- 1GFZ German Research Centre for Geosciences, Helmholtz Centre Potsdam, Section 5.3 Geomicrobiology, Potsdam, Germany
- 2GFZ German Research Centre for Geosciences, Helmholtz Centre Potsdam, Section 3.2 Organic Geochemistry, Potsdam, Germany
- 3Institute of Earth and Environmental Science, University of Potsdam, Potsdam, Germany
More than 41% of the Earth’s land area is covered by permanent or seasonally arid dryland ecosystems. Global development and human activity have led to an increase in aridity, resulting in ecosystem degradation and desertification around the world. The objective of the present work was to investigate and compare the microbial community structure and geochemical characteristics of two geographically distinct saline pan sediments in the Kalahari Desert of southern Africa. Our data suggest that these microbial communities have been shaped by geochemical drivers, including water content, salinity, and the supply of organic matter. Using Illumina 16S rRNA gene sequencing, this study provides new insights into the diversity of bacteria and archaea in semi-arid, saline, and low-carbon environments. Many of the observed taxa are halophilic and adapted to water-limiting conditions. The analysis reveals a high relative abundance of halophilic archaea (primarily Halobacteria), and the bacterial diversity is marked by an abundance of Gemmatimonadetes and spore-forming Firmicutes. In the deeper, anoxic layers, candidate division MSBL1, and acetogenic bacteria (Acetothermia) are abundant. Together, the taxonomic information and geochemical data suggest that acetogenesis could be a prevalent form of metabolism in the deep layers of a saline pan.
Introduction
Extreme environments were once thought to be incapable of sustaining a variety of life; however, organisms have developed various methods of adapting to the harshest of environments, ranging from hot springs and hydrothermal vents (Kallmeyer, 2017) to permafrost (Mitzscherling et al., 2017) and hypersaline lakes (Ventosa et al., 2011). The present study focuses on the microbial community structure and geochemical characteristics of two different saline pan sediments in the Kalahari Desert in southern Africa. Saline pans are common geomorphic formations in arid and sandy interdune desert systems. These pan structures have been described in many arid and semiarid environments, such as Australia (Magee et al., 1995), China (Bowler et al., 1986), Africa (Lancaster, 1986; Goudie and Wells, 1995), and the southwestern United States (Handford, 1982), but they also exist in cold drylands, such as in Antarctica (Lyons et al., 1998). The drylands comprise the major terrestrial biome of the Earth’s land surface (Reynolds et al., 2007). Due to the desiccation of surface waters and high evaporation rates, aridification in the Kalahari region is a fast process occurring on a seasonal scale. High aridity and daily temperature fluctuations together with intense solar radiation contribute to an extreme habitat for the living organisms. The key microorganisms involved in the biogeochemical cycles of saline sediments and a large number of microbial processes, such as nitrogen fixation and sulfur metabolism, have been insufficiently characterized (Keshri et al., 2013).
All over the world, hypersaline environments – including athalassohaline lakes, evaporation ponds, deserts, and hypersaline environments with marine origins – are inhabited by a variety of microorganisms (DasSarma and Arora, 2001). Halophiles are salt-loving organisms from all three domains of the biological classification of life. They are adapted to grow in high-salt ecosystems, although their overall microbial diversity decreases with increasing salt concentrations (Oren, 1999). Prokaryotes are adapted to resist osmotic stress caused by the high concentration of ions in the external environment by using two main adaptive strategies. To prevent desiccation, first, the “high-salt-in” strategy enables the microorganisms to accumulate potassium and chloride in their cytoplasm. Second, the “low-salt, organic-solutes-in” strategy refers to the accumulation of organic osmotic solutes (Ma et al., 2010), such as ectoine, betaine, and sugars. In saline environments, the microbial life forms face thermodynamic limitations and hence require an appropriate mechanism of osmotic adaption and a form of metabolism that yields enough energy for biomass synthesis and osmoregulation (Oren, 1999, 2011). The majority of halophiles found in salt deposits are archaea, while bacteria form the minority groups (McGenity et al., 2000). The archaea that require the most saline conditions fall under the class Halobacteria (Oren, 2008; Youssef et al., 2012). These Halobacteria are often found in a diverse range of hypersaline environments, such as salt marshes (Bowen et al., 2012), solar salterns (Sørensen et al., 2005), and hypersaline soils (Crits-Christoph et al., 2013; Keshri et al., 2013). Halophiles within the bacterial community are known to exist in the phyla Cyanobacteria, Proteobacteria, Bacteroidetes, Firmicutes, Chloroflexi, and Actinobacteria (Oren, 2008).
Several bacterial species have been isolated from the hypersaline Lake Chaka in China via culture-dependent techniques (Jiang et al., 2006), whereas other studies have used molecular methods, such as 16S rRNA gene clone library sequencing, denaturing gradient gel electrophoresis (DGGE), or terminal restriction fragment length polymorphism (T-RFLP) band sequencing, and have highlighted the idea that the microbial diversity significantly varies between different saline pan systems (Lefebvre et al., 2006; Montoya et al., 2013). In recent years, the next-generation sequencing technologies have expanded our knowledge of the diversity and composition of microbial communities in saline ecosystems (Bowen et al., 2012; Youssef et al., 2012). In the present study, we applied Illumina 16S rRNA gene sequencing and geochemical methods to analyze the microbial diversity and the distribution patterns in Witpan in northwestern South Africa. In a second step, we compared the microbial communities and geochemical features of Witpan with those of the Omongwa pan (Genderjahn et al., 2018). This comparison allowed the identification of the microbial key community occurring at both sites as well as site-specific specialists that occur in southern African saline pans. Multivariate statistics were applied to identify the major community-shaping environmental factors. This study aims to provide a better understanding of the saline and dry habitats and their diverse microbial ecosystems.
Materials and Methods
Study Site and Sampling
In the Kalahari region, precipitation is regulated by the seasonal shift of the Intertropical Convergence Zone and the migration of the westerlies in the Southern Hemisphere (Ahrens and Samson, 2010). Seasonally strong precipitation leads to transient runoff into ephemeral rivers, and pans are temporally filled with water. Pans (also called playa) are the predominant geomorphic feature of the Kalahari. They are closed systems in terms of their surface hydrology because they have no surface outflow. The high evaporation exceeds the rainfall during all months (Lancaster, 1976; Goudie and Wells, 1995); therefore, these bodies of water are often highly saline (Shaw and Bryant, 2011). The hydrological input comes from direct precipitation, surface, or subsurface inflow, and standing surface water occurs ephemerally.
In autumn 2013, a field campaign to southern Africa was conducted in cooperation with the Helmholtz Centre Potsdam GFZ German Research Centre for Geosciences, the German Centre for Marine Biodiversity Research (DZMB) – Senckenberg am Meer, and the Institute for Chemistry and Biology of the Marine Environment (ICBM) of the University Oldenburg, Germany. Sample material was collected from the pan sediments of Witpan (S 26° 40, 658′ E 020° 09, 45′) in northwestern South Africa and from the Omongwa pan (S 23° 42, 59′ E 019° 22, 15′) in Namibia in the western Kalahari (Figure 1). The Witpan deposits display great variability in grain-size distribution. Silt and evaporite crystals dominate the top layers (0–14 cm), followed by a mixture of silt and sand. Fine- and medium-grained sand make up the largest proportion of the sediment, which is between 25 and 119 cm. Clay and silt occur between 119 and 180 cm and dominate Witpan sediment composition at this depth interval (see Supplementary Figure S1). The groundwater table lies at a depth of 230 cm. The Omongwa pan is characterized by the presence of little organic matter, dispersed evaporated crystals, and low-porosity, fine-grained sediments, which mostly consist of silt and gypsum crystals (Supplementary Figure S1, Schüller et al., 2018). During sampling, the Omongwa pan was covered by a thin saline crust. According to Milewski et al. (2017), the main mineralogy components of the top layer are halite (NaCl, 94%) and gypsum (CaSO4⋅2H2O, 3%).
FIGURE 1. Map of the study sites: Witpan (S 26° 40, 658′ E 020° 09, 45′) in South Africa, Omongwa pan (S 23° 42, 59′ E 019° 22, 15′) in eastern Namibia, Kalahari Desert. Quantum GIS 2.18 was used to produce the map (http://www.qgis.org).
Samples were taken from a 50 cm deep trench excavated from the sediment. Between the surface and the top 15 cm, samples were taken at 3-cm intervals, followed by 5-cm intervals between 15 and 50 cm. A short core (50–180 cm) was drilled with an Eijkelkamp hand auger and sampling continued at 15-cm intervals in this section. The samples for molecular biological analyses were taken from the inner parts of the drill core using a sterilized spatula. The samples for biomarker analyses were immediately frozen in liquid nitrogen, and the samples for molecular studies were cooled during the field work and were kept at -24°C after their arrival at GFZ Potsdam.
Quantification of Phospholipid-Derived Fatty Acids
The samples were freeze-dried and homogenized. They were ground using a disk-mill with stainless steel grinding set and extracted with a modified Bligh and Dyer (1959) method that included solvent extraction and phase separation. A sample preparation method after Zink and Mangelsdorf (2004) was used to separatethe total the lipid extract into a low polar fraction, a free fatty acid fraction, a glycolipid fraction, and an intact phospholipid (PL) fraction. The internal standard 1-myristoy-(D27)-2-hydroxy-sn-glycerol-3-phosphocholine was added to quantify the phospholipids. Half of the PL fraction was saponicated to obtain the phospholipid-derived fatty acids (PLFAs) (Müller et al., 1990). The samples were measured on a DSQ Thermo Scientific Quadrupole mass spectrometry (Thermo Fisher Scientific) coupled with a gas chromatograph (TRACE GC Ultra, Thermo Fisher Scientific) equipped with a cold injection system BPX5 (SGE) column (50 m length, 0.22 mm inner diameter, 0.25 μm film thickness). The gas chromatograph (GC) was run in splitless mode with the following settings: injector temperature from 50 to 300°C with 10°C/s, oven temperature from 50°C (1 min isothermal) with a heating step with a rate of 3°C min-1 to 310°C finally held for 30 min. Helium was utilized as a carrier gas at a constant flow rate of 1 mL min-1. The gas chromatography-mass spectrometer (GC-MS) was run in electron impact (EI) ionization mode at 70 eV. Full-scan mass spectra were recorded from m/z 50–650 amu at a scan rate of 1.5 scans s-1.
Sediment Properties
Since the sediment samples for measuring the ionic composition contained too little pore water, they were leached, as indicated by Blume et al. (2011). An aliquot of 5 g of each sample was suspended in 25 mL of deionized water, shaken for 90 min, and centrifuged to remove all the solids. The ion concentrations of anions and organic acids were measured by ion chromatography (IC). The leached samples were investigated for the following ions: chloride, nitrate, sulfate, acetate, and formate. The specifics of the method to detect organic acids have been described by Vieth et al. (2008); for inorganic acids, see Noah et al. (2014). Analytical settings are shown in the Supplementary Table S1. Furthermore, the total organic carbon (TOC) content of each sediment sample was measured according to DIN EN 1484-H3 by Potsdamer Wasser und Umweltlabor GmbH & Co. KG, Germany.
DNA Extraction and Preparation of Next-Generation Sequencing
The total genomic DNA was extracted in triplicate from 0.3–0.5 g sediment material using the Power SoilTM DNA Isolation Kit (Mo Bio Laboratories Inc., Carlsbad, CA, United States) in compliance with the company’s protocol. To enhance the efficiency of the DNA extraction, all the samples were heated up for 10 min to 70°C after step four. The DNA triplicates were pooled for downstream analysis.
The hypervariable region V4 of the 16S rRNA gene was targeted for a subsequent amplification using the primer pair 515F and 806R (Caporaso et al., 2011). The polymerase chain reactions (PCR) were carried out at least in technical triplicates. The 50 μl PCR reaction mix consisted of 25 μl Mango-Mix (including a MangoTaqTM DNA Polymerase, MgCl2, and ultra-pure dNTPs manufactured by Bioline GmbH, Luckenwalde, Germany) and 1 μl of each primer (10 mM), and 5 μl of template. The mix was filled up to 50 μl with PCR-clean water (MO BIO Laboratories, Inc., Carlsbad, CA, United States). Afterward, the PCR products were run on a 1% agarose gel in a 1× Tris-acetate-EDTA buffer stained with GelRedTM Nucleic Acid Gel Stain (Biotium, United States) to check the samples for sequencing. The PCR products were pooled and purified by the Genomic DNA Clean & ConcentratorTM-10 (Zymo Research, United States) and quantified by the Qubit Fluorometer (InvitrogenTM, Thermo Fisher Scientific, United States) for library preparation. The samples were sent to the Illumina MiSeq platform Eurofins Genomics, 85560 Ebersberg, Germany. Sequence libraries were generated by a 2 × 250-bp paired-end approach. The primer sequence consisted of a 6-bp tag and spanned the V4 region. Data were received as raw FASTQ files and deposited into the European Nucleotide Archive (sample accession: ERS1599441 – ERS1599469).
