
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 23 October 2018
Sec. Fungi and Their Interactions
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.02518
Stress activated MAP kinases (SAPKs) of the Hog1/Sty1/p38 family are specialized in transducing stress signals. In contrast to what is seen in animal cells, very few fungal species contain more than one SAPK. Aspergillus nidulans and other Aspergilli contain two SAPKs called SakA/HogA and MpkC. We have shown that SakA is essential for conidia to maintain their viability and to survive high H2O2 concentrations. H2O2 induces SakA nuclear accumulation and its interaction with transcription factor AtfA. Although SakA and MpkC show physical interaction, little is known about MpkC functions. Here we show that ΔmpkC mutants are not sensitive to oxidative stress but in fact MpkC inactivation partially restores the oxidative stress resistance of ΔsakA mutants. ΔmpkC mutants display about twofold increase in the production of fully viable conidia. The inactivation of the SakA upstream MAPKK PbsB or the simultaneous elimination of sakA and mpkC result in virtually identical phenotypes, including decreased radial growth, a drastic reduction of conidiation and a sharp, progressive loss of conidial viability. SakA and to a minor extent MpkC also regulate cell-wall integrity. Given the roles of MpkC in conidiation and oxidative stress sensitivity, we used a functional MpkC::GFP fusion to determine MpkC nuclear localization as an in vivo indicator of MpkC activation during asexual development and stress. MpkC is mostly localized in the cytoplasm of intact conidia, accumulates in nuclei during the first 2 h of germination and then becomes progressively excluded from nuclei in growing hyphae. In the conidiophore, MpkC nuclear accumulation increases in vesicles, metulae and phialides and decreases in older conidia. Oxidative and osmotic stresses induce MpkC nuclear accumulation in both germinating conidia and hyphae. In all these cases, MpkC nuclear accumulation is largely dependent on the MAPKK PbsB. Our results indicate that SakA and MpkC play major, distinct and sometimes opposing roles in conidiation and conidiospore physiology, as well as common roles in response to stress. We propose that two SAPKs are necessary to delay (MpkC) or fully stop (SakA) mitosis during conidiogenesis and the terminal differentiation of conidia, in the highly prolific phialoconidiation process characteristic of the Aspergilli.
Eukaryotic cells use MAP kinase cascades, composed of a MAPK, a MAPK kinase (MAPKK), and a MAPKK kinase (MAPKKK), to transmit environmental signals. Active phosphorylated MAPKs phosphorylate multiple targets, including other enzymes and usually translocate from cytoplasm to nucleus to phosphorylate nuclear targets such as transcription factors. In contrast, upstream MAPKK and MAPKKK phosphorylate only the immediate downstream kinase in the cascade. The topology of this basic module is enough to achieve transient, sustained and oscillatory responses. However, input/response dynamics is greatly affected by the presence of negative/positive feedback loops, scaffolding proteins and spatial gradients of kinases and phosphatases. Moreover, the range of responses is further expanded by the presence of several isoforms (i.e., p38 in animal cells) of a specific type of kinase (see Kholodenko and Birtwistle, 2009 for a review).
Classical stress-activated protein kinases or SAPKs are MAP kinases specialized in transducing multiple stress signals. In fungi, SAPK input typically involves a phosphorelay signal transduction system. Other MAP kinases, members of the MpkA and MpkB families, transduce specific signals such as cell-wall stress and hormone signals, through membrane sensors or G-protein coupled receptors (Grice et al., 2013). Saccharomyces cerevisiae Hog1, the first SAPK identified (Brewster et al., 1993) has been studied in great detail mainly as a pathway connected to osmoresistance and cell-cycle regulation (Escote et al., 2004). Likewise, Schizosaccharomyces pombe Sty1/Spc1 has been extensively characterized as a multi-stress responding SAPK involved in stress resistance and in cell-cycle control, mainly through the MAP kinase-activated protein kinase (MAPKAP) Srk1 (Lopez-Aviles et al., 2008; Shiozaki, 2009; Smith et al., 2010).
In filamentous fungi, Magnaporthe grisea OSM1, was the first HOG1/Spc1/p38 homolog studied, and shown to be required for normal asexual sporulation (mutants producing about 10 times less conidia), osmoresistance and arabitol biosynthesis (Dixon et al., 1999). Later, two independent groups cloned the Aspergillus nidulans HOG1 homolog and named it hogA (Han and Prade, 2002) and sakA (Kawasaki et al., 2002). Han and Prade (2002), reported that hogA expression was transiently induced by high osmolarity and that ΔhogA mutants showed decreased growth in the presence of 1–1.5 M NaCl at low (30°C) but not at A. nidulans normal growth temperature (37°C). Kawasaki et al. (2002), reported that SakA was transiently phosphorylated in response to both osmotic and oxidative stress, as well as early after the induction of asexual sporulation (conidiation), and that while ΔsakA mutants were not sensitive to osmotic stress, they produced asexual spores that progressively lost their viability and were sensitive to oxidative and heat shock stress (Kawasaki et al., 2002). In most fungi the constitutive activation of the SAPK pathway results in lethality and in fact, this is the action mechanism of common fungicides such as fludioxonil. In filamentous fungi in which the HOG1 pathway is solely responsible for providing resistance to osmotic stress, its elimination is enough to confer resistance to fludioxonil. In contrast, in A. nidulans and other filamentous fungi (Izumitsu et al., 2007) osmoresistance is regulated by both SakA and response regulator SrrA, and it is necessary to eliminate either the common upstream histidine kinase NikA or both, SakA and SrrA, to produce osmosensitivity (Vargas-Perez et al., 2007).
In A. nidulans, SakA is also phosphorylated in response to nutrient starvation and hypoxia stress (Lara-Rojas et al., 2011), and it mediates light responses (Fischer et al., 2016; Yu et al., 2016). When phosphorylated, it translocates to nuclei, where it physically interacts with transcription factor AtfA, to regulate the expression of several genes in response to oxidative (Lara-Rojas et al., 2011) and osmotic stress (Hagiwara et al., 2009). SakA also interacts with the MAPKAP SrkA, a homolog of S. pombe Srk1, and mediates its nuclear localization in response to oxidative stress. Also in response to H2O2, SakA interacts with several other proteins, some related to cell-cycle regulation (Jaimes-Arroyo et al., 2015).
SakA also links stress environmental sensing and development, playing essential roles in the transition between growth and differentiation. ΔsakA mutants show a strong de-repression of NADPH oxidase gene noxA, essential for sexual development (Lara-Ortiz et al., 2003), and a highly exacerbated sexual development (Kawasaki et al., 2002). During asexual development, ΔsakA intact conidia progressively lose their viability and this is consistent with the developmental phosphorylation and nuclear accumulation of SakA in intact conidia. Moreover, SakA needs to be dephosphorylated for germination of conidia to take place (Lara-Rojas et al., 2011). In many other fungi where the single SakA ortholog present has been studied, it has been linked to stress sensing and the regulation of development or pathogenicity (Segmuller et al., 2007; Lamb et al., 2012; Nimmanee et al., 2015; Esquivel-Naranjo et al., 2016).
The presence of more than one SAPK in a single fungal species was first documented in A. nidulans, where the mpkC gene was identified (GenBank accession numbers: AF195773 and AN4668) and the protein compared to SakA (Kawasaki et al., 2002). SakA (379 amino-acids) and MpkC (415 amino-acids) are 62% identical, both being substrates of the upstream MAPKK PbsB (Furukawa et al., 2005) and showing physical interaction (Jaimes-Arroyo et al., 2015). Unexpectedly, the deletion mpkC did not generate any clear phenotype (Jun et al., 2011).
More recently, two SAPKs have been reported in the obligatory halophilic basidiomycetous Wallemia ichthyophaga and the ascomycetous yeast-like fungus Hortaea werneckii. Having virtually the same size and being 69% identical, W. ichthyophaga WiHog1A and WiHog1B genes are differentially induced by high osmolarity and show different degrees of complementation of a S. cerevisiae Δhog1 mutant (Konte and Plemenitas, 2013). With evidence supporting an ancestral duplication of its entire genome, H. werneckii contains the two nearly identical (95%) and functionally redundant SAPKs HwHog1A and HwHog1B, which show osmolyte-type-dependent phosphorylation (Kejzar et al., 2015).
To evaluate the relative contribution of SakA and MpkC in stress sensing and development, we decided to characterized single and double ΔmpkC and ΔsakA null mutants and compare them with mutants in which the upstream MAPKK gene pbsB was deleted. In addition, we studied the nuclear localization of a functional MpkC::GFP fusion during stress and development in wild type and ΔpbsB genetic backgrounds, as a visual tool to detect MpkC activation in vivo.
Aspergillus nidulans strains used in this work are listed in Supplementary Table S1 (McCluskey et al., 2010). All strains were grown at 37°C in glucose minimal nitrate medium (Hill and Käfer, 2001), plus supplements. H2O2 was added to agar medium at ∼50°C before solidification. H2O2-containing plates were used the day they were prepared or stored at 4°C for no more than 24 h. 6 cm diameter plates were used in solid media experiments, except in Figure 1, where we used 10 cm plates. For mycelial stress sensitivity assays, mycelial plugs of the same area (diameter, 0.5 cm) were cut from the growing edge of 5-day colonies using a cork borer. Agar excess was removed and the mycelial mat was transferred to the testing medium.
