods herein described, exceeded the alarm thresholds (Bacci et al., 2018, unpublished). Thus, it is likely that some sort of water treatment should be considered to comply with the proposed reference values.
In conclusion, our results allowed identifying warning and alarm threshold values based on the assessment of the total microbial load, thus providing a backbone dataset to develop and test innovative water monitoring approaches for space and Earth-based applications. Cultivation-independent techniques could help in managing the spacecraft on-board water quality in (near) real time, and ultimately enhance the crew responsiveness by providing an immediate alert of microbial load peaks.
All authors have equally contributed to the experimental design. SA, CL, and SR wrote and edited the manuscript.
This work was supported by the European project BIOWYSE (H2020-COMPET-2015-687447).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02903/full#supplementary-material
Allen, C. S., Giraudo, M., Moratto, C., and Yamaguchi, N. (2018). “Spaceflight environment,” in Space Safety and Human Performance, eds B. G. Kanki, Clervoy J-F, and G. Sandal (Amsterdam: Elsevier), 87–138. doi: 10.1016/B978-0-08-101869-9.00004-2
Allen, M. J., Edberg, S. C., and Reasoner, D. J. (2004). Heterotrophic plate count bacteria — what is their significance in drinking water? Int. J. Food Microbiol. 92, 265–274. doi: 10.1016/j.ijfoodmicro.2003.08.017
Amalfitano, S., Del Bon, A., Zoppini, A., Ghergo, S., Fazi, S., Parrone, D., et al. (2014). Groundwater geochemistry and microbial community structure in the aquifer transition from volcanic to alluvial areas. Water Res. 65, 384–394. doi: 10.1016/j.watres.2014.08.004
Amalfitano, S., Fazi, S., Ejarque, E., Freixa, A., Romaní, A. M., and Butturini, A. (2018). Deconvolution model to resolve cytometric microbial community patterns in flowing waters. Cytometry Part A 93, 194–200. doi: 10.1002/cyto.a.23304
APHA (2005). Standard Methods for the Examination of Water and Wastewater. Stand. Methods Exam. Water Wastewater. Denver, CO: APHA, 541.
Besmer, M. D., Hammes, F., Sigrist, J. A., and Ort, C. (2017). Evaluating monitoring strategies to detect precipitation-induced microbial contamination events in karstic springs used for drinking water. Front. Microbiol. 8:2229. doi: 10.3389/fmicb.2017.02229
Biddau, R., Cidu, R., Lorrai, M., and Mulas, M. G. (2017). Assessing background values of chloride, sulfate and fluoride in groundwater: a geochemical-statistical approach at a regional scale. J. Geochem. Explor. 181, 243–255. doi: 10.1016/j.gexplo.2017.08.002
Boguraev, A.-S., Christensen, H. C., Bonneau, A. R., Pezza, J. A., Nichols, N. M., Giraldez, A. J., et al. (2017). Successful amplification of DNA aboard the International Space Station. NPJ Microgravity 3:26. doi: 10.1038/s41526-017-0033-39
Boi, P., Amalfitano, S., Manti, A., Semprucci, F., Sisti, D., Rocchi, M. B., et al. (2016). Strategies for water quality assessment: a multiparametric analysis of microbiological changes in river waters. River Res. Appl. 32, 490–500. doi: 10.1002/rra.2872
Boitard, L., Cottinet, D., Bremond, N., Baudry, J., and Bibette, J. (2015). Growing microbes in millifluidic droplets. Eng. Life Sci. 15, 318–326. doi: 10.1002/elsc.201400089
Bottari, B., Santarelli, M., and Neviani, E. (2015). Determination of microbial load for different beverages and foodstuff by assessment of intracellular ATP. Trends Food Sci. Technol. 44, 36–48. doi: 10.1016/j.tifs.2015.02.012
Casentini, B., Falcione, F. T., Amalfitano, S., Fazi, S., and Rossetti, S. (2016). Arsenic removal by discontinuous ZVI two steps system for drinking water production at household scale. Water Res. 106, 135–145. doi: 10.1016/j.watres.2016.09.057
Castro, V. A., Thrasher, A. N., Healy, M., Ott, C. M., and Pierson, D. L. (2004). Microbial characterization during the early habitation of the international space station. Microb. Ecol. 47, 119–126. doi: 10.1007/s00248-003-1030-y
Chapman, D. V. (1996). Water Quality Assessments: A Guide to the Use of Biota, Sediments, and Water in Environmental Monitoring, 2 Edn. London: D. V Chapman. doi: 10.4324/NOE0419216001
Coil, D. A., Neches, R. Y., Lang, J. M., Brown, W. E., Severance, M., Cavalier, D. D., et al. (2016). Growth of 48 built environment bacterial isolates on board the International Space Station (ISS). PeerJ 4, 1–11. doi: 10.7287/peerj.preprints.1654v1