Processing Next-Generation Sequencing Data
Assembling the reads was performed using PEAR (Zhang et al., 2014). Standardizing the nucleotide sequence orientation, trimming (see also Supplementary Table S2) and filtering the low-quality sequences were performed using Trimmomatic, which has been described in detail by Bolger et al. (2014). Afterward, all the chimeras were removed, and the sequences were clustered into OTUs (QIIME pipeline). A taxonomic classification was assigned by the SILVA database (Version 128) (Quast et al., 2013) with a cutoff value of 97% using the QIIME open-source software package and by choosing the open-reference OTUs (Caporaso et al., 2010). The following filters were applied: removing singletons and eliminating all operational taxonomic units (OTUs), which had an occurrence of less than 0.5% in each sample. Overall, the diversity was estimated using the statistic program PAST 3.15 with a taxonomic method (Shannon index, H). The Shannon index is a phylotype-based method created with OTU grouping (McCann et al., 2016). Statistics were carried out using CANOCO 5. The species response to environmental variations was modeled with the help of a canonical correspondence analysis (CCA) (Ramette, 2007).
Quantitative Polymerase Chain Reaction Analysis of Archaeal and Bacterial SSU rRNA Genes
A quantitative polymerase chain reaction (qPCR) was used to quantify the total bacterial and archaeal abundances (Supplementary Figure S2). For bacteria, the forward primer Eub 331-F 5′-TCCTACGGGAGGCAGCAGT-3′ and the reverse primer Eub 797-R 5′-GGACTA CCAGGG-TATCTAATCCTGTT-3′ (Nadkarni et al., 2002) were used to amplify the fragments from the bacterial SSU rRNA genes. The quantification of the archaeal 16S rRNA gene was based on the primers A751F 5′-CCgACGGTGAGRGRYGAA-3′ and UA1204R 5′-TTMGGGGCATRCIKACCT-3′ (Baker et al., 2003). All the qPCR analyses were performed in analytical triplicates in a thermal cycler (CFX ConnectTM Real-Time PCR Detection System, Bio-Rad Laboratories, United States) instrument using the polymerase iTaqTM Universal SYBR® Green Supermix (Qiagen). Each PCR essay contained 12.5 ml of Universal SYBR® Green Supermix SYBR® – including the polymerase iTaqTM, 0.5 μl of each primer (20 mM), and 5 μl template (diluted, 1:7) – and was filled up to 25 ml with PCR-clean water (MO BIO Laboratories, Inc., Carlsbad, CA, United States). After the initial denaturation phase of 30 s at 95°C, the annealing phase followed at 58.5°C for 1 min. Elongation was performed at 72°C for 30 s and then at 80°C for 3 s. In total, 40 cycles were run. To generate a standard curve, known dilutions (101–107 gene copies) of the target fragments amplified from Methanosarcina barkeri (for archaea) and Bacillus subtilis (for bacteria) were used. Finally, the melting curve analyses were performed to ensure correct amplification.
Results
Abiotic and Biotic Parameters of Witpan and Omongwa Pan
In Witpan, the values of the deposits of the TOC are rather low and range from 0.03 to 0.26 wt% (Figure 2A). Between 14 and 50 cm, the TOC content is below the detection limit (0.01 wt%). A slight increase in TOC was detected from 80–160 cm. In contrast, the TOC of the Omongwa pan ranges from 0.3 to 1.7 wt%, with the highest values in the top 20 cm and a maximum value at a depth of 75 cm (Figure 2A).
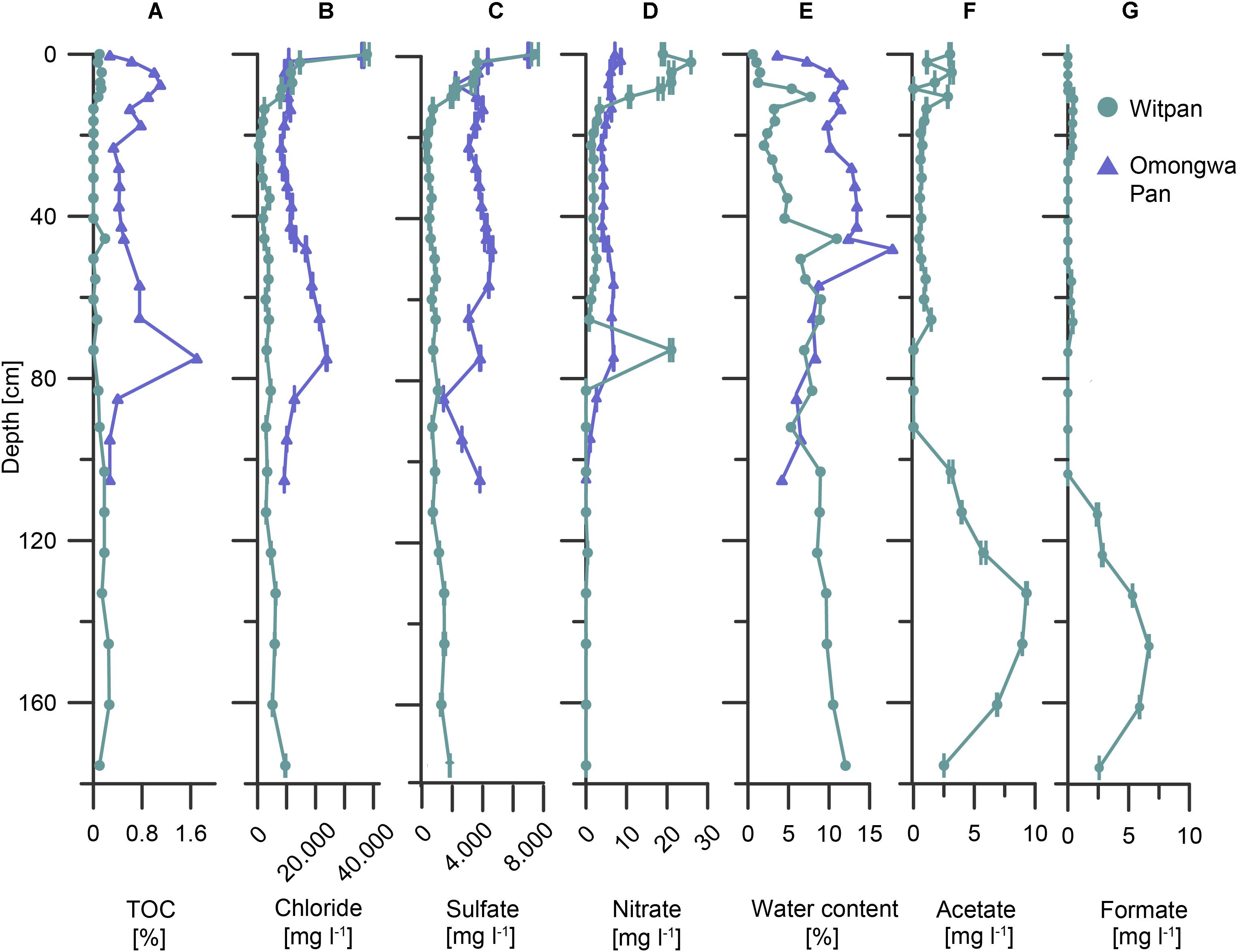
FIGURE 2. Abiotic and biotic parameters of Witpan (turquoise) and the Omongwa pan (purple) with depth. (A) Total organic carbon (TOC), (B) chloride, (C) sulfate, (D) nitrate, (E) water content, (F) acetate, and (G) formate [(B–D,F,G) were obtained from sample leaching]. Note different x-scales.
Chloride, sulfate, and nitrate are the predominant anions in the near-surface layers of both pans. In Witpan sediments, the concentrations increase up to 38,000 mg l-1 of chloride, up to 7,500 mg l-1 of sulfate, and up to 26 mg l-1 of nitrate (Figures 2B–D). Below the surface layer, chloride and sulfate concentrations decrease to 480 and 370 mg l-1, respectively, before beginning to slightly but progressively increase again to 9,560 mg -1 (chloride) and 1,880 mg l-1 (sulfate) at 175 cm. The nitrate concentration is quite low (around 2 mg l-1) or absent between 120 and 175 cm, with an exception at a depth of 70 cm (21 mg l-1). In Omongwa pan sediments, the chloride concentrations range from 8,200 to 36,300 mg l-1, and the sulfate concentrations vary between 1,500 and 7,100 mg l-1. Both concentrations are greatest in samples close to the surface, followed by an abrupt decrease with depth. Between 45 and 80 cm, chloride increases again. The nitrate concentrations in the Omongwa pan decrease with depth and are significantly lower (up to 8.6 mg l-1, Figure 2D) than those of chloride or sulfate. The water content of Witpan is around 0.6% in the top layers and increases slowly but more-or-less steadily with depth up to ∼12%. In the Omongwa pan sediments, the water content is around 4% in the upper layer and increases to 18% at a depth of 50 cm. After this peak, the water content decreases again with depth to 4% (Figure 2E). Low molecular weight acids such as acetate and formate could only be detected in Witpan in varying concentrations between 0.2 and ∼9 mg l-1 (Figures 2F,G). The acetate and formate concentrations increase at a depth between 100 and 160 cm compared with the upper part of Witpan.
Microbial Community Composition of Witpan
In the dataset described here, 407 different OTUs were found to be distributed between the Omongwa pan and Witpan. Detailed taxonomic information was assigned to the relative abundance (rel. ab.) of OTUs within the microbial community. Classified sequences from Witpan showed forms of life from 20 phyla, with bacteria accounting to 36–100% of the microbial community (Figure 3A). The majority of archaeal sequences could be assigned to Euryarchaeota (17 OTUs) and detected almost throughout the entire depth profile. Fourteen OTUs of Euryarchaeota belong to the class Halobacteria, including the genera Halobiforma, Natronococcus, Halococcus, Halomicrobium, and a high proportion of uncultured genera. Halobacteria were observed from the near-surface layers down to 103 cm and represent up to 65% (at 22.5 cm) of the microbial community. Archaeal sequences in deeper layers (from 123 to 160 cm) are affiliated with candidate division MSBL1 (2 OTUs) and form up to 10% of the combined bacterial and archaeal community. Only at 133 cm was one archaeal OTU of Hadesarchaea detectable (less than 1.8% of the community).
