
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 29 October 2018
Sec. Microbial Symbioses
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.02565
Microbes play a crucial role in sustaining the coral holobiont’s functions and in particular under the pressure of environmental stressors. The effect of a changing environment on coral health is now a major branch of research that relies heavily on aquarium experiments. However, the effect of captivity on the coral microbiome remains poorly known. Here we show that different cold-water corals species have different microbiome responses to captivity. For both the DNA and the RNA fraction, Madrepora oculata bacterial communities were maintained for at least 6 months of aquarium rearing, while Lophelia pertusa bacteria changed within a day. Interestingly, bacteria from the genus Endozoicomonas, a ubiquitous symbiont of numerous marine hosts, were resilient and remained active in M. oculata for several months. Our results demonstrate that a good knowledge of the coral microbiome and an understanding of the ecological strategy of the holobiont is needed before designing aquarium experiments.
Corals and microbes form together an holobiont that is built upon the complex and intricate relationship between the cnidarian host and its associated microbial community. The role of the microbiome in sustaining the coral holobiont’s function is still poorly known but it certainly plays an important role for the coral nutrition and health (Bourne et al., 2016). Corals health is particularly sensitive to environmental changes. Water temperature, quality, and acidification are, among others, factors that strongly impact the corals’ ecology and physiology, and that have been the focus of a number of studies. Recent works have emphasis the need to characterize the microbial communities associated to the host to better understand the impact of environmental conditions on corals (Kelly et al., 2014).
Studying the effects of environmental changes on corals often requires manipulating the corals surrounding environment. Such operations are often easier to achieve under controlled conditions in aquaria. Aquaria experiments require the maintenance of coral in captivity for acclimation during long periods. During that time, the aquarium conditions of rearing, although mimicking natural conditions, are always different from the ones found in nature. Nevertheless, captive corals can be kept alive and remain apparently healthy for long periods of time (Carlson, 1987; Borneman, 2008), including deep-sea corals that exhibit growth rates comparable to the in situ ones (Orejas et al., 2011; Form and Riebesell, 2012; Lartaud et al., 2014). However, as the importance of taking into account the entire holobiont in coral studies has emerged, the question remains as whether the coral associated microbes are strongly impacted by the captivity of the host. If the microbiome is indeed impacted, results obtained in aquaria should be extrapolated to the natural coral colonies with caution. To date, the few existing comparisons between in situ and aquaria coral microbiomes suggest that captivity always changes the coral’s bacterial communities (Kooperman et al., 2007; Schöttner et al., 2009; Pratte et al., 2015; Röthig et al., 2017). However, studies remain scarce and they often encompass a few samples only, analyzed with different protocols and gathered with diverse sampling strategies.
The importance of aquaria experiments for studying corals is critical for species that are hard to sample and monitor in situ. It’s the case for deep-sea corals, also named cold-water corals, which are in most cases found at depths not accessible by divers. Cold-water corals provide important ecosystem services by forming deep-sea reefs that represent hot spots of biodiversity in many regions of the world (Roberts et al., 2006). Among cold-water corals, Lophelia pertusa and Madrepora oculata are the two key species found globally. They often grow in the same sites, but they can show different responses to environmental changes such as temperature variations (Naumann et al., 2014). The physiological differences may be related to their distinct reproductive strategy (Waller and Tyler, 2005), feeding or assimilation/storage efficiencies (Kiriakoulakis et al., 2005) or association to different bacterial communities (Hansson et al., 2009; Schöttner et al., 2012; Meistertzheim et al., 2016). The different communities could reflect different ecological strategies with a resilient bacterial consortium associated to M. oculata, which fits the strict definition of holobiont, and a more versatility microbiome for L. pertusa, which suggests an association that is more influenced by environmental conditions or nutritional status (Meistertzheim et al., 2016).
The goal of this study was to test if the microbiomes associated to L. pertusa and M. oculata changed under captivity. We chose these two species as models representing the contrasted cases of an “unfaithful” versus a “loyal” microbiome. We maintained the two cold-water coral species in aquaria and monitored their associated bacterial communities at different time scales ranging from 1 day to 2 years. We targeted both the 16S ribosomal RNA genes (DNA) and the 16S ribosomal RNA (RNA) to compare the entire bacterial assemblage to the active fraction of the community (Galand et al., 2015).
Corals were sampled in the Lacaze-Duthiers submarine canyon off the coast of Banyuls-sur-Mer in the northwestern Mediterranean Sea (42°32′0.72′′ N; 03°25′0.26′′ W) at ∼530 m depth. Healthy looking coral fragments were randomly collected from three colonies of M. oculata and three colonies of L. pertusa growing on a small coral reef area (2,400 m2) located at the base of the western flank of the canyon. The corals were collected sequentially using a Remotely Operated Vehicle (ROV) deployed from the R/V Minibex Vessel (COMEX SA). They were placed in separate polypropylene boxes that were closed in situ to maintain the ambient bottom water temperature (∼13°C) during the transport to the surface, and avoid cross contamination between samples. On board, the coral fragments meant as time 0 samples were flash frozen in liquid nitrogen and the rest of the coral fragments were transferred to aerated 30 L seawater tanks maintained in the dark at 13°C.
Once in the laboratory, live corals were fixed to cement blocks using an aquatic epoxy resin and positioned in a 80 L aquarium in the dark in a thermo-regulated room that received continuous flow (>1 renewal day-1) of filtered (5 μm) Mediterranean seawater pumped from 5 m depth. To mimic deep-sea temperature stability, the aquarium sea water was maintained year-round at 13°C ±0.5°C by controlling the room temperature and by using a storage tank coupled to a chiller for regulating the incoming sea water temperature. Corals were fed three times a week with freshly hatched Artemia salina nauplii (1000 A. salina L-1) (Lartaud et al., 2013).
Coral fragments were sampled after 1, 5, 20, 60, 180, 270, and 730 days of captivity. For L. pertusa, we had fewer fragments and did not sample at 180 days. Coral samples were flash frozen in liquid nitrogen and then stored at -80°C. In addition, 5 L of aquarium water were sampled at time 1, 5, 20, and 60 days, and filtered on 0.22-μm pore-size polycarbonate filters (Millipore) after prefiltration through 3-μm pore-size polycarbonate filters (Millipore). Filters were stored at -80°C until nucleic acid extraction.