Colwell, R. R. (2009). “Viable but not cultivable bacteria,” in Uncultivated Microorganisms, ed. S. S. Epstein (Berlin: Springer), 121–129. doi: 10.1007/978-3-540-85465-4_1
Crucian, B., and Sams, C. (2005). Reduced gravity evaluation of potential spaceflight-compatible flow cytometer technology. Cytometry B Clin. Cytom. 9, 1–9. doi: 10.1002/cyto.b.20057
Crucian, B., and Sams, C. (2012). Flow Cytometry Methods to Monitor Immune Dysregulation Associated With Spaceflight, ed. A. C. Berlin (Berlin: Springer), doi: 10.1007/978-3-642-22272-6
Czekalski, N., Berthold, T., Caucci, S., Egli, A., and Bürgmann, H. (2012). Increased levels of multiresistant bacteria and resistance genes after wastewater treatment and their dissemination into Lake Geneva, Switzerland. Front. Microbiol. 3:106. doi: 10.3389/fmicb.2012.00106
Di Cesare, A., Eckert, E. M., Teruggi, A., Fontaneto, D., Bertoni, R., Callieri, C., et al. (2015). Constitutive presence of antibiotic resistance genes within the bacterial community of a large subalpine lake. Mol. Ecol. 24, 3888–3900. doi: 10.1111/mec.13293
Diduch, M., Polkowska, Z., and Namieśnik, J. (2016). The role of heterotrophic plate count bacteria in bottled water quality assessment. Food Control 61, 188–195. doi: 10.1016/j.foodcont.2015.09.024
Dubeau-Laramée, G., Rivière, C., Jean, I., Mermut, O., and Cohen, L. Y. (2014). Microflow1, a sheathless fiber-optic flow cytometry biomedical platform: demonstration onboard the international space station. Cytometry. Part A 85, 322–331. doi: 10.1002/cyto.a.22427
Duncan, J. M., Bogomolov, V. V., Castrucci, F., Koike, Y., Comtois, J. M., and Sargsyan, A. E. (2008). Organization and management of the International Space Station (ISS) multilateral medical operations. Acta Astronaut. 63, 1137–1147. doi: 10.1016/j.actaastro.2007.12.001
European Union (1998). 98/83/EC on the quality of water intented for human consumption. Adopt. Counc. 3, 32–54.
Foladori, P., Bruni, L., Tamburini, S., and Ziglio, G. (2010). Direct quantification of bacterial biomass in influent, effluent and activated sludge of wastewater treatment plants by using flow cytometry. Water Res. 44, 3807–3818. doi: 10.1016/j.watres.2010.04.027
Gensberger, E. T., Gössl, E.-M., Antonielli, L., Sessitsch, A., and Kostić, T. (2015). Effect of different heterotrophic plate count methods on the estimation of the composition of the culturable microbial community. PeerJ 3:e862. doi: 10.7717/peerj.862
Grégori, G., Denis, M., Sgorbati, S., and Citterio, S. (2018). Resolution of viable and membrane-compromised free bacteria in aquatic environments by flow cytometry. Curr. Protoc. Cytometry. 85:e42. doi: 10.1002/cpcy.42
Guarnieri, V., Gaia, E., Battocchio, L., Pitzurra, M., Savino, A., Pasquarella, C., et al. (1997). New methods for microbial contamination monitoring: an experiment on board the mir orbital station. Acta Astronaut. 40, 195–201. doi: 10.1016/S0094-5765(97)00102-1
Hammer, Ø, Harper, D. A. T. A. T., and Ryan, P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron 4, 1–9.
Hammes, F., Berney, M., Wang, Y., Vital, M., Köster, O., and Egli, T. (2008). Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 42, 269–277. doi: 10.1016/j.watres.2007.07.009
Hammes, F., Goldschmidt, F., Vital, M., Wang, Y., and Egli, T. (2010). Measurement and interpretation of microbial adenosine tri-phosphate ( ATP ) in aquatic environments. Water Res. 44, 3915–3923. doi: 10.1016/j.watres.2010.04.015
Harry, I. S. K., Ameh, E., Coulon, F., and Nocker, A. (2016). Impact of treated sewage effluent on the microbiology of a small brook using flow cytometry as a diagnostic tool. Water Air Soil Pollut. 227:57. doi: 10.1007/s11270-015-2723-2729
Horneck, G., Klaus, D. M., and Mancinelli, R. L. (2010). Space Microbiology. Microbiol. Mol. Biol. Rev 74, 121–156. doi: 10.1128/MMBR.00016-19
Huang, B., Li, D.-G., Huang, Y., and Liu, C.-T. (2018). Effects of spaceflight and simulated microgravity on microbial growth and secondary metabolism. Mil. Med. Res. 5:18. doi: 10.1186/s40779-018-0162-9
International Organization for Standardization (1999). Water Quality: Enumeration of Culturable Micro-organisms: Colony Count by Inoculation in a Nutrient Agar Culture Medium. Geneva: International Organization for Standardization.