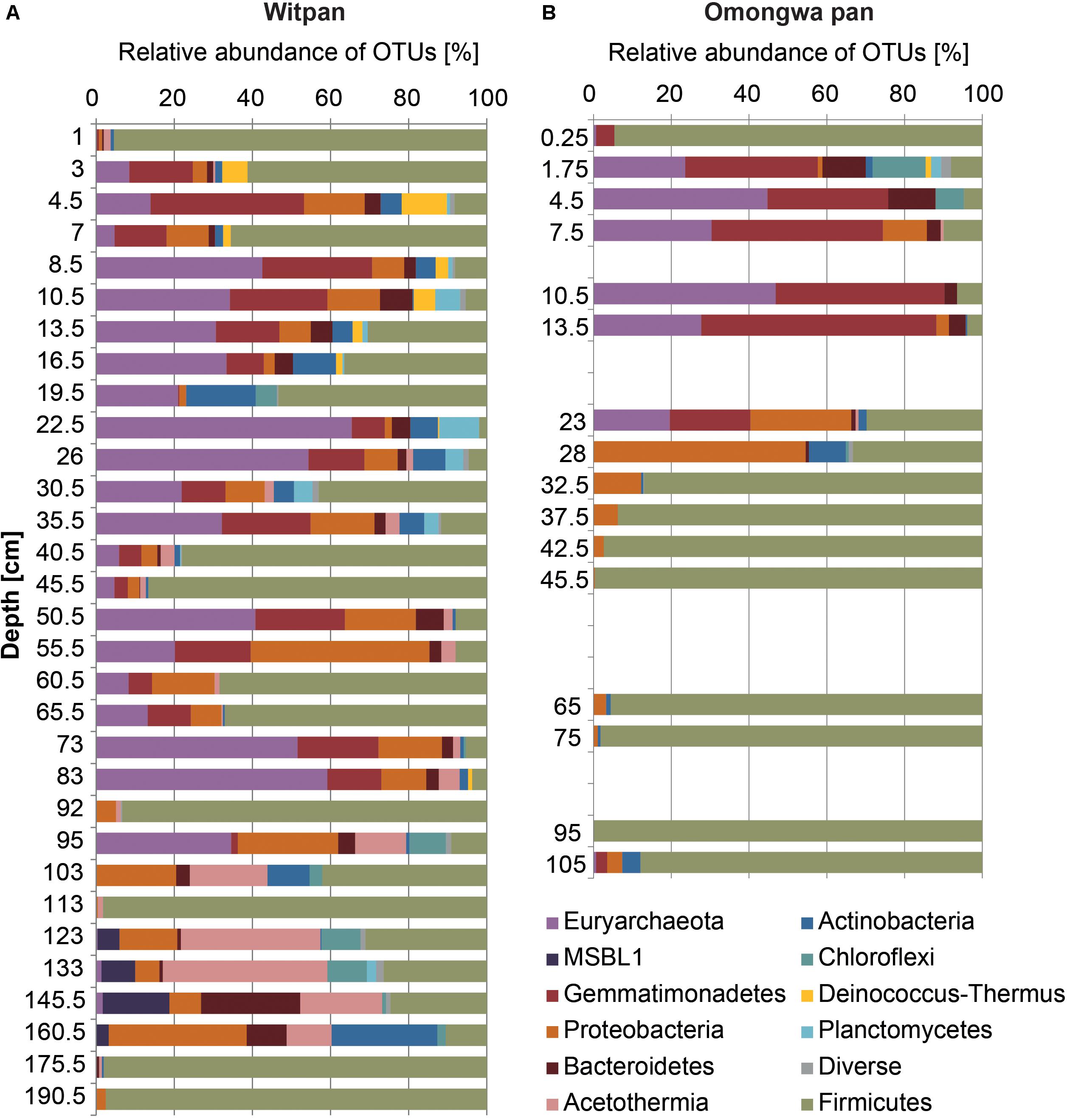
FIGURE 3. Microbial communities of Witpan (A) and Omongwa pan (B). Depth profile shows different bacterial and archaeal phyla obtained using high-throughput Illumina sequencing. Color codes are indicated on the right.
Sequences of Firmicutes were detected throughout the entire depth profile. Several layers are dominated by Firmicutes such as the surface layer and layers at 40 to 45.5 cm, 92 cm, 113 cm, and 175 cm, where they represent more than 95% of the community. The genus Bacillus dominates the phylum Firmicutes and occurs in every depth range. In addition, Clostridia could be found mainly between 103 and 160.5 cm (up to 13% rel. ab.). The bacterial sequences of Gemmatimonadetes (7 OTUs) are abundant from the top layers down to 95 cm and form up to 38% of the microbial community (e.g., at 4.5 cm). Four orders could be identified: Longimicrobia (2–16.5 cm), S0134 (13.5–16.5 cm), PAUC43f (19.5–83 cm), and BDS-11 (22–83 cm). Bacteroidetes can be assigned to 6 different classes including Bacteroidia (1 OTU), Cytophagia (5 OTUs), Flavobacteria (3 OTUs), Sphingobacteria (2 OTUs), and Bacteroidetes Incertae Sedis Order III (5 OTUs, formerly assigned to Rhodothermales). Down to 83 cm, Bacteroidetes Incertae Sedis (1–8% rel. ab. of all sequences) dominates the phylum Bacteroidetes, whereas in the deep layers from 123–160 cm, Bacteroidia are predominant (up to 23% of the microbial community). Proteobacteria (59 OTUs) are present all over the depth profile and consists of Alpha-, Beta-, Gamma-, and Delta-proteobacteria. At a depth of 123–175 cm, the families Methylophilaceae, Comamonadaceae, Desulfohalobiaceae, and the order Xanthomonadales become predominant within the phylum Proteobacteria. In layers from 24–34 cm, the order Rhodospirillales comprises 1–4% of the microbial community. The order Rhizobiales (including mostly Salinarimonas and Methylobacteriaceae) occurs infrequently, and sequences could mainly be detected between 2 and 7 cm, forming 1 to 4% of the community. In the deep layers from 95–175 cm, Rhizobiales make up to 1% of the microbial community. Actinobacteria sequences that can be assigned the class Nitriliruptoria (6 OTUs) were found between 7 and 45.5 cm in Witpan (1–4% rel. ab.). Among the sequences assigned to Actinobacteria, Acidimicrobiales (3 OTUs) occur infrequently between 2 and 83 cm (0.5–5%), and Actinomycetales (13 OTUs) could be detected at 13.5–19.5 cm, 40.5 cm, 95 cm, and 160 cm, with relative abundances between 0.4–8%.
Sequences affiliated with the phylum Chloroflexi and the phylum Acetothermia – formerly known as OP1 (Rinke et al., 2013; Nigro et al., 2016) – become dominant in the deep layers, especially from 95–160 cm. Chloroflexi belong to the class Dehalococcoidetes (5 OTUs) and comprise the candidate groups GIF 9 and MSBL5. Dehalococcoides sequences occur especially often in the deeper sections (92–145 cm, 2–8% of the total sequence abundance). Sequences related to Acetothermia are highly abundant between 95 and 160 cm and make up 12–40% of the total microbial community.
The phylum Deinococcus-Thermus (1 OTU) occurs in the upper layers between 2 and 16.5 cm (2–12%), and the OTUs belong to the genus Truepera. Planctomycetes (4 OTUs) are present from 4.5 to 40 cm, with up to 10% of the microbial community at a depth of 23 cm. Planctomycetes are represented by the classes of Phycisphaerae (3 OTUs) and Planctomycetia (4 OTUs). Additionally, Spirochaetaceae sequences (1 OTU) could be found between 26 and 35 cm and make up less than 2% of the microbial community. The abundance of recovered Verrucomicrobia (2 OTUs) occurs between 4.5 and 10 cm as well as between 95 and 103 cm depth and form ∼1% of the microbial community. Fourteen OTUs could not be assigned.
Microbial Community of Omongwa Pan
In total, 121 OTUs from 13 different bacterial and archaeal phyla were detected using high-throughput sequencing. Absolute read counts were transformed to relative abundances in order to standardize the data. Firmicutes dominate the depth profile of the Omongwa pan from 32.5–105 cm as well as the surface layer (85–98% of the microbial community, Figure 3B). Within this phylum, three different orders are represented: Lactobacillales (2 OTUs), Thermoanaerobacterales (1 OTU), and Bacillales (2 OTUs). Bacillales can be assigned to the genus Bacillus. Between 1.75 and 23 cm, a high proportion of Euryarchaeota (20–47%) and Gemmatimonadetes (21–61%, Figure 3B) were detected. Three orders of Gemmatimonadetes were identified: Longimicrobia, PAUC43f, and BDS-11. Around 25 OTUs of Euryarchaeota can be assigned to Halobacteria (Gupta et al., 2016); hence, a few were identified at the genus level: Natronomonas (3 OTUs), Natronococcus (1 OTU), Haloterrigena (1 OTU), and Halorhabdus (2 OTUs). The OTUs assigned to Proteobacteria dominate between 23 and 28 cm (26–54%, Figure 3B). A high abundance of Bacteroidetes (2–14%) sequences were detected between 2 and 30 cm (Figure 3B). Almost all Bacteroidetes can be attributed to the family Rhodothermaceae (2 OTUs), whereby three OTUs were identified as being from the family Salinibacter. Additionally, the phyla with less than 10% of relative abundance were found (Figure 3B), such as Actinobacteria (7 OTUs), Chloroflexi (3 OTU), Fusobacteria (1 OTU), Planctomycetes (1 OTU), Deinococcus-Thermus (1 OTU), Acetothermia (1 OTU), slight traces of Thaumarcheaota (1 OTU), and 2 OTUs of unassigned phyla.
Statistical Analyses and Description of Core Taxa Among Two Different Continental Pans
The Shannon index (H-index) demonstrates the species diversity within a community (Colwell, 2009) and was calculated based on OTU sequences. The h-index of Witpan decreases with depth. At 103 and 175.5 cm, the h-index is below 0.4. For all other depths, the h-index varies between 1 and 3. In the Omongwa pan, the h-index varies from 0 to 2.2, and two distinct microbial communities could be observed. Within the top layers (1.8–28 cm), the community is characterized by higher species diversity (Shannon index Ø 1.8), whereas the second community (32.5–105 cm) consists of only a few species (Shannon index Ø 0.19, Figure 4A).
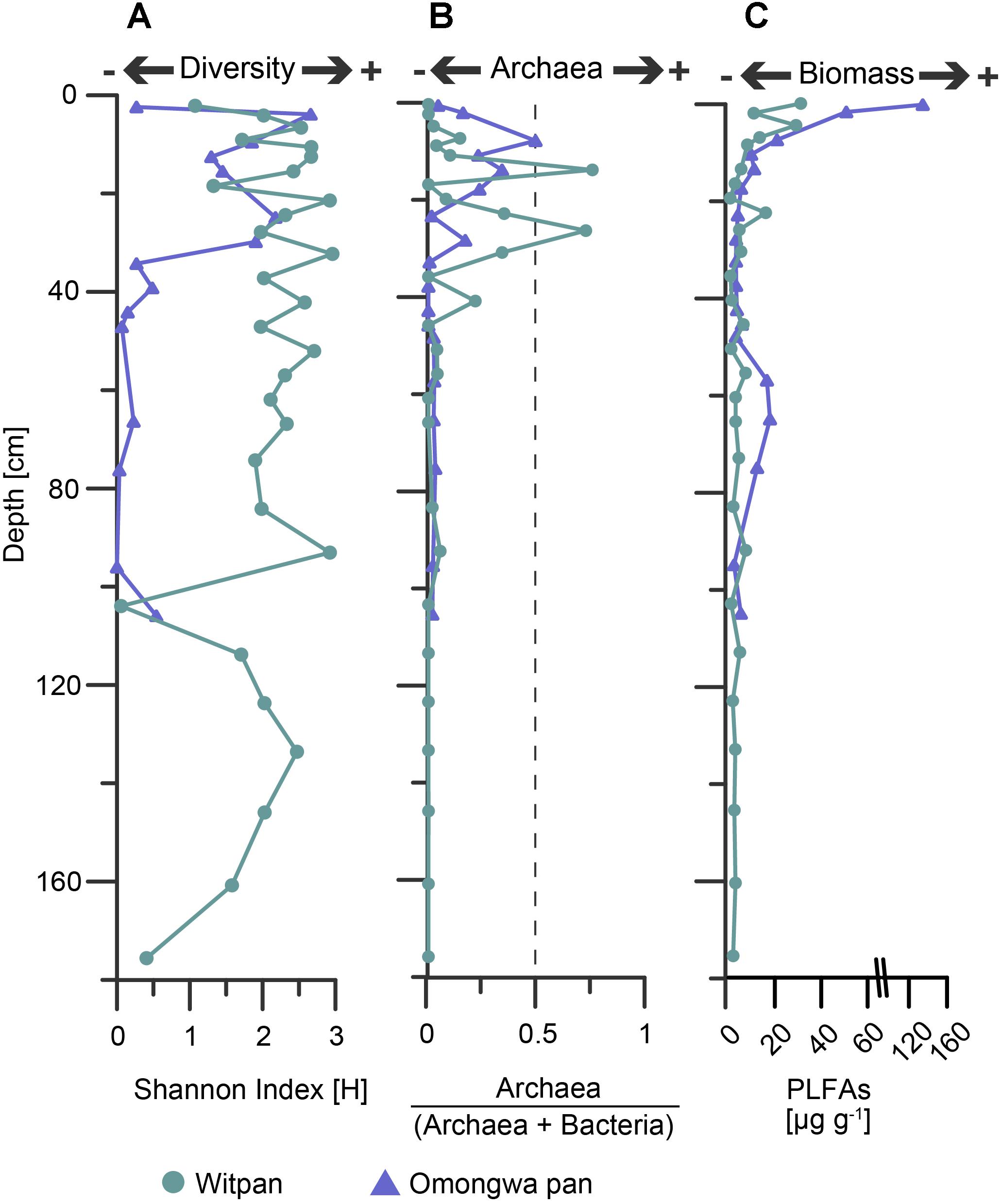
FIGURE 4. (A) Shannon index [H] based on OTU sequencing data, (B) abundance of archaea vs. archaea + bacteria based on archaeal and bacterial 16S rRNA genes 16S, and (C) biomass input was calculated from phospholipid fatty acids (PLFAs). Witpan, turquoise; Omongwa pan, purple.