For each sampling time, DNA and RNA was extracted from three different polyps for each species. Polyps were crushed separately using a hammer and the tissues were homogenized in tubes containing a garnet matrix using a FastPrep Instrument (MP Biomedical, Santa Ana, CA, United States). The samples were then divided into two tubes, one for RNA extraction and one for DNA extraction. RNA and DNA were extracted using, respectively, the Maxwell® simply RNA Tissues Kit LEV and the Maxwell® Blood DNA Purification Kit LEV (Promega, Madison, WI, United States) on a Maxwell 16 MDx Instrument (Promega) following the manufacturer instructions. For the water bacteria communities, DNA and RNA were extracted from filters following our standard protocol (Galand et al., 2015). Briefly, cells were lysed with freshly prepared lysozyme solution applied directly to Sterivex cartridges, and a second incubation after adding proteinase K, followed by extraction using the AllPrep DNA/RNA kit (Qiagen). DNA and RNA concentrations were measured by spectrophotometry (Nanodrop ND-1000, Thermo Fisher Scientific, Waltham, MA, United States). The RNA samples were reverse-transcribed to cDNA with random primers using the RevertAid™ H Minus First Strand cDNA Synthesis kit (Life Technologies).
For both the DNA and cDNA, the V1–V3 region of the bacterial 16S rRNA genes were amplified using bacteria specific primers 27F AGRGTTTGATCMTGGCTCAG and 519R GTNTTACNGCGGCKGCTG with a single step and 28 cycles of PCR using the HotStarTaq Plus Master Mix Kit (Qiagen, Valencia, CA, United States). Following the PCR, all the amplicon products from the different samples were mixed in equal concentrations and purified using Agencourt Ampure beads (Agencourt Bioscience Corporation, MA, United States). Purified PCR products were used to prepare a DNA library by following the Illumina TruSeq DNA library preparation protocol. All samples were sequenced on the same Miseq Illumina sequencer run (Illumina, San Diego, CA, United States) using Miseq reagent kit V3 (Illumina) producing 2 × 300-bp long reads. PCR and sequencing were conducted in a commercial laboratory (MR DNA, Shallowater, TX, United States). All sequences were deposited in GenBank under SRA accession number SRP156494.
All the reads that had a mismatch with the 16S rRNA primers, contained ambiguous nucleotides (N) or were <300 bp long beyond the forward primer were removed. In addition, a stringent quality trimming criteria was applied to remove reads that had ≥10% of bases with Phred values <27. This procedure is recommended to ensure that when clustering at 97% or more, the influence of erroneous reads is minimized (Huse et al., 2010; Kunin et al., 2010). The sequences were then de-replicated and clustered at a 97% threshold using UCLUST (Edgar, 2010) for de novo operational taxonomic unit (OTU) picking. Representative sequences were classified against the SILVA v.128 database (Quast et al., 2013). Putative chimeric sequences were identified as sequences having a best Blast alignment <90% of the trimmed read length to the reference database, >90% sequence identity to the best Blast match and OTU size ≤2. Sequence analysis were performed with the PyroTagger pipeline (Kunin and Hugenholtz, 2010).
To compare the bacterial communities in the diversity analysis, all the samples were randomly re-sampled to match the size of the sample containing the fewest sequences (n = 8855). Possible differences in bacterial community composition were assessed by correspondence analysis (CA) with the vegan package in R (Oksanen et al., 2013) and significant differences were tested with ANOSIM. Possible differences between DNA and RNA community composition were measured by comparing the DNA versus the RNA samples scores along the first CA axes. The linear increase in diversity was tested using ordinary least squares regression (OLS) models and the statistical significance of models described with F statistics in R. The Bray Curtis index was computed between all samples with the vegan package in R.
Our experiment shows that the temporal dynamics of the coral microbiome, for both the active fraction (RNA) and the standing stock of bacteria (DNA), differed between L. pertusa and M. oculata (Figure 1). M. oculata maintained its associated community for the first 6 months of captivity, and the pattern was similar for both the active fraction and the standing stock. Inversely, the bacterial communities associated with L. pertusa changed rapidly and differences between wild and captive corals were seen already after 1 day of rearing (Figure 1). We also observed that for both the RNA and DNA fractions, L. pertusa and M. oculata had different bacterial community composition, and that the coral communities were different from the water communities (Supplementary Table 1). Interestingly, the community composition of L. pertusa and M. oculata converged with captivity time, and after 730 days there was no significant difference in community composition between the two coral species for both the DNA and RNA fractions (ANOSIM, R = 0.33 and 0.19 and p = 0.1 for the DNA and RNA fraction, respectively) (Figure 1).