Kacena, M. A., Merrell, G. A., Manfredi, B., Smith, E. E., Klaus, D. M., and Todd, P. (1999). Bacterial growth in space flight: logistic growth curve parameters for Escherichia coli and Bacillus subtilis. Appl. Microbiol. Biotechnol. 51, 229–234. doi: 10.1007/s002530051386
Karouia, F., Peyvan, K., and Pohorille, A. (2017). Toward biotechnology in space: high-throughput instruments for in situ biological research beyond Earth. Biotechnol. Adv. 35, 905–932. doi: 10.1016/j.biotechadv.2017.04.003
Klappenbach, J. A. (2001). rrndb: the ribosomal rna operon copy number database. Nucleic Acids Res. 29, 181–184. doi: 10.1093/nar/29.1.181
La Duc, M. T., Kern, R., and Venkateswaran, K. (2004). Microbial monitoring of spacecraft and associated environments. Microb. Ecol. 47, 150–158. doi: 10.1007/s00248-003-1012-0
Liu, G., Ling, F. Q., van der Mark, E. J., Zhang, X. D., Knezev, A., Verberk, J. Q. J. C., et al. (2016). Comparison of particle-associated bacteria from a drinking water treatment plant and distribution reservoirs with different water sources. Sci. Rep. 6:20367. doi: 10.1038/srep20367
Lopez-Roldan, R., Tusell, P., Cortina, J. L., Courtois, S., and Cortina, J. L. (2013). On-line bacteriological detection in water. Trends Anal. Chem. 44, 46–57. doi: 10.1016/j.trac.2012.10.010
Matturro, B., Heavner, G. L., Richardson, R. E., and Rossetti, S. (2013). Quantitative estimation of Dehalococcoides mccartyi at laboratory and field scale: comparative study between CARD-FISH and real time PCR. J. Microbiol. Methods 93, 127–133. doi: 10.1016/j.mimet.2013.02.011
McCleskey, C., Rhodes, R., Lepsch, R., Henderson, E., and Robinson, J. (2012). “Approach to an Affordable and Productive Space Transportation System,” in Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit Joint Propulsion Conferences, (Los Angeles, CA: American Institute of Aeronautics and Astronautics). doi: 10.2514/6.2012-4153
Moissl-eichinger, C., Cockell, C., and Rettberg, P. (2016). venturing into new realms? microorganisms in space international space station. FEMS Microbiol. Rev. 40, 722–737. doi: 10.1093/femsre/fuw015
NASA (2005). Microbial Analysis of ISS Water Using the Water Microbiology Kit (WMK) and the Microbiology Water Analysis Kit. Houston: NASA.
Osman, S., Duc, M. T., La Dekas, A., and Newcombe, D. (2008). Microbial burden and diversity of commercial airline cabin air during short and long durations of travel. ISME J. 2, 482–497. doi: 10.1038/ismej.2008.11
Parra, M., Jung, J., Boone, T. D., Tran, L., Blaber, E. A., Brown, M., et al. (2017). Microgravity validation of a novel system for RNA isolation and multiplex quantitative real time PCR analysis of gene expression on the International Space Station. PLoS One 12:e0183480. doi: 10.1371/journal.pone.0183480
Phipps, W. S., Yin, Z., Bae, C., Sharpe, J. Z., Bishara, A. M., Nelson, E. S., et al. (2014). Reduced-gravity environment hardware demonstrations of a prototype miniaturized flow cytometer and companion microfluidic mixing technology. J. Vis. Exp. 13:e51743. doi: 10.3791/51743
Pierson, D. L., Botkin, D. J., Bruce, R. J., Castro, V. A., Smith, M. J., Oubre, C. M., et al. (2013). “Microbial monitoring of the international space station,” in Environmental Monitoring: A Comprehensive Handbook, ed. J. Moldenhauer (River Grove: DHI Publishing), 1–27.