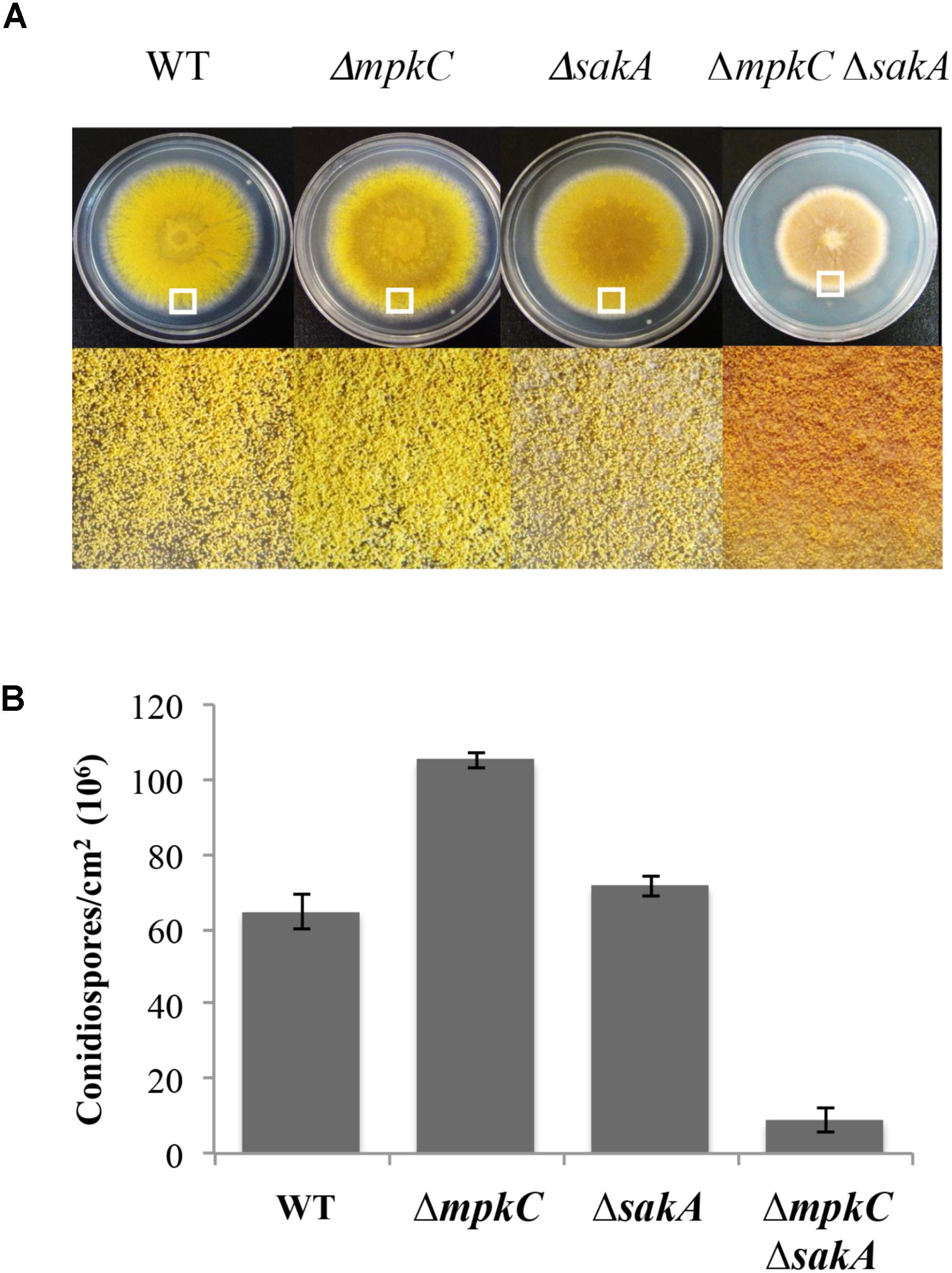
FIGURE 1. sakA and mpkC deletion show opposing effects on conidiation but both genes are needed for normal conidiation. (A) Asexual spores (1 × 104) from strains CLK43 (WT), CFL10 (ΔmpkC), TOL1 (ΔsakA) and CFL12 (ΔmpkC ΔsakA) were inoculated on supplemented MM plates and incubated at 37°C during 5 days. (B) Total conidiospores per colony were harvested, counted, and the count divided by the colony area to obtain the number of conidiospores per square centimeter. Bars indicate standard deviation from three independent experiments. A representative experiment is shown. The white squares in panel (A) indicate the colony regions enlarged at the bottom of the figure. See Supplementary Table S1 for strain full genotypes.
The different gene-deletion constructs were produced by double joint PCR (Yu et al., 2004) using genomic DNA as template and different primer combinations. Primers are listed in Supplementary Table S2. To delete the mpkC gene (AN4668), PCR fragments were generated with primers 5′For-mpkC/5′Rev-mpkC and 3′For-mpkC/3′Rev-mpkC. Aspergillus fumigatus pyrG marker was amplified with primers pyrGforward and pyrGreverse, using plasmid PFNO3 as template (Nayak et al., 2006). These three fragments were purified, mixed and used in a fusion PCR with primers 5′Nest-mpkC and 3′Nest-mpkC. The final 4300 bp mpkC–AfpyrG–mpkC cassette was purified and used to transform A. nidulans strain MH11035 by electroporation (Sánchez and Aguirre, 1996; Sánchez et al., 1998). One PyrG+ transformant was obtained, analyzed by Southern blot to confirm mpkC elimination and named TFL8. TFL8 was crossed with strain CFL3 to remove the ΔnkuA mutation, and progeny strains CFL8 and CFL10 were confirmed by PCR and used in further experiments. To obtain ΔmpkC strain COS0020ΔmpkC, strains TFL8 and CLK43 were crossed to remove the nkuA deletion. COS0020ΔmpkC was confirmed by PCR and used in further experiments. To obtain ΔsakA ΔmpkC double mutants, strains TFLΔsakA-03 and CFL8 were crossed and the progeny analyzed by PCR to confirm the presence of both gene deletions.
To delete pbsB gene (AN0931), genomic DNA was used as template to amplify pbsB fragments with primers pbsB5′Fw/pyrGpbsB5′Rv and pbsB3′pyrGFw/pbsB3′Rv. A. fumigatus pyrG marker was amplified with primers pyrGforward/pyrGreverse, as before. These three fragments were purified and mixed with primers RealNestedpbsB5′/RealNestedpbsBRev to produce a final 4307 bp pbsB–AfpyrG–pbsB cassette, which was then used to transform A. nidulans strain MH11035 by electroporation. Five PyrG+ transformants were obtained and analyzed by PCR to confirm the elimination of pbsB. Strain TOSΔpbsB03 was chosen and crossed to strain CLK43 to get rid of nkuA deletion. Progeny strain COSΔpbsB05 was confirmed by PCR and used in further experiments.
To constitutively express MpkC::GFP from the gpdA promoter, a PCR construct biA-pyroA-gpdA-mpkC-GFP-biA, was used to transform strain TFL22 (Jaimes-Arroyo et al., 2015). PyroA+ BiA- transformants were analyzed for GFP signal and strain TRJ12 was selected for further experiments. The absence of mutations in TRJ12 mpkC ORF fused to GFP was confirmed by DNA sequencing. The MpkC::GFP construct was derived from a pyroA bearing plasmid containing the gpdA promoter fused to mpkC cDNA and GFP, cloned in the middle of the biA gene (Bayram et al., 2012). Strain TRJ12 was transformed with PCR construct gpdA-h2A-mrfp-phleo, which confers resistance to phleomycin and labels nuclei with Histone H2A fused to mRFP (Bayram et al., 2012), and transformant TRJ13 was chosen for further experiments.
The gpdA(p)::h2A::mrfp allele was introduced into a ΔsakA genetic background by crossing strains TRJ7 and CRJ1. The presence of labeled nuclei and ΔsakA deletion was confirmed by Epifluorescence microscopy and PCR, respectively, and strain CVG18 was selected. Crosses between strains CRJ11 × TRJ13 and CRJ11 × TRJ7 were carried out to introduce biA::pyroA::gpdA(p)::mpkC::GFP::pyroA::biA and/or gpdA(p)::h2A::mrfp alleles into ΔmpkC, ΔsakA, or ΔmpkC ΔsakA backgrounds. Selected progeny were first tested for the presence of mRFP and/or GFP signal using Epifluorescence microscopy, while the presence of ΔmpkC and/or ΔsakA deletions was confirmed by PCR. Strains CVG17 and CVG18 were selected for additional experiments. The same strategy was used to introduce biA::pyroA::gpdA(p)::mpkC::GFP::pyroA::biA and gpdA(p)::h2A::mrfp markers into a ΔpbsB background, starting by crossing strains TRJ7 or TRJ13 with strain COSΔpbsB05. Strains CVG10 and CVG20 were selected and the presence of ΔpbsB mutation was confirmed by PCR.
Fluorescence microscopy images were captured in vivo. For MpkC::GFP detection during germination, conidia were germinated for 2, 4, or 7 h at 37°C and observed using confocal microscopy. For stress treatments, 6 h germinated conidia were treated or not with 10 mM H2O2 for 10 min and observed within the next 10 min, or were germinated for 6 h in the presence of 1.2 M sorbitol. Image processing and fluorescence quantification were made using Image J and ZEN 2012 (Carl Zeiss, Jena, Germany). To observe conidiophores, the growing edge of a MpkC::GFP colony grown for 3 days at 37°C was sectioned, a drop of water was added and the section was carefully covered with a coverslip. Different fields in which conidiophore structure was better preserved were chosen for observation using confocal microscopy. To observe growing hyphae, 14 h grown mycelia was treated or not with 10 mM H2O2 for 20 min or grown for 14 h in MM containing 1.2 M sorbitol and then observed using confocal microscopy. All images were acquired using a Zeiss LSM800 inverted laser scanning confocal microscope using a Plan-Apochromat 63×/1.4 oil immersion objective and 488 and 561 nm laser lines. Maximum intensity projections were obtained from Z-stack images collected every 15 μm through entire cell volume. Images were processed using software ZEN 2012 (Carl Zeiss, Jena, Germany).