A ratio (archaea / [archaea + bacteria]) based on the total abundances of bacterial and archaeal 16S rRNA genes was calculated (Figure 4B). In both the pan sediment profiles, the abundance of archaea decreases with increasing depth. In the Omongwa pan, a larger number of archaea within the top 23 cm (up to 49%, Figure 3B) could be observed. The microbial communities of Witpan are partly dominated by bacteria that comprise 28–100% of the sediment. In the near-surface layers (0–7 cm) archaea are less abundant (0–14%). Archaea become more abundant and partly dominate the microbial community at 13.5 cm and 26 cm, where they comprise up to 75% of the sediment (Figure 3A). The archaeal SSU genes were not detected in the deeper layers of Witpan (92–175 cm, Figure 4B).
At the order level, 29 shared taxa from Witpan and the Omongwa pan (Figure 5) are distributed among nine different phyla. This core community is dominated by Bacillus, Halobacteria, and Gemmatimonadetes (Figure 5). The remaining sequences can be assigned to Actinobacteria, Bacteroidetes, and Proteobacteria. Within the core community, a few sequences closely related to Planctomycetes and Acetothermia could also be identified.
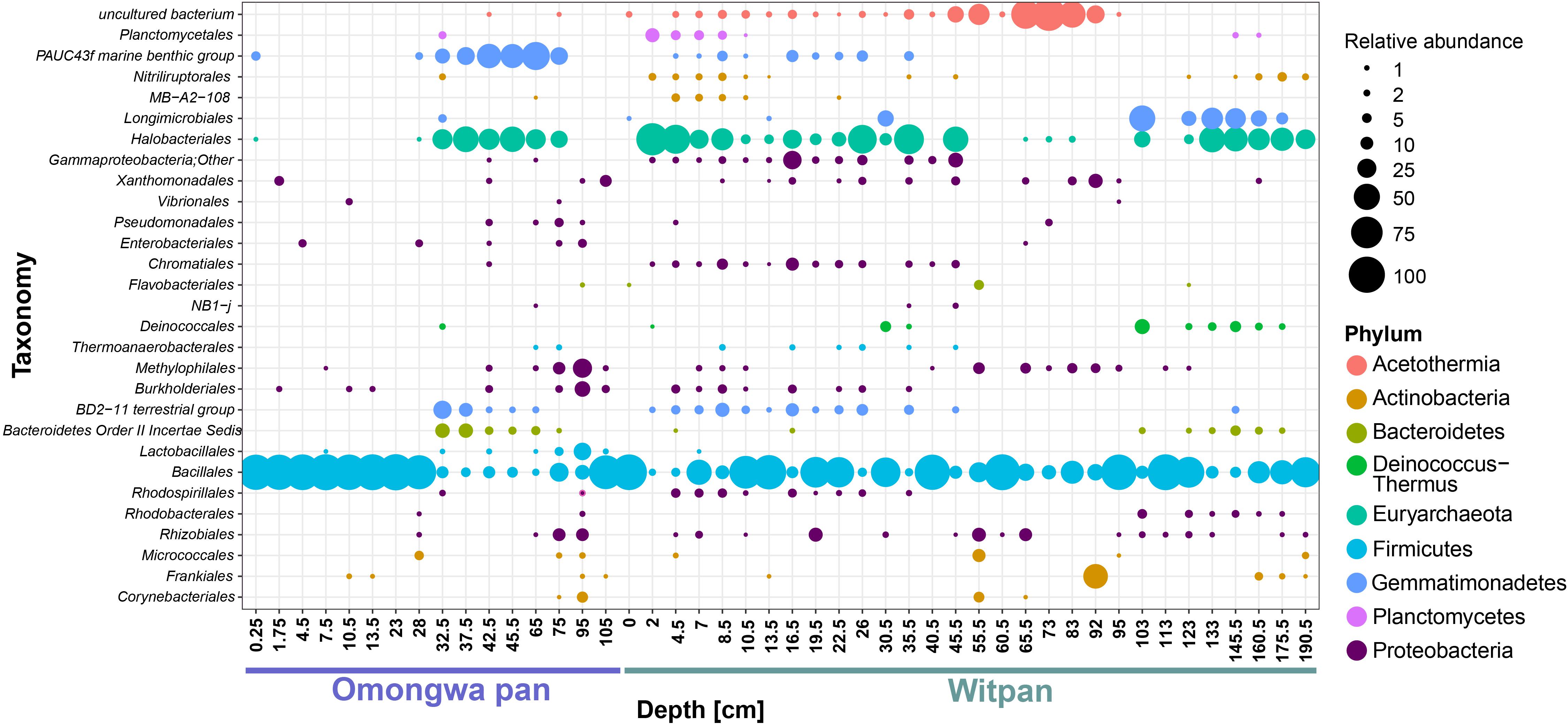
FIGURE 5. Identification of shared OTUs between Omongwa pan and Witpan is based on order level taxonomic distribution in sediment depth profile. Bubble size indicates the relative abundance. Different phyla are indicated via color-coding.
Statistical analysis was applied to identify the correlations between the environmental data and the identified OTUs using CCA (Figure 6). The CCA log file of Witpan revealed a total variation of 6.079, while explanatory variables account for 25.4% of the total variance in bacterial and archaeal distribution. Axes 1 and 2 explain 38.5 and 20.5% of the total variance, respectively. The CCA results of Witpan reveal a grouping of deep layers (103–175.5 cm) according to the acetate, formate, as well as water content. The upper layers (0–19.5 cm) of Witpan are characterized by high chloride and sulfate concentrations. The microbial variation of the Omongwa pan is also explained by the environmental parameters using a CCA plot. 31.4% of the total variance in bacterial and archaeal distribution can be accounted for by environmental parameters (total variation: 3.403). Axes 1 and 2 explain 52.6 and 33.5% of the total variance, respectively. Chloride and sulfate concentrations are related to the surface layer and to the deeper layers (42.5–105 cm) of the Omongwa pan. In contrast, the layers between 25 and 75 cm can be grouped according to the water content. P-values of all the descriptive variables are below 0.05.
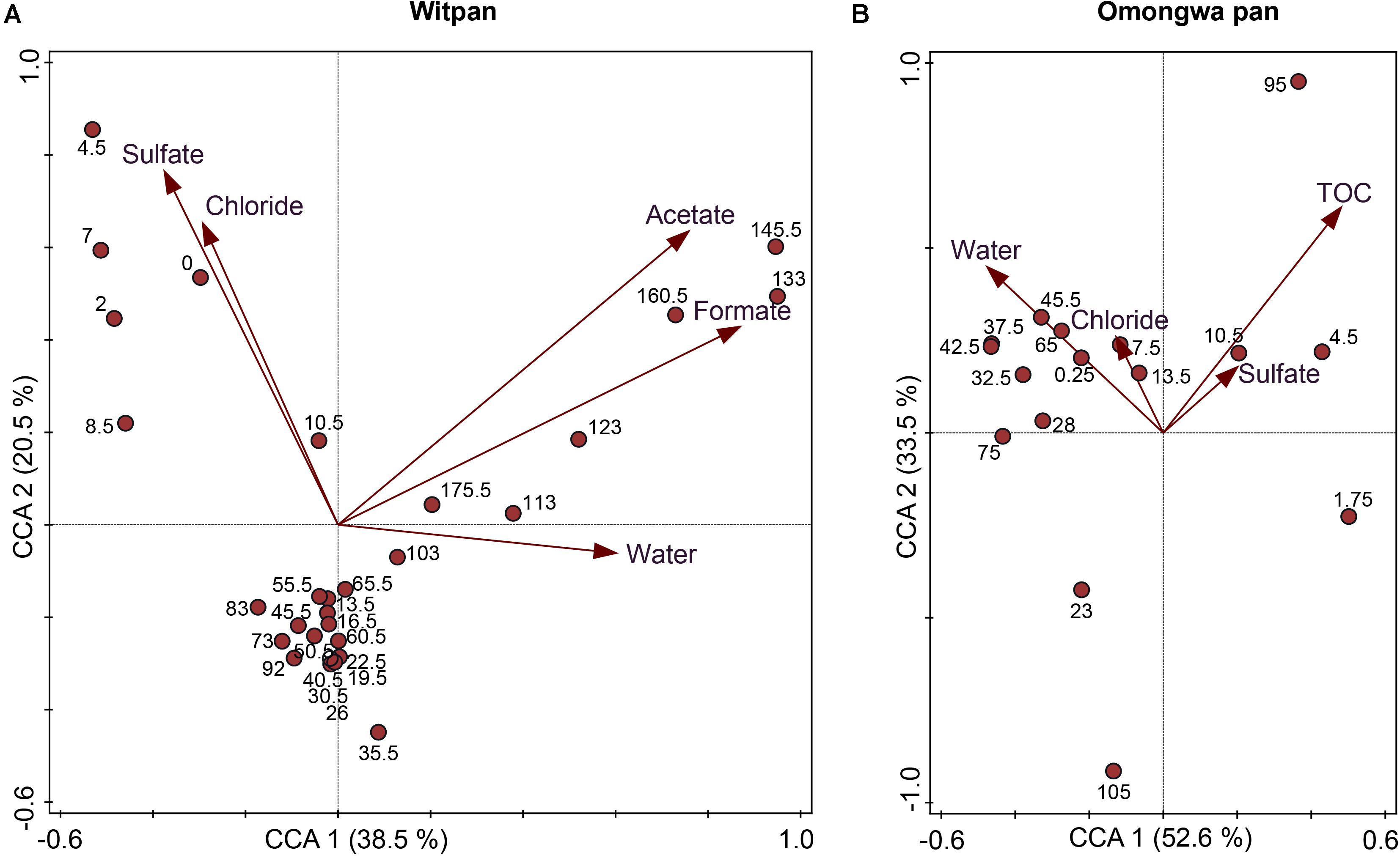
FIGURE 6. Canonical correspondence analysis (CCA) correlates the environmental parameters (chloride, sulfate, TOC, water content, acetate, and formate) with OTU sequencing data and different sampling depths (cm) in (A) Witpan and (B) Omongwa pan. All p-values are <0.05.
Analyses of Phospholipid Fatty Acids (PLFAs) From Witpan and Omongwa Pan
In the Witpan sediments, saturated (C14:0 to C20:0), branched (iso/anteiso-C15:0, iso-C16:0, iso/ai-C17:0, 10Me-10Me-C16:0), unsaturated (C16:1ω9, C16:1ω7, C16:1ω5, C18:1ω7, and C18:1ω9), and cyclopropyl (cy-C17:1 and cy-C19:1) PLFAs were identified (Genderjahn et al., 2018). Molar amounts (nmol g-1 of dry weight, dw) of the saturated PLFAs were used as an estimation of the microbial biomass. The highest values were found in the surface layer (31 μg g-1) and decreased with depth down to 0.9 μg g-1 at 35.5 cm and 2 μg g-1 at 175 cm (Figure 4C).
In the Omongwa sediments, saturated (C12:0 to C22:0), branched/saturated (iso-C14:0, iso/ai-C15:0, iso-C16:0, iso/ai-C17:0, 10Me-C16:0), unsaturated (C16:1ω7, C16:1ω5, C18:1ω7, and C18:1ω9, branched C19:1), and cy-C17:1 PLFAs were identified. The highest concentration of PLFAs was identified in the uppermost layers at a sediment depth from 0–3 cm (135 μg g-1) and decreased significantly with depth (down to 3.2 μg g-1 at 32.5 cm). A slight increase in the estimated biomass based on the saturated PLFA concentrations was observed between 60 and 80 cm (Figure 4C).