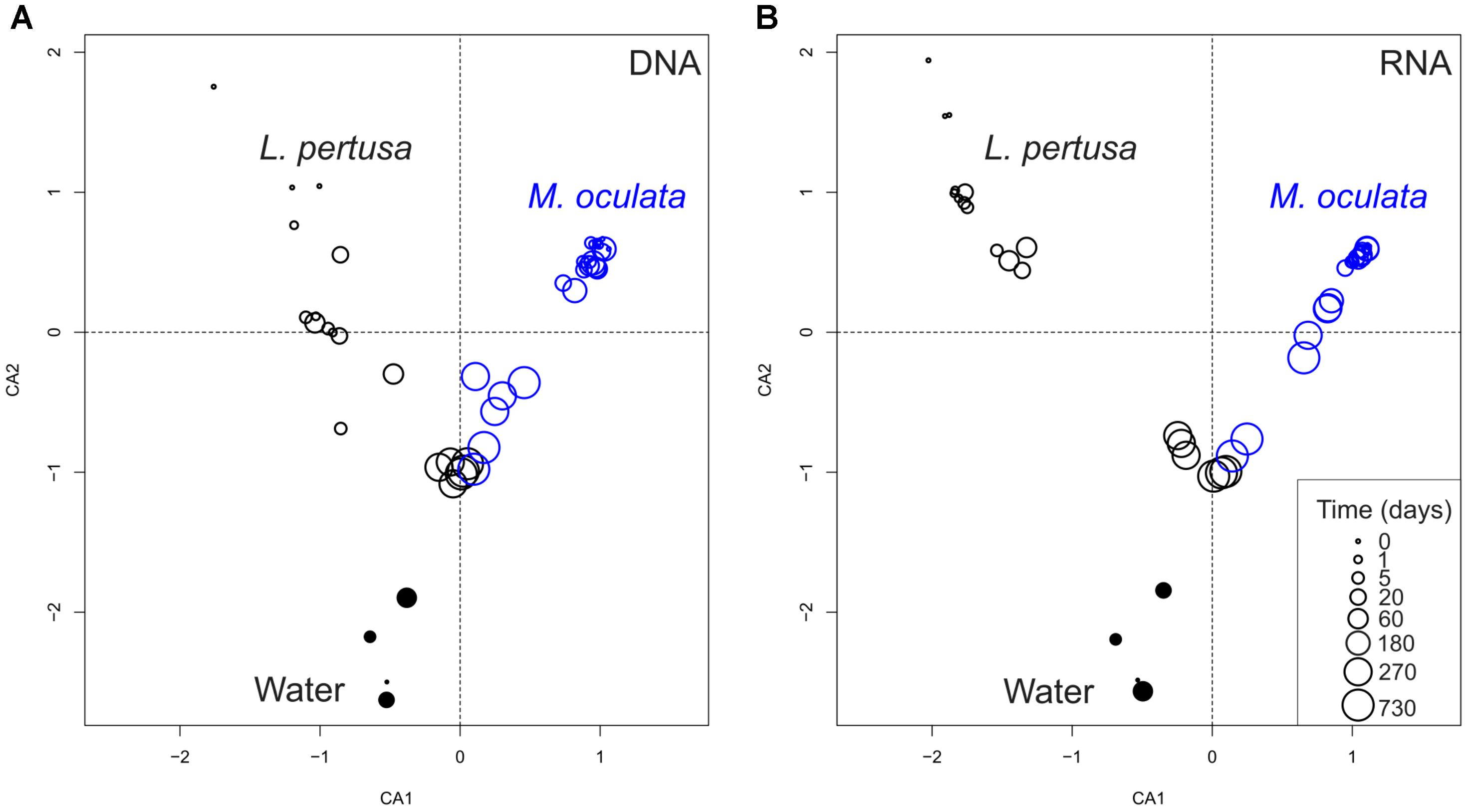
FIGURE 1. Correspondence analysis of bacterial communities based on 16S rDNA (DNA) (A) and 16S rRNA (RNA) (B). L. pertusa communities are marked as black open circles, M. oculata as blue open circles, and water as filled circles. The size of the circles corresponds to the time in captivity.
Our results cast a new light on the effect of captivity on the coral holobiont. We show that captivity can rapidly impact the associated bacterial communities of one coral species but not of the other one at a 6 months’ time scale. After 1 year of captivity, however, both coral microbiomes had changed and lost their species-specific characteristics. The observation of different dynamics is interesting because captivity has been thought to always transform bacterial communities, as seen earlier for skeletal and mucus associated bacteria of L. pertusa (Schöttner et al., 2009) or during long term rearing of the deep-sea coral Eguchipsammia fistula (Röthig et al., 2017). It should, however, be noted that the differences observed in earlier studies may have been caused, in part, by sampling at different seasons (Schöttner et al., 2009), or by the use of different fixation methods (Schöttner et al., 2009). A few studies on tropical corals have also showed changes with captivity for Siderastrea siderea (Pratte et al., 2015), and Fungia granulosa, but on two samples only for the later (Kooperman et al., 2007).
In order to pinpoint the pattern lying behind the changes in community composition we further identified the main bacteria associated to both cold-water corals species (Figure 2 and Supplementary Table 1). The six most abundant species specific bacteria represented 76 and 75% of the sequences in L. pertusa and M. oculata, respectively. Our results show that different bacterial taxa had different patterns of responses at the OTU level (Figure 2). Endozoicomonas, a typical symbiont found in various marine hosts (Neave et al., 2016), has been shown to have a stable relationship with M. oculata (Meistertzheim et al., 2016). In the RNA fraction, the number of Endozoicomonas sequences dropped significantly after 1 day of M. oculata captivity, but increased again after 5 days and hold for 6 months (Figure 2). It suggests that collection and the subsequent change of environment had an immediate effect on the activity of Endozoicomonas. However, this bacterium seems to rapidly adapt to the conditions found in aquarium and to be able to thrive for the first 6 months of captivity. This observed resilience is in line with the recent report of persistent Endozoicomonas phylotypes under coral bleaching and colony mortality (Pogoreutz et al., 2018). Other M. oculata bacteria were not as successful and showed a sharp decline in their relative number of RNA sequences during captivity (Figure 2). For L. pertusa, we also identified species specific bacteria that had been found earlier associated to the coral species in situ (Figure 2 and Supplementary Table 1). Some of these bacteria showed a sharp decrease in their RNA sequence abundance, or even a disappearance after 1 day of captivity, while other increased in relative sequence abundance (Figure 2). We hypothesize that the loss of some bacterial taxa released ecological niches that were quickly occupied by other bacteria, the ones that showed an increase in sequence number. We also identified bacteria that were found only at the end of the experiment (Figure 3), and which we considered as signature for the aquaria effect on the microbiome. These bacteria had never been found earlier associated to hosts (Supplementary Table 1), and their representative sequences were similar to sequences from bacteria growing on sediment or mineral substrates in the sea. It suggests that opportunist bacteria colonized the coral surface during captivity. Our observation of a significant increase in bacterial community richness with time of captivity for L. pertusa and M. oculata (F = 50.25, P < 0.001 and F = 14.59, P < 0.001, respectively) (Figure 4), which was observed earlier for L. pertusa (Schöttner et al., 2009), confirms the appearance of additional coral associated bacterial species during captivity. It shows that corals lost their ability to strongly select specific bacteria, which in turn may reflect a poorer health status. The fact that corals held in captivity for 10 months showed similar growth rates as corals in situ (Lartaud et al., 2014) suggests, however, a limited impact of captivity. Captivity’s effect on coral health remains to be tested exhaustively.