To compare SakA and MpkC functions in A. nidulans, we characterized mutants carrying deletions of mpkC and sakA genes. First, a ΔmpkC mutant was generated and single and double mutants were obtained by crosses (see section “Materials and Methods” and Supplementary Figure S1). In contrast to previous results showing that MpkC is necessary for polyalcohol sugar utilization in A. fumigatus (Reyes et al., 2006), we found that ΔmpkC and ΔsakA mutants grew as well as the wild type strain on different carbon sources, including mannitol and sorbitol (Supplementary Figure S2A). As reported before (Jun et al., 2011), ΔmpkC mutants did not display any obvious phenotype (Supplementary Figure S2B and Figure 2B). However, ΔmpkC colonies looked a little brighter, suggesting higher conidiation levels. To examine MpkC and SakA interactions during stress and conidiation, we generated a double ΔmpkC ΔsakA mutant and compared it with single ΔmpkC and ΔsakA mutants. While osmosentitivity was not increased by the simultaneous inactivation of MpkC and SakA (Supplementary Figure S2B), MpkC inactivation did affect conidiation. When compared to the wild type strain ΔmpkC mutants showed an increase in the production conidia, in sharp contrast with the ΔmpkC ΔsakA mutant, which presented a drastic reduction in conidiation as well as a clear reduction in radial growth (Figures 1A,B). While MpkC functions in conidiation were not evaluated in A. fumigatus, our results indicate that MpkC functions in polyalcohol sugar utilization are different in A. fumigatus (Reyes et al., 2006) and A. nidulans.
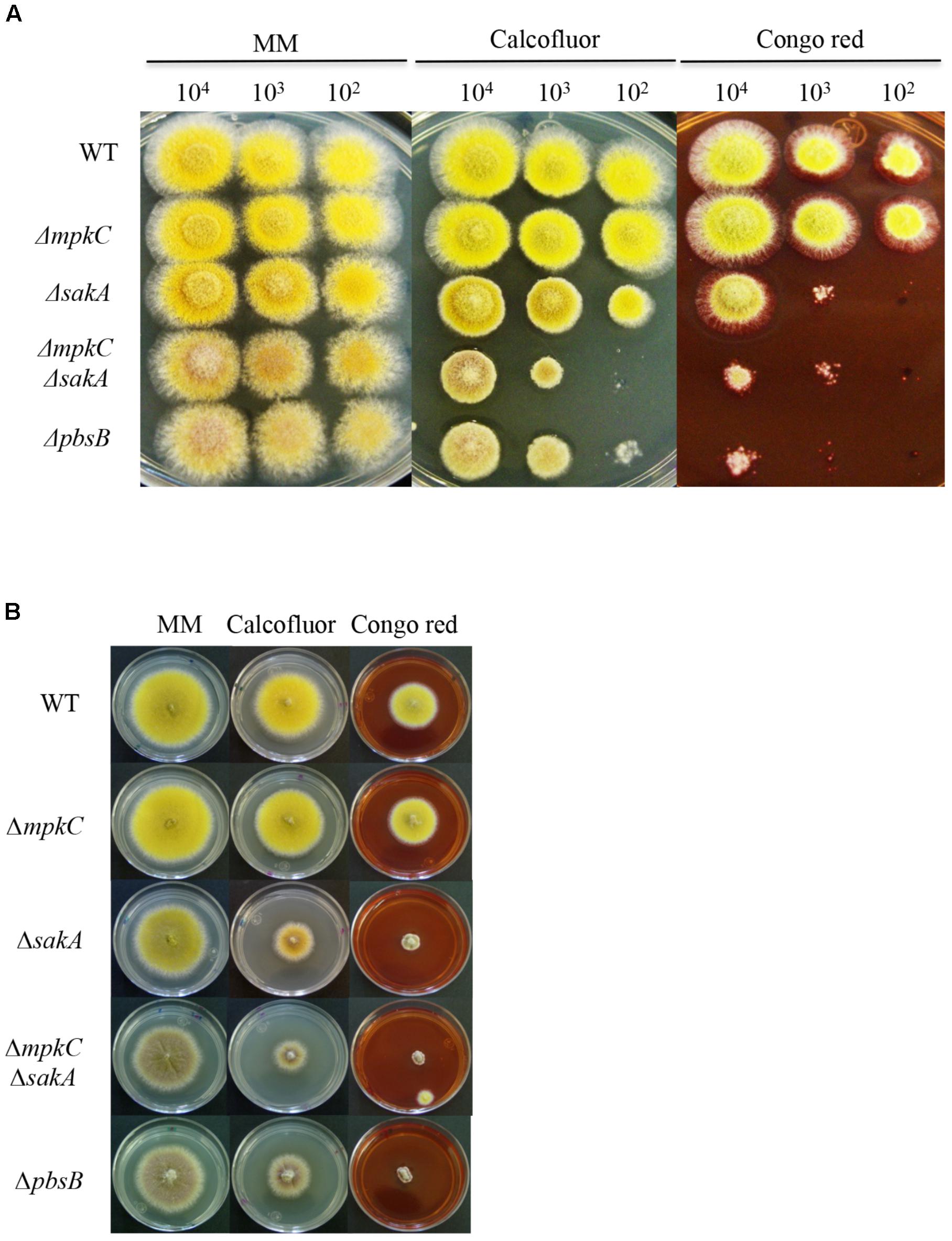
FIGURE 2. SakA and MpkC inactivation results in sensitivity to cell wall damaging in conidia and mycelia. (A) Spores (1 × 104) from strains CLK43 (WT), COS0020ΔmpkC (ΔmpkC), CRJ1 (ΔsakA), CRJ11 (ΔmpkC ΔsakA) and COSΔpbsB05 (ΔpbsB) were inoculated on supplemented MM plates containing cell wall damaging compounds Calcofluor (20 μg/ml) or Congo red (30 μg/ml) and incubated at 37°C for 2 days. (B) Mycelial plugs cut from the growing edge of 5-day colonies from strains CLK43 (WT), COS0020ΔmpkC (ΔmpkC), CRJ1 (ΔsakA), CRJ11 (ΔmpkC ΔsakA), and COSΔpbsB05 (ΔpbsB) were transferred to plates containing Calcofluor (20 μg/ml) or Congo red (30 μg/ml) and incubated at 37°C for 4 days.
To better understand the relationship between MpkC and SakA, we generated a mutant in which the upstream MAPKK gene pbsB was deleted (see section “Materials and Methods” and Supplementary Figure S3), as it has been shown that PbsB is necessary for SakA and MpkC phosphorylation (Furukawa et al., 2005). As shown in Figures 2, 3, ΔpbsB and ΔmpkC ΔsakA mutant growth and conidiation phenotypes were very similar, both producing similarly low amounts of conidia (Supplementary Figure S4A). Indeed, PbsB inactivation and the simultaneous inactivation of SakA and MpkC resulted in additional similar phenotypes (see further).
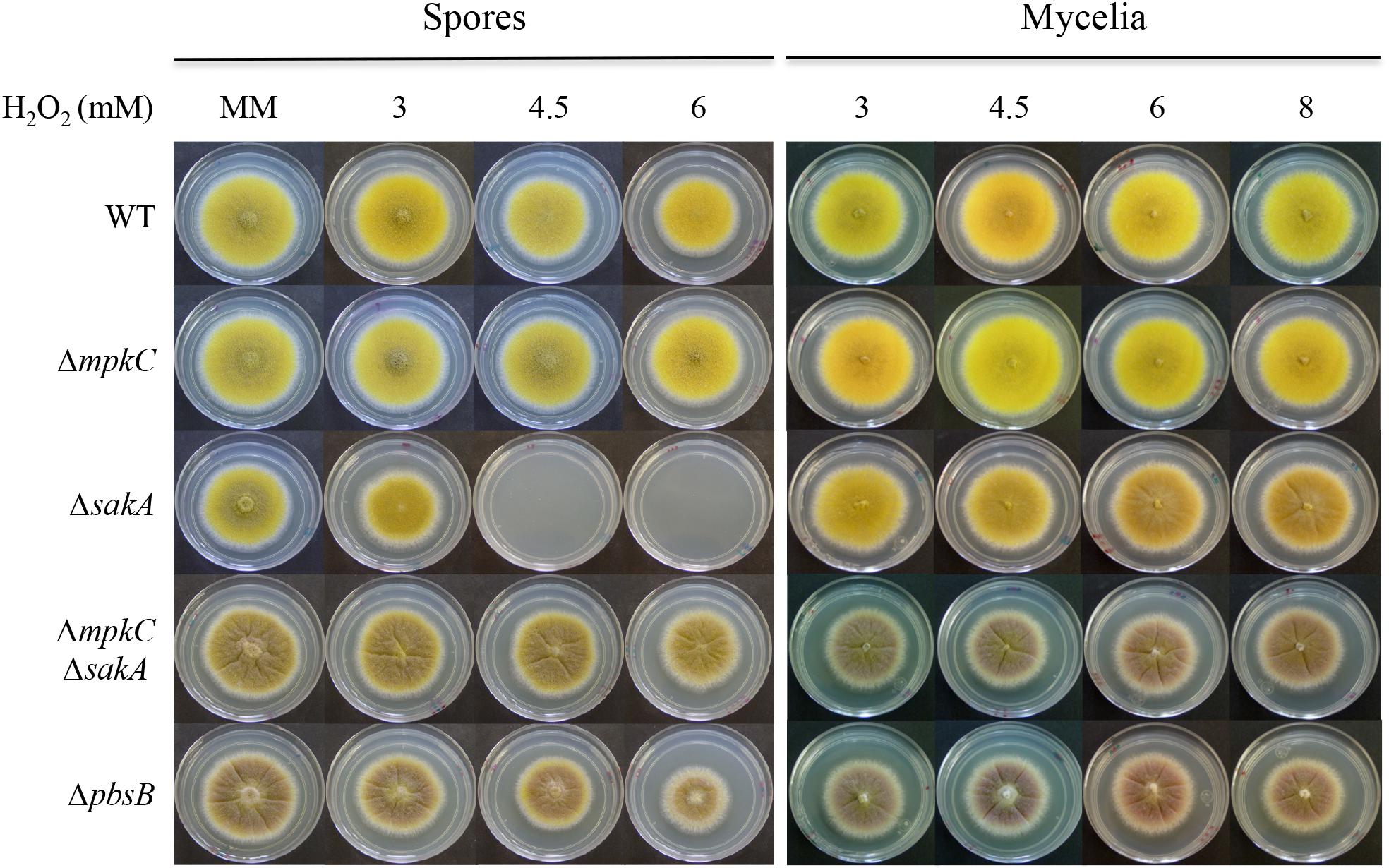
FIGURE 3. SakA and MpkC regulate conidia oxidative stress resistance in opposite ways. Conidia (1 × 104) or mycelial plugs cut from the growing edge of 5-day colonies from strains CLK43 (WT), COS0020ΔmpkC (ΔmpkC), CRJ1 (ΔsakA), CRJ11 (ΔmpkC ΔsakA), and COSΔpbsB05 (ΔpbsB) were used to inoculate supplemented MM plates containing H2O2 at the indicated concentrations, and incubated at 37°C for 4 days.