Discussion
Kalahari Pans as a Habitat for Microorganisms
Desert environments are ecosystems with limited nutrient availability and high desiccation potential (Pointing and Belnap, 2012). A detailed analysis of the Kalahari pan sediments provide insights into the microbial abundance and diversity. Both Witpan and the Omongwa pan (see also Genderjahn et al., 2018) are characterized by a low amount of total organic carbon (Figure 2A), but display differences in their texture (see Supplementary Figure S1). The Omongwa pan consists mainly of silt, whereas the Witpan sediments are more heterogonous geneous in terms of lithological texture. Witpan is primarily composed of a mixture of clay and silt in the near-surface layers followed by sandy layers from 25–120 cm, while the deeper layers are dominated by clay. Sand fractions in Witpan can act as a “skeleton,” and water can quickly infiltrate the deeper layers of the sediment. The coarser sediment structure of Witpan might explain the fluctuations in the microbial richness found there (Figure 4A). Sediment texture, in terms of the composition of clay, silt, and sand, as well as the pore size distribution, affects the kinetic of the available nutrients as well as electron acceptors and water retention (Rhodes, 2012). Our data on Witpan deposits suggest an increased substrate potential (electron donors and acceptors) for microorganisms in the near-surface layers and in deeper layers from 110–180 cm, whereas in the Omongwa pan, the formate and acetate were not measurable (Figures 2F,G). Buried organic matter is the main source of carbon and energy for microorganisms in the sedimentary systems (Schaechter, 2009), and acids with low molecular weight, such as formate and acetate, are the key intermediates in microbial heterotrophic metabolism (Schulz and Zabel, 2013). Based on our CCA analyses, the microbial community composition is influenced by salinity and the availability of water in Witpan and in the Omongwa pan (Figure 6). In arid systems, the salinity naturally increases due to evaporation after rainfall (Youssef et al., 2012). The dominance of chloride and sulfate in the investigated pans (Figures 2B,C) indicates that salinity is a key factor that affects the pan microbiomes, and specialized microorganisms were found to occur.
Microbial Community Structure in Witpan Deposits
The Kalahari pans are extreme habitats in the sense that they are highly limited in moisture and salinity is usually extremely high. The present study provides insights into the complex microbial community structure of Witpan for the first time. Firmicutes is the most abundant phylum in the present dataset (up to 95%, Figure 5). This taxon is characterized by fast spore germination and a short doubling time. Among the sequences assigned to Firmicutes, the dominant genus is Bacillus. A large number of Bacillus have been isolated from saline and alkaline soil habitats (Ren and Zhou, 2005; Jiang et al., 2006). In the deep anaerobic layers of Witpan, Firmicutes of the families Natranaerobiaceae and Syntrophomonadaceae occur. Natranaerobiaceae are halophilic alkalithermophilic bacteria and are model organisms for evaluating theories on the origin of life (Mesbah et al., 2007), including the hypothesis that life evolved in the shallow, heated saline and alkaline pools (Wiegel and Michael, 2002). Syntrophomonadaceae can convert fatty acids produced by fermentative bacteria to acetate, formate, or hydrogen, which can then be consumed by methanogens (Dworkin et al., 2006). In general, the syntrophic bacteria are found when the organic matter is degraded and inorganic electron acceptors are missing.
Gemmatimonadetes are found in low frequency in all types of soils, including marine and lake sediments (DeBruyn et al., 2011; Fierer et al., 2012). They are predominant in hyper- and semi-arid soils with very low biomass, as in the Sahara Desert (Meola et al., 2015), the Atacama Desert (Schulze-Makuch et al., 2018), as well as Arctic polar deserts (McCann et al., 2016) and Antarctic glacier forefields (Bajerski and Wagner, 2013). In Witpan, Gemmatimonadetes form up to 38% of the microbial community and occur between 0–95 cm depth (Figure 3A). Slowly growing Gemmatimonadetes (Zhang et al., 2003) are important colonists that adapt well to low soil moisture (DeBruyn et al., 2011), pointing to a tolerance for desiccation.
Archaea comprise up to 65% of the entire microbial community. The majority of the archaeal sequences are related to halophilic archaea, namely Halobacteria. These findings are consistent with several other studies on the archaeal community composition in hypersaline environments (Maturrano et al., 2006; Youssef et al., 2012; Weigold et al., 2016). Halobacteria perform the salt-in strategy for osmoregulation, which generally requires less metabolic energy compared with the synthesis of compatible solutes.
Trueperaceae affiliated to the phylum Deinococcus-Thermus can be found in the near-surface layers (2–16.5 cm). Deinococcus-related bacteria are isolated from hot, arid environments, such as the Tunisian Sahara (Chanal et al., 2006; Theodorakopoulos et al., 2013), hot springs (Albuquerque et al., 2005), as well as radioactive sites (Asker et al., 2011) and industry water (Kämpfer et al., 2008). They are extremely ionizing-radiation-resistant, slightly thermophilic, chemoorganotrophic, and aerobic (Albuquerque et al., 2005; Slade and Radman, 2011).
At depths of 13.5–35.5 cm, between 6 and 17% of the taxa belong to the phylum Actinobacteria. Chemo-organotrophic Actinobacteria have developed diverse strategies for survival, including sporulation; wide metabolic, degradation capacity; synthesis of secondary metabolites; and various UV repair mechanisms (Ensign, 1978; McCarthy and Williams, 1992). Actinobacteria have been isolated from saline soils in Mexico (Valenzuela-Encinas et al., 2009) and described as a dominant phylum in arid environments, such as the Namib Desert (Makhalanyane et al., 2013). In Witpan, actinobacterial sequences can be mainly assigned to the class Nitriliruptoria (Ludwig et al., 2012) and the order Actinomycetes. In the upper layers, the sequences are related to Nitriliruptoria, which has been isolated from soda lake sediments of the Kulunda Steppe (Altai, Russia) (Sorokin et al., 2009). Sorokin et al. (2009) have demonstrated the capability of the species Niriliruptor alkaliphilus to degrade complex, naturally occurring nitriles that can be produced during the anaerobic degradation of amino acids (Harper and Gibbs, 1979). Different families of Actinomycetes occur infrequently in the depth profile and perform specific mechanisms of adapting to saline and alkaline habitats (Singh et al., 2012). At a depth of 4.5–40 cm, up to 10% of the microbial community can be assigned to the phylum Planctomycetes, which comprises aerobic organoheterotrophic Phycisphaerae and Planctomycetaceae. Phycisphaerae were originally isolated from marine algae (Fukunaga et al., 2009) and play an important role in the rhizome-associated concretions (Fernandez et al., 2016). Planctomycetaceae are of particular interest for their eukaryotic-like cell structures and their properties of resistance to extreme environmental conditions (Guo et al., 2012).
The deepest layers of Witpan (103–175.5 cm) displayed higher concentrations of acetate and formate as well as higher water content (Figures 2E–G). Compared with the overlying sediment layers, the changes in the microbial community structure likely reflect anoxic conditions. Acetothermia, Chloroflexi, and the family Bacteroidales of the phylum Bacteroidetes become more abundant. Members of Bacteroidetes are adapted to saline conditions and can be observed in a variety of hypersaline systems, including the Atacama Desert (Fernandez et al., 2016; Schulze-Makuch et al., 2018), salterns (Oren et al., 2009), microbial mats (Sørensen et al., 2005), and evaporates (Farías et al., 2014). Chloroflexi is also found in hypersaline environments, such as high-saline soils and hypersaline wastewater. Most of the Chloroflexi sequences can be found in the deep layers of Witpan and assigned to the class Dehalococcoidia. These organohalide-respiring bacteria were first isolated from chloroethene-contaminated terrestrial aquifer environments (Maymo-Gatell, 1997). Dehalococcoidia are strictly anaerobic, slow-growing, and highly niche-adapted toward reductive dehalogenation. They might be linked to the accumulation of halogenated organic compounds as a consequence of the humification of plant matter (Hug et al., 2013). Increased concentrations of acetate in the deep layers of Witpan (Figure 2F) probably originate from highly active Acetothermia, which are chemolithotrophic bacteria that perform acetogenesis as a primary energy- and carbon metabolic pathway (Takami et al., 2012). Acetothermia has hardly been described in environmental studies; however, in single-cell amplified genome studies, its potential to adapt to osmotic stress in hypersaline environments has been demonstrated (Nigro et al., 2016). Acetogens utilize the reductive acetyl-CoA pathway (Wood–Ljungdahl pathway) for carbon fixation that is known to be prevalent in the subsurface ecosystems, such as in goldmine boreholes in the Witwatersrand basin in South Africa (Magnabosco et al., 2016). Acetogens only produce acetate as a fermentation product (Ragsdale and Pierce, 2008), but they have a higher threshold for hydrogen than do most methanogens. The archaeal community at deeper levels was mainly formed by euryarchaeal candidate division MSBL1 (previously assigned to Methanobacteriales) instead of Halobacteria, indicating a community shift due to the changes in the geochemical sediment properties.
DNA-based approaches such as high-throughput sequencing are appropriate for characterizing the microbial communities in detail, while the PLFA method has the advantage of providing quantitative information on the total microbial biomass (Frostegård et al., 2011). The intact phospholipid esters are essential membrane components of living bacterial cells. Since phospholipid esters rapidly degrade after cell death. They are good indicators for viable microorganisms (Zelles, 1999). In this study all PLFAs are summed up as an index of microbial biomass (Quideau et al., 2016; Figure 4A). The biomass input decreases with increasing sediment depth that is consistent with other PLFA analyses from soil depth profiles (Bischoff et al., 2013; Stapel et al., 2016). The PLFA profile showed a few peaks at the surface at 45 and 113 cm depth. These peaks are characterized by low species diversity as represented by the Shannon index H. The h-index for Witpan reveals no clear trend, but its diversity index is quite low, similar to the saline shallow lakes of the Monegros Desert in Spain (Casamayor et al., 2013) and the saline sediments such as the Great Salt Plains in Oklahoma (h-Index ∼5) (Youssef et al., 2012).
We were able to show that the carbon-limited and nutrient-poor sediments of Witpan consist mainly of halophilic archaea (e.g., Halobacteria) and bacteria (e.g., Actinobacteria) as well as microorganisms that are known to be well-adapted to semi-arid conditions (e.g., Gemmatimonadetes, Firmicutes). Integrating the results from molecular biology with data on soil chemistry suggests a significant correlation between the sulfate- and chloride concentrations in the near-surface layers of Witpan (Figure 6). In the deep layers of Witpan, the microbial community structure changes due to increased concentrations of acetate and formate as well as the TOC supply. Acetothermales become highly abundant, and acetogenesis might also play an important role.
Comparative Analysis of Microbial Communities in Kalahari Pans
Microorganisms are involved in different physicochemical and biological processes, such as nutrient cycling, mineralization, and soil aggregation. The microbial diversity is therefore an important component to functioning ecosystems (Keshri et al., 2013). Shared taxa of different study sites can reveal the drivers of the microbial community structure across habitats and help researchers to identify the microbial taxa that have central functions in an ecosystem (Shade and Handelsman, 2012).
We compared the results of high-throughput sequencing data from Witpan in the southern Kalahari with the existing sequencing data on the Omongwa pan, a saline pan in the western Kalahari (Genderjahn et al., 2018). Twenty-nine taxonomic orders are shared between two different pan sediments, and nine phyla can be identified, comprising Actinobacteria, Bacteroidetes, Euryarchaeota (mainly Halobacteria), Firmicutes, Gemmatimonadetes, Acetothermia, Planctomycetes, and Proteobacteria (Figure 5). The sequences related to Actinomycetes and spore-forming Bacillus are highly abundant in both pan sediments. These microorganisms withstand harsh environmental conditions in a state of dormancy or sporulation or as an inactive but viable cell. Once the environmental conditions become more favorable and water has been available for an appropriate amount of time, the cells are able to divide again (Jones and Lennon, 2010; Crits-Christoph et al., 2013).