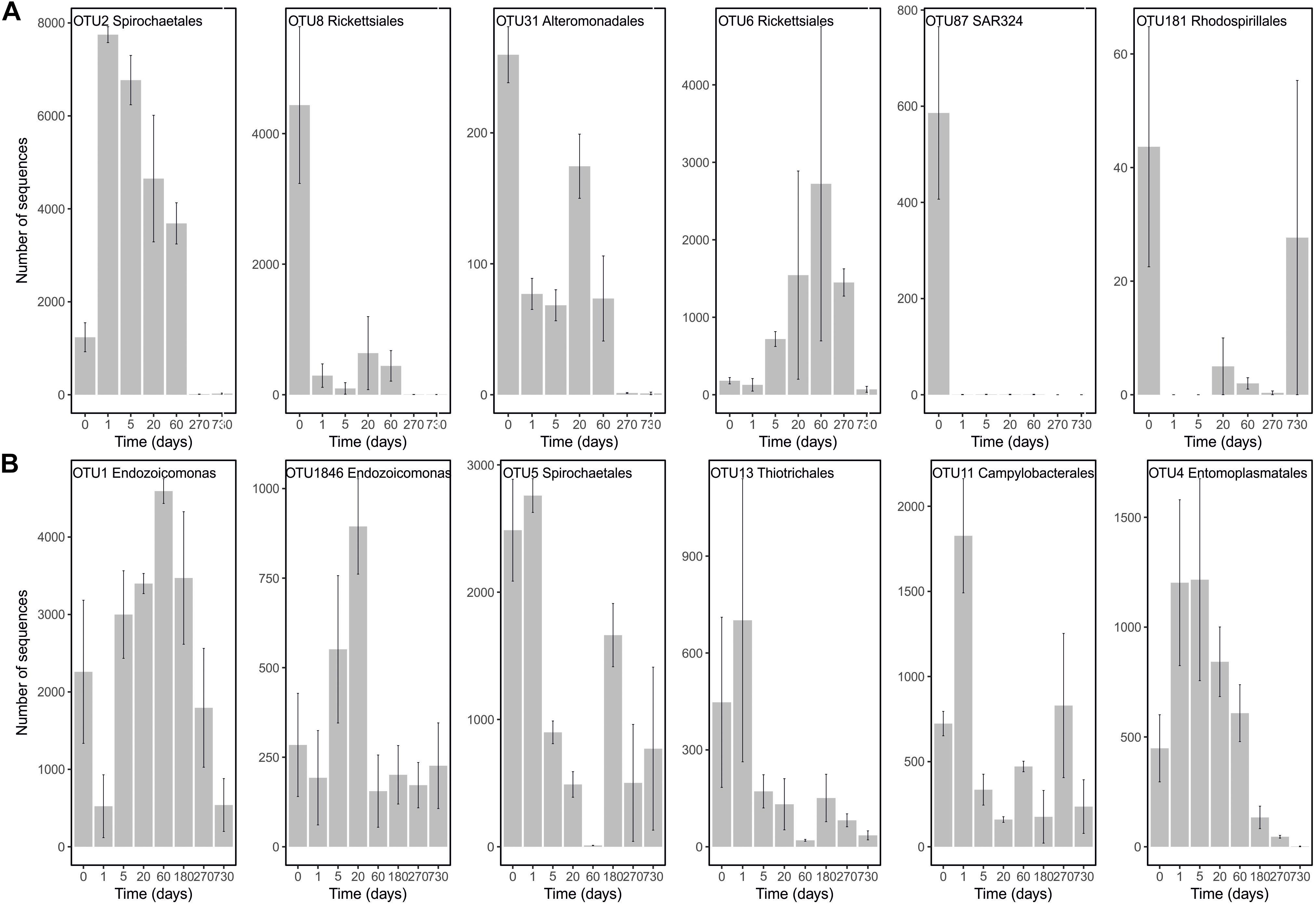
FIGURE 2. Number of sequences in the RNA fraction of the most abundant OTUs present in L. pertusa (A) and M. oculata (B) at different times of captivity. Detailed taxonomic affiliation for each OTUs is given in Supplementary Table 1.
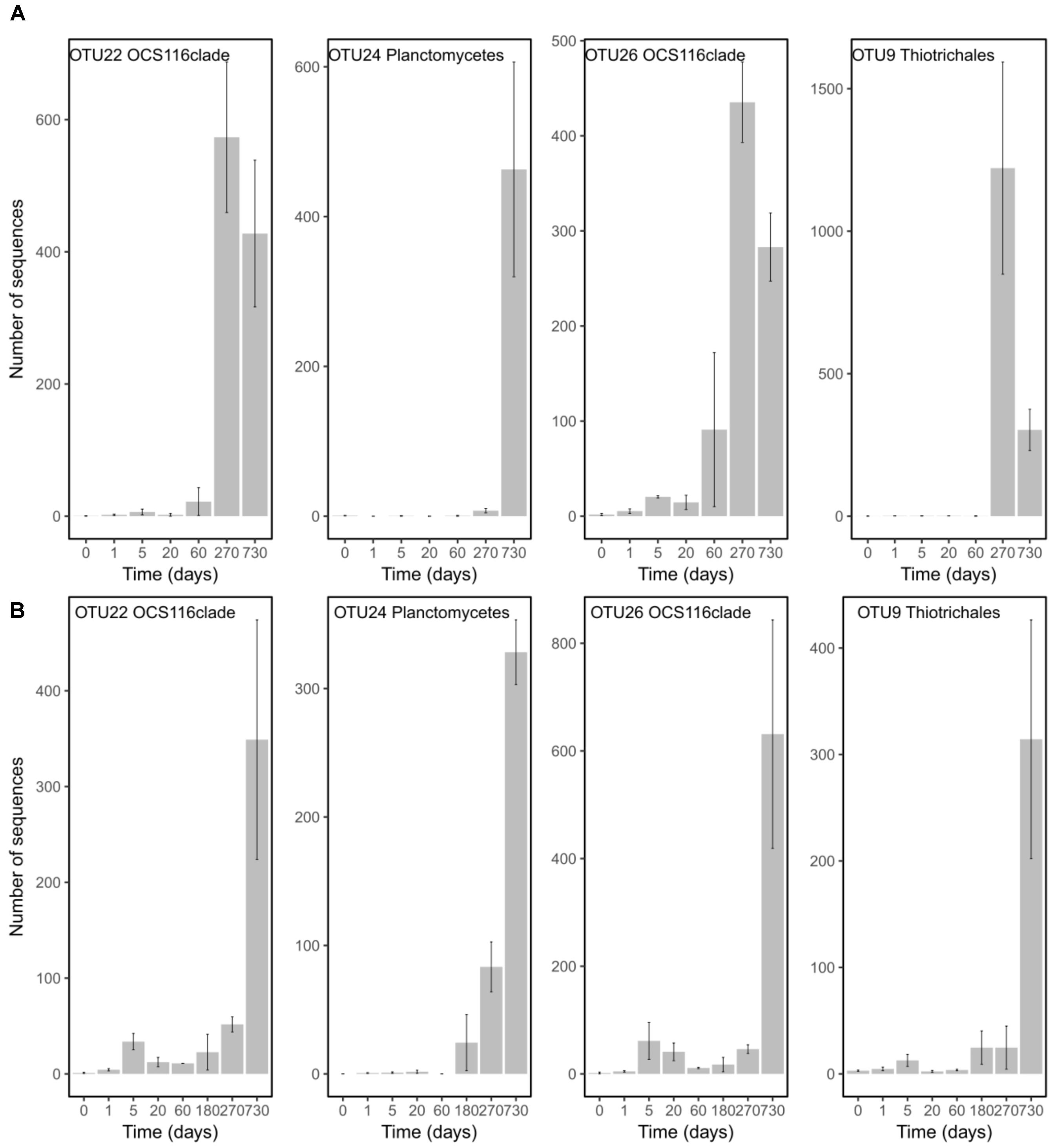
FIGURE 3. Number of sequences in the RNA fraction of OTUs characterizing time 270 and 730 and that were present in both species. Abundances are given for L. pertusa (A) and M. oculata (B) at different time of captivity. Detailed taxonomic affiliation for each OTUs is given in Supplementary Table 2.