Given that ΔsakA conidia suffer a progressive and dramatic loss of viability after they are formed (Kawasaki et al., 2002), we tested the viability of conidia produced by ΔmpkC, ΔmpkC ΔsakA, and ΔpbsB mutants. Results in Supplementary Figure S4B show that, like WT, ΔmpkC conidia remained fully viable for at least 40 days. In contrast, ΔmpkC ΔsakA conidia lost their viability at a much faster rate than conidia from the ΔsakA mutant. Consistent with this, conidia from the mutant lacking the MAPKK PbsB showed a viability loss rate similar to the one observed for ΔmpkC ΔsakA conidia.
In view of the growth reduction observed in the ΔmpkC ΔsakA and ΔpbsB mutants, we tested the role of these SAPKs in maintaining cell-wall integrity by plating conidia or mycelia from ΔmpkC, ΔsakA, ΔmpkC ΔsakA, and ΔpbsB mutants on media containing the cell-wall disturbing compounds calcofluor or Congo red. As shown in Figure 2A, the germination and growth of the ΔmpkC mutant was not affected by the presence of these compounds. In contrast, ΔsakA mutant growth was clearly reduced, particularly in the presence of Congo red. Moreover, ΔmpkC ΔsakA double mutant was even more sensitive to these compounds, clearly indicating that although SakA plays a more important role than MpkC, both SAPKs contribute to proper cell-wall biosynthesis. Again, ΔmpkC ΔsakA mutant phenotype was very similar to the one displayed by ΔpbsB mutant. When the same experiment was carried out using mycelia instead of conidia, ΔsakA, ΔmpkC ΔsakA, and ΔpbsB mutants showed similar sensitivity to calcofluor and Congo red suggesting that MpkC contribution to cell-wall integrity in mycelia is less important than in conidia (Figure 2B).
In contrast to their mycelial insensitivity to H2O2, ΔsakA mutants produce conidia that are sensitive to H2O2 (Kawasaki et al., 2002). To analyze MpkC contribution to this phenotype, we compared oxidative stress sensitivity of conidia and mycelia from ΔmpkC, ΔsakA, ΔmpkC ΔsakA, and ΔpbsB mutants. As shown in Figure 3, ΔsakA conidia were unable to grow at 4.5 and 6 mM H2O2, while ΔmpkC conidia showed wild type resistance to H2O2. Unexpectedly, MpkC inactivation restored the ability to ΔsakA mutants to grow at 4.5 and 6 mM H2O2 and a similar result was observed in the presence of tert-Butyl hydroperoxide (not shown). In contrast, a lack of mpkC or pbsB did not affect the sensitivity of mycelia to H2O2 (Figure 3). This indicates that SakA and MpkC regulate the response of intact conidia to oxidative stress in opposite ways. In line with this result, conidia from the ΔpbsB mutant, unable to activate both SakA and MpkC, were able to grow at 4.5 and 6 mM H2O2 (Figure 3). This indicates that PbsB is an upstream regulator of both SakA and MpkC, confirms the interactions observed in ΔmpkC ΔsakA mutants and show that in the absence of SakA, MpkC mediates a higher sensitivity of conidia to H2O2.
Overall, our results indicate that MpkC and SakA show complex interactions in opposing (conidiation and oxidative stress sensitivity), as well as concurrent (cell-wall biosynthesis) pathways, to regulate A. nidulans stress responses, growth, and development.
Since mpkC gene is expressed at very low basal levels in A. nidulans, PbsB requirement for MpkC phosphorylation was shown expressing mpkC from an A. oryzae constitutive promoter (Furukawa et al., 2005). Recently, MpkC nuclear localization induced by osmotic stress was reported in A. fumigatus germinated conidia, using a MpkC::GFP fusion expressed form its native promoter (Bruder Nascimento et al., 2016). However, with such fusion GFP signal is virtually undetectable in the absence of stress. Here we decided to constitutively express MpkC tagged with GFP from the gpdA gene promoter, to examine the effects of MpkC expression and the role of PbsB in MpkC nuclear localization, under stressed and non-stressed conditions. To test that MpkC::GFP fusion was functional, we introduced it in WT, ΔmpkC and ΔmpkC ΔsakA backgrounds (Supplementary Figure S5). As shown in Supplementary Figures S6A,B, MpkC::GFP expression in a wild type background did not seem to affect growth, conidiation or stress sensitivity. In contrast, MpkC::GFP expression in ΔmpkC and ΔmpkC ΔsakA backgrounds was able to restore conidiation to wild type levels and to partially restore calcofluor and Congo red resistance of ΔsakA and ΔmpkC ΔsakA mutants (Supplementary Figure S6C). These results indicated that this MpkC::GFP fusion was functional.
Next, we decided to follow MpkC::GFP localization during asexual development and under stress conditions. As shown in Figure 4A, MpkC::GFP was found mostly in the cytoplasm of intact conidia, which contain a single G1-arrested nuclei. Notably, during germination an increased accumulation of MpkC::GFP was detected in nuclei, labeled with histone H2A::mCherry, during spore swelling (Figure 4B) and the establishment of polar growth and mitosis (Figures 4B, 5). Moreover, MpkC::GFP nuclear localization was further increased when germinated spores were treated with H2O2 or germinated in sorbitol medium (Figure 5). This observation was supported after measuring and comparing MpkC::GFP nuclear fluorescence intensity in non-stressed and stressed germlings (Supplementary Figure S7). These results suggest that MpkC nuclear localization is regulated during asexual development and indicate that oxidative and osmotic stress induce MpkC nuclear localization. To further explore this, we detected MpkC::GFP signal in intact conidiophores. Despite the difficulties in signal detection due to the overlap of several layers of cells, a clear red signal was observed in nuclei located in hyphae around foot cells, confirming very low levels of nuclear MpkC::GFP in hyphae (Figure 6), while different red and green intensities were observed in the different conidiophore cell-types. A clear nuclear orange color, indicative of increased MpkC nuclear localization, was observed in vesicles. Although mRFP signal decreased in metulae and phialides, green signal was clearly more intense in the center of the cell than in the periphery, suggesting a high MpkC::GFP nuclear/cytoplasmic ratio in these cells. Young conidia displayed orange and yellow signals, indicating partial nuclear localization of MpkC::GFP, while nuclear green signal decreased in older conidia, as it was also observed in isolated mature conidia (Figure 4A). This pattern of fluorescence signal suggests that while MpkC is mostly localized in the cytoplasm of hyphae, during normal conidiophore development MpkC is gradually translocated to nuclei in vesicles, metulae, and phialides, and then mostly re-localized to cytoplasm in mature conidia.
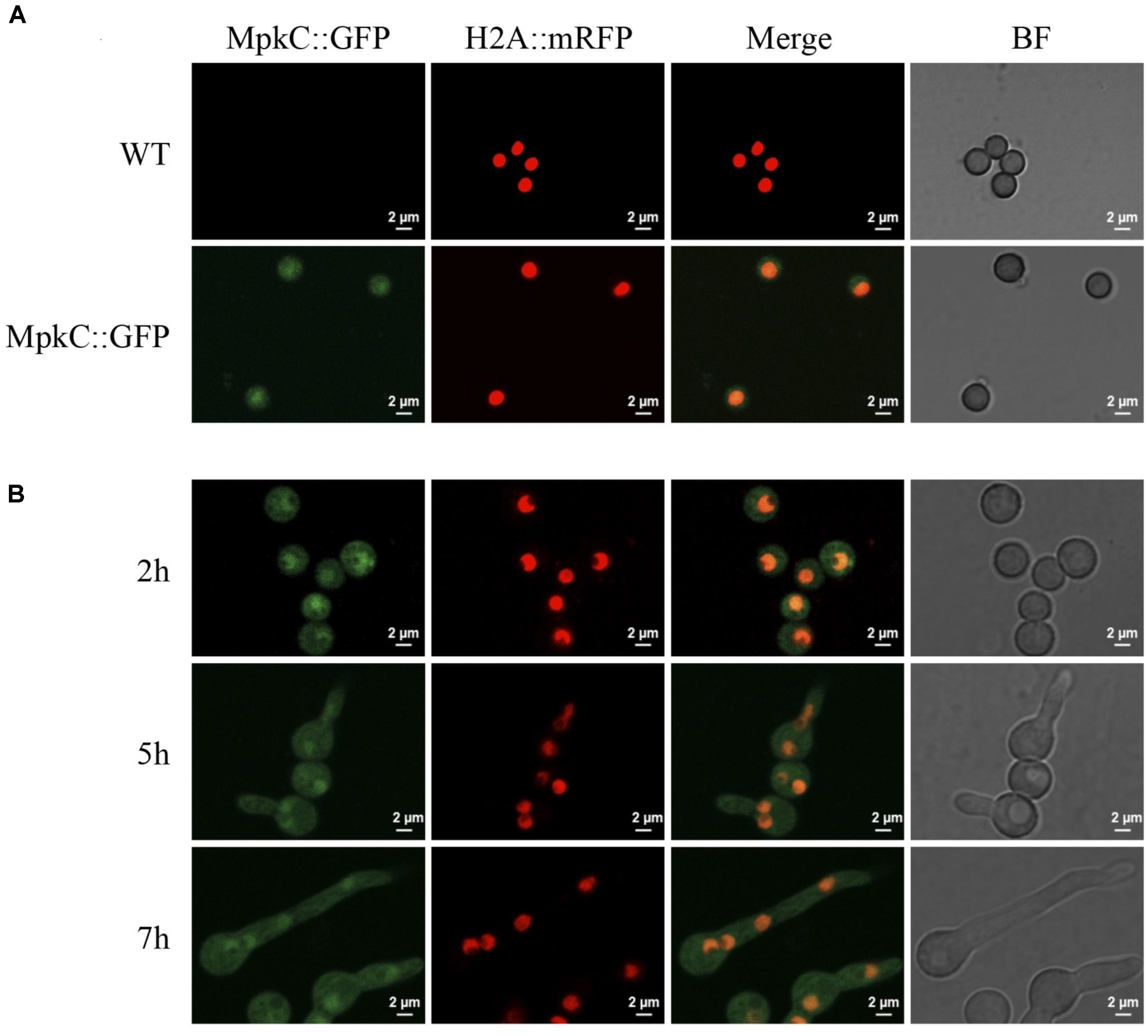
FIGURE 4. Constitutively expressed MpkC::GFP shows nuclear localization during spore germination, in the absence of stress. (A) Intact conidia from strains TRJ7 (WT H2A::mRFP) and TRJ13 (MpkC::GFP H2A::mRFP) were observed using confocal microscopy. (B) Conidia from strain TRJ13 were germinated for the indicated times and observed using confocal microscopy.