In contrast to the microbial community of Witpan described above, the Omongwa pan displays two distinct microbial communities in its depth profile. The near-surface community (down to 33 cm) is characterized by a specialized consortium of microorganisms that are attributed to fast-changing conditions and may represent an important refuge for Gemmatimonadetes and Bacteroidetes as well as for halophilic archaeal Halobacteria (Figure 3B). In the deeper layers of the Omongwa pan (33 to 105 cm), the microbial diversity decreases compared with the near-surface layers, and only a few species can be identified. The deeper sections are dominated by Bacillus (Figure 3B), whereas in Witpan, the relative abundance of Bacillus is very high in irregular intervals. Representatives of Firmicutes are widely distributed in saline habitats, such as a hypersaline crater lake (Paul et al., 2015) or a saline desert in India (Pandit et al., 2014). During sampling, the Omongwa pan was characterized by a distinct halite crust that had been formed due to the evaporation of water and precipitation of chloride and sulfate ions. This salt crust influences the microbial structure and might protect microorganisms against UV radiation. High-throughput sequencing results reveal that the halophilic archaea represent a large proportion of the microbial community, especially in the near-surface sediments of the Kalahari pans (Figure 3B). A large percentage of archaea could be observed in the upper layers of the Omongwa pan from 4.5–10.5 cm and in Witpan between 10.5 and 30.5 cm (Figure 3). This observation was also described by Maturrano et al. (2006) for salterns of the Peruvian Andes, where archaea dominate over bacteria and a large population of different Halobacteria are harbored. The aerobic halophilic archaea form the main microbial biomass in bodies of water with a concentration of sodium chloride approaching saturation, such as soda lakes and crystallizer ponds of solar salterns (Oren, 2002). Different physiological and molecular adaption mechanisms enable the microorganisms to survive under water scarcity. Genderjahn et al. (2017) have described the presence of dialkyl glycerol diethers (DGDs) in the Witpan sediments. DGD lipid membranes might be involved in the ‘salt-in’ strategy and in balancing the osmotic stress by reducing the membrane permeability to H+, Na+, and other solutes (Dawson et al., 2012). Halobacteria can therefore adapt to different salinity conditions and quickly repopulate sediments when the water levels rise and the salinity decreases, such as after rainfall (Kulp et al., 2007).
The relative abundance of Bacteroidetes, Chloroflexi, and Proteobacteria is comparably low in Witpan. The sequences related to Bacteroidetes Incertae Sedis – known as Rhodothermales and Cytophagia – can be classified within Bacteroidetes and have recently been identified in alkaline and saline soils (de Leon-Lorenzana et al., 2017) as well as in microbial mats (Fernandez et al., 2016). In pan sediments, the members of thermophilic Chloroflexi have been assigned to Dehalococcoidetes, which uses organohalide respiration for energy conservation (Kaster et al., 2014). In contrast to the Omongwa pan, Dehalococcoidetes mainly occur in the deep anoxic sediments of Witpan. Within the phylum Proteobacteria, taxa related to Ectothiorhodospiraceae, Rhodobacteraceae, and Rhodospirillales are particularly prone to occur. Isolates of Ectothiorhodospiraceae are found in marine environments as well as in hypersaline and alkaline lakes (Imhoff and Süling, 1996). They are adapted to saline and alkaline growth conditions and grow via anoxygenic photosynthesis by using reduced sulfur compounds, hydrogen, organic compounds, or arsenite as electron donors (Hoeft McCann et al., 2016). Casamayor et al. (2013) have reported the presence of Rhodobacteraceae and Rhodospirillaceae in shallow saline lakes in the Spanish Monegros Desert. Sequences related to Planctomycetes and Actinobacteria in Kalahari pan sediments are rare and do not occur frequently.
In contrast, the shared taxa of Gemmatimonadetes, Bacillus, and Halobacteria occur both in Witpan and the Omongwa pan. Gemmatimonadetes are always identified with the extreme halophilic Halobacteria and Bacteroidetes. Gemmatimonadetes have not yet been identified as a halophile or halotolerant organism. Studies by DeBruyn et al. (2011) and Fawaz (2013) revealed that a higher abundance of Gemmatimonadetes is significantly correlated with low soil moisture. In the investigated pans, Gemmatimonadetes were frequently abundant confirming that they are important colonists in arid environments. Additionally, in this study, a high proportion of archaeal sequences could be found, leading to a unique structural depiction of the microbial population in Kalahari pans. Halobacteria can also be observed in salt pans on the Namibian coastline, where fog and rainfall are the main sources of water (Cloete, 2015). In general, the metagenomic studies in arid areas reveal a higher prevalence of genes related to osmoregulation, dormancy, and stress response than in non-arid environments. This finding might be a consequence of evolutionary adjustment due to moisture- and hot-stress events (Fierer et al., 2012). In previous studies, Prestel et al. (2008) and Ronca et al. (2015) have described the microbial community studies of the Namib Desert. Sequences of 16S rRNA gene were extracted from Namib Desert surface sands and indicated a high proportion of Firmicutes, especially of the genus Bacillus (Prestel et al., 2008), which could also be observed in the investigated pan sediments. Furthermore, Prestel et al. (2008) detected members of Bacteroidetes, Planctomycetes, Chloroflexi, and Deltaproteobacteria. Makhalanyane et al. (2015) and Ronca et al. (2015) showed a predominance of Proteobacteria, Actinobacteria, and Bacteroidetes within the microbial community in desert systems. These bacterial sequences were found in both pan sediments, but their relative frequency was rather low. One reason for this finding could be technical in nature as high-throughput sequencing allows for a more detailed description of the bacterial and archaeal community.
Our observations point to different localized microbial populations. In the deep layers of Witpan (95–180 cm), the microbial consortia vary widely compared with the upper layers. Anaerobic archaea and bacteria in the deep layers of Witpan dominate the microbial community structure which may be due to lack of oxygen. Additionally, the archaeal community shifts from Halobacteria to MSBL1. The relative abundance of sequences related to Acetothermales ranges from 10–44%. Increased concentrations of acetate (Figure 2F) suggested highly active Acetothermales.
High-throughput sequencing allows for deep insights to be made into the microbial community structure of Kalahari pans. When comparing two different study sites, saline- and desiccation-tolerant microorganisms were emphasized and described as dominating taxa. Their present existence in pan sediments is closely related to the surface processes that control water, substrate-, and nutrient availability.
Conclusion
Despite the possibility of the occurrence of novel microorganisms in hypersaline environments with high economic and industrial potential, only a few detailed reports on the microbial diversity in the Kalahari have been produced thus far. Our study provides new insights into the archaeal and bacterial diversity of desert ecosystems. In both the continental pans, halophilic and desiccation-tolerant taxa were found. Analyses of high-throughput sequencing data from two different pan sediments identified a core community dominated by Firmicutes, Gemmatimonadetes, and Halobacteria. The high abundance of halophilic archaea and their influence on biogeochemical cycles remain largely unexplored yet represent a major aspect of the desert ecosystem. The deep, anoxic layers of Witpan are characterized by a special consortium of microorganisms. Higher concentrations of acetate indicate the presence of highly active Acetothermia, which perform acetogenesis. In addition, Dehalococcoides are also present in the deeper layers of Witpan. This new knowledge might be useful for the treatment of wastewater. An investigation of novel niches that harbor dehalorespiring microorganisms could broaden our knowledge of organohalide biodegradation applications.
Author Contributions
SG performed all experiments, interpreted the results, and wrote the manuscript. MA substantially contributed to data interpretation and revised the manuscript. KM and SG sampled during the field campaign. KM contributed to the development of the concept and helped with valuable discussion. FH processed next generation sequencing data. DW provided financial and technical support and revised the manuscript.
Funding
The project “Signals of climate and landscape change preserved in southern African GeoArchives” (Project 03G0838B/C) is part of the SPACES program (Science Partnerships for the Assessment of Complex Earth System Processes), which is financially supported by the German Federal Ministry of Education and Research.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Namibian Geological Survey for its logistical and administrative support. Thanks also go to Dr. Sizhong Yang (German Research Centre for Geosciences, GFZ) for his bioinformatics expertise. Finally, we wish to extend our gratitude to all GeoArchive project partners, particularly to Irka Schüller (Institute Senckenberg am Meer), who provided the sedimentological profiles. We also thank C-EW and AO for reviewing our manuscript and the Editor JD for his helpful comments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02082/full#supplementary-material
References
Ahrens, C. D., and Samson, P. J. (2010). Extreme Weather and Climate. Belmont, MA: Brooks/Cole. doi: 10.1016/0031-0182(95)00060-Y
Albuquerque, L., Simões, C., Nobre, M. F., Pino, N. M., Battista, J. R., Silva, M. T., et al. (2005). Truepera radiovictrix gen. nov., sp. nov., a new radiation resistant species and the proposal of Trueperaceae fam. nov. FEMS Microbiol. Lett. 247, 161–169. doi: 10.1016/j.femsle.2005.05.002
Asker, D., Awad, T. S., McLandsborough, L., Beppu, T., and Ueda, K. (2011). Deinococcus depolymerans sp. nov., a gamma- and UV-radiation-resistant bacterium, isolated from a naturally radioactive site. Int. J. Syst. Evol. Microbiol. 61(Pt 6), 1448–1453. doi: 10.1099/ijs.0.013482-0
Bajerski, F., and Wagner, D. (2013). Bacterial succession in antarctic soils of two glacier forefields on Larsemann Hills, East Antarctica. FEMS Microbiol. Ecol. 85, 128–142. doi: 10.1111/1574-6941.12105.
Baker, G. C., Smith, J. J., and Cowan, D. A. (2003). Review and re-analysis of domain-specific 16S primers. J. Microbiol. Methods 55, 541–555. doi: 10.1016/j.mimet.2003.08.009
Bischoff, J., Mangelsdorf, K., Gattinger, A., Schloter, M., Kurchatova, A. N., Herzschuh, U., et al. (2013). Response of methanogenic archaea to late pleistocene and holocene climate changes in the siberian arctic. Glob. Biogeochem. Cycles 27, 305–317. doi: 10.1029/2011gb004238
Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. doi: 10.1139/o59-099
Blume, H. P., Stahr, K., and Leinweber, P. (2011). Bodenkundliches Praktikum: Eine Einführung in pedologisches Arbeiten für Ökologen, Land- und Forstwirte, Geo- und Umweltwissenschaftler. Heidelberg: Spektrum Akademischer Verlag.
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Bowen, J. L., Morrison, H. G., Hobbie, J. E., and Sogin, M. L. (2012). Salt marsh sediment diversity: a test of the variability of the rare biosphere among environmental replicates. ISME J. 6, 2014–2023. doi: 10.1038/ismej.2012.47.
Bowler, J. M., Qi, H., Kezao, C., Head, M. J., and Baoyin, Y. (1986). Radiocarbon dating of playa-lake hydrologic changes: examples from northwestern China and central Australia. Palaeogeogr. Palaeocol. 54, 241–260. doi: 10.1016/0031-0182(86)90127-6
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Lozupone, C. A., Turnbaugh, P. J., et al. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl. 1), 4516–4522. doi: 10.1073/pnas.1000080107
Casamayor, E. O., Triadó-Margarit, X., and Castañeda, C. (2013). Microbial biodiversity in saline shallow lakes of the Monegros Desert, Spain. FEMS Microbiol. Ecol. 85, 503–518. doi: 10.1111/1574-6941.12139
Chanal, A., Chapon, V., Benzerara, K., Barakat, M., Christen, R., Achouak, W., et al. (2006). The desert of tataouine: an extreme environment that hosts a wide diversity of microorganisms and radiotolerant bacteria. Environ. Microbiol. 8, 514–525. doi: 10.1111/j.1462-2920.2005.00921.x
Cloete, M. (2015). Microbial Diversity Of The Namib Desert Salt Pans. Master’s thesis, University of Western Cape, Cape.
Colwell, R. K. (2009). Biodiversity: Concepts, Patterns And Measurement. New Jersey, NJ: Princeton University Press.
Crits-Christoph, A., Robinson, C. K., Barnum, T., Fricke, W. F., Davila, A. F., Jedynak, B., et al. (2013). Colonization patterns of soil microbial communities in the Atacama Desert. Microbiome 1:28. doi: 10.1186/2049-2618-1-28
Dawson, K. S., Freeman, K. H., and Macalady, J. L. (2012). Molecular characterization of core lipids from halophilic archaea grown under different salinity conditions. Org. Geochem. 48, 1–8. doi: 10.1016/j.orggeochem.2012.04.003
de Leon-Lorenzana, A. S., Delgado-Balbuena, L., Dominguez-Mendoza, C., Navarro-Noya, Y. E., Luna-Guido, M., and Dendooven, L. (2017). Reducing salinity by flooding an extremely alkaline and saline soil changes the bacterial community but Its effect on the archaeal community is limited. Front. Microbiol. 8:466. doi: 10.3389/fmicb.2017.00466
DeBruyn, J. M., Nixon, L. T., Fawaz, M. N., Johnson, A. M., and Radosevich, M. (2011). Global biogeography and quantitative seasonal dynamics of gemmatimonadetes in soil. Appl. Environ. Microbiol. 77, 6295–6300. doi: 10.1128/AEM.05005-11
Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K. H., and Stackebrandt, E. (2006). The Prokaryotes: Vol. 4: Bacteria: Firmicutes, Cyanobacteria. New York, NY: Springer Science & Business Media.