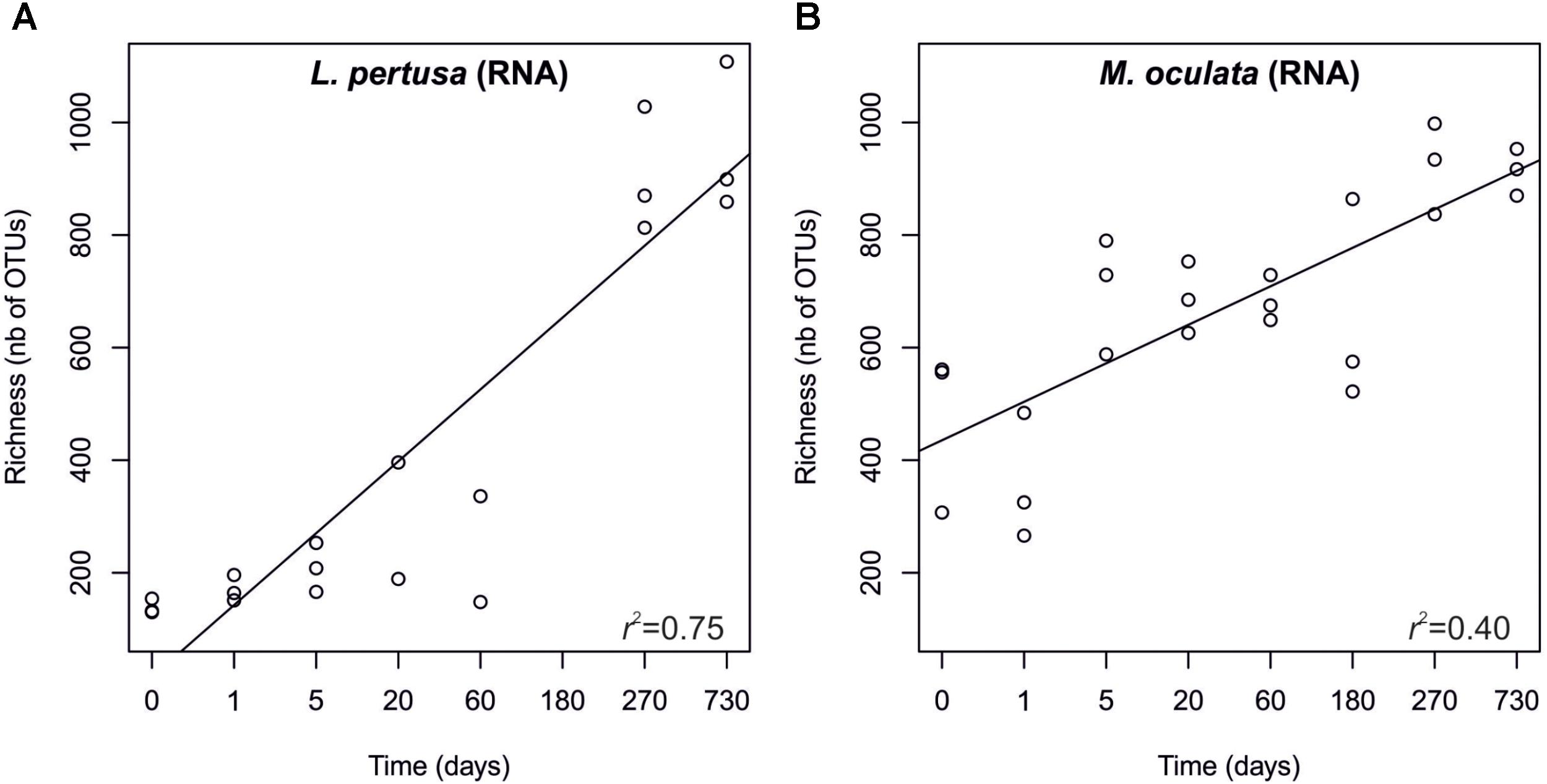
FIGURE 4. Richness of the RNA fraction of the bacterial communities in L. pertusa (A) and M. oculata (B). Sampling time corresponds to the number of days during which the corals were maintained in captivity.
To further assess the effect of captivity on the corals’ microbiomes we measured beta diversity between samples at each sampling time. We observed that for the RNA fraction in L. pertusa the beta-diversity dispersion was low early in captivity, increased after 3 weeks and remained high for the rest of the experiment (Figure 5). For M. oculata, the beta-diversity dispersion was stable for the first 6 months of captivity and then increased (Figure 5). Such patterns of microbial community change can theoretically be used to understand how certain stressors affect host associated microorganisms (Zaneveld et al., 2017). In particular, the Anna Karenina Principle (AKP) predicts that certain stressors have stochastic rather than deterministic effects on community composition (Zaneveld et al., 2017). In the case of cold water corals, our results indicate that, if we consider capture and captivity as a stressor, the effect on L. pertusa was deterministic during the first week. The communities in the different polyps all changed toward a similar composition. Later, the effect became stochastic with communities having different composition in the different polyps. In M. oculata, the stabile beta diversity patterns during the first 6 months of captivity reflected the stabile community composition.