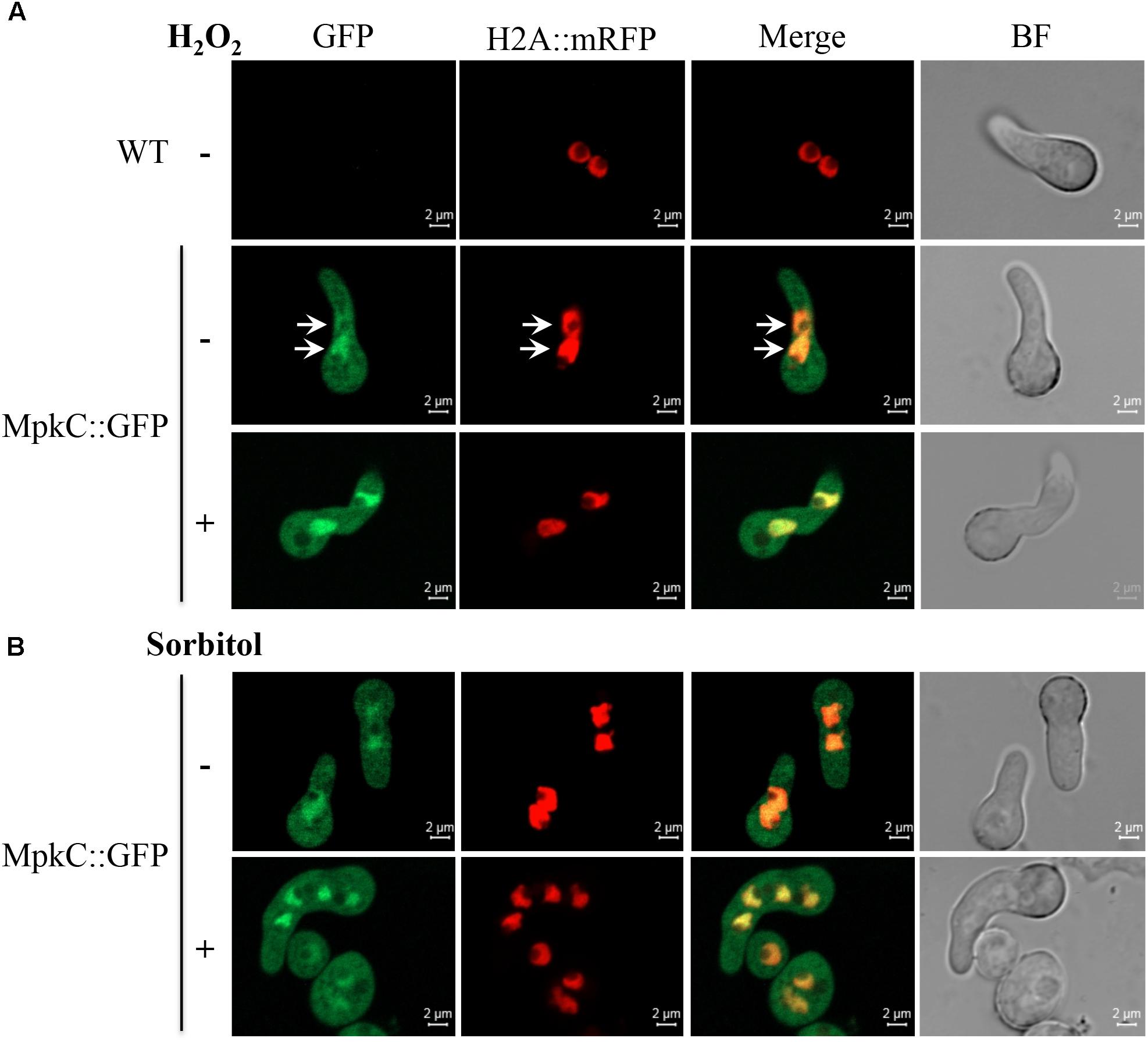
FIGURE 5. Oxidative and osmotic stresses increase the nuclear accumulation of MpkC during spore germination. (A) Conidia from strains TRJ7 (WT H2A::mRFP) and TRJ13 (MpkC::GFP H2A::mRFP) were germinated for 6 h in minimal medium (Top), treated or not with 10 mM H2O2 for 10 min and then observed using confocal microscopy. (B) Conidia from strain TRJ13 (MpkC::GFP H2A::mRFP) were germinated for 6 h in MM containing or lacking 1.2 M sorbitol and observed using confocal microscopy. Arrows in panel (A) point to nuclear signal.
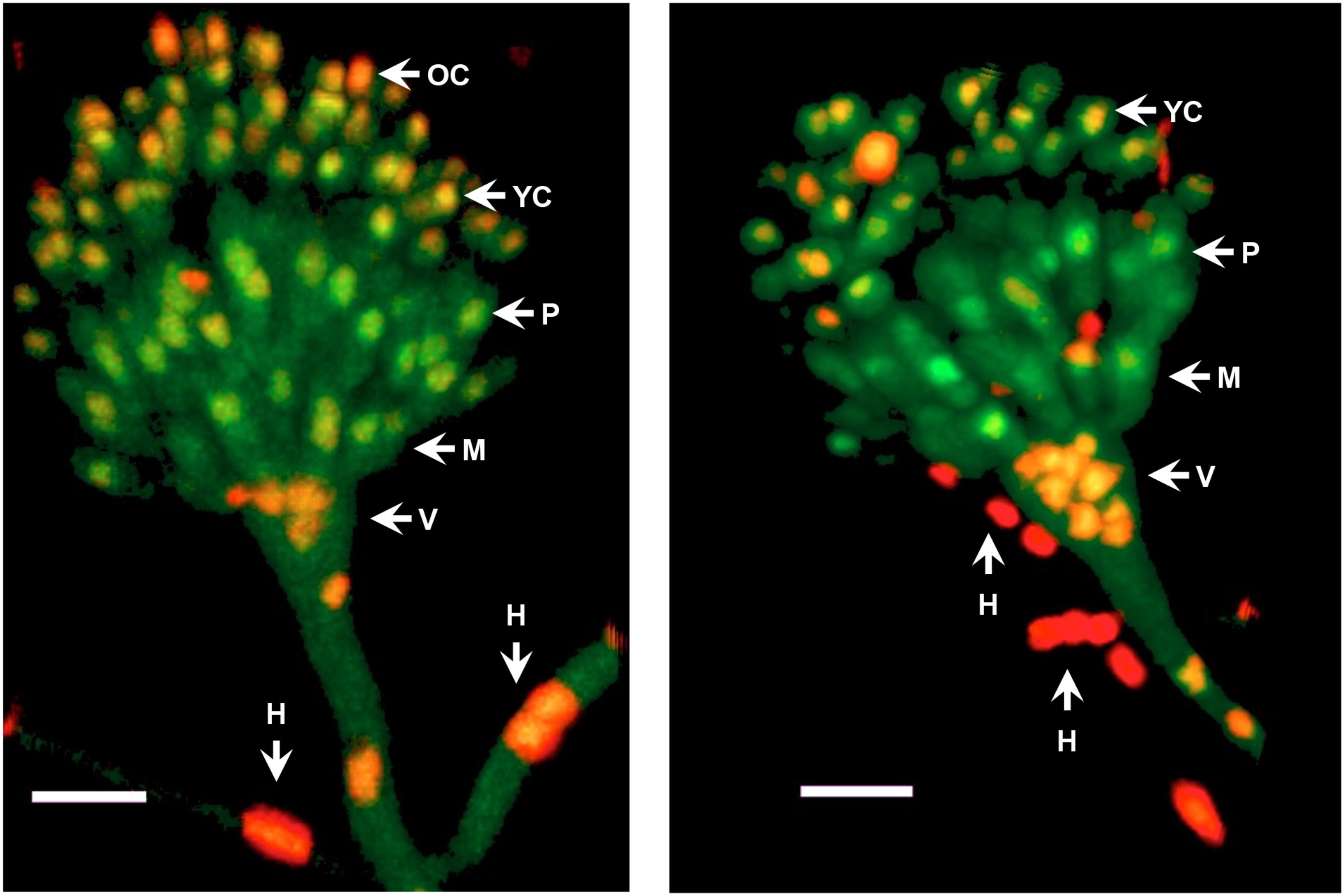
FIGURE 6. MpkC shows different nuclear localization patterns in different conidiophore cell-types. The growing edge of a colony grown for 3 days at 37°C was sectioned and observed directly by confocal microscopy. Two different conidiophores are shown. The different indicated structures are: H, hyphae; V, vesicle; M, metulae; P, phialide; YC, young conidia; and OC, older conidia. Images correspond to maximum intensity Z-stack projections. Scale bar corresponds to 5 μm.