Ensign, J. C. (1978). Formation, properties, and germination of actinomycete spores. Annu. Rev. Microbiol. 32, 185–219. doi: 10.1146/annurev.mi.32.100178.001153
Farías, M. E., Contreras, M., Rasuk, M. C., Kurth, D., Flores, M. R., Poiré, D. G., et al. (2014). Characterization of bacterial diversity associated with microbial mats, gypsum evaporites and carbonate microbialites in thalassic wetlands: tebenquiche and La Brava, Salar de Atacama, Chile. Extremophiles 18, 311–329. doi: 10.1007/s00792-013-0617-6
Fawaz, M. N. (2013). Revealing the Ecological Role of Gemmatimonadetes Through Cultivation and Molecular Analysis of Agricultural Soils. Master’s Thesis, University of Tennessee, Knoxville, Knoxville, TN.
Fernandez, A. B., Rasuk, M. C., Visscher, P. T., Contreras, M., Novoa, F., Poire, D. G., et al. (2016). Microbial diversity in sediment ecosystems (evaporites domes, microbial mats, and crusts) of hypersaline laguna Tebenquiche, Salar de Atacama, Chile. Front. Microbiol. 7:1284. doi: 10.3389/fmicb.2016.01284
Fierer, N., Leff, J. W., Adams, B. J., Nielsen, U. N., Bates, S. T., Lauber, C. L., et al. (2012). Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. U.S.A. 109, 21390–21395. doi: 10.1073/pnas.1215210110
Frostegård, A., Tunlid, A., and Bååth, E. (2011). Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 43, 1621–1625. doi: 10.1016/j.soilbio.2010.11.021
Fukunaga, Y., Kurahashi, M., Sakiyama, Y., Ohuchi, M., Yokota, A., and Harayama, S. (2009). Phycisphaera mikurensis gen. nov., sp. nov., isolated from a marine alga, and proposal of Phycisphaeraceae fam. nov., Phycisphaerales ord. nov. and Phycisphaerae classis nov. in the phylum Planctomycetes. J. Gen. Appl. Microbiol. 55, 267–275. doi: 10.2323/jgam.55.267
Genderjahn, S., Alawi, M., Kallmeyer, J., Belz, L., Wagner, D., and Mangelsdorf, K. (2017). Present and past microbial life in continental pan sediments and its response to climate variability in the southern Kalahari. Org. Geochem. 108, 30–42. doi: 10.1016/j.orggeochem.2017.04.001
Genderjahn, S., Alawi, M., Wagner, D., Schuller, I., Wanke, A., and Mangelsdorf, K. (2018). Microbial community responses to modern environmental and past climatic conditions in omongwa pan, Western Kalahari: a paired 16S rRNA gene profiling and lipid biomarker approach. J. Geophys. Res. Biogeo. 123, 1333–1351. doi: 10.1002/2017JG004098
Goudie, A. S., and Wells, G. L. (1995). The nature, distribution and formation of pans in arid zones. Earth Sci. Rev. 38, 1–69. doi: 10.1016/0012-8252(94)00066-6
Guo, M., Han, X., Jin, T., Zhou, L., Yang, J., Li, Z., et al. (2012). Genome sequences of three species in the family Planctomycetaceae. J. Bacteriol. 194, 3740–3741. doi: 10.1128/JB.00639-12
Gupta, R. S., Naushad, S., Fabros, R., and Adeolu, M. (2016). A phylogenomic reappraisal of family-level divisions within the class Halobacteria: proposal to divide the order Halobacteriales into the families Halobacteriaceae, Haloarculaceae fam. nov., and Halococcaceae fam. nov., and the order Haloferacales into the families, Haloferacaceae and Halorubraceae fam nov. Antonie Van Leeuwenhoek 109, 565–587. doi: 10.1007/s10482-016-0660-2
Handford, C. R. (1982). Sedimentology and evaporite genesis in a holocene continental-sabkha playa basin-bristol dry lake, California. Sedimentology 29, 239–253. doi: 10.1111/j.1365-3091.1982.tb01721.x
Harper, D. B., and Gibbs, P. A. (1979). Identification of isobutyronitrile and isobutyraldoxime O-methyl ether as volatile microbial catabolites of valine. Biochem. J. 182, 609–611. doi: 10.1042/bj1820609
Hoeft McCann, S., Boren, A., Hernandez-Maldonado, J., Stoneburner, B., Saltikov, C. W., Stolz, J. F., et al. (2016). Arsenite as an electron donor for anoxygenic photosynthesis: description of three strains of Ectothiorhodospira from mono lake, California and big soda lake, Nevada. Life 7:1. doi: 10.3390/life7010001
Hug, L. A., Castelle, C. J., Wrighton, K. C., Thomas, B. C., Sharon, I., Frischkorn, K. R., et al. (2013). Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 1:22. doi: 10.1186/2049-2618-1-22
Imhoff, J. F., and Süling, J. (1996). The phylogenetic relationship among Ectothiorhodospiraceae: a reevaluation of their taxonomy on the basis of 16S rDNA analyses. Arch. Microbiol. 165, 106–113. doi: 10.1007/s002030050304
Jiang, H., Dong, H., Zhang, G., Yu, B., Chapman, L. R., and Fields, M. W. (2006). Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in northwestern China. Appl. Environ. Microbiol. 72, 3832–3845. doi: 10.1128/AEM.02869-05
Jones, S. E., and Lennon, J. T. (2010). Dormancy contributes to the maintenance of microbial diversity. Proc. Natl. Acad. Sci. U.S.A. 107, 5881–5886. doi: 10.1073/pnas.0912765107
Kämpfer, P., Lodders, N., Huber, B., Falsen, E., and Busse, H. J. (2008). Deinococcus aquatilis sp. nov., isolated from water. Int. J. Syst. Evol. Microbiol. 58(Pt 12), 2803–2806. doi: 10.1099/ijs.0.2008/001206-0
Kaster, A. K., Mayer-Blackwell, K., Pasarelli, B., and Spormann, A. M. (2014). Single cell genomic study of Dehalococcoidetes species from deep-sea sediments of the Peruvian Margin. ISME J. 8, 1831–1842. doi: 10.1038/ismej.2014.24
Keshri, J., Mishra, A., and Jha, B. (2013). Microbial population index and community structure in saline-alkaline soil using gene targeted metagenomics. Microbiol. Res. 168, 165–173. doi: 10.1016/j.micres.2012.09.005
Kulp, T. R., Han, S., Saltikov, C. W., Lanoil, B. D., Zargar, K., and Oremland, R. S. (2007). Effects of imposed salinity gradients on dissimilatory arsenate reduction, sulfate reduction, and other microbial processes in sediments from two California soda lakes. Appl. Environ. Microbiol. 73, 5130–5137. doi: 10.1128/AEM.00771-07
Lancaster (1986). Pans in the southwestern Kalahari: a preliminary report. Palaeoecol. Afr. 17, 59–67.
Lefebvre, O., Vasudevan, N., Thanasekaran, K., Moletta, R., and Godon, J. J. (2006). Microbial diversity in hypersaline wastewater: the example of tanneries. Extremophiles 10, 505–513. doi: 10.1007/s00792-006-0524-1
Ludwig, W., Euzéby, J., and Whitman, W. B. (2012). “Taxonomic outline of the phylum actinobacteria,” in Bergey’s Manual® of Systematic Bacteriology: Volume Five The Actinobacteria, Part A and B, eds M. Goodfellow, P. Kämpfer, H.-J. Busse, M. E. Trujillo, K.-I. Suzuki, W. Ludwig, and W. B. Whitman (New York, NY: Springer), 29–31. doi: 10.1007/978-0-387-68233-4_2
Lyons, W. B., Welch, K. A., Neumann, K., Toxey, J. K., McArthur, R., Williams, C., et al. (1998). Geochemical linkages among glaciers, streams and lakes within the taylor valley, Antartica. Antarct. Res. Ser. 72, 77–92. doi: 10.1029/AR072p0077
Ma, Y., Galinski, E. A., Grant, W. D., Oren, A., and Ventosa, A. (2010). Halophiles 2010: life in saline environments. Appl. Environ. Microbiol. 76, 6971–6981. doi: 10.1128/AEM.01868-10
Magee, J. W., Bowler, J. M., Miller, G. H., and Williams, D. L. G. (1995). Stratigraphy, sedimentology, chronology and paleohydrology of quaternary lacustrine deposits at Madigan Gulf, lake eyre, South Australia. Palaeogeogr. Palaeocol. 113, 3–42. doi: 10.1016/0031-0182(95)00060-Y
Magnabosco, C., Ryan, K., Lau, M. C., Kuloyo, O., Sherwood Lollar, B., Kieft, T. L., et al. (2016). A metagenomic window into carbon metabolism at 3 km depth in Precambrian continental crust. ISME J. 10, 730–741. doi: 10.1038/ismej.2015.150.