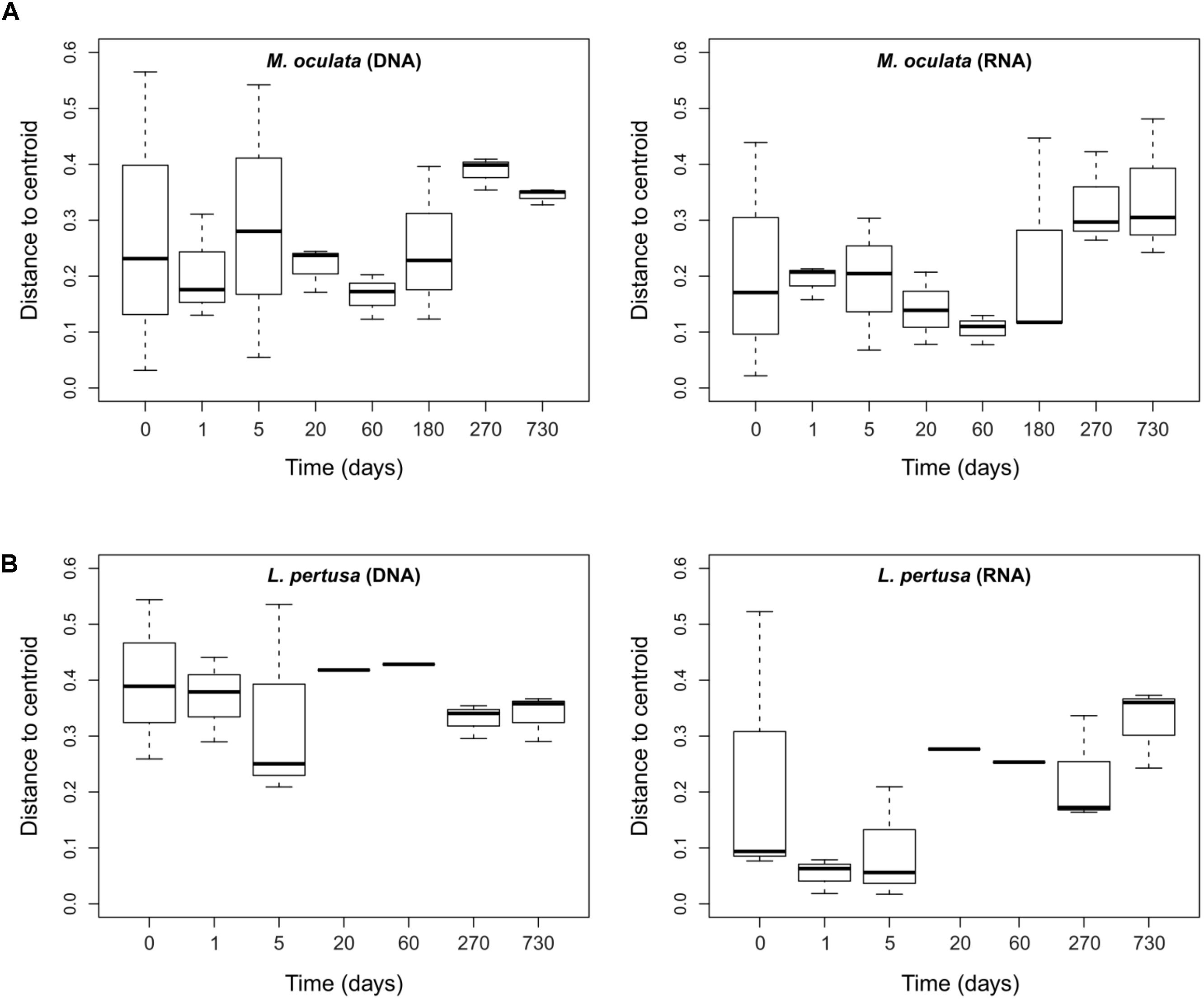
FIGURE 5. Dispersion of the bacterial communities’ beta-diversity at different times of captivity for the DNA and RNA fraction in M. oculata (A) and L. pertusa (B). The dispersion is expressed as the distance to the centroid for each Bray Curtis values computed between replicates of a same category.
The differences observed between L. pertusa and M. oculata confirm that they have species specific bacterial communities even though they share the same habitat (Hansson et al., 2009; Schöttner et al., 2012; Meistertzheim et al., 2016). The difference was hypothesized to correspond to different ecological strategies between the two species with a resilient consortium for M. oculata that fits the definition of holobiont, and more versatility for L. pertusa, which suggests an association that is more influenced by environmental conditions or nutritional status (Meistertzheim et al., 2016). Our results appear to validate that hypothesize. The difference seen between the RNA and DNA fraction for L. pertusa indicates that not all the bacteria that are present on the coral are active (Figure 6). It suggests the presence of opportunist bacteria, probably sticking to the coral, that are not active and thus probably without ecological relevance. Inversely, the similar DNA and RNA structure in M. oculata (Figure 6) may reflect a strong selection for a specific group of bacteria that is active, and the elimination of the less fit inactive organisms.