In support of this, we found that without stress MpkC was virtually absent from nuclei in growing hyphae, while oxidative and osmotic stress induced its nuclear localization (Figure 7A). In the absence of the MAPKK PbsB, the stress-induced nuclear localization of MpkC was drastically reduced (Figure 7B). The lack of PbsB also resulted in decreased MpkC nuclear localization during conidia germination as well as during oxidative or osmotic stress (Figure 8).
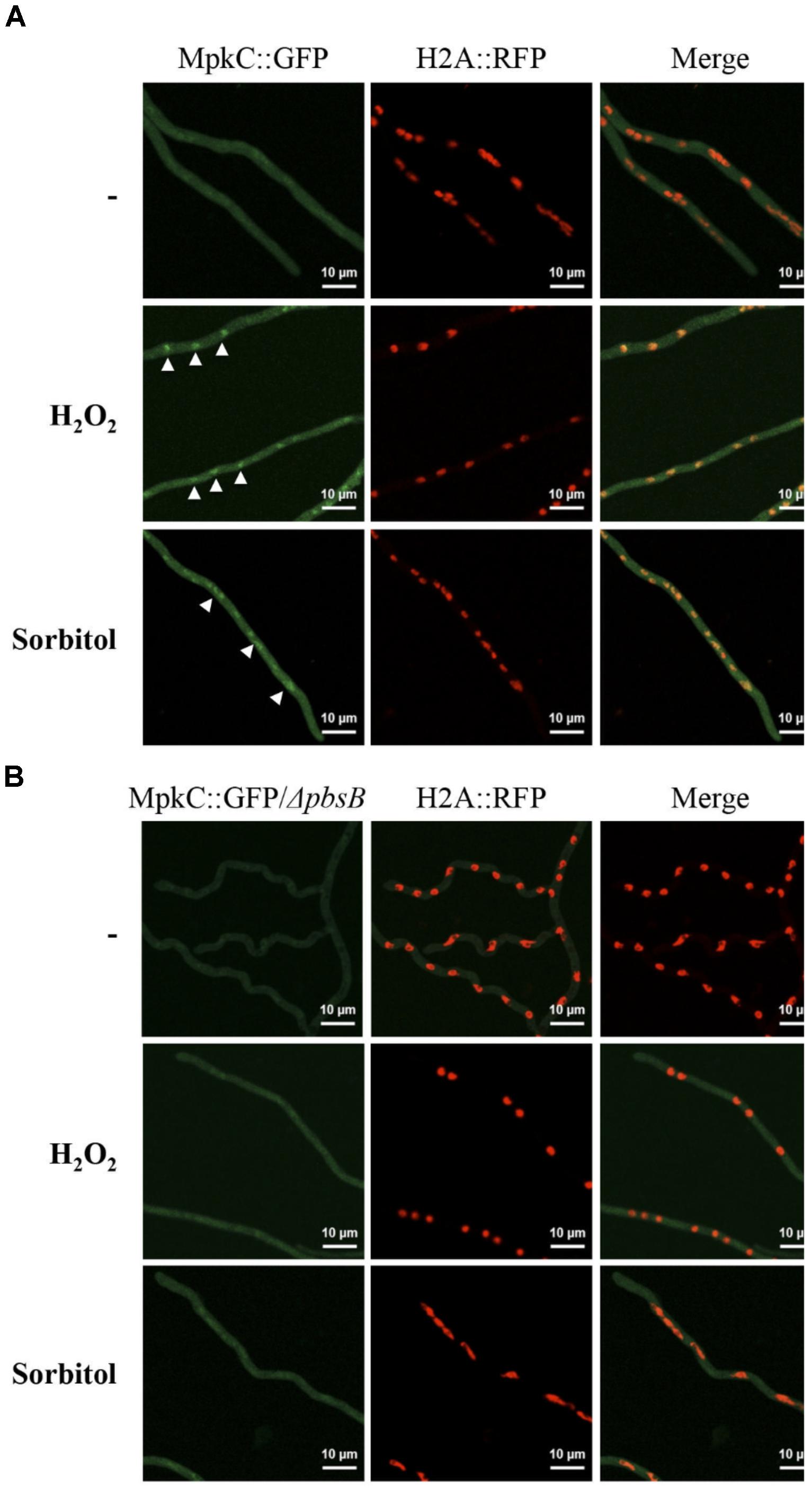
FIGURE 7. In mycelia the MAPKK PbsB is necessary for MpkC nuclear accumulation in response to oxidative and osmotic stress. (A) Mycelia from strain TRJ13 (MpkC::GFP H2A::mRFP) grown for 14 h in minimal medium (Top) was treated or not with 10 mM H2O2 for 20 min (Middle) or grown for 14 h on MM containing 1.2 M sorbitol and then observed using confocal microscopy. (B) Mycelia from strain CVG10 (ΔpbsB MpkC::GFP H2A::mRFP) grown for 14 h in minimal medium (Top) was treated or not with 10 mM H2O2 for 20 min (Middle) or grown for 14 h on MM containing 1.2 M sorbitol and then observed using confocal microscopy. White arrowheads in panel (A) point to some of the nuclei with MpkC::GFP signal.
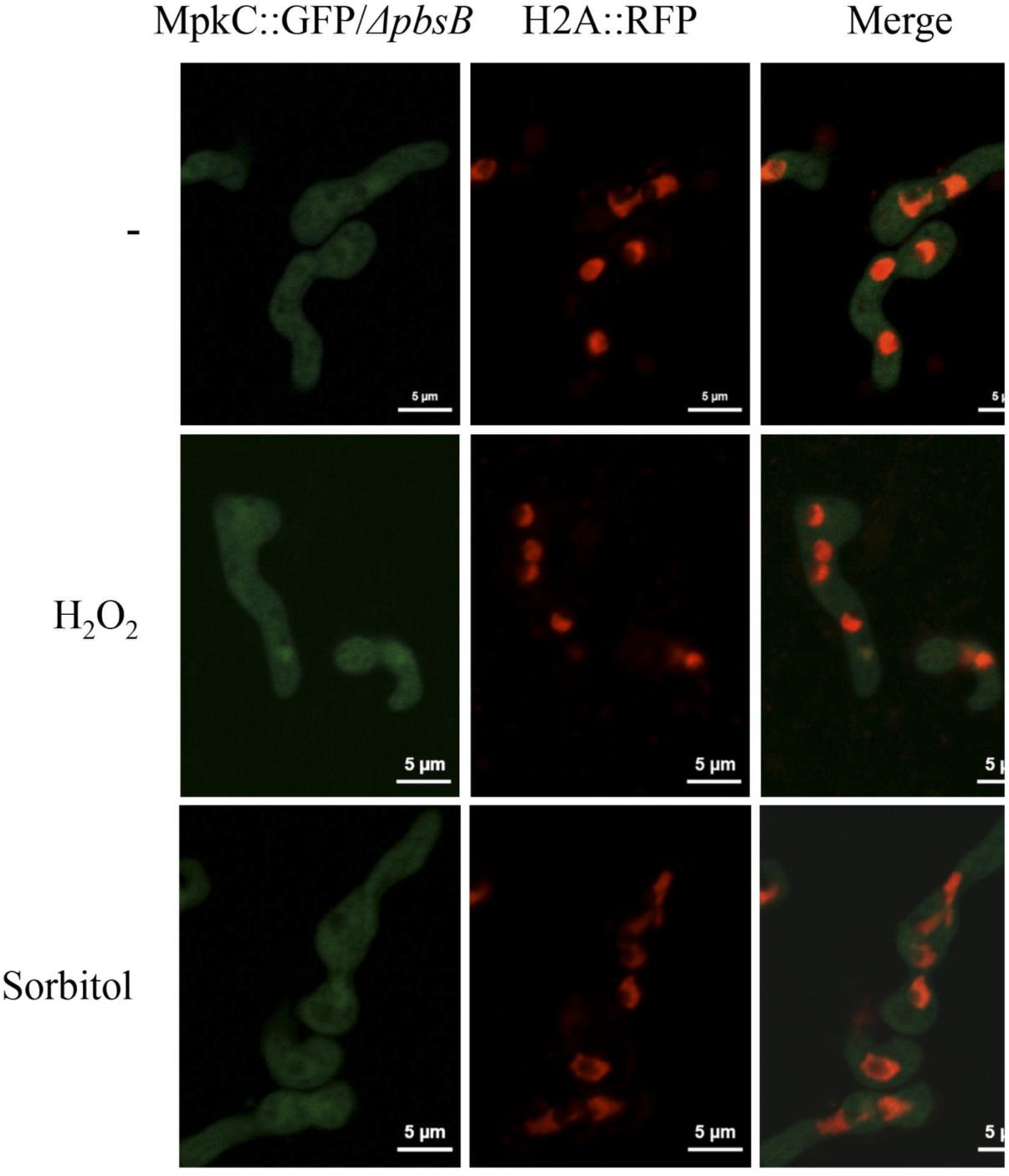
FIGURE 8. The MAPKK PbsB is necessary for MpkC nuclear accumulation during spore germination and in response to oxidative and osmotic stress. Conidia from strain CVG10 (ΔpbsB MpkC::GFP H2A::mRFP) were germinated for 6 h in minimal medium (Top) and treated with 10 mM H2O2 for 10 min (Middle) or germinated for 6 h in MM containing 1.2 M sorbitol and then observed using confocal microscopy.
In summary, these results support a model in which MpkC nuclear localization is regulated during A. nidulans asexual development. In this model MpkC is localized in the cytoplasm during hyphal growth, it accumulates in nuclei during conidiophore development and is largely re-localized to the cytoplasm in mature conidia. During germination, MpkC would be enriched in nuclei until hyphal growth is fully established, where MpkC becomes cytoplasmic again. Our results also show that in addition to this developmental regulation, MpkC accumulates in nuclei in response to oxidative and osmotic stress.
SakA nuclear accumulation is also regulated during development and in response to oxidative and osmotic stress. However, the developmental regulation reported for SakA (Kawasaki et al., 2002; Lara-Rojas et al., 2011; Jaimes-Arroyo et al., 2015) is opposite to what we report here for MpkC.