Porter, K. G., and Feig, Y. S. (1980). The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25, 943–948. doi: 10.4319/lo.1980.25.5.0943
Porter, T. M., and Hajibabaei, M. (2018). Scaling up: A guide to high-throughput genomic approaches for biodiversity analysis. Mol. Ecol. 27, 313–338. doi: 10.1111/mec.14478
Preziosi, E., Parrone, D., Bon, A., and Del Ghergo, S. (2014). Natural background level assessment in groundwaters: probability plot versus pre-selection method. J. Geochem. Explor. 143, 43–53. doi: 10.1016/j.gexplo.2014.03.015
Reimann, C., Filzmoser, P., and Garrett, R. G. (2005). Background and threshold: critical comparison of methods of determination. Sci. Total Environ. 346, 1–16. doi: 10.1016/j.scitotenv.2004.11.023
Robinson, J. P., and Roederer, M. (2015). Flow cytometry strikes gold. Science 350, 739–740. doi: 10.1126/science.aad6770
Siebel, E., Wang, Y., Egli, T., and Hammes, F. (2008). Correlations between total cell concentration, total adenosine tri-phosphate concentration and heterotrophic plate counts during microbial monitoring of drinking water. Drink. Water Eng. Sci. Discuss 1, 71–86. doi: 10.5194/dwesd-1-71-2008
Smith, C. J., and Osborn, A. M. (2009). Advantages and limitations of quantitative PCR ( Q-PCR ) -based approaches in microbial ecology. FEMS Microbiol. Ecol. 67, 6–20. doi: 10.1111/j.1574-6941.2008.00629.x
Sohier, D., Pavan, S., Riou, A., Combrisson, J., and Postollec, F. (2014). Evolution of microbiological analytical methods for dairy industry needs. Front. Microbiol. 5:16. doi: 10.3389/fmicb.2014.00016
Stefania, G. A., Zanotti, C., Bonomi, T., Fumagalli, L., and Rotiroti, M. (2018). Determination of trigger levels for groundwater quality in landfills located in historically human-impacted areas. Waste Manag. 75, 400–406. doi: 10.1016/j.wasman.2018.01.043
U. S. Environmental Protection Agency [USEPA] (2002). Calculating Upper Confidence Limits for Exposure Point Concentrations at Hazardous Waste Sites. Washington, DC: USEPA.
van der Wielen, P., and van der Kooij, D. (2010). Effect of water composition, distance and season on the adenosine triphosphate concentration in unchlorinated drinking water in the Netherlands. Water Res. 44, 4860–4867. doi: 10.1016/j.watres.2010.07.016
Van Houdt, R., Mijnendonckx, K., and Leys, N. (2012). Microbial contamination monitoring and control during human space missions. Planet. Space Sci. 60, 115–120. doi: 10.1016/j.pss.2011.09.001
Van Nevel, S., Koetzsch, S., Proctor, C. R., Besmer, M. D., Prest, E. I., Vrouwenvelder, J. S., et al. (2017). Flow cytometric bacterial cell counts challenge conventional heterotrophic plate counts for routine microbiological drinking water monitoring. Water Res. 113, 191–206. doi: 10.1016/j.watres.2017.01.065
Vang, ÓK., Corfitzen, C. B., Smith, C., and Albrechtsen, H.-J. (2014). Evaluation of ATP measurements to detect microbial ingress by wastewater and surface water in drinking water. Water Res. 64, 309–320. doi: 10.1016/j.watres.2014.07.015
Vital, M., Dignum, M., Magic-knezev, A., Ross, P., Rietveld, L., and Hammes, F. (2012). Flow cytometry and adenosine tri-phosphate analysis: alternative possibilities to evaluate major bacteriological changes in drinking water treatment and distribution systems. Water Res. 46, 4665–4676. doi: 10.1016/j.watres.2012.06.010
Wand, M. P. (1997). Data-based choice of histogram bin width. Am. Stat. 51, 59–64. doi: 10.1080/00031305.1997.10473591
Wang, Y., Hammes, F., De Roy, K., Verstraete, W., and Boon, N. (2010). Past, present and future applications of flow cytometry in aquatic microbiology. Trends Biotechnol. 28, 416–424. doi: 10.1016/j.tibtech.2010.04.006
Keywords: water quality, confidence values, microbial contamination, aquatic bacteria, HPC, ATP, qPCR, flow cytometry
Citation: Amalfitano S, Levantesi C, Garrelly L, Giacosa D, Bersani F and Rossetti S (2018) Water Quality and Total Microbial Load: A Double-Threshold Identification Procedure Intended for Space Applications. Front. Microbiol. 9:2903. doi: 10.3389/fmicb.2018.02903
Received: 06 August 2018; Accepted: 13 November 2018;
Published: 06 December 2018.
Edited by:
Kartik Chandran, Columbia University, United StatesReviewed by:
Yonghong Bi, Chinese Academy of Sciences, ChinaCopyright © 2018 Amalfitano, Levantesi, Garrelly, Giacosa, Bersani and Rossetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Amalfitano, YW1hbGZpdGFub0BpcnNhLmNuci5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.