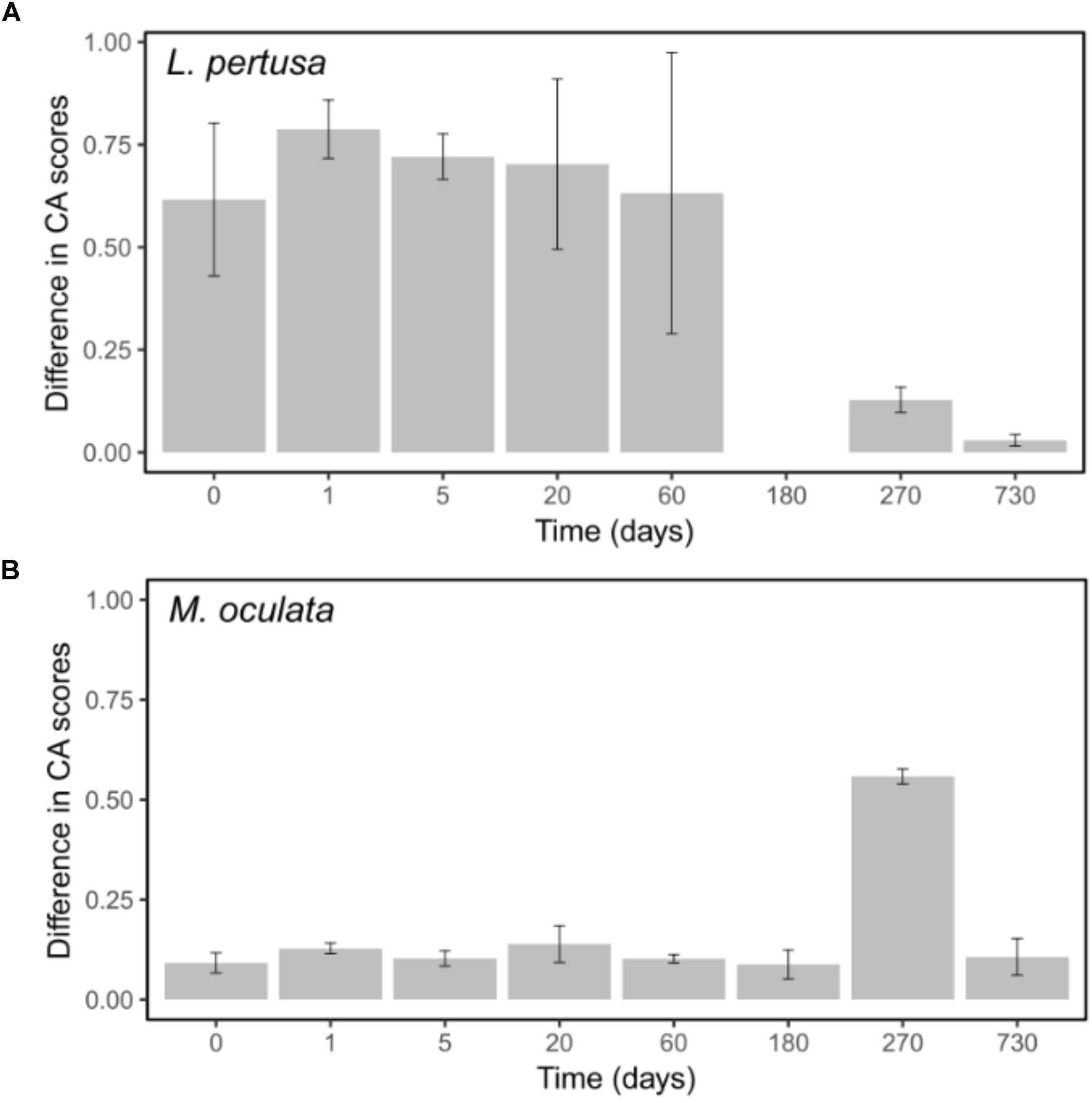
FIGURE 6. Differences between DNA and RNA sample scores on the first CA axes for L. pertusa (A) and M. oculata (B). Bars represent the standard error of the mean.
It should be noted that although the use of RNA has been shown to be a good indicator of the metabolic state for certain marine microbes (Salter et al., 2015), its use has been criticized (Blazewicz et al., 2013). However, in our study, the marked difference that we observed in DNA versus RNA communities between the L. pertusa and M. oculata (Figure 6) is a strong indication that the two species have very different types of relationship to their associated microbiome. The fact that the M. oculata microbiome is maintained during several months in aquaria argues for a stable relationship between the bacteria and the host. Inversely, the L. pertusa microbiome appears to change with changes in its surrounding environment. It remains to be shown if the plasticity of the L. pertusa holobiont can give competitive advantage or better fitness in a changing environment.
We show that for some coral species, maintenance in captivity does not alter significantly the associated bacterial communities for at least 6 months. For others, the microbiome composition and activity change very quickly, as fast as within a day. Our results also highlight that different coral species have different types of associations to bacterial communities, which can be coined as “unfaithful” versus “loyal.” It demonstrates that a good knowledge of the coral microbiome and an understanding of the ecological strategy of the holobiont is needed before designing aquarium experiments. The choice of the species to study and the duration of the experiment is crucial. Finally, we also reveal the notable resilience under aquarium conditions of the Endozoicomonas bacteria known as key symbionts in many marine hosts.
PG and FL designed the study. PG, FL, LC, A-LM, and EP conducted the study, analyzed data, and wrote the paper.
PG was supported by the Agence Nationale de la Recherche (ANR) through the project EUREKA (ANR-14-CE02-0004-01). LC’s Ph.D. grant was funded by the French Ministry of Higher Education, Research and Innovation through the Doctoral School “Sciences de l’Environnement d’Ile de France-ED129.” The coral sampling was conducted as part of the Chair “Biodiversity, extreme marine environment, and global change” supported by Foundation TOTAL, UPMC, and CNRS, coordination N. Le Bris.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge the support of the Banyuls Oceanological Observatory (FR3724) through the federative axes “Marine Biotic Interactions” and the facilities of the Marine Biodiversity and Biotechnology (Bio2Mar) platform.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02565/full#supplementary-material
Blazewicz, S. J., Barnard, R. L., Daly, R. A., and Firestone, M. K. (2013). Evaluating rRNA as an indicator of microbial activity in environmental communities: limitations and uses. ISME J. 7, 2061–2068. doi: 10.1038/ismej.2013.102
Borneman, E. (2008). Introduction to the husbandry of corals in aquariums: a review. Public Aquar. Husb. Ser. 2, 3–14.
Bourne, D. G., Morrow, K. M., and Webster, N. S. (2016). Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annu. Rev. Microbiol. 70, 317–340. doi: 10.1146/annurev-micro-102215-095440
Carlson, B. A. (1987). Aquarium systems for living corals. Int. Zoo Yearbook 26, 1–9. doi: 10.1111/j.1748-1090.1987.tb03126.x
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. doi: 10.1093/bioinformatics/btq461
Form, A. U., and Riebesell, U. (2012). Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob. Chang. Biol. 18, 843–853. doi: 10.1111/j.1365-2486.2011.02583.x
Galand, P., Salter, I., and Kalenitchenko, D. (2015). Microbial productivity is associated with phylogenetic distance in surface marine waters. Mol. Ecol. 24, 5785–5795. doi: 10.1111/mec.13347
Hansson, L., Agis, M., Maier, C., and Weinbauer, M. G. (2009). Community composition of bacteria associated with cold-water coral Madrepora oculata: within and between colony variability. Mar. Ecol. Prog. Ser. 397, 89–102. doi: 10.3354/meps08429
Huse, S. M., Welch, D. M., Morrison, H. G., and Sogin, M. L. (2010). Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ. Microbiol. 12, 1889–1898. doi: 10.1111/j.1462-2920.2010.02193.x
Kelly, L. W., Williams, G. J., Barott, K. L., Carlson, C. A., Dinsdale, E. A., Edwards, R. A., et al. (2014). Local genomic adaptation of coral reef-associated microbiomes to gradients of natural variability and anthropogenic stressors. Proc. Natl. Acad. Sci. U.S.A. 111, 10227–10232. doi: 10.1073/pnas.1403319111
Kiriakoulakis, K., Fisher, E., Wolff, G. A., Freiwald, A., Grehan, A., and Roberts, J. M. (2005). “Lipids and nitrogen isotopes of two deep-water corals from the North-East Atlantic: initial results and implications for their nutrition,” in Cold-Water Corals and Ecosystems, eds A. Freiwald and J. M. Roberts (Berlin: Springer), 715–729.