Makhalanyane, T. P., Valverde, A., Gunnigle, E., Frossard, A., Ramond, J. B., and Cowan, D. A. (2015). Microbial ecology of hot desert edaphic systems. FEMS Microbiol. Rev. 39, 203–221. doi: 10.1093/femsre/fuu011
Makhalanyane, T. P., Valverde, A., Lacap, D. C., Pointing, S. B., Tuffin, M. I., and Cowan, D. A. (2013). Evidence of species recruitment and development of hot desert hypolithic communities. Environ. Microbiol. Rep. 5, 219–224. doi: 10.1111/1758-2229.12003
Maturrano, L., Santos, F., Rosselló-Mora, R., and Antón, J. (2006). Microbial diversity in Maras salterns, a hypersaline environment in the Peruvian Andes. Appl. Environ. Microbiol. 72, 3887–3895. doi: 10.1128/AEM.02214-05
Maymo-Gatell, X. (1997). Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276, 1568–1571. doi: 10.1126/science.276.5318.1568
McCann, C. M., Wade, M. J., Gray, N. D., Roberts, J. A., Hubert, C. R., and Graham, D. W. (2016). Microbial communities in a high arctic polar desert landscape. Front. Microbiol. 7:419. doi: 10.3389/fmicb.2016.00419
McCarthy, A. J., and Williams, S. T. (1992). Actinomycetes as agents of biodegradation in the environment - a review. Gene 115, 189–192. doi: 10.1016/0378-1119(92)90558-7
McGenity, T. J., Gemmell, R. T., Grant, W. D., and Stan-Lotter, H. (2000). Origins of halophilic microorganisms in ancient salt deposits. Environ. Microbiol. 2, 243–250. doi: 10.1046/j.1462-2920.2000.00105.x
Meola, M., Lazzaro, A., and Zeyer, J. (2015). Bacterial composition and survival on Sahara dust particles transported to the European Alps. Front. Microbiol. 6:1454. doi: 10.3389/fmicb.2015.01454
Mesbah, N. M., Hedrick, D. B., Peacock, A. D., Rohde, M., and Wiegel, J. (2007). Natranaerobius thermophilus gen. nov., sp. nov., a halophilic, alkalithermophilic bacterium from soda lakes of the Wadi An Natrun, Egypt, and proposal of Natranaerobiaceae fam. nov. and Natranaerobiales ord. nov. Int. J. Syst. Evol. Microbiol. 57, 2507–2512. doi: 10.1099/ijs.0.65068-0
Milewski, R., Chabrillat, S., and Behling, R. (2017). Analyses of recent sediment surface dynamic of a Namibian Kalahari salt pan based on multitemporal landsat and hyperspectral hyperion data. Remote Sens. Basel 9:170. doi: 10.3390/Rs9020170
Mitzscherling, J., Winkel, M., Winterfeld, M., Horn, F., Yang, S. Z., Grigoriev, M. N., et al. (2017). The development of permafrost bacterial communities under submarine conditions. J. Geophys. Res. Biogeo. 122, 1689–1704. doi: 10.1002/2017JG003859
Montoya, L., Vizioli, C., Rodriguez, N., Rastoll, M. J., Amils, R., and Marin, I. (2013). Microbial community composition of Tirez lagoon (Spain), a highly sulfated athalassohaline environment. Aquat. Biosyst. 9:19. doi: 10.1186/2046-9063-9-19
Müller, K. D., Husmann, H., and Nalik, H. P. (1990). A new and rapid method for the assay of bacterial fatty-acids using high-resolution capillary gas-chromatography and trimethylsulfonium hydroxide. Zentralbl. Bakteriol. 274, 174–182. doi: 10.1016/S0934-8840(11)80100-3
Nadkarni, M. A., Martin, F. E., Jacques, N. A., and Hunter, N. (2002). Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148, 257–266. doi: 10.1099/00221287-148-1-257
Nigro, L. M., Hyde, A. S., MacGregor, B. J., and Teske, A. (2016). Phylogeography, salinity adaptations and metabolic potential of the candidate division KB1 bacteria based on a partial single cell genome. Front. Microbiol. 7:1266. doi: 10.3389/fmicb.2016.01266
Noah, M., Lappé, M., Schneider, B., Vieth-Hillebrand, A., Wilkes, H., and Kallmeyer, J. (2014). Tracing biogeochemical and microbial variability over a complete oil sand mining and recultivation process. Sci. Total Environ. 499, 297–310. doi: 10.1016/j.scitotenv.2014.08.020
Oren, A. (2002). Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J. Ind. Microbiol. Biotechnol. 28, 56–63. doi: 10.1038/sj/jim/7000176
Oren, A. (2008). Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Systems 4:2. doi: 10.1186/1746-1448-4-2
Oren, A. (2011). Thermodynamic limits to microbial life at high salt concentrations. Environ. Microbiol. 13, 1908–1923. doi: 10.1111/j.1462-2920.2010.02365.x
Oren, A., Sørensen, K. B., Canfield, D. E., Teske, A. P., Ionescu, D., Lipski, A., et al. (2009). Microbial communities and processes within a hypersaline gypsum crust in a saltern evaporation pond (Eilat, Israel). Hydrobiologia 626, 15–26. doi: 10.1007/s10750-009-9734-8
Pandit, A. S., Joshi, M. N., Bhargava, P., Ayachit, G. N., Shaikh, I. M., Saiyed, Z. M., et al. (2014). Metagenomes from the saline desert of kutch. Genome Announc. 2:e00439-14. doi: 10.1128/genomeA.00439-14
Paul, D., Kumbhare, S. V., Mhatre, S. S., Chowdhury, S. P., Shetty, S. A., Marathe, N. P., et al. (2015). Exploration of microbial diversity and community structure of lonar lake: the only hypersaline meteorite crater lake within basalt rock. Front. Microbiol. 6:1553. doi: 10.3389/fmicb.2015.01553
Pointing, S. B., and Belnap, J. (2012). Microbial colonization and controls in dryland systems. Nat. Rev. Microbiol. 10, 551–562. doi: 10.1038/nrmicro2831
Prestel, E., Salamitou, S., and DuBow, M. S. (2008). An examination of the bacteriophages and bacteria of the Namib desert. J. Microbiol. 46, 364–372. doi: 10.1007/s12275-008-0007-4
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Quideau, S. A., McIntosh, A. C., Norris, C. E., Lloret, E., Swallow, M. J., and Hannam, K. (2016). Extraction and analysis of microbial phospholipid fatty acids in soils. J. Vis. Exp. 114:e54360. doi: 10.3791/54360
Ragsdale, S. W., and Pierce, E. (2008). Acetogenesis and the wood-ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 1784, 1873–1898. doi: 10.1016/j.bbapap.2008.08.012
Ramette, A. (2007). Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 62, 142–160. doi: 10.1111/j.1574-6941.2007.00375.x
Ren, P. G., and Zhou, P. J. (2005). Tenuibacillus multivorans gen. nov., sp. nov., a moderately halophilic bacterium isolated from saline soil in Xin-Jiang, China. Int. J. Syst. Evol. Microbiol. 55(Pt 1), 95–99. doi: 10.1099/ijs.0.63180-0
Reynolds, J. F., Smith, D. M., Lambin, E. F., Turner, B. L. II, Mortimore, M., Batterbury, S. P., et al. (2007). Global desertification: building a science for dryland development. Science 316, 847–851. doi: 10.1126/science.1131634
Rhodes, C. J. (2012). Feeding and healing the world: through regenerative agriculture and permaculture. Sci. Prog. 95(Pt 4), 345–446. doi: 10.3184/003685012X13504990668392
Rinke, C., Schwientek, P., Sczyrba, A., Ivanova, N. N., Anderson, I. J., Cheng, J. F., et al. (2013). Insights into the phylogeny and coding potential of microbial dark matter. Nature 499, 431–437. doi: 10.1038/nature12352
Ronca, S., Ramond, J.-B., Jones, B. E., Seely, M., and Cowan, D. A. (2015). Namib desert dune/interdune transects exhibit habitat-specific edaphic bacterial communities. Front. Microbiol. 6:845. doi: 10.3389/fmicb.2015.00845
Schüller, I., Belz, L., Wilkes, H., and Wehrmann, A. (2018). Late quaternary shift in southern African rainfall zones: sedimentary and geochemical data from Kalahari pans. Z. Geomorphol. 61, 339–362. doi: 10.1127/zfg/2018/0556
Schulz, H. D., and Zabel, M. (2013). Marine Geochemistry. Berlin: Springer Science & Business Media.
Schulze-Makuch, D., Wagner, D., Kounaves, S. P., Mangelsdorf, K., Devine, K. G., de Vera, J. P., et al. (2018). Transitory microbial habitat in the hyperarid Atacama Desert. Proc. Natl. Acad. Sci. U.S.A. 115, 2670–2675. doi: 10.1073/pnas.1714341115
Shade, A., and Handelsman, J. (2012). Beyond the venn diagram: the hunt for a core microbiome. Environ. Microbiol. 14, 4–12. doi: 10.1111/j.1462-2920.2011.02585.x
Shaw, P. A., and Bryant, R. G. (2011). “Pans, playas and salt lakes,” in Arid Zone Geomorphology, ed. D. S. G. Thomas (Hoboken, NJ: John Wiley & Sons, Ltd.), 373–401. doi: 10.1002/9780470710777.ch15
Singh, S. P., Raval, V. H., Purohit, M. K., Thumar, J. T., Gohel, S. D., Pandey, S., et al. (2012). “Haloalkaliphilic bacteria and actinobacteria from the saline habitats: new opportunities for biocatalysis and bioremediation,” in Microorganisms in Environmental Management: Microbes and Environment, eds T. Satyanarayana and B. N. Johri (Dordrecht: Springer Netherlands), 415–429.
Slade, D., and Radman, M. (2011). Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 75, 133–191. doi: 10.1128/MMBR.00015-10
Sørensen, K. B., Canfield, D. E., Teske, A. P., and Oren, A. (2005). Community composition of a hypersaline endoevaporitic microbial mat. Appl. Environ. Microbiol. 71, 7352–7365. doi: 10.1128/AEM.71.11.7352-7365.2005
Sorokin, D. Y., van Pelt, S., Tourova, T. P., and Evtushenko, L. I. (2009). Nitriliruptor alkaliphilus gen. nov., sp. nov., a deep-lineage haloalkaliphilic actinobacterium from soda lakes capable of growth on aliphatic nitriles, and proposal of Nitriliruptoraceae fam. nov. and Nitriliruptorales ord. nov. Int. J. Syst. Evol. Microbiol. 59(Pt 2), 248–253. doi: 10.1099/ijs.0.002204-0
Stapel, J. G., Schirrmeister, L., Overduin, P. P., Wetterich, S., Strauss, J., Horsfield, B., et al. (2016). Microbial lipid signatures and substrate potential of organic matter in permafrost deposits: implications for future greenhouse gas production. J. Geophys. Res. Biogeosci. 121, 2652–2666. doi: 10.1002/2016JG003483
Takami, H., Noguchi, H., Takaki, Y., Uchiyama, I., Toyoda, A., Nishi, S., et al. (2012). A deeply branching thermophilic bacterium with an ancient acetyl-CoA pathway dominates a subsurface ecosystem. PLoS One 7:e30559. doi: 10.1371/journal.pone.0030559
Theodorakopoulos, N., Bachar, D., Christen, R., Alain, K., and Chapon, V. (2013). Exploration of deinococcus-thermus molecular diversity by novel group-specific PCR primers. Microbiologyopen 2, 862–872. doi: 10.1002/mbo3.119
Valenzuela-Encinas, C., Neria-González, I., Alcántara-Hernández, R. J., Estrada-Alvarado, I., Zavala-Díaz de la Serna, F. J., Dendooven, L., et al. (2009). Changes in the bacterial populations of the highly alkaline saline soil of the former lake Texcoco (Mexico) following flooding. Extremophiles 13, 609–621. doi: 10.1007/s00792-009-0244-4
Ventosa, A., Oren, A., and Ma, Y. (2011). Halophiles and Hypersaline Environments: Current Research and Future Trends. Heidelberg: Springer-Verlag Berlin and Heidelberg.
Vieth, A., Mangelsdorf, K., Sykes, R., and Horsfield, B. (2008). Water extraction of coals - potential for estimating low molecular weight organic acids as carbon feedstock for the deep terrestrial biosphere. Org. Geochem. 39, 985–991. doi: 10.1016/j.orggeochem.2008.02.012
Weigold, P., Ruecker, A., Loesekann-Behrens, T., Kappler, A., and Behrens, S. (2016). Ribosomal tag pyrosequencing of DNA and RNA reveals “rare” taxa with high protein synthesis potential in the sediment of a hypersaline lake in western Australia. Geomicrobiol. J. 33, 426–440. doi: 10.1080/01490451.2015.1049304
Wiegel, J., and Michael, A. W. W. (2002). Thermophiles: The Keys to the Molecular Evolution and the Origin of Life? London: Taylor & Francis.
Youssef, N. H., Ashlock-Savage, K. N., and Elshahed, M. S. (2012). Phylogenetic diversities and community structure of members of the extremely halophilic Archaea (order Halobacteriales) in multiple saline sediment habitats. Appl. Environ. Microbiol. 78, 1332–1344. doi: 10.1128/AEM.07420-11
Zelles, L. (1999). Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol. Fertil. Soils 29, 111–129. doi: 10.1007/s003740050533
Zhang, H., Sekiguchi, Y., Hanada, S., Hugenholtz, P., Kim, H., Kamagata, Y., et al. (2003). Gemmatimonas aurantiaca gen. nov., sp. nov., a gram-negative, aerobic, polyphosphate-accumulating micro-organism, the first cultured representative of the new bacterial phylum Gemmatimonadetes phyl. nov. Int. J. Syst. Evol. Microbiol. 53(Pt 4), 1155–1163. doi: 10.1099/ijs.0.02520-0
Zhang, J., Kobert, K., Flouri, T., and Stamatakis, A. (2014). PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30, 614–620. doi: 10.1093/bioinformatics/btt593
Keywords: saline pan, Kalahari, Halobacteria, Gemmatimonadetes, Firmicutes
Citation: Genderjahn S, Alawi M, Mangelsdorf K, Horn F and Wagner D (2018) Desiccation- and Saline-Tolerant Bacteria and Archaea in Kalahari Pan Sediments. Front. Microbiol. 9:2082. doi: 10.3389/fmicb.2018.02082
Received: 29 March 2018; Accepted: 14 August 2018;
Published: 20 September 2018.
Edited by:
Jesse G. Dillon, California State University, Long Beach, United StatesReviewed by:
Carl-Eric Wegner, Friedrich-Schiller-Universität Jena, GermanyAharon Oren, Hebrew University of Jerusalem, Israel
Copyright © 2018 Genderjahn, Alawi, Mangelsdorf, Horn and Wagner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steffi Genderjahn, c2dlbmRlckBnZnotcG90c2RhbS5kZQ==
 Steffi Genderjahn
Steffi Genderjahn Mashal Alawi
Mashal Alawi Kai Mangelsdorf2
Kai Mangelsdorf2 Fabian Horn
Fabian Horn Dirk Wagner
Dirk Wagner