Together, our results show that SakA and MpkC have opposite as well as common functions during the A. nidulans life cycle and thus contribute to understand the functional relationship between these two SAPKs. Indeed, SakA and MpkC regulate conidia oxidative stress resistance in opposite ways (Figure 3) and this is consistent with their opposite nuclear localization in intact conidia, where MpkC is mostly localized in the cytoplasm (Figure 4A), and SakA is mainly localized in nuclei (Lara-Rojas et al., 2011). We ignore the mechanism by which in the absence of SakA, MpkC mediates a higher sensitivity of conidia to H2O2. Under these conditions, perhaps H2O2 induces a hyper activation of the MpkC pathway, resulting in the killing of the cell. During germination MpkC is enriched in nuclei, while SakA localize in the cytoplasm and in fact it needs to be dephosphorylated for germination to take place (Lara-Rojas et al., 2011). Moreover, SakA activity is essential while MpkC is dispensable to maintain the viability of conidia (Lara-Rojas et al., 2011; Supplementary Figure S4B). In addition to its developmental regulation, MpkC nuclear accumulation in germlings is increased by oxidative and osmotic stress treatments. MpkC roles in conidiation and stress responses can be both related to the transient regulation of cell-cycle arrest under these conditions (see further).
SakA and MpkC regulate conidiation in different ways. The lack of SakA has no major effects in conidiation, while the lack of MpkC results in an increased production of conidia, and the lack of both MAPKs and PbsB results in a drastic reduction in conidiation (Figures 1–3 and Supplementary Figure S4A). ΔmpkC and ΔmpkC ΔsakA mutant conidiophores, developed on solid media or induced by glucose or nitrogen starvation (Skromne et al., 1995) in liquid media, display normal morphology (not shown). Their respective increased and decreased conidiation, seems to result from differences in the number of conidia produced per phialide and the density of conidiophores, parameters that are difficult to evaluate. While it is clear that SakA is necessary to fully arrest mitosis in dormant conidia (Lara-Rojas et al., 2011), we propose that low kinase levels of nuclear (active) MpkC, due to low MpkC intrinsic kinase activity and/or its low expression, are necessary to delay mitosis during conidia development. In S. pombe, SakA ortholog Spc1/Sty1 mediates cell-cycle arrest in response to stress by phosphorylating the MAPKAP Srk1, and regulating its translocation to the nucleus (Lopez-Aviles et al., 2008). In A. nidulans, SakA interacts with MpkC, the Srk1 ortholog SrkA and other proteins involved in cell-cycle regulation, and also regulate SrkA nuclear localization (Jaimes-Arroyo et al., 2015). This suggests that MpkC might also regulate mitosis trough SrkA. In unicellular S. pombe, under poor nutrient conditions, low and high kinase levels of a single SAPK (Spc1/Sty1) suffice to regulate the advancement or the delay of mitosis, respectively (Hartmuth and Petersen, 2009; Shiozaki, 2009). In filamentous fungi cell size-mitosis control must be critical during single-cell (conidia) differentiation. Two different SAPKs might be necessary to modulate mitosis in the complex multicellular conidiophore produced by the Aspergilli, and to maintain the final dormant state of conidia. Indeed, in these fungi phialoconidiation first involves nuclei proliferation without division at the vesicle stage and later a single cell, the phialide, should undergo mitosis to produce two nuclei, one that migrates to the nascent conidia and remains arrested at G1, and the one retained by the phialide, which will undergo mitosis again in a process that in the Aspergilli is repeated many times, to produce chains of up to 120 conidia (Oliver, 1972; Mims et al., 1988; Sewall et al., 1990). In coenocytic hyphae, MpkC nuclear localization is induced by osmotic and oxidative stress, playing a minor and mostly redundant function with SakA, which explains why mpkC initial inactivation did not produce any clear phenotype (Jun et al., 2011).
The MAPKK PbsB mediates both, developmental and stress-induced localization of SakA and MpkC and therefore mediates SakA and MpkC phosphorylation levels. However, very low levels of MpkC nuclear signal were observed in the absence of PbsB, particularly under sorbitol treatment (Figure 8). This could be a secondary effect derived from the use of a constitute promoter to express MpkC, or it could indicate the existence of PbsB-independent mechanisms to activate MpkC. Autophosphorylation is one possibility to be explored. Although MAPKs in general do not show spontaneous autophosphorylation, such capability has been reported for p38β and in other MAPKs it could be de-repressed under specific conditions (Beenstock et al., 2014; Tesker et al., 2016).
SakA and MpkC molecular differences can easily account for their functional differences. Indeed, human CSBP2 and CSBP1, two SakA/MpkC/Hog1 homologs that are splice variants differing only in an internal 25-amino acid sequence, contrast in their ability to complement a Δhog1 mutant and are differentially activated by salt in yeast. CSBP2 but not CSPB1 complemented a Δhog1 yeast phenotype and yet CSPB1 was constitutively active in a PbsB2 MAPKK-dependent fashion. Notably, a CSPB1 mutant with about 3 times lower kinase activity was able to complement a Δhog1 mutant (Kumar et al., 1995). Although both regulated by PbsB, SakA and MpkC might show differences in basal and activated kinase activity, the extent of activation by PbsB, their sensitivity to phosphatases and also in their interactions with common and different substrates.
In contrast to the different roles that SakA and MpkC play during development, they play common roles in maintaining cell-wall integrity in conidia. In A. fumigatus both MAPKs were shown to be individually required for resistance to osmotic, oxidative and cell-wall stress, with the simultaneous disruption of both MAPKs showing additive defects in these processes (Bruder Nascimento et al., 2016). Although we do not observe an additive effect of SakA and MpkC inactivation in oxidative or osmotic stress sensitivity, we do observe that both MAPKs contribute to cell wall integrity in conidia. In a previous report, MpkC constitutively expressed in mycelia grown for 24 h at 30°C, was not detected as phosphorylated, while a 10 min treatment with 0.5 M NaCl resulted in its phosphorylation (Furukawa et al., 2005). This is in agreement with our results, as we find that in mycelia MpkC is largely absent from nuclei in the absence of stress, and that both oxidative and osmotic stress treatments induce its nuclear accumulation.
JA designed the experiments, wrote the manuscript, and obtained funding. VG-B, RJ-A, OS, and FL-R performed and designed the experiments, and contributed to manuscript writing.
This work was supported by CONACYT grants CB-2014-01-238492, Investigación en Fronteras de la Ciencia 2015-I-319, and PAPIIT-UNAM IN208916.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
VG-B received a doctoral fellowship from CONACYT and this work is part of her Ph.D. thesis, as student in the program Maestría y Doctorado en Ciencias Biológicas from Universidad Nacional Autónoma de México. We are grateful to Ozgür Bayram, Özlem S. Bayram, and Gerhard Braus (Georg-August University, Germany) for the mpkC::gfp construct. We also thank IFC-UNAM Molecular Biology and Computer Units and are particularly grateful to Dr. Fernando García from IFC Imagenology unit for his help with confocal microscopy.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02518/full#supplementary-material
Bayram, O., Bayram, O. S., Ahmed, Y. L., Maruyama, J., Valerius, O., Rizzoli, S. O., et al. (2012). The Aspergillus nidulans MAPK module AnSte11-Ste50-Ste7-Fus3 controls development and secondary metabolism. PLoS Genet. 8:e1002816. doi: 10.1371/journal.pgen.1002816
Beenstock, J., Ben-Yehuda, S., Melamed, D., Admon, A., Livnah, O., Ahn, N. G., et al. (2014). The p38beta mitogen-activated protein kinase possesses an intrinsic autophosphorylation activity, generated by a short region composed of the alpha-G helix and MAPK insert. J. Biol. Chem. 289, 23546–23556. doi: 10.1074/jbc.M114.578237
Brewster, J. L., de Valoir, T., Dwyer, N. D., Winter, E., and Gustin, M. C. (1993). An osmosensing signal transduction pathway in yeast. Science 259, 1760–1763. doi: 10.1126/science.7681220
Bruder Nascimento, A. C., Dos Reis, T. F., de Castro, P. A., Hori, J. I., Bom, V. L., de Assis, L. J., et al. (2016). Mitogen activated protein kinases SakA(HOG1) and MpkC collaborate for Aspergillus fumigatus virulence. Mol. Microbiol. 100, 841–859. doi: 10.1111/mmi.13354
Dixon, K. P., Xu, J. R., Smirnoff, N., and Talbot, N. J. (1999). Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant Cell 11, 2045–2058. doi: 10.1105/tpc.11.10.2045
Escote, X., Zapater, M., Clotet, J., and Posas, F. (2004). Hog1 mediates cell-cycle arrest in G1 phase by the dual targeting of Sic1. Nat. Cell Biol. 6, 997–1002. doi: 10.1038/ncb1174ncb1174