Kooperman, N., Ben-Dov, E., Kramarsky-Winter, E., Barak, Z., and Kushmaro, A. (2007). Coral mucus-associated bacterial communities from natural and aquarium environments. FEMS Microbiol. Lett. 276, 106–113. doi: 10.1111/j.1574-6968.2007.00921.x
Kunin, V., Engelbrektson, A., Ochman, H., and Hugenholtz, P. (2010). Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ. Microbiol. 12, 118–123. doi: 10.1111/j.1462-2920.2009.02051.x
Kunin, V., and Hugenholtz, P. (2010). PyroTagger: a fast, accurate pipeline for analysis of rRNA amplicon pyrosequence data. Open J. 1–8.
Lartaud, F., Pareige, S., De Rafélis, M., Feuillassier, L., Bideau, M., Peru, E., et al. (2013). A new approach for assessing cold-water coral growth in situ using fluorescent calcein staining. Aquat. Living Resour. 26, 187–196. doi: 10.1051/alr/2012029
Lartaud, F., Pareige, S., De Rafélis, M., Feuillassier, L., Bideau, M., Peru, E., et al. (2014). Temporal changes in the growth of two Mediterranean cold-water coral species, in situ and in aquaria. Deep Sea Res. II Top. Stud. Oceanogr. 99, 64–70. doi: 10.1016/j.dsr2.2013.06.024
Meistertzheim, A.-L., Lartaud, F., Arnaud-Haond, S., Kalenitchenko, D., Bessalam, M., Le Bris, N., et al. (2016). Patterns of bacteria-host associations suggest different ecological strategies between two reef building cold-water coral species. Deep Sea Res. I Oceanogr. Res. Pap. 114, 12–22. doi: 10.1016/j.dsr.2016.04.013
Naumann, M. S., Orejas, C., and Ferrier-Pagès, C. (2014). Species-specific physiological response by the cold-water corals Lophelia pertusa and Madrepora oculata to variations within their natural temperature range. Deep Sea Res. II Top. Stud. Oceanogr. 99, 36–41. doi: 10.1016/j.dsr2.2013.05.025
Neave, M. J., Apprill, A., Ferrier-Pagès, C., and Voolstra, C. R. (2016). Diversity and function of prevalent symbiotic marine bacteria in the genus Endozoicomonas. Appl. Microbiol. Biotechnol. 100, 8315–8324. doi: 10.1007/s00253-016-7777-0
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O’hara, R., et al. (2013). Package ‘vegan’. Community Ecology Package, version 2.
Orejas, C., Ferrier-Pagès, C., Reynaud, S., Gori, A., Beraud, E., Tsounis, G., et al. (2011). Long-term growth rates of four Mediterranean cold-water coral species maintained in aquaria. Mar. Ecol. Prog. Ser. 429, 57–65. doi: 10.3354/meps09104
Pogoreutz, C., Rädecker, N., Cárdenas, A., Gärdes, A., Wild, C., and Voolstra, C. R. (2018). Dominance of Endozoicomonas bacteria throughout coral bleaching and mortality suggests structural inflexibility of the Pocillopora verrucosa microbiome. Ecol. Evol. 8, 2240–2252. doi: 10.1002/ece3.3830
Pratte, Z. A., Richardson, L. L., and Mills, D. K. (2015). Microbiota shifts in the surface mucopolysaccharide layer of corals transferred from natural to aquaria settings. J. Invertebr. Pathol. 125, 42–44. doi: 10.1016/j.jip.2014.12.009
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Roberts, J. M., Wheeler, A. J., and Freiwald, A. (2006). Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312, 543–547. doi: 10.1126/science.1119861
Röthig, T., Roik, A., Yum, L. K., and Voolstra, C. R. (2017). Distinct bacterial microbiomes associate with the deep-sea coral Eguchipsammia fistula from the Red Sea and from Aquaria Settings. Front. Mar. Sci. 4:259. doi: 10.3389/fmars.2017.00259
Salter, I., Galand, P. E., Fagervold, S. K., Lebaron, P., Obernosterer, I., Oliver, M. J., et al. (2015). Seasonal dynamics of active SAR11 ecotypes in the oligotrophic Northwest Mediterranean Sea. ISME J. 9, 347–360. doi: 10.1038/ismej.2014.129
Schöttner, S., Hoffmann, F., Wild, C., Rapp, H. T., Boetius, A., and Ramette, A. (2009). Inter-and intra-habitat bacterial diversity associated with cold-water corals. ISME J. 3, 756–759. doi: 10.1038/ismej.2009.15
Schöttner, S., Wild, C., Hoffmann, F., Boetius, A., and Ramette, A. (2012). Spatial scales of bacterial diversity in cold-water coral reef ecosystems. PLoS One 7:e32093. doi: 10.1371/journal.pone.0032093
Waller, R. G., and Tyler, P. A. (2005). The reproductive biology of two deep-water, reef-building scleractinians from the NE Atlantic Ocean. Coral Reefs 24, 514–522. doi: 10.1007/s00338-005-0501-7
Keywords: Lophelia pertusa, Madrepora oculata, DNA/RNA, bacteria, aquaria experiment
Citation: Galand PE, Chapron L, Meistertzheim A-L, Peru E and Lartaud F (2018) The Effect of Captivity on the Dynamics of Active Bacterial Communities Differs Between Two Deep-Sea Coral Species. Front. Microbiol. 9:2565. doi: 10.3389/fmicb.2018.02565
Received: 03 August 2018; Accepted: 08 October 2018;
Published: 29 October 2018.
Edited by:
David William Waite, University of Auckland, New ZealandReviewed by:
Julie L. Meyer, University of Florida, United StatesCopyright © 2018 Galand, Chapron, Meistertzheim, Peru and Lartaud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pierre E. Galand, cGllcnJlLmdhbGFuZEBvYnMtYmFueXVscy5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.