Esquivel-Naranjo, E. U., Garcia-Esquivel, M., Medina-Castellanos, E., Correa-Perez, V. A., Parra-Arriaga, J. L., Landeros-Jaime, F., et al. (2016). A Trichoderma atroviride stress-activated MAPK pathway integrates stress and light signals. Mol. Microbiol. 100, 860–876. doi: 10.1111/mmi.13355
Fischer, R., Aguirre, J., Herrera-Estrella, A., and Corrochano, L. M. (2016). The Complexity of fungal vision. Microbiol. Spectr. 4:FUNK-0020-2016. doi: 10.1128/microbiolspec.FUNK-0020-2016
Furukawa, K., Hoshi, Y., Maeda, T., Nakajima, T., and Abe, K. (2005). Aspergillus nidulans HOG pathway is activated only by two-component signalling pathway in response to osmotic stress. Mol. Microbiol. 56, 1246–1261. doi: 10.1111/j.1365-2958.2005.04605.x
Grice, C. M., Bertuzzi, M., and Bignell, E. M. (2013). Receptor-mediated signaling in Aspergillus fumigatus. Front. Microbiol. 4:26. doi: 10.3389/fmicb.2013.00026
Hagiwara, D., Asano, Y., Marui, J., Yoshimi, A., Mizuno, T., and Abe, K. (2009). Transcriptional profiling for Aspergillus nidulans HogA MAPK signaling pathway in response to fludioxonil and osmotic stress. Fungal Genet. Biol. 46, 868–878. doi: 10.1016/j.fgb.2009.07.003
Han, K. H., and Prade, R. A. (2002). Osmotic stress-coupled maintenance of polar growth in Aspergillus nidulans. Mol. Microbiol. 43, 1065–1078. doi: 10.1046/j.1365-2958.2002.02774.x
Hartmuth, S., and Petersen, J. (2009). Fission yeast Tor1 functions as part of TORC1 to control mitotic entry through the stress MAPK pathway following nutrient stress. J. Cell Sci. 122(Pt 11), 1737–1746. doi: 10.1242/jcs.049387
Hill, T. W., and Käfer, E. (2001). Improved protocols for Aspergillus minimal medium: trace element and minimal medium salt stock solutions. Fungal Genet. Rep. 48, 20–21. doi: 10.4148/1941-4765.1173
Izumitsu, K., Yoshimi, A., and Tanaka, C. (2007). Two-component response regulators Ssk1p and Skn7p additively regulate high-osmolarity adaptation and fungicide sensitivity in Cochliobolus heterostrophus. Eukaryot. Cell 6, 171–181. doi: 10.1128/EC.00326-06
Jaimes-Arroyo, R., Lara-Rojas, F., Bayram, O., Valerius, O., Braus, G. H., and Aguirre, J. (2015). The SrkA kinase is part of the SakA Mitogen-activated protein kinase interactome and regulates stress responses and development in Aspergillus nidulans. Eukaryot. Cell 14, 495–510. doi: 10.1128/EC.00277-14
Jun, S. C., Lee, S. J., Park, H. J., Kang, J. Y., Leem, Y. E., Yang, T. H., et al. (2011). The MpkB MAP kinase plays a role in post-karyogamy processes as well as in hyphal anastomosis during sexual development in Aspergillus nidulans. J. Microbiol. 49, 418–430. doi: 10.1007/s12275-011-0193-3
Karos, M., and Fischer, R. (1999). Molecular characterization of HymA, an evolutionarily highly conserved and highly expressed protein of Aspergillus nidulans. Mol. Gen. Genet. 260, 510–521. doi: 10.1007/s004380050924
Kawasaki, L., Sanchez, O., Shiozaki, K., and Aguirre, J. (2002). SakA MAP kinase is involved in stress signal transduction, sexual development and spore viability in Aspergillus nidulans. Mol. Microbiol. 45, 1153–1163. doi: 10.1046/j.1365-2958.2002.03087.x
Kejzar, A., Grotli, M., Tamas, M. J., Plemenitas, A., and Lenassi, M. (2015). HwHog1 kinase activity is crucial for survival of Hortaea werneckii in extremely hyperosmolar environments. Fungal Genet. Biol. 74, 45–58. doi: 10.1016/j.fgb.2014.11.004
Kholodenko, B. N., and Birtwistle, M. R. (2009). Four-dimensional dynamics of MAPK information processing systems. Wiley Interdiscip. Rev. Syst. Biol. Med. 1, 28–44. doi: 10.1002/wsbm.16
Konte, T., and Plemenitas, A. (2013). The HOG signal transduction pathway in the halophilic fungus Wallemia ichthyophaga: identification and characterisation of MAP kinases WiHog1A and WiHog1B. Extremophiles 17, 623–636. doi: 10.1007/s00792-013-0546-4
Kumar, S., McLaughlin, M. M., McDonnell, P. C., Lee, J. C., Livi, G. P., and Young, P. R. (1995). Human mitogen-activated protein kinase CSBP1, but not CSBP2, complements a hog1 deletion in yeast. J. Biol. Chem. 270, 29043–29046. doi: 10.1074/jbc.270.49.29043
Lamb, T. M., Finch, K. E., and Bell-Pedersen, D. (2012). The Neurospora crassa OS MAPK pathway-activated transcription factor ASL-1 contributes to circadian rhythms in pathway responsive clock-controlled genes. Fungal Genet. Biol. 49, 180–188. doi: 10.1016/j.fgb.2011.12.006
Lara-Ortiz, T., Riveros-Rosas, H., and Aguirre, J. (2003). Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol. Microbiol. 50, 1241–1255. doi: 10.1046/j.1365-2958.2003.03800.x
Lara-Rojas, F., Sanchez, O., Kawasaki, L., and Aguirre, J. (2011). Aspergillus nidulans transcription factor AtfA interacts with the MAPK SakA to regulate general stress responses, development and spore functions. Mol. Microbiol. 80, 436–454. doi: 10.1111/j.1365-2958.2011.07581.x
Lopez-Aviles, S., Lambea, E., Moldon, A., Grande, M., Fajardo, A., Rodriguez-Gabriel, M. A., et al. (2008). Activation of Srk1 by the mitogen-activated protein kinase Sty1/Spc1 precedes its dissociation from the kinase and signals its degradation. Mol. Biol. Cell 19, 1670–1679. doi: 10.1091/mbc.E07-07-0639
McCluskey, K., Wiest, A., and Plamann, M. (2010). The fungal genetics stock center: a repository for 50 years of fungal genetics research. J. Biosci. 35, 119–126. doi: 10.1007/s12038-010-0014-6
Mims, C. W., Richardson, E. A., and Timberlake, W. E. (1988). Ultrastructural analysis of conidiophore development in the fungus Aspergillus-nidulans using freeze-substitution. Protoplasma 144, 132–141. doi: 10.1007/Bf01637246
Nayak, T., Szewczyk, E., Oakley, C. E., Osmani, A., Ukil, L., Murray, S. L., et al. (2006). A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172, 1557–1566. doi: 10.1534/genetics.105.052563
Nimmanee, P., Woo, P. C., Kummasook, A., and Vanittanakom, N. (2015). Characterization of sakA gene from pathogenic dimorphic fungus Penicillium marneffei. Int. J. Med. Microbiol. 305, 65–74. doi: 10.1016/j.ijmm.2014.11.003
Oliver, P. T. (1972). Conidiophore and spore development in Aspergillus nidulans. J. Gen. Microbiol. 73, 45–54. doi: 10.1099/00221287-73-145
Reyes, G., Romans, A., Nguyen, C. K., and May, G. S. (2006). Novel mitogen-activated protein kinase MpkC of Aspergillus fumigatus is required for utilization of polyalcohol sugars. Eukaryot. Cell 5, 1934–1940. doi: 10.1128/EC.00178-06
Sánchez, O., and Aguirre, J. (1996). Efficient transformation of Aspergillus nidulans by electroporation of germinated conidia. Fungal Genet. Rep. 43,48–51.
Sánchez, O., Navarro, R. E., and Aguirre, J. (1998). Increased transformation frequency and tagging of developmental genes in Aspergillus nidulans by restriction enzyme-mediated integration (REMI). Mol. Gen. Genet. 258, 89–94. doi: 10.1007/s004380050710
Segmuller, N., Ellendorf, U., Tudzynski, B., and Tudzynski, P. (2007). BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot. Cell 6, 211–221. doi: 10.1128/EC.00153-06
Sewall, T. C., Mims, C. W., and Timberlake, W. E. (1990). abaA controls phialide differentiation in Aspergillus nidulans. Plant Cell 2, 731–739. doi: 10.1105/tpc.2.8.731
Shiozaki, K. (2009). Nutrition-minded cell cycle. Sci. Signal. 2:pe74. doi: 10.1126/scisignal.296pe74
Skromne, I., Sanchez, O., and Aguirre, J. (1995). Starvation stress modulates the expression of the Aspergillus nidulans brlA regulatory gene. Microbiology 141(Pt 1), 21–28. doi: 10.1099/00221287-141-1-21
Smith, D. A., Morgan, B. A., and Quinn, J. (2010). Stress signalling to fungal stress-activated protein kinase pathways. FEMS Microbiol. Lett. 306, 1–8. doi: 10.1111/j.1574-6968.2010.01937.x
Tesker, M., Selamat, S. E., Beenstock, J., Hayouka, R., Livnah, O., and Engelberg, D. (2016). Tighter alpha C-helix-alpha L16-helix interactions seem to make p38 alpha less prone to activation by autophosphorylation than Hog1. Biosci. Rep. 36:e00324. doi: 10.1042/BSR20160020
Vargas-Perez, I., Sanchez, O., Kawasaki, L., Georgellis, D., and Aguirre, J. (2007). Response regulators SrrA and SskA are central components of a phosphorelay system involved in stress signal transduction and asexual sporulation in Aspergillus nidulans. Eukaryot. Cell 6, 1570–1583. doi: 10.1128/EC.00085-07
Yu, J. H., Hamari, Z., Han, K. H., Seo, J. A., Reyes-Dominguez, Y., and Scazzocchio, C. (2004). Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41, 973–981. doi: 10.1016/j.fgb.2004.08.001
Keywords: oxidative stress, cell-wall stress, spore germination, spore development, spore viability, MAPK nuclear localization, MAPKK
Citation: Garrido-Bazán V, Jaimes-Arroyo R, Sánchez O, Lara-Rojas F and Aguirre J (2018) SakA and MpkC Stress MAPKs Show Opposite and Common Functions During Stress Responses and Development in Aspergillus nidulans. Front. Microbiol. 9:2518. doi: 10.3389/fmicb.2018.02518
Received: 12 July 2018; Accepted: 03 October 2018;
Published: 23 October 2018.
Edited by:
Luis F. Larrondo, Pontificia Universidad Católica de Chile, ChileReviewed by:
Iran Malavazi, Universidade Federal de São Carlos, BrazilCopyright © 2018 Garrido-Bazán, Jaimes-Arroyo, Sánchez, Lara-Rojas and Aguirre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jesús Aguirre, amFndWlycmVAY29ycmVvLmlmYy51bmFtLm14